Abstract
Hereby we communicate the first autochthon isolation of Cryptococcus gattii VGIII in Chile, which was obtained from a nasal injury in a domestic cat in the Valparaíso region in Chile. The strain was identified using morphophysiological tests, MALDI-TOF, and URA5 gene PCR-RFLP analysis.
Keywords: Cryptococcus gattii,; VGIII; Nasal; Cat; URA5
1. Introduction
C. neoformans/gattii species complex are causal agents in most dogs, cats and human cryptococcosis [1]. C. neoformans is distributed worldwide, being the most frequent etiological agent isolated. C. gattii was regarded as geographically restricted to tropical and subtropical area, however it has been currently recognized as an emergent pathogen in immunocompetent population of North America´s temperate regions, particularly the west coast [2]. It has also been isolated in the southwest region of British Columbia, Vancouver Island from humans and animals, as well as in the southeast coast of Vancouver island from air, soil and vegetation samples [3]. C. gattii has been also reported in Argentina, Austria, Canada, China, Congo, India, Italy, Japan, South Korea, Netherlands, Spain, South Africa, United Kingdom, USA, and Democratic Republic of Congo. Infections in domestic animals such as goats, dogs, cats and horses commonly occurred in Australia, New Zealand, Canada and Brazil. In contrast to human cases, veterinary infections are underdiagnosed and less reported, which suggests a higher incidence of C. gattii infections in animals than known [4]. The aim of this study is to report the first autochthonous isolation of C. gattii VGIII type in Chile. The strain was found in a nasal injury of a domestic cat from Limache, Valparaíso region in Chile.
2. Case presentation
A 14-years-old female domestic longhair cat, body condition scoring (BCS) 2/5 was brought to a veterinary clinic in Limache with a two month history of a nasal injury of a diameter of 4 × 4 cm. (Fig. 1). Mucocutaneous cryptococcosis was suspected and nasal samples of secretion were taken for mycological studies. The cat was treated with oral itraconazole syrup (10 mg/kg for 8 weeks). No auspicious clinical response was observed; hence, considering the age and general condition of the cat, euthanasia was practiced.
Fig. 1.
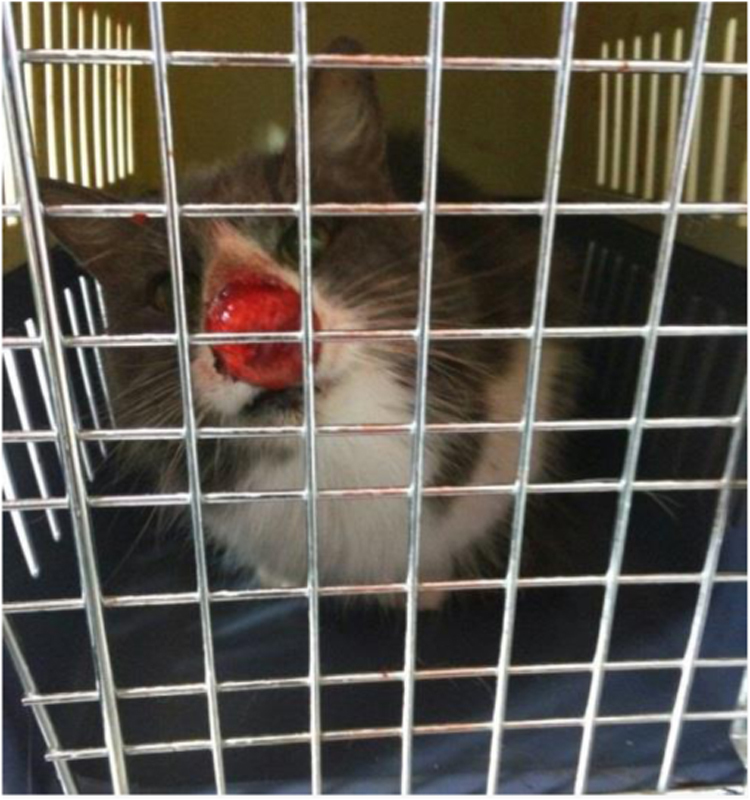
Mucocutaneous nasal injury.
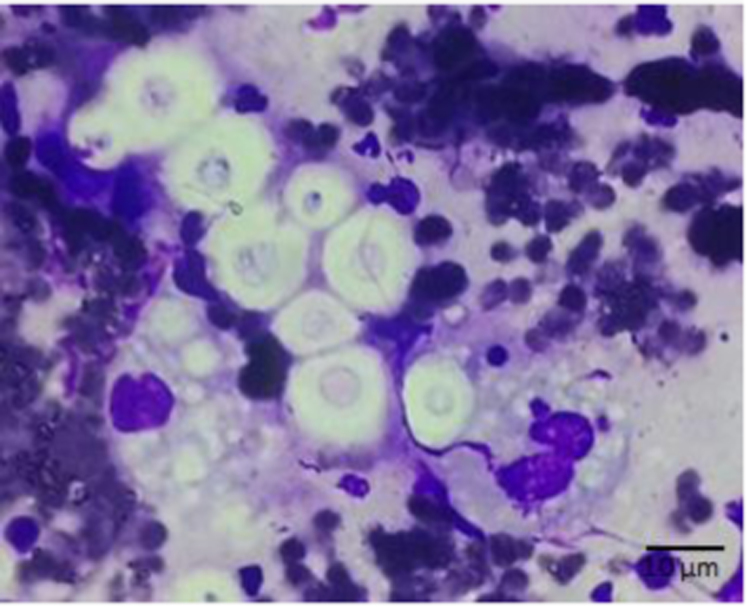
The nasal swab was submitted to the Mycology Laboratory of Universidad de Valparaíso. The sample was cultured on Sabouraud dextrose agar, supplemented with chloramphenicol (peptone 1 gr., glucose 2 gr., agar 16 gr., chloramphenicol 125 mg., distilled water 1000 ml.) and incubated at 37 °C. Cytological smears were performed and stained with Giemsa (Merck Millipore). Microscopically, abundant capsulated yeast and large amounts of inflammatory cells were observed (Fig. 2). After 48 h cultures showed whitish, mucoid, bright colonies. Microscopical analysis with cotton blue revealed globose capsulated yeast cells of 5 µm diameter.
Fig. 2.
Abundant yeasts with capsule and large amounts of inflammatory cells (40×).
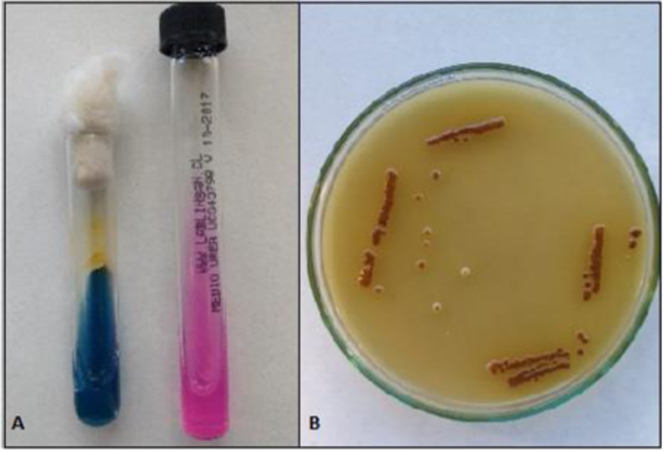
Physiological identification was carried out with Staib agar [5], Christenesen's urea agar (Merck Millipore) and Canavanine-glycine-bromothymol blue (CGB) agar [6]. The isolate was cultured and incubated at 30 °C, after a period of 7 days positive test results were obtained from the three media (Fig. 3) and phenotypically classified as C. gattii.
Fig. 3.
A: Positivity for CGB agar and urea agar; B: Staib agar, brown colonies for melanin production.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry MALDI-TOF MS (Vitek MS®, BioMérieux) identified the strain as C. gattii with an identification score of 99.9.
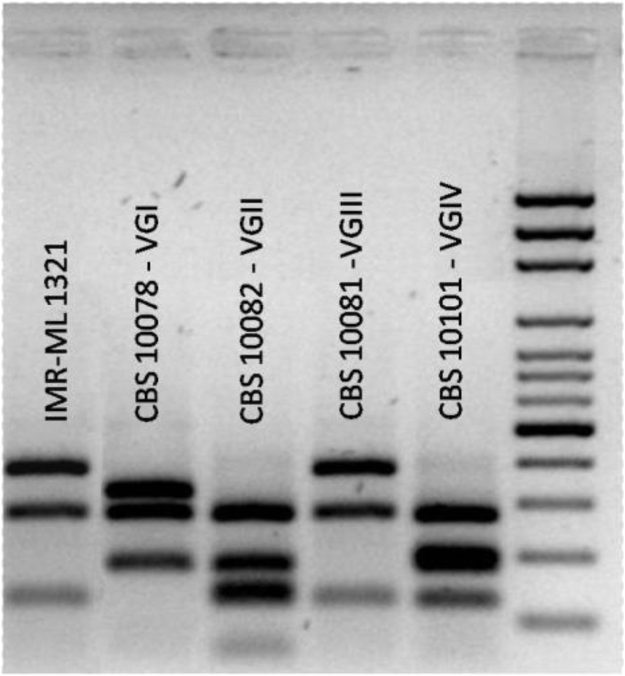
Genotype identification was performed by restriction fragment length polymorphisms of URA5 (PCR – RFLP) at the Departamento de Micología, Instituto de Medicina Regional, Universidad Nacional del Nordeste, Argentina. DNA extraction was performed according to Bosco et al. [7]. Polymerase chain reaction (PCR) was performed according Meyer et al. [8]. PCR products were double digested with the restriction enzymes Sau 96I and HhaI. Restriction fragments were subjected to electrophoresis by 3% agarose gel at 100 V during 5 h. The genotype was determined by comparing the URA5-RFLP pattern to those of the Cryptococcus reference strains (C. neoformans var. grubii: CBS 10085 VNI and CBS 10084 VNII; C. neoformans hybrid AD: CBS 10080 VNIII; C. neoformans var. neoformans: CBS 10079 VNIV; C. gattii: CBS 10078 VGI; CBS 10082 VGII; CBS 10081 VGIII and CBS 10101 VGIV) (Fig. 4). This method classified the strain as C. gattii VGIII.
Fig. 4.
: PCR - RFLP URA5. Line 1: IMR-ml-1321; line 2: CBS 10078 - VGI; line 3: CBS 10082 - VGII; line 4: CBS 10081 -VGIII; line 5: CBS 10101 – VGIV.
3. Discussion
The distribution of C. gattii was considered to be restricted to tropical and subtropical regions of Australia, South America, Southeast Asia (New Guinea, Thailand), and few locations of Africa [9]. However, since 1999 the species emerged in temperate climates such as Vancouver Island in British Columbia, Canada, causing outbreaks, which affected animals as well as immunocompetent humans with over 200 documented cases [10].
Environmental positive samples have been collected from barks Oregon white oak, maple, cedar and pine; from air, fresh water and sea water [1], [2].
In 2015, in the O’Higgins and Maule regions in Chile, three presumptive C. gattii isolates from Prunus cerasifera artropurpurea (purple plum) and Eucalyptus sp. were reported. No other test than CGB agar was performed to identify those strains [14]. Although phenotypical analyses are useful as a first line approach for identification, false positives and false negatives were reported using canavanine-bromothymol blue-glycine (CGB), emphasizing the need for molecular tests in order confirm such diagnoses [15], [16].
In Australia, the VGI type, whose reservoirs are eucalyptus and koalas, has a higher prevalence than the VGII type, which prevails in the southwest and northern areas [1], [9]. In Canada and USA, VGII type has shown higher virulence than other genotypes, with around 95% of infections being caused by this type. The presence in temperate climates suggests a possible further endemic expansion [2], [11].
A study published in 2003 including 340 human, veterinary, and environmental samples collected from Argentina, Brazil, Chile, Colombia, Mexico, Peru, Venezuela, Guatemala, and Spain showed that the predominant molecular type of C. gattii isolates was VGIII, corresponding to 9,1% of all isolates [8].
North and northeast Brazil is endemic for C. gattii. Martins et al. evaluated 63 cryptococcal meningitis cases in the Piauí and Maranhao regions, of which 24 were C. gattii, 3 VGI type from HIV positive patients and 21 VGII types from HIV negative patients [12]. In Argentina, during 2013, a genetic characterization of Cryptococcus isolated from clinical samples in the main hospital of the Chaco province was performed. In this study, one out of 26 isolates was identified as C. gatti VGI and came from a HIV positive patient. This was the first genotypic report about C. neoformans/gattii complex in clinical samples from Northeast Argentina [13].
Since up to now there are no reports of VGIII in Chile, our case complements the complex epidemiology described in Ibero-America.
In animals, the most common site of localized infections is the nasal cavity. Nasal cryptococcosis is frequently accompanied by clinical manifestations such as sneezing, snoring or snorting, dyspnea, and nasal deformities [1]. Our case presented all the above mentioned signs and symptoms.
Treatment of localized disease is generally successful using azole antifungal drugs. Some cats require long-term (> 1 year) treatment or indefinite therapy [1]. Our case received only 8 weeks of treatment; however, because of the lack of response and the clinical situation, euthanasia was practiced.
The epidemiology of fungal species constantly changes and C. neoformans/C. gatti is not an exception. Rigorous identification of both clinical and environmental isolates is necessary to determine the presence or absence of a particular fungal species in a determined geographical area.
Acknowledgements
We thank Lorena Porte, microbiologist of the Clinical Laboratory of Clínica Alemana Santiago for identification by MALDI-TOF and Isabel Varas Cruz for English editing.
Acknowledgments
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Refai M., El-Hariri M., Alarousy R. Cryptococcosis in animals and birds: a review. Eur. J. Acad. Essays. 2017;4(8):202–223. [Google Scholar]
- 2.Kronstad J.W., Attarian R., Cadieux B., Choi J., D'Souza C.A., Griffiths E.J. Expanding fungal pathogenesis: cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 2011;9(3):193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan C.G., Stephen C., Campbell J. Evaluation of risk factors for Cryptococcus gattii infection in dogs and cats. J. Am. Vet. Med. Assoc. 2006;228(3):377–382. doi: 10.2460/javma.228.3.377. [DOI] [PubMed] [Google Scholar]
- 4.Springer D.J., Chaturvedi V. Projecting global occurrence of Cryptococcus gattii. Emerg. Infect. Dis. 2010;16(1):14–20. doi: 10.3201/eid1601.090369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staib F., Seibold M., Antweiler E., Frohlich B., Weber S., Blisse A. The brown colour effect (BCE) of Cryptococcus neoformans in the diagnosis, control and epidemiology of C. neoformans infections in AIDS patients. Zent. Bakteriol. Mikrobiol. Hyg. A. 1987;266(1–2):167–177. doi: 10.1016/s0176-6724(87)80030-5. [DOI] [PubMed] [Google Scholar]
- 6.Kwon-Chung K.J., Polacheck I., JE B. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C) J. Clin. Microbiol. 1982;15(3):535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosco Borgeat M.E., Mazza M., Refojo N., Tipificación M.T. Molecular de especies del género Cryptococcus. In: Merino L.A.G.G., editor. Manual de métodos moleculares para estudios microbiológicos. 1st ed. Asociación Argentina de Microbiología; Buenos Aires: 2011. pp. 164–166. [Google Scholar]
- 8.Meyer W., Castaneda A., Jackson S., Huynh M., Castaneda E., IberoAmerican Cryptococcal Study G. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg. Infect. Dis. 2003;9(2):189–195. doi: 10.3201/eid0902.020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lester S.J., Malik R., Bartlett K.H., Duncan C.G. Cryptococcosis: update and emergence of Cryptococcus gattii. Vet. Clin. Pathol. 2011;40(1):4–17. doi: 10.1111/j.1939-165X.2010.00281.x. [DOI] [PubMed] [Google Scholar]
- 10.Galanis E., Macdougall L., Kidd S., Morshed M. British Columbia Cryptococcus gattii working G. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg. Infect. Dis. 2010;16(2):251–257. doi: 10.3201/eid1602.090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrnes E.J., Li W., Lewit Y., Ma H., Voelz K., Ren P. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 2010;6(4):e1000850. doi: 10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins L.M., Wanke B., Lazera Mdos S., Trilles L., Barbosa G.G., Macedo R.C. Genotypes of Cryptococcus neoformans and Cryptococcus gattii as agents of endemic cryptococcosis in Teresina, Piaui (northeastern Brazil) Membr. Inst. Oswaldo Cruz. 2011;106(6):725–730. doi: 10.1590/s0074-02762011000600012. [DOI] [PubMed] [Google Scholar]
- 13.Cattana M.E., Tracogna M.F., Fernandez M.S., Carol Rey M.C., Sosa M.A., Giusiano G.E. [Genotyping of Cryptococcus neoformans/Cryptococcus gattii complex clinical isolates from Hospital "Dr. Julio C. Perrando", Resistencia city (Chaco, Argentina)] Rev. Argent. Microbiol. 2013;45(2):89–92. doi: 10.1016/s0325-7541(13)70005-1. [DOI] [PubMed] [Google Scholar]
- 14.Toro V., Brevis P. Aislamiento presuntivo y caracterización de Cryptococcus neoformans y Cryptococcus gattii desde árboles en la región de O′Higgins y Maule. Chile Bol. Micol. 2015;30(2):6–15. [Google Scholar]
- 15.Khan Z.U., Al-Anezi A.A., Chandy R., Xu J. Disseminated cryptococcosis in an AIDS patient caused by a canavanine-resistant strain of Cryptococcus neoformans var. grubii. J. Med. Microbiol. 2003;52(Pt 3):271–275. doi: 10.1099/jmm.0.05097-0. [DOI] [PubMed] [Google Scholar]
- 16.Leal A.L., Faganello J., Bassanesi M.C., Vainstein M.H. Cryptococcus species identification by multiplex PCR. Med. Mycol. 2008;46(4):377–383. doi: 10.1080/13693780701824429. [DOI] [PubMed] [Google Scholar]