Abstract
Having served as a symbolic fruit since ancient times, pomegranate (Punica granatum) has also gained considerable recognition as a functional food in the modern era. A large body of literature has linked pomegranate polyphenols, particularly anthocyanins (ATs) and hydrolyzable tannins (HTs), to the health-promoting activities of pomegranate juice and fruit extracts. However, it remains unclear as to how, and to what extent, the numerous phytochemicals in pomegranate may interact and exert cooperative activities in humans. In this review, we examine the structural and analytical information of the diverse phytochemicals that have been identified in different pomegranate tissues, to establish a knowledge base for characterization of metabolite profiles, discovery of novel phytochemicals, and investigation of phytochemical interactions in pomegranate. We also assess recent findings on the function and molecular mechanism of ATs as well as urolithins, the intestinal microbial derivatives of pomegranate HTs, on human nutrition and health. A better understanding of the structural diversity of pomegranate phytochemicals as well as their bioconversions and bioactivities in humans will facilitate the interrogation of their synergistic/antagonistic interactions and accelerate their applications in dietary-based cancer chemoprevention and treatment in the future.
Keywords: anthocyanin, ellagic acid, ellagitannin, functional food, hydrolyzable tannin, polyphenol, pomegranate, Punica granatum, urolithin
1. Introduction
Cultivation and consumption of pomegranate (Punica granatum) can be dated back to at least 3000 BC. Historically, pomegranate has served as a symbol of fertility and prosperity. In addition, various parts of the pomegranate have been used in traditional medicine for treating a wide variety of illness. Pomegranate fruits have purported use for expelling parasites [1], seeds and fruit peels for treating diarrhea [2,3], flowers for managing diabetes [4], tree barks and roots for stopping bleeding and healing ulcers [5], and leaves for controlling inflammation and treating digestive system disorders [5].
Due to its reported benefits to human health, the pomegranate has drawn great interest from the consumers in recent years. Nowadays, the pomegranate is used for functional food ingredients and dietary supplements in various forms, such as fresh fruit and juice, powdered capsules and tablets that contain extracts of different pomegranate tissues, tea brewed from pomegranate leaves, jam, jelly, juice and wine produced from pomegranate fruits, as well as spices prepared from dried seeds [6].
With the advancement of technologies and the expansion of experimental inquiries into the bioactivities of pomegranate phytochemicals, many new discoveries have been made in this ancient fruit within the last decade. To date, over 1500 articles have been published on the subject “pomegranate”, of which 1259 articles were published between 2006 and 2016. Although the pomegranate produces and accumulates a wide variety of phytochemicals with diverse structures in different tissues (Table 1), investigative efforts thus far have been given mainly to the bioactivities of polyphenols in pomegranate fruits, in particular anthocyanins (ATs) and hydrolyzable tannins (HTs), which are assessed in this review. Specifically, various health-promoting activities of urolithins, a group of phenolic metabolites transformed from ellagic acid (EA) (a hydrolysis product of pomegranate ellagitannins (ETs)) by the human gut microbiota, will be reviewed.
Table 1.
Different classes of phytochemicals identified from pomegranate. Detailed descriptions of the chemical structures, molecular formulas, molecular weights, tissues of identification, and representative references are presented in Supplementary Table S1.
| Classes | Phytochemicals |
|---|---|
| Ellagitannins, gallotannins and derivatives | Brevifolin, Brevifolin carboxylic acid, Brevifolin carboxylic acid 10-monopotassium sulphate, Castalagin, Casuariin, Casuarinin, Corilagin, Isocorilagin, Hippomanin A, Gemin D, Diellagic acid rhamnosyl(1→4) glucopyranoside, 1,2-Di-O-galloyl-4,6-O-(S)-hexahydroxydiphenoyl β-d-glucopyranoside, Ellagic acid, 3,3′-Di-O-methylellagic acid, 3,3′,4′-Tri-O-methylellagic acid, 3-O-Methylellagic acid, 4,4′-Di-O-methylellagic acid, 3′-O-Methyl-3,4-methylenedioxy-ellagic acid, Eschweilenol C (Ellagic acid 4-O-α-l-rhamnopyranoside), Ethyl brevifolincarboxylate, Eucalbanin B, Eucarpanin T1, Pomegraniin A, Pomegraniin B, Gallagic acid, Gallic acid 3-O-β-d-(6′-O-galloyl)-glucopyranoside, 6-O-Galloyl-2,3-(S)-hexahydroxydiphenoyl-d-glucose, 5-Galloylpunicacortein D, 2-O-Galloylpunicalin (2-O-Galloyl-4,6-(S,S)-gallagyl-d-glucose), Granatin A, Granatin B, 2,3-(S)-Hexahydroxydiphenoyl-d-glucose, Lagerstannin B, Lagerstannin C, 3-O-Methylellagic acid 4-O-α-l-rhamnopyranoside, 3,4′-O-Dimethylellagic acid 4-O-α-l-rhamnopyranoside, Oenothein B, Pedunculagin I, Pedunculagin II, 1,2,3,4,6-Penta-O-galloyl-β-d-glucose, 3,4,8,9,10-Pentahydroxydibenzo [b,d] pyran-6-one (Urolithin M-5), Phyllanthusiin E, Pomegranatate, Punicacortein A, Punicacortein B, Punicacortein C, Punicacortein D, Punicafolin, Punicalagin A, Punicalagin B, Punicalin, Punicatannin A, Punicatannin B, Punigluconin, Strictinin [1-O-Galloyl-4,6-(S)-hexahydroxydiphenoyl-d-glucose], Tellimagrandin I, Tercatain [1,4-Di-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-β-glucopyranose], Terminalin (Gallagyl dilactone), 1,2,4,6-Tetra-O-galloyl-β-d-glucose, 1,2,3-Tri-O-galloyl-β-glucopyranose, 1,2,4-Tri-O-galloyl-β-glucopyranose, 1,2,6-Tri-O-galloyl-β-glucopyranose, 1,3,4-Tri-O-galloyl-β-glucopyranose, 1,4,6-Tri-O-galloyl-β-glucopyranose, 3,4,6-Tri-O-galloyl-β-glucopyranose, Valoneic acid dilactone |
| Flavonoids | Hovetrichoside C, Phloretin, Phlorizin, Eriodictyol-7-O-α-l-arabinofuranosyl (1-6)-β-d-glucoside, Granatumflavanyl xyloside, Naringin (Naringenin-7-O-rhamnoglucoside), Naringenin-4′methyl ether 7-O-α-l-arabinofuranosyl(1-6)-β-d-glucoside, Pinocembrin, Punicaflavanol, Apigenin, Apigenin 4′-O-β-glucopyranoside, Luteolin, Luteolin 3′-O-β-glucopyranoside, Luteolin 4′-O-β-glucopyranoside, Cynaroside (Luteolin 7-O-glycoside), Luteolin 3′-O-β-xylopyranoside, Tricetin, Daidzein, Genistein, Amurensin (Noricaritin 7-β-d-glucopyranoside), Kaempferol, Astragalin (Kaempferol 3-O-glucoside), Kaempferol-3-O-rhamnoglucoside, Myricetin, Phellatin, Quercetin, Hirsutrin (Quercetin-3-O-glucoside), Quercimeritrin (Quercetin-7-O-glucoside), Quercetin 3-O-rhamnoside, Rutin (Quercetin-3-O-rutinoside), Quercetin-3,4′-dimethyl ether 7-O-α-l-arabinofuranosyl(1-6)-β-d-glucoside, Cyanidin, Chrysanthemin (Cyanidin-3-O-glucoside), Cyanin (Cyanidin-3,5-di-O-glucoside), Antirrhinin (Cyanidin-3-O-rutinoside), Catechin-cyanidin-3-hexoside, Delphinidin, Myrtillin (Delphinidin-3-O-glucoside), Delphinidin-3,5-di-O-glucoside, Pelargonidin, Callistephin (Pelargonidin-3-O-glucoside), Pelargonin (Pelargonidin-3,5-di-O-glucoside), Catechin, Epicatechin, Epicatechin gallate, Epigallocatechin-3-O-gallate, Gallocatechin-(4→8)-catechin, Gallocatechin-(4→8)-gallocatechin, Catechin-(4→8)-gallocatechin, Procyanidin A2, Procyanidin B1, Procyanidin B2, Procyanidin B3 |
| Lignans | Conidendrin, Isohydroxymatairesinol, Isolariciresinol, Matairesinol, Medioresinol, Phylligenin, Pinoresinol, Secoisolariciresinol, Syringaresinol, Pomegralignan, Punicatannin C |
| Triterpenoids and phytosterols | Asiatic acid, Betulinic acid (Betulic acid), Friedooleanan-3-one (Friedelin), Maslinic acid, Oleanolic acid, Punicanolic acid, Ursolic acid, Campesterol, Cholesterol, Daucosterol, β-Sitosterol, β-Sitosterol laurate, β-Sitosterol myristate, Stigmasterol |
| Alkaloids and indolamines | N-(2′,5′-Dihydroxyphenyl)pyridinium chloride, Hygrine, Norhygrine, Pelletierine, N-Methylpelletierine, Norpseudopelletierine, Pseudopelletierine, 2-(2′-Hydroxypropyl)-∆1piperideine, 2-(2′-Propenyl)-∆1piperideine, Punigratane (2,5-Diheptyl-N-methylpyrrolidine), Sedridine, Melatonin, Serotonin, Tryptamine |
| Fatty acids and lipids | Caproic acid (Hexanoic acid), Caprylic acid (Octanoic acid), Capric acid (Decanoic acid), Lauric acid (Dodecanoic acid), Myristic acid (Tetradecanoic acid), Myristoleic acid (9-cis-Tetradecanoic acid), Palmitic acid (Hexadecanoic acid), Palmitoleic acid (Hexadec-9-enoic acid), Punicic acid (9Z, 11E, 13Z-octadecatrienoic acid), Linoleic acid (cis, cis-9,12-Octadecadienoic acid), α-Linolenic acid (All-cis-9,12,15-octadecatrienoic acid), γ-Linolenic acid (All-cis-6,9,12-octadecatrienoic acid), Oleic acid (9Z-octadecenoic acid), Stearic acid (Octadecanoic acid), α-Eleostearic acid (9Z, 11E, 13E-octadecatrienoic acid), β-Eleostearic acid (9E, 11E, 13E-octadecatrienoic acid), Catalpic acid (9E, 11E, 13Z-octadecatrienoic acid), Arachidic acid (Eicosanoic acid), Gadoleic acid (9Z-icosenoic acid), Behenic acid (Docosanoic acid), Nervonic acid (cis-15-Tetracosenoic acid), 1-O-9E,11Z,13E-Octadecatrienoyl glycerol, 1-O-Isopentyl-3-O-octadec-2-enoyl glycerol, Tri-O-punicylglycerol, Di-O-punicyl-O-octadeca-8Z, 11Z, 13E-trienylglycerol, N-palmitoyl cerebroside |
| Organic acids and phenolic acids | Ascorbic acid, Citric acid, Fumaric acid, l-Malic acid, Oxalic acid, Quinic acid, Succinic acid, Tartaric acid, Caffeic acid, Chlorogenic acid, Cinnamic acid, o-Coumaric acid, p-Coumaric acid, cis-p-Coumaric acid, Coutaric acid, 7,8-Dihydroxy-3-carboxymethylcoumarin-5-carboxylic acid, Ferulic acid, Gallic acid, Methyl gallate, Neochlorogenic acid (5-O-Caffeoylquinic acid), Protocatechuic acid, Vanillic acid, Coniferyl 9-O-[β-d-apiofuranosyl(1→6)]-O-β-d-glucopyranoside, Sinapyl 9-O-[β-d-apiofuranosyl(1→6)]-O-β-d-glucopyranoside |
| Other compounds | Catechol, Coumestrol, Icariside D1, Phenylethylrutinoside, Syringaldehyde |
Development of cutting-edge analytical techniques has enabled the acquisition of large-scale metabolic datasets, which requires careful analysis and interpretation. To facilitate characterization of metabolite profiling data in pomegranate, we examine the phytochemicals that have been identified in pomegranate, including detailed information on the chemical structures, molecular formulas, molecular weights, analytical methods (reflecting confidence/accuracy of the identifications), and tissues of identification (Table 1 and Table S1). Knowledge of phytochemicals present in different pomegranate tissues will also help assess the structural determinants of their bioactivities as well as the additive, antagonistic or synergistic interactions of these phytochemicals in complex mixtures.
2. Occurrence and Structure of Diverse Phytochemicals in Pomegranate
Numerous phytochemicals have been (tentatively) identified in different pomegranate tissues using diode array detection (DAD), electron spin resonance (ESR), fluorescence detection (FD), flame ionization detection (FID), infrared spectroscopy (IR), mass spectrometry (MS), nuclear magnetic resonance (NMR), and thin layer chromatography (TLC; by comparing the Rf values of pomegranate phytochemicals to those of the authentic standards) (Table 1 and Table S1). It should be noted that disparities regarding the presence/absence of phytochemicals in specific tissues have been observed in different pomegranate cultivars [7,8,9,10]. In addition, the quantities of phytochemicals vary among the pomegranate cultivars [11].
2.1. Ellagitannins, Gallotannins, and Their Derivatives
HTs are among the most studied phytochemicals in pomegranate; they can be further grouped into ETs and gallotannins (GTs) based on the different phenolic acids that are esterified to the core cyclic polyol molecule (it is often a glucose molecule). Overall, more than 60 HTs have been identified from pomegranate using MS and/or NMR (Table 1). Pomegranate fruit peel is rich in HTs, particularly ETs. Punicalagin isomers (ETs) constitute up to 85% (w/w) of total tannins extracted from pomegranate fruit peel [12]. EA, methylated EA, and their glycosidic derivatives have also been found in fruit peel and other pomegranate tissues (Table 1). Although punicalagin isomers represent the major ETs in pomegranate roots, they accumulate at much lower levels in roots than fruit peel [13].
Besides fruit peel, pomegranate stem barks are also abundant in HTs and have been used historically in tanneries for making leather. In addition to the HTs identified in fruit peel, stem barks also contain ET C-glycosides, punicacorteins A–D (ETs with a gallagic acid component), and punigluconin (an ET with a gluconic acid core) [14]. The dense inner part of pomegranate tree trunk (i.e., heartwood) contains brevifolin carboxylic acid, EA rutinoside, diellagic acid rutinoside, methyl-EA, methyl-EA rutinoside, punicalin, galloylpunicalin, and galloylpunicacortein D [15,16,17].
The composition of HTs in pomegranate leaves is largely different from that in fruit peel. In leaves, the major HTs are granatins A and B, whereas punicalagins and punicalins are present at negligible levels [18]. Additional ETs with galloyl and/or hexahydroxydiphenoyl (HHDP) substitutions have also been identified in leaves [18,19,20]. Interestingly, derivatives of EA and ETs, including urolithin M-5, brevifolin, and brevifolin carboxylic acid, have been isolated from pomegranate leaves [19].
In pomegranate flowers, EA and two oxidized derivatives of EA, pomegranatate and phyllanthusiin E, were discovered [21]. Punicatannins A and B, two ETs that contain an unusual 3-oxol,3,3a,8b-tetrahydrofuro[3,4-b]benzofuran functional group, together with a structurally related compound isocorilagin, were also found in pomegranate flowers [22]. In addition, brevifolin carboxylic acid, ethylbrevifolin carboxylate, as well as glucose with various galloyl and/or HHDP substitutions, including hippomanin A, gemin D, digalloyl-diHHDP-glucose, trigalloyl glucose, and gallic acid 3-O-β-d-(6′-O-galloyl)-glucopyranoside showed measurable accumulations in pomegranate flowers [21,23,24].
2.2. Flavonoids
Pomegranate fruit peel, aril, and juice are abundant in flavonoids of diverse structures, including the aglycones and glycosides of chalcones, flavanones, flavones, flavonols, ATs, flavan-3-ols, and procyanidins (Table 1). Two flavones, luteolin and tricetin, were found in a methanolic extract of pomegranate flowers [23]. Structures of two flavanones, punicaflavanol (a misnomer; it is a flavanone) and granatumflavanyl xyloside, were elucidated by NMR [25], while hovetrichoside C (a glycoside of an auronol) and phlorizin (a glycoside of the dihydrochalone phloretin) were identified by IR in pomegranate flowers [24].
Similar to other plants, leaves of pomegranate also accumulate high levels of flavone (e.g., apigenin and luteolin) glycosides [19]. Two flavanone diglycosides and one flavonol diglycoside isolated from pomegranate stem barks were shown to be eriodictyol-7-O-α-l-arabinofuranosyl(1-6)-β-d-glucoside, naringenin-4′methyl ether 7-O-α-l-arabinofuranosyl(1-6)-β-d-glucoside, and quercetin-3,4′-dimethyl ether 7-O-α-l-arabinofuranosyl(1-6)-β-d-glucoside, respectively, by NMR analysis [26,27]. High performance liquid chromatography (HPLC)-DAD studies revealed that two isoflavones, genistein and daidzein, as well as a flavonol quercetin, are present in pomegranate seeds [9,28].
2.3. Lignans
Plant lignans are a group of phytoestrogens that can be metabolized into mammalian lignans by the gut microbiota. Furofuran-, dibenzylbutane-, and dibenzylbutyrolactone-type lignans have been identified in different pomegranate tissues based on liquid chromatography (LC)-MSn studies (Table 1), while isolariciresinol is the most abundant lignan present in pomegranate fruit peel [29]. In addition to the above-mentioned lignans, pomegralignan, a dihydrobenzofuran-type neolignan glycoside, was discovered in the aril and fruit peel of pomegranate [30]. Another neolignan, punnicatannin C, was isolated from pomegranate flowers and structurally characterized by NMR analysis [24].
2.4. Triterpenoids and Phytosterols
Triterpenoids (C30) are the biosynthetic precursors of steroids in plants (phytosterols) and animals (steroid hormones). Triterpenoids and phytosterols have been found in pomegranate seed, leaf, flower, fruit peel, and bark tissues (Table 1). The presence of human steroid hormones, including estrone, estriol, estradiol, and testosterone, in pomegranate seeds was reported previously based on TLC separations and colorimetric assays [31,32]. However, HPLC-DAD- and gas chromatography (GC)-MS-based analysis showed that these steroid hormones could not be identified in pomegranate seeds using the more sensitive analytical methods [33].
2.5. Alkaloids and Indolamines
Pelletierine, pseudopelletierine, and N-methylpelletierine comprise the major alkaloids in pomegranate stem and root barks [34]. Sedridine, 2-(2′-hydroxypropyl)-∆1piperideine, 2-(2′-propenyl)-∆1piperideine, norpseudopelletierine, and the pyrrolidine alkaloids (with a five-membered N-containing ring) hygrine and norhygrine, were also found in root barks at low quantities [34]. In addition to the alkaloids that accumulate in root and stem barks, N-(2′,5′-dihydroxyphenyl)pyridinium chloride was identified in pomegranate leaves [19], and a pyrrolidine-type alkaloid punigratane (2,5-diheptyl-N-methylpyrrolidine) was recently characterized in pomegranate fruit peel [35]. Besides alkaloids, low levels of indolamines (amine derivatives of indole), including tryptamine, melatonin, and serotonin, were present in the extract of pomegranate fruit [36].
2.6. Fatty Acids and Lipids
Fatty acids (FAs) of medium (C6, C8, C10, and C12), long (C14, C16, C18, and C20), and very long (C22 and C24) chain length have been identified from pomegranate seeds, juice, and fruit using GC-FID, MS, or NMR analysis (Table 1). The polyunsaturated FA punicic acid (9Z, 11E, 13Z-octadecatrienoic acid) represents the most abundant FA in pomegranate seeds, accounting for over 60% of seed oil. Triacylglycerols (TAGs) containing 9E, 11Z, 13E-octadecatrienoic acid, 3-O-octadec-2-enoic acid, 9Z, 11E, 13Z-octadecatrienoic acid, and 8Z, 11Z, 13E-octadecatrienoic acid are produced in pomegranate seeds and their structures were determined by NMR [37,38,39]. In addition, a glycosphingolipid N-palmitoyl cerebroside was identified from pomegranate (cv. Nana) seed oil by TLC and GC-FID analyses [40].
2.7. Organic Acids and Phenolic Acids
The major organic acids in pomegranate juice are citric acid and malic acid [41]. Pomegranate juice also contains ascorbic acid, fumaric acid, oxalic acid, quinic acid, succinic acid, and tartaric acid, some of which have also been identified in the leaf, fruit peel, and seed tissues [8,9,41,42,43]. Phenolic acids (aromatic acids), primarily benzoic acid and cinnamic acid derivatives, are usually found in pomegranate fruit peel, juice, and flowers (Table 1). In addition, the structure of a substituted coumarin, 7,8-dihydroxy-3-carboxymethylcoumarin-5-carboxylic acid, was characterized in pomegranate flowers by NMR [24].
2.8. Other Compounds
Phenolic compounds that do not belong to HTs, flavonoids, and phenolic acids were also identified in pomegranate juice and seeds using DAD and MS (Table 1). These include catechol (a dihydroxybenzene), coumestrol (a coumestan), syringaldehyde (an aromatic aldehyde), as well as icariside D1 and phenylethylrutinoside (two phenylethanoid glycosides) [28,41,43,44].
3. Interactions of Pomegranate Phytochemicals
The synergy principle of phytochemicals has long been employed in traditional herbal medicine. Multi-target drugs derived from mixtures of plant natural products have increasingly been pursued nowadays to contradict drug tolerance and resistance in cancer therapy. Although several studies (discussed below) have suggested synergistic interactions among pomegranate phytochemicals, this is a promising, but currently under-explored topic.
Fresh arils and juice products of pomegranate fruit, as well as seeds of the soft-seeded cultivars, are mostly consumed. Phytochemicals in pomegranate fruit peel extracts, fermented pomegranate juice, and pomegranate seed oil exhibited cooperative interactions toward limiting the proliferation, metastasis, and invasiveness of human prostate cancer cells in vitro [45]. A subsequent analysis with pure phytochemicals, including EA, caffeic acid, luteolin, and punicic acid, also showed synergistic interactions on suppressing the invasion of prostate cancer cells [46]. Interestingly, commercial pomegranate juice demonstrated more antioxidant and anti-proliferative activities than the purified pomegranate polyphenols in colon cancer cells, suggesting that inherent synergies exist among polyphenols and other phytochemicals in pomegranate juice [47].
When the commercial pomegranate-nectarine juice was separated into predominately sugar, organic acid, neutral phenol, and AT fractions, complex antagonistic or synergistic effects were observed among different fractions on the total phenol or total antioxidant content [48]. The antagonistic or synergistic interactions depended on the concentrations of the chemical constituents in the juice product [49]. The polyphenol extracts of pomegranate fruit also synergistically interacted with the antibacterial drug ciprofloxacin, though various bacterial strains responded differently to the phytochemical-drug synergy, and the underlying mechanism of such synergy remains unknown [50].
4. Functions of Pomegranate ATs in Human Nutrition and Health
ATs are colorful, water-soluble polyphenol pigments that are found in many plant foods, such as berries and pomegranate fruits. Plant ATs are often investigated collectively as a group of phytochemicals for their bioactivities, and have been linked to many aspects of human disease prevention and treatment [51,52,53,54,55,56]. The anti-inflammatory and cardioprotective activities of ATs are attributed by their antioxidant properties via various underlying mechanisms [51]. ATs can quench free radicals, inhibit the activity of xanthine oxidase that generates free radicals, and chelate metal ions that are involved in oxidation of low-density lipoproteins (LDLs) [57]. In addition, ATs induce the expression of nuclear factor-erythroid 2-related factor-2 (Nrf2) that regulates the expression of endogenous antioxidant enzymes, such as hemeoxygenase-1 (HO-1) [51,55]. Besides reactive oxygen species (ROS), ATs also inhibit the production of reactive nitrogen species (RNS), particularly nitric oxide (NO), as well as their associated oxidative processes [58]. Furthermore, release of pro-inflammatory mediators and adhesion molecules is suppressed by ATs via targeting of the respective signaling pathways, e.g., the arachidonic acid and the tumor necrosis factor (TNF)-α, nuclear factor (NF)-κB pathways [59,60].
Aside from cardioprotection, the anti-inflammatory property of ATs also contributes to their anti-obesity effect and action in adipose tissue [53]. Studies using AT-rich fruit extracts showed that ATs prevented the upregulation of inflammatory response in adipose tissue, when triggered with consumption of a high-fat diet, by regulating the NF-κB stress signaling pathway [61]. ATs also reduce the level of pro-inflammatory cytokines (mediators of cell-to-cell communications in immune response) [62] and modulate the expression of adipocytokines (mediators linking adipose tissue, inflammation and immunity) [62,63]. Although studies using cell lines and animal models support the anti-obesity effect of ATs, a causal relationship between AT consumption and reduction of body mass index has yet to be conclusively established by clinical studies [64]. This could be due to one or more parameters that are associated with different clinical studies, such as the amount and types of ATs ingested, the food matrix of ATs, and the inherent difference of the study subjects (e.g., different age groups of the participants) [53].
In addition to their antioxidant and anti-inflammatory activities, ATs act in cancer chemoprevention by inducing terminal differentiation of tumor cells and thus impeding tumorigenesis [65]. Prevention of malignant cell transformation and inhibition of cancer cell proliferation by ATs have also been reported [66]. ATs can further interfere with cancer development by activating caspases and inducing apoptosis of cancer cells [67]. However, the cancer chemotherapeutic effect of ATs still requires strong supporting evidence from clinical studies. In addition, the bioconversion of ATs by human intestinal microbiome should be rigorously investigated.
Similar to other AT-rich fruits, the pomegranate also has a cardioprotective effect by targeting two major causal factors of atherosclerotic lesion and cardiovascular disease, namely the accumulation of cholesterol and oxidized lipids, and arterial macrophage foam cell formation [6]. Pomegranate juice and fruit extracts are also associated with antidiabetic activities, largely due to the antioxidant properties of HTs and ATs in reducing oxidative stress and lipid peroxidation [4]. Supplements of pomegranate juice or fruit extracts (including juice, fruit peel and seeds) at 1 g/kg/day for five weeks, effectively enhanced endothelial NO synthase (eNOS) expression, as well as plasma nitrate and nitrite (end products of NO) levels in plasma in obese Zucker rats fed with an atherogenic diet [68]. In addition, supplements of juice (extracted from pomegranate arils) at 100 mg/kg/day or 300 mg/kg/day for four weeks prevented the development of high blood pressure in diabetic rats [69]. Consumption of pomegranate juice rich in ATs and HTs also lowered systolic and diastolic blood pressure in hypertensive patients [70].
The bioactivities of pomegranate ATs have mostly been studied in the context of pomegranate juice and fruit extracts. However, these AT-rich sources are abundant in vitamin C, carotenoids, and HTs, which can also contribute to the ascribed bioactivities. To this end, “white” (AT-less) pomegranate cultivars are available, which lack the accumulation of ATs in leaves, fruits (including peels and arils) and flowers [71,72]. Comparative analysis of the white and AT-rich pomegranate cultivars will be informative as to the specific role of ATs in the health-promoting activities of pomegranate juice and fruit extracts.
5. Roles of Urolithins, the ET-Derived Metabolites, in Human Nutrition and Health
ETs are abundant in pomegranate fruit peel, as well as juice and extracts that are produced commercially from the whole fruit. Besides pomegranate, ETs are also present in a wide range of medicinal and food plants, such as many berries, nuts, and herbs [73,74]. As such, the knowledge of biotransformation and bioconversion of pomegranate ETs by human and microbial enzymes (discussed in this section) will inform nutritional and pharmacological studies on ETs that are isolated from other plants.
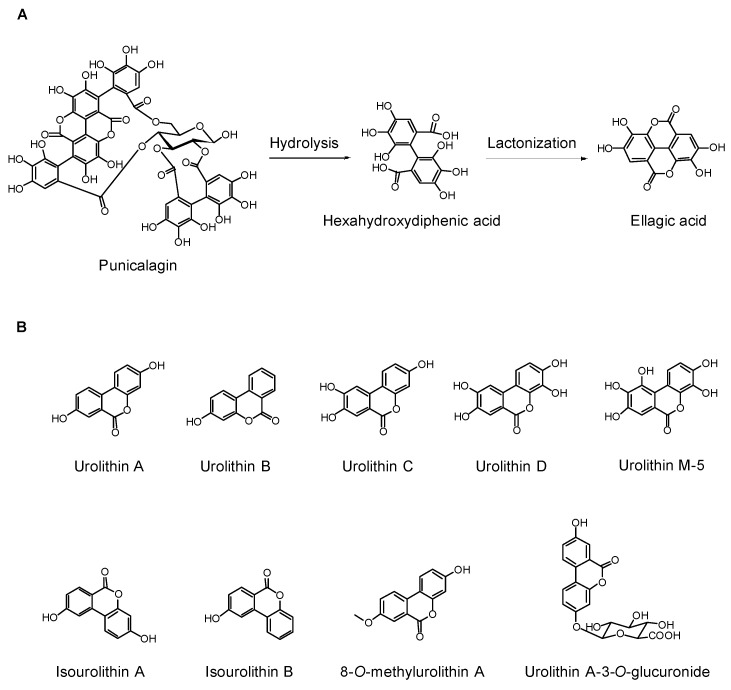
EA, a hydrolysis product of ETs (Figure 1A), can be detected (at a maximum concentration of 0.06 μM) in the blood circulation of healthy volunteers within half an hour of ingesting pomegranate juice or extracts [75]. The human gut microbiota can further convert EA into urolithins prior to their absorption by the intestinal cells. It was shown that urolithin concentrations reached up to 18.6 μM in the plasma of healthy volunteers after consuming pomegranate juice for five consecutive days [76].
Figure 1.
Pomegranate ellagitannin-derived metabolites. (A) Punicalagin (a pomegranate ellagitannin) is hydrolyzed to hexahydroxydiphenic acid, which is lactonized to yield ellagic acid; (B) Chemical structures of representative urolithins, the microfloral transformation products of ellagic acid.
Urolithins contain a common core structure of dibenzopyranone and are more hydrophobic than EA (Figure 1B). The microfloral transformation of EA to urolithins likely involves cleavage of the lactone ring of EA by an esterase, followed by removal of the carboxyl group resulted from the ring opening by a decarboxylase, and then removal of one or more hydroxyl groups by an oxidoreductase [77]. Urolithin A (UA), urolithin B (UB), hydroxyl-UA, UA-glucuronide, and dimethyl EA-glucuronide have been identified in the plasma of healthy individuals after taking pomegranate extracts [78]. Additional urolithins, such as isourolithin A (isoUA), have also been reported in human urine or stool samples [79]. Emerging experimental evidence has suggested that urolithins play a major role in the anti-cancer, anti-inflammatory, and anti-aging activities of pomegranate fruit and products, which are discussed in the following sections.
5.1. Breast and Endometrial Cancers
Aromatase that converts androgens to estrogens has been considered to be a therapeutic target for treating the hormone-sensitive type of breast cancer [80]. Although several urolithins (at 47 μM) exhibited anti-aromatase activities in a placental microsome-based enzyme assay, only UB competitively inhibited and most effectively suppressed the aromatase activity in an aromatase-overexpressing breast cancer cell line (MCF-7aro) [81]. Consistent with “in cell” aromatase-inhibitory activity, UB also significantly arrested testosterone-induced proliferation of MCF-7aro cells, suggesting an underlying mechanism of aromatase inhibition. Interestingly, estrogen-induced proliferation of MCF-7aro cells was also inhibited by urolithins. However, inhibition was was likely effected through mechanisms independent of the aromatase activity [81].
Besides breast cancer, urolithins can also reduce the risks of another sex steroid hormone-related cancer, endometrial cancer. UA and UB (at 10 μM for 48 h) caused over 50% reduction in the proliferation of human endometrial cancer cells [82]. UA (at 10 μM and 50 μM for 48 h) arrested cell cycle at the G2/M phase and regulated the expression of cell cycle-related proteins at this phase. On the other hand, UA was shown to act as an estrogen agonist and modulate the estrogen-receptor α (ERα)-dependent gene expression in the ER-positive endometrial cancer cells. It remains to be determined whether and how estrogen signaling could be involved in the suppression of endometrial cancer cell growth by urolithins [82].
5.2. Prostate Cancer
UA (IC50 = 35.2 ± 3.7 μM), UB (IC50 > 40 μM), and urolithin C (UC; IC50 = 46.5 ± 1.6 μM) impaired the proliferation of the “androgen-sensitive” prostate cancer cells (LNCaP) [83]. UA and UC also attenuated the release of the prostate specific antigen (PSA) by the cancer cells, and inhibited the activities of arginase (a hydrolase that converts arginine to ornithine and urea and is critical for the proliferation of prostate cancer cells) [83]. Further investigations of the regulatory mechanism of urolithins indicated that UA and UB repressed the transcription and translation of the androgen receptor (AR), and subsequently reduced PSA transcripts and proteins in the LNCaP cells [84]. Urolithins also induced apoptosis in the LNCaP cells, which correlated with a decrease in the anti-apoptotic B-cell lymphoma 2 (Bcl-2) proteins, an increase in the apoptotic-protective Cyclin Dependent Kinase Inhibitor 1A (CDKN1A) transcripts and proteins, and an activation of the apoptotic cysteine proteases caspases 3 and 7 [84,85].
Cytochrome P450 1 (CYP1) family members activate pro-carcinogens and often exhibit enhanced expression in tumor and cancer cells [86]. UA (IC50 = 1.15 ± 0.65 μM), UB (IC50 = 1.55 ± 0.49 μM), and methyl-UA (mUA; IC50 = 1.49 ± 0.39 μM) effectively inhibited the CYP1-mediated ethoxy resorufin-O-deethylase (EROD) activities and were selective toward CYP1-B1 over CYP1-A1 (CYP1-B1 and CYP1-A1 are two members of the CYP1 family) in in vitro assays [87]. Incubation with UA (IC50 = 32 ± 8.9 μM) and UB (IC50 = 38 ± 3.9 μM) also led to inhibition of the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced CYP1 activities and decreased CYP1-B1 protein accumulation in the “androgen-responsive” 22Rv1 prostate cancer cells. However, the anti-proliferative effects of UC, dimethyl-UC, and mUA toward 22Rv1 cells were due to their cytotoxic activities rather than inhibition of the TCDD-induced CYP1 expression [87].
In the “androgen-independent” DU145 and PC3 prostate cancer cell lines, EA and UA synergistically inhibited cancer cell proliferation [88]. However, EA and UA exerted differential impacts on cell cycle control and induction of apoptosis in DU145 and PC3 cancer cells, suggesting that different molecular targets may exist for these pomegranate-derived metabolites [88]. Caspase-dependent cell apoptosis was induced in DU145 cells by mUA treatment [89]. Upon exposure to mUA (at 40 μM or 60 μM), expression of the oncogenic microRNA-21 (miR-21) was reduced, whereas protein levels of the miR-21 targets, including phosphatase and tensin homolog (PTEN), programmed cell death 4 (Pdcd4), and forkhead box O3a (FOXO3a), were elevated. In addition, the expression of protein kinase B (Akt), as well as β-catenin and its downstream oncogenic transcription factors, was decreased by the mUA treatment [89]. These data suggest that the mUA suppression of DU145 cell growth is mediated by regulation of miR-21 expression and the downstream PTEN/Akt and Wnt/β-catenin signaling pathways.
The Eph receptor-Ephrin ligand signaling system is implicated in an array of cellular processes, such as cancer development. UC and urolithin D (UD) reduced specifically the EphA2-Ephrin-A1 interaction (this receptor-ligand pair is known to be involved in cancer development and progression) of the Eph-Ephrin system [90]. UD acts as a protein-protein antagonist of EphA2 (a receptor kinase) by interfering its phosphorylation mediated by Ephrin-A1, without inhibiting the kinase domain of EphA2, and using a mechanism other than cytotoxicity or anti-proliferative effect in the PC3 human prostate adenocarcinoma cells [90].
Overall, these studies collectively suggest that urolithins may find applications as chemo-preventive agents against prostate cancers, through different mechanisms in androgen-sensitive, -responsive, and -independent cells.
5.3. Colon and Bladder Cancers
The canonical Wnt/β-catenin signaling pathway has been shown to activate T-cell factor transcription and function in colon carcinogenesis [91]. To determine the potential involvement of urolithins in colon carcinogenesis through Wnt signaling, phenolic extracts of several fruits rich in ETs, including pomegranate, strawberry (Fragaria ananassa), and Jamun berry (Eugenia jambolana), as well as pure EA and UA compounds, were tested in the human embryonic kidney 293T cells that express a reconstructed canonical Wnt signaling pathway [92]. Although the fruit extracts and chemicals all inhibited Wnt signaling, only UA exhibited an IC50 value (at 39 μM) that was physiologically relevant in the lumen of colons when taking enterohepatic circulation of urolithins into consideration [92].
During the initiation stage of the HT-29 colon cancer cells, urolithins A–D inhibited TCDD-induced, CYP1-mediated EROD activities [93]. At the cancer progression stage, UA and UB (at 30 μg mL−1 for 48 h) impaired the proliferation of HT-29 cells and led to cell cycle arrest at the G2/M phase, as well as activation of CDKN1A expression. In addition, treatment with urolithins resulted in activation of caspases 3, 8, and 9, suggesting that urolithins induced both the extrinsic (death receptor-mediated; where caspase 8 is activated) and intrinsic (mitochondrial mediated; where caspase 9 is activated) apoptotic pathways in HT-29 cells [94].
UA, UB, and mUA inhibited the proliferation of the T24 human bladder cancer cells in vitro, with IC50 values of 43.9, 35.2, and 46.3 μM, respectively [10], comparable to the UA inhibition of Wnt signaling at an IC50 of 39 μM [92]. The transcript and protein levels of Phospho-p38 mitogen-activated protein kinase (MAP kinase; MAPK) were increased by the urolithin treatment, while those of MAP kinase kinase kinase1 (MEKK1) and Phospho-c-Jun were decreased in the T24 cells. Furthermore, these urolithins reduced the level of H2O2-induced oxidative stress and induced apoptosis through activation of caspase 3 and PPAR-γ protein expression [10].
5.4. Cardiovascular Diseases
Inflammatory responses involving the activation of neutrophils and monocytes play a central role in the development of cardiovascular diseases [95]. Intriguingly, the number of free hydroxyl functional groups on urolithins appeared to impact how they modulate the inflammatory functions of neutrophils [96]. Among the urolithins tested (including UA, UB, UC, mUA, and methyl-urolithin C/mUC), UA (with two free hydroxyl groups; at 1 μM) exhibited the most potent antioxidant activities against the release of ROS from the pro-inflammatory triggered neutrophils. UB (with one free hydroxyl group; at 20 μM) significantly affected several inflammatory biomarkers that are associated with cardiovascular events, by inhibiting the production of interleukin 8 (IL8) and metalloproteinase-9 (MMP-9), and preventing the shedding of selectin CD62L triggered by the pro-inflammatory factor cytochalasin A/formyl-met-leu-phenylalanine (f-MLP). UC (with three free hydroxyl groups; at 5 μM), on the other hand, inhibited the release of elastase, a pro-inflammatory mediator responsible for extracellular matrix (ECM) degradation, from the f-MLP-stimulated neutrophils [96].
In addition to recruitment of neutrophils, monocyte adhesion to endothelial cells represents another key event in inflammatory responses [95]. A mixture of UA and UB (each at 10 μM) restricted the adhesion of Tamm-Horsfall protein-1 (THP-1) monocytes to the human umbilical vein endothelial cells (HUVECs) [97]. UA glucuronide (at 15 μM), but not UA, UB or UB glucuronide, inhibited monocyte adhesion to TNFα-stimulated human aortic endothelial cells (HAECs); a dosage-dependent inhibition of TNFα-induced migration of endothelial cells was also shown for the above-mentioned urolithins [98].
NO plays multifaceted roles in combatting cardiovascular diseases [99]. Although UA, UB, and UB glucuronide did not show any effect individually at 15 μM on NO bioavailability, a mixture of the three urolithins at equal concentrations (total 15 μM) activated the expression of eNOS after a 5-min incubation and increased NO production in primary HAECs after a 24-h incubation [100]. Overall, these in vitro studies with neutrophils, monocytes, and NO suggested the potential anti-inflammatory and cardiovascular-protective functions of urolithins.
5.5. Obesity
Based on the urinary excretion of urolithins by healthy volunteers after ingesting ET-rich foods or fruit extracts, three urolithin metabolic types (metabotypes) have been defined, including A (excretes only UA), B (excretes UA, UB, and isoUA), and 0 (does not excrete urolithins) [79]. Interestingly, the population of the human gut bacteria Gordonibacter urolithinfaciens correlated positively with the in vivo production of UA, but inversely with that of UB and isoUA [101]. A recent study observed an interlinked relationship among gut dysbiosis (i.e., microbial imbalance), ET metabolism, and obesity [102]. A relatively high percentage of metabotype B was found in the overweight-obese group, while metabotype A had a higher presentation in the normoweight (i.e., normal weight) than the overweight-obese group. In addition, G. urolithinfaciens levels were higher in the metabotype A than the metabotype B individuals [102]. Further investigations should provide a mechanistic understanding of how consuming the polyphenol precursors of urolithins, in the presence of UA-producing bacteria, may reduce the risks of diseases associated with obesity.
Large inter-individual variations in the cardiovascular risk biomarkers were observed in healthy overweight-obese individuals after consuming pomegranate supplement [103]. However, after clustering the different urolithin metabotypes in these individuals, improved blood lipid profiles were evident in the metabotype B group, in a dose-dependent fashion, while there was no significant effect in the metabotype A group. Interestingly, several metabotype 0 (urolithin non-producers; according to baseline analysis) individuals shifted to metabotype A or B (urolithin producers) after consuming pomegranate extracts [103]. Together with the study by Selma et al. (2016), these results suggest that consumption of pomegranate extracts may have personalized effects that are associated with the gut microbiota and the urolithin metabotypes of the individuals.
5.6. Aging
Of the various pomegranate phenolic metabolites exhibiting anti-Alzheimer activities in in vitro assays, only urolithins, including UA, UB, mUA, and methyl-urolithin B (mUB), were predicted to be capable of crossing the blood-brain barrier [104]. Methylation of urolithins by the mammalian enzymes may further improve their lipophilicity and facilitate the penetration of the blood-brain barrier. Corroborating with the computational predictions, urolithins reduced the production of the neurotoxic, fibrillogenic β-amyloid (Aβ) peptide in vitro. Significant improvement of the survival/mobility of Caenorhabditis elegans (post-induction by Aβ1-42 for muscular paralysis) was also achieved by mUB treatment [104]. However, empirical evidence is still needed to verify the roles of urolithins in preventing Alzheimer’s disease (age-related memory loss) in vivo.
Advanced protein glycation (non-enzymatic glycosylation) products have been implicated in the development of chronic diseases, such as diabetes and Alzheimer’s disease. The inhibitory effects of pomegranate fruit extract, punicalagin, EA, gallic acid (GA), UA, and UB toward the early, middle, and late stage of protein glycation were compared using in vitro assays [105]. Although all phenolic metabolites showed anti-glycation activities, pomegranate extract, punicalagin, and EA were more effective than GA, UA, and UB in preventing the glycation of proteins by fructose [105].
The mechanistic basis underlying UA’s anti-aging activities was explored recently using C. elegans, mammalian cells, and rodent models [106]. Feeding C. elegans with UA led to a dosage-dependent extension of the worm’s lifespan. It was shown that UA could activate mitophagy (degradation of mitochondria by autophagy) and eliminate damaged mitochondria in C. elegans, mammalian cells derived from muscle or intestinal tissues, and muscles of young and old rodents. UA was also found to be bioavailable in the skeletal muscle and could improve muscle function in mice and rats [106]. The role of urolithins in enhancing mitochondrial function and muscle quality presents promising dietary leads (e.g., ET-rich foods) for improved mobility in the elderly population.
6. Future Perspectives
Pomegranate fruits, leaves, flowers, and seeds have all been used in traditional herbal medicine for treating various illness. However, bioactive metabolites present in pomegranate fruit peel and juice have received considerable more attention than those found in other tissues. Bioactivities of the phytochemicals accumulating in tissues other than fruit should be investigated more rigorously in the future. In addition, detailed metabolite profiling and characterization of different pomegranate cultivars, grown under non-standard conditions (e.g., subjected to biotic and abiotic stresses), can be carried out to explore further the phytochemical diversity in pomegranate.
Metabolic enzymes and pathways that confer the structural diversity of pomegranate phytochemicals also warrant further investigation. For example, the production of diverse HT monomers and polymers has been suggested to involve the activity of multiple laccase enzymes. However, the HT-forming laccases have not been cloned and characterized from pomegranate or any other plant species. Elucidation of the laccase family enzymes in pomegranate that bring about the great diversity of HT structures will have broad implications in dissecting the biological functions of HTs.
In vitro assays and animal model studies have demonstrated various health benefits of urolithins, the ET derivatives. It is critical to obtain clinically relevant data on their efficacies prior to widespread applications in human disease interventions. Overall, exploration of the diversity and interactions of pomegranate phytochemicals, as well as preclinical and clinical investigations of their bioactivities, holds great promise of fully realizing the potential of this ancient fruit and modern functional food. Since a majority of the phytochemicals identified in pomegranate have also been found in other plants, examining the interactions and health-promoting functions of pomegranate phytochemicals will also have a far-reaching impact on exploiting the bioactive components of various edible medicinal plants and functional plant food.
Acknowledgments
We are grateful for the support provided by the Science and Technology Commission of Shanghai Municipality (14DZ2260400), the National Science Foundation (MCB1120323 to L.T.), and the Binational Agricultural Research and Development Fund (BARD) (IS-4822-15R to L.T.).
Supplementary Materials
The following are available online, Table S1: Phytochemicals identified from different pomegranate tissues.
Author Contributions
S.W. and L.T. conceived and wrote the review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dell’Agli M., Galli G.V., Corbett Y., Taramelli D., Lucantoni L., Habluetzel A., Maschi O., Caruso D., Giavarini F., Romeo S., et al. Antiplasmodial activity of Punica granatum L. fruit rind. J. Ethnopharmacol. 2009;125:279–285. doi: 10.1016/j.jep.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Qnais E.Y., Elokda A.S., Abu Ghalyun Y.Y., Abdulla F.A. Antidiarrheal activity of the aqueous extract of Punica granatum (pomegranate) peels. Pharm. Biol. 2007;45:715–720. doi: 10.1080/13880200701575304. [DOI] [Google Scholar]
- 3.Das A.K., Mandal S.C., Banerjee S.K., Sinha S., Das J., Saha B.P., Pal M. Studies on antidiarrhoeal activity of Punica granatum seed extract in rats. J. Ethnopharmacol. 1999;68:205–208. doi: 10.1016/S0378-8741(99)00102-6. [DOI] [PubMed] [Google Scholar]
- 4.Banihani S., Swedan S., Alguraan Z. Pomegranate and type 2 diabetes. Nutr. Res. 2013;33:341–348. doi: 10.1016/j.nutres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Bhowmik D., Gopinath H., Kumar B., Duraivel S., Aravind G., Kumar K. Medicinal uses of Punica granatum and its health benefits. J. Pharmacogn. Phytochem. 2013;1:28–35. [Google Scholar]
- 6.Newman R., Lansky E., Block M. Pomegranate: The Most Medicinal Fruit. Basic Health Publications; Laguna Beach, CA, USA: 2007. [Google Scholar]
- 7.Kaufman M., Wiesman Z. Pomegranate oil analysis with emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J. Agric. Food Chem. 2007;55:10405–10413. doi: 10.1021/jf072741q. [DOI] [PubMed] [Google Scholar]
- 8.Melgarejo P., Salazar D.M., Artés F. Organic acids and sugars composition of harvested pomegranate fruits. Eur. Food Res. Technol. 2000;211:185–190. doi: 10.1007/s002170050021. [DOI] [Google Scholar]
- 9.Pande G., Akoh C.C. Antioxidant capacity and lipid characterization of six Georgia-grown pomegranate cultivars. J. Agric. Food Chem. 2009;57:9427–9436. doi: 10.1021/jf901880p. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Z., Zhou B., Jin L., Yu H., Liu L., Liu Y., Qin C., Xie S., Zhu F. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem. Toxicol. 2013;59:428–437. doi: 10.1016/j.fct.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Tzulker R., Glazer I., Bar-Ilan I., Holland D., Aviram M., Amir R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007;55:9559–9570. doi: 10.1021/jf071413n. [DOI] [PubMed] [Google Scholar]
- 12.Seeram N., Lee R., Hardy M., Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005;41:49–55. doi: 10.1016/j.seppur.2004.04.003. [DOI] [Google Scholar]
- 13.Ono N., Bandaranayake P.C.G., Tian L. Establishment of pomegranate (Punica granatum) hairy root cultures for genetic interrogation of the hydrolyzable tannin biosynthetic pathway. Planta. 2012;236:931–941. doi: 10.1007/s00425-012-1706-y. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T., Nonaka G.-I., Nishioka I. Tannins and related compounds. XLI.: Isolation and characterization of novel ellagitannins, punicacorteins A, B, C, and D, and punigluconin from the bark of Punica granatum L. Chem. Pharm. Bull. Tokyo. 1986;34:656–663. doi: 10.1248/cpb.34.656. [DOI] [Google Scholar]
- 15.El-Toumy S., Marzouk M., Rauwald H. Ellagi- and gallotannins from Punica granatum heartwood. Pharmazie. 2001;56:823–824. [PubMed] [Google Scholar]
- 16.El-Toumy S., Rauwald H. Two new ellagic acid rhamnosides from Punica granatum heartwood. Planta Med. 2003;69:682–684. doi: 10.1055/s-2003-41107. [DOI] [PubMed] [Google Scholar]
- 17.El-Toumy S.A.A., Rauwald H.W. Two ellagitannins from Punica granatum heartwood. Phytochemistry. 2002;61:971–974. doi: 10.1016/S0031-9422(02)00435-1. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T., Nonaka G.-I., Nishioka I. Punicafolin, an ellagitannin from the leaves of Punica granatum. Phytochemistry. 1985;24:2075–2078. doi: 10.1016/S0031-9422(00)83125-8. [DOI] [Google Scholar]
- 19.Nawwar M.A.M., Hussein S.A.M., Merfort I. Leaf phenolics of Punica granatum. Phytochemistry. 1994;37:1175–1177. doi: 10.1016/S0031-9422(00)89552-7. [DOI] [Google Scholar]
- 20.Hussein S.A.M., Barakat H.H., Merfort I., Nawwar M.A.M. Tannins from the leaves of Punica granatum. Phytochemistry. 1997;45:819–823. doi: 10.1016/S0031-9422(96)00888-6. [DOI] [Google Scholar]
- 21.Wang R., Wei W., Wang L., Liu R., Yi D., Du L. Constituents of the flowers of Punica granatum. Fitoterapia. 2006;77:534–537. doi: 10.1016/j.fitote.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Yuan T., Ding Y., Wan C., Li L., Xu J., Liu K., Slitt A., Ferreira D., Khan I.A., Seeram N.P. Antidiabetic ellagitannins from pomegranate flowers: Inhibition of α-glucosidase and lipogenic gene expression. Org. Lett. 2012;14:5358–5361. doi: 10.1021/ol302548c. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y., Morikawa T., Ninomiya K., Imura K., Muraoka O., Yuan D., Yoshikawa M. Medicinal flowers. XXIII. New taraxastane-type triterpene, punicanolic acid, with tumor necrosis factor-a inhibitory activity from the flowers of Punica granatum. Chem. Pharm. Bull. Tokyo. 2008;56:1628–1631. doi: 10.1248/cpb.56.1628. [DOI] [PubMed] [Google Scholar]
- 24.Yuan T., Wan C., Ma H., Seeram N.P. New phenolics from the flowers of Punica granatum and their in vitro α-glucosidase inhibitory activities. Planta Med. 2013;79:1674–1679. doi: 10.1055/s-0033-1350925. [DOI] [PubMed] [Google Scholar]
- 25.Bagri P., Ali M., Sultana S., Aeri V. New flavonoids from Punica granatum flowers. Chem. Nat. Compd. 2010;46:201–204. doi: 10.1007/s10600-010-9568-7. [DOI] [Google Scholar]
- 26.Chauhan D., Chauhan J.S. Flavonoid diglycoside from Punica granatum. Pharm. Biol. 2001;39:155–157. doi: 10.1076/phbi.39.2.155.6254. [DOI] [Google Scholar]
- 27.Srivastava R., Chauhan D., Chauhan J. Flavonoid diglycosides from Punica granatum. Indian J. Chem. Sect. B. 2001;40B:170–172. [Google Scholar]
- 28.Moneam N.M.A., El Sharaky A.S., Badreldin M.M. Oestrogen content of pomegranate seeds. J. Chromatogr. 1988;438:438–442. doi: 10.1016/S0021-9673(00)90278-4. [DOI] [PubMed] [Google Scholar]
- 29.Fischer U.A., Jaksch A.V., Carle R., Kammerer D.R. Determination of lignans in edible and nonedible parts of pomegranate (Punica granatum L.) and products derived therefrom, particularly focusing on the quantitation of isolariciresinol using HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 2012;60:283–292. doi: 10.1021/jf203598m. [DOI] [PubMed] [Google Scholar]
- 30.Ito H., Li P., Koreishi M., Nagatomo A., Nishida N., Yoshida T. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 2014;152:323–330. doi: 10.1016/j.foodchem.2013.11.160. [DOI] [PubMed] [Google Scholar]
- 31.El Wahab S., El Fiki N., Mostafa F., Hassan A. Characterization of certain steroid hormones in Punica granatum L. seeds. Bull. Fac. Pharm. 1998;36:11–15. [Google Scholar]
- 32.Heftmann E., Ko S.-T., Bennett R.D. Identification of estrone in pomegranate seeds. Phytochemistry. 1966;5:1337–1339. doi: 10.1016/S0031-9422(00)86133-6. [DOI] [Google Scholar]
- 33.Choi D.W., Kim J.Y., Choi S.H., Jung H.S., Kim H.J., Cho S.Y., Kang C.S., Chang S.Y. Identification of steroid hormones in pomegranate (Punica granatum) using HPLC and GC-mass spectrometry. Food Chem. 2006;96:562–571. doi: 10.1016/j.foodchem.2005.03.010. [DOI] [Google Scholar]
- 34.Neuhofer H., Witte L., Gorunovic M., Czygan F. Alkaloids in the bark of Punica granatum L. (pomegranate) from Yugoslavia. Pharmazie. 1993;48:389–391. [Google Scholar]
- 35.Rafiq Z., Narasimhan S., Vennila R., Vaidyanathan R. Punigratane, a novel pyrrolidine alkaloid from Punica granatum rind with putative efflux inhibition activity. Nat. Prod. Res. 2016;25:1–6. doi: 10.1080/14786419.2016.1146883. [DOI] [PubMed] [Google Scholar]
- 36.Badria F. Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J. Med. Food. 2004;5:153–157. doi: 10.1089/10966200260398189. [DOI] [PubMed] [Google Scholar]
- 37.Fatope M.O., Al Burtomani S.K.S., Takeda Y. Monoacylglycerol from Punica granatum seed oil. J. Agric. Food Chem. 2002;50:357–360. doi: 10.1021/jf010711w. [DOI] [PubMed] [Google Scholar]
- 38.Lal C., Sharma M., Shakyawar D., Raja A., Sharma K., Pareek P. Natural Dye constituents from rind of Punica granatum and its application on Pashmina fabrics. Arch. Appl. Sci. Res. 2011;3:350–357. [Google Scholar]
- 39.Yusuph M., Mann J. A triglyceride from Punica granatum. Phytochemistry. 1997;44:1391–1392. doi: 10.1016/S0031-9422(96)00742-X. [DOI] [Google Scholar]
- 40.Tsuyuki H., Ito S., Nakatsukasa Y. Studies on the lipids in pomegranate seeds. Bull. Coll. Agric. Vet. Med. Nihon Univ. 1981;38:141–148. [Google Scholar]
- 41.Mena P., Calani L., Dall’Asta C., Galaverna G., García-Viguera C., Bruni R., Crozier A., Del Rio D. Rapid and comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules. 2012;17:14821–14840. doi: 10.3390/molecules171214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poyrazoğlu E., Gökmen V., Artιk N. Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Compos. Anal. 2002;15:567–575. doi: 10.1016/S0889-1575(02)91071-9. [DOI] [Google Scholar]
- 43.Artik N., Murakami H., Mori T. Determination of phenolic compounds in pomegranate juice by using HPLC. Fruit Process. 1998;8:492–499. [Google Scholar]
- 44.Wang R.-F., Xie W.-D., Zhang Z., Xing D.-M., Ding Y., Wang W., Ma C., Du L.-J. Bioactive compounds from the seeds of Punica granatum (pomegranate) J. Nat. Prod. 2004;67:2096–2098. doi: 10.1021/np0498051. [DOI] [PubMed] [Google Scholar]
- 45.Lansky E., Jiang W., Mo H., Bravo L., Froom P., Yu W., Harris N., Neeman I., Campbell M. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Investig. New Drugs. 2005;23:11–20. doi: 10.1023/B:DRUG.0000047101.02178.07. [DOI] [PubMed] [Google Scholar]
- 46.Lansky E., Harrison G., Froom P., Jiang W. Pomegranate (Punica granatum) pure chemical show possible synergistic inhibition of human PC-3 prostate cancer cell invasion across Matrigel. Investig. New Drugs. 2005;23:121–122. doi: 10.1007/s10637-005-5856-7. [DOI] [PubMed] [Google Scholar]
- 47.Seeram N.P., Adams L.S., Henning S.M., Niu Y., Zhang Y., Nair M.G., Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Bolling B.W., Chen Y.-Y., Chen C.Y.O. Contributions of phenolics and added vitamin C to the antioxidant capacity of pomegranate and grape juices: Synergism and antagonism among constituents. Int. J. Food Sci. Technol. 2013;48:2650–2658. doi: 10.1111/ijfs.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolling B.W., Chen Y.-Y., Kamil A.G., Chen C.Y.O. Assay dilution factors confound measures of total antioxidant capacity in polyphenol-rich juices. J. Food Sci. 2012;77:H69–H75. doi: 10.1111/j.1750-3841.2011.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dey D., Debnath S., Hazra S., Ghosh S., Ray R., Hazra B. Pomegranate pericarp extract enhances the antibacterial activity of ciprofloxacin against extended-spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) producing Gram-negative bacilli. Food Chem. Toxicol. 2012;50:4302–4309. doi: 10.1016/j.fct.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Reis J.F., Monteiro V.V.S., de Souza Gomes R., do Carmo M.M., da Costa G.V., Ribera P.C., Monteiro M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016;14:315. doi: 10.1186/s12967-016-1076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin B.-W., Gong C.-C., Song H.-F., Cui Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017;174:1226–1243. doi: 10.1111/bph.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azzini E., Giacometti J., Russo G.L. Antiobesity effects of anthocyanins in preclinical and clinical studies. Oxid. Med. Cell. Longev. 2017;2017:2740364. doi: 10.1155/2017/2740364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morais C.A., de Rosso V.V., Estadella D., Pisani L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016;33:1–7. doi: 10.1016/j.jnutbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Liobikas J., Skemiene K., Trumbeckaite S., Borutaite V. Anthocyanins in cardioprotection: A path through mitochondria. Phytother. Res. 2016;113:808–815. doi: 10.1016/j.phrs.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 56.Li D., Wang P., Luo Y., Zhao M., Chen F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017;57:1729–1741. doi: 10.1080/10408398.2015.1030064. [DOI] [PubMed] [Google Scholar]
- 57.Borges F., Fernandes E., Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr. Med. Chem. 2002;9:195–217. doi: 10.2174/0929867023371229. [DOI] [PubMed] [Google Scholar]
- 58.Pergola C., Rossi A., Dugo P., Cuzzocrea S., Sautebin L. Inhibition of nitric oxide biosynthesis by anthocyanin fraction of blackberry extract. Nitric Oxide. 2006;15:30–39. doi: 10.1016/j.niox.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Dreiseitel A., Korte G., Schreier P., Oehme A., Locher S., Hajak G., Sand P.G. sPhospholipase A2 is inhibited by anthocyanidins. J. Neural Transm. 2009;116:1071–1077. doi: 10.1007/s00702-009-0268-z. [DOI] [PubMed] [Google Scholar]
- 60.Speciale A., Canali R., Chirafisi J., Saija A., Virgili F., Cimino F. Cyanidin-3-O-glucoside protection against TNF-α-induced endothelial dysfunction: Involvement of nuclear factor-κB signaling. J. Agric. Food Chem. 2010;58:12048–12054. doi: 10.1021/jf1029515. [DOI] [PubMed] [Google Scholar]
- 61.DeFuria J., Bennett G., Strissel K.J., Perfield J.W., Milbury P.E., Greenberg A.S., Obin M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009;139:1510–1516. doi: 10.3945/jn.109.105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mykkänen O.T., Huotari A., Herzig K.-H., Dunlop T.W., Mykkänen H., Kirjavainen P.V. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PLoS ONE. 2014;9:e114790. doi: 10.1371/journal.pone.0114790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baum J., Howard L., Prior R., Lee S. Effect of Aronia melanocarpa (black chokeberry) supplementation on the development of obesity in mice fed a high-fat diet. J. Berry Res. 2016;6:203–212. doi: 10.3233/JBR-160134. [DOI] [Google Scholar]
- 64.Wright O.R.L., Netzel G.A., Sakzewski A.R. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: The QUENCH trial. Can. J. Physiol. Pharmacol. 2013;91:480–488. doi: 10.1139/cjpp-2012-0349. [DOI] [PubMed] [Google Scholar]
- 65.Fimognari C., Berti F., Nüsse M., Cantelli-Forti G., Hrelia P. Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin-3-O-β-glucopyranoside. Biochem. Pharmacol. 2004;67:2047–2056. doi: 10.1016/j.bcp.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 66.Malik M., Zhao C., Schoene N., Guisti M., Moyer M., Magnuson B. Anthocyanin-rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer. 2003;46:186–196. doi: 10.1207/S15327914NC4602_12. [DOI] [PubMed] [Google Scholar]
- 67.Lee S., Park S., Park S., Park J., Shin D., Kim G., Ryu C., Shin S., Jung J., Kang H., Lee W., Choi Y. Induction of apoptosis in human leukemia U937 cells by anthocyanins through down-regulation of Bcl-2 and activation of caspases. Int. J. Oncol. 2009;34:1077–1083. doi: 10.3892/ijo_00000234. [DOI] [PubMed] [Google Scholar]
- 68.De Nigris F., Balestrieri M.L., Williams-Ignarro S., D’Armiento F.P., Fiorito C., Ignarro L.J., Napoli C. The influence of pomegranate fruit extract in comparison to regular pomegranate juice and seed oil on nitric oxide and arterial function in obese Zucker rats. Nitric Oxide. 2007;17:50–54. doi: 10.1016/j.niox.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Mohan M., Waghulde H., Kasture S. Effect of pomegranate juice on Angiotensin II-induced hypertension in diabetic wistar rats. Phytother. Res. 2010;24:S196–S203. doi: 10.1002/ptr.3090. [DOI] [PubMed] [Google Scholar]
- 70.Asgary S., Sahebkar A., Afshani M.R., Keshvari M., Haghjooyjavanmard S., Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014;28:193–199. doi: 10.1002/ptr.4977. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Simhon Z., Judeinstein S., Trainin T., Harel-Beja R., Bar-Ya’akov I., Borochov-Neori H., Holland D. A “white” anthocyanin-less pomegranate (Punica granatum L.) caused by an insertion in the coding region of the leucoanthocyanidin dioxygenase (LDOX; ANS) gene. PLoS ONE. 2015;10:e0142777. doi: 10.1371/journal.pone.0142777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao X., Yuan Z., Feng L., Fang Y. Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J. Plant Res. 2015;128:687–696. doi: 10.1007/s10265-015-0717-8. [DOI] [PubMed] [Google Scholar]
- 73.Okuda T., Yoshida T., Hatano T. Ellagitannins as active constituents of medicinal plants. Planta Med. 1989;55:117–122. doi: 10.1055/s-2006-961902. [DOI] [PubMed] [Google Scholar]
- 74.Wilson A.E., Matel H.D., Tian L. Glucose ester enabled acylation in plant specialized metabolism. Phytochem. Rev. 2016;15:1057–1074. doi: 10.1007/s11101-016-9467-z. [DOI] [Google Scholar]
- 75.Seeram N.P., Henning S.M., Zhang Y., Suchard M., Li Z., Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 76.Cerdá B., Espín J.C., Parra S., Martínez P., Tomás-Barberán F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004;43:205–220. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 77.Selma M.V., Espín J.C., Tomás-Barberán F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 78.Mertens-Talcott S.U., Jilma-Stohlawetz P., Rios J., Hingorani L., Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agric. Food Chem. 2006;54:8956–8961. doi: 10.1021/jf061674h. [DOI] [PubMed] [Google Scholar]
- 79.Tomás-Barberán F.A., García-Villalba R., González-Sarrías A., Selma M.V., Espín J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014;62:6535–6538. doi: 10.1021/jf5024615. [DOI] [PubMed] [Google Scholar]
- 80.Lephart E.D. Modulation of aromatase by phytoestrogens. Enzyme Res. 2015;2015:594656. doi: 10.1155/2015/594656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adams L.S., Zhang Y., Seeram N.P., Heber D., Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells. Cancer Prev. Res. 2010;3:108–113. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang W., Chen J.-H., Aguilera-Barrantes I., Shiau C.-W., Sheng X., Wang L.-S., Stoner G.D., Huang Y.-W. Urolithin A suppresses the proliferation of endometrial cancer cells by mediating estrogen receptor-α-dependent gene expression. Mol. Nutr. Food Res. 2016;60:2387–2395. doi: 10.1002/mnfr.201600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stolarczyk M., Piwowarski J.P., Granica S., Stefańska J., Naruszewicz M., Kiss A.K. Extracts from Epilobium sp. herbs, their components and gut microbiota metabolites of epilobium ellagitannins, urolithins, inhibit hormone-dependent prostate cancer cells-(LNCaP) proliferation and PSA secretion. Phytother. Res. 2013;27:1842–1848. doi: 10.1002/ptr.4941. [DOI] [PubMed] [Google Scholar]
- 84.Sanchez-Gonzalez C., Ciudad C.J., Noe V., Izquierdo-Pulido M. Walnut polyphenol metabolites, urolithins A and B, inhibit the expression of the prostate-specific antigen and the androgen receptor in prostate cancer cells. Food Funct. 2014;5:2922–2930. doi: 10.1039/C4FO00542B. [DOI] [PubMed] [Google Scholar]
- 85.Sánchez-González C., Ciudad C.J., Izquierdo-Pulido M., Noé V. Urolithin A causes p21 up-regulation in prostate cancer cells. Eur. J. Nutr. 2016;55:1099–1112. doi: 10.1007/s00394-015-0924-z. [DOI] [PubMed] [Google Scholar]
- 86.Go R.-E., Hwang K.-A., Choi K.-C. Cytochrome P450 1 family and cancers. J. Steroid Biochem. Mol. Biol. 2015;147:24–30. doi: 10.1016/j.jsbmb.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Kasimsetty S.G., Bialonska D., Reddy M.K., Thornton C., Willett K.L., Ferreira D. Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells. J. Agric. Food Chem. 2009;57:10636–10644. doi: 10.1021/jf902716r. [DOI] [PubMed] [Google Scholar]
- 88.Vicinanza R., Zhang Y., Henning S.M., Heber D. Pomegranate juice metabolites, ellagic acid and urolithin A, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis. Evid. Based Complement. Altern. Med. 2013;2013:247504. doi: 10.1155/2013/247504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou B., Wang J., Zheng G., Qiu Z. Methylated urolithin A, the modified ellagitannin-derived metabolite, suppresses cell viability of DU145 human prostate cancer cells via targeting miR-21. Food Chem. Toxicol. 2016;97:375–384. doi: 10.1016/j.fct.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Giorgio C., Mena P., DelRio D., Brighenti F., Barocelli E., Hassan-Mohamed I., Callegari D., Lodola A., Tognolini M. The ellagitannin colonic metabolite urolithin D selectively inhibits EphA2 phosphorylation in prostate cancer cells. Mol. Nutr. Food Res. 2015;59:2155–2167. doi: 10.1002/mnfr.201500470. [DOI] [PubMed] [Google Scholar]
- 91.Krausova M., Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell. Signal. 2014;26:570–579. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 92.Sharma M., Li L., Celver J., Killian C., Kovoor A., Seeram N.P. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J. Agric. Food Chem. 2010;58:3965–3969. doi: 10.1021/jf902857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kasimsetty S.G., Bialonska D., Reddy M.K., Ma G., Khan S.I., Ferreira D. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J. Agric. Food Chem. 2010;58:2180–2187. doi: 10.1021/jf903762h. [DOI] [PubMed] [Google Scholar]
- 94.Cho H., Jung H., Lee H., Yi H.C., Kwak H.-K., Hwang K.T. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 2015;6:1675–1683. doi: 10.1039/C5FO00274E. [DOI] [PubMed] [Google Scholar]
- 95.Nasimudeen R.J., Shams T. Cardiovascular disease management through restrained inflammatory responses. Curr. Pharm. Des. 2016;22:940–946. doi: 10.2174/1381612822666151209153823. [DOI] [PubMed] [Google Scholar]
- 96.Piwowarski J.P., Granica S., Kiss A.K. Influence of gut microbiota-derived ellagitannins’ metabolites urolithins on pro-inflammatory activities of human neutrophils. Planta Med. 2014;80:887–895. doi: 10.1055/s-0034-1368615. [DOI] [PubMed] [Google Scholar]
- 97.Mele L., Mena P., Piemontese A., Marino V., López-Gutiérrez N., Bernini F., Brighenti F., Zanotti I., Del Rio D. Antiatherogenic effects of ellagic acid and urolithins in vitro. Arch. Biochem. Biophys. 2016;599:42–50. doi: 10.1016/j.abb.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 98.Giménez-Bastida J.A., González-Sarrías A., Larrosa M., Tomás-Barberán F., Espín J.C., García-Conesa M.-T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. Food Res. 2012;56:784–796. doi: 10.1002/mnfr.201100677. [DOI] [PubMed] [Google Scholar]
- 99.Naseem K.M. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 100.Spigoni V., Mena P., Cito M., Fantuzzi F., Bonadonna R., Brighenti F., Dei Cas A., Del Rio D. Effects on nitric oxide production of urolithins, gut-derived ellagitannin metabolites, in human aortic endothelial cells. Molecules. 2016;21:1009. doi: 10.3390/molecules21081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Romo-Vaquero M., García-Villalba R., González-Sarrías A., Beltrán D., Tomás-Barberán F.A., Espín J.C., Selma M.V. Interindividual variability in the human metabolism of ellagic acid: Contribution of Gordonibacter to urolithin production. J. Funct. Food. 2015;17:785–791. doi: 10.1016/j.jff.2015.06.040. [DOI] [Google Scholar]
- 102.Selma M.V., Romo-Vaquero M., Garcia-Villalba R., Gonzalez-Sarrias A., Tomas-Barberan F.A., Espin J.C. The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism. Food Funct. 2016;7:1769–1774. doi: 10.1039/C5FO01100K. [DOI] [PubMed] [Google Scholar]
- 103.González-Sarrías A., García-Villalba R., Romo-Vaquero M., Alasalvar C., Örem A., Zafrilla P., Tomás-Barberán F.A., Selma M.V., Espín J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomised clinical trial. Mol. Nutr. Food Res. 2017;61:1600830. doi: 10.1002/mnfr.201600830. [DOI] [PubMed] [Google Scholar]
- 104.Yuan T., Ma H., Liu W., Niesen D.B., Shah N., Crews R., Rose K.N., Vattem D.A., Seeram N.P. Pomegranate’s neuroprotective effects against Alzheimer’s disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem. Neurosci. 2016;7:26–33. doi: 10.1021/acschemneuro.5b00260. [DOI] [PubMed] [Google Scholar]
- 105.Liu W., Ma H., Frost L., Yuan T., Dain J.A., Seeram N.P. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food Funct. 2014;5:2996–3004. doi: 10.1039/C4FO00538D. [DOI] [PubMed] [Google Scholar]
- 106.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-dit-Felix A.A., Williams E.G., Jha P., Lo Sasso G., Huzard D., et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.