Abstract
A series of sulfanilamide-1,2,3-triazole hybrids were designed by a molecular hybridization strategy and evaluated for antiproliferative activity against three selected cancer cell lines (MGC-803, MCF-7 and PC-3). The detailed structure-activity relationships for these sulfanilamide-1,2,3-triazole hybrids were investigated. All these sulfanilamide-1,2,3-triazole hybrids exhibited moderate to potent activity against all cell lines. In particular 4-methyl-N-((1-(3-phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)benzenesulfonamide (11f) showed the most potent inhibitory effect against PC-3 cells, with an IC50 value of 4.08 μM. Furthermore, the tubulin polymerization inhibitory activity in vitro of compound 11f was 2.41 μM. These sulfanilamide hybrids might serve as bioactive fragments for developing more potent antiproliferative agents.
Keywords: sulfanilamide-1,2,3-triazole; molecular hybridization strategy; antiproliferative activity; structure-activity relationships; tubulin polymerization
1. Introduction
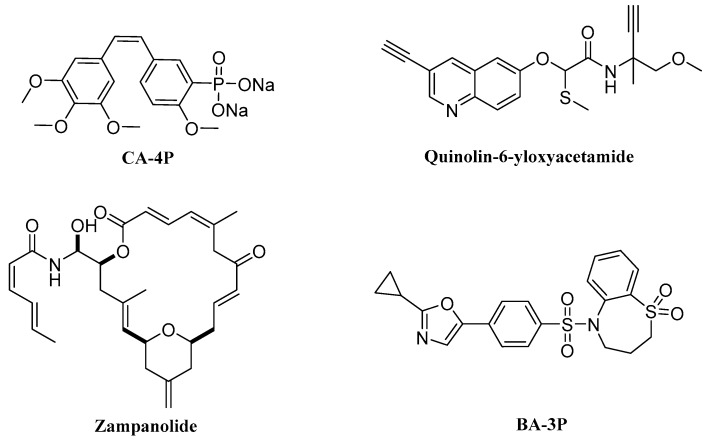
Microtubules, as a key component of the cytoskeleton, play important roles in many cellular events, including the maintenance of cell shape, cell migration, cell division and intracellular transport [1,2,3,4]. Recently, many tubulin polymerization inhibitors were designed and synthesized. For example, combretastatin A-4P (CA-4P) was used as a clinical drug for the treatment of cancers [5]. Quinolin-6-yloxyacetamide (Figure 1) displayed potent antiproliferative activity against the A2780AD cell line, with an IC50 value of 262 nM by disrupting the microtubule cytoskeleton [6]. Zampanolide as a microtubule-stabilizing agent was active in resistant cancer cells and inhibited cell migration [7]. Benzenesulfonamide derivative BA-3P was designed as a potential anticancer agent with an IC50 value of 5.882 μM against PC3 cells targeting tubulin [8]. However, the major problem existing in clinical use of these anti-tubulin agents is their undesirable side effects, like neurotoxicity and cardiovascular toxicities [6,8]. Therefore, it is necessary to develop novel tubulin polymerization inhibitors for cancer therapy.
Figure 1.
Potent tubulin-targeting agents.
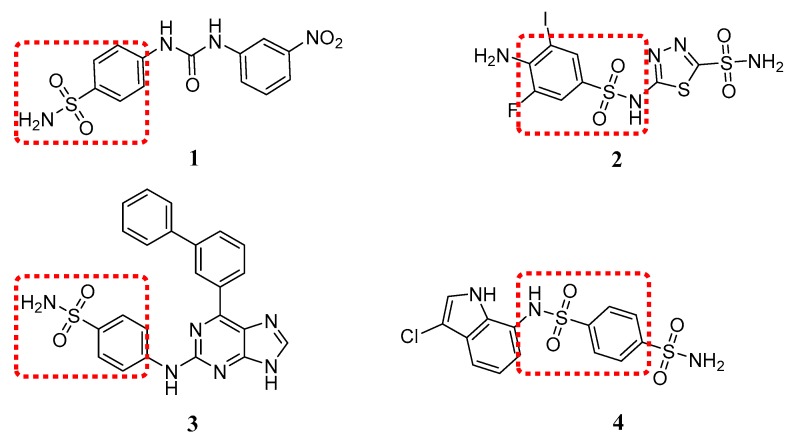
Sulfanilamide is a potential scaffold in antitumor drug discovery. Sulfanilamide derivative 1 (Figure 2) significantly inhibited the formation of metastases by the highly aggressive 4T1 mammary tumor cells at pharmacologic concentrations of 45 mg/kg [9]. 5-((4-Amino-3-fluoro-5-iodo-phenyl)sulfonamido)-1,3,4-thiadiazole-2-sulfonamide (2) was designed as a carbonic anhydrase inhibitor [10]. Benzenesulfonamide 3 as a selective cyclin-dependent kinase (CDK) inhibitor exhibited high potency toward CDK2 (IC50 0.044 μM), but was ~2000-fold less active toward CDK1 (IC50 86 μM) [11]. N-(3-chloro-7-indolyl)-1,4-benzenedisulfonamide 4 showed significant antitumor activity against HCT116 human colon carcinoma, with an IC50 value of 0.11 μg/mL in a cell proliferation assay [12].
Figure 2.
Antitumor sulfanilamides.
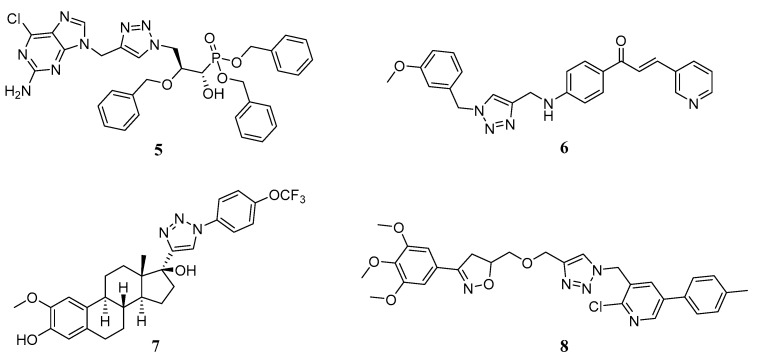
1,2,3-Triazoles were widely used as an anticancer scaffold in medicinal chemistry to design novel antitumor agents. (1R,2S)-1,2,3-triazole derivative 5 (Figure 3) displayed potent inhibitory effects against the proliferation of murine leukemia with an IC50 value of 21 μM [13]. Chalcone-1,2,3-triazole 6 designed in our group could inhibit the proliferation of SK-N-SH cancer cells by inducing apoptosis and arresting the cell cycle at the G1 phase [14]. On the other hand, the 1,2,3-triazole moiety has also been used as an important linker to design novel tubulin polymerization inhibitors. For example, the 1,4-disubstituted 1,2,3-triazole analog 7 based on 2-methoxyestradiol exhibited anti-proliferative effects at low micromolar concentrations and inhibited tubulin assembly with an IC50 value of 5.9 μM [15]. Pyridinyl-1H-1,2,3-triazolyldihydroisoxazole 8 was designed as a tubulin polymerization inhibitor with a concomitant accumulation of cells in the G2/M phase of the cell cycle [16].
Figure 3.
Antitumor 1,2,3-triazoles.
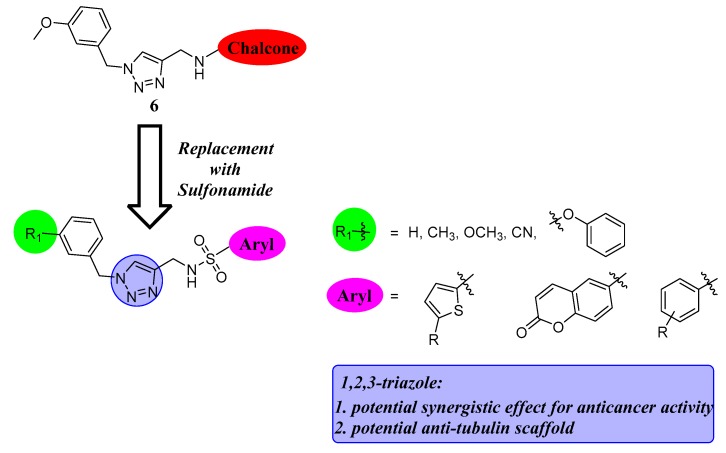
These interesting findings and our continuous quest to identify more potent anticancer agents, led us to use the molecular hybridization of 1,2,3-triazole and sulfonamide groups to generate a novel hybrid with potent antiproliferative activity. As shown in Figure 4, the novel hybrid based on the structures of 1,2,3-triazole and sulfonamide has three parts: an aryl sulfonamide (thiophenesulfonamide, coumarin sulfonamide, benzenesulfonamide), the potential anti-tubulin 1,2,3-triazole scaffold and various phenyl units. Importantly, detailed structure activity relationships (SARs) of these three regions were investigated in this work. These novel sulfonamide-1,2,3-triazole hybrids might serve as bioactive fragments and lead compounds for developing more potent cytotoxic agents and tubulin polymerization inhibitors.
Figure 4.
Rational design strategy for novel sulfonamide-1,2,3-triazoles.
2. Results and Discussion
2.1. Chemistry
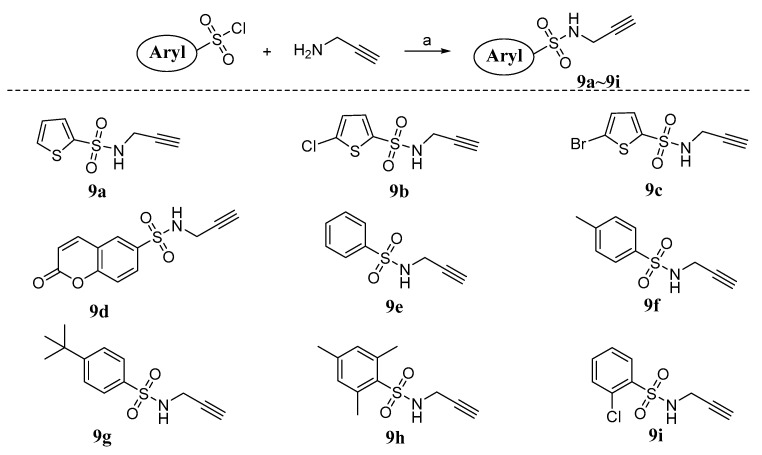
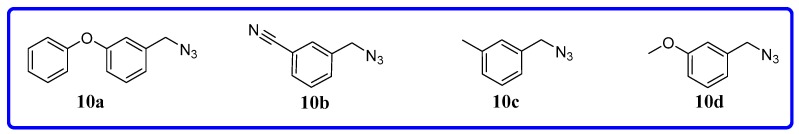
Alkyne intermediates 9a~i were synthesized as shown in Scheme 1. Commercially available aryl sulfonyl chlorides (thiophenesulfonyl chloride, coumarinsulfonyl chloride, benzenesulfonyl chloride) were treated with propynylamine in the presence of potassium carbonate to provide 9a~i. Azide derivatives 10a~d used in this work (Figure 5) were purchased from Zhengzhou Research Biotechnology Co., Ltd. (Zhengzhou, China).
Scheme 1.
Synthesis of alkyne intermediates 9a~i. Reagents and conditions: (a) K2CO3, dichloromethane, r.t.
Figure 5.
Azides 10a~d in the present study.
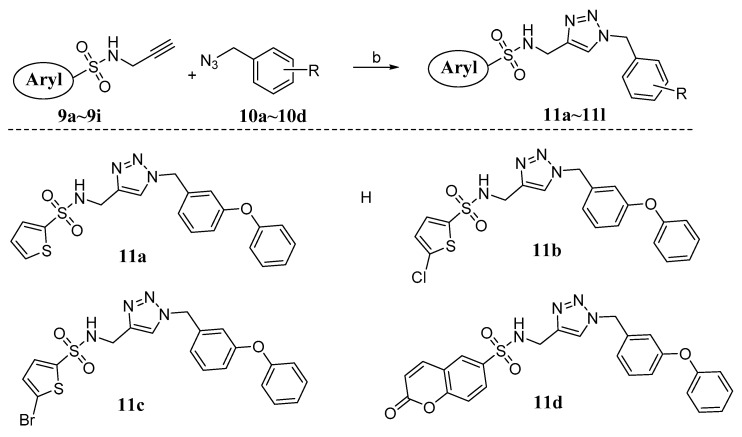
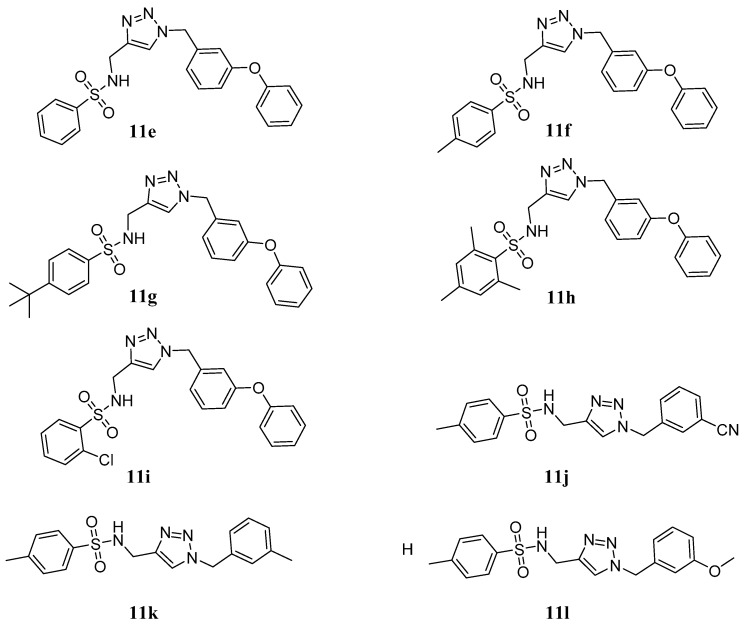
The applications of click chemistry are increasingly found in all aspects of drug discovery, ranging from lead discovery through combinatorial chemistry and target-templated in situ chemistry, to proteomics and DNA research, using bioconjugation reactions [17,18,19,20]. The sulfonamide-1,2,3-triazole hybrids 11a~l were synthesized through a click reaction between alkyne intermediates 9a~i and derivatives 10a~d using copper sulfate and sodium ascorbate in THF/H20 (Scheme 2).
Scheme 2.
Synthesis of target hybrids 11a~l. Reagents and conditions: (b) CuSO4·5H2O, sodium ascorbate, THF:H2O (1:1), r.t.
2.2. Antiproliferative Activity and SARs Analysis
All synthesized sulfonamide-1,2,3-triazole hybrids were evaluated for their anticancer activity against three cancer cell lines: MGC-803 (human gastric cancer cell line), MCF-7 (human breast cancer cell line), and PC-3 (human prostate cancer cell line) using a MTT (4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) assay [21,22,23]. The well-known tubulin polymerization inhibitor CA-4P was used as a control. In [24], 5-fluorouracil (5-Fu) was selected as the control in the antiproliferative evaluation assay of 1,2,3-triazole derivatives. Therefore, 5-Fu was also used as the reference drug in this MTT assay.
The antiproliferative results are summarized in Table 1. In order to investigate the effect of the inhibitory activity of 1,2,3-triazole moiety, the antiproliferative activity of the non-1,2,3-triazole-sulfonamide derivatives 9a, 9d and 9f and the 1,2,3-triazole-sulfonamide hybrids 11a~l was investigated. Removal of the 1,2,3-triazole skeleton was clearly detrimental for the inhibitory activity against the three cancer cell lines, as shown by the results of compounds 9a, 9d and 9f. The introduction of 1,2,3-triazole scaffold resulted in a significant improvement of inhibitory activity, suggesting that the 1,2,3-triazole moiety displays a synergistic role in determining activity. In particular, compound 11f showed more potent inhibitory effects against three cell lines than 5-Fu, with IC50 values ranging from 4.1 μM to 15.7 μM.
Table 1.
Antiproliferative results of the target compounds.
| Compound | IC50 (μM) a | ||
|---|---|---|---|
| MGC-803 | MCF-7 | PC-3 | |
| 9a | >100 | >100 | >100 |
| 9d | >100 | >100 | >100 |
| 9f | >100 | >100 | >100 |
| 11a | 17.0 ± 1.8 | 78.5 ± 2.2 | 24.6 ± 0.9 |
| 11b | 15.3 ± 0.8 | 49.9 ± 1.2 | 19.8 ± 0.6 |
| 11c | 31.0 ± 2.2 | 34.7 ± 2.6 | 49.4 ± 1.0 |
| 11d | 88.8 ± 0.5 | 36.4 ± 2.0 | 34.1 ± 0.3 |
| 11e | 21.7 ± 0.1 | 39.6 ± 5.0 | 6.4 ± 1.2 |
| 11f | 13.7 ± 2.9 | 15.7 ± 0.2 | 4.1 ± 1.4 |
| 11g | >100 | 54.9 ± 1.7 | 7.1 ± 0.5 |
| 11h | 18.4 ± 0.7 | 24.6 ± 1.6 | 24.7 ± 3.0 |
| 11i | 15.3 ± 0.5 | 77.5 ± 0.7 | 22.7 ± 0.5 |
| 11j | 41.3 ± 2.3 | >100 | >100 |
| 11k | 75.8 ± 0.6 | >100 | >100 |
| 11l | 77.2 ± 7.8 | >100 | >100 |
| 5-Fu | 15.6 ± 0.4 | 21.2 ± 3.6 | 13.9 ± 1.5 |
| CA-4P | 0.027 ± 0.003 | 0.039 ± 0.005 | nd b |
a Inhibitory activity was assayed by exposure for 48 h to substances and expressed as concentration required to inhibit tumor cell proliferation by 50% (IC50). Data are presented as the means ± SDs of three independent experiments. b Not determined.
In order to complete the structure activity relationship study, compounds with a thiophene ring (11a~c), a coumarin ring (11d), a phenyl ring (11i), a formononetin scaffold (11j) and coumarin scaffolds (11e~h) were synthesized to explore the effect of aryl rings for their antiproliferative activity. Among all the thiophenesulfonamide derivatives 11a~c, compound 11b with a 4-Cl-thiophene ring displayed the most potent antiproliferative activity against three selected cell lines. Coumarin-1,2,3-triazole-sulfonamide hybrid 11d showed weak antiproliferative activity with IC50 values ranging from 34.1 μM to 88.8 μM. These results suggested that heteroaromatic rings play an important role in the inhibitory activity.
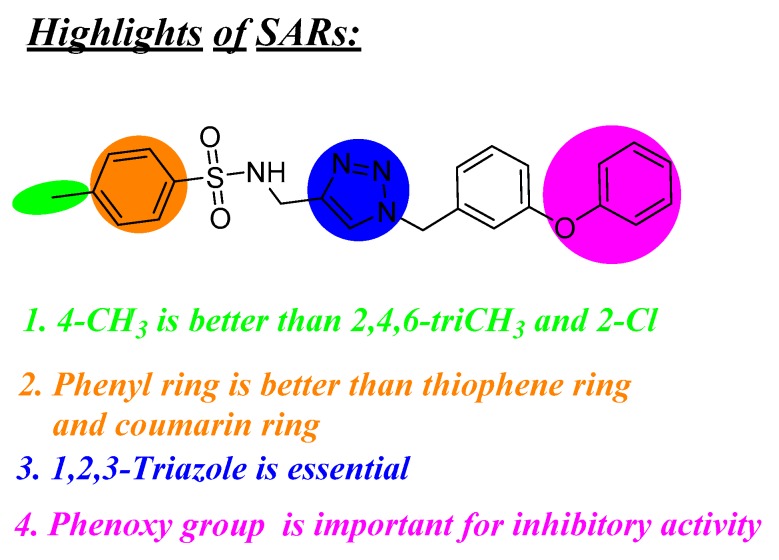
We also found that the phenyl ring was important for the activity, shown by an improvement for inhibitory activity against PC-3 cells, when the thiophene ring and coumarin ring were replaced with a phenyl ring (compounds 11a~d vs. 11e). In addition, the substituent on the phenyl ring bearing the sulfonamide may affected the antiproliferative activity. Replacement of the hydrogen atom of compound 11e with a tertiary butyl group as in compound 11g led to a decrease of the activity. However, changing the 2,4,6-triCH3 substitution pattern (compound 11h) and 2-Cl (compound 11i) to 4-CH3 (compound 11f) led to an improvement of activity against all three cell lines, indicating the significance of the 4-CH3 group on the phenyl ring attaching sulfonamide in their antiproliferative activity.
Furthermore, a phenoxy group on the phenyl attaching the 1,2,3-triazole played a key role in the antiproliferative activity. Removal of the phenoxy group (11f) was clearly detrimental for the inhibitory activity against the three cancer cell lines, as shown by compounds 11j~l without a phenoxy group which displayed no inhibitory activity against MCF-7 cells and PC-3 cells, with IC50 values of >100 μM.
2.3. In Vitro Tubulin Polymerization Inhibitory Activity Assay
Compound 11f was further examined for possible inhibitory effects against GES-1 (normal human gastric epithelial cell line). We found that compound 11f exhibited no significantly inhibitory effect against GES-1 (>64 μM). This fact, combined with its more potent antiproliferative activity against the selected MGC-803 cancer cell line than 5-Fu, with an IC50 value of 13.7 μM, indicated that compound 11f had good selectivity between cancer and normal cells. An illustration summarizing the structure activity relationships of the target derivatives in provided in Figure 6.
Figure 6.
Summary of SARs.
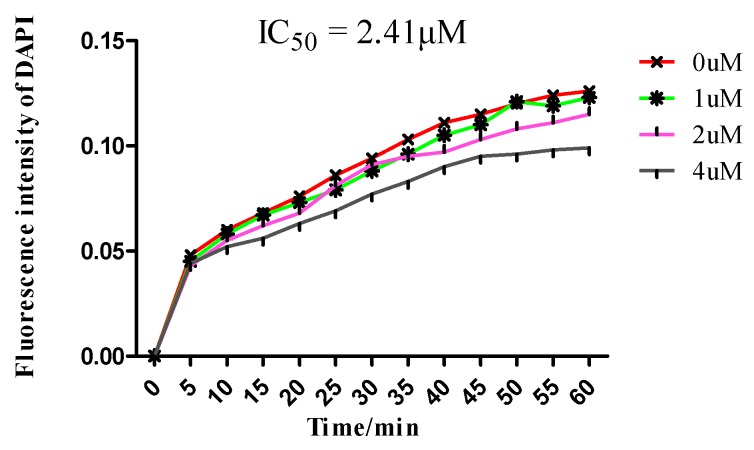
The inhibitory concentrations that reduced the polymerized tubulin by 50% of CA-4P was 2.40 μM [25]. To investigate whether the synthesized 1,2,3-triazole-sulfonamide hybrids target the tubulin-microtubule system, the in vitro tubulin polymerization inhibition activity of compound 11f was evaluated because of its best antiproliferative activity results among all the tested compounds in the initial cytotoxicity screening. When tubulin was incubated with the tested 1,2,3-triazole-sulfonamide 11f at the indicated concentrations, an increased fluorescence intensity was obvious. The inhibitory concentrations that reduced the polymerized tubulin by 50% (IC50) of compound 11f was 2.41 μM (Figure 7). This revealed 11f was a novel tubulin polymerization inhibitor.
Figure 7.
Tubulin polymerization inhibitory activity of compound 11f.
3. Materials and Methods
3.1. General Chemical Experimental Procedures
All reagents and solvents were of analytical grade and purchased from commercial sources. Thin-layer chromatography was carried out on glass plates coated with silica gel and visualized by UV light (254 nm). All NMR spectra were recorded with a DPX 400 MHz spectrometer (Bruker, Switzerland). Mass spectra (MS) were recorded on an Esquire 3000 mass spectrometer by electrospray ionization (ESI) (Micromass UK Limited, Manchester, England).
3.1.1. General Procedure for the Synthesis of Compounds 9a~i
To a stirred solution of the appropriate sulfonyl chloride (5 mmol) in dichloromethane (20 mL), propargylamine (5 mmol) and K2CO3 (5 mmol) were added carefully and the reaction mixture was stirred at room temperature for 6 h. The system was dissolved in dichloromethane (20 mL) and washed with water, brine, dried over anhydrous Na2SO4 and concentrated under vacuum to afford compounds 9a~i, which were used in the next reaction without further purification. Copies of 1H- and 13C-NMR spectras for compounds 9a~i were shown in supplementary materials.
N-(Prop-2-yn-1-yl)thiophene-2-sulfonamide (9a). Yellow Liquid, yield: 49.2%. 1H-NMR (400 MHz, CDCl3) δ 7.59 (dt, J = 9.3, 4.7 Hz, 1H, Ar), 7.55 (dd, J = 5.0, 1.3 Hz, 1H, Ar), 7.03 (dd, J = 5.0, 3.8 Hz, 1H, Ar), 5.26 (s, 1H, NH), 3.83 (dd, J = 6.0, 2.5 Hz, 2H, CH2), 2.12–2.03 (m, 1H, CCH). 13C-NMR (100 MHz, CDCl3) δ 139.33, 131.82, 131.33, 126.44 (Ar), 76.69, 71.91 (CCH), 32.05 (CH2). HRMS (ESI) calcd. for C7H8NO2S2 [M + H]+: 201.9999, found: 201.9996. IR: 3264, 1426, 1327, 1161, 1067, 878, 715, 666, 595 cm−1.
5-Chloro-N-(prop-2-yn-1-yl)thiophene-2-sulfonamide (9b). Orange liquid, yield: 67.9%. 1H-NMR (CDCl3) δ 7.39 (d, J = 4.0 Hz, 1H, Ar), 6.87 (d, J = 4.0 Hz, 1H, Ar), 5.09 (s, 1H, NH), 3.84 (dd, J = 5.6, 2.4 Hz, 2H, CH2), 2.12 (dd, J = 5.1, 2.6 Hz, 1H, CCH). 13C-NMR (CDCl3) δ 137.23, 136.79, 131.32, 125.79 (Ar), 76.41, 72.26 (CCH), 32.04 (CH2). HRMS (ESI) calcd. for C7H7ClNO2S2 [M + H]+: 235.9608, found: 235.9607. IR: 3273, 1413, 1330, 1152, 1084, 994, 801, 647, 616 cm−1.
5-Bromo-N-(prop-2-yn-1-yl)thiophene-2-sulfonamide (9c). Orange liquid, yield: 45.0%. 1H-NMR (CDCl3) δ 7.35 (d, J = 4.0 Hz, 1H, Ar), 7.01 (dd, J = 3.9, 1.8 Hz, 1H, Ar), 4.93 (d, J = 77.0 Hz, 1H, NH), 3.84 (d, J = 2.1 Hz, 2H, CH2), 2.13 (t, J = 2.5 Hz, 1H, CCH). 13C-NMR (CDCl3) δ 140.12, 132.00, 129.42, 119.47 (Ar), 72.32, 72.27 (CCH), 32.08 (CH2). HRMS (ESI) calcd. for C7H7BrNO2S2 [M + H]+: 279.9107, found: 279.9102. IR: 3303, 3266, 1428, 1401, 1354, 1329, 1156, 1060, 969, 805, 676, 629, 572 cm−1.
2-Oxo-N-(prop-2-yn-1-yl)-2H-chromene-6-sulfonamide (9d). White solid, yield: 20.7%, m.p.: 193–196 °C. 1H-NMR (DMSO-d6) δ 8.25 (dt, J = 12.8, 7.8 Hz, 3H, Ar), 7.99 (dd, J = 8.7, 2.2 Hz, 1H, Ar), 7.60 (d, J = 8.7 Hz, 1H, Ar), 6.64 (d, J = 9.6 Hz, 1H, NH), 3.86–3.65 (m, 2H, CH2), 3.02 (t, J = 2.5 Hz, 1H, CCH). 13C-NMR (CDCl3) δ 164.53, 160.92, 148.91, 141.78, 135.21, 133.00, 132.91, 123.97, 122.79, 122.67 (Ar), 84.38, 80.17 (CCH), 37.14 (CH2). HRMS (ESI) calcd. for C12H10NO4S [M + H]+: 264.0335, found: 264.0331. IR: 3287, 3216, 1703, 1598, 1336, 1161, 1116, 1074, 834, 675, 598 cm−1.
N-(Prop-2-yn-1-yl)benzenesulfonamide (9e). Colorless liquid, yield: 93.8%. 1H-NMR (CDCl3) δ 8.02–7.73 (m, 2H, Ar), 7.65–7.34 (m, 3H, Ar), 5.08 (s, 1H, NH), 3.77 (dd, J = 6.1, 2.5 Hz, 2H, CH2), 2.00 (t, J = 2.5 Hz, 1H, CCH). 13C-NMR (CDCl3) δ 138.50, 131.97, 128.08, 126.33 (Ar), 76.90, 71.98 (CCH), 31.80 (CH2). HRMS (ESI) calcd. for C9H10NO2S [M + H]+: 196.0434, found: 196.0432. IR: 3278, 3254, 1445, 1166, 1093, 1067, 759, 723, 657, 597 cm−1.
4-Methyl-N-(prop-2-yn-1-yl)benzenesulfonamide (9f). White solid, yield: 41.4%, m.p.: 70–75 °C. 1H-NMR (CDCl3) δ 7.71 (d, J = 8.1 Hz, 2H, Ar), 7.23 (t, J = 10.6 Hz, 2H, Ar), 4.66 (s, 1H, NH), 3.76 (dd, J = 5.9, 2.4 Hz, 2H, CH2), 2.36 (s, 3H, CH3), 2.03 (t, J = 2.2 Hz, 1H, CCH). 13C-NMR (CDCl3) δ 142.85, 135.51, 128.70, 126.39 (Ar), 76.94, 71.98 (CCH), 31.85 (CH2), 20.54 (CH3). HRMS (ESI) calcd. for C10H12NO2S [M + H]+: 210.0588, found: 210.0589. IR: 3273, 1325, 1158, 1069, 703, 669, 576, 547 cm−1.
4-(Tert-butyl)-N-(prop-2-yn-1-yl)benzenesulfonamide (9g). White solid, yield: 30.9%, m.p.: 58–63 °C. 1H-NMR (CDCl3) δ 7.74 (d, J = 8.5 Hz, 2H, Ar), 7.46 (d, J = 8.5 Hz, 2H, Ar), 4.68 (s, 1H, NH), 3.76 (d, J = 2.4 Hz, 2H, CH2), 2.02 (s, 1H, CCH), 1.27 (s, 9H, C(CH3)3). 13C-NMR (CDCl3) δ 155.90, 135.31, 126.21, 125.09 (Ar), 76.96, 71.91 (CCH), 34.16 (C(CH3)3), 31.90 (CH2), 30.06 (C(CH3)3). HRMS (ESI) calcd. for C13H18NO2S [M + H]+: 252.1059, found: 252.1058. IR: 3314, 3266, 2961, 2869, 1596, 1327, 1162, 1112, 822, 636, 570, 539 cm−1.
2,4,6-Trimethyl-N-(prop-2-yn-1-yl)benzenesulfonamide (9h). White solid, yield: 74.2%, m.p.: 93–96 °C. 1H-NMR (CDCl3) δ 6.89 (s, 2H, Ar), 4.64 (s, 1H, NH), 3.71 (s, 2H, CH2), 2.58 (s, 6H, (CH3)2), 2.23 (s, 3H, CH3), 2.02 (t, J = 2.5 Hz, 1H, CCH). 13C-NMR (CDCl3) δ 141.53, 138.31, 132.27 , 130.95 (Ar), 76.79, 71.68 (CCH), 31.32 (CH2), 21.95, 19.94 (CH3). HRMS (ESI) calcd. for C12H16NO2S [M + H]+: 238.0906, found: 238.0902. IR: 3319, 1429, 1321, 1162, 1076, 816, 691, 660, 529 cm−1.
2-Chloro-N-(prop-2-yn-1-yl)benzenesulfonamide (9i). White solid, yield: 67.0%, m.p.: 90–92 °C. 1H-NMR (CDCl3) δ 8.03 (s, 1H, Ar), 7.40 (d, J = 36.7 Hz, 3H, Ar), 5.26 (s, 1H, NH), 3.79 (s, 2H, CH2), 1.90 (s, 1H, CCH). 13C-NMR (CDCl3) δ 136.18, 132.93, 130.80, 130.46, 130.30, 126.17 (Ar), 76.46, 71.86 (CCH), 31.95 (CH2). HRMS (ESI) calcd. for C9H9ClNO2S [M + H]+: 230.0047, found: 230.0043. IR: 3277, 1455, 1428, 1330, 1168, 1074, 1043, 765, 666, 569, 553 cm−1.
3.1.2. General Procedure for the Synthesis of Compounds 11a~l
Alkyne intermediates 9a~i (2 mmol), azide derivatives 10a~d (2 mmol), CuSO4·5H2O (0.4 mmol) and sodium ascorbate (0.2 mmol) were dissolved in THF/H2O (8 mL/8 mL) and stirred for 10 h at room temperature. Upon completion of the reactions, the precipitated product was filtered to afford products 11a~l, which were purified with column chromatography on silica gel (hexane/EtOAc = 8/1). Copies of 1H- and 13C-NMR spectras for compounds 9a~i were shown in Supplementary materials.
N-((1-(3-Phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)thiophene-2-sulfonamide (11a). White solid, yield: 60.1%, m.p.: 104–106 °C. 1H-NMR (DMSO-d6) δ 8.34 (s, 1H, NH), 7.95 (s, 1H, Ar), 7.88 (dd, J = 5.0, 1.3 Hz, 1H, Ar), 7.57 (dd, J = 3.7, 1.3 Hz, 1H, Ar), 7.40 (tt, J = 9.4, 5.1 Hz, 3H, Ar), 7.15 (ddd, J = 11.0, 6.2, 2.3 Hz, 2H, Ar), 7.08–6.97 (m, 4H, Ar), 6.94 (dd, J = 8.1, 1.8 Hz, 1H, Ar), 5.54 (s, 2H, PhCH2), 4.12 (s, 2H, NHCH2). 13C-NMR (DMSO-d6) δ 157.38, 156.69, 144.03, 141.65, 138.60, 132.96, 132.18, 130.90, 130.61, 128.10, 124.21, 123.98, 123.34, 119.27, 118.53, 118.43 (Ar), 52.77 (PhCH2), 38.75 (NHCH2). HRMS (ESI) calcd. for C20H19N4O3S2 [M + H]+: 427.0898, found: 427.0899. IR: 3176, 3100, 1489, 1331, 1253, 1215, 1151, 750, 688, 593, 529 cm−1.
5-Chloro-N-((1-(3-phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)thiophene-2-sulfonamide (11b). White solid, yield: 68.3%, m.p.: 110–111 °C. 1H-NMR (DMSO-d6) δ 8.52 (s, 1H, NH), 8.00 (s, 1H, Ar), 7.55–7.29 (m, 4H, Ar), 7.25–7.10 (m, 2H, Ar), 7.07–6.97 (m, 4H, Ar), 6.98–6.88 (m, 1H, Ar), 5.55 (s, 2H, PhCH2), 4.15 (s, 2H, NHCH2). 13C-NMR (DMSO-d6) δ 157.39, 156.68, 143.77, 140.18, 138.60, 134.89, 132.04, 130.91, 130.61, 128.30, 124.21, 124.09, 123.28, 119.27, 118.52, 118.43 (Ar), 52.78 (PhCH2), 38.66 (NHCH2). HRMS (ESI) calcd. for C20H18ClN4O3S2 [M + H]+: 461.0512, found: 461.0509. IR: 3120, 1488, 1412, 1328, 1252, 1159, 786, 685, 612, 523 cm−1.
5-Bromo-N-((1-(3-phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)thiophene-2-sulfonamide (11c). White solid, yield: 59.1%, m.p.: 111–112 °C. 1H-NMR (DMSO-d6) δ 8.50 (s, 1H, NH), 8.00 (s, 1H, Ar), 7.48–7.33 (m, 4H, Ar), 7.29 (d, J = 4.0 Hz, 1H, Ar), 7.16 (t, J = 7.4 Hz, 1H, Ar), 7.08–6.98 (m, 4H, Ar), 6.94 (dd, J = 8.1, 1.8 Hz, 1H, Ar), 5.55 (s, 2H, PhCH2), 4.14 (s, 2H, NHCH2). 13C-NMR (DMSO-d6) δ 157.39, 156.68, 143.79, 142.76, 138.60, 132.79, 131.69, 130.91, 130.61, 124.21, 124.09, 123.28, 119.28, 118.70, 118.52, 118.43 (Ar), 52.78 (PhCH2), 38.67 (NHCH2). HRMS (ESI) calcd. for C20H18BrN4O3S2 [M + H]+: 505.0007, found: 505.0004. IR: 3118, 1489, 1403, 1328, 1253, 1158, 1086, 819, 786, 749, 605, 522 cm−1.
2-Oxo-N-((1-(3-phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)-2H-chromene-6-sulfonamide (11d). White solid, yield: 61.9%, m.p.: 144–146 °C. 1H-NMR (DMSO-d6) δ 8.28 (s, 1H, NH), 8.19 (dd, J = 9.1, 5.9 Hz, 2H, Ar), 8.00–7.87 (m, 2H, Ar), 7.53 (d, J = 8.7 Hz, 1H, Ar), 7.38 (dt, J = 16.3, 7.9 Hz, 3H, Ar), 7.16 (t, J = 7.4 Hz, 1H, Ar), 7.06–6.96 (m, 4H, Ar), 6.93 (d, J = 8.2 Hz, 1H, Ar), 6.63 (d, J = 9.6 Hz, 1H, Ar), 5.51 (s, 2H, PhCH2), 4.10 (s, 2H, NHCH2). 13C-NMR (DMSO-d6) δ 159.75, 157.37, 156.67, 156.01, 144.08, 144.01, 138.57, 136.90, 130.85, 130.60, 130.12, 127.96, 124.21, 124.00, 123.19, 119.27, 119.25, 118.46, 118.40, 118.07, 117.93 (Ar), 52.72 (PhCH2), 38.55 (NHCH2). HRMS (ESI) calcd. for C25H21N4O5S [M + H]+: 489.1237, found: 489.1233. IR: 3256, 3137, 1744, 1488, 1321, 1252, 1157, 1107, 834, 751, 601 cm−1.
N-((1-(3-Phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)benzenesulfonamide (11e). White solid, yield: 56.8%, m.p.: 127–130 °C. 1H-NMR (DMSO-d6) δ 8.14 (s, 1H, NH), 7.89 (s, 1H, Ar), 7.77 (dd, J = 5.3, 3.4 Hz, 2H, Ar), 7.69–7.47 (m, 3H, Ar), 7.47–7.27 (m, 3H, Ar), 7.16 (t, J = 7.4 Hz, 1H, Ar), 7.09–6.84 (m, 5H, Ar), 5.51 (s, 2H, PhCH2), 4.05 (s, 2H, NHCH2). 13C-NMR (DMSO-d6) δ 157.37, 156.69, 144.17, 140.82, 138.58, 132.86, 130.89, 130.61, 129.57, 126.97, 124.21, 123.92, 123.32, 119.26, 118.52, 118.42 (Ar), 52.74 (PhCH2), 38.55 (NHCH2). HRMS (ESI) calcd. for C22H21N4O3S [M + H]+: 421.1338, found: 421.1334. IR: 3269, 3123, 1587, 1495, 1322, 1253, 1215, 1157, 690, 587 cm−1.
4-Methyl-N-((1-(3-phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)benzenesulfonamide (11f). White solid, yield: 66.5%, m.p.: 118–121 °C. 1H-NMR (DMSO-d6) δ 8.03 (s, 1H, NH), 7.91 (s, 1H, Ar), 7.66 (d, J = 8.2 Hz, 2H, Ar), 7.52–7.27 (m, 5H, Ar), 7.16 (t, J = 7.4 Hz, 1H, Ar), 7.08–6.97 (m, 4H, Ar), 6.94 (dd, J = 8.0, 2.1 Hz, 1H, Ar), 5.52 (s, 2H, PhCH2), 4.01 (s, 2H, NHCH2), 2.37 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 156.88, 156.20, 143.76, 142.62, 138.11, 137.45, 130.37, 130.10, 129.51, 126.56, 123.70, 123.42, 122.78, 118.76, 118.01, 117.92 (Ar), 52.25 (PhCH2), 38.07 (NHCH2), 20.93 (CH3). HRMS (ESI) calcd. for C23H23N4O3S [M + H]+: 435.1497, found: 435.1491. IR: 3294, 3251, 1590, 1491, 1328, 1265, 1160, 819, 755, 687, 557 cm−1.
4-(Tert-butyl)-N-((1-(3-phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)benzenesulfonamide (11g). White solid, yield: 49.3%, m.p.: 137–139 °C. 1H-NMR (DMSO-d6) δ 8.05 (s, 1H, NH), 7.95 (s, 1H, Ar), 7.72 (d, J = 8.5 Hz, 2H, Ar), 7.59 (d, J = 8.6 Hz, 2H, Ar), 7.39 (dt, J = 11.7, 8.1 Hz, 3H, Ar), 7.16 (t, J = 7.4 Hz, 1H, Ar), 7.10–6.84 (m, 5H, Ar), 5.53 (s, 2H, PhCH2), 4.02 (s, 2H, NHCH2), 1.30 (s, 9H, CH3). 13C-NMR (DMSO-d6) δ 157.39, 156.68, 155.88, 144.32, 138.64, 137.88, 130.89, 130.60, 126.94, 126.46, 124.21, 123.98, 123.26, 119.27, 118.46, 118.41 (Ar), 52.76 (PhCH2), 38.61 (NHCH2), 35.30 (C(CH3)3), 31.28 (C(CH3)3). HRMS (ESI) calcd. for C26H29N4O3S [M + H]+: 477.1967, found: 477.1960. IR: 3268, 1593, 1488, 1322, 1242, 1163, 1047, 754, 570 cm−1.
2,4,6-Trimethyl-N-((1-(3-phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)benzenesulfonamide (11h). White solid, yield: 78.7%, m.p.: 111–113 °C. 1H-NMR (DMSO-d6) δ 7.95 (s, 1H, NH), 7.70 (s, 1H, Ar), 7.48–7.28 (m, 3H, Ar), 7.17 (dd, J = 10.6, 4.2 Hz, 1H, Ar), 7.06–6.89 (m, 7H, Ar), 5.48 (s, 2H, PhCH2), 4.04 (s, 2H, NHCH2), 2.50 (s, 6H, CH3), 2.22 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 157.34, 156.71, 144.46, 141.83, 138.74, 138.60, 134.94, 132.01, 130.87, 130.61, 124.20, 123.61, 123.26, 119.22, 118.47, 118.43 (Ar), 52.65 (PhCH2), 37.67 (NHCH2), 22.98 (CH3), 20.84(CH3). HRMS (ESI) calcd. for C25H27N4O3S [M + H]+: 463.1807, found: 463.1804. IR: 3307, 3269, 1587, 1488, 1326, 1257, 1243, 1154, 736, 657 cm−1.
2-Chloro-N-((1-(3-phenoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl) benzenesulfonamide (11i). White solid, yield: 80.8%, m.p.: 97–99 °C. 1H-NMR (DMSO-d6) δ 8.41 (s, 1H, NH), 7.91 (dd, J = 7.8, 1.5 Hz, 1H, Ar), 7.83 (s, 1H, Ar), 7.61–7.48 (m, 2H, Ar), 7.48–7.32 (m, 4H, Ar), 7.17 (t, J = 7.4 Hz, 1H, Ar), 7.10–6.89 (m, 5H, Ar), 5.50 (s, 2H, PhCH2), 4.17 (s, 2H, NHCH2). 13C-NMR (DMSO-d6) δ 157.36, 156.70, 144.14, 138.53, 138.49, 134.27, 131.99, 131.05, 130.89, 130.79, 130.62, 127.91, 124.21, 123.83, 123.34, 119.26, 118.54, 118.44 (Ar), 52.67 (PhCH2), 38.31 (NHCH2). HRMS (ESI) calcd. for C22H20ClN4O3S [M + H]+: 455.0948, found: 455.0945. IR: 3137, 1593, 1489, 1451, 1332, 1253, 1212, 1157, 758, 688, 585 cm−1.
N-((1-(3-Cyanobenzyl)-1H-1,2,3-triazol-4-yl)methyl)-4-methylbenzenesulfonamide (11j). White solid, yield: 58.1%, m.p.: 145–147 °C. 1H-NMR (DMSO-d6) δ 8.03 (s, 1H, NH), 7.98 (s, 1H, Ar), 7.83 (td, J = 4.6, 1.4 Hz, 1H, Ar), 7.77 (s, 1H, Ar), 7.66 (d, J = 8.2 Hz, 2H, Ar), 7.60 (d, J = 5.0 Hz, 2H, Ar), 7.34 (d, J = 8.0 Hz, 2H, Ar), 5.61 (s, 2H, PhCH2), 4.02 (s, 2H, NHCH2), 2.37 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 143.89, 142.62, 137.55, 137.44, 132.88, 131.95, 131.56, 130.03, 129.50, 126.56, 123.62, 118.40, 111.64 (Ar), 51.70 (PhCH2), 38.05 (NHCH2), 20.93 (CH3). HRMS (ESI) calcd. for C18H18N5O2S [M + H]+: 368.1181, found: 368.1181. IR: 3265, 2217, 1430, 1323, 1160, 1049, 889, 709, 546 cm−1.
4-Methyl-N-((1-(3-methylbenzyl)-1H-1,2,3-triazol-4-yl)methyl)benzenesulfonamide (11k). White solid, yield: 67.7%, m.p.: 145–148 °C. 1H-NMR (DMSO-d6) δ 8.01 (t, J = 6.0 Hz, 1H, NH), 7.87 (s, 1H, Ar), 7.66 (d, J = 8.1 Hz, 2H, Ar), 7.34 (d, J = 8.0 Hz, 2H, Ar), 7.25 (t, J = 7.5 Hz, 1H, Ar), 7.14 (dd, J = 15.2, 7.5 Hz, 2H, Ar), 7.06 (d, J = 7.6 Hz, 1H, Ar), 5.47 (s, 2H, PhCH2), 4.00 (d, J = 6.0 Hz, 2H, NHCH2), 2.37 (s, 3H, CH3), 2.29 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 143.70, 142.61, 137.92, 137.44, 135.83, 129.51, 128.73, 128.61, 128.55, 126.57, 125.06, 123.27 (Ar), 52.69 (PhCH2), 38.09 (NHCH2), 20.94 (CH3), 20.89 (CH3). HRMS (ESI) calcd. for C18H21N4O2S [M + H]+: 357.1388, found: 357.1385. IR: 3258, 3121, 1452, 1325, 1160, 1098, 772, 669, 558 cm−1.
N-((1-(3-Methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)-4-methylbenzenesulfonamide (11l). White solid, yield: 82.8%, m.p.: 112–114 °C. 1H-NMR (DMSO-d6) δ 8.02 (s, 1H, NH), 7.89 (s, 1H, Ar), 7.66 (d, J = 8.2 Hz, 2H, Ar), 7.34 (d, J = 8.1 Hz, 2H, Ar), 7.28 (t, J = 7.9 Hz, 1H, Ar), 6.99–6.85 (m, 2H, Ar), 6.82 (d, J = 7.6 Hz, 1H, Ar), 5.49 (s, 2H, PhCH2), 4.01 (s, 2H, NHCH2), 3.74 (s, 3H, OCH3), 2.37 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 159.40, 143.72, 142.62, 137.45, 137.38, 129.84, 129.51, 126.56, 123.34, 120.00, 113.79, 113.37 (Ar), 55.08 (OCH3), 52.60 (PhCH2), 38.08 (NHCH2), 20.92 (CH3). HRMS (ESI) calcd. for C18H21N4O3S [M + H]+: 373.1339, found: 373.1334. IR: 3294, 3247, 1600, 1322, 1286, 1265, 1091, 1040, 775, 668, 557 cm−1.
3.2. MTT Assay
MGC-803 cells (human gastric cancer), MCF-7 (human breast cancer), PC-3 (human prostate cancer) and GES-1 (human gastric epithelial cell) were cultured in RPMI 1640 medium with 10% FBS and 100 U/mL penicillin and 0.1 mg/mL streptomycin in the 37 °C in an atmosphere containing 5% CO2. All cell lines were purchased from the China Center for Type Culture Collection (CCTCC, Shanghai, China). For pharmacological investigations, 10 mM stock solutions of the tested compounds were prepared with dimethyl sulfoxide (DMSO). The highest DMSO concentration of the medium (0.1%) did not have any substantial effect on the determined cellular functions. The MTT assay was prepared according to the previous method [26,27,28].
3.3. In Vitro Tubulin Polymerization Inhibition Assay
Tubulin polymerization in vitro was assyed with 100 μL samples and monitored by absorbance at 350 nm (Filter A00019x) in 96-well plates with an Appliskan plate reader (Thermo Fisher Scientific, Waltham, MA, USA). An amount of 5.6 mg/mL tubulin was resuspended in PEM buffer (80 mM PIPES (pH 6.9), 1 mM EGTA, 0.5 mM MgCl2, 1 mM ATP, 10.2% (v/v) glycerol) and then was preincubated with compound 11f or vehicle DMSO on ice. Data were exported using the Thermo Scientific SkanIt software of the Appliskan (version 2.3) and Excel software was used to generate plots. For convenience, data were normalized to the minimum absorbance value of the initial stable plateau. This tubulin polymerization inhibition assay was measured according to the method originally [6,25].
4. Conclusions
In summary, a series of novel sulfanilamide-1,2,3-triazole hybrids were designed, synthesized and evaluated for their antiproliferative activity against three selected cancer cell lines (MGC-803, MCF-7 and PC-3). Most of the synthesized compounds exhibited moderate to good activity against all the selected cancer cell lines. Particularly, compound 11f showed the most excellent antiproliferative activity, with an IC50 value of 4.1 μM against PC-3 cancer cells. The tubulin polymerization inhibition activity of compound 11f was 2.41 μM. Highlights of structure activity relationships were the importance of 1,2,3-triazole scaffold and phenoxy group. Efforts to optimize the structure of compound 11f to further improve its potency are ongoing.
Although CA-4P targeting the colchicine binding site was designated with orphan drug status for the treatment of anaplastic thyroid cancer and ovarian cancer by the FDA [29], two major problems are still encountered during its use as therapeutics: (1) the isomerization to the inactive trans configuration during storage by heat and light [30]; (2) the undesirable side effects and potent toxicity against normal cells [31]. Compared to CA-4P, there is no isomerized double bond in compound 11f. Importantly, compound 11f exhibited no significant cytotoxicity against GES-1 (>64 μM), suggesting that sulfanilamide-1,2,3-triazole hybrid 11f could be a potent tubulin polymerization inhibitor for potential clinical application.
Acknowledgments
This work was supported by the National Natural Sciences Foundations of China (No. 81673322). Dong-Jun Fu is grateful for the financial support from the Outstanding PhD Training Program of Zhengzhou University and the China Scholarship Council (CSC).
Abbreviations
| CA-4P | Combretastatin A-4P |
| CDK | Cyclin-dependent kinase |
| 5-Fu | 5-Fluorouracil |
| CLog P | Lipo-hydro partition coefficient |
| SAR | Structure-activity relationship |
Supplementary Materials
Supplementary materials are available online.
Author Contributions
Dong-Jun Fu designed the research; Dong-Jun Fu, Ji-Feng Liu, Ruo-Han Zhao, Jia-Huan Li performed experiments. Sai-Yang Zhang and Yan-Bing Zhang were also responsible for the correspondence of the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Dong M., Liu F., Zhou H., Zhai S., Yan B. Novel natural product- and privileged scaffold-based tubulin inhibitors targeting the colchicine binding site. Molecules. 2016;21:1375. doi: 10.3390/molecules21101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang W.-T., Liu W., Chiu Y.-H., Chen B.-H., Chuang S.-C., Chen Y.-C., Hsu Y.-T., Lu M.-J., Chiou S.-J., Chou C.-K., et al. A 4-phenoxyphenol derivative exerts inhibitory effects on human hepatocellular carcinoma cells through regulating autophagy and apoptosis accompanied by downregulating α-tubulin expression. Molecules. 2017;22:854. doi: 10.3390/molecules22050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohena C., Risinger A., Devambatla R., Dybdal-Hargreaves N., Kaul R., Choudhary S., Gangjee A., Mooberry S. Janus compounds, 5-chloro-N4-methyl-N4-aryl-9H-pyrimido[4,5-b]indole-2,4-diamines, cause both microtubule depolymerizing and stabilizing effects. Molecules. 2016;21:1661. doi: 10.3390/molecules21121661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandić B., Simić M., Vučković I., Vujisić L., Novaković M., Trifunović S., Nikolić-Mandić S., Tešević V., Vajs V., Milosavljević S. Pyrrolizidine alkaloids and fatty acids from the endemic plant species rindera umbellata and the effect of lindelofine-N-oxide on tubulin polymerization. Molecules. 2013;18:10694–10706. doi: 10.3390/molecules180910694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker A.L., Teo W.S., McCarroll J.A., Kavallaris M. An emerging role for tubulin isotypes in modulating cancer biology and chemotherapy resistance. Int. J. Mol. Sci. 2017;18:1434. doi: 10.3390/ijms18071434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A., Sáez-Calvo G., Olieric N., de Asís Balaguer F., Barasoain I., Lamberth C., Díaz J., Steinmetz M. Quinolin-6-yloxyacetamides are microtubule destabilizing agents that bind to the colchicine site of tubulin. Int. J. Mol. Sci. 2017;18:1336. doi: 10.3390/ijms18071336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field J., Northcote P., Paterson I., Altmann K.-H., Díaz J., Miller J. Zampanolide, a microtubule-stabilizing agent, is active in resistant cancer cells and inhibits cell migration. Int. J. Mol. Sci. 2017;18:971. doi: 10.3390/ijms18050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Yang S., Zhou S., Lu D., Ji L., Li Z., Yu S., Meng X. Synthesis, anti-cancer evaluation of benzenesulfonamide derivatives as potent tubulin-targeting agents. Eur. J. Med. Chem. 2016;122:488–496. doi: 10.1016/j.ejmech.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Pacchiano F., Carta F., McDonald P.C., Lou Y., Vullo D., Scozzafava A., Dedhar S., Supuran C.T. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J. Med. Chem. 2011;54:1896–1902. doi: 10.1021/jm101541x. [DOI] [PubMed] [Google Scholar]
- 10.Ilies M.A., Vullo D., Pastorek J., Scozzafava A., Ilies M., Caproiu M.T., Pastorekova S., Supuran C.T. Carbonic Anhydrase Inhibitors. Inhibition of Tumor-Associated Isozyme IX by Halogenosulfanilamide and Halogenophenylaminobenzolamide Derivatives. J. Med. Chem. 2003;46:2187–2196. doi: 10.1021/jm021123s. [DOI] [PubMed] [Google Scholar]
- 11.Coxon C.R., Anscombe E., Harnor S.J., Martin M.P., Carbain B., Golding B.T., Hardcastle I.R., Harlow L.K., Korolchuk S., Matheson C.J., et al. Cyclin-Dependent Kinase (CDK) inhibitors: Structure-activity relationships and insights into the CDK-2 selectivity of 6-substituted 2-arylaminopurines. J. Med. Chem. 2017;60:1746–1767. doi: 10.1021/acs.jmedchem.6b01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owa T., Yoshino H., Okauchi T., Yoshimatsu K., Ozawa Y., Sugi N.H., Nagasu T., Koyanagi N., Kitoh K. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J. Med. Chem. 1999;42:3789–3799. doi: 10.1021/jm9902638. [DOI] [PubMed] [Google Scholar]
- 13.Głowacka I., Andrei G., Schols D., Snoeck R., Piotrowska D. Phosphonylated acyclic guanosine analogues with the 1,2,3-triazole linker. Molecules. 2015;20:18789–18807. doi: 10.3390/molecules201018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu D.-J., Zhang S.-Y., Liu Y.-C., Yue X.-X., Liu J.-J., Song J., Zhao R.-H., Li F., Sun H.-H., Zhang Y.-B., et al. Design, synthesis and antiproliferative activity studies of 1,2,3-triazole-chalcones. MedChemComm. 2016;7:1664–1671. doi: 10.1039/C6MD00169F. [DOI] [Google Scholar]
- 15.Solum E.J., Vik A., Hansen T.V. Synthesis, cytotoxic effects and tubulin polymerization inhibition of 1,4-disubstituted 1,2,3-triazole analogs of 2-methoxyestradiol. Steroids. 2014;87:46–53. doi: 10.1016/j.steroids.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Suman P., Murthy T.R., Rajkumar K., Srikanth D., Dayakar C., Kishor C., Addlagatta A., Kalivendi S.V., Raju B.C. Synthesis and structure-activity relationships of pyridinyl-1H-1,2,3-triazolyldihydroisoxazoles as potent inhibitors of tubulin polymerization. Eur. J. Med. Chem. 2015;90:603–619. doi: 10.1016/j.ejmech.2014.11.063. [DOI] [PubMed] [Google Scholar]
- 17.Kolb H.C., Sharpless K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 2003;8:1128–1137. doi: 10.1016/S1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S.Y., Fu D.J., Yue X.X., Liu Y.C., Song J., Sun H.H., Liu H.M., Zhang Y.B. Design, synthesis and structure-activity relationships of novel chalcone-1,2,3-triazole-azole derivates as antiproliferative agents. Molecules. 2016;21:653. doi: 10.3390/molecules21050653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasdev R., Preston D., Scottwell S., Brooks H., Crowley J., Schramm M. Oxidatively locked [Co2L3]6+ cylinders derived from bis(bidentate) 2-pyridyl-1,2,3-triazole “click” ligands: Synthesis, stability, and antimicrobial studies. Molecules. 2016;21:1548. doi: 10.3390/molecules21111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelriheem N., Mohamed A., Abdelhamid A. Synthesis of some new 1,3,4-thiadiazole, thiazole and pyridine derivatives containing 1,2,3-triazole moiety. Molecules. 2017;22:268. doi: 10.3390/molecules22020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu D.J., Li Z., Jian S., Mao R.W., Zhao R.H., Liu Y.C., Hou Y.H., Li J.H., Yang J.J., Jin C.Y. Design and synthesis of formononetin-dithiocarbamate hybrids that inhibit growth and migration of PC-3cells via MAPK/Wnt signaling pathways. Eur. J. Med. Chem. 2016;127:87–99. doi: 10.1016/j.ejmech.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu D.-J., Zhang S.-Y., Liu Y.-C., Song J., Zhao R.-H., Mao R.-W., Liu H.-M., Zhang Y.-B. Design, synthesis and antiproliferative evaluation of 3-aminopropyloxy derivatives of chalcone. J. Chem. Res. 2016;40:624–627. doi: 10.3184/174751916X14740434083371. [DOI] [Google Scholar]
- 23.Fu D.-J., Zhang S.-Y., Liu Y.-C., Zhang L., Liu J.-J., Song J., Zhao R.-H., Li F., Sun H.-H., Liu H.-M., et al. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate-chalcone derivates. Bioorg. Med. Chem. Lett. 2016;26:3918–3922. doi: 10.1016/j.bmcl.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Fu D.-J., Song J., Zhao R.-H., Liu Y.-C., Zhang Y.-B., Liu H.-M. Synthesis of novel antiproliferative 1,2,3-triazole hybrids using the molecular hybridisation approach. J. Chem. Res. 2016;40:674–677. doi: 10.3184/174751916X14761050193688. [DOI] [Google Scholar]
- 25.Bonne D., Heuséle C., Simon C., Pantaloni D. 4′,6-Diamidino-2-phenylindole, a fluorescent probe for tubulin and microtubules. J. Biol. Chem. 1985;260:2819–2825. [PubMed] [Google Scholar]
- 26.Kelley J.L., Soroko F.E. 9-(2-Fluorobenzyl)-6-(methylamino)-9H-purine hydrochloride. Synthesis and anticonvulsant activity. J. Med. Chem. 1986;29:1133–1134. doi: 10.1021/jm00157a003. [DOI] [PubMed] [Google Scholar]
- 27.Li Z.-H., Yang D.-X., Geng P.-F., Zhang J., Wei H.-M., Hu B., Guo Q., Zhang X.-H., Guo W.-G., Zhao B., et al. Design, synthesis and biological evaluation of [1,2,3]triazolo[4,5-d]pyrimidine derivatives possessing a hydrazone moiety as antiproliferative agents. Eur. J. Med. Chem. 2016;124:967–980. doi: 10.1016/j.ejmech.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Li Z.-H., Zhang J., Liu X.-Q., Geng P.-F., Ma J.-L., Wang B., Zhao T.-Q., Zhao B., Wei H.-M., Wang C., et al. Identification of thiazolo[5,4-d]pyrimidine derivatives as potent antiproliferative agents through the drug repurposing strategy. Eur. J. Med. Chem. 2017;135:204–212. doi: 10.1016/j.ejmech.2017.04.056. [DOI] [PubMed] [Google Scholar]
- 29.Grosios K., Holwell S.E., McGown A.T., Pettit G.R., Bibby M.C. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br. J. Cancer. 1999;81:1318–1327. doi: 10.1038/sj.bjc.6692174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greene T.F., Wang S., Greene L.M., Nathwani S.M., Pollock J.K., Malebari A.M., McCabe T., Twamley B., O’Boyle N.M., Zisterer D.M., et al. Synthesis and biochemical evaluation of 3-phenoxy-1,4-diarylazetidin-2-ones as tubulin-targeting antitumor agents. J. Med. Chem. 2016;59:90–113. doi: 10.1021/acs.jmedchem.5b01086. [DOI] [PubMed] [Google Scholar]
- 31.Rustin G.J., Galbraith S.M., Anderson H., Stratford M., Folkes L.K., Sena L., Gumbrell L., Price P.M. Phase I clinical trial of weekly combretastatin A4 phosphate: Clinical and pharmacokinetic results. J. Clin. Oncol. 2003;21:2815–2822. doi: 10.1200/JCO.2003.05.185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.