Abstract
Development of a curative local treatment for large hepatocellular carcinoma (HCC) is an important issue. Here, we investigated the dose homogeneity, safety and antitumor effectiveness of proton beam therapy (PBT) using a patch-field technique for large HCC. Data from nine patients (aged 52–79 years) with large HCC treated with patch-field PBT were investigated. The cranial–caudal diameters of the clinical target volumes (CTVs) were 15.0–18.6 cm (median 15.9). The CTV was divided cranially and caudally while both isocenters were aligned along the cranial–caudal axis and overlap of the cranial and caudal irradiation fields was set at 0–0.5 mm. Multileaf collimators were used to eliminate hot or cold spots. Total irradiation doses were 60–76.4 Gy equivalents. Irradiation doses as a percentage of the prescription dose (from the treatment planning system) around the junction were a minimum of 93–105%, a mean of 99–112%, and a maximum of 105–120%. Quality assurance (QA) was assessed in the cranial and caudal irradiation fields using imaging plates. Acute adverse effects of Grade 3 were observed in one patient (hypoalbuminemia), and a late adverse effect of Grade 3 was observed in one patient (liver abscess). Child–Pugh class elevations were observed in four patients (A to B: 3; B to C: 1). Overall survival rates at 1 and 2 years were 55 and 14%, respectively, with a median overall survival of 13.6 months. No patients showed local recurrence. Patch-field PBT supported by substantial QA therefore is one of the treatment options for large HCC.
Keywords: hepatocellular carcinoma, proton beam therapy, patch-field technique, dose homogeneity
INTRODUCTION
Proton beams, with their ability to emit high energy after penetration to a certain depth, are used for the treatment of various kinds of cancer, and hepatocellular carcinomas (HCCs) are especially amenable to proton beam therapy (PBT). The main benefits of PBT are superior localization control and lower toxicity [1–8]. Moreover, PBT maintains its efficacy in the treatment of larger tumors when compared with other less invasive local treatments such as radiofrequency ablation (RFA) and/or stereotactic body radiotherapy (SBRT). For example, the treatable tumor size is <5 cm for RFA or SBRT, but PBT achieves sufficient results, even for tumors >5 cm in diameter [2, 9]. Moreover, we previously investigated PBT applications for large HCC (defined as those >10 cm in diameter) without the use of the patch-field technique, but using a single irradiation field, and reported a 2-year local control (LC) rate of 87% without severe late treatment-related toxicity [10].
In recent years, there has been a trend toward widening the PBT irradiation field, since technical advances in the development of wobbler methods or electromagnetic scanning techniques have enabled a wider-range beam delivery than with the traditional double-scattering method [11–13]. Moreover, it is expected that the use of wide-field irradiation (e.g. for whole-brain and spinal cord irradiation) will increase due to insurance adoption of PBT for pediatric cancer patients. However, as of 2017, only 5 of 12 Japanese facilities have an irradiation field of >20 cm, and even our hospital has a field size of only 15 × 15 cm (width and length). Thus, it is necessary to establish clear methods for treating those patients who require large irradiation fields to encourage the adoption of the more effective wider-beam techniques.
We previously investigated geometric and dose distribution accuracies of the PBT patch-field technique and showed that irradiation doses at a junction calculated by a treatment-planning system (TPS) had an error range within 4.3% of the actual measured dose in esophageal cancer patients [14]. Before this study, we have used the PBT patch-field technique to treat esophageal cancer patients, as well as to conduct whole-brain and spinal cord irradiation of pediatric cancer patients in clinical practice.
We have recently treated patients with large HCC using our novel patch-field protocol, and here we present our method and investigate the dose homogeneity, safety and antitumor effectiveness.
METHODS AND MATERIALS
Patient and tumor data
We retrospectively reviewed the patients with HCC who had received PBT at our institute and whose irradiation field was beyond the limit of our institute (15 × 15 cm) from April 2011 to May 2016. All study procedures involving human participants were conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. All treatments were discussed at in-hospital conferences, and study-specific informed consent was obtained from the individual participants. The study received institutional review board approval. The study population was comprised of nine patients (six men and three women) of ages 52–79 years (median: 66). Six patients had solitary tumors and three patients had multiple tumors. The maximum tumor diameter was 11–20 cm (median: 15). All multiple tumors were combined into a single clinical target volume (CTV) and treated at the same time. The cranial–caudal diameters of the CTV ranged from 15–19 cm (median: 16). There were two patients whose maximum tumor diameter was <15 cm but CTV was CTV>15 cm due to inclusion of multiple tumors in the CTV. No patients had distant metastasis. The Eastern Cooperative Oncology Group performance status (PS) was as follows: four patients were in Category 0 (fully active), three were in Category 1 (ambulatory but restricted) and two were in Category 2 (up and about >50% of waking hours). Two patients had hepatitis C virus and four patients had alcoholic liver damage. Six patients were categorized as Child–Pugh Class A and three patients as Class B. A total of four patients had tumor thrombosis (portal vein: two; hepatic vein and inferior vena cava: one; both: one). A total of four patients received transcatheter arterial chemoembolization/infusion before PBT. Patient and tumor characteristics are shown in Table 1.
Table 1.
Characteristics of patients
| Age | 52–79 (66) |
|---|---|
| Men/Women | 6/3 |
| Performance status: 0/1/2 | 4/3/2 |
| Child–Pugh classification: A/B | 6/3 |
| Causes of liver damage: HBV/HCV/Alcohol | 0/2/4 |
| Solitary/Multiple | 6/3 |
| Tumor size (cm) | 11–20 (15) |
| Clinical target volume (cm3) | 918–2988 (1348) |
| Prior treatment: TACE/TAI | 3/1 |
| Total irradiation dose (GyE) | 60–76.4 (72.6) |
Numbers in parentheses are median values. HBV = hepatitis B virus, HCV = hepatitis C virus, TACE = transcatheter arterial chemoembolization, TAI = transcatheter arterial infusion, GyE = Gray equivalent.
Treatment
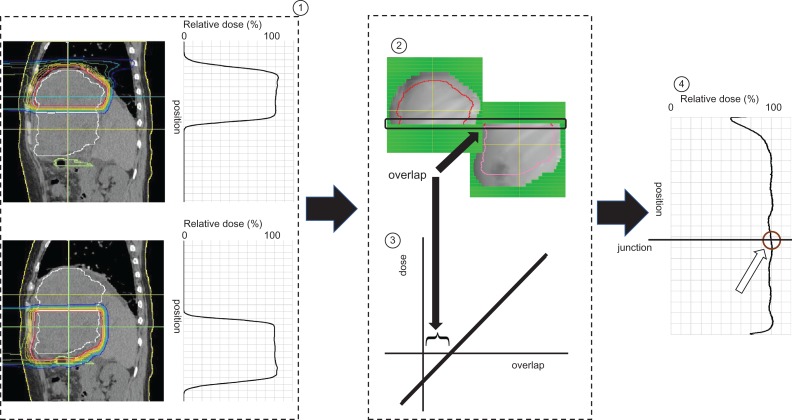
Each patient’s body was immobilized using a custom-made body cast. Computed tomography (CT) images were taken at 2.5 or 5 mm intervals during the end of expiratory phase under a respiratory gating system [15]. During treatment planning, an aperture margin of 5–12.5 mm and a margin of 0–5 mm on the caudal axes were added to cover the entire CTV. Patch-field irradiation was then performed as previously reported [14]. Briefly, the CTV was divided into two sections covered by a single PBT field (cranial CTV and caudal CTV), and both isocenters of the CTVs were aligned along the cranial–caudal axis, while the leaves of multileaf collimators (MLCs) were moved to eliminate hot or cold spots. The overlap of the cranial and caudal irradiation fields (calculated geometrically from treatment device parameters such as distance between the two isocenters, snout position, and aperture size of the MLC) was set to 0–0.5 mm. In our previous examination, we found the regression model [dose (%) = 6.7 × overlap (mm) + 94.0] in our PBT and TPS system, and we routinely use this model in the clinical setting to determine the overlap (Fig. 1).
Fig. 1.
Schema of the treatment planning and the quality assurance of the junction. Treatment plans for the cranial and caudal irradiation fields were designed, respectively. The overlap of the irradiation fields was set at between 0 and 0.5 mm. We developed a regression model of the overlap and irradiation dose at the junction. The irradiation dose at the junction was confirmed from the dose profile determined by using imaging plates.
We used the PROBEAT series in the PBT, and the treatment plans were generated in a TPS, VQA, version 2 (both Hitachi, Ltd, Japan). Proton beams of 155 to 250 MeV were generated using a synchrotron accelerator. The beams were delivered using a rotational gantry. The dose distribution was calculated using a pencil-beam algorithm. The beam delivery devices, including a ridge filter and a fine degrader, were selected automatically by the TPS and then adjusted manually. A collimator to shape the lateral edge of the field was produced using a brass array. A range-compensating bolus was fabricated with a material mainly made of acrylonitrile-butadiene-styrene resin. The beam delivery system created a 100% dose level homogeneously, using the spread-out Bragg peak of the proton beams. Since the prescription dose of each field is equal, it is not difficult to set the reference point on either side. At our hospital, we routinely set the reference point of the prescribed dose as the whole reference point on the cranial side.
The dosing and fractionation were decided according to the tumor location and the treatment strategy. The total irradiation dose was 60–76.4 Gray equivalents (GyE) (median 72.6). The most frequent dosage was 72.6 GyE with 22 fractions in four patients. The maximum cumulative dose was set below 50 GyE for the spinal cord, stomach and duodenum, and below 60 GyE for the colon. The dose for the skin was set to make the area where a higher dose (such as 95% dose) was delivered as narrow as possible, while balancing other possible toxicities. The relative biological effectiveness of the PBT was assumed to be 1.1 [16].
No patients received adjuvant therapy after PBT, but five patients received additional drug therapy for recurrence.
Quality assurance at the junction
Quality assurance was assessed by irradiating imaging plates placed on a water-equivalent phantom under actual treatment conditions. The measurement depth in the phantom was decided from the range of the beams penetrating the bolus when the center of the spread-out Bragg peak and CTV were matched at the junction level. The installed phantom was tilted according to the beam angles, and dose profiling was performed along the cranial–caudal axis crossing the center of the junction. (Fig. 1).
Follow-up procedures and evaluation criteria
Acute treatment-related toxicities were assessed during the treatment course. Physical examinations, blood tests, and CT or magnetic resonance imaging (MRI) scans were performed and quality of life was checked at 3-month intervals after PBT. For the patients who could not routinely come to our hospital, we obtained the medical information as far as possible from the main doctor and from letters from the patients and their families. Adverse events were assessed after every procedure, according to the Common Terminology Criteria for Adverse Effects (version 4.03) [17]. Assessment of the responses was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1) [18].
Data analysis
We examined dose homogeneity, safety, and antitumor effectiveness. For analysis of dose homogeneity, a circular region of interest (ROI) of 5-cm diameter was located within a range of 2.5 cm up and down from the center of junction level, and the irradiation dose was calculated. For the analysis of safety, treatment completion rates and adverse effects were examined. For the analysis of antitumor effectiveness, overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan–Meier method.
RESULTS
Dose homogeneity
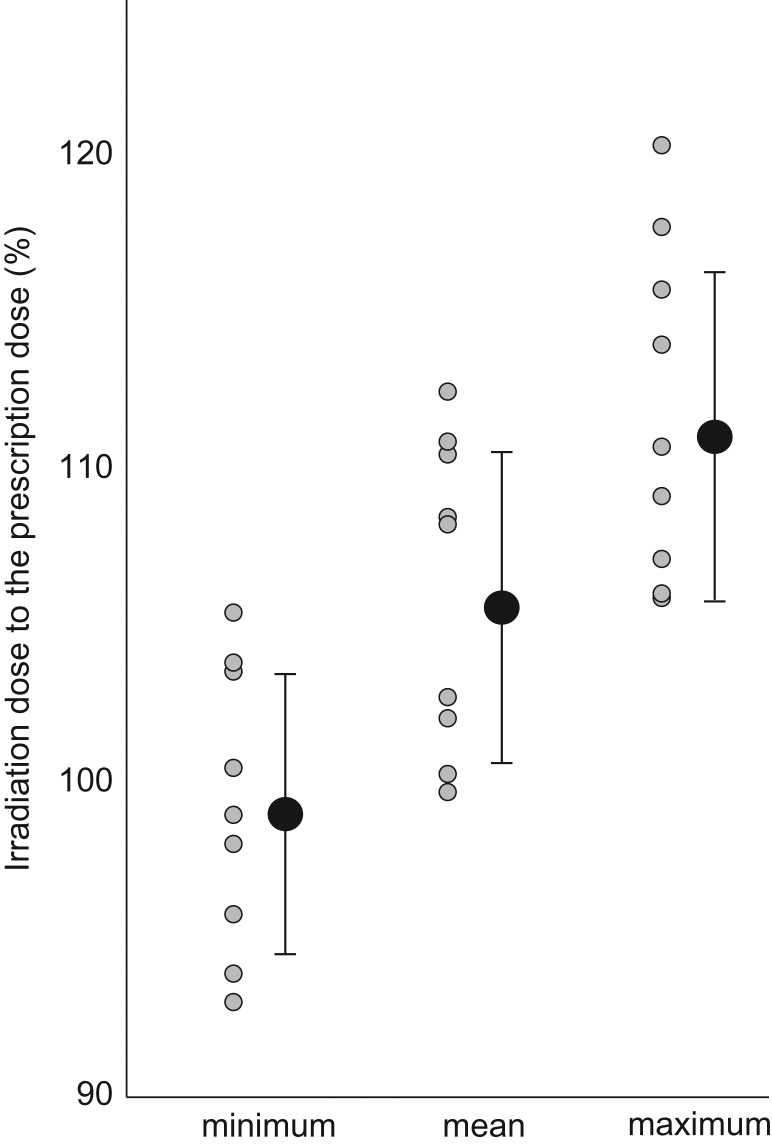
Irradiation dosing around the junction with respect to the TPS-prescribed dosage was a minimum of 93–105% (99.3 ± 4.5), a mean of 99–112% (106.2 ± 4.9) and a maximum of 105–120% (111.9 ± 5.3) (Fig. 2).
Fig. 2.
Irradiation dose at the junction. The minimum, mean and maximum irradiation doses in the region of interest (5 cm diameter circle, located within a range of 2.5 cm up and down from the center of junction) are displayed as percentages of the prescription dose.
Safety
The follow-up period was 2.5–27.3 months (median: 13.7). More than 1 year follow-up was carried out for three patients. Acute dermatitis of Grade 1–2 was observed in all patients. An acute adverse effect of Grade 3 was observed in one patient (hypoalbuminemia), and irradiation was discontinued in that one patient during their treatment courses at 7 days. However, all patients completed their planned dose and number of treatments. A late adverse effect of Grade 3 was observed in one patient (hepatic abscess). A total of four patients showed a Child–Pugh score elevation during the follow-up period (+1: three patients; +2: one patient) and the Child–Pugh class was changed from A to B in three patients and from B to C in one patient.
Antitumor effectiveness
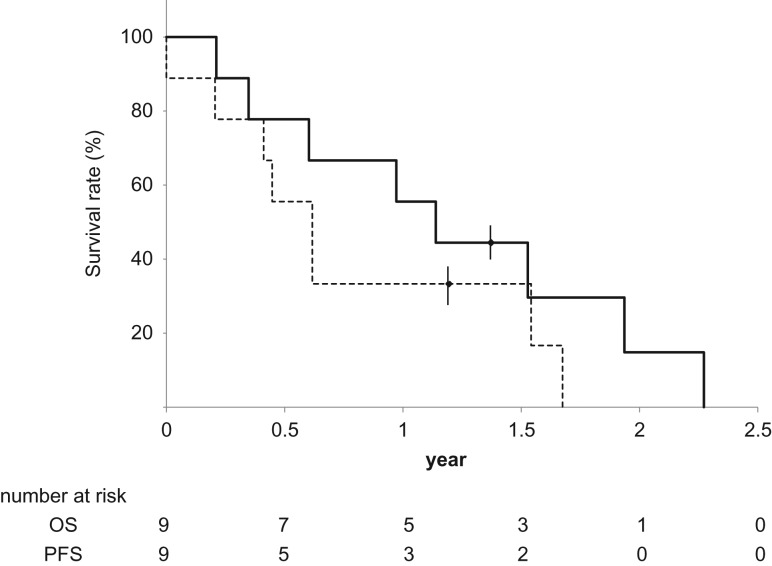
Of the nine patients, one was still alive 16.5 months later at the final follow-up. The OS rates at 1 and 2 years were 55 and 14%, respectively, with a median of 13.6 months. No patients showed recurrence within the PBT field, but six patients showed recurrence outside of the irradiation field. The most frequent organ affected by recurrence was the lung (five patients), followed by the liver, bone, spleen, lymph node, and brain (with a total of ten lesions in five patients) (Table 2). Four of nine patients had tumor thrombosis. Lung metastasis was observed in two of the four patients who had tumor thrombosis, and in three of the five patients who did not have tumor thrombosis. The PFS rates at 1 and 2 years were 33% and 0%, respectively, with a median of 6.1 months (Fig. 3).
Table 2.
Recurrence
| Location | number |
|---|---|
| Lung | 5 |
| Liver (out of the irradiation field) | 1 |
| Bone | 1 |
| Spleen | 1 |
| Lymph node | 1 |
| Brain | 1 |
| Total | 10 lesions in 5 patients |
Fig. 3.
Survival curve. Straight line: overall survival (OS). Dotted line: progression-free survival (PFS).
Case presentation
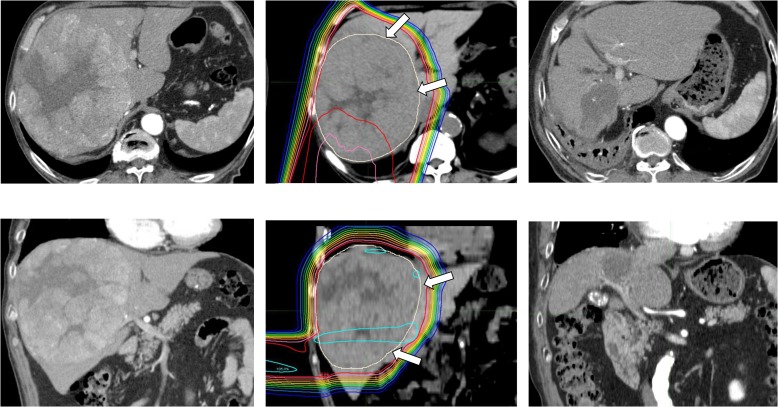
Figure 4 presents an example of a large HCC treated using the patch-field technique. This patient received PBT of 74 GyE with 37 fractions. The minimum and maximum dosing around the junction on the TPS was 93 and 117% of the prescription dose, respectively. The tumor size was reduced from a diameter of 17 cm to 6.7 cm in 14 months, without any severe toxicity. Although the patient died of cerebral infarction 27 months after treatment, no recurrence or liver dysfunction was observed at the final follow-up 20 months after PBT.
Fig. 4.
Dose distribution and computed tomography (CT) before and after PBT. Left: CT before PBT; middle: dose distribution; right: CT 14 months after PBT. Upper: axial image; lower: coronal image. Dose lines represent from 105 to 10% lines relative to the prescription dose from inside to outside. Arrows indicate the clinical target volume (CTV).
DISCUSSION
We previously presented the use of the PBT patch-field technique in esophageal cancer patients in 2012 and found that the minimum and maximum doses were 95.9 ± 3.2% and 105.3 ± 4.1%, respectively, around the TPS junction [14]. Because new CT and TPS units were installed after that study, we conducted a preliminary check of the dose distribution around the junction in recently treated esophageal cancer patients before this study. The irradiation dose around the junction of the recently treated 10 esophageal cancer patients was a minimum of 90–101% (mean ± SD; 95.3 ± 3.7), a mean of 103–112% (106.6 ± 3.5) and a maximum of 103–119% (111.7 ± 4.6) of the TPS. It is important to note that treatment of esophageal cancer is always finely tuned so that the actual irradiation dose does not exceed 100% of the prescribed dose at the center of the junction. As general practice, we have strict safeguards against excess irradiation, and thus a range of 0–0.5 mm overlap is comparable with 6–2.6% below the prescription dose (from our regression model), which complies with our safeguards. We have considerable institutional experience in treating esophageal cancers.
We then turned our attention to other applications for our patch-field technique based on the data and results for esophageal cancer patients. As shown in Fig. 2, a TPS-prescribed minimum, mean and maximum dosage around the junction for large HCC showed a similar pattern to that of recently treated esophageal cancer patients mentioned above, when planning was performed under the same conditions. Therefore, we posited that the patch-field technique could also be applied to liver cancer treatments since the dose distribution data was similar. Moreover, we routinely modify the junction location during a treatment course, so as to reduce any inhomogeneous dose distribution and accomplish safe and efficient treatment.
Although one of nine patients discontinued treatment during the planned treatment course due to acute adverse effects, it is not likely that the hypoalbuminemia that patient experienced was caused by the PBT and the full therapy course was completed. Given that all patients had exhausted other safe and effective treatment options, we were satisfied that no case was accomplished with discontinuation. Furthermore, we were confident that liver function would not be impaired compared with the effects of other treatments because PBT has been established as a modality that causes less liver damage [1–3]. Our previous report of 150 HCC patients treated by PBT supports this, since changes in Child–Pugh scores were elevated in only 11% of patients [19]. Moreover, in another study for large HCC (10–14 cm diameter), only 1 out of 22 patients treated by PBT without using the patch-field technique died of liver failure within 6 months, and that was not due to radiation-induced liver damage, and we thus concluded that large HCC irradiation would not be lethal [10]. However, in the current study, Child–Pugh score elevation was observed in 6 out of 9 patients, and Child–Pugh class elevation was also observed in 4 patients. Although severe liver damage was not observed, our current findings bring into question the concept that PBT is less toxic to the liver than photon therapy, as has been previously reported [5]. One factor that might have affected our results is the size of the tumors we treated, because tumors that require the patch-field technique are obviously larger than any of the reported HCCs treated by PBT. We cannot be certain that the liver damage we saw was directly attributable to the beam, or whether large tumors in general negatively affect the liver due to inflammation from the massive die-off of tumor cells. Therefore, we must recommend that any large tumor therapy with patch-field PBT be carefully and closely monitored to allow for rapid response and re-evaluation of treatment.
The prognosis of large HCC is pessimistic. Mok et al. reported that the OS rate of patients with HCC >10 cm in diameter treated with multimodality non-surgical therapies was 23.3 and 9.6% at 1 and 3 years, respectively [20]. This may reflect both the incidence of microscopic vascular invasion being elevated as size increases, and that the organ hosting the most extrahepatic lesions was the lungs [21–23]. This is in line with our study, in which we found that the most frequent extrahepatic metastases were in the lungs. Although it could not be ruled out that microscopic vascular invasion had already occurred in the patients who developed lung metastasis after PBT, no obvious relation was observed in our study between the presence of tumor thrombosis and the occurrence of lung metastasis. Although it may seem futile to locally treat a large HCC that is likely to metastasize, there was serious concern that the growing tumors would cause high risk of rupture or sudden liver dysfunction due to compression of the portal vein/bile duct. The therapy was well tolerated and no local recurrence was observed. This treatment was considerably challenging either safe and treatment effect point. Under the condition, after treatment with PBT using the patch-field technique, more than half of the patients were still alive after 1 year, and one patient was still alive after 2 years without severe adverse effects. We believe that PBT using the patch-field technique is one of the treatment options for large HCC.
There are some limitations to this study, and there is room to improve the treatment method. First, our follow-up period was short because of the relatively short survival times. Some patients could have died before local recurrence happened, thereby making it difficult to determine local tumor control in long term. Second, we set a constant ROI around the junction to estimate dose homogeneity. Ideally, the dose of the whole CTV should be calculated, but some patients whose tumors extended beyond the field size could not receive enough dose at the margin of the large tumors. The reduced irradiation doses in these cases would not be from the dose inhomogeneity of the junction, but rather from insufficient field size. Thus, we set the ROI (5-cm diameter) as around the junction to remove the bias of the reduced dose at the margin of large tumors. Regarding the treatment, our patch-field technique covered the CTV in only the cranial–caudal direction. However, enlarged tumors often exceed these boundaries along a left–right or anterior–posterior axis, and such tumors would require a three-dimensional enlarged irradiation field, and would require a considerably different therapeutic approach such as esophageal cancer therapy or craniospinal irradiation in pediatric patients. Although it is possible to create irradiation fields that combine the field in the cranial–caudal direction with the fields in other directions on the TPS, the high complexity raises questions as to whether or not the PBT can be performed safely when the further complication of respiratory movement is added. Therefore, we do not combine the field in the cranial–caudal direction with the fields in other directions in routine patch-field treatment.
Recent PBT systems can cover larger targets, leading to increased clinical demands for applications that use the less toxic proton beams. Therefore, we predict that the demand for the patch-field technique will further increase as new protocols are established and verified. Given that situation, more advanced methods for fine alignment of the patch-field technique in three-dimensions, and to accommodate respiratory movement, would be highly desirable, and would probably drive this technology to the next stage.
CONCLUSION
Nine patients with large HCC treated with PBT using the patch-field technique showed no severe adverse events, while favorable local control was established in all cases. PBT using the patch-field technique is one of the treatment options for large HCC.
ACKNOWLEDGEMENTS
This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan, and also supported by the Japan Agency for Medical Research and Development, AMED (15H04901). We thank Bryan J. Mathis, Medical English Communications Center, University of Tsukuba, for grammatical revision of the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1. Mizumoto M, Okumura T, Hashimoto T et al. . Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys 2011;81:1039–45. [DOI] [PubMed] [Google Scholar]
- 2. Fukumitsu N, Sugahara S, Nakayama H et al. . A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2009; 74:831–6. [DOI] [PubMed] [Google Scholar]
- 3. Kawashima M, Furuse J, Nishio T et al. . Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol 2005; 23:1839–46. [DOI] [PubMed] [Google Scholar]
- 4. Bush DA, Kayali Z, Grove R et al. . The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer 2011;117:3053–9. [DOI] [PubMed] [Google Scholar]
- 5. Qi WX, Fu S, Zhang Q et al. . Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol 2015;114:289–95. [DOI] [PubMed] [Google Scholar]
- 6. Hata M, Tokuuye K, Sugahara S et al. . Proton beam therapy for aged patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2007; 69(): 805–12. [DOI] [PubMed] [Google Scholar]
- 7. Fukumitsu N, Okumura T, Takizawa D et al. . Proton beam therapy for metastatic liver tumors. Radiother Oncol 2015;117:322–7. [DOI] [PubMed] [Google Scholar]
- 8. Hong TS, Wo JY, Yeap BY et al. . Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mizumoto M, Tokuuye K, Sugahara S et al. . Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys 2008;71:462–7. [DOI] [PubMed] [Google Scholar]
- 10. Sugahara S, Oshiro Y, Nakayama H et al. . Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2010;76:460–6. [DOI] [PubMed] [Google Scholar]
- 11. Takada Y. Optimum solution of dual-ring double-scattering system for an incident beam with given phase space for proton beam spreading. Nucl Instrum Methods Phys Res 2002;A485:255–76. [Google Scholar]
- 12. Tansho R, Takada Y, Kohno R et al. . Experimental verification of dose calculation using the simplified Monte Carlo method with an improved initial beam model for a beam-wobbling system. Phys Med Biol 2013;58:6047–64. [DOI] [PubMed] [Google Scholar]
- 13. Weber DC, Trofimov AV, Delaney TF et al. . A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys 2004;58:1596–606. [DOI] [PubMed] [Google Scholar]
- 14. Okonogi N, Hashimoto T, Ishida M et al. . Designed-seamless irradiation technique for extended whole mediastinal proton-beam irradiation for esophageal cancer. Radiat Oncol 2012;7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukumitsu N, Ishida M, Terunuma T et al. . Reproducibility of image quality for moving objects using respiratory-gated computed tomography: a study using a phantom model. J Radiat Res 2012;53:945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paganetti H, Niemierko A, Ancukiewicz M et al. . Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407–21. [DOI] [PubMed] [Google Scholar]
- 17. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0. US Department of Health and Human Services, National Cancer Institute, 2010. [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 19. Mizumoto M, Okumura T, Hashimoto T et al. . Evaluation of liver function after proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;82:E529–35. [DOI] [PubMed] [Google Scholar]
- 20. Mok KT, Wang BW, Lo GH et al. . Multimodality management of hepatocellular carcinoma larger than 10 cm. J Am Coll Surg 2003;197:730–8. [DOI] [PubMed] [Google Scholar]
- 21. Paw TM, Delman KA, Vauthey JN et al. . Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086–92. [DOI] [PubMed] [Google Scholar]
- 22. Uchino K, Tateishi R, Shiina S et al. . Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 2011;117:4475–83. [DOI] [PubMed] [Google Scholar]
- 23. Spolverato G, Ejaz A, Kim Y et al. . Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg 2014;18:1284–91. [DOI] [PubMed] [Google Scholar]