Abstract
The development of head and neck squamous cell carcinoma (HNSCC) is closely associated with inflammation. Tumor associated macrophages (TAMs), the largest population of inflammatory cells in the tumor stroma, serve an important role in accelerating cancer progression. The present study aimed to investigate the role of TAMs in the metastasis of HNSCC. TAM biomarkers and epithelial to mesenchymal transition (EMT)-associated proteins were detected using immunohistochemical and immunofluorescence staining in HNSCC. Then, direct and indirect co-culture systems of TAMs and HNSCC cells were established. The EMT-associated proteins and associated signaling pathways in HNSCC cells of the co-culture system were measured by reverse transcription-quantitative polymerase chain reaction and western blotting. Finally, hierarchical clustering was performed to analyze associations among TAM biomarkers, epidermal growth factor receptor (EGFR), activated extracellular signal-regulated protein kinase 1/2 (ERK1/2) and EMT-associated proteins in HNSCC tissues. The results indicated that the expression of EMT-associated proteins was positively associated with M2 macrophage biomarkers in HNSCC tissues. Cal27 cells were isolated from the co-culture system by fluorescence-activated cell sorting, and it was identified that E-cadherin was downregulated in Cal27 cells, while Vimentin and Slug were upregulated. Furthermore, the results indicated that EGF released by M2 macrophages in the co-culture served an important role by activating ERK1/2. The correlation and cluster analyses indicated that activated ERK1/2 was positively correlated with cluster of differentiation-163, EGFR, Vimentin and Slug. This suggested that TAMs may induce the EMT of cancer cells by activating the EGFR/ERK1/2 signaling pathway in HNSCC, which may be a promising approach to suppressing cancer metastasis.
Keywords: HNSCC, EMT, metastasis, invasion, tumor associated macrophages, EGF, ERK
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the most common types of cancer worldwide (1). Approximately 550,000 new patients are diagnosed, and 30,000 patients succumb to this disease each year (2). HNSCC has varying levels of differentiation, and a propensity for lymph node metastasis and poor prognosis (3,4). Over the past few years, there has been little improvement in the five-year survival rate using the current therapies available. Therefore, in light of the high recurrence rates and limited treatment options, it is necessary to investigate the invasive molecular mechanism of HNSCC.
Tumor-associated macrophages (TAMs) are a key component of the tumor microenvironment and are crucial for cancer development (5). The majority of macrophages originate from monocytes in the circulation and form an extremely heterogeneous population. Activated macrophages are classified into M1 and M2 macrophages. M1 is classically activated, while M2 is activated via an alternative pathway (6,7). TAMs, similar to the M2 phenotype, promote cancer progression by accelerating new blood cell formation and mediating immunoregulation (8,9). High levels of TAMs are often associated with a poor prognosis (10). Therefore, studying the mechanism of the interaction between TAMs and tumors may provide important targets for cancer therapy.
Epithelial to mesenchymal transition (EMT) increases the motility of cancer cells by altering epithelial cell morphology and apicobasal polarity, changing cellular contact patterns and reducing cell adhesion (11,12). It has been reported that macrophages may directly induce the EMT of breast cancer cells in a juxtacrine manner, thereby establishing an environment suitable for the growth of tumor stem cells (13). TAMs are often located in the invasive front of cancer cells, where the majority of cancer cells are undergoing EMT (14). Our previous study indicated that HNSCC cells could induce monocytes to differentiate into M2 macrophages via a C-C motif chemokine ligand 2 and C-C chemokine receptor type 2 pathway (15). In addition, it was identified that macrophages were likely to induce the EMT of HNSCC cells, as the majority of macrophages were located at the edge of the scratch in the wound healing assay. Pirilä et al (16) reported that M2 macrophages co-cultured with HSC-3 cells increased the expression of epidermal growth factor (EGF), transforming growth factor-β (TGF-β) and macrophage colony-stimulating factor (M-CSF). Activation of the EGF and/or TGF-β signaling pathways and their downstream cascade may trigger the EMT process in various types of cancer cells (17,18). However, the mechanism by which TAMs in HNSCC induce the EMT of tumor cells remains unknown.
In the present study, the expression of TAMs and EMT-associated proteins in the HNSCC tissues were detected, and the correlations between them were evaluated. Direct and indirect co-culture systems of TAMs and HNSCC cells were established, and the involved extracellular and intracellular signaling pathways were examined. To the best of our knowledge, this is the first study to suggest that TAMs induce the EMT of HNSCC cells primarily by activating the EGF receptor (EGFR)/extracellular signal-regulated kinase1/2 (ERK1/2) signaling pathway. This may provide a potential therapeutic strategy for suppressing tumor invasion and migration in HNSCC.
Materials and methods
Patient samples
A total of 56 paraffin-embedded human HNSCC specimens and 10 normal adjacent mucous samples that were histopathologically diagnosed at Second Hospital of Dalian Medical University (Dalian, China) from January 2010 to December 2014 were included in the present study. The detailed pathological and clinical data for all of the samples are presented in Table I. The use of human tissues was approved by the Medical Ethics Committee of Dalian Medical University and written informed consent was provided by each patient. Specimens that were obtained from patients treated with radiotherapy and chemotherapy were excluded from the present study. The procedure followed the USA National Institutes of Health guidelines (19) regarding use of human tissues.
Table I.
Clinical characteristics of patients and the 56 HNSCC and 10 normal tissues.
| Macrophages infiltration | ||||
|---|---|---|---|---|
| Clinical characteristic | Total cases (n) | Negative | Low | High |
| Normal and adjacent tissue | 10 | 7 | 3 | – |
| HNSCC | 56 | – | 25 | 31 |
| Age, years | ||||
| ≤45 | 12 | – | 4 | 8 |
| >45 | 44 | – | 21 | 23 |
| Sex | ||||
| Male | 36 | – | 12 | 24 |
| Female | 20 | – | 13 | 7 |
| TNM grading | ||||
| Stage I | 21 | – | 14 | 7 |
| Stage II | 24 | – | 8 | 16 |
| Stage III | 8 | – | 3 | 5 |
| Stage IV | 3 | – | 0 | 3 |
| Histological differentiation | ||||
| Well | 33 | – | 18 | 15 |
| Moderately | 18 | – | 6 | 12 |
| Poorly | 5 | – | 1 | 4 |
HNSCC, head and neck squamous cell carcinoma; TNM, tumor-node-metastasis.
Cell culture
THP1 [human acute monocytic leukemia cell line; China Center for Type Culture Collection (CCTCC), Wuhan, China] cells were maintained in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Cal27 (oral tongue squamous carcinoma cell line; CCTCC) cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.). SCC25 [oral tongue squamous carcinoma cell line; American Type Culture Collection (ATCC), Manassas, VA, USA] cells were cultured in a 1:1 mixture of DMEM and Ham's F12 medium (Thermo Fisher Scientific, Inc.) and Fadu (hypopharyngeal squamous carcinoma cell line; ATCC) cells were cultured in DMEM. All the cells were cultured at 37°C in a 5% CO2 humidified atmosphere with medium containing 10% fetal bovine serum (FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.).
Induction of macrophage polarization
According to our previous study, M0, M1 and M2 macrophages were induced from THP1 cells (15,20). During this induction process, cells were cultured at 37°C in a 5% CO2 humidified atmosphere. First, phorbol-12-myristate-13-acetate (PMA; 320 nM; Cell Signaling Technology, Inc., Danvers, MA, USA) was added to 1×106/ml THP1 cells. Following 24 h, THP1 cells were induced into the M0 phenotype. For M1 and M2 macrophages, THP1 cells were treated with PMA for 6 h, and then induced into M1 macrophages by interferon-γ (IFN-γ; 20 ng/ml) and lipopolysaccharide (LPS; 100 ng/ml) for 18 h, or induced into M2 macrophages by interleukin-4 (IL-4; 20 ng/ml) and IL-13 (20 ng/ml) for 18 h. Following a further 24 h culture of each macrophage phenotype in FBS-free medium, the macrophage conditional medium was collected for use in the next step.
Immunohistochemistry and evaluation
Immunohistochemical staining was performed according to the method described previously (21). Briefly, tissues were fixed with 4% paraformaldehyde for 48 h at room temperature. Then 4-µm thick tissue sections were cut, dried, deparaffinized with xylene and rehydrated using a descending series of alcohol following standard procedures. Then, the sections were subjected to heat-induced antigen retrieval at 98°C for 10 min with citrate buffer (Abcam, Cambridge, MA, USA) and washed thrice with PBS for 3 min each. The sections were then blocked with 10% non-immune goat serum (Gibco; Thermo Fisher Scientific, Inc.) for 1 h at room temperature. Immunohistochemical staining was performed overnight a 4°C with the following primary antibodies: Mouse monoclonal anti-cluster of differentiation (CD)-68 antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA; dilution, 1:50; cat. no. sc-17832), mouse monoclonal anti-CD163 antibodies (Santa Cruz Biotechnology, Inc.; dilution, 1:50; cat. no. sc-33715) and antibodies against EMT markers, including mouse anti-Vimentin (Abcam; dilution, 1:200; cat. no. ab8978) and rabbit anti-E-cadherin (Abcam; dilution, 1:100; cat. no. ab40772). Tissues were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Abcam; dilution, 1:500; cat. nos. ab6112 and ab97046) for 1 h at room temperature. The macrophage markers of CD68 and CD163 were semi-quantitatively evaluated in HNSCC on the basis of staining intensity and distribution using the immunoreactive score (IRS) (22): IRS = staining intensity × percentage of positive cells. The staining intensity was defined as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The percentage of positive cells was defined as follows: 0, negative; 1, 1–20% positive cells; 2, 21–50% positive cells; and 3, 51–100% positive cells. The IRS ranged from 0 to 9, which was divided into 3 groups on the basis of the final score: i) IRS 0, negative; ii) IRS 1–4, low immunoreactivity; and iii) IRS >4, high immunoreactivity. The percentage of positive cells was quantified by 3 pathologists who counted in ten different visual fields at random using a light microscope (Leica Microsystems, Inc., Buffalo Grove, IL, USA; magnification, ×200) and the average IRS was calculated as the final value. HNSCC cells with high immunoreactivity for CD68 and CD163 were classified as high macrophage HNSCC (HM-SCC); cells with low immunoreactivity for one or both of the macrophage markers (CD68 and CD163) were classified as low macrophage HNSCC (LM-SCC). All of the HNSCC samples were divided into HM-SCC and LM-SCC, as shown in Table I.
Immunofluorescence
Tissue section processing was similar to that performed for immunohistochemistry. The treated tissue was incubated overnight with primary antibodies against EMT markers (E-cadherin and Vimentin) and M2 macrophage markers (CD68 and CD163) at 4°C (all antibodies as aforementioned). Then, the tissue was incubated with secondary anti-mouse or anti-rabbit antibodies conjugated with Alexa Fluor 488 (Invitrogen; Thermo Fisher Scientific, Inc.; dilution, 1:400; cat. no. A-11034) or Alexa Fluor 568 (Invitrogen; Thermo Fisher Scientific, Inc.; dilution, 1:400; cat. no. A-21144) for 1 h at room temperature. Finally, the nuclei were stained with mounting medium containing DAPI for 5 min at room temperature. The tissues were observed and photographed with a fluorescence microscope (Leica Microsystems, Inc.). For the evaluation, as described previously (23), the images were analyzed using the Image-Pro Plus 7.0 version software (Media Cybernetics, Inc., Rockville, MD, USA). Briefly, five representative high-power fields were selected randomly. The reference value that identified the positive staining was set. Then, the sum integrated optical density (IOD) and the sum area (area) would be generated automatically. The semi-quantitative value of each marker was expressed as a mean density (IOD/area).
Flow cytometry
To analyze the macrophage marker expression in the induced THP1 cells, flow cytometry was performed. Different phenotype macrophages were harvested, washed in ice-cold PBS and blocked with PBS containing 3% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) on ice for 30 min. The cells were centrifuged at 300 × g for 5 min at 4°C and resuspended with cold PBS to a final concentration of 2×107 cells/ml. Then 1×106 cells/tube in 100 µl were prepared and incubated with fluorescein isothiocyanate conjugated CD68 antibody (BD Biosciences, Franklin Lakes, NJ, USA; cat. no. 562117; dilution, 1:20) and phycoerythrin-conjugated antibody CD163 (BD Biosciences; cat. no. 563887; dilution, 1:20) for 20 min on ice avoiding light. Cells were washed 3 times in PBS, and detected on Fluorescence-Activated Cell Sorting (FACS) Calibur flow cytometer (BD Biosciences). The data was analyzed using BD Cell Quest 5.1 (BD Biosciences) and WinMDI 2.9 software (The Scripps Institute, Flow Cytometry Core Facility, La Jolla, CA, USA; www.cyto.purdue.edu/flowcyt/software/Winmdi.htm).
Cell invasion and migration assay
A 24-well Transwell chamber (pore size, 8 mm) with or without Matrigel coating (BD Biosciences) was used to detect tumor cell invasion and migration, respectively. Tumor cells (1×105) co-cultured with or without TAMs were plated in the upper chamber, and different conditional media (CM) containing 10% FBS (Thermo Fisher Scientific, Inc.) with or without U0126 [1,4-diamino-2, 3-dicyano-1,4-bis (2-aminophenylmercapto) butadiene; ERK1/2 inhibitor; 10 µg/ml; Cell Signaling Technology, Inc.] were added to the lower chamber. Once the cells were incubated for 24 h, the non-penetrated cells were scraped from the upper surface of the filter, and the penetrated cells, representing invaded or migrated cells, were stained with crystal violet solution (Sigma-Aldrich; Merck KGaA) at room temperature for 10 min. Cells were counted under a phase microscope, photographed and analyzed using ImageJ 1.42 software (National Institutes of Health, Bethesda, MD, USA).
Wound healing assay
Cal27 cells (1×106) were seeded in 6-well plates with or without TAM-CM and M2 macrophages. When tumor cells reached 70–80% confluence, a straight scratch was made using a 200 µl pipette tip, and an artificial wound was formed. Following 18 h at 37°C in a 5% CO2 humidified atmosphere, the migration of tumor cells across this artificial wound was photographed using a light microscope (Leica Microsystems, Inc.) and assessed using ImageJ 1.42 software (National Institutes of Health).
Enzyme-linked immunosorbent assay (ELISA)
Cytokine secretion of EGF and TGF-β in the THP1 M0, M1 and M2 macrophage culture supernatant was measured using EGF ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA; cat. no. #DY236) and TGF-β ELISA kits (R&D Systems, Inc.; cat. no. #DY240) according to the manufacturer's protocol. Briefly, 100 µl of prepared standard and samples were added to the 96-wells, then the plate was covered and incubated at room temperature for 2 h. The samples were removed and the plate was washed 3 times with 200 µl PBS. Then, 100 µl diluted antibody was added into each well and tapped gently on the side of the plate to mix. Following incubation for 1 h at room temperature, the liquid was discarded and the wells were washed 3 times. Then, 100 µl of diluted HRP conjugate was added to each well and incubated at room temperature for 30 min. The liquid was removed and washed 3 times. Then, 100 µl chromogenic substrate was added to each well at room temperature in the dark for 30 min. Following the addition of 100 µl of stop solution to each well, the absorbance of each sample was evaluated using a microplate reader at 450 and 550 nm. By comparing the absorbance of the standard points against the standard concentrations, a standard curve was constructed, and the results for the test samples were calculated.
Western blot analysis
Cal27 cells labeled with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Thermo Fisher Scientific, Inc.; cat. no. V12883) were added to the co-culture system. Following treatment with the EGFR inhibitors Cetuximab (10 µg/ml; Merck KGaA) or U0126 (10 µg/ml; Cell Signaling Technology, Inc.) for the indicated times 24 h, Cal27 cells were sorted from the co-culture by FACS. Tumor cells (2×106) were then lysed with radioimmunoprecipitation assay lysis and extraction buffer (Thermo Fisher Scientific, Inc.; cat. no. #89900) with Halt Protease and Phosphatase Inhibitor (Thermo Fisher Scientific, Inc.; cat. no. #78447) at 4°C, and the concentration of protein in each cell lysate was measured using the bicinchoninic acid method. Then, 20 µg of protein was separated by 10% SDS-PAGE and electroblotted onto polyvinylidene fluoride membranes. The blots were blocked with 5% nonfat dry milk at room temperature for 1 h, and probed overnight at 4°C with primary antibodies, including rabbit anti-total ERK1/2 (1:1,000; cat. no. 4695), rabbit anti-total P65 (1:500; cat. no. 8242), rabbit anti-protein kinase B (Akt; 1:1,000; cat. no. 9272), anti-phospho-Akt (p-Akt; 1:1,000; cat. no. 9611), rabbit anti-Mothers against decapentaplegic homolog 3 (Smad3; 1:1,000; cat. no. 9513), anti-p-Smad3 antibodies (1:500; cat. no. 9520; all from Cell Signaling Technologies, Inc.), mouse anti-α-smooth muscle actin (α-SMA; 1:1,000; cat. no. ab7817), mouse anti-GADPH antibody (1:1000; cat. no. ab8245; both Abcam), rabbit anti-p-ERK1/2 (1:500; cat. no. MAB18251) and anti-p-P65 antibodies (1:500; cat. no. MAB72261; both R&D Systems, Inc.). Then, the immunoblots were incubated with HRP-conjugated secondary antibodies (1:5,000; cat. nos. 32230 and 32260; Pierce; Thermo Fisher Scientific, Inc.) at room temperature for 2 h. The protein bands were visualized using an enhanced chemiluminescence detection kit (Pierce; Thermo Fisher Scientific, Inc.). ImageJ 1.42 software (National Institutes of Health) was used for densitometric analysis.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and reverse transcribed using PrimeScript RT (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's protocol. Briefly, the template RNA/Primer Mixture was incubated at 65°C for 5 min, then immediately cooled on ice. PrimeScript Buffer and Reverse Transcriptase were then added to the reaction mixture, creating a 20-µl reaction, and incubated at 42°C for 50 min and 70°C for 15 min, followed by cooling on ice. qPCR was performed using the 7500 Fast System (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the Fast SYBR-Green Master Mix (Thermo Fisher Scientific, Inc.). RT-qPCR was performed with the following thermocycling conditions: 40 cycles of 95°C for 5 sec, 60°C for 20 sec and 72°C for 20 sec. The internal control of the experiment was GADPH, and the primer sequences used for qPCR are presented in Table II. The relative expression levels were analyzed using the 2−ΔΔCq method (24).
Table II.
Primer sequences used to determine gene expression by reverse transcription-quantitative polymerase chain reaction.
| Gene | Type | Primers sequence (5′-3′) |
|---|---|---|
| TNF-α | Sense | TCTTCTCGAACCCCGAGTGA |
| Antisense | CCTCTGATGGCACCACCAG | |
| IL-1β | Sense | TACGAATCTCCGACCACCACTACAG |
| Antisense | TGGAGGTGGAGAGCTTTCAGTTCATATG | |
| TGF-β | Sense | GGGACTATCCACCTGCAAGA |
| Antisense | CCTCCTTGGCGTAGTAGTCG | |
| IL-10 | Sense | AGAACAGCTGCACCCACTTC |
| Antisense | GCATCACCTCCTCCAGGTAA | |
| EGF | Sense | GTGCAGCTTCAGGACCACAA |
| Antisense | AAATGCATGTGTCGAATATCTTGAG | |
| GAPDH | Sense | AGTCCTTCCACGATACCAAAGT |
| Antisense | CATGAGAAGTATGACAACAGCCT |
TNF-α, tumor necrosis factor-α; IL, interleukin; TGF-β, transforming growth factor-β; EGF, epithelial growth factor.
Statistical analysis
The results of immunohistochemistry and immunofluorescence were assessed using Image Pro Plus 7.0 software (Media Cybernetics, Inc.). Western blotting and wound healing assay results were analyzed using ImageJ 1.42 software (National Institutes of Health). The hierarchical analysis was conducted on the basis of Pearson's correlation using Cluster version 3.0 (Eisen Lab, University of California, Berkeley, CA, USA; bonsai.hgc.jp/~mdehoon/software/cluster/) and visualized as a heatmap using the Java Tree View version 1.0.5 software (Eisen Lab, University of California; bitbucket.org/TreeView3Dev/treeview3/). All experiments included at least 3 independent repeats. The data were analyzed using GraphPad Prism 6 for Windows (GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of variance followed by the post hoc Tukey's multiple comparison test was performed for analysis of 3 or more groups. Two-tailed Pearson's statistics was performed for correlation analysis of EMT and macrophage markers. P<0.05 was considered to indicate a statistically significant difference.
Results
TAMs are associated with EMT markers in HNSCC
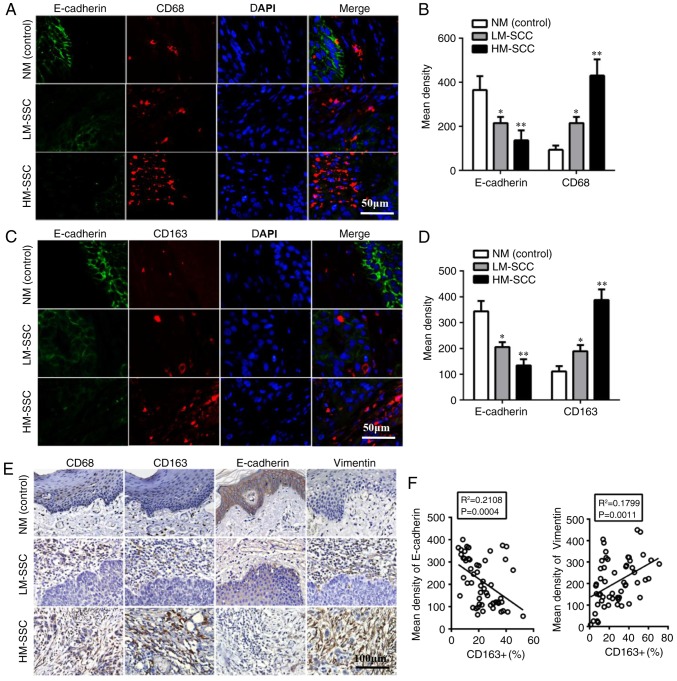
The 57 HNSCC specimens were classified into two groups of HM-SCC and LM-SCC on the basis of CD68 and CD163 immunohistochemical staining using the immunoreactive score (Table I). To explore the association between TAMs and EMT in HNSCC, the expression levels of EMT-associated proteins were evaluated. Using immunofluorescence, the results indicated that the epithelial marker, E-cadherin, was significantly downregulated in HM-SCC compared with LM-SCC (P<0.01) and normal mucosa (NM; P<0.005, Fig. 1A-D). Immunohistochemistry was also performed to confirm the expression of EMT-associated proteins in HNSCC and NM samples. The results indicated higher expression of Vimentin and lower expression of E-cadherin in HM-SCC when compared with LM-SCC and NM (Fig. 1E). These results suggested that HNSCC cells with high infiltration of M2 macrophages may undergo EMT. Furthermore, Pearson correlation analysis revealed correlations between EMT-associated proteins (E-cadherin and Vimentin) and M2-macrophage markers (CD68 and CD163). The results indicated that the mean density of E-cadherin were negatively correlated with CD163+ cells (P=0.0004; Fig. 1F), while the expression of Vimentin in HNSCC was positively correlated with CD163+ cells (P=0.0011; Fig. 1F). These results indicated that the EMT of HNSCC cells may be induced by TAMs, which may contribute to the aggressive behaviors of HNSCC.
Figure 1.
Tumor associated macrophages biomarkers are associated with EMT-associated proteins in HNSCC. (A) Detection of CD68 and E-cadherin expression by double immunofluorescent staining in HNSCC tissues. Magnification, ×200. (B) Quantitative data of CD68 and E-cadherin expression from immunofluorescent staining in HNSCC tissues. (C) Detection of CD163 and E-cadherin by double immunofluorescent staining in HNSCC tissues. Magnification, ×200. (D) Quantitative data of CD163 and E-cadherin from immunofluorescent staining in HNSCC tissues. Data are expressed as the mean ± standard error of the mean. *P<0.05 and **P<0.01 vs. NM (control). (E) Detection of CD68, CD163 and EMT-associated protein expression by immunohistochemical staining in HNSCC tissues. Magnification, ×200. (F) Pearson correlation analysis of EMT-associated protein and CD163+ expression. HNSCC, head and neck squamous cell carcinoma; EMT, epithelial to mesenchymal transition; CD, cluster of differentiation; HM-SCC, HNSCC with high macrophages; LM-SCC, HNSCC with low macrophages; NM, normal mucosa.
TAMs induce the EMT of HNSCC cells
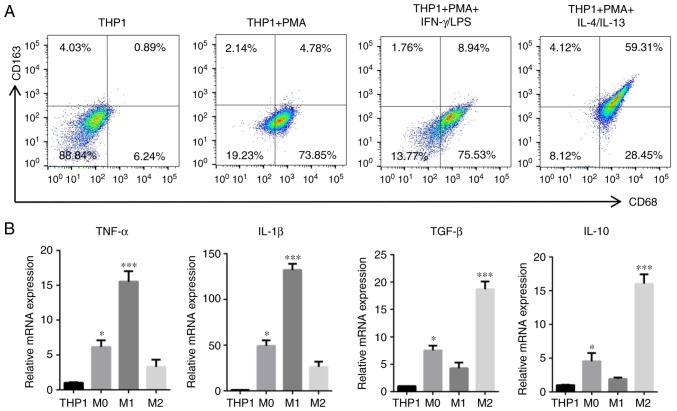
To investigate whether TAMs induce EMT in HNSCC cells, direct and indirect co-culture systems were established between HNSCC cells and TAMs. Human THP-1 cells are widely used as models for monocyte/macrophage differentiation; when treated with PMA (320 nM) for 24 h, THP-1 cells quickly stop proliferating, become attached and differentiate into macrophages (25). According to this method, THP1 cells were induced into M0, M1 and M2 phenotype macrophages. Then, the surface markers of macrophages were detected by flow cytometry. The results indicated that M0 (PMA only) and M1 (PMA/IFN-γ/LPS) cells were positive for CD68, and M2 (PMA/IL-4/IL-13) cells were positive for CD68 and CD163 (Fig. 2A). To further confirm the different polarization of macrophages, the cytokine profiles of THP1, M0-phenotype, M1-polarized and M2-polarized THP-1 cells were assessed using RT-qPCR. The results indicated that M1-polarized THP-1 macrophages expressed high levels of TNF-α and IL-1β mRNA and low levels of TGF-β and IL-10 mRNA, while the opposite results were observed in M2-polarized THP-1 macrophages (Fig. 2B). In PMA-treated THP1 cells (M0 macrophages), the expression of TNF-α, IL-1β, TGF-β and IL-10 was slightly increased when compared with the THP1 control. The cytokine profiles and surface markers provided evidence that the PMA-treated, M1-polarized and M2-polarized THP-1 groups had been established successfully.
Figure 2.
Induction and acquirement of macrophage polarization. Macrophages with M0, M1 and M2 functional profiles were acquired from THP1 cells following treatment with PMA, PMA/LPS/IFN-γ and PMA/IL-4/IL-13, respectively. (A) Expression of classical M0, M1 and M2 polarized macrophage surface markers (CD68 and CD163) was evaluated by flow cytometry. (B) mRNA expression levels of TNF-α, IL-1β, TGF-β and IL-10 in differentiated cells following induction were detected by reverse transcription-quantitative polymerase chain reaction. Data are expressed as the mean ± standard error of the mean. *P<0.05 and ***P<0.001 vs. THP1. PMA, phorbol-12-myristate-13-acetate; LPS, lipopolysaccharide; CD, cluster of differentiation; IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β.
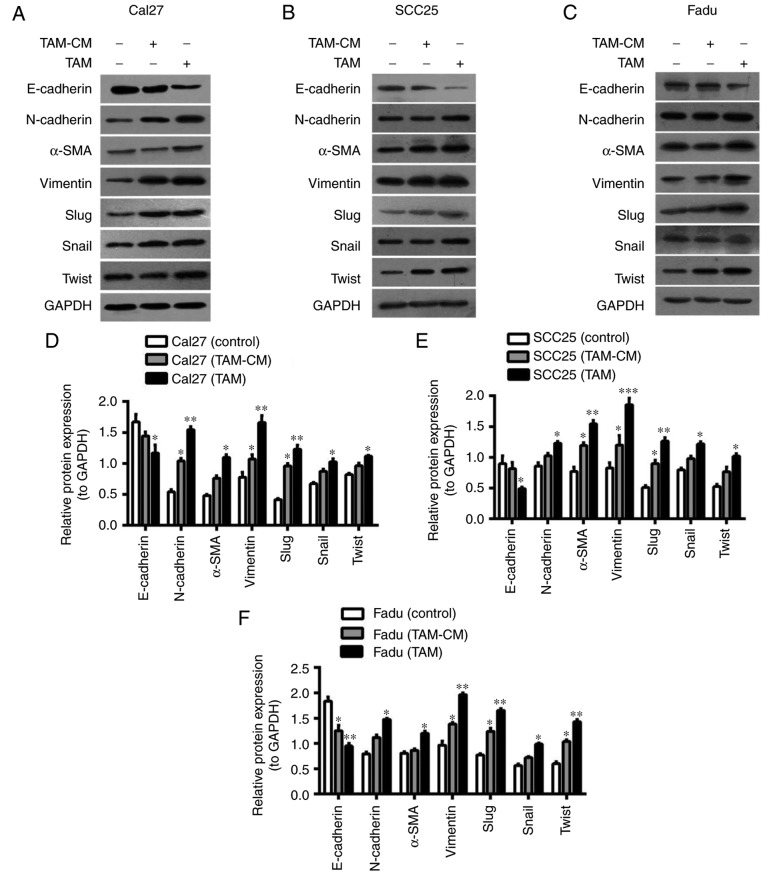
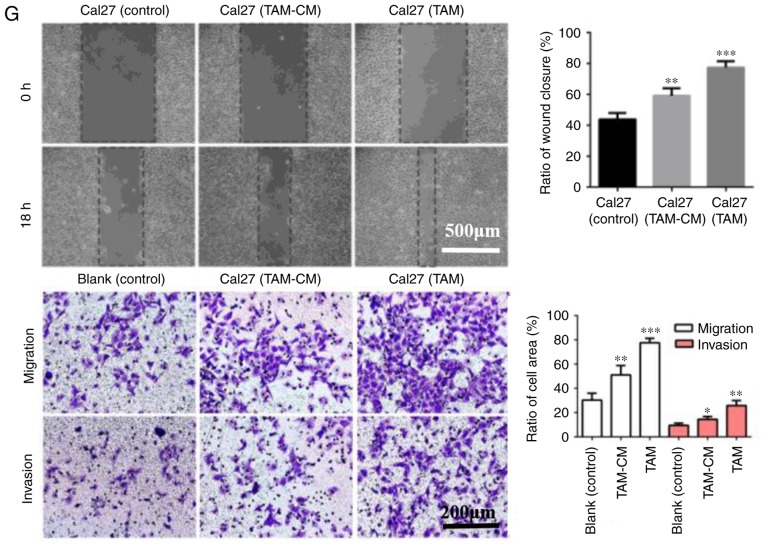
In the direct co-culture system, THP1 cells were first induced into M2 macrophages, then Cal27 cells labeled with CFDA-SE were added to the dishes. Following co-culture for 24 h, the Cal27 cells were separated by FACS. In the indirect co-culture system, TAMs-CM was added to the Cal27 dishes for 24 h. Then, the proteins of HNSCC cells were extracted and assessed (Fig. 3). The results from western blot analysis indicated that E-cadherin in co-cultured Cal27 cells (direct and indirect) was downregulated, while N-cadherin, Vimentin and α-SMA were upregulated (Fig. 3A and D). Similar results were obtained in SCC25 and Fadu cells (Fig. 3B, C, E and F). Furthermore, the protein expression of EMT-associated transcription factors, including Slug, Snail and Twist, was increased in HNSCC cells that were co-cultured with TAMs, particularly Slug (Fig. 3D-F). As the EMT of cancer cells accelerates their migration, invasion and metastasis, the migration and invasion ability of Cal27 cells was assessed using wound healing and Transwell assays. In the wound healing assay, the horizontal migration ability of Cal27 cells co-cultured with TAM-CM or TAMs was markedly enhanced when compared with Cal27 only. In the Transwell assay, for the Cal27 (TAM) group, 1×105 THP-1 cells were first seeded into upper inserts, treated with PMA for 6 h, then induced into M2-polarized macrophages with IL-4/IL-13 for 18 h. The inserts with M2-macrophages were washed to remove all PMA and co-cultured with Cal27 cells (1×105 cells/well) in 24-well plates for a further 24 h. The results indicated that the vertical migratory and invasive abilities of Cal27 were increased by TAMs-CM or TAMs, particularly by TAMs (Fig. 3G). These results suggested that TAMs induced EMT-like transformation of HNSCC cells in the direct and indirect co-culture systems.
Figure 3.
TAMs induce the EMT of HNSCC cells. Cal27 cells were labeled by carboxyfluorescein diacetate succinimidyl ester, then added to the dishes that M2-macrophages had been seeded in for 24 h. Following co-culture for 24 h, the cells were sorted by fluorescence-activated cell sorting. (A-C) Western blot analysis of the expression of EMT-associated proteins (E-cadherin, N-cadherin, α-SMA, Vimentin, Slug, Snail and Twist) in HNSCC cells: (A) Cal27, (B) SCC25 and (C) Fadu cells were co-cultured with TAMs and TAM-CM. GADPH was used as an internal control. (D-F) Statistical analysis of EMT-associated protein expression of (D) Cal27, (E) SCC25 and (F) Fadu cells in the co-culture system. (G) The migratory and invasive abilities of Cal27 cells were measured by wound healing and Transwell assay (magnification, ×200). Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 and ***P<0.001 vs. the associated control. TAMs, tumor associated macrophages; EMT, epithelial to mesenchymal transition; α-SMA, α-smooth muscle actin; CM, conditional media.
Macrophages secrete EGF and TGF-β depending on the co-culture system in HNSCC cells
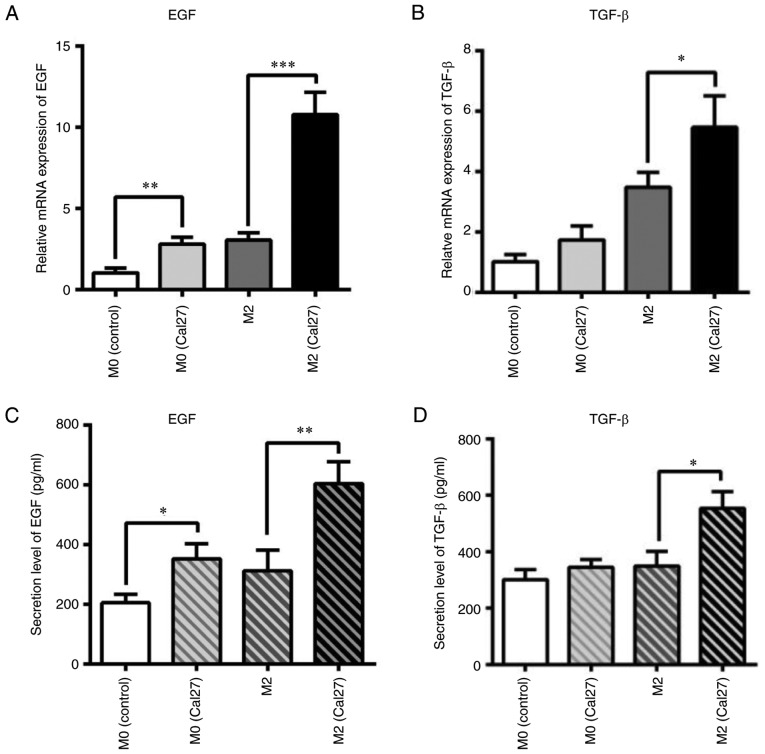
The aforementioned results suggested that TAMs may promote an EMT-like process by secreting growth factors and cytokines in the direct and indirect co-culture systems. Thus, macrophages were labeled with CFDA-SE and sorted from the co-cultured system. The mRNA expression of EMT-associated growth factors and cytokines, including EGF and TGF-β, was measured by qPCR. M0 macrophages induced from THP1 cells by treatment with PMA were used as control. As shown in Fig. 4A and B, the mRNA expression of EGF and TGF-β was significantly increased in the co-cultured macrophages when compared with the control group. In particular, EGF was upregulated by 5-fold (P<0.001). Furthermore, the secreted EGF and TGF-β were analyzed by ELISA. The results were similar to those for mRNA expression levels (Fig. 4C and D). The EGF protein level in the direct co-culture system increased from ~400 to ~700 pg/ml when compared with the control (P<0.01). TGF-β level in the co-culture was also upregulated from ~400 to ~500 pg/ml when compared with the control (P<0.05).
Figure 4.
TAMs secreting EGF and TGF-β depend on HNSCC cells. (A and B) Detection of EMT-associated cytokines, (A) EGF and (B) TGF-β, in TAMs co-cultured with Cal27 cells by reverse transcription-quantitative polymerase chain reation. M0 macrophages were used as the control. (C) EGF and (D) TGF-β cytokines in the co-culture system were detected by ELISA. Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 and ***P<0.001, as indicated. TAMs, tumor associated macrophages; EMT, epithelial to mesenchymal transition; TGF-β, transforming growth factor-β; EGF, epidermal growth factor.
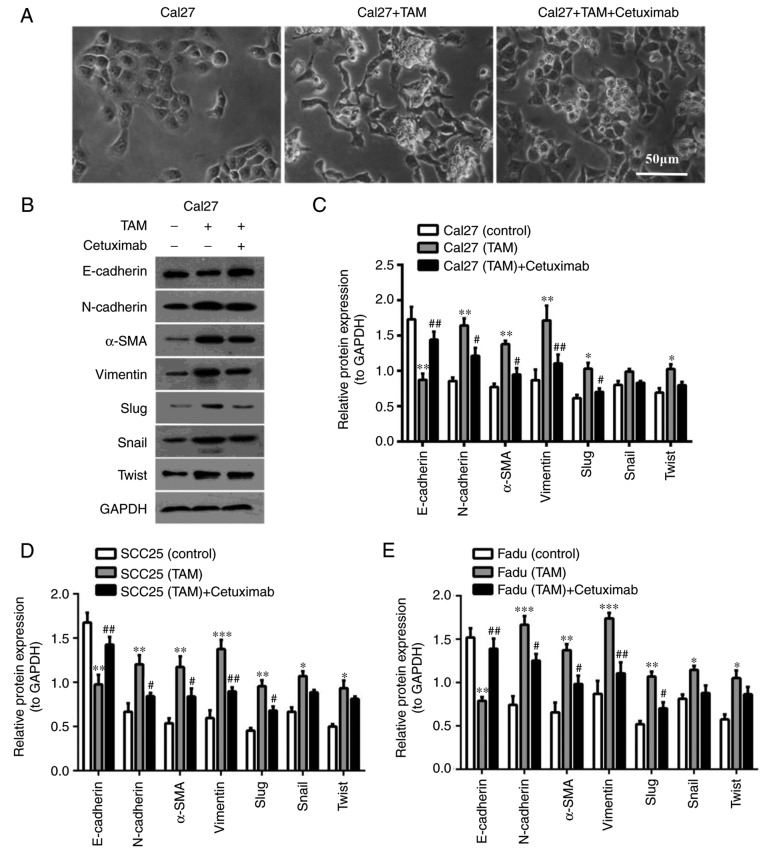
To investigate the function of EGF in this process, the EGFR inhibitor Cetuximab (10 µg/ml) was added into the co-culture system. As shown in Fig. 5A, the morphology of HNSCC cells was transformed from an elongated, spindle-shaped appearance to cobblestones in the direct co-culture with Cetuximab. Following co-culture for 24 h, the Cal27 cells were sorted. Western blot analysis of protein expression indicated that E-cadherin was slightly increased, while N-cadherin, Vimentin, α-SMA and transcription factors (Slug, Snail and Twist) were significantly decreased in the co-cultured Cal27 cells treated with Cetuximab (Fig. 5B and C). In the co-culture with SCC25 or Fadu cells, the epithelial marker E-cadherin was also upregulated and the mesenchymal markers were downregulated with Cetuximab treatment (Fig. 5D and E). These results suggested that EGF/EGFR may contribute to the TAM-induced EMT of HNSCC cells.
Figure 5.
TAMs induce the EMT of HNSCC cells via the EGFR signaling pathway. (A) Following co-culture for 24 h with or without the EGFR mono-antibody Cetuximab (10 µg/ml), the morphological changes of Cal27 cells were observed. Magnification, ×200. (B) Using western blot analysis, the protein expression of EMT-associated markers was measured in Cal27 cells co-cultured with TAMs. (C-E) Quantitative analysis of western blots in (C) Cal27, (D) SCC25 and (E) Fadu cells. Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 and ***P<0.001 vs. Control; #P<0.05 and ##P<0.01 vs. tumor cells co-cultured with TAMs. TAMs, tumor associated macrophages; EMT, epithelial to mesenchymal transition; EGFR, epidermal growth factor receptor; α-SMA, α-smooth muscle actin.
TAMs induce EMT via the ERK1/2 signaling pathway in HNSCC cells
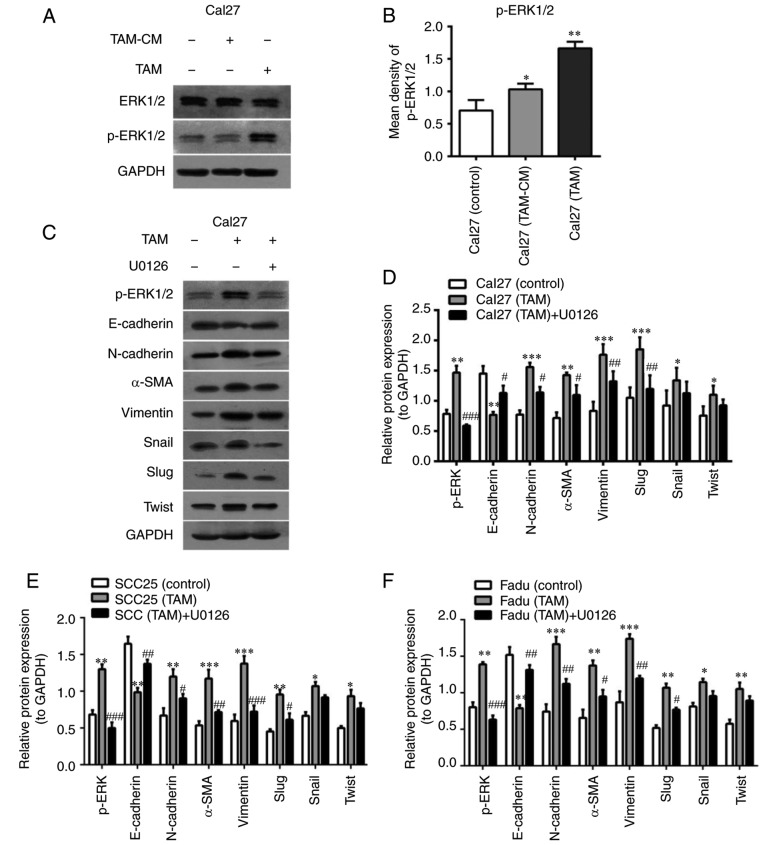
Previous reports have suggested that certain signaling pathways that are upregulated by EGF and TGF-β activation lead to EMT and cellular migration (17,18,26). Thus, signaling pathways including mitogen-activated protein kinase (MAPK), Akt, Smad3 and nuclear factor-kB P65 (P65) were evaluated to identify possible cascades involved in macrophage-mediated EMT. Western blot analysis indicated that the p-ERK1/2 pathway was significantly activated in the co-cultured HNSCC cells (Fig. 6A and B), while the signaling of Akt, Smad3 and P65 was not markedly activated (data not shown).
Figure 6.
TAMs induce EMT in HNSCC cells via the ERK1/2 signaling pathway. (A) The ERK1/2 signaling pathway was detected in Cal27 cells in the direct and indirect co-culture system by western blot analysis. (B) Expression of the ERK1/2 signaling pathway was quantified. (C) A specific inhibitor of ERK1/2 (U0126; 10 µg/ml) was added to the co-culture system of TAMs and Cal27 cells for 24 h. Cal27 cells were labeled with carboxyfluorescein diacetate succinimidyl ester and sorted using fluorescence-activated cell sorting. ERK1/2 pathway activating and EMT-associated proteins were measured in Cal27 cells by western blotting. (D-F) Quantitative analysis of p-ERK1/2 and EMT-associated proteins in (D) Cal27, (E) SCC25 and (F) Fadu cells. Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 and ***P<0.001 vs. Control; #P<0.05, ##P<0.01 and ###P<0.001 vs. tumor cells co-cultured with TAMs. TAMs, tumor associated macrophages; EMT, epithelial to mesenchymal transition; ERK1/2, extracellular signal-regulated protein kinase 1/2; p-, phosphorylated; α-SMA, α-smooth muscle actin; CM, conditional media.
U0126 is commonly used as an MAPK inhibitor. U0126 may selectively inhibit MAPK kinase 1 (MEK1) and MEK2, thereby suppressing the phosphorylation and activation of ERK1/2. In the present study, U0126 (10 mM) was added into the co-culture system, which abolished the increase of N-cadherin, α-SMA and Vimentin expression and restored E-cadherin levels in HNSCC cells induced by TAMs (Fig. 6C and D). Furthermore, the protein expression of transcription factors that repress epithelial genes, including Slug, Snail and Twist, was also assessed in the presence of U0126. The results indicated that the expression of Slug was significantly downregulated (P<0.01), and Snail and Twist were slightly decreased in the co-cultured Cal27 cells with U0126. The results from the co-cultured SCC25 and Fadu cells were similar to those of Cal27 cells (Fig. 6E and F).
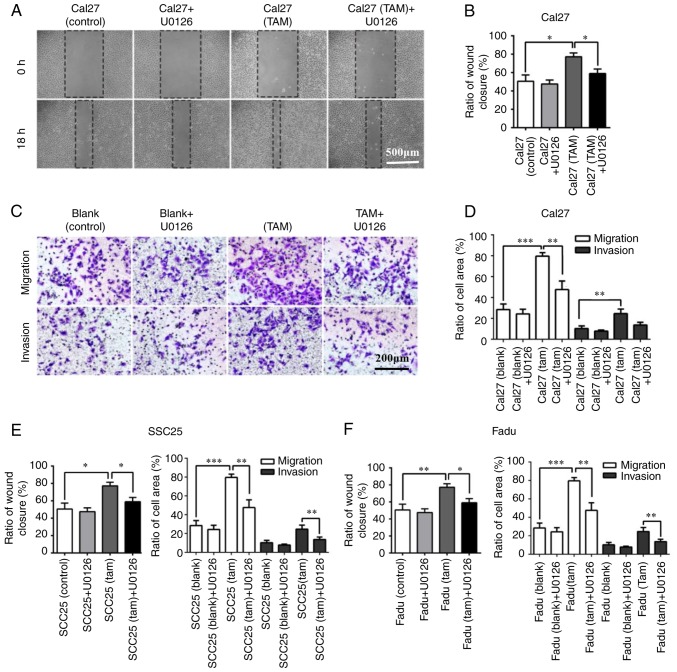
In the wound healing and Transwell assay, the horizontal invasive and migratory abilities of HNSCC cells were significantly impaired following 18 h of U0126 (10 µg/ml) treatment in the co-culture system (Fig. 7A and B). In addition, the vertical migratory and invasive abilities of Cal27 cells were also inhibited following 24 h incubation with U0126 (10 µg/ml; Fig. 7C and D). The results of SCC25 and Fadu cells were consistent with the results observed in Cal27 cells (Fig. 7E and F). In conclusion, these results indicated that TAMs induced the EMT of HNSCC cells via the ERK1/2 signaling pathway, and suppression of ERK1/2 markedly inhibited the EMT process of HNSCC cells and diminished the potential motility of tumor cells in the co-culture system.
Figure 7.
U0126 suppresses the invasion and migration abilities of Cal27 cells. The concentration of U0126 was 10 µg/ml, and Cal27 cells without U0126 were used as the control group. (A) In wound healing assays, the horizontal migration ability of Cal27 cells with U0126 in the co-culture system was markedly suppressed when compared with the control group. Magnification, ×200. (B) Quantitative analysis of wound healing assay results. (C) In Transwell chamber assays, the vertical migration and invasion abilities of Cal27 cells with U0126 in the co-culture system were also markedly inhibited when compared with the Cal27 control. Magnification, ×200. (D) Quantitative analysis of Transwell chamber assays. (E and F) Quantitative data of wound healing and Transwell assays in (E) SCC25 and (F) Fadu cells. Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 and ***P<0.001. TAMs, tumor associated macrophages.
Activation of the ERK1/2 signaling pathway is positively correlated with EMT in HNSCC induced by TAMs
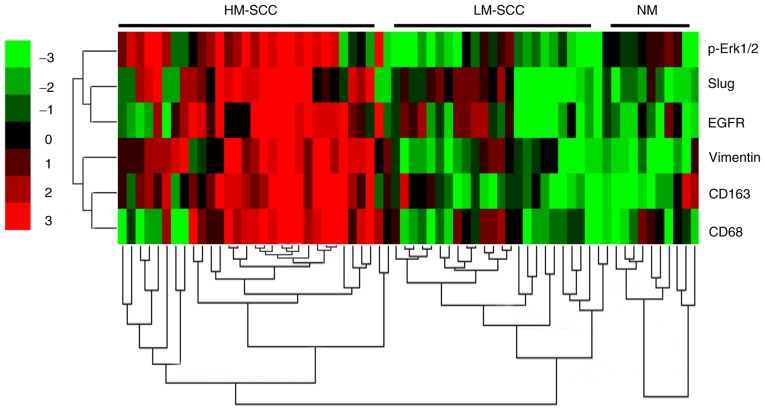
The expression of p-ERK1/2, as well as the biomarkers of EMT-associated genes, was evaluated in patient tissues. These results were subjected to cluster analysis and then visualized as a heatmap. The NM samples were strongly positive for E-cadherin and negative or weakly positive for Vimentin, Slug and p-ERK1/2. By contrast, the expression levels of E-cadherin were significantly downregulated, while the expression levels of Vimentin, α-SMA, Slug and p-ERK1/2 were markedly upregulated in the majority of HNSCC samples, particularly the samples with high infiltration of macrophages (data not shown). In Fig. 8, the correlation between tested markers (left) and samples (top) was indicated by the length and subdivision of the branches in the heatmap. The TAM marker CD163 and EMT-associated gene Vimentin were closely clustered, which was consistent with the results from the Pearson correlation analysis. In addition, the cluster analysis demonstrated the closest association between EGFR and Slug. Furthermore, a close association between Slug and p-ERK1/2 was also verified. All of the HNSCC samples were clustered and could be divided into two groups, with high and low infiltration of macrophages. Collectively, the cluster analysis revealed that the EGFR/ERK1/2 signaling pathway was closely associated with the EMT-associated markers and the infiltration of TAMs.
Figure 8.
Activation of the ERK1/2 signaling pathway was positively correlated with the epithelial to mesenchymal transition of tumor cells induced by tumor associated macrophages in HNSCC tissues. Hierarchical clustering analysis of CD68, CD163, Vimentin, EGFR, Slug and p-ERK1/2 in HNSCC and normal tissues. ERK1/2, extracellular signal-regulated protein kinase 1/2; CD, cluster of differentiation; EGFR, epidermal growth factor receptor; p-, phosphorylated; HNSCC, head and neck squamous cell carcinoma; HM-SCC, HNSCC with high macrophages; LM-SCC, HNSCC with low macrophages; NM, normal mucosa.
Discussion
HNSCC is thought to be caused by chronic inflammation through smoking, repeated injuries or human papillomavirus infection (27–29). A number of previous studies have indicated that poor prognosis in patients with HNSCC is associated with macrophage infiltration, and the amount of macrophage infiltration may potentially be used as a prognostic marker (21,30). Recently, we investigated the interaction between monocytes and HNSCC cells (15). The results revealed that HNSCC cells could recruit and differentiate monocytes into M2 macrophages when HNSCC cells were co-cultured with monocytes. This process, in turn, promoted the formation of aggressive invadopodia of cancer cells (15). According to these results, the aim of the present study was to investigate whether HNSCC cells underwent EMT-like transformation induced by TAMs, so as to promote invasion and metastasis. Furthermore, the underlying mechanism by which TAMs induce the EMT of tumor cells was evaluated, which may reveal novel targets for HNSCC therapy.
In the present study, M2 macrophage markers were detected in HNSCC and normal adjacent mucosa. The results confirmed our previous findings that the expression of CD68 and CD163 in HNSCC was significantly higher when compared with NM, which indicated that M2 macrophages are a primary cellular component in the tumor stroma in HNSCC. M2-polarized macrophages may facilitate tumor maintenance and growth by enhancing tumor-associated angiogenesis, immunosuppression, invasion and metastasis (31,32). EMT is a powerful metastasis process that describes a change from polarized immotile epithelial cells to motile mesenchymal cells, which leads to an increase in cell mobilization. This transition is usually accompanied by a decrease in intercellular adhesive molecules such as E-cadherin and β-catenin, and an increase in mesenchymal cell markers such as Vimentin and N-cadherin, as well as the upregulation of matrix metalloproteinase (12,33,34). EMT is not only critical for appropriate embryonic development, wound healing, tissue regeneration, organ fibrosis and cancer progression, but also an important process in tumor invasion, metastasis and tumor drug resistance (11,35,36). A large body of evidence has indicated that TAMs also serve an important role during the EMT of cancer cells, through which cancer cells could disperse from primary sites and form metastatic lesions (14,37–39). In the present study, a close association between TAM infiltration and the EMT of tumor cells was demonstrated in HNSCC, and the mechanism of interaction between them was evaluated.
In the present study, EMT-associated markers were detected in the human HNSCC samples, and then the correlation between EMT and TAM infiltration was analyzed. The results indicated that the expression of E-cadherin was downregulated, while Vimentin and α-SMA were upregulated in the samples with high infiltration of macrophages when compared with those with low infiltration of macrophages and NM. Through correlation analysis, the immunohistochemical staining of E-cadherin and Vimentin was identified to be correlated with CD163+ cells. These results indicated that EMT induced by TAMs may serve a role in the aggressive behavior of HNSCC. To investigate the association between TAMs and EMT in HNSCC cell lines, a direct and indirect co-culture system was established between TAMs and HNSCC cells, including Cal27, SCC25 and Fadu cells; the direct co-culture may better reflect the real situation in tumor tissues. Tumor cells were sorted from the co-culture system by FACS for further analysis. All 3 HNSCC cell lines exhibited a significant decrease in the epithelial marker E-cadherin, and an increase in the mesenchymal markers N-cadherin, Vimentin and α-SMA. In conclusion, these results indicated that TAMs could induce the EMT of HNSCC cells in the indirect and direct co-culture system. Notably, the EMT-like transformation appeared to be stronger in the direct co-culture system when compared with the indirect co-culture system, indicating that direct contact between cancer cells and macrophages also contributes to the process.
Subsequently, the underlying mechanism was explored further. A previous study has reported that TAMs promote the EMT of tumor cells by secreting growth factors and cytokines (16). In the present study, it was observed that the cytokines EGF and TGF-β in M2-macrophages directly co-cultured with Cal27 were significantly increased when compared with M2-macrophages alone. EGF is a growth factor highly associated with the progression of HNSCC (18). TGF-β is associated with M2 macrophages and tumor cell proliferation, tissue fibrosis, immunosuppressive activity and EMT. The natural ligands of EGFR include EGF and TGF-β (40). Therefore, Cetuximab, a monoclonal antibody that binds EGFR, was added to the co-culture system. The data revealed that Cetuximab could reverse the expression of EMT-associated proteins in Cal27 cells co-cultured with TAMs. These results indicated that the EGFR signaling pathway may serve an important role in the EMT process in HNSCC.
According to these results, the indirect and direct co-culture systems could induce HNSCC cells to undergo the EMT process. That is to say, TAMs induced the EMT of tumor cells, via juxtacrine and paracrine signaling. Therefore, it was important to evaluate intracellular signaling pathways in the present study. EMT is governed by multiple molecular mechanisms, leading to the phosphorylation and activation of intracellular signaling events, including the MAPK, phosphoinositide 3-kinase/Akt, Smad3 and protein kinase C signaling pathways. Together, these signaling pathways result in the loss of apico-basal polarity, and the acquisition of the motile and invasive phenotype (26,41). In the present study, it was observed that the ERK1/2 signaling pathway acted as the major regulator for EMT-like transformation in HNSCC cells induced by TAMs. Signaling by receptor tyrosine kinases to RAS-ERK-MAPK has been demonstrated to participate in the EMT of various types of tumor cells (42,43). In the present study, it was further revealed that pretreatment with the ERK1/2-specific inhibitor U0126 reversed the EMT-like process induced by TAMs due to the upregulation of E-cadherin and downregulation of Vimentin and α-SMA. Simultaneously, the EMT-associated transcription factors Slug, Snail and Twist were also downregulated, particularly Slug in all 3 HNSCC cell lines. In addition, it was demonstrated that U0126 in the co-culture system could prevent the tumor cell migration and invasion of human HNSCC cells. Collectively, these results indicated that activation of the ERK1/2 signaling pathway served a major role in the induction of EMT by TAMs in HNSCC cells. As the RAF-MEK-ERK cascade serves a central role in the regulation of EMT, and cell migration and invasion, together with the frequent deregulation of this pathway in human cancer, it makes an attractive target for drug development (44).
To improve the clinical relevance of these findings, the expression status of p-ERK1/2 signaling and the EMT inducer Slug was evaluated in HNSCC specimens, as well as EGFR, Vimentin and CD163 expression. Hierarchical clustering analysis indicated a close association between p-ERK1/2 and Slug, and also indicated that the expression of TAM marker CD163 and EMT marker Vimentin were closely associated with the expression of p-ERK1/2 and Slug. This suggested that ERK1/2 may serve an important role in the EMT process induced by TAMs in HNSCC, and could be a promising target for the prevention of cancer metastasis.
In conclusion, the present study provides evidence that TAMs could induce the EMT of HNSCC cells via the secretion of EGF and TGF-β, which further enhanced the invasive ability of HNSCC cells. In addition, it was indicated that the EMT process of HNSCC cells induced by TAMs was partly via the ERK1/2 signaling pathway, which may provide a novel target for cancer therapy in HNSCC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- TAMs
tumor associated macrophages
- EMT
epithelial to mesenchymal transition
- HNSCC
head and neck squamous cell carcinoma
- EGF
epithelial growth factor
- ERK
extracellular regulated protein kinases
Funding
The present study was partially supported by the National Natural Science Foundation of China (grant nos. 81570994, 81371159 and 81602780). It was also supported by the Young Elite Scientist Sponsorship Program by China Association for Science and Technology (CAST; grant no. 2015QNRC001) and the Open Research Fund Program of Hubei-MOST KLOS & KLOBM (grant no. 201803).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YZ, WZ and LG conceived and designed the study. LG, WqZ, ZL and HL performed the experiments. LG drafted the article. ZY was also involved in the conception of the study and performed the experiment. YZ and WZ reviewed and edited the manuscript. All authors have read and approved the manuscript to be published.
Ethics approval and consent to participate
All procedures were approved by the Medical Ethics Committee of Wuhan University (Hubei, China). Experiments involving human were approved by the Medical Ethics Committee of Dalian Medical University (Dalian, China) and written informed consent was provided by each patient.
Patient consent for publication
Patient consent for publication was obtained from each patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Siegel R, Xu J, War E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jou A, Hess J. Epidemiology and molecular biology of head and neck cancer. Oncol Res Treat. 2017;40:328–332. doi: 10.1159/000477127. [DOI] [PubMed] [Google Scholar]
- 3.Thompson L. World health organization classification of tumours: Pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74. [PubMed] [Google Scholar]
- 4.Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8:11884–11894. [PMC free article] [PubMed] [Google Scholar]
- 5.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 9.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 10.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H, Clauser KR, Tam WL, Fröse J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014;16:1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Wang FQ, Li HM, Yang JG, Ren JG, He KF, Liu B, Zhang W, Zhao YF. CCL2/EGF positive feedback loop between cancer cells and macrophages promotes cell migration and invasion in head and neck squamous cell carcinoma. Oncotarget. 2016;7:87037–87051. doi: 10.18632/oncotarget.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirilä E, Väyrynen O, Sundquist E, Päkkilä K, Nyberg P, Nurmenniemi S, Pääkkönen V, Pesonen P, Dayan D, Vered M, et al. Macrophages modulate migration and invasion of human tongue squamous cell carcinoma. PLoS One. 2015;10:e0120895. doi: 10.1371/journal.pone.0120895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan J, Elhousiny M, Johnson NW, Gao J. Transforming growth factor-β1 treatment of oral cancer induces epithelial-mesenchymal transition and promotes bone invasion via enhanced activity of osteoclasts. Clin Exp Metastasis. 2013;30:659–670. doi: 10.1007/s10585-013-9570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Q, Zhang Q, Ishida Y, Hajjar S, Tang X, Shi H, Dang CV, Le AD. EGF induces epithelial-mesenchymal transition and cancer stem-like cell properties in human oral cancer cells via promoting Warburg effect. Oncotarget. 2017;8:9557–9571. doi: 10.18632/oncotarget.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health (NIH): HIPAA Authorization for Research. Bethesda, MD: Apr, 2004. NIH Publication No. 04-5529. [Google Scholar]
- 20.Zhang W, Chen G, Wang FQ, Ren JG, Zhu JY, Cai Y, Zhao JH, Jia J, Zhao YF. Macrophages contribute to the progression of infantile hemangioma by regulating the proliferation and differentiation of hemangioma stem cells. J Invest Dermatol. 2015;135:3163–3172. doi: 10.1038/jid.2015.321. [DOI] [PubMed] [Google Scholar]
- 21.He KF, Zhang L, Huang CF, Ma SR, Wang YF, Wang WM, Zhao ZL, Liu B, Zhao YF, Zhang WF, Sun ZJ. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res Int. 2014;2014:838632. doi: 10.1155/2014/838632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chui X, Egami H, Yamashita J, Kurizaki T, Ohmachi H, Yamamoto S, Ogawa M. Immunohistochemical expression of the c-kit proto-oncogene product in human malignant and non-malignant breast tissues. Br J Cancer. 1996;73:1233–1236. doi: 10.1038/bjc.1996.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong WQ, Chen G, Zhang W, Xiong XP, Ren JG, Zhao Y, Liu B, Zhao YF. Down-regulation of connexin43 and connexin32 in keratocystic odontogenic tumours: Potential association with clinical features. Histopathology. 2015;66:798–807. doi: 10.1111/his.12569. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 26.Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29:4947–4958. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 27.Lambert R, Sauvaget C, de Camargo Cancela M, Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:633–641. doi: 10.1097/MEG.0b013e3283484795. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Wawrose JS, Gooding WE, Garraway LA, Lui VW, Peyser ND, Grandis JR. Genomic analysis of head and neck squamous cell carcinoma cell lines and human tumors: A rational approach to preclinical model selection. Mol Cancer Res. 2014;12:571–582. doi: 10.1158/1541-7786.MCR-13-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh Y, Amelio I, Urbano Guerrero T, Tavassoli M. Clinical update on cancer: Molecular oncology of head and neck cancer. Cell Death Dis. 2014;5:e1018. doi: 10.1038/cddis.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji WT, Chen HR, Lin CH, Lee JW, Lee CC. Monocyte chemotactic protein 1 (MCP-1) modulates pro-survival signaling to promote progression of head and neck squamous cell carcinoma. PLoS One. 2014;9:e88952. doi: 10.1371/journal.pone.0088952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Garg M. Epithelial-mesenchymal transition-activating transcription factors-multifunctional regulators in cancer. World J Stem Cells. 2013;5:188–195. doi: 10.4252/wjsc.v5.i4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mego M, Gao H, Lee BN, Cohen EN, Tin S, Giordano A, Wu Q, Liu P, Nieto Y, Champlin RE, et al. Prognostic value of EMT-circulating tumor cells in metastatic breast cancer patients undergoing high-dose chemotherapy with autologous hematopoietic stem cell transplantation. J Cancer. 2012;3:369–380. doi: 10.7150/jca.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ, Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, Fan J. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol. 2015;46:587–596. doi: 10.3892/ijo.2014.2761. [DOI] [PubMed] [Google Scholar]
- 38.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Techasen A, Loilome W, Namwat N, Dokduang H, Jongthawin J, Yongvanit P. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13(Suppl):S115–S118. [PubMed] [Google Scholar]
- 40.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 41.Liu ZC, Chen XH, Song HX, Wang HS, Zhang G, Wang H, Chen DY, Fang R, Liu H, Cai SH, et al. Snail regulated by PKC/GSK-3β pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell Tissue Res. 2014;358:491–502. doi: 10.1007/s00441-014-1953-2. [DOI] [PubMed] [Google Scholar]
- 42.Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, Zhao L, Qu H, Fan Y, Wu C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One. 2012;7:e39520. doi: 10.1371/journal.pone.0039520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie YX, Liao R, Pan L, Du CY. ERK pathway activation contributes to the tumor-promoting effects of hepatic stellate cells in hepatocellular carcinoma. Immunol Lett. 2017;188:116–123. doi: 10.1016/j.imlet.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.