Abstract
Schistosomiasis, which is caused by helminth trematode blood flukes of the genus Schistosoma, is a serious health and economic problem in tropical areas, and the second most prevalent parasitic disease after malaria. Currently, there is no effective vaccine available and treatment is entirely dependent on a single drug, praziquantel (PZQ), raising a significant potential public health threat due to the emergence of PZQ drug resistance. It is thus urgent and necessary to explore novel therapeutic targets for the treatment of schistosomiasis. Previous studies demonstrated that acetylcholinesterase (AChE) and nicotinic acetylcholine receptors (nAChRs) play important roles in the schistosome nervous system and ion channels, both of which are targeted by a number of currently approved and marketed anthelminthic drugs. To improve understanding of the functions of the cholinergic system in schistosomes, this article reviews previous studies on AChE and nAChRs in schistosomes and other helminths and discusses their potential as suitable targets for vaccine development and drug design against schistosomiasis.
Keywords: schistosome, acetylcholinesterase, nicotinic acetylcholine receptors, drug targets, vaccine targets
1. Introduction
Schistosomiasis, caused by parasitic flatworms of the genus Schistosoma, remains one of the most insidious and serious of the tropical parasitic diseases of clinical and public health significance. Currently, there is no effective vaccine to prevent schistosomiasis [1] and treatment relies heavily on a single drug, praziquantel (PZQ), despite the fact that it is ineffective in killing the intra-mammalian larval schistosomula stages and that PZQ drug resistance may emerge in the future in endemic areas because of its wide-spread use. Therefore, it is urgent and necessary to explore novel therapeutic targets for the treatment of schistosomiasis. Helminth parasites depend on fast-synaptic transmission in their neuromusculature to interact with the outside environment and respond to it. The neuromuscular system is targeted by a number of currently approved and marketed anthelminthics [2], including levamisole, pyrantel, monepantel [2,3] and metrifonate [4]. Inhibition of neuromuscular activity may lead to loss of muscle function and essential activities, including host attachment, feeding and mating, thereby interfering with parasite maturation [5], and, finally, parasite killing in the host. Metrifonate, targeting schistosome acetylcholinesterase (AChE) was shown to be effective in killing Schistosoma haematobium [6] but not S. mansoni; it was withdrawn from the World Health Organization’s (WHO) list of essential drugs for treating schistosomiasis [7] because of unacceptable toxicity to the host and the variable efficacy against different schistosome species [8].
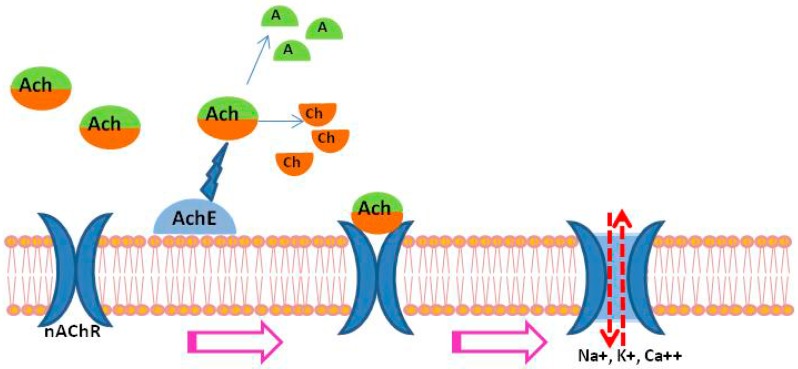
In the cholinergic system of flatworms, AChE plays an important role in regulating the interaction between acetylcholine (ACh) and the parasite nicotinic acetylcholine receptors (nAChRs) [9] by hydrolysing ACh to choline and acetate, allowing ions to pass down electro-chemical gradients into or out of cells [10] (Figure 1). The major role of AChE is termination of transmission at cholinergic synapses by rapid hydrolysis of the neurotransmitter, ACh, with high catalytic activity (at a turnover number of about 10,000/s) [11]. Signaling through cation-selective nAChRs, ACh mediates muscular contraction via membrane depolarization due to an influx of Na+, K+ or Ca++ which may produce clear effects on the worms, typically paralysis [12]. These processes are responsible for the long-term maintenance of low red-cell-membrane potential [13]. Based on a previous study showing exogenous cholinergic, agonists could cause flaccid paralysis of adult trematodes [14], a causal relationship was established between the activation of nAChR in S. mansoni muscle fibers and the flaccid paralysis caused by ACh in whole worms [15]. On the other hand, when AChE activity became inhibited, the higher levels of ACh caused excessive stimulation of nAChR leading to its desensitisation and closing of ion channels [9]. In mammalian cells, 17 known homologous subunits (α1–α10, β1–β4, γ, δ, and ε) assemble into different nAChR subtypes [16], including muscle nAChRs and neuronal nAChR subtypes. Muscle nAChRs are heteropentamers with a stoichiometry of α12β1γδ subunits and are able to be activated by binding to ACh leading to a slow metabolic response. In contrast, neuronal nAChR subtypes are pentamers and are either homomers (α7, α8, α9 and α10) or heteromers of α and β subunits (α4β2, α3β4 and α4α2β3) and also of two different α subunits (α7α8, α9α10) [16]. Neuronal nAChRs are ligand-gated ion channels mediated by fast synaptic transmission. nAChRs contain two or more alpha subunits, and a dimer formed by the alpha subunits with adjacent subunit are critical for ACh binding. The transmembrane domain of each nAChR subunit consists of four helicoidal transmembrane domains (M1 to M4) [16]. The M2 domain of the nAChR subunits form the ion channel, which is only opened by the allosteric conformation change triggered by the binding of ACh. This channel is equally permeable to Na+ and K+, and Ca++ contributes approximately 2.5% to its total permeability [17]. New evidence has indicated direct coupling between G proteins and nAChRs in neurons, leading to a new hypothesis that binding to G proteins modulates the activity and signaling of nAChRs in cells, a process structurally associated with the opening of ligand-gated ion channels [18]. However, it remains to be determined how the binding between ACh and parasite AChRs activates AChRs, leading to opening of the ion channels. Given the studies on AChE and nAChRs in schistosomes and based on the research in mammalian cells mentioned above, the potential cholinergic signaling pathway predicted for schistosomes is shown in Figure 1.
Figure 1.
Predicted cholinergic signaling in schistosomes.
The AChE of schistosomes, located on the surface of worms, breaks down host ACh in the blood into acetate (A) and choline (Ch), preventing overstimulation and blockage of the parasite AChRs. The AChRs of schistosomes having the same location as AChE may consist of pentameric proteins that form an ion channel embedded in the tegument of the parasites [19]. The ACh binds to the ligand binding domain (extracellular part) of nAChR subunits and the activated nAChR causes structural changes in the pentameric proteins, leading to the opening of the ligand-gated ion channels.
2. Acetylcholinesterase (AChE) in Schistosomes
AChE was first identified as a potential drug candidate in adult S. mansoni in 1952 by Bueding [20]. Further study showed that AChE is involved in the motor activity of S. mansoni [14], indicating that the cholinergic mechanisms are associated with neuromuscular function [21,22]. To date, AChE has been characterised from S. mansoni, S. haematobium and S. bovis [9,23], and S. japonicum [24], showing that the enzyme is present on the parasite tegument membrane and in the musculature, both in blood dwelling adults and schistosomula. Importantly, it was shown that AChE activity was highly enriched in the isolated external membrane [24,25] and its presence on the schistosome surface raised the possibility of a role other than termination of synaptic transmission and also suggested that it might prove to be an effective immunological target. A recent schistosome protein microarray study showed a predicted S. japonicum AChE precursor (AY810792) was significantly targeted by protective IgG1 immune responses in S. haematobium-exposed individuals that had acquired drug-induced resistance to schistosomiasis after PZQ treatment [26], thereby supporting consideration of AChE as a suitable anti-schistosomiasis vaccine candidate. Importantly, the absence of cross-reactivity with human AChE further supports schistosome AChE as a suitable target for immunological attack [22]. In vitro studies have shown that polyclonal anti-AChE antibodies are cytotoxic and cause complement-dependent killing 85% of schistosome parasites [22,27]. Most monoclonal antibodies raised in mice against S. mansoni AChE have been shown to interact only with parasite AChE, and not with the vertebrate enzyme, suggesting that the enzymes have different epitopes and that the specific schistosome AChE epitopes might be suitable candidates for drug and vaccine design [28].
Previous studies showed that circulating concentrations of ACh can result in an increase in glucose uptake in schistosomes in vitro [29]. Exposure of S. haematobium or S. bovis, but not S. mansoni, to low concentrations of ACh (10−8 to 10−9 M, the same concentration found in host blood) enhanced glucose uptake by the parasites, whereas at higher concentrations (10−5 to 10−6 M) ACh inhibited glucose uptake from host blood into the parasites. However, the influence of ACh on glucose uptake can also be reduced through inhibition of either tegumental AChE [30] or tegumental nAChR [9] of adult worms. It has been shown that the basal rate of glucose uptake in adult S. haematobium and S. bovis is about twice that in S. mansoni [29]. Indicative of the higher metabolic requirements for glucose in S. haematobium and S. bovis, relatively higher amounts of AChE activity are present on their teguments compared with S. mansoni [31]. These higher levels of AChE activity result in the recorded higher susceptibility to metrifonate [32], and this might explain why metrifonate mediates killing of S. haematobium but not S. mansoni. A recent report showed that in S. japonicum 90% of AChE activity occurs on the tegument [24], indicating the possibility of developing an effective inhibitor targeting AChE in S. japonicum as well. However, tegumental AChE could cause hydrolysis of local host glycogen stores or inhibit glycogen synthesis, either of which would induce more glucose available for the parasite in the local environment [33]. It also has been reported that AChE can increase by 60% glucose uptake by schistosomes from host blood by inhibiting the desensitisation of tegumental nAChR [29].
More recently, genomic studies of S. mansoni [34], S. haematobium [35] and S. japonicum [36] have stimulated further interest in the functional characterisation of cholinergic chloride channels and in revisiting the unusual inhibitory activity of AChE and the interaction between ACh and nAChRs in schistosomes. Previous studies and recent bioinformatics analyses identified different AChE amino acid sequences in S. haematobium and S. japonicum, but only a single AChE amino acid sequence in S. mansoni (Table 1). However, further functional and structural studies of these different types of AChE in schistosomes need to be undertaken, as it is known that multiple isoforms of AChE occur in other parasites [37]. Indeed, early studies showed that two principal molecular forms (both globular) of AChE are present in S. mansoni, whereas very limited information is available on different isoforms of AChE in S. japonicum and S. haematobium. The two forms of S. mansoni AChE (SmAChE), present in approximately equal amounts, with sedimentation coefficients of 6.5S and 8S [38], differ in their solubility characteristics and quaternary structure [39]. One form of SmAChE is internalised in the muscle which interacts with heparin and is involved in cholinergic processes. The other form of SmAChE is surface-localized and is anchored to the membrane via a covalently attached glycophosphatidylinositol (GPI) anchor and may be involved in non-cholinergic processes and in signal transduction [40]. This GPI-anchored AChE could be released from the schistosome surface membranes and attach to a PI-specific phospholipase C (PIPL-C), which can remove considerable quantities of AChE from the tegument of schistosomula in vitro, without impairing the viability of the parasite [41]. The released AChE was considered to trigger immediate replenishment of the surface enzyme. However, this process does occur with another GPI-anchored protein, alkaline phosphatase, which is also present on the schistosome surface [27].
Table 1.
AChEs and nAChRs identified in S. haematobium, S. mansoni and S. japonicum.
| Schistosome Species | AChE | nAChR | ||||
|---|---|---|---|---|---|---|
| Protein ID | Description | Protein ID | Conserved Domains/Motifs | |||
| Ligand Domain | Trans-Membrane Region | Cys-Loop | ||||
| S. haematobium | A_03825 | AChR 1alpha | AAR84357 * | √ | √ | √ |
|
KGB33661 * (XP_012793429 *) |
AChR 2beta | AAR84358 * | √ | √ | √ | |
| AChR 2beta | AAX59989 * | √ | √ | √ | ||
|
KGB33101 * (XP_012792873 *; A_04487 ~) |
nAChR beta 3 (Dbeta 3) subunit | A_01504 ~ | ||||
| A_01761 ~ | √ | |||||
|
KGB37011 * (XP_012796773 *; A_02007 ~) |
Putative nAChR alpha 9b subunit | B_00805 ~ | √ | √ | ||
| AChR beta, putative | A_05298 ~ | √ | ||||
|
AAO49838 * (AAO62355 *, AAQ14322 *) |
AChR-related | A_06497 ~ | √ | √ | ||
| ACh-gated chloride channel-1 (ShACC-1) | A_02378 ~ | √ | √ | √ | ||
| ACh-gated chloride channel-2 (ShACC-2) | A_06346 ~ | |||||
| S. mansoni |
AAQ14321 * (CCD58664 * XP_018645024 *) |
AChR alpha subunit precursor | AAR84361 * | √ | ||
| AChR non-alpha subunit precursor | AAR84362 * | √ | ||||
| ACh-gated chloride channel-1 (SmACC-1) | Smp_176310 # | √ | √ | √ | ||
| ACh-gated chloride channel-2 (SmACC-2) | Smp_142690 # | √ | √ | √ | ||
| Putative nAChR alpha 9b subunit | Smp_135040 # | |||||
| AChR-related | Smp_012000 # | √ | √ | √ | ||
| Putative nAChR subunit | Smp_101990 # | |||||
| Smp_037960 # | √ | √ | √ | |||
| Smp_157790 # | √ | √ | √ | |||
| Smp_132070 # | √ | |||||
| Smp_180570 # | √ | √ | √ | |||
| Smp_197600 # | √ | √ | ||||
| Smp_142700 # | √ | √ | ||||
| Smp_031680 # | √ | √ | √ | |||
| Smp_139330 # | √ | √ | √ | |||
| S. japonicum | Sjp_0045440 # | Putative nAChR alpha 9b subunit | Sjp_0071780 # | |||
| Sjp_0034800 # | √ | √ | √ | |||
| Neuronal AChR subunit alpha-7 | Sjp_0082390 # | √ | √ | |||
| Sjp_0070510 # | AChR-related | Sjp_0131150 # | √ | √ | √ | |
| Sjp_0036280 # | Putative nAChR subunit | Sjp_0015560 # | √ | √ | ||
| Sjp_0066940 # | √ | |||||
| ANH56887 [24] * | ACh-gated chloride channel (SjACC-1) | Sjp_0115170 # | ||||
Note: * protein ID cited from PubMed (https://www.ncbi.nlm.nih.gov/pubmed); # cited from GeneBD (http://www.genedb.org/Homepage); ~ cited from WormBase Parasite (http://parasite.wormbase.org/index.html). Amino acid sequences of all proteins listed in this Table can be found in Supplementary Materials Table S1.
3. Nicotinic Acetylcholine Receptors (nAChRs) in Schistosomes
As indicated earlier, the neuromuscular effects of acetylcholine are typically mediated by gated cation channels of the nicotinic receptor (nAChR) family within the nervous system of parasites. Bentley et al. (2003) identified and reported (https://www.ncbi.nlm.nih.gov/pubmed/) the sequences of two types of nAChRs from S. mansoni, including nAChR alpha subunit precursor (AAR84361) and nAChR non-alpha subunit precursor (AAR84362) but undertook no further characterization of these molecules. Recent bioinformatics analyses identified numerous nAChR subunits in the S. mansoni genome and showed that approximately half of these subunits represented a motif involved in chloride-selectivity [42]. These putative S. mansoni acetylcholine gated chloride channels (SmACCs) form a unique clade within the larger family of nAChRs [42]. Two types of SmACC (SmACC-1 and 2) have been characterized from S. mansoni, both of which localize to regions of the peripheral nervous system that innervate the body wall muscles. RNA interference, targeting 5 putative nAChR subunits (Smp_155790, Smp_0.37960, Smp_132070, Smp_176310 (SmACC-1) and Smp_142690 (SmACC-2); Table 1), was used to knockdown nAChRs in larval schistosomula, and this resulted in 90% and 60% reductions in the transcription levels of SmACC-1 and 2, respectively. Furthermore, SmACC-1 has been shown to play an important role in forming a functional homomeric chloride channel and is activated selectively by a panel of cholinergic agonists [42]. This novel clade of nicotinic chloride channels may act as inhibitory modulators of schistosome neuromuscular function.
Three types of nAChRs have been isolated and partially characterized from S. haematobium, namely ShAR1α (AAR84357) [19,43], ShAR1β (AAR8435) [19] and ShAR2β (AAX59989) [44]. ShAR1α is located on the parasite surface and may contribute to the potentiation of the uptake of glucose from host blood in response to circulating concentrations of ACh [19]. AChE and nAChR are both found to be predominantly concentrated on the dorsal surface of adult male S. haematobium [43], with only low representation on the male head, tail, and ventral surface and gynaecophoric canal of the males and the entire female tegument [45].
Recently, additional putative nAChR subunits have been identified from S. mansoni [34], S. haematobium [35] and S. japonicum [36] (Table 1), but no further functional characterization of these components has been undertaken. The motif analysis (http://www.genome.jp/tools/motif/), that we have undertaken, indicates a highly conserved neurotransmitter-gated ion-channel ligand binding domain and transmembrane region present in the majority of these schistosome nAChR subunits. Both extracellular and intracellular domains occur in the nAChRs of S. haematobium (AAR84357 *, AAR84358 *, AAX59989 *, B_00805, A_02378), S. japonicum (Sjp_0034800 #, Sjp_0131150 and Sjp_0015560) and S. mansoni (AAR84361 *, AAR84362 *, Smp_139330, Smp_031680, Smp_142700, Smp_180570, Smp_132070.1, Smp_132070.2, Smp_012000, Smp_037960, Smp_157790, Smp_142690, Smp_176310). A conserved Cys-loop motif is present in the neurotransmitter-gated ion-channel ligand binding domain of the majority of the S. haematobium, S. mansoni and S. japonicum nAChR subunits as shown in Table 2 and suggests the schistosome nAChRs are members of a Cys-loop ligand-gated ion channel superfamily where the Cys-loop is a 13 amino acid sequence linked by a cysteine disulfide bond contained within the extracellular domain. This Cys-loop domain plays an important role in the structural binding between ACh and nAChR [46] and may be involved in inhibitory amine neurotransmission in parasites [47].
Table 2.
Cys-loop motif sequences present in the nAChR subunits of S. haematobium, S. mansoni and S. japonicum.
| Schistosome Species | nAChR | Cys-Loop Sequence |
|---|---|---|
| S. haematobium | AAR84357 | CNIDILWFPFDEQSC |
| AAR84358 | CDIEVNWFPFDSQNC | |
| AAX59989 | CQVEITYFPFDSQVC | |
| A_01761 | CSVDIKYFPFDRQKC | |
| A_06497 | CPLDVSFFPFDYQTC | |
| A_02378 | CPVKIKYFPYDKQVC | |
| S. mansoni | AAR84362 | CDIEVNWFPFDSQNC |
| Smp_139330 | CDIEVNWFPFDSQNC | |
| AAR84361 | CNIDILWFPFDEQSC | |
| Smp_031680 | CNIDILWFPFDEQSC | |
| Smp_197600 | CKIDITYFPFDDQSC | |
| Smp_157790 | CKIDIKSFPFDEQTC | |
| Smp_132070.1 | CPIDIKNFPFDYQHC | |
| Smp_132070.2 | CPIDIKNFPFDYQHC | |
| Smp_012000 | CPLDVSFFPFDYQTC | |
| Smp_142690 | CQVEITYFPFDSQVC | |
| Smp_037960 | CEVEITYFPFDTQIC | |
| Smp_176310 | CPVKIKYFPYDKQVC | |
| Smp_180570 | CQVDITLFPFDQQNC | |
| S. japonicum | Sjp_0034800 | CNVDVLYFPFDHQLC |
| Sjp_0131150 | CPLDVSFFPFDYQTC | |
| Sjp_0082390 | CDIEVNWFPFDSQNC |
Note: The Cys-loop sequence is shown with the cysteine residues and conserved FP-D-Q highlighted in black shading while gray shading indicates conserved residues.
4. AChE and AChRs in Other Helminths
AChE has been identified in a number of other helminth parasites [48]. ACh and AChE inhibitors relax worm musculature, decrease worm motility and eventually produce paralysis in, for example, Fasciola hepatica [49], Dipylidium caninum [48] and the nematode, Nippostrongylus brasiliensis [50]. An early study showed that N. brasiliensis actively secretes AChE during infection and that this enzyme is able to induce an immune response in infected rats [51]. More recent research indicated that one of the potential functions of AChE in Trypanosoma musculiv is to alter the host cytokine environment and depress the development of M2 macrophages which are deleterious to worm survival [52]. AChE activity has also been detected in the cell bodies and extracellularly in the neuropile of the cerebral ganglia of adult F. hepatica [53]. Further, that study showed an AChE reaction product associated with synaptic endings that localized at the site of synaptic contact between the zone of apposition of the pre- and postsynaptic terminals, suggesting an important role in synaptic transmission in this liver fluke [53]. Changes in the molecular forms and activity of AChE during different life cycle stages in parasites may reflect the presence of a complex nervous system, a situation apparently evident in the cyclophyllidean cestode, Mesocestoides corti, during development of the reproductive apparatus in segmented and adult worms, which opens up the possibility of developing an anti-AChE intervention as an effective therapeutic strategy against this and other tapeworms [54].
AChRs have received considerable recent attention as potential anthelmintic drug targets in nematodes. AChRs represent major targets for cholinergic agonist or antagonist anthelmintic drugs such as levamisole, pyrantel, tribendimidine and derquantel [55]. Despite the wide diversity of nAChRs present in nematodes, which are located not only at the neuromuscular junction but also in the nerve ring and in the pharynx [56], only a few receptor subtypes have so far been characterized. The model free living nematode, Caenorhabditis elegans, has been shown to possess two stimulatory AChRs, one sensitive to levamisole (L-AChR) and the other sensitive to nicotine (N-AChR) [57]. The latter is a homopentamer of the ACR-16 subunit in C. elegans [58], which is highly conserved in a variety of other parasitic nematode species representing different clades. In contrast, L-AChR is composed of five subunits including three α-subunits (unc38, unc-63, and lev-8), and two non-α-subunits (unc-29 and lev-1) [59]. The parasitic nematodes have lost specific subunits and functional receptors, and with different pharmacology, have been produced with different subunit combinations in each species [60]. An analysis of genomic data from 25 nematode parasite species indicated multiple independent duplications of unc-29 as positive, directional selections acting on amino acid positions associated with subunit assembly. It has been demonstrated that functional divergence in AChRs with novel pharmacology occurs more rapidly and may be mediated by alteration of receptor assembly, providing a foundation for understanding the broader context of changing anthelmintic drug targets across the parasitic nematodes [61]. Furthermore, identification of four gene copies of AChRs from the sheep nematode, Haemonchus contortus, also demonstrated that each copy has acquired unique functional characteristics [61].
Functional AChRs have been reported in the pig nematodes Ascaris suum and Oesophagostomum dentatum, and in H. contortus Muscle movement in A. suum is driven by activation of the nAChRs that are found synaptically and extra-synaptically over the surface of the muscle cells. In vitro expression in Xenopus oocytes of two of the A. suum AChR subunits [62], which share similar sequence to unc-29 and unc-38 in C. elegan, showed that changing the expression level of a single receptor subunit dramatically modified the effectiveness of a number of anthelmintics drugs. This information may prove useful in the future development of screens to identifying novel anti-parasite compounds.
Three subtypes of AChR isolated from A. suum have been named as N (for nicotine), L (for levamisole) and B (for bephenium) [12]. In vitro reconstitution experiments showed that receptors with properties similar to those of the ‘N’ and ‘L’ subtypes could be produced by expression of the Asu-unc-29 and Asu-unc-38 subunits [62]. Changing the ratio of the two subunits resulted in differences in sensitivity to both the anthelmintics pyrantel and oxantel, demonstrating that the latter acts on the ‘N’ AChR and the former is more active on the ‘L’ sub-type [63]. However, the lev-8 and lev-1 genes are not detectable in H. contortus. A study on in vitro expression of H. contortus levamisole-sensitive receptors (acr-8, unc-29, unc-38 and unc-63 subunits) in Xenopus oocytes [64] showed that the increased expression of receptors lacking acr-8 might be associated with levamisole resistance [65]. The two well-conserved AChR subunits, unc-38 and unc-29, identified in H. contortus and A. Suum play important roles in rescuing levamisole-resisteance [66].
Four biophysical subtypes of AChR (Ode-unc-38, Ode-unc-63, Ode-unc-29 and Ode-acr-8) have been identified in Oesophagostomum dentatum nAChR and expressed functionally in Xenopus oocytes [60]. The results obtained further supported previous studies showing that a differential combination of these AChRs subunits can affect sensitivity and resistance to the cholinergic anthelmintics pyrantel, tribendimidine and derquantel.
5. Conclusions
AChE and nAChRs play important roles in the neuromuscular and other signaling systems of parasitic helminths and are targets of a number of currently marketed anthelminthics. AChE is an important metabolic enzyme in schistosomes present in the musculature and on the surface of the intra-mammalian blood stages where it has been implicated in the modulation of glucose scavenging from host blood. Targeting schistosome AChE, metrifonate was used historically as an effective drug for the treatment of urinary schistosomiasis, strongly emphasising the important role of the enzyme in the neuromuscular or cholinergic systems. Subsequent studies in the 1980/90s on AChE in S. haematobium and S. mansoni revealed further the neuromuscular effects of AChE through studies inhibiting the interaction between ACh and the nAChR subunits regulating the gated cation channels within the nervous system of both parasites. Three types of nAChRs were initially partially characterized from S. haematobium with two nAChRs isolated from S. mansoni [19,43,44]. Characterisation of ACh-gated chloride channels (SmACCs) in S. mansoni followed in 2014 [42]. These studies emphasised the important role of nAChRs in inhibitory neurotransmission in schistosomes and their potential as major targets for cholinergic agonists. However, our limited understanding of the neuromuscular and cholinergic systems in the blood flukes has hampered the development of effective drugs targeting these pathways. Given the availability of the extensive genomic data for the three major clinically relevant schistosome species now available and recent research advances on AChE/AChRs in other helminths, future research can be expected to result in the discovery of novel drug or vaccine candidates targeting these critical components.
Acknowledgments
We are grateful for funding supported by an Australian Infectious Disease Research Centre Seed Grant, a Program Grant from the National Health and Medical Research Council (NHMRC) of Australia (APP 1037304) and a scholarship from China Scholarship Council (CSC).
Abbreviations
The following abbreviations are used in this manuscript
| PZQ | praziquantel |
| AChE | acetylcholinesterase |
| ACh | acetylcholine |
| nAChRs | nicotinic acetylcholine receptors |
| GPI | glycophosphatidylinositol |
| PIPL-C | PI-specific phospholipase C |
| SmACCs | S. manosni acetylcholine gated chloride channels |
| ShAR1α | S. haematobium nicotinic acetylcholine receptor 1 α |
| ShAR1β | S. haematobium nicotinic acetylcholine receptor 1 β |
| ShAR2β | S. haematobium nicotinic acetylcholine receptor 2 β |
Supplementary Materials
Supplementary materials are available online. Table S1: Amino acid sequences of AChEs and nAChRs identified in S. haematobium, S. mansoni and S. japonicum.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Beaumier C.M., Gillespie P.M., Hotez P.J., Bottazzi M.E. New vaccines for neglected parasitic diseases and dengue. Transl. Res. 2013;162:144–155. doi: 10.1016/j.trsl.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Robertson A.P., Martin R.J. Ion-channels on parasite muscle: Pharmacology and physiology. Invert. Neurosci. 2007;7:209–217. doi: 10.1007/s10158-007-0059-x. [DOI] [PubMed] [Google Scholar]
- 3.Kaminsky R., Gauvry N., Weber S.S., Skripsky T., Bouvier J., Wenger A., Schroeder F., Desaules Y., Hotz R., Goebel T., et al. Identification of the amino-acetonitrile derivative monepantel (AAD 1566) as a new anthelmintic drug development candidate. Parasitol. Res. 2008;103:931–939. doi: 10.1007/s00436-008-1080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueding E., Liu C.L., Rogers S.H. Inhibition by metrifonate and dichlorvos of cholinesterases in schistosomes. Br. J. Pharmacol. 1972;46:480–487. doi: 10.1111/j.1476-5381.1972.tb08145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabtree J.E., Wilson R.A. Schistosoma mansoni: A scanning electron microscope study of the developing schistosomulum. Parasitology. 1980;81:553–564. doi: 10.1017/S003118200006193X. [DOI] [PubMed] [Google Scholar]
- 6.El Ridi R.A., Tallima H.A. Novel therapeutic and prevention approaches for schistosomiasis: Review. J. Adv. Res. 2013;4:467–478. doi: 10.1016/j.jare.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergquist R. Strategies for Control of Infection and Disease: Current Practice and Future Potential. In: Mahmoud A.A.F., editor. Schistosomiasis. Imperial College Press; London, UK: 2001. [Google Scholar]
- 8.Salafsky B., Fusco A.C., Whitley K., Nowicki D., Ellenberger B. Schistosoma mansoni: Analysis of cercarial transformation methods. Exp. Parasitol. 1988;67:116–127. doi: 10.1016/0014-4894(88)90014-8. [DOI] [PubMed] [Google Scholar]
- 9.Jones A.K., Bentley G.N., Parra W.G.O., Agnew A. Molecular characterization of an acetylcholinesterase implicated in the regulation of glucose scavenging by the parasite Schistosoma. FASEB J. 2002;16:441–443. doi: 10.1096/fj.01-0683fje. [DOI] [PubMed] [Google Scholar]
- 10.Thompson A.J., Lester H.A., Lummis S.C. The structural basis of function in Cys-loop receptors. Q. Rev. Biophys. 2010;43:449–499. doi: 10.1017/S0033583510000168. [DOI] [PubMed] [Google Scholar]
- 11.Quinn D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics and virtual transition states. Chem. Rev. 1987;87:955–979. doi: 10.1021/cr00081a005. [DOI] [Google Scholar]
- 12.Wolstenholme A.J. Ion channels and receptor as targets for the control of parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2011;1:2–13. doi: 10.1016/j.ijpddr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennekou P. The voltage-gated non-selective cation channel from human red cells is sensitive to acetylcholine. Biochim. Biophys. Acta. 1993;1147:165–167. doi: 10.1016/0005-2736(93)90328-W. [DOI] [PubMed] [Google Scholar]
- 14.Barker L.R., Bueding E., Timms A.R. The possible role of acetylcholine in Schistosoma mansoni. Br. J. Pharmacol. 1966;26:656–665. doi: 10.1111/j.1476-5381.1966.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day T.A., Chen G.Z., Miller C., Tian M., Bennett J.L., Pax R.A. Cholinergic inhibition of muscle fibres isolated from Schistosoma mansoni (Trematoda:Digenea) Parasitology. 1996;113:55–61. doi: 10.1017/S0031182000066270. [DOI] [PubMed] [Google Scholar]
- 16.Zouridakis M., Zisimopoulou P., Poulas K., Tzartos S.J. Recent advances in understanding the structure of nicotinic acetylcholine receptors. IUBMB Life. 2009;61:407–423. doi: 10.1002/iub.170. [DOI] [PubMed] [Google Scholar]
- 17.Naguib M., Flood P., McArdle J.J., Brenner H.R. Advances in neurobiology of the neuromuscular junction: Implications for the anesthesiologist. Anesthesiology. 2002;96:202–231. doi: 10.1097/00000542-200201000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Kabbani N., Nordman J.C., Corgiat B.A., Veltri D.P., Shehu A., Seymour V.A., Adams D.J. Are nicotinic acetylcholine receptors coupled to G proteins? Bioessays. 2013;35:1025–1034. doi: 10.1002/bies.201300082. [DOI] [PubMed] [Google Scholar]
- 19.Bentley G.N., Jones A.K., Oliveros Parra W.G., Agnew A. ShAR1alpha and ShAR1beta: Novel putative nicotinic acetylcholine receptor subunits from the platyhelminth blood fluke Schistosoma. Gene. 2004;329:27–38. doi: 10.1016/j.gene.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Bueding E. Acetylcholinesterase activity of Schistosoma mansoni. Br. J. Pharmacol. 1952;7:563–566. doi: 10.1111/j.1476-5381.1952.tb00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pax R.A., Day T.A., Miller C.L., Bennett J.L. Neuromuscular physiology and pharmacology of parasitic flatworms. Parasitology. 1996;113(Suppl. 1):S83–S96. doi: 10.1017/S003118200007791X. [DOI] [PubMed] [Google Scholar]
- 22.Espinoza B., Tarrab-Hazdai R., Himmeloch S., Arnon R. Acetylcholinesterase from Schistosoma mansoni: Immunological characterization. Immunol. Lett. 1991;28:167–174. doi: 10.1016/0165-2478(91)90116-R. [DOI] [PubMed] [Google Scholar]
- 23.Bentley G.N., Jones A.K., Agnew A. Expression and comparative functional characterisation of recombinant acetylcholinesterase from three species of Schistosoma. Mol. Biochem. Parasitol. 2005;141:119–123. doi: 10.1016/j.molbiopara.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 24.You H., Gobert G.N., Du X., Pali G., Cai P., Jones M.K., McManus D.P. Functional characterisation of Schistosoma japonicum acetylcholinesterase. Parasit. Vectors. 2016;9:328. doi: 10.1186/s13071-016-1615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levi-Schaffer F., Tarrab-Hazdai R., Schryer M.D., Arnon R., Smolarsky M. Isolation and partial characterization of the tegumental outer membrane of schistosomula of Schistosoma mansoni. Mol. Biochem. Parasitol. 1984;13:283–300. doi: 10.1016/0166-6851(84)90120-8. [DOI] [PubMed] [Google Scholar]
- 26.Pearson M.S., Becker L., Driguez P., Young N.D., Gaze S., Mendes T., Li X.H., Doolan D.L., Midzi N., Mduluza T., et al. Of monkeys and men: Immunomic profiling of sera from humans and non-human primates resistant to schistosomiasis reveals novel potential vaccine candidates. Front. Immunol. 2015;6:213. doi: 10.3389/fimmu.2015.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnon R., Silman I., Tarrab-Hazdai R. Acetylcholinesterase of Schistosoma mansoni—Functional correlates. Contributed in honor of Professor Hans Neurath’s 90th birthday. Protein Sci. 1999;8:2553–2561. doi: 10.1110/ps.8.12.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espinoza B., Parizade M., Ortega E., Tarrab-Hazdai R., Zilberg D., Arnon R. Monoclonal antibodies against acetylcholinesterase of Schistosoma mansoni: Production and characterization. Hybridoma. 1995;14:577–586. doi: 10.1089/hyb.1995.14.577. [DOI] [PubMed] [Google Scholar]
- 29.Camacho M., Agnew A. Schistosoma: Rate of glucose import is altered by acetylcholine interaction with tegumental acetylcholine receptors and acetylcholinesterase. Exp. Parasitol. 1995;81:584–591. doi: 10.1006/expr.1995.1152. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro-dos-Santos G., Verjovski-Almeida S., Leite L.C. Schistosomiasis—A century searching for chemotherapeutic drugs. Parasitol. Res. 2006;99:505–521. doi: 10.1007/s00436-006-0175-2. [DOI] [PubMed] [Google Scholar]
- 31.Camacho M., Tarrab-Hazdai R., Espinoza B., Arnon R., Agnew A. The amount of acetylcholinesterase on the parasite surface reflects the differential sensitivity of schistosome species to metrifonate. Parasitology. 1994;108:153–160. doi: 10.1017/S0031182000068244. [DOI] [PubMed] [Google Scholar]
- 32.Harder A. Chemotherapeutic approaches to schistosomes: Current knowledge and outlook. Parasitol. Res. 2002;88:395–397. doi: 10.1007/s00436-001-0587-y. [DOI] [PubMed] [Google Scholar]
- 33.Lee D.L. Why do some nematode parasites of the alimentary tract secrete acetylcholinesterase? Int. J. Parasitol. 1996;26:499–508. doi: 10.1016/0020-7519(96)00040-9. [DOI] [PubMed] [Google Scholar]
- 34.Berriman M., Haas B.J., LoVerde P.T., Wilson R.A., Dillon G.P., Cerqueira G.C., Mashiyama S.T., Al-Lazikani B., Andrade L.F., Ashton P.D., et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young N.D., Jex A.R., Li B., Liu S., Yang L., Xiong Z., Li Y., Cantacessi C., Hall R.S., Xu X., et al. Whole-genome sequence of Schistosoma haematobium. Nat. Genet. 2012;44:221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 36.Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massoulie J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu. Rev. Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- 38.Tarrab-Hazdai R., Toker L., Silman I., Arnon R. Acetylcholinesterase from Schistosoma mansoni: Interaction of globular species with heparin. Biochem. J. 1999;344:945–951. doi: 10.1042/bj3440945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarrab-Hazdai R., Levi-Schaffer F., Gonzales G., Arnon R. Acetylcholinesterase of Schistosoma mansoni. Molecular forms of the solubilized enzyme. Biochim. Biophys. Acta. 1984;790:61–69. doi: 10.1016/0167-4838(84)90332-7. [DOI] [PubMed] [Google Scholar]
- 40.Espinoza B., Silman I., Arnon R., Tarrab-Hazdai R. Phosphatidylinositol-specific phospholipase C induces biosynthesis of acetylcholinesterase via diacylglycerol in Schistosoma mansoni. Eur. J. Biochem. 1991;195:863–870. doi: 10.1111/j.1432-1033.1991.tb15776.x. [DOI] [PubMed] [Google Scholar]
- 41.Espinoza B., Tarrab-Hazdai R., Silman I., Arnon R. Acetylcholinesterase in Schistosoma mansoni is anchored to the membrane via covalently attached phosphatidylinositol. Mol. Biochem. Parasitol. 1988;29:171–179. doi: 10.1016/0166-6851(88)90072-2. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald K., Buxton S., Kimber M.J., Day T.A., Robertson A.P., Ribeiro P. Functional characterization of a novel family of acetylcholine-gated chloride channels in Schistosoma mansoni. PLoS Pathog. 2014;10:e1004181. doi: 10.1371/journal.ppat.1004181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camacho M., Alsford S., Jones A., Agnew A. Nicotinic acetylcholine receptors on the surface of the blood fluke Schistosoma. Mol. Biochem. Parasitol. 1995;71:127–134. doi: 10.1016/0166-6851(94)00039-P. [DOI] [PubMed] [Google Scholar]
- 44.Bentley G.N., Jones A.K., Agnew A. ShAR2beta, a divergent nicotinic acetylcholine receptor subunit from the blood fluke Schistosoma. Parasitology. 2007;134:833–840. doi: 10.1017/S0031182006002162. [DOI] [PubMed] [Google Scholar]
- 45.Camacho M., Agnew A. Glucose uptake rates by Schistosoma mansoni, S. haematobium, and S. bovis adults using a flow in vitro culture system. J. Parasitol. 1995;81:637–640. doi: 10.2307/3283866. [DOI] [PubMed] [Google Scholar]
- 46.Dani J.A. Neuronal Nicotinic Acetylcholine receptor structure and function and response to nicotine. Int. Rev. Neurobiol. 2015;124:3–19. doi: 10.1016/bs.irn.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beech R.N., Callanan M.K., Rao V.T., Dawe G.B., Forrester S.G. Characterization of cys-loop receptor genes involved in inhibitory amine neurotransmission in parasitic and free living nematodes. Parasitol. Int. 2013;62:599–605. doi: 10.1016/j.parint.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Mansour T.E. Chemotherapeutic Targets in Parasites Contemporary Strategies. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- 49.Sukhdeo S.C., Sangster N.C., Mettrick D.F. Effects of cholinergic drugs on longitudinal muscle contractions of Fasciola hepatica. J. Parasitol. 1986;72:858–864. doi: 10.2307/3281834. [DOI] [PubMed] [Google Scholar]
- 50.Rapson E.B., Chilwan A.S., Jenkins D.C. Acetylcholinesterase secretion—A parameter for the interpretation of in vitro anthelmintic screens. Parasitology. 1986;92:425–430. doi: 10.1017/S0031182000064180. [DOI] [PubMed] [Google Scholar]
- 51.Ogilvie B.M., Rothwell T.L., Bremner K.C., Schnitzerling H.J., Nolan J., Keith R.K. Acetylcholinesterase secretion by parasitic nematodes—I. Evidence for secretion of the enzyme by a number of species. Int. J. Parasitol. 1973;3:589–597. doi: 10.1016/0020-7519(73)90083-0. [DOI] [PubMed] [Google Scholar]
- 52.Vaux R., Schnoeller C., Berkachy R., Roberts L.B., Hagen J., Gounaris K., Selkirk M.E. Modulation of the Immune Response by Nematode Secreted Acetylcholinesterase Revealed by Heterologous Expression in Trypanosoma musculi. PLoS Pathog. 2016;12:e1005998. doi: 10.1371/journal.ppat.1005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sukhdeo S.C., Sukhdeo M.V., Mettrick D.F. Histochemical localization of acetylcholinesterase in the cerebral ganglia of Fasciola hepatica, a parasitic flatworm. J. Parasitol. 1988;74:1023–1032. doi: 10.2307/3282227. [DOI] [PubMed] [Google Scholar]
- 54.Kemmerling U., Cabrera G., Campos E.O., Inestrosa N.C., Galanti N. Localization, specific activity, and molecular forms of acetylcholinesterase in developmental stages of the cestode Mesocestoides corti. J. Cell Physiol. 2006;206:503–509. doi: 10.1002/jcp.20495. [DOI] [PubMed] [Google Scholar]
- 55.Martin R.J. Modes of action of anthelmintic drugs. Vet. J. 1997;154:11–34. doi: 10.1016/S1090-0233(05)80005-X. [DOI] [PubMed] [Google Scholar]
- 56.Jones A.K., Sattelle D.B. Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays. 2004;26:39–49. doi: 10.1002/bies.10377. [DOI] [PubMed] [Google Scholar]
- 57.Richmond J.E., Jorgensen E.M. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Touroutine D., Fox R.M., Von Stetina S.E., Burdina A., Miller D.M., 3rd, Richmond J.E. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J. Biol. Chem. 2005;280:27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- 59.Boulin T., Gielen M., Richmond J.E., Williams D.C., Paoletti P., Bessereau J.L. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buxton S.K., Charvet C.L., Neveu C., Cabaret J., Cortet J., Peineau N., Abongwa M., Courtot E., Robertson A.P., Martin R.J. Investigation of acetylcholine receptor diversity in a nematode parasite leads to characterization of tribendimidine- and derquantel-sensitive nAChRs. PLoS Pathog. 2014;10:e1003870. doi: 10.1371/journal.ppat.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duguet T.B., Charvet C.L., Forrester S.G., Wever C.M., Dent J.A., Neveu C., Beech R.N. Recent Duplication and Functional Divergence in Parasitic Nematode Levamisole-Sensitive Acetylcholine Receptors. PLoS Negl. Trop. Dis. 2016;10:e0004826. doi: 10.1371/journal.pntd.0004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williamson S.M., Robertson A.P., Brown L., Williams T., Woods D.J., Martin R.J., Sattelle D.B., Wolstenholme A.J. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: Formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathog. 2009;5:e1000517. doi: 10.1371/journal.ppat.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin R.J., Clark C.L., Trailovic S.M., Robertson A.P. Oxantel is an N-type (methyridine and nicotine) agonist not an L-type (levamisole and pyrantel) agonist: Classification of cholinergic anthelmintics in Ascaris. Int. J. Parasitol. 2004;34:1083–1090. doi: 10.1016/j.ijpara.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Boulin T., Fauvin A., Charvet C.L., Cortet J., Cabaret J., Bessereau J.L., Neveu C. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br. J. Pharmacol. 2011;164:1421–1432. doi: 10.1111/j.1476-5381.2011.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fauvin A., Charvet C., Issouf M., Cortet J., Cabaret J., Neveu C. cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Mol. Biochem. Parasitol. 2010;170:105–107. doi: 10.1016/j.molbiopara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Sloan M.A., Reaves B.J., Maclean M.J., Storey B.E., Wolstenholme A.J. Expression of nicotinic acetylcholine receptor subunits from parasitic nematodes in Caenorhabditis elegans. Mol. Biochem. Parasitol. 2015;204:44–50. doi: 10.1016/j.molbiopara.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.