Abstract
Pyrazinamide, the first-line antitubercular drug, has been regarded the basic component of tuberculosis treatment for over sixty years. Researchers have investigated its effect on Mycobacterium tuberculosis for this long time, and as a result, new potential targets of pyrazinamide or its active form, pyrazinoic acid, have been found. We have designed and prepared 3-(phenyl-carbamoyl)pyrazine-2-carboxylic acids as more lipophilic derivatives of pyrazinoic acid. We also prepared methyl and propyl derivatives as prodrugs with further increased lipophilicity. Antimycobacterial, antibacterial and antifungal growth inhibiting activity was investigated in all prepared compounds. 3-[(4-Nitrophenyl)carbamoyl]pyrazine-2-carboxylic acid (16) exerted high antimycobacterial activity against Mycobacterium tuberculosis H37Rv with MIC = 1.56 μg·mL−1 (5 μM). Propyl 3-{[4-(trifluoromethyl)phenyl]carbamoyl}pyrazine-2-carboxylate (18a) showed also high antimycobacterial activity against Mycobacterium tuberculosis H37Rv with MIC = 3.13 μg·mL−1. In vitro cytotoxicity of the active compounds was investigated and no significant cytotoxic effect was observed. Based to structural similarity to known inhibitors of decaprenylphosphoryl-β-d-ribose oxidase, DprE1, we performed molecular docking of the prepared acids to DprE1. These in silico experiments indicate that modification of the linker connecting aromatic parts of molecule does not have any negative influence on the binding.
Keywords: anilides, antimycobacterial activity, cytotoxicity, DprE1, pyrazinamide, pyrazinoic acid, RpsA
1. Introduction
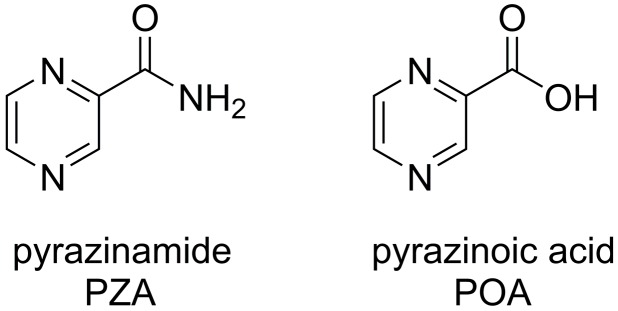
Pyrazinamide (PZA, Figure 1) is one of the fist-line drugs used in the treatment of tuberculosis (TB). Due to its unique sterilizing effect on semi-dormant tubercle bacilli and ability to shorten the duration of therapy, PZA has been used for over sixty years in combination with other antituberculotics [1,2,3]. Although PZA has been applied for such a long time, the mechanism of action is still not yet completely understood. PZA is known to be a prodrug that is converted to its active form, pyrazinoic acid (POA), by the enzyme pyrazinamidase/nicotinamidase inside the mycobacterial cell [4].
Figure 1.
Structures of pyrazinamide and its active form, pyrazinoic acid.
Its non-specific mechanism of action is based on a cyclic expulsion of POA anion to the extracellular space, protonation and penetration of the uncharged POA back to the cell. This cycle results in proton accumulation and potentially to cytoplasmic acidification along with the possible collapse of membrane potential and membrane transport [5,6]. With respect to previous observations and the pKa of POA, an acidic pH is considered important for the PZA/POA activity. However, recent experiments by Peterson et al. [7] disputed the acidification of cytoplasm as a mechanism of action of PZA and POA and showed that the acidification of intracellular compartment required PZA/POA concentrations at least 1–2 orders higher than minimum inhibitory concentrations (MICs). Specific targets of PZA/POA were suggested as Fatty Acid Synthase (FAS) I (involved in mycolic acid biosynthesis) [8], aspartate decarboxylase (biosynthetic pathway of coenzyme A) [9,10] and ribosomal protein S1 (RpsA, important for trans-translation) [11].
RpsA, a 30S ribosomal protein S1, is essential for translation and is involved in trans-translation, which is the process of rescuing ribosomes stalled in the translation of mRNA [12]. Shi et al. [11] proposed that POA binds directly to the C-terminus region and disrupts the formation of RpsA-tmRNA complex. Experiments of Yang et al. [13] have pointed to the fourth S1 domain of RpsA as the binding site for POA, where the surface is supposed to interact with tmRNA [13,14].
POA itself cannot penetrate through the lipid-rich mycobacterial cell wall easily due to its low lipophilicity and significant acidity (pKa = 2.9) [5] According to the performed experiments, Yang et al. [13] suggested a substitution with any lipophilic moiety in the position 5 or 6 on the pyrazine ring. This hypothesis is based on the interactions observed in a co-crystallized complex of POA-RpsA, where the substituents at C-5 and C-6 of the pyrazine ring seem to be less critical for POA binding to S1 domain.
Our research group has focused on the synthesis of N-phenylpyrazine-2-carboxamides for several years. Many of these compounds exerted antimycobacterial effect comparable to PZA (MIC = 6.25–12.5 μg·mL−1, Šula´s medium, pH = 5.6 [15,16,17]). Considering the previous results of N-phenylcarboxamides (anilides), we decided to choose the phenylcarbamoyl moiety as a lipophilic substituent for the synthesis of more lipophilic POA derivatives as Yang et al. have suggested. We intend to prepare derivatives substituted in positions 5, 6 and even 3 to study the influence of positional isomerism. First, we decided to prepare substituted derivatives of POA with phenylcarbamoyl substituent in position 3.
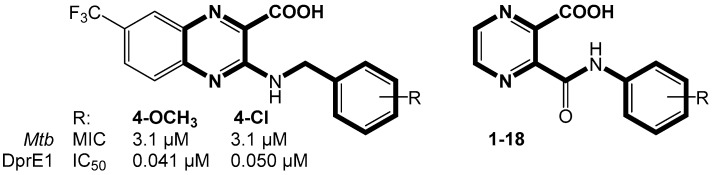
We thus prepared a series of 18 substituted POA derivatives and also 17 propyl esters and 12 methyl esters from the synthesized acids to increase their lipophilicity and possibly enhance the penetration through the lipid-rich mycobacterial cell wall. After synthesis of these derivatives, we went through literature and found an article of Neres et al. [18] describing 2-carboxyquinoxalines as inhibitors of mycobacterial decaprenylphosphoryl-β-d-ribose oxidase (DprE1). DprE1 is an essential enzyme involved in the biosynthesis of arabinogalactan, a basic component of mycobacterial cell wall [19,20]. Nitrobenzothiazinones have become the first effective inhibitors of DprE1 [21]. Since then, the modification and design of new potential inhibitors continue. Discovered inhibitors belong to many structural classes, namely azaindoles [22], 4-aminoquinolone piperidine amides [23], pyrazolopyridones [24], 8-pyrrolobenzothiazinones [25], benzothiazolylpyrimidine-5-carboxamides [26], and 2-carboxyquinoxalines [18], among others.
Although the 3-substituted derivatives may fail to bind to the RpsA for the reasons mentioned above, their structural similarity to known inhibitors of DprE1 (Figure 2) led us to perform a molecular docking to DprE1. We intended to study the effect of structural differences between reported inhibitors and our compounds, that is, the absence of the second condensed ring and alteration of -NH-CH2- linker.
Figure 2.
Comparison of the basic scaffold of previously prepared DprE1 inhibitors and structures presented in this paper.
2. Results and Discussion
2.1. Chemistry
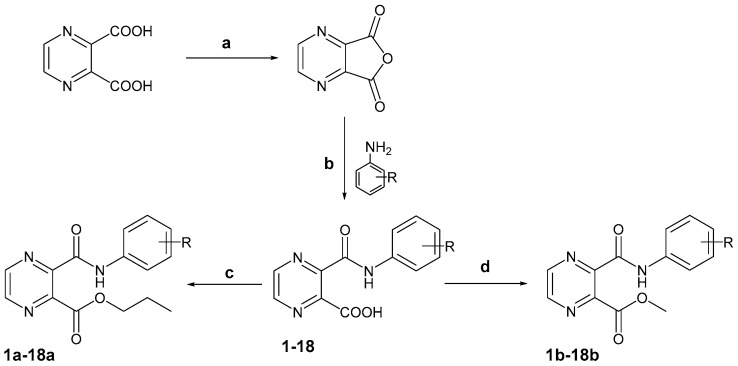
In this project 18 compounds 1–18 from a series of substituted 3-(phenylcarbamoyl)pyrazine-2-carboxylic acids, 17 related propyl esters 1a–18a and 12 related methyl esters 1b, 4b–6b, 8b, 11b–15b and 17b, 18b were prepared. Compounds 1, 7, 9, 11, 16 and 18 were published previously as semi-products—see Section 3.2.3 for references.
The majority of compounds from the acid series were prepared starting from commercially available pyrazine-2,3-dicarboxylic anhydride and the synthetic procedure was performed according to [27] with some modifications, see Scheme 1. Several compounds were prepared using pyrazine-2,3-dicarboxylic acid as a starting compound. Pyrazine-2,3-dicarboxylic acid was treated in acetic acid anhydride at reflux to convert to pyrazine-2,3-dicarboxylic anhydride; the procedure was modified on the basis of the same literature. The analytical characteristics of pyrazine-2,3-dicarboxylic anhydride were in accordance with published literature data. Subsequently the anhydride was dissolved in tetrahydrofuran and a corresponding substituted aniline was added. The reaction mixture was stirred for 1 h at RT. Afterwards water was poured in and a saturated aqueous solution of NaHCO3 was added dropwise to pH 6 in order to form crystals of the desired substituted 3-(phenylcarbamoyl)pyrazine-2-carboxylic acids 1–18 in 58–98% yield. Propyl esters 1a–18a were prepared via esterification in propanol with a catalytic amount of H2SO4 in 35–85% yield. Methyl esters 1b–18b of several of the acids (1, 4–6, 8, 11–15 and 17, 18) were also prepared using the same procedure as propyl esters with methanol instead as the solvent in 52–76% yield. The esterification was carried out under microwave irradiation to accelerate the progress of the reaction. The reaction was performed under mild conditions in a microwave reactor to facilitate the forming of the ester bond and to prevent a decomposition of the carboxylic moiety. Despite the mild conditions and an effort to avoid decarboxylation, we have not been successful in the synthesis of propyl ester of compound 16 (the presence of the decarboxylation product was confirmed by NMR). Subsequently, synthesis of the ester 16a was attempted by conversion of the acid 16 to its acyl chloride by conventional addition of SOCl2, followed by alcoholysis by propanol in the presence of pyridine as a base. However, this was not successful either, due to the low yields.
Scheme 1.
Synthetic procedures of prepared series. Reagents and Conditions: (a) acetic anhydride, reflux, 1 h; (b) 1. tetrahydrofuran, RT, 1 h, 2. water, NaHCO3 sol.; (c) propanol, H2SO4, MW: 120 °C, 20 min, 50 W; (d) methanol, H2SO4, MW: 120 °C, 20 min, 50 W.
All compounds were characterized by 1H- and 13C-NMR, IR, elemental analysis, and melting point. The 1H-NMR spectra showed the proton peaks of the carboxylic groups in the range of 13.91–13.73 ppm. In case of some acids, this peak was not visible due to exchange with D2O. Peaks for the pyrazine hydrogens were in the range of 8.99–8.86 in DMSO-d6 and 8.82–8.68 in CDCl3. They were detected as one peak or as two peaks with coupling constant J ranging between 2.2–2.7 Hz, which is in accordance with the literature [28]. The IR spectroscopy confirmed the presence of the expected characteristic functional groups. The C=O stretching bands were located at 1697–1611 cm−1 for amidic moiety, 1757–1701 cm−1 for carboxylic acids, and 1748–1727 cm−1 for esters. Amidic N-H stretching bands were at 3379–3106 cm−1 and carboxylic O-H stretching at 3187–2584 cm−1. Esters showed an alkyl C-H stretching in the range of 2983–2919 cm−1.
The stability of the prepared esters in DMSO solution was studied. Samples were kept in the refrigerator (7 °C) for one month and subsequently the stability was verified by TLC (mobile phase: propanol/30% aq. sol. of ammonia 3:1) in comparison with the original acid. The temperature used for stability validation was based on storage conditions of dissolved samples before biological testing. No presence of the original acid was observed in the studied samples.
2.2. Lipophilicity
Lipophilicity is an important physico-chemical property of drugs or biologically active compounds. This property determines the penetration through biological membranes by passive diffusion. A successful biological effect depends on an appropriate ratio between the hydrophilic and hydrophobic properties of the substance. This aspect is very important especially for antitubercular drugs due to the presence of the lipid-rich mycobacterial wall. Log P values, mentioned in Table 1, were calculated by ChemBioDraw Ultra 14.0. The log P value of POA is −0.66. The average increase of lipophilicity of acids 1–18, compared to POA, was 1.59 ± 0.58 (n = 18). Further esterification of 1–18 increased log P by 0.28 ± 0.06 (n = 12) in the case of methyl esters (1b, 4b–6b, 8b, 11b–15b, 17b, 18b) and 1.09 ± 0.03 (n = 17) for propyl esters 1a–18a.
Table 1.
Prepared compounds with lipophilicity parameter log P and antimycobacterial activity against Mtb in μg·mL−1 and in μM in brackets.
| R | Cpd. | Log P | MIC Mtb (μg·mL−1) | Cpd. | Log P | MIC Mtb (μg·mL−1) | Cpd. | Log P | MIC Mtb (μg·mL−1) |
|---|---|---|---|---|---|---|---|---|---|
| H | 1 | 0.57 | >100 | 1a | 1.66 | >100 | 1b | 0.83 | >100 |
| 2-OH | 2 | 0.18 | 50 (193) | 2a | 1.27 | 50 (160) | - | - | - |
| 4-OCH3 | 3 | 0.44 | >100 | 3a | 1.53 | >100 | - | - | - |
| 2,4-diOCH3 | 4 | 0.32 | >100 | 4a | 1.40 | >100 | 4b | 0.58 | >100 |
| 2,5-diCH3 | 5 | 1.54 | >100 | 5a | 2.63 | >100 | 5b | 1.81 | >100 |
| 4-CH2CH3 | 6 | 1.47 | >100 | 6a | 2.56 | >100 | 6b | 1.74 | >100 |
| 4-F | 7 | 0.73 | >100 | 7a | 1.81 | >100 | - | - | - |
| 2,4-diF | 8 | 0.89 | >100 | 8a | 1.97 | >100 | 8b | 1.15 | >100 |
| 4-Cl | 9 | 1.13 | >100 | 9a | 2.21 | >100 | - | - | - |
| 3,4-diCl | 10 | 1.69 | 100 | 10a | 2.77 | >100 | - | - | - |
| 4-Br | 11 | 1.40 | 50 (155) | 11a | 2.49 | >100 | 11b | 1.66 | >100 |
| 5-F-2-CH3 | 12 | 1.21 | >100 | 12a | 2.30 | >100 | 12b | 1.48 | >100 |
| 2-Cl-5-CH3 | 13 | 1.61 | >100 | 13a | 2.70 | >100 | 13b | 1.88 | >100 |
| 5-Cl-2-OH | 14 | 0.74 | 100 | 14a | 1.83 | >100 | 14b | 1.00 | >100 |
| 2-OH-5-NO2 | 15 | −0.26 | >100 | 15a | 0.96 | >100 | 15b | 0.21 | >100 |
| 4-NO2 | 16 | 0.13 | 1.56 (5) | - | - | - | - | - | - |
| 3-CF3 | 17 | 1.49 | 100 | 17a | 2.58 | >100 | 17b | 1.75 | >100 |
| 4-CF3 | 18 | 1.49 | >100 | 18a | 2.58 | 3.13 (10) | 18b | 1.75 | >100 |
| - | PZA | −1.31 | 100 | POA | −0.66 | 100 | INH | −0.64 | 0.1–0.2 (0.7–1.5) |
INH—isoniazid; PZA—pyrazinamide.
2.3. Antimycobacterial Evaluation
Antimycobacterial in vitro screening was performed on four mycobacterial strains—Mtb, M. avium, M. kansasii and fast growing M. smegmatis—using a Microplate Alamar Blue Assay (MABA; see the Supplementary Material for the experimental details). The antimycobacterial activity results were expressed as the minimum inhibitory concentration (MIC) in μg·mL−1. Three standards were used—POA, PZA and isoniazid (INH).
Only six compounds from 1–18 exerted certain antimycobacterial activity against Mtb, the results are presented in Table 1. Compounds 10, 14 and 17 showed activity comparable with PZA and POA (MIC = 100 μg·mL−1). The antimycobacterial activity of PZA and POA is dependent on the conditions of biological assays; the activity increases with a decrease of the environmental pH [29,30]. For example, in acidic pH = 5.6 PZA exerts MIC of 12.5–25 µg·mL−1 [16] yet is only weakly active at pH = 6 with MIC = 200 µg·mL−1 [30]. Compounds 2 and 11 showed moderate activity with MIC = 50 μg·mL−1. The most active compound from the series of acids was compound 16 with 4-NO2 substitution on phenyl ring with MIC = 1.56 μg·mL−1 (5 μM). Although this activity can be considered as high, it does not achieve the antimycobacterial activity of isoniazid (MIC = 0.1–0.2 μg·mL−1; 0.7–1.5 μM).
In general, the increase of lipophilicity via conversion of the acids to their propyl or methyl esters did not cause any significant improvement in antimycobacterial activity against Mtb. The only exception was compound 18a, which displayed activity with MIC = 3.13 μg·mL−1 (10 μM). Compound 2 with 2-OH substitution kept its antimycobacterial effect after conversion to the propyl ester 2a (MIC = 50 μg·mL−1). Methyl esters did not exert any antimycobacterial activity at the tested concentrations. None of the presented compounds proved any significant activity against M. kansasii, M. avium and M. smegmatis.
2.4. Antibacterial and Antifungal Evaluation
All prepared compounds were tested for antibacterial and antifungal activity using the microdilution broth method. These biological assays were performed on eight bacterial and eight fungal strains of clinical importance. See the Supplementary Material for the experimental details. None of compounds 1–18 displayed any antibacterial or antifungal activity up to the highest tested concentration of 500 µM. Propyl esters 1a–18a did not show any antifungal and antibacterial activity, except for compound 11a. This compound had low antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa with MIC = 250 µM. Only one compound among the methyl esters, 8b, showed antibacterial activity against Staphylococcus aureus with MIC = 250 µM, and the rest of the methyl esters were inactive against the tested bacterial and fungal strains.
2.5. Cytotoxicity
The active compounds 16 and 18a were studied for their in vitro cytotoxic effect in the HepG2 cell line. The results of the experiments are presented as the inhibitory concentration that reduces the viability of the cell population to 50% of the maximal viability, IC50. None of the tested compounds (16: 4-NO2; 18a: 4-CF3) exerted significant cytotoxic effect. Compound 16 showed IC50 > 750 µM (precipitation at higher concentration) and ester 18a showed IC50 = 252.4 µM. The values of selectivity index (SI = IC50 / MIC) related to Mtb were SI > 150 for compound 16 and for 18a SI = 25.24.
2.6. In Silico Docking Study
We performed molecular docking studies on DrpE1 of Mtb H37Rv. DprE1 was chosen as a potential new target involved in mycobacterial cell wall synthesis. We studied only acids 1–18, as the methyl and propyl esters are considered as prodrugs which are hydrolyzed in mycobacterium. Molecular Operating Environment (MOE) 2016.08 (Chemical Computing Group, Montreal, QC, Canada) was used to conduct the in silico study. To verify the docking procedure, the originally co-crystalized ligand (quinoxaline) was removed and redocked again with RMSD = 0.24 Å.
PDB structure 4P8N (chain A) was chosen for in silico study of DprE1. The predicted poses of individual ligands 1–18 were evaluated with regard to the ligand-receptor interactions and position of original ligand. Eight ligands (3, 4, 6, 9, 10, 11, 16, 18) combining the best docking score, similarity in interactions to the original ligand, and overlapping with the original ligand were considered as the best candidates for DprE1 inhibition. In comparison to the original structure of 2-carboxyquinoxalines, the replacement of -NH-CH2- linker by -CONH- group does not radically change the character of binding mode. On the other hand, the loss of the condensed ring along with large CF3 substituent seems to decrease the antimycobacterial activity. Probably the large lipophilic substituent is needed for the filling of the hydrophobic binding sub-pocket and will be considered for further investigation. The interactions and binding scores are described and depicted in Supplementary Material.
3. Experimental
3.1. General Information
All chemicals were of reagent or higher grade of purity and were purchased from Sigma-Aldrich (Steinheim, Germany). The progress of reactions was monitored by Thin Layer Chromatography (TLC; TLC Silica gel 60 F254, Merck, Darmstadt, Germany) with UV detection using a wavelength of 254 nm. Microwave-assisted reactions were performed in a CEM Discover microwave reactor with a focused field (CEM Corporation, Matthews, NC, USA) connected to an Explorer 24 autosampler (CEM Corporation) and this equipment was run under CEM’s Synergy™ software (version 1.38) for setting and monitoring the reaction conditions. The temperature of reactions was monitored by an internal infrared sensor. Propyl esters and methyl esters were purified by preparative flash chromatograph CombiFlash® Rf (Teledyne Isco Inc., Lincoln, NE, USA). The type of elution was gradient, using the mixture of hexane (LachNer, Neratovice, Czech Republic) and ethyl acetate (Penta, Prague, Czech Republic) as mobile phase. Silica gel (0.040–0.063 nm, Merck) was used as the stationary phase. NMR spectra were taken in DMSO-d6 with a Varian VNMR S500 spectrometer (499.87 MHz for 1H and 125.71 MHz for 13C; Varian Corporation, Palo Alto, CA, USA). Chemical shifts were reported in ppm (δ) and were referred indirectly to tetramethylsilane. Infrared spectra were recorded with a FT-IR Nicolet 6700 spectrometer (Thermo Scientific, Waltham, MA, USA) using attenuated total reflectance (ATR) on Ge crystal. Elemental analyses were measured using Micro Cube Elemental Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). Melting points were assessed by SMP3 Stuart Scientific (Bibby Sterling Ltd., Staffordshire, UK) in an open capillary and are uncorrected. Lipophilicity parameter log P was calculated by software CS ChemBioDraw Ultra 14.0 (CambridgeSoft, Cambridge, MA, USA).
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of Acids 1–18
Pyrazine-2,3-dicarboxylic acid (4.0 g, 23.8 mmol) was dissolved in acetic anhydride (30 mL). The reaction mixture was refluxed for one hour, and subsequently cooled down to 0 °C in ice bath. The obtained crystals of pyrazine-2,3-dicarboxylic anhydride were filtered off (yield 70%).
Pyrazine-2,3-dicarboxylic anhydride (1.0 g, 6.7 mmol) was dissolved in tetrahydrofuran (40 mL) in an Erlenmeyer flask and the corresponding substituted aniline (6.7 mmol, 1 equiv.) was added in one dose. The reaction mixture was stirred for 1 hour at laboratory temperature. Water (30 mL) was added into the mixture followed by the saturated aqueous solution of NaHCO3 until pH 6 to form the corresponding 3-(phenylcarbamoyl)pyrazine-2-carboxylic acid 1–18. Obtained crystals were filtered off and washed with water. The progress of the procedure was monitored by TLC eluted with the system water/butanol/acetic acid 5:4:1.
3.2.2. General Procedure for the Synthesis of Propyl and Methyl Esters
Propyl esters 1a–15a, 17a and 18a were prepared using microwave irradiation. Substituted acid 1–18 (300 mg), propanol (3 mL) and one drop of H2SO4 were put to a thick-wall vial for the microwave reactions. Conditions for the synthesis were 120 °C, 20 min, and 50 W in the pressurized vial. The progress of the reactions was monitored by TLC in system propanol/30% aq. sol. of ammonia 3:1. The reaction mixture was adsorbed on silica and purified by flash chromatography using gradient elution with ethyl-acetate (50–90%) in hexane. Methyl esters 1b, 4b–6b, 8b, 11b–15b and 17b, 18b were prepared using the same procedure as propyl esters using methanol instead as the solvent.
3.2.3. Analytical Data of Prepared Compounds
3-(Phenylcarbamoyl)pyrazine-2-carboxylic acid (1) [31]. Pale yellow solid. Yield 91%; m.p. 163.1–164.6 °C; IR (cm−1): 3341 (N-H, CONH), 3051 (O-H, COOH), 1712 (C=O, COOH), 1682 (C=O, CONH); 1H-NMR δ 13.77 (bs, 1H, COOH), 10.75 (s, 1H, NH), 8.92–8.86 (m, 2H, pyr.), 7.80–7.75 (m, 2H, Ar), 7.40–7.34 (m, 2H, Ar), 7.16–7.11 (m, 1H, Ar); 13C-NMR δ 166.45, 162.61, 146.40, 145.85, 145.80, 144.69, 138.57, 128.98, 124.39, 120.25; Elemental analysis: calc. for C12H9N3O3 (MW 243.22): 59.26% C, 3.73% H, 17.28% N; found 59.34% C, 3.61% H, 17.24% N.
3-[(2-Hydroxyphenyl)carbamoyl]pyrazine-2-carboxylic acid (2). Yellow solid. Yield 95%; m.p. 266.4–269.3 °C; IR (cm−1): 3278 (O-H, OH), 3106 (N-H, CONH), 2854 (O-H, COOH), 1713 (C=O, COOH), 1611 (C=O, CONH); 1H-NMR δ 13.74 (bs, 1H, COOH), 10.31 (s, 1H, OH), 10.15 (s, 1H, NH), 8.93 (d, J = 2.5 Hz, 1H, pyr.), 8.90 (d, J = 2.5 Hz, 1H, pyr.), 8.24–8.20 (m, 1H, Ar), 7.03–6.93 (m, 2H, Ar), 6.88–6.83 (m, 1H, Ar); 13C-NMR δ 166.92, 160.10, 147.86, 147.12, 146.85, 144.27, 141.64, 125.81, 125.03, 119.93, 119.48, 115.13; Elemental analysis: calc. for C12H9N3O4 (MW 259.22): 55.60% C, 3.50% H, 16.21% N; found 55.77% C, 3.39% H, 16.07% N.
3-[(4-Methoxyphenyl)carbamoyl]pyrazine-2-carboxylic acid (3). Yellow solid. Yield 98%; m.p. 167.1–168.0 °C; IR (cm−1): 3330 (N-H, CONH), 3169 (O-H, COOH), 1757 (C=O, COOH), 1665 (C=O, CONH), 1243 (C-O, OCH3); 1H-NMR δ 13.74 (bs, 1H, COOH), 10.64 (s, 1H, CONH), 8.89–8.86 (m, 2H, pyr.), 7.73–7.68 (m, 2H, Ar), 6.96–6.92 (m, 2H, Ar), 3.74 (s, 3H, CH3); 13C-NMR δ 166.56, 161.97, 156.06, 146.67, 145.76, 145.47, 144.51, 131.65, 121.79, 114.08, 55.40; Elemental analysis: calc. for C13H11N3O4 (MW 273.25): 57.14% C, 4.06% H, 15.38% N; found 56.68% C, 3.93% H, 15.13% N.
3-[(2,4-Dimethoxyphenyl)carbamoyl]pyrazine-2-carboxylic acid (4). Yellow solid. Yield 85%; m.p. 199.6–200.3 °C; IR (cm−1): 3309 (N-H, CONH), 3129 (O-H, COOH), 1741 (C=O, COOH), 1667 (C=O, CONH); 1H-NMR δ 13.73 (bs, 1H, COOH), 9.95 (s, 1H, NH), 8.91 (d, J = 2.4 Hz, 1H, pyr.), 8.87 (d, J = 2.4 Hz, 1H, pyr.), 8.11 (d, J = 8.8 Hz, 1H, Ar), 6.70 (d, J = 2.6 Hz, 1H, Ar), 6.58–6.55 (m, 1H, Ar), 3.89 (s, 3H, CH3), 3.77 (s, 3H, CH3); 13C-NMR δ 166.87, 160.06, 157.18, 150.63, 147.69, 146.60, 144.22, 142.07, 121.24, 119.89, 104.50, 99.14, 56.27, 55.56; Elemental analysis: calc. for C14H13N3O5 (MW 303.27): 55.45% C, 4.32% H, 13.86% N; found 55.46% C, 4.31% H, 13.84% N.
3-[(2,5-Dimethylphenyl)carbamoyl]pyrazine-2-carboxylic acid (5). Grey solid. Yield 81%; m.p. 177.4–178.8 °C; IR (cm−1): 3339 (N-H, CONH), 2918 (O-H, COOH), 1729(C=O, COOH),1687 (C=O, CONH); 1H-NMR δ 13.75 (bs, 1H, COOH), 10.15 (s, 1H, NH), 8.91–8.87 (m, 2H, pyr.), 7.40–7.38 (m, 1H, Ar), 7.14 (d, J = 7.7 Hz, 1H, Ar), 6.99–6.95 (m, 1H, Ar), 2.28 (s, 3H, CH3), 2.22 (s, 3H, CH3); 13C-NMR δ 166.53, 162.37, 146.50, 145.90, 145.30, 144.69, 135.48, 135.36, 130.39, 129.06, 126.63, 125.43, 20.80, 17.39; Elemental analysis: calc. for C14H13N3O3 (MW 271.28): 61.99% C, 4.83% H, 15.49% N; found 62.46% C, 4.73% H, 15.53% N.
3-[(4-Ethylphenyl)carbamoyl]pyrazine-2-carboxylic acid (6). Beige solid. Yield 98%; m.p. 159.1–159.9 °C; IR (cm−1): 3314 (N-H, CONH), 2972 (O-H, COOH), 1704 (C=O, COOH), 1664 (C=O, CONH); 1H-NMR δ 10.68 (s, 1H, NH), 8.90–86 (m, 2H, pyr.), 7.71–7.65 (m, 2H, Ar), 7.23–7.16 (m, 2H, Ar), 2.58 (q, J = 7.6 Hz, 2H, CH2), 1.17 (t, J = 7.6 Hz, 3H, CH3); 13C-NMR δ 166.50, 162.32, 146.52, 145.79, 145.67, 144.60, 139.87, 136.24, 128.16, 120.32, 27.85, 15.88; Elemental analysis: calc. for C14H13N3O3 (MW 271.28): 61.99% C, 4.83% H, 15.49% N; found 61.51% C, 5.24% H, 15.08% N.
3-[(4-Fluorophenyl)carbamoyl]pyrazine-2-carboxylic acid (7) [27]. Grey solid. Yield 90%; m.p. 168.7–169.6 °C; IR (cm−1): 3358 (N-H, CONH), 3069 (O-H, COOH), 1730 (C=O, COOH), 1683 (C=O, CONH); 1H-NMR δ 13.79 (bs, 1H, COOH), 10.85 (s, 1H, CONH), 8.92–8.87 (m, 2H, pyr.), 7.84–7.78 (m, 2H, Ar), 7.24–7.18 (m, 2H, Ar); 13C-NMR δ 166.46, 162.48, 158.75 (d, J = 240.8 Hz), 146.53, 145.93, 145.51, 144.66, 134.95 (d, J = 2.5 Hz), 122.16 (d, J = 8.0 Hz), 115.59 (d, J = 22.3 Hz); Elemental analysis: calc. for C12H8FN3O3 (MW 261.21): 55.18% C, 3.09% H, 16.09% N; found 54.96% C, 3.15% H, 15.85% N.
3-[(2,4-Difluorophenyl)carbamoyl]pyrazine-2-carboxylic acid (8). Grey solid. Yield 94%; m.p. 187.0–188.0 °C; IR (cm−1): 3346 (N-H, CONH), 2906 (O-H, COOH), 1702 (C=O, COOH), 1654 (C=O, CONH);1H-NMR δ 13.79 (bs, 1H, COOH), 10.54 (s, 1H, NH), 8.91 (d, 1H, J = 2.7 Hz, pyr.), 8.89 (d, 1H, J = 2.7 Hz, pyr.), 7.86–7.79 (m, 1H, Ar), 7.42–7.35 (m, 1H, Ar), 7.17–7.12 (m, 1H, Ar); 13C-NMR δ 165.4, 162.7, 159.5 (dd, J = 245.1 Hz, J = 11.4 Hz), 155.1 (dd, J = 249.9 Hz, J = 12.4 Hz), 146.4, 146.2, 144.8, 144.6, 126.7 (dd, J = 9.6 Hz, J = 2.9 Hz), 122.0 (dd, J = 12.4 Hz, J = 3.9 Hz), 111.5 (dd, J = 21.9 Hz, J = 3.8 Hz), 104.6 (dd, J = 26.6 Hz, J = 23.9 Hz); Elemental analysis: calc. for C12H7F2N3O3 (MW 279.20): 51.62% C, 2.53% H, 15.05% N; found 51.41% C, 2.05% H, 14.72% N.
3-[(4-Chlorophenyl)carbamoyl]pyrazine-2-carboxylic acid (9) [32]. White solid. Yield 98%; m.p. 171.1–173.2 °C; IR (cm−1): 3331 (N-H, CONH), 2584 (O-H, COOH), 1708 (C=O, COOH), 1692 (C=O, CONH); 1H-NMR δ 13.81 (bs, 1H, COOH), 10.92 (s, 1H, CONH), 8.91–8.88 (m, 2H, pyr.), 7.84–7.80 (m, 2H, Ar), 7.45–7.41 (m, 2H, Ar); 13C-NMR δ 166.38, 162.70, 146.41, 145.97, 145.52, 144.70, 137.53, 128.90, 128.05, 121.83; Elemental analysis: calc. for C12H8ClN3O3 (MW 277.66): 51.91% C, 2.90% H, 15.13% N; found 52.24% C, 2.83% H, 15.25% N.
3-[(3,4-Dichlorophenyl)carbamoyl]pyrazine-2-carboxylic acid (10). White solid. Yield 85%; m.p. 290.0–292.1 °C; IR (cm−1): 3274 (N-H, CONH), 2898 (O-H, COOH), 1724 (C=O, COOH), 1671 (C=O, CONH); 1H-NMR δ 11.10 (s, 1H, NH), 8.92 (d, J = 2.5 Hz, 1H, pyr.), 8.90 (d, J = 2.5 Hz, 1H, pyr.), 8.16 (d, J = 2.4 Hz, 1H, Ar), 7.77–7.71 (m, 1H, Ar), 7.63 (d, J = 8.8 Hz, 1H, Ar); 13C-NMR δ 166.35, 162.94, 146.51, 146.23, 145.03, 144.76, 138.65, 131.26, 130.96, 126.00, 121.49, 120.37; Elemental analysis: calc. for C12H7Cl2N3O3 (MW 312.11): 46.18% C, 2.26% H, 13.46% N; found 46.18% C, 2.24% H, 13.32% N.
3-[(4-Bromophenyl)carbamoyl]pyrazine-2-carboxylic acid (11) [33]. White solid. Yield 84%; m.p. 171.1–171.9 °C; IR (cm−1): 3312 (N-H, CONH), 2973 (O-H, COOH), 1706 (C=O, COOH), 1662 (C=O, CONH); 1H-NMR δ 10.92 (s, 1H, NH), 8.92–8.86 (m, 2H, pyr.), 7.80–7.72 (m, 2H, Ar), 7.59–7.52 (m, 2H, Ar); 13C-NMR δ 166.39, 162.75, 146.43, 145.98, 145.55, 144.71, 137.96, 131.82, 122.20, 116.16; Elemental analysis: calc. for C12H8BrN3O3 (MW 322.12): 44.75% C, 2.50% H, 13.05% N; found 44.30% C, 2.62% H, 12.55% N.
3-[(5-Fluoro-2-methylphenyl)carbamoyl]pyrazine-2-carboxylic acid (12). Beige solid. Yield 92%; m.p. 185.0–186.1 °C; IR (cm−1): 3301 (N-H, CONH), 3187 (O-H, COOH), 1746 (C=O, COOH), 1670 (C=O, CONH); 1H-NMR δ 13.83 (bs, 1H, COOH), 10.26 (s, 1H, NH), 8.92 (d, 1H, J = 2.4 Hz, pyr.), 8.90 (d, 1H, J = 2.4 Hz, pyr.), 7.51 (dd, 1H, J = 9.1 Hz J = 2.9 Hz, Ar), 7.01 (dd, 1H, J = 9.1 Hz J = 6.9 Hz, Ar), 7.00 (dt, 1H, J = 9.1 Hz J = 2.9 Hz, Ar), 2.25 (s, 3H, CH3); 13C-NMR δ 166.4, 162.7, 160.4 (d, J = 240.3 Hz), 146.3, 146.1, 145.2, 144.9, 136.9 (d, J = 10.4 Hz), 131.8 (d, J = 8.5 Hz), 127.5 (d, J = 2.9 Hz), 112.3 (d, J = 21.0 Hz), 111.0 (d, J = 24.8 Hz), 17.1; Elemental analysis: calc. for C13H10FN3O3 (MW 275.24): 56.73% C, 3.66% H, 15.27% N; found 56.84% C, 3.81% H, 14.93% N.
3-[(2-Chloro-5-methylphenyl)carbamoyl]pyrazine-2-carboxylic acid (13). Yellow solid. Yield 90%; m.p. 190.1–191.2 °C; IR (cm−1): 3342 (N-H, CONH), 2964 (O-H, COOH), 1701 (C=O, COOH), 1664 (C=O, CONH); 1H-NMR δ 13.79 (bs, 1H, COOH), 10.34 (s, 1H, NH), 8.94 (d, J = 2.5 Hz, 1H, pyr.), 8.91 (d, J = 2.5 Hz, 1H, pyr.), 7.91 (d, J = 2.0 Hz, 1H, Ar), 7.43 (d, J = 8.2 Hz, 1H, Ar), 7.09–7.05 (m, 1H, Ar), 2.33 (s, 3H, CH3); 13C-NMR δ 166.56, 161.54, 147.20, 146.73, 144.57, 142.86, 137.65, 133.83, 129.34, 127.35, 124.58, 122.67, 20.86; Elemental analysis: calc. for C13H10ClN3O3 (MW 291.69): 53.53% C, 3.46% H, 14.41% N; found 53.88% C, 3.37% H, 14.32% N.
3-[(5-Chloro-2-hydroxyphenyl)carbamoyl]pyrazine-2-carboxylic acid (14). Yellow solid. Yield 97%; m.p. 263.1–265.1 °C; IR (cm−1): 3271 (O-H, OH), 3116 (N-H, CONH), 3048 (O-H, COOH), 1709 (C=O, COOH), 1610 (C=O, CONH); 1H-NMR δ 13.78 (bs, 1H, COOH), 10.68 (s, 1H, OH), 10.16 (s, 1H, NH), 8.94 (d, J = 2.5 Hz, 1H, pyr.), 8.90 (d, J = 2.4 Hz, 1H, pyr.), 8.29 (d, J = 2.6 Hz, 1H, Ar), 7.07–7.03 (m, 1H, Ar), 6.95 (d, J = 8.7 Hz, 1H, Ar); 13C-NMR δ 166.74, 160.59, 147.63, 146.97, 146.02, 144.37, 141.58, 126.94, 124.38, 122.70, 119.30, 116.21; Elemental analysis: calc. for C12H8ClN3O4 (MW 293.66): 49.08% C, 2.75% H, 14.31% N; found 49.57% C, 2.69% H, 14.17% N.
3-[(2-Hydroxy-5-nitrophenyl)carbamoyl]pyrazine-2-carboxylic acid (15). Pale yellow solid. Yield 90%; m.p. 290.0–291.3 °C; IR (cm−1): 3566 (O-H, OH), 3363 (N-H, CONH), 2972 (O-H, COOH), 1744 (C=O, COOH), 1676 (C=O, CONH), 1545 (N-O, NO2), 1349 (N-O, NO2); 1H-NMR δ 13.73 (bs, 1H, COOH), 12.08 (bs, 1H, OH), 10.25 (s, 1H, NH), 9.16 (d, J = 2.8 Hz, 1H, Ar), 8.95 (d, J = 2.4 Hz, 1H, pyr.), 8.91 (d, J = 2.4 Hz, 1H, pyr.), 8.00–7.96 (m, 1H, Ar), 7.11 (d, J = 9.0 Hz, 1H, Ar); 13C-NMR δ 166.66, 161.15, 153.61, 147.46, 147.04, 144.49, 141.76, 139.49, 126.02, 121.49, 115.12, 114.78; Elemental analysis: calc. for C12H8N4O6 (MW 304.22): 47.38% C, 2.65% H, 18.42% N; found 46.99% C, 3.13% H, 18.15% N.
3-[(4-Nitrophenyl)carbamoyl]pyrazine-2-carboxylic acid (16) [34]. Yellow solid. Yield 58%; m.p. 224.5–227.2°C; IR (cm−1): 3281 (N-H, CONH), 3065 (O-H, COOH), 1713 (C=O, COOH), 1693 (C=O, CONH), 1543 (N-O, NO2), 1339 (N-O, NO2); 1H-NMR δ 13.91 (bs, 1H, COOH), 11.36 (s, 1H, CONH), 8.95–8.92 (m, 2H, pyr.), 8.30–8.25 (m, 2H, Ar), 8.06–8.02 (m, 2H, Ar); 13C-NMR δ 166.18, 163.52, 146.24, 146.06, 145.61, 144.96, 144.69, 143.13, 125.12, 120.06; Elemental analysis: calc. for C12H8N4O5 (MW 288.22): 50.01% C, 2.80% H, 19.44% N; found 50.28% C, 2.82% H, 19.35% N.
3-{[3-(Trifluoromethyl)phenyl]carbamoyl}pyrazine-2-carboxylic acid (17). Pale yellow solid. Yield 97%; m.p. 176.3–177.1°C; IR (cm−1): 3356 (N-H, CONH), 3064 (O-H, COOH), 1718 (C=O, COOH), 1694 (C=O, CONH); 1H-NMR δ 11.14 (s, 1H, NH), 8.93 (d, 1H, J = 2.4 Hz, pyr.), 8.91 (d, 1H, J = 2.4 Hz, pyr.), 8.30–8.28 (m, 1H, Ar), 8.01 (d, 1H, J = 7.9 Hz, Ar), 7.62 (t, 1H, J = 7.9 Hz, Ar), 7.50 (d, 1H, J = 7.9 Hz, Ar); 13C-NMR δ 166.4, 163.1, 146.5, 146.2, 145.2, 144.8, 139.4, 130.3, 129.7 (q, J = 31.4 Hz), 124.3 (q, J = 271.8 Hz), 123.9, 120.8 (q, J = 3.8 Hz), 116.4 (q, J = 3.8 Hz); Elemental analysis: calc. for C13H8F3N3O3 (MW 311.22): 50.17% C, 2.59% H, 13.50% N; found 49.86% C, 2.75% H, 13.42% N.
3-{[4-(Trifluoromethyl)phenyl]carbamoyl}pyrazine-2-carboxylic acid (18) [35]. White solid. Yield 90%; m.p. 153.5–154.7 °C; IR (cm−1): 3306, 1706 (C=O, COOH), 1669 (C=O, CONH); 1H-NMR δ 11.13 (s, 1H, NH), 8.92–8.91 (m 1H, pyr.), 8.91–8.89 (m, 1H, pyr.), 8.08–7.96 (m, 2H, Ar), 7.77–7.70 (m, 2H, Ar); 13C-NMR δ 166.4, 163.3, 146.4, 146.1, 145.7, 144.8, 142.2, 126.3 (q, J = 3.8 Hz), 124.5 (q, J = 270.9 Hz), 124.4 (q, J = 31.4 Hz), 120.2; Elemental analysis: calc. for C13H8F3N3O3 (MW 311.22): 50.17% C, 2.59% H, 13.50% N; found 50.42% C, 2.21% H, 13.84% N.
Propyl 3-(phenylcarbamoyl)pyrazine-2-carboxylate (1a). White solid. Yield 85%; m.p. 74.9–76.5 °C; IR (cm−1): 3355 (N-H, CONH), 2961 (C-H), 1734 (C=O, COO), 1686 (C=O, CONH), 1112, 1076 (C-O, COO); 1H-NMR δ 10.82 (s, 1H, CONH), 8.94 (s, 2H, pyr.), 7.80–7.77 (m, 2H, Ar), 7.407.35 (m, 2H, Ar), 7.17–7.12 (m, 1H, Ar), 4.26 (t, J = 6.6 Hz, 2H, CH2), 1.70–1.61 (m, 2H, CH2), 0.89 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.04, 162.19, 146.14, 145.68, 145.49, 145.11, 138.36, 128.94, 124.47, 120.32, 67.46, 21.45, 10.38; Elemental analysis: calc. for C15H15N3O3 (MW 285.30): 63.15% C, 5.30% H, 14.73% N; found 63.41% C, 5.40% H, 14.61% N.
Propyl 3-[(2-hydroxyphenyl)carbamoyl]pyrazine-2-carboxylate (2a). Pale yellow solid. Yield 64%; m.p. 156.8–159.6 °C; IR (cm−1): 3348 (N-H, CONH), 2924 (C-H), 1741 (C=O, COO), 1678 (C=O, CONH), 1111, 1086 (C-O, COO); 1H-NMR δ 10.33 (s, 1H, CONH), 10.16 (s, 1H, OH), 8.96 (d, J = 2.5 Hz, 1H, pyr.), 8.95 (d, J = 2.4 Hz, 1H, pyr.), 8.22–8.17 (m, 1H, Ar), 7.04–6.93 (m, 2H, Ar), 6.89–6.82 (m, 1H, Ar), 4.31 (t, J = 6.6 Hz, 2H, CH2), 1.76–1.67 (m, 2H, CH2), 0.93 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.50, 159.83, 147.16, 146.98, 146.63, 144.83, 142.09, 125.63, 125.11, 120.01, 119.45, 115.10, 67.41, 21.49, 10.41; Elemental analysis: calc. for C15H15N3O4 (MW 301.30): 59.80% C, 5.02% H, 13.95% N; found 59.83% C, 5.15% H, 13.52% N.
Propyl 3-[(4-methoxyphenyl)carbamoyl]pyrazine-2-carboxylate (3a). White solid. Yield 43%; m.p. 102.0–102.9 °C; IR (cm−1): 3284 (N-H, CONH), 2968 (C-H), 1745 (C=O, COO), 1652 (C=O, CONH), 1159, 1089 (C-O, COO); 1H-NMR δ 10.71 (s, 1H, CONH), 8.92 (s, 2H, pyr.), 7.72–7.68 (m, 2H, Ar), 6.96–6.92 (m, 2H, Ar), 4.26 (t, J = 6.6 Hz, 2H, CH2), 3.75 (s, 3H, CH3), 1.70–1.62 (m, 2H, CH2), 0.89 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.18, 161.58, 156.12, 146.06, 145.74, 145.43, 144.97, 131.44, 121.88, 114.06, 67.40, 55.37, 21.46, 10.39; Elemental analysis: calc. for C16H17N3O4 (MW 315.33): 60.94% C, 5.43% H, 13.33% N; found 60.90% C, 5.47% H, 13.21% N.
Propyl 3-[(2,4-dimethoxyphenyl)carbamoyl]pyrazine-2-carboxylate (4a). Yellow solid. Yield 75%; m.p. 116.4–118.5 °C; IR (cm−1): 3371 (N-H, CONH), 2962 (C-H), 1736 (C=O, COO), 1677 (C=O, CONH) ), 112 , 1084 (C-O, COO); 1H-NMR δ 9.97 (s, 1H, CONH), 8.76 (d, J = 2.4 Hz, 1H, pyr.), 8.68 (d, J = 2.4 Hz, 1H, pyr.), 8.43 (d, J = 8.6 Hz, 1H, Ar), 6.54–6.50 (m, 2H, Ar), 4.47 (t, J = 6.8 Hz, 2H, CH2), 3.93 (s, 3H, CH3), 3.82 (s, 3H, CH3), 1.89–1.80 (m, 2H, CH2), 1.03 (t, J = 7.5 Hz, 3H, CH3); 13C-NMR δ 166.02, 158.94, 157.01, 149.92, 148.00, 145.69, 143.06, 142.49, 120.76, 120.42, 103.79, 98.67, 68.05, 55.84, 55.51, 21.77, 10.31; Elemental analysis: calc. for C17H19N3O5 (MW 345.36): 59.12% C, 5.55% H, 12.17% N; found 58.70% C, 5.42% H, 11.87% N.
Propyl 3-[(2,5-dimethylphenyl)carbamoyl]pyrazine-2-carboxylate (5a). White solid. Yield 78%; m.p. 111.8–112.9 °C; IR (cm−1): 3354 (N-H, CONH), 2976 (C-H), 1741 (C=O, COO), 1686 (C=O, CONH), 1145, 1079 (C-O, COO); 1H-NMR δ 9.54 (s, 1H, CONH), 8.80 (d, J = 2.4 Hz, 1H, pyr.), 8.68 (d, J = 2.5 Hz, 1H, pyr.), 8.02–8.00 (m, 1H, Ar), 7.11 (d, J = 7.6 Hz, 1H, Ar), 6.93 (d, J = 7.4 Hz, 1H, Ar), 4.47 (t, J = 6.8 Hz, 2H, CH2), 2.35 (d, J = 6.1 Hz, 6H, CH3), 1.90–1.80 (m, 2H, CH2), 1.03 (t, J = 7.5 Hz, 3H, CH3); 13C-NMR δ 165.87, 159.33, 148.23, 146.08, 143.00, 142.07, 136.74, 134.76, 130.23, 126.04, 125.13, 122.31, 68.13, 21.76, 21.17, 17.16, 10.29; Elemental analysis: calc. for C17H19N3O3 (MW 313.36): 65.16% C, 6.11% H, 13.41% N; found 64.93% C, 5.91% H, 13.47% N.
Propyl 3-[(4-ethylphenyl)carbamoyl]pyrazine-2-carboxylate (6a). White solid. Yield 80%; m.p. 59.8–61.9 °C; IR (cm−1): 3332 (N-H, CONH), 2971 (C-H), 1743 (C=O, COO), 1659 (C=O, CONH), 1154, 1090 (C-O, COO); 1H-NMR δ 10.75 (s, 1H, CONH), 8.93 (s, 2H, pyr.), 7.69 (d, J = 8.1 Hz, 2H, Ar), 7.20 (d, J = 8.1 Hz, 2H, Ar), 4.26 (t, J = 6.6 Hz, 2H, CH2), 2.58 (q, J = 7.6 Hz, 2H, CH2), 1.74–1.61 (m, 2H, CH2), 1.17 (t, J = 7.6 Hz, 3H, CH3), 0.89 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.11, 161.90, 146.10, 145.62, 145.56, 145.03, 139.97, 136.04, 128.13, 120.41, 67.42, 27.83, 21.46, 15.82, 10.38; Elemental analysis: calc. for C17H19N3O3 (MW 313.36): 65.16% C, 6.11% H, 13.41% N; found 64.97% C, 6.02% H, 12.95% N.
Propyl 3-[(4-fluorophenyl)carbamoyl]pyrazine-2-carboxylate (7a). Beige solid. Yield 72%; m.p. 100.9–103.1 °C; IR (cm−1): 3351 (N-H, CONH), 2981 (C-H), 1736 (C=O, COO), 1690 (C=O, CONH), 1159, 1103 (C-O, COO); 1H-NMR δ 10.92 (s, 1H, CONH), 8.94 (s, 2H, pyr.), 7.84–7.80 (m, 2H, Ar), 7.24–7.19 (m, 2H, Ar), 4.26 (t, J = 6.5 Hz, 2H, CH2), 1.70–1.62 (m, 2H, CH2), 0.88 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.07, 162.08, 158.80 (d, J = 241.2 Hz), 146.24, 145.63, 145.37, 145.09, 134.76 (d, J = 2.5 Hz), 122.25 (d, J = 8.0 Hz), 115.57 (d, J = 22.3 Hz), 67.46, 21.46, 10.37; Elemental analysis: calc. for C15H14FN3O3 (MW 303.29): 59.40% C, 4.65% H, 13.85% N; found 59.04% C, 4.51% H, 13.59% N.
Propyl 3-[(2,4-difluorophenyl)carbamoyl]pyrazine-2-carboxylate (8a). White solid. Yield 74%; m.p. 93.3–96.1 °C; IR (cm−1): 3358 (N-H, CONH), 2976 (C-H), 1742 (C=O, COO), 1694 (C=O, CONH), 1141, 1099 (C-O, COO); 1H-NMR δ 10.61 (bs, 1H, CONH), 8.97–8.93 (m, 2H, pyr.), 7.84–7.77 (m, 1H, Ar), 7.42–7.36 (m, 1H, Ar), 7.18–7.12 (m, 1H, Ar), 4.25 (t, J = 6.6 Hz, 2H, CH2), 1.71–1.62 (m, 2H, CH2), 0.89 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.00, 162.29, 159.62 (dd, J = 244.9, 11.6 Hz), 155.18 (dd, J = 249.7, 12.7 Hz), 146.54, 145.58, 145.24, 144.32, 126.84 (dd, J = 9.9, 2.7 Hz), 121.79 (dd, J = 12.3, 3.7 Hz), 111.54 (dd, J = 22.0, 3.9 Hz), 104.61 (dd, J = 27.0, 23.8 Hz), 67.46, 21.45, 10.35.; Elemental analysis: calc. for C15H13F2N3O3 (MW 321.28): 56.08% C, 4.08% H, 13.08% N; found 55.94% C, 4.12% H, 13.21% N.
Propyl 3-[(4-chlorophenyl)carbamoyl]pyrazine-2-carboxylate (9a). White solid. Yield 65%; m.p. 121.0–122.1 °C; IR (cm−1): 3352 (N-H, CONH), 2981 (C-H), 1737 (C=O, COO), 1690 (C=O, CONH), 1146, 1104 (C-O, COO); 1H-NMR δ 10.99 (s, 1H, CONH), 8.94 (s, 2H, pyr.), 7.83 (d, J = 8.6 Hz, 2H, Ar), 7.44 (d, J = 8.5 Hz, 2H, Ar), 4.26 (t, J = 6.5 Hz, 2H, CH2), 1.65 (h, J = 7.0 Hz, 2H, CH2), 0.88 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 164.98, 162.31, 146.29, 145.50, 145.40, 145.15, 137.34, 128.89, 128.18, 121.91, 67.49, 21.45, 10.37; Elemental analysis: calc. for C15H14ClN3O3 (MW 319.75): 56.35% C, 4.41% H, 13.14% N; found 56.50% C, 4.39% H, 13.20% N.
Propyl 3-[(3,4-dichlorophenyl)carbamoyl]pyrazine-2-carboxylate (10a). White solid. Yield 34%; m.p. 101.4–102.2 °C; IR (cm−1): 3336 (N-H, CONH), 2975 (C-H), 1735 (C=O, COO), 1697 (C=O, CONH), 1148, 1082 (C-O, COO); 1H-NMR δ 11.17 (s, 1H, CONH), 8.97–8.95 (m, 2H, pyr.), 8.16 (d, J = 2.5 Hz, 1H, Ar), 7.77 (dd, J = 8.8, 2.5 Hz, 1H, Ar), 7.64 (d, J = 8.8 Hz, 1H, Ar), 4.27 (t, J = 6.5 Hz, 2H, CH2), 1.72–1.60 (m, 2H, CH2), 0.89 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 164.92, 162.54, 146.52, 145.56, 145.18, 144.91, 138.45, 131.20, 130.93, 126.11, 121.58, 120.43, 67.53, 21.45, 10.36; Elemental analysis: calc. for C15H13Cl2N3O3 (MW 354.19): 50.87% C, 3.70% H, 11.86% N; found 50.77% C, 3.71% H, 11.49% N.
Propyl 3-[(4-bromophenyl)carbamoyl]pyrazine-2-carboxylate (11a). White solid. Yield 68%; m.p. 86.6–88.6 °C; IR (cm−1): 3337 (N-H, CONH), 2966 (C-H), 1740 (C=O, COO), 1693 (C=O, CONH), 1142, 1083 (C-O, COO); 1H-NMR δ 10.99 (s, 1H, CONH), 8.94 (s, 2H, pyr.), 7.79–7.75 (m, 2H, Ar), 7.58–7.54 (m, 2H, Ar.), 4.26 (t, J = 6.5 Hz, 2H, CH2), 1.70–1.61 (m, 2H, CH2), 0.88 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 164.97, 162.33, 146.29, 145.48, 145.41, 145.15, 137.76, 131.80, 122.26, 116.30, 67.49, 21.45, 10.38; Elemental analysis: calc. for C13H14BrN3O3 (MW 364.20): 49.47% C, 3.87% H, 11.54% N; found 49.01% C, 3.75% H, 11.18% N.
Propyl 3-[(5-fluoro-2-methylphenyl)carbamoyl]pyrazine-2-carboxylate (12a). White solid. Yield 33%; m.p. 124.8–127.0 °C; IR (cm−1): 3358 (N-H, CONH), 2973 (C-H), 1739 (C=O, COO), 1690 (C=O, CONH), 1135, 1106 (C-O, COO); 1H-NMR δ 10.34 (s, 1H, CONH), 8.96 (s, 2H, pyr.), 7.53–7.49 (m, 1H, Ar), 7.33–7.28 (m, 1H, Ar), 7.03–6.98 (m, 1H, Ar), 4.28 (t, J = 6.6 Hz, 2H, CH2), 2.24 (s, 3H, CH3), 1.72–1.64 (m, 2H, CH2), 0.91 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.09, 162.07, 160.31 (d, J = 240.5 Hz), 146.48, 145.64, 145.20, 144.59, 136.76 (d, J = 10.7 Hz), 131.77 (d, J = 9.0 Hz), 127.50 (d, J = 3.2 Hz), 112.38 (d, J = 20.7 Hz), 111.04 (d, J = 24.7 Hz), 67.47, 21.49, 17.06, 10.38; Elemental analysis: calc. for C16H16FN3O3 (MW 317.32): 60.56% C, 5.08% H, 13.24% N; found 60.14% C, 5.01% H, 12.82% N.
Propyl 3-[(2-chloro-5-methylphenyl)carbamoyl]pyrazine-2-carboxylate (13a). White solid. Yield 53%; m.p. 110.9–111.4 °C; IR (cm−1): 3323 (N-H, CONH), 2974 (C-H), 1736 (C=O, COO), 1697 (C=O, CONH), 1141, 1087 (C-O, COO); 1H-NMR δ 10.21 (s, 1H, CONH), 8.82 (d, J = 2.4 Hz, 1H, pyr.), 8.73 (d, J = 2.4 Hz, 1H, pyr.), 8.42–8.40 (m, 1H, Ar), 7.32–7.26 (m, 1H, Ar), 6.94–6.90 (m, 1H, Ar), 4.49 (t, J = 6.8 Hz, 2H, CH2), 2.38 (s, 3H, CH3), 1.90–1.82 (m, 2H, CH2), 1.04 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.79, 159.54, 148.23, 146.28, 143.18, 141.68, 138.03, 133.47, 128.72, 126.10, 121.84, 120.47, 68.17, 21.78, 21.28, 10.30; Elemental analysis: calc. for C16H16ClN3O3 (MW 333.77): 57.58% C, 4.83% H, 12.59% N; found 57.72% C, 4.87% H, 12.62% N.
Propyl 3-[(5-chloro-2-hydroxyphenyl)carbamoyl]pyrazine-2-carboxylate (14a). Beige solid. Yield 43%; m.p. 196.6–199.2 °C; IR (cm−1): 3369 (N-H, CONH), 2983 (C-H), 1689, 1542, 1339 (N-O, NO2), 1156, 1116 (C-O, COO); 1H-NMR δ 10.70 (bs, 1H, OH), 10.18 (s, 1H, CONH), 8.97 (d, J = 2.4 Hz, 1H, pyr.), 8.95 (d, J = 2.5 Hz, 1H, pyr.), 8.25 (d, J = 2.7 Hz, 1H, Ar), 7.07–7.04 (m, 1H, Ar), 6.95 (d, J = 8.6 Hz, 1H, Ar), 4.32 (t, J = 6.6 Hz, 2H, CH2), 1.75–1.68 (m, 2H, CH2), 0.93 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.34, 160.33, 147.13, 146.43, 146.09, 144.94, 141.99, 126.78, 124.50, 122.65, 119.33, 116.22, 67.48, 21.50, 10.40; Elemental analysis: calc. for C15H14ClN3O4 (MW 335.74): 53.66% C, 4.20% H, 12.52% N; found 53.24% C, 3.85% H, 12.39% N.
Propyl 3-[(2-hydroxy-5-nitrophenyl)carbamoyl]pyrazine-2-carboxylate (15a). Beige solid. Yield 58%; m.p. 171.4 °C decomposition; IR (cm−1): 3324 (N-H, CONH), 3208 (O-H, OH), 2968 (C-H), 1727 (C=O, COO), 1678 (C=O, CONH), 1094, 1071 (C-O, COO); 1H-NMR δ 12.16 (bs, 1H, OH), 10.29 (s, 1H, CONH), 9.12 (d, J = 2.9 Hz, 1H, Ar), 8.99 (d, J = 2.4 Hz, 1H, pyr.), 8.96 (d, J = 2.4 Hz, 1H, pyr.), 8.01–7.97 (m, 1H, Ar), 7.11 (d, J = 9.0 Hz, 1H, Ar), 4.33 (t, J = 6.6 Hz, 2H, CH2), 1.77–1.67 (m, 2H, CH2), 0.94 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.23, 160.90, 153.75, 147.20, 146.22, 145.06, 142.17, 139.44, 125.87, 121.63, 115.21, 114.81, 67.55, 21.52, 10.41; Elemental analysis: calc. for C15H14N4O6 (MW 346.30): 52.03% C, 4.08% H, 16.18% N; found 51.60% C, 4.31% H, 16.60% N.
Propyl 3-{[3-(trifluoromethyl)phenyl]carbamoyl}pyrazine-2-carboxylate (17a). White solid. Yield 53%; m.p. 80.7–83.3 °C; IR (cm−1): 3347 (N-H, CONH), 2974 (C-H), 1745 (C=O, COO), 1697 (C=O, CONH), 1148, 1105 (C-O, COO); 1H-NMR δ 9.69 (s, 1H, CONH), 8.82 (d, J = 2.4 Hz, 1H, pyr.), 8.68 (d, J = 2.4 Hz, 1H, pyr.), 8.02 (d, J = 1.9 Hz, 1H, Ar), 7.96–7.93 (m, 1H, Ar), 7.51 (t, J = 8.0 Hz, 1H, Ar), 7.45–7.42 (m, 1H, Ar), 4.48 (t, J = 6.8 Hz, 2H, CH2), 1.90–1.82 (m, 2H, CH2), 1.04 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.68, 159.76, 148.33, 146.49, 142.98, 141.19, 137.48, 131.55 (q, J = 32.7 Hz), 129.70, 123.74 (q, J = 272.4 Hz), 122.90, 121.48 (q, J = 3.8 Hz), 120.49, 116.62 (q, J = 4.1 Hz), 68.27, 21.76, 10.29; Elemental analysis: calc. for C16H14F3N3O3 (MW 353.30): 54.39% C, 3.99% H, 11.89% N; found 53.95% C, 4.03% H, 11.65% N.
Propyl 3-{[4-(trifluoromethyl)phenyl]carbamoyl}pyrazine-2-carboxylate (18a). White solid. Yield 60%; m.p. 102.0–102.9 °C; IR (cm−1): 3308 (N-H, CONH), 2965 (C-H), 1741 (C=O, COO), 1677 (C=O, CONH), 1110, 1070 (C-O, COO); 1H-NMR δ 11.20 (s, 1H, CONH), 8.97 (s, 2H, pyr.), 8.01 (d, J = 8.4 Hz, 2H, Ar), 7.75 (d, J = 8.6 Hz, 2H, Ar), 4.27 (t, J = 6.6 Hz, 2H, CH2), 1.69–1.61 (m, 2H, CH2), 0.88 (t, J = 7.4 Hz, 3H, CH3); 13C-NMR δ 165.16, 163.12, 146.70, 145.75, 145.62, 145.56, 142.25, 126.56 (q, J = 3.8 Hz), 124.78 (q, J = 32.1 Hz), 124.72 (q, J = 271.4 Hz), 120.61, 67.82, 21.73, 10.63; Elemental analysis: calc. for C16H14F3N3O3 (MW 353.30): 54.39% C, 3.99% H, 11.89% N; found 54.06% C, 4.05% H, 11.97% N.
Methyl 3-(phenylcarbamoyl)pyrazine-2-carboxylate (1b). Pale yellow solid. Yield 71%; m.p. 81.8–82.7 °C; IR (cm−1): 3349 (N-H, CONH), 2960 (C-H), 1735 (C=O, COO), 1678 (C=O, CONH), 1142, 1087 (C-O, COO); 1H-NMR δ 10.83 (s, 1H, NH), 8.96–8.93 (m, 2H, pyr.), 7.81–7.76 (m, 2H, Ar), 7.40–7.35 (m, 2H, Ar), 7.18–7.12 (m, 1H, Ar), 3.89 (s, 3H, CH3); 13C-NMR δ 165.58, 161.96, 146.65, 146.24, 145.52, 145.15, 138.24, 128.95, 124.56, 120.52, 53.07; Elemental analysis: calc. for C13H11N3O3 (MW 257.25): 60.70% C, 4.31% H, 16.33% N; found 60.35% C, 4.55% H, 16.79% N.
Methyl 3-[(2,4-dimethoxyphenyl)carbamoyl]pyrazine-2-carboxylate (4b). Yellow solid. Yield 59%; m.p. 192.5–194.2 °C; IR (cm−1): 3367 (N-H, CONH), 2965 (C-H), 1740 (C=O, COO), 1676 (C=O, CONH), 1124, 1084 (C-O, COO); 1H-NMR δ 9.99 (s, 1H, NH), 8.95 (d, J = 2.4 Hz, 1H, pyr.), 8.94 (d, J = 2.4 Hz, 1H, pyr.), 8.06 (d, J = 8.8 Hz, 1H, Ar), 6.71 (d, J = 2.6 Hz, 1H, Ar), 6.59–6.55 (m, 1H, Ar), 3.90 (d, J = 6.9 Hz, 6H, CH 3), 3.77 (s, 3H, CH 3); 13C-NMR δ 165.88, 159.72, 157.33, 150.75, 146.81, 146.26, 144.91, 142.39, 121.52, 119.60, 104.56, 99.12, 56.27, 55.56, 53.00; Elemental analysis: calc. for C15H15N3O5 (MW 317.30): 56.78% C, 4.77% H, 13.24% N; found 56.83% C, 4.56% H, 13.44% N.
Methyl 3-[(2,5-dimethylphenyl)carbamoyl]pyrazine-2-carboxylate (5b). Pale yellow solid. Yield 67%; m.p. 132.1–134.6 °C; IR (cm−1): 3379 (N-H, CONH), 2953 (C-H), 1735 (C=O, COO), 1683 (C=O, CONH), 1121, 1082 (C-O, COO); 1H-NMR δ 10.26 (s, 1H, NH), 8.96–8.93 (m, 2H, pyr.), 7.38–7.35 (m, 1H, Ar), 7.15 (d, J = 7.7 Hz, 1H, Ar), 7.01–6.96 (m, 1H, Ar), 3.88 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.21 (s, 3H, CH3); 13C-NMR δ 165.65, 161.75, 146.33, 145.63, 145.19, 144.69, 135.44, 135.29, 130.39, 129.19, 126.81, 125.52, 53.02, 20.77, 17.34; Elemental analysis: calc. for C15H15N3O3 (MW 285.30): 63.15% C, 5.30% H, 14.73% N; found 62.71% C, 5.49% H, 14.92% N.
Methyl 3-[(4-ethylphenyl)carbamoyl]pyrazine-2-carboxylate (6b). Pale brown solid. Yield 58%; m.p. 89.1–90.7 °C; IR (cm−1): 3356 (N-H, CONH), 2962 (C-H), 1737 (C=O, COO), 1686 (C=O, CONH), 1147, 1083 (C-O, COO); 1H-NMR δ 10.76 (s, 1H, NH), 8.95–8.92 (m, 2H, pyr.), 7.72–7.67 (m, 2H, Ar), 7.23–7.18 (m, 2H, Ar), 3.88 (s, 3H, CH3), 2.58 (q, J = 7.6 Hz, 2H, CH2), 1.19–1.14 (m, 3H, CH3); 13C-NMR δ 165.63, 161.69, 146.19, 145.62, 145.12, 145.08, 140.07, 135.92, 128.14, 120.59, 53.04, 27.84, 15.84; Elemental analysis: calc. for C15H15N3O3 (MW 285.30): 63.15% C, 5.30% H, 14.73% N; found 62.88% C, 5.23% H, 14.92% N.
Methyl 3-[(2,4-difluorophenyl)carbamoyl]pyrazine-2-carboxylate (8b). White solid. Yield 72%; m.p. 165.9–167.4 °C; IR (cm−1): 3348 (N-H, CONH), 2965 (C-H), 1739 (C=O, COO), 1693 (C=O, CONH), 1143, 1103, 1082 (C-O, COO); 1H-NMR δ 10.63 (bs, 1H, NH), 8.96 (s, 2H, pyr.), 7.82–7.74 (m, 1H, Ar), 7.43–7.36 (m, 1H, Ar), 7.18–7.11 (m, 1H, Ar), 3.87 (s, 3H, CH3); 13C-NMR δ 165.5, 162.2, 159.7 (dd, J = 245.1 Hz, J = 13.7 Hz), 155.4 (dd, J = 249.9 Hz, J = 13.5 Hz), 146.7, 145.5, 143.4, 144.1, 127.2 (dd, J = 9.6 Hz, J = 1.9 Hz), 121.7 (dd, J = 12.4 Hz, J = 3.9 Hz), 111.6 (dd, J = 22.0 Hz, J = 3.9 Hz), 104.7 (dd, J = 26.6 Hz, J = 23.8 Hz), 53.1; Elemental analysis: calc. for C13H9F2N3O3 (MW 293.23): 53.25% C, 3.09% H, 14.33% N; found 53.36% C, 2.76% H, 14.53% N.
Methyl 3-[(4-bromophenyl)carbamoyl]pyrazine-2-carboxylate (11b). White solid. Yield 66%; m.p. 128.5–130.4 °C; IR (cm−1): 3326 (N-H, CONH), 2956 (C-H), 1742 (C=O, COO), 1686 (C=O, CONH), 1174, 1071 (C-O, COO); 1H-NMR δ 10.99 (s, 1H, NH), 8.96–8.94 (m, 2H, pyr.), 7.80–7.76 (m, 2H, Ar), 7.58–7.55 (m, 2H, Ar), 3.88 (s, 3H, CH 3); 13C-NMR δ 165.51, 162.07, 146.64, 146.39, 145.18, 144.87, 137.64, 131.79, 122.45, 116.39, 53.10; Elemental analysis: calc. for C13H10BrN3O3 (MW 336.15): 46.45% C, 3.00% H, 12.50% N; found 46.11% C, 2.76% H, 12.19% N.
Methyl 3-[(5-fluoro-2-methylphenyl)carbamoyl]pyrazine-2-carboxylate (12b). White solid. Yield 76%; m.p. 174.2–176.9 °C; IR (cm−1): 3375 (N-H, CONH), 2955 (C-H), 1741 (C=O, COO), 1692 (C=O, CONH), 1142, 1112 (C-O, COO); 1H-NMR δ 10.36 (bs, 1H, NH), 8.97–8.95 (m, 2H, pyr.), 7.49 (dd, 1H, J = 9.7 Hz, J = 2.9 Hz, Ar), 7.33–7.28 (1H, m, Ar), 7.01 (dt, 1H, J = 9.7 Hz J = 2.9 Hz, Ar), 3.89 (s, 3H, CH3), 2.24 (s, 3H, CH3); 13C-NMR δ 165.5, 162.0, 160.3 (d, J = 240.3 Hz), 146.5, 145.3, 144.5, 144.1, 136.7 (d, J = 10.4 Hz), 131.8 (d, J = 9.4 Hz), 127.7 (d, J = 2.9 Hz), 112.5 (d, J = 21.0 Hz), 111.2 (d, J = 24.8 Hz), 53.1, 17.0; Elemental analysis: calc. for C14H12FN3O3 (MW 289.27): 58.13% C, 4.18% H, 14.53% N; found 57.74% C, 3.97% H, 14.26% N.
Methyl 3-[(2-chloro-5-methylphenyl)carbamoyl]pyrazine-2-carboxylate (13b). Pale yellow solid. Yield 74%; m.p. 166.7–168.8 °C; IR (cm−1): 3349 (N-H, CONH), 2956 (C-H), 1737 (C=O, COO), 1689 (C=O, CONH), 1144, 1088 (C-O, COO); 1H-NMR δ 10.43 (s, 1H, NH), 8.99–8.97 (m, 2H, pyr.), 7.86 (d, J = 2.0 Hz, 1H, Ar), 7.44 (d, J = 8.1 Hz, 1H, Ar), 7.12–7.05 (m, 1H, Ar), 3.91 (s, 3H, CH3), 2.33 (s, 3H, CH3); 13C-NMR δ 165.63, 161.15, 147.02, 146.00, 145.19, 142.85, 137.73, 133.66, 129.36, 127.60, 124.87, 123.02, 53.13, 20.83; Elemental analysis: calc. for C14H12ClN3O3 (MW 305.72): 55.00% C, 3.96% H, 13.75% N; found 55.43% C, 3.85% H, 13.88% N.
Methyl 3-[(5-chloro-2-hydroxyphenyl)carbamoyl]pyrazine-2-carboxylate (14b). Yellow solid. Yield 72%; m.p. 174.2–176.9 °C; IR (cm−1): 3316 (N-H, CONH), 2963 (C-H), 1735 (C=O, COO), 1681 (C=O, CONH), 1148, 1091 (C-O, COO); 1H-NMR δ 10.71 (s, 1H, OH), 10.19 (s, 1H, NH), 8.98 (d, J = 2.4 Hz, 1H, pyr.), 8.96 (d, J = 2.5 Hz, 1H, pyr.), 8.25 (d, J = 2.5 Hz, 1H, Ar), 7.09–7.03 (m, 1H, Ar), 6.95 (d, 1H, Ar), 3.92 (d, J = 9.0 Hz, 3H, CH3); 13C-NMR δ 165.80, 160.17, 147.20, 146.66, 146.10, 145.02, 141.77, 126.72, 124.55, 122.69, 119.34, 116.23, 53.13; Elemental analysis: calc. for C13H10ClN3O4 (MW 307.69): 50.75% C, 3.28% H, 13.66% N; found 50.39% C, 3.19% H, 13.72% N.
Methyl 3-[(2-hydroxy-5-nitrophenyl)carbamoyl]pyrazine-2-carboxylate (15b). Pale yellow solid. Yield 60%; m.p. 226.4–228.6 °C; IR (cm−1): 3317 (N-H, CONH), 2969 (C-H), 1732 (C=O, COO), 1683 (C=O, CONH), 1267, 1095 (C-O, COO); 1H-NMR δ 12.10 (bs, 1H, OH), 10.29 (s, 1H, NH), 9.10 (d, J = 2.9 Hz, 1H, Ar), 8.99 (d, J = 2.4 Hz, 1H, pyr.), 8.97 (d, J = 2.4 Hz, 1H, pyr.), 8.01–7.94 (m, 1H, Ar), 7.10 (d, J = 9.0 Hz, 1H, Ar), 3.95 (s, 3H, CH3); 13C-NMR δ 165.71, 160.66, 153.69, 147.28, 146.16, 145.12, 141.83, 139.46, 125.81, 121.65, 115.15, 114.79, 53.19; Elemental analysis: calc. for C13H10N4O6 (MW 318.25): 49.06% C, 3.17% H, 17.61% N; found 48.76% C, 3.05% H, 17.52% N.
Methyl 3-{[3-(trifluoromethyl)phenyl]carbamoyl}pyrazine-2-carboxylate (17b). Yellow solid. Yield 52%; m.p. 106.3–108.5 °C; IR (cm−1): 3357 (N-H, CONH), 2919 (C-H), 1748 (C=O, COO), 1693 (C=O, CONH), 1110, 1081 (C-O, COO); 1H-NMR δ 9.71 (bs, 1H, NH), 8.82 (d, 1H, J = 2.2 Hz, pyr.), 8.69 (d, 1H, J = 2.2 Hz, pyr.), 8.08 (s, 1H, Ar), 7.93 (d, 1H, J = 8.0 Hz, Ar), 7.51 (t, 1H, J = 8.0 Hz, Ar), 7.43 (d, 1H, J = 8.0 Hz, Ar), 4.10 (s, 3H, CH3); 13C-NMR δ 166.0, 159.8, 148.0, 146.5, 145.5, 143.1, 137.4, 131.6 (q, J = 33.3 Hz), 129.7, 123.0, 123.7 (q, J = 271.9 Hz), 121.6 (q, J = 3.8 Hz), 116.7 (q, J = 3.9 Hz), 53.4.
Methyl 3-{[4-(trifluoromethyl)phenyl]carbamoyl}pyrazine-2-carboxylate (18b). White solid. Yield 50%; m.p. 146.8–148.2 °C; IR (cm−1): 3297 (N-H, CONH), 2961 (C-H), 1746 (C=O, COO), 1691 (C=O, CONH), 1112, 1063 (C-O, COO); 1H-NMR δ 9.73 (bs, 1H, NH), 8.82 (d, 1H, J = 2.2 Hz, pyr.), 8.69 (d, 1H, J = 2.2 Hz, pyr.), 7.87–7.84 (m, 2H, Ar), 7.65–7.62 (m, 2H, Ar), 4.09 (s, 3H, CH3); 13C-NMR δ 166.0, 159.8, 148.0, 146.5, 145.5, 143.1, 141.2, 126.8 (q, J = 33.8 Hz), 126.4 (q, J = 3.8 Hz), 123.9 (q, J = 270.9 Hz), 119.7, 53.4; Elemental analysis: calc. for C14H10F3N3O3 (MW 325.25): 51.70% C, 3.10% H, 12.92% N; found 51.95% C, 3.41% H, 12.83% N.
4. Conclusions
In this research project, we focused on the synthesis of 18 POA derivatives with increased lipophilicity achieved by substitution with phenylcarbamoyl moiety in position 3 of the pyrazine ring. Furthermore, we prepared 17 propyl esters and 12 methyl esters as prodrugs to further increase the lipophilicity and enhance the penetration of these derivatives through the mycobacterial cell wall. The recently uncovered antimycobacterial target, DprE1, was evaluated as potential target for our new series of POA derivatives, on the basis of structural similarities with previously published 2-carboxyquinoxalines (Figure 2). The results from a molecular docking study to DprE1 showed that the alteration of -NH-CH2- linker of the original scaffold to -CONH- group (title compounds of this study) did not alter the position of the scaffold in DprE1.
Prepared compounds were tested for in vitro growth inhibition activity against M. tuberculosis H37Rv and three nontuberculous strains. Five compounds showed moderate activity against Mtb (MIC = 50 or 100 μg·mL−1) and compound 16 exerted high efficiency with MIC = 1.56 μg·mL−1. The whole cell activity of 16 (MIC = 5.0 μM) was fully comparable with the activity of the best template 2-carboxyquinoxalines (Figure 1, MIC = 3.1 μM). With respect to our docking and biological results and the activities of 2-carboxyquinoxalines published previously (Figure 2), a bulky lipophilic substituent on the pyrazine ring, complimentary to the lipophilic cavity, is suggested to be important for the inhibition of DprE1. The lowered antimycobacterial activity of our compounds in whole cell assay could be caused by insufficient penetration through the cell wall (insufficient lipophilicity), low intrinsic affinity to the enzyme, or both. The most lipophilic compound from our series was propyl ester 10a with log P = 2.77, which is significantly lower in comparison with the model 2-carboxyquinoxaline derivative (log P = 4.05).
The increase of lipophilicity by the conversion to propyl and methyl esters did not produce any improvement in biological activity in general. Only propyl ester 18a showed antimycobacterial activity against Mtb with MIC = 3.13 μg·mL−1, a value similar to its parent acid 18. Antibacterial and antifungal assays did not reveal any compound with significant activity. As next research, we will focus on the design of compounds with lipophilic/bulky substituents at C-5 or C-6 of the pyrazine core as an attempt to fill in the lipophilic cavity of DprE1.
Acknowledgments
This study was supported by the Grant Agency of Charles University B-CH/1594214, SVV 260 401 and by the Czech Science Foundation project No. 17-27514Y.
Supplementary Materials
Supplementary Materials are available online. Docking study and methodologies of biological assays.
Author Contributions
M.D. set the topic and designed the study. J.Z. designed the study and assisted to the molecular docking studies. L.S. designed the structures, evaluated the biological activity, and wrote the paper. L.S., P.J., C.F. and G.B. synthetized the compounds. O.J. and P.P. performed the antimycobacterial assays. K.K. performed the antibacterial and antifungal assays. L.N. conceived and performed the cytotoxicity. J.K. interpreted the NMR spectra.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Singh P., Mishra A.K., Malonia S.K., Chauhan D.S., Sharma V.D., Venkatesan K., Katoch V.M. The paradox of pyrazinamide: An update on the molecular mechanisms of pyrazinamide resistance in Mycobacteria. J. Commun. Dis. 2006;38:288–298. [PubMed] [Google Scholar]
- 2.World Health Organization . Global Tuberculosis Report 2016. World Health Organization; Geneva, Switzerland: 2016. WHA68/2015/REC/1. [Google Scholar]
- 3.Heifets L., Lindholm-Levy P. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am. Rev. Respir. Dis. 1992;145:1223–1225. doi: 10.1164/ajrccm/145.5.1223. [DOI] [PubMed] [Google Scholar]
- 4.Stehr M., Elamin A.A., Singh M. Filling the pipeline—New drugs for an old disease. Curr. Top. Med. Chem. 2014;14:110–129. doi: 10.2174/1568026613666131113152908. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Mitchison D. The curious characteristics of pyrazinamide: A review. Int. J. Tuberc. Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 6.Zhang Y., Wade M.M., Scorpio A., Zhang H., Sun Z. Mode of action of pyrazinamide: Disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 2003;52:790–795. doi: 10.1093/jac/dkg446. [DOI] [PubMed] [Google Scholar]
- 7.Peterson N.D., Rosen B.C., Dillon N.A., Baughn A.D. Uncoupling environmental pH and intrabacterial acidification from pyrazinamide susceptibility in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015;59:7320–7326. doi: 10.1128/AAC.00967-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayahi H., Pugliese K.M., Zimhony O., Jacobs W.R., Jr., Shekhtman A., Welch J.T. Analogs of the antituberculous agent pyrazinamide are competitive inhibitors of NADPH binding to M. tuberculosis fatty acid synthase I. Chem. Biodivers. 2012;9:2582–2596. doi: 10.1002/cbdv.201200291. [DOI] [PubMed] [Google Scholar]
- 9.Shi W., Chen J., Feng J., Cui P., Zhang S., Weng X., Zhang W., Zhang Y. Aspartate decarboxylase (PanD) as a new target of pyrazinamide in Mycobacterium tuberculosis. Emerg. Microbes Infect. 2014;3:e58. doi: 10.1038/emi.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillon N.A., Peterson N.D., Rosen B.C., Baughn A.D. Pantothenate and pantetheine antagonize the antitubercular activity of pyrazinamide. Antimicrob. Agents Chemother. 2014;58:7258–7263. doi: 10.1128/AAC.04028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi W., Zhang X., Jiang X., Yuan H., Lee J.S., Barry C.E., III, Wang H., Zhang W., Zhang Y. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science. 2011;333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keiler K.C. Biology of trans-translation. Annu. Rev. Microbiol. 2008;62:133–151. doi: 10.1146/annurev.micro.62.081307.162948. [DOI] [PubMed] [Google Scholar]
- 13.Yang J., Liu Y., Bi J., Cai Q., Liao X., Li W., Guo C., Zhang Q., Lin T., Zhao Y., et al. Structural basis for targeting the ribosomal protein S1 of Mycobacterium tuberculosis by pyrazinamide. Mol. Microbiol. 2015;95:791–803. doi: 10.1111/mmi.12892. [DOI] [PubMed] [Google Scholar]
- 14.Salah P., Bisaglia M., Aliprandi P., Uzan M., Sizun C., Bontems F. Probing the relationship between Gram-negative and Gram-positive S1 proteins by sequence analysis. Nucleic Acids Res. 2009;37:5578–5588. doi: 10.1093/nar/gkp547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolezal M., Zitko J., Kesetovicova D., Kunes J., Svobodova M. Substituted N-phenylpyrazine-2-carboxamides: Synthesis and antimycobacterial evaluation. Molecules. 2009;14:4180–4189. doi: 10.3390/molecules14104180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Servusova B., Vobickova J., Paterova P., Kubicek V., Kunes J., Dolezal M., Zitko J. Synthesis and antimycobacterial evaluation of N-substituted 5-chloropyrazine-2-carboxamides. Bioorg. Med. Chem. Lett. 2013;23:3589–3591. doi: 10.1016/j.bmcl.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Zitko J., Franco F., Paterova P. Synthesis and anti-infective evaluation of 5-amino-N-phenylpyrazine-2-carboxamides. Ceska Slov. Farm. 2015;64:19–24. [PubMed] [Google Scholar]
- 18.Neres J., Hartkoorn R.C., Chiarelli L.R., Gadupudi R., Pasca M.R., Mori G., Venturelli A., Savina S., Makarov V., Kolly G.S., et al. 2-Carboxyquinoxalines kill Mycobacterium tuberculosis through noncovalent inhibition of DprE1. ACS Chem. Biol. 2015;10:705–714. doi: 10.1021/cb5007163. [DOI] [PubMed] [Google Scholar]
- 19.Riccardi G., Pasca M.R., Chiarelli L.R., Manina G., Mattevi A., Binda C. The DprE1 enzyme, one of the most vulnerable targets of Mycobacterium tuberculosis. Appl. Microbiol. Biotechnol. 2013;97:8841–8848. doi: 10.1007/s00253-013-5218-x. [DOI] [PubMed] [Google Scholar]
- 20.Wolucka B.A. Biosynthesis of D-arabinose in mycobacteria—A novel bacterial pathway with implications for antimycobacterial therapy. FEBS J. 2008;275:2691–2711. doi: 10.1111/j.1742-4658.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- 21.Makarov V., Manina G., Mikusova K., Mollmann U., Ryabova O., Saint-Joanis B., Dhar N., Pasca M.R., Buroni S., Lucarelli A.P., et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science. 2009;324:801–804. doi: 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirude P.S., Shandil R., Sadler C., Naik M., Hosagrahara V., Hameed S., Shinde V., Bathula C., Humnabadkar V., Kumar N., et al. Azaindoles: Noncovalent DprE1 inhibitors from scaffold morphing efforts, kill Mycobacterium tuberculosis and are efficacious in vivo. J. Med. Chem. 2013;56:9701–9708. doi: 10.1021/jm401382v. [DOI] [PubMed] [Google Scholar]
- 23.Naik M., Humnabadkar V., Tantry S.J., Panda M., Narayan A., Guptha S., Panduga V., Manjrekar P., Jena L.K., Koushik K., et al. 4-Aminoquinolone piperidine amides: Noncovalent inhibitors of DprE1 with long residence time and potent antimycobacterial activity. J. Med. Chem. 2014;57:5419–5434. doi: 10.1021/jm5005978. [DOI] [PubMed] [Google Scholar]
- 24.Panda M., Ramachandran S., Ramachandran V., Shirude P.S., Humnabadkar V., Nagalapur K., Sharma S., Kaur P., Guptha S., Narayan A., et al. Discovery of pyrazolopyridones as a novel class of noncovalent DprE1 inhibitor with potent anti-mycobacterial activity. J. Med. Chem. 2014;57:4761–4771. doi: 10.1021/jm5002937. [DOI] [PubMed] [Google Scholar]
- 25.Makarov V., Neres J., Hartkoorn R.C., Ryabova O.B., Kazakova E., Sarkan M., Huszar S., Piton J., Kolly G.S., Vocat A., et al. The 8-pyrrole-benzothiazinones are noncovalent inhibitors of DprE1 from Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015;59:4446–4452. doi: 10.1128/AAC.00778-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chikhale R., Menghani S., Babu R., Bansode R., Bhargavi G., Karodia N., Rajasekharan M.V., Paradkar A., Khedekar P. Development of selective DprE1 inhibitors: Design, synthesis, crystal structure and antitubercular activity of benzothiazolylpyrimidine-5-carboxamides. Eur. J. Med. Chem. 2015;96:30–46. doi: 10.1016/j.ejmech.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Saudi M., Zmurko J., Kaptein S., Rozenski J., Neyts J., Van Aerschot A. Synthesis and evaluation of imidazole-4,5-and pyrazine-2,3-dicarboxamides targeting dengue and yellow fever virus. Eur. J. Med. Chem. 2014;87:529–539. doi: 10.1016/j.ejmech.2014.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holzer W., Eller G.A., Datterl B., Habicht D. Derivatives of pyrazinecarboxylic acid: H-1, C-13 and N-15 NMR spectroscopic investigations. Magn. Reson. Chem. 2009;47:617–624. doi: 10.1002/mrc.2437. [DOI] [PubMed] [Google Scholar]
- 29.Salfinger M., Heifets L.B. Determination of Pyrazinamide MICs for Mycobacterium-tuberculosis at different pHs by the radiometric method. Antimicrob. Agents Chemother. 1988;32:1002–1004. doi: 10.1128/AAC.32.7.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Permar S., Sun Z.H. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 2002;51:42–49. doi: 10.1099/0022-1317-51-1-42. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan G.B., Rose J.C., Brown G.P. Polyimides Based on Pyrazinetetracarboxylic dianhydride and some related model compounds. J. Polym. Sci. A1. 1971;9:1117–1138. doi: 10.1002/pol.1971.150090422. [DOI] [Google Scholar]
- 32.Roehrig S., Jeske M., Akbaba M., Rosentreter U., Boyer S., Fischer K., Pohlmann J., Tuch A., Perzborn E., Gerdes C., et al. Pyrazine Dicarboxamides and the Use Thereof. WO2006061116 (A1) Patent. 2006 Jun 15;
- 33.Mackerell A., Jr., Zhang H., Osterman A., Kolhatkar R. Targeting NAD Biosynthesis in Bacterial Pathogens. WO2011006158 (A2) Patent. 2011 Jan 13;
- 34.Lui Y., Li J., Bi K., Liu L. Preparing Method for N-substituted Pyrrolo [3,4-B] pyrazine-5,7(6H)-diketone. CN106220630 (A) Patent. 2016 Dec 14;
- 35.Leban J., Kramer B., Saeb W., Garcia G. Novel Compounds as Anti-Inflammatory, Immunomodulatory and Anti-Proliferatory Agents. 2003203951 (A1) U.S. Patent. 2003 Jan 23;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.