Abstract
A non-targeted detection method using near-infrared (NIR) spectroscopy combined with chemometric modeling was developed for the rapid screening of commercial milk powder (MP) products as authentic or potentially mixed with known and unknown adulterants. Two benchtop FT-NIR spectrometers and a handheld NIR device were evaluated for model development. The performance of SIMCA classification models was then validated using an independent test set of genuine MP samples and a set of gravimetrically prepared mixtures consisting of MPs spiked with each of eleven potential adulterants. Classification models yielded 100% sensitivities for the benchtop spectrometers. Better specificity, which was influenced by the nature of the adulterant, was obtained for the benchtop FT-NIR instruments than for the handheld NIR device, which suffered from lower spectral resolution and a narrower spectral range. FT-NIR spectroscopy and SIMCA classification models show promise for the rapid screening of commercial MPs for the detection of potential adulteration.
Keywords: Analytical chemistry, Food safety, Food analysis
1. Introduction
Milk is a rich nutrient source that contains proteins, carbohydrates, vitamins, and minerals, and the associated nutritional value of milk has led to higher consumption and production of milk and milk-derived products worldwide (Nascimento et al., 2017). However, due in part to this demand, milk powder is a target of adulteration, ranking second only to olive oil according to the USP database on food fraud and economic adulteration (Moore et al., 2012). Milk powder can be diluted and/or mixed with cheap, readily available, relatively odorless, colorless, and tasteless substances to mask inferior quality, increase volume, or substitute natural constituents with cheaper adulterants for economic gain (MacMahon et al., 2012; Nascimento et al., 2017).
Unfortunately, economically motivated adulteration (EMA) of milk powder (MP) sometimes may cause adverse health effects. For example, EMA of food ingredients received worldwide attention by two incidents of milk and wheat gluten adulteration with nitrogen-rich melamine. In 2007, the melamine and cyanuric acid co-contamination of a wheat gluten ingredient caused pet-food associated renal failure in a number of cats and dogs in the US (Brown et al., 2007; Qin et al., 2013). In 2008, melamine adulteration of infant formula in China caused thousands of cases of renal complications in children, at least six confirmed deaths, and precipitated mass product recalls from multiple countries (MacMahon et al., 2012; Qin et al., 2013; Xin and Stone, 2008). The widespread global attention on the melamine scandal, the increased regulations by government agencies, and the availability of numerous analytical methods have decreased the risk of recurrence of melamine incidents (Draher et al., 2014; MacMahon et al., 2012; Turnipseed et al., 2008; U.S. FDA, 2017a,b,c). However, EMA of MP with many other nitrogen-rich adulterants remains a serious concern.
Based on the criteria for a potential milk powder adulterant, the Canadian Food Inspection Agency's Food Safety Division composed a list of possible economic adulterants (MacMahon et al., 2012). This list, combined with other intelligence information, led FDA to develop a targeted LC-MS method for six possible economic adulterants: dicyandiamide, urea, biuret, triuret, cyromazine, and amidinourea (MacMahon et al., 2012). Additionally, potential nitrogen-rich adulterants include cyanuric acid (Draher et al., 2014), ammonium sulfate, and aminotriazole (DeVries et al., 2017; Finete et al., 2013). According to reports from the European Union, there are indications of falsified MP contents due the addition of plant proteins (Luykx et al., 2007; Maraboli et al., 2002; Scholl et al., 2014). The low prices and availability of proteins, such as those from soybeans and peas, make them attractive as potential adulterants in MP. These plant protein isolates can be added to adjust MP physical characteristics such as viscosity, flavor, and nutrition. In general, EMA of these foreign proteins at concentrations below 1% in MP is not expected to occur for commercial gain (Haasnoot et al., 2001; Luykx et al., 2007). Interestingly, the development of reliable analytical test methods to detect EMA of MP products with plant proteins has been overshadowed by the melamine scandals (Scholl et al., 2014).
Additionally, polysaccharides/disaccharides such as starch, sucrose, and maltodextrin are potential MP adulterants (Borin et al., 2006). These adulterants could be added to adjust the density and freezing point of the adulterated product (de Almeida et al., 2012). In general, EMA from these food additives is expected to range from 20 to 25% of total weight; however, levels suspected to be as high as 60% have been suggested (Borin et al., 2006). Finally, some inorganic salts, such as carbonates and bicarbonates, can be added as neutralizers to adjust the pH of badly preserved milk to pass off the soured milk as fresh (Handford et al., 2016).
Both targeted and untargeted analytical approaches have been explored to detect economically motivated milk powder fraud. Targeted analysis is focused on specific adulterants and their quantification, whereas untargeted approaches provide less information on the type of adulterant and focus on classification of unknown products as belonging to the class of authentic products or not (as non-conforming samples). Numerous targeted analytical methods have been developed to detect the type and quantity of specific adulterants in milk; liquid and gas chromatography (LC, GC) coupled with mass spectrometry (MS) have been widely used for highly sensitive determinations of different types of MP adulterants (Abernethy and Higgs, 2013; Ehling et al., 2007; MacMahon et al., 2012; Pan et al., 2013). Despite their high sensitivity, these methods often entail time consuming extraction and derivatization steps and are thus potentially poorly suited for rapidly screening large numbers of food samples in the analytical laboratory and the field.
Spectroscopic methods are highly desirable for the analysis of MP adulterants because they are easy to use, provide rapid analysis, require little or no sample preparation, and multiple analyses are possible on a single test portion due to the methods' non-destructive nature. Spectroscopic methods, including mid infrared (MIR), near infrared (NIR), and Raman spectroscopies, combined with chemometric approaches have been evaluated as targeted analytical tools for reliable quantitative detection of specific adulterants in MP (Balabin and Smirnov, 2011; Jawaid et al., 2013; Lim et al., 2016; Lou et al., 2011; Ma et al., 2013; Santos et al., 2013). Development of non-targeted analytical tools for food surveillance to ensure consumer protection is urgently needed because as laboratories improve targeted detection methods specific to particular adulterants, fraudulent producers might introduce previously unknown adulterants that can evade detection with the targeted techniques. As such the non-targeted analysis of MP adulteration based on vibrational spectroscopic methods and chemometrics are being developed to qualitatively classify unknown samples as authentic or potentially adulterated.
Our research team recently evaluated the non-targeted detection of skim milk powder (SMP) and non-fat dry milk (NFDM) adulteration using melamine as an example adulterant (Karunathilaka et al., 2016, 2017). In those studies, spectral data collected from a benchtop Raman spectrometer were qualitatively processed by a soft independent modeling of class analogy (SIMCA) classification to discriminate wet-blended (WB) and dry-blended (DB) MPs with melamine from unspiked, authentic MP products produced on a commercial scale.
An international collaborative project led by the United States Pharmacopeial Convention (USP) further evaluated the potential of a NIR spectroscopic procedure for the non-targeted detection of MP adulteration using melamine as a potential adulterant (Scholl et al., 2017). In this study, the SIMCA classification model was based on a single class, principal component analysis (PCA) model that was developed using a diverse set of non-adulterated, authentic MP samples. These non-adulterated MPs in the training set belonged to a controlled set of samples that contained only the natural milk ingredients such as proteins, fat, and carbohydrates (e.g. lactose). However, commercially available milk powder products contain, according to their ingredient lists, additional components, such as low levels of vitamins and minerals. Upon further investigation, it was clear that the SIMCA model developed with these well-controlled authentic MPs did not perform well with commercially sourced MPs which was likely due to matrix differences. Therefore, the training set in the current study employed a variety of commercial MP products purchased from small to large scale producers. Further, this research investigated the detection abilities of the non-targeted classification model with eleven potential MP adulterants, versus a model solely tested with melamine, and includes plant proteins, sugars, inorganic salts, and additional nitrogen-rich chemicals. As the current study was not limited to a specific type of adulterant and included commercially available products, this technique holds greater potential for truly non-targeted detection capabilities for milk powder fraud, and, to our knowledge, is the most robust study on commercial milk powder products performed to date.
2. Materials and methods
2.1. Samples
Commercially available, diverse genuine milk powder samples were purchased from local stores and online sources. All the products selected for this study were NFDM powders and were manufactured in at least 16 states in the USA to allow for geographic diversity. Thirty-five samples were procured that were produced by 24 separate companies/brands with one company represented by 12 different packages from various retailers. Among the 24 brands, 6 contained only dry milk, 17 listed only vitamin A (palmitate) and D (or D3) as additional components, and one product listed lactose as well as vitamins A and D in their ingredient lists. The products with enhanced vitamin contents listed that one serving provided percent daily values (PDV) of 10–11% (one at 2%) for vitamin A and 25% (one at 10%, two had no value) for vitamin D. Additionally, calcium, when listed as percentage of daily serving size, was reported at 20–35%.
Eleven potential MP adulterants, that can be organized into four categories, were selected based on their history of use as adulterants or their potential for future use as adulterants in milk. The four adulterant categories were as follows: (1) low molecular weight, nitrogen-rich compounds (melamine (MEL), dicyandiamide (DC), aminotriazole (AMT), biuret (BU), and cyanuric acid (CA) from Sigma-Aldrich, St. Louis, MO), (2) plant proteins (soy protein isolate (SPI) and pea protein isolate (PPI) from Now Foods, Bloomingdale, IL), (3) inorganic salts (ammonium sulfate (AS) and calcium carbonate (CC) from Sigma-Aldrich), (4) non-fat solids (sucrose (SC) and maltodextrin (MD) from Sigma-Aldrich). Series of 0.0–2.0% (w/w) gravimetric blends of MP and the low molecular weight, nitrogen-rich compounds or inorganic salts were prepared by accurately mixing the required amounts of adulterants and MP in a 50 mL Nalgene Oak Ridge PPCO centrifuge tube (Thermo Scientific, Rochester, NY) to achieve a 10.0 g total weight. The mixtures were then homogenized by geno-grinding for 1 min at 1500 rpm (SPEX Sample Prep LLC, New Jersey). An initial study was performed to evaluate if geno-grinding had an impact on the spectra of the commercial MP samples, and no significant spectral differences in the genuine MP samples were observed. Further, for every set of MP adulterants, the MP used in spiking was evaluated without and with geno-grinding to evaluate any impacts on model performance. These two types of samples were observed to have similar model prediction results. Hence, geno-grinding was used solely for the gravimetric sample preparation to allow for more uniform sample distribution in those mixtures. A similar procedure was followed for the preparation of gravimetric mixtures for the other classes of adulterants. Blended samples for the two types of plant proteins were prepared in the range of 0.0–20.0 % (w/w), while, to ensure the scope of potential adulteration, the concentrations of maltodextrin and sucrose in mixtures ranged from 0.0 to 20.0 % (w/w) and 0.0 to 50.0 % (w/w), respectively. The sample information, including concentrations and number of replicate spectral measurements for each adulterant, is given in in Table 1. All the stock powdered milk samples and dry-blended (DB) adulterant samples were stored in sealed polypropylene centrifuge tubes and in a desiccator under nitrogen.
Table 1.
SIMCA classification results for the genuine milk powder and gravimetric blends in the validation set. Results are shown for the two benchtop FT-NIR instruments investigated.
| Validation sample | Adulterant % (w/w)a |
% of Correct Classification (No. of correct classifications/No. of replicates) |
|
|---|---|---|---|
| Bruker MPA FT-NIRb |
PE Frontier FT-NIRb |
||
| MP controls | 0 | 100 (67/67) | 100 (53/53) |
| 0.2 | 20 (1/5) | 50 (2/4) | |
| 0.4 | 80 (4/5) | 100 (4/4) | |
| Melamine | 0.6 | 100 (5/5) | 100 (4/4) |
| 0.8 | 100 (5/5) | 100 (4/4) | |
| 1 | 100 (5/5) | 100 (4/4) | |
| 2 | 100 (5/5) | 100 (4/4) | |
| 0.2 | 0 (0/5) | 0 (0/4) | |
| 0.4 | 0 (0/5) | 0 (0/4) | |
| Dicyandiamide | 0.6 | 20 (1/5) | 0 (0/4) |
| 0.8 | 80 (4/5) | 25 (1/4) | |
| 1 | 60 (3/5) | 0 (0/4) | |
| 2 | 100 (5/5) | 50 (2/4) | |
| 0.2 | 50 (3/6) | 25 (1/4) | |
| 0.4 | 100 (6/6) | 100 (4/4) | |
| Aminotriazole | 0.6 | 100 (6/6) | 100 (4/4) |
| 0.8 | 100 (6/6) | 100 (4/4) | |
| 1 | 100 (6/6) | 100 (4/4) | |
| 2 | 100 (6/6) | 100 (4/4) | |
| 0.2 | 100 (3/3) | 0 (0/3) | |
| 0.4 | 100 (3/3) | 100 (3/3) | |
| Biuret | 0.6 | 100 (3/3) | 100 (3/3) |
| 0.8 | 100 (3/3) | 100 (3/3) | |
| 1 | 100 (3/3) | 100 (3/3) | |
| 2 | 100 (3/3) | 100 (3/3) | |
| 0.2 | 0 (0/2) | 0 (0/2) | |
| 0.4 | 0 (0/3) | 0 (0/3) | |
| Cyanuric acid | 0.6 | 33 (1/3) | 0 (0/3) |
| 0.8 | 0 (0/3) | 0 (0/3) | |
| 1 | 0 (0/3) | 0 (0/3) | |
| 2 | 0 (0/3) | 0 (0/2) | |
| 1 | 33 (1/3) | 33 (1/3) | |
| 2 | 33 (1/3) | 100 (3/3) | |
| Soy Protein Isolate | 5 | 100 (3/3) | 100 (3/3) |
| 10 | 100 (3/3) | 100 (3/3) | |
| 15 | 100 (3/3) | 100 (3/3) | |
| 20 | 100 (3/3) | 100 (3/3) | |
| 1 | 33 (1/3) | 33 (1/3) | |
| 2 | 67 (2/3) | 100 (3/3) | |
| Pea Protein Isolate | 5 | 100 (3/3) | 100 (3/3) |
| 10 | 100 (3/3) | 100 (3/3) | |
| 15 | 100 (3/3) | 100 (3/3) | |
| 20 | 100 (3/3) | 100 (3/3) | |
| 0.2 | 0 (0/6) | 0 (0/4) | |
| 0.4 | 0 (0/6) | 0 (0/4) | |
| Ammonium sulfate | 0.6 | 0 (0/6) | 0 (0/4) |
| 0.8 | 17 (1/6) | 0 (0/4) | |
| 1 | 17 (1/6) | 0 (0/4) | |
| 2 | 67 (4/6) | 0 (0/4) | |
| 0.2 | 0 (0/3) | 33 (1/3) | |
| 0.4 | 0 (0/3) | 0 (0/3) | |
| Calcium carbonate | 0.6 | 0 (0/3) | 0 (0/3) |
| 0.8 | 33 (1/3) | 0 (0/3) | |
| 1 | 0 (0/3) | 0 (0/3) | |
| 2 | 33 (1/3) | 0 (0/2) | |
| 1 | 33 (1/3) | 0 (0/3) | |
| 2 | 100 (3/3) | 0 (0/3) | |
| Maltodextrin | 5 | 100 (3/3) | 100 (3/3) |
| 10 | 100 (3/3) | 100 (3/3) | |
| 15 | 100 (3/3) | 100 (3/3) | |
| 20 | 100 (3/3) | 100 (3/3) | |
| 1 | 0 (0/3) | 33 (1/3) | |
| 2 | 33 (1/3) | 0 (0/3) | |
| 3 | 0 (0/3) | 0 (0/3) | |
| 5 | 0 (0/3) | 33 (1/3) | |
| Sucrose | 7 | 100 (3/3) | 67 (2/3) |
| 10 | 100 (3/3) | 100 (3/3) | |
| 15 | 100 (3/3) | 100 (3/3) | |
| 20 | 100 (3/3) | 100 (3/3) | |
| 25 | 100 (3/3) | 100 (3/3) | |
| 50 | 100 (3/3) | 100 (3/3) | |
Percentage as-is basis from gravimetric preparation.
Samples with 100% correct classification are highlighted in bold.
2.2. NIR spectral acquisition
NIR diffuse reflectance and absorbance spectra (12,500 to 4,000 cm−1) for commercial MP and DB samples were acquired with a Bruker MPA benchtop FT-NIR spectrometer (Billerica, Massachusetts) fitted with a diffuse reflectance accessory. A 12-mm diameter spot was illuminated on the sampling interface, and each resulting percent reflectance (% R) NIR spectrum was an average of 64 scans collected at 16 cm−1 resolution. A background scan was acquired at the beginning of the experiment, and spectra from the reference standards were used to monitor the wavelength accuracy and photometric intensity; NIR spectra of a USP NIR suitability reference standard (USP, Rockville, MD) and a 99% spectralon diffuse reflectance standard (Labsphere, North Sutton, NH) were collected at the beginning of data collection and at specified intervals on each day of analysis. For each sample measurement, an approximately 10 g sample portion was placed in a rotating cup over a 50-mm diameter quartz window. Three replicate measurements for each MP and DB sample were acquired on three different days of analysis, unless otherwise specified. DB samples were remixed by multiple inversions between each NIR measurement. Since the same sample cup was used for each successive sample measurement, it was thoroughly cleaned between measurements by pouring out the sample powder and removing the remaining MP particles by vacuum suction.
A second benchtop spectrometer, a Frontier FT-NIR system (PerkinElmer (PE), Waltham, MA) fitted with a rotatory diffuse reflectance accessory (PE) was also used to collect the spectra of the MP and DB samples. FT-NIR spectra of the samples placed in 60-mm diameter, manufacturer recommended, glass petri dishes (approximately 1 mm thick) were acquired at an average of 32 scans and 16 cm−1 resolution in the 10,000–4000 cm−1 spectral range. The instrument performance was verified at the beginning of each day of data collection using PE-specific reference standards. For spectral measurements, approximately 10 g sample portions were evenly distributed in the petri dish and placed in a 60-mm sample spinner. Similar to Bruker data collections, replicate measurements were acquired on three different days, and samples were remixed by inversions before sampling.
To explore the potential of using a portable device for the non-targeted detection of milk powder adulteration, NIR spectra for the same sample set were collected using a Phazir handheld NIR device (Thermo Fisher Scientific, Waltham, MA). Spectral data were collected at the available optical resolution of 11 nm, over the spectral range of 6266–4167 cm−1, and under experimental conditions recommended by the manufacturer (background: 10 scans; sample: 10 scans). Approximately 3 g of sample distributed in a 40-mm, manufacturer recommended petri dish (approximately 1 mm thick) was placed on the sampling area (reflectance probe) of the device for spectral data collection. The fully integrated software (version 3.1) that is configured to control data collection was also used for spectral file manipulation. Three replicate measurements for each sample were acquired on three different days of analysis, unless otherwise specified.
2.3. Chemometrics analyses
An unsupervised pattern recognition method based on PCA and supervised classification models based on SIMCA were performed using PLS_Toolbox_8.0.1 (Eigenvector Research Inc., Wenatchee, WA) that runs in a MATLAB computational environment (MATLAB 8.6, Natick, MA). Raw NIR spectral data were observed to consist of unrelated spectral variations between samples due to uncontrollable physical variation in the powders such as non-homogeneous distribution of the particles, particle size variations, and sample morphology differences (surface roughness/shape). These physical variations lead to light scattering effects which contribute to varying sample/effective path length and result in additive, multiplicative, and wavelength-dependent baseline effects (Huang et al., 2010). Different spectral pre-treatments, namely derivatives (e.g. Savitzky-Golay first and second derivatives), standard normal variate (SNV), and multiplicative scatter correction (MSC), were evaluated individually or in combinations to minimize the adulterant-unrelated variability. PCA was then performed separately on genuine MP spectral data from each of the three instruments to identify potential spectral outliers by applying a 95% confidence level to the Q residuals and Hoteling's T2 scores. In the present study, sensitivity was measured by the ability of the classification model developed with the MP samples to identify its samples as such (true positives), while specificity was measured by the ability of the model to distinguish external samples (true negatives) (de Souza Gondim et al., 2017). Therefore, the sensitivity indicated the ability of the model to detect truly genuine MP samples, and the specificity was a measure of the model's ability to identify the adulterated or atypical samples. Accordingly, samples in the validation set that were “adulterated” MPs but classified as genuine were termed false-positives, while any genuine MP sample that was not classified as belonging to its MP class by the model was termed a false-negative (Wiki.eigenvector, 2017).
3. Results and discussion
3.1. Visual evaluation of FT-NIR spectra
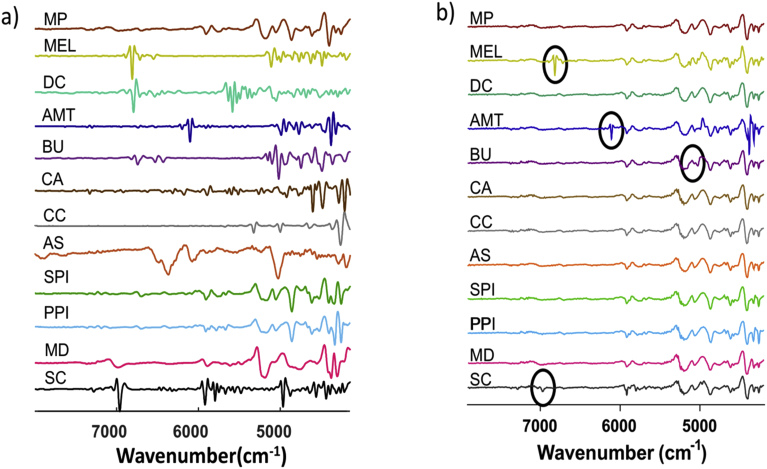
Fig. 1a illustrates the representative second derivative FT-NIR spectra collected using the PE Frontier FT-NIR spectrometer for a representative MP sample and each of the eleven, pure component, potential MP adulterants, while Fig. 1b shows second derivative spectra for gravimetric blends of each adulterant at their highest concentrations in MP. The displayed spectra in Fig. 1a and b were similar to those collected with the second benchtop Bruker MPA FT-NIR spectrometer. Unique second derivative spectral bands were visually observed in Fig. 1a, and these distinct bands were highlighted in the mixture spectra in Fig. 1b at 6812, 6102, ∼5100–4900, and 6950 cm−1 for the gravimetric blends of adulterants MEL, AMT, BU, and SC, respectively. Hence, a classification model would be expected to be specific for the detection of adulteration of genuine MP with these four adulterants.
Fig. 1.
a) NIR second derivative spectra for a representative MP sample and for each of the eleven, pure component, potential MP adulterants collected with the PE Frontier benchtop FT-NIR spectrometer. Second derivative spectra are in arbitrary units and spatially offset for visual clarity. b) Second derivative spectra for each type of gravimetric blend at their highest concentrations with a MP spectrum shown for comparison.
The second derivative spectra for the pure adulterants and the gravimetric blends for SPI, PPI, and MD are also illustrated in Fig. 1a and b. While a genuine MP sample and these adulterants exhibited many overlapping NIR spectral features, minor spectral differences and band intensity variations were still observed in the lower wavenumber region, namely the 5300–4900 cm−1 range. For the two inorganic salts, AS exhibited broad, but weak, spectral features between 6100–6500 cm−1, while CC only weakly absorbed in the NIR region (Fig. 1a). As such, the second derivative spectra for the blends of these inorganic salts at ≤2% concentrations were visually indistinguishable from the MP spectrum in Fig. 1b. Unique NIR fingerprints were observed for pure CA and DC when compared to that of a MP sample (Fig. 1a); however, the second derivative NIR spectra for CA and DC blends at ≤2% were visually similar to that of a MP spectrum (Fig. 1b). This visual inspection and evaluation indicates the potential for success in authentication of MP but also elucidates the need for more advanced data-processing and analysis than solely spectral matching.
3.2. Principal component analysis (PCA)
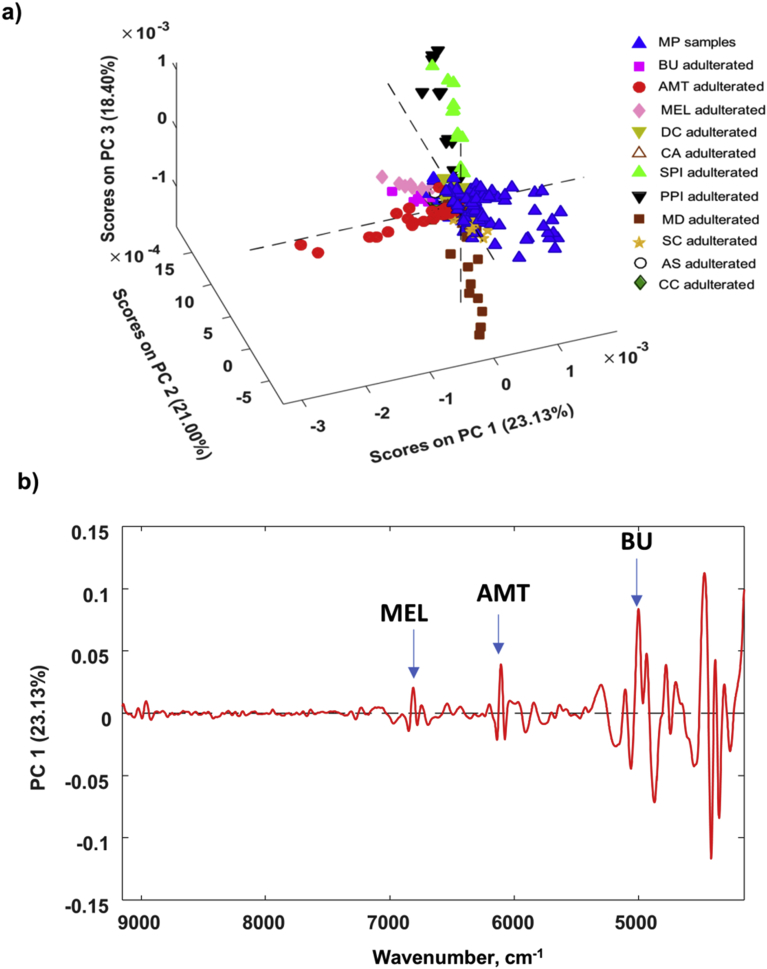
An unsupervised pattern recognition method, PCA, was used to explore the possibility of differentiating adulterated MP samples from non-adulterated ones and to evaluate the effect of different types of adulterants on their detection in spiked MP. A spectral data matrix consisting of all the sample spectra for MP and MP blends (383 spectra total) from the PE Frontier FT-NIR spectrometer in the 9150–4150 cm−1 wavenumber region was submitted to PCA. Prior to PCA, raw FT-NIR spectra were transformed using a SNV correction combined with a second derivative transformation (Savitsky-Golay algorithm; width size of 35 points, third-order polynomial fit) followed by mean center preprocessing. Fig. 2a illustrates the PCA scores of the first three principal components (PCs) that explained 62.53% of the cumulative spectral variance. The first three PC scores for the non-adulterated MP samples (blue triangles) formed a cluster with spread likely due to composition differences. Specifically, replicate measurements from two MP samples which had comparatively lower declared amounts of vitamin A, D, and C as well as replicate measurements for a sample that had no declared vitamin contents were separated from the rest of the MP samples along the co-variance of PC1 and 3.
Fig. 2.
a) PCA scores plot for genuine and adulterated MP spectral data collected with PE FT-NIR spectrometer. Spectra were pretreated by the use of SNV followed by a second derivative transformation. b) Loading plot for PC1 with arrows and labels for unique spectral bands attributed to three MP adulterants.
Three nitrogen-rich compounds (MEL, BU and AMT) were separated from the non-adulterated MP samples along PC1 (Fig. 2a). Additionally, the higher concentrated blends were observed to separate furthest from the genuine MP samples in the scores plot. The PC loadings plots (Fig. 2b) were evaluated to explore the spectral features that explained the clustering patterns observed in the scores plot. Higher positive PC1 loadings observed at 6812, 6102 and ∼5100–4900 cm−1 were characteristic of these three adulterants and indicated that these unique spectral regions were essential for discriminating these adulterants from authentic MP samples (see labeled arrows in Fig. 2b). However, two additional adulterants that belonged to the same adulterant category for low molecular weight, nitrogen-rich compounds (e.g. DC and CA) were clustered together with the MP samples, as the spectra for their blends were indistinguishable from that of a genuine MP sample as noted in Section 3.1.
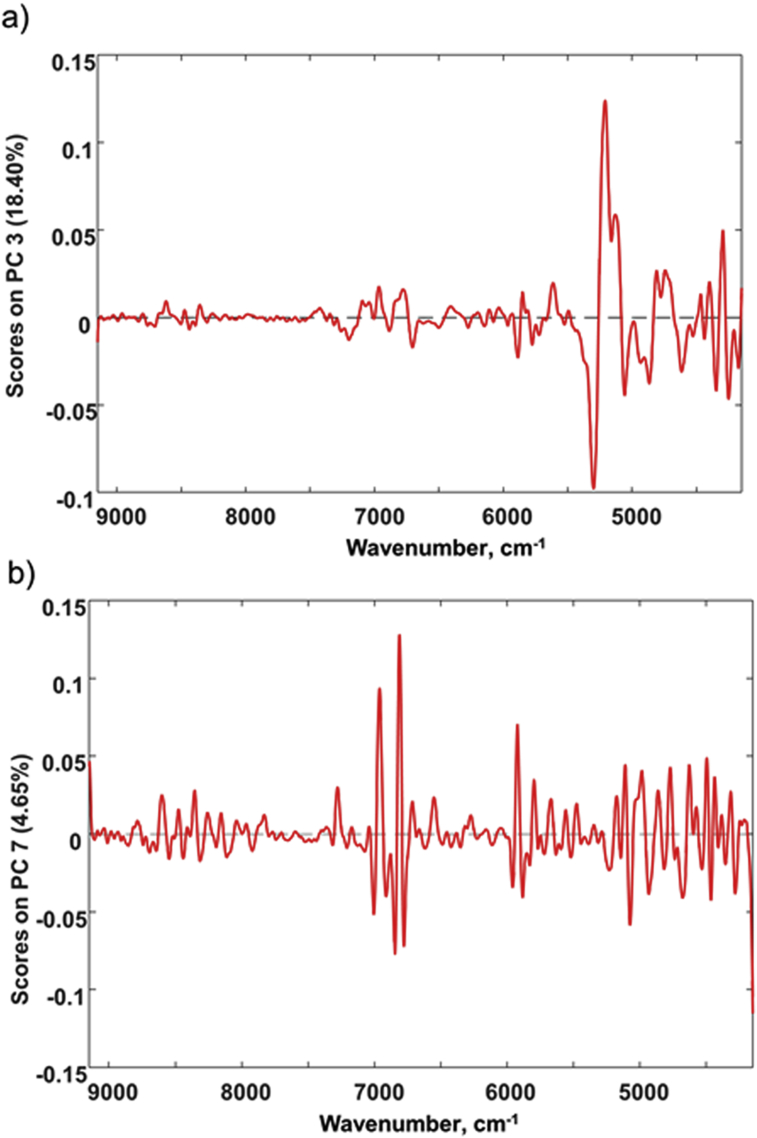
The separation along PC3 of blends for the two plant proteins, SPI and PPI, and MD from non-adulterated MPs could be evaluated with the corresponding loading plot (Fig. 3a). PC3 had higher positively and negatively loaded bands at 5300–4900 cm−1, which appeared to be caused by the minor spectral feature differences observed in these regions (Fig. 1a and b). The bands resulted in the separation of the plant proteins and MD blends at the opposite sides on the PC3 axis with the proteins on the positive side and maltodextrin on the negative one. With regard to sucrose, clear separation for SC samples was not observed in the first three PC scores plot. However, PC7, which explained 4.65% of the variance, showed higher positive loadings at approximately 6950 cm−1 (Fig. 3b) and was important for the unsupervised discrimination of SC from MP samples. The two inorganic salts exhibited weak absorption in the NIR range, and spectra for their blends at 2% concentration were indistinguishable from a MP FT-NIR spectrum. Hence, blends for inorganic salts were clustered together with MP samples in the PC scores plot. As observed in our previous Raman spectroscopic work, the separation between MP and adulterated samples at low concentrations was less discriminative with the PCA, unsupervised pattern recognition method than with SIMCA analysis (Karunathilaka et al., 2017). Hence, the supervised classification method SIMCA, which was based on a single class PCA model trained for the genuine MPs, was subsequently investigated herein as a potentially superior discriminator method for adulteration detection in NIR evaluation.
Fig. 3.
Principal component (PC) loading plots for the non-supervised principal component analysis (PCA) performed for the data collected from the PE FT-NIR spectrometer. a) Loading plot for PC3 that illustrates higher positively and negatively loaded bands at 5300–4900 cm−1. b) Loading plot for PC7 that has higher positive loadings at approximately 6950 cm−1.
3.3. Non-targeted classification using SIMCA
Using SIMCA, a single class PCA model was developed for a library of NIR spectra collected from the diverse set of genuine MP samples. Adulterated samples, when tested against the PCA model boundaries, would be classified as outliers if spectral differentiation was possible thus enabling a non-targeted, abnormality testing of MPs. A venetian blind cross-validation method was used to retain a sufficient number of PCs to ensure a robust calibration model (Karunathilaka et al., 2017). After the development of the PCA calibration model, the NIR spectrum of a new test sample was projected into the PC space of the calibration, and the Q residual for the test sample was calculated and compared with a threshold confidence limit value. This Q residual described the portion of the sample variance that was not modelled by the calibration set of genuine MP samples. The differences might be due to adulteration or sample anomaly. Hence, if a test sample Q residual value was lower than the pre-defined confidence limit for the calibration set, the sample was then considered as belonging to the MP class and assigned as a genuine MP, while those samples with Q residual values higher than the confidence limit were labelled as suspect samples.
In the present study, the single PCA model for SIMCA classification was developed with 25 genuine MP samples after removing outliers. In general, for all the data collected with three NIR spectrometers, four outlier samples were found and were due to either low vitamin/mineral amounts or samples without declared vitamin contents, and these were removed prior to SIMCA classification. The calibration set of spectra from all the instruments were collected in three replicate measurements on three different days (75 total spectra) to include potential day-to-day variance into the model. Once the calibration models were developed, model performances were evaluated by using validation samples that consisted of replicate measurements from an independent test set of six genuine MP samples and gravimetric mixtures of the eleven potential MP adulterants. The data for the validation samples were collected in multiple days of data collections, two of which were completely independent of the calibration days, unless otherwise specified, to evaluate the robustness of the developed models.
Among the investigated spectral pre-treatments, only the optimal one for each instrument, which was chosen based on model performance in terms of sensitivity and specificity, are reported here. The SIMCA model for the Bruker MPA instrument was developed using SNV and first derivative transformation (2nd order polynomial fit, 15 points) FT-NIR spectra. For the PE Frontier FT-NIR spectrometer, SNV followed by a second derivative transformation (2nd order polynomial fit, 35 points) was found to be the optimum processing procedure. For the two benchtop FT-NIR spectrometers, the classification models were developed for the pre-treated spectra in the 9150–4150 cm−1 range. Based on cross-validation, six and four PCs that explained 99.72 and 94.76% of the total variance were used for Bruker and PE instruments, respectively.
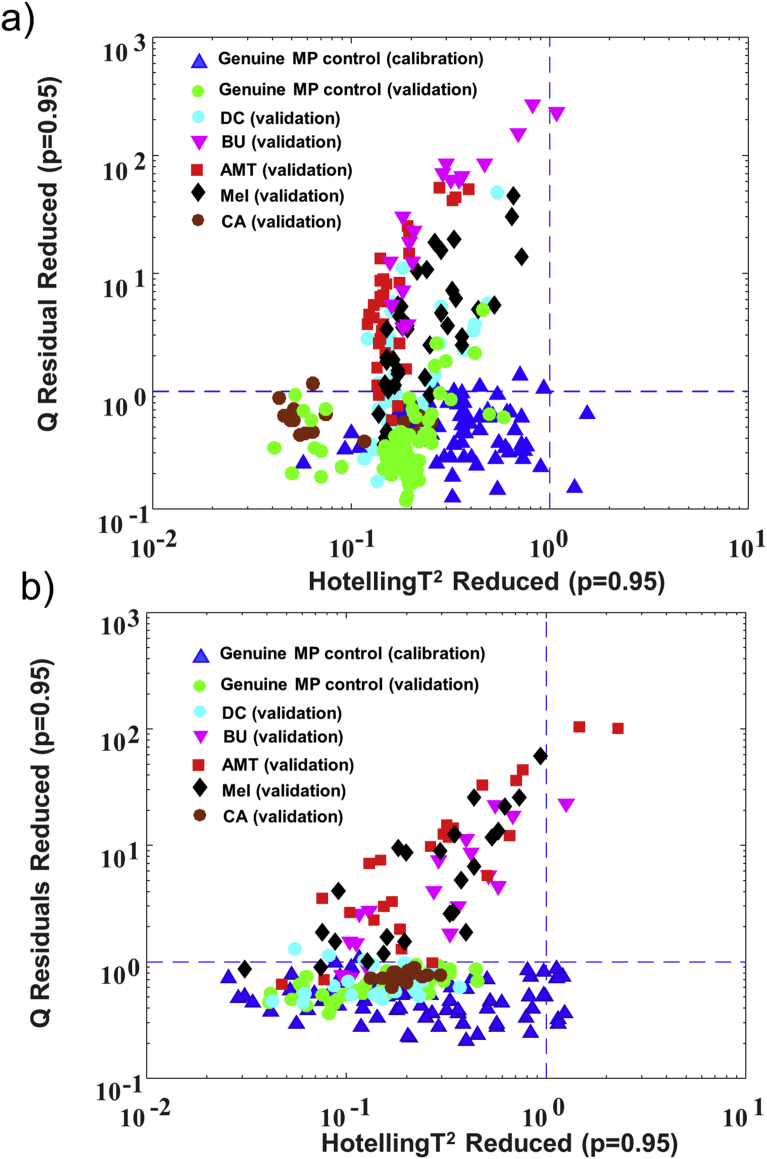
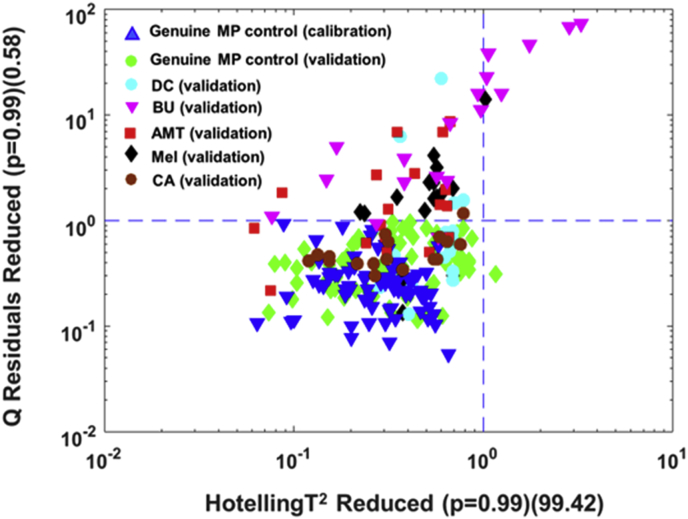
The number of correct classification per total number of replicate measurements for each type of validation sample is listed in Table 1. For the non-targeted models developed for the two benchtop spectrometers, all the replicate measurements for the genuine MP samples in the validation set were correctly predicted as belonging to the genuine milk powder class and thus had no false-negatives at a 95% confidence limit for Q residuals. The reduced Q vs Hotelling's T2 plots for the model predictions for the two benchtop FT-NIR spectrometers for the genuine MP samples in the calibration and validation sets along with five representative nitrogen-rich MP adulterants (instead of all adulterants for easier readability), are illustrated in Fig. 4a and b. From the five nitrogen-rich compounds, MEL, AMT and BU yielded the highest level of specificities at the 95% confidence limit for Q residuals, as seen by the data points being well-separated from the genuine MP cluster (Fig. 4a and b) and the associated high prediction capability (Table 1). Both benchtop NIR models correctly classified these blends at ≥ 0.4%, except for MEL by the Bruker MPA instrument that yielded one misclassified measurement at 0.4% of adulteration. Interestingly, a similar level of detection was reported for a set of DB MEL samples in a recent non-targeted Raman spectroscopy and SIMCA study based on the use of a library calibration set of Raman spectra collected from 27 well-controlled MP samples (Karunathilaka et al., 2017). The higher specificities for these compounds in comparison to other adulterants was likely owed to the unique FT-NIR spectral features at 6812, 6102, ∼5100–4900 cm−1 for MEL, AMT, and BU, respectively, versus the lack of specificity seen for the spectrally weak-blends of DC and CA (Fig. 1a and b). With regard to the two inorganic salts, CC and AS, they were also inadequately distinguished from the genuine MP samples at the concentrations (i.e. ≤ 2%) investigated in the present study due to the weak and poorly resolved spectral features of these salts (Fig. 1a).
Fig. 4.
Q residuals (reduced) versus Hotelling's T2 (reduced) plots for the SIMCA models developed for the data collected from the two benchtop FT-NIR spectrometers. Data are shown for genuine MP and five MP samples each spiked with a low molecular weight, nitrogen-rich adulterant. a) Bruker MPA and b) PE Frontier FT-NIR spectrometers. The optimized 95% confidence limits are indicated by blue lines.
For the non-targeted models developed on the two benchtop FT-NIR spectrometers, 100% correct classification with no false-positives for the prediction of two plant protein isolates, SPI and PPI, at ≥5% concentrations was achieved. Minor spectral differences observed in the lower wavenumber region contributed to the successful detection of these adulterants. As such, the developed NIR/SIMCA detection methodology is well suited to the detection of EMA of these plant proteins, as lower concentrations (e.g. less than 5%) are generally not considered economically viable (Haasnoot et al., 2001; Luykx et al., 2007). Regarding the two non-fat solids used, blends of SC at ≥10% were correctly identified as adulterated with 100% correct classification of spectra collected on the two benchtop FT-NIR spectrometers. MD, on the other hand, was better discriminated from genuine MP samples, as samples at ≥5% were correctly classified with no false-positive results. For SC, a weak, but unique, FT-NIR spectral feature that was observed at 6950 cm−1 (Figs. 1a, b and 3b) was responsible for the observed discrimination from typical, commercial MPs. For MD, minor, yet characteristic, spectral differences were observed in the 5300–4900 cm−1 range. In general, adulteration with these food additives can range from 20 to 25% of total weight; however, concentrations as high as 60% have been reported (Borin et al., 2006). Hence, the developed non-targeted method is well suited to detect EMA with these food additives. The specificity of the non-targeted method was, however, dependent on the type of adulterant and associated NIR activity. That is to say, an adulterant that had unique and/or intense FT-NIR spectral features from that of MP resulted in a higher specificity.
The performance of a portable NIR device for the non-targeted detection of MP adulteration was also studied in an effort to further drive this methodology to point-of-production or importation analysis. Data were preprocessed with MSC followed by first derivative transformation (2nd order polynomial fit, 13 points), and the reduced Q vs the Hotelling's T2 plots for the SIMCA model are in Fig. 5. All the replicate measurements for the genuine MP test samples in the validation set were correctly predicted as such, with no false-negatives at a 99% confidence limit. However, the portable NIR instrument exhibited lower specificity, as observed in the adulterant-spiked samples clustering with the non-spiked MP samples. Satisfactory results were obtained with BU; the developed model correctly classified ≥0.4% biuret blends, which was a similar level of performance as observed for the two benchtop FT-NIR spectrometers. This was not unexpected, as the classification model for the handheld NIR device was developed for the 6005–4406 cm−1 spectral range which contained the unique NIR spectral features for BU observed at ∼5100–4900 cm−1. When compared to the two benchtop spectrometers, the portable device showed lower specificities for the prediction of adulteration from the other adulterants investigated. We attribute this result, in part, to the limited spectral range offered by the portable device, which does not include the distinctive NIR spectral features observed for some adulterants (e.g., MEL, AMT, SC), and the lower spectral resolution which likely adversely impacted the specificity of the portable device for other adulterants (e.g., SPI, PP, MD). As such, the portable device in the current instrument configuration would be of limited utility for the non-targeted detection of adulteration of this food commodity.
Fig. 5.
Q residuals (reduced) versus Hotelling's T2 (reduced) plot for a SIMCA model built in the 6005–4406 cm−1 range for the handheld NIR device. The 99% confidence limits are indicated by blue lines. In the plot, MP = milk powder; DC = dicyandiamide; BU = biuret; AMT = aminotriazole; Mel = melamine; CA = cyanuric acid. The optimized 99% confidence limits are indicated by blue lines.
There are many recent studies on the detection of adulteration in milk powder using NIR or NIR-imaging combined with chemometrics (Balabin and Smirnov, 2011; Lim et al., 2016; Fu et al., 2014; Wu et al., 2016). Balabin and Smirnov (2011) developed several nonlinear regression methods, such as Poly-PLS, artificial neural networks (ANN) and support vector regression (SVR), and least squares-support vector machine (LS-SVM), to correctly predict the melamine content of milk products, and the authors reported a limit of detection below 1 ppm. Lim et al. (2016) used a NIR hyperspectral imaging technique combined with a partial least squares regression (PLSR) model to detect melamine adulterant particles in milk powder at 0.02% melamine. A hyperspectral NIR imaging method to detect low-concentration melamine in dry milk (at 200 ppm) was also reported (Fu et al., 2014). In another study, the feasibility of using NIR spectroscopy and chemometrics for identifying and quantifying melamine in liquor milk was evaluated by Wu et al. (2016). In all cases, however, such NIR approaches were targeted and only applicable to detecting a known, singular adulterant. In contrast, the non-targeted method developed herein was not limited to a specific type of known adulterant and holds greater potential for truly non-targeted capabilities for detecting milk powder fraud.
In the area non-targeted detection using spectroscopy, Capuano et al. (2015) recently developed targeted and untargeted approaches for the detection of acid whey, starch, and maltodextrin adulteration of skim milk powder by NIR. In 2017, de Souza Gondim et al. further evaluated the detection of several adulterants in raw, liquid milk by mid-infrared spectroscopy and chemometrics. The method developed in this study furthers non-targeted detection by evaluating a variety of adulterant classes in a diversity of commercially available MPs. As such, this technique holds greater potential for truly non-targeted detection capabilities for milk powder fraud, and, to our knowledge, is the most robust study on commercial skim milk powder products performed to date.
4. Conclusions
This research focused on the development of a non-targeted method using NIR spectroscopy and chemometrics to screen for known and potentially unknown adulterants in MPs. The effects of different types of NIR instruments, benchtop and portable, as well as different types of milk powder adulterants were investigated. In general, the portable NIR method had comparatively poorer detection capability for all potential MP adulterants studied, likely due to both the lower spectral resolution and the narrower spectral range offered by the device. Most noteworthy in this work, the simultaneous detection of multiple MP adulterants was demonstrated on the benchtop FT-NIR instruments, indicating the power of these developed SIMCA models to perform non-targeted screening for NIR active molecules. This study further demonstrated that the specificity of a non-targeted method was dependent on the type of adulterant and associated NIR activity, and the use of complimentary methods, such as Raman spectroscopy, should be investigated to fully cover the adulterant classes.
Declarations
Author contribution statement
Sanjeewa R. Karunathilaka: Analyzed and interpreted the data; Wrote the paper.
Betsy J. Yakes: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Keqin He, Jin Kyu Chung: Performed the experiments.
Magdi Mossoba: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Keqin He was supported by the University of Maryland, Joint Institute for Food Safety and Applied Nutrition through a cooperative agreement with the FDA, (#FDU001418).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
SRK acknowledges the support provided by an appointment to the Research Participation Program at the Center for Food Safety and Applied Nutrition, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the US Food and Drug Administration (US FDA). We thank Robert Packer, Perkin Elmer (PE), for the use of PE instrumentation.
References
- Abernethy G., Higgs K. Rapid detection of economic adulterants in fresh milk by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2013;1288:10–20. doi: 10.1016/j.chroma.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Balabin R., Smirnov S.V. Melamine detection by mid-and near-infrared (MIR/NIR) spectroscopy: a quick and sensitive method for dairy products analysis including liquid milk, infant formula, and milk powder. Talanta. 2011;85:562–568. doi: 10.1016/j.talanta.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Borin A., Ferrao M.F., Mello C., Maretto D.A., Poppi R.J. Least-squares support vector machines and near infrared spectroscopy for quantification of common adulterants in powdered milk. Anal. Chim. Acta. 2006;579:25–32. doi: 10.1016/j.aca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Brown C.A., Jeong K.S., Poppenga R.H., Puschner B., Miller D.M., Ellis A.E., Kang K.I., Sum S., Cistola A.M., Brown S.A. Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J. Vet. Diagn. Invest. 2007;19:525–531. doi: 10.1177/104063870701900510. [DOI] [PubMed] [Google Scholar]
- Capuano E., Boerrigter-Eenling R., Koot A., van Ruth S.M. Targeted and untargeted detection of skim milk powder adulteration by near-infrared spectroscopy. Food Anal. Meth. 2015;8:2125–2134. [Google Scholar]
- de Almeida M.R., de Sá Oliveira K., Stephani R., Cappa de Oliveira L.F. Application of FT-Raman spectroscopy and chemometric analysis for determination of adulteration in milk powder. Anal. Lett. 2012;45:2589–2602. [Google Scholar]
- de Souza Gondim C., Junqueira R.G., de Souza S.V.C., Ruisánchez I., Callao M.P. Detection of several common adulterants in raw milk by Mid-infrared spectroscopy and one-class and multi-class multivariate strategies. Food Chem. 2017;230:68–75. doi: 10.1016/j.foodchem.2017.03.022. [DOI] [PubMed] [Google Scholar]
- DeVries J.W., Greene G.W., Payne A., Zbylut S., Scholl P.F., Wehling P., Evers J.M., Moore J.C. Non-protein nitrogen determination: a screening tool for nitrogenous compound adulteration of milk powder. Int. Dairy J. 2017;68:46–51. [Google Scholar]
- Draher J., Pound V., Reddy T.M. Validation of a rapid method of analysis using ultrahigh-performance liquid chromatography-tandem mass spectrometry for nitrogen-rich adulterants in nutritional food ingredients. J. Chromatogr. A. 2014;1373:106–113. doi: 10.1016/j.chroma.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Ehling S., Tefera S., Ho I. High-performance liquid chromatographic method for the simultaneous detection of the adulteration of cereal flours with melamine and related triazine by-products ammeline, ammelide, and cyanuric acid. Food Addit. Contam. 2007;24:1319–1325. doi: 10.1080/02652030701673422. [DOI] [PubMed] [Google Scholar]
- Finete V.D.L.M., Gouvêa M.M., de Carvalho Marques F.F., Netto A.D.P. Is it possible to screen for milk or whey protein adulteration with melamine, urea and ammonium sulphate, combining Kjeldahl and classical spectrophotometric methods? Food Chem. 2013;141:3649–3655. doi: 10.1016/j.foodchem.2013.06.046. [DOI] [PubMed] [Google Scholar]
- Fu X., Kim M.S., Chao K., Qin J., Lim J., Lee H., Garrido-Varo A., Pérez-Marín D., Ying Y. Detection of melamine in milk powders based on NIR hyperspectral imaging and spectral similarity analyses. J. Food Eng. 2014;124:97–104. [Google Scholar]
- Haasnoot W., Olieman K., Cazemier G., Verheijen R. Direct biosensor immunoassays for the detection of nonmilk proteins in milk powder. J. Agric. Food Chem. 2001;49:5201–5206. doi: 10.1021/jf010440p. [DOI] [PubMed] [Google Scholar]
- Handford C.E., Campbell K., Elliott C.T. Impacts of milk fraud on food safety and nutrition with special emphasis on developing countries. Compr. Rev. Food Sci. Food Saf. 2016;15:130–142. doi: 10.1111/1541-4337.12181. [DOI] [PubMed] [Google Scholar]
- Huang J., Romero-Torres S., Moshgbar M. Practical considerations in data pre-treatment for NIR and Raman spectroscopy. Am. Pharmaceut. Rev. 2010;13:116. https://www.americanpharmaceuticalreview.com/Featured-Articles/116330-Practical-Considerations-in-Data-Pre-treatment-for-NIR-and-Raman-Spectroscopy/ [Google Scholar]
- Jawaid S., Talpur F.N., Sherazi S., Nizamani S.M., Khaskheli A.A. Rapid detection of melamine adulteration in dairy milk by SB-ATR–Fourier transform infrared spectroscopy. Food Chem. 2013;141:3066–3071. doi: 10.1016/j.foodchem.2013.05.106. [DOI] [PubMed] [Google Scholar]
- Karunathilaka S.R., Farris S., Mossoba M.M., Moore J.C., Yakes B.J. Characterising variances of milk powder and instrumentation for the development of a non-targeted, Raman spectroscopy and chemometrics detection method for the evaluation of authenticity. Food Addit. Contam. 2016;33:921–932. doi: 10.1080/19440049.2016.1188437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunathilaka S.R., Farris S., Mossoba M.M., Moore J.C., Yakes B.J. Non-targeted detection of milk powder adulteration using Raman spectroscopy and chemometrics: melamine case study. Food Addit. Contam. 2017;34:170–182. doi: 10.1080/19440049.2016.1260168. [DOI] [PubMed] [Google Scholar]
- Lim J., Kim G., Mo C., Kim M.S., Chao K., Qin J., Fu X., Baek I., Cho B.K. Detection of melamine in milk powders using near-infrared hyperspectral imaging combined with regression coefficient of partial least square regression model. Talanta. 2016;151:183–191. doi: 10.1016/j.talanta.2016.01.035. [DOI] [PubMed] [Google Scholar]
- Lou T., Wang Y., Li J., Peng H., Xiong H., Chen L. Rapid detection of melamine with 4-mercaptopyridine-modified gold nanoparticles by surface-enhanced Raman scattering. Anal. Bioanal. Chem. 2011;401:333–338. doi: 10.1007/s00216-011-5067-3. [DOI] [PubMed] [Google Scholar]
- Luykx D.M., Cordewener J.H., Ferranti P., Frankhuizen R., Bremer M.G., Hooijerink H., America A.H. Identification of plant proteins in adulterated skimmed milk powder by high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A. 2007;1164:189–197. doi: 10.1016/j.chroma.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Ma P., Liang F., Sun Y., Jin Y., Chen Y., Wang X., Zhang H., Gao D., Song D. Rapid determination of melamine in milk and milk powder by surface-enhanced Raman spectroscopy and using cyclodextrin-decorated silver nanoparticles. Microchim. Acta. 2013;180:1173–1180. [Google Scholar]
- MacMahon S., Begley T.H., Diachenko G.W., Stromgren S.A. A liquid chromatography–tandem mass spectrometry method for the detection of economically motivated adulteration in protein-containing foods. J. Chromatogr. A. 2012;1220:101–107. doi: 10.1016/j.chroma.2011.11.066. [DOI] [PubMed] [Google Scholar]
- Maraboli A., Cattaneo T.M.P., Giangiacomo R. Detection of vegetable proteins from soy, pea and wheat isolates in milk powder by near infrared spectroscopy. J. Near Infrared Spectrosc. 2002;10:63–69. [Google Scholar]
- Moore J.C., Spink J., Lipp M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J. Food Sci. 2012;77:R118–R126. doi: 10.1111/j.1750-3841.2012.02657.x. [DOI] [PubMed] [Google Scholar]
- Nascimento C.F., Santos P.M., Pereira-Filho E.R., Rocha F.R. Recent advances on determination of milk adulterants. Food Chem. 2017;221:1232–1244. doi: 10.1016/j.foodchem.2016.11.034. [DOI] [PubMed] [Google Scholar]
- Pan X.D., Wu P.G., Yang D.J., Wang L.Y., Shen X.H., Zhu C.Y. Simultaneous determination of melamine and cyanuric acid in dairy products by mixed-mode solid phase extraction and GC–MS. Food Contr. 2013;30:545–548. [Google Scholar]
- Qin J., Chao K., Kim M.S. Simultaneous detection of multiple adulterants in dry milk using macro-scale Raman chemical imaging. Food Chem. 2013;138:998–1007. doi: 10.1016/j.foodchem.2012.10.115. [DOI] [PubMed] [Google Scholar]
- Santos P., Pereira-Filho E., Rodriguez-Saona L. Rapid detection and quantification of milk adulteration using infrared microspectroscopy and chemometrics analysis. Food Chem. 2013;138:19–24. doi: 10.1016/j.foodchem.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Scholl P.F., Farris S.M., Mossoba M.M. Rapid turbidimetric detection of milk powder adulteration with plant proteins. J. Agric. Food Chem. 2014;62:1498–1505. doi: 10.1021/jf405617f. [DOI] [PubMed] [Google Scholar]
- Scholl P.F., Bergana M.M., Yakes B.J., Xie Z., Zbylut S., Downey G., Mossoba M.M., Jablonski J.E., Magaletta R., Holroyd S.E. Effects of adulteration technique on the NIR detection of melamine in milk powder. J. Agric. Food Chem. 2017;65:5799–5809. doi: 10.1021/acs.jafc.7b02083. [DOI] [PubMed] [Google Scholar]
- Turnipseed S., Casey C., Nochetto C., Heller D.N. Determination of melamine and cyanuric acid residues in infant formula using LC-MS/MS. Lab. Inf. Bull. 2008;4421:496–549. https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071637.htm [Google Scholar]
- U.S. FDA . 2017. Import Alert 99-29.https://www.accessdata.fda.gov/cms_ia/importalert_267.html [Google Scholar]
- U.S. FDA . 2017. Import Alert 99-30.https://www.accessdata.fda.gov/cms_ia/importalert_401.html [Google Scholar]
- U.S. FDA . 2017. Import Alert 72-05.https://www.accessdata.fda.gov/cms_ia/importalert_421.html [Google Scholar]
- Wiki. Eigenvector . 2017. Confusion Matrix.http://wiki.eigenvector.com/index.php?title=Confusionmatrix [Google Scholar]
- Wu T., Chen H., Lin Z., Tan C. Identification and quantitation of melamine in milk by near-infrared spectroscopy and chemometrics. J. Spectrosc. 2016;2016 [Google Scholar]
- Xin H., Stone R. Chinese probe unmasks high-tech adulteration with melamine. Science. 2008;322:1310–1311. doi: 10.1126/science.322.5906.1310. http://science.sciencemag.org/content/322/5906/1310 [DOI] [PubMed] [Google Scholar]