Abstract
The leaves of Perilla frutescens var. crispa (Lamiaceae)—known as ‘Jureum-soyeop’ or ‘Cha-jo-ki’ in Korean, ‘ZI SU YE’ in Chinese, and ‘Shiso’ in Japan—has been used as a medicinal herb. Recent gamma irradiated mutation breeding on P. frutescens var. crispa in our research group resulted in the development of a new perilla cultivar, P. frutescens var. crispa (cv. Antisperill; PFCA), which has a higher content of isoegomaketone. The leaves of PFCA were extracted by supercritical carbon dioxide (SC-CO2) extraction, and phytochemical investigation on this extract led to the isolation and identification of a new compound, 9-hydroxy-isoegomaketone [(2E)-1-(3-furanyl)-4-hydroxy-4-methyl-2-penten-1-one; 1]. Compound 1 exhibited inhibitory activity on nitric oxide (NO) production in lipopolysaccharide (LPS)-activated RAW264.7 cells with an IC50 value of 14.4 μM. The compounds in the SC-CO2 extracts of the radiation mutant cultivar and the original plant were quantified by high-performance liquid chromatography with diode array detection.
Keywords: Perilla frutescens var. crispa, radiation mutant cultivar, 9-Hydroxy-isoegomaketone, anti-inflammation
1. Introduction
Perillae Folium is the dried leaves of Perilla species (Lamiaceae) including P. frutescens var. frutescens, P. frutescens var. crispa, and P. frutescens var. acuta. Perillae Folium has a pungent taste and a warm property acting on the lung and spleen channels, and it has been also used as a diuretic, sedative, detoxifying, and antipyretic agent in the traditional medicine of East Asia [1]. The leaves of Perilla frutescens var. crispa (Lamiaceae) have been ethnopharmacologically used to protect the digestive tract from inflammatory diseases and to prevent sea-food poisoning [2]. Monoterpenes in the essential oil of P. frutescens var. crispa are the major components [3], with diverse biological effects including antifungal [4], neuroprotective [5], anticancer [6,7], angiogenesis inhibitory [8], anti-inflammatory [9], and antioxidant activities [10]. In addition, various types of anthocyanin [11], phenolics [12,13], polysaccharides [14], and flavonoids [15] have also been isolated from this plant. Some of these compounds were found to have diverse biological activities such as antioxidant [12], immune adjuvant [14], anti-inflammatory [15,16], and antioxidant effects [17].
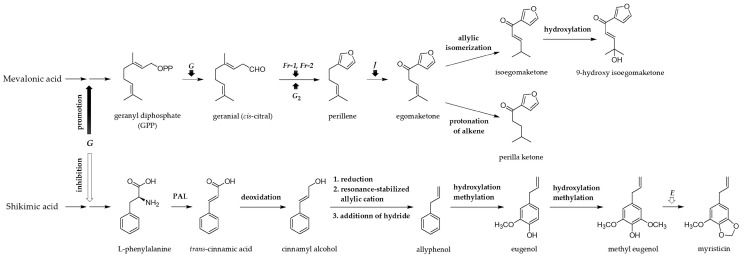
Mutation breeding generated by ionizing generation has been used to improve crop productivity and quality for many years and has led to more than 3000 mutant varieties of plants [18]. Our research group has developed a gamma irradiated mutant cultivar of P. frutescens var. crispa (cv. Antisperill; PFCA), with a high content of isoegomaketone (2) compared to the original cultivar [13] (Figure 1). In our previous biological evaluations on 2, this compound was found to have anticancer [7], anti-inflammatory [9], and antioxidant activities [10]. Recently, we reported that the PFCA extract by supercritical carbon dioxide (SC-CO2) extraction method contained the increased content of monoterpenes and enhanced anti-inflammatory effect, compared to those of the SC-CO2 extract of the original cultivar (PFC) [19] as well as the ethanol extract of PFCA [20]. SC-CO2 extraction technology has been recognized as a powerful technique for essential oils from oil bearing plant sources [21], with advantages of being non-toxic, non-flammable, time and cost-effective, and more environmentally friendly [22]. Other than 2 and perilla ketone (3), two unknown compounds were found in the SC-CO2 extracts of PFCA and PFC by HPLC analysis [19,20]. Therefore, repeated column chromatography of the SC-CO2 extracts of PFCA and PFC led to the isolation of a new compound 1 and myristicin (4) [23], respectively (Figure 2). The structure elucidation and biological evaluation of 1 and quantitative analyses of the components of PFCA and PFC are described herein.
Figure 1.
Perilla frutescens var. crispa (Lamiaceae): (a) A mutant cultivar, P. frutescens var. crispa (cv. Antisperill; PFCA), which developed by 200 Gy gamma irradiation from a labeled Cobalt (60Co) source on seeds of an original cultivar; (b) an original cultivar (PFC) that has red-purple leaves.
Figure 2.
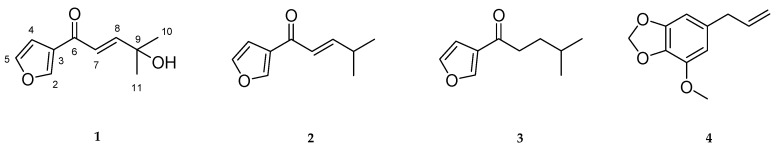
Chemical structures of compounds from the SC-CO2 extracts of PFCA and PFC.
2. Results
2.1. Structure Elucidation of Compound 1
Compound 1 was obtained as a colorless oil, with a molecular ion peak [M]− at m/z 180.0788 in the HRESIMS spectrum, corresponding to an elemental formula of C10H12O3. The UV absorption maxima at 230 and 254 nm indicated the presence of aromatic ring and conjugated enone group [24]. The 1H- and 13C-NMR spectra of 1 were similar to those of isoegomaketon [25], except for the absence of signals for a methine group and the presence of an oxygenated tertiary carbon signal at δC 71.3 (C-9). The 1H- and 13C-NMR spectra of 1 displayed signals for an aromatic system at δH 7.46 (1H, s, H-2)/δC 144.4 (C-2), 6.84 (1H, s, H-3)/109.1 (C-3), and 8.81 (1H, s, H-5)/147.6 (C-5), and a conjugated carbonyl carbon at δC 184.6 (C-6), suggesting that 1 has a 3-furylketone, supported by the 1H-13C HMBC correlations of H-2/C-4, C-5, H-4/C-2, C-5, and H-5/C-2, C-3, C-4 (Figure 3). The 1H NMR signals for two uncoupled methyl groups at δH 1.40 (6H, s, H-10 and H-11), a trans-olefinic group at 6.77 (1H, d, J = 15.3 Hz, H-7) and 7.08 (1H, d, J = 15.3 Hz, H-8), and the 13C-NMR signal for an oxygenated tertiary carbon at δC 71.3 (C-9) showed the presence of an isoprenyl alcohol group, positioned at the C-6 as evidenced by the 1H-13C HMBC correlations of H-7/C-6, C-9 and H-8/C-6, C-9 (Figure 3). Further detailed analysis of 1H-1H COSY, 1H-13C HSQC, and 1H-13C HMBC NMR data allowed unambiguous assignments for all of the 1H- and 13C-NMR signals of 1 (Figures S1−S5). Therefore, the structure of 1 was determined as (2E)-1-(3-furanyl)-4-hydroxy-4-methyl-2-penten-1-one and this compound was named as 9-hydroxy-isoegomaketone.
Figure 3.
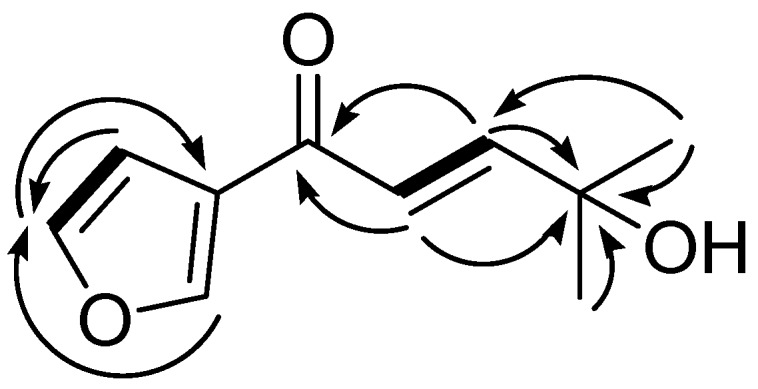
Key 1H-1H COSY (▬) and 1H-13C HMBC (→) correlations of 1.
2.2. Effect of Compound 1 on NO production in LPS-Activatied RAW 264.7 Cells
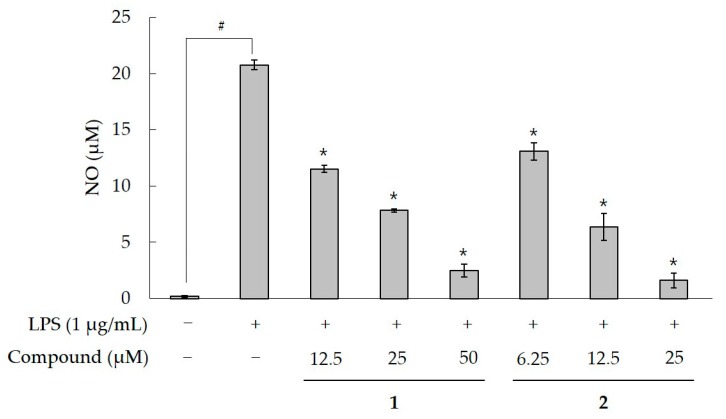
NO is a gaseous free radical produced by the oxidation of l-arginine catalyzed by NO synthases (NOS), with a wide range of physiological and pathological actions. Among the isoforms of NOS—neural (nNOS), endotherial (eNOS), and inducible (iNOS)—iNOS is induced in response to bacterial LPS and proinflammatory cytokines. Therefore, the inhibition of LPS-induced NO production is considered as an inhibitor of iNOS, which is a mediator of inflammation and carcinogenesis [26]. Compound 1 decreased the LPS-stimulated NO production in a concentration-dependent manner (Figure 4) and showed inhibitory activity with an IC50 value of 14.4 μM. However, it inhibited the LPS-induced NO production less effectively than 2 (IC50, 8.8 μM). Cytotoxicities of compounds were also determined by using the EZ-Cytox cell viability assay kit. Compound 1 did not affect cell viability at any of the tested concentrations, but 2 was found too toxic at the concentrations ≥25 μM (data not shown) [8].
Figure 4.
Effects of 1 and 2 on NO production in RAW 264.7 cells. Data are presented as means ± SD (n = 6). # p < 0.01 vs. negative control. * p < 0.05 vs. the LPS-alone group.
2.3. Quantitative Analysis of Four Compounds from Two Perilla Cultivars
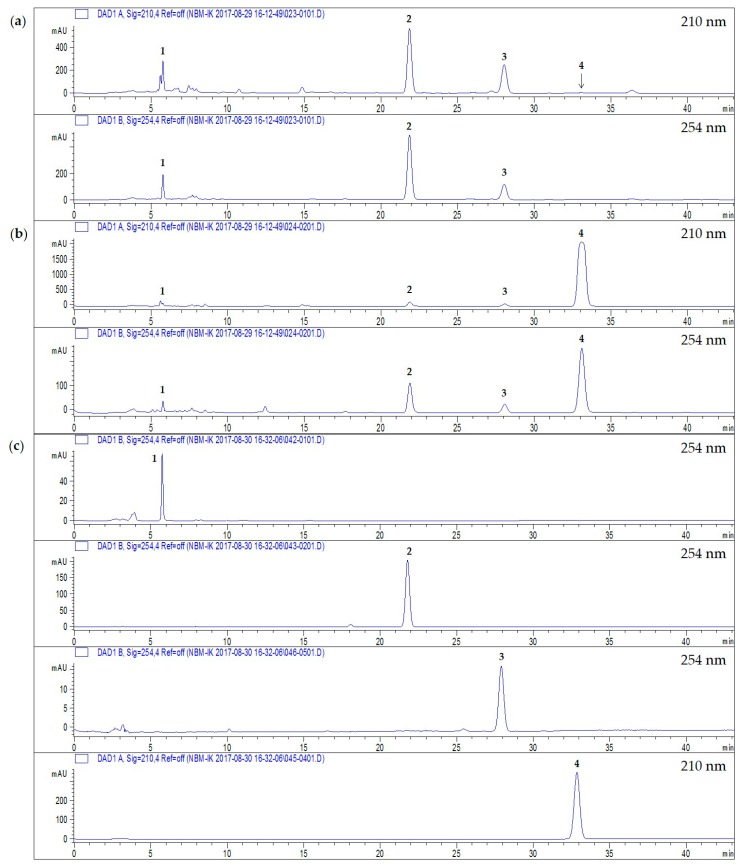
The HPLC-DAD analytical method established in our previous studies [19,27] was applied to the simultaneous determination of four compounds in the SC-CO2 extracts of PFCA and PFC (Figure 5). The four components were separated on a reverse phase analytical column using a gradient solvent system of acetonitrile and water. The ultraviolet wavelength used for detection was 254 nm. The linearity of this analytical method was evaluated based on the correlation coefficient (r2) value of the calibration curves of each compound. The calibration curves of the four compounds were obtained by the assessment of the peak areas of the standard solutions at four different concentrations. The calibration curves showed a high degree of linearity with an r2 > 0.9991 over the concentration ranges 20−100 μg/mL for 1 and 3, 20−164 μg/mL for 2, and 100−1000 μg/mL for 4 (Table 1). The limits of detection (LOD) and limits of quantitation (LOQ) for the four components were calculated using the slope of the calibration curve and the standard deviation (SD) of the intercept. The LODs and LOQs for the compounds 1–4 were in the range 0.006−0.124 μg/mL and 0.116−0.952 μg/mL, respectively. The amounts of the four compounds are listed in Table 2. The contents of 1–3 were approximately 9-, 5-, and 4-fold higher, respectively, in the SC-CO2 extracts of PFCA than in those of PFC. Compound 4 was not observed in the SC-CO2 extracts of PFCA; however, it was the most abundant in the SC-CO2 extracts of PFC.
Figure 5.
HPLC chromatograms of (a) the SC-CO2 extract of PFCA at 210 and 254 nm; (b) the SC-CO2 extract of PFC at 210 and 254 nm; (c) the four standards: 9-hydroxy-isoegomaketone (1), isoegomaketone (2), and perilla ketone (3) at 254 nm and myristicin (4) at 210 nm.
Table 1.
Linear range, regression equation, correlation coefficients, LODs, and LOQs for compounds.
| Compound | tR (min) | Linear Range (μg/mL) | Regression Equation (y = ax + b) 1 | Correlation Coefficient (r2) | LOD 2 (μg/mL) | LOQ 3 (μg/mL) |
|---|---|---|---|---|---|---|
| 1 | 5.78 | 20−100 | y = 13.154x − 8.1524 | 0.9991 | 0.103 | 0.641 |
| 2 | 21.87 | 20−164 | y = 44.574x − 27.463 | 0.9992 | 0.067 | 0.952 |
| 3 | 28.04 | 20−100 | y = 7.8236x − 0.9952 | 0.9992 | 0.006 | 0.116 |
| 4 | 33.10 | 100−1000 | y = 10.824x − 67.536 | 0.9992 | 0.124 | 0.375 |
1 y = peak area, x = concentration (μg/mL), a = slope, b = intercept; 2 LOD: 3.3 × (SD of the response/slope of the calibration curve); 3 LOQ: 10 × (SD of the response/slope of the calibration curve).
Table 2.
The contents of compounds in the leaves of PFCA and PFC.
| Peak | Compound | Contents (mg/g) | |
|---|---|---|---|
| PFCA | PFC | ||
| 1 | 9-hydroxy-isoegomaketone (1) | 1.33 ± 0.07 | 0.15 ± 0.02 |
| 2 | isoegomaketone (2) | 2.76 ± 0.05 | 0.51 ± 0.02 |
| 3 | perilla ketone (3) | 6.96 ± 0.17 | 1.71 ± 0.12 |
| 4 | myristicin (4) | 0.042 ± 0.003 | 36.77 ± 5.60 |
3. Discussion
In the comparison studies of LPS-induced anti-inflammatory activities and chemical profiles of the SC-CO2 extract of PFCA versus PFC [19] and the SC-CO2 versus the ethanol extracts of PFCA [20], the SC-CO2 extract of PFCA showed greater potency and higher content of 2 and 3 than the SC-CO2 extract of PFC and the ethanol extract of PFCA. The HPLC chromatogram of these three extracts showed an unknown peak besides the two main peaks for 2 and 3, and thus it was identified as compound 1. While, another unknown peak, which appeared in the chromatogram of the SC-CO2 extract of PFC, but not in the chromatograms of the SC-CO2 and the ethanol extracts of PFCA, was identified as compound 4. Therefore, the accumulation of 1–3 and the degradation of 4 in PFCA leaves are thought to be associated with the variation in the metabolic pathways by gamma irradiation effect. The biosynthesis of new compound 1 can be inferred from several studies on the biosynthetic pathway of monoterpene and phenylpropanoid proposed from their genetic analysis in P. frutescens (Figure 6) [28,29,30,31]. Compounds 2 and 3 were synthesized from a precursor, geranyl diphosphate (GPP) through the mevalonate pathway. In this reaction, GPP gave rise to a linear monoterpene, geranial (cis-citral) by promotion of dominant gene G which is considered to be essential for the initiation of monoterpene biosynthesis [29]. Geranial (cis-citral) was converted to perillene through the furan formation controlled by the polymeric genes (Fr1 and Fr2) [30] and (or) the oxidation of cis-citral controlled by gene G2, and then the conversion of perillene into egomaketone was promoted by the dominant gene (J) that controlled the oxidation of C-6 position of perillene [31]. Based on the construction mechanisms [32], 2 and 3 would be expected to be synthesized by the allylic isomerization and the protonation of alkene, respectively, of the precursor egomaketone. Further hydroxylation of 2 was assumed to create 9-hydroxy-isoegomaketone (1) of a new structure type (Figure 6). While, in the absence of gene G, phenylpropanoids accumulated instead of monoterpenes in the perilla plants [28]. Compound 4 was known to be produced in the biosynthesis via the shikimate pathway [32]. Shikimic acid has a role in the formation of aromatic acids, l-phenylalanine, l-tyrosine, and l-tryptophan. Among them, l-phenylalanine generates trans-cinnamic acid by the elimination of ammonia from its side-chain via phenylalanine ammonia lyase (PAL) which is an enzyme to catalyze the reaction converting l-phenylalanine to trans-cinnamic acid. Deoxidation from trans-cinnamic acid generates cinnamyl alcohol, and then eugenol is derived from cinnamyl alcohol by the following steps: loss of hydroxyl as leaving group, resonance form of the allylic cation, and addition of hydride [32]. Tabata (2000) suggested that a possible precursor—methyleugenol—was metabolized to compound 4 by the formation of a methylenedioxy group, in the absence of a dominant gene E which is involved in the production of elemicin. However, to understand the mechanisms responsible for the accumulation of monoterpenes in the gamma-irradiated mutant cultivar PFCA, further enzymological and gene function studies are still necessary.
Figure 6.
Proposed biosynthetic pathway for four components found in PFCA and PFC [28,29,30,31,32].
Medicinal plants have historically been valuable sources of therapeutic agents, and still an interest in the discovery of novel drug leads from natural products is on the increase [33,34,35]. In continuation of our search to find new active compounds from plants and their improved varieties (cultivars), a new compound 1 was isolated from a new cultivar—PFCA—and showed the inhibitory activity in the measurement of NO production on LPS-stimulated macrophage cells. Compound 2 showed greater inhibitory activity with an IC50 value of 8.8 μM than that of 1 (IC50, 14.4 μM), but its inhibitory activity on NO production seems to be related to its concomitant cytotoxic effect. Thus, Compound 1 may be considered as an active and safe component of PFCA for targeting inflammation disease. Further mechanistic studies using in vitro and in vivo models of inflammation disease will be performed to verify the anti-inflammatory activity of 1.
4. Materials and Methods
4.1. General Procedures
The NMR experiments were performed using a JNM-ECA 500 MHz NMR instrument (JEOL Ltd., Tokyo, Japan). HRESIMS was carried out on a JMS-700 MStation Mass Spectrometer (JEOL Ltd., Tokyo, Japan). UV spectra were recorded on an Evolution 260 Bio UV–Visible spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA). Thin-layer chromatographic (TLC) analysis was performed on Kieselgel 60 F254 (Merck, Darmstadt, Germany) and Kieselgel 60 RP-18 F254S (Merck), with visualization performed under UV light (254 and 365 nm) and 10% (v/v) sulfuric acid spray followed by heating (200 °C, 2 min). YMC Gel ODS-A (12 nm, S-150 μm; YMC Co., Kyoto, Japan) was used for column chromatography (CC). Preparative HPLC was performed using a Gilson Preparative HPLC system (Gilson Inc., Middleton, WI, USA) equipped with YMC Pack Pro C18 (5 μm, 250 × 20 mm, YMC Co.). Analytical HPLC was performed using an Agilent 1100 series system (Agilent Technologies, Palo Alto, CA, USA) equipped with YMC-Triart C18 (5 μm, 250 × 4.6 mm, YMC Co.). A [60Co] γ-irradiator (150 TBq capacity; AECL, Ottawa, Canada) was used for gamma irradiation. All other chemicals and solvents used in this study were of analytical grade.
4.2. Plant Materials
Perilla frutescens var. crispa (cv. Antisperill; PFCA) is a mutant perilla cultivar that produces green leaf with a high content of isoegomaketone (2). It was developed by 200 Gy gamma irradiation from a labeled Cobalt (60Co) source on seeds of the original plant, Perilla frutescens var. crispa, with a red-purple leaf and examined as stable inheritance of phenotype for three years (1995–1998) at the Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute (Jeongeup-si, Jeollabuk-do, Korea). PFCA leaves were collected each year shortly before the flowering time. PFCA seeds have been deposited for the purpose of patent procedures in the Korean Collection for Type Cultures, Biological Resource Center, Korea Research Institute of Bioscience and Biotechnology (August, 2016). The voucher specimens have been deposited at the Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute (Jeongeup-si, Jeollabuk-do, Korea).
4.3. Extraction and Isolation
The dried leaves of PFCA (300 g) were pulverized and then extracted by SC-CO2 extraction method using a laboratory-scale supercritical fluid extraction system (Ilshin Autoclave Co., Daejeon, Korea). The powdered sample was placed into the extraction column of SC-CO2 extractor. The predetermined conditions are as follows: pressure, 400 bar; temperature, 40 °C. The flow rate of CO2 (99.9%) was constant at 60 mL/min, for an extraction time of 3 h. The oil was collected 13.81 g (4.6% w/w) and stored in a refrigerator at 4 °C. A part (2 g) of the extract was subjected to RP-C18 CC (MeOH–water, 1:1 to 9:1, v/v) to yield 11 fractions (F01–F11). Fraction F03 (25 mg) was chromatographed on sephadex LH-20 using 100% MeOH to give five sub-fractions (F0301-F0305). The third fraction (F0303, 11 mg) was purified by preparative HPLC (YMC Triart C18, MeOH–water = 2:3, 4 mL/min, UV 280 nm) to yield 1 (tR 21.03 min, 6 mg). The dried leaves of PFC (300 g) were extracted by the SC-CO2 extraction method under the same conditions as mentioned above, affording 14.84 g of oil extract (4.9% w/w) was obtained. A part (1 g) of the extract was subjected to RP-C18 CC (MeOH–water, 2:1 to 4:1, v/v) to yield twenty fractions (F01–F20) and pure compounds 2 (2 mg), 3 (5 mg), and 4 (9 mg).
9-Hydroxy-isoegomaketone ((2E)-1-(3-furanyl)-4-hydroxy-4-methyl-2-penten-1-one; 1). Colorless oil. UV (MeOH) λmax (log ε) 230 (3.69), 254 (3.44) nm; 1H-NMR (CDCl3, 500 MHz) δ 8.81 (1H, br s, H-5), 7.46 (1H, s, H-2), 7.08 (1H, d, J = 15.3 Hz, H-8), 6.84 (1H, br s, H-4), 6.77 (1H, d, J = 15.3 Hz, H-7), 1.40 (6H, s, H-10 and H-11); 13C-NMR (CDCl3, 125 MHz) δ 184.6 (C-6), 153.4 (C-8), 147.6 (C-5), 144.4 (C-2), 128.4 (C-3), 122.7 (C-7), 109.1 (C-4), 71.3 (C-9), 29.6 (C-10 and C-11); HREIMS m/z 180.0788 [M]− (calcd. for C10H12O3, 180.0786).
4.4. HPLC-DAD Anlysis
Quantitative analysis was conducted using an Agilent 1200 series LC system. Data acquisition and processing were performed using the ChemStation software (version B.04.). The chromatographic separation of four compounds was performed at room temperature using an YMC-Triart C18 column (5 μm, 250 × 4.6 mm, YMC Co.) with a gradient solvent system of acetonitrile and water (45:55–55:45). The flow rate was maintained at 1 mL/min and the injection volume was 10 μL. Chromatograms were acquired at 254 nm for 1–3 and 210 nm for 4 using a DAD detector.
All the four compounds were weighed accurately, dissolved in methanol at 1.0 mg/mL, and diluted to yield a series of standard solutions at four different concentrations for quantitative analysis. The SC-CO2 extracts of PFCA and PFC were weighed accurately and dissolved in methanol at a concentration of 20 mg/mL. The standard and sample solutions were filtered through a syringe filter (0.45 μm) before HPLC analysis.
4.5. Measurement of NO Production on LPS-Stimulated RAW 264.7 Cells
NO formation was measured in cultured RAW 264.7 cells. RAW 264.7 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/mL), and streptomycin (100 μg/mL) and incubated at 37 °C in 5% CO2 of humidified air. The cells were plated in a 96-well plate and then incubated for 24 h. The cells were pre-treated with various concentrations of compounds (6.25−50 μM) for 2 h, and then incubated in the medium with 1 μg/mL of LPS in the presence or absence of test samples for an additional 18 h. The media were collected and analyzed for nitrite accumulation by the Griess reaction. Briefly, 100 μL of Griess reagent—0.1% N-(1-naphthyl)ethylenediamine dihydrochloride in H2O and 1% sulfanilamide in 5% H3PO4—was added to 100 μL of each supernatant from LPS or sample-treated cells in 96-well plates. The absorbance was measured at 540 nm using an ELISA reader, and the nitrite concentration was determined by comparison with a sodium nitrite standard curve. The percentage inhibition was expressed as [1 − (NO level of test samples/NO level of vehicle-treated control)] × 100. The IC50 value, the sample concentration resulting in 50% inhibition of NO production, was determined by non-linear regression analysis (% inhibition versus concentration).
4.6. Cytotoxicity Assay
The EZ-Cytox cell viability assay kit was used to measure the cell viability. The cells were cultured in a 96-well plate at a density of 2 × 105 cells/mL for 24 h. Compounds were dissolved in DMSO and incubated with the cells at the concentrations of 6.25, 12.5, 25, and 50 µM for an additional 24 h. After the incubation period, 10 μL solution of cell viability assay kit was added to each well and incubated for 4 h at 37 °C and 5% CO2. The index of cell viability was determined by measuring the formazan production using a spectrophotometer at an absorbance of 480 nm with a reference wavelength of 650 nm.
5. Conclusions
Our phytochemical investigation on the SC-CO2 extracts of the radiation mutant cultivar, PFCA, and the original cultivar, PFC, successfully led to the isolation of a new monoterpene, 9-hydroxy-isoegomaketone (1) and myricitrin (4), respectively, for the identification of unknown peaks of the HPLC chromatograms of PFCA and PFC. Compound 1 exhibited inhibitory activity on LPS-induced NO production in RAW 264.7 cells. Thus, 1 might be a potential standard for efficacious use of PFCA in inflammation disease, together with isoegomaketone (2), which is known as the major active standard in the SC-CO2 extract of PFCA. Our findings will be helpful for the quality control of PFCA to develop the medicine and dietary supplement related to anti-inflammation.
Acknowledgments
This research was supported by Radiation Technology R&D program (No. 2017M2A2A6A05018541) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning.
Supplementary Materials
Supplementary Materials are available online. Figure S1: 1H-NMR (500 MHz, CDCl3) spectrum of compound 1, Figure S2: 13C-NMR (125 MHz, CDCl3) spectrum of compound 1, Figure S3: 1H-1H COSY NMR spectrum of compound 1, Figure S4: 1H-13C HMQC NMR spectrum of compound 1, Figure S5: 1H-13C HMBC NMR spectrum of compound 1.
Author Contributions
A.-R.H. and C.H.J. conceived and designed the experiments; B.N. and A.-R.H. wrote the paper; B.N. performed the phytochemical experiments and analyzed the data; Y.S. and H.Y.K. performed the biological experiments and analyzed the data; J.-B.K. provided the plant materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Zhu Y.-P. Chinese Materia Medica: Chemistry, Pharmacology and Applications. CRC Press; Boca Raton, FL, USA: 1988. pp. 54–55. [Google Scholar]
- 2.Tan F., Chen Y., Tan X., Ma Y., Peng Y. Chinese materia medica used in medicinal diets. J. Ethnopharmcol. 2017;206:40–54. doi: 10.1016/j.jep.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Bumblauskienl L., Jakstas V., Janulis V., Mazdzieriene R., Ragazinskiene O. Preliminary analysis on essential oil composition of Perilla L. cultivated in Lithuania. Acta Pol. Pharm. 2009;66:409–413. [PubMed] [Google Scholar]
- 4.Tian J., Wang Y., Lu Z., Sun C., Zhang M., Zhu A., Peng X. Perillaldehyde, a promising antifungal agent used in food preservation, triggers apoptosis through a metacaspase-dependent pathway in Aspergillus flavus. J. Agric. Food Chem. 2016;64:7404–7413. doi: 10.1021/acs.jafc.6b03546. [DOI] [PubMed] [Google Scholar]
- 5.Xu L., Li Y., Fu Q., Ma S. Perillaldehyde attenuates cerebral ischemia-reperfusion injury-triggered overexpression of inflammatory cytokines via modulating Akt/JNK pathway in the rat brain cortex. Biochem. Biophys. Res. Commun. 2014;454:65–70. doi: 10.1016/j.bbrc.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Chung B.H., Lee H., Lee J.S., Young C.Y.F. Perillyl alcohol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett. 2006;236:222–228. doi: 10.1016/j.canlet.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Cho B.O., Jin C.H., Park Y.D., Ryu H.W., Byun M.W., Seo K.I., Jeong I.Y. Isoegomaketone induces apoptosis through caspase-dependent and caspase-independent pathways in human DLD1 cells. Biosci. Biotechnol. Biochem. 2011;75:1306–1311. doi: 10.1271/bbb.110088. [DOI] [PubMed] [Google Scholar]
- 8.Loutrari H., Hatziapostolou M., Skouridou V., Papadimitriou E., Roussos C., Kolisis F.N., Papapetropoulos A. Perillyl alcohol is an angiogenesis inhibitor. J. Pharmacol. Exp. Ther. 2004;311:568–575. doi: 10.1124/jpet.104.070516. [DOI] [PubMed] [Google Scholar]
- 9.Jin C.H., Lee H.J., Park Y.D., Choi D.S., Kim D.S., Kang S.-Y., Seo K.-I., Jeong I.Y. Isoegomaketone inhibits lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages through the heme oxygenase-1 induction and inhibition of the interferon-β-STAT-1 pathway. J. Agric. Food Chem. 2010;58:860–867. doi: 10.1021/jf9033333. [DOI] [PubMed] [Google Scholar]
- 10.Jin C.H., So Y., Kim J.-B., Han S.N. Isoegomaketone upregulates heme oxygenase-1 in RAW264.7 cells via ROS/p38 MAPK/Nrf2 pathway. Biomol. Ther. 2016;24:510–516. doi: 10.4062/biomolther.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida K., Kondo T., Kameda K., Goto T. Structure of anthocyanins isolated from purple leaves of Perilla ocimoides L. var. crispa Benth and their isomerization by irradiation of light. Agric. Biol. Chem. 1990;54:1745–1751. [Google Scholar]
- 12.Tada M., Matsumoto R., Yamaguchi H., Chiba K. Novel antioxidants isolated from Perilla frutescens Britton var. crispa (Thunb.) Biosci. Biotechnol. Biochem. 1996;60:1093–1095. doi: 10.1271/bbb.60.1093. [DOI] [PubMed] [Google Scholar]
- 13.Park Y.D., Lee Y.M., Kang M.A., Lee H.J., Jin C.H., Choi D.S., Kim D.S., Kang S.-Y., Kim W.-G., Jeong I.Y. Phytochemical profiles and in vitro anti-inflammatory properties of Perilla frutescens cv. Chookyoupjaso mutants induced by mutagenesis with γ-ray. Food Sci. Biotechnol. 2010;19:305–311. doi: 10.1007/s10068-010-0044-8. [DOI] [Google Scholar]
- 14.Kwon K.H., Kim K.I., Jun W.J., Shin D.H., Cho H.Y., Hong B.S. In vitro and in vivo effects of macrophage-stimulatory polysaccharide from leaves of Perilla frutescens var. crispa. Biol. Pharm. Bull. 2002;25:367–371. doi: 10.1248/bpb.25.367. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima A., Yamamoto Y., Yoshinaka N., Namba M., Matsuo H., Okuyama T., Yoshigai E., Okumura T., Nishizawa M., Ikeya Y. A new flavanone and other flavonoids from green perilla leaf extract inhibit nitric oxide production in interleukin 1β-treated hepatocytes. Biosci. Biotechnol. Biochem. 2015;79:138–146. doi: 10.1080/09168451.2014.962474. [DOI] [PubMed] [Google Scholar]
- 16.So Y., Lee S.Y., Han A.-R., Kim J.-B., Jeong H.G., Jin C.H. Rosmarinic acid methyl ester inhibits LPS-induced NO production via suppression of MyD88-dependent and -independent pathways and induction of HO-1 in RAW 264.7 cells. Molecules. 2016;21:1083. doi: 10.3390/molecules21081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin C.H., Yang H.S., Choi D.S., Byun M.W., Kim W.G., Jeong I.Y. Rosmarinic acid attenuated SIN-1-induced cytotoxicity in HepG2 cells through the HO-1 induction and radical scavenging activity. Food Sci. Biotechnol. 2013;22:549–556. doi: 10.1007/s10068-013-0113-x. [DOI] [Google Scholar]
- 18.The Joint FAO/IAEA Mutant Variety Database. [(accessed on 4 September 2017)]; Available online: https://mvd.iaea.org.
- 19.Park H.C., So Y., Kim J.-B., Yuk H.S., Jin C.H. Comparison study of anti-inflammatory activity of extracts with supercritical carbon dioxide from radiation mutant Perilla frutescens (L.) Britton and wild-type. J. Radiat. Ind. 2016;10:97–104. [Google Scholar]
- 20.Jin C.H., Park H.C., So Y., Nam B., Han S.N., Kim J.-B. Comparison of the anti-inflammatory activities of supercritical carbon dioxide versus ethanol extracts from leaves of Perilla frutescens Britt. radiation mutant. Molecules. 2017;22:311. doi: 10.3390/molecules22020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S., Lee W., Yeo S., Choi Y. Supercritical carbon dioxide extraction of Perilla seed oil. Food Sci. Biotechnol. 1996;5:300–304. [Google Scholar]
- 22.Chiappini L., Perraudin E., Durand-Jolibois R., Doussin J.F. Development of a supercritical fluid extraction-gas chromatography mass spectrometry method for the identification of highly polar compounds in secondary organic aerosols formed from biogenic hydrocarbons in smog chamber experiments. Anal. Bioanal. Chem. 2006;386:1749–1759. doi: 10.1007/s00216-006-0744-3. [DOI] [PubMed] [Google Scholar]
- 23.You C.X., Jiang H.Y., Zhang W.J., Guo S.S., Yang K., Lei N., Ma P., Geng Z.F., Du S.S. Contact toxicity and repellency of the main components from the essential oil of Clausena anisum-olens against two stored product insects. J. Insect Sci. 2015;15:87. doi: 10.1093/jisesa/iev071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavia D.L., Lampman G.M., Kriz G.S. Introduction to Spectroscopy. 3rd ed. Thomson Learning, Ltd.; London, UK: 2001. pp. 353–386. [Google Scholar]
- 25.Park Y.D., Jin C.H., Choi D.S., Byun M.-W., Jeong I.Y. Biological evaluation of isoegomaketone isolated from Perilla frutescens and its synthetic derivatives as anti-inflammatory agents. Arch. Pharm. Res. 2011;34:1277–1282. doi: 10.1007/s12272-011-0806-8. [DOI] [PubMed] [Google Scholar]
- 26.Zamora R., Vodovotz Y., Billiar T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000;6:347–373. [PMC free article] [PubMed] [Google Scholar]
- 27.Nam B., Lee S.Y., Kim J.-B., Kang S.-Y., Jin C.H. Simultaneous determination of isoegomaketone and perillaketone in Perilla frutescens (L.) Britton leaves by HPLC-DAD. korean J. Pharmacogn. 2016;47:79–83. [Google Scholar]
- 28.Tabata M. Genetics of monoterpene biosynthesis in Perilla plants. Plant Biotechnol. 2000;17:273–280. doi: 10.5511/plantbiotechnology.17.273. [DOI] [Google Scholar]
- 29.Koezuka Y., Honda G., Tabata M. Genetic control of the chemical composition of volatile oils in Perilla frutescens. Phytochemistry. 1986;25:859–863. doi: 10.1016/0031-9422(86)80017-6. [DOI] [Google Scholar]
- 30.Yuba A., Honda G., Koezuka Y., Tabata M. Genetic analysis of essential oil variants in Perilla frutescens. Biochem. Genet. 1995;33:341–348. doi: 10.1007/BF02399932. [DOI] [PubMed] [Google Scholar]
- 31.Nishizawa A., Honda G., Tabata M. Genetic control of perillene accumulation in Perilla frutescens. Phytochemisty. 1990;29:2873–2875. doi: 10.1016/0031-9422(90)87094-B. [DOI] [Google Scholar]
- 32.Dewick P.M. Medicinal Natural Products: A Biosynthetic Approach. 2nd ed. John Wiley & Sons Ltd.; West Sussex, UK: 2002. pp. 7–34, 121–140. [Google Scholar]
- 33.Waltenberger B, Mocan A., Šmejkal K., Heiss E.H., Atanasov A.G. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules. 2016;21:807. doi: 10.3390/molecules21060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinghorn A.D., Pan L., Fletcher J.N., Chai H. The relevance of higher plants in lead compound discovery programs. J. Nat. Prod. 2011;74:1539–1555. doi: 10.1021/np200391c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.