Abstract
Bruton’s tyrosine kinase (BTK) is a clinically validated target for B-cell leukemias and lymphomas with FDA-approved small-molecule inhibitors ibrutinib and acalabrutinib. Tirabrutinib (GS-4059/ONO-4059, Gilead Sciences, Inc., Foster City, CA) is a second-generation, potent, selective, irreversible BTK inhibitor in clinical development for lymphoid malignancies, including chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL). An accurate pharmacodynamic assay to assess tirabrutinib target coverage in phase 1/2 clinical studies will inform dose and schedule selection for advanced clinical evaluation. We developed a novel duplex homogeneous BTK occupancy assay based on time-resolved fluorescence resonance energy transfer (TR-FRET) to measure free and total BTK levels in a multiplexed format. The dual-wavelength emission property of terbium-conjugated anti-BTK antibody served as the energy donor for two fluorescent energy acceptors with distinct excitation and emission spectra. The assay was characterized and qualified using full-length purified recombinant human BTK protein and peripheral blood mononuclear cells derived from healthy volunteers and patients with CLL. We demonstrated assay utility using cells derived from lymph node and bone marrow samples from patients with CLL and DLBCL. Our TR-FRET-based BTK occupancy assay provides accurate, quantitative assessment of BTK occupancy in the clinical trial program for tirabrutinib and is in use in ongoing clinical studies.
Keywords: Bruton’s tyrosine kinase, BTK inhibitor, lymphoid malignancies, assay development
Introduction
Bruton’s tyrosine kinase (BTK), a member of the tyrosine kinase expressed in the hepatocellular carcinoma (TEC) family of cytoplasmic protein tyrosine kinases,1,2 was initially identified as the pathogenic protein in X-linked agammaglobulinemia, a human primary immune deficiency disease.3 Expressed in B cells and myeloid cells, BTK plays an essential role in the B-cell receptor (BCR) signaling pathway.4,5 BTK promotes development and maturation of B cells through activation of cell cycle regulators and differentiation factors, and directs B-cell proliferation and survival through regulation of apoptosis.6–8 With its central role in BCR signaling and survival, BTK is an oncogenic driver in human chronic lymphocytic leukemia (CLL) and activated B-cell-like subtype of diffuse large B-cell lymphoma (ABC-DLBCL).
Development of selective adenosine triphosphate (ATP)-competitive kinase inhibitors is hampered by highly conserved ATP binding sites within this family. The presence of a nucleophilic cysteine at residue 481 (C481) in its ATP binding pocket has enabled the design of electrophilic BTK inhibitors that react with the proximal cysteine side chain, forming a covalent bond.9 With only 10 out of 491 kinases possessing a cysteine at the same position, this targeted approach was used to achieve selectivity against the majority of kinases. Pharmacological inhibition of BTK by the first-in-class BTK inhibitor, ibrutinib, showed effective clinical antileukemic activity10,11 and led to its approval for CLL, mantle cell lymphoma (MCL), and Waldenstrom’s macroglobulinemia.11–14 However, ibrutinib also inhibits other kinases, including epidermal growth factor kinase (EGFR), interleukin-2-inducible T-cell kinase (ITK), and TEC, with BTK selectivity of 11-, 21-, and 156-fold, respectively.15 This lack of target selectivity may contribute to clinical adverse events associated with ibrutinib.11,16,17 The recent approval of the more selective BTK inhibitor acalabrutinib for MCL therapy further validates BTK as a target for B-cell malignancies.18,19
Tirabrutinib (GS-4059/ONO-4059, Gilead Sciences, Inc., Foster City, CA) is a second-generation, potent BTK inhibitor that irreversibly binds to C481 of BTK with greater target selectivity.20–23 Compared with ibrutinib, tirabrutinib showed 15-, 2.6-, and 2.3-fold greater selectivity for BTK over EGFR, ITK, and bone marrow kinase on chromosome X (BMX), respectively.21 Selectivity for BTK over TEC was 2.3-fold for tirabrutinib and 5.6-fold for ibrutinib.21 In a phase 1 clinical study, tirabrutinib showed high response rates in CLL (96%) and MCL (92%) and modest response in nongerminal center DLBCL (35%), with an overall favorable tolerability profile.24 Further clinical development of tirabrutinib required dose-ranging studies to establish appropriate drug dosing and inform the pharmacokinetic/pharmacodynamic (PK/PD) relationship. To support these studies, a biomarker assay was needed that accurately assessed target coverage based on drug exposure in circulating peripheral blood mononuclear cells (PBMCs) as well as in lymph node and bone marrow tissues where the diseased cells may reside. Here, we report the development of a novel, homogenous BTK occupancy assay to assess PD BTK engagement in patient samples. Our assay is based on time-resolved fluorescence resonance energy transfer (TR-FRET) that simultaneously measures free and total BTK levels in a multiplexed format, reducing sample requirement while also enhancing the accuracy of measuring target occupancy and the ease of assay execution. This TR-FRET-based BTK occupancy assay was validated and qualified for clinical samples, including PBMCs and lymph node- and bone marrow-derived cells from patients with CLL and DLBCL. This assay will be used to evaluate target engagement as a PD biomarker in an ongoing clinical study with tirabrutinib.
Materials and Methods
Compounds
Tirabrutinib and biotinylated tirabrutinib were synthesized at Gilead Sciences, Inc. (Branford, CT). Compounds were serially diluted (1:3) in DMSO to generate 10-point dose–response curves, including zero and ranging from 10 µM to 1.5 nM. Twenty nanoliters of serial diluted compound was transferred to a 384-well, low-volume, white nonbinding surface (NBS) assay plate (Corning, Corning, NY) using Echo acoustic liquid dispensing technology (Labcyte, Inc., San Jose, CA). The final DMSO concentration was 0.5%.
Biochemical Enzyme Activity Assay
Compound dilutions were prepared using an HP D300 liquid dispenser (Hewlett-Packard, Palo Alto, CA) into a 96-well NBS assay plate (Corning). Reactions were carried out in TR-FRET dilution buffer (Life Technologies, Carlsbad, CA) containing 50 mM HEPES (pH 7.5), 0.01% brij-35, 10 mM MgCl2, 1 mM EGTA, and 0.5 mg/mL BSA. Fifty microliters of 2× recombinant glutathione-S-transferase (GST)-BTK protein (produced at Gilead Sciences, Inc., Foster City, CA) in TR-FRET dilution buffer was added to the assay plate containing 1 µL of compound solution in DMSO. The compound/enzyme mixture was preincubated for 30 min, and reactions were initiated with the addition of 50 µL of 2× fluorescein-Poly GT substrate (Life Technologies). Final assay conditions were 200 pM GST-BTK, 0.2 µM fluorescein-Poly GT substrate, and 40 µM ATP (2× Km) in TR-FRET dilution buffer. Reactions were terminated with 100 µL of 2× EDTA/LanthaScreen terbium (Tb)-PY20 antibody mixture (Life Technologies) with final concentrations of 10 mM EDTA and 2 nM antibody. Fluorescence intensity (λ excitation 332 nm/λ emission 486/515 nm) was read on a TECAN Infinite M1000 Pro Multimode reader (Tecan, Männedorf, Switzerland). The ratio of fluorescence at 515 nm to that at 486 nm was the measure of product formation. The TR-FRET ratio was plotted against the inhibitor concentration and normalized to enzyme/no enzyme controls. Half maximal inhibitory concentration (IC50) values were calculated with a four-parameter logistic fit using GraphPad Prism (version 7; La Jolla, CA).
Assay Reagents
Custom Tb-labeled and D2-labeled anti-BTK antibodies, as well as G2-conjugated streptavidin, were purchased from Cisbio US (Bedford, MA). NP40 cell lysis buffer was obtained from Thermo Fisher Scientific (Waltham, MA) and used at 0.34× concentration. Cell lysis buffer supplemented with blocking reagent (Cisbio) was used for preparing cell lysates and the recombinant BTK standard curve.
PBMC, Ramos, and Jurkat Cell Titration
Cell Preparation for Cell Titration Curves
Cryopreserved PBMCs from healthy volunteers were purchased from StemCell Technologies (Vancouver, BC, Canada). Freshly harvested whole blood was obtained from AllCells (Alameda, CA). Ramos and Jurkat cell lines were obtained from the American Type Culture Collection (Manassas, VA). Ramos and Jurkat cells were maintained in RPMI 1640 medium supplemented with 100 units/mL penicillin, 10 μg/mL streptomycin, and 10% fetal bovine serum (FBS).
Cryopreserved PBMCs were thawed in a 37 °C water bath and rinsed in phosphate-buffered saline (PBS). Viable cells were counted using a Cellometer instrument (Nexcelom Bioscience, LLC, Lawrence, MA), pelleted by centrifugation at 250g, and resuspended in PBS at 125,000 cells/µL. Cells were serially diluted from 125,000 to 5000 cells/µL at a dilution factor of 2. Cells (4 µL) were added to a 384-well, low-volume, white NBS assay plate (Corning) with a manual pipet.
Similarly, Ramos and Jurkat cells were harvested and rinsed once in PBS, counted using a Cellometer instrument, pelleted by centrifugation at 250g, and resuspended in PBS at 100,000 cells/µL. Cells were serially diluted from 100,000 to 4000 cells/µL at a dilution factor of 2, and 4 µL of cells were added to the 384-well assay plate with a manual pipet.
Cell Lysate Preparation
Cryopreserved PBMCs were thawed in a 37 °C water bath, rinsed in PBS, counted, centrifuged, and resuspended in lysis buffer 2 at 25,000 cells/µL for 1 h at room temperature. Similarly, Ramos and Jurkat cells were harvested, rinsed in PBS, counted, centrifuged, and resuspended in lysis buffer 2 at 12,500 cells/µL for 1 h at room temperature. Four microliters of lysate was added to the 384-well assay plate already containing serial diluted tirabrutinib with a manual pipet.
Recombinant BTK Protein Titration
Recombinant full-length human BTK (rBTK) was purchased from Carna Biosciences (Kobe, Japan) and serially diluted (1:2) in lysis buffer 2 to generate a 10-point concentration standard curve including zero and ranging from 2500 to 9.8 ng/mL. Four microliters of serially diluted protein was added to a 384-well assay plate with a manual pipet.
Preparation of Samples from Patients
PBMCs and Bone Marrow Cells
Cryopreserved PBMCs from CLL patients were purchased from BioreclamationIVT (Chestertown, MD) and lysates were prepared for healthy volunteer PBMCs as described above. Additionally, matched PBMCs and bone marrow mononuclear cells (BMMCs) from two patients with CLL were acquired from Conversant Biologics, Inc. (Huntsville, AL). Cells were thawed in a 37 °C water bath for 2 min and transferred to a 15 mL centrifuge tube containing 10 mL of RPMI 1640 with 10% FBS (heat-inactivated). Cells were centrifuged at 250g for 10 min, and supernatant was removed by aspiration without disturbing the cell pellet. Five microliters of media was used to resuspend the cell pellet, and cells were counted on a Cellometer K2 (Nexcelom). Cells were seeded at 2 million/mL in a T25 flask and incubated to recover overnight in a 37 °C incubator with 5% CO2. Cells were counted the next day, and 1.5 mL cells were aliquoted to three 14 mL, round-bottom tubes (BD FALCON; BD Biosciences, Bedford, MA). Cells were treated by spiking 110, 8, and 0 nM tirabrutinib with a final DMSO concentration of 0.1%. After incubating for 2 h in a 37 °C incubator with 5% CO2, the cells were transferred to a 1.5 mL microcentrifuge tube and centrifuged at 250g for 10 min. Supernatant was removed and the cells were resuspended in 1 mL of Dulbecco’s phosphate-buffered saline (DPBS) and counted. Cells were centrifuged at 250g for 10 min. The supernatant was removed by aspiration. A half volume of DPBS and 2× lysis mixture (0.68× lysis buffer [Invitrogen, Carlsbad, CA] + 4× blocking reagent from Total BTK kit [Cisbio]) were added to the cells at a concentration of 1.25 × 107 cells/mL. The tube was vortexed for 2 s and the cells were incubated at room temperature for 1 h. Cell lysate was then diluted to 6.25 × 106 cells/mL and 3.125 × 106 cells/mL, and the BTK occupancy assay was performed.
Lymph Node Tissue
Two lymph node tissue samples from patients with DLBCL were purchased from Folio Biosciences (Powell, OH). The cold block, dissection tools, and 2 mL Safe-Lock tubes (Eppendorf Biotech, Hamburg, Germany) were prechilled on dry ice. The lymph node tissue samples on a weighting bowl on top of the cold block were cut into 30–100 mg pieces with a razor blade in a biosafety cabinet. The tissue pieces were placed into a 2 mL tube for lysis using a Tissuelyser II (QIAGEN, Hilden, Germany) per the manufacturer’s instructions. Briefly, one 5 mm stainless steel bead (QIAGEN) and 600 µL of 0.34× lysis buffer (Invitrogen, cat. FNN0021) + 2× blocking reagent from the Total BTK kit (Cisbio) were added to each tube containing lymph node tissue. The tissues were homogenized at a current frequency of 20 for 2 min in the Tissuelyser II. The process was repeated once. The lysates were centrifuged at 10,000 rpm in an Eppendorf microcentrifuge for 10 min at 4 °C. The supernatant was then transferred to a 1.5 mL microcentrifuge tube, followed by protein measurement with QIAxper (QIAGEN). The free and total BTK in the lymph node tissue lysates were measured using the BTK occupancy assay.
Multiplexed Assay for Free and Total BTK
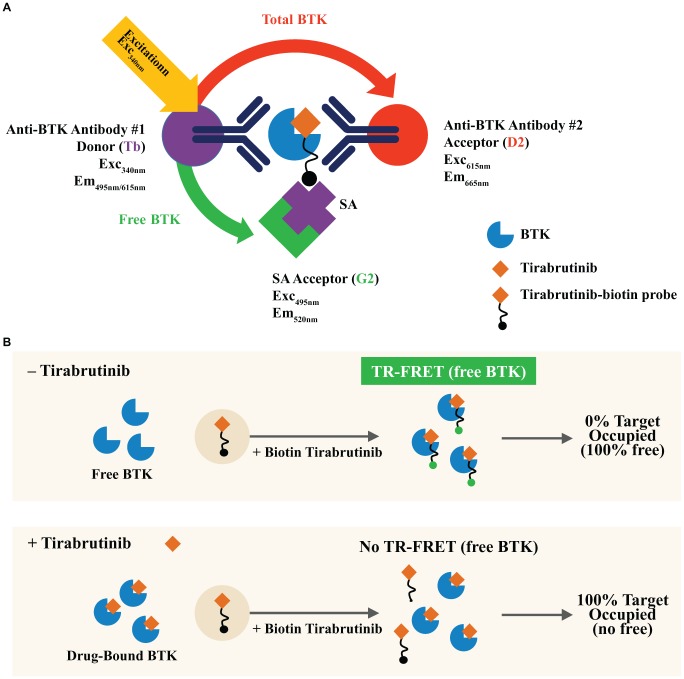
Samples (cells, cell lysates, or rBTK standard curve, prepared as described above) were plated into a 384-well assay plate at 4 μL/well, followed by addition of 2 μL/well probe solution (5 μM in lysis buffer 1, biotinylated probe [ Fig. 1A ]), and incubated for 1 h. Detection solution composed of anti-BTK-Tb (1:32 dilution), anti-BTK-D2 (1:32 dilution), and SA-G2 (1:24.57 dilution) in 0.5× detection buffer was added to the plate at 4 μL/well. Plates were sealed and equilibrated for 30 min at room temperature before storing at 4 °C overnight and reading with an EnVision laser-based reader (PerkinElmer, Waltham, MA) the next day. Plates were centrifuged at 250g for 1 min after every addition step, and all incubation steps were done at room temperature unless stated otherwise.
Figure 1.
(A) Chemical structure of tirabrutinib (left) and probe (right) consisting of tirabrutinib conjugated to biotin (yellow circle). (B) Activity of probe on recombinant BTK activity. The biotinylated tirabrutinib probe (red) functionally inhibits enzymatic activity of human BTK in vitro with similar potency as tirabrutinib (blue).
Readout values were the ratio of acceptor to donor emissions for total BTK (665 nm/615 nm) and for free BTK (520 nm/615 nm) ( Fig. 2A ). These values were then used for interpolating concentrations of BTK from the standard curves. The assay used rBTK to generate independent standard curves for free and total BTK. The concentration of BTK was derived by interpolating data from the standard curves using four-parameter logistic regressions after log transforming both the concentration and emission ratio data. Interpolated values were correspondingly exponentiated to return back-calculated concentration values, which are given as nanograms per milliliter.
Figure 2.
(A) TR-FRET-based detection of free and total BTK. The distinct, nonoverlapping emission spectra of Tb allows it to serve as a common fluorescence donor for two spectrally distinct acceptors: G2-streptavidin (SA)-bound biotinylated tirabrutinib (to detect free BTK) and D2-coupled anti-BTK antibody that binds to a different BTK epitope (to detect total BTK). TR-FRET readout values are the ratio of acceptor to donor emissions for free BTK (520 nm/615 nm) and total BTK (665 nm/615 nm). This multiplexed assay allows detection of both free and total BTK simultaneously in the same sample. (B) Principle of the BTK occupancy assay. In the absence of tirabrutinib, 100% of BTK in the sample is unbound. The free BTK is captured by the biotinylated tirabrutinib probe and measured by the Tb-to-G2 TR-FRET readout. In the presence of a saturating dose of tirabrutinib, all BTK in the sample is drug bound. The lack of TR-FRET signal for free BTK indicates 100% BTK occupancy.
Assay Validation and Qualification
The parameters assessed to validate the quantitative performance of the assay included range of quantitation, sensitivity, specificity, accuracy, and precision. BTK occupancy was determined following data normalization to vehicle-treated samples and was calculated using the following formula:
where freeBTKx or totalBTKx indicates the respective concentration at the dose or time point of interest and freeBTKy or totalBTKy indicates the respective concentration in 0-dose or predose condition. In principle, the TR-FRET binding assay will detect 100% free BTK in the absence of tirabrutinib and 100% occupied BTK in the presence of a saturating dose of tirabrutinib ( Fig. 2B ).
Stability of Tirabrutinib-Treated Whole Blood
Whole-blood spike-in experiments were performed using whole-blood samples from three donors acquired from AllCells. The blood samples (10 mL/tube) were treated by spiking in at final concentrations of 110, 8, and 0 nM tirabrutinib or 110 nM acalabrutinib (ACP-196) with a final DMSO concentration of 0.1%. After incubating for 2 h in a 37 °C incubator with 5% CO2, the blood samples were further incubated at either 4 °C or room temperature for up to 48 h. At various time points (0–48 h), the blood samples were processed to purify PBMCs using Lymphoprep tubes. Purified PBMCs (20–40 million/tube recovered) were cryopreserved at 5–10 × 106 cells/mL in Recovery cell freezing medium (Thermo Fisher Scientific).
For the BTK occupancy assay, cryopreserved PBMCs were thawed in a 37 °C water bath for 2 min and washed once with 1 mL DPBS. PBMCs were centrifuged at 250g for 10 min to remove the wash buffer. PBMC pellets were resuspended in 1 mL of DPBS and counted using a Cellometer. PBMCs were centrifuged at 250g for 10 min to collect the cell pellets. PBMC lysates (12,500 cells/µL) were made using lysis buffer (0.34× NP40 lysis buffer + 2× blocking reagent from the Total BTK kit). Cell lysates were then diluted to 6250 and 3125 cells/µL using lysis buffer as the diluent. Cell lysates were transferred into a 384-well assay plate at 4 μL/well, followed by addition of 2 μL/well probe solution (5 μM in 0.34× NP40 lysis buffer, biotinylated probe [ Fig. 1A ]), and incubated for 1 h. Detection solution composed of anti-BTK-Tb (1:32 dilution), anti-BTK-D2 (1:32 dilution), and SA-G2 (1:24.57 dilution) in 0.5× detection buffer was added to the plate at 4 μL/well. Plates were sealed and equilibrated for 30 min at room temperature before storing at 4 °C overnight and reading with an EnVision laser-based reader (PerkinElmer) the next day. Plates were centrifuged at 250g for 1 min after every additional step, and all incubation steps were done at room temperature unless stated otherwise.
Results
Tirabrutinib Probe Design and Evaluation
Tirabrutinib (GS-4059/ONO-4059) binds irreversibly to C481 within the ATP binding pocket of BTK.20,21 The chemical structure of tirabrutinib is shown in Figure 1A . A biotinylated probe was designed that binds in the same BTK pocket as the parent tirabrutinib molecule. Biotin was conjugated to tirabrutinib via a linker on a part of the molecule that does not interfere with BTK binding and covalent bond formation ( Fig. 1A ). BTK binding by biotinylated tirabrutinib was assessed in a biochemical assay. Biotinylated tirabrutinib inhibited BTK enzymatic activity at similar potency as the parent molecule, with IC50 values of 4.7 and 9.7 nM, respectively ( Fig. 1B ). The observed IC50 values are consistent with the tirabrutinib potency reported previously,25 suggesting that the probe binds to the same BTK pocket with similar affinity as the parent molecule and is suitable for use as a probe to measure BTK occupancy by tirabrutinib.
Principle of Multiplexed Assay for Free and Total BTK
Target engagement or occupancy is a measure of drug binding to its target. It is determined by quantifying the fraction of target bound to drug that induces a biological effect. The accuracy of target occupancy measurement depends on quantification of both total and drug-bound target. To obtain high-accuracy data, we developed a multiplexed homogeneous assay that measures total and tirabrutinib-free BTK simultaneously in the same well ( Fig. 2A ). The assay is based on TR-FRET, taking advantage of the dual-wavelength emission property of Tb to serve as the common energy donor for two fluorescent energy acceptors with distinct excitation and emission spectra: (1) G2 small-molecule green acceptor and (2) D2 small-molecule red acceptor. To detect free BTK, a Tb-coupled anti-BTK antibody was used as the FRET energy donor and G2-streptavidin-bound biotinylated tirabrutinib was used as the energy acceptor. Total BTK was detected in the same sample with a second D2-coupled anti-BTK antibody that binds to a different BTK epitope as the FRET energy acceptor. The multiplexed assay avoided sampling variability in traditional single-analyte assays, and the use of a common antibody as the FRET donor further served to normalize the detection of total and free BTK with respect to each other. Additionally, the multiplexed assay format reduced the clinical sample requirement for analysis.
Assay Validation
The TR-FRET BTK occupancy assay was validated using purified human rBTK and cell extracts. Based on a set of six experiments with quadruplicate samples using rBTK, the assay dynamic range was 9.75–312 ng/mL free and total BTK. The lower and upper limits of quantitation were 12 ng/mL (limit of detection 6 ng/mL) and 166 ng/mL, respectively ( Fig. 3A ). Variability in free and total BTK levels within the full assay dynamic range was very low, with interassay coefficient of variation (CV) ≤6% for free BTK and ≤3% for total BTK. Variation in percent free BTK, as defined by the ratio of free/total BTK levels, was similarly low, with an average CV of 1.6%. In addition to demonstrating precision in interassay performance, the assay also demonstrated accuracy; samples were spiked at varying levels and showed acceptable recovery variation (CV ≤5%) ( Fig. 3B ). Based on the variability and error limits of measuring two analytes that are combined to derive target occupancy, the percent occupancy range for this assay is 10%–90%. Full target engagement, defined as >90% occupancy as the upper limit of quantitation, is achieved when the free BTK level is below the lower limit of detection. The variability of free and total BTK detection in the inhibitor-free condition establishes the lower limit of quantitation at <10% occupancy for fully free BTK.
Figure 3.
(A) Assay range of quantitation. A set of six experiments (in quadruplicate) using full-length purified rBTK protein was used to generate independent standard curves for free and total BTK. The lower limit of quantitation of the assay was 12 ng/mL, and the upper limit was 166 ng/mL. The BTK concentration in cell samples in subsequent experiments was interpolated from these standard curves. (B) Assay accuracy and precision. Low percent CV (solid lines) in interassay performance demonstrated precision of the assay, and percent recovery (dotted lines) after spikes of 110, 8, and 0 nM tirabrutinib demonstrated accuracy of the assay. Data represent mean values from four independent experiments in quadruplicate. (C) Specificity of the assay for BTK inhibition was demonstrated by detection of free and total BTK in titrated lysates of Ramos B cells (light purple dots) but not Jurkat T cells (pink dots). BTK expression was lower in PBMCs (dark purple dots) than in Ramos cells. The decrease in TR-FRET at high Ramos cell concentration reflects analyte saturation. Representative data are shown from three or more experiments.
The specificity of the assay for BTK inhibition was demonstrated by the detection of free and total BTK in titrated lysates of Ramos B cells, which express an intact BCR signaling pathway, but not in lysates of Jurkat T cells, which do not express BTK ( Fig. 3C ). At high concentrations of Ramos B-cell lysates, a decrease in TR-FRET relative fluorescence units was observed, reflecting analyte saturation. BTK expression in human PBMCs was lower than that in Ramos cells, as expected.
Assay Qualification and Utility
The TR-FRET assay was used to detect free and total BTK using rBTK, Ramos B cells, purified human PBMCs, and whole blood from healthy volunteers treated with increasing concentrations of tirabrutinib. Values were normalized to vehicle-treated samples to obtain percent BTK occupancy ( Fig. 4 ). Tirabrutinib demonstrated dose-dependent, competitive inhibition of biotinylated tirabrutinib binding. The half maximal effective concentration (EC50) of tirabrutinib on rBTK in the BTK occupancy assay was 5.9 nM, consistent with its IC50 on rBTK enzyme activity ( Fig. 1B ). The concentration of tirabrutinib required for 50% occupancy of BTK after 1 h of incubation was 72 nM in Ramos B cells and 92 nM in PBMCs, and 99 nM in whole blood following 2 h of incubation. Full BTK occupancy was achieved at 100 nM tirabrutinib for rBTK and at 500–1000 nM tirabrutinib in the tested Ramos B cells, human PBMCs, and whole blood.
Figure 4.
Inhibition of BTK by tirabrutinib. The TR-FRET assay was used to detect free and total BTK using rBTK, Ramos B cells, purified human PBMCs, and whole blood (WB) samples preincubated for 2 h with increasing concentrations of tirabrutinib. Values were normalized to vehicle-treated samples to obtain percent BTK occupancy. The top left panel shows the standard dose–response curve generated using recombinant BTK. In Ramos B cells, purified human PBMCs, and WB samples, BTK binding to the biotinylated tirabrutinib probe was competitively inhibited in a dose-dependent manner by tirabrutinib. The EC50 of tirabrutinib as measured by the TR-FRET assay is shown in the table (mean ± SD of n = 3 experiments, each in quadruplicate).
BTK Expression in Healthy Donor and CLL Patient Samples
PBMCs in patients with B-cell malignancies can show very high BTK levels due to enrichment of B cells. We therefore use three cell densities (12,500–50,000 cells/mL) in testing clinical samples to ensure that BTK is within the dynamic range of this assay. Since BTK occupancy in this assay is determined by measuring the fraction of total BTK that is tirabrutinib-free, and percent free/total BTK is independent of cell density or BTK levels, we used samples with optimal BTK levels from each patient or subject. The BTK occupancy assay was developed to determine tirabrutinib target coverage in clinical samples from patients with lymphoid malignancies. We therefore tested PBMCs isolated from nine patients with CLL along with those from four healthy volunteers in this assay. All PBMC samples were normalized to 106 viable cells/mL. As shown in Figure 5A , total and free BTK were readily detected from the CLL patient samples. In addition, we observed that total BTK expression in CLL patient PBMCs spanned a broad range, from 33 to 174 ng/mL, and was generally higher than total BTK expression in PBMCs from healthy volunteers (average 30 ng/mL). The variable and relatively higher BTK expression in the patient samples tested is consistent with the enrichment of BTK-expressing B lymphocytes to varying degrees among the CLL patient population.26 These results indicate that the BTK occupancy assay is suitable for use on patient PBMC samples.
Figure 5.
Assay utility in CLL and DLBCL patient samples: (A) PBMCs, (B) lymph nodes, and (C) bone marrow. (A) The concentration of total BTK in PBMCs from nine patients with CLL ranged from 33 to 174 ng/mL. The average total BTK expression in PBMCs from four healthy volunteers (HV, gray bar) was 30 ng/mL. Data represent the average and standard error of quadruplicate samples from each subject or from four healthy donors tested in quadruplicate. (B) The TR-FRET assay detected robust levels of free (light blue bars) and total (dark blue bars) BTK in lymph node (LN) tissue lysates from two patients with DLBCL. Data represent the average and standard error from samples tested in triplicate. (C) Matched BMMCs and PBMCs from two patients with CLL were treated by spiking 110, 8, and 0.1 nM tirabrutinib with a final DMSO concentration of 0.1%. The TR-FRET assay showed dose-dependent BTK binding after ex vivo treatment with tirabrutinib. BTK occupancy reached nearly 100% at a dose of 110 nM tirabrutinib. Data represent the average and standard error from triplicate samples.
CLL is characterized by malignant B cells that are enriched in circulating blood, in lymphoid tissues, and in the bone marrow.27 Assessment of tirabrutinib target coverage in clinical studies requires testing of not just patient PBMCs derived from whole blood, but testing of lymph node and/or bone marrow samples may also be desirable. We determined whether free and total BTK in cells from lymph node and bone marrow biopsies of patients with DLBCL and CLL, respectively, could be detected in the BTK occupancy assay. As shown in Figure 5B , total and free BTK were detected from 4.5 mg/mL total protein in the lymph node DLBCL biopsy sample. In addition, BMMCs and PBMCs from patients with CLL showed dose-dependent BTK binding after ex vivo treatment with tirabrutinib ( Fig. 5C ). BTK occupancy reached nearly >80% at a dose of 110 nM tirabrutinib, slightly higher than that observed in PBMCs from healthy volunteers ( Fig. 4 ).
Stability of Tirabrutinib-Treated Whole Blood
Whole-blood samples collected from subjects in tirabrutinib clinical studies are shipped to a testing lab for PD evaluation. Drug-treated samples must therefore be stable for 24–48 h. Stability in the case of the described occupancy assay for the irreversible BTK inhibitor tirabrutinib also includes the need to ensure that additional tirabrutinib receptor binding in the sample will not occur once the sample is removed from the patient, as this would artificially increase the measured occupancy. The stability of whole-blood samples from healthy volunteers stored at 4 °C and at room temperature was examined following ex vivo incubation with three submaximal concentrations of tirabrutinib for 2 h to evaluate the best shipping conditions for clinical samples. While BTK occupancy in the samples stored at 4 °C was maintained at the same level for up to 48 h, significantly higher target occupancy was observed in samples following 24 h at room temperature ( Fig. 6A , B ). To determine if the observed difference in stability of tirabrutinib samples at the tested storage temperatures is unique to tirabrutinib, another irreversible BTK inhibitor, ACP-196 (acalabrutinib), was evaluated under the same conditions. The effect of room temperature storage was also observed for acalabrutinib ( Fig. 6C ), suggesting that time-dependent target binding is an intrinsic property of covalent inhibitors.
Figure 6.
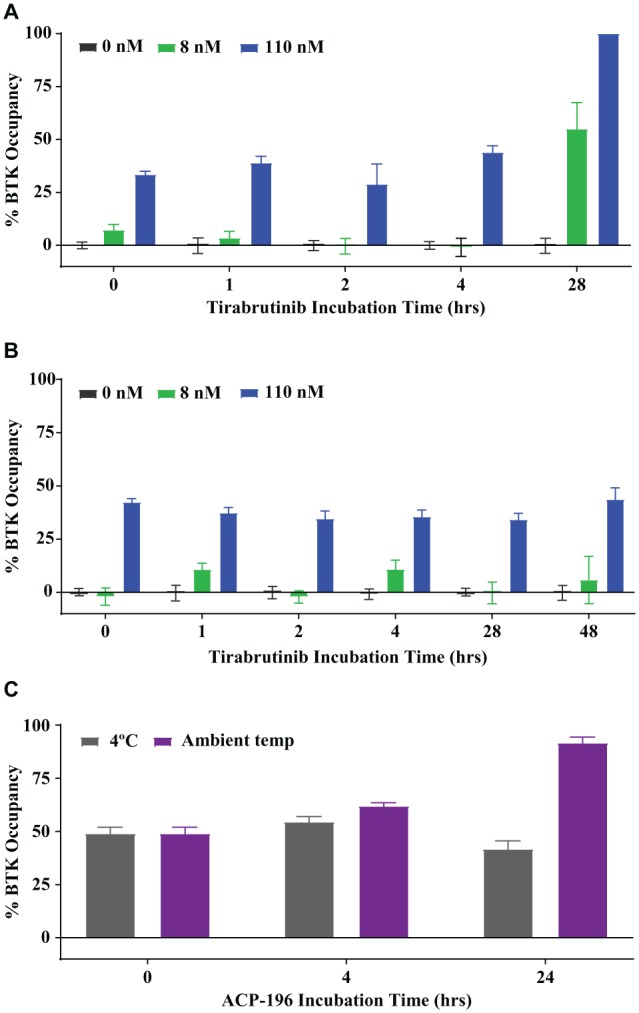
Storage temperature effect on BTK occupancy in whole blood. (A,B) Human whole blood was pretreated with the indicated tirabrutinib concentrations for 2 h, followed by (A) incubation at ambient temperature for up to 28 h or (B) being chilled and stored at 4 °C for up to 48 h. (C) Human whole blood was incubated with 110 nM acalabrutinib (ACP-196) for 2 h, followed by storage at 4 °C or ambient room temperature for the indicated times. Data represent average values and standard error from three healthy donors tested in quadruplicate (A,B) or two donors tested in triplicate (C).
Discussion
BTK plays a key role in B-cell malignancies associated with dysregulated BCR signaling and is an important therapeutic drug target for these diseases.4,5 In CLL, BTK is essential for B-cell migration, survival, and proliferation in lymph nodes.28,29 Similarly, BTK plays a role in the constitutive activation of BCR signaling that supports B-cell proliferation in MCL30 and DLBCL.31
Tirabrutinib is an orally bioavailable, selective, irreversible small-molecule BTK inhibitor that covalently interacts with C481 in the ATP binding pocket of BTK.20 It shows in vitro antiproliferative activity on follicular lymphoma, MCL, and CLL cell lines and on ABC-DLBCL in TMD8 mouse xenograft models.32,33 Tirabrutinib is currently in clinical development for CLL, MCL, and DLBCL and has shown promising clinical activity in patients with B-cell malignancies.23 Correlation of tirabrutinib target engagement at the molecular level with pharmacological and phenotypic disease observations is crucial for establishing the appropriate clinical dose.
Target occupancy assays, conducted in vitro on clinical samples, are used to determine target engagement. Irreversible binders such as tirabrutinib are more amenable for assessing target occupancy due to the stability of the drug–target complex formed. To determine in vivo tirabrutinib BTK occupancy, the cell population harboring the target is harvested and samples are collected and tested for target occupancy in clinically dosed subjects. In the present report, we describe the development of a TR-FRET-based BTK occupancy assay that can measure target engagement in PBMCs and in lymph node and bone marrow samples to support tirabrutinib clinical studies. We designed and synthesized a tirabrutinib-based biotinylated affinity probe that showed equivalent BTK inhibitory potency as the parent molecule in a biochemical assay and selective binding to BTK in cell extracts. The use of a common fluorescence donor to detect free and total BTK delivers sampling accuracy, while time-resolved fluorescence technology provides low assay background for assay sensitivity.
The EC50 of BTK occupancy from dose–response curves in Ramos B cells was 72 nM for tirabrutinib, and 90% occupancy was achieved at a concentration of 110 nM in bone marrow and peripheral blood samples from patients with CLL. In a phase 1 dose escalation study of tirabrutinib in patients with relapsed/refractory non-Hodgkin’s lymphoma and CLL, maximum observed plasma concentrations (Cmax) within the first 24 h following a single orally administered dose of 20–600 mg were 63.7–1509.8 ng/mL, corresponding to 140–3300 nM.23 At these doses, high target coverage was likely achieved based on tirabrutinib in vitro EC50 in the BTK occupancy assay, resulting in objective responses observed at all doses for the CLL cohort.
Furthermore, we evaluated how the biomarker analysis was affected by the time between blood draw and analysis, as well as sample storage and handling. In clinical trials, biomarker samples are obtained at multiple trial sites from patients and are usually processed and analyzed in central laboratories. Shipping times depend on the distance from the clinical site to the processing laboratory; if not tightly controlled, temperatures can vary during shipment. This is of particular importance for the measurement of receptor binding and occupancy of a compound that is an irreversible inhibitor of a receptor, such as tirabrutinib. Unbound tirabrutinib at the time of sample collection may continue to bind during storage and shipment to the processing laboratory, leading to an overestimation of occupied receptor. Our data indicate that additional binding of tirabrutinib to BTK occurred when the blood was stored at room temperature for more than 24 h, leading to a higher BTK occupancy. In contrast, BTK occupancy remained at the same level for 48 h when blood samples were stored at 4 °C. Our findings suggest that blood samples drawn from patients for this assay should be shipped cooled to avoid the overestimation of BTK binding in patient samples at the time of analysis. Our data further show that the additional binding of the inhibitor to BTK when the blood sample is stored at room temperature is not restricted to tirabrutinib but was also observed with acalabrutinib, another irreversible inhibitor of BTK. BTK occupancy reported for other irreversible BTK inhibitors may be affected in a similar manner.34
Integrated drug discovery and clinical development programs increasingly emphasize target engagement as a translational approach to inform PD and efficacy assessments.35 An important feature of our homogenous BTK occupancy assay for tirabrutinib is the multiplexed format that allows simultaneous measurement of free and total BTK levels. This is a key advantage over other BTK occupancy assays that require two independent methods for the detection of free versus total BTK levels. Thus, our assay avoids sampling error and requires less sample volume for analysis.
In summary, we developed, validated, and qualified a TR-FRET-based homogeneous duplex assay to measure BTK occupancy in PBMCs from tirabrutinib (GS-4059/ONO-4059) clinical studies. Utility of the assay also was demonstrated using cells derived from lymph node and bone marrow samples from patients with CLL and DLBCL. Tirabrutinib is currently in phase 1/2 clinical development and the assay is in use in an ongoing clinical study.
Acknowledgments
We thank Nam Bui for help with sourcing the tissues and CLL samples. We also thank Yuanyuan Xiao and members of the tirabrutinib team for constructive comments and discussions in preparing this manuscript. Medical writing support was provided by Robin L. Stromberg, PhD, of Impact Communications Partners, Inc., and funded by Gilead Sciences, Inc.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employees of Gilead Sciences, Inc.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Qiu Y., Kung H. J. Signaling Network of the BTK Family Kinases. Oncogene 2000, 19, 5651–5661. [DOI] [PubMed] [Google Scholar]
- 2. Kurosaki T., Hikida M. Tyrosine Kinases and Their Substrates in B Lymphocytes. Immunol. Rev. 2009, 228, 132–148. [DOI] [PubMed] [Google Scholar]
- 3. Tsukada S., Saffran D. C., Rawlings D. J., et al. Deficient Expression of a B Cell Cytoplasmic Tyrosine Kinase in Human X-Linked Agammaglobulinemia. Cell 1993, 72, 279–290. [DOI] [PubMed] [Google Scholar]
- 4. Genevier H. C., Hinshelwood S., Gaspar H. B., et al. Expression of Bruton’s Tyrosine Kinase Protein within the B Cell Lineage. Eur. J. Immunol. 1994, 24, 3100–3105. [DOI] [PubMed] [Google Scholar]
- 5. Rickert R. C. New Insights into Pre-BCR and BCR Signalling with Relevance to B Cell Malignancies. Nat. Rev. Immunol. 2013, 13, 578–591. [DOI] [PubMed] [Google Scholar]
- 6. Uckun F. M. Bruton’s Tyrosine Kinase (BTK) as a Dual-Function Regulator of Apoptosis. Biochem. Pharmacol. 1998, 56, 683–691. [DOI] [PubMed] [Google Scholar]
- 7. Kawakami Y., Kitaura J., Hata D., et al. Functions of Bruton’s Tyrosine Kinase in Mast and B Cells. J. Leukoc. Biol. 1999, 65, 286–290. [DOI] [PubMed] [Google Scholar]
- 8. Akinleye A., Chen Y., Mukhi N., et al. Ibrutinib and Novel BTK Inhibitors in Clinical Development. J. Hematol. Oncol. 2013, 6, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan Z., Scheerens H., Li S. J., et al. Discovery of Selective Irreversible Inhibitors for Bruton’s Tyrosine Kinase. ChemMedChem 2007, 2, 58–61. [DOI] [PubMed] [Google Scholar]
- 10. Davis R. E., Ngo V. N., Lenz G., et al. Chronic Active B-Cell-Receptor Signalling in Diffuse Large B-Cell Lymphoma. Nature 2010, 463, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrd J. C., Furman R. R., Coutre S. E., et al. Targeting BTK with Ibrutinib in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2013, 369, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Treon S. P., Tripsas C. K., Yang G., et al. A Prospective Multicenter Study of the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib in Patients with Relapsed or Refractory Waldenstrom’s Macroglobulinemia. Blood 2013, 122, Abstract 251. [Google Scholar]
- 13. Wang M. L., Rule S., Martin P., et al. Targeting BTK with Ibrutinib in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2013, 369, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burger J. A., Tedeschi A., Barr P. M., et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Honigberg L. A., Smith A. M., Sirisawad M., et al. The Bruton Tyrosine Kinase Inhibitor PCI-32765 Blocks B-Cell Activation and Is Efficacious in Models of Autoimmune Disease and B-Cell Malignancy. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Byrd J. C., Brown J. R., O’Brien S., et al. Ibrutinib Versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N. Engl. J. Med. 2014, 371, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Brien S., Furman R. R., Coutre S. E., et al. Ibrutinib as Initial Therapy for Elderly Patients with Chronic Lymphocytic Leukaemia or Small Lymphocytic Lymphoma: An Open-Label, Multicentre, Phase 1b/2 Trial. Lancet Oncol. 2014, 15, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CALQUENCE (Acalabrutinib) Prescribing Information; AstraZeneca Pharmaceuticals LP: Wilmington, DE. [Google Scholar]
- 19. Wang M., Rule S., Zinzani P. L., et al. Acalabrutinib in Relapsed or Refractory Mantle Cell Lymphoma (ACE-LY-004): A Single-Arm, Multicentre, Phase 2 Trial. Lancet 2018, 391, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yasuhiro T., Yoshizawa T., Daub H., et al. ONO-WG-307, a Novel, Potent and Selective Inhibitor of Bruton’s Tyrosine Kinase (BTK), Results in Sustained Inhibition of the ERK, AKT and PKD Signaling Pathways. Cancer Res. 2012, 72 (8 Suppl.), Abstract 2021. [Google Scholar]
- 21. Liclican A., Xing W., Serafini L., et al. Biochemical Characterization of GS-4059 as a Potent and Selective Covalent Irreversible Inhibitor of Bruton’s Tyrosine Kinase. Blood 2016, 128, Abstract 1594. [Google Scholar]
- 22. Hendriks R. W., Yuvaraj S., Kil L. P. Targeting Bruton’s Tyrosine Kinase in B Cell Malignancies. Nat. Rev. Cancer 2014, 14, 219–232. [DOI] [PubMed] [Google Scholar]
- 23. Czerwieniec G., Serafini L., Liclican A., et al. Characterization of Covalent Inhibitors GS-4059 and Ibrutinib to Bruton’s Tyrosine Kinase (BTK) by Mass Spectrometry. In 65th ASMS Conference on Mass Spectrometry and Allied Topics, Indianapolis, IN, June 4–8, 2017. [Google Scholar]
- 24. Walter H. S., Rule S. A., Dyer M. J., et al. A Phase 1 Clinical Trial of the Selective BTK Inhibitor ONO/GS-4059 in Relapsed and Refractory Mature B-Cell Malignancies. Blood 2016, 127, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshizawa T., Yasuhiro T., Honda H., et al. ONO-4059—A Potent and Selective Reversible Bruton’s Tyrosine Kinase (BTK) Inhibitor: Single Agent, Twice Daily (BD) Dosing and Dosing with Food Results in Sustained, High Trough Levels of ONO-4059, Translating into 100% Tumour Remission in a TMD-8 Xenograft Model. Blood 2014, 124, Abstract 4502. [Google Scholar]
- 26. Herman S. E., Gordon A. L., Hertlein E., et al. Bruton Tyrosine Kinase Represents a Promising Therapeutic Target for Treatment of Chronic Lymphocytic Leukemia and Is Effectively Targeted by PCI-32765. Blood 2011, 117, 6287–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kipps T. J., Stevenson F. K., Wu C. J., et al. Chronic Lymphocytic Leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Rooij M. F., Kuil A., Geest C. R., et al. The Clinically Active BTK Inhibitor PCI-32765 Targets B-Cell Receptor- and Chemokine-Controlled Adhesion and Migration in Chronic Lymphocytic Leukemia. Blood 2012, 119, 2590–2594. [DOI] [PubMed] [Google Scholar]
- 29. Ponader S., Chen S. S., Buggy J. J., et al. The Bruton Tyrosine Kinase Inhibitor PCI-32765 Thwarts Chronic Lymphocytic Leukemia Cell Survival and Tissue Homing In Vitro and In Vivo. Blood 2012, 119, 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cinar M., Hamedani F., Mo Z., et al. Bruton Tyrosine Kinase Is Commonly Overexpressed in Mantle Cell Lymphoma and Its Attenuation by Ibrutinib Induces Apoptosis. Leuk. Res. 2013, 37, 1271–1277. [DOI] [PubMed] [Google Scholar]
- 31. Compagno M., Lim W. K., Grunn A., et al. Mutations of Multiple Genes Cause Deregulation of NF-kappaB in Diffuse Large B-Cell Lymphoma. Nature 2009, 459, 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kozaki R., Yoshizawa T., Tohda S., et al. Development of a Bruton’s Tyrosine Kinase (BTK) Inhibitor, ONO-WG-307: Efficacy in ABC-DLBCL Xenograft Model—Potential Treatment for B-cell Malignancies. Blood 2011, 118, Abstract 3731. [Google Scholar]
- 33. Kozaki R., Yoshizawa T., Yasuhiro T., et al. Development of a Bruton’s Tyrosine Kinase (BTK) Inhibitor—ONO-WG-307, a Potential Treatment for B-Cell Malignancies. Cancer Res. 2012, 72 (8 Suppl.), Abstract 857. [Google Scholar]
- 34. Byrd J. C., Harrington B., O’Brien S., et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Durham T. B., Blanco M. J. Target Engagement in Lead Generation. Bioorg. Med. Chem. Lett. 2015, 25, 998–1008. [DOI] [PubMed] [Google Scholar]