Abstract
Background
Chemoradiation (CRT) or short-course radiotherapy (SCRT) are standard treatments for locally advanced rectal cancer (LARC). We evaluated the efficacy/safety of two neoadjuvant chemotherapy (NACT) regimens as an alternative prior to total mesorectal excision (TME).
Methods/design
This multi-centre, phase II trial in patients with magnetic resonance imaging (MRI) defined high-risk LARC (>cT3b, cN2+ or extramural venous invasion) randomised patients (1:1) to FOLFOX + Bevacizumab (Arm 1) or FOLFOXIRI + bevacizumab (Arm 2) every 14 days for 6 cycles prior to surgery. Patients were withdrawn if positron emission tomography (PET) standardised uptake value (SUV) after 3 cycles failed to decrease by >30% or increased compared to baseline. Primary endpoint was pathological complete response rate (pCR). Secondary endpoints included adverse events (AE) and toxicity. Neoadjuvant rectal (NAR) scores based on “T” and “N” downstaging were calculated.
Findings
Twenty patients aged 18–75 years were randomised. The trial stopped early because of poor accrual. Seventeen patients completed all 6 cycles of NACT. One stopped due to myocardial infarction, 1 poor response on PET (both received CRT) and 1 committed suicide. 11 patients had G3 AE, 1 G4 AE (neutropenia), and 1 G5 (suicide). pCR (the primary endpoint) was 0/10 for Arm 1 and 2/10 for Arm 2 i.e. 2/20 (10%) overall. Median NAR score was 14·9 with 5 (28%), 7 (39%), and 6 (33%) having low, intermediate, or high scores. Surgical morbidity was acceptable (1/18 wound infection, no anastomotic leak/pelvic sepsis/fistulae). The 24-month progression-free survival rate was 75% (95% CI: 60%–85%).
Interpretation
The primary endpoint (pCR rate) was not met. However, FOLFOXIRI and bevacizumab achieved promising pCR rates, low NAR scores and was well-tolerated. This regimen is suitable for testing as the novel arm against current standards of SCRT and/or CRT in a future trial.
Keyword: Oncology
1. Background
Preoperative CRT followed by TME has become the international standard treatment for patients with LARC. CRT improves local control, but has failed to enhance overall survival (OS). Tumor down-staging is achieved in only 50%, and a pCR in 10–25%. CRT delivers low doses of chemotherapy, and delays administration of effective systemic chemotherapy by 4–6 months Up to 30% of patients with LARC still subsequently develop metastatic disease [1]. Pelvic radiotherapy is associated with an increased risk of postoperative wound complications and long-term adverse late-effects (gastrointestinal, urological, psycho-sexual symptoms and chronic pain) and an increased risk of second malignancy. Recent improvements in the quality of surgery have also led to low local recurrence rates without radiation.
MRI can define the maximal extramural depth (EMD) of radial tumor spread from the breached muscularis propria in a sub-classification of T3 tumors i.e. mrT3a = <1 mm, mrT3b = 1·01-5·00 mm, mrT3c = 5·01-15·00 mm and mrT3d = >15·01 mm, the distance to the mesorectal fascia and extramural venous invasion (EMVI). Hence, risk adaptive strategies might be envisaged whereby chemotherapy or radiotherapy is selected according to the relative risks of local or distant recurrence.
In contrast to postoperative adjuvant chemotherapy, compliance with NACT is high [2]. In the Grupo Cáncer de Recto 3 study [3] 92% patients received full systemic doses in the induction arm prior to CRT, compared with only 51% in postoperative adjuvant arm (p = 0.0001).
In colon cancer, the earlier the adjuvant chemotherapy following surgery, the more effective it is [4]. In rectal cancer, the optimal time to start chemotherapy may be within 5–6 weeks [5], but surgical morbidity can delay delivery and increase the risk of distant metastases [6].
Triplet schedules (FOLFOXIRI) with or without biological agents demonstrate high response rates in metastatic disease, which led us to randomise between FOLFOX and bevacizumab, and FOLFOXIRI and bevacizumab in the neoadjuvant setting. The BACCHUS study examines the potential benefit of doublet (FOLFOX) and triplet (FOLFOXIRI) chemotherapy regimens in combination with bevacizumab, and investigated whether intensive NACT alone without radiotherapy can be tolerable with acceptable toxicity. We hoped to achieve a pCR rate in primary rectal cancer sufficient to warrant further investigation in a phase III study comparing current standards of SCRT and/or 5FU based chemoradiotherapy with NACT alone.
2. Methods
This multicentre, open-label, prospective, randomised phase II study (NCT01650428) was approved by Riverside l Research Ethics Committee (ref:12/LO/1158), and sponsored by University College London. All participants provided written informed consent before inclusion in the trial.
In terms of the population of the study, patients with histologically confirmed MRI-defined high-risk resectable adenocarcinoma of the rectum and World Health Organisation (WHO) performance status of 0–1 with no distant metastatic disease were recruited. High-resolution thin-slice MRI (3 mm) was mandated for loco-regional staging. Other eligibility criteria included distal tumour 4–12 cm from the anal verge, with a predicted penetration of the muscularis propria by >1 mm extension (i.e. minimum of cT3b) or T4a; cN2, and EMVI. Patients with tumour or suspected involved lymph nodes extending to within ≤1 mm from, or breaching the circumferential resection margin (CRM) were excluded.
The study objective was to demonstrate the efficacy and safety of NACT alone in LARC. The primary endpoint was pCR. Secondary endpoints included safety, tolerability and feasibility of delivering FOLFOX/FOLFOXIRI/Bevacizumab; overall response rate (ORR), CRM negative (R0) resection rate, T and N stage down-staging, Progression-free survival (PFS), Disease-free Survival (DFS), OS, local control, 1 year colostomy rate, adverse events, compliance with chemotherapy treatment, and tumour regression grade (TRG).
The completion rate of the neo-adjuvant treatment, pCR frequency, number of patients with a R0 resection were recorded. Adverse events were recorded and graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI-CTCAE) version v4.03.
We also used NAR score as a composite endpoint [7, 8] using a weighted combination of final pathological nodal stage (ypN) and down-staging of T stage (mrT stage to ypT stage) representing a pseudo-continuous variable with 24 possible discrete scores, ranging from 0–100 [8].
Patient randomisation was performed centrally at the UCL trials centre and patients were randomly assigned to one of two treatment arms using a minimisation algorithm in a 1:1 ratio and stratified according to treating centre, gender and presence or absence of EMVI.
The primary endpoint was pCR (ypT0N0). We considered a likely pCR rate of 15–20 % after standard fluoropyrimidine–based CRT and 4% with radiotherapy alone. The study was powered on the assumption that NACT would achieve a pCR rate of 20%. Hence, with a type I error α = 0·05 and a type II error β = 0·8, then 27 patients for the FOLFOX/Bevacizumab arm were required, and the same number for the FOLFOXIRI/Bevacizumab arm. Assuming 10 % of patients will be non-evaluable, 30 patients were to be recruited to each arm (i.e. a total of 60 patients). NACT would be considered promising and worth exploring further in a randomised phase III trial if at least 4/27 pCRs (15%) were observed in each arm. The trial was not powered to perform any direct comparisons between the two arms.
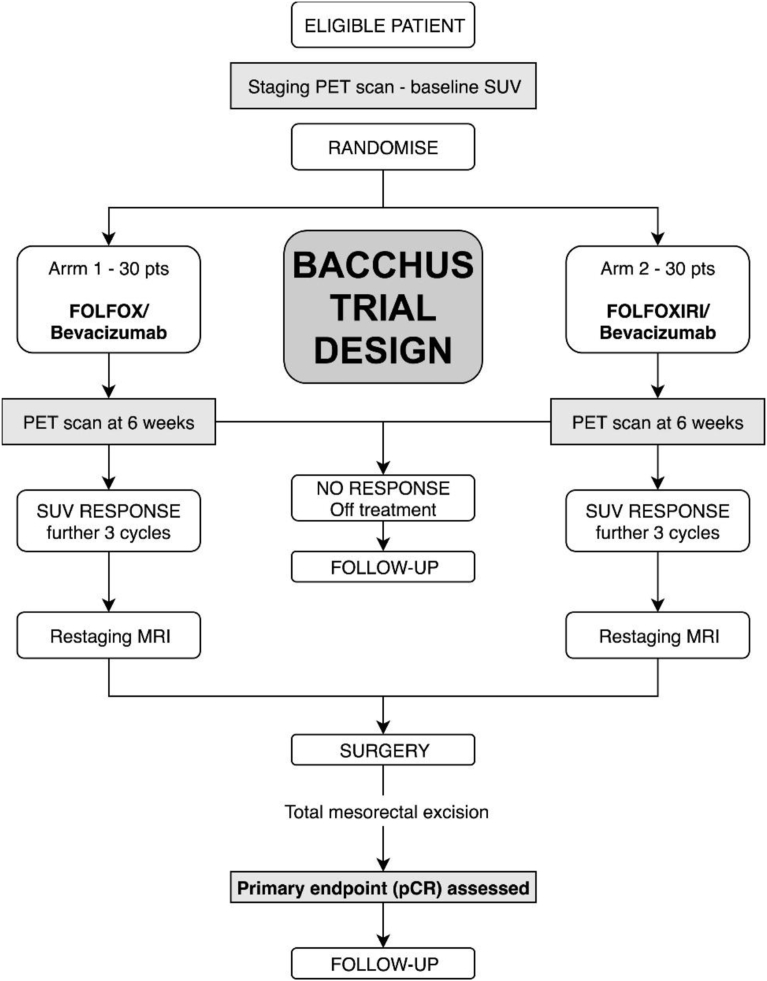
Both arms delivered chemotherapy (FOLFOXIRI or FOLFOX) preoperatively with bevacizumab every 2 weeks to a total of 6 cycles (bevacizumab omitted during cycle 6 i.e. the final chemotherapy cycle before resection). See Fig. 1 for Treatment schedule.
Fig. 1.
Treatment schedule as planned.
Arm 1 –consisted of Bevacizumab 5 mg/kg IV over 30–90 minutes (cycles 1–5), Oxaliplatin 85 mg/m2 IV over 2 hours, Folinic acid 350 mg IV over 2 hours, 5FU 3200 mg/m2 IV continuous infusion over 48 hours given every 2 weeks for 12 weeks.
Arm 2–consisted of Bevacizumab 5 mg/kg IV over 30–90 minutes (cycles 1–5), Irinotecan 165 mg/m2 IV over 1 hour, Oxaliplatin 85 mg/m2 IV over 2 hours, Folinic acid 350 mg IV over 2 hours and 5FU 3200 mg/m2 IV continuous infusion over 48 hours given every 2 weeks for 12 weeks.
Dose modifications for toxicity were permitted according to specified protocol guidelines. Adverse events were monitored from informed consent to 3 months after surgery.
The assessment of response and progression was based on investigator-reported measurements, which were subsequently centrally reviewed. Evaluation of SUV changes in primary tumour with PET/CT was mandated prior to cycle 4. Response was defined as a decrease in SUV by ≥30% after 3 cycles compared to baseline. Patients who failed to respond came off trial, and were treated at investigators discretion, but were expected to receive CRT prior to surgery. Patients also underwent clinical response evaluation with MRI prior to cycle 4 and prior to surgery according to the Response Evaluation Criteria in Solid Tumours (RECIST 1.1).
Surgery was specified as TME and performed between a minimum of 6 weeks after the end of chemotherapy or 8 weeks after bevacizumab (>2 half-lives of bevacizumab) to a maximum of 10 weeks after last administration of trial treatment. Surgical morbidity was recorded with particular emphasis on anastomotic leakage and perineal wound complications.
Pathological evaluation of resected specimens was performed according to the 3rd edition of the Royal College of Pathologists' guidelines using the 5th edition of TNM [9]. In addition, to compare with mrT-substaging, ypT3 disease was sub-divided into ypT3a, ypT3b, ypT3c and ypT3d disease according to the radial outgrowth from the breached muscularis propria. pTRG is presented as data categorised into five groups- pTRG 0, pTRG 1, pTRG 2, pTRG 3, and pTRG 4 using the Dworak categories. Also, the quality of the resected specimen was evaluated with separate scoring for the mesorectum and the anal canal (in abdominoperineal excisions). pCR was defined as complete regression in the primary tumour and associated lymph nodes (ypT0 ypN0) following embedding of the entire scar and examination of at least three deeper levels per block, in keeping with RCPath guidance.
Postoperative adjuvant chemotherapy was permitted according to local protocols. Patients were followed every 6 months to 42 months after randomisation, to document recurrence and survival. Postoperative investigations/surveillance were performed according to local practice, but with a minimum of 2 CT scans in the first 2 years. Progression-free survival (PFS) was defined as time from randomisation to disease progression or death, whichever occurs first. Disease-free survival (DFS) was defined as the time from surgery with complete resection (R0) to relapse, second colorectal primary or death from any cause, whichever occurred first.
3. Results
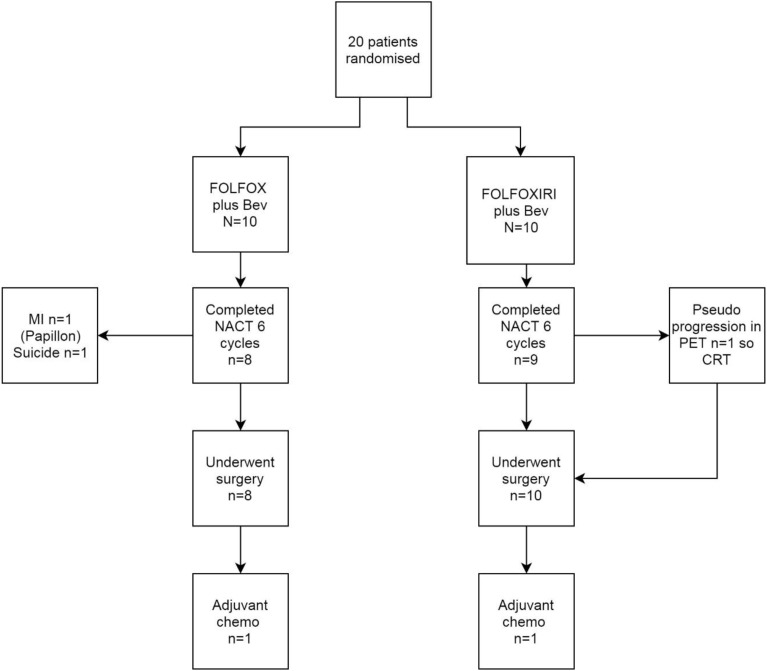
The study intended to recruit 27 patients in each arm. In the event seven sites in the UK randomised 20 patients (10 in each arm) between May 2013 and August 2015. Median age was 58 years. The trial stopped early because of poor accrual. Patient baseline characteristics in the two groups are similar (Table 1). See Fig. 2 for Consort diagram.
Table 1.
Baseline characteristics.
| Baseline characteristics | Arm 1 |
Arm 2 |
Total |
|---|---|---|---|
| FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
||
| N = 10 |
N = 10 |
N = 20 |
|
| N (%) | N (%) | N (%) | |
| Age in years | |||
| Median (range) | 58 (36–70) | 58 (34–70) | 58 (34–70) |
| Sex | |||
| Female | 4 (40%) | 4 (40%) | 8 (40%) |
| Male | 6 (60%) | 6 (60%) | 12 (60%) |
| cT stage | |||
| mrT3b | 5 (50%) | 5 (50%) | 10 (50%) |
| mrT3c | 5 (50%) | 3 (30%) | 8 (40%) |
| mrT3d | 0 (0%) | 1 (10%) | 1 (5%) |
| mrT4 (peritoneal involvement) | 0 (0%) | 1 (10%) | 1 (5%) |
| cN stage | |||
| mrN0 | 4 (40%) | 0 (0%) | 4 (20%) |
| mrN1 | 2 (20%) | 5 (50%) | 7 (35%) |
| mrN2 | 4 (40%) | 5 (50%) | 9 (45%) |
| mrEMVI | |||
| Absent | 5 (50%) | 6 (60%) | 11 (55%) |
| Present | 5 (50%) | 4 (40%) | 9 (45%) |
| ECOG performance status | |||
| Fully active (0) | 8 (80%) | 8 (80%) | 16 (80%) |
| Ambulatory (1) | 2 (20%) | 2 (20%) | 4 (20%) |
Note: All patients were Class I for New York Heart Association Classification at baseline.
Fig. 2.
Bacchus consort diagram.
A total of 8 (80%) patients in Arm 1 and 9 (90%) in Arm 2 completed all 6 intended cycles of NACT. Three patients discontinued treatment early: one patient (Arm 2) had not responded after 3 cycles, one (Arm 1) stopped treatment because of a myocardial infarction during cycle 1 and one (Arm 1) committed suicide after 4 cycles of treatment. There were no toxicity related deaths. The percentage dose delivered in relation to the planned dose for chemotherapy and bevacizumab was good (see compliance in Table 2). Table 3 shows the percentage total dose delivered by chemotherapy drug. Median duration of NACT treatment was 72·5 days (range: 2–107) and 86·5 days (range: 30–117) in Arm 1 and Arm 2 respectively. Table 4 shows that treatment delays due to adverse events were more common amongst patients in Arm 2 (60% versus 40%) but this did not impact on the delivered total dose for any patient.
Table 2.
Treatment compliance.
| Treatment summary | Arm 1 |
Arm 2 |
|---|---|---|
| FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
|
| N = 10 | N = 10 | |
| Total number of chemo cycles given | ||
| 1 | 1 (10%) | 0 (0%) |
| 3 | 0 (0%) | 1 (10%) |
| 4 | 1 (10%) | 0 (0%) |
| 6 | 8 (80%) | 9 (90%) |
| Total number of Bevazizumab cycles given | ||
| 1 | 1 (10%) | 0 (0%) |
| 3 | 0 (0%) | 1 (10%) |
| 4 | 1 (10%) | 0 (0%) |
| 6 | 8 (80%) | 9 (90%) |
| Early treatment discontinuation | ||
| No | 8 (80%) | 9 (90%) |
| Yes | 2 (20%) | 1 (10%) |
| SAE (Myocardial infarction) | 1 (10%) | 0 (0%) |
| No response following 3 cycles | 0 (0%) | 1 (10%) |
| Suicide | 1 (10%) | 0 (0%) |
Table 3.
Percentage total dose delivered by chemotherapy drug.
| Percentage total dose delivered | Arm 1 |
Arm 2 |
|---|---|---|
| FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
|
| N = 10 |
N = 10 |
|
| Median (range) | Median (range) | |
| 5FU | 100% (16·7%–100%) | 100% (50%–100%) |
| Bevacizumab | 100% (20%–100%) | 100% (60%–100%) |
| Folinic acid | 100% (16·7%–100%) | 100% (50%–100%) |
| Oxaliplatin | 100% (16·7%–100%) | 95·8% (50%–100%) |
| Irinotecan | Not applicable | 91·7% (50%–100%) |
Table 4.
Reasons for chemotherapy delays.
| Reasons for chemotherapy delays | Arm 1 |
Arm 2 |
|---|---|---|
| FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
|
| N = 10 | N = 10 | |
| Administrative reason/error | 3 (30%) | 4 (40%) |
| Patient choice | - | 3 (30%) |
| Neutropenia | 3 (30%) | 6 (60%) |
| Febrile neutropenia | - | 1 (10%) |
| Respiratory tract infection | - | 1 (10%) |
| Thrombocytopenia | 2 (20%) | - |
| Other adverse event | 1 (10%) | - |
| Any adverse event related reason | 4 (40%) | 6 (60%) |
| Any reason | 6 (60%) | 8 (80%) |
Table 5 lists all the treatment-related grade 1–4 adverse events during the study. Twelve patients had at least 1 Grade 3 adverse event, and two patients G4 (one G4 neutropenia). Table 6 shows the Acute Toxicity from NACT Bevacizumab.
Table 5.
Worst grade experienced during the study (grade 1 & 2 and grade 3 & 4) by arm and by treatment.
| Worst grade experienced during the study CTCAE term v4.03 | Grade 1 & 2 |
Grade 3 & 4 |
||
|---|---|---|---|---|
| Arm 1 |
Arm 2 |
Arm A |
Arm B |
|
| FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
|
| N = 10 | N = 10 | N = 10 | N = 10 | |
| Blood and lymphatic system disorders | 5 (50%) | 8 (80%) | - | 2 (20%) |
| Anemia | 5 (50%) | 9 (90%) | - | - |
| Bone marrow hypocellular | - | - | - | 1 (10%) |
| Febrile neutropenia | 1 (10%) | 1 (10%) | - | 1 (10%) |
| Leukocytosis | - | 2 (20%) | - | - |
| Cardiac disorders | - | - | 1 (10%) | - |
| Acute coronary syndrome | - | - | 1 (10%) | - |
| Atrial fibrillation | 1 (10%) | - | - | - |
| Eye disorders | 1 (10%) | - | - | - |
| Blurred vision | 1 (10%) | - | - | - |
| Gastrointestinal disorders | 5 (50%) | 8 (80%) | 5 (50%) | 2 (20%) |
| Abdominal distension | - | 1 (10%) | - | - |
| Abdominal pain | 4 (40%) | 8 (80%) | 2 (20%) | 2 (20%) |
| Anal pain | 1 (10%) | - | - | - |
| Colonic obstruction | - | - | 1 (10%) | - |
| Constipation | 2 (20%) | 8 (80%) | 1 (10%) | - |
| Diarrhea | 5 (50%) | 8 (80%) | 2 (20%) | - |
| Gastroesophageal reflux disease | 1 (10%) | 1 (10%) | - | - |
| Mucositis oral | 4 (40%) | 3 (30%) | - | - |
| Nausea | 5 (50%) | 8 (80%) | - | - |
| Rectal haemorrhage | 1 (10%) | - | - | - |
| Rectal pain | 1 (10%) | - | - | - |
| Small intestinal obstruction | - | - | 1 (10%) | - |
| Vomiting | 5 (50%) | 5 (50%) | 1 (10%) | - |
| General disorders and administration site conditions | 8 (80%) | 8 (80%) | - | 2 (20%) |
| Fatigue | 8 (80%) | 8 (80%) | - | 2 (20%) |
| Fever | - | 1 (10%) | - | - |
| Pain | 1 (10%) | 1 (10%) | - | - |
| Immune system disorders | 1 (10%) | 1 (10%) | - | - |
| Allergic reaction | 1 (10%) | 1 (10%) | - | - |
| Infections and infestations | 3 (30%) | 4 (40%) | - | 1 (10%) |
| Bronchial infection | 1 (10%) | - | - | - |
| Catheter related infection | 1 (10%) | 1 (10%) | - | - |
| Pelvic infection | 1 (10%) | - | - | - |
| Sepsis | 1 (10%) | - | - | - |
| Upper respiratory infection | - | 3 (30%) | - | - |
| Urinary tract infection | - | 2 (20%) | - | - |
| Wound infection | - | 1 (10%) | - | 1 (10%) |
| Injury, poisoning and procedural complications | 2 (20%) | 1 (10%) | - | 1 (10%) |
| Wound complication | 2 (20%) | 1 (10%) | - | 1 (10%) |
| Wound dehiscence | - | - | - | 1 (10%) |
| Investigations | 7 (70%) | 7 (70%) | 1 (10%) | 3 (30%) |
| Activated partial thromboplastin time prolonged | 2 (20%) | 3 (30%) | - | - |
| Alanine aminotransferase increased | 4 (40%) | 4 (40%) | - | - |
| Alkaline phosphatase increased | 1 (10%) | 3 (30%) | - | - |
| Aspartate aminotransferase increased | 3 (30%) | 3 (30%) | - | - |
| Blood bilirubin increased | 1 (10%) | 1 (10%) | - | - |
| Creatinine increased | 1 (10%) | 2 (20%) | - | - |
| GGT increased | 2 (20%) | 4 (40%) | - | 1 (10%) |
| Lymphocyte count decreased | 1 (10%) | 3 (30%) | - | - |
| Lymphocyte count increased | - | 1 (10%) | - | - |
| Neutrophil count decreased | 4 (40%) | 6 (60%) | 1 (10%) | 2 (20%) |
| Other investigations | - | 1 (10%) | - | - |
| Platelet count decreased | 3 (30%) | 4 (40%) | - | - |
| Weight loss | 1 (10%) | - | - | - |
| White blood cell decreased | 3 (30%) | 5 (50%) | - | - |
| Metabolism and nutrition disorders | 4 (40%) | 7 (70%) | - | 1 (10%) |
| Anorexia | 2 (20%) | 5 (50%) | - | - |
| Hypoalbuminemia | 1 (10%) | 3 (30%) | - | - |
| Hypokalemia | 1 (10%) | 2 (20%) | - | - |
| Hyponatremia | 2 (20%) | 2 (20%) | - | 1 (10%) |
| Musculoskeletal and connective tissue disorders | 1 (10%) | 2 (20%) | - | - |
| Arthralgia | - | 1 (10%) | - | - |
| Back pain | - | 1 (10%) | - | - |
| Other musculoskeletal and connective tissue disorders | 1 (10%) | - | - | - |
| Nervous system disorders | 7 (70%) | 8 (80%) | - | - |
| Dysesthesia | 2 (20%) | 4 (40%) | - | - |
| Headache | - | 2 (20%) | - | - |
| Paresthesia | 1 (10%) | - | - | - |
| Peripheral sensory neuropathy | 6 (60%) | 8 (80%) | - | - |
| Psychiatric disorders | 1 (10%) | 1 (10%) | - | - |
| Anxiety | 1 (10%) | - | - | - |
| Insomnia | - | 1 (10%) | - | - |
| Renal and urinary disorders | 3 (30%) | 2 (20%) | 1 (10%) | - |
| Acute kidney injury | - | - | 1 (10%) | - |
| Proteinuria | 2 (20%) | 1 (10%) | - | - |
| Urinary retention | 1 (10%) | 1 (10%) | - | - |
| Reproductive system and breast disorders | 2 (20%) | 2 (20%) | - | - |
| Ejaculation disorder | 1 (10%) | - | - | - |
| Pelvic pain | 1 (10%) | 2 (20%) | - | - |
| Respiratory, thoracic and mediastinal disorders | 1 (10%) | 2 (20%) | - | - |
| Cough | - | 1 (10%) | - | - |
| Dyspnea | - | 1 (10%) | - | - |
| Epistaxis | 1 (10%) | - | - | - |
| Laryngospasm | 1 (10%) | - | - | - |
| Other respiratory, thoracic and mediastinal disorders | 1 (10%) | 1 (10%) | - | - |
| Pharyngeal mucositis | - | 1 (10%) | - | - |
| Sore throat | - | 1 (10%) | - | - |
| Skin and subcutaneous tissue disorders | - | 4 (40%) | - | - |
| Alopecia | - | 4 (40%) | - | - |
| Palmar-plantar erythrodysesthesia syndrome | - | 1 (10%) | - | - |
| Vascular disorders | 1 (10%) | 2 (20%) | 1 (10%) | 5 (50%) |
| Hot flashes | - | 1 (10%) | - | - |
| Hypertension | 1 (10%) | 2 (20%) | 1 (10%) | 4 (40%) |
| Hypotension | - | 1 (10%) | - | 1 (10%) |
| Any adverseevent | 4 (40%) | 2 (20%) | 6 (60%) | 8 (80%) |
Table 6.
Toxicity from BevacizumaB (BVZ) within NACT – acute toxicity.
| CTCAE 4.03 AE term worst grade | Grade 1–2 |
Grade 3–5 |
||
|---|---|---|---|---|
| FOLFOX/BVZ | FOLFOXIRI/BVZ | FOLFOX/BVZ | FOLFOXIRI/BVZ | |
| Bleeding/haemorrhage | ||||
| Rectal haemorrhage | 1 | - | - | - |
| Fistula | - | - | - | - |
| None | - | - | - | - |
| Gastrointestinal perforation | - | - | - | - |
| None | - | - | - | - |
| Heartfailure | - | - | - | - |
| Acute coronary syndrome | - | - | 1 | - |
| Hypertension (grades 3 – 5 only) | - | - | - | - |
| Hypertension | - | - | 1 | 4 |
| Proteinuria | - | - | - | - |
| Proteinuria | 2 | 1 | - | - |
| Reversible Posterior Leukoencephalopathy Syndrome (RPLS) | - | - | - | - |
| None | - | - | - | - |
| Thromboembolic events (arterial andvenous) | - | - | - | - |
| None | - | - | - | - |
| Woundcomplication | - | - | - | - |
| Wound complication | 2 | 1 | - | 1 |
| Wound dehiscence | - | - | - | 1 |
| Pelvic sepsis | 1 | - | - | - |
Note: numbers represent frequency of patients.
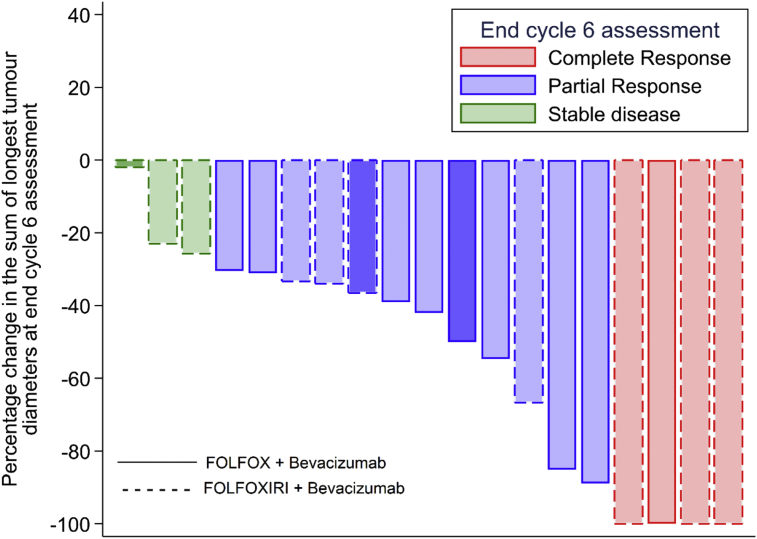
A best response assessment (RECIST v1.1) at end of cycle 3 and/or end cycle 6 was available for 19 patients. Of the patients who had at least one response assessment, a total of 4 (21%) achieved a complete response (1 FOLFOX and 3 FOLFOXIRI), 12 (63%) achieved a partial response (8 FOLFOX and 4 FOLFOXIRI), and 3 (16%) had stable disease as their best response (all FOLFOXIRI). There is no evidence of association between the best overall response obtained and treatment group (Fishers' p = 0·09). The waterfall plot Fig. 3 shows the percentage change in the sum of the longest tumour diameters observed along with the clinical response classification at end of NACT (cycle 6) compared to baseline. Of the 18 patients assessed by PET/CT after cycle 3 in terms of the SUV, 7/8 (88%) in FOLFOX and 8/10 (80%) in FOLFOXIRI had a reduction equal or greater than 30% in the SUV after cycle 3 compared with baseline.
Fig. 3.
Waterfall plot of changes in the tumour size at end of cycle 6 assessment by treatment. Only 16 patients had an assessment for response at end of cycle 6. The patients marked in bold colours (3 patients) did not have an assessment for response at end of cycle 6. For these patients, the response showed in this graph was the one assessed at end of cycle 3. There are 19 patients with a response assessment at either end of cycle 3 or cycle 6.
Seventeen patients had tumour in the mid-rectum (>5–10 cm) and 3 in the upper rectum (from >10 cm). Radical surgery was performed in 18/20 patients: anterior resection (14), abdominoperineal excision of the rectum (3) and Hartmann' procedure (1). Surgery was not performed in one patient due to suicide and one patient refused. Both had received FOLFOX and bevacizumab. Amongst patients who had surgery in the FOLFOX arm, the median time from end of chemotherapy to surgery was 59·5 days (range: 43–154 days). All patients in the FOLFOXIRI arm had surgery with a median interval of 52·5 days (40–191 days). Of the 18 patients who proceeded to surgery –see Fig. 2 for consort diagram – 17/18 (94%) achieved an R0 resection. A total of 2/18 (11%) patients had a pCR (both in the FOLFOXIRI arm).
There were no life-threatening episodes from surgical morbidity (Table 7), and no post-surgical deaths, although one patient developed an adhesional bowel obstruction 1 month after surgery. There was no evidence the addition of bevacizumab adversely affected surgical morbidity.
Table 7.
Complications within 3 months post-surgery by arm and severity.
| Reported post-surgical complications (48 hours, 1 month or 3 months post-surgery | Grade 1 & 2 |
Grade 3 & 4 |
||
|---|---|---|---|---|
| FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
|
| N | N | N | N | |
| Post-surgical complication | ||||
| Abdominal pain | 1 | - | - | - |
| Bowel obstruction | - | - | 1 | - |
| Breakdown of perineal wounda | - | - | - | 1 |
| Diarrhoea | - | - | 1 | - |
| Hypotension | - | 2 | - | - |
| Loss of leg mobility | 1 | - | - | - |
| Parastomal hernia | 1 | - | - | - |
| Pelvic sepsis | 1 | - | - | - |
| Perineal hernia | - | - | - | 1 |
| Pulmonary complications | - | 1 | - | - |
| Urinary tract infection | - | 2 | - | - |
| Urinary retention | - | 1 | - | - |
| Vomiting | 1 | - | - | - |
| Wound infection/complication | 1 | - | - | 1 |
At 3 months post-surgery.
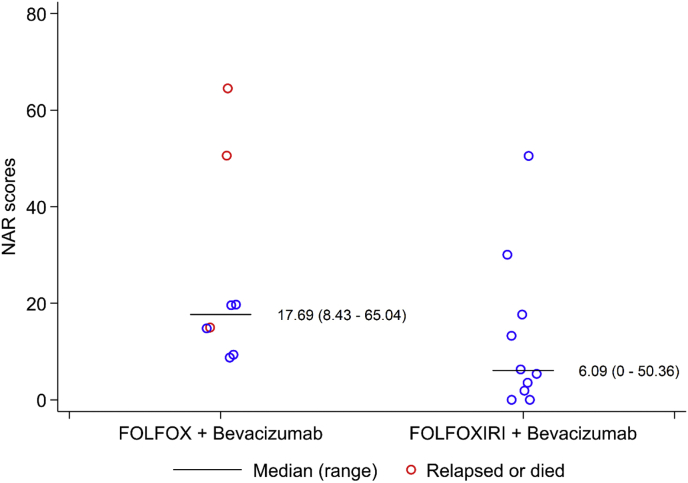
T and N down-staging were observed. The median NAR score in evaluable patients was 14·9 with 5 (28%), 7 (39%), and 6 (33%) patients having low, intermediate, and high scores. NAR scores after NACT are shown for each arm in Table 8. The observed median NAR score for patients receiving FOLFOX was higher in comparison to those receiving FOLFOXIRI but not statistically significant (FOLFOX: 17·69, range: 8·43–65·04; FOLFOXIRI: 6·09, range: 0–50·36, p = 0·07). A comparison of clinical stage, pathological stage and NAR scores is shown in Table 9, Fig. 4.
Table 8.
NAR status after NACT for each arm.
| NAR status | Arm 1 |
Arm 2 |
|---|---|---|
| FOLFOX + Bevacizumab |
FOLFOXIRI + Bevacizumab |
|
| N = 10 | N = 10 | |
| NAR status | ||
| Low (NAR<8) | 0 (0%) | 5 (50%) |
| Intermediate (NAR ≥8 – NAR ≤16) | 4 (40%) | 3 (30%) |
| High (NAR>16) | 4 (40%) | 2 (20%) |
| Not evaluablea | 2 (20%) | 0 (0%) |
Note: Patients 1 (Arm B FOLFOXIRI + Bevacizumab) and 17 (Arm A FOLFOX + Bevacizumab) did not receive all planned cycles of chemotherapy.
NAR score for patient 7 and 9 could not be calculated as they did not undergo surgery.
Table 9.
Clinical versus pathological staging and interval for NAR scoring.
| Id | Arm | Baseline |
Best OR | Wks from end of NACT to surgery | Resect | ypT | ypN | Regression | NAR | Relapsed or died | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cT stage | cN stage | MRI EMVI | ||||||||||
| 2 | 1 | T3c | N2 | Y | PR | 6·3 | R0 | ypT2 | pN1 | Good | 20·4 | NO |
| 3 | 1 | T3b | N2 | N | PR | 6·4 | R0 | ypT2 | pN0 | Minimal | 8·4 | NO |
| 6 | 1 | T3b | N2 | N | PR | 11·9 | R0 | ypT3a | pN0 | None | 15·0 | Yes |
| 7 | 1 | T3b | N0 | N | PR | Refused surgery | Yes | |||||
| 9 | 1 | T3c | N0 | Y | PR | No surgery suicide | Yes | |||||
| 11 | 1 | T3c | N0 | N | PR | 10·9 | R0 | ypT3a | pN0 | Good | 15·0 | NO |
| 14 | 1 | T3b | N1 | N | CR | 7·6 | R0 | ypT2 | pN0 | Moderate | 8·4 | NO |
| 16 | 1 | T3c | N2 | Y | PR | 6·6 | R2 | ypT4 | pN2 | Minimal | 65·0 | Yes |
| 17 | 1 | T3c | N0 | Y | PR | 22·1 | R0 | ypT2 | pN1 | Moderate | 20·4 | NO |
| 18 | 1 | T3b | N1 | Y | PR | 9·7 | R0 | ypT3a | pN2 | Minimal | 50·4 | Yes |
| 1 | 2 | T3c | N2 | Y | SD | 27·4 | R0 | ypT2 | pN1 | Moderate | 30·1 | NO |
| 4 | 2 | T3b | N1 | N | PR | 6·1 | R0 | ypT2 | pN0 | Moderate | 8·4 | NO |
| 5 | 2 | T3c | N2 | Y | PR | 7·9 | R0 | ypT3b | pN0 | Moderate | 15·0 | NO |
| 8 | 2 | T3b | N2 | N | CR | 9·4 | R0 | ypT0 | pN0 | Total | 0·9 | NO |
| 10 | 2 | T3b | N2 | Y | SD | 7·1 | R0 | ypT3c | pN0 | Minimal | 15·0 | NO |
| 12 | 2 | T4 | N1 | N | CR | 5·9 | R0 | ypT0 | pN0 | Total | 0 | NO |
| 13 | 2 | T3b | N1 | N | PR | 7·4 | R0 | ypT1 | pN0 | Moderate | 3·7 | NO |
| 15 | 2 | T3b | N1 | Y | CR | 8·7 | R0 | ypT1 | pN0 | Good | 3·7 | NO |
| 19 | 2 | T3c | N1 | N | SD | 7·4 | R0 | ypT3b | pN2 | Moderate | 50·4 | NO |
| 20 | 2 | T3d | N2 | Y | PR | 9·7 | R0 | ypT1 | pN0 | Good | 3·7 | NO |
Three patients discontinued treatment early. Patient 1 in FOLFOXIRI arm did not respond according to SUV after 3 cycles; patient 17 in the FOLFOX arm stopped treatment in cycle 1 due to myocardial infarction and patient 9 committed suicide after 4 cycles of treatment.
Arm 1 = FOLFOX plus Bevacizumab, Arm 2 = FOLFOXIRI plus Bevacizumab; cT stage = clinical Tumour stage (TNM); cN = clinical nodal stage (TNM); MR EMVI = extramural vascular invasion defined on staging MRI; OR = overall response; PR = clinical partial response; CR = clinical complete response; R0 = curative resection with margin >1 mm; ypT = pathological tumour T stage after treatment; ypN = pathological nodal stage after treatment; NAR = neoadjuvant rectal score.
Fig. 4.
NAR scores by treatment group. A nonparametric K-sample test on the equality of medians p-value is 0·066. This suggests no evidence of a difference in the medians of NAR scores between treatment groups at a 5% significance level.
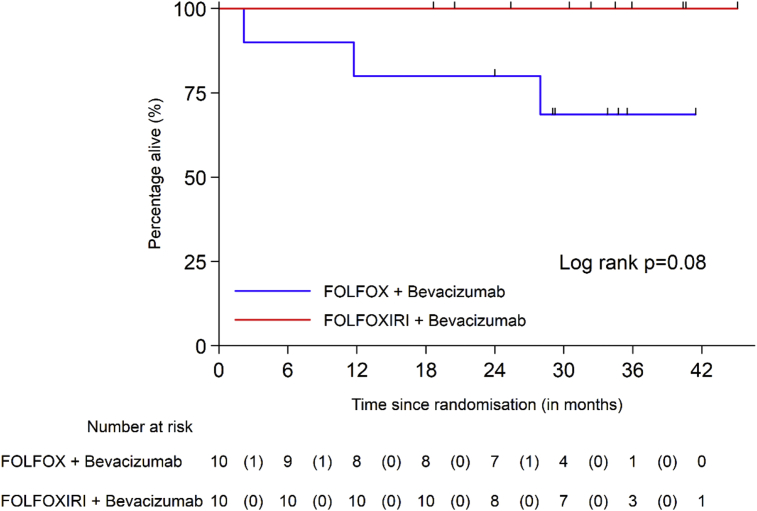
At the time of the analysis, the median follow-up time was 33·7 months In Arm 1, three deaths were reported, of which one was suicide and two due to disease progression, one of which related to a second malignancy (malignant melanoma). Two other patients in Arm 1 progressed, one patient refused surgery and received brachytherapy but subsequently relapsed at the primary site, and the other in liver. In Arm 1, the 2-year OS rate was 80% (95% CI: 41%–95%) and the 2-year PFS rate 60% (95% CI: 25%–83%).
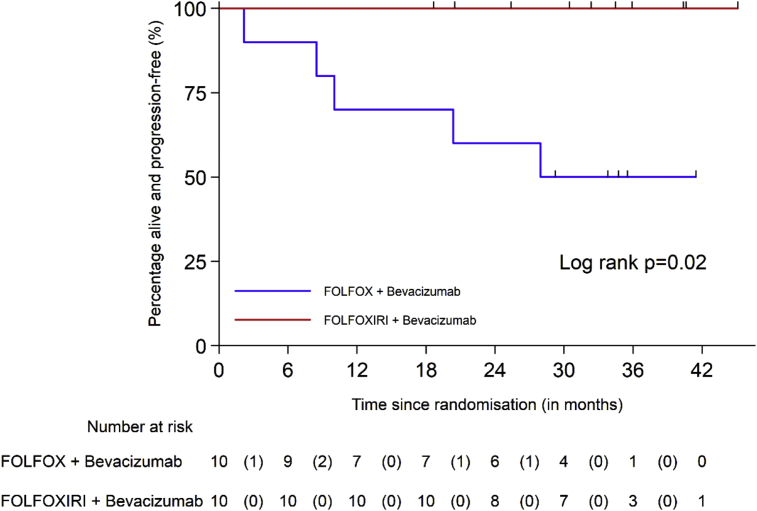
No deaths or progressions were reported in Arm 2. OS and PFS survival curves by arm are shown in Figs. 5 and 6.
Fig. 5.
Overall survival according to treatment arm.
Fig. 6.
Progression-free survival according to treatment arm.
The results of imaging with PET, mriTRG and MRI diffusion weighted imaging and RECIST, TRG and tumour cell density along with the translational results will be presented in a separate report.
4. Discussion
To our knowledge, the BACCHUS trial is the only NACT clinical trial which has investigated the combination of a triplet-chemotherapy backbone with bevacizumab in patients with rectal cancer. Because accrual ended early, neither of the 2 study arms met the primary endpoint of achieving a pCR of 4/27 (15%). On this basis, neither of the investigational strategies appear to merit further investigation. Nevertheless, the trial provides important data, not least because Arm 2 (FOLFOXIRI and bevacizumab) showed a pCR of 2/10 (20%) in a high-risk group where 5/10 (50%) had baseline mrEMVI (an independent prognostic factor for poor outcomes in rectal cancer).
The FOLFOXIRI plus bevacizumab arm is also promising in terms of RECIST response, pathological down-staging, pCR and the low NAR scores. Other retrospective data indicate better clinical and pathological responses after NACT combined with bevacizumab compared with NACT alone [10]. A recent meta-analysis of targeted agents added to neoadjuvant therapy in rectal cancer suggested that bevacizumab enhances the pCR rate with a pooled estimate of 27% (95% CI, 21–34%) [11].
We have shown that NACT with FOLFOX and bevacizumab or FOLFOXIRI and bevacizumab without preoperative SCRT or CRT in patients with localised but high-risk rectal cancer is safe, with acceptable toxicity. There have been concerns regarding excessive surgical morbidity with intensive NACT – if bevacizumab is administered, and particularly when combined with preoperative radiation, but in BACCHUS there was no increase in anastomotic leaks, pelvic sepsis or fistulae, even with the addition of bevacizumab. Neither diarrhoea nor neutropenia were enhanced with the addition of irinotecan in Arm 2 (FOLFOXIRI + Bevacizumab), although 4/10 experienced some hair loss.
The primary endpoint was pCR in the TME specimen. We had hoped the BACCHUS regimens would produce a pCR rate comparable to CRT, but only 2/18 (11%) achieved pCR (both received FOLFOXIRI + bevacizumab). FOLFOXIRI and bevacizumab appears active as 4/10 (40%) achieved a complete clinical response. Other studies of NACT alone with and without bevacizumab in rectal cancer have reported higher pCR rates after NACT alone with a range of 7%–25% (Table 10) [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24], but some have included more liberal entry criteria, less advanced tumours and less in-depth pathology assessment without a defined dissection protocol for pCR.
Table 10.
Studies of neoadjuvant chemotherapy without radiation.
| No of ptsa | Eligibility | NACT | Acute Toxicity | PCRb | Ro resection | Outcomes | |
|---|---|---|---|---|---|---|---|
| Ishii 2010 [12] | 26 | T3/T4 N0-2 | Irinotecan (80 mg/m2), FUFA days 1, 8, and 15 for 4 weeks | Not stated | 1/15 (7%) | Not stated | 5 year RFS 74% OS 84% |
| Fernandez-Martos 2014 [13] | 46 | T3 middle third tumors ≥2 mm from the mesorectal fascia | Capox + bev | 2 acute toxic deaths 13% rate of anastomotic leak higher than expected (1 death) | 9/46 (19·5%) | 96·4% | No data |
| Uehara 2011, 2013 [14,15] | 32 | T3 >5 mm, T4, N2, CRM involved/at risk | Capox + bev | Postop complication in 43% | 8/32 (12.5%) | 84·3% | No data |
| Schrag 2014 [16] | 32 | T2N1, T3 any N (not N2 bulky) Not T4 5–12 cm from anal verge | FOLFOX + bev (6 cycles bev 1–4) | 2 pts withdrew (angina arrhythmia) | 8/32 (25%) | 100% | RFS 92% OS 91% |
| AlGizawy 2015 [17] | 45 | C Stage II and III | 6 cycles of FOLFOX 6 | 3 pelvic collections 2 delayed wound healing | 8/45 (17·8% | No data | 3 year DFS 68% 3 year OS 81% |
| Hasegawa 2017 [18] (UMIN000005654) | 60 | C Stage II and III | mFOLFOX6) + bev or cetuximab, depending on KRAS status | Postop complication rate (≥grade 2) 21·7% |
10/60 (16·7%) | 98·3% | No data |
| Matsumoto 2015 [19] retro-spective | 15 | cT3/cT4a, cN+ | FOLFOX (60%) IRIS FOLFIRI | 3/15 (20%) grade 3/4 adverse events | 2/15 (13·3%) | 100% | 5-year RFS rate 66·7% and 62·6% in NAC non-NAC groups |
| Ueki 2016 [20] | 31 | Clinical stage II/III lower rectal cancer | XELOX | Grade 3–4 adverse events in 9/31 (31%). | 3/29 (10·3%) | 96·5% | No data |
| Kamiya 2016 [21] CORONA 1 trial phase II | 41 | cT3/T4 cN+ | XELOX | Major complication in 6/40 patients (15·0%). | 5/41 (12·2%) | 37/41 90·3% |
No data |
| FOWARC trial Deng 2016 [22] | 163 | MRI or CT + EUS stage II (T3-4/N0) or stage III (T1-4/N1-2), M0, <12 cm above anal verge | modified FOLFOX6 alone | Low | 10/152 (6·6%) | 136/152 (89%) | No data |
| FACT trial Koike 2017 [23] | 52 | T3 or T4 stage II/III rectal cancer | FOLFOX | Safe | 5/42 resected (11·9%) 5/52 overall | 91% | No data |
| GRECCAR 4 Rouanet 2017 [24] | 10 | Mri defined cT3 ≥ c, cT4 or predicted CRM ≤ 1 mm | FOLFIRINOX | Grade 3–4 toxicity in 7/11 (63·6%) | 1/10 (10%) | 10/10 (100%) | No data |
| BACCHUS present study | 20 | Mri defined high risk > T3b | Modified FOLFOX6 alone FOLFOXIRI plus Bevacizumab | 1 pelvic sepsis 2 wound infections no leaks |
2/20 10% |
17/18 (94%) resected | 2 year OS 80% |
Number entering study.
Number having had surgery.
The NAR score was developed from the NSABP-R04 trial data as a measure of down-staging and as a surrogate endpoint for clinical trials involving CRT [7]. Data from 1,479 patients within the NSABP R04 trial, showed that low, intermediate and high risk of death categories, based on tertiles of the NAR score, were significantly associated with 5-year OS (p < 0.0001) – giving values of 92%, 89%, and 68 %, respectively [7]. Others have also shown lower NAR scores correlate with improved 5-year OS (p < 0·0001) [25], and outperform pCR [26]. Further studies have shown 5-year overall survival of 84%, 71%, and 59% for low-, intermediate-, and high-risk NAR scores respectively (P = ·004) [27]. The NAR score after CRT has recently been validated with individual-level surrogacy according to Prentice criteria for DFS within the CAO/ARO/AIO-04 randomized phase 3 trial, and is approved by the National Cancer Institute as a surrogate primary endpoint in phase II rectal cancer clinical trials assessing neoadjuvant CRT. However, the NAR score has not previously been used to assess outcomes from NACT alone and will require validation.
In BACCHUS, median NAR score in evaluable pts was 14·9 with 5 (28%), 7 (41%), and 5 (30%) patients having low, intermediate, and high NAR scores. These compare favourably with a recent retrospective analysis in patients treated with preoperative CRT, which showed 193/522 (37·0%) had low, 183/522 (35·0%) intermediate, and 146/522 (28·0%) showed high NAR scores [28]. FOLFOXIRI and bevacizumab was particularly active in terms of the NAR score (See Table 6 and Fig. 3).
The strengths of BACCHUS reflect the MRI-defined high risk entry criteria, an intensive triplet chemotherapy regimen, the high quality of surgery and a clear definition of the primary endpoint. In this selected high-risk population (making up about 40% of rectal cancers overall) the risk of distant relapse predominates over local recurrence. FOLFOXIRI and bevacizumab is a highly active regimen. A recent meta-analysis of FOLFOXIRI-Bevacizumab studies involving 877 patients in colorectal cancer with initially unresectable metastatic disease reported an objective response rate of 69% (95% CI, 65%–72%; I2 = 25%) [29]. The Neoadjuvant FOLFOX 6 Chemotherapy With or Without Radiation in Rectal Cancer (FOWARC) trial also suggests that NACT causes less late functional effects than CRT [22, 30] Hence late effects may be expected to be less severe with NACT than after CRT.
However, there are limitations. The trial stopped early because of poor accrual after only 20 patients. Some centres were unwilling to forego CRT. The design of the BACCHUS trial could be criticised for the restrictive patient selection, which limits its applicability to the “real world”. An upper age limit of 75 years was mandated with PS 0–1, and current smokers were ineligible. The average of only 1–3 patients per year in individual centres could indicate a significant selection bias towards younger fitter patients.
In BACCHUS, a long interval (6–8 weeks) was mandated between NACT and surgery for safety reasons. The median interval from the end of cytotoxic treatment until surgery was 56 days, but from the final dose of bevacizumab was 72 days (range 20–107 days) in Arm 1 and 86 days (range 30–117 days) in Arm 2 respectively. This delay could have been responsible for less surgical morbidity, there were no anastomotic leaks or pelvic sepsis. However, this interval could also be criticized as being too long, allowing regrowth of the tumour in some patients.
The results of BACCHUS compare favourably with previous NACT studies. The Grupo Español Multidisciplinar en Cáncer Digestivo (Gemcad 0801) study achieved a 15% pCR with XELOX + bevacizumab [13] in an MRI defined population in 46 patients without any radiotherapy. mrEMVI, at baseline was defined in 23/46 (50%) patients compared with 9/20 (45%) in BACCHUS.
However, the GEMCAD 0801 trial reported a higher than expected anastomotic leak rate of 13%, with a 3- to 4-week interval (21–28 days) between completion of chemotherapy and surgery [13]. In a Japanese study, a total 10/30 (43%) patients with LARC treated with XELOX and bevacizumab NACT also developed surgical morbidities [15].
A feasibility study in a less restricted group of patients (WHO 0–2, no age limit) with clinical stage II-III rectal cancer (but not T4 tumours) used NACT alone with FOLFOX + Bevacizumab without radiotherapy [16]. R0 resection rate was the primary outcome and pCR was reported in 8/29 resected patients (27%). Surgical morbidity is not described but there was a single postoperative death attributed to dehydration from high-volume ileostomy output. The 4-year DFS rate was 84% and local recurrence was 0% [16].
Based on these results, a large multi-centre ongoing Phase III study (CALGB PROSPECT/Allianz N1048 trial) compares standard CRT against chemotherapy using FOLFOX, and examines the selective use of CRT, depending on response to FOLFOX alone (NCT01515787). The primary endpoints are time to local recurrence and DFS.
5. Conclusions
As the quality of TME improves, fewer patients with rectal cancer benefit from radiotherapy (which is a local treatment). Hence, the ‘blanket use’ of radiotherapy is outdated. We need to validate preoperative biomarkers, which predict a high risk of systemic recurrence such as EMVI, and can be imaged on MRI. The potential advantages of NACT in place of RT include the ability to reduce the risk of micrometastases, and to spare patients from the morbidity of pelvic radiotherapy. In future trials, we need to test these biomarkers to estimate prospectively the relative merits of CRT and NACT for the individual.
Previous phase II trials in NACT have had heterogeneous inclusion criteria, few patients and uncertain surgical and MRI quality. The BACCHUS trial shows the delivery of FOLFOXIRI + Bevacizumab is feasible, safe, and effective in MRI defined high-risk LARC. This triplet combination allows early exposure to an effective systemic regimen, without impacting on compliance or surgical morbidity. Early oncological outcomes in this small number of patients seem promising even in the context of adverse features with EMVI. These findings support ongoing efforts to shift systemic treatments in LARC into the neoadjuvant setting and suggest that delivering neoadjuvant chemotherapy could be as effective as chemoradiation. If high proportions of complete responses and similar NAR scores can be replicated, high-risk patients with locally advanced rectal cancer could have improved survival and simultaneously avoid the harmful effects of pelvic radiation. For this reason, FOLFOXIRI + bevacizumab should be explored in a large phase III trial in patients at high risk of systemic relapse and low risk of local relapse against the current standard of routine SCPRT or CRT, and if validated can be translated into an alternative in everyday practice.
Declarations
Author contribution statement
Rob Glynne-Jones: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Marcia Hall, Mark Harrison: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Andre Lopes, Sarah Pearce, Nicholas West, Philip Quirke, Sandy Beare, Natasha Hava, Marian Duggan: Analyzed and interpreted the data; Wrote the paper.
Vicky Goh, Wailup Wong: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sam Bosompem: Conceived and designed the experiments; Wrote the paper.
John Bridgewater, Ian Chau, Harpreet Wasan, Lucinda Melcher: Performed the experiments; Wrote the paper.
Brendan Moran: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
This work was supported by CRUK and Roche Products Ltd. CRUK funded the trial. Roche products provided bevacizumab and funded PET scans.
Competing interest statement
The authors declare the following conflict of interests:
The trial was funded by CRUK. Roche products provided the bevacizumab and extra funding for PET scans.
Rob Glynne-Jones has received honoraria from Roche, Sanofi-Aventis, Merck KgaA, Eli-Lilly and Amgen. He has received research funding from Merck, KgaA, Roche and Sanofi.
Ian Chau has participated in advisory boards and provided consultancy for Bristol Myers Squibb, Roche, Sanofi Oncology, Merck Serono, Eli-Lilly, Novartis and Gilead Science. He has received honoraria from Sanofi-Oncology, Eli-Lilly and Taiho. He has received research funding from Sanofi-Oncology, Roche, Novartis and Merck-Serono.
Marcia Hall has received honoraria for advisory boards and consultancy from Roche, Clovis Oncology, Astra Zeneca and Tesaro.
Philip Quirke and Nicholas West are supported by programme grants from Yorkshire Cancer Research.
Philip Quirke is a National Institute of Health Research Senior Investigator and has received honoraria from Amgen. Consultancy from Roche, Eisai. Research funding from Roche and Amgen.
Additional information
The clinical trial described in this paper was registered at clinicaltrials.gov under the registration number NCT01650428.
Acknowledgements
The Pathology and Tumour Biology laboratory at the University of Leeds is supported by grants from Yorkshire Cancer Research, the Pathological Society of Great Britain and Ireland, the Academy of Medical Sciences, The Medical Research Council and a National Institute of Health Research Senior Investigator Award.
We thank all the patients who participated in this trial. We are grateful for the hard work of the research team from each of the participating centres and the UCL trial centre. We also thank members of the Independent Data Monitoring Committee and the Independent Trial Steering Committee for their oversight of the trial.
Roche Products Ltd. was not involved in the preparation, drafting, or editing of this manuscript. Roche Products Ltd. has conducted a factual accuracy check on the final article, but any decisions to incorporate comments were made solely at the discretion of the authors.
References
- 1.Sauer R., Liersch T., Merkel S. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012 Jun 1;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 2.Dewdney A., Cunningham D., Tabernero J. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J. Clin. Oncol. 2012;30(14):1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Martos C., Pericay C., Aparicio J., Salud A., Safont M., Massuti B. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J. Clin. Oncol. 2010;28(5):859–865. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 4.Biagi J.J., Raphael M.J., Mackillop W.J. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 5.Gresham G., Cheung W.Y., Speers C., Woods R., Kennecke H. Time to adjuvant chemotherapy and survival outcomes among patients with stage 2 to 3 rectal cancer treated with preoperative chemoradiation. Clin. Colorectal Cancer. 2015 Mar;14(1):41–45. doi: 10.1016/j.clcc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Tevis S.E., Kohlnhofer B.M., Stringfield S. Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis. Colon Rectum. 2013 Dec;56(12):1339–1348. doi: 10.1097/DCR.0b013e3182a857eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yothers G., George T.J., Petrelli N.J. Neoadjuvant rectal cancer (RC) score to predict survival: potential surrogate endpoint for early phase trials. J. Clin. Oncol. 2014;32(5s) (suppl; abstract 3533) [Google Scholar]
- 8.George T.J., Jr., Allegra C.J., Yothers G. Neoadjuvant rectal (NAR) score: a new surrogate endpoint in rectal cancer clinical trials. Curr. Colorectal Cancer Rep. 2015;11(5):275–280. doi: 10.1007/s11888-015-0285-2. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobin L.H., Wittekind Ch. fifth ed. Wiley–Liss; New York: 1997. UICC TNM Classification of Malignant Tumors. [Google Scholar]
- 10.Arimoto A., Uehara K., Tsuzuki T. Role of bevacizumab in neoadjuvant chemotherapy and its influence on microvessel density in rectal cancer. Int. J. Clin. Oncol. 2015 Oct;20(5):935–942. doi: 10.1007/s10147-015-0818-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhong X., Wu Z., Gao P., Shi J., Sun J., Guo Z., Wang Z., Song Y. The efficacy of adding targeted agents to neoadjuvant therapy for locally advanced rectal cancer patients: a meta-analysis. Cancer Med. 2018 Mar;7(3):565–582. doi: 10.1002/cam4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii Y., Hasegawa H., Endo T. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur. J. Surg. Oncol. 2010;36(11):1061–1065. doi: 10.1016/j.ejso.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Martos C., Brown G., Estevan Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014 Oct;19(10):1042–1043. doi: 10.1634/theoncologist.2014-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uehara K., Ishiguro S., Sakamoto E. Phase II trial of neoadjuvant chemotherapy with XELOX plus bevacizumab for locally advanced rectal cancer. Jpn. J. Clin. Oncol. 2011;41(8):1041–1044. doi: 10.1093/jjco/hyr084. [DOI] [PubMed] [Google Scholar]
- 15.Uehara K., Hiramatsu K., Maeda A. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II trial. Jpn. J. Clin. Oncol. 2013;43:964–971. doi: 10.1093/jjco/hyt115. [DOI] [PubMed] [Google Scholar]
- 16.Schrag D., Weiser M.R., Goodman K.A., Gonen M., Hollywood E., Cercek A. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J. Clin. Oncol. 2014 Feb 20;32(6):513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Gizawy S.M., Essa H.H., Ahmed B.M. Chemotherapy alone for patients with stage II/III rectal cancer undergoing radical surgery. Oncologist. 2015 Jul;20(7):752–757. doi: 10.1634/theoncologist.2015-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa S., Goto S., Matsumoto T. A multicenter phase 2 study on the feasibility and efficacy of neoadjuvant chemotherapy without radiotherapy for locally advanced rectal cancer. Ann. Surg. Oncol. 2017 Nov;24(12):3587–3595. doi: 10.1245/s10434-017-5967-3. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T., Hasegawa S., Zaima M., Inoue N., Sakai Y. Outcomes of neoadjuvant chemotherapy without radiation for rectal cancer. Dig. Surg. 2015;32:275–283. doi: 10.1159/000430469. [DOI] [PubMed] [Google Scholar]
- 20.Ueki T., Manabe T., Inoue S., Ienaga J., Yamanaka N., Egami T. A feasibility study of neoadjuvant XELOX without radiotherapy for locally advanced lower rectal cancer. Anticancer Res. 2016 Feb;36(2):741–747. [PubMed] [Google Scholar]
- 21.Kamiya T., Uehara K., Nakayama G. Early results of multicenter phase II trial of perioperative oxaliplatin and capecitabine without radiotherapy for high-risk rectal cancer: CORONA I study. Eur. J. Surg. Oncol. 2016;42:829–835. doi: 10.1016/j.ejso.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Deng Y., Chi P., Lan P. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J. Clin. Oncol. 2016;4:3300–3307. doi: 10.1200/JCO.2016.66.6198. [DOI] [PubMed] [Google Scholar]
- 23.Koike J., Funahashi K., Yoshimatsu K. Efficacy and safety of neoadjuvant chemotherapy with oxaliplatin, 5-fluorouracil, and levofolinate for T3 or T4 stage II/III rectal cancer: the FACT trial. Cancer Chemother. Pharmacol. 2017 Mar;79(3):519–525. doi: 10.1007/s00280-017-3243-7. [DOI] [PubMed] [Google Scholar]
- 24.Rouanet P., Rullier E., Lelong B. Tailored treatment strategy for locally advanced rectal carcinoma based on the tumor response to induction chemotherapy: preliminary results of the French phase II multicenter GRECCAR4 trial. Dis. Colon Rectum. 2017;60:653–663. doi: 10.1097/DCR.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 25.Roy A., Olsen J.R., Myerson R.J. Short-term endpoints for neoadjuvant rectal cancer therapy: pathologic complete response or neoadjuvant rectal cancer score? Int. J. Radiat. Oncol. Biol. Phys. 2016;96:E201. [Google Scholar]
- 26.Raissouni S., Mercer J., Gresham G. External validation of the neoadjuvant rectal (NAR) score and Valentini prediction nomogram (VPN): a multicenter study. J. Clin. Oncol. 2014;32 (suppl; abstr 3532) [Google Scholar]
- 27.Roselló S., Frasson M., García-Granero E., Roda D., Jordá E., Navarro S. Integrating downstaging in the risk assessment of patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy: validation of Valentini's Nomograms and the neoadjuvant rectal score. Clin. Colorectal Cancer. 2017 Oct 28 doi: 10.1016/j.clcc.2017.10.014. pii: S1533-0028(17)30212–8. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y., Zhang Y., Wu X., Lin H., Lu X., Huang Y. Prognostic significance of neoadjuvant rectal score in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and construction of a prediction model. J. Surg. Oncol. 2017 Dec 11 doi: 10.1002/jso.24907. [DOI] [PubMed] [Google Scholar]
- 29.Tomasello G., Petrelli F., Ghidini M., Russo A., Passalacqua R., Barni S. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: a systematic review and pooled analysis. JAMA Oncol. 2017 Jul 13;3(7):e170278. doi: 10.1001/jamaoncol.2017.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang M., Lin J., Yu X., Chen S., Kang L., Deng Y., Zheng J., Luo Y., Wang L., Lan P., Wang J. Erectile and urinary function in men with rectal cancer treated by neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy alone: a randomized trial report. Int. J. Colorectal Dis. 2016 Jul;31(7):1349–1357. doi: 10.1007/s00384-016-2605-7. [DOI] [PubMed] [Google Scholar]