Abstract
The efficacy of Roux-en-Y gastric-bypass (RYGB) and other bariatric surgeries in the management of obesity and type 2 diabetes mellitus and novel developments in gastrointestinal (GI) endocrinology have renewed interest in the roles of GI hormones in the control of eating, meal-related glycemia, and obesity. Here we review the nutrient-sensing mechanisms that control the secretion of four of these hormones, ghrelin, cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), and peptide tyrosine tyrosine [PYY(3–36)], and their contributions to the controls of GI motor function, food intake, and meal-related increases in glycemia in healthy-weight and obese persons, as well as in RYGB patients. Their physiological roles as classical endocrine and as locally acting signals are discussed. Gastric emptying, the detection of specific digestive products by small intestinal enteroendocrine cells, and synergistic interactions among different GI loci all contribute to the secretion of ghrelin, CCK, GLP-1, and PYY(3–36). While CCK has been fully established as an endogenous endocrine control of eating in healthy-weight persons, the roles of all four hormones in eating in obese persons and following RYGB are uncertain. Similarly, only GLP-1 clearly contributes to the endocrine control of meal-related glycemia. It is likely that local signaling is involved in these hormones' actions, but methods to determine the physiological status of local signaling effects are lacking. Further research and fresh approaches are required to better understand ghrelin, CCK, GLP-1, and PYY(3–36) physiology; their roles in obesity and bariatric surgery; and their therapeutic potentials.
I. INTRODUCTION
A. Background
The first hormones, secretin (66), gastrin (229), and cholecystokinin (CCK) (362), were discovered in the early 20th century. These discoveries provided a novel signaling mechanism for the control of gastrointestinal (GI) physiology, which supplanted Pavlov's “nervism” doctrine (44, 401, 566a). From this beginning, endocrinology rapidly grew into a discipline crucial to virtually all of physiology and medicine.
The contributions of GI hormones to insulin secretion and glycemic regulation were identified in the 1960s (109, 782). The discovery of CCK's satiating effect in the 1970s (278) ushered GI hormones into the physiology of eating. By the 2000s, at least a dozen GI hormones had been hypothesized to contribute to eating (836). GI hormones secreted in response to eating, however, were mainly considered to be phasic signals sculpting the timing and size of individual meals and were not thought to be relevant for the tonic control of total energy intake and body-weight regulation (e.g., Refs. 172, 521, 672, 837). This view soon changed. In 2002, Cummings et al. (174) reported that levels of the gastric hormone ghrelin, which had been shown to increase eating when infused intravenously in humans (839), were inversely related to body adiposity in healthy-weight, obese, and weight-reduced humans, consistent with a tonic signaling function. Recent clinical trials indicate that treatment with long-acting glucagon-like peptide-1 (GLP-1) receptor agonists such as liraglutide [Victoza for type 2 diabetes mellitus (T2DM) and Saxenda for weight control, Novo Nordisk, Bagsvaerd, Denmark] leads to weight loss and amelioration of T2DM (335, 414, 581). Finally, changes in GI hormone secretion provide plausible mechanisms for the remarkable therapeutic efficacy of bariatric surgery, especially Roux-en-Y gastric bypass (RYGB), to reduce adiposity and improve glycemic control (113, 310, 421, 513, 551, 712).
In light of this, we review, 1) the secretion of ghrelin, CCK, GLP-1, and peptide tyrosine tyrosine [PYY(3–36)] around meals; 2) the contributions of these hormones to the control of meal size, meal timing, and meal-related glycemia, but as explained below, not to hedonics; 3) because it is an increasingly important issue in GI endocrinology, whether the hormones' mode of signaling in these situations is classically endocrine or local; 4) given the close relationship of GI endocrine and GI motor physiology, the role of GI motility in the hormones' effects; 5) whether obesity [i.e., body mass index (BMI); weight in kg/(height in m)2 ≥30 kg/m2], alters the hormones' effects on eating or glycemic control, and 6) because of the marked alterations in nutrient delivery into the small intestines and contact with enteroendocrine cells after RYGB (Figure 1), the hormones' contributions to the effects of RYGB on eating and glycemic control.
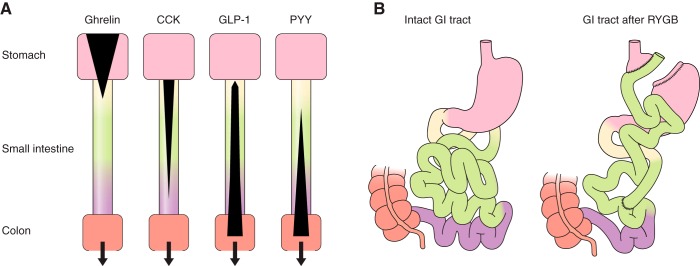
FIGURE 1.
Schematic depictions of the localization of enteroendocrine cells and changes after RYGB. A: distribution of enteroendocrine cells secreting ghrelin, CCK, GLP-1, and PYY in the stomach (pink), duodenum (yellow), jejunum (green), and ileum (violet). Black areas indicate the relative densities of expression of enteroendocrine cells producing the hormones indicated. Enteroendocrine cells secreting particular hormones were initially categorized histologically, e.g., I cells for CCK, L cells for enteroglucagons and PYY, etc. (166, 567, 591). It is now clear, however, that this categorization is not a reliable guide to hormone secretion. Rather, individual enteroendocrine cells secrete variable mixtures of hormones (231, 303, 597, 738). Bottom salmon rectangle, proximal large intestine. B: intact gastrointestinal tract (left) and gastrointestinal rearrangement after RYGB (right). Pink areas are stomach, salmon areas are large intestine (∼1.5 m long in healthy adults), yellow is duodenum (typically ∼25 cm long), green is jejunum (∼2–3 m), and violet is ileum (∼3–4 m). For RYGB, the stomach is divided into a small upper pouch with a volume of ∼25 ml and an isolated gastric remnant, the small intestine is divided ∼50 cm from the pylorus, and the distal limb of the small intestine (Roux or alimentary limb) is brought up to the gastric pouch and connected to it by an end-to-side gastroenterostomy. As a result, ingested food enters the small gastric pouch and empties directly into the jejunum. The gastric remnant and isolated ∼50 cm of small intestine (“biliopancreatic limb”) is connected to the jejunum ∼150 cm distal to the gastroenterostomy. The small intestine distal to the anastomosis is called the common channel.
B. Approach
1. Why focus on meals?
Total amount eaten and glycemic control are critically dependent on the control of and physiological responses to individual meals, and a significant component of these functions is thought to be mediated by ghrelin, CCK, GLP-1, and PYY(3–36) secretion, as schematized in Figure 2.
FIGURE 2.
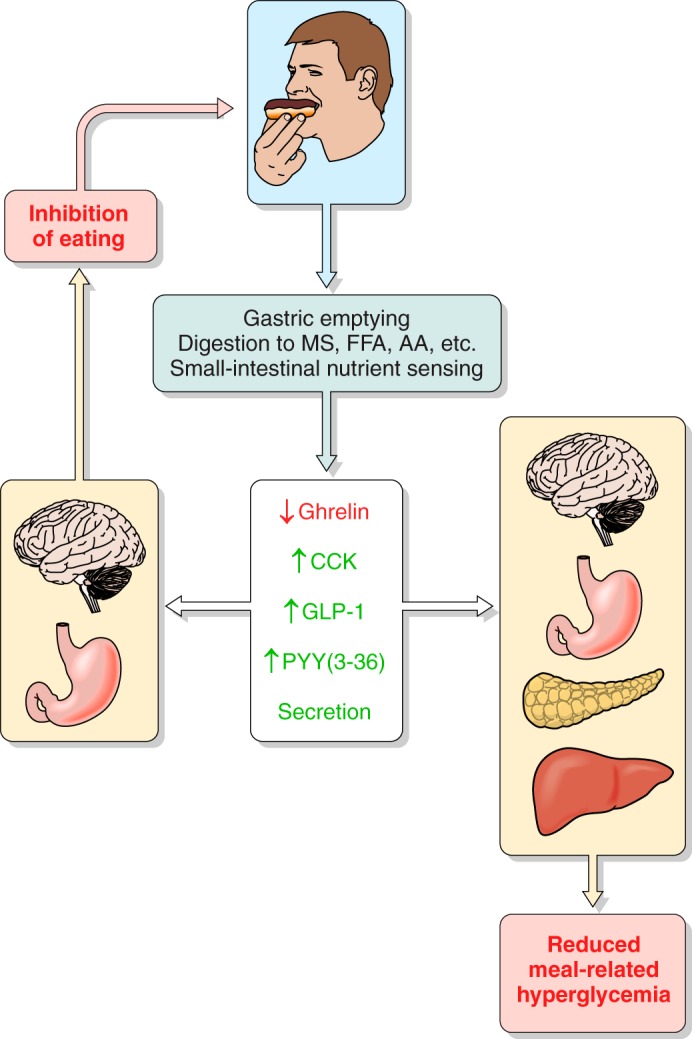
Overview of the hypothesized physiological roles of ghrelin, CCK, GLP-1, and PYY(3–36) in the control of eating and of meal-related glycemia. Gastric emptying, which controls the rate of appearance of ingested food in the small intestine, intestinal transit, rate of digestion, and small intestinal nutrient sensing are the major determinants of the inhibition of ghrelin secretion and the stimulation of CCK, GLP-1, and PYY(3–36) secretion during and after meals. Left: changes in hormone levels lead to GI and central nervous system events whose outcome is to inhibit eating. Right: changes in hormone levels lead GI, pancreatic, hepatic, and central nervous system events whose outcome is to dampen postprandial increases in blood glucose. All four hormones have been hypothesized to contribute to each type of outcome. MS, monosaccharides; FFA, free fatty acids; AA, amino acids.
The timing, size, and content of meals provide a complete description of what, when, and how much (in terms of g, kcal, or macronutrients) is eaten. Meal patterns are produced by species-specific physiological controls as well as environmental, social, and cultural contingencies. The control of meal size is disturbed in psychiatric eating disorders (271, 483, 743). In addition, obese individuals eat larger meals than healthy-weight individuals (5, 74, 189, 497) (healthy body weight is BMI ≥18.5 and <25 kg/m2). Thus the physiology of individual meals is crucial for understanding normal and disordered eating, including the chronic overeating that has led to the obesity epidemic (368, 741, 792). Smith (702) referred to the recognition of the central role of meals in the physiology of eating as “a paradigm shift from nutritional homeostasis to behavioral neuroscience.”
Ghrelin, CCK, GLP-1, and PYY(3–36) contribute to three of the putative motivational processes that provide the basic unconditioned control of meal initiation and meal size (85, 86, 271): 1) hunger, which refers to the process energizing the acquisition of food and meal initiation; 2) satiation, which leads to ending the meal; and 3) postprandial satiety, which inhibits eating after meals and prolongs the intermeal interval. The hormones' possible roles in a fourth meal-control process, flavor hedonics, and the central neural mechanisms integrating their effects are not reviewed, as these topics have been adequately reviewed elsewhere (for reviews of the hormones' hedonic effects, see Refs. 24, 212, 467, 499, 571, 699, 700, 827; for reviews of their central processing, see Refs. 75, 76, 291, 524, 623, 625).
It has long been known that meal-stimulated insulin release accounts for about half of total daily insulin secretion (408, 592, 593). More recently, measurement of glycated hemoglobin A1c (HbA1c) in T2DM patients with well-controlled glucose levels (HbA1c <7.3%), i.e., patients most closely resembling metabolically healthy persons, revealed that meal-related increases in blood glucose account for ∼70% of the total increment in diurnal blood glucose levels over fasting levels (509, 621). (“Meal related” indicates both during and after meals and is clearer than “prandial,” which sometimes is used to indicate only during meals.) Two of the principal factors related to meal-related increases in blood glucose are GI functions: 1) gastric emptying, which determines the rate of appearance of glucose in the small intestines, and, ordinarily, in the blood, and 2) the release of incretin hormones, i.e., GI hormones that stimulate insulin secretion (472). Thus, because CCK and PYY(3–36) contribute to gastric emptying, because GLP-1 is one of the principal incretin hormones (together with glucose-dependent insulinotropic peptide, GIP), and because ghrelin, CCK, GLP-1, and PYY(3–36) may have other effects that influence meal-related glycemia, meal physiology is an integral component of glycemic control.
2. Why a “physiological” approach?
Since its beginning, endocrinology has been organized around specific empirical criteria to identify hormones and their normal physiological functions (59, 156, 293, 489, 833). The first criteria were stated implicitly by Bayliss and Starling in 1902 (66) in their description of the discovery of secretin (Table 1). In their “crucial experiment,” they observed that pancreatic secretion was stimulated both by acid introduced into a denervated loop of an anesthetized dog's jejunum and by intravenous injection of an extract of jejunal mucosa. This effectively began a new chapter in physiology. Starling coined the term hormone, from the Greek for I arouse or excite, in 1905 (711) based on the secretin work, on earlier studies of the pressor effect of adrenal epinephrine by Oliver and Schäfer (556), and on his belief in the importance of chemical control in physiology (320). In the subsequent decades, isolating hormones from gland tissue was a major enterprise and was organized around additional criteria, such as Doisy's (218, 833) (Table 1). The development of radioimmunoassay and other accurate assay methods beginning in the mid-20th century brought additional criteria, based on appropriate changes in plasma hormone levels, such as Grossman's (293) (Table 1). Radioactive (and other) molecular-labeling methods also enabled the study of hormone receptors, and criteria based on receptor function were added (267, 270, 703), as discussed further below.
Table 1.
Evolution of endocrine criteria
| A. William Bayliss and Ernest H. Starling (1902) |
| 1. The adequate stimulus produces the response after complete denervation of the hormone-producing tissue. |
| 2. Intravenous injection of an extract of the hormone-producing tissue produces the response. |
| B. Edward A. Doisy (1936) |
| 1. Identification of the tissue that produces a hormone. |
| 2. Development of bioassay methods to identify the hormone. |
| 3. Preparation of active extracts that can be purified, using the relevant bioassay. |
| 4. Isolation, identification of structure, and synthesis of the hormone. |
| C. Morton I. Grossman (1973) |
| 1. The adequate stimulus produces a response in a distant target. |
| 2. The response persists after cutting all nerves connecting the site of stimulation and the target. |
| 3. The response is produced by an extract of the hormone-producing tissue. |
| 4. The effect is produced by infusing exogenous hormone in amounts and molecular forms that copy the increase in blood concentrations produced by the adequate stimulus for endogenous release. |
See text for references and discussion.
It is now clear that many hormones signal locally as well as via the classic blood-borne, endocrine mode (163, 463, 611). Local signaling in the GI tract can take three forms. First, hormones may act in a paracrine mode, i.e., be released as usual into the GI lamina propria and act on neighboring nonneural cells before absorption. Second, they may act in a neuroendocrine-like mode if they affect neural afferents in the laminal propria. Third, they may act in a neurocrine-like mode following release from axonlike cytoplasmic extensions of the enteroendocrine cells, called neuropods (90, 418). CCK- and PYY-containing neuropods, ending mainly in close apposition to glial cells of the enteric nervous system, were recently described in mice (92, 93). Neuropods appear to have a synapse-like function because there are accumulations of secretory vesicles near the appositions, the neuropods and target cells express characteristic pre- and postsynaptic proteins, and rabies virus moves retrogradely through them. This neuropod mode of action presumably mediates more specific cell-to-cell signaling than paracrine mechanisms. One possibility is that this signaling contributes to enteric nervous system reflexes linking the proximal and distal small intestine, which, as described below, appear important in the control of GI hormone secretion. Figure 3 summarizes the signaling modes of GI hormones.
FIGURE 3.
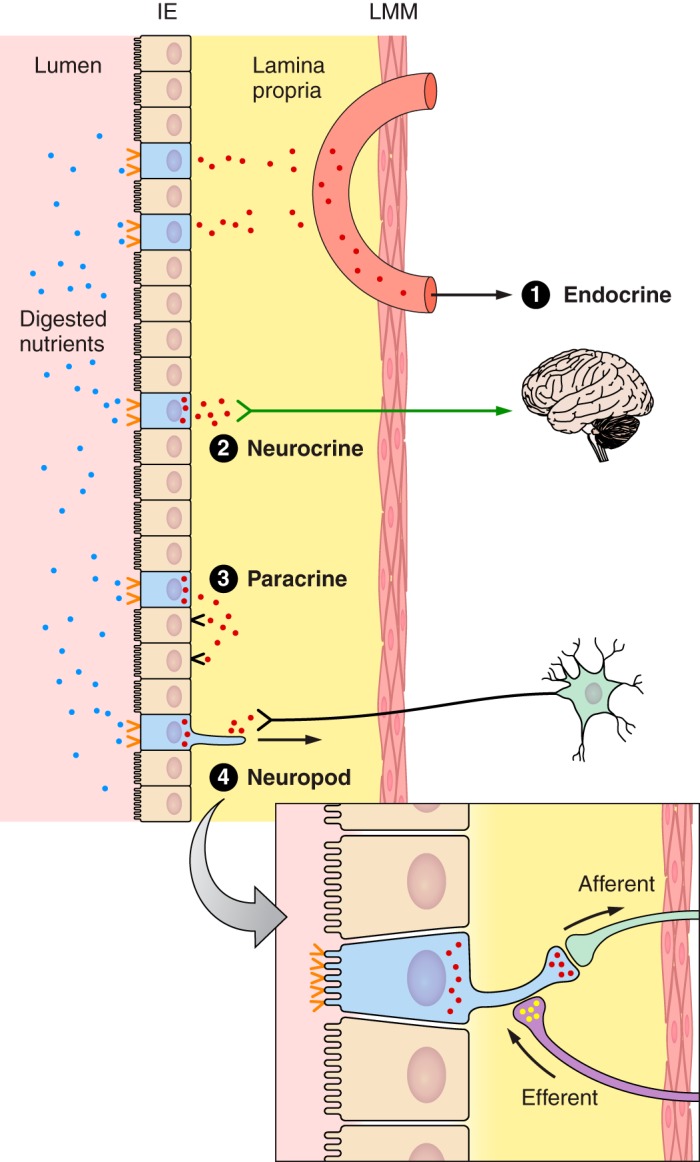
Schematic of the small intestinal mucosa showing potential modes of action of CCK, GLP-1, and PYY. The mucosa includes the epithelial cell layer (IE) on the luminal side, the lamina propria, and the lamina muscularis mucosae (LMM), which limns the submucosa and underlying serosa (not shown). The epithelium consists of enterocytes (tan), which are specialized for nutrient absorption, enteroendocrine cells (blue, villi not shown), which secrete GI hormones, and other cell types (not shown). Digested nutrients activate specific nutrient receptors and transporters (orange <) expressed on the apical surface of enteroendocrine cells, leading to secretion of CCK, GLP-1, and PYY from the basolateral side of enteroendocrine cells. Four modes of action are diagrammed. Mode 1 is the classical endocrine mode, in which hormones diffuse from the lamina propria into mesenteric capillaries (salmon), which drain into the hepatic-portal vein and finally the systemic circulation, allowing hormones to act on distant targets. Modes 2–4 show variations of local actions. Mode 2 is a neuroendocrine mode, in which hormones in the lamina propria activate vagal afferents (green arrow), which in turn stimulate brain-mediated responses. Mode 3 is the paracrine mode, in which hormones in the lamina propria act on receptors (black <) on nearby cells, either neuroendocrine cells or other cell types. Mode 4 shows the anatomical basis for a neuropod mode of action, which has been described for enteroendocrine CCK and PYY cells, and may exist for other GI hormones. This involves hormone release from enteroendocrine-cell neuropods that end in synapse-like appositions to glial cells of the enteric nervous system and other cell types. Note that the hormone concentrations involved in these different modes vary: hormone concentrations in the small gap between neuropods and adjacent cells are likely to be highest, paracrine and vagal neuroendocrine signaling may be the next highest hormone concentrations, endocrine signaling in the liver involves moderate hormone concentrations, and endocrine signaling in which hormones reach their receptors via the systemic circulation involves relatively low hormone concentrations. Hormones also enter the lymph from the lamina propria via bulk flow (not shown), but this is not known to be functionally relevant. Although ghrelin secretion is not stimulated directly by nutrients, secreted ghrelin may act in the modes shown here.
The criteria used here for normal, endogenous physiological function are listed in Table 2. Criteria 1 and 2 address the plausibility that the candidate signal controls a particular function, criteria 3–5 concern the candidate signal's sufficiency, and criterion 6 concerns its necessity. For hormones acting via an endocrine mode, endocrine tests of criterion 1 may be based on concentrations of the molecule in the blood and at its site of action, and criteria 3, 4, and 6 may be tested with intravenous infusions (unless blood-borne agonists or antagonists do not readily access the receptors, for example because of the blood-brain barrier). Plasma levels and intravenous infusions, however, do not provide adequate tests of paracrine or neuropod signaling. This is because intravenous infusion of a hormone, even if it matches the hormone's meal-related changes in the blood, may not mimic its concentration at paracrine or neuropod sites of action, i.e., in the lamina propria or at the site of close appositions with other cells, respectively. The same goes for agonist or antagonist administration. Thus, for paracrine or neuropod modes of action, the criteria remain theoretical possibilities, at least in humans, because, at present, there are no validated means to deliver hormones locally into the lamina propria or the close appositions formed by neuropods, or to measure their concentrations at such sites. In animals, however, these limitations soon may be surmounted, for example, by targeting the lamina propria with infusions into intestinal lumen (147).
Table 2.
Criteria to assess physiological status of GI hormones in meal-related functions
| 1. Concentrations of the hormone change at the site of action in a pattern consistent with the effect.* |
| 2. Cognate receptors for the hormone are expressed at its site(s) of action. |
| 3. Exogenous administration of the hormone in amounts duplicating the meal-related changes in endogenous patterns at the site of action produces the effect. |
| 4. Administration of secretagogues for the hormone produce effects similar to the effect of the hormone. |
| 5. The hormone's effect occurs in the absence of abnormal behavioral, physiological, or subjective effects. |
| 6. Administration of selective agonists and antagonists of the hormone's receptors produce effects that are consistent with their receptor pharmacologies.†‡ |
These criteria extend earlier versions (265, 268, 696) to accommodate paracrine and neuropod signaling as well as endocrine signaling, as described in the text.
At a minimum, the change in concentrations of the proposed signal should precede the effect; see Geary (265) for discussion. For example, administration of specific and potent receptor antagonists should delay or reduce eating in the case of a hunger signal or increase eating in the case of a satiation signal.
We do not include phenotypic evaluation of global transgenic or spontaneous genetic loss-of-function models in this criterion. These are valuable research tools, but complications due to developmental compensatory effects, pleiotropic actions, and species differences preclude their use as a “necessity” criterion for physiological function. Rapidly inducible, tissue-specific reductions in gene function, however, may complement the use of receptor antagonists in establishing necessity.
A related issue is that multiple parameters of hormone secretion other than plasma concentrations may encode feedback signals controlling eating. These include times of onset of changes in plasma levels, rates of change, pulsatility, and effects of sustained or integrated levels versus momentary levels. Unfortunately, the parameters that actually serve as endogenous physiological signals have not been intensively studied. Rather, researchers have modeled mainly a single parameter, the peak plasma level, and peaks have been modeled only crudely by continuous infusions that do not consider the duration or timing of the peaks. Therefore, for the purposes of evaluating criterion 1, “physiological” endocrine doses are provisionally defined as those reproducing the peak plasma levels produced by mixed-nutrient meals (Table 3). As limited as this definition is, it is at present the state of the art and has proven quite useful.
Table 3.
Physiological endocrine doses of ghrelin, CCK, GLP-1, and PYY(3–36) in healthy-weight humans
| Hormone | Physiological Dose, pmol·kg−1·min−1 | Reference Nos. |
|---|---|---|
| Ghrelin | ? (<0.3)* | 441, 759 |
| CCK | 0.2–0.7 | 54, 297, 433, 435 |
| GLP-1 | 0.3–0.90† | 69, 253, 299, 659 |
| PYY(3–36) | ? (<0.2)‡ | 10, 192, 196, 421 |
Physiological endocrine doses (pmol·kg−1·min−1) are those reported to reproduce the peak plasma levels produced by mixed-nutrient meals [CCK, GLP-1, PYY(3–36) or premeal levels (ghrelin)].
In one study, infusion of 0.3 pmol·kg−1·min−1 acyl ghrelin increased plasma total ghrelin levels 2.2-fold to about the fasting level (759); in another study, infusion of the same dose after breakfast increased acyl ghrelin 2.4-fold above the fasting level (441); the effects of lower doses have not been reported.
Physiological GLP-1 doses are based on across-study comparisons.
The ability to analyze hormone function with agonists and antagonists (criterion 6) is linked to developments in receptor pharmacology and receptor-subtype analyses. The use of antagonists in particular is now considered one of the cardinal criteria for physiological function. These tools also demand careful interpretation if the biological half-life, receptor affinity, relative access to receptors beyond the blood-brain barrier, etc., differ between the hormone and the agonist or antagonist. For example, the eating-inhibitory effects of the long-lasting GLP-1 agonist exendin-4 differ markedly from those of native GLP-1.
3. Why consider GI motor function?
GI endocrine function and GI motor function are so closely related that one cannot be understood without the other. Gastric emptying and intestinal transit determine which enteroendocrine cells are exposed to chyme and for how long. This in turn affects GI hormone secretion, which feeds back onto gastric emptying. Therefore, the review begins with an introduction to the effects of GI motor function on eating and glycemia in health, obesity, and after RYGB.
II. GI MOTOR FUNCTION
A. Gastric Accommodation and Emptying
Physical digestion of solid food begins in the mouth, but is primarily a gastric function (126, 127, 318, 341, 366, 481, 578, 724). Gastric volume during the meal usually exceeds the volume of ingesta due to gastric secretions and swallowed saliva and air (118, 282). Vago-vagal gastric-accommodation reflexes increase gastric volume as meals progress, avoiding significant increases in intragastric pressure or gastric-wall tension (43, 405). The lack of stimulation of gastric-tension receptors ensures that accommodation does not lead to aversive sensations, although they do appear sufficient to elicit a pleasant sensation of fullness (215, 464). Accommodation reflexes are triggered mainly by gastric mechanoreceptors and intestinal nutrient receptors and are mediated in part by CCK (43, 246).
Ingested liquids are distributed evenly throughout the stomach and begin emptying almost immediately. In contrast, ingested solids are initially restricted mainly to the fundus and move gradually to the antrum, where they are mixed with gastric secretions and reduced in size by antral trituration, i.e., by churning and grinding movements that produce semi-solid chyme. When chyme particles reach a size of ∼1–2 mm, they are emptied through the pylorus into the duodenum, a process that involves coordinated antropyloric propulsive pressure waves, pyloric-sphincter relaxation, and duodenal pressure waves. The delay until the first emptying of solid food, known as the lag phase, can last from a few minutes to over an hour, depending on the physical characteristics of the food. Once in the proximal small intestine, chyme initiates several neural and GI-hormonal reflexes that decelerate emptying.
When gastric emptying is measured for intervals approximating the normal intermeal interval, beginning after the lag phase for solids and ignoring pulsatile pyloric chyme propulsion, exponential curves provide good fits (Figure 4) (118, 123, 126, 129, 158, 314, 349, 481, 663, 693, 744, 795, 855). The Weibull or “power-exponential” function fits the lag phase as well (118, 126, 234, 347, 372, 452, 645, 772).
FIGURE 4.
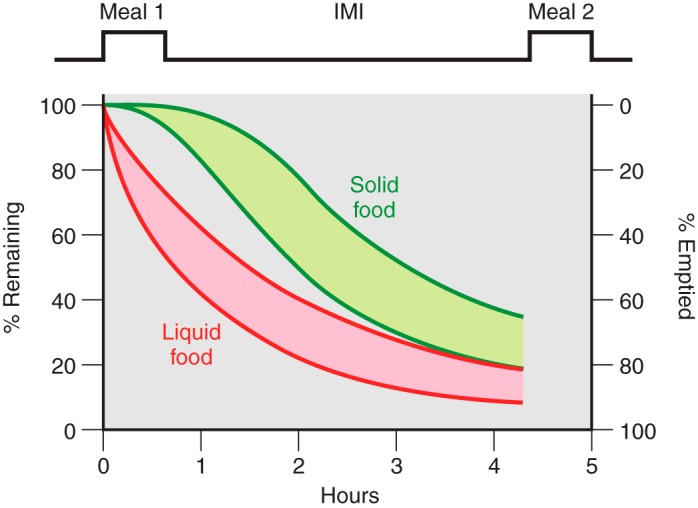
Typical patterns of gastric emptying of solid (green) and liquid (red) foods in relation to meals and intermeal intervals. Depending on the physical digestibility of solid foods, emptying during the first several minutes is very slow (the lag phase), whereas it is uncontrolled and rapid for liquids. The overall shapes of the gastric emptying curves for solid food after the lag phase and for liquid foods are exponential, although significant extents of each approximate linear functions. As described in the text, gastric emptying plays important roles in the control of eating and meal-related glycemia.
Emptying patterns are affected by meal volume, osmotic pressure, energy density, digestibility, and macronutrient adaptation (97, 162, 175, 176, 340). When meals contain both liquids and solids, the two phases empty differentially, although each affects the other, with liquids generally emptying faster (345, 481, 643). Gastric emptying is somewhat slower in women than men (73, 187, 200, 295, 350, 394), although whether it is affected by the menstrual-cycle phase is not clear (101, 200). Normal aging apparently has little effect (606). As a result of these factors, there is considerable interindividual variability in gastric emptying rate. In contrast, intraindividual variability is low under laboratory conditions (532).
Neural and endocrine reflexes are generally thought to synergize in the control of gastric emptying (126, 216, 555). The importance of the vagal contribution is underscored by the decrease in emptying of solid foods after vagotomy (405). The roles of ghrelin, CCK, GLP-1, and PYY(3–36) are reviewed in section IIC.
B. Small Intestinal Motility
The contributions of segmentation, mixing, and propulsion of chyme in the small intestine to intestinal nutrient sensing and the control of eating and glycemia are not well understood. Challenges include: 1) present methods to measure segmentation, mixing, and propulsion of chyme are limited, although novel approaches may soon accelerate progress (30, 33, 87, 220); and 2) the simultaneous changes in gastric accommodation, gastric emptying, and GI-hormone secretion are difficult to control (641, 681, 682, 718). Multivariate statistics, such as the approaches of Seimon et al. (683) and Acosta et al. (5), provide a useful strategy to dissect these diverse factors functionally.
Two pharmacological studies suggest an important role for small intestinal motility in incretin hormone secretion. In both, healthy-weight subjects received intraduodenal infusions of glucose, and intraduodenal pressure and flow events were assessed by manometry and impedance measurements. Pretreatment with hyoscine butylbromide (137) increased intraduodenal waves for 10 min and reduced flow events for 60 min. This was associated with decreased plasma level GIP at 10 and 20 min, suggesting that normal GIP release depends on the spread of glucose through duodenum and proximal jejunum. In contrast, pretreatment with metoclopramide (411) stimulated duodenal pressure waves, but did not affect flow events. Metoclopramide produced marked increases in plasma GLP-1 and GIP, suggesting that increased mixing of the luminal contents increased their contact with enteroendocrine cells, thus increasing incretin secretion. By extension, small intestinal motility may also affect ghrelin, CCK, and PYY secretion.
When the stomach is empty, small intestinal peristaltic activity is controlled by phase III activity of the migrating motor complex (MMC), which originates in the stomach in humans, rats, and mice (203, 266, 748, 853) and appears to be stimulated by motilin (203).
C. Roles of Ghrelin, CCK, GLP-1, and PYY(3–36)
Supraphysiological doses of ghrelin accelerated gastric emptying (429) and reversed the inhibition of gastric emptying elicited by intragastric lipid infusion (374), but whether reproducing endogenous amounts and patterns of ghrelin is sufficient to stimulate gastric emptying and whether ghrelin antagonists inhibit gastric emptying have not been tested in humans. Thus ghrelin does not yet fulfill criteria 3 and 6 (Table 2) for having an endocrine role in gastric emptying. Similarly, a supraphysiological ghrelin infusion elicited phase III MMC activity, but smaller doses did not (203, 748), and endogenous phase III MMC activity was not temporally associated with plasma ghrelin concentrations (although phase III MMC activity was associated with motilin levels) (204). Thus these tests did not produce evidence that ghrelin fulfills criteria 1 or 3 (Table 2) for an endocrine effect on GI motility.
Several studies indicate that CCK meets both criteria 1 and 6 (Table 2) for having an endocrine role in gastric emptying of liquid food (262, 263, 436, 447, 495, 674; but see Ref. 433 for a negative report). Animal studies indicate that CCK slows gastric emptying via vago-vagal reflexes stimulated by both endocrine and paracrine signaling (216, 555, 823). CCK also fulfilled criteria 1, 4, and 6 for endocrine roles in the increases in tonic and phasic pyloric pressures and reductions in antral and duodenal pressures stimulated by intraduodenal lipids (102, 190, 259, 316, 584), responses that presumably contribute to CCK's inhibitory effect on gastric emptying (382). One study in humans failed to detect an effect of CCK-receptor antagonism on small intestinal transit time (495).
There is also support for GLP-1 as an endocrine control of gastric emptying. Physiological doses of GLP-1 slowed emptying of liquid meals (541, 822), supporting criterion 3 (Table 2), and administration of the GLP-1 receptor antagonist exendin(9–39) accelerated gastric emptying in two studies (41, 196), supporting criterion 6. Exendin(9–39) failed to affect gastric emptying in three other studies (531, 546, 650), however, suggesting that differences in test meal characteristics, plasma glucose levels, or other situational variable may contribute to GLP-1's influence on gastric emptying. Exendin(9–39) also stimulates PYY(3–36) secretion, which may slow gastric emptying and contribute to these variable results (41, 230, 665, 670, 721, 843). Intravenous infusion of physiological doses of GLP-1 also stimulated tonic and phasic pyloric pressures and reduced antral and duodenal pressure waves (662), and infusion of exendin(9–39) abolished glucose-induced changes in antropyloroduodenal pressures (664), indicating a role for GLP-1 in small intestinal motility.
Supraphysiological PYY(3–36) infusions slowed gastric emptying in two studies (26, 834). Savage et al. (659) reported that emptying of a non-nutrient liquid meal was slowed by infusion of 0.4 pmol·kg−1·min−1 PYY(3–36). They measured only 0.18 pmol·kg−1·min−1 PYY(3–36) at the tip of the catheter, however, PYY(3–36) may meet the physiological-dose criterion (criterion 2, Table 2) for having a physiological endocrine role in gastric emptying. Studies with PYY(3–36) antagonists have not yet been reported. PYY(3–36) has been hypothesized to mediate the “ileal brake” on gastric emptying, a term referring to the ability of nutrients in the ileum to slow gastric emptying. In support of this, intra-ileal infusions of triglycerides, free fatty acids, sucrose, or casein increased plasma PYY(3–36) levels and slowed gastric emptying in several studies (585, 708, 709, 787). Whether endocrine, paracrine, or neuropod PYY(3–36) signaling mediates this effect is not known. Whether PYY(3–36) affects small intestinal motility has not been studied in humans. Intravenous infusion of PYY(3–36) and ileal infusion of a mixed-nutrient solution similarly increased the cycle length of phase III MMC in dogs (819), but the relevance of this for humans is uncertain because the control of the phase III MMC differs in dogs and humans (554).
In summary, different degrees of evidence support the physiological roles for ghrelin, CCK, GLP-1, and PYY(3–36) in GI motility. An important feature of these relationships is that gastric volume, gastric emptying, intestinal-nutrient sensing, and the secretion of these four hormones are linked in negative-feedback loops (Figure 5).
FIGURE 5.
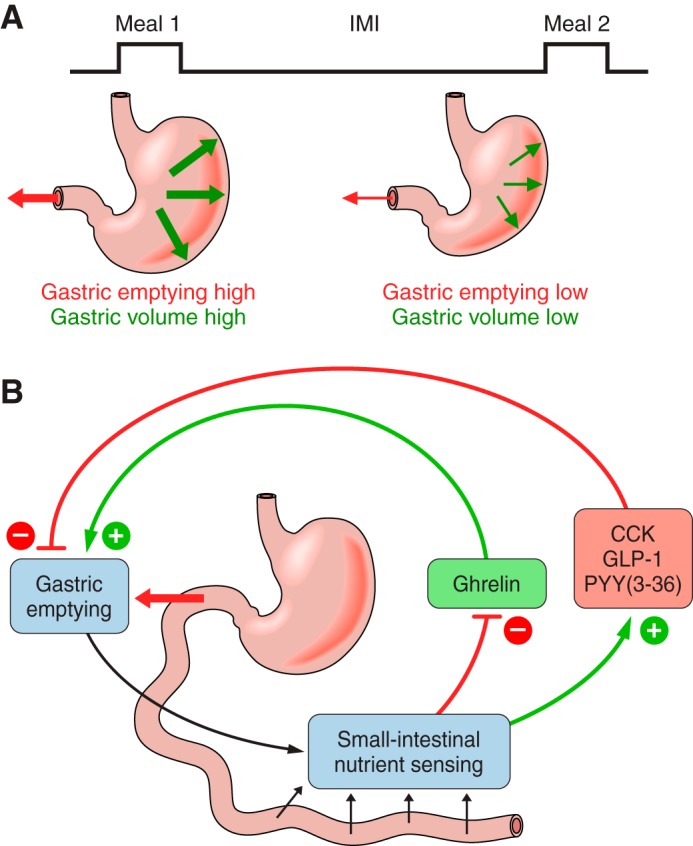
Gastric volume, gastric emptying, and ghrelin, CCK, GLP-1, and PYY(3–36) secretion in relation to meals. A: eating a meal increases gastric volume-related mechanoreception (bold green arrows), which increases satiation signaling via neural afferents, and increases gastric emptying and the delivery of ingested nutrients into the small intestine (bold red arrow), which increases satiation and satiety signaling and decreases hunger signaling. As the intermeal interval (IMI) progresses, volume sensing and gastric emptying progressively decrease (thin red and green arrows). B: gastric emptying determines the rate of appearance of nutrients into the small intestine and, together with the rate of digestion and small-intestinal motility, controls small intestinal-nutrient sensing. For most meals, small intestinal-nutrient sensing inhibits ghrelin secretion (red arrow, −) and stimulates CCK, GLP-1, and PYY secretion (green arrows, +). In turn, ghrelin stimulates (green arrow, +) and CCK, GLP-1, and PYY(3–36) inhibit (red arrows, −) gastric emptying. Note that each feedback loop is negative, as indicated by the change in sign (e.g., red to green) between (small intestinal-nutrient sensing)–(hormone secretion) and (hormone secretion)–(gastric emptying).
D. Eating
Perhaps the oldest mechanistic explanation for eating is Galen's suggestion that hunger results from gastric “pangs and gnawing sensations” (7, 482). These sensations may result from phase III MMC. 1) In 12 h-fasted subjects, subjective hunger ratings were closely associated with the intensity of gastric motility during both spontaneous phase III MMC and phase III MMC elicited by erythromycin, a motilin agonist. 2) Erythromycin infusion elicited meal requests (205, 746). When spontaneous eating was measured in another study, however, no premeal increases in gastric motility occurred (728). In addition, under other conditions, erythromycin decreased rather than increased food intake (770). Furthermore, phase III MMC develop only when the stomach is nearly empty, which usually occurs only after overnight fasts, yet subjective hunger is usually quite low in the morning (660) and breakfast is typically the smallest meal of the day. Thus the role of gastric motility in hunger remains doubtful.
Several lines of evidence support the hypothesis that increased gastric volume contributes to satiation. 1) Ratings of fullness were correlated with total gastric volume and with antral cross-sectional area after both liquid and solid test meals (201, 282, 351, 373, 474, 655, 730, 794). 2) Manipulations designed to selectively increase gastric volume increased fullness ratings and decreased eating (331, 475, 630–632). 3) Inflation of an intragastric balloon during meals also increased fullness ratings and decreased meal size (273). 4) Pharmacological inhibition of the gastric-accommodation reflex decreased gastric volume during a meal and decreased meal size (747). 5) Rapid intragastric glucose infusions increased fullness more than intraduodenal infusions roughly matched to the rate of gastric emptying (719), although it was not clear whether this was due to gastric-volume detection because the intragastric infusions also increased GLP-1 and PYY(3–36) secretion more than the intraduodenal infusions. Interestingly, gastric-volume effects appear to synergize with postgastric satiation signals because oral nutrient preloads together with intraduodenal nutrient infusions (133, 480, 552), CCK infusions (528), or GLP-1 infusions (199) each decreased eating more than the individual manipulations.
Gastric-volume signals are thought to control eating via mechanoreceptors that are tuned to both tension and stretch or length (294, 366, 560, 579) and are linked to the CNS by vagal and spinal visceral (splanchnic) afferents (272, 791, 809). In rats, these include polymodal vagal afferents whose response can be increased severalfold by combinations of gastric fill and CCK infusion (671) or influenced oppositely by CCK and ghrelin treatment (217). Thus neural information processing controlling GI function and eating appears to begin at the level of the vagal afferents.
As mentioned above, negative-feedback loops link gastric volume and gastric emptying to intestinal nutrient sensing and to ghrelin, CCK, GLP-1, and PYY(3–36) secretion (Figure 5). The relationship of these feedback loops to satiation is complex because both gastric and postgastric signals contribute to satiation. Thus, depending on the circumstances, accelerating gastric emptying may either decrease satiation by decreasing gastric-volume signals or increase satiation by increasing postgastric signals. This point is underscored by the report (770) that erythromycin accelerated gastric emptying and decreased rather than increased meal size in a group of overweight (i.e., BMI ≥25 and <30 m/kg2) and obese (BMI ≥30 kg/m2) subjects. Finally, recent data suggest functional roles for gastric nutrient-sensing receptors, which may include roles in eating (see sect. IIIC).
E. Glycemic Control
Gastric emptying contributes importantly to the regulation of meal-related glycemia and, thus, to overall glucose homeostasis (472). For example, in both healthy subjects and patients with T2DM, intersubject variability in emptying of an oral glucose load accounted for significant amounts of the variability in plasma glucose increments (342, 470). In addition, pharmacological manipulation of gastric emptying of a solid-liquid mixed-nutrient meal produced corresponding glycemic changes in patients with T2DM (285). Variation in factors that affect gastric emptying, such as decreasing dietary fiber content (369, 771), would presumably increase the relative contribution of gastric emptying to meal-related glycemia; conversely, manipulating postgastric factors, such as incretin-hormone secretion, would reduce it. Small intestinal nutrient transport also may affect meal-related glycemia. For example, pharmacological slowing of intestinal flow of intraduodenally infused glucose slowed glucose absorption and reduced blood glucose (137).
F. Obesity
In several small-scale studies, gastric emptying was comparable (562, 681), faster (295, 794, 797), or slower (338, 497) in obese relative to healthy-weight people. These inconsistent results may be related to several differences among the studies, including differences in the nutrient composition of the test meals and methodological differences (e.g., scintigraphy vs. less direct measures). In contrast, a large scintigraphic study (389 subjects) demonstrated clearly that overweight and obesity are associated with increased gastric emptying rates of both solid and liquid foods (5). Interestingly, the degrees of increase were similar in overweight, obese, and morbidly obese (i.e., BMI ≥35 kg/m2) subjects (decreases of ∼20% in solid-meal half-emptying time and ∼30% in liquid-meal half-emptying time in each group). This suggests increased gastric-emptying rate is more likely to be a permissive rather than an effective cause of obesity.
Manipulations of gastric volume may contribute to obesity therapy. Consistent with this possibility, some data relate the eating-inhibitory, weight-reducing, and glycemic effects of the GLP-1 receptor agonist liraglutide to reduced gastric emptying (343, 789). Furthermore, implantable devices designed to electrically stimulate the vagus in a way that blocks vagal signaling reduced subjective hunger, increased fullness, decreased body weight, and improved glycemic regulation in clinical trials (89, 134, 353, 654, 656, 689). The most compelling of these was a randomized, double-blind, sham-controlled trial involving 239 obese patients (353). Those receiving vagal blockade lost 24% of their excess weight in 1 year, versus 16% in the sham-operated group. The mechanism underlying the efficacy of vagal blockade is unknown. One possibility is that slowed gastric-emptying rate is involved (134, 405). In the patients described above, gastric emptying was reportedly unchanged, but because gastric emptying was measured after the patients had undergone more than a year of vagal stimulation, it is possible that there was tachyphylaxis of an earlier effect (658). In other studies, vagal blockade increased ghrelin secretion and reduced secretion of CCK and GLP-1 (153, 154), effects that presumably would oppose any decrease in eating. An alternative hypothesis that deserves investigation is that vagal blockade reduces gastric accommodation during meals, leading to increased distension and early satiation. Because the blockade prevents vagal afferent signaling, this hypothesis requires that distension is adequately sensed by spinal-visceral afferents (82, 146).
G. RYGB
Due to a greatly reduced gastric lumen (Figure 1), only a fraction of the normal gastric volume can be accommodated, and antral trituration and pyloric control of emptying are absent after RYGB. As a result, RYGB markedly accelerates gastric emptying of liquids and solids (although emptying of small, solid meals with volumes not exceeding the pouch volume may be slower) (213, 244, 339, 518, 536, 802, 808). This, in turn, often leads to bloating, nausea, and dumping in RYGB patients (307, 377, 545, 695, 745). Glucose solutions, as used in glucose-tolerance tests, may empty almost immediately in RYGB patients, leading to the appearance of ∼300 kcal in the jejunum within 1–2 min (544). Such rapid increases in small intestinal nutrient content are likely to contribute to the increased meal-related secretion of CCK, GLP-1, PYY(3–36), and insulin after RYGB, as described in the next sections. For example, infusion of glucose at a high physiological rate (4 kcal/min) into the Roux limb of RYGB patients and into the duodenum of healthy subjects elicited comparable increases in GLP-1, whereas oral glucose loads (200 kcal/150 ml) produced larger GLP-1 responses in the RYGB patients (544).
Three additional studies reveal further contributions of RYGB-induced alterations of GI function to changes in eating and body weight: 1) faster pouch emptying on postoperative day 1 was associated with a ∼4 kg increase in 1 year weight loss (21); 2) pouch size was negatively correlated with weight loss after 6 months and 1 year (626); and 3) thresholds for detection of inflation of a balloon placed in the Roux limb were negatively correlated with spontaneous meal sizes 6 months and 1 year postoperatively (81). Relearning to eat comfortably is likely to be important in some of these effects, but such learning has not yet been studied much in either humans (108, 177) or animals (424, 690). For example, a questionnaire follow-up indicated that meat was the food most often linked to food aversions after RYGB (288), which may be due to the challenge of digesting meat without a stomach.
H. Summary
GI motor function and gastric emptying are closely regulated. Neural gastric-volume detection contributes to the inhibitory control of eating, and gastric emptying contributes to meal-related glycemic control. CCK, GLP-1, and PYY(3–36) contribute to the control gastric emptying, and ghrelin may do so. Intestinal-nutrient sensing links the secretion of ghrelin, CCK, GLP-1, and PYY(3–36) to gastric emptying and gastric volume (Figure 5). Loss of normal gastric accommodation, food storage, food trituration, and emptying are likely to play important roles in the effects of RYGB on eating and glycemic control.
III. GHRELIN
A. Introduction
Ghrelin is a 28-amino acid peptide hormone discovered in 1999. Ghrelin is produced by closed-type enteroendocrine cells in the oxyntic glands of the gastric fundus (184, 397) (Figure 6A), as well as by some small intestinal enteroendocrine cells, pancreatic-islet cells, and neurons in various brain areas, including the arcuate nucleus of the hypothalamus (Arc) (184, 398, 524, 597, 742, 799, 826). Ghrelin O-acyltransferase (GOAT) catalyzes the conversion of ghrelin into its biologically active acylated forms, octanoyl- and decanoyl-ghrelin (together referred to as acyl-ghrelin, in contrast to unacylated or des-acyl-ghrelin). GOAT physiology has emerged as an important modulator of ghrelin function (58, 389, 524, 847). Less than 10% of circulating ghrelin is acyl-ghrelin, which together with the difficulty in assaying it, complicates studies of endogenous ghrelin (390, 596, 707). The ghrelin receptor was described in 1996 as the growth hormone-secretagogue receptor-1A (GHSR1A) (348). It is widely expressed peripherally and centrally (398, 524, 799). Des-acyl-ghrelin has little affinity for GHSR1A but may have metabolic effects via other receptors (524).
FIGURE 6.
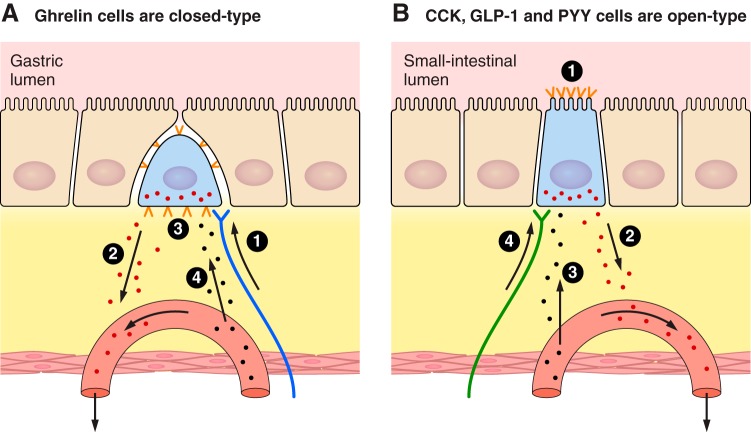
Schematic of the organization of ghrelin, CCK, GLP-1, and PYY entroendocrine cells. A: gastric ghrelin cells (blue) are closed-type. Their apical aspects are enclosed by epithelial cells (tan) so that they have no direct contact with the gastric lumen. 1) Neural signals provide the major stimulatory control of ghrelin secretion. 2) Secreted ghrelin (red dots) diffuses through the lamina propria (yellow) into gastric capillaries (salmon) and is transported into the hepatic-portal vein and systemic circulation. 3) Ghrelin cells express a number of nutrient receptors, mainly on the basal and lateral aspects (orange <). These are probably stimulated mainly by metabolites reaching them by diffusion from the gastric capillaries through the lamina propria, although some nutrients may reach them directly from the stomach. 4) CCK, PYY(3–36), perhaps other small intestinal hormones, and other humoral stimuli reach ghrelin cells via the circulation and inhibit ghrelin secretion. Paracrine signals (not shown) may also be involved. B: CCK, GLP-1, and PYY cells (blue) are open-type, with direct contact with the small intestinal lumen. 1) Each expresses a number of nutrient receptors, mainly on the apical and lateral aspects (orange <). These are probably the major controls of secretion of these hormones. The nutrient receptors expressed by ghrelin, CCK, GLP-1, and PYY cells are listed in Table 4, which also indicates the extensive overlap in the nutrient receptors expressed by the these cell types. 2) Secreted hormones (red dots) diffuse through the lamina propria (yellow) into small-intestinal capillaries (salmon) and are transported into the hepatic-portal vein and systemic circulation. 3) Metabolites, hormones, and other humoral factors reach CCK, GLP-1, and PYY cells by diffusion from mesenteric capillaries through the lamina propria (yellow) or from nearby small-intestinal epithelial cells (tan). 4) Neural inputs also control CCK, GLP-1, and PYY secretion.
B. Secretion
Plasma concentrations of total and acyl-ghrelin increase before meals, decline precipitously after meals, and then increase gradually until the next meal (173, 326, 707). For example, when acyl-ghrelin was sampled frequently throughout the day in subjects adhering to a controlled sleep-wake, activity and meal protocol (707), acyl-ghrelin maxima were ∼110 pM before breakfast and ∼100 pM before lunch and dinner, and post-meal minima were ∼70 pM. Importantly, the ratio of circulating acyl- to total ghrelin may change around meals (707). Because ghrelin's plasma half-life is ∼30 min (20, 800), postprandial acyl-ghrelin dynamics presumably primarily reflect inhibition of secretion. There is also a gradual decrease in acyl-ghrelin after midnight, which probably reflects an inhibitory effect of sleep; the pre-breakfast increase begins only after awakening (707). These patterns suggest that habitual sleep-wake cycles and the timing of breakfast modulate morning ghrelin levels. This complicates any definition of “basal” plasma ghrelin and indicates that across-group comparisons of pre-meal plasma ghrelin concentrations should consider the times of sampling with respect to habitual meal times. Average daily ghrelin levels might provide a useful alternative. Cummings et al. (173) reported 1) a correlation of 0.95 between the 0930 h ghrelin level (i.e., the post-breakfast minimum) and the 24 h ghrelin area under the curve (AUC), and 2) a correlation of 0.87 between the 0600 h ghrelin level (the pre-breakfast minimum) and the 24 h AUC, indicating that both measures accurately reflect the integrated or average daily ghrelin level.
The mechanisms stimulating ghrelin secretion during fasting are poorly understood. In one study, ghrelin levels were elevated after a 3-day fast and did not change around meals, although the nocturnal increase was unaffected (139). Autonomic efferents contribute to the stimulation of ghrelin secretion in both humans (107) and animals (346, 852). Cephalic-phase reflexes activated by the sight, smell, and taste of food (i.e., elicited by modified sham feeding) were reported to both stimulate (511, 512, 696) and inhibit ghrelin secretion (35) in humans. Time cues also increase pre-meal ghrelin levels in schedule-fed rats (180, 494).
Ghrelin secretion after meals is inhibited by GI signals that are recruited rapidly by nutrient ingestion. Conditioned (167) and cephalic-phase reflexes (315) may contribute. There appears to be no gastric phase to post-meal ghrelin inhibition because 1) intragastric water or liquid-diet infusions had no effect on ghrelin levels in rats when infusates were confined to the stomach with a pyloric cuff (558, 829), 2) plasma ghrelin concentrations were reduced comparably by intragastric and intraduodenal glucose infusions in healthy-weight men and women (563, 719), and 3) in contrast to most enteroendocrine cells, gastric ghrelin cells are closed type, i.e., do not directly contact to the GI lumen (Figure 6A). Nevertheless, gastric ghrelin cells express several nutrient-sensing receptors that may affect ghrelin secretion (207, 237, 311, 367, 457, 524, 824, 825) (Table 4, which includes the full and the former names of the nutrient receptors discussed below). Because these are expressed mainly on the basolateral aspects of ghrelin cells, they are probably stimulated mainly by metabolites entering the laminal propria from the circulation, as discussed below. There is some evidence, however, that they can be stimulated by gastric contents. Lu et al. (457), for example, found that mouse ghrelin cells express the free fatty acid receptor 4 (Ffar4), that fatty acids inhibited ghrelin secretion in vitro, and that intragastric lipid loads reduced serum ghrelin levels in mice with ligated pylori. Similar results were obtained in rat gastric explants (22, 692). These data are inconsistent with the rat pyloric cuff data described above (558, 829), and relevant studies remain to be done in humans. Few human enteroendocrine ghrelin cells express GNAT3, TAS1R1/TAS1R3, or FFAR4, although nearby cells do, suggesting the possibility of a gastric paracrine chemosensory control of ghrelin secretion (825).
Table 4.
Nutrient receptors expressed by enteroendocrine ghrelin, CCK, GLP-1, and PYY cells
| Nutrient Receptor | Ghrelin | CCK | GLP-1 | PYY |
|---|---|---|---|---|
| CASR (CaR) | X | X | X | X |
| CD36 | X | |||
| FFAR1 (GPR40) | X | |||
| FFAR2 (GPR43) | X | X | ||
| FFAR3 (GPR41) | X | |||
| FFAR4 (GPR120) | X | X | X | |
| GNAT3 (gustducin) | X | X | ||
| GPR119 | X | |||
| HCAR1 (GPR81) | X | |||
| LPAR5 (GPR93) | X | |||
| SLC2A1 (GLUT1) | X | |||
| SLC2A2 (GLUT2) | X | |||
| SLC2A5 (GLUT5) | X | |||
| SLC5A1 (SGLT1) | X | |||
| SLC15A1 (PEPT1) | X | X | ||
| TAS1R1/TAS1R3 (T1R1/T1R3) | X | X | ||
| TAS1R2/TAS1R3 (T1R2/T1R3) | X | X | ||
| TAS1R3 (T1R3) | X |
The table is based on the evidence of receptor expression in mice, rats, or humans discussed in the text. Former names of the receptors are given in parentheses. CaR, calcium receptor; CASR, calcium-sensing receptor; CD36, thrombospondin receptor; FFAR, free fatty acid receptor; GLUT, glucose transporter; GNAT3, guanine nucleotide-binding protein, alpha transducing 3; GPR, G protein-coupled receptor; HCAR1, hydroxycarboxylic acid receptor 1; LPAR5, lysophosphatidic acid receptor 5; PEPT1 and SLC15A1, solute carrier family 15 (oligopeptide transporter), member 1; TAS1R1 and T1R1, taste receptor, member 1; TAS1R2 and T1R2, taste receptor, member 2; TAS1R3 and T1R3, taste receptor, member 3 (T1R1/T1R3). Note that abbreviations are for the human genes, although many of the receptors indicated have been identified on the respective enteroendocrine cells so far only in mice or rats.
The intestinal phase of post-meal ghrelin inhibition is well established (169, 248, 563, 640, 719). The critical site for inhibition by glucose appears to be distal to the duodenum and proximal jejunum because ghrelin secretion (60 min AUC) was not inhibited by intraduodenal infusions of glucose that were limited to only the proximal 60 cm of the small intestine to glucose by an inflated balloon, but was inhibited when glucose was also allowed access to the more distal small intestine (445). The underlying mechanisms are unknown.
Circulating metabolites and hormones may also contribute to the inhibition of ghrelin secretion. Intravenous glucose infusion, alone or together with insulin, reduced ghrelin levels under several conditions (254, 506, 526, 644, 688). Insulin may be the key factor, as meals did not reduce ghrelin levels in patients with type 1 diabetes mellitus (T1DM) in the absence of insulin therapy, but did so following reinstatement of basal euinsulinemia (526). In contrast to glucose infusions, intravenous lipid infusions failed to affect plasma ghrelin levels (506). Increases in peripheral concentrations of lactate and short-chain fatty acids resulting from colonic fermentation of poorly digestible carbohydrates (46, 523, 753, 758) may be sensed by hydrocarboxylic acid receptor 1 (HCAR1) and FFAR2, respectively, because the corresponding receptors are expressed by gastric ghrelin cells in mice (237). Plasma lactate also increases following many meals (694, 734) as well as during exercise and hypoxia (135, 286, 815), and both exercise and hypoxia decrease plasma ghrelin levels in rats and humans (135, 815). Finally, circulating amino acids may inhibit ghrelin secretion via calcium-sensing receptor (CASR) (237).
All three macronutrients inhibit ghrelin secretion after meals. Consumption of carbohydrate and protein reduced ghrelin levels during the next 3 h more than did isoenergetic lipid loads (258, 510). Whether carbohydrates and proteins differentially affect ghrelin secretion is less clear. 1) In overweight and obese men, ∼250 kcal oral loads containing 80% energy as lactose, whey or casein reduced ghrelin levels more than similar glucose loads 120–180 min after ingestion (96). 2) In healthy-weight and overweight men and women, ∼500 kcal oral protein and glucose loads reduced ghrelin levels similarly for ∼3 h, but protein reduced ghrelin levels more effectively subsequently (258). 3) In healthy-weight women, no differences in ghrelin levels were detected during 24 h trials comparing a 10% protein-energy diet, a 60% carbohydrate-energy diet, and a 30% protein- and 40% carbohydrate-energy diet (427). Carbohydrate type is also important: oral glucose reduced ghrelin levels less than lactose (96), but more than fructose (755). Because none of the studies reviewed above assessed gastric emptying, differential rates of small intestinal appearance of ingested nutrients may have contributed as well as direct effects of specific intestinal nutrient sensors.
Lipids and di- or polysaccharides require digestion to inhibit ghrelin secretion fully because tetrahydrolipstatin, a lipase inhibitor, and arcabose, an α-glucosidase inhibitor, decreased the inhibition of ghrelin secretion by intraduodenal lipid infusions and sucrose drinks, respectively (197, 248, 250, 749). These studies also revealed that only fatty acids with a chain length greater than or equal to C12 inhibit ghrelin secretion (197, 250).
The neuroendocrine reflexes mediating post-meal ghrelin inhibition by intestinal nutrient sensing are poorly understood. The vagus nerve seems unnecessary in rats because vagotomy did not affect post-meal ghrelin inhibition in rats (830). CCK and PYY(3–36) may be involved because intravenous infusions of each reduced plasma ghrelin levels in humans (61, 104, 198), whereas GLP-1 infusion did not (104). We are aware of only one test of the physiological relevance of these potential endocrine controls of ghrelin secretion: CCKA-receptor blockade abolished long-chain fatty acid-induced ghrelin inhibition in healthy subjects, suggesting that the mechanism involves CCK (197). Finally, although fasting plasma ghrelin levels correlated with basal leptin levels (240), leptin infusion failed to reinstate normal meal-related ghrelin patterns in healthy-weight men who had fasted 3 days (139).
C. Eating
Changes in plasma ghrelin levels around meals fulfill criterion 1 of Table 2 for an endocrine role in hunger signaling. 1) Plasma ghrelin levels increase progressively before meal onset and fall precipitously afterwards (173, 276, 390, 707, 778). 2) Hunger ratings were closely related to the drops and subsequent increases of total ghrelin levels between lunch and a spontaneous dinner in healthy-weight, time-blinded men (171) as well as between breakfast and a lunch offered at a set time in overweight and obese men and women (276). 3) Breakfast-to-lunch intermeal intervals in healthy-weight, time-blinded men who were served dinner upon request were correlated with post-breakfast decreases in total ghrelin and with the AUC of the breakfast-to-lunch ghrelin response (84) [although these correlations were not detected in non-time-blinded men (124)]. 4) Ghrelin concentrations at meal onset correlated with meal size in healthy-weight and overweight men and women offered lunch at a set time (276). Tests of ghrelin infusions, however, have hitherto failed to fulfill criterion 3 of Table 2. Intravenous infusion of 0.3 pmol·kg−1·min−1 ghrelin, a near-physiological dose (Table 3), that began 1 h after a standard meal did not affect subjective hunger, the spontaneous intermeal interval, or the size of the following spontaneous meal (444). Pre-meal infusion of 1–5 pmol·kg−1·min−1 ghrelin, however, did stimulate eating in two tests in which meals were offered at set times (221, 839). Interestingly, supraphysiological ghrelin infusions also increased neural activity in response to pictures of food, as detected by functional magnetic-resonance imaging (fMRI), in brain regions associated with food reward (284, 465). This suggests that ghrelin may affect eating primarily via changes in food hedonics rather than hunger, a hypothesis supported by neuropharmacological data in animals (211, 370). For example, in rats and mice, injection of ghrelin into the ventral tegmental area, a reward area, activated dopamine neurons, and injection of a ghrelin-receptor antagonist into the ventral tegmental area prevented the stimulation of eating by peripheral ghrelin administration (4).
Animal studies also link ghrelin signaling to brain networks thought to be related primarily to homeostatic eating. For example, in mice, ghrelin administration into the Arc acutely stimulated eating and altered the activities of Arc neuropeptide Y, agouti-related peptide, and pro-opiomelanocortin neurons (145, 164, 810). Ghrelin also appears to act in the Arc to reduce serotonin 2C receptor-mediated inhibition of eating (661). Finally, initial reports that the vagus nerve was required for ghrelin to stimulate eating (36, 185, 186) were not replicated when a more selective lesion method was used, which also supports a central action of ghrelin on eating (34).
An unresolved challenge to the hypothesis that ghrelin signals hunger is that transgenic mice with reduced ghrelin signaling do not display a tonic increase in eating (524). Some such transgenics do develop obesity, especially when fed a high-fat diet (524), but this may be secondary to decreases in fatty acid oxidation and increases in lipid deposition in response to changes in autonomic nervous system activity (484, 524, 572, 757). As a consequence, ghrelin is currently considered to be a stronger candidate for the development of pharmacotherapies for metabolic disease than for overeating.
D. Glycemic Control
Ghrelin may affect glycemic control by accelerating gastric emptying, inhibiting insulin secretion, or stimulating secretion of glucagon or other counterregulatory hormones (106, 152, 170, 202, 524, 530, 750, 799). In one study, intravenous infusion of a near-physiological dose of 0.3 pmol·kg−1·min−1 ghrelin, reduced insulin levels in response to intravenous glucose infusion and increased growth hormone and cortisol, but not glucagon, epinephrine, or norepinephrine, levels (767). Studies in mice indicate that the insulin-inhibitory effect of ghrelin is mediated by a direct action on pancreatic β-cells (208, 413). The modulation of ghrelin acylation by dietary levels of C8 and C10 fatty acids may provide a mechanism for brain nutrient sensing and the neural regulation of glucose metabolism (389), although given the low levels of these fatty acids in most diets, this seems unlikely to be a physiological endocrine effect under most conditions.
E. Obesity
GHRL polymorphisms were associated with BMI variation in several human populations (430). Although significant, the effects are quite small [for example, a GHRL polymorphism at rs35683 accounted for <0.3% of the variance in BMI in a sample of 2,000 European-Americans (430)], and the functional pathways that contribute to the effects are unknown.
Fasting plasma ghrelin levels are decreased in obese subjects and increased by diet-induced weight loss (174, 240, 390, 779). Because obesity increases fasting insulin levels, the inhibitory effect of insulin on ghrelin secretion (see sect. IIID) may contribute to obesity's effect on fasting ghrelin. Shiiya et al. (688), however, did not detect any effect of T2DM on fasting plasma ghrelin in obese subjects. Postprandial drops in plasma ghrelin were reduced in some (239, 240, 497, 574), but not all (103, 174, 403), studies of obese subjects.
We are aware of one study of the effect of ghrelin on eating in obese humans (221), which was inconclusive. Acute intravenous infusions of supraphysiological doses of ghrelin (1 and 5 pmol·kg−1·min−1) appeared to increase eating more in obese than in healthy-weight subjects, but whether the differences were statistically significant was not tested.
F. RYGB
Fasting and post-meal ghrelin levels are reduced in the first 2 wk after RYGB, but the longer-term effects are controversial (174, 310, 390, 403, 574, 712). Peterli et al. (574) reported that in obese subjects who had elevated fasting ghrelin levels and no post-meal ghrelin drops, RYBG initially reduced fasting ghrelin, but that by 1 year post-RYGB, fasting ghrelin levels were no longer reduced and there were typical post-meal drops. Such gradual normalization of ghrelin secretion after RYGB may result either from weight loss or from dynamic adaptation of the GI tract (678). RYGB increased ghrelin levels in some rodent studies (31, 780, 854), but decreased them in others (731, 735). Stylopoulos et al. (731) suggested that this apparent discrepancy may be attributable to an effect of the initial rapid postsurgical weight loss to increase ghrelin levels combined with a sustained decrease in ghrelin secretory capacity due to the gastric resection. Interestingly, in their rat model, weight loss 3 months after surgery was correlated with the pre- to postsurgery decrease in ghrelin levels (731). In another rat study (691), in which there were no consistent changes in pre-meal ghrelin levels tested 12–16 wk after RYGB, ghrelin levels decreased more after meals in RYGB than control rats, and the magnitude of the effect was correlated with weight loss.
G. Summary
Figure 7 summarizes ghrelin physiology around meals. Ghrelin secretion increases during fasting and is inhibited by cephalic- and intestinal-phase reflexes during and after meals. Sensing of all three macronutrients contributes to the intestinal phase of ghrelin inhibition. Pre-meal ghrelin levels are correlated with hunger sensations and meal size, but if ghrelin has a causal endocrine role in hunger is unclear. Ghrelin may contribute to glycemic control via several mechanisms. Indeed, it has been hypothesized that ghrelin's major function is to prepare the organism for the nutrient repletion and storage (389, 524). Studies to date of ghrelin physiology in obese individuals and after RYGB have not produced consistent results. Ghrelin antagonists and inverse agonists suitable for human use (78, 125) may soon resolve many of these outstanding questions.
FIGURE 7.
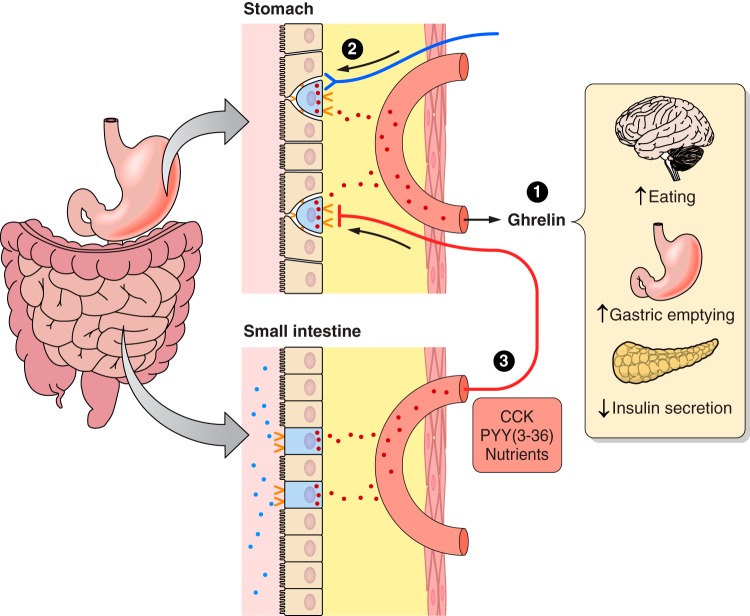
Some features of ghrelin physiology. Ghrelin is secreted from closed-type enteroendocrine cells (blue) dispersed in the epithelial layer (tan) of the gastric mucosa. Ghrelin diffuses through the lamina propria (yellow) and into gastric capillaries (salmon). 1) Ghrelin's potential physiological effects include acting in the brain to stimulate eating, acting in the stomach to stimulate gastric emptying, and acting on the pancreatic β-cells to inhibit insulin secretion. 2) Ghrelin secretion is stimulated mainly by neural controls. 3) Feedback from small-intestinal nutrient sensing, mediated in part by open-type CCK and PYY(3–36) cells, inhibits ghrelin secretion during and after meals.
IV. CHOLECYSTOKININ
A. Introduction
CCK cells are open-type cells, i.e., their apical surfaces are exposed to the intestinal lumen (Figure 6B). Initial electron microscopy and immunocytochemistry studies suggested that they were a unique species of enteroendocrine cells, called I-cells (590). Contemporary methods, however, indicate that, at least in rodents, many enteroendocrine CCK cells also express and secrete ghrelin, GLP-1, PYY, GIP, neurotensin, or secretin (231, 303, 597, 738, 742). In humans, swine, and rats, enteroendocrine CCK cells are densely expressed in the duodenum and proximal jejunum, less dense in the distal jejunum, and sparse in the ileum (45, 478, 503).
CCK circulates predominantly in a 58-amino acid form (CCK-58) (243, 431, 612, 722). Importantly, many CCK assays that involve plasma formation recover <20% of endogenous CCK, so they provide accurate relative, but not absolute, levels (243, 431, 722). Additionally, most tests of exogenous CCK use CCK-8, which is rare or absent in the plasma. This may be important because the liver clears CCK-8 faster than larger forms (287, 404) and CCK-8 had slightly different effects than CCK-33 or CCK-58 in animal models (607, 608), including in tests of eating in rats (232, 279, 281).
There are two CCK receptors, CCKAR (or CCK1R) and CCKBR (CCK2R) (216, 514, 515, 612). CCKAR is more abundant peripherally than centrally and requires the seven-amino carboxy-terminal segment and sulfation of the tyrosine residue at position 7 for full activation. CCKBR, or the gastrin receptor, is sensitive to unsulfated CCK hexapeptides and is abundant both peripherally and centrally, where CCK-8 is a neurotransmitter.
B. Secretion
Mixed-nutrient meals increase CCK secretion. Using a well validated radioimmunoassay, Rehfeld et al. (609) found that a 1,470 kcal mixed-nutrient meal increased plasma CCK from a fasting level of ∼1 to ∼3 pM at 30 min and ∼5 pM at 60–90 min. Similarly, using the state-of-the-art RAPID method, Eysselein et al. (242) found a 1,600 kcal mixed-nutrient meal increased plasma CCK from a fasting level ∼2.5 to ∼7 pM at 60 min. A number of studies involving isoenergetic loads of highly digestible nutrients that were infused intraduodenally to control gastric-emptying effects indicate that, with respect to both peak values and AUC, 1) oral lipids stimulate CCK secretion most per kcal, proteins are intermediate, and carbohydrates stimulate CCK secretion least; and 2) plasma levels increase in 10–15 min (327, 337, 446, 584, 641, 682).
Hydrolysis of proteins and triglycerides is required for normal CCK secretion (55, 159, 247, 325, 479, 718). Additionally, fatty acids with chain length greater than or equal to C12 stimulate CCK secretion much more than fatty acids less than C12 (249, 479, 486, 487), and less saturated long-chain fatty acids stimulated CCK secretion more than highly saturated fatty acids (67). Carbohydrate digestion may not be required, as the α-glucosidase inhibitor acarbose had little or no effect on the CCK response to mixed-nutrient meals (236, 751, 784).
Consistent with the higher density of enteroendocrine CCK cells in the proximal small intestine, intraduodenal glucose infusions that were prevented from transiting more than 60 cm distal to the pylorus by an inflated balloon stimulated CCK secretion as much as infusions done without balloon inflation (445). This is likely also to be the case for fat and protein. Intraileal lipid infusion also increased CCK secretion (466), but whether this was due to a direct action on ileal CCK cells or to an indirect, presumably endocrine, distal-to-proximal reflex is unknown.
Intraluminal nutrients directly and indirectly stimulate CCK secretion. Direct nutrient effects are mediated by a variety of nutrient receptors expressed on the apical surface of CCK cells (Figure 6B and Table 4, which includes the full and the former names of the nutrient receptors discussed below). In humans, free fatty acids act on FFAR1 (443), FFAR4 (752), and the fatty-acid transporter CD36 (733); oligopeptides and amino acids act on CASR (161, 328, 811), LPAR5 (149), TAS1R1/TAS1R3 (160, 182, 543) and, perhaps, SLC15A1 (183). The presence of transcripts for TAS1R2/TAS1R3 and GNAT3 on CCK-secreting mouse enteroendocrine STC-1 cells suggests that sweet-receptor signaling may contribute to glucose-induced CCK release in mice (228, 849). This may not be the case in humans, however, because intragastric and intraduodenal infusions of the sweet-receptor inhibitor lactisole that reduced glucose-induced GLP-1 and PYY secretion did not affect CCK secretion (275). Intraluminal nutrients also stimulate CCK secretion indirectly via the CCK-releasing factors “pancreatic monitor peptide” and “intestinal luminal CCK-releasing factor” (456, 504, 812). This occurs in part due to binding of proteases to proteins and lipids, which reduces protease-induced degradation of CCK-releasing factors (168, 432).
C. Eating
CCK is the best-established GI endocrine satiation signal in humans. First, in three studies (54, 299, 438), intravenous infusions of physiological doses of CCK reduced meal size without adverse physical or subjective effects in men and women, which fulfills criteria 3 and 5 of Table 2. The study by Lieverse et al. (438) is especially interesting because the test food, bananas, did not elicit CCK secretion under their conditions (440), so that the infused CCK did not synergize with endogenous CCK, as probably happens in most satiation tests. Second, intravenous infusions of the CCKAR antagonist loxiglumide increased premeal hunger feelings, reduced fullness feelings during the meal, increased meal size, and blocked the satiating effects of intraduodenal lipid infusion (70, 439, 480), which fulfills criteria 4 and 6 of Table 2. These studies, summarized in Figure 8, have made CCK paradigmatic for the study of the endocrine control of eating.
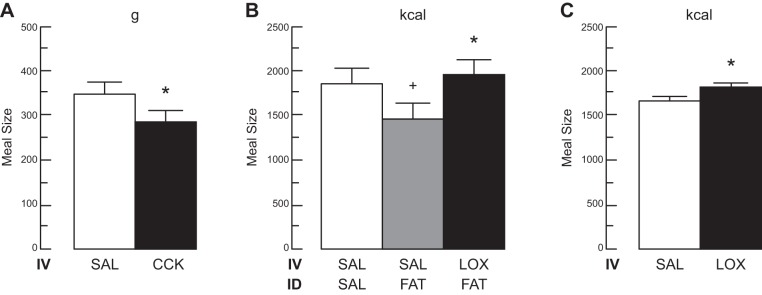
FIGURE 8.
Evidence that endogenous CCK signals satiation in healthy humans. A: intravenous infusion of a physiological dose of CCK inhibited eating. Ten healthy-weight women [body mass index (BMI) 22 ± 3 kg/m2] and 8 obese women (BMI 39 ± 2 kg/m2) received 60 min intravenous (IV) infusions of 0.24 pmol·kg ideal body weight−1·min−1 CCK-33 or saline (SAL) beginning at 0800 after an overnight fast. At 0900, a 132 kcal preload of bananas was served, and at 0915, a banana-shake meal was served in excess; bananas were used because they did not elicit CCK secretion. CCK significantly reduced meal size (*) without physical or subjective side effects. [From Lieverse et al. (438), with permission from BMJ Publishing Group Ltd.] B: the CCKA receptor antagonist loxiglumide (LOX) antagonized the satiating action of endogenous CCK stimulated by intraduodenal (ID) infusion of a fat emulsion. Healthy-weight adult males began a midday lunch buffet 4 h after a standard breakfast, 90 min after onset of an IV infusion of 10 μmol·kg−1·h−1 LOX or SAL, 60 min after an ID infusion of 0.4 ml/min corn oil (FAT) or SAL, and 20 min after an oral preload of 400 ml of a low-fat banana milkshake. Infusions were continued throughout the meal. ID fat infusion significantly reduced the size of the lunch meal (+), and that this was reversed by LOX (*); no physical or subjective side effects occurred in any condition. [From Matzinger et al. (480).] C: antagonism of CCK signaling with the CCKA receptor antagonist LOX stimulated eating. Healthy-weight adult males began a midday lunch buffet 4 h after a standard breakfast and 60 min after beginning an IV infusion of 22 μmol·kg−1·h−1 LOX or SAL. Infusions were continued throughout the meal. LOX significantly increased meal size (*) without physical or subjective side effects. [From Beglinger et al. (70).]
In addition, 1) human CCKAR polymorphisms are associated with increased meal size, increased food intake, and obesity (192, 473, 501), suggesting that endogenous CCK is also important for the tonic control of eating. 2) fMRI following intragastric lauric acid loads with or without loxiglumide indicated that CCK signaling is crucial for normal brain responses to this fatty acid (419) (because lauric acid is uncommon in Western diets, the generality of this finding is uncertain). 3) CCK doses substantially above physiological (i.e., ∼1.8 to ∼3.5 pmol·kg−1·min−1, Table 3) inhibited eating without eliciting adverse effects (100, 269, 290, 391). Interestingly, CCK infusions reduced meal size ∼30–50% in these studies without affecting fullness or other meal-related sensations compared with the control condition, suggesting that CCK had an effect on consciousness indistinguishable from the presumably more complex afferent activation produced by the larger quantity of food eaten in the control condition. 4) In most of these studies, CCK infusions began after a small preload to capitalize on the synergy between gastric mechanoreception and CCK (528), described in section IIC.
Attempts to relate endogenous CCK levels with subjective measures of appetite have been less informative than studies of manipulation of CCK. 1) In the sole study of intrameal effects, plasma CCK increased more during meals in women than in men, but hunger and fullness ratings did not differ; women gave higher ratings of “sickness” early in the meal, but not later when CCK levels increased more, nor did they spontaneously report illness or display signs of illness (549). The small sample size (four of each sex) further limits this study. 2) Postprandial CCK levels and hunger and fullness ratings were significantly correlated in a group of nine men, but the relationships were not detected in all individuals (3 of 9 for hunger and 4 of 9 for fullness) (260). 3) Meals containing different fats differentially increased postprandial plasma CCK levels in eight women, and these were mirrored by subjective hunger and fullness ratings; but neither CCK responses nor appetite ratings differed in seven men (119). 4) Meals containing different fat-to-carbohydrate ratios differentially increased postprandial plasma CCK levels in 16 overweight and obese men and women, but no associations with subjective appetite were detected; there was also no difference in the size of meals offered 3 h later, but by this time CCK levels had returned to basal (277). Because CCK appears to signal satiation, but not postprandial satiety, it is unfortunate that there are not more studies of the relationships among differential intrameal plasma CCK levels, appetite, and meal size.
Reproductive physiology may affect CCK satiation. Women spontaneously eat progressively less during the follicular phase of the menstrual cycle, reaching a nadir in daily food intake during the periovulatory phase that is ∼275 kcal/day less than the luteal-phase maximum (38). Rats and mice also display a decrease in food intake during the periovulatory phase, due in part to an increase in the satiating potency of CCK related to estrogen signaling in the nucleus of the solitary tract (NTS) (38).
Studies in rodents suggest that CCK inhibits eating via both local and endocrine modes of action. In support of local action, intravenous infusion of the small-molecule CCKAR antagonist devazepide, which presumably diffused from the capillaries into the small intestinal-lamina propria, increased food intake, but infusions of a CCK antibody, which would not escape the vasculature so as to selectively block endocrine effects, did not (616). Infusion studies indicate that the most likely physiological site of CCKAR mediating satiation is the proximal small intestine (165, 814). In addition, the satiating action of intraperitoneal injections of CCK is mediated by vagal afferent fibers (165, 426, 704, 705), and most small intestinal vagal afferents terminate within the crypt and villous lamina propria, but not in close apposition to enteroendocrine CCK cells, indicative of a paracrine action (77). Nevertheless, some vagal afferents terminate with 5 μm of CCK cells (77), and CCK neuropods appear to signal via enteric glial cells (90) (described in section IB3 and Figure 3) so that neurocrine or neuropod satiation signaling is also possible.
Other data in rats and mice support an endocrine mode of action. 1) CCKAR in the pyloric muscle layers contribute to the satiating effect of exogenous CCK (516). As little or no food reaching the pylorus is digested sufficiently to stimulate CCK secretion and the CCKAR are localized in the muscle layer rather than the mucosal layer (565), the pyloric contribution to CCK satiation is likely to be endocrine. 2) Vagotomy studies suggest that endocrine CCK may also act in the brain to inhibit eating (615, 850). For example, intravenous infusions of devazepide, which enters the brain, stimulated eating after vagotomy, whereas infusions of a larger-molecule CCKAR antagonist that penetrates peripheral capillaries, but not the blood-brain barrier, did not (615). The site of the brain CCKAR mediating these effects is not known. The NTS (83, 330), to which vagal afferents project, and the dorsomedial nucleus of the hypothalamus (79) are candidates.
Whether CCK's physiological satiating effect in humans involves local or endocrine action is unclear. That infusions mimicking systemic levels reached during meals are sufficient to reduce eating even when endogenous CCK secretion is minimized (438, 440) suggests, but does not prove, that local signaling is not responsible. This is because GI hormones diffuse down a steep concentration gradient from the lamina propria into the mesenteric veins and are then successively diluted in the hepatic-portal circulation and systemic circulation (Figure 3). Thus, although the exact difference between lamina propria and systemic CCK concentrations is unknown, it seems likely that physiological intravenous CCK infusions are not sufficient to reproduce the CCK concentrations in the lamina propria that occur during meals.
D. Glycemic Control
No direct role has been established for CCK in glycemic control in humans. Infusion of a physiological CCK-8 dose (0.4 pmol·kg−1·min−1) reduced plasma glucose after an oral glucose load, but not after an intraduodenal glucose infusion that mimicked the gastric-emptying rate of the oral glucose (437), suggesting that CCK reduces blood glucose indirectly via inhibition of gastric emptying. In two studies, however, the CCKAR antagonist loxiglumide failed to affect plasma glucose despite accelerating gastric emptying (263, 323). Physiological levels of CCK do not appear to lower blood glucose by increasing insulin secretion because infusion of 0.2–0.4 pmol·kg−1·min−1 CCK-8 did not increase the insulin response to co-infusion of glucose (637). Physiological infusions of CCK did, however, increase the insulin response produced by amino-acid infusions in two (324, 637) of three (253) studies. But several attempts to demonstrate a direct insulinotropic effect of CCK using various CCKAR antagonists and various nutrient stimuli failed (65, 263, 323, 324, 400, 434, 547, 667, 673).
CCK secretion was reduced in patients with longstanding T2DM (112, 636), perhaps due to the autonomic neuropathy and reduced rates of gastric emptying typical of these patients (344). CCK-8 infusion further slowed gastric emptying and improved postprandial insulinemia and glycemia in patients with T2DM (18, 580, 636). Thus CCK agonists may be useful in diabetes therapy.
Studies in rats indicate that CCK affects glucose metabolism by reducing hepatic glucose production via a vagal-vagal reflex (98, 99, 147, 605). CCK was infused intraduodenally in amounts that failed to increase CCK concentration in the hepatic-portal vein to mimic the local action of CCK in the lamina propria, thus providing unique evidence for a paracrine action. This method seems feasible for human studies and may lead to better understanding of the relative roles of local and endocrine signaling in GI hormone function.
E. Obesity
Whether obesity affects CCK secretion is controversial. Fasting CCK levels were reduced in obese subjects in one study (57), but not two others (103, 725). CCK responses to intraduodenal oleic acid infusions tended to be delayed and reduced in overweight or obese compared with healthy-weight subjects in one study (725), but CCK responses to high-fat, high-carbohydrate, and high-protein meals were comparable in obese and healthy-weight subjects in another (103), and CCK responses to ingestion of high-fat meals were larger in obese than healthy-weight subjects in a third study (261). Whether these contrasting results were due to differences in the specific nutrient stimuli used, in gastric emptying, which was not assessed, or other factors is not known.
Some defects in CCK signaling can lead to obesity. As mentioned above, human CCKAR polymorphisms are associated with increased meal size, increased food intake, and obesity (192, 473, 501), suggesting that CCK-signaling defects can contribute to obesity etiology. In addition, allelic variations in CCK were significantly more prevalent in obese persons who habitually ate very large meals than those who did not (the “extreme discordant phenotype” approach) (192).
We know of only one study comparing the satiating action of CCK infusions in healthy-weight and obese humans (438). No difference was obtained (infusion of 0.24 pmol·kg−1·min−1 CCK-33 reduced meal size ∼18% in 10 healthy-weight women and ∼20% in 8 obese women).
Obesity produced by high-fat feeding may interfere with CCK satiation. 1) CCK injections and balloon distension in isolated jejunal segments elicited smaller vagal-afferent electrophysiological responses in mice made obese by feeding a high-fat diet than in chow-fed mice (181). 2) The CCK responsivity of vagal afferents was reduced in high-fat fed, leptin-resistant rats (193). 3) Fat-induced CCK secretion, the satiating effect of exogenous CCK, and the de-satiating effect of devazepide were reduced in rats fed a high-fat diet (225, 739).
Reports that rats that received intraperitoneal infusions of CCK during eve ry spontaneous meal for 6 days (821) and mice with transgenic null mutations of Cckar (402) increased meal frequency and failed to gain weight contributed to the views that CCK (and by extension other GI hormones) does not contribute significantly to tonic energy homeostasis and is a poor candidate for obesity pharmacotherapy. [The OLETF rat, which also bears a Cckar null mutation, is obese, but this is apparently due to loss of hypothalamic Cckar rather than vagal Cckar (79).] Recent preclinical data are more promising. First, stabilized forms of CCK that resist enzymatic degradation reduced food intake and body weight in various mouse obesity models (357, 359, 582). Second, native and stabilized CCK and CCKAR agonists increased the anorectic and weight-lowering actions of a GLP-1 receptor agonist, amylin, leptin, or amylin plus leptin in various rat and mouse obesity models (358, 776, 777), as did a CCK/GLP-1 agonist hybrid peptide (360). These promising results suggest that increased CCK signaling may contribute effectively to obesity pharmacotherapy.
F. RYGB
CCK has not been a focus of RYGB research because RYGB prevents ingesta from contacting the majority of CCK-secreting cells. Nevertheless, intraileal lipid infusions increased CCK secretion in healthy-weight subjects (466), and postprandial CCK levels were normal (383, 622, 634) or increased (364, 574) following RYGB. For example, Peterli et al. (574) found that CCK levels 30 min after a mixed-nutrient meal were increased approximately twofold 1 wk, 3 mo, and 1 yr after RYGB. That the increase occurred just 1 wk post-RYGB suggests that it does not require proliferation of CCK cells. The larger increases at later points may be related to the proliferation of CCK cells, which was reported in the Roux and common limbs of RYGB rats (525). The effect of RYGB on CCK secretion is an interesting and under-researched phenomenon that may lead to new opportunities for obesity therapy.
RYGB reduced food intake and body weight in obese OLETF rats (305), indicating that Cckar signaling is not necessary for some response to RYGB in this model. As no ad libitum-fed, genetically normal RYGB rats were included in the experiment, however, it is unclear whether the OLETF rats were normally responsive to RYGB. We know of only one test of acute CCKAR antagonism in RYGB rats (37), which failed to reveal any effect of RYGB on endogenous CCK satiation.
G. Summary
CCK is secreted in response to the products of carbohydrate, lipid, and protein digestion. It has been clearly established as a satiation signal in humans and may contribute to the control of meal-related glycemia both indirectly, via its effect on gastric emptying, and directly via control of hepatic glucose production. The satiating effect in humans may result from both local and endocrine actions, although the latter appear more likely. These actions of CCK are summarized in Figure 9. Pathophysiology of CCK signaling may contribute to overeating, to obesity and T2DM in some patients, and to early satiation after RYGB. Preclinical studies indicate that CCK is a candidate for obesity pharmacotherapy, especially in combination with other endocrine-based therapies.
FIGURE 9.
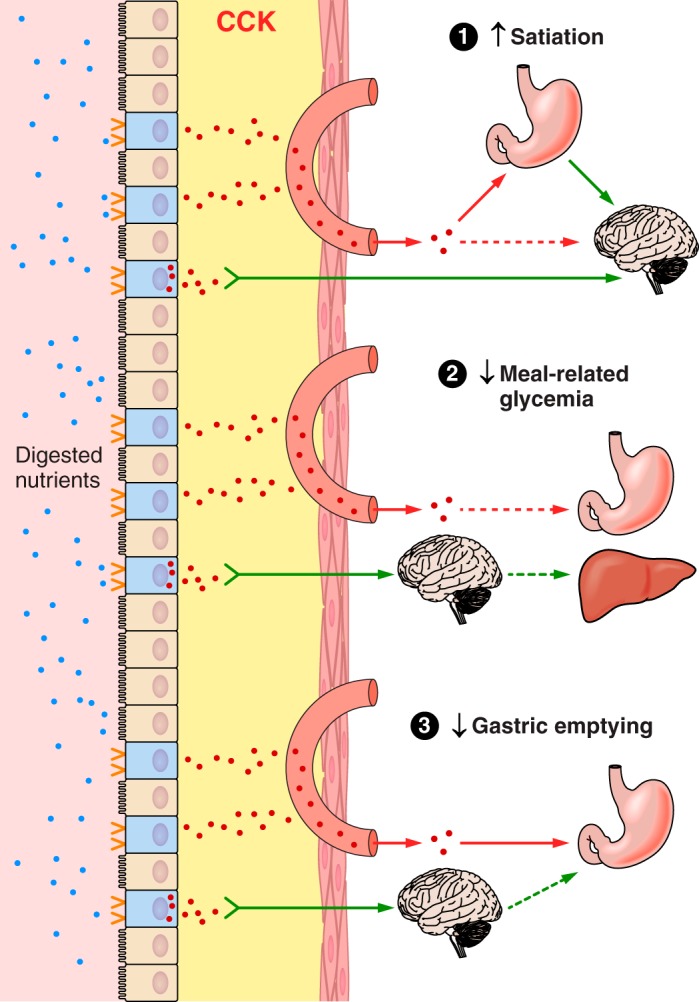
Some features of CCK physiology. CCK (red dots) secretion is stimulated by the digestive products of all three macronutrients acting on nutrient receptors on the apical aspects of enteroendocrine CCK cells (blue) dispersed in the epithelial layer (tan) of the small intestinal mucosa. CCK acts in an endocrine mode by diffusing through the lamina propria (yellow) and into intestinal capillaries (salmon) to reach distant target organs (red arrows), or acts locally. 1) CCK's physiological effects include stimulating satiation. This may occur via endocrine actions in the pyloric area of the stomach that produce signals relayed to the brain via vagal afferents (green arrow) or via local actions on vagal afferents in the lamina propria. An endocrine action in the brain may also contribute. 2) CCK lowers meal-related glycemia via an endocrine effect on gastric emptying and perhaps via a vagal-vagal reflex. 3) Similarly, CCK slows gastric emptying via a direct endocrine effect and perhaps via a vagal-vagal reflex. Solid lines indicate well-established effects, and dashed lines indicate less well established effects.
V. GLUCAGON-LIKE PEPTIDE-1
A. Introduction
GLP-1 is secreted by open-type enteroendocrine cells (Figure 6B), originally identified as L cells (114, 589), located in both the small and the large intestine (336, 597). Most GLP-1 cells in the distal jejunum and ileum coexpress and secrete PYY; in addition, some GLP-1 cells coexpress CCK, GIP neurotensin, or secretin (231, 303, 568, 597, 738, 742). GLP-1 is also produced by a small group of neurons located in the NTS (72, 128, 222, 336). Two equipotent molecular forms circulate, GLP-1(7–37) and GLP-1(7-36NH2); the latter predominates in humans. Dipeptidyl peptidase-4 (DPP-4), a proline/alanine-specific peptidase found on the luminal surface of capillary endothelial cells, in the liver, and in the blood, rapidly degrades active GLP-1 to inactive forms, GLP-1(9–37) and GLP-1(9-36NH2). In swine, only ∼25% of active GLP-1 secreted from the intestine reaches the portal circulation, and only ∼10–15% reaches the systemic circulation (336).
GLP-1 receptors (GLP-1R) are expressed in the GI tract, pancreas, cardiac atrium, abdominal vagal afferents, and many brain areas (28, 115, 128, 336, 450, 623, 783). In some animals, GLP-1 degradation products appear to signal via non-GLP-1 receptors (766). Whether these peptides have physiological functions in humans is not known.
B. Secretion
Available GLP-1 assay methods usually yield similar relative changes, but often different absolute concentrations (49, 410). Due to the rapid degradation of active GLP-1, GLP-1 secretion is best estimated by the sum of active and inactive GLP-1 in plasma (336, 508). Plasma GLP-1 concentrations are at their lowest levels after overnight fasts, increase rapidly during meals, and usually do not return to the morning level between meals (130, 235, 322, 557, 681, 804, 805). For example, when healthy-weight men and women ate 524 kcal breakfasts and, 4 h later, mixed-nutrient lunches containing 511, 743, or 1034 kcal, active GLP-1 increased from ∼5 pM to ∼8, ∼12, and ∼16 pM, respectively, after 30 min and then decreased to ∼7 pM after 180 min (27).
Oral loads of glucose and several other carbohydrates usually result in monophasic increases in plasma GLP-1, with onsets after 5–15 min, peak values after 15–30 min, and initial values regained after 3–4 h (130, 235, 322, 399, 663, 714, 759, 793, 805). Oral fructose, however, was only about half as potent a GLP-1 secretogogue as oral glucose, both on a molar basis (409) and when matched for perceived sweetness (716). Oral protein and lipid typically produce slower-onset, more sustained increases than does glucose (95, 122, 128, 131, 195, 235, 336, 442, 600, 762). Due to the complex patterns of GLP-1 secretion described below, however, it is unclear whether any macronutrient should be considered to be the most potent GLP-1 secretagogue. Both fasting and glucose-stimulated GLP-1 secretion are pulsatile, with a period of ∼8 min (50); the controls and consequences of this are not known.
GLP-1 levels after mixed-nutrient meals sometimes display biphasic patterns (130, 235, 604), with secondary peaks after 60–120 min. Differences in rates of gastric emptying and other differences in digestibility of the meal components are likely to contribute to this, but are not their sole cause because biphasic patterns also occurred after oral loads of individual nutrients (130, 322).
There may be sex-specific effects on GLP-1 secretion. Oral glucose and mixed-nutrient meals increased GLP-1 levels more in women than men in two studies (763, 805), but not in two others (132, 385). In addition, oral glucose increased GLP-1 levels less during the follicular phase than during the luteal phase, apparently due to slower gastric emptying (101).
Several mechanisms may contribute to the rapid initial GLP-1 response: 1) GLP-1-cells are expressed in the duodenum and proximal jejunum (148, 365, 738, 759), so direct stimulation of GLP-1 secretion occurs as soon as ingesta pass the pylorus. 2) The initial rate of gastric emptying of glucose solutions, especially in fasted subjects, may produce glucose concentrations that exceed the absorption capacity of the proximal small intestine, so that glucose may reach more distal GLP-1 cells within 5–10 min after meals (50, 663). 3) The rate of increase in plasma GLP-1 may exceed the rate of increase of plasma glucose, suggesting that neuroendocrine reflexes may stimulate GLP-1 secretion in addition to direct stimulation of GLP-1 cells by glucose (508).
Intraduodenal-infusion studies, similar to oral-loading studies, indicate that carbohydrate increases plasma GLP-1 levels faster than either protein or lipid (71, 247, 249, 412, 446, 550, 583) and that biphasic responses sometimes occur (446, 583). In addition, 1) for each macronutrient, increasing caloric load increased GLP-1 levels (446, 583, 640). 2) GLP-1 responses were greater during initially faster and subsequently slower nutrient infusions compared with identical loads infused at constant rates, suggesting that increases in the initial intraluminal nutrient load disproportionally increase GLP-1 secretion (136, 550). Because gastric emptying rates are highest initially, this suggests that gastric empting contributes to the control of GLP-1 secretion.
The distal small intestine usually plays the leading role in sustained GLP-1 secretion. This was first indicated by comparisons of GLP-1 secretion and glucose absorption. The threshold intraduodenal glucose infusion rate for sustained increases in plasma GLP-1 was between 1 and 2 kcal/min in two studies (663, 774) and between 2 and 4 kcal/min in another study (583). Because the absorptive capacity of the duodenum and first 25–30 cm of jejunum is ∼0.9–1.4 kcal/min (334, 505, 575), glucose probably reached the more distal jejunum in these studies only when at least ∼1.5 kcal/min glucose was infused, which suggests that stimulation of more distal GLP-1 cells is required to elicit sustained GLP-1 secretion. Two experiments confirm this suggestion. The first (159) involved intraduodenal infusion of 3.5 kcal/min glucose 2 cm distal to the pylorus, aspiration of the intestinal contents 60 cm distal to the pylorus, inflation of an occluding balloon just distal to the aspiration site, and intrajejunal infusion of glucose or saline distal to the occlusion, 75 cm distal to the pylorus. Plasma GLP-1 levels increased when glucose was infused intrajejunally in amounts matching the aspirated glucose, but not when saline was infused intrajejunally. In the second (844), 2 kcal/min glucose was infused either via intraduodenal catheters that ended 12 cm distal to the pylorus or via intrajejunal catheters that ended 50 cm distal to the pylorus; in this condition a balloon was inflated 30 cm distal to the pylorus to exclude reflux. Plasma GLP-1 levels increased markedly more when glucose was infused intrajejunally than intraduodenally. These two experiments demonstrate that small intestinal glucose stimulation ≥50–75 cm distal to the pylorus is necessary for GLP-1 secretion and that small intestinal glucose stimulation <50–60 cm distal to the pylorus is not sufficient for it.
Further observations also attest to the importance of distal small intestinal GLP-1 cells in GLP-1 secretion. 1) Reducing intestinal nutrient transit with hyoscine decreased GLP-1 secretion in response to glucose (137). 2) GLP-1 secretion was increased when carbohydrates were administered with the acarbose, which slows digestion of starch and disaccharides and increases the amounts of carbohydrates reaching the more distal small intestine (274, 601, 680, 784, 785). 3) GLP-1 secretion was increased more by lower glycemic load foods, i.e., less digestible, than by higher glycemic load foods (635), again presumably by increasing the amounts of carbohydrates reaching the more distal small intestine. 4) Delivery of small amounts of lauric acid to the ileum and colon by enteric-coated pellets increased meal-induced GLP-1 secretion (459). 5) GLP-1 responses to intraduodenal infusions of amino acids and fatty acids are slow in onset (71, 718), which, because no digestion is required in these situations, indicates that GLP-1 cells in the proximal small intestine are not sufficient for the responses. 6) That GLP-1 cells are more dense in the distal jejunum and ileum than in the more proximal small intestine in humans (233) also supports the importance of the distal small intestine in GLP-1 secretion.
Many products of digestive hydrolysis directly stimulate GLP-1 secretion via membrane receptors on the apical surfaces of enteroendocrine GLP-1 cells (Table 4, which includes the full and the former names of the nutrient receptors discussed below). Intragastric infusion of lactisole, an inhibitor of the TAS1R2/TAS1R3 sweet receptor, reduced the GLP-1 response to intragastric glucose in humans (275, 717), suggesting that GLP-1 cells express these receptors (228). TAS1R2/TAS1R3 receptors do not appear to be sufficient for GLP-1 secretion, however, because artificial sweeteners that stimulate them had no effect (716, 845, 849). Glucose absorption via the glucose transporters SLC5A1 and SLC2A2 may be required because selective inhibitors of both transporters reduced GLP-1 secretion in animals (461, 519). SLC2A1 and SLC2A5 have also been found on GLP-1 cells (618); the latter suggests a mechanism for the stimulation of GLP-1 secretion by fructose mentioned above.
There is indirect evidence that protein hydrolysis is required for GLP-1 secretion (142, 302, 322, 461, 718, 760). Oligopeptides and amino acids may be sensed by CASR (210, 461). Phenylalanine, tryptophan, asparagine, arginine, and glutamine each stimulated GLP-1 in isolated rat small intestine, and this was abolished in the absence of extracellular Ca2+ or the presence of a CASR inhibitor (461).
As for ghrelin and CCK, the effect of lipids on GLP-1 secretion depends on digestive hydrolysis and the presence of fatty acids with chain length greater than or equal to C12 (71, 249). The free fatty-acid receptors FFAR2, FFAR3, and FFAR4 are densely expressed on distal small intestinal GLP-1 cells (329, 380, 715, 754), which may contribute to the slower-onset, more sustained GLP-1 responses after lipid ingestion. Carbohydrates reaching the large intestine are fermented to short-chain fatty acids, which are sensed by FFAR2 and FFAR3 (206, 617) and contribute to the later phase of GLP-1 secretion (376, 765). For example, oral loads of xylose, a poorly absorbed sugar, led to greater increases in GLP-1 than did glucose from 60 to 180 min after loading (793). GLP-1 cells also express GPR119, which mediates responses to oleoethylamine (151, 420, 618), a long-chain fatty acid derivative formed during absorption.
Nutrients may also stimulate GLP-1 secretion indirectly by increasing bile secretion. Bile acids appear to control GLP-1 secretion in two ways. First, circulating bile acids diffuse into the lamina propria and reach GPBAR1 on the basolateral aspects of GLP-1 cells, which stimulates GLP-1 secretion in mice (105, 564, 761). Second, the nuclear bile-acid receptor FXR appears to inhibit GLP-1 production because treatment with a bile-acid sequestrant improved glucose tolerance and increased ileal GLP-1 expression in wild-type mice, but not Fxr knockout mice (773). The importance of bile acids for human GLP-1 secretion is not clear. 1) Bile acid and GLP-1 responses were correlated in one study (627). 2) Intrajejunal infusion of taurocholic acid did not affect GLP-1 secretion by itself, but increased glucose-stimulated GLP-1 secretion beginning after ∼90 min (841). 3) Intraduodenal infusion of chenodeoxycholic acid had only a small effect on plasma GLP-1 levels (496).
There appear to be several reflexive controls of GLP-1 secretion. 1) The nicotinic cholinergic antagonist trimethaphan did not reduce the early increase in GLP-1 after a mixed-nutrient meal, but did reduce the increases in insulin and pancreatic polypeptide, suggesting that cephalic-phase reflexes did not contribute to the GLP-1 response (17). A preprandial cephalic-phase GLP-1 reflex, however, was demonstrated in rats (180, 786). 2) The muscarinic cholinergic antagonist atropine reduced the GLP-1 response to an oral glucose load (50). 3) Mouse GLP-1 cells express the melanocortin 4 receptor (Mcr4), whose activation increased GLP-1 secretion (561).
The afferent limbs of GLP-1 reflexes may involve gastric-phase signals because intragastric infusion of glucose or a mixed-nutrient solution led to greater plasma GLP-1 levels than matched intraduodenal infusions (719), although it is also possible that the initial rate of gastric emptying may have exceeded the rate of intraduodenal infusions, leading to greater direct stimulation of GLP-1 release. An intestinal-phase reflex appears to contribute to GLP-1 secretion in response to intraduodenal fat infusions because GLP-1 did not increase after CCKAR antagonism (71). Finally, research in rats indicates that vagal signaling contributes importantly to reflexive GLP-1 release (29, 110, 628).
C. Eating
GLP-1 fulfills criterion 3 of Table 2 for an endocrine satiation signal in humans because intravenous infusion of physiological doses of GLP-1 (0.3–0.9 pmol·kg−1·min−1) reduced meal size in the absence of adverse effects in healthy-weight men (199, 255, 299, 301). Test context may affect GLP-1 satiation, however, because a physiological dose failed to inhibit eating in another test (100), and supraphysiological doses (1.2–1.5 pmol·kg−1·min−1 GLP-1) reduced meal size in one (301), but not in another study done under different conditions (454). The site(s) of the GLP-1R mediating this satiating effect is unknown. As explained in section IVC, we conclude that positive results with physiological endocrine doses suggest that GLP-1 acts via an endocrine mode of action, rather than signaling locally, to inhibit eating in these tests.
With respect to criterion 6 of Table 2, GLP-1R antagonism with exendin(9–39) failed to increase test meal size in healthy humans tested under five distinct experimental conditions (492, 721) and in two groups of RYGB patients tested 3–12 mo after surgery, when their mean BMI was ∼34 kg/m2 (737). In contrast, exendin(9–39) did increase test meal size in one of the RYGB-patient groups in a preoperative test (737). Several factors may have contributed to these disparate results. 1) The positive result was obtained with a primed infusion and a higher maintenance dose of exendin(9–39). 2) Meal-related GLP-1 secretion was markedly increased in the patients tested after RYGB, so even the higher exendin(9–39) dose may not have been sufficient. 3) Patients were heavier before RYGB (∼40 kg/m2) than after (∼34 kg/m2), suffered from T2DM, and were receiving insulin treatment (after RYGB only 2 of 12 patients had T2DM, and treatment was not specified). 4) Exendin(9–39) led to unusually high levels of PYY(3–36) in healthy subjects (721) and in patients tested after RYGB, but failed to increase PYY(3–36) in patients tested before RYGB (737).
Intravenous GLP-1 infusions failed to inhibit eating in men with truncal vagotomy and pyloroplasty (588), suggesting that GLP-1 acts in the abdomen to inhibit eating. Many data in rats also support a vagal mechanism (1, 313, 407, 639), although capsaicin lesions of unmyelinated abdominal afferents failed to block GLP-1's eating-inhibitory effect in one study (613).
Additional rat and mouse data also indicate that intestinal GLP-1 acts locally to inhibit eating in these species. 1) Meals failed to increase systemic active GLP-1 levels in rats (598, 691) [although meals did lead to GLP-1 increases in mice (15, 297)]. 2) Infusion of 0.5 nmol/kg GLP-1 via the cranial-mesenteric artery, which supplies much of the small and large intestines, reduced meal size more than identical infusions via the hepatic-portal vein or femoral artery in rats (832). 3) Hepatic-portal vein infusion of 1 nmol/kg GLP-1, which was near the threshold for an eating-inhibitory effect under the conditions tested in rats, increased active GLP-1 in portal-vein plasma to ∼20-fold the maximum level observed after a meal under the same conditions (599, 639). 4) Intraperitoneal injections of an GLP-1-albumin conjugate, which is unlikely to enter the brain, inhibited eating in mice (48). 5) In chow-fed rats, intravenous infusions of GLP-1R antagonists failed to increase eating (387, 638, 832), whereas intraperitoneal injections of GLP-1R antagonists did so in several (37, 828, 831), although not all (3, 179, 832), tests. The inconsistent eating-stimulatory effect of intraperitoneal GLP-1-antagonist administration suggests that locally acting GLP-1 may be an endogenous satiation signal in rats only under particular conditions. Dietary fat content may be one factor that downregulates the eating-inhibitory effect of GLP-1 (224, 595, 831).
Animal studies have also identified a number of brain sites where GLP-1 may act to inhibit eating, including the area postrema, NTS, lateral parabrachial nucleus, ventral tegmental area, paraventricular nucleus of the hypothalamus, and nucleus accumbens (23, 219, 499, 500, 599, 619, 620, 652). Because the data reviewed above suggest that GLP-1 does not increase in the systemic circulation after meals in rats and that intestinal GLP-1 does not control eating in rats via an endocrine mechanism, however, these sites are probably physiologically stimulated by neuronal GLP-1 in rats, which originates in neurons in the caudal brain stem that project to all the areas listed above and more (450, 623). Because postprandial GLP-1 levels are relatively high in the systemic circulation in humans, it is possible that endocrine GLP-1 does affect these brain areas in humans. GLP-1 appears to enter the brain by simple diffusion (381). Finally, rat studies indicate that GLP-1 signaling in the NTS affects satiation in part by modulating the processing of signals related to gastric fill (312, 806), although whether endocrine or neurocrine GLP-1 is involved is unclear.
GLP-1 may contribute to postprandial satiety as well as to satiation. 1) As described above, GLP-1 levels are often increased for several hours postprandially. 2) In a test of healthy-weight subjects who consumed fixed-size, high-fat, low-carbohydrate test meals (276), plasma GLP-1 levels 60–180 min later were correlated with both hunger ratings and the size of meals offered at 180 min (neither correlation was present 0–60 min after the fixed-size meals). 3) In rats, chronic intrajejunal infusions of linoleic acid increased GLP-1 levels and selectively reduced meal frequency, and intraperitoneal exendin(9–39) infusion reversed the reduction in meal frequency (179).
GLP-1 may affect eating in other ways. 1) GLP-1 may contribute to flavor hedonics (24, 212, 499, 699, 827). Many of the effects on rats' eating produced by central GLP-1 manipulations described above related to “hedonic eating,” and the effects of GLP-1 on human fMRI responses to pictures of foods also occurred in brain areas related to the generation of hedonic judgments (788). 2) GLP-1 signaling in the caudal brain stem (624) and in the amygdala (388) may be involved in the aversive control of eating in rats. 3) GLP-1 may have physiological roles in thirst and in sodium and water homeostasis (298, 485), which may influence eating under some conditions. Future research should carefully consider these possibilities.
There are two interesting distinctions between the eating-inhibitory effects of chronic GLP-1 treatments in animals versus in humans. 1) The site of action in humans is unknown. Chronic GLP-1-agonist treatments that produce weight loss in rats do so by acting centrally (379, 677, 698), whereas the long-lasting GLP-1 agonist liraglutide had low uptake into the cerebrospinal fluid in humans (150). 2) Visceral illness is not a serious side effect of GLP-1-agonist treatment in humans (335, 414, 581), but reliably accompanies the reductions in food intake and body weight produced by chronic GLP-1-agonist treatment in rats (379). GLP-1 agonist-induced illness in rats is apparently mediated by a subset of central GLP-1R sites (378). Better understanding of these phenomena is important for advancing GLP-1 obesity pharmacotherapy.
D. Glycemic Control
GLP-1 appears to contribute to meal-related glycemic control by stimulating insulin secretion, inhibiting glucagon secretion, slowing gastric emptying, and reducing hepatic glucose metabolism (128, 222, 336, 653). GLP-1 may also contribute to glycemic control in the fasting state. The latter is suggested by recent studies employing pancreatic clamps, i.e., somatostatin infusion and glucagon and insulin replacement, that suggest that GLP-1 reduces endogenous glucose production during the fasting state in both metabolically healthy individuals (594) and those with T2DM (679). Studies in mice suggest that these effects are due in part to an insulin-independent effect GLP-1R in the hepatic portal vein or liver (117).
GLP-1, together with GIP, mediates the incretin effect by exerting dose-related, glucose-dependent insulinotropic effects on β-cells (128, 223, 336, 406). Infusions of physiological endocrine doses of GLP-1 are sufficient to increase insulin secretion in fasting subjects and during glucose infusions (406, 537, 803). Furthermore, a physiological dose of GLP-1 infused during isoglycemic glucose infusions, i.e., infusions leading to identical glycemic profiles as oral glucose, reproduced the insulin response to oral glucose (537). These data indicate that GLP-1 meets criterion 3 of Table 2 for a physiological endocrine incretin effect. Infusion of the GLP-1R antagonist exendin(9–39) decreased insulin secretion after oral glucose loads, after meals, during intraduodenal glucose infusions, and during hyperglycemic glucose clamps (546, 650, 664, 721), indicating that GLP-1 meets also criterion 6 for a physiological endocrine incretin effect.
Additional important aspects of the incretin effect in metabolically healthy individuals include 1) comparisons of insulin or C-peptide secretion in response to oral glucose or isoglycemic intravenous glucose infusions indicate that the incretin effect increases with increasing glucose loads and, thus, limits meal-related glucose excursions even after large glucose loads (47, 490, 539). 2) GLP-1 synergizes with GIP to increase insulin secretion (537, 803), but 3) intraduodenal glucose infusions at rates in the physiological range of gastric emptying of glucose solutions indicated that GIP was the predominant incretin during infusion of ≤2 kcal/min glucose, whereas GLP-1 predominated during infusion of either 3 or 4 kcal/min glucose (471, 774).
Although glucose stimulated GLP-1 and GIP secretion normally in patients with T2DM (47, 121, 460, 542), the incretin effect was diminished (47, 128, 223, 472). This apparently reflects a decrease in the β-cell response to GLP-1 and, more importantly, a near lack of response to GIP (332, 392, 476, 491, 493, 538, 764, 842). As a result, GLP-1 may contribute relatively more to the incretin effect in T2DM patients than in metabolically healthy persons (835). The defect in the incretin effect in T2DM is reversible: improving glucose levels in patients with T2DM for only 4 wk improved C-peptide secretion in response to both GLP-1 and GIP infusions markedly (332). Thus GLP-1 agonists hold great promise for the treatment of T2DM. GLP-1-based therapy, however, may not be advantageous for all T2DM patients. For example, the discovery of gene polymorphisms that affect incretin hormone secretion and action in T2DM patients (527) and that affect GLP-1's insulinotropic effect in healthy individuals (657) suggest that defects in incretin function may be a primary pathophysiology in some patients.
GLP-1's glucagonostatic action also contributes to meal-related glycemic control. Physiological infusions of 0.25–0.4 pmol·kg−1·min−1 GLP-1 inhibited glucagon secretion and reduced glucose levels in both healthy subjects and T2DM patients (309, 352), and exendin(9–39) markedly elevated glucagon secretion and increased glucose levels in healthy subjects (230, 546, 721).
As described in section IIC, GLP-1 slows gastric emptying, which also improves glycemic control (448, 541, 659, 834). Comparisons of the relative contributions of GLP-1's diverse effects on glycemia suggest that its glucagonostatic and gastric-emptying inhibitory effects are more important than its insulinotropic effect in healthy subjects (541, 546). Several aspects of GLP-1's effect on GI motility are relevant for the treatment of T2DM. 1) The slowing of gastric emptying by exogenous GLP-1 displayed tachyphylaxis during sustained (>24 h) GLP-1 infusions in healthy subjects (540, 781). 2) Shorter-acting GLP-1 agonists have a more sustained effect on gastric emptying and thereby reduce meal-related glycemia more than longer-acting GLP-1 agonists (577). 3) A short-acting GLP-1 agonist also reduced duodenal motility and flow, suggesting an additional mechanism by which GLP-1 may reduce meal-related glycemia (756).
Additional mechanisms also may contribute to GLP-1's glycemic effects. A study in truncally vagotomized human subjects indicated that the vagus is involved in GLP-1's effect on GI glucose disposal, but not in its incretin effect, although a limitation was that the subjects had pyloroplasty together with vagotomy (587). This effect of GLP-1 on glucose disposal may involve GLP-1R in the hepatic-portal vein, which animal studies indicate activate neural reflexes that increase glucose clearance without affecting insulin secretion (117, 355, 548). The role of these reflexes in normal meal-related glucose control remains unclear. Mice with global deletion of GLP-1R in which GLP-1R were selectively reconstituted in the pancreas displayed normal oral and intraperitoneal glucose tolerance (417), suggesting the endocrine mechanism is sufficient and neural and other mechanisms are not necessary. Other data suggest that brain GLP-1R also may contribute GLP-1's glycemic effects: 1) GLP-1 infusions into the third cerebral ventricle increased insulin secretion in rats and mice (393, 652) and decreased gastric-emptying rates in rats (354); 2) exendin(9–39) infusions into the third cerebral ventricle increased muscle glucose utilization independent of insulin signaling in mice (393); and 3) GLP-1 infusions directly into the Arc reduced hepatic glucose production in rats (652). Whether these central effects reflect actions of enteroendocrine GLP-1 or neural GLP-1, however, is unclear.
E. Obesity
The peak and AUC of meal-stimulated GLP-1 secretion were reduced in several studies of obese persons (6, 130, 458, 497, 603, 798), although not all (245, 681, 794). These decreases appear to be secondary to obesity because meal-stimulated GLP-1 secretion increased to near that of healthy-weight individuals in obese patients following weight loss (from mean BMI of 39 to 33 kg/m2) achieved by caloric restriction (798). Obesity does not appear to influence GLP-1's incretin effect because the β-cell response to GLP-1 infusion during hyperglycemic clamps increased in proportion to insulin resistance in individuals without T2DM identically in healthy-weight and morbidly obese subjects (42). Postprandial circulating bile-acid levels are reduced in obesity (570), which may contribute to the reduction in GLP-1 secretion.
A polymorphism in GLP1R at rs2268641 was associated with BMI in a European-American sample, although it accounted for <0.3% of the variance (430), suggesting that defects in GLP-1 signaling contribute to obesity risk in some individuals. Tests of GLP-1's effects on eating in children who are at high familial risk for obesity before they become hyperphagic and overweight (see Ref. 726) would be useful in analyzing this.
Intravenous infusion of physiological doses of GLP-1 inhibited eating in obese subjects in three studies (256, 300, 534) and failed to do so in one, apparently underpowered study (535). Although these studies did not include nonobese control groups, an across-study analysis of 72 normal-weight and 43 overweight and obese subjects, some with T2DM, failed to detect any change in the dose-effect relations of infusions of 0.4–1.5 pmol·kg−1·min−1 GLP-1 (796). As described in section VC, exendin(9–39) increased test meal size in morbidly obese patients with T2DM, but no longer did so after RYGB (737).
F. RYGB
Meal-related GLP-1 secretion is substantially increased after RYGB (94, 310, 574, 846). Although fasting GLP-1 levels are usually unchanged, meal-related GLP-1 increases within 2 days of surgery, increases progressively for at least 6 mo, and persists apparently indefinitely. As described in section IIG, increased gastric empting may account for these increases in GLP-1 secretion when highly digestible nutrients enter the Roux limb rapidly, as in the case of oral glucose challenges (544). Increases in meal-related GLP-1 secretion may contribute to the beneficial effects of RYGB on eating, body weight, and glycemic regulation: 1) the magnitude of meal-related GLP-1 responses has been associated with weight loss (214, 425) and remission of T2DM (533); and 2) patients bearing the MC4R variant I251L lost more weight after RYGB (502), which may reflect increased GLP-1 secretion (469).
Tests of acute somatostatin treatment support the involvement of GLP-1 and PYY(3–36) on RYGB's eating effects. 1) Somatostatin treatment decreased meal-related GLP-1 and PYY(3–36) levels, decreased fullness rating during a test meal, and increased test-meal size in RYGB patients; it was not established that these effects were specific to RYGB, however, as no unoperated control subjects were tested (191, 425). 2) Somatostatin failed to affect pre-meal hunger ratings but did increase progressive-ratio responding for chocolate sweets in RYGB patients more than it did in control subjects, an effect the authors interpreted in terms of hedonics rather that satiation (283). 3) In a rat study, somatostatin injection appeared to increase eating more in RYGB than in control animals, although the difference was not tested statistically (251). In view of the myriad effects of somatostatin, whether these effects were due to changes in GLP-1, PYY(3–36), or other effects is uncertain. Similarly, simultaneous antagonism of GLP-1R with exendin(9–39) and inhibition of PYY(3–36) synthesis with sitagliptin (Januvia or Sitagliptin, Merck, Kenilworth, NJ) increased test meal size ∼20% in RYGB patients, although neither treatment alone affected test meal size (737). These data suggest that GLP-1 and PYY(3–36) have a synergistic satiating action after RYGB. Again, however, it was not established that the effect of the combination treatment was increased by RYGB because no unoperated control subjects were tested.
Studies of the role of GLP-1 in RYGB in rodents produced mainly negative results. 1) In RYGB rats, acute administration of exendin(9–39) increased eating in one test (3), but not in two others (37, 477). 2) The GLP-1R agonist exendin-4 had comparable effects in RYGB and sham-operated rats (251). 3) Meals increased systemic GLP-1 levels in RYGB rats (691), suggesting that brain GLP-1R may mediate RYGB's eating-inhibitory effect. But chronic infusions of exendin(9–39) into the lateral cerebral ventricle increased food intake and weight gain similarly in RYGB and sham-operated rats, suggesting that central GLP-1R are not involved in the effects of RYGB (848). Unfortunately, the effects of peripheral GLP-1 were not assessed, so it was possible that peripheral GLP-1 signaling contributed to the observed effects of RYGB. 4) RYGB had comparable weight-loss and eating-inhibitory effects in mice with transgenic deletions of GLP-1R (Glp1r−/−) or Gnat3 (α-Gust−/−), which do not secrete GLP-1 in response to oral glucose, and wild-type mice (507, 848). Thus the preponderance of evidence from animal models argues strongly against the hypothesis that GLP-1 contributes importantly to the eating-inhibitory and weight-loss effects of RYGB (the Glp1r−/− and α-Gust−/− models are discussed further below).
In marked contrast to the eating data, there is compelling evidence that GLP-1 contributes in several ways to the beneficial effects of RYGB on glycemic regulation in humans. The clearest evidence is that exendin(9–39) markedly reduced insulinemia and increased glycemia after consumption of mixed-nutrient meals or glucose solutions tested 1 wk to 5 yr after RYGB (371, 375, 648–650, 684, 736). This also occurred in RYGB patients with T2DM (371, 375). Additionally, exendin(9–39) increased glucagon secretion (375, 650, 736) and accelerated gastric emptying rate in one study (684), although not another (650). In contrast to these increased effects of GLP-1, RYGB did not appear to increase the contribution of GIP to meal-related glycemic control (736). A dual-isotope glucose-tracing study indicated that GLP-1 was not involved in the reduction of endogenous glucose production and the increase in glucose disposal after RYGB (375). Increased GLP-1 secretion after RYGB also contributed to the development of meal-related hyperinsulinemic hypoglycemia in some patients (647–649; for reviews of these and related data, see Refs. 468, 646, 653). It is also important to note that reduced eating also contributes importantly to the improvements in glycemic control after RYGB (361, 363, 415).
Exendin(9–39) treatment also reversed the improvements in glucose tolerance and insulin secretion after RYGB in a rat model (138). There is a disconnect, however, between the demonstrations with exendin(9–39) of the importance of GLP-1 for glycemic regulation after RYGB and a report (507) that Glp1r−/− and α-Gust−/− mice with RYGB and wild-type RYGB mice had similar glucose tolerance, insulin tolerance, and glucose-stimulated insulin release [α-Gust−/− mice, which do not secrete measureable GLP-1, were used to test the role of GLP-1's degradation products GLP-1(9–36)amide and GLP-1(28–36)amide, which may improve glucose homeostasis via GLP-1R-independent mechanisms]. The resolution of this apparent paradox is unclear. It is possible that there are important species differences. In addition, because these were germline transgenic animals, mechanisms compensating for the lack of GLP-1 signaling may have developed during the animals' maturation. In any case, these data provide an important challenge to the human literature.
RYGB increases meal-stimulated circulating bile-acid levels (157, 570, 740), which might contribute to RYGB-induced increases in GLP-1 secretion and to RYGB's therapeutic effects. Consistent with this hypothesis, meal-stimulated bile-acid and GLP-1 responses were associated in several studies done 4 mo or more after RYGB (396, 566, 668, 820). But meal-stimulated bile-acid levels did not increase in tests 1 wk, 1 mo, or 3 mo after RYGB (16, 720). Thus, because meal-related GLP-1 levels are markedly increased at these times, any contribution of elevated bile-acid levels to GLP-1 secretion or the effects of RYGB are likely to be late-developing mechanisms. Finally, mouse models support the hypothesis that changes in bile acids contribute to the effects of bariatric surgery. 1) Diversion of bile to the ileum increased circulating bile acids 10-fold and led to decreases in food intake, glycemia, and body weight that were similar to those produced by RYGB and appeared to be at least in part independent of fat malabsorption; unfortunately, the role of GLP-1 was not assessed (257). 2) A transgenic mouse-model study (642) implicated FXR in the efficacy of vertical-sleeve gastrectomy; again, the importance of GLP-1 was not assessed. Furthermore, although vertical-sleeve gastrectomy increased circulating bile acids in mice (529), the human data are mixed (157).
G. Summary
GLP-1 is secreted in response to the products of carbohydrate, lipid, and protein digestion. It may act as an endocrine satiation signal in healthy humans, but antagonist studies have not yet confirmed this. Intestinal and brain GLP-1 also may have other effects on ingestive behavior, but these are not established in humans. Intestinal GLP-1 reduces meal-related increases in glycemia by stimulating insulin secretion (i.e., acting as an incretin), by inhibiting glucagon secretion, by slowing gastric emptying, and, perhaps, other effects. The effects of GLP-1 on eating and glycemic control are summarized in Figure 10. Defects in GLP-1 secretion or signaling may contribute to overeating in obesity. Although GLP-1's effectiveness in glycemic control decreases in individuals with insulin resistance or T2DM, it remains a crucial contributor, and GLP-1 agonists are already in use in T2DM and obesity therapy. GLP-1 contributes to improved glycemic control after RYGB; its role in eating after RYGB is unclear.
FIGURE 10.
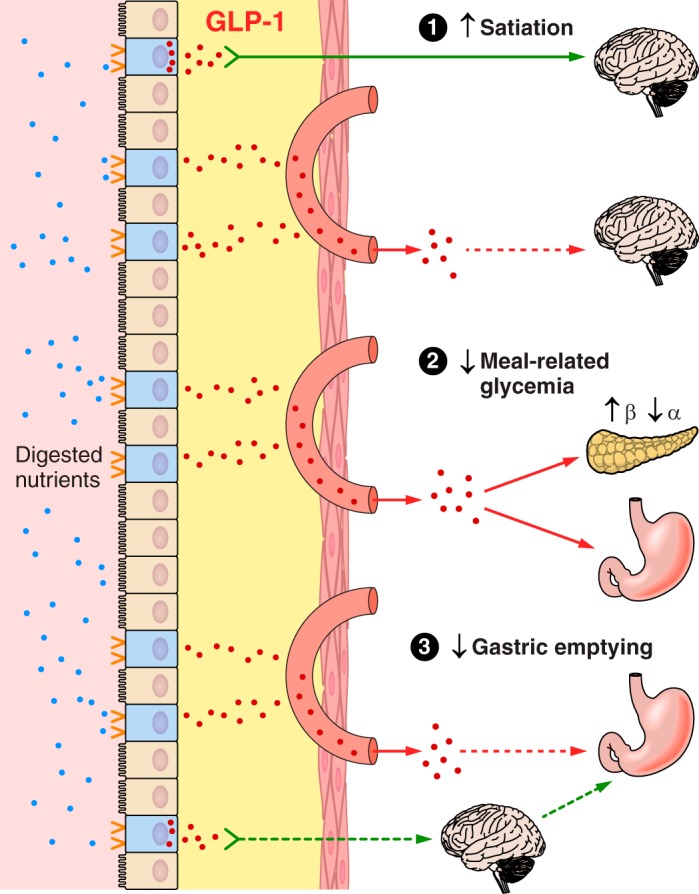
Some features of GLP-1 physiology. GLP-1 secretion is stimulated by the digestive products of all three macronutrients acting on nutrient receptors on the apical aspects of enteroendocrine GLP-1 cells (blue) dispersed in the epithelial layer (tan) of the small intestinal mucosa. GLP-1 acts in an endocrine mode by diffusing through the lamina propria (yellow) and into intestinal capillaries (salmon) to reach distant target organs (red arrows), or acts locally. 1) GLP-1 stimulates satiation. Data in rats indicate GLP-1 signals satiation via a local action on vagal afferents (green arrow) in the lamina propria. GLP-1 may also act in the brain to affect satiation or postprandial satiety. 2) GLP-1 improves meal-related glycemia by increasing pancreatic β-cell insulin secretion in a glucose-dependent manner, by inhibiting pancreatic α-cell glucagon secretion, and by inhibiting gastric emptying; all three appear to be endocrine effects of GLP-1. 3) GLP-1 slows gastric emptying via a direct endocrine effect and perhaps via a vagal-vagal reflex. Solid lines indicate well established effects, and dashed lines indicate less well established effects.
VI. PYY(3–36)
A. Introduction
Endocrine PYY is synthesized and secreted by open-type enteroendocrine cells (Figure 6B). Enteroendocrine PYY cells are located predominately in the distal small intestine and colon and often coexpress and secrete GLP-1 and, less frequently, CCK, GIP, neurotensin, or secretin (8, 10, 25, 231, 303, 597, 738, 742). Plasma PYY is a mix of PYY(1–36), the secreted form, PYY(3–36), the active endocrine form, which results from cleavage of the tyrosine-proline residue from the NH2-terminal of PYY(1–36) by DPP-4 in the lamina propria (296, 451), capillary endothelial cells, liver and blood (51, 289, 488), and some breakdown fragments (768, 769). As discussed in section IB2, mouse intestinal PYY cells appear to release some PYY from axon-like cytoplasmic extensions, or neuropods, that end in close apposition to glial cells of the enteric nervous system (92, 93) . PYY is also expressed in the endocrine pancreas, where PYY(1–36) may have paracrine intraislet actions (573). Finally, PYY is expressed by neurons in the gigantocellular reticular nucleus of the rostral medulla, which have widespread central projections (280).
PYY(1–36) activates several neuropeptide Y-family receptors, including NPY1R (or Y1R), NPY2R, NPY4R, and NPY5R, whereas PYY(3–36) is selective for NPY2R (120, 226, 697, 807). NPY2R are expressed throughout the body, including in several brain regions, the GI tract, and vagal afferents.
B. Secretion
Difficulties in assaying PYY(3–36) complicate studies of its physiology (467, 768, 769). Therefore, the plasma levels described here refer to total PYY. Plasma PYY levels generally begin to increase ∼15–30 min after meals, reach maxima ∼60–90 min after meals, and remain elevated for several hours so that morning fasting levels are not reached until several hours after evening meals (51, 61, 64, 198, 241, 276, 326, 453, 713). Morning fasting PYY levels are typically 10–20 pM, and peak levels after moderate-size meals are ∼15–30 pM (10, 198, 276). Postprandial PYY and GLP-1 profiles are often dissimilar because DPP-4 activates PYY, but inactivates GLP-1 (233, 520), and because, at least in rodents, the enteroendocrine cells that express PYY are located more distally than those that express GLP-1 (597, 738).
Orally ingested lipids lead to larger and more sustained elevations in plasma PYY than glucose ingestion (10, 103, 241, 276, 319, 462); the relative effect of protein is less clear (10, 64, 317, 319, 787). The PYY effects in these studies were relatively variable, possibly reflecting assay variability, differences in gastric emptying, time of day (317), test-food digestibility, or subject variables, including differences in DPP-4 activity (467).
Lipid-induced PYY secretion is dependent on hydrolysis and fatty-acid chain length greater than or equal to C12 (197, 250). Amino acids stimulated PYY release (718), but whether PYY release requires protein hydrolysis has not been assessed directly. Acarbose increased PYY levels after a mixed-nutrient meal (265), suggesting that secretion is increased when carbohydrates reach the distal small intestine or proximal colon, where the densities of PYY cells are higher. This suggestion is consistent with the increased PYY levels in patients with dumping syndrome, tropical sprue, small-intestinal resection, or RYGB (12–14, 467, 574).
The stimulation of enteroendocrine PYY secretion via membrane nutrient receptors has been less extensively studied than for ghrelin, CCK, and GLP-1 (Table 4, which includes the full and the former names of the nutrient receptors discussed below). Carbohydrate (glucose in most studies) appears to stimulate PYY secretion in part via stimulation of TAS1R1/TAS1R3 sweet receptors (228, 275, 717), but because equally sweet artificial sweeteners did not trigger PYY release, other mechanisms must be also involved (461, 716, 732, 849). Whether FFAR1 or FFAR4 contributes to PYY secretion has not been studied to our knowledge, but given that many enteroendocrine cells produce both GLP-1 and PYY (304), it is likely that they do so. CASR appears to contribute to the stimulation of PYY secretion by oligopeptides and amino acids because their effects on PYY secretion in isolated loops of rat small intestine were reduced by a CASR inhibitor and dependent on extracellular Ca2+ (461).
Neurohumoral reflexes also appear to contribute to PYY release, especially its early phase (51, 227, 586). 1) Intravenous CCK infusions increased plasma PYY in humans (102). 2) GLP-1 infusion decreased (104) and exendin(9–39) infusion increased (230, 721) PYY secretion, perhaps reflecting an autoregulatory mechanism in GLP-1/PYY cells. 3) Bile acids may be an important mediator, particularly of lipid-stimulated PYY secretion (9, 11, 569, 840). 4) Animal studies implicated vasoactive intestinal polypeptide and the vagus nerve in PYY secretion (9, 51–53, 264, 685). 5) Some mouse PYY cells express Mc4r, which appears to facilitate PYY secretion (561).
C. Eating
The eating-inhibitory effect of peripheral PYY is mediated by PYY(3–36) (62, 144, 172, 676). 1) Intravenous infusion of PYY(3–36) inhibited eating ∼10-fold more potently than infusions of PYY(1–36) in rats and, comparing across experiments, ∼4- to 8-fold more potently in humans (69, 198, 701). 2) Central administration of PYY(1–36) stimulated eating in rats.
Potential roles of PYY(3–36) in both satiation and postprandial satiety have been investigated. Four studies (62, 194, 423, 701) modeled PYY(3–36)'s postprandial satiety effect using intermeal infusions that began after standard meals (62, 194, 423, 701) or after an overnight fast (701) and ended before the test meal. 1) In one test, infusion of a supraphysiological dose of 0.8 pmol·kg−1·min−1 PYY(3–36) for 90 min increased peak plasma PYY(3–36) concentration from ∼8 to ∼44 pM and reduced the size of a buffet meal presented 2 h after the infusion ended (62). The authors concluded that this was a physiological effect because in a previous study, meals of 530, 870, and 4500 kcal increased PYY to from ∼8–10 pM to ∼12, ∼25, and ∼55 pM, respectively (10). 2) In another test, the threshold for a significant decrease in meal size was 0.7 pmol·kg−1·min−1 PYY(3–36), although infusion of 0.5 and 0.6 pmol·kg−1·min−1 PYY(3–36) increased fullness and tended to decrease eating. These infusions increased peak plasma PYY to ∼45–60 pM, more than the ∼40 pM produced by a 3000 kcal meal in the same study (423). 3) Infusion of lower PYY(3–36) doses, 0.2–0.3 pmol·kg−1·min−1, failed to affect subsequent meal size (194, 701). Thus doses of PYY(3–36) that inhibited eating in these studies increased plasma PYY levels more than all but extremely large meals, suggesting that the effects of PYY(3–36) on postprandial satiety do not meet criterion 3 of Table 2 for a physiological endocrine dose. This conclusion is consistent with the report (276) that the 3 h total PYY AUC after high-carbohydrate, low-fat breakfasts or low-carbohydrate, high-fat breakfasts were not significantly correlated with the sizes of the following lunches, even though ghrelin and GLP-1 AUC after each breakfast were significantly correlated with lunch sizes. In addition, although adverse effects were not reported when 0.8 pmol·kg−1·min−1 PYY(3–36) was infused following standard meals (62, 194, 423), when infused in fasting subjects, this dose elicited “severe malaise or nausea” in half the subjects in two studies (701, 768). These data suggest that criterion 5 of Table 2, that the effect of PYY(3–36) on satiety effect be selective, requires further testing. In addition, in all these studies the test meals were offered at fixed times; thus future tests in which subjects are asked to report when they wish to initiate meals may reveal effects of PYY(3–36) on the duration of the intermeal interval, which is hypothesized to be under the control of postprandial satiety processes (see sect. IB1).
Only one study of the satiating effect of intrameal PYY(3–36) infusions has been reported (198). This revealed 1) the threshold dose for a significant reduction in meal size was between 0.2 and 0.4 pmol·kg−1·min−1 PYY(3–36); 2) infusion of 0.2 and 0.4 pmol·kg−1·min−1 PYY(3–36) increased plasma total PYY from the fasting level of ∼10 to ∼25 and ∼31 pM, respectively, which was more than the level of ∼13 pM achieved after a 1500 kcal mixed-nutrient meal; and 3) 0.4 pmol·kg−1·min−1 PYY(3–36) often led to nausea. These data suggest that the eating-inhibitory effects of intra-meal PYY(3–36) infusions in this experiment were pharmacological, rather than physiological, and were due in part to aversive effects, i.e., they failed to meet criteria 3 and 5 of Table 2. Furthermore, because only slightly supraphysiological intravenous doses of PYY(3–36) elicited illness and because such doses presumably elicit infraphysiological paracrine levels of PYY(3–36) in the lamina propria, it is difficult to see how PYY(3–36) could have a physiological paracrine action on eating. Perhaps, however, current methods either fail to mimic a crucial aspect of endogenous PYY(3–36) dynamics or fail to provide some aspect of normal meals that prevents endogenous PYY(3–36) from provoking illness.
Studies in animals do not strongly support a physiological role for PYY(3–36) in eating. Intramuscular injections of PYY(3–36) reduced eating in monkeys, but how the doses administered compared with endogenous levels was unclear (517). In one study, hepatic-portal infusions of PYY(3–36) inhibited eating in rats without signs of illness (710), but systemic intravenous infusions of PYY(3–36) that inhibited eating in rats did produce illness (143, 144), as did intraperitoneal injections of PYY(3–36) in mice (306). Interestingly, however, intravenous infusions of a peripherally acting NPY2R antagonist reduced the eating-inhibitory effects of intravenous infusions of PYY(3–36) and of smaller, but not larger, intragastric loads of protein and fat in rats (614), suggesting that PYY(3–36) fulfills criterion 6 of Table 2 under some conditions. Infusion of the NPY2R antagonist by itself failed to increase eating, however, which fails to provide support for criterion 6 of Table 2. Studies of mice with transgenic deletions of Pyy also provide only weak support for a role in eating (441). In one Pyy−/− mouse line, male mice were hyperphagic and females were not tested (64); in another, females but not males were hyperphagic (88); and in two others no eating phenotype was detected (669, 838). Ectopic Pyy overexpression in adult mice failed to affect body weight, but slightly decreased eating after a 24 h fast (686). As none of these transgenic methods discriminated between effects of PYY(1–36) and PYY(3–36), it is possible that more refined molecular genetic tools will provide more useful information. Finally, although knockout of peripheral NPY2R in mice increased eating under some conditions, it appeared that this was secondary to metabolic effects (687, 851).
Whether PYY(3–36) acts peripherally or centrally to inhibit eating is unclear. In support of peripheral action, 1) subdiaphragmatic vagotomy reduced or abolished the eating-inhibitory effect of peripherally administered PYY(3–36) in rats (56, 395), 2) conjugating PYY(3–36) to albumin to prevent it from crossing the blood-brain barrier reduced its eating-inhibitory potency (56), and 3) an NPY2R antagonist that does not cross the blood-brain barrier blocked the eating-inhibitory effect of peripherally administered PYY(3–36) (614). In support of central action, 1) injections of PYY(3–36) directly into the Arc reduced eating in rats (2), 2) injections of an NPY2R antagonist into the Arc reduced the eating-inhibitory effect of peripherally administered PYY(3–36) in rats (2), 3) PYY(3–36) inhibited eating in vagotomized mice (306), and 4) PYY(3–36) inhibited eating in rats with capsaicin lesions of unmyelinated abdominal afferents (613).
In conclusion, present data fail to support the hypothesis that PYY(3–36) physiologically inhibits eating in humans or animals. Further efforts to determine whether PYY(3–36) infusions that better model the dynamics of human and animal PYY secretion around meals have physiological eating-inhibitory effects are required to determine whether PYY(3–36) is a plausible candidate physiological satiation or postprandial satiety signal. Studies of antagonism of PYY(3–36)-NPY2R signaling are also necessary, but specific antagonists for human use are not available. Furthermore, as described in section IB2, some PYY is apparently released from neuropods (92, 93), and analysis of the potential effects of this is beyond available physiological methods. Finally, Batterham and colleagues (64, 467) hypothesized that PYY is involved in protein-induced satiety and in exercise-induced anorexia, which have not been extensively tested, and in food reward, which has been tested in a number of human functional brain imaging studies that we do not review (63, 194, 818).
D. Glycemic Control
There is little evidence that PYY(3–36) affects insulin secretion in humans. Infusion of 1 or 5 pmol·kg−1·min−1 PYY(3–36) failed to affect the insulin response to a bolus intravenous glucose infusion in fasting women (19), and PYY(3–36) infusions during meals failed to increase plasma insulin levels (61, 62), except when the doses elicited illness (701, 834).
In contrast, animal studies suggest that PYY affects glycemic regulation in two apparently opposing ways. 1) PYY(3–36) may indirectly stimulate nutrient-induced insulin secretion in rodents. PYY(3–36) reduced postprandial glycemia without affecting fasting glycemia, an effect mimicked by a NPY2R agonist, and this was blocked by peripheral, but not central, administration of a NPY2R antagonist (140, 467). This effect of PYY(3–36) appeared to be mediated by GLP-1 because exendin(9–39) blocked the glucose-lowering effects of PYY(3–36) (140, 467). 2) PYY(1–36) may directly inhibit insulin secretion via a paracrine mode of action. In mice, PYY is expressed in pancreatic α- and δ-cells, Npy1r and Npy4r, but not Npy2r, are expressed in β-cells, and PYY(1–36), but not PYY(3–36), dose-dependently reduced glucose-stimulated insulin release from β-cells in vitro (116, 140, 467, 573, 651, 790). Furthermore, this was absent in cells derived from Pyy−−/− or Npy1r−/− mice, and both mutants were hyperinsulinemic (88, 116).
PYY may contribute to glycemic regulation via two further actions. 1) Endocrine intestinal PYY(3–36) may improve insulin sensitivity, at least under some conditions, because intravenous infusion of PYY(3–36) increased glucose uptake in muscle and adipose tissue of high-fat fed mice during a hyperinsulinemic-euglycemic clamp (790). 2) Paracrine pancreatic PYY(1–36) may tonically stimulate islet-cell proliferation and inhibit β-cell apoptosis in mice (573, 651). 3) Any decrease in gastric emptying produced by PYY(3–36) may lead to reductions in glycemia.
Interestingly, oral fat loads and mixed-nutrient meals appear to stimulate less PYY secretion in patients with T2DM (238, 252, 856). To investigate whether this precedes T2DM, Viardot et al. (801) compared subjects with strong family histories of T2DM with subjects matched for insulin sensitivity, age, and BMI, but without family histories of T2DM. PYY responses to high-carbohydrate meals were impaired in the subjects at risk for T2DM, suggesting that defective PYY secretion may be causally linked with T2DM.
E. Obesity
The relationship between obesity and PYY secretion is unclear. 1) Although several studies detected decreases in fasting total PYY levels in obese patients (61, 64, 423, 633, 856), other similarly powered studies did not (333, 385, 576, 727, 794). 2) Weight reduction was reported to increase fasting total PYY in obese children (633), but to decrease it in obese adults (576), although in both studies the weight and PYY changes were small. 3) Postprandial PYY secretion was reduced in obese patients in six studies (61, 64, 422, 423, 497, 727, 856), but not in four others (103, 333, 385, 794). 4) In the one comparison of PYY(3–36)'s eating-inhibitory effect in obese and healthy-weight subjects to date, no difference was detected (61).
F. RYGB
There are several reports that postprandial plasma PYY levels increase at various times after RYGB, with little or no change in fasting levels (39, 213, 310, 425, 846). As yet, however, there is a dearth of knowledge concerning the time courses or physiological consequences of these increases. For example, in one study (94), 3 h AUC of PYY after a 420 kcal mixed-nutrient meal was not significantly increased until 3 mo after RYGB, whereas in another (574), both maximum postprandial PYY levels and 3 h PYY AUC after 424 kcal mixed-nutrient meals were increased more 1 wk postoperatively than 3 or 12 mo postoperatively.
We know of only one test of the eating-inhibitory effect of PYY(3–36) in RYGB patients, in which inhibition of PYY(3–36) synthesis with sitagliptin failed to increase test meal size (737). But somatostatin treatment (191, 425) and simultaneous exendin(9–39) and sitagliptin treatment (737) both increased test meal size in RYGB patients, suggesting that GLP-1 and PYY(3–36) synergize to decrease eating after RYGB (these data were reviewed in sect. VF). Because neither test included unoperated control subjects, whether RYGB increased the effects is uncertain.
Two additional rodent studies of PYY(3–36)'s involvement in RYGB produced mixed results: 1) chronic infusions of a NPY2R antagonist into the lateral cerebral ventricle failed to affect food intake or weight gain in either RYGB or sham-operated rats (848). Although this fails to support a role for central NPY2R signaling in the effects of RYGB, it should be noted that the test was done 5 mo after surgery, when RYGB and sham-operated animals were eating similar amounts. It is possible that the outcome may have been different if tested when RYGB reduced eating. 2) Pyy−/− mice lost less weight than wild-type mice during the initial 10 days after surgical bypass of the duodenum and proximal jejunum; unfortunately, food intakes were not reported (141). Finally, as noted in section VIF, increases in PYY(3–36) secretion in patients bearing the MC4R variant I251L (469) could explain their better weight-loss outcomes after RYGB (502).
G. Summary
PYY(3–36) is secreted in response to the products of carbohydrate, lipid, and protein digestion during and after meals. As summarized in Figure 11, PYY(3–36) may contribute to gastric emptying via the ileal brake mechanism, to the inhibition of eating, and to the control of meal-related glycemia, but the evidence that these are physiological actions remains thin. Similarly, PYY(3–36)'s role in RYGB remains unclear. This modest progress may be due in part to the difficulties of PYY(3–36) research, including the low threshold for eliciting illness with PYY(3–36) infusions, the lack of NPY2R antagonists for human use, and the possibility of neuropod PYY signaling.
FIGURE 11.
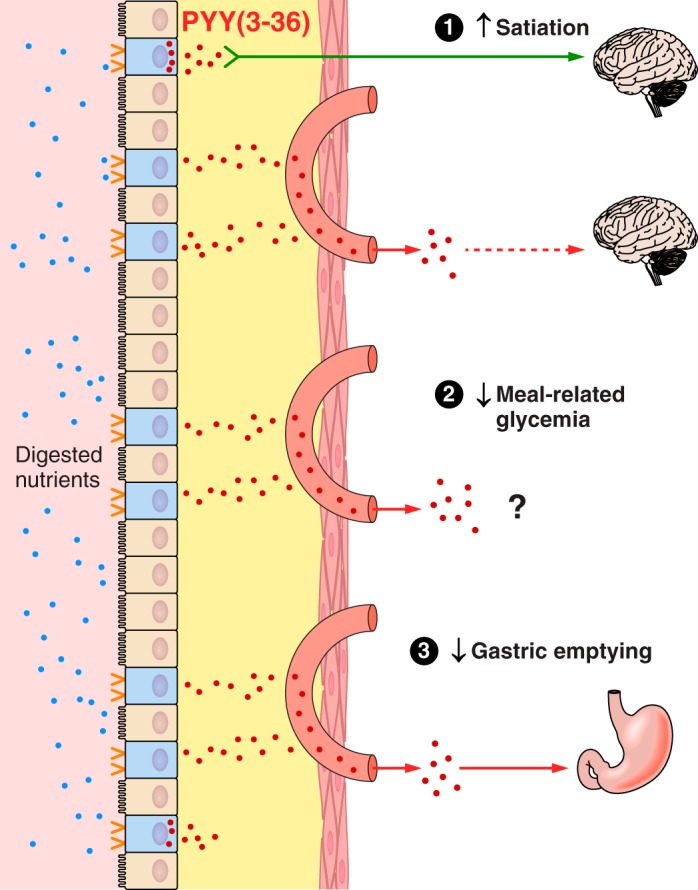
Some features of PYY(3–36) physiology. PYY secretion is stimulated by the digestive products of all three macronutrients acting on nutrient receptors on the apical aspects of enteroendocrine PYY cells (blue) dispersed in the epithelial layer (tan) of the small-intestinal mucosa. PYY is transformed into PYY(3–36) beginning in the lamina propria. PYY(3–36) acts in an endocrine mode by diffusing through the lamina propria (yellow) and into intestinal capillaries (salmon) to reach distant target organs (red arrows), or acts locally. 1) The role of PYY(3–36) in eating is uncertain. It may inhibit eating via a local action on vagal afferents (green arrow) in the lamina propria or by acting directly in the brain. 2) Whether PYY(3–36) improves meal-related glycemic control is uncertain. 3) PYY(3–36) appears to slow gastric emptying via a direct endocrine effect on the stomach; whether vagal-vagal reflexes contribute is unknown. Solid lines indicate well established effects, and dashed lines indicate less well established effects.
VII. DISCUSSION
The act of eating sets in motion an intricately coordinated series of GI responses that, via central and peripheral influences, contribute importantly to the control of eating and meal-related glycemia. The control of secretion of GI hormones by small-intestinal nutrient sensing is a cornerstone of these functions. The hormones control exposure of the small intestine to nutrients via their effects on GI motility, especially gastric emptying, and thereby modulate their own secretion. Here we reviewed the nutrient-secretory controls and contributions to eating, meal-related glycemia, and GI motility of ghrelin, CCK, GLP-1, and PYY(3–36), in healthy-weight and obese humans as well as in RYGB patients. The primary focus was on normal endogenous or “physiological” endocrine function in humans because currently available methods make its determination feasible (Table 2).
As Table 5 indicates, despite considerable research effort, at present there are many more questions than answers regarding ghrelin, CCK, GLP-1, and PYY(3–36) as physiological endocrine signals in humans in the functions reviewed. Indeed, only CCK has been fully established as a physiological endocrine control of eating and only GLP-1 as a physiological endocrine control of meal-related glycemia in healthy-weight individuals. There is incomplete support for endocrine roles of CCK in meal-related glycemia and gastric emptying, for GLP-1 in eating and gastric emptying, and for PYY(3–36) in gastric emptying in humans. Moreover, animal research fills only a few of the gaps indicated in Table 5 (an important exception is that GLP-1R antagonism does increase eating in rats under many conditions; see sect. VD).
Table 5.
Physiological status of ghrelin, CCK, GLP-1, and PYY(3–36) in the endocrine control of eating, GI motility, and meal-related glycemic control in healthy humans
| Eating PD/A | GI Motility* PD/A | Meal-Related Glycemic Control PD/A | |
|---|---|---|---|
| Ghrelin | ?/? | ?/? | ?/? |
| CCK | YES/YES | Yes/Yes† | YES/No |
| GLP-1 | YES/No‡ | Yes/Yes | YES/YES |
| PYY(3–36) | No/? | Yes/? | ?/? |
Experimental support for the two cardinal criteria of physiological endocrine function, i.e., the “physiological-dose” criterion (PD) and “antagonism” criterion (A), is rated as convincing (YES), partial (Yes), negative (No), or unknown (?) for each hormone and function. See text for details and references.
GI motility refers to gastric emptying and small-intestinal motor function.
Cholecystokinin (CCK) antagonism slowed gastric emptying of liquid food, but not solid food.
In view of this decidedly modest estimation of the state of proof of physiological endocrine function for ghrelin, CCK, GLP-1, and PYY(3–36), one may question whether the criteria for physiological function (Table 2) are overly rigorous or whether the criterion-based approach is misguided. The answer to each question is no. As reflected in Table 1, criteria for endocrine function have evolved in step with advances in understanding and methodology during the century-plus history of endocrinology and have shaped the logical and programmatic course of endocrinology and its contributions to medical diagnosis and treatment (59, 292, 449, 489, 610, 833). That knowledge at each stage is hard won is not a criticism of the strategy. Rather, criteria for physiological function should continue to guide GI hormone research. Identifying truly physiological endocrine functions of GI hormones can only facilitate understanding of eating, GI motor function, and meal-related glycemic control and development of therapies for their disorders.
Perhaps the most pressing issue facing ghrelin, CCK, GLP-1, and PYY(3–36) physiology is the need to determine the roles of non-endocrine, i.e., local, signaling, which has been implicated in several of the effects reviewed. The need to develop methods enabling tests of local-signaling hypotheses against the criteria of Table 2 is especially urgent. As mentioned in section IVD, intraintestinal hormone infusions might selectively target the lamina propria (147), and hormone concentrations in the lamina propria can be estimated from assays of lymph. The temporal resolution of lymph assays, however, is poor due to its slow flow. Nor have the results of tests of meal-related hormone changes in the lymph been straightforward. For example, post-meal concentrations of GLP-1 were reported to be ∼6-fold higher in lymph than in hepatic portal-vein plasma in rats (178) and ∼8-fold higher in lymph than in orbital-plexus plasma in mice (553), but ∼10-fold lower in lymph in hepatic portal-vein plasma in swine (308).
An additional, related challenge for GI-hormonal physiology is to encompass the emerging picture of integrated hormonal and electric signaling in the GI tract. Electrically excitable GI cells form what Bohórquez and Liddle (91) call the gut connectome, comprised of enteroendocrine cells, neurons and glia of the enteric nervous system, intrinsic GI neurons, and peripheral ganglia innervating the GI tract. Finally, a third challenge is to better link GI-hormonal physiology to the study of information processing in the brain, as understood using functional imaging methods in humans and neuropharmacological and molecular-genetic methods in animals. Present progress in humans is limited largely to studies of the telencephalic mechanisms of food hedonics, which have been reviewed elsewhere (references are given in sect. IA). Advances in the spatial resolution of functional imaging methods should allow this kind of work to address the diencephalic and brain-stem mechanisms that are clearly crucial for the effects of GI hormones on other aspects of eating as well as for the control of GI motility and metabolic function. Increased back-and-forth translation between human and animal studies should also accelerate progress in unraveling central mechanisms mediating the functions of ghrelin, CCK, GLP-1, and PYY(3–36).
Future research should also widen the range of designs used in GI hormone physiology. Studies of meal size and timing in particular have used remarkably similar experimental approaches, which may contribute to some of the negative data reviewed. As discussed in section IB2, few GI hormone-infusion studies have interrogated parameters other than peak plasma levels. Studies of rate ascent of plasma hormone concentration, the timing of the infusion in relation to the course of the meal or intermeal interval, or other parameters may unveil physiological effects or explain some apparent paradoxes, such as why apparently physiological doses of PYY(3–36) elicit illness (see sect. VID). Similarly, few studies address synergistic effects (268). Gastric volume may synergize with pharmacological doses of CCK or GLP-1 to elicit satiation (see sect. IID), but whether this reflects a physiological synergy has not been studied. Synergy analyses may also illuminate some failures of antagonist effects. For example, failures of GLP-1R antagonism to increase eating in humans may be due to increases in glucagon or PYY(3–36) rather than the absence of a satiating effect of GLP-1 (see sect. VD), and multi-antagonist approaches may provide useful tests of this hypothesis. Adaptation to consumption of particular food types can affect gastric emptying (see sect. IIA) and, presumably, other GI hormone-mediated responses. Finally, experience eating leads to learning of several types (e.g., Refs. 68, 80, 111, 155, 188, 321, 602, 675, 723, 813). Such learning may overshadow unconditioned GI-hormonal effects, despite the fact that these were probably the basis for the learning in the first place. A hypothetical example is shown in Figure 12. In sum, both improved methods and more varied experimental approaches are likely to enlarge the current restricted view of the contributions of ghrelin, CCK, GLP-1, and PYY(3–36) to eating and meal-related glycemic control.
FIGURE 12.
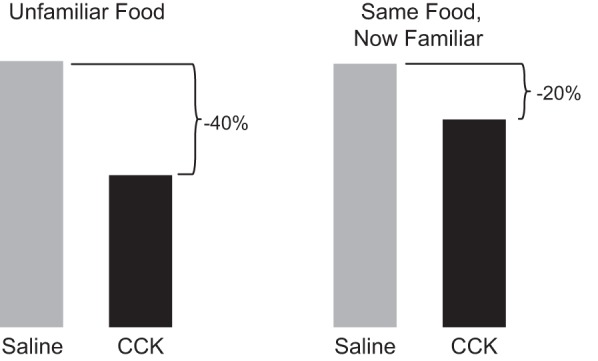
A thought experiment depicting how learning may influence the control of eating by GI hormones. Left: when an individual is served a palatable but unfamiliar food, meal size is determined mainly by unconditioned satiation signals related to gastric volume, CCK and GLP-1 secretion, etc., as discussed in the review. Under these conditions, CCK infusion during the meal might exert its full unconditioned effect, indicated by the 40% reduction in meal size. Right: if the same individual is tested after extensive experience eating the test food, meal size might be the same as initially, but will now be under the control of conditioned responses such as expected satiation (111), portion-size estimation (629), etc., that override unconditioned signals, and because conditioned eating controls are resistant to physiological feedback, the same CCK infusion might now reduce meal size less, indicated by the 20% effect.
We find little evidence that pathophysiology of ghrelin, CCK, GLP-1, or PYY(3–36) function contributes to obesity, with the exception of rare individuals bearing genetic polymorphisms. But the extremely heterogeneous nature of human obesity should caution against a strong interpretation of these negative data. The participants in obesity studies often have wide ranges in several variables that may influence the GI responses under study, including BMI range, sex, age, race, duration of obesity, age of obesity onset, pattern of adipose-tissue distribution (intra-abdominal, abdominal subcutaneous, gluteo-femoral subcutaneous, etc.), gustatory capacity, dietary habits (large meals, snacking, habitual levels of sugar and fat intake, etc.), eating traits (dietary restraint, binge-eating propensity, etc.), and a host of additional psychological traits (in addition to the discussion of these in the preceding sections, see Refs. 32, 38, 40, 60, 498, 522, 706, 729, 817). Although studies with sufficient power to resolve the influence of such a farrago of factors are rare, positive results (e.g., Ref. 5) encourage the view that the issues are tractable. Alternatively, one may isolate and study specific subsets of individuals. One approach to this is exemplified by the study of de Krom et al. (192) of obese individuals who habitually took unusually large meals or unusually frequent snacks. A number of multivariate subgroup-analysis methods also can be used to search for reliable variation in the absence of a phenotypic or genetic starting point (384). One may also search directly for consistent individual differences in responses to GI hormones. This can be done using the repeated-randomization design, in which trials are repeated in the same individuals to identify subgroups with consistently larger or smaller responses (455), which then can be used in mechanistic follow-up studies. Although not a focus here, synergistic interactions involving GI hormones, such as those among CCK, GLP-1, amylin, and leptin (775, 776), are promising platforms for development of obesity therapies. Similarly, the expanding roster of molecular nutrient sensors that control secretion of multiple GI hormones (Table 4) seems to present especially attractive targets. Finally, as mentioned in several sections, the potential of GI hormones in obesity pharmacotherapy does not depend on whether or not they contribute to obesity pathophysiology.
RYGB and related bariatric-surgery procedures substantially decrease ghrelin secretion and increase CCK, GLP-1, and PYY(3–36) secretion, especially in the first months after surgery. But whether these changes mediate the procedures' therapeutic effects remains uncertain, with the exception of the contribution of increased GLP-1 secretion to improved meal-related glycemia after RYGB (see sect. VF) or vertical-sleeve gastrectomy (678). In particular, extensive tests of GLP-1's role in reduced eating after RYGB in animals have failed to produce positive evidence. Some promising effects (see sect. VF) suggest that several hormones may contribute synergistically to the reduction in eating after RYGB, but this remains to be tested.
In conclusion, although research aimed at understanding the physiological effects of GI hormones in humans is expensive, technically demanding, and labor-intensive, it should remain a high priority. Animal research indicates that local signaling plays a key role in the effects of GI hormones. Although there are presently few methods to study the physiology of such effects, emerging technologies for miniaturization, telemetry, and molecular-genetic methods applicable to humans (209, 416, 428, 559, 816) may soon create new opportunities. These, as well as more sophisticated testing designs, should be exploited to expand basic physiological knowledge and to help meet the continuing challenges of the epidemics of obesity and T2DM.
GRANTS
R. E. Steinert was supported by a Mary Overton Early Career Fellowship, C. Feinle-Bisset by a Senior Research Fellowship from the National Health & Medical Research Council of Australia (Grant 627002), L. Asarian by U.S. National Institutes of Health Grant DK-092608, and C. Beglinger by Swiss National Science Foundation Grant 320030-132960/1.
DISCLOSURES
R. E. Steinert is employed by DSM Nutritional Products. There is no conflict of interest with regard to the content of the paper.
ACKNOWLEDGMENTS
We thank Timothy H. Moran, Johns Hopkins University School of Medicine; Gerard P. Smith, Weill Medical College of Cornell University; Stephen C. Woods, University of Cincinnati; Guillaume de Lartigue, John B. Pierce Laboratory; and Richard L. Young and Tongzhi Wu, Discipline of Medicine, The University of Adelaide, for helpful comments.
Address for reprint requests and other correspondence: R. E. Steinert, DSM Nutritional Products Ltd., R&D Human Nutrition and Health, Basel, Switzerland (e-mail: re.steinert@gmail.com).
REFERENCES
- 1.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res : 127–131, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res : 139–144, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Abegg K, Schiesser M, Lutz TA, Bueter M. Acute peripheral GLP-1 receptor agonism or antagonism does not alter energy expenditure in rats after Roux-en-Y gastric bypass. Physiol Behav : 70–78, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest : 3229–3239, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, O'Neill J, Eckert D, Zinsmeister AR. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology : 537–546, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adam TC, Westerterp-Plantenga MS. Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. Br J Nutr : 845–851, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Adolph EF. Early concepts of physiological regulations. Physiol Rev : 737–770, 1961. [DOI] [PubMed] [Google Scholar]
- 8.Adrian TE, Bacarese-Hamilton AJ, Smith HA, Chohan P, Manolas KJ, Bloom SR. Distribution and postprandial release of porcine peptide YY. J Endocrinol : 11–14, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Adrian TE, Ballantyne GH, Longo WE, Bilchik AJ, Graham S, Basson MD, Tierney RP, Modlin IM. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut : 1219–1224, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology : 1070–1077, 1985. [DOI] [PubMed] [Google Scholar]
- 11.Adrian TE, Gariballa S, Parekh KA, Thomas SA, Saadi H, Al Kaabi J, Nagelkerke N, Gedulin B, Young AA. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia : 2343–2347, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Adrian TE, Long RG, Fuessl HS, Bloom SR. Plasma peptide YY (PYY) in dumping syndrome. Dig Dis Sci : 1145–1148, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Adrian TE, Savage AP, Bacarese-Hamilton AJ, Wolfe K, Besterman HS, Bloom SR. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology : 379–384, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Adrian TE, Savage AP, Fuessl HS, Wolfe K, Besterman HS, Bloom SR. Release of peptide YY (PYY) after resection of small bowel, colon, or pancreas in man. Surgery : 715–719, 1987. [PubMed] [Google Scholar]
- 15.Ahlkvist L, Vikman J, Pacini G, Ahren B. Synergism by individual macronutrients explains the marked early GLP-1 and islet hormone responses to mixed meal challenge in mice. Regul Pept : 29–35, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes : 1553–1559, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahrén B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes : 1030–1038, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Ahren B, Holst JJ, Efendic S. Antidiabetogenic action of cholecystokinin-8 in type 2 diabetes. J Clin Endocrinol Metab : 1043–1048, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Ahren B, Larsson H. Peptide YY does not inhibit glucose-stimulated insulin secretion in humans. Eur J Endocrinol : 362–365, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, Tada H, Miura K, Shimizu A, Fukushima M, Yokode M, Tanaka K, Kangawa K. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol : 447–455, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Akkary E, Sidani S, Boonsiri J, Yu S, Dziura J, Duffy AJ, Bell RL. The paradox of the pouch: prompt emptying predicts improved weight loss after laparoscopic Roux-Y gastric bypass. Surg Endosc : 790–794, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Al Massadi O, Pardo M, Roca-Rivada A, Castelao C, Casanueva FF, Seoane LM. Macronutrients act directly on the stomach to regulate gastric ghrelin release. J Endocrinol Invest : 599–602, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ. Glucagon-like Peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology : 2233–2243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology : 647–658, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali-Rachedi A, Varndell IM, Adrian TE, Gapp DA, Van Noorden S, Bloom SR, Polak JM. Peptide YY (PYY) immunoreactivity is co-stored with glucagon-related immunoreactants in endocrine cells of the gut and pancreas. Histochemistry : 487–491, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Allen JM, Fitzpatrick ML, Yeats JC, Darcy K, Adrian TE, Bloom SR. Effects of peptide YY and neuropeptide Y on gastric emptying in man. Digestion : 255–262, 1984. [DOI] [PubMed] [Google Scholar]
- 27.Alsalim W, Omar B, Pacini G, Bizzotto R, Mari A, Ahren B. Incretin and islet hormone responses to meals of increasing size in healthy subjects. J Clin Endocrinol Metab : 561–568, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, Sanz C, Vazquez P, Maldonado A, de Caceres J, Desco M, Pozo MA, Blazquez E. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem : 798–806, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology : 2420–2426, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Antoniou AJ, Raja S, El-Khouli R, Mena E, Lodge MA, Wahl RL, Clarke JO, Pasricha P, Ziessman HA. Comprehensive radionuclide esophagogastrointestinal transit study: methodology, reference values, and initial clinical experience. J Nucl Med : 721–727, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Aprahamian CJ, Tekant G, Chen M, Yagmurlu A, Yang YK, Loux T, Harmon CM. A rat model of childhood diet-induced obesity: Roux-en-Y gastric bypass induced changes in metabolic parameters and gastric peptide ghrelin. Pediatr Surg Int : 653–657, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Ard J, Cannon A, Lewis CE, Lofton H, Vang Skjoth T, Stevenin B, Pi-Sunyer X. Efficacy and safety of liraglutide 30 mg for weight management are similar across races: subgroup analysis across the SCALE and phase II randomized trials. Diabetes Obes Metab : 430–435, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arkwright JW, Blenman NG, Underhill ID, Maunder SA, Spencer NJ, Costa M, Brookes SJ, Szczesniak MM, Dinning PG. A fibre optic catheter for simultaneous measurement of longitudinal and circumferential muscular activity in the gastrointestinal tract. J Biophotonics : 244–251, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci : 11052–11060, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arosio M, Ronchi CL, Beck-Peccoz P, Gebbia C, Giavoli C, Cappiello V, Conte D, Peracchi M. Effects of modified sham feeding on ghrelin levels in healthy human subjects. J Clin Endocrinol Metab : 5101–5104, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology : 337–345, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Asarian L, Abegg K, Geary N, Schiesser M, Lutz TA, Bueter M. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology : 325–327, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol : R1215–R1267, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashrafian H, le Roux CW. Metabolic surgery and gut hormones: a review of bariatric entero-humoral modulation. Physiol Behav : 620–631, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Atalayer D, Astbury NM. Anorexia of aging and gut hormones. Aging Dis : 264–275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aulinger BA, Bedorf A, Kutscherauer G, de Heer J, Holst JJ, Goke B, Schirra J. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes (T2D) utilizing DPP-4 inhibition and GLP-1-receptor blockade. Diabetes : 1079–1092, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Aulinger BA, Vahl TP, Wilson-Perez HE, Prigeon RL, D'Alessio DA. β-Cell sensitivity to GLP-1 in healthy humans is variable and proportional to insulin sensitivity. J Clin Endocrinol Metab : 2489–2496, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azpiroz F, Feinle-Bisset C, Grundy D, Tack J. Gastric sensitivity and reflexes: basic mechanisms underlying clinical problems. J Gastroenterol : 206–218, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Babkin BP. Pavlov—a Biography. Chicago, IL: Univ. of Chicago Press, 1949. [Google Scholar]
- 45.Bacarese-Hamilton AJ, Adrian TE, Bloom SR. Distribution and heterogeneity of immunoreactive cholecystokinin (CCK) in the mucosa of the porcine gastrointestinal tract. Regul Pept : 289–298, 1984. [DOI] [PubMed] [Google Scholar]
- 46.Bach Knudsen KE, Jorgensen H, Canibe N. Quantification of the absorption of nutrients derived from carbohydrate assimilation: model experiment with catheterised pigs fed on wheat- or oat-based rolls. Br J Nutr : 449–458, 2000. [PubMed] [Google Scholar]
- 47.Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsbøll T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab : 737–745, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes : 2492–2500, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Bak MJ, Wewer Albrechtsen NJ, Pedersen J, Knop FK, Vilsboll T, Jorgensen NB, Hartmann B, Deacon CF, Dragsted LO, Holst JJ. Specificity and sensitivity of commercially available assays for glucagon-like peptide-1 (GLP-1): implications for GLP-1 measurements in clinical studies. Diabetes Obes Metab : 1155–1164, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Balks HJ, Holst JJ, von zur Muhlen A, Brabant G. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab : 786–790, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Ballantyne GH. Peptide YY(1–36) and peptide YY(3–36): Part I. Distribution, release and actions. Obes Surg : 651–658, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Ballantyne GH, Goldenring JR, Savoca PE, Kranz HK, Adrian TE, Bilchik AJ, Modlin IM. Cyclic AMP-mediated release of peptide YY (PYY) from the isolated perfused rabbit distal colon. Regul Pept : 117–126, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Ballantyne GH, Longo WE, Savoca PE, Adrian TE, Vukasin AP, Bilchik AJ, Sussman J, Modlin IM. Deoxycholate-stimulated release of peptide YY from the isolated perfused rabbit left colon. Am J Physiol Gastrointest Liver Physiol : G715–G724, 1989. [DOI] [PubMed] [Google Scholar]
- 54.Ballinger A, McLoughlin L, Medbak S, Clark M. Cholecystokinin is a satiety hormone in humans at physiological post-prandial plasma concentrations. Clin Sci : 375–381, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Ballinger AB, Clark ML. l-Phenylalanine releases cholecystokinin (CCK) and is associated with reduced food intake in humans: evidence for a physiological role of CCK in control of eating. Metabolism : 735–738, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Baraboi ED, Michel C, Smith P, Thibaudeau K, Ferguson AV, Richard D. Effects of albumin-conjugated PYY on food intake: the respective roles of the circumventricular organs and vagus nerve. Eur J Neurosci : 826–839, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Baranowska B, Radzikowska M, Wasilewska-Dziubinska E, Roguski K, Borowiec M. Disturbed release of gastrointestinal peptides in anorexia nervosa and in obesity. Diabetes Obes Metab : 99–103, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, Lin YY, Bowers EM, Mukherjee C, Song WJ, Longo PA, Leahy DJ, Hussain MA, Tschop MH, Boeke JD, Cole PA. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science : 1689–1692, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrington EJW. An Introduction to General and Comparative Endocrinology. Oxford, UK: Clarendon, 1963, p. 33–35. [Google Scholar]
- 60.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci : 1137–1148, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med : 941–948, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature : 650–654, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SCR. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature : 106–109, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab : 223–233, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Baum F, Nauck MA, Ebert R, Cantor P, Hoffmann G, Choudhury AR, Schmidt WE, Creutzfeldt W. Role of endogenously released cholecystokinin in determining postprandial insulin levels in man: effects of loxiglumide, a specific cholecystokinin receptor antagonist. Digestion : 189–199, 1992. [DOI] [PubMed] [Google Scholar]
- 66.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol : 325–353, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beardshall K, Frost G, Morarji Y, Domin J, Bloom SR, Calam J. Saturation of fat and cholecystokinin release: implications for pancreatic carcinogenesis. Lancet : 1008–1010, 1989. [DOI] [PubMed] [Google Scholar]
- 68.Beauchamp GK, Moran M. Dietary experience and sweet taste preference in human infants. Appetite : 139–152, 1982. [DOI] [PubMed] [Google Scholar]
- 69.Beglinger C, Degen L. Gastrointestinal satiety signals in humans—physiologic roles for GLP-1 and PYY? Physiol Behav : 460–464, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Beglinger C, Degen L, Matzinger D, D'Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am J Physiol Regul Integr Comp Physiol : R1149–R1154, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Beglinger S, Drewe J, Schirra J, Göke B, D'Amato M, Beglinger C. Role of fat hydrolysis in regulating glucagon-like Peptide-1 secretion. J Clin Endocrinol Metab : 879–886, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature : 368–371, 1983. [DOI] [PubMed] [Google Scholar]
- 73.Bennink R, Peeters M, Van den Maegdenbergh V, Geypens B, Rutgeerts P, De Roo M, Mortelmans L. Comparison of total and compartmental gastric emptying and antral motility between healthy men and women. Eur J Nucl Med : 1293–1299, 1998. [DOI] [PubMed] [Google Scholar]
- 74.Berg C, Lappas G, Wolk A, Strandhagen E, Toren K, Rosengren A, Thelle D, Lissner L. Eating patterns and portion size associated with obesity in a Swedish population. Appetite : 21–26, 2009. [DOI] [PubMed] [Google Scholar]
- 75.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev : 393–428, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc : 478–487, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat : 123–131, 1996. [DOI] [PubMed] [Google Scholar]
- 78.Bhattacharya SK, Andrews K, Beveridge R, Cameron KO, Chen C, Dunn M, Fernando D, Gao H, Hepworth D, Jackson VM, Khot V, Kong J, Kosa RE, Lapham K, Loria PM, Londregan AT, McClure KF, Orr ST, Patel J, Rose C, Saenz J, Stock IA, Storer G, VanVolkenburg M, Vrieze D, Wang G, Xiao J, Zhang Y. Discovery of PF-5190457, a potent, selective, and orally bioavailable ghrelin receptor inverse agonist clinical candidate. ACS Med Chem Lett : 474–479, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology : 3873–3880, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Birch LL, Doub AE. Learning to eat: birth to age 2 y. Am J Clin Nutr : 723S–728S, 2014. [DOI] [PubMed] [Google Scholar]
- 81.Bjorklund P, Laurenius A, Een E, Olbers T, Lonroth H, Fandriks L. Is the Roux limb a determinant for meal size after gastric bypass surgery? Obes Surg : 1408–1414, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil : 1–19, 2007. [DOI] [PubMed] [Google Scholar]
- 83.Blevins JE, Stanley BG, Reidelberger RD. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res : 1–10, 2000. [DOI] [PubMed] [Google Scholar]
- 84.Blom WA, de Graaf C, Lluch A, Stafleu A, Schaafsma G, Hendriks HF. Postprandial ghrelin responses are associated with the intermeal interval in time-blinded normal weight men, but not in obese men. Physiol Behav : 742–748, 2009. [DOI] [PubMed] [Google Scholar]
- 85.Blundell J. Pharmacological approaches to appetite suppression. Trends Pharmacol Sci : 147–157, 1991. [DOI] [PubMed] [Google Scholar]
- 86.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H, Westerterp M. Appetite control: methodological aspects of the evaluation of foods. Obes Rev : 251–270, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boesmans W, Hao MM, Vanden Berghe P. Optical tools to investigate cellular activity in the intestinal wall. J Neurogastroenterol Motil : 337–351, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, Couzens M, Slack K, Dallmann R, Sainsbury A, Herzog H. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia : 1360–1370, 2006. [DOI] [PubMed] [Google Scholar]
- 89.Bohdjalian A, Ludvik B, Guerci B, Bresler L, Renard E, Nocca D, Karnieli E, Assalia A, Prager R, Prager G. Improvement in glycemic control by gastric electrical stimulation (TANTALUS) in overweight subjects with type 2 diabetes. Surg Endosc : 1955–1960, 2009. [DOI] [PubMed] [Google Scholar]
- 90.Bohorquez DV, Liddle RA. Axon-like basal processes in enteroendocrine cells: characteristics and potential targets. Clin Transl Sci : 387–391, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bohorquez DV, Liddle RA. The gut connectome: making sense of what you eat. J Clin Invest : 888–890, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bohorquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One : e89881, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest : 782–786, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg : 210–215, 2006. [DOI] [PubMed] [Google Scholar]
- 95.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab : 2913–2919, 2006. [DOI] [PubMed] [Google Scholar]
- 96.Bowen J, Noakes M, Trenerry C, Clifton PM. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab : 1477–1483, 2006. [DOI] [PubMed] [Google Scholar]
- 97.Boyd KA, O'Donovan DG, Doran S, Wishart J, Chapman IM, Horowitz M, Feinle C. High-fat diet effects on gut motility, hormone, and appetite responses to duodenal lipid in healthy men. Am J Physiol Gastrointest Liver Physiol : G188–G196, 2003. [DOI] [PubMed] [Google Scholar]
- 98.Breen DM, Rasmussen BA, Cote CD, Jackson VM, Lam TK. Nutrient-sensing mechanisms in the gut as therapeutic targets for diabetes. Diabetes : 3005–3013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Breen DM, Yue JT, Rasmussen BA, Kokorovic A, Cheung GW, Lam TK. Duodenal PKC-delta and cholecystokinin signaling axis regulates glucose production. Diabetes : 3148–3153, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brennan IM, Feltrin KL, Horowitz M, Smout AJ, Meyer JH, Wishart J, Feinle-Bisset C. Evaluation of interactions between CCK and GLP-1 in their effects on appetite, energy intake, and antropyloroduodenal motility in healthy men. Am J Physiol Regul Integr Comp Physiol : R1477–R1485, 2005. [DOI] [PubMed] [Google Scholar]
- 101.Brennan IM, Feltrin KL, Nair NS, Hausken T, Little TJ, Gentilcore D, Wishart JM, Jones KL, Horowitz M, Feinle-Bisset C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol : G602–G610, 2009. [DOI] [PubMed] [Google Scholar]
- 102.Brennan IM, Little TJ, Feltrin KL, Smout AJ, Wishart JM, Horowitz M, Feinle-Bisset C. Dose-dependent effects of cholecystokinin-8 on antropyloroduodenal motility, gastrointestinal hormones, appetite, and energy intake in healthy men. Am J Physiol Endocrinol Metab : E1487–E1494, 2008. [DOI] [PubMed] [Google Scholar]
- 103.Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, Feinle-Bisset C. Effects of fat, protein and carbohydrate, and protein load, on appetite, plasma cholecystokinin, peptide YY and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol : G129–G140, 2012. [DOI] [PubMed] [Google Scholar]
- 104.Brennan IM, Otto B, Feltrin KL, Meyer JH, Horowitz M, Feinle-Bisset C. Intravenous CCK-8, but not GLP-1, suppresses ghrelin and stimulates PYY release in healthy men. Peptides : 607–611, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, Gribble FM, Reimann F. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology : 3961–3970, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab : 5083–5086, 2001. [DOI] [PubMed] [Google Scholar]
- 107.Broglio F, Gottero C, Van Koetsveld P, Prodam F, Destefanis S, Benso A, Gauna C, Hofland L, Arvat E, van der Lely AJ, Ghigo E. Acetylcholine regulates ghrelin secretion in humans. J Clin Endocrinol Metab : 2429–2433, 2004. [DOI] [PubMed] [Google Scholar]
- 108.Brolin RE, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg : 782–790, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brown JC, Otte SC. Gastrointestinal hormones and the control of insulin secretion. Diabetes : 782–787, 1978. [DOI] [PubMed] [Google Scholar]
- 110.Brubaker PL, Anini Y. Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can J Physiol Pharmacol : 1005–1012, 2003. [DOI] [PubMed] [Google Scholar]
- 111.Brunstrom JM. Mind over platter: pre-meal planning and the control of meal size in humans. Int J Obes Suppl 1: S9–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bucceri AM, Calogero AE, Brogna A. Gallbladder and gastric emptying: relationship to cholecystokininemia in diabetics. Eur J Intern Med : 123–128, 2002. [DOI] [PubMed] [Google Scholar]
- 113.Bueter M, le Roux CW. Gastrointestinal hormones, energy balance and bariatric surgery. Int J Obes Suppl 3: S35–39, 2011. [DOI] [PubMed] [Google Scholar]
- 114.Buffa R, Capella C, Fontana P, Usellini L, Solcia E. Types of endocrine cells in the human colon and rectum. Cell Tissue Res : 227–240, 1978. [DOI] [PubMed] [Google Scholar]
- 115.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology : 2968–2978, 1996. [DOI] [PubMed] [Google Scholar]
- 116.Burcelin R, Brunner H, Seydoux J, Thorensa B, Pedrazzini T. Increased insulin concentrations and glucose storage in neuropeptide Y Y1 receptor-deficient mice. Peptides : 421–427, 2001. [DOI] [PubMed] [Google Scholar]
- 117.Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes : 1720–1728, 2001. [DOI] [PubMed] [Google Scholar]
- 118.Burton DD, Kim HJ, Camilleri M, Stephens DA, Mullan BP, O'Connor MK, Talley NJ. Relationship of gastric emptying and volume changes after a solid meal in humans. Am J Physiol Gastrointest Liver Physiol : G261–G266, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Burton-Freeman B, Davis PA, Schneeman BO. Interaction of fat availability and sex on postprandial satiety and cholecystokinin after mixed-food meals. Am J Clin Nutr : 1207–1214, 2004. [DOI] [PubMed] [Google Scholar]
- 120.Cabrele C, Beck-Sickinger AG. Molecular characterization of the ligand-receptor interaction of the neuropeptide Y family. J Pept Sci : 97–122, 2000. [DOI] [PubMed] [Google Scholar]
- 121.Calanna S, Christensen M, Holst JJ, Laferrere B, Gluud LL, Vilsboll T, Knop FK. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia : 965–972, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr : 127–139, 2004. [DOI] [PubMed] [Google Scholar]
- 123.Calbet JA, MacLean DA. Role of caloric content on gastric emptying in humans. J Physiol : 553–559, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab : 1319–1324, 2004. [DOI] [PubMed] [Google Scholar]
- 125.Cameron KO, Bhattacharya SK, Loomis AK. Small molecule ghrelin receptor inverse agonists and antagonists. J Med Chem : 8671–8691, 2014. [DOI] [PubMed] [Google Scholar]
- 126.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology : 640–658, 2006. [DOI] [PubMed] [Google Scholar]
- 127.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol : 18–37, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab : 819–837, 2013. [DOI] [PubMed] [Google Scholar]
- 129.Carbonnel F, Lemann M, Rambaud JC, Mundler O, Jian R. Effect of the energy density of a solid-liquid meal on gastric emptying and satiety. Am J Clin Nutr : 307–311, 1994. [DOI] [PubMed] [Google Scholar]
- 130.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahren B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared with lean, nondiabetic men. J Clin Endocrinol Metab : 872–878, 2010. [DOI] [PubMed] [Google Scholar]
- 131.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, Ahren B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab : E779–E784, 2008. [DOI] [PubMed] [Google Scholar]
- 132.Carroll JF, Kaiser KA, Franks SF, Deere C, Caffrey JL. Influence of BMI and gender on postprandial hormone responses. Obesity : 2974–2983, 2007. [DOI] [PubMed] [Google Scholar]
- 133.Castiglione KE, Read NW, French SJ. Food intake responses to upper gastrointestinal lipid infusions in humans. Physiol Behav : 141–145, 1998. [DOI] [PubMed] [Google Scholar]
- 134.Cha R, Marescaux J, Diana M. Updates on gastric electrical stimulation to treat obesity: systematic review and future perspectives. World J Gastrointest Endosc : 419–431, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chaiban JT, Bitar FF, Azar ST. Effect of chronic hypoxia on leptin, insulin, adiponectin, and ghrelin. Metabolism : 1019–1022, 2008. [DOI] [PubMed] [Google Scholar]
- 136.Chaikomin R, Doran S, Jones KL, Feinle-Bisset C, O'Donovan D, Rayner CK, Horowitz M. Initially more rapid small intestinal glucose delivery increases plasma insulin, GIP, and GLP-1 but does not improve overall glycemia in healthy subjects. Am J Physiol Endocrinol Metab : E504–E507, 2005. [DOI] [PubMed] [Google Scholar]
- 137.Chaikomin R, Wu KL, Doran S, Jones KL, Smout AJ, Renooij W, Holloway RH, Meyer JH, Horowitz M, Rayner CK. Concurrent duodenal manometric and impedance recording to evaluate the effects of hyoscine on motility and flow events, glucose absorption, and incretin release. Am J Physiol Gastrointest Liver Physiol : G1099–G1104, 2007. [DOI] [PubMed] [Google Scholar]
- 138.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology : 950–958, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chan JL, Bullen J, Lee JH, Yiannakouris N, Mantzoros CS. Ghrelin levels are not regulated by recombinant leptin administration and/or three days of fasting in healthy subjects. J Clin Endocrinol Metab : 335–343, 2004. [DOI] [PubMed] [Google Scholar]
- 140.Chandarana K, Gelegen C, Irvine EE, Choudhury AI, Amouyal C, Andreelli F, Withers DJ, Batterham RL. Peripheral activation of the Y2-receptor promotes secretion of GLP-1 and improves glucose tolerance. Mol Metab : 142–152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, Viollet B, Andreelli F, Withers DJ, Batterham RL. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes : 810–818, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang J, Wu T, Greenfield JR, Samocha-Bonet D, Horowitz M, Rayner CK. Effects of intraduodenal glutamine on incretin hormone and insulin release, the glycemic response to an intraduodenal glucose infusion, and antropyloroduodenal motility in health and type 2 diabetes. Diabetes Care : 2262–2265, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3–36) on conditioned taste aversion in rats. Peptides : 3193–3201, 2006. [DOI] [PubMed] [Google Scholar]
- 144.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology : 879–888, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology : 2607–2612, 2004. [DOI] [PubMed] [Google Scholar]
- 146.Chen JH, Song GQ, Yin J, Sun Y, Chen JD. Gastric electrical stimulation reduces visceral sensitivity to gastric distention in healthy canines. Auton Neurosci : 16–20, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheung GWC, Kokorovic A, Lam CKL, Chari M, Lam TKT. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab : 99–109, 2009. [DOI] [PubMed] [Google Scholar]
- 148.Cho HJ, Kosari S, Hunne B, Callaghan B, Rivera LR, Bravo DM, Furness JB. Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res : 693–698, 2015. [DOI] [PubMed] [Google Scholar]
- 149.Choi S, Lee M, Shiu AL, Yo SJ, Halldén G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol : G1366–G1375, 2007. [DOI] [PubMed] [Google Scholar]
- 150.Christensen M, Sparre-Ulrich AH, Hartmann B, Grevstad U, Rosenkilde MM, Holst JJ, Vilsboll T, Knop FK. Transfer of liraglutide from blood to cerebrospinal fluid is minimal in patients with type 2 diabetes. Int J Obes : 1651–1654, 2015. [DOI] [PubMed] [Google Scholar]
- 151.Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, Chen R, Jones RM, Behan DP, Leonard J. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology : 2038–2047, 2008. [DOI] [PubMed] [Google Scholar]
- 152.Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol : 1600–1611, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res : 1456–1462, 2003. [DOI] [PubMed] [Google Scholar]
- 154.Cigaina V, Hirschberg AL. Plasma ghrelin and gastric pacing in morbidly obese patients. Metabolism : 1017–1021, 2007. [DOI] [PubMed] [Google Scholar]
- 155.Cohen DA. Neurophysiological pathways to obesity: below awareness and beyond individual control. Diabetes : 1768–1773, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cohen S. “What is physiological?” An Answer! Gastroenterology 66 479–480, 1974. [PubMed] [Google Scholar]
- 157.Cole AJ, Teigen LM, Jahansouz C, Earthman CP, Sibley SD. The influence of bariatric surgery on serum bile acids in humans and potential metabolic and hormonal implications: a systematic review. Curr Obes Rep : 441–450, 2015. [DOI] [PubMed] [Google Scholar]
- 158.Collins PJ, Horowitz M, Cook DJ, Harding PE, Shearman DJ. Gastric emptying in normal subjects–a reproducible technique using a single scintillation camera and computer system. Gut : 1117–1125, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Colombel JF, Sutton A, Chayvialle JA, Modigliani R. Cholecystokinin release and biliopancreatic secretion in response to selective perfusion of the duodenal loop with aminoacids in man. Gut : 1158–1166, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Conigrave AD, Hampson DR. Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. Pharmacol Ther : 252–260, 2010. [DOI] [PubMed] [Google Scholar]
- 161.Conigrave AD, Quinn SJ, Brown EM. l-Amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA : 4814–4819, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Corvilain B, Abramowicz M, Fery F, Schoutens A, Verlinden M, Balasse E, Horowitz M. Effect of short-term starvation on gastric emptying in humans: relationship to oral glucose tolerance. Am J Physiol Gastrointest Liver Physiol : G512–G517, 1995. [DOI] [PubMed] [Google Scholar]
- 163.Cote CD, Zadeh-Tahmasebi M, Rasmussen BA, Duca FA, Lam TK. Hormonal signaling in the gut. J Biol Chem : 11642–11649, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron : 649–661, 2003. [DOI] [PubMed] [Google Scholar]
- 165.Cox JE. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol Regul Integr Comp Physiol : R1390–R1396, 1998. [DOI] [PubMed] [Google Scholar]
- 166.Creutzfeldt W, Gregory RA, Grossman MI, Pearse AGE. Origin, Chemistry, Physiology and Pathophysiology of the Gastrointestinal Hormones. Proc Int Symp Wiesbaden : 95, 1970. [Google Scholar]
- 167.Crum AJ, Corbin WR, Brownell KD, Salovey P. Mind over milkshakes: mindsets, not just nutrients, determine ghrelin response. Health Psychol : 424–429, 2011. [DOI] [PubMed] [Google Scholar]
- 168.Cuber JC, Bernard G, Fushiki T, Bernard C, Yamanishi R, Sugimoto E, Chayvialle JA. Luminal CCK-releasing factors in the isolated vascularly perfused rat duodenojejunum. Am J Physiol Gastrointest Liver Physiol : G191–G197, 1990. [DOI] [PubMed] [Google Scholar]
- 169.Cukier K, Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL, Horowitz M, Feinle-Bisset C. Effect of small intestinal glucose load on plasma ghrelin in healthy men. Am J Physiol Regul Integr Comp Physiol : R459–R462, 2008. [DOI] [PubMed] [Google Scholar]
- 170.Cummings DE, Foster-Schubert KE, Overduin J. Ghrelin and energy balance: focus on current controversies. Curr Drug Targets : 153–169, 2005. [DOI] [PubMed] [Google Scholar]
- 171.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab : E297–E304, 2004. [DOI] [PubMed] [Google Scholar]
- 172.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest : 13–23, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes : 1714–1719, 2001. [DOI] [PubMed] [Google Scholar]
- 174.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med : 1623–1630, 2002. [DOI] [PubMed] [Google Scholar]
- 175.Cunningham KM, Daly J, Horowitz M, Read NW. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut : 483–486, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cunningham KM, Horowitz M, Read NW. The effect of short-term dietary supplementation with glucose on gastric emptying in humans. Br J Nutr : 15–19, 1991. [DOI] [PubMed] [Google Scholar]
- 177.Cushing CC, Benoit SC, Peugh JL, Reiter-Purtill J, Inge TH, Zeller MH. Longitudinal trends in hedonic hunger after Roux-en-Y gastric bypass in adolescents. Surg Obes Relat Dis : 125–130, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.D'Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol : R2163–R2169, 2007. [DOI] [PubMed] [Google Scholar]
- 179.Dailey MJ, Moghadam AA, Moran TH. Jejunal linoleic acid infusions require GLP-1 receptor signaling to inhibit food intake: implications for the effectiveness of Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab : E1184–E1190, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dailey MJ, Stingl KC, Moran TH. Disassociation between preprandial gut peptide release and food-anticipatory activity. Endocrinology : 132–142, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol : 2857–2870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, Shirazi-Beechey SP. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol : G271–G282, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Darcel NP, Liou AP, Tome D, Raybould HE. Activation of vagal afferents in the rat duodenum by protein digests requires PepT1. J Nutr : 1491–1495, 2005. [DOI] [PubMed] [Google Scholar]
- 184.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology : 4255–4261, 2000. [DOI] [PubMed] [Google Scholar]
- 185.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology : 1120–1128, 2002. [DOI] [PubMed] [Google Scholar]
- 186.Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology : 3518–3525, 2005. [DOI] [PubMed] [Google Scholar]
- 187.Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med : 1204–1207, 1987. [PubMed] [Google Scholar]
- 188.Davis JD, Smith GP. Learning to sham feed: behavioral adjustments to loss of physiological postingestional stimuli. Am J Physiol Regul Integr Comp Physiol : R1228–R1235, 1990. [DOI] [PubMed] [Google Scholar]
- 189.De Castro JM, King GA, Duarte-Gardea M, Gonzalez-Ayala S, Kooshian CH. Overweight and obese humans overeat away from home. Appetite : 204–211, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De Giorgio R, Stanghellini V, Ricci Maccarini M, Morselli-Labate AM, Barbara G, Franzoso L, Rovati LC, Corinaldesi R, Barbara L, Go VL. Effects of dietary fat on postprandial gastrointestinal motility are inhibited by a cholecystokinin type A receptor antagonist. Ann NY Acad Sci : 226–231, 1994. [DOI] [PubMed] [Google Scholar]
- 191.De Hollanda A, Casals G, Delgado S, Jimenez A, Viaplana J, Lacy AM, Vidal J. Gastrointestinal hormones and weight loss maintenance following Roux-en-Y gastric bypass. J Clin Endocrinol Metab : 4677–4684, 2015. [DOI] [PubMed] [Google Scholar]
- 192.De Krom M, van der Schouw YT, Hendriks J, Ophoff RA, van Gils CH, Stolk RP, Grobbee DE, Adan R. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes : 276–280, 2007. [DOI] [PubMed] [Google Scholar]
- 193.De Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS One . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JD, Dhillo WS. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab : 700–706, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Deacon CF, Ahren B. Physiology of incretins in health and disease. Rev Diabet Stud : 293–306, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Deane AM, Nguyen NQ, Stevens JE, Fraser RJ, Holloway RH, Besanko LK, Burgstad C, Jones KL, Chapman MJ, Rayner CK, Horowitz M. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab : 215–221, 2010. [DOI] [PubMed] [Google Scholar]
- 197.Degen L, Drewe J, Piccoli F, Grani K, Oesch S, Bunea R, D'Amato M, Beglinger C. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. Am J Physiol Regul Integr Comp Physiol : R1391–R1399, 2007. [DOI] [PubMed] [Google Scholar]
- 198.Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology : 1430–1436, 2005. [DOI] [PubMed] [Google Scholar]
- 199.Degen L, Oesch S, Matzinger D, Drewe J, Knupp M, Zimmerli F, Beglinger C. Effects of a preload on reduction of food intake by GLP-1 in healthy subjects. Digestion : 78–84, 2006. [DOI] [PubMed] [Google Scholar]
- 200.Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut : 299–305, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Delgado-Aros S, Camilleri M, Castillo EJ, Cremonini F, Stephens D, Ferber I, Baxter K, Burton D, Zinsmeister AR. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacologic study. Clin Gastroenterol Hepatol : 997–1006, 2005. [DOI] [PubMed] [Google Scholar]
- 202.Delhanty PJ, van der Lely AJ. Ghrelin and glucose homeostasis. Peptides : 2309–2318, 2011. [DOI] [PubMed] [Google Scholar]
- 203.Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol : 271–285, 2012. [DOI] [PubMed] [Google Scholar]
- 204.Deloose E, Vos R, Corsetti M, Depoortere I, Tack J. Endogenous motilin, but not ghrelin plasma levels fluctuate in accordance with gastric phase III activity of the migrating motor complex in man. Neurogastroenterol Motil : 63–71, 2015. [DOI] [PubMed] [Google Scholar]
- 205.Deloose E, Vos R, Janssen P, Van den Bergh O, Van Oudenhove L, Depoortere I, Tack J. The motilin receptor agonist erythromycin stimulates hunger and food intake through a cholinergic pathway. Am J Clin Nutr : 730–737, 2016. [DOI] [PubMed] [Google Scholar]
- 206.Delzenne N, Blundell J, Brouns F, Cunningham K, De Graaf K, Erkner A, Lluch A, Mars M, Peters HP, Westerterp-Plantenga M. Gastrointestinal targets of appetite regulation in humans. Obes Rev : 234–250, 2010. [DOI] [PubMed] [Google Scholar]
- 207.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut : 179–190, 2014. [DOI] [PubMed] [Google Scholar]
- 208.Dezaki K, Damdindorj B, Sone H, Dyachok O, Tengholm A, Gylfe E, Kurashina T, Yoshida M, Kakei M, Yada T. Ghrelin attenuates cAMP-PKA signaling to evoke insulinostatic cascade in islet beta-cells. Diabetes : 2315–2324, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dhillo WS, Murphy KG, Bloom S. Endocrinology: the next 60 years. J Endocrinol : 7–10, 2006. [DOI] [PubMed] [Google Scholar]
- 210.Diakogiannaki E, Pais R, Tolhurst G, Parker HE, Horscroft J, Rauscher B, Zietek T, Daniel H, Gribble FM, Reimann F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia : 2688–2696, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol : 80–87, 2011. [DOI] [PubMed] [Google Scholar]
- 212.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci : 4812–4820, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dirksen C, Damgaard M, Bojsen-Moller KN, Jorgensen NB, Kielgast U, Jacobsen SH, Naver LS, Worm D, Holst JJ, Madsbad S, Hansen DL, Madsen JL. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil : 346–e255, 2013. [DOI] [PubMed] [Google Scholar]
- 214.Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Jacobsen SH, Clausen TR, Worm D, Hartmann B, Rehfeld JF, Damgaard M, Madsen JL, Madsbad S, Holst JJ, Hansen DL. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes : 1452–1459, 2013. [DOI] [PubMed] [Google Scholar]
- 215.Distrutti E, Azpiroz F, Soldevilla A, Malagelada JR. Gastric wall tension determines perception of gastric distention. Gastroenterology : 1035–1042, 1999. [DOI] [PubMed] [Google Scholar]
- 216.Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes : 8–12, 2012. [DOI] [PubMed] [Google Scholar]
- 217.Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol : 2927–2941, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Doisy EA. Sex Hormones. The Porter Lectures, 1936. Lawrence, KS: Univ. of Kansas Press, 1936. [Google Scholar]
- 219.Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab : E1314–E1320, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Drozdzal M, Segui S, Radeva P, Malagelada C, Azpiroz F, Vitria J. Motility bar: a new tool for motility analysis of endoluminal videos. Comput Biol Med : 320–330, 2015. [DOI] [PubMed] [Google Scholar]
- 221.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Ghatei MA, Small C, Bloom SR. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes : 1130–1136, 2005. [DOI] [PubMed] [Google Scholar]
- 222.Drucker DJ. The biology of incretin hormones. Cell Metab : 153–165, 2006. [DOI] [PubMed] [Google Scholar]
- 223.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet : 1696–1705, 2006. [DOI] [PubMed] [Google Scholar]
- 224.Duca FA, Sakar Y, Covasa M. Combination of obesity and high-fat feeding diminishes sensitivity to GLP-1R agonist exendin-4. Diabetes : 2410–2415, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Duca FA, Zhong L, Covasa M. Reduced CCK signaling in obese-prone rats fed a high fat diet. Horm Behav : 812–817, 2013. [DOI] [PubMed] [Google Scholar]
- 226.Dumont Y, Fournier A, St-Pierre S, Quirion R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31,Pro34]peptide YY and [125I]peptide YY3-36 as selective Y1 and Y2 radioligands. J Pharmacol Exp Ther : 673–680, 1995. [PubMed] [Google Scholar]
- 227.Dumoulin V, Dakka T, Plaisancie P, Chayvialle JA, Cuber JC. Regulation of glucagon-like peptide-1-(7–36) amide, peptide YY, and neurotensin secretion by neurotransmitters and gut hormones in the isolated vascularly perfused rat ileum. Endocrinology : 5182–5188, 1995. [DOI] [PubMed] [Google Scholar]
- 228.Dyer J, Salmon KSH, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans : 302–305, 2005. [DOI] [PubMed] [Google Scholar]
- 229.Edkins JS. The chemical mechanism of gastric secretion. J Physiol : 133–144, 1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes : 86–93, 1999. [DOI] [PubMed] [Google Scholar]
- 231.Egerod KL, Engelstoft MS, Grunddal KV, Nohr MK, Secher A, Sakata I, Pedersen J, Windelov JA, Fuchtbauer EM, Olsen J, Sundler F, Christensen JP, Wierup N, Olsen JV, Holst JJ, Zigman JM, Poulsen SS, Schwartz TW. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology : 5782–5795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Eisen S, Phillips RJ, Geary N, Baronowsky EA, Powley TL, Smith GP. Inhibitory effects on intake of cholecystokinin-8 and cholecystokinin-33 in rats with hepatic proper or common hepatic branch vagal innervation. Am J Physiol Regul Integr Comp Physiol : R456–R462, 2005. [DOI] [PubMed] [Google Scholar]
- 233.Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Goke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest : 283–291, 1992. [DOI] [PubMed] [Google Scholar]
- 234.Elashoff JD, Reedy TJ, Meyer JH. Analysis of gastric emptying data. Gastroenterology : 1306–1312, 1982. [PubMed] [Google Scholar]
- 235.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol : 159–166, 1993. [DOI] [PubMed] [Google Scholar]
- 236.Enc FY, Imeryuz N, Akin L, Turoglu T, Dede F, Haklar G, Tekesin N, Bekiroglu N, Yegen BC, Rehfeld JF, Holst JJ, Ulusoy NB. Inhibition of gastric emptying by acarbose is correlated with GLP-1 response and accompanied by CCK release. Am J Physiol Gastrointest Liver Physiol : G752–G763, 2001. [DOI] [PubMed] [Google Scholar]
- 237.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab : 376–392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.English PJ, Ashcroft A, Patterson M, Dovey TM, Halford JC, Harrison J, Eccleston D, Bloom SR, Ghatei MA, Wilding JP. Fasting plasma peptide-YY concentrations are elevated but do not rise postprandially in type 2 diabetes. Diabetologia : 2219–2221, 2006. [DOI] [PubMed] [Google Scholar]
- 239.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab : 2984, 2002. [DOI] [PubMed] [Google Scholar]
- 240.Erdmann J, Lippl F, Wagenpfeil S, Schusdziarra V. Differential association of basal and postprandial plasma ghrelin with leptin, insulin, and type 2 diabetes. Diabetes : 1371–1378, 2005. [DOI] [PubMed] [Google Scholar]
- 241.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab : 4052–4055, 2007. [DOI] [PubMed] [Google Scholar]
- 242.Eysselein VE, Eberlein GA, Hesse WH, Schaeffer M, Grandt D, Williams R, Goebell H, Reeve JR Jr. Molecular variants of cholecystokinin after endogenous stimulation in humans: a time study. Am J Physiol Gastrointest Liver Physiol : G951–G957, 1990. [DOI] [PubMed] [Google Scholar]
- 243.Eysselein VE, Eberlein GA, Schaeffer M, Grandt D, Goebell H, Niebel W, Rosenquist GL, Meyer HE, Reeve JR Jr. Characterization of the major form of cholecystokinin in human intestine: CCK-58. Am J Physiol Gastrointest Liver Physiol : G253–G260, 1990. [DOI] [PubMed] [Google Scholar]
- 244.Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab : 2227–2235, 2011. [DOI] [PubMed] [Google Scholar]
- 245.Feinle C, Chapman IM, Wishart J, Horowitz M. Plasma glucagon-like peptide-1 (GLP-1) responses to duodenal fat and glucose infusions in lean and obese men. Peptides : 1491–1495, 2002. [DOI] [PubMed] [Google Scholar]
- 246.Feinle C, D'Amato M, Read NW. Cholecystokinin-A receptors modulate gastric sensory and motor responses to gastric distension and duodenal lipid. Gastroenterology : 1379–1385, 1996. [DOI] [PubMed] [Google Scholar]
- 247.Feinle C, O'Donovan D, Doran S, Andrews JM, Wishart J, Chapman I, Horowitz M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol : G798–G807, 2003. [DOI] [PubMed] [Google Scholar]
- 248.Feinle-Bisset C, Patterson M, Ghatei MA, Bloom SR, Horowitz M. Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metab : E948–E953, 2005. [DOI] [PubMed] [Google Scholar]
- 249.Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJPM, Wishart J, Pilichiewicz AN, Rades T, Chapman IM, Feinle-Bisset C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol : R524–R533, 2004. [DOI] [PubMed] [Google Scholar]
- 250.Feltrin KL, Patterson M, Ghatei MA, Bloom SR, Meyer JH, Horowitz M, Feinle-Bisset C. Effect of fatty acid chain length on suppression of ghrelin and stimulation of PYY, GLP-2 and PP secretion in healthy men. Peptides : 1638–1643, 2006. [DOI] [PubMed] [Google Scholar]
- 251.Fenske WK, Bueter M, Miras AD, Ghatei MA, Bloom SR, le Roux CW. Exogenous peptide YY3-36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int J Obes : 379–384, 2012. [DOI] [PubMed] [Google Scholar]
- 252.Fernandez-Garcia JC, Murri M, Coin-Araguez L, Alcaide J, El Bekay R, Tinahones FJ. GLP-1 and peptide YY secretory response after fat load is impaired by insulin resistance, impaired fasting glucose and type 2 diabetes in morbidly obese subjects. Clin Endocrinol : 671–676, 2014. [DOI] [PubMed] [Google Scholar]
- 253.Fieseler P, Bridenbaugh S, Nustede R, Martell J, Orskov C, Holst JJ, Nauck MA. Physiological augmentation of amino acid-induced insulin secretion by GIP and GLP-I but not by CCK-8. Am J Physiol Endocrinol Metab : E949–E955, 1995. [DOI] [PubMed] [Google Scholar]
- 254.Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab : E313–E316, 2003. [DOI] [PubMed] [Google Scholar]
- 255.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest : 515–520, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord : 781–792, 2001. [DOI] [PubMed] [Google Scholar]
- 257.Flynn CR, Albaugh VL, Cai S, Cheung-Flynn J, Williams PE, Brucker RM, Bordenstein SR, Guo Y, Wasserman DH, Abumrad NN. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun : 7715, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab : 1971–1979, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fraser R, Fone D, Horowitz M, Dent J. Cholecystokinin octapeptide stimulates phasic and tonic pyloric motility in healthy humans. Gut : 33–37, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.French SJ, Murray B, Rumsey RD, Sepple CP, Read NW. Is cholecystokinin a satiety hormone? Correlations of plasma cholecystokinin with hunger, satiety and gastric emptying in normal volunteers. Appetite : 95–104, 1993. [DOI] [PubMed] [Google Scholar]
- 261.French SJ, Murray B, Rumsey RD, Sepple CP, Read NW. Preliminary studies on the gastrointestinal responses to fatty meals in obese people. Int J Obes Relat Metab Disord : 295–300, 1993. [PubMed] [Google Scholar]
- 262.Fried M, Erlacher U, Schwizer W, Lochner C, Koerfer J, Beglinger C, Jansenr JB, Lamers CB, Harder F, Bischof-Delaloye A, et al. Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology : 503–511, 1991. [DOI] [PubMed] [Google Scholar]
- 263.Fried M, Schwizer W, Beglinger C, Keller U, Jansen JB, Lamers CB. Physiological role of cholecystokinin on postprandial insulin secretion and gastric meal emptying in man. Studies with the cholecystokinin receptor antagonist loxiglumide. Diabetologia : 721–726, 1991. [DOI] [PubMed] [Google Scholar]
- 264.Fu-Cheng X, Anini Y, Chariot J, Castex N, Galmiche JP, Roze C. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: neural regulation by proximal gut. Pflügers Arch : 571–579, 1997. [DOI] [PubMed] [Google Scholar]
- 265.Fuessl HS, Adrian TE, Uttenthal LO, Bloom SR. Peptide YY in diabetics treated chronically with an intestinal glucosidase inhibitor. Klin Wochenschr : 985–989, 1988. [DOI] [PubMed] [Google Scholar]
- 266.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol : 227–240, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Geary N. Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol Behav : 719–733, 2004. [DOI] [PubMed] [Google Scholar]
- 268.Geary N. Understanding synergy. Am J Physiol Endocrinol Metab : E237–E253, 2013. [DOI] [PubMed] [Google Scholar]
- 269.Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am J Physiol Regul Integr Comp Physiol : R975–R980, 1992. [DOI] [PubMed] [Google Scholar]
- 270.Geary N, Moran TH. Basic science of appetite. In: Comprehensive Textbook of Psychiatry (9th ed), edited by Sadock BJ, Sadock VA, Ruiz P. New York: Wolters Kluwer/Lippincott Williams & Wilkens, 2009, p. 375–387. [Google Scholar]
- 271.Geary N, Moran TH. Basic science of appetite. In: Comprehensive Textbook of Psychiatry (10th ed.), edited by Sadock BJ, Sadock VA, Ruiz P. New York: Wolters Kluwer/Lippincott Williams & Wilkens. In press. [Google Scholar]
- 272.Geeraerts B, Van Oudenhove L, Dupont P, Vanderghinste D, Bormans G, Van Laere K, Tack J. Different regional brain activity during physiological gastric distension compared to balloon distension: a H2 15O-PET study. Neurogastroenterol Motil : 533–e203, 2011. [DOI] [PubMed] [Google Scholar]
- 273.Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr : 592–594, 1988. [DOI] [PubMed] [Google Scholar]
- 274.Gentilcore D, Bryant B, Wishart JM, Morris HA, Horowitz M, Jones KL. Acarbose attenuates the hypotensive response to sucrose and slows gastric emptying in the elderly. Am J Med : 1289, 2005. [DOI] [PubMed] [Google Scholar]
- 275.Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab : E317–E325, 2011. [DOI] [PubMed] [Google Scholar]
- 276.Gibbons C, Caudwell P, Finlayson G, Webb DL, Hellstrom PM, Naslund E, Blundell JE. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab : 847–855, 2013. [DOI] [PubMed] [Google Scholar]
- 277.Gibbons C, Finlayson G, Caudwell P, Webb DL, Hellstrom PM, Naslund E, Blundell JE. Postprandial profiles of CCK after high fat and high carbohydrate meals and the relationship to satiety in humans. Peptides : 3–8, 2016. [DOI] [PubMed] [Google Scholar]
- 278.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol : 488–495, 1973. [DOI] [PubMed] [Google Scholar]
- 279.Glatzle J, Raybould HE, Kueper MA, Reeve JR Jr, Zittel TT. Cholecystokinin-58 is more potent in inhibiting food intake than cholecystokinin-8 in rats. Nutr Neurosci : 69–74, 2008. [DOI] [PubMed] [Google Scholar]
- 280.Glavas MM, Grayson BE, Allen SE, Copp DR, Smith MS, Cowley MA, Grove KL. Characterization of brainstem peptide YY (PYY) neurons. J Comp Neurol : 194–210, 2008. [DOI] [PubMed] [Google Scholar]
- 281.Goebel-Stengel M, Stengel A, Wang L, Ohning G, Tache Y, Reeve JR Jr. CCK-8 and CCK-58 differ in their effects on nocturnal solid meal pattern in undisturbed rats. Am J Physiol Regul Integr Comp Physiol : R850–R860, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Goetze O, Steingoetter A, Menne D, van der Voort IR, Kwiatek Ma, Boesiger P, Weishaupt D, Thumshirn M, Fried M, Schwizer W. The effect of macronutrients on gastric volume responses and gastric emptying in humans: a magnetic resonance imaging study. Am J Physiol Gastrointest Liver Physiol : G11–G17, 2007. [DOI] [PubMed] [Google Scholar]
- 283.Goldstone AP, Miras AD, Scholtz S, Jackson S, Neff KJ, Penicaud L, Geoghegan J, Chhina N, Durighel G, Bell JD, Meillon S, le Roux CW. Link between increased satiety gut hormones and reduced food reward after gastric bypass surgery for obesity. J Clin Endocrinol Metab : 599–609, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, Deliran SS, Beckmann C, Ghatei MA, Ashby DR, Waldman AD, Gaylinn BD, Thorner MO, Frost GS, Bloom SR, Bell JD. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr : 1319–1330, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gonlachanvit S, Hsu CW, Boden GH, Knight LC, Maurer AH, Fisher RS, Parkman HP. Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig Dis Sci : 488–497, 2003. [DOI] [PubMed] [Google Scholar]
- 286.Goodwin ML, Harris JE, Hernandez A, Gladden LB. Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol : 558–569, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gores GJ, Kost LJ, Miller LJ, LaRusso NF. Processing of cholecystokinin by isolated liver cells. Am J Physiol Gastrointest Liver Physiol : G242–G248, 1989. [DOI] [PubMed] [Google Scholar]
- 288.Graham L, Murty G, Bowrey DJ. Taste, smell and appetite change after Roux-en-Y gastric bypass surgery. Obes Surg : 1463–1468, 2014. [DOI] [PubMed] [Google Scholar]
- 289.Grandt D, Schimiczek M, Struk K, Shively J, Eysselein VE, Goebell H, Reeve JR Jr. Characterization of two forms of peptide YY, PYY(1–36) and PYY(3–36), in the rabbit. Peptides : 815–820, 1994. [DOI] [PubMed] [Google Scholar]
- 290.Greenough A, Cole G, Lewis J, Lockton A, Blundell J. Untangling the effects of hunger, anxiety, and nausea on energy intake during intravenous cholecystokinin octapeptide (CCK-8) infusion. Physiol Behav : 303–310, 1998. [DOI] [PubMed] [Google Scholar]
- 291.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab : 296–309, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Grossman MI. Gastrointestinal hormones. Physiol Rev : 33–90, 1950. [DOI] [PubMed] [Google Scholar]
- 293.Grossman MI. Physiological effects of gastrointestinal hormones. Federation Proc : 1930–1932, 1977. [PubMed] [Google Scholar]
- 294.Grundy D. Signalling the state of the digestive tract. Auton Neurosci : 76–80, 2006. [DOI] [PubMed] [Google Scholar]
- 295.Gryback P, Naslund E, Hellstrom PM, Jacobsson H, Backman L. Gastric emptying of solids in humans: improved evaluation by Kaplan-Meier plots, with special reference to obesity and gender. Eur J Nucl Med : 1562–1567, 1996. [DOI] [PubMed] [Google Scholar]
- 296.Gu N, Tsuda M, Matsunaga T, Adachi T, Yasuda K, Ishihara A, Tsuda K. Glucose regulation of dipeptidyl peptidase IV gene expression is mediated by hepatocyte nuclear factor-1alpha in epithelial intestinal cells. Clin Exp Pharmacol Physiol : 1433–1439, 2008. [DOI] [PubMed] [Google Scholar]
- 297.Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, Ahren B. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology : 3173–3180, 2006. [DOI] [PubMed] [Google Scholar]
- 298.Gutzwiller JP, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, Gutmann H, Stanga Z, Vogel D, Beglinger C. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion : 142–150, 2006. [DOI] [PubMed] [Google Scholar]
- 299.Gutzwiller JP, Degen L, Matzinger D, Prestin S, Beglinger C. Interaction between GLP-1 and CCK-33 in inhibiting food intake and appetite in men. Am J Physiol Regul Integr Comp Physiol : R562–R567, 2004. [DOI] [PubMed] [Google Scholar]
- 300.Gutzwiller JP, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol Regul Integr Comp Physiol : R1541–R1544, 1999. [DOI] [PubMed] [Google Scholar]
- 301.Gutzwiller JP, Göke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut : 81–86, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Häberer D, Tasker M, Foltz M, Geary N, Westerterp M, Langhans W. Intragastric infusion of pea-protein hydrolysate reduces test-meal size in rats more than pea protein. Physiol Behav : 1041–1047, 2011. [DOI] [PubMed] [Google Scholar]
- 303.Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, Morley TC, Yeo GS, Reimann F, Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology : 3054–3065, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia : 1413–1416, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol : G967–G979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab : 159–168, 2005. [DOI] [PubMed] [Google Scholar]
- 307.Hammer HF. Medical complications of bariatric surgery: focus on malabsorption and dumping syndrome. Dig Dis : 182–186, 2012. [DOI] [PubMed] [Google Scholar]
- 308.Hansen M, Hjollund KR, Hartmann B, Plamboeck A, Deacon CF, Wewer Albrechtsen NJ, Holst JJ. Important species differences regarding lymph contribution to gut hormone responses. Peptides : 28–31, 2015. [DOI] [PubMed] [Google Scholar]
- 309.Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, Vilsboll T. Preserved inhibitory potency of GLP-1 on glucagon secretion in type 2 diabetes mellitus. J Clin Endocrinol Metab : 4679–4687, 2009. [DOI] [PubMed] [Google Scholar]
- 310.Harvey EJ, Arroyo K, Korner J, Inabnet WB. Hormone changes affecting energy homeostasis after metabolic surgery. Mt Sinai J Med : 446–465, 2010. [DOI] [PubMed] [Google Scholar]
- 311.Hass N, Schwarzenbacher K, Breer H. A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem Cell Biol : 457–471, 2007. [DOI] [PubMed] [Google Scholar]
- 312.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology : 2654–2659, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hayes MR, Kanoski SE, De Jonghe BC, Leichner TM, Alhadeff AL, Fortin SM, Arnold M, Langhans W, Grill HJ. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol : R1479–R1485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Heading RC, Tothill P, McLoughlin GP, Shearman DJ. Gastric emptying rate measurement in man. A double isotope scanning technique for simultaneous study of liquid and solid components of a meal. Gastroenterology : 45–50, 1976. [PubMed] [Google Scholar]
- 315.Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol : 273–281, 2004. [DOI] [PubMed] [Google Scholar]
- 316.Heddle R, Dent J, Read NW, Houghton LA, Toouli J, Horowitz M, Maddern GJ, Downton J. Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Physiol Gastrointest Liver Physiol : G671–G679, 1988. [DOI] [PubMed] [Google Scholar]
- 317.Heden TD, Liu Y, Sims L, Kearney ML, Whaley-Connell AT, Chockalingam A, Dellsperger KC, Fairchild TJ, Kanaley JA. Liquid meal composition, postprandial satiety hormones, and perceived appetite and satiety in obese women during acute caloric restriction. Eur J Endocrinol : 593–600, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Hellstrom PM, Gryback P, Jacobsson H. The physiology of gastric emptying. Best Pract Res Clin Anaesthesiol : 397–407, 2006. [DOI] [PubMed] [Google Scholar]
- 319.Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3–36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab : 188–195, 2008. [DOI] [PubMed] [Google Scholar]
- 320.Henderson J. Ernest Starling and ‘Hormones’: an historical commentary. J Endocrinol : 5–10, 2005. [DOI] [PubMed] [Google Scholar]
- 321.Herman CP, Polivy J. Normative influences on food intake. Physiol Behav : 762–772, 2005. [DOI] [PubMed] [Google Scholar]
- 322.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion : 117–126, 1995. [DOI] [PubMed] [Google Scholar]
- 323.Hidalgo L, Clave P, Estorch M, Rodriguez-Espinosa J, Rovati L, Greeley GH, Capella G, Lluis F. Effect of cholecystokinin-A receptor blockade on postprandial insulinaemia and gastric emptying in humans. Neurogastroenterol Motil : 519–525, 2002. [DOI] [PubMed] [Google Scholar]
- 324.Hildebrand P, Ensinck JW, Ketterer S, Delco F, Mossi S, Bangerter U, Beglinger C. Effect of a cholecystokinin antagonist on meal-stimulated insulin and pancreatic polypeptide release in humans. J Clin Endocrinol Metab : 1123–1129, 1991. [DOI] [PubMed] [Google Scholar]
- 325.Hildebrand P, Petrig C, Burckhardt B, Ketterer S, Lengsfeld H, Fleury A, Hadvary P, Beglinger C. Hydrolysis of dietary fat by pancreatic lipase stimulates cholecystokinin release. Gastroenterology : 123–129, 1998. [DOI] [PubMed] [Google Scholar]
- 326.Hill BR, De Souza MJ, Wagstaff DA, Sato R, Williams NI. 24-hour profiles of circulating ghrelin and peptide YY are inversely associated in normal weight premenopausal women. Peptides : 159–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Himeno S, Tarui S, Kanayama S, Kuroshima T, Shinomura Y, Hayashi C, Tateishi K, Imagawa K, Hashimura E, Hamaoka T. Plasma cholecystokinin responses after ingestion of liquid meal and intraduodenal infusion of fat, amino acids, or hydrochloric acid in man: analysis with region specific radioimmunoassay. Am J Gastroenterol : 703–707, 1983. [PubMed] [Google Scholar]
- 328.Hira T, Nakajima S, Eto Y, Hara H. Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells. FEBS J : 4620–4626, 2008. [DOI] [PubMed] [Google Scholar]
- 329.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Med : 90–94, 2005. [DOI] [PubMed] [Google Scholar]
- 330.Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP-1 neurons involves alpha1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes : 2701–2709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hoad CL, Rayment P, Spiller RC, Marciani L, Alonso Bde C, Traynor C, Mela DJ, Peters HP, Gowland PA. In vivo imaging of intragastric gelation and its effect on satiety in humans. J Nutr : 2293–2300, 2004. [DOI] [PubMed] [Google Scholar]
- 332.Hojberg PV, Vilsboll T, Rabol R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia : 199–207, 2009. [DOI] [PubMed] [Google Scholar]
- 333.Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes : 1640–1646, 2008. [DOI] [PubMed] [Google Scholar]
- 334.Holdsworth CD, Dawson AM. The absorption of monosaccharides in man. Clin Sci : 371–379, 1964. [PubMed] [Google Scholar]
- 335.Holst JJ. Incretin hormones and the satiation signal. Int J Obes (Lond) : 1161–1168, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev : 1409–1439, 2007. [DOI] [PubMed] [Google Scholar]
- 337.Hopman WP, Jansen JB, Lamers CB. Comparative study of the effects of equal amounts of fat, protein, and starch on plasma cholecystokinin in man. Scand J Gastroenterol : 843–847, 1985. [DOI] [PubMed] [Google Scholar]
- 338.Horowitz M, Collins PJ, Shearman DJ. Effect of increasing the caloric/osmotic content of the liquid component of a mixed solid and liquid meal on gastric emptying in obese subjects. Hum Nutr Clin Nutr : 51–56, 1986. [PubMed] [Google Scholar]
- 339.Horowitz M, Cook DJ, Collins PJ, Harding PE, Hooper MJ, Walsh JF, Shearman DJ. Measurement of gastric emptying after gastric bypass surgery using radionuclides. Br J Surg : 655–657, 1982. [DOI] [PubMed] [Google Scholar]
- 340.Horowitz M, Cunningham KM, Wishart JM, Jones KL, Read NW. The effect of short-term dietary supplementation with glucose on gastric emptying of glucose and fructose and oral glucose tolerance in normal subjects. Diabetologia : 481–486, 1996. [DOI] [PubMed] [Google Scholar]
- 341.Horowitz M, Dent J. Disordered gastric emptying: mechanical basis, assessment and treatment. Baillieres Clin Gastroenterol : 371–407, 1991. [DOI] [PubMed] [Google Scholar]
- 342.Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia : 857–862, 1993. [DOI] [PubMed] [Google Scholar]
- 343.Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, Doran S, Jax T, Zdravkovic M, Chapman IM. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract : 258–266, 2012. [DOI] [PubMed] [Google Scholar]
- 344.Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, Shearman DJ. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia : 151–159, 1989. [DOI] [PubMed] [Google Scholar]
- 345.Horowitz M, Maddox A, Bochner M, Wishart J, Bratasiuk R, Collins P, Shearman D. Relationships between gastric emptying of solid and caloric liquid meals and alcohol absorption. Am J Physiol Gastrointest Liver Physiol : G291–G298, 1989. [DOI] [PubMed] [Google Scholar]
- 346.Hosoda H, Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regul Pept : 12–18, 2008. [DOI] [PubMed] [Google Scholar]
- 347.Hou Q, Lin Z, Dusing RW, Gajewski BJ, McCallum RW. A Bayesian hierarchical assessment of gastric emptying with the linear, power exponential and modified power exponential models. Neurogastroenterol Motil : 1308–1317, 2010. [DOI] [PubMed] [Google Scholar]
- 348.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science : 974–977, 1996. [DOI] [PubMed] [Google Scholar]
- 349.Hunt JN, Stubbs DF. The volume and energy content of meals as determinants of gastric emptying. J Physiol : 209–225, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology : 11–17, 1989. [DOI] [PubMed] [Google Scholar]
- 351.Hveem K, Jones KL, Chatterton BE, Horowitz M. Scintigraphic measurement of gastric emptying and ultrasonographic assessment of antral area: relation to appetite. Gut : 816–821, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Hvidberg A, Nielsen MT, Hilsted J, Orskov C, Holst JJ. Effect of glucagon-like peptide-1 (proglucagon 78-107amide) on hepatic glucose production in healthy man. Metabolism : 104–108, 1994. [DOI] [PubMed] [Google Scholar]
- 353.Ikramuddin S, Blackstone RP, Brancatisano A, Toouli J, Shah SN, Wolfe BM, Fujioka K, Maher JW, Swain J, Que FG, Morton JM, Leslie DB, Brancatisano R, Kow L, O'Rourke RW, Deveney C, Takata M, Miller CJ, Knudson MB, Tweden KS, Shikora SA, Sarr MG, Billington CJ. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA : 915–922, 2014. [DOI] [PubMed] [Google Scholar]
- 354.Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol Gastrointest Liver Physiol : G920–G927, 1997. [DOI] [PubMed] [Google Scholar]
- 355.Ionut V, Hucking K, Liberty IF, Bergman RN. Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia : 967–975, 2005. [DOI] [PubMed] [Google Scholar]
- 357.Irwin N, Frizelle P, Montgomery IA, Moffett RC, O'Harte FP, Flatt PR. Beneficial effects of the novel cholecystokinin agonist (pGlu-Gln)-CCK-8 in mouse models of obesity/diabetes. Diabetologia : 2747–2758, 2012. [DOI] [PubMed] [Google Scholar]
- 358.Irwin N, Montgomery IA, Flatt PR. Comparison of the metabolic effects of sustained CCK1 receptor activation alone and in combination with upregulated leptin signalling in high-fat-fed mice. Diabetologia : 1425–1435, 2013. [DOI] [PubMed] [Google Scholar]
- 359.Irwin N, Montgomery IA, O'Harte FP, Frizelle P, Flatt PR. Comparison of the independent and combined metabolic effects of subchronic modulation of CCK and GIP receptor action in obesity-related diabetes. Int J Obes (Lond) : 1058–1063, 2013. [DOI] [PubMed] [Google Scholar]
- 360.Irwin N, Pathak V, Flatt PR. A novel CCK-8/GLP-1 hybrid peptide exhibiting prominent insulinotropic, glucose-lowering, and satiety actions with significant therapeutic potential in high-fat-fed mice. Diabetes : 2996–3009, 2015. [DOI] [PubMed] [Google Scholar]
- 361.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care : 1438–1442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ivy AC, Oldberg E. A hormone mechanism for gallbladder contraction and evacuation. Am J Physiol : 599–613, 1928. [Google Scholar]
- 363.Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell function in type 2 diabetic patients. Diabetes : 3027–3032, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Worm D, Almdal T, Naver LS, Hvolris LE, Rehfeld JF, Wulff BS, Clausen TR, Hansen DL, Holst JJ, Madsbad S. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg : 1084–1096, 2012. [DOI] [PubMed] [Google Scholar]
- 365.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA : 15069–15074, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther : 880–894, 2011. [DOI] [PubMed] [Google Scholar]
- 367.Janssen S, Laermans J, Verhulst Pj Thijs T, Tack J, Depoortere I. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA : 2094–2099, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Jeffery RW, Harnack LJ. Evidence implicating eating as a primary driver for the obesity epidemic. Diabetes : 2673–2676, 2007. [DOI] [PubMed] [Google Scholar]
- 369.Jenkins AL, Jenkins DJ, Zdravkovic U, Wursch P, Vuksan V. Depression of the glycemic index by high levels of beta-glucan fiber in two functional foods tested in type 2 diabetes. Eur J Clin Nutr : 622–628, 2002. [DOI] [PubMed] [Google Scholar]
- 370.Jerlhag E, Janson AC, Waters S, Engel JA. Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS One : e49557, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Jimenez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care : 2062–2069, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Jonderko K. Comparative analysis of quantitative gastric emptying indices and power-exponential modelling of gastric emptying curves. Clin Phys Physiol Meas : 161–170, 1989. [DOI] [PubMed] [Google Scholar]
- 373.Jones KL, Doran SM, Hveem K, Bartholomeusz FD, Morley JE, Sun WM, Chatterton BE, Horowitz M. Relation between postprandial satiation and antral area in normal subjects. Am J Clin Nutr : 127–132, 1997. [DOI] [PubMed] [Google Scholar]
- 374.Jones RB, McKie S, Astbury N, Little TJ, Tivey S, Lassman DJ, McLaughlin J, Luckman S, Williams SR, Dockray GJ, Thompson DG. Functional neuroimaging demonstrates that ghrelin inhibits the central nervous system response to ingested lipid. Gut : 1543–1551, 2012. [DOI] [PubMed] [Google Scholar]
- 375.Jorgensen NB, Dirksen C, Bojsen-Moller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes : 3044–3052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kaji I, Karaki S, Kuwahara A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion : 31–36, 2014. [DOI] [PubMed] [Google Scholar]
- 377.Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD. Self-report of gastrointestinal side effects after bariatric surgery. Surg Obes Relat Dis : 1202–1207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol : R885–R895, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology : 1916–1927, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res : 353–360, 2006. [DOI] [PubMed] [Google Scholar]
- 381.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci : 7–14, 2002. [DOI] [PubMed] [Google Scholar]
- 382.Katschinski M, Schirra J, Begliner C, Langbein S, Wank U, D'Amato M, Arnold R. Intestinal phase of human antro-pyloro-duodenal motility: cholinergic and CCK-mediated regulation. Eur J Clin Invest : 574–583, 1996. [DOI] [PubMed] [Google Scholar]
- 383.Kellum JM, Kuemmerle JF, O'Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg : 763–770, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials : 85, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kim BJ, Carlson OD, Jang HJ, Elahi D, Berry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J Clin Endocrinol Metab : 6665–6671, 2005. [DOI] [PubMed] [Google Scholar]
- 387.Kim DH, D'Alessio DA, Woods SC, Seeley RJ. The effects of GLP-1 infusion in the hepatic portal region on food intake. Regul Pept : 110–114, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci : 10470–10476, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, Heiman ML, Tschop MH. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med : 741–745, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kirchner H, Heppner KM, Tschop MH. The role of ghrelin in the control of energy balance. Handb Exp Pharmacol 161–184, 2012. [DOI] [PubMed] [Google Scholar]
- 391.Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr : 154–160, 1981. [DOI] [PubMed] [Google Scholar]
- 392.Kjems LL, Holst JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes : 380–386, 2003. [DOI] [PubMed] [Google Scholar]
- 393.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest : 3554–3563, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Knight LC, Parkman HP, Brown KL, Miller MA, Trate DM, Maurer AH, Fisher RS. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol : 968–975, 1997. [PubMed] [Google Scholar]
- 395.Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K, Nakazato M. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology : 2369–2375, 2005. [DOI] [PubMed] [Google Scholar]
- 396.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab : E708–712, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature : 656–660, 1999. [DOI] [PubMed] [Google Scholar]
- 398.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev : 495–522, 2005. [DOI] [PubMed] [Google Scholar]
- 399.Kong MF, Chapman I, Goble E, Wishart J, Wittert G, Morris H, Horowitz M. Effects of oral fructose and glucose on plasma GLP-1 and appetite in normal subjects. Peptides : 545–551, 1999. [DOI] [PubMed] [Google Scholar]
- 400.Konturek JW, Stoll R, Gutwinska-Konturek M, Konturek SJ, Domschke W. Cholecystokinin in the regulation of gastric acid and endocrine pancreatic secretion in humans. Scand J Gastroenterol : 401–407, 1993. [DOI] [PubMed] [Google Scholar]
- 401.Konturek PC, Konturek SJ. The history of gastrointestinal hormones and the Polish contribution to elucidation of their biology and relation to nervous system. J Physiol Pharmacol Suppl 3: 83–98, 2003. [PubMed] [Google Scholar]
- 402.Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest : 383–391, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) : 786–795, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kost LJ, Gores GJ, Sayles JM, Miller LJ, Lemasters JJ, Herman B, LaRusso NF. Lack of metabolic effects of cholecystokinin on hepatocytes. Hepatology : 301–305, 1990. [DOI] [PubMed] [Google Scholar]
- 405.Kral JG, Paez W, Wolfe BM. Vagal nerve function in obesity: therapeutic implications. World J Surg : 1995–2006, 2009. [DOI] [PubMed] [Google Scholar]
- 406.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet : 1300–1304, 1987. [DOI] [PubMed] [Google Scholar]
- 407.Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes : 34–43, 2016. [DOI] [PubMed] [Google Scholar]
- 408.Kruszynska YT, Home PD, Hanning I, Alberti KG. Basal and 24-h C-peptide and insulin secretion rate in normal man. Diabetologia : 16–21, 1987. [DOI] [PubMed] [Google Scholar]
- 409.Kuhre RE, Gribble FM, Hartmann B, Reimann F, Windelov JA, Rehfeld JF, Holst JJ. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am J Physiol Gastrointest Liver Physiol : G622–G630, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Kuhre RE, Wewer Albrechtsen NJ, Hartmann B, Deacon CF, Holst JJ. Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J Diabetes Complications : 445–450, 2015. [DOI] [PubMed] [Google Scholar]
- 411.Kuo P, Bellon M, Wishart J, Smout AJ, Holloway RH, Fraser RJ, Horowitz M, Jones KL, Rayner CK. Effects of metoclopramide on duodenal motility and flow events, glucose absorption, and incretin hormone release in response to intraduodenal glucose infusion. Am J Physiol Gastrointest Liver Physiol : G1326–G1333, 2010. [DOI] [PubMed] [Google Scholar]
- 412.Kuo P, Chaikomin R, Pilichiewicz A, O'Donovan D, Wishart JM, Meyer JH, Jones KL, Feinle-Bisset C, Horowitz M, Rayner CK. Transient, early release of glucagon-like peptide-1 during low rates of intraduodenal glucose delivery. Regul Peptides : 1–3, 2008. [DOI] [PubMed] [Google Scholar]
- 413.Kurashina T, Dezaki K, Yoshida M, Sukma Rita R, Ito K, Taguchi M, Miura R, Tominaga M, Ishibashi S, Kakei M, Yada T. The beta-cell GHSR and downstream cAMP/TRPM2 signaling account for insulinostatic and glycemic effects of ghrelin. Sci Rep : 14041, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Ladenheim EE. Liraglutide and obesity: a review of the data so far. Drug Des Dev Ther : 1867–1875, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab : 2479–2485, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Lamberts SW, Romijn JA, Wiersinga WM. The future endocrine patient. Reflections on the future of clinical endocrinology. Eur J Endocrinol : 169–175, 2003. [DOI] [PubMed] [Google Scholar]
- 417.Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest : 388–402, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Larsson LI, Goltermann N, de Magistris L, Rehfeld JF, Schwartz TW. Somatostatin cell processes as pathways for paracrine secretion. Science : 1393–1395, 1979. [DOI] [PubMed] [Google Scholar]
- 419.Lassman DJ, McKie S, Gregory LJ, Lal S, D'Amato M, Steele I, Varro A, Dockray GJ, Williams SC, Thompson DG. Defining the role of cholecystokinin in the lipid-induced human brain activation matrix. Gastroenterology : 1514–1524, 2010. [DOI] [PubMed] [Google Scholar]
- 420.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes : 1058–1066, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lonroth H, Fandriks L, Olbers T. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond) : 348–355, 2012. [DOI] [PubMed] [Google Scholar]
- 422.Le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg : 108–114, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology : 3–8, 2006. [DOI] [PubMed] [Google Scholar]
- 424.Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Lowenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol : R1057–R1066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg : 780–785, 2007. [DOI] [PubMed] [Google Scholar]
- 426.Le Sauter J, Goldberg B, Geary N. CCK inhibits real and sham feeding in gastric vagotomized rats. Physiol Behav : 527–534, 1988. [DOI] [PubMed] [Google Scholar]
- 427.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr : 89–94, 2006. [DOI] [PubMed] [Google Scholar]
- 428.Lepage R, Albert C. Fifty years of development in the endocrinology laboratory. Clin Biochem : 542–557, 2006. [DOI] [PubMed] [Google Scholar]
- 429.Levin F, Edholm T, Schmidt PT, Gryback P, Jacobsson H, Degerblad M, Hoybye C, Holst JJ, Rehfeld JF, Hellstrom PM, Naslund E. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab : 3296–3302, 2006. [DOI] [PubMed] [Google Scholar]
- 430.Li P, Tiwari HK, Lin WY, Allison DB, Chung WK, Leibel RL, Yi N, Liu N. Genetic association analysis of 30 genes related to obesity in a European American population. Int J Obes (Lond) : 724–729, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Liddle RA. Cholecystokinin cells. Annu Rev Physiol : 221–242, 1997. [DOI] [PubMed] [Google Scholar]
- 432.Liddle RA. Regulation of cholecystokinin secretion by intraluminal releasing factors. Am J Physiol Gastrointest Liver Physiol : G319–G327, 1995. [DOI] [PubMed] [Google Scholar]
- 433.Liddle RA, Gertz BJ, Kanayama S, Beccaria L, Coker LD, Turnbull TA, Morita ET. Effects of a novel cholecystokinin (CCK) receptor antagonist, MK-329, on gallbladder contraction and gastric emptying in humans. Implications for the physiology of CCK. J Clin Invest : 1220–1225, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Liddle RA, Gertz BJ, Kanayama S, Beccaria L, Gettys TW, Taylor IL, Rushakoff RJ, Williams VC, Coker LD. Regulation of pancreatic endocrine function by cholecystokinin: studies with MK-329, a nonpeptide cholecystokinin receptor antagonist. J Clin Endocrinol Metab : 1312–1318, 1990. [DOI] [PubMed] [Google Scholar]
- 435.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest : 1144–1152, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Liddle RA, Morita ET, Conrad CK, Williams JA. Regulation of gastric emptying in humans by cholecystokinin. J Clin Invest : 992–996, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Liddle RA, Rushakoff RJ, Morita ET, Beccaria L, Carter JD, Goldfine ID. Physiological role for cholecystokinin in reducing postprandial hyperglycemia in humans. J Clin Invest : 1675–1681, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Lieverse RJ, Jansen JB, Masclee AA, Lamers CB. Satiety effects of a physiological dose of cholecystokinin in humans. Gut : 176–179, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Lieverse RJ, Jansen JB, Masclee AA, Rovati LC, Lamers CB. Effect of a low dose of intraduodenal fat on satiety in humans: studies using the type A cholecystokinin receptor antagonist loxiglumide. Gut : 501–505, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Lieverse RJ, Jansen JB, van de Zwan A, Samson L, Masclee AA, Lamers CB. Effects of a physiological dose of cholecystokinin on food intake and postprandial satiation in man. Regul Pept : 83–89, 1993. [DOI] [PubMed] [Google Scholar]
- 441.Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides : 189–200, 2004. [DOI] [PubMed] [Google Scholar]
- 442.Lindgren O, Carr RD, Holst JJ, Deacon CF, Ahren B. Dissociated incretin hormone response to protein versus fat ingestion in obese subjects. Diabetes Obes Metab : 863–865, 2011. [DOI] [PubMed] [Google Scholar]
- 443.Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology : 903–912, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Lippl F, Erdmann J, Steiger A, Lichter N, Czogalla-Peter C, Bidlingmaier M, Tholl S, Schusdziarra V. Low-dose ghrelin infusion–evidence against a hormonal role in food intake. Regul Pept : 26–31, 2012. [DOI] [PubMed] [Google Scholar]
- 445.Little TJ, Doran S, Meyer JH, Smout AJ, O'Donovan DG, Wu KL, Jones KL, Wishart J, Rayner CK, Horowitz M, Feinle-Bisset C. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab : E647–E655, 2006. [DOI] [PubMed] [Google Scholar]
- 446.Little TJ, Feltrin KL, Horowitz M, Smout AJ, Rades T, Meyer JH, Pilichiewicz AN, Wishart J, Feinle-Bisset C. Dose-related effects of lauric acid on antropyloroduodenal motility, gastrointestinal hormone release, appetite, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol : R1090–R1098, 2005. [DOI] [PubMed] [Google Scholar]
- 447.Little TJ, Gopinath A, Patel E, McGlone A, Lassman DJ, D'Amato M, McLaughlin JT, Thompson DG. Gastric emptying of hexose sugars: role of osmolality, molecular structure and the CCK1 receptor. Neurogastroenterol Motil : 1183–1190, 2010. [DOI] [PubMed] [Google Scholar]
- 448.Little TJ, Pilichiewicz AN, Russo A, Phillips L, Jones KL, Nauck MA, Wishart J, Horowitz M, Feinle-Bisset C. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: relationships with postprandial glycemic and insulinemic responses. J Clin Endocrinol Metab : 1916–1923, 2006. [DOI] [PubMed] [Google Scholar]
- 449.Liu EH, Oberg K. The history and development of the gastroenteropancreatic endocrine axis. Endocrinol Metab Clin North Am : 697–711, 2010. [DOI] [PubMed] [Google Scholar]
- 450.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience : 111–121, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Lo CM, Xu M, Yang Q, Zheng S, Carey KM, Tubb MR, Davidson WS, Liu M, Woods SC, Tso P. Effect of intraperitoneal and intravenous administration of cholecystokinin-8 and apolipoprotein AIV on intestinal lymphatic CCK-8 and apo AIV concentration. Am J Physiol Regul Integr Comp Physiol : R43–R50, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Locatelli I, Mrhar A, Bogataj M. Gastric emptying of pellets under fasting conditions: a mathematical model. Pharm Res : 1607–1617, 2009. [DOI] [PubMed] [Google Scholar]
- 453.Lomenick JP, Clasey JL, Anderson JW. Meal-related changes in ghrelin, peptide YY, and appetite in normal weight and overweight children. Obesity : 547–552, 2008. [DOI] [PubMed] [Google Scholar]
- 454.Long SJ, Sutton Ja Amaee WB, Giouvanoudi a Spyrou NM, Rogers PJ, Morgan LM. No effect of glucagon-like peptide-1 on short-term satiety and energy intake in man. Br J Nutr : 273–279, 1999. [PubMed] [Google Scholar]
- 455.Loop MS, Frazier-Wood AC, Thomas AS, Dhurandhar EJ, Shikany JM, Gadbury GL, Allison DB. Submitted for your consideration: potential advantages of a novel clinical trial design and initial patient reaction. Front Genet : 145, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Lu L, Louie D, Owyang C. A cholecystokinin releasing peptide mediates feedback regulation of pancreatic secretion. Am J Physiol Gastrointest Liver Physiol : G430–G435, 1989. [DOI] [PubMed] [Google Scholar]
- 457.Lu X, Zhao X, Feng J, Liou AP, Anthony S, Pechhold S, Sun Y, Lu H, Wank S. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. Am J Physiol Gastrointest Liver Physiol : G367–G376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Lugari R, Dei Cas A, Ugolotti D, Barilli AL, Camellini C, Ganzerla GC, Luciani A, Salerni B, Mittenperger F, Nodari S, Gnudi A, Zandomeneghi R. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. Horm Metab Res : 111–115, 2004. [DOI] [PubMed] [Google Scholar]
- 459.Ma J, Checklin HL, Wishart JM, Stevens JE, Jones KL, Horowitz M, Meyer JH, Rayner CK. A randomised trial of enteric-coated nutrient pellets to stimulate gastrointestinal peptide release and lower glycaemia in type 2 diabetes. Diabetologia : 1236–1242, 2013. [DOI] [PubMed] [Google Scholar]
- 460.Ma J, Pilichiewicz AN, Feinle-Bisset C, Wishart JM, Jones KL, Horowitz M, Rayner CK. Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet Med : 604–608, 2012. [DOI] [PubMed] [Google Scholar]
- 461.Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol : 2917–2936, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.MacIntosh CG, Andrews JM, Jones KL, Wishart JM, Morris HA, Jansen JB, Morley JE, Horowitz M, Chapman IM. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr : 999–1006, 1999. [DOI] [PubMed] [Google Scholar]
- 463.Makhlouf GM. Neural and hormonal regulation of function in the gut. Hosp Pract : 79–87, 1990. [DOI] [PubMed] [Google Scholar]
- 464.Malagelada C, Accarino A, Molne L, Mendez S, Campos E, Gonzalez A, Malagelada JR, Azpiroz F. Digestive, cognitive and hedonic responses to a meal. Neurogastroenterol Motil : 389–396, 2015. [DOI] [PubMed] [Google Scholar]
- 465.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab : 400–409, 2008. [DOI] [PubMed] [Google Scholar]
- 466.Maljaars PW, Symersky T, Kee BC, Haddeman E, Peters HP, Masclee AA. Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int J Obes (Lond) : 1633–1639, 2008. [DOI] [PubMed] [Google Scholar]
- 467.Manning S, Batterham RL. The role of gut hormone peptide yy in energy and glucose homeostasis: twelve years on. Annu Rev Physiol : 585–608, 2014. [DOI] [PubMed] [Google Scholar]
- 468.Manning S, Pucci A, Batterham RL. GLP-1: a mediator of the beneficial metabolic effects of bariatric surgery? Physiology : 50–62, 2015. [DOI] [PubMed] [Google Scholar]
- 469.Manning S, Pucci A, Batterham RL. Roux-en-Y gastric bypass: effects on feeding behavior and underlying mechanisms. J Clin Invest : 939–948, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Marathe CS, Horowitz M, Trahair LG, Wishart JM, Bound M, Lange K, Rayner CK, Jones KL. Relationships of “early” and “late” glycemic responses with gastric emptying during an oral glucose tolerance test. J Clin Endocrinol Metab : 3565–3571, 2015. [DOI] [PubMed] [Google Scholar]
- 471.Marathe CS, Rayner CK, Bound M, Checklin H, Standfield S, Wishart J, Lange K, Jones KL, Horowitz M. Small intestinal glucose exposure determines the magnitude of the incretin effect in health and type 2 diabetes. Diabetes : 2668–2675, 2014. [DOI] [PubMed] [Google Scholar]
- 472.Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care : 1396–1405, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Marchal-Victorion S, Vionnet N, Escrieut C, Dematos F, Dina C, Dufresne M, Vaysse N, Pradayrol L, Froguel P, Fourmy D. Genetic, pharmacological and functional analysis of cholecystokinin-1 and cholecystokinin-2 receptor polymorphism in type 2 diabetes and obese patients. Pharmacogenetics : 23–30, 2002. [DOI] [PubMed] [Google Scholar]
- 474.Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, Fillery-Travis AJ. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am J Physiol Gastrointest Liver Physiol : G1227–G1233, 2001. [DOI] [PubMed] [Google Scholar]
- 475.Marciani L, Hall N, Pritchard SE, Cox EF, Totman JJ, Lad M, Hoad CL, Foster TJ, Gowland PA, Spiller RC. Preventing gastric sieving by blending a solid/water meal enhances satiation in healthy humans. J Nutr : 1253–1258, 2012. [DOI] [PubMed] [Google Scholar]
- 476.Mari A, Bagger JI, Ferrannini E, Holst JJ, Knop FK, Vilsboll T. Mechanisms of the incretin effect in subjects with normal glucose tolerance and patients with type 2 diabetes. PLoS One : e73154, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Mathes CM, Bueter M, Smith KR, Lutz TA, le Roux CW, Spector AC. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am J Physiol Regul Integr Comp Physiol : R751–R767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Maton PN, Selden AC, Chadwick VS. Differential distribution of molecular forms of cholecystokinin in human and porcine small intestinal mucosa. Regul Pept : 9–19, 1984. [DOI] [PubMed] [Google Scholar]
- 479.Matzinger D, Degen L, Drewe J, Meuli J, Duebendorfer R, Ruckstuhl N, D'Amato M, Rovati L, Beglinger C. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut : 688–693, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Matzinger D, Gutzwiller JP, Drewe J, Orban A, Engel R, D'Amato M, Rovati L, Beglinger C. Inhibition of food intake in response to intestinal lipid is mediated by cholecystokinin in humans. Am J Physiol Regul Integr Comp Physiol : R1718–R1724, 1999. [DOI] [PubMed] [Google Scholar]
- 481.Maurer AH. Advancing gastric emptying studies: standardization and new parameters to assess gastric motility and function. Semin Nucl Med : 101–112, 2012. [DOI] [PubMed] [Google Scholar]
- 482.Mayer J, Thomas DW. Regulation of food intake and obesity. Science : 328–337, 1967. [DOI] [PubMed] [Google Scholar]
- 483.Mayer LE, Schebendach J, Bodell LP, Shingleton RM, Walsh BT. Eating behavior in anorexia nervosa: before and after treatment. Int J Eat Disord : 290–293, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab : 54–60, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.McKay NJ, Galante DL, Daniels D. Endogenous glucagon-like peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. J Neurosci : 16417–16423, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.McLaughlin J, Grazia Luca M, Jones MN, D'Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology : 46–53, 1999. [DOI] [PubMed] [Google Scholar]
- 487.McLaughlin JT, Lomax RB, Hall L, Dockray GJ, Thompson DG, Warhurst G. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J Physiol : 11–18, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Medeiros MD, Turner AJ. Processing and metabolism of peptide-YY: pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology : 2088–2094, 1994. [DOI] [PubMed] [Google Scholar]
- 489.Medvei VC. A History of Endocrinology. Lancaster, PA: MTP Press, 1982. [Google Scholar]
- 490.Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev : 91–117, 2005. [DOI] [PubMed] [Google Scholar]
- 491.Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes : 1117–1125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Melhorn SJ, Tyagi V, Smeraglio A, Roth CL, Schur EA. Initial evidence that GLP-1 receptor blockade fails to suppress postprandial satiety or promote food intake in humans. Appetite : 85–90, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Mentis N, Vardarli I, Kothe LD, Holst JJ, Deacon CF, Theodorakis M, Meier JJ, Nauck MA. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes : 1270–1276, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Merkestein M, Brans MA, Luijendijk MC, de Jong JW, Egecioglu E, Dickson SL, Adan RA. Ghrelin mediates anticipation to a palatable meal in rats. Obesity : 963–971, 2012. [DOI] [PubMed] [Google Scholar]
- 495.Meyer BM, Werth BA, Beglinger C, Hildebrand P, Jansen JB, Zach D, Rovati LC, Stalder GA. Role of cholecystokinin in regulation of gastrointestinal motor functions. Lancet : 12–15, 1989. [DOI] [PubMed] [Google Scholar]
- 496.Meyer-Gerspach AC, Steinert RE, Keller S, Malarski A, Schulte FH, Beglinger C. Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans. J Clin Endocrinol Metab : 3351–3358, 2013. [DOI] [PubMed] [Google Scholar]
- 497.Meyer-Gerspach AC, Wolnerhanssen B, Beglinger B, Nessenius F, Napitupulu M, Schulte FH, Steinert RE, Beglinger C. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav : 265–271, 2014. [DOI] [PubMed] [Google Scholar]
- 498.Michaliszyn SF, Lee S, Bacha F, Tfayli H, Farchoukh L, Mari A, Ferrannini E, Arslanian S. Differences in beta-cell function and insulin secretion in Black vs. White obese adolescents: do incretin hormones play a role? Pediatr Diabetes. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, Roitman MF, Hayes MR. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J Neurosci : 6985–6992, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab : E1367–E1374, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Miller LJ, Holicky EL, Ulrich CD, Wieben ED. Abnormal processing of the human cholecystokinin receptor gene in association with gallstones and obesity. Gastroenterology : 1375–1380, 1995. [DOI] [PubMed] [Google Scholar]
- 502.Mirshahi UL, Still CD, Masker KK, Gerhard GS, Carey DJ, Mirshahi T. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab : E2088–2096, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Miyamoto Y, Miyamoto M. Immunohistochemical localizations of secretin, cholecystokinin, and somatostatin in the rat small intestine after acute cisplatin treatment. Exp Mol Pathol : 238–245, 2004. [DOI] [PubMed] [Google Scholar]
- 504.Miyasaka K, Guan DF, Liddle RA, Green GM. Feedback regulation by trypsin: evidence for intraluminal CCK-releasing peptide. Am J Physiol Gastrointest Liver Physiol : G175–G181, 1989. [DOI] [PubMed] [Google Scholar]
- 505.Modigliani R, Bernier JJ. Absorption of glucose, sodium, and water by the human jejunum studied by intestinal perfusion with a proximal occluding balloon and at variable flow rates. Gut : 184–193, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Mohlig M, Spranger J, Otto B, Ristow M, Tschop M, Pfeiffer AF. Euglycemic hyperinsulinemia, but not lipid infusion, decreases circulating ghrelin levels in humans. J Endocrinol Invest : RC36–38, 2002. [DOI] [PubMed] [Google Scholar]
- 507.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab : 191–201, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Moller JB, Jusko WJ, Gao W, Hansen T, Pedersen O, Holst JJ, Overgaard RV, Madsen H, Ingwersen SH. Mechanism-based population modelling for assessment of L-cell function based on total GLP-1 response following an oral glucose tolerance test. J Pharmacokinet Pharmacodyn : 713–725, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care : 881–885, 2003. [DOI] [PubMed] [Google Scholar]
- 510.Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab : 5510–5514, 2003. [DOI] [PubMed] [Google Scholar]
- 511.Monteleone P, Serritella C, Martiadis V, Maj M. Deranged secretion of ghrelin and obestatin in the cephalic phase of vagal stimulation in women with anorexia nervosa. Biol Psychiatry : 1005–1008, 2008. [DOI] [PubMed] [Google Scholar]
- 512.Monteleone P, Serritella C, Scognamiglio P, Maj M. Enhanced ghrelin secretion in the cephalic phase of food ingestion in women with bulimia nervosa. Psychoneuroendocrinology : 284–288, 2010. [DOI] [PubMed] [Google Scholar]
- 513.Moran TH, Dailey MJ. Intestinal feedback signaling and satiety. Physiol Behav : 77–81, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol Regul Integr Comp Physiol : R618–R625, 1998. [DOI] [PubMed] [Google Scholar]
- 515.Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res : 175–179, 1986. [DOI] [PubMed] [Google Scholar]
- 516.Moran TH, Shnayder L, Hostetler AM, McHugh PR. Pylorectomy reduces the satiety action of cholecystokinin. Am J Physiol Regul Integr Comp Physiol : R1059–R1063, 1988. [DOI] [PubMed] [Google Scholar]
- 517.Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE. Peptide YY(3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol : R384–R388, 2005. [DOI] [PubMed] [Google Scholar]
- 518.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab : 1735–1740, 2006. [DOI] [PubMed] [Google Scholar]
- 519.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab : E1358–E1365, 2009. [DOI] [PubMed] [Google Scholar]
- 520.Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept : 189–196, 2003. [DOI] [PubMed] [Google Scholar]
- 521.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature : 289–295, 2006. [DOI] [PubMed] [Google Scholar]
- 522.Moss C, Dhillo WS, Frost G, Hickson M. Gastrointestinal hormones: the regulation of appetite and the anorexia of ageing. J Hum Nutr Diet : 3–15, 2012. [DOI] [PubMed] [Google Scholar]
- 523.Muir JG, Lu ZX, Young GP, Cameron-Smith D, Collier GR, O'Dea K. Resistant starch in the diet increases breath hydrogen and serum acetate in human subjects. Am J Clin Nutr : 792–799, 1995. [DOI] [PubMed] [Google Scholar]
- 524.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D'Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Dieguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrere B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschop MH. Ghrelin. Mol Metab : 437–460, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil : e70–79, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Murdolo G, Lucidi P, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Fanelli CG, Bolli GB, Santeusanio F, De Feo P. Insulin is required for prandial ghrelin suppression in humans. Diabetes : 2923–2927, 2003. [DOI] [PubMed] [Google Scholar]
- 527.Mussig K, Staiger H, Machicao F, Haring HU, Fritsche A. Genetic variants affecting incretin sensitivity and incretin secretion. Diabetologia : 2289–2297, 2010. [DOI] [PubMed] [Google Scholar]
- 528.Muurahainen NE, Kissileff HR, Lachaussée J, Pi-Sunyer FX. Effect of a soup preload on reduction of food intake by cholecystokinin in humans. Am J Physiol Regul Integr Comp Physiol : R672–R680, 1991. [DOI] [PubMed] [Google Scholar]
- 529.Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity : 390–400, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol : R1483–R1487, 2001. [DOI] [PubMed] [Google Scholar]
- 531.Nagell CF, Pedersen JF, Holst JJ. The antagonistic metabolite of GLP-1, GLP-1 (9–36)amide, does not influence gastric emptying and hunger sensations in man. Scand J Gastroenterol : 28–33, 2007. [DOI] [PubMed] [Google Scholar]
- 532.Nair NS, Brennan IM, Little TJ, Gentilcore D, Hausken T, Jones KL, Wishart JM, Horowitz M, Feinle-Bisset C. Reproducibility of energy intake, gastric emptying, blood glucose, plasma insulin and cholecystokinin responses in healthy young males. Br J Nutr : 1094–1102, 2009. [DOI] [PubMed] [Google Scholar]
- 533.Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab : 4391–4399, 2013. [DOI] [PubMed] [Google Scholar]
- 534.Naslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rossner S, Hellstrom PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord : 304–311, 1999. [DOI] [PubMed] [Google Scholar]
- 535.Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr : 525–530, 1998. [DOI] [PubMed] [Google Scholar]
- 536.Naslund I, Beckman KW. Gastric emptying rate after gastric bypass and gastroplasty. Scand J Gastroenterol : 193–201, 1987. [DOI] [PubMed] [Google Scholar]
- 537.Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7–36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab : 912–917, 1993. [DOI] [PubMed] [Google Scholar]
- 538.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest : 301–307, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab : 492–498, 1986. [DOI] [PubMed] [Google Scholar]
- 540.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes : 1561–1565, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol Endocrinol Metab : E981–E988, 1997. [DOI] [PubMed] [Google Scholar]
- 542.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia : 10–18, 2011. [DOI] [PubMed] [Google Scholar]
- 543.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature : 199–202, 2002. [DOI] [PubMed] [Google Scholar]
- 544.Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, Rayner CK, Horowitz M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption, and postprandial symptoms after gastric bypass. Obesity : 2003–2009, 2014. [DOI] [PubMed] [Google Scholar]
- 545.Nguyen NQ, Debreceni TL, Burgstad CM, Neo M, Bellon M, Wishart JM, Standfield S, Bartholomeusz D, Rayner CK, Wittert G, Horowitz M. Effects of fat and protein preloads on pouch emptying, intestinal transit, glycaemia, gut hormones, glucose absorption, blood pressure and gastrointestinal symptoms after Roux-en-Y gastric bypass. Obes Surg : 77–84, 2016. [DOI] [PubMed] [Google Scholar]
- 546.Nicolaus M, Brödl J, Linke R, Woerle HJ, Göke B, Schirra J. Endogenous GLP-1 regulates postprandial glycemia in humans: relative contributions of insulin, glucagon, and gastric emptying. J Clin Endocrinol Metab : 229–236, 2011. [DOI] [PubMed] [Google Scholar]
- 547.Niederau C, Schwarzendrube J, Luthen R, Niederau M, Strohmeyer G, Rovati L. Effects of cholecystokinin receptor blockade on circulating concentrations of glucose, insulin, C-peptide, and pancreatic polypeptide after various meals in healthy human volunteers. Pancreas : 1–10, 1992. [DOI] [PubMed] [Google Scholar]
- 548.Nishizawa M, Moore MC, Shiota M, Gustavson SM, Snead WL, Neal DW, Cherrington AD. Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. Am J Physiol Endocrinol Metab : E1027–E1036, 2003. [DOI] [PubMed] [Google Scholar]
- 549.Nolan LJ, Guss JL, Liddle RA, Pi-Sunyer FX, Kissileff HR. Elevated plasma cholecystokinin and appetitive ratings after consumption of a liquid meal in humans. Nutrition : 553–557, 2003. [DOI] [PubMed] [Google Scholar]
- 550.O'Donovan DG, Doran S, Feinle-Bisset C, Jones KL, Meyer JH, Wishart JM, Morris HA, Horowitz M. Effect of variations in small intestinal glucose delivery on plasma glucose, insulin, and incretin hormones in healthy subjects and type 2 diabetes. J Clin Endocrinol Metab : 3431–3435, 2004. [DOI] [PubMed] [Google Scholar]
- 551.Odstrcil EA, Martinez JG, Santa Ana CA, Xue B, Schneider RE, Steffer KJ, Porter JL, Asplin J, Kuhn JA, Fordtran JS. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr : 704–713, 2010. [DOI] [PubMed] [Google Scholar]
- 552.Oesch S, Degen L, Beglinger C. Effect of a protein preload on food intake and satiety feelings in response to duodenal fat perfusions in healthy male subjects. Am J Physiol Regul Integr Comp Physiol : R1042–R1047, 2005. [DOI] [PubMed] [Google Scholar]
- 553.Ohlsson L, Kohan AB, Tso P, Ahren B. GLP-1 released to the mesenteric lymph duct in mice: effects of glucose and fat. Regul Pept : 40–45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Ohno T, Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H. Ghrelin does not stimulate gastrointestinal motility and gastric emptying: an experimental study of conscious dogs. Neurogastroenterol Motil : 129–135, 2006. [DOI] [PubMed] [Google Scholar]
- 555.Okano-Matsumoto S, McRoberts JA, Tache Y, Adelson DW. Electrophysiological evidence for distinct vagal pathways mediating CCK-evoked motor effects in the proximal versus distal stomach. J Physiol : 371–393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Oliver G, Schafer EA. The physiological effects of extracts of the suprarenal capsules. J Physiol : 230–276, 1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol : 665–670, 1996. [DOI] [PubMed] [Google Scholar]
- 558.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology : 845–850, 2005. [DOI] [PubMed] [Google Scholar]
- 559.Padmanabhan P, Kumar A, Kumar S, Chaudhary RK, Gulyas B. Nanoparticles in practice for molecular-imaging applications: an overview. Acta Biomater : 1–16, 2016. [DOI] [PubMed] [Google Scholar]
- 560.Page AJ, Symonds E, Peiris M, Blackshaw LA, Young RL. Peripheral neural targets in obesity. Br J Pharmacol : 1537–1558, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Panaro BL, Tough IR, Engelstoft MS, Matthews RT, Digby GJ, Moller CL, Svendsen B, Gribble F, Reimann F, Holst JJ, Holst B, Schwartz TW, Cox HM, Cone RD. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab : 1018–1029, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Park MI, Camilleri M, O'Connor H, Oenning L, Burton D, Stephens D, Zinsmeister AR. Effect of different macronutrients in excess on gastric sensory and motor functions and appetite in normal-weight, overweight, and obese humans. Am J Clin Nutr : 411–418, 2007. [DOI] [PubMed] [Google Scholar]
- 563.Parker BA, Doran S, Wishart J, Horowitz M, Chapman IM. Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin Endocrinol : 539–546, 2005. [DOI] [PubMed] [Google Scholar]
- 564.Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol : 414–423, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Patterson LM, Zheng H, Ward SM, Berthoud HR. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res : 11–23, 2001. [DOI] [PubMed] [Google Scholar]
- 566.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity : 1671–1677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566a.Pavlov IP. The Work of the Digestive Glands, translated by Thompson WH. London, UK: Griffin, 1910. [Google Scholar]
- 567.Pearse AG, Coulling I, Weavers B, Friesen S. The endocrine polypeptide cells of the human stomach, duodenum, and jejunum. Gut : 649–658, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Pedersen J, Ugleholdt RK, Jorgensen SM, Windelov JA, Grunddal KV, Schwartz TW, Fuchtbauer EM, Poulsen SS, Holst PJ, Holst JJ. Glucose metabolism is altered after loss of L cells and alpha-cells but not influenced by loss of K cells. Am J Physiol Endocrinol Metab : E60–E73, 2013. [DOI] [PubMed] [Google Scholar]
- 569.Penagini R, Misiewicz JJ, Frost PG. Effect of jejunal infusion of bile acids on small bowel transit and fasting jejunal motility in man. Gut : 789–794, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Penney NC, Kinross J, Newton RC, Purkayastha S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes (Lond) : 1565–1574, 2015. [DOI] [PubMed] [Google Scholar]
- 571.Perello M, Dickson SL. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol : 424–434, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Perez-Tilve D, Hofmann SM, Basford J, Nogueiras R, Pfluger PT, Patterson JT, Grant E, Wilson-Perez HE, Granholm NA, Arnold M, Trevaskis JL, Butler AA, Davidson WS, Woods SC, Benoit SC, Sleeman MW, DiMarchi RD, Hui DY, Tschop MH. Melanocortin signaling in the CNS directly regulates circulating cholesterol. Nat Neurosci : 877–882, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Persaud SJ, Bewick GA. Peptide YY: more than just an appetite regulator. Diabetologia : 1762–1769, 2014. [DOI] [PubMed] [Google Scholar]
- 574.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg : 740–748, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Pfeiffer A, Schmidt T, Vidon N, Kaess H. Effect of ethanol on absorption of a nutrient solution in the upper human intestine. Scand J Gastroenterol : 515–521, 1993. [DOI] [PubMed] [Google Scholar]
- 576.Pfluger PT, Kampe J, Castaneda TR, Vahl T, D'Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AF, Koebnick C, Weickert MO, Otto B, Spranger J, Tschop MH. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab : 583–588, 2007. [DOI] [PubMed] [Google Scholar]
- 577.Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol : 112–128, 2015. [DOI] [PubMed] [Google Scholar]
- 578.Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol : 112–128, 2015. [DOI] [PubMed] [Google Scholar]
- 579.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res : 1–26, 2000. [DOI] [PubMed] [Google Scholar]
- 580.Phillips WT, Schwartz JG, McMahan CA. Reduced postprandial blood glucose levels in recently diagnosed non-insulin-dependent diabetics secondary to pharmacologically induced delayed gastric emptying. Dig Dis Sci : 51–58, 1993. [DOI] [PubMed] [Google Scholar]
- 581.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP, Obesity S, Prediabetes NNSG. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med : 11–22, 2015. [DOI] [PubMed] [Google Scholar]
- 582.Pierson ME, Comstock JM, Simmons RD, Kaiser F, Julien R, Zongrone J, Rosamond JD. Synthesis and biological evaluation of potent, selective, hexapeptide CCK-A agonist anorectic agents. J Med Chem : 4302–4307, 1997. [DOI] [PubMed] [Google Scholar]
- 583.Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL, Smout AJPM, Horowitz M, Feinle-Bisset C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab : E743–E753, 2007. [DOI] [PubMed] [Google Scholar]
- 584.Pilichiewicz AN, Papadopoulos P, Brennan IM, Little TJ, Meyer JH, Wishart JM, Horowitz M, Feinle-Bisset C. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol : R2170–R2178, 2007. [DOI] [PubMed] [Google Scholar]
- 585.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, Tosetti C, Poggioli G, Morselli Labate AM, Monetti N, et al. . Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology : 733–739, 1993. [DOI] [PubMed] [Google Scholar]
- 586.Plaisancie P, Bernard C, Chayvialle JA, Cuber JC. Release of peptide YY by neurotransmitters and gut hormones in the isolated, vascularly perfused rat colon. Scand J Gastroenterol : 568–574, 1995. [DOI] [PubMed] [Google Scholar]
- 587.Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, Meisner S, Hovendal C, Knop FK, Vilsboll T, Holst JJ. Characterisation of oral and iv glucose handling in truncally vagotomised subjects with pyloroplasty. Eur J Endocrinol : 187–201, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, Meisner S, Hovendal C, Vilsboll T, Knop FK, Holst JJ. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol : G1117–G1127, 2013. [DOI] [PubMed] [Google Scholar]
- 589.Polak JM, Bloom S, Coulling I, Pearse AG. Immunofluorescent localization of enteroglucagon cells in the gastrointestinal tract of the dog. Gut : 311–318, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Polak JM, Bloom SR, Rayford PL, Pearse AG, Buchan AM, Thompson JC. Identification of cholecystokinin-secreting cells. Lancet : 1016–1018, 1975. [DOI] [PubMed] [Google Scholar]
- 591.Polak JM, Pearse AG, Heath CM. Complete identification of endocrine cells in the gastrointestinal tract using semithin-thin sections to identify motilin cells in human and animal intestine. Gut : 225–229, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Polonsky KS, Given BD, Hirsch L, Shapiro ET, Tillil H, Beebe C, Galloway JA, Frank BH, Karrison T, Van Cauter E. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest : 435–441, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest : 442–448, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Prigeon RL, Quddusi S, Paty B, D'Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab : E701–E707, 2003. [DOI] [PubMed] [Google Scholar]
- 595.Pritchett CE, Hajnal A. Glucagon-like peptide-1 regulation of carbohydrate intake is differentially affected by obesogenic diets. Obesity : 313–317, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Prudom C, Liu J, Patrie J, Gaylinn BD, Foster-Schubert KE, Cummings DE, Thorner MO, Geysen HM. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. J Clin Endocrinol Metab : 2351–2358, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest : 908–917, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Punjabi M, Arnold M, Geary N, Langhans W, Pacheco-Lopez G. Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol Behav : 71–76, 2011. [DOI] [PubMed] [Google Scholar]
- 599.Punjabi M, Arnold M, Ruttimann E, Graber M, Geary N, Pacheco-Lopez G, Langhans W. Circulating glucagon-like peptide-1 (GLP-1) inhibits eating in male rats by acting in the hindbrain and without inducing avoidance. Endocrinology : 1690–1699, 2014. [DOI] [PubMed] [Google Scholar]
- 600.Raben A, Agerholm-Larsen L, Flint A, Holst JJ, Astrup A. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am J Clin Nutr : 91–100, 2003. [DOI] [PubMed] [Google Scholar]
- 601.Radziuk J, Kemmer F, Morishima T, Berchtold P, Vranic M. The effects of an alpha-glucoside hydrolase inhibitor on glycemia and the absorption of sucrose in man determined using a tracer method. Diabetes : 207–213, 1984. [DOI] [PubMed] [Google Scholar]
- 602.Ramsay DS, Woods SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev : 225–247, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut : 916–919, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Rask E, Olsson T, Soderberg S, Johnson O, Seckl J, Holst JJ, Ahren B, Northern Sweden Monitoring of Trends and Determinants in Cardiovascular Disease. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care : 1640–1645, 2001. [DOI] [PubMed] [Google Scholar]
- 605.Rasmussen BA, Breen DM, Luo P, Cheung GW, Yang CS, Sun B, Kokorovic A, Rong W, Lam TK. Duodenal activation of cAMP-dependent protein kinase induces vagal afferent firing and lowers glucose production in rats. Gastroenterology : 834–843, 2012. [DOI] [PubMed] [Google Scholar]
- 606.Rayner CK, Horowitz M. Physiology of the ageing gut. Curr Opin Clin Nutr Metab Care : 33–38, 2013. [DOI] [PubMed] [Google Scholar]
- 607.Reeve JR Jr, Eysselein VE, Rosenquist G, Zeeh J, Regner U, Ho FJ, Chew P, Davis MT, Lee TD, Shively JE, Brazer SR, Liddle RA. Evidence that CCK-58 has structure that influences its biological activity. Am J Physiol Gastrointest Liver Physiol : G860–G868, 1996. [DOI] [PubMed] [Google Scholar]
- 608.Reeve JR Jr, Wu SV, Keire DA, Faull K, Chew P, Solomon TE, Green GM, Coskun T. Differential bile-pancreatic secretory effects of CCK-58 and CCK-8. Am J Physiol Gastrointest Liver Physiol : G395–G402, 2004. [DOI] [PubMed] [Google Scholar]
- 609.Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem : 991–1001, 1998. [PubMed] [Google Scholar]
- 610.Rehfeld JF. Beginnings: a reflection on the history of gastrointestinal endocrinology. Regul Pept Suppl: S1–5, 2012. [DOI] [PubMed] [Google Scholar]
- 611.Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res : 735–741, 2004. [DOI] [PubMed] [Google Scholar]
- 612.Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TV. The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem : 1154–1165, 2007. [DOI] [PubMed] [Google Scholar]
- 613.Reidelberger R, Haver A, Anders K, Apenteng B. Role of capsaicin-sensitive peripheral sensory neurons in anorexic responses to intravenous infusions of cholecystokinin, peptide YY-(3–36), and glucagon-like peptide-1 in rats. Am J Physiol Endocrinol Metab : E619–E629, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Reidelberger R, Haver A, Chelikani PK. Role of peptide YY(3–36) in the satiety produced by gastric delivery of macronutrients in rats. Am J Physiol Endocrinol Metab : E944–E950, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol : R1005–R1012, 2004. [DOI] [PubMed] [Google Scholar]
- 616.Reidelberger RD, Varga G, Liehr RM, Castellanos DA, Rosenquist GL, Wong HC, Walsh JH. Cholecystokinin suppresses food intake by a nonendocrine mechanism in rats. Am J Physiol Regul Integr Comp Physiol : R901–R908, 1994. [DOI] [PubMed] [Google Scholar]
- 617.Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J : 236–242, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab : 532–539, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Richard JE, Anderberg RH, Goteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One : e0119034, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson JO, Liposits Z, Skibicka KP. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology : 4356–4367, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care : 2508–2514, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Rieu PN, Jansen JB, Hopman WP, Joosten HJ, Lamers CB. Effect of partial gastrectomy with Billroth II or Roux-en-Y anastomosis on postprandial and cholecystokinin-stimulated gallbladder contraction and secretion of cholecystokinin and pancreatic polypeptide. Dig Dis Sci : 1066–1072, 1990. [DOI] [PubMed] [Google Scholar]
- 623.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res : 18–34, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Rinaman L. Hindbrain contributions to anorexia. Am J Physiol Regul Integr Comp Physiol : R1035–R1036, 2004. [DOI] [PubMed] [Google Scholar]
- 625.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol : R222–R235, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Roberts K, Duffy A, Kaufman J, Burrell M, Dziura J, Bell R. Size matters: gastric pouch size correlates with weight loss after laparoscopic Roux-en-Y gastric bypass. Surg Endosc : 1397–1402, 2007. [DOI] [PubMed] [Google Scholar]
- 627.Roberts RE, Glicksman C, Alaghband-Zadeh J, Sherwood RA, Akuji N, le Roux CW. The relationship between postprandial bile acid concentration, GLP-1, PYY and ghrelin. Clin Endocrinol : 67–72, 2011. [DOI] [PubMed] [Google Scholar]
- 628.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology : 1687–1694, 1999. [DOI] [PubMed] [Google Scholar]
- 629.Rolls BJ. What is the role of portion control in weight management? Int J Obes Suppl 1: S1–8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Rolls BJ, Bell EA, Thorwart ML. Water incorporated into a food but not served with a food decreases energy intake in lean women. Am J Clin Nutr : 448–455, 1999. [DOI] [PubMed] [Google Scholar]
- 631.Rolls BJ, Bell EA, Waugh BA. Increasing the volume of a food by incorporating air affects satiety in men. Am J Clin Nutr : 361–368, 2000. [DOI] [PubMed] [Google Scholar]
- 632.Rolls BJ, Roe LS. Effect of the volume of liquid food infused intragastrically on satiety in women. Physiol Behav : 623–631, 2002. [DOI] [PubMed] [Google Scholar]
- 633.Roth CL, Enriori PJ, Harz K, Woelfle J, Cowley MA, Reinehr T. Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J Clin Endocrinol Metab : 6386–6391, 2005. [DOI] [PubMed] [Google Scholar]
- 634.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg : 236–242, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Runchey SS, Valsta LM, Schwarz Y, Wang C, Song X, Lampe JW, Neuhouser ML. Effect of low- and high-glycemic load on circulating incretins in a randomized clinical trial. Metabolism : 188–195, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Rushakoff RA, Goldfine ID, Beccaria LJ, Mathur A, Brand RJ, Liddle RA. Reduced postprandial cholecystokinin (CCK) secretion in patients with noninsulin-dependent diabetes mellitus: evidence for a role for CCK in regulating postprandial hyperglycemia. J Clin Endocrinol Metab : 489–493, 1993. [DOI] [PubMed] [Google Scholar]
- 637.Rushakoff RJ, Goldfine ID, Carter JD, Liddle RA. Physiological concentrations of cholecystokinin stimulate amino acid-induced insulin release in humans. J Clin Endocrinol Metab : 395–401, 1987. [DOI] [PubMed] [Google Scholar]
- 638.Ruttimann EB, Arnold M, Geary N, Langhans W. GLP-1 antagonism with exendin (9–39) fails to increase spontaneous meal size in rats. Physiol Behav : 291–296, 2010. [DOI] [PubMed] [Google Scholar]
- 639.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology : 1174–1181, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Ryan AT, Feinle-Bisset C, Kallas A, Wishart JM, Clifton PM, Horowitz M, Luscombe-Marsh ND. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr : 474–482, 2012. [DOI] [PubMed] [Google Scholar]
- 641.Ryan AT, Luscombe-Marsh ND, Saies AA, Little TJ, Standfield S, Horowitz M, Feinle-Bisset C. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr : 300–311, 2013. [DOI] [PubMed] [Google Scholar]
- 642.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature : 183–188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes : 2691–2700, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, Boyadjian R. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab : 3997–4000, 2002. [DOI] [PubMed] [Google Scholar]
- 645.Salaun PY, Querellou S, Nguyen JM, Bodet-Milin C, Carlier T, Turzo A, Bizais Y, Couturier O. Comparison of gastric emptying scintigraphy based on the geometric mean of the gastric proportion of the abdominal radioactivity or on the geometric mean of the intragastric radioactivity. Nucl Med Commun : 431–437, 2006. [DOI] [PubMed] [Google Scholar]
- 646.Salehi M, D'Alessio DA. Effects of glucagon like peptide-1 to mediate glycemic effects of weight loss surgery. Rev Endocr Metab Disord : 171–179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Salehi M, Gastaldelli A, D'Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab : 2008–2017, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology : 669–680, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes : 2308–2314, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Salehi M, Vahl TP, D'Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab : 4909–4916, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Sam AH, Gunner DJ, King A, Persaud SJ, Brooks L, Hostomska K, Ford HE, Liu B, Ghatei MA, Bloom SR, Bewick GA. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology : 459–468, 2012. [DOI] [PubMed] [Google Scholar]
- 652.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes : 2046–2054, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Sandoval DA, D'Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev : 513–548, 2015. [DOI] [PubMed] [Google Scholar]
- 654.Sanmiguel CP, Conklin JL, Cunneen SA, Barnett P, Phillips EH, Kipnes M, Pilcher J, Soffer EE. Gastric electrical stimulation with the TANTALUS System in obese type 2 diabetes patients: effect on weight and glycemic control. J Diabetes Sci Technol : 964–970, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Santangelo A, Peracchi M, Conte D, Fraquelli M, Porrini M. Physical state of meal affects gastric emptying, cholecystokinin release and satiety. Br J Nutr : 521–527, 1998. [DOI] [PubMed] [Google Scholar]
- 656.Sarr MG, Billington CJ, Brancatisano R, Brancatisano A, Toouli J, Kow L, Nguyen NT, Blackstone R, Maher JW, Shikora S, Reeds DN, Eagon JC, Wolfe BM, O'Rourke RW, Fujioka K, Takata M, Swain JM, Morton JM, Ikramuddin S, Schweitzer M, Chand B, Rosenthal R, Group ES. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg : 1771–1782, 2012. [DOI] [PubMed] [Google Scholar]
- 657.Sathananthan A, Man CD, Micheletto F, Zinsmeister AR, Camilleri M, Giesler PD, Laugen JM, Toffolo G, Rizza RA, Cobelli C, Vella A. Common genetic variation in GLP1R and insulin secretion in response to exogenous GLP-1 in nondiabetic subjects: a pilot study. Diabetes Care : 2074–2076, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Sathananthan M, Ikramuddin S, Swain JM, Shah M, Piccinini F, Dalla Man C, Cobelli C, Rizza RA, Camilleri M, Vella A. The effect of vagal nerve blockade using electrical impulses on glucose metabolism in nondiabetic subjects. Diabetes Metab Syndr Obes : 305–312, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut : 166–170, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity : 421–423, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Schellekens H, De Francesco PN, Kandil D, Theeuwes WF, McCarthy T, van Oeffelen WE, Perello M, Giblin L, Dinan TG, Cryan JF. Ghrelin's orexigenic effect is modulated via a serotonin 2C receptor interaction. ACS Chem Neurosci : 1186–1197, 2015. [DOI] [PubMed] [Google Scholar]
- 662.Schirra J, Houck P, Wank U, Arnold R, Goke B, Katschinski M. Effects of glucagon-like peptide-1(7–36)amide on antro-pyloro-duodenal motility in the interdigestive state and with duodenal lipid perfusion in humans. Gut : 622–631, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest : 92–103, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Goke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut : 243–251, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Schirra J, Nicolaus M, Woerle HJ, Struckmeier C, Katschinski M, Goke B. GLP-1 regulates gastroduodenal motility involving cholinergic pathways. Neurogastroenterol Motil : 609–618, 2009. [DOI] [PubMed] [Google Scholar]
- 666.Schirra J, Wank U, Arnold R, Goke B, Katschinski M. Effects of glucagon-like peptide-1(7–36)amide on motility and sensation of the proximal stomach in humans. Gut : 341–348, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Schmidt WE, Creutzfeldt W, Hocker M, Nustede R, Choudhury AR, Schleser A, Rovati LC, Folsch UR. Cholecystokinin receptor antagonist loxiglumide modulates plasma levels of gastro-entero-pancreatic hormones in man. Feedback control of cholecystokinin and gastrin secretion. Eur J Clin Invest : 501–511, 1991. [DOI] [PubMed] [Google Scholar]
- 668.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, Vincent RP, Alaghband-Zadeh J, Ghatei MA, Waldman AD, Frost GS, Bell JD, le Roux CW, Goldstone AP. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut : 891–902, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Schonhoff S, Baggio L, Ratineau C, Ray SK, Lindner J, Magnuson MA, Drucker DJ, Leiter AB. Energy homeostasis and gastrointestinal endocrine differentiation do not require the anorectic hormone peptide YY. Mol Cell Biol : 4189–4199, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Schvarcz E, Palmer M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology : 60–66, 1997. [DOI] [PubMed] [Google Scholar]
- 671.Schwartz GJ, McHugh PR, Moran TH. Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am J Physiol Regul Integr Comp Physiol : R872–R876, 1993. [DOI] [PubMed] [Google Scholar]
- 672.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature : 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 673.Schwarzendrube J, Niederau M, Luthen R, Niederau C. Effects of cholecystokinin-receptor blockade on pancreatic and biliary function in healthy volunteers. Gastroenterology : 1683–1690, 1991. [DOI] [PubMed] [Google Scholar]
- 674.Schwizer W, Borovicka J, Kunz P, Fraser R, Kreiss C, D'Amato M, Crelier G, Boesiger P, Fried M. Role of cholecystokinin in the regulation of liquid gastric emptying and gastric motility in humans: studies with the CCK antagonist loxiglumide. Gut : 500–504, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Sclafani A. Gut-brain nutrient signaling. Appetition vs satiation. Appetite : 454–458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3-36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol : 452–457, 2005. [DOI] [PubMed] [Google Scholar]
- 677.Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, Schaffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest : 4473–4488, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Seeley RJ, Chambers AP, Sandoval DA. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab : 369–378, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Seghieri M, Rebelos E, Gastaldelli A, Astiarraga BD, Casolaro A, Barsotti E, Pocai A, Nauck M, Muscelli E, Ferrannini E. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia : 156–161, 2013. [DOI] [PubMed] [Google Scholar]
- 680.Seifarth C, Bergmann J, Holst JJ, Ritzel R, Schmiegel W, Nauck MA. Prolonged and enhanced secretion of glucagon-like peptide 1 (7–36 amide) after oral sucrose due to alpha-glucosidase inhibition (acarbose) in Type 2 diabetic patients. Diabet Med : 485–491, 1998. [DOI] [PubMed] [Google Scholar]
- 681.Seimon RV, Brennan IM, Russo A, Little TJ, Jones KL, Standfield S, Wishart JM, Horowitz M, Feinle-Bisset C. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. Am J Physiol Endocrinol Metab : E294–E300, 2013. [DOI] [PubMed] [Google Scholar]
- 682.Seimon RV, Feltrin KL, Meyer JH, Brennan IM, Wishart JM, Horowitz M, Feinle-Bisset C. Effects of varying combinations of intraduodenal lipid and carbohydrate on antropyloroduodenal motility, hormone release, and appetite in healthy males. Am J Physiol Regul Integr Comp Physiol : R912–R920, 2009. [DOI] [PubMed] [Google Scholar]
- 683.Seimon RV, Lange K, Little TJ, Brennan IM, Pilichiewicz AN, Feltrin KL, Smeets AJ, Horowitz M, Feinle-Bisset C. Pooled-data analysis identifies pyloric pressures and plasma cholecystokinin concentrations as major determinants of acute energy intake in healthy, lean men. Am J Clin Nutr : 61–68, 2010. [DOI] [PubMed] [Google Scholar]
- 684.Shah M, Law JH, Micheletto F, Sathananthan M, Man CD, Cobelli C, Rizza RA, Camilleri M, Zinsmeister AR, Vella A. The contribution of endogenous glucagon-like peptide-1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes : 483–493, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Sheikh SP, Holst JJ, Orskov C, Ekman R, Schwartz TW. Release of PYY from pig intestinal mucosa; luminal and neural regulation. Regul Pept : 253–266, 1989. [DOI] [PubMed] [Google Scholar]
- 686.Shi YC, Hammerle CM, Lee IC, Turner N, Nguyen AD, Riepler SJ, Lin S, Sainsbury A, Herzog H, Zhang L. Adult-onset PYY overexpression in mice reduces food intake and increases lipogenic capacity. Neuropeptides : 173–182, 2012. [DOI] [PubMed] [Google Scholar]
- 687.Shi YC, Lin S, Castillo L, Aljanova A, Enriquez RF, Nguyen AD, Baldock PA, Zhang L, Bijker MS, Macia L, Yulyaningsih E, Zhang H, Lau J, Sainsbury A, Herzog H. Peripheral-specific y2 receptor knockdown protects mice from high-fat diet-induced obesity. Obesity : 2137–2148, 2011. [DOI] [PubMed] [Google Scholar]
- 688.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab : 240–244, 2002. [DOI] [PubMed] [Google Scholar]
- 689.Shikora S, Toouli J, Herrera MF, Kulseng B, Zulewski H, Brancatisano R, Kow L, Pantoja JP, Johnsen G, Brancatisano A, Tweden KS, Knudson MB, Billington CJ. Vagal blocking improves glycemic control and elevated blood pressure in obese subjects with type 2 diabetes mellitus. J Obes : 245683, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Shin AC, Berthoud HR. Food reward functions as affected by obesity and bariatric surgery. Int J Obes Suppl 3: S40–44, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology : 1588–1597, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Shrestha YB, Wickwire K, Giraudo SQ. Direct effects of nutrients, acetylcholine, CCK, and insulin on ghrelin release from the isolated stomachs of rats. Peptides : 1187–1191, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Siegel JA, Urbain JL, Adler LP, Charkes ND, Maurer AH, Krevsky B, Knight LC, Fisher RS, Malmud LS. Biphasic nature of gastric emptying. Gut : 85–89, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Silberbauer C, Frey-Rindova P, Langhans W. Breakfasts with different fiber and macronutrient contents do not differentially affect timing, size or microstructure of the subsequent lunch. Z Ernahrungswiss : 356–368, 1996. [DOI] [PubMed] [Google Scholar]
- 695.Sima E, Hedberg J, Sundbom M. Gastrointestinal symptoms, weight loss and patient satisfaction 5 years after gastric bypass: a study of three techniques for the gastrojejunal anastomosis. Surg Endosc : 1553–1558, 2016. [DOI] [PubMed] [Google Scholar]
- 696.Simonian HP, Kresge KM, Boden GH, Parkman HP. Differential effects of sham feeding and meal ingestion on ghrelin and pancreatic polypeptide levels: evidence for vagal efferent stimulation mediating ghrelin release. Neurogastroenterol Motil : 348–354, 2005. [DOI] [PubMed] [Google Scholar]
- 697.Simpson K, Parker J, Plumer J, Bloom S. CCK, PYY and PP: the control of energy balance. Handb Exp Pharmacol 209–230, 2012. [DOI] [PubMed] [Google Scholar]
- 698.Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest : 2456–2463, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci : 181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Skibicka KP, Shirazi RH, Hansson C, Dickson SL. Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward. Endocrinology : 1194–1205, 2012. [DOI] [PubMed] [Google Scholar]
- 701.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab : E1062–E1068, 2007. [DOI] [PubMed] [Google Scholar]
- 702.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition : 814–820, 2000. [DOI] [PubMed] [Google Scholar]
- 703.Smith GP. Gut hormone hypothesis of postprandial satiety. In: Eating and Its Disorders, edited by Stunkard AJ, Stella E. New York: Raven, 1984, p. 67–75. [PubMed] [Google Scholar]
- 704.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science : 1036–1037, 1981. [DOI] [PubMed] [Google Scholar]
- 705.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol Regul Integr Comp Physiol : R638–R641, 1985. [DOI] [PubMed] [Google Scholar]
- 706.Snyder DJ, Bartoshuk LM. Epidemiological studies of taste function: discussion and perspectives. Ann NY Acad Sci : 574–580, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab : 486–493, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Spiller RC, Trotman IF, Adrian TE, Bloom SR, Misiewicz JJ, Silk DB. Further characterisation of the “ileal brake” reflex in man–effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut : 1042–1051, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake–inhibition of jejunal motility after ileal fat perfusion in man. Gut : 365–374, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Stadlbauer U, Arnold M, Weber E, Langhans W. Possible mechanisms of circulating PYY-induced satiation in male rats. Endocrinology : 193–204, 2013. [DOI] [PubMed] [Google Scholar]
- 711.Starling EH. The Croonian lectures on the chemical correlation of the functions of the body. Lancet 339–341, 1905. [Google Scholar]
- 712.Stefater MA, Wilson-Perez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev : 595–622, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Steinert RE, Poller B, Castelli M, Drewe J, Beglinger C. Oral administration of glucagon-like peptide 1 or peptide YY 3–36 affects food intake in healthy male subjects. Am J Clin Nutr : 810–817, 2010. [DOI] [PubMed] [Google Scholar]
- 714.Steinert RE, Poller B, Castelli M, Friedman K, Huber A, Drewe J, Beglinger C. Orally administered glucagon-like peptide-1 affects glucose homeostasis following an oral glucose tolerance test in healthy male subjects. Clin Pharmacol Ther : 644–650, 2009. [DOI] [PubMed] [Google Scholar]
- 715.Steinert RE, Beglinger C. Nutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol Behav : 62–70, 2011. [DOI] [PubMed] [Google Scholar]
- 716.Steinert RE, Frey F, Toepfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr : 1320–1328, 2011. [DOI] [PubMed] [Google Scholar]
- 717.Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr : 524–532, 2011. [DOI] [PubMed] [Google Scholar]
- 718.Steinert RE, Luscombe-Marsh ND, Little TJ, Standfield S, Otto B, Horowitz M, Feinle-Bisset C. Effects of intraduodenal infusion of l-tryptophan on ad libitum eating, antropyloroduodenal motility, glycemia, insulinemia and gut peptide secretion in healthy men. J Clin Endocrinol Metab : 3275–32, 2014. [DOI] [PubMed] [Google Scholar]
- 719.Steinert RE, Meyer-Gerspach AC, Beglinger C. The role of the stomach in the control of appetite and the secretion of satiation peptides. Am J Physiol Endocrinol Metab : E666–E673, 2012. [DOI] [PubMed] [Google Scholar]
- 720.Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, Beglinger C. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity : E660–E668, 2013. [DOI] [PubMed] [Google Scholar]
- 721.Steinert RE, Schirra J, Meyer-Gerspach AC, Kienle P, Fischer H, Schulte F, Goeke B, Beglinger C. Effect of glucagon-like peptide-1 receptor antagonism on appetite and food intake in healthy men. Am J Clin Nutr : 514–523, 2014. [DOI] [PubMed] [Google Scholar]
- 722.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Tache Y, Reeve JR Jr. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology : 5113–5118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Sterling P. Allostasis: a model of predictive regulation. Physiol Behav : 5–15, 2012. [DOI] [PubMed] [Google Scholar]
- 724.Stevens JE, Jones KL, Rayner CK, Horowitz M. Pathophysiology and pharmacotherapy of gastroparesis: current and future perspectives. Expert Opin Pharmacother : 1171–1186, 2013. [DOI] [PubMed] [Google Scholar]
- 725.Stewart JE, Seimon RV, Otto B, Keast RS, Clifton PM, Feinle-Bisset C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr : 703–711, 2011. [DOI] [PubMed] [Google Scholar]
- 726.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci : 4360–4366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR, Chanoine JP. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab : 2161–2168, 2005. [DOI] [PubMed] [Google Scholar]
- 728.Stunkard AJ, Fox S. The relationship of gastric motility and hunger. A summary of the evidence. Psychosom Med : 123–134, 1971. [DOI] [PubMed] [Google Scholar]
- 729.Sturm K, MacIntosh CG, Parker BA, Wishart J, Horowitz M, Chapman IM. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J Clin Endocrinol Metab : 3747–3755, 2003. [DOI] [PubMed] [Google Scholar]
- 730.Sturm K, Parker B, Wishart J, Feinle-Bisset C, Jones KL, Chapman I, Horowitz M. Energy intake and appetite are related to antral area in healthy young and older subjects. Am J Clin Nutr : 656–667, 2004. [DOI] [PubMed] [Google Scholar]
- 731.Stylopoulos N, Davis P, Pettit JD, Rattner DW, Kaplan LM. Changes in serum ghrelin predict weight loss after Roux-en-Y gastric bypass in rats. Surg Endosc : 942–946, 2005. [DOI] [PubMed] [Google Scholar]
- 732.Sugiyama K, Manaka H, Kato T, Yamatani K, Tominaga M, Sasaki H. Stimulation of truncated glucagon-like peptide-1 release from the isolated perfused canine ileum by glucose absorption. Digestion : 24–28, 1994. [DOI] [PubMed] [Google Scholar]
- 733.Sundaresan S, Shahid R, Riehl TE, Chandra R, Nassir F, Stenson WF, Liddle RA, Abumrad NA. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J : 1191–1202, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Surina DM, Langhans W, Pauli R, Wenk C. Meal composition affects postprandial fatty acid oxidation. Am J Physiol Regul Integr Comp Physiol : R1065–R1070, 1993. [DOI] [PubMed] [Google Scholar]
- 735.Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery : 283–290, 2005. [DOI] [PubMed] [Google Scholar]
- 736.Svane MS, Bojsen-Moller KN, Nielsen S, Jorgensen NB, Dirksen C, Bendtsen F, Kristiansen VB, Hartmann B, Holst JJ, Madsbad S. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab : E505–E514, 2016. [DOI] [PubMed] [Google Scholar]
- 737.Svane MS, Jorgensen NB, Bojsen-Moller KN, Dirksen C, Nielsen S, Kristiansen VB, Torang S, Wewer Albrechtsen NJ, Rehfeld JF, Hartmann B, Madsbad S, Holst JJ. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond). In press. [DOI] [PubMed] [Google Scholar]
- 738.Svendsen B, Pedersen J, Albrechtsen NJ, Hartmann B, Torang S, Rehfeld JF, Poulsen SS, Holst JJ. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology : 847–857, 2015. [DOI] [PubMed] [Google Scholar]
- 739.Swartz TD, Savastano DM, Covasa M. Reduced sensitivity to cholecystokinin in male rats fed a high-fat diet is reversible. J Nutr : 1698–1703, 2010. [DOI] [PubMed] [Google Scholar]
- 740.Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol : 727–740, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr : 1453–1456, 2009. [DOI] [PubMed] [Google Scholar]
- 742.Sykaras AG, Demenis C, Cheng L, Pisitkun T, McLaughlin JT, Fenton RA, Smith CP. Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology : 3339–3351, 2014. [DOI] [PubMed] [Google Scholar]
- 743.Sysko R, Devlin MJ, Walsh BT, Zimmerli E, Kissileff HR. Satiety and test meal intake among women with binge eating disorder. Int J Eat Disord : 554–561, 2007. [DOI] [PubMed] [Google Scholar]
- 744.Szarka LA, Camilleri M. Methods for measurement of gastric motility. Am J Physiol Gastrointest Liver Physiol : G461–G475, 2009. [DOI] [PubMed] [Google Scholar]
- 745.Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol : 741–749, 2014. [DOI] [PubMed] [Google Scholar]
- 746.Tack J, Deloose E, Ang D, Scarpellini E, Vanuytsel T, Van Oudenhove L, Depoortere I. Motilin-induced gastric contractions signal hunger in man. Gut : 214–224, 2016. [DOI] [PubMed] [Google Scholar]
- 747.Tack J, Demedts I, Meulemans A, Schuurkes J, Janssens J. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut : 219–224, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther : 847–853, 2005. [DOI] [PubMed] [Google Scholar]
- 749.Tai K, Hammond AJ, Wishart JM, Horowitz M, Chapman IM. Carbohydrate and fat digestion is necessary for maximal suppression of total plasma ghrelin in healthy adults. Appetite : 407–412, 2010. [DOI] [PubMed] [Google Scholar]
- 750.Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab : 4908–4911, 2000. [DOI] [PubMed] [Google Scholar]
- 751.Takei I, Miyamoto K, Funae O, Ohashi N, Meguro S, Tokui M, Saruta T. Secretion of GIP in responders to acarbose in obese Type 2(NIDDM) patients. J Diabetes Complications : 245–249, 2001. [DOI] [PubMed] [Google Scholar]
- 752.Tanaka T, Katsuma S, Adachi T, Koshimizu Ta Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn-Schmiedebergs Arch Pharmacol : 523–527, 2008. [DOI] [PubMed] [Google Scholar]
- 753.Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab : 9–16, 2010. [DOI] [PubMed] [Google Scholar]
- 754.Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol Suppl 2: 251–262, 2008. [PubMed] [Google Scholar]
- 755.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D'Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab : 2963–2972, 2004. [DOI] [PubMed] [Google Scholar]
- 756.Thazhath SS, Marathe CS, Wu T, Chang J, Khoo J, Kuo P, Checklin HL, Bound MJ, Rigda RS, Crouch B, Jones KL, Horowitz M, Rayner CK. The glucagon-like peptide 1 receptor agonist exenatide inhibits small intestinal motility, flow, transit, and absorption of glucose in healthy subjects and patients with type 2 diabetes: a randomized controlled trial. Diabetes : 269–275, 2016. [DOI] [PubMed] [Google Scholar]
- 757.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schurmann A, Szanto I, Tschop MH, Rohner-Jeanrenaud F. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest : 1983–1993, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Theil PK, Jorgensen H, Serena A, Hendrickson J, Bach Knudsen KE. Products deriving from microbial fermentation are linked to insulinaemic response in pigs fed breads prepared from whole-wheat grain and wheat and rye ingredients. Br J Nutr : 373–383, 2011. [DOI] [PubMed] [Google Scholar]
- 759.Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab : E550–E559, 2006. [DOI] [PubMed] [Google Scholar]
- 760.Thimister PW, Hopman WP, Sloots CE, Rosenbusch G, Willems HL, Trijbels FJ, Jansen JB. Role of intraduodenal proteases in plasma cholecystokinin and pancreaticobiliary responses to protein and amino acids. Gastroenterology : 567–575, 1996. [DOI] [PubMed] [Google Scholar]
- 761.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab : 167–177, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, Hermansen K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr : 1135–1143, 1999. [DOI] [PubMed] [Google Scholar]
- 763.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab : 3717–3723, 2001. [DOI] [PubMed] [Google Scholar]
- 764.Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab : 3853–3860, 2001. [DOI] [PubMed] [Google Scholar]
- 765.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes : 364–371, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab : 59–67, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Tschop MH, D'Alessio D. Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans. J Clin Endocrinol Metab : 2536–2543, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Torang S, Bojsen-Moller KN, Svane MS, Hartmann B, Rosenkilde MM, Madsbad S, Holst JJ. In vivo and in vitro degradation of peptide YY3-36 to inactive peptide YY3-34 in humans. Am J Physiol Regul Integr Comp Physiol : R866–R874, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Torang S, Veedfald S, Rosenkilde MM, Hartmann B, Holst JJ. The anorexic hormone Peptide YY3-36 is rapidly metabolized to inactive Peptide YY3-34 in vivo. Physiol Rep : 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Torra S, Ilzarbe L, Malagelada JR, Negre M, Mestre-Fusco A, Aguade-Bruix S, Florensa E, Sune P, Gras B, Hernandez JJ, Casamitjana R, Garcia MA, Ros FB, Delgado-Aros S. Meal size can be decreased in obese subjects through pharmacological acceleration of gastric emptying (The OBERYTH trial). Int J Obes (Lond) : 829–837, 2011. [DOI] [PubMed] [Google Scholar]
- 771.Tosh SM. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr : 310–317, 2013. [DOI] [PubMed] [Google Scholar]
- 772.Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, Hocking MP, Quigley EM, Koch KL, Tokayer AZ, Stanghellini V, Chen Y, Huizinga JD, Ryden J, Bourgeois I, McCallum RW. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol : 1456–1462, 2000. [DOI] [PubMed] [Google Scholar]
- 773.Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, Perino A, Brighton CA, Sebti Y, Kluza J, Briand O, Dehondt H, Vallez E, Dorchies E, Baud G, Spinelli V, Hennuyer N, Caron S, Bantubungi K, Caiazzo R, Reimann F, Marchetti P, Lefebvre P, Backhed F, Gribble FM, Schoonjans K, Pattou F, Tailleux A, Staels B, Lestavel S. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun : 7629, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Trahair LG, Horowitz M, Rayner CK, Gentilcore D, Lange K, Wishart JM, Jones KL. Comparative effects of variations in duodenal glucose load on glycemic, insulinemic, and incretin responses in healthy young and older subjects. J Clin Endocrinol Metab : 844–851, 2012. [DOI] [PubMed] [Google Scholar]
- 775.Trevaskis JL, Parkes DG, Roth JD. Insights into amylin-leptin synergy. Trends Endocrinol Metab : 473–479, 2010. [DOI] [PubMed] [Google Scholar]
- 776.Trevaskis JL, Sun C, Athanacio J, D'Souza L, Samant M, Tatarkiewicz K, Griffin PS, Wittmer C, Wang Y, Teng CH, Forood B, Parkes DG, Roth JD. Synergistic metabolic benefits of an exenatide analogue and cholecystokinin in diet-induced obese and leptin-deficient rodents. Diabetes Obes Metab : 61–73, 2015. [DOI] [PubMed] [Google Scholar]
- 777.Trevaskis JL, Turek VF, Griffin PS, Wittmer C, Parkes DG, Roth JD. Multi-hormonal weight loss combinations in diet-induced obese rats: therapeutic potential of cholecystokinin? Physiol Behav : 187–195, 2010. [DOI] [PubMed] [Google Scholar]
- 778.Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest : RC19–21, 2001. [DOI] [PubMed] [Google Scholar]
- 779.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes : 707–709, 2001. [DOI] [PubMed] [Google Scholar]
- 780.Uchida A, Zechner JF, Mani BK, Park WM, Aguirre V, Zigman JM. Altered ghrelin secretion in mice in response to diet-induced obesity and Roux-en-Y gastric bypass. Mol Metab : 717–730, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Umapathysivam MM, Lee MY, Jones KL, Annink CE, Cousins CE, Trahair LG, Rayner CK, Chapman MJ, Nauck MA, Horowitz M, Deane AM. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide-1 receptor on gastric emptying and glycaemia. Diabetes : 785–790, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Unger RH, Eisentraut AM. Entero-insular axis. Arch Intern Med : 261–266, 1969. [PubMed] [Google Scholar]
- 783.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev : 187–215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Uttenthal LO, Ukponmwan OO, Ghiglione M, Bloom SR. Acute and short term effects of intestinal alpha-glucosidase inhibition on gut hormone responses in man. Dig Dis Sci : 139–144, 1987. [DOI] [PubMed] [Google Scholar]
- 785.Uttenthal LO, Ukponmwan OO, Wood SM, Ghiglione M, Ghatei MA, Trayner IM, Bloom SR. Long-term effects of intestinal alpha-glucosidase inhibition on postprandial glucose, pancreatic and gut hormone responses and fasting serum lipids in diabetics on sulphonylureas. Diabet Med : 155–160, 1986. [DOI] [PubMed] [Google Scholar]
- 786.Vahl TP, Drazen DL, Seeley RJ, D'Alessio DA, Woods SC. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology : 569–575, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Van Avesaat M, Troost FJ, Ripken D, Hendriks HF, Masclee AA. Ileal brake activation: macronutrient-specific effects on eating behavior? Int J Obes (Lond) : 235–243, 2015. [DOI] [PubMed] [Google Scholar]
- 788.Van Bloemendaal L, IJzerman RG, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, Veltman DJ, Diamant M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes : 4186–4196, 2014. [DOI] [PubMed] [Google Scholar]
- 789.Van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) : 784–793, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Van den Hoek AM, Heijboer AC, Corssmit EP, Voshol PJ, Romijn JA, Havekes LM, Pijl H. PYY3-36 reinforces insulin action on glucose disposal in mice fed a high-fat diet. Diabetes : 1949–1952, 2004. [DOI] [PubMed] [Google Scholar]
- 791.Vandenbergh J, Dupont P, Fischler B, Bormans G, Persoons P, Janssens J, Tack J. Regional brain activation during proximal stomach distention in humans: a positron emission tomography study. Gastroenterology : 564–573, 2005. [DOI] [PubMed] [Google Scholar]
- 792.Vandevijvere S, Chow CC, Hall KD, Umali E, Swinburn BA. Increased food energy supply as a major driver of the obesity epidemic: a global analysis. Bull World Health Organ : 446–456, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Vanis L, Hausken T, Gentilcore D, Rigda RS, Rayner CK, Feinle-Bisset C, Horowitz M, Jones KL. Comparative effects of glucose and xylose on blood pressure, gastric emptying and incretin hormones in healthy older subjects. Br J Nutr : 1644–1651, 2011. [DOI] [PubMed] [Google Scholar]
- 794.Vazquez Roque MI, Camilleri M, Stephens DA, Jensen MD, Burton DD, Baxter KL, Zinsmeister AR. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology : 1717–1724, 2006. [DOI] [PubMed] [Google Scholar]
- 795.Velchik MG, Reynolds JC, Alavi A. The effect of meal energy content on gastric emptying. J Nucl Med : 1106–1110, 1989. [PubMed] [Google Scholar]
- 796.Verdich C, Flint A, Gutzwiller JP, Naslund E, Beglinger C, Hellstrom PM, Long SJ, Morgan LM, Holst JJ, Astrup A. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab : 4382–4389, 2001. [DOI] [PubMed] [Google Scholar]
- 797.Verdich C, Madsen JL, Toubro S, Buemann B, Holst JJ, Astrup A. Effect of obesity and major weight reduction on gastric emptying. Int J Obes Relat Metab Disord : 899–905, 2000. [DOI] [PubMed] [Google Scholar]
- 798.Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety–effect of obesity and weight reduction. Int J Obes Relat Metab Disord : 1206–1214, 2001. [DOI] [PubMed] [Google Scholar]
- 799.Verhulst PJ, Depoortere I. Ghrelin's second life: from appetite stimulator to glucose regulator. World J Gastroenterol : 3183–3195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Vestergaard ET, Hansen TK, Gormsen LC, Jakobsen P, Moller N, Christiansen JS, Jorgensen JO. Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. Am J Physiol Endocrinol Metab : E1829–E1836, 2007. [DOI] [PubMed] [Google Scholar]
- 801.Viardot A, Heilbronn LK, Herzog H, Gregersen S, Campbell LV. Abnormal postprandial PYY response in insulin sensitive nondiabetic subjects with a strong family history of type 2 diabetes. Int J Obes (Lond) : 943–948, 2008. [DOI] [PubMed] [Google Scholar]
- 802.Villar HV, Wangenteen SL, Burks TF, Patton DD. Mechanisms of satiety and gastric emptying after gastric partitioning and bypass. Surgery : 229–236, 1981. [PubMed] [Google Scholar]
- 803.Vilsboll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept : 115–121, 2003. [DOI] [PubMed] [Google Scholar]
- 804.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab : 2706–2713, 2003. [DOI] [PubMed] [Google Scholar]
- 805.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes : 678–687, 2008. [DOI] [PubMed] [Google Scholar]
- 806.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol : R470–R478, 2003. [DOI] [PubMed] [Google Scholar]
- 807.Walther C, Morl K, Beck-Sickinger AG. Neuropeptide Y receptors: ligand binding and trafficking suggest novel approaches in drug development. J Pept Sci : 233–246, 2011. [DOI] [PubMed] [Google Scholar]
- 808.Wang G, Agenor K, Pizot J, Kotler DP, Harel Y, Van Der Schueren BJ, Quercia I, McGinty J, Laferrere B. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP). Obes Surg : 1263–1267, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, Korner J, Bauman A, Fowler JS, Thanos PK, Volkow ND. Gastric distention activates satiety circuitry in the human brain. Neuroimage : 1824–1831, 2008. [DOI] [PubMed] [Google Scholar]
- 810.Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab : 64–72, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Wang Y, Chandra R, Samsa LA, Gooch B, Fee BE, Cook JM, Vigna SR, Grant AO, Liddle RA. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol : G528–G537, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Wang Y, Prpic V, Green GM, Reeve JR Jr, Liddle RA. Luminal CCK-releasing factor stimulates CCK release from human intestinal endocrine and STC-1 cells. Am J Physiol Gastrointest Liver Physiol : G16–G22, 2002. [DOI] [PubMed] [Google Scholar]
- 813.Wansink B. From mindless eating to mindlessly eating better. Physiol Behav : 454–463, 2010. [DOI] [PubMed] [Google Scholar]
- 814.Washington MC, Williams K, Sayegh AI. The feeding responses evoked by endogenous cholecystokinin are regulated by different gastrointestinal sites. Horm Behav : 79–85, 2015. [DOI] [PubMed] [Google Scholar]
- 815.Wasse LK, Sunderland C, King JA, Batterham RL, Stensel DJ. Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. J Appl Physiol (1985) : 552–559, 2012. [DOI] [PubMed] [Google Scholar]
- 816.Waters MJ. Endocrinology: the next 60 years–the helix and the chip. J Endocrinol : 11–12, 2006. [DOI] [PubMed] [Google Scholar]
- 817.Wehrwein EA, Carter JR. The mind matters: psychology as an overlooked variable within physiology studies. Physiology : 74–75, 2016. [DOI] [PubMed] [Google Scholar]
- 818.Weise CM, Thiyyagura P, Reiman EM, Chen K, Krakoff J. Postprandial plasma PYY concentrations are associated with increased regional gray matter volume and rCBF declines in caudate nuclei–a combined MRI and H2(15)O PET study. Neuroimage : 592–600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Wen J, Phillips SF, Sarr MG, Kost LJ, Holst JJ. PYY and GLP-1 contribute to feedback inhibition from the canine ileum and colon. Am J Physiol Gastrointest Liver Physiol : G945–G952, 1995. [DOI] [PubMed] [Google Scholar]
- 820.Werling M, Vincent RP, Cross GF, Marschall HU, Fandriks L, Lonroth H, Taylor DR, Alaghband-Zadeh J, Olbers T, Le Roux CW. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol : 1257–1264, 2013. [DOI] [PubMed] [Google Scholar]
- 821.West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol Regul Integr Comp Physiol : R776–R787, 1984. [DOI] [PubMed] [Google Scholar]
- 822.Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci : 665–673, 1993. [DOI] [PubMed] [Google Scholar]
- 823.Wickbom J, Herrington MK, Permert J, Jansson A, Arnelo U. Gastric emptying in response to IAPP and CCK in rats with subdiaphragmatic afferent vagotomy. Regul Pept : 21–25, 2008. [DOI] [PubMed] [Google Scholar]
- 824.Widmayer P, Breer H, Hass N. Candidate chemosensory cells in the porcine stomach. Histochem Cell Biol : 37–45, 2011. [DOI] [PubMed] [Google Scholar]
- 825.Widmayer P, Kuper M, Kramer M, Konigsrainer A, Breer H. Altered expression of gustatory-signaling elements in gastric tissue of morbidly obese patients. Int J Obes (Lond) : 1353–1359, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Wierup N, Bjorkqvist M, Westrom B, Pierzynowski S, Sundler F, Sjolund K. Ghrelin and motilin are cosecreted from a prominent endocrine cell population in the small intestine. J Clin Endocrinol Metab : 3573–3581, 2007. [DOI] [PubMed] [Google Scholar]
- 827.Williams DL. Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiol Behav : 194–199, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology : 1680–1687, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology : 2765–2767, 2003. [DOI] [PubMed] [Google Scholar]
- 830.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology : 5184–5187, 2003. [DOI] [PubMed] [Google Scholar]
- 831.Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, Torregrossa AM. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav : 557–564, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Williams KE, Washington MC, Johnson-Rouse T, Johnson RE, Freeman C, Reed C, Heath J, Sayegh AI. Exogenous glucagon-like peptide-1 acts in sites supplied by the cranial mesenteric artery to reduce meal size and prolong the intermeal interval in rats. Appetite : 254–259, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Wilson JD. The evolution of endocrinology. Clin Endocrinol : 389–396, 2005. [DOI] [PubMed] [Google Scholar]
- 834.Witte AB, Gryback P, Holst JJ, Hilsted L, Hellstrom PM, Jacobsson H, Schmidt PT. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul Pept : 57–62, 2009. [DOI] [PubMed] [Google Scholar]
- 835.Woerle HJ, Carneiro L, Derani A, Goke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes : 2349–2358, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836.Woods SC. Gastrointestinal satiety signals. I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol : G7–G13, 2004. [DOI] [PubMed] [Google Scholar]
- 837.Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab : S37–50, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Wortley KE, Garcia K, Okamoto H, Thabet K, Anderson KD, Shen V, Herman JP, Valenzuela D, Yancopoulos GD, Tschop MH, Murphy A, Sleeman MW. Peptide YY regulates bone turnover in rodents. Gastroenterology : 1534–1543, 2007. [DOI] [PubMed] [Google Scholar]
- 839.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab : 5992, 2001. [DOI] [PubMed] [Google Scholar]
- 840.Wu T, Bound MJ, Standfield SD, Gedulin B, Jones KL, Horowitz M, Rayner CK. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab : 474–477, 2012. [DOI] [PubMed] [Google Scholar]
- 841.Wu T, Bound MJ, Standfield SD, Jones KL, Horowitz M, Rayner CK. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab : E718–722, 2013. [DOI] [PubMed] [Google Scholar]
- 842.Wu T, Ma J, Bound MJ, Checklin H, Deacon CF, Jones KL, Horowitz M, Rayner CK. Effects of sitagliptin on glycemia, incretin hormones, and antropyloroduodenal motility in response to intraduodenal glucose infusion in healthy lean and obese humans, and patients with type 2 diabetes treated with or without metformin. Diabetes : 2776–2787, 2014. [DOI] [PubMed] [Google Scholar]
- 843.Wu T, Rayner CK, Young RL, Horowitz M. Gut motility and enteroendocrine secretion. Curr Opin Pharmacol : 928–934, 2013. [DOI] [PubMed] [Google Scholar]
- 844.Wu T, Thazhath SS, Marathe CS, Bound MJ, Jones KL, Horowitz M, Rayner CK. Comparative effect of intraduodenal and intrajejunal glucose infusion on the gut-incretin axis response in healthy males. Nutr Diabetes : e156, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.Wu T, Zhao BR, Bound MJ, Checklin HL, Bellon M, Little TJ, Young RL, Jones KL, Horowitz M, Rayner CK. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr : 78–83, 2012. [DOI] [PubMed] [Google Scholar]
- 846.Yan W, Polidori D, Yieh L, Di J, Wu X, Moreno V, Li L, Briscoe CP, Shankley N, Dohm GL, Pories WJ. Effects of meal size on the release of GLP-1 and PYY after Roux-en-Y gastric bypass surgery in obese subjects with or without type 2 diabetes. Obes Surg : 1969–1974, 2014. [DOI] [PubMed] [Google Scholar]
- 847.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell : 387–396, 2008. [DOI] [PubMed] [Google Scholar]
- 848.Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Munzberg H, Morrison CD, Drucker DJ, Berthoud HR. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol : R352–R362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849.Young RL. Sensing via intestinal sweet taste pathways. Front Neurosci : 23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Zhang J, Ritter RC. Circulating GLP-1 and CCK-8 reduce food intake by capsaicin-insensitive, nonvagal mechanisms. Am J Physiol Regul Integr Comp Physiol : R264–R273, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.Zhang L, Riepler SJ, Turner N, Enriquez RF, Lee IC, Baldock PA, Herzog H, Sainsbury A. Y2 and Y4 receptor signaling synergistically act on energy expenditure and physical activity. Am J Physiol Regul Integr Comp Physiol : R1618–R1628, 2010. [DOI] [PubMed] [Google Scholar]
- 852.Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci USA : 15868–15873, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Zheng J, Ariga H, Taniguchi H, Ludwig K, Takahashi T. Ghrelin regulates gastric phase III-like contractions in freely moving conscious mice. Neurogastroenterol Motil : 78–84, 2009. [DOI] [PubMed] [Google Scholar]
- 854.Zhou D, Jiang X, Ding W, Zhang D, Yang L, Zhen C, Lu L. Impact of bariatric surgery on ghrelin and obestatin levels in obesity or type 2 diabetes mellitus rat model. J Diabetes Res : 569435, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Ziessman HA, Fahey FH, Atkins FB, Tall J. Standardization and quantification of radionuclide solid gastric-emptying studies. J Nucl Med : 760–764, 2004. [PubMed] [Google Scholar]
- 856.Zwirska-Korczala K, Konturek SJ, Sodowski M, Wylezol M, Kuka D, Sowa P, Adamczyk-Sowa M, Kukla M, Berdowska A, Rehfeld JF, Bielanski W, Brzozowski T. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol Suppl 1: 13–35, 2007. [PubMed] [Google Scholar]