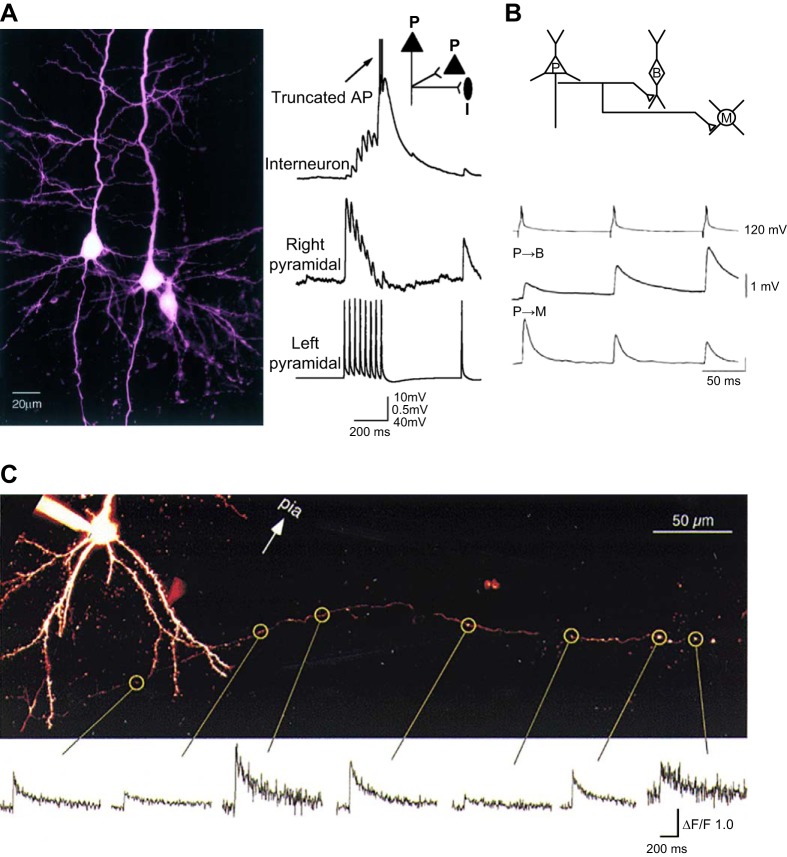
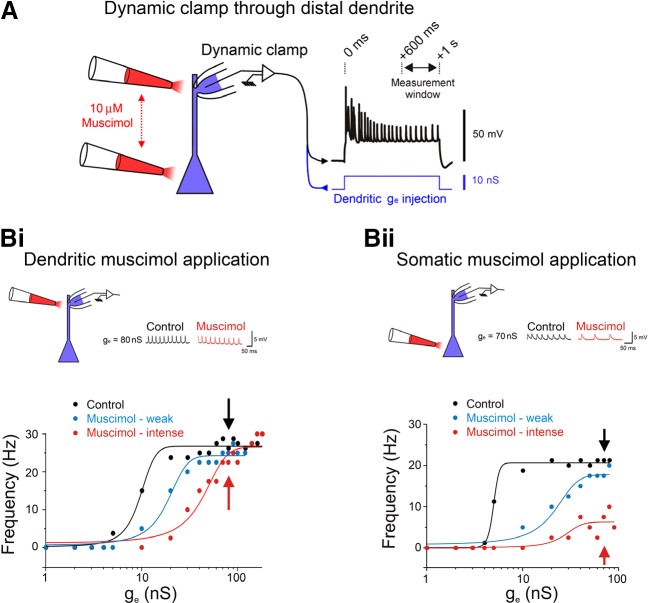
Abstract
In the hippocampus GABAergic local circuit inhibitory interneurons represent only ~10–15% of the total neuronal population; however, their remarkable anatomical and physiological diversity allows them to regulate virtually all aspects of cellular and circuit function. Here we provide an overview of the current state of the field of interneuron research, focusing largely on the hippocampus. We discuss recent advances related to the various cell types, including their development and maturation, expression of subtype-specific voltage- and ligand-gated channels, and their roles in network oscillations. We also discuss recent technological advances and approaches that have permitted high-resolution, subtype-specific examination of their roles in numerous neural circuit disorders and the emerging therapeutic strategies to ameliorate such pathophysiological conditions. The ultimate goal of this review is not only to provide a touchstone for the current state of the field, but to help pave the way for future research by highlighting where gaps in our knowledge exist and how a complete appreciation of their roles will aid in future therapeutic strategies.
I. INTRODUCTION
In hippocampus, GABAergic local circuit inhibitory interneurons account for ~10–15% of the total neuronal cell population. In a 30-day-old Wistar rat it has been estimated that the total CA1 hippocampal neuronal population is ~350,000, which contains a conservative estimate of ~38,500 inhibitory interneurons (102). Despite being in the minority, this diverse neuronal population serves as a major determinant of virtually all aspects of cortical circuit function and regulation. Across all subfields of the hippocampus, the cell bodies of glutamatergic pyramidal neurons are organized in a three- to five-cell-deep laminar arrangement in stratum pyramidale (s.p.) and have orthogonal dendrites that span from the deep stratum oriens (s.o.) to the superficial layers of the stratum lacunosum moleculare (s.l.m.). This organization permits pyramidal neurons to receive afferent input from a variety of both intrinsic and extrinsic sources across well-defined dendritic domains. In contrast, inhibitory interneurons, which by definition release the neurotransmitter GABA, have their cell bodies scattered throughout all major subfields, and the positioning of their somatodendritic arbors allows them to integrate from a more restricted intrinsic and extrinsic afferent input repertoire than their pyramidal cell counterparts. The axons of many interneuron subtypes can remain local to the subfield housing their soma and dendrites, although some interneurons possess axons that cross considerable distances to innervate distinct subcellular compartments or alternatively form long range projections that extend beyond their original central location to ramify within both cortical and subcortical structures. Their axons can target well-defined narrow postsynaptic domains (i.e., soma and proximal dendrites) or can provide widespread input to large portions of target cell dendrites. This innervation of different postsynaptic cellular compartments ensures that virtually all domains of their principal cell targets receive extensive coverage and importantly introduces the concept that each interneuron subtype performs a distinct role in the hippocampal circuit. Interneurons are primarily providers of inhibitory GABAergic synaptic input, a physiological role that utilizes Cl− influx or K+ efflux via cognate GABAA or GABAB receptor activation, respectively, to transiently hyperpolarize or shunt the cell membrane away from action potential threshold. They play major roles in not only the regulation of single cell excitability, but provide well-timed inhibitory input that dictates the temporal window for synaptic excitation, and subsequent action potential initiation, thus shaping the timing of afferent and efferent information flow. In addition, they harness and synchronize both local and distributed cortical circuits to facilitate oscillatory activity across broad frequency domains.
In 1996 Freund and Buzsaki (352) published a seminal and comprehensive review of the state of the field of inhibitory interneuron research, which served as a manifesto for subsequent research in the decades that followed. Rereading their review today we are struck by the observation that at that time the field was dominated by careful and precise anatomical investigations, with only a small number of laboratories performing any cellular electrophysiological or circuit analysis of their function either in vitro or in vivo. Moreover, little was known about interneuron embryogenesis and development, and our appreciation of the roles inhibitory interneurons played in neuronal circuit disorders was primarily focused on their role in the epilepsies. Indeed, a PUBMED search of the term inhibitory interneuron up to 1996 reveals a little under 1,000 relevant publications. In contrast, between 2011 and 2016, there were >2,500 publications on hippocampal interneurons. This surge in interest has precipitated development and adoption of exciting new tools that are being used to interrogate the roles played by specific interneuron cohorts in virtually every aspect of cortical development and circuit function as well as their participation in a number of cortical circuit disorders. Indeed, this is an exciting time for inhibitory interneuron research.
During the planning phase of this review it became clear that this might be one of the last times that any attempt should be made to provide a compendium of the field of hippocampal inhibitory interneurons. Indeed, the request to write a review on “inhibitory interneurons” seems in hindsight somewhat ridiculous given their role in virtually all aspects of cortical development and function and would be akin to asking someone to write a review of pyramidal cells; the literature is simply too vast. However, we decided that we would indeed make an attempt, to document what we consider to be the most important aspects of interneuron research, to highlight the appreciation of the important roles played by this diverse cell population, and to posit questions we feel are important for future research. Accordingly, we have endeavored to cover as many aspects of hippocampal interneuron anatomy, intrinsic and synaptic physiology, circuit connectivity, and their roles in oscillations and nervous system disorders as we possibly could. However, it is impossible to discuss every aspect of these extensive fields in full, and where possible we have indicated other review articles or original research that we consider important for a true appreciation of many of these topics. It is often hard to comprehend how far this field has come in such a short time. However, as will be evident to anybody reading this review, it is also clear that only a very small number of cell types have been systematically explored in great depth, leaving the study of many other interneurons incomplete but tantalizingly tractable given the numerous emerging tools and mouse reporter lines.
II. ANATOMY
One of the most striking features of cortical GABAergic interneurons is their remarkable anatomical diversity. The variety in morphological features of interneurons originally revealed by Golgi impregnation inspired Santiago Ramon y Cajal in the early 20th century to suggest that “. . . the functional superiority of the human brain is intimately bound up with the prodigious abundance and unusual wealth of forms of the so-called neurons with short axon . . .” (933). Cajal argued that the particular elaboration of diverse interneurons in higher primates was responsible for more complex brain functions and characterized interneurons as “butterflies of the soul.” The evaluation of diverse Golgi-stained interneurons performed by Cajal, and later his pupil Lorente de No, provided the earliest evidence for a functional significance of morphological diversity (694, 933). By meticulously cataloging the characteristic laminar distributions of dendritic processes and axonal arborizations, it was possible to predict likely sources of afferent input and postsynaptic target selection, respectively, providing a morphological basis to predict circuit function. Indeed, it is now recognized that anatomical specialization among cortical interneuron subtypes allows for a division of labor that affords inhibitory networks exquisite spatiotemporal control over principal cell activity, rather than providing generalized inhibition (586, 1036).
Though imperfect on its own as a means for successful classification of interneuron subtypes (264, 902), neuroanatomical profiling as the primary basis for understanding interneuron diversity endured for more than half a century and remains a core feature of all modern day polythetic classification schemes. Typically, contemporary interneuron taxonomical approaches complement anatomical features with 1) developmental origins, 2) molecular expression profiles, 3) intrinsic electrophysiological membrane properties, and 4) in vivo temporal firing distributions. In this section we summarize information regarding major anatomical features including typical cell soma localization, dendritic arborizations, postsynaptic target cell-type/domain specificity, and quantitative estimates of cell numbers/output synapse numbers with relation to basic developmental origins and molecular expression profiles for widely recognized and studied interneuron subsets. Other sections deal explicitly with lineage-driven genetic programs specifying interneuron fate (sect. III), intrinsic electrophysiological features (sect. IV), and in vivo firing properties of distinct interneuron cohorts (sect. XV). Descriptions are primarily based on findings from the rodent hippocampal CA1 region, which has a highly simplified laminar architecture that significantly aids in interneuron identification and where interneuron diversity is arguably best appreciated (schematically summarized in FIGURE 1). However, the majority of interneurons described have homologs throughout the remaining hippocampus and isocortex of mouse, rat, cat, monkey, and human. Our goal in this section is not to provide a comprehensive historical survey of the existing literature describing interneuron anatomical diversity but instead to provide an updated and consolidated snapshot of the current state of knowledge for widely studied interneuron subtypes. Essentially, we adopt the molecularly and anatomically defined hippocampal interneuron subtypes outlined in previous reviews (352, 1036, 586), layer in quantitative estimates produced by Bezaire and Soltesz (102) along with developmental origins, and provide additional relevant updated information related to these features. For simplicity, we use the term interneuron generally to refer to GABAergic nonpyramidal cells.
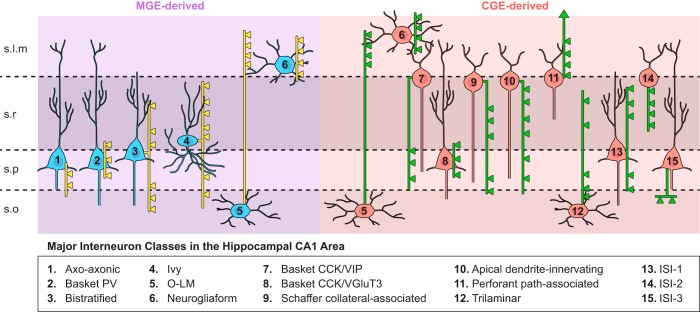
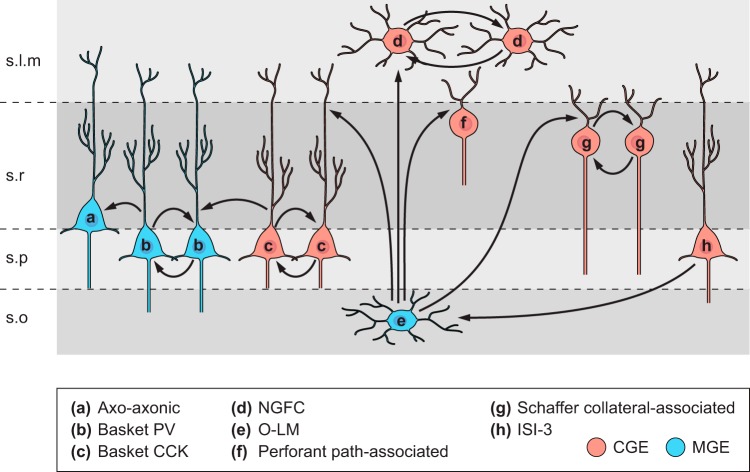
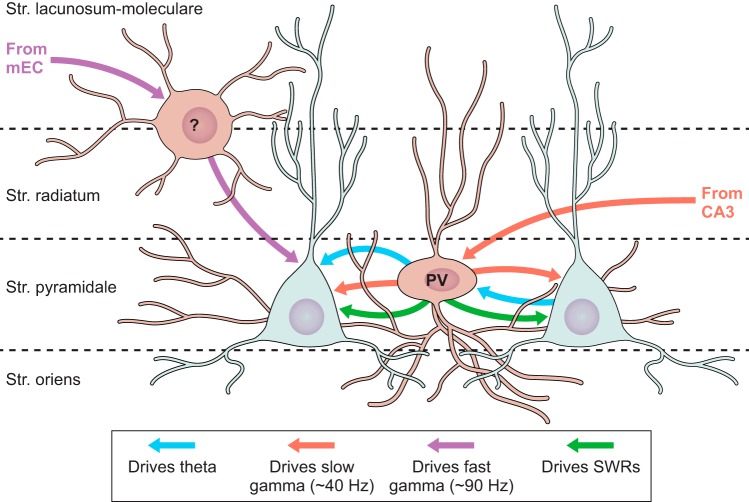
FIGURE 1.
Schematic representation of hippocampal interneuron subtypes highlighted in this review. Interneuron subtypes are parsed according to origin within the medial ganglionic eminence (MGE) or caudal ganglionic eminence (CGE). Cells with dual origins are represented in both cohorts. Somato-dendritic profiles are represented in uniform color (blue for MGE, red for CGE). Thinner axon trajectories are illustrated in yellow (MGE-derived cells) and green (CGE-derived cells) with boutons (triangles) illustrating the dominantly targeted domains of CA1 pyramidal cells innervated by each interneuron subtype.
A. Axo-axonic or Chandelier Cells
Axo-axonic cells (AACs) are estimated to make up ~4% (~1,500 cells) of CA1 hippocampal interneurons, thus representing 0.04% of all CA1 neurons assuming interneurons account for 11% of the total neuronal population (102). Cell somas typically reside within (70%) or immediately adjacent to the s.p. both in s.o. (24%) and stratum radiatum (s.r.) (6%). Their mostly aspiny, radially oriented dendrites frequently span all layers from the alveus to the s.l.m. with minimal branching in s.r. and prominent tufts in the alveus and s.l.m., positioning them to receive excitatory input from all excitatory afferent projections innervating the hippocampal CA1 region (FIGURE 2A) (135, 582, 676). However, a subset of AACs possess exclusively or dominantly horizontally oriented dendrites within s.o. (381, 1163). The axon originates from the soma or a primary dendrite and densely arborizes throughout s.p. and superficial s.o. with terminals exclusively targeting the axon initial segments of up to 1,200 pyramidal cells (FIGURE 2A) (135, 676, 1037). Main axonal branches run horizontally along the s.p.-s.o. border and drop collaterals into s.p. where terminals are arranged in vertical or oblique rows of 2–15 boutons with each row innervating a single pyramidal cell postsynaptic axon initial segment (FIGURE 2B). These characteristic candlestick-like terminal arrays or axon cartridges are the major distinguishing anatomical feature of AACs and underlie their alternative nomenclature as Chandelier cells. CA3 hippocampal AACs exhibit anatomical features similar to those in CA1 though their axonal spread may be greater (441, 1175). In the dentate gyrus (DG), AACs reside within or immediately adjacent to the granule cell layer and typically extend dendritic trees towards the hippocampal fissure to receive afferent input throughout the molecular layer with only very sparse dendritic targeting towards the hilus (136, 461, 1040). As in the CA regions, DG AAC terminals are arranged in rows of boutons that exclusively target the axon initial segments of postsynaptic targets in the hilus and proximal CA3 including granule cells, hilar mossy cells, and displaced CA3 pyramidal cells. Neocortical AACs exhibit similar candlestick-like axonal cartridges innervating neighboring pyramidal cell axon initial segments and are found in all cortical layers with a bias towards upper layer 2/3 (540, 1033, 1079, 1097, 1218). Thus, throughout hippocampus and neocortex, selective innervation of axon initial segments by AACs provides exquisite control over principal cell spike generation.
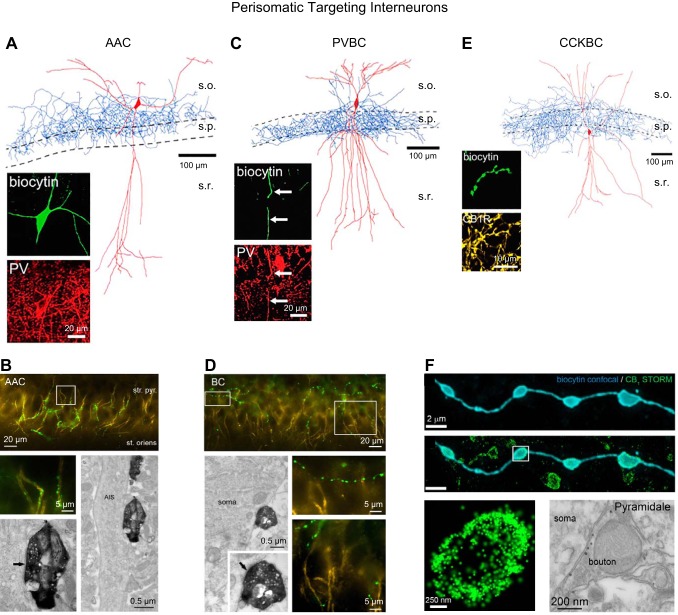
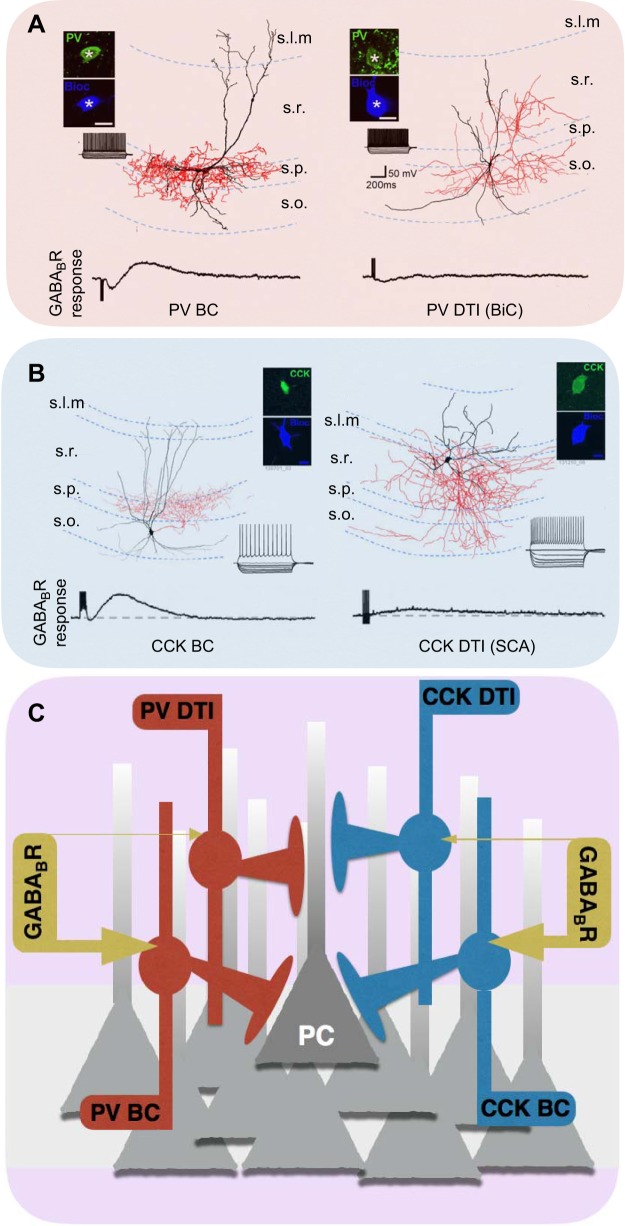
FIGURE 2.
Perisomatic targeting interneurons. A: morphological reconstruction of a representative axo-axonic cell (AAC). Inset shows that the dye-filled AAC is immunopositive for parvalbumin (PV). B: immunohistochemistry and electron microscopy images illustrating that the cartridges and axon terminals of a dye filled AAC (green) target ankyrin G-positive (yellow) axon initial segments (AISs) of principal cells. C: morphological reconstruction of a representative PVBC with inset confirming PV immunoreactivity within a dendritic segment of the dye filled PVBC. D: immunohistochemistry and electron microscopy images illustrating that PVBC terminals (green) target principal cell somas and avoid ankyrin G-positive AISs. E: morphological reconstruction of a representative CCKBC with inset showing CB1R immunolabeling within a segment of dye filled axon. F: superresolution STORM imaging illustrates intense CB1R immunolabeling of dye-filled CCKBC terminals. Also shown is an electron micrograph highlighting CB1R expression in perisomatic targeting GABAergic terminals. [Reconstructions with inset immunohistochemistry presented in A, C, and E are modified with permission from Nissen et al. (838) and Journal of Neuroscience. Images B and D are modified with permission from Gulyás et al. (443) and Journal of Neuroscience. STORM images in F were kindly provided by Dr. Katona while the electron micrograph was modified with permission from Dudok et al. (289) and Nature Neuroscience.]
Generally, the calcium binding protein parvalbumin (PV) is considered a core molecular marker for mature AACs with immunolabeling detectable throughout their somatodendritic and axonal compartments (FIGURE 2A) (74, 352, 563, 1036, 1041). However, while PV expression is limited to GABAergic interneurons in the cortex and hippocampus (183, 600), it is not restricted to AACs. Further complicating matters, recent evidence indicates that only 15–50% of neocortical AACs are PV immunopositive depending on cortical region examined (475, 1097). Though PV-negative AACs have not been reported in the hippocampus (e.g., Refs. 344, 582, 1163, 1175), cellular PV expression levels themselves are reportedly plastic (280). In CA1, AACs are estimated to represent 15% of PV+ interneurons with the remainder made up mostly of PV+ basket cells (PVBCs) and bistratified cells (BiCs) (discussed below) (74). Anatomically, PV+ axon terminals of AACs can be difficult to differentiate from neighboring PV expressing terminals of perisomatic targeting basket cells. However, AAC terminal identification can be confirmed by inspecting for bouton alignment with ankyrin G-expressing principal cell axon initial segments (FIGURE 2B) (e.g., Refs. 344, 1163, 1175). Among PV-expressing hippocampal interneurons, mature AACs are further distinguished by the absence of immunoreactivity for the transcription factor SATB1, which is present in most medial ganglionic eminence (MGE)-derived interneurons including the other PV expressing populations (209, 267, 1163, 1175).
Both hippocampal and neocortical AACs have origins in MGE with specification driven by the homeodomain transcription factor Nkx2.1 (1097, 1137, 1218). With the assumption that MGE-derived interneurons account for ~60% of all CA1 interneurons with relative contributions of 35/25/40% from PV, somatostatin, and neurogliaform/Ivy interneuron cohorts, respectively, AACs account for ~5% of MGE-derived hippocampal interneurons since AACs represent 15% of all PV interneurons (102, 1137). Interestingly, a large percentage of neocortical AACs are derived late in gestation (~E15–17) from Nkx2.1-expressing progenitors contrasting with other MGE-derived interneurons that are generated earlier in gestation (~E9–13) (1097). Thus using an Nkx2.1-CreER driver line with temporally late tamoxifen administration allows genetic access to large numbers of neocortical AACs (1097). Whether hippocampal AACs can also be selectively genetically targeted with this strategy has not been explicitly investigated. However, preliminary evidence indicates that hippocampal AACs are born with temporal profiles that are distinct from their neocortical counterparts (Hiroki Taniguchi, personal communication). For in depth evaluation and discussion of common transgenic mouse driver/reporter lines available for dissecting distinct interneuron cohorts, including the Nkx2.1-CreER driver line along with others outlined below, readers are referred to References 475, 1096.
B. Parvalbumin-Expressing Basket Cells
PVBCs are estimated to comprise ~14% (~5,530 cells) of CA1 interneurons (1.5% of CA1 neurons) (102). PVBCs frequently have large pyramidal shaped or fusiform somas typically residing within (70%) or immediately adjacent to s.p. both in s.o. (24%) and s.r. (6%). PVBCs predominantly display mostly aspiny, pyramidal-shaped, or bitufted dendritic trees spanning from the alveus to s.l.m., positioning them to receive input from all excitatory afferent projections innervating the hippocampal CA1 region (FIGURE 2C) (135, 768, 1014). The axon emerges from the soma or a primary dendrite and ultimately gives rise to large numbers of collaterals forming basketlike dense pericellular arrays of synaptic boutons primarily innervating the soma and proximal dendrites of pyramidal cells (99% of output synapses), with a minority of outputs (1%) forming autapses or contacting other interneurons (FIGURE 2, C and D) (102, 211, 884, 1014). Individual PVBCs contact up to 2,500 pyramidal cells with an average of 6 synapses onto each one (102, 340, 352, 1014). The axonal arbor is highly concentrated within s.p. but can spread to varying degrees into s.o. and s.r. (885), and recent evidence suggests that output targets may be biased towards deep versus superficial pyramidal cells (656). Cell bodies of PVBCs in the DG tend to localize among deep granule cells at the hilar border extending both basal and apical dendrites that sample inputs from the hilus to the outer molecular layer (30, 593, 998, 983). The axons of PVBCs in the DG primarily innervate perisomatic regions of granule cells yielding a dense axon cloud throughout stratum granulosum (s.g.). In the neocortex PVBCs have multipolar dendrites with cell somas that tend to concentrate in deeper layers (569, 1134). As in hippocampus, neocortical PVBCs innervate the perisomatic regions of postsynaptic principal cells; however, the lack of clear lamination makes this anatomical assessment difficult and at the gross level the axon may appear to be randomly distributed. Indeed, neocortical PVBC axonal arborization territories can remain local, translaminar, and even transcolumnar.
PVBCs have origin in the MGE with specification driven by the homeodomain transcription factor Nkx2.1 (21% of MGE-derived interneurons, see assumptions above) (147, 575, 1138, 1233). As the name implies, mature PVBCs characteristically express PV, which can be detected throughout the somatodendritic and axonal compartments. Perisomatic targeting nerve terminals of mature PVBCs also express, and immunocytochemically label for, synaptotagmin 2 (386, 1030). However, while this marker is selective for perisomatic targeting PV expressing terminals, it is unclear whether it differentiates between PVBC and AAC terminals. PVBCs are estimated to represent ~60% of all PV-containing interneurons within CA1 (74). Importantly, PV expression in both hippocampus and neocortex is minimal before postnatal day (PN) 10 then increases to mature levels between PN12-P30 in rodents (14, 260, 840). Thus PV expression probed with immunocytochemistry or reported genetically based on PV promoter activity (using PV-Cre driver lines) is undetectable during embryonic and early postnatal stages. In addition, at mature stages, the degree of PV expression itself within PVBCs is reportedly plastic according to activity levels within the circuits they are embedded in, perhaps explaining in part differential staining intensities between individual cells (280).
Presently there is no genetic strategy that allows for selective targeting and manipulation of PVBCs. Though PV-Cre driver lines allow genetic access to PVBCs, these lines will additionally exhibit recombination in AACs and some PV-expressing dendrite targeting interneuron populations (see below). Moreover, addition of other commonly used metrics to confirm PVBC identity such as post hoc confirmation of PV expression immunocytochemically or spike properties also fail to differentiate among these distinct PV expressing interneuron cohorts (discussed at length in Ref. 1163). Thus caution is warranted when interpreting circuit and whole animal level experiments (e.g., optogenetic, DREADDS, or conditional knockouts) following genetic manipulation using PV-Cre driver lines. An additional complication in using PV-Cre driver lines relates to the delayed temporal expression of PV itself, which precludes genetic access at early developmental time points. Based on their MGE origin, cells destined to become PV-expressing interneurons can be targeted using Nkx2.1-Cre driver lines allowing for early genetic manipulation. However, the Nkx2.1 lineage also gives rise to dendrite targeting somatostatin (SST)-expressing interneurons, and thus use of Nkx2.1-Cre lines in isolation will result in recombination within a wide variety of perisomatic and dendrite targeting interneurons (147, 339, 1138, 1233, 1235). For reporting purposes a recently described intersectional approach using Nkx2.1-Cre in combination with SST-Flp and a newly generated intersectional/subtractive reporter line allows for simultaneous but segregated reporting of PV- and SST-expressing interneuron cohorts in red and green channels respectively (475).
C. Bistratified Cells
BiCs are estimated to make up roughly 6% (~2,200 cells) of CA1 interneurons (0.7% of all CA1 neurons) (102). The cell bodies of most BiCs cells reside within s.p. (70%) and widely extend multipolar dendrites throughout s.o. and s.r. (FIGURE 3A) (135, 455, 561, 583, 790, 885, 1142). In contrast to PVBCs and AACs, the aspiny dendrites of these BiCs avoid s.l.m. While only a small percentage of BiCs have cell somas within s.r. (6%), nearly a quarter (24%) can be found within s.o. adjacent to s.p. and deeper towards the alvear border. Typically deeper s.o. residing BiCs extend horizontal dendritic trees that are restricted to s.o. (721, 1138, 1163). BiCs are named for their dense axonal arborizations with fine varicose collaterals split above and below s.p. simultaneously innervating both the basal and apical dendritic territories of pyramidal cells in almost equal proportion (~50% in s.o. to ~40% in s.r) (FIGURE 3A) (135, 455, 561, 583, 790, 885, 1142). While axon collaterals sparsely travel through s.p. (~10% of the axon) to connect those in s.o. and s.r., BiCs essentially avoid synapsing with the perisomatic domains of principal cells. Collaterals of individual BiCs fill the entire depth of s.o. but concentrate in deeper s.r., innervating ~1,600 postsynaptic cells with 5–10 synapses each. BiC terminals primarily target pyramidal cell dendrites with ~20% of synapses made onto spines (455). In addition, a minority of BiC terminals (~8%) make synaptic contact with other interneurons including PVBCs (455, 884).
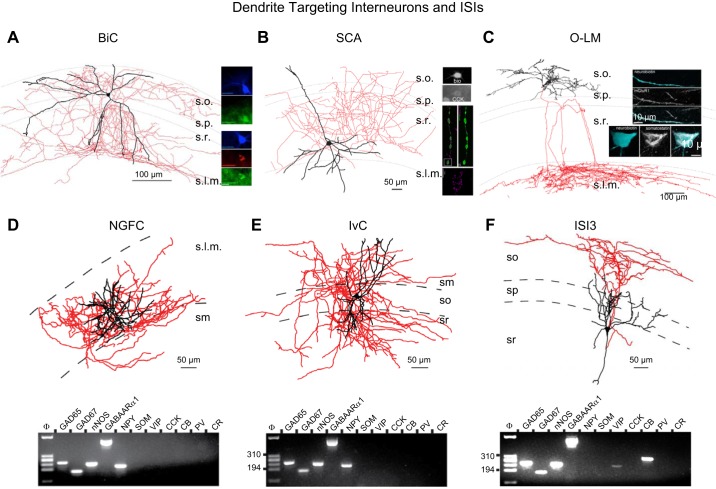
FIGURE 3.
Dendrite targeting interneurons and interneuron selective interneurons (ISIs). A: morphological reconstruction of a representative bistratified cell (BiC). At right, the reconstructed cell is illustrated to be immunopositive for SST and NPY while a different BiC highlights PV expression in this interneuron subtype. B: morphological reconstruction of a representative SCA with top inset showing that the cell is CCK immunopositive. Also shown at right (bottom) are STORM images illustrating strong CB1R immunolabeling within terminals of a separate dendrite targeting CCK interneuron. [Bottom right panel modified with permission from Dudok et al. (289).] C: morphological reconstruction of a representative O-LM with insets illustrating SST and mGluR1α immunoreactivity in the soma and along a dendritic segment, respectively. D–F: morphological reconstructions of representative NGFC (D), IvC (E), and ISI3 (F) cells along with single cell RT-PCR profiles probing for mRNA expression of the indicated markers. [A modified with permission from Klausberger et al. (583) and Nature Neuroscience. B modified with permission from Lee et al. (654) and Journal of Neuroscience. C modified with permission from Katona et al. (561) and Neuron. D–F modified with permission from Tricoire et al. (1137) and Journal of Neuroscience.]
BiCs represent ~25% of PV-expressing hippocampal interneurons with prominent immunosignal evident throughout the somatodendritic compartments (74). However, in contrast to AACs and PVBCs, BiCs coexpress neuropeptide Y (NPY) and SST, allowing them to be molecularly distinguished from the other prominent PV+ interneuron populations (75, 561, 583, 1163). Complicating interpretation of PV levels detected by immunodetection is a report that BiCs can exhibit significantly weaker PV immunoreactivity compared with AACs and PVBCs (324). Though not extensively investigated, BiCs appear to have origin in the MGE with specification driven by the homeodomain transcription factor Nkx2.1 (9% of MGE-derived interneurons) (1138).
D. Cholecystokinin-Expressing Basket Cells
Cholecystokinin-expressing basket cells (CCKBCs) are estimated to comprise roughly 9% (~3,600 cells) of all CA1 hippocampal interneurons (1% of all CA1 neurons) (102). Like PVBCs, the defining anatomical feature of CCKBCs is preferential perisomatic axonal targeting within s.p. to surround the soma and proximal dendrites of pyramidal cells (FIGURE 2E) (224, 352, 584, 1036, 1172). Individual CCKBCs contact roughly half as many pyramidal cells as PVBCs (~1,250), and it is estimated that the ratio of PVBC to CCKBC terminals on individual postsynaptic pyramidal cell targets is 1.6:1 (102, 340, 654, 1225). A minority of CCKBC synapses (~8%) innervate interneurons, typically other CCK-expressing interneurons, though cross-talk between CCKBCs and PVBCs has also been reported (5224, 554, 584). Despite the overlapping axonal profiles, CCKBCs are much less uniform than PVBCs in their somatodendritic architecture. While the majority of CCKBC somas reside within s.r., with a significant concentration at the s.r/s.l.m. border, substantial numbers can also be found in s.p. and s.o. (224, 258, 340, 584, 654, 1172). Moreover, while many CCKBCs have radially oriented bitufted or multipolar dendrites spanning all lamina from the alveus to s.l.m., others display horizontally oriented dendrites limited to s.r. or s.o. (181, 224, 258, 340, 584, 654, 721, 885, 1138, 1172). Though less studied, homologous cortical CCKBCs are primarily found in layer 2/3 and target the perisomatic regions of postsynaptic targets from layer 1 through 5 (569, 570, 611, 1134).
In the dentate gyrus, CCKBC somas almost exclusively reside at the s.g.-hilar border and subgranular polymorphic layer (352, 477, 499, 980). Much like dentate PVBCs, these dentate CCKBCs are typically pyramidal shaped with bitufted radially oriented aspiny dendrites. Basal dendrites branch throughout the hilus, and a prominent apical dendrite extends through s.g. then branches in the molecular layer and radially extends towards the pial surface. In contrast to other basket cells (all PVBCs and CA1–3 CCKBCs), dentate CCKBC terminals preferentially innervate proximal dendrites with minimal contacts onto postsynaptic target somas. Indeed, CCKBC axons traverse through most of s.g. to provide a dense band of terminals in the outer third of s.g. and the adjacent narrow band of the inner molecular layer innervating primarily the proximal dendrites of granule cells (352, 477, 499, 980). This anatomical profile overlaps with cells originally described as hilar commissural-associational pathway-related interneurons (HICAPs; Refs. 461, 1013, 457), suggesting that HICAPs and dentate CCKBCs are one and the same (499). However, inner molecular layer targeting HICAP-like cells display variable firing properties from accommodating action potential trains with slow afterhyperpolarizations (AHPs) (CCKBC like) to nonaccommodating with fast deep AHPs (PVBC, AAC, BiC like), suggesting that the term HICAP may encompass multiple diverse subtypes of interneurons (461, 499, 980, 812).
All hippocampal and cortical CCKBCs have origin in the caudal ganglionic eminence (CGE) (203, 651, 805, 1138, 1204). Assuming that CGE-derived interneurons account for ~40% of all CA1 interneurons with relative contributions of 30/30/25/10% from CCK+ interneurons, interneuron selective interneurons, neurogliaform interneurons, SST+ interneurons, respectively, and a remaining 5% of unidentified interneurons, CCKBCs account for 20% of CGE-derived interneurons as CCKBC comprise 65% of all CCK+ interneurons (102, 203, 1138). As the name implies, the defining molecular characteristic of CCKBCs is the expression of the octapeptide form of the CCK neuropeptide (CCK-8) throughout the somatodendritic and axonal compartments of CCKBCs (352, 355, 467, 843). However, not all CCK immunoreactive neurons are CCKBCs, as CCK can also be detected in glutamatergic principal cell subsets (1096, 494) and additional subpopulations of dendrite targeting interneurons (352, 1036). Molecularly, CCKBCs are considered to parse into at least two subtypes: those that coexpress vasoactive intestinal peptide (VIP) and those that coexpress vesicular glutamate transporter 3 (VGluT3), though again neither of these markers is specific on their own for CCKBCs and a substantial proportion of CCKBCs express neither of them (5, 102, 1031). The terminals of CCKBCs are heavily endowed with cannabinoid type 1 receptors (CB1Rs) which serve as the molecular substrate responsible for depressing CCKBC GABAergic output in response to endogenous and exogenous cannabinoids (FIGURE 2F; Refs. 108, 350, 559, 1139) (see sect. VIII). CCKBC terminals have also been suggested to selectively express GABAB receptors (GABABRs)in comparison with terminals from PVBCs potentially limiting GABA-mediated presynaptic inhibition of perisomatic inhibition to CCKBCs (47, 350, 354, 858). However, recent evidence from paired recordings indicates that PVBC-mediated inhibition is strongly depressed by pharmacological GABABR activation (116, 204) similar to findings for CCKBCs (657, 833). Thus, despite weak GABABR immunoreactivity in PVBCs compared with CCKBCs (1024), presynaptic release from both terminal subsets is functionally depressed by GABABR activation.
CCKBCs can be genetically targeted using CCK-Cre driver mice; however, additional CCK-expressing interneurons and even some glutamatergic principal cell subsets will also exhibit recombination (68, 1095, 1096). Intersectional approaches combining CCK-Cre mice with other interneuron specific marker or transcription factor driver lines can be used to selectively target CCK-expressing interneurons over principal cells. For example, CCK-Cre has been used in combination with Dlx5/6-Flp or VIP-Flp and dually conditional reporters to selectively reveal subsets of CCK-expressing interneurons (1095, 1096). Similarly, a novel recombinant adeno-associated virus that restricts gene expression to GABAergic interneurons based on Dlx5/6 enhancer elements can be used in combination with CCK-Cre drivers to selectively introduce recombinant proteins into CCK expressing interneurons minimizing the need for complicated breeding strategies (274). This latter approach also offers the opportunity to introduce exogenous proteins for activity monitoring (e.g., GCaMPs) and functional manipulation (e.g., ChR2, DREADDS) selectively into CCK expressing interneurons, without the need to generate dually conditional reporter lines for each protein of interest (274). However, as exciting as these refinements are, they do not allow for selective targeting of CCKBCs as a number of CCK-expressing dendrite-innervating interneurons will also be targeted using these combinatorial genetic approaches (see below). Thus again caution is warranted in interpreting circuit and whole animal observations following genetic manipulation involving CCK-Cre driver mice.
E. Dendrite Targeting CCK Expressing Interneurons
In addition to CCKBCs, CCK expression can also be detected in a variety of dendrite targeting inhibitory interneurons that largely parse on the basis of axon termination zones across distinct pyramidal cell dendritic compartments (102, 581, 1036). CCK-expressing dendrite targeting interneurons together comprise between 3 and 5% (~1,500 cells) of CA1 interneurons (~0.4% of all CA1 neurons), largely reside in s.r., with some tendency to concentrate at the s.r./s.l.m. border. They generally extend multipolar dendrites spanning all layers, though horizontally oriented dendritic arbors mostly confined to s.r. have also been observed (181, 224, 452, 584, 654, 885, 1172). The axonal projections of Schaffer collateral-associated cells (SCAs) co-align with glutamatergic inputs from CA3, ramifying dominantly within s.r. and to a lesser extent in s.o. to primarily target the oblique and basal dendrites of pyramidal cells (FIGURE 3B). Apical dendrite targeting interneurons (ADIs) are similar to SCAs but preferentially innervate the main apical shaft of pyramidal cells avoiding oblique and basal dendrites (584). The axons of perforant path-associated cells (PPAs) concentrate within s.l.m. overlapping with excitatory inputs from the entorhinal cortex and nucleus reuniens targeting the distal apical tufts of CA1 pyramidal cells (584, 1172). However, PPAs also extend axon collaterals across the hippocampal fissure to target the dendrites of granule cells further aligning their output with excitatory entorhinal input to the hippocampus (452, 584, 1172). All CCK-expressing dendrite targeting cells dominantly target principal cell postsynaptic targets with ~6 synapses per connection; however, a minority of terminals (~8%) innervate other interneurons (102, 224, 584, 1172). Dendrite targeting CCK-expressing interneurons are also found in the neocortex, most notably the vertically oriented bitufted double bouquet cells with ascending and descending tight radial axonal bundles targeting principal cell dendritic shafts and spines (263, 355, 556, 569, 1034, 1085, 1150).
The developmental origins for each subset of CCK expressing dendrite-targeting cell have not been systematically examined. However, in both neocortex and hippocampus, all CCK-expressing interneurons have so far been found to arise from the CGE (10% of CGE-derived interneurons based on assumptions outlined above) (102, 203, 651, 1138). Like CCKBCs, dendrite targeting CCK interneuron subtypes typically express CB1Rs (FIGURE 3B) (108, 289, 584, 594, 665, 1194). Additional molecular markers commonly associated with hippocampal and neocortical CCK-expressing dendrite targeting interneurons are calbindin (CB) (hippocampus and neocortex), calretinin (CR) (neocortex), and VIP (neocortex), though none of these markers is specific for or ubiquitously expressed by CCK-expressing dendrite targeting interneurons (224, 556, 569, 584, 611, 612). While VGluT3 expression was originally described as being limited to CCKBCs (1031), it has also been detected in ADIs (584).
F. Oriens Lacunosum-Moleculare Interneurons
Oriens lacunosum-moleculare interneurons (O-LMs) are estimated to comprise ~4.5% (~1650 cells) of hippocampal CA1 interneurons (0.5% of all CA1 neurons) (102). O-LMs are named for their striking anatomy with soma and dendrites restricted to s.o. and the alveus while the axon ascends with minimal branching through s.p. and s.r. to densely collateralize within s.l.m. (FIGURE 3C) (352, 719, 721, 768, 1014). This anatomical arrangement optimally positions O-LMs to function in a prototypical feedback inhibitory circuit. Indeed, the restriction of horizontally oriented O-LM soma and spiny dendrites within s.o. and the alveus dictates that the dominant source of excitatory recruitment is from CA1 pyramidal cell collaterals while the preferential axonal targeting to s.l.m. then distributes inhibition back to the distal apical dendritic tufts of CA1 pyramidal cells to gate excitatory input from the entorhinal cortex and nucleus reuniens (20, 106, 649, 720, 923, 1067). After emerging from the soma or a proximal dendrite, ~7% of an O-LM axon remains in s.o. while greater than 90% is targeted to s.l.m. (1014). The number of branches ascending through s.p. and s.r. is variable (typically between 1 and 5), but all branches generally continue to s.l.m. before collateralizing into a dense axonal cloud with fine varicosities dominantly innervating pyramidal cell spines and dendritic shafts (e.g., compare cell recoveries from References 324, 561, 642, 721, 768). In contrast to CCK-expressing PPA cells (see above) and the recently described CA1 SOM-containing back projection neuron (562), the axon of O-LM cells does not cross the hippocampal fissure to invade the dentate gyrus in the healthy brain; however, O-LM axon sprouting into the dentate gyrus has been reported in an animal model of epilepsy (894). Individual O-LMs are estimated to contact around 1,450 pyramidal cells with an average of 10 synapses per connection (102, 1014). A minority of O-LM terminals (~10%) innervate other interneurons in s.o., at the s.r/s.l.m border, and within s.l.m. (102, 299, 559, 592).
While O-LMs with classic horizontally oriented dendrites restricted to s.o. can be found in CA3, there are additional populations with multipolar dendrites extending across all strata except s.l.m. (352, 441, 442). The dentate gyrus O-LM equivalent is the hilar perforant path associated cell (HIPP) (352, 461, 499, 980, 1013). As in CA1, HIPPs are optimally positioned to participate in a feedback inhibitory loop gating excitatory drive from extrahippocampal projections to the distal dendrites of their principal cell targets. Thus HIPP fusiform cell bodies and their multipolar dendrites remain confined to the hilus optimally positioning them for recruitment by granule cell mossy fiber collaterals while the axon crosses s.g. to extensively arborize throughout the outer two-thirds of the molecular layer aligning with entorhinal cortex inputs to granule cell dendrites. The neocortical homolog of the O-LM cell is the Martinotti cell typically encountered in layers 2/3 and 5/6 with ascending axon forming a plexus in layer 1 targeting the distal dendritic tufts of pyramidal cells (569, 571, 1134, 1154, 1192).
The defining molecular characteristic of mature O-LM, HIPP, and Martinotti cells is the expression of SST (FIGURE 3C) (352, 571, 581, 716, 721, 862, 980, 1134, 1154). However, SST is not restricted to these cell populations, and in the hippocampus, O-LMs are estimated to comprise only 40% of SST-expressing interneurons (102, 324, 716, 862, 1154). Reelin is frequently coexpressed with SST in O-LM and Martinotti cells, but is not selective for or necessarily comprehensively expressed by these interneurons (203, 367, 651, 796, 899). Based on the frequent coexpression of NPY with SST, it is often assumed that O-LMs, HIPPs, and Martinotti cells represent subsets of NPY expressing interneurons (352, 610, 612, 1134). However, while limited evidence supports NPY expression in morphologically identified Martinotti and HIPP cells (352, 549, 612, 716, 1192), anatomically identified O-LMs repeatedly fail to immunostain for NPY (561, 862, 344). Further divergence in O-LM and Martinotti cell molecular profiles is evidenced by the frequent coexpression of CR with SST in Martinotti cells, a molecular signature that is absent from the hippocampus (203, 339, 1042, 1238). On the other hand, O-LMs express PV, albeit at significantly lower levels than AACs/PVBCs/BiCs, while PV and SST are considered mutually exclusive in the neocortex (324, 344, 561, 568, 582, 721, 1142, 1237). In the hippocampus, a conspicuously high level of metabotropic glutamate receptor 1α (mGluR1α) immunoreactivity along their horizontal dendrites further helps to molecularly identify O-LM cells (75, 324, 581, 582, 1142). Similarly, somatodendritic labeling for the extracellular leucine-rich repeat fibronectin containing 1 protein (Elfn1) is suggested to be specific for O-LMs (561, 1073).
Interestingly, a subset of hippocampal SST-expressing interneurons, including a subpopulation of O-LMs, expresses serotonin 3A receptors (5-HT3ARs) consistent with developmental origins in the CGE (O-LMs comprise 4% of CGE-derived interneurons based on assumptions outlined above combined with the fact that O-LMs represent 40% of SST interneurons) (102, 203, 651, 1179). In contrast, Martinotti cells and 5-HT3AR-lacking O-LM cells arise from MGE progenitors with specification driven by the homeodomain transcription factor Nkx2.1 (10% of MGE-derived hippocampal interneurons based on assumptions outlined above combined with the fact that O-LMs represent 40% of SST interneurons) (102, 203, 651, 794, 1042, 1134, 1138, 1213). Thus hippocampal SST-expressing interneurons, including O-LMs, appear to have dual MGE and CGE origins while neocortical SST expressing interneurons arise from the MGE only.
In general, SST-expressing interneurons can be genetically accessed in developing and mature brains using SST-Cre driver lines (702, 1096). However, given the diversity in SST-expressing interneurons throughout the neocortex and hippocampus, these lines cannot be used for selective targeting of O-LM, HIPP, and Martinotti cells (102, 535, 536, 716, 862, 1154). Moreover, off-target recombination in non-SST-expressing interneurons and even in pyramidal cells has been reported using SST-Cre lines (501, 787). In the hippocampus, preferential targeting for O-LMs has been reported using a driver line based on nicotinic acetylcholine receptor α2 subunit promoter activity (Chrna2-Cre mice) (649, 787).
G. Neurogliaform and Ivy Cells
Neurogliaform cells (NGFCs) are estimated to make up just over 9% (~3,600 cells) of the entire CA1 hippocampal interneuron population (1% of all CA1 neurons) (102). In CA1, the small spherical somas (~15 µm in diameter) of NGFCs are typically found in s.l.m. with a minority of cells residing at the s.l.m./s.r. border and superficial s.r. (46, 165, 870). Several primary dendrites extend from the soma then branch extensively in a stellate fashion yielding a compact somatodendritic profile reminiscent of glial cells that is typically fully contained within the NGFC’s local axon cloud (FIGURE 3D; Refs. 299, 367, 550, 578, 606, 926, 1137, 1172, 1280). The axon arises from the soma or a primary dendrite and extensively collateralizes giving rise to a remarkably dense local fine axonal plexus (FIGURE 3D). Indeed, despite occupying a relatively small tissue volume, the total axon length of an individual NGFC is estimated to be greater than three times that of a typical PVBC (102, 870). The strong affiliation of most CA1 NGFC input and output elements with s.l.m. optimally positions them to primarily serve a feedforward inhibitory role in gating excitatory input from the entorhinal cortex and nucleus reuniens. However, NGFC dendrites and axons can also cross the hippocampal fissure into the molecular layer of the dentate gyrus and penetrate into superficial s.r., indicating that CA1 NGFC recruitment and distribution of inhibition extend beyond s.l.m. (367, 550, 926, 927). Moreover, some NGFCs near the s.r./s.l.m border appear to demonstrate stronger affiliation with the Schaffer collateral pathway (606, 1138, 1172). In the dentate gyrus, NGFCs frequently reside in the outer molecular layer where they also exhibit dense axonal clouds that can penetrate the hippocampal fissure to invade the CA1 and subiculum subfields (48, 184). In addition, dentate gyrus NGFCs have been found in the hilus near s.g. (751). While neocortical NGFCs can be found throughout all layers, they are particularly enriched in supragranular layers and form a major constituent cell population in L1 (564, 858, 1017, 1074, 1086, 1134).
An unusual anatomical feature of NGFCs is their remarkably high density of small area en passant boutons that in many cases (50–75% of terminals) do not have clear postsynaptic targets (102, 858, 1172). Even at synaptic junctions with identifiable postsynaptic elements (typically principal cell spines, spine necks, or dendritic shafts), NGFC inputs exhibit a strikingly wide synaptic cleft (858). These features combined with the dense local ramification of NGFC axons are considered to underlie the ability of NGFCs to mediate volume transmission leading to slow dual component (GABAAR- and GABABR-mediated) inhibition at virtually any postsynaptic element within their dense axonal plexus (550, 926, 927, 1074, 1086). In addition, the cloud of GABA generated from NGFC release can also activate presynaptic GABABR to mediate homosynaptic and heterosynaptic depression of GABAergic and glutamatergic release (48, 858, 859, 926, 927) sometimes with remarkable target cell specificity (204). Interestingly, NGFCs exhibit a remarkably high degree of chemical and electrical connectivity with each other and promiscuously with additional non-NGFC interneuron subtypes allowing individual NGFCs to extend their influence well beyond their local dense axonal cloud through disinhibition and coordination of inhibitory networks (926, 1017, 1280, 1281).
Molecular markers associated with NGFCs include NPY, reelin, neuronal nitric oxide synthase (nNOS), α-actinin 2, and COUP transcription factor 2 (COUPTF2) (48, 367, 549, 796, 858, 926, 1137). However, molecular identification of NGFCs is complicated by considerable heterogeneity such that no one marker or combination of markers uniquely or comprehensively reveals the entire NGFC cohort. In both the hippocampus and neocortex, NPY and reelin are likely expressed by all NGFCs, although neither of these markers or their combination is selective for NGFCs. In CA1 hippocampus differential expression of nNOS by NGFCs correlates to distinct embryonic origins. Thus, in general, nNOS-expressing NGFCs derive from the MGE (4% of all MGE-derived interneurons based on assumtions outlined above and a 10% contribution of NGFCs to nNOS+ MGE-derived interneurons) while nNOS-lacking NGFCs derive from the CGE (25% of all CGE-derived interneurons based on assumptions outlined above) (1137, 1138). In contrast, neocortical NGFCs have singular origins within the CGE (651, 796, 1179).
Ivy cells (IvCs) are closely related to NGFCs and are estimated to be the largest cohort of hippocampal interneurons making up almost a quarter (23%, 8,800 cells) of the entire CA1 interneuron population (2.5% of all CA1 neurons) (102, 366, 1137). IvCs are named for the English ivy-like appearance of their axons which branch profusely close to their origin giving rise to a dense cloud of fine thin collaterals with frequent small en passant boutons much like those of NGFCs but targeting more proximal oblique and basal CA1 pyramidal cell dendrites (FIGURE 3E) (366). In contrast to NGFCs, IvC somas avoid s.l.m. and are dominantly found in and around s.p. but also populate s.o. and s.r. to lesser extents (366, 367, 1032, 1075, 1137). The aspiny multipolar dendrites of IvCs are less compact than those of NGFCs and frequently extend beyond their axonal span to largely inhabit s.o. and s.r., positioning them for feedforward recruitment by CA3 Schaffer collateral inputs and also for feedback recruitment by CA1 pyramidal cell collaterals (FIGURE 3E). However, a subset of IvCs located in superficial s.r. has been found to extend significant dendritic and axonal process into s.l.m. blurring the lines between the input and output domains of IvCs and NGFCs (1032). IvCs are generated from MGE progenitors (36% of MGE-derived interneurons based on assumptions outlined above and a 90% contribution of IvCs to nNOS+ MGE-derived interneurons) and their molecular signature is similar to MGE-derived NGFCs with prominent expression of NPY, nNOS, and COUPTF2, but unlike NGFCs, IvCs do not express reelin (366, 367, 606, 1137).
H. Interneuron Selective Interneurons
While each of the interneuron subtypes described thus far exhibits some degree of connectivity among themselves and other interneurons, these homotypic and heterotypic interneuron-interneuron connections represent a minority of their outputs (~5–15%) compared with their innervation of pyramidal cells. In contrast, a distinct family of interneurons selectively or preferentially innervates other interneurons providing a cellular substrate specialized for network disinhibition. Initially, such interneuron selective interneurons (ISIs) were anatomically identified and characterized through immunostaining for CR or VIP with correlated light microscopy and ultrastructural analyses revealing that the major postsynaptic elements targeted by CR and VIP terminals were dendrites and somas of dendrite targeting GABAergic cells (5, 6, 425, 439, 449, 1046). More recently, a number of functional studies in both hippocampus and neocortex have taken advantage of transgenic animals and modern circuit mapping tools to functionally confirm that subsets of CR- and VIP-expressing interneurons do indeed mediate circuit disinhibition by selectively targeting other interneurons over pyramidal cells (185, 363, 652, 906, 908, 1145, 1270). In the hippocampus such ISIs are estimated to comprise almost 20% of all CA1 interneurons (2.2% of all CA1 neurons) and are separable into three subsets based on unique morphological and neurochemical signatures outlined below (102, 186, 352, 353).
Type 1 ISIs (ISI-1s) are CR-expressing multipolar cells with somas found throughout s.r, s.p., and s.o. (439, 1145). The smooth dendritic trees of ISI-1s arborize most extensively within s.r. but also infiltrate all other strata. A unique feature of ISI-1s is the regular occurrence of long dendrodendritic junctions in which two to seven dendrites from separate ISI-1s are intermingled for more than 100 µm frequently with varicose axons of additional ISI-1s (439). These braidlike structures connect clusters of ~15 ISI-1s and likely serve to synchronize their activity through electrical and chemical synapses. The axons of ISI-1s ramify within s.r., s.p., and sparsely within s.o. seeking out somatodendritic compartments of interneuron targets. In contrast to pyramidal cell targeting interneurons, terminal distribution along ISI-1 axons (and all ISIs) is highly uneven with large lengths of axon exhibiting few boutons interspersed with sections of high bouton density upon encountering appropriate GABAergic postsynaptic elements. The preferred targets of ISI-1s are CB-expressing dendrite targeting interneurons (likely SCAs, PPAs, and CCK-expressing interneurons described above), other CR-expressing ISI-1s, and VIP-expressing CCKBCs. Upon encountering these cells, individual axons frequently form multiple contacts in a climbing fiber-like manner along their dendrites or soma. In contrast, anatomical observations indicate that ISI-1s essentially avoid PV expressing interneurons and pyramidal cells. Thus recruitment of ISI-1s by Schaffer collateral, entorhinal, reuniens, or CA1 pyramid collateral input is expected to preferentially disinhibit the apical dendrites of CA1 pyramidal cells within the termination zone of CA3 Schaffer collateral input.
Type 2 ISIs (ISI-2s) are VIP-expressing interneurons with cell somas typically found at the border of s.r. and s.l.m. (5, 6). The majority of these cells have a characteristic smooth or sparsely spiny dendritic tree comprised of a tuft restricted to s.l.m. positioning them for recruitment primarily by entorhinal and reuniens input. The axons of ISI-2s descend in the opposite direction to ramify in a mostly radial orientation throughout s.r. giving rise to a number of fine branches again with highly uneven terminal distributions. Like ISI-1s, the VIP-expressing terminals of ISI-2s preferentially form multiple synaptic contacts on the somas and dendrites of CB-expressing dendrite targeting interneurons (i.e., CCK-expressing dendrite targeting interneurons) and also innervate other VIP-expressing interneurons (likely CCKBCs and other ISI-2s or ISI-3s, see below) located throughout s.r. The output of ISI-2s further resembles that of ISI-1s in essentially avoiding PV-expressing interneurons and pyramidal cells as postsynaptic partners. Based on these features, ISI-2s appear to provide a disinhibitory network that largely overlaps with that of ISI-1s in the CA3 Schaffer collateral termination zone of CA1 pyramidal cell apical dendrites, but that is preferentially recruited by entorhinal and reuniens inputs. However, a subpopulation of ISI-2s exhibit bipolar radially oriented dendrites spanning all strata, suggesting overlap in function with ISI-1s. Further complication in parsing this subpopulation from ISI-1s is the fact ~50% of the bipolar ISI-2s coexpress CR with VIP while ISI-2s with dendrites restricted to s.l.m. do not express CR.
VIP and CR coexpressing type 3 ISIs (ISI-3s) have fusiform cell bodies typically residing in s.p. and deep s.r. (5, 6, 185, 439, 1145). ISI-3 dendritic trees are typically bipolar spanning all strata with a prominent tuft of several horizontally running branches in s.l.m. However, in some cases, all primary dendrites (up to 3) ascend towards s.l.m. (1145). In contrast to the other ISIs, the main axon primarily descends to s.o. where it emits several long horizontal collaterals producing a dense plexus within deep s.o. and the alveus co-aligning with the horizontal dendrites of oriens residing interneurons (FIGURE 3F). Indeed, initial anatomical studies revealed that the major postsynaptic targets of ISI-3s are the horizontal running dendrites of SST- and mGluR1α-expressing O-LMs with which individual ISI-3s form multiple synaptic contacts. Recent functional investigations have confirmed O-LMs as the preferred target of ISI-3s and additionally revealed less frequent connections between ISI-3s with other interneurons including BiCs and PVBCs but not with CA1 pyramidal neurons (185, 1145). Thus recruitment of ISI-3s is expected to primarily disinhibit O-LM-mediated feedback inhibition of the most distal apical dendritic elements of CA1 pyramidal cells in the termination zone of entorhinal and reuniens afferent input.
In the dentate gyrus, VIP and CR coexpressing interneurons homologous to ISI-3s are the most frequently encountered ISIs (352, 449). These dentate ISIs are typically bipolar with fusiform cell somas often located in s.g. or in the molecular layer and emit axons that primarily target the dendrites of SST-expressing HIPP cells located in the hilus. Thus, like ISI-3s, such dentate ISIs appear specialized to disinhibit the distal dendrites of local principal cells, the granule cells, in the termination zone of perforant path inputs from the entorhinal cortex. This disinhibitory motif is also common to the neocortex where a majority of vertically oriented bipolar VIP and CR coexpressing interneurons reside in layer2/3 and emit axons that preferentially innervate dendrite targeting SST expressing interneurons within the same column (168, 363, 425, 475, 553, 652, 906, 928, 1046, 1134, 1270).
In both neocortex and hippocampus, CR- and VIP-expressing ISIs represent subsets of 5-HT3AR-expressing interneurons and have singular origin in the CGE (combined ISIs represent 30% of CGE-derived interneurons based on assumptions outlined above) (102, 203, 651, 796, 1138, 1179). Genetic access for manipulation of ISIs is available through use of VIP-Cre and CR-Cre driver lines of mice (1096). Indeed, these driver lines have been successfully used in a number of recent studies to dissect circuit functions of ISIs in both hippocampus and neocortex (363, 553, 652, 906, 908, 1145, 1270). In both regions the inclusion of VIP-expressing CCKBCs when using VIP-Cre driver mice is a potential confound to data interpretation at circuit and whole animal level investigations. Similarly in the neocortex, the expression of CR by subsets of SST expressing Martinotti cells could complicate interpretation of studies using CR-Cre driver mice. The recent development of tools that allow for an intersectional approach reliant upon VIP and CR coexpression (i.e., VIP-flp with CR-Cre driver lines) offers the potential to significantly refine genetic targeting of ISIs (475).
III. DEVELOPMENT AND EMBRYONIC ORIGINS
The remarkable diversity of cortical interneurons directly relates to their spatiotemporal origins from discrete progenitor pools within the developing embryonic telencephalon (71, 227, 1213). Indeed, although distinct interneurons require weeks of postnatal maturation to fully attain their subtype-defining characteristics, evidence accumulated over the past two decades has revealed striking correlations between the place and time of birth of interneurons and their ultimate subtype identity in the mature neocortex and hippocampus. This suggests that genetic restriction of neuronal potential at the progenitor stage is a major determinant of interneuron diversity. Interestingly, neocortical and hippocampal interneurons are generated from progenitor pools in the ventral subcortical telencephalon (subpallium) far removed from their ultimate target locations in the cortical circuits of the dorsal telencephalon (pallium). This contrasts with excitatory principal neurons that are generated in the dorsal telencephalon in close proximity to the cortical circuits they will establish by invading the cortical plate through radial migration. Thus nascent interneurons must undergo long-range ventral to dorsal (subpallial-to-pallial) tangential migration before invading target cortical circuits via radial migration. Then, through a combination of intrinsic genetic programs and local circuit activity, a given interneuron undergoes terminal differentiation towards its mature identity to fulfill its role within the circuit. In this section we briefly highlight the genetic programs, cell and molecular mechanisms, as well as local circuit activities underlying this broad developmental program of cortical interneurons starting with features of the subpallium associated with generating interneuron diversity. Due to the richness of available data, our discussion is based on findings from mouse; however, recent evidence indicates a dominantly subpallial origin for cortical interneurons in humans and primates as well (464, 714).
The developing telencephalon is first evident as a simple neuroepithelial sheet around embryonic day (E) 8.5 in mouse just as the neural tube is closing. Shortly after initial dorsoventral patterning orchestrated in part by gradients of extrinsic morphogens such as Sonic Hedgehog and fibroblast growth factors (Shh/FGFs, ventralizing influence) regulating transcription factors such as Gli3 (dorsalizing influence), a group of highly proliferative germinal zones known as the ganglionic eminences (GEs) emerge in the subpallium next to the lateral ventricle (FIGURE 4A) (476). There are three GEs morphologically defined according to their anatomical locations in the dorsoventral, rostrocaudal, and mediolateral extents of the embryonic subpallium: the medial ganglionic eminence (MGE), the lateral ganglionic eminence (LGE), and the caudal ganglionic eminence (CGE). These GEs arise in a discrete temporal order with the ventral MGE appearing first around E9 followed by the dorsal LGE around E10 with a prominent sulcus separating the two at more anterior levels. The CGE arises around E11 and was proposed as a discrete entity as the eminence that is posterior to the fusion of the MGE and LGE and, thus, is not physically separated from the MGE and LGE. Importantly, these GEs are only transiently distinguishable based on morphological criteria. For example, the prominent sulcus separating the LGE from the MGE disappears beyond E15.5 and ultimately all of the GEs fuse and give way to basal ganglia structures in the mature telencephalon. However, a more reliable map of these distinct germinal territories is revealed by gene expression profiles (see below).
FIGURE 4.
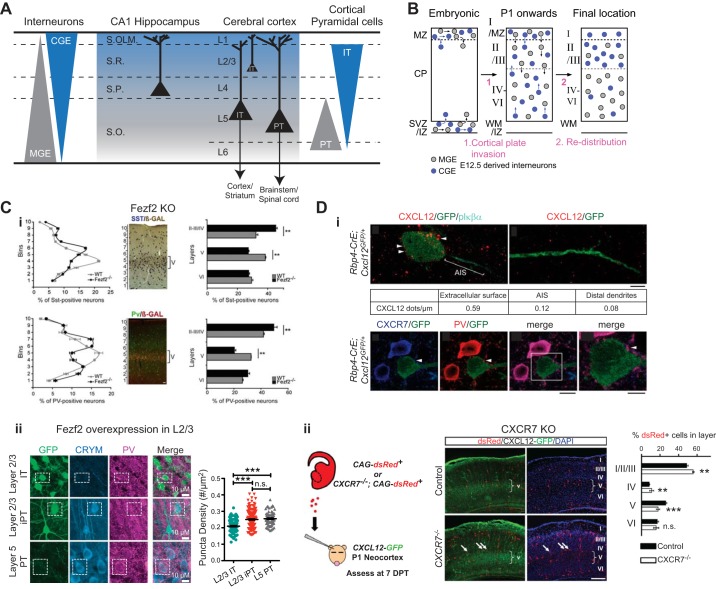
Subpallial embryonic origins, and genetic programs directing genesis/migration/circuit integration of cortical interneurons. A, left panel: schematic illustrating pathways of migration for cortical and striatal interneurons from the GEs. Right panel: diagram of embryonic brain with the subdivisions of the ventral telencephalon in the coronal plane. The three regions where striatal, neocortical, and hippocampal interneurons originate are the medial ganglionic eminence (MGE) (including the dorsal MGE-dMGE), the caudal ganglionic eminence (CGE), and the preoptic area (POA). The lateral ganglionic eminence (LGE) largely gives rise to basal forebrain neurons and striatal medium spiny neurons. Note that the vast majority of cortical interneurons are derived from MGE and CGE. B: genetic programs controlling neurogenesis, cell commitment, tangential, and radial migration as well as maturation of cortical interneurons. The subdivision of the neuroepithelium can be identified by combinatorial expression patterns of transcription factors involved at different stages of cortical interneuron development. Some of these factors participate broadly in interneuron development such as Dlx and CoupTF gene families. Some transcription factors are unique to specific domains and/or stages of differentiation. Nkx2.1 defines the MGE and activates a cascade of genes including Lhx6, Sox6, and Satb1. Nkx6.2 and Gli1 are enriched in the dMGE. Prox1 and Sp8 are expressed in CGE-derived cortical interneurons at all stages of their development. Note that it is unclear whether Sox6 and SatB1 are necessary for the development of nNOS expressing Ivy cells. [Adapted with permission from Kessaris et al. (577) with permission from Current Opinions in Neurobiology and from Wonders and Anderson (1213) with permission from Nature Reviews Neuroscience.]
Studies demonstrating that DilC18 (also known as DiI) fluorescently labeled GE derived cells migrate dorsally to the developing cortical telencephalon provided the first direct evidence for subpallial origins of cortical interneurons (35). Moreover, mutant mice lacking the homeodomain transcription factors Distaless 1 and 2 (Dlx1/2) that are expressed by most subpallial progenitors exhibited a severe reduction in subpallium-to-pallium interneuron migration resulting in a 70% reduction in neocortical interneurons (35). Following these observations, a great number of studies using transplantation techniques, analyses of knockout mice, and genetically inducible fate mapping (GIFM) have confirmed the ventral origins of cortical interneurons and significantly refined our understanding of the discrete spatiotemporal origins for specific interneuron subtypes (147, 203, 335, 339, 391, 519, 651, 681, 794, 796, 797, 829, 914, 1042, 1068, 1097, 1137, 1138, 1179, 1202, 1214, 1233, 1273). In general, these studies have found that the vast majority (~90%) of cortical interneurons arise from progenitor pools in the MGE and CGE primarily between E9 and birth (schematically summarized in FIGURE 4B). In addition, a small diverse population of cortical interneurons is generated in a proliferative zone ventral to the MGE called the preoptic area (POA).
A. The Medial Ganglionic Eminence
The MGE produces ~60% of neocortical and hippocampal interneurons (147, 914, 1138, 1213). In neocortex, the vast majority of PV (e.g., PVBCs and AACs) and SST (e.g., Martinotti cells) expressing interneurons are derived from MGE progenitors (147, 339, 914, 1213, 1235). In the hippocampus, MGE-derived interneurons include PV-expressing interneurons (PVBCs, BiCs, AACs), IvCs, ~60% of SST-expressing cells (e.g., O-LMs), and a subset of NGFCs (203, 914, 1137, 1138) (FIGURES 1, 4B, and 5). The majority of progenitors within the MGE, but not CGE or LGE, are molecularly defined by expression of the homeodomain transcription factor Nkx2.1, which is necessary for the generation and proper specification of mature interneuron subtypes derived from MGE progenitors. Indeed, Nkx2.1 null mice have a 50% reduction in cortical interneurons; however, death at birth precludes finer analysis of specific interneuron subtypes in mature brains (1068). Subsequent studies investigating postnatal mice with conditional loss of Nkx2.1 within MGE progenitors confirmed dramatic reductions in PV and SST interneuron populations in both neocortex and hippocampus as well as nNOS expressing IvCs and NGFCs in the hippocampus (148, 1137). Moreover, GIFM studies using Nkx2.1-Cre driver lines have confirmed that mature MGE-derived interneurons comprise these populations consistent with earlier findings from MGE transplantations (147, 212, 335, 339, 1137, 1138, 1202, 1214, 1235).
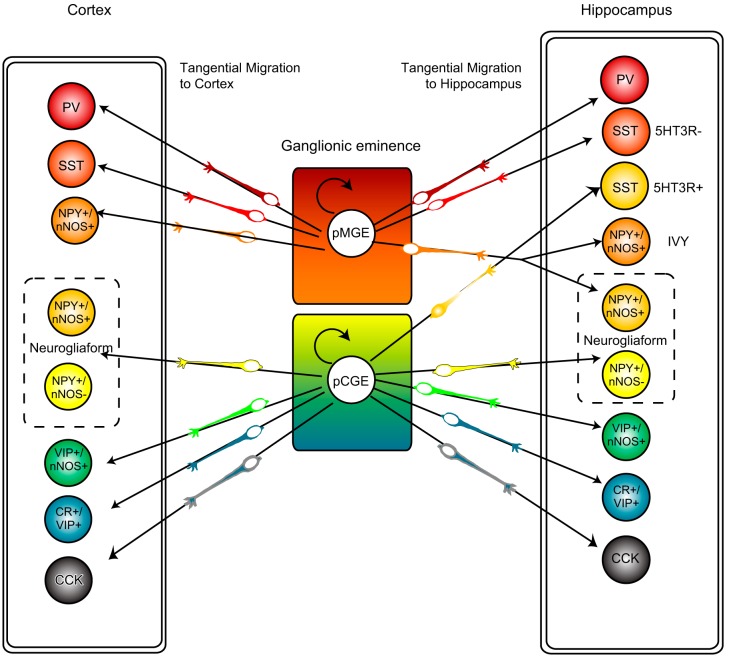
FIGURE 5.
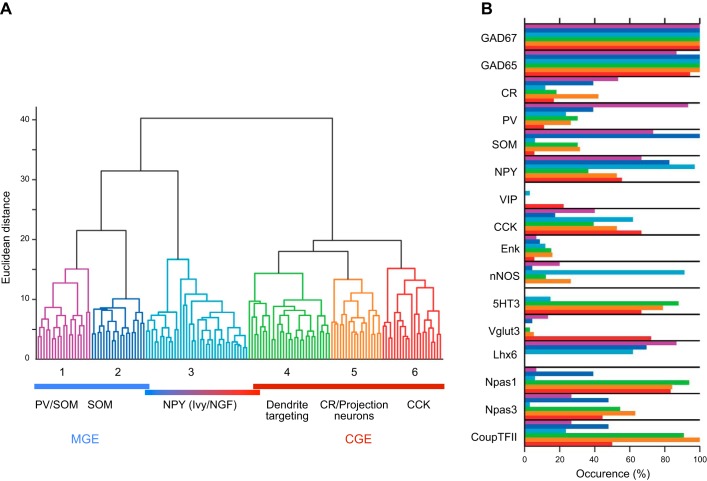
The ganglionic eminence origins for hippocampal interneurons often deviate from the rules underlying neocortical interneuron embryogenesis. In cortex, all PV- and SST-containing (as well as a minor population of NPY-positive/nNOS-positive) interneurons are derived from the MGE, while the remaining populations (including VIP, CCK, and NPY-containing interneurons) are derived from the CGE. While these rules are true for many hippocampal interneuron subpopulations, 5HTR3AR-positive SST-containing interneurons are derived from the CGE. Furthermore, all NGFCs destined to reside in cortex have their origins in the CGE, whereas in hippocampus NPY-positive/nNOS-containing NGFC have their origins in the MGE and NPY-positive, nNOS-negative NGFC arise from CGE origins.
The transcription factor Lhx6 is an essential downstream effector of Nkx2.1 that is upregulated in MGE-progenitors upon exiting the ventricular zone (VZ) and persists through adulthood in most MGE-derived interneurons (212, 288, 339, 426, 433, 639, 1068) (FIGURE 4B). Loss of Lhx6 function produces deficits in MGE-derived interneurons by disrupting migration and specification to PV and SST fates (681, 1273). Downstream of Lhx6, the transcription factors Sox6 and SatB1 direct the migration, survival, specification, and functional maturation of PV and SST interneurons, respectively (52, 72, 209, 267, 826). Combined these studies highlight the critical contributions of transcription factor cascades initiated within discrete pools of subpallial progenitors in the generation of distinct cortical interneuron subtypes. Indeed, in the mice with conditional loss of Nkx2.1 mentioned above, MGE-derived interneurons are respecified towards subtype fates typically associated with CGE or LGE origins reflecting a dorsalization of the MGE progenitor zone (148, 1068). Thus Nkx2.1 serves as an example of a master regulator gene expressed in a defined proliferative region of the subpallium responsible for driving cell fate decisions by serving as a molecular switch that favors MGE over CGE/LGE fates. Importantly, Nkx2.1 expression within the MGE is driven by Shh signaling, and conditional loss of Shh replicates the MGE-derived interneuron deficits observed in Nkx2.1 mutants illustrating the continued requirement of this critical morphogen in establishing proliferative zone identity beyond initial pallial-subpallial patterning (1233, 1234, 1236).
The fact that multiple distinct cell subtypes are generated from the MGE suggests the possibility of smaller subdivisions within this progenitor pool. Indeed, based on the combinatorial expression profiles of several transcription factors within the MGE ventricular zone, it has been proposed that this region consists of up to five distinct progenitor domains (335). Thus, in a manner akin to generating cellular diversity within the spinal cord (531), spatially segregated progenitor lineages with restricted fate potential dedicated to producing particular interneuronal subtypes was considered to underlie cortical interneuron diversity. However, microtransplantation fate-mapping studies revealed mixed populations of mature interneuron subtypes generated from individual MGE subregions (335, 519, 1214). Moreover, recent clonal analysis revealed that individual MGE progenitors can give rise to both PV- and SST-expressing interneurons that disperse widely throughout the neocortex and hippocampus revealing that progenitors are not restricted to making one interneuron subtype (129, 207, 469, 766).
Though spatially segregated subdivisions of MGE progenitors are not absolutely deterministic of mature interneuron fate, transplant and GIFM studies consistently revealed biases for SST-expressing interneurons to originate from the dorsal MGE (dMGE) and for PV expressing interneurons to originate from the ventral MGE (vMGE) (335, 336, 339, 519, 1042, 1214, 1234) (FIGURE 4B). This spatial bias is nicely illustrated by GIFM studies based on Nkx6.2 which is strongly expressed in dMGE progenitors near the CGE border but absent in vMGE (339, 1042, 1214). In the mature neocortex interneurons from the Nkx6.2 lineage adopt primarily SST fates, particularly SST/CR coexpressing Martinotti cells, and rarely exhibit PV interneuron fates (339, 1042). In contrast, microtransplantations revealed that PV AACs derive almost exclusively from the ventral-most MGE progenitors (519). This dorsoventral bias in SST versus PV interneuron generation relates to the level of Shh signaling such that high Shh signaling favors SST over PV interneuron generation (1146, 1234). Indeed, a number of Shh effectors beyond Nkx2.1 are enriched in dMGE over vMGE including the above-mentioned Nkx6.2 (1214, 1258). Curiously, a major source of Shh within the subpallium is the ventricular zone of the POA far removed from the dMGE where SST interneurons are dominantly generated and close to the vMGE (FIGURE 4A). However, recent evidence indicates that dMGE progenitors receive additional Shh signal secreted from nascent MGE-derived neurons in the mantle zone of the MGE induced to express Shh by Lhx6/Lhx7 transcriptional regulation (337). Thus immature early postmitotic neurons exiting the proliferative zone themselves provide feedback signaling instructive for dMGE progenitor developmental programs and as such influence the balance of SST versus PV interneuron production (FIGURE 4B).
Complementing the spatial control of interneuron specification within the GEs is a temporal dynamic that has significant impact on mature interneuron fate (147, 519, 794, 796, 909, 1097, 1138). Within the MGE, SST-expressing interneurons destined for the neocortex exhibit a peak in neurogenesis around E11.5 while neocortical PV expressing interneuron production peaks around E13.5. In the hippocampus SST and PV interneurons both exhibit peaks in their genesis around E11.5; however, nNOS expressing IvCs/NGFCs exhibit a later peak in production around E13.5. The distinct temporal windows for different interneuron subtype generation are particularly striking when comparing PV-expressing AACs with SST-expressing hub cells which serve as organizers of early patterned network activity. Indeed, SST hub cells are generated from Nkx2.1 progenitors at the earliest time points of interneuron production (E9.5) before the MGE is even morphologically distinguishable while PV-expressing AAC neurogenesis preferentially occurs beyond E15.5 after the MGE has morphologically flattened out into a ventral germinal zone of the lateral ventricle (475, 519, 909, 1097). Remarkably, when E16.5 Nkx2.1 expressing donor cells were transplanted into the somatosensory cortex of P3 hosts, they reliably differentiated into AACs implying that late MGE progenitors are fate committed to generating AACs by an intrinsic genetic program late in embryogenesis (1097). Thus there are clear correlations between birthdate and ultimate interneuron subtype identity. However, as for spatial influences, temporal dynamics only partially predict ultimate interneuron identity as both SST- and PV-expressing interneurons are produced in varying proportions during the dominant period of MGE neurogenesis between E9.5 and E15.5 (794, 1138).
Potentially related to temporal influences on interneuron specification are recent findings illustrating that the mode of progenitor mitosis critically influences MGE-derived interneuron fate determination (904). The periventricular proliferative zone of the subpallium consists of both the VZ and subventricular zone (SVZ) populated with apical progenitors (ApPs) and basal progenitors (BPs), respectively. In vivo manipulation of these progenitor pools combined with fate mapping revealed that ApP neurogenesis within the MGE VZ is strongly biased to produce SST interneurons, while BP neurogenesis within the SVZ yields primarily PV interneurons (904). These findings are consistent with prior evidence that loss of the cell cycle regulator cyclin D2, which is enriched within SVZ BPs of the MGE, results in a 30–40% reduction in PV-expressing neocortical interneurons with no change in SST interneuron numbers (405, 406). It has been postulated that VZ ApPs could undergo asymmetric cell division to yield SST interneurons and SVZ BPs (which will produce PV interneurons), thus neatly explaining why SST interneurons tend to be born earlier and how individual progenitors give rise to mixed clones of SST- and PV-expressing interneurons (58, 904).
B. The Caudal Ganglionic Eminence
The CGE produces ~30% of neocortical and hippocampal interneurons (203, 651, 796, 963, 966, 1138, 1179). In the neocortex NGFCs (reelin-expressing, SST negative), ISIs (VIP- and CR-expressing), and CCK-expressing interneurons (e.g., CCKBCs) are derived from CGE progenitors (651, 796, 963, 1179) (FIGURE 5). In the hippocampus CGE-derived interneurons include CCK-expressing interneurons (CCKBCs, SCAs, ADIs, PPAs), ISIs (VIP and CR expressing), a subset of NGFCs, and ~40% of SST-expressing interneurons (e.g., O-LMs) (203, 1137, 1138) (FIGURES 1, 4B, AND 5). Though a specific master regulator gene, analogous to Nkx2.1 in the MGE, has yet to be discovered for CGE progenitors, a number of transcription factors such as Gsx2, CoupTF1/2, and SP8 are enriched within the CGE and have been implicated in the generation, migration, specification, and maturation of CGE-derived interneurons (547, 688, 715, 797, 964, 1234) (FIGURE 4B). Interestingly, while the homeodomain transcription factor Prox1 is present in the SVZs of all GEs, its expression is selectively maintained in CGE-derived, and downregulated in MGE-derived, interneuron precursors (797, 964). Loss-of-function studies indicate that Prox1 is differentially required embryonically and postnatally for CGE-derived interneuron migration and differentiation/circuit integration, respectively (797). Further work should reveal the genetic programs responsible for promoting the selective maintenance of Prox1 in CGE-derived interneurons and, hence, delineate genetic cascades that instruct CGE interneuron fate determination.
Initially, in vitro culture and in vivo transplant studies implicated the CGE as a source of cortical interneurons that assumed mature fates distinct from MGE-derived interneurons (147, 829, 1233). Subsequently, elucidation of the full complement of CGE-derived interneurons within mature cortical circuits was achieved through GIFM using a Mash1-CreER driver line with expression fortuitously restricted to LGE/CGE-derived populations (203, 796, 1137, 1138). With the use of this inducible line, CGE interneuronogenesis was demonstrated to lag that of the MGE both for initial (E12.5 for CGE vs. E9.5 for MGE) and peak (E16.5 for CGE vs. E13.5 for MGE) generation (796). Thus general comparison of CGE and MGE interneuron production further highlights that distinct interneuron subtypes exhibit unique spatiotemporal neurogenesis profiles within the ventral telencephalon. However, in contrast to the MGE, the subtypes of neocortical interneurons generated within the CGE do not change significantly over time with similar proportions of reelin-expressing NGFCs and VIP/CR-expressing ISIs generated across CGE neurogenesis (796). Similarly, in the hippocampus, VIP- and reelin-expressing subsets of CGE derived interneurons are consistently produced between E12.5 and E16.5 (1138). However, CCK-expressing interneurons exhibit an early peak in production around E12.5, while CR-expressing interneuron genesis peaks late at E16.5 (1138). Thus, given that VIP is coexpressed by a subset of CCKBCs and also by CR-expressing ISIs, the relatively stable genesis of hippocampal VIP-expressing interneurons does not necessarily reflect consistent production of a single VIP-containing interneuron subtype throughout CGE neurogenesis.
The full complement of CGE-derived interneurons can also be investigated using constitutive GFP reporter lines based on serotonin 3A receptor promoter activity (5-HT3AR-GFP mice) (203, 651, 1179). Indeed, GFP expression in these lines is largely confined to the CGE and is initiated in neuronal precursors shortly after exiting the cell cycle as they begin tangential migration. Moreover, in the mature neocortex and hippocampus, 5-HT3AR-GFP reported cells are GABAergic interneurons that largely overlap (>90%) with those reported by the Mash1-CreER driver line described above, and have minimal overlap, to the populations reported in the Nkx2.1 lineage (203, 651). However, potential overlap between 5-HT3AR-GFP/Mash1-CreER reported interneurons with populations from the dMGE reported by the Nkx6.2-CreER driver line has not yet been examined. This would seem important, as the dMGE is morphologically contiguous with the CGE. In the neocortex it seems unlikely that there would be extensive overlap between 5-HT3AR-GFP/Mash1-CreER reported interneurons with populations from the dMGE as the vast majority of Nkx6.2 lineage interneurons express SST which is only minimally detected in 5-HT3AR-GFP/Mash1-CreER reported neocortical interneurons (651, 796, 1042, 1179). Nonetheless, a large cohort of SST Martinotti cells generated from dMGE progenitors coexpress CR, a neurochemical marker typically associated with interneurons derived from CGE progenitors that is not observed in more vMGE generated Martinotti cells reported by the Nkx2.1-Cre line (SST positive and CR negative) (339, 1042). Thus it is possible that progenitors near the dMGE/CGE boundary specify interneurons with some hybrid MGE/CGE properties.
Nkx6.2 lineally related interneurons from the dMGE have not been fate mapped in the mature hippocampus, and a SST/CR coexpressing neurochemical signature is not detected in hippocampal interneurons (FIGURE 4B). However, ~40% of hippocampal SST-expressing interneurons are reported in 5-HT3AR-GFP mice, and these cells do not overlap with SST interneurons in the Nkx2.1 lineage, suggesting dual MGE and CGE origins for hippocampal SST interneurons (203) (FIGURE 5). Surprisingly, this evidence for dual origins even extends to interneurons that are typically considered homogeneous with regard to their anatomical and basic electrophysiological profiles as O-LM cells significantly contribute to the SST cohorts of both lineages (203). Similarly, hippocampal NGFCs exhibit dual MGE/CGE origins (1137, 1138). In contrast, neocortical NGFCs and SST interneurons (e.g., Martinotti cells) have singular origins within the CGE and MGE, respectively (FIGURE 5). Why CGE-derived SST cells (including O-LMs) and MGE-derived NGFCs selectively populate the hippocampus and avoid the neocortex is presently unknown. However, these observations of dual origins for some hippocampal interneuron populations greatly complicate logical schemes devised around a parsing of interneuron subtypes based on spatially segregated progenitor pools.
C. The Preoptic Area
The POA is estimated to produce ~10% of neocortical and hippocampal interneurons (389, 391). Mature cortical interneurons derived from the POA are highly diverse consisting of small fractions of PV-, SST-, NPY-, and reelin-expressing interneurons and may explain the small residual scattered populations of PV- and SST-expressing interneurons remaining in mice lacking Lhx6 (389, 391, 681, 1273). Indeed, though POA progenitors lying ventral to the MGE also express Nkx2.1, most interneuron precursors emerging from the POA do not rely on downstream activation of Lhx6 to specify PV and SST interneurons (335, 389, 391). Two subdomains are found in the POA based on nonoverlapping expression profiles for Dbx1 and Nkx5.1 (335, 389, 391). GIFM studies using Nkx5.1-Cre mice revealed primarily superficial multipolar mature cortical interneurons expressing NPY and reelin, but not other typical interneuron markers such as PV, SST, CR, and VIP (391, 390). In contrast, GIFM studies using Dbx1-Cre mice revealed that the ventral POA region generates minor populations of deep PV- and SST-expressing interneurons (389). Thus the POA contributes a minor yet highly diverse population of cortical interneurons some of which are MGE-like while others display more CGE-like mature phenotypes.
D. Circuit Assembly and Connectivity
Upon exiting the ganglionic eminences, newborn interneurons must travel long distances to reach their final destination in either the neocortex or hippocampus (reviewed in Refs. 444, 744, 746). This is in stark contrast to excitatory principal cells (PCs), which are born locally in the VZ and SVZ and are tasked purely with radial migration to an appropriate laminar position (reviewed in Ref. 432). As described in detail below, an emerging model of cortical circuit formation suggests an important interplay between PCs and interneurons. Interneurons are channeled to developing neocortical and hippocampal regions and then allowed considerable freedom within migratory streams to disperse across the entire telencephalon. After leaving these streams they migrate radially among neighboring PCs, which in turn provide cues that influence their final position and synaptic connectivity. Importantly, immature interneurons are not passive participants in circuit formation, but play a key role in generating early network activity that promotes synaptogenesis. Subsequently, they are necessary for critical period plasticity, which refines functional circuits and sensory maps. Although the mechanisms are still being elucidated, the end result is mature circuitry composed of interneurons and PCs that demonstrate preferential connectivity and distinct synaptic dynamics dependent on the subtype identity of both the pre- and postsynaptic partners.
E. Migration of Interneurons From the Ganglionic Eminences to the Telencephalon
Nascent interneurons exit the GEs via several migratory routes, which they follow before dispersal throughout the neocortex and hippocampus (38, 829, 1123, 1202, 1254) (and see reviews in Refs. 444 and 744). MGE- and CGE-derived interneurons reach the dorsal telencephalon via separate pathways: those from the MGE travel via dorsolateral routes while those from the CGE follow three distinct routes laterally, medially, and caudally. The choice of migratory path appears to be an intrinsic property of cells dependent on their region of origin and ultimate destination, and governed by different transcription factors (547, 829, 842, 1123, 1254). For example, MGE-derived interneurons transplanted into the CGE do not join native CGE-derived cells along the caudal pathway, but rather migrate laterally and rostrally (1254). However, they can be induced to follow the caudal pathway when forced to overexpress COUPTF2, a transcription factor preferentially expressed in the CGE (547). CGE-derived interneurons themselves express differential combinations of the transcription factors SP8, PROX1, COUPTF1, and COUPTF2 dependent on which of the three migratory paths they take (1123). Finally, as mentioned, MGE progenitors preferentially express the transcription factor Nkx2.1 (147, 148, 829, 1233), which must be downregulated in migrating postmitotic cells in order for them to avoid the striatum and reach the cortex (842). Expression of Nkx2.1 represses transcription of neuropilin-2 (842), a receptor necessary for migrating interneuron repulsion by semaphorin 3F expressed in the striatum (748).
As they enter the developing neocortex and hippocampus, interneurons from both GEs converge and then disperse widely via two streams running in parallel, separated by the cortical plate (CPl) or hippocampal primordium: a deep stream in the intermediate/subventricular zones (IZ/SVZ) and a superficial one in the marginal zone (MZ) (639, 736, 795, 829, 1138). It is presently unresolved why two streams exist or whether their segregation is of specific functional importance. Antypa et al. (42) found differential gene expression profiles of migrating interneurons dependent on stream location, suggesting the choice is not random. However, choice of stream is not dependent on lineage, as both MGE- and CGE-derived interneurons can be found in either (795, 963, 1138). Furthermore, although it has been suggested that interneurons segregate by future subtype (such as CCK+ interneurons being confined to the MZ), the evidence for this is conflicting (159, 805). Interestingly, interneurons from both the CGE and MGE initially enter via the IZ/SVZ, with a subset crossing to the MZ before then resettling within the CPl or hippocampus to begin radial migration (794–796, 963, 1088, 1089, 1091). Signaling mechanisms involving netrin-1 and GABABRs attract interneurons specifically to the MZ (689, 1047), suggesting directed movement to this stream. Within the MZ, interneurons have been observed to migrate in multiple directions, seemingly at random, over long distances (469, 1088, 1089, 1091). Thus it has been proposed that movement to the MZ serves to facilitate the balancing of subtype distributions throughout different regions of the telencephalon (1089, 1091).
Multiple mechanisms act in concert to induce interneurons to leave the tangential migratory streams and take up permanent residence in the CPl and early hippocampus. The most thoroughly described involves chemokine signaling via Cxcl12, which acts as an attractant to keep interneurons within the IZ/SVZ and MZ until they reach appropriate maturity to begin radial migration (672, 690, 973, 1191). Cxcl12 is expressed at high levels within the IZ/SVZ and MZ, and mutations of the receptors Cxcr4 and Cxcr7 result in premature accumulation of interneurons within the CPl (672, 690). Cxcr7 regulates the local concentrations of Cxcl12 sensed by Cxcr4 and controls Cxcr4 protein levels (973). As interneurons mature, Cxcr7 is downregulated, leading to Cxcr4 protein degradation, which in turn acts as a permissive cue for interneurons to leave the tangential migratory streams (672, 973, 1191). Calcium signaling, modulated by neurotransmitters, also plays an important role in the transition to radial migration. Glutamate, via AMPA and NMDA receptors, activates voltage-gated calcium channels to promote invasion of the CPl and hippocampus (78, 117, 736). In CGE-derived interneurons, depolarization via ionotropic 5-HT3ARs serves as an additional mechanism (819). Importantly, GABA either promotes or arrests radial migration dependent on cell maturity. This is due to developmental regulation of the chloride gradient (91, 872, 947, 1241), which renders GABAA depolarizing during early time points (for detailed reviews, see Refs. 89, 92). This is accomplished by regulated expression of the K/Cl cotransporter KCC2, which extrudes chloride, and Na-K-2Cl cotransporter, NKCC1, which accumulates chloride. Migrating interneurons initially express low levels of KCC2, which allows GABA to increase motility via depolarization and activation of voltage-gated calcium channels (117). However, KCC2 levels increase as interneurons mature which renders GABAA hyperpolarizing to reduce calcium influx and stop radial migration within the cortex (117, 795).
F. Establishment of Laminar Position During Radial Migration
Developing interneurons must properly integrate within local circuits, and an emerging model proposes that PCs instruct their radial migration and synaptic connectivity. In the cortex it is well established that PCs laminate in an inside-out manner according to their birthdate: early born cells take up residence in deep layers while those born later populate the superficial layers (40). Furthermore, the PCs are functionally organized among the lamina: corticocortical projection neurons are predominantly found in the superficial layers while subcerebral and corticothalamic projection neurons are found only in deep layers. This organization is likely due to temporal restriction of progenitor potential and specialized progenitor pools within the VZ (reviewed in Ref. 432). In the hippocampus there also appears to be a laminar organization to PCs within the tightly packed s.p., and, similar to cortex, early born cells are deeper (closer to the s.o.) with respect to those born later (reviewed in Ref. 1023). As described in detail below, the lamination of different interneuron subtypes correlates with multiple variables, including birth order and embryonic origin from either the MGE or CGE. However, such intrinsic properties may be primarily important for restricting the response of interneurons to local extrinsic signals from PCs, which invade the CPl and early hippocampus first (39, 179, 432, 796, 913, 1138).
Choice of layer in both the cortex and hippocampus most strikingly correlates with embryonic origin (FIGURE 6A). In cortex, MGE- and CGE-derived cells are biased towards the deep and superficial layers, respectively (795, 796). Similarly, in the hippocampus, MGE-derived interneurons are biased towards the deeper s.o. and s.p., while those from the CGE are primarily found in the more superficial s.r. and s.l.m. (1138). Furthermore, MGE-derived interneurons laminate in the cortex according to birth order in the same inside-out manner as PCs: early born cells migrate deeper than those born later (310, 794, 913, 1155). In contrast, CGE-derived interneurons lack any such correlation with birth order; rather, their attraction to the superficial layers is dominant regardless of birthdate (796). These findings imply that intrinsic factors play an important role in determining laminar position. However, in a key study, Miyoshi et al. (795) found that all interneurons, regardless of birthdate or embryonic origin, are initially distributed evenly throughout all layers of cortex and only later migrate to their final laminar position. Specifically, MGE- and CGE-derived cells, born on either E12.5 or 16.5, are all indistinguishable regarding their laminar position at PN1. They only begin to sort by layer around PN3 and do not take up the stereotyped positions described above until the end of the first postnatal week (FIGURE 6B). This suggests that coordination between intrinsic cell identity and extrinsic cues found locally in the cortex is necessary to guide interneuron migration.
FIGURE 6.
PCs influence the radial migration of interneurons in the cortex. A: MGE- and CGE-derived interneurons are biased in their distributions between deep and superficial lamina, respectively. In the hippocampus, lamination depth is relative to the direction of the apical dendrite (i.e., s.o. is deep and s.l.m. is superficial). In the cortex, the distribution of interneurons correlates with that of pyramidal cell subtypes: intratelencephalic (IT) type are found primarily in superficial layers while pyramidal tract (PT) type are found only in deep layers. B: MGE- and CGE-derived interneurons born on the same day are initially unsorted among the cortical layers and only achieve their stereotyped laminar distributions over the course of the first postnatal week. C, i: knockout of the transcription factor Fezf2 results in the loss of PT-type projection neurons in deep cortical layers, which are replaced by IT type. In these mice, SST+ and PV+ interneurons accumulate to a greater degree in superficial than deep layers. ii: Layer 5 PT-type projection neurons receive greater PVBC inhibition than layer 2/3 IT type. Overexpression of Fezf2 in layer 2/3 results in the conversion of IT type cells to PT type with concomitant increase in PVBC inhibition. PV puncta density was compared between layer 2/3 IT, layer 2/3-induced PT (iPT), and layer 5 PT-type projection neurons. CRYM is a marker for PT type cells. D, i, top: in layer 5 pyramidal cells CXCL12 is localized to the cell bodies but not AIS or dendrites. Bottom: CXCR7, the receptor for CXCL12, is localized to the soma and axon terminals of PVBCs, which target layer 5 pyramidal cells. Pyramidal cells are labeled with GFP. plkBa is a marker for the AIS. ii: Interneurons expressing dsRed were transplanted from the MGE of control or CXCR7 knockout mice at embryonic day 13.5 (E13.5) into the cortex of CXCL12-GFP mice at postnatal day 1 (P1). Interneurons lacking CXCR7 accumulated in the superficial layers, rather than being attracted to CXCL12 in the deep layers. Bracket indicates the location of layer 5 cell bodies expressing GFP from the CXCL12 gene locus. [B from Miyoshi and Fishell (795) with permission from Cerebral Cortex. Ci from Lodato et al. (687) and Cii from Ye et al. (1249) modified with permission from Neuron. Di from Wu et al. (1221) modified with permission from Cerebral Cortex. Dii from Vogt et al. (1176) with permission from Neuron.]
Several lines of evidence implicate PCs as an important source of such guidance cues. In mutant mice in which PC lamination is disrupted, interneurons are correspondingly displaced. For example, in the reeler mouse model, in which expression of reelin or associated proteins is disrupted, the lamination of PCs in cortex is inverted and the s.p. of the hippocampus is split in region CA1 (178–180, 945). In the cortex of reeler mice, MGE-derived interneurons are inverted similar to PCs: early-born cells are found ectopically in superficial layers while those born later are found in the deep layers (489, 913). Furthermore, there is a corresponding inversion of common interneuron molecular markers among the layers (913). Importantly, interneurons lacking the reelin receptor Dab1 migrate to the appropriate layer when transplanted into the cortex of wild-type mice (913). Thus loss of reelin signaling does not disrupt interneuron lamination in a cell-autonomous manner; rather, it disrupts PC lamination, which in turn instructs interneuron migration. In the hippocampus of wild-type mice, the somas of PV-expressing interneurons commonly reside close to or within s.p. Interestingly, in reeler mutants, these interneurons maintain this laminar profile and are often found within the split bands of PCs in region CA1 (121). A very similar phenomenon is observed for PV-expressing interneurons in Lis1 mutant mice, in which the PCs also split into multiple bands (338). Thus the soma and axons of PVBCs appear to be attracted to the somas of hippocampal PCs, whereas the somas of PCs may be repulsive for the axons of PV-expressing BICs. Finally, in utero knockout of doublecortin arrests radial migration of neocortical PCs out of the VZ, resulting in an ectopic cluster of PCs within the white matter below layer 6, and interneurons are specifically attracted to these clusters (935).
Recent work in the neocortex has demonstrated that PCs can modulate interneuron migration in a subtype specific manner and has begun to identify potential molecular mechanisms. In particular, Lodato et al. (687) studied the effects of knocking out Fezf2, a transcription factor necessary for the specification of subcerebral projection neurons in deep cortical layers (195, 196, 798). The loss of subcerebral-projecting PCs correlated with a shift of PV- and SST-expressing interneurons from deep to superficial layers (687) (FIGURE 6Ci). Conversely, overexpression of Fezf2 in utero produced ectopic subcerebral PCs below the white matter that preferentially attracted PV- and SST-expressing interneurons, but not subtypes primarily found in the superficial layers (687). Chemokine-signaling at least in part mediates this attraction of deep layer interneurons to subcerebral projection PCs. As described above, during embryonic development Cxcl12 interacts with the receptors Cxcr7 and Cxcr4 to maintain tangential migrating streams within the IZ/SVZ and MZ. In the postnatal cortex, Cxcl12 and Cxcr7 expression are maintained preferentially in layer 5 (991, 1176). Importantly, Cxcl12 is expressed selectively on the somas of subcerebral projection neurons and Cxcr7 is expressed on the axon terminals of PVBCs (1221) (FIGURE 6Di). Knockout of Cxcl12 results in reduced perisomatic inhibition onto this specific subtype of PC in layer 5. Furthermore, when embryonic MGE-derived interneurons lacking Cxcr7 are transplanted into the cortex at PN1, they fail to migrate to layer 5 and instead accumulate in the superficial layers in adult animals (1176) (FIGURE 6Dii). Finally, when PCs are induced to take on a subcerebral projection identity via Fezf2 overexpression, the number of synapses they receive from local PVBCs increases (1249) (FIGURE 6Cii). Together, these data clearly demonstrate that the patterns of radial migration and synaptic connectivity of interneurons strongly depend on their own subtype identity and that of local PCs.
It was recently proposed that clonal lineage also impacts interneuron radial positioning and potential synaptic connectivity (129, 207, 1065). This was inspired by work on the development of cortical columns and recurrent connectivity among neighboring PCs. Specifically, clonally related sister PCs were found to be radially aligned in the cortex and to preferentially form synaptic connections (1259, 1260). Subsequent work attempted to identify clonally related interneurons by sparse labeling of progenitors with fluorescent markers. Colabeled interneurons in the mature cortex were assumed to represent clonally related sister cells. These studies described radially or horizontally aligned local clusters of labeled interneurons (129, 207), and strongly implied that interneurons from the same progenitor tangentially migrate together to integrate within a common local circuit. However, Ciceri et al. (207) noted that experiments using two different fluorophores suggested that many such identified clusters were likely composed of cells from different progenitors. Subsequent work revisited this hypothesis using bar-coded retroviral libraries, which allows sibling cells to be unambiguously identified (419). These studies revealed that clonally related interneurons disperse widely across the telencephalon and that spatially restricted clusters of interneurons are in fact comprised of cells unrelated by a common progenitor (469, 765, 766, 1143). These data support the general model described above in which interneurons are distributed broadly across the telencephalon and utilize local guidance cues, at least in part from neighboring cells, during circuit assembly.
G. Contribution of Interneurons to Early Intrinsic Network Activity and Circuit Formation
Although molecular cues guide neurons to their proper location and aid in the selection of synaptic partners, the formation of neural circuits is crucially dependent on activity to trigger early plasticity mechanisms. Indeed, structures throughout the entire developing nervous system generate network activity intrinsically before the arrival of mature external sensory stimuli (4, 105). The resulting calcium influx through NMDA receptors (NMDARs) and voltage-gated calcium channels is important for dendritic arborization and establishment of early synaptic connections (597, 1044). In both the hippocampus and neocortex, the earliest activity occurs in the absence of synaptic connections in the form of spontaneous calcium plateaus, which are mediated by voltage-gated calcium channels and coordinated among cells by gap junction coupling (25, 26, 241, 384, 1262). However, this activity is quickly replaced by synapse-driven network events in which interneurons play a key role via depolarizing actions of GABA on PCs during early development (25, 60, 91, 241, 872). This is due to developmental regulation of the chloride gradient via differential expression of the cotransporters KCC2 and NKCC1, described above (91, 947, 1241). Thus, as detailed in this section, GABA acts initially as an excitatory neurotransmitter to drive network activity important for synaptogenesis and circuit formation.
In both the neocortex and hippocampus, interneurons send and receive the earliest synaptic connections, with important consequences for circuit assembly. In PCs, the earliest synaptic currents detected postnatally are mediated by GABAARs and NMDARs; AMPA receptor (AMPAR)-mediated currents are initially absent, only appearing later during the first postnatal week (33, 92, 292, 873, 1149, 1185). In contrast, interneurons demonstrate AMPAR-mediated synaptic currents by the first postnatal day (34, 430, 480). Furthermore, at least in the hippocampus, interneurons demonstrate functional GABAergic synapses very early during postnatal development (430, 480). Thus, during the first postnatal week, interneurons and PCs form excitatory circuits that precede those between PCs. This is functionally significant, as interneurons are positioned to promote early network oscillations, which in turn provide depolarization necessary for glutamatergic synapse development via calcium influx through NMDARs (292, 1219). Indeed, perturbations of GABAergic excitation in vivo disrupt PC glutamatergic synaptogenesis, which can be rescued by increased NMDAR-mediated transmission. For example, Wang et al. (1185) knocked down NKCC1 (which accumulates Cl−) using RNA interference to render GABA hyperpolarizing/shunting throughout development, and this stunted the arrival of AMPAR-mediated synaptic events as well as dendritic branching and spine density in PCs. Similar results were obtained by blocking NKCC1 with bumetanide injections (1184, 1185) or forcing early overexpression of KCC2 (which extrudes Cl−) (161). Importantly, Wang et al. (1185) rescued the development of AMPAR-mediated synaptic transmission by coexpressing a mutant NMDAR that is less susceptible to Mg2+ block. Thus they functionally demonstrated that GABAergic depolarization and NMDARs act in concert to promote PC synaptogenesis (664).
In both the neocortex and hippocampus, network oscillations dependent on glutamatergic and GABAergic synapses emerge near the middle of the first postnatal week (25, 27, 88, 91, 92, 241, 423, 663). This activity has been most extensively studied in vitro, where it is referred to as giant depolarizing potentials (GDPs). GDPs occur as rhythmic bouts of reverberating activity within a local circuit lasting hundreds of milliseconds and travel as a wave (FIGURE 7A). While they involve recurrent excitation between interneurons and PCs, blockade of GABARs alone or optogenetic silencing of interneurons dramatically reduces or abolishes the occurrence of GDPs (FIGURE 7C) (25, 91, 241, 1198). Interestingly, GABAergic synapses between immature interneurons in the hippocampus remain hyperpolarizing and shunting throughout development (60), which may provide a means of suppressing runaway excitation. In the hippocampus GDPs are most readily initiated in area CA3, where depolarization from local interneurons leads to burst firing among recurrently connected PCs, followed by propagation to CA1 (112, 783, 1019, 1020). However, GDPs can also be initiated in CA1, where neonatal recurrent excitation is driven almost exclusively by interneurons, and propagate back to CA3 (383, 783, 1198). In the neocortex, GDPs emerge a couple of days later than those observed in the hippocampus and are primarily generated in the deep layers (25). Furthermore, they may be preceded by or coincide with a separate network rhythm that is primarily dependent on NMDARs and referred to as neocortical early network oscillations (25, 384) (FIGURE 7B). Importantly, in both the hippocampus and neocortex, these in vitro neonatal network activity patterns have in vivo counterparts (217, 423, 663).
FIGURE 7.
Interneurons contribute to the generation of early network activity in the hippocampus and cortex. A, top: spontaneous GDPs recorded intracellularly in hippocampal CA3 pyramidal cells occur rhythmically and are sensitive to the GABAA antagonist bicuculline (Bic). Bottom: expansion of individual GDP examples. B: early network activity in the cortex recorded with calcium imaging of a large population of cells. Left: cortical GDPs (cGDPs) occur rhythmically, engage ~30% of imaged cells, and are sensitive to bicuculline. Calcium signals from three representative cells are shown below the population histograms. Right: early cortical network oscillations (eCNOs) are also observed but are not sensitive to bicuculline. C: optogenetic inhibition of MGE- or CGE-derived interneurons with archearhodopsin (green box) in hippocampal CA1 at postnatal day 5. Inhibiting MGE- but not CGE-derived interneurons greatly reduces the frequency of spontaneous GDPs recorded in pyramidal cells. Traces from multiple overlaid trials are shown. D, i: example of a hub cell in hippocampal CA3 that is highly interconnected with neighboring cells and is capable of triggering a GDP when stimulated to fire action potentials. Axon depicted in red. ii: A subset of hub cells generated early during embryonic development project a long-range axon out of the fimbria (indicated by arrow), and thus may coordinate early network activity across different brain regions. In mature animals these interneurons target the septum. [A from Ben-Ari et al. (91) with permission from Journal of Physiology. B from Allene et al. (25) modified with permission from Journal of Neuroscience. C from Wester and McBain (1198) modified with permission from Journal of Neuroscience. D from Bonifazi et al. (114) modified with permission from Science.]
Although all interneuron subtypes have the potential to contribute to GDP generation, those derived from the MGE may play a more prominent role than those from the CGE. In CA1 of the hippocampus, optogenetic silencing of MGE-derived interneurons reduces the frequency of spontaneous GDPs to a greater extent than silencing those from the CGE (1198) (FIGURE 7C). In the neocortex, GDPs are primarily observed in deep cortical layers, where MGE-derived interneurons are in the majority (25). Furthermore, the time course of neocortical GDP onset correlates with that of synapse development between fast spiking PV-containing interneurons (which are MGE-derived) and neighboring PCs in deep layers (25, 880). Finally, the participation of interneurons in GDPs correlates with their morphological maturity (27). At least in the hippocampus, MGE-derived interneurons appear to be morphologically and synaptically more mature than those from the CGE during the first postnatal week (1198). These data make sense in context of the fact that the MGE begins producing interneurons before the CGE and produces a majority of the total number of interneurons (651, 796, 966, 1138). However, CGE-derived interneurons certainly play a role in GDP generation. It has long been noted that endocannabinoid agonists suppress GDPs while antagonists increase their frequency, strongly implying the participation of CGE-derived CCK-containing interneurons (98, 886).
Finally, a subset of interneurons referred to as hub cells are highly interconnected within local hippocampal circuits and powerfully modulate GDPs, with some capable of triggering GDPs on their own (114, 909, 1173) (FIGURE 7Di). These cells are diverse morphologically, including both perisomatic- and dendrite-targeting interneurons that project widely within the hippocampus (114). Interestingly, a subset of hub cells born early during embryonic development also projects a long-range axon outside of the hippocampus via the fimbria (909) (FIGURE 7Dii). In mature animals, these cells target the septum and in turn receive inputs from major neuromodulatory centers as well as the septum and entorhinal cortex (1173). Thus they likely contribute to the coordination of early network oscillations across multiple related structures.
H. The Transition to Inhibitory Circuits and the Role of Interneurons in Critical Period Plasticity
As neurons mature, the reversal potential for chloride becomes shunting/hyperpolarizing, leading to inhibitory GABAergic transmission (872). This is of course crucial for the normal operation of mature circuits, which requires tight coupling between excitation and inhibition (for reviews see Refs. 268, 271, 522). However, this transition also promotes the next stage of circuit development: critical periods during which maps of external sensory stimuli are refined and established (see reviews in Refs. 481, 668, 1082, 1127). Critical period plasticity has been most thoroughly studied in the visual system, where transiently blocking input to one eye [monocular deprivation (MD)] during a limited developmental time window weakens cortical responsiveness to that eye relative to other (309, 428, 508, 1205). Importantly, the maturation of inhibitory circuits, in particular mediated by PVBCs, is necessary for the expression of this early competitive plasticity (265, 307, 308, 482, 507, 589, 1043). The emergence of inhibition dampens intrinsically generated spontaneous activity while regulating spike-timing dependent plasticity in response to externally driven sensory input (253, 474, 613, 1125). Thus, as interneurons become inhibitory, they sculpt early circuits by supporting competition among nascent synapses.
The onset and closure of critical periods appears to depend on different threshold levels of inhibition that are achieved during postnatal development (1045). In transgenic mice with disrupted GABA synthesis at synaptic terminals, the critical period for MD sensitivity fails to open unless GABA agonists are applied (308, 482). Conversely, acceleration of interneuron circuit development by overexpressing brain-derived neurotrophic factor (BDNF) closes the critical period early (463, 507). BDNF promotes inhibitory synapse development by reducing GABAAR endocytosis, and its release is stimulated by calcium entry through voltage-dependent Ca2+ channels (VDCCs) activated by early depolarizing GABA (919). Rendering GABA hyperpolarizing during the first postnatal week by block of NKCC1 leads to reduced BDNF release and results in late closure of the critical period (265). Thus inhibition is necessary to open critical periods, but once it reaches a threshold level of maturity, the potential for plasticity is diminished and the critical period ends. However, this potential remains in mature circuits, as sensitivity to MD can be reestablished in adult mice by suppressing GABA synthesis or reducing PVBC firing rates (465, 614). Interestingly, closure of the critical period coincides with the establishment of perineuronal nets (PNNs), which are structures of the extracellular matrix that form around PVBCs and stabilize synapses (for reviews, see Refs. 1039, 1183). PNNs also facilitate the capture and uptake of the transcription factor Otx2, which in turn promotes PVBC maturation as well as further PNN development (100, 173, 912, 1063). Importantly, degrading PNNs or blocking their formation reopens or extends the critical period into adulthood (173, 912). Similarly, blocking the ability of Otx2 to bind to PNNs results in their degradation and reopens the critical period in adults (100). Thus PNNs are an important candidate mechanism for the establishment of mature inhibitory circuits and plasticity rules.
I. PCs and Interneurons Form Selective Microcircuits Dependent on Subtype
The diversity of interneuron subtypes leads to sophisticated control of synaptic integration along the somatodendritic axis of PCs, as they differentially target distinct subcompartments (see sect. VIID) (586). However, an important question is to what extent interneurons are also selective in their targeting of neighboring PCs to form specific microcircuits. Some subtypes, such as PVBCs and NGFCs, demonstrate very high rates of connectivity to PCs: connection probabilities in paired recordings are in the range of ~50–90% (204, 331, 876, 880). Thus it has been proposed that nonselective targeting of inhibition is a general rule of cortical circuits and the identities of the pre- and postsynaptic cells are irrelevant (552, 876). However, multiple studies have challenged this view and strongly suggest that subtype specific microcircuits indeed exist, even among PVBCs (650, 656, 1230, 1252). For example, in the neocortex, PVBCs are more likely to innervate and evoke larger amplitude IPSCs in neighboring PCs that provide reciprocal excitation (1252). Furthermore, in the hippocampus, PVBCs evoke larger amplitude IPSCs in PCs located near s.o. than those near s.r. (i.e., deep vs. superficial) (656) (FIGURE 8A). Importantly, PCs can be segregated into distinct subtypes based on the projection target of their long-range axons (313, 432, 466), and recent work suggests this informs connectivity rules with local interneurons (607). In the neocortex, PVBCs are more likely to innervate PCs that project to subcerebral targets (e.g, brain stem and spinal cord) than those that project within the telencephalon (e.g., callosal) (650, 1249) (FIGURE 8B). A similar connectivity rule exists for SST-containing Martinotti cells in deep cortical layers, as they form circuits preferentially with neighboring subcerebral projecting PCs (647, 1015) (FIGURE 8C). In medial entorhinal cortex, CCKBCs innervate PCs that project to the contralateral cortex but avoid neighboring PCs that project to the hippocampal dentate gyrus (1162) (FIGURE 8D). Finally, in CA1 of the hippocampus, PVBCs evoke larger IPSCs in PCs that project to the amygdala than those that project to the prefrontal cortex; conversely, PVBCs are more likely to be innervated by and receive the largest excitatory drive from prefrontal cortex projecting PCs (656) (FIGURE 8E). Thus, while many interneuron subtypes are certainly promiscuous in their targeting of PCs, it is likely that subtype specific microcircuits exist to carry out different cortical and hippocampal computations.
FIGURE 8.
PCs and interneurons form subtype specific microcircuits in both the hippocampus and cortex. A: paired whole cell recordings in hippocampal CA1 between a PVBC (black) and a neighboring deep PC (green) and superficial PC (blue). The probability of observing a connection from a PVBC to either a deep or superficial PC is the same; however, deep PCs demonstrate larger amplitude inhibitory currents. This finding is consistent along the entire axis of the hippocampus (septal to temporal poles). B: in prefrontal cortex, PVBCs connect to PT-type pyramidal cells with greater probability than neighboring IT type. C: in somatosensory cortex, SST+ Martinotti cells mediate disynaptic inhibition between neighboring recurrently connected PT type PCs. D: in medial entorhinal cortex, CCKBCs selectively target PCs that project to the contralateral entorhinal cortex but not neighboring PCs that project to the dentate gyrus of the hippocampus. E, left: in CA1 hippocampus, PVBCs evoke larger amplitude inhibitory currents in PCs that project to the amygdala (AMG) vs. the media prefrontal cortex (mPFC). Right: PCs projecting to the mPFC are more likely to provide synaptic input to neighboring PVBCs than PCs projecting to the AMG or medial entorhinal cortex (MEC). [A and E from Lee et al. (656) modified with permission from Neuron. B from Lee et al. (650) modified with permission from Neuron. C from Silberberg and Markram (1015) modified with permission from Neuron. D from Varga et al. (1162) modified with permission from Nature Neuroscience.]
IV. PHYSIOLOGICAL PROPERTIES OF INHIBITORY INTERNEURONS
The last two decades have strengthened early observations suggesting that the complement of both voltage- and ligand-gated channels expressed on hippocampal inhibitory interneurons differs markedly from those expressed on their glutamatergic principal cell counterparts (502, 641, 770). Given the myriad of inhibitory interneuron types (see sect. II), and the differing roles they play in their respective circuits, it is perhaps not surprising that each particular subtype of inhibitory neuron would express its own unique combination of channels and proteins to fulfill these roles. However, the repertoire of both voltage- and ligand-gated channels is extensive, and their documentation in particular cell types is largely incomplete. In fact, only a handful of studies have tackled the task of unraveling the molecular identities of channels expressed on particular inhibitory interneuron subtypes, allowing only a few channels and their physiological roles in specific subpopulations to be unequivocally identified. For example, much is known about the repertoire of intrinsic conductances present in the PVBC population; the identities and roles of fast Na+ (Nav1.1 and Nav1.6), K+ (Kv1 and Kv3), and Ca2+ (Cav2.1) conductances, the leakage conductances as well as a number of other voltage- and ligand-gated channels in PVBCs are well documented (see below for further discussion; for review, see Ref. 502). Furthermore, how the interplay of these channels regulate PV-containing interneuron excitability, action potential phenotype, and contribute to the generation of coherent oscillations within and between neuronal circuits is reasonably well understood (67, 502). However little, if anything, is known about the identity of equivalent conductances on lesser studied interneuron subpopulations. Although there was an intense avenue of research in the early 1990s, the lack of adequate pharmacological tools has significantly hampered attempts to assign particular roles to molecularly identified voltage-gated channels within discrete interneuron populations. However, despite the absence of the true molecular identity of many of these proteins, much can be learned from the study of a cell’s intrinsic conductances. In addition to providing the molecular and biophysical underpinnings of the cell’s physiological phenotype, they also inform response profiles to a particular neuromodulator or transmitter and provide clues for potential drug targets to treat central nervous system circuit disorder pathologies.
A. Action Potential Firing Patterns
Importantly, cell firing properties in response to electrotonic current injections are frequently used to aid interneuron classification. Thus, before embarking on a detailed discussion of the various passive and active membrane properties in relation to specific ion channels of discrete interneuron subtypes, we briefly describe the most common features of action potential firing associated with the interneurons outlined in section II. Fast-spiking behavior, typically exhibited by PV-expressing AACs, PVBCs, and BiCs, refers to repetitive firing without obvious frequency adaptation/accomodation (i.e., no change in interspike interval) from the beginning to end of a sustained electrotonic depolarizing current pulse beyond threshold (FIGURE 9, A–D). Indeed, in some cases, acceleration of firing with interspike interval shortening can even occur. Individual action potentials are brief with short half-widths (~0.5 ms) that remain fairly constant from the first to last spike and are followed by large/deep, fast/narrow afterhyperpolarizations. The discharge frequency of fast-spiking cells increases steeply as a function of the injected current and can reach well in excess of 100 Hz making them particularly well suited to pace and coordinate high-frequency network activity. In contrast, many interneurons, including OLMs, CCKBCs, SCAs, PPAs, and ADIs, exhibit considerable spike frequency adaptation (with increasing inter spike intervals) that limits maximal firing frequencies to levels much lower than those in fast-spiking cells (typically <50 Hz) (FIGURE 9, E–H). Such “regular spiking” accommodating cells display characteristically wider spikes with longer half-widths (~0.8 ms) that increase from the beginning to end of the discharge. Hippocampal NGFCs and IvCs exhibit only modest frequency accommodation, with broader spikes (~0.8 ms), and achieve significantly lower maximal firing frequencies than fast-spiking cells. Indeed, in these cells, threshold is often reached by a slow ramplike depolarization beyond the initial voltage deflection driven by a sustained square wave current injection (FIGURE 9, I–K). Thus, at such threshold levels of depolarization, NGFCs/IvCs exhibit a “late-spiking” phenotype typified by considerable delay from depolarization onset to action potential discharge of the first action potential. Finally, ISIs (and in some cases members of the other families, particularly at early developmental stages) can exhibit a variety of spiking phenotypes including 1) “irregular” with single action potentials discharged randomly throughout a sustained suprathreshold current injection resulting in highly variable inter spike intervals; 2) “bursting” with three to five spikes discharged at high frequency at the beginning of the depolarizing pulse followed by single spikes of variable interevent intervals; and 3) “stuttering” with clusters of spikes separated by unpredictable periods of silence of varying durations.
FIGURE 9.
Multiparametric analysis of MGE-derived hippocampal interneurons. A–K: neurolucida reconstructions of GFP-containing interneurons recorded in slices from P15–P30 Nkx2–1Cre:RCE pups (dendrites and soma in black; axon in red). Scale bar: 100 μm. The dashed lines indicate the approximate boundaries of s.o., s.p., s.r., and s.l.m. Under each camera lucida drawing is the molecular profile obtained from single-cell PCR analysis for the recorded cell with filled boxes indicating transcripts detected. Also shown are the electrophysiological responses of the cells to the indicated square wave current pulses (bottom) from a resting potential near −60 mV. Depolarizing current pulses and corresponding responses are for near-threshold and 2x-threshold stimulation (scale bars shown in K are for all traces). Phase plots of the APs arising from 2x-threshold stimulation are shown at right, with the first AP phase plot colored red and subsequent APs progressing from warm to cool colors ending in violet. L: histogram summarizing the frequency of occurrence for 16 transcripts probed by scPCR among the MGE cohort of recorded cells. GAD65, GAD67 glutamic acid dehydrogenase; nNOS, neuronal nitric oxide synthase; CR, calretinin; PV, parvalbumin; SOM, somatostatin; NPY, neuropeptide Y; VIP, vasoactive intestinal peptide; CCK, cholecystokinin; VGlut3, vesicular glutamate transporter type 3; enk, preproencephalin; Lhx6, LIM/homeoboxprotein 6; Npas1, Npas3 neuronal PAS domain 1 and 3; COUPTFII, chicken ovalbumin upstream promoter transcription factor II. [From Tricoire et al. (1138) with permission from Society for Neuroscience.]
B. Resting Membrane Potential
Early electrophysiological studies of inhibitory interneurons using sharp microelectrode recording techniques were quick to demonstrate that the resting conductances and physiological firing patterns of inhibitory interneurons deviated markedly from their excitatory cell counterparts (567, 623, 624). Indeed, most studies agreed that interneuron resting membrane potentials were more depolarized and that many cells demonstrated rapid and nonaccommodating firing patterns in response to electrotonic depolarizing current steps (1130, 1131). However, these studies also immediately revealed that many divergent firing patterns existed between inhibitory interneurons, and consequently limited cell classification schemes were built around anatomical and neurochemical features in combination with the nuances of firing patterns typically driven by steady-state depolarizing currents. With the advent of whole cell patch-clamp recording techniques from cells in in vitro slice preparations becoming commonplace in the 1980s, much of these data were systematically reinvestigated. The increased resolution afforded by this technique demonstrated that many of the resting parameters (resting potential, input resistance, membrane capacitance) in subpopulations of inhibitory interneurons possessed few common features. Before considering these data, it is worthwhile pointing out that it is often difficult to get an absolute measure of these parameters when comparing data obtained across different manuscripts from different laboratories. Small changes in slice preparations, intracellular solution composition, and recording quality and resolution (e.g., access resistance, junction potentials) often alter ion reversal potentials, channel availability, and open probability, making measurement of a “true” resting potential (Vm) or other intrinsic conductances problematic.
Two approaches have emerged that facilitate an accurate measure of resting membrane potential that exploit the cell’s intrinsic voltage- or ligand-gated conductances. With the use of a loose cell-attached configuration, an accurate measure of Vm can be established by exploiting the K+ reversal potential (EK+) (359, 1029, 1168). By setting the K+ concentration in the recording pipette approximately equal to the intracellular K+ concentration, EK+ approximates 0 mV. Thus voltage-gated K+ currents will reverse direction when the pipette potential equals Vm, which is determined using a series of depolarizing ramp protocols (1029, 1168). This approach revealed that resting potentials of specific cell types were often more hyperpolarized than original estimates provided by the whole cell recording configuration. For example, in interneurons of the CA1 s.r., this approach yielded Vm values that ranged from −59 to −93 mV (mean −74 mV) (cf. with CA1 pyramidal cells Vm = −84mV; Ref. 359). In contrast, using whole cell recording techniques, the same authors found that electrodes filled with either a high Cl− solution or gluconate-containing solution resulted in Vm measurements that were ~15mV more depolarized. This approach also allows extraction of both the true resting potential and the threshold for firing (359).
Tricoire et al. (1138) used this approach to measure the resting potentials of ~150 anatomically identified MGE- and CGE-derived interneuron subtypes of the CA1 subfield. Cells of the neurogliaform family (NGFC, IvC) typically possess the most hyperpolarized Vm (mean −70 mV, cluster 3 in TABLE 1), whereas PV-containing cells (PVBCs, BiCs, AACs) and virtually all CGE-derived cell types (CCK-containing, long range projecting, VIP-containing) have the most depolarized resting potentials (approximately −55 to −60 mV). SST-containing cells (e.g., O-LM cells) have resting potentials intermediate to all other cells (Vm = −65 mV). Despite possessing varied resting potentials, virtually all cells had similar action potential thresholds close to −35mV (range −32 to −38 mV) (TABLE 1). A second, similar approach, that exploits the known reversal potential of NMDAR-gated single-channel currents, has also been effective in accurately determining resting and threshold properties of cells (90, 943, 1148).
Table 1.
Electrophysiological properties of the identified interneuron clusters
| Cluster 1 (n = 15) | Cluster 2 (n = 23) | Cluster 3 (n = 34) | Cluster 4 (n = 33) | Cluster 5 (n = 19) | Cluster 6 (n = 18) | Comparison | |
|---|---|---|---|---|---|---|---|
| Resting potential, mV | −57 ± 5 | −64 ± 7 | −70 ± 10 | −57 ± 10 | −59 ± 10 | −54 ± 6 | 3<<1,2,4,5,6 |
| Input resistance, MΩ | 116 ± 63 | 216 ± 124 | 302 ± 139 | 431 ± 193 | 401 ± 212 | 219 ± 98 | 1<<2,3,4,5,6, |
| Time constant, ms | 13 ± 8 | 46 ± 18 | 25 ± 8 | 44 ± 15 | 38 ± 13 | 22 ± 9 | 1<<3,6<<<2,4,5 |
| Sag index | 0.84 ± 0.06 | 0.79 ± 0.09 | 0.88 ± 0.06 | 0.80 ± 0.10 | 0.80 ± 0.07 | 0.71 ± 0.15 | 1,2,4,5,6 < 3 |
| Frequency at 2X threshold, Hz | 70 ± 26 | 19 ± 9 | 27 ± 10 | 17 ± 7 | 18 ± 6 | 27 ± 13 | 2,4,5<<3,6<<<1 |
| Adaption ratio at 2X threshold | 0.88 ± 0.13 | 0.64 ± 0.22 | 0.80 ± 0.12 | 0.56 ± 0.17 | 0.44 ± 0.17 | 0.46 ± 0.17 | 4,5,6 < 2<<<3 < 1 |
| 1st Spike threshold, mV | −32 ± 4 | −38 ± 3 | −34 ± 4 | −35 ± 3 | −36 ± 3 | −38 ± 4 | 2,4,5,6 < 3,1 |
| 2nd Spike threshold, mV | −32 ± 4 | −37 ± 3 | −34 ± 4 | −35 ± 3 | −35 ± 4 | −37 ± 4 | 1,2,3,4,5,6 |
| 1st Spike amplitude, mV | 47 ± 7 | 62 ± 8 | 52 ± 9 | 62 ± 6 | 48 ± 10 | 59 ± 8 | 1,3,5 < 2,4,6 |
| 2nd Spike amplitude, mV | 48 ± 8 | 61 ± 7 | 52 ± 9 | 61 ± 6 | 48 ± 10 | 60 ± 7 | 1,5 < 3<<2,4,6 |
| 1st Spike half-width, ms | 0.54 ± 0.11 | 0.74 ± 0.11 | 1.06 ± 0.20 | 0.81 ± 0.09 | 1.04 ± 0.15 | 0.72 ± 0.10 | 1<<<2,6<<4<<<3,5 |
| 2nd Spike half-width, ms | 0.54 ± 0.11 | 0.77 ± 0.12 | 1.08 ± 0.21 | 0.86 ± 0.10 | 1.12 ± 0.14 | 0.77 ± 0.10 | 1<<<2,6<<4<<<3,5 |
| 1st Time to repolarize, ms | 3.1 ± 1.2 | 4.3 ± 1.6 | 9.6 ± 4.1 | 10.8 ± 7.8 | 20.4 ± 12.7 | 11.5 ± 6.8 | 1 < 2<<<3,4,6 < 5 |
| 2nd Time to repolarize, ms | 3.1 ± 1.1 | 4.7 ± 1.5 | 9.9 ± 3.7 | 13.1 ± 7.7 | 23.2 ± 10.1 | 16.3 ± 10.0 | 1<<2<<<3,4,6<<5 |
| 1st Maximal decay slope, mV/ms | −112 ± 24 | −96 ± 17 | −55 ± 13 | −79 ± 16 | −50 ± 12 | −83 ± 14 | 1 < 2 <4,6<<<3,5 |
| 2nd Maximal decay slope, mV/ms | −113 ± 26 | −91 ± 18 | −55 ± 13 | −74 ± 15 | −46 ± 10 | −78 ± 14 | 1<<2<<4,6<<<3<<5 |
| 1st AHP amplitude, mV | 25 ± 3 | 21 ± 3 | 20 ± 3 | 19 ± 3 | 13 ± 3 | 14 ± 3 | 5,6<<<4<<2,3<<1 |
| 2nd AHP amplitude, mV | 25 ± 4 | 22 ± 3 | 21 ± 4 | 20 ± 4 | 15 ± 4 | 15 ± 3 | 5,6<<<2,3,4<<1 |
| 1st AHP half-width, ms | 29 ± 16 | 55 ± 36 | 72 ± 31 | 124 ± 76 | 85 ± 41 | 63 ± 31 | 1 < 2,3,4,5,6 |
| 2nd AHP half-width, ms | 27 ± 15 | 76 ± 47 | 69 ± 33 | 129 ± 50 | 116 ± 63 | 82 ± 32 | 1<<<2,3,6 < 4,5 |
n, Number of cells; < indicates significantly smaller with P ≤ 0.05; << indicates significantly smaller with P ≤ 0.01; <<< indicates significantly smaller with P ≤ 0.001. [From Tricoire et al. (1138) with permission from Society for Neuroscience.]
C. Voltage-Gated K+ Conductances
In the early 1990s successful cloning of many voltage-gated ion channels promised a new era where one could study the biophysics of channels expressed in heterologous systems as a means to identify the molecular identity of conductances underlying a particular aspect of cell physiology, e.g., the action potential waveform, and threshold and subthreshold conductances. This optimism was short lived as it became apparent that few channels expressed in recombinant systems perfectly matched the biophysical properties of native channels (967). We now appreciate that native channels are often modified by phosphorylation and other posttranslational processes or are in intimate association with auxiliary and accessory proteins that shape their trafficking, availability, and biophysical features in unpredictable ways. Early attempts to match expression patterns of recent cloned channel transcripts with biophysical properties measured by whole cell recording techniques in in vitro slices did, however, yield some modest successes, particularly with channels formed by the Kv3 family of voltage-gated potassium channels (967) as discussed below.
Early electrophysiological recordings were quick to highlight that the physiological properties of hippocampal inhibitory interneurons differed markedly from principal cells. The rapid rise, fall, and brevity of action potentials, together with their deep AHPs, provided the first clues that the conductances underlying these important physiological features were likely distinct from those observed in their pyramidal cell counterparts. Subsequent studies provided important but limited evidence for the roles played by specific voltage-gated conductances in particular subpopulations of inhibitory interneurons, with the best characterized being the diverse repertoire of voltage-gated potassium channels. However, like many aspects of interneuron physiology, the story is largely incomplete for the vast majority of cell types with the conductances of only a small number of identified interneuron types being studied in any great detail. However, what is evident is that the spatial arrangement of numerous voltage-gated K+ conductances across the somatodendritic and axonal compartments are key determinants of their spatial and temporal signal processing capabilities.
Early recordings from interneurons located within the s.o. and s.r. indicated that both transient “A-type” and numerous sustained “delayed rectifier” currents were common to virtually all interneurons (202, 678, 756, 1268, 1269). The kinetic properties of these conductances were typically rapid and possessed pharmacologies that diverged from those observed in pyramidal cells. Outside-out nucleated patch recordings from identified CA1 SST-positive O-LM cells revealed that three voltage-gated K+ current types predominate in this cell type (TABLE 2) (678). A fast “delayed rectifier”, highly sensitive to 4-aminopyridine (4-AP) and tetraethylammonium (TEA) (IC50 values <100 μM), a slow delayed rectifier blocked by high concentrations of TEA, but insensitive to 4-AP and a rapidly inactivating A-type current that was blocked by high concentrations of 4-AP but resistant to TEA. These currents contributed 57, 25, and 19% to the total macroscopic current. Single-cell reverse transcription polymerase chain reaction (RT-PCR) revealed that the fast delayed rectifier and the A-type current components were mediated by homomeric Kv3.2 and Kv4.3 channels, respectively (678).
Table 2.
Functional properties of major K+ current components in O-LM and PVBCs
| O-LM Interneurons | PVBC |
|---|---|
| Fast delayed rectifier | |
| 57 ± 5% | 58 ± 6% |
| Activation curve | Activation curve |
| Midpoint V: −8.0 ± 2.1 mV | Midpoint V: −7.1 ± 0.9 mV |
| k: 16.1 ± 0.7 mV | k: 11.5 ± 0.8 mV |
| Inactivation curve | |
| Midpoint V: −40.6 ± 2.4 mV | |
| k: 7.8 ± 0.8 mV | |
| Noninact. comp.: 8 ± 4% | |
| Deactiv. time constant (−40 mV): 11.1 ± 0.9 ms | |
| Slow delayed rectifier | |
| 25 ± 6% | 26 ± 5% |
| Activation curve | Activation curve |
| Midpoint V: −3.6 ± 4.2 mV | Midpoint V: −3.3 ± 4.9 mV |
| k: 23.1 ± 1.0 mV | k: 17.3 ± 1.5 mV |
| Inactivation curve | Inactivation curve |
| Midpoint V: −52.2 ± 7.7 mV | Midpoint V: −63.8 ± 6.2 mV |
| k: 15.2 ± 1.7 mV | k: 11.1 ± 2.0 mV |
| Noninact. comp.: 7 ± 2% | Noninact. comp.: 37 ± 5% |
| Deactiv. time constant (−40 mV): 21.0 ± 1.7 ms | |
| A-type | |
| 19 ± 2% | 17 ± 4% |
| Activation curve | Activation curve |
| Midpoint V: −0.2 ± 3.0 mV | Midpoint V: −6.2 ± 3.3 mV |
| k: 26.8 ± 0.8 mV | k: 23.0 ± 0.7 mV |
| Inactivation curve | Inactivation curve |
| Midpoint V: −78.5 ± 2.4 mV | Midpoint V: −75.5 ± 2.5 mV |
| k: 6.0 ± 1.2 mV (7) | k: 8.5 ± 0.8 mV (7) |
| Recovery from inactivation time constant: 39.3 ± 18.5 ms | Recovery from inactivation time constant: 30.1 ± 6.4 ms |
| Amplitude: 42 ± 13% | Amplitude: 22 ± 4% |
| Time constant: 329 ± 317 ms | Time constant: 165 ± 49 ms |
| Amplitude: 36 ± 12% | Amplitude: 55 ± 5% |
Values are means ± SE. Noninact., noninactivating; deactiv., deactivation. Data were extracted from References 678 and 756.
Similar electrophysiological recordings from PVBCs revealed that macroscopic currents were dominated (~58%) by a fast non-inactivating delayed rectifier current component sensitive to low concentrations of 4-AP and TEA (IC50 = <100 mM), similar (but not identical) to that observed in dendrite targeting SST-containing interneurons (FIGURE 10 and TABLE 2) (678). The kinetic properties of this current are remarkably rapid with the 20–80% rise time being <1 ms and showing little, if any, inactivation during a 100-ms voltage step. A second slower delayed rectifier (20–80% rise time ~6 ms) component with intermediate sensitivity to TEA and a rapid inactivating A-type current component blocked by 4-AP but resistant to TEA were also identified (756). Single-cell RT-PCR revealed a high prevalence of Kv3.1 and Kv3.2 in all PVBCs with a lower abundance of Kv4.2 and Kv4.3 transcripts.
FIGURE 10.
Voltage-gated potassium and sodium currents in PVBCS. A, top left: a train of action potentials evoked by a 1-s depolarizing current pulse in a fast spiking basket cell in current-clamp configuration. Bottom left: voltage-gated K+ currents evoked in a nucleated patch isolated from a FSBC (holding potential −90 mV and test potentials delivered between −80 and 70 mV in 10-mV increments). Right: current subtraction analysis reveals three kinetically and pharmacologically distinct K+ current components in nucleated patches from FSBC. Top: a fast delayed rectifier K+ current component, [isolated by Icontrol – I0.5 mM 4-AP (top trace) or Icontrol – I1mM TEA (bottom trace)]. Middle: a slow delayed rectifier K+ current component (isolated by I0.5mM 4-AP – I0.5mM 4-AP + 20mM TEA or I1mM TEA – I20mM TEA). Bottom: an A-type K+ current component (isolated in the presence of I20 mM TEA). Currents were evoked by test pulses to 70 mV (from a holding potential of −90 mV). B: voltage-gated Na+ channel spatial distribution profiling in FSBCs. Channel density measured in the outside-out patch configuration is plotted against distance, with negative values indicating dendritic location (red) and positive values indicating axonal location (blue). Note the absence of an appreciable Na+ conductance in the dendrites and a stepwise increase of Na+ channel density from the soma to the proximal axon, followed by a gradual increase to the distal axon. [A from Martina et al. (756) with permission from Society for Neuroscience. B from Hu et al. (502) with permission from Science.]
Delayed rectifier channels assembled from Kv3 subunits are instrumental components in conferring high-frequency action potential firing in numerous hippocampal and cortical interneuron subtypes (304, 967, 1187). Their rapid activation ensures efficient spike repolarization, and their rapid deactivation allows minimal K+ current accumulation during the interspike interval that would interfere with generation of subsequent action potentials. Importantly, the rapid activation kinetics enforces a narrow temporal window that minimizes Na+ channel inactivation, and the consequent deep hyperpolarization serves to deinactivate other voltage-gated channels ensuring a high fraction are available for activation during trains of action potentials (967).
Although it is widely recognized that Kv3 containing channels are synonymous with cells that fire action potentials at high frequency (967), Kv3.2 is highly expressed in both fast spiking basket cells and SST-containing O-LM cells. Although O-LM cells can fire at reasonably high frequencies, their maximal firing frequency is significantly lower than that of PVBCs. A comparison of the data from two publications from Peter Jonas’ laboratory (678, 756), who used identical recording techniques in O-LM and PVBCs, reveals important quantitative differences between the rapidly activating sustained K+ currents in the two cell types (TABLE 2). Although many of the kinetic properties were similar, the activation and inactivation curves are less steep in O-LM cells compared with PVBCs, and the deactivation of the predominantly Kv3.2 channels in the O-LM cells is slower than the equivalent Kv3.1 containing channels in PVBCs. These subtle differences likely set the upper firing frequency of O-LM cells to be lower than PVBCs.
Kv3.2 subunits, but not Kv3.1 subunits, possess a consensus sequence for protein kinase A (PKA) phosphorylation (803, 965). cAMP and PKA phosphorylation inhibits current through Kv3.2 channels but not Kv3.1b. PKA phosphorylation of Kv3.2 channels in both PVBCs and SST-containing interneurons broadens the action potential waveform and reduces the maximal firing frequency in both cell types consequently modulating network oscillatory activity (49). Despite lacking a PKA phosphorylation site, Kv3.1 subunits have 11 protein kinase C (PKC) and 10 casein kinase sites (1166), suggesting that the phosphorylated state of both Kv3.1 and Kv3.2 could have a major impact on the firing properties of a number of interneuron populations.
In hippocampus, both Kv3.1 and Kv3.2 protein expression are developmentally upregulated between PN7 and PN21 (287, 1098). Immunohistochemistry reveals Kv3.2 expression is primarily across the somatodendritic arbor of virtually all PV-containing cells, ~85% of nNOS-positive cells, and ~50% of SST-containing interneurons. Kv3.1b is largely expressed across the soma, dendrites, as well as the axons of PV-containing interneurons and is completely absent in SST-containing or any other interneuron type (287). Kv3 expression is highest in dendrites of cells that typically possess low dendritic Na+ channel densities (502, 504). Dendritic Kv3 currents act to accelerate the decay time course of excitatory postsynaptic potentials (EPSPs), imposing a narrow window for temporal integration (504, 967), and minimize the impact of clustered excitatory input to favor distributed synaptic inputs (504). In the axonal compartment, Kv3 channels ensure brief and rapid action potential firing and repolarization. Rowan et al. (957) recently demonstrated that Kv3 channels are clustered at individual presynaptic boutons and are usually spared from axonal shafts. These axonal Kv3 channels control local spike repolarization and act as key determinants of the compartmentalized control of action potential width in a synapse by synapse manner (957).
Postnatal upregulation of Kv3 containing currents in PV-containing cells is part of a coordinated sequence of events that tune PV cells for their ultimate participation in gamma oscillogenesis (277, 287, 1098) (see sect. XV). Gamma oscillations in the young hippocampus are poorly organized in part due to the immature state of PV-containing interneurons. At early postnatal stages, action potential firing is slow and unable to sustain periods of high-frequency activity. In addition, action potential propagation and transmitter release components cannot support the rapid throughput typically observed in mature cells. Consequently, during the first two to three postnatal weeks of life, action potential duration, propagation time, and duration of the transmitter release period decrease by ~50% as a result of the concurrent functional maturation of cell morphology, voltage-gated Na+ and K+ currents, as well as refinement of the presynaptic release machinery (277, 502).
In addition to the Kv3 subunits, both Kv1.1 and Kv1.2 are expressed in axons of PV-containing interneurons. Both Kv1.1 and Kv1.2 have low voltage activation thresholds and slower activation when compared with the Kv3 family and underlie the conductance known as the D-type K+ current (245, 415). Both Kv1.1 and Kv1.2 are highly enriched at the axon initial segment where they colocalize with the voltage-gated sodium channel Nav1.6 (415, 695). When compared with the axon initial segments of principal cells, Kv1.1 and Kv1.2 are expressed at a much higher density (695), suggesting that they may play a role in establishing an increased threshold for action potential initiation in PV-containing cells. Goldberg et al. (415) observed that the slow inactivation of Kv1 containing channels in PV-containing cells of the barrel cortex causes a shift in the threshold for action potential initiation. Moreover, Kv1.1 likely functions to permit responses primarily to large events that can temporally exceed its rate of activation. In support of this, blockade of Kv1.1 eliminates the delay to first spike typically observed in these cells and converts the cell to a continuous high-frequency firing mode. In consideration of these data, Hu et al. (502) reasoned that Kv1 channels may impose a fast coincidence detection mechanism in PV-containing interneurons, allowing fast EPSPs to beat Kv1 activation leading to a rapid action potential initiation with little delay. Finally, brief repetitive stimulation of Schaffer collateral inputs to PV-containing cells results in a novel form of long-term intrinsic plasticity (160). This intrinsic plasticity results in increased excitability and recruitment of PV-containing cells, which arises through an mGluR5-dependent downregulation of currents through Kv1 channels.
Kv4.3 subunits are highly enriched in neocortical and hippocampal interneurons (678, 756, 782, 944, 999) and are thought to underlie the 4-AP-sensitive, A-type inactivating K+ conductance in these cells. In hippocampus, Kv4.3 expression is highest in the somatodendritc compartments of CCK-containing, and CB-containing interneurons located in both s.r. and s.l.m. as well as SST-containing interneurons in s.p. and s.o. (119, 678, 782). In contrast, Kv4.3 is expressed in only a small percentage of PV-containing cells (119). Both Kv4.1 and Kv4.2 channels are largely absent from hippocampal interneurons (944). Native Kv4 containing channels typically associate with auxiliary subunits, which act to modify their expression levels and biophysical properties (530). The potassium channel interacting proteins (KChIPs) are Ca2+-binding proteins, which interact with the cytoplasmic NH2-terminal domain of Kv4 channels and KChIP1 is found exclusively in hippocampal interneurons where it overlaps with Kv4.3 (782, 944). Of the total Kv4.3/KChIP1 coexpression, 26% was contributed by PV-containing neurons, ~18% by SST-containing, ~20% by CB-containing, and ~34% by CR-containing interneurons (782). It is unclear what this partial overlap is actually telling us about the roles played by Kv4.3/KChIP channels in these cells, and it is possible that KChIP1 may interact with another as yet unidentified surface expressed protein.
Application of the muscarinic acetylcholine receptor (mAChR) agonist carbachol to in vitro hippocampal slices generates theta frequency subthreshold membrane potential oscillations in inhibitory interneurons (191) that are generated by the interplay of voltage-dependent K+ (IA, IKfast, IKslow, and ID) and persistent Na+ conductances (191). Using a small interfering RNA knock down approach, Bourdeau et al. (119) implicated Kv4.3 containing channels in the generation of these subthreshold membrane potential oscillations, suggesting that significant A-type current can be active at potentials close to rest and have roles in physiological processes other than action potential repolarization.
D. Voltage-Gated Na+ Conductances
Unlike voltage-gated K+ conductances, relatively little is known about voltage-gated Na+ channels and conductances expressed on inhibitory interneurons. What information does exist is almost exclusively derived from studies of PVBCs (for review, see Ref. 502) and SST-containing O-LM cells (FIGURE 10). The earliest study of Na+ conductances in fast spiking interneurons revealed significant differences between interneurons and their principal cell counterparts (755). Although the voltage dependence of activation was similar between the two cell types, the deactivation kinetics differed, being more rapid in basket cells (~0.13 vs. 0.2 ms at −40 mV). However, the greatest differences occurred in their steady-state inactivation properties, which were steeper and shifted to more positive voltages in PVBCs (755). The molecular identities of Na+ channels in interneurons suggest that at least in PVBCs the vast majority of Na+ channels are formed by Nav1.1 and Nav1.6 subunits with a lesser contribution from Nav1.2, Nav1.4, and Nav1.7 (502, 695, 857, 852). In contrast to hippocampal principal cells, Na+ channels are largely excluded from the dendrites of PVBCs where K+ channels predominate. Na+ channels instead are clustered in high density at the axon initial segment, which in PVBCs originates at locations extremely close to the soma (~20 μm away), and steadily increase in density towards distal axon sites (FIGURE 10B). Calculations have suggested that 99% of PVBC Na+ channels are contained within the axonal compartment (503).
Na+ channels in PV-containing cells are targets for neuromodulation and have been implicated in pathophysiological states. While the data regarding neuromodulation of Na+ channels in interneurons are sparse, Janssen et al. (528) directly demonstrated that application of exogenous neuregulin (NRG1) reduces the excitability of Erb4-expressing interneurons (of which PV-containing cells are a subset) in primary culture by raising action potential threshold and decreasing Na+ channel activity by an as yet unknown mechanism. Transient cerebral ischemia also impacts Na+ channel activation and surface expression in PV-containing interneurons rendering cells less excitable (1265). Reduced Nav1.1 levels and PV-containing interneuron dysfunction critically contribute to abnormalities in oscillations, synchrony, and memory in human amyloid precursor protein transgenic mice and possibly in humans with Alzheimers disease (1170). Finally, a dominant loss of function mutation in Nav1.1 causes Dravet syndrome, an intractable form of childhood-onset epilepsy that arises from a reduction in Na+ channel density in hippocampal interneurons (1255). In mouse models of Dravets syndrome cortical interneurons cannot sustain high-frequency firing, leading to circuit disinhibition, seizures, and premature death (194, 852).
Unlike PV-containing interneurons, SST-containing cells, particularly O-LM cells, have a comparatively high density of Na+ channels on their somatodendritic axis. Outside-out patch-clamp recordings from different portions of the somatodendritic domain revealed a reasonably uniform high density of Na+ channels regardless of the compartment (757). The peak Na+ conductance density in O-LM cell dendrites was measured at ~110 pS/μm2 at −10 mV (757), about three times that seen on cortical pyramidal cell dendrites (1059, but cf. with PV-containing axons described above). Of interest, the midpoint for Na+ channel activation was observed at more negative potentials in the dendrites compared with somas. However, the time constant for recovery from inactivation was identical at both locations (757). This negatively shifted activation range undoubtedly has a major role to play in lowering the threshold for spike initiation in SST-containing cells whose axons often emerge from portions of the dendritic tree.
E. Voltage-Dependent Ca2+ Conductances
VDCCs play myriad roles in central neurons. VDCCs located on the soma and dendrites can regulate dendritic excitability and shape the spatial and temporal properties of incoming synaptic inputs. The subsequent increase in intracellular Ca2+ triggers a multitude of second messenger cascades and regulates transcription and translation events. Ca2+ channels located in the axon have a pivotal role in establishing the mechanisms behind neurotransmitter regulation and release. The identities and biophysical properties of VDCCs in principal cells are well characterized, and a clear picture of their roles in these cells is firmly established. The picture is less clear for Ca2+ channels in inhibitory interneurons. Much of what we know concerning Ca2+ channels in interneurons has come from immunohistochemical and pharmacological studies with few studies reporting the biophysical properties of Ca2+ channels in interneuron subtypes.
High-resolution immunohistochemical analyses of Ca2+ channel subunit expression reveals a marked heterogeneity throughout mouse hippocampal interneuron subtypes (1174). Cav2.1 (responsible for P/Q-type VDCCs) and Cav3.1 (T-type VDCCs) subunits are uniformly expressed in almost all interneuron subtypes. The L-type VDCC subunits, Cav1.2 and Cav1.3, colocalize to CR-containing interneurons, while Cav1.3 expression is highest in PV-containing and SST-containing interneurons. Cav2.2 (N-type VDCCs) is expressed in all interneurons with the exception of CB-containing cells. Cav2.3 (R-type VDCCs) is also uniformly expressed in all interneurons except PV-containing and CR-containing where expression is much lower (1174). Surprisingly, no study has tackled an extensive characterization of the biophysical properties of any of these channel types in particular subtypes of interneurons; therefore, it is unclear whether Ca2+ channel properties are similar or distinct from those of hippocampal principal cells.
Ca2+ imaging studies were first to show the presence of functional dendritic VDCCs that mediate Ca2+ influx during synaptic depolarizations and back-propagating action potentials in numerous interneuron subtypes (416, 418, 545, 961). The first direct demonstration of a high-density expression of low-voltage-activated Ca2+ channels came from imaging the dendrites of SST-containing low threshold spiking cortical interneurons (416). In these cells dendritic T-type channels trigger low-threshold spikes and subsequent recruitment of other Ca2+ channel types including R- and L-type channels. During trains of activity, the dendrites of low-threshold spiking interneurons behave as a single nonlinear compartment generating global Ca2+ elevation across the entire dendritic tree (416). This is in marked contrast to Ca2+ dynamics in PVBCs and cortical “irregular-spiking” supragranular interneurons where the voltage-gated transient potassium current IA restricts action potential propagation and limits dendritic Ca2+ channel activation (417, 418).
In hippocampus, imaging of Ca2+ transients triggered by back-propagating action potentials in SST-containing O-LM cells revealed contributions of T, L, N, and P/Q channel types (1111). Dendritic L-type VDCCs are implicated in Hebbian long-term potentiation (LTP) at excitatory synapses onto interneurons (see sect. VIB) (1110, 1112), and high-frequency synaptic activity of mGluR5 induces a selective and compartmentalized potentiation of L-type channels by a PKC-dependent mechanism and release of Ca2+ from ryanodine-sensitive intracellular stores (1111).
As discussed above, the L-type Ca2+ channel subunit Cav1.3 (and to a lesser extent Cav1.2) is enriched in PV-containing interneurons where it contributes to the low-voltage-activated Ca2+ conductance. Both Cav1.2 and Cav1.3 are largely expressed on the cell bodies and dendrites of PV-containing interneurons and are absent from the axonal compartment (533). Functional maturation of PV-containing interneurons is retarded by antagonists and accelerated by agonists of L-type VDCCs, indicating a critical role for L-type channels in cell maturation. Moreover, currents through L-type channels facilitate CREB phosphorylation important for excitatory transcription coupling in PV-containing cells (214). In cortex and hippocampus, general expression of T-type channel subunits Cav3.1 and Cav3.2 increases during development to peak at P21 (10). In contrast, Cav3.1 expression in G42-positive cortical fast spiking basket cells is downregulated during the first few weeks of postnatal life, likely contributing to a decrease in cell excitability upon maturation (857). Although tetrodotoxin-insensitive depolarizations and action potentials are common in young (PN5–7) PV-containing cells, they are largely absent by PN25 consistent with the loss of functional T-type channels in these cells (857).
Cav2.1 (P/Q channels) and Cav2.2 (N-type channels) are widely expressed throughout all interneuron subtypes and contribute to a class of Ca2+ channels known as high-voltage-activated channels (HVA). HVA channels are largely responsible for controlling neurotransmitter release and are intimately associated with the presynaptic machinery at individual release sites. Cav2.1 channels (P/Q type) are particularly enriched in the axon terminals of PV-containing interneurons, whereas Cav2.2 (N-type channels) are preferentially expressed in CCK-containing cell axons (see sect. IVL for their roles in neurotransmitter release mechanisms). In addition to HVA channels, Cav3 (T-type) channels have also been identified at presynaptic sites on PV-containing axons (1093). Activation of T-type channels on PV-containing axons by nicotinic acetylcholine receptors can trigger asynchronous transmitter release, which can be augmented by a concomitant Ca2+ release from presynaptic intracellular stores (1093).
F. The Hyperpolarization-Activated Cation Current
Hyperpolarization-activated cation channels (Ih or HCN channels) are widely expressed in neurons of the mammalian central nervous system. Formed from four subunits, HCN1-HCN4, they have been most widely studied in principal excitatory neurons within the hippocampus and cortex where they regulate cell excitability and synaptic integration (709, 881, 1210). In situ hybridization analysis suggests that the majority of Ih channels in inhibitory neurons are formed from HCN1 and HCN2 subunits with a smaller contribution of HCN4 subunits (976). In principal neurons, Ih channel density increases as a function of distance from the soma (730). Although it is unclear whether the Ih density is similarly distributed in interneuron dendrites, a multicomparment modeling study suggests that Ih is dendritically expressed in O-LM cells (994).
Only a small number of studies have formally demonstrated the Ih current under voltage-clamp conditions in inhibitory neurons (see below). However, its presence is often assumed based on a voltage-dependent sag that manifests during electrotonic hyperpolarizing current pulses in current clamp recordings (881, 949). Tricoire et al. (1138) noted that in recordings from ~150 CA1 interneurons that while virtually all cells displayed a voltage-dependent sag during current pulses, this sag was largest in the SST- and CCK-containing interneurons (FIGURE 9 and TABLE 1).
The first formal demonstration of Ih in hippocampal interneurons came from recordings from O-LM interneurons of the CA1 s.o.-alveus (718) (FIGURE 11A). Ih in these cells is activated at potentials close to −50 mV, with a mid activation point of approximately −80 mV and makes a major contribution to the total membrane conductance at rest. Ih is blocked by both external Cs+ and ZD7288 (FIGURE 11, A and B) and potentiated by the monoamine adrenergic agonists norepinephrine and isoprenaline (718). Ih amplitude and activation kinetics are enhanced by ethanol in s.l.m. interneurons (1244). Ih in O-LM interneurons is inhibited by activation of mu and delta opioid receptors (1071) as well as nicotinic receptors (435). Since Ih is active at potentials close to rest, its blockade results in membrane hyperpolarization accompanied by increased cell input resistance. Block of Ih also decreases spontaneous action potential frequency by prolonging the interspike trajectory (718).
FIGURE 11.
Ih and IM in O-LM interneurons. A: whole cell voltage-clamp recordings from CA1 O-LM interneurons showing a family of Ih traces elicited by hyperpolarizing test pulses (Vh = −35 mV) in the range −50 to −120 mV in control (left panel) and after addition of extracellular Cs+ (5 mM, middle panel). The Cs+-sensitive current was obtained by digital subtraction (right panel). Bottom traces: similar results were obtained using the Ih antagonist ZD7288 (100 μM). B: time course of ZD7288 block: Ih was activated with repetitive steps to −120 mV (Vh = −40 mV). ZD7288 blocks the time-dependent inward current leaving only the leak and capacitive artifact (inset: i, control; ii, ZD7288; and i-ii, subtracted). Bottom right: under current-clamp recording conditions, ZD7288 induces a hyperpolarization of the cell, concomitant with a block of the sag and the rebound depolarization (arrow) elicited by hyperpolarizing current steps (insets: i, control; and ii, in the presence of ZD7288) (Vh = −60 mV). C: IM in SO interneurons can be identified using the antagonists, linopirdine, XE-991, and retigabine. Under whole cell voltage-clamp conditions, steps from −30 to −50 mV at 15-s intervals activate the time-dependent current IM (traces 1, 3, and 5). Addition of linopirdine, XE-991, and retigabine removes the time-dependent component. Traces enumerated in each condition are the average of three traces. Control traces (gray) are overlaid for comparison with drug conditions (black). Bottom panels: isolated IM amplitudes and changes in holding current (Ihold) in the presence of linopirdine, XE-991, and retigabine conditions. GFP-positive s.o. interneurons, anatomically identified O-LM cells, and unidentified s.o. interneurons are indicated by black symbols, gray symbols, and open symbols, respectively. [A and B from Maccaferri and McBain (718) with permission from Journal of Physiology. C from Lawrence et al. (645) with permission from the Society for Neuroscience.]
In a study of PV-containing fast spiking basket cells in the DG, Aponte et al. (43) observed that the biophysical properties of Ih were somewhat similar to those described in O-LM cells and had a role to play in establishing resting potential, input resistance, and the membrane time constant. In PV-containing cells, RT-PCR identified Ih as the heteromeric assembly of HCN1 and HCN2 subunits. Of particular interest Ih not only has a role to play in somatodendritic processes but also determines axonal properties (43, 977). In DG PVBCs, Ih channels depolarize the axon and reduce the threshold for action potential initiation thereby increasing spike reliability (43). Moreover, blockade of Ih by ZD7288 reduces the frequency, but not the amplitude, of mIPSCs, suggesting that Ih channels are located close to individual transmitter release sites and can influence transmitter release probability (Pr).
G. The M-Current
The M-current (IM) is a noninactivating voltage-gated K+ conductance comprised of the Kv7 (aka KCNQ) channels Kv7.2 and Kv7.3. IM is widely expressed in central neurons and plays a major role in regulating cell excitability, action potential firing, and accommodation as well as contributing to a medium-duration AHP in many cells. The voltage dependence of activation is such that IM is active close to Vm, and since it operates at subthreshold potentials, it is an important target for a number of neuromodulators in regulating cell excitability (223, 459). Although widely studied in hippocampal principal cells, only a handful of studies have investigated this current in inhibitory interneurons. Immunohistochemistry has shown Kv7 channel expression on the soma and dendritic compartments of PV-containing and SST-containing interneuron populations (222, 645). Although somewhat controversial, there is little evidence for axonal expression of Kv7 channels in interneurons.
IM has been best studied in CA1 SST-containing O-LM cells, which express Kv7.2 and Kv7.3 protein on their soma and dendrites, suggesting that native IM arises from their heteromeric assembly (645, 646) (FIGURE 11C). IM in O-LM cells is active at resting potentials close to −50 mV and is blocked by the antagonists linopirdine, TEA, and XE-991 and enhanced by the Kv7 channel opener retigabine (645) (FIGURE 11C). Using standard depolarizing test pulses, IM contributes ~15% of the total outward current activated at +40 mV. Blockade of IM increases spontaneous firing frequency by reducing the interspike interval without impacting action potential half-width. Enhancing IM activity with retigabine ceases spontaneous action potential firing (645). A current with similar properties has also been described in hippocampal fast spiking cells maintained in primary culture (434).
In the presence of mAChR agonists, SST-containing O-LM cells and CCKBCs exhibit an M1/M3-receptor dependent acceleration of action potential firing frequency concomitant with the emergence of a prominent afterdepolarization (ADP). Emergence of the ADP results from an inhibition of IM and the slow AHP, concomitant with activation of a nonselective cation conductance (182, 640, 645). A similar (but not identical) excitatory effect of muscarinic receptor activation via M1 receptors on PV-containing cells has also been observed (182, 1251). Although not formally tested, the change in excitability triggered by M1 receptor activation in PV-containing cells is consistent with a reduction in IM. Finally, BDNF strongly modulates the firing of PV-containing cells via an action on IM availability (837). Application of BDNF decreases the firing frequency and input resistance of PV-containing cells by a mechanism involving a TrkB-mediated increase of IM.
H. Acid Sensing Ion Channels
Members of the degenerin/epithelial Na+ channel superfamily show wide expression throughout central neurons, with the subfamily member of acid-sensing ion channels (ASICs) being the most widely studied (1181, 1195). ASIC channels are comprised of six family members (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4) encoded by four genes (Accn1–4). ASIC currents possess a steep pH dependence of activation, are Na+ selective, and are classically blocked by external Ca2+ and amiloride (1181). ASIC1a, unlike other family members, is permeable to Ca2+ ions (1195). In hippocampus, although ASICs are expressed in both principal cells and inhibitory interneurons, the latter have the larger current density (113, 205, 1196, 1277). Although the role of ASICs in inhibitory interneurons has received relatively little attention, they have been implicated in neurological disorders such as stroke, ischemic cell damage, and termination of electrographic seizure events. Two ASIC family members ASIC1a and ASIC2 are differentially expressed in hippocampal interneurons. SST-containing O-LM cells typically express transcripts for both ASIC1a and ASIC2 channels, whereas only ASIC1a is expressed in DG PVBCs (1196). In neocortex, ASIC4 expression is restricted to CR- and VIP-positive cells; however, its expression in hippocampus has not been reported (680).
Rapid acidification of the extracellular environment triggers activation of an inward ASIC channel-mediated current (at −60 mV) in O-LM and PVBCs (1196), as well as unidentified interneurons of s.r. and s.l.m. (113, 1102). In side-by-side recordings, ASIC currents were sixfold greater in O-LM cells (current density 0.75 pA/μm2) compared with PVBCs (0.12 pA/μm2, a density similar to that observed in principal cells). ASIC currents in O-LM cells possess markedly rapid recoveries from desensitization (0.96 s) compared with PVBC currents (42.8 s), suggesting that the latter may accumulate in a desensitized state more readily (1196). Unidentified cells types referred to as dentate gyrus “stuttering” and “accommodating” cells had current amplitudes intermediate to O-LM and PVBCs (1196). Psalmotoxin-1, a selective inhibitor of ASIC1a channels, inhibited currents in PVBCs, consistent with the expression of only ASIC1a mRNA transcripts in these cells. In contrast, ASIC-mediated currents in O-LM cells were largely resistant to psalmotoxin-1 consistent with their expression of both ASIC1a and ASIC2 transcripts (1196).
Although ASIC channels are predominantly expressed on the soma and dendrites of interneurons, there is evidence for presynaptic expression of ASICs (516). Block of presynaptic ASIC channels by an orthosteric noncompetitive antagonist 2-oxo-2H-chromene-3-carboxamidine (aka compound 5), triggers an increase in the frequency of spontaneous IPSCs onto principal cells, suggesting that presynaptically active ASICs on as yet unidentified interneurons normally act to dampen spontaneous inhibitory tone in the hippocampal circuit (516).
Classically ASIC channels are blocked by amiloride; however, native ASIC channels on s.r. and s.l.m. interneurons are also blocked by the mAChR M1 receptor agonist oxotremorine (282) and potentiated by monoamine compounds known to block N-methyl-d-aspartate (NMDA) receptor-gated channels (IEM-1921, IEM-2117, and memantine), albeit at concentrations ~10-fold higher than typically used to block NMDAR-gated currents (1102). In mouse models of electrographic seizures (kainate acid lesioning, PTZ, and reduced extracellular Mg2+), termination of the electrographic event depends on the activity of ASIC1a channels (1277). Given that seizures trigger an extracellular acidification, it is not unreasonable to assume that this acidification activates ASIC channels on both principal and inhibitory interneurons. Indeed, Zeimann et al. (1277) demonstrated that disruption of the Asic1a gene or pharmacological block of ASIC1 increased seizure severity. Acidification of the extracellular fluid to levels achieved during electrographic events triggers activation of ASIC1a channels on interneurons, increases interneuron cell firing, and promotes increased inhibitory tone onto principal cells (1277). However, selective block of ASIC1a on inhibitory neuron presynaptic terminals actually reduces electrographic seizure activity in low Mg2+ and kainate-induced models of seizures by a mechanism involving presynaptic ASIC channel modulation of inhibitory tone (1102), suggesting that interneuron presynaptic ASICs are not the molecular substrate responsible for terminating electrographic seizures.
I. TASK Channels
Twik-related acid-sensitive channels (TASK) are voltage-independent, potassium-selective channels that belong to the two-pore domain potassium channel family (76, 422). TASK-1 (KCNK3) and TASK-3 (KCNK) are the predominant subtypes found in the central nervous system and can exist as either homomers or heteromers (1083). Although both channel types are widely expressed in principal cells, higher levels of TASK3 and lower levels of TASK1 are typically expressed in hippocampal interneurons (1083, 1099). Single-cell RT-PCR and immunohistochemistry have demonstrated that TASK3 is expressed in ~50% of GAD67-positive interneurons. Of these, most (97%) PV-containing interneurons, as well as a subpopulation of SST-containing interneurons including O-LM cells, are heavily decorated by TASK3 immunoreactivity (1099, 1113). Pharmacological tools are sparse for TASK-related channels; however, they are typically activated by alkalinization and inhibited by acidification (76). Accordingly, acidification or alkalinization of the extracellular fluid impacts the input resistances and holding currents required to voltage-clamp at rest diverse populations of CA1 interneurons. Both of these effects were blocked by the anesthetic bupivacaine consistent with a role for TASK channels in establishing intrinsic resting parameters (1113). Moreover, activation of 5HT2A receptors of entorhinal cortex interneurons increases their excitability by a mechanism involving inhibition of TASK3 channels (269).
J. Na+/Ca2+ Exchanger
Very little is known concerning the identities of the diverse family of membrane transporters and pumps typically found on neurons throughout the central nervous system and which, if any, are present on specific inhibitory interneuron subpopulations. However, one landmark study highlighted an essential role for the Na+/Ca2+ exchanger in providing input specific dendritic compartmentalization of synaptic signaling in neocortical fast spiking interneurons (417). Most excitatory synapses are made onto spine-free, smooth dendrites of inhibitory interneurons (1035) (see sect. V). This arrangement suggests that unlike principal cells, inhibitory interneurons lack a mechanism to compartmentalize Ca2+ transients generated by single synapses. Goldberg and colleagues (417, 418) used imaging techniques to monitor single synaptic inputs arriving onto GluA2-lacking Ca2+-permeable AMPAR synapses of neocortical layer II/III and V fast spiking basket cells. Surprisingly, they observed that synaptic input generated Ca2+ transients that were remarkably localized despite the absence of physical structures to limit the Ca2+ spread (417). This “compartmentalization” of the synaptic signal resulted from the unique combination of both the kinetics of the underlying Ca2+-permeable AMPARs and a fast extrusion mechanism provided by a proximally located low-affinity, high-capacity Na+/Ca2+ exchanger. Indeed, blockade of the Na+/Ca2+ exchanger with benzamil increased the spatial spread of Ca2+ microdomains (417). Such an arrangement provides the molecular underpinnings of Ca2+ microdomains in interneuron subtypes.
K. Cluster Analysis of Physiological Parameters
As stated above, comparing results from different cell types obtained from different laboratories using different recording conditions is often problematic. Moreover, consideration of one or a few physiological feature (s) (e.g., resting potential or firing threshold) alone is usually not particularly informative in trying to determine the intrinsic properties or identity of any particular cell type. However, several recent studies have made recordings from large numbers of identified cell types using identical recording conditions (providing an internal control), thus allowing comparisons of basic membrane and active properties across interneuron subtypes derived from either both MGE and CGE (1138), only those derived from the CGE (796), cells restricted to a particular hippocampal subfield (499), or cortical NPY-expressing interneurons (549). Although briefly discussed here, readers are encouraged to seek out these original papers to truly digest the complexities and value of these approaches.
Using a polythetic classification scheme, Tricoire et al. (1138) compared electrophysiological, molecular, and lineage parameters of identified MGE- and CGE-derived hippocampal interneurons. Unsupervised cluster analyses (Wards and K-means) that included 20 features of intrinsic excitability and firing properties were performed (FIGURES 9 and 12 and TABLE 1). This data set, obtained from in excess of 140 cells, resulted in identification of six robust cell clusters. Clusters 1 and 2 were comprised solely of MGE-derived inhibitory neurons, whereas clusters 4, 5, and 6 comprised only cells of CGE origin (FIGURE 12). Cluster 1 comprised cells expressing PV- and SST-mRNA transcripts and possessed the fastest membrane time constants (13 ms) and steady-state firing frequencies (mean 70 Hz) (TABLE 1). Action potentials were brief (half-widths of 0.5 ms) followed by large-amplitude (25 mV) and short-duration AHPs (29 ms). Cells fire action potentials at high frequency with limited accommodation compared with all other cells in clusters 2 through 6 (1138). This cell cluster comprised PVBCs, BiCs, and AACs.
FIGURE 12.
Unsupervised cluster analyses of hippocampal GABAergic interneurons based on developmental, electrophysiological, and molecular properties. A: Ward’s clustering applied to a sample of 142 recorded MGE- and CGE-derived interneurons. In this dendrogram, the x-axis represents individual cells, and the y-axis represents the average Euclidean within-cluster linkage distance. B: histogram summarizing the frequency of occurrence of each of the 16 transcripts probed by single cell PCR within each cluster obtained with the K-means clustering (K 6). See A for cluster color code. [Data from Tricoire et al. (1138) with permission the Society for Neuroscience.]
Cells in cluster 2 possessed slower membrane time constants (46 ms) and firing frequencies (~20 Hz), typically exhibited significant voltage-dependent sag indicative of Ih, and expressed SST-mRNA transcripts. Cluster 3 was the only group to comprise cells derived from both the MGE and CGE that were characterized as expressing NPY-mRNA and possessed a late spiking firing phenotype consistent with Ivy and NGFC populations (TABLE 1). Action potential amplitudes are relatively small, duration is moderate and typically followed by a brief but large AHPs. Firing patterns are largely nonaccommodating and often accelerate as the depolarizing pulse proceeds (1137, 1138). The inability of the cluster analyses to separate NGFCs derived from either the MGE or CGE underscores the common physiological features despite being derived from disparate embryonic eminences (1137). Cells in clusters 4 and 5 had similar electrophysiological properties with high input resistances (430 and 401 MΩ, respectively) and slow membrane time constants (57 and 5 ms, respectively). Cells in cluster 4 were typically CGE-derived, dendrite targeting cells, such as SCA and associational collateral associated CCK-cells. Cells in cluster 5 possessed axons with little to no ramification within the hippocampus and were considered to represent long range projection or interneuron-targeting interneurons (5, 439). Finally, cells in cluster 6 had the highest incidence of CCK expression (typically in combination with VGluT3 and VIP) and anatomies indicative of CCKBCs (1031, 584) and mossy fiber associated cells (1138) with intrinsic features that were often intermediate to cells in all other clusters (TABLE 1).
Close inspection of these data begs consideration of whether these intrinsic physiological parameters are largely genetically determined or induced at their final settling positions within the hippocampus. The observation of no overlap of functional characteristics between MGE-versus CGE-derived cells within five of the six clusters suggests that the cell origin has high discriminative power in the cluster analyses (FIGURE 12). Similarly, some electrophysiological properties such as time constants and spike kinetic/frequency parameters possessed very strong discriminative power, and randomization of these parameters significantly degraded the clusters. This observation is most likely influenced by the unique physiological features that PV-containing cells of cluster 1 possess, which are among the earliest born MGE-derived interneurons. Cluster 1 cells have the lowest input resistance, fastest membrane time constants, and rapid action potential kinetics/frequency parameters. The remaining MGE-derived interneurons of clusters 2 and 3 have physiological features that diverge from the PV-cell population and suggest that spatiotemporal changes in the production of MGE-derived cells can have significant influence over their ultimate physiological properties. Fine-grained inspection of electrophysiological properties of clusters 2 through 6 (in absence of cluster 1) yields few features that discriminate between the cells of these clusters, suggesting that no obvious single genetic or transcriptional feature determines the overall physiological intrinsic properties of hippocampal interneurons.
Using a GAD67-eGFP mouse line, Hosp et al. (499) took a similar morphophysiological approach to determine whether interneurons of the hippocampal DG could be “broken” into functionally distinct groups or exist in a continuum. Cluster analysis based on morphometric variables of 114 sampled cells revealed 5 distinct classes of interneurons each with distinct morphophysiological features. Based on dendritic and axonal morphological criteria alone, this approach revealed clear separation of interneurons into five well-defined subcategories. Of these morphological features, the layer specific distribution of the axon together with its length and density in the transverse plane were critical determinant features. Like the studies described above, perisomatic targeting cells (PVBCs and presumed AACs) formed a major cluster (M1). However, with the exception of molecular layer cells (ML, corresponding to MOPP cells and NGFCs) analysis of electrophysiological parameters alone were insufficient to clearly delineate interneuron subtypes, confirming our hypothesis that there is a continuum for sampled electrophysiological parameters that are often insufficient to permit fine grain definition of individual interneuron subtypes. Combining both morphological and electrophysiological features revealed five cell clusters comprising PVBCs (BC, cluster), HICAPs, total molecular layer cells, HIPPs, and molecular layer cells. Comparison of these with the clusters identified in CA1 subfield by Tricoire et al. (1138) reveal some remarkable consistencies between cluster identities from both studies. Both the BC cluster (499) and cluster 1 of Tricoire et al. (1138) comprise almost exclusively PV-containing interneurons. Similarly, HIPP cells form a cluster equivalent to that of cluster 2 SST-containing cells in Tricoire et al. (1138). DG HIPP cells have previously been suggested to correspond to O-LM cells of the CA subfields (352). Similarly, cluster 3 of Tricoire et al. (1138) contains cells of the NGFC family as did the ML cluster of Hosp et al. (499). The HICAP cluster comprises CCK-containing interneurons similar to cluster 4 in the CA1 hippocampus. Taken together, these two independent studies using morphological and physiological features of randomly selected neurons yield cell cluster identities with remarkable consistency across different hippocampal subfields.
A similar study by Miyoshi et al. (796) probed the intrinsic properties of neocortical inhibitory neurons derived solely from the CGE. Using the 5HT3aR-eGFP mouse reporter line, they attempted to unravel the diversity of CGE-derived cell types and determine whether distinct subpopulations of cells could be grouped according to anatomical, transcriptional, and electrophysiological features. They observed that this divergent population of CGE-derived cells parsed into nine distinct subtypes based on morphology, cell layer positioning, firing patterns, and other intrinsic properties. Of these, the largest groups were either reelin-positive cells, characterized by cells with a late spiking phenotype or VIP-containing cells. The reelin cells comprised two subgroups based on cell size (small vs. large soma-dendritic-axonal arborizations) and position (primarily layer I/II vs. layers I/II/III), together with late spiking firing phenotypes in response to depolarizing stimuli. The majority of CGE-derived interneurons that did not express reelin expressed VIP. These cells were found primarily in layers II/III and fire strongly accommodating trains of brief duration action potentials. Interestingly, this study also revealed several other classes of interneurons that possessed unique anatomical and electrophysiological properties distinct from either reelin or VIP cells, underscoring that CGE-derived neocortical interneurons comprise a diverse and larger population of inhibitory interneurons than initially predicted. Close inspection of the electrophysiological properties of these cells, however, highlight few features that are distinct to one or few cell types, with only minor exceptions, and all nine identified cell types largely exhibit a continuum of intrinsic parameters. It is possible that spatiotemporal changes in the production of CGE-derived subtypes are less prominent than observed in MGE-derived interneurons, consistent with the observation that the ratio of reelin- to VIP-containing interneurons remains constant throughout development. However, two functionally distinct cell types argue against this simple notion: the “sigmoid intrinsic bursting” interneuron types (high input resistance, bursting firing pattern) are among the earliest CGE-derived interneurons, whereas the fast adapting interneurons [electrically small, high input resistance and fire spikes with short half widths (~1.0ms)] are preferentially produced at the peak of CGE-interneuron production (147, 796). It is unclear at this time whether shifts in the production of particular subtypes and their intrinsic properties represent temporal alterations in the progenitor populations or shifts in intrinsic cues within the cortex as development progresses (796).
Finally, Karagiannis et al. (549) also used unsupervised cluster analysis based on morphological properties, laminar positioning, and 32 electrophysiological features to interrogate the diversity of NPY-expressing interneurons in the rat barrel cortex. Their extensive data analysis from 200 cells suggested three main populations of NPY-expressing cells. 1) The first class are nNOS-expressing NGFCs with a dense axonal plexus and a late-spiking phenotype. This adapting NPY cluster exhibited the lowest firing frequencies of the cells tested. Action potentials were followed by biphasic AHPs comprising early and late components. This cell cluster had a remarkably diverse array of firing phenotypes perhaps suggestive of different cell types captured within the cluster that have some overlapping features. 2) The second population are SST-containing Martinotti-like cells with axons that ascend into layer 1 and represent the most excitable cell type. These cells exhibited a relatively depolarized Vm, were electrically more excitable than PVBCs, and exhibited pronounced sag during hyperpolarizing current steps indicative of Ih. These cells fired action potentials of durations intermediate between those observed in PVBCs and pyramidal cells and demonstrated slow accommodation. 3) PVBCs with low input resistance, short membrane time constant, and high rheobase comprised the third class of distinct NPY-containing interneurons. These cells also fired brief action potentials of small amplitude followed by sharp monophasic afterhyperpolarizations. Action potentials showed an acceleration of their firing rate when depolarized above threshold and could sustain high steady-state firing frequencies. Each of these cell types possessed distinct repertoires of mRNA transcripts that correlated well with functional physiological parameters.
L. Axonal and Presynaptic Release Properties
Although all inhibitory interneurons by definition release GABA as their primary neurotransmitter, the structural and functional properties of the axonal compartments of these cells are far from homogeneous. Neurotransmitter release is generally considered a “point to point” process i.e., liberation of vesicular neurotransmitter occurs from a well-defined presynaptic structure onto clusters of receptors housed in a postsynaptic specialization. Neurotransmitter release profiles are dictated by the combined properties of the axonal architecture, action potential duration and propagation, the biophysical properties of presynaptic voltage-gated Ca2+ channels, and the particular neurotransmitter release machinery used to liberate synaptic vesicles (1282). Consequently, neurotransmitter release profiles can vary considerably due to the highly divergent nature of these parameters across discrete cell types and synapses.
As described in section II, the axons of many interneurons can remain local to the subfield housing their soma and dendrites (e.g., IvCs and NGFCs), or possess axons that cross considerable distances to innervate distinct subcellular compartments (e.g., CCKBCs, O-LM, and backprojecting cells) or, alternatively, form long-range projections that extend beyond their original central location to ramify within both cortical and subcortical structures. At the anatomical level, the properties of many interneuron subtypes have been studied and the length and trajectories of these cells well documented (102). However, the functional properties of axons of only a small number of cells have been described in any great detail. However, it is becoming clear that the functional properties of axons that are often excessively lengthy, branch often, and taper to extremely small diameters require functional specializations that are not typical of principal excitatory cell axons. Of particular relevance for our discussion here are cells of the PV-containing, CCK-containing, and NGFC subpopulations. These three subfamilies have axonal and synaptic neurotransmitter release properties that differ considerably from each other and serve to exemplify synchronous, asynchronous, and a hybrid form of phasic/tonic neurotransmission mechanisms used by inhibitory interneurons (FIGURE 13). The increasing understanding of their release biophysics highlights the remarkable diversity that exists even at the level of inhibitory neurotransmission.
FIGURE 13.
The three modes of GABA release from PV-containing, CCK-containing and NGFC interneurons. A and B: superimposed IPSCs (black) evoked by single presynaptic action potentials (red) in a PV-containing interneuron-granule cell (GC) pair (A) and a CCK interneuron-granule cell pair (B). Insets: histograms of IPSC latency. A and B, bottom panels: superimposed IPSCs (black) evoked by trains of 10 action potentials (red) in a PV interneuron-granule cell pair (left) show a predominant synchronous mode of inhibitory output. In contrast, repetitive transmission in a CCK interneuron-granule cell pair (right) shows a large asynchronous component of transmission as the train proceeds. Green: average IPSCs. Insets: presynaptic action potentials aligned to the stimulus onset at expanded time scale (scale bars: 2 ms, 50 mV). C: GABAA IPSCs evoked by NGFCs (top right panel) are comparatively slower than those observed at FSBC synapses. Top: 10 consecutive IPSCs (gray) and their average (black) in a layer 2/3 PC after single action potentials in a layer 1 NGFC (top). Middle traces show equivalent IPSCs evoked by a layer 2 FSBC for comparison. Bottom right panel: comparison of the kinetics of postsynaptic responses evoked by NGFCs (black squares) and FSBCs (gray circles). Each point represents an individual connection. D: modulation of GABA responses evoked by NGFCs by GABA uptake. Fast IPSCs evoked from FSBC are not sensitive to the GABA uptake inhibitor NO711. In contrast, GABAA,slow IPSCs arising from NGFCs are markedly prolonged in the presence of NO711, demonstrating a differential sensitivity of the two modes of transmission to inhibition of GABA uptake. [A and B from Hefft and Jonas (477) with permission from Nature Neuroscience. C and D from Szabadics et al. (1074).]
1. PV-containing interneurons
The best studied of these three interneuron subtypes is the PV-containing interneuron subfamily (see sect. IIB). The axons of single PV interneurons extensively arborize to form en passant boutons (~10,000 in the CA1 hippocampus), which provide synchronous robust inhibitory control of their downstream targets (FIGURES 2C, 9A, and 13). The axons of PVBCs typically innervate the soma and proximal dendrites of principal cells, while those of AACs form “chandelier”-like strings that selectively innervate the axon initial segments of principal cell targets (352) (FIGURE 2, A–D). To enforce such strong inhibitory output onto downstream targets the PV axon has incorporated numerous functional specialties worthy of discussion (502). Action potential initiation and propagation in PV cells possess features distinct from those of excitatory pyramidal cells. The action potential initiation site is extremely close to the soma (within 2 0μm), and spike initiation of the AP is reliable and seldom fails. Action potentials are brief (~0.5 ms) (TABLE 1), and propagation speeds are extraordinarily fast with orthodromic speeds of ~0.25–1.5ms-1 being reported using direct axonal recordings (277, 503). In considering action potential propagation through this thin extensively diverging axonal compartment, it is hard to fathom that equivalent transmission could exist at the farthest reaches of this axon (~50 mm in CA1). However, a unique arrangement of voltage-gated Na+ channels facilitates conduction throughout this extensive compartment. In a remarkable series of patch-clamp recordings, Hu and Jonas (503) demonstrated that the dendrites and somas of PV cells possess a low density of Na+ channels (~1% of total). The Na+ channel density progressively increases (~100-fold) with distance along the axon (FIGURE 10B). At distal locations the Na+ conductance density was reported as ~600 pS/μm2. This “supercritical” density of Na+ channels likely ensures failsafe and rapid action potential propagation. The biophysical properties of these Na+ channels (primarily Nav1.1 and Nav1.6), coupled with the axonal expression of Kv3-containing voltage-gated K+ channels, ensures brief-duration action potentials that depolarize and repolarize rapidly, and importantly ensure rapid and efficient channel deinactivation such that a large fraction of channels are available for subsequent action potential initiation. These biophysical properties allow firing at high frequencies and rapid propagation through an increasingly small axonal compartment to ensure high fidelity of transmission to downstream targets (65, 503, 755).
On reaching presynaptic en passant boutons, the action potential wavefront activates rapidly activating Cav2.1 P/Q-type voltage-gated Ca2+ channels. Ca2+ entry through P/Q channels is tightly coupled to the exocytotic release machinery via formation of specialized “nanodomains” (296). The short physical distance between the site of Ca2+ entry and the synaptic release machinery enforces a tight coupling between action potential invasion and neurotransmitter release with rapid and precise temporal dynamics (132, 477). The synaptotagmin family member syt2, which possesses rapid Ca2+ binding kinetics (1232), is highly enriched in PV cell axons (576). The Ca2+ binding properties of syt2 likely serve to minimize the time required between Ca2+ entry and the liberation of neurotransmitter (502). The measured half duration of quantal release at these synapses is ~300 μs, and Pr is high (~0.6) (602). Thus, through a combination of anatomical and molecular specializations, action potential initiation, propagation, and presynaptic invasion are “tuned” to ensure rapid and reliable neurotransmission to downstream targets, consistent with a role for these cells in enforcing temporally precise feedforward/feedback-inhibitory control as well as the generation of coherent oscillations within cortical ensembles (FIGURES 13A and 20).
FIGURE 20.
Common motifs of inhibition within cortical and hippocampal circuits. A, left: feedforward (a) and feedback (b) inhibitory control of principal (PC) cell circuits are the most common circuits within hippocampus and cortex. In feedforward inhibitory circuits, a common afferent (PCI) makes monosynaptic connections to both PCII and inhibitory interneurons. The inhibitory interneuron then makes a monosynaptic inhibitory connection onto PCII. Thus the same principal cell (PCI) drives monosynaptic excitation and disynaptic inhibition onto a common PC (B and D). In feedback inhibitory circuits, the output of PCII makes a monosynaptic excitatory input onto interneurons which then return monosynaptic inhibition onto the same cell or population of cells. A, right: some interneuron subpopulations make only inhibitory connections with other interneurons. In this configuration afferent excitatory drive onto these types of interneurons drives disinhibition of principal cells by an inhibition of inhibitory input. B–D: a schematic representation of feedforward inhibitory control of the temporal window for excitation. Inset shows a hypothetical electrophysiological recording from PCII (inset). Monosynaptic excitation of the principal cells (red trace) results in a prolonged excitatory synaptic event that has a long temporal window in which to exceed threshold. The concomitant activation of the disynaptic inhibitory input (IPSP) summates with the EPSP and narrows the temporal window (black trace) for the EPSP to exceed threshold. C: under current-clamp conditions, stimulation of this feedforward inhibitory circuit triggers an early EPSP and a later IPSP, both of which are blocked by the AMPAR antagonist NBQX confirming that the EPSP-IPSP sequence is being driven by glutamatergic afferents. D: voltage traces for current-clamp recordings from CA1 PCs upon stimulation of two Schaffer collateral pathways under control (left) and in the presence of a GABAA receptor antagonist, illustrating that inhibitory input enforces a narrow temporal window for coincidence detection, which is lost in the absence of inhibitory control. [C and D from Pouille and Scanziani (922) with permission from Science.]
The brevity of action potentials combined with rapid Ca2+ entry that is tightly coupled to release machinery ensures that neurotransmission from PVBCs to their downstream targets will proceed with high efficiency. As a consequence of these molecular specializations, the small number of Ca2+ channels at each release site coupled to syt2-mediated neurotransmitter release favors fast temporally precise “synchronous” phasic transmitter release, thus making them rapid signaling devices capable of providing precisely timed inhibition onto their postsynaptic cells (64, 502) (FIGURE 13). Despite the specializations designed to ensure high fidelity across the axonal arborization, PVBCs can display distance-dependent scaling. In a recent study, Bartos and colleagues (1058) observed that single axons of DG PVBCs differentially inhibited close versus distant principal cell targets. Inhibitory strength declined (presumably due to lower contact numbers) and signal duration was increased with distance (likely due to changes in postsynaptic GABAA receptor subunit composition) between the presynaptic cell and the downstream targets. Of particular interest, this distance-scaling feature appears to facilitate the ability of PV-containing interneurons to synchronize large principal cell populations during gamma frequency oscillations (1058).
2. CCK-containing cells
CCK-containing cells show an altogether distinct mode of neurotransmission that favors looser coupling and an asynchronous mode of transmitter release compared with PV-containing cells (FIGURE 13B). In addition, CCK cells fire action potentials with longer durations (>0.5 ms), which lengthen upon repetitive activation during trains further contrasting with their PV cell counterparts (182). Although studies similar to those described above for PV-containing cells have not been made for CCK cells (or for any other interneuron subtype for that matter), their action potential conduction velocities are thought to be slower than those of PVBCs since evoked unitary IPSCs occur with longer delays than that seen at PVBC synapses (FIGURE 13, A and B) (477). CCK interneuron unitary IPSC amplitudes and latencies show large fluctuations (258, 477), due to weak coupling between their presynaptic N-type Ca2+ channels and release machinery (64, 477) (FIGURE 13B). An important feature of transmission at CCK-containing cells and their downstream targets is the transition from largely synchronous neurotransmitter release to asynchronous release during repetitive activation (21, 258, 477). Indeed, synchronicity ratios of transmission for CCK cells (the ratio between synchronous vs. asynchronous release during a train of 25 events at 50 Hz) were calculated to be between 1.1 (CCK-dendrite targeting cells) to 2.1 (CCKBCs), which are significantly lower values than those calculated for PVBCs (6.2) (258). Asynchronous release appears to be a general property of CCK-containing interneurons and correlates with the class of presynaptic CCK interneuron but is largely independent of postsynaptic target identity (258, 554). However, Ali and Todorova (21) observed that the synchronicity ratio for CCK cells residing at the s.l.m.-s.r. border was larger for postsynaptic interneurons than CA1 pyramidal cells, suggesting that postsynaptic targets may influence synchronicity of release for certain CCK cell subtypes. The precise mechanism underlying the transition from synchronous to asynchronous modes of transmitter release is presently unclear. However, asynchronous release at a number of synapses has been attributed to the buildup of residual Ca2+ during periods of high activity (706, 868). Elimination of dominant presynaptic Ca2+ sensor synaptotagmin isoforms (Syt2 at the Calyx of Held or Syt1 at DG cell synapses) (576, 1066) selectively eliminates synchronous release revealing an increase in asynchronous release, suggesting that separate Ca2+ sensors may underlie the two modes of release. At CCK cell synapses, asynchronous release increases with elevation of extracellular Ca2+ concentration (258). The difference in Ca2+ dependence between synchronous and asynchronous release observed at CCK synapses is consistent with the presence of two Ca2+ sensors at these presynaptic terminals (256, 258). Functionally one can imagine that an asynchronous mode of transmitter release will endow CCK cells with prolonged inhibitory control over their postsynaptic targets, a mechanism distinct from the temporal precision enforced by PVBCs. Indeed, in paired recordings, one striking feature of asynchronous release is the long temporal window of transmitter release that continues many 100s of milliseconds after termination of presynaptic firing (258, 477), underscoring that this form of inhibition is prolonged and temporally imprecise (FIGURE 13B).
3. NGFCs
The above examples highlight two well-established distinct modes of phasic synaptic signaling for discrete interneurons both with clearly identifiable synaptic structures consisting of anatomically well defined pre- and postsynaptic specializations, i.e., point-to-point transmission. Transmitter concentration profiles during such phasic forms of neurotransmission favor postsynaptic receptor activation and deactivation, with few if any roles for receptor desensitization. In contrast, tonic synaptic signaling results in the prolonged and persistent activation of receptors by ambient GABA in the extracellular space, which drives an equilibrium between desensitized and open states (870). Interestingly, NGFCs mediate a third form of GABAergic transmission intermediate between phasic and tonic signaling (165) (FIGURE 13C). This atypical form of neurotransmission arises from the unique axonal arborizations of NGFCs that emerges from the soma or dendrites to form a small dense plexus around the parent cell soma (FIGURES 3, 9I, and 13C). The axonal arborization has been calculated to approximate 140,000 μm in length, compared with ~46,000 μm for a typical PVBC. Importantly, the presynaptic bouton density of NGFCs is among the highest of all hippocampal interneurons with an average bouton density close to 42 per 100 μm of axon (interbouton separation 2.5 μm) (102). Indeed, the bouton density of a single NGFC matches the release site density of five or six 6 overlapping basket cell axons (858). One feature of NGFC axons separating them from all other inhibitory interneurons is that the vast majority of presynaptic boutons are spatially located at a larger than usual distance from their target dendrites and are often not in register with any clear postsynaptic structure (858, 870). In somatosensory cortex, the separation between NGFC boutons and their dendrite targets averages 2.7 μm (range 1.1–5.0 μm) (858). This arrangement has led to the hypothesis that NGFCs release GABA in a target independent, volume- or “cloud-like”- manner to generate a nonspecific form of inhibitory control. Action potentials in NGFC cells are of small amplitudes and moderate duration. Their firing patterns are largely nonaccommodating and often accelerate during depolarizing pulses. Little is known however about the functional properties of NGFC axons and the properties underlying their neurotransmitter release. Of particular interest, in contrast to the rapid and synchronous IPSCs produced by PVBCs, transmission from NGFCs generates long-lasting IPSCs resulting from prolonged GABA transients (550, 1074, 1086) that arise from the unusual synaptic architecture where bouton density is high but close apposition to specialized postsynaptic densities is low (550, 751, 858, 1074) (FIGURE 13C). This low and prolonged GABA transient favors postsynaptic receptor desensitization and results in use-dependent synaptic depression as receptors accumulate in desensitized states (550). Second, in contrast to other interneuron subtypes that precisely target distinct subcellular domains, inhibition mediated by NGFCs generally lacks target cell and synaptic specificity. Moreover, the cloud of GABA released from NGFCs is strongly influenced by GABA uptake mechanisms (FIGURE 13D) and acts on GABAA and GABAB receptors located on any nearby neuronal element, including the releasing cell itself, potentially producing suppression of neural activity in a widespread area dictated by their dense NGFC axonal arbor (870).
M. Gap Junctions
The observation that sensory stimuli could trigger synchronous inhibitory activity across large populations of cortical cells suggested that networks of certain interneuron subtypes were highly interconnected (220, 541, 1072). Moreover, the observations that specific interneuron subtypes are necessary for the generation of coherent cortical and hippocampal oscillations (333, 1200) and that interneuron subtypes fire at specific phases of the oscillatory cycle (586) suggest that synchronization among interneurons is critical for cortical circuit function (151). Although GABAergic interactions between interneurons have been implicated in the generation of synchronous activity (1190), the observation that many interneuron types were highly interconnected by connexin-36-containing electrical synapses (497, 1167) or gap junctions provided an important clue as to how large populations can be synchronized during oscillatory activity (220, 488). Within hippocampus and neocortex, dendrodendritic or dendrosomatic electrical synapses are a common feature of many interneurons (80, 370, 371, 376, 398, 1084). In contrast, electrical synapses are rarely seen between principal cells (901). Electrical synapses between interneurons are made typically only between cells of the same class or subtype. Almost all cardinal classes of interneuron (PV-containing, SST-containing, CB1R-positive, NGFCs) exhibit within class electrical synapses making extended and independent gap junction coupled networks. One interesting deviation from this canonical rule are NGFCs of layer 2/3 (but not layer 1) (206) of rat somatosensory cortex. Although these cells were highly interconnected via electrical synapses, they also made widespread electrical synapses with PVBCs, regular spiking nonpyramidal cells, AACs as well as many other unidentified interneuron cell types (1017). Estimates of connectivity suggest that >50% of cells make within class electrical synapses. PVBCs are connected via both chemical and electrical synapses. In contrast, SST-containing cells make few GABAergic synapses onto each other but are highly connected via electrical synapses. Of particular importance electrical synapses are essentially bidirectional and rapidly transmit voltage changes at speeds slightly faster than chemical synapses (970). Electrical synapses have unusual low pass filtering properties that provide essential clues to their function. The low pass coupling coefficient of connections is highly variable but is typically between 1 and 40% (with a mean coupling conductance of 1.6 nS) (220, 488). The low pass nature of the coupling severely truncates rapid events such as action potentials and favors slower depolarizations/ hyperpolarizations such as AHPs. Action potentials when propagated across gap junctions are severely truncated and manifest themselves as “spikelets” of a few millivolts that are capable of triggering rapid depolarizations that can facilitate activation of voltage-dependent conductances and trigger coordinated spiking across clusters of coupled interneurons. Such coupling is rapid and bidirectional facilitating coordinated inhibitory synaptic transmission onto downstream principal cells, as well as triggering synchronous oscillatory activity across neural networks (80, 375, 1128). Events such as the action potential AHP, which are faithfully transmitted across electrical synapses, serve to deinactivate intrinsic voltage-gated conductances across the many connected cells. This allows for simultaneous cell activation once the AHP relaxes, further enhancing synchronous activity (107, 397, 671).
N. Persistent Action Potential Firing
In addition to fast, regular, bursting, accommodating, delayed and stuttering action potential firing patterns, an altogether unique form of firing has been observed in a small number of cell types. This novel form of slow integration is triggered in response to prolonged action potential activity and is known as persistent or retroaxonal barrage firing (300, 606, 1000, 1001, 1069, 300). In IvC, NGFCs [called perforant path associated HTr5b-GFP cells of SR/SLM border in (1000)], and PVBCs of several cortical and hippocampal regions persistent firing is generated within the distal axon compartment, requires Ca2+ elevation and gap junction coupling, and persists for several minutes on cessation of the trigger. During persistent firing, cells fire at frequencies ranging from 20 to 130 Hz, depending on the particular region (1069). In PV-containing interneurons, steady-state persistent firing dominates at gamma frequencies close to 50 Hz (300). Although the mechanism underlying the generation of persistent firing is at present unidentified, it is not blocked by GABAA, GABAB, AMPA, or NMDA receptor antagonists (1000). In CA1 PPA cells, persistent firing is attenuated by blocking voltage-gated Ca2+ channels (1001). In contrast, in PV-containing interneurons of the DG, application of Cd2+ or inclusion of the intracellular Ca2+ chelator BAPTA in the recording pipette boosts the duration and number of action potentials during persistent firing (300), suggesting interneuron-type specific or brain region specific differences in the contributions of Ca2+ to persistent firing. In PV-containing cells, persistent firing depends on Ih activation (300). Induction is inhibited (at least in hippocampal NPY-expressing Ivy cells) by activation of μ-opioid peptide receptors (606), which act to either hyperpolarize the NGFC or inhibit the locally connected gap-junction-network between cells. Although axonal action potential firing is required to trigger persistent firing, somatic depolarization is not. In paired recordings, persistent firing was not restricted to the stimulated neuron; it could also be produced in the unstimulated cell, suggesting that activity can percolate through the network via electrical coupling. Consistent with this observation are the manifestation of action potential “spikelets,” indicative of activity being registered by the recorded neuron through gap junction coupling to other cells in the network undergoing persistent firing. Importantly, persistent firing, which likely provides a global “brake” on local excitability, occurs in NGFCs both in vitro and in vivo, although in vivo it occurs less frequently and requires more prolonged barrages of action potential activity for initiation in neocortical NGFCs (1069).
V. GLUTAMATE RECEPTORS
Inhibitory interneurons are embedded within complex neuronal networks of interconnected principal glutamatergic cells and GABAergic interneurons and thus participate in almost all forms of network activity. The principal form of excitation onto interneurons arises via activation of ionotropic and metabotropic glutamate receptors by glutamate, the primary neurotransmitter within the central nervous system. Despite having many common features with glutamate receptor containing synapses on principal cells, a number of notable differences and exceptions exist making them worthy of discussion here.
Ionotropic glutamate receptors are divided into three main subtypes: AMPARs, NMDAR, and kainate (KA) receptors. Historically these receptors have been defined based on agonist and antagonist pharmacological properties (1133). Each of these three receptor subtypes is composed of distinct subunits whose combinations confer unique biophysical properties to the native receptor. For more extensive discussion on glutamate receptors and specifically those found on interneurons, interested readers are pointed to recent reviews (11, 1133).
A. AMPA Receptors
Throughout the central nervous system, the vast majority of fast synaptic neurotransmission arises via AMPARs (1133). Native AMPARs are typically homomeric or heteromeric assemblies of the four subunits, GluA1–4 (572, 822, 955). Of these subunits, incorporation of GluA2 is key to the overall biophysical properties of the receptor. The M2 reentrant transmembrane loop of the AMPAR subunit forms the lining of the channel pore, and amino acids in the so-called “QRN” site determine the ion selectivity of the channel (1133). Unlike GluA1, GluA3 and GluA4 subunits which contain an unedited glutamine (Q) most GluA2 subunits contain a fully edited arginine (R) at this site which has important consequences for single channel conductance, Ca2+-permeability, and channel block by polyamines (1133).
Although most mature AMPAR on principal neurons are typically comprised of GluA1/2 or 2/3 heteromers, which are Ca2+-impermeable (CI) and possess nearly linear current-voltage relationships, early evidence indicated that many interneurons expressed AMPARs that differed markedly in these properties from their principal neuron counterparts (517, 874). In situ hybridization and RT-PCR coupled with electrophysiological approaches subsequently revealed the presence of both inwardly rectifying, GluA2-lacking Ca2+-permeable (CP) AMPARs and GluA2-containing CI-AMPARs on inhibitory interneurons throughout the cortex and hippocampal formation (11, 51, 107, 388, 539, 769, 960). Until recently, little attention was paid to what interneuron type (with the exception of noting which hippocampal or cortical subfield the cell body resided in) expressed a particular AMPAR subtype. Moreover, both CP- and CI-impermeable AMPARs had been observed in recordings from single CA3 stratum lucidum (s.l.) interneurons, which targeted each receptor subtype to postsynaptic sites innervated by distinct afferent projections (1119). Mossy fiber axons of DG granule cells innervated GluA2-lacking CP-AMPAR dominated synapses, whereas inputs from associational fibers of CA3 pyramidal cells onto the same cell innervated GluA2-containing, CI-AMPAR dominated synapses. The mechanisms whereby single interneurons can manufacture these distinct AMPARs and target them to specific dendritic domains remain poorly understood, but such selective targeting has been observed at numerous cells throughout the mammalian central nervous system (for review, see Ref. 1120).
Early quantitative electron microscopy studies indicated that asymmetric synapses onto interneuron dendrites were enriched for GluA subunits, with little evidence for the “silent” synapses observed on principal cell dendrites that lacked AMPAR subunits and appear critical for synapse maturation (846). Quantitative immunogold electron microscopy of the AMPAR subunits expressed at Schaffer collateral inputs onto CA1 s.r. interneurons revealed no differences in GluA1, GluA2, or GluA3 subunit expression between synapses onto PV-containing interneurons versus “non-PV” synapses (1242). However, GluA4 expression was significantly higher at PV synapses than non-PV synapses. It is unclear exactly what population (s) are captured by the “non-PV” cell data set, which likely comprised both MGE-derived (e.g., SST- and nNOS/NPY-containing) and CGE-derived (e.g., CCK-, NPY, VIP-containing) interneuron types. Subsequent analysis of mRNA transcripts using fluorescent in situ hybridization revealed a high abundance of GluA1 and GluA3 in PV cells, equivalent to expression levels on principal cells. However, GluA4 was significantly higher and GluA2 significantly lower at PV cell synapses than observed at principal cell synapses. In contrast, both CCK-containing and NOS/NPY-containing interneurons showed comparatively low but consistent levels of all four GluA transcripts compared with principal cell synapses (1242).
The expression profile of GluA4 is particularly interesting since it imparts novel kinetic properties to the AMPAR. Unlike mature hippocampal and cortical principal cells, which do not typically express GluA4, PV-containing interneurons possess a high level of GluA4 and a lower level of GluA2 subunit expression (388, 630, 886, 1242). However, immature synapses onto PV-containing interneurons lack GluA4 and are comprised of GluA1 homomers (886). GluA4 expression increases over the first two postnatal weeks to generate receptors comprised of GluA1/GluA4 heteromers by adulthood (99, 388, 800, 886). These GluA1/4 containing AMPARs generate synaptic currents that possess unusually rapid kinetics, have high Ca2+ permeability, and show strong inward rectification due to block by intracellular polyamines (644). This receptor arrangement is critical for circuit recruitment of PV-containing interneurons to enforce temporal control over principal cells for efficient generation of large scale oscillatory activity (for review, see Refs. 67, 502). GluA1 or GluA4 loss-of-function experiments show compromised gamma oscillations and impairments in hippocampal-dependent working memory (364). Similarly, overexpression of the GluA2 subunit slows the AMPAR kinetics and disrupts long range synchrony underscoring the importance of synaptic AMPARs with rapid kinetics in these cells (365).
Using a combination of genetic and electrophysiological approaches, Matta et al. (763) provided a comprehensive picture of AMPAR expression profiles at Schaffer collateral synapses onto CA1 interneuron populations based on their embryonic origins within either the MGE or CGE throughout development (FIGURE 14). Schaffer collateral synapses onto MGE-derived interneurons (e.g., PV-, SST-, and nNOS-containing interneurons) typically express CP-AMPARs, whereas CGE-derived interneurons (CR, VIP, CCK, reelin, and some nNOS interneurons) mostly express CI-AMPARs. These origin specific AMPAR profiles are consistent across a broad developmental age range and importantly demonstrate that, at least at SC synapses onto CA1 subfield inhibitory interneurons, GluA2 expression is not developmentally regulated as seen at some principal cell synapses (492). Whether this MGE- versus CGE-origin specific AMPAR expression exists at other synapses remains to be demonstrated, but suggests that embryonic origin provides a strong predictor of glutamate receptor expression.
FIGURE 14.
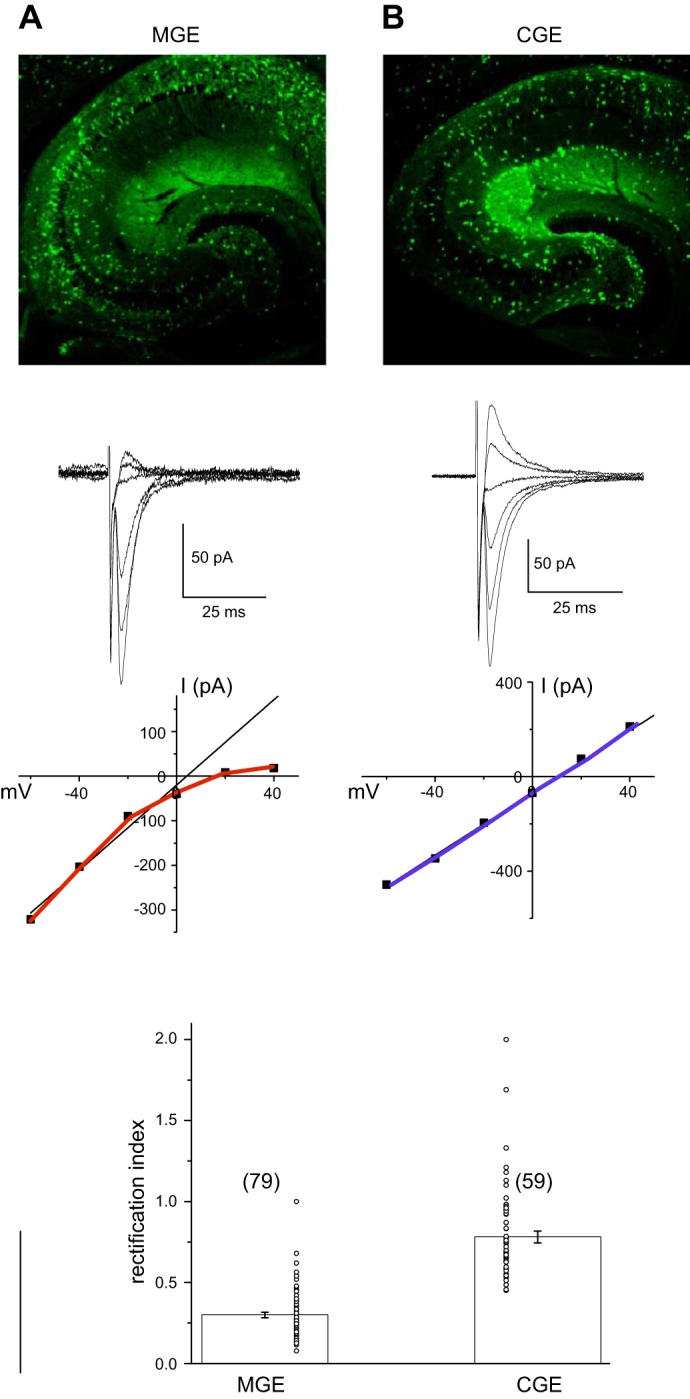
MGE and CGE-dependent expression of synaptic AMPAR-preferring glutamate receptors. A and B, top panels: interneurons that were targeted for recording using hippocampal slices derived from Nkx2–1-cre:RCE GFP and Htr3a-GFP reporter mouse lines, respectively (Scale bars, 100 μm). Middle panels: representative current-voltage relationships of Schaffer collateral-evoked AMPAR-mediated EPSCs in MGE- vs. CGE-derived CA1 interneurons. MGE-derived interneurons typically possess GluA2-lacking CP-AMPARs that possess strong inward rectification (left bottom panels). CGE-derived interneurons typically expressed GluA2-containing CI-AMPARs which possess near linear rectification properties. Individual dots in bottom panels represent data from a single recording; numbers in parentheses represent the number of cells recorded. [Data from Matta et al. (763).]
B. AMPAR Auxiliary/Interacting Proteins; TARPs
Most if not all AMPAR subunits exist in a complex with a number of channel auxiliary subunits that bind to a variety of targets on the pore forming subunit to modify receptor expression and recycling, as well as their biophysical and plastic properties (1243). Of these accessory proteins, most AMPARs complex with transmembrane AMPA regulatory proteins (TARPs) (1107). The TARP family comprises six isoforms, which differentially modify almost every function of native AMPARs (1133). In hippocampal principal cells, the vast majority of AMPARs interact with the TARP type 1 γ2 and γ8 isoforms, which alter surface expression, synaptic targeting kinetics, and plasticity (524, 525). Surprisingly, very little is known about the role of TARPs in regulating AMPAR function in inhibitory interneurons. A recent study combining FISH and immunohistochemistry and quantitative electron microscopy illustrated that TARPs γ2 and γ3 are enriched in PV-containing interneurons compared with CCK- and nNOS/NPY-containing interneurons. In contrast, only low expression of γ8 TARP was observed across all interneuron subtypes tested (1242). In γ2 knockout mice, AMPAR density was markedly reduced at excitatory synapses onto PV-containing interneurons but surprisingly was unaffected at synapses onto the cell cohort labeled as “non-PV,” suggesting a TARP functional redundancy at the latter synapses. In contrast, AMPAR number was unchanged at synapses onto both PV- and non-PV cell groups in both γ3 and γ8 knockouts (1242). These data suggest a prominent role for the γ2 in AMPAR expression at excitatory synapses onto PV-containing neurons. In contrast, it is unclear what role if any TARPs are having at excitatory synapses onto other non-PV interneuron subtypes.
C. AMPAR Auxiliary/Interacting Proteins; Neuronal Pentraxins
The neuronal pentraxin family (NPTX1, NPTX2, and NPTXR) is a family of Ca2+-dependent lectins that are highly enriched at excitatory synapses throughout the adult brain (1140). All three NPTXs form disulfide-linked assemblies that bind to the NH2-terminal domain of AMPARs and promote receptor clustering (1008, 1229). Of these, NPTX1 and NPTX2 (or Narp) are secreted proteins, whereas NPTXR possesses a transmembrane domain and can function to anchor NPTX complexes to plasma membranes. NPTX2 is an immediate early gene highly enriched at excitatory synapses onto PV-containing interneurons and promotes activity dependent accumulation of GluA4-containing AMPARs (189). This regulation by NPTX2 critically dictates PV-containing interneuron recruitment to maintain E/I balance in the face of perturbations in network activity (189). However, studies of NPTX2 in regulating excitatory drive onto PV-containing cells revealed only a homeostatic role for NPTX2 and required mice to be pretreated with stimuli that induce NPTX2 expression. In a subsequent study, the developmental upregulation of GluA4 in PV-containing interneurons was observed to require the coordinated expression of both NPTX2 and NPTXR (886). Loss of function of both pentraxins prevents the developmental incorporation of GluA4, resulting in small amplitude and slower AMPAR mediated EPSCs, which disrupted both downstream feedforward inhibitory drive and circuit maturation with a prolonged critical period for giant depolarizing potentials (GDPs) and perturbations in gamma and sharp wave oscillations in vivo, together with a lower threshold for electrographic seizure activity (886).
D. AMPAR Auxiliary/Interacting Proteins; SAP97
The membrane-associated guanylate kinase, MAGUK, family member synapse associated protein 97 (SAP97) is typically associated with components of the postsynaptic density at glutamatergic synapses onto principal cells where its interaction with GluA1 (666) and NMDARs (233) regulates both presynaptic activity and postsynaptic receptor expression levels (937, 968). SAP97 is developmentally expressed in cortical PV- and SST-containing interneurons, with virtually all cells expressing SAP97 in early postnatal periods (P15) followed by a decline to ~40% of cells in adulthood (12). SAP97-positive PV cells in visual cortex receive a twofold higher frequency of mEPSCs with more rapid kinetics than SAP97-negative PV-containing interneurons (12). Overexpression of exogenous SAP97 increases the frequency of fast mEPSCs, which is correlated with dendritic complexity and altered passive and active membrane properties (12). Although the precise mechanism is at present unclear, SAP97 regulates both synaptic input and intrinsic excitability of cortical interneurons. Given the role for SAP97 in regulating GluA1 expression, it is highly likely that early in development excitatory synapses onto PV-interneurons are comprised of GluA1 homomers that require SAP97 for their correct expression. As development progresses, the role of SAP97 may be minimized as its expression diminishes and increased expression of the neuronal pentraxins NPTX2/NPTXR coordinates the incorporation of GluA4 into GluA1/GluA4 heteromeric receptors observed at more mature synapses (886).
E. AMPAR Auxiliary/Interacting Proteins; Erb4/Neuregulin
The receptor tyrosine kinase Erb4 binds members of the NRG and epidermal growth factor families to regulate a variety of cellular processes including migration, proliferation, synaptic transmission, and plasticity (142). In hippocampus, Erb4 primarily localizes to the postsynaptic density of glutamatergic synapses contacting dendrites of CA1 s.o. and s.r inhibitory interneurons. (319, 1104, 1180). Erb4 expression is highest in PVBCs and AACs while expression is observed only in a small fraction of CR- and SST-containing interneurons (319). Erb4 colocalizes with PSD95 and interacts with GluA4 (886); however, it is unclear what instructive role, if any, Erb4 has for AMPAR expression. Erb4 knockouts show a marked reduction in miniature EPSC frequency (but not amplitude), suggesting a role for Erb4 in controlling glutamatergic synapse formation on interneurons (319) but not necessarily a role in AMPAR trafficking or function. Similarly NRG1, which binds to Erb4, plays a role in stimulating formation of new glutamatergic synapses as well as strengthening existing synapses onto interneurons by a mechanism thought to involve PSD95 stabilization (1104).
F. N-Methyl-d-Aspartate Receptors
The NMDAR family represents the second major ionotropic glutamate receptor type found at virtually all central synapses and is involved in fast synaptic transmission. Unlike AMPARs, they are typically activated and deactivated with slower kinetics and consequently are involved in a second prolonged component of synaptic transmission. Their unique kinetic properties, coupled to a strong voltage-dependent block by extracellular Mg2+ at negative membrane potentials, have placed them center stage as coincidence detectors involved in many forms of synaptic plasticity at excitatory principal cell synapses (1133). Similar to AMPARs, NMDARs are heteromeric assemblies formed from five principal subunits, GluN1, GluN2A, GluN2B, GluN2C, and GluN2D. GluN1 is a core component of all native NMDARs and is an absolute requirement for channel function. Heterotetramers formed between GluN1 and differing combinations of the four GluN2 subunits provide a myriad of channel types each with distinct biophysical properties, pharmacology and expression profiles (1133). NMDARs are unique among glutamate receptors in that they require the simultaneous binding of two agonists to activate the native receptors; glycine (or d-serine) to GluN1 and glutamate to GluN2 (587). Native receptor subunits are also subject to many forms of alternative splicing and posttranslational modifications making for a dizzying array of receptor possibilities expressed at central neuron synapses (interested readers are directed to the comprehensive review in Ref. 1133).
Like AMPARs, NMDAR subunit expression and receptor composition varies between interneuron subtypes (261, 661, 763, 1048, 1177) and in many cases directly correlates with the type of AMPARs expressed at the synapse (11, 661, 769). Early studies of NMDAR function in interneurons recognized a diversity of kinetic profiles and relative contributions of NMDARs at synapses onto specific interneuron types (769, 799). However, as for AMPARs, very little attempt was made to correlate this with particular interneuron subtypes until more recently. In the hippocampal CA1 subfield, NMDARs at Schaffer collateral synapses onto MGE-derived interneurons express GluN2B-containing receptors early in development (763). These receptors possess slow kinetics and are blocked by the GluN2B preferring antagonist ifenprodil. Of particular interest GluN2B-containing receptors are coexpressed with GluA2-lacking CP-AMPARs (FIGURE 15). The AMPA:NMDA amplitude ratio at these synapses is high (~5), suggesting that NMDARs make only a relatively small current contribution at these synapses (FIGURE 15A). Indeed, of all interneurons studied, MGE-derived PV-containing interneurons have the smallest observable NMDAR conductance (763). Later in development synaptic GluN2B subunits are replaced by GluN2A subunits as indicated by a speeding up of receptor kinetics together with a loss of ifenprodil sensitivity (763) (FIGURE 15B). This developmental subunit switch is also triggered by high-frequency synaptic stimulation and requires a rise in intracellular Ca2+ through CP-AMPARs, but intriguingly not through NMDARs themselves or by activation of mGluRs, both of which are required for a similar subunit switch in pyramidal cell synapses (762, 763). In contrast, Schaffer collateral synapses onto almost all CGE-derived interneuron subtypes express GluN2B-containing NMDARs across all developmental stages tested that persist through adulthood and do not exhibit any plastic subunit switch following high frequency stimulation (763) (FIGURE 15, A and B). The one exception to this rule so far identified is CGE-derived CCK-containing SCA interneurons, which also demonstrate the GluN2B-GluN2A switch (763). At CGE cell synapses GluN2B-containing receptors associate with GluA2-containing, CI-AMPARs. The AMPA:NMDA amplitude ratio at these synapses is close to unity, indicating a greater role for NMDARs at synapses onto CGE-derived interneurons (763).
FIGURE 15.
MGE and CGE-dependent expression of synaptic NMDAR-preferring glutamate receptors and the cell type developmental expression of synaptic GluN2 receptors. A, left: MGE-derived interneurons typically possess small NMDAR-mediated EPSCs that have rapid kinetics. Schaffer collateral evoked synaptic traces show AMPAR-mediated inward currents and NMDAR-mediated outward currents (Vh = −70 and +40 mV, respectively). A, middle: in contrast, CGE-derived interneurons typically possess large and kinetically slow evoked NMDAR-mediated currents. Right panel: AMPA/NMDAR amplitude ratios to be ~0.25 for MGE-derived interneurons and close to unity for CGE-derived interneurons. B: synaptic NMDARs at Schaffer collateral synapses onto CA1 MGE-derived interneurons undergo a developmental switch in NMDAR subunit expression. Left column: MGE-derived interneurons transition from GluN2B containing NMDARs to GluN2A-containing in juvenile receptors as evidenced by a loss of ifenprodil sensitivity and decrease in the time constant of decay. Middle panels: in contrast, CGE-derived interneurons possess GluN2B-containing receptors that persist through both neonate and juvenile states. Right panels, top: summary plot for the NMDAR EPSC decay kinetic weighted time constant (τw) for both neonate and juvenile MGE- and CGE-derived identified interneurons. Bottom panels: summary graph of the developmental regulation of ifenprodil sensitivity expressed as the ratio of the NMDAR EPSC peak amplitude measured in the presence of ifenprodil divided by the control NMDA EPSC peak amplitude. [Data taken from Matta et al. (763).]
The expression of GluN2C and GluN2D subunits on interneurons is less clear. Recent evidence from in situ hybridization studies shows GluN2D expression in cortical and hippocampal PV-, SST-, CB- and CR-positive interneurons as well as in VIP-positive irregular spiking interneurons (799, 920, 1177). Indeed, functional GluN2D-containing NMDARs are found at synapses onto a number of hippocampal interneurons (898, 1177). Early in development, blocking NMDAR activity by ifenprodil increases the decay of synaptic currents in wild-type mice, consistent with the uncovering of NMDARs containing the GluN2D subunit, a phenomenon absent in GluN2D loss of function mice (1177). Expression of slow gating GluN2B and GluN2D dominated NMDARs early in development likely provides a wider temporal window for synaptic integration, potentially important for network maturation, whereas GluN2A expression at later ages enhances the precision of synaptic responses.
Synaptically driven Ca2+ entry into cells is undoubtedly a tightly controlled feature, and the above discussion highlights a major divergence in glutamate receptor-mediated calcium influx between interneurons with distinct origins. In MGE-derived interneurons, the expression of GluA2-lacking CP-AMPARs together with a low NMDAR conductance suggests that the primary route of Ca2+ entry is through AMPARs, with a smaller contribution of GluN2B or GluN2D NMDARs. In contrast, at synapses onto CGE-derived interneurons, the primary route of Ca2+ entry is likely through GluN2B-containing NMDARs. The consequences of these differential routes for Ca2+ entry during phasic transmission are at present unclear but may provide clues to the types of plasticity and second messenger mechanisms that are triggered in MGE- versus CGE-derived interneurons (see sect. VI) (214).
Important physiological roles for interneuron NMDARs have been described at cellular (573, 574, 661, 763), network (599, 574), and behavioral levels (82). In MGE-derived hippocampal interneurons of young mice, GluN2B-containing NMDARs play an important role in modulating excitation-spike coupling. The slower kinetics of GluN2B-containing NMDARs influence the synaptic integration properties to regulate both the summation and timing of action-potential generation (763). Similarly at mossy fiber synapses onto CI-AMPAR interneuron synapses, which exhibit high NMDA/AMPAR ratios, a train of stimuli triggers multiple action potentials at each stimulus followed by a large late NMDAR-dependent depolarizing envelope that persists long after the stimulus. In contrast, in synapses with low NMDA/AMPAR ratios, EPSPs trigger only single action potential firing with no substantial after depolarizing phase (661).
Integration of developing interneurons into the nascent circuit critically depends on NMDAR activity. Genetic deletion of GluN2B in hippocampal interneurons leads to a reduction in the frequency of AMPAR-mediated mEPSCs observed onto CA1 s.o. interneurons (574). This decreased excitatory drive promotes hippocampal seizures and subsequent lethality (574). In contrast, elimination of GluN1 from CGE-derived CA1 s.l.m. NGFCs arrests maturation of both pre- and postsynaptic elements such that a higher frequency and amplitude of EPSCs is observed, coupled to an exuberance of cell morphology (Chittajallu and McBain, unpublished observation). Selective ablation of NMDARs later in development in PV-positive interneurons alters theta and gamma oscillations with consequent impairments in spatial and short- and long-term memory tests, indicating a critical role for NMDARs in PV-interneuron-mediated circuit entrainment (82, 599). Loss of NMDARs in PV-containing interneurons at early postnatal ages triggers behavioral deficits associated with schizophrenia including psychomotor agitation, anhedonia, reduced pre-pulse inhibition of acoustic startle, deficits in nesting/mating, and social withdrawal (82), supporting an NMDAR hypofunction theory of schizophrenia (for reviews, see Refs. 234, 235). However, given that PV-containing interneurons possess the smallest NMDAR currents (763), it is hard to reconcile the hypothesis that PV interneuron synaptic NMDAR hypofunction is an underpinning for schizophrenia (825), suggesting that alternative roles for NMDARs other than conventional synaptic transmission must exist in these cells early in development. Indeed, tonic extrasynaptic NMDAR-mediated currents have been identified on PV-containing interneurons of the prefrontal cortex (925) and CA1 s.r. (946), suggesting a novel role for extrasynaptic NMDARs on interneurons in addition to their role in synaptic transmission.
As described in section III, cortical interneurons originate in the ventral telencephalon and migrate tangentially into the neocortex and hippocampus (747). A number of in vitro (78) and in vivo (117, 736) studies have proposed a role for NMDARs in interneuron migration based on changes in cell migration following pharmacological antagonism of NMDA receptors (117). However, the density of PV-positive interneurons in somatosensory cortex of transgenic mice with selective deletion of GluN2B from GAD67-positive interneurons was comparable to wild-type mice (574). In contrast, similar genetic manipulation resulted in increased cell death of adult born olfactory bulb granule cells (573), indicating that NMDARs play different roles in cell survival across different brain regions or developmental stages. Elimination of GluN1 and GluN2B, but not GluN2A, in cortical CGE derived interneurons produces cell type specific (reelin-positive, but not VIP-containing cells) stunting of dendritic and axonal morphology (261). In contrast, elimination of GluN1 in CA1 s.l.m. NGFCs results in cells with more complex dendritic arbors, with dendrites shifting from a stellate form to one polarized towards the termination zones of afferents from the entorhinal cortex and thalamic nucleus reuniens (Chittajallu and McBain, unpublished observation).
G. Kainate Receptors
Kainate receptors (KRs) are homo- or heteromeric tetramers assembled from GluK1, GluK2, GluK3, GluK4, and GluK5 (1133). Although all subunits are widely expressed throughout the central nervous system, interneurons predominantly express GluK1 and GluK2 (with lower levels of GluK3) at either pre- and postynaptic sites (143, 332; for review, see Refs. 11, 171).
KR-mediated currents through either homomeric or heteromeric GluK1 or GluK2-containing KRs have been observed in numerous interneuron subtypes (230, 332, 348, 867, 883, 920). Functional mapping of KRs using uncaging methods revealed a continuous density of KRs across the dendrites of CA1 s.o. O-LM and “trilaminar” (sic) interneurons (1245). Of interest, BiCs have KRs on all dendrites residing in the s.o. but not on those that extended into s.r., while other interneurons in s.o. had “hotspots” or were completely devoid of KRs.
In CA1 s.r. interneurons, exogenous kainate evokes inward currents in both GluK1 or GluK2 knockouts, suggesting functional receptor subunit redundancy (816). In contrast, KR-mediated inward currents in CA3 s.r. interneurons are absent in GluK2 loss-of-function mice but not GluK1 knockouts, consistent with a role for GluK2 containing KRs on specific CA3 interneuron populations (332). KR activation by exogenous kainate robustly increases interneuron excitability, triggers action potential firing, and boosts spontaneous inhibitory drive onto pyramidal cells (230, 348). Despite this evidence for functional KRs on various interneurons, only a few studies have identified KR-mediated synaptic currents on interneurons. In principal cells KR mediated EPSCs tend to be kinetically slow, are activated by repetitive high frequency stimulation, and manifest as slow tail currents typically following the AMPAR mediated component at negative holding potentials. Similarly, evoked or spontaneous KR-mediated synaptic events onto inhibitory interneurons often appear as small slow tail currents in the EPSC waveform (229, 230, 348, 349, 1215). However, Cossart and colleagues (229, 420) observed spontaneous KR-mediated synaptic events with slow kinetics (decay tau’s ~10 ms) onto CA1 s.o. interneurons, primarily O-LM cells, that occurred in the absence of a detectable AMPAR component. These studies were the first to suggest that KR synaptic events may exist in isolation from synapses containing AMPARs, and importantly these events comprise a significant fraction of the total spontaneous EPSC population (~30%). Similar KR-mediated synaptic events were observed in s.r. and s.l.m. interneurons (1215). Goldin et al. (420) suggested that KRs, but not AMPARs, on SST-positive O-LM cells entrain spiking at theta frequencies, suggesting an important role in oscillatory activity. Indeed, GluK1 antagonists reduce the frequency of hippocampal theta oscillations in vivo (515). However, Oren et al. (867) found no evidence for spontaneous KR-mediated events in recordings from the same cell population and concluded that the vast majority of spontaneous EPSCs in s.o. O-LM cells were AMPAR mediated (1215). Indeed, evoked GluK1-containing KR synaptic events make only a modest contribution (~10% of the total current) when synaptic transmission is driven by repetitive activity (867).
KRs are also expressed on axonal presynaptic terminals of certain interneuron types, where they serve to regulate neurotransmitter release. In hippocampus, exogenous kainate reduces the amplitude and Pr of evoked IPSCs onto CA1 pyramidal cells (208, 231, 734, 951) (however cf. with Refs. 231, 332). Lerma et al. (952) have suggested that this occurs via a metabotropic KR mechanism, although little corroboration of this hypothesis exists. In paired electrophysiological recordings, GluK1 containing KR activation decreases presynaptic release from CCK-containing but not PV-containing interneurons that is observed at release sites onto pyramidal but not interneuron targets (256). As discussed in section IVK, CCK-containing interneurons release transmitter via a combination of synchronous and asynchronous modes. Activation of presynaptic KRs preferentially reduces synchronous, but spares asynchronous transmitter release (256). Such a mechanism may act as a switch to move between phasic modes of transmitter to prolonged inhibition when glutamate levels are elevated during periods of intense activity. Presynaptic modulation by KRs has also been linked to the endocannabinoid (eCB) system (701, 793). High-frequency stimulation of CA1 Schaffer collateral afferents transiently depresses inhibitory transmission onto pyramidal cells via a mechanism involving glutamate spillover activating presynaptic GluK1-containing receptors on CB1R-positive interneuron presynaptic terminals (701). This spillover mechanism requires the concerted activation of the eCB system, presynaptic CB1Rs and GluK1-containing KRs (701).
H. Kainate Receptor Auxiliary/Interacting Proteins; Neuropilin- and Tolloid-like Proteins
Neuropilin- and tolloid-like proteins (Neto1/Neto2) are auxiliary KR subunits capable of regulating almost every parameter of receptor function. In overexpression studies in heterologous cells, Netos regulate KR desensitization and deactivation kinetics, channel open probability, ligand affinity, and subcellular localization (225, 226, 1057, 1243). At dentate gyrus mossy fiber axons to CA3 pyramidal cell synapses, Neto1 regulates binding affinity, kinetics, and synaptic targeting of native GluK2-containing postsynaptic KRs (1056, 1094, 1224). Direct evidence for Neto2-mediated regulation of endogenous KR function remains lacking despite association with native KR complexes. Neto1 and Neto2 colocalize with GluK subunits (1/2/5) in SST-, CCK-, and PV-containing interneurons (Wyeth, Pelkey, and McBain, unpublished observation). Neto1, but not Neto2, regulates postsynaptic KR currents in all three interneuron subtypes as well as the KR-mediated recruitment of inhibitory drive onto pyramidal cells. Presynaptic GluK1-containing KRs on CCK/CB1R-containing interneurons are regulated by both Neto1 and Neto2, with Neto1 being required for presynaptic KR function and Neto2 modulating KR affinity (Wyeth, Pelkey, and McBain, unpublished observation).
I. Delta Glutamate Receptors
The delta subfamily of glutamate receptors comprises two family members, GluD1 and GluD2. Although they share some sequence homology with other glutamate receptors, they do not bind glutamate and normally do not function as ionotropic receptors but rather serve as scaffolding proteins (1133). The GluD2 subunit is expressed predominantly in cerebellar Purkinje cells and plays an important role in long-term depression at parallel fiber-Purkinje cell synapses (595), and acts as a synaptic organizer via interactions with both pre- and postsynaptic elements (546). Although GluD2 is not thought to act as an ion channel, it does interact with the metabotropic glutamate receptor mGluR1, which can trigger currents through GluD2 (9). The role of GluD1 is poorly understood but also possesses a channel pore domain and promotes synapse formation in vitro (621). Hepp et al. (484) showed wide expression of both GluD1 and GluD2 throughout the hippocampal formation. RT-PCR from interneurons of the s.r. and s.l.m. revealed highest expression of GluD1 (representing >90% of GluD transcripts) with a much lower expression of GluD2. In ~30% of cells tested, transcripts for both GluD1 and GluD2 were detected (484). Despite this widespread distribution in interneurons, the role(s) of either subunits is completely unexplored at this time; however, global knockout of GluD1 results in mice that exhibit hyperaggresiveness and deficits in social interaction consistent with features of schizophrenic behavior (1239).
J. Metabotropic Glutamate Receptors
mGluRs are a family of G protein-coupled receptors that are widely expressed throughout the central nervous system. The eight members of this family are grouped into three classes based on sequence homology, agonist/antagonist selectivity, and G protein coupling characteristics (for reviews, see Ref. 839). Group I mGluRs comprise mGluR1 and mGluR5, group II includes mGluR2 and mGluR3, and group III includes mGluRs 4, 6, 7, and 8. In general terms, group I mGluRs are expressed in the postsynaptic domain and signal via Gq/G11 to activate phospholipase C, resulting in the generation of inositol 1,4,5-trisphosphate and diacylglycerol to mobilize intracellular Ca2+ and activate PKC. Groups II and III are usually expressed in presynaptic or axonal compartments and are predominantly coupled to Gi/o proteins, which downregulate adenylyl cyclase formation. Liberation of the Gβ/γ subunits positively or negatively modulate a number of ion channels (e.g., activation of K+ channels and inhibition of Ca2+ channels) and other downstream effector mechanisms. Of course, many exceptions exist to this general activation scheme, and it is becoming widely appreciated that many other distinct downstream pathways (e.g., JNK1, MAPK, cGMP, ERK, mTOR/p70 S6 kinase) are triggered by mGluRs in a cell type specific manner (for a more complete discussion, see Ref. 839).
1. Group I mGluRs
Before the advent of reasonably selective ligands, activation of mGluRs was observed to directly depolarize interneurons and alter the frequency of sIPSCs and sEPSCs onto cells, suggesting complex pre- and postsynaptic roles for mGluRs in regulating interneuron excitability (270, 393, 768, 789, 869, 917). Application of the nonselective mGluR ligand trans-ACPD to CA1 s.o. SST-containing O-LM interneurons (768) and CB-positive interneurons (1156) triggered slow oscillatory inward currents (768, 1156, 1217). Somogyi and colleagues subsequently demonstrated that the mGluR1α isoform is particularly enriched in CA1 SST-containing interneurons (75, 326); however, the overlap between mGluR1α and SST is incomplete. In addition, VIP-, CR-, and dendrite targeting CCK-containing interneurons also express mGluR1α (176, 324). Whether PV-containing interneurons express mGluR1α has been controversial (75); however, a recent study using both immunohistochemistry and quantitative immunoelectron microscopy has shown both mGluR1α and mGluR5 to be expressed on both DG and CA1/CA3 PVBCs (447). The splice variant mGluR1b is enriched in unidentified interneurons of the CA3 subfield but not CA1 (325). mGluR5 is expressed on the soma and dendrites of subsets of SST-containing interneurons and PVBCs (1156). Group I mGluRs exhibit highest expression around the perisynaptic annulus of glutamate synapses (710) and have been implicated in the generation of slow oscillatory activity and long lasting plasticity of interneurons (see sect. VI).
2. Group II and III mGluRs
GABA release from interneuron axons is modulated by presynaptic mGluRs. Electron microscopic immunocytochemistry has revealed the expression of group III family members, mGluR4, mGluR7a, mGluR7b, and mGluR8a in the presynaptic active zone of GABAergic terminals onto hippocampal CA1 and CA3 interneurons (but not onto pyramidal cells) (592, 1004). Early studies revealed that inhibitory postsynaptic currents were strongly depressed by the group III agonist l-(+)-2-amino phosphonobutyric acid (l AP4) in unidentified CA1 s.r. interneurons (995). Kogo et al. (592) demonstrated that both low concentrations (selective for mGluR4 and mGluR8) and high concentrations (thought to activate mGluR4, 7, and 8) of l-AP4 depressed evoked IPSCs onto SST-containing O-LM interneurons to a similar degree by a presynaptic mechanism, suggesting that several group III mGluRs are involved in presynaptic depression of inhibitory transmitter release onto interneurons. The high variability of block of IPSCs observed likely indicated that different GABAergic terminals onto interneurons express different combinations of group III mGluRs.
Activation of presynaptic mGluRs reduces transmitter release by altering Ca2+ entry into the presynaptic terminals (283, 514). Transmission at most central synapses is supported by Ca2+ entry through either P/Q- or N-type voltage-gated Ca2+-channels. Modulation of inhibitory transmission by group III mGluRs occurs via a reduction of Ca2+ influx through N-type channels but not P/Q channels (969). Imaging of interneuron axonal boutons demonstrated that neighboring varicosities often showed heterogeneous sensitivity to group III mGluR activation, consistent with the target cell dependence of mGluR expression (969, 1004, 1005). mGluR-mediated depression of inhibitory transmission is accompanied by a reduction in presynaptic Pr, thus enhancing paired pulse facilitation at these synapses. Such a mechanism is likely important during patterned activity where pooled glutamate from the surrounding neuropil can activate mGluRs on GABAergic terminals to directly modulate the inhibitory tone of the network. This mechanism would depend not only on the inhibitory cell types activated but the rate and pattern of excitatory activity flowing through the network and the type of mGluR expressed on the presynaptic terminal (700).
mGluRs show exquisite targeting to distinct interneuron terminals. Ferraguti et al. (326) demonstrated that mGluR8 had a unique expression profile among all interneuron types. mGluR8-positive boutons, which also expressed VIP or mGluR7, are selectively targeted to muscarinic receptor M2-containing interneurons. In vivo recording of an mGluR8 decorated, M2-positive interneuron revealed a new trilaminar cell type with complex spike bursts during theta oscillations and firing during sharp wave ripples. The trilaminar cell had a strong projection to the subiculum as well as innervating both pyramidal cells and interneurons in the CA1 subfield.
In contrast to group III mGluRs, very little is known about the expression profile of group II mGluRs in interneurons. Poncer et al. (916) demonstrated that transmission between CA3 s.r. interneurons and pyramidal cells was reduced by the group II agonist DCG-IV. Although the interneuron cell type (s) within the s.r. cohort were not identified, the action of DCG-IV was precluded by prior application of an N-type Ca2+-channel blocker, suggesting that they were recording transmission from CCK-containing interneurons. In contrast, inhibitory transmission from s.o. interneurons (which use P/Q Ca2+ channels for transmission) was insensitive to DCG-IV, indicating a lack of group II mGluRs on this cell type. Little information exists regarding the localization of mGluR2/3 on interneuron axons; however, on glutamatergic axons, mGluR2 is typically expressed outside the presynaptic active zone at extrasynaptic locations (839).
Surprisingly, since this flurry of papers on mGluRs in the late 1990s/early 2000s, there have been few studies (if any) that have systematically probed pre- or postsynaptic mGluR expression on specific cohorts of well-defined interneurons in mouse reporter lines, leaving a large gap in our understanding of mGluR regulation of specific interneuron excitability and synaptic transmission.
VI. SYNAPTIC PLASTICITY OF GLUTAMATERGIC TRANSMISSION ONTO INTERNEURONS
A. Short-Term Pre- and Postsynaptic Mechanisms
Repetitive activation of most central synapses results in a short-term increase (facilitation) or decrease (depression) of subsequent postsynaptic events (938). The mechanisms underlying this short-term plasticity can have origins in presynaptic or postsynaptic elements or both. Short-term plasticity is an important feature of network dynamics and whether a synapse facilitates or depresses in response to repetitive activation has significant impact on information flow. At the simplest level, short-term facilitation of glutamatergic synaptic responses transiently brings the postsynaptic membrane voltage closer to threshold for action potential firing. Furthermore, synaptic facilitation will act to increase the recruitment of NMDARs by removing the voltage-dependent Mg2+ block, expanding the temporal window of excitation as well as recruiting an essential route for Ca2+ entry. In contrast, short-term depression acts to enforce a narrow temporal window for successive synaptic events to summate and to trigger action potential activity, thereby progressively weakening any influence that the presynaptic cell may have during repetitive activation. How these features of transmission impact circuit dynamics will be discussed in greater detail in section VIID.
Whether a synapse weakens or strengthens during repetitive activity is determined in part by the identities of both the post- and presynaptic cells. Numerous reviews have tackled the issue of target cell specificity of short-term synaptic transmission and interested readers are directed to these for a more detailed discussion (104, 636, 772, 817, 888, 1120). There is now overwhelming evidence that a single axon possesses different release properties at adjacent boutons onto differing postsynaptic targets (888). Markram et al. (749) using triple patch-clamp recordings were first to demonstrate that a presynaptic train of action potentials in a cortical layer 5 pyramidal cell simultaneously triggered short-term facilitation of transmission onto an interneuron target and depressing synaptic events onto a pyramidal cell target (FIGURE 16A). This differential mode of transmission was not simply a consequence of the postsynaptic target cell type (i.e., pyramidal cell vs. inhibitory interneuron) since a similar study demonstrated that a cortical layer 2/3 pyramidal cell presynaptic to two different inhibitory interneuron targets provided the same basic observation (FIGURE 16B). A train of action potentials in the presynaptic pyramidal cell triggered short-term facilitation onto a bitufted interneuron and depression of transmission onto a multipolar interneuron (940). That such exquisite regulation of transmitter release properties could exist in a single axon raises the question of what could be the local mechanism responsible? Whether a synapse facilitates or depresses in response to a train of stimuli is generally determined by the Pr. In general, synapses with a low initial Pr show facilitation upon repetitive activation, whereas those with high Prs typically demonstrate short-term depression (938). The type of presynaptic machinery and the interterminal Ca2+ dynamics are largely thought to determine release at individual synapses. An elegant study by Koester and Sakmann (591) demonstrated that frequency-dependent facilitation between pyramidal cells and bitufted cells is more sensitive to presynaptic Ca2+ buffering than is depression onto multipolar cells. This likely reflects differing diffusional distances between Ca2+ entry sites and the release machinery or disparities between Ca2+ channel types or densities. Indeed, a 10-fold variation in single action potential evoked Ca2+ transients was observed across different boutons of a single layer 2/3 pyramidal neuron despite all boutons using P/Q-type Ca2+ channels (591) (FIGURE 16C). Ca2+ transients were larger at high Pr synapses onto multipolar cells than those observed at low Pr synapses onto bitufted cells (590). A recent study by Rowan et al. (957) demonstrated that axons of cerebellar stellate cell (SC) interneurons possess variable action potential widths at presynaptic bouton sites within the same axon branch. Localized expression of Kv3-containing voltage-gated potassium channels at individual boutons dictate site-specific spike repolarization at each release site. Thus the clustering and variable density of channels that shape the action potential waveform endows axons with exquisite control of transmission in a bouton to bouton manner (957).
FIGURE 16.
Target-cell-dependent inhibitory transmission. A, left: image of three biocytin-filled neurons in layer 5 somatosensory cortex. The pyramidal neuron on the left innervated another pyramidal neuron and a bipolar interneuron, both on the right. A, right: single-trial responses (30 Hz) to the same action potential train (evoked in the presynaptic left pyramidal cell) for the simultaneously recorded postsynaptic interneuron and pyramidal cell targets. Note the strong frequency facilitation and depression of synaptic events in the interneuron and pyramidal postsynaptic targets, respectively. B: data from a triple-patch recording in layer2/3 somatosensory cortex, revealing differential short-term plasticity in two classes of interneurons innervated by a single pyramidal neuron. Three action potentials evoked at 10 Hz in the presynaptic pyramidal cell (top trace), evoked short-term facilitation of unitary EPSPs evoked in the bitufted cell (middle trace, P-B connection), whereas the amplitude of EPSPs evoked simultaneously in the multipolar cell decreased (bottom trace, P-M connection). C: presynaptic Ca2+ transients at divergent release sites of the same axon exhibit target-cell dependence. A single layer 2/3 pyramidal cell loaded with Ca2+ indicator (upper fluorescence image) displays a large degree of heterogeneity in single action potential-evoked Ca2+ transients (bottom traces) at various boutons (circles) along a single axon collateral. [A from Markram et al. (749). B from Reyes et al. (940) with permission from Nature Neuroscience. C from Koester and Sakmann (591) with permission from Journal of Physiology.]
In the CA1 hippocampus, transmission between CA1 pyramidal cells and PV-containing interneurons shows marked short-term depression during repetitive activation, while the same presynaptic input to SST-containing interneurons shows facilitation. Elfn1 is expressed at pyramidal cell synapses onto SST-containing cells but not PV-containing interneurons in both the CA1 and dentate gyrus (1073, 1106). Although the mechanism is not completely understood, Elfn1 is thought to act as a retrograde signaling device to determine presynaptic Pr. Viral vector-mediated Elfn1 knock down (1073), or knockout of Elfn1 (1106) reduces the magnitude of short-term facilitation, confirming a role for Elfn1 in establishing a low Pr. However, the absence of Elfn1 does not convert pyramidal neuron-SST-containing interneuron synaptic activity to match that of pyramidal neuron-basket cell connections, which have an extremely high initial Pr and show marked paired pulse depression. Rather, loss of Elfn1 normalizes transmission across the train of stimuli, with only weak facilitation remaining (1073), suggesting that other factors must be involved in establishing the high Pr of pyramidal neuron-basket cell synapses.
Elfn1 expression increases during postnatal development and is instructive in recruiting presynaptic mGluR7-containing glutamatergic processes onto SST-containing interneurons (1106). Elfn1 directly interacts with mGluR7 and may function as an endogenous transynaptic activator of mGluR7 (1106). Indeed, loss of Elfn1 reduces the amount of presynaptic mGluR7 which leads to an increased Pr (172). However, it is unclear how mGluR7 and Elfn1 interact to modulate transmitter availability. Losonczy et al. (700) suggested that excitatory inputs onto O-LM cells are tonically modulated by persistently activated mGluR2/3/8; however, they failed to implicate mGluR7 in this process. Elfn1 may also act by engaging a GluK2-dependent KR-mediated mechanism to regulate facilitation (1073). Given the myriad proteins that are expressed in the pre- and postsynaptic elements during developmental maturation, there likely exist a large number of as yet unidentified candidates that determine the short-term dynamics of transmitter release.
An entirely postsynaptic form of short-term plasticity exists at glutamatergic synapses made onto CP-AMPARs. As described in section VA, CP-AMPARs are both Ca2+ permeable and blocked in a voltage-dependent manner by cytoplasmic polyamines, such as spermine and spermidine (1133). This intracellular block imparts inward rectification to the current-voltage relationship, precluding current flow at more depolarized potentials. The relief of polyamine block is both use and voltage dependent, providing these synapses with a novel form of postsynaptic short-term plasticity (959, 1121). Repeated activation of the receptor during a train of activity essentially “flushes” the polyamine from the pore allowing greater current flow and a larger resulting EPSC. Block of the channel pore by polyamines is sensitive to cytoplasmic levels of ATP, which chelates polyamines, suggesting an additional mechanism to modulate postsynaptic channel availability (57, 1121). In the CA1 hippocampus, CP-AMPARs are primarily expressed at synapses onto interneurons derived from the MGE (PV-, SST-, and nNOS/NPY-containing interneurons) and largely absent from CGE-derived cells (763), suggesting that this novel form of plasticity is intimately linked to the circuit functions provided by MGE-derived interneurons.
B. CA1 Hippocampal Interneuron LTP
Long-lasting potentiation and depression (LTP and LTD) of glutamatergic synaptic transmission onto glutamatergic principal cells has been a major focus of neuroscience research over the last 30 years (431, 487, 509). Although a number of potentially distinct, but overlapping mechanisms have been identified at synapses onto principal cells, the vast majority of these plastic changes rely on activation of the NMDAR, which acts as a coincidence detector, and the primary route of Ca2+ entry that triggers downstream second messenger cascades to ultimately influence AMPAR trafficking (509). In contrast, studies of long-term plasticity of glutamatergic synaptic transmission onto inhibitory interneurons have been less intense and the results often less than clear in determining the underpinning mechanism(s). Although NMDAR-dependent plasticity has been observed at synapses onto interneurons (see below for further discussion), interneurons lack Ca2+/calmodulin-dependent kinase IIa (686, 1012), which is an essential component of the NMDAR-dependent cascade that underlies LTP at glutamatergic synapses onto principal cells (682), suggesting that alternative mechanisms must exist for LTP/LTD onto interneurons. Instead, the major “types” of interneuron plasticity center on activation of mGluRs and CP-AMPARs. Interested readers are directed to several excellent reviews that discuss the history and the nuances of this research (619, 620, 625, 802).
As discussed in section II, many distinct types of interneurons can be found in the CA1 hippocampus alone. This diversity in part has hampered a clear description of long-lasting plasticity of glutamatergic synaptic transmission onto inhibitory neurons since it now appears likely that different cell types and classes possess distinct and nonoverlapping types of plasticity. This together with the complication that few studies have rigorously identified the cell types being studied much beyond what subfield they were recorded from, as well as the differing recording techniques and conditions, has made for slow progress in our understanding of the types of plasticity observed onto identified interneuron subtypes.
Two predominant types of LTP exist at glutamatergic synapses onto CA1 interneurons. The first, an NMDAR-independent form of LTP was identified in SST-containing interneurons of the s.o. (primarily O-LM cells) and subsequently observed in PVBCs, and AACs (867, 896). High-frequency afferent stimulation or a 5-Hz stimulation coupled with postsynaptic hyperpolarization triggers an LTP that requires a postsynaptic Ca2+ elevation, but appears to have a presynaptic expression locus coupled to increased Pr (867). This form of LTP requires cooperative interaction between group I mGluRs or M1 mAChRs by an as yet unknown mechanism (648). This LTP predominates at synapses comprising CP-AMPARs and has been termed “anti-Hebbian” (633). The “anti-Hebbian” tag is derived from the observation that CP-AMPARs act as coincidence detectors between an excitatory afferent input and synapses that are hyperpolarized or inactive (617, 618, 625). The requirement for hyperpolarization during the induction period serves to increase the driving force for Ca2+ entry, as well as remove the use- and voltage-dependent polyamine block typical of these receptors (see sect. VIA for further discussion). One can imagine a scenario where an interneuron simultaneously receiving high-frequency excitatory input concomitant with a hyperpolarizing GABA or neuromodulatory input would trigger such an “anti-Hebbian” form of plasticity. However, it does suggest that a complex interplay of presumably multicellular events must be at play to satisfy all of the requirements for plasticity induction.
The same presynaptically expressed LTP can be triggered at these synapses by theta-burst stimulation and postsynaptic depolarization and requires activation of group I mGluRs (896). This induction paradigm triggers mGluR1 and mGluR5 activation and also requires postsynaptic Ca2+ elevation together with Src/ERK and TRP channel activation (634, 896, 1110). The postsynaptic locus for induction and presynaptic locus for expression of this NMDAR-independent form of LTP implies retrograde signaling between the post- and presynaptic sites. At this time it is unclear what this signaling mechanism may be, but the use of nitric oxide, cannabinoid, and TRPV1 receptor antagonists has failed to implicate any of the more “conventional” retrograde messenger systems (834). So far, NMDAR-independent LTP has largely been observed at synapses between CA1 pyramidal cells and their downstream interneuron targets and does not typically occur at Schaffer collateral synapses onto interneurons residing in the s.r. (867).
An NMDA-dependent form of LTP exists at synapses onto some s.o. interneurons (869), low-threshold spiking interneurons of the somatosensory cortex (705), and at Schaffer collateral synapses onto a subset of aspiny interneurons residing in the CA1 s.r. (631). This form of LTP shares many of the features observed at Schaffer collateral synapses onto principal cells and appears to have its primary induction and expression loci within the postsynaptic compartment. However, as mentioned above, interneurons lack the Ca2+/calmodulin-dependent kinase IIa pathway essential for LTP in principal cells (686, 1012). Indeed, CA1 s.o. interneurons in the αCaMKII T286A mutant possess normal NMDAR-dependent LTP unlike principal cells (632). Potential Ca2+ sensors may be βCaMKII (1186), CaMKI, and CaMKIV (1026) or perhaps the Ca2+ binding proteins PV, CB, and CR themselves (144), although evidence for this is sparse.
C. CA1 Hippocampal Interneuron LTD
One of the first reports of long-lasting plasticity at excitatory synapses onto hippocampal interneurons came from observations that a high-frequency stimulation paradigm that typically triggers LTP at synapses onto principal cells induced LTD at synapses onto s.r. interneurons (774). This LTD was heterosynaptic, i.e., unstimulated synapses onto the same interneuron were also depressed (774). Like LTP at synapses onto interneurons, this LTD required group I mGluRs. Activation of group I mGluRs triggers arachidonic acid formation and release of 12-(S)-HPETE by a mechanism involving 12-lipoxygenase. 12-(S)-HPETE functions as a retrograde signaling messenger, and its liberation from the postsynaptic compartment allows it to diffuse to the presynaptic site and activate the nonselective cation channel TRPV1 (395). This LTD and sensitivity to 12-(S)-HPETE are absent in TRPV1 loss-of-function mice, confirming a role for TRPV1 channels in interneuron LTD. It is worthwhile pointing out that these same interneurons that demonstrate high-frequency stimulus-induced LTD also show NMDAR-dependent LTP. However, these two mechanisms are not simply the reverse of each other and appear to share few common cellular features for induction and expression. How they interact to regulate interneuron circuit function has yet to be formally tested.
D. CA3 Hippocampal Interneuron Plasticity
1. CA3 pyramidal–s.r. interneuron synapses
In a manner similar to that observed at synapses onto CA1 inhibitory interneurons, high-frequency stimulation of afferents in CA3 triggers differing forms of plasticity at excitatory synapses onto CA3 inhibitory interneurons. High-frequency stimulation of CA3 afferents onto unidentified s.r. interneurons triggers an NMDAR-independent form of LTD at CP-AMPAR synapses that arises only when the postsynaptic membrane potential is hyperpolarized in a manner akin to that described as “anti-Hebbian” in CA1 (627). Induction of this plasticity requires Ca2+ entry through postsynaptic CP-AMPARs and activation of presynaptic mGluR7 (for review, see Ref. 625). Intriguingly, with NMDARs unblocked, the same induction paradigm triggers an NMDAR-dependent form of LTP or LTD that is entirely dependent on the postsynaptic membrane potential (626). Taken together, these studies suggest a complex interplay by Ca2+ entry via CP-AMPARs, NMDARs, and pre- and postsynaptic mGluRs to trigger either LTD or LTP all modulated by modest changes in postsynaptic membrane potential.
2. Mossy fiber-stratum lucidum interneuron synapses
The axons of DG granule cells, the so-called mossy fibers, form somewhat unique synapses compared with others within the mammalian cortex and hippocampus. Mossy fiber boutons onto downstream principal cell targets (primarily CA3 pyramidal cells) are extremely large (~10 μm in diameter) and possess numerous well-defined presynaptic release sites with low Pr, which engulf their postsynaptic dendritic targets to form the so-called thorny excrescence synapse (7, 201). In contrast, small filopodial extensions emanate from these larger mossy fiber boutons to form either en passant or terminal synapses onto inhibitory interneurons within the hilar or CA3 subfields. These filopodial extensions are small in diameter and possess only a single high Pr release site (641, 662). High-frequency stimulation of mossy fibers triggers a presynaptic form of LTP at synapses onto CA3 pyramidal cells but triggers LTD at naive synapses onto s.l. interneurons (722, 889). At CI-AMPARs, this LTD is NMDAR dependent and possesses a postsynaptic locus of induction and expression that relies on NSF/AP2-dependent AMPAR internalization (662). At CP-AMPAR synapses, the same induction protocol triggers presynaptic NMDAR-independent LTD (662). Like the many forms of interneuron plasticity described above, mGluRs are key regulators of this form of plasticity. mGluR7b, which is highly enriched at mossy fiber synapses onto s.l. interneurons (but not CA3 pyramidal cells), functions as a metaplastic switch controlling bidirectional plasticity. Glutamate liberated by the induction paradigm triggers activation of presynaptic mGluR7b, which reduces presynaptic Ca2+ entry through P/Q Ca2+ channels by a mechanism involving PKC (887, 890; for reviews, see Ref. 772), resulting in LTD. As a consequence of binding glutamate, mGluR7b is rapidly internalized, and subsequent rounds of the induction protocol now induce a presynaptic form of LTP via a cAMP-dependent mechanism identical to that observed at mossy fiber principal cell synapses (891). Thus the presence or absence of mGluR7b at these synapses acts as a bidirectional switch that dictates the direction of plasticity at these synapses by governing whether a PKC- or cAMP-mediated LTD or LTP is triggered, respectively.
3. Mossy fiber-stratum lacunosum moleculare interneuron synapses
At mossy fiber synapses onto CA3 s.l.m interneurons, high-frequency stimulation induces a bidirectional form of NMDAR-independent plasticity. Stimulation of afferents onto CI-AMPAR synapses triggers an LTD, which relies on postsynaptic mGluR1 activation and requires an IP3-mediated elevation of intracellular Ca2+, or entry through L-type Ca2+ channels and PKA and PKC activation. When postsynaptic mGluR1s are blocked, the same induction paradigm now triggers LTD, which requires Ca2+ entry through L-type Ca2+ channels (377, 378).
In CA3 s.r. and s.l.m. interneurons that receive both CA3 pyramidal cell recurrent collateral and mossy fiber inputs, there appears to be a compartmentalization of synaptic plasticity (379). NMDAR-dependent plasticity at s.r. inputs on CI-AMPAR synapses requires postsynaptic Ca2+ elevation and activation of Ca2+/calmodulin-dependent protein kinase II and PKC. While mossy fiber LTP onto the same cells also requires PKC formation, this LTP is independent of CaMKII activation instead of relying upon a cAMP PKA signaling cascade.
4. Mossy fiber-dentate gyrus basket cell synapses
To complicate matters even further, mossy fiber inputs onto CP-AMPAR containing synapses on DG PVBCs express an NMDAR-independent form of LTP in response to high-frequency stimulation (23). LTP induction requires a postsynaptic elevation in Ca2+ and PKC activation but is expressed presynaptically. Pharmacological block of either perisynaptic mGluR1 or mGluR5 prevents this Hebbian form of LTP; however, exogenous application of either mGluR1 or mGluR5 agonists alone is insufficient to induce LTP, suggesting that convergent activity via CP-AMPARs and mGluRs are required for its induction (447).
Like many interneurons, PVBCs receive afferent inputs from converging sources. In the DG, PVBCs receive feedforward excitation via entorhinal cortex perforant pathway inputs and feedback excitation from mossy fiber afferents. Repeated coactivation of the two inputs triggers associative LTP at mossy fiber synapses, which requires Ca2+ entry via CP-AMPARs (972). In contrast, perforant pathway synapses made onto CI-AMPARs do not show associative LTP. Sambandan et al. (972) suggest that this associative form of plasticity is an essential mechanism that acts to adjust inhibition to maintain sparse cell activation and an acceptable signal-noise level in the circuit.
It is worthwhile pointing out that although LTP and LTD undoubtedly exist at glutamatergic synapses onto interneurons, they are not universally observed in all interneuron types. Indeed, progress has been extremely slow in defining clear rules for interneuron plasticity, since synapses onto many interneuron types lack any clear form of plasticity and simply follow the “passive propagation” of plasticity induced at excitatory synapses onto principal cells, which acts to increase their afferent output into the hippocampal network (619, 720, 771). This together with the differing recording techniques used and the ease with which whole cell recordings flush essential components out of the postsynaptic compartment have made the study of interneuron plasticity difficult. In the future it will be essential to revisit many of these earlier studies and determine the true identity of each cell type to determine whether logical rules exist at particular cell types. For example, consideration of the literature suggests an intriguing possibility about the rules underlying which cells will express which type of plasticity in the CA1 hippocampus. Kullmann and Lamsa (619) recognized that the cells in which NMDAR-dependent LTP was most readily observed tended to possess the largest NMDAR-mediated conductances. In addition, presynaptically expressed NMDAR-independent “anti-Hebbian” LTP requires either mGluR or mACh receptor activation and occurs primarily at synapses comprised of CP-AMPARs. Of interest, these two forms of LTP appear to largely correlate with cellular origins in either the MGE or CGE. As described in section V, Matta et al. (763) observed that MGE-derived CA1 interneurons exclusively express CP-AMPARs at Schaffer collateral synapses, and this subset comprises PV-, SOM-, and NPY-containing interneurons, all of which express “anti-Hebbian” NMDAR-independent LTP. Cells derived from the CGE typically possess large synaptic NMDAR conductances through GluN2B-containing NMDARs. In hippocampus, CGE-derived interneurons comprise both CCK- and VIP-containing interneurons and largely reside in the CA1 s.r. (763), consistent with those cells that express the NMDAR-dependent LTP. This differential expression of LTP between MGE- versus CGE-derived interneurons has not been rigorously determined, but given the known synaptic properties of these cells, it seems extremely likely that developmental origin will determine which type of LTP an interneuron expresses.
VII. GABA RECEPTORS
Since the discovery of the γ-aminobutyric acid (GABA) in the central nervous system over 60 years ago, its role as a major inhibitory neurotransmitter has been well established. GABA exerts its cellular actions predominantly via the ionotropic GABAA receptors (GABAARs) and metabotropic GABABRs (863). The vast majority of studies have focused on the functional properties and physiological roles of GABAR signaling on principal excitatory cells. However, it is evident that interneurons also impinge on each other providing inhibitory control of inhibition itself (186). As highlighted previously, the CA1 hippocampus contains many different subtypes of interneurons that together serve as activity dependent sources of GABA. Therefore, the possible interactions between pre- and post-synaptic interneuron partners are potentially vast and like the delineation of their subtypes, providing a complete description of all these interactions is by no means a trivial endeavor. Here, we will briefly summarize the current state of knowledge regarding the known connectivity between interneurons, particularly in the hippocampus, as evidenced by GABAR mediated responses on the varied subtypes (FIGURE 17). In this section we will highlight the functional properties of GABAergic inhibitory transmission between interneurons and describe some of the physiological roles attributed to the resulting disinhibition produced by such interactions within neural circuits.
FIGURE 17.
Schematic illustrating the major GABAAR-mediated functional connectivity among hippocampal interneurons. This schematic summarizes the known GABAAR-mediated cross-talk, as assessed by paired electrophysiological recordings in identified CA1/CA3 hippocampal interneuron subtypes. It must be noted that anatomical studies have demonstrated additional putative interactions between interneuron subtypes that have not been included in this summary. s.o., Stratum oriens; s.p., stratum pyramidale; s.r., stratum radiatum; s.l.m., stratum lacunosum moleculare.
GABAARs belong to the cys-loop family and are pentameric hetero-oligomers that assemble to form anion selective channels and predominantly flux chloride. To date 19 individual mammalian genes encoding homologous GABAAR subunits have been identified consisting of α1–α6, β1– β3, γ1-γ3, ε, δ, θ, π, ρ1−3 (for review see (1011). GABAARs in the brain, including the hippocampus, are pentameric and generally consist of two α subunits, two β subunits and either a γ or δ subunit (828, 1011). As will be outlined, the inclusion of certain subunits can influence the cellular localization and fine-tune numerous biophysical and pharmacological properties of the GABAAR complex thus shaping the physiological nature of inhibition.
A. GABAAR-Mediated Inhibition Between Interneurons
1. Interneuron targets of PV-expressing cells
PV-expressing interneurons in CA1 and DG innervate each other as evidenced by the identification of synaptic boutons originating from this subtype at symmetric synapses, indicative of an inhibitory phenotype, along the somatodendritic axis of postsynaptic PV labeled interneurons (8, 440). This mutual connectivity occurs in various cortical regions (374, 455, 906) and appears to be a generalized circuit phenomenon. In hippocampus, PVBCs provide GABAAR inhibition onto each other (65, 211, 258, 594, 980, 1257), whereas there is no such connectivity between their AAC counterparts (594). GABAAR responses can be observed between PVBCs and AACs but due to their lack of connectivity never in the reverse direction (594). Thus, within the various PV-expressing populations of interneurons, specific connectivity rules exist.
The α1 subunit is prevalent in all subfields and lamina of the hippocampus (382) and is the most common GABAAR subunit. Some of the highest expression levels are encountered in PVBCs, AACs, and BiC populations (74, 382, 692, 788), and this subunit is particularly enriched at PV-PV interneuron synapses (585). The α1 subunit influences the activation and deactivation kinetics of GABAAR-mediated responses, and the unitary GABAAR IPSCs between mature PVBCs posseses fast decay kinetics (65, 258). The expression of α1 increases during postnatal development of PV interneurons and is accompanied by speeding of the decay of GABAAR IPSCs (277). Furthermore, in α1 knockout mice, GABAAR IPSCs onto CA1 interneurons possess significantly slower decay times that in part maybe due to “compensatory” increases in α2 and α3 subunit expression in numerous interneurons including PV subtypes that normally express little or no α2 and α3 (421, 990).
Benzodiazepines (BZs) are positive allosteric modulators of GABAAR function (i.e., require the presence of GABA to exert their actions) and their binding site is located between the α (either 1, 2, 3, or 5 isoforms) and γ subunits (for review, see Ref. 1010). Zolpidem, a non-BZ, also binds to the BZ site to act as a positive allosteric modulator of GABAAR function. However, it has higher affinity for receptors containing the α1 subunit than those with α2, α3, or α5 subunits. Thus, at low nanomolar concentrations, the actions of zolpidem are selective for α1-containing GABAARs, and this agent has served as an experimental tool to probe for expression of this subunit. Indeed, the effect of low nanomolar concentrations of zolpidem on GABAAR-mediated responses onto interneurons is abolished or largely reduced following genetic ablation of α1 subunits or mutation of the histidine residue [replaced by arginine, a1 (H101R)] in this subunit that is critical for binding of zolpidem (54, 421). In agreement with immunohistochemical studies demonstrating enriched α1 subunit expression in PV interneurons (382, 692, 788), zolpidem potentiates GABAAR-mediated responses between members of this subtype (277, 972).
The fast uIPSC kinetics of α1-containing GABAAR-mediated responses results in precise temporal inhibition between PVBCs similar to that imposed by PVBCs onto principal cells (64). PVBCs are fast signaling devices that enable large ensembles of PCs to be precisely temporally coordinated, thus generating network activity patterns (see sect. XV). Under certain modeling conditions, networks of PV interneurons are sufficient to drive oscillations (66, 1190), and the propensity to do so is particularly sensitive to the strength and prevalence of their inhibitory interconnectivity (323). Additionally, altering the kinetics of the GABAAR-mediated conductance onto PVBCs in this computational network influences the coherence and frequency of the resultant oscillatory behavior (66, 67). Empirically, conditional genetic deletion of the γ2 subunit in PV-expressing interneurons results in loss of fast α1-containing synaptic GABAAR-mediated IPSCs and disrupts the coupling of theta and gamma oscillations in the CA1 hippocampus (1222), resulting in deficits of hippocampal-dependent spatial memory tasks (667). Thus fast α1γ2-containing GABAAR-mediated inhibitory input onto PV interneurons is critical for the generation and characteristics of coordinated network activity that underlie varying cognitive processes.
Anatomically defined inhibitory synaptic inputs originating from PV cells have also been observed targeting other interneuron subtypes such as CCK interneurons (8, 554). In DG, PVBCs mediate GABAAR responses onto CCK HICAP cells (980), but functional evidence of an analogous connection in CA1 is yet to be described. Interestingly, a subpopulation of entorhinal cortical interneurons, some of which express PV, send long-range inhibitory projections into the hippocampus and elicit GABAAR-mediated responses onto various hippocampal interneurons (69, 781). Conditional expression of channel rhodopsin has revealed that PV-long range mediated inhibition of CCK-expressing interneurons provides a temporally defined gating of Schaffer-collateral excitatory input onto CA1 PCs, rendering them permissive for the expression of synaptic plasticity underlying various learning behaviors (69). Additionally, the prevalence and role of local interactions between PV-expressing interneurons in CA1, a subpopulation of which are perisomatic targeting, have also been examined with conditional optogenetic approaches. Functional GABAAR-mediated inhibitory inputs are observed onto BiCs and O-LM interneurons with a higher synaptic strength onto the former (703). Inhibiting PV interneuron activity increases the firing rate of BiCs, resulting in a switch from perisomatic to dendritic inhibition of CA1 PCs (703). Thus such interneuron interactions result in dynamic shifts between distinct modes of inhibition impinging on PCs impacting the processing of CA3 Schaffer-collateral inputs.
2. Interneuron targets of CCK-expressing cells
GABAAR-mediated inhibitory transmission between homologous pairs of CCKBCs (258, 594), CCK SCAs (19, 21), and CCK HICAPs (980) have all been identified. Thus, as observed for PV subtypes, homotypic interactions between CCK-expressing interneurons are also prevalent within the hippocampus. In contrast to the temporally precise PV interneuron inhibition, CCK interneurons produce marked asynchronous GABAAR-mediated responses onto connected principal cells due to a relatively weak coupling between N-type calcium channels and the GABA release machinery in their presynaptic terminals (21, 64, 477). Interestingly, this mode of release is also evident at CCK-CCK synapses including those between CCKBCs resulting in a more temporally variable, prolonged inhibition compared with that seen at PV-PV synapses (258). In CA1 and dentate gyrus, axonal boutons originating from CCK interneurons are closely apposed to somatic, dendritic, and axonal compartments of PV interneurons (8, 554). Indeed, GABAAR-mediated IPSCs can be elicited on PV cells by CCK interneuron activity in both these regions (506, 980, 1257) and possess the archetypal asynchronicity (506). Disruption of CCK interneuron firing results in decreased inhibitory drive onto these postsynaptic targets altering the temporal characteristics of PV interneuron firing (506). It is hypothesized that this interaction desynchronizes pyramidal cell output to manifest as a deficit in gamma oscillations (506).
3. Interneuron targets of SST-expressing cells
Unlike the prevalent homotypic interactions of PV and CCK interneurons within the CA1, it remains unclear whether SST-expressing subtypes are mutually connected. However, in dentate gyrus, the O-LM equivalent, SST-expressing HIPP interneurons impart GABAAR inhibition onto each another, albeit with a lower connectivity rate when compared with other homotypic interactions between CCK HICAP-CCK HICAP and PVBC-PVBC pairs that occur in this region (980). However, SST, O-LM, and HIPP subtypes anatomically contact other interneurons such as PV, CCK, and CR expressing subtypes (557). Paired electrophysiological recordings of presynaptic identified O-LM interneurons reveal action potential-dependent GABAAR-mediated responses on various interneuron subtypes including CCKBCs, SCAs, PPAs, and NGFCs (299). This relatively widespread inhibitory influence of SST interneurons has also been described in cortical circuits (906). Manipulations of SST interneuron activity can modulate the rate and precision of PVBC firing (980), likely influencing information flow through the dentate gyrus. Furthermore, the disinhibition of CA1 NGFCs by O-LM interneurons modulates the balance between feed-forward and feedback inhibition of the distal dendrites of PCs (299), revealing a role for interneuron connectivity in gating modes of inhibition onto PCs.
4. Interneuron targets of NGFCs
Electrophysiological recordings from pairs of NGFCs demonstrate a marked functional connectivity with each other (48, 550, 926, 1074). Like PV-expressing interneurons, NGFCs express high levels of α1 subunit, and unsurprisingly, the GABAAR-mediated responses impinging on this subtype are sensitive to low nanomolar concentrations of zolpidem (367, 550). However, in contrast to the fast synaptic inhibitory responses that occur on PV cells, inhibition between NGFCs results in GABAAR-mediated IPSCs with decay times an order of magnitude larger that have been termed GABAA,slow (48, 167, 870, 1086). These remarkably slow kinetics are not fully attributable to dendritic filtering, asynchronous release, spillover, nor the properties of the GABAARs themselves (however, see Refs. 483, 1264) but are primarily due to the spatiotemporal profile of GABA release from this interneuron subtype (see sect. IVL) (167, 550, 870, 1074). In CA1, NGFCs and their related IvCs (366) are the most abundant subgroup (102) of interneurons and are distributed in the s.l.m., s.r., and s.p. layers (see sect. IIG). Together, their cumulative prodigious axonal arbors cover large regions of the hippocampal real estate. To what extent NGFCs/IvCs communicate with other specific interneuron subtypes requires further investigation, although in CA1 and dentate gyrus they have been shown to mediate GABAA,slow on unidentified s.l.m. and molecular layer interneurons, respectively (48, 926). Modeling of networks consisting of interconnected interneurons mediating GABAA,slow and GABAA,fast is sufficient to produce theta and gamma rhythms, thus implicating NGFC cross-talk to other interneuron subtypes in such network phenomenon (1199). Furthermore, the role of GABAA,slow in the generation of network oscillations has been described (167, 483), but the relative contributions of NGFC-mediated GABAA,slow onto principal cells versus that onto other interneurons in mediating these phenomenon has not been directly or individually assessed.
5. Interneuron specific cells
One of the most compelling observations illustrating the potential importance of inhibitory control of interneurons lies in the existence of a defined subpopulation of so-called ISI cells. As the name suggests, ISI cells display a remarkable selectivity of their postsynaptic partners primarily targeting other interneurons whilst typically avoiding principal cells. In the CA1, ISI cells can be further subdivided based on anatomical location and immunocytochemical marker profile (see sect. IIH). ISI-3 interneurons express VIP and CR, and recently, the postsynaptic partners of ISI-3 interneurons have been identified at a functional level. Employing transgenic reporter mice and conditional optogenetic expression strategies, ISI-3 cells located in s.p. and s.r. possess a relatively high connectivity to O-LM interneurons (185, 1145) whilst providing no input onto principal cells (1145). This interaction paces O-LM interneuron output, modulating their firing rate and timing with implications for dendritic electrogenesis, burst firing in PCs, and the generation of oscillatory activity (703, 1145). The targeting of SST interneurons by VIP cells appears to be a generalized motif across brain structures including somatosensory, visual, and auditory cortices, and this specific disinhibitory microcircuit is important in sensory processing that, in some circumstances, demonstrates behavioral state dependence (363, 553, 660, 905, 906, 908, 1246, 1270). In addition to entorhinal cortical long-range inhibitory projections described earlier, a population of GABAergic medial septal neurons specifically innervate hippocampal interneurons via long-range projections providing regulation of theta frequency synchronization (351, 438, 1118). This additional dialogue provides an alternative long-range mode of disinhibition to that locally imparted by ISI-3 cells which together modulate the function of O-LM cells (185).
B. Extrasynaptic Tonic Inhibition of Hippocampal Interneurons
Although GABAARs are prevalent at synaptic sites, a significant population exists at peri- and extrasynaptic locations. The subunit composition dictates the trafficking of the receptor complex, and generally speaking, GABAARs containing α1–3 and γ subunits versus those comprised of α4–6 and δ subunits are predominantly located at synaptic and extrasynaptic locations, respectively (81, 317, 410). The α5 and δ subunits containing extrasynaptic GABAARs possess a much higher affinity and minimal desensitization to GABA than synaptic receptors. Thus, as opposed to fast phasic inhibition mediated by relatively large GABA transients at synaptic GABAARs, ambient low concentrations of GABA can persistently activate this extrasynaptic population of receptors permitting the presence of an ongoing inhibitory tone. GABA found in the extracellular space can be derived from a number of nonvesicular mechanisms including astrocytic release and reversal of GABA transporters. It has also been suggested that vesicular release during trains of action potential discharges provide an appreciable source of GABA resulting in tonic inhibition via GABAARs (411).
Extrasynaptic GABAAR subunits are highly expressed in dentate granule cells and in hippocampal interneurons (893). However, with respect to the latter, subtype specificity of expression is apparent. The δ subunit is particularly abundant in PV interneurons (320, 788, 1256) and NGFCs (316, 858) with little or no detectable levels observed in SST-, CB-, or CR-expressing subtypes (788). The extent to which a given neuron is influenced by tonic inhibition can be experimentally assayed by monitoring changes in membrane potential (or holding current required to maintain a specific membrane potential) upon application of GABAAR antagonists such as bicuculline, gabazine, or picrotoxin (660). Additionally, 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) possesses an extremely high efficacy for δ-containing GABAARs that is much greater than that of GABA itself. This “superagonist” serves as a useful experimental tool to test for the presence of tonic inhibition. This distinct inhibition mode has been described in interneurons with soma located in the m.l. of DG and CA1 s.r. (409, 659, 1038, 1256). In agreement with the selective expression of δ subunits in certain hippocampal interneuron subtypes, THIP-mediated currents are present in dentate gyrus PV-containing but not other DG interneurons including HIPP and other SST-containing interneuron populations (1109, 1160, 1256). As expected, ablation of the δ subunit reduces THIP-mediated currents in interneuron populations, including PV subtypes (739). Ectopic expression of the δ-subunit in SST HIPP cells results in the presence of GABAARs containing this subunit at nonsynaptic locations, including perisynaptic sites near symmetric synapses and an emergence of THIP sensitivity (1109).
Tonic inhibition controls neuronal excitability and hence action potential output in pyramidal and dentate granule cells (317, 660, 996). The role of tonic inhibition of interneurons has been experimentally determined by conditional ablation of the δ subunit. In DG, removal of this subunit increases inhibitory input onto granule cells and reduces the susceptibility to kainate-induced seizures (659). Conversely, increasing tonic inhibition in hilar SST cells via ectopic expression of α6 and δ subunits reduces experimentally induced bursting of IPSCs impinging on granule cells (1109). Conditional δ ablation specifically from PV-expressing interneurons increases the frequency of γ oscillatory activity in the CA3, illustrating a negative modulation of such oscillatory activity by tonic inhibition (320, 739). Therefore, changes in tonic inhibition of interneurons can regulate excitability and oscillatory behavior throughout the hippocampal circuit. The cellular effect of tonic inhibition is likely more complex than to simply provide a dampening of interneuron activity resulting in disinhibition of downstream principal cells. For example, in unidentified CA1 interneurons of the s.r., tonic inhibition is biphasic resulting in an increase or decrease in action potential output due to depolarizing and shunting responses, respectively, that are in turn dependent on the conductance level of tonic inhibition (1038). Whether this is a generalized phenomenon found in all interneurons that are susceptible to tonic inhibition remains to be determined.
The most potent endogenous modulators of GABAARs are the neurosteroids allopregnanolone (ALLO) and tetrahydrodeoxycorticosterone (THDOC). Although the binding site is located between α and β subunits (498), it is the δ subunit that generally dictates sensitivity to these neurosteroids (629). Thus THDOC-mediated effects are seen in interneuron subtypes expressing δ such as NGFCs and PV interneurons (316, 320, 788, 858, 1256) with little or no effect in SST cells that are devoid of this subunit (1160). Interestingly, expression of the δ subunit itself can be altered in part due to changes in the levels of circulating neurosteroids that occur during stress, puberty, menstrual cycle, and pregnancy (726, 732, 733, 807). In particular, plasticity of δ subunits on PV-expressing interneurons is linked to alterations of γ oscillatory activity that may explain memory and cognitive dysfunctions known to occur during estrus and pregnancy (320, 321).
C. GABAB Receptor-Mediated Inhibition
In contrast to the ligand-gated GABAA receptors, GABABRs are heterodimers of seven transmembrane G protein (Gi/Go)-linked proteins that are comprised of a GABAB1 (either GABAB1a or GABAB1b) and a GABAB2 subunit. Activation of postsynaptic GABABRs and subsequent direct G protein interaction with GIRK (Kir3) channels predominantly results in membrane hyperpolarization inhibiting neural excitability. Immunoreactivity for GABABRs demonstrates a relatively ubiquitous distribution on somatodendritic compartments of varying interneuron subtypes including SST-, NPY-, CCK-, CR-, and CB-expressing subtypes, although differences in relative expression levels are apparent (115, 116, 345, 616, 691, 926, 1024). PVBCs of the dentate gyrus, but not their dendrite-targeting counterparts, express functional postsynaptic GABABRs (116) (FIGURE 18). Computational modeling predicts the possible importance of this signaling in modulating the temporal nature of output in the former subtype (116), perhaps explaining the differing firing patterns observed between PVBCs and dendritic targeting interneurons during network oscillations. Particularly high levels of GABABRs are found on somatodendritic regions of CCK cells (115, 1024). However, the relative size of GABABR-mediated currents among the various CCK subtypes significantly differ with larger responses noted in CCKBCs compared with those observed in SCAs or PPAs (FIGURE 18) (115). Thus, as with PV cells, postsynaptic GABABR-mediated signaling preferentially occurs in perisomatic versus dendritic targeting CCK subtypes (FIGURE 18).
FIGURE 18.
Selectivity of postsynaptic GABABR-mediated responses at perisomatic vs. dendritic targeting PV- and CCK-containing interneuron subtypes. A and B: trains of stimuli elicit robust GABABR-mediated postsynaptic IPSCs (traces at bottom of cell reconstructions) in PV- and CCK-BC subtypes (see insets for post hoc immunocytochemistry and membrane voltage responses including action potential firing patterns in response to hyperpolarizing and depolarizing current steps) with minimal or no response at their dendrite targeting (DTI) counterparts. Based on axonal arborization, the PV and CCK DTIs in these examples correspond to bistratified (BiC) and Schaffer-collateral associated cells, respectively (SCA). C: schematic summarizing this selectivity as described in these studies. PC, pyramidal cell. [PV cell reconstructions and traces from Booker et al. (116) with permission from Journal of Neuroscience. CCK cell reconstructions and traces from Booker et al. (115) with permission from Cerebral Cortex.]
In general, postsynaptic GABABR activation occurs via spillover of GABA from the synapse that in experimental paradigms requires either strong electrical stimulation/trains of stimuli resulting in GABA release from multiple neurons to overcome GABA uptake mechanisms or stimulation under conditions where GABA uptake is pharmacologically blocked (427, 523, 982). GABABR postsynaptic potentials/currents have been identified in numerous interneuron subtypes (115, 116, 137, 578, 622, 811). Not all interneuron subtypes exhibit postsynaptic GABABR responses, and a division of labor between subtypes in providing putative GABAAR- or GABABR-mediated IPSPs (628, 993) indicates the presence of selective microcircuits responsible for activation of postsynaptic GABABRs. Thus it is unclear whether the functional connectivity rules as defined by GABAAR signaling outlined above also apply to interactions between interneurons that are mediated via GABABR signaling (844).
Activation of presynaptic GABABRs on various interneurons results in negative modulation of transmission of varying degrees due to activation of GIRK channels and/or inhibition of voltage-gated calcium channels coupled to vesicular release. Such modulatory mechanisms occur on various interneuron subtypes resulting in decreased GABA output at synapses onto both excitatory and inhibitory neurons (116, 478, 916), the former providing another synaptic route for disinhibition. Intriguingly, NMDAR-dependent LTP of excitatory inputs onto CA1 pyramidal cells and dentate gyrus granule cells is sensitive to a concomitant presynaptic GABABR-mediated decrease of GABA release resulting in disinhibition of pyramidal cells and enhanced NMDAR activation (255, 810, 939). Although the interneuron subtype(s) involved remains to be determined, these data confirm a potential role for disinhibition in gating synaptic plasticity, albeit via a distinct mechanism from that described previously. On the other hand, this form of disinhibition can result in hyperexcitability of principal cells (345, 808, 809) and may play a part in pathological discharges akin to those seen in epilepsy. Again, the interneuron subtype(s) involved remains a mystery, but recently CCK-expressing interneurons have been implicated in this proconvulsant phenomenon at least in CA3 (290). Thus short-term dynamic and more persistent changes in presynaptic GABABR-mediated inhibition of GABA release are implicated in both physiological and pathophysiological scenarios.
NGFCs, by virtue of their ability to elicit volume transmission (see sect. IVL3), are a major source of GABA that culminates in GABABR activation. Therefore, this interneuron subtype may play a significant role in some of the disinhibitory mechanisms involving this receptor. It is evident that NGFCs mediate postsynaptic GABABR-mediated responses on other NGFCs and principal cells even after a single action potential (48, 927, 1086). As mentioned, dentate gyrus NGFCs impart GABAA,slow responses on molecular layer interneurons (48), but whether these same interactions result in additional postsynaptic GABABR responses was not tested. Volume transmission by NGFCs also results in presynaptic GABABR activation on axon terminals of both PCs and other interneurons in a paracrine manner to modulate neurotransmitter release in cortical circuits (204, 858). More recently, ongoing activity of SST interneurons in the somatosensory cortex was shown to mediate depression of glutamate release from pyramidal cells via presynaptic GABABR activation (1154). However, whether similar modulatory mechanisms exist in the hippocampus is unclear.
The rich tapestry of interconnectivity mediated by GABAR signaling between diverse interneuron subtypes, including both local and long range, provide additional arrays of layered microcircuits. Although this intercommunication has the potential to be widespread, it is clear that specific connectivity rules exist serving discrete network functions. As is the case for principal cells, varied inhibitory modalities coexist even onto a single interneuron subtype. This is dependent not only on the identity of the presynaptic interneuron but also on the functional properties of postsynaptic GABARs, with the latter being dictated by subtype (i.e., GABAA vs. GABAB), cellular location (i.e., synaptic vs. extrasynaptic; pre- vs. postsynaptic), and subunit composition. Dynamic modulation of such interactions serves to gate the mode and timing of inhibition impinging on principal cells, hence sculpting their input/output relationships central for synaptic plasticity induction/expression and generation of network oscillations.
D. Circuit Dynamics of Dendritic Versus Perisomatic Inhibition
As described in section II, local circuit interneuron axons possess considerable diversity in the targeting of their downstream postsynaptic domains (352, 582, 1036) (FIGURE 1). At one end of this continuum are two subsets of PV-containing perisomatic targeting interneurons. PVBCs innervate the soma and first 20–30 μm of the proximal dendrites of principal cells, and AACs target the pyramidal neuron axon initial segment (FIGURE 2). In contrast, SST-containing hippocampal O-LM cells or cortical Martinotti cells send their largely unbranching axons across several subfields to target the most distal dendritic portions of pyramidal cells (FIGURE 3). The target-specific diversity of local circuit interneurons ensures that virtually every compartment of the dendrites-soma and axon initial segment are selectively targeted. But to what end? Transmission onto perisomatic versus dendritic targets provides fundamentally different forms of inhibitory synaptic control. Perisomatic inhibition efficiently controls Na+-dependent action potential generation, whereas dendritic inhibition influences local voltage-gated conductances, shunts excitatory inputs lying distal to their location, and regulates the generation of Ca2+-dependent action potentials and synaptic plasticity (522, 790, 721).
While principal neurons of the hippocampus and cortex are highly enriched for both inhibitory and excitatory synaptic inputs, these inputs are not distributed evenly across cells. The vast majority of excitatory synapses onto pyramidal cells are made onto spines located on the mid proximal to distal dendritic portions of the cell. In contrast, as stated above, inhibitory inputs are made onto almost all portions of the dendritic tree as well as the perisomatic regions including the axon initial segment. This synaptic arrangement undoubtedly has consequences for the functional control of principal cell outputs. Pouille et al. (924) combined optogenetics and dynamic patch-clamp techniques to explore the consequences of distal dendritic versus proximal GABAergic inputs on excitatory synaptic integration and action potential firing in hippocampal and cortical pyramidal cells. Of interest, they observed that when inhibition and excitation arrived at the same dendritic compartment, inhibition caused a rightward shift in the firing rate function related to excitatory conductance (FIGURE 19). In other words, the postsynaptic cell could still achieve the same maximal firing rate, albeit in response to a greater excitatory conductance input. When inhibition was placed at locations proximal to the excitatory conductance, the firing rate of the cell is again reduced; however, proximal inhibition alters the electrotonic properties (i.e., shunting) of the path between the excitatory conductance input and the axon initial segment output to both increase threshold and decrease the maximal firing rate for a given excitatory conductance input. This rather elegant example serves to illustrate the different functional impact inhibitory input location can have on principal cell output (924).
FIGURE 19.
The differential impact of inhibitory inputs arriving at distal vs. proximal dendritic locations. A: schematic showing the two different experimental protocols, with application of the GABAA agonist muscimol either to the soma and proximal apical dendritic trunk, or to the more distal apical trunk close to the location of a patch pipette. A steady-state excitatory conductance (Erev = 0 mV) is simulated by dynamic clamp over a 1-s period, and the steady-state firing rate is derived from the final 400 ms of this. B: input-output (IO) functions at different levels of ambient muscimol for dendritic (Bi) and somatic (Bii) applications. Muscimol is applied by a continual series of low pressure puffs, and the concentration is varied by changing the frequency of pressure puffs. Note that in the configuration where muscimol is applied to the distal dendritic compartment, the maximal attainable firing frequency is unchanged in contrast to when muscimol is applied to the somatic/proximal dendritic compartment. [Data from Pouille et al. (924) with permission from Physiological Reports.]
This anatomic arrangement of dendritic and perisomatic inhibition, together with the particular cell intrinsic properties (i.e., the complement of voltage-gated conductances), action potential dynamics, neurotransmitter release properties, and short-term plasticity of both excitatory and inhibitory transmission provides the anatomic and functional basis for almost all feedforward and feedback inhibitory control of cortical networks (FIGURE 20). In the CA1 hippocampus, excitatory afferents (of both intrahippocampal and subcortical origins) target both excitatory and inhibitory neurons. In general, excitatory afferent inputs onto interneurons are stronger than the equivalent inputs onto principal cells (522). This arrangement ensures that any input that activates monosynaptic excitatory input onto principal cells will almost always trigger disynaptic inhibitory input to generate feedforward inhibition of the principal cells (FIGURE 20). In most circuits the short latency of feedforward inhibition enforces a narrow temporal window for action potential firing in principal cells (922). In a series of experiments combining somatic and dendritic recordings from hippocampal CA1 pyramidal cells, Pouille and Scanziani (922) elegantly demonstrated that the bulk of feedforward inhibitory inputs arise through perisomatic fast spiking basket cells and that inhibition onto dendritic compartments was modest by comparison. This differential inhibitory input provides two integration windows for EPSP summation with a broader integration window existing for EPSPs arriving at dendritic sites. In fact, this is a motif shared across many cortical and hippocampal circuits (257, 372, 403, 404, 922). Recently, Basu et al. (68) challenged the narrow hypothesis that PVBCs are the main providers of feedforward inhibition and demonstrated that CCK-containing interneurons can also play a prominent role in providing feedforward inhibition driven by perforant path and Schaffer collateral inputs to CA1 pyramidal cells (see also Ref. 1165). Given the surprising number of interneurons whose cell bodies and dendrites occupy space in the CA1 s.r. and can therefore be targeted by both intra- and extrahippocampal afferents, it is highly likely that many interneuron subtypes contribute in discrete ways to enforce different aspects of feedforward inhibitory control over both principal and interneuron targets.
In most cortical networks, the dynamic between excitatory and inhibitory inputs is strongly modulated by the short-term plasticity properties of the respective components, such that progressive changes in the excitation:inhibition ratio results in a progressive degradation of the excitation and inhibition balance (372, 404). For example, in somatosensory cortex, although both EPSCs and feedforward IPSCs depress in response to repetitive stimulation, the magnitude of the depression is greater for inhibition than excitation resulting in an increased action potential jitter and a loss of temporal precision (372). In contrast in CA3 circuits, mossy fiber synapses onto CA3 pyramidal cells provide a strong monosynaptic drive that demonstrates unusually strong facilitation in response to repetitive stimulation (538, 641). Mossy fiber-driven feedforward inhibition facilitates to maintain a fixed excitatory:inhibitory balance during trains of activity. Of interest, in contrast to other cortical circuits, erosion of this feedforward inhibitory input does not lead to a loss of temporal precision of action potential generation in CA3 pyramidal cells, but can trigger a prolonged plateau depolarization following action potential firing (1114). This suggests that temporal precision in the mossy fiber-CA3 circuit is primarily determined by the rapid kinetics of the excitatory input itself and that feedforward inhibition serves largely to prevent excessive depolarization triggered by this large and rapid excitatory conductance (1114).
The output of principal cells is similarly differentially influenced by distinct inhibitory interneuron subpopulations via feedback inhibitory control of spiking activity. The output features of principal cells, i.e., spike frequency and timing, are critical for correct cortical functioning and are regulated by recurrent excitatory drive onto interneurons that then target the very same principal cell populations. Pouille and Scanziani (923) provided the first demonstration that recurrent inhibition onto CA1 pyramidal cells occurs via a dynamic rerouting of inhibitory drive that commences via perisomatic inhibitory input followed by a delayed primarily dendritic inhibition. This circuit arrangement is predicated on several pre- and postsynaptic features of the small circuit loop: excitatory synaptic kinetics, properties of short-term plasticity of excitation onto different interneurons, intrinsic properties of interneurons, and the temporal dynamics of disynaptic inhibition. Trains of action potentials from CA1 pyramidal cells drive excitation onto two populations of interneurons termed “onset transient” and “late persistent” interneurons (923). Synaptic events with rapid kinetics exhibiting short-term depression in response to trains of action potentials drive the early-onset transient form of perisomatic inhibition, presumably primarily via PV-containing interneurons back onto CA1 pyramidal cells. The rapid depression of excitatory drive onto this interneuron population ensures that they are active for only short periods, providing a coincidence detection mode of inhibitory control. In contrast, slowly facilitating excitatory input onto a different population of “late persistent” interneurons recruits dendritic inhibition onto CA1 pyramidal cells. As a result of slow facilitation, these interneurons [presumably SST-containing O-LM cells, BiCs and CB1R-positive CCK-containing cells (404)] are engaged in a later inhibitory epoch reflecting an integration mode that drives distal dendritic inhibitory inputs proportional to spike rates late in the series.
All of the above discussion treats inhibitory control as a point-to-point function in a seemingly Euclidean or linear manner. Of course, this is an extraordinarily simplistic point of view since multiple converging afferent excitatory and local inhibitory inputs are likely to be simultaneously engaged during any period of ongoing activity. Moreover, as discussed above, interneurons make highly extensive reciprocal connections between each other (374, 398, 522). The combination of afferent and efferent synaptic temporal dynamics coupled with each interneuron’s intrinsic properties will have many functional consequences for cortical integration and function. Indeed, the very idea that inhibition exists as a precise regulator of specific cell types and domains has been challenged by a number of studies.
In many cortical circuits, lateral inhibition is thought to sharpen stimulus selectivity by sharpening the tuning of neuronal receptive fields in a cell type and circuit specific manner (522). However, recent evidence suggests that certain forms of inhibition are promiscuous and less selective in downstream targeting than initially thought (331, 552, 876, 918). Using a combination of optogenetics, high-resolution imaging, and electrophysiological techniques, Yuste and colleagues (552) found that neocortical PV- and SST-containing interneurons indiscriminately target virtually all principal cells within a 200-μm radius of their cell body, to provide what has been termed “blanket inhibition.” Such an arrangement would ensure that individual or small groups of interneurons would target overlapping domains to globally inhibit large numbers of pyramidal cells within their vicinity, thus diminishing excitatory output. In keeping with the discussion above, one can envision that recruitment of PV- versus SST-mediated blanket inhibition would have significantly different temporal dynamics with distinct influences on cortical information processing (see sects. IIB and IIII for further discussion). PV-containing interneurons would provide rapid and early blanket inhibition of the somatic and axonal initial segment domains of principal cells, whereas the slowly facilitating inputs onto SST-containing interneurons would ensure a later shift toward global dendritic forms of inhibition (552). In this scenario NGFCs would present a novel, nonspecific form of blanket inhibition. In the CA1 hippocampus, the vast majority of NGFCs are located within the s.l.m., where they appear to tile the entire extent of the region to cover the distal dendrites of principal cells (1137, 1138). Given that NGFCs release GABA in a hybrid manner with features of both synchronous and volume transmission (see sect. IVL3), large areas of the hippocampal dendritic landscape could become bathed in GABA yielding massive indiscriminant inhibition, suggesting a more permissive binary role for inhibition in sculpting excitation than one designed around its timing. Given that the s.l.m. is the target subfield for excitatory afferents from both the temporoammonic entorhinal cortex and thalamic nucleus reuniens, such blanket inhibition could act to shunt the distal dendrites of principal cells functioning as a gate to block entorhinal/thalamic input and favoring activity arising through the Schaffer collateral pathway into s.r.
Based on the discussions above, the very notion of blanket inhibition provided by either PV-perisomatic targeting or SST-dendritic targeting cells seems counterintuitive to the primary roles typically assigned to these cells. Moreover, how could excitatory principal cells respond to incoming afferent activity if they are constantly blanketed by inhibition? A recent series of manuscripts have shed potential light on this apparent conundrum. VIP-containing interneurons selectively target other inhibitory interneurons (see sect. IIH) with SST-containing interneurons serving as a primary target, while principal cells are largely spared VIP-containing interneuron input (553, 652, 906). VIP-containing interneurons therefore provide a mechanism for lateral disinhibition, i.e., inhibition of inhibitory interneurons, which allow principal cells temporary relief from inhibitory control. Karnani et al. (553) rather elegantly show that activation of VIP-containing interneurons in vivo makes “local and transient holes in the inhibitory blanket” provided by SST-containing interneurons, thereby allowing patterns of principal neuron activation to propagate within specific networks. The radial extent of single VIP-cell disinhibition of SOM-interneuron driven lateral inhibition is ~120 μm, which is consistent with the known axonal arborization of VIP-containing inhibitory neurons (569). Such a mechanism whereby small numbers of VIP-containing interneurons disinhibit “pockets” of principal cells provides an attractive mechanism to permit transient activation of precise selective circuits within normally inhibited cortical circuits.
VIII. ENDOCANNABINOIDS
The endogenous cannabinoid (eCB) system primarily consists of the lipid-derived messengers N-arachidonoyl-ethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG) that exert their cellular actions through two major receptor subtypes, CB1R and CB2R (17, 272). These G protein-coupled receptors are predominantly expressed in the brain and immune system, respectively (727). The CB1R is one of the most highly expressed G protein-coupled receptors in the CNS (485, 486) mainly located at synaptic terminals on PCs and interneurons in the hippocampus (559, 560, 754). In fact, CB1R expression in hippocampal interneurons shows a remarkable selectivity for the CCK-expressing subtypes (559). Anandamide and 2-AG are partial and full CB1R agonists, respectively, with the latter being present at much higher concentrations and therefore is the major known contributor to eCB signaling in the brain (1053). In this section we aim to give a brief introduction to the distinct mechanisms by which eCBs modulate neural circuits with particular focus on those pertaining to the control of interneuron function within the hippocampus and highlight the pathophysiological consequences of dysregulation of eCB system. We also refer readers to a number of excellent and recent reviews regarding this topic (17, 175, 558, 704, 1028).
A. Cellular Mechanisms of eCB/CB1R-Mediated Regulation of CCK Interneurons
One of the most well-described synaptic mechanisms resulting from activation of CB1Rs is termed depolarization-induced suppression of inhibition (DSI) (18, 343, 855, 1212). Depolarization of postsynaptic neurons that express the necessary cellular machinery for eCB synthesis initiates an as yet unknown cellular cascade to produce diacylglycerol lipase-α (DAGLα) in response to increases in [Ca2+]i through VDCCs. DAGLα enzymatically converts DAG to 2-AG that is released in a retrograde manner to activate presynaptic CB1Rs reducing GABAAR-mediated transmission from CCK interneurons by inhibition of presynaptic N-type VDCCs (1077) (for reviews, see Refs. 548, 1028). DSI is regulated by enzymatic degradation of 2-AG via monoacylglycerol lipase (MAGL) expressed in presynaptic terminals and astrocytes (275, 437, 1171). Thus, under most conditions, it is relatively short-lived, lasting on the order of seconds (18, 1212). Activation of glutamatergic afferents and NMDAR-mediated Ca2+ influx is itself sufficient to trigger the eCB signaling cascade even in the absence of postsynaptic VDCC activity (854). However, physiologically DSI is likely triggered through a combination of postsynaptic VDCC and NMDAR activation due to NMDAR-mediated depolarization of the postsynaptic membrane.
Another route of 2-AG generation involves metabotropic receptor signaling coupled to the Gq/11 effector protein, for example, via mGluR5, CCK2R, and M1 mAChR subtypes (558). Sometimes referred to as metabotropic-induced suppression of inhibition (MSI) (704), this alternative pathway, unlike DSI, does not require an increase in postsynaptic [Ca2+]i but instead occurs via PLCβ1 activation to generate DAG, thus initiating the eCB cascade. Although these distinct pathways exist, under many physiological contexts they can act in concert since PLCβ1 function is in itself regulated by [Ca2+]i therefore representing a form of coincidence detection between DSI and MSI to ultimately control CCK interneuron output (470, 472).
Similar eCB-mediated cellular mechanisms, particularly involving activation of group I mGluRs, can also depress GABA release from CCK interneurons over more sustained periods of time and various presynaptic cellular cascades must concomitantly occur for the successful expression of such eCB LTD (199, 200, 729). Many forms of long-lasting synaptic plasticity require de novo protein synthesis for their maintenance (907). In fact, the presence of ribosomal machinery in CCK-containing interneuron boutons provides a rapid and local ability to respond to CB1R activity, independent of transcription, resulting in long-term changes of CCK interneuron function (1253). Interestingly, eCB-LTD of CCK interneuron output effectively primes dendritic regions of the postsynaptic PC allowing for greater excitation-spike coupling and LTP induction of their excitatory inputs (198, 199), and such metaplasticity is critical for temporal memory encoding (1231).
In addition to “on-demand” eCB production, CCK interneuron inhibitory release is also susceptible to a combination of on-going basal eCB synthesis and constitutively active CB1Rs, which both promote tonic inhibition of GABA release (21, 654, 699, 833, 1275). At its extreme, this can be rather powerful resulting in virtual silencing of CCK-interneuron inhibition (699). The balance between synthesis and degradation of 2-AG, for example, which occurs via changes in presynaptic MAGL activity (471, 833, 878, 1055) finetunes this tonic influence. Thus eCB signaling can be triggered by a number of distinct pathways that synergistically interact and via retrograde action ultimately converge onto presynaptic CB1 receptors to impact synaptic transmission of CCK interneurons over a wide variety of timescales.
B. Specificity of CB1 Receptor Regulation Among CCK Interneuron Subtypes
Although the overwhelming majority of CCK interneuron boutons in the hippocampus express CB1Rs (559), the susceptibility to DSI, mGluR-mediated, and tonic eCB regulation of GABA release appears to be more pronounced in CCK-perisomatic subtypes (basket cells) than in their dendritic-targeting counterparts (e.g., SCAs) (654). Furthermore, increases in [Ca2+]i upon CB1R antagonist application is restricted to somatically opposed boutons with no response in their dendritic targeting counterparts, thus extending this differing propensity to the tonic inhibitory influence of eCBs (665). High-resolution imaging of CCK boutons indicates a larger abundance of CB1Rs and the effectors of GABA release (VDCCs and bassoon) at perisomatic- versus dendritic-targeting CCK interneuron boutons (289). Although care must be taken when extrapolating anatomical data to function (665), these differences provide a possible explanation for the dichotomy of CB1R-mediated synaptic modulation between these CCK interneuron populations. Furthermore, distinct mechanisms and sensitivity to CB1R presynaptic modulation at VGlut3-positive versus VGlut3-negative CCKBC inputs to PCs has been postulated as a consequence of differing neurochemical signatures and morphologies of these respective synapses (864). Thus, taken together, this selectivity enables eCBs to control particular aspects of hippocampal network behavior that are sculpted by specific subsets of CCK interneurons.
C. “Noncanonical” Modulation of Interneuron Function by eCB Signaling
Within the population of hippocampal interneurons, the selective expression of CB1Rs in CCK cells indicates a privileged role of eCBs in regulating inhibition mediated by this cell type. The additional presence of CB1Rs at PC synaptic terminals, albeit at lower expression levels, nevertheless renders them susceptible to eCB-mediated depolarization- and metabotropic-induced suppression of excitation (704, 856). Thus network inhibition can also be affected via heterosynaptic CB1R-mediated modulation of excitatory afferents onto a potentially wide variety of interneuron subtypes (495, 948). Interestingly, disruption of cholinergic induced γ oscillations in the CA3 by CB1R activation occurs in part via decreases of excitatory recruitment of putative PVBCs (495). Furthermore, interneurons including CCK-, PV-, and SST-containing subtypes express low levels of DAGLα providing a route by which CB1R-mediated negative-feedback regulation of their excitatory input may be controlled (490, 785, 900).
The reach of eCBs can also circumvent the classic CB1R pathway. Anandamide is also an agonist at the Ca2+-permeable vanilloid receptor of the transient receptor potential family (TRPV) (1117). Although the presence of TRPV1 receptors in hippocampus is controversial (177; but see Refs. 162, 785, 929), TRPV1 and TRPV3 receptor-dependent presynaptic forms of LTD have been described at excitatory inputs onto the majority of s.r. interneurons tested (130, 395). In addition, plasticity of postsynaptic GABAAR expression contributes to anandamide/TRPV1 receptor-mediated changes in the inhibitory influence on PCs (193). In combination, these TRPV1 receptor-mediated plasticity mechanisms serve to disinhibit PCs. As described previously for CB1R eCB-LTD of inhibitory input onto PCs (198, 199), the TRPV receptor-mediated disinhibition also magnifies the predisposition of PCs to undergo LTP of their excitatory inputs, demonstrating similar roles for both 2-AG and anandamide in mediating metaplasticity (93, 130), hence regulating information transfer within the hippocampus. Interestingly, 2-AG is not active at the TRPV1 receptor, and therefore a complex relationship between eCBs and network inhibition likely exists (272). For example, anandamide/TRPV1 receptors activation antagonizes 2-AG/CB1R-mediated tonic reduction of GABA release from CCKBCs (655). Finally, an unknown CB1R/TRPV1 receptor-independent alteration of the excitatory drive onto interneurons has been described (294). Together, these “noncanonical” signaling modes contribute further to the possible cellular pathways mediating eCB regulation of interneuron and hence hippocampal function.
D. Physiological Implications and Abnormal eCB Signaling in Neurological Disorders
During development, CB1R activity regulates proliferation, migration, as well as morpho- and synaptogenesis (95, 96, 723, 815). Indeed, CB1Rs are expressed at early embryonic stages in a CGE progenitor population destined to become CCK interneurons (805, 1165). Of particular societal relevance are the findings that placental transfer of the main psychoactive ingredient of marijuana delta-9 tetrahydrocannabinol (Δ9THC) during perinatal pregnancy results in numerous behavioral deficits in offspring, some of which (e.g., abnormal social behavior) may relate to deficits in hippocampal CCK interneuron function including cell loss and alterations of CB1R-mediated tonic inhibition in the surviving CCK interneurons (1165). The availability of conditional CB1R knockout mouse lines permits specific neural components to be examined (1278). Interestingly, intersex social interactions of mice are decreased or increased following CB1R ablation specifically in glutamatergic versus GABAergic neurons, respectively (13, 1100), thus demonstrating remarkably distinct roles of eCB signaling dependent on cell type.
Again with particular relevance to hippocampal function, cognitive decline including memory impairment represents a well-characterized effect of cannabis use in humans (131). In rodents, administration of Δ9THC or genetic CB1R knockout disrupts hippocampal-dependent tasks requiring various forms of learning and memory (for review, see Ref. 1278). Interestingly, single in vivo exposure to Δ9THC precipitates a reversible decrease in the propensity to initiate eCB-LTD of hippocampal inhibitory transmission (761). Cannabis use in humans and Δ9THC administration in rodents results in a reduction in CB1R expression (289, 491). Furthermore, CB1R knockout specifically in GABAergic interneurons results in deficits in spatial memory acquisition and novel object recognition performance (13). However, conditional knockout of CB1Rs in hippocampal astrocytes that express low levels of this receptor (827) completely abolishes the Δ9THC-induced deficits in spatial working memory, suggesting an indispensible role for eCB signaling in this glial cell population with respect to such hippocampal-dependent behaviors (13, 460). Taken together, it is evident that CB1R-mediated signaling is critical for both the deleterious effects of Δ9THC and the physiological role of eCBs in learning and memory, but further clarification of the cellular players is clearly necessary.
Changes in CB1R expression have also been observed in various neurological disorders. For example, in the pilocarpine model of temporal lobe epilepsy, an initial downregulation of CB1R expression is followed by a long-lasting upregulation, with the latter corresponding to the temporal emergence of seizures (312, 731). The chronic effects are characterized by a permanent redistribution of CB1R expression between hippocampal subfields (311). At the cellular level, febrile-induced seizures precipitate an increase in CB1R expression at CCKBC terminals with no change at excitatory synapses (197). These cell-specific alterations are accompanied by a potentiation of DSI and increased eCB-mediated tonic inhibition of inhibitory output that likely precipitate hyperexcitability of the network (197). Interestingly, brain region and cell-type specific temporally biphasic changes of CB1R expression are observed in humans with epilepsy (413, 707, 731).
In addition to changes in CB1R expression, alterations at other loci within the eCB pathway are also apparent. Fragile X syndrome resulting from FMR1 gene silencing is a major hereditary cause of intellectual retardation and autism precipitated by a plethora of circuit abnormalities (221). In a mouse model of Fragile X, an increased eCB-LTD of inhibitory input onto hippocampal and striatal principal cells is precipitated that is due to altered coupling between mGlu5 and DAGLα leading to increased 2-AG synthesis (724, 1092, 1266). The resulting enhanced disinhibition of PCs amplifies the predisposition to LTP of their excitatory inputs precipitating enhanced excitability (198, 1266), although additional cellular mechanisms independent of the eCB system cannot be discounted (221). Nevertheless, CB1R antagonism effectively normalizes the augmented eCB-LTD of GABA release (424) and can ameliorate memory impairments found in this mouse model (145, 424). In contrast, eCB-LTD of striatal and prefrontal cortical excitatory synapses is absent due to a reduction in mGlu5-mediated 2-AG synthesis (543), illustrating brain region and synapse specific changes. Intriguingly, inhibition of MAGL to increase levels of 2-AG was sufficient to rescue eCB-LTD at these synapses and also reverses deficits in open-field exploratory and elevated plus maze behaviors normally observed in Fragile X mice (543). Thus pharmacological interventions leading to opposing effects on eCB signaling reveals a complex scenario with regards to possible therapeutic intervention to treat the numerous behavioral deficits associated with mouse models of this neurological disorder.
Mice with neuroligin-3 (NLG-3) mutations associated with autism display social deficits and altered spatial learning (305, 529). At a synaptic level these mice possess deficits of tonic but not phasic eCB-mediated inhibition of CCKBC output demonstrating an interaction between NLG-3 and eCB pathways (342). Recently, another protein implicated in autism, Alzheimer’s disease, and Huntington’s disease called p21-activated kinase (PAK) (401, 713) was found to indirectly and negatively control basal anandamide but not 2-AG levels (1227). PAK1 knockout mice show impaired tonic inhibition onto hippocampal PCs due to enhanced presynaptic CB1R activation (1227). Interestingly, in both studies, the changes in tonic eCB signaling are specific to inhibitory synapses with their excitatory counterparts being spared (342, 1227). Further investigation of the interactions between eCBs and other cellular signaling pathways should reveal additional targets for therapeutic intervention. Finally, on a celestial note, a recent study in which rodents exposed to irradiation mimicking cosmic ray exposure of astronauts during space travel reveal a decrease in eCB-mediated tonic inhibition of CCK interneuron transmission in the hippocampus that are not caused by changes in CB1R expression but partly due to lower levels of basal 2-AG (653). Therefore, these changes may underlie the emergence of cognitive deficits that potentially could jeopardize mission performance during prolonged space exploration (882).
IX. ACETYLCHOLINE RECEPTORS
A. Muscarinic Receptors
Basal forebrain cholinergic input to the neocortex and hippocampal formation plays a major role in regulating arousal and sleep wake cycles, attention, and memory formation as well as being a primary regulator of oscillatory activity (473). The hippocampal formation primarily receives its cholinergic input from the medial septum and diagonal band of Broca (MS/DBB) (293). Fibers from the MS/DBB extensively ramify throughout all layers of the hippocampus to target principal cells, inhibitory interneurons, and astrocytes. In the mouse, cholinergic fibers densely innervate the CA1 subfield with the highest density of fibers innervating s.p. and the border between s.r. and s.l.m. The bulk (~90%) of cholinergic axons lack any specialized presynaptic release sites, and it is thought that transmission influences its downstream targets by volume transmission, suggesting that areas of high axonal density will “pool” the highest concentrations of released ACh (1152). ACh acts through both pre- and postsynaptic metabotropic muscarinic (mAChR) and ionotropic nicotinic (nAChR) receptors to regulate both principal cell and inhibitory interneuron activity (640). Early studies highlighted that the increased inhibitory tone onto pyramidal cells following ACh application was due to direct excitation of inhibitory neurons instead of a secondary increased excitatory drive from pyramidal cells (79, 911). Convergence of local interneuron activity and cholinergic modulation of multiple cell types has a complex role to play in the modulation of neocortical and hippocampal principal neuron oscillatory activity that relates to behavioral state (138).
Early studies of mAChR activation on interneurons recognized that application of ACh agonists triggered complex changes in cell excitability that did not always clearly correspond to cell identity. In neocortex, application of cholinergic agonists strongly depolarizes SST-containing Martinotti cells, but not PVBCs, and triggers biphasic responses in CCK-containing interneurons (566). Similar experiments in hippocampal CA1 using exogenous agonists or extracellular stimulation of cholinergic fibers triggers either a membrane hyperpolarization, depolarization, or biphasic responses often accompanied by changes in the AHP/ADP that does not correspond to specific anatomical subtypes of interneurons. These data suggest a complex interplay of muscarinic receptor (s) modulation of temporally overlapping intrinsic conductances in different subpopulations of interneurons (777, 778, 1203).
The advent of numerous mouse interneuron reporter lines coupled with more thorough anatomical recovery and cell identification has however begun to reveal stereotypic responses to mAChR activation in a handful of hippocampal interneuron subtypes. In CA1 hippocampus, in response to muscarinic agonist exposure, SST-containing O-LM cells show an acceleration in firing frequency that is coupled to conversion of the spike AHP to an ADP. This arises primarily via M1/M3 mAChR activation of a nonselective cation current (ICAT) and inhibition of both IM and the slow AHP (IAHP) currents (646). This mAChR modulation tunes the intrinsic oscillatory properties of O-LM cells to increase firing reliability (642, 646).
mAChRs on CA1 CCKBCs and SCAs increase action potential duration and frequency, reduce spike adaptation, and promote conversion of the AHP to an ADP via activation of both M1 and M3 mAChRs (181, 182). In these cells activation of M3 mAChRs controls the spike frequency increase and M1/M3 receptor activation triggers conversion of the AHP to an ADP. CCKBCs typically demonstrate an M1/M3 membrane depolarization, whereas SCA cells undergo a biphasic membrane voltage change driven separately by M1 and M3 receptor activation of GIRK channels and inhibition of IM, respectively (181). Of interest, mAChR activation triggers long-lasting repetitive firing in both cell types in response to synaptic stimulation as well as amplifying 0.5–2.0 Hz subthreshold membrane oscillatory activity facilitating recruitment at theta frequencies.
mAChRs differentially modulate somatodendritic and axonal mAChRs on CA1 hippocampal and prefrontal cortex PVBCs and BiCs (182, 1250). Activation of soma-dendritic M1 mAChRs depolarizes and increases the firing frequency of PV-containing interneurons (182). While CA1 PV-containing interneurons also show a conversion of the AHP to an ADP, PV-containing interneurons in the prefrontal cortex do not (1250). M2 mAChRs are expressed on the presynaptic terminals of PV-containing interneurons (454). IPSCs generated at synapses between PVBCs and cortical pyramidal cells (608), DG granule cells (478), and hippocampal CA3 (1076) and CA1 pyramidal cells (643) are all inhibited by M2 mAChR-mediated reductions in presynaptic Ca2+-transients leading to decreased Pr (643).
All of the above studies relied primarily on agonist application to trigger mAChR. Electrical or optogenetic stimulation of ACh release in mouse interneuron reporter lines have added another perspective to the role played by both mAChR and nAChR modulation of interneurons. Widmer et al. (1203) demonstrated that bulk electrical stimulation of cholinergic fibers in hippocampus triggered complex responses in interneurons that included hyperpolarization, depolarization, or both, with no clean segregation between anatomically distinct subtypes. However, optogenetic studies using channelrhodopsin to trigger release of ACh and archaerhodopsin to suppress excitation of specific interneuron subtypes demonstrated that VIP/CCK-containing perisomatic targeting interneurons depolarize and increase their firing rate on mAChR activation, leading to an increase in sIPSCs onto pyramidal cells (84, 85, 821). In contrast, PVBCs depolarize, hyperpolarize, or produced biphasic responses following optogenetic release of ACh. The extent of the depolarization is smaller in PV-containing interneurons than that observed in VIP-containing CCKBCs, consistent with the observation that PV-containing cells are not the primary target for cholinergic driven increases in PC sIPSCs (84, 85, 821). Of interest, depolarizing responses required greater cholinergic terminal stimulation than did muscarinic mediated hyperpolarization, suggesting that presynaptic firing properties coupled to axon densities and pooled ACh concentrations may be important determinants of the type of cholinergic modulation observed on specific interneuron subtypes (86). While M4-mAChRs were responsible for the hyperpolarizing response via activation of an inward rectifying K+ conductance, the depolarizing response was resistant to block of M1, M4, and M5 receptors consistent with a role for M3 mAChRs in generating the membrane depolarization as shown previously in the agonist experiments described above (86).
B. Nicotinic Receptors
Like mAChRs, ionotropic nAChRs are differentially expressed across inhibitory interneuron populations. Although 11 nicotinic receptor subunits have been identified (1062), only α7 and α4β2 receptors have been identified on inhibitory interneurons. Early studies in the neocortex demonstrated that nAChR agonists strongly depolarize and excite CCK- and VIP-containing interneurons, while only weakly exciting PV-containing cells (565, 921). In hippocampus, PVBCs are similarly resistant to nicotinic receptor agonists. α7-Containing receptors are enriched in interneurons of the s.r., including CCK-containing interneurons (22, 542, 779). It has been suggested that nAChR expression is a hallmark feature of cortical interneurons derived from the CGE (651). In the s.o., both MGE- and CGE-derived SST-containing O-LM cells express both α7 and non-α 7 receptors (139, 779), suggesting that at least some MGE-derived interneurons also express nAChRs (203, 1138). Electrical stimulation of cholinergic axons generates rapid kinetic synaptic events onto CA1 interneurons that is thought to arise primarily via activation of α7 receptors (22, 347). However, subsequent studies indicate that the pharmacological profile of the responses indicates α4β2 containing receptors rather than α7-containing (322, 921). Consistent with this observation, nicotine-sensitive neocortical CCK-containing interneurons express transcripts for α4/5 and β2 but only low levels of α7. In contrast, hippocampal CCK-containing interneurons primarily express α7 mRNA transcripts (801), indicating potential cell type and regional heterogeneity in receptor expression profiles. A recent study using optogenetic techniques to release ACh has challenged this observation and suggests that α4β2 nicotinic receptors are the primary target for acetylcholine release onto hippocampal CCK- and VIP-containing interneurons leaving the role of α7 nicotinic receptors in question (83, 84). Synaptic events triggered by light-activated ACh release have significantly slower kinetics compared with the presumed α7 events evoked by extracellular electrical stimulation and were small and subthreshold in nature. Whether this reflects a technical issue related to the use of optogenetics (which does not trigger neurotransmitter release in a manner identical to electrical stimulation) remains to be determined. Interneuron nAChR activation by optogenically released ACh is modulated by presynaptic M2 mAChRs, suggesting a dynamic interplay between mAChRs and nAChRs controls ACh regulation of inhibitory interneurons. A recent study has added a new dimension to our understanding of cholinergic modulation of inhibitory transmission. Pabst et al. (875) demonstrated that optogenetic release of ACh from septohippocampal projections triggers an increase in GABAergic tone onto DG granule cells. However, released ACh does not excite inhibitory interneurons directly but instead activates hilar astrocyte intermediaries, which subsequently release glutamate onto downstream hilar interneuron targets to drive an increase in inhibition. It is unclear if this mechanism is shared across other hippocampal and cortical subfields, but if so it would act to expand the repertoire of modulatory pathways available to cholinergic pathways in the mammalian CNS.
nAChRs also modulate transmitter release directly from inhibitory interneuron terminals (725). Activation of presynaptic nAChRs on perisomatic basket cells, presumably PV-containing interneurons, triggers a robust action potential independent release of GABA via α3/β4 nAChRs coupled to T-type Ca2+-channels and Ca2+-induced Ca2+ release from intracellular stores.
X. DOPAMINE RECEPTORS
Subcortical dopaminergic axons from the ventral tegmental area ramify widely throughout the hippocampal formation (1169), and a role for dopamine in hippocampal novel memory formation and synaptic plasticity is well established (684). However, much of what we know concerning dopamine receptor distribution in the hippocampus and cortex is biased towards studies of principal cell expression of D1 and D2 receptors. In contrast, only a handful of studies have tackled the distribution of dopamine receptors on specific inhibitory interneuron subpopulations.
Although a number of autoradiographic and mRNA studies have indicated dopamine receptor subtype expression in “scattered” cells outside of the pyramidal cell layers (741), a general lack of reliable antibodies for dopamine receptor subtypes has hampered elucidation of their expression patterns. With the use of a D1R-eGFP mouse line, D1 receptor expression has been shown in DG presumed molecular layer PPA cells, as well as presumed PVBCs and AACs in the hilus (380). Within the CA1 and CA3 hippocampus proper, the vast majority of D1R-expressing interneurons reside in s.r., with lower numbers in s.p., s.o., and s.l.m. Although the exact identities of these interneurons are unknown, many are CR- or CB-positive, but PV-negative (380). Although a similarly labeled D2R-eGFP mouse line failed to reveal D2 receptor expression in inhibitory neurons (380), Drd2-Cre:riboTag and Drd2-Cre:RCE mouse lines show multiple cell populations expressing D2R in the CA1 and CA3 subfields. Combined immunohistochemistry reveals D2R-expressing interneurons primarily localized to s.o. and s.l.m. comprised of PV-, CB-, CR-, NPY-, SST- and nNOS/reelin, coupTFII-containing cell types, indicating widespread expression of D2Rs throughout diverse interneuron populations (930).
The D4 receptor is of particular interest because of its suggested role in schizophrenia and high affinity for the antipsychotic drug clozapine. D4Rs are expressed at extremely low levels throughout the hippocampus, with in situ hybridization and immunohistochemistry approaches revealing D4R expression in a small number of presumed interneurons (36, 44, 262). Within the CA1 hippocampus, ~25% of PV-containing interneurons are D4R-immunopositive and ~50% of D4R-expressing interneurons are positive for the neuregulin ErbB4 (36). Triple immunofluorescence indicates that D4R- and ErbB4-immunoreactivity overlap within a subset of PV-containing interneurons and converge to positively modulate KR-induced gamma oscillations (36). D4Rs are also enriched in interneurons within the prefrontal cortex (813). D4R activation of PFC layer I-V interneurons in a GAD-GFP transgenic mouse line (862) reduces excitatory transmission onto these cells via a mechanism involving calcineurin-dependent AMPAR trafficking (1261). One can imagine that a D4R-mediated reduction in the excitatory drive of as yet unidentified PFC interneurons would result in an imbalance in the excitation:inhibition dynamics within circuits essential for executive function.
XI. SEROTONIN RECEPTORS
Subcortical serotonergic innervation of the hippocampal formation originates from the median raphe nucleus, with a somewhat minor projection coming from the dorsal raphe. These two projections are thought to give rise to two distinct afferent fiber types (601, 677): one with large boutons that cluster along dendritic branches and a second with thin axonal projections with numerous small evenly distributed varicosities, respectively. The projection from the dorsal raphe is thought to release serotonin at nonsynaptic sites to target cells expressing the metabotropic G protein-coupled 5-HT1 and 5-HT2 receptor subtypes, whereas the medial raphe nucleus afferent pathway innervates specific interneuron subtypes expressing the ionotropic 5-HT3A receptors (352). Studies using a 5-HT3AR-eGFP mouse line demonstrated that neocortical and hippocampal interneurons derived from the CGE, but not the MGE, express 5-HT3Rs (651, 1179). 5-HT3AR expression is observed in early embryonic stages ~E14.5 and persists throughout the animals lifespan making it an excellent marker for cells of CGE origin. Indeed, Rudy and colleagues (651, 1134) have gone so far as to use this 5-HT3AR expression as a defining feature for classification schemes of the CGE-derived group of neocortical interneurons. It is important to point out, however, that the CGE-derived cohort of neocortical and hippocampal interneurons comprises a broad and functionally diverse collection of interneurons types, including VIP-, CCK-, CR-, reelin-, NPY-, and in hippocampus SST-containing cell types (see sect. III).
In vivo recordings revealed that stimulation of medial raphe fibers elicits a rapid and robust modulation of ongoing hippocampal activity (841). Early studies in vitro showed complex responses to exogenously applied serotonin, suggesting that multiple overlapping receptor subtypes may contribute to the observed response. Recordings from CA1 pyramidal cells showed an increased frequency and amplitude of unitary IPSPs, which primarily resulted from a 5-HT3AR-mediated depolarization of select but unidentified s.r. interneurons (954). Indeed, subsequent voltage-clamp recordings from s.r. interneurons revealed a rapid inward current triggered by serotonin application that was G protein independent (773). The current-voltage relationship of this current possessed a region of negative slope conductance reminiscent of that seen at NMDARs, that was insensitive to extracellular Mg2+ concentrations (in contrast to NMDAR) but linearized when extracellular Ca2+ was reduced. More recently, optogenetic activation of median raphe fibers was shown to rapidly recruit hippocampal interneurons via glutamate/serotonin cotransmission through AMPARs and 5-HT33Rs, respectively, with high spatial and temporal resolution (1164). These data suggest that unlike other subcortical inputs into the hippocampus and neocortex, the median raphe serotonin projections can trigger a rapid modulation of ongoing activity via ionotropic receptors.
As described in section IIF, a subset of CGE-derived SST-containing O-LM interneurons in CA1 express 5-HT3ARs (203). The presence of 5-HT3ARs on these cells endows them with a divergent role from their MGE-derived counterparts in kainate-induced gamma frequency oscillatory activity in vitro (203). CGE-derived O-LM cells exhibit a mean firing frequency probability per gamma cycle of ~0.033, which is significantly lower than that observed in their MGE-derived counterparts (mean firing probability per cycle is ~0.16). Of particular interest, 5HT3AR activation of CGE-derived O-LM cells doubles their firing probability during each gamma cycle without altering their phase preference (203). These data suggest that MGE- and CGE-derived O-LM cells are differentially recruited during hippocampal gamma oscillations in acute slices and that participation of CGE-derived O-LM interneurons in synchronized hippocampal network activity can be rapidly modified by serotonergic tone. This observation is consistent with a report that 5-HT3AR antagonism with ondansetron reduces the recruitment of some hippocampal interneurons in network oscillations recorded in freely moving rats (942).
Much less is known about the expression patterns of metabotropic serotonin receptors on specific interneuron populations. Exogenous application of serotonin hyperpolarizes unidentified hippocampal CA1 interneurons and reduces evoked fast and slow IPSPs onto CA1 pyramidal cells via 5-HT1ARs (989). Evoked EPSPs onto interneurons of both the CA1 hippocampus and layer I and II entorhinal cortex are similarly reduced by activation of 5-HT1ARs, suggesting that the reduced inhibition onto principal cells results in part from reduced excitatory drive onto interneurons (988, 989). Paired recordings of EPSPs between CA1 pyramidal neurons and O-LM cells are similarly inhibited by activation of presynaptic 5-HT receptors (110). GABAB-mediated slow IPSPs (but not GABAA-mediated IPSPs) onto CA3 pyramidal cells are also reduced by exogenous serotonin application via a presynaptic modulation of inhibitory transmitter release (860).
With the use of an Htr2A-eGFP mouse line, 5-HT2ARs were observed to be highly enriched in interneurons localized primarily to the CA1 s.r. and s.l.m. border, with a few additional cells observed in the s.o. of CA1 and CA3 (1226). Activation of pharmacologically isolated 5-HT2ARs generates a robust inward current and depolarization of CA1 s.r. interneurons (1226), leading to an increased inhibitory drive onto principal cells (1003). The identities of 5-HT2AR expressing interneurons from which the recordings were made are somewhat unclear, but anatomical reconstructions of these cells are consistent with PPA, CCKBC, and SCA morphologies. In situ hybridization studies have also shown 5-HT1A, 5-HT2A, 5-HT2C receptor mRNA expression in prefrontal cortex interneurons including PV-containing cells (975). 5-HT5BRs, like 5-HT2ARs, are most commonly observed in interneurons at the s.r. and s.l.m. border (1000). Anatomical reconstruction of these cells suggests that they are most likely NGFCs or IvCs, capable of showing persistent firing properties (1000, 1001). In situ hybridization analysis of the recently described metabotropic 5-HT6R revealed expression in ~15% of hippocampal and layer I/II neocortical interneurons. Of these, ~50% of 5-HT3AR-positive interneurons expressed 5-HT6R, while negligible levels were observed in PV- and SST-containing interneurons (479).
XII. OPIOID RECEPTORS
The three opioid receptors mu-, delta- and kappa- belong to the superfamily of G protein-coupled receptors and together with the endogenous opioid peptides play a major role in nociception, learning and memory, anxiety, as well as neuroendocrine and autonomic function.
A. Mu-Opioid Receptors
Modulation of hippocampal network activity by opioid peptides has been long recognized (228). Early studies in hippocampal slices in vitro demonstrated that enkephalin generally excites the hippocampal network (836) by a mechanism involving a reduction in inhibitory drive (835, 1276). Using blind sharp microelectrode recordings from CA1 inhibitory interneurons, Madison and Nicoll (728) demonstrated that the enkephalin analog, d-Ala-Met-enkephalinamide, hyperpolarized interneurons by increasing a potassium conductance which led to a disinhibition of both pyramidal cells and other interneurons. Subsequent studies demonstrated that mu-opioid receptor activation decreased presynaptic GABA release onto CA3 pyramidal cells by a G protein-mediated mechanism (166). This reduction in GABAergic tone acts to increase network excitability resulting in increased seizure susceptibility and lowered threshold for synaptic plasticity (124, 776, 806).
Mu-opioid receptors are expressed on a small number of discrete inhibitory interneuron subtypes, which include PV-containing and NPY-containing cells (285, 1060) and appear to be absent in CB1R-expressing CCK-containing interneurons (833). Accordingly, electrophysiological studies revealed that mu-opioid receptor activation by the exogenous agonist DAMGO hyperpolarizes the membrane potential and reduces inhibitory transmission between PVBCs and their downstream pyramidal cell targets (1070). The mechanism of mu-receptor modulation of synaptic transmission is consistent with a reduction in presynaptic transmitter release probability (403, 1070). In contrast, unitary connections between CB1R expressing CCK-containing interneurons and CA1 pyramids were largely unaffected (403). Evoked IPSCs onto PVBCs and CCKBCs are also modulated by mu-opioid receptor activation, with transmission onto the former being suppressed to a larger extent (decreased by ~60% compared with ~30%, respectively) (403). Consistent with this observation the feedforward component of inhibition is strongly modulated by mu-opioid receptor activation, whereas the feedback component largely driven by CCK-containing interneurons is largely resistant.
Mu-opioid receptor modulation of presynaptic transmitter release has also been observed at synapses responsible for the GABABR-mediated inhibitory input onto pyramidal cells. These synapses are dendrite targeting and use N-type Ca2+ channels for transmitter release but are insensitive to eCB modulation, implicating IvCs and NGFCs (628, 775). Indeed, direct recordings from IvCs and NGFCs revealed sensitivity to mu-opioid receptor modulation (606). Similar to PVBCs, paired recordings between synaptically coupled IvCs and CA1 pyramidal cells revealed mu-opioid receptor modulation of presynaptic transmitter release (606). Of particular interest, induction of persistent firing (see sect. IVM for discussion) in IvCs is inhibited by mu-opioid receptor activation (606), and although the exact mechanism of action is unclear, it may be linked to general mu-opioid receptor-mediated hyperpolarization of gap junction connected neurogliaform family cells. Mu-opioid receptor mRNA has also been detected in SST-containing HIPP cells in the dentate gyrus as well as a small number of CR-containing interneurons in the granule cell layer (284). However, the physiological roles played by mu-opioid receptors on these cell types have not been explored.
Ovarian sex steroid hormones modulate mu-opioid receptor expression in select interneuron populations. Elevated levels of estrogens in proestrus females increase mu-opioid receptor trafficking and expression in dentate gyrus PVBCs (1115). Thus the magnitude of mu-opioid receptor modulation of inhibitory networks is likely tuned by fluctuations in steroid hormones.
B. Delta-Opioid Receptors
Delta-opioid receptors play important roles in anxiety, depression, control of emotional responses, and spatial memory (218, 329, 950). Immunohistochemistry has shown highest delta receptor expression in NPY-containing and a subset of SST-containing interneurons (218). Using a knock-in mouse expressing a functional delta-opioid receptor fused at its carboxy terminal with eGFP mouse, the highest GFP signal was found in the GABAergic axonal fibers terminating throughout the pyramidal cell layer, suggesting presynaptic expression of delta receptors; however, colocalization interneuron neurochemical markers was not studied (941).
Despite the findings in delta-opioid GFP mice, delta receptor expression is generally accepted to be highest on dendrite projecting interneuron subtypes and appears to be complementary to mu-opioid receptor expression (1070). Early electrophysiological studies showed that mu-opioid receptor activation attenuated electrically evoked IPSCs in principal cells, whereas delta-opioid receptor agonists had no effect (711, 1193). Consistent with these early observations, electrophysiological recordings combined with anatomical reconstruction demonstrated that dendrite targeting, BiCs and O-LMs, are hyperpolarized by agonists selective for delta receptors but are largely insensitive to mu-receptor agonists (1070). Thus, rather than regulating perisomatic inhibition and action potential timing in principal cells, delta-opioid receptors are positioned to modulate feedback dendritic inhibitory input to principal cells. Moreover, given the role that O-LM cells play in modulating the influence of temporoammonic inputs onto the distal dendrites of CA1 pyramidal cells (720), it is possible that delta-receptor-mediated inhibition of these cells would act as a gate to minimize their influence on temporoammonic inputs, thus strengthening enthorinal cortex input into the CA1 hippocampus. Like mu-opioid receptors, delta-opioid receptor availability is modulated by ovarian hormones. In NPY-containing interneurons, ovarian hormones present in normal cycling females alter the number of soma containing delta-opioid receptor immunoreactivity in CA1 and CA3 s.o., without influencing the number of axon terminals containing delta-opioid receptors in CA1 s.r. (1211).
C. Kappa-Opioid Receptors
Significantly less is known about kappa-opioid receptor expression and modulation of inhibitory interneurons. Kappa-opioid receptor immunoreactivity has been observed on subsets of both NPY- and SST-containing interneuron populations (456, 931), reminiscent of the same cell populations expressing delta-opioid receptors (1070). The functional role(s) of kappa-opioid receptors has to date been completely unexplored in specific interneuron populations. However, by analogy to observations for delta-opioid receptors, inhibition of SST- and NPY-containing interneurons by kappa-opioid receptor activation to regulate dendritic inhibition seems plausible.
XIII. OXYTOCIN RECEPTORS
The neurohypophysial peptide oxytocin is released from axon terminals of the hypothalamo-extrahypophysial pathway to exert a diverse array of actions throughout the central nervous system (140, 141). Light microscopic autoradiography and in situ hybridization approaches have shown high-affinity oxytocin receptors in hippocampus and subiculum that appear to be targeted to nonprincipal cells, presumably inhibitory interneurons (357, 1136). Early physiological recordings indicated that exogenously applied oxytocin hyperpolarized the membrane potential and increased spontaneous inhibitory input to pyramidal cells while directly exciting unidentified nonpyramidal cells in the CA1 subfield (814). Application of the oxytocin receptor agonist [Thr4, Gly7]-oxytocin (TGOT) was also shown to excite s.p. interneurons, as well as a subset of s.o. cells but failed to excite s.r. interneurons (1263), indicating cellular heterogeneity in oxytocin receptor expression. More recently, a study by Tsien and colleagues (871) demonstrated that TGOT directly increases the firing frequency of anatomically identified CA1 PVBCs. Most remarkably, while this increase in PVBC activity suppresses spontaneous pyramidal cell firing, it also enhances spike fidelity and sharpens spike timing for evoked inputs, thus dramatically improving signal-to-noise in the network. The authors intelligently asked how could this paradoxical increase in basket cell activity and resulting inhibitory tone result in a higher fidelity of principal cell action potential throughput and timing? Using paired recordings they then demonstrated that PVBC unitary IPSCs were reduced by oxytocin receptor activation when the PVBC was allowed to depolarize in response to TGOT, thus shifting the excitation:inhibition balance onto pyramidal cells in the favor of excitation (871). As discussed in section VII, transmission between PVBCs and their downstream targets undergoes profound short-term depression in response to repetitive activation. Owen et al. (871) demonstrated that the TGOT-induced increase in basket cell firing causes a use-dependent depression of the IPSC that is both necessary and sufficient for the enhancement of excitation-spike coupling in pyramidal cells. Finally, the authors demonstrated the generality of this phenomenon by using two other unrelated agents, CCK and channelrhodopsin, to drive increases in fast spiking interneuron action potential activity. Importantly, many studies have demonstrated that particular agents or modulators increase the firing rates of particular subtypes of interneuron and simplistically concluded that such recruitment will ultimately result in an increased inhibitory output that is presumed to silence or reduce the excitability of the particular downstream targets. The study by Owen et al. (871) brings into sharp focus that one has to also consider the downstream temporal dynamics of the resulting inhibition to fully appreciate the outcome of increasing the excitability of a particular interneuron type.
In addition to direct excitation of interneurons, oxytocin appears to play an important role in influencing GABAergic synaptic transmission in fetal neurons during delivery (579). As discussed in section III, GABA has a depolarizing and excitatory action during development and early postnatal life. This depolarizing action of GABA relates to developmental changes in the two cation-chloride cotransporters Na+-K+-Cl− cotransporter (NKCC1) and K+/Cl− cotransporter (KCC2). In early development, the high expression of NKCC1 and low expression of KCC2 ensure that cytoplasmic chloride concentrations are high and that under normal conditions, activation of synaptic GABA receptors will trigger a Cl− efflux and a membrane depolarization (92). Tyzio et al. (1147) observed that while GABA is indeed depolarizing during fetal and early postnatal life, there is a window of ~48–72 h around the period of term (E20-P0) when the proportion of cells excited by GABA decrease. This transient perinatal loss of GABAergic excitation results from an oxytocin-dependent negative shift in the Cl− reversal potential via a mechanism involving inhibition of NKCC1 cotransporter activity (579, 1147). These observations suggest that one of the roles for the massive release of oxytocin at term and during delivery may be a form of neuroprotection by limiting neural network activity during perinatal hypoxia (579).
XIV. CHOLECYSTOKININ
Cholecystokinin is one of the most abundant peptides in the mammalian central nervous system and is implicated in a broad range of central functions including feeding/satiety, anxiety, nociception, and learning and memory. Despite such broad participation, surprisingly little is known, beyond the most basic information, about the precise mechanisms and circuit functions of CCK. As described in section II, CCK-containing inhibitory neurons represent a large subpopulation of CGE-derived inhibitory neurons, whose GABAergic synaptic mechanisms have been well studied. However, like most neuropeptide-containing central neurons, it has been extraordinarily difficult to ascertain the mechanism(s) underlying CCK release from these cells and more importantly what CCK does once liberated. In the CNS, the CCK peptide exists primarily as the sulfated 8-amino acid CCK8S, which binds primarily to G protein-coupled CCK2 (or CCKB) receptors, although a few central areas also express the CCK1 receptor (657). Application of exogenous CCK8S to hippocampal neurons in vitro was originally shown to increase action potential-dependent sIPSCs onto CA1 pyramidal cells and DG granule cells (269, 341, 555, 792). This increase in sIPSCs was blocked by prior application of agatoxin-TK, a blocker of P/Q Ca2+ channels, on perisomatic targeting PVBC terminals. Subsequent studies demonstrated a direct CCK-mediated excitation of PVBCs (but not BiCs) via activation of the CCK2 receptors (341). CCK2 receptor activation on PVBCs triggers a pertussis toxin-sensitive pathway coupled to ryanodine receptor-mediated intracellular Ca2+ release (658). This stands in marked contrast to the canonical Gq-PLC pathway typically associated with CCK2 receptor activation. In paired recordings between PVBCs and CA1 pyramidal cells, CCK had no effect on the unitary IPSP (cf. Ref. 871), this discrepancy is most likely due to the holding of the PVBC at a fixed holding potential in the study of (341). In contrast, in paired recordings between CCKBCs and CA1 pyramidal neurons, exogenous CCK significantly reduced the unitary IPSP by altering presynaptic transmitter release (341). This presynaptic reduction in GABA release was not a consequence of activation of presynaptic CCK receptors but rather by activation of postsynaptic CCK2 receptors on pyramidal cells, which then triggers PLC/DAG lipase-mediated eCB formation and liberation, that results in the activation of a canonical retrograde suppression of GABA release from CCK interneurons via presynaptic CB1R activation (see sect. VIII).
Perhaps the most surprising aspect of this data set is the narrowness of the cellular targets for CCK influence: primarily perisomatic PV- and CB1R-positive, CCK-containing interneurons. Such an arrangement will favor simultaneous excitation of PVBCs but inhibition of release from CCKBCs. Thus CCK acts as a gate to switch between the two modes of perisomatic inhibition to favor tasks that require precision and timing such as oscillatory activity (47, 657). As described above, in addition to targeting perisomatic regions, CCK-containing interneurons also target the proximal and distal dendrites of principal cells as well as targeting other interneurons, suggesting a complex role for CCK in sculpting network dynamics. It is worthwhile to point out that most studies of neuropeptide modulation of central neurons, including CCK, rely primarily on exogenous application of CCK, which will target numerous sites simultaneously, perhaps masking the subtleties of CCK-dependent modulation of circuit function. However, it has proven difficult to determine the conditions necessary to trigger endogenous CCK release, which coupled to local degradation mechanisms make it problematic to study intrinsic release of neuromodulators.
XV. INTERNEURONS AND NETWORK FUNCTION
Information processing in neuronal networks is critically dependent on the precise synchronization of ensembles of neurons. Interneurons play a key role in coordinating network activity both within local networks and across the relatively long distances that separate different brain regions. The synchronous firing of neurons gives rise to neuronal oscillations, characterized by waves of electrical activity that can be observed in extracellular field recordings or electroencephalograms (EEGs). The first EEGs were reported by Hans Berger in 1929 (94), where he described oscillations below 12 Hz as alpha waves, and those above 12 Hz as beta waves. The convention of naming neuronal oscillations by their frequency band is still followed today and are categorized by their frequency band, such as slow oscillations (<1 Hz), theta oscillations (4–10 Hz), or gamma oscillations (30–90 Hz) (152). The exact frequency bands reported can vary between species; for example, theta oscillations occur at 6–12 Hz in rodents, but slow down to 4–6 Hz in carnivores, and occur at 1–4 Hz in humans (for review, see Ref. 154). Interestingly, recent work found that 7–9 Hz theta oscillations do indeed occur in human hippocampus during real-world navigation, with the slower observed 3- to 4-Hz theta oscillation likely being an experimental artifact from having humans navigating in a virtual reality environment (109). However, while the exact frequency range may vary between species, each oscillation band consistently correlates with different behavioral states and, by controlling the timing of principal cell output, inhibitory interneurons are key to generating these rhythms of the brain (FIGURE 21).
FIGURE 21.
Entrainment of oscillations in CA1. CA1 pyramidal cells are important for generating theta oscillations in CA1, although fast-spiking PV basket cells play an important role in entraining CA1 network oscillations at theta frequencies. Slow gamma oscillations are driven by inputs from CA3 and, as such, show peak amplitude in stratum radiatum. The slow CA3 gamma oscillation recruits CA1 fast-spiking PV basket cells, which in turn drive slow gamma rhythms in CA1 pyramidal cells and other interneurons. Fast gamma oscillations are driven by inputs arriving from the medial entorhinal cortex and appear to drive an as yet unidentified group of interneurons that, in turn, entrain the local network to this faster rhythm. Remarkably, sharp wave-ripples can be driven by a single fast-spiking PV basket cell.
A. Gamma Oscillations
Gamma oscillations are associated with numerous cognitive functions such as memory and spatial navigation (e.g., Refs. 67, 302, 1189) and correlate with working memory load in humans (500). While gamma oscillations occur in all cortical regions (152), many of the mechanistic insights into their genesis come from hippocampal studies. Early in vivo studies of nonanesthetised rats revealed that gamma oscillations (40–100 Hz) occurred in all hippocampal subfields, correlated with theta oscillations, and had the highest power in the dentate gyrus (123). Interestingly, this study found that bilateral lesions of the entorhinal cortex attenuated or ablated the oscillation in the dentate gyrus but revealed a slower (25–50 Hz), larger amplitude gamma oscillation occurring in the CA3 to CA1 network (123). This study demonstrated that there are two hippocampal gamma oscillators, one in the dentate gyrus and one in the CA3-CA1 network, with the former requiring input from extrahippocampal regions. A follow-up study of the intrahippocampal oscillator found that gamma oscillations were generated within the recurrent network of CA3 by interactions between pyramidal cells and interneurons, and that gamma oscillations in CA1 were entrained by CA3 pyramidal cells driving the activity of CA1 interneurons (244).
1. Interneurons and gamma oscillations in CA3
In vivo experiments demonstrated the essential role of interneurons in driving intrahippocampal gamma oscillations, but the precise cellular mechanisms generating the oscillation can be better dissected using in vitro slice preparations. This intrahippocampal gamma oscillation can also be evoked in hippocampal slices through bath application of the cholinomimetic carbachol, where it occurs at a peak frequency of 40 Hz and appears to be generated in CA3 (138). Carbachol-evoked oscillations are remarkably stable over time (occurring on the order of hours), and persistent gamma oscillations can also be observed through bath application of kainate, both in CA1 (1129) and CA3 (332). Brief epochs of gamma oscillations can also be evoked in vitro by electrical stimulation (1200) or by local application of kainate to s.r. in both CA3 (291, 408) or CA1 (238).
The earliest studies of hippocampal gamma oscillations revealed that inhibitory neurotransmission was essential for their generation. Electrical stimulation of CA1 afferents revealed that gamma oscillations were associated with rhythmic barrages of IPSCs and could be blocked by bicuculline (1200). Persistent carbachol-evoked gamma oscillations observed in vitro require both GABAA and non-NMDA ionotropic glutamate receptors for their genesis (333). In vivo recordings show that pyramidal cells do not fire on every gamma cycle (123, 244, 997), with in vitro slice experiments suggesting that an individual pyramidal cell fires during ~5% of gamma cycles (333). Further in vitro slice studies of persistent gamma oscillations in CA3 confirmed this and reported that PVBCs were strongly phase-locked to the oscillation (453). This study also found that interneuron-selective interneurons in CA3 were strongly phase-locked with the oscillation; morphological reconstructions of these ISIs revealed cells with dendrites restricted to s.o. and the axon targeting both s.o. and s.r (453). Another in vitro study of kainate-evoked gamma oscillations in CA3 confirmed that individual pyramidal cells fired infrequently while PVBCs and BiCs were both strongly phase-locked to the oscillation (408). Subsequent in vitro slice studies strongly indicated that fast-spiking, perisomatic targeting interneurons were essential for generating gamma rhythms in CA3 (740, 865, 866). Two landmark studies using optogenetic control of PV-expressing interneurons confirmed that silencing PV interneurons impaired gamma oscillations, while driving the cells was sufficient to evoke a gamma-frequency oscillation in vivo in various cortical regions (169, 1027).
2. Other hippocampal gamma oscillators
Although the term gamma oscillation is given to any oscillation occurring between 30 and 90 Hz, it is becoming clear that this broad term encompasses several distinct network rhythms (154, 216). Gamma oscillations are also apparent in CA1 and can be further parsed by peak frequency into two bands [slow (~40 Hz) and fast gamma (~90 Hz) (215)] or into three bands [slow (30–50 Hz), mid-frequency (50–90 Hz), and fast/epsilon (90–150 Hz) (87)]. Slow gamma in CA1 is coherent with CA3 gamma (215) and is strongest in s.r. (87), indicating that it is driven by CA3, while mid-frequency (90 Hz) gamma is coherent with entorhinal gamma (215) and is strongest in s.l.m. (87), indicating that it is driven by the entorhinal cortex. Gamma oscillations generated in CA3 are critically dependent on the interaction between pyramidal cells and PVBCs, as are gamma oscillations in a number of other cortical regions (154), but is this mechanism common to all forms of gamma oscillation?
Theoretically, gamma oscillations can be generated purely through networks of coupled interneurons (interneuron network gamma oscillations, or the ING model) or through reciprocally coupled networks of pyramidal cells and interneurons (pyramidal-interneuron network gamma oscillations, or the PING model) (1201). PING-type oscillations require phasic synaptic excitatory input onto interneurons, while ING-type oscillations can be observed in sparsely connected interneuron networks with just tonic excitatory drive (1132, 1200). In vitro studies of CA3-generated persistent gamma oscillations (332, 333, 408, 1129) show that this gamma rhythm is of the PING type, as is the mid-frequency gamma oscillation observed in isolated slices of medial entorhinal cortex (mEC) (246). It should be noted that, unlike carbachol-induced gamma (333), the kainate-evoked persistent gamma oscillation persists when AMPARs are blocked (332), showing that both kainate and AMPARs can provide sufficient excitation to generate a gamma rhythm. In CA1, the slow CA3-driven gamma oscillations can be observed using in vitro slices (910). When CA1 is isolated from the rest of the hippocampal network, another gamma oscillation emerges, which is ~10 Hz faster and can be evoked using either carbachol (910) or kainate (786). The carbachol-evoked intrinsic gamma oscillation in CA1 appears also to be a PING-type oscillation with peak pyramidal cell firing slightly preceding peak interneuron firing (910), although no interneuron subtype-specific data were reported in this study.
Craig and colleagues (238) conducted an in vitro survey of the behavior of CA1 interneurons during gamma oscillations, using local application of kainate to evoke brief epochs of gamma oscillation without chronic application of drugs. This experimental paradigm produces a mid-frequency gamma oscillation intrinsically generated in CA1, which is 11 Hz faster but of a lower power than CA3 gamma and, unlike CA3, could still be evoked during optogenetic inhibition of pyramidal cell firing (238), consistent with an ING-type gamma. PVBCs displayed strong, phase-locked firing in CA3, but had a much lower firing frequency in CA1, and exhibited no phase preference. In CA1, they found that MGE-derived BiCs and AACs were strongly phase-locked to the oscillation, as were CGE-derived putative trilaminar cells and back-projecting interneurons (238). Unlike other forms of gamma oscillation studied in vitro, they found that PVBCs were not essential for kainate-evoked CA1 gamma. Similar results can be seen in CA1 in vivo, where PVBCs drive gamma oscillations around s.p. but not in distal s.r. or s.l.m. (637). Indeed, in vivo recordings show that CA1 PVBCs are only weakly modulated by gamma oscillations (1142). In mEC, PVBCs modulate slow NMDAR-dependent, but not mid-frequency NMDAR-independent gamma oscillations (786). Mid-frequency ING-like gamma oscillations can be evoked using local application of extracellular potassium in DG slices, and fast gamma oscillations can be evoked in DG even in the absence of synaptic transmission (1124). Together, these studies reinforce the observation that several qualitatively different gamma oscillations coexist (154) and that PVBCs are often, but not always, a key driver of gamma rhythms. Interestingly, in vivo recordings show that CA1 dendrite-targeting BiCs show the strongest depth of gamma modulation (1142). The preferred in vivo gamma firing phase of identified CA1 interneurons is summarized in FIGURE 22H.
FIGURE 22.
Firing of different interneurons during network oscillations. A: reconstruction of a CA1 PVBC recorded juxtacellularly in vivo from an awake rat. B: firing rate of CA1 PV basket cells during different network oscillations. C: reconstruction of a CA1 ivy cell recorded juxtacellularly in vivo from an awake rat. D: firing rate of CA1 ivy cells during different network oscillations. Example traces showing spiking activity of PV basket cell (E) and ivy cell (F) during sharp wave-ripple oscillations. G: preferred firing phase of CA1 interneurons during the theta cycle. Projecting interneurons are not shown, but most tend to fire around the trough of the theta cycle (537). H: preferred firing phase of CA1 interneurons during the gamma cycle. O-LM cells do not significantly phase-lock to the gamma cycle. Note that while all cell types shown have a phase preference, the depth of gamma modulation varies significantly between cell types, with the firing rate of bistratified cells being most strongly modulated by gamma (1141). [A–F adapted from Lapray et al. (635) with permission from Nature Neuroscience.]
While gamma oscillations are strongly correlated with numerous cognitive tasks such as attention, working memory, and conceptual categorization (302), some authors argue that gamma oscillations serve no functional role beyond homeostatic matching of inhibition in response to increased excitation (784). However, the consensus opinion in the field is that gamma oscillations are essential for neural computation and that they provide a temporal window that allows functionally coupled neurons or ensembles to interact both locally and across long distances (302, 360, 361, 1018). Regardless of whether gamma oscillations are essential to cognition or epiphenomenal, deficits in gamma oscillations correlate with deficits in interneuron and circuit function seen in many pathological conditions (670, 1151). Thus gamma oscillations provide a useful assay of network function that can be carried out in vitro, which can reliably predict deficits in behavioral tasks.
B. Sharp Wave-Ripple Oscillations
Hippocampal sharp wave-ripples (SWRs) are irregular, synchronous bursts found in the hippocampus, which occur as slow sharp waves (0.1–3 Hz, most prominent in s.r.) and associated ripples (~200 Hz). SWRs occur most frequently during slow-wave sleep, but can also be observed during periods of immobility in freely moving animals (149, 848). Early work found that SWRs were associated with population bursts of pyramidal cells along with increased firing of interneurons and dentate granule cells (149). The first in vivo studies supported the notion that SWRs generally inititate from a population burst of CA3 pyramidal cells, which then triggers a subsequent burst in CA1 (243); it seems that individual ripples do not propagate to CA1 but that a burst of activity in CA3 provides sufficient excitation to locally generate a ripple in CA1 (118, 1064). A recent study confirmed that SWRs occur in CA3 before “spreading” to CA1, but found that pyramidal cells in CA2 became active before synchronous firing in CA3, stongly implying that CA2 is the site of SWR generation in vivo, especially during awake states (861). In CA1, neurons that fire together during exploratory behavior also coactivate in SWRs occurring during periods of slow-wave sleep following the behavioral task (851), suggesting that SWRs during sleep represent a reactivation, and thus consolidation, of waking memories (850). Blocking SWR generation in CA3 reduces the incidence of SWRs seen in CA1 and impairs consolidation of memory (823).
Early studies of SWRs revealed that interneurons were active during this oscillation (149), but the first interneuron subtype-specific information came from elegant in vivo juxtacellular recordings in anesthetized rats spearheaded by Peter Somogyi, Thomas Klausberger, and colleagues. Of the dendrite targeting interneurons, O-LM cells (582), and CCK-expressing PPA interneurons (584) are silent while PV/SOM-expressing BiCs (583) fire during SWRs. Of the perisomatic-targeting interneurons, AACs are silent during SWRs (582) while PVBCs are very active (582, 584); CCKBCs do not display a homogeneous firing pattern during SWRs, with some becoming more active and others become less (584). These in vivo experiments in anesthetized animals reveal that pyramidal cells, PVBCs, and BiCs are the most active during SWRs (586). Later work in unanesthetized animals (FIGURE 22, A–F) confirmed that PVBCs are very active during SWRs (635, 1161) but additionaly that O-LM cells do fire during SWRs in awake states (1161). IvCs do not seem to participate in SWRs (366, 1081).
While in vivo juxtacellular recordings provide the gold standard for observing interneuron behavior in network activity, in vitro models provide a complementary system that allows manipulation of neural circuitry. Like gamma oscillations, SWRs can be observed in vitro in CA3 in interface conditions (609), or in submerged conditions when adequately oxygenated (450). SWRs can originate from any CA3 subfield, although they are most likely to begin in CA3b (301). In CA3, generation of SWRs depends on both AMPA and GABAA receptors, with only a small number of pyramidal cells firing during an individual SWR (301, 451). PVBCs greatly increase their firing during SWRs (451) and, remarkably, stimulating a single PVBC is sufficient to generate a SWR in vitro (301). Of the other interneuron types in CA3, IvCs are silent but most other interneuron types fire, with no overall significant difference in firing rate between perisomatic targeting interneurons (PVBCs, AACs, and CCKBCs) and dendritic targeting cells (O-LM cells, s.o.-s.o. cells, s.o.-s.r. cells, and s.r. cells) (451). During SWRs, the predominant synaptic input to pyramidal cells is inhibitory while the dominant input to all firing interneurons is excitatory (451). Perisomatic inhibition via PVBCs is one of the main sources of the large extracellular field signal seen in s.r. during SWRs (451).
For SWRs generated in CA1, pyramidal cell activity is also essential both in vitro (56) and in vivo (1050). The firing of CA1 perisomatic targeting interneurons is phase-locked to SWRs in vitro (56), and local GABAA receptor-mediated inhibition is critical for SWR generation. Optogenetic activation of pyramidal cells can induce a SWR in vivo, and while activation of PV-containing interneurons does not generate a ripple, they can pace the rate of ensemble firing (1050). Interestingly, while O-LM cells are silent during SWRs in CA3 (582), they are recruited into CA1 SWRs in vivo (879), so it seems that similar, but not identical, mechanisms drive the generation of SWRs in CA3 and CA1.
C. Slow Oscillations
In the hippocampus, SWRs are the prominent oscillation associated with slow-wave sleep and memory consolidation. In cortical areas, including the entorhinal cortex, the dominant network rhythm during slow-wave sleep is the slow oscillation. During the slow oscillation, cortical neurons display synchronous bursts of depolarization (Up states) punctuated by periods of relative quiescence (Down states) and oscillate between up and down states at frequencies of <1 Hz (1054). Cortical up and down states modulate SWR activity during slow-wave sleep (73, 445, 532, 1009, 1021), and the slow oscillation is ubiquitous to all cortical regions. Up states can initiate in any layer in the cortex, likely due to spontaneous synaptic events (192), and although slow oscillations can be generated within the cortex alone (1103), the thalamus is also important for their generation in vivo (242). As might be expected for an oscillation associated with sleep, in vivo activation of cholinergic neurons in the medial septum strongly reduces the power of neocortical slow oscillations (1158).
Many mechanistic insights into the cellular circuitry that generates up and down states have come from in vitro slice experiments, where the oscillation can be observed using “in vivo-like” artificial cerebrospinal fluid containing low concentrations of calcium and magnesium (974). Up states are sustained through a balance of recurrent fast ionotropic excitatory and inhibitory neurotransmission, where inhibitory conductances dynamically scale to match excitation, both in vitro (219, 1007) and in vivo (446). Slow GABABR-mediated inhibition is also important for terminating up states in vitro (738), with postsynaptic GABABRs needed for afferent-evoked termination of up states (236); although this mechanism has yet to be confirmed in vivo, a number of converging lines of evidence suggest that it should exist (237).
During up states, principal cells receive strong inhibitory conductances in ferret prefrontal cortex (1007), rat mEC (738), and mouse barrel cortex (831). PVBCs have the highest firing rate of any interneuron subtype during up states in both barrel cortex and mEC and receive more excitatory than inhibitory conductances (831). In barrel cortex, SST-expressing interneurons are moderately active during up states in barrel cortex (315, 831), where they regulate the excitability of pyramidal cells (830), but fire only sparsely in mEC (831, 1080). Similarly, VIP-expressing cells are active during up states in the barrel cortex (831), although they do not affect pyramidal cell firing (830). Unlike in the barrel cortex, VIP-expressing interneurons are almost completely silent during mEC up states, while NPY-expressing interneurons are rarely active during cortical up states in either mEC or barrel cortex (831, 1080). The role of ionotropic glutamate receptors in sustaining up states is also divergent between barrel cortex and mEC, with NMDAR important for the former, while kainate receptors are important in the latter (273). While the slow oscillation is common to all cortical regions, these studies advise caution when trying to make generalizations about interneuron function during network states. Given the unusual circuitry of the entorhinal cortex when compared with other cortical regions (163), it is perhaps not surprising that interneuron behavior during network activity is different.
In vivo recordings show that, in PFC, pyramidal cells and PVBCs fire rapidly at the beginning of up states, while AACs fire more than 200 ms after their onset, at around half the frequency of basket cells (760). Similarly, PV-containing interneurons are also very active during up states in both barrel (392) and visual (915) cortices, with SST cells also participating in slow oscillations in vivo (915). Earlier in vivo studies also reported that PVBCs are very active during up states in ferret prefrontal cortex (446). Deleting GAD67 from PV-containing interneurons leads to shorter up states with more multiunit firing, while deleting GAD67 from SST-expressing interneurons has the opposite effect (615), supporting in vitro observations that these two groups of interneurons are likely important for controlling excitation during up states.
D. Theta Oscillations
The hippocampal theta oscillation (4–10 Hz) is apparent during voluntary movements and REM sleep, where the oscillation modulates the timing of action potentials (848, 936, 1159). Gamma oscillations are often nested within theta oscillations in vivo, such that the amplitude of the gamma oscillation varies in phase with the theta rhythm (153). Indeed, optogenetic stimulation of CA1 pyramidal cells at theta frequencies is sufficient to evoke gamma rhythms in vitro (146). In vivo, theta oscillations are most regular and have the highest amplitude in CA1 s.l.m., require glutamatergic input from the entorhinal cortex and cholinergic input from the medial septum, and can also be generated in CA3 (reviewed in Ref. 150). However, in vitro experiments using isolated whole hippocampus preparations show that CA1 can intrinsically generate spontaneous theta oscillations without the application of drugs (429). Surprisingly, the same group also found that the subiculum can generate theta oscillations that “reverse propagate” through CA1 to entrain network rhythms in CA3 (526).
Excitatory pyramidal cells make an important contribution to generating theta oscillations in vivo, with both recurrent connectivity and intrinsic membrane oscillations playing a role (150). However, interneurons also appear to be critical (153), and a single PVBC can phase-lock the firing of CA1 pyramidal cells at theta frequencies (210). BiCs and PVBCs are both phase-locked to CA1 theta oscillations, as are AACs and O-LM cells (582, 583). IvCs fire sparsely during theta oscillations but are phase-locked to the trough (366), and modulate their firing rate in response to the frequency of the theta oscillation (635). CCK-expressing interneurons, although representing a morphologically diverse group of neurons, seem to have a relatively homogeneous, but similarly phase-locked, firing behavior during theta oscillations in CA1 (584, 1142). However, in CA3, CCK-expressing interneurons display divergent behaviors with CCKBCs- and dendrite targeting-cells firing at the peak, and PPA interneurons firing at the trough, of the CA1 theta oscillation (638). Inhibitory input onto PV-containing interneurons is essential for the coupling of gamma and theta oscillations in CA1; deleting GABAA receptors from PV-containing interneurons also greatly reduces the amplitude of CA1 theta oscillations but has no effect on gamma oscillations (1222). More direct evidence for PV-containing interneuron involvement in the generation of theta oscillations come from optogenetic experiments, which show that PV-containing interneurons can entrain hippocampal networks more effectively than pyramidal cells to resonate at theta frequencies in vivo (1049). Inhibiting PV-containing interneurons strongly impairs intrinsically generated CA1 theta oscillations in whole hippocampal preparations in vitro, while inhibiting SST interneurons has only a modest effect (31). The preferred in vivo theta firing phase of identified CA1 interneurons is summarized in FIGURE 22G.
While we tend to think of transmission through the hippocampus as being a unidirectional flow of excitatory projections, the subiculum-generated theta oscillation can propagate “backward” through the hippocampus via inhibitory mechanisms, with optogenetic activation of subicular PV-containing interneurons sufficient to drive network activity in CA3 (526). This result shouldn’t be too surprising, given that in vivo labeling studies describe interneurons in CA1 that project to both CA3 and the subiculum, and which show firing phase-locked with the theta rhythm and SWRs (536, 1014). At least some of these back-projecting interneurons are CGE-derived and also participate in network rhythms in vitro (238). The spike timing of pyramidal cell firing relative to the phase of the theta rhythm is believed to encode spatially-relevant information: as an animal progresses through the place field for a particular pyramidal cell, the cell will fire at progressively earlier points in the theta cycle, a phenomenon that is termed “phase precession” (849). This was initially assumed to be a feature unique to pyramidal cells, but later studies demonstrated that interneurons in CA1 also display phase-precession (297), implying that interneurons also have an important role in information processing.
So far, we have considered the role of interneurons in shaping neuronal oscillations, which is a key aspect of how interneurons behave and influence network function. We will now broaden our focus to consider how interneurons regulate the development of neural circuits, and how different interneuron types can influence network function in the context of behavior.
E. Interneuron Broad Subtypes and Behavior
The advent of optogenetic (266) and pharmacogenetic (1153) tools, combined with interneuron-specific Cre driver lines (1096), means that it is now possible to study the effects of selectively manipulating identified groups of interneurons in vivo on circuit function and behavior. For in vivo experiments, interneurons are often parsed into three groups that, in the cortex at least, are nonoverlapping: PV containing, SST containing, or VIP/5-HT3AR expressing (966, 1237). In the hippocampus, this grouping does not quite hold, as some interneurons express both SST and 5HT3a receptors (e.g., subset of O-LM cells) and some interneurons express both PV- and SST (e.g., BiCs) (74, 203, 1138). Two recent reviews discuss how these broad interneuron subtypes alter their activity during behavior and how neuromodulators affect their activity in vivo (1197), and discuss the function of interneurons in intact circuits (956). Instead of recapitulating much of the content of these reviews, we will briefly discuss the consequences of manipulating interneuron activity during hippocampus-dependent behavioral tasks.
Global reductions in excitatory input onto PV-containing interneurons impair performance in cognitive functions dependent on the hippocampus such as spatial working memory (365, 886) without affecting reference memory (365). Directly inhibiting synaptic release from PV-containing interneurons locally in CA1 confirms that PV-containing interneurons in this region are essential for spatial working memory but do not contribute to reference memory (818). Recent evidence shows that hippocampal PVBCs can be further subdivided by their birth date during embryogenesis, with each subtype having different roles in learning: early-born PVBCs contribute to learning associations while late-born PVBCs contribute to acquiring new knowledge (279). PV-containing interneurons are also important for maintaining memory, as mice with enhanced PV-containing interneuron function show reduced extinction of fear memory in behavioral tasks, as well as increased frequency of SWRs in hippocampal slices (158). However, PV-containing interneurons in CA1 are not required for memory acquisition in contextual fear conditioning (CFC) tasks, which instead is dependent on SST-expressing interneurons in s.o. that target s.l.m. (702). During CFC acquisition, these SST interneurons (presumably O-LM cells) become activated by cholinergic inputs from the medial septum and seem to block nonsalient sensory information arriving from glutamatergic entorhinal projections onto the distal dendrites of CA1 pyramidal cells (702). The entorhinal cortex also sends inhibitory projections to the hippocampus (781), and inhibitory projections from lateral entorhinal cortex (LEC) are important for efficient learning of context in CFC and novel object recognition tasks, where they inhibit the activity of CA1 s.r. and s.l.m. CCK-expressing interneurons to increase excitation of pyramidal cells by disinhibition (69). Interestingly, LEC inhibitory projections to CA1 were not required for either hippocampus-dependent learning task, but blocking their activity caused overgeneralization of contextual cues (69). Additionally, SST-expressing interneurons in the dentate gyrus control which granule cells become incorporated into the ensemble that encodes a memory trace in CFC, while PV-containing interneurons are not involved in this process (1051).
In addition to memory, one of the other main functions attributed to the hippocampus is spatial navigation (847). Pyramidal cells are well-known to be place cells, so are most associated with the encoding of spatially-relevant information, but emerging evidence suggests that interneurons also have an important function in navigation (reviewed in Refs. 239, 407). Interneurons also show discrete place fields (1207), which can be inverted relative to pyramidal cells (462), implying that the interneurons are not just driven by principal cells but can actively shape place fields through disinhibitory mechanisms. The latter study did not unequivocally identify the interneurons involved, but their firing properties and theta phase preference implied that they were perisomatic-targeting interneurons (462). Loss of gap junctions between interneurons is sufficient to interfere with short-term spatial memory and the relative firing of place cells relative to the theta oscillation (24). Optogenetic silencing shows that PV-containing interneurons control the spike timing of place cells relative to the theta oscillation and that SST interneurons control the firing rate of place cells (958), implying different functions in the coding of spatial information. Glutamatergic projections from the medial septum to interneurons located in the alveus/s.o. are also important for entraining place cell firing to the theta oscillation during locomotion (368). Interestingly, evidence suggests that while interneurons are important for controlling principal cell firing within their place field, inhibitory interneurons appear not to affect the overall spatial scale of place fields (513, 958), with evidence also suggesting that PV-containing interneurons do not contribute to grid formation in mEC (134).
Hippocampal interneurons play an important role in controlling network rhythms and in cognitive processes such as memory and spatial navigation, especially MGE-derived interneurons such as those expressing PV and SST. Indeed, it is hard not to assume that PVBCs do all the heavy lifting in terms of network function, given how much research focuses on them. While these perisomatic targeting interneurons are clearly essential to many processes, questions remain over what role the other subtypes play in network function, especially CGE-derived interneurons. Identifying how CCK-expressing interneurons modulate behavior will be particularly challenging using genetic targeting approaches, as interneurons from this broad subtype display remarkably diverse firing patterns in vivo (FIGURE 23). Future experiments, combined with more specific genetic tools to improve subtype-specific selectivity, should help address these questions.
FIGURE 23.
Firing of CA3 CCK-positive interneurons during network oscillations. The advent of optogenetic tools and interneuron-specific Cre driver lines has led to a wealth of data on how different interneuron “subtypes” can influence behavior. However, these data, taken from juxtacellular recordings of CCK-expressing CA3 interneurons, show that cells expressing the same neuropeptides or Ca2+-binding proteins can behave very differently during rhythmic activity. A: firing pattern of a CCK-expressing basket cell relative to theta oscillations in CA1. Note that the cell tended to fire at the peak of the theta oscillation. B: juxtacellular recordings from other CCK-expressing interneurons in CA3 show that different cell types display remarkably divergent behaviors during both theta and sharp wave-ripple oscillations. [Adapted from Lasztoczi et al. (638) with permission from the Society for Neuroscience.]
XVI. INTERNEURONS AND NEURAL CIRCUIT DISORDERS
Considering the critical role that interneurons have in controlling neuronal network function, it should not come as a surprise that dysfunctional inhibitory neurotransmission has been implicated in numerous neurological and psychiatric disorders. From neurodegenerative conditions like Alzheimer’s disease through to psychiatric disorders such as schizophrenia, disruptions in the function, or a specific loss of, inhibitory interneurons appears to underlie at least some of the cognitive impairments associated with these conditions. In this section, we will discuss pathologies affecting interneurons by parsing disorders into three broad groups: developmental disorders (such as autism), neurological disorders (such as epilepsy, stroke, or dementia), or psychiatric disorders (such as major depression). However, it should be borne in mind that some disorders resist such a simple categorization. The most obvious example is schizophrenia, which is a complex psychiatric disorder that clearly has a developmental component, but where environmental factors also contribute to its etiology.
A. Developmental Disorders of Interneuron Circuits
One of the most sensitive periods for the developing brain is during embryogenesis and early postnatal development, when perturbations during phases of rapid neuronal proliferation and migration can have profound consequences for the developing brain. Glutamatergic neurons undergo radial migration during development, and disruption of this process has been associated with a number of human conditions such as lissencephaly and periventricular heterotopia (402). Similarly, disruptions to interneuron development can cause severe congenital brain injury. In addition to functioning as a neurotransmitter, GABA is also an important neurotrophic signaling molecule in the developing brain, acting through both inotropic GABAA and metabotropic GABABRs (373), so disruptions in GABAergic signaling can severely affect brain development. This is highlighted by conditions such as fetal alcohol syndrome, where prenatal exposure to alcohol can cause abnormal gross morphology of the brain and intellectual disability (953); animal models show that early exposure to ethanol disrupts the tangential migration of MGE-derived interneurons by increasing GABA concentrations in the embryonic brain (248), and can cause shrinkage of the dendrites of PV-containing interneurons (259). Antiepileptic drugs (AEDs) are also known to be teratogens that increase the likelihood of neurological problems, with valproate being one of the AEDs most likely to cause fetal anticonvulsant syndrome (895). Animal models suggest that AEDs that increase concentrations of GABA in the developing brain are more likely to cause neurological defects through the mechanisms of impaired migration and cell death (737). Numerous other genes and trophic factors can affect interneuron migration (see sect. III). One such gene is X-linked aristaless homeobox gene (ARX), mutations in which cause several neurological syndromes in humans (685). While ARX is most associated with migration of glutamatergic neurons, it also controls interneuron migration, and interneuron-specific deletion of ARX causes a developmental epilepsy phenotype in mice resembling that observed in heterozygous human carriers of ARX mutations (753).
1. Autism and related disorders
The term Autism Spectrum Disorder (ASD) is given to a wide range of neurodevelopmental disorders of complex or unknown etiology, but which share a number of common features including an onset in the early developmental period, impaired social interaction and communication, and stereotyped or repetitive behaviors; these features can vary in the degree of severity (693). Fragile X syndrome and Rett syndrome both cause autistic phenotypes, but they are often not considered ASDs (1). Many of those with Fragile X syndrome who display autistic-like phenotypes do not meet the DSM criteria for ASD (133), and Rett syndrome was removed from the ASD category in DSM-V (1182). The pathophysiology of ASD is believed to involve perturbations in synaptogenesis and synaptic pruning during critical developmental periods, which disrupt the assembly and function of neural circuits and lead to alterations in excitatory-inhibitory balance; many of those with ASD exhibit seizures and abnormal EEG activity (120, 962). Genome-wide association studies have found that ASD has a strong genetic link (520), with recent work suggesting that a large number of ASD cases are caused by de novo mutations in genes that are highly expressed in the brain during embryogenesis (521). Several genes acting on the mTOR pathway, such as NF1, Tsc1/Tsc2, and PTEN, have been implicated in ASD, as have mutations in the NRXN-NLGN-SHANK pathway (120, 962). The brains of those with ASD tend to be larger than normal, and although they frequently have more neurons, the dendritic arbors of those neurons are often less complex (reviewed in Ref. 693).
Animal models carrying mutations or deletions of genes implicated in ASD have provided valuable insights into circuit-level disruptions in ASD. For example, deleting Tsc1 from CA1 pyramidal cells increases mTOR activity and causes a reduction in inhibitory input, leading to hyperexcitability in the network (70). Similarly, selectively deleting Tsc1 from MGE interneurons also increases excitability in hippocampal networks, due to both a loss of interneurons and disrupted migration during development (362). EphA7, a receptor tyrosine kinase, is essential for stabilizing inhibitory synapses from PVBCs onto dentate granule cells, through mTOR-dependent mechanisms; knocking down EphA7 activity in adults is sufficient to impair learning (101). Mutations in EphA7 and EphA3 have been linked to ASD in humans, and mice lacking the ligands for these receptors also display behavioral deficits consistent with ASD (1223). Mutations in the SHANK family of genes are also strongly associated with ASD (534). Shank1 is highly expressed in PV-containing interneurons and regulates their excitatory input; mice lacking Shank1 show reduced basal firing of hippocampal PV-containing interneurons and an excitatory shift in excitation/inhibition balance (742). Recent evidence suggests that Shank1 mice have an altered excitation/inhibition balance because of a loss of the PV protein itself, as opposed to a loss of neurons (327). Mice lacking CNTNAP2, another gene associated with ASD, show hyperexcitability, impaired neuron migration, and a loss of NPY-, PV-, and CR-expressing interneurons, along with behavioral deficits including impaired social interactions, stereotypic movements, behavioral inflexibility, and hyperactivity (892). Hippocampal slices from mice lacking CNTNAP2 show normal excitatory neurotransmission; however, CA1 pyramidal cells show deficits in perisomatic, but not dendritic, evoked IPSCs (544).
Both Fragile X syndrome and Rett syndrome are associated with impaired inhibitory function, with each caused by a single mutant gene (FMR1 and MECP2, respectively), which leads to circuit dysfunction due to impaired excitation/inhibition balance (387). Given that both syndromes are caused by a mutation in a single gene, they can be reliably modeled using transgenic mice. Most of those with Fragile X syndrome show a degree of developmental delay or intellectual disability, particularly in short-term memory and executive function (385, 458), and up to 60% of Fragile X syndrome cases show some autistic features (133). Mouse models show that loss of FMR1 leads to reductions in tonic but not phasic GABAergic inhibition onto pyramidal cells, implying a loss of extrasynaptic GABA receptors (247). FMR1 knockout mice also show reduced excitatory input onto fast-spiking interneurons but increased inhibitory input onto SST-expressing interneurons (396). The net effect at the network level is hyperexcitability, which is apparent through prolonged Up states that show impaired synchrony of IPSCs (396). Studies on Drosophila show that the protein encoded by FMR1 differently regulates intracellular calcium signaling in interneurons and excitatory cells during a critical developmental period that is essential for synaptogenesis and achieving the appropriate excitation/inhibition balance (278). As may be expected with a disrupted excitation/inhibition balance, those with Fragile X syndrome have an increased risk of epilepsy (385).
Rett sydrome is caused by mutations in the X-linked gene MECP2 that cause a loss of function in the protein MeCP2 and is characterized by normal development up until 18 mo of age, followed by a gradual loss of language and motor skills, along with the development of microcephaly, autistic features, seizures, and ataxia (32). Mouse models of Rett syndrome show that cortical networks display altered excitation/inhibition balance, with reduced excitation in both cortical (254) and hippocampal (1267) networks. Principal cells in MECP2 knockout mice do not show a reduction in spontaneous inhibitory inputs (254), but electrical stimulation in CA3 reveals a hyperexcitable phenotype as it evokes spontaneous sharp-wave like events in knockout, but not wild-type, mice (1267). The onset of hippocampal hyperexcitability in MECP2 knockout mice occurs in early adulthood and correlates with the onset of Rett Syndrome symptoms (156). Hyperexcitability suggests that interneuron-specific deficits may occur in Rett syndrome. This was confirmed by the finding that selectively deleting MECP2 from inhibitory interneurons recapitulates many features of Rett syndrome including impaired motor function and autism-like behaviors, as well as reduced GABA release and hyperexcitability in slices (190). PVBCs in CA3 of MECP2 knockout mice show reduced excitatory input, which points towards a failure in recruitment of inhibition being the cause of hyperexcitability in Rett syndrome (157). Compared with glutamatergic cells and SST-expressing interneurons, MeCP2 is highly expressed in cortical PV- and calretinin-containing interneurons (1105), and biochemical fractionation experiments show that MeCP2 is found in the postsynaptic domain (3). MeCP2 is phosphorylated in neurons in an activity-dependent manner, and this phosphorylation regulates dendritic growth and spine maturation (1274). These lines of evidence support the hypothesis that the cognitive deficits in Rett syndrome are, at least in part, caused by a failure in maturation of excitatory synapses onto inhibitory interneurons, particularly PVBCs. This is supported by recent evidence showing that deleting MECP2 from PV-containing interneurons is sufficient to induce the same circuit-level deficits in visual cortex that occur in a global MECP2 knockout mouse (59).
In summary, while ASD and disorders such as Fragile X syndrome and Rett syndrome each have different underlying genetic causes, it seems that these multiple developmental pathways converge upon similar mechanisms of disrupted inhibitory interneuron function. Studying the commonalities between these disorders may lead to greater understanding both of the pathological changes occurring in inhibitory circuits and how these circuits relate to behavior in healthy, physiological conditions.
2. Schizophrenia
Schizophrenia is a complex psychiatric disorder or spectrum of disorders that generally has an onset in early adulthood and is associated with psychosis and hallucinations (positive symptoms), as well as loss of motivation, social withdrawal, cognitive impairments (negative symptoms), and affective dysregulation (where the patient experiences manic and depressive episodes). Although schizophrenia has a very high heritability (80%), the disorder does not have a single genetic cause, but rather appears to require further environmental factors to precipitate the illness in those with a genetic predisposition (1157). Numerous genes have been proposed to have a strong role in causing schizophrenia, including DISC1 (791), Neuregulin1 (1052), and COMT (295), but no significant linkage of historical schizophrenia candidate genes has emerged from large genome-wide association studies (318). The genetics of schizophrenia remain highly contentious. Recent insights from rare but highly penetrant mutations suggest that changes in levels of gene expression or gene dose, as opposed to mutations in individual genes, can have a causal role in schizophrenia. Variations in the copy number of genes (CNVs) are widespread in normal genomes and can be caused by de novo microdeletions or duplications of small chromosomal regions. It is becoming clear that multiple CNVs can confer a very high risk for disorders such as schizophrenia or autism (735). For example, up to one-third of those carrying microdeletions at the 22q11.2 region develop schizophrenia or schizoaffective disorder (reviewed in Ref. 551). CNVs at both the 22q11.2 and 16p11.2 loci confer a very high risk of both ASD and schizophrenia (286, 496, 551, 1078), which strongly implies that common developmental mechanisms underlie the pathology in at least some forms of these disorders. For example, the regulation of interneuron migration by Cxcr4 is disrupted in 22q11.2 deletion syndrome (780), and this gene is also involved in the correct targeting of thalamocortical axons (2), so this provides at least one mechanism that could underlie the etiology of both ASD and schizophrenia. While the causes of schizophrenia remain controversial, a wealth of evidence points towards deficits in neural circuits and interneuron function as being key factors in the pathophysiology of the disorder (670, 683, 745).
Reductions in CB levels in schizophrenic post-mortem brains have been reported for prefrontal cortex (PFC) (77), anterior cingulate cortex (ACC) (232), and planum temporale (187). Postmortem studies of PV expression in schizophrenic patients are more controversial, with studies showing either decreases (77) or no change (1216) for PFC, no changes being reported in ACC (232), but with large decreases in PV levels reported for the hippocampus (1272) (FIGURE 24, A–D). These differences could be due to differences in methodology or medication of patients, or may suggest that schizophrenia-related changes to interneurons are restricted to specific circuits. Interestingly, rearing rats in social isolation from weaning is sufficient to elicit behavioral changes reminiscent of schizophrenia and leads to reductions in hippocampal levels of PV and CB (468), raising questions about the causal relationship between neurochemical changes and behavioral symptoms in schizophrenia. Although the results of post-mortem studies vary in the detail of interneuron types affected, there are clear overall deficits in inhibitory interneurons: one of the most consistent findings in post-mortem brains of schizophrenics is a reduction in prefrontal expression of GAD67 (but not GAD65), as well as lower levels of CB and reelin (reviewed in Ref. 588). Functional studies of schizophrenic patients also point to clear deficits in circuits involving interneurons, with decreases in gamma oscillation power and synchrony reported in many studies of schizophrenia, including those carried out in unmedicated patients and nonschizophrenic first-order relatives (reviewed in Ref. 1209).
FIGURE 24.
Parvalbumin-containing interneurons in schizophrenia. One of the most consistent findings from postmortem studies of patients with psychiatric disease is a reduced density of PV interneurons in cases of schizophrenia and, to lesser extent, bipolar disorder. PV-expressing neurons from human prefrontal cortex (A) and hippocampal region CA1 (controls) (B). PV cell density is significantly reduced in prefrontal cortex (C) and hippocampus in human psychiatric disease (D). Deleting the NMDAR subunit NR1 from GAD67-expressing interneurons (including PV interneurons) early in development causes a resistance to MK801-induced hyperlocomotion (E) and a reduction in prepulse inhibition (PPI) (F), implying that reduced NMDAR function in these cells is a cause of schizophrenia-like behaviors. However, deleting NR1 from the same neurons in adulthood fails to cause the same behavioral phenotypes (G), and PV interneuron-specific deletions of NR1 fail to cause deficits in PPI (blue: controls, red: KOs) (H). Mice with PV interneuron-specific deletions of NR1 show lower levels of hyperlocomotion than controls when dosed with MK801 at both 0.2 mg/kg (I) and 0.5 mg/kg (J), but this appears to be due to these animals displaying a greater sensitivity to MK801 and spending a large amount of time in a cataleptic state. These data suggest that NMDAR hypofunction in PV-containing interneurons is not an underlying factor in schizophrenia-like behavioral deficits, but that loss of functional NMDAR in PV-containing interneurons may actually be a risk factor instead of a cause of schizophrenia, by making neural circuits more susceptible to impaired NMDAR function in other types of neuron. [A and C adapted from Beasley et al. (77), with permission from Biological Psychiatry. B and D adapted from Zhang and Reynolds (1272), with permission from Schizophrenia Research. E– G from Belforte et al. (82), with permission from Nature Neuroscience. H–K from Bygrave et al. (155), with permission from Translational Psychiatry.]
A key outstanding unknown in the pathophysiology of schizophrenia is the relationship between the observed changes in interneuron numbers to and dysfunction in neural circuits. At the circuit level, schizophrenic patients consistently show deficits in gamma oscillations (reviewed in Refs. 683, 745), consistent with deficits in PV-containing interneuron function. Furthermore, numerous studies report that schizophrenic patients have impaired synchrony between the hippocampus and prefrontal cortex, both at rest and during working memory tasks, and the degree of impairment correlates with the severity of some symptoms (reviewed in Ref. 412). Hyperactivity in the dopaminergic system was initially believed to be an underlying causal factor in schizophrenia as drugs that activate this system mimic positive symptoms of schizophrenia, but more recent theories suggest that impaired glutamatergic neurotransmission (NMDA hypofunction model) is causative because drugs such as phencyclidine, which are NMDAR antagonists, reproduce both positive and negative symptoms in humans (683).
Disruption of NMDAR function specifically in PV-containing interneurons has been suggested as a causal factor in schizophrenia. Deleting GluN1 from a mixed population of neurons (around 70% of which express PV) early in development causes schizophrenia-related behaviors, including hyperactivity and impaired social memory, spatial working memory, and prepulse inhibition (82). However, this model also deleted GluN1 from NPY-expressing interneurons and a small number of pyramidal cells, and deficits were only observed if the deletion occurred early in development, as opposed to in adult mice (82) (FIGURE 24, E–G). Another study where GluN1 was deleted specifically from PV-containing interneurons also found impairments in neural synchrony and spatial working memory (599). However, additional studies report that mice lacking NMDARs in PV-containing cells show only modest impairments (170) or are indistinguishable from controls (103, 155, 979) in tests of working memory and other behaviors relevant to schizophrenia. One of the main arguments in favor of a PV-containing interneuron-specific deficit in NMDAR function is the finding that, unlike wild-type controls, mice lacking NMDARs in these interneurons do not show increased hyperlocomotion when exposed to the NMDAR antagonist MK801 (82, 170). However, more recent work has shown that mice with PV-specific deletions of GluN1 are actually more sensitive to the effects of MK801, compared with wild-type control. MK801 induces stereotyped behaviors and catalepsy in these mice (FIGURE 24, H–J), which confounds experimental measures of hyperlocomotive behavior, as well as impairments in working memory and large amplitudes in delta oscillations in PFC (155). This later study presents evidence against the hypothesis that a PV-containing interneuron-specific dysfunction of NMDARs underlies schizophrenia-related behavioral deficits, but does support the model that NMDARs on other neurons are important to the pathophysiology. Indeed, PV-containing interneurons show very small NMDA currents relative to other interneuron types (763). The studies showing more modest or no behavioral deficits used the PV promoter to drive deletion of NMDARs (103, 155, 170, 979), so NMDARs would function in these neurons during early postnatal development, while the report showing the strongest effect was when NMDARs were deleted from multiple cell types early in development (82). Together, these data suggest that dysfunction in NMDAR signaling during development is more likely than a mature PV-containing interneuron specific deficit in NMDAR function to cause schizophrenia-related behavioral deficits.
B. Neurological Disorders Involving Interneuron Function
In addition to developmental disorders such as ASD, the pathophysiology of myriad neurological conditions, from epilepsy to Alzheimer’s disease, involves dysfunction of interneuron function. Indeed, even diseases not commonly considered neurological diseases can affect interneuron function. For example, human immunodeficiency virus (HIV) is most commonly associated with the immune system, but some patients also develop HIV-associated neurocognitive disorders (41, 767). This syndrome is associated with hippocampal pathology, and expression of an HIV protein, HIV-1 Tat, in mice causes impaired cognitive function and a selective loss of several MGE-derived interneurons, including those expressing PV, SST, and nNOS (750). In addition to HIV, infection by several other neurotropic viruses, as well as the associated inflammatory immune response, can lead to neurological pathology (708); herpes simplex virus 1 preferentially targets the hippocampus, and infection increases hippocampal excitability and the risk of seizures (1220). Maternal infection with influenza or other viruses during pregnancy is believed to increase the risk of schizophrenia in offspring (111), with mouse models showing similar deficits in schizophrenia-related behaviors and reduced expression of mGluR2 and 5-HT2A receptors (804).
1. Epilepsy
Epilepsy is a broad term applied to neurological conditions in which the patient suffers from a recurrence of epileptic seizures (334), which can vary from almost undetectable brief absence seizures to long tonic-clonic seizures, but always involve abnormal rhythmic firing of large ensembles of neurons. Epilepsy can be caused by acute traumas such as head injury or stroke, or can occur due to genetic mutations and/or as a comorbidity in other neurodevelopmental disorders (188). While AEDs can control seizures in many patients, they are associated with a range of adverse effects, and up to 35% of patients do not respond to treatment (987). Numerous animal models of epilepsy exist, and commonly used models include kindling via repeated electrical stimulation, or through use of excitotoxic agents such as systemic injection of pilocarpine or intrahippocampal injection of kainic acid; in all cases, animals develop spontaneous recurrent seizures after a latency period (696). While these models do have limitations (e.g., Refs. 436, 697), they have provided useful insights into the circuit mechanisms underlying seizure genesis. Hippocampal atrophy, especially in CA1, is a common feature in human temporal lobe epilepsy patients (53, 743) and animal models confirm that the dentate gyrus and CA1 are the most vulnerable regions (356).
In a rat kindling model, development of spontaneous seizures was associated with a loss of CCK-expressing interneurons in the dentate gyrus (981), and a rat pilocarpine model reported a loss of interneurons expressing either SST or PV in CA1 s.o. (276). Interestingly, this latter study found no loss of interneurons that expressed both SST and PV, or SST and CB, and found no significant loss of interneurons in other layers of CA1 (276). Another study, using pilocarpine in mice, found that animals displaying recurrent seizures had a loss of inhibition from CCKBCs onto CA1 pyramidal cells, but that perisomatic inhibition from PVBCs remained intact (1225). In contrast to this pilocarpine model, a study using intrahippocampal injection of kainic acid found a loss of PV-expressing interneurons that extended beyond the injection site, along with ectopic expression of NPY (759). Interneuron activity also changes in response to epileptogenesis: hippocampal expression of NRG1 increases after electrical stimulation or dosing of pilocarpine and intracerebral infusion of NRG1 delays the onset of seizures (1087). Furthermore, inhibiting endogenous NRG1 or deleting its receptor, ErbB4, from PV-containing interneurons alone is sufficient to exacerbate epileptogenesis in both kindling and pilocarpine models (1087) (FIGURE 25, A–J). A separate study found that NRG1 acts to make PVBCs more excitable by reducing the activity of Kv1.1 and lowering action potential threshold, and this study also found that deleting ErbB4 from PV-containing interneurons increased susceptibility to epilepsy in the pilocarpine model (674) (FIGURE 25, K–N).
FIGURE 25.
PV interneurons become more excitable via neuregulin1/ErbB4 signaling during epileptogenesis. A: in situ hybridization shows increased expression of neuregulin1 (NRG1) in the hours after seizure kindling or exposure to pilocarpine in rats. B and C: increased NRG1 expression is associated with increased activation of its receptor, ErbB4, measured through increased levels of phosphorylated ErbB4 (p-ErbB4). D: intracerebroventricular infusion of NRG1 delayed kindling-induced epileptogenesis. E: inhibiting ErbB4 activity with the tyrosine kinase inhibitor PD158780 exacerbated the effects of kindling. F: representative traces. G and H: deleting ErbB4 from PV interneurons is sufficient to increase susceptibility to kindling. I: the incidence of spontaneous seizures in kindled mice. J: example traces. K: NRG1 increases firing rate of PV interneurons in both cortex and hippocampus, while neutralizing endogenous NRG1 with the Ecto-ErbB4 peptide reduces the firing rate. L: example traces. M: application of NRG1 enhances initiation of action potentials in PV interneurons. N: neutralizing endogenous NRG with Ecto-ErbB4 increases K+ currents in PV interneurons, and this increase is completely blocked by the Kv1.1-specific blocker DTx-K. These data together show that NRG1 increases the excitability of PV interneurons through inhibition of Kv1.1 potassium channels, providing a homeostatic response to increase inhibition in the network during epileptogenesis. [A–J adapted from Tan et al. (1087). K–N adapted from Li et al. (674). Figures used with permission from Nature Neuroscience.]
Inhibitory neurotransmission also influences the spread of an ictal event through cortical networks, with in vitro slice models showing that feedforward inhibition acts as a “brake” to slow down the spread of ictal events (1135). Neurons in cortical areas adjacent to ictal regions display large barrages of inhibitory events that become excitatory as the ictal event invades that region, with the transition to ictal activity being marked by a large increase in coherence between cells (984). Extracellular recordings from human epileptic patients show a similar EEG pattern to these in vitro models, implying that similar mechanisms may underlie ictal spread in humans (984). Changes in chloride reversal potential due to intracellular Cl− accumulations may underlie seizure propagation, by switching GABAA receptor-mediated events from inhibitory to depolarizing and helping drive runaway excitation. Pyramidal cells have been shown to accumulate chloride at the start of a seizure (679), and excitatory GABAergic events driven by PV-containing interneurons onto CA1 pyramidal cells are apparent at the end of epileptiform discharges in in vitro slice models (369). Blockade of KCC2 is sufficient to induce seizure-like activity in hippocampal networks, both in vitro and in vivo (1022), and optogenetic perturbation of intracellular chloride levels can alter the propagation of epileptiform activity (15, 16). Activation of potassium currents by adenosine receptors (A1Rs) can also affect GABAA receptor-mediated currents in in vitro models of epileptiform activity (518). A1R-dependent activation of potassium channels increases the cell's membrane conductance and acts as a shunting effect on GABAAR currents to limit the spread of seizure activity. Altogether, these studies show that interneurons are important both in the pathophysiology of epilepsy and in regulating homeostatic responses to hyperexcitability and suggest possible circuit mechanisms that could be targeted to provide more specific treatments for the disorder.
2. Traumatic brain injury
Traumatic brain injury (TBI) occurs when the brain is damaged by an external force and is associated with two phases: the primary injury and a secondary phase associated with continuing damage and loss of neurons that develops in the days after the injury (717). The secondary injury is caused by several factors, including inflammatory, excitotoxic, and oxidative stresses, caused in part by excessive calcium entry through ionotropic glutamate receptors (1061). The time course of the secondary injury can be very long: in human patients, an initial loss of hippocampal pyramidal cells can be seen in both CA1 and CA3 within 1 wk of TBI, with CA1 but not CA3 progressively showing further cell death 6 mo after the initial injury (764). Animal models of TBI show that inhibitory circuits are also affected. Excitatory neurotransmission increases in injured cortex, and spontaneous inhibitory neurotransmission decreases, and these functional deficits are associated with a selective loss of PV- and SST-expressing interneurons (164). Similarly, reduced inhibitory input to dentate gyrus granule cells occurs after TBI (1122) even though excitatory input to hilar interneurons increases (512), suggesting that compensatory changes in inhibitory circuits occur in response to the injury. Interestingly, TBI to the neocortex is associated with a loss of DG CCK- and PV-containing interneurons, but only in the hilus and not in the granular layer, with no obvious change apparent in CA1 (1122). Even mild TBI is sufficient to reduce the amplitude and frequency of spontaneous IPSCs onto CA1 pyramidal cells, and while no overall loss of neurons occurs, there is also a reduction in GAD67 immunoreactivity apparent 7 days after the injury (28).
3. Ischemia
Ischemic injury to the brain is associated with a similar pattern of hippocampal damage to that seen in temporal lobe epilepsy in both humans (903) and animal models (580), with a loss of neurons occurring after a delay, particularly in CA1. Immediately after injury, CA1 appears normal, but pyramidal cells gradually die over the next 4 days (580). In addition to excitatory cells, SST- and NPY-expressing interneurons are lost from the hilus in the days following ischemic injury (97). In contrast to pyramidal cells, the total number of interneurons does not change in CA1 following ischemic injury, but the expression of SST and NPY does decrease, implying functional changes in the interneurons (97). Overall, interneurons are significantly more resistant to hypoxic/ischemic injury than glutamatergic cells (346, 985), perhaps due to differential expression of hypoxia-inducible factor 1α (932). In addition to cell death, ischemic injury is also associated with increased neurogenesis. In rats, ischemic injury induces neurogenesis in the neonatal striatum, with CR-expressing interneurons being born (1248). Newborn interneurons following ischemic injury can also been seen in the neocortex in adults, where proliferating progenitor cells form interneurons expressing NPY, SST, and CR (but not PV) in layer 1 of rat primary somatosensory cortex (853). Although there are no reports of proliferating interneuron progenitors in the hippocampus, ischemic injury in the hippocampus increases proliferation of neuronal progenitors destined to become adult-born granule cells (1240), and interestingly, activity-dependent differentiation of these progenitors appears to be driven by depolarizing GABAergic inputs from hippocampal interneurons (1126). Similarly, after ischemic injury, neuronal progenitors infiltrate CA1 from the periventricular region and become pyramidal cells that functionally integrate into the hippocampal network (824).
4. Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder where patients experience a progressive loss of memory and cognitive function. AD is associated with extracellular deposits of β-amyloid (Aβ) plaques and intracellular accumulations of neurofibrillary tangles of hyperphosphorylated tau protein in a number of structures associated with memory, including the hippocampus and entorhinal cortex (122). A wide variety of animal models of AD exist, most of which involve overexpressing forms of human amyloid precursor protein (APP, the protein from which Aβ is cleaved) and/or mutant tau that are found in familial forms of AD (298). Some AD models also involve coexpression with mutant forms of presenilin 1 or 2 (PS1 or PS2), and some use triple transgenic animals that express mutant Aβ, tau, and PS1 (298).
Human AD patients show an increased risk of epileptic seizures (877), and mouse models that overexpress human forms of APP reveal increases in the intrinsic excitability of neurons, through decreased Na+ currents in both CA1 pyramidal cells (128) and interneurons (1170) which lead to circuit disruption and a generalized increase in network excitability. Mice overexpressing APP show significant reductions in the voltage-gated sodium channel Nav1.1, as do human AD patients, and Nav1.1 expression is mostly restricted to PV-containing interneurons in both mice (1170) and humans (1188). The APP mice displayed impaired inhibitory function and gamma oscillations, as well as cognitive deficits, and these deficits could be reversed by overexpressing Nav1.1 (1170). Mice overexpressing APP/PS1 show a significant loss of both SST- and NPY-containing interneurons early in the disease progression, at 4 and 6 mo, respectively, but with no associated loss in the number PV-containing interneurons (934). APP/PS1 mice also show an early loss of CR-positive interneurons, with significant reductions in both CA1 and CA3 by 4 mo (55). A recent study using APP/PS1 mice found a progressive loss of axon in s.l.m., and in vivo calcium imaging reveals O-LM cell-specific deficits in activation during fear conditioning (986). Mice overexpressing mutant tau also display cognitive deficits that are associated with a loss of hippocampal interneurons expressing SST and PV (669). A study using mice carrying the human AD risk gene apolipoprotein E4 (ApoE4) also reported significant losses of SST interneurons, but from the DG and not CA1, which was associated with impaired spatial learning on the Morris water maze (37). At least three mechanistically independent mouse models of AD show a loss of SST interneurons, so targeting this cell type may prove a fruitful strategy for future treatment of AD. Improving inhibitory neurotransmission alone may be sufficient to improve function, as the ApoE4 study reported that daily treatment with the GABAA receptor agonist pentobarbital for 4 wk was sufficient to rescue deficits in learning and memory (37). Deleting nitric oxide synthase2 (NOS2) from APP mice greatly exacerbates AD pathology, leading to extensive neuron loss, particularly NPY-expressing interneurons in the hippocampus (1206), so these neurons may also provide a fruitful therapeutic target.
C. Interneurons and Psychiatric Disease
So far, we have considered the role that interneurons play in the pathophysiology of developmental and neurological disorders. In this section, we will consider how hippocampal interneuron function in neuronal circuits is affected by psychiatric conditions such as mood disorders (e.g., major depression and bipolar disorder), as well as conditions such as anxiety disorders, post-traumatic stress disorder (PTSD), and addiction. Psychiatric illnesses are widespread, affecting one in five adults annually, placing a significant burden both on individuals and healthcare systems. Major depression, anxiety disorders, and stress disorders such as PTSD are frequently comorbid, with chronic activation of the stress response via the hypothalamic-pituitary-adrenal axis possibly having a causal role in depression (127, 400, 414).
1. Mood disorders
A substantial body of evidence is accumulating in support of the hypothesis that dysfunction of the GABAergic system underlies major depressive disorder (reviewed in Ref. 712). At the anatomical level, human patients with major depression show a reduction in hippocampal volume (127, 1002), and stress also reduces hippocampal volume in mice (249). However, recent work in primates shows that depression-induced changes in hippocampal volume are due to loss of glia and not neurons (1208). Bipolar disorder is not usually associated with a reduction in hippocampal volume, but a recent meta-analysis concluded that this is due to neuroprotective effects of lithium treatment and found that untreated bipolar patients do show reduced hippocampal volume when compared with treated patients and controls (448). Interneurons are also affected in mood disorders: a post-mortem study comparing brains of patients with schizophrenia, bipolar disorder, and major depression found that all three groups displayed significant reductions in hippocampal reelin levels but, unlike bipolar disorder or schizophrenia, depression was not associated with a reduction in levels of PV or GAD67 (1116). A later post-mortem study of human bipolar disorder patients found that, while there was no overall change in number of neurons in hippocampus, there was a reduction in volume of nonpyramidal layers along with reduced numbers of SST- and PV-containing interneurons, and reduced mRNA for SST, PV and GAD1 (596). These post-mortem studies suggest that, in the hippocampus at least, the pathophysiology of bipolar disorder is more similar to schizophrenia than major depression. While the lack of animal models of bipolar disorder prevent detailed study of interneuron function in the hippocampus in this disorder (832), evidence gathered from human studies suggests that the hippocampus is an important region in bipolar disorder (reviewed in Ref. 358).
Unlike bipolar disorder, robust models of depression do exist in rodents, which use chronic stress to induce depressive-like symptoms in rodents that respond only to chronic and not acute treatment with antidepressant medications (832). Given the links between stress and major depression (127, 400, 414), we will consider these models in the next section. Additionally, some transgenic mouse strains also show depressive behavior. Mice carrying a mutated form of DISC1, which is associated with mental illness in Scotland, show depressive-like behavior and impaired PVBC function, along with impaired theta and gamma oscillations in CA1 (978). Humans with a loss-of-function mutation in the CYP2C19 isoform of cytochrome P-450 have a lower incidence of depressive symptoms (1016), and expressing the human CYP2C19 isoform in mice causes depressive behavior, reduced hippocampal volume, and fewer interneurons immunoreactive for PV or double-cortin (897). Interestingly, experimental diabetes also induces a depressive phenotype in mice linked to reduced hippocampal neurogenesis, and these deficits are reversed with insulin (493).
2. Anxiety disorders, stress, and PTSD
Anxiety disorders are the most prevalent mental health condition, experienced by one in nine people, with symptoms including excessive fear, anxiety, or avoidance of perceived environmental threats such as social interaction or unfamiliar surroundings (240). Disturbances in GABAergic neurotransmission, especially in the amygdala, are believed to underlie much of the pathology of anxiety disorders, as GABAergic agonists and allosteric modulators are anxiolytic (845). In addition to the amygdala, the ventral hippocampus also plays an important role in anxiety (62). Stress responses are modulated via the hypothalamic-pituitary-adrenal axis, through release of glucocorticoids from the adrenal cortex, and negative feedback from the hippocampus plays an important role in terminating the stress response (1025). Impaired feedback from the hippocampus to the hypothalamic-pituitary-adrenal axis and amygdala has been implicated in anxiety disorders and PTSD (reviewed in Refs. 126, 527, 1006).
Chronic stress reduces the number of neurons that are PV-immunoreactive in the dentate gyrus and CA3 in the tree shrew hippocampus, and these reductions can be prevented by fluoxetine or treatment with antagonists for substance P (NK1) receptors (250). In rats, acute and chronic stress both increase the frequency but not amplitude of spontaneous IPSCs onto CA1 pyramidal cells via glucocorticoid receptors activating mechanisms that appear to involve both CCK- and PV-containing interneurons (505). This study also found a decrease in PV immunoreactivity across all hippocampal subfields, but without a change in CCK immunoreactivity (505). Further evidence of a role of PV-containing interneurons in regulating the stress response comes from the observation that pharmacogenetic activation of PV-containing interneurons specifically in the dentate gyrus has an anxiolytic effect, without affecting locomotor or depression-related behaviors (1279).
In humans, reduced levels of NPY are associated with PTSD while soldiers with high NPY plasma levels are resilient to stress (reviewed in Ref. 971), and stress also leads to reductions in hippocampal NPY levels in animal models (213, 675). A recent study examined the effects of chronic mild stress on different interneuron populations in rat hippocampus, where they could parse rats into groups that displayed anhedonic (depressive) behavior and those that were resilient. They found similar reductions in PV and NPY immunoreactivity for both anhedonic and resilient groups in all subfields, while reductions in SST and CR immunoreactivity were much more pronounced in the anhedonic group than resilient group, particularly in CA1; no change in CCK or CB immunoreactivity was observed (251). This suggests that, in addition to PV and NPY, studying the activity of hippocampal SST and CR-expressing interneurons in anxiety models may provide further insights to the circuit mechanisms associated with pathological change.
3. Addiction
While the dopaminergic system is one of the key brain regions implicated in addiction, circuit-level changes in the hippocampus also play an important role (reviewed in Refs. 174, 598). Stimulation of the hippocampus at theta frequency is sufficient to reinstate cocaine-seeking behavior in rats after extinction of self-administration, suggesting a possible role for hippocampal activity in relapse (1178). Blocking mGluR1 receptors within the dorsal hippocampus can suppress reinstatement of cocaine-seeking behavior in rats (1228), although the authors could not determine whether the affected receptors were on glutamatergic or GABAergic neurons. In vivo rat studies show that nicotine induces LTP in dentate granule cells, but that this LTP is preceded by reduced local circuit GABAergic inhibition onto granule cells, making them more responsive to perforant path inputs and possibly reinforcing drug-associated memories (1271). Few studies have examined changes to hippocampal interneurons in addiction, but recent work comparing gene expression in the hippocampi of cocaine or alcohol-addicted humans to alcohol-preferring rats found that all three groups displayed reductions in GABAB1R mRNA expression, as well as changes in other genes related to GABAergic neurotransmission (303). This reduction in GABABR expression is interesting in the light of evidence suggesting that activation of GABABRs may provide a useful treatment for addiction in humans (reviewed in Ref. 1144). Therefore, we suggest that further study of the role of GABAergic interneurons, in a subtype-specific manner, may provide new insights into circuit-based treatments for addiction.
In this section, we have reviewed the role that hippocampal interneurons have in the pathophysiology of developmental, neurological, and psychiatric disorders. While PV-expressing interneurons have received much attention (understandable, given the lead role that PVBCs have in rhythm generation), it is clear that dysfunction in other interneuron subtypes also occurs in numerous disorders of the brain. With the exception of ASD, many studies on interneurons and disease focus on changes in immunoreactivity for interneuron markers. However, plasticity in interneurons, both synaptic and structural, is an important aspect of their function within circuits (63, 618, 820), so it is important for researchers to consider also disease-related morphological changes that could cause changes in interneuron function without affecting the overall number of cells. In the next section, we will discuss the therapeutic potential of circuit-based strategies that specifically target interneurons.
D. Therapeutic Potential of Interneurons
The early observation that application of penicillin to the surface of the exposed cortex precipitates electrographic seizures led to a rapid appreciation that erosion of GABAergic control has dire consequences for cortical circuit function. The corollary of this however is that augmentation of GABAergic influence (e.g., pharmacological use of benzodiazepines, closed loop, or deep brain stimulation) or frank replenishment of lost inhibitory interneurons to damaged or compromised circuits might rescue or limit runaway excitation. The concept that GABAergic inhibitory interneurons provide an essential brake on cellular and circuit excitability has existed for over 50 years. In recent years, demonstrations of finely tuned excitatory/inhibitory balances within virtually all cortical circuits has refined our thinking about the nature of GABAergic inhibitory control (522). Indeed, a matched excitatory/inhibitory ratio, or a controlled temporal or spatial shift in their relative dynamics during trains of activity, enables proper cortical and hippocampal function. This underscores the concept that proper cortical circuit function requires both appropriate timing and magnitude of inhibitory input and that even small changes in the level of inhibitory control can have significant consequences for the nature and function of the circuit.
1. Brain stimulation and closed loop optogenetics
The hypothesis that a compromised excitatory/inhibitory balance may underlie a multitude of neural circuit disorders has caught the attention of many researchers and importantly suggests that restoring this balance may be a tractable therapeutic strategy. This imbalance may result from an erosion or loss of interneuron circuit efficacy and has led to the suggestion that brain stimulation strategies that preferentially drive the remaining inhibitory circuit could be used as tractable treatment strategy. Indeed, brain stimulation is increasingly used in patients with intractable epilepsy to provide palliative care and improve quality of life. Strategies that stimulate either vagus nerve to activate brain stem nuclei, or stimulation of targeted network hubs either within or outside of the seizure focus are becoming routinely explored as alternative strategies for seizure control often combined with pharmacological strategies (992). Although the precise mechansims underlying their effectiveness are largely unexplored, it is hypothesized that these strategies work by preferentially activating inhibitory circuits and the consequent desynchronization of network activity (992).
Brain implanted devices and in particularly the use of closed loop feedback strategies have received considerable attention in the last few years (45, 604). Closed loop strategies rely on feedback between the output and input signals of an implanted device, which then rapidly reacts to influence an actuator that then delivers an appropriate subsequent intervention. To be effective closed loop systems must detect ongoing neural circuit activity, recognize and extract the “aberrant” signal, which is then fedback and used to rapidly activate “corrective” patterned activity to the target neurons, ideally converting pathological signals into physiological patterned activity. Although at this time closed loop systems have only limited clinical applications (particularly in movement disorders and epilepsy), their utility has been convincingly explored in a number of animal models. Ivan Soltesz and colleagues (603, 605) used an “on-demand” optogenetic closed loop approach to control temporal lobe seizures in a mouse model of epilepsy. Using a tunable, closed loop seizure detection program, which then feedbacks to trigger light delivery, they could arrest seizure activity by either optically inhibiting excitatory principal cells using halorhodopsin, or by activating PV-containing interneurons using channelrhodopsin. That seizure control was effective when only a small (~5%) population of interneurons were light-activated suggests that a cell type approach to neural ciruit disorders represent a viable strategy for tackling neural circuit disorders.
2. Tissue grafts, transplantation, and stem cell strategies
Early approaches to implant fetal tissue grafts containing GABAergic neurons to limit electrographic seizure events had limited success (330, 698). While the transplanted cells often survived, they typically remained close to the transplantation site, and only limited evidence suggested that they made functional connections in a manner that would appropriately contain electrographic events. Similarly, transplanted genetically engineered cells or cell lines that were selective for “GABA secreting” cells also offered only marginal anticonvulsant potential (394, 1101). However, observations that inhibitory neurons could be harvested from the embryonic ganglionic eminences and subsequently successfully transplanted into the developing cortex (29, 71, 829, 1202) offered the first promise that such a manipulation could be used to correct damaged or compromised circuits. These transplanted cells not only survive transplantation, they also migrate considerable distances and integrate into functional circuits where they establish and receive appropriate synaptic connections with host pyramidal cells (71). However, its has been noted that targeting of transplanted inhibitory cells to host interneuron subtypes (and other GFP-transplanted interneurons) was limited, and it is uncertain whether appropriate connections are made between ganglionic eminence derived interneurons and other interneurons (61, 511; for review, see Refs. 510).
Clear evidence of inhibitory interneuron transplantation as a potential therapeutic strategy for electrographic seizure activity came from two landmark studies (61, 511; for review, see Ref. 510). Using a mouse lacking the voltage-gated potassium channel subunit Kv1.1, a model of congenital epilepsy, Baraban et al. (61) transplanted MGE-derived interneurons into neocortex at postnatal day 2. Approximately 1 mo later, mice receiving grafts showed an ~90% reduction in spontaneous seizure incidence, consistent with the observed increase of inhibitory tone onto glutamatergic principal cells. In a second study using the pilocarpine model of epilepsy, MGE-derived cells were transplanted once spontaneous seizures had been established. Again mice receiving MGE-derived interneuron transplants showed a remarkable 90% reduction in seizure incidence 60 or more days after receiving transplantation (511). Numerous subsequent studies have also reported amelioration of a number of behavioral and circuit deficits following MGE-derived interneuron transplantation including animal models of stroke (252), AD (1108), schizophrenia (399, 1090), spinal cord injury and neuropathic pain (125, 306), Parkinson’s disease (758), and fear erasure (1247). In a mouse model of AD-induced learning and memory deficits, knock-in of apoE4 with or without Aβ accumulation results in hippocampal hyperactivity and a concomitant loss of hilar GABAergic interneurons (328, 673). Transplantation of mouse MGE-derived inhibitory interneuron progenitors into the hilar region restores normal learning and memory in both apoE4 knock-in mice and apoE4 mice expressing amyloid precursor protein (1108).
Taken together, these studies all underscore the importance of appropriate excitation:inhibition balance for correct circuit function. However, the actual consequences of interneuron transplant have not been studied at the circuit level much beyond monitoring the frequency of spontaneous IPSCs. All of these studies exploit the ability of MGE-derived precursor interneurons to survive and integrate into the target circuit. However, it is unclear what aspect of interneuron function is critical for the apparent reversal of all of these diverse circuit disorders. What trophic and environmental factors influence the extent of interneuron migration away from the transplantation site? Do these cells make appropriate cell surface domain specific synaptic contacts or do they form random contacts with their principal cell targets? Is temporal phasic structure in their inhibitory synaptic output important or is an increased generalized inhibitory tone sufficient? MGE-derived cells are a mixed population of diverse interneuron subclasses; are particular subtypes (i.e., PVBCs) better than others in the success of such transplantation studies? Obviously many questions remain regarding exactly what features of the transplanted cells are critically important once they enter their target tissue, and our ability to improve on and exploit these features can only add to the potential for their future use in treatment strategies for neural circuit disorders.
All of the above studies have relied on transplanted GABAergic interneurons harvested from fetal ganglionic eminences and transplanted into the relevant murine host site. In order for this to be a tractable approach in human therapeutic strategies, tissue would have to be similarly extracted from fetal human tissue, which would seem to be an unlikely scenario at this time. However, several alternative strategies exist. Ye et al. (1249) demonstrated that in vivo lineage reprogramming of mouse cortical excitatory projection neurons dictated the degree of inhibitory innervation. Reprogramming layer 2/3 callosal projection neurons into Fezf2-induced corticofugal projection neurons results in an increased innervation by PVBCs (1249). These induced corticofugal projection neurons not only took on the molecular identity of the endogenous cells, they were able to influence the nature of the inhibitory connectivity that they received, providing a clue that cell type specificity could be used to rewire damaged tissue and circuits.
A second and more promising approach has been to differentiate embryonic stem cells into inhibitory interneuron populations. Although cortical interneurons can be generically produced from stem cells, the lack of understanding of specific transcriptional programs has hampered the generation of specific interneuron subpopulaitons. Au et al. (50) utilized the directed differentiation of stem cells into specific subpopulations of cortical interneurons. With the use of transient expression of Nkx2–1 and Dlx2, two factors required for the generation of inhibitory interneurons, they successfully improved the differentiation efficiency and generation of cell type specificity. They then extended this approach to establish a “modular system” that allowed additional transcription factors to be introduced. Using this approach, they then identified Lmo3 and Pou3f4 as genes that augment the differentiation and/or subtype specificity of inhibitory interneurons (50). A second paradigm shifting study by Maroof et al. (752) similarly exploited the known genetics of cortical interneurons to manipulate the timing of sonic hedgehog activation in an NKX2.1:GFP human embryonic stem cell reporter line to produce enriched populations of PV- and SOM-expressing inhibitory interneurons.
Using rat embryonic stem cells in vitro, Donegan et al. (281) generated enriched populations of either PV-containing or SST-containing interneurons and transplanted them into the hippocampus of a rodent methylazoxymethanol model of schizophrenia. Both interneuron types successfully integrated into the surrounding tissue and circuitry to reduce hippocampal hyperactivity and correct aberrant dopaminergic neuron activity with PV-containing cell transplants (but not SST-containing) reversing social interaction deficits and extradimensional set shifting behavior. In a second rather beautiful study, Fandel et al. (314) transplanted “MGE-like interneurons” derived from human embryonic stem cells into mice harboring spinal cord injury. These transplanted cells survive extended periods of time, differentiate into GABAergic inhibitory interneurons, and fully integrate into the host spinal cord to alleviate pain-related symptoms and ameliorate bladder dysfunction which arises from excessive glutamatergic activity and GABAergic hypofunction (314). Importantly, these cells both receive and make appropriate synaptic contacts with the local circuitry. A major concern for the use of embryonic stem cells is the potential for tumor formation arising from pluripotent cells in the transplant. In the study by Fandel et al. (314), they observed that the vast majority of cells differentiated into interneurons (CR-, CB-, SST-, and PV-containing cell types) with only a small percentage of cells acquiring oligodendrocyte phenotype. Importantly, they found no evidence for tumors in any of their grafts. Going forward such a strategy will undoubtedly be of importance not only in treating spinal cord injury but also manipulating damaged central circuits.
XVII. SUMMARY
As can be seen from the topics covered in this broad review of hippocampal inhibitory interneurons, we have learned much about the myriad roles played by these cells over the last few decades. However, while our understanding of their cellular physiology and circuit roles played by some cells is extensive, in particular the parvalbumin-containing FSBCs, research into many other interneuron types has languished or we have only rudimentary insight into their basic properties and roles in the cortical circuit. Of course, the emergence and development of new techniques and mouse lines will hopefully facilitate their study in greater depth, and we hope that by highlighting here what is known and what is not that this will shine a necessary light onto these cells so that a more complete understanding of all interneuron types can be achieved.
Over the course of writing this review, many “holes” in our understanding became apparent to us. The most general, yet critical, of these is our lack of confident resolution of the number and types of interneurons that populate the hippocampal formation. The emergence of new tools and genetic approaches has allowed us to parse cells types into ever increasing numbers. There is no reason to doubt that as we come to greater understand the genetic programs that drive interneuron development that these numbers will continue to grow. However, from a pragmatic point of view, we need to adopt strategies that allow us to better understand the roles played by these cells and to ask whether small differences in anatomy, physiology, or protein expression that split interneurons into ever increasing silos truly aid our understanding of their roles. Tools such as the Allen Cell Type Database and neuroelectro.org, which take an agnostic approach to cell feature classification, will greatly facilitate our extraction of the true identity, nature, and roles played by this important cell class. It is important that we as a field continue to explore the properties of channels and receptors of the lesser-studied cell types and when possible make the data available in public databases. The converse of this argument is the unnecessary oversimplification of cell types when using transgenic mouse lines for elimination, overexpression, or optogenetic experiments. In many cases, many of these mouse lines do not report activity in a single interneuron population. For example, using a PV-cre or equivalent line impacts not only FSBCs, but also AACs and BiCs, confounding clean interpretations of the roles played by specific subpopulations. The literature is littered with these kinds of erroneous interpretations, and we urge care and caution when designing experimental strategies and interpreting data from such lines.
A second, critical open question is whether every hippocampal principal cell is innervated by every interneuron subtype? With more than 20 types of interneuron in the CA1 hippocampus alone, is it reasonable to expect that they will all target the same principal cell or do specialized subcircuits exist within this subfield? It is becoming clear that multiple distinct pyramidal cells exist, that project to differing target areas outside of the hippocampal formation. It is not unreasonable to consider that each of these distinct projection neuron types will have its own repertoire of inhibitory cells innervating it. This would suggest that numerous interdigitated parallel circuits exist within the CA1 hippocampus, which could receive common or unique patterns of interneuron innervation, significantly reducing the number of inhibitory interneuron types that innervate a particular population of cells.
In our opinion, one of the single most important successes over the last decade has been to attract developmental cell biologists to this field of research. From their work we have learned much about the genetic and transcription regulation and determination of cortical and hippocampal inhibitory interneurons. This has not only informed a greater understanding of the roles played by interneuron subtypes, but it has facilitated development of numerous mouse lines permitting manipulation of relevant genes. However, many fundamental questions remain. What temporal and spatial rules determine whether an MGE-derived interneuron will adopt a PV-containing or SST-containing fate or any cell subtype for that matter? Are interneurons generated in a stochastic manner, dictated by simultaneous regulation of multiple genes and transcription factors? If so, what and when is the tipping point for dictating cell identity and fate? What roles do the differing migratory streams play, and what determines when an interneuron will choose to leave and enter the cortex or carry on towards hippocampus? What is the true nature of clonal cell lineages and what dictates their ultimate positional destination in the nascent cortical and hippocampal circuit and beyond? Can cell duplication during evolution explain why there are so many similar cell types? We now know that the MGE and the CGE give rise to distinct basket cells, O-LM cells, bistratified cells, and NGFCs that share many anatomical and physiological features and differ in expression of only a small number of mRNAs and protein. Consideration of the evolution and development of both the hippocampus and cortex could shed light onto this question.
In the last few years there has been an extraordinary proliferation of papers that indict interneurons in a number of neural circuit disorders. In hindsight, this is of course not particularly surprising given the multiple roles interneurons play in shaping and coordinating network activity. Small perturbations in cell and circuit excitability likely have major consequences for network function. We are encouraged by the burgeoning number of cell transplantation, grafts, stem cells, and optogenetic papers that point toward a tenable strategy for therapeutic intervention and consider this to be a major beacon for future research that will attract new investigators and pharmaceutical companies to this field of research. We also hope that these approaches will usher in a new epoch where studies in human inhibitory interneurons will be commonplace, since it is likely that many secrets are harbored in the human interneuron population.
Finally, from a personal standpoint, it is almost 30 years since the senior author of this review made his first electrophysiological recordings from hippocampal inhibitory interneurons. I can still remember the jubilation in Dr. Ray Dingledine’s laboratory at that time as we began to explore this new frontier. These early, tentative steps took my research life down the path of inhibitory interneurons for better or for worse, and I would urge any young scientist reading this review who is remotely interested in this field to jump in with both feet and have fun doing so because we still have much to learn!
GRANTS
The work was supported by a National Institute of Child Health and Human Development Intramural Research Award (to C. J. McBain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: C. J. McBain, Porter Neuroscience Center, Eunice Kennedy-Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892 (e-mail: mcbainc@mail.nih.gov).
REFERENCES
- 1.Abbeduto L, McDuffie A, Thurman AJ. The fragile X syndrome-autism comorbidity: what do we really know? Front Genet : 355, 2014. doi: 10.3389/fgene.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe P, Molnár Z, Tzeng YS, Lai DM, Arnold SJ, Stumm R. Intermediate Progenitors Facilitate Intracortical Progression of Thalamocortical Axons and Interneurons through CXCL12 Chemokine Signaling. J Neurosci : 13053–13063, 2015. doi: 10.1523/JNEUROSCI.1488-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aber KM, Nori P, MacDonald SM, Bibat G, Jarrar MH, Kaufmann WE. Methyl-CpG-binding protein 2 is localized in the postsynaptic compartment: an immunochemical study of subcellular fractions. Neuroscience : 77–80, 2003. doi: 10.1016/S0306-4522(02)00586-9. [DOI] [PubMed] [Google Scholar]
- 4.Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Curr Opin Neurobiol : 166–175, 2014. doi: 10.1016/j.conb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acsády L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience : 299–315, 1996. doi: 10.1016/0306-4522(95)00610-9. [DOI] [PubMed] [Google Scholar]
- 6.Acsády L, Görcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience : 317–334, 1996. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- 7.Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci : 3386–3403, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acsády L, Katona I, Martínez-Guijarro FJ, Buzsáki G, Freund TF. Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus. J Neurosci : 6907–6919, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ady V, Perroy J, Tricoire L, Piochon C, Dadak S, Chen X, Dusart I, Fagni L, Lambolez B, Levenes C. Type 1 metabotropic glutamate receptors (mGlu1) trigger the gating of GluD2 delta glutamate receptors. EMBO Rep : 103–109, 2014. doi: 10.1002/embr.201337371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguado C, García-Madrona S, Gil-Minguez M, Luján R. Ontogenic Changes and Differential Localization of T-type Ca(2+) Channel Subunits Cav3.1 and Cav3.2 in Mouse Hippocampus and Cerebellum. Front Neuroanat : 83, 2016. doi: 10.3389/fnana.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akgül G, McBain CJ. Diverse roles for ionotropic glutamate receptors on inhibitory interneurons in developing and adult brain. J Physiol : 5471–5490, 2016. doi: 10.1113/JP271764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akgul G, Wollmuth LP. Synapse-associated protein 97 regulates the membrane properties of fast-spiking parvalbumin interneurons in the visual cortex. J Neurosci : 12739–12750, 2013. doi: 10.1523/JNEUROSCI.0040-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albayram Ö, Passlick S, Bilkei-Gorzo A, Zimmer A, Steinhäuser C. Physiological impact of CB1 receptor expression by hippocampal GABAergic interneurons. Pflugers Arch : 727–737, 2016. doi: 10.1007/s00424-015-1782-5. [DOI] [PubMed] [Google Scholar]
- 14.Alcántara S, Ferrer I, Soriano E. Postnatal development of parvalbumin and calbindin D28K immunoreactivities in the cerebral cortex of the rat. Anat Embryol (Berl) : 63–73, 1993. doi: 10.1007/BF00191452. [DOI] [PubMed] [Google Scholar]
- 15.Alfonsa H, Lakey JH, Lightowlers RN, Trevelyan AJ. Cl-out is a novel cooperative optogenetic tool for extruding chloride from neurons. Nat Commun : 13495, 2016. doi: 10.1038/ncomms13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfonsa H, Merricks EM, Codadu NK, Cunningham MO, Deisseroth K, Racca C, Trevelyan AJ. The contribution of raised intraneuronal chloride to epileptic network activity. J Neurosci : 7715–7726, 2015. doi: 10.1523/JNEUROSCI.4105-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alger BE. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know. J Physiol : 2203–2212, 2012. doi: 10.1113/jphysiol.2011.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alger BE, Pitler TA, Wagner JJ, Martin LA, Morishita W, Kirov SA, Lenz RA. Retrograde signalling in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J Physiol : 197–209, 1996. doi: 10.1113/jphysiol.1996.sp021677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali AB. Presynaptic inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J Neurophysiol : 861–869, 2007. doi: 10.1152/jn.00156.2007. [DOI] [PubMed] [Google Scholar]
- 20.Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. J Physiol : 185–199, 1998. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci : 1196–1207, 2010. doi: 10.1111/j.1460-9568.2010.07165.x. [DOI] [PubMed] [Google Scholar]
- 22.Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther : 1396–1411, 1997. [PubMed] [Google Scholar]
- 23.Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber-interneuron synapse. Proc Natl Acad Sci USA : 14708–14713, 2001. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen K, Fuchs EC, Jaschonek H, Bannerman DM, Monyer H. Gap junctions between interneurons are required for normal spatial coding in the hippocampus and short-term spatial memory. J Neurosci : 6542–6552, 2011. doi: 10.1523/JNEUROSCI.6512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allène C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, Cossart R. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci : 12851–12863, 2008. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allene C, Cossart R. Early NMDA receptor-driven waves of activity in the developing neocortex: physiological or pathological network oscillations? J Physiol : 83–91, 2010. doi: 10.1113/jphysiol.2009.178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allene C, Picardo MA, Becq H, Miyoshi G, Fishell G, Cossart R. Dynamic changes in interneuron morphophysiological properties mark the maturation of hippocampal network activity. J Neurosci : 6688–6698, 2012. doi: 10.1523/JNEUROSCI.0081-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida-Suhett CP, Prager EM, Pidoplichko V, Figueiredo TH, Marini AM, Li Z, Eiden LE, Braga MF. GABAergic interneuronal loss and reduced inhibitory synaptic transmission in the hippocampal CA1 region after mild traumatic brain injury. Exp Neurol : 11–23, 2015. doi: 10.1016/j.expneurol.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, Rubenstein JL, Alvarez-Buylla A, Baraban SC. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci : 7380–7389, 2006. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol : 851–914, 1978. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- 31.Amilhon B, Huh CY, Manseau F, Ducharme G, Nichol H, Adamantidis A, Williams S. Parvalbumin Interneurons of Hippocampus Tune Population Activity at Theta Frequency. Neuron : 1277–1289, 2015. doi: 10.1016/j.neuron.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet : 185–188, 1999. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 33.Anastasiades PG, Butt SJ. A role for silent synapses in the development of the pathway from layer 2/3 to 5 pyramidal cells in the neocortex. J Neurosci : 13085–13099, 2012. doi: 10.1523/JNEUROSCI.1262-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anastasiades PG, Marques-Smith A, Lyngholm D, Lickiss T, Raffiq S, Kätzel D, Miesenböck G, Butt SJ. GABAergic interneurons form transient layer-specific circuits in early postnatal neocortex. Nat Commun : 10584, 2016. doi: 10.1038/ncomms10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science : 474–476, 1997. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 36.Andersson RH, Johnston A, Herman PA, Winzer-Serhan UH, Karavanova I, Vullhorst D, Fisahn A, Buonanno A. Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc Natl Acad Sci USA : 13118–13123, 2012. doi: 10.1073/pnas.1201011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci : 13707–13717, 2010. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ang ES Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci : 5805–5815, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angevine JB., Jr Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp Neurol Suppl : 2, 1965. [PubMed] [Google Scholar]
- 40.Angevine JB Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature : 766–768, 1961. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 41.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology : 1789–1799, 2007. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antypa M, Faux C, Eichele G, Parnavelas JG, Andrews WD. Differential gene expression in migratory streams of cortical interneurons. Eur J Neurosci : 1584–1594, 2011. doi: 10.1111/j.1460-9568.2011.07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aponte Y, Lien CC, Reisinger E, Jonas P. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol : 229–243, 2006. doi: 10.1113/jphysiol.2005.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR. Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res : 26–34, 1997. doi: 10.1016/S0006-8993(96)01422-9. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong C, Krook-Magnuson E, Oijala M, Soltesz I. Closed-loop optogenetic intervention in mice. Nat Protoc : 1475–1493, 2013. doi: 10.1038/nprot.2013.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong C, Krook-Magnuson E, Soltesz I. Neurogliaform and Ivy Cells: A Major Family of nNOS Expressing GABAergic Neurons. Front Neural Circuits : 23, 2012. doi: 10.3389/fncir.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong C, Soltesz I. Basket cell dichotomy in microcircuit function. J Physiol : 683–694, 2012. doi: 10.1113/jphysiol.2011.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong C, Szabadics J, Tamás G, Soltesz I. Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward γ-aminobutyric acidergic modulators of entorhinal-hippocampal interplay. J Comp Neurol : 1476–1491, 2011. doi: 10.1002/cne.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atzori M, Lau D, Tansey EP, Chow A, Ozaita A, Rudy B, McBain CJ. H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking. Nat Neurosci : 791–798, 2000. doi: 10.1038/77693. [DOI] [PubMed] [Google Scholar]
- 50.Au E, Ahmed T, Karayannis T, Biswas S, Gan L, Fishell G. A modular gain-of-function approach to generate cortical interneuron subtypes from ES cells. Neuron : 1145–1158, 2013. doi: 10.1016/j.neuron.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Audinat E, Lambolez B, Rossier J. Functional and molecular analysis of glutamate-gated channels by patch-clamp and RT-PCR at the single cell level. Neurochem Int : 119–136, 1996. doi: 10.1016/0197-0186(95)00075-5. [DOI] [PubMed] [Google Scholar]
- 52.Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci : 1238–1247, 2009. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babb TL, Pretorius JK, Kupfer WR, Crandall PH. Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. J Neurosci : 2562–2574, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci : 9664–9674, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baglietto-Vargas D, Moreno-Gonzalez I, Sanchez-Varo R, Jimenez S, Trujillo-Estrada L, Sanchez-Mejias E, Torres M, Romero-Acebal M, Ruano D, Vizuete M, Vitorica J, Gutierrez A. Calretinin interneurons are early targets of extracellular amyloid-beta pathology in PS1/AbetaPP Alzheimer mice hippocampus. J Alzheimers Dis : 119–132, 2010. doi: 10.3233/JAD-2010-100066. [DOI] [PubMed] [Google Scholar]
- 56.Bähner F, Weiss EK, Birke G, Maier N, Schmitz D, Rudolph U, Frotscher M, Traub RD, Both M, Draguhn A. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc Natl Acad Sci USA : E607–E616, 2011. doi: 10.1073/pnas.1103546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bähring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol : 575–589, 1997. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bandler RC, Mayer C, Fishell G. Cortical interneuron specification: the juncture of genes, time and geometry. Curr Opin Neurobiol : 17–24, 2017. doi: 10.1016/j.conb.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banerjee A, Rikhye RV, Breton-Provencher V, Tang X, Li C, Li K, Runyan CA, Fu Z, Jaenisch R, Sur M. Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc Natl Acad Sci USA : E7287–E7296, 2016. doi: 10.1073/pnas.1615330113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci : 11720–11725, 2006. doi: 10.1523/JNEUROSCI.2887-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, García-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci USA : 15472–15477, 2009. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol : 49–56, 2010. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartos M, Alle H, Vida I. Role of microcircuit structure and input integration in hippocampal interneuron recruitment and plasticity. Neuropharmacology : 730–739, 2011. doi: 10.1016/j.neuropharm.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Bartos M, Elgueta C. Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol : 669–681, 2012. doi: 10.1113/jphysiol.2011.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartos M, Vida I, Frotscher M, Geiger JR, Jonas P. Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci : 2687–2698, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci USA : 13222–13227, 2002. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci : 45–56, 2007. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 68.Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron : 1208–1221, 2013. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, Siegelbaum SA. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science : aaa5694, 2016. doi: 10.1126/science.aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron : 510–522, 2013. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol : 81–118, 2009. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron : 466–481, 2009. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem : 697–704, 2004. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baude A, Bleasdale C, Dalezios Y, Somogyi P, Klausberger T. Immunoreactivity for the GABAA receptor alpha1 subunit, somatostatin and Connexin36 distinguishes axoaxonic, basket, and bistratified interneurons of the rat hippocampus. Cereb Cortex : 2094–2107, 2007. doi: 10.1093/cercor/bhl117. [DOI] [PubMed] [Google Scholar]
- 75.Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron : 771–787, 1993. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 76.Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv : 205–219, 2003. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- 77.Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry : 708–715, 2002. doi: 10.1016/S0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- 78.Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu QY, Colton CA, Barker JL. Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci : 4449–4461, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Behrends JC, ten Bruggencate G. Cholinergic modulation of synaptic inhibition in the guinea pig hippocampus in vitro: excitation of GABAergic interneurons and inhibition of GABA-release. J Neurophysiol : 626–629, 1993. [DOI] [PubMed] [Google Scholar]
- 80.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci : 904–910, 2000. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 81.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci : 12757–12763, 2009. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci : 76–83, 2010. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bell KA, Shim H, Chen CK, McQuiston AR. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain α4 and β2 subunits. Neuropharmacology : 1379–1388, 2011. doi: 10.1016/j.neuropharm.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bell LA, Bell KA, McQuiston AR. Acetylcholine release in mouse hippocampal CA1 preferentially activates inhibitory-selective interneurons via α4β2* nicotinic receptor activation. Front Cell Neurosci : 115, 2015. doi: 10.3389/fncel.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bell LA, Bell KA, McQuiston AR. Activation of muscarinic receptors by ACh release in hippocampal CA1 depolarizes VIP but has varying effects on parvalbumin-expressing basket cells. J Physiol : 197–215, 2015. doi: 10.1113/jphysiol.2014.277814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bell LA, Bell KA, McQuiston AR. Synaptic muscarinic response types in hippocampal CA1 interneurons depend on different levels of presynaptic activity and different muscarinic receptor subtypes. Neuropharmacology : 160–173, 2013. doi: 10.1016/j.neuropharm.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsáki G. Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. J Neurosci : 423–435, 2012. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci : 353–360, 2001. doi: 10.1016/S0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 89.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci : 728–739, 2002. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 90.Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience : 187–219, 2014. doi: 10.1016/j.neuroscience.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol : 303–325, 1989. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev : 1215–1284, 2007. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 93.Bennion D, Jensen T, Walther C, Hamblin J, Wallmann A, Couch J, Blickenstaff J, Castle M, Dean L, Beckstead S, Merrill C, Muir C, St Pierre T, Williams B, Daniel S, Edwards JG. Transient receptor potential vanilloid 1 agonists modulate hippocampal CA1 LTP via the GABAergic system. Neuropharmacology : 730–738, 2011. doi: 10.1016/j.neuropharm.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 94.Berger H. Uber das Elektrenkephalogram des Menchen. Arch Psychiatr Nervenkr : 527–570, 1929. doi: 10.1007/BF01797193. [DOI] [Google Scholar]
- 95.Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci USA : 19115–19120, 2005. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science : 1212–1216, 2007. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 97.Bering R, Draguhn A, Diemer NH, Johansen FF. Ischemia changes the coexpression of somatostatin and neuropeptide Y in hippocampal interneurons. Exp Brain Res : 423–429, 1997. doi: 10.1007/PL00005712. [DOI] [PubMed] [Google Scholar]
- 98.Bernard C, Milh M, Morozov YM, Ben-Ari Y, Freund TF, Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci USA : 9388–9393, 2005. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bettler B, Boulter J, Hermans-Borgmeyer I, O’Shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron : 583–595, 1990. doi: 10.1016/0896-6273(90)90213-Y. [DOI] [PubMed] [Google Scholar]
- 100.Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci : 9429–9437, 2012. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beuter S, Ardi Z, Horovitz O, Wuchter J, Keller S, Saha R, Tripathi K, Anunu R, Kehat O, Kriebel M, Richter-Levin G, Volkmer H. Receptor tyrosine kinase EphA7 is required for interneuron connectivity at specific subcellular compartments of granule cells. Sci Rep : 29710, 2016. doi: 10.1038/srep29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bezaire MJ, Soltesz I. Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus : 751–785, 2013. doi: 10.1002/hipo.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, Morita S, Featherstone RE, Ortinski PI, Gandal MJ, Lin R, Liang Y, Gur RE, Carlson GC, Hahn CG, Siegel SJ. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology : 1603–1613, 2014. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blackman AV, Abrahamsson T, Costa RP, Lalanne T, Sjöström PJ. Target-cell-specific short-term plasticity in local circuits. Front Synaptic Neurosci : 11, 2013. doi: 10.3389/fnsyn.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci : 18–29, 2010. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blasco-Ibáñez JM, Freund TF. Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur J Neurosci : 2170–2180, 1995. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 107.Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron : 805–817, 2003. doi: 10.1016/S0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 108.Bodor AL, Katona I, Nyíri G, Mackie K, Ledent C, Hájos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci : 6845–6856, 2005. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bohbot VD, Copara MS, Gotman J, Ekstrom AD. Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nat Commun : 14415, 2017. doi: 10.1038/ncomms14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Böhm C, Pangalos M, Schmitz D, Winterer J. Serotonin Attenuates Feedback Excitation onto O-LM Interneurons. Cereb Cortex : 4572–4583, 2015. doi: 10.1093/cercor/bhv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boksa P. Maternal infection during pregnancy and schizophrenia. J Psychiatry Neurosci : 183–185, 2008. [PMC free article] [PubMed] [Google Scholar]
- 112.Bolea S, Sanchez-Andres JV, Huang X, Wu JY. Initiation and propagation of neuronal coactivation in the developing hippocampus. J Neurophysiol : 552–561, 2006. doi: 10.1152/jn.00321.2005. [DOI] [PubMed] [Google Scholar]
- 113.Bolshakov KV, Essin KV, Buldakova SL, Dorofeeva NA, Skatchkov SN, Eaton MJ, Tikhonov DB, Magazanik LG. Characterization of acid-sensitive ion channels in freshly isolated rat brain neurons. Neuroscience : 723–730, 2002. doi: 10.1016/S0306-4522(01)00582-6. [DOI] [PubMed] [Google Scholar]
- 114.Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, Represa A, Ben-Ari Y, Cossart R. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science : 1419–1424, 2009. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- 115.Booker SA, Althof D, Gross A, Loreth D, Müller J, Unger A, Fakler B, Varro A, Watanabe M, Gassmann M, Bettler B, Shigemoto R, Vida I, Kulik Á. KCTD12 Auxiliary Proteins Modulate Kinetics of GABAB Receptor-Mediated Inhibition in Cholecystokinin-Containing Interneurons. Cereb Cortex : 2318–2334, 2017. doi: 10.1093/cercor/bhw090. [DOI] [PubMed] [Google Scholar]
- 116.Booker SA, Gross A, Althof D, Shigemoto R, Bettler B, Frotscher M, Hearing M, Wickman K, Watanabe M, Kulik Á, Vida I. Differential GABAB-receptor-mediated effects in perisomatic- and dendrite-targeting parvalbumin interneurons. J Neurosci : 7961–7974, 2013. doi: 10.1523/JNEUROSCI.1186-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron : 53–71, 2009. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Both M, Bähner F, von Bohlen und Halbach O, Draguhn A. Propagation of specific network patterns through the mouse hippocampus. Hippocampus : 899–908, 2008. doi: 10.1002/hipo.20446. [DOI] [PubMed] [Google Scholar]
- 119.Bourdeau ML, Morin F, Laurent CE, Azzi M, Lacaille JC. Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. J Neurosci : 1942–1953, 2007. doi: 10.1523/JNEUROSCI.3208-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol : 231–234, 2009. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 121.Boyle MP, Bernard A, Thompson CL, Ng L, Boe A, Mortrud M, Hawrylycz MJ, Jones AR, Hevner RF, Lein ES. Cell-type-specific consequences of Reelin deficiency in the mouse neocortex, hippocampus, and amygdala. J Comp Neurol : 2061–2089, 2011. doi: 10.1002/cne.22655. [DOI] [PubMed] [Google Scholar]
- 122.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol : 239–259, 1991. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 123.Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci : 47–60, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bramham CR, Sarvey JM. Endogenous activation of mu and delta-1 opioid receptors is required for long-term potentiation induction in the lateral perforant path: dependence on GABAergic inhibition. J Neurosci : 8123–8131, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bráz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron : 663–675, 2012. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci : 445–461, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry : 115–118, 2000. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 128.Brown JT, Chin J, Leiser SC, Pangalos MN, Randall AD. Altered intrinsic neuronal excitability and reduced Na+ currents in a mouse model of Alzheimer’s disease. Neurobiol Aging : 2109.e1–2109.e14, 2011. doi: 10.1016/j.neurobiolaging.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 129.Brown KN, Chen S, Han Z, Lu CH, Tan X, Zhang XJ, Ding L, Lopez-Cruz A, Saur D, Anderson SA, Huang K, Shi SH. Clonal production and organization of inhibitory interneurons in the neocortex. Science : 480–486, 2011. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown TE, Chirila AM, Schrank BR, Kauer JA. Loss of interneuron LTD and attenuated pyramidal cell LTP in Trpv1 and Trpv3 KO mice. Hippocampus : 662–671, 2013. doi: 10.1002/hipo.22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biol Psychiatry : 557–567, 2016. doi: 10.1016/j.biopsych.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 132.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron : 536–545, 2008. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 133.Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Dev Neurosci : 379–394, 2011. doi: 10.1159/000330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Buetfering C, Allen K, Monyer H. Parvalbumin interneurons provide grid cell-driven recurrent inhibition in the medial entorhinal cortex. Nat Neurosci : 710–718, 2014. doi: 10.1038/nn.3696. [DOI] [PubMed] [Google Scholar]
- 135.Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature : 823–828, 1994. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- 136.Buhl EH, Han ZS, Lörinczi Z, Stezhka VV, Karnup SV, Somogyi P. Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. J Neurophysiol : 1289–1307, 1994. [DOI] [PubMed] [Google Scholar]
- 137.Buhl EH, Szilágyi T, Halasy K, Somogyi P. Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus : 294–305, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 138.Buhl EH, Tamás G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol : 117–126, 1998. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buhler AV, Dunwiddie TV. Regulation of the activity of hippocampal stratum oriens interneurons by alpha7 nicotinic acetylcholine receptors. Neuroscience : 55–67, 2001. doi: 10.1016/S0306-4522(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 140.Buijs RM, Swaab DF. Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res : 355–365, 1979. doi: 10.1007/BF00233648. [DOI] [PubMed] [Google Scholar]
- 141.Buijs RM, Van Heerikhuize JJ. Vasopressin and oxytocin release in the brain--a synaptic event. Brain Res : 71–76, 1982. doi: 10.1016/0006-8993(82)90979-9. [DOI] [PubMed] [Google Scholar]
- 142.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol : 287–296, 2001. doi: 10.1016/S0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 143.Bureau I, Bischoff S, Heinemann SF, Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci : 653–663, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burgoyne RD, O’Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV. Neuronal Ca2+-sensor proteins: multitalented regulators of neuronal function. Trends Neurosci : 203–209, 2004. doi: 10.1016/j.tins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 145.Busquets-Garcia A, Gomis-González M, Guegan T, Agustín-Pavón C, Pastor A, Mato S, Pérez-Samartín A, Matute C, de la Torre R, Dierssen M, Maldonado R, Ozaita A. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med : 603–607, 2013. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- 146.Butler JL, Mendonça PR, Robinson HP, Paulsen O. Intrinsic Cornu Ammonis Area 1 Theta-Nested Gamma Oscillations Induced by Optogenetic Theta Frequency Stimulation. J Neurosci : 4155–4169, 2016. doi: 10.1523/JNEUROSCI.3150-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron : 591–604, 2005. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 148.Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron : 722–732, 2008. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buzsáki G. Hippocampal sharp waves: their origin and significance. Brain Res : 242–252, 1986. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- 150.Buzsáki G. Theta oscillations in the hippocampus. Neuron : 325–340, 2002. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- 151.Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol : 504–510, 1995. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 152.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science : 1926–1929, 2004. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 153.Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res : 139–171, 1983. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 154.Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci : 203–225, 2012. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bygrave AM, Masiulis S, Nicholson E, Berkemann M, Barkus C, Sprengel R, Harrison PJ, Kullmann DM, Bannerman DM, Kätzel D. Knockout of NMDA-receptors from parvalbumin interneurons sensitizes to schizophrenia-related deficits induced by MK-801. Transl Psychiatry : e778, 2016. doi: 10.1038/tp.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Calfa G, Hablitz JJ, Pozzo-Miller L. Network hyperexcitability in hippocampal slices from Mecp2 mutant mice revealed by voltage-sensitive dye imaging. J Neurophysiol : 1768–1784, 2011. doi: 10.1152/jn.00800.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Calfa G, Li W, Rutherford JM, Pozzo-Miller L. Excitation/inhibition imbalance and impaired synaptic inhibition in hippocampal area CA3 of Mecp2 knockout mice. Hippocampus : 159–168, 2015. doi: 10.1002/hipo.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Çaliskan G, Müller I, Semtner M, Winkelmann A, Raza AS, Hollnagel JO, Rösler A, Heinemann U, Stork O, Meier JC. Identification of Parvalbumin Interneurons as Cellular Substrate of Fear Memory Persistence. Cereb Cortex : 2325–2340, 2016. doi: 10.1093/cercor/bhw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Calvigioni D, Máté Z, Fuzik J, Girach F, Zhang MD, Varro A, Beiersdorf J, Schwindling C, Yanagawa Y, Dockray GJ, McBain CJ, Hökfelt T, Szabó G, Keimpema E, Harkany T. Functional Differentiation of Cholecystokinin-Containing Interneurons Destined for the Cerebral Cortex. Cereb Cortex : 2453–2468, 2017. doi: 10.1093/cercor/bhw094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Campanac E, Gasselin C, Baude A, Rama S, Ankri N, Debanne D. Enhanced intrinsic excitability in basket cells maintains excitatory-inhibitory balance in hippocampal circuits. Neuron : 712–722, 2013. doi: 10.1016/j.neuron.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 161.Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci : 5224–5235, 2007. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Canduela MJ, Mendizabal-Zubiaga J, Puente N, Reguero L, Elezgarai I, Ramos-Uriarte A, Gerrikagoitia I, Grandes P. Visualization by high resolution immunoelectron microscopy of the transient receptor potential vanilloid-1 at inhibitory synapses of the mouse dentate gyrus. PLoS One : e0119401, 2015. doi: 10.1371/journal.pone.0119401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast : 381243, 2008. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cantu D, Walker K, Andresen L, Taylor-Weiner A, Hampton D, Tesco G, Dulla CG. Traumatic Brain Injury Increases Cortical Glutamate Network Activity by Compromising GABAergic Control. Cereb Cortex : 2306–2320, 2015. doi: 10.1093/cercor/bhu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Capogna M. Neurogliaform cells and other interneurons of stratum lacunosum-moleculare gate entorhinal-hippocampal dialogue. J Physiol : 1875–1883, 2011. doi: 10.1113/jphysiol.2010.201004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Capogna M, Gähwiler BH, Thompson SM. Mechanism of mu-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol : 539–558, 1993. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Capogna M, Pearce RA. GABA A,slow: causes and consequences. Trends Neurosci : 101–112, 2011. doi: 10.1016/j.tins.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 168.Caputi A, Rozov A, Blatow M, Monyer H. Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cereb Cortex : 1345–1359, 2009. doi: 10.1093/cercor/bhn175. [DOI] [PubMed] [Google Scholar]
- 169.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature : 663–667, 2009. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Carlén M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Rühlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry : 537–548, 2012. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carta M, Fièvre S, Gorlewicz A, Mulle C. Kainate receptors in the hippocampus. Eur J Neurosci : 1835–1844, 2014. doi: 10.1111/ejn.12590. [DOI] [PubMed] [Google Scholar]
- 172.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem : 889–907, 2000. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 173.Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain : 2331–2347, 2010. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 174.Castilla-Ortega E, Serrano A, Blanco E, Araos P, Suárez J, Pavón FJ, Rodríguez de Fonseca F, Santín LJ. A place for the hippocampus in the cocaine addiction circuit: Potential roles for adult hippocampal neurogenesis. Neurosci Biobehav Rev : 15–32, 2016. doi: 10.1016/j.neubiorev.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 175.Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron : 70–81, 2012. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci USA : 6144–6149, 2000. doi: 10.1073/pnas.97.11.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci : 5067–5077, 2011. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res : 293–302, 1982. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- 179.Caviness VS., Jr Time of neuron origin in the hippocampus and dentate gyrus of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol : 113–120, 1973. doi: 10.1002/cne.901510203. [DOI] [PubMed] [Google Scholar]
- 180.Caviness VS Jr, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol : 141–151, 1973. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 181.Cea-del Rio CA, Lawrence JJ, Erdelyi F, Szabo G, McBain CJ. Cholinergic modulation amplifies the intrinsic oscillatory properties of CA1 hippocampal cholecystokinin-positive interneurons. J Physiol : 609–627, 2011. doi: 10.1113/jphysiol.2010.199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cea-del Rio CA, Lawrence JJ, Tricoire L, Erdelyi F, Szabo G, McBain CJ. M3 muscarinic acetylcholine receptor expression confers differential cholinergic modulation to neurochemically distinct hippocampal basket cell subtypes. J Neurosci : 6011–6024, 2010. doi: 10.1523/JNEUROSCI.5040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Celio MR. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science : 995–997, 1986. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- 184.Ceranik K, Bender R, Geiger JR, Monyer H, Jonas P, Frotscher M, Lübke J. A novel type of GABAergic interneuron connecting the input and the output regions of the hippocampus. J Neurosci : 5380–5394, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chamberland S, Salesse C, Topolnik D, Topolnik L. Synapse-specific inhibitory control of hippocampal feedback inhibitory circuit. Front Cell Neurosci : 130, 2010. doi: 10.3389/fncel.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chamberland S, Topolnik L. Inhibitory control of hippocampal inhibitory neurons. Front Neurosci : 165, 2012. doi: 10.3389/fnins.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chance SA, Walker M, Crow TJ. Reduced density of calbindin-immunoreactive interneurons in the planum temporale in schizophrenia. Brain Res : 32–37, 2005. doi: 10.1016/j.brainres.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 188.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med : 1257–1266, 2003. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 189.Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci : 1090–1097, 2010. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature : 263–269, 2010. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chapman CA, Lacaille JC. Cholinergic induction of theta-frequency oscillations in hippocampal inhibitory interneurons and pacing of pyramidal cell firing. J Neurosci : 8637–8645, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex : 2660–2674, 2010. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chávez AE, Hernández VM, Rodenas-Ruano A, Chan CS, Castillo PE. Compartment-specific modulation of GABAergic synaptic transmission by TRPV1 channels in the dentate gyrus. J Neurosci : 16621–16629, 2014. doi: 10.1523/JNEUROSCI.3635-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cheah CS, Yu FH, Westenbroek RE, Kalume FK, Oakley JC, Potter GB, Rubenstein JL, Catterall WA. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA : 14646–14651, 2012. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA : 17184–17189, 2005. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci USA : 11382–11387, 2008. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen K, Ratzliff A, Hilgenberg L, Gulyás A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron : 599–611, 2003. doi: 10.1016/S0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 198.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron : 871–881, 2004. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 199.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron : 461–472, 2003. doi: 10.1016/S0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 200.Chevaleyre V, Heifets BD, Kaeser PS, Südhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron : 801–812, 2007. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol : 169–182, 1992. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- 202.Chikwendu A, McBain CJ. Two temporally overlapping “delayed-rectifiers” determine the voltage-dependent potassium current phenotype in cultured hippocampal interneurons. J Neurophysiol : 1477–1490, 1996. [DOI] [PubMed] [Google Scholar]
- 203.Chittajallu R, Craig MT, McFarland A, Yuan X, Gerfen S, Tricoire L, Erkkila B, Barron SC, Lopez CM, Liang BJ, Jeffries BW, Pelkey KA, McBain CJ. Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT(3A)R expression. Nat Neurosci : 1598–1607, 2013. doi: 10.1038/nn.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chittajallu R, Pelkey KA, McBain CJ. Neurogliaform cells dynamically regulate somatosensory integration via synapse-specific modulation. Nat Neurosci : 13–15, 2013. doi: 10.1038/nn.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cho JH, Askwith CC. Presynaptic release probability is increased in hippocampal neurons from ASIC1 knockout mice. J Neurophysiol : 426–441, 2008. doi: 10.1152/jn.00940.2007. [DOI] [PubMed] [Google Scholar]
- 206.Chu Z, Galarreta M, Hestrin S. Synaptic interactions of late-spiking neocortical neurons in layer 1. J Neurosci : 96–102, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marín O. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci : 1199–1210, 2013. doi: 10.1038/nn.3485. [DOI] [PubMed] [Google Scholar]
- 208.Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature : 599–603, 1997. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- 209.Close J, Xu H, De Marco García N, Batista-Brito R, Rossignol E, Rudy B, Fishell G. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci : 17690–17705, 2012. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature : 75–78, 1995. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 211.Cobb SR, Halasy K, Vida I, Nyiri G, Tamás G, Buhl EH, Somogyi P. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience : 629–648, 1997. doi: 10.1016/S0306-4522(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 212.Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci : 1059–1068, 2005. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 213.Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathé AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology : 350–363, 2012. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cohen SM, Ma H, Kuchibhotla KV, Watson BO, Buzsáki G, Froemke RC, Tsien RW. Excitation-Transcription Coupling in Parvalbumin-Positive Interneurons Employs a Novel CaM Kinase-Dependent Pathway Distinct from Excitatory Neurons. Neuron : 292–307, 2016. doi: 10.1016/j.neuron.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature : 353–357, 2009. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 216.Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) : 319–329, 2010. doi: 10.1152/physiol.00021.2010. [DOI] [PubMed] [Google Scholar]
- 217.Colonnese MT, Khazipov R. “Slow activity transients” in infant rat visual cortex: a spreading synchronous oscillation patterned by retinal waves. J Neurosci : 4325–4337, 2010. doi: 10.1523/JNEUROSCI.4995-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Commons KG, Milner TA. Cellular and subcellular localization of delta opioid receptor immunoreactivity in the rat dentate gyrus. Brain Res : 181–195, 1996. doi: 10.1016/S0006-8993(96)00774-3. [DOI] [PubMed] [Google Scholar]
- 219.Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol : 2707–2725, 2003. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- 220.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci : 393–418, 2004. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 221.Contractor A, Klyachko VA, Portera-Cailliau C. Altered Neuronal and Circuit Excitability in Fragile X Syndrome. Neuron : 699–715, 2015. doi: 10.1016/j.neuron.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci : 9529–9540, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cooper EC, Jan LY. M-channels: neurological diseases, neuromodulation, and drug development. Arch Neurol : 496–500, 2003. doi: 10.1001/archneur.60.4.496. [DOI] [PubMed] [Google Scholar]
- 224.Cope DW, Maccaferri G, Márton LF, Roberts JD, Cobden PM, Somogyi P. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience : 63–80, 2002. doi: 10.1016/S0306-4522(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 225.Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J Neurosci : 7334–7340, 2011. doi: 10.1523/JNEUROSCI.0100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Copits BA, Swanson GT. Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nat Rev Neurosci : 675–686, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Corbin JG, Butt SJ. Developmental mechanisms for the generation of telencephalic interneurons. Dev Neurobiol : 710–732, 2011. doi: 10.1002/dneu.20890. [DOI] [PubMed] [Google Scholar]
- 228.Corrigall WA. Opiates and the hippocampus: a review of the functional and morphological evidence. Pharmacol Biochem Behav : 255–262, 1983. doi: 10.1016/0091-3057(83)90371-4. [DOI] [PubMed] [Google Scholar]
- 229.Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y, Crépel V. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron : 147–159, 2002. doi: 10.1016/S0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 230.Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci : 470–478, 1998. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- 231.Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron : 497–508, 2001. doi: 10.1016/S0896-6273(01)00221-5. [DOI] [PubMed] [Google Scholar]
- 232.Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry : 377–386, 2002. doi: 10.1016/S0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- 233.Cousins SL, Papadakis M, Rutter AR, Stephenson FA. Differential interaction of NMDA receptor subtypes with the post-synaptic density-95 family of membrane associated guanylate kinase proteins. J Neurochem : 903–913, 2008. doi: 10.1111/j.1471-4159.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- 234.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry : 241–253, 1996. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 235.Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr Bull : 920–926, 2012. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Craig MT, Mayne EW, Bettler B, Paulsen O, McBain CJ. Distinct roles of GABAB1a- and GABAB1b-containing GABAB receptors in spontaneous and evoked termination of persistent cortical activity. J Physiol : 835–843, 2013. doi: 10.1113/jphysiol.2012.248088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Craig MT, McBain CJ. The emerging role of GABAB receptors as regulators of network dynamics: fast actions from a ‘slow’ receptor? Curr Opin Neurobiol : 15–21, 2014. doi: 10.1016/j.conb.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Craig MT, McBain CJ. Fast gamma oscillations are generated intrinsically in CA1 without the involvement of fast-spiking basket cells. J Neurosci : 3616–3624, 2015. doi: 10.1523/JNEUROSCI.4166-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Craig MT, McBain CJ. Navigating the circuitry of the brain’s GPS system: future challenges for neurophysiologists. Hippocampus : 736–743, 2015. doi: 10.1002/hipo.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Craske MG, Stein MB. Anxiety. Lancet : 3048–3059, 2016. doi: 10.1016/S0140-6736(16)30381-6. [DOI] [PubMed] [Google Scholar]
- 241.Crépel V, Aronov D, Jorquera I, Represa A, Ben-Ari Y, Cossart R. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron : 105–120, 2007. doi: 10.1016/j.neuron.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 242.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci : 9–17, 2010. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Csicsvari J, Hirase H, Mamiya A, Buzsáki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron : 585–594, 2000. doi: 10.1016/S0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- 244.Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron : 311–322, 2003. doi: 10.1016/S0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 245.Cudmore RH, Fronzaroli-Molinieres L, Giraud P, Debanne D. Spike-time precision and network synchrony are controlled by the homeostatic regulation of the D-type potassium current. J Neurosci : 12885–12895, 2010. doi: 10.1523/JNEUROSCI.0740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cunningham MO, Davies CH, Buhl EH, Kopell N, Whittington MA. Gamma oscillations induced by kainate receptor activation in the entorhinal cortex in vitro. J Neurosci : 9761–9769, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Curia G, Papouin T, Séguéla P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex : 1515–1520, 2009. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci : 1854–1864, 2008. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA : 12796–12801, 2001. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology : 67–79, 2005. doi: 10.1038/sj.npp.1300581. [DOI] [PubMed] [Google Scholar]
- 251.Czéh B, Varga ZK, Henningsen K, Kovács GL, Miseta A, Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus : 393–405, 2015. doi: 10.1002/hipo.22382. [DOI] [PubMed] [Google Scholar]
- 252.Daadi MM, Lee SH, Arac A, Grueter BA, Bhatnagar R, Maag AL, Schaar B, Malenka RC, Palmer TD, Steinberg GK. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant : 815–826, 2009. doi: 10.3727/096368909X470829. [DOI] [PubMed] [Google Scholar]
- 253.Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron : 23–30, 2004. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 254.Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA : 12560–12565, 2005. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature : 609–611, 1991. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- 256.Daw MI, Pelkey KA, Chittajallu R, McBain CJ. Presynaptic kainate receptor activation preserves asynchronous GABA release despite the reduction in synchronous release from hippocampal cholecystokinin interneurons. J Neurosci : 11202–11209, 2010. doi: 10.1523/JNEUROSCI.6334-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Daw MI, Scott HL, Isaac JT. Developmental synaptic plasticity at the thalamocortical input to barrel cortex: mechanisms and roles. Mol Cell Neurosci : 493–502, 2007. doi: 10.1016/j.mcn.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci : 11112–11122, 2009. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.De Giorgio A, Comparini SE, Intra FS, Granato A. Long-term alterations of striatal parvalbumin interneurons in a rat model of early exposure to alcohol. J Neurodev Disord : 18, 2012. doi: 10.1186/1866-1955-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.De Lecea L, del Río JA, Soriano E. Developmental expression of parvalbumin mRNA in the cerebral cortex and hippocampus of the rat. Brain Res Mol Brain Res : 1–13, 1995. doi: 10.1016/0169-328X(95)00056-X. [DOI] [PubMed] [Google Scholar]
- 261.De Marco García NV, Priya R, Tuncdemir SN, Fishell G, Karayannis T. Sensory inputs control the integration of neurogliaform interneurons into cortical circuits. Nat Neurosci : 393–401, 2015. doi: 10.1038/nn.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Defagot MC, Malchiodi EL, Villar MJ, Antonelli MC. Distribution of D4 dopamine receptor in rat brain with sequence-specific antibodies. Brain Res Mol Brain Res : 1–12, 1997. doi: 10.1016/S0169-328X(96)00235-5. [DOI] [PubMed] [Google Scholar]
- 263.DeFelipe J, Hendry SH, Jones EG. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res : 49–54, 1989. doi: 10.1016/0006-8993(89)91702-2. [DOI] [PubMed] [Google Scholar]
- 264.DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Anderson S, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, González-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvárday Z, Kubota Y, Lewis DA, Marín O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamás G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci : 202–216, 2013. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Deidda G, Allegra M, Cerri C, Naskar S, Bony G, Zunino G, Bozzi Y, Caleo M, Cancedda L. Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nat Neurosci : 87–96, 2015. doi: 10.1038/nn.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Deisseroth K. Optogenetics. Nat Methods : 26–29, 2011. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Denaxa M, Kalaitzidou M, Garefalaki A, Achimastou A, Lasrado R, Maes T, Pachnis V. Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Reports : 1351–1362, 2012. doi: 10.1016/j.celrep.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Denève S, Machens CK. Efficient codes and balanced networks. Nat Neurosci : 375–382, 2016. doi: 10.1038/nn.4243. [DOI] [PubMed] [Google Scholar]
- 269.Deng PY, Lei S. Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci : 273–284, 2008. doi: 10.1016/j.mcn.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Desai MA, Conn PJ. Excitatory effects of ACPD receptor activation in the hippocampus are mediated by direct effects on pyramidal cells and blockade of synaptic inhibition. J Neurophysiol : 40–52, 1991. [DOI] [PubMed] [Google Scholar]
- 271.Destexhe A, Rudolph M, Paré D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci : 739–751, 2003. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- 272.Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci : 3216–3228, 2012. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Digby RJ, Bravo DS, Paulsen O, Magloire V. Distinct mechanisms of Up state maintenance in the medial entorhinal cortex and neocortex. Neuropharmacology , Pt A: 543–555, 2017. doi: 10.1016/j.neuropharm.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi GA, Guo L, Xu Q, Liu R, Lu C, Chu J, Grimley JS, Krostag AR, Kaykas A, Avery MC, Rashid MS, Baek M, Jacob AL, Smith GB, Wilson DE, Kosche G, Kruglikov I, Rusielewicz T, Kotak VC, Mowery TM, Anderson SA, Callaway EM, Dasen JS, Fitzpatrick D, Fossati V, Long MA, Noggle S, Reynolds JH, Sanes DH, Rudy B, Feng G, Fishell G. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci : 1743–1749, 2016. doi: 10.1038/nn.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA : 10819–10824, 2002. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Dinocourt C, Petanjek Z, Freund TF, Ben-Ari Y, Esclapez M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J Comp Neurol : 407–425, 2003. doi: 10.1002/cne.10622. [DOI] [PubMed] [Google Scholar]
- 277.Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci : 12956–12968, 2008. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Doll CA, Broadie K. Neuron class-specific requirements for Fragile X Mental Retardation Protein in critical period development of calcium signaling in learning and memory circuitry. Neurobiol Dis : 76–87, 2016. doi: 10.1016/j.nbd.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Donato F, Chowdhury A, Lahr M, Caroni P. Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron : 770–786, 2015. doi: 10.1016/j.neuron.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 280.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature : 272–276, 2013. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 281.Donegan JJ, Tyson JA, Branch SY, Beckstead MJ, Anderson SA, Lodge DJ. Stem cell-derived interneuron transplants as a treatment for schizophrenia: preclinical validation in a rodent model. Mol Psychiatry 2016. doi: 10.1038/mp.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Dorofeeva NA, Karpushev AV, Nikolaev MV, Bolshakov KV, Stockand JD, Staruschenko A. Muscarinic M1 modulation of acid-sensing ion channels. Neuroreport : 1386–1391, 2009. doi: 10.1097/WNR.0b013e3283318912. [DOI] [PubMed] [Google Scholar]
- 283.Doze VA, Cohen GA, Madison DV. Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. J Neurophysiol : 43–53, 1995. [DOI] [PubMed] [Google Scholar]
- 284.Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res : 245–263, 2007. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- 285.Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus : 119–136, 2002. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- 286.Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, Mukai J, Fenelon K, Hsu PK, Gogos JA, Karayiorgou M. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci : 259–281, 2011. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Du J, Zhang L, Weiser M, Rudy B, McBain CJ. Developmental expression and functional characterization of the potassium-channel subunit Kv3.1b in parvalbumin-containing interneurons of the rat hippocampus. J Neurosci : 506–518, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development : 1559–1567, 2008. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- 289.Dudok B, Barna L, Ledri M, Szabó SI, Szabadits E, Pintér B, Woodhams SG, Henstridge CM, Balla GY, Nyilas R, Varga C, Lee SH, Matolcsi M, Cervenak J, Kacskovics I, Watanabe M, Sagheddu C, Melis M, Pistis M, Soltesz I, Katona I. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci : 75–86, 2015. doi: 10.1038/nn.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Dugladze T, Maziashvili N, Börgers C, Gurgenidze S, Häussler U, Winkelmann A, Haas CA, Meier JC, Vida I, Kopell NJ, Gloveli T. GABA(B) autoreceptor-mediated cell type-specific reduction of inhibition in epileptic mice. Proc Natl Acad Sci USA : 15073–15078, 2013. doi: 10.1073/pnas.1313505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Dugladze T, Schmitz D, Whittington MA, Vida I, Gloveli T. Segregation of axonal and somatic activity during fast network oscillations. Science : 1458–1461, 2012. doi: 10.1126/science.1222017. [DOI] [PubMed] [Google Scholar]
- 292.Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature : 71–75, 1996. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 293.Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev : 393–427, 1995. [DOI] [PubMed] [Google Scholar]
- 294.Edwards JG, Gibson HE, Jensen T, Nugent F, Walther C, Blickenstaff J, Kauer JA. A novel non-CB1/TRPV1 endocannabinoid-mediated mechanism depresses excitatory synapses on hippocampal CA1 interneurons. Hippocampus : 209–221, 2012. doi: 10.1002/hipo.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA : 6917–6922, 2001. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci : 7–21, 2011. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ego-Stengel V, Wilson MA. Spatial selectivity and theta phase precession in CA1 interneurons. Hippocampus : 161–174, 2007. doi: 10.1002/hipo.20253. [DOI] [PubMed] [Google Scholar]
- 298.Elder GA, Gama Sosa MA, De Gasperi R. Transgenic mouse models of Alzheimer’s disease. Mt Sinai J Med : 69–81, 2010. doi: 10.1002/msj.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Elfant D, Pál BZ, Emptage N, Capogna M. Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur J Neurosci : 104–113, 2008. doi: 10.1111/j.1460-9568.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- 300.Elgueta C, Köhler J, Bartos M. Persistent discharges in dentate gyrus perisoma-inhibiting interneurons require hyperpolarization-activated cyclic nucleotide-gated channel activation. J Neurosci : 4131–4139, 2015. doi: 10.1523/JNEUROSCI.3671-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ellender TJ, Nissen W, Colgin LL, Mann EO, Paulsen O. Priming of hippocampal population bursts by individual perisomatic-targeting interneurons. J Neurosci : 5979–5991, 2010. doi: 10.1523/JNEUROSCI.3962-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci : 704–716, 2001. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 303.Enoch MA, Zhou Z, Kimura M, Mash DC, Yuan Q, Goldman D. GABAergic gene expression in postmortem hippocampus from alcoholics and cocaine addicts; corresponding findings in alcohol-naïve P and NP rats. PLoS One : e29369, 2012. doi: 10.1371/journal.pone.0029369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol : 2476–2489, 1999. [DOI] [PubMed] [Google Scholar]
- 305.Etherton M, Földy C, Sharma M, Tabuchi K, Liu X, Shamloo M, Malenka RC, Südhof TC. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci USA : 13764–13769, 2011. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Etlin A, Bráz JM, Kuhn JA, Wang X, Hamel KA, Llewellyn-Smith IJ, Basbaum AI. Functional Synaptic Integration of Forebrain GABAergic Precursors into the Adult Spinal Cord. J Neurosci : 11634–11645, 2016. doi: 10.1523/JNEUROSCI.2301-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Fagiolini M, Fritschy JM, Löw K, Möhler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science : 1681–1683, 2004. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 308.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature : 183–186, 2000. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 309.Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res : 709–720, 1994. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 310.Fairén A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol : 67–83, 1986. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- 311.Falenski KW, Blair RE, Sim-Selley LJ, Martin BR, DeLorenzo RJ. Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience : 1232–1244, 2007. doi: 10.1016/j.neuroscience.2007.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Falenski KW, Carter DS, Harrison AJ, Martin BR, Blair RE, DeLorenzo RJ. Temporal characterization of changes in hippocampal cannabinoid CB(1) receptor expression following pilocarpine-induced status epilepticus. Brain Res : 64–72, 2009. doi: 10.1016/j.brainres.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Fame RM, MacDonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends Neurosci : 41–50, 2011. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, Noble-Haeusslein LJ, Kriegstein AR. Transplanted Human Stem Cell-Derived Interneuron Precursors Mitigate Mouse Bladder Dysfunction and Central Neuropathic Pain after Spinal Cord Injury. Cell Stem Cell : 544–557, 2016. doi: 10.1016/j.stem.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 315.Fanselow EE, Connors BW. The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP-DOWN states of mouse neocortex. J Neurophysiol : 596–606, 2010. doi: 10.1152/jn.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Faragó N, Kocsis AK, Lovas S, Molnár G, Boldog E, Rózsa M, Szemenyei V, Vámos E, Nagy LI, Tamás G, Puskás LG. Digital PCR to determine the number of transcripts from single neurons after patch-clamp recording. Biotechniques : 327–336, 2013. doi: 10.2144/000114029. [DOI] [PubMed] [Google Scholar]
- 317.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci : 215–229, 2005. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 318.Farrell MS, Werge T, Sklar P, Owen MJ, Ophoff RA, O’Donovan MC, Corvin A, Cichon S, Sullivan PF. Evaluating historical candidate genes for schizophrenia. Mol Psychiatry : 555–562, 2015. doi: 10.1038/mp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Fazzari P, Paternain AV, Valiente M, Pla R, Luján R, Lloyd K, Lerma J, Marín O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature : 1376–1380, 2010. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 320.Ferando I, Mody I. Altered gamma oscillations during pregnancy through loss of δ subunit-containing GABA(A) receptors on parvalbumin interneurons. Front Neural Circuits : 144, 2013. doi: 10.3389/fncir.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ferando I, Mody I. In vitro gamma oscillations following partial and complete ablation of δ subunit-containing GABAA receptors from parvalbumin interneurons. Neuropharmacology : 91–98, 2015. doi: 10.1016/j.neuropharm.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Férézou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci : 7389–7397, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ferguson KA, Huh CY, Amilhon B, Williams S, Skinner FK. Experimentally constrained CA1 fast-firing parvalbumin-positive interneuron network models exhibit sharp transitions into coherent high frequency rhythms. Front Comput Neurosci : 144, 2013. doi: 10.3389/fncom.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ferraguti F, Cobden P, Pollard M, Cope D, Shigemoto R, Watanabe M, Somogyi P. Immunolocalization of metabotropic glutamate receptor 1alpha (mGluR1alpha) in distinct classes of interneuron in the CA1 region of the rat hippocampus. Hippocampus : 193–215, 2004. doi: 10.1002/hipo.10163. [DOI] [PubMed] [Google Scholar]
- 325.Ferraguti F, Conquet F, Corti C, Grandes P, Kuhn R, Knopfel T. Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J Comp Neurol : 391–407, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 326.Ferraguti F, Klausberger T, Cobden P, Baude A, Roberts JD, Szucs P, Kinoshita A, Shigemoto R, Somogyi P, Dalezios Y. Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J Neurosci : 10520–10536, 2005. doi: 10.1523/JNEUROSCI.2547-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Filice F, Vörckel KJ, Sungur AO, Wöhr M, Schwaller B. Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol Brain : 10, 2016. doi: 10.1186/s13041-016-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA : 7209–7214, 2009. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet : 195–200, 2000. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 330.Fine A, Meldrum BS, Patel S. Modulation of experimentally induced epilepsy by intracerebral grafts of fetal GABAergic neurons. Neuropsychologia : 627–634, 1990. doi: 10.1016/0028-3932(90)90038-P. [DOI] [PubMed] [Google Scholar]
- 331.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron : 1188–1203, 2011. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci : 9658–9668, 2004. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature : 186–189, 1998. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 334.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshé SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia : 475–482, 2014. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 335.Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marín O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci : 9682–9695, 2007. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Flandin P, Kimura S, Rubenstein JL. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci : 2812–2823, 2010. doi: 10.1523/JNEUROSCI.4228-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Flandin P, Zhao Y, Vogt D, Jeong J, Long J, Potter G, Westphal H, Rubenstein JL. Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron : 939–950, 2011. doi: 10.1016/j.neuron.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Fleck MW, Hirotsune S, Gambello MJ, Phillips-Tansey E, Suares G, Mervis RF, Wynshaw-Boris A, McBain CJ. Hippocampal abnormalities and enhanced excitability in a murine model of human lissencephaly. J Neurosci : 2439–2450, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Fogarty M, Grist M, Gelman D, Marín O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci : 10935–10946, 2007. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Földy C, Lee SH, Morgan RJ, Soltesz I. Regulation of fast-spiking basket cell synapses by the chloride channel ClC-2. Nat Neurosci : 1047–1049, 2010. doi: 10.1038/nn.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Földy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci : 1128–1130, 2007. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- 342.Földy C, Malenka RC, Südhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron : 498–509, 2013. doi: 10.1016/j.neuron.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Földy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci : 1465–1469, 2006. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Forro T, Valenti O, Lasztoczi B, Klausberger T. Temporal organization of GABAergic interneurons in the intermediate CA1 hippocampus during network oscillations. Cereb Cortex : 1228–1240, 2015. doi: 10.1093/cercor/bht316. [DOI] [PubMed] [Google Scholar]
- 345.Foster JD, Kitchen I, Bettler B, Chen Y. GABAB receptor subtypes differentially modulate synaptic inhibition in the dentate gyrus to enhance granule cell output. Br J Pharmacol : 1808–1819, 2013. doi: 10.1111/bph.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Frahm C, Haupt C, Witte OW. GABA neurons survive focal ischemic injury. Neuroscience : 341–346, 2004. doi: 10.1016/j.neuroscience.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 347.Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci : 8228–8235, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci : 479–486, 1998. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- 349.Frerking M, Ohliger-Frerking P. AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci : 7434–7443, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci : 489–495, 2003. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 351.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature : 170–173, 1988. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 352.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus : 347–470, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 353.Freund TF, Gulyás AI. Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol : 479–487, 1997. doi: 10.1139/y97-033. [DOI] [PubMed] [Google Scholar]
- 354.Freund TF, Katona I. Perisomatic inhibition. Neuron : 33–42, 2007. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 355.Freund TF, Maglóczky Z, Soltész I, Somogyi P. Synaptic connections, axonal and dendritic patterns of neurons immunoreactive for cholecystokinin in the visual cortex of the cat. Neuroscience : 1133–1159, 1986. doi: 10.1016/0306-4522(86)90129-6. [DOI] [PubMed] [Google Scholar]
- 356.Freund TF, Ylinen A, Miettinen R, Pitkänen A, Lahtinen H, Baimbridge KG, Riekkinen PJ. Pattern of neuronal death in the rat hippocampus after status epilepticus. Relationship to calcium binding protein content and ischemic vulnerability. Brain Res Bull : 27–38, 1992. doi: 10.1016/0361-9230(92)90227-O. [DOI] [PubMed] [Google Scholar]
- 357.Freund-Mercier MJ, Stoeckel ME, Palacios JM, Pazos A, Reichhart JM, Porte A, Richard P. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience : 599–614, 1987. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- 358.Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F. The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol : 419–430, 2007. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- 359.Fricker D, Verheugen JA, Miles R. Cell-attached measurements of the firing threshold of rat hippocampal neurones. J Physiol : 791–804, 1999. doi: 10.1111/j.1469-7793.1999.0791s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci : 474–480, 2005. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 361.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci : 209–224, 2009. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 362.Fu C, Cawthon B, Clinkscales W, Bruce A, Winzenburger P, Ess KC. GABAergic interneuron development and function is modulated by the Tsc1 gene. Cereb Cortex : 2111–2119, 2012. doi: 10.1093/cercor/bhr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell : 1139–1152, 2014. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Fuchs EC, Doheny H, Faulkner H, Caputi A, Traub RD, Bibbig A, Kopell N, Whittington MA, Monyer H. Genetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation. Proc Natl Acad Sci USA : 3571–3576, 2001. doi: 10.1073/pnas.051631898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron : 591–604, 2007. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 366.Fuentealba P, Begum R, Capogna M, Jinno S, Márton LF, Csicsvari J, Thomson A, Somogyi P, Klausberger T. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron : 917–929, 2008. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Fuentealba P, Klausberger T, Karayannis T, Suen WY, Huck J, Tomioka R, Rockland K, Capogna M, Studer M, Morales M, Somogyi P. Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci : 1595–1609, 2010. doi: 10.1523/JNEUROSCI.4199-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S. Locomotion, Theta Oscillations, and the Speed-Correlated Firing of Hippocampal Neurons Are Controlled by a Medial Septal Glutamatergic Circuit. Neuron : 1253–1264, 2015. doi: 10.1016/j.neuron.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 369.Fujiwara-Tsukamoto Y, Isomura Y, Imanishi M, Ninomiya T, Tsukada M, Yanagawa Y, Fukai T, Takada M. Prototypic seizure activity driven by mature hippocampal fast-spiking interneurons. J Neurosci : 13679–13689, 2010. doi: 10.1523/JNEUROSCI.1523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Fukuda T, Kosaka T. The dual network of GABAergic interneurons linked by both chemical and electrical synapses: a possible infrastructure of the cerebral cortex. Neurosci Res : 123–130, 2000. doi: 10.1016/S0168-0102(00)00163-2. [DOI] [PubMed] [Google Scholar]
- 371.Fukuda T, Kosaka T. Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J Neurosci : 1519–1528, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron : 315–327, 2005. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 373.Gaiarsa JL, Porcher C. Emerging neurotrophic role of GABAB receptors in neuronal circuit development. Front Cell Neurosci : 206, 2013. doi: 10.3389/fncel.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA : 12438–12443, 2002. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci : 425–433, 2001. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- 376.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature : 72–75, 1999. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 377.Galván EJ, Calixto E, Barrionuevo G. Bidirectional Hebbian plasticity at hippocampal mossy fiber synapses on CA3 interneurons. J Neurosci : 14042–14055, 2008. doi: 10.1523/JNEUROSCI.4848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Galván EJ, Cosgrove KE, Mauna JC, Card JP, Thiels E, Meriney SD, Barrionuevo G. Critical involvement of postsynaptic protein kinase activation in long-term potentiation at hippocampal mossy fiber synapses on CA3 interneurons. J Neurosci : 2844–2855, 2010. doi: 10.1523/JNEUROSCI.5269-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Galván EJ, Pérez-Rosello T, Gómez-Lira G, Lara E, Gutiérrez R, Barrionuevo G. Synapse-specific compartmentalization of signaling cascades for LTP induction in CA3 interneurons. Neuroscience : 332–345, 2015. doi: 10.1016/j.neuroscience.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Gangarossa G, Longueville S, De Bundel D, Perroy J, Hervé D, Girault JA, Valjent E. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus : 2199–2207, 2012. doi: 10.1002/hipo.22044. [DOI] [PubMed] [Google Scholar]
- 381.Ganter P, Szücs P, Paulsen O, Somogyi P. Properties of horizontal axo-axonic cells in stratum oriens of the hippocampal CA1 area of rats in vitro. Hippocampus : 232–243, 2004. doi: 10.1002/hipo.10170. [DOI] [PubMed] [Google Scholar]
- 382.Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the alpha 1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci : 837–853, 1994. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 383.Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol : 219–236, 1998. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci : 452–459, 2000. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- 385.Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet : 666–672, 2008. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.García-Junco-Clemente P, Cantero G, Gómez-Sánchez L, Linares-Clemente P, Martínez-López JA, Luján R, Fernández-Chacón R. Cysteine string protein-alpha prevents activity-dependent degeneration in GABAergic synapses. J Neurosci : 7377–7391, 2010. doi: 10.1523/JNEUROSCI.0924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Gatto CL, Broadie K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front Synaptic Neurosci : 4, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron : 193–204, 1995. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 389.Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marín O. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci : 16570–16580, 2011. doi: 10.1523/JNEUROSCI.4068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Gelman DM, Marín O. Generation of interneuron diversity in the mouse cerebral cortex. Eur J Neurosci : 2136–2141, 2010. doi: 10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- 391.Gelman DM, Martini FJ, Nóbrega-Pereira S, Pierani A, Kessaris N, Marín O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci : 9380–9389, 2009. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron : 422–435, 2010. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 393.Gereau RW IV, Conn PJ. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci : 6879–6889, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Gernert M, Thompson KW, Löscher W, Tobin AJ. Genetically engineered GABA-producing cells demonstrate anticonvulsant effects and long-term transgene expression when transplanted into the central piriform cortex of rats. Exp Neurol : 183–192, 2002. doi: 10.1006/exnr.2002.7914. [DOI] [PubMed] [Google Scholar]
- 395.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron : 746–759, 2008. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol : 2615–2626, 2008. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol : 467–480, 2005. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- 398.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature : 75–79, 1999. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 399.Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S, Chuhma N, Glickstein S, Merker RJ, Xu Q, Small SA, Anderson SA, Ross ME, Moore H. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc Natl Acad Sci USA : 7450–7455, 2014. doi: 10.1073/pnas.1316488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci : 1242–1247, 2002. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron : 898–907, 2011. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Gleeson JG, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci : 352–359, 2000. doi: 10.1016/S0166-2236(00)01607-6. [DOI] [PubMed] [Google Scholar]
- 403.Glickfeld LL, Atallah BV, Scanziani M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci : 1824–1832, 2008. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci : 807–815, 2006. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Glickstein SB, Monaghan JA, Koeller HB, Jones TK, Ross ME. Cyclin D2 is critical for intermediate progenitor cell proliferation in the embryonic cortex. J Neurosci : 9614–9624, 2009. doi: 10.1523/JNEUROSCI.2284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Glickstein SB, Moore H, Slowinska B, Racchumi J, Suh M, Chuhma N, Ross ME. Selective cortical interneuron and GABA deficits in cyclin D2-null mice. Development : 4083–4093, 2007. doi: 10.1242/dev.008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Gloveli T. Hippocampal spatial navigation: interneurons take responsibility. J Physiol : 4609–4610, 2010. doi: 10.1113/jphysiol.2010.200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol : 131–147, 2005. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci : 1421–1426, 2008. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron : 763–770, 2007. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 411.Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol : 1163–1178, 2007. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol : 1165–1181, 2013. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 413.Goffin K, Van Paesschen W, Van Laere K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain : 1033–1040, 2011. doi: 10.1093/brain/awq385. [DOI] [PubMed] [Google Scholar]
- 414.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry : 254–275, 2002. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 415.Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron : 387–400, 2008. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Goldberg JH, Lacefield CO, Yuste R. Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting. J Physiol : 465–478, 2004. doi: 10.1113/jphysiol.2004.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron : 807–821, 2003. doi: 10.1016/S0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 418.Goldberg JH, Tamas G, Yuste R. Ca2+ imaging of mouse neocortical interneurone dendrites: Ia-type K+ channels control action potential backpropagation. J Physiol : 49–65, 2003. doi: 10.1113/jphysiol.2003.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Golden JA, Fields-Berry SC, Cepko CL. Construction and characterization of a highly complex retroviral library for lineage analysis. Proc Natl Acad Sci USA : 5704–5708, 1995. doi: 10.1073/pnas.92.12.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crépel V, Cossart R. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci : 9560–9572, 2007. doi: 10.1523/JNEUROSCI.1237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Goldstein PA, Elsen FP, Ying SW, Ferguson C, Homanics GE, Harrison NL. Prolongation of hippocampal miniature inhibitory postsynaptic currents in mice lacking the GABA(A) receptor alpha1 subunit. J Neurophysiol : 3208–3217, 2002. doi: 10.1152/jn.00885.2001. [DOI] [PubMed] [Google Scholar]
- 422.Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci : 175–184, 2001. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 423.Golshani P, Gonçalves JT, Khoshkhoo S, Mostany R, Smirnakis S, Portera-Cailliau C. Internally mediated developmental desynchronization of neocortical network activity. J Neurosci : 10890–10899, 2009. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Gomis-González M, Busquets-Garcia A, Matute C, Maldonado R, Mato S, Ozaita A. Possible Therapeutic Doses of Cannabinoid Type 1 Receptor Antagonist Reverses Key Alterations in Fragile X Syndrome Mouse Model. Genes (Basel) : 56, 2016. doi: 10.3390/genes7090056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Gonchar Y, Burkhalter A. Connectivity of GABAergic calretinin-immunoreactive neurons in rat primary visual cortex. Cereb Cortex : 683–696, 1999. doi: 10.1093/cercor/9.7.683. [DOI] [PubMed] [Google Scholar]
- 426.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature : 917–925, 2003. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 427.Gonzalez-Burgos G. GABA transporter GAT1: a crucial determinant of GABAB receptor activation in cortical circuits? Adv Pharmacol : 175–204, 2010. doi: 10.1016/S1054-3589(10)58008-6. [DOI] [PubMed] [Google Scholar]
- 428.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci : 3274–3286, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Goutagny R, Jackson J, Williams S. Self-generated theta oscillations in the hippocampus. Nat Neurosci : 1491–1493, 2009. doi: 10.1038/nn.2440. [DOI] [PubMed] [Google Scholar]
- 430.Gozlan H, Ben-Ari Y. Interneurons are the source and the targets of the first synapses formed in the rat developing hippocampal circuit. Cereb Cortex : 684–692, 2003. doi: 10.1093/cercor/13.6.684. [DOI] [PubMed] [Google Scholar]
- 431.Granger AJ, Nicoll RA. Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on. Philos Trans R Soc Lond B Biol Sci : 20130136, 2013. doi: 10.1098/rstb.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Custo Greig LF, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci : 755–769, 2013. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Grigoriou M, Tucker AS, Sharpe PT, Pachnis V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development : 2063–2074, 1998. [DOI] [PubMed] [Google Scholar]
- 434.Grigorov A, Moskalyuk A, Kravchenko M, Veselovsky N, Verkhratsky A, Fedulova S. Kv7 potassium channel subunits and M currents in cultured hippocampal interneurons. Pflugers Arch : 1747–1758, 2014. doi: 10.1007/s00424-013-1406-x. [DOI] [PubMed] [Google Scholar]
- 435.Griguoli M, Maul A, Nguyen C, Giorgetti A, Carloni P, Cherubini E. Nicotine blocks the hyperpolarization-activated current Ih and severely impairs the oscillatory behavior of oriens-lacunosum moleculare interneurons. J Neurosci : 10773–10783, 2010. doi: 10.1523/JNEUROSCI.2446-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Grone BP, Baraban SC. Animal models in epilepsy research: legacies and new directions. Nat Neurosci : 339–343, 2015. doi: 10.1038/nn.3934. [DOI] [PubMed] [Google Scholar]
- 437.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci : 441–458, 2004. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 438.Gulyás AI, Görcs TJ, Freund TF. Innervation of different peptide-containing neurons in the hippocampus by GABAergic septal afferents. Neuroscience : 31–44, 1990. doi: 10.1016/0306-4522(90)90189-B. [DOI] [PubMed] [Google Scholar]
- 439.Gulyás AI, Hájos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci : 3397–3411, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Gulyás AI, Megías M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci : 10082–10097, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Gulyás AI, Miles R, Hájos N, Freund TF. Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur J Neurosci : 1729–1751, 1993. doi: 10.1111/j.1460-9568.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 442.Gulyás AI, Miles R, Sík A, Tóth K, Tamamaki N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature : 683–687, 1993. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- 443.Gulyás AI, Szabó GG, Ulbert I, Holderith N, Monyer H, Erdélyi F, Szabó G, Freund TF, Hájos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci : 15134–15145, 2010. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Guo J, Anton ES. Decision making during interneuron migration in the developing cerebral cortex. Trends Cell Biol : 342–351, 2014. doi: 10.1016/j.tcb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Hahn TT, Sakmann B, Mehta MR. Differential responses of hippocampal subfields to cortical up-down states. Proc Natl Acad Sci USA : 5169–5174, 2007. doi: 10.1073/pnas.0700222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci : 4535–4545, 2006. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Hainmüller T, Krieglstein K, Kulik A, Bartos M. Joint CP-AMPA and group I mGlu receptor activation is required for synaptic plasticity in dentate gyrus fast-spiking interneurons. Proc Natl Acad Sci USA : 13211–13216, 2014. doi: 10.1073/pnas.1409394111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Hajek T, Kopecek M, Höschl C, Alda M. Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. J Psychiatry Neurosci : 333–343, 2012. doi: 10.1503/jpn.110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Hajos N, Acsady L, Freund TF. Target selectivity and neurochemical characteristics of VIP-immunoreactive interneurons in the rat dentate gyrus. Eur J Neurosci : 1415–1431, 1996. doi: 10.1111/j.1460-9568.1996.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 450.Hájos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF, Paulsen O. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci : 319–327, 2009. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Hájos N, Karlócai MR, Németh B, Ulbert I, Monyer H, Szabó G, Erdélyi F, Freund TF, Gulyás AI. Input-output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro. J Neurosci : 11677–11691, 2013. doi: 10.1523/JNEUROSCI.5729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Hájos N, Mody I. Synaptic communication among hippocampal interneurons: properties of spontaneous IPSCs in morphologically identified cells. J Neurosci : 8427–8442, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci : 9127–9137, 2004. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Hájos N, Papp EC, Acsády L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience : 355–376, 1998. doi: 10.1016/S0306-4522(97)00300-X. [DOI] [PubMed] [Google Scholar]
- 455.Halasy K, Buhl EH, Lörinczi Z, Tamás G, Somogyi P. Synaptic target selectivity and input of GABAergic basket and bistratified interneurons in the CA1 area of the rat hippocampus. Hippocampus : 306–329, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 456.Halasy K, Rácz B, Maderspach K. Kappa opioid receptors are expressed by interneurons in the CA1 area of the rat hippocampus: a correlated light and electron microscopic immunocytochemical study. J Chem Neuroanat : 233–241, 2000. doi: 10.1016/S0891-0618(00)00068-5. [DOI] [PubMed] [Google Scholar]
- 457.Halasy K, Somogyi P. Subdivisions in the multiple GABAergic innervation of granule cells in the dentate gyrus of the rat hippocampus. Eur J Neurosci : 411–429, 1993. doi: 10.1111/j.1460-9568.1993.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 458.Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with Fragile X syndrome. J Abnorm Child Psychol : 927–939, 2008. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res : 71–92, 1982. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- 460.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, Liu Q, Bai G, Wang W, Xiong L, Ren W, Marsicano G, Zhang X. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell : 1039–1050, 2012. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 461.Han ZS, Buhl EH, Lörinczi Z, Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci : 395–410, 1993. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 462.Hangya B, Li Y, Muller RU, Czurkó A. Complementary spatial firing in place cell-interneuron pairs. J Physiol : 4165–4175, 2010. doi: 10.1113/jphysiol.2010.194274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci : RC40, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci : 1576–1587, 2013. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Viegi A, Berardi N, Maffei L. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci : 361–371, 2010. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Harris KD, Shepherd GM. The neocortical circuit: themes and variations. Nat Neurosci : 170–181, 2015. doi: 10.1038/nn.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Harris KM, Marshall PE, Landis DM. Ultrastructural study of cholecystokinin-immunoreactive cells and processes in area CA1 of the rat hippocampus. J Comp Neurol : 147–158, 1985. doi: 10.1002/cne.902330202. [DOI] [PubMed] [Google Scholar]
- 468.Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm (Vienna) : 893–898, 2007. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- 469.Harwell CC, Fuentealba LC, Gonzalez-Cerrillo A, Parker PR, Gertz CC, Mazzola E, Garcia MT, Alvarez-Buylla A, Cepko CL, Kriegstein AR. Wide Dispersion and Diversity of Clonally Related Inhibitory Interneurons. Neuron : 999–1007, 2015. doi: 10.1016/j.neuron.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca(2+)-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol : 360–365, 2007. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 471.Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci : 1211–1219, 2007. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Hashimotodani Y, Ohno-Shosaku T, Watanabe M, Kano M. Roles of phospholipase Cbeta and NMDA receptor in activity-dependent endocannabinoid release. J Physiol : 373–380, 2007. doi: 10.1113/jphysiol.2007.137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol : 710–715, 2006. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Hayama T, Noguchi J, Watanabe S, Takahashi N, Hayashi-Takagi A, Ellis-Davies GC, Matsuzaki M, Kasai H. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat Neurosci : 1409–1416, 2013. doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, Levine JM, Kelly SM, Krugikov I, Wu P, Chen Y, Gong L, Hou Y, Osten P, Rudy B, Huang ZJ. Strategies and Tools for Combinatorial Targeting of GABAergic Neurons in Mouse Cerebral Cortex. Neuron : 555, 2016. doi: 10.1016/j.neuron.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 476.Hébert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci : 678–685, 2008. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci : 1319–1328, 2005. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 478.Hefft S, Kraushaar U, Geiger JR, Jonas P. Presynaptic short-term depression is maintained during regulation of transmitter release at a GABAergic synapse in rat hippocampus. J Physiol : 201–208, 2002. doi: 10.1113/jphysiol.2001.013455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Helboe L, Egebjerg J, de Jong IE. Distribution of serotonin receptor 5-HT6 mRNA in rat neuronal subpopulations: A double in situ hybridization study. Neuroscience : 442–454, 2015. doi: 10.1016/j.neuroscience.2015.09.064. [DOI] [PubMed] [Google Scholar]
- 480.Hennou S, Khalilov I, Diabira D, Ben-Ari Y, Gozlan H. Early sequential formation of functional GABA(A) and glutamatergic synapses on CA1 interneurons of the rat foetal hippocampus. Eur J Neurosci : 197–208, 2002. doi: 10.1046/j.1460-9568.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- 481.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci : 877–888, 2005. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 482.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science : 1504–1508, 1998. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Hentschke H, Benkwitz C, Banks MI, Perkins MG, Homanics GE, Pearce RA. Altered GABAA,slow inhibition and network oscillations in mice lacking the GABAA receptor beta3 subunit. J Neurophysiol : 3643–3655, 2009. doi: 10.1152/jn.00651.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Hepp R, Hay YA, Aguado C, Lujan R, Dauphinot L, Potier MC, Nomura S, Poirel O, El Mestikawy S, Lambolez B, Tricoire L. Glutamate receptors of the delta family are widely expressed in the adult brain. Brain Struct Funct : 2797–2815, 2015. doi: 10.1007/s00429-014-0827-4. [DOI] [PubMed] [Google Scholar]
- 485.Herkenham M. Characterization and localization of cannabinoid receptors in brain: an in vitro technique using slide-mounted tissue sections. NIDA Res Monogr : 129–145, 1991. [PubMed] [Google Scholar]
- 486.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA : 1932–1936, 1990. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Herring BE, Nicoll RA. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol : 351–365, 2016. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- 488.Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci : 304–309, 2005. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 489.Hevner RF, Daza RA, Englund C, Kohtz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience : 605–618, 2004. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 490.Hill EL, Gallopin T, Férézou I, Cauli B, Rossier J, Schweitzer P, Lambolez B. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol : 2580–2589, 2007. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- 491.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry : 642–649, 2012. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Ho MT, Pelkey KA, Topolnik L, Petralia RS, Takamiya K, Xia J, Huganir RL, Lacaille JC, McBain CJ. Developmental expression of Ca2+-permeable AMPA receptors underlies depolarization-induced long-term depression at mossy fiber CA3 pyramid synapses. J Neurosci : 11651–11662, 2007. doi: 10.1523/JNEUROSCI.2671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Ho N, Balu DT, Hilario MR, Blendy JA, Lucki I. Depressive phenotypes evoked by experimental diabetes are reversed by insulin. Physiol Behav : 702–708, 2012. doi: 10.1016/j.physbeh.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Hökfelt T, Blacker D, Broberger C, Herrera-Marschitz M, Snyder G, Fisone G, Cortés R, Morino P, You ZB, Ogren SO. Some aspects on the anatomy and function of central cholecystokinin systems. Pharmacol Toxicol : 382–386, 2002. doi: 10.1034/j.1600-0773.2002.910617.x. [DOI] [PubMed] [Google Scholar]
- 495.Holderith N, Németh B, Papp OI, Veres JM, Nagy GA, Hájos N. Cannabinoids attenuate hippocampal γ oscillations by suppressing excitatory synaptic input onto CA3 pyramidal neurons and fast spiking basket cells. J Physiol : 4921–4934, 2011. doi: 10.1113/jphysiol.2011.216259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, Krieger AM, Buja A, Henkelman RM, Wigler M, Mills AA. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci USA : 17076–17081, 2011. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron : 487–495, 2001. doi: 10.1016/S0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 498.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature : 486–489, 2006. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 499.Hosp JA, Strüber M, Yanagawa Y, Obata K, Vida I, Jonas P, Bartos M. Morpho-physiological criteria divide dentate gyrus interneurons into classes. Hippocampus : 189–203, 2014. doi: 10.1002/hipo.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex : 1369–1374, 2003. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 501.Hu H, Cavendish JZ, Agmon A. Not all that glitters is gold: off-target recombination in the somatostatin-IRES-Cre mouse line labels a subset of fast-spiking interneurons. Front Neural Circuits : 195, 2013. doi: 10.3389/fncir.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science : 1255263, 2014. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- 503.Hu H, Jonas P. A supercritical density of Na(+) channels ensures fast signaling in GABAergic interneuron axons. Nat Neurosci : 686–693, 2014. doi: 10.1038/nn.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Hu H, Martina M, Jonas P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science : 52–58, 2010. doi: 10.1126/science.1177876. [DOI] [PubMed] [Google Scholar]
- 505.Hu W, Zhang M, Czéh B, Flügge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology : 1693–1707, 2010. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Huang Y, Yoon K, Ko H, Jiao S, Ito W, Wu JY, Yung WH, Lu B, Morozov A. 5-HT3a Receptors Modulate Hippocampal Gamma Oscillations by Regulating Synchrony of Parvalbumin-Positive Interneurons. Cereb Cortex : 576–585, 2016. doi: 10.1093/cercor/bhu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell : 739–755, 1999. doi: 10.1016/S0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 508.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol : 419–436, 1970. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron : 704–717, 2013. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Hunt RF, Baraban SC. Interneuron Transplantation as a Treatment for Epilepsy. Cold Spring Harb Perspect Med : a022376, 2015. doi: 10.1101/cshperspect.a022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci : 692–697, 2013. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Hunt RF, Scheff SW, Smith BN. Synaptic reorganization of inhibitory hilar interneuron circuitry after traumatic brain injury in mice. J Neurosci : 6880–6890, 2011. doi: 10.1523/JNEUROSCI.0032-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Hussaini SA, Kempadoo KA, Thuault SJ, Siegelbaum SA, Kandel ER. Increased size and stability of CA1 and CA3 place fields in HCN1 knockout mice. Neuron : 643–653, 2011. doi: 10.1016/j.neuron.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Huston E, Cullen GP, Burley JR, Dolphin AC. The involvement of multiple calcium channel sub-types in glutamate release from cerebellar granule cells and its modulation by GABAB receptor activation. Neuroscience : 465–478, 1995. doi: 10.1016/0306-4522(95)00172-F. [DOI] [PubMed] [Google Scholar]
- 515.Huxter JR, Zinyuk LE, Roloff E, Clarke VR, Dolman NP, More JC, Jane DE, Collingridge GL, Muller RU. Inhibition of kainate receptors reduces the frequency of hippocampal theta oscillations. J Neurosci : 2212–2223, 2007. doi: 10.1523/JNEUROSCI.3954-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Ievglevskyi O, Isaev D, Netsyk O, Romanov A, Fedoriuk M, Maximyuk O, Isaeva E, Akaike N, Krishtal O. Acid-sensing ion channels regulate spontaneous inhibitory activity in the hippocampus: possible implications for epilepsy. Philos Trans R Soc Lond B Biol Sci : 20150431, 2016. doi: 10.1098/rstb.2015.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Iino M, Ozawa S, Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol : 151–165, 1990. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Ilie A, Raimondo JV, Akerman CJ. Adenosine release during seizures attenuates GABAA receptor-mediated depolarization. J Neurosci : 5321–5332, 2012. doi: 10.1523/JNEUROSCI.5412-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Inan M, Welagen J, Anderson SA. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex : 820–827, 2012. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.International Molecular Genetic Study of Autism Consortium (IMGSAC) A genomewide screen for autism: strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet : 570–581, 2001. doi: 10.1086/323264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Iossifov I, Levy D, Allen J, Ye K, Ronemus M, Lee YH, Yamrom B, Wigler M. Low load for disruptive mutations in autism genes and their biased transmission. Proc Natl Acad Sci USA : E5600–E5607, 2015. doi: 10.1073/pnas.1516376112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron : 231–243, 2011. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Isaacson JS, Solís JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron : 165–175, 1993. doi: 10.1016/0896-6273(93)90308-E. [DOI] [PubMed] [Google Scholar]
- 524.Jackson AC, Milstein AD, Soto D, Farrant M, Cull-Candy SG, Nicoll RA. Probing TARP modulation of AMPA receptor conductance with polyamine toxins. J Neurosci : 7511–7520, 2011. doi: 10.1523/JNEUROSCI.6688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Jackson AC, Nicoll RA. Stargazing from a new vantage--TARP modulation of AMPA receptor pharmacology. J Physiol : 5909–5910, 2011. doi: 10.1113/jphysiol.2011.223495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Jackson J, Amilhon B, Goutagny R, Bott JB, Manseau F, Kortleven C, Bressler SL, Williams S. Reversal of theta rhythm flow through intact hippocampal circuits. Nat Neurosci : 1362–1370, 2014. doi: 10.1038/nn.3803. [DOI] [PubMed] [Google Scholar]
- 527.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev : 118–134, 1991. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 528.Janssen MJ, Leiva-Salcedo E, Buonanno A. Neuregulin directly decreases voltage-gated sodium current in hippocampal ErbB4-expressing interneurons. J Neurosci : 13889–13895, 2012. doi: 10.1523/JNEUROSCI.1420-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Jaramillo TC, Liu S, Pettersen A, Birnbaum SG, Powell CM. Autism-related neuroligin-3 mutation alters social behavior and spatial learning. Autism Res : 264–272, 2014. doi: 10.1002/aur.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Jerng HH, Qian Y, Pfaffinger PJ. Modulation of Kv4.2 channel expression and gating by dipeptidyl peptidase 10 (DPP10). Biophys J : 2380–2396, 2004. doi: 10.1529/biophysj.104.042358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet : 20–29, 2000. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 532.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci : 100–107, 2007. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 533.Jiang M, Swann JW. A role for L-type calcium channels in the maturation of parvalbumin-containing hippocampal interneurons. Neuroscience : 839–850, 2005. doi: 10.1016/j.neuroscience.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 534.Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron : 8–27, 2013. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Jinno S. Structural organization of long-range GABAergic projection system of the hippocampus. Front Neuroanat : 13, 2009. doi: 10.3389/neuro.05.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsáki G, Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci : 8790–8804, 2007. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsáki G, Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci : 8790–8804, 2007. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol : 615–663, 1993. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron : 1281–1289, 1994. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 540.Jones EG. Varieties and distribution of non-pyramidal cells in the somatic sensory cortex of the squirrel monkey. J Comp Neurol : 205–267, 1975. doi: 10.1002/cne.901600204. [DOI] [PubMed] [Google Scholar]
- 541.Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol : 1505–1518, 2000. [DOI] [PubMed] [Google Scholar]
- 542.Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol : 603–610, 1997. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun : 1080, 2012. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Jurgensen S, Castillo PE. Selective Dysregulation of Hippocampal Inhibition in the Mouse Lacking Autism Candidate Gene CNTNAP2. J Neurosci : 14681–14687, 2015. doi: 10.1523/JNEUROSCI.1666-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Kaiser KM, Zilberter Y, Sakmann B. Back-propagating action potentials mediate calcium signalling in dendrites of bitufted interneurons in layer 2/3 of rat somatosensory cortex. J Physiol : 17–31, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Kakegawa W, Miyazaki T, Emi K, Matsuda K, Kohda K, Motohashi J, Mishina M, Kawahara S, Watanabe M, Yuzaki M. Differential regulation of synaptic plasticity and cerebellar motor learning by the C-terminal PDZ-binding motif of GluRdelta2. J Neurosci : 1460–1468, 2008. doi: 10.1523/JNEUROSCI.2553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci : 13582–13591, 2008. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev : 309–380, 2009. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 549.Karagiannis A, Gallopin T, Dávid C, Battaglia D, Geoffroy H, Rossier J, Hillman EM, Staiger JF, Cauli B. Classification of NPY-expressing neocortical interneurons. J Neurosci : 3642–3659, 2009. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Karayannis T, Elfant D, Huerta-Ocampo I, Teki S, Scott RS, Rusakov DA, Jones MV, Capogna M. Slow GABA transient and receptor desensitization shape synaptic responses evoked by hippocampal neurogliaform cells. J Neurosci : 9898–9909, 2010. doi: 10.1523/JNEUROSCI.5883-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci : 402–416, 2010. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Karnani MM, Agetsuma M, Yuste R. A blanket of inhibition: functional inferences from dense inhibitory connectivity. Curr Opin Neurobiol : 96–102, 2014. doi: 10.1016/j.conb.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Karnani MM, Jackson J, Ayzenshtat I, Hamzehei Sichani A, Manoocheri K, Kim S, Yuste R. Opening Holes in the Blanket of Inhibition: Localized Lateral Disinhibition by VIP Interneurons. J Neurosci : 3471–3480, 2016. doi: 10.1523/JNEUROSCI.3646-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci : 4140–4154, 2009. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Karson MA, Whittington KC, Alger BE. Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology : 117–128, 2008. doi: 10.1016/j.neuropharm.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Karube F, Kubota Y, Kawaguchi Y. Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J Neurosci : 2853–2865, 2004. doi: 10.1523/JNEUROSCI.4814-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Katona I, Acsády L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience : 37–55, 1999. doi: 10.1016/S0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- 558.Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci : 529–558, 2012. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci : 4544–4558, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Katona I, Urbán GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci : 5628–5637, 2006. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Katona L, Lapray D, Viney TJ, Oulhaj A, Borhegyi Z, Micklem BR, Klausberger T, Somogyi P. Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron : 872–886, 2014. doi: 10.1016/j.neuron.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Katona L, Micklem B, Borhegyi Z, Swiejkowski DA, Valenti O, Viney TJ, Kotzadimitriou D, Klausberger T, Somogyi P. Behavior-dependent activity patterns of GABAergic long-range projecting neurons in the rat hippocampus. Hippocampus : 359–377, 2017. doi: 10.1002/hipo.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Katsumaru H, Kosaka T, Heizmann CW, Hama K. Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res : 347–362, 1988. [DOI] [PubMed] [Google Scholar]
- 564.Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci : 2638–2655, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol : 1743–1747, 1997. [DOI] [PubMed] [Google Scholar]
- 566.Kawaguchi Y, Aosaki T, Kubota Y. Cholinergic and GABAergic interneurons in the striatum. Nihon Shinkei Seishin Yakurigaku Zasshi : 87–90, 1997. [PubMed] [Google Scholar]
- 567.Kawaguchi Y, Hama K. Physiological heterogeneity of nonpyramidal cells in rat hippocampal CA1 region. Exp Brain Res : 494–502, 1988. doi: 10.1007/BF00250594. [DOI] [PubMed] [Google Scholar]
- 568.Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol : 277–287, 2002. doi: 10.1023/A:1024126110356. [DOI] [PubMed] [Google Scholar]
- 569.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex : 476–486, 1997. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 570.Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience : 677–701, 1998. doi: 10.1016/S0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- 571.Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci : 2701–2715, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Keinänen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science : 556–560, 1990. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- 573.Kelsch W, Li Z, Eliava M, Goengrich C, Monyer H. GluN2B-containing NMDA receptors promote wiring of adult-born neurons into olfactory bulb circuits. J Neurosci : 12603–12611, 2012. doi: 10.1523/JNEUROSCI.1459-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Kelsch W, Li Z, Wieland S, Senkov O, Herb A, Göngrich C, Monyer H. GluN2B-containing NMDA receptors promote glutamate synapse development in hippocampal interneurons. J Neurosci : 16022–16030, 2014. doi: 10.1523/JNEUROSCI.1210-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature : 318–326, 2014. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Kerr AM, Reisinger E, Jonas P. Differential dependence of phasic transmitter release on synaptotagmin 1 at GABAergic and glutamatergic hippocampal synapses. Proc Natl Acad Sci USA : 15581–15586, 2008. doi: 10.1073/pnas.0800621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Kessaris N, Magno L, Rubin AN, Oliveira MG. Genetic programs controlling cortical interneuron fate. Curr Opin Neurobiol : 79–87, 2014. doi: 10.1016/j.conb.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Khazipov R, Congar P, Ben-Ari Y. Hippocampal CA1 lacunosum-moleculare interneurons: modulation of monosynaptic GABAergic IPSCs by presynaptic GABAB receptors. J Neurophysiol : 2126–2137, 1995. [DOI] [PubMed] [Google Scholar]
- 579.Khazipov R, Tyzio R, Ben-Ari Y. Effects of oxytocin on GABA signalling in the foetal brain during delivery. Prog Brain Res : 243–257, 2008. doi: 10.1016/S0079-6123(08)00421-4. [DOI] [PubMed] [Google Scholar]
- 580.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res : 57–69, 1982. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 581.Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci : 947–957, 2009. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- 582.Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature : 844–848, 2003. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 583.Klausberger T, Márton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci : 41–47, 2004. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- 584.Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci : 9782–9793, 2005. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci : 2513–2521, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science : 53–57, 2008. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science : 835–837, 1988. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 588.Knable MB, Barci BM, Bartko JJ, Webster MJ, Torrey EF. Molecular abnormalities in the major psychiatric illnesses: Classification and Regression Tree (CRT) analysis of post-mortem prefrontal markers. Mol Psychiatry : 392–404, 2002. doi: 10.1038/sj.mp.4001034. [DOI] [PubMed] [Google Scholar]
- 589.Kobayashi Y, Ye Z, Hensch TK. Clock genes control cortical critical period timing. Neuron : 264–275, 2015. doi: 10.1016/j.neuron.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science : 863–866, 2005. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- 591.Koester HJ, Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J Physiol : 625–646, 2000. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Kogo N, Dalezios Y, Capogna M, Ferraguti F, Shigemoto R, Somogyi P. Depression of GABAergic input to identified hippocampal neurons by group III metabotropic glutamate receptors in the rat. Eur J Neurosci : 2727–2740, 2004. doi: 10.1111/j.0953-816X.2004.03394.x. [DOI] [PubMed] [Google Scholar]
- 593.Koh DS, Geiger JR, Jonas P, Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol : 383–402, 1995. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Kohus Z, Káli S, Rovira-Esteban L, Schlingloff D, Papp O, Freund TF, Hájos N, Gulyás AI. Properties and dynamics of inhibitory synaptic communication within the CA3 microcircuits of pyramidal cells and interneurons expressing parvalbumin or cholecystokinin. J Physiol : 3745–3774, 2016. doi: 10.1113/JP272231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Kondo T, Kakegawa W, Yuzaki M. Induction of long-term depression and phosphorylation of the delta2 glutamate receptor by protein kinase C in cerebellar slices. Eur J Neurosci : 1817–1820, 2005. doi: 10.1111/j.1460-9568.2005.04319.x. [DOI] [PubMed] [Google Scholar]
- 596.Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry : 340–350, 2011. doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Konur S, Ghosh A. Calcium signaling and the control of dendritic development. Neuron : 401–405, 2005. doi: 10.1016/j.neuron.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 598.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : 217–238, 2010. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron : 557–569, 2010. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 600.Kosaka T, Katsumaru H, Hama K, Wu JY, Heizmann CW. GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res : 119–130, 1987. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- 601.Kosofsky BE, Molliver ME. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse : 153–168, 1987. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- 602.Kraushaar U, Jonas P. Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J Neurosci : 5594–5607, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun : 1376, 2013. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Krook-Magnuson E, Gelinas JN, Soltesz I, Buzsáki G. Neuroelectronics and Biooptics: Closed-Loop Technologies in Neurological Disorders. JAMA Neurol : 823–829, 2015. doi: 10.1001/jamaneurol.2015.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Krook-Magnuson E, Ledri M, Soltesz I, Kokaia M. How might novel technologies such as optogenetics lead to better treatments in epilepsy? Adv Exp Med Biol : 319–336, 2014. doi: 10.1007/978-94-017-8914-1_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Krook-Magnuson E, Luu L, Lee SH, Varga C, Soltesz I. Ivy and neurogliaform interneurons are a major target of μ-opioid receptor modulation. J Neurosci : 14861–14870, 2011. doi: 10.1523/JNEUROSCI.2269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Krook-Magnuson E, Varga C, Lee SH, Soltesz I. New dimensions of interneuronal specialization unmasked by principal cell heterogeneity. Trends Neurosci : 175–184, 2012. doi: 10.1016/j.tins.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron : 911–924, 2008. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Kubota D, Colgin LL, Casale M, Brucher FA, Lynch G. Endogenous waves in hippocampal slices. J Neurophysiol : 81–89, 2003. doi: 10.1152/jn.00542.2002. [DOI] [PubMed] [Google Scholar]
- 610.Kubota Y. Untangling GABAergic wiring in the cortical microcircuit. Curr Opin Neurobiol : 7–14, 2014. doi: 10.1016/j.conb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 611.Kubota Y, Karube F, Nomura M, Kawaguchi Y. The Diversity of Cortical Inhibitory Synapses. Front Neural Circuits : 27, 2016. doi: 10.3389/fncir.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex : 1803–1817, 2011. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- 613.Kuhlman SJ, Lu J, Lazarus MS, Huang ZJ. Maturation of GABAergic inhibition promotes strengthening of temporally coherent inputs among convergent pathways. PLOS Comput Biol : e1000797, 2010. doi: 10.1371/journal.pcbi.1000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature : 543–546, 2013. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Kuki T, Fujihara K, Miwa H, Tamamaki N, Yanagawa Y, Mushiake H. Contribution of parvalbumin and somatostatin-expressing GABAergic neurons to slow oscillations and the balance in beta-gamma oscillations across cortical layers. Front Neural Circuits : 6, 2015. doi: 10.3389/fncir.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Kulik A, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci : 11026–11035, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Kullmann DM, Lamsa KP. Interneurons go plastic. Neuropharmacology : 711, 2011. doi: 10.1016/j.neuropharm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 618.Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci : 687–699, 2007. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- 619.Kullmann DM, Lamsa KP. LTP and LTD in cortical GABAergic interneurons: emerging rules and roles. Neuropharmacology : 712–719, 2011. doi: 10.1016/j.neuropharm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 620.Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron : 951–962, 2012. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 621.Kuroyanagi T, Yokoyama M, Hirano T. Postsynaptic glutamate receptor delta family contributes to presynaptic terminal differentiation and establishment of synaptic transmission. Proc Natl Acad Sci USA : 4912–4916, 2009. doi: 10.1073/pnas.0900892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Lacaille JC. Postsynaptic potentials mediated by excitatory and inhibitory amino acids in interneurons of stratum pyramidale of the CA1 region of rat hippocampal slices in vitro. J Neurophysiol : 1441–1454, 1991. [DOI] [PubMed] [Google Scholar]
- 623.Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci : 1979–1993, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci : 1411–1424, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Laezza F, Dingledine R. Induction and expression rules of synaptic plasticity in hippocampal interneurons. Neuropharmacology : 720–729, 2011. doi: 10.1016/j.neuropharm.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Laezza F, Dingledine R. Voltage-controlled plasticity at GluR2-deficient synapses onto hippocampal interneurons. J Neurophysiol : 3575–3581, 2004. doi: 10.1152/jn.00425.2004. [DOI] [PubMed] [Google Scholar]
- 627.Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science : 1411–1414, 1999. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- 628.Lafourcade CA, Alger BE. Distinctions among GABAA and GABAB responses revealed by calcium channel antagonists, cannabinoids, opioids, and synaptic plasticity in rat hippocampus. Psychopharmacology (Berl) : 539–549, 2008. doi: 10.1007/s00213-007-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology , Suppl 1: S48–S58, 2009. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 630.Lambolez B, Ropert N, Perrais D, Rossier J, Hestrin S. Correlation between kinetics and RNA splicing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in neocortical neurons. Proc Natl Acad Sci USA : 1797–1802, 1996. doi: 10.1073/pnas.93.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci : 916–924, 2005. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- 632.Lamsa K, Irvine EE, Giese KP, Kullmann DM. NMDA receptor-dependent long-term potentiation in mouse hippocampal interneurons shows a unique dependence on Ca(2+)/calmodulin-dependent kinases. J Physiol : 885–894, 2007. doi: 10.1113/jphysiol.2007.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science : 1262–1266, 2007. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Lapointe V, Morin F, Ratté S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol : 125–135, 2004. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Lapray D, Lasztoczi B, Lagler M, Viney TJ, Katona L, Valenti O, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T. Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci : 1265–1271, 2012. doi: 10.1038/nn.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Larsen RS, Sjöström PJ. Synapse-type-specific plasticity in local circuits. Curr Opin Neurobiol : 127–135, 2015. doi: 10.1016/j.conb.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Lasztóczi B, Klausberger T. Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron : 1126–1139, 2014. doi: 10.1016/j.neuron.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 638.Lasztóczi B, Tukker JJ, Somogyi P, Klausberger T. Terminal field and firing selectivity of cholecystokinin-expressing interneurons in the hippocampal CA3 area. J Neurosci : 18073–18093, 2011. doi: 10.1523/JNEUROSCI.3573-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci : 7881–7888, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Lawrence JJ. Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci : 317–327, 2008. doi: 10.1016/j.tins.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 641.Lawrence JJ, Grinspan ZM, McBain CJ. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J Physiol : 175–193, 2004. doi: 10.1113/jphysiol.2003.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Lawrence JJ, Grinspan ZM, Statland JM, McBain CJ. Muscarinic receptor activation tunes mouse stratum oriens interneurones to amplify spike reliability. J Physiol : 555–562, 2006. doi: 10.1113/jphysiol.2005.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Lawrence JJ, Haario H, Stone EF. Presynaptic cholinergic neuromodulation alters the temporal dynamics of short-term depression at parvalbumin-positive basket cell synapses from juvenile CA1 mouse hippocampus. J Neurophysiol : 2408–2419, 2015. doi: 10.1152/jn.00167.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends Neurosci : 631–640, 2003. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 645.Lawrence JJ, Saraga F, Churchill JF, Statland JM, Travis KE, Skinner FK, McBain CJ. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci : 12325–12338, 2006. doi: 10.1523/JNEUROSCI.3521-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Lawrence JJ, Statland JM, Grinspan ZM, McBain CJ. Cell type-specific dependence of muscarinic signalling in mouse hippocampal stratum oriens interneurones. J Physiol : 595–610, 2006. doi: 10.1113/jphysiol.2005.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Le Bé JV, Silberberg G, Wang Y, Markram H. Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb Cortex : 2204–2213, 2007. doi: 10.1093/cercor/bhl127. [DOI] [PubMed] [Google Scholar]
- 648.Le Duigou C, Savary E, Kullmann DM, Miles R. Induction of Anti-Hebbian LTP in CA1 Stratum Oriens Interneurons: Interactions between Group I Metabotropic Glutamate Receptors and M1 Muscarinic Receptors. J Neurosci : 13542–13554, 2015. doi: 10.1523/JNEUROSCI.0956-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Leão RN, Mikulovic S, Leão KE, Munguba H, Gezelius H, Enjin A, Patra K, Eriksson A, Loew LM, Tort AB, Kullander K. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci : 1524–1530, 2012. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Lee AT, Gee SM, Vogt D, Patel T, Rubenstein JL, Sohal VS. Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron : 61–68, 2014. doi: 10.1016/j.neuron.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci : 16796–16808, 2010. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci : 1662–1670, 2013. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Lee SH, Dudok B, Parihar VK, Jung KM, Zöldi M, Kang YJ, Maroso M, Alexander AL, Nelson GA, Piomelli D, Katona I, Limoli CL, Soltesz I. Neurophysiology of space travel: energetic solar particles cause cell type-specific plasticity of neurotransmission. Brain Struct Funct : 2345–2357, 2017. doi: 10.1007/s00429-016-1345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Lee SH, Földy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci : 7993–8000, 2010. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Lee SH, Ledri M, Tóth B, Marchionni I, Henstridge CM, Dudok B, Kenesei K, Barna L, Szabó SI, Renkecz T, Oberoi M, Watanabe M, Limoli CL, Horvai G, Soltesz I, Katona I. Multiple Forms of Endocannabinoid and Endovanilloid Signaling Regulate the Tonic Control of GABA Release. J Neurosci : 10039–10057, 2015. doi: 10.1523/JNEUROSCI.4112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Lee SH, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, Soltesz I. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron : 1129–1144, 2014. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Lee SH, Soltesz I. Requirement for CB1 but not GABAB receptors in the cholecystokinin mediated inhibition of GABA release from cholecystokinin expressing basket cells. J Physiol : 891–902, 2011. doi: 10.1113/jphysiol.2010.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Lee SY, Soltesz I. Cholecystokinin: a multi-functional molecular switch of neuronal circuits. Dev Neurobiol : 83–91, 2011. doi: 10.1002/dneu.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Lee V, Maguire J. Impact of inhibitory constraint of interneurons on neuronal excitability. J Neurophysiol : 2520–2535, 2013. doi: 10.1152/jn.00047.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Lee V, Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits : 3, 2014. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron : 921–933, 2002. doi: 10.1016/S0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- 662.Lei S, McBain CJ. Two Loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J Neurosci : 2112–2121, 2004. doi: 10.1523/JNEUROSCI.4645-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsáki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science : 2049–2052, 2002. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- 664.Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron : 243–255, 1997. doi: 10.1016/S0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- 665.Lenkey N, Kirizs T, Holderith N, Máté Z, Szabó G, Vizi ES, Hájos N, Nusser Z. Tonic endocannabinoid-mediated modulation of GABA release is independent of the CB1 content of axon terminals. Nat Commun : 6557, 2015. doi: 10.1038/ncomms7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem : 19518–19524, 1998. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 667.Leppä E, Linden AM, Vekovischeva OY, Swinny JD, Rantanen V, Toppila E, Höger H, Sieghart W, Wulff P, Wisden W, Korpi ER. Removal of GABA(A) receptor γ2 subunits from parvalbumin neurons causes wide-ranging behavioral alterations. PLoS One : e24159, 2011. doi: 10.1371/journal.pone.0024159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Levelt CN, Hübener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci : 309–330, 2012. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 669.Levenga J, Krishnamurthy P, Rajamohamedsait H, Wong H, Franke TF, Cain P, Sigurdsson EM, Hoeffer CA. Tau pathology induces loss of GABAergic interneurons leading to altered synaptic plasticity and behavioral impairments. Acta Neuropathol Commun : 34, 2013. doi: 10.1186/2051-5960-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci : 312–324, 2005. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 671.Lewis TJ, Rinzel J. Dynamics of spiking neurons connected by both inhibitory and electrical coupling. J Comput Neurosci : 283–309, 2003. doi: 10.1023/A:1023265027714. [DOI] [PubMed] [Google Scholar]
- 672.Li G, Adesnik H, Li J, Long J, Nicoll RA, Rubenstein JL, Pleasure SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci : 1085–1098, 2008. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell : 634–645, 2009. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Li K-X, Lu Y-M, Xu Z-H, Zhang J, Zhu J-M, Zhang J-M, Cao S-X, Chen X-J, Chen Z, Luo J-H, Duan S, Li X-M. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci : 267–273, 2011. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- 675.Li Q, Bartley AF, Dobrunz LE. Endogenously Released Neuropeptide Y Suppresses Hippocampal Short-Term Facilitation and Is Impaired by Stress-Induced Anxiety. J Neurosci : 23–37, 2017. doi: 10.1523/JNEUROSCI.2599-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Li XG, Somogyi P, Tepper JM, Buzsáki G. Axonal and dendritic arborization of an intracellularly labeled chandelier cell in the CA1 region of rat hippocampus. Exp Brain Res : 519–525, 1992. doi: 10.1007/BF00230934. [DOI] [PubMed] [Google Scholar]
- 677.Lidov HG, Grzanna R, Molliver ME. The serotonin innervation of the cerebral cortex in the rat--an immunohistochemical analysis. Neuroscience : 207–227, 1980. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- 678.Lien CC, Martina M, Schultz JH, Ehmke H, Jonas P. Gating, modulation and subunit composition of voltage-gated K+ channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol : 405–419, 2002. doi: 10.1113/jphysiol.2001.013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Lillis KP, Kramer MA, Mertz J, Staley KJ, White JA. Pyramidal cells accumulate chloride at seizure onset. Neurobiol Dis : 358–366, 2012. doi: 10.1016/j.nbd.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Lin SH, Chien YC, Chiang WW, Liu YZ, Lien CC, Chen CC. Genetic mapping of ASIC4 and contrasting phenotype to ASIC1a in modulating innate fear and anxiety. Eur J Neurosci : 1553–1568, 2015. doi: 10.1111/ejn.12905. [DOI] [PubMed] [Google Scholar]
- 681.Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci : 3078–3089, 2007. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci : 169–182, 2012. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci : 234–242, 2008. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron : 703–713, 2005. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 685.Liu JS. Molecular genetics of neuronal migration disorders. Curr Neurol Neurosci Rep : 171–178, 2011. doi: 10.1007/s11910-010-0176-5. [DOI] [PubMed] [Google Scholar]
- 686.Liu XB, Jones EG. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc Natl Acad Sci USA : 7332–7336, 1996. doi: 10.1073/pnas.93.14.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, Hensch TK, Arlotta P. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron : 763–779, 2011. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Lodato S, Tomassy GS, De Leonibus E, Uzcategui YG, Andolfi G, Armentano M, Touzot A, Gaztelu JM, Arlotta P, Menendez de la Prida L, Studer M. Loss of COUP-TFI alters the balance between caudal ganglionic eminence- and medial ganglionic eminence-derived cortical interneurons and results in resistance to epilepsy. J Neurosci : 4650–4662, 2011. doi: 10.1523/JNEUROSCI.6580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.López-Bendito G, Luján R, Shigemoto R, Ganter P, Paulsen O, Molnár Z. Blockade of GABA(B) receptors alters the tangential migration of cortical neurons. Cereb Cortex : 932–942, 2003. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- 690.López-Bendito G, Sánchez-Alcañiz JA, Pla R, Borrell V, Picó E, Valdeolmillos M, Marín O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci : 1613–1624, 2008. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.López-Bendito G, Shigemoto R, Kulik A, Vida I, Fairén A, Luján R. Distribution of metabotropic GABA receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus during prenatal and postnatal development. Hippocampus : 836–848, 2004. doi: 10.1002/hipo.10221. [DOI] [PubMed] [Google Scholar]
- 692.Lopez-Tellez JF, Vela J, del Rio JC, Ramos B, Baglietto-Vargas D, Santa-Maria C, Ruano D, Gutierrez A, Vitorica J. Postnatal development of the alpha1 containing GABAA receptor subunit in rat hippocampus. Brain Res Dev Brain Res : 129–141, 2004. doi: 10.1016/j.devbrainres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 693.Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron : 355–363, 2000. doi: 10.1016/S0896-6273(00)00115-X. [DOI] [PubMed] [Google Scholar]
- 694.Lorente de No R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol : 113–177, 1934. [Google Scholar]
- 695.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci : 14329–14340, 2008. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Löscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res : 105–123, 2002. doi: 10.1016/S0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- 697.Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure : 359–368, 2011. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 698.Löscher W, Ebert U, Lehmann H, Rosenthal C, Nikkhah G. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res : 196–209, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 699.Losonczy A, Biró AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA : 1362–1367, 2004. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Losonczy A, Somogyi P, Nusser Z. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J Neurophysiol : 1910–1919, 2003. doi: 10.1152/jn.00842.2002. [DOI] [PubMed] [Google Scholar]
- 701.Lourenço J, Cannich A, Carta M, Coussen F, Mulle C, Marsicano G. Synaptic activation of kainate receptors gates presynaptic CB(1) signaling at GABAergic synapses. Nat Neurosci : 197–204, 2010. doi: 10.1038/nn.2481. [DOI] [PubMed] [Google Scholar]
- 702.Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science : 857–863, 2014. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci : 423–430, 2012. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- 704.Lu HC, Mackie K. An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry : 516–525, 2016. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Lu JT, Li CY, Zhao JP, Poo MM, Zhang XH. Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J Neurosci : 9711–9720, 2007. doi: 10.1523/JNEUROSCI.2513-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Lu T, Trussell LO. Inhibitory transmission mediated by asynchronous transmitter release. Neuron : 683–694, 2000. doi: 10.1016/S0896-6273(00)81204-0. [DOI] [PubMed] [Google Scholar]
- 707.Ludányi A, Eross L, Czirják S, Vajda J, Halász P, Watanabe M, Palkovits M, Maglóczky Z, Freund TF, Katona I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci : 2976–2990, 2008. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Ludlow M, Kortekaas J, Herden C, Hoffmann B, Tappe D, Trebst C, Griffin DE, Brindle HE, Solomon T, Brown AS, van Riel D, Wolthers KC, Pajkrt D, Wohlsein P, Martina BEE, Baumgärtner W, Verjans GM, Osterhaus ADME. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol : 159–184, 2016. doi: 10.1007/s00401-015-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature : 587–591, 1998. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 710.Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci : 1488–1500, 1996. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 711.Lupica CR, Proctor WR, Dunwiddie TV. Dissociation of mu and delta opioid receptor-mediated reductions in evoked and spontaneous synaptic inhibition in the rat hippocampus in vitro. Brain Res : 226–238, 1992. doi: 10.1016/0006-8993(92)91312-3. [DOI] [PubMed] [Google Scholar]
- 712.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry : 383–406, 2011. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Ma QL, Yang F, Frautschy SA, Cole GM. PAK in Alzheimer disease, Huntington disease and X-linked mental retardation. Cell Logist : 117–125, 2012. doi: 10.4161/cl.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, Liu F, You Y, Chen C, Campbell K, Song H, Ma L, Rubenstein JL, Yang Z. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci : 1588–1597, 2013. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- 715.Ma T, Zhang Q, Cai Y, You Y, Rubenstein JL, Yang Z. A subpopulation of dorsal lateral/caudal ganglionic eminence-derived neocortical interneurons expresses the transcription factor Sp8. Cereb Cortex : 2120–2130, 2012. doi: 10.1093/cercor/bhr296. [DOI] [PubMed] [Google Scholar]
- 716.Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci : 5069–5082, 2006. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol : 728–741, 2008. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 718.Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol : 119–130, 1996. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci : 5334–5343, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron : 137–145, 1995. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 721.Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol : 91–116, 2000. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Maccaferri G, Tóth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science : 1368–1370, 1998. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- 723.Maccarrone M, Guzmán M, Mackie K, Doherty P, Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci : 786–801, 2014. doi: 10.1038/nrn3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A, Bernardi G, Bagni C, Centonze D. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology : 1500–1509, 2010. doi: 10.1038/npp.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci : 443–485, 1999. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 726.MacKenzie G, Maguire J. The role of ovarian hormone-derived neurosteroids on the regulation of GABAA receptors in affective disorders. Psychopharmacology (Berl) : 3333–3342, 2014. doi: 10.1007/s00213-013-3423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol , Suppl 1: 10–14, 2008. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 728.Madison DV, Nicoll RA. Enkephalin hyperpolarizes interneurones in the rat hippocampus. J Physiol : 123–130, 1988. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron : 463–475, 2001. doi: 10.1016/S0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 730.Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci : 7613–7624, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Maglóczky Z, Tóth K, Karlócai R, Nagy S, Eross L, Czirják S, Vajda J, Rásonyi G, Kelemen A, Juhos V, Halász P, Mackie K, Freund TF. Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia , Suppl 3: 115–120, 2010. doi: 10.1111/j.1528-1167.2010.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron : 207–213, 2008. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci : 797–804, 2005. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 734.Maingret F, Lauri SE, Taira T, Isaac JT. Profound regulation of neonatal CA1 rat hippocampal GABAergic transmission by functionally distinct kainate receptor populations. J Physiol : 131–142, 2005. doi: 10.1113/jphysiol.2005.089474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell : 1223–1241, 2012. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Manent JB, Jorquera I, Ben-Ari Y, Aniksztejn L, Represa A. Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. J Neurosci : 5901–5909, 2006. doi: 10.1523/JNEUROSCI.1033-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Manent JB, Jorquera I, Mazzucchelli I, Depaulis A, Perucca E, Ben-Ari Y, Represa A. Fetal exposure to GABA-acting antiepileptic drugs generates hippocampal and cortical dysplasias. Epilepsia : 684–693, 2007. doi: 10.1111/j.1528-1167.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 738.Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci : 7513–7518, 2009. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci : 205–212, 2010. doi: 10.1038/nn.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron : 105–117, 2005. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 741.Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci : 2587–2600, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Mao W, Watanabe T, Cho S, Frost JL, Truong T, Zhao X, Futai K. Shank1 regulates excitatory synaptic transmission in mouse hippocampal parvalbumin-expressing inhibitory interneurons. Eur J Neurosci : 1025–1035, 2015. doi: 10.1111/ejn.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain : 499–530, 1966. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 744.Marín O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur J Neurosci : 2019–2029, 2013. doi: 10.1111/ejn.12225. [DOI] [PubMed] [Google Scholar]
- 745.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci : 107–120, 2012. [DOI] [PubMed] [Google Scholar]
- 746.Marín O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci : 780–790, 2001. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 747.Marín O, Valiente M, Ge X, Tsai LH. Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol : a001834, 2010. doi: 10.1101/cshperspect.a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Marín O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science : 872–875, 2001. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 749.Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci USA : 5323–5328, 1998. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Marks WD, Paris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, Knapp PE, Hauser KF. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol : 747–762, 2016. doi: 10.1007/s13365-016-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Markwardt SJ, Dieni CV, Wadiche JI, Overstreet-Wadiche L. Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat Neurosci : 1407–1409, 2011. doi: 10.1038/nn.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, Widmer HR, Eggan K, Goldstein PA, Anderson SA, Studer L. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell : 559–572, 2013. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Marsh E, Fulp C, Gomez E, Nasrallah I, Minarcik J, Sudi J, Christian SL, Mancini G, Labosky P, Dobyns W, Brooks-Kayal A, Golden JA. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain : 1563–1576, 2009. doi: 10.1093/brain/awp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci : 4213–4225, 1999. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 755.Martina M, Jonas P. Functional differences in Na+ channel gating between fast-spiking interneurones and principal neurones of rat hippocampus. J Physiol : 593–603, 1997. doi: 10.1111/j.1469-7793.1997.593ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci : 8111–8125, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Martina M, Vida I, Jonas P. Distal initiation and active propagation of action potentials in interneuron dendrites. Science : 295–300, 2000. doi: 10.1126/science.287.5451.295. [DOI] [PubMed] [Google Scholar]
- 758.Martínez-Cerdeño V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell : 238–250, 2010. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Marx M, Haas CA, Häussler U. Differential vulnerability of interneurons in the epileptic hippocampus. Front Cell Neurosci : 167, 2013. doi: 10.3389/fncel.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Massi L, Lagler M, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T. Temporal dynamics of parvalbumin-expressing axo-axonic and basket cells in the rat medial prefrontal cortex in vivo. J Neurosci : 16496–16502, 2012. doi: 10.1523/JNEUROSCI.3475-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci : 585–586, 2004. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- 762.Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JT. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron : 339–351, 2011. doi: 10.1016/j.neuron.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci : 1032–1041, 2013. doi: 10.1038/nn.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Maxwell WL, Dhillon K, Harper L, Espin J, MacIntosh TK, Smith DH, Graham DI. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J Neuropathol Exp Neurol : 272–279, 2003. doi: 10.1093/jnen/62.3.272. [DOI] [PubMed] [Google Scholar]
- 765.Mayer C, Bandler RC, Fishell G. Lineage Is a Poor Predictor of Interneuron Positioning within the Forebrain. Neuron : 45–51, 2016. doi: 10.1016/j.neuron.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Mayer C, Jaglin XH, Cobbs LV, Bandler RC, Streicher C, Cepko CL, Hippenmeyer S, Fishell G. Clonally Related Forebrain Interneurons Disperse Broadly across Both Functional Areas and Structural Boundaries. Neuron : 989–998, 2015. doi: 10.1016/j.neuron.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol : 699–714, 2010. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 768.McBain CJ, DiChiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J Neurosci : 4433–4445, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.McBain CJ, Dingledine R. Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol : 373–392, 1993. doi: 10.1113/jphysiol.1993.sp019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci : 11–23, 2001. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 771.McBain CJ, Freund TF, Mody I. Glutamatergic synapses onto hippocampal interneurons: precision timing without lasting plasticity. Trends Neurosci : 228–235, 1999. doi: 10.1016/S0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- 772.McBain CJ, Kauer JA. Presynaptic plasticity: targeted control of inhibitory networks. Curr Opin Neurobiol : 254–262, 2009. doi: 10.1016/j.conb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.McMahon LL, Kauer JA. Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol : 2493–2502, 1997. [DOI] [PubMed] [Google Scholar]
- 774.McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron : 295–305, 1997. doi: 10.1016/S0896-6273(00)80269-X. [DOI] [PubMed] [Google Scholar]
- 775.McQuiston AR. Layer selective presynaptic modulation of excitatory inputs to hippocampal cornu Ammon 1 by mu-opioid receptor activation. Neuroscience : 209–221, 2008. doi: 10.1016/j.neuroscience.2007.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.McQuiston AR. Mu opioid receptor activation normalizes temporo-ammonic pathway driven inhibition in hippocampal CA1. Neuropharmacology : 472–479, 2011. doi: 10.1016/j.neuropharm.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.McQuiston AR, Madison DV. Muscarinic receptor activity has multiple effects on the resting membrane potentials of CA1 hippocampal interneurons. J Neurosci : 5693–5702, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.McQuiston AR, Madison DV. Muscarinic receptor activity induces an afterdepolarization in a subpopulation of hippocampal CA1 interneurons. J Neurosci : 5703–5710, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci : 2887–2896, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci USA : 18601–18606, 2012. doi: 10.1073/pnas.1211507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science : 1506–1510, 2012. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- 782.Menegola M, Misonou H, Vacher H, Trimmer JS. Dendritic A-type potassium channel subunit expression in CA1 hippocampal interneurons. Neuroscience : 953–964, 2008. doi: 10.1016/j.neuroscience.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Menendez de la Prida L, Bolea S, Sanchez-Andres JV. Origin of the synchronized network activity in the rabbit developing hippocampus. Eur J Neurosci : 899–906, 1998. doi: 10.1046/j.1460-9568.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- 784.Merker B. Cortical gamma oscillations: the functional key is activation, not cognition. Neurosci Biobehav Rev : 401–417, 2013. doi: 10.1016/j.neubiorev.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 785.Merrill CB, McNeil M, Williamson RC, Poole BR, Nelson B, Sudweeks S, Edwards JG. Identification of mRNA for endocannabinoid biosynthetic enzymes within hippocampal pyramidal cells and CA1 stratum radiatum interneuron subtypes using quantitative real-time polymerase chain reaction. Neuroscience : 89–99, 2012. doi: 10.1016/j.neuroscience.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Middleton S, Jalics J, Kispersky T, Lebeau FE, Roopun AK, Kopell NJ, Whittington MA, Cunningham MO. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci USA : 18572–18577, 2008. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Mikulovic S, Restrepo CE, Hilscher MM, Kullander K, Leão RN. Novel markers for OLM interneurons in the hippocampus. Front Cell Neurosci : 201, 2015. doi: 10.3389/fncel.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Milenkovic I, Vasiljevic M, Maurer D, Höger H, Klausberger T, Sieghart W. The parvalbumin-positive interneurons in the mouse dentate gyrus express GABAA receptor subunits α1, β2, and δ along their extrasynaptic cell membrane. Neuroscience : 80–96, 2013. doi: 10.1016/j.neuroscience.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 789.Miles R, Poncer JC. Metabotropic glutamate receptors mediate a post-tetanic excitation of guinea-pig hippocampal inhibitory neurones. J Physiol : 461–473, 1993. doi: 10.1113/jphysiol.1993.sp019605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron : 815–823, 1996. doi: 10.1016/S0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 791.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet : 1415–1423, 2000. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 792.Miller KK, Hoffer A, Svoboda KR, Lupica CR. Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons. J Neurosci : 4994–5003, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Min MY, Melyan Z, Kullmann DM. Synaptically released glutamate reduces gamma-aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors. Proc Natl Acad Sci USA : 9932–9937, 1999. doi: 10.1073/pnas.96.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci : 7786–7798, 2007. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex : 845–852, 2011. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci : 1582–1594, 2010. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Miyoshi G, Young A, Petros T, Karayannis T, McKenzie Chang M, Lavado A, Iwano T, Nakajima M, Taniguchi H, Huang ZJ, Heintz N, Oliver G, Matsuzaki F, Machold RP, Fishell G. Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons. J Neurosci : 12869–12889, 2015. doi: 10.1523/JNEUROSCI.1164-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron : 817–831, 2005. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 799.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron : 529–540, 1994. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 800.Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron : 799–810, 1991. doi: 10.1016/0896-6273(91)90176-Z. [DOI] [PubMed] [Google Scholar]
- 801.Morales M, Hein K, Vogel Z. Hippocampal interneurons co-express transcripts encoding the alpha7 nicotinic receptor subunit and the cannabinoid receptor 1. Neuroscience : 70–81, 2008. doi: 10.1016/j.neuroscience.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Moreau AW, Kullmann DM. NMDA receptor-dependent function and plasticity in inhibitory circuits. Neuropharmacology : 23–31, 2013. doi: 10.1016/j.neuropharm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 803.Moreno H, Kentros C, Bueno E, Weiser M, Hernandez A, Vega-Saenz de Miera E, Ponce A, Thornhill W, Rudy B. Thalamocortical projections have a K+ channel that is phosphorylated and modulated by cAMP-dependent protein kinase. J Neurosci : 5486–5501, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Moreno JL, Kurita M, Holloway T, López J, Cadagan R, Martínez-Sobrido L, García-Sastre A, González-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J Neurosci : 1863–1872, 2011. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Morozov YM, Torii M, Rakic P. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb Cortex , Suppl 1: i78–i89, 2009. doi: 10.1093/cercor/bhp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Morris BJ, Johnston HM. A role for hippocampal opioids in long-term functional plasticity. Trends Neurosci : 350–355, 1995. doi: 10.1016/0166-2236(95)93927-P. [DOI] [PubMed] [Google Scholar]
- 807.Mostallino MC, Sanna E, Concas A, Biggio G, Follesa P. Plasticity and function of extrasynaptic GABA(A) receptors during pregnancy and after delivery. Psychoneuroendocrinology , Suppl 1: S74–S83, 2009. doi: 10.1016/j.psyneuen.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 808.Motalli R, Louvel J, Tancredi V, Kurcewicz I, Wan-Chow-Wah D, Pumain R, Avoli M. GABA(B) receptor activation promotes seizure activity in the juvenile rat hippocampus. J Neurophysiol : 638–647, 1999. [DOI] [PubMed] [Google Scholar]
- 809.Mott DD, Bragdon AC, Lewis DV, Wilson WA. Baclofen has a proepileptic effect in the rat dentate gyrus. J Pharmacol Exp Ther : 721–725, 1989. [PubMed] [Google Scholar]
- 810.Mott DD, Lewis DV. Facilitation of the induction of long-term potentiation by GABAB receptors. Science : 1718–1720, 1991. doi: 10.1126/science.1675489. [DOI] [PubMed] [Google Scholar]
- 811.Mott DD, Li Q, Okazaki MM, Turner DA, Lewis DV. GABAB-receptor-mediated currents in interneurons of the dentate-hilus border. J Neurophysiol : 1438–1450, 1999. [DOI] [PubMed] [Google Scholar]
- 812.Mott DD, Turner DA, Okazaki MM, Lewis DV. Interneurons of the dentate-hilus border of the rat dentate gyrus: morphological and electrophysiological heterogeneity. J Neurosci : 3990–4005, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature : 245–248, 1996. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 814.Mühlethaler M, Dreifuss JJ. Excitation of hippocampal neurones by posterior pituitary peptides: vasopressin and oxytocin compared. Prog Brain Res : 147–151, 1983. doi: 10.1016/S0079-6123(08)64382-4. [DOI] [PubMed] [Google Scholar]
- 815.Mulder J, Aguado T, Keimpema E, Barabás K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzmán M, Lu HC, Galve-Roperh I, Harkany T. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci USA : 8760–8765, 2008. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Mulle C, Sailer A, Swanson GT, Brana C, O’Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron : 475–484, 2000. doi: 10.1016/S0896-6273(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 817.Müller C, Remy S. Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Front Synaptic Neurosci : 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Murray AJ, Sauer J-F, Riedel G, McClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci : 297–299, 2011. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Murthy S, Niquille M, Hurni N, Limoni G, Frazer S, Chameau P, van Hooft JA, Vitalis T, Dayer A. Serotonin receptor 3A controls interneuron migration into the neocortex. Nat Commun : 5524, 2014. doi: 10.1038/ncomms6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Nacher J, Guirado R, Castillo-Gómez E. Structural plasticity of interneurons in the adult brain: role of PSA-NCAM and implications for psychiatric disorders. Neurochem Res : 1122–1133, 2013. doi: 10.1007/s11064-013-0977-4. [DOI] [PubMed] [Google Scholar]
- 821.Nagode DA, Tang AH, Karson MA, Klugmann M, Alger BE. Optogenetic release of ACh induces rhythmic bursts of perisomatic IPSCs in hippocampus. PLoS One : e27691, 2011. doi: 10.1371/journal.pone.0027691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Nakanishi N, Shneider NA, Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron : 569–581, 1990. doi: 10.1016/0896-6273(90)90212-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron : 781–787, 2009. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell : 429–441, 2002. doi: 10.1016/S0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 825.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology : 1574–1583, 2012. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Narboux-Nême N, Goïame R, Mattéi MG, Cohen-Tannoudji M, Wassef M. Integration of H-2Z1, a somatosensory cortex-expressed transgene, interferes with the expression of the Satb1 and Tbc1d5 flanking genes and affects the differentiation of a subset of cortical interneurons. J Neurosci : 7287–7300, 2012. doi: 10.1523/JNEUROSCI.6068-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Navarrete M, Díez A, Araque A. Astrocytes in endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci : 20130599, 2014. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Nayeem N, Green TP, Martin IL, Barnard EA. Quaternary structure of the native GABAA receptor determined by electron microscopic image analysis. J Neurochem : 815–818, 1994. doi: 10.1046/j.1471-4159.1994.62020815.x. [DOI] [PubMed] [Google Scholar]
- 829.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci : 1279–1287, 2002. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 830.Neske GT, Connors BW. Synchronized gamma-frequency inhibition in neocortex depends on excitatory-inhibitory interactions but not electrical synapses. J Neurophysiol : 351–368, 2016. doi: 10.1152/jn.00071.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Neske GT, Patrick SL, Connors BW. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J Neurosci : 1089–1105, 2015. doi: 10.1523/JNEUROSCI.2279-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci : 1161–1169, 2010. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Neu A, Földy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol : 233–247, 2007. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Nicholson E, Kullmann DM. Long-term potentiation in hippocampal oriens interneurons: postsynaptic induction, presynaptic expression and evaluation of candidate retrograde factors. Philos Trans R Soc Lond B Biol Sci : 20130133, 2013. doi: 10.1098/rstb.2013.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Nicoll RA, Alger BE, Jahr CE, Morley JS. Peptides as putative excitatory neurotransmitters: carnosine, enkephalin, substance P and TRH. Proc R Soc Lond B Biol Sci : 133–149, 1980. doi: 10.1098/rspb.1980.0124. [DOI] [PubMed] [Google Scholar]
- 836.Nicoll RA, Siggins GR, Ling N, Bloom FE, Guillemin R. Neuronal actions of endorphins and enkephalins among brain regions: a comparative microiontophoretic study. Proc Natl Acad Sci USA : 2584–2588, 1977. doi: 10.1073/pnas.74.6.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Nieto-Gonzalez JL, Jensen K. BDNF Depresses Excitability of Parvalbumin-Positive Interneurons through an M-Like Current in Rat Dentate Gyrus. PLoS One : e67318, 2013. doi: 10.1371/journal.pone.0067318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Nissen W, Szabo A, Somogyi J, Somogyi P, Lamsa KP. Cell type-specific long-term plasticity at glutamatergic synapses onto hippocampal interneurons expressing either parvalbumin or CB1 cannabinoid receptor. J Neurosci : 1337–1347, 2010. doi: 10.1523/JNEUROSCI.3481-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol : 295–322, 2010. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Nitsch R, Soriano E, Frotscher M. The parvalbumin-containing nonpyramidal neurons in the rat hippocampus. Anat Embryol (Berl) : 413–425, 1990. doi: 10.1007/BF02433788. [DOI] [PubMed] [Google Scholar]
- 841.Nitz DA, McNaughton BL. Hippocampal EEG and unit activity responses to modulation of serotonergic median raphe neurons in the freely behaving rat. Learn Mem : 153–167, 1999. [PMC free article] [PubMed] [Google Scholar]
- 842.Nóbrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marín O. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron : 733–745, 2008. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843.Nunzi MG, Gorio A, Milan F, Freund TF, Somogyi P, Smith AD. Cholecystokinin-immunoreactive cells form symmetrical synaptic contacts with pyramidal and nonpyramidal neurons in the hippocampus. J Comp Neurol : 485–505, 1985. doi: 10.1002/cne.902370406. [DOI] [PubMed] [Google Scholar]
- 844.Nurse S, Lacaille JC. Do GABAA and GABAB inhibitory postsynaptic responses originate from distinct interneurons in the hippocampus? Can J Physiol Pharmacol : 520–525, 1997. doi: 10.1139/y97-064. [DOI] [PubMed] [Google Scholar]
- 845.Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat : 165–175, 2015. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron : 545–559, 1998. doi: 10.1016/S0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 847.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res : 171–175, 1971. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 848.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon, 1978. [Google Scholar]
- 849.O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus : 317–330, 1993. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 850.O’Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci : 220–229, 2010. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 851.O’Neill J, Senior T, Csicsvari J. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron : 143–155, 2006. doi: 10.1016/j.neuron.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 852.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci : 5903–5914, 2007. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci : 173–179, 2010. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- 854.Ohno-Shosaku T, Hashimotodani Y, Ano M, Takeda S, Tsubokawa H, Kano M. Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol : 407–418, 2007. doi: 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron : 729–738, 2001. doi: 10.1016/S0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 856.Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci : 3864–3872, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 857.Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci : 7040–7052, 2009. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Oláh S, Füle M, Komlósi G, Varga C, Báldi R, Barzó P, Tamás G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature : 1278–1281, 2009. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859.Oláh S, Komlósi G, Szabadics J, Varga C, Tóth E, Barzó P, Tamás G. Output of neurogliaform cells to various neuron types in the human and rat cerebral cortex. Front Neural Circuits : 4, 2007. doi: 10.3389/neuro.04.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Oleskevich S, Lacaille JC. Reduction of GABAB inhibitory postsynaptic potentials by serotonin via pre- and postsynaptic mechanisms in CA3 pyramidal cells of rat hippocampus in vitro. Synapse : 173–188, 1992. doi: 10.1002/syn.890120302. [DOI] [PubMed] [Google Scholar]
- 861.Oliva A, Fernández-Ruiz A, Buzsáki G, Berényi A. Role of Hippocampal CA2 Region in Triggering Sharp-Wave Ripples. Neuron : 1342–1355, 2016. doi: 10.1016/j.neuron.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Oliva AA Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci : 3354–3368, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev : 243–260, 2008. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Omiya Y, Uchigashima M, Konno K, Yamasaki M, Miyazaki T, Yoshida T, Kusumi I, Watanabe M. VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. J Neurosci : 4215–4228, 2015. doi: 10.1523/JNEUROSCI.4681-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Oren I, Hájos N, Paulsen O. Identification of the current generator underlying cholinergically induced gamma frequency field potential oscillations in the hippocampal CA3 region. J Physiol : 785–797, 2010. doi: 10.1113/jphysiol.2009.180851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Oren I, Mann EO, Paulsen O, Hájos N. Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci : 9923–9934, 2006. doi: 10.1523/JNEUROSCI.1580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.Oren I, Nissen W, Kullmann DM, Somogyi P, Lamsa KP. Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J Neurosci : 939–950, 2009. doi: 10.1523/JNEUROSCI.3251-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J Neurosci : 420–433, 2004. doi: 10.1523/JNEUROSCI.4452-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.Ouardouz M, Lacaille JC. Mechanisms of selective long-term potentiation of excitatory synapses in stratum oriens/alveus interneurons of rat hippocampal slices. J Neurophysiol : 810–819, 1995. [DOI] [PubMed] [Google Scholar]
- 870.Overstreet-Wadiche L, McBain CJ. Neurogliaform cells in cortical circuits. Nat Rev Neurosci : 458–468, 2015. doi: 10.1038/nrn3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature : 458–462, 2013. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci : 6414–6423, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Owens DF, Liu X, Kriegstein AR. Changing properties of GABA(A) receptor-mediated signaling during early neocortical development. J Neurophysiol : 570–583, 1999. [DOI] [PubMed] [Google Scholar]
- 874.Ozawa S, Iino M, Tsuzuki K. Two types of kainate response in cultured rat hippocampal neurons. J Neurophysiol : 2–11, 1991. [DOI] [PubMed] [Google Scholar]
- 875.Pabst M, Braganza O, Dannenberg H, Hu W, Pothmann L, Rosen J, Mody I, van Loo K, Deisseroth K, Becker AJ, Schoch S, Beck H. Astrocyte Intermediaries of Septal Cholinergic Modulation in the Hippocampus. Neuron : 853–865, 2016. doi: 10.1016/j.neuron.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 876.Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci : 13260–13271, 2011. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol : 435–440, 2009. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 878.Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci : 13420–13430, 2011. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879.Pangalos M, Donoso JR, Winterer J, Zivkovic AR, Kempter R, Maier N, Schmitz D. Recruitment of oriens-lacunosum-moleculare interneurons during hippocampal ripples. Proc Natl Acad Sci USA : 4398–4403, 2013. doi: 10.1073/pnas.1215496110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 880.Pangratz-Fuehrer S, Hestrin S. Synaptogenesis of electrical and GABAergic synapses of fast-spiking inhibitory neurons in the neocortex. J Neurosci : 10767–10775, 2011. doi: 10.1523/JNEUROSCI.6655-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 881.Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol : 299–327, 1996. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 882.Parihar VK, Allen B, Tran KK, Macaraeg TG, Chu EM, Kwok SF, Chmielewski NN, Craver BM, Baulch JE, Acharya MM, Cucinotta FA, Limoli CL. What happens to your brain on the way to Mars. Sci Adv : e1400256, 2015. doi: 10.1126/sciadv.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J Neurosci : 196–205, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.Pawelzik H, Hughes DI, Thomson AM. Modulation of inhibitory autapses and synapses on rat CA1 interneurones by GABA(A) receptor ligands. J Physiol : 701–716, 2003. doi: 10.1113/jphysiol.2002.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Pawelzik H, Hughes DI, Thomson AM. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. J Comp Neurol : 346–367, 2002. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- 886.Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, Mitchell RM, Wyeth MS, Petralia RS, Chittajallu R, Karlsson RM, Cameron HA, Murata Y, Colonnese MT, Worley PF, McBain CJ. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron : 1257–1272, 2015. doi: 10.1016/j.neuron.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron : 89–102, 2005. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 888.Pelkey KA, McBain CJ. Differential regulation at functionally divergent release sites along a common axon. Curr Opin Neurobiol : 366–373, 2007. doi: 10.1016/j.conb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 889.Pelkey KA, McBain CJ. Target-cell-dependent plasticity within the mossy fibre-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. J Physiol : 1495–1502, 2008. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Pelkey KA, Topolnik L, Lacaille JC, McBain CJ. Compartmentalized Ca(2+) channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron : 497–510, 2006. doi: 10.1016/j.neuron.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 891.Pelkey KA, Topolnik L, Yuan XQ, Lacaille JC, McBain CJ. State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit. Neuron : 980–987, 2008. doi: 10.1016/j.neuron.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell : 235–246, 2011. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 893.Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol : 179–197, 2002. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 894.Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci : 14392–14405, 2013. doi: 10.1523/JNEUROSCI.2045-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Pennell PB. Using current evidence in selecting antiepileptic drugs for use during pregnancy. Epilepsy Curr : 45–51, 2005. doi: 10.1111/j.1535-7597.2005.05201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci USA : 9401–9406, 2001. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897.Persson A, Sim SC, Virding S, Onishchenko N, Schulte G, Ingelman-Sundberg M. Decreased hippocampal volume and increased anxiety in a transgenic mouse model expressing the human CYP2C19 gene. Mol Psychiatry : 733–741, 2014. doi: 10.1038/mp.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898.Perszyk RE, DiRaddo JO, Strong KL, Low CM, Ogden KK, Khatri A, Vargish GA, Pelkey KA, Tricoire L, Liotta DC, Smith Y, McBain CJ, Traynelis SF. GluN2D-Containing N-Methyl-d-Aspartate Receptors Mediate Synaptic Transmission in Hippocampal Interneurons and Regulate Interneuron Activity. Mol Pharmacol : 689–702, 2016. doi: 10.1124/mol.116.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 899.Pesold C, Liu WS, Guidotti A, Costa E, Caruncho HJ. Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete reelin into perineuronal nets, nonsynaptically modulating gene expression. Proc Natl Acad Sci USA : 3217–3222, 1999. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Péterfi Z, Urbán GM, Papp OI, Németh B, Monyer H, Szabó G, Erdélyi F, Mackie K, Freund TF, Hájos N, Katona I. Endocannabinoid-mediated long-term depression of afferent excitatory synapses in hippocampal pyramidal cells and GABAergic interneurons. J Neurosci : 14448–14463, 2012. doi: 10.1523/JNEUROSCI.1676-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Peters A. Morphological correlates of epilepsy: cells in the cerebral cortex. Adv Neurol : 21–48, 1980. [PubMed] [Google Scholar]
- 902.Petilla Interneuron Nomenclature Group, Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvárday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Muñoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci : 557–568, 2008. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology : 1281–1286, 1987. doi: 10.1212/WNL.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 904.Petros TJ, Bultje RS, Ross ME, Fishell G, Anderson SA. Apical Versus Basal Neurogenesis Directs Cortical Interneuron Subclass Fate. Cell Reports : 1090–1095, 2015. doi: 10.1016/j.celrep.2015.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 905.Pfeffer CK. Inhibitory neurons: vip cells hit the brake on inhibition. Curr Biol : R18–R20, 2014. doi: 10.1016/j.cub.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 906.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci : 1068–1076, 2013. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 907.Pfeiffer BE, Huber KM. Current advances in local protein synthesis and synaptic plasticity. J Neurosci : 7147–7150, 2006. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 908.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature : 521–524, 2013. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909.Picardo MA, Guigue P, Bonifazi P, Batista-Brito R, Allene C, Ribas A, Fishell G, Baude A, Cossart R. Pioneer GABA cells comprise a subpopulation of hub neurons in the developing hippocampus. Neuron : 695–709, 2011. doi: 10.1016/j.neuron.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 910.Pietersen AN, Ward PD, Hagger-Vaughan N, Wiggins J, Jefferys JG, Vreugdenhil M. Transition between fast and slow gamma modes in rat hippocampus area CA1 in vitro is modulated by slow CA3 gamma oscillations. J Physiol : 605–620, 2014. doi: 10.1113/jphysiol.2013.263889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.Pitler TA, Alger BE. Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J Physiol : 127–142, 1992. doi: 10.1113/jphysiol.1992.sp019119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science : 1248–1251, 2002. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 913.Pla R, Borrell V, Flames N, Marín O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci : 6924–6934, 2006. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 914.Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, Rubenstein JL. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron : 727–740, 2000. doi: 10.1016/S0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 915.Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci : 1331–1339, 2013. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916.Poncer JC, McKinney RA, Gahwiler BH, Thompson SM. Differential control of GABA release at synapses from distinct interneurons in rat hippocampus. J Physiol : 123–130, 2000. doi: 10.1111/j.1469-7793.2000.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Poncer JC, Miles R. Fast and slow excitation of inhibitory cells in the CA3 region of the hippocampus. J Neurobiol : 386–395, 1995. doi: 10.1002/neu.480260310. [DOI] [PubMed] [Google Scholar]
- 918.Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron : 850–861, 2009. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 919.Porcher C, Hatchett C, Longbottom RE, McAinch K, Sihra TS, Moss SJ, Thomson AM, Jovanovic JN. Positive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J Biol Chem : 21667–21677, 2011. doi: 10.1074/jbc.M110.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Porter JT, Cauli B, Staiger JF, Lambolez B, Rossier J, Audinat E. Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur J Neurosci : 3617–3628, 1998. doi: 10.1046/j.1460-9568.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- 921.Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci : 5228–5235, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science : 1159–1163, 2001. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 923.Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature : 717–723, 2004. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- 924.Pouille F, Watkinson O, Scanziani M, Trevelyan AJ. The contribution of synaptic location to inhibitory gain control in pyramidal cells. Physiol Rep : e00067, 2013. doi: 10.1002/phy2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925.Povysheva NV, Johnson JW. Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons. J Neurophysiol : 2232–2243, 2012. doi: 10.1152/jn.01017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 926.Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci : 6775–6786, 2005. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927.Price CJ, Scott R, Rusakov DA, Capogna M. GABA(B) receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J Neurosci : 6974–6982, 2008. doi: 10.1523/JNEUROSCI.4673-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Prönneke A, Scheuer B, Wagener RJ, Möck M, Witte M, Staiger JF. Characterizing VIP Neurons in the Barrel Cortex of VIPcre/tdTomato Mice Reveals Layer-Specific Differences. Cereb Cortex : 4854–4868, 2015. doi: 10.1093/cercor/bhv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929.Puente N, Reguero L, Elezgarai I, Canduela MJ, Mendizabal-Zubiaga J, Ramos-Uriarte A, Fernández-Espejo E, Grandes P. The transient receptor potential vanilloid-1 is localized at excitatory synapses in the mouse dentate gyrus. Brain Struct Funct : 1187–1194, 2015. doi: 10.1007/s00429-014-0711-2. [DOI] [PubMed] [Google Scholar]
- 930.Puighermanal E, Biever A, Espallergues J, Gangarossa G, De Bundel D, Valjent E. drd2-cre:ribotag mouse line unravels the possible diversity of dopamine d2 receptor-expressing cells of the dorsal mouse hippocampus. Hippocampus : 858–875, 2015. doi: 10.1002/hipo.22408. [DOI] [PubMed] [Google Scholar]
- 931.Rácz B, Halasy K. Kappa opioid receptor is expressed by somatostatin- and neuropeptide Y-containing interneurons in the rat hippocampus. Brain Res : 50–55, 2002. doi: 10.1016/S0006-8993(02)02259-X. [DOI] [PubMed] [Google Scholar]
- 932.Ramamoorthy P, Shi H. Ischemia induces different levels of hypoxia inducible factor-1α protein expression in interneurons and pyramidal neurons. Acta Neuropathol Commun : 51, 2014. doi: 10.1186/2051-5960-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 933.Ramon y Cajal S. Histology of the Nervous System. New York: Oxford University Press, 1995. [Google Scholar]
- 934.Ramos B, Baglietto-Vargas D, del Rio JC, Moreno-Gonzalez I, Santa-Maria C, Jimenez S, Caballero C, Lopez-Tellez JF, Khan ZU, Ruano D, Gutierrez A, Vitorica J. Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1xAPP transgenic model of Alzheimer’s disease. Neurobiol Aging : 1658–1672, 2006. doi: 10.1016/j.neurobiolaging.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 935.Ramos RL, Bai J, LoTurco JJ. Heterotopia formation in rat but not mouse neocortex after RNA interference knockdown of DCX. Cereb Cortex : 1323–1331, 2006. doi: 10.1093/cercor/bhj074. [DOI] [PubMed] [Google Scholar]
- 936.Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol : 461–531, 1973. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 937.Regalado MP, Terry-Lorenzo RT, Waites CL, Garner CC, Malenka RC. Transsynaptic signaling by postsynaptic synapse-associated protein 97. J Neurosci : 2343–2357, 2006. doi: 10.1523/JNEUROSCI.5247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938.Regehr WG. Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol : a005702, 2012. doi: 10.1101/cshperspect.a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 939.Remondes M, Schuman EM. Molecular mechanisms contributing to long-lasting synaptic plasticity at the temporoammonic-CA1 synapse. Learn Mem : 247–252, 2003. doi: 10.1101/lm.59103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940.Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci : 279–285, 1998. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- 941.Rezaï X, Faget L, Bednarek E, Schwab Y, Kieffer BL, Massotte D. Mouse δ opioid receptors are located on presynaptic afferents to hippocampal pyramidal cells. Cell Mol Neurobiol : 509–516, 2012. doi: 10.1007/s10571-011-9791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 942.Reznic J, Staubli U. Effects of 5-HT3 receptor antagonism on hippocampal cellular activity in the freely moving rat. J Neurophysiol : 517–521, 1997. [DOI] [PubMed] [Google Scholar]
- 943.Rheims S, Minlebaev M, Ivanov A, Represa A, Khazipov R, Holmes GL, Ben-Ari Y, Zilberter Y. Excitatory GABA in rodent developing neocortex in vitro. J Neurophysiol : 609–619, 2008. doi: 10.1152/jn.90402.2008. [DOI] [PubMed] [Google Scholar]
- 944.Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci : 7903–7915, 2004. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 945.Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci : 1005–1039, 2001. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- 946.Riebe I, Seth H, Culley G, Dósa Z, Radi S, Strand K, Fröjd V, Hanse E. Tonically active NMDA receptors--a signalling mechanism critical for interneuronal excitability in the CA1 stratum radiatum. Eur J Neurosci : 169–178, 2016. doi: 10.1111/ejn.13128. [DOI] [PubMed] [Google Scholar]
- 947.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature : 251–255, 1999. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 948.Rivera P, Arrabal S, Cifuentes M, Grondona JM, Pérez-Martín M, Rubio L, Vargas A, Serrano A, Pavón FJ, Suárez J, Rodríguez de Fonseca F. Localization of the cannabinoid CB1 receptor and the 2-AG synthesizing (DAGLα) and degrading (MAGL, FAAH) enzymes in cells expressing the Ca(2+)-binding proteins calbindin, calretinin, and parvalbumin in the adult rat hippocampus. Front Neuroanat : 56, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 949.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol : 453–480, 2003. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 950.Robles Y, Vivas-Mejía PE, Ortiz-Zuazaga HG, Félix J, Ramos X, Peña de Ortiz S. Hippocampal gene expression profiling in spatial discrimination learning. Neurobiol Learn Mem : 80–95, 2003. doi: 10.1016/S1074-7427(03)00025-X. [DOI] [PubMed] [Google Scholar]
- 951.Rodríguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron : 893–901, 1997. doi: 10.1016/S0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- 952.Rodríguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron : 1211–1218, 1998. doi: 10.1016/S0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 953.Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res : 339–344, 1998. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- 954.Ropert N, Guy N. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol : 121–136, 1991. doi: 10.1113/jphysiol.1991.sp018742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 955.Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science : 1596–1599, 1998. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- 956.Roux L, Buzsáki G. Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology : 10–23, 2015. doi: 10.1016/j.neuropharm.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 957.Rowan MJ, DelCanto G, Yu JJ, Kamasawa N, Christie JM. Synapse-Level Determination of Action Potential Duration by K(+) Channel Clustering in Axons. Neuron : 370–383, 2016. doi: 10.1016/j.neuron.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 958.Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci : 769–775, 2012. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 959.Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature : 594–598, 1999. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- 960.Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J Neurosci : 8062–8071, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961.Rozsa B, Zelles T, Vizi ES, Lendvai B. Distance-dependent scaling of calcium transients evoked by backpropagating spikes and synaptic activity in dendrites of hippocampal interneurons. J Neurosci : 661–670, 2004. doi: 10.1523/JNEUROSCI.3906-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav : 255–267, 2003. doi: 10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Rubin AN, Alfonsi F, Humphreys MP, Choi CK, Rocha SF, Kessaris N. The germinal zones of the basal ganglia but not the septum generate GABAergic interneurons for the cortex. J Neurosci : 12050–12062, 2010. doi: 10.1523/JNEUROSCI.6178-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 964.Rubin AN, Kessaris N. PROX1: a lineage tracer for cortical interneurons originating in the lateral/caudal ganglionic eminence and preoptic area. PLoS One : e77339, 2013. doi: 10.1371/journal.pone.0077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 965.Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal MS, Hernandez-Pineda R, Hernandez-Cruz A, Erisir A, Leonard C, Vega-Saenz de Miera E. Contributions of Kv3 channels to neuronal excitability. Ann N Y Acad Sci : 304–343, 1999. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- 966.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol : 45–61, 2011. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 967.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci : 517–526, 2001. doi: 10.1016/S0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 968.Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci : 4567–4576, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969.Rusakov DA, Wuerz A, Kullmann DM. Heterogeneity and specificity of presynaptic Ca2+ current modulation by mGluRs at individual hippocampal synapses. Cereb Cortex : 748–758, 2004. doi: 10.1093/cercor/bhh035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 970.Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature : 170–172, 1996. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- 971.Sah R, Geracioti TD. Neuropeptide Y and posttraumatic stress disorder. Mol Psychiatry : 646–655, 2013. doi: 10.1038/mp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 972.Sambandan S, Sauer JF, Vida I, Bartos M. Associative plasticity at excitatory synapses facilitates recruitment of fast-spiking interneurons in the dentate gyrus. J Neurosci : 11826–11837, 2010. doi: 10.1523/JNEUROSCI.2012-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Sánchez-Alcañiz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, López-Bendito G, Stumm R, Marín O. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron : 77–90, 2011. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 974.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci : 1027–1034, 2000. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 975.Santana N, Artigas F. Expression of Serotonin2C Receptors in Pyramidal and GABAergic Neurons of Rat Prefrontal Cortex: A Comparison with Striatum. Cereb Cortex : 3125–3139, 2017. doi: 10.1093/cercor/bhw148. [DOI] [PubMed] [Google Scholar]
- 976.Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci : 5264–5275, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell : 717–729, 1998. doi: 10.1016/S0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- 978.Sauer JF, Strüber M, Bartos M. Impaired fast-spiking interneuron function in a genetic mouse model of depression. eLife : e04979, 2015. doi: 10.7554/eLife.04979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Saunders JA, Tatard-Leitman VM, Suh J, Billingslea EN, Roberts TP, Siegel SJ. Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Res : 69–77, 2013. doi: 10.1002/aur.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.Savanthrapadian S, Meyer T, Elgueta C, Booker SA, Vida I, Bartos M. Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J Neurosci : 8197–8209, 2014. doi: 10.1523/JNEUROSCI.5433-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Sayin U, Osting S, Hagen J, Rutecki P, Sutula T. Spontaneous seizures and loss of axo-axonic and axo-somatic inhibition induced by repeated brief seizures in kindled rats. J Neurosci : 2759–2768, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 982.Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron : 673–681, 2000. doi: 10.1016/S0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 983.Scharfman HE. Electrophysiological diversity of pyramidal-shaped neurons at the granule cell layer/hilus border of the rat dentate gyrus recorded in vitro. Hippocampus : 287–305, 1995. doi: 10.1002/hipo.450050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984.Schevon CA, Weiss SA, McKhann G Jr, Goodman RR, Yuste R, Emerson RG, Trevelyan AJ. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun : 1060, 2012. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985.Schlander M, Hoyer S, Frotscher M. Glutamate decarboxylase-immunoreactive neurons in the aging rat hippocampus are more resistant to ischemia than CA1 pyramidal cells. Neurosci Lett : 241–246, 1988. doi: 10.1016/0304-3940(88)90687-8. [DOI] [PubMed] [Google Scholar]
- 986.Schmid LC, Mittag M, Poll S, Steffen J, Wagner J, Geis HR, Schwarz I, Schmidt B, Schwarz MK, Remy S, Fuhrmann M. Dysfunction of Somatostatin-Positive Interneurons Associated with Memory Deficits in an Alzheimer’s Disease Model. Neuron : 114–125, 2016. doi: 10.1016/j.neuron.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 987.Schmidt D. Drug treatment of epilepsy: options and limitations. Epilepsy Behav : 56–65, 2009. doi: 10.1016/j.yebeh.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 988.Schmitz D, Empson RM, Gloveli T, Heinemann U. Serotonin blocks different patterns of low Mg2+-induced epileptiform activity in rat entorhinal cortex, but not hippocampus. Neuroscience : 449–458, 1997. doi: 10.1016/S0306-4522(96)00302-8. [DOI] [PubMed] [Google Scholar]
- 989.Schmitz D, Empson RM, Heinemann U. Serotonin and 8-OH-DPAT reduce excitatory transmission in rat hippocampal area CA1 via reduction in presumed presynaptic Ca2+ entry. Brain Res : 249–254, 1995. doi: 10.1016/0006-8993(95)01005-5. [DOI] [PubMed] [Google Scholar]
- 990.Schneider Gasser EM, Duveau V, Prenosil GA, Fritschy JM. Reorganization of GABAergic circuits maintains GABAA receptor-mediated transmission onto CA1 interneurons in alpha1-subunit-null mice. Eur J Neurosci : 3287–3304, 2007. doi: 10.1111/j.1460-9568.2007.05558.x. [DOI] [PubMed] [Google Scholar]
- 991.Schönemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol : 207–220, 2008. doi: 10.1002/cne.21780. [DOI] [PubMed] [Google Scholar]
- 992.Schulze-Bonhage A. Brain stimulation as a neuromodulatory epilepsy therapy. Seizure : 169–175, 2017. doi: 10.1016/j.seizure.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 993.Segal M. A subset of local interneurons generate slow inhibitory postsynaptic potentials in hippocampal neurons. Brain Res : 163–164, 1990. doi: 10.1016/0006-8993(90)90236-5. [DOI] [PubMed] [Google Scholar]
- 994.Sekulić V, Chen TC, Lawrence JJ, Skinner FK. Dendritic distributions of I h channels in experimentally-derived multi-compartment models of oriens-lacunosum/moleculare (O-LM) hippocampal interneurons. Front Synaptic Neurosci : 2, 2015. doi: 10.3389/fnsyn.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 995.Semyanov A, Kullmann DM. Modulation of GABAergic signaling among interneurons by metabotropic glutamate receptors. Neuron : 663–672, 2000. doi: 10.1016/S0896-6273(00)81068-5. [DOI] [PubMed] [Google Scholar]
- 996.Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci : 484–490, 2003. [DOI] [PubMed] [Google Scholar]
- 997.Senior TJ, Huxter JR, Allen K, O’Neill J, Csicsvari J. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci : 2274–2286, 2008. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 998.Seress L, Pokorny J. Structure of the granular layer of the rat dentate gyrus. A light microscopic and Golgi study. J Anat : 181–195, 1981. [PMC free article] [PubMed] [Google Scholar]
- 999.Serôdio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol : 1081–1091, 1998. [DOI] [PubMed] [Google Scholar]
- 1000.Sheffield ME, Best TK, Mensh BD, Kath WL, Spruston N. Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. Nat Neurosci : 200–207, 2011. doi: 10.1038/nn.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1001.Sheffield ME, Edgerton GB, Heuermann RJ, Deemyad T, Mensh BD, Spruston N. Mechanisms of retroaxonal barrage firing in hippocampal interneurons. J Physiol : 4793–4805, 2013. doi: 10.1113/jphysiol.2013.258418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1002.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA : 3908–3913, 1996. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1003.Shen RY, Andrade R. 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J Pharmacol Exp Ther : 805–812, 1998. [PubMed] [Google Scholar]
- 1004.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci : 7503–7522, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1005.Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature : 523–525, 1996. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- 1006.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology : 169–191, 2010. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1007.Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature : 288–293, 2003. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 1008.Sia GM, Béïque JC, Rumbaugh G, Cho R, Worley PF, Huganir RL. Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron : 87–102, 2007. doi: 10.1016/j.neuron.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 1009.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron : 1123–1128, 1998. doi: 10.1016/S0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 1010.Sigel E, Lüscher BP. A closer look at the high affinity benzodiazepine binding site on GABAA receptors. Curr Top Med Chem : 241–246, 2011. doi: 10.2174/156802611794863562. [DOI] [PubMed] [Google Scholar]
- 1011.Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem : 40224–40231, 2012. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Sík A, Hájos N, Gulácsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci USA : 3245–3250, 1998. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1013.Sik A, Penttonen M, Buzsáki G. Interneurons in the hippocampal dentate gyrus: an in vivo intracellular study. Eur J Neurosci : 573–588, 1997. doi: 10.1111/j.1460-9568.1997.tb01634.x. [DOI] [PubMed] [Google Scholar]
- 1014.Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci : 6651–6665, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron : 735–746, 2007. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 1016.Sim SC, Nordin L, Andersson TM, Virding S, Olsson M, Pedersen NL, Ingelman-Sundberg M. Association between CYP2C19 polymorphism and depressive symptoms. Am J Med Genet B Neuropsychiatr Genet : 1160–1166, 2010. [DOI] [PubMed] [Google Scholar]
- 1017.Simon A, Oláh S, Molnár G, Szabadics J, Tamás G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci : 6278–6285, 2005. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci : 555–586, 1995. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 1019.Sipilä ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci : 5280–5289, 2005. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1020.Sipilä ST, Huttu K, Voipio J, Kaila K. Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+-activated K+ current. Eur J Neurosci : 2330–2338, 2006. doi: 10.1111/j.1460-9568.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- 1021.Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA : 2065–2069, 2003. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1022.Sivakumaran S, Cardarelli RA, Maguire J, Kelley MR, Silayeva L, Morrow DH, Mukherjee J, Moore YE, Mather RJ, Duggan ME, Brandon NJ, Dunlop J, Zicha S, Moss SJ, Deeb TZ. Selective inhibition of KCC2 leads to hyperexcitability and epileptiform discharges in hippocampal slices and in vivo. J Neurosci : 8291–8296, 2015. doi: 10.1523/JNEUROSCI.5205-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1023.Slomianka L, Amrein I, Knuesel I, Sørensen JC, Wolfer DP. Hippocampal pyramidal cells: the reemergence of cortical lamination. Brain Struct Funct : 301–317, 2011. doi: 10.1007/s00429-011-0322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1024.Sloviter RS, Ali-Akbarian L, Elliott RC, Bowery BJ, Bowery NG. Localization of GABA(B) (R1) receptors in the rat hippocampus by immunocytochemistry and high resolution autoradiography, with specific reference to its localization in identified hippocampal interneuron subpopulations. Neuropharmacology : 1707–1721, 1999. doi: 10.1016/S0028-3908(99)00132-X. [DOI] [PubMed] [Google Scholar]
- 1025.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci : 383–395, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026.Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci : 232–236, 1999. doi: 10.1016/S0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- 1027.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature : 698–702, 2009. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1028.Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, Watanabe M. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci : 264–277, 2015. doi: 10.1038/nrn3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029.Soltesz I, Mody I. Patch-clamp recordings reveal powerful GABAergic inhibition in dentate hilar neurons. J Neurosci : 2365–2376, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Sommeijer JP, Levelt CN. Synaptotagmin-2 is a reliable marker for parvalbumin positive inhibitory boutons in the mouse visual cortex. PLoS One : e35323, 2012. doi: 10.1371/journal.pone.0035323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031.Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, Somogyi P. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci : 552–569, 2004. doi: 10.1111/j.0953-816X.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- 1032.Somogyi J, Szabo A, Somogyi P, Lamsa K. Molecular analysis of ivy cells of the hippocampal CA1 stratum radiatum using spectral identification of immunofluorophores. Front Neural Circuits : 35, 2012. doi: 10.3389/fncir.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1033.Somogyi P. A specific ‘axo-axonal’ interneuron in the visual cortex of the rat. Brain Res : 345–350, 1977. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- 1034.Somogyi P, Cowey A. Combined Golgi and electron microscopic study on the synapses formed by double bouquet cells in the visual cortex of the cat and monkey. J Comp Neurol : 547–566, 1981. doi: 10.1002/cne.901950402. [DOI] [PubMed] [Google Scholar]
- 1035.Somogyi P, Kisvárday ZF, Martin KA, Whitteridge D. Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience : 261–294, 1983. doi: 10.1016/0306-4522(83)90133-1. [DOI] [PubMed] [Google Scholar]
- 1036.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol : 9–26, 2005. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1037.Somogyi P, Nunzi MG, Gorio A, Smith AD. A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res : 137–142, 1983. doi: 10.1016/0006-8993(83)91076-4. [DOI] [PubMed] [Google Scholar]
- 1038.Song I, Savtchenko L, Semyanov A. Tonic excitation or inhibition is set by GABA(A) conductance in hippocampal interneurons. Nat Commun : 376, 2011. doi: 10.1038/ncomms1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1039.Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JC, Miquel M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J Neurosci : 11459–11468, 2016. doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1040.Soriano E, Frotscher M. A GABAergic axo-axonic cell in the fascia dentata controls the main excitatory hippocampal pathway. Brain Res : 170–174, 1989. doi: 10.1016/0006-8993(89)91722-8. [DOI] [PubMed] [Google Scholar]
- 1041.Soriano E, Nitsch R, Frotscher M. Axo-axonic chandelier cells in the rat fascia dentata: Golgi-electron microscopy and immunocytochemical studies. J Comp Neurol : 1–25, 1990. doi: 10.1002/cne.902930102. [DOI] [PubMed] [Google Scholar]
- 1042.Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex , Suppl 1: i1–i10, 2009. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043.Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science : 1145–1148, 2010. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1044.Spitzer NC. Electrical activity in early neuronal development. Nature : 707–712, 2006. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 1045.Spolidoro M, Sale A, Berardi N, Maffei L. Plasticity in the adult brain: lessons from the visual system. Exp Brain Res : 335–341, 2009. doi: 10.1007/s00221-008-1509-3. [DOI] [PubMed] [Google Scholar]
- 1046.Staiger JF, Masanneck C, Schleicher A, Zuschratter W. Calbindin-containing interneurons are a target for VIP-immunoreactive synapses in rat primary somatosensory cortex. J Comp Neurol : 179–189, 2004. doi: 10.1002/cne.10953. [DOI] [PubMed] [Google Scholar]
- 1047.Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci USA : 7595–7600, 2009. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1048.Standaert DG, Landwehrmeyer GB, Kerner JA, Penney JB Jr, Young AB. Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Brain Res Mol Brain Res : 89–102, 1996. doi: 10.1016/S0169-328X(96)00117-9. [DOI] [PubMed] [Google Scholar]
- 1049.Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, Buzsáki G. Inhibition-induced theta resonance in cortical circuits. Neuron : 1263–1276, 2013. doi: 10.1016/j.neuron.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsáki G. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron : 467–480, 2014. doi: 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1051.Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P. Hippocampal Somatostatin Interneurons Control the Size of Neuronal Memory Ensembles. Neuron : 1074–1085, 2016. doi: 10.1016/j.neuron.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 1052.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet : 877–892, 2002. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1053.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature : 773–778, 1997. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 1054.Steriade M, Nuñez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci : 3252–3265, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1055.Straiker A, Mackie K. Cannabinoid signaling in inhibitory autaptic hippocampal neurons. Neuroscience : 190–201, 2009. doi: 10.1016/j.neuroscience.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056.Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, Tomita S. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci : 866–873, 2011. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057.Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr Opin Neurobiol : 488–495, 2012. doi: 10.1016/j.conb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1058.Strüber M, Jonas P, Bartos M. Strength and duration of perisomatic GABAergic inhibition depend on distance between synaptically connected cells. Proc Natl Acad Sci USA : 1220–1225, 2015. doi: 10.1073/pnas.1412996112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059.Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature : 69–72, 1994. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- 1060.Stumm RK, Zhou C, Schulz S, Höllt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol : 107–118, 2004. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- 1061.Su E, Bell M. Diffuse Axonal Injury. In: Translational Research in Traumatic Brain Injury, edited by Laskowitz D, Grant G. Boca Raton, FL: CRC, 2016. [Google Scholar]
- 1062.Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol : 515–528, 2000. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1063.Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell : 508–520, 2008. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 1064.Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsáki G. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J Neurosci : 8605–8616, 2011. doi: 10.1523/JNEUROSCI.0294-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1065.Sultan KT, Han Z, Zhang XJ, Xianyu A, Li Z, Huang K, Shi SH. Clonally Related GABAergic Interneurons Do Not Randomly Disperse but Frequently Form Local Clusters in the Forebrain. Neuron : 31–44, 2016. doi: 10.1016/j.neuron.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1066.Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature : 676–682, 2007. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1067.Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, Callaway EM, Xu X. Cell-type-specific circuit connectivity of hippocampal CA1 revealed through Cre-dependent rabies tracing. Cell Reports : 269–280, 2014. doi: 10.1016/j.celrep.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1068.Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development : 3359–3370, 1999. [DOI] [PubMed] [Google Scholar]
- 1069.Suzuki N, Tang CS, Bekkers JM. Persistent barrage firing in cortical interneurons can be induced in vivo and may be important for the suppression of epileptiform activity. Front Cell Neurosci : 76, 2014. doi: 10.3389/fncel.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1070.Svoboda KR, Adams CE, Lupica CR. Opioid receptor subtype expression defines morphologically distinct classes of hippocampal interneurons. J Neurosci : 85–95, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1071.Svoboda KR, Lupica CR. Opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (Ih) currents. J Neurosci : 7084–7098, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1072.Swadlow HA. Thalamocortical control of feed-forward inhibition in awake somatosensory ‘barrel’ cortex. Philos Trans R Soc Lond B Biol Sci : 1717–1727, 2002. doi: 10.1098/rstb.2002.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1073.Sylwestrak EL, Ghosh A. Elfn1 regulates target-specific release probability at CA1-interneuron synapses. Science : 536–540, 2012. doi: 10.1126/science.1222482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1074.Szabadics J, Tamás G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci USA : 14831–14836, 2007. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1075.Szabo A, Somogyi J, Cauli B, Lambolez B, Somogyi P, Lamsa KP. Calcium-permeable AMPA receptors provide a common mechanism for LTP in glutamatergic synapses of distinct hippocampal interneuron types. J Neurosci : 6511–6516, 2012. doi: 10.1523/JNEUROSCI.0206-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1076.Szabó GG, Holderith N, Gulyás AI, Freund TF, Hájos N. Distinct synaptic properties of perisomatic inhibitory cell types and their different modulation by cholinergic receptor activation in the CA3 region of the mouse hippocampus. Eur J Neurosci : 2234–2246, 2010. doi: 10.1111/j.1460-9568.2010.07292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1077.Szabó GG, Lenkey N, Holderith N, Andrási T, Nusser Z, Hájos N. Presynaptic calcium channel inhibition underlies CB1 cannabinoid receptor-mediated suppression of GABA release. J Neurosci : 7958–7963, 2014. doi: 10.1523/JNEUROSCI.0247-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1078.Szatkiewicz JP, O’Dushlaine C, Chen G, Chambert K, Moran JL, Neale BM, Fromer M, Ruderfer D, Akterin S, Bergen SE, Kähler A, Magnusson PK, Kim Y, Crowley JJ, Rees E, Kirov G, O’Donovan MC, Owen MJ, Walters J, Scolnick E, Sklar P, Purcell S, Hultman CM, McCarroll SA, Sullivan PF. Copy number variation in schizophrenia in Sweden. Mol Psychiatry : 762–773, 2014. doi: 10.1038/mp.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1079.Szentágothai J, Arbib MA. Conceptual models of neural organization. Neurosci Res Program Bull : 305–510, 1974. [PubMed] [Google Scholar]
- 1080.Tahvildari B, Wölfel M, Duque A, McCormick DA. Selective functional interactions between excitatory and inhibitory cortical neurons and differential contribution to persistent activity of the slow oscillation. J Neurosci : 12165–12179, 2012. doi: 10.1523/JNEUROSCI.1181-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1081.Takács VT, Klausberger T, Somogyi P, Freund TF, Gulyás AI. Extrinsic and local glutamatergic inputs of the rat hippocampal CA1 area differentially innervate pyramidal cells and interneurons. Hippocampus : 1379–1391, 2012. doi: 10.1002/hipo.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1082.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res : 3–34, 2013. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 1083.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci : 7491–7505, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084.Tamás G, Buhl EH, Lörincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci : 366–371, 2000. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 1085.Tamás G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J Neurosci : 6352–6364, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1086.Tamás G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science : 1902–1905, 2003. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 1087.Tan G-H, Liu Y-Y, Hu X-L, Yin D-M, Mei L, Xiong Z-Q. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat Neurosci : 258–266, 2011. doi: 10.1038/nn.3005. [DOI] [PubMed] [Google Scholar]
- 1088.Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development : 5803–5813, 2003. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- 1089.Tanaka DH, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development : 2167–2176, 2006. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- 1090.Tanaka DH, Toriumi K, Kubo K, Nabeshima T, Nakajima K. GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. J Neurosci : 14116–14125, 2011. doi: 10.1523/JNEUROSCI.2786-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1091.Tanaka DH, Yanagida M, Zhu Y, Mikami S, Nagasawa T, Miyazaki J, Yanagawa Y, Obata K, Murakami F. Random walk behavior of migrating cortical interneurons in the marginal zone: time-lapse analysis in flat-mount cortex. J Neurosci : 1300–1311, 2009. doi: 10.1523/JNEUROSCI.5446-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1092.Tang AH, Alger BE. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome. J Neurosci : 3938–3945, 2015. doi: 10.1523/JNEUROSCI.4499-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1093.Tang AH, Karson MA, Nagode DA, McIntosh JM, Uebele VN, Renger JJ, Klugmann M, Milner TA, Alger BE. Nerve terminal nicotinic acetylcholine receptors initiate quantal GABA release from perisomatic interneurons by activating axonal T-type (Cav3) Ca2+ channels and Ca2+ release from stores. J Neurosci : 13546–13561, 2011. doi: 10.1523/JNEUROSCI.2781-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1094.Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, McInnes RR. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci : 10009–10018, 2011. doi: 10.1523/JNEUROSCI.6617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1095.Taniguchi H. Genetic dissection of GABAergic neural circuits in mouse neocortex. Front Cell Neurosci : 8, 2014. doi: 10.3389/fncel.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1096.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron : 995–1013, 2011. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1097.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science : 70–74, 2013. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098.Tansey EP, Chow A, Rudy B, McBain CJ. Developmental expression of potassium-channel subunit Kv3.2 within subpopulations of mouse hippocampal inhibitory interneurons. Hippocampus : 137–148, 2002. doi: 10.1002/hipo.1104. [DOI] [PubMed] [Google Scholar]
- 1099.Taverna S, Tkatch T, Metz AE, Martina M. Differential expression of TASK channels between horizontal interneurons and pyramidal cells of rat hippocampus. J Neurosci : 9162–9170, 2005. doi: 10.1523/JNEUROSCI.2454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1100.Terzian AL, Micale V, Wotjak CT. Cannabinoid receptor type 1 receptors on GABAergic vs. glutamatergic neurons differentially gate sex-dependent social interest in mice. Eur J Neurosci : 2293–2298, 2014. doi: 10.1111/ejn.12561. [DOI] [PubMed] [Google Scholar]
- 1101.Thompson K, Anantharam V, Behrstock S, Bongarzone E, Campagnoni A, Tobin AJ. Conditionally immortalized cell lines, engineered to produce and release GABA, modulate the development of behavioral seizures. Exp Neurol : 481–489, 2000. doi: 10.1006/exnr.1999.7305. [DOI] [PubMed] [Google Scholar]
- 1102.Tikhonova TB, Nagaeva EI, Barygin OI, Potapieva NN, Bolshakov KV, Tikhonov DB. Monoamine NMDA receptor channel blockers inhibit and potentiate native and recombinant proton-gated ion channels. Neuropharmacology : 1–10, 2015. doi: 10.1016/j.neuropharm.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 1103.Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex : 1185–1199, 2000. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 1104.Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC, Mei L. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci : 15–25, 2011. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1105.Tomassy GS, Morello N, Calcagno E, Giustetto M. Developmental abnormalities of cortical interneurons precede symptoms onset in a mouse model of Rett syndrome. J Neurochem : 115–127, 2014. doi: 10.1111/jnc.12803. [DOI] [PubMed] [Google Scholar]
- 1106.Tomioka NH, Yasuda H, Miyamoto H, Hatayama M, Morimura N, Matsumoto Y, Suzuki T, Odagawa M, Odaka YS, Iwayama Y, Won Um J, Ko J, Inoue Y, Kaneko S, Hirose S, Yamada K, Yoshikawa T, Yamakawa K, Aruga J. Elfn1 recruits presynaptic mGluR7 in trans and its loss results in seizures. Nat Commun : 4501, 2014. doi: 10.1038/ncomms5501. [DOI] [PubMed] [Google Scholar]
- 1107.Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature : 1052–1058, 2005. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 1108.Tong LM, Djukic B, Arnold C, Gillespie AK, Yoon SY, Wang MM, Zhang O, Knoferle J, Rubenstein JL, Alvarez-Buylla A, Huang Y. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Aβ accumulation. J Neurosci : 9506–9515, 2014. doi: 10.1523/JNEUROSCI.0693-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1109.Tong X, Peng Z, Zhang N, Cetina Y, Huang CS, Wallner M, Otis TS, Houser CR. Ectopic Expression of α6 and δ GABAA Receptor Subunits in Hilar Somatostatin Neurons Increases Tonic Inhibition and Alters Network Activity in the Dentate Gyrus. J Neurosci : 16142–16158, 2015. doi: 10.1523/JNEUROSCI.2853-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1110.Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille JC. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J Physiol : 115–131, 2006. doi: 10.1113/jphysiol.2006.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1111.Topolnik L, Chamberland S, Pelletier JG, Ran I, Lacaille JC. Activity-dependent compartmentalized regulation of dendritic Ca2+ signaling in hippocampal interneurons. J Neurosci : 4658–4663, 2009. doi: 10.1523/JNEUROSCI.0493-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1112.Topolnik L, Congar P, Lacaille JC. Differential regulation of metabotropic glutamate receptor- and AMPA receptor-mediated dendritic Ca2+ signals by presynaptic and postsynaptic activity in hippocampal interneurons. J Neurosci : 990–1001, 2005. doi: 10.1523/JNEUROSCI.4388-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1113.Torborg CL, Berg AP, Jeffries BW, Bayliss DA, McBain CJ. TASK-like conductances are present within hippocampal CA1 stratum oriens interneuron subpopulations. J Neurosci : 7362–7367, 2006. doi: 10.1523/JNEUROSCI.1257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1114.Torborg CL, Nakashiba T, Tonegawa S, McBain CJ. Control of CA3 output by feedforward inhibition despite developmental changes in the excitation-inhibition balance. J Neurosci : 15628–15637, 2010. doi: 10.1523/JNEUROSCI.3099-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1115.Torres-Reveron A, Williams TJ, Chapleau JD, Waters EM, McEwen BS, Drake CT, Milner TA. Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp Neurol : 319–327, 2009. doi: 10.1016/j.expneurol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1116.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry : 252–260, 2005. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 1117.Tóth A, Blumberg PM, Boczán J. Anandamide and the vanilloid receptor (TRPV1). Vitam Horm : 389–419, 2009. doi: 10.1016/S0083-6729(09)81015-7. [DOI] [PubMed] [Google Scholar]
- 1118.Tóth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol : 463–474, 1997. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1119.Tóth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci : 572–578, 1998. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- 1120.Tóth K, McBain CJ. Target-specific expression of pre- and postsynaptic mechanisms. J Physiol : 41–51, 2000. doi: 10.1111/j.1469-7793.2000.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1121.Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci : 8279–8289, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1122.Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci : 8106–8117, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1123.Touzot A, Ruiz-Reig N, Vitalis T, Studer M. Molecular control of two novel migratory paths for CGE-derived interneurons in the developing mouse brain. Development : 1753–1765, 2016. doi: 10.1242/dev.131102. [DOI] [PubMed] [Google Scholar]
- 1124.Towers SK, LeBeau FE, Gloveli T, Traub RD, Whittington MA, Buhl EH. Fast network oscillations in the rat dentate gyrus in vitro. J Neurophysiol : 1165–1168, 2002. doi: 10.1152/jn.00495.2001. [DOI] [PubMed] [Google Scholar]
- 1125.Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK, Miller KD. A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron : 51–63, 2013. doi: 10.1016/j.neuron.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron : 803–815, 2005. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 1127.Trachtenberg JT. Competition, inhibition, and critical periods of cortical plasticity. Curr Opin Neurobiol : 44–48, 2015. doi: 10.1016/j.conb.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1128.Traub RD, Bibbig R, Piechotta A, Draguhn R, Schmitz D. Synaptic and nonsynaptic contributions to giant ipsps and ectopic spikes induced by 4-aminopyridine in the hippocampus in vitro. J Neurophysiol : 1246–1256, 2001. [DOI] [PubMed] [Google Scholar]
- 1129.Traub RD, Cunningham MO, Gloveli T, LeBeau FE, Bibbig A, Buhl EH, Whittington MA. GABA-enhanced collective behavior in neuronal axons underlies persistent gamma-frequency oscillations. Proc Natl Acad Sci USA : 11047–11052, 2003. doi: 10.1073/pnas.1934854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130.Traub RD, Miles R. Neuronal Networks of the Hippocampus. New York: Cambridge University Press, 1992, p. 281. [Google Scholar]
- 1131.Traub RD, Miles R, Wong RK. Model of the origin of rhythmic population oscillations in the hippocampal slice. Science : 1319–1325, 1989. doi: 10.1126/science.2646715. [DOI] [PubMed] [Google Scholar]
- 1132.Traub RD, Whittington MA, Colling SB, Buzsáki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol : 471–484, 1996. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1133.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev : 405–496, 2010. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1134.Tremblay R, Lee S, Rudy B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron : 260–292, 2016. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1135.Trevelyan AJ, Sussillo D, Yuste R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci : 3383–3387, 2007. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1136.Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res : 105–118, 1988. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- 1137.Tricoire L, Pelkey KA, Daw MI, Sousa VH, Miyoshi G, Jeffries B, Cauli B, Fishell G, McBain CJ. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci : 2165–2176, 2010. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1138.Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci : 10948–10970, 2011. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139.Tsou K, Mackie K, Sañudo-Peña MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience : 969–975, 1999. doi: 10.1016/S0306-4522(99)00086-X. [DOI] [PubMed] [Google Scholar]
- 1140.Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci : 2463–2478, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1141.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci : 8184–8189, 2007. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1142.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci : 8184–8189, 2007. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1143.Turrero García M, Mazzola E, Harwell CC. Lineage Relationships Do Not Drive MGE/PoA-Derived Interneuron Clustering in the Brain. Neuron : 52–58, 2016. doi: 10.1016/j.neuron.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1144.Tyacke RJ, Lingford-Hughes A, Reed LJ, Nutt DJ. GABAB receptors in addiction and its treatment. Adv Pharmacol : 373–396, 2010. doi: 10.1016/S1054-3589(10)58014-1. [DOI] [PubMed] [Google Scholar]
- 1145.Tyan L, Chamberland S, Magnin E, Camiré O, Francavilla R, David LS, Deisseroth K, Topolnik L. Dendritic inhibition provided by interneuron-specific cells controls the firing rate and timing of the hippocampal feedback inhibitory circuitry. J Neurosci : 4534–4547, 2014. doi: 10.1523/JNEUROSCI.3813-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1146.Tyson JA, Goldberg EM, Maroof AM, Xu Q, Petros TJ, Anderson SA. Duration of culture and sonic hedgehog signaling differentially specify PV versus SST cortical interneuron fates from embryonic stem cells. Development : 1267–1278, 2015. doi: 10.1242/dev.111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1147.Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hübner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science : 1788–1792, 2006. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 1148.Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben-Ari Y, Khazipov R. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J Neurophysiol : 2964–2972, 2003. doi: 10.1152/jn.00172.2003. [DOI] [PubMed] [Google Scholar]
- 1149.Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci : 10372–10382, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1150.Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, Obata K, Yoshida S, Hirabayashi M, Yanagawa Y, Kawaguchi Y. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex : 315–330, 2008. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- 1151.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron : 155–168, 2006. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 1152.Umbriaco D, Garcia S, Beaulieu C, Descarries L. Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus : 605–620, 1995. doi: 10.1002/hipo.450050611. [DOI] [PubMed] [Google Scholar]
- 1153.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol : 399–417, 2015. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- 1154.Urban-Ciecko J, Barth AL. Somatostatin-expressing neurons in cortical networks. Nat Rev Neurosci : 401–409, 2016. doi: 10.1038/nrn.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1155.Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci : 5113–5122, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1156.Van Hooft JA, Giuffrida R, Blatow M, Monyer H. Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J Neurosci : 3544–3551, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1157.Van Os J, Kapur S. Schizophrenia. Lancet : 635–645, 2009. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 1158.Vandecasteele M, Varga V, Berényi A, Papp E, Barthó P, Venance L, Freund TF, Buzsáki G. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc Natl Acad Sci USA : 13535–13540, 2014. doi: 10.1073/pnas.1411233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1159.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol : 407–418, 1969. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 1160.Vardya I, Drasbek KR, Dósa Z, Jensen K. Cell type-specific GABA A receptor-mediated tonic inhibition in mouse neocortex. J Neurophysiol : 526–532, 2008. doi: 10.1152/jn.01224.2007. [DOI] [PubMed] [Google Scholar]
- 1161.Varga C, Golshani P, Soltesz I. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc Natl Acad Sci USA : E2726–E2734, 2012. doi: 10.1073/pnas.1210929109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1162.Varga C, Lee SY, Soltesz I. Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci : 822–824, 2010. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1163.Varga C, Oijala M, Lish J, Szabo GG, Bezaire M, Marchionni I, Golshani P, Soltesz I. Functional fission of parvalbumin interneuron classes during fast network events. eLife : e04006, 2014. doi: 10.7554/eLife.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1164.Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science : 449–453, 2009. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- 1165.Vargish GA, Pelkey KA, Yuan X, Chittajallu R, Collins D, Fang C, McBain CJ. Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Mol Psychiatry : 56–67, 2017. doi: 10.1038/mp.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Vega-Saenz de Miera E, Moreno H, Fruhling D, Kentros C, Rudy B. Cloning of ShIII (Shaw-like) cDNAs encoding a novel high-voltage-activating, TEA-sensitive, type-A K+ channel. Proc Biol Sci : 9–18, 1992. doi: 10.1098/rspb.1992.0036. [DOI] [PubMed] [Google Scholar]
- 1167.Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci USA : 10260–10265, 2000. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1168.Verheugen JA, Fricker D, Miles R. Noninvasive measurements of the membrane potential and GABAergic action in hippocampal interneurons. J Neurosci : 2546–2555, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1169.Verney C, Baulac M, Berger B, Alvarez C, Vigny A, Helle KB. Morphological evidence for a dopaminergic terminal field in the hippocampal formation of young and adult rat. Neuroscience : 1039–1052, 1985. doi: 10.1016/0306-4522(85)90275-1. [DOI] [PubMed] [Google Scholar]
- 1170.Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell : 708–721, 2012. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1171.Viader A, Blankman JL, Zhong P, Liu X, Schlosburg JE, Joslyn CM, Liu QS, Tomarchio AJ, Lichtman AH, Selley DE, Sim-Selley LJ, Cravatt BF. Metabolic Interplay between Astrocytes and Neurons Regulates Endocannabinoid Action. Cell Reports : 798–808, 2015. doi: 10.1016/j.celrep.2015.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1172.Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol : 755–773, 1998. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1173.Villette V, Guigue P, Picardo MA, Sousa VH, Leprince E, Lachamp P, Malvache A, Tressard T, Cossart R, Baude A. Development of early-born γ-aminobutyric acid hub neurons in mouse hippocampus from embryogenesis to adulthood. J Comp Neurol : 2440–2461, 2016. doi: 10.1002/cne.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1174.Vinet J, Sík A. Expression pattern of voltage-dependent calcium channel subunits in hippocampal inhibitory neurons in mice. Neuroscience : 189–212, 2006. doi: 10.1016/j.neuroscience.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 1175.Viney TJ, Lasztoczi B, Katona L, Crump MG, Tukker JJ, Klausberger T, Somogyi P. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat Neurosci : 1802–1811, 2013. doi: 10.1038/nn.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1176.Vogt D, Hunt RF, Mandal S, Sandberg M, Silberberg SN, Nagasawa T, Yang Z, Baraban SC, Rubenstein JL. Lhx6 directly regulates Arx and CXCR7 to determine cortical interneuron fate and laminar position. Neuron : 350–364, 2014. doi: 10.1016/j.neuron.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1177.Von Engelhardt J, Bocklisch C, Tönges L, Herb A, Mishina M, Monyer H. GluN2D-containing NMDA receptors-mediate synaptic currents in hippocampal interneurons and pyramidal cells in juvenile mice. Front Cell Neurosci : 95, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1178.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science : 1175–1178, 2001. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 1179.Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis T. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex : 2333–2347, 2010. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1180.Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci : 12255–12264, 2009. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1181.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature : 173–177, 1997. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 1182.Walker HC, Lawrence JJ, McBain CJ. Activation of kinetically distinct synaptic conductances on inhibitory interneurons by electrotonically overlapping afferents. Neuron : 161–171, 2002. doi: 10.1016/S0896-6273(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 1183.Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res : 147–160, 2012. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- 1184.Wang DD, Kriegstein AR. Blocking early GABA depolarization with bumetanide results in permanent alterations in cortical circuits and sensorimotor gating deficits. Cereb Cortex : 574–587, 2011. doi: 10.1093/cercor/bhq124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185.Wang DD, Kriegstein AR. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci : 5547–5558, 2008. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1186.Wang JH, Kelly P. Calcium-calmodulin signalling pathway up-regulates glutamatergic synaptic function in non-pyramidal, fast spiking rat hippocampal CA1 neurons. J Physiol : 407–422, 2001. doi: 10.1111/j.1469-7793.2001.0407a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1187.Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol : 183–194, 1998. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1188.Wang W, Takashima S, Segawa Y, Itoh M, Shi X, Hwang SK, Nabeshima K, Takeshita M, Hirose S. The developmental changes of Na(v)1.1 and Na(v)1.2 expression in the human hippocampus and temporal lobe. Brain Res : 61–70, 2011. doi: 10.1016/j.brainres.2011.02.083. [DOI] [PubMed] [Google Scholar]
- 1189.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev : 1195–1268, 2010. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1190.Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci : 6402–6413, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1191.Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, Pleasure SJ, Behrens T, Rubenstein JL. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron : 61–76, 2011. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1192.Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol : 65–90, 2004. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1193.Watson GB, Lanthorn TH. Electrophysiological actions of delta opioids in CA1 of the rat hippocampal slice are mediated by one delta receptor subtype. Brain Res : 129–135, 1993. doi: 10.1016/0006-8993(93)91703-U. [DOI] [PubMed] [Google Scholar]
- 1194.Wedzony K, Chocyk A. Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin- but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol Rep : 1000–1007, 2009. doi: 10.1016/S1734-1140(09)70161-6. [DOI] [PubMed] [Google Scholar]
- 1195.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci : 578–586, 2006. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 1196.Weng JY, Lin YC, Lien CC. Cell type-specific expression of acid-sensing ion channels in hippocampal interneurons. J Neurosci : 6548–6558, 2010. doi: 10.1523/JNEUROSCI.0582-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1197.Wester JC, McBain CJ. Behavioral state-dependent modulation of distinct interneuron subtypes and consequences for circuit function. Curr Opin Neurobiol : 118–125, 2014. doi: 10.1016/j.conb.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1198.Wester JC, McBain CJ. Interneurons Differentially Contribute to Spontaneous Network Activity in the Developing Hippocampus Dependent on Their Embryonic Lineage. J Neurosci : 2646–2662, 2016. doi: 10.1523/JNEUROSCI.4000-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1199.White JA, Banks MI, Pearce RA, Kopell NJ. Networks of interneurons with fast and slow gamma-aminobutyric acid type A (GABAA) kinetics provide substrate for mixed gamma-theta rhythm. Proc Natl Acad Sci USA : 8128–8133, 2000. doi: 10.1073/pnas.100124097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1200.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature : 612–615, 1995. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 1201.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol : 315–336, 2000. doi: 10.1016/S0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 1202.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development : 3759–3771, 2001. [DOI] [PubMed] [Google Scholar]
- 1203.Widmer H, Ferrigan L, Davies CH, Cobb SR. Evoked slow muscarinic acetylcholinergic synaptic potentials in rat hippocampal interneurons. Hippocampus : 617–628, 2006. doi: 10.1002/hipo.20191. [DOI] [PubMed] [Google Scholar]
- 1204.Wierenga CJ, Müllner FE, Rinke I, Keck T, Stein V, Bonhoeffer T. Molecular and electrophysiological characterization of GFP-expressing CA1 interneurons in GAD65-GFP mice. PLoS One : e15915, 2010. doi: 10.1371/journal.pone.0015915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1205.Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol : 1003–1017, 1963. [DOI] [PubMed] [Google Scholar]
- 1206.Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci : 1537–1545, 2008. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1207.Wilent WB, Nitz DA. Discrete place fields of hippocampal formation interneurons. J Neurophysiol : 4152–4161, 2007. doi: 10.1152/jn.01200.2006. [DOI] [PubMed] [Google Scholar]
- 1208.Willard SL, Riddle DR, Forbes ME, Shively CA. Cell number and neuropil alterations in subregions of the anterior hippocampus in a female monkey model of depression. Biol Psychiatry : 890–897, 2013. doi: 10.1016/j.biopsych.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1209.Williams S, Boksa P. Gamma oscillations and schizophrenia. J Psychiatry Neurosci : 75–77, 2010. doi: 10.1503/jpn.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1210.Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol : 3177–3182, 2000. [DOI] [PubMed] [Google Scholar]
- 1211.Williams TJ, Akama KT, Knudsen MG, McEwen BS, Milner TA. Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in CA1 pyramidal cell dendrites. Exp Neurol : 186–196, 2011. doi: 10.1016/j.expneurol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1212.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature : 588–592, 2001. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 1213.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci : 687–696, 2006. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 1214.Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol : 127–136, 2008. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1215.Wondolowski J, Frerking M. Subunit-dependent postsynaptic expression of kainate receptors on hippocampal interneurons in area CA1. J Neurosci : 563–574, 2009. doi: 10.1523/JNEUROSCI.4788-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1216.Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry : 1013–1015, 1997. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 1217.Woodhall G, Gee CE, Robitaille R, Lacaille JC. Membrane potential and intracellular Ca2+ oscillations activated by mGluRs in hippocampal stratum oriens/alveus interneurons. J Neurophysiol : 371–382, 1999. [DOI] [PubMed] [Google Scholar]
- 1218.Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits : 15, 2009. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1219.Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science : 972–976, 1996. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- 1220.Wu HM, Huang CC, Chen SH, Liang YC, Tsai JJ, Hsieh CL, Hsu KS. Herpes simplex virus type 1 inoculation enhances hippocampal excitability and seizure susceptibility in mice. Eur J Neurosci : 3294–3304, 2003. doi: 10.1111/j.1460-9568.2003.03075.x. [DOI] [PubMed] [Google Scholar]
- 1221.Wu PR, Cho KK, Vogt D, Sohal VS, Rubenstein JL. The Cytokine CXCL12 Promotes Basket Interneuron Inhibitory Synapses in the Medial Prefrontal Cortex. Cereb Cortex : 4303–4313, 2016. doi: 10.1093/cercor/bhw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1222.Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bähner F, Both M, Tort AB, Kopell NJ, Wisden W, Monyer H. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci USA : 3561–3566, 2009. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1223.Wurzman R, Forcelli PA, Griffey CJ, Kromer LF. Repetitive grooming and sensorimotor abnormalities in an ephrin-A knockout model for Autism Spectrum Disorders. Behav Brain Res : 115–128, 2015. doi: 10.1016/j.bbr.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1224.Wyeth MS, Pelkey KA, Petralia RS, Salter MW, McInnes RR, McBain CJ. Neto auxiliary protein interactions regulate kainate and NMDA receptor subunit localization at mossy fiber-CA3 pyramidal cell synapses. J Neurosci : 622–628, 2014. doi: 10.1523/JNEUROSCI.3098-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1225.Wyeth MS, Zhang N, Mody I, Houser CR. Selective reduction of cholecystokinin-positive basket cell innervation in a model of temporal lobe epilepsy. J Neurosci : 8993–9006, 2010. doi: 10.1523/JNEUROSCI.1183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1226.Wyskiel DR, Andrade R. Serotonin excites hippocampal CA1 GABAergic interneurons at the stratum radiatum-stratum lacunosum moleculare border. Hippocampus : 1107–1114, 2016. doi: 10.1002/hipo.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1227.Xia S, Zhou Z, Leung C, Zhu Y, Pan X, Qi J, Morena M, Hill MN, Xie W, Jia Z. p21-activated kinase 1 restricts tonic endocannabinoid signaling in the hippocampus. eLife : e14653, 2016. doi: 10.7554/eLife.14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1228.Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) : 1–11, 2010. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1229.Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O’Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron : 513–528, 2003. doi: 10.1016/S0896-6273(03)00463-X. [DOI] [PubMed] [Google Scholar]
- 1230.Xu HT, Han Z, Gao P, He S, Li Z, Shi W, Kodish O, Shao W, Brown KN, Huang K, Shi SH. Distinct lineage-dependent structural and functional organization of the hippocampus. Cell : 1552–1564, 2014. doi: 10.1016/j.cell.2014.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1231.Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, Contractor A. Hippocampal metaplasticity is required for the formation of temporal associative memories. J Neurosci : 16762–16773, 2014. doi: 10.1523/JNEUROSCI.2869-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232.Xu J, Mashimo T, Südhof TC. Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron : 567–581, 2007. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 1233.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci : 2612–2622, 2004. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1234.Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron : 328–340, 2010. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1235.Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol : 16–29, 2008. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- 1236.Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development : 4987–4998, 2005. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- 1237.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol : 389–404, 2010. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1238.Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol : 144–160, 2006. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- 1239.Yadav R, Gupta SC, Hillman BG, Bhatt JM, Stairs DJ, Dravid SM. Deletion of glutamate delta-1 receptor in mouse leads to aberrant emotional and social behaviors. PLoS One : e32969, 2012. doi: 10.1371/journal.pone.0032969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1240.Yagita Y, Kitagawa K, Ohtsuki T, Takasawa Ki, Miyata T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke : 1890–1896, 2001. doi: 10.1161/01.STR.32.8.1890. [DOI] [PubMed] [Google Scholar]
- 1241.Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol : 829–841, 2004. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1242.Yamasaki M, Fukaya M, Yamazaki M, Azechi H, Natsume R, Abe M, Sakimura K, Watanabe M. TARP γ-2 and γ-8 Differentially Control AMPAR Density Across Schaffer Collateral/Commissural Synapses in the Hippocampal CA1 Area. J Neurosci : 4296–4312, 2016. doi: 10.1523/JNEUROSCI.4178-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1243.Yan D, Tomita S. Defined criteria for auxiliary subunits of glutamate receptors. J Physiol : 21–31, 2012. doi: 10.1113/jphysiol.2011.213868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1244.Yan H, Li Q, Fleming R, Madison RD, Wilson WA, Swartzwelder HS. Developmental sensitivity of hippocampal interneurons to ethanol: involvement of the hyperpolarization-activated current, Ih. J Neurophysiol : 67–83, 2009. doi: 10.1152/jn.90557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1245.Yang EJ, Harris AZ, Pettit DL. Variable kainate receptor distributions of oriens interneurons. J Neurophysiol : 1683–1689, 2006. doi: 10.1152/jn.01332.2005. [DOI] [PubMed] [Google Scholar]
- 1246.Yang GR, Murray JD, Wang XJ. A dendritic disinhibitory circuit mechanism for pathway-specific gating. Nat Commun : 12815, 2016. doi: 10.1038/ncomms12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1247.Yang WZ, Liu TT, Cao JW, Chen XF, Liu X, Wang M, Su X, Zhang SQ, Qiu BL, Hu WX, Liu LY, Ma L, Yu YC. Fear Erasure Facilitated by Immature Inhibitory Neuron Transplantation. Neuron : 1352–1367, 2016. doi: 10.1016/j.neuron.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 1248.Yang Z, You Y, Levison SW. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J Comp Neurol : 19–33, 2008. doi: 10.1002/cne.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1249.Ye Z, Mostajo-Radji MA, Brown JR, Rouaux C, Tomassy GS, Hensch TK, Arlotta P. Instructing Perisomatic Inhibition by Direct Lineage Reprogramming of Neocortical Projection Neurons. Neuron : 475–483, 2015. doi: 10.1016/j.neuron.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1250.Yi F, Ball J, Stoll KE, Satpute VC, Mitchell SM, Pauli JL, Holloway BB, Johnston AD, Nathanson NM, Deisseroth K, Gerber DJ, Tonegawa S, Lawrence JJ. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol : 3463–3494, 2014. doi: 10.1113/jphysiol.2014.275453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1251.Yi F, DeCan E, Stoll K, Marceau E, Deisseroth K, Lawrence JJ. Muscarinic excitation of parvalbumin-positive interneurons contributes to the severity of pilocarpine-induced seizures. Epilepsia : 297–309, 2015. doi: 10.1111/epi.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1252.Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci : 1552–1559, 2005. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- 1253.Younts TJ, Monday HR, Dudok B, Klein ME, Jordan BA, Katona I, Castillo PE. Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release. Neuron : 479–492, 2016. doi: 10.1016/j.neuron.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1254.Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci : 7268–7277, 2005. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1255.Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci : 1142–1149, 2006. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 1256.Yu J, Proddutur A, Elgammal FS, Ito T, Santhakumar V. Status epilepticus enhances tonic GABA currents and depolarizes GABA reversal potential in dentate fast-spiking basket cells. J Neurophysiol : 1746–1763, 2013. doi: 10.1152/jn.00891.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1257.Yu J, Proddutur A, Swietek B, Elgammal FS, Santhakumar V. Functional Reduction in Cannabinoid-Sensitive Heterotypic Inhibition of Dentate Basket Cells in Epilepsy: Impact on Network Rhythms. Cereb Cortex : 4299–4314, 2015. doi: 10.1093/cercor/bhv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1258.Yu W, Wang Y, McDonnell K, Stephen D, Bai CB. Patterning of ventral telencephalon requires positive function of Gli transcription factors. Dev Biol : 264–275, 2009. doi: 10.1016/j.ydbio.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 1259.Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature : 501–504, 2009. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1260.Yu YC, He S, Chen S, Fu Y, Brown KN, Yao XH, Ma J, Gao KP, Sosinsky GE, Huang K, Shi SH. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature : 113–117, 2012. doi: 10.1038/nature10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1261.Yuen EY, Yan Z. Dopamine D4 receptors regulate AMPA receptor trafficking and glutamatergic transmission in GABAergic interneurons of prefrontal cortex. J Neurosci : 550–562, 2009. doi: 10.1523/JNEUROSCI.5050-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1262.Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science : 665–669, 1992. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- 1263.Zaninetti M, Raggenbass M. Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur J Neurosci : 3975–3984, 2000. doi: 10.1046/j.1460-9568.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 1264.Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor alpha5 subunits contribute to GABAA,slow synaptic inhibition in mouse hippocampus. J Neurophysiol : 1179–1191, 2009. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1265.Zhan RZ, Nadler JV, Schwartz-Bloom RD. Impaired firing and sodium channel function in CA1 hippocampal interneurons after transient cerebral ischemia. J Cereb Blood Flow Metab : 1444–1452, 2007. doi: 10.1038/sj.jcbfm.9600448. [DOI] [PubMed] [Google Scholar]
- 1266.Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci : 5724–5729, 2010. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1267.Zhang L, He J, Jugloff DG, Eubanks JH. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus : 294–309, 2008. doi: 10.1002/hipo.20389. [DOI] [PubMed] [Google Scholar]
- 1268.Zhang L, McBain CJ. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J Physiol : 661–672, 1995. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1269.Zhang L, McBain CJ. Voltage-gated potassium currents in stratum oriens-alveus inhibitory neurones of the rat CA1 hippocampus. J Physiol : 647–660, 1995. doi: 10.1113/jphysiol.1995.sp020997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1270.Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science : 660–665, 2014. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1271.Zhang TA, Tang J, Pidoplichko VI, Dani JA. Addictive nicotine alters local circuit inhibition during the induction of in vivo hippocampal synaptic potentiation. J Neurosci : 6443–6453, 2010. doi: 10.1523/JNEUROSCI.0458-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1272.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res : 1–10, 2002. doi: 10.1016/S0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 1273.Zhao Y, Flandin P, Long JE, Cuesta MD, Westphal H, Rubenstein JL. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. J Comp Neurol : 79–99, 2008. doi: 10.1002/cne.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1274.Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron : 255–269, 2006. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1275.Zhu PJ, Lovinger DM. Developmental alteration of endocannabinoid retrograde signaling in the hippocampus. J Neurophysiol : 1123–1129, 2010. doi: 10.1152/jn.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1276.Zieglgänsberger W, French ED, Siggins GR, Bloom FE. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science : 415–417, 1979. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]
- 1277.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA III, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci : 816–822, 2008. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1278.Zimmer A. Genetic Manipulation of the Endocannabinoid System. Handb Exp Pharmacol : 129–183, 2015. doi: 10.1007/978-3-319-20825-1_5. [DOI] [PubMed] [Google Scholar]
- 1279.Zou D, Chen L, Deng D, Jiang D, Dong F, McSweeney C, Zhou Y, Liu L, Chen G, Wu Y, Mao Y. DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr Mol Med : 91–102, 2016. doi: 10.2174/1566524016666151222150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1280.Zsiros V, Maccaferri G. Electrical coupling between interneurons with different excitable properties in the stratum lacunosum-moleculare of the juvenile CA1 rat hippocampus. J Neurosci : 8686–8695, 2005. doi: 10.1523/JNEUROSCI.2810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1281.Zsiros V, Maccaferri G. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci : 1804–1815, 2008. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1282.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol : 355–405, 2002. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]