Abstract
One new cassane diterpene possessing an unusual N bridge between C-19 and C-20 named caesalsappanin R (1), as well as another new diterpene caesalsappanin S (2), were isolated from the seeds of Caesalpinia sappan with methanol extract. Their structures were determined by spectroscopic analysis and examined alongside existing data from prior studies. Their biological activities were profiled by their antiplasmodial activity.
Keywords: Caesalpinia sappan, cassane diterpenes, N bridge, antiplasmodial activity
1. Introduction
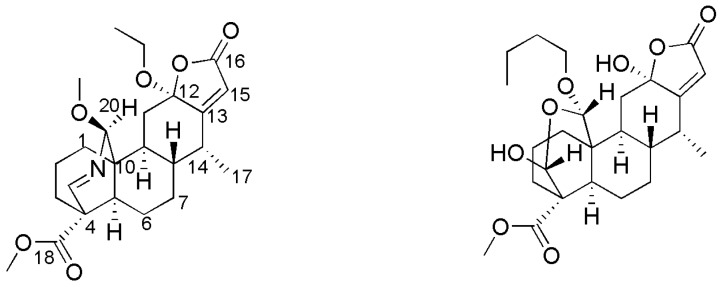
Caesalpinia sappan has been a part of traditional Chinese herbal medicine and is widely used in the treatment of dysmenorrheal, blood stagnation, and tetanus. Previous phytochemical investigations indicated that this genus contains an abundant source of cassane diterpenes with different structure types, and most of them showed in vitro or in vivo pharmacological impacts such as antiproliferative [1,2,3], antiplasmodial [4,5], antibacterial [6], antihelmintic, and antineoplastic activity [7]. As a continuation of our project towards new bioactive diterpenes discovery from the genus Caesalpinia [2,3,8], we examined the chemical constituents of C. sappan and obtained two new cassane diterpenes, designated caesalsappanin R (1) and caesalsappanin S (2) (Figure 1). Compound 1 is a rather unusual cassane diterpenoid lactone-type skeleton, consisting of an N bridge between C-19 and C-20. In this paper, we detail the separation and structural determination of the novel agents and the examination of their antiplasmodial activity.
Figure 1.
The structures of compounds 1–2.
2. Results and Discussion
2.1. Purification of Compounds 1–2
The seeds of C. sappan were extracted with MeOH three times. The two cassane-type diterpenoids were isolated and purified via silica gel chromatography, Sephadex LH-20 gel chromatography and semi-HPLC.
2.2. Structure Elucidation of Compounds 1–2
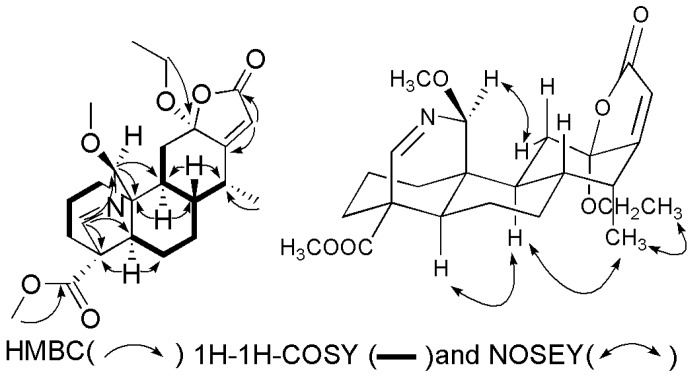
Compound 1 was acquired as a white shapeless powder. The HRESIMS spectrum demonstrated a quasi-molecular ion at m/z 454.2199 (Calcd. for C24H33NO6Na, 454.2206), which in connection with the NMR data, confirmed that the molecular formula was C24H33NO6. The IR and UV spectra revealed absorptions for an amidogen (3190 cm−1), a carbonyl (1735 cm−1), and an α,β-unsaturated butenolide unit (210 nm; 1749 cm−1) [2]. The 1H and 13C APT NMR spectra (Table 1) displayed the olefinic proton signal at δH 5.86 (H-15, s) and four downfield-shifted carbon signals at δC 107.4 (C-12), 171.0 (C-13), 115.9 (C-15), and 179.9 (C-16), which also confirmed the presence of the α,β-unsaturated butenolide ring. Additionally, the 1H-NMR spectrum exhibited signals for a methyl at δH 1.14 (d, J = 7.2 Hz, H3-17), two methoxys at δH 3.74 (s, 18-OCH3) and 3.72 (s, 20-OCH3), an ethoxy group at δH 3.30, 3.58 (m, OCH2CH3) and 1.21 (d, J = 7.2 Hz, OCH2CH3), a nitrogen oxymethylene proton at δH 5.07 (d, J = 2.4 Hz), and a nitrogen alkenyl at δH 7.53 (s). Except for the methoxy (δC 52.2, 57.1) and ethoxy (δC 59.3, 15.0) substituents, the 13C APT NMR spectrum showed 20 carbons including one methyl (δC 12.2), six methylenes (δC 19.3, 25.6, 29.1, 30.2, 33.1, and 37.2), seven methines (δC 37.0, 42.2, 43.4, 47.0, 91.2, 115.9, and 169.9), and six quaternary carbons (δC 44.1, 49.8, 107.4, 169.9, 171.0, and 175.3). The HSQC spectrum displayed all of the proton signals assigned to the corresponding carbons through direct 1H and 13C correlations. The overall 1H- and 13C-NMR spectroscopic data confirmed that 1 is an oxynitride diterpene possessing a fused butenolide unit [9,10], and its entire structure was connected, as confirmed using HSQC, HMBC, and 1H-1H-COSY spectra (Figure 2). The nitrogen oxymethylene proton at δH 5.07 (d, J = 2.4 Hz, H-20) showed long-range correlations with carbons at δC 30.2 (C-1), 49.8 (C-10), 162.6 (C-19), and 57.1 (20-OCH3), which suggested that C-1, C-10, C-19, and −OCH3 were connected through the nitrogen oxymethylene carbon C-20. The quaternary carbon C-4 (δC 44.1) was connected to C-3 (δC 33.1), C-5 (δC 47.0), C-18 (δC 175.3), and C-19 (δC 162.6) due to the HMBC correlations of H-19, H2-3, and H-5 to C-4 and C-18. Moreover, the nitrogen bridge between C-19 and C-20 was confirmed by the downfield chemical shifts of C-19 (δC 162.6) and C-20 (δC 91.2) together with the HMBC correlations between H-19 and C-20. Finally, the α,β-unsaturated butenolide moiety was connected to C-11 and C-14 based on the HMBC correlations from H2-11 to C-12 (δC 107.4) and H-14 to C-13 (δC 171.0). The proton H3-17 (δH 1.14, d, J = 7.2 Hz) showed long-range correlations with carbons C-14 (δC 37.0), which indicated that the methyl group of C-17 was connected to C-14. The methoxyl and ethoxyl groups were attached to C-18 and C-12, respectively, based on the HMBC correlations between δH 3.74 (s, −OCH3) and δC 175.3 (C-18), δH 3.30, 3.58 (m, OCH2CH3) and δC 107.4 (C-12). The NOESY experiment established the relative configuration of compound 1, the correlations of H-20 (δH 5.07)/H-1α (δH 1.69–1.72), H3-17 (δH 1.14)/H-9 (δH 1.78), OCH2CH3-12 (δH 3.30)/H3-17 (δH 1.14) showed that the hydroxyl group was β-oriented at C-20, and the methyl group at C-14 and the ethoxy group at C-12 were all α-oriented. The same carbon skeleton with the trans/anti/trans system of three six-membered rings A, B, and C, and the oriented proton at C-8 was β-axial and the oriented protons at C-5/C-9 was α-axial, which are well established on all cassane diterpenes isolated so far from the genus Caesalpinia [3,8,11]. Considering the biosynthetic relationship and comparing with the literature of cassane diterpenoids [12], the absolute configurations of the chiral carbons were determined to be 4S, 5R, 8S, 9S, 10S, 12S, 14R in 1 and are shown in Figure 2. Therefore, the structure of 1 was determined and it was named caesalsappanin R (Figure 1). Compound 1 is representative of a new cassane diterpenoid lactone-type skeleton with an N bridge between C-19 and C-20.
Table 1.
NMR spectral data of 1–2 (CDCl3, 600 and 150 MHz).
| No. | 1 | 2 | Caesalsappanin H | ||
|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | |
| 1 | 30.2 CH2 | 1.69–1.72 (m) | 37.7 CH2 | 1.28–1.30 (m) | 37.8 CH2 |
| 2.17–2.21 (m) | 2.07–2.09 (m) | ||||
| 2 | 19.3 CH2 | 1.38–1.41 (m) | 20.6 CH2 | 1.58–1.60 (m) | 20.6 CH2 |
| 2.59–2.63 (m) | 2.23–2.25 (m) | ||||
| 3 | 33.1 CH2 | 1.28–1.32 (m) | 28.5 CH2 | 1.82–1.83 (m) | 28.6 CH2 |
| 1.89–1.93 (m) | 2.25–2.30 (m) | ||||
| 4 | 44.1 C | 50.3 C | 50.4 C | ||
| 5 | 47.0 CH | 1.73 (m) | 47.2 CH | 1.68–1.71 (m) | 47.2 CH |
| 6 | 25.6 CH2 | 1.18–1.20 (m) | 24.2 CH2 | 1.19–1.21 (m) | 24.2 CH2 |
| 1.39–1.42 (m) | 2.00–2.02 (m) | ||||
| 7 | 29.1 CH2 | 1.69–1.72 (m) | 29.5 CH2 | 1.25–1.28(m) | 29.5 CH2 |
| 2.19–2.23 (m) | 1.60–1.62 (m) | ||||
| 8 | 43.4 CH | 1.49 (m) | 41.5 CH | 2.19–2.21 (m) | 41.1 CH |
| 9 | 42.2 CH | 1.78 (m) | 41.3 CH | 1.51–1.53 (m) | 41.3 CH |
| 10 | 49.8 C | 38.6 C | 38.7 C | ||
| 11 | 37.2 CH2 | 1.68–1.70 (m) | 38.0 CH2 | 1.36–1.38 (m) | 38.1 CH2 |
| 2.75 (dd, 12.0,2.4) | 2.51–2.53 (m) | ||||
| 12 | 107.4 C | 105.5 C | 105.9 C | ||
| 13 | 171.0 C | 173.4 C | 173.7 C | ||
| 14 | 37.0 CH | 2.99 (qd, 7.2, 2.4) | 37.1 CH | 2.91 (qd, 7.2, 2.4) | 37.1 CH |
| 15 | 115.9 CH | 5.86 (s) | 113.5 CH | 5.69 (s) | 113.8 CH |
| 16 | 169.9 C | 170.7 C | 170.7 C | ||
| 17 | 12.2 CH3 | 1.14 (d, 7.2) | 12.0 CH3 | 1.13 (d, 7.2) | 12.1 CH3 |
| 18 | 175.3 C | 175.6 C | 175.5 C | ||
| 19 | 162.6 CH | 7.53, s | 90.1 CH | 5.60 (s) | 90.1 CH |
| 20 | 91.2 CH | 5.07 (d, 2.4) | 104.2 CH | 4.49 (s) | 105.4 CH |
| 59.3, CH2 | 3.30 (m) | ||||
| 3.58 (m) | |||||
| OCH2CH3-12 | 15.0, CH3 | 1.21 (t, 7.2) | |||
| OCH3-18 | 52.2 | 3.74 (s) | 52.0 | 3.71 (s) | 51.7 |
| OCH3-20 | 57.1 | 3.72 (s) | 55.7 | ||
| OCH2CH2CH2CH3-20 | 67.8 | 3.22 (ddd, 9.6, 6.0, 3.0) 3.80 (ddd, 9.6, 6.0, 3.0) | |||
| OCH2CH2CH2CH3-20 | 31.9 | 1.32 (m) | |||
| 1.47 (m) | |||||
| OCH2CH2CH2CH3-20 | 20.0 | 1.28 (m) | |||
| 1.43 (m) | |||||
| OCH2CH2CH2CH3-20 | 13.7 | 1.87 (t, 7.2) | |||
Figure 2.
Key 2D NMR correlations of compound 1.
Compound 2 was separated as a white shapeless powder, [α] −47.3 (c = 0.1, MeOH). Its molecular formula was determined to be C25H36O8 by HRESIMS (observed m/z 487.2332 [M + Na]+). The 1H- and 13C-NMR data displayed a cassane diterpene skeleton with an oxygen bridge between C-19 and C-20, which was very similar to the reported compound caesalsappanin H [3]. In fact, the only difference between them was that the methoxy group at C-20 in caesalsappanin H was replaced with a butoxy group in 2. The 1H- and 13C-NMR spectra displayed the signals at δH 3.22, 3.80 (2H, ddd, J = 9.6, 6.0, 3.0 Hz, CH2), 1.32, 1.47 (2H, m, CH2), 1.28, 1.43 (2H, m, CH2), 1.87 (3H, t, J = 7.2 Hz, CH3) and δC 67.8 (CH2), 31.9 (CH2), 20.0 (CH2), 13.7 (CH3), which suggested the presence of a butoxy group. Also, the HMBC correlations from CH2 (δH 3.22, 3.80) to C-20 (δC 104.2) supported the position of the butoxy group at C-20. Taken along with 1H-1H COSY, HSQC, HMBC, and NOE spectra, the structure of compound 2 was determined and it was named caesalsappanin S (Figure 1).
2.3. In Vitro Antiplasmodial and Larvicidal Activities of Compounds 1–2
The two compounds were tested against the chloroquine-resistant strain K1 of P. falciparum (Table 2). Compound 1 exhibited relatively good antiplasmodial activity in vitro with IC50 values of 3.6 μM, compared with chloroquine. On the other hand, compound 2 showed only weak activity against the chloroquine-resistant K1 strain of P. falciparum. It appears that the presence of the N bridge in cassane-type diterpenoids may play an important role in enhancing activity against the chloroquine-resistant K1 strain of P. falciparum in vitro. Furthermore the toxic activity of compounds 1 and 2 against mosquito larvae was evaluated. Both compounds displayed only low activity.
Table 2.
In vitro antiplasmodial and Larvicidal activities of compounds 1–2.
| Compounds | IC50 (μM) a | LC50 (μM) b |
|---|---|---|
| 1 | 3.60 ± 1.2 | 60.2 ± 2.3 |
| 2 | 25.1 ± 1.3 | 262.0 ± 8.7 |
| Chloroquine c | 0.19 ± 0.05 | 38.6 ± 2.1 |
a IC50 = inhibitory concentration 50%; b LC50 = lethal concentration 50%. Values are means ± SD of triplicate experiments. c Positive control substance.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotation data were measured with a Perkin-Elmer 341 digital polarimeter (PerkinElmer, Norwalk, CT, USA). UV and IR data spectra were recorded on Shimadzu UV2550 and FTIR-8400S spectrometers (Shimadzu, Kyoto, Japan). NMR spectra were obtained using a Bruker AV III 600 NMR spectrometer with chemical shift values presented as δ values having TMS (Tetramethylsilane) as the internal standard. HRESIMS was performed using an LTQ-Orbitrap XL spectrometer (Thermo Fisher Scientific, Boston, MA, USA). Column–chromatography (CC) was performed using silica gel (100–200 and 300–400 mesh, Qingdao Marine Chemical Plant, Qingdao, China), Sephadex LH-20 (Pharmacia, Uppsala, Sweden). Precoated silica gel GF254 plates (Zhi Fu Huang Wu Pilot Plant of Silica Gel Development, Yantai, China) were used for TLC. All solvent used was of analytical grade (Beijing Chemical Plant, Beijing, China).
3.2. Plant Material
The seeds of C. sappan were collected from Nanning, Guangxi Province, People’s Republic of China, in April 2013, and identified by Professor Jing Quan Yuan of the Department of Pharmaceutical Chemistry, Guangxi Botanical Garden of Medicinal Plants. A voucher specimen (NO. 13418) was deposited at the Guangxi Botanical Garden of Medical Plants.
3.3. Isolation and Purification of Compounds 1–2
The air-dried seeds of C. sappan (5.0 kg) were extracted with MeOH (3 × 40 L, 3 h each) at room temperature. Removal of the MeOH under reduced pressure yielded a MeOH-soluble extract (1267 g). The residue was suspended to H2O (3 L) and partitioned with petroleum ether (3 × 3 L), CH2Cl2 (3 × 3 L), EtOAc (3 × 3 L), and n-BuOH (3 × 3 L), successively. The EtOAc fraction (164 g) was subjected to CC (column–chromatography) over silica gel (100–200 mesh, 15 × 60 cm) eluting with a stepwise gradient of CH2Cl2–MeOH (from 1:0 to 0:1, 100:0, 90:1, 70:1, 50:1, 30:1, 20:1, 10:1, 5:1, 2:1, 1:1, 0:1, v/v) to afford fractions A–G. Fraction E (3.1 g) was subjected to chromatographed repeatedly over silica gel CC eluting with CH2Cl2–MeOH (50:0, 40:1, 30:1, 20:1, 10:1, v/v) to obtained sub-fractions Fractions E1–E5. Fraction E3 was separated using silica gel CC eluting with CH2Cl2–MeOH (40:1, 30:1, 0:100, v/v) to obtained sub-fractions I-III. Sub-fraction II was purified by semi-HPLC of MeOH–H2O (55:45, v/v) as the mobile phase to yield compound 1 (6.3 mg, 0.000146%, tR = 28.4 min). Fraction E2 was separated using silica gel CC eluting with CH2Cl2–MeOH (50:1, v/v), yielding compound 2 (8.6 mg, 0.000172%).
3.4. Characterization of Compounds 1–2
Caesalsappanin R (1), White powder (MeOH); [α] −24.2 (c = 0.05, MeOH); UV (MeOH) λmax (log ε) 210 (3.86) nm; IR (film) νmax 3190, 1745, 1735 cm−1; 1H- and 13C-NMR data (CDCl3), see (Table 1); HR-ESI-MS m/z 454.2199 [M + Na]+. (Calcd. for. 454.2206 C24H33NO6Na).
Caesalsappanin S (2), White powder (MeOH); [α] −47.3 (c = 0.1, MeOH); UV (MeOH) λmax (log ε) 213 (3.94) nm; IR (film) νmax 3450, 1730 cm−1; 1H- and 13C-NMR data (CDCl3), see (Table 1); HR-ESI-MS m/z 487.2332 [M + Na]+. (Calcd. for. 487.2308 C24H34O8Na).
3.5. Antiplasmodial Assays of Compounds 1–2
Antiplasmodial activity in vitro was determined by means of the microculture radioisotope technique based on the method described by Desjardins [13]. The parasite P. falciparum (K1, multidrug-resistant strain) was cultured continuously according to the method of Trager and Jensen [14]. Three preparations were used for each experiment. The determination of IC50 values against the erythrocytic stages of P. falciparum was carried out in duplicate using the [3H]-hypoxanthine incorporation assay [15]. Laboratory colonies of mosquito larvae/pupae (Culex quinquefasciatus Say, Diptera, Culicidae) were used for the larvicidal/pupicidal activity. Twenty-five numbers of first to fourth instars larvae and puape were introduce into 500 mL glass beaker containing 249 mL of de-chlorinated water and 1 mL of desired concentrations of ethanolic leaf extract were added. Larval food was given for the test larvae. At each tested concentration two to five trials were made and each trial consisted of five replicates. The control was setup by mixing 1 mL of acetone with 249 mL of dechlorinated water. The larvae and pupae were exposed to dechlorinated water without acetone served as control. The control mortalities were corrected by using Abbott’s formula [16,17]. The LC50 were calculated from toxicity data by using probit analysis [18]. Chloroquine was included as a standard for comparison. Data are presented as means ± SEM. Statistical analyses were done by means of the Student’s t-test. A P value of less than 0.05 was considered a significant difference.
4. Conclusions
In conclusion, two new cassane-type diterpenoids (1 and 2) were isolated and characterized by spectrometric analysis (1 and 2D NMR, HRESIMS). Compound 1 exhibited active antiplasmodial activity in vitro with IC50 at 3.60 μM. In addition, the compounds that we had reported also showed antiplasmodial activities; caesalsappanins A, G, H, and I displayed antiplasmodial activities with IC50 values of 7.4, 0.78, 0.52, and 2.5 μM, respectively [3]. Therefore, we believe that this plant is an important source for the diverse structure of cassane-type diterpenoids and should be further investigated for the antiplasmodial activity.
Acknowledgments
The work was financially supported by the Technological Large Platform for Comprehensive Research and Development of New Drugs in the Twelfth Five-Year “Significant New Drugs Created” Science and Technology Major Projects (No. 2012ZX09301-002-001-026), the National Science and Technology Support Program (No. 2012BA127B06), the Innovation Capacity Building in Guangxi Science and Technology Agency (No. 10100027-3), the National Natural Sciences Foundation of China (No. 81502945), and the PUMC Youth Fund (No. 3332015141), Key Medical Research Projects in Guangxi (2011029) and Research projects of Shenzhen Science and Technology Program (GCZX 2015050411225563).
Author Contributions
Guo-Xu Ma, Xu-Dong Xu, and Jun-Shan Yang conceived and designed the experiments; Nai-Liang Zhu, Zhong-Hao Sun, Mei-Geng Hu, Tong-Yu Wu, Jing-Quan Yuan, and Hai-Feng Wu performed the experiments; Yu Tian and Peng-Fei Li analyzed the data; Nai-Liang Zhu and Guo-Xu Ma wrote the paper.
Conflicts of Interest
There is no conflict of interest associated with the authors of this paper.
Footnotes
Sample Availability: Samples of the compounds 1–2 are available from the authors.
References
- 1.Consolacion Y., Ragasa J.G.H., John A.R. New Furanoid Diterpenes from Caesalpinia pulcherrima. J. Nat. Prod. 2002;65:1107–1110. doi: 10.1021/np0201523. [DOI] [PubMed] [Google Scholar]
- 2.Ma G.X., Yuan J.Q., Wu H.F., Cao L., Zhang X.P., Xu L.J., Wei H., Wu L.Z., Zheng Q.X., Li L.Y., et al. Caesalpins A–H, Bioactive Cassane-Type Diterpenes from the Seeds of Caesalpinia minax. J. Nat. Prod. 2013;76:1025–1031. doi: 10.1021/np300918q. [DOI] [PubMed] [Google Scholar]
- 3.Ma G.X., Wu H.F., Chen D.L., Zhu N.L., Zhu Y.D., Li P.F., Sun Z.H., Yang J.S., Xu X.D. Antimalarial and Antiproliferative Cassane Diterpenes of Caesalpinia sappan. J. Nat. Prod. 2015;78:2364–2371. doi: 10.1021/acs.jnatprod.5b00317. [DOI] [PubMed] [Google Scholar]
- 4.Yodsaoue O., Cheenpracha S., Karalai C., Ponglimanont C., Chantrapromma S., Fun H.K., Kanjana O.A. Phanginin A–K, diterpenoids from seeds of Caesalpinia sappan Linn. Phytochemistry. 2008;69:1242–1249. doi: 10.1016/j.phytochem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Pudhom K., Sommit D., Suwankitti N., Petsom A. Cassane Furano-diterpenoids from the Seed Kernels of Caesalpinia bonduc from Thailand. J. Nat. Prod. 2007;70:1542–1544. doi: 10.1021/np070330y. [DOI] [PubMed] [Google Scholar]
- 6.Dickson R.A., Houghton P.J., Hylands P.J. Antibacterial and antioxidant cassanediterpenoids from Caesalpinia benthamiana. Phytochemistry. 2007;68:1436–1441. doi: 10.1016/j.phytochem.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Cheenpracha S., Karalai C., Ponglimanont C., Chantrapromma K., Laphookhieo S. Cassane-type diterpenes from the seeds of Caesalpinia crista. Helv. Chim. Acta. 2006;89:1062–1066. doi: 10.1002/hlca.200690083. [DOI] [Google Scholar]
- 8.Xu X.D., Yang J.Q., Zhou X.Y., Li W.P., Zhu N.L., Wu H.F., Li P.F., Sun Z.H., Yang J.S., Ma G.X. Cassane diterpenes with oxygen bridge from the seeds of Caesalpinia sappan. Fitoterapia. 2016;112:205–210. doi: 10.1016/j.fitote.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang W.X., Xiong J., Tang Y., Zhu J.J., Li M., Zhao Y., Yang G.X., Xia G., Hu J.F. Rearranged abietane diterpenoids from the roots of Clerodendrum trichotomum and their cytotoxicities against human tumor cells. Phytochemistry. 2013;89:89–95. doi: 10.1016/j.phytochem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Rayanil K., Limpanawisut S., Tuntiwachwuttikul P. Entpimarane and enttrachylobane diterpenoids from Mitrephora alba and their cytotoxicity against three human cancer cell lines. Phytochemistry. 2013;89:125–130. doi: 10.1016/j.phytochem.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen H.X., Nguyen N.T., Dang P.H., Ho P.T., Nguyen M.T.T., Can M.V., Dibwe D.F., Ueda J.Y., Awale S. Cassane ditrpenes from the seed kernels of Caesalpinia sappan. Phytochemistry. 2016;122:286–293. doi: 10.1016/j.phytochem.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J.Y., Abdel-Mageed W.M., Liu M.M. Caesanines A-D, New Cassane Diterpenes with Unprecedented N Bridge from Caesalpinia sappan. Org. Lett. 2013;15:4726–4729. doi: 10.1021/ol402058z. [DOI] [PubMed] [Google Scholar]
- 13.Desjardins R.E., Canfield C.J., Haynes J.D., Chulay J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979;16:710–718. doi: 10.1128/AAC.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trager W., Jensen J.B. Human Malaria in Continuous Culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 15.Vennerstrom J.L., Arbe-Barnes S., Brun R., Charman S.A., Chiu F.C.K., Chollet J., Dong Y., Dorn A., Hunziker D., Matile H., et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 16.Kovendan K., Murugan K., Panneerselvam C., Aarthi N., Mahesh P.K., Subramaniam J., Amerasan D., Kalimuthu K., Vincent S. Antimalarial activity of Carica papaya (Family: Caricaceae) leaf extract against Plasmodium falciparum. Asian Pac. J. Trop. Dis. 2012;2:S306–S311. doi: 10.1016/S2222-1808(12)60171-6. [DOI] [Google Scholar]
- 17.Abbott W.S. A method of computing the effectiveness of insecticides. J. Econ. Entomol. 1925:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 18.Finney D.J. Probit Analysis. Cambridge University; London, UK: 1971. pp. 68–78. [Google Scholar]