Abstract
The hypothesis that the small conducting airways were the major site of obstruction to airflow in normal lungs was introduced by Rohrer in 1915 and prevailed until Weibel introduced a quantitative method of studying lung anatomy in 1963. Green repeated Rohrer's calculations using Weibels new data in 1965 and found that the smaller conducting airways offered very little resistance to airflow. This conflict was resolved by seminal experiments conducted by Macklem and Mead in 1967, which confirmed that a small proportion of the total lower airways resistance is attributable to small airways <2 mm in diameter. Shortly thereafter, Hogg, Macklem, and Thurlbeck used this technique to show that small airways become the major site of obstruction in lungs affected by emphysema. These and other observations led Mead to write a seminal editorial in 1970 that postulated the small airways are a silent zone within normal lungs where disease can accumulate over many years without being noticed. This review provides a progress report since the 1970s on methods for detecting chronic obstructive pulmonary disease, the structural nature of small airways' disease, and the cellular and molecular mechanisms that are thought to underlie its pathogenesis.
I. INTRODUCTION AND HISTORICAL PERSPECTIVE
By the 1950s it was well understood that chronic bronchitis occurring either with or without emphysema was a major cause of death in the Western world, but little was known about its beginnings and trajectory. Fletcher and his associates set out to fill this gap by initiating a prospective study designed to establish the natural history of what was then termed chronic bronchitis and emphysema. They conducted an observational study of lung function, smoking habits, cough, sputum production, and episodes of acute infection in 1,136 working men between 30–59 years of age, of whom 792 completed an 8-yr follow up between 1961 and 1969 (35, 48). A unique feature of this important study was that it was based on a population of working men who were healthy at the time of entry into the study. Their results were summarized in an important monograph entitled “The natural history of chronic bronchitis and emphysema” in 1976 (34). Although the terms chronic bronchitis and emphysema were in use long before Fletcher's study began in 1961, the causal links between smoking and these two “diseases” were largely based on opinion rather than data. At that time the small conducting airways that connect the bronchi to the gas exchanging surface were thought to be the major site of resistance to airflow in the lower respiratory tract of normal individuals based on the work of the great Swiss physiologist Fritz Rohrer (1888–1926). Rohrer employed aerodynamic principles to calculate the resistance to airflow through the mouth and nose, across the larynx, and along the airways leading to the alveoli, using measurements he made from casts of human lungs. This work was first published in German in 1915 (92), reached a wider audience following its republication in a popular German handbook in 1925 (93), and was well known and widely accepted by physiologists and clinicians up until the 1960s. The first major challenge to Rohrer's conclusion that the small airways accounted for the majority of lower airway resistance came with the publication of Ewald Weibel's now classic monograph on the morphometry of the human lung (137). Weibel provided quantitative anatomic information showing that the total cross-sectional area of the conducting airways increased exponentially as the gas exchanging surface was approached. The increase he attributed to a rapid increase in the total number of airways in this semi-dichotomous branching system, as well as a greater cross-sectional area of the two daughter branches compared with their parent branch beyond generation 5–6. Approximately one year after Weibel's monograph appeared, Green (39) used these new data to repeat Rohrer's calculations and found that the small airways offered much less resistance to airflow than Rohrer estimated. Shortly thereafter, Macklem and Mead (61) provided the first experimental data in support of this new concept based on a novel method they developed to provide direct measurements of peripheral airway resistance by positioning a catheter in the airways <2 mm in diameter and measuring the resistance offered by the airways located central and peripheral to the catheter tip. This seminal study showed that instead of accounting for the bulk of the lower airways resistance as Roher had calculated, airways <2 mm in diameter actually accounted for <10% of the total resistance to flow below the larynx (61). Macklem completed this work with Mead while he was a visiting scientist at the Harvard School of Public Health between 1965 and 1966. He then returned to McGill where he initiated and directed a very productive research program designed to explore small airways structure and function in both health and disease. This work included collaboration with William Thurlbeck, a pathologist interested in chronic bronchitis and emphysema, and one of the authors of this manuscript (JCH) as a post-doctoral fellow. Those studies confirmed Macklem and Mead's original results, and added the important discovery that the same airways that offered so little resistance in normal lungs became the major site of obstruction to airflow in post mortem lungs affected by emphysema (42). In addition, they also described the nature of the disease in the small airways using a combination of post mortem bronchograms to show that the lumens of smaller bronchi and bronchioles were narrowed and distorted, and histology that showed the presence of a mixture of chronic inflammation and fibrosis in the airway walls as well as plugging of the airway lumen by mucus exudates (42). This report (42) introduced the term small airways disease because the disease affected both the smaller bronchi defined by the presence of cartilage in their walls as well as the bronchioles. Two years later, the same group reported additional studies on the effect of lung development on the distribution of lower airways resistance, which showed that the small conducting airways accounted for a much greater proportion of total lower airway resistance in the first few years of life than they did in older children and adults (44). These observations were consistent with the hypothesis that abnormalities in early lung development might influence the development of airway disease in adulthood. In the same issue of New England Journal of Medicine, a second manuscript from Macklem's group showed that gas exchange deteriorated in patients with mild bronchitis and asthma before routine pulmonary function tests became abnormal (58). Importantly, these two articles were accompanied by an influential editorial in which Mead postulated that the airways <2 mm in diameter represent a “quiet” zone within normal adult lung where disease can accumulate over many years without being noticed by either the persons affected or the physicians responsible for their care (64).
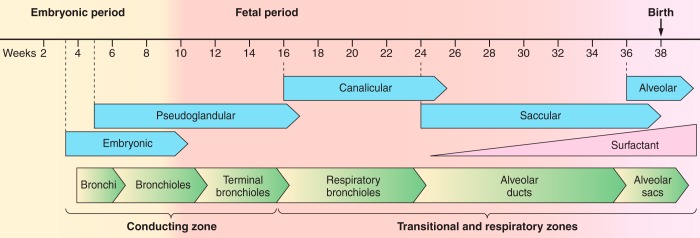
Figure 1 provides an overview of the major events associated with intrauterine lung development. The bronchial tree and acini are formed by about the 16th week of intrauterine life, and the distal generations of the conducting airways including the alveolar ducts and sacs are in place by the end of the canalicular period at the 24th week of intrauterine life. These events are then followed by a saccular period of growth between the 24th and 36th week of gestation, during which the existing airspaces expand, and a final period when alveoli form by septation of the existing alveolar ducts and sacs between the 36th week of gestation and the first few years of extrauterine life. Based on this knowledge, the McGill group reasoned that the smaller, purely conducting airways, which are completely surrounded by alveolar tissue, maintain a relatively fixed diameter during the period when the lung is growing by adding new alveoli. Moreover, they also reasoned that the completion of lung growth by alveolar addition and the start of lung growth by an increase in alveolar and airway size would be associated with sharp reduction in peripheral airways resistance. This prediction was based on Poiseuille's equation (see below), which shows that the flow rate through tubes is determined by the pressure drop along the tube divided by the resistance to flow through it, and that the resistance of the tube is proportional to the inverse of its radius raised to the 4th power.
FIGURE 1.
The embryonic phase of lung development (4–8 wk) begins with the formation of a groove (sulcus laryngotrachealis) in the ventral lower pharynx, after which the lung bud (true lung primodium) forms which further subdivides into the two main bronchi of which the left bronchus is directed more laterally establishing the asymmetry of the main stem bronchi that is present in adults. In the pseudoglandular phase (5–16 wk), all conducting airways are established (bronchi, bronchioles, and terminal bronchioles). These are lined with cuboidal cells which are the precursors of ciliated cells that can be found in humans from the 13th week of pregnancy. The canalicular phase (16–24 wk) is associated with the development of the pulmonary parenchyma consisting of respiratory bronchioles, alveolar ducts, alveolar sacs, and an invasion of capillaries around the acini for later gas exchange. There is also a significant differentiation of type II pneumocytes into attenuated type I pneumocytes. By the beginning of the saccular phase (25 wk), a large part of the amniotic fluid is produced by the lung epithelium. From this time the maturity of the lung can be measured clinically by the production of surfactant by type II pneumocytes. At the end of this phase, interstitial fibroblasts begin production of extracellular matrix in the interductal and intersaccular space. Over the last few weeks prior to birth, the first alveoli form, and after birth become increasingly complex through alveolarization with formation of secondary septae. Depending on the study, this process continues to the first or eighth year of life.
where F is flow rate, P is pressure, R is resistance, η is viscosity, L is length, r is radius, and π is the mathematical constant Pi.
Furthermore, the McGill group also suggested that the small airways of infants and children were vulnerable to disease as they showed that the small airways contributed substantially to airway resistance in the post mortem lungs of children who died of cystic fibrosis (which was then termed fibrocystic disease of the pancreas) (44). These data are consistent with a recent report based on micro computed tomography (micro CT) studies of explanted lungs from patients with cystic fibrosis treated by lung transplantation, which showed extensive destruction of the terminal bronchioles (7). These results are also consistent with the notion that severe bronchiolitis is a precursor to the devastating bronchiectasis observed in older children and adults with this disease.
Northway et al. (79) established the link between the respiratory failure associated with premature birth and fixed airflow limitation with abnormalities in lung structure in survivors who reach late childhood and early adulthood. It has also been well established that childhood respiratory infections and exposure to second-hand smoke contribute to the risk of developing chronic obstructive pulmonary disease (COPD) in later life (111). These findings are consistent with a recent report on the trajectory of the development of COPD (54), confirming that some adults have airflow limitation because they fail to reach their maximal expected forced expiratory volume in 1 s (FEV1) by age 25. How much of the apparent increase in the incidence in COPD in nonsmokers is related to the improved survival of infants who experience respiratory failure associated with premature birth, suffer from repeated respiratory infections, or are exposed to second-hand smoke in the perinatal period, remains to be seen.
The rapid expansion of the literature on the structure and function of the small airways in health and disease since the 1960s is truly remarkable. A Google search for “small airways disease” performed May 2016 produced 2,450,000 results, and a similar search of PubMed identified 63,333 articles in the medical literature that mention small airways disease in either the title or selected key words by the authors. The sheer volume of literature has made it difficult, if not impossible, to review everything written about small airways disease in a coherent, chronological fashion. Therefore, this review will take a more traditional approach by beginning with a discussion of the anatomy of the small conducting airways <2 mm in diameter, with special reference to the branching patterns and lumen areas at each generation of branching. That is followed by a brief discussion of the basic processes of bulk flow and diffusion of gases to illustrate why the smallest conducting airways and the respiratory bronchioles are particularly vulnerable to the injury caused by inhaling toxic particles and gases. We then discuss the contribution of the small airways to both the mechanics of breathing and distribution of ventilation within the lungs so as to provide a suitable background for the discussion of the physiological tests and imaging procedures that have been, and are being, developed to detect disease in the small airways. That is then followed by a more detailed description of how the lungs are normally protected from inhalational injury by a cooperative response of the innate and adaptive host immune systems.
II. ANATOMY OF THE SMALL CONDUCTING AIRWAYS
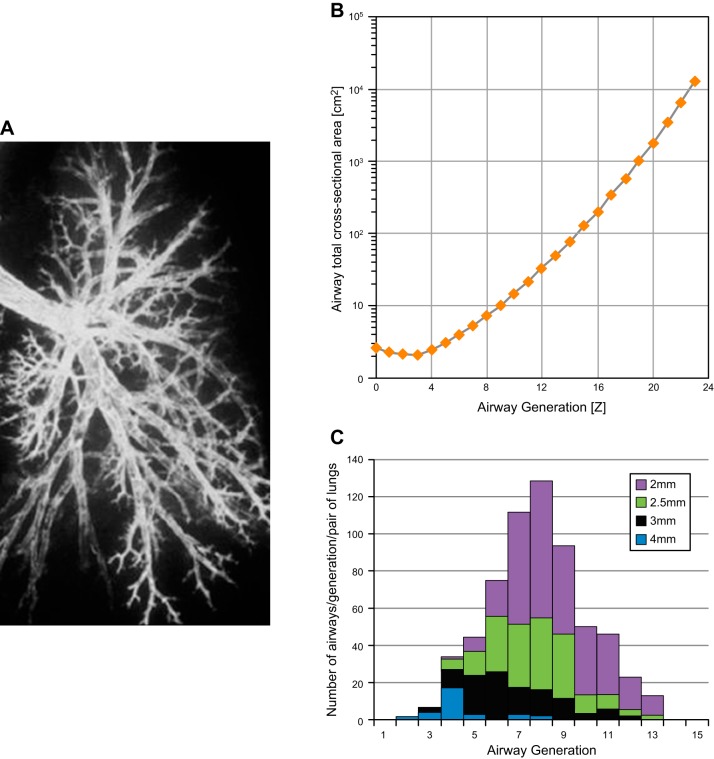
The human bronchogram in Figure 2A illustrates the striking variance in pathway length between the main stem bronchus and gas exchanging tissue. Data replotted from Weibel's classic monograph (137) (Figure 2B) shows that the difference in pathway length allows airways with a lumen diameter of <2 mm to be reached in as few as 4 and as many as 14 generations of branching (mean = 8 generations). Figure 2C shows the data from Weibel replotted again as a color coded map which shows that airways of a particular size tend to be normally distributed within the branching system, which means each generation of airway branching contains airways of several different sizes (137).
FIGURE 2.
A: bronchogram of a left lung to demonstrate the different pathway lengths to the periphery of the lung. B: distribution of airways of a given size in each generation of branching to demonstrate that each generation contains airways of several different sizes. [Data redrawn from Weibel (137).] C: total lumen cross-sectional area of all the branches decreases between generation 0–3 and then increases exponentially toward the periphery of the lung.
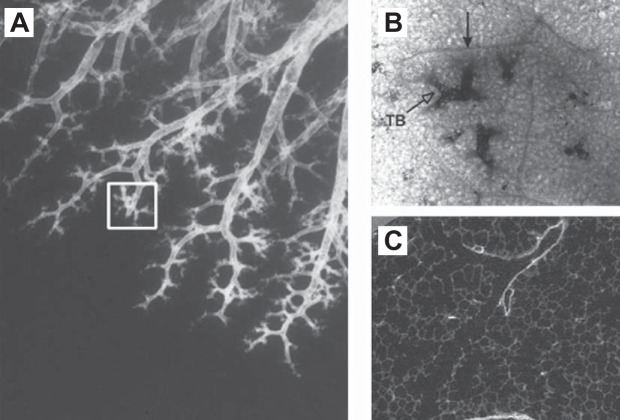
Reid's (91) studies of human bronchograms showed that the distal conducting airways end in clusters of branches located only millimeters apart (Figure 3A), and she was the first to recognize that each of these clusters is consistent with a secondary lobule (Figure 3B), a unit of lung surrounded by a fibrous connective tissue septum. Horsfield and Cumming (45) used the term lobular airways to describe the most distal airway branches in their exhaustive study of a single lung cast because they were not confident they could recognize terminal bronchioles in casts of peripheral airway lumens. In addition, they showed that these lobular branches are normally distributed between the 8th and 22nd generations of branching with a mean of ∼14. Figure 3B shows a lobule (closed arrowhead) that contains several terminal bronchioles, each supplying a single acinus, and it has been estimated that there are between 3 and 25 individual acini in each secondary lobule. Figure 3C shows a micro CT image of a single terminal bronchiole branching into two first-order respiratory bronchioles where alveoli first appear (termed transitional bronchioles by Weibel). This transition can be appreciated in Figure 3B (open arrowhead) and is shown at higher magnification in Figure 3C. Although the acinus has been defined in several different ways, this review will follow the convention that an acinus is a unit of lung supplied by a single terminal bronchiole, because this definition clearly separates the last purely conducting airways (i.e., the terminal bronchioles) from the airways that participate in gas exchange.
FIGURE 3.
A: bronchogram of the distal human lung to demonstrate Reid's original observation that in an individual pathway the airways branch points are initially centimeters apart, become closer to each other near the periphery of the lung, and end in clusters of branches (white rectangle) that are only millimeters apart. B: an image of one of these clusters taken through the pleural surface to show that it represents a secondary lobule first described by Miller as a group of preterminal bronchioles surrounded by fibrous connective tissue septa (black arrow) and a terminal bronchiole (white arrowhead) that opens into tuft-shaped respiratory bronchioles. C: a micro CT image at much higher magnification clearly demonstrates the junction between the terminal bronchiole and two first-order respiratory bronchioles (also termed transitional bronchioles) where the alveoli opening from them are visible. [From Hogg et al. (42a), with permission from Elsevier.]
III. MOVEMENT OF GAS INTO AND OUT OF THE LUNG
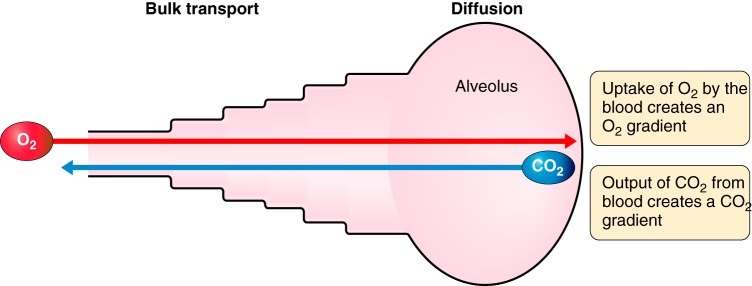
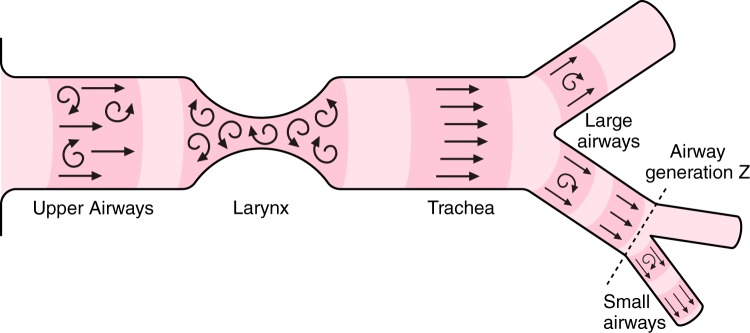
The transport of gas from the airway opening at the mouth and nose to the gas exchanging surface involves two separate and distinct physical processes as illustrated by the simple model in Figure 4. The first involves the bulk transport of gas along a pressure gradient developed by the action of the respiratory muscles. The second involves the diffusion of gases along a concentration gradient created by the uptake of oxygen (O2) from alveolar gas into the pulmonary capillary blood, and the down loading of carbon dioxide (CO2) from the pulmonary capillary blood into alveolar gas. At steady state, bulk flow remains constant at all levels of the tracheobronchial tree, meaning that the velocity of gas flow is very high through the narrow laryngeal orifice and along the trachea and main stem bronchi, and then rapidly slows as the total cross-sectional area of the airways widens exponentially.
FIGURE 4.
A simple model to illustrate that gas transport from the atmosphere to the blood circulating through the pulmonary capillaries depends on two fundamentally different processes: the bulk movement of gas along a pressure gradient developed by the respiratory muscles and the diffusion of gases along a concentration gradient developed by the exchange of oxygen and carbon dioxide at the alveolar surface. This creates an interface between bulk flow and diffusion that renders the smaller conducting airways and proximal respiratory bronchioles vulnerable to the deposition of fine particulates that remain suspended in the gas reaching the peripheral lung. Because these small particles do not diffuse as readily as the gases they are suspended in, they tend to settle and deposit on the lung surface in the regions where the shift from bulk flow to diffusion occurs.
The position of the interface created between the freshly inhaled and residual gas depends on the tidal volume (Vt) breathed in and its relationship to the ∼3 liters of gas present in the lung at functional residual capacity (FRC). Importantly, mixing between each tidal breath of fresh air and the residual gas remaining in the lungs is almost entirely dependent on diffusion. The effectiveness of the diffusion process for gas mixing and exchange is illustrated by the fact that arterial blood O2 saturation can be maintained even with the very low tidal volumes associated with high-frequency ventilation (22), although arterial CO2 increases as the dead space to tidal volume ratio increases. Alteration in the rate and depth of breathing shifts the anatomic location of this interface between bulk flow and diffusion. But, the large increase in volume at the level of the alveoli keeps the interface close to the boundary between the purely conducting airways and the alveolated tissue. These facts make the smallest purely conducting airways and proximal respiratory bronchioles vulnerable to the deposition of fine particulates that remain suspended in the incoming air because the suspended particles diffuse much more slowly than the gases they are suspended in, and the fine particles tend to settle on the airway surface at this interface.
As previously mentioned, the bulk flow of gas through tubes under fully developed laminar flow conditions is described by the Poiseuille equation. During laminar flow (indicated by straight lines in Figure 5) concentric layers of increasing gas velocity occur from the outer layer, which is slowed by friction with the airway wall, to the inner layers, where each concentric layer moves with progressively increasing velocity, without mixing between the layers, because friction decreases due to the reduction in surface area between the concentric layers. In contrast, turbulent flow is associated with higher flow velocities, where the gas molecules develop sufficient momentum to move between layers, especially at orifices and branch points in the airway tree (indicated by curved arrows in Figure 5). Under laminar flow conditions, the pressure drop along the airways for a given flow is linearly related to the flow. However, when the flow shifts from laminar to turbulent conditions, the pressure-flow relationship becomes nonlinear and the pressure drop is more closely related to flow squared rather than flow. Figure 5 shows that turbulence develops in the larynx, trachea, and central conducting airways, where the velocities of flow increase the intertial forces in relation to the viscous forces and raise the Reynolds number into the range where turbulence occurs.1 Once past the larynx and central conducting airways, the steep increase in total cross-sectional area slows the flow velocity reducing the inertial forces and Reynolds number to a point consistent with the establishment of fully developed laminar flow conditions (Figure 5). The importance of fully developed laminar flow in the periphery of the lung is that it minimizes convection and increases the diffusion of gases into and out of the depths of the lungs. Conversely, turbulent conditions contribute to a higher resistance in the central airways.
FIGURE 5.
The laryngeal orifice creates turbulent airflow (curly arrows) which extends into the trachea and is reinforced at branch points in the central airways. The airflow through the trachea is divided among an ever increasing number of airway branches, causing the velocity of airflow through the individual branches to diminish until a fully developed pattern of laminar flow is established.
IV. MECHANICS OF BREATHING
The bulk movement of gas into and out of the lungs is determined by the total resistance of the respiratory system and the compliance of the tissue in the lungs and chest wall. The total resistance to flow in the respiratory system (RRS) includes the resistance to flow through the airways, plus resistance to the movement of the lung and chest wall tissues that is in phase with flow (27). Upper airway resistance is the resistance between the atmosphere and the opening of the trachea below the larynx, while lower airway resistance is that between the trachea and alveoli. The resistance of the upper airways can be measured by relating flow in and out of the lung to the pressure drop from the mouth or nose to a point in the trachea just below the larynx, and it normally contributes ∼50% of RRS. The resistance to flow through the lower airways is measured by relating flow to the pressure difference between the trachea and the pleural space, which accounts for the remaining 50% of RRS. Airway resistance (Raw) can be measured in a body plethysmograph and excludes both lung and chest wall resistance (28, 36).
The development of the retrograde catheter technique by Macklem and Mead (61) provided the first opportunity to partition lower pulmonary resistance into central and peripheral components. The McGill group, led by Macklem, used this technique to both confirm and extend these findings by showing the same small airways that offer so little resistance in normal lungs become the major site of airway obstuction in COPD (41). Sometime later a Belgian group (120) used the retrograde catheter technique to show that small airways resistance in normal lungs was more consistent with Rohrer's original calculations than with those reported by Macklem and Mead and the McGill group. However, they did confirm the same marked increase in small airways resistance in lungs affected by COPD reported by the McGill group. Later still, a group from Japan (140) provided the first measurements in living humans, by measuring the pressure difference between a catheter placed in the peripheral airways through a bronchoscope and a catheter placed in the esophagus, to estimate intrapleural pressure. This study confirmed the relatively low resistance to flow through the small airways in persons with normal lung function, as well as the marked increase in small airways resistance in living persons with COPD. Moreover, two subsequent studies performed by Wagner and associates (132, 133), using a completely different approach based on a modification of a technique originally developed by Terry et al (116), confirmed that the resistance of the small conducting airways was low in normal living humans. Collectively, five of the six studies that have partitioned airway resistance within human lungs have shown that the resistance of the small airways <2 mm in diameter is low in the normal lung, and all three of the studies performed on subjects with COPD have shown that these same small airways become the major site of resistance to airflow in disease.
V. PHYSIOLOGICAL TESTS FOR SMALL AIRWAYS DISEASE
The rate at which a lung unit (i.e., alveolus, acinus, segment, or lobe) fills and empties is determined by the resistance to airflow offered by the airways leading to the unit, and the elastic properties (compliance) of the alveolar tissue in the unit. The product of the units of resistance (cmH2O·liter−1·s−1) and compliance (cmH2O/liter) simplify to time, because all the other units cancel. The time constant τ is defined by the time taken for the respiratory system to come to 63% of static equilibrium after a step change in pressure is applied to the lung in either inspiration or expiration.
where τ is time constant, R is resistance, and C is compliance.
From this relationship, it follows that when the time required to empty a lung unit exceeds the time between breaths, the gas that remains in the lung will be “trapped” in the lung. Moreover, in subjects who have small airways disease, the increase in breathing frequency associated with exercise shortens the time available to empty their lungs, to the point where the lung fails to empty before the next breath is taken, resulting in dynamic hyperinflation of the lungs.
The recognition that the same small airways represent a silent zone in the normal lung where disease can accumulate without being noticed (41) stimulated a search for new and better tests of small airways function, to detect disease before symptoms begin, and routine tests of lung function become abnormal. Many of the tests developed for this purpose in the late 1960s and early 1970s had previously been used to investigate the mechanical properties of the lungs in both health and disease. These included the measurement of the frequency dependence of dynamic compliance (Cdyn), the single-breath nitrogen washout (ΔN2/liter, closing volume and closing capacity), the density dependence of maximal expiratory flow, and maximal flows at lower lung volumes including the average forced expiratory flow between 25 and 75% of expired vital capacity (FEF25–75) and the instantaneous FEF rates at 50% and 25% of vital capacity (i.e., V̇max50 and V̇max25). Other than the FEF25–75, V̇max25 and V̇max50, which are measured on spirometric tracings and flow-volume curves respectively, these tests are no longer performed on a routine basis.
An amazing feature of normal lung function is that dynamic compliance (i.e., the change in volume/change in transpulmonary pressure at the points of zero flow at the beginning and end of each tidal breath) remains constant over breathing frequencies that range from 8–16 breaths/min at rest to 60–120 breaths/min during heavy exercise. This means that the time constants (τ) determined by the resistance (R) and compliance (C) of the many thousands of peripheral lung units that fill and empty synchronously are small and not very different from each other. Macklem and Mead (61) estimated that the τ of the airways <2 mm in diameter must be on the order of 0.01 s to allow the peripheral lung to empty normally, in the 0.5 s available at a breathing frequency of 120 breaths/min. Moreover, they estimated that a fourfold difference in τ between lung units would be sufficient to cause dynamic compliance to fall as breathing frequency increased. Woolcock, Vincent, and Macklem were the first to use measurements of dynamic compliance to diagnose small airways obstruction in persons with either mild chronic bronchitis or asthma when the subjects had nearly normal routine lung function tests (138).
A. Single and Multiple Breath Nitrogen Washout Techniques
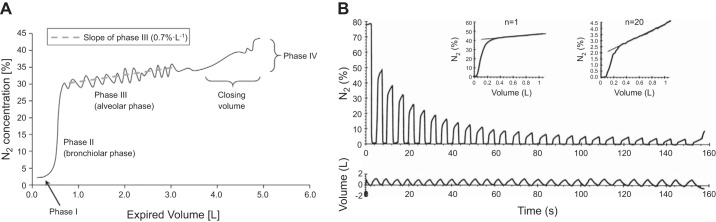
Early tests for small airways disease that used inert gas washout techniques were primarily based on the single breath nitrogen washout (SBNW), which is accomplished by taking a vital capacity breath of pure oxygen and measuring exhaled nitrogen (N2) during a subsequent slow exhalation to residual volume. During the SBNW (Figure 6A), N2 is absent in the washout of the dead space where no gas exchange takes place (phase 1) followed by a rapidly increasing slope in N2 contributed from units that do and do not exchange gas (phase 2), subsequently a prolonged slowly increasing slope occurs (phase 3), derived from alveolar units which is referred to as the alveolar plateau and is quantified by ΔN2/liter. Phase 3 is followed by a sudden increase in N2 (phase 4).
FIGURE 6.
A: an example of the changes in nitrogen concentration measured at the mouth during a single breath nitrogen washout (SBNW). The expiration that follows the inspiration of a single deep breath of 100% oxygen produces a flat phase 1 as the dead space empties. This is followed by an upward sloping phase 2 where the nitrogen concentration rises swiftly followed by a much more slowly rising alveolar plateau (phase 3). That is followed by phase 4 produced by closure of the airways supplying the better ventilated regions of the lung and a greater contribution from the regions that are less well ventilated which have a higher concentration of nitrogen. B: a multiple breath nitrogen washout curve (MBNW). Tidal volume (bottom panel) and expired nitrogen (top panel) are plotted against breath number. Each expiration has its own alveolar plateau. The alveolar plateaus for the 1st and 20th breath are shown in the inset normalized for the mean expired nitrogen concentration. The initial phase 3 slope is influenced by inhomogeneity in the acinar compartment, and the progressive increase in slope as a function of breath number is influenced by convective inhomogeneity. Calculating Sacin and Scond can indicate whether the origin of the inhomogeneity is in the peripheral or more central airways. [B from Verbanck et al. (127).]
More than 40 years ago, Dollfuss et al. (25) investigated the source of this abrupt change in N2 concentration by injecting boluses of radioactive xenon gas at different time points during an inspiratory maneuver between residual volume (RV) to total lung capacity (TLC). They found that a bolus delivered near RV goes preferentially to the upper regions of the lung because these airways remain open at RV, whereas boluses delivered at higher lung volumes were more evenly distributed to the mid and lower regions of the lungs as the airways in these regions open during lung inflation. During expiration, the gentle increasing slope of the N2 concentration in phase 3 is due to sequential emptying of regions of the lung that have different N2 concentrations at full inflation. The sudden rapid rise in N2 concentration which marks phase 4 occurs when airways in the gravity-dependent lung regions, which have the lowest N2 concentration, close. This transition occurs in normal individuals near RV, but with small airways disease, there is both an increase in the upward slope of phase 3 as well as an increase in the volume at which the airways close. The volume above RV at which phase 4 starts is termed closing volume, and can be expressed as a percent of vital capacity.
Closing capacity is the absolute volume at which closure starts (i.e., closing volume + residual volume). Because it is the small airways that close at low lung volumes (73), SBNW (closing volume and closing capacity) has the potential to provide information about early abnormalities in the small airways in COPD. The single-breath test also provides information about the evenness of lung emptying. The slope of the alveolar plateau (phase 3) would be flat if all lung units emptied homogeneously. The increasing slope of this curve indicates there is sequential emptying of units that have different N2 concentrations. The pleural pressure gradient contributes to the regional differences in N2 concentration, and sequential emptying is partially attributable to regional differences in small airway resistance and air-space compliance, that create regional differences in the time required to fill and empty different lung regions. Both the closing volume and the phase 3 slope are abnormal in some smokers (10). Moreover, these derangements have good correlation with pathological abnormalities in both purely conducting (membranous) as well as respiratory bronchioles (18, 139).
In contrast, the multiple breath nitrogen washout (MBNW) (124, 126) has recently regained popularity and is performed while breathing at functional residual capacity (FRC) (50). On the basis of theoretical models of the lung, it has been proposed that the MBNW (Figure 6B) can be used to distinguish between ventilation inhomogeneities originating in the acinar compartment (Sacin), as well as in the more proximal conducting airways (Scond), respectively, (51) where Scond is calculated as the increase in the normalized N2 phase 3 slope that occurs during progressive washout of N2, and Sacin is calculated from the initial N2 phase 3 slope corrected by Scond (123). Although Scond and Sacin have not been directly correlated with pathological changes in the airways and parenchyma, various modeling studies suggest that the measures reflect changes in the acinar and conducting airway compartments. Verbank et al. (123) have shown that patients with COPD who show evidence for emphysema based on diffusing capacity of the lung for carbon monoxide (DLCO) and CT have a higher Sacin than those with similar levels of airflow obstruction without evidence of emphysema. In contrast, bronchoconstriction produced by inhaled histamine increased Scond but had no effect on Sacin (127). In addition, King et al. (51) have shown that Scond, but not Sacin, is related to the volume of trapped gas following a methacholine challenge. Smokers who quit show a sustained increase in Scond but not Sacin, suggesting that abnormalities in the small conducting airways, but not the parenchyma, are partially reversible (125). Scond relates to the degree of airway closure measured by scintigraphy after inhalation of methacholine in asthmatics (32).
B. The Flow Volume Curve
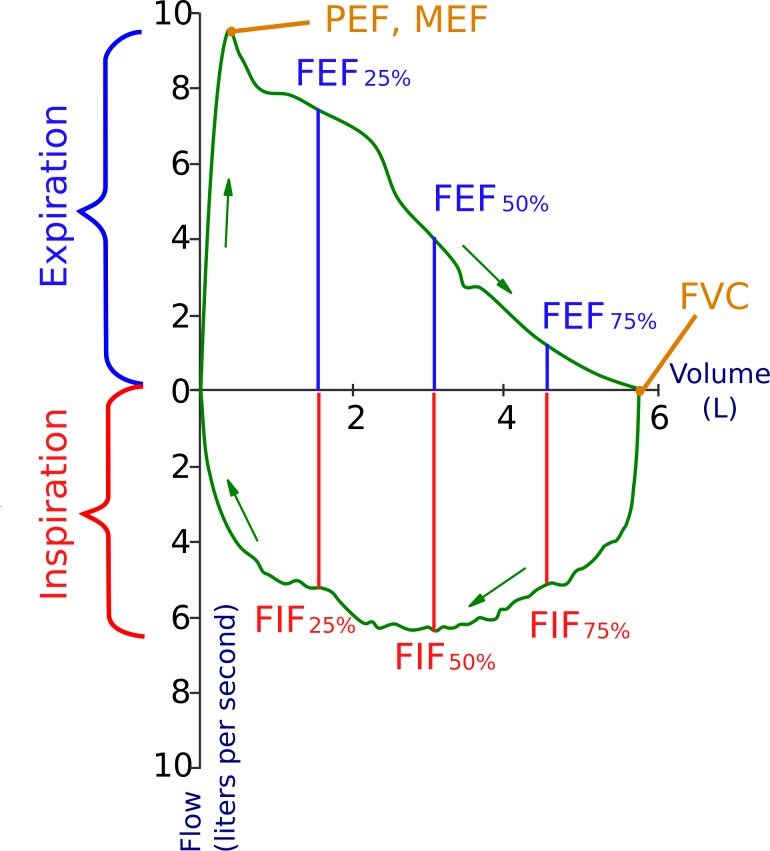
Figure 7 shows the flow of gas into and out of the lung during a maximal inspiration from RV to TLC, and a maximal expiration from TLC to RV. The major difference between inspiratory and expiratory flow is that airflow into the lung is entirely dependent on effort during an inspiratory maneuver, whereas airflow out of the lung is only dependent on effort over the first 10–15% of the expiratory maneuver. For the remaining 85% of the forced expiration, the relationship between flow and volume decreases linearly (in healthy subjects) with a relatively constant slope until RV is reached, irrespective of effort. This difference is explained by the fact that the inspiratory effort exerts forces that enlarge the airways throughout inspiration, whereas expiratory effort compresses the intrathoracic airways and limits flow. The reason for the decrease in expiratory flow is a progressive increase in the time constant of the lung. The units of the slope of the flow volume (FV) curve are liters/time/liters, which simplifies to 1/time (1/τ). As the lung empties, the lung's elastic recoil decreases and the caliber of the intraparenchymal airways decreases (i.e., both resistance and compliance increase). This change in τ is accompanied by a peripheral movement of the choke point in the lung.2 The reason that normal individuals can empty ∼80% of their vital capacity within 1 s is related to the relatively low resistance of the airways, and thus a short time constant. At high lung volumes, the choke point is in the trachea or major bronchi; however, as the airways narrow and recoil decreases, the choke point moves into the smaller bronchi and bronchioles. Because of this movement, flows at lower lung volume are disproportionately influenced by small airway dimensions. Thus forced expiratory flow values at 50 and 75% of the expired vital capacity (FEF50 and FEF75) are more sensitive to small airways disease. In disease, small airways are narrowed and/or lost, which contributes to peripheral movement of the choke point because of higher frictional pressure losses during expiration. These factors are reflected in the characteristic convex shape of the slope of the FV curve in disease. Because τ is influenced by both resistance and compliance, an increase in either airway's resistance or lung compliance will lengthen the time constant for lung emptying and cause scalloping of the lower third of the flow volume curve.
FIGURE 7.
The maximum flow volume diagram. Inspiratory flow is unrestricted and entirely dependent on effort over the whole vital capacity range. Peak expiratory flow (PEF) or maximal expiratory flow (MEF) is also effort dependent, but after ∼10% of the vital capacity is exhaled, flow becomes fixed irrespective of the effort and measures derived from this portion of the curve include instantaneous flows at 75, 50, and 25% of vital capacity and maximal mid expiratory flow rate (MMEFR) or MEF 25–75. Note that the flow rates achieved at the end of the forced expiration are much lower than the flow rates achieved at the same lung volume on inspiration due to dynamic compression of the intrathoracic airways. (Copyright: “Flow-volume-loop” by SPhotographer–Own work. Licensed under CC BY-SA 3.0 via Wikimedia Commons: https://commons.wikimedia.org/wiki/File:Flow-volume-loop.svg#/media/File:Flow-volume-loop.svg)
These same aerodynamic factors are relevant to tests of small airways function that are based on breathing a low gas density mixture, such as 80% helium/20% oxygen. Such mixtures have a lower density than air and will increase maximal achievable expiratory flow from regions of the lung where flow at the choke point is turbulent, but will have no effect if the flow regime at the choke point is laminar. Because laminar flow predominates in the peripheral small airways, the density dependence of maximal expiratory flow should theoretically decrease in patients in whom small airway narrowing moves the choke point from large to small airways. However, in practice, the density dependence of maximal expiratory flow did not prove to be an effective screening test and did not relate to pathological abnormalities in small airways (82). In patients with established COPD and definite bronchiolar pathology, density dependence may be preserved, possibly because flow-limiting segments remain in central airways despite the increase in peripheral resistance, or because turbulence develops in the peripheral airways. Additionally, the test has a large coefficient of variation when repeated on the same subject (94).
The basic premise behind the hypothesis that small airway tests will predict later decline in forced expiratory volume in 1 second (FEV1) is that these tests are more sensitive than simple spirometry; however, there is considerable evidence that this may not be correct. In fact, it has been shown that changes in FEV1 and FEV1/forced vital capacity (FVC) parallel those in the small airway tests (24, 26, 78), and although the absolute changes in FEV1 may be less than those in small airway test values, they are of equal or greater significance because the coefficients of variation of FEV1 and FVC are much smaller. The results of longitudinal studies have, in general, not confirmed the hypothesis that abnormalities in these originally proposed tests of small airway function predict longitudinal decline in FEV1 (11, 80). Changes in the forced expiratory spirogram occur in young smokers and probably identify those at risk for later decline as well, or better, than these so-called small airway tests (134). Because spirometry is easier and cheaper to perform, it has, to date, remained the most useful test in the clinical management and epidemiologic investigation of patients who have, or are at risk of, COPD (106).
C. The Forced Oscillation Technique
More recently, developments in the forced oscillatory technique and detailed analysis of the MBNW technique have suggested novel and easier ways to measure small airway abnormalities. Airway and lung tissue properties can be assessed using impedance oscillometry which includes the forced oscillation technique and impulse oscillometry. With the use of these methods, pressure fluctuations are applied to the airway opening, using sound waves, and the resulting flow signals are related to the pressure signals to determine the impedance of the respiratory system, which includes airway and parenchymal properties. A range of frequencies of pressure oscillations between 0.1 and 40 Hz can be used. Although first described in 1956 by Arthur Dubois (29), impedance oscillometry was not used extensively until the last decade when, advances in computing power and methods to analyze the signals, enabled more extensive research and clinical use of the technique. Advantageously, it can be applied during normal tidal breathing, at a variety of lung volumes, and requires little or no effort from the subject or patient.
The relationship between the applied pressure and flow is impedance, which can be separated into the resistance and the reactance of the respiratory system. Resistance is the part of the pressure signal which is in phase with flow and represents the pressure required to overcome the friction generated by gas flow within the conducting airway as well as the viscous properties of the chest wall, parenchyma, and airways. The reactance is related to the elastic properties of the system and to inertial forces required to accelerate and decelerate the gas. The relative contribution of the different components of impedance, and the contribution of small and larger airways to resistance, vary as a function of the oscillation frequency. At high oscillation frequencies (20 Hz), the signal is dominated by the properties of the upper airways, while at low frequencies (below 10 Hz), it reflects the resistance of the entire trachea-bronchial tree, including the small airways. At lower oscillation frequencies, reactance represents the elastic properties of the parenchyma, airways, and chest wall, whereas at higher frequencies inertial forces predominate. At resonant frequency (∼8 Hz in normal subjects), the elastic and inertial forces are equal and opposite and thus cancel each other. The pressure-flow relationship at this frequency solely reflects the resistance of the system.
However, reactance does not only reflect elastic properties of the system; it is influenced by inhomogeneity of airway caliber and ventilation and by airway closure or flow limitation (60). The frequency dependence of reactance measured during the forced oscillatory technique is thus analogous to the frequency dependence of dynamic compliance that was originally proposed as an early test for small airway obstruction (138). Steeper slopes of resistance, and reactance versus frequency, are believed to be sensitive indicators of small airways disease. Reactance has also been suggested as a method to detect the development of flow limitation during tidal breathing and is sensitive to airway closure, which can happen in the small airways of subjects with disease (21, 81).
VI. IMAGING THE PERIPHERAL LUNG
The introduction of X-ray computed tomography (CT) of the entire thorax was one of the most significant advances in chest medicine in the 20th century. Rapid development of multidetector computed tomography (MDCT) scanners has enabled volumetric CT images of the entire thorax to be obtained within a single breath hold (<10 s) with spatial resolution of 600–800 μm in the absence of motion artifact, and spatial resolutions of ∼1,000 μm (1 mm) in the presence of motion artifacts introduced by pulsating vessels and a beating heart. This technology has made it possible to observe and quantify the extent and severity of the emphysematous destruction of the lungs in living persons as well as locate the regions of the lung where the gas is trapped when vital capacity is reduced.
Although the small airways <2 mm in diameter are too small to be visualized by conventional MDCT scanning, various features derived from CT images indicate the presence of small airway disease. Nakano et al. (76) found that the dimensions of relatively large airways assessed using conventional MDCT reflect small airway dimensions measured histologically in the same lungs. This finding suggests that the process which thickens and narrows the peripheral airways in disease also acts on the larger airways which can be visualized on CT, a conclusion that is supported by an earlier, purely histological study by Tiddens et al. (118). The other feature that suggests small airways disease can be appreciated on conventional MDCT is “gas trapping.” Gas trapping behind closed airways is indicated by areas of relatively low lung density on expiratory scans. It has been suggested that the sum of all CT voxels with a density less than −856 Houndsfield units on an expiratory CT scan, normalized by total lung volume, can be used as a measure of gas trapping (90). However, such regions may also reflect areas of emphysema. The ability to distinguish between the contribution of small airways disease and emphysema has been improved by the recent development of parametric response mapping (PRM) techniques, which allow information from all the lung voxels in an inspiratory MDCT scan to be registered to the voxels present on an expiratory MDCT scan (38). This innovation has made it possible to identify regions of lung with functional small airways disease (fSAD), and to distinguish these regions from those affected by emphysema when examined near TLC. Voxels affected by emphysema have low attenuation values on both inspiratory and expiratory scans, while those with functional small airways disease only have lower than expected attenuation on expiratory scans because they trap gas excessively on expiration. In a recent study, Boes et al. (6) used PRM analysis to monitor the progression of fSAD and emphysema over a 1-yr period. The results strongly suggest that fSAD represents a transitional phase between normal lung and emphysematous tissue destruction. This conclusion is consistent with earlier micro CT data suggesting that terminal bronchioles are destroyed prior to the onset of emphysematous destruction in COPD (63).
The introduction of micro CT has made it possible to achieve radiological images of the lung at a spatial resolution of 10 μm, so terminal and respiratory bronchioles as well as alveoli can be accurately seen in three dimensions and their number per unit lung determined (63, 128). Micro CT studies of the small airways have also confirmed that the terminal bronchioles have a catenoid shape in which the lumen at both ends is larger than in the middle, and that their minimal diameter in an inflated lung specimen is 424.0 ± 48.0 μm (63, 128). Moreover, McDonough et al. (63) have shown that there are 6.9 ± 1 terminal bronchioles/ml normal human lung in which the average distance between alveolar walls (i.e., the mean linear intercept Lm) ranged from 225 to 600 μm with an upper 95% confidence interval of 489 μm (63). Additionally, the combined use of MDCT scans of fully inflated rapidly frozen human lung specimens to compute total lung volume and micro CT scans of specimens removed from these lungs to count the mean number of terminal bronchioles per milliliter lung, has enabled the estimation of the total number of terminal bronchioles per lung. There are approximately 40,000 terminal bronchioles per lung pair in the lungs of control subjects, and this number is reduced by ∼90% in lungs from patients with very severe COPD treated by transplantation (63). Furthe rmore, direct comparison of the number of terminal bronchioles per sample to measurements of Lm in the same lung samples indicate that destruction of the terminal bronchioles begins before there is any micro CT evidence of emphysematous destruction, and long before the emphysematous lesions become large enough to be visualized on MDCT scans (63). Moreover, preliminary data based on micro CT studies of paraffin-embedded lung tissue samples obtained from patients who required lung resection for cancer (52) indicate that the destruction of terminal bronchioles occurs in mild (Global initiative for Obstructive Lung Disease, GOLD1) and moderate (GOLD2) stages of COPD.
VII. EFFECT OF ATMOSPHERIC PARTICULATE MATTER ON THE LUNGS
The particulate matter (PM) suspended in the atmosphere that is 10 μm or less in diameter (PM10) is primarily generated from plants, tree pollens, road dust, the burning of biomass fuel for cooking and heating, the cleaning and harvesting grain, and industrial processes that generate smoke and dusts. Smaller particles which are 2.5 μm and less in diameter (PM2.5) are primarily generated by internal combustion engines, industrial processes such as the purification (smelting) and processing of metals, and the natural burning of biomass fuels and forest fires. As particles enter the atmosphere, they are picked up by the wind and remain suspended for varying periods of time depending on their mass and size. For example, particles with a diameter between 10 and 2.5 μm remain suspended for relatively short periods (minutes to hours) and travel relatively short distances, whereas PM2.5 can remain suspended in the air for days or weeks and travel hundreds of miles. The deposition of these suspended particles onto the surface of the lung is largely dependent on the PM volume, mass, and the time that they have to settle. During inspiration, larger particles that develop the greatest momentum (i.e., mass x velocity) leave the flowing stream of inspired gas at bends and branch points, because they are deposited on the surface of the more central airways by impaction. In contrast, the finer particulates (PM2.5) have less momentum and remain suspended in the inhaled air until they reach regions of the lung where gas moves primarily by diffusion. This means that PM2.5 preferentially deposit in the transitional zone because even the smallest suspended particles diffuse much more slowly than the gases they are suspended in.
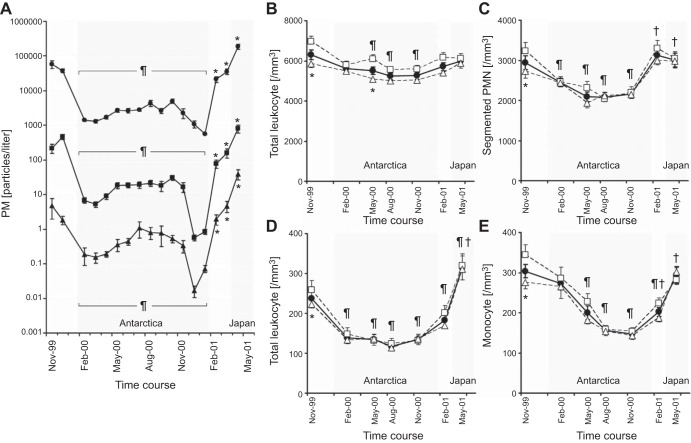
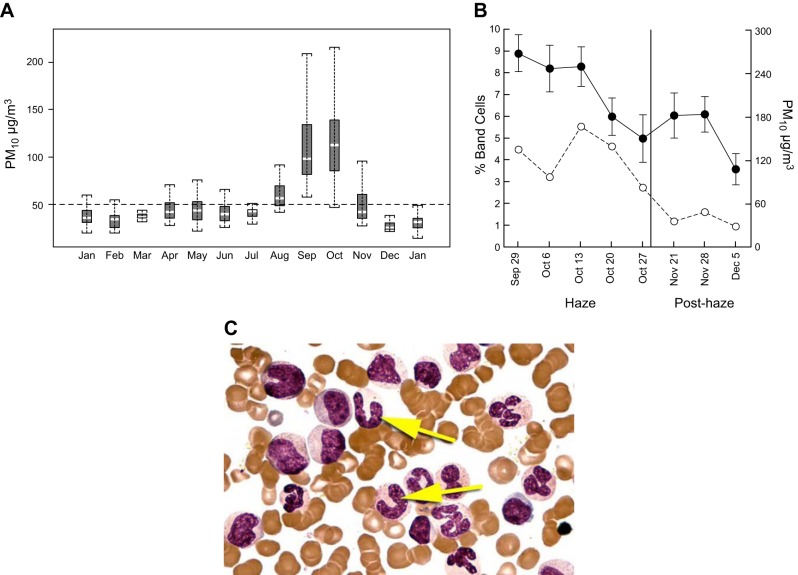
The phagocytosis of particles within the lung has been shown to induce both a local and systemic response that stimulates the bone marrow to increase the release of leukocytes into the circulating blood. A striking example of this relationship between atmospheric PM and the level of circulating leukocytes was reported from an observational study of 39 healthy persons participating in the 41st Japanese Antarctic Research Expedition (JARE41) (99). The scientists measured the levels of air pollution surrounding the ship that carried the participants from Japan to Antarctica, during the 12 months they remained in Antarctica, and on their return voyage to Japan. During the entire expedition, a physician scientist responsible for the health of the group collected blood samples and performed spirometry on all 39 subjects at regular intervals. These data show (Figure 8A) that the concentration of small atmospheric particles <2 μm in diameter fell from ∼100,000/liter in Japan to ∼1,000/liter in Antarctica, remained near that level for the entire 12 months the group remained in Antarctica, and returned to the initial values as they returned to Japan (99). A similar reduction and return to baseline values was observed for both medium-sized (2–5 μm) and larger (>5 μm) atmospheric particles. Importantly, these substantial changes in atmospheric PM were associated with modest reductions in the total circulating white blood cell (WBC) counts (Figure 8B) and more prominent reductions in circulating polymorphonuclear leukocytes (PMN) (Figure 8C) and band cell PMNs (Figure 8D) that were used to monitor the output of PMN from the bone marrow. Similar changes to those observed for the PMN were also observed in the circulating monocyte counts (Figure 8E), which are the main source of the pulmonary macrophages. A surprising feature of this study was that, although the smokers WBC remained high, contrary to everyone's prediction, they also like nonsmokers showed a similar decrease in marrow output with a decrease in atomospheric PM exposure.
FIGURE 8.
The changes in atmospheric particulate air pollution during the 41st Japanese expedition to the south pole. A: PM 0.3-0.2, PM 2.0–5.0, and PM 5.0 levels during the entire journey from Japan to Antarctica and return. B: total leukocyte count. C: segmented polymorphonuclear leukocyte (PMN) count. D: band-form PMN count that indicates the early release of PMN of the marrow. E: monocyte counts, over the same time periods that the air pollution was measured. Although there were differences in leukocyte counts between smokers (open squares) and nonsmokers (open triangles), these differences tended to disappear in Antarctica where the levels of air pollution were very low (please see the original report for the complete statistical analysis). [Modified from Sakai et al. (99).]
A study on the effect of increasing atmospheric PM air pollution on circulating leukocyte counts was conducted in Singapore where widespread forest fires in the Indonesian islands of Southern Kalimantan, Sumatra, and Java in 1997 provided Tan and colleagues with the opportunity to explore the effect of acute atmospheric PM air pollution on a group of healthy young persons undergoing military training in Singapore (114, 122). For an 8-wk period between late August and early November, the prevailing winds enveloped Singapore with a biomass-generated smoke containing high levels of gases (NO2, O3, SO2) and ultrafine particles capable of passing through the filters of most air-conditioned buildings and penetrating deep into the lungs (Figure 8A). This natural event allowed Tan and associates to examine the hypothesis that a defined increase in air pollution stimulates a systemic host response. The subjects were 30 male volunteers between 19 and 24 years of age that were engaged in an intensive physical training programs as part of the national service in Singapore. These results showed that the pollution was associated with an increased level of mature PMNs and their band cell precursors (Figure 9,B and C) in the peripheral blood. There was a progressive increase in the circulating band cell PMNs until the height of the haze, followed by a steady return to control values as the haze cleared.
FIGURE 9.
A: box and whisker plot of the levels of PM 10 air pollution in Singapore from January 1996 to January 1997, to illustrate the sharp but still modest increase in air pollution that occurred between August and November due to the South East Asian haze of 1997. B: data from a group of military recruits that were undergoing basic training during the entire period of the haze where the data show a clear association between circulating PMN band cell forms and the level of particulate air pollution as the haze cleared. C: photomicrograph of the human bone marrow to illustrate the clear difference between band cell PMNs (yellow arrows) and mature PMNs. Importantly, the band cell PMNs only leave the marrow in small numbers compared with mature PMNs, and the rise in band cell counts shown in Figures 8 and 9 provides clear evidence that marrow output has been increased. Please see the original reference for the statistical analysis. [From Tan et al. (114). Reprinted with permission of The American Thoracic Society. Copyright 2016 American Thoracic Society.]
Collectively, these two field studies provide direct evidence in support of the hypothesis that changes in the levels of PM air pollution are associated with changes in the levels of circulating leukocytes in the blood. Furthermore, experiments in rabbits have shown that the signal for this change is derived from the phagocytosis of the particulate matter by alveolar macrophages, in that either placing particles in the lungs or feeding particles to alveolar macrophages in vitro and placing the supernatant down into the lungs, produces a similar effect (122).
VIII. EFFECT OF SMOKING TOBACCO ON THE SMALL AIRWAYS
In contrast to the relatively small effects produced by inhaling atmospheric PM, the repetitive inhalation of tobacco smoke 20–30 times each day over 20–30 years is a well-established risk factor for the development of disease in the lung, as well as cardiovascular and other organ systems. However the extent and severity of the lung injury produced by smoking depends on several factors including the blend of tobacco, the type of paper used to contain the tobacco while it is burned, and the type of tip used to filter the smoke before it is inhaled into the lungs. It has been estimated that ∼4,700 different toxic substances are in tobacco smoke (8), and these substances include milligram quantities of carbon oxides, nitrogen oxides, nicotine and picrogram levels of amides, free radicals, heavy metals, volatile compounds, ketones, and nitriles. In addition, the storage and curing of tobacco is associated with the rapid growth of a range of fungi and bacteria that contribute microbial toxins to the inhaled smoke (55). Metagenomic studies of the microbial content of tobacco have revealed that at least 15 different classes of bacteria can be present, including a broad range of pathogens (83, 102).
There is also substantial experimental evidence that lung epithelial cells and alveolar macrophages generate a rich mixture of inflammatory mediators when exposed to atmospheric particles, and a review of the evidence that these products can be measured in induced sputum, BAL fluid, and blood has been presented elsewhere (4). Moreover, a single puff of cigarette smoke is estimated to contain more than 1014 oxidative free radicals such as superoxide radicals, nitric oxide, or tar-semiquinone (86). Cigarette smoke also activates macrophages and neutrophils to release reactive oxygen species (ROS) that can cause lipid peroxidation, leading to disruption of cellular membranes and inactivation of receptors, transcription factors, and enzymes (87). For example, a number of products of oxidative stress have been shown to inactivate histone deacetylase (HDAC)-2, an epigenetic enzyme integral to the regulation of inflammatory gene transcription (88). HDACs deacetylate lysine residues on histones, causing a conformational change of the chromatin that makes local DNA inaccessible to transcription factor binding (3). Upregulation of HDAC activity is one of the mechanisms by which corticosteroids inhibit inflammation. The activities of HDAC-2, -3, -5, and -8 (46, 88) have all been demonstrated to be reduced in COPD subjects, suggesting that oxidant inactivation of HDACs may be particularly relevant to steroid insensitivity, a well-recognized phenomenon in COPD. Therefore, these data provide evidence in support of the hypothesis that increased oxidative stress may upregulate the normal inflammatory response to cigarette smoke and other environmental stimuli in COPD. However, it remains unclear why only certain cigarette smokers develop COPD, when exposure to inhaled oxidants from cigarette smoke would be fairly consistent between individuals with comparable smoking histories. Future work to answer this question could contribute to an improved understanding of the nongenetic factors (e.g., diet) that may modify the antioxidant balance. The closing sections of this manuscript will attempt to review the progress that has been made with respect to the cellular and molecular mechanisms associated with bronchiolar and alveolar tissue remodeling and destruction in COPD.
IX. THE EPITHELIAL BARRIER AND MUCUS SECRETION
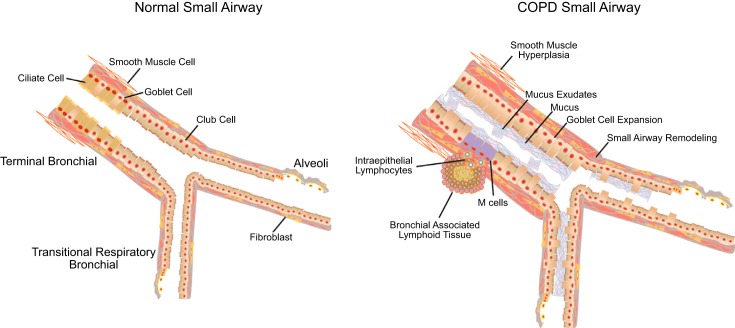
The airways are lined with an array of pseudostratified columnar cells that include ciliated, serous, goblet, basal, and Club (previously known as Clara) cells that are joined together by tight junctions, adherens junctions, and desmosomes, which form a formidable barrier to transport across the epithelial surface. In the large conducting airways, ciliated cells comprise ∼60% of the cells, but in the smaller airways of the normal lung both ciliated and goblet cells diminish in number and eventually disappear as the serous, and to a lesser extent the Club cells become the common cell lining the airway surface (59, 136). The inhalation of toxic particles and gases associated with cigarette smoke damages the epithelium and disrupts tight junctions in ways that both reduce mucociliary clearance and increase epithelial permeability. In the bronchi, these structural changes include goblet cell and squamous cell metaplasia, and a reduction in ciliated cell number and submucosal gland hypertrophy (Figure 10). Electron microscopic studies have shown that the discrete patches of mucus observed in healthy airways are replaced by continuous layers of mucus that cover the surface of the airways, in subjects with chronic bronchitis (47). In COPD, the small conducting airways also show evidence of epithelial disruption and goblet cell metaplasia (19, 97), as well as inflammatory immune cell infiltration (96), and fibrosis (131). Moreover, these pathological changes are associated with thickening of the walls of the small conducting airways by a process that includes thickening of the epithelium, the bronchial smooth muscle, and adventitia (40).
FIGURE 10.
Structural alterations that can be seen in the small airways in COPD. These include increased numbers of goblet cells and decreased numbers of Club (Clara cells) leading to mucus plugging, hyperplasia of smooth muscle, fibrosis of the airway wall, and bronchial-associated lymphoid tissue with intraepithelial lymphocytes that potentially capture antigens by interacting with microfold (M) cells or by extending transepithelial projections into the lumen.
The mucus hypersecretion induced by inhalation injury is a complex and incompletely understood phenomenon, leading to adverse effects on the structure and function of cilia (57, 113, 129). It is known that compounds in cigarette smoke, such as acrolein, increase mucus production (23), and it has also been shown that many stimuli (13) including cigarette smoke activate epidermal growth factor receptors (EGFR) inducing mucus overproduction (112). The exact molecular pathways that alter the number of goblet cells is currently under active investigation. What is clear is that the appearance of goblet cells within small airways where they are normally either sparse or absent, as well as the disappearance of ciliated cells and reduced function of the cilia that remain, contribute to the accumulation of mucus exudates within the small airways of patients with COPD.
Airway mucus consists of secreted polymeric mucin proteins, MUC5AC and MUC5B, which serve as the organizing framework of a mucus gel containing carbohydrate ligands that sequester pathogens and form a sink for host-protective proteins and peptides (i.e., IgA, lysozymes). In chronic lung diseases such as COPD, the composition and macromolecular structure of airway mucus changes due to an increase in solids of 15% versus the normal 3%, which changes its biophysical properties to a highly viscous and elastic mucus that is not easily cleared (17, 31, 53, 117).
On the basis of these observations, it is not surprising that this combination of increased production of highly viscous mucus in combination with reduced clearance caused by partial destruction of the epithelial surface and damage to the cilia of the remaining cells, first narrows and then closes the lumen of the smaller conducting airways. A major consequence of this occlusion is that it provides an opportunity for replication of bacteria that can no longer be cleared toward the mouth. Moreover, competition for space from within the expanding microbiome might account for the maintenance of density with loss of diversity that was observed when the microbiome of control lung tissue was compared to the microbiome of lung tissue from patients with COPD (112). The combination of increased replication and loss of diversity could contribute to the emergence of different stains of pathogenic bacteria that Sethi and Murphy and their colleagues have observed (103, 104). Pathogenic bacteria, such as Hemophilus influenza, Streptococcus pneumoniae, and Moraxella catarrhalis, have been detected in 25% of stable COPD patients and in over 50% of COPD patients during exacerbation (101). Similarly, viruses can be detected in 10–15% of sputum samples from persons with stable COPD, and 30–60% of patients during acute exacerbation of COPD (105). Irrespective of whether the infection is viral or bacterial in origin, it is well established in the literature (70, 72) that COPD exacerbations are associated with a variety of infiltrating cells (i.e., neutrophils, eosinophils, macrophages, CD4+, and CD8+ T cells), as well as a variety of enzymes (neutrophil elastase and MMP-9) and cytokines (CXCL8, TNF-α) in sputum and bronchoalveolar lavage (BAL) fluid (103). However, it is probably premature to attribute these changes to infection with either bacteria or viruses unless there is direct evidence that one of these microbes is present in the sputum or BAL fluid (62, 71). Furthermore, although acute exacerbations are recognized as a characteristic feature of the natural history of COPD, which occur with an average frequency of one to two exacerbations annually. This increase in exacerbation frequency is only associated with a small but statistically significant reduction in FEV1 of ∼5 ml/yr (13).
Reports from several laboratories have shown that the decline in FEV1 in COPD is linked to the infiltration of both large and small airways and the gas exchanging tissue by macrophages, PMN, CD4, CD8, and B-cell lymphocytes. Moreover, one study that had the opportunity to compare tissue from control lungs (i.e., smokers with normal lung function) with tissue from persons at all four stages of the GOLD classification of COPD severity (GOLD 1–4) showed that this inflammatory-immune cell infiltration is associated with a sharp increase in tertiary lymphoid organ formation in severe (GOLD 3) and very severe (GOLD 4) COPD (76). Although this histological response was once thought to be a curiosity, developments over the past decade have shown that this pattern of histopathology that is currently termed “lymphoid neogenesis with tertiary lymphoid organ formation” represents an important pathophysiological process. This histological process has the potential to link persistent innate and adaptive immune inflammatory responses to abnormal tissue repair in a variety of different disease states that include rheumatoid arthritis, Hashimoto's thyroiditis, Sjogren's syndrome, chronic Helicobacter gastritis, and chronic Lyme disease (2).
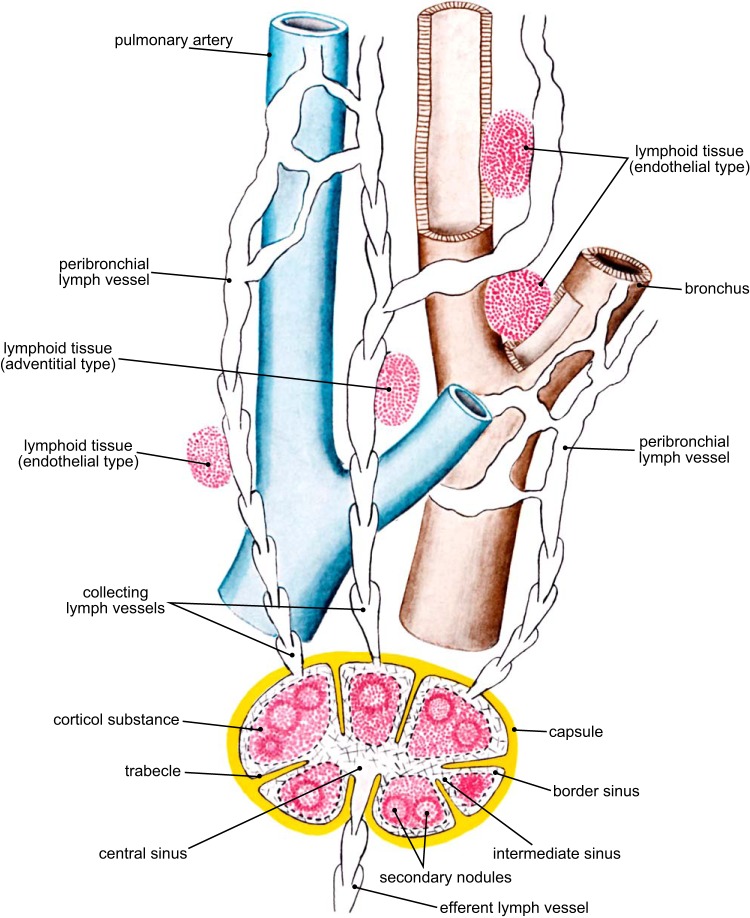
Lymphoid neogenesis with tertiary lymphoid organ formation represents a combined response from both the innate and adaptive immune cells that have their origin in the bone marrow. The innate immune response in the lung is initiated when bone marrow-derived macrophages phagocytose the fine particulates deposited on the lung surface by atmospheric pollution (68,69) and/or repetitive tobacco smoke inhalation. Normally, when released from the marrow, T lymphocytes further develop in the thymus, where the T cells that recognize host antigens are destroyed, and the T cells that recognize foreign antigens are allowed to mature, reenter the circulation, and relocate to secondary and tertiary lymphoid organs. These cells function as either helper T cells that stimulate B cells to become plasma cells that produce antibodies, cytotoxic T cells that provide the cellular component of the adaptive immune response, or regulatory T cells (T regs) that control the immune response. In contrast, B cells produced in the marrow reach maturity in secondary lymphoid structures (i.e., the lymph nodes and spleen) and in the tertiary lymphoid organs, where they develop as mucosal associated lymphoid tissue (MALT). In the lung these accumulations of immune cells in tertiary lymphoid organs are commonly referred to as bronchial associated lymphoid tissue (BALT) or inducible BALT (iBALT), even though they are also known to develop in alveolar tissue, around vessels, and in the lymphatic endothelium. A drawing from Nagaishi's text book on the functional anatomy of the lung describes these lymphoid structures which have been recognized since the 1930s (Figure 11) (74).
FIGURE 11.
A diagram from Nagaishi's text book on the functional anatomy of the lung showing a secondary lymphoid organ (i.e., a lymph node) that has a capsule and afferent and efferent lymphatics, as well as tertiary lymphoid organs that are located beneath the epithelium of airways and endothelium of lymphatic vessels. The cellular content of the tertiary lymphoid organs is similar to that observed in lymph nodes, and they are capable of forming germinal centers and may be involved in local immunoregulation. [From Nagaishi (74).]
Figure 11 also shows that the secondary lymphoid organs (i.e., true lymph nodes) have afferent lymphatics that penetrate their capsule and empty into the marginal sinus which allows the lymph to percolate through the cortical substance before collecting in a central sinus and exiting the node through the efferent lymphatic vessel. The lymph nodes are usually located close to the hilum, but occasionally appear as isolated nodules in the periphery of the lung that are mistaken for lung cancer and surgically removed. In contrast, tertiary lymphoid organs lack both a capsule and afferent lymphatic vessels and are primarily located away from the hilum in the periphery of the lung. In the lung, these BALT structures extend from the lumen of the airway surface and are covered with an epithelium containing microfold (M) cells capable of transporting antigens from the airway lumen into the tertiary lymphatic collections (Figure 12). Although relatively sparse in the healthy lung, tertiary lymphoid organs increase sharply in number in both small airways (40) and lung parenchyma (121), and especially in severe (GOLD 3) and very severe (GOLD 4) categories of COPD (40). In COPD, these tertiary lymphoid follicles often form germinal centers that are the hallmark of an adaptive immune response to either foreign or auto-antigens (89). Baraldo et al. (2) have convincingly demonstrated that patients with pan-lobular emphysema related to alpha-one antitrypsin deficiency develop similar accumulations of lymphoid collections to that observed in smokers with normal alpha-one antitrypsin levels who develop centrilobular emphysema (69). Collectively, these data indicate that destruction of bronchiolar and alveolar tissue is closely associated with lymphoid neogenesis, and tertiary lymphoid organ formation associated with an adaptive immune response in both of these phenotypes of COPD. However, the antigen(s) driving this adaptive immune response could be quite different in the two types of disease or even in different individuals. Exactly how this adaptive immune response might occur remains to be clarified. Similarly, it is also not known whether this response is involved in the pathogenesis of tissue destruction in COPD or more simply part of the response to repeated infections that occur in the later stages of both of these phenotypes of COPD.
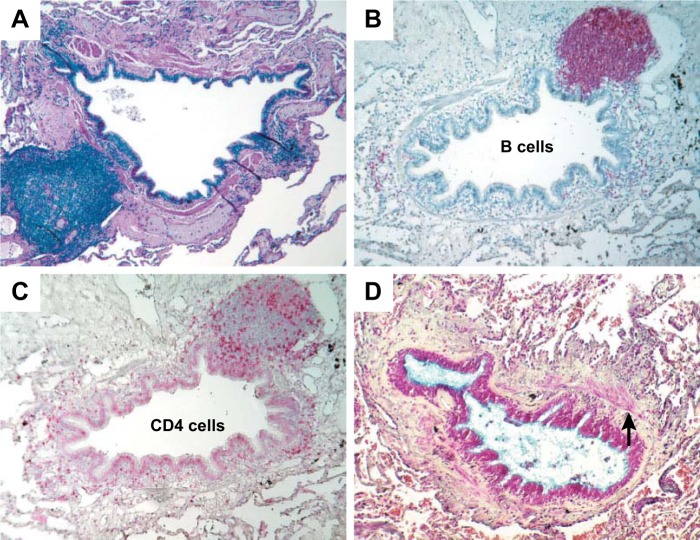
FIGURE 12.
A: a collection of bronchial lymphoid tissue with a lymphoid follicle containing a germinal center (GC) surrounded by a rim of darker-staining lymphocytes that extend to the epithelium of both the small airway and alveolar surface (Movat's stain, ×6). B: another follicle, in which the germinal center stains strongly for B cells (×6). C: a serial section of the same airway stained for CD4 cells, which are scattered around the edge of the follicle and in the airway wall (×6.5). D: an airway that has been extensively remodeled by connective tissue deposition in the subepithelial and adventitial compartments of the airway wall. The arrow points to the smooth muscle that separates the subepithelial from the adventitial compartments (Movat's stain, ×6). [From Hogg et al. (40), with permission from Massachusetts Medical Society.]
BALT follicles are anatomically and functionally well organized, consisting of memory and naive B cells, T cells, plasmacytoid and follicular dendritic cells, which allow for T cell and B cell priming and clonal expansion (9). Through their response to antigens arriving from the airway surface, these follicles are thought to mediate local immune responses and maintain memory cells within the lung tissue. The observation that B cell activating factor (BAFF), a member of the tumor necrosis factor family, is increased in lymphoid follicles in the lungs of patients affected by COPD compared with controls, suggests that BAFF expression may maintain the B-cell population providing a possible mechanism for the development and maintenance of lymphoid follicles in COPD (84). Importantly, many of the B cell follicles within the BALT of COPD patients show evidence of clonal B cell populations and ongoing somatic hypermutation, suggesting that these B cells are responding to an antigen (121). At present, little is known about the antigens that actually drive this response, but microbial antigens, cigarette-smoke derived antigens, breakdown products from the extracellular matrix, and other auto-antigens have all been suggested (9).
The development of local adaptive immunity is usually beneficial to the host by combating an invasive tumor or infection, but conversely can be detrimental if it destroys the joint tissue of patients with rheumatoid arthritis, the gut in inflammatory bowel disease, or the small airways and alveoli in patients with COPD (1). Recent studies in our laboratory support that the local adaptive immune response may be important in the development of COPD. We have reported a unique 127-gene expression signature for emphysematous tissue destruction measured by micro CT (14). Suzuki et al. (110) examined the overlap of the expression of these 127 genes with the expression signatures associated with infiltrating inflammatory immune cells in the lungs of subjects with advanced but heterogeneous emphysema. There was substantial overlap of the emphysema signature and that of infiltrating macrophages, CD4, and B cell lymphocytes but no overlap of the signatures for emphysema and neutrophil infiltration. Because the former cell types are those found in lymphoid follicles, these data support the hypothesis that the adaptive immune response is playing an important role in tissue destruction. Although this hypothesis is attractive, it is inconsistent with animal experiments reported by D'Hulst et al. (115) who demonstrated in severe combined immunodeficiency (SCID) mice, which completely lack B cells and are unable to form germinal centers, still develop emphysema when exposed to smoke. Thus, although BALT is associated with severe COPD and cigarette smoke induces BALT in animal models, the causal link between BALT and COPD remains unclear. Furthermore, it is unknown if BALT is beneficial or harmful in the progression of COPD (9), and strongly indicates that further research is needed into this difficult problem before firm conclusions are reached.
Irrespective of whether the development of BALT is beneficial or harmful, and the nature of the antigens driving their development in COPD, it is relevant to ask what are the mechanisms by which antigens are processed and presented to the lymphoid tissue. In the last 5 years, innate lymphoid cells (ILCs) have been identified as regulators of hematopoietic and nonhematopoietic cells in immunity, inflammation, and homestatisis of the body's tissues (30, 107, 108). The ILC family encompasses cytotoxic natural killer (NK) cells, lymphoid tissue inducer (LTi cells), and noncytotoxic ILC populations (30, 65, 68, 77, 85), which are now known to play an important role in innate immune responses to viruses, bacteria, fungi, and parasites at mucosal surface barriers. ILCs have been shown to play a role in a number of chronic inflammatory diseases including inflammatory bowel disease (IBD) (12), (5, 37), chronic rhinosinusitis (67), atopic dermatitis (100), and psoriasis vulgaris (130). A recent study used a smoke exposure model in mice to follow the response of ILC cells following viral infection to model an acute COPD exacerbation (49). In this model, smoke decreased IL-33 receptor (ST2) expression on ILC2 cells, while elevating ST2 expression on macrophages and NK cells, thus altering IL-33 responsiveness within the lung. Abrogation of IL-33 signaling (using IL-33 and ST2 receptor knockout mice) conferred complete protection during the viral induced exacerbations and prevented inflammation and weight loss (49). Based on these findings, the authors concluded that smoke alters the lung microenvironment to facilitate an alternative, IL-33-dependent, exaggerated proinflammatory response to infection, which exacerbates the disease process. The recognition that ILC cells have the capacity to communicate with a variety of both hematopoietic and nonhematopoietic cells, and their location both within lymphoid and nonlymphoid tissues, confers a remarkable potential for ILCs to regulate physiological processes within the body. A very recent publication by De Grove et al. (20) has shown that ILC cells are present and change their distribution in human lungs affected by COPD. A clearer understanding of how ILC responses are dysregulated in chronic inflammatory conditions might offer therapeutic potential to regulate the pathological responses that occur in the lungs of COPD patients.
X. SMALL AIRWAYS INFLAMMATION AND REMODELING
The repair process that follows tissue injury involves the activation and suppression of a number of fundamental physiological processes including the coagulation process to initiate damage control. This is then followed by the development of an acute inflammatory process that brings in PMN and macrophages into the injury site to protect against infection and perform the initial demolition process that removes the debris formed by dead and dying tissues. These important early steps prepare the site for activation of both local fibroblasts and migrating myofibroblasts and fibrocytes that remodel collagenous and noncollagenous tissues into scar tissue. Following a single injury the PMN cells have usually left the site before the resident fibroblasts and migrating myofibroblasts and fibrocytes are activated to drive the tissue repair process. In sharp contrast, repetitive injury is associated with persistence of inflammatory immune cell infiltration during the remodeling of the connective tissue that increases the potential for the development of an abnormal repair process creating further injury with abnormal scar formation and potentially permanent destruction of damaged tissue. As it is difficult to imagine a better example of repetitive injury than the inhalation of the smoke from 20–30 cigarettes/day for 30–40 years, it is not surprising that several groups have reported associations between infiltration of the lung tissue by inflammatory/immune cells and the decline in lung function associated with smoking (19, 40, 43, 47, 69, 95–98). The study by Finkelstein et al. (33) stands out as the first to have compared inflammatory immune cell infiltration into the lung tissue directly with histological estimates of emphysematous destruction. Much more recently, preliminary data reported by Suzuki et al. (110) show that the increase in Lm that occurs with emphysematous destruction is associated with a progressive infiltration of both the bronchiolar and alveolar tissues by macrophages, CD4 and B cell lymphocytes, with the additional infiltration of the alveolar tissue by CD8 lymphocytes and eosinophils. Moreover, these changes occurs in association with a measurable remodeling of collagen and elastin that includes an increase in collagen I and a decrease in collagen III consistent with scar formation (63, 110). Furthermore, these new data indicate that these changes precede the appearance micro CT evidence of emphysematous destruction in lungs from patients with very severe COPD treated by lung transplantation. It is clear that the cells listed above are not only present but increase with disease severity in and around the small airways in COPD (40), and persist even after the removal of the original noxious insult.
In addition to persistent inflammation, COPD is characterized by mucus hypersecretion as discussed above, and peribronchial fibrosis, but little is known about how the tissue of the small airways becomes remodeled. Fibroblasts function as the major interstitial mesenchymal cell within the lung and are an important source of growth factors, extracellular proteins, and the metalloproteinases and a host of cytokines that remodel the interstitium of the airway and alveolar tissue (109). Exposure of cultured human lung fibroblasts to cigarette smoke extract (CSE) leads to the induction of markers of oxidative stress and inflammation (16). Cigarette smoke has also been shown to adversely affect the ability of fibroblasts to proliferate and synthesize fibronectin and contract collagen I (15, 75, 135), all of which are important features of both normal and abnormal tissue repair. Many of the genes that were downregulated in association with emphysematous destruction in the study of Campbell et al. (14), that linked micro CT measurements of emphysematous destruction to a 127-gene expression signature, were associated with wound repair pathways including integrin signaling, cytoskeletal contraction, extracellular matrix (ECM) production, vascular endothelial growth factor (VEGF), and transforming growth factor (TGF)-β signaling. That these gene expression changes may have functional consequences was demonstrated by the finding that fibroblasts from ex-smokers with severe COPD showed deficient formation of focal adhesions and reduced ability to contract collagen I gels compared with fibroblasts from former smokers without COPD (14).
Defective collagen contraction by fibroblasts derived from COPD donors has been replicated by other groups (56, 119). Fibrilar collagens such as collagen I form nanoscale ropes that are essential for providing tensile strength within the tissue but also act as a scaffold for elastic fibers as well as fibronectin, decorin, and associated proteins in the ECM. If defective radial collagen fibers are laid down within the small airways or parenchyma during repair, as these data suggest, a disruption of the mechanism by which the small airways are radially tethered by the alveolar walls would result. However, the loss of terminal bronchioles in regions of nonemphysematous tissue in end-stage COPD patients (63), and the preliminary data by our group (52), which show a 33% loss of small airways within the lungs of mild to moderate COPD patients, with no evidence of emphysematous destruction either radiographically or histologically, suggest that loss of terminal bronchioles occurs prior to emphysematous destruction. An hypothesis for this observation was suggested by Mitzner (66), in that the axial fibers that provide the structural linkage between large and small airways could be lost at the distal ends leading to axial recoil of the airways. This shortening would contribute to the obstruction by shortening the small airways and decreasing lumen size. Tanabe et al. (115) have recently provided preliminary micro CT evidence in support of this concept by showing that the reduction in the numbers of radial alveolar attachments to the bronchioles is associated with a shortening of the preterminal bronchioles and a reduction in bronchiolar lumen size in lungs with end-stage centrilobular and panlobular emphysema. These observations suggest the hypothesis that fibroblasts may lose their ability to arrange collagen into fibers in COPD and that this defect influences both radial and axial traction exerted on the airway wall.
XI. SUMMARY AND CONCLUSIONS
The discovery that the small conducting airways are a silent zone within the lungs where disease could accumulate over time combined with epidemiological evidence that for a susceptible minority of “healthy” working men, cigarette smoke accelerates the age-related decline in FEV1, led to the search for specialized physiological tests to detect disease abnormalities early, to prevent disease progression. This hypothesis initiated development of tests of small airways disease to detect early abnormalities, but these were largely abandoned by the 1980s when it became apparent that the variance in the results, both within and between individuals, was substantial and that none of them could reliably identify the minority of subjects who would subsequently develop COPD. Newer tests including forced oscillation and multiple breath washout offer some promise as predictors of early disease but remain to be tested longitudinally.
With the introduction of thoracic CTs in the 1970s and the subsequent wide availability of whole body scanners by the mid 1980s, investigators focused on imaging the airways. Although the resolution of MDCT scanners does not allow for the direct visualization of small airways, its combination with micro CT scanning of samples removed from inflated lung specimens has revealed that end-stage COPD is associated with a 90% loss of terminal bronchioles even in areas of lung unaffected by emphysema. In addition, paramagnetic response mapping, accomplished by registering CT scans obtained at full inspiration and expiration, has allowed the separation of regions of lung that trap gas behind narrowed and obliterated small airways from regions affected by emphysema. Moreover, serial studies show that the areas of trapped gas develop emphysema over time.
The integration of micro CT imaging of multiple samples, from heterogeneously affected diseased lungs, with gene expression profiling led to the discovery of a 127-gene expression signature for emphysematous destruction, that when integrated with histological analysis of the tissue was associated with infiltration of the tissue by macrophages, CD4, and B cell lymphocytes. These results are concordant with the observation that the progression of COPD is associated with the increased appearance of tertiary lymphoid organs containing CD4, CD8, and B cell lymphocytes in the peripheral lung. Collectively these data suggest that destruction of the bronchiolar and alveolar tissue is closely associated with lymphoid neogenesis and tertiary lymphoid organ formation, which is consistent with an adaptive immune response.
Although much remains to be done, novel functional and imaging approaches offer promise that the process that destroys the small conducting airways of the lung can be detected at an earlier stage, and powerful new cellular and molecular studies can suggest therapeutic targets to treat this devastating disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01HL118542 and Candian Institutes of Health Research Grant 119374.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: J. C. Hogg, Centre for Heart Lung Innovation, St Paul's Hospital, 1081 Burrard St., Vancouver, B.C. V6Z 1Y6, Canada (e-mail: Jim.Hogg@hli.ubc.ca).
Footnotes
Reynolds number is a dimensionless number that determines whether flow is laminar or turbulent. It is related to gas density X gas velocity X airway diameter/gas viscosity.
The choke point, previously referred to as the equal pressure point, is the location in the tracheobronchial tree during forced expiration where the pressure outside the airway equals the pressure inside the airway. The choke point divides the airway into two segments: the upstream segment and the downstream segment. There is a tendency for the airway to be compressed downstream of the choke point leading to flow limitation.
REFERENCES
- 1.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nature Rev Immunol : 205–217, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Baraldo S, Turato G, Lunardi F, Bazzan E, Schiavon M, Ferrarotti I, Molena B, Cazzuffi R, Damin M, Balestro E, Luisetti M, Rea F, Calabrese F, Cosio MG, Saetta M. Immune activation in alpha1-antitrypsin-deficiency emphysema. Beyond the protease-antiprotease paradigm. Am J Respir Crit Care Med : 402–409, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J : 552–563, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol Appl Pharmacol : 269–275, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjosberg JM, Spits H. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nature Immunol : 221–229, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Boes JL, Hoff BA, Bule M, Johnson TD, Rehemtulla A, Chamberlain R, Hoffman EA, Kazerooni EA, Martinez FJ, Han MK, Ross BD, Galban CJ. Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD Study (SPIROMICS). Academic Radiol : 186–194, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon M, Verleden SE, Bosch B, Lammertyn E, McDonough JE, Mai C, Verschakelen J, Kemner-van de Corput M, Tiddens H, Proesmans M, Vermeulen FL, Verbeken EK, Cooper J, Decramer M, Verleden GM, Hogg JC, Vanaudenaerde BM, Dupont LJ, De Boeck K. Morphometric analysis of explant lungs in cystic fibrosis. Am J Respir Cell Mol Biol : 516–526, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Borgerding M, Klus H. Analysis of complex mixtures–cigarette smoke. Exp Toxicol Pathol Suppl 1: 43–73, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Brusselle GG, Demoor T, Bracke KR, Brandsma CA, Timens W. Lymphoid follicles in (very) severe COPD: beneficial or harmful? Eur Respir J : 219–230, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Buist AS, Ghezzo H, Anthonisen NR, Cherniack RM, Ducic S, Macklem PT, Manfreda J, Martin RR, McCarthy D, Ross BB. Relationship between the single-breath N test and age, sex, and smoking habit in three North American cities. Am Rev Respir Dis : 305–318, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Buist AS, Vollmer WM, Johnson LR, McCamant LE. Does the single-breath N2 test identify the smoker who will develop chronic airflow limitation? Am Rev Respir Dis : 293–301, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature : 1371–1375, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax : 992–996, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell JD, McDonough JE, Zeskind JE, Hackett TL, Pechkovsky DV, Brandsma CA, Suzuki M, Gosselink JV, Liu G, Alekseyev YO, Xiao J, Zhang X, Hayashi S, Cooper JD, Timens W, Postma DS, Knight DA, Lenburg ME, Hogg JC, Spira A. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med : 67, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnevali S, Nakamura Y, Mio T, Liu X, Takigawa K, Romberger DJ, Spurzem JR, Rennard SI. Cigarette smoke extract inhibits fibroblast-mediated collagen gel contraction. Am J Physiol Lung Cell Mol Physiol : L591–L598, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Carnevali S, Petruzzelli S, Longoni B, Vanacore R, Barale R, Cipollini M, Scatena F, Paggiaro P, Celi A, Giuntini C. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol : L955–L963, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Cone RA. Barrier properties of mucus. Adv Drug Delivery Rev : 75–85, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med : 1277–1281, 1978. [DOI] [PubMed] [Google Scholar]
- 19.Cosio MG, Hale KA, Niewoehner DE. Morphologic and morphometric effects of prolonged cigarette smoking on the small airways. Am Rev Respir Dis : 265–221, 1980. [DOI] [PubMed] [Google Scholar]
- 20.De Grove KC, Provoost S, Verhamme FM, Bracke KR, Joos GF, Maes T, Brusselle GG. Characterization and quantification of innate lymphoid cell subsets in human lung. PloS One : e0145961, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellaca RL, Santus P, Aliverti A, Stevenson N, Centanni S, Macklem PT, Pedotti A, Calverley PM. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J : 232–240, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Carlin B, Lowson S, Granton J, Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial Study I . High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med : 801–808, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, Korfhagen TR, Corradi M, Nadel JA, Borchers MT, Leikauf GD. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol : 446–454, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detels R, Tashkin DP, Simmons MS, Carmichael HE Jr, Sayre JW, Rokaw SN, Coulson AH Sayre JW, Rokaw SN, Coulson AH. The UCLA population studies of chronic obstructive respiratory disease 5 Agreement and disagreement of tests in identifying abnormal lung function. Chest : 630–638, 1982. [DOI] [PubMed] [Google Scholar]
- 25.Dollfuss RE, Milic-Emili J, Bates DV. Regional ventilation of the lung, studied with boluses of 133Xenon. Respir Physiol : 234–246, 1967. [Google Scholar]
- 26.Dosman JA, Cotton DJ, Graham BL, Hall DL, Li R, Froh F, Barnett GD. Sensitivity and specificity of early diagnostic tests of lung function in smokers. Chest : 6–11, 1981. [DOI] [PubMed] [Google Scholar]
- 27.Dubois AB. Resistance to Breathing. Baltimore, MD: Williams and Wilkins, 1964. [Google Scholar]
- 28.Dubois AB, Botelho SY, Comroe JH Jr. A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. J Clin Invest : 327–335, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois AB, Brody AW, Lewis DH, Burgess BF Jr. Oscillation mechanics of lungs and chest in man. J Appl Physiol : 587–594, 1956. [DOI] [PubMed] [Google Scholar]
- 30.Eberl G. Development and evolution of RORgammat+ cells in a microbe's world. Immunol Rev : 177–188, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med : 2233–2247, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrow CE, Salome CM, Harris BE, Bailey DL, Bailey E, Berend N, Young IH, King GG. Airway closure on imaging relates to airway hyperresponsiveness and peripheral airway disease in asthma. J Appl Physiol (1985) : 958–966, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med : 1666–1672, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher CM, Peto R, Tinker C, Speizer FE. The Natural History of Chronic Bronchitis and Emphysema. Oxford, UK: Oxford Univ. Press, 1976, p. 150. [Google Scholar]
- 35.Fletcher CM. Letter: Natural history of chronic bronchitis. Br Med J : 1592–1593, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fredberg JJ, Keefe DH, Glass GM, Castile RG, Frantz ID 3rd. Alveolar pressure nonhomogeneity during small-amplitude high-frequency oscillation. J Appl Physiol : 788–800, 1984. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity : 769–781, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galban CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, Galban S, Rehemtulla A, Kazerooni EA, Martinez FJ, Ross BD. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nature Med : 1711–1715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green M. How big are the bronchioles? In: St. Thomas Hospital Gazette. London: St. Thomas’s Hospital, 1965, p. 136–139. [Google Scholar]
- 40.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med : 2645–2653, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Hogg JC, Macklem PT, Thurlbeck WM. The resistance of small airways in normal and diseased human lungs. Aspen Emphysema Conf : 433–441, 1967. [PubMed] [Google Scholar]
- 42.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med : 1355–1360, 1968. [DOI] [PubMed] [Google Scholar]
- 42a.Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest : 1436–1443, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol : 435–459, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Hogg JC, Williams J, Richardson JB, Macklem PT, Thurlbeck WM. Age as a factor in the distribution of lower-airway conductance and in the pathologic anatomy of obstructive lung disease. N Engl J Med : 1283–1287, 1970. [DOI] [PubMed] [Google Scholar]
- 45.Horsfield K, Cumming G. Morphology of the bronchial tree in man. J Appl Physiol : 373–383, 1968. [DOI] [PubMed] [Google Scholar]
- 46.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med : 1967–1976, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Jeffery PK. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax : 129–136, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston RN, McNeill RS, Smith DH, Legge JS, Fletcher F. Chronic bronchitis–measurements and observations over 10 years. Thorax : 25–29, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kearley J, Silver JS, Sanden C, Liu Z, Berlin AA, White N, Mori M, Pham TH, Ward CK, Criner GJ, Marchetti N, Mustelin T, Erjefalt JS, Kolbeck R, Humbles AA. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity : 566–579, 2015. [DOI] [PubMed] [Google Scholar]
- 50.King GG. Cutting edge technologies in respiratory research: lung function testing. Respirology : 883–890, 2011. [DOI] [PubMed] [Google Scholar]
- 51.King GG, Downie SR, Verbanck S, Thorpe CW, Berend N, Salome CM, Thompson B. Effects of methacholine on small airway function measured by forced oscillation technique and multiple breath nitrogen washout in normal subjects. Respir Physiol Neurobiol : 165–177, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Koo HK, Vasilescu DM, Scott AE, Orestis K, Warner JA, Sinclair I, Hogg JC, Hackett TL. Micro-CT analysis of paraffin embedded lung tissue: is small airway obstruction an early feature Of COPD? Am J Respir Crit Care Med : A5573, 2015. [Google Scholar]
- 53.Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Delivery Rev : 86–100, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez-Camblor P, Meek P, Owen CA, Petersen H, Pinto-Plata V, Schnohr P, Sood A, Soriano JB, Tesfaigzi Y, Vestbo J. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med : 111–122, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Larsson L, Szponar B, Ridha B, Pehrson C, Dutkiewicz J, Krysinska-Traczyk E, Sitkowska J. Identification of bacterial and fungal components in tobacco and tobacco smoke. Tobacco Induced Dis : 4, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsson-Callerfelt AK, Hallgren O, Andersson-Sjoland A, Thiman L, Bjorklund J, Kron J, Nihlberg K, Bjermer L, Lofdahl CG, Westergren-Thorsson G. Defective alterations in the collagen network to prostacyclin in COPD lung fibroblasts. Respir Res : 21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leopold PL, O'Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PloS One : e8157, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levine G, Housley E, MacLeod P, Macklem PT. Gas exchange abnormalities in mild bronchitis and asymptomatic asthma. N Engl J Med : 1277–1282, 1970. [DOI] [PubMed] [Google Scholar]
- 59.Lozewicz S, Wells C, Gomez E, Ferguson H, Richman P, Devalia J, Davies RJ. Morphological integrity of the bronchial epithelium in mild asthma. Thorax : 12–15, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lutchen KR, Gillis H. Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. J Appl Physiol (1985) : 1192–1201, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Macklem PT, Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol : 395–401, 1967. [DOI] [PubMed] [Google Scholar]
- 62.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, Johnston SL. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med : 734–742, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Pare PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med : 1567–1575, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mead J. The lung's “quiet zone.” N Engl J Med : 1318–1319, 1970. [DOI] [PubMed] [Google Scholar]
- 65.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity : 493–504, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Mitzner W. Emphysema–a disease of small airways or lung parenchyma? N Engl J Med : 1637–1639, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature Immunol : 1055–1062, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature : 540–544, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Mullen JB, Wright JL, Wiggs BR, Pare PD, Hogg JC. Reassessment of inflammation of airways in chronic bronchitis. Br Med J : 1235–1239, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med : 853–860, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med : 195–199, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med : 266–272, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Murtagh PS, Proctor DF, Permutt S, Kelly BL, Evering S. Bronchial mechanics in excised dog lobes. J Appl Physiol : 403–408, 1971. [DOI] [PubMed] [Google Scholar]
- 74.Nagaishi C. Functional Anatomy and Histology of the Lung. Baltimore: University Park Press, 1972. [Google Scholar]
- 75.Nakamura Y, Romberger DJ, Tate L, Ertl RF, Kawamoto M, Adachi Y, Mio T, Sisson JH, Spurzem JR, Rennard SI. Cigarette smoke inhibits lung fibroblast proliferation and chemotaxis. Am J Respir Crit Care Med : 1497–1503, 1995. [DOI] [PubMed] [Google Scholar]
- 76.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Pare PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med : 142–146, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature : 1367–1370, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nemery B, Moavero NE, Brasseur L, Stanescu DC. Significance of small airway tests in middle-aged smokers. Am Rev Respir Dis : 232–238, 1981. [DOI] [PubMed] [Google Scholar]
- 79.Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease bronchopulmonary dysplasia. N Engl J Med : 357–368, 1967. [DOI] [PubMed] [Google Scholar]
- 80.Olofsson J, Bake B, Svardsudd K, Skoogh BE. The single breath N2-test predicts the rate of decline in FEV1. The study of men born in 1913 and 1923. Eur J Respir Dis : 46–56, 1986. [PubMed] [Google Scholar]
- 81.Oppenheimer BW, Goldring RM, Berger KI. Distal airway function assessed by oscillometry at varying respiratory rate: comparison with dynamic compliance. COPD : 162–170, 2009. [DOI] [PubMed] [Google Scholar]
- 82.Pare PD, Brooks LA, Coppin CA, Wright JL, Kennedy S, Dahlby R, Mink S, Hogg JC. Density-dependence of maximal expiratory flow and its correlation with small airway disease in smokers. Am Rev Respir Dis : 521–526, 1985. [DOI] [PubMed] [Google Scholar]
- 83.Pauly JL, Smith LA, Rickert MH, Hutson A, Paszkiewicz GM. Review: is lung inflammation associated with microbes and microbial toxins in cigarette tobacco smoke? Immunol Res : 127–136, 2010. [DOI] [PubMed] [Google Scholar]
- 84.Polverino F, Baraldo S, Bazzan E, Agostini S, Turato G, Lunardi F, Balestro E, Damin M, Papi A, Maestrelli P, Calabrese F, Saetta M. A novel insight into adaptive immunity in chronic obstructive pulmonary disease: B cell activating factor belonging to the tumor necrosis factor family. Am J Respir Crit Care Med : 1011–1019, 2010. [DOI] [PubMed] [Google Scholar]
- 85.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA : 11489–11494, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann NY Acad Sci : 12–27, 1993. [DOI] [PubMed] [Google Scholar]
- 87.Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys : 167–188, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol : 1255–1267, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Randall TD. Bronchus-Associated Lymphoid Tissue (BALT): Structure and Function. Rochester, NY: Elsevier, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD : 32–43, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reid L. The secondary lobule in the adult human lung, with special reference to its appearance in bronchograms. Thorax : 110–115, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roher F. Der Stromungswiderstand in der menschlichen Atemwegen und der Einfluss der unregelmassigen Verzweigung es Bronchial-systems auf der Atmungsverlauf in vershiedenen Lungenbezinken. Arch Ges Physiol : 225–229, 1915. [Google Scholar]
- 93.Rohrer F. Physiologie der Atembewegung. Berlin, Germany: Springer, 1925. [Google Scholar]
- 94.Rossoff LJ, Csima A, Zamel N. Reproducibility of maximum expiratory flow in severe chronic obstructive pulmonary disease. Bull Eur Physiopathol Respir : 1129–1136, 1979. [PubMed] [Google Scholar]
- 95.Saetta M, Di Stefano A, Maestrelli P, Ferraresso A, Drigo R, Potena A, Ciaccia A, Fabbri LM. Activated T-lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. Am Rev Respir Dis : 301–306, 1993. [DOI] [PubMed] [Google Scholar]
- 96.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med : 822–826, 1998. [DOI] [PubMed] [Google Scholar]
- 97.Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp CE, Maestrelli P, Cavallesco G, Papi A, Fabbri LM. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med : 1016–1021, 2000. [DOI] [PubMed] [Google Scholar]
- 98.Saetta M, Turato G, Facchini FM, Corbino L, Lucchini RE, Casoni G, Maestrelli P, Mapp CE, Ciaccia A, Fabbri LM. Inflammatory cells in the bronchial glands of smokers with chronic bronchitis. Am J Respir Crit Care Med : 1633–1639, 1997. [DOI] [PubMed] [Google Scholar]
- 99.Sakai M, Sato Y, Sato S, Ihara S, Onizuka M, Sakakibara Y, Takahashi H. Effect of relocating to areas of reduced atmospheric particulate matter levels on the human circulating leukocyte count. J Appl Physiol (1985) : 1774–1780, 2004. [DOI] [PubMed] [Google Scholar]
- 100.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, Fallon PG, Ogg GS. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med : 2939–2950, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature : 1253–1257, 2007. [DOI] [PubMed] [Google Scholar]
- 102.Sapkota AR, Berger S, Vogel TM. Human pathogens abundant in the bacterial metagenome of cigarettes. Environ Health Perspect : 351–356, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med : 2355–2365, 2008. [DOI] [PubMed] [Google Scholar]
- 104.Sethi S, Muscarella K, Evans N, Klingman KL, Grant BJ, Murphy TF. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest : 1557–1565, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Singh M, Lee SH, Porter P, Xu C, Ohno A, Atmar RL, Greenberg SB, Bandi V, Gern J, Amineva S, Aminev A, Skern T, Smithwick P, Perusich S, Barrow N, Roberts L, Corry DB, Kheradmand F. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol : 1369–1378 e1362, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Solomon DA. Are small airways tests helpful in the detection of early airflow obstruction? Chest : 567–569, 1978. [DOI] [PubMed] [Google Scholar]
- 107.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells–a proposal for uniform nomenclature. Nature Rev Immunol : 145–149, 2013. [DOI] [PubMed] [Google Scholar]
- 108.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nature Immunol : 21–27, 2011. [DOI] [PubMed] [Google Scholar]
- 109.Stone PJ, Morris SM, Thomas KM, Schuhwerk K, Mitchelson A. Repair of elastase-digested elastic fibers in acellular matrices by replating with neonatal rat-lung lipid interstitial fibroblasts or other elastogenic cell types. Am J Respir Cell Mol Biol : 289–301, 1997. [DOI] [PubMed] [Google Scholar]
- 110.Suzuki M, Elliott WM, McDonough JE, Hayashi S, Sanchez PG, Cooper JD, Hogg JC. The quantitative relationship between collagen deposition and lung structure in COPD (Abstract). Am J Respir Cell Mol Biol : A6808, 2010. [Google Scholar]
- 111.Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, de Marco R, Norback D, Raherison C, Villani S, Wjst M, Svanes K, Anto JM. Early life origins of chronic obstructive pulmonary disease. Thorax : 14–20, 2010. [DOI] [PubMed] [Google Scholar]
- 112.Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF, Protin U, Kroschel P, Nadel JA. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol : L165–L172, 2001. [DOI] [PubMed] [Google Scholar]
- 113.Tamashiro E, Xiong G, Anselmo-Lima WT, Kreindler JL, Palmer JN, Cohen NA. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy : 117–122, 2009. [DOI] [PubMed] [Google Scholar]
- 114.Tan WC, Qiu D, Liam BL, Ng TP, Lee SH, van Eeden SF, D'Yachkova Y, Hogg JC. The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med : 1213–1217, 2000. [DOI] [PubMed] [Google Scholar]
- 115.Tanabe N, Vasilescu DM, McDonough JE, Kinose D, Suzuki M, Cooper JD, Pare PD, Hogg JC. MicroCT comparison of preterminal bronchioles in centrilobular and panlobular emphysema. Am J Respir Crit Care Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Terry PB, Traystman RJ, Newball HH, Batra G, Menkes HA. Collateral ventilation in man. N Engl J Med : 10–15, 1978. [DOI] [PubMed] [Google Scholar]
- 117.Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thoracic Soc : 54–61, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Tiddens HA, Pare PD, Hogg JC, Hop WC, Lambert R, de Jongste JC. Cartilaginous airway dimensions and airflow obstruction in human lungs. Am J Respir Crit Care Med : 260–266, 1995. [DOI] [PubMed] [Google Scholar]
- 119.Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, Kawasaki S, Ahn Y, Fredriksson K, Skold CM, Mueller KC, Branscheid D, Welker L, Watz H, Magnussen H, Rennard SI. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med : 248–260, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van Brabandt H, Cauberghs M, Verbeken E, Moerman P, Lauweryns JM, Van de Woestijne KP. Partitioning of pulmonary impedance in excised human and canine lungs. J Appl Physiol : 1733–1742, 1983. [DOI] [PubMed] [Google Scholar]
- 121.Van der Strate BW, Postma DS, Brandsma CA, Melgert BN, Luinge MA, Geerlings M, Hylkema MN, van den Berg A, Timens W, Kerstjens HA. Cigarette smoke-induced emphysema: a role for the B cell? Am J Respir Crit Care Med : 751–758, 2006. [DOI] [PubMed] [Google Scholar]
- 122.Van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants [PM(10)]. Am J Respir Crit Care Med : 826–830, 2001. [DOI] [PubMed] [Google Scholar]
- 123.Verbanck S, Schuermans D, Meysman M, Paiva M, Vincken W. Noninvasive assessment of airway alterations in smokers: the small airways revisited. Am J Respir Crit Care Med : 414–419, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Verbanck S, Schuermans D, Noppen M, Van Muylem A, Paiva M, Vincken W. Evidence of acinar airway involvement in asthma. Am J Respir Crit Care Med : 1545–1550, 1999. [DOI] [PubMed] [Google Scholar]
- 125.Verbanck S, Schuermans D, Paiva M, Meysman M, Vincken W. Small airway function improvement after smoking cessation in smokers without airway obstruction. Am J Respir Crit Care Med : 853–857, 2006. [DOI] [PubMed] [Google Scholar]
- 126.Verbanck S, Schuermans D, Van Muylem A, Melot C, Noppen M, Vincken W, Paiva M. Conductive and acinar lung-zone contributions to ventilation inhomogeneity in COPD. Am J Respir Crit Care Med : 1573–1577, 1998. [DOI] [PubMed] [Google Scholar]
- 127.Verbanck S, Schuermans D, Van Muylem A, Paiva M, Noppen M, Vincken W. Ventilation distribution during histamine provocation. J Appl Physiol (1985) : 1907–1916, 1997. [DOI] [PubMed] [Google Scholar]
- 128.Verleden SE, Vasilescu DM, Willems S, Ruttens D, Vos R, Vandermeulen E, Hostens J, McDonough JE, Verbeken EK, Verschakelen J, Van Raemdonck DE, Rondelet B, Knoop C, Decramer M, Cooper J, Hogg JC, Verleden GM, Vanaudenaerde BM. The site and nature of airway obstruction after lung transplantation. Am J Respir Crit Care Med : 292–300, 2014. [DOI] [PubMed] [Google Scholar]
- 129.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Cremoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med : 630–634, 1995. [DOI] [PubMed] [Google Scholar]
- 130.Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK, Chapman A, Smith CH, Di Meglio P, Nestle FO. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol : 984–991, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vlahovic G, Russell ML, Mercer RR, Crapo JD. Cellular and connective tissue changes in alveolar septal walls in emphysema. Am J Respir Crit Care Med : 2086–2092, 1999. [DOI] [PubMed] [Google Scholar]
- 132.Wagner EM, Bleecker ER, Permutt S, Liu MC. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med : 447–452, 1998. [DOI] [PubMed] [Google Scholar]
- 133.Wagner EM, Liu MC, Weinmann GG, Permutt S, Bleecker ER. Peripheral lung resistance in normal and asthmatic subjects. Am Rev Respir Dis : 584–588, 1990. [DOI] [PubMed] [Google Scholar]
- 134.Walter S, Nancy NR, Collier CR. Changes in the forced expiratory spirogram in young male smokers. Am Rev Respir Dis : 717–724, 1979. [DOI] [PubMed] [Google Scholar]
- 135.Wang H, Liu X, Umino T, Skold CM, Zhu Y, Kohyama T, Spurzem JR, Romberger DJ, Rennard SI. Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am J Respir Cell Mol Biol : 772–779, 2001. [DOI] [PubMed] [Google Scholar]
- 136.Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med : 1868–1902, 1996. [DOI] [PubMed] [Google Scholar]
- 137.Weibel ER. Morphometry of the Human Lung. New York: Academic, 1963. [Google Scholar]
- 138.Woolcock AJ, Vincent NJ, Macklem PT. Frequency dependence of compliance as a test for obstruction in the small airways. J Clin Invest : 1097–1106, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wright JL, Lawson LM, Pare PD, Kennedy S, Wiggs B, Hogg JC. The detection of small airways disease. Am Rev Respir Dis : 989–994, 1984. [DOI] [PubMed] [Google Scholar]
- 140.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol (1985) : 1016–1023, 1992. [DOI] [PubMed] [Google Scholar]