Abstract
The dose distribution of passive and scanning irradiation for carbon-ion radiotherapy for breast cancer was compared in order to determine the preferred treatment method. Eleven Japanese patients who received carbon-ion radiotherapy for breast cancer were retrospectively analyzed. The original clinical plans were used for the passive irradiation method, while the plans for the scanning irradiation method were more recently made. Statistical analysis suggested that there was no significant difference in superiority in terms of dose distribution between the passive and scanning irradiation methods. The present study found that the scanning irradiation method was not always superior to the passive method, despite a previous study having reported the superiority of scanning irradiation. The present result is considered to arise from characteristics of breast cancer treatment, such as the simplicity of the organ at risk and the shallow depth point of the target from the skin. It is noteworthy that the present study suggests that the passive irradiation method can provide better dose distribution, depending on the case.
Keywords: carbon-ion radiotherapy, breast cancer, scanning irradiation, passive irradiation
INTRODUCTION
Dose distribution in radiotherapy is desired not only for localization on the tumor but also to avoid damage to organs at risk (OARs). Charged particle therapy, proton or carbon ion, provides better dose distribution compared with conventional radiotherapy or intensity-modulated radiation therapy (IMRT) using high-energy X-rays. This is because charged particles exhibit dose localization at the Bragg peak. If proton and carbon ion beams are compared, the penumbra of carbon-ion beams in the lateral component is sharper than that of proton beams owing to the heaviness of the particle mass. Carbon-ion radiotherapy therefore provides superior physical dose distribution within the external radiation therapy clinically available.
Clinical evidence for the superiority of carbon-ion radiotherapy was established [1] at the Heavy-Ion Medical Accelerator in Chiba (HIMAC) [2] at the National Institute of Radiological Sciences (NIRS), Japan. Two kinds of carbon-ion beam delivery systems have been developed; they are the passive and scanning irradiation methods. Passive irradiation is provided by the use of wobbling magnets, which present a broadened beam to cover the target area in two dimensions (2D) [3], while the scanning method actively scans the target volume in three dimensions (3D) using scanning magnets [4]. Passive beam irradiation has been performed since 1994 at HIMAC, and scanning irradiation was initiated in 2011 as a new carbon-ion radiotherapy method. Since 3D irradiation usually realizes a more flexible dose distribution compared with the 2D method, scanning irradiation is expected to be superior to passive irradiation.
Breast cancer is one of the commonest cancers for women. The standard treatment for early breast cancer is breast-conserving therapy, which consists of breast-conserving surgery and whole-breast irradiation with high-energy X-ray [5–7]. Non-surgical treatments such as radiofrequency ablation (RFA) [8–10], cryoablation therapy [11–13], and high-intensity focused ultrasound (HIFU) [14–16] have been developed in order to achieve a positive cosmetic outcome. With the aim of an even better cosmetic outcome for breast cancer, carbon-ion external radiotherapy without surgery has been underway as a clinical trial at NIRS since 2013 [17, 18]. Here, the passive irradiation method was being applied because scanning irradiation had not matured well enough by 2013 except for prostate cancer treatment. Adaptation of the scanning irradiation method, however, was intended to be expanded. Shiomi et al. studied suitable irradiation methods for pancreatic cancer using carbon-ion radiotherapy; they reported that the scanning irradiation method provided superior dose distribution compared with passive irradiation [19]. However, it has remained questionable as to which irradiation method is the most suitable for the treatment of breast cancer using carbon-ion radiotherapy.
This study compares the dose distribution of carbon-ion radiotherapy using the passive and scanning irradiation methods for early breast cancer. Eleven Japanese patients enrolled in the clinical trial or the advanced medical program at NIRS were analyzed. Evidence for which is the best irradiation method for early breast cancer is discussed.
MATERIALS AND METHODS
Enrolled patients and treatments
Eleven Japanese patients who had carbon-ion radiotherapy using passive beams for breast cancer were retrospectively analyzed. The treatments were conducted at HIMAC between October 2013 and December 2014. The patients were considered to be at low risk, based on Clinical Stage T1N0M0 classification. Surgery was ruled out for physical or mental reasons. A required condition for the carbon-ion radiotherapy was a distance of >5 mm between tumor and skin (for the sake of low skin toxicity). Institutional approval was granted, and informed consent was obtained from all patients before treatment. Characteristics of the enrolled patients are summarized in Table 1.
Table 1.
Details of enrolled patients
| Patient | Age | LRa | Location | Major axis | Distance to skin | Total dose |
|---|---|---|---|---|---|---|
| (year) | (mm) | (mm) | [Gy(RBE)] | |||
| #1 | 64 | R | A | 9 | 20 | 48.0 |
| #2 | 61 | R | C | 20 | 30 | 48.0 |
| #3 | 72 | R | A | 17 | 10 | 52.8 |
| #4 | 76 | R | B | 14 | 15 | 52.8 |
| #5 | 61 | L | A | 4 | 15 | 52.8 |
| #6 | 66 | R | D | 13 | 20 | 52.8 |
| #7 | 56 | R | A | 18 | 29 | 60.0 |
| #8 | 44 | L | C | 14 | 5 | 60.0 |
| #9 | 79 | R | C | 9 | 5 | 60.0 |
| #10 | 70 | R | A | 10 | 20 | 60.0 |
| #11 | 81 | L | C | 20 | 10 | 60.0 |
| Median | 66 | 14 | 15 |
aL = left, R = right.
Treatment planning CT was obtained with a slice thickness of 2.5 mm. A supine treatment position was used due to the physical limitations of the treatment couch. The patients were immobilized by fixation body shells (Shellfitter®; Kuraray, Japan) and a cast (Moldcarer®; Alcare, Japan) on the treatment couch. Carbon-ion beams were accelerated up to 290 MeV/u. The total prescription dose was 48.0, 52.8 and 60.0 Gy (RBE), because a study of dose-escalation trials on breast cancer was concurrently in progress [18]. The total dose was delivered in four fractions, so the treatment was completed in four days. Each fraction was delivered from three ports. The dose weight of the ports was 2:1:1, and the incident directions were from vertical, horizontal and horizontal angles, respectively. The irradiations were performed using respiratory gating [20]. Details of the treatment have been reported by Akamatsu et al. [17], although the patient described in that paper was not included in the present study.
Treatment planning
The gross tumor volume (GTV) was defined as the gross tumor and intraductal components detected by MR (magnetic resonance) images. The cinical target volume (CTV) included the GTV and the region suspected to contain the tumor by MR images. The planning target volume (PTV) was created by adding a margin of 5 mm to the CTV. Only skin was selected as an OAR, since a high radiation dose on the skin often causes skin toxicity, as has been reported in the case of proton radiotherapy [21]. ‘Skin’ was contoured so as to have a thickness of 1 mm from the surface. Healthy breast, heart, ribs, and lung were not included as OARs because they received a negligible dose due to good localization by the carbon-ion beam, even though they are usually considered to be OARs when conventional treatment using high-energy X-rays is performed.
Treatment plans for passive irradiation were prepared by XiO-N software (Mitsubishi, Japan). The original plan used for clinical treatment was used as the passive irradiation plan. A range compensator (made of polyethylene) and a patient collimator (made of brass) were used in order to have appropriate dose depth and to reduce unnecessary exposure owing to lateral components of the beam, respectively [3]. For safety reasons, the original plans were based on the skin dose not reaching half of the prescribed dose; the present planning policy accepted that the dose concentration on the PTV sometimes did not satisfy 95% of the prescribed dose. Tumor control was, however, achieved in all patients [18]. If the skin dose exceeded the limitation, the multileaf collimator was adjusted for the field size to be squeezed and for the skin dose to decrease.
Dose distributions by scanning irradiation were calculated using the treatment planning software XiDose, which was developed and clinically used at NIRS as part of a collaboration with ELEKTA [22–25]. Scanning irradiation plans were newly prepared: the PTV, incident port angles, and dose constraint were copied from those of the passive irradiation plan. Neither range compensator nor patient collimator was used. A pencil-beam algorithm with triple Gaussian forms was employed for calculation. Scanning irradiation has three kinds of range-adjusting methods: range-shifter scanning, hybrid-depth scanning, and active-energy scanning; hybrid-depth scanning is a combination method of range-shifter and active-energy scanning [26]. Since usage of range shifter increases beam spot size owing to multiple scattering, better dose distribution is obtained when hybrid-depth or active-energy scanning is employed. The present study employed active-energy scanning as the scanning irradiation method. A typical beam spot size in the body in the case of a 290 MeV/u carbon beam was 3–4 mm in sigma. If the skin dose exceeded the limitation, the priority of the optimal skin dose was adjusted for a decreased skin dose.
Plan evaluation
Treatment plans were evaluated by dose concentration on the target using dose–volume histograms (DVHs). Dose concentration on the target was assessed by D95, which denotes the dose in Gy (RBE) delivered to 95% of the PTV. The dose to the OAR was taken to be acceptable as long as the skin dose did not reach half of the prescribed dose.
A conformity index is often used for evaluation of the dose concentration at the target. A hot spot within the breast, however, does not clinically matter because the OAR around the PTV is only skin. Therefore, a conformity index was not employed for plan evaluation in the present study.
RESULTS
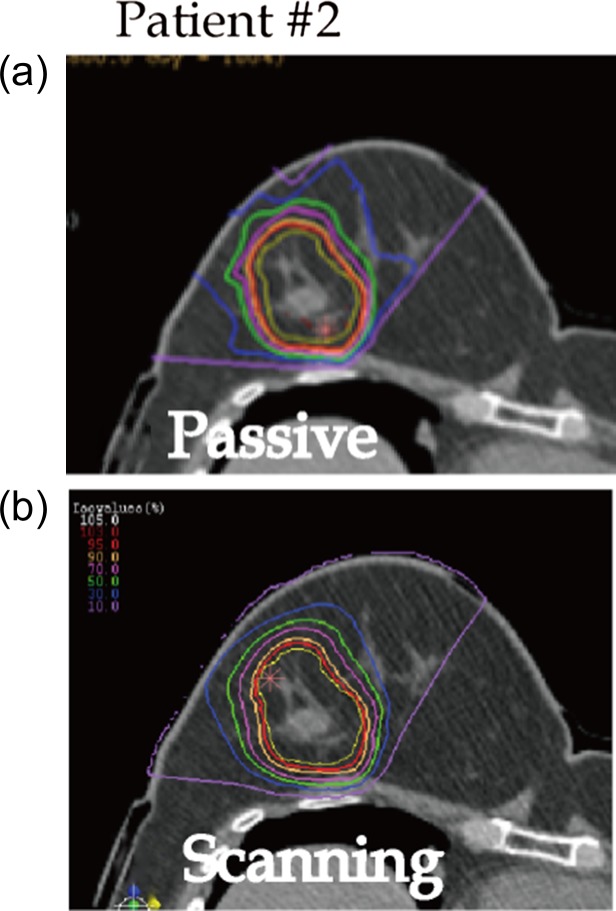
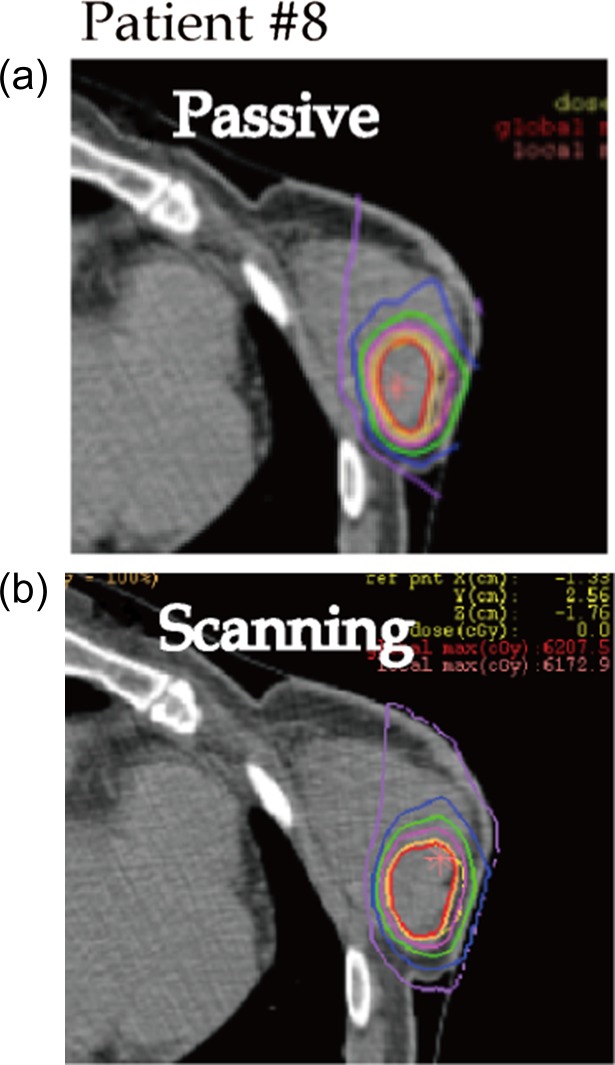
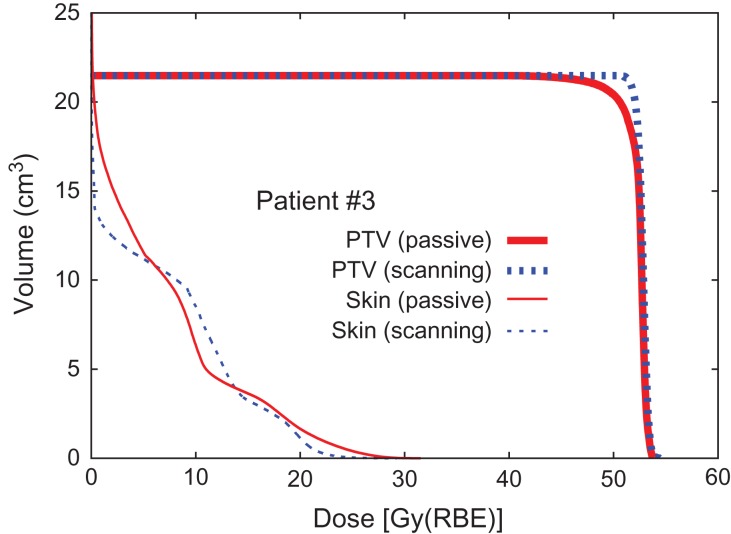
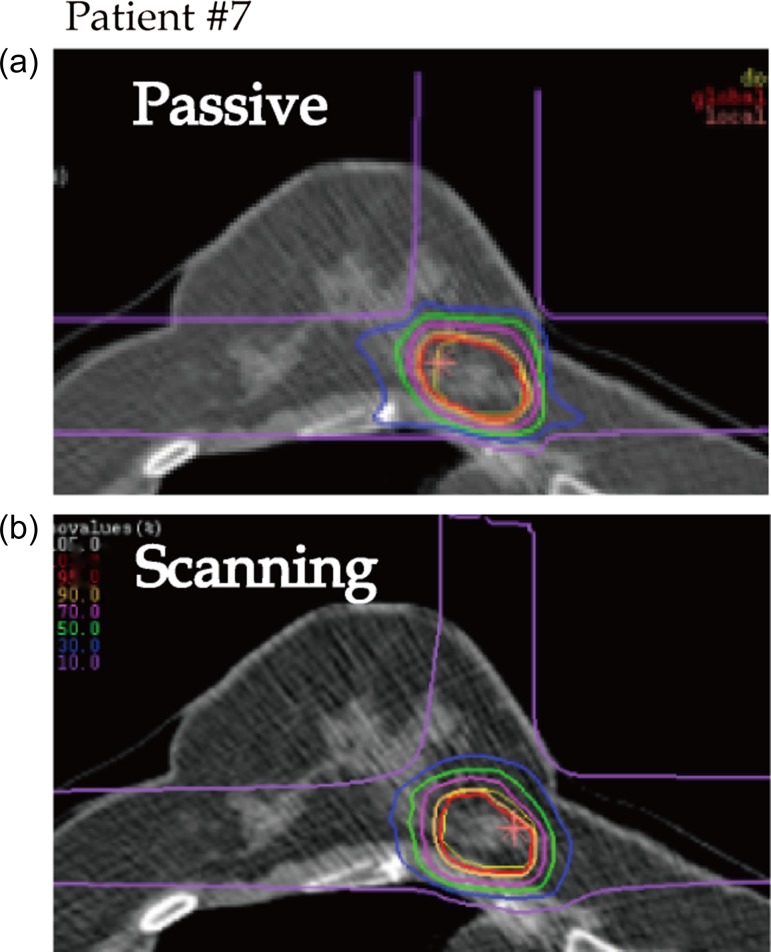
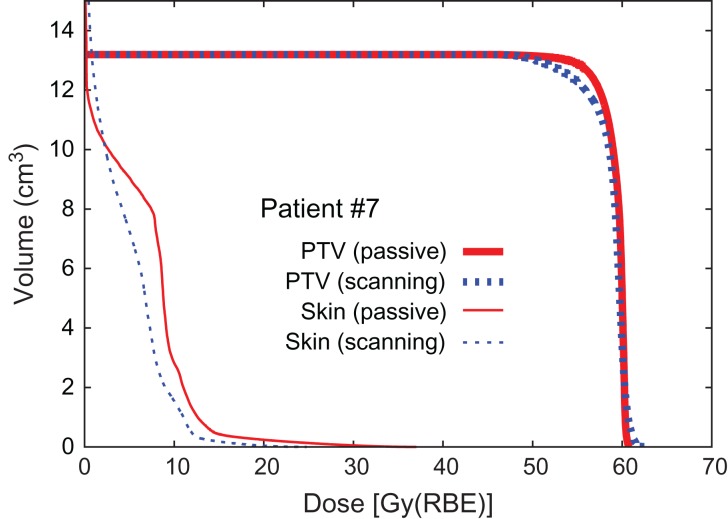
Dose distribution and DVHs for Patients #2, #8 and #3 are shown in Figs 1, 2, 3, 4, 5 and 6, respectively, as typical examples. They correspond to ‘best case’, ‘worst case’, and ‘most differing case’, respectively: the ‘best case’ was that in which both plans achieved a high dose in terms of D95; the ‘worst case’ was that with a low dose for both of the plans; and the ‘most differing case’ occurred when the difference in D95 between the two plans was greatest. In addition to the above, the dose distribution and the DVH for patient #7 are drawn in Figs 7 and 8 in order to show the case for which carbon-ion radiotherapy with passive irradiation was better. Dose distributions by passive and scanning irradiation methods at the same axial slice are shown in the top and bottom panels, respectively, in Fig. 1 for Patient #2, Fig. 3 for Patient #8, in Fig. 5 for Patient #3, and in Fig. 7 for Patient #7. The red star symbol in the figures of dose distribution denotes the highest dose point in the slice.
Fig. 1.
Typical dose distributions of ‘best case’ (Patient #2). Yellow line denotes PTV. Isodose lines of 95%, 90%, 70%, 50%, 30% and 10% for the prescribed dose are colored in red, orange, pink, green, blue and purple, respectively. Red star denotes the highest dose point in the slice.
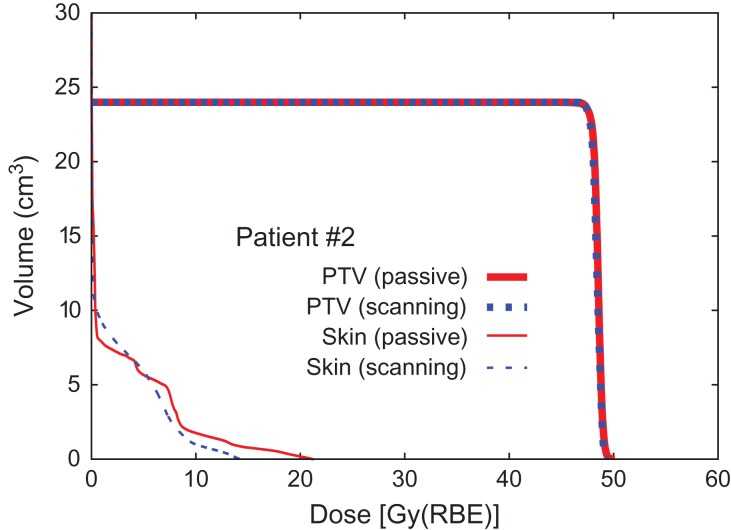
Fig. 2.
Dose–volume histogram (Patient #2).
Fig. 3.
Typical dose distributions of ‘worst case’ (Patient #8). Lines are colored as in Fig. 1.
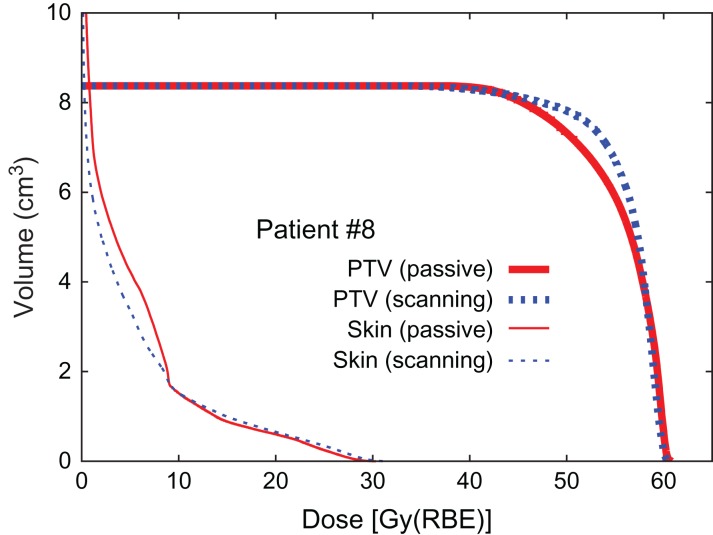
Fig. 4.
Dose–volume histogram (Patient #8).
Fig. 5.
Typical dose distributions of ‘most differing case’ (Patient #3). Lines are colored as in Fig. 1.
Fig. 6.
Dose–volume histogram (Patient #3).
Fig. 7.
Typical dose distributions (Patient #7). Lines are colored as in Fig. 1.
Fig. 8.
Dose–volume histogram (Patient #7).
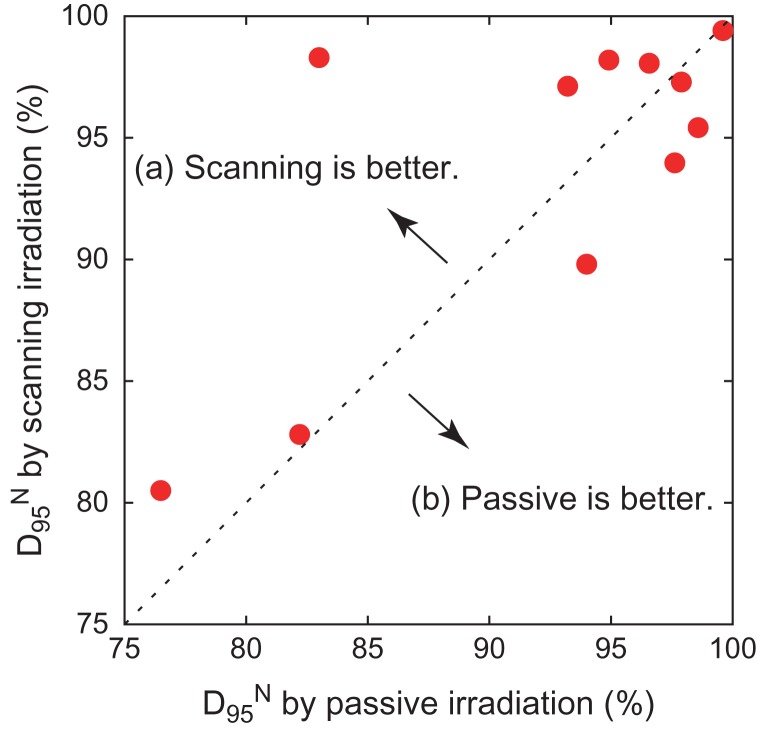
The results for dose concentration at the target for all patients are summarized in Table 2. Here, D95 is expressed as a percentage of the prescribed dose as normalized D95 (D95N) in order to ignore the variety of prescribed doses among the patients. The results of D95N are also visually shown in Fig. 9 as a 2D scatter plot, where data plotted in the left-upper panel (a) denote that scanning irradiation is better than the passive kind, while data in the right-bottom panel (b) indicate the opposite. The results shown in Fig. 9 make it difficult to reach a conclusion in terms of method superiority, since the data are scattered in both panels (a) and (b), although the median values of D95N suggest that the scanning irradiation method is slightly superior to the passive one. Statistical analysis, thus, was applied to the D95N data in order to clarify which of the two irradiation methods was superior. It was found that the P value obtained when comparing passive and scanning irradiation methods was 0.58, when a two-sided Wilcoxon signed-rank test was employed for comparison, suggesting that there was no significant difference in superiority between the passive and scanning irradiation methods for breast cancer. Although four patients with a distance to skin of ≤10 mm were separated off to one side (a) in Fig. 9, a significant difference was again not observed because the derived P value was 0.49.
Table 2.
Summary of DVH values
| D95[Gy(RBE)] | D95N(%) | |||
|---|---|---|---|---|
| Patient | Pass. | Scan. | Pass. | Scan. |
| #1 | 47.0 | 46.7 | 97.9 | 97.3 |
| #2 | 47.8 | 47.7 | 99.6 | 99.4 |
| #3 | 43.8 | 51.9 | 83.0 | 98.3 |
| #4 | 52.0 | 50.3 | 98.5 | 95.3 |
| #5 | 50.1 | 51.8 | 94.9 | 98.1 |
| #6 | 51.6 | 49.7 | 97.7 | 94.1 |
| #7 | 56.4 | 53.9 | 94.0 | 89.8 |
| #8 | 45.9 | 48.3 | 76.5 | 80.5 |
| #9 | 49.3 | 49.7 | 82.2 | 82.8 |
| #10 | 57.8 | 58.8 | 96.3 | 98.0 |
| #11 | 56.1 | 58.3 | 93.5 | 97.2 |
| Median | 94.9 | 97.2 | ||
| P value | 0.58 | |||
The dose delivered to the PTV is expressed in Gy (RBE) and the percentage of the prescribed dose, as D95 and D95N, respectively.
Fig. 9.
Two-dimensional scatter plot of D95N.
DISCUSSION
The present result based on statistical analysis suggests that there is no significant difference in superiority between the passive and scanning irradiation methods. Since scanning irradiation is usually expected to provide better dose distribution, as reported [19], the present result was unexpected. Two reasons arising from the characteristics of the treatment for breast cancer are believed to explain the result: the simplicity of the OAR, and the shallow location of target.
First, skin was the only organ treated as an OAR for the treatment of breast cancer. This corresponds to simplicity of the spatial configuration between target and OAR. In addition to that, the shape of the target of breast cancer is simply globular. As a result, breast cancer treatment does not require the complicated dose distribution that can only be realized by the scanning irradiation method.
Second, the target is located at a shallow position, which means that a low-energy carbon-ion beam can be employed for the treatment. It should be noted that the beam spot size tends to be wider as the beam energy decreases or as the range in the body becomes shorter [26]. As a result, the dose distribution quality by the scanning irradiation method deteriorates owing to blurring of the lateral component of the beam, but the quality of the passive method does not change because of the usage of the patient collimator.
Taking the above two reasons into account, it can be said that the advantage of the scanning irradiation method is more or less neutralized in the case of breast cancer treatment. In contrast, for example, pancreatic cancer is located at a deeper point inside the body and has several OARs, so scanning irradiation provides better dose distribution than passive irradiation [19]. In fact, this would be true for most types of cancer, but not for breast cancer.
It must be emphasized that the present analysis shows the results for just 11 Japanese patients. A significant difference could appear from statistical analysis of a larger sample group. This issue should be studied further, as the overall trend of the present data implies slight superiority of the scanning method. Another possibility for significant difference may be if the study were applied to non-Japanese patients, whose target might generally be located at a somewhat deeper level. Nevertheless, it is valuable to know that the scanning irradiation method may not always be superior to the passive method, and that such superiority would be case-dependent. The present results suggest that carbon-ion external radiotherapy for breast cancer without surgery could be performed at any carbon-ion facility, not necessarily one with the scanning irradiation system, which could contribute to the elimination of regional disparities in cancer care.
CONCLUSION
We investigated whether the passive or scanning irradiation method for carbon-ion radiotherapy for breast cancer was superior. However, no significant difference in dose concentration at the target was found when comparing the two methods. This result was thought to be due to the characteristics of the treatment of breast cancer in contrast to those of other types of cancer. Points of difference from other general treatment cases are (i) the simplicity of the spatial configuration of the OAR and (ii) the shallowness of the target depth. Although further study is still desired, it is worth noting that the present study suggests that the passive irradiation method still provides good dose distribution, depending on the case.
CONFLICT OF INTEREST
The authors state that they have no conflicts of interest.
FUNDING
None
REFERENCES
- 1. Tsujii H, Mizoe J, Kamada T et al. Clinical results of carbon ion radiotherapy at NIRS. J Radiat Res 2007;48:A1–13. [DOI] [PubMed] [Google Scholar]
- 2. Hirao Y, Ogawa H, Yamada S et al. Heavy ion synchrotron for medical use HIMAC project at NIRS-Japan. Nucl Phys A 1992;538:541–50. [Google Scholar]
- 3. Torikoshi M, Minohara S, Kanematsu N et al. Irradiation system for HIMAC. J Radiat Res 2007;48:A15–25. [DOI] [PubMed] [Google Scholar]
- 4. Furukawa T, Inaniwa T, Sato S et al. Performance of the NIRS fast scanning system for heavy-ion radiotherapy. Med Phys 2010;37:5672–82. [DOI] [PubMed] [Google Scholar]
- 5. Clarke M, Collins R, Darby S et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet Oncol 2005;366:2087–106. [DOI] [PubMed] [Google Scholar]
- 6. Haviland JS, Owen JR, Agrawal RK et al. The UK standardization of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomized controlled trials. Lancet Oncol 2013;14:1086–94. [DOI] [PubMed] [Google Scholar]
- 7. Karasawa K, Kunogi H, Hirai T et al. Comparison of hypofractionated and conventionally fractionated whole breast irradiation for early breast cancer patients: a single-institute study of 1,098 patients. Breast Cancer 2014;21:402–8. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto N, Fujimoto H, Nakamura R et al. Pilot study of radiofrequency ablation therapy without surgical excision for T1 breast cancer: evaluation with MRI and vacuum-assisted core needle biopsy and safety management. Breast Cancer 2011;18:3–9. [DOI] [PubMed] [Google Scholar]
- 9. Kinoshita T, Iwamoto E, Tsuda H et al. Radiofrequency ablation as local therapy for early breast carcinomas. Breast Cancer 2011;18:10–17. [DOI] [PubMed] [Google Scholar]
- 10. Noguchi M, Motoyoshi A, Earashi M et al. Long-term outcome of breast cancer patients treated with radiofrequency ablation. Eur J Surg Oncol 2012;38:1036–42. [DOI] [PubMed] [Google Scholar]
- 11. Tozaki M, Fukuma E, Suzuki T et al. Ultrasound-guided cryoablation of invasive ductal carcinoma inside the MR room. Magn Reson Med Sci 2010;9:31–6. [DOI] [PubMed] [Google Scholar]
- 12. Lanza E, Palussiere J, Buy X et al. Percutaneous image-guided cryoablation of breast cancer: a systematic review. J Vasc Interv Radiol 2015;26:1652–7. [DOI] [PubMed] [Google Scholar]
- 13. Simmons RM, Ballman KV, Cox C et al. A phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: results from ACOSOG (Alliance) Z1072. Ann Surg Oncol 2016;23:2438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li S, Wu PH. Magnetic resonance image–guided versus ultrasound-guided high-intensity focused ultrasound in the treatment of breast cancer. Chin J Cancer 2013;32:441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deckers R, Merckel LG, Denis de Senneville B et al. Performance analysis of a dedicated breast MR-HIFU system for tumor ablation in breast cancer patients. Phys Med Biol 2015;60:5527–42. [DOI] [PubMed] [Google Scholar]
- 16. Merckel LG, Knuttel FM, Deckers R et al. First clinical experience with a dedicated MRI-guided high-intensity focused ultrasound system for breast cancer ablation. Eur Radiol 2016;26:4037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akamatsu H, Karasawa K, Omatsu T et al. First experience of carbon-ion radiotherapy for early breast cancer. Jpn J Radiol 2014;32:288–95. [DOI] [PubMed] [Google Scholar]
- 18. Karasawa K, Omatsu T, Wakatsuki M et al. Carbon ion radiation therapy for stage I breast cancer. Int J Radiat Oncol Biol Phys 2016;96:E7. [Google Scholar]
- 19. Shiomi M, Mori S, Shinoto M et al. Comparison of carbon-ion passive and scanning irradiation for pancreatic cancer. Radiother Oncol 2016;119:326–30. [DOI] [PubMed] [Google Scholar]
- 20. Minohara S, Kanai T, Endo M et al. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:1097–103. [DOI] [PubMed] [Google Scholar]
- 21. Whaley JT, Kirk M, Cengel K et al. Protective effect of transparent film dressing on proton therapy induced skin reaction. Radiat Oncol 2013;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inaniwa T, Kanematsu N, Hara Y et al. Implementation of a triple Gaussian beam model with subdivision and redefinition against density heterogeneities in treatment planning for scanned carbon-ion radiotherapy. Phys Med Biol 2014;59:5361–86. [DOI] [PubMed] [Google Scholar]
- 23. Inaniwa T, Kanematsu N. A trichrome beam model for biological dose calculation in scanned carbon-ion radiotherapy treatment planning. Phys Med Biol 2015;60:437–51. [DOI] [PubMed] [Google Scholar]
- 24. Inaniwa T, Kanematsu N, Hara Y et al. Nuclear-interaction correction of integrated depth dose in carbon-ion radiotherapy treatment planning. Phys Med Biol 2015;60:421–35. [DOI] [PubMed] [Google Scholar]
- 25. Inaniwa T, Kanematsu N, Matsufuji N et al. Reformulation of clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys Med Biol 2015;60:3271–86. [DOI] [PubMed] [Google Scholar]
- 26. Inaniwa T, Furukawa T, Kanematsu N et al. Evaluation of hybrid depth scanning for carbon-ion radiotherapy. Med Phys 2012;39:2820–25. [DOI] [PubMed] [Google Scholar]