Abstract
Anti-tumor immunity modulates the local effects of radiation therapy. High mobility group box 1 (HMGB1) plays a pivotal role in activating antigen-specific T-cell responses. Here, we examined the relationship between linear energy transfer (LET) and HMGB1 release. We assessed the proportions of KYSE-70, HeLa and SiHa cells surviving after carbon-ion (C-ion) beam irradiation with different LET values, using a clonogenic assay. The D10, the dose at which 10% of cells survived, was calculated using a linear–quadratic model. HMGB1 levels in the culture supernatants of C-ion beam–irradiated tumor cells were assessed by enzyme-linked immunosorbent assay. The D10 doses for 13 keV/μm of C-ion irradiation in KYSE-70, HeLa and SiHa cells were 2.8, 3.9 and 4.1 Gy, respectively, whereas those for 70 keV/μm C-ion irradiation were 1.4, 1.9 and 2.3 Gy, respectively. We found that 70 keV/μm of C-ion irradiation significantly increased HMGB1 levels in the culture supernatants of all cell lines 72 h after irradiation compared with non-irradiated controls. Furthermore, 70 keV/μm of C-ion irradiation significantly increased HMGB1 levels in the culture supernatants of all cell lines 72 h after irradiation compared with 13 keV/μm. The results suggest that HMGB1 release from several cancer cell lines increases with increased LET.
Keywords: high mobility group box 1, carbon-ion beams, anti-tumor immunity, linear energy transfer, human cancer cells
INTRODUCTION
Radiotherapy (RT) activates anti-tumor immunity. Immunogenic cell death induced by radiation releases danger signals and inflammatory cytokines that promote the ability of dendritic cells (DCs) to present released tumor antigens to T lymphocytes [1–4]. High mobility group box 1 (HMGB1) is one of the key danger signals. HMGB1 acts as a damage-associated molecular marker and triggers the activation of an adoptive immunity by binding to toll-like receptor 4 on DCs [5, 6]. We recently demonstrated that tumor antigen–specific T-cell responses were induced in esophageal cancer patients both during and after chemoradiotherapy, that HMGB1 was significantly increased in the tumor microenvironments of patients with esophageal cancer after preoperative chemoradiotherapy, and that the HMGB1 level was positively correlated with patient survival [7]. Thus, HMGB1 is an important molecule in RT-induced anti-tumor immunity.
Carbon-ion (C-ion) RT is an emerging breakthrough technique in the RT field. C-ion beams have a sharp dose profile based on a Bragg peak, enabling radiation to be applied with a highly concentrated dose distribution while sparing surrounding normal tissues [8]. In addition, C-ion beams at the Bragg peak show high linear energy transfer (LET), thereby providing a high density of energy deposition per unit length [9]. This results in a cell-killing effect two to three times greater than that with X-rays [10]. Several studies have demonstrated favorable clinical outcomes for C-ion RT, especially in X-ray–resistant tumors [11, 12].
HMGB1 is one of the major chromatin-associated non-histone proteins in the nucleus [13]. Heavy ions with high-LET, such as C-ions, induce highly complex DNA lesions comprising double-strand breaks (DSBs) [14, 15]. Hence, it is thought that C-ion irradiation effectively induces HMGB1 release from human cancer cell lines. To test this hypothesis, we previously examined the ability of C-ion beams and X-rays to induce the in vitro release of HMGB1 from human cancer cell lines [16]. There was no significant difference in the amount of HMGB1 released by iso-survival doses of X-rays and C-ion beams, except in NCI-H460 cells; thus, we concluded that comparable levels of HMGB1 were detected after irradiation with X-rays and C-ion beams. In that study, however, the C-ion RT conditions were the same as in the clinical setting, meaning that they included various levels of LET [16]. Thus, it remains unclear whether the amount of HMGB1 released depends on the LET value. Here, we examined the ability of C-ion beams with different LET values to induce in vitro release of HMGB1 from human cancer cell lines originating from different organs.
MATERIALS AND METHODS
Cell lines and cell culture
The human cervical cancer cell lines HeLa and SiHa were purchased from American Type Culture Collection (Manassas, VA). The human esophageal squamous cancer cell line, KYSE-70, was purchased from Health Science Research Resources Bank (Osaka, Japan). Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 5% fetal calf serum (FCS), 50 U/ml penicillin, and 2 mM L-glutamine, and grown at 37°C with 5% CO2. RPMI 1640 and FCS were purchased from Invitrogen (Carlsbad, CA), and penicillin was purchased from Sigma-Aldrich (St Louis, MO).
Carbon-ion beam irradiation
C-ion irradiation was performed at the National Institute of Radiological Sciences (NIRS), Japan, and C-ions were accelerated by the heavy-ion medical accelerator in Chiba (HIMAC). The initial energy of the C-ion beam was 290 MeV per nucleon, and the LET values were 13 and 70 keV/μm, corresponding to a mono-energetic beam with narrow Bragg peak at a depth of 10 cm.
Colony-formation assay
Cells were seeded in 25 cm2 flasks in specified numbers and allowed to attach overnight. The cells were irradiated with C-ion beams and then cultured for 10–14 days. To count colonies, the flasks were washed with saline, fixed with methanol, stained with methylene blue, washed with water, and air-dried. Colonies containing >50 cells were counted. The D10 value, which represents the radiation dose required for reducing the surviving fraction to 10%, was calculated from each survival dataset by a curve-fitting method using the linear–quadratic (LQ) model: SF = exp(−αD−βD2), where SF is the surviving fraction and D is the dose.
HMGB1 enzyme-linked immunosorbent assay (ELISA)
Cells were seeded at 2 × 105 cells per 25 cm2 flask and allowed to attach overnight. The cells were irradiated with C-ion beams at D10 doses, and the medium was exchanged with 3 ml of fresh culture medium. After incubation for the indicated times, the culture supernatants were collected and centrifuged, and the supernatants were collected. The HMGB1 concentrations were measured using an ELISA kit (Shinotest, Tokyo, Japan) according to the manufacturer’s instructions.
Viable and dead cell counting
To assess the number of viable and dead cancer cells at the time of HMGB1 measurement, we counted the number of viable and dead cells 72 h after irradiation. Cells were treated as for the HMGB1 measurements, and the number of viable cells was determined by counting cells excluding trypan blue' stained cells under a microscope. The trypan blue–positive dead cells were counted by an automated cell counter (BIO RAD, Hercules, CA).
Statistical analysis
Statistical analyses were performed by one-way analysis of variance and Tukey’s post hoc test for multiple comparisons. Error bars represent the standard deviation (SD). P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 24.0 for Mac (SPSS, Chicago, IL, USA).
RESULTS
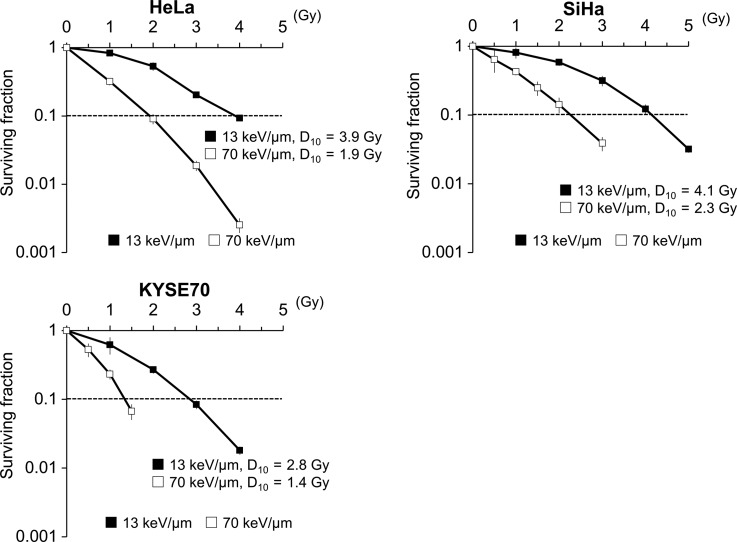
Three human cancer cell lines were treated with C-ion beams, and the surviving fractions were determined by colony-formation assay (Fig. 1). The surviving fractions decreased in a dose-dependent manner with both 13 and 70 keV/μm of C-ion irradiation. The D10 doses for the 13 keV/μm C-ion beam were 2.8, 3.9 and 4.1 Gy for KYSE-70, HeLa and SiHa cells, respectively, whereas those for the 70 keV/μm C-ion beam were 1.4, 1.9 and 2.3 Gy, respectively.
Fig. 1.
Survival curves of irradiated cancer cells. Three human cancer cell lines were treated with C-ion beams (13 or 70 keV/μm) at the indicated doses and then cultured for 10–14 days. The surviving fractions were calculated as ratios relative to non-irradiated controls. The results are expressed as the mean ± SD of three independent experiments.
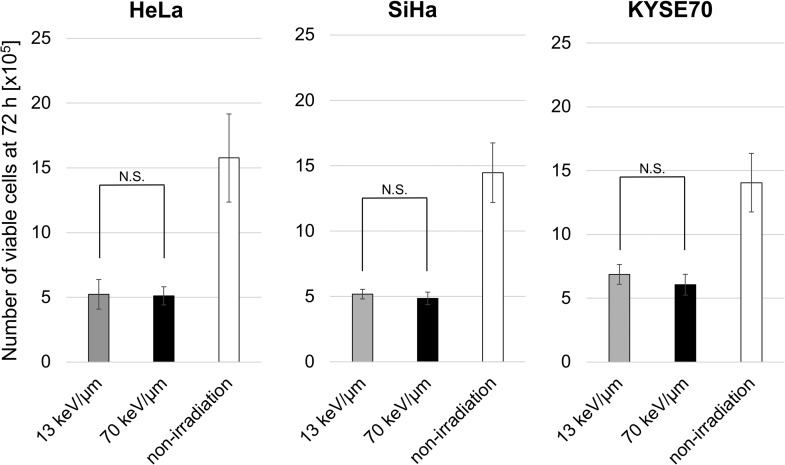
We first assessed the viability of the cells at the time of HMGB1 measurement to examine whether HMGB1 release depends on LET or cell death. The D10 doses of the 13 and 70 keV/μm C-ion beams equally affected the viability of each line at 72 h post irradiation (Fig. 2), confirming that the D10 doses calculated in the clonogenic assay were equivalent in the conditions used for ELISA.
Fig. 2.
Viability of irradiated cancer cells 72 h after irradiation. Three human cancer cell lines were treated with a D10 dose of C-ion beams and then cultured for 72 h. The results are expressed as the mean ± SD of four independent experiments. N.S., not significant.
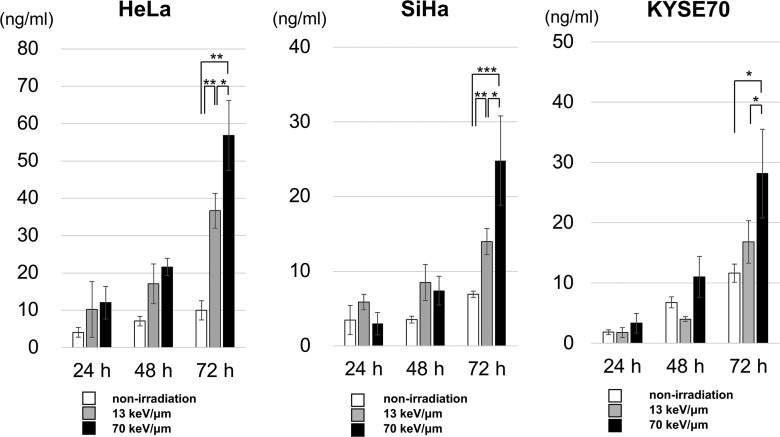
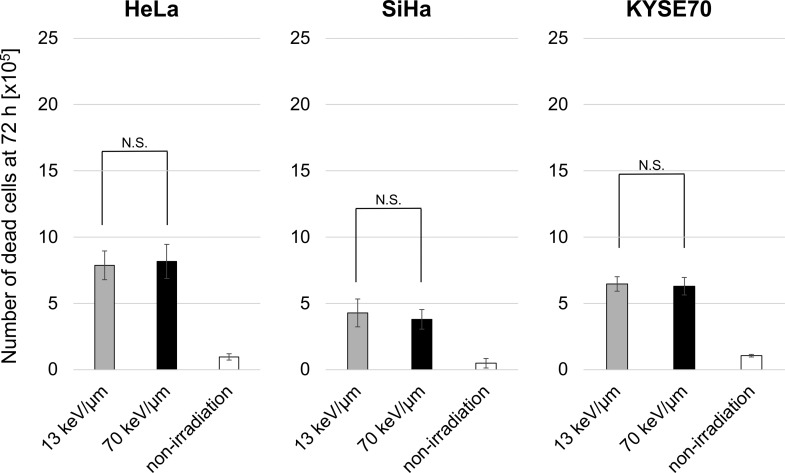
To examine HMGB1 release, the cell lines were treated with D10 doses of the 13 or 70 keV/μm C-ion beams, and ELISA was used to measure the HMGB1 concentrations in the culture supernatant (Fig. 3). We found that 70 keV/μm of C-ion irradiation significantly increased HMGB1 levels in the culture supernatants of all cell lines 72 h after irradiation compared with non-irradiated controls. Furthermore, 70 keV/μm of C-ion irradiation significantly increased HMGB1 levels in the culture supernatants of all cell lines 72 h after irradiation compared with 13 keV/μm. There was no statistically significant difference between any of the cell lines regarding the number of dead cells (Fig. 4). The results indicate that HMGB1 release from cancer cells increased dose dependently with increased LET values at iso-survival doses.
Fig. 3.
HMGB1 release from irradiated cancer cells. Three human cancer cell lines were treated with a D10 dose of 13 or 70 keV/μm C-ion beams and cultured for the indicated times. ELISA was used to measure the HMGB1 concentrations in the culture supernatants. The results are expressed as the mean ± SD of four independent experiments. *, P < 0.05;**, P < 0.01;***, P < 0.001.
Fig. 4.
Number of dead cancer cells 72 h after irradiation. Three human cancer cell lines were treated with a D10 dose of C-ion beams and then cultured for 72 h. The numbers shown are dead cells expressed as the mean ± SD of four independent experiments. N.S., not significant.
DISCUSSION
To the best of our knowledge, this is the first study to demonstrate the relationship between HMGB1 release and the LET value of C-ion RT. Our previous study showed that the levels of HMGB1 released by irradiation were relatively higher than those induced by chemotherapy, despite the presence of nearly equal amounts of dying esophageal cancer cells [7]. In the present study, a high-LET C-ion beam significantly increased HMGB1 release from multiple cancer cell lines compared with low-LET C-ion beams; nevertheless, there was no statistically significant difference in the number of dead cells. Our results herein demonstrate that a high-LET C-ion beam significantly increased HMGB1 release from multiple cancer cell lines compared with low-LET C-ion beams when administered at iso-survival doses. Because HMGB1 stimulates DCs to activate immature T lymphocytes [5, 6], the present study suggests that high-LET irradiation may induce more efficient specific anti-tumor immunity than low-LET irradiation, even if the iso-survival dose is administered. Furusawa et al. reported the LET dependence of cell killing among accelerated ion species and cell strains [17]. They demonstrated that the oxygen enhancement ratio started to decrease at a LET of ~50 keV/μm, and passed below 2 at ~100 keV/μm [17]. This suggested that the characteristic biological advantages of high-LET particles appear from ~50 keV/μm. In fact, the C-ion beam LET value in a previous study by Yoshimoto et al. was <50 keV/μm [16]. Considering these data and the results of the present study, irradiation with higher (>50 keV/μm) LET may be more effective in terms of HMBG1 release from cancer cells. Recently, Oike et al. demonstrated the formation of complex DNA DSBs in a human tumor clinically treated with C-ion RT [18]. Furthermore, their study suggested that the complexity of RT-induced DSBs is related to LET [18]. These irreparable complex DSBs are more likely to lead to cell death than the single DSBs induced by X-rays [19–21]. Thus, C-ion RT with high LET may have dual advantages, i.e. eradicating cancer cells by both inducing severe DNA damage and stimulating anti-tumor immunity.
There are two ways for cells to release HMGB1 into the extracellular environment: passive and active release [22]. In damaged cells, HMGB1 is passively released from the nucleus into the extracellular space through leaky plasma membranes [22]. Wang et al. demonstrated that radiation induces DSBs and induces cytoplasmic HMGB1 translocation and passive release [23]. However, the exact mechanisms of how HMBG1 is released by irradiation remain unclear at the molecular level. A recent study showed that checkpoint kinase 1, which coordinates the DNA damage and cell cycle checkpoint responses, is activated by ~3-fold after C-ion irradiation compared with after an equal physical dose of X-rays [24]. Notably, the cell cycle checkpoint response showed LET dependence. In addition, Meyer et al. demonstrated that the clustered DNA damage caused by C-ion beams induces pan-nuclear H2AX phosphorylation, mediated by the kinases ataxia telangiectasia mutated and DNA-dependent protein kinase [25]. Thus, C-ion irradiation causes nuclear-wide DNA signal expansion, which may cause substantial upregulation of signaling leading to HMGB1 release, especially with high-LET. A limitation of the present study is the lack of proof of the relationship between levels of HMGB1 release and number of DSBs in tumor cells. By quantification of clustered DSBs using a super-high-resolution microscope system, Hagiwara et al. suggested that closely localized DSBs are a risk factor for the formation of chromosomal rearrangement after C-ion RT [26]. The use of such a high-resolution microscope system may facilitate understanding of HMGB1 release under high-LET radiation.
Although C-ion beams with various levels of LET are used clinically, our preclinical study suggests the superiority of more homogeneous high-LET beams. Recently, Bassler et al. proposed a new concept termed ‘LET painting’, which involves the redistribution of high-LET C-ion beams to certain regions of the tumor by field arrangements [27]. They reported that LET painting increases the tumor control probability in hypoxic tumors by localizing the high-LET region to the hypoxic compartment of the tumor [28]. This would induce higher levels of HMGB1 release from tumor cells, which is advantageous for anti-tumor immunity. Thus far, no studies have evaluated the relationship between the LET of C-ion RT and tumor antigen–specific T-cell responses via DC activation by HMGB1 in vivo. Considering the complexity of immune-related factors such as regulatory T cells, myeloid-derived suppressor cells, and programmed death-1/programmed death ligand-1, further studies using animal models are necessary to examine the immunostimulating activities of HMGB1.
In conclusion, we have demonstrated that higher LET C-ion RT induces larger amounts of HMGB1 release, which may lead to more efficient DC-mediated anti-tumor immunity. Further study is required in order to examine the immunological anti-tumor effects of HMGB1 release induced by C-ion RT in animal models.
Supplementary Material
CONFLICT OF INTEREST
The authors state that there are no conflicts of interest.
FUNDING
This work was supported by the Research Project with Heavy Ions at NIRS-HIMAC [Project No. 16J115] and the Japan Society for the Promotion of Science KAKENHI [Grant No. 17K15763].
REFERENCES
- 1. Kalbasi A, June CH, Haas N et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frey B, Rubner Y, Kulzer L et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother 2014;63:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis 2013;4:e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hannani D, Sistigu A, Kepp O et al. Prerequisites for the antitumor vaccine-like effect of chemotherapy and radiotherapy. Cancer J 2011;17:351–8. [DOI] [PubMed] [Google Scholar]
- 5. Apetoh L, Ghiringhelli F, Tesniere A et al. Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13:1050–9. [DOI] [PubMed] [Google Scholar]
- 6. Apetoh L, Tesniere A, Ghiringhelli F et al. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 2008;68:4026–30. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki Y, Mimura K, Yoshimoto Y et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 2012;72:3967–76. [DOI] [PubMed] [Google Scholar]
- 8. Loeffler JS, Durante M. Charged particle therapy—optimization, challenges and future directions. Nat Rev Clin Oncol 2013;10:411–24. [DOI] [PubMed] [Google Scholar]
- 9. Okayasu R. Repair of DNA damage induced by accelerated heavy ions—a mini review. Int J Cancer 2012;130:991–1000. [DOI] [PubMed] [Google Scholar]
- 10. Ando K, Kase Y. Biological characteristics of carbon-ion therapy. Int J Radiat Biol 2009;85:715–28. [DOI] [PubMed] [Google Scholar]
- 11. Shinoto M, Yamada S, Terashima K et al. Carbon ion radiation therapy with concurrent gemcitabine for patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2016;95:498–504. [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto K, Imai R, Kamada T et al. Impact of carbon ion radiotherapy for primary spinal sarcoma. Cancer 2013;119:3496–503. [DOI] [PubMed] [Google Scholar]
- 13. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005;5:331–42. [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Wang X, Zhang P et al. The Ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair 2008;7:725–33. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe R, Wada S, Funayama T et al. Monte Carlo simulation of radial distribution of DNA strand breaks along the C and Ne ion paths. Radiat Prot Dosimetry 2011;143:186–90. [DOI] [PubMed] [Google Scholar]
- 16. Yoshimoto Y, Oike T, Okonogi N et al. Carbon-ion beams induce production of an immune mediator protein, high mobility group box 1, at levels comparable with X-ray irradiation. J Radiat Res 2015;56:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furusawa Y, Fukutsu K, Aoki M et al. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated 3He-, 12C- and 20Ne-ion beams. Radiat Res 2000;154:485–96. [DOI] [PubMed] [Google Scholar]
- 18. Oike T, Niimi A, Okonogi N et al. Visualization of complex DNA double-strand breaks in a tumor treated with carbon ion radiotherapy. Sci Rep 2016;6:22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakajima NI, Hagiwara Y, Oike T et al. Pre-exposure to ionizing radiation stimulates DNA double strand break end resection, promoting the use of homologous recombination repair. PLoS ONE 2015;10:e0122582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amornwichet N, Oike T, Shibata A et al. The EGFR mutation status affects the relative biological effectiveness of carbon-ion beams in non-small cell lung carcinoma cells. Sci Rep 2015;5:11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amornwichet N, Oike T, Shibata A et al. Carbon-ion beam irradiation kills X-ray–resistant p53-null cancer cells by inducing mitotic catastrophe. PLoS ONE 2014;9:e115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:191–5. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, He L, Bao G et al. Ionizing radiation induces HMGB1 cytoplasmic translocation and extracellular release. Guo Ji Fang She Yi Xue He Yi Xue Za Zhi 2016;40:91–9. [PMC free article] [PubMed] [Google Scholar]
- 24. Xue L, Furusawa Y, Okayasu R et al. The complexity of DNA double strand break is a crucial factor for activating ATR signaling pathway for G2/M checkpoint regulation regardless of ATM function. DNA Repair 2015;25:72–83. [DOI] [PubMed] [Google Scholar]
- 25. Meyer B, Voss KO, Tobias F et al. Clustered DNA damage induces pan-nuclear H2AX phosphorylation mediated by ATM and DNA-PK. Nucleic Acids Res 2013;41:6109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hagiwara Y, Niimi A, Isono M et al. 3D-structured illumination microscopy reveals clustered DNA double-strand break formation in widespread γH2AX foci after high LET heavy-ion particle radiation. Oncotarget 2017;8:109370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bassler N, Jäkel O, Søndergaard CS et al. Dose- and LET-painting with particle therapy. Acta Oncol 2010;49:1170–6. [DOI] [PubMed] [Google Scholar]
- 28. Bassler N, Toftegaard J, Lühr A et al. LET-painting increases tumour control probability in hypoxic tumours. Acta Oncol 2014;53:25–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.