Abstract
Objective:
Anticonvulsants have been studied for many indications, including posttraumatic stress disorder (PTSD). The limited efficacy research on anticonvulsants for PTSD is mixed. However, anticonvulsants are prescribed widely to Veterans with PTSD. Our objective was to measure trends and factors associated with anticonvulsant prescription among Veterans with PTSD.
Method:
We obtained administrative and pharmacy data for Veterans who initiated PTSD treatment in the Department of Veterans Affairs (VA) between 2004 and 2013 (n=731,520). We identified those who received anticonvulsants during the year following their initial clinical PTSD diagnosis and examined common indications for anticonvulsant use, patient characteristics, and service use characteristics. Using logistic regression, we determined the predictors of anticonvulsant initiation among those without an indication.
Results:
Although 24.9% of patients in the cohort received an anticonvulsant during their initial year of PTSD treatment, 94.6% had an indication unrelated to PTSD and 51.2% initiated anticonvulsant use before their PTSD diagnosis. While there was growth in anticonvulsant initiation over the 10-year period, this was explained both by growth in indications unrelated to PTSD and increased use of anticonvulsants for these indications. The rate of anticonvulsant initiation without an indication was stable at approximately 5% throughout the period, with patient and service use characteristics driving the selection of individual agents.
Conclusion:
A large and increasing proportion of Veterans with PTSD receive anticonvulsant prescriptions. However, this may be appropriate use driven by increased prevalence of comorbid conditions that may be an indication for anticonvulsant use, including pain and headache disorders.
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a mental health condition that sometimes follows exposure to a traumatic event.1 Symptoms include reexperiencing the trauma, avoidance of reminders of the trauma, hyperarousal, and negative cognitions. PTSD affects approximately 6% of the United States (US) population during their lifetime.2 Rates are higher in combat or military-exposed populations such as Veterans who use health services provided by the US Department of Veterans Affairs (VA).3, 4
Convergent findings from recent meta-analyses indicate that four antidepressant medications are effective treatments for PTSD, including the selective serotonin reuptake inhibitors (SSRI) fluoxetine, sertraline, and paroxetine, as well as the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine.5, 6 Despite the availability of a large number of antidepressants to treat PTSD, many patients do not respond to these agents.7–10 Therefore, there has been research into other drug classes such as atypical antipsychotics, adrenergic antagonists, and anticonvulsants as potential treatments for PTSD.5, 6
Anticonvulsants are medications developed to inhibit propagation of seizures in the brain by suppressing the rapid and excessive firing of neurons.11 Typically, anticonvulsants enhance the function of inhibitory ϒ-aminobutyric acid (GABA) or reduce the release of excitatory glutamate through action at sodium or potassium channels.12 The theoretical model relating PTSD to seizure disorder is derived from the similarities between two neurobiological phenomena: kindling and behavioral sensitization.13 In kindling, an electrical or chemical stimulus that was previously subconvulsant elicits a seizure after repeated intermittent administration to the same animal’s brain. In behavioral sensitization, repeated exposure to a noxious environmental stimulus that initially produced little or no response eventually produces a profound response. Therefore, both processes have in common the ability to increase physiological or behavioral responsivity to repeated presentation of the same inducing stimulus.14 As a result of the similarities in proposed mechanisms of hypersensitivity in seizure disorder and in PTSD, anticonvulsants have been suggested as potential treatments for PTSD.15
There have been randomized placebo-controlled trials (RCTs) of at least five different anticonvulsants for the treatment of PTSD. This includes one of the GABA reuptake inhibitor tiagabine,16 one RCT of the sodium channel antagonist lamotrigine,17 one of the calcium channel modulator pregabalin,18 two of the sodium channel antagonist divalproex,19, 20 and four of topiramate,21–24 which has multiple mechanisms of action at sodium channels, calcium channels, and GABA receptors. In summary, a small pilot study indicates that lamotrigine may be a promising medication for PTSD, a single study of pregabalin indicates a significant advantage that may not be clinically meaningful, and topiramate appears to confer a statistically significant and clinically meaningful advantage versus placebo. Current evidence does not support the efficacy of tiagabine or divalproex in the treatment of PTSD. Current practice guidelines do not recommend anticonvulsants as first or second-line treatments for PTSD.25–28
Despite mixed results in clinical trials, one existing study indicates that VA patients with PTSD commonly received anticonvulsants in clinical practice. In a national survey of 482 VA users with new PTSD diagnoses between 2006 and 2007, 81% received treatment with any psychotropic medication.29 Of those who received a medication, 22% were treated with a mood stabilizer (a larger category of medications generally including both lithium and anticonvulsants) in the year following their diagnosis. Even after excluding patients with seizure disorder and bipolar disorder, it appeared that 18% of patients received a mood stabilizer as PTSD treatment during their first year of care. However, many questions remain. Given that anticonvulsants are commonly prescribed for indications other than seizure disorder and bipolar disorder, including pain syndromes, headaches, alcohol use disorder, impulse control disorders, and restless legs syndrome, it is important to account for these additional conditions.
As the evidence is stronger for some agents than others, it is critical to understand which specific anticonvulsants patients with PTSD receive. The publication of RCT results may have affected clinical practice and because some agents have only recently become available, understanding longitudinal use is important. Finally, survey may not be the most appropriate way to understand overall prescription practices in the VA. While survey methods allow researchers to add important explanatory variables not easily abstracted from the electronic medical record such as PTSD severity and interest in various treatments, low response rates may introduce biases.
In this study, we use pharmacy and electronic medical records to understand anticonvulsant receipt for all patients with new episodes of PTSD care from 2004 to 2013. Our objective was to measure trends in anticonvulsant prescriptions among VA users with PTSD and to determine factors associated with initiation of these agents in Veterans without a clear non-PTSD anticonvulsant indication.
Method
Data Source
We used the VA corporate data warehouse (CDW) to identify patients with new PTSD treatment episodes from fiscal year 2004 through fiscal year 2013. We obtained patient demographic information as well as encounter, diagnostic, and pharmacy data from the CDW. The Dartmouth College Committee for the Protection of Human Subjects, the White River Junction VA Medical Center Research and Development Committee, and VA National Data Systems approved this study.
Patients
We included VA users who received a primary diagnosis of PTSD at two or more outpatient encounters, at least one of which occurred in a mental health setting, over the course of 90 days between October 1, 2003 and September 30, 2013, and had not met this criterion during the prior two years. We examined one year of treatment receipt following the first diagnosis of the two qualifying diagnoses. When patients met the cohort inclusion criteria multiple times over the 10-year period, only their first episode was included.
Anticonvulsant Use
We examined all medications dispensed by VA pharmacies during the year following the index PTSD diagnosis. Anticonvulsant drug names were classified into categories for individual agents and an overall category. The anticonvulsant drug class label was used to confirm our coding. We determined both whether patients received each anticonvulsant and whether they received any anticonvulsant during the year.
Potential Moderators: We developed three groups of potential moderators. First, we examined clinical uses for which anticonvulsants are indicated or widely recommended by assessing comorbid diagnoses for the year before and year following the index PTSD diagnosis. These included impulse control disorders, alcohol use disorders, bipolar disorder, schizoaffective disorder, restless legs syndrome, headache disorders, pain disorders, and seizure disorders. We also identified patients who received the plurality of their anticonvulsant prescriptions from neurology or rehabilitation as a marker that the medication might be primarily intended for a non-psychiatric indication. Second, we examined patient factors including age, gender, military service-connected disability for PTSD, psychiatric comorbidity, and history of traumatic brain injury during the year following the index PTSD diagnosis. For history of traumatic brain injury, we also examined the two years before the index PTSD diagnosis. Third, we examined treatment receipt factors during the year following the index PTSD diagnosis. This included number of outpatient mental health visits, whether patients had any inpatient mental health admissions, whether patients received any psychotropic medication, total number of psychotropic medications, and whether patients received the plurality of their anticonvulsant prescriptions from mental health or primary care. To determine whether clinicians attempted to follow relevant treatment recommendations, we also determined whether patients also received a medication recommended for PTSD in clinical practice guidelines from the US Department of Veterans Affairs and Defense (VA/DoD CPGs).27
Analysis
There were five steps to our analysis. First, we summarized cohort characteristics and compared patients who received anticonvulsants with those who did not using t-test or χ2 analysis, as appropriate. Second, we mapped patients’ anticonvulsant prescriptions to diagnostic data indicating possible indications for prescription unrelated to PTSD. We indicated when the anticonvulsant-diagnosis match aligned with a US Food and Drug Administration (FDA) indication. We also indicated when the anticonvulsant-diagnosis match aligned with common off-label prescription recommendations. Because no agency had uniform clinical practice guidelines across all possible indications, we used recommendations from UpToDate®, the clinical decision support tool most commonly used by physicians at the point of care.30. For the last three steps, we focused on anticonvulsant initiation by excluding patients who received anticonvulsants prior to their index PTSD diagnosis. In the third step, we calculated initiation rates for any anticonvulsant and for each individual agent for the overall 10-year period and for each individual fiscal year. We then calculated the absolute and relative change in prescription frequency from 2004 to 2013. In the fourth step, we calculated anticonvulsant initiation rates for patients without any anticonvulsant indication and among those with the most common indications. We then calculated the absolute and relative change in prescription frequency for these groups from 2004 to 2013. In the fifth step, we developed a logistic regression model to predict initiation of any anticonvulsant and the most commonly prescribed anticonvulsants among patients without an indication for anticonvulsant use using multivariate models controlling for patient and service use characteristics. All analyses were completed in SAS (Version 9.4, Carey NC).
Results
Over the 10-year period, 731,520 Veterans met our inclusion criteria, and a quarter received an anticonvulsant during the year following their index PTSD diagnosis. Those who received anticonvulsants differed from those who did not on all patient and service use characteristics (Table 1). Patients who received an anticonvulsant were more likely to be diagnosed with each of the indications for anticonvulsant use. The most common indications were pain disorders, headache disorders, alcohol use disorders, and bipolar and schizoaffective disorders. Patients who received an anticonvulsant were slightly younger, more likely to be women, and had lower rates of military service-connected disability for PTSD. In addition, they had higher rates of psychiatric comorbidities and more commonly had a history of traumatic brain injury. They had more mental health visits, were more likely to be admitted to inpatient psychiatric units, and received a greater number of psychotropic medications, including those recommended for PTSD.
Table 1.
VA Users with New Episodes of PTSD Care from 2004–2013 by Anticonvulsant Use
| Group, % (n) | All Patients (731,520) |
Received AC 24.9 (182,077) |
No AC 75.1 (549,443) |
|---|---|---|---|
| Common Indications for Anticonvulsants | |||
| Any Indication, % (n) | 84.2 (615,587) | 94.6 (172,385) | 80.7 (443,202) |
| Any Indication or Plurality from Neurology/Rehabilitation, % (n) | 84.2 (615,762) | 94.8 (172,560) | 80.7 (443,202) |
| Impulse Control Disorders, % (n) | 1.4 (10,516) | 2.7 (4,982) | 1.0 (5,534) |
| Alcohol Use Disorders, % (n) | 27.1(198,116) | 32.2 (58,586) | 25.4 (139,530) |
| Bipolar & Schizoaffective Disorders, % (n) | 8.0 (58,836) | 19.4 (35,233) | 4.3 (23,603) |
| Restless Legs Syndrome, % (n) | 1.1 (8,284) | 2.0 (3,708) | 0.8 (4,576) |
| Headache Disorders, % (n) | 29.4 (214,780) | 45.4 (82,661) | 24.1 (132,119) |
| Pain Disorders, % (n) | 73.3 (536,172) | 84.9 (154,491) | 69.5 (381,681) |
| Seizure Disorders, % (n) | 3.2 (23,014) | 7.7 (14,049) | 1.6 (8,965) |
| Plurality of AC Prescriptions from Neurology, % (n) | - | 4.0 (7,211) | - |
| Plurality of AC Prescriptions from Rehabilitation, % (n) | - | 1.7 (3,159) | - |
| Indication established before first AC Prescription, % (n) | - | 94.6 (171,872) | - |
| Indication established before Index PTSD Diagnosis, % (n) | 67.5 (493,889) | 79.5 (144,721) | 63.6 (349,168) |
| No non-PTSD Indication, % (n) | 15.8 (115,718) | 5.2 (9,517) | 19.3 (106,241) |
| Patient Characteristics | |||
| Age, M (SD) | 49.9 (15.4) | 49.3 (14.4) | 50.1 (15.7) |
| Men, % (n) | 91.5 (669,663) | 89.4 (162,757) | 92.3 (506,906) |
| Military Service-Connected Disability for PTSD, % (n) | 65.9 (482,048) | 60.7 (110,592) | 67.6 (371,456) |
| Psychotic Disorders (Non-Schizoaffective), % (n) | 4.0 (29,350) | 6.5 (11,792) | 3.2 (17,558) |
| Unipolar Depressive Disorders, % (n) | 58.9 (430,467) | 66.4 (120,843) | 56.4 (309,624) |
| Anxiety Disorders (Non-PTSD), % (n) | 26.1 (191,098) | 31.4 (57,202) | 24.4 (133,896) |
| Personality Disorders, % (n) | 4.0 (29,272) | 7.7 (14,060) | 2.8 (15,212) |
| Substance Use Disorders (Non-Alcohol), % (n) | 33.7 (246,180) | 42.7 (77,710) | 30.7 (168,470) |
| History of Traumatic Brain Injury, % (n) | 9.3 (67,756) | 14.0 (25,554) | 7.7 (42,202) |
| Service Use Characteristics | |||
| Mental Health Visits, M (SD) | 17.6 (22.4) | 24.1 (27.7) | 15.4 (19.8) |
| Inpatient Mental Health Admissions, % (n) | 8.9 (65,040) | 17.0 (31,024) | 6.2 (34,016) |
| Received Any Psychiatric Medication, % (n) | 90.5 (661,904) | 100 (182,077) | 87.3 (479,827) |
| Number of Psychotropic Medications, M (SD) | 3.9 (2.5) | 5.7 (2.9) | 3.2 (2.0) |
| Received AC before Index PTSD Diagnosis, % (n) | 16.9 (123,744) | 51.2 (93,291) | 5.5 (30,453) |
| Any Recommended Medication for PTSD, % (n) | 76.9 (562,773) | 85.2 (155,170) | 74.2 (407,603) |
| Plurality of AC Prescriptions from Mental Health, % (n) | - | 42.5 (77,441) | - |
| Plurality of AC Prescriptions from Primary Care, % (n) | - | 20.0 (36,471) | - |
Note. AC=Anticonvulsant, M=mean, SD=standard deviation; All differences significant at p<0.0001
Gabapentin was by far the most commonly prescribed agent. Most patients who received it were also diagnosed with a pain disorder (Table 2). Gabapentin was also the most-commonly prescribed agent in all other indication groups except impulse control disorders and bipolar and schizoaffective disorders, where valproic acid was most commonly used. An anticonvulsant-diagnosis match aligned with an indication in 79.9% of cases and that percentage rose to 90.0% when including additional recommendations. The most common agents prescribed to the 9,517 Veterans who received an anticonvulsant with no identifiable indication (and thus may have been used as a primary PTSD treatment) were gabapentin, valproic acid, lamotrigine, topiramate, and carbamazepine.
Table 2.
Individual Patients with PTSD Receiving Prescriptions for Each Anticonvulsant by Non-PTSD Indicationa
| By Individual Indication | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anticonvulsant Rank |
Overall | No Indication Identified |
Impulse Control Disorders |
Alcohol Use Disorders |
Bipolar & Schizoaffective Disorders |
Restless Legs Syndrome |
Headache Disorders |
Pain Disorders | Seizure Disorders |
| 1 | Gabapentin 102,791 |
Gabapentin 4,605 |
Valproic Acid 2,464 |
Gabapentin 32,192 |
Valproic Acid 17,567 |
Gabapentin 2,650 |
Gabapentin 47,076 |
Gabapentin 92,459 |
Gabapentin 5,976 |
| 2 | Valproic Acid 49,197 |
Valproic Acid 2,761 |
Gabapentin 2,127 |
Valproic Acid 19,128 |
Gabapentin 12,205 |
Valproic Acid 640 |
Valproic Acid 21,146 |
Valproic Acid 39,312 |
Valproic Acid 3,737 |
| 3 | Topiramate 22,803 |
Lamotrigine 915 |
Lamotrigine 543 |
Topiramate 6,516 |
Lamotrigine 8,198 |
Topiramate 426 |
Topiramate 16,414 |
Topiramate 19,471 |
Levetiracetam 2,048 |
| 4 | Lamotrigine 17,009 |
Topiramate 670 |
Topiramate 486 |
Lamotrigine 5,902 |
Topiramate 3,794 |
Lamotrigine 319 |
Lamotrigine 7,196 |
Lamotrigine 13,552 |
Topiramate 1,936 |
| 5 | Carbamazepine 8,627 |
Carbamazepine 482 |
Carbamazepine 418 |
Carbamazepine 3,007 |
Carbamazepine 2,841 |
Pregabalin 207 |
Carbamazepine 3,794 |
Carbamazepine 6,973 |
Phenytoin 1,930 |
| 6 | Pregabalin 4,748 |
Phenytoin 340 |
Phenytoin 108 |
Phenytoin 1,717 |
Oxcarbazepine 651 |
Carbamazepine 134 |
Pregabalin 3,056 |
Pregabalin 4,528 |
Lamotrigine 1,684 |
| 7 | Phenytoin 4,631 |
Levetiracetam 143 |
Oxcarbazepine 102 |
Levetiracetam 1,253 |
Phenytoin 597 |
Levetiracetam 95 |
Levetiracetam 1,798 |
Phenytoin 3,574 |
Primidone 1,488 |
| 8 | Levetiracetam 3,746 |
Primidone 116 |
Pregabalin 86 |
Pregabalin 1,129 |
Pregabalin 594 |
Primidone 94 |
Phenytoin 1,696 |
Levetiracetam 3,033 |
Carbamazepine 1,201 |
| 9 | Primidone 2,637 |
Pregabalin 86 |
Levetiracetam 83 |
Primidone 613 |
Levetiracetam 513 |
Phenytoin 57 |
Primidone 804 |
Primidone 2,122 |
Pregabalin 431 |
| 10 | Oxcarbazepine 1,482 |
Oxcarbazepine 65 |
Primidone 39 |
Oxcarbazepine 570 |
Primidone 331 |
Oxcarbazepine 25 |
Oxcarbazepine 722 |
Oxcarbazepine 1,204 |
Oxcarbazepine 262 |
| 11 | Zonisamide 230 |
Tiagabine 19 |
Zonisamide 3 |
Tiagabine 59 |
Tiagabine 36 |
Zonisamide 6 |
Zonisamide 150 |
Zonisamide 190 |
Zonisamide 100 |
| 12 | Tiagabine 194 |
Zonisamide 6 |
Tiagabine 2 |
Zonisamide 48 |
Zonisamide 31 |
Tiagabine 0 |
Tiagabine 83 |
Tiagabine 151 |
Tiagabine 22 |
| Total | 182,077 | 9,517 | 4,982 | 58,586 | 35,233 | 3,708 | 82,661 | 154,491 | 14,049 |
Note. All patients are included.
FDA approved indication 79.7% (145,155/182,077)
Add additional recommendations 90.0% (163,848/182,077)
Among the 607,776 Veterans who did not receive an anticonvulsant prior to their index PTSD diagnosis, 14.6% initiated any anticonvulsant in the subsequent year. This proportion grew over the 10-year period from 11.6% to 17.5%, a 50.9% relative increase (Figure 1). Most of the increase in anticonvulsant initiation was due to gabapentin, which almost doubled from 5.4% to 10.2%. Whereas initiation of valproic acid was nearly as common as initiation of gabapentin in the early years of the cohort, initiation of both valproic acid (4.3% to 4.1%) and carbamazepine (0.6% to 0.5%) decreased slightly over time. There were large relative increases in initiation of topiramate (1.2% to 2.8%; a 141.7% relative increase) and lamotrigine (0.6% to 1.9%; a 185.9% relative increase) over time, but use of these medications accounted for relatively few anticonvulsant starts.
Figure 1.
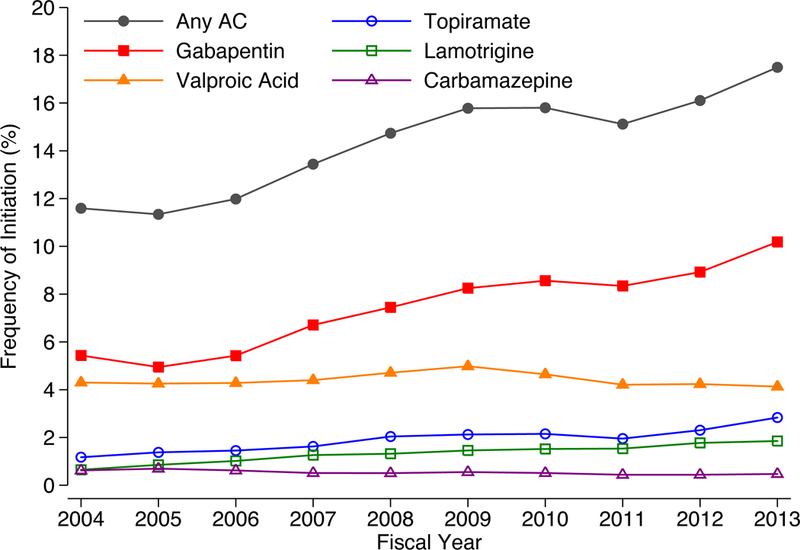
Frequency of Anticonvulsant Initiation Among Veterans with New Episodes of PTSD Carea
The proportion of patients without an indication for anticonvulsants decreased over time (22.0% to 15.8%; a 28.2% relative decrease) and initiation of anticonvulsants in this group was stable, ranging between 4.8% and 5.2% during the 10-year period (Table 3). However, growing populations of patients with comorbid pain and headache disorders were over 50% more likely to receive an anticonvulsant in 2013 than in 2003. While there was little growth in alcohol use disorders and a decrease in bipolar and schizoaffective disorders, initiation of anticonvulsants also became more common in these groups.
Table 3.
Frequency of Most Common Anticonvulsant Indication and Initiation Among Veterans with New Episodes of PTSD Care, 2004–2013a
| Frequency of Medication Use by Fiscal Year (number of veterans), % |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | Change | ||
| (607,776) | (43,080) | (50,822) | (50,019) | (59,179) | (65,517) | (69,127) | (70,338) | (68,915) | (64,354) | (66,425) | Abs.b | Rel.c | |
| No Indication Identified | |||||||||||||
| Patients in Category | 18.1% | 22.0% | 21.2% | 20.8% | 22.5% | 18.0% | 16.8% | 16.8% | 17.1% | 16.7% | 15.8% | −6.2% | −28.2% |
| Received Anticonvulsant | 5.0% | 5.2% | 5.1% | 5.0% | 4.8% | 5.1% | 5.1% | 5.0% | 4.8% | 5.1% | 5.2% | 0.0% | −0.4% |
| Comorbid Pain Disorders | |||||||||||||
| Patients in Category | 70.5% | 66.0% | 67.6% | 68.0% | 82.3% | 70.7% | 71.4% | 71.9% | 71.5% | 72.3% | 73.1% | 7.0% | 10.7% |
| Received Anticonvulsant | 17.0% | 13.4% | 12.9% | 13.9% | 15.7% | 17.1% | 18.5% | 18.5% | 17.6% | 18.6% | 20.3% | 6.9% | 51.4% |
| Comorbid Headache Disorders | |||||||||||||
| Patients in Category | 26.3% | 20.4% | 20.9% | 21.7% | 28.0% | 27.4% | 28.8% | 28.2% | 27.6% | 28.8% | 30.4% | 10.0% | 49.1% |
| Received Anticonvulsant | 25.1% | 19.0% | 20.0% | 20.0% | 23.2% | 25.4% | 26.6% | 26.6% | 25.5% | 27.3% | 29.0% | 10.0% | 52.4% |
| Comorbid Alcohol Use Disorders | |||||||||||||
| Patients in Category | 26.2% | 25.9% | 24.8% | 24.7% | 29.6% | 25.9% | 27.5% | 27.7% | 27.1% | 26.7% | 26.0% | 0.1% | 0.4% |
| Received Anticonvulsant | 18.4% | 14.5% | 14.1% | 15.7% | 17.4% | 18.4% | 19.7% | 19.3% | 19.1% | 20.6% | 21.9% | 7.4% | 51.2% |
| Comorbid Bipolar and Schizoaffective Disorders | |||||||||||||
| Patients in Category | 5.2% | 5.9% | 5.3% | 5.4% | 6.4% | 5.1% | 5.3% | 5.2% | 4.8% | 5.0% | 5.0% | −0.9% | −14.5% |
| Received Anticonvulsant | 40.7% | 34.5% | 35.7% | 36.5% | 40.4% | 40.5% | 42.0% | 44.1% | 42.9% | 43.5% | 43.1% | 8.6% | 24.9% |
Note. Excludes patients who received an anticonvulsant before their index PTSD diagnosis and comorbidities were established before the initiation of the anticonvulsant.
Absolute change in frequency of use from fiscal years 2004 through 2013; negative values represent decreased frequency over time.
Relative change in frequency of use from fiscal years 2004 through 2013; negative values represent decreased frequency over time.
Among the group of 87,626 patients without an identifiable indication for an anticonvulsant who were treated with any psychotropic medication, 6.3% initiated an anticonvulsant. Multivariate logistic regression models to predict the initiation of anticonvulsants in general and of specific agents had good discriminative ability, with c-statistics of approximately 0.7 (Table 4). Compared to those who received any psychotropic medication, patients who initiated an anticonvulsant were likely to be younger men with personality disorders and a history of traumatic brain injury who did not receive one of the recommended medications for PTSD despite being prescribed a high number of psychotropic medications.
Table 4.
Predictors of Anticonvulsant Initiation Among Patients who Received a Psychotropic Medication and had no Obvious Indication for an Anticonvulsanta
| Regression Model Characteristics | ||||||
|---|---|---|---|---|---|---|
| Inclusion Criteria for Model | All (n=87,626) | Received Specific AC (n=5,532) | ||||
| Dependent Variable (Received Agent) | Any AC | Gabapentin | Valproic Acid | Lamotrigine | Topiramate | Carbamazepine |
| Prescription Status, % (n) | 6.31% (5,532) | 41.6% (2302) | 35.6% (1,969) | 12.0% (665) | 8.4% (465) | 3.7%(204) |
| Model Performance | c=0.74 | c=0.75 | c=0.72 | c=0.72 | c=0.67 | c=0.65 |
| Notation | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Patient Characteristics | ||||||
| Age >= 55 |
0.81 (0.76–0.86) |
2.21 (1.94–2.52) |
0.54 (0.48–0.62) |
0.84 (0.70–1.01) |
0.76 (0.61–0.94) |
0.90 (0.67–1.23) |
| Men |
1.20 (1.07–1.36) |
1.15 (0.87–1.51) |
2.79 (2.12–3.67) |
0.38 (0.29–0.50) |
0.31 (0.23–0.43) |
1.47 (0.75–2.87) |
| Psychotic Disorders (Non-Schizoaffective) |
0.81 (0.69–0.96) |
0.49 (0.33–0.71) |
1.55 (1.11–2.17) |
1.15 (0.72–1.83) |
0.91 (0.50–1.63) |
1.07 (0.50–2.25) |
| Unipolar Depressive Disorders | 1.02 (0.96–1.08) |
1.00 (0.88–1.13) |
0.77 (0.68–0.87) |
1.70 (1.41–2.06) |
1.03 (0.84–1.26) |
0.85 (0.63–1.15) |
| Anxiety Disorders | 0.96 (0.90–1.03) |
1.04 (0.90–1.20) |
1.03 (0.90–1.18) |
0.86 (0.71–1.05) |
1.02 (0.82–1.28) |
1.24 (0.90–1.71) |
| Personality Disorders |
1.53 (1.28–1.84) |
0.71 (0.47–1.05) |
1.64 (1.15–2.34) |
1.16 (0.73–1.84) |
0.69 (0.37–1.29) |
1.75 (0.90–3.41) |
| Non-Alcohol Substance Use Disorders |
0.91 (0.86–0.98) |
1.22 (1.06–1.41) |
0.99 (0.85–1.14) |
0.98 (0.80–1.19) |
0.73 (0.57–0.94) |
0.68 (0.47–0.98) |
| History of Traumatic Brain Injury |
1.35 (1.18–1.56) |
0.77 (0.57–1.05) |
1.38 (1.05–1.80) |
0.63 (0.41–0.97) |
2.27 (1.58–3.27) |
1.34 (0.73–2.45) |
| Service Use Characteristics | ||||||
| Fiscal Years 2008–2013 (v. 2004–2007) | 1.03 (0.97–1.09) |
1.49 (1.32–1.70) |
0.65 (0.57–0.73) |
1.71 (1.41–2.06) |
0.76 (0.62–0.93) |
0.65 (0.47–0.87) |
| 5–13 Mental Health Visits (v. 4 or fewer) |
1.41 (1.30–1.53) |
1.04 (0.87–1.24) |
1.06 (0.89–1.27) |
1.13 (0.87–1.49) |
1.88 (0.67–1.17) |
0.73 (0.49–1.07) |
| >= 14 Mental Health Visits (v. 4 or fewer) |
1.82 (1.66–1.98) |
1.25 (1.04–1.52) |
0.95 (0.78–1.15) |
1.41 (1.06–1.87) |
0.84 (0.62–1.14) |
0.60 (0.39–0.93) |
| One or More Inpatient Mental Health Admission |
1.70 (1.48–1.94) |
0.74 (0.56–0.99) |
1.12 (0.86–1.45) |
0.67 (0.46–1.00) |
0.81 (0.51–1.27) |
2.26 (1.39–3.68) |
| Three or More Psychiatric Medications (v. 1 or 2) |
6.15 (5.73–6.60) |
0.81 (0.69–0.96) |
1.12 (0.94–1.32) |
1.36 (1.06–1.74) |
1.37 (1.01–1.84) |
1.73 (1.12–2.68) |
| Any Recommended Medication for PTSD |
0.41 (0.38–0.44) |
1.22 (1.03–1.45) |
1.08 (0.91–1.28) |
0.51 (0.41–0.64) |
1.29 (0.96–1.73) |
0.73 (0.49–1.07) |
| Measures that Apply to AC Population Only | ||||||
| Plurality of AC Prescriptions from Mental Health | - |
0.24 (0.21–0.28) |
3.92 (3.29–4.67) |
3.69 (2.74–4.97) |
1.49 (1.13–1.96) |
1.36 (0.92–1.99) |
| Plurality of AC Prescriptions from Primary Care | - |
1.64 (1.34–2.02) |
0.70 (0.54–0.91) |
0.81 (0.52–1.28) |
0.66 (0.43–1.01) |
0.48 (0.25–0.94) |
Note. AC=Anticonvulsant, M=mean, SD=standard deviation, C=C-statistic
OR and 95% CI less than 1
OR and 95% CI greater than 1
Different patterns emerged in predicting the choice of specific agents among the subgroup of 5,532 patients who initiated any anticonvulsant in the absence of an identifiable indication. Patients who received gabapentin were older non-psychotic Veterans with substance use disorders. They tended to receive their gabapentin from their primary care doctors. These patients also were more often prescribed recommended medications for PTSD. While they took fewer psychotropic medications and were less likely to be admitted to inpatient psychiatry, a high number of mental health visits was also associated with increased odds of receiving gabapentin. Patients who received valproic acid tended to be younger men with psychotic and personality disorders as well as traumatic brain injuries that were seen by psychiatrists. Patients who received lamotrigine tended to be women with unipolar depressive disorders who were seen by psychiatrists and did not receive a recommended medication for PTSD. Patients who received topiramate tended to be younger women with traumatic brain injury who were seen by a psychiatrist. The small and declining number of patients who received carbamazepine tended to receive their anticonvulsants from their primary care physicians, although they had higher odds of inpatient psychiatric admission and receiving a high number of psychiatric medications.
Discussion
Using administrative and pharmacy data, we confirmed prior survey results suggesting that a large proportion of patients with PTSD receive an anticonvulsant. Furthermore, we showed that this practice is increasing over time. However, we differ from prior research in finding that almost all patients receiving these agents have an indication for anticonvulsant use unrelated to PTSD. Thus, it appears most anticonvulsant prescriptions in patients with PTSD are given appropriately for other disorders or health problems. By examining the many reasons for anticonvulsant initiation over our 10-year window, we have found an increase in prevalence of conditions that may be an indication for anticonvulsant use. However, it is important to note that using administrative data, we cannot tell whether anticonvulsants were prescribed specifically for these indications. At the same time, neuropsychiatric comorbidity appears to drive anticonvulsant initiation in the small number of Veterans without an identifiable indication. While it is possible that PTSD contributes to a physician’s choice to prescribe an anticonvulsant, there is every indication that VA physicians are choosing these agents parsimoniously based on complex constellations of medical and psychiatric problems.
It is important to highlight the complexity of Veterans initiating treatment for PTSD in the VA and to note that many of these Veterans may be refractory to the first-line recommended medications for PTSD. Regardless of whether they receive an anticonvulsant, 84.2% had a diagnosis for which anticonvulsants are indicated or recommended. This includes 73.3% with a pain disorder, 29.4% with a headache disorder and 27.1% with an alcohol use disorder. For most patients in our cohort, PTSD is one of many concerns. It is possible that in these cases, physicians chose medications they hope will have a positive effect on as many problems as possible. For example, recent evidence indicates that in addition to helping decrease alcohol consumption,31 topiramate decreases PTSD symptoms in patients with comorbid alcohol use disorders and PTSD.32 Future anticonvulsant trials should address other major comorbidity groups such as patients with PTSD and pain or headaches.
Even in the case of patients without an identifiable indication for anticonvulsant use, comorbidity appears to drive the selection of individual agents. For example, lamotrigine is indicated for the treatment and prevention of depressive episodes among patients with bipolar disorder.33 While there is some indication that this medication is helpful for PTSD symptoms,17 it was prescribed preferentially to patients with a comorbid unipolar depressive disorder in our cohort. This perhaps represents clinicians’ application of the evidence in bipolar depression to unipolar depression, though two recently published negative RCTs of lamotrigine for unipolar depression indicate this is an incorrect extension of the literature.34,35. It is unclear why gabapentin was the most frequently initiated agent among patients without an identifiable indication for an anticonvulsant. A case series of 30 patients treated in a PTSD specialty clinic at one VA medical center between 1997 and 2000 indicated that gabapentin was most commonly initiated for PTSD-related sleep difficulties, with clinicians reporting positive results.36. Given our finding that gabapentin is the most popularly prescribed anticonvulsant among Veterans with PTSD and that there have been no RCTs of gabapentin for PTSD,37 the authors’ call for prospective, controlled studies continues to be well-justified.
There are several limitations to this study. Firstly, it is a retrospective cohort study. We were not able to ask patients and clinicians why they chose to use anticonvulsants as part of their treatment plans. While we believe our work to be the most detailed analysis in this area, it is possible that both our choices around cohort construction and clinical coding errors could have unduly influenced our findings. Secondly, we included only patients with PTSD in our cohort. Therefore, it is not possible to tell how a PTSD diagnosis influences the decision to prescribe an anticonvulsant. Finally, while FDA indications for several anticonvulsants are in place for a relatively narrow spectrum of pain disorders (trigeminal, glossopharyngeal, post-herpetic, and diabetic neuralgia as well as fibromyalgia), it appears that these agents are being prescribed to patients with a wider range of pain disorders. There has not been extensive study of anticonvulsants for those other pain conditions.
In summary, our findings indicate that most anticonvulsant prescribing among VA PTSD patients appears to be reasonable and justified by high levels of comorbidity. In the small group of patients with PTSD who have no identifiable indication for an anticonvulsant, comorbidity appears to drive the selection of individual agents. Overall, it appears that when treating highly complex patients with PTSD, VA physicians try to use medications likely to have a positive effect on as many conditions as possible. Additional prospective research on the use of anticonvulsants in major comorbidity groups, such as patients with pain and PTSD is indicated.
Clinical Points:
-
-
Despite mixed results in clinical trials, existing data indicate that VA patients with PTSD commonly receive anticonvulsants in clinical practice.
-
-
However, given that anticonvulsants are commonly prescribed for other indications, is important to account for comorbidity.
-
-
While it is possible that PTSD contributes to a physician’s choice to prescribe an anticonvulsant, there is every indication that VA physicians are choosing these agents parsimoniously based on complex constellations of medical and psychiatric problems.
acknowledgements:
This project was supported was supported by a Department of Veterans Affairs Health Services Research and Development Career Development Award (CDA11–263), VA Office of Research and Development, Washington, DC (Dr. Shiner). The sponsor did not have any role in the study design, methods, analysis, and interpretation of results, or in preparation of the manuscript and the decision to submit it for publication. The views expressed in this article are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs.
Footnotes
Previous Presentations: None
Disclosures: The authors report no conflicts of interest.
Additional Information: The VA Corporate Data Warehouse (CDW) contains electronic medical record data compiled from individual VA facilities and is described at http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm. Data are stored on geographically dispersed server farms. To access the CDW, researchers generally need to have an employment relationship with the VA. After local institutional review board approval, requests for data are submitted to VA National Data Systems using the Data Access Request Tracker. Datasets are then built and analyzed in secure virtual project workspaces within the VA Informatics and Computing Infrastructure environment. Researchers with VA network access can obtain descriptions of CDW data at http://vaww.virec.research.va.gov/.
Note. Excludes patients who received an anticonvulsant before their index PTSD diagnosis. AC=Anticonvulsant
Contributor Information
Brian Shiner, Veterans Affairs Medical Center, White River Junction, VT, and Assistant Professor of Psychiatry, Geisel School of Medicine at Dartmouth, Hanover, NH.
Christine Leonard Westgate, Veterans Affairs Medical Center, White River Junction, VT.
Nancy C. Bernardy, National Center for PTSD, White River Junction, VT, and Associate Professor of Psychiatry, Geisel School of Medicine at Dartmouth, Hanover, NH.
Paula P. Schnurr, National Center for PTSD, White River Junction, VT, and Research Professor of Psychiatry, Geisel School of Medicine at Dartmouth, Hanover, NH.
Bradley V. Watts, National Center for Patient Safety, Ann Arbor, MI, and Assistant Professor of Psychiatry, Geisel School of Medicine at Dartmouth, Hanover, NH.
REFERENCES
- 1.APA. Diagnostic and statistical manual of mental disorders : DSM-5 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of anxiety disorders 2011. April;25(3):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry 2005. May-Jun;27(3):169–179. [DOI] [PubMed] [Google Scholar]
- 4.Shiner B, Drake RE, Watts BV, Desai RA, Schnurr PP. Access to VA services for returning veterans with PTSD. Military medicine 2012. July;177(7):814–822. [DOI] [PubMed] [Google Scholar]
- 5.Jonas DE, Cusack K, Forneris CA, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder (PTSD) Rockville (MD); 2013. [PubMed] [Google Scholar]
- 6.Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. The Journal of clinical psychiatry 2013. June;74(6):e541–550. [DOI] [PubMed] [Google Scholar]
- 7.Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. Jama 2000. April 12;283(14):1837–1844. [DOI] [PubMed] [Google Scholar]
- 8.Connor KM, Sutherland SM, Tupler LA, Malik ML, Davidson JR. Fluoxetine in post-traumatic stress disorder. Randomised, double-blind study. The British journal of psychiatry : the journal of mental science 1999. July;175:17–22. [DOI] [PubMed] [Google Scholar]
- 9.Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Archives of general psychiatry 2006. October;63(10):1158–1165. [DOI] [PubMed] [Google Scholar]
- 10.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. The American journal of psychiatry 2001. December;158(12):1982–1988. [DOI] [PubMed] [Google Scholar]
- 11.Lado FA, Moshe SL. How do seizures stop? Epilepsia 2008. October;49(10):1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nature medicine 2004. July;10(7):685–692. [DOI] [PubMed] [Google Scholar]
- 13.Friedman MJ. Neurobiological sensitization models of post-traumatic stress disorder: their possible relevance to multiple chemical sensitivity syndrome. Toxicology and industrial health 1994. Jul-Oct;10(4–5):449–462. [PubMed] [Google Scholar]
- 14.Post RM, Weiss SRB, Smith MA. Sensitization and Kindling: Implications for the Evolving Neural Substrates of Post-Traumatic Stress Disorder. In: Friedman MJ, Charney DS, Deutch AY, eds. Neurbiological and Clinical Consequences of Stress Philadelphia: Lippincott-Raven Publishers; 1995: 203–224. [Google Scholar]
- 15.Post RM, Weiss SR, Li H, Leverich GS, Pert A. Sensitization components of post-traumatic stress disorder: implications for therapeutics. Seminars in clinical neuropsychiatry 1999. Oct;4(4):282–294. [DOI] [PubMed] [Google Scholar]
- 16.Davidson JR, Brady K, Mellman TA, Stein MB, Pollack MH. The efficacy and tolerability of tiagabine in adult patients with post-traumatic stress disorder. Journal of clinical psychopharmacology 2007. February;27(1):85–88. [DOI] [PubMed] [Google Scholar]
- 17.Hertzberg MA, Butterfield MI, Feldman ME, et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biological psychiatry 1999. May 1;45(9):1226–1229. [DOI] [PubMed] [Google Scholar]
- 18.Baniasadi M, Hosseini G, Fayyazi Bordbar MR, Rezaei Ardani A, Mostafavi Toroghi H. Effect of pregabalin augmentation in treatment of patients with combat-related chronic posttraumatic stress disorder: a randomized controlled trial. Journal of psychiatric practice 2014. November;20(6):419–427. [DOI] [PubMed] [Google Scholar]
- 19.Davis LL, Davidson JR, Ward LC, Bartolucci A, Bowden CL, Petty F. Divalproex in the treatment of posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial in a veteran population. Journal of clinical psychopharmacology 2008. February;28(1):84–88. [DOI] [PubMed] [Google Scholar]
- 20.Hamner MB, Faldowski RA, Robert S, Ulmer HG, Horner MD, Lorberbaum JP. A preliminary controlled trial of divalproex in posttraumatic stress disorder. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists 2009. Apr-Jun;21(2):89–94. [PubMed] [Google Scholar]
- 21.Yeh MS, Mari JJ, Costa MC, Andreoli SB, Bressan RA, Mello MF. A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD. CNS neuroscience & therapeutics 2011. October;17(5):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tucker P, Trautman RP, Wyatt DB, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. The Journal of clinical psychiatry 2007. February;68(2):201–206. [DOI] [PubMed] [Google Scholar]
- 23.Lindley SE, Carlson EB, Hill K. A randomized, double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. Journal of clinical psychopharmacology 2007. December;27(6):677–681. [DOI] [PubMed] [Google Scholar]
- 24.Akuchekian S, Amanat SA. A comparison of topiramate and placebo in the treatment of posttraumatic stress disorder: a randomized, double-blind study. J Res Med Sci 2004;5(5):240–244. [Google Scholar]
- 25.Bisson J, Ehlers A. Clinical Guideline 26: Post-traumatic stress disorder (PTSD): the management of PTSD in adults and children in primary and secondary care. National Institute for Clinical Excellence, http://www.nice.org.uk/nicemedia/pdf/CG026NICEguideline.pdf; 2005. [Google Scholar]
- 26.Foa EB, Keane TM, Friedman MJ, Cohen JA, eds. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. Second Edition ed. New York: The Guilford Press; 2010. [Google Scholar]
- 27.Friedman MJ, Lowry P, Ruzek J. VA/DoD clinical practice guidelines for the management of post-traumatic stress (page 150). United States Departments of Veterans Affairs and Defense, http://www.healthquality.va.gov; 2010. [Google Scholar]
- 28.Benedek DM, Friedman MJ, Zatzick D, Ursano RJ. Guideline Watch (March 2009): Practice Guideline for the Treatment of Patients with Acute Stress Disorder and Posttraumatic Stress Disorder 2009. [cited May 31, 2016]; https://psychiatry.org/psychiatrists/practice/clinical-practice-guidelines]. Available from: [PubMed] [Google Scholar]
- 29.Jain S, Greenbaum MA, Rosen C. Concordance between psychotropic prescribing for veterans with PTSD and clinical practice guidelines. Psychiatric services 2012. Feb 1;63(2):154–160. [DOI] [PubMed] [Google Scholar]
- 30.Marshall JG, Sollenberger J, Easterby-Gannett S, et al. The value of library and information services in patient care: results of a multisite study. Journal of the Medical Library Association : JMLA 2013. January;101(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcoholism, clinical and experimental research 2014. June;38(6):1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batki SL, Pennington DL, Lasher B, et al. Topiramate treatment of alcohol use disorder in veterans with posttraumatic stress disorder: a randomized controlled pilot trial. Alcoholism, clinical and experimental research 2014. August;38(8):2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhavalkar KS, Poovanpallil NB, Bhatt LK. Management of bipolar depression with lamotrigine: an antiepileptic mood stabilizer. Frontiers in pharmacology 2015;6:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos MA, Rocha FL, Hara C. Efficacy and safety of antidepressant augmentation with lamotrigine in patients with treatment-resistant depression: a randomized, placebo-controlled, double-blind study. Primary care companion to the Journal of clinical psychiatry 2008;10(3):187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbee JG, Thompson TR, Jamhour NJ, et al. A double-blind placebo-controlled trial of lamotrigine as an antidepressant augmentation agent in treatment-refractory unipolar depression. The Journal of clinical psychiatry 2011. October;72(10):1405–1412. [DOI] [PubMed] [Google Scholar]
- 36.Hamner MB, Brodrick PS, Labbate LA. Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists 2001. September;13(3):141–146. [DOI] [PubMed] [Google Scholar]
- 37.Wang HR, Woo YS, Bahk WM. Anticonvulsants to treat post-traumatic stress disorder. Human psychopharmacology 2014. September;29(5):427–433. [DOI] [PubMed] [Google Scholar]


