Abstract
Unique to striated muscle cells, transverse tubules (t-tubules) are membrane organelles that consist of sarcolemma penetrating into the myocyte interior, forming a highly branched and interconnected network. Mature t-tubule networks are found in mammalian ventricular cardiomyocytes, with the transverse components of t-tubules occurring near sarcomeric z-discs. Cardiac t-tubules contain membrane microdomains enriched with ion channels and signaling molecules. The microdomains serve as key signaling hubs in regulation of cardiomyocyte function. Dyad microdomains formed at the junctional contact between t-tubule membrane and neighboring sarcoplasmic reticulum are critical in calcium signaling and excitation-contraction coupling necessary for beat-to-beat heart contraction. In this review, we provide an overview of the current knowledge in gross morphology and structure, membrane and protein composition, and function of the cardiac t-tubule network. We also review in detail current knowledge on the formation of functional membrane subdomains within t-tubules, with a particular focus on the cardiac dyad microdomain. Lastly, we discuss the dynamic nature of t-tubules including membrane turnover, trafficking of transmembrane proteins, and the life cycles of membrane subdomains such as the cardiac BIN1-microdomain, as well as t-tubule remodeling and alteration in diseased hearts. Understanding cardiac t-tubule biology in normal and failing hearts is providing novel diagnostic and therapeutic opportunities to better treat patients with failing hearts.
I. INTRODUCTION
Cardiac transverse tubules (t-tubules) are highly branched invaginations of cardiomyocyte sarcolemma that are rich in ion channels important for excitation-contraction (EC) coupling, maintenance of resting membrane potential, action potential initiation and regulation, and signaling transduction. Specific to striated muscle cells, t-tubules are invaginations of the sarcolemma, penetrating into the intracellular space of myocytes. These tubular invaginations form a complex network with transverse tubules that are interconnected within the cytoplasm by longitudinal tubules. The transverse tubules occur around myofilaments, anchoring to sarcomeric z-discs through costameres. The phospholipid-rich t-tubule lipid bilayers are shaped and maintained by membrane scaffolding proteins and intracellular cytoskeleton, as well as surrounding extracellular matrix. T-tubule membrane contains microdomains that compartmentalize transmembrane ion handling proteins and signaling molecules. Together, t-tubules are effective organelles that serve as a centralized signaling hub controlling cardiac contractile function and electrophysiology.
The most recognized function of t-tubules is regulation of cardiac EC coupling by concentrating voltage-gated L-type calcium channels (LTCCs) and positioning them in close proximity to calcium sense and release channels, ryanodine receptors (RyRs), at the junctional membrane of sarcoplasmic reticulum (jSR). The closely approximated LTCCs and RyRs form calcium releasing units, dyads, where calcium transients are initiated following each beat-to-beat action potential. With recent advances in high-resolution imaging technologies, detailed t-tubule microdomain structural organization and newly recognized functions are starting to emerge. For instance, the t-tubular invagination is not smooth, but consists of extensive membrane microfolds sculpted by a cardiac isoform of a BAR domain containing protein bridging integrator 1 (BIN1 or amphiphysin 2) (91). Cardiac BIN1 (cBIN1) organized membrane microfolds generate diffusion barriers which slow t-tubule-associated extracellular ionic diffusion, preserving electrical stability of myocytes during tachycardia-related fluctuations of ionic concentrations in the local extracellular and possibly intracellular environment (91). In addition, cBIN1-microfolds also organize local microdomains for efficient and dynamic regulation of cardiac dyad function, regulating EC coupling (66, 93, 94).
Cardiac t-tubules are also substantially remodeled during heart failure (38, 52, 89, 97, 98, 108, 130-134, 180, 202, 207, 208, 220, 224, 230). In addition to the observed loss and gross diminishment of the t-tubule network, protein components at t-tubules including ion channels and signaling molecules are reported to be either reduced at the t-tubule surface or redistributed elsewhere to non-t-tubule sarcolemma (20, 93, 134). Loss of complex t-tubules in heart failure not only causes impaired contractile function due to disrupted dyads and the resultant EC uncoupling, but also alters local concentration gradients and increases susceptibility to ventricular arrhythmia (66, 85, 91, 157, 163, 179, 181, 213). The molecular and cellular mechanisms of t-tubule remodeling in failing cardiomyocytes, in particular the alterations in t-tubule membrane ultrastructure and subdomains, is an active area of interest among cardiac biologists.
This review summarizes and cites the current knowledge of cardiac t-tubule morphology, structure, components, and physiological functions, with emphasis on the organization and function of microdomains within t-tubules. The dynamic life cycle of t-tubule membrane and protein components, as well as the current understanding of microdomain remodeling in heart failure, will also be discussed.
II. CARDIAC T-TUBULE STRUCTURE
A. History of T-Tubule Structure-Related Research
Striated muscle cells have a unique membrane system, the transverse-tubules (t-tubules), or more precisely, the transverse-axial-tubular system. T-tubules are sarcolemma invaginations formed by membrane tubules that are primarily perpendicular to myocyte longitudinal edges. These membrane tubular structures are interconnected in a complex network. Retzius first proposed the existence of t-tubules over 130 years ago, in 1881, when exploring the quick inward spread of action potentials penetrating into muscle cells (see the 1967 Croonian lecture by Huxley) (95). The first visual evidence of tubular structures around myofilament was in 1897 by light microscopy in Nystrom's study (95), when he injected mammalian heart muscle with India ink to track extracellular space. Later, in 1924, Tiegs visualized Z-lines as a spiral structures where inward spread of action potential occurs (95). However, it was not until the 1950s that Huxley and his peers reported, in various muscle cells, the structure for inward spread as tubular membrane structures at the I band (which spans the Z-line) (96). The primary element of cardiac t-tubules is transverse to the long axis of myocytes and occurs periodically at sarcomeric z-discs of myofilament (126). By using transmission electron microscopy (TEM) imaging methods in 1957, Lindner (126) identified that these transverse cardiac t-tubules are open to extracellular space. The longitudinal axial component of t-tubules was identified later in 1970s (201), adding the complexity of the t-tubule system. The 1960s and 1970s was a period of improved TEM and sample preparation technology which led to prolific t-tubule morphology research (60, 62, 153, 190, 191, 201), revealing the organization of t-tubule networks (see details in sect. IIB). The discovery of dyads and calcium releasing units at t-tubules in late 1980s and early 1990s (31, 59) led to function-driven research of cardiac t-tubules. In the past 5 years, with advance in super-resolution fluorescent microscopy and three-dimensional TEM tomography reconstruction, direct visualization into membrane ultrastructure and protein localization has provided unprecedented details of membrane microdomains within cardiac t-tubules, which will be discussed in more detail in section IIC.
Present studies of cardiac t-tubules include myocytes from the atria (109), ventricles, and conducting system (3) and are performed across different species including small murine, large mammals, and human hearts (20). Cardiac t-tubules have classic features that distinguish them from skeletal t-tubules. It is well documented that t-tubules of some form exist in all cardiomyocytes and that the t-tubule system in mammalian ventricular cardiomyocytes is the most extensive (see Figure 1 for a schematic illustration of ventricular cardiomyocyte organziation and t-tubule membrane structure occurring near z-discs). Recently identified t-tubule membrane subdomains also provide a structural foundation and clearer understanding of t-tubule regulation of cardiomyocyte function. This section reviews the gross morphology, membrane ultrastructure and microdomains, and unique features of cardiac t-tubules.
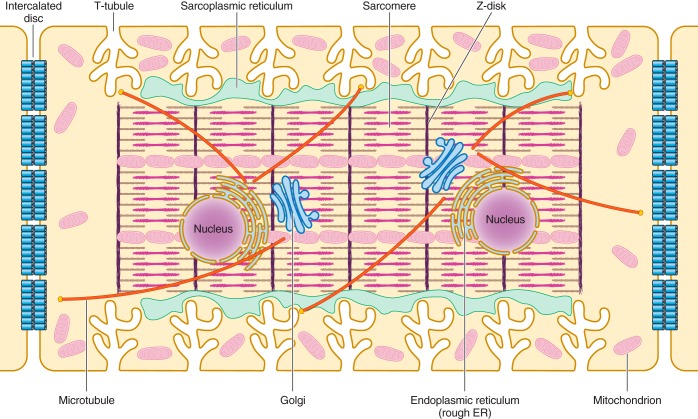
FIGURE 1.
Schematic illustration of the internal structures of an adult ventricular cardiomyocyte. T-tubules, which are enriched with voltage-gated L-type calcium channels, are positioned closely near the sarcoplasmic reticulum, the primary internal calcium store. Sarcomeres form myofibrils, which are responsible for cardiomyocyte contraction upon calcium release. The Golgi apparatus and microtubules serve as the “loading dock” and “highways,” respectively, to deliver ion channels to specific subdomains on the plasma membrane. Mitochondria provide the energy needed for the contraction of cardiomyocytes. Intercalated discs located at the longitudinal sides of each ventricular cardiomyocyte mediate the cell-to-cell propagation of action potentials.
B. Gross Morphology and Geometry
Early TEM images in glutaraldehyde-fixed cardiac tissues infiltrated with tracers such as ferritin, lanthanum salts, or horseradish peroxidase identified some of the key features of cardiac t-tubule system (60, 62, 153, 190, 191, 201) including 1) t-tubules are continuous extension of sarcolemma with openings clearly connected to extracellular space; 2) orientation of t-tubules indicates these membrane tubules are primarily transverse and perpendicular to myocyte longitudinal edges, with an axial portion running in parallel between myofilaments connecting the transverse tubules; and 3) t-tubules wrap around the z-disc and form tubular structures with the flat ends of sarcoplasmic reticulum known as terminal cisternae.
Since the 1990s, fast development of modern fluorescent light microscopy techniques has made it feasible to image cardiac t-tubules in intact cardiac tissue and live cardiomyocytes. Consistent with the earlier TEM findings, two-photon and confocal images of membrane fluorescent dye-labeled t-tubules reveal that these membrane invaginations occur orderly at every ∼1.8- to 2-μm intervals perpendicular to the long axis of cardiomyocytes, which forms a radial “spokelike” organization in transverse section of myofilament near Z-disc regions (198). Three-dimensional (3D) volume reconstruction of stepper motor acquired z-stacks of two-dimensional XY frame images acquired at incremental depth provide spatial visualization of the t-tubule gross morphology and network, allowing global analysis of t-tubule localization, size, and branching points. On the basis of studies using these imaging techniques together with electrophysiological tools, current knowledge of t-tubule morphology and geometry includes the ratio between transverse and axial elements (60 vs. 40%) (198), luminal diameter (ranges from 20 to 450 nm with an average of 200–300 nm for rat cardiomyocytes), average length between branching points (∼6.7 μm), total volume percent of cardiomyocyte (varies between 0.8% in mouse and 3.6% in rat cardiomyocytes), and percentage of membrane capacitance (varies from 21 to 64% with an average around 30%) (159, 161–163, 198).
The advent of super-resolution fluorescent microscopy imaging techniques can, in theory, improve XY spatial resolution from 250 nm with confocal microscopy to 10–20 nm, while retaining robust protein localization (227). In a study using stimulated emission depletion (STED) imaging, t-tubule structure and lumen are visualized in mouse cardiomyocytes with new structural details (220). In addition to the fluorescent microscopy, scanning ion conductance microscopy (SICM) has also been used to image live cardiomyocyte surface topology, providing visualization of openings of transverse tubular invaginations (77, 154). Meanwhile, the new two-axis tilted electron microscopy imaging in combination with three-dimensional tomography reconstruction also provides new TEM features of t-tubule ultrastructure, including the identification of variable t-tubule luminal diameter (87) with dilation near junction (228), ryanodine receptor feet at the dyad junctional membrane (43, 87), and membrane microfolds (91) (see more details in sect. IIC). The use of super-resolution microscopy helps identify LTCC and RyR localization within these microfolds (66).
C. Membrane Ultrastructure and Microdomains
The meshlike cardiac t-tubule network with interconnected transverse and longitudinal tubules is now increasingly appreciated as a nonuniform tubular network consisting of variable lumen diameters, differential membrane ultrastructure, and multiple types of frequently occurring subdomains. These membrane ultrastructure and subdomains not only add spatial complexity to t-tubule lumen, but also provide structural foundation necessary to fulfill the functional needs of t-tubules in regulation of EC coupling, extracellular ion diffusion, membrane excitability, and cellular signaling. In this section, we discuss main membrane microdomains within t-tubule membrane invaginations.
1. cBIN1 microfolds: microdomains supporting LTCC-RyR dyads
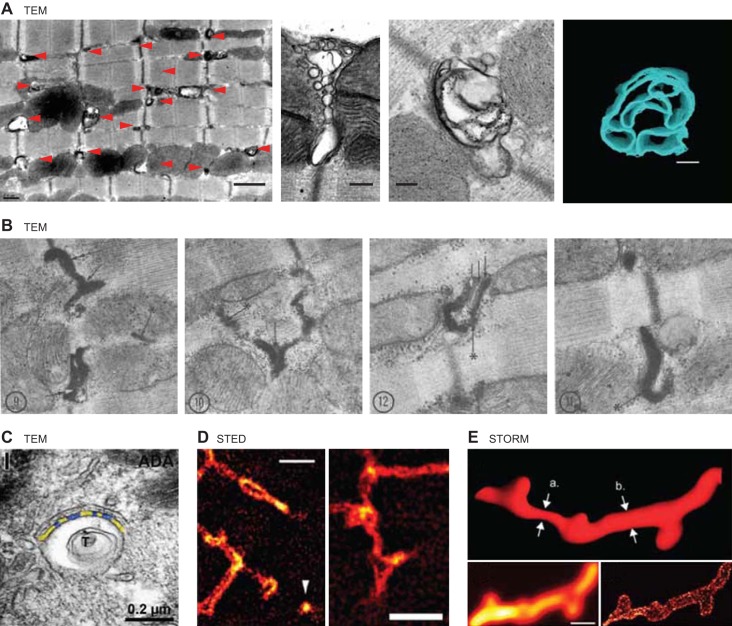
Recently, a t-tubule subdomain was found to consist of membrane microfolds sculpted by cBIN1. With the use of 3D confocal and TEM imaging, it was identified that t-tubule invaginations are actually not formed by simply straight and smooth membrane on the sides, but rather with complex and layered membrane microfolds (91). As shown in Figure 2A, low-magnification 2D TEM image (left) indicates that t-tubules are contoured tubules with calcium-dependent electron-dense precipitations along tubular membrane. High-magnification 2D TEM images and 3D tomography (Figure 2A, right panels) of transverse and axial cross sections of cardiac t-tubules reveal that redundant membrane layers and microfolds exist along tubular membrane (91). While it is intriguing to some readers that such dramatic membrane structural feature received sparse attention, reports of contoured t-tubules in mammalian cardiomyocytes have been reported for decades. As early as in 1970, Forssmann and Girardier (62) found in rat cardiomyocytes that t-tubules are extensively tortuous with hairpin-like bends (Figure 2B). Following this initial study, contoured cardiac t-tubules with extra membrane layers and microfolds can be identified in multiple cardiac t-tubule studies including a recent study from Franzini-Armstrong's group in 2015 (Figure 2C) (117), as well as the apparent variable luminal areas within t-tubules and extended membrane subdomains by super-resolution fluorescent imaging with STED (Figure 2D) (220) and stochastic reconstructive optical reconstruction microscopy (STORM) (Figure 2E) (101). By inspection, cBIN1 generated microfolds are more concentrated at the neck of t-tubules close to tubular openings at the sarcolemma (Figure 1E in Ref. 91). Removal of these microfolds in cardiac conditional Bin1-deleted adult mouse ventricular cardiomyocytes not only increases t-tubule luminal diameter, but also increases the visibility of t-tubule openings via SICM imaging of cardiomyocyte surface topology (91). While increasing evidence in support of the existence of microfolds within t-tubules starts to emerge, in our experience their fragility places an emphasis on cardiomyocyte preservation protocols for electron microscopy. We have found that, for TEM imaging (Figure 2A), freshly extracted whole mouse hearts need to be maintained with immediate perfusion on a Langendorff system and then, while the heart is still beating, the myocytes should be fixed in calcium-containing buffer introduced via the perfusion needle securing the aorta directing flow into the coronaries and retrograde through the heart. The variation in sample preparation and imaging techniques can possibly explain the time it has taken to fully appreciate the complexity of microfolds within t-tubule invaginations.
FIGURE 2.
Images of t-tubule microfolds. A: TEM images reproduced from Hong et al. (91). B: TEM images reproduced from Forssmann and Girardier (62). C: TEM image reproduced from Lavorato et al. (117), with permission from Springer. D: STED images reproduced from Wagner et al. (220). E: STORM images reproduced from Jayasinghe et al. (101).
Sculpting of microfolds within cardiac t-tubules is dependent on BIN1, which is a phospholipid-adherent membrane scaffolding protein. BIN1, alternatively known as amphiphysin 2, is a member of the BIN1-Amphiphysin-Rvs (BAR) domain containing protein superfamily, which consists of membrane deformation proteins involved in multiple cellular processes across different tissues and organs. BIN1, and in general the BAR domain protein superfamily, contributes to membrane trafficking and remodeling, cytoskeleton dynamics, DNA repair, cell cycle progression, and apoptosis (173). With 49% sequence homology (64), BIN1 and its closest cousin amphiphysin 1 both belong to the N-BAR subfamily containing an NH2-terminal BAR domain. One of the most well-studied functions of amphiphysins is their involvement in membrane trafficking and endocytosis (9). Interestingly, BIN1 was first identified as a MYC oncoprotein interacting protein (36, 47), which serves as a tumor suppressor by inhibiting MYC function (221). Follow-up studies identified that more than 10 BIN1 protein isoforms are encoded by a single gene with 20 exons (Figure 3). All the BIN1 protein isoforms contain a conserved NH2-terminal lipid binding BAR domain encoded by exons 1–9 (exon 7 is skipped outside of the neuronal system), an alternatively spliced linkage coiled-coil region, the MYC binding domain encoded by a ubiquitously alternatively spliced exon 17, a constitutive exon 18, and a conserved COOH-terminal exons 19–20 encoded Src homology 3 (SH3) domain critical for interaction with cytoskeleton and other intracellular protein partners. The middle linkage region is heavily alternatively spliced with tissue and organ specificity including a skeletal specific exon 11 encoding the phosphoinositide (PI) binding domain, a cardiac alternatively spliced exon 13 encoding proline-rich domains, and neuronal co-spliced exons 13–16 encoding a clathrin and AP2 binding domain (CLAP) (22, 72).
FIGURE 3.
Bin1 splice variants in adult mouse cardiomyocytes. Cartoon of Bin1 exons and the splice variants found in adult mouse cardiomyocytes, as well as brain and skeletal muscle. BAR, Bin-amphiphysin-Rvs domain; PI, phosphoinositide binding domain; CLAP, clathrin/AP2 binding region; MBD, myc binding domain; SH3, SH3 domain. For details, see Reference 89.
Cardiac t-tubule membrane microfold formation requires the cardiac isoform BIN1+13+17 with inclusion of both the cardiac alternatively spliced exon 13 and the ubiquitously alternatively spliced exon 17 (91, 222) (Figure 3). The ability of BIN1+13+17 to induce microfolds is likely through its NH2-terminal BAR domain together with the proline-rich domains encoded by cardiac exon 13 because the NH2-terminal BAR domains oligomerize into concave-shaped surfaces with positively charged patches crucial for interactions with membrane lipids. Through these interactions membrane is sculpted around BIN1 organized curvature. The crystal structure of the amphiphysin N-BAR domain reveals a dimeric interaction between helixes from each monomer to form a 6-helix bundle (169). With the elongated banana shape of the dimers (169) and the general function in membrane curvature formation, BIN1 has thus been referred to as the “banana” molecule (28, 79, 174). The interface between the monomers is largely hydrophobic, and the curvature of the dimer results from how the monomers intersect at highly conserved kinks (28, 225) at the end of the 6-helix bundle. These kinks are dependent on proline residues, isomerization of which represents a rate-limiting step in dimer formation (79). Domains immediately following the N-BAR in BIN1 can further strengthen its binding affinity to phospholipids for curvature formation. For instance, cardiac isoform BIN1+13+17 contains proline-rich domains encoded by the cardiac spliced exon 13. These extra proline residues likely create additional kinks in BIN1+13+17 dimers, increasing concavity of the surface of the banana molecules needed for microfold formation within t-tubules. On the other hand, inclusion of exon 17 can further separate exon 13-encoded proline-rich domains from the NH2-terminal SH3 domain, preventing intramolecular binding between the two domains as observed in BIN1+13. As a result, the BIN1+13+17 isoform is particularly well suited to promote N-WASP (neuronal Wiskott-Aldrich syndrome protein)-dependent actin polymerization to maintain and stabilize t-tubule membrane microfolds to sarcomeric z-discs (91). Interestingly, the skeletal muscle specific splicing of exon 11 encodes highly positively charged amino acids, increasing skeletal BIN1-BAR's binding affinity to phospholipids. A recent study of exon 11 skipping in zebrafish skeletal muscle reported a dilated t-tubule pattern similar to the cardiac phenotype, indicating a similar function of exon 11 containing skeletal BIN1 in organizing microdomains in skeletal t-tubules (194).
Together with the previously identified ability of BIN1 in localizing and clustering LTCCs to cardiac t-tubules, these cardiac BIN1+13+17 created microfolds are likely the membrane microdomains supporting cardiac dyad (LTCC-RyR) formation. The role of BIN1 in facilitating microtubule-dependent targeted delivery of CaV1.2 channels to t-tubule membrane (94) will be discussed in section III. Confocal imaging and biochemical results identified that Cav1.2 is localized to BIN1 molecules at cardiac t-tubules. BIN1 decreases occur in human (93) and animal models of heart failure (23, 134), which affects LTCC surface expression at the t-tubules of failing cardiomyocytes. New super-resolution STORM imaging further identified that BIN1-microfolds are not only enriched with Cav1.2 channel clusters but also attract RyRs from the jSR membrane (see supplemental movie on the Physiological Reviews website), further supporting the role of BIN1 in organizing cardiac dyads (65, 66). Thus BIN1-induced t-tubule microfolds create local microdomains bringing sarcolemmal LTCCs to couple with RyRs from jSR membrane. BIN1-regulated coupling of LTCCs to RyRs also helps explain previous observed allosteric activation of RyR following calcium channel activation independent of Ca2+ influx (73). In summary, BIN1 microdomains provide a physical understanding of how cardiac calcium releasing units are organized (Figure 4). More detailed discussion into the multimodal role of BIN1 in formation of LTCC-RyR dyads and extracellular ion diffusion will be further discussed in sections IV and V.
FIGURE 4.
Schematic illustration of BIN1-microdomain organization within cardiac t-tubules. Top: the “banana”-shaped molecule cBIN1 regulates cardiac t-tubule function through: 1) facilitating microtubule-dependent forward trafficking of Cav1.2 channels (LTCC, L-type calcium channels) to cBIN1 organized membrane microfolds at t-tubules (targeted delivery); 2) clustering of LTCCs and RyRs at cBIN1-microfolds based microdomains; 3) creating extracellular ion slow diffusion zone within t-tubule lumen; 4) organizing microdomains for dyad formation and regulation by β-adrenergic signaling. Bottom: cartoon of mammalian adult ventricular cardiomyocytes, which are rod-shaped striated muscle cells with t-tubule invaginations occurring periodically near z-discs of myofilaments.
2. Caveoli (caveolae): β-AR/LTCC signaling hubs
The other well-identified membrane subdomains are caveoli, which are flask-shaped membrane invaginations with diameter typically around 90 nm (123). In muscle cells, caveoli are detergent-resistant membrane structures organized by the plasma membrane binding protein caveolin-3, rich in cholesterol and sphingomyelin. Caveoli are found to be attached to both general sarcolemma (0.04 μm2 membrane area/μm3 cell volume) and t-tubule membrane (0.03 μm2 membrane area /μm3 cell volume) in cardiomyocytes (159), increasing total plasma membrane area by 14–21% (159). Within t-tubules of cardiomyocytes, caveoli cluster at necks of the t-tubules in the subsarcolemmal space (151, 238). Attachment of caveolae to t-tubules not only increases the spatial complexity of t-tubule lumen, but also differentially concentrates membrane ion channels and signaling molecules for localized regulation at these membrane subdomains. In cardiomyocytes, caveolae can house ion channels HCN4, Cav1.2, Kv1.5, Kir6.2/Sur2a, Nav1.5, and NCX1, and signaling molecules such as β-adrenergic receptors (β-AR). For example, caveolin-3 is critical in concentrating β2-AR-cAMP signaling to the t-tubules (229). A subpopulation of LTCCs at caveolae, which is under the regulation of β2-AR stimulation (14), is reported to be important in hypertrophic signaling but not muscle contraction (137). Thus caveolae have a critical role in myocyte function by creating microdomains with compartmentalized specific lipid species, membrane proteins, and signaling molecules, facilitating functional regulation.
The membrane scaffolding proteins responsible for caveolae formation are caveolins, which are a family of proteins with binding affinity to plasma membrane lipids particularly cholesterol and sphingomyelin. There are three caveolin isoforms, caveolin-1, caveolin-2, and caveolin-3, with caveolin-3 specific to the muscle cells (200). Caveolin-3 has been indicated in muscle t-tubule development and is associated with developing skeletal t-tubules, while knockout of cavolin-3 can induce abnormal t-tubules in skeletal muscle (70). Furthermore, as caveolin-3 organized caveloae is critical in compartmentalized regulation of ion channels, mutations in caveolin-3 are associated with channelopathies including long QT syndrome (5).
3. Ankyrin B microdomains: NCX/NKA/InsP3 macromolecular complex
In addition to BIN1-microfolds and caveoli, a third set of microdomains organized by the membrane scaffolding protein ankyrin B has been identified to be essential in the formation of the macromolecular complex of sodium calcium exchanger (NCX), sodium-potassium-ATPase (NKA), and inositol trisphosphate receptor (InsP3) (147). Distinct from the dyad macromolecular complex, the NCX/NKA/InsP3 complex is critical in removing calcium from the luminal clefts between the t-tubule and jSR membrane, facilitating calcium decline during muscle relaxation (147). Therefore, by balancing calcium entry through LTCC-RyR dyads and calcium removal through NCX anchored at ankyrin B-microdomains, intracellular calcium equilibrium can be achieved to maintain coordinated beat-to-beat heart contraction.
The ankyrin B membrane microdomains are formed by ankyrin B protein, which belongs to a family of intracellular proteins with binding affinity to actin and spectrins. Three separate genes ANK1, ANK2, and ANK3 encode ankyrin R, ankyrin B, and ankyrin G, respectively, that are ubiquitously expressed with multiple alternatively spliced forms of each ankyrin. However, cardiomyocytes express three ankyrin B isoforms including the ankyrin B-220 kDa and two recently identified isoforms ankyrin B-188 kDa and ankyrin B-212 kDa (231), and one 190-kDa ankyrin G, with distinct functions in organizing NCX/NKA/InsP3 complex at t-tubules or trafficking Nav1.5 to intercalated discs, respectively [see more details in reviews (39, 146)]. A loss-of-function mutation in human ankyrin B as well as heterozygous ankyrin B knockout mouse are associated with cardiac features including sinus bradycardia, abnormal heart rate variability, defects in cardiac conduction, and increased ventricular arrhythmia (148). The ankyrin B syndromes are distinct from classical long QT syndrome, but can manifest as sick sinus syndrome with bradycardia and increased risk of sudden cardiac death. Results from cell biological studies indicate that lack of ankyrin B microdomains alters membrane ion channel localization to their physiological subdomains, disrupting intracellular calcium homeostasis and promoting arrhythmias (24, 146, 148, 149).
The composition, organization, function, regulation, and turnover of these t-tubule-related membrane microdomains remain active areas of research. Further understanding of microdomain content and regulation may provide new insight into the biological functions of t-tubules in striated muscles, both in normal physiology and during disease development.
D. Peri-Membrane Cytoskeleton Scaffolds at Cardiac T-Tubules
T-tubule membrane is supported by a unique set of peri-membrane scaffolds formed by both extracellular cell adhesion molecules and intracellular membrane scaffolding proteins tethered through cortical cytoskeleton to costamere and sarcomere structural proteins. Different from skeletal t-tubules, cardiac t-tubule membrane is anchored to the Z-discs of sarcomeres. In this section, peri-t-tubule membrane scaffolds and cytoskeleton as well as the t-tubule membrane anchors, including F-actin/N-WASP/tropomysin, costamere dystrophin, and t-tubule membrane-associated dysferlin, will be discussed.
1. Actin filaments, tropomyosin, and N-WASP
Actin filaments have been indicated in the organization and maintenance of t-tubule structure and function. Stabilization of cardiac actin filament by cytochalasin D preserves t-tubules in rodent ventricular cardiomyocytes during extended in vitro culture, which normally loses t-tubule invaginations (91, 118, 214). Actin depolymerization by latrunculin A, on the other hand, disrupts membrane microfolds at t-tubules (91). The integrity of actin filaments is heavily regulated by many actin-regulating proteins including G-actin monomer binding proteins, nucleating proteins, depolymerizing proteins, capping proteins, and polymerizing proteins. These regulatory proteins are important in the organization and function of the myocyte contractile apparatus, cardiac sarcomeres that are primarily composed of cardiac actin and myosin. For example, muscle specific tropomyosin isoforms are associated with sarcomeric actins to form thin filaments, responsible for myocyte contraction following intracellular calcium elevation. Interestingly, in addition to sarcomere organization, recent studies indicate that nonsarcomeric actin is involved in vesicle trafficking (197), and a non-muscle tropomyosin (Tm5NM1) localized adjacent to Z-line can define actin filaments that are associated with t-tubules, contributing to t-tubule organization and function (219).
Furthermore, BIN1+13+17 facilitates N-WASP-dependent actin polymerization, responsible for the maintenance of BIN1-microfolds at t-tubules. N-WASP is a ubiquitously expressed protein involved in transduction of signals from receptors to actin cytoskeleton. N-WASP is bound to the actin nucleator Arp2/3 protein complex, and remains inactive through autoinhibition. Upon activation by small GTPase Cdc42, PIP2, amphiphysin 1, or BIN1, the functional VCA domain of N-WASP is released from autoinhibition, which subsequently activates Arp2/3-dependent actin polymerization (203). In cardiomyocytes, N-WASP is localized at the z-discs (211). When interacting with t-tubule membrane associated BIN1+13+17, N-WASP activation promotes F-actin polymer formation, stabilizing BIN1+13+17-membrane microfolds to z-discs (91).
2. Costamere complex and t-tubule membrane-associated scaffolds
Costameres are cytoskeleton-rich structures surrounding the sarcomere Z-discs right below t-tubule membrane, serving as anchor points between sarcolemma and myofilament. Costameres consist of two major protein complexes, the dystrophin-glycoprotein (DAG) complex and the vinculin-talin-integrin complex. The vinculin-talin-integrin complex is involved in the bidirectional transmission of contractile force between cardiomyocytes and extracellular matrix, with vinculin as the protein linking membrane and actin cytoskeleton. By clustering with vinculin complex (106), dystrophin also has a primary mechanical role in maintenance of cell membrane integrity. Dystrophin is a large 427-kDa protein product encoded by the Duchenne muscular dystrophy gene. In addition to the full-length dystrophin Dp427, a short isoform Dp71 is also expressed in mouse cardiomyocytes (141). Dystrophin is present at cardiac t-tubules from the time tubules develop. Dp427 is found in both general sarcolemma and t-tubules, whereas Dp71 is specifically expressed in t-tubules (58). The dystrophin-DAG complex provides a structural link between cytoskeleton and extracellular matrix. Sarcolemma fragility secondary to dystrophin degradation has been observed in dilated cardiomyopathy (107).
As discussed in other sections, t-tubule membrane-associated scaffolds such as BIN1 (sect. IIC), caveolin-3 (sect. IIC), and junctophilin (sect. VA) are important to maintain normal cardiac t-tubule structure and function. Additionally, another membrane-inserted protein, dysferlin, is also understood to be an important regulator of t-tubule membrane trafficking and repair during injury (6, 7, 46, 54). Dysferlin is a 230-kDa membrane inserted protein with binding affinity to both calcium and phospholipid (44), which is enriched in subsarcolemma vesicles and can be quickly recruited to the membrane injury site (7) to facilitate membrane repair (54). In cardiomyocytes, dysferlin is found to be present in both sarcolemma t-tubules and intercalated discs (30). For t-tubule membrane repair, it has been proposed that upon insult, dysferlin can be recruited to t-tubules along with its interacting partners including BIN1, caveolin-3, and EHDs to induce repair (46). Without dysferlin, abnormal lipid vesicle trafficking impairs membrane repair and accumulates neutral lipids like glycerol, which further promotes detubulation and myopathy (46). Dysferlin deficiency is associated with dilated cardiomyopathy (84, 114, 226), further indicating its important role in maintenance of normal cardiac t-tubule function.
3. Myofilament structural proteins
The Z-disc organizing protein α-actinin, with the cardiac isoform being α-actinin 2, is the structural protein at both Z-disks and peri-Z-disc costameres. Using F-actin filament, α-actinin is linked to the t-tubule membrane-associated cardiac BIN1+13+17 for the stabilization of t-tubule microdomains around myofilament Z-discs. Whether sarcomere α-actin, costameric γ-actin, and/or cortical β-actin fibers are involved in α-actinin-dependent microfold stabilization to Z-discs remains unidentified. Tcap is another myofilament aligning protein (86, 111) that is critical in the maintenance of the integrity of t-tubule network. Mutations of Tcap have been associated with abnormal t-tubule morphology (111) and development of hypertrophic and dilated cardiomyopathies.
Supported by these numerous membrane-associated proteins, scaffolding proteins, cytoskeleton, costamere linking proteins, sarcomere structural proteins, and extracellular matrix, t-tubules are well-organized rigid membrane organelle with limited fluidity. Yet, t-tubule membrane system can also be extremely dynamic and labile (see more detailed discussion in sect. V). It remains to be understood how these regulatory proteins work with each other to maintain a rigid yet dynamic membrane system.
E. Variation in Cardiac T-Tubule Geometry
A high degree of variability in t-tubule geometry has been reported in myocytes from different cardiac chambers and across species. T-tubules have been found in cardiomyocytes from all mammalian species studied so for (mouse, rat, rabbit, dog, sheep, pig, human), but are inconsistently reported or devoid from other phyla including amphibians, reptiles, and birds (see review in Ref. 19). Across mammalian species, it is well accepted that ventricular cardiomyocytes have the most developed and mature t-tubule system (see reviews in Refs. 19, 20, 80), whereas atrial myocytes have more scarce and irregular t-tubules (109, 121, 175, 215). Occasionally, t-tubules are identified to be present in the Purkinje conducting system (51, 160). In mammalian ventricular cardiomyocytes, although the Z-line localization of transverse tubules remains conserved across species, large variability is found in the geometry and size of t-tubules. It appears that t-tubules in rodents and small mammals are denser, deeper, and narrower with more structural complexity than t-tubules from large mammals (i.e., rabbit, pig, and human). Quantitative measurements of overall t-tubule morphology and spacing, based on traditional fluorescent microscopy which cannot discern membrane microfolds but is excellent and quantifying the larger structures, estimate segment branch length (L) to be ∼9 μm and diameter (D) to be ∼200 nm in mouse (91), ∼7 μm (L) and ∼250 nm (D) in rat (198), and ∼2 μm (L) and ∼400 nm (D) in rabbit (183) and human (26) myocytes. The size and complexity of t-tubule network seem to correlate with the heart rate of each species (20). Conceptually, higher heart rates as in rodents require faster action potential propagation into cell interior, allowing for efficient intracellular Ca2+ diffusion to SR to maintain the synchronicity and efficacy of beat-to-beat myofilament contraction (16, 20, 237). Humans and large mammals have resting heart rates less than 100 beats/min, so relatively larger and more widely spaced t-tubules (20, 90) can fulfill the same functional needs to maintain synchronized ventricular contraction during each heartbeat.
Although a t-tubule network is a membrane structure common to the striated muscles, cardiac t-tubules have unique characteristics that are distinct with regard to tubular size, location, and numerical count. Compared with the small skeletal tubules with diameter averaged at 20–40 nm, cardiac t-tubules are much larger with estimated diameter to be 100–400 nm depending on species. Meanwhile, cardiac t-tubules are present within 0.5 μm of Z-discs (198), whereas skeletal t-tubules are localized to the interface between A band and I band of the sarcomere. Thus cardiac t-tubules appear once every sarcomere length spaced out at ∼1.8–2 μm along the longitudinal edge of the myocytes. The skeletal t-tubules, on the other hand, consist of two sets of transverse tubules at each A band/I band interface within individual sarcomeres of skeletal myofilaments.
III. TRANSMEMBRANE PROTEINS AT CARDIAC T-TUBULES
Continuously extended from surface sarcolemma, t-tubules are lipid bilayers embedded with transmembrane or lipid-associated proteins. Studies indicate t-tubules have a different lipid composition relative to general sarcolemma. Previous studies from skeletal muscle identified that t-tubules are enriched in cholesterol (27) and phospholipids, and have a higher phospholipids/cholesterol ratio (167, 178, 204), with phosphotidylserine and sphingomyelin as the two most abundant phospholipid species. The enrichment of phospholipids may be enhanced by the BIN1 organized membrane microfolds which preferentially bind to and concentrate negatively charged phosphoinositides (PIP2) (120). The full lipid profile of cardiac t-tubules awaits careful lipidomic studies. In contrast, the protein components of t-tubules are well studied. This section will focus on the current knowledge of the transmembrane protein composition at t-tubule membrane, focusing on ion handling proteins and signaling molecules.
A. Ion Handling Proteins (Channels/Transporters/Pumps)
The expression of transmembrane ion channels, ion transporters, and pumps have been well characterized in cardiac t-tubules. The calcium handling proteins that are important in cardiac EC coupling, in particular the LTCCs, are mostly enriched in t-tubules. Other Na+ and K+ channels and handling proteins are also found to be present in t-tubules, although to a lesser degree of enrichment. Electrophysiology studies in normal and osmotic shock detubulated cardiomyocytes identified the relative amount of membrane currents at t-tubule membrane versus non-t-tubule sarcolemma (see review in Ref. 19).
1. Ca2+ handling proteins
The most cardiac t-tubule “signature” protein is the calcium transient initiator LTCC, consisting of one α subunit with multiple auxiliary β, α2δ1, and γ subunits. The ICa conducting pore is formed by the large 24 transmembrane domain containing an α-subunit, whose trafficking and gating properties are regulated by the auxiliary subunits (42). The predominant splice variant of α-subunit expressed in cardiomyocytes is α1c, or CaV1.2 (212). CaV1.2 LTCCs are critical in cardiac development and function (186). Global deletion of CaV1.2 induces abnormal cardiac morphogenesis with embryonic lethality (186). On the other hand, in postdevelopment adult hearts, inducible deletion of CaV1.2 leads to reduced contractility and increased susceptibility to stress-induced heart failure (76). As the cell surface dyadic channel, LTCCs need to be concentrated to t-tubules for better coupling with RyRs from jSR membrane, allowing efficient CICR and normal EC coupling (163). Immunocytochemistry data indicate that LTCCs are concentrated at t-tubules, and electrophysiology measurement identified that 80% of the surface calcium current mediated by LTCC is at t-tubules. Such a high concentration of LTCCs at t-tubule membrane is achieved by microtubule-dependent targeted delivery of LTCCs to t-tubules, a process facilitated by the t-tubule membrane curvature protein BIN1 (94) (Figure 4). Furthermore, LTCCs at t-tubules are not distributed evenly, but rather clustered to membrane microdomains formed by BIN1-microfolds. These LTCCs clusters will couple with RyRs channels on the nearby jSR membrane to form functional calcium releasing units bridged by BIN1 molecules (Figure 4). These TEM visible electron-dense feetlike structures, dyads, are the initiation sites where beat-to-beat calcium transient starts. During each action potential, membrane depolarization activates voltage-gated LTCCs to induce calcium influx. The initial calcium ions entered through LTCCs at the junctional dyads diffuse a short distance to activate the nearby calcium sensing and releasing RyRs at jSR membrane, inducing a large release of calcium from SR lumen to cytosol. This is a sequence of events known as calcium-induced calcium release (CICR). How LTCC-RyR dyads are organized by BIN1-microfolds to control CICR and EC coupling (66) will be further discussed in section IV focusing on the functional regulation of dyads.
During relaxation, distal SR membrane localized sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) reuptake elevated cytosol calcium into SR storage. Meanwhile, the elevated intracellular calcium at the cleft between t-tubule and SR membrane will also be extruded through sarcolemmal NCX1, a calcium handling protein anchored at ankyrin-B microdomains within t-tubules that is critical for intracellular calcium homeostasis.
2. Na+ handling proteins
In most excitable cells, the voltage-gated sodium channel-mediated Na+ current is responsible for the rapid action potential upstroke. Sodium channels consist of four pore-forming α-subunits and one or two auxiliary β-subunits. Ventricular cardiomyocytes express several different pore-forming subunits of the voltage-gated Na+ channels including the primary cardiac isoform Nav1.5 and the brain-type isoforms Nav1.1, Nav1.3, and Nav1.6 (136), with different intracellular localization and functional importance. Sodium current measured in control and detubulated rat ventricular cardiomyocytes indicate more uniform distribution of cardiac Nav1.5 current along sarcolemma and t-tubules (in channels/μm2, 11 on total sarcolemma vs. 10 on t-tubules), and a significant t-tubule concentration of current mediated by neuronal sodium channel isoforms (in channels/μm2, 1 on total sarcolemma vs. 2.5 on t-tubules).
Although Nav1.5 is also localized to cardiac t-tubules (55), this primary cardiac α-subunit Nav1.5 is preferentially localized to intercalated discs with auxiliary β2- and/or β4-subunits, mediating rapid action potential propagation from cell to cell across the myocardium. In contrast, the brain isoforms of Nav1.1, Nav1.3, and Nav1.6 together with β1- and/or β3-subunits, are localized to t-tubules (135) and near t-tubule openings (56). These t-tubule localized brain Nav isoforms have more negative voltage dependence of gating and more rapid activation and inactivation kinetics, facilitating fast action potential inward penetration from myocyte surface to interior via t-tubule invagination. However, the role of these channels in EC coupling remains controversial. T-tubule localized neuronal isoforms of sodium channels have been indicated as contributors to more effective CICR in some studies (136), and removal of Nav1.6 has been shown to prolong calcium transient duration (155). Other studies indicate that, although these neuronal sodium channels are concentrated at cardiac t-tubules, they are not required for EC coupling (21).
NKA uses ATP hydrolysis-derived energy to pump 3 Na+ and 2 K+ ions across membrane, hence maintaining membrane potential in cardiomyocytes. Three catalytic α (α1, α2, α3) subunits of NKA are expressed in the heart, with α1 as the dominant isoform (206). However, the α1 isoform is more uniformly expressed, whereas isoforms α2 and α3 are mainly located in the junctional membrane within t-tubules (105). Studies have shown that isoform α2 expression is four times higher in t-tubules versus non-t-tubule sarcolemma (10). Within t-tubules, NKA colocalizes with NCX1 at ankyrin B microdomains, contributing to the regulation of CICR and calcium transient. NKA and NCX1 and the jSR localized InsP3 receptors are clustered to a junctional subdomain organized by a membrane scaffolding protein ankyrin B. Distinct from BIN1-organzied LTCC-RyR dyads, ankryin B similarly tethers cytoskeleton to facilitate targeted delivery of NCX1 and NKA (147), resulting in the concentrated expression of the functional NKA and NCX1 at t-tubules (∼3- to 3.5-fold higher than external sarcolemma) (48).
3. K+ handling proteins
Most potassium channels are more uniformly distributed between t-tubules and surface sarcolemma in cardiomyocytes. Detubulation of cardiomyocytes only decreases the steady-state current Iss [mediated by Kv1.2/2.1 (184) or TASK-1 (104)], without affecting the density of other potassium currents including transient outward current Ito (mediated by Kv4.2/Kv4.3), inward rectifier current Ik1 (mediated by Kir2.x), and delayed rectifier current Ik (Ikr mediated by HERG, and Iks mediated by KvLQT1) (113). Interestingly, immunofluorescence and TEM imaging data demonstrated that the Ito generating Kv4.2 is concentrated at both t-tubules and intercalated discs (8). The lack of current density change after detubulation may be due to the Ito current at the intercalated discs. Antibody labeling also supports that Ik1 generating Kir2.x, including Kir2.1 (35), Kir2.2 (122), and Kir2.3 (143) channels, are localized to t-tubules. Other ATP-sensitive inward rectifiers (Kir6.x) are also localized to t-tubules. Both IKATP and Ik1 decrease along with t-tubule membrane loss when cardiomyocytes are cultured in vitro (34, 145). Interestingly, KATP channel release from the Golgi can be dependent on stress-induced PKA activation, resulting in rapid insertion of KATP channels into t-tubule membrane (1). If the cargo is in the close proximity to the membrane, there may a direct vesicle-mediated Golgi to membrane translocation pathway (172). Immunofluorescence data also indicate that the cardiac Ikr channels, which consist of two α-subunits hERG1a and hERG1b, are localized to t-tubules (103).
B. Signaling Molecules at Cardiac T-Tubules
The most well-studied signaling pathway at t-tubules is the G protein-coupled β-adrenergic receptor (β-AR) pathway, which mediates the sympathetic control of myocardial function. Cardiomyocytes express both β1-AR and β2-AR, which are coupled to the Gs-AC-cAMP cascade (see review in Ref. 233). Through the activation of cAMP-dependent protein kinase A (PKA) and the subsequent phosphorylation by PKA of critical proteins involved in calcium handling and contractile function, β-ARs modulate catecholamine-dependent rate, force, and speed of myocyte contraction. The protein targets of β-AR-activated PKA include sarcolemmal t-tubule LTCCs (67, 68), SR membrane RyRs (139) and phospholamban (PLB) (125), and intracellular myofilament proteins (troponin I and C proteins) (12). In addition to the stimulatory Gs pathway which β1-AR is solely coupled to, β2-AR is also known to couple to the inhibitory Gi protein (234, 235). Thus, within β2-AR-enriched caveolae (14, 229) and t-tubule (77), the β2-AR to Gi pathway can negate β2-AR-Gs mediated cAMP-PKA signaling.
In the t-tubule system, several factors affect the compartmentalization of the β-AR signaling. First, the differential distribution pattern of β1-AR and β2-AR reveals that β1-AR is homogeneously spread throughout sarcolemma and t-tubules, whereas β2-AR is more selectively confined to t-tubules (77, 154). Second, LTCCs, the ion channel targets of Gs-activated PKA, are concentrated to t-tubule membrane with particular clustering to BIN1-microfolds (94). Third, a number of signaling molecules in the β-AR-Gs-PKA cascade are also localized to t-tubules, including phosphodiesterase 3 (PDE3), PKA, AKAPs, and AC. The β1/2-ARs mediated Gs-cAMP signaling at t-tubules is therefore more confined to subsarcolemmal microenvironment right below the t-tubule openings, where most BIN1-microfold clustered dyadic LTCCs and RyRs are also enriched. The structural restriction of AKAP on PKA diffusion (78, 110) further confines β-AR-Gs-PKA signals close to submembrane microdomains to more effectively modulate the phosphorylation and activity of surface LTCCs without affecting intracellular protein targets. Compartmentalization of β-AR signaling to cardiac dyads is also linked by cBIN1-microdomains, which both organize LTCC-RyR dyads and undergo fast reassembly upon acute β-adrenergic activation (66).
Other than the secondary messenger cAMP-dependent function in contractile regulation, β-AR signaling pathway also contributes to regulation in apoptosis and cell survival. The β1/2-AR-cAMP-PKA pathway stimulates apoptosis by activating MAPK family, particularly ERK1 and ERK2 (32, 41, 241). The antiapoptotic effect of β2-AR activation, on the other hand, is more likely mediated through the Gβγ subunits, which are released from Gi upon β2-AR activation by catecholamine (240). Thus t-tubule localized β2-AR also signal through Gi-Gβγ pathway to activate the pro-survival PI3K and AKT signals (240).
Given the differential role of β1-AR/β2-AR in regulating cell contractile function and apoptosis/cell survival, t-tubule-dependent-compartmentalized β2-ARs play a critical role in regulating these signaling pathways to maintain normal cardiac function. In fact, redistribution of β2-AR with altered β2-AR-cAMP signaling occurs during heart failure (77, 134, 154), indicating the importance of t-tubule compartmentalized β2-AR-cAMP signaling in normal cardiac function and during disease development.
IV. FUNCTION OF CARDIAC T-TUBULES
Exploration of the functional importance of t-tubules in cardiac calcium transients has been a very active field of research since late 1980s. The dyad membrane structure formed by t-tubules and jSR terminal cisternae is critical in organizing EC coupling of heart muscle. In 1989, Fabiato (59) proposed the model of CICR as the molecular mechanism underlying EC coupling, which is further supported by the later identification of calcium sparks as the elementary events (31). The sarcolemma voltage-gated LTCCs, which are activated during membrane depolarization following each action potential, conduct the initial calcium influx to induce the subsequent series of events of intracellular CICR. The intracellular calcium sense and release units are RyRs on the jSR membrane. In addition to dyad formation for efficient CICR and normal EC coupling, t-tubules also create a diffusion barrier for extracellular ions, protecting electrical stability of the cardiomyocytes during fluctuations in extracellular bulk environment. The recently identified BIN1 microdomains contribute to both dyad microdomain formation (65, 94) and generation of a “fuzzy space” (119) restricted diffusion zone for extracellular ions, thus serving as a major regulator of the primary functions of cardiac t-tubules (Figure 2).
A. Dyad and EC Coupling
Normal ventricular EC coupling requires a calcium transient initiated at the cardiac t-tubules. As discussed earlier, the current accepted model of intracellular calcium transient development is CICR (59). At each heartbeat, in response to action potential-triggered inward sodium current, sarcolemmal membrane depolarization activates voltage-gated LTCCs at the t-tubule membrane. The initial calcium influx mediated by activated LTCC subsequently induces a massive calcium release from SR stores. Proposed as early as in late 1980s, this CICR model was further supplemented by the identification of the jSR membrane localized RyRs (100, 168). In the early 1990s, calcium sparks from the SR were then identified as the “elementary units” of the calcium transient (31). The complexes consisting of LTCCs at t-tubules and RyRs at jSR membrane (∼1:4 ratio) form cardiac dyads (13, 25). Close physical association of jSR membrane localized RyRs with sarcolemma LTCCs (31, 59, 100, 168) is required for optimal CICR needed for efficient EC coupling. Such a close association between the two dyadic channels is achieved by LTCC localization to t-tubules (11, 185) and clustering to microdomainds induced by BIN1 microfolds, which also recruit RyRs to jSR membrane for LTCC-RyR couplon formation (Figure 3). Upon membrane depolarization, the initial calcium influx through LTCCs and the close association between LTCCs and RyRs (∼15 nm) at dyads permit efficient CICR and subsequent sarcomeric contraction (156).
1. LTCC trafficking and clustering to dyads through BIN1
The calcium transient initiator LTCCs need to be concentrated and clustered to t-tubule microdomains for better coupling with RyRs to form functional dyads (163). Precise t-tubule localization of LTCCs relies on the membrane scaffolding protein BIN1 for several reasons. First of all, LTCC trafficking from the Golgi apparatus occurs on microtubules which deliver LTCCs to BIN1 containing membrane (94). This microtubule-dependent channel delivery highway with specificity, known as targeted delivery, was introduced via previous studies using connexin 43 trafficking to intercalated discs as a model system (93, 94, 188, 195). Briefly, targeted delivery describes that, after exiting the Golgi, membrane vesicles containing transmembrane ion channels are rapidly directed on microtubule highways across the cytoplasm to their specific membrane microdomains (Figure 5). The microtubules with negative ends are anchored at the microtubule organizing center (MTOC) near Golgi, and the fast growing positive ends are approaching outward ready to be captured by membrane anchor protein complexes at specific plasma membrane subdomains. Specificity of delivery requires a combination of the individual channel protein or truncated isoform (Figure 6), the plus-end-tracking proteins at the growing ends of microtubules, and the membrane anchor protein complex responsible for capturing. In the case of Cav1.2 channel delivery, t-tubule-associated BIN1 anchors growing microtubules with Cav1.2 cargoes, allowing the delivery of Cav1.2 to occur at BIN1 molecules (94). BIN1 contains a membrane curvature BAR domain, the middle coiled-coil regions, and an SH3 protein-protein interaction domain (see sect. II). Compelling data are that deletion of the coiled-coil and SH3 domains abrogates the ability of BIN1 in facilitating Cav1.2 trafficking without altering membrane invagination (94). Thus targeted delivery of Cav1.2 is achieved through interaction with the BIN1 molecules at t-tubules, rather than the t-tubule structures themselves. The non-BAR domains in BIN1 molecules likely attract Cav1.2 channels through interaction with the COOH terminus of the channel. The involvement of BIN1 in trafficking Cav1.2 is further confirmed in a series of subsequent studies using BIN1-deficient ventricular cardiomyocytes (91, 93). Alternative translation of connexin 43 provides smaller isoforms (Figure 6) that assist with forward trafficking of the full-length protein (196). Fragments of the LTCC COOH terminus occur and have been attributed to cleavage (69). It is not known if these fragments could be formed by alternative translation instead, if whether they assist with forward trafficking.
FIGURE 5.
Schematic representation of ion channel targeted delivery. Channel proteins are sorted into vesicular carriers and docked onto microtubules at the trans-Golgi network (TGN) and subsequently delivered to their subcellular destinations in cooperation with actin “rest stops” along the route. Microtubule plus-end binding proteins interact with anchor proteins of specific membrane subdomains, allowing targeted delivery of cargo proteins. In the case of connexin 43 (Cx43) trafficking, the interaction between the microtubule plus-end binding protein EB1, the channel itself, and the adherens junction complex ensures the targeted delivery of Cx43 hemichannels to the intercalated discs. For LTCC delivery to t-tubules, key components are the LTCC channels, a +TIP protein, and cardiac bridging integrator 1 (cBIN1). The microtubule +TIP protein associated with LTCC delivery has not yet been identified.
FIGURE 6.
Alternative translation of connexin 43. In addition to full-length 43-kDa connexin 43 (GJA1-43K), there are six NH2-terminal truncated smaller Cx43 isoforms (GJA1-32K, GJA1-29K, GJA1-26K, GJA1-20K, GJA1-11K, and GJA1-7K) resulting from internal methionine residues that initiate ribosomal translation, a process known as alternative translation. At least one of the isoform, GJA1-20k, has been identified as necessary for the full-length channel to traffick to the surface membrane.
In addition to facilitate forward trafficking of Cav1.2 channels, BIN1 also plays a critical role in clustering Cav1.2 channels that are already localized at the t-tubule membrane. Through formation of BIN1 microfolds, these unique membrane structures form curved membrane microdomains enriched with Cav1.2 channels. As indicated in our recent super-resolution STORM imaging (XY-resolution at 10–20 nm, Z-resolution at 50 nm), Cav1.2 form discrete clusters at cuplike microdomains created by BIN1 microfolds. The role of BIN1 in clustering Cav1.2 channels is further supported by the observed smaller Cav1.2 clusters in adult mouse ventricular cardiomyocytes with Bin1 heterozygous deletion (91). Clustering of Cav1.2 channels at these t-tubule microfolds alters channel kinetic properties including gating and open probability (53, 152). How BIN1 microdomains regulated Cav1.2 clustering alters coupled gating of LTCCs at the t-tubule surface is an interesting and important area of future consideration.
2. RyR recruitment to dyads through BIN1
The other critical dyadic component is the RyR homotetramer at the jSR membrane apposing LTCCs at t-tubules. In the RyR tetramer, the COOH-terminal transmembrane pore domain of each RyR monomer comes together to form a calcium-conducting pore across the SR membrane. The four NH2-terminal cytoplasmic domains from the tetramer constitute a large mushroomlike platform containing binding sites (182) for various modulators including calstabin (216), PKA and mAKAP (muscle A kinase anchoring protein), phosphatases PP1 and PP2A (139, 140), as well as calmodulin (236) and Ca2+/calmodulin kinase II (CaMKII) (223). Biophysical properties of RyR can be regulated by these modulators via alteration in receptor posttranslational modifications including phosphorylation states, luminal calcium (81), or stabilization of receptor closed states. The macromolecular complex centering on RyR channels facilitates efficient spatial and timely regulation of channel function.
It is important to note that the arrangement of RyR at dyad is neither uniform nor static. The receptor cluster contains 10–300 RyR homotetromers (63) with a spatial separation of 0.6–1 μm between clusters (199), the organization of which can be altered on a time scale of minutes by Mg2+ concentration, receptor phosphorylation, as well as reorganization of BIN1 microdomains (2). It was recently identified that t-tubule-attached BIN1 recruits RyRs into dyads, an interaction that is enhanced after RyR phosphorylation (66). The enrichment of positively charged residues within the BAR domain of BIN1 may explain its preferential binding to negatively charged hyperphosphorylated receptors, in a way similar to the known binding affinity of BIN1 to negatively charged phospholipids. Interestingly, upon acute β-AR activation, BIN1 is redistributed to t-tubules, a result likely due to β-AR activation-induced phospholipid accumulation at the t-tubule lipid bilayer. Subsequently, phosphorylated RyRs are further recruited to BIN1 microdomains, allowing efficient RyR coupling with LTCC for functional dyad formation, improving EC coupling with preserved electrical stability during acute stress (Figure 7) (66).
FIGURE 7.
Cartoon of BIN1-dependent recruitment of phosphorylated ryanodine receptors (RyR) to dyads during acute stress. Acute isoproterenol (ISO) redistributes BIN1 to cardiac t-tubules and subsequently recruits phosphorylated RyRs to couple with LTCCs at dyads, increasing excitation-contraction coupling gain while maintaining electrical stability. In Bin1 HT cardiomyocytes with less BIN1 available, insufficient RyR recruitment leads to accumulation of hyperactive phosphorylated RyRs outside of dyads, increasing spontaneous calcium release and promoting arrhythmias.
3. Junctophilin-2 in t-tubule-jSR junctional membrane complex formation
Another well-studied protein family involved in t-tubule and jSR organization is the junctophilins (JPs), which have the apparently unique ability to directly bind to both t-tubule and SR membranes. In mammalian tissue, three junctophilin proteins JP-1, JP-2, and JP-3 are encoded by different genes, with JP-2 expressed in heart. JP-2 has a COOH-terminal hydrophobic segment spanning the SR membrane, a linkage α-helical domain, and the remaining cytoplasmic region consisting of eight lipophilic “membrane occupation and recognition nexus (MORN)” domains (115). The MORN domains are associated with t-tubules, approximating t-tubule sarcolemma to the jSR membrane. It remains unclear whether JP-2 facilitates BIN1 bridged LTCC-RyR dyad formation and function. Whether JP-2 is critical in EC coupling and contributes to heart failure progression is also controversial. Some studies indicate that JP-2 is downregulated in animal models of hypertrophic and dilated cardiomyopathy, and conditional knockdown of JP-2 in the heart causes systolic heart failure in murine models (218). Other studies report no significant changes of JP-2 expression in failing hearts (23), or with recovery of heart function (134).
B. Ion Diffusion and Membrane Excitability
One of the functional attributes of t-tubules is the presence of slow diffusion zones close to t-tubule sarcolemma (164, 189, 209). Restricted diffusion occurs both at extracellular and intracellular spaces near the t-tubule membrane with accumulated ions such as calcium, which was originally observed as a theoretical “fuzzy space” (119). Growing evidence indicates that extracellular ions diffuse slowly within the t-tubule network, compared with extracellular bulk environment. In fact, ion diffusion coefficients within the t-tubule network are normally 5–10 times slower than those in the extracellular bulk environment (189, 209). Fluctuations in environmental extracellular ion concentrations therefore occur with delayed lag phase before reaching to t-tubule membrane and its complement of ion channels. For instance, the change of extracellular calcium is delayed by up to 2.3 s to reach the t-tubules in guinea pig myocytes (16). This lag phase due to restricted diffusion within the t-tubules can have a significant effect on muscle cell homeostasis and stability, particularly during environmental fluctuations induced by acute stress. Increased heart rate and quick transmembrane ion flux together with limited diffusion can rapidly accumulate outward current ions like potassium (209) and deplete inward current ions like calcium (164, 165), affecting the driving force of ion channels at t-tubule membrane and shortening the action potential duration (166). Shortened action potential limits t-tubule membrane excitability and electrical instability, serving as a crucial protective mechanism in preventing detrimental ventricular arrhythmias.
Although t-tubule forms a complex interconnected network (198), this network feature does not explain the highly restricted diffusion near the t-tubule sarcolemma. The structural foundation of the restricted diffusion zone was not revealed until the recent discovery of t-tubule membrane microfolds created by cardiac BIN1+13+17. In wild-type adult mouse ventricular cardiomyocytes, along with tortuous t-tubule (91) path meandering into the myocyte interior, BIN1 microfolds are present at t-tubule membrane, bringing spatial complexity to the lumen. These spatial divided subdomains with narrow connecting points to the central lumen help trap extracellular ions, preventing ionic diffusion. For example, the small extracellular gaps between layered membrane microfolds can trap Ca2+ and K+. Thus, in addition to dyad microdomain organization, BIN1 microfolds also create spatial pockets, serving as reservoirs for extracellular ions and other signaling molecules. In BIN1-deficient cardiomyocytes, these microfolds and so formed spatial pockets are lost and tortuous t-tubules become more straight and dilated, normalizing rapid ion diffusion at t-tubules. Mathematical modeling further confirms that removal of this diffusion barrier increases fast ion diffusion from t-tubule network to outside bulk environment. When BIN1 is reduced as occurs in heart failure, loss of BIN1 microfolds removes the protective ion diffusion barrier, increasing membrane excitability and promoting arrhythmias (91). In short, the microfold-forming cardiac BIN1+13+17 plays a pivotal role in maintaining and organizing t-tubule ultrastructure essential for cardiomyocyte electrical stability and homeostasis. Interestingly, recent studies in zebrafish skeletal muscle indicate that skeletal BIN1 may also have a similar function in maintaining t-tubule ultrastructure (194). Whether a similar membrane microdomain-formed slow diffusion zone exists in narrow skeletal tubular network with functional significance remains to be identified.
C. Other Microdomain-Related Functions
Other t-tubule microdomains, in addition to dyads, serve as signaling centers by concentrating transmembrane receptors, ion channels, and signaling molecules. For instance, caveolae are enriched with a subset of LTCCs (4) as well as β2-AR (229), permitting efficient calcium signaling regulation by the β-adrenergic system (5). In addition, ankyrin B-organized NCX/NKA/InsP3 macromolecular complex (147) facilitates calcium removal during muscle relaxation. Coordination of these t-tubule microdomains is necessary for achieving timely intracellular calcium equilibrium required for beat-to-beat heart contraction. Recent evidence indicates that crosstalk between BIN1 microdomains and caveolae likely exists for better control of β-adrenergic-regulated calcium entry through LTCCs, especially during acute stress as well as chronic dysregulation in heart failure. It is likely that caveloae microdomains are indeed related to BIN1 microfolds, since intracellular localization of caveolin-3 is altered in cardiomyocytes missing the Bin1 allele (116). The role of BIN1 in regulation of caveolae localization and function remains to be tested. Nevertheless, in addition to organizing microfolds-based dyads, BIN1 may have a significant role in other t-tubule microdomains as well.
V. LIFE CYCLE OF CARDIAC T-TUBULES
Despite their structural complexity, t-tubules are extremely labile (112). T-tubules are absent in embryonic myocytes (187); develop only after birth (82, 187); dedifferentiate in cultured myocytes (118, 130, 145), a process that can be slowed by actin stabilization (118, 214); remodel during heart hypertrophy and failing (130, 132, 133, 224); and can recover during functional recovery of the hearts (133). In addition to membrane turnover, other t-tubule components including transmembrane ion channels and receptors are also extremely dynamic. By regulating protein trafficking and recycling, cardiomyocytes maintain a steady state of continuously replenished t-tubule pool of ion channels and signaling molecules for functional homeostasis. Meanwhile, intracellular cytoskeleton components including cortical actin, microtubules, and costameres are actively regulated to control membrane integrity and protein function. This flexible function of t-tubules allows them to accommodate both healthy and stress environments.
A. Membrane Biogenesis, Maintenance, and Turnover
Unlike the sarcoplasmic reticulum membrane system, which develops in the fetal stage, cardiac t-tubules are absent in embryonic hearts. In rodent and rabbit hearts, the process of t-tubule biogenesis initiates a few days after birth (82, 83, 187), along with the rise of the left ventricular pressure and working volume. Mature t-tubules are only fully developed by the end of the first month of life. The mechanism of cardiac t-tubule development remains unclear. The Franzini-Armstrong group proposed in 2007 that the inward penetration of t-tubule invagination is driven by accrual of membrane lipids and specific proteins (50). Up to this date, limited progress has been made to further understand the lipids, proteins, and other molecules involved in biogenesis process of t-tubules. The need to resolve the molecular mechanisms of postnatal cardiac t-tubule development becomes even more urgent since the fast development in the stem cell research field over the last few years. In 2007, Yamanaka's group successfully reprogrammed differentiated human somatic cells into an undifferentiated pluripotent state for the generation of induced pluripotent stem (iPS) cells (210), which can then be redifferentiated into most any terminally differentiated cell types including cardiomyocytes. These iPS-induced cardiomyocytes contain essential contractile apparatus including sarcomeres, yet they do not have a mature t-tubule network. It is of great interest to know how to mature these iPS-differentiated cardiomyocytes into adult phenotype with characteristic interconnected t-tubules and efficient EC coupling machineries.
T-tubule networks and especially BIN1 organized microdomains can also be extremely dynamic. The gross t-tubule network in isolated cardiomyocytes loses integrity when cardiac origin cells are cultured in vitro, and t-tubule loss and remodeling are also a signature of failing cardiomyocytes. On one hand, the SH3 domain of BIN1 binds to dynamin 2 and is involved in dynamin 2-dependent endocytosis (22, 171), indicating a possible intracellular recycling of BIN1 microfolds through endocytic vesicle formation (Figure 8, adapted from BAR-regulated endocytosis in non-cardiac cells in Ref. 232). On the other hand, the recently identified BIN1 microfolds are lost in isolated cardiomyocytes during extended in vitro culture (91), indicating external release of BIN1 microfolds. Loss of BIN1 microfolds can be rescued by replenishment of the microfold-forming BIN1 isoform and stabilization of the filamentous actin cytoskeleton. Furthermore, BIN1 is blood available, and plasma BIN1 correlates with cardiac function (92). It is possible that plasma BIN1 originates from cardiac t-tubules as a result of BIN1-microfold turnover and release. In addition to stabilizing membrane microfolds to Z-discs, BIN1 may also regulate cytoskeleton dynamics for microparticle release (see speculative release mechanism in Figure 9), balancing the amount of microfolds and microfold-based calcium regulatory microdomains at t-tubule membrane. The equilibrium between microfold organization and turnover is achieved by the amount of BIN1 protein at the t-tubule membrane. The released BIN1-containing microparticles therefore carry a signature of cardiomyocyte t-tubule membrane, which can be quantified in plasma level, and the resulted plasma cardiac BIN1 level can be used as a marker of myocardial health (92). How this BIN1-mediated membrane turnover and release is linked to other membrane vesicle trafficking processes, such as injury-related membrane repair (45, 46) especially during acute stress and insult, will be interesting to pursue in the future.
FIGURE 8.
Schematic of endocytosis from cBIN1 microdomains. Adapting BAR domain regulated endocytosis from noncardiac cells (229), we expect that for t-tubules, cBIN1 interacts with cortical actin to generate an endocytic neck, and the SH3 domain of BIN1 the binds to dynamin 2 for scission and internalization of the endocytic vesicle.
FIGURE 9.
BIN1-microdomain release from cardiac t-tubules. A schematic of how fission can potentially occur between two neighboring invaginated cBIN1 microfolds, resulting in external release of cBIN1 microfolds that result in blood-borne microparticles.
B. Ion Channel Trafficking and Half-Life
Transmembrane ion channels and ion handling proteins are dynamically turned over to maintain a functional pool at the t-tubule surface. In cardiomyocytes, the half-life of individual ion channels is remarkably short, on the order of hours. The half-lives of t-tubule localized calcium and potassium channels, and sodium-calcium exchangers, are all on the order of 10 h (33, 37, 49, 57). Even the more extended half-life of sodium channels is ∼35 h (138). For an overall turnover scale of several to tens of hours, the bulk of the ion channels in each cardiomyocyte are regenerated and have to be newly positioned to t-tubule membrane in the course of any day. To maintain such a steady state and continuously replenished t-tubule pool for functional homeostasis, ion channels at the t-tubule membrane need to be tightly regulated at both forward trafficking and recycling pathways. In fact, ion channels often contain multiple signaling sequences that regulate forward trafficking including the rate of their synthesis, organization at endoplasmic reticulum such as multimerization with auxiliary subunits, and traffic through Golgi apparatus before arrival at the plasma membrane (144, 205, 239). On the other hand, t-tubule pool of ion channels is also regulated by retrograde trafficking including internalization, recycling, and degradation. Furthermore, it is possible that ion channel half-life at plasma membrane may occur at a different time scale. For instance, the half-life of active NCX in the plasma membrane is likely to be under 12 h (57, 127), which is shorter than the long half-life of 33 h measured using metabolic labeling in lysates from neonatal cardiomyocytes (193). Similarly, the functional half-life of the Cav1.2 channel is ∼3 h (33), whereas the total protein turnover occurs at a much slower rate of 25 h (29). The same might be true for other ion channels including the potassium and sodium channels. The precise measurement of channel life cycles at differential plasma membrane subdomains is limited in the literature, which will require a combination of pulse-chase experiments with biochemical fractionation and extensive use of life cell imaging.
To provide more insights into ion channel turnover in t-tubules versus sarcolemmal membrane, future studies are required in primary cardiomyocytes from adult ventricles. The results will also help determine how microdomains in t-tubules affect ion channel stability compared with non-t-tubule plasma membrane. Nevertheless, it is fair to conclude from studies to date that dynamic turnover of ion channels at t-tubule membrane is essential for normal function of cardiomyocytes, and allows timely regulation of functional channels under normal physiological conditions. Furthermore, it has already been established that mutations which affect forward trafficking, recycling, and degradation of ion channels, or their interaction with microdomain-organizing proteins such as caveolin-3 and, ankyrin B can result in major cardiac diseases (17; review in Ref. 40).
VI. ALTERATION OF T-TUBULE STRUCTURE AND FUNCTION IN HEART FAILURE
Heart failure is the fastest growing cardiovascular disorder with a high mortality and morbidity affecting more 40 million patients worldwide (129, 176, 177), particularly in the developed countries including the United States. The primary mortality involves cardiac pump failure as a consequence of reduced cardiac contractility and sudden cardiac death due to detrimental ventricular arrhythmias. The definitive therapy for end-stage heart failure is a heart transplant, which unfortunately remains as a limited option due to poor organ availability (102), and a need for subsequent care and monitoring. The alternative therapy for end-stage heart failure is left ventricular assist devices (LVAD) (124, 192), and for arrhythmia management are implantable cardioverter defibrillators (ICDs) (217). However, the cost of device implantation of either LVAD or ICD and the morbidity associated with implanted devices are enormous. Lack of an accurate understanding of cardiomyocyte health and recovery potential limits the ability in making critical decisions with regard to heart transplant priorities and criteria for LVAD and ICD implantation (18, 170). New diagnostic and prognostic tests that accurately evaluate myocardial health and arrhythmogenic potential at the cellular level could significantly improve decision making for LVAD and ICD implantation with improvement in patient care. New therapeutic tools with the ability to break pathophysiological cycling of heart failure progression are acutely needed to help postpone and rescue disease development in millions of patients with heart failure.
For efficient development of new diagnostic, prognostic, and therapeutic tools of human heart failure, a more complete understanding of the biology of failing cardiomyocytes is required. The pathophysiological hallmark of failing cardiomyocytes is weakened calcium transients (74, 75, 128). Normal transients are initiated at cardiac dyads, where t-tubule LTCCs (11, 185) are closely associated with SR RyRs (31, 59, 100, 168) for optimal CICR following each action potential. Failing calcium transients can be attributed to t-tubule remodeling (132, 133, 224) and dyad uncoupling (15, 74, 75, 88, 131, 132). T-tubule remodeling has been considered as a characteristic mark of the transition from hypertrophy to failure (224) and a contributing mechanism of heart failure progression (see reviews in Refs. 19, 20, 61, 80, 99, 132). In addition to the destruction of the gross t-tubule network, protein components at t-tubules including ion channels and signaling molecules are also reported to be either reduced or redistributed out of t-tubules. Loss of complex t-tubules in heart failure not only desynchronizes CICR and impairs contractility, but also impairs local electrophysiological homeostasis and increases susceptibility to ventricular arrhythmia.
Of heart failure-associated molecules, the cardiac t-tubule membrane deformation protein BIN1 is emerging as a crucial master regulator of healthy t-tubule function, which when reduced can promote heart failure progression (93, 116). The role of BIN1 as a cardiac t-tubule adaptor protein was first noted when constitutive Bin1 knockout in mice caused perinatal lethality due to cardiomyopathy (150). Later it was identified that BIN1 localizes to cardiac t-tubules, facilitating microtubule-dependent forward delivery of Cav1.2 channels to t-tubule membrane (94). Follow-up studies from independent research groups identified that BIN1 is transcriptionally decreased in acquired human heart failure (93), as well as multiple animal models of heart failure (23, 134). Decreased BIN1 level in failing cardiomyocytes reduces LTCC surface expression (23, 93, 134), contributing to the pathophysiology of diseased calcium transients and electrical instability in failing hearts (Figure 10). In zebrafish, morpholino-mediated knockdown of the Bin1 gene induces significant contractile dysfunction and cardiomyopathy (93). Mice with cardiac conditional Bin1 knockout after birth have increased tendency to develop cardiomyopathy with either older age or pressure overload (116). Of the four BIN1 isoforms, cardiac specific BIN1+13+17 with inclusion of exons 13 and 17 is the primary BIN1 isoform localized to cardiac t-tubules and responsible for cardiac t-tubule membrane ultrastructure formation (91). Whether aberrant splicing and mutation in cardiac spliced exons plays a role in cardiomyopathy development remains to be observed. Although the mechanism of reduced BIN1 expression in heart failure remains to be determined, BIN1-based microdomain is a potential therapeutic target for the development of new heart failure therapies.
FIGURE 10.
Reduced cBIN1 microdomains in failing cardiomyocytes. In failing cardiomyocytes with reduced cBIN1 and cBIN1-induced microdomains, expression of ion channels on the cell surface and the morphology of t-tubules are altered. As highlighted in the cartoon, changes following reduced BIN1 transcription include 1) impaired microtubule-dependent targeted delivery of LTCC results in reduced t-tubule surface expression of LTCCs; 2) disruption of cBIN1 microdomains impairs sufficient LTCC clustering within t-tubules; 3) loss of dense membrane microfolds and resultant t-tubule dilation cause removal of the protective slow diffusion zone within t-tubule lumen; and 4) impaired recruitment of RyRs causes accumulation of hyperphosphorylated RyRs outside dyads, increasing orphaned leaky RyRs.
In addition to serving as a target for therapeutic development, BIN1 is a potential biomarker of myocardial health and reserve. BIN1 is blood available, and plasma BIN1 correlates with cardiac health (92), through continuous turnover of BIN1-microdomains released from the t-tubule membrane. In a cohort of arrhythmogenic right ventricular cardiomyopathy patients, plasma BIN1 decreases in patients developing symptomatic heart failure (92). Low plasma levels of BIN1 correlates with heart failure status and predicts future arrhythmia incidences (92). The recently cloned microdomain forming cBIN1 (91) provides an opportunity to develop a cardiac specific BIN1 blood test to aid in the diagnosis and management of patients with failing hearts.
In the clinical literature, a clear distinction now exists between heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), with HFpEF incidence exceeding that of HFrEF (158). The diagnosis of HFpEF is made in patients who present with clinical heart failure despite the absence of left ventricular systolic dysfunction. Unlike HFrEF, HFpEF has few available treatment options (142, 158). Newer therapies such as cardiosphere-derived cell-based treatments are still in the research phase (71). In unpublished studies we have found that plasma-based cBIN1 decreases in patients with HFpEF just as it does for patients with HFrEF. These types of studies suggest that future classification of failing hearts will be based less on measures of overall organ function such as ejection fraction, and more on the molecular details of myocyte, and in particular t-tubule, pathophysiology.
VII. CONCLUSIONS
In this review, we provided an overview of the structural and functional organization of the cardiomyocyte t-tubule system that is required for normal contractility and electrical stability of the heart. The life cycle of t-tubule membrane and proteins, as well as t-tubule remodeling during heart failure, is also discussed. The membrane microdomains within the cardiac t-tubular invaginations are highlighted with particular focus on the recently discovered microdomains sculpted by the cardiac isoform of the membrane curvature formation protein BIN1 (cBIN1). Evidence is emerging that cBIN1-formed membrane microfolds are needed for both normal dyad formation and creation of a “fuzzy space” of slow ionic diffusion zone within the t-tubule network. The reversible reduction of BIN1 transcription in failing myocardium further indicates the crucial role of BIN1 in heart failure pathophysiology. Measurement of cBIN1 levels at the cardiac t-tubule membrane can potentially be monitored through a simple blood test, likely due to the continuous turnover of BIN1 microfolds into circulation. Thus BIN1 microdomain is a promising new target for the development of novel diagnostic and therapeutic tools for human acquired heart failure. More generally, t-tubules are now being approached as structurally complex, multifaceted organelles vital to cardiomyocyte health and function.
GRANTS
This work is funded by National Heart, Lung, and Blood Institute Grants R00-HL10975 (to T. Hong) and R01-HL094414 (to R. M. Shaw) and by American Heart Association Grants IRG-16IRG27780031 and BGIA-16BGIA27770151 (to T. Hong) and EIA-13EIA4480016 (to R. M. Shaw).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgments
We acknowledge the members of our research groups, as well as members of the Cedars-Sinai Heart Institute, in particular Eduardo Marbán, MD, PhD, for the stimulating environment which integrates bench research with patient care. We also thank Janet Iwasa, PhD, for the scientific illustrations and Leon Fine, MD, for helpful discussions.
Address for reprint requests and other correspondence: R. M. Shaw, Wasserman Endowed Chair in Cardiology, 8700 Beverly Blvd., Davis Bldg. 1016, Los Angeles, CA 90048 (e-mail: robin.shaw@cshs.org).
REFERENCES
- 1.Arakel EC, Brandenburg S, Uchida K, Zhang H, Lin YW, Kohl T, Schrul B, Sulkin MS, Efimov IR, Nichols CG, Lehnart SE, Schwappach B. Tuning the electrical properties of the heart by differential trafficking of KATP ion channel complexes. J Cell Sci : 2106–2119, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asghari P, Scriven DR, Sanatani S, Gandhi SK, Campbell AI, Moore ED. Nonuniform and variable arrangements of ryanodine receptors within mammalian ventricular couplons. Circ Res : 252–262, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Ayettey AS, Navaratnam V. The T-tubule system in the specialized and general myocardium of the rat. J Anat : 125–140, 1978. [PMC free article] [PubMed] [Google Scholar]
- 4.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci USA : 7500–7505, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol : 149–160, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol : 206–213, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature : 168–172, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Barry DM, Trimmer JS, Merlie JP, Nerbonne JM. Differential expression of voltage-gated K+ channel subunits in adult rat heart. Relation to functional K+ channels? Circ Res : 361–369, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem : 30984–30992, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase alpha1 and alpha2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res : 92–100, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Bers DM. Cardiac excitation-contraction coupling. Nature : 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, Netherlands: Kluwer Academic, 2001. [Google Scholar]
- 13.Bers DM, Stiffel VM. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am J Physiol Cell Physiol : C1587–C1593, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Best JM, Kamp TJ. Different subcellular populations of L-type Ca2+ channels exhibit unique regulation and functional roles in cardiomyocytes. J Mol Cell Cardiol : 376–387, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bito V, Heinzel FR, Biesmans L, Antoons G, Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodelling. Cardiovasc Res : 315–324, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Blatter LA, Niggli E. Confocal near-membrane detection of calcium in cardiac myocytes. Cell Calcium : 269–279, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Bohm J, Yis U, Ortac R, Cakmakci H, Kurul SH, Dirik E, Laporte J. Case report of intrafamilial variability in autosomal recessive centronuclear myopathy associated to a novel BIN1 stop mutation. Orphanet J Rare Dis : 35, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braunwald E. Biomarkers in heart failure. N Engl J Med : 2148–2159, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology : 167–173, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res : 1182–1192, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Brette F, Orchard CH. No apparent requirement for neuronal sodium channels in excitation-contraction coupling in rat ventricular myocytes. Circ Res : 667–674, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Butler MH, David C, Ochoa GC, Freyberg Z, Daniell L, Grabs D, Cremona O, De Camilli P. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol : 1355–1367, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caldwell JL, Smith CE, Taylor RF, Kitmitto A, Eisner DA, Dibb KM, Trafford AW. Dependence of cardiac transverse tubules on the BAR domain protein amphiphysin II (BIN-1). Circ Res : 986–996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camors E, Mohler PJ, Bers DM, Despa S. Ankyrin-B reduction enhances Ca spark-mediated SR Ca release promoting cardiac myocyte arrhythmic activity. J Mol Cell Cardiol : 1240–1248, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J : 1942–1956, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannell MB, Crossman DJ, Soeller C. Effect of changes in action potential spike configuration, junctional sarcoplasmic reticulum micro-architecture and altered t-tubule structure in human heart failure. J Muscle Res Cell Motil : 297–306, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Carozzi AJ, Ikonen E, Lindsay MR, Parton RG. Role of cholesterol in developing T-tubules: analogous mechanisms for T-tubule and caveolae biogenesis. Traffic : 326–341, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Casal E, Federici L, Zhang W, Fernandez-Recio J, Priego EM, Miguel RN, DuHadaway JB, Prendergast GC, Luisi BF, Laue ED. The crystal structure of the BAR domain from human Bin1/amphiphysin II and its implications for molecular recognition. Biochemistry : 12917–12928, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol : 923–933, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chase TH, Cox GA, Burzenski L, Foreman O, Shultz LD. Dysferlin deficiency and the development of cardiomyopathy in a mouse model of limb-girdle muscular dystrophy 2B. Am J Pathol : 2299–2308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science : 740–744, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3'-kinase. Circ Res : 1172–1179, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem : 30036–30044, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Christe G. Localization of K+ channels in the tubules of cardiomyocytes as suggested by the parallel decay of membrane capacitance, IK(1) and IK(ATP) during culture and by delayed IK(1) response to barium. J Mol Cell Cardiol : 2207–2213, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Clark RB, Tremblay A, Melnyk P, Allen BG, Giles WR, Fiset C. T-tubule localization of the inward-rectifier K+ channel in mouse ventricular myocytes: a role in K+ accumulation. J Physiol : 979–992, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole MD. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet : 361–384, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Colley BS, Biju KC, Visegrady A, Campbell S, Fadool DA. Neurotrophin B receptor kinase increases Kv subfamily member 1.3 (Kv13) ion channel half-life and surface expression. Neuroscience : 531–546, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crossman DJ, Ruygrok PN, Soeller C, Cannell MB. Changes in the organization of excitation-contraction coupling structures in failing human heart. PloS One : e17901, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunha SR, Mohler PJ. Cardiac ankyrins: essential components for development and maintenance of excitable membrane domains in heart. Cardiovasc Res : 22–29, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Curran J, Mohler PJ. Alternative paradigms for ion channelopathies: disorders of ion channel membrane trafficking and posttranslational modification. Annu Rev Physiol : 505–524, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature : 88–91, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev : 411–452, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das T, Hoshijima M. Adding a new dimension to cardiac nano-architecture using electron microscopy: coupling membrane excitation to calcium signaling. J Mol Cell Cardiol : 5–12, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem : 22883–22888, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Demonbreun AR, Quattrocelli M, Barefield DY, Allen MV, Swanson KE, McNally EM. An actin-dependent annexin complex mediates plasma membrane repair in muscle. J Cell Biol : 705–718, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demonbreun AR, Rossi AE, Alvarez MG, Swanson KE, Deveaux HK, Earley JU, Hadhazy M, Vohra R, Walter GA, Pytel P, McNally EM. Dysferlin and myoferlin regulate transverse tubule formation and glycerol sensitivity. Am J Pathol : 248–259, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DePinho RA, Schreiber-Agus N, Alt FW. myc family oncogenes in the development of normal and neoplastic cells. Adv Cancer Res : 1–46, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Despa S, Brette F, Orchard CH, Bers DM. Na/Ca exchange and Na/K-ATPase function are equally concentrated in transverse tubules of rat ventricular myocytes. Biophys J : 3388–3396, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Biase V, Tuluc P, Campiglio M, Obermair GJ, Heine M, Flucher BE. Surface traffic of dendritic CaV1.2 calcium channels in hippocampal neurons. J Neurosci : 13682–13694, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Maio A, Karko K, Snopko RM, Mejia-Alvarez R, Franzini-Armstrong C. T-tubule formation in cardiacmyocytes: two possible mechanisms? J Muscle Res Cell Motil : 231–241, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Di Maio A, Ter Keurs HE, Franzini-Armstrong C. T-tubule profiles in Purkinje fibres of mammalian myocardium. J Muscle Res Cell Motil : 115–121, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, Trafford AW. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circulation Heart Failure : 482–489, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Dixon RE, Moreno CM, Yuan C, Opitz-Araya X, Binder MD, Navedo MF, Santana LF. Graded Ca2+/calmodulin-dependent coupling of voltage-gated CaV1.2 channels. eLife : 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doherty KR, McNally EM. Repairing the tears: dysferlin in muscle membrane repair. Trends Mol Med : 327–330, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Dominguez JN, de la Rosa A, Navarro F, Franco D, Aranega AE. Tissue distribution and subcellular localization of the cardiac sodium channel during mouse heart development. Cardiovasc Res : 45–52, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Duclohier H. Neuronal sodium channels in ventricular heart cells are localized near T-tubules openings. Biochem Biophys Res Commun : 1135–1140, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Egger M, Porzig H, Niggli E, Schwaller B. Rapid turnover of the “functional” Na+-Ca2+ exchanger in cardiac myocytes revealed by an antisense oligodeoxynucleotide approach. Cell Calcium : 233–243, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Fabbrizio E, Nudel U, Hugon G, Robert A, Pons F, Mornet D. Characterization and localization of a 77 kDa protein related to the dystrophin gene family. Biochem J : 359–365, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol Cell Physiol : C1–C14, 1983. [DOI] [PubMed] [Google Scholar]
- 60.Fawcett DW, McNutt NS. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol : 1–45, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrantini C, Crocini C, Coppini R, Vanzi F, Tesi C, Cerbai E, Poggesi C, Pavone FS, Sacconi L. The transverse-axial tubular system of cardiomyocytes. Cell Mol Life Sci : 4695–4710, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forssmann WG, Girardier L. A study of the T system in rat heart. J Cell Biol : 1–19, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franzini-Armstrong C, Protasi F, Ramesh V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann NY Acad Sci : 20–30, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell : 191–196, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu Y, Hong T. BIN1 regulates dynamic t-tubule membrane. Biochim Biophys Acta : 1839–1847, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu Y, Shaw SA, Naami R, Vuong CL, Basheer WA, Guo X, Hong T. Isoproterenol promotes rapid ryanodine receptor movement to bridging integrator 1 (BIN1)-organized dyads. Circulation : 388–397, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu Y, Westenbroek RE, Scheuer T, Catterall WA. Basal and beta-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700. Proc Natl Acad Sci USA : 16598–16603, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu Y, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc Natl Acad Sci USA : 19621–19626, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu Y, Westenbroek RE, Yu FH, Clark JP 3rd, Marshall MR, Scheuer T, Catterall WA. Deletion of the distal C terminus of CaV1.2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J Biol Chem : 12617–12626, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem : 21425–21433, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Gallet R, de Couto G, Simsolo E, Valle J, Sun B, Liu W, Tseliou E, Zile MR, Marban E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci : 14–28, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ge K, DuHadaway J, Du W, Herlyn M, Rodeck U, Prendergast GC. Mechanism for elimination of a tumor suppressor: aberrant splicing of a brain-specific exon causes loss of function of Bin1 in melanoma. Proc Natl Acad Sci USA : 9689–9694, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gez LS, Hagalili Y, Shainberg A, Atlas D. Voltage-driven Ca2+ binding at the L-type Ca2+ channel triggers cardiac excitation-contraction coupling prior to Ca2+ influx. Biochemistry : 9658–9666, 2012. [DOI] [PubMed] [Google Scholar]
- 74.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation : 688–693, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science : 800–806, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, Sargent MA, Hofmann F, Moosmang S, Marks AR, Houser SR, Bers DM, Molkentin JD. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J Clin Invest : 280–290, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorelik J, Wright PT, Lyon AR, Harding SE. Spatial control of the betaAR system in heart failure: the transverse tubule and beyond. Cardiovasc Res : 216–224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR 3rd, Scheuer T, Catterall WA, Murphy BJ. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron : 1017–1026, 1998. [DOI] [PubMed] [Google Scholar]
- 79.Gruber T, Balbach J. Protein folding mechanism of the dimeric amphiphysinII/Bin1 N-BAR domain. PloS One : e0136922, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo A, Zhang C, Wei S, Chen B, Song LS. Emerging mechanisms of T-tubule remodelling in heart failure. Cardiovasc Res : 204–215, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J : 2121–2128, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, Jafri MS, Artman M. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circ Res : 415–427, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Hamaguchi S, Kawakami Y, Honda Y, Nemoto K, Sano A, Namekata I, Tanaka H. Developmental changes in excitation-contraction mechanisms of the mouse ventricular myocardium as revealed by functional and confocal imaging analyses. J Pharmacol Sci : 167–175, 2013. [DOI] [PubMed] [Google Scholar]
- 84.Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, McNeil PL, Campbell KP. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J Clin Invest : 1805–1813, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harris DM, Mills GD, Chen X, Kubo H, Berretta RM, Votaw VS, Santana LF, Houser SR. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circ Res : 543–550, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Hayashi T, Arimura T, Itoh-Satoh M, Ueda K, Hohda S, Inagaki N, Takahashi M, Hori H, Yasunami M, Nishi H, Koga Y, Nakamura H, Matsuzaki M, Choi BY, Bae SW, You CW, Han KH, Park JE, Knoll R, Hoshijima M, Chien KR, Kimura A. Tcap gene mutations in hypertrophic cardiomyopathy and dilated cardiomyopathy. J Am Coll Cardiol : 2192–2201, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Hayashi T, Martone ME, Yu Z, Thor A, Doi M, Holst MJ, Ellisman MH, Hoshijima M. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J Cell Sci : 1005–1013, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res : 298–307, 2001. [DOI] [PubMed] [Google Scholar]
- 89.Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, Claus P, Dymarkowski S, Maes F, Bogaert J, Rademakers F, D'Hooge J, Sipido K. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res : 338–346, 2008. [DOI] [PubMed] [Google Scholar]
- 90.Heinzel FR, Bito V, Volders PG, Antoons G, Mubagwa K, Sipido KR. Spatial and temporal inhomogeneities during Ca2+ release from the sarcoplasmic reticulum in pig ventricular myocytes. Circ Res : 1023–1030, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marban E, Jan LY, Shaw RM. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nature Med : 624–632, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong TT, Cogswell R, James CA, Kang G, Pullinger CR, Malloy MJ, Kane JP, Wojciak J, Calkins H, Scheinman MM, Tseng ZH, Ganz P, De Marco T, Judge DP, Shaw RM. Plasma BIN1 correlates with heart failure and predicts arrhythmia in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm : 961–967, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong TT, Smyth JW, Chu KY, Vogan JM, Fong TS, Jensen BC, Fang K, Halushka MK, Russell SD, Colecraft H, Hoopes CW, Ocorr K, Chi NC, Shaw RM. BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm : 812–820, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol : e1000312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huxley AF. The activation of striated muscle and its mechanical response. Proc R Soc Lond Ser B Biol Sci : 1–27, 1971. [DOI] [PubMed] [Google Scholar]
- 96.Huxley AF, Taylor RE. Function of Krause's membrane. Nature : 1068, 1955. [DOI] [PubMed] [Google Scholar]
- 97.Ibrahim M, Al Masri A, Navaratnarajah M, Siedlecka U, Soppa GK, Moshkov A, Al-Saud SA, Gorelik J, Yacoub MH, Terracciano CM. Prolonged mechanical unloading affects cardiomyocyte excitation-contraction coupling, transverse-tubule structure, and the cell surface. FASEB J : 3321–3329, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ibrahim M, Navaratnarajah M, Siedlecka U, Rao C, Dias P, Moshkov AV, Gorelik J, Yacoub MH, Terracciano CM. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca2+-induced Ca2+release in a rodent model of heart failure. Eur J Heart Failure : 571–580, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ibrahim M, Terracciano CM. Reversibility of T-tubule remodelling in heart failure: mechanical load as a dynamic regulator of the T-tubules. Cardiovasc Res : 225–232, 2013. [DOI] [PubMed] [Google Scholar]
- 100.Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem : 15637–15642, 1987. [PubMed] [Google Scholar]
- 101.Jayasinghe ID, Clowsley AH, Munro M, Hou Y, Crossman DJ, Soeller C. Revealing t-tubules in striated muscle with new optical super-resolution microscopy techniques. Eur J Transl Myol : 15–26, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson MR, Meyer KH, Haft J, Kinder D, Webber SA, Dyke DB. Heart transplantation in the United States, 1999–2008. Am J Transplant : 1035–1046, 2010. [DOI] [PubMed] [Google Scholar]
- 103.Jones EM, Roti Roti EC, Wang J, Delfosse SA, Robertson GA. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem : 44690–44694, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Jones SA, Morton MJ, Hunter M, Boyett MR. Expression of TASK-1, a pH-sensitive twin-pore domain K+ channel, in rat myocytes. Am J Physiol Heart Circ Physiol : H181–H185, 2002. [DOI] [PubMed] [Google Scholar]
- 105.Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci USA : 1800–1805, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaprielian RR, Stevenson S, Rothery SM, Cullen MJ, Severs NJ. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation : 2586–2594, 2000. [DOI] [PubMed] [Google Scholar]
- 107.Kawada T, Hemmi C, Fukuda S, Tezuka A, Iwasawa K, Nakazawa M, Sato H, Toyo-Oka T. Sarcolemmal fragility secondary to the degradation of dystrophin in dilated cardiomyopathy, as estimated by electron microscopy. Exp Clin Cardiol : 67–70, 2003. [PMC free article] [PubMed] [Google Scholar]
- 108.Kemi OJ, Hoydal MA, Macquaide N, Haram PM, Koch LG, Britton SL, Ellingsen O, Smith GL, Wisloff U. The effect of exercise training on transverse tubules in normal, remodeled, and reverse remodeled hearts. J Cell Physiol : 2235–2243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kirk MM, Izu LT, Chen-Izu Y, McCulle SL, Wier WG, Balke CW, Shorofsky SR. Role of the transverse-axial tubule system in generating calcium sparks and calcium transients in rat atrial myocytes. J Physiol : 441–451, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science : 1589–1592, 1996. [DOI] [PubMed] [Google Scholar]
- 111.Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell : 943–955, 2002. [DOI] [PubMed] [Google Scholar]
- 112.Kohl T, Lehnart SE. Imaging T-tubules: dynamic membrane structures for deep functions. Cardiovasc Res : 162–164, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komukai K, Brette F, Yamanushi TT, Orchard CH. K+ current distribution in rat sub-epicardial ventricular myocytes. Pflügers Arch : 532–538, 2002. [DOI] [PubMed] [Google Scholar]
- 114.Kuru S, Yasuma F, Wakayama T, Kimura S, Konagaya M, Aoki M, Tanabe M, Takahashi T. [A patient with limb girdle muscular dystrophy type 2B (LGMD2B) manifesting cardiomyopathy]. Rinsho Shinkeigaku : 375–378, 2004. [PubMed] [Google Scholar]
- 115.Landstrom AP, Beavers DL, Wehrens XH. The junctophilin family of proteins: from bench to bedside. Trends Mol Med : 353–362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laury-Kleintop LD, Mulgrew JR, Heletz I, Nedelcoviciu RA, Chang MY, Harris DM, Koch WJ, Schneider MD, Muller AJ, Prendergast GC. Cardiac-specific disruption of Bin1 in mice enables a model of stress- and age-associated dilated cardiomyopathy. J Cell Biochem : 2541–2551, 2015. [DOI] [PubMed] [Google Scholar]
- 117.Lavorato M, Huang TQ, Iyer VR, Perni S, Meissner G, Franzini-Armstrong C. Dyad content is reduced in cardiac myocytes of mice with impaired calmodulin regulation of RyR2. J Muscle Res Cell Motil : 205–214, 2015. [DOI] [PubMed] [Google Scholar]
- 118.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell Calcium : 515–526, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Lederer WJ, Niggli E, Hadley RW. Sodium-calcium exchange in excitable cells: fuzzy space. Science : 283, 1990. [DOI] [PubMed] [Google Scholar]
- 120.Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science : 1193–1196, 2002. [DOI] [PubMed] [Google Scholar]
- 121.Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D'Hooge J, Heidbuchel H, Sipido KR, Willems R. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res : 876–885, 2009. [DOI] [PubMed] [Google Scholar]
- 122.Leonoudakis D, Mailliard W, Wingerd K, Clegg D, Vandenberg C. Inward rectifier potassium channel Kir2.2 is associated with synapse-associated protein SAP97. J Cell Sci : 987–998, 2001. [DOI] [PubMed] [Google Scholar]
- 123.Levin KR, Page E. Quantitative studies on plasmalemmal folds and caveolae of rabbit ventricular myocardial cells. Circ Res : 244–255, 1980. [DOI] [PubMed] [Google Scholar]
- 124.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation : 497–505, 2007. [DOI] [PubMed] [Google Scholar]
- 125.Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem : 464–471, 1983. [PubMed] [Google Scholar]
- 126.Lindner E. [Submicroscopic morphology of the cardiac muscle]. Zeitschrift fur Zellforschung und mikroskopische Anatomie : 702–746, 1957. [PubMed] [Google Scholar]
- 127.Lipp P, Schwaller B, Niggli E. Specific inhibition of Na-Ca exchange function by antisense oligodeoxynucleotides. FEBS Lett : 198–202, 1995. [DOI] [PubMed] [Google Scholar]
- 128.Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ Res : 1040–1047, 2000. [DOI] [PubMed] [Google Scholar]
- 129.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation : e46–e215, 2010. [DOI] [PubMed] [Google Scholar]
- 130.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res : 63–73, 2004. [DOI] [PubMed] [Google Scholar]
- 131.Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol : 519–533, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Louch WE, Sejersted OM, Swift F. There goes the neighborhood: pathological alterations in T-tubule morphology and consequences for cardiomyocyte Ca2+ handling. J Biomed Biotechnol : 503906, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci USA : 6854–6859, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lyon AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, Hulot JS, Kohlbrenner E, Hajjar RJ, Peters NS, Korchev YE, Macleod KT, Harding SE, Gorelik J. Plasticity of surface structures and beta(2)-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circulation Heart Failure : 357–365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation : 1421–1427, 2004. [DOI] [PubMed] [Google Scholar]
- 136.Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci USA : 4073–4078, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR. A caveolae-targeted L-type Ca2+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ Res : 669–674, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maltsev VA, Kyle JW, Mishra S, Undrovinas A. Molecular identity of the late sodium current in adult dog cardiomyocytes identified by Nav1.5 antisense inhibition. Am J Physiol Heart Circ Physiol : H667–H676, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol : 699–708, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell : 365–376, 2000. [DOI] [PubMed] [Google Scholar]
- 141.Masubuchi N, Shidoh Y, Kondo S, Takatoh J, Hanaoka K. Subcellular localization of dystrophin isoforms in cardiomyocytes and phenotypic analysis of dystrophin-deficient mice reveal cardiac myopathy is predominantly caused by a deficiency in full-length dystrophin. Exp Anim/Jpn Assoc Lab Anim Sci : 211–217, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J : 1787–1847, 2012. [DOI] [PubMed] [Google Scholar]
- 143.Melnyk P, Zhang L, Shrier A, Nattel S. Differential distribution of Kir2.1 and Kir23 subunits in canine atrium and ventricle. Am J Physiol Heart Circ Physiol : H1123–H1133, 2002. [DOI] [PubMed] [Google Scholar]
- 144.Misonou H, Trimmer JS. Determinants of voltage-gated potassium channel surface expression and localization in Mammalian neurons. Crit Rev Biochem Mol Biol : 125–145, 2004. [DOI] [PubMed] [Google Scholar]
- 145.Mitcheson JS, Hancox JC, Levi AJ. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflügers Arch : 814–827, 1996. [DOI] [PubMed] [Google Scholar]
- 146.Mohler PJ, Bennett V. Ankyrin-based cardiac arrhythmias: a new class of channelopathies due to loss of cellular targeting. Curr Opin Cardiol : 189–193, 2005. [DOI] [PubMed] [Google Scholar]
- 147.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol : e423, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature : 634–639, 2003. [DOI] [PubMed] [Google Scholar]
- 149.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA : 9137–9142, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muller AJ, Baker JF, DuHadaway JB, Ge K, Farmer G, Donover PS, Meade R, Reid C, Grzanna R, Roach AH, Shah N, Soler AP, Prendergast GC. Targeted disruption of the murine Bin1/Amphiphysin II gene does not disable endocytosis but results in embryonic cardiomyopathy with aberrant myofibril formation. Mol Cell Biol : 4295–4306, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murphy RM, Mollica JP, Lamb GD. Plasma membrane removal in rat skeletal muscle fibers reveals caveolin-3 hot-spots at the necks of transverse tubules. Exp Cell Res : 1015–1028, 2009. [DOI] [PubMed] [Google Scholar]
- 152.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res : 748–756, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nelson DA, Benson ES. On the structural continuities of the transverse tubular system of rabbit and human myocardial cells. J Cell Biol : 297–313, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science : 1653–1657, 2010. [DOI] [PubMed] [Google Scholar]
- 155.Noujaim SF, Kaur K, Milstein M, Jones JM, Furspan P, Jiang D, Auerbach DS, Herron T, Meisler MH, Jalife J. A null mutation of the neuronal sodium channel NaV1.6 disrupts action potential propagation and excitation-contraction coupling in the mouse heart. FASEB J : 63–72, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Orchard C, Brette F. t-Tubules and sarcoplasmic reticulum function in cardiac ventricular myocytes. Cardiovasc Res : 237–244, 2008. [DOI] [PubMed] [Google Scholar]
- 157.Orchard CH, Pasek M, Brette F. The role of mammalian cardiac t-tubules in excitation-contraction coupling: experimental and computational approaches. Exp Physiol : 509–519, 2009. [DOI] [PubMed] [Google Scholar]
- 158.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med : 251–259, 2006. [DOI] [PubMed] [Google Scholar]
- 159.Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol Cell Physiol : C147–C158, 1978. [DOI] [PubMed] [Google Scholar]
- 160.Page E. Tubular systems in Purkinje cells of the cat heart. J Ultrastruct Res : 72–83, 1967. [DOI] [PubMed] [Google Scholar]
- 161.Page E, McCallister LP, Power B. Sterological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proc Natl Acad Sci USA : 1465–1466, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Page E, Surdyk-Droske M. Distribution, surface density, and membrane area of diadic junctional contacts between plasma membrane and terminal cisterns in mammalian ventricle. Circ Res : 260–267, 1979. [DOI] [PubMed] [Google Scholar]
- 163.Pasek M, Brette F, Nelson A, Pearce C, Qaiser A, Christe G, Orchard CH. Quantification of t-tubule area and protein distribution in rat cardiac ventricular myocytes. Prog Biophys Mol Biol : 244–257, 2008. [DOI] [PubMed] [Google Scholar]
- 164.Pasek M, Simurda J, Christe G. The functional role of cardiac T-tubules explored in a model of rat ventricular myocytes. Philos Trans Ser A Math Phys Eng Sci : 1187–1206, 2006. [DOI] [PubMed] [Google Scholar]
- 165.Pasek M, Simurda J, Orchard CH. Role of t-tubules in the control of trans-sarcolemmal ion flux and intracellular Ca2+ in a model of the rat cardiac ventricular myocyte. Eur Biophys J : 491–503, 2012. [DOI] [PubMed] [Google Scholar]
- 166.Pasek M, Simurda J, Orchard CH, Christe G. A model of the guinea-pig ventricular cardiac myocyte incorporating a transverse-axial tubular system. Prog Biophys Mol Biol : 258–280, 2008. [DOI] [PubMed] [Google Scholar]
- 167.Pediconi MF, Donoso P, Hidalgo C, Barrantes FJ. Lipid composition of purified transverse tubule membranes isolated from amphibian skeletal muscle. Biochim Biophys Acta : 398–404, 1987. [DOI] [PubMed] [Google Scholar]
- 168.Pessah IN, Waterhouse AL, Casida JE. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun : 449–456, 1985. [DOI] [PubMed] [Google Scholar]
- 169.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science : 495–499, 2004. [DOI] [PubMed] [Google Scholar]
- 170.Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, Vuillomenet A, Jeker U, Dubach P, Beer H, Yoon SI, Suter T, Osterhues HH, Schieber MM, Hilti P, Schindler R, Brunner-La Rocca HP, Investigators TC. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA : 383–392, 2009. [DOI] [PubMed] [Google Scholar]
- 171.Picas L, Viaud J, Schauer K, Vanni S, Hnia K, Fraisier V, Roux A, Bassereau P, Gaits-Iacovoni F, Payrastre B, Laporte J, Manneville JB, Goud B. BIN1/M-Amphiphysin2 induces clustering of phosphoinositides to recruit its downstream partner dynamin. Nature Commun : 5647, 2014. [DOI] [PubMed] [Google Scholar]
- 172.Posey AD Jr, Swanson KE, Alvarez MG, Krishnan S, Earley JU, Band H, Pytel P, McNally EM, Demonbreun AR. EHD1 mediates vesicle trafficking required for normal muscle growth and transverse tubule development. Dev Biol : 179–190, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prokic I, Cowling BS, Laporte J. Amphiphysin 2 (BIN1) in physiology and diseases. J Mol Med : 453–463, 2014. [DOI] [PubMed] [Google Scholar]
- 174.Qualmann B, Koch D, Kessels MM. Let's go bananas: revisiting the endocytic BAR code. EMBO J : 3501–3515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Richards MA, Clarke JD, Saravanan P, Voigt N, Dobrev D, Eisner DA, Trafford AW, Dibb KM. Transverse tubules are a common feature in large mammalian atrial myocytes including human. Am J Physiol Heart Circ Physiol : H1996–H2005, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation : e18–e209, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation : e69–171, 2007. [DOI] [PubMed] [Google Scholar]
- 178.Rosemblatt M, Hidalgo C, Vergara C, Ikemoto N. Immunological and biochemical properties of transverse tubule membranes isolated from rabbit skeletal muscle. J Biol Chem : 8140–8148, 1981. [PubMed] [Google Scholar]
- 179.Sacconi L, Ferrantini C, Lotti J, Coppini R, Yan P, Loew LM, Tesi C, Cerbai E, Poggesi C, Pavone FS. Action potential propagation in transverse-axial tubular system is impaired in heart failure. Proc Natl Acad Sci USA : 5815–5819, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sachse FB, Torres NS, Savio-Galimberti E, Aiba T, Kass DA, Tomaselli GF, Bridge JH. Subcellular structures and function of myocytes impaired during heart failure are restored by cardiac resynchronization therapy. Circ Res : 588–597, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sah R, Ramirez RJ, Backx PH. Modulation of Ca2+ release in cardiac myocytes by changes in repolarization rate: role of phase-1 action potential repolarization in excitation-contraction coupling. Circ Res : 165–173, 2002. [DOI] [PubMed] [Google Scholar]
- 182.Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol : 206–222, 2015. [DOI] [PubMed] [Google Scholar]
- 183.Savio-Galimberti E, Frank J, Inoue M, Goldhaber JI, Cannell MB, Bridge JH, Sachse FB. Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. Biophys J : 2053–2062, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Scamps F. Characterization of a beta-adrenergically inhibited K+ current in rat cardiac ventricular cells. J Physiol : 81–97, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J : 2682–2691, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem : 39193–39199, 2000. [DOI] [PubMed] [Google Scholar]
- 187.Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, Tohse N. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res : 535–548, 2003. [DOI] [PubMed] [Google Scholar]
- 188.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell : 547–560, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shepherd N, McDonough HB. Ionic diffusion in transverse tubules of cardiac ventricular myocytes. Am J Physiol Heart Circ Physiol : H852–H860, 1998. [DOI] [PubMed] [Google Scholar]
- 190.Simpson FO. The transverse tubular system in mammalian myocardial cells. Am J Anat : 1–17, 1965. [DOI] [PubMed] [Google Scholar]
- 191.Simpson FO, Oertelis SJ. The fine structure of sheep myocardial cells; sarcolemmal invaginations and the transverse tubular system. J Cell Biol : 91–100, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH, HeartMate III. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med : 2241–2251, 2009. [DOI] [PubMed] [Google Scholar]
- 193.Slodzinski MK, Blaustein MP. Na+/Ca2+ exchange in neonatal rat heart cells: antisense inhibition and protein half-life. Am J Physiol Cell Physiol : C459–C467, 1998. [DOI] [PubMed] [Google Scholar]
- 194.Smith LL, Gupta VA, Beggs AH. Bridging integrator 1 (Bin1) deficiency in zebrafish results in centronuclear myopathy. Hum Mol Genet : 3566–3578, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest : 266–279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Smyth JW, Shaw RM. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Reports : 611–618, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, Shaw RM. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res : 978–989, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res : 266–275, 1999. [DOI] [PubMed] [Google Scholar]
- 199.Soeller C, Crossman D, Gilbert R, Cannell MB. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc Natl Acad Sci USA : 14958–14963, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem : 15160–15165, 1996. [DOI] [PubMed] [Google Scholar]
- 201.Sperelakis N, Rubio R. An orderly lattice of axial tubules which interconnect adjacent transverse tubules in guinea-pig ventricular myocardium. J Mol Cell Cardiol : 211–220, 1971. [DOI] [PubMed] [Google Scholar]
- 202.Stolen TO, Hoydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisloff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res : 527–536, 2009. [DOI] [PubMed] [Google Scholar]
- 203.Suetsugu S, Miki H, Takenawa T. Identification of another actin-related protein (Arp) 2/3 complex binding site in neural Wiskott-Aldrich syndrome protein (N-WASP) that complements actin polymerization induced by the Arp2/3 complex activating (VCA) domain of N-WASP. J Biol Chem : 33175–33180, 2001. [DOI] [PubMed] [Google Scholar]
- 204.Sumnicht GE, Sabbadini RA. Lipid composition of transverse tubular membranes from normal and dystrophic skeletal muscle. Arch Biochem Biophys : 628–637, 1982. [DOI] [PubMed] [Google Scholar]
- 205.Sun XX, Hodge JJ, Zhou Y, Nguyen M, Griffith LC. The eag potassium channel binds and locally activates calcium/calmodulin-dependent protein kinase II. J Biol Chem : 10206–10214, 2004. [DOI] [PubMed] [Google Scholar]
- 206.Sweadner KJ, Herrera VL, Amato S, Moellmann A, Gibbons DK, Repke KR. Immunologic identification of Na+,K+-ATPase isoforms in myocardium. Isoform change in deoxycorticosterone acetate-salt hypertension. Circ Res : 669–678, 1994. [DOI] [PubMed] [Google Scholar]
- 207.Swift F, Birkeland JA, Tovsrud N, Enger UH, Aronsen JM, Louch WE, Sjaastad I, Sejersted OM. Altered Na+/Ca2+-exchanger activity due to downregulation of Na+/K+-ATPase alpha2-isoform in heart failure. Cardiovasc Res : 71–78, 2008. [DOI] [PubMed] [Google Scholar]
- 208.Swift F, Franzini-Armstrong C, Oyehaug L, Enger UH, Andersson KB, Christensen G, Sejersted OM, Louch WE. Extreme sarcoplasmic reticulum volume loss and compensatory T-tubule remodeling after Serca2 knockout. Proc Natl Acad Sci USA : 3997–4001, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Swift F, Stromme TA, Amundsen B, Sejersted OM, Sjaastad I. Slow diffusion of K+ in the T tubules of rat cardiomyocytes. J Appl Physiol : 1170–1176, 2006. [DOI] [PubMed] [Google Scholar]
- 210.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell : 861–872, 2007. [DOI] [PubMed] [Google Scholar]
- 211.Takano K, Watanabe-Takano H, Suetsugu S, Kurita S, Tsujita K, Kimura S, Karatsu T, Takenawa T, Endo T. Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science : 1536–1540, 2010. [DOI] [PubMed] [Google Scholar]
- 212.Takimoto K, Li D, Nerbonne JM, Levitan ES. Distribution, splicing and glucocorticoid-induced expression of cardiac alpha 1C and alpha 1D voltage-gated Ca2+ channel mRNAs. J Mol Cell Cardiol : 3035–3042, 1997. [DOI] [PubMed] [Google Scholar]
- 213.Tao W, Shi J, Dorn GW 2nd, Wei L, Rubart M. Spatial variability in T-tubule and electrical remodeling of left ventricular epicardium in mouse hearts with transgenic Galphaq overexpression-induced pathological hypertrophy. J Mol Cell Cardiol : 409–419, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tian Q, Pahlavan S, Oleinikow K, Jung J, Ruppenthal S, Scholz A, Schumann C, Kraegeloh A, Oberhofer M, Lipp P, Kaestner L. Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement. J Mol Cell Cardiol : 113–124, 2012. [DOI] [PubMed] [Google Scholar]
- 215.Tidball JG, Cederdahl JE, Bers DM. Quantitative analysis of regional variability in the distribution of transverse tubules in rabbit myocardium. Cell Tissue Res : 293–298, 1991. [DOI] [PubMed] [Google Scholar]
- 216.Timerman AP, Jayaraman T, Wiederrecht G, Onoue H, Marks AR, Fleischer S. The ryanodine receptor from canine heart sarcoplasmic reticulum is associated with a novel FK-506 binding protein. Biochem Biophys Res Commun : 701–706, 1994. [DOI] [PubMed] [Google Scholar]
- 217.Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA 3rd, Ferguson TB Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol : 1297–1313, 2012. [DOI] [PubMed] [Google Scholar]
- 218.Van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation : 979–988, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vlahovich N, Kee AJ, Van der Poel C, Kettle E, Hernandez-Deviez D, Lucas C, Lynch GS, Parton RG, Gunning PW, Hardeman EC. Cytoskeletal tropomyosin Tm5NM1 is required for normal excitation-contraction coupling in skeletal muscle. Mol Biol Cell : 400–409, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, Luther S, Hasenfuss G, Parlitz U, Jafri MS, Hell SW, Lederer WJ, Lehnart SE. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res : 402–414, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wechsler-Reya R, Elliott K, Herlyn M, Prendergast GC. The putative tumor suppressor BIN1 is a short-lived nuclear phosphoprotein, the localization of which is altered in malignant cells. Cancer Res : 3258–3263, 1997. [PubMed] [Google Scholar]
- 222.Wechsler-Reya R, Sakamuro D, Zhang J, Duhadaway J, Prendergast GC. Structural analysis of the human BIN1 gene. Evidence for tissue-specific transcriptional regulation and alternate RNA splicing. J Biol Chem : 31453–31458, 1997. [DOI] [PubMed] [Google Scholar]
- 223.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res : e61–70, 2004. [DOI] [PubMed] [Google Scholar]
- 224.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res : 520–531, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Weissenhorn W. Crystal structure of the endophilin-A1 BAR domain. J Mol Biol : 653–661, 2005. [DOI] [PubMed] [Google Scholar]
- 226.Wenzel K, Geier C, Qadri F, Hubner N, Schulz H, Erdmann B, Gross V, Bauer D, Dechend R, Dietz R, Osterziel KJ, Spuler S, Ozcelik C. Dysfunction of dysferlin-deficient hearts. J Mol Med : 1203–1214, 2007. [DOI] [PubMed] [Google Scholar]
- 227.Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature : 935–939, 2006. [DOI] [PubMed] [Google Scholar]
- 228.Wong J, Baddeley D, Bushong EA, Yu Z, Ellisman MH, Hoshijima M, Soeller C. Nanoscale distribution of ryanodine receptors and caveolin-3 in mouse ventricular myocytes: dilation of t-tubules near junctions. Biophys J : L22–24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wright PT, Nikolaev VO, O'Hara T, Diakonov I, Bhargava A, Tokar S, Schobesberger S, Shevchuk AI, Sikkel MB, Wilkinson R, Trayanova NA, Lyon AR, Harding SE, Gorelik J. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J Mol Cell Cardiol : 38–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu CY, Jia Z, Wang W, Ballou LM, Jiang YP, Chen B, Mathias RT, Cohen IS, Song LS, Entcheva E, Lin RZ. PI3Ks maintain the structural integrity of T-tubules in cardiac myocytes. PloS One : e24404, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wu HC, Yamankurt G, Luo J, Subramaniam J, Hashmi SS, Hu H, Cunha SR. Identification and characterization of two ankyrin-B isoforms in mammalian heart. Cardiovasc Res : 466–477, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, De Camilli P. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nature Cell Biol : 902–908, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xiao RP. Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to Gs and Gi proteins. Science's STKE : re15, 2001. [DOI] [PubMed] [Google Scholar]
- 234.Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, Lefkowitz RJ, Koch WJ, Lakatta EG. Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res : 43–52, 1999. [DOI] [PubMed] [Google Scholar]
- 235.Xiao RP, Ji X, Lakatta EG. Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol : 322–329, 1995. [PubMed] [Google Scholar]
- 236.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca2+ release channel (ryanodine receptor). J Biol Chem : 23480–23486, 2003. [DOI] [PubMed] [Google Scholar]
- 237.Yao A, Spitzer KW, Ito N, Zaniboni M, Lorell BH, Barry WH. The restriction of diffusion of cations at the external surface of cardiac myocytes varies between species. Cell Calcium : 431–438, 1997. [DOI] [PubMed] [Google Scholar]
- 238.Zampighi G, Vergara J, Ramon F. On the connection between the transverse tubules and the plasma membrane in frog semitendinosus skeletal muscle. Are caveolae the mouths of the transverse tubule system? J Cell Biol : 734–740, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhu J, Watanabe I, Gomez B, Thornhill WB. Heteromeric Kv1 potassium channel expression: amino acid determinants involved in processing and trafficking to the cell surface. J Biol Chem : 25558–25567, 2003. [DOI] [PubMed] [Google Scholar]
- 240.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA : 1607–1612, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zou Y, Komuro I, Yamazaki T, Kudoh S, Uozumi H, Kadowaki T, Yazaki Y. Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. J Biol Chem : 9760–9770, 1999. [DOI] [PubMed] [Google Scholar]