Abstract
Background
Studies on the chondrocyte inflammatory injury are very important for understanding the pathogenesis and clinical treatment of osteoarthritis (OA). Evidence suggests that N-methyl pyrrolidone (NMP) may be used as an adjuvant therapy alongside established methods of OA treatment. This study investigated the effect of NMP on chondrocyte inflammatory injury and explored the underlying molecular mechanism.
Material/Methods
To mimic the inflammatory injury in vitro, the articular chondrocyte line ATDC5 was simulated with lipopolysaccharide (LPS). ATDC5 cells were treated with various concentrations of NMP (0, 5, and 10 nM). Cell viability was measured using CCK-8 assay; cell apoptosis was detected using FCM; related protein and mRNA expressions were determined using Western blot assay and qRT-PCR assay; and inflammatory factors (tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8) productions were measured by performing ELISA assay.
Results
The results showed that LPS simulation repressed ATDC5 cell viability, prompted cell apoptosis, and enhanced the secretion of inflammatory factors. NMP treatment reduced inflammatory injury induced by LPS in a dose-dependent manner. Furthermore, NMP inhibited the activation of JNK and p38 pathways. In addition, inhibition of NF-κB activation was observed following NMP treatment.
Conclusions
NMP prevents inflammatory reaction of articular chondrocytes via repressing the MAPK/NF-κB pathway. Our findings provide a promising therapeutic agent for OA treatment.
MeSH Keywords: Chondrocytes, Inflammation, Osteoarthritis
Background
Osteoarthritis (OA) is a frequently-occurring disease and inflammatory joint disease in orthopedics [1,2]. The main pathological features of OA are the reduction of chondrocytes, the metabolic disorder of extracellular matrix (ECM), the inflammatory reaction of synovial membrane, and the remodeling of subchondral bone in joint tissues [3]. These pathological changes will eventually lead to joint deformities and joint dysfunction in patients with OA, and even lead to long-term disability in the patient, resulting in an ultimate mortality rate of 53% [4]. As one of the joint disorders that seriously affect human health and life, OA is more common in middle-aged and elderly people over 50 years of age, seriously endangering the physical health of the middle-aged and elderly people and greatly affecting their quality of life, as well as imposing a heavy burden on individuals, families, and the community [5]. The incidence of OA increases with age and has thus become a major problem in the aging population [6].
Articular cartilage shows abnormal pathological changes earlier in the process of OA. Its pathological changes are related to excessive degradation of structural proteins such as proteoglycan and type II collagen in cartilage extracellular matrix, and its degradation metabolism far exceeds that of anabolism [7–10]. Chondrocytes are the only cells in the articular cartilage tissue and their abnormal changes in cellular physiology are inextricably linked with the development of OA [11]. Chondrocytes are important components involved in the balance of cartilage metabolism and play a key role in maintaining the stability of their physicochemical properties and integrity of the structure and function of cartilage ECM [12]. As chondrocytes play a pivotal role in the pathogenesis of OA, they have become the preferred cells for OA research. To study the inflammatory injury of chondrocytes is important for understanding the pathogenesis and clinical treatment of OA [13].
N-methyl pyrrolidone (NMP), a small bioactive molecule, plays a key role in osteoblast and osteoclast differentiation [14,15]. NMP has been used as a constituent in guided tissue regeneration membranes, guided bone regeneration membranes, and bone substitute materials [16–19]. NMP has been found to be a functional low-affinity acetyl lysine mimic and Bromodomain inhibitor with anti-multiple myeloma and immunomodulatory activity [20]. Recently, a study reported that NMP could preserve both mass and quality of long bones in ovariectomized rats [21]. Studies have demonstrated that NMP ameliorates the hypoxia-reduced osteoblast differentiation and inhibits inflammation by repressing the NF-κB pathway [22,23]. These results indicate that NMP may be used as an adjuvant therapy alongside established methods of OA treatment. However, the exact effect and underlying mechanism of NMP in OA, especially chondrocyte inflammatory injury, is still unclear. Therefore, the purpose of current study was to investigate the effect of NMP on chondrocyte inflammatory injury and to explore the underlying molecular mechanism.
Material and Methods
Cell culture and cell treatment
The murine articular chondrocyte line ATDC5 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). ATDC5 cells were grown in Dulbecco’s modified Eagle’s medium/nutrient mixture F12 (DMEM/F12; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 2 mM Glutamine (Sigma-Aldrich, St. Louis, MO, USA) and 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA). Cells were incubated at 37°C with 5% CO2. The cell culture medium was replaced with fresh medium every 2–3 days.
ATDC5 cells were cultured with various concentrations (0, 1, 5, and 10 μg/ml) of lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, Mo, USA) for 6 h and then collected for further analyses. Cells were divided into 4 different groups: 1) control group (ATDC5 cells without any treatment); 2) 5 μg/ml LPS induced group (ATDC5 cells were treated with 5 μg/ml LPS for 6 h at 37°C); 3) 5 μg/ml LPS + 5 mM NMP group (ATDC5 cells were treated with 5 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C); and 4) 5 μg/ml LPS + 10 mM NMP group (ATDC5 cells were treated with 10 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C). The NMP concentrations in the current study were chosen according to a previous study [23].
Cell viability assay
Cell counting kit-8 (CCK-8, Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to detect cell viability according to the manufacturer’s instructions. Briefly, after treatment, ATDC5 cells were plated into a 6-well plate (5×103 cells/well) and then incubated at 37°C for 24 h. Subsequently, 20 μl CCK-8 solution (5 g/L) was added to each culture well and then incubated for 3 h under standard conditions. Finally, a plate reader (Bio-Rad Laboratories, Tokyo, Japan) was used to measure the absorbance at 450 nm. Tests were repeated at least 3 times.
Apoptotic assay
Apoptotic cells were analyzed using the Annexin V-FITC/PI apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA) per the manufacturer’s instructions. Shortly after specific treatment, 1×106 ATDC5 cells were dyed with 5 μl Annexin V-FITC (BD Biosciences) at room temperature for 20 min without light. Then, each sample was incubated with 10 μl PI (5 μg/ml) in 1×binding buffer for another 15 min without light. Flow cytometry (FACSCalibur; Becton-Dickinson, Franklin Lakes, NJ, USA) was used to analyzed the apoptosis of cells. Tests were repeated at least 3 times.
Enzyme-linked immunosorbent assay (ELISA) assay
After specific treatment, the ATDC5 cell culture supernatant was harvested from each sample and concentrations of inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8, were detected using an ELISA kit (R&D Systems, Abingdon, UK) following the manufacturer’s protocols.
Real-time quantitative PCR (qRT-PCR)
Total RNA from ATDC5 cells was isolated using Trizol reagent (Life Technologies Corporation, Carlsbad, CA, USA) in strict accordance with the product specification. The extracted RNA was reversed into cDNAs using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) according to the manufacturer’s protocols. QRT-PCR was conducted using the TaqMan® Universal PCR Master Mix kit (Thermo Fisher Scientific, Inc.) on the ABI PRISM 7500 Real-time PCR System (Applied Biosystems, Foster City, CA). All reactions were performed at least 3 times. Relative gene expression was quantified using the 2–ΔΔCq method (24) and GAPDH was used as an internal reference. All primer sequences used in the present study are listed in Table 1.
Table 1.
Primer sequence for PCR.
| Sequence (5′-3′) | |
|---|---|
| TNF-α-forward: | 5′-CGTCAGCCGATTTGCTATCT-3′ |
| TNF-α-reverse: | 5′-CGGACTCCGCAAAGTCTAAG-3′ |
| IL-1β-forward: | 5′-AAGATGAAGGGCTGCTTCCAAACC-3′ |
| IL-1β-reverse: | 5′-ATACTGCCTGCCTGAAGCTCTTGT-3′ |
| IL-6-forward: | 5′-CATCCAGTTGCCTTCTTGGGA-3′ |
| IL-6-reverse: | 5′-CTGAAGGACTCTGGCTTGTC-3′ |
| IL-8-forward: | 5′-CATCTTCGTCCGTCCCTGTG-3′ |
| IL-8-reverse: | 5′-GCCAACAGTAGCCTTCACCCA-3′ |
| COX-2-forward: | 5′-TCCATTGACCAGAGCAGAGA-3′ |
| COX-2-reverse: | 5′-TCTGGACGAGGTTTTTCCAC-3′ |
| iNOS-forward: | 5′-CACCTTGGAGTTCACCCAGT-3′ |
| iNOS-reverse: | 5′-ACCACTCGTACTTGGGATGC-3′ |
| GAPDH-forward: | 5′-CTTTGGTATCGTGGAAGGACTC-3′ |
| GAPDH-reverse: | 5′-GTAGAGGCAGGGATGATGTTCT-3′ |
Western blot assay
The total protein from ATDC5 cells was isolated using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) according to the product specification. Protein samples were quantified by using the BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA) in line with the manufacturer’s instructions. Equal amounts of protein samples (2 μg/lane) were loaded on 12% SDS-PAGE and blotted onto polyvinylidene fluoride (PVDF) membranes in accordance with the instructions. Following blocking with 5% non-fat milk at room temperature for 2 h, the membranes were incubated with primary antibodies [Cleaved Caspase3 (ab2302); Cleaved Caspase 9 (ab2324); Caspase 9 (#9508); Caspase 3 (#9668); Bcl-2 (sc509); Bax (sc20067); COX-2 (#4842), iNOS (#13120), p-p38 (#1170), p-JNK (#4668), Phospho-NF-κB p65 (#3033) and β-actin (#4970)] at 4°C overnight, then the membranes were incubated with the anti-rabbit immunoglobulin G, and horseradish peroxidase-linked antibody (HRP, ab131368 and ab1575332, Abcam, USA) at room temperature for 1 h. Finally, protein blots were developed with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce; Thermo Fisher Scientific, Inc.) and the ChemiDoc XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was applied for protein band observation per the manufacturer’s protocols.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). SPSS software version 20.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Comparisons between groups were assessed by using a two-way analysis of variance (ANOVA) followed by Bonferroni test or t test. A value of p<0.05 was considered as statistically significant.
Results
LPS induced inflammatory reaction of ATDC5 cells
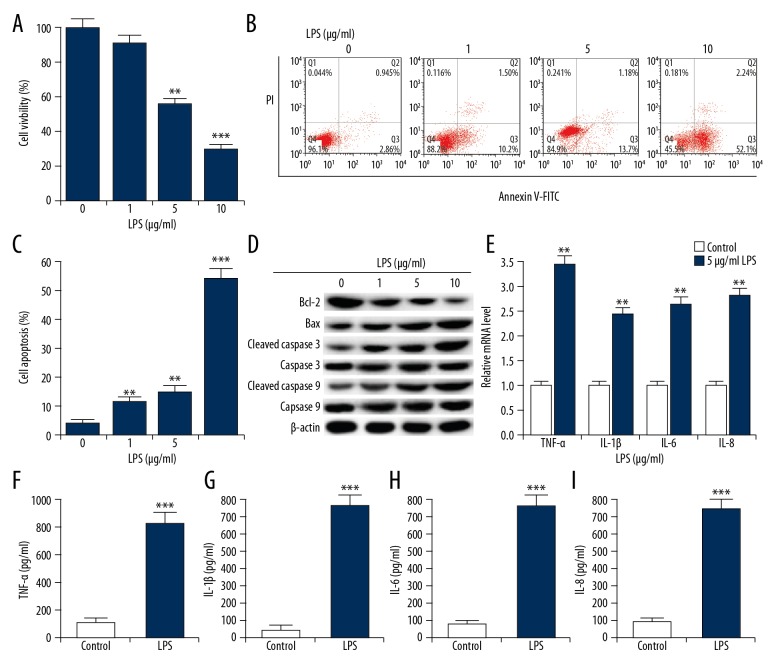
After treatment with various concentrations of LPS (0, 1, 5, and 10 μg/ml), cell viability of ATDC5 cells was detected using CCK-8. As shown in Figure 1A, compared with control groups, 5 and 10 μg/ml LPS treatment significantly reduced ATDC5 cell viability. We also found that compared with the control group, cell apoptosis in LPS-treated groups significantly increased (Figure 1B, 1C). We also observed that, compared with groups without LPS treatment, Bcl-2 protein level was decreased in LPS-treated ATDC5 cells, while protein levels of Bax, cleaved Caspase 3, and cleaved Caspase 9 were all significantly enhanced (Figure 1D). No significant differences were observed in the expression of total Caspase 9 and Caspase 3 in different groups. The data showed that LPS administration prevented the cell viability of ATDC5 cells, and cell apoptosis was promoted. Based on the effect of LPS on ATDC5 cell viability, we selected 5 μg/ml LPS for the following experiments.
Figure 1.
LPS induced chondrocyte inflammatory injury in ATDC5 cells. ATDC5 cells were treated with various doses of LPS (1, 5, and 10 μg/ml) to simulate inflammatory lesions. Cells without LPS administration (0 μg/ml) were used as control. (A) CCK-8 was performed to measure cell viability; (B, C) Relative apoptotic cells were measured by flow cytometry (cell apoptosis=early apoptosis late apoptosis); (D) The protein levels of apoptosis-related factors were detected by Western blotting. (E) The mRNA expression levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) were detected by qRT-PCR. (F–I) ELISA was used to measure the productions of TNF-α, IL-1β, IL-6 and IL-8. *, **, *** p<0.05, 0.01, 0.001 compared with control.
The protein and mRNA levels of TNF-α, IL-1β, IL-6, and IL-8 were determined using qRT-PCR analysis and ELISA assay, respectively. As shown in Figure 1E–1I, compared with control group, both mRNA and protein expression levels of TNF-α, IL-1β, IL-6, and IL-8 were all markedly increased in LPS-treated ATDC5 cells. The data suggested that LPS effectively enhanced inflammatory factors production and induced inflammatory response of ATDC5 cells.
NMP treatment reduced the inflammatory injury induced by LPS
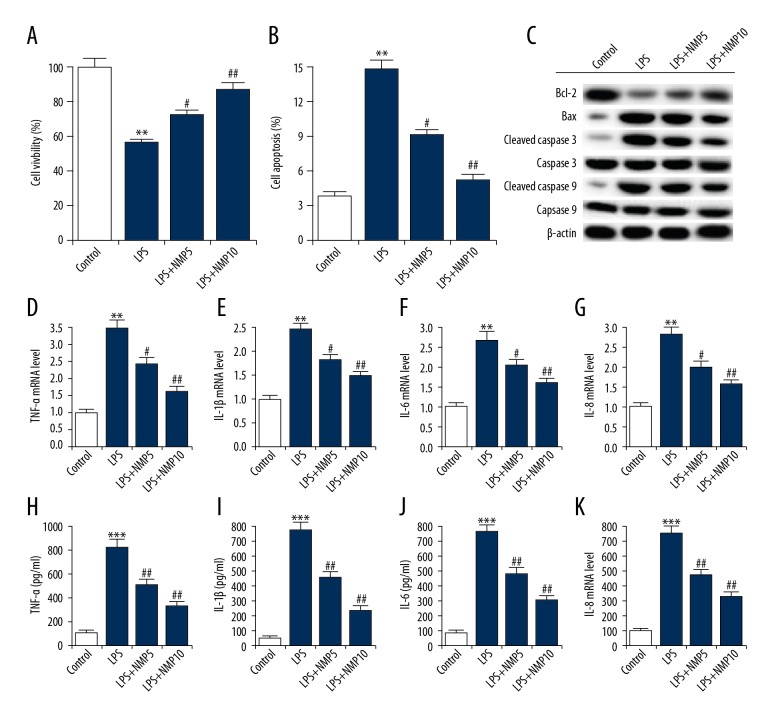
ATDC5 cells were pre-treated with various concentrations of NMP (0, 5, and 10 nM) and then treated with 5 μg/ml LPS. Then, ATDC5 cell viability and apoptosis were assessed. As shown in Figure 2A and 2B, compared with the LPS alone treatment group, NMP increased ATDC5 cell viability and decreased cell apoptosis in a dose-dependent manner. Moreover, in the LPS treatment group, NMP treatment increased the expression of Bcl-2, and decreased the expressions of Bax, cleaved Caspase 3, and cleaved Caspase 9 in a dose-dependent way (Figure 2C). No significant differences were observed in the expression of total Caspase 9 and Caspase 3 in the different groups. In addition, compared with the LPS treatment alone group, the protein and mRNA levels of TNF-α, IL-1β, IL-6, and IL-8 were all significantly decreased in NMP-treated ATDC5 cells (Figure 2D–2K). In summary, the data suggested that NMP reduced inflammatory reaction of ATDC5 cells.
Figure 2.
NMP reduced LPS induced inflammatory injury. ATDC5 cells were pre-treated with various concentrations of NMP (0, 5, and 10 nM) and then treated with 5 μg/ml LPS. (A) CCK-8 was performed to measure cell viability; (B) Relative apoptotic cells were measured by flow cytometry (cell apoptosis=early apoptosis late apoptosis); (C) The protein levels of apoptosis-related factors were detected by Western blotting. (D–G) The mRNA expression levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) were detected by qRT-PCR; (H–K) ELISA was used to measure the productions of TNF-α, IL-1β, IL-6, and IL-8. Control: cells without any treatment; LPS: cells were treated with 5 μg/ml LPS for 6 h at 37°C; LPS+NMP5: cells were treated with 5 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C; LPS+NMP10: cells were treated with 10 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C. ** p<0.01 vs. control; #, ## p<0.05, 0.01 vs. LPS group.
NMP significantly inhibited the LPS induced increase of COX-2 and iNOS
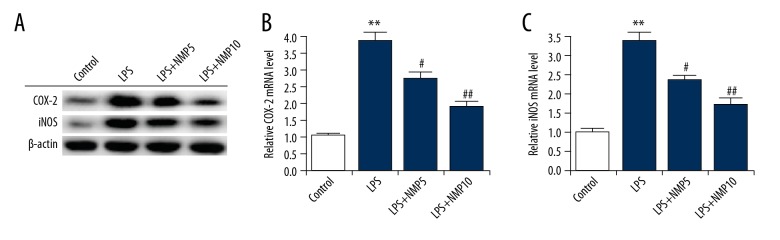
ATDC5 cells were pre-treated with various concentrations of NMP (0, 5, and 10 nM) and then treated with 5 μg/ml LPS. After treatment, the protein and mRNA level of COX-2 and iNOS in ATDC5 cells was assessed using Western blot and qRT-PCR assay, respectively. The results indicated that compared with the control group, both the protein and mRNA level of COX-2 and iNOS were significantly increased in the LPS treatment alone group, and these increases in COX-2 and iNOS levels were notably reversed by treatment with 5 mM and 10 mM NMP (Figure 3).
Figure 3.
NMP significantly inhibits the LPS-induced increase of COX-2 and iNOS expression. ATDC5 cells were pre-treated with various concentrations of NMP (0, 5, and 10 nM) and then treated with 5 μg/ml LPS. The protein (A) and mRNA (B, C) levels of COX-2 and iNOS was determined using Western blot and qRT-PCR, respectively. Control: cells without any treatment; LPS: cells were treated with 5 μg/ml LPS for 6 h at 37°C; LPS+NMP5: cells were treated with 5 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C; LPS+NMP10: cells were treated with 10 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C. ** p<0.01 vs. control; #, ## p<0.05, 0.01 vs. LPS group.
NMP markedly inhibited the LPS induced over-activation of JNK and p38
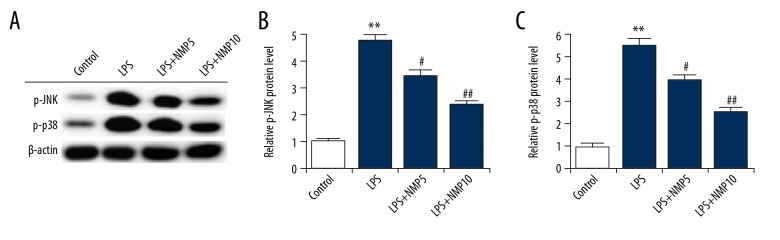
To determine the underlying molecular mechanism of the effect of NMP on LPS-treated ATDC5 cells, the MAPK pathway was analyzed. Compared with the control group, the expressions of p-JNK and p-p38 were markedly enhanced in the LPS treatment alone group. Treatment with NMP (5 and 10 nM) significantly inhibited the LPS induced up-regulation of p-JNK and p-p38 expression (Figure 4).
Figure 4.
NMP significantly inhibits the LPS-induced over-activation of JNK and p38. ATDC5 cells were pre-treated with various concentrations of NMP (0, 5, and 10 nM) and then treated with 5 μg/ml LPS. The protein level of p-JNK and p-p38 was determined using Western blot assay. Control: cells without any treatment; LPS: cells were treated with 5 μg/ml LPS for 6 h at 37°C; LPS+NMP5: cells were treated with 5 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C; LPS+NMP10: cells were treated with 10 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C. ** p<0.01 vs. control; #, ## p<0.05, 0.01 vs. LPS group.
NMP markedly inhibited the LPS-induced over-activation of NF-κB pathway
NMP inhibits inflammation by repressing the NF-κB pathway [23]. Thus, in the present study, we investigated whether NMP affected the NF-κB pathway in ATDC5 cells through analyzing p-p65 expression. As shown in Figure 5, NMP significantly decreased the level of p-p65, which was significantly enhanced by LPS administration.
Figure 5.
NMP significantly inhibits the LPS-induced over-activation of NF-κB pathway. ATDC5 cells were pre-treated with various concentrations of NMP (0, 5, and 10 nM) and then treated with 5 μg/ml LPS. The protein level of p-p65 was determined using Western blot assay. Control: cells without any treatment; LPS: cells were treated with 5 μg/ml LPS for 6 h at 37°C; LPS+NMP5: cells were treated with 5 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C; LPS+NMP10: cells were treated with 10 mM NMP for 48 h at 37°C and then 5 μg/ml LPS for 6 h at 37°C. ** p<0.01 vs. control; #, ## p<0.05, 0.01 vs. LPS group.
Discussion
In the present study, we determined that NMP inhibited the cell viability, induced apoptosis, and prevented the production of inflammatory factors (TNF-α, IL-1β, IL-6, and IL-8) in articular chondrocytes via repressing the MAPK/NF-κB pathway. We revealed that NMP may act as a potential therapeutic agent for the treatment of OA.
There is a poor intrinsic capacity for repair in articular cartilage, and even a small defect resulting from mechanical damage can fail to heal, thus degenerating to OA [25]. OA, one of the most common degenerative disorders in joints worldwide, is characterized by slow destruction and loss of articular cartilage, which mainly impairs the hips and knees [26]. Specifically, in China, the morbidity of OA (symptomatic knee) is as high as 8.1% [27]. Unfortunately, the precise pathogenesis of OA remains unclear and the treatment is not satisfactory.
Studies show that NMP plays a key role in bone formation, osteoblast differentiation, and inflammation response [22,23], indicating that NMP may have a potential therapeutic effect on OA. Therefore, we conducted the present study, in which we found that NMP inhibits the inflammatory injury of chondrocytes and regulates activation of the MAPK/NF-κB signaling pathway, which provides a promising agent for the treatment of cartilage defection. Firstly, ATDC5 cells were treated with LPS to mimic inflammatory model in vitro, and the results confirmed that LPS effectively enhanced the production of inflammatory factors and induced the inflammatory response of ATDC5 cells. Then, we investigated the effect of NMP on inflammatory injury of chondrocytes and the results showed that compared with the LPS treatment alone group, NMP dose-dependently increased ATDC5 cell viability and decreased cell apoptosis. NMP administration increased Bcl-2 expression level and decreased the expressions of Bax, cleaved Caspase 3, and cleaved Caspase 9 in a dose-dependent manner. Moreover, the protein and mRNA levels of TNF-α, IL-1β, IL-6, and IL-8 were all significantly reduced in NMP-treated cells compared with that in the LPS treatment alone group. All these data indicate that NMP treatment reduces LPS-induced inflammatory injury.
As an enzyme encoded by the prostaglandin endoperoxide synthase 2 gene [28], COX-2 is not expressed or is under-expressed in most cells in normal physiological conditions but is over-expressed in inflammatory conditions [29]. Nitric oxide synthases (NOSs) are a family of enzymes catalyzing the production of nitric oxide (NO) from L-arginine. NO is an important cellular signaling molecule. Nitric oxide is mediated in mammals by the calcium-calmodulin-controlled isoenzymes eNOS (endothelial NOS) and nNOS (neuronal NOS). The inducible isoform, iNOS, is involved in immune response, binds calmodulin at physiologically relevant concentrations, and produces NO as an immune defense mechanism. iNOS is calcium-insensitive and the gene that codes for iNOS is located on chromosome 17 [30]. Activation of the NF-κB-dependent iNOS promoter supports inflammation-mediated iNOS transcription stimulation [31]. In addition, iNOS stimulates production of large amounts of NO through IL-1 and TNF-α [32]. Studies suggested that LPS may induce iNOS and COX-2 expression in RAW264.7 macrophages [23]. Therefore, the present study evaluated whether NMP significantly reverses LPS induced up-regulation of iNOS and COX-2 in ATDC5 cells. Compared with the control group, LPS significantly increased both mRNA and protein levels of COX-2 and iNOS, which were significantly inhibited by treatment with NMP.
p38 and JNK, but not ERK, participate in important signal-transducing pathways in the interactions between kinins and IL-1β that lead to the enhancement of COX-2 expression [33]. To determine the underlying molecular mechanism of the effect of NMP on LPS-treated ATDC5 cells, the p38 and JNK pathways were analyzed. The results showed that NMP markedly repressed the LPS induced up-regulation of p-JNK and p-p38 expression. A previous study indicated that NMP inhibits inflammation response via preventing NF-κB pathway activation [23]. Thus, in the present study, we investigated whether NMP affected the NF-κB pathway in ATDC5 cells, and the findings indicated that NMP significantly repressed NF-κB pathway activation, which was significantly enhanced by LPS administration.
Taken together, our findings suggest that NMP inhibits the inflammatory injury of chondrocytes via regulating activation of the MAPK/NF-κB signaling pathway. However, there are some limitations to our research. For example, we only selected 3 concentrations of NMP for the experiments, and our experiments were only in vitro. Therefore, the effect of NMP on osteoarthritis progression needs further in-depth study. In the future, we will continue to conduct in-depth studies on the role of NMP in osteoarthritis.
Conclusions
Our study results indicate that NMP inhibits the inflammatory injury of chondrocytes via the regulation of the MAPK/NF-κB signaling pathway and suggest that NMP may be a promising agent for OA treatment, providing a stronger theoretical basis for the clinical treatment of OA.
Acknowledgment
Thanks to Dr. Xiaoxiao Zhou from Zhoupu Hospital for guidance during the experiments, and to Dr. Xiaoping Yang from Pudong Hospital Affiliated to Fudan University for guidance in this study.
Footnotes
Conflict of interests
None.
Source of support: The present study was supported by 2017 Zhejiang Taizhou Science and Technology Plan B Project (No. 1702ky19)
References
- 1.Pelletier JP, Mantel-Pelletier JF, Malemud CJ. Immunological analysis of proteoglycan changes in the early stage of osteoarthritic canine cartilage lesions. J Orthop Res. 1992;10:511–23. doi: 10.1002/jor.1100100406. [DOI] [PubMed] [Google Scholar]
- 2.Colen S, Bekerom MPJVD, Mulier M. Intra-articular viscosupplementation in patients with hip osteoarthritis. Eur Musculoskelet Rev. 2011;6:79–82. [Google Scholar]
- 3.Arden N, Blanco F, Cooper C, et al. Atlas of osteosteoarthritis. Springer Healthcare Ltd; 2014. pp. 69–82. [Google Scholar]
- 4.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: Its prevalence and relationship to physical function. Arthritis Rheum. 2004;51(6):941–46. doi: 10.1002/art.20825. [DOI] [PubMed] [Google Scholar]
- 6.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12:216. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oakley SP, Portek I, Szomor Z, et al. Arthroscopy – a “gold standard” for the diagnosis of the chondropathy of early osteoarthritis. Osteoarthritis Cartilage. 2005;13:368–78. doi: 10.1016/j.joca.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Madry H, Luyten FP, Facchini A. Biological aspects of early osteoarthritis. Knee Sung Sports Traumatol Arthrosc. 2012;20:407–22. doi: 10.1007/s00167-011-1705-8. [DOI] [PubMed] [Google Scholar]
- 9.Rolauffs B, Rothdiener M, Bahrs C, et al. Onset of preclinical osteoarthritis: The angular spatial organization permits early diagnosis. Arthritis Rheum. 2011;63:1637–47. doi: 10.1002/art.30217. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand J, Cromme C, Umlauf D, et al. Molecular mechanisms of cartilage remodelling in osteoarthritis. Int J Biochem Cell Biol. 2010;42:1594–601. doi: 10.1016/j.biocel.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Carames B, Taniguchi N, Otsuki S, et al. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8:333–45. doi: 10.2174/138945007779940025. [DOI] [PubMed] [Google Scholar]
- 13.Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–20. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghayor C, Correro RM, Lange K, et al. Inhibition of osteoclast differentiation and bone resorption by N-methylpyrrolidone. J Biol Chem. 2011;286:24458–66. doi: 10.1074/jbc.M111.223297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miguel BS, Ghayor C, Ehrbar M, et al. N-methyl pyrrolidone as a potent bone morphogenetic protein enhancer for bone tissue regeneration. Tissue Eng Part A. 2009;15:2955–63. doi: 10.1089/ten.TEA.2009.0009. [DOI] [PubMed] [Google Scholar]
- 16.Southard GL, Dunn RL, Garrett S. The drug delivery and biomaterial attributes of the ATRIGEL technology in the treatment of periodontal disease. Expert Opin Investig Drugs. 1998;7:1483–91. doi: 10.1517/13543784.7.9.1483. [DOI] [PubMed] [Google Scholar]
- 17.Zwahlen RA, Cheung LK, Zheng LW, et al. Comparison of two resorbable membrane systems in bone regeneration after removal of wisdom teeth: A randomized-controlled clinical pilot study. Clin Oral Implants Res. 2009;20:1084–91. doi: 10.1111/j.1600-0501.2009.01751.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmidlin PR, Nicholls F, Kruse A, et al. Evaluation of moldable, in situ hardening calcium phosphate bone graft substitutes. Clin Oral Implants Res. 2013;24:149–57. doi: 10.1111/j.1600-0501.2011.02315.x. [DOI] [PubMed] [Google Scholar]
- 19.Schneider D, Weber FE, Grunder U, et al. A randomized controlled clinical multicenter trial comparing the clinical and histological performance of a new, modified polylactide-co-glycolide acid membrane to an expanded polytetrafluorethylene membrane in guided bone regeneration procedures. Clin Oral Implants Res. 2014;25:150–58. doi: 10.1111/clr.12132. [DOI] [PubMed] [Google Scholar]
- 20.Shortt J, Hsu AK, Martin BP, et al. The drug vehicle and solvent N-methylpyrrolidone is an immunomodulator and antimyeloma compound. Cell Rep. 2014;7:1009–19. doi: 10.1016/j.celrep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Gjoksi B, Ghayor C, Siegenthaler B, et al. The epigenetically active small chemical N-methyl pyrrolidone (NMP) prevents estrogen depletion induced osteoporosis. Bone. 2015;78:114–21. doi: 10.1016/j.bone.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Liu R, Zhao J, Lu Q. N-methyl pyrrolidone (NMP) ameliorates the hypoxia-reduced osteoblast differentiation viainhibiting the NF-κB signaling. J Toxicol Sci. 2016;41:701–9. doi: 10.2131/jts.41.701. [DOI] [PubMed] [Google Scholar]
- 23.Ghayor C, Gjoksi B, Siegenthaler B, Weber FE. N-methyl pyrrolidone (NMP) inhibits lipopolysaccharide-induced inflammation by suppressing NF-κB signaling. Inflamm Res. 2015;64:527–36. doi: 10.1007/s00011-015-0833-x. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TDL. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. doi: 10.22203/ecm.v009a04. [DOI] [PubMed] [Google Scholar]
- 26.Tsezou A. Osteoarthritis year in review 2014: Genetics and genomics. Osteoarthritis Cartilage. 2014;22:2017–24. doi: 10.1016/j.joca.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Tang X, Wang S, Zhang Y, et al. The prevalence of symptomatic knee osteoarthritis in China: Results from China Health and Retirement Longitudinal Study. Arthritis Rheumatol. 2016;68(3):648–53. doi: 10.1002/art.39465. [DOI] [PubMed] [Google Scholar]
- 28.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–88. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurumbail RG, Kiefer JR, Marnett LJ. Cyclooxygenase enzymes: Catalysis and inhibition. Curr Opin Struct Biol. 2001;11:752–60. doi: 10.1016/s0959-440x(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 30.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calegari-Silva TC, Pereira RM, De-Melo LD, et al. NF-kappaB-mediated repression of iNOS expression in Leishmania amazonensis macrophage infection. Immunol Lett. 2009;127:19–26. doi: 10.1016/j.imlet.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Green SJ, Scheller LF, Marletta MA, et al. Nitric oxide: Cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol Lett. 1994;43:87–94. doi: 10.1016/0165-2478(94)00158-8. [DOI] [PubMed] [Google Scholar]
- 33.Brechter AB, Lerner UH. Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of Cyclooxygenase 2, resulting in increased RANKL expression. Arthritis Rheum. 2007;56:910–23. doi: 10.1002/art.22445. [DOI] [PubMed] [Google Scholar]