Abstract
The catalytic activity of enzymes produced by an entomopathogenic filamentous fungus (Isaria fumosorosea KCh J2) towards selected steroid compounds (androstenedione, adrenosterone, progesterone, 17α-methyltestosterone and dehydroepiandrosterone) was investigated. All tested substrates were efficiently transformed. The structure of the substrate has a crucial impact on regio- and stereoselectivity of hydroxylation since it affects binding to the active site of the enzyme. Androstenedione was hydroxylated in the 7α-position to give a key intermediate in the synthesis of the diuretic-7α-hydroxyandrost-4-ene-3,17-dione with 82% conversion. Adrenosterone and 17α-methyltestosterone were hydroxylated in the 6β-position. Hydroxylated derivatives such as 15β-hydroxy-17α-methyltestosterone and 6β,12β-dihydroxy-17α-methyltestosterone were also observed. In the culture of Isaria fumosorosea KCh J2, DHEA was effectively hydroxylated in the C-7 position and then oxidized to give 7-oxo-DHEA, 3β,7α- and 3β,7β-dihydroxy-17a-oxa-d-homo-androst-5-ene-17-one. We obtained 7β-OH-DHEA lactone with 82% yield during 3 days transformation of highly concentrated (5 g/L) DHEA.
Keywords: Isaria fumosorosea, biotransformation, dehydroepiandrosterone, DHEA, steroid lactones
1. Introduction
Steroid compounds are very common in pharmacy and medicine because of their variety of biological activities [1,2,3]. Hydroxylation of steroid compounds by microbial monooxygenases, which are similar to mammalian cytochromes P450, is a source of molecules of high biological activity and key intermediates in chemical synthesis [4,5,6,7]. The first known hydroxylated corticosteroid is cortisol (hydrocortisone), which is produced by the adrenal glands from cortisone by the 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in response to stress and low blood glucose concentrations. Reduction of the carbonyl group attached to C-11 increases the anti-inflammatory properties relative to cortisone and allows it to be used in dermatology as a topical steroid [8]. These findings caused increased demand for this drug and hence the need to improve the process for obtaining it. Hydroxylation of Reichstein’s S substance in the 11β-position in a culture of Curvularia lanata significantly decreased the number of chemical steps (from 36 to 11) and the price of hydrocortisone to less than 1 $ [9] An additional hydroxyl group in the 16α-position eliminates the salt-retaining activity—the most common adverse effect of steroids. These discoveries resulted in obtaining chloro and fluoro derivatives, with much stronger anti-inflammatory activity and fewer side effects, which are used nowadays in medicine [10]. In many cases, biotransformation products have higher biological activity than their precursors. The enzyme selectivity and mild biotransformation conditions contributed to the development of this field of research [11].
Dehydroepiandrosterone (DHEA) is a natural steroid hormone produced by the adrenal cortex. Its hydroxylated derivatives have immunomodulatory, antiproliferative, neuroprotective and anti-inflammatory properties. They are responsible for anti-glucocorticoid action [12,13,14,15,16]. 7α- and 7β-hydroxy derivatives have neuroprotective effects in an animal model of Alzheimer’s disease, improve memory in old mice and affect the mood during the menstrual cycle [15,17,18,19]. 7α-Hydroxy-DHEA extends its antioxidant effect to oxidative damage in the liver earlier than DHEA [13]. 7α-Hydroxyepiandrosterone, another DHEA derivative, is also a very potent anti-inflammatory agent in colitis as well as neuroprotective in cerebral ischemia [20]. Due to high biological activity of 7-hydroxy derivatives of DHEA, 481 strains from different genera were tested for 7-hydroxylase activity by Lobastova et al. [21]. Strains from 64 tested genera were able to perform hydroxylation in this position, the majority giving products with the 7α-configuration. Methods for achieving high yields of 7α-hydroxy-DHEA in cultures of Absidia coerulea AM93, Fusarium culmorum, Mortierella isabellina AM212, Gelasinospora retispora, Mucor silvaticus as well as 7β-hydroxy-DHEA in the cultures of Cephalosporium aphidicola, Aspergillus wentii MRC 200316, and Mortierella isabellina AM212 are described in the literature [4,22,23,24,25,26,27]. 7α-Hydroxyandrostenedione is also a compound of practical significance used in the pharmaceutical industry in the production of diuretic agents. It can be obtained in cultures of Chaetomium sp. KCH 6651, Didymosphearia igniaria KCH 6670, Paecilomyces victoriae or Absidia glauca [7,28,29,30]. 7-Oxo-DHEA affects the levels of thyroid hormones and thermogenesis and can be used to control obesity [31,32,33,34]. Moreover, steroid lactones can inhibit aromatase—an enzyme that catalyzes aromatization of androgens into estrogens, overexpression of which may be observed in hormone-dependent breast cancers [35]. The first steroid aromatase inhibitor used in treatment was testolactone [36]. High activity of Baeyer-Villiger monooxygenase are characteristic to strains from the genera Penicillium, e.g., P. simplicissimum, P. lanosocoeruleum, P. commune or P. chrysogenum [6,37,38].
The biocatalyst used in this work, an Isaria fumosorosea strain, belongs to an interesting group of about 700 species (from >100 genera) of entomopathogenic (insect-pathogenic) fungi that constitute a unique, highly specialized trophic subgroup [39,40]. Fungal pathogens of insects are found within every ecosystem and all major fungal lineages with the principal exception of the higher basidiomycetes [40,41,42]. Isaria fumosorosea (previously Paecilomyces fumosoroseus [43,44]) is a promising mycoinsecticide for the control of the diamondback moth (Plutella xylostella), the Asian citrus psyllid (Diaphorina citri), whiteflies and other pest insects [45,46,47,48]. Like most fungal pathogens, I. fumosorosea directly penetrates the host through the exoskeleton, with the help of a range of hydrolytic enzymes secreted by growing hyphae. These enzymes include chitinases, proteases, and lipases, which destroy the complex and variable structure of the insect cuticle [49,50,51,52,53]. Among the extensive group of entomopathogenic fungi, strains of mainly one species, Beauveria bassiana, have been used as a biocatalyst. The species is able to provide highly effective glucosylation of aromatic compounds, as well as polyphenols [54,55,56,57,58,59,60]. It is a well-known biocatalyst for the transformation of steroid compounds. Hydroxylations in the C-7α and C-11α positions are described in the literature [61,62,63,64,65]. Strains from this genus also produce Baeyer-Villiger oxidations to afford D-homo lactones [66,67]. There are various publications describing the transformations of different B. bassiana strains to multiple metabolites [61,67].
2. Results and Discussion
The purpose of this study was to investigate the catalytic ability of entomopathogenic filamentous fungus of the Isaria fumosorosea KCh J2 strain against selected steroid compounds. The strain was isolated from a spider cadaver. Substrates in this study were androstenedione, adrenosterone, progesterone, 17α-methyltestosterone and dehydroepiandrosterone. All substrates were transformed with high conversion in a short period of time. The effect of molecular structure on regio- and stereoselectivity of hydroxylation was observed. An additional goal was to obtain lactone derivatives of DHEA and to optimize the process. The course of biotransformation was established by a screening procedure. The structure of products was determined by analysis of pure fractions obtained from preparative biotransformations.
Product structures were determined by proton nuclear magnetic resonance (1H-NMR), carbon-13 nuclear magnetic resonance (13C-NMR) (Table 1), heteronuclear multiple-bond correlation spectroscopy (HMBC), heteronuclear multiple-quantum correlation spectroscopy (HMQC), gas chromatography (GC) and thin-layer chromatography (TLC). The spectral characteristics of obtained compounds were in agreement with literature data [4,28,68,69,70].
Table 1.
13C-NMR chemical shifts of products in CDCl3.
| Atom Number | Products | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7α-OH-AD | 6β-OH-Adr | 6β-OH-17mT | 6β-OH-17mT a | 15β-OH-17mT | 6β,12β-OH-17mT b | 7α-OH-DHEA | 7β-OH-DHEA | 7α-OH-DHEA-Lactone | 7β-OH-DHEA-Lactone | 7-oxo-DHEA | |
| 1 | 35.40 | 36.10 | 38.36 | 38.50 | 34.11 | 36.58 | 37.07 | 37.02 | 36.84 | 36.72 | 36.60 |
| 2 | 33.89 | 34.11 | 34.39 | 35.08 | 32.87 | 33.89 | 31.39 | 31.60 | 31.32 | 31.53 | 31.25 |
| 3 | 198.83 | 200.67 | 200.53 | 198.91 | 199.71 | 199.22 | 71.25 | 71.30 | 71.20 | 71.11 | 70.44 |
| 4 | 127.03 | 127.17 | 126.55 | 126.46 | 124.07 | 125.21 | 42.06 | 41.77 | 42.02 | 41.47 | 41.99 |
| 5 | 167.25 | 166.61 | 168.38 | 169.45 | 171.20 | 168.84 | 146.64 | 143.78 | 146.29 | 144.00 | 166.27 |
| 6 | 41.01 | 72.13 | 73.25 | 73.40 | 32.78 | 71.07 | 123.67 | 125.65 | 123.43 | 125.15 | 126.06 |
| 7 | 67.01 | 38.10 | 39.05 | 40.11 | 32.22 | 37.52 | 64.37 | 72.91 | 63.44 | 72.21 | 201.20 |
| 8 | 39.37 | 30.65 | 30.70 | 31.82 | 35.94 | 29.34 | 37.32 | 40.54 | 40.24 | 43.42 | 44.47 |
| 9 | 45.31 | 63.13 | 53.77 | 55.32 | 54.32 | 51.68 | 42.72 | 48.37 | 41.76 | 48.13 | 50.22 |
| 10 | 38.55 | 37.68 | 38.22 | 39.14 | 38.95 | 38.27 | 37.63 | 36.78 | 37.54 | 36.52 | 38.54 |
| 11 | 20.15 | 207.41 | 20.78 | 21.85 | 20.70 | 29.54 | 20.19 | 20.51 | 21.70 | 22.07 | 20.72 |
| 12 | 30.98 | 50.50 | 31.54 | 32.79 | 31.47 | 71.83 | 31.18 | 31.36 | 38.53 | 39.15 | 30.85 |
| 13 | 47.31 | 50.44 | 45.60 | 46.58 | 44.89 | 48.41 | 47.23 | 47.89 | 83.50 | 83.46 | 48.00 |
| 14 | 45.64 | 49.71 | 50.29 | 51.57 | 54.77 | 49.35 | 45.05 | 51.34 | 40.15 | 47.20 | 45.88 |
| 15 | 21.25 | 21.69 | 23.30 | 24.37 | 69.16 | 22.67 | 22.02 | 24.31 | 19.86 | 21.55 | 24.31 |
| 16 | 35.71 | 36.03 | 37.29 | 39.89 | 51.97 | 38.02 | 35.91 | 36.10 | 28.86 | 29.26 | 35.77 |
| 17 | 220.41 | 217.24 | 81.73 | 80.90 | 81.40 | 80.42 | 221.30 | 221.31 | 171.83 | 171.79 | 220.52 |
| 18 | 13.49 | 14.91 | 14.11 | 14.71 | 16.67 | 8.75 | 13.39 | 13.70 | 20.08 | 20.38 | 13.89 |
| 19 | 17.01 | 19.32 | 19.68 | 19.94 | 17.47 | 18.86 | 18.38 | 19.29 | 18.33 | 19.07 | 17.57 |
| 20 | 25.91 | 26.64 | 25.41 | 25.83 | |||||||
a Compound 6β-OH-mT in THF-d8; b Compound 6β,12β-mT in DMSO-d6.
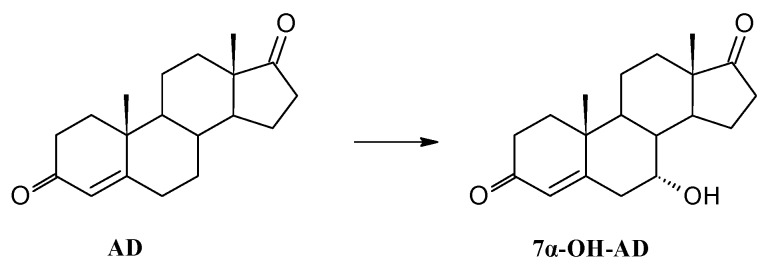
Biotransformation of androstenedione (AD) in Isaria fumosorosea KCh J2 culture gave only one monohydroxylation product, 7α-hydroxyandrost-4-ene-3,17-dione (7α-OH-AD), in high yield (Scheme 1). All the added substrate was transformed in less than 24 h (Table 2). Prolongation of the process caused product degradation. There are known microbial methods of obtaining 7α-OH-AD using Didymosphearia igniaria KCH 6670, Chaetomium sp. KCH 6651, Neurospora crassa or Mucor racemosus, but their efficiency is low and the number of by-products is substantial [7,28,71,72]. The product can be used in the pharmaceutical industry in the synthesis of diuretics [73].
Scheme 1.
Transformation of androstenedione in Isaria fumosorosea KCh J2 culture.
Table 2.
Product accumulation during transformation in Isaria fumosorosea KCh J2 culture.
| Substrate | Compounds Found in the Reaction Mixture (%) | Biotransformation Time (Days) | ||
|---|---|---|---|---|
| 1 | 3 | 7 | ||
| Androstenedione | AD | - | - | - |
| 7α-OH-AD | 76 | 71 | 64 | |
| Adrenosterone | Adr | 38 | 28 | 11 |
| 6β-OH-Adr | 57 | 67 | 84 | |
| 17α-Methyltestosterone | 17mT | - | - | - |
| 6β-OH-17mT | 76 | 49 | 20 | |
| 15β-OH-17mT | 11 | 9 | 8 | |
| 6β,12β-OH-17mT | 4 | 67 | 84 | |
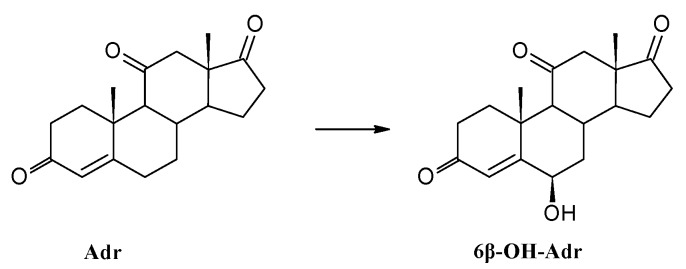
In contrast, the transformation of adrenosterone (Adr), another substrate with a 3-on-4-ene moiety and a carbonyl group attached to C-17, did not result in the formation of the corresponding 7α-hydroxy derivative (Scheme 2). Probably, the carbonyl group at C-11 is a sterical hindrance and prevents access to the active site of the enzyme and formation of 7α-hydroxyadrenosterone. Instead of this, 6β-hydroxyadrenosterone (6β-OH-Adr) is produced but much more slowly (complete transformation of the substrate in more than 7 days) compared to products of other substrates (Table 2). Reduction of the C-17 carbonyl group of adrenosterone, known from the transformation in cultures of Cunninghamella elegant, Aspergillus tamarii Kita, Cephalosporium aphidicola, Fusarium lini or Trichothecium roseum [74,75,76], did not happen after 7 days of transformation by Isaria fumosorosea KCh J2.
Scheme 2.
Hydroxylation of adrenosterone by Isaria fumosorosea KCh J2 strain.
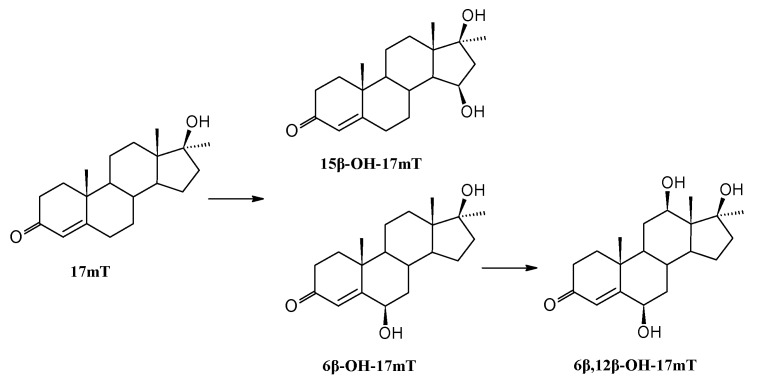
In the culture of Isaria fumosorosea KCh J2 17α-methyltestosterone (17mT) was transformed into two monohydroxylated derivatives: 6β-OH-17mT and 15β-OH-17mT, in less than 24 h (Table 2). Additionally, 6β-OH-17mT was hydroxylated in the 12β position, giving the dihydroxylated derivative 6β,12β-OH-17mT (Scheme 3). 6β-Hydroxy- and dihydroxy derivatives were obtained in the culture of Acremonium strictum [70].
Scheme 3.
Transformation of 17α-methyltestosterone in Isaria fumosorosea KCh J2 culture.
Ten mg of progesterone was transformed in the culture of Isaria fumosorosea KCh J2 strain in less than 24 h, giving many products. Eight fractions from the crude mixture were separated as a result of the transformation of 100 mg of progesterone, but the number of fractions and their purity did not allow the chemical structure of any product to be determined. The number of fractions was astonishing considering the one or three products seen in the transformations of other 3-oxo-4-ene-steroids (AD, Adr, 17mT) used in this study.
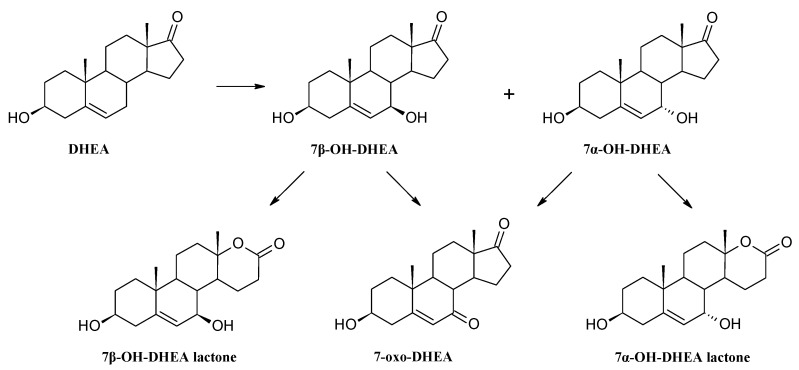
Dehydroepiandrosterone (DHEA) was transformed in less than 24 h in the culture of Isaria fumosorosea KCh J2 strain (Table 3). To establish the pathway of DHEA transformation we modified the screening procedure (Section 3.3). This modification confirmed that the substrate was firstly regioselectively transformed to C-7 position monohydroxylated derivatives (7α-OH-DHEA and 7β-OH-DHEA) and then oxidized, to a small extent, to 7-oxo-DHEA. Furthermore, both hydroxy derivatives were transformed to the corresponding D-lactones (Scheme 4). Additional transformations of intermediate products were conducted to verify possible interconversion between isomers. Transformation of 7α-OH-DHEA led to the formation of 7β-OH-DHEA and 7α-OH-DHEA lactone. The addition of 7β-OH-DHEA to the culture of Isaria fumosorosea KCh J2 gave an analogous result. Surprisingly, in the transformation of both alcohols, we did not observe any amount of 7-oxo-DHEA. This compound as a substrate was not reduced to any 7-hydroxy derivatives after 3 days of transformation. The total amount of 7-alcohol came from hydroxylation of DHEA, not from the reduction of 7-oxo-DHEA. Milecka-Tronina et al. and Kołek et al. also observed, in cultures of different genera—Absidia coerulea AM93 and Mortierella isabellina AM212— the oxidation of stereoisomers of 7OH-DHEA to 7-oxo-DHEA but not a stereoselective reduction to the opposite 7-alcohols [4,22]. However, such an interconversion was successfully tested in human, pig and rat liver microsomal fractions containing 11β-hydroxysteroid dehydrogenase (11βHSD) as well as human 11βHSD1 expressed in Saccharomyces cerevisiae [77,78,79,80,81]. Isoenzymes of the 11βHSD family catalyze the reaction of activation of cortisone and inactivation of cortisol, but the spectrum of substrates is broader (corticosterone, 7-hydroxy-cholesterol, 7OH-DHEA) and oxidoreductase activity also occurs in the C-7 position [77]. Thus, interconversion of 7OH-DHEA via 7-oxo-DHEA is probably specific only to these animals’ cytochrome P450s. In addition, 7-oxo-DHEA was characterized as an 11β-HSD inhibitor, which can explain the lack of reduction to 7OH-DHEA [77]. In our study interconversion of 7-hydroxy-DHEA stereoisomers was observed but not via the 7-oxo form. We assume that this interconversion is not catalyzed by 11βHSD. Moreover, transformations of intermediates were slower than the transformation of DHEA. In the transformation of DHEA lactones were observed after 1 day of incubation, in contrast to 3 days incubation of the 7β-hydroxy derivative. 7α-OH-DHEA lactone was not transformed to the corresponding lactone in 3 days incubation. Such results indicate that DHEA acts as an inductor for this reaction cascade.
Table 3.
Compositions of crude mixtures obtained in transformations of different amounts of DHEA.
| Concentration of Substrate (g/L) | Compounds Found in the Reaction Mixture (%) | Biotransformation Time (h) | ||||
|---|---|---|---|---|---|---|
| 3 | 12 | 24 | 72 | 168 | ||
| 0.1 | DHEA | 7 | - | - | - | - |
| 7α-OH-DHEA | 15 | 2 | - | - | - | |
| 7β-OH-DHEA | 75 | 34 | 18 | 8 | - | |
| 7-oxo-DHEA | 2 | 9 | 6 | - | - | |
| 7α-OH-DHEA lactone | - | 13 | 20 | 22 | 22 | |
| 7β-OH-DHEA lactone | - | 41 | 52 | 60 | 58 | |
| 0.5 | DHEA | 74 | 2 | - | - | - |
| 7α-OH-DHEA | 5 | 1 | - | - | - | |
| 7β-OH-DHEA | 21 | 16 | - | - | - | |
| 7-oxo-DHEA | - | 1 | - | - | - | |
| 7α-OH-DHEA lactone | - | 15 | 18 | 17 | 8 | |
| 7β-OH-DHEA lactone | - | 62 | 76 | 72 | 74 | |
| 1.0 | DHEA | 75 | 1 | - | - | - |
| 7α-OH-DHEA | 5 | 2 | - | - | - | |
| 7β-OH-DHEA | 20 | 36 | - | - | - | |
| 7-oxo-DHEA | - | 2 | - | - | - | |
| 7α-OH-DHEA lactone | - | 12 | 19 | 20 | 15 | |
| 7β-OH-DHEA lactone | - | 45 | 76 | 70 | 66 | |
| 2.0 | DHEA | 89 | 8 | - | - | - |
| 7α-OH-DHEA | 2 | 7 | - | - | - | |
| 7β-OH-DHEA | 9 | 58 | 2 | - | - | |
| 7-oxo-DHEA | - | 4 | - | - | - | |
| 7α-OH-DHEA lactone | - | 1 | 16 | 14 | 12 | |
| 7β-OH-DHEA lactone | - | 20 | 78 | 78 | 79 | |
| 5.0 | DHEA | 96 | 56 | 26 | - | - |
| 7α-OH-DHEA | 1 | 5 | 6 | - | - | |
| 7β-OH-DHEA | 3 | 33 | 37 | - | - | |
| 7-oxo-DHEA | - | 1 | 3 | - | - | |
| 7α-OH-DHEA lactone | - | - | 2 | 12 | 11 | |
| 7β-OH-DHEA lactone | - | 4 | 23 | 81 | 75 | |
Scheme 4.
Putative transformation of DHEA in the culture of Isaria fumosorosea KCh J2 strain.
Due to the rapid transformation of DHEA, an experiment with different DHEA concentrations was performed. Various amounts of the substrate were added to a grown culture of Isaria fumosorosea KCh J2 to give a final medium concentration of DHEA in the range of 0.1 to 5.0 g/L. Analysis of the composition of the reaction mixtures indicates that the 7β-OH product is formed in the largest amount (Table 3). Along with the increase of DHEA concentration the ratio of 7α-OH-DHEA to 7β-OH-DHEA decreases. Also, transformation of hydroxy derivatives into the corresponding lactones is faster at a higher concentration of substrate. As we suggested earlier, DHEA seems to be an inductor of the cascade, but this requires further investigation.
3. Materials and Methods
3.1. Materials
The substrates androstenedione, adrenosterone, progesterone, 17α-methyltestosterone and dehydroepiandrosterone (DHEA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3β,7β-Dihydroxyandrost-5-ene-17-one (7β-OH-DHEA), 3β,7β-dihydroxyandrost-5-ene-17-one (7β-OH-DHEA) and 3β-hydroxyandrost-5-ene-7,17-dione (7-oxo-DHEA) were isolated as a products of DHEA transformation in the culture of Isaria fumosorosea KCh J2 strain.
The microorganism Isaria fumosorosea KCh J2 was obtained from the collection of the Department of Chemistry, Wrocław University of Environmental and Life Sciences (Wrocław, Poland). Isolation and identification procedures were described in our previous paper [82]. The strain was maintained on Sabouraud 4% dextrose-agar slopes and freshly subcultured before use in the transformation experiments.
3.2. Screening Procedure
Erlenmeyer flasks (300 mL), each containing 100 mL of the cultivation medium (3% glucose, 1% aminobac), were inoculated with a suspension of I. fumosorosea KCh J2 strain and then incubated for 3 days at 24 °C on a rotary shaker. Then 10 mg of a substrate dissolved in 1 mL of tetrahydrofuran (THF) was added. Samples were taken on the 1st, 3rd and 7th day of the process and products were subsequently extracted using chloroform and analyzed using TLC and GC.
3.3. Screening Procedure for DHEA
Incubation of DHEA was carried out in Erlenmeyer flasks (300 mL) containing 100 mL of the cultivation medium. Ten mg of DHEA was added in THF (1 mL) to a 3-day-old culture of the investigated strain. The transformation conditions were the same as in the standard experiment (Section 3.2). Samples were taken at 3, 6, 9, 12 h and on the 1st, 3rd and 7th day of the process, and products were subsequently extracted using chloroform and analyzed using TLC and GC.
3.4. Establishing the DHEA Transformation Pathway
To the 3-day-old culture, cultivated as described in Section 3.2, Ten mg of 7α-OH-DHEA, 7β-OH-DHEA, 7-oxo-DHEA and DHEA (control) dissolved in 1 mL of THF was added to each flask. Samples were taken at 6 and 12 h and on the 1st and 3rd day of the process and products were subsequently extracted using chloroform and analyzed using TLC.
3.5. Transformation Procedure for Different DHEA Concentrations
To each flask containing 100 mL of the 3-day-old culture of Isaria fumosorosea KCh J2, cultivated as described in Section 3.2, DHEA (50, 100, 200 or 500 mg) dissolved in THF (2 mL) was added. The final concentration of DHEA in the culture was 0.5, 1.0, 2.0 and 5.0 g/L, respectively. Samples were taken at 6 and 12 h and on the 1st and 3rd day of the process and products were subsequently extracted using chloroform and analyzed using TLC and GC.
3.6. Preparative Biotransformation
The same transformations were performed on the preparative scale in 2000 mL flasks, each containing 500 mL of the cultivation medium. The culture of I. fumosorosea KCh J2 was incubated under the same conditions as in the screening procedure and then 100 mg of substrate dissolved in 2 mL of THF was added to the 3-day-old culture. After the complete transformation of the substrate, the mixture was extracted with CHCl3 (3 × 300 mL), dried (MgSO4) and concentrated in vacuo. The crude mixture obtained this way was separated by preparative TLC and analyzed (TLC, GC).
3.7. Analytical Methods
The course of the biotransformation was monitored by means of TLC. The composition of product mixtures was established by GC. The crude mixture was separated by preparative TLC (Silica Gel GF, 500 μm, Analtech, Newark, DE, USA) and hexane/acetone mixture (2:1, v/v) as an eluent. After elution products were detected under UV light (365 nm) then scraped from the plate and eluted with acetone to give fractions. Analytical TLC was carried out on silica gel G (Merck, Darmstadt, Germany). Compounds were detected by spraying the plates with a H2SO4/CH3OH mixture (1:1, v/v) and visualized under UV light (254 nm). GC analysis was performed using a Hewlett-Packard 5890A (Series II) GC instrument fitted with a flame ionization detector (FID) (nowadays Agilent, Santa Clara, CA, USA). The DB-5MS (cross-linked phenyl- methylsiloxane) capillary column (30 m × 0.32 mm × 0.25 μm) was used to determine the composition of product mixtures. The following temperature program was used: 220 °C (1 min)/4 °C/min/260 °C (1 min)/30 °C/min/300 °C (5 min). For gas chromatography – mass spectrometry GC-MS analysis, a GCMS-SATURN 2000 instrument (Varian, nowadays Agilent, Santa Clara, CA, USA) was used with a ZB-1 (crosslinked phenyl-methylsiloxane) capillary column (30 m × 0.25 mm × 0.25 μm). The following temperature programme was used: 220 °C (1 min)/5 °C/1 min/300 °C (7 min) (Supplementary Materials). The NMR spectra were recorded on a DRX 600 MHz spectrometer (Bruker, Bruker, Billerica, MA, USA) and measured in CDCl3. Products poorly soluble in chloroform were dissolved in DMSO-d6 or THF-d8. The products’ structures were determined by means of elemental analysis, 1H-NMR, 13C-NMR and correlation spectroscopy (HMBC, HMQC).
3.8. Spectral Data of Isolated Metabolites
3.8.1. Transformation of Androstenedione (AD)
After 24 h transformation of 100 mg of androstenedione in Isaria fumosorosea KCh J2 culture 42.3 mg (42%) of 7α-hydroxyandrost-4-ene-3,17-dione (7α-OH-AD) was isolated as the sole product (Supplementary Materials).
7α-Hydroxyandrost-4-ene-3,17-dione (7α-OH-AD). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 0.89 (s, 3H, 18-H); 1.20 (s, 3H, 19-H); 1.26 (td, 1H, J = 13.1, 3.6 Hz, 12-Hα); 1.45 (td, 1H, J = 13.0, 3.5 Hz, 11-Hβ); 1.50–1.60 (m, 2H, 9-H, 15-Hβ); 1.68–1.79 (m, 4H, 1-Hα, 8-H, 11-Hα, 14-H); 1.82 (dm, 1H, J = 13.0 Hz, 12-Hβ); 2.01–2.13 (m, 3H, 1-Hb, 15-Hα, 16-Hα); 2.35 (dm, 1H, J = 16.6 Hz, 2-Hα); 2.37–2.49 (m, 3H, 2-Hβ, 6-Hα, 16-Hβ); 2.65 (d, 1H, J = 14.9 Hz, 6-Hβ); 4.09 (s, 1H, 7-Hβ); 5.78 (s, 1H, 4-H).
3.8.2. Transformation of Adrenosterone (Adr)
After 24 h incubation of 100 mg adrenosterone in Isaria fumosorosea KCh J2 culture 33.1 mg (33%) of 6β-hydroxyandrost-4-ene-3,11,17-trione (6β-OH-Adr) was isolated as the sole product (Supplementary Materials).
6β-Hydroxyandrost-4-ene-3,11,17-trione (6β-OH-Adr). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 0.89 (s, 3H, 18-H); 1.50 (ddd, 1H, J = 14.1, 11.6, 2.6 Hz, 7-Hα); 1.60 (s, 3H, 19-H); 1.62 (td, 1H, J = 14.5, 4.0 Hz, 1-Hα); 1.73 (ddd, 1H, J = 21.4, 12.1, 9.4 Hz, 15-Hβ); 1.86 (d, 1H, J = 11.5 Hz, 9-H); 1.89 (td, 1H, J = 11.8, 5.7 Hz, 14-H); 2.12–2.18 (m, 1H, 15-Hα); 2.20–2.37 (m, 4H, 2-Hα, 7-Hβ, 12-Hα, 16-Hα); 2.44–2.60 (m, 4H, 2-Hβ, 8-H, 12-Hβ, 16-Hβ); 2.80 (dm, 1H, J = 13.4 Hz, 1-Hβ); 4.39 (br s, 1H, 6-Hα); 5.79 (br s, 1H, 4-H).
3.8.3. Transformation of 17α-Methyltestosterone (17mT)
After 7 days transformation of 100 mg of 17α-methyltestosterone in Isaria fumosorosea KCh J2 culture 8.4 mg (8%) of 6β-hydroxy-17α-methyltestosterone (6β-OH-17mT), 5.1 mg (5%) of 15β-hydroxy-17α-methyltestosterone (15β-OH-17mT) and 34.3 mg (34%) of 6β,12β-dihydroxy-17α-methyltestosterone (6β,12β-OH-17mT) were isolated (Supplementary Materials).
6β-Hydroxy-17α-methyltestosterone (6β-OH-17mT). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 0.89 (td, 1H J = 11.3, 4.3 Hz, 9-H); 0.94 (s, 3H, 18-H); 1.19–1.23 (m, 2H, 7-Hα, 14-H); 1.22 (s, 3H, 20-H); 1.28–1.38 (m, 2H, 12-Hα, 15-Hβ); 1.40 (s, 3H, 19-H); 1.50 (qd, 1H, J = 12.7, 4.0 Hz, 11-Hα); 1.55–1.64 (m, 3H, 11-Hβ, 12-Hβ, 15-Hα); 1.69 (ddd, 1H, J = 14.5, 13.4, 4.2 Hz, 1-Hα); 1.76 (ddd, 1H, J = 14.2, 9.6, 6.4 Hz, 16-Hα); 1.84 (qd, 1H, J = 14.1, 3.5 Hz, 16-Hβ); 2.01 (dt, 1H, J = 13.5, 3.0 Hz, 7-Hβ); 2.03–2.07 (m, 2H, 1-Hβ, 8-H); 2.39 (dddd, 1H, J = 16.9, 4.0, 2.8, 0.7 Hz, 2-Hα); 2.52 (ddd, 1H, J = 16.8, 15.1, 5.0 Hz, 2-Hβ); 4.35 (t, 1H, J = 2.8 Hz, 6-Hα); 5.82 (s, 1H, 4-H).
Due to the low solubility of the resulting product in CDCl3, the compound was dissolved in the deuterated THF(THF-d8) and NMR analysis was performed again (Supplementary Materials).
6β-Hydroxy-17α-methyltestosterone (6β-OH-17mT). 1H-NMR (600 MHz) (ppm) (THF-d8) δ: 0.87 (td, 1H J = 11.3, 4.3 Hz, 9-H); 0.90 (s, 3H, 18-H); 1.13 (s, 3H, 20-H); 1.13 (tdd, 1H, J = 12.7, 3.1, 1.3 Hz, 7-Hα); 1.21 (d, 1H, J = 11.9, 10.8, 4.3 Hz, 14-H); 1.25–1.33 (m, 2H, 12-Hα, 15-Hβ); 1.38 (s, 3H, 19-H); 1.48–1.55 (m, 2H, 11-Hα, 12-Hβ); 1.56–1.62 (m, 3H, 11-Hβ, 15-Hα, 16-Hα); 1.66 (ddd, 1H, J = 14.6, 13.4, 4.3 Hz, 1-Hα); 1.80–1.86 (m, 1H, 16-Hβ); 1.93 (dt, 1H, J = 13.5, 3.0 Hz, 7-Hβ); 2.01 (ddd, 1H, J = 13.1, 4.9, 2.8 Hz, 1-Hβ); 2.08 (qd, 1H, J = 11.0, 3.2 Hz, 8-H); 2.22 (dddd, 1H, J = 16.9, 3.9, 2.8, 0.8 Hz, 2-Hα); 2.44 (ddd, 1H, J = 16.8, 15.2, 5.0 Hz, 2-Hβ); 3.29 (s, 1H, 17-OH); 4.17 (q, 1H, J = 2.8 Hz, 6-Hα); 4.29 (dd, 1H, J = 2.7, 1.4 Hz, 6-OH); 5.66 (s, 1H, 4-H).
15β-Hydroxy-17α-methyltestosterone (15β-OH-17mT). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 0.99 (td, 1H, J = 12.2, 3.9 Hz, 9-H); 1.06–1.13 (m, 2H, 7-Hα, 14-H); 1.15 (s, 3H, 18-H); 1.18 (s, 3H, 19-H); 1.24 (s, 3H, 20-H); 1.29 (td, 1H, J = 13.7, 4.7 Hz, 12-Hα); 1.46 (qd, 1H, J = 12.9, 3.3 Hz, 11-Hβ); 1.52 (td, 1H, J = 12.4, 3.6 Hz, 12-Hβ); 1.63 (qd, 1H, J = 11.6, 4.2 Hz, 11-Hα); 1.74 (td, 1H, J = 13.9, 4.8 Hz, 1-Hα); 2.00 (qd, 1H, J = 11.0, 3.2 Hz, 8-H); 2.05 (ddd, 1H, J = 13.7, 5.2, 3.3 Hz, 1-Hβ); 2.10 (ddt, 1H, J = 12.5, 5.5, 2.9 Hz, 7-Hβ); 2.27 (dd, 1H, J = 14.4, 3.9, 2.7 Hz, 6-Hα); 2.30-2.37 (m, 3H, 2-Hα, 16-Hα, 16-Hβ); 2.43 (ddd, 1H, J = 19.5, 11.8, 5.2 Hz, 2-Hβ); 2.48 (td, 1H, J = 14.4, 1.7 Hz; 6-Hβ); 4.20 (ddd, 1H, J = 7.9, 5.8, 2.4 Hz, 15-Hα); 5.74 (s, 1H, 4-H).
Due to the low solubility of the resulting product in CDCl3, the compound was dissolved in the deuterated DMSO (DMSO-d6) and NMR analysis was performed again (Supplementary Materials).
6β,12β-Dihydroxy-17α-methyltestosterone (6β,12β-OH-17mT). 1H-NMR (600 MHz) (ppm) (DMSO-d6) δ: 0.81 (s, 3H, 18-H); 0.90 (ddd, 1H, J = 11.7, 10.1, 4.2 Hz, 9-H); 0.98–1.09 (m, 2H, 7-Hα, 14-H); 1.21 (s, 3H, 20-H); 1.22–1.27 (m, 1H, 15-Hβ); 1.28 (s, 3H, 19-H); 1.32 (q, 1H, J = 12.0 Hz, 11-Hα); 1.48–1.55 (m, 3H, 11-Hβ, 15-Hα, 16-Hα); 1.60 (ddd, 1H, J = 14.6, 13.4, 4.1 Hz, 1-Hα); 1.73 (t, 1H, J = 11.2 Hz, 16-Hβ); 1.78 (dt, 1H, J = 13.6, 3.0 Hz, 7-Hβ); 1.82 (qd, 1H, J = 11.2, 2.8 Hz, 8-H); 1.90 (ddd, 1H, J = 13.0, 4.7, 2.6 Hz, 1-Hβ); 2.20 (ddd, 1H, J = 16.9, 3.9, 2.8 Hz, 2-Hα); 2.45 (ddd, 1H, J = 16.9, 15.4, 4.7 Hz, 2-Hβ); 3.52–3.56 (m, 1H, 12-Hα); 3.57 (s, 1H, 17-OH); 4.00 (d, 1H, J = 3.6 Hz, 12-OH); 5.11 (q, 1H, J = 2.5 Hz, 6-Hα); 5.11 (d, 1H, J = 2.5 Hz, 6-OH); 5.66 (s, 1H, 4-H).
3.8.4. Transformation of Progesterone (P)
The complete transformation of 100 mg of progesterone by Isaria fumosorosea KCh J2 culture in 48 h gave many products. Eight fractions from crude mixture were separated by preparative TLC. NMR analysis of each fraction exposed a mixture of products. Analysis of NMR, TLC and GC data ensured that the biotransformation of progesterone was effective although the quantity of products makes identification impossible.
3.8.5. Transformation of dehydroepiandrosterone (DHEA)
After 12 h transformation of 200 mg of dehydroepiandrosterone in Isaria fumosorosea KCh J2 culture 10.2 mg (5%) of 3β,7α-dihydroxyandrost-5-ene-17-one (7α-OH-DHEA), 86.4 mg (43%) of 3β,7β-dihydroxyandrost-5-ene-17-one (7β-OH-DHEA), 5.8 mg (3%) of 3β-hydroxyandrost-5-ene-7,17-dione (7-oxo-DHEA) and 31.7 mg (16%) of 3β,7β-dihydroxy-17a-oxa-d-homo-androst-5-ene-17-one (7β-OH-DHEA lactone) were isolated. After 1 day transformation of 200 mg of dehydroepiandrosterone in Isaria fumosorosea KCh J2 culture 30.1 mg (15%) 3β,7α-dihydroxy-17a-oxa-d-homo-androst-5-ene-17-one (7α-OH-DHEA lactone) and 119.8 mg (60%) of 3β,7β-dihydroxy-17a-oxa-d-homo-androst-5-ene-17-one (7β-OH-DHEA lactone) were isolated (Supplementary Materials).
3β,7α-Dihydroxyandrost-5-ene-17-one (7α-OH-DHEA). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 0.87 (s, 3H, 18-H); 1.01 (s, 3H, 19-H); 1.11 (td, 1H, J = 13.4, 3.8 Hz, 1-Hα); 1.23–1.31 (m, 2H, 9-H, 12-Hα); 1.49 (td, 1H, J = 13.1, 4.3 Hz, 11-Hα); 1.50–1.60 (m, 2H, 2-Hα, 15-Hα); 1.64–1.72 (m, 2H, 8-H, 11-Hβ); 1.78 (td, 1H, J = 12.1, 5.3 Hz, 14-H); 1.80–1.89 (m, 3H, 1-Hβ, 2-Hβ, 12-Hβ); 2.07–2.17 (m, 2H, 15-Hβ, 16-Hα); 2.29 (br t, 1H, J = 12.3 Hz, 4-Hα); 2.35 (ddd, 1H, J = 13.3, 4.8, 2.0 Hz, 4-Hβ); 2.33 (dd, 1H, J = 13.1, 4.6 Hz, 16-Hβ); 3.56 (tt, 1H, J = 11.3, 4.7 Hz, 3-Hα); 3.96 (br t, 1H, J = 3.8 Hz, 7-Hβ); 5.63 (dd, 1H, J = 5.1, 1.2 Hz, 6-H).
3β,7β-Dihydroxyandrost-5-ene-17-one (7β-OH-DHEA). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 0.88 (s, 3H, 18-H); 1.05 (td, 1H, J = 13.4, 3.7 Hz, 1-Hα); 1.06 (s, 3H, 19-H); 1.10 (td, 1H, J = 12.1, 4.6 Hz, 9-H); 1.25 (td, 1H, J = 13.7, 4.7 Hz, 12-Hα); 1.44 (ddd, 1H, J = 12.5, 11.0, 5.9 Hz, 14-H); 1.47–1.55 (m, 2H, 2-Hα, 11-Hα); 1.57 (td, 1H, J = 11.2, 8.2 Hz, 8-H); 1.70 (dtd, 1H, J = 13.8, 4.3, 2.9 Hz, 11-Hβ); 1.81–1.89 (m, 4H, 1-Hβ, 2-Hβ, 12-Hβ, 15-Hβ); 2.11 (dt, 1H, J = 19.0, 9.1 Hz, 16-Hα); 2.24 (ddd, 1H, J = 12.3, 8.7, 6.0 Hz, 15-Hα); 2.26 (ddt, 1H, J = 13.4, 11.3, 2.1 Hz, 4-Ha); 2.35 (ddd, 1H, J = 13.2, 4.9, 2.1 Hz, 4-Hβ); 2.45 (dd, 1H, J = 19.4, 8.6 Hz, 16-Hβ); 3.55 (tt, 1H, J = 11.3, 4.4 Hz, 3-Hα); 3.95 (dt, 1H, J = 8.1, 2.2 Hz, 7-Hα); 5.31 (t, 1H, J = 1.9 Hz, 6-H).
3β-Hydroxyandrost-5-ene-7,17-dione (7-oxo-DHEA). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 0.89 (s, 3H, 18-H); 1.22 (s, 3H, 19-H); 1.23–1.34 (m, 2H, 1-Hα, 12-Hα); 1.54–1.68 (m, 4H, 2-Hα, 9-H, 11-Hα, 14-H); 1.70–1.91 (m, 3H, 11-Hβ, 12-Hβ, 15-Hα); 1.91–2.00 (m, 2H, 1-Hβ, 2-Hβ); 2.14 (dd, 1H, J = 18.8, 9.8 Hz, 16-Hα); 2.35–2.45 (m, 2H, 4-Ha, 8-H); 2.46 (dd, 1H, J = 18.8, 8.3 Hz, 16-Hβ); 2.54 (ddd, 1H, J = 13.9, 4.6, 2,2 Hz, 4-Hβ); 2.81 (ddd, 1H, J = 13.4, 8.7, 7.2 Hz, 15-Hβ); 3.68 (tt, 1H, J = 11.3, 4.8 Hz, 3-Hα); 5.74 (br s, 1H, 6-H)
3β,7α-Dihydroxy-17a-oxa-d-homo-androst-5-ene-17-one (7α-OH-DHEA lactone). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 0.97 (s, 3H, 19-H); 1.13 (td, 1H, J = 13.6, 3.7 Hz, 1-Hα); 1.33 (s, 3H, 18-H); 1.34–1.40 (m, 3H, 9-H, 11-Hα, 14-H); 1.50–1.56 (m, 2H, 2-Hα, 15-Hα); 1.67 (td, 1H, J = 12.8, 4.0 Hz, 12-Hα); 1.81 (m, 1H, 11-Hβ); 1.86-1.91 (m, 2H, 1-Hβ, 2-Hβ); 1.94–2.02 (m, 2H, 8-H, 12-Hβ); 2.11 (dddd, 1H, J = 13.1, 8.5, 4.6, 3.0 Hz, 15-Hβ); 2.24 (ddt, 1H, J = 13.2, 11.5, 1.7 Hz, 4-Ha); 2.37 (dd, 1H, J = 13.4, 5.1, 2.1 Hz, 4-Hβ); 2.63 (td, 1H, J = 18.8, 8.9 Hz, 16-Hα); 2.69 (ddd, 1H, J = 18.8, 8.7, 2.5 Hz, 16-Hβ); 3.59 (tt, 1H, J = 11.4, 4.6 Hz, 3-Hα); 3.99 (br s, 1H, 7-Hβ); 5.62 (dd, 1H, J = 5.2, 1.7 Hz, 6-H).
3β,7β-Dihydroxy-17a-oxa-d-homo-androst-5-ene-17-one (7β-OH-DHEA lactone). 1H-NMR (600 MHz) (ppm) (CDCl3) δ: 1.03 (s, 3H, 19-H); 1.10 (td, 1H, J = 13.2, 3.5 Hz, 1-Hα); 1.25 (td, 1H, J = 12.2, 3.9 Hz, 9-H); 1.32 (td, 1H J = 7.3, 3.5 Hz, 8-H); 1.34 (s, 3H, 18-H); 1.51 (ddd, 1H, J = 13.6, 9.6, 2.9 Hz, 2-Hα); 1.59 (ddd, 1H, J = 12.2, 10.5, 4.3 Hz, 14-H); 1.65 (td, 1H, J = 13.2, 4.0 Hz, 12-Hα); 1.74–1.83 (m, 2H, 11-Hα, 11-Hβ, 15-Hα); 1.85–1.91 (m, 2H, 1-Hβ, 2-Hβ); 1.98 (dt, 1H, J = 12.6, 3.4 Hz, 12-Hβ); 2.24 (ddt, 1H, J = 13.4, 11.5, 2.0 Hz, 4-Ha); 2.37 (ddd, 1H, J = 13.4, 4.8, 2.4 Hz, 4-Hβ); 2.44 (dddd, 1H, J = 9.4, 7.1, 4.7, 1.9 Hz, 15-Hβ); 2.57 (dt, 1H, J = 18.8, 9.3 Hz, 16-Hα); 2.69 (ddd, 1H, J = 18.9, 8.4, 1.9 Hz, 16-Hβ); 3.57 (tt, 1H, J = 11.3, 4.5 Hz, 3-Hα); 3.95 (br d, 1H, J = 5.2 Hz, 7-Hα); 5.27 (t, 1H, J = 2.3 Hz, 6-H).
4. Conclusions
The entomopathogenic fungus Isaria fumosorosea KCh J2 has broad ability to transform steroid substrates into the corresponding hydroxylated derivatives. Transformations of androstenedione gave 7α-OH-AD as the sole product in high yield after a short period of time. Transformation of DHEA gave hydroxylated D ring lactones. The strain is able to transform the substrate in a concentration of 5.0 g/L in less than 72 h. With such a large amount of substrate, we observed higher specificity of hydroxylation and faster transformation to the corresponding lactones. The high substrate specificity and acceptance of a high substrate concentration by this strain could be utilized in the production of steroid compounds on an industrial scale. These results encourage further investigation of metabolic activity of Isaria fumosorosea KCh J2 and other entomopathogenic fungi.
Acknowledgments
Publication supported by the Wroclaw Centre of Biotechnology under the Leading National Research Centre (KNOW) programme for years 2014–2018.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
Ewa Kozłowska and Tomasz Janeczko conceived and designed the experiments; Ewa Kostrzewa-Susłow performed the biotransformations; Monika Dymarska performed microbiological examination; Tomasz Janeczko and Edyta Kostrzewa-Susłow analyzed the spectral data; Ewa Kostrzewa-Susłow and Tomasz Janeczko interpreted the results; Ewa Kostrzewa-Susłow wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all the compounds are available from the authors.
References
- 1.Crabb T.A., Saul J.A., Williams R.O. Microbiological transformations. Part 4. Microbiological transformations of 5α-androstan-17-ones and of 17a-aza-d-homo-5α -androstan-17-ones with the fungus Cunninghamella elegans. J. Chem. Soc. Perk. Trans. 1. 1981:1041–1045. doi: 10.1039/P19810001041. [DOI] [Google Scholar]
- 2.Žnidaršič-Plazl P., Plazl I. Development of a continuous steroid biotransformation process and product extraction within microchannel system. Catal. Today. 2010;157:315–320. doi: 10.1016/j.cattod.2010.01.042. [DOI] [Google Scholar]
- 3.Schmid A., Dordick J.S., Hauer B., Kiener A., Wubbolts M., Witholt B. Industrial biocatalysis today and tomorrow. Nature. 2001;409:258–268. doi: 10.1038/35051736. [DOI] [PubMed] [Google Scholar]
- 4.Kołek T., Milecka N., Świzdor A., Panek A., Białońska A. Hydroxylation of DHEA, androstenediol and epiandrosterone by Mortierella isabellina AM212. Evidence indicating that both constitutive and inducible hydroxylases catalyze 7α- as well as 7β-hydroxylations of 5-ene substrates. Org. Biomol. Chem. 2011;9:5414–5422. doi: 10.1039/c1ob05350g. [DOI] [PubMed] [Google Scholar]
- 5.Bhatti H.N., Khera R.A. Biological transformations of steroidal compounds: A review. Steroids. 2012;77:1267–1290. doi: 10.1016/j.steroids.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Kozłowska E., Urbaniak M., Kancelista A., Dymarska M., Kostrzewa-Susłow E., Stępień Ł., Janeczko T. Biotransformation of dehydroepiandrosterone (DHEA) by environmental strains of filamentous fungi. RSC Adv. 2017;7:31493–31501. doi: 10.1039/C7RA04608A. [DOI] [Google Scholar]
- 7.Janeczko T., Świzdor A., Dmochowska-Gładysz J., Białońska A., Ciunik Z., Kostrzewa-Susłow E. Novel metabolites of dehydroepiandrosterone and progesterone obtained in Didymosphearia igniaria KCH 6670 culture. J. Mol. Catal. B Enzym. 2012;82:24–31. doi: 10.1016/j.molcatb.2012.05.009. [DOI] [Google Scholar]
- 8.Katz M., Gans E.H. Topical corticosteroids, structure-activity and the glucocorticoid receptor: Discovery and development—A process of “Planned Serendipity”. J. Pharm. Sci. 2008;97:2936–2947. doi: 10.1002/jps.21222. [DOI] [PubMed] [Google Scholar]
- 9.Janeczko T., Milecka N., Kostrzewa-Susłow E. Industrial importance of microbial hydroxylation of steroids. Przem. Chem. 2012;91:767–771. [Google Scholar]
- 10.Brazzini B., Pimpinelli N. New and established topical corticosteroids in dermatology: Clinical pharmacology and therapeutic use. Am. J. Clin. Dermatol. 2002;3:47–58. doi: 10.2165/00128071-200203010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Borges K.B., de Souza Borges W., Durán-Patrón R., Pupo M.T., Bonato P.S., Collado I.G. Stereoselective biotransformations using fungi as biocatalysts. Tetrahedron-Asymmetry. 2009;20:385–397. doi: 10.1016/j.tetasy.2009.02.009. [DOI] [Google Scholar]
- 12.Loria R.M. Immune up-regulation and tumor apoptosis by androstene steroids. Steroids. 2002;67:953–966. doi: 10.1016/S0039-128X(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 13.Pelissier M.-A., Trap C., Malewiak M.-I., Morfin R. Antioxidant effects of dehydroepiandrosterone and 7alpha-hydroxy-dehydroepiandrosterone in the rat colon, intestine and liver. Steroids. 2004;69:137–144. doi: 10.1016/j.steroids.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Hampl R., Lapčík O., Hill M., Klak J., Kasal A., Nováček A., Šterzl I., Šterzl J., Stárka L. 7-Hydroxydehydroepiandrosterone-a natural antiglucocorticoid and a candidate for steroid replacement therapy? Physiol. Res. 2000;49:S107–S112. [PubMed] [Google Scholar]
- 15.Duskova M., Simunkova K., Hill M., Starka L. 7-Hydroxylated derivatives of dehydroepiandrosterone as possibly related to menstrual mood change in healthy women. Endocr. Regul. 2011;45:131–137. doi: 10.4149/endo_2011_03_131. [DOI] [PubMed] [Google Scholar]
- 16.El Kihel L. Oxidative metabolism of dehydroepiandrosterone (DHEA) and biologically active oxygenated metabolites of DHEA and epiandrosterone (EpiA)-Recent reports. Steroids. 2012;77:10–26. doi: 10.1016/j.steroids.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Segura L.M., Balthazart J. Steroids and neuroprotection: New advances. Front. Neuroendocrinol. 2009;30:v–ix. doi: 10.1016/j.yfrne.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huppert F.A., Van Niekerk J.K. Dehydroepiandrosterone (DHEA) supplementation for cognitive function. Cochrane Database Syst. Rev. 2001;2 doi: 10.1002/14651858.CD000304. [DOI] [PubMed] [Google Scholar]
- 19.Bazin M.-A., El Kihel L., Boulouard M., Bouët V., Rault S. The effects of DHEA, 3β-hydroxy-5α-androstane-6,17-dione, and 7-amino-DHEA analogues on short term and long term memory in the mouse. Steroids. 2009;74:931–937. doi: 10.1016/j.steroids.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Hennebert O., Pelissier M.A., Le Mee S., Wülfert E., Morfin R. Anti-inflammatory effects and changes in prostaglandin patterns induced by 7β-hydroxy-epiandrosterone in rats with colitis. J. Steroid Biochem. Mol. Biol. 2008;110:255–262. doi: 10.1016/j.jsbmb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Lobastova T.G., Gulevskaya S.A., Sukhodolskaya G.V., Turchin K.F., Donova M.V. Screening of mycelial fungi for 7α- and 7β-hydroxylase activity towards dehydroepiandrosterone. Biocatal. Biotransform. 2007;25:434–442. doi: 10.1080/10242420701568492. [DOI] [Google Scholar]
- 22.Milecka-Tronina N., Kołek T., Świzdor A., Panek A. Hydroxylation of DHEA and its analogues by Absidia coerulea AM93. Can an inducible microbial hydroxylase catalyze 7α- and 7β-hydroxylation of 5-ene and 5α-dihydro C19-steroids? Bioorgan. Med. Chem. 2014;22:883–891. doi: 10.1016/j.bmc.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 23.Kołek T. Biotransformation XLVII: Transformations of 5-ene steroids in Fusarium culmorum culture. J. Steroid Biochem. Mol. Biol. 1999;71:83–90. doi: 10.1016/S0960-0760(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 24.Koshimura M., Utsukihara T., Hara A., Mizobuchi S., Horiuchi C.A., Kuniyoshi M. Hydroxylation of steroid compounds by Gelasinospora retispora. J. Mol. Catal. B Enzym. 2010;67:72–77. doi: 10.1016/j.molcatb.2010.07.008. [DOI] [Google Scholar]
- 25.Wang Y., Sun D., Chen Z., Ruan H., Ge W. Biotransformation of 3β-hydroxy-5-en-steroids by Mucor silvaticus. Biocatal. Biotransform. 2013;31:168–174. doi: 10.3109/10242422.2013.813490. [DOI] [Google Scholar]
- 26.Bensasson C.M., Hanson J.R., Hunter A.C. The hydroxylation of Δ5-androstenes by Cephalosporium aphidicola. Phytochemistry. 1998;49:2355–2358. doi: 10.1016/S0031-9422(98)00312-4. [DOI] [Google Scholar]
- 27.Yildirim K. Microbial hydroxylation of some steroids by Aspergillus wentii MRC 200316. Collect. Czechoslov. Chem. Commun. 2010;75:1273–1281. doi: 10.1135/cccc2010112. [DOI] [Google Scholar]
- 28.Janeczko T., Dmochowska-Gładysz J., Kostrzewa-Susłow E., Białońska A., Ciunik Z. Biotransformations of steroid compounds by Chaetomium sp. KCH 6651. Steroids. 2009;74:657–661. doi: 10.1016/j.steroids.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Shen G.J., Zhou B., Lai T.C., Su H.L., Yang H.Y. Study on Biotransformation Products of Androstenedione by Paecilomyces victoriae. Adv. Mater. Res. 2013;807:414–417. doi: 10.4028/www.scientific.net/AMR.807-809.414. [DOI] [Google Scholar]
- 30.Huszcza E., Dmochowska-Gladysz J. Transformations of testosterone and related steroids in Absidia glauca culture. J. Basic Microbiol. 2003;43:113–120. doi: 10.1002/jobm.200390011. [DOI] [PubMed] [Google Scholar]
- 31.Zenk J.L., Helmer T.R., Kassen L.J., Kuskowski M.A. The effect of 7-Keto Naturalean™ on weight loss: A randomized, double-blind, placebo-controlled trial. Curr. Ther. Res. Clin. Exp. 2002;63:263–272. doi: 10.1016/S0011-393X(02)80031-5. [DOI] [Google Scholar]
- 32.Ihler G., Chami-Stemmann H. 7-oxo-DHEA and Raynaud’s phenomenon. Med. Hypotheses. 2003;60:391–397. doi: 10.1016/S0306-9877(02)00409-7. [DOI] [PubMed] [Google Scholar]
- 33.Kalman D., Colker C., Swain M., Torina G. A randomized, double-blind, placebo-controlled study of 3-acetyl-7-oxo-dehydroepiandrosterone in healthy overweight adults. Curr. Ther. Res. 2000;61:435–442. doi: 10.1016/S0011-393X(00)89034-7. [DOI] [Google Scholar]
- 34.Hampl R., Sulcová J., Bílek R., Hill M. How short-term transdermal treatment of men with 7-oxo-dehydroepiandrosterone influence thyroid function. Physiol. Res. 2006;55:49–54. doi: 10.33549/physiolres.930759. [DOI] [PubMed] [Google Scholar]
- 35.Yadav M.R., Barmade M.A., Tamboli R.S., Murumkar P.R. Developing steroidal aromatase inhibitors-an effective armament to win the battle against breast cancer. Eur. J. Med. Chem. 2015;105:1–38. doi: 10.1016/j.ejmech.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Cepa M.M.D.S., Tavares da Silva E.J., Correia-da-Silva G., Roleira F.M.F., Teixeira N.A.A. Structure-activity relationships of new A,D-ring modified steroids as aromatase inhibitors: Design, synthesis, and biological activity evaluation. J. Med. Chem. 2005;48:6379–6385. doi: 10.1021/jm050129p. [DOI] [PubMed] [Google Scholar]
- 37.Yang B., Wang Y., Chen X., Feng J., Wu Q., Zhu D. Biotransformations of steroids to testololactone by a multifunctional strain Penicillium simplicissimum WY134-2. Tetrahedron. 2014;70:41–46. doi: 10.1016/j.tet.2013.11.039. [DOI] [Google Scholar]
- 38.Świzdor A. Baeyer-Villiger oxidation of some C19 steroids by Penicillium lanosocoeruleum. Molecules. 2013;18:13812–13822. doi: 10.3390/molecules181113812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samson R.A., Evans H.C., Latgé J.-P. Atlas of Entomopathogenic Fungi. 1st edition. Springer; Berlin/Heidelberg, Germany: 1988. pp. 5–16. [Google Scholar]
- 40.Gibson D.M., Donzelli B.G.G., Krasnoff S.B., Keyhani N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014;31:1287–1305. doi: 10.1039/C4NP00054D. [DOI] [PubMed] [Google Scholar]
- 41.Hibbett D.S., Binder M., Bischoff J.F., Blackwell M., Cannon P.F., Eriksson O.E., Huhndorf S., James T., Kirk P.M., Lücking R., et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Heitman J. Microbial pathogens in the fungal kingdom. Fungal Biol. Rev. 2011;25:48–60. doi: 10.1016/j.fbr.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantone F.A., Vandenberg J.D. Intraspecific diversity in Paecilomyces fumosoroseus. Mycol. Res. 1998;102:209–215. doi: 10.1017/S0953756297004590. [DOI] [Google Scholar]
- 44.Luangsa-Ard J.J., Hywel-Jones N.L., Manoch L., Samson R.A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 2005;109:581–589. doi: 10.1017/S0953756205002741. [DOI] [PubMed] [Google Scholar]
- 45.Huang Z., Hao Y., Gao T., Huang Y., Ren S., Keyhani N.O. The Ifchit1 chitinase gene acts as a critical virulence factor in the insect pathogenic fungus Isaria fumosorosea. Appl. Microbiol. Biotechnol. 2016;100:5491–5503. doi: 10.1007/s00253-016-7308-z. [DOI] [PubMed] [Google Scholar]
- 46.Avery P.B., Wekesa V.W., Hunter W.B., Hall D.G., Cindy L., Osborne L.S., Powell C.A., Rogers M.E. Effects of the fungus Isaria fumosorosea (Hypocreales : Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (emiptera : Psyllidae) Biocontrol. Sci. Technol. 2011;21:1065–1078. doi: 10.1080/09583157.2011.596927. [DOI] [Google Scholar]
- 47.Hunter A.W.B., Avery P.B., Pick D., Powell C.A. Broad spectrum potential of Isaria fumosorosea against insect pests of citrus. Sci. Notes. 2011;94:1051–1054. [Google Scholar]
- 48.Pick D.A., Avery P.B., Hunter W.B., Powell C.A., Arthurs S.P. Effect of Isaria fumosorosea (Hypocreales: Cordycipitaceae) and Lysiphlebus testaceipes, (Hymenoptera: Braconidae) on the brown citrus aphid: Preliminary assessment of a compatibility study. Fla. Entomol. 2012;95:764–766. doi: 10.1653/024.095.0328. [DOI] [Google Scholar]
- 49.Wang C., Gao T., Huang Y., Huang Z. Effect of Ifchit1 gene of Isaria fumosorosea on mortality, oviposition and oxidase activities of Bemisia tabaci. Biocontrol. Sci. Technol. 2017;27:485–495. doi: 10.1080/09583157.2017.1315052. [DOI] [Google Scholar]
- 50.Ali S., Huang Z., Ren S. Media composition influences on growth, enzyme activity, and virulence of the entomopathogen hyphomycete Isaria fumosoroseus. Entomol. Exp. Appl. 2009;131:30–38. doi: 10.1111/j.1570-7458.2009.00833.x. [DOI] [Google Scholar]
- 51.Ali S., Huang Z., Ren S. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J. Pest Sci. 2010;83:361–370. doi: 10.1007/s10340-010-0305-6. [DOI] [Google Scholar]
- 52.Ortiz-Urquiza A., Keyhani N. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects. 2013;4:357–374. doi: 10.3390/insects4030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pedrini N., Ortiz-Urquiza A., Huarte-Bonnet C., Zhang S., Keyhani N.O. Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: Hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol. 2013;4:1–18. doi: 10.3389/fmicb.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhan J., Gunatilaka A.A.L. Microbial metabolism of 1-aminoanthracene by Beauveria bassiana. Bioorgan. Med. Chem. 2008;16:5085–5089. doi: 10.1016/j.bmc.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 55.Olivo H.F., Peeples T.L., Ríos M.-Y., Velázquez F., Kim J.-W., Narang S. Microbial C-hydroxylation and β-4-O-methylglucosidation of methyl-benzamide 7-azanorbornane ethers with Beauveria bassiana. J. Mol. Catal. B Enzym. 2003;21:97–105. doi: 10.1016/S1381-1177(02)00081-4. [DOI] [Google Scholar]
- 56.Tronina T., Bartmańska A., Milczarek M., Wietrzyk J., Popłoński J., Rój E., Huszcza E. Antioxidant and antiproliferative activity of glycosides obtained by biotransformation of xanthohumol. Bioorgan. Med. Chem. Lett. 2013;23:1957–1960. doi: 10.1016/j.bmcl.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 57.Bartmańska A., Huszcza E., Tronina T. Transformation of isoxanthohumol by fungi. J. Mol. Catal. B Enzym. 2009;61:221–224. doi: 10.1016/j.molcatb.2009.07.008. [DOI] [Google Scholar]
- 58.Bartmańska A., Tronina T., Huszcza E. Transformation of 8-prenylnaringenin by Absidia coerulea and Beauveria bassiana. Bioorgan. Med. Chem. Lett. 2012;22:6451–6453. doi: 10.1016/j.bmcl.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 59.Sordon S., Popłoński J., Tronina T., Huszcza E. Microbial glycosylation of daidzein, genistein and biochanin A: Two new glucosides of biochanin A. Molecules. 2017;22:81. doi: 10.3390/molecules22010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tronina T., Strugała P., Popłoński J., Włoch A., Sordon S., Bartmańska A., Huszcza E. The influence of glycosylation of natural and synthetic prenylated flavonoids on binding to human serum albumin and inhibition of cyclooxygenases COX-1 and COX-2. Molecules. 2017;22:1230. doi: 10.3390/molecules22071230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong Z., Wei Q., Chen H., Chen S., Xu W., Qiu G., Liang S., Hu X. Microbial transformation of androst-4-ene-3,17-dione by Beauveria bassiana. Steroids. 2006;71:979–983. doi: 10.1016/j.steroids.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez R., Nicolau F., Peeples T.L. Optimization of the 11α-hydroxylation of steroid DHEA by solvent-adapted Beauveria bassiana. Biocatal. Biotransform. 2017;35:103–109. doi: 10.1080/10242422.2017.1289183. [DOI] [Google Scholar]
- 63.Gonzalez R., Nicolau F., Peeples T. N-alkane solvent-enhanced biotransformation of steroid DHEA by Beauveria bassiana as biocatalyst. J. Adv. Biol. Biotechnol. 2015;2:30–37. doi: 10.9734/JABB/2015/13541. [DOI] [Google Scholar]
- 64.Gao Q., Qiao Y., Shen Y., Wang M., Wang X., Liu Y. Screening for strains with 11α-hydroxylase activity for 17α-hydroxy progesterone biotransformation. Steroids. 2017;124:67–71. doi: 10.1016/j.steroids.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Huszcza E., Dmochowska-Gładysz J., Bartmańska A. Transformations of steroids by Beauveria bassiana. Z. Naturforsch. C. 2005;60:103–108. doi: 10.1515/znc-2005-1-219. [DOI] [PubMed] [Google Scholar]
- 66.Świzdor A., Kołek T., Panek A., Białońska A. Microbial Baeyer-Villiger oxidation of steroidal ketones using Beauveria bassiana: Presence of an 11α-hydroxyl group essential to generation of d-homo lactones. Biochim. BBA-Mol. Cell Biol. Lipids. 2011;1811:253–262. doi: 10.1016/j.bbalip.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Świzdor A., Panek A., Milecka-Tronina N. Microbial Baeyer-Villiger oxidation of 5α-steroids using Beauveria bassiana. A stereochemical requirement for the 11α-hydroxylation and the lactonization pathway. Steroids. 2014;82:44–52. doi: 10.1016/j.steroids.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Lobastova T.G., Khomutov S.M., Donova M.V. Formation of hydroxylated steroid lactones from dehydroepiandrosterone by Spicaria fumoso-rosea F-881. Appl. Biochem. Microbiol. 2015;51:180–187. doi: 10.1134/S000368381502012X. [DOI] [PubMed] [Google Scholar]
- 69.Urech J., Vischer E., Wettstein A. Substratspezifische hydroxylierungen von steroiden mittels pilz-stämmen der gattung Gibberella. Mikrobiologische Reaktionen, 9. Mitteilung. Helv. Chim. Acta. 1960;43:1077–1086. doi: 10.1002/hlca.19600430417. [DOI] [Google Scholar]
- 70.Nassiri-Koopaei N., Mogharabi M., Amini M., Shafiee A., Faramarzi M.A. Fungal transformation of methyltestosterone by the soil ascomycete Acremonium strictum to some hydroxy derivatives of 17-methylsteroid. Chem. Nat. Compd. 2013;49:665–670. doi: 10.1007/s10600-013-0703-0. [DOI] [Google Scholar]
- 71.Faramarzi M.A., Aghelnejad M., Tabatabaei Yazdi M., Amini M., Hajarolasvadi N. Metabolism of androst-4-en-3,17-dione by the filamentous fungus Neurospora crassa. Steroids. 2008;73:13–18. doi: 10.1016/j.steroids.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Faramarzi M.A., Badiee M., Yazdi M.T., Amini M., Torshabi M. Formation of hydroxysteroid derivatives from androst-4-en-3,17-dione by the filamentous fungus Mucor racemosus. J. Mol. Catal. B Enzym. 2008;50:7–12. doi: 10.1016/j.molcatb.2007.09.017. [DOI] [Google Scholar]
- 73.Lehman L.R., Stewart J.D. Filamentous fungi: Potentially useful catalysts for the biohydroxylations of non-activated carbon centers. Curr. Org. Chem. 2001;5:439–470. doi: 10.2174/1385272013375490. [DOI] [Google Scholar]
- 74.Choudhary M.I., Khan N.T., Musharraf S.G., Anjum S., Atta-ur-Rahman Biotransformation of adrenosterone by filamentous fungus, Cunninghamella elegans. Steroids. 2007;72:923–929. doi: 10.1016/j.steroids.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Brannon D.R., Parrish F.W., Wiley B.J., Long Jr L. Microbial transformation of a series of andogens with Aspergillus tamarii. J. Org. Chem. 1967;32:1521–1527. doi: 10.1021/jo01280a048. [DOI] [PubMed] [Google Scholar]
- 76.Musharraf S.G., Atta-Ur-Rahman, Choudhary M.I., Sultan S. Microbial transformation of (+)-adrenosterone. Nat. Prod. Lett. 2002;16:345–349. doi: 10.1080/10575630290033105. [DOI] [PubMed] [Google Scholar]
- 77.Robinzon B., Prough R.A. A novel NADP+-dependent dehydrogenase activity for 7α/β- and 11β-hydroxysteroids in human liver nuclei: A third 11β-hydroxysteroid dehydrogenase. Arch. Biochem. Biophys. 2009;486:170–176. doi: 10.1016/j.abb.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muller C., Pompon D., Urban P., Morfin R. Inter-conversion of 7α- and 7β-hydroxy-dehydroepiandrosterone by the human 11β-hydroxysteroid dehydrogenase type 1. J. Steroid Biochem. Mol. Biol. 2006;99:215–222. doi: 10.1016/j.jsbmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 79.Marwah A., Marwah P., Lardy H. Ergosteroids: VI. Metabolism of dehydroepiandrosterone by rat liver in vitro : A liquid chromatographic-mass spectrometric study. J. Chromatogr. B. 2002;767:285–299. doi: 10.1016/S1570-0232(01)00570-0. [DOI] [PubMed] [Google Scholar]
- 80.Hennebert O., Montes M., Favre-Reguillon A., Chermette H., Ferroud C., Morfin R. Epimerase activity of the human 11β-hydroxysteroid dehydrogenase type 1 on 7-hydroxylated C19-steroids. J. Steroid Biochem. Mol. Biol. 2009;114:57–63. doi: 10.1016/j.jsbmb.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 81.Chalbot S. Human liver S9 fractions: Metabolism of dehydroepiandrosterone, epiandrosterone, and related 7-hydroxylated derivatives. Drug Metab. Dispos. 2005;33:563–569. doi: 10.1124/dmd.104.003004. [DOI] [PubMed] [Google Scholar]
- 82.Dymarska M., Grzeszczuk J., Urbaniak M., Janeczko T., Pląskowska E., Stępień Ł., Kostrzewa-Susłow E. Glycosylation of 6-methylflavone by the strain Isaria fumosorosea KCH J2. PLoS ONE. 2017;12:e.0184885. doi: 10.1371/journal.pone.0184885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.