Abstract
High blood pressure is present in more than one billion adults worldwide and is the most important modifiable risk factor of death resulting from cardiovascular disease. While many factors contribute to the pathogenesis of hypertension, a role of the immune system has been firmly established by a large number of investigations from many laboratories around the world. Immunosuppressive drugs and inhibition of individual cytokines prevent or ameliorate experimental hypertension, and studies in genetically-modified mouse strains have demonstrated that lymphocytes are necessary participants in the development of hypertension and in hypertensive organ injury. Furthermore, immune reactivity may be the driving force of hypertension in autoimmune diseases. Infiltration of immune cells, oxidative stress, and stimulation of the intrarenal angiotensin system are induced by activation of the innate and adaptive immunity. High blood pressure results from the combined effects of inflammation-induced impairment in the pressure natriuresis relationship, dysfunctional vascular relaxation, and overactivity of the sympathetic nervous system. Imbalances between proinflammatory effector responses and anti-inflammatory responses of regulatory T cells to a large extent determine the severity of inflammation. Experimental and human studies have uncovered autoantigens (isoketal-modified proteins and heat shock protein 70) of potential clinical relevance. Further investigations on the immune reactivity in hypertension may result in the identification of new strategies for the treatment of the disease.
I. INTRODUCTION
Hypertension is defined as blood pressure equal to or greater than 140/90 mmHg and occurs in 25–43% of the world population older than 18 yr, representing the leading modifiable risk factor for death resulting from cardiovascular disease (321). High blood pressure may be secondary to a number of causes, but in the vast majority of the patients, it does not have a recognized etiology. These patients are collectively grouped in what is known as primary or essential hypertension that, despite improved therapeutic options, is uncontrolled in 8–12% of the patients (36).
The interaction of environmental, genetic, anatomical, neural, endocrine, humoral, and hemodynamic factors plays a role in essential hypertension. These factors have been condensed in the Page Mosaic theory (202), about which it has been said that “its weakness is that it cannot be proved wrong” (73). Immunity and autoimmunity do not appear in the octagonal Page Mosaic; nevertheless, Harrison (92) has noted that inflammation is present upstream or downstream of each one of the interconnected factors.
The initial studies that examined the role of immune cells in hypertension were done nearly half a century ago (196, 305), but research on immunity in the pathogenesis of hypertension was rare until 16–18 yr ago when a reawakened interest fueled an exponential increase in the number of publications in this topic (228). This paper reviews the research that has established the pivotal contribution of the immune system in the pathogenesis of essential hypertension.
II. IMMUNITY IN EXPERIMENTAL MODELS OF HYPERTENSION
A. Genetic Models of Hypertension
Experimental interventions directed to suppress immune reactivity in genetic models of hypertension are shown in TABLE 1 that includes the magnitude of the antihypertensive effects of each treatment.
Table 1.
Hypertensive animal strains in which suppression of immunity/inflammation ameliorated or prevented hypertension
| Blood Pressure, mmHg |
|||
|---|---|---|---|
| Strain | Experimental Strategy/Treatment | Untreated | Treated |
| Spontaneously hypertensive rat (SHR) | Cyclophosphamide (132) | 172 | 153 |
| Mycophenolate mofetil (222) | 198 | 147 | |
| Inhibition of NFκB activation (218) | 198 | 127 | |
| Suppression of oxidative stress (189) | 195 | 149 | |
| (223) | 207 | 179 | |
| (316) | 175 | 149 | |
| Stroke-prone SHR | Increase CD4+CD25+Foxp3 cells (Treg cells) by sympathetic denervation (128) | 175 | 150 |
| Dahl’s salt sensitive rat | Inhibition of pyrimidine synthesis (283) | 250 | 200 |
| Mycophenolate mofetil (174) | 139* | 122* | |
| Tacrolimus (52) | 170* | 150* | |
| Tempol (52) | 140* | 126* | |
| Etanercept (104) | 165* | 145* | |
| Anti-TGF-β antibody (44) | 190* | 177* | |
| (187) | 200* | 179* | |
| Anti-IL-6 antibody (94) | 149* | 138* | |
| Genetic deletion of p67phox (69) | 180* | 140* | |
| Mesenchymal stem cell transplantation (103) | 185* | 150* | |
| Mutation of RAG 1 gene (175) | 180* | 150* | |
| Deletion of the CD247 gene (225) | 151* | 134* | |
| Mutation in the SH2B3 gene (226) | 175* | 135* | |
| Lyon rat | Neonatal thymectomy (8) | 122 | 111 |
| Cyclophosphamide (9) | 175 | 135 | |
| Fawn Hooded rat | Inhibition of NFκB activation (137) | 159 | 128 |
| dTGF rat | Inhibition of NFκB activation (185) | 185 | 162 |
| Cyclosporine A (180) | 210 | 178 | |
| Inhibition of p38 MAPK (204) | 203 | 165 | |
| NZB mice | Cyclosporine A (263) | 155* | 147* |
| NZBW mice | Anti-IL-4 antibody (285) | 87* | 68* |
| Rosiglitazone (291) | 139* | 127* | |
| Etanercept (290) | 150* | 130* | |
| Anti-CD20 antibody (170) | 141* | 127* | |
Data are systolic blood pressure or mean arterial pressure (*).
There is considerable variation in the antihypertensive effect of the various treatments. Reference numbers are given in parentheses. See text for definitions.
1. Spontaneously hypertensive rat (SHR)
In 1963, Okamoto and Aoki (194) described a strain of rats that are normotensive 3–4 wk after birth and develop progressive hypertension as they age. The finding of arteriolar inflammation in the kidneys of adult SHR suggested the participation of immunity, and early studies documented reduced delayed-type hypersensitivity suggesting impaired cell mediated immunity. This led to a series of investigations aimed to modify cell-mediated immunity with thymocytotoxic antibodies, thymosin (extract of calf thymus), thymus grafting, and administration of interleukin (IL)-2. These investigations had conflicting results with some studies ameliorating, others aggravating, and yet others having no effect on hypertension (6, 266, 267). These mixed results were likely due to the lack of discrimination between proinflammatory and immune suppressor T cells in the early experimental studies.
Subsequent experiments evaluated the effects of immunosuppression on blood pressure. Administration of cyclophosphamide (132), mofetil mycophenolate (MMF) (222), or suppressing the overactivity of the proinflamatory transcription factor NFκB with pyrrolidine dithiocarbamate (PDTC) (218) reduced the renal infiltration of immune cells in tubulointerstitial areas, suppressed the oxidative stress, and corrected the hypertension. Amelioration of hypertension in the SHR was also found in association with reduction of renal inflammation resulting from treatments directed to correct oxidative stress. These treatments included administration of melatonin (189) and antioxidant-rich diets (223) or improving nitric oxide (NO) availability with sildenafil (316), which inhibits the degradation of cGMP (mediator of NO biological actions).
In summary, these studies are all suggestive that renal inflammation plays a role in the pathogenesis of hypertension of the SHR.
2. Stroke-prone spontaneously hypertensive rat (SPSHR)
The stroke-prone strain was established as a substrain of the SHR rats with severe hypertension and high stroke susceptibility (195). The SPSHR has increased sympathetic nervous system (SNS) activity (317) and a relative reduction of anti-inflammatory CD4+CD25+Foxp3 regulatory T (Treg) cells in the spleen. Splenic denervation increased the proportion of Treg cells and delayed the development of hypertension in the SPSHR (128). Inflammation in the central nervous system is less likely responsible for the hypertension since reduction in microglial activation with minocycline does not modify the blood pressure (268).
3. Dahl salt-sensitive rat
In 1962, Dahl et al. (43) utilized selective outbreeding of Sprague-Dawley rats to generate two lines of rats that differed by their blood pressure response to a high-salt diet: a salt-sensitive (SS) line that developed hypertension and salt-resistant (SR) line that remained normotensive.
Early studies by Dahl’s group noted that intrarenal inflammation was present in SS rats and became more prominent on a high-salt diet (117). While glomerular injury and sclerosis developed in these rats (254), hypertension was strongly correlated with tubulointerstitial inflammation (173, 297). CD4+ (helper) and CD8+ (cytotoxic) T lymphocytes infiltrate in equal numbers and are primarily located in areas surrounding damaged glomeruli and blood vessels (51).
Early investigators administered the compound HR325 (cyanocyclopropyl trifluoromethyl acrylamide), an immunosuppressive drug that inhibits pyrimidine synthesis and suppresses both humoral and cellular immunity, to Dahl SS rats. Salt-induced hypertension was improved in association with reduced tissue inflammation, and the authors concluded that a “hyperimmune state” was responsible for the susceptibility of Dahl SS rats to hypertension (283). Mattson and co-workers (51, 174) later administered MMF or Tacrolimus and obtained 50–60% reduction in the renal tubulointerstitial immune cell infiltration with amelioration of hypertension and proteinuria. Subsequent studies demonstrated that a high-salt diet induced intrarenal activation of NFκB and that T cells infiltrating the kidney had increased mRNA expression of proinflammatory cytokines (271). SS hypertension was associated with overexpression of the p67phox, gp91phox, and p47phx subunits of NADPH oxidase (52), and genetic deletion of p67phox ameliorated hypertension (69). The interaction between inflammation and oxidative stress was highlighted by the reduction in NFκB activation, immune cell infiltration, proteinuria, and hypertension resulting from antioxidant treatments with vitamin C and vitamin E (272, 273). Reduction in renal tubulointerstitial inflammation with MMF also prevents the hypertension resulting from a high-protein (53) and a high-fat diet (250) in Dahl SS rats. Anti-inflammatory agents without immune suppressive activity do not modify SS hypertension (98, 308).
The role played by tubulointerstitial inflammation was also demonstrated in studies where mesenchymal stem (immunosuppressive) cells were transplanted into the renal medulla of uninephrectomized Dahl’s SS rats and obtained improvement of the inflammatory infiltrate and correction of hypertension (103).
Recent research has used Dahl SS rats to identify the role of genes associated with hypertension in genome-wide association studies (GWAS). In particular, the CD247 gene and the SH2B3 (LNK) gene have been carefully evaluated. The CD247 gene is part of the T cell receptor complex, and its deletion in the Dahl rat (225) gave evidence of the importance of antigen recognition in SS hypertension (discussed in sect. VII). SH2B3 plays a suppressive role in the activation of immune responses and cytokine signaling (54). Saleh et al. (230) demonstrated that deficiency in SH2B3 gene resulted in increased inflammation in the kidneys and aorta and exaggerated response to angiotensin II infusions. Bone marrow transplantation experiments showed that loss of SH2B3 gene in endothelial cells was responsible for these findings. Rudemiller et al. (226) induced a mutation in the SH2B3 that was predicted to affect a phosphotyrosine-binding site in the SH2 domain and thereby suppress signal transduction. The mutation, in fact, ameliorated hypertension and inflammation. These findings could be explained in part by a selective increase in Tregs in the mutant rats associated with a high-salt diet. A higher ratio of Tregs to proinflammatory T cells could be a factor in the attenuation of inflammation. Bone marrow cross-transplantation demonstrated that the findings were dependent on SH2B3 mutant bone marrow cells. Contraction and dilatation of resistance vessels were unmodified in the mutant rats; therefore, the improvement in hypertension could not be explained by changes in vascular function.
4. Lyon rat
The Lyon hypertensive (LH) and normotensive (LN) rats resulted from selective breeding from the same Sprague-Dawley colony. The LH rats present with both spontaneous and SS hypertension, low circulating renin, increased body weight, hyperlipidemia, proteinuria, and increased insulin-to-glucose ratio (70, 232). The participation of the immune system in the hypertension of LH rats was suggested by the finding that neonatal thymectomy and treatment with cyclophosphamide improved hypertension (8, 9).
5. Fawn hooded (FH) rat
The FH rat strain was introduced as an outbred stock of rats with a hemorrhagic tendency due to a platelet defect (281). Selective mating among FH siblings resulted in the breeding of a hypertensive (FHH) strain and a normotensive strain. The FHH rats showed a correlation between the severity of hypertension and the glomerular and tubulointerstitial injury as well as with plasma renin levels (144).
The participation of renal inflammation in the pathogenesis of hypertension in the FHH rats was suggested by the demonstration that activation of NFκB early in life was a critical factor in the development of hypertension; indeed, perinatal PDTC treatment ameliorated hypertension in association with reduction in renal immune cell infiltration, albuminuria, and glomerulosclerosis (137).
6. dTGF rat
Double transgenic rats (dTGF) for human angiotensinogen and renin were developed by crossing a transgenic strain for human angiotensinogen and a transgenic strain for human renin (19). Involvement of the immune system in the dTGR is an early event, as noted by complement activation in the blood vessel walls with inflammatory cell infiltration that occurs before albuminuria develops (240). The dTGF rats have been used for studying the effects of severe angiotensin II-mediated hypertension and tissue damage. In this model of hypertension, the administration of dexamethasone, MMF, or etanercept [a recombinant fusion protein of the extracellular ligand-binding domain of tumor necrosis factor (TNF) receptor type 2], could reduce the immune cell infiltration in the kidneys, resulting in less fibrosis and albuminuria but without effect on hypertension, thus separating the tissue injury and the blood pressure effects of angiotensin II (186). The independence of hypertension and the tissue injury in this model were further shown in studies that normalized blood pressure with triple antihypertensive treatment (hydralazine, reserpine, and hidrochorothiazide) with only minimal improvement in histological renal damage (181). Nevertheless, other studies from the same group found that suppression of inflammation by inhibition of NFκB as well as by treatment with cyclosporin A, could ameliorate both angiotensin II-induced inflammatory damage and hypertension (180, 185). Park et al. (204) blocked the proinflammatory mitogen-activated protein kinase (MAPK) pathway and found amelioration of hypertension in association with reduction in the inflammatory cell infiltration in the kidneys and heart.
Taken together, these studies suggest that high levels of angiotensin and associated proinflammatory cytokines may induce renal injury independently of hypertension. Nevertheless, once injury is established, inflammation-induced damage contributes to the development and severity of hypertension
7. Sabra rat
The Sabra rats have SS and SR phenotypes genetically different from Dahl strains (16). In the Sabra rat, oxidative stress and inflammation both precede and accompany hypertension (237).
8. New Zealand Black (NZB) mouse
Initial studies on the blood pressure of the NZB mouse strain gave contrasting results. Svendsen (263), using intracarotid determination of blood pressure, found the NZB mice to be hypertensive and reported that high blood pressure was improved with cyclophosphamide treatment and that athymic NZB mice did not develop hypertension. In contrast, Rudofsky et al. (227), using tail-cuff methodology, reported the NZB mice to be normotensive despite the presence of renal disease. Nevertheless, the offspring (NZBWF1) of the cross of NZB mice with the New Zealand White mice were hypertensive in association with nephritis (227) and had many features resembling systemic lupus erythematosus (SLE) in humans (102, 169, 170, 179).
B. Experimentally Induced Hypertensive Models
1. Renal infarct model
The first studies that associated immunity and hypertension were done in rats with partial renal infarct and contralateral nephrectomy that developed anti-kidney and anti-artery antibodies. Furthermore, “suppressants of antibody reactions” (cortisone and 6-mercaptopurine), as well as thymectomy, ameliorated hypertension (305). Hypertension could be transferred by spleen cells of hypertensive rats to normotensive recipients (196). The same model was used by Svendsen for a series of investigations (260, 261) in mice with normal thymus (haired mice) and athymic (nude) mice. He described an early hypertension (30–40 days postoperatively) that was similar in the haired and nude mice. The early hypertension was followed by a late, chronic hypertension in the haired mice that was absent in athymic mice. The administration of cyclophosphamide did not modify the early hypertension but ameliorated the late hypertension and corrected the renal “round cell” infiltration that accompanied hypertension in the haired mice. Thymus grafts restored the late hypertension and the renal immune cell infiltration in the nude mice. He concluded that in this model there existed an early thymus-independent hypertension and a chronic thymus-dependent hypertension. This was one of the earliest and best documented studies of role of T cells in experimental hypertension.
2. Deoxycorticosterone acetate (DOCA)-salt hypertension
In 1970, Gardner et al. (79) studied a model of severe hypertension induced by a combination of subcutaneous implantation of DOCA pellets, high-salt diet, and unilateral nephrectomy. They showed that cyclophosphamide prevented vascular lesions, although in association with a high mortality. This work did not report if immunosuppressive treatment improved hypertension. Subsequently, Olsen (197) found that hypertension could be transferred to normotensive rats by spleen cells of rats that had been hypertensive for 3 mo or more and suggested that hypertension resulted from delayed hypersensitivity directed against arterial walls. In this model, Svendsen (262) showed that, as in the experiments with the renal infarct model, the initial hypertension was independent of the thymus, whereas athymic mice did not develop the late salt-driven hypertension. Grafting of thymus in the athymic mice restored the capacity to develop the late hypertension and intrarenal inflammation.
3. Prenatally programmed hypertension
Reduction in the nephron number resulting from maternal protein and caloric malnutrition during pregnancy is a risk factor for adult hypertension (159). Stewart et al. (255) examined the role of renal inflammation and oxidative stress in prenatally programmed hypertension. They gave a low-protein diet to pregnant Sprague-Dawley rats during the last 9 days of gestation and the resulting offspring became hypertensive between 6 and 8 wk of life. At 4 wk of age, before they became hypertensive, the rats developed renal infiltration of immune cells and oxidative stress. The administration of MMF or tempol (SOD mimetic) at days 21–42 of age suppressed the lymphocyte and macrophage infiltration of the kidney and the oxidative stress and prevented hypertension. The blood pressure remained normal after discontinuation of the drugs. This study showed that renal inflammation is a critical element in the pathogenesis of prenatally programmed hypertension.
4. Cellophane-wrapped kidneys
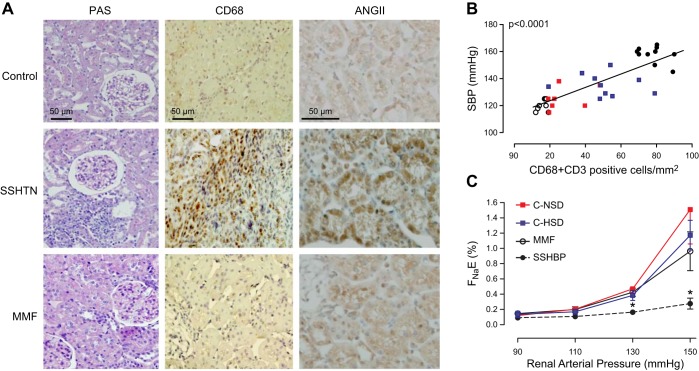
In 1939, Irving Page reported that cellophane wrapping of the kidneys caused hypertension (201). He postulated that the cellophane-induced fibrocollagenous perinephritic reaction compressed the kidney and caused intrarenal ischemia, thus resembling the hypertension obtained by Goldblatt with constriction of the renal arteries. Concordant with this view, hypertension in the Page kidney was traditionally attributed to increased levels of systemic angiotensin II (95). However, investigations by Vanegas et al. (286) showed that cellophane-wrapped kidneys developed intense tubulointerstitial inflammation and increased intrarenal angiotensin II concentration while plasma angiotensin II levels were unmodified. Administration of MMF suppressed the immune cell infiltration, reduced the intrarenal angiotensin II, and prevented hypertension. These results strongly suggest a role of immune cell activation in this model.
5. Chronic low-dose lead exposure
Chronic lead exposure results in adrenergic overactivity (279) and increased oxidative stress (288) and has been associated with hypertension in humans (21, 33). The role of tubulointerstitial inflammation in the pathogenesis of lead-induced hypertension was evaluated in rats that received 14 wk of a low dose of lead acetate (100 ppm in the drinking water). These rats developed progressive hypertension, oxidative stress, interstitial accumulation of lymphocytes and macrophages, activation of NFκB, and increased intrarenal angiotensin II. The administration of MMF corrected the renal inflammation and the oxidative stress, normalized the renal angiotensin II, and prevented the development of hypertension (24).
6. Angiotensin II infusion
Angiotensin II infusions have been given to mice or rats in doses ranging from 0.694 (41) to 3,600 ng·kg−1·min−1 (147) to evaluate the various aspects of the pathogenesis of angiotensin II-induced hypertension, endothelial dysfunction, and tissue injury. Administration of angiotensin II is usually made by subcutaneously placed osmotic minipumps and the duration of the infusion is 2–4 wk. Wilcox’s group (129) studied the slow pressor response of low doses of angiotensin. They have shown that a subcutaneous infusion of angiotensin II at a rate of 400 ng·kg−1·min−1 in mice does not elevate the blood pressure by day 6, but induces hypertension after 10 days. This slow pressor response is caused, at least in part, by oxidative stress, because it is corrected with tempol. Infusion rates of 800–1,000 ng·kg−1·min−1 cause an abrupt increase in blood pressure that is sustained with a tendency to plateau after 2 wk. Effects related to the immune activation induced by angiotensin II infusions are investigated in 2–4 wk studies. Angiotensin II doses in the range of 800–1,000 ng·kg−1·min−1 are generally used in investigations directed to evaluate renal, vascular, or heart injury because these doses cause more reproducible histological damage. However, it should be kept in mind that after 2 wk high infusion rates of angiotensin II cause hypokalemia, likely resulting from angiotensin II-stimulated aldosterone production (129), and renal tubulointerstitial damage associated with potassium depletion may be a confounding feature (257).
Doses in the intermediate range (490–600 ng·kg−1·min−1) are administered in investigations that focus on the nature and characteristics of the immune cell infiltration (90, 135). Surprisingly, very few data exist in relation to the levels of circulating angiotensin II levels induced by angiotensin infusions. Doses of 200 ng·kg−1·min−1 in mice are reported to result in angiotensin II plasma concentrations of 51 ± 8 fmol/ml, which correspond to levels observed in the physiological increase resulting from a low-salt diet. Doses 4 times higher (800 ng·kg−1·min−1) result in a 10-fold increment in plasma angiotensin II concentration, but pharmacokinetics were not analyzed (162). Therefore, it appears likely that the doses used in the majority of the studies done with angiotensin II infusions result in plasma levels of angiotensin II substantially higher than those present in physiological responses or in physiopathological conditions.
It is interesting that in a newly developed mouse model in which the murine immune system is replaced by a human immune system, activation and tissue infiltration of immune cells after angiotensin II infusions occurs independently of angiotensin II. Correction of hypertension with hydralazine and hydrochlorothiazide prevents accumulation of T cells in the kidney. Therefore, human T cells infiltrate tissues in response to high blood pressure in this model (113).
7. SS hypertension induced by transient angiotensin II infusions
Lombardi et al. (156) showed that 2 wk of angiotensin II administration in rats resulted in tubulointerstitial inflammation and subtle renal injury associated with loss of peritubular capillaries. Subsequent administration of a high-salt diet resulted in hypertension. Suppressing the inflammatory response induced by angiotensin II with the administration of MMF (220) during the time when angiotensin was infused did not modify the hypertension induced by the hemodynamic effects of angiotensin II but resulted in a substantial reduction of the immune cell infiltration, oxidative stress, and tubulointerstitial injury and prevented the development of post-angiotensin salt-induced hypertension.
8. SS hypertension induced by transient l-NAME administration
Inhibition of nitric oxide synthase (NOS) with l-NAME is an experimental model of hypertension described in 1992 by Baylis et al. (15) and Ribeiro et al. (215). The sympathetic nervous system (231), the renal renin-angiotensin system (127), and endothelin (292) participate in the development of l-NAME-induced hypertension. Histologically, suppression of NOS is associated with tubulointerstitial immune cell infiltration and fibrosis, glomerulosclerosis, and arteriolar lesions. Administration of l-NAME for 3 wk to normotensive rats results in a progressive elevation of the blood pressure that returns to normal levels a week after discontinuation of l-NAME. Subsequent administration of a high-salt diet results in hypertension. The role of renal inflammation resulting from l-NAME treatment in the subsequent development of SS hypertension was investigated by administering MMF in association with l-NAME. MMF did not modify the hypertension that occurred during the administration of l-NAME-induced hypertension but suppressed the renal injury and immune cell infiltration and the subsequent salt-induced hypertension (213). In the l-NAME-induced model of hypertension, effector memory cells accumulate in the kidney, and CD70-deficient mice that cannot develop memory T cells are protected from post-l-NAME salt-driven hypertension (114).
9. SS hypertension induced by overload proteinuria
Systemic administration of protein results in proteinuria that is associated with structural glomerular changes (47) and intense tubulointerstitial inflammation (63) and with the development of hypertension in response to a high-salt diet (1). Treatment with MMF during BSA overload did not modify the proteinuria but suppressed the tubulointerstitial infiltration of lymphocytes and macrophages and prevented the salt-driven hypertension (1).
In summary, immunosuppressive interventions associated with reduction of inflammation, improvement of oxidative stress, and reduction in renal angiotensin II activity have been shown to prevent, improve, or correct hypertension in genetic and experimentally induced models of hypertension. T and B lymphocytes, monocytes/macrophages, natural killer cells, and dendritic cells are the central cellular elements in immune-driven reactivity. Their participation in the pathogenesis of hypertension results from the activation of the innate and adaptive pathways of immune reactivity.
III. OVERVIEW OF THE IMMUNE RESPONSE
Innate immunity is a system of immediate response against danger signals. These signals correspond to molecular patterns in pathogenic microorganisms (pathogen-associated molecular patterns or PAMPs) or endogenously generated cellular stress signals (danger-associated molecular patterns or DAMPs). These signals are recognized by pattern recognition receptors (PRR) that engage intracellular pathways that induce the assembly of caspase-1-activating complexes called inflammasomes. The inflammasomes induce the processing and secretion of a common set of proinflammatory cytokines that aim to suppress the harmful element and induce a form of cell death called pyroptosis (235). There are four inflammasomes defined by their NLR protein (NLRP1, NLRC4, NLRP3, and AIM2) of which the NLRP3 is the one studied in relation to the activation of the innate immunity in hypertension. Among the PRR, the Toll-like receptors (TLRs) are, up to the present time, the only group that has been shown to be involved in the inflammation associated with hypertension. TLRs are expressed by T and B lymphocytes, monocytes, dendritic cells, and other somatic cells, such as endothelial and vascular smooth muscle cells. The TLRs engage the inflammasome pathway that is activated by two signals. Signal I includes upregulation of NF-κB, AP-1, and interferon-regulatory factors which result in the upregulation of genes that control inflammasome components, such as the sensor molecule NLRP3, procaspase, pro-IL-1β, and pro-IL-18. The priming of NLRP3 is a requisite for inflammasome activation except when there is constitutive NLRP3 expression, as in macrophages (10, 11). Signal II in the canonical inflammasome activation consists of the detection of PAMPs and DAMPs by NRLP3, which in turn engages a caspase recruitment domain (ASC) and procaspase which heterodimerize to form active caspase. The role of caspase in the inflammasome is to catalyze the intracellular processing of pro-IL-1β and pro-IL-18 to their biologically active forms (IL-1β and IL-18). The active forms are released to the extracellular space and drive the inflammation. In addition to offering an immediate defense response, the inflammasome supports an effective antigen presentation to naive T cells and thereby facilitates a subsequent acquired (adaptive) immune response directed specifically to the corresponding antigen (166). The delivery of signal II for inflammasome activation may come from cellular efflux of potassium ions, production of mitochondrial ROS, or release of mitochondrial DNA and lysosomal destabilization (323).
The adaptive immune system is characterized by specific immune response directed to exogenous or endogenous antigens. The most important effector cells of the adaptive immune system are T and B lymphocytes. Activation of T cells requires that antigens are presented in the context of MHC by APCs. In order for the T cells to be activated, they need two signals: first, an antigen in the MHC of the APC that is recognized by a specific TCR and second, independent costimulation by B7 ligands (CD80 or CD86) that link with CD28 in the T cell. In addition, the clonal expansion of activated T cells requires a third signal to proliferate that is provided by cytokines (42). The generation of memory T cells requires the interaction of the CD27 molecule in the T lymphocyte with costimulatory molecule CD70 in antigen presenting cells and is also a central feature of adaptive immunity. Memory T cells are responsible for the accelerated protective response to subsequent antigen exposure. Cytokines produced by activated CD4+ T cells generate and maintain B-cell humoral immune responses.
The naive CD4+ T cell, depending on the cytokine environment, polarizes to Th1, Th2, Th17, or Treg phenotypes. The Th1 phenotype is generated in environment of IL-12 and interferon (IFN)-γ and predominantly secretes IL-2, TNF-α, and IFN-γ. The Th2 phenotype is generated in IL-4 environment and predominantly secretes IL-4 and IL-10. The Th17 phenotype requires IL-6, IL-21, IL-23, transforming growth factor (TGF)-β, and IL-1β; is activated by aldosterone; and secretes IL-17A, IL-17F, IL-21, and IL-22 (77). The Treg phenotype is generated in TGF-β1 environment with low concentration of IL-6, and its anti-inflammatory activity is exerted by secretion of immunosuppressive immune factors such as IL-9, IL-10, TGF-β, and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and by direct cell-to-cell contact (324).
In the following sections we will discuss the roles of immune cells, cytokines, and innate and adaptive immunity in experimental hypertension.
IV. IMMUNE CELLS AND HYPERTENSION
A. T Lymphocytes
The definite demonstration of a specific role for T cells in the pathogenesis of experimental angiotensin II-induced hypertension was obtained by Guzik et al. (90) using the rag1−/− mouse that lacks T and B lymphocytes. Angiotensin II-induced hypertension is associated with T-cell infiltration in perivascular tissue; oxidative stress; expression of intercellular adhesion molecule 1 (ICAM-1), RANTES, and TNF-α; and impairment in endothelial-dependent vasodilatation. All these features were suppressed in the rag1−/− mouse in association with a blunted blood pressure response to angiotensin II or to DOCA-salt. Adoptive transfer of T cells, but not B cells, restored the response to angiotensin II, including hypertension, in the rag1−/− mouse.
The role of T lymphocytes in Dahl SS rats was subsequently demonstrated by Mattson et al. (175) who used zinc finger-nuclease technology to induce a mutation of the exon 1 of rag1 in Dahl rats that resulted in deletion of immunoreactive rag1 protein in the thymus and a significant reduction of T and B lymphocytes. The mutant Dahl SS rats showed amelioration of salt-induced hypertension in association with reduced T-cell infiltration in the kidneys.
The participation of lymphocytes was also examined by Crowley et al. (40) who showed that the scid mice with impaired lymphocyte function responded to angiotensin II with enhanced natriuresis resulting from upregulated renal expression of eNOS and COX-2, and increased generation of NO and prostaglandins. As a consequence, the scid mice had a blunted response to the late (after 5 days) hypertension induced by angiotensin II infusion. Another important finding in this study (40) was the demonstration that lymphocyte deficiency suppressed pressure-independent heart and kidney injury induced by angiotensin II.
1. T helper (CD4+) and T cytotoxic (CD8+) cells
Many studies have shown that cytokines activated in a Th1 response play a role in hypertension, and Treg cells ameliorate hypertension (see later). Therefore, it was widely assumed that CD4+ T cells were the key elements in the pathogenesis of hypertension. Surprisingly, elegant investigations of Trott et al. (278) showed that CD8+ T cells played a central role in hypertension. Their comprehensive studies included TCR Vβ spectratyping of the CD4+ and CD8+ cells isolated form target organs, adoptive transfer of CD4+ and CD8+ T cells to rag 1 −/− mice, and evaluation of the response to sodium and volume challenge in CD4−/− and CD8−/− mice. Since T-cell receptors (TCR) are necessary for the development of adaptive immunity, the authors examined the TCR Vβ region looking for a dominant transcript length that would be indication of clonal expansion. In the control mice, as expected, there was a Gaussian distribution of TCR Vβ families. However, in angiotensin II-infused mice, there was a dominant transcript length Vβ 3, 8.1, and 17 families in the CD8+T cells present in the kidneys. Deep sequencing of the TCR CD8+ T cells revealed three clonotypes shared by the majority of angiotensin II-infused mice and absent in controls. The low frequency of unique clonotypes in the hypertensive kidney suggested that a group of clones is first activated and, as inflammation develops and new neoantigens are originated, a different group of clones is added. The role of CD4+ and CD8+ cells was studied evaluating the blood pressure response to angiotensin II and DOCA-salt in CD4−/− and CD8−/− mice. Wild-type and CD4−/− mice responded with similar hypertension and retained sodium and water when infused with angiotensin II. In contrast, CD8−/− mice did not and had a blunted hypertension. Then, the blood pressure response to angiotensin of rag 1−/− mouse, without lymphocytes, was evaluated. As in previous studies (90), rag 1−/− mouse had a diminished hypertensive response. Adoptive transfer of CD4+ T cells did not modify the blunted blood pressure response, while adoptive transfer of CD8+ T cells resulted in a full restoration of the angiotensin II-induced hypertension. These findings conclusively established a role for the CD8+ cells in the pathogenesis of angiotensin-induced hypertension.
2. Th17 cells
Th17 cells are involved in adaptive and innate immune responses, and dysregulation of Th17 cells has been associated with autoimmune disorders (322). Th17 polarization is suppressed by Tregs (330), and Amador et al. (2) showed that DOCA-salt hypertension is associated with activation of Th17 cells and downregulation of Treg mRNA in heart and kidneys. Spironolactone (but not other antihypertensive treatment) prevented Th17 activation and increased the numbers of Treg cells, and treatment with anti-IL-17A antibody ameliorated hypertension and fibrotic injury in heart and kidneys. Therefore, IL-17 is an important factor in mineralocorticoid-induced hypertension, and an alteration in the IL-17/ Treg balance plays a role in DOCA-induced hypertension. Similar proinflammatory imbalance has been found to be caused by tacrolimus and is probably causally related to the hypertension observed during treatment with this drug (34).
Two recent studies have simultaneously demonstrated that a high-salt diet is capable of inducing Th17 cells and production of IL-17. These investigations demonstrated that salt induces SGK1 which is a critical modulator of cellular Na transport and NaCl homeostasis. Kleinewietfeld et al. (136) found that salt concentrations in the physiological range activate the p38 MAPK pathway during cytokine-induced Th17 polarization. The salt-induced p38 MAPK activation resulted from engaging the tonicity-responsive enhancer binding protein (TonEBP/NFAT5) and SGK1. Wu et al. (312) examined the way by which IL-23 stabilizes and reinforces a Th17 response. They found that modest increments in salt concentration induce SGK1, promote IL-23R expression, and stimulate Th17 differentiation in vitro and in vivo. They demonstrated that SGK1 deactivates FoxO1, which is a suppressor of IL-23R expression. SGK1 was therefore identified as critical downstream element for regulating IL23R expression and, thereby, stabilization of Th17. Both papers show how a high-salt diet may induce IL-17, thereby suggesting a direct link between autoimmunity and salt-driven hypertension.
More recently, Norlander et al. (193) found IL-17 deficiency suppressed angiotensin-induced activation of sodium-chloride cotransporter and the epithelial sodium channel in the distal tubule. Interestingly, they also found that distal tubular cells produce IL-17 and showed that IL-17 deficiency protected from glomerular and tubular injury caused by angiotensin II.
3. Regulatory T cells
The role of Tregs in hypertension was evaluated in angiotensin-induced and mineralocorticoid-induced hypertension. Administration of a single dose or weekly injections of Tregs improve cardiac hypertrophy, electrically induced arrhythmias, endothelial relaxation, oxidative stress, and inflammation in angiotensin II-induced and mineralocorticoid-induced hypertension, but blood pressure was not significantly modified (126, 146). However, mice given a higher dose of Tregs (3 weekly doses of Tregs for 2 wk) developed a sustained reduction in blood pressure in association with reduction in immune cell infiltration (7, 171). The effectiveness of repeated Treg administration is likely due to repopulation of Tregs that are depleted by angiotensin-induced apoptosis (7).
More recently, Majeed et al. (161) used a different strategy to increase Tregs in angiotensin II-infused mice. They took advantage of investigations that showed that the administration of immune complexes of IL-2 and anti-IL-2 monoclonal antibody (IL-2/mAbCD25) result in binding of IL-2 to CD25 expressing cells that induces a selective and rapid expansion of Tregs with anti-inflammatory activity in vivo (300). Using optimal doses and molar ratios, they obtained a fivefold expansion of the Treg phenotype in spleen with only minimal changes in CD4+ and CD8+ T cell numbers. IL-2/mAbCD25 was given intraperitoneally for 5 consecutive days before angiotensin II infusion and three times weekly thereafter. Treatment resulted in a suppression of angiotensin II-induced IL-17 gene expression and reduced infiltration and activation of immune cells in the aorta. However, stimulation of natural expansion of the Treg population did not modify angiotensin II-induced hypertension.
Mian et al. (182) used a different strategy to evaluate the role of Tregs. They worked with Scurfy mice that are deficient in Tregs because of a mutation in the FoxP3 gene. These mice die at 4–6 wk of age, and the study involved adoptive transfer of T cells from Scurfy and wild-type mice into rag 1−/− mice that lack T and B lymphocytes. The lack of Treg in the rag 1−/− mice that received T cells from Scurfy mice resulted in an exaggerated response to angiotensin-induced hypertension and microvascular injury.
Other workers have been interested in myeloid-derived suppressor cells that are one of the ways by which the immune system limits inflammatory injury. The myeloid suppressor cells are a heterogeneous group of immature myeloid cells that suppress T-cell activation. These cells express myeloid markers CD11b and Gr1 and have been found to be increased in the circulation and in the spleen in several models of experimentally induced hypertension. Shah et al. (241) showed that treatment with gemcitabine, an immunosuppressive agent that selectively depletes myeloid-derived suppressor cells, increased the severity of hypertension. Conversely, adoptive transfer of myeloid suppressor cells ameliorated hypertension (241).
Other investigations have uncovered an important interrelation between Treg functionality and sympathetic activity. The SPSHR have sympathetic overactivity (discussed earlier) and reduced proportions of Tregs cells that precede the development of hypertension. Splenic denervation increased the Tregs in spleen and in peripheral blood in approximately the same proportion as did the administration of the IL-2/mAbCD25 immune complex (see before) and delayed and ameliorated the hypertension of the SPSHR (128). The increment in Treg cells induced by splenic sympathetic denervation suggests an additional mechanism for the hypertension improvement resulting from blockade of sympathetic activity.
B. B Lymphocytes
B cells are essential players in adaptive immunity. The role of B lymphocytes in hypertension has been largely unexplored because the experiments in the rag 1−/− mouse, lacking T and B lymphocytes, showed that only the adoptive transfer of T cells of restored the hypertensive response to angiotensin II and DOCA-salt. However, Chan et al. (32) recently showed that angiotensin infusions increase the activation of B cells and plasma cells in lymphoid tissues and induced aortic IgG deposition. Depletion of B cells with the administration of anti-CD20 antibody, as well as genetic deficiency of B cells (BAFF-R−/− mice) protected mice from the chronic pressor effects of angiotensin II. Furthermore, angiotensin II-induced aortic infiltration of macrophages and CD4+ T cells and arterial wall remodeling are suppressed in the BAF-FR−/− mice. Since B cells by themselves do not modify hypertension in the rag1−/− mouse (90), the important experiments of Chan et al. (32) raise a new line of inquiry concerning the participation of B cells in the pathogenesis of hypertension within a normal (intact) immunological environment.
C. Natural Killer Cells
Natural killer (NK) cells are non-T, non-B lymphocytes with the capacity for spontaneous or “natural,” antigen-independent cytotoxic activity. They are part of the group of innate lymphoid cells that play a central role in the innate immune system (295). Kossmann et al. (138) have shown that there is a mutual activation between NK cells and monocytes in angiotensin II-induced hypertension. The role of NK cells in hypertension and vascular remodeling was investigated by Taherzadeh et al. (265) who studied a congenic strain in which the NK gene complex of the C57BL/6 (Th1 biased) was introduced in the BALB/c (Th2 biased) background and found that strains that shared the same NK gene complex had similar blood pressure response to chronic l-NAME-induced hypertension. These studies underline the role of NK cells in the sensitivity to develop hypertension induced by inhibition of NOS.
D. Monocytes/Macrophages
Macrophages are always present in vessel walls and in the kidney in hypertension. They are involved in innate immunity and participate in adaptive immunity acting as antigen-presenting cells (APC). Macrophages are closely related to the dendritic cells, and several classifications of macrophages are presently in use. One classification uses the LysM marker to separate tissue resident macrophages and inflammatory macrophages. Another and more common classification differentiates M1 and M2 macrophage subtypes. The M1 subtype is proinflammatory and is activated when exposed to IFN-γ and TNF-α. The M2 macrophages are anti-inflammatory and play an important role in salt and water homeostasis. Investigations without selective depletion of M1 or M2 macrophages are difficult to interpret because results may respond to unidentified alteration of their balance. Moreover, there is a continuum between the M1 and M2 cell types, and a clear polarization of M1 and M2 macrophages is often impossible.
Some studies have evaluated the effects of suppressing macrophage infiltration in the tissues by inhibiting monocyte chemoattractant protein (MCP-1) or blocking the MCP-1 receptor C-C chemokine receptor 2 (CCR2). With the use of this strategy, reduction in macrophage infiltration and reduction in blood pressure were observed in angiotensin II-induced (67, 109) and DOCA-salt hypertension (31). Other investigators studied the osteopetrotic mice (Op/Op) that are deficient in macrophage colony-stimulating factor (m-CSF). They found that the Op/Op mice were protected from angiotensin II and DOCA-salt hypertension and showed less endothelial dysfunction, arterial remodeling, and oxidative stress than the control heterozygous (Op-/+) and wild-type mice (48). More recently, elegant investigations by Wenzel et al. (304) used the cre-lox technology to induce the diphtheria toxin receptor in LysM-positive macrophages. The subsequent administration of low-dose diphtheria toxin-depleted myelomonocytic cells reduced the number of circulating monocytes and of macrophages infiltrating vascular walls. This treatment corrected the hypertension, vascular dysfunction, and oxidative stress induced by angiotensin II infusion. Adoptive transfer of normal LysM-positive cells restored the angiotensin II-induced effects and hypertension.
In addition to the proinflammatory characteristics of the M1 macrophage, a series of investigations have highlighted the role of M2 macrophages in sodium and water homeostasis. Initial observations from Titze’s group showed that regions of the dermis serve as a site of water free sodium storage (275). Subsequent investigations demonstrated that interstitial hypertonicity stimulates tonicity-responsive enhancer binding protein (TONEBP) production by the macrophages. TONEBP-stimulated overproduction of VEGF-C drives lymphangiogenesis in the dermis (158). Depletion of macrophages, depletion of TONEBP, blockade of vascular endothelial growth factor (VEGF) receptor or deletion of VEGF resulted in salt-sensitive hypertension, demonstrating that the macrophage-orchestrated system attenuates the hypertensive response to sodium retention (306). More recent studies demonstrated that high salt has a proinflammatory effects and stimulates M1 macrophages and suppress the activation of M2 macrophages (17).
E. Dendritic Cells
Dendritic cells (DCs) are immunocompetent cells closely related to the macrophages. The central function of the DC is to accept antigenic molecules, process them to peptides, migrate to lymphoid organs, and present them in the context of MHC to T cells with the receptor that recognizes the specific peptide. Recent investigations indicate that in specific areas, such as the kidney, DCs have intravascular processes that may capture antigens and direct T-cell migration into the tissues (319). Intracellular antigens are processed in the proteosome and presented by the MHC class I to CD8+ T cells, and extracellular antigens processed in the lysosome are presented in MHC class II to CD4+ T cells. Extracellular antigens may also be presented to CD8+ T cells via MHC I by cross presentation. There are several subtypes of DCs that preferentially activate CD4+ or CD8+ T cells. The kidneys have an extensive net of DC especially in tubulointerstitium areas, and only 5% of them belong to the CD8-like subtype (302). Selective depletion of DCs is not possible, and therefore, studies examining antigen presentation are focused on suppression of stimulation signals in antigen recognition (see sect. VII).
V. CYTOKINES IN EXPERIMENTAL HYPERTENSION
Cytokines that are particularly relevant to hypertension are produced by T cells, B cells, mast cells, macrophages, and DCs. Studies focusing on specific cytokines in experimental models of hypertension are shown in TABLE 2. Several considerations are important. First, it should be kept in mind that not only the individual values of the cytokines but also their balance is important. For example, angiotensin-induced hypertensive renal damage is associated with increase in Th1 cytokine INF-γ and reduction in Th2 cytokine IL-4 (242). The T-bet deficient mice are unable to produce a Th1 response, and Zhang et al. (327) showed in the T-bet−/− mice that Th1 proinflammatory response was necessary for angiotensin II-induced renal injury but not for hypertension. It is also important to recognize that the antihypertensive result of suppressing a specific cytokine may depend on the experimental model used in the investigation. For instance, as depicted in TABLE 2, DOCA-salt hypertension is unmodified by TNF-α, IL-6, or IL-17 deficiency which is in contrast to the amelioration observed in of angiotensin II-induced hypertension. Finally, cytokines have frequently overlapping functions, which presents a challenge in studies that target individual cytokines to evaluate their role in hypertension and tissue injury (39). All these circumstances are responsible for the variability in the amelioration of hypertension attributed to suppression of specific cytokines. The reduction in blood pressure in TABLE 2 ranges from 19% (160) to no significant antihypertensive effect (55, 66, 139, 164, 183, 186, 256).
Table 2.
Effects of suppressing individual cytokines in experimental models of hypertension
| Cytokine | Hypertension Model | Treatment/Immune Deficiency | Results | Reference Nos. |
|---|---|---|---|---|
| IFN-γ | ANG II infusion | IFN-γ−/− | Hypertension ameliorated (SBP: WT = 170 mmHg; IFN-γ−/− = 148 mmHg) | 125 |
| ANG II infusion | IFN-γ R−/− | Injury improved, hypertension unchanged | 164 | |
| TNF-α | ANG II infusion | TNF-α−/− | Hypertension ameliorated (MAP: WT = 151 mmHg; TNF-α−/− = 113 mmHg) | 252 |
| TNF-α−/− | Hypertension ameliorated (MAP: WT = 183 mmHg; TNF-α−/− = 166 mmHg) | 326 | ||
| dTGF | Etanercept | Injury improved, hypertension unchanged | 186 | |
| DOCA-salt | Etanercept | Injury improved, hypertension unchanged | 66 | |
| RANTES | ANG II infusion | RANTES−/− | Suppressed perivascular immune infiltration, improved endothelial dysfunction, hypertension unchanged | 183 |
| IL-1 | ANG II infusion | IL-1r−/− mice | Sustained (late) hypertension improved (MAP: WT = 180 mmHg; IL-1r−/− = 165 mmHg) | 328 |
| IL-4 | NZBF1 rats | Anti-IL-4 antibodies | Hypertension ameliorated (MAP: not treated = 87 mmHg; treated = 68 mmHg) | 285 |
| IL-6 | ANG II infusion | IL-6−/− mice | Hypertension ameliorated (MAP: WT = 160 mmHg; IL-6−/− = 134 mmHg) | 147 |
| DOCA-salt | IL-6−/− mice | Hypertension unchanged | 256 | |
| Cold-induced hypertension | IL-6 knockdown | Hypertension improved (MAP: WT = 140 mmHg; IL-6 deficient = 120 mmHg) | 38 | |
| IL-10 | ANG II infusion | IL-10−/− mice | Increased ROS and vascular dysfunction, hypertension unchanged | 55 |
| DOCA-salt treated pregnant (DSP) rats | Injections of IL-10 | Hypertension improved (SBP: DSP = 135 mmHg; DSP+IL-10 = 115 mmHg), endothelial dysfunction improved | 274 | |
| IL-17 | ANG II infusion | IL 17−/− mice | Late (>2 wk) hypertension ameliorated (MAP: WT = 150 mmHg; IL 17−/− = 128 mmHg) | 160 |
| DOCA-salt | Anti-IL-17 antibodies | Hypertension ameliorated (SBP: not treated = 150 mmHg; treated = 123 mmHg), mineralocorticoid receptor modulates inflammation | 2 | |
| DOCA-salt +ANG II | IL 17−/− mice | Hypertension unmodified/injury worse | 139 | |
| IL-17 administration | C57BL/6 mice | Blood pressure increment mediated by Rho-kinase | 192 |
SBP, systolic blood pressure; MAP, mean arterial pressure; WT, wild type with the corresponding hypertension model. See text for other definitions.
A. IFN-γ
IFN-γ is a member of the type II cytokine family that is produced by T cells. It has two receptors (IFNGR1 and IFNGR2) and induces polarization to the Th1 phenotype and activation of macrophages and B cells. IFN-γ is critical for the development of renal injury induced by angiotensin II infusion in studies done with IFN-γ−/− and IFN-γR−/− mice. Nevertheless, hypertension was either unmodified (164) or improved in association with suppressed renal sodium transporter activation (125).
B. TNF-α
TNF-α belongs to the tumor necrosis factor superfamily. It is produced by macrophages, NK cells, and T cells and has two receptors: CD120a and CD120b. TNF-α activates endothelial cells and neutrophils and causes fever and catabolism of fat and muscle. Studies examining the role of TNF-α in hypertension have used etanercept or the TNF-α−/− mouse. Etanercept administration in the dTDF rats (186) and in DOCA-salt hypertension failed to modify the blood pressure levels despite the reduction in albuminuria, cortical NFκB activity, and cell adhesion molecules (66). In contrast, TNFα−/− mice have increased eNOS production and were protected from developing hypertension and from the sodium and water retention induced by 2 wk of angiotensin infusion (252, 326). Intrarenal TNF-α is increased by a high-salt diet in the Dahl SS rat, and administration of intrarenal etanercept improved SS hypertension and renal injury (104). Since TNF-α reduces renal blood flow and inhibits the sodium potassium 2-chloride (NKCC2) transporter, Ryan (228) has noted that blood pressure effects of TNF-α likely depend on the balance between vasoconstriction and natriuretic activities.
C. CCL5 (RANTES)
CCL5 is a chemoattractant for inflammatory cells that is produced by T cells, resident vascular cells, and adipose tissue. It has three receptors (CCR1, CCR3, and CCR5). CCL5−/− mice have suppressed T-cell infiltration in perivascular tissue and less endothelial dysfunction following angiotensin II infusion. These effects likely result from a diminished infiltration of IFN-γ producing T cells (183).
D. TGF-β
TGF-β is produced mostly by Tregs and macrophages. It has three receptors (TGF-βR1, R2, and R3), stimulates collagen production by fibroblasts, and inhibits proliferation and activation of T cells, B cells, and macrophages. TGF-β is stimulated in SS hypertension, and reduction of TGF-β improves several models of experimental glomerulonephritis. Administration of anti-TGF-β antibodies resulted in amelioration of salt induced hypertension in the Dahl SS rat in association with a reduction in renal and cardiac fibrosis (44, 187).
E. IL-1
IL-1 is a member of the IL-1 cytokine family that is produced by macrophages, DCs, fibroblasts, endothelial cells, keratinocytes, and hepatocytes. IL-1 induces activation of endothelial cells, fever, and synthesis of acute-phase proteins. IL-1 has two isoforms, IL-1α and IL-1β, and both bind a single receptor (IL-1r). Both isoforms of IL-1 are increased in the kidney in angiotensin II-induced hypertension (40), and deficiency of IL-1r ameliorates the hypertension resulting from 3 wk of angiotensin II infusion (328). The attenuation of hypertension in the IL-1r−/− mice results from increased natriuresis due to absence of angiotensin II-induced hyperactivity of the NKCC2 transporter and to elevated NO levels due to preferential differentiation of immature Ly6C+Ly6G+ myeloid cells to the NO-producing Ly6C+Ly6G- macrophage phenotype (328).
F. IL-2
IL-2 is member of the type I family of cytokines. It is secreted by T cells activated by TCR-antigen presenting cell interactions and additionally costimulated via CD80/CD86. IL-2 drives the proliferation of effector T cells. Proliferating T cells deprived of IL-2 undergo apoptosis. In addition, IL-2 signals are essential for the generation and survival of Tregs. In turn, Tregs regulate IL-2 availability by directly inhibiting IL-2 production, as well as by consumption of IL-2 and by blocking CD80/86 costimulation (50). Therefore, IL-2 is a key player in the development of both effector T-cell and regulatory T-cell responses. The administration of IL-2 in doses ranging from 5,000 to 100,000 units/kg to SHR (206, 282) and Dahl SS rats (82, 110) was found to ameliorate hypertension (110, 282) or to have no effect on blood pressure (82, 206). Effector T cells and Tregs were not examined in these early studies, and it seems likely that the complex interactions of IL-2, resulting in either expansion or suppression of immune reactivity, are the explanation, at least in part, for the discrepancies in experimental studies.
G. IL-4
IL-4 is a member of the type I cytokine family produced mainly by CD4+ T cells and mast cells. IL-4 has two receptors (CD124 and CD132) and mediates the differentiation to the Th2 phenotype. IL-4 production is suppressed in angiotensin II-induced hypertension (242), but to our knowledge, the only investigations of the role of IL-4 were done in female NZBW rats by van Heuven et al. (285) who showed that intraperitoneal administration of anti-IL-4 antibody at 6, 8, and 10 wk of age suppressed hypertension in the NZBW rats.
H. IL-6
IL-6 is a member of type I cytokine family produced by macrophages, endothelial cells, and T cells. It has two receptors (CD126 and CD130) and induces proliferation of B cells and acute-phase protein synthesis. Since IL-6 is increased in plasma by angiotensin II infusions, Brands’group (147, 256) studied IL-6−/− mice to establish the role played by IL-6 in angiotensin II-induced hypertension. In a series of elegant studies they showed that the increment in IL-6 required the presence of aldosterone and hypertension induced by 800 ng·kg−1·min−1 of angiotensin II (but not higher doses) was prevented in the IL-6−/− mice, while renal vasoconstriction was unaffected. Angiotensin II-induced phosphorylation of JAK2 and signal STAT 3 were completely suppressed in the IL-6−/− mice (22). Superoxide generation, vascular remodeling, and endothelial dysfunction induced by angiotensin II are all dependent on IL-6 generation (234). In more recent studies it has been shown that administration of IL-6 neutralizing antibody attenuated SS hypertension, renal inflammation, and injury in the Dahl SS rat (94). IL-6 is produced not only in T cells and macrophages, but also in cells that play a role in hemodynamic physiology, such as endothelial cells (84), vascular smooth muscle cells (91), and sympathetic nerves (168); therefore, blood pressure-lowering effects resulting from suppression of IL-6 may result, at least in part, from effects other than immune modulation.
An important issue is whether IL-6 plays a role in the physiological increase in angiotensin II levels. This appears unlikely since during a low-salt diet IL-6 levels are not increased and JAK2 is not required to maintain blood pressure (22, 30).
I. IL-10
IL-10 is a member of type II cytokine family that has anti-inflammatory activity. It is produced by monocytes, Th2 lymphocytes, mast cells, subsets of B cells, and Tregs. IL-10 has two receptors: CD210 (IL-10Rα) and IL-10Rβ. In experimental preeclampsia, IL-10 administration ameliorates hypertension and albuminuria (274), and IL-10 deficiency aggravates angiotensin II-induced endothelial dysfunction and superoxide production (55).
The role played by immunosuppressive cytokines produced by Tregs has been shown in studies of a consomic strain of rats (SSBN2) that have chromosome 2 of normotensive Brown Norway rats transferred to the genome of hypertensive salt-sensitive Dahl rats (293). SSBN2 rats have increased expression of FoxP3, TGF-β, and IL-10 and a reduced blood pressure response to high-salt diet.
J. IL-17
The IL-17 family of cytokines comprises six members (A, B, C, D, E and F) of which IL-17A is the prototype. It is produced by the T helper lymphocyte subtype Th17 and also by immune activation of DCs, macrophages, natural killer cells, CD8+ cells, and gamma-delta T cells. Interestingly, gamma/delta T cells are the major source of IL-17 in the inflammatory damage induced by angiotensin II infusions, and its production is regulated by monocyte-derived IL-1β (150).
IL-17 facilitates the infiltration of inflammatory cells in tissues by the induction of adhesion molecules and chemokines and has been implicated in the pathogenesis of autoimmune diseases (133). Madhur et al. (160) found that angiotensin II infusions caused a severalfold increment of IL-17 in circulating T cells, accumulation of IL-17 protein in the medial layer of thoracic aorta, and vascular dysfunction. Similar results were not observed in the IL-17−/− mouse injected with angiotensin II. Deficiency in IL-17 did not modify the initial increase in blood pressure induced by angiotensin II infusions but significantly ameliorated the sustained hypertension observed after 2 wk. Interestingly, gene array studies made in human aortic smooth muscle cells revealed that IL-17 by itself induced little gene changes, but in association with TNF-α modulated the expression of more than 30 genes, a value several times higher than what was obtained with TNF-α alone. Despite the synergy of TNF α and IL-17 in the modulation of proinflammatory genes, the endothelial vascular dysfunction induced by IL-17 requires no additional cooperation because IL-17 causes impairment of NO production due to suppressed eNOS activity (160). Nguyen et al. (192) demonstrated that IL-17 increases phosphorylation of the inhibitory eNOS residue threonine 495 (eNOS Thr495). Of the various kinases known to activate eNOS Thr495, only the Rho-kinase activator was responsible for IL-17-induced depression of the vascular relaxation response. Administration of IL-17 increased blood pressure that was prevented by an inhibitor of Rho-kinase.
Recent investigations have added complexity to the role played by IL-17 in the development of inflammation. In a model of hypertension that combines DOCA-salt and angiotensin II, Krebs et al. (139) found that, contrary to their expectations, deficiency in IL-17/IL-23 axis did not modify the hypertension and actually worsened the renal and cardiac injury. There is no explanation for these findings at the present time, and the authors (139) raised the possibility of a biphasic response to IL-17 that would confer protection early in hypertensive disease and cause aggravation in more advances stages.
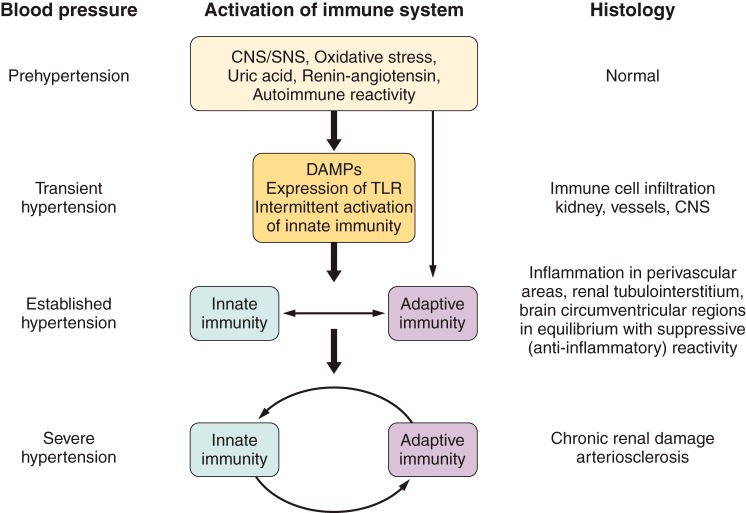
VI. INNATE IMMUNITY IN HYPERTENSION
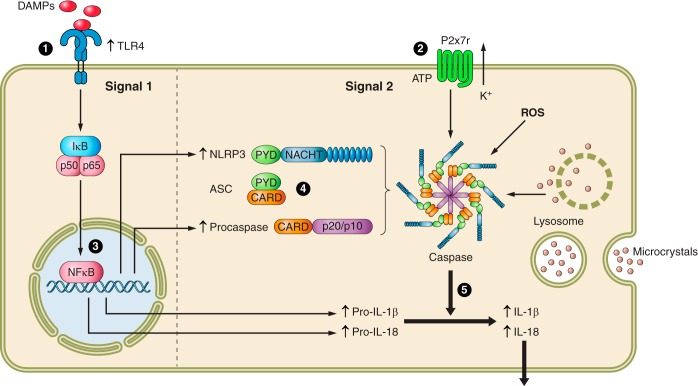
Several investigations have studied the role played by the NLRP3 inflammasome in experimental models of hypertension (FIGURE 1).
FIGURE 1.
Participation of the NLRP3 inflammasome in the pathogenesis of experimental hypertension. 1) Suppression or deficiency of TLR4 ameliorates or prevents hypertension (20, 46, 247). 2) Inactivation or deficiency of the P2x7 receptor ameliorates hypertension in the Dahl SS rat (120). 3) Suppression of NFκB activation ameliorates hypertension in SHR (218), Fawn Hooded rat (137), and the dTGF rat (185). 4) Deficiency of ASC ameliorates DOCA-salt hypertension (140). 5) NLRP3 inflammasome components overexpressed in the SHR (see FIGURE 2) and inhibition of inflammasome activation ameliorate DOCA-salt hypertension (140).
A. Toll-like Receptors in Hypertension
The associations of TLRs and inflammation in relation to hypertension have been recently reviewed (177). Activation of TLR in models of hypertension was first suggested by studies demonstrating that TLR4 is increased in the kidneys of SHR and by the finding that cytokine production by splenocytes from SHR rats is increased following stimulation of TLR 7/8 or 9. Furthermore, splenocytes of the SHR treated with TLR ligands showed an enhanced cytokine production in the presence of nicotine (an ACh agonist), in contrast to the reduction observed in the splenocytes from control WKY rats (93). Direct evidence of the role of TLR4 in hypertension was later shown by the ability of anti-TLR4 antibody treatment to ameliorate hypertension in the SHR (20) and by the failure of TLR4−/− mice to develop l-NAME-induced hypertension (247). The importance of TLRs in specific areas of the brain is suggested by the demonstration that TLR4 is upregulated in the paraventricular nucleus of the hypothalamus in the SHR, but not in the normotensive WKY rats. Furthermore, injection of a specific TLR4 blocker to this brain area lowered blood pressure; reduced mRNA and protein abundance of TNF-α, IL-1β, and inducible NOS; and suppressed NFκB activity in SHR (46).
Hypertension-related DAMPs are capable of activating TLR2 and TLR4 signaling. These include angiotensin (119), C-reactive protein (CRP) (151), uric acid (153), and heat shock proteins 60 (49) and 70 (4).
The activation of TLR4 and TLR2 in essential hypertension is discussed later.
B. Activators of the Inflammasome in Hypertension
Elements potentially responsible for activation of the inflammasome in hypertension include both soluble (315) and crystalline (165) urate, reactive oxygen radicals, and ATP-induced activation of the P2x7 receptor.
Monosodium urate crystals are recognized danger signals of stressed cells that are capable of activating the NLRP3 inflammasome (108, 165). Mazzali et al. (176) showed that the induction of hyperuricemia resulted in hypertension in rats. Reducing the levels of uric acid with allopurinol improves the blood pressure in adolescent patients with hypertension (68). It is possible that the activation of specific urate transporters may increase intracellular urate and formation of microcrystals. It remains to be determined if microcrystal-induced inflammasome activation represents a mechanism of pathogenic importance in essential hypertension.
Oxidative stress has been repeatedly shown to play a role in vascular dysfunction and hypertension (277, 307), and excessive production of reactive oxygen species (ROS) plays a central role in driving signal I in the process of activation of the inflammasome. Experts have argued that ROS is likely the common signal for inflammasome activation (280) that is likely situated upstream of NLRP3 induction (10). Overexpression of TLRs resulting from excess generation of ROS in complicated pregnancies has been proposed to be a factor in the adult development of hypertension (269).
Extracellular ATP acting at P2X7 receptor is a stimulus for NLRP3 inflammasome activation. The P2X7 receptor is an ion-gated channel that generates K+ efflux when activated and recruits the pore-forming protein pannexin-1 to the plasma membrane. Potassium efflux triggers inflammasome activation (216).
Angiotensin II infusion induces overexpression of P2X7 (296) and inactivation or suppression of the P2X7 receptor as well as ameliorates the vicious circle of inflammation and SS hypertension in the Dahl rat (120).
C. Inflammasome Components in Hypertension
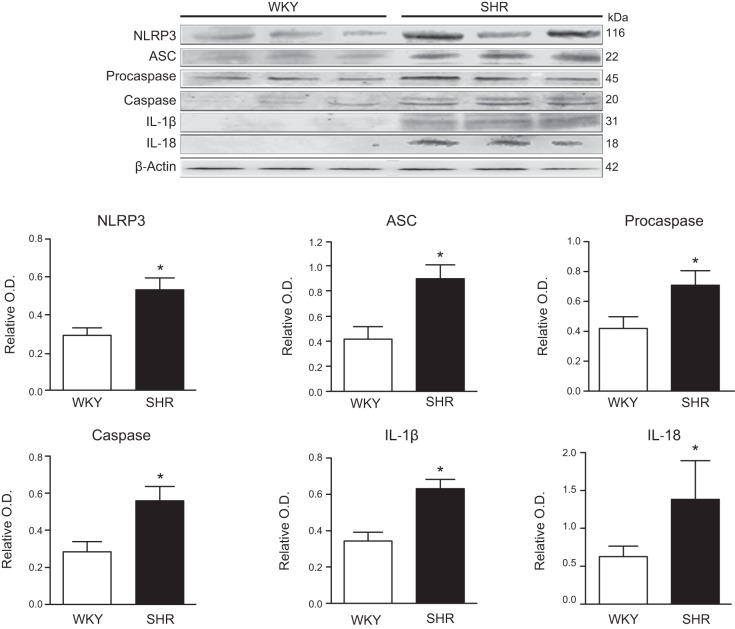
NLRP3 inflammasome is activated in the SHR. FIGURE 2 compares the protein abundance of the components of the NLRP3 inflammasome in SHR with control normotensive WKY at 40 wk of age. The involvement of the inflammasome in the pathogenesis of hypertension has been strongly suggested by several studies that have reported that blocking inflammasome components ameliorates hypertension in animal models (TABLE 3).
FIGURE 2.
NLRP3 inflammasome components are increased in the kidney of SHR. Relative abundance of NLRP3, ASC, procaspase, caspase, IL-1β, and IL-18 in 40-wk-old SHR and normotensive WKY rats. See text for definitions.
Table 3.
Results of interventions in mechanisms of the innate immunity in experimental models of hypertension
| Experimental Model | Intervention | Results | Reference Nos. |
|---|---|---|---|
| SHR | Anti-TLR4 | Hypertension ameliorated (MAP: not treated = 160 mmHg; treated = 140 mmHg), reduced vascular contractility | 20 |
| Brain (PVN) blockade of TLR4 | Hypertension ameliorated (MAP: not treated = 170 mmHg; treated = 142 mmHg), cardiac hypertrophy ameliorated, reduction of HMGB1 | 46 | |
| SPSHR | IL-1β administration | Increase in stroke incidence, blood pressure not modified | 35 |
| Dahl SS | P2X7 receptor antagonist | Amelioration of SS hypertension, (SBP: not treated = 195 mmHg; treated = 165 mmHg), reduction of inflammation and albuminuria | 120 |
| ANG II | Anti-TLR4 antibody | Reduced inflammation in VSMC | 119 |
| DOCA-salt | C5a receptor antagonist | Reduction of heart inflammation and fibrosis, hypertension unchanged | 116 |
| ASC−/− | Amelioration of hypertension (SBP: WT = 155 mmHg; ASC −/− = 140 mmHg), reduced inflammation | 140 | |
| Inhibition of inflammasome (MCC960) | Amelioration of hypertension (SBP: NT = 160 mmHg; Treated = 140 mmHg), reduced Inflammation | 140 | |
| l-NAME | TLR4−/− | Amelioration of hypertension (MAP: WT = 125 mmHg; TLR4−/− = 100 mmHg), reduction in arterial contractility, reduced inflammation | 247 |
| Unilateral uretheral obstruction | C3−/− | Amelioration of hypertension (SBP: WT = 120 mmHg; C3−/− = 105 mmHg), reduction in intrarenal ANG II, reduction in EMT | 329 |
The majority experimental interventions on elements of innate immunity ameliorate hypertension. TLR, Toll-like receptor, PVN, paraventricular nuclei; ASC, adapter protein, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain; EMT, epithelial mesenchymal transition; SS, salt sensitive; SBP, systolic blood pressure; MAP, mean arterial pressure; WT, wild type receiving the corresponding hypertensive treatment.
The binding of inflammasome end products, IL-1β and IL-18, to their specific receptors, IL-1 type 1 receptor (IL-1RI) and the IL-18 receptor α chain (IL-18Rα), is critical for their activity. These receptors are expressed in lymphocytes, monocytes, vascular endothelial cells, vascular smooth muscle cells, and renal tubular epithelial cells. The receptor-ligand binding recruits accessory proteins and adaptor molecules that, in turn, activate signaling pathways, transcription factors such as NF-κB and AP-1, and downstream proinflammatory cytokines.
In addition to proinflamatory activity, IL-1β and IL-18 have direct effects on the vessels that may contribute to hypertension. Rat resistance arteries incubated with IL-1β have increased generation of superoxide and impaired ACh-induced vasodilatation that can be reversed partially with SOD (121). IL-18 induces proliferation and migration of vascular smooth muscle cells also driven by ROS overproduction (284).
The activation of innate immunity in the SPSHR has been suggested by the demonstration of increased plasma levels of the IL-1β in association with gene overexpression of IL-1β, IL-1 receptors, and caspase-1. The administration of IL-1β using an osmotic pump increased hypertension and the incidence of stroke (35).
As will be discussed later, circulating levels of IL-1β (45), IL-18 (214), and IL-1 receptor (IL-1Ra) (207) are increased in hypertensive patients, and the severity of hypertension is correlated with the levels of IL-18 (214). The possible roles of endogenous antagonists of IL-1 receptor (3) and of IL-33, a recently identified cytokine with anti-inflammatory activity (208) in the pathogenesis of hypertension, are undefined at the present time.
D. The Complement System
The complement system is a network of plasma- and membrane-associated proteins involved in the development of inflammatory and cytolytic responses and represents a major effector mechanism of the innate and adaptive immunity (60). It is activated by three pathways: the classical pathway, activated when C1q binds to an antibody attached to an antigen; the lectin pathway, activated when mannose-binding lectin binds to carbohydrate motifs; and the alternative pathway, activated when C3 undergoes hydrolysis and, in the presence of specific factors, presents additional C3 cleavage. The three pathways result in the formation of convertases that are responsible for the generation of anaphylotoxins, opsonins and the membrane attack complex, that are responsible for the major effects of the complement system. Complement is activated early in the dTGF rat (240), but only a few studies have examined the role played by the complement system in hypertension. In DOCA-salt hypertension, treatment with a C5a receptor antagonist improved cardiac remodeling but did not modify blood pressure. Zhou et al. (329) studied the model of unilateral ureteral obstruction and showed that C3 deficiency prevented hypertension and renal injury and demonstrated that C3 activates the renin-angiotensin system and is central in the development of epithelial-to-mesenchymal transition and fibrosis in this model.
VII. ADAPTIVE IMMUNITY IN HYPERTENSION
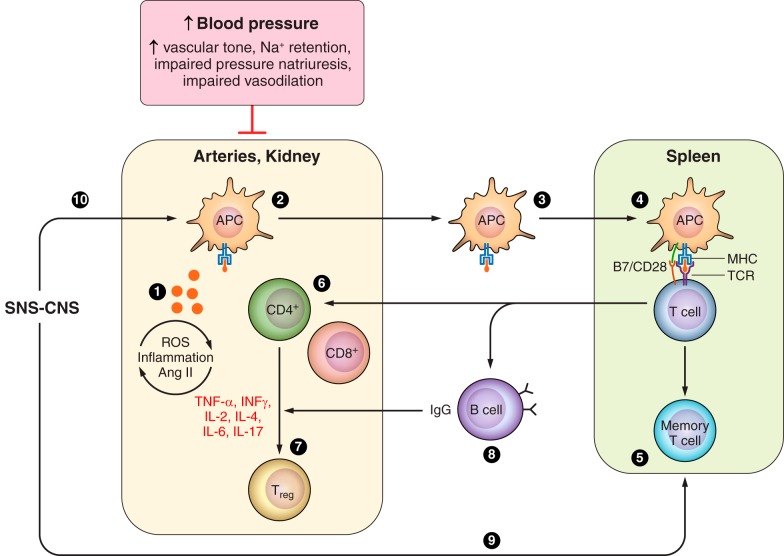
Numerous investigations have established the involvement of several aspects of adaptive immunity in experimental hypertension (FIGURE 3).
FIGURE 3.
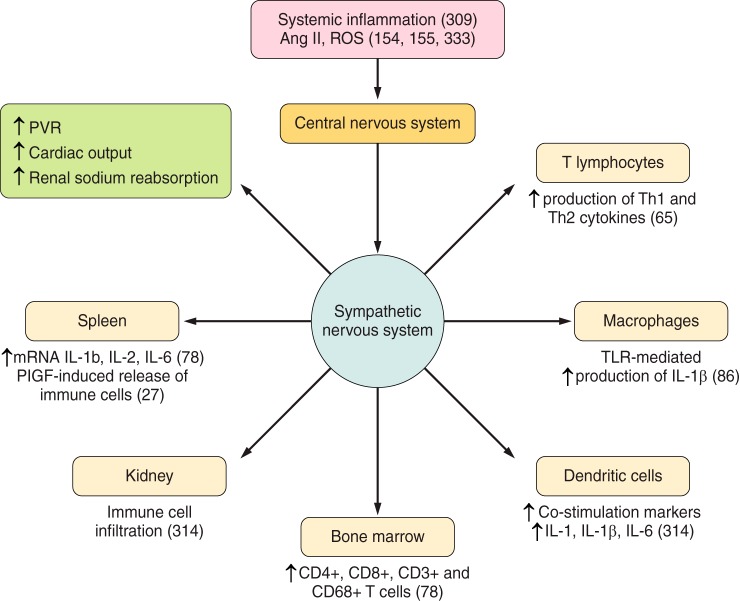
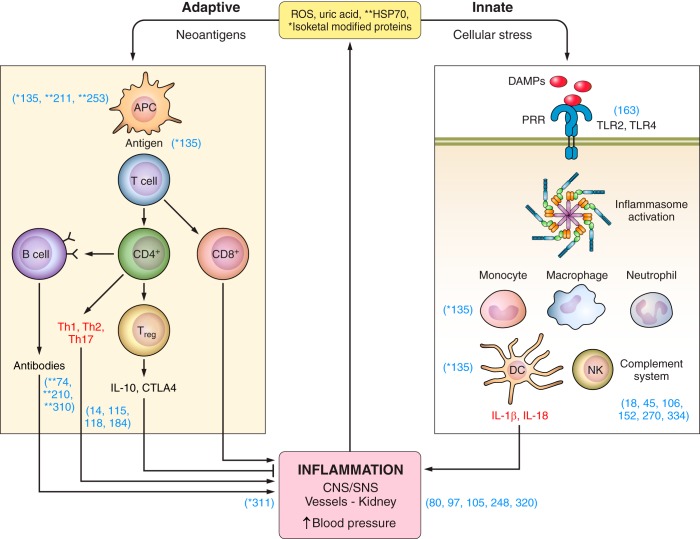
Adaptive immunity in experimental hypertension. Investigations demonstrating involvement of adaptive immunity in experimental models of hypertension include evidence that isoketal–modified proteins (135) and overexpression of HSP70 (25, 111, 211) are potential antigens in hypertension-associated immune reactivity (1). Dendritic cells process the antigen (135) (2), travel to lymphoid organs (135) (3), and present it to the TCR in T cells (90, 175) in the context of the MHC (225) in association with costimulatory signals (294) (4). Memory T cells (5) are developed and stored for inducing accelerated responses to subsequent antigenic challenge (114, 205) and activation and expansion of effector T cells (6) that result in proinflammatory cytokine responses (see TABLE 2) and regulatory T cells (7, 126, 146, 161, 182, 241) (7). B-cell activation (8) is necessary for the development of hypertension when the immune system is intact (32). The CNS-SNS axis is recruited by oxidative stress (154, 155, 251, 333) involving angiotensin II receptors (100, 244, 251) and results in SNS-induced stimulation of the release of activated T cells from the spleen (9) (27, 28) and stimulation of target organ immune infiltration and reactivity (314) (10). APC, antigen presenting cells; MHC, major histocompatibility complex; TCR, T cell receptor; CNS, central nervous system; SNS, sympathetic nervous system.
Vinh et al. (294) focused on costimulatory mechanisms of antigen presentation. They demonstrated that angiotensin II infusion induces overexpression of CD86 in DCs. Blockade of the B7 costimulatory pathway with CTLA4-IgG or by genetic deletion of B7 ligands (CD80 and CD86) ameliorates angiotensin-induced and DOCA-salt hypertension (294). Furthermore, the hypertensive response could be restored in the B7−/− mice by adoptive transfer of bone marrow from the wild-type mice.
The critical role of antigen presentation was also demonstrated in the elegant experiments of Rudemiller et al. (225) who focused on CD247 gene that encodes the CD3 ζ chain, involved in the assembly of the T-cell receptor of antigen recognition. Population studies in hypertensive patients had revealed an association between high blood pressure and a single polymorphism variant in intron 1 of CD247 (64). This finding led Rudemiller et al. (225) to delete CD247 in the genetic background of the Dahl SS rat to examine the importance of T-cell activation in salt-induced hypertension. The CD247−/− Dahl SS rat had almost complete disappearance of circulating CD3+T cells, a drastic reduction of the infiltration of T cells in the kidney and amelioration in the SS hypertension and albuminuria.
Itani et al. (114) explored the generation of memory T cells in angiotensin II and l-NAME models of hypertension to demonstrate the participation of adaptive immunity. Mice given a low dose of angiotensin II (140 ng·kg−1·min−1) or a high-salt diet do not develop hypertension. However, hypertension developed if mice had previously received a high dose of angiotensin II (490 ng·kg−1·min−1) or 2 wk of l-NAME treatment before the administration of the high-salt diet. The development of hypertension in response to secondary subthreshold stimulation in these mice was the result of activation of memory T cells demonstrated in the kidney and in the bone marrow. Mice lacking CD70, thereby incapable of generating memory T cells, did not develop hypertension on secondary stimulation. Adoptively transferred memory T cells nested in the bone marrow and the spleen of the recipient and expanded in response to antigen exposure.
VIII. AUTOIMMUNITY IN EXPERIMENTAL HYPERTENSION
Recent studies have focused on autoantigens of potential relevance in activating the adaptive immune system in hypertension. Pons et al. (211) studied the role of HSP70 in autoimmune reactivity in SS hypertension. HSPs, and particularly HSP70, are immunodominant molecules that, in addition to functioning as chaperones of nascent proteins and driving the immune response to microorganisms, play a well-recognized role in autoimmunity either by themselves or binding to misfolded proteins (276). HSP70 is overexpressed in the kidney of in l-NAME-induced and angiotensin-induced hypertension (25, 111), and T cells obtained in experimental models of SS hypertension develop a proliferative reaction when challenged with HSP70 (205). In the adult SHR, HSP70 and HSP72 are overexpressed in the kidney. Furthermore, overexpression of HSP72 is already present in the SHR at 4 wk of age, before hypertension develops (FIGURE 4).
FIGURE 4.
Relative content of HSP70 and HSP72 in SHR and WKY at 4 and 40 wk of age. SHR are normotensive at 4 wk of age and have overexpression of HSP72. At 40 wk of age, HSP70 and HSP72 are overexpressed in the SHR. Histology corresponds to immunoperoxidase staining for HSP70 in WKY and SHR 40 wks of age.
Since a specific amino acid sequence in mycobacterial HSP70 induces an IL-10 response capable of preventing autoimmune arthritis in rats (212, 303), we used the same peptide to induce immune tolerance to HSP70 in rats given high-salt diet after transient exposure to l-NAME (213). In this model, HSP70 is overexpressed in the kidney, and T cells present a clonal CD4 response when exposed to HSP70. Immune tolerance resulted from the generation of IL-10 producing Tregs and was associated with reduced renal inflammation and prevention of salt-driven hypertension. Adoptive transfer of T cells from tolerized rats into rats with SS hypertension corrected hypertension. In additional experiments, we transfected the kidneys with a plasmid-HSP70 construct (pCMVSPORT6-human HSP70) injected in both renal veins that were occluded during the procedure. This procedure led to renal overexpression of HSP70 and in rats previously sensitized to HSP70 resulted in renal inflammation and salt-induced increase in blood pressure (211).
In 2014, Kirabo et al. (135) examined autoimmune phenomena resulting from oxidative stress in hypertension. In their comprehensive studies, they showed that angiotensin II infusion and DOCA-salt administration induce hypertension in association with generation of ROS in dentritic cells. Since reduction of the blood pressure with hydralazine did not prevent the generation of ROS, the oxidative stress is not caused by the increase in blood pressure. Oxidative stress resulted in the formation of γ-ketoaldehydes (isoketals) that bind to lysine residues and crosslink proteins that accumulate in DCs resulting in neoantigens. The formation of isoketal-protein adducts drives DCs to produce IL-6, IL-1β, and IL-23 and to increase costimulatory proteins CD80 and CD86. Activated DCs induced proliferation of T cells, especially CD8+T cells with the production of IFN-γ and IL-17A and hypertension. All these events were suppressed with the administration of the isoketal scavenger 2-HOBA. Adoptive transfer of DCs activated with isoketals induced hypertension in wild-type mice but not in rag 1 −/− mice that lack T cells.
Subsequent studies done in mice with excessive vascular production of ROS by overexpression of NADPH oxidase subunit p22(phox) or deletion of SOD have demonstrated isoketal-protein adducts in aortas, DCs, and macrophages that are capable of activating T cells. Treatment with tempol (SOD mimetic) or 2-HOBA prevented T-cell and dendritic cell activation, vascular inflammation, and hypertension (311). These important studies (135, 311) established a missing and important link between oxidative stress, adaptive immunity, and hypertension.
IX. PATHOPHYSIOLOGY OF HYPERTENSION INDUCED BY IMMUNITY
Elevation of the blood pressure is the result of many factors. The participation of immunity is a consequence of a complex interaction between activated immune cells, oxidative stress, and angiotensin II activity that drives a low-grade inflammation in the kidney, arteries, and central nervous system.
A. Interactions Between Oxidative Stress, Inflammation, and Angiotensin II
Oxidative stress, inflammation, and angiotensin II activity are inextricably linked in the pathogenesis of hypertension (272, 289, 307). Recent studies have added insight on the role of mitochondrial oxidative stress in hypertension. The redox activation of CypD, a regulatory subunit of the mitochondrial permeability transition pore implicated in cell death, drives the generation of ROS in angiotensin II-induced hypertension in cooperation with IL-17.
CypD deficiency and inhibition of CypD, overexpression of mitochondrial SOD2, or scavenging of mitochondrial ROS with mitochondria-targeted antioxidants are all capable of attenuating angiotensin-induced hypertension (57, 59, 112).
Oxidative stress and inflammation support one another. Oxidative stress activates proinflammatory transduction pathways (JNK, p38 MAPK) and transcription factors (AP-1 and NFκB) and is a potent stimulus for lymphocyte function (157). Conversely, inflammation induces and amplifies oxidative stress by the generation of increased amounts of ROS that represent a necessary component of innate immunity.
Angiotensin II is a third element that interacts with oxidative stress and inflammation. All components of the renin-angiotensin system (RAS) are present in the kidney. Navar’s group has shown that the intrarenal RAS system is regulated differently from the circulating RAS and that intrarenal RAS activation has a key role in the pathogenesis of hypertension (190, 191). An example of the independence of the renal and systemic RAS is the Page (cellophane wrap) kidney model of hypertension in which the plasma angiotensin II is unaltered but the renal angiotensin II is increased in association with interstitial renal inflammation (286). In SS hypertension, the severity of hypertension is directly correlated with renal concentration of angiotensin II and inversely correlated with plasma concentration of angiotensin II, which, as expected, is suppressed by a high-salt diet (71). While most intrarenal RAS is generated in intrinsic cells in the kidney, 20–40% of lymphocytes and macrophages infiltrating the kidney stain positive for angiotensin II (FIGURE 5). These findings are consistent in several studies (213, 220, 222) and were unexplained until investigations by Hoch et al. (101) elegantly showed lymphocytes produced angiotensinogen, angiotensin converting enzyme (ACE), and renin and produced angiotensin II that contributed to T-cell activation.
FIGURE 5.
Lymphocytes staining positive for angiotensin II in tubulointerstitial areas of the SHR. Double staining methodology used to demonstrate by indirect immunofluorescence (A) lymphocytes (fluorescein-labeled CD5 positive cells) expressing angiotensin II (rhodamine-labeled angiotensin II positive cells) (B). [From Rodriguez-Iturbe et al. (222).]
The complex interplay between the immune cells and the RAS in hypertension has been highlighted in the studies of Crowley’s group (41, 325, 327) who demonstrated that the type of cell expressing AT1r is critically important in hypertension induced by angiotensin II. Specifically, bone marrow chimeras lacking AT1r had normal baseline blood pressure and, surprisingly, presented an augmented blood pressure response to angiotensin II, thereby documenting a protective role of AT1r in bone marrow-derived cells against the hypertensive actions of angiotensin II. To confirm these results, Zhang et al. (327) removed the AT1r from CD4+T lymphocytes using the Cre-lox gene targeting technology. They showed that the CD4+ T cells lacking AT1r have increased expression of T-bet transcription factor which drives a Th1 commitment (264), whereas T cells expressing AT1r have a suppressed Th1 response and protect the kidney from angiotensin-induced injury. Suppressing AT1r from LysM expressing macrophages did not modify the chronic hypertensive response to angiotensin II but increased tubulointerstitial fibrosis, indicating that the AT1r attenuated the expression of M1-type proinflammatory cytokines (325).
These important studies highlight the independence and complex interrelation of proinflammatory and hemodynamic responses to angiotensin II mediated by AT1r. A large body of experimental and clinical evidence has shown that angiotensin II receptor blockers ameliorate hypertensive renal damage and kidney disease progression. Therefore, the results of global AT1r blockade are anti-inflammatory. Rudemiller and Crowley (224) have suggested that AT1r in nonimmune cells may modify the function of AT1r in immune cells or, alternatively, that AT1r activation in immune cells would improve renal injury by driving overactivated leukocytes to apoptosis. At any rate, the findings of Crowley’s group suggest the possibility that selective suppression of the RAS in the kidney and the cardiovascular system, in association with preservation or enhancement of AT1r expression in the immune system, may result in greater benefits than global AT1r blockade in the treatment of progressive renal damage.
B. Renal Inflammation in Hypertension
The pressure-natriuresis relationship defines a renal adaptive response to maintain sodium balance. Impairment in the pressure-natriuresis response implies that elevation of the blood pressure is necessary to induce the natriuresis required to maintain water and sodium homeostasis (89).
In the kidney, the renal infiltration of immune cells in the SHR precedes the development of hypertension (221). Renal inflammation is associated with impairment in the pressure-natriuresis response (219), and the intensity of immune cell infiltration in the kidneys has been found to be correlated with the severity of hypertension in the SHR (222) (FIGURE 6), in SS hypertension (71), and in autopsies of patients with hypertension (105). In SS hypertension, determinations made at similar (controlled) renal perfusion pressure show that accumulation of immune cells is inversely correlated with fractional sodium excretion (72) (FIGURE 6).
FIGURE 6.
Immune cell infiltration in tubulointerstitial areas impairs pressure natriuresis. A: salt-sensitive hypertension following l-NAME administration (SSHBP) is associated with immune cell infiltration and angiotensin positive cells in tubulointerstitial areas that are suppressed with mofetil mycophenolate (MMF) treatment. B: the severity of salt-induced hypertension is directly related to the intensity of immune cells (CD68+ and CD3+ cells) infiltration. C: pressure natriuresis is impaired in SSHBP. MMF treatment increases pressure natriuresis in l-NAME-treated rats to the values found in control rats on a high (C-HSD) and normal (C-NSD) sodium diet. SBP, systolic blood pressure; FNaE, fractional sodium excretion. [Data from Franco et al. (72).]
Renal inflammation is associated with increased intrarenal angiotensin II activity that plays a major role in the inflammation-induced impairment in the pressure-natriuresis (71, 72). Vascular AT1r are critical for the renal actions of angiotensin II (249) that include glomerular vasoconstriction, upregulation of tubuloglomerular feedback, and stimulation of tubular sodium reabsorption (190).
Other elements of the renal RAS system are necessary for the chronic blood pressure responses to angiotensin II. Recent studies in inbred mice deficient in renal ACE have demonstrated that renal ACE is indispensable for the development of sustained hypertension induced by angiotensin infusions and for the SS hypertension resulting from inflammatory injury. Modulation of glomerular filtration rate and activation of proximal and distal tubular sodium transport require a critical level of local angiotensin II that is not obtained in the absence of renal ACE (81, 83).
Renal inflammation has additional characteristics that have a negative impact on pressure natriuresis. Asghar et al. (5) have shown that the function of dopamine D1 receptors that are involved in urinary sodium excretion is compromised by inflammation, and Johnson and Schreiner (122) proposed that loss of peritubular capillaries critically impairs pressure natriuresis.
C. Vascular Inflammation in Hypertension
A number of studies have demonstrated in experimental models of hypertension the infiltration of T cells, monocytes, and DCs in perivascular and adventitia of large and medium-sized vessels (7, 90, 126). IL-6 plays a role in perivascular immune cell infiltration since RNA interference knockdown of IL-6 ameliorates the lymphocyte and macrophage infiltration in the aorta (38). Similar reduction in angiotensin II-induced perivascular inflammation was demonstrated in MAPK2-deficient mice (61).
Perivascular inflammation is associated with increased vasoconstriction in response to norepinephrine and impairment of endothelial-dependent (ACh-induced) vasorelaxation (7, 90, 126, 293). The mechanism of periarterial accumulation of immune cells is not completely defined, but sympathetic nerve endings are present in these areas and the central nervous system plays a major role in the activation, homing, and infiltration of immune cells in vascular areas (167).
Gratze et al. (85) showed that a functional transcription factor Id2 is necessary for angiotensin II-induced hypertension and organ damage. The Id−/− mouse is deficient in DCs, NK cells, and memory CD8+ cells, but extensive bone marrow and kidney transplant experiments suggested that alterations in immune cells, by themselves, were not responsible for angiotensin resistance. Rather, the authors posited that alterations in the vessel wall were responsible for the findings in the Id2−/− mice (85). The immune cell infiltration may trigger the remodeling of the vessel walls and thereby contribute to the increased vascular resistance and hypertension (233).
D. Central and Autonomic Nervous System, Immunity, and Hypertension
The interrelation between the central nervous system (CNS) and the immune system depends on the extensive sympathetic innervations existing in lymphoid organs (65). Several investigations have examined the participation of the CNS in angiotensin II-induced hypertension, and research has centered on the anteroventral third ventricle (AV3V) region because this area has a poorly developed blood-brain barrier and is capable of responding to angiotensin II (96). Angiotensin II increases superoxide production in subfornical organs (332). Deletion of SOD in circumventricular organs augments the perivascular infiltration of activated T cells, the sympathetic outflow, and the hypertensive response (154). Conversely, reducing oxidative stress by intracerebral administration of adenovirus encoding for SOD (333) or by suppression of p22 (phox) subunit of NADPH oxidase in the subfornical organ inhibits accumulation of leukocytes in vascular walls and ameliorates hypertension (155). These data give strong support for the role of oxidative stress in the CNS in the pathogenesis of angiotensin II-induced hypertension.
Angiotensin II directly or by pathways driven by generation of ROS increases proinflammatory cytokines in the brain (244), which led Sriramula et al. (251) to examine if the central actions of angiotensin II were mediated by TNF-α. Intracerebroventricular injections of etanercept reversed angotensin-induced increments in TNF-α, IL-6, and IL-1β; suppressed the expression of AT1r; and attenuated hypertension (252).
AT1r in the CNS are important in other models of hypertension. Targeted deletion of AT1r in the subfornical organ results in a blunted hypertensive response to DOCA-salt in association with reduction in polydipsia, polyuria, and sodium intake (100).
Since hypertension is associated with systemic markers of inflammation, it is important to define if systemic inflammation may drive pro-hypertensive responses in the CNS. Wu et al. (309) used intraperitoneal minipumps to infuse low dose (1.2 mg·kg−1·day−1) of lipopolysaccharide for 2 wk. As a result, blood levels of CRP, TNF-α, and IL-1β increased and hypertension developed. The participation of the CNS was demonstrated because these effects were suppressed by intracisternal administration of minocycline or by the inhibitor of cytokine synthesis pentoxifylline.
The SNS is the major interconnecting pathway linking the CNS and the immune system (FIGURE 7). Stimulation of the SNS results in an increase in blood pressure due to an increase in peripheral vascular resistance, cardiac output, and renal sodium reabsorption. In addition, immune cells express adrenoreceptors and may release norepinephrine that influences the traffic of lymphocytes and, depending on the preexisting state of the stimulated lymphoid cells, induce production of Th1 or Th2 cytokines (65). While adrenergic stimulation increases the TLR-mediated production of proinflammatory cytokines by macrophages (86), the sympathetic stimulation of immune cells may induce both anti-inflammatory or a proinflammatory responses depending on the perceived requirement of the occasion (65).
FIGURE 7.
Participation of the sympathetic nervous system in the immune responses (key references in parentheses). Most of the immune-related effects of the sympathetic nervous system have been identified using angiotensin infusions. Hemodynamic actions of the sympathetic nervous system favoring hypertension are included in a box. PVP, peripheral vascular resistance; PIGF, placental growth factor.
Changes in immune reactivity resulting from sympathetic nerve discharge have been investigated in the bone marrow and in the spleen because these organs have dense sympathetic innervations. Angiotensin II infusion induces a 46% decrease in bone marrow-derived endothelial progenitor cells (EPC) and a 250% increase in bone marrow-derived inflammatory cells. This proinflammatory modification was corrected by intracerebroventricular administration of mitochondria-targeted antioxidant that also ameliorated hypertension. Retrograde labeling of the paraventricular nucleus neurons of the brain after injecting the bone marrow with green fluorescent protein-tagged pseudorabies virus confirmed the brain-bone marrow interaction (124). This brain-immune system interaction is also evident by the increase in the mRNA expression of IL-1β, IL-2, and IL-6 in the spleen resulting from intracerebroventricular administration of angiotensin II (78).
More recent studies have added insight to the mechanisms driven by the SNS stimulation that result in activation and release of T cells from the spleen. Extensive investigations by Carnevale et al. (27) reported that sympathetic signals stimulate norepinephrine release and placental growth factor (PIGF, a member of the angiogenesis family related to the VEGF) from the spleen. Genetic deletion of PIGF completely prevents angiotensin II-induced hypertension, vascular and renal immune cell infiltration, and organ damage. Increased PIGF suppresses the expression of tissue inhibitor of metalloproteinases 3 (Timp3). The suppression of Timp3 is mediated through the transcriptional Sirt1-p53 axis and allows costimulation via CD68 of T cells and their migration from the spleen to the vascular walls and the kidney. These comprehensive studies (27) further demonstrated that ablation of the splenic nerve, or the celiac ganglion, or splenectomy prevented the influx of pathogenic T cells to target organs, thus showing, first, that sympathetic innervation is a requisite for target organ inflammation and, second, that immune cells infiltrating target organs are of splenic origin. Reimplantation of the spleen from wild-type donors reestablished angiotensin II-induced hypertension while reimplantation of spleens from PIGF-deficient donors did not change the resistance to angiotensin II resulting from splenectomy. The role of the Sirt1 was demonstrated because its selective inhibitor Ex-527 and genetic silencing of Sirt1 in PIGF-deficient splenocytes restored the hypertensive response to angiotensin II. These elegant studies uncover previously unidentified roles for the SNS in the immune pathogenesis of hypertension.
The role of the SNS as a mediator of the renal inflammation induced by angiotensin II was also studied by Xiao et al. (314) who showed that denervation of renal arteries blunts the hypertension induced by angiotensin II and suppresses the immune cell infiltration in the kidneys. Other effects of angiotensin, such as the formation of isoketal-protein adducts in DCs and the expression of costimulation markers and production of cytokines are also prevented by renal denervation. DCs have RNA for α1D, α2A, α2B, α2C, β1, and β2 adrenergic receptors. These receptors are downregulated by angiotensin II, with the exception of β2 adrenoreceptors that are increased by angiotensin II infusion. However, angiotensin-induced effects are not mediated by adrenergic β2 receptors because they are not suppressed in mice lacking these receptors (314).
The parasympathetic nervous system also participates in the regulation of the immune reactivity driving anti-inflammatory responses. T cells express ACh receptors (AChR), and stimulation of the vagus nerve inhibits the secretion of TNF-α from the macrophages and reduces systemic inflammation. The receptor of this ACh-mediated response to vagal stimulation is the nicotinic AChR alpha7 subunit (299), and the AChR−/− mice have increased severity in angiotensin II-induced inflammation (148). It is interesting that dysfunctional cholinergic stimulation of the innate immunity has been demonstrated in the SHR. Harwani et al. (93) have shown that SHR has an inflammatory innate immune response to nicotine/cholinergic stimulation. TLR-mediated response to nicotine, both in vivo and in splenocytes, resulted in overproduction of IL-6 and IL-1β and increase in CD161+ activated macrophages in the prehypertensive SHR. This contrasts with the pronounced anti-inflammatory nicotininc/cholinergic modulation of the innate immune response in the WKY rat. These investigations raise the intriguing possibility that reverse modulation of the immune system by the autonomic nervous system could play a role in the development of genetic hypertension.
In a recent paper, Carnevale et al. (28) reported a vagus-splenic nerve pathway, mediated by nicotinic cholinergic receptors, that links the brain and spleen. Interestingly, thermoablation of the splenic nerve prevents T-cell egression and protects against hypertension.
The clinical relevance of the wealth of experimental data associating hypertension and immunity is supported by data obtained in patients with essential hypertension that will be discussed in the following sections.
X. IMMUNE REACTIVITY IN PATIENTS WITH HYPERTENSION
A. Hypertension in Autoimmune Diseases
The increased incidence of hypertension in autoimmune diseases is well recognized. In SLE the median age of development of hypertension is 24 yr (229a). The prevalence of hypertension in rheumatoid arthritis is 52–73% (203), in dermatomyositis/polymyositis is 70% (56), and in systemic scleroderma with renal involvement is 90% (143). Despite the accumulated evidence that associates autoimmune disease and hypertension, the question of a causal relationship remains unanswered. In NZBW mice that have a disease resembling SLE in humans (102, 179), Ryan and McLemore (229) found that increase in anti-DNA titers and mean arterial pressure both occurred at 36 wk of age. Hypertension was associated with a shift to the right of the pressure natriuresis relationship (169), and administration of rosiglitazone (an insulin sensitizing agent that stimulates peroxisome proliferator-activated receptors) ameliorated hypertension in association with a reduction in renal immune cell infiltration (291). A reduction of blood pressure was obtained by TNF-α blockade with etanercept (290), and administration of anti-CD20 antibody reduced the formation of autoantibodies, decreased TNF-α expression, and ameliorated hypertension (170). While a causal relationship cannot be definitely established, these investigations strengthen the association between autoimmunity and hypertension and strongly suggest that in autoimmune diseases the immune reactivity plays a key role in the development of hypertension.
B. Immunoglobulins and Inflammatory Markers in Essential Hypertension
In 1970, Ebringer and Doyle (62) reported that 118 severely hypertensive patients (standing mean blood pressure ≥130 mmHg) had higher levels of serum IgG than 163 age-and sex-matched normotensive blood donors. The authors suggested that autoimmune reactivity was driven by vascular damage induced by hypertension. Several subsequent studies documented increased IgG levels in patients with essential hypertension (88, 142, 198). More recent studies have analyzed the relationship between markers of inflammation and blood pressure in patients with essential hypertension. IL-6 levels were found to be significantly associated with blood pressure in a study of healthy men (29). Increasing quartiles of CRP have been reported to be associated with increasing prevalence of hypertension in a population survey (13) and with blood pressure levels in a case-control study (12). A larger cross-sectional population study that included 4,813 men and 3,534 women ≥20 yr of age arrived to similar results: the prevalence of hypertension increased in each one of the increasing quartiles of CRP (258). Prospective studies in 20,525 normotensive female health professionals aged 40 yr or older, followed for a median of 7.8 yr, demonstrated a linear trend in the association between the levels of CRP and the development of hypertension in all prespecified subgroups (238). Since increased levels of inflammatory markers precede hypertension, inflammation was not the result of hypertension. The same authors (239) subsequently did a nested case-control study of 400 women developing hypertension during 10 yr of follow-up in the Women's Health Study and an equal number of age-matched normotensive controls. All the participants initially had a normal blood pressure. After multivariate adjustment, the increased risk of hypertension was strongly associated with CRP levels and weakly, if at all, with IL-6. Higher levels of CRP have also been found in prehypertensive individuals (BP = 120–139/80–89 mmHg) (134) and in patients with isolated systolic hypertension (172). Nevertheless, despite an association between high CRP levels and hypertension, a causal relationship has not been demonstrated. In fact, Smith et al. (246) used a Mendelian randomization approach to examine a possible causal relationship analyzing the association of the 1059G/C polymorphism in the human CRP gene with pulse pressure and hypertension. The expectation was that a higher incidence and prevalence of hypertension would be found in subjects carrying the polymorphism that results in higher CRP levels. This was not the case, and the work failed to confirm a causal relationship between CRP and blood pressure.
Mirhafez et al. (184) examined the blood levels of 12 cytokines and growth factors in 155 hypertensive and 148 normotensive individuals and found that hypertensive patients had higher concentrations of IL-1α, IL-2, IL-8, VEGF, IFN-γ, TNF-α, and MCP-1 and lower levels of IL-10. No differences were found in IL-4, IL-6, and IL-1β levels. Other studies have found that IL-6 and TNF-α blood concentrations were high in hypertensive patients (14, 76, 115, 118) or similar in hypertensive and normotensive subjects (207, 243). Prospective studies fail to find a significant association between IL-6 levels and the risk of hypertension (239). Taken together, these studies have a reasonable agreement in the association between high CRP values and prevalence and incidence of hypertension. Other inflammatory markers have given less consistent results.
C. Renal Infiltration of Immune Cells in Human Hypertension
The renal infiltration of immune cells in patients with essential hypertension has been known for a long time. In 1958, Sommers et al. (248) published a seminal paper detailing the renal biopsy findings of 1,346 subjects with essential hypertension in whom tissues were obtained during sympathectomies that were, at the time, an accepted treatment of essential hypertension. Collections of lymphocytes were found infiltrating interstitial areas in the vast majority of biopsies, including in 20% of specimens graded normal or with only minor vascular and glomerular changes (grade 0–1). Heptinstall (97) also reported lymphocyte infiltration in 16 of 37 renal biopsies obtained during sympathectomies, and Gareau et al. (80) described interstitial nephritis in 7 of 55 patients and noted that it was impossible by the histological examination of the biopsies to define if the “interstitial nephritis influence the progression to nephrosclerosis or if it is the cause of hypertension.” Similar interstitial lymphocyte infiltrations are observed in protocol biopsies taken from hypertensive donors in renal transplantation (217). Hughson et al. (105) examined autopsied kidneys of 107 African Americans and 87 whites aged 18 to 65 yr. Of them, 59 African Americans and 39 white patients were classified as hypertensive from the chart review. There were no associations between hypertension and glomerular number or birth weight and, therefore, the data did not support the notion that a congenital under endowment of nephrons was a common factor in the pathogenesis of essential hypertension (26, 130). Instead, the data showed a significant correlation (P < 0.001) between immune cell infiltration (CD68+ cells) and mean arterial pressure. Additional data were obtained in seven hypertensive patients by Youn et al. (320) who found significant interstitial infiltration of CD4+ and CD8+ T cells in association with increased expression in the proximal and distal tubules of I-TAC, one of the CXCR3 chemokines that initiates T cell-driven inflammation.
D. Evidence for Innate Immunity in Human Hypertension
Marketou et al. (163) investigated gene expression of TLR4 and TLR2 in the peripheral blood monocytes of 43 nondiabetic patients with grade I or II hypertension and in 16 normotensive controls. Despite the small sample number, they found increased mRNA levels of TLR4 in hypertensive patients. They also found that intense (but not standard) blood pressure treatment reduced TLR4 and TLR2 mRNA levels.
Dalekos et al. (45) compared the serum levels of IL-1β of 28 untreated patients with essential hypertension, 31 normotensive patients with familial hypercholesterolemia, and 35 healthy controls. Hypertensive patients had serum levels of IL-1β that were three to five times higher than the other two groups. Circulating monocytes isolated from hypertensive patients have exaggerated IL-β production in response to angiotensin II and lipopolysaccharide stimulation, both at the protein and at the mRNA level, suggesting a state of inflammasome preactivation (58).
IL-18 has also been studied in essential hypertension. While the PRIME European study found no difference in circulating IL-18 levels in patients with and without hypertension (18), the MONICA/KORA in Germany (270), the CUDAS study in Perth (106), and the Dallas Heart study (334) all found that hypertensive patients had higher levels of IL-18.
Finally, studies have examined genes that participate in the activation of the inflammasome. The association between susceptibility to essential hypertension and the cold-induced autoinflammatory syndrome 1 (CIAS1) gene was studied by Omi et al. (200) in 987 patients and 924 controls. C1AS1 is a member of the NLRP3/ PYPAF subfamily of the CATERPILLER protein family that regulates inflammasome-induced activation of NFκB and caspase. Subjects with 12–12 genotype of the C1AS1 42 bp-VNTR were more frequently hypertensive than controls (P = 0.006). This study suggests that genetic influences play a role in the activation of innate immune responses in patients with essential hypertension.
Other elements of the innate immune response may play a role in hypertension. A relative increase in neutrophil count, evaluated as the neutrophil-to-lymphocyte ratio, was recently found to be associated with the risk of hypertension (152).
E. Agonistic Autoantibodies in Hypertension
Autoantibodies directed against G protein-coupled receptors (GPCR) have a functional response (stimulatory, inhibitory, or synergistic) when they bind to their targets. Stimulatory (agonistic) autoantibodies recognize epitopes in the first or second extracellular loops of the receptor, and their effects are associated with stabilization of the receptor-antibody conformation after binding. As a result, they promote sustained receptor activation resistant to the downregulation and to protective mechanisms associated with the binding with the natural ligands. Agonistic antibodies have been authoritatively reviewed (298, 313) and will not be discussed here. In essential hypertension, anti-AT1r antibodies are present in nearly 60% of the patients with refractory hypertension (331), but their clinical relevance remains unproven. Wei et al. (301) compared the effectiveness of treatment with angiotensin receptor blockers (candesartan) and angiotensin converting enzyme inhibitors (imidapril) in patients with moderate to severe essential hypertension. The assumption was that the treatment with angiotensin receptor blockers would be more effective if anti-AT1r antibodies had a relevant agonistic activity. In a study of 512 subjects, half of which were given candesartan and half imidapril, those patients who had anti-AR1r antibodies had a more pronounced reduction in blood pressure with candesartan than with imidapril. However, the study did not find correlation between the titer of anti-AT1r antibodies and the efficacy of candesartan-based therapy.
F. Autoantigens in Essential Hypertension
The generation of autoantigens in arteries of hypertensive patients (75, 199) and their association with vascular complications (141) were reported several decades ago. Subsequently, investigation of autoantigens in hypertension was largely suspended. More recent studies have found preliminary evidence of autoantigenic reactivity to HSP70 and to isoketal-modified proteins in subjects with essential hypertension and will be discussed in the following sections.
1. HSP 70
Plasma anti-HSP70 antibodies have been reported in patients with borderline hypertension (74) and hypertensive individuals working under stressful conditions (310). Pockely et al. (210) directly addressed the issue of antibodies against HSPs and blood pressure in a study of 111 patients with hypertension from the European Lacidipine Study on Atherosclerosis (ELSA) and 75 normotensive controls from a population screening program. They found that anti-HSP65 and anti-HSP70 antibody titers were higher in hypertensive patients independently of age, smoking habits, or blood lipids. Our group studied the proliferative response to HSP70 in lymphocytes from 10 patients with essential hypertension grade I or II and in 12 normotensive age- and sex-matched controls. Peripheral blood lymphocytes were cultured for 7 days with the HSP70 peptide used in tolerization and sensitization experiments described previously (211). The lymphocytes of patients with essential hypertension responded with a high proliferation index, while the lymphocytes from controls were unresponsive (211). These studies, in a limited number of patients, raise the possibility that a cellular immune response to HSP70 is activated in essential hypertension. HSP70 mRNA was found increased in lymphocytes of hypertensive patients submitted to heat stress (145). In very recent studies, Srivastava et al. (253) studied the blood of 132 patients with essential hypertension and 132 control individuals and found that hypertensive patients had a 6.4-fold higher HSP70 gene expression (P < 0.0001) as well as increased HSP70 protein abundance (P < 0.0001). HSP70 mRNA correlated significantly with circulating levels of TNFα, IL-6, and CRP.
Finally, some studies have shown that polymorphisms in the HSP70 gene family are associated with hypertension. Li et al. (149) studied 415 subjects (211 hypertensives) of the Uygur ethnic minority in China. This population is highly homogeneous and has unique diet and life styles. They evaluated the association between five polymorphisms in three genes (HSPA1A, HSPA1B, and HSPA1L) of the HSP 70 family and essential hypertension. In haplotype analyses using the haplotype H1 as a reference, haplotype H4 had a 40% reduced risk, while haplotypes H5 and H8 had a 5.00- and 3.75-fold, respectively, increased risk for essential hypertension. Nevertheless, as acknowledged by the authors (149), the case-control characteristic of the study renders it unsuitable for demonstrating a causality relationship.
2. Isoketal-modified proteins
As discussed earlier, Harrison’s group (135) has presented compelling evidence on the role of γ-ketoaldehydes (isoketal)-modified proteins in activating DCs for antigen presentation in experimental models of hypertension. In addition to the experimental data, the authors studied 16 normotensive subjects, 44 patients with well-controlled hypertension, and 86 patients with resistant hypertension to examine the participation of isoketals in the pathogenesis of essential hypertension. They found that plasma F2-isoprostanes that are formed in concert with isoketals are increased in patients with refractory hypertension, compared with controlled hypertension and normotensive subjects (P < 0.05). In a second group of 12 patients with essential hypertension and 8 normotensive controls, the isoketal-protein adduct content in circulating mononuclear cells was analyzed by flow cytometry. Their data demonstrated that isoketal adducts in CD14+ cells and in CD83+ DCs from hypertensive patients were severalfold higher than in normotensive controls. Highly significant correlations were found between the systolic blood pressure and the percentage of isoketal-positive CD14+ and CD83+ cells (135). More recently, it has been shown that the aortic content of isoketal-protein adducts is correlated with fibrosis and inflammation severity (311).
Investigations in humans supporting engagement of the innate and adaptive immunity in the pathogenesis of essential hypertension are shown in FIGURE 8.
FIGURE 8.
Innate and adaptive immune system in clinical hypertension. The innate and adaptive immunity play a role in establishing inflammation in the kidney, arteries (perivascular), and central nervous system (CNS)/sympathetic nervous system (SNS). Danger-associated molecular patterns (DAMPs) recognized by pattern recognition receptors (PRR), specifically, Toll-like receptors (TLR), activate the inflammasome and drive an innate immune response with the participation of monocytes (Mo), macrophages (M¢), dendritic cells (DC), neutrophils (N), and natural killer cells (NK). Neoantigens generated (isoketal-modified proteins) and overexpressed immunodominant molecules (HSP70) are taken up by antigen presenting cells (APCs), presented for recognition to the naive T lymphocytes in the context of the MHC to the specific T cell receptor. Stimulation of antibody producing B cells and of helper (CD4+) and cytotoxic (CD8+) T cells takes place. CD4+ T cells generate a proinflammatory Th1, Th2, and IL-17 cytokine responses and T regulatory cells (Tregs), required for limiting inflammation. Numbers in parentheses indicate the references that support the finding in human hypertension (*data for isoketal-modified proteins, **data for HSP70).
G. Clinical Hypertension and Immune Suppression
Studies performed three decades ago reported that the absolute number of CD4+ T cells was reduced during treatment with ACE inhibitors and suggested that suppression of the RAS had an effect on cellular immunity (245). These findings have not been investigated subsequently. More recently, the association of immune suppression with amelioration of hypertension has been reported. Data from the Multicenter AIDS Cohort study indicate that untreated HIV-positive patients with chronically low numbers of CD4+ T cells have a lower prevalence of systolic hypertension than treated HIV patients and uninfected control subjects (236). Grome et al. (87) reported reduced brachial artery flow-mediated vasodilatation associated with a higher percentage of activated (CD38+) CD8+T cells in 70 HIV-infected adults. Higher ICAM-1 and VCAM-1 levels were associated with activated macrophages. These findings suggested a relationship between CD8+T cell activation and impairment in arterial relaxation and between monocyte activation and immune cell vascular infiltration. The role of immune system in human hypertension is supported in a study by Herrera et al. (99) in which eight patients with essential hypertension received MMF for coexisting rheumatoid arthritis or psoriasis without modification of antihypertensive treatment or diet. Blood pressure fell during MMF treatment in association with reduction in urinary RANTES and TNF-α. When MMF was discontinued, blood pressure returned to previously high levels, suggesting that amelioration of hypertension was the result of immune suppression.
XI. GUT MICROBIOTA AND HYPERTENSION
The interaction between the gut microbiota and the immune system in hypertension has been recently reviewed (123). Reduction in gut microbiota has been associated with systemic inflammation and hypertension, and the administration of probiotics improves hypertension in eclampsia (23). However, the mechanisms involved are largely unknown. Specific immunomodulatory effects have been demonstrated in the Bacteroides fragilis where the production of a polysaccharide is able to restore the Th1/Th2 balance in the germ-free mice and affect natural killer cells. Lactobacilli produce peptides capable of inhibiting ACE1 (188), and Clostridium-related microorganisms can induce Treg production in the colonic lamina propria and IL-17 production in the small intestinal lamina propria (259). Olfactory receptors are expressed in the juxtaglomerular apparatus in the kidney and respond to short-chain fatty acids produced by gut microbiota that enter the circulation by colonic absorption. The modulation of blood pressure by propionate generated by gut microorganisms likely depends on the balance of two olfactory receptors: OLfr 48 and Gpr41 (209).
In the SHR there is a reduction in the richness of the gut microbiota and a high Firmicutes-to-Bacteroidetes ratio. Restoring this ratio with the administration of minocycline improved hypertension. Differences in the constitution of the gut microbiota have also been shown to exist in the Dahl SS and SR rats. Surprisingly, a single bolus of fecal content of SS rats administered to SR rats increased the blood pressure of SR rats for the rest of their lives (178).
In a recent study, gut dysbiosis was found in a small group of 7 hypertensive patients when compared with 10 normotensive controls (318), and a meta-analysis of 9 trials reported a minor reduction in blood pressure with the administration of probotics (131). Future studies will likely define characteristics of the gut microbiota that, in conjunction with other variables, may play a role in the modulation of blood pressure.
XII. CONCLUDING REMARKS
High blood pressure is a hemodynamic consequence of many factors, and there is now definitive evidence for a role of immunity in the pathogenesis of essential hypertension. As shown in FIGURE 9, the development and aggravation of hypertension may be considered a stepwise process of engagement of the immune system. This process originates from stimuli that activate innate immunity, first transiently and then permanently, in conjunction with an adaptive immune response to neoantigens. The addition of chronic renal damage and arteriosclerosis increases the severity of hypertension and its complications.
FIGURE 9.
Activation of the immune system and the natural history of essential hypertension. Prehypertension is associated with irregular generation of stimulatory signals associated with rise in blood pressure. Transient episodes of hypertension are associated with episodic generation of danger-associated molecular patterns (DAMPs) and expression of Toll-like receptors (TLRs) that activate intermittently the innate immune system with episodic inflammatory infiltration in target organs. Established hypertension results from the activation of both the innate and the adaptive immunity that support one another and drive a permanent renal and vascular inflammation that is, nevertheless, in a state of unsteady equilibrium with the suppressive (anti-inflammatory) responses. This balance is capable of maintaining a well-preserved renal function. The development of chronic renal damage and arteriosclerosis, resulting from persistent and increasing inflammation fueled by the unchecked generation of neoantigens, is manifested by hypertension of increased severity and resistance to treatment.
We suggest that major issues that need to be addressed at the present time include the following.
-
1.
Identification of genetic traits that have increased frequency in hypertensive patients and, additionally, are related to the immune response. The relevance of a given trait may be explored experimentally. This approach has already proved successful in several studies (226, 230).
-
2.
Identification of antigenic determinants in hypertension capable of activating T and B responses. These studies may lead to strategies directed to minimize their generation or to suppress the immune responses driven by these antigens.
-
3.
Evaluation of mechanisms of engagement of innate immunity in hypertension and determine if suppressing inflammasome activation results in prevention or correction of experimental hypertension. The availability of compounds that inhibit the activation of the inflammasome with relatively minor adverse effects (37) makes this line of investigation attractive.
-
4.
Investigation of the potential use of a short period of immune suppression as valuable adjunct to antihypertensive medication in selected cases of severe resistant hypertension. To be sure, presently available antihypertensive drugs offer an effective and safe treatment in the vast majority of hypertensive patients without the complications of long-term immunosuppression. Nevertheless, there are exceptional cases of severe hypertension that are resistant to treatment. In these patients, renal denervation has been used with variable success (107). We recently reported a patient with resistant hypertension, considered for renal denervation, in whom 2 mo of MMF treatment resulted in control of the blood pressure with regular doses of antihypertensive medication (217). While this anecdotal report needs confirmation, it is not unreasonable to evaluate if a few weeks of immunosuppression avoids or postpones the need of an invasive procedure that is not devoid of complications (107).
In moving forward, we need to be aware that although the mouse is conveniently used in experimental studies of hypertension, the SHR and the Dahl SS rats are genetic strains that resemble better human hypertension. Furthermore, the use of intravenous infusions of angiotensin II is an excellent way to dissect pathogenetic mechanisms, but the doses frequently used likely result in circulating levels of angiotensin that are several times higher than those present in physiological or physiopathological conditions (162) and therefore of uncertain clinical relevance.
Much has been learned about the immune basis of hypertension in the last decade, and further research in the immune mechanisms participating in the pathogenesis of hypertension may open new strategies in the treatment of the disease.
GRANTS
The work in the authors’ laboratories is funded by grants from FONACYT, Venezuela (FC-2005000283, to B. Rodriguez-Iturbe) and Asociación de Amigos del Riñón (to B. Rodriguez-Iturbe) and by National Heart, Lung, and Blood Institute Grant HL-68607 (to R. J. Johnson).
DISCLOSURES
R. J. Johnson is on the Scientific Board of Amway and XORT Therapeutics and has patent and patent applications related to lowering uric acid or blocking fructose metabolism in the treatment of hypertension and metabolic disorders. H. Pons and B. Rodriguez-Iturbe have no conflicts of interest, financial or otherwise.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: B. Rodriguez-Iturbe, Hospital Universitario de Maracaibo, Servicio de Nefrología, Ave. Goajira, Maracaibo, Venezuela (e-mail: brodrigueziturbe@gmail.com).
REFERENCES
- 1.Alvarez V, Quiroz Y, Nava M, Pons H, Rodríguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol 283: F1132–F1141, 2002. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 2.Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, Carrasco L, Alzamora R, Figueroa F, Kalergis AM, Michea L. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 63: 797–803, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02883. [DOI] [PubMed] [Google Scholar]
- 3.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 223: 20–38, 2008. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 4.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034, 2002. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 5.Asghar M, Chugh G, Lokhandwala MF. Inflammation compromises renal dopamine D1 receptor function in rats. Am J Physiol Renal Physiol 297: F1543–F1549, 2009. doi: 10.1152/ajprenal.00366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol 128: 1211–1216, 1982. [PubMed] [Google Scholar]
- 7.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 8.Bataillard A, Freiche JC, Vincent M, Sassard J, Touraine JL. Antihypertensive effect of neonatal thymectomy in the genetically hypertensive LH rat. Thymus 8: 321–330, 1986. [PubMed] [Google Scholar]
- 9.Bataillard A, Vincent M, Sassard J, Touraine JL. Antihypertensive effect of an immunosuppressive agent, cyclophosphamide, in genetically hypertensive rats of the Lyon strain. Int J Immunopharmacol 11: 377–384, 1989. doi: 10.1016/0192-0561(89)90084-2. [DOI] [PubMed] [Google Scholar]
- 10.Bauernfeind F, Bartok E, Rieger A, Franchi L, Núñez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol 187: 613–617, 2011. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791, 2009. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bautista LE, Atwood JE, O’Malley PG, Taylor AJ. Association between C-reactive protein and hypertension in healthy middle-aged men and women. Coron Artery Dis 15: 331–336, 2004. doi: 10.1097/00019501-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bautista LE, López-Jaramillo P, Vera LM, Casas JP, Otero AP, Guaracao AI. Is C-reactive protein an independent risk factor for essential hypertension? J Hypertens 19: 857–861, 2001. doi: 10.1097/00004872-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 19: 149–154, 2005. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 15.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 90: 278–281, 1992. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Ishay D, Saliternik R, Welner A. Separation of two strains of rats with inbred dissimilar sensitivity to Doca-salt hypertension. Experientia 28: 1321–1322, 1972. doi: 10.1007/BF01965321. [DOI] [PubMed] [Google Scholar]
- 17.Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinewietfeld M, Voelkl J, Schatz V, Linker RA, Lang F, Voehringer D, Wright MD, Hubner N, Dechend R, Jantsch J, Titze J, Müller DN. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest 125: 4223–4238, 2015. doi: 10.1172/JCI80919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blankenberg S, Luc G, Ducimetière P, Arveiler D, Ferrières J, Amouyel P, Evans A, Cambien F, Tiret L, PRIME Study Group . Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 108: 2453–2459, 2003. doi: 10.1161/01.CIR.0000099509.76044.A2. [DOI] [PubMed] [Google Scholar]
- 19.Bohlender J, Fukamizu A, Lippoldt A, Nomura T, Dietz R, Ménard J, Murakami K, Luft FC, Ganten D. High human renin hypertension in transgenic rats. Hypertension 29: 428–434, 1997. doi: 10.1161/01.HYP.29.1.428. [DOI] [PubMed] [Google Scholar]
- 20.Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 122: 535–543, 2012. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bost L, Primatesta P, Dong W, Poulter N. Blood lead and blood pressure: evidence from the Health Survey for England 1995. J Hum Hypertens 13: 123–128, 1999. doi: 10.1038/sj.jhh.1000771. [DOI] [PubMed] [Google Scholar]
- 22.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 56: 879–884, 2010. doi: 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brantsaeter AL, Myhre R, Haugen M, Myking S, Sengpiel V, Magnus P, Jacobsson B, Meltzer HM. Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian Mother and Child Cohort Study. Am J Epidemiol 174: 807–815, 2011. doi: 10.1093/aje/kwr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodríguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol 293: F616–F623, 2007. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 25.Bravo J, Quiroz Y, Pons H, Parra G, Herrera-Acosta J, Johnson RJ, Rodríguez-Iturbe B. Vimentin and heat shock protein expression are induced in the kidney by angiotensin and by nitric oxide inhibition. Kidney Int Suppl 64: S46–S51, 2003. doi: 10.1046/j.1523-1755.64.s86.9.x. [DOI] [PubMed] [Google Scholar]
- 26.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994. doi: 10.1016/S0272-6386(12)80967-X. [DOI] [PubMed] [Google Scholar]
- 27.Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M, Lembo G. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 41: 737–752, 2014. doi: 10.1016/j.immuni.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Carnevale D, Perrotta M, Pallante F, Fardella V, Iacobucci R, Fardella S, Carnevale L, Carnevale R, De Lucia M, Cifelli G, Lembo G. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat Commun 7: 13035, 2016. doi: 10.1038/ncomms13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension 38: 399–403, 2001. doi: 10.1161/01.HYP.38.3.399. [DOI] [PubMed] [Google Scholar]
- 30.Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, Jeunemaitre X, Thomas A. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens 24: 1143–1148, 2011. doi: 10.1038/ajh.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan CT, Moore JP, Budzyn K, Guida E, Diep H, Vinh A, Jones ES, Widdop RE, Armitage JA, Sakkal S, Ricardo SD, Sobey CG, Drummond GR. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension 60: 1207–1212, 2012. doi: 10.1161/HYPERTENSIONAHA.112.201251. [DOI] [PubMed] [Google Scholar]
- 32.Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A, Drummond GR. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66: 1023–1033, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05779. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the Normative Aging Study. Am J Epidemiol 153: 164–171, 2001. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- 34.Chiasson VL, Talreja D, Young KJ, Chatterjee P, Banes-Berceli AK, Mitchell BM. FK506 binding protein 12 deficiency in endothelial and hematopoietic cells decreases regulatory T cells and causes hypertension. Hypertension 57: 1167–1175, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiba T, Itoh T, Tabuchi M, Nakazawa T, Satou T. Interleukin-1β accelerates the onset of stroke in stroke-prone spontaneously hypertensive rats. Mediators Inflamm 2012: 701976, 2012. doi: 10.1155/2012/701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chobanian AV. Shattuck Lecture. The hypertension paradox–more uncontrolled disease despite improved therapy. N Engl J Med 361: 878–887, 2009. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- 37.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21: 248–255, 2015. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crosswhite P, Sun Z. Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension 55: 1484–1491, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowley SD, Jeffs AD. Targeting cytokine signaling in salt-sensitive hypertension. Am J Physiol Renal Physiol 311: F1153–F1158, 2016. doi: 10.1152/ajprenal.00273.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 298: R1089–R1097, 2010. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55: 99–108, 2010. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22: 333–340, 2010. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahl LK, Heine M, Tassinari L. Effects of chronia excess salt ingestion. Evidence that genetic factors play an important role in susceptibility to experimental hypertension. J Exp Med 115: 1173–1190, 1962. doi: 10.1084/jem.115.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283: R757–R767, 2002. doi: 10.1152/ajpregu.00098.2002. [DOI] [PubMed] [Google Scholar]
- 45.Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med 129: 300–308, 1997. doi: 10.1016/S0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- 46.Dange RB, Agarwal D, Teruyama R, Francis J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation 12: 31, 2015. doi: 10.1186/s12974-015-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies DJ, Brewer DB, Hardwicke J. Urinary proteins and glomerular morphometry in protein overload proteinuria. Lab Invest 38: 232–243, 1978. [PubMed] [Google Scholar]
- 48.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 25: 2106–2113, 2005. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- 49.De Graaf R, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect 8: 1859–1865, 2006. doi: 10.1016/j.micinf.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 50.De la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 34: 2480–2488, 2004. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 51.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 57: 269–274, 2011. doi: 10.1161/HYPERTENSIONAHA.110.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devallière J, Charreau B. The adaptor Lnk (SH2B3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem Pharmacol 82: 1391–1402, 2011. doi: 10.1016/j.bcp.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 55.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension 54: 619–624, 2009. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diederichsen LP, Diederichsen AC, Simonsen JA, Junker P, Søndergaard K, Lundberg IE, Tvede N, Gerke O, Christensen AF, Dreyer L, Petersen H, Ejstrup L, Kay SD, Jacobsen S. Traditional cardiovascular risk factors and coronary artery calcification in adults with polymyositis and dermatomyositis: a Danish multicenter study. Arthritis Care Res (Hoboken) 67: 848–854, 2015. doi: 10.1002/acr.22520. [DOI] [PubMed] [Google Scholar]
- 57.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassègue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20: 281–294, 2014. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dörffel Y, Lätsch C, Stuhlmüller B, Schreiber S, Scholze S, Burmester GR, Scholze J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 34: 113–117, 1999. doi: 10.1161/01.HYP.34.1.113. [DOI] [PubMed] [Google Scholar]
- 59.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 60.Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res 20: 34–50, 2010. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 61.Ebrahimian T, Li MW, Lemarié CA, Simeone SM, Pagano PJ, Gaestel M, Paradis P, Wassmann S, Schiffrin EL. Mitogen-activated protein kinase-activated protein kinase 2 in angiotensin II-induced inflammation and hypertension: regulation of oxidative stress. Hypertension 57: 245–254, 2011. doi: 10.1161/HYPERTENSIONAHA.110.159889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebringer A, Doyle AE. Raised serum IgG levels in hypertension. BMJ 2: 146–148, 1970. doi: 10.1136/bmj.2.5702.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eddy AA. Interstitial nephritis induced by protein-overload proteinuria. Am J Pathol 135: 719–733, 1989. [PMC free article] [PubMed] [Google Scholar]
- 64.Ehret GB, O’Connor AA, Weder A, Cooper RS, Chakravarti A. Follow-up of a major linkage peak on chromosome 1 reveals suggestive QTLs associated with essential hypertension: GenNet study. Eur J Hum Genet 17: 1650–1657, 2009. doi: 10.1038/ejhg.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595–638, 2000. [PubMed] [Google Scholar]
- 66.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II-salt hypertension. Hypertension 50: 1069–1076, 2007. doi: 10.1161/HYPERTENSIONAHA.107.098806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 300: 924–932, 2008. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O’Connor PM, Cowley AW Jr. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–208, 2012. doi: 10.1016/j.cmet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Florin M, Lo M, Liu KL, Sassard J. Salt sensitivity in genetically hypertensive rats of the Lyon strain. Kidney Int 59: 1865–1872, 2001. doi: 10.1046/j.1523-1755.2001.0590051865.x. [DOI] [PubMed] [Google Scholar]
- 71.Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 72.Franco M, Tapia E, Bautista R, Pacheco U, Santamaria J, Quiroz Y, Johnson RJ, Rodriguez-Iturbe B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am J Physiol Renal Physiol 304: F982–F990, 2013. doi: 10.1152/ajprenal.00463.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frohlich ED, Dustan HP, Bumpus FM. Irvine H. Page: 1901-1991. The celebration of a leader. Hypertension 18: 443–445, 1991. doi: 10.1161/01.HYP.18.4.443. [DOI] [PubMed] [Google Scholar]
- 74.Frostegård J, Lemne C, Andersson B, van der Zee R, Kiessling R, de Faire U. Association of serum antibodies to heat-shock protein 65 with borderline hypertension. Hypertension 29: 40–44, 1997. doi: 10.1161/01.HYP.29.1.40. [DOI] [PubMed] [Google Scholar]
- 75.Frostegård J, Wu R, Gillis-Haegerstrand C, Lemne C, de Faire U. Antibodies to endothelial cells in borderline hypertension. Circulation 98: 1092–1098, 1998. doi: 10.1161/01.CIR.98.11.1092. [DOI] [PubMed] [Google Scholar]
- 76.Furumoto T, Saito N, Dong J, Mikami T, Fujii S, Kitabatake A. Association of cardiovascular risk factors and endothelial dysfunction in Japanese hypertensive patients: implications for early atherosclerosis. Hypertens Res 25: 475–480, 2002. doi: 10.1291/hypres.25.475. [DOI] [PubMed] [Google Scholar]
- 77.Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol 23: 613–619, 2011. doi: 10.1016/j.coi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol 289: H1683–H1691, 2005. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 79.Gardner DL, Quagliata F, Drossman M, Kalish M, Schimmer B. Attempted prevention of arteriolar lesions in accelerated rat hypertension by immunosuppression. Br J Exp Pathol 51: 242–252, 1970. [PMC free article] [PubMed] [Google Scholar]
- 80.Gareau RJ, Cartier GE. Etude histologique de 55 biopsies rénales prélevées chez des malades hypertendus. Union Med Can 84: 1134–1142, 1955. [PubMed] [Google Scholar]
- 81.Giani JF, Bernstein KE, Janjulia T, Han J, Toblli JE, Shen XZ, Rodriguez-Iturbe B, McDonough AA, Gonzalez-Villalobos RA. Salt sensitivity in response to renal injury requires renal angiotensin-converting enzyme. Hypertension 66: 534–542, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Given MB, Lowe RF, Williams DL, Sander GE, Giles TD. Failure of interleukin-2 to alter systolic blood pressure in Dahl salt-sensitive rats. Am J Hypertens 5: 203–204, 1992. doi: 10.1093/ajh/5.3.203. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ACE protects against hypertension. J Clin Invest 123: 2011–2023, 2013. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gornikiewicz A, Sautner T, Brostjan C, Schmierer B, Függer R, Roth E, Mühlbacher F, Bergmann M. Catecholamines up-regulate lipopolysaccharide-induced IL-6 production in human microvascular endothelial cells. FASEB J 14: 1093–1100, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Gratze P, Dechend R, Stocker C, Park JK, Feldt S, Shagdarsuren E, Wellner M, Gueler F, Rong S, Gross V, Obst M, Plehm R, Alenina N, Zenclussen A, Titze J, Small K, Yokota Y, Zenke M, Luft FC, Müller DN. Novel role for inhibitor of differentiation 2 in the genesis of angiotensin II-induced hypertension. Circulation 117: 2645–2656, 2008. doi: 10.1161/CIRCULATIONAHA.107.760116. [DOI] [PubMed] [Google Scholar]
- 86.Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, Porter JE. α1-Adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. J Pharmacol Exp Ther 338: 648–657, 2011. doi: 10.1124/jpet.110.178012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of T cell and macrophage activation with arterial vascular health in HIV. AIDS Res Hum Retroviruses 33: 181–186, 2017. doi: 10.1089/aid.2016.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gudbrandsson T, Hansson L, Herlitz H, Lindholm L, Nilsson LA. Immunological changes in patients with previous malignant essential hypertension. Lancet 1: 406–408, 1981. doi: 10.1016/S0140-6736(81)91790-6. [DOI] [PubMed] [Google Scholar]
- 89.Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 90.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-κ B transcription factors. Circ Res 84: 695–703, 1999. doi: 10.1161/01.RES.84.6.695. [DOI] [PubMed] [Google Scholar]
- 92.Harrison DG. The mosaic theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J Am Soc Hypertens 7: 68–74, 2013. doi: 10.1016/j.jash.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res 111: 1190–1197, 2012. doi: 10.1161/CIRCRESAHA.112.277475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F555–F561, 2016. doi: 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haydar A, Bakri RS, Prime M, Goldsmith DJ. Page kidney–a review of the literature. J Nephrol 16: 329–333, 2003. [PubMed] [Google Scholar]
- 96.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 288: H680–H685, 2005. doi: 10.1152/ajpheart.00823.2004. [DOI] [PubMed] [Google Scholar]
- 97.Heptinstall RH. Renal biopsies in hypertension. Br Heart J 16: 133–141, 1954. doi: 10.1136/hrt.16.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hermann M, Shaw S, Kiss E, Camici G, Bühler N, Chenevard R, Lüscher TF, Gröne HJ, Ruschitzka F. Selective COX-2 inhibitors and renal injury in salt-sensitive hypertension. Hypertension 45: 193–197, 2005. doi: 10.1161/01.HYP.0000153053.82032.bf. [DOI] [PubMed] [Google Scholar]
- 99.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17, Suppl 3: S218–S225, 2006. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 100.Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, Sigmund CD. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension 61: 716–722, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296: R208–R216, 2009. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holmes MC, Burnet FM. The natural history of autoimmune disease in NZB mice. A comparison with the pattern of human autoimmune manifestations. Ann Intern Med 59: 265–276, 1963. doi: 10.7326/0003-4819-59-3-265. [DOI] [PubMed] [Google Scholar]
- 103.Hu J, Zhu Q, Xia M, Guo TL, Wang Z, Li PL, Han WQ, Yi F, Li N. Transplantation of mesenchymal stem cells into the renal medulla attenuated salt-sensitive hypertension in Dahl S rat. J Mol Med (Berl) 92: 1139–1145, 2014. doi: 10.1007/s00109-014-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang B, Cheng Y, Usa K, Liu Y, Baker MA, Mattson DL, He Y, Wang N, Liang M. Renal tumor necrosis factor α contributes to hypertension in Dahl salt-sensitive Rats. Sci Rep 6: 21960, 2016. doi: 10.1038/srep21960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hughson MD, Gobe GC, Hoy WE, Manning RD Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 52: 18–28, 2008. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 106.Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol 25: 1268–1273, 2005. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 107.Iliescu R, Lohmeier TE, Tudorancea I, Laffin L, Bakris GL. Renal denervation for the treatment of resistant hypertension: review and clinical perspective. Am J Physiol Renal Physiol 309: F583–F594, 2015. doi: 10.1152/ajprenal.00246.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Isaka Y, Takabatake Y, Takahashi A, Saitoh T, Yoshimori T. Hyperuricemia-induced inflammasome and kidney diseases. Nephrol Dial Transplant 31: 890–896, 2016. doi: 10.1093/ndt/gfv024. [DOI] [PubMed] [Google Scholar]
- 109.Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, Tsuchihashi M, Sugaya T, Charo IF, Kura S, Tsuzuki T, Ishibashi T, Takeshita A, Egashira K. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res 94: 1203–1210, 2004. doi: 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- 110.Ishimitsu T, Uehara Y, Numabe A, Tsukada H, Ogawa Y, Yagi S. Antihypertensive effect of interleukin-2 in salt-sensitive Dahl rats. Hypertension 23: 68–73, 1994. doi: 10.1161/01.HYP.23.1.68. [DOI] [PubMed] [Google Scholar]
- 111.Ishizaka N, Aizawa T, Ohno M, Usui S, Mori I, Tang SS, Ingelfinger JR, Kimura S, Nagai R. Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension 39: 122–128, 2002. doi: 10.1161/hy1201.096818. [DOI] [PubMed] [Google Scholar]
- 112.Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, Dikalov SI. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension 67: 1218–1227, 2016. doi: 10.1161/HYPERTENSIONAHA.115.07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of Human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68: 123–132, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res 118: 1233–1243, 2016. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ito H, Ohshima A, Tsuzuki M, Ohto N, Takao K, Hijii C, Yanagawa M, Ogasawara M, Nishioka K. Association of serum tumour necrosis factor-alpha with serum low-density lipoprotein-cholesterol and blood pressure in apparently healthy Japanese women. Clin Exp Pharmacol Physiol 28: 188–192, 2001. doi: 10.1046/j.1440-1681.2001.03429.x. [DOI] [PubMed] [Google Scholar]
- 116.Iyer A, Woodruff TM, Wu MC, Stylianou C, Reid RC, Fairlie DP, Taylor SM, Brown L. Inhibition of inflammation and fibrosis by a complement C5a receptor antagonist in DOCA-salt hypertensive rats. J Cardiovasc Pharmacol 58: 479–486, 2011. doi: 10.1097/FJC.0b013e31822a7a09. [DOI] [PubMed] [Google Scholar]
- 117.Jaffé D, Sutherland LE, Barker DM, Dahl LK. Effects of chronic excess salt ingestion. Morphologic findings in kidneys of rats with differing genetic susceptibilities to hypertension. Arch Pathol 90: 1–16, 1970. [PubMed] [Google Scholar]
- 118.Jekell A, Malmqvist K, Wallén NH, Mörtsell D, Kahan T. Markers of inflammation, endothelial activation, and arterial stiffness in hypertensive heart disease and the effects of treatment: results from the SILVHIA study. J Cardiovasc Pharmacol 62: 559–566, 2013. doi: 10.1097/FJC.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 119.Ji Y, Liu J, Wang Z, Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem 23: 265–276, 2009. doi: 10.1159/000218173. [DOI] [PubMed] [Google Scholar]
- 120.Ji X, Naito Y, Hirokawa G, Weng H, Hiura Y, Takahashi R, Iwai N. P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res 35: 173–179, 2012. doi: 10.1038/hr.2011.153. [DOI] [PubMed] [Google Scholar]
- 121.Jiménez-Altayó F, Briones AM, Giraldo J, Planas AM, Salaices M, Vila E. Increased superoxide anion production by interleukin-1beta impairs nitric oxide-mediated relaxation in resistance arteries. J Pharmacol Exp Ther 316: 42–52, 2006. doi: 10.1124/jpet.105.088435. [DOI] [PubMed] [Google Scholar]
- 122.Johnson RJ, Schreiner GF. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int 52: 1169–1179, 1997. doi: 10.1038/ki.1997.442. [DOI] [PubMed] [Google Scholar]
- 123.Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens 24: 403–409, 2015. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jun JY, Zubcevic J, Qi Y, Afzal A, Carvajal JM, Thinschmidt JS, Grant MB, Mocco J, Raizada MK. Brain-mediated dysregulation of the bone marrow activity in angiotensin II-induced hypertension. Hypertension 60: 1316–1323, 2012. doi: 10.1161/HYPERTENSIONAHA.112.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ-/- and interleukin-17A-/- mice. Hypertension 65: 569–576, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59: 324–330, 2012. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 127.Kashiwagi M, Shinozaki M, Hirakata H, Tamaki K, Hirano T, Tokumoto M, Goto H, Okuda S, Fujishima M. Locally activated renin-angiotensin system associated with TGF-β1 as a major factor for renal injury induced by chronic inhibition of nitric oxide synthase in rats. J Am Soc Nephrol 11: 616–624, 2000. [DOI] [PubMed] [Google Scholar]
- 128.Katsuki M, Hirooka Y, Kishi T, Sunagawa K. Decreased proportion of Foxp3+ CD4+ regulatory T cells contributes to the development of hypertension in genetically hypertensive rats. J Hypertens 33: 773–783, 2015. doi: 10.1097/HJH.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 129.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol 13: 2860–2868, 2002. doi: 10.1097/01.ASN.0000035087.11758.ED. [DOI] [PubMed] [Google Scholar]
- 130.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 131.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64: 897–903, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 132.Khraibi AA, Norman RA Jr, Dzielak DJ. Chronic immunosuppression attenuates hypertension in Okamoto spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 247: H722–H726, 1984. [DOI] [PubMed] [Google Scholar]
- 133.Kim BS, Park YJ, Chung Y. Targeting IL-17 in autoimmunity and inflammation. Arch Pharm Res 39: 1537–1547, 2016. doi: 10.1007/s12272-016-0823-8. [DOI] [PubMed] [Google Scholar]
- 134.King DE, Egan BM, Mainous AG III, Geesey ME. Elevation of C-reactive protein in people with prehypertension. J Clin Hypertens (Greenwich) 6: 562–568, 2004. doi: 10.1111/j.1524-6175.2004.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J II, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Müller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koeners MP, Wesseling S, Sánchez M, Braam B, Joles JA. Perinatal inhibition of NF-KappaB has long-term antihypertensive and renoprotective effects in Fawn-Hooded hypertensive rats. Am J Hypertens 29: 123–131, 2016. doi: 10.1093/ajh/hpv065. [DOI] [PubMed] [Google Scholar]
- 138.Kossmann S, Schwenk M, Hausding M, Karbach SH, Schmidgen MI, Brandt M, Knorr M, Hu H, Kröller-Schön S, Schönfelder T, Grabbe S, Oelze M, Daiber A, Münzel T, Becker C, Wenzel P. Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol 33: 1313–1319, 2013. doi: 10.1161/ATVBAHA.113.301437. [DOI] [PubMed] [Google Scholar]
- 139.Krebs CF, Lange S, Niemann G, Rosendahl A, Lehners A, Meyer-Schwesinger C, Stahl RA, Benndorf RA, Velden J, Paust HJ, Panzer U, Ehmke H, Wenzel UO. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin II-induced hypertension. Hypertension 63: 565–571, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02620. [DOI] [PubMed] [Google Scholar]
- 140.Krishnan SM, Dowling JK, Ling YH, Diep H, Chan CT, Ferens D, Kett MM, Pinar A, Samuel CS, Vinh A, Arumugam TV, Hewitson TD, Kemp-Harper BK, Robertson AA, Cooper MA, Latz E, Mansell A, Sobey CG, Drummond GR. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol 173: 752–765, 2016. doi: 10.1111/bph.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kristensen BO, Andersen PL, Wiik A. Autoantibodies and vascular events in essential hypertension: a five-year longitudinal study. J Hypertens 2: 19–24, 1984. doi: 10.1097/00004872-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 142.Kristensen BO, Sølling K. Serum concentrations of immunoglobulins and free light chains before and after vascular events in essential hypertension. Acta Med Scand 213: 15–20, 1983. doi: 10.1111/j.0954-6820.1983.tb03682.x. [DOI] [PubMed] [Google Scholar]
- 143.Kronbichler A, Mayer G. Renal involvement in autoimmune connective tissue diseases. BMC Med 11: 95, 2013. doi: 10.1186/1741-7015-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuijpers MH, de Jong W. Relationship between blood pressure level, renal histopathological lesions and plasma renin activity in fawn-hooded rats. Br J Exp Pathol 68: 179–187, 1987. [PMC free article] [PubMed] [Google Scholar]
- 145.Kunes J, Poirier M, Tremblay J, Hamet P. Expression of hsp70 gene in lymphocytes from normotensive and hypertensive humans. Acta Physiol Scand 146: 307–311, 1992. doi: 10.1111/j.1748-1716.1992.tb09424.x. [DOI] [PubMed] [Google Scholar]
- 146.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 119: 2904–2912, 2009. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 147.Lee DL, Sturgis LC, Labazi H, Osborne JB Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290: H935–H940, 2006. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 148.Li DJ, Evans RG, Yang ZW, Song SW, Wang P, Ma XJ, Liu C, Xi T, Su DF, Shen FM. Dysfunction of the cholinergic anti-inflammatory pathway mediates organ damage in hypertension. Hypertension 57: 298–307, 2011. doi: 10.1161/HYPERTENSIONAHA.110.160077. [DOI] [PubMed] [Google Scholar]
- 149.Li JX, Tang BP, Sun HP, Feng M, Cheng ZH, Niu WQ. Interacting contribution of the five polymorphisms in three genes of Hsp70 family to essential hypertension in Uygur ethnicity. Cell Stress Chaperones 14: 355–362, 2009. doi: 10.1007/s12192-008-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Y, Wu Y, Zhang C, Li P, Cui W, Hao J, Ma X, Yin Z, Du J. γδT cell-derived interleukin-17A via an interleukin-1β-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension 64: 305–314, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02604. [DOI] [PubMed] [Google Scholar]
- 151.Liu N, Liu J, Ji Y, Lu P. Toll-like receptor 4 signaling mediates inflammatory activation induced by C-reactive protein in vascular smooth muscle cells. Cell Physiol Biochem 25: 467–476, 2010. doi: 10.1159/000303052. [DOI] [PubMed] [Google Scholar]
- 152.Liu X, Zhang Q, Wu H, Du H, Liu L, Shi H, Wang C, Xia Y, Guo X, Li C, Bao X, Su Q, Sun S, Wang X, Zhou M, Jia Q, Zhao H, Song K, Niu K. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens 28: 1339–1346, 2015. doi: 10.1093/ajh/hpv034. [DOI] [PubMed] [Google Scholar]
- 153.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52: 2936–2946, 2005. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 154.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 55: 277–283, 2010. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension 61: 382–387, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to angiotensin II. Hypertension 33: 1013–1019, 1999. doi: 10.1161/01.HYP.33.4.1013. [DOI] [PubMed] [Google Scholar]
- 157.Los M, Dröge W, Stricker K, Baeuerle PA, Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol 25: 159–165, 1995. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 158.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15: 545–552, 2009. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 159.Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: the fetal flaw in essential hypertension? Kidney Int Suppl 55: S30–S34, 1996. [PubMed] [Google Scholar]
- 160.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Majeed B, Tawinwung S, Eberson LS, Secomb TW, Larmonier N, Larson DF. Interleukin-2/Anti-Interleukin-2 immune complex expands regulatory T cells and reduces angiotensin II-induced aortic stiffening. Int J Hypertens 2014: 126365, 2014. doi: 10.1155/2014/126365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Manhiani MM, Seth DM, Banes-Berceli AKL, Satou R, Navar LG, Brands MW. The role of IL-6 in the physiologic versus hypertensive blood pressure actions of angiotensin II. Physiol Rep 3: e12595, 2015. doi: 10.14814/phy2.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marketou ME, Kontaraki JE, Zacharis EA, Kochiadakis GE, Giaouzaki A, Chlouverakis G, Vardas PE. TLR2 and TLR4 gene expression in peripheral monocytes in nondiabetic hypertensive patients: the effect of intensive blood pressure-lowering. J Clin Hypertens (Greenwich) 14: 330–335, 2012. doi: 10.1111/j.1751-7176.2012.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Markó L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Müller DN. Interferon-γ signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension 60: 1430–1436, 2012. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 165.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 166.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117: 561–574, 2004. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 167.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 107: 263–270, 2010. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.März P, Cheng JG, Gadient RA, Patterson PH, Stoyan T, Otten U, Rose-John S. Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci USA 95: 3251–3256, 1998. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mathis KW, Venegas-Pont M, Masterson CW, Wasson KL, Ryan MJ. Blood pressure in a hypertensive mouse model of SLE is not salt-sensitive. Am J Physiol Regul Integr Comp Physiol 301: R1281–R1285, 2011. doi: 10.1152/ajpregu.00386.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mathis KW, Wallace K, Flynn ER, Maric-Bilkan C, LaMarca B, Ryan MJ. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension 64: 792–800, 2014. doi: 10.1161/HYPERTENSIONAHA.114.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Matrougui K, Zakaria AE, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol 178: 434–441, 2011. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mattace-Raso FU, Verwoert GC, Hofman A, Witteman JC. Inflammation and incident-isolated systolic hypertension in older adults: the Rotterdam study. J Hypertens 28: 892–895, 2010. doi: 10.1097/HJH.0b013e328336ed26. [DOI] [PubMed] [Google Scholar]
- 173.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307: F499–F508, 2014. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 175.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 177.McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol 306: H184–H196, 2014. doi: 10.1152/ajpheart.00328.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mellors RC. Autoimmune disease in NZB/B1 mice. I. Pathology and pathogenesis of a model system of spontaneous glomerulonephritis. J Exp Med 122: 25–40, 1965. doi: 10.1084/jem.122.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mervaala E, Müller DN, Park JK, Dechend R, Schmidt F, Fiebeler A, Bieringer M, Breu V, Ganten D, Haller H, Luft FC. Cyclosporin A protects against angiotensin II-induced end-organ damage in double transgenic rats harboring human renin and angiotensinogen genes. Hypertension 35: 360–366, 2000. doi: 10.1161/01.HYP.35.1.360. [DOI] [PubMed] [Google Scholar]
- 181.Mervaala E, Müller DN, Schmidt F, Park JK, Gross V, Bader M, Breu V, Ganten D, Haller H, Luft FC. Blood pressure-independent effects in rats with human renin and angiotensinogen genes. Hypertension 35: 587–594, 2000. doi: 10.1161/01.HYP.35.2.587. [DOI] [PubMed] [Google Scholar]
- 182.Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens 34: 97–108, 2016. doi: 10.1097/HJH.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 183.Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D, Sagan A, Wu J, Vinh A, Marvar PJ, Guzik B, Podolec J, Drummond G, Lob HE, Harrison DG, Guzik TJ. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J 30: 1987–1999, 2016. doi: 10.1096/fj.201500088R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mirhafez SR, Mohebati M, Feiz Disfani M, Saberi Karimian M, Ebrahimi M, Avan A, Eslami S, Pasdar A, Rooki H, Esmaeili H, Ferns GA, Ghayour-Mobarhan M. An imbalance in serum concentrations of inflammatory and anti-inflammatory cytokines in hypertension. J Am Soc Hypertens 8: 614–623, 2014. doi: 10.1016/j.jash.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 185.Müller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension 35: 193–201, 2000. doi: 10.1161/01.HYP.35.1.193. [DOI] [PubMed] [Google Scholar]
- 186.Müller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Murphy SR, Dahly-Vernon AJ, Dunn KM, Chen CC, Ledbetter SR, Williams JM, Roman RJ. Renoprotective effects of anti-TGF-β antibody and antihypertensive therapies in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 303: R57–R69, 2012. doi: 10.1152/ajpregu.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nakamura Y, Yamamoto N, Sakai K, Takano T. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J Dairy Sci 78: 1253–1257, 1995. doi: 10.3168/jds.S0022-0302(95)76745-5. [DOI] [PubMed] [Google Scholar]
- 189.Nava M, Quiroz Y, Vaziri N, Rodríguez-Iturbe B. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol 284: F447–F454, 2003. doi: 10.1152/ajprenal.00264.2002. [DOI] [PubMed] [Google Scholar]
- 190.Navar LG. The intrarenal renin-angiotensin system in hypertension. Kidney Int 65: 1522–1532, 2004. doi: 10.1111/j.1523-1755.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 191.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316–322, 2002. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 97: 696–704, 2013. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 68: 167–174, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27: 282–293, 1963. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 195.Okamoto K, Hazama F, Yamori Y, Haebara H, Nagaoka A. Pathogenesis and prevention of stroke in spontaneously hypertensive rats. Clin Sci Mol Med Suppl 2: 161s–163s, 1975. [DOI] [PubMed] [Google Scholar]
- 196.Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med 25: 257–264, 1967. [PubMed] [Google Scholar]
- 197.Olsen F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol Microbiol Scand C 88: 1–5, 1980. [DOI] [PubMed] [Google Scholar]
- 198.Olsen F, Hilden M, Ibsen H. Raised levels of immunoglobulins in serum of hypertensive patients. Acta Pathol Microbiol Scand B Microbiol Immunol 81: 775–778, 1973. [DOI] [PubMed] [Google Scholar]
- 199.Olsen F, Loft B. Delayed hypersensitivity directed against arterial antigens in the hypertensive disease in man. Acta Pathol Microbiol Scand A 81: 498–500, 1973. [DOI] [PubMed] [Google Scholar]
- 200.Omi T, Kumada M, Kamesaki T, Okuda H, Munkhtulga L, Yanagisawa Y, Utsumi N, Gotoh T, Hata A, Soma M, Umemura S, Ogihara T, Takahashi N, Tabara Y, Shimada K, Mano H, Kajii E, Miki T, Iwamoto S. An intronic variable number of tandem repeat polymorphisms of the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies gene expression and is associated with essential hypertension. Eur J Hum Genet 14: 1295–1305, 2006. doi: 10.1038/sj.ejhg.5201698. [DOI] [PubMed] [Google Scholar]
- 201.Page IH. The production of persistent arterial hypertension by cellophane perinephritis. JAMA 113: 2046–2048, 1939. doi: 10.1001/jama.1939.02800480032008. [DOI] [Google Scholar]
- 202.Page IH. Pathogenesis of arterial hypertension. J Am Med Assoc 140: 451–458, 1949. doi: 10.1001/jama.1949.02900400005002. [DOI] [PubMed] [Google Scholar]
- 203.Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, Kitas GD. Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 47: 1286–1298, 2008. doi: 10.1093/rheumatology/ken159. [DOI] [PubMed] [Google Scholar]
- 204.Park JK, Fischer R, Dechend R, Shagdarsuren E, Gapeljuk A, Wellner M, Meiners S, Gratze P, Al-Saadi N, Feldt S, Fiebeler A, Madwed JB, Schirdewan A, Haller H, Luft FC, Müller DN. p38 mitogen-activated protein kinase inhibition ameliorates angiotensin II-induced target organ damage. Hypertension 49: 481–489, 2007. doi: 10.1161/01.HYP.0000256831.33459.ea. [DOI] [PubMed] [Google Scholar]
- 205.Parra G, Quiroz Y, Salazar J, Bravo Y, Pons H, Chavez M, Johnson RJ, Rodriguez-Iturbe B. Experimental induction of salt-sensitive hypertension is associated with lymphocyte proliferative response to HSP70. Kidney Int Suppl 74: S55–S59, 2008. doi: 10.1038/ki.2008.513. [DOI] [PubMed] [Google Scholar]
- 206.Pascual DW, Jin HK, Bost KL, Oparil S. Interleukin-2 does not attenuate hypertension in spontaneously hypertensive rats. Hypertension 16: 468–471, 1990. doi: 10.1161/01.HYP.16.4.468. [DOI] [PubMed] [Google Scholar]
- 207.Peeters AC, Netea MG, Janssen MC, Kullberg BJ, Van der Meer JW, Thien T. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Invest 31: 31–36, 2001. doi: 10.1046/j.1365-2362.2001.00743.x. [DOI] [PubMed] [Google Scholar]
- 208.Pei C, Barbour M, Fairlie-Clarke KJ, Allan D, Mu R, Jiang H-R. Emerging role of interleukin-33 in autoimmune diseases. Immunology 141: 9–17, 2014. doi: 10.1111/imm.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 5: 202–207, 2014. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegård J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens 20: 1815–1820, 2002. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 211.Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, Rodriguez-Iturbe B. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol 304: F289–F299, 2013. doi: 10.1152/ajprenal.00517.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Prakken BJ, Wendling U, van der Zee R, Rutten VP, Kuis W, van Eden W. Induction of IL-10 and inhibition of experimental arthritis are specific features of microbial heat shock proteins that are absent for other evolutionarily conserved immunodominant proteins. J Immunol 167: 4147–4153, 2001. doi: 10.4049/jimmunol.167.8.4147. [DOI] [PubMed] [Google Scholar]
- 213.Quiroz Y, Pons H, Gordon Kl, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ, Rodríguez-Iturbe B. Mycophenolate mofetil prevents the salt-sensitive hypertension resulting from short-term nitric oxide synthesis inhibition. Am J Physiol Renal Physiol 281: F38–F47, 2001. [DOI] [PubMed] [Google Scholar]
- 214.Rabkin SW. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat Clin Pract Cardiovasc Med 6: 192–199, 2009. doi: 10.1038/ncpcardio1453. [DOI] [PubMed] [Google Scholar]
- 215.Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 20: 298–303, 1992. doi: 10.1161/01.HYP.20.3.298. [DOI] [PubMed] [Google Scholar]
- 216.Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis 3: e403, 2012. doi: 10.1038/cddis.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rodriguez-Iturbe B. Autoimmunity in the pathogenesis of hypertension. Hypertension 67: 477–483, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06418. [DOI] [PubMed] [Google Scholar]
- 218.Rodríguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther 315: 51–57, 2005. doi: 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]
- 219.Rodriguez-Iturbe B, Franco M, Johnson RJ. Impaired pressure natriuresis is associated with interstitial inflammation in salt-sensitive hypertension. Curr Opin Nephrol Hypertens 22: 37–44, 2013. doi: 10.1097/MNH.0b013e32835b3d54. [DOI] [PubMed] [Google Scholar]
- 220.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 221.Rodríguez-Iturbe B, Quiroz Y, Ferrebuz A, Parra G, Vaziri ND. Evolution of renal interstitial inflammation and NF-kappaB activation in spontaneously hypertensive rats. Am J Nephrol 24: 587–594, 2004. doi: 10.1159/000082313. [DOI] [PubMed] [Google Scholar]
- 222.Rodríguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201, 2002. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 223.Rodríguez-Iturbe B, Zhan C-D, Quiroz Y, Sindhu RK, Vaziri ND. Antioxidant-rich diet relieves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension 41: 341–346, 2003. doi: 10.1161/01.HYP.0000052833.20759.64. [DOI] [PubMed] [Google Scholar]
- 224.Rudemiller NP, Crowley SD. Interactions between the immune and the renin-angiotensin systems in hypertension. Hypertension 68: 289–296, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL, PhysGen Knockout Program . CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 65: 1111–1117, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rudofsky UH, Dilwith RL, Roths JB, Lawrence DA, Kelley VE, Magro AM. Differences in the occurrence of hypertension among (NZB X NZW)F1, MRL-lpr, and BXSB mice with lupus nephritis. Am J Pathol 116: 107–114, 1984. [PMC free article] [PubMed] [Google Scholar]
- 228.Ryan MJ. Immune mechanisms in hypertension. In: Colloquium Series on Integrated system Physiology, edited by Granger ND, Granger J. San Rafael, CA: Morgan & Claypool Life Sciences, 2013, p. 33–44. [Google Scholar]
- 229.Ryan MJ, McLemore GR Jr. Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 292: R736–R742, 2007. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 229a.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, Mediavilla JD, Jiménez-Jáimez J, Díaz-Chamorro A, Jiménez-Alonso J, Grupo Lupus Virgen de las Nieves . Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol 38: 1026–1032, 2011. doi: 10.3899/jrheum.101132. [DOI] [PubMed] [Google Scholar]
- 230.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sander M, Hansen PG, Victor RG. Sympathetically mediated hypertension caused by chronic inhibition of nitric oxide. Hypertension 26: 691–695, 1995. doi: 10.1161/01.HYP.26.4.691. [DOI] [PubMed] [Google Scholar]
- 232.Sassard J, Lo M, Liu KL. Lyon genetically hypertensive rats: an animal model of “low renin hypertension”. Acta Pharmacol Sin 24: 1–6, 2003. [PubMed] [Google Scholar]
- 233.Schiffrin EL. Mechanisms of remodelling of small arteries, antihypertensive therapy and the immune system in hypertension. Clin Invest Med 38: E394–E402, 2015. [DOI] [PubMed] [Google Scholar]
- 234.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol 27: 2576–2581, 2007. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 235.Schroder K, Tschopp J. The inflammasomes. Cell 140: 821–832, 2010. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 236.Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP, Multicenter AIDS Cohort Study . Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 19: 953–960, 2005. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 237.Sela S, Mazor R, Amsalam M, Yagil C, Yagil Y, Kristal B. Primed polymorphonuclear leukocytes, oxidative stress, and inflammation antecede hypertension in the Sabra rat. Hypertension 44: 764–769, 2004. doi: 10.1161/01.HYP.0000144480.10207.34. [DOI] [PubMed] [Google Scholar]
- 238.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA 290: 2945–2951, 2003. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 239.Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension 49: 304–310, 2007. doi: 10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]
- 240.Shagdarsuren E, Wellner M, Braesen JH, Park JK, Fiebeler A, Henke N, Dechend R, Gratze P, Luft FC, Müller DN. Complement activation in angiotensin II-induced organ damage. Circ Res 97: 716–724, 2005. doi: 10.1161/01.RES.0000182677.89816.38. [DOI] [PubMed] [Google Scholar]
- 241.Shah KH, Shi P, Giani JF, Janjulia T, Bernstein EA, Li Y, Zhao T, Harrison DG, Bernstein KE, Shen XZ. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ Res 117: 858–869, 2015. doi: 10.1161/CIRCRESAHA.115.306539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42: 31–38, 2003. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- 243.Sheu WHH, Lee WJ, Chang RL, Chen YT. Plasma tumor necrosis factor alpha levels and insulin sensitivity in hypertensive subjects. Clin Exp Hypertens 22: 595–606, 2000. doi: 10.1081/CEH-100100094. [DOI] [PubMed] [Google Scholar]
- 244.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension 56: 297–303, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Smart YC, Gillies AH, Waga SW, Carney SL, Smith AJ, Burton RC. Effects of captopril on circulating T lymphocyte subsets. Int J Clin Pharmacol Ther Toxicol 25: 389–391, 1987. [PubMed] [Google Scholar]
- 246.Smith GD, Lawlor DA, Harbord R, Timpson N, Rumley A, Lowe GD, Day IN, Ebrahim S. Association of C-reactive protein with blood pressure and hypertension: life course confounding and mendelian randomization tests of causality. Arterioscler Thromb Vasc Biol 25: 1051–1056, 2005. doi: 10.1161/01.ATV.0000160351.95181.d0. [DOI] [PubMed] [Google Scholar]
- 247.Sollinger D, Eißler R, Lorenz S, Strand S, Chmielewski S, Aoqui C, Schmaderer C, Bluyssen H, Zicha J, Witzke O, Scherer E, Lutz J, Heemann U, Baumann M. Damage-associated molecular pattern activated Toll-like receptor 4 signalling modulates blood pressure in l-NAME-induced hypertension. Cardiovasc Res 101: 464–472, 2014. doi: 10.1093/cvr/cvt265. [DOI] [PubMed] [Google Scholar]
- 248.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol 34: 685–715, 1958. [PMC free article] [PubMed] [Google Scholar]
- 249.Sparks MA, Stegbauer J, Chen D, Gomez JA, Griffiths RC, Azad HA, Herrera M, Gurley SB, Coffman TM. Vascular type 1A angiotensin II receptors control blood pressure by regulating renal blood flow and urinary sodium excretion. J Am Soc Nephrol 26: 2953–2962, 2015. doi: 10.1681/ASN.2014080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Spradley FT, De Miguel C, Hobbs J, Pollock DM, Pollock JS. Mycophenolate mofetil prevents high-fat diet-induced hypertension and renal glomerular injury in Dahl SS rats. Physiol Rep 1: e00137, 2013. doi: 10.1002/phy2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS One 8: e63847, 2013. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-α in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 51: 1345–1351, 2008. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Srivastava K, Narang R, Bhatia J, Saluja D. Expression of heat shock protein 70 gene and its correlation with inflammatory markers in essential hypertension. PLoS One 11: e0151060, 2016. doi: 10.1371/journal.pone.0151060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int 33: 1119–1129, 1988. doi: 10.1038/ki.1988.120. [DOI] [PubMed] [Google Scholar]
- 255.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int 68: 2180–2188, 2005. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 256.Sturgis LC, Cannon JG, Schreihofer DA, Brands MW. The role of aldosterone in mediating the dependence of angiotensin hypertension on IL-6. Am J Physiol Regul Integr Comp Physiol 297: R1742–R1748, 2009. doi: 10.1152/ajpregu.90995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Suga SI, Phillips MI, Ray PE, Raleigh JA, Vio CP, Kim YG, Mazzali M, Gordon KL, Hughes J, Johnson RJ. Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am J Physiol Renal Physiol 281: F620–F629, 2001. [DOI] [PubMed] [Google Scholar]
- 258.Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, Park JR, Kim SW. High sensitivity C-reactive protein as an independent risk factor for essential hypertension. Am J Hypertens 16: 429–433, 2003. doi: 10.1016/S0895-7061(03)00566-1. [DOI] [PubMed] [Google Scholar]
- 259.Surana NK, Kasper DL. Deciphering the tête-à-tête between the microbiota and the immune system. J Clin Invest 124: 4197–4203, 2014. doi: 10.1172/JCI72332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Svendsen UG. Studies elucidating the importance of thymus on the degree of increased blood pressure and vascular disease in renal hypertensive mice. A comparison of the disease in nude and haired littermates. Acta Pathol Microbiol Scand A 83: 568–572, 1975. [DOI] [PubMed] [Google Scholar]
- 261.Svendsen UG. The role of thymus for the development and prognosis of hypertension and hypertensive vascular disease in mice following renal infarction. Acta Pathol Microbiol Scand A 84: 235–243, 1976. [DOI] [PubMed] [Google Scholar]
- 262.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A 84: 523–528, 1976. [DOI] [PubMed] [Google Scholar]
- 263.Svendsen UG. Spontaneous hypertension and hypertensive vascular disease in the NZB strain of mice. Acta Pathol Microbiol Scand A 85: 548–554, 1977. [DOI] [PubMed] [Google Scholar]
- 264.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669, 2000. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 265.Taherzadeh Z, VanBavel E, de Vos J, Matlung HL, van Montfrans G, Brewster LM, Seghers L, Quax PH, Bakker EN. Strain-dependent susceptibility for hypertension in mice resides in the natural killer gene complex. Am J Physiol Heart Circ Physiol 298: H1273–H1282, 2010. doi: 10.1152/ajpheart.00508.2009. [DOI] [PubMed] [Google Scholar]
- 266.Takeichi N, Ba D, Kobayashi H. Natural cytotoxic autoantibody against thymocytes in spontaneously hypertensive rats. Cell Immunol 60: 181–190, 1981. doi: 10.1016/0008-8749(81)90258-6. [DOI] [PubMed] [Google Scholar]
- 267.Takeichi N, Suzuki K, Okayasu T, Kobayashi H. Immunological depression in spontaneously hypertensive rats. Clin Exp Immunol 40: 120–126, 1980. [PMC free article] [PubMed] [Google Scholar]
- 268.Takesue K, Kishi T, Hirooka Y, Sunagawa K. Activation of microglia within paraventricular nucleus of hypothalamus is NOT involved in maintenance of established hypertension. J Cardiol 69: 84–88, 2017. doi: 10.1016/j.jjcc.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 269.Thompson JA, Webb RC. Potential role of Toll-like receptors in programming of vascular dysfunction. Clin Sci (Lond) 125: 19–25, 2013. doi: 10.1042/CS20120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Thorand B, Kolb H, Baumert J, Koenig W, Chambless L, Meisinger C, Illig T, Martin S, Herder C. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984-2002. Diabetes 54: 2932–2938, 2005. doi: 10.2337/diabetes.54.10.2932. [DOI] [PubMed] [Google Scholar]
- 271.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD Jr. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 292: H1018–H1025, 2007. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- 272.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD Jr. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 273.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD Jr. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension 45: 934–939, 2005. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 274.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 298: R713–R719, 2010. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]
- 275.Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, Dietsch P, Hilgers KF. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol 287: H203–H208, 2004. doi: 10.1152/ajpheart.01237.2003. [DOI] [PubMed] [Google Scholar]
- 276.Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology 110: 1–9, 2003. doi: 10.1046/j.1365-2567.2003.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122: 339–352, 2004. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 278.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64: 1108–1115, 2014. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tsao DA, Yu HS, Cheng JT, Ho CK, Chang HR. The change of beta-adrenergic system in lead-induced hypertension. Toxicol Appl Pharmacol 164: 127–133, 2000. doi: 10.1006/taap.1999.8871. [DOI] [PubMed] [Google Scholar]
- 280.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215, 2010. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 281.Tschopp TB, Zucker MB. Hereditary defect in platelet function in rats. Blood 40: 217–226, 1972. [PubMed] [Google Scholar]
- 282.Tuttle RS, Boppana DP. Antihypertensive effect of interleukin-2. Hypertension 15: 89–94, 1990. doi: 10.1161/01.HYP.15.1.89. [DOI] [PubMed] [Google Scholar]
- 283.Uehara Y, Hirawa N, Kawabata Y, Akie Y, Ichikawa A, Funahashi N, Omata M. Immunosuppressant HR-325 attenuates progression of malignant arteritis in the kidney of Dahl salt-sensitive rats. Hypertens Res 20: 91–97, 1997. doi: 10.1291/hypres.20.91. [DOI] [PubMed] [Google Scholar]
- 284.Valente AJ, Yoshida T, Murthy SN, Sakamuri SS, Katsuyama M, Clark RA, Delafontaine P, Chandrasekar B. Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18. Am J Physiol Heart Circ Physiol 303: H282–H296, 2012. doi: 10.1152/ajpheart.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Van Heuven-Nolsen D, De Kimpe SJ, Muis T, van Ark I, Savelkoul H, Beems RB, van Oosterhout AJ, Nijkamp FP. Opposite role of interferon-gamma and interleukin-4 on the regulation of blood pressure in mice. Biochem Biophys Res Commun 254: 816–820, 1999. doi: 10.1006/bbrc.1998.8742. [DOI] [PubMed] [Google Scholar]
- 286.Vanegas V, Ferrebuz A, Quiroz Y, Rodríguez-Iturbe B. Hypertension in Page (cellophane-wrapped) kidney is due to interstitial nephritis. Kidney Int 68: 1161–1170, 2005. doi: 10.1111/j.1523-1755.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 288.Vaziri ND, Lin CY, Farmand F, Sindhu RK. Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension. Kidney Int 63: 186–194, 2003. doi: 10.1046/j.1523-1755.2003.00711.x. [DOI] [PubMed] [Google Scholar]
- 289.Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 582–593, 2006. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 290.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56: 643–649, 2010. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296: R1282–R1289, 2009. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Verhagen AMG, Rabelink TJ, Braam B, Opgenorth TJ, Gröne HJ, Koomans HA, Joles JA. Endothelin A receptor blockade alleviates hypertension and renal lesions associated with chronic nitric oxide synthase inhibition. J Am Soc Nephrol 9: 755–762, 1998. [DOI] [PubMed] [Google Scholar]
- 293.Viel EC, Lemarié CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol 298: H938–H944, 2010. doi: 10.1152/ajpheart.00707.2009. [DOI] [PubMed] [Google Scholar]
- 294.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122: 2529–2537, 2010. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Vivier E, van de Pavert SA, Cooper MD, Belz GT. The evolution of innate lymphoid cells. Nat Immunol 17: 790–794, 2016. doi: 10.1038/ni.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Vonend O, Turner CM, Chan CM, Loesch A, Dell’Anna GC, Srai KS, Burnstock G, Unwin RJ. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int 66: 157–166, 2004. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 297.Wade B, Abais-Battad JM, Mattson DL. Role of immune cells in salt-sensitive hypertension and renal injury. Curr Opin Nephrol Hypertens 25: 22–27, 2016. doi: 10.1097/MNH.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wallukat G, Schimke I. Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Semin Immunopathol 36: 351–363, 2014. doi: 10.1007/s00281-014-0425-9. [DOI] [PubMed] [Google Scholar]
- 299.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 300.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 206: 751–760, 2009. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wei F, Jia XJ, Yu SQ, Gu Y, Wang L, Guo XM, Wang M, Zhu F, Cheng X, Wei YM, Zhou ZH, Fu M, Liao YH, SOT-AT1 Study Group . Candesartan versus imidapril in hypertension: a randomised study to assess effects of anti-AT1 receptor autoantibodies. Heart 97: 479–484, 2011. doi: 10.1136/hrt.2009.192104. [DOI] [PubMed] [Google Scholar]
- 302.Weisheit CK, Engel DR, Kurts C. Dendritic cells and macrophages: sentinels in the kidney. Clin J Am Soc Nephrol 10: 1841–1851, 2015. doi: 10.2215/CJN.07100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol 164: 2711–2717, 2000. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- 304.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381, 2011. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 305.White FN, Grollman A. Autoimmune factors associated with infarction of the kidney. Nephron 1: 93–102, 1964. doi: 10.1159/000179322. [DOI] [PubMed] [Google Scholar]
- 306.Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Müller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123: 2803–2815, 2013. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 308.Worou ME, Liao TD, D’Ambrosio M, Nakagawa P, Janic B, Peterson EL, Rhaleb NE, Carretero OA. Renal protective effect of N-acetyl-seryl-aspartyl-lysyl-proline in dahl salt-sensitive rats. Hypertension 66: 816–822, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wu KLH, Chan SHH, Chan JYH. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation 9: 212–227, 2012. doi: 10.1186/1742-2094-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wu T, Ma J, Chen S, Sun Y, Xiao C, Gao Y, Wang R, Poudrier J, Dargis M, Currie RW, Tanguay RM. Association of plasma antibodies against the inducible Hsp70 with hypertension and harsh working conditions. Cell Stress Chaperones 6: 394–401, 2001. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ II, Madhur MS, Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest 126: 50–67, 2016. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517, 2013. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res 113: 78–87, 2013. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J II, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res 117: 547–557, 2015. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Xiao J, Zhang XL, Fu C, Han R, Chen W, Lu Y, Ye Z. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med 35: 1347–1354, 2015. doi: 10.3892/ijmm.2015.2148. [DOI] [PubMed] [Google Scholar]
- 316.Yaguas K, Bautista R, Quiroz Y, Ferrebuz A, Pons H, Franco M, Vaziri ND, Rodriguez-Iturbe B. Chronic sildenafil treatment corrects endothelial dysfunction and improves hypertension. Am J Nephrol 31: 283–291, 2010. doi: 10.1159/000279307. [DOI] [PubMed] [Google Scholar]
- 317.Yamori Y, Ikeda K, Kulakowski EC, McCarty R, Lovenberg W. Enhanced sympathetic-adrenal medullary response to cold exposure in spontaneously hypertensive rats. J Hypertens 3: 63–66, 1985. doi: 10.1097/00004872-198502000-00010. [DOI] [PubMed] [Google Scholar]
- 318.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yatim KM, Gosto M, Humar R, Williams AL, Oberbarnscheidt MH. Renal dendritic cells sample blood-borne antigen and guide T-cell migration to the kidney by means of intravascular processes. Kidney Int 90: 818–827, 2016. doi: 10.1016/j.kint.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 320.Youn J-C, Yu HT, Lim BJ, Koh MJ, Lee J, Chang D-Y, Choi YS, Lee S-H, Kang S-M, Jang Y, Yoo OJ, Shin E-C, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62: 126–133, 2013. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 321.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364: 937–952, 2004. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 322.Zambrano-Zaragoza JF, Romo-Martínez EJ, Durán-Avelar MJ, García-Magallanes N, Vibanco-Pérez N. Th17 cells in autoimmune and infectious diseases. Int J Inflamm 2014: 651503, 2014. doi: 10.1155/2014/651503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zewinger S, Schumann T, Fliser D, Speer T. Innate immunity in CKD-associated vascular diseases. Nephrol Dial Transplant 31: 1813–1821, 2016. doi: 10.1093/ndt/gfv358. [DOI] [PubMed] [Google Scholar]
- 324.Zhang J, Crowley SD. Role of T lymphocytes in hypertension. Curr Opin Pharmacol 21: 14–19, 2015. doi: 10.1016/j.coph.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhang JD, Patel MB, Griffiths R, Dolber PC, Ruiz P, Sparks MA, Stegbauer J, Jin H, Gomez JA, Buckley AF, Lefler WS, Chen D, Crowley SD. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest 124: 2198–2203, 2014. doi: 10.1172/JCI61368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD. Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64: 1275–1281, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, Spurney RF, Coffman TM, Crowley SD. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res 110: 1604–1617, 2012. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 23: 360–368, 2016. doi: 10.1016/j.cmet.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Zhou X, Fukuda N, Matsuda H, Endo M, Wang X, Saito K, Ueno T, Matsumoto T, Matsumoto K, Soma M, Kobayashi N, Nishiyama A. Complement 3 activates the renal renin-angiotensin system by induction of epithelial-to-mesenchymal transition of the nephrotubulus in mice. Am J Physiol Renal Physiol 305: F957–F967, 2013. doi: 10.1152/ajprenal.00344.2013. [DOI] [PubMed] [Google Scholar]
- 330.Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453: 236–240, 2008. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zhu F, Sun Y, Wang M, Ma S, Chen X, Cao A, Chen F, Qiu Y, Liao Y. Correlation between HLA-DRB1, HLA-DQB1 polymorphism and autoantibodies against angiotensin AT(1) receptors in Chinese patients with essential hypertension. Clin Cardiol 34: 302–308, 2011. doi: 10.1002/clc.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002. doi: 10.1161/01.RES.0000043501.47934.FA. [DOI] [PubMed] [Google Scholar]
- 333.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 334.Zirlik A, Abdullah SM, Gerdes N, MacFarlane L, Schönbeck U, Khera A, McGuire DK, Vega GL, Grundy S, Libby P, de Lemos JA. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol 27: 2043–2049, 2007. doi: 10.1161/ATVBAHA.107.149484. [DOI] [PubMed] [Google Scholar]