Abstract
The compatibility between Danggui (Angelicae Sinensis Radix) and Honghua (Carthami Flos) is a known herb pair, which could activate blood circulation and dissipate blood stasis effects. In this paper, we quantified seven main bio-active components (hydroxysafflor yellow A, caffeic acid, p-coumaric acid, kaempferol-3-O-rutinoside, ferulic acid, 3-n-butylphthalide, and ligustilide) in plasma samples in vivo by UPLC-TQ/MS method and investigatedwhether the pharmacokinetic (PK) behaviors of the seven components could be altered in blood stasis rats after oral administration of the Gui-Hong extracts. It was found that the Cmax and AUC0-t of these components in blood stasis rats had increasing tendency compared with normal rats. Most components in model and normal rats had significant difference in some pharmacokinetic parameters, which indicated that the metabolism enzymes and transporters involved in the metabolism and disposition of these bio-active componentsmay bealtered in blood stasis rats. This study was the first report about the pharmacokinetic investigation between normal and blood stasis rats after oral administrationof Gui-Hong extracts, and these results are important and valuable for better clinical applications of Gui-Hong herb pair and relatedTCM formulae.
Keywords: Danggui, Angelica sinensis, honghua, Carthamustinctorius, herb pair, pharmacokinetics
1. Introduction
Danggui (Angelicae Sinensis Radix) is the dried radix of Angelica sinensis (Oliv.) Diels (Umbelliferae) and Honghua (CarthamiFlos) is the dried flower of Carthamus tinctorius L. (Compositae). The compatibility between Danggui and Honghua could be found in Danggui-Honghua Decoction of Shanghandabai. It is an ancient and classic formula comprised of Danggui and Honghua, and it is also a famous herb pair (Gui-Hong), which has the activating blood circulation and dissipating blood stasis effects [1].
To date, pharmacological studies and chemical investigation have found that the aromatic acids, phthalides and quinochalcone C-glycosides were the main constitutes responsible for the herb pair’bio-activities [2,3]. Pharmacokinetic studieson these components havebeen partly demonstrated [4,5,6]. However, there arefew reports about the pharmacokinetic characteristics ofthe herb pair. The pharmacokinetic process of herbs will be affectedby the co-usedherbs when used as an herb pair or herb formulae [7].
Blood stasis syndrome isan important cause of many diseases, including myocardial ischemia anddysmenorrheal.There is no doubt that these pathological alternationsmay significantlyinfluence theprotein/mRNA expressions or activities of metabolic enzymes and transportersinvolved in the absorption or disposition of these active components in the Gui-Hong herb pair [8]. Considering the popular use of the Gui-Hong herb pair in the treatment of blood stasis syndrome, in this study, a sensitive and reliable ultra-high-performance liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry (UPLC-MS/MS) method which can be used tosimultaneously quantify seven main bio-active components (hydroxysafflor yellow A, caffeic acid, p-coumaric acid, kaempferol-3-O-rutinoside, ferulic acid, 3-n-butylphthalide, and ligustilide) in plasma samples was developed. Additionally, the pharmacokinetic (PK) parameters of the seven components in normal and blood stasis rats after oral administration of Gui-Hong extracts were compared.
2. Results and Discussion
2.1. The Component Contents in Gui-Hong Extracts
A validated UPLC-TQ/MS method was employed to quantifythe contents of the main constitutes in Gui-Hong extracts. The results showed that Gui-Hong extracts (0.405 g/mL) contained 144.075 μg/mL of HSYA, 3.758 μg/mL of caffeic acid, 4.350 μg/mL of p-coumaric acid, 33.844 μg/mL of kaempferol-3-O-rutinoside, 31.647 μg/mL of ferulic acid, 1.583 μg/mL of 3-n-butylphthalide, and 3.175 μg/mL of ligustilide.
2.2. The Observation on Haemorheology of Rats
The haemorheological indices of the rats in these two groups are shown in Table 1, including whole blood viscosity (WBV), plasma viscosity (PV), erythrocyte sedimentation rate (ESR), andhematocrit (HCT). These indiceswere the main diagnostic criteria of blood stasis syndrome. Results showed that WBV (200 s−1, 30 s−1, 5 s−1), PV, ESR, and HCT in blood stasis rats increased significantly (p< 0.05, p< 0.01) when compared with the normal rats, which indicatedthat the blood stasis animal model was successfully established.
Table 1.
The haemorheological indices of normal and blood stasis rats.
| Group | WBV/mPa·s | PV/mPa·s (200 s−1) | ESR/mm·h−1 | HCT/L·L−1 | |||
|---|---|---|---|---|---|---|---|
| 200 s−1 | 30 s−1 | 5 s−1 | 1 s−1 | ||||
| Control | 3.22 ± 0.16 | 3.78 ± 0.36 | 5.30 ± 1.01 | 9.35 ± 3.02 | 1.33 ± 0.06 | 1.00 ± 0.00 | 0.33 ± 0.03 |
| Model | 4.36 ± 0.35 ∆∆ | 5.27 ± 0.50 ∆∆ | 7.53 ± 1.51∆ | 14.44 ± 3.74 | 1.90 ± 0.27 ∆∆ | 5.13 ± 1.93 ∆∆ | 0.42 ± 0.05 ∆∆ |
∆ p < 0.05, ∆∆ p < 0.01, compared with normal group.
2.3. Method Validation
2.3.1. Specificity
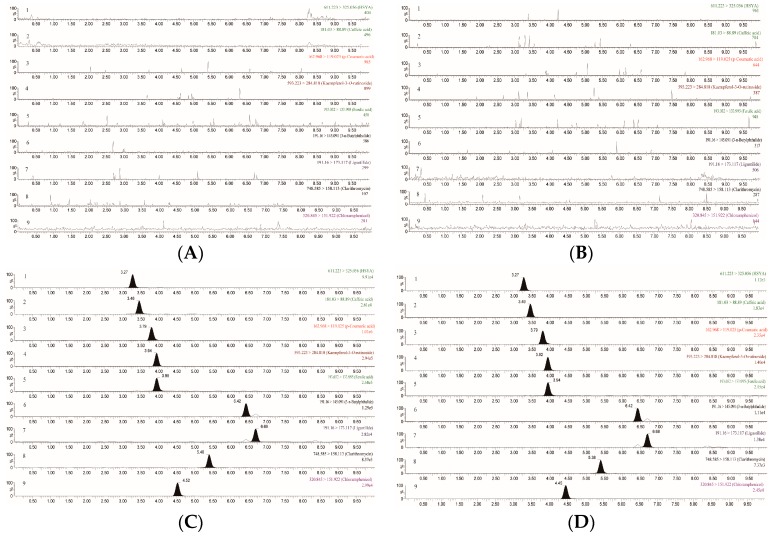
No interference could beobserved at the elutingtimes of either analytes or IS in blankplasma samples from normal or blood stasis rats, which indicated thatthe method exhibited good specificity. Typical MRM chromatograms were shown in Figure 1.
Figure 1.
Typical MRM chromatograms of the seven components in rats: (A) blank plasma sample from a normal rat; (B) blank plasma sample from a model rat; (C) blank plasma samples spiked with standard mixtures and internal standards; and (D) ratplasma samples collected after oral administration of Gui-Hong extracts. Note: hydroxysafflor yellow A (1), caffeic acid (2), p-coumaric acid (3), kaempferol-3-O-rutinoside (4), ferulic acid (5), 3-n-butylphthalide (6), ligustilide (7), chloramphenicol (8), and clarithromycin (9).
2.3.2. Linearity and LLOQ
Linear equation ofthese seven calibration curves, linear ranges and LLOQs of these seven analytes using blank plasma samples from normal rats arepresented in Table 2.
Table 2.
The regression equation, linear range, and LLOQ of the seven components in rat plasma samples.
| Components | Regression Equation | R2 | Linear Range (ng mL−1) | LLOQ (ng mL−1) |
|---|---|---|---|---|
| hydroxysafflor yellow A | Y = 1.96 × 10−4X + 2.43 × 10−4 | 0.9959 | 1.125–225 | 1.125 |
| caffeic acid | Y = 1.71 × 10−3X − 3.77 × 10−3 | 0.9988 | 1.145–229 | 1.145 |
| p-coumaric acid | Y = 2.19 × 10−3X + 3.90 × 10−4 | 0.9987 | 1.065–1065 | 1.065 |
| kaempferol-3-O-rutinoside | Y = 1.20 × 10−3X + 5.62 × 10−3 | 0.9989 | 1.37–685 | 1.37 |
| ferulic acid | Y = 1.00 × 10−3X + 9.83 × 10−4 | 0.9975 | 4.5–900 | 4.5 |
| 3-n-butylphthalide | Y = 3.08 × 10−2X − 1.41 × 10−1 | 0.9986 | 1.23–246 | 1.23 |
| ligustilide | Y = 4.45 × 10−3X − 1.20 × 10−3 | 0.9943 | 0.965–386 | 0.965 |
2.3.3. Precision and Accuracy
The developed method showed an acceptable precision and accuracy inthe intra-day and inter-day test. All of these results were expectedto meet the required variable limits and the detailed data areshown in Table 3.
Table 3.
Precision, accuracy, extraction recovery, matrix effect, and stability of the seven components in rat plasma samples (n = 6).
| Components | Concentration (ng mL−1) | Inter-Day | Intra-Day | Extraction Recovery (%, Mean ± SD) | Matrix Effect (%, Mean ± SD) | Stability (RE/RSD%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Precision (RSD%) | Accuracy (RE%) | Precision (RSD%) | Accuracy (RE%) | Short-Term | Three Freeze-Thaw Cycles | Long-Term | ||||
| 1 | 180 | 1.15 | 0.13 | 1.52 | −0.40 | 86.83 ± 3.04 | 88.99 ± 1.86 | 0.55/1.25 | −0.01/1.26 | 1.12/1.40 |
| 22.5 | 1.72 | 0.58 | 2.39 | −0.16 | 93.79 ± 3.79 | 87.06 ± 1.91 | 0.50/1.09 | 1.09/1.25 | 0.59/2.04 | |
| 2.25 | 4.04 | −1.31 | 3.52 | 1.52 | 88.13 ± 3.10 | 88.99 ± 3.82 | 2.15/5.19 | −1.01/3.96 | 0.41/5.29 | |
| 2 | 183.2 | 1.88 | 0.77 | 1.24 | 0.23 | 91.42 ± 5.86 | 88.44 ± 2.98 | 1.12/1.93 | 0.27/2.28 | 1.28/1.72 |
| 22.9 | 2.56 | −0.82 | 3.38 | −1.01 | 93.42 ± 5.63 | 86.91 ± 0.91 | −2.01/3.12 | −2.78/2.73 | −3.21/2.92 | |
| 2.29 | 5.42 | −4.27 | 4.22 | −1.18 | 88.56 ± 1.70 | 87.56 ± 1.44 | −6.50/8.48 | −6.97/8.76 | −5.68/7.73 | |
| 3 | 852 | 1.84 | 1.69 | 1.74 | 2.11 | 87.76 ± 2.10 | 86.18 ± 2.11 | 1.64/1.62 | 1.87/2.27 | 1.78/1.94 |
| 106.5 | 0.73 | −2.97 | 3.62 | −1.02 | 88.56 ± 0.91 | 89.83 ± 4.58 | −0.53/3.65 | −0.09/4.43 | 1.04/2.04 | |
| 2.13 | 3.67 | 1.02 | 3.60 | 0.37 | 95.72 ± 2.47 | 87.29 ± 1.99 | −2.27/7.63 | −2.22/8.02 | −6.33/8.79 | |
| 4 | 548 | 1.83 | −0.03 | 2.49 | 0.85 | 87.72 ± 2.63 | 85.97 ± 1.51 | 2.18/2.14 | 1.26/2.61 | 1.51/2.76 |
| 68.5 | 4.70 | 2.53 | 2.63 | 3.05 | 87.51 ± 1.66 | 87.24 ± 1.95 | 0.28/4.30 | 1.95/3.17 | 3.60/3.68 | |
| 2.74 | 4.19 | −1.00 | 6.34 | −3.22 | 89.91 ± 2.84 | 85.08 ± 7.66 | −0.11/6.65 | −3.67/9.31 | −6.24/7.05 | |
| 5 | 720 | 1.40 | 0.49 | 2.18 | −0.53 | 86.68 ± 1.62 | 86.92 ± 3.29 | 1.27/2.22 | −1.20/1.99 | 0.73/2.14 |
| 90 | 1.47 | 2.33 | 3.62 | −0.16 | 86.39 ± 5.10 | 88.07 ± 3.18 | 0.11/2.56 | 2.76/1.28 | 0.74/3.72 | |
| 9 | 2.47 | 2.10 | 4.86 | 0.45 | 87.03 ± 3.94 | 86.02 ± 1.96 | 14.13/7.65 | −9.21/7.66 | −0.43/7.72 | |
| 6 | 196.8 | 1.85 | 0.69 | 1.04 | 1.52 | 88.68 ± 2.00 | 89.15 ± 4.60 | 1.23/1.64 | 2.18/1.13 | 0.89/2.03 |
| 24.6 | 2.04 | 0.64 | 2.90 | −0.09 | 88.42 ± 0.82 | 85.77 ± 2.99 | 0.48/3.14 | −2.12/3.72 | 0.60/3.10 | |
| 2.46 | 3.40 | −0.75 | 5.42 | −0.13 | 87.45 ± 1.55 | 87.67 ± 3.36 | 2.41/6.57 | −8.06/7.65 | 0.96/9.26 | |
| 7 | 308.8 | 2.14 | 0.67 | 2.59 | 0.62 | 90.20 ± 0.86 | 86.28 ± 2.03 | 0.04/1.00 | 0.59/1.67 | 0.39/1.23 |
| 38.6 | 2.08 | 2.15 | 2.56 | 0.84 | 88.30 ± 1.09 | 86.73 ± 4.23 | 1.60/1.90 | 1.84/2.46 | 1.33/2.07 | |
| 1.93 | 6.50 | 1.25 | 5.26 | −1.25 | 87.61 ± 0.62 | 89.80 ± 8.21 | 3.17/9.21 | 7.58/8.45 | 4.13/6.92 | |
2.3.4. Extraction Recovery and Matrix Effect
Extraction recovery and matrix effect of the seven components could be seen in Table 3. Average extraction recovery of the two ISs, chloramphenicol and clarithromycin, were 86.11 ± 1.20% and 85.85 ± 2.74%, respectively.The matrix effect of these ISs were 87.67 ± 1.01% and 86.15 ± 1.81%.
2.3.5. Stability
The stability of all these seven analytes in rat plasma under different storage conditions was evaluated. As shown in Table 3, the results indicatedthat these analytes were all stable in these different conditions.
2.4. Pharmacokinetic Study
2.4.1. Comparison of Pharmacokinetic Parameters of Each Gui-Hong Component in Normal and Blood Stasis Rats
Blood stasis syndrome is a kind of pathological state in blood circulation and causes the development of many diseases. Based on TCM theory, it is characterized with a slowing or pooling blood mainly owingto the disruption of heart Qi, and now it is often understood to be the hematological disorders (e.g., a rise in whole blood and plasma viscosity) [9,10]. Thus, we investigate the pharmacokinetic behaviors of the main bioactive constitutes in Gui-Hong herb pair in blood stasis rats induced by subcutaneously injecting with adrenaline hydrochloride.
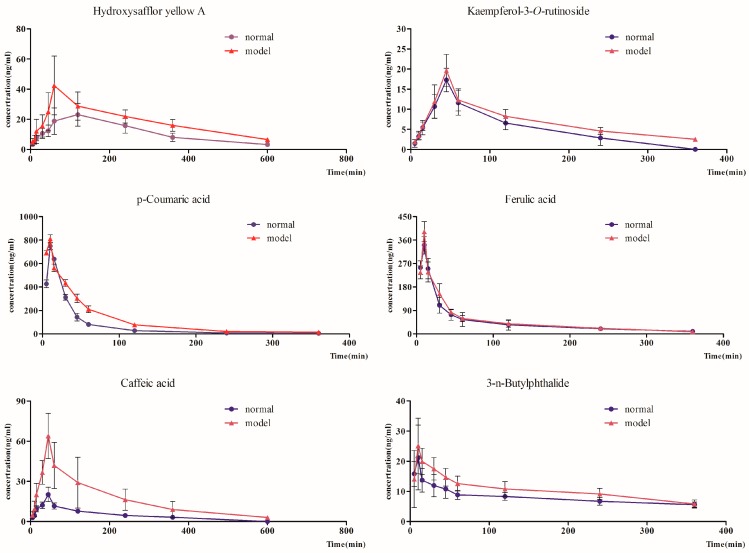
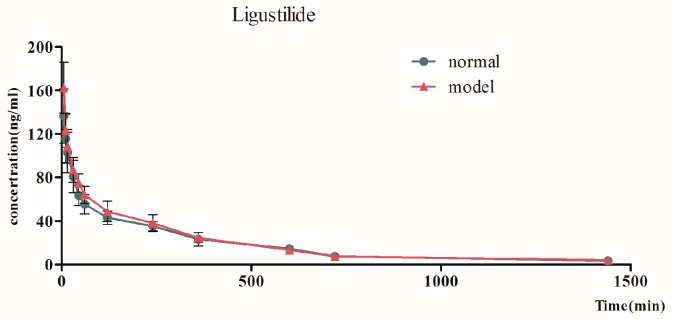
The mean plasma concentration-time profiles of the seven components were illustrated in Figure 2 and the main pharmacokinetic parameters were calculated and summarized in Table 4, respectively.
Figure 2.
Mean plasma concentration-time curves of seven components after oral administration of Gui-Hong extracts to normal and blood stasis rats.
Table 4.
NCA pharmacokinetic parameters of seven components of Gui-Hong extracts between normal and blood stasis rats (n = 6).
| Components | Group | Cmax (ng mL−1) | Tmax (h) | T1/2z (h) | MRT0-t (h) | AUC0-t (ng mL−1 h) | AUC0-∞(ng mL−1 h) |
|---|---|---|---|---|---|---|---|
| 1 | normal | 24.937 ± 8.883 | 1.833 ± 0.408 | 2.591 ± 0.3 | 3.653 ± 0.272 | 116.415 ± 31.299 | 126.972 ± 31.524 |
| model | 43.62 ± 19.132 | 1.167 ± 0.408 * | 4.075 ± 0.855 ** | 3.846 ± 0.27 | 187.687 ± 45.794 * | 234.492 ± 69.678 ** | |
| 2 | normal | 20.165 ± 5.345 | 0.75 ± 0.000 | 2.7 ± 1.161 | 2.212 ± 0.206 | 41.309 ± 3.209 | 53.374 ± 10.783 |
| model | 63.905 ± 16.832 ** | 0.75 ± 0.000 | 2.721 ± 0.703 | 2.89 ± 0.267 ** | 165.157 ± 77.583 ** | 177.222 ± 76.372 ** | |
| 3 | normal | 749.709 ± 28.526 | 0.167 ± 0.000 | 1.038 ± 0.374 | 0.759 ± 0.109 | 430.468 ± 33.622 | 434.338 ± 35.425 |
| model | 811.579 ± 34.445 ** | 0.167 ± 0.000 | 1.104 ± 0.447 | 1.079 ± 0.135 ** | 706.649 ± 45.829 ** | 720.461 ± 53.934 ** | |
| 4 | normal | 17.277 ± 2.931 | 0.75 ± 0.000 | 0.995 ± 0.527 | 1.727 ± 0.214 | 31.132 ± 7.886 | 32.162 ± 7.77 |
| model | 19.686 ± 3.955 | 0.75 ± 0.000 | 2.253 ± 0.254 ** | 2.197 ± 0.104 ** | 41.06 ± 5.99 * | 49.353 ± 5.514 ** | |
| 5 | normal | 340.535 ± 34.027 | 0.167 ± 0.000 | 2.379 ± 1.096 | 1.431 ± 0.191 | 272.75 ± 65.418 | 311.633 ± 77.29 |
| model | 392.586± 38.43 * | 0.167 ± 0.000 | 1.85 ± 0.275 | 1.417 ± 0.236 | 297.676 ± 27.747 | 319.448 ± 31.097 | |
| 6 | normal | 21.227 ± 10.74 | 0.167 ± 0.000 | 7.604 ±1.731 | 2.547 ±0.079 | 48.091 ± 8.745 | 108.678 ± 24.651 |
| model | 25.127 ± 9.177 | 0.167 ± 0.000 | 5.338 ± 1.724 * | 2.47 ± 0.166 | 62.826 ± 11.975 * | 112.425 ± 27.906 | |
| 7 | normal | 136.395 ± 25.05 | 0.083 ± 0.000 | 6.325 ± 1.453 | 5.693 ± 0.758 | 432.488 ± 71.117 | 463.314 ± 79.027 |
| model | 162.474 ± 23.267 | 0.083 ± 0.000 | 5.772 ± 1.862 | 5.57 ± 0.262 | 461.85 ± 72.942 | 489.422 ± 92.129 |
* p < 0.05, ** p < 0.01, compared with the normal group.
Pharmacokinetic parameters of HSYA of blood stasis rats exhibited higher Cmax, T1/2z, AUC0-t, MRT0-t, and AUC0-∞, and lower Tmax. Among these data, Tmax, AUC0-t, AUC0-∞, and T1/2z had significant difference. As previously work indicated that HSYA was mainly absorbed through the small intestine, while poor blood circulation might prolong the retention time of HSYA in the small intestine, thus eventually led to an increased bioavailability of HSYA [5]. Additionally, slowed blood circulation may decrease the liver perfusion which may lead to decreased hydroxylation, methylation, acetylation, and glucuronidation of the HSYA in liver. This decreased drug metabolism may significantly increase the bioavailability of HSYA in blood stasis rats [11].
For caffeic acid, the plasma samples from the blood stasis rats showed higher Cmax, AUC0-t, MRT0-t, T1/2z, and AUC0-∞. Tmax of caffeic acid in normal and blood stasis rats was same. Meanwhile, the Cmax, AUC0-t, MRT0-t, and AUC0-∞ of caffeic acid had significant difference. Catechol-O-methyltransferase (COMT) mediated O-methylation plays an important role in the metabolism of caffeic acid in rat hepatocytes. Blood stasis syndromesinduced by adrenaline hydrochloride or occlusion of the left anterior descending coronary arteryhave shown decreased COMT activity in liver plasma and the heart [12,13]. The increased bioavailability of caffeic acid may partially contribute to the decreased liver and blood metabolism [14,15].
For p-coumaric acid, the model samples revealed higher Cmax, AUC0-t, MRT0-t, T1/2z, and AUC0-∞. And the Cmax, AUC0-t, MRT0-t, and AUC0-∞ of p-coumaric acid had a significant difference. Our previously work has demonstrated that the pharmacokinetic behavior of p-coumaric acid may be altered after compatibility in rats [16]. While in this study, the result indicated that the absorption of p-coumaric acid could be increased and the process of elimination was slowed in thepathological state.
As for kaempferol-3-O-rutinoside, the model samples showed higher Cmax, T1/2z, AUC0-t, MRT0-t, and AUC0-∞ compared with normal rats, and the T1/2z, AUC0-t, MRT0-t, and AUC0-∞ of kaempferol-3-O-rutinoside had significant difference. Kaempferol-3-O-rutinoside is a kind of flavonoid glycosides. A series of reasons such as high polarity, large molecules, etc., will result in the low bioavailability of kaempferol-3-O-rutinosideafter oral administration [17].
For ferulic acid with similar chemical structure of p-coumaric acid, the model rats implied higher Cmax, AUC0-t, and AUC0-∞, and lower T1/2z and MRT0-t. The Cmax of ferulic acid had a significant difference. Additionally, Tmax of ferulic acid in these two groups was the same, which implied that ferulic acid absorbed more rapidly in blood stasis rats.
When looking at 3-n-butylphthalide, the model rats showed lower T1/2z and MRT0-t, and higher Cmax, AUC0-t, and AUC0-∞. Moreover, the T1/2z and AUC0-t of 3-n-butylphthalide had a significant difference. Since 3-n-butylphthalide was mainly metabolized by CYP2E1, 2C11 and 3A1/2 in rats [18], which referred that model rats could change the eliminated time of 3-n-butylphthalide may due to the changes in the enzymes. Also, Gui-Hong herb pair also could up-regulate the CYP1A and CYP3A mRNA expression, but decrease the CYP2E1 mRNA expression [19]. Thus, further work need to be conducted to find whether the mRNA or protein expression of these metabolism enzymes were changed in this pathological states, especially when fed with the Gui-Hong herb pair.
Finally, for ligustilide, the model rats exhibited higher Cmax, AUC0-t, and AUC0-∞, and lower MRT0-t and T1/2z. The result implied that blood stasis model rats could speed up the time and extent of absorption of ligustilide, however, there was no significant difference between normal and model rats.Further studies may be helpful in providing additional insight.
In this study, we mainly focused on the changed pharmacokinetic behavior of these seven active components in the herb pair in blood stasis model rats. In order to uncover the underlying mechanisms, the activity, mRNA and protein expression of these enzymes and transporters need to further studied in our ongoing works.
2.4.2. The Pharmacokinetic Comparison of Different Types of Gui-Hong Components in Normal and Blood Stasis Rats
For further study, we divided the seven major bio-active components into three different types. The total Tmax and AUC0-t calculated from the three different types of components in Gui-Hong extracts as two integrated PK parameters were used in order to compare their pharmacokinetic profiles of normal and blood stasis rats. The total Tmax and AUC0-t of these aromatic acids, including caffeic acid, ferulic acid, and p-coumaric acid, in normal rats were 2.583 h, 147.547 ng mL−1 h while that in model rats were 1.917 h, 228.747 ng mL−1 h, respectively. The total Tmax and AUC0-t of flavonoid glycosides (HSYA, kaempferol-3-O-rutinoside) were 1.084 h, 744.527 ng mL−1 h in normal rats and 1.084 h, 1169.482 ng mL−1 h in model rats, respectively. The total Tmax and AUC0-t ofphthalides (3-n-butylphthalide, ligustilide) were 0.25 h, 480.579 ng mL−1 h in normal rats and 0.25 h, 524.676 ng mL−1 h in model rats, respectively. It was found that only the Tmax of aromatic acids in model rats was shorter than that in normal rats. The AUC0-t of the three different types of components in the model rats were all larger than in normal rats, which suggested that these components had higher exposure levels in model rats than in normal rats.
3. Experimental
3.1. Chemicals and Regents
The radix of Angelica sinensis (Oliv.) Diels (Umbelliferae) was collected at Min County, Gansu Province, China, in July 2014. The flos of Carthamus tinctorius L. (Compositae) was collected at Yili County, Xinjiang Province, China, in March 2014. All of these herb medicineswere identified by Dr. Hui Yan in our group.The voucher specimens (Nos. NJUTCM-20111009 and NJUTCM-20111018) were deposited in the Herbarium of Nanjing University of Chinese Medicine.
Reference standards of hydroxysafflor yellow A (HSYA) (1, 98%), caffeic acid (2, 98%), p-coumaric acid (3, 98%), kaempferol-3-O-rutinoside (4, 98%), ferulic acid (5, 98%), 3-n-butylphthalide (6, 98%), and ligustilide (7, 98%) were supplied by Chengdu Chroma-Biotechnology Co., Ltd. (Chengdu, China). Chloramphenicol (8, IS1) and clarithromycin (9, IS2) were purchased from the National Institute for the Control of Pharmaceutical and Biological products (Beijing, China). Their chemical structures arepresented in Figure 3. Acetonitrile, methanol, and acetic acid (HPLC grade) were purchased from Merck KGaA (Darmstadt, Germany). Ultra-pure water was purified by an EPED super purification system (Nanjing, China). Adrenaline hydrochloride was purchased from Harvest pharmaceutical Co., Ltd., Shanghai, China (batch number: W150102, Shanghai, China).
Figure 3.
Chemical structures of hydroxysafflor yellow A (1), caffeic acid (2), p-coumaric acid (3), kaempferol-3-O-rutinoside (4), ferulic acid (5), 3-n-butylphthalide (6), ligustilide (7), chloramphenicol (8), and clarithromycin (9).
3.2. Extract Preparation
A total 500 g Gui-Hong (1:1, w/w) cut into pieces were extracted with boiling water (1:8, w/v) for once with a total time of 2 h, and filtered through gauze. Then the residue was refluxed with boiling water (1:6, w/v) twice, with 1.5 h for each time. Allthree filtrates were merged and evaporated with rotary evaporation under vacuum at 50 °C, and then the Gui-Hong extract could be obtained.
3.3. Pharmacokinetic Study
Female Sprague-Dawley (SD) rats (200 ± 20 g) were purchasedfrom Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). The rats were kept in a controlledenvironmental condition (temperature: 20 ± 2 °C, humidity: 60 ± 5%) for oneweek untilthe experiments started. Animal welfare and experimental procedures were strictly in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996) and the related ethics regulations of Nanjing University of Chinese Medicine.
Rats were randomly divided into two groups (n = 6): a normal control group administrated with Gui-Hong extracts, and an acute blood stasis model group also orally administered with Gui-Hong extracts. The acute blood stasis rats were induced by injectingwith adrenaline hydrochloride (0.8 mg/kg). 2 hlater, the rats were soaked in ice-water for 4 min with their headskeeping above the surface. After 2 h again, the rats were then injected with adrenaline hydrochloride again [9]. All rats were fasted for 12 h with free access to water prior to the experiments. Both normal and blood stasis rats were orally administered Gui-Hong extracts at a dosage of 4.05 g/kg (4.05 g crude herbs per 1 kg rat body weight) which was homogeneously dissolved and dispersed in ultrapure water. The animal dose of the Gui-Hong extracts was extrapolated from the human daily dose, using the body surface area normalization method. The formula for dose translation was as follows: human dose of crude herbs in clinic × 0.018/200 × 1000 × the multiple of clinical equivalency dose [9]. The dose of Gui-Hong extracts was equivalent to three times the adult daily dose of the Gui-Hong herb pair (15 g, from Tao-Hong-Si-Wu-Tang in which Danggui and Honghua were 9 g and 6 g, respectively) crude herbs based on the TCM prescription. Then 0.3 mL blood samples were collected into the centrifuge tubes which contained 0.03 mL EDTA-2Na from each rat at different time points (5, 10, 15, 30, 45, 60, 120, 240, 360, 600, 720, and 1440 min). Blood samples were centrifuged at 3000 rpm for 10 min at 4 °C and the supernatant was transferred and stored at −70 °C until analysis.
3.4. Chromatography and Mass Spectrometry Conditions
Chromatographic separation was carried out on a Thermo Hypersil Gold C18 column (2.1 mm × 50 mm, 1.9 μm) maintained at 35 °C. The mobile phase gradient conditions consisted of 0.1% aqueous acetic acid (A) and acetonitrile (B): 5% B (1.0 min)-40%B (5.0 min)-95% B (8.0 min)-95% B (9.0 min)-5% B (9.2 min)-5% B (10.0 min). The flow rate was set at 0.4 mL/min. The auto-sampler was conditioned at 4 °C and the sample injection volume was 2 μL. The ESI source was set in both positive and negative ionization mode. The scanning mode was set multiple reaction monitoring (MRM) mode and the retention time (tR), optimized MS/MS transitions, cone voltage and collision energy of each component were shown in Table 5. The TQ mass spectrometer was operated with a capillary voltage of 3 kV, a source temperature of 150 °C, and a desolvation temperature of 500 °C. MassLynx and DAS 2.0 (Waters, Corp., Milford, MA, USA) software were employed to analyze the data.
Table 5.
The retention time (tR), optimized MS/MS transitions, cone voltage, and collision energy for each component.
| Components | Retention Time (min) | ESI Mode | MRM Transitions (Precursor-Product) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| 1 | 3.27 | − | 611.223→325.056 | 32 | 28 |
| 2 | 3.49 | + | 181.03→88.89 | 12 | 26 |
| 3 | 3.79 | − | 162.968→119.025 | 20 | 14 |
| 4 | 3.92 | − | 593.223→284.818 | 40 | 28 |
| 5 | 3.94 | − | 193.032→133.995 | 22 | 14 |
| 6 | 6.42 | + | 191.16→145.091 | 14 | 14 |
| 7 | 6.68 | + | 191.16→173.117 | 22 | 16 |
| 8 | 3.78 | − | 320.845→151.922 | 20 | 20 |
| 9 | 4.86 | + | 748.585→158.113 | 28 | 26 |
3.5. Preparation of Calibration Standards and Quality Control (QC) Samples
The stock solutions of IS1 (189 μg/mL), IS2 (181 μg/mL), HSYA (225 μg/mL), caffeic acid (229 μg/mL), p-coumaric acid (213 μg/mL), kaempferol-3-O-rutinoside (137 μg/mL), ferulic acid (180 μg/mL), 3-n-butylphthalide (246 μg/mL), and ligustilide (386 μg/mL) were prepared in methanol, respectively. Then the series of working solutionswere obtained by further dilutionwith methanol.
Low, middle, and high concentrations of quality control (QC) samples were independently prepared at concentrations of 2.25, 22.5, and 180 ng/mL for HSYA; 2.29, 22.9, and 183.2 ng/mL for caffeic acid; 2.13, 106.5, and 852 ng/mL for p-coumaric acid; 2.74, 68.5, and 548 ng/mL for kaempferol-3-O-rutinoside; 9, 90, and 720 ng/mL for ferulic acid; 2.46, 24.6, and 196.8 ng/mL for 3-n-butylphthalide; and 1.93, 38.6, and 308.8 ng/mL for ligustilide using the same method as for the calibration samples. All stock solutions and working solutions were stored at −20 °C in dark until use.
3.6. Plasma Sample Preparation
Plasma samples were thawed at 37°Cbefore analysis. Then the 60 μL mixture of IS1 and IS2 solution (4.725 μg/mL of IS1 and 90.5 ng/mL of IS2) and 540 μL of methanol were added to the plasma sample (200 μL) in a 1.5 mL Eppendorf tube. The mixture was vortexed for 5 min and centrifuged at 13,000 rpm for 10 min at 4 °C to deproteinize. The supernatant was then transferred into a new 1.5 mL centrifuge tube and evaporated to dryness under vacuum with a Labconco CentriVap concentrator (Kansas City, MO, USA). The residue was reconstituted in 200 μL 80% methanol solution, and the mixtures were vortexed for 5 min, and centrifuged at 13,000 rpm for 10 min at 4 °C. Finally, 2 μL of the supernatant was injected into the UPLC-TQ/MS system for analysis.
3.7. Method Validation
3.7.1. Specificity
The specificity was calculated by comparing blank plasma samples from normal rats and blood stasis rats, blank plasma samples spiked with standards and internal standards, and plasma samplescollected after oral administration of Gui-Hong extracts and spiked with ISs.
3.7.2. Linearity and Lower Limit of Quantification (LLOQ)
For the calibration curve, the mixture stock solution was diluted with methanol to make a series of working solutions. The calibration samples were prepared independently by adding a series of different concentration working solutions (200 μL), IS solution (60 μL), and 340 μL methanol to blank rat plasma (200 μL) to determine linearity and the lower limit of quantification (LLOQ).
3.7.3. Precision and Accuracy
The precision and accuracy of the method wereevaluated by analysisof three QC samples. The precision was determined from inter-day and intra-day using low, middle, and high concentrations. The precision was expressed by relative standard deviation (RSD%), and the accuracy by relative error (RE%).
3.7.4. Recovery and Matrix Effects
The extraction recoveries of analytes were determined by comparing the peak areas of the QC samples pre-spiked in blank plasma with those post-spiked in blank plasma (n = 6). The matrix effect was determined by comparing the peak areas of the QC samples pre-spiked in blank plasma with those in the mobile phase (n = 6).
3.7.5. Stability
The stability of analytes in the plasma was performed byusing the QC samples under three conditions: (1) short-term stability, QC samples (n = 6) werestored at room temperature for 12 h and at refrigerated (4 °C) for 24 h; (2) long-term stability, QC samples (n = 6) were stored at −80 °C for 20 days; (3) three freeze–thaw cycles stability, QC samples (n = 6) were detected after three cycles of freezing (−20 °C) and thawing (ambient temperature).
4. Conclusions
In this study, the developed UPLC–MS/MS method for the quantification of HSYA, caffeic acid, p-coumaric acid, kaempferol-3-O-rutinoside, ferulic acid, 3-n-butylphthalide, and ligustilide in normal and model rat plasma is suitable for pharmacokinetic study. Pharmacokinetic results indicated that the seven bio-active components had obvious differences in some pharmacokinetic characteristics, suggesting that the drug metabolism enzymes might be altered in animals with blood stasis syndrome. The changed pharmacokinetic processes of the herb pair in normal and pathological conditions could help us explain and predict various events related to the efficacy and adverse reaction of Gui-Hong extract, and for better clinical applications of the Gui-Hong herb pair and relatedTCM formulae.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81573714, 81603257, 81603258, 81773882), 333 Highlevel Talents Training Project Funded by Jiangsu Province(No. BRA2016387). This work was also supported by the Open Project Program of Jiangsu Key Laboratory for High Technology Research of TCM Formulae and Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization (No. FJGJS-2015-11). This research was also financially supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author Contributions
Yi Jin, Yu-Ping Tang, An Kang and Jin-Ao Duan conceptualized the project and Jin-Ao Duan, Shao-Ping Li, Zhi-shu Tang, An Kang and Yu-Ping Tang headed the project. Yi Jin, Yu-Ping Tang, Zhen-Hua Zhu, Er-Xin Shang, Han-Qing Pang, Xu-Qin Shi, Gui-Sheng Zhou and An Kang performed experiments. Yi Jin, Yu-Ping Tang, Jin Wang, Xing Chang, An Kang, Jin Sun and Jin-Ao Duan were involved in writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds hydroxysafflor yellow A, caffeic acid, p-coumaric acid, kaempferol-3-O-rutinoside, ferulic acid and ligustilide are available from the authors.
References
- 1.Li S.J., Lin H., Qu C., Tang Y.P., Shen J., Li W.X., Yue S.J., Kai J., Shang G.X., Zhu Z.H., et al. Urine and plasma metabonomics coupled with UHPLC-QTOF/MS and multivariate data analysis on potential biomarkers in anemia and hematinic effects of herb pair Gui-Hong. J. Ethnopharmacol. 2015;170:175–183. doi: 10.1016/j.jep.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y., Qu C., Tang Y.P., Pang H.Q., Liu L.L., Zhu Z.H., Shang E.X., Huang S.L., Sun D.Z., Duan J.A. Herb pairs containing Angelicae Sinensis Radix (Danggui): A review of bio-active constituents and compatibility effects. J. Ethnopharmacol. 2016;181:158–171. doi: 10.1016/j.jep.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Yue S.J., Tang Y.P., Li S.J., Duan J.A. Chemical and biological properties of quinochalcone C-glycosides from the florets of Carthamus tinctorius. Molecules. 2013;18:15220–15254. doi: 10.3390/molecules181215220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X.Y., Li W., Ma X.H., Chu Y., Li S.M., Guo J.H., Jia Y.M., Zhou S.P., Zhu Y.H., Liu C.X. Simultaneous determination of caffeic acid and its major pharmacologically active metabolites in rat plasma by LC-MS/MS and its application in pharmacokinetic study. Biomed. Chromatogr. 2014;29:552. doi: 10.1002/bmc.3313. [DOI] [PubMed] [Google Scholar]
- 5.Tian Y., Yang Z.F., Li Y., Qiao Y., Yang J., Jia Y.Y., Wen A.D. Pharmacokinetic comparisons of hydroxysafflower yellow A in normal and blood stasis syndrome rats. J. Ethnopharmacol. 2010;129:1–4. doi: 10.1016/j.jep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Zeng M.F., Zhang J., Yang Y.F., Jin Y., Xiao W., Wang Z.Z., Ding G., Yan R.J. An automated dual-gradient liquid chromatography–MS/MS method for the simultaneous determination of ferulic acid, ligustrazine and ligustilide in rat plasma and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2014;88:354–363. doi: 10.1016/j.jpba.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Li W.X., Guo J.M., Tang Y.P., Wang H., Huang M.Y., Qian D.W., Duan J.A. Pharmacokinetic comparison of ferulic acid in normal and blood deficiency rats after oral administration of Angelica sinensis, Ligusticum chuanxiong and their combination. Int. J. Mol. Sci. 2012;13:3583–3597. doi: 10.3390/ijms13033583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong D., Sun H., Wu Z.F., Wu B.J., Xue Y.X., Li Z.J. A validated ultra-performance liquid chromatography-tandem mass spectrometry method to identify the pharmacokinetics of SR8278 in normal and streptozotocin-induced diabetic rats. J. Chromatogr. B. 2016;1020:142–147. doi: 10.1016/j.jchromb.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Li S.J., Lin H., Tang Y.P., Li W.X., Shen J., Kai J., Yue S.J., Shang G.X., Zhu Z.H., Shang E.X., et al. Comparative metabolomics analysis on invigorating blood circulation for herb pair Gui-Hong by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry and pattern recognition approach. J. Pharm. Biomed. Anal. 2015;107:456–463. doi: 10.1016/j.jpba.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Chiu C.C., Lan C.Y., Chang Y.H. Objective assessment of blood stasis using computerized inspection of sublingual veins. Comput. Methods Programs Biomed. 2002;69:1–12. doi: 10.1016/S0169-2607(01)00181-X. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y., Wu L., Tang Y.P., Cao Y.J., Li S.J., Shen J., Yue S.J., Qu C., Shan C.X., Cui X.B., et al. UFLC-Q-TOF/MS based screening and identification of the metabolites in plasma, bile, urine and feces of normal and blood stasis rats after oral administration of hydroxysafflor yellow A. J. Chromatogr. B. 2016;1012–1013:124–129. doi: 10.1016/j.jchromb.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Grohmann M. The activity of the neuronal and extra neuronal catecholamine-metabolizing enzymes of the perfused rat heart. Naunyn-Schmiedebergs Arch. Pharmacol. 1987;336:139–147. doi: 10.1007/BF00165797. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J., Zhang J.B., Cao Y.G., Chen Y.C., Yu D., Liu X.Q. Effect of acute myocardial ischemia on methylation of danshensu in rats. J. Chin. Pharm. Univ. 2009;40:72–76. [Google Scholar]
- 14.Azuma K., Ippoushi K., Nakayama M., Ito H., Higashio H., Terao J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000;48:5496–5500. doi: 10.1021/jf000483q. [DOI] [PubMed] [Google Scholar]
- 15.Lafay S., Morand C., Manach C., Besson C., Scalbert A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006;96:39–46. doi: 10.1079/BJN20061714. [DOI] [PubMed] [Google Scholar]
- 16.Zeng H.T., Xue P., Su S.L., Huang X.C., Shang E.X., Guo J.M., Qian D.W., Tang Y.P., Duan J.A. Comparative pharmacokinetics of three major bioactive components in rats after oral administration of Typhae Pollen-Trogopterus Feces drug pair before and after compatibility. Daru. 2016;20:2. doi: 10.1186/s40199-016-0140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang S.P., Liu F., Du F.F., Niu W., Sun Y., Li C., Guo M.L. A new method for determination of kaempferol-3-O-rutinoside in plasma: Application to pharmacokinetic study in Sprague-Dawley rats. Chin. J. Clin. Pharmacol. Therap. 2006;11:491–496. [Google Scholar]
- 18.Zhao Q., Hu J.P., Jiang J., Li Y., Hu P. Interaction of butylphthalide with rat and human liver CYP450 isoenzymes. Acta Pharm. Sin. 2015;50:541–546. [PubMed] [Google Scholar]
- 19.Li Y.H., Zhang Y.Q., Li L., Wang Q., Wang N.S. Effect of Danggui and Honghua on Cytochrome P450 1A2, 2C11, 2E1 and 3A1 mRNA Expression in Liver of Rats. Am. J. Chin. Med. 2008;36:1071–1081. doi: 10.1142/S0192415X08006454. [DOI] [PubMed] [Google Scholar]