Abstract
Four new flavones with modified prenyl groups, namely (E)-5-hydroxytephrostachin (1), purleptone (2), (E)-5-hydroxyanhydrotephrostachin (3), and terpurlepflavone (4), along with seven known compounds (5–11), were isolated from the CH2Cl2/MeOH (1:1) extract of the stem of Tephrosia purpurea subsp. leptostachya, a widely used medicinal plant. Their structures were elucidated on the basis of NMR spectroscopic and mass spectrometric evidence. Some of the isolated compounds showed antiplasmodial activity against the chloroquine-sensitive D6 strains of Plasmodium falciparum, with (E)-5-hydroxytephrostachin (1) being the most active, IC50 1.7 ± 0.1 μM, with relatively low cytotoxicity, IC50 > 21 μM, against four cell-lines.
Keywords: Tephrosia purpurea subsp. leptostachya, stem, flavone, antiplasmodial, cytotoxicity
1. Introduction
Tephrosia purpurea (family Leguminosae) is one of the most widely distributed Tephrosia species and is found in tropical, subtropical, and other arid parts of the world. It consists of the four subspecies purpurea, leptostachya, appolinea, and barbigera, and four varieties, namely under subsp. leptostachya var. leptostachya and var. pubescens, and under subsp. barbigera var. barbigera and var. rufescens [1,2,3,4,5]. In Africa, a decoction of roots, leaves, and fruits of Tephrosia purpurea is given as a diuretic, for blood purification, and for the treatment of a cough and cold [6]. Its macerated leaves are used for curing diarrhoea and whooping cough in children [6]. In East Africa, its roots are used against stomach pains, while its leaves are used to treat snake bites and headaches. A decoction of its leaves and roots is used as a purgative [7], whereas that of the roots of T. purpurea subsp. leptostachya is employed for the treatment of schistosomiasis [6].
Phytochemical studies on T. purpurea collected from different parts of the world have resulted in the isolation of a wide variety of flavonoids; flavones [8,9], rotenoids [10], chalcones [11], and flavanones [12]. The crude extracts and pure compounds obtained from T. purpurea have shown a wide range of biological activities including antiplasmodial [12,13], anticancer [14], antacid [15], antidiabetic [16], analgesic and anti-inflammatory [17], and hepatoprotective [18] activities, and were also shown to be applicable to treat Helicobacter pylori infection [19]. Despite the presence of several subspecies and varieties of the taxa T. purpurea, the ethnobotanical, bioactivity, and phytochemical reports available so far have not been specific on the particular subspecies and variety. In order to better understand the relationship between T. purpurea and other species, the chemical variability among its subspecies and varieties has to be documented. With this in mind, the first phytochemical and biological report on T. purpurea subsp. leptostachya is reported here.
2. Results and Discussion
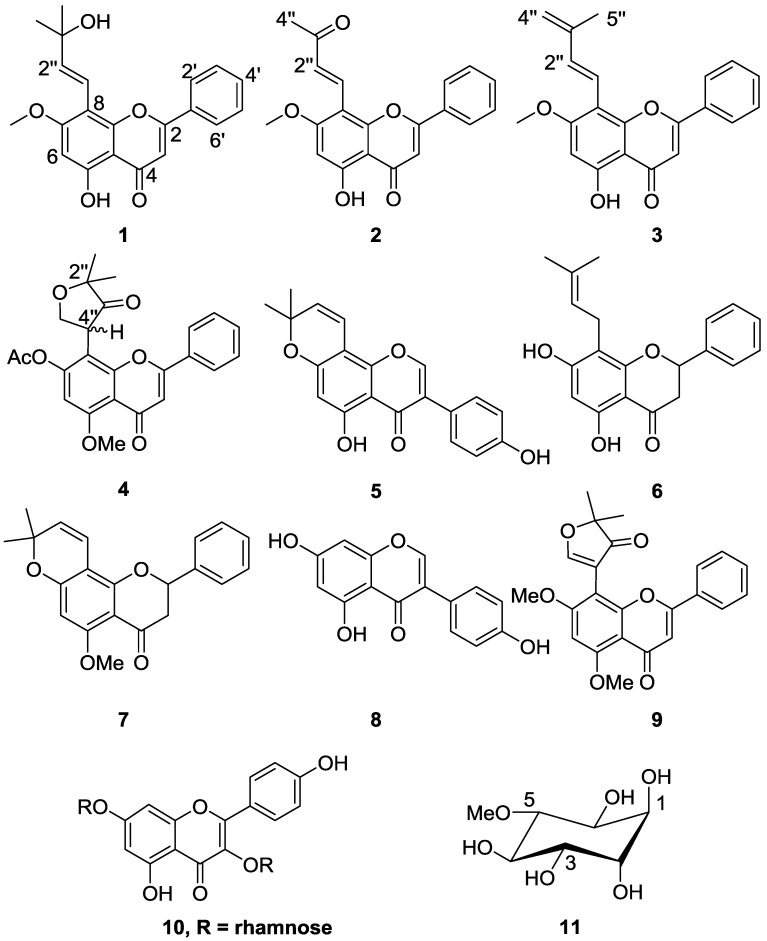
Extraction of the air dried stem of T. purpurea subsp. leptostachya with CH2Cl2/MeOH (1:1) at room temperature, followed by a combination of chromatographic separations, gave four new (1–4) and seven known (5–11) compounds (Figure 1).
Figure 1.
Structures of compounds isolated from T. purpurea subsp. leptostachya.
Compound 1 was isolated as yellow crystals, and its molecular formula C21H20O5 was established from HRMS (m/z 352.1315) and 1H- and 13C-NMR data (Table 1, Figures S1–S6). The UV (λmax 230, 270 and 310 nm), 1H (δH 6.67 for H-3), and 13C (δC 164.2 for C-2, 105.5 for C-3, and 182.9 for C-3) NMR spectral data suggested that this compound is a flavone derivative substituted with methoxy (δH 3.92; δC 56.1), hydrogen bonded hydroxyl (δH 13.08), and 2-methylbut-3-en-2-ol (Table 1, Tables S1–S6) substituents. The HMBC correlation of H-3 (δH 6.67) with C-2 (δC 164.2), C-4 (δC 182.9), and C-4a (δC 105.2) further supported the proposed flavone structure. Three sets of mutually coupled protons resonating at δH 7.91 (H-2′/6′), 7.52 (H-3′/5′), and 7.55 (H-4′) with corresponding carbons at δC 126.5 (C-2′/6′), 129.1 (C-3′/5′), and 131.9 (C-4′), respectively, were assigned to ring-B, which is unsubstituted (Table 1). The 1H-NMR data (Table 1) of 1 possesses a singlet at δH 6.40 (δC 95.3) on ring-A, which is hence trisubstituted with a methoxy (at C-7), a hydrogen bonded hydroxy (at C-5), and a (E)-2-methylbut-3-en-2-ol group. The HMBC correlations of the singlet at δH 6.40 with C-4a (δC 105.2), C-5 (δC 161.3), C-7 (δC 163.1), and C-8 (δC 105.3) allowed its assignment to H-6. Based on HMBC correlations, the methoxy group (δH 3.92, δC 56.1) was placed at C-7 (δC 163.1) and the hydrogen bonded hydroxy group (δH 13.08) at C-5, and the 2-methylbut-3-en-2-ol group could only be placed at C-8. This regiochemistry was confirmed by the HMBC correlation of OH-5 (δH 13.08) to C-4a (δC 105.2), C-5 (δC 161.3), and C-6 (δC 95.3)], and of the olefinic proton H-1″ (δH 6.85) to C-7 (δC 163.1) and C-8a (δC 154.1). The J = 16.5 Hz coupling between H-1″ (δH 6.85) and H-2″ (δH 6.70) is consistent with the E-configuration of the double bond of the 2-methylbut-3-en-2-ol group [20]. Therefore, compound 1 was characterized as (E)-5-hydroxy-8-(3-hydroxy-3-methylbut-1-en-1-yl)-7- methoxy-2-phenyl-4H-chromen-4-one. It is a 5-hydroxy derivative of trans-tephrostachin [20] and hence was given the trivial name (E)-5-hydroxytephrostachin.
Table 1.
1H- (800 MHz) and 13C- (200 MHz) NMR data for compounds 1, 2, and 3 (in CDCl3) at 25 °C.
| Position | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δC (ppm) | δH, m (J in Hz) | HMBC (H→C) | δC | δH, m (J in Hz) | HMBC (H→C) | δC | δH, m (J in Hz) | HMBC (H→C) | |
| 2 | 164.2 | 164.6 | 164.2 | ||||||
| 3 | 105.5 | 6.57 s | C-2, C-4, C-4a, C-1′ | 106.2 | 6.74 s | C-2, C-4, C-4a, C-1′ | 105.5 | 6.71 s | C-2, C-4, C-4a, C-1′ |
| 4 | 182.9 | 182.6 | 183.0 | ||||||
| 4a | 105.2 | 105.4 | 105.3 | ||||||
| 5 | 161.3 | 164.2 | 161.4 | ||||||
| 5-OH | 13.08 s | C-4a, C-5, C-6 | 13.41 s | C-4a, C-5, C-6 | 13.11 s | C-4a, C-5, C-6 | |||
| 6 | 95.3 | 6.40 s | C-4a, C-5, C-7, C-8 | 95.6 | 6.40 s | C-4a, C-5, C-7, C-8 | 95.4 | 6.45 s | C-4a, C-5, C-7, C-8 |
| 7 | 163.1 | 165.0 | 163.2 | ||||||
| 8 | 105.3 | 103.4 | 106.0 | ||||||
| 8a | 154.1 | 156.0 | 154.2 | ||||||
| 1′ | 131.5 | 131.5 | 131.5 | ||||||
| 2′,6′ | 126.5 | 7.91 m | C-2, C-4′, C-2′, C-6′ | 126.5 | 7.92 m | C-2, C-4′, C-2′, C-6′ | 126.4 | 7.93 m | C-2, C-4′, C-2′, C-6′ |
| 3′,5′ | 129.1 | 7.52 m | C-1′, C-3′, C-5′ | 129.4 | 7.59 m | C-1′, C-3′, C-5′ | 129.2 | 7.54 m | C-1′, C-3′, C-5′ |
| 4′ | 131.9 | 7.55 m | C-2′, C-6′ | 132.2 | 7.59 m | C-2′, C-6′ | 132.0 | 7.56 m | C-2′, C-6′ |
| 1″ | 114.9 | 6.85, d (16.5) | C-7, C-8a, C-2″, C-3″ | 132.0 | 8.06, d (16.4) | C-7, C-8a, C-2″, C-3″ | 117.5 | 6.83, d (16.5) | C-7, C-8a, C-2″, C-3″ |
| 2″ | 141.3 | 6.70, d (16.5) | C-8, C-3″, 3″-Me2 | 128.8 | 7.18, d (16.4) | C-8, C-3″, C-4″ | 135.4 | 6.29, d (16.5) | C-8, C-3″, C-4″, C-5″ |
| 3″ | 71.5 | 199.1 | 142.9 | ||||||
| 3″-Me2 | 30.0 | 1.50 s | C-2″, C-3″, 3″-Me2 | ||||||
| 4″ | 27.8 | 2.41 s | C-2″, C-3″ | 116.8 | 5.10 s | C-2″, C-3″, C-5″ | |||
| 5″ | 18.2 | 2.06 s | C-2″, C-3″, C-4″ | ||||||
| 7(OMe) | 56.1 | 3.92 s | C-7 | 56.4 | 4.01 s | C-7 | 56.2 | 3.97 s | C-7 |
The molecular formula of compound 2 was established as C20H16O5 from HRMS (m/z 336.0980), and 1H- and 13C-NMR data (Table 1, Figures S9–S13). Its UV spectrum (λmax 230, 290, and 330 nm), along with its NMR spectra (Table 1), suggested that 2 had a flavone skeleton. Its 1H- and 13C-NMR spectra (Table 1) showed high similarities to those of 1. Thus, ring-B of 2 is unsubstituted, while its ring-A is trisubstituted, with a hydroxy at C-5, a methoxy at C-7, and a modified prenyl group at C-8 (Table 1). The 1H-NMR spectral data further suggested the presence of trans-oriented and mutually coupled (J = 16.4 Hz) olefinic protons, which are deshielded (δH 8.06, H-1″, and δH 7.18, H-2″), suggesting a different substituent at C-8 of 2 as compared to 1. Furthermore, a single, deshielded methyl signal (δH 2.41; δC 27.8) was observed, which along with an additional carbonyl signal (δC 199.1) showing HMBC correlations to H-1″ (δH 8.06) and H-2″ (δH 7.18), suggests that the C-8 substituent is the rare (E)-but-3-en-2-one group, similar to that reported for (2S)-5-hydroxy-7-methoxy-8-[(E)-3-oxo-1-butenyl]flavanone [21], and for erylivingstone F [22]. Based on the above spectroscopic data, compound 2 was characterized as (E)-5-hydroxy-7-methoxy-8-(3-oxobut-1-en-1-yl)-2-phenyl-4H-chromen-4-one and was given the trivial name purleptone.
Compound 3 ([M + 1]+ m/z 335.1227, C21H18O4) was also found to be a flavone derivative (λmax 230, 280 and 310 nm), whose 1H- and 13C-NMR spectra (Table 1, Figures S16–S21) showed close similarities to those of 1 and 2. It was found to have an unsubstituted ring-B, and trisubstituted ring-A with hydroxy at C-5, methoxy at C-7, and a modified prenyl group at C-8. The structure of the latter substituent was established to be (E)-3-methylbuta-1,3-dien-1-yl from the 1H- and 13C-NMR spectral data (Table 1), and was confirmed by the HMBC correlations of CH2-4″ (δH 5.10) with C-2″ (δC 135.4), C-3″ (δC 142.9), and C-5″ (δC 18.2). The placement of this group at C-8 was established from the HMBC correlations of H-2″ (δH 6.29) to C-8 (δC 106.0), C-3″ (δC 142.9), C-4″ (δC 116.8), and C-5″ (δC 18.2), and of H-5″ (δH 2.06) with C-2″ (δC 135.4), C-3″ (δC 142.9), and C-4″ (δC 116.8). In agreement with this, H-1″ also showed HMBC correlation with C-7 (δC 163.2), C-8a (δC 154.2), C-2″ (δC 135.4), and C-3″ (δC 142.9). Compound 3 was therefore characterized as (E)-5-hydroxy-7-methoxy-8-(3- methylbuta-1,3-dien-1-yl)-2-phenyl-4H-chromen-4-one, and was given the trivial name (E)-5-hydroxyanhydrotephrostachin as it is structurally closely related to anhydrotephrostachin [20].
The structure of compound 4 ([M + 1]+, m/z 423.1465, C24H22O7), also a flavone, was established from 1H- and 13C-NMR data (Table 2, Figures S24–S29), as well as from its UV spectrum (λmax 230, 260, and 310 nm). Its NMR spectra (Table 2) revealed the presence of an unsubstituted ring-B (δH 7.70, δC 126.3 (H-2′/6′), δH 7.45, δC 128.7 (H-3′/5′), and δH 7.49, δC 131.1 (H-4′ m)), a methoxy (δH 3.96, δC 56.7) at C-5, an acetate [(δH 2.11, δC 21.4 (Me), δC 170.0 (C=O)] at C-7, and a modified prenyl group in the form of a tetrahydrofuran ring at C-8 (Table 2), similar to terpurinflavone [12] and tephroglabrin [23]. The presence of an additional carbonyl (δC 206.1) and two geminal methyl groups (δH 1.57, δC 24.0 and δH 1.65, δC 23.9), and three mutually coupled protons at δH 4.95 (dd, J = 6.1, 10.2 Hz), δH 4.90 (dd, J = 6.1, 8.8) and δH 4.84 (dd, J = 6.1, 8.8 Hz) indicated that the C-8 substituent was a 5,5-dimethyl-4-oxo-tetrahydrofuran-3-yl group. In agreement with this, H-4″ (δH 4.95), H-5″ (δH 4.90), and 2″-(Me)2 (δH 1.57 and 1.65) showed HMBC correlations to the carbonyl carbon C-3″ (δC 206.1). The HMBC correlation of H-4″ (δH 4.95) with C-7; H-6 (δH 6.41) with C-4a (δC 109.1), C-5 (δC 162.9), C-7 (δC 166.3), and C-8 (δC 103.9); and the OMe (δH 3.96) with C-5 (δC 162.9) confirmed the substitution pattern of this ring. The coupling constant J = 10.2 Hz of H-4″ and H-5″ indicated a 1,2-diaxial orientation of these protons [12]. Hence, compound 4 was characterized as 8-(5,5-dimethyl-4-oxotetrahydrofuran-3-yl)-5-methoxy-4-oxo-2-phenyl-4H-chromen-7-yl acetate and was given the trivial name terpurlepflavone.
Table 2.
1H- (800 MHz) and 13C- (200 MHz) spectroscopic data for compound 4 (CDCl3) at 25 °C.
| Position | δC | δH, m (J in Hz) | HMBC (H→C) |
|---|---|---|---|
| 2 | 160.6 | ||
| 3 | 110.1 | 6.55 s | C-2, C-4, C-4a, C-1′ |
| 4 | 177.2 | ||
| 4a | 109.1 | ||
| 5 | 162.9 | ||
| 6 | 91.1 | 6.41 s | C-4a, C-5, C-7, C-8 |
| 7 | 166.3 | ||
| 8 | 103.9 | ||
| 8a | 154.9 | ||
| 1′ | 131.7 | ||
| 2′,6′ | 126.3 | 7.70 m | C-2, C-4′, C-2′, C-6′ |
| 3′,5′ | 128.7 | 7.45 m | C-1′, C-3′, C-5′ |
| 4′ | 131.1 | 7.49 m | C-2′, C-6′ |
| 2″ | 83.9 | ||
| 3″ | 206.1 | ||
| 4″ | 47.7 | 4.95 dd (10.2, 6.1) | C-7, C-8, C-8a, C-2″, C-3″, C-5″ |
| 5″ | 75.8 | 4.90 dd (10.2, 8.8) | C-7, C-8, C-3″, C-4″ |
| 4.84 dd (6.1, 8.8) | C-7, C-8, C-3″, C-4″ | ||
| 2″-Me | 24.0 | 1.57 s | C-2″, C-3″, 2″-Me |
| 2″-Me | 23.9 | 1.65 s | C-2″, C-3″, 2″-Me |
| 5-OMe | 56.7 | 3.96 s | C-5 |
| 7-COMe | 170.0 | ||
| 7-COMe | 21.4 | 2.11 s | 7-COMe |
The known compounds were identified as derrone (5) [24], glabranin (6) [25], obovatin methyl ether (7) [26], genistein (8) [27], tachrosin (9) [28], kaempferitrin (10) [29], and d-pinitol (11) [30] by a comparison of their spectroscopic data (Tables S1 to S7) with that available in the literature. The major flavones of this plant were tested for antiplasmodial activity against the D6 strain of Plasmodium falciparum (Table 3). Among these, (E)-5-hydroxytephrostachin (1) showed good activity, IC50 1.7 μM), while terpurlepflavone (4) and tachrosin (9) showed low antiplasmodial activities. The compounds were also tested for cytotoxicity against two non-tumoral and two cancerous cell-lines (Table 3). Most of these did not show cytotoxicity (IC50 > 100 μM), while compound 1 showed IC50 between 21–100 μM, which is still significatly lower than its antiplasmodial activity with a selectivity index > 12. The results observed here demonstrate the potential of flavones as antiplasmodial agents, parallel to the in vitro and in vivo antiplasmodial activites reported earlier for some flavones [12,31].
Table 3.
In vitro antiplasmodial activity and cytototoxicity of compounds 1, 2, 4 and 9 (IC50, μM).
| Samples | Antiplasmodial Activity against P. falciparum | Cytotoxicity | |||
|---|---|---|---|---|---|
| D6 | LO2 * | BEAS * | A549 ** | HepG2 ** | |
| (E)-5-Hydroxytephrostachin (1) | 1.7 ± 0.1 | 21.7 ± 4.8 | 24.5 ± 2.7 | 76.1 ± 2.9 | >100 |
| Purleptone (2) | NT | >100 | >100 | >100 | >100 |
| Terpurlepflavone (4) | 14.8 ± 3.2 | >100 | >100 | >100 | >100 |
| Tachrosin (9) | 27.1 ± 3.2 | >100 | >100 | >100 | >100 |
| Chloroquine | 0.037 ± 0.003 | ||||
| Artesunate-Mefloquine | 0.075 ± 0.006 | ||||
* Non-tumoral cell: LO2, Immortal human hepatic cell line; BEAS, Lung/bronchus cell line (epithelial virus transformed); ** Cancer cell: A549, adenocarcinomic human alveolar basal epithelial cells; HepG2, human liver cancer cell line; NT = Not Tested.
3. Materials and Methods
3.1. General Experimental Procedure
UV spectra were recorded on a Specord S600 (Analytik Jena AG, Jena, Germany) spectrophotometer. Melting points were obtained on a Büchi Melting point B-545 (Flawil, Switzerland) apparatus, and optical rotations were measured on Perkin Elmer 341-LC (Perkin Elmer, Wellesley, MA, USA), whereas CD experiments were run on a Jasco J-715 spectropolarimeter (Jasco, Corp., Tokyo, Japan). NMR spectra were acquired on a Bruker Avance III HD 800 spectrometer (Bruker BioSpin AG, Fallanden, Switzerland) equipped with a TXO cryogenic probe using the residual solvent peak as the reference. Analytical reversed phase liquid chromatography (RP-HPLC)—mass spectrometry (MS) was performed on a API SCIEX 150 EX Perkin Elmer (Perkin Elmer, Waltham, MA, USA) ESI-MS (30 eV) connected to a Perkin Elmer gradient pump system and a C8 column (120 Å, 4 μm, 4.6 mm × 50 mm) using gradients of acetonitrile/water (CH3CN/H2O) with 1% formic acid (HCOOH) as the mobile phase at a flow rate of 1 mL/min. TLC was carried out on Merck pre-coated silica gel 60 F254 plates (Merck, Darmstadt, Germany). Column chromatography was run on silica gel 60 (70–230 mesh). Gel filtration was done on Sephadex LH-20 (Fluka, Buchs, Switzerland). Preparative HPLC was carried out on a Waters 600E instrument using the Chromulan (Pikron Ltd., Praha, Czech Republic) software and a RP-C8 Kromasil® (250 mm × 55 mm, Kromasil, Bohus, Sweden) column with an H2O/MeOH solvent system for elution. HRESIMS were obtained with a Q-TOF-LC/MS spectrometer (Stenhagen Analyslab AB, Gothenburg, Sweden) using a 2.1 mm × 30 mm, 1.7 μm RPC18 column and a H2O–CH3CN gradient system (5:95–95:5 gradient and 0.2% formic acid).
3.2. Plant Material
The stems of Tephrosia purpurea subsp. leptostachya were collected in April 2015 from the Kilungu hills in Makueni County, Kenya. The plant specimen was identified by Mr. Patrick C. Mutiso of the Herbarium, School of Biological Sciences, University of Nairobi, where a voucher specimen (Mutiso-841/April 2015) was deposited.
3.3. Extraction and Isolation
The air dried and ground stems (2 kg) of T. purpurea subsp. leptostachya were extracted with CH2Cl2/MeOH (1:1) for seven days at 20–25 °C by percolation (3 × 2 L) to yield a dark yellow paste (80 g, 4%). Hence, it was soaked for 24 h with 2 L solvent, filtered, and concentrated using a rotatory evaporator. This procedure was then repeated three times. A portion of the extract (31 g) was subjected to column chromatography over silica gel (300 g) eluting with iso-hexane containing increasing amounts of EtOAc. The fraction that eluted with 3% EtOAc in iso-hexane was purified by gel filtration on Sephadex LH-20 (eluent: CH2Cl2/MeOH; 1:1) to give 2 (16.2 mg, ≥97% purity) and 3 (23.4 mg, ≥97% purity). The eluent with 5% EtOAc in iso-hexane was first separated over Sephadex LH-20 (CH2Cl2/MeOH; 1:1) followed by preparative HPLC (20:80 MeOH/H2O–100% MeOH gradient elution for 20 min with flow rate 8 mL/min) to give 5 (derrone, 28 mg, ≥98% purity) [24], 6 (glabranin, 52 mg, ≥98% purity) [25], 7 (obovatin methyl ether, 47 mg, ≥99% purity) [26], and 8 (genistein, 53 mg, ≥98% purity) [27]. Elution with 6% EtOAc in iso-hexane gave a yellow solid which was recrystallized from CH2Cl2/MeOH (1:1) to give 1 (550 mg, ≥99% purity). Further elution with 8% EtOAc in iso-hexane gave 4 (67.5 mg, ≥99% purity); the eluent with 9% EtOAc in iso-hexane gave 9 (tachrosin, 158 mg, >99% purity) [28]; and the 10% EtOAc in iso-hexane eluent gave 10 (kaempferitrin, 97 mg, >99% purity) [29]. Fraction elution with 15% EtOAc in iso-hexane gave 11 (d-pinitol, 650 mg, >99% purity) [30].
(E)-5-Hydroxytephrostachin (1): Yellow crystals (CH2Cl2/MeOH; 1:1). mpt 160–162 °C. UV λmax (CH2Cl2): 230, 270 and 310 nm. 1H- and 13C-NMR (Table 1). EIMS m/z (rel. int.) 353.6 [M]+ (100). HRMS [M]+ m/z 352.1315 C21H20O5 (Calculated: 352.1311).
Purleptone (2): Colourless amorphous solid. UV λmax (CH2Cl2): 230, 290 and 330 nm.1H- and 13C-NMR (Table 1). EIMS m/z (rel. int.) 337 [M]+ (100). HRMS [M]+ m/z 336.0980 C20H16O5 (Calculated: 336.0998).
(E)-5-Hydroxyanhydrotephrostachin (3): Colourless amorphous solid. UV λmax (CH2Cl2): 230, 280 and 310 nm. 1H- and 13C-NMR (Table 1). EIMS m/z (rel. int.) 336.1276 [M]+. HRMS [M + 1]+ m/z 335.1227 C21H18O4 (Calculated: 335.1283).
Terpurlepflavone (4): White amorphous solid. m.pt 210–214 °C. UV λmax(CH2Cl2): 230, 260 and 310 nm. CD (MeOH) λ nm (Δε; M−1·cm−1): (122.83)221; (−58.17)212.[α]D20 +14.00° (c 0.001, MeOH). 1H- and 13C-NMR (Table 2). EIMS m/z (rel. int.) 423 [M]+. HRMS [M + 1]+ m/z 423.1465 C24H22O7 (Calculated: 423.1444).
3.4. In Vitro Antiplasmodial Activity
The pure compounds were assayed using a non-radioactive assay technique as described by Smilkstein et al., 2004 [32] with modifications given in the literature [12,33].
3.5. Cell Culture
A549, HepG2, and non-tumoral cells were all purchased from ATCC. Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics penicillin (50 U/mL) and streptomycin (50 μg/mL; Invitrogen, Paisley, Scotland, UK). All cell cultures were incubated at 37 °C in a 5% humidified CO2 incubator.
3.6. Cytotoxicity Assay
All tested compounds were dissolved in DMSO at a final concentration of 50 mmol/L and stored at −20 °C before use. Cytotoxicity was assessed by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5.0 mg/mL) assay as previously described [34]. Briefly, 4 × 103 cells per well were seeded in 96-well plates before drug treatments. After overnight culture, the cells were then exposed to different concentrations of selected compounds (0.039–100 μmol/L) for 72 h. Cells without drug treatment were used as the control. Subsequently, MTT (10 μL) solution was added to each well and incubated at 37 °C for 4 h followed by the addition of 100 μL solubilization buffer (10% SDS in 0.01 mol/L HCl) and overnight incubation. A570 nm was then determined in each well on the next day. The percentage of cell viability was calculated using the following formula: Cell viability (%) = Atreated/Acontrol × 100. Data were obtained from three independent experiments and the standard error was calculated.
4. Conclusions
Four new prenylflavones with seven known compounds were isolated from the stem of Tephrosia purpurea subsp. leptostachya. The isolated flavones were tested for antiplasmodial activity against the D6 strain of Plasmodium falciparum. Among these, (E)-5-hydroxytephrostachin (1) showed good activity (IC50 1.7 μM). The compounds were also tested for cytotoxicity against two non-tumoral and two cancerous cell-lines. Most of these did not show cytotoxicity (IC50 > 100 μM), while compound 1 showed IC50 between 21–100 μM.
Acknowledgments
Yoseph Atilaw is grateful to the German Academic Exchange Services (DAAD) for a scholarship which was offered through the Natural Products Research Network for Eastern and Central Africa (NAPRECA). The Swedish Research Council (Swedish Research Links, 2016-05857), the International Science Program (ISP Sweden, grant KEN-02), the Adlerbertska Research Foundation and the Royal Society of Arts and Sciences in Göteborg are acknowledged for financial support. Matthias Heydenreich is thanked for measuring the HRMS for two compounds. Gao Jiaying is acknowledged for technical assistance in the cytotoxicity assay.
Supplementary Materials
The Supplementary Materials are available online. NMR, UV and MS spectra for all new compounds and spectral data for the known compounds are available as Supporting Information.
Author Contributions
The list of authors contributed to this work as follows: Extraction and isolation of compounds was done by Y. Atilaw and L. Muiva-Mutisya; spectroscopic characterization was carried out by Y. Atilaw, A. Yenesew, A. Ndakala, and M. Erdélyi; antiplasmodial activity assays were done by H.M. Akala and R. Yeda; and cytotoxicity assays were done by Y.J. Wu, P. Coghi, and V.K.W. Wong. All authors contributed to the preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of compounds 1, 4–11 are available from the authors.
References
- 1.De Queiroz R.T., Goulart de Azevedo Tozzi A.M., Lewis G.P. Seed morphology: An addition to the taxonomy of Tephrosia (Leguminosae, Papilionoideae, Millettieae) from South America. Plant Syst. Evol. 2013;299:459–470. doi: 10.1007/s00606-012-0735-0. [DOI] [Google Scholar]
- 2.Al-Ghamdi F.A., Al-Zahrani R.M. Seed morphology of some species of Tephrosia Pers. (Fabaceae) from Saudi Arabia Identification of species and systematic significance. Feddes Repert. 2010;121:59–65. doi: 10.1002/fedr.201011128. [DOI] [Google Scholar]
- 3.Hosni H., El-Karemy Z. Systematic revision of Leguminosae in Egypt. 1. Tephrosia Pers. Sendtnera. 1993;1:245–257. [Google Scholar]
- 4.Bosman M.T.M., De Haas A.J.P. A revision of the genus Tephrosia (Leguminosae-Papilionoideae) in Malesia. Blumea-Biodivers. Evol. Biogeogr. Plants. 1983;28:421–487. [Google Scholar]
- 5.Gillett J.B. Notes on Tephrosia in Tropical Africa. Kew Bull. 1958;13:111. doi: 10.2307/4117636. [DOI] [Google Scholar]
- 6.Neuwinger H.D. African Traditional Medicine. MedPharm Scientific Publishers; Stuttgart, Germany: 2000. pp. 515–516. [Google Scholar]
- 7.Kokwaro J.O. Medicinal Plants of East Africa. 3rd ed. University of Nairobi Press; Nairobi, Kenya: 2009. pp. 185–187. [Google Scholar]
- 8.Chang L.C., Daniel C., Song L.L., Fransworth N.R., Pezzuto J.M., Kinghorn A.D. Absolute configuration of novel bioactive flavonoids from Tephrosia purpurea. Org. Lett. 2000;2:515–518. doi: 10.1021/ol990407c. [DOI] [PubMed] [Google Scholar]
- 9.Hegazy M.E., Abd el-Razek M.H., Nagashima F., Asakawa Y., Pare P.W. Rare prenylated flavonoids from Tephrosia purpurea. Phytochemistry. 2009;70:1474–1477. doi: 10.1016/j.phytochem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad V.U., Ali Z., Hussaini S.R., Iqbal F., Zahid M., Abbas M., Saba N. Flavonoids of Tephrosia purpurea. Fitoterapia. 1999;70:443–445. doi: 10.1016/S0367-326X(99)00046-5. [DOI] [Google Scholar]
- 11.Sinha B., Natu A.A., Nanavati D.D. Prenylated favonoids from Tephrosia purpurea seeds. Phytochemistry. 1982;21:1468–1470. doi: 10.1016/0031-9422(82)80177-5. [DOI] [Google Scholar]
- 12.Juma W.P., Akala H.M., Eyase F.L., Muiva L.M., Heydenreich M., Okalebo F.A., Gitu P.M., Peter M.G., Walsh D.S., Imbuga M., et al. Terpurinflavone: An antiplasmodial flavone from the stem of Tephrosia purpurea. Phytochem. Lett. 2011;4:176–178. doi: 10.1016/j.phytol.2011.02.010. [DOI] [Google Scholar]
- 13.Muiva-Mutisya L., Bernard M., Matthias H., Andreas K., Akala H.M., Derese S., Omosa L.K., Yusuf A.O., Edwin K., Yenesew A. 6α-Hydroxy-α-toxicarol and (+)-tephrodin with antiplasmodial activities from Tephrosia species. Phytochem. Lett. 2014;10:179–183. doi: 10.1016/j.phytol.2014.09.002. [DOI] [Google Scholar]
- 14.Gulecha V., Sivakuma T. Anticancer activity of Tephrosia purpurea and Ficus religiosa using MCF 7 cell lines. Asian Pac. J. Trop. Med. 2011;4:526–529. doi: 10.1016/S1995-7645(11)60139-9. [DOI] [PubMed] [Google Scholar]
- 15.Sandhya S., Venkata K.R., Vinod K.R., Rsnakk C. Assessment of in vitro antacid activity of different root extracts of Tephrosia purpurea (L) Pers by modified artificial stomach model. Asian Pac. J. Trop. Med. 2012;2:S1487–S1492. doi: 10.1016/S2221-1691(12)60442-0. [DOI] [Google Scholar]
- 16.Jain A., Nahata A., Santram L., Singhai A.K. Effects of Tephrosia purpurea and Momordica dioica on streptozotocin-induced diabetic nephropathy in rats. Biomed. Prev. Nutr. 2014;4:383–389. doi: 10.1016/j.bionut.2014.06.003. [DOI] [Google Scholar]
- 17.Shenoy S., Shwetha K., Prabhu K., Maradi R., Bairy K.L., Shanbhag T. Evaluation of antiinflammatory activity of Tephrosia purpurea in rats. Asian Pac. J. Trop. Med. 2010;3:193–195. doi: 10.1016/S1995-7645(10)60007-7. [DOI] [Google Scholar]
- 18.Khatri A., Garg A., Agrawal S.S. Evaluation of hepatoprotective activity of aerial parts of Tephrosia purpurea L. and stem bark of Tecomella undulata. J. Ethnopharm. 2009;122:1–5. doi: 10.1016/j.jep.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Chinniah A., Mohapatra S., Goswami S., Mahapatra A., Kar S.K., Mallavadhani U.V., Das P.K. On the potential of Tephrosia purpurea as anti-Helicobacter pylori agent. J. Ethnopharm. 2009;124:642–645. doi: 10.1016/j.jep.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Khalid S.A., Waterman P.G. 8-C-Prenylflavonoids from the seed of Tephrosia bracteolata. Phytochemistry. 1981;20:1719–1720. doi: 10.1016/S0031-9422(00)98562-5. [DOI] [Google Scholar]
- 21.Jang D.S., Park E.J., Kang Y.-H., Hawthorne M.E., Vigo J.S., Graham J.G., Cabieses F., Fong H.H., Mehta R.G., Pezzuto J.M. Potential cancer chemopreventive flavonoids from the stems of Tephrosia toxicaria. J. Nat. Prod. 2003;66:1166–1170. doi: 10.1021/np0302100. [DOI] [PubMed] [Google Scholar]
- 22.Bedane K.G., Kusari S., Masesane I.B., Spiteller M., Majinda R.R. Flavanones of Erythrina livingstoniana with antioxidant properties. Fitoterapia. 2016;108:48–54. doi: 10.1016/j.fitote.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Waterman P.G., Khalid S.A. The major flavonoids of the seed of Tephrosia apollinea. Phytochemsitry. 1980;19:909–915. doi: 10.1016/0031-9422(80)85137-5. [DOI] [Google Scholar]
- 24.Lin C.-F., Liu Y.-W., Kuo Y.-H., Shen C.-C., Chiou W.-F., Chen C.-C. Two new isoflavones from the tubers of Apios taiwanianus. Phytochem. Lett. 2016;15:164–167. doi: 10.1016/j.phytol.2016.01.001. [DOI] [Google Scholar]
- 25.Yuldashev M.P., Batirov E.S., Vdovin A.D., Abdullaev N.D. Flavonoids from the aerial parts of Glycyrrhiza glabra L. Izv. Minist. Obraz. Nauki Resp. Kaz., Nats. Akad. Nauk Resp. Kaz., Ser. Khim. 2000;2:67–71. [Google Scholar]
- 26.Chen Y.-L., Wang Y.-S., Lin Y.-L., Munakata K., Ohta K. Obovatin, obovatin methyl ether and obovatachalcone, new piscicidal flavonoids from Tephrosia obovata. Agric. Biol. Chem. 1978;42:2431–2432. doi: 10.1271/bbb1961.42.2431. [DOI] [Google Scholar]
- 27.Gao J.Y., Jiang Y.L., Niu L.L., Li H.D., Yin W.P. Novel isoflavone from the cockroach Periplaneta americana. Chem. Nat. Compd. 2016;52:413–416. doi: 10.1007/s10600-016-1661-0. [DOI] [Google Scholar]
- 28.Smalberger T., Vleggaar R., De Waal H.L. Tachrosin: A new flavone from Tephrosia polystachyoides. J. S. Afr. Chem. Inst. 1971;24:1–12. [Google Scholar]
- 29.Yin R., Han K., Heller W., Albert A., Dobrev P.I., Zazimalova E., Schaeffner A.R. Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. New Phytol. 2014;201:466–475. doi: 10.1111/nph.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raya-Gonzalez D., Pamatz-Bolanõs T., Rio-Torres R.E.D., Martinez-Munõz R.E., Ron-Echeverria O., Martinez-Pacheco M.M. D-(+)-Pinitol, a component of the heartwood of Enterolobium cyclocarpum (Jacq.) Griseb. Z. Naturforsch. C. 2008;63:922. doi: 10.1515/znc-2008-11-1225. [DOI] [PubMed] [Google Scholar]
- 31.Nogueira C.R., Lopes L.M.X. Antiplasmodial natural products. Molecules. 2011;16:2146–2190. doi: 10.3390/molecules16032146. [DOI] [Google Scholar]
- 32.Smilkstein M., Sriwilaijaroen N., Kelly J.X., Wilairat P., Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okoth D.A., Akala H.M., Johnson J.D., Koorbanally N.A. Alkyl phenols, alkenyl cyclohexenones and other phytochemical constituents from Lannea rivae (chiov) Sacleux (Anacardiaceae) and their bioactivity. Med. Chem. Res. 2016;25:690–703. doi: 10.1007/s00044-016-1521-2. [DOI] [Google Scholar]
- 34.Wong V.K., Li T., Law B.Y., Ma E.D., Yip N.C., Michelangeli F., Law C.K., Zhang M.M., Lam K.Y., Chan P.L., et al. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 2013;4:e720. doi: 10.1038/cddis.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.