Abstract
This study retrospectively evaluated the long-term results of neoadjuvant chemoradiotherapy (NCRT) followed by esophagectomy for the patients with resectable, locally advanced esophageal squamous cell carcinoma (ESCC). Altogether, 49 patients treated from 2008 to 2012 were analyzed. Chemotherapy consisted of 5-fluorouracil and cisplatin. Radiotherapy was performed with a total dose of 40 Gy in 20 fractions for primary tumor, metastatic lymph nodes, and elective nodal area. Subsequently, transthoracic esophagectomy with extensive lymphadenectomy was performed. The median follow-up time for the survivors was 86 (range, 55–111) months. Pathological complete response from NCRT was observed in 17 (35%) patients. The 5-year overall survival and relapse-free survival rates were 56% [95% confidence interval (CI): 43–71%] and 55% (95% CI: 41–69%), respectively. The 5-year locoregional control rate was 84% (95% CI: 74–95%). Multivariate analyses revealed body mass index, N-factor, and %ΔSUVmax as significant factors for overall survival. Recurrences and within–irradiation field failure were observed in 16 (31%) and 4 (8%) patients, respectively. Toxicities of NCRT were generally mild. Postoperative Grade IIIb or worse complications were seen in 14% of patients, including one Grade V case (2%). The 5-year incidence rate of late complications of Grade 3 or worse was 22% (95% CI: 7–36%). The cumulative 5-year incidence rate of metachronous malignancies was 13% (95% CI: 1–26%). NCRT followed by esophagectomy for patients with resectable, locally advanced ESCC showed favorable locoregional control and overall survival, with acceptable postoperative complications. Long-term careful follow-up for late complications and metachronous malignancies is needed.
Keywords: esophageal squamous cell carcinoma (ESCC), neoadjuvant chemoradiotherapy, complications, esophagectomy
INTRODUCTION
For resectable, locally advanced esophageal cancer, neoadjuvant chemoradiotherapy (NCRT) followed by surgery has been the standard treatment in Western countries because several randomized trials and meta-analyses revealed the superior survival benefit of NCRT over surgery alone [1–4]. On the other hand, the Japan Clinical Oncology Group (JCOG) has continuously conducted randomized controlled trials in this cohort. The recent JCOG9907 study [5] showed a better survival benefit in the preoperative chemotherapy group than the postoperative chemotherapy group. However, the superiority of the survival benefits of NCRT over preoperative chemotherapy are still controversial to date [6–8].
Recently, late complications (including cardiopulmonary complications after definitive chemoradiotherapy) have become critical issues [9]. However, the details of late complications of NCRT followed by surgery are not well understood. In our institution, we have performed NCRT with cisplatin (CDDP) and 5-fluorouracil (5-FU) followed by surgery as a protocol treatment for resectable, locally advanced esophageal cancer since 2008. In this study, we retrospectively investigated the long-term results of this treatment, including late complications.
MATERIALS AND METHODS
Eligibility criteria
NCRT followed by surgery was adopted for patients with resectable, locally advanced diseases, with medically operable conditions, and wishing to receive this treatment. Eligibility for this analysis was based on the following criteria: a histologically confirmed thoracic esophageal or esophagogastric junction (EGJ) cancer; Stage IB to IIIC without T4 lesions (according to the 7th edition of the Union for International Cancer Control – TNM Classification) diagnosed via endoscopic ultrasonography, computed tomography (CT) and 18F-fluorodeoxyglucose–positron emission tomography (FDG-PET) CT; age ≤75 years; a performance status of 0 to 2 according to the World Health Organization scale; no prior treatment for previous malignant tumor within 5 years and no synchronous malignant tumor (excluding gastric or esophageal cancer that was controlled via endoscopic resection alone); and NCRT with CDDP and 5-FU followed by surgery performed from 2008 to 2012. There were 88 patients who received NCRT followed by surgery in this study duration, and 49 patients met the eligibility criteria. All patients provided written informed consent, and our institutional review board approved this retrospective study (E-853).
Radiotherapy
Three-dimensional radiotherapy treatment planning was performed for all patients. The gross tumor volume (GTV) included the primary tumor and lymph node (LN) metastasis. The clinical target volume (CTV) was defined as the GTV with a 5-mm margin in all directions plus elective nodal areas, and was adjusted with consideration of the potential spread of microscopic disease according to the anatomical barrier. The elective nodal areas were determined according to primary tumor subsites as follows: supraclavicular to middle mediastinal regions for upper thoracic tumors; upper mediastinal to perigastric regions for middle and lower thoracic tumors; and lower mediastinal to celiac regions for EGJ tumors. Margins of 8–12 mm were added to the CTV to determine the planning target volume (PTV). Multiportal beams were used for reducing the dose to the heart, if possible. A total irradiation dose of 40 Gy in 20 fractions was administered for PTV.
Chemotherapy
The chemotherapy regimen consisted of a combination of CDDP (70 mg/m2 on Days 1 and 29) and 5-FU (700 mg/m2/day on Days 1–4 and 29–32). Our protocol initially consisted of one course of concurrent chemotherapy (15 patients), but was changed to two courses afterward (34 patients).
Surgery
Surgery was planned for 4–8 weeks after the completion of NCRT. The main surgical procedure was a right transthoracic esophagectomy and two- or three-field LN dissection. Patients with upper and middle thoracic esophageal lesions or LN metastasis in the upper mediastinum underwent cervical lymphadenectomy.
Follow-up protocol
Regarding the post-treatment follow-up protocol, enhanced CT for the cervix, chest and abdomen was evaluated at least every 4 months for the first 2 years, every 6 months for the following 3 years and once every year thereafter. Endoscopy for the pharynx, cervical esophagus and gastric tube was evaluated at least every 6 months for the first year and once every year thereafter.
Analysis
Overall survival (OS) was defined as the time from the initiation of NCRT to death from any cause. Relapse-free survival (RFS) was defined as the time from the initiation of NCRT to relapse of disease and/or death from any cause. Locoregional control (LRC) was defined as the absence of local and/or regional recurrence or progression. The Kaplan–Meier method was used to calculate survival rates. The log-rank test was used to compare survival curves in univariate analysis. Multivariate analysis was performed using Cox’s proportional hazards model. Factors with P < 0.10 in the univariate analysis were entered into the multivariate analysis. We used the stepwise forward selection method as the variable selection procedure. A P-value < 0.05 was considered to indicate statistical significance. The adverse events of NCRT were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [10]. Postoperative complications were defined as complications that occurred within 90 days from surgery, and were graded from I to V based on the Clavien–Dindo classification [11]. Late complications were defined as adverse events that occurred beyond 90 days from surgery, and were graded according to CTCAE version 4.0. Statistical analysis was performed via Bell Curve for Excel (Social Survey Research Information Co., Ltd).
RESULTS
Patient and tumor characteristics
The patient and tumor characteristics are summarized in Table 1. Eighteen patients had a history of cardiovascular diseases: hypertension in 14, angina in 2, arrhythmia in 1 and valvular disorder in 1. All patients had squamous cell carcinoma (SCC) histology.
Table 1.
Patient and tumor characteristics
| Characteristics | |
|---|---|
| Gender | |
| Male | 42 |
| Female | 7 |
| Age (years) | |
| Range | 50–75 |
| Median | 66 |
| Performance status | |
| 0 | 41 |
| 1 | 8 |
| 2 | 0 |
| Body mass index | |
| Range | 16.2–31.1 |
| Median | 20.4 |
| DM | |
| Yes | 6 |
| No | 43 |
| CVD history | |
| Yes | 18 |
| No | 31 |
| SCC antigen | |
| Normal | 24 |
| Elevation | 25 |
| Histology | |
| SCC | 49 |
| Tumor location | |
| Upper | 12 |
| Middle | 20 |
| Lower/EGJ | 17 |
| Tumor length (cm) | |
| Range | 2.5–9 |
| Median | 5 |
| T-factor | |
| 1 | 1 |
| 2 | 7 |
| 3 | 41 |
| N-factor | |
| 0 | 10 |
| 1 | 29 |
| 2 | 10 |
| 3 | 0 |
| Stage | |
| IB | 3 |
| IIA/B | 3/8 |
| IIIA/B/C | 26/9/0 |
DM = diabetes mellitus, CVD = cardiovascular disease, SCC = squamous cell carcinoma, EGJ = esophagogastric junction.
Feasibility of NCRT
All patients accomplished the planned radiotherapy. Of the 34 patients who were scheduled to undergo two courses of chemotherapy, 33 (98%) completed the course. Table 2 shows the acute hematologic and non-hematologic toxicities due to NCRT. Principal toxicities of Grade 3 or worse were leukopenia and neutropenia, and Grade 4 leukopenia and neutropenia were observed in 2 (4%) and 4 (8%) cases, respectively. One (2%) patient suffered Grade 3 lung infection. All these toxicities resolved with conservative management.
Table 2.
Acute toxicity by neoadjuvant chemoradiotherapy
| No. of patients (%) | |||||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | |
| Hematologic | |||||
| Anemia | 15 (31) | 8 (16) | 2 (4) | 0 (0) | 0 (0) |
| Leucopenia | 2 (4) | 22 (45) | 18 (37) | 2 (2) | 0 (0) |
| Neutropenia | 7 (14) | 19 (39) | 14 (28) | 4 (8) | 0 (0) |
| Febrile neutropenia | 2 (4) | 0 (0) | 0 (0) | ||
| Thrombocytopenia | 10 (20) | 3 (6) | 2 (4) | 0 (0) | 0 (0) |
| Non-hematologic | |||||
| Nausea | 4 (8) | 10 (20) | 5 (10) | 0 (0) | 0 (0) |
| Esophagitis | 6 (12) | 13 (27) | 6 (12) | 0 (0) | 0 (0) |
| Dermatitis | 8 (16) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Renal function | 5 (10) | 3 (6) | 0 (0) | 0 (0) | 0 (0) |
| Liver function | 1 (2) | 2 (4) | 0 (0) | 0 (0) | 0 (0) |
| Lung infection | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
Assessment of %ΔSUVmax
All patients received a FDG-PET scan before NCRT and before surgery. Each patient had their pre-NCRT scan and their pre-surgical scan carried out at the same institution. The pre-NCRT scan was carried out 1–32 days (median 11 days) before NCRT, and the pre-surgical scan was carried out 5–32 days (median 12 days) before surgery. The rate of decrease in the maximum standardized uptake value (SUVmax) of FDG-PET of the primary tumor before surgery (%ΔSUVmax) was assessed for a preoperative prognostic factor, where %ΔSUVmax = (SUVmax before NCRT – SUVmax after NCRT)/SUVmax before NCRT × 100. Then, the median %ΔSUVmax was 76.2% (range, 11–95%).
Surgical outcomes and pathological effects of NCRT
The median duration between the end of NCRT and surgery was 6 weeks (range, 4–23 weeks). Delay of the planned surgery was observed in 3 patients. One patient suffered persistent bone marrow depression, and underwent surgery 9 weeks after NCRT. Another patient was diagnosed with a liver mass by pre-surgical CT exam. The liver mass was diagnosed as an RT-induced alteration by MRI, and this patient received surgery 12 weeks after NCRT. The other patient suffered Grade 3 lung infection. Surgery was postponed for treatment, and was performed at 23 weeks after NCRT. These 3 patients were alive with no sign of recurrence at the time of the latest follow-up.
Right transthoracic esophagectomy was performed in 48 patients (98%), and video-assisted thoracoscopic esophagectomy was performed in 1 patient (2%). Thirty-seven patients (76%) and 12 (24%) received three-field and two-field LN dissections, respectively. Regarding resection margins, R0, R1 and R2 resection was observed in 46 (94%), 0 (0%) and 3 (6%) patients, respectively. Among the 3 patients with R2 resection, 2 had invasion of the trachea and 1 had metastasis on the surface of the liver. With respect to the pathological responses, pathological complete response (pCR) of primary tumors was observed in 21 patients (43%) and pCR of both primary tumors and LN metastasis was observed in 17 patients (35%).
Postoperative complications
The duration of hospitalization from surgery ranged 17–111 days (median 25 days), and a duration of more than 90 days was observed in 3 patients (6%). Table 3 lists the postoperative complications. Overall, postoperative complications of Grade II or worse were seen in 26 (53%) patients. Among them, severe Grade IIIb or worse complications were observed in 7 (14%) patients as follows: Grade IIIb chylothorax (n = 1), pneumothorax (n = 1) and bleeding (n = 1) from the surgical site; Grade IVa acute respiratory distress syndrome (n = 1), bilateral recurrent nerve paralysis (n = 1), and bleeding from the respiratory tract (n = 1); and Grade V non-occlusive mesenteric ischemia at 3 days from surgery and death at 37 days (n = 1).
Table 3.
Postoperative complications
| n (%) | |
|---|---|
| Postoperative complications | |
| Anastomotic leakage: major | 10 (20) |
| Pleural effusion | 7 (14) |
| Pneumonia | 5 (10) |
| Atrial fibrillation | 5 (10) |
| Recurrent nerve paralysis | 4 (8) |
| Pneumothorax | 4 (8) |
| Bleeding | 3 (6) |
| Chylothorax | 2 (4) |
| Thoracic empyema | 2 (4) |
| Gastric tube ulcer | 1 (2) |
| Wound infection | 1 (2) |
| Acute respiratory distress syndrome | 1 (2) |
| Pneumonitis | 1 (2) |
| Non-occlusive mesenteric ischemia | 1 (2) |
| The most severe grade | |
| 0 | 20 (41) |
| I | 3 (6) |
| II | 7 (14) |
| IIIa | 12 (24) |
| IIIb | 3 (6) |
| IVa | 3 (6) |
| IVb | 0 (0) |
| V | 1 (2) |
Survival, prognostic factors, and patterns of recurrence
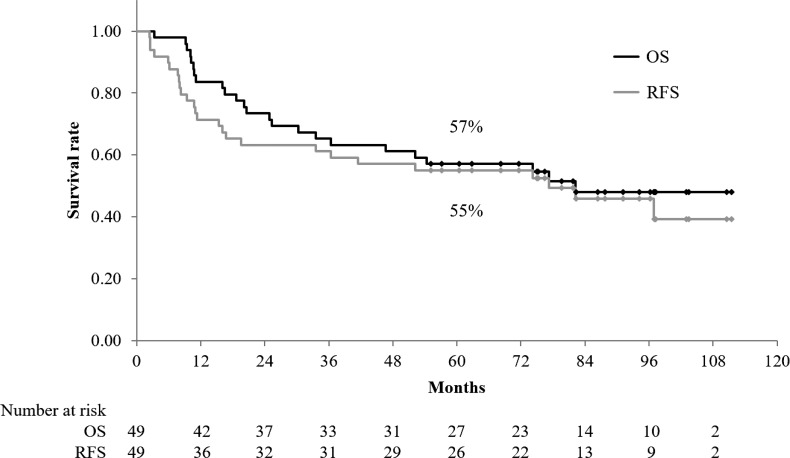
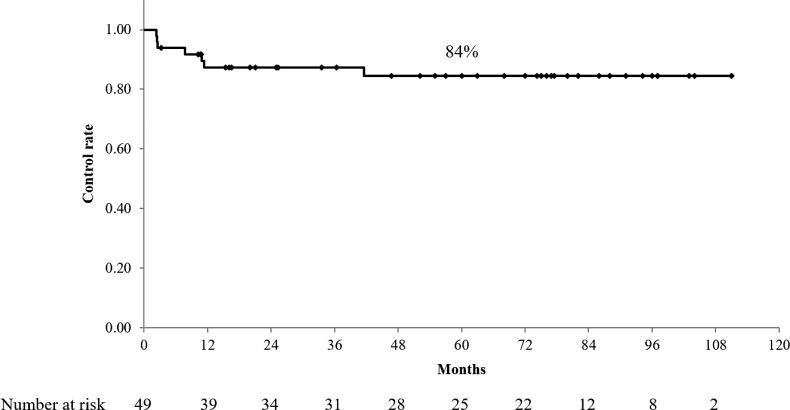
With a median follow-up time for survivors of 86 months (range, 55–111 months), the median OS and RFS were 82 months and 77 months, respectively. The 5-year OS, RFS, and LRC rates were 57% (95% confidence interval [CI]: 43–71%), 55% (95% CI: 41–69%) and 84% (95% CI: 74–95%), respectively (Figs 1 and 2). No significant difference in OS, RFS and LRC was noted between the patients who received one course of chemotherapy and those who received two courses. The prognostic preoperative factors are summarized in Table 4. Univariate analysis showed that age, body mass index (BMI), N-factor, clinical stage, and %ΔSUVmax were significant factors for OS. Meanwhile, multivariate analyses revealed BMI, N-factor and %ΔSUVmax as significant factors for OS. Recurrences were observed in 16 (31%) patients. Regarding the patterns of initial recurrence, locoregional recurrence, locoregional recurrence concurrent with distant metastasis, and distant metastasis were observed in 1 (2%), 3 (6%) and 12 (24%) patients, respectively. Within–irradiation field recurrences were observed in 4 (8%) patients.
Fig. 1.
Overall survival (OS) and relapse-free survival (RFS) for all patients. The 5-year OS and RFS rates were 57% (95% CI, 43–71%) and 55% (95% CI, 41–69%), respectively.
Fig. 2.
Locoregional control (LRC) rate. The 5-year LRC rate was 84% (95% CI, 74–95%).
Table 4.
Prognostic factors
| Factors | n | 5-year OS | P-value | |
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Age (years) | ||||
| <66 | 24 | 67% | 0.036 | 0.145 |
| ≥66 | 25 | 48% | ||
| Performance status score | ||||
| 0 | 41 | 61% | 0.052 | 0.102 |
| 1 | 8 | 38% | ||
| Gender | ||||
| Male | 42 | 57% | 0.834 | |
| Female | 7 | 57% | ||
| Body mass index | ||||
| <20.5 | 26 | 38% | 0.012 | 0.007 |
| ≥20.5 | 23 | 78% | ||
| DM history | ||||
| Yes | 6 | 67% | 0.535 | |
| No | 43 | 56% | ||
| CVD history | ||||
| Yes | 18 | 56% | 0.407 | |
| No | 31 | 58% | ||
| Tumor location | ||||
| Upper/Middle | 32 | 56% | 0.408 | |
| Lower/EGJ | 17 | 59% | ||
| Tumor length (cm) | ||||
| <5.0 | 26 | 54% | 0.772 | |
| ≥5.0 | 23 | 61% | ||
| T-factor | ||||
| 1–2 | 8 | 75% | 0.473 | |
| 3 | 41 | 53% | ||
| N-factor | ||||
| Negative | 10 | 90% | 0.013 | 0.008 |
| Positive | 39 | 49% | ||
| Clinical stage | ||||
| I–II | 14 | 79% | 0.070 | 0.442 |
| III | 35 | 49% | ||
| CEA value | ||||
| Normal | 44 | 59% | 0.624 | |
| High | 5 | 40% | ||
| SCC antigen value | ||||
| Normal | 24 | 63% | 0.506 | |
| High | 25 | 52% | ||
| Hemoglobin value | ||||
| Normal | 36 | 61% | 0.272 | |
| Low | 13 | 46% | ||
| Albumin value | ||||
| Normal | 39 | 62% | 0.116 | |
| Low | 10 | 40% | ||
| Initial SUVmax value | ||||
| <10 | 24 | 54% | 0.939 | |
| ≥10 | 25 | 60% | ||
| %ΔSUVmax (%) | ||||
| <72 | 23 | 39% | 0.034 | 0.020 |
| ≥72 | 26 | 73% | ||
| Course of chemotherapy | ||||
| 1 course | 15 | 50% | 0.706 | |
| 2 courses | 34 | 61% | ||
OS = overall survival, DM = diabetes mellitus, CVD = cardiovascular disease, CEA = carcinoembryonic antigen, SCC = squamous cell carcinoma, SUV = standardized uptake value
Late complications
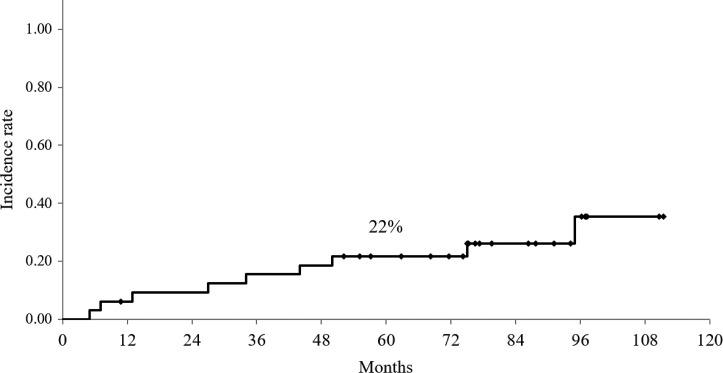
For assessment of late complications and metachronous malignancies, we evaluated 33 patients, excluding those who died of esophageal cancer or who died of a Grade V postoperative complication (described above). Table 5 shows the details of late complications. A Grade 2 anastomotic stricture that needed endoscopic dilatation was observed in 10 patients (30%). Regarding Grade 3 or worse complications, 12 were observed in 10 patients, and the 5-year incidence rate was 22% (95% CI: 7–36%) (Fig. 3). Among them, 2 patients had a gastric tube ulcer that developed 20 and 44 months after surgery. Although one needed management in the intensive care unit for perforation, this patient recovered and was alive at 60 months after surgery. Two patients had heart failure: one needed a pacemaker implantation at 95 months after surgery, and the other died of heart failure at 13 months after surgery. One patient had Grade 3 atrial fibrillation and received ablation at 75 months after surgery. Two patients had Grade 3 pleural effusion that needed drainage at 5 and 7 months after surgery. Three patients had aspiration pneumonia at 4, 27 and 50 months after surgery. One died of food aspiration at 34 months after surgery. One patient suffered myelodysplastic syndrome (MDS) at 30 months after surgery and died at 34 months.
Table 5.
Late complications beyond 90 days from surgery
| Number of events (%) | |||||
|---|---|---|---|---|---|
| Grade 0/1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Anastomotic stricture | 23 (70) | 10 (30) | 0 (0) | 0 (0) | 0 (0) |
| Gastric tube ulcer | 31 (94) | 0 (0) | 1 (3) | 1 (3) | 0 (0) |
| Heart failure | 31 (94) | 0 (0) | 1 (3) | 0 (0) | 1 (3) |
| Atrial fibrillation | 30 (91) | 2 (6) | 1 (3) | 0 (0) | 0 (0) |
| Pericardial effusion | 33 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pleural effusion | 28 (85) | 3 (9) | 2 (6) | 0 (0) | 0 (0) |
| Aspiration | 29 (88) | 0 (0) | 3 (9) | 0 (0) | 1 (3) |
| Myelodysplastic syndrome | 32 (97) | 1 (3) | |||
Fig. 3.
Cumulative incidence rate of late toxicity. The 5-year incidence rate of Grade 3 or worse late toxicity was 22% (95% CI, 7–36%).
Metachronous malignancies
Five patients were diagnosed with metachronous malignancies after treatment, namely, gastric tube cancer at 14 and 37 months after surgery (n = 2), cervical esophageal cancer at 52 months (n = 1), bladder cancer at 26 months (n = 1) and malignant lymphoma of the colon at 87 months (n = 1). The cumulative 5-year incidence rate was 13% (95% CI: 1–26%). Two patients with gastric tube cancer and one patient with cervical esophageal cancer underwent salvage endoscopic resection. One patient with bladder cancer was salvaged via transurethral resection and intravesical chemotherapy. One patient with malignant lymphoma was under treatment at the last follow-up.
DISCUSSION
We retrospectively analyzed the outcomes of NCRT followed by surgery in patients with resectable, locally advanced esophageal SCC. After a median follow-up of 86 months for survivors, we found favorable LRC and OS rates, with acceptable postoperative complications. In addition, our results indicate that long-term careful follow-up for late complications and metachronous malignancies is needed.
For resectable, locally advanced esophageal cancer, higher locoregional control and survival benefits of NCRT plus surgery over surgery alone have been shown [1–4]. The CROSS trial confirmed the superior survival benefits of NCRT over surgery alone for both SCC and adenocarcinoma [3]. In particular, the NCRT group showed a favorable median OS of 81.6 months for patients with SCC. In our study, all patients had SCC histology, and the median OS of 82 months was extremely close to that of the CROSS trial. Our results showed a 5-year LRC rate of 84%, and within–irradiation field recurrences were observed in only 4 (8%) patients. We believe that LRC via NCRT contributed to the favorable survival. As a prognostic factor, Hamai et al. [12] in our institution reported the usefulness of a %ΔSUVmax for esophageal SCC patients treated by NCRT followed by surgery. In this study cohort, %ΔSUVmax was also revealed as one of the significant prognostic factors for OS.
At present, the survival benefits of NCRT over preoperative chemotherapy remain controversial [6–8]. Stahl et al. [6] and Burmeister et al. [7] reported the results of randomized trials comparing preoperative chemotherapy with NCRT. Although both trials showed significantly higher pCR rates and lower locoregional recurrence rates in the NCRT groups, both did not show statistically significant survival benefits. In Japan, preoperative chemotherapy followed by surgery has been the standard therapy for patients with resectable, locally advanced esophageal SCC based on the results of the JCOG9907 trial [5]. This trial showed a better survival benefit in the preoperative chemotherapy group than in the postoperative chemotherapy group (5-year OS: 55% versus 43%, P = 0.04). A recent topic of preoperative chemotherapy is the chemotherapeutic regimen of docetaxel, CDDP and 5-FU (DCF). The efficacy of the DCF regimen in induction chemotherapy for locally advanced head and neck cancer has been reported [13]. For esophageal cancer, Hara et al. [14] reported the results of preoperative chemotherapy with DCF. This study reported a high completion rate of treatment (90.5%), tolerable incidence of operative morbidity, and promising antitumor activity (pCR rate of 17%). To validate the superiority of NCRT over preoperative chemotherapy, two on-going randomized controlled trials have been conducted. One is the Japanese three-arm trial (JCOG1109 NeXT trial) [15] comparing preoperative DCF, preoperative CDDP and 5-FU, and NCRT with CDDP and 5-FU for esophageal SCC patients. The other is the Irish Neo-AEGIS trial (ICORG10–14) [16] comparing preoperative and postoperative chemotherapy with etoposide, CDDP, and 5-FU versus NCRT with carboplatin and paclitaxel for esophageal adenocarcinoma patients.
The use of NCRT has been reported to increase postoperative complications, despite the clinical benefit. Bosh et al. [17] reported that NCRT was significantly associated with an increased risk of pneumonia, pleural effusion, and cardiac arrhythmia, but this did not increase the mortality risk. Morita et al. [18] reported that both pulmonary complications and anastomotic leakage more frequently developed in the NCRT group than in the surgery-alone group. In the analysis of adverse events in the CROSS trial [19], respiratory complications were the most common, followed by anastomotic leakage and cardiac arrhythmias. Grade IIIb or worse Clavien–Dindo postoperative complications were observed in 33% of the NCRT group and 41% in the surgery-alone group. Meanwhile, no difference in the frequency of complications and postoperative mortality was noted. Similar to these reports, the main postoperative morbidities in our study were anastomotic leakage, respiratory complications, and atrial fibrillation. Grade IIIb or worse complications were observed in 14% of patients. This occurrence rate is lower than that of the CROSS trial, and we consider this result as acceptable.
Only a few studies have investigated the late complications after NCRT plus surgery. For a while, serious cardiopulmonary toxicities after definitive CRT for esophageal cancer have been reported [9]. In our study, we examined late complications. Grade 2 anastomotic stricture was the most common event and was observed in 30% of the 33 patients. A total of 12 events of Grade 3 or worse complications were observed in 10 patients, including gastric tube ulcer, cardiac complications, and pulmonary complications. The 5-year incidence rate was 22%, and three patients died of the late complications. One died of heart failure at 14 months after surgery, another died of food aspiration at 34 months, and the other died of MDS. Long-term follow-up to monitor the development of any late complications is needed. The definition of late complications is controversial. We defined late complications as adverse events that occurred beyond 90 days from surgery. The Clavien–Dindo classification is used for early post-surgical complications occurring until the day of initial discharge from hospital, as a rule. Forty-six of 49 patients (94%) were discharged from hospital within 90 days from surgery. In consideration of those, we determined the definition of late complication in this study. However, there is no consensus about the definition of late post-surgical complications. This issue needs to be considered in future reports.
The occurrence of metachronous malignancies of remnant esophagus or other organs are relatively common in patients with esophageal cancer [20–22]. In our study, we observed 5 metachronous malignancies, including remnant esophageal cancer (n = 1), gastric tube cancer (n = 2), bladder cancer (n = 1) and malignant lymphoma (n = 1). The cumulative 5-year incidence rate was 13%, and these malignancies developed within 14–87 months. Thus, long-term follow-up for metachronous malignancies is needed.
This study was limited by its retrospective nature, the small numbers of patients included, and differing numbers of chemotherapeutic courses. However, as there are few studies on the long-term results, including late complications of NCRT followed by surgery for resectable, locally advanced esophageal SCC at a single institution, we consider that the results of this study are of great significance.
In conclusion, NCRT followed by esophagectomy for patients with resectable, locally advanced esophageal SCC showed favorable 5-year LRC and OS rates, with acceptable postoperative complications. Long-term careful follow-up for late complications and metachronous malignancies is needed.
CONFLICT OF INTEREST
The authors state that there are no conflicts of interest.
FUNDING
None
REFERENCES
- 1. Gebski V, Burmeister B, Smithers BM et al. . Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34. [DOI] [PubMed] [Google Scholar]
- 2. Van Hagen P, Hulshof MC, van Lanschot JJ et al. . Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro J, van Lancchot JJ, Hulshof MC et al. . Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–8. [DOI] [PubMed] [Google Scholar]
- 4. Sjoquist KM, Burmeister BH, Smithers BM et al. . Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681–92. [DOI] [PubMed] [Google Scholar]
- 5. Ando N, Kato H, Igaki H et al. . A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68–74. [DOI] [PubMed] [Google Scholar]
- 6. Stahl M, Walz MK, Stuschke M et al. . Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851–6. [DOI] [PubMed] [Google Scholar]
- 7. Burmeister BH, Thomas JM, Burmeister EA et al. . Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer 2011;47:354–60. [DOI] [PubMed] [Google Scholar]
- 8. Hamilton E, Vohra RS,Griffiths EA. What is the best neoadjuvant regimen prior to oesophagectomy: chemotherapy or chemoradiotherapy? Int J Surg 2014;12:196–9. [DOI] [PubMed] [Google Scholar]
- 9. Ishikura S, Nihei K, Ohtsu A et al. . Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 2003;21:2697–702. [DOI] [PubMed] [Google Scholar]
- 10. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, 2010. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (14 June 2018, date last accessed).
- 11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamai Y, Hihara J, Emi M et al. . Ability of fluorine-18 fluorodeoxyglucose positron emission tomography to predict outcomes of neoadjuvant chemoradiotherapy followed by surgical treatment for esophageal squamous cell carcinoma. Ann Thorac Surg 2016;102:1132–9. [DOI] [PubMed] [Google Scholar]
- 13. Blanchard P, Bourhis J, Lacas B et al. . Taxane–cisplatin–fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol 2013;31:2854–60. [DOI] [PubMed] [Google Scholar]
- 14. Hara H, Tahara M, Daiko H et al. . Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 2013;104:1455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura K, Kato K, Igaki H et al. . Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for resectable locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752–5. [DOI] [PubMed] [Google Scholar]
- 16. Keegan N, Keane F, Cuffe S et al. . ICORG 10–14: Neo-AEGIS: a randomized clinical trial of neoadjuvant and adjuvant chemotherapy (modified MAGIC regimen) versus neoadjuvant chemoradiation (CROSS protocol) in adenocarcinoma of the esophagus and esophagogastric junction. Proc Am Soc Clin Oncol 2014;32(5 suppl):abstr TPS4145.http://ascopubs.org/doi/abs/10.1200/jco.2014.32.15_suppl.tps4145 (14 June 2018, date last accessed). [Google Scholar]
- 17. Bosch DJ, Muijs CT, Mul VE et al. . Impact of neoadjuvant chemoradiotherapy on postoperative course after curative-intent transthoracic esophagectomy in esophageal cancer patients. Ann Surg Oncol 2014;21:605–11. [DOI] [PubMed] [Google Scholar]
- 18. Morita M, Masuda T, Okada S et al. . Preoperative chemoradiotherapy for esophageal cancer: factors associated with clinical response and postoperative complications. Anticancer Res 2009;29:2555–62. [PubMed] [Google Scholar]
- 19. Nederlof N, Slaman AE, van Hagen P et al. . Using the Comprehensive complication index to assess the impact of neoadjuvant chemoradiotherapy on complication severity after esophagectomy for cancer. Ann Surg Oncol 2016;23:3964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Urabe Y, Hiyama T, Tanaka S et al. . Metachronous multiple esophageal squamous cell carcinomas and Lugol-voiding lesions after endoscopic mucosal resection. Endoscopy 2009;41:304–9. [DOI] [PubMed] [Google Scholar]
- 21. Morita M, Saeki H, Ito S et al. . Surgical strategies for esophageal cancer associated with head and neck cancer. Surg Today 2013;44:1603–10. [DOI] [PubMed] [Google Scholar]
- 22. Chuang SC, Hashibe M, Scelo G et al. . Risk of second primary cancer among esophageal cancer patients: a pooled analysis of 13 cancer registries. Cancer Epidemiol Biomarkers Prev 2008;17:1543–49. [DOI] [PubMed] [Google Scholar]