Abstract
Radiotherapy for head and neck cancers commonly causes damage to salivary gland tissue, resulting in xerostomia (dry mouth) and numerous adverse medical and quality-of-life issues. Amifostine is the only Food and Drug Administration–approved radioprotective drug used clinically to prevent xerostomia. However, systemic administration of amifostine is limited by severe side effects, including rapid decrease in blood pressure (hypotension), nausea, and a narrow therapeutic window. In this study, we demonstrate that retroductal delivery of amifostine and its active metabolite, WR-1065, to murine submandibular glands prior to a single radiation dose of 15 Gy maintained gland function and significantly increased acinar cell survival. Furthermore, in vivo stimulated saliva secretion was maintained in retrograde-treated groups at levels significantly higher than irradiated-only and systemically treated groups. In contrast to intravenous injections, retroductal delivery of WR-1065 or amifostine significantly attenuated hypotension. We conclude that localized delivery to salivary glands markedly improves radioprotection at the cellular level, as well as mitigates the adverse side effects associated with systemic administration. These results support the further development of a localized delivery system that would be compatible with the fractionated dose regimen used clinically.
Keywords: submandibular gland, salivary ducts, radiation, saliva, acinar cells, hypotension
Introduction
Globally, over 630,000 people are diagnosed with head and neck cancer each year, many of whom require radiation treatment (Vigneswaran and Williams 2014). Salivary gland dysfunction is a common side effect of radiation therapy to this region, stemming from loss of the secretory cells that produce saliva in the parotid gland and submandibular gland (SMG). Clinically, this injury manifests as radiation-induced hyposalivation (RIH) and xerostomia (dry mouth). Reduced saliva secretion predisposes patients to oral and systemic infection, loss of teeth, impaired swallowing, and speech, resulting in significantly diminished quality of life (Berk et al. 2005).
Amifostine (Am), the only Food and Drug Administration (FDA)–approved drug in use for xerostomia prophylaxis, is administered intravenously (IV) prior to fractionated radiotherapy. Am, a prodrug, is dephosphorylated to its active metabolite, WR-1065 (WR), by alkaline phosphatases on vascular cell membranes (Eisbruch 2011). Both compounds act as reactive oxygen species scavengers and are able to mitigate DNA damage to healthy tissue (Grdina et al. 2000). Am pretreatment can rescue saliva secretion in patients after radiotherapy (McDonald et al. 1994).
Am induces systemic side effects, which include nausea, vomiting, and a rapid decrease in blood pressure (BP; hypotension) that occur within an hour following administration (Yuhas 1980; Ryan et al. 1996; Antonadou et al. 2002; Rades et al. 2004). Due to rapid clearance, the onset of off-target effects interferes with the timing of radiation therapy. Thus, despite the efficacy of Am in reducing xerostomia, patients are either unlikely to complete treatment or often decrement to a better tolerated but less effective dose (Rades et al. 2004).
Retrograde injection through the Wharton’s excretory duct enables localized delivery to the SMG (Kuriki et al. 2011). Retrograde delivery was used in clinical trials to administer adenoviral vectors to the SMG for xerostomia relief (Baum et al. 2010; Baum et al. 2012). Retroductal injection has also been used in numerous murine models to deliver growth factors, primary cells, small interfering RNA (siRNA), and cytokines to the salivary glands (Redman et al. 2009; Arany et al. 2013; Marmary et al. 2016).
We hypothesized that retroductal delivery of Am or its activated form, WR, directly to the SMG can enhance radioprotection and long-term secretory function in comparison to systemic administration. We further proposed that adverse side effects, namely hypotension, can be diminished by retroductal versus systemic delivery. The results of this study support the development of a localized delivery system that can be applied clinically to fractionated dosing regimens.
Materials and Methods
Animals
Adult female C57/BL6 mice, 8 to 10 wk of age, were purchased from Jackson Laboratory. Food and water were provided ad libitum. All procedures were approved by the University Committee on Animal Resources at the University of Rochester. This study conforms to ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (supplemental information).
Compounds and Study Design
The irradiation studies were performed using 8 groups of mice (n ≥ 4) to test systemic versus local delivery of either Am (A5922; Sigma-Aldrich) or its aminothiol metabolite WR (W2020; Sigma-Aldrich) versus saline controls (Fig. 1A, B). Experimental groups are as listed (Appendix Table 1). All groups, except nonirradiated, received a single radiation dose of 15 Gy and were analyzed at 48 h, 2 wk, 6 wk, or 12 wk following treatment (Fig. 1C). Based on pilot toxicity studies, intraperitoneal (IP) dosing of WR was 50 mg/kg, and the retroductal dose of both WR and Am was 50 mg/kg. The Am dose (150 mg/kg) administered IP was based on previous studies (Okumura et al. 2009).
Figure 1.
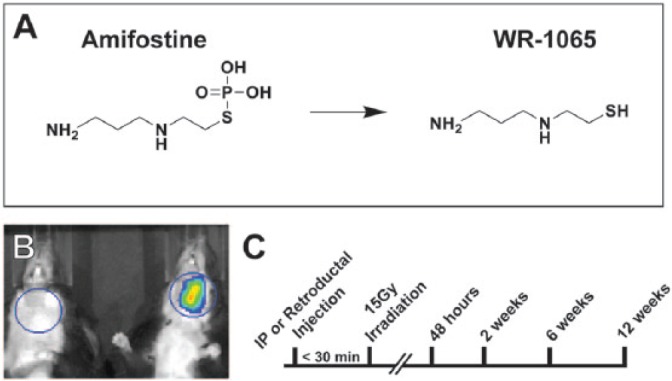
Experimental overview. (A) Structures of amifostine (Am) (left) and its dephosphorylated active metabolite, WR-1065 (WR) (right). (B) Unilateral retroductal (Ret) injection of Texas Red solution (50 µg) into the left submandibular gland (SMG) is detected using IVIS imaging (right) and noninjected control (left). (C) Experimental timeline. Injections of Am or WR were completed less than 30 min prior to administration of 15 Gy IR. Tissue was harvested at 48 h, 2 wk, 6 wk, or 12 wk (timeline not to scale). IP, intraperitoneal; IR, irradiation; Ret, retroductal; WR, WR-1065.
Retroductal Injections
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). Following ductal cannulation with 32-gauge tubing (CS-32; Braintree Scientific), 1 mg/kg muscarinic antagonist atropine was administered IP to reduce salivation. Using a 50-µL Hamilton syringe, retroductal injection was performed and injection pressure maintained for 1 min to improve retention. Mice received unilateral SMG injection of Am, WR, or saline (Sal) (1 µL/g body weight).
Cardiovascular Measurements
Mice were anesthetized with isoflurane and flow was adjusted to target heart rate (HR) above 450 beats/min. Animals were catheterized for BP monitoring and compound administration. For continuous recordings of BP and HR, a pressure transducer (1F Mikro-tip; Millar Instruments) was placed into the descending aorta through the femoral artery. For IV injections, a saline-filled catheter (CS-32; Braintree Scientific) was inserted into the femoral vein and advanced into the inferior vena cava. Experimental groups are as listed (Appendix Table 2). Hemodynamic parameters were acquired for 5 min before and 12 min after injections. At the end of each experiment, a single dose of phenylephrine (Phe; 0.6 mg/kg; IV) was given to assess BP pressor responses, as previously described (Peotta et al. 2001). Pulsatile and mean arterial pressure (MAP), as well as HR parameters, were electronically recorded and analyzed using PowerLab (AD Instruments).
Irradiation
Mice were anesthetized with ketamine/xylazine. SMG received gamma radiation at a single dose of 15.0 Gy, using a JL Shepherd 137Cs irradiator with single-slit collimator (Appendix Fig. 1) as previously described (Arany et al. 2013). Mice were irradiated within 30 min of IP or retroductal injections.
Saliva Collection
Mice were anesthetized with ketamine/xylazine. Tracheotomy was performed to ensure a patent airway. An IP injection of muscarinic agonist pilocarpine (100 mg/kg) was given to stimulate saliva secretion. Total saliva was collected by fluid aspiration for 10 min. Following euthanasia, SMGs were excised and weighed. Saliva secretion measurements are reported as saliva weight/gland weight, assuming a saliva density of 1.0 g/mL (Arany et al. 2013). Saliva collection was performed by 2 blinded, independent researchers on up to 5 randomly selected mice at a time.
Histology and Immunofluorescence
SMG tissue was fixed in 4% paraformaldehyde overnight, embedded in paraffin, and 6-micron sections cut. Sections were stained with hematoxylin and eosin (H&E) for gross morphological assessment. Antigen retrieval was performed in Tris-EDTA (pH 9.0) or citrate (pH 6.0) HIER buffer for Mist-1 and γH2AX immunofluorescence (IF), respectively. Ten percent normal donkey serum in 0.1% PBSA or CAS block (casein blocking buffer) (00-8120; Thermo Fisher Scientific) was used to block for 1 h for Mist-1 or γH2AX, respectively. Primary antibodies (rabbit–anti-Mist1 [1:200, ab187978; Abcam] and mouse–anti-γH2AX [1:100, 05-636; Millipore]) were applied overnight at 4°C. Secondary staining was performed using donkey–anti-rabbit Alexa 594 conjugated secondary antibody (1:500, A21207; Thermo Fisher Scientific) or donkey–anti-mouse 594 conjugated secondary antibody (1:500, A21203; Thermo Fisher Scientific). DAPI (D1306; Thermo Fisher Scientific) was used (1:1,000) as a nuclear counterstain. Quantification of Mist-1–positive cells and γH2AX staining, on representative images at ×200 and ×1,575 magnification (n = 5 mice, 5 images per mouse), respectively, was performed by a blinded, independent observer and was automated using ImageJ (National Institutes of Health). Quantification of γH2AX was performed as previously reported (Marmary et al. 2016).
Statistics
All statistical analyses were performed using GraphPad Prism 6.0 software. One-way analysis of variance (ANOVA) was used with appropriate post hoc testing to correct for multiple comparisons as indicated to assess significant differences between means with α = 0.05. For all plots, the mean is represented with standard error shown as error bars.
Results
Retroductal Injection of Amifostine or WR-1065 Significantly Mitigates Radiation-Induced DNA Damage
Am and WR have been shown to decrease double-strand breaks (DSBs) following irradiation (Hofer et al. 2016). To address the effect of drug treatment on DNA damage, we measured the accumulation of γH2AX, a histone modification that occurs as a reaction to DSBs, by immunofluorescence (Paull et al. 2000). SMG tissues were isolated and fixed at 48 h postirradiation. Immunological staining showed increased DNA damage concentrated in the ducts in all irradiated samples, in contrast to nonirradiated controls (Fig. 2A–D). Systemically (IP) administered WR was not associated with a significant decrease in DSBs per duct cell (Fig. 2C, G), whereas IP Am demonstrated a reduction in DSBs compared to irradiated and sham controls (Fig. 2D, G). Retrograde Am and WR cohorts showed significant 2- to 3-fold reductions in DSBs versus irradiated, sham, and systemic intervention groups (Fig. 2E–G). The data indicate that Am and WR pretreatment can dampen acute genotoxic stress following irradiation, and this effect is significantly enhanced when the drugs are administered retroductally.
Figure 2.
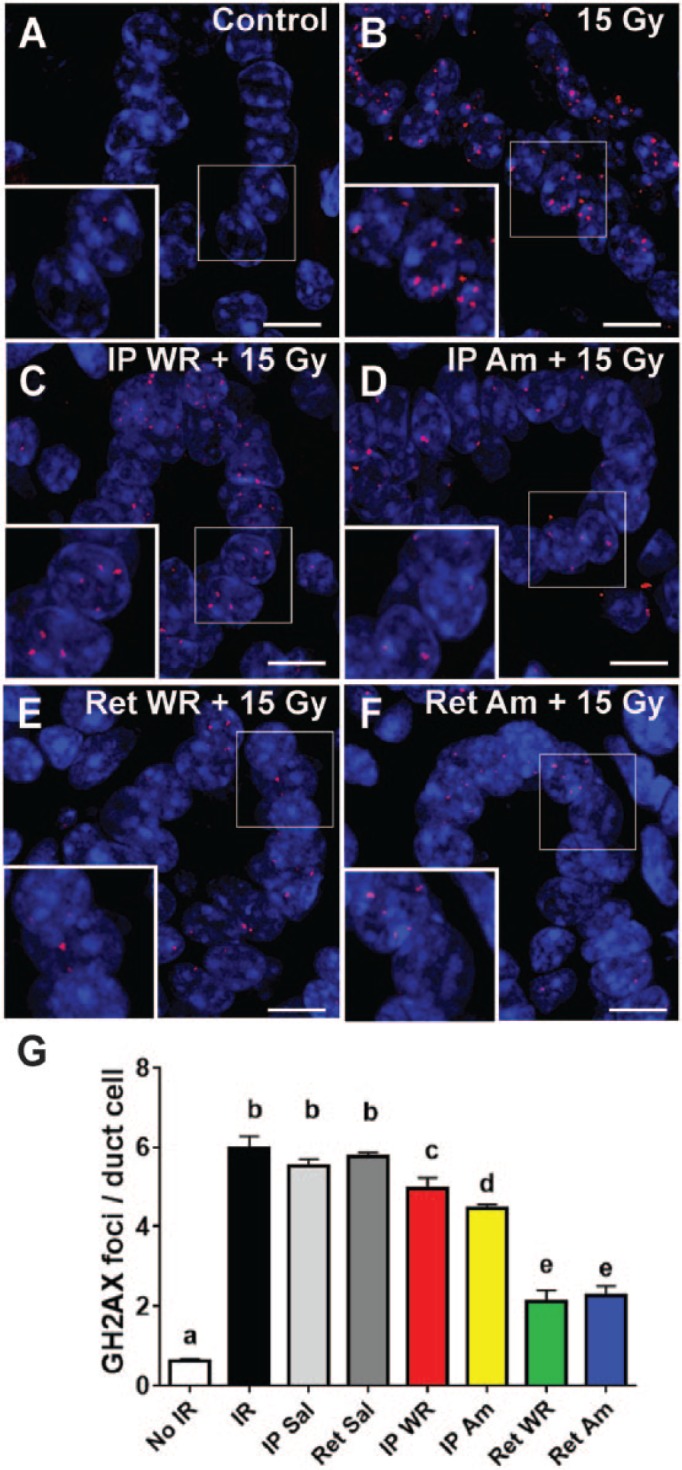
Immunofluorescent staining for γH2AX (red punctate dots) localizes to sites of DNA damage in nuclei of duct cells at 48 h after 15 Gy irradiation (scale bars = 5 μm). Sections of submandibular gland isolated from control (A), 15 Gy (B), IP WR+ 15 Gy (C), IP Am + 15 Gy (D), Ret WR + 15 Gy (E), and Ret Am + 15 Gy (F) labeled with antibody to γH2AX. Nuclei stained with DAPI. (G) Quantification of γH2AX foci, per duct cell per field. All groups are significantly different from group a. Group e is significantly different from all other groups. Group c is not different from all members of group b. Group d is significantly different from all groups except c (mean ± SEM, n = 5, 1-way analysis of variance with Tukey’s post hoc test for multiple comparisons). Am, amifostine; IP, intraperitoneal; IR, irradiation; Ret, retroductal; Sal, saline; WR, WR-1065.
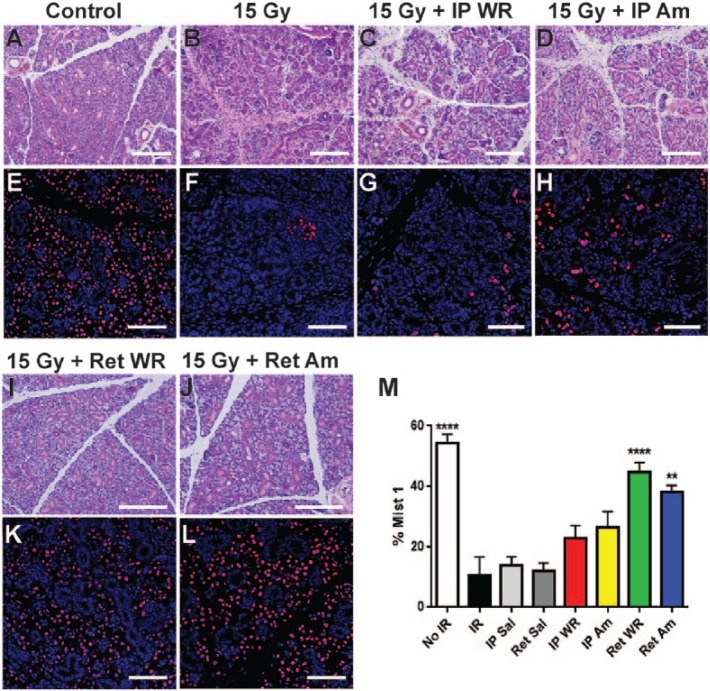
Localized Delivery of Radioprotectant Improves Acinar Cell Survival in Irradiated SMGs
Radiation treatment results in a dramatic but gradual loss of salivary gland acinar cells (Konings et al. 2005). To evaluate and compare the ability of systemic and localized delivery of Am and WR to preserve SMG secretory cells, SMG sections isolated at 12 wk following radiation were stained with H&E or antibody to Mist1, an acinar cell–specific transcription factor (Pin et al. 2000; Aure et al. 2015). Notably, SMG acinar cell depletion does not occur by 2 or 6 wk following irradiation (Appendix Fig. 2). As expected, at 12 wk postirradiation, H&E staining showed a loss of acinar cells in irradiated tissue versus nonirradiated controls (Fig. 3A, B). Tissue from mice administered Am or WR by IP showed similar acinar cell loss (Fig. 3C, D). In contrast, acinar area was widely preserved in sections from retrograde-treated groups, and SMG morphology can be compared to nonirradiated controls (Fig. 3I, J; Appendix Fig. 3). To corroborate this observation, we stained with Mist-1 antibody to detect acinar cells, which demonstrated an approximately 4.5-fold decrease in the percentage of Mist-1–positive cells in irradiated and saline-treated groups versus nonirradiated controls (Fig. 3E, F, M). IP-treated groups of WR and Am show a 2-fold decrease in Mist-1–positive cells from nonirradiated controls (Fig. 3G, H, M). In contrast, the percentage of Mist1-positive cells in retrograde-treated WR and Am groups was not statistically different from nonirradiated controls (Fig. 3K–M). These data show that retrograde administration of WR and Am increases the long-term preservation of secretory acinar cells up to 12 wk following irradiation.
Figure 3.
Gland histology and Mist1 immunofluorescence at 12 weeks postirradiation. (A–D, I, J) Hematoxylin and eosin staining shows gross morphology of acini and ducts in submandibular gland at 12 wk after IR (scale bars = 200 μm). (E–H, K, L) Corresponding immunofluorescence shows nuclei stained with antibody to Mist-1 (red) in acinar cells with nuclear counterstain (DAPI) at 12 wk after IR (scale bars = 75 μm). Quantification of acinar cell areas is shown (M). Mean ± SEM, n = 5, ****P < 0.0001, **P < 0.01 versus IR, IP Saline (Sal), and Ret Sal, using 1-way analysis of variance with Tukey’s post hoc test for multiple comparisons. Am, amifostine; IP, intraperitoneal; IR, irradiation; Ret, retroductal; Sal, saline; WR, WR-1065.
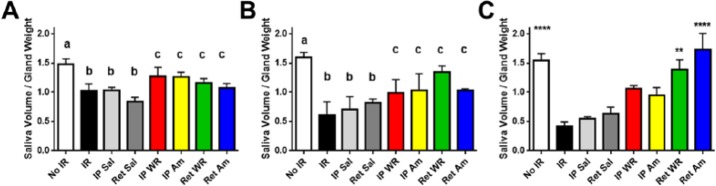
Localized Delivery of Amifostine or WR-1065 Significantly Improves Saliva Secretion in Irradiated Glands
To examine whether retroductally administered Am or WR preserves functional secretion, stimulated saliva secretion was evaluated at 2, 6, and 12 wk postirradiation. As expected, salivary function was significantly decreased in the irradiated and saline-treated groups in comparison to nonirradiated controls at all 3 time points (Fig. 4A–C). Saliva secretion in IP Am- and WR-treated groups was not significantly different from nonirradiated or all irradiated controls across all time points (Fig. 4A–C). Retrograde-treated groups receiving Am or WR likewise showed no difference from either nonirradiated or irradiated and Sal-treated groups at 2 and 6 wk (Fig. 4A, B). However, at 12 wk postirradiation, saliva function in retrograde-treated WR and Am groups was maintained at levels significantly higher than irradiated controls (Fig. 4C). These functional data are consistent with the increased number of acinar cells present at 12 wk in the irradiated SMG following retroductal administration of Am or WR.
Figure 4.
Measurement of stimulated saliva secretion normalized by gland weight at (A) 2 wk, (B) 6 wk, and (C) 12 wk after 15 Gy IR. Superscripts (a, b, c) are used to group cohorts for statistical comparisons. At 2 and 6 wk, b groups are significantly different from a, and c groups are not significantly different from a or all b groups. At 12 wk, control and Ret WR and Am groups are significantly different from all 3 IR and Sal groups (mean ± SEM, n ≥ 4, ****P < 0.0001, **P < 0.01 versus IR, IP Sal, and Ret Sal, using 1-way analysis of variance with Tukey’s post hoc test for multiple comparisons). Am, amifostine; IP, intraperitoneal; IR, irradiation; Ret, retroductal; Sal, saline; WR, WR-1065.
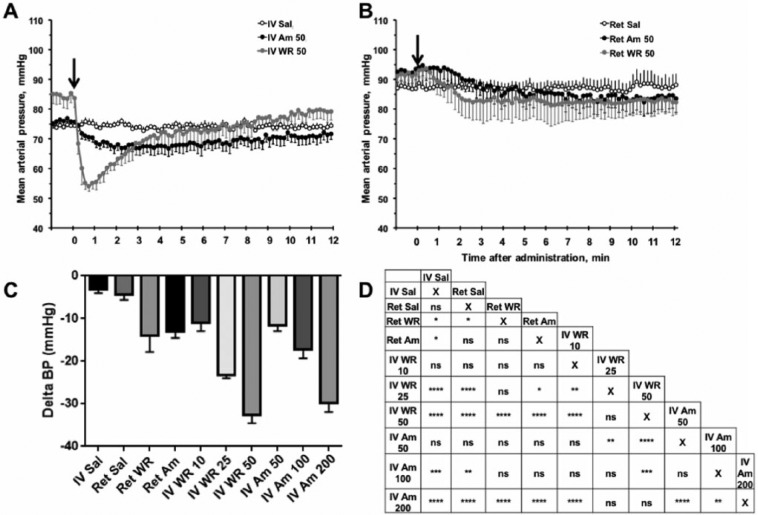
Localized WR-1065 Delivery Alleviates Systemic Drop in Mean Arterial Pressure
The current therapeutic prophylaxis for xerostomia is IV administration of Am, which causes a profound and rapid BP decrease (Eisbruch 2011). The functional consequences of this severe hypotension limit the clinical use of Am. To determine if localized delivery by retroductal injection could mitigate this effect, we compared localized to systemic drug delivery, using a pressure transducer introduced into the aorta through femoral catheterization to measure hypotension. Averaged pulsatile BP traces are shown in Figure 5A, B, with arrows indicating the timepoint of drug or Sal infusion. Injections of Sal (IV and Ret) had a minimal effect on MAP (Fig. 5). IV injection of Am resulted in a gradual decrease in MAP (maximal at 200 mg/kg), while WR (maximal at 50 mg/kg) caused a rapid and significant decrease followed by recovery (Fig. 5A, C, D). No significant MAP changes were observed with IV or Ret administration of Am at the 50-mg/kg dose compared to the Sal controls (Fig. 5). However, Ret WR attenuated the hypotensive effect by more than 2-fold compared to IV injection of WR at the 50-mg/kg dose (Fig. 5C, D). Altogether, our results demonstrate that localized delivery may reduce the detrimental decrease in MAP, which occurs upon systemic administration.
Figure 5.
Mean arterial pressure (MAP) change after intravenous (IV) or Ret injection compared to no treatment baseline (Delta MAP is the difference in pretreatment baseline MAP and minimum posttreatment MAP). (A, B) Representative MAP traces over time after IV injection or Ret injection (marked by arrow) of Sal, Am, or WR. (C) MAP decrease from baseline per treatment group (mean ± SEM). Ret WR and Ret Am were administered at 50 mg/kg; IV WR was administered at 10, 25, and 50 mg/kg; IV Am at 50, 100, and 200 mg/kg. (D) Pairwise comparisons between Delta BP values to show significance (mean ± SEM, n ≥ 5, ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 using 1-way analysis of variance with Tukey’s post hoc test for multiple comparisons). Am, amifostine; IV, intravenous; Ret, retroductal; Sal, saline; WR, WR-1065.
Discussion
The major finding of this study is that localized delivery of Am or WR to the murine SMG significantly preserves secretory cells and protects against radiation-induced hyposalivation compared to systemic administration. Specifically, we have demonstrated that 1) net DNA damage is reduced in treated glands directly after radiation; 2) acinar cell number is maintained in treated glands up to 12 wk postirradiation; 3) saliva secretion levels are maintained in both Am- and WR-treated glands at 2, 6, and 12 wk following radiation; and 4) local WR-1065 administration reduces the hypotensive response observed following systemic delivery.
Retroductal cannulation and injection provide direct SMG access, bypassing the systemic circulation (Kuriki et al. 2011). This can also reduce or obviate the side effects that limit clinical use of Am, most notably, hypotension. Our findings underscore the efficacy of a localized delivery strategy and the advantages it provides over systemic drug administration, including reducing the risk of unintended tumor protection.
Consistent with previous reports, we observed that net DNA damage is mitigated through administration of Am or WR (Hofer et al. 2016). Both compounds act in the setting of radiation as radical scavengers and are hypothesized to stabilize DNA strands through charge interactions and potentiation of double-strand break repair (Grdina et al. 2000). While there is disagreement on the exact mechanism of salivary gland radiosensitivity, mitigating the net genotoxic stress of radiation using these and similar compounds has been shown to enhance cell viability and tissue function (Konings et al. 2005; Soref et al. 2012). Our data demonstrate that localized delivery of both drugs significantly lowers the number of γH2AX foci in comparison to all other experimental cohorts. IP WR did not show a significant decrease in DSBs relative to irradiated controls. This may be due to its rapid metabolism to the disulfide WR-33278 and subsequent clearance from the circulatory system, preventing adequate SMG accumulation (Newton et al. 1996).
The retrograde dosages of 50 mg/kg for Am and WR were based on pilot toxicity and efficacy studies. It is important to note that this Am dose would be subtherapeutic if given IV against 15 Gy irradiation. Thus, although the IP Am dosage was 3 times higher, the retrograde doses were more effective at preserving salivary function. Biodistribution studies have shown 0.5% uptake of Am in the salivary glands following systemic administration (400 mg/kg IP), demonstrating that drug accumulation of 40 µg per gland is sufficient to protect secretory function (Rasey et al. 1984). Through retroductal injection, we administer approximately 25 times this dose without significant deleterious effects. Our data also show that substances injected retrogradely are cleared, likely via normal gland secretion (Appendix Fig. 4).
Severe radiation injury to the salivary glands results in depletion of acinar cells, concomitant with ductal expansion, and an increase in fibrosis and inflammation (Cheng et al. 2011; Nam et al. 2016). Secretory cell loss appears to be a late effect, as SMG acinar cell depletion is not obvious at 2 or 6 wk following irradiation (Appendix Fig. 2). Immunofluorescence and histology reveal significant preservation of acinar cells at 12 wk postirradiation in glands treated with retrograde Am or WR. As in earlier studies of radioprotective compounds or strategies, the targets are unclear. The maintenance of secretion could be through direct action upon acinar cells, indirect preservation and protection of supporting cells, or likely a combination of the two (Zheng et al. 2011; Arany et al. 2013). Protection of the microvasculature has also been shown to contribute to the maintenance of salivary gland function (Mizrachi et al. 2016).
In long-term studies, Am has a radiation dose modification factor (DMF) greater than 2, meaning that a 15-Gy salivary gland dose can be mitigated to an equivalent of <7.5 Gy (Sodicoff et al. 1978). DMF is a function of amifostine concentration, which we have maximized through local delivery (Stewart and Rojas 1982). It has been shown that saliva flow from the rat SMG at 3 mo following doses <7.5 Gy is not significantly lower than baseline (Nagler et al. 1998). In this study, acinar cell numbers are preserved on histology, although ductal structures sustain DNA damage at 48 h (Fig. 2). This is consistent with published reports indicating that Am and WR can effectively reduce but not block an absorbed radiation dose (Junn et al. 2012; Vasin and Ushakov 2015).
Saliva production progressively decreases in irradiated control cohorts from 2 to 12 wk after irradiation. However, saliva secretion is maintained by Am or WR delivered retroductally, with the protective effect most apparent at 12 wk. At the selected dosages, Am and WR delivered IP are not as effective. Higher systemic doses may be used but with greater risk of side effects.
While there is not a significant difference in acinar cell maintenance or gland function between Ret Am and Ret WR groups, contralateral glands do show differential radioprotection. Namely, in Ret Am–treated mice, contralateral glands show preserved acinar area, whereas in Ret WR–treated mice, they do not (Appendix Fig. 5). This may be attributable to the 2-step metabolism of Am versus the 1-step metabolism of WR (Newton et al. 1996).
Retrograde WR injection elicits a low hypotensive effect at 50 mg/kg, in contrast to a 2-fold decrease in MAP following IV administration. At 50 mg/kg, WR is more potent than Am in its hypotensive effect, and when administered retroductally, WR significantly attenuates MAP decrease compared to IV treatment. Interestingly, we also observed no decrease in pressor response to the α-1 agonist phenylephrine (Appendix Fig. 6) following preadministration of either Am or WR (IV or Ret). This supplements prior work using epinephrine (α- and β-agonist) following Am pretreatment in rats and provides mechanistic evidence to suggest that clinical hypotension following Am or WR could be reversed with an appropriate pressor (Soref et al. 2012).
Our MAP results are consistent with clinical trials that showed improvement in hypotensive effects after subcutaneous Am administration compared to IV administration (Koukourakis et al. 2000). Although subcutaneous administration reduced radiation-induced oral toxicities, including mucositis and esophagitis, rates of xerostomia were not reduced (Koukourakis et al. 2000; Bardet et al. 2011).
Our results demonstrate that localized Am, retroductally administered to the SMG, is protective against RIH even though the compound itself is not delivered in its active form. Oral Am application has likewise been shown to confer protection against radiation-induced mucositis in guinea pigs, demonstrating drug efficacy without introduction into the vasculature (Li et al. 2014). It is possible that alkaline phosphatase activity, present in saliva, may result in conversion of Am to WR following retroductal injection (Patel et al. 2016). We note that given the slight, though insignificant, decrease in blood pressure following retroductal amifostine administration, we cannot rule out diffusion from the main excretory duct into the proximal SMG blood supply.
Am is the only FDA-approved drug in use for xerostomia prophylaxis. As more candidate drugs become available, the retroductal delivery strategy coupled with localized SMG irradiation (Appendix Fig. 1) is a practical preclinical platform to test in vivo radioprotective efficacy. Moreover, localized delivery may allow higher doses of these compounds to be delivered to increase the concentrations available to gland parenchyma. When first discovered, Am was found to be better tolerated than WR, as reflected by its higher LD50 value (Sweeney 1979). Our pilot toxicity studies confirmed this finding and may suggest a dosing limitation on WR-1065, both local and systemic.
We have demonstrated that localized, noninvasive delivery of the known radioprotective agent, Am, to the SMG markedly improves radioprotection at the cellular level and mitigates off-target side effects. Previous work has explored the radioprotective efficacy of localized Am delivery, but this study is the first to do so in the salivary gland (France et al. 1986; Koukourakis et al. 2000; Li et al. 2014). The significant protection of salivary gland function following a single dose of radiation motivates further development of this strategy for use with a fractionated dose scheme in a clinical setting.
Author Contributions
J.J. Varghese, S.D. Newlands, V.A. Korshunov, D.S.W. Benoit, C.E. Ovitt, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; I.L. Schmale, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; D. Mickelsen, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; M.E. Hansen, contributed to data acquisition, analysis, and interpretation, drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518767408 for Localized Delivery of Amifostine Enhances Salivary Gland Radioprotection by J.J. Varghese, I.L. Schmale, D. Mickelsen, M.E. Hansen, S.D. Newlands, D.S.W. Benoit, V.A. Korshunov, and C.E. Ovitt in Journal of Dental Research
Acknowledgments
We thank Dr. Jonathan Axelrod (Hadassah Hebrew University) for guidance with γH2AX antibody staining and Andrew Hollomon (University of Rochester) for image quantification. We also thank Dr. Elaine Smolock and Emily Wu for critical reading of this manuscript.
Footnotes
A supplemental appendix to this article is available online.
This work was funded by the IADR Innovation in Oral Care Award (CEO). Research reported in this publication was also supported by the National Institute of Dental and Craniofacial Research (NIDCR) and the National Cancer Institute (NCI) of the National Institutes of Health under award number R56 DE025098 (C.E.O., D.S.W.B.), UG3 DE027695 (D.S.W.B., C.E.O.), and F30 CA206296 (J.J.V.). V.A.K. has received research support from Novartis Pharmaceuticals Corp.
The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Antonadou D, Pepelassi M, Synodinou M, Puglisi M, Throuvalas N. 2002. Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 52(3):739–747. [DOI] [PubMed] [Google Scholar]
- Arany S, Benoit DS, Dewhurst S, Ovitt CE. 2013. Nanoparticle-mediated gene silencing confers radioprotection to salivary glands in vivo. Mol Ther. 21(6):1182–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure MH, Konieczny SF, Ovitt CE. 2015. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell. 33(2):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardet E, Martin L, Calais G, Alfonsi M, Feham NE, Tuchais C, Boisselier P, Dessard-Diana B, Seng S-H, Garaud P, et al. 2011. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: final results of the Gortec2000-02 phase III randomized trial. J Clin Oncol. 29(2):127–133. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, et al. 2012. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci U S A. 109(47):19403–19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum BJ, Zheng C, Alevizos I, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, McDermott N, Chiorini JA, Nikolov NP, et al. 2010. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncol. 46(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk LB, Shivnani AT, Small W., Jr. 2005. Pathophysiology and management of radiation-induced xerostomia. J Support Oncol. 3(3):191–200. [PubMed] [Google Scholar]
- Cheng SC, Wu VW, Kwong DL, Ying MT. 2011. Assessment of post-radiotherapy salivary glands. Br J Radiol. 84(1001):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisbruch A. 2011. Amifostine in the treatment of head and neck cancer: intravenous administration, subcutaneous administration, or none of the above. J Clin Oncol. 29(2):119–121. [DOI] [PubMed] [Google Scholar]
- France HG, Jirtle RL, Mansbach CM. 1986. Intracolonic WR 2721 protection of the rat colon from acute radiation injury. Gastroenterology. 91(3):644–650. [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Kataoka Y, Murley JS. 2000. Amifostine: mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Interact. 16(4):237–279. [DOI] [PubMed] [Google Scholar]
- Hofer M, Falk M, Komurkova D, Falkova I, Bacikova A, Klejdus B, Pagacova E, Stefancikova L, Weiterova L, Angelis KJ, et al. 2016. Two new faces of amifostine: protector from DNA damage in normal cells and inhibitor of DNA repair in cancer cells. J Med Chem. 59(7):3003–3017. [DOI] [PubMed] [Google Scholar]
- Junn JC, Sciubba JJ, Bishop JA, Zinreich E, Tang M, Levine MA, Palermo RA, Fakhry C, Blanco RG, Saunders JR, et al. 2012. The effect of amifostine on submandibular gland histology after radiation. Int J Otolaryngol. 2012:508279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings AWT, Coppes RP, Vissink A. 2005. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 62(4):1187–1194. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Kyrias G, Kakolyris S, Kouroussis C, Frangiadaki C, Giatromanolaki A, Retalis G, Georgoulias V. 2000. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. J Clin Oncol. 18(11):2226–2233. [DOI] [PubMed] [Google Scholar]
- Kuriki Y, Liu Y, Xia D, Gjerde EM, Khalili S, Mui B, Zheng C, Tran SD. 2011. Cannulation of the mouse submandibular salivary gland via the Wharton’s duct. J Vis Exp. (51): 3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Wang SZ, Wang SY, Zhang YP. 2014. Assessment of the effect of local application of amifostine on acute radiation-induced oral mucositis in guinea pigs. J Radiat Res. 55(5):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmary Y, Adar R, Gaska S, Wygoda A, Maly A, Cohen J, Eliashar R, Mizrachi L, Orfaig-Geva C, Baum BJ, et al. 2016. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer Res. 76(5):1170–1180. [DOI] [PubMed] [Google Scholar]
- McDonald S, Meyerowitz C, Smudzin T, Rubin P. 1994. Preliminary results of a pilot study using WR-2721 before fractionated irradiation of the head and neck to reduce salivary gland dysfunction. Int J Radiat Oncol Biol Phys. 29(4):747–754. [DOI] [PubMed] [Google Scholar]
- Mizrachi A, Cotrim AP, Katabi N, Mitchell JB, Verheij M, Haimovitz-Friedman A. 2016. Radiation-induced microvascular injury as a mechanism of salivary gland hypofunction and potential target for radioprotectors. Radiat Res. 186(2):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler RM, Baum BJ, Miller G, Fox PC. 1998. Long-term salivary effects of single-dose head and neck irradiation in the rat. Arch Oral Biol. 43(4):297–303. [DOI] [PubMed] [Google Scholar]
- Nam K, Maruyama CL, Trump BG, Buchmann L, Hunt JP, Monroe MM, Baker OJ. 2016. Post-irradiated human submandibular glands display high collagen deposition, disorganized cell junctions, and an increased number of adipocytes. J Histochem Cytochem. 64(6):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton GL, Aguilera JA, Kim T, Ward JF, Fahey RC. 1996. Transport of aminothiol radioprotectors into mammalian cells: passive diffusion versus mediated uptake. Radiat Res. 146(2):206–215. [PubMed] [Google Scholar]
- Okumura H, Nasu M, Yosue T. 2009. Effects of amifostine administration prior to irradiation to the submandibular gland in mice: autoradiographic study using 3H-leucine. Okajimas Folia Anat Jpn. 85(4):151–160. [DOI] [PubMed] [Google Scholar]
- Patel RM, Varma S, Suragimath G, Zope S. 2016. Estimation and comparison of salivary calcium, phosphorous, alkaline phosphatase and pH levels in periodontal health and disease: a cross-sectional biochemical study. J Clin Diagn Res. 10(7):ZC58–ZC61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 10(15):886–895. [DOI] [PubMed] [Google Scholar]
- Peotta VA, Vasquez EC, Meyrelles SS. 2001. Cardiovascular neural reflexes in L-NAME–induced hypertension in mice. Hypertension. 38(3):555. [DOI] [PubMed] [Google Scholar]
- Pin CL, Bonvissuto AC, Konieczny SF. 2000. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec. 259(2):157–167. [DOI] [PubMed] [Google Scholar]
- Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. 2004. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 70(3):261–264. [DOI] [PubMed] [Google Scholar]
- Rasey JS, Nelson NJ, Mahler P, Anderson K, Krohn KA, Menard T. 1984. Radioprotection of normal tissues against gamma rays and cyclotron neutrons with WR-2721: LD50 studies and 35S-WR-2721 biodistribution. Radiat Res. 97(3):598–607. [PubMed] [Google Scholar]
- Redman RS, Ball WD, Mezey E, Key S. 2009. Dispersed donor salivary gland cells are widely distributed in the recipient gland when infused up the ductal tree. Biotech Histochem. 84(6):253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SV, Carrithers SL, Parkinson SJ, Skurk C, Nuss C, Pooler PM, Owen CS, Lefer AM, Waldman SA. 1996. Hypotensive mechanisms of amifostine. J Clin Pharmacol. 36(4):365–373. [DOI] [PubMed] [Google Scholar]
- Sodicoff M, Conger AD, Pratt NE, Trepper P. 1978. Radioprotection by WR-2721 against long-term chronic damage to the rat parotid gland. Radiat Res. 76(1):172–179. [PubMed] [Google Scholar]
- Soref CM, Hacker TA, Fahl WE. 2012. A new orally active, aminothiol radioprotector-free of nausea and hypotension side effects at its highest radioprotective doses. Int J Radiat Oncol Biol Phys. 82(5):e701–e707. [DOI] [PubMed] [Google Scholar]
- Stewart FA, Rojas A. 1982. Radioprotection of mouse skin by WR-2721 in single and fractionated treatments. Br J Radiol. 55(649):42–47. [DOI] [PubMed] [Google Scholar]
- Sweeney TR. 1979. A survey of compounds from the antiradiation drug development program of the U.S. Army medical research and development command/T. R. Sweeney. Washington (DC): Department of Defense, Department of the Army, Surgeon General’s Office, Army Medical Research and Development Command, Walter Reed Army Institute of Research. [Google Scholar]
- Vasin MV, Ushakov IB. 2015. Comparative efficacy and the window of radioprotection for adrenergic and serotoninergic agents and aminothiols in experiments with small and large animals. J Radiat Res. 56(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran N, Williams MD. 2014. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 26(2):123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas JM. 1980. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by s-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 40(5):1519–1524. [PubMed] [Google Scholar]
- Zheng C, Cotrim AP, Rowzee A, Swaim W, Sowers A, Mitchell JB, Baum BJ. 2011. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin Cancer Res. 17(9):2842–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518767408 for Localized Delivery of Amifostine Enhances Salivary Gland Radioprotection by J.J. Varghese, I.L. Schmale, D. Mickelsen, M.E. Hansen, S.D. Newlands, D.S.W. Benoit, V.A. Korshunov, and C.E. Ovitt in Journal of Dental Research