Abstract
International travel is rising quickly worldwide. Many people travel to tropical and subtropical areas, where there has been increasing exposure of travelers to infectious pathogens. Ocular parasitic infections are more prevalent in these geographical areas and they can lead to morbidity and mortality, often due to late or misdiagnosis due to the unfamiliarity of health staff with these diseases. This is an up-to-date comprehensive review article that familiarizes physicians with ocular signs and symptoms, treatment, prevention, and geographic distribution of some parasites associated with travel.
Keywords: Acanthamoeba, American trypanosomiasis, eye, giardiasis, leishmaniasis, malaria, parasitosis, protozoan infection, toxoplasmosis, travel
Introduction
International travel is rising quickly worldwide.[1] In 2015, the number of travelers exceeded 1.2 billion and it is estimated that it may reach 1.6 billion in 2020 in all countries.[2,3,4] Many of them travel to tropical and subtropical areas and participate in outdoor activities.[3] Travel to such places and these behavioral habits cause increasing exposure to infectious pathogens and travelers can easily acquire and transmit these diseases.[5,6] Ocular infections caused by various parasites in humans are more prevalent in tropical and subtropical geographical areas. These infections can lead to ocular morbidity and mortality largely because of misdiagnosis, often from unfamiliarity of healthcare workers with the disease course.
For identification of the causes of ocular infection, matching general and ocular symptoms and signs with travel history to endemic areas, dietary history, and advances in diagnosis are important.[7] This article familiarizes ophthalmologists and general physicians with some ocular parasites. For each disease, we describe the causative agent, geographic distribution, clinical ocular presentation, diagnostic methods, treatment, and prevention in travelers.
Methods
A literature search was performed using PubMed Medline until 2016. To collect material for this study, a detailed search of the PubMed database was performed using any term related to ophthalmology. The terms were as follows: “eye,” “vision,” “visual,” “optic,” “lens,” “retina,” “uvea,” “choroid,” “cilia,” “cornea,” “sclera” AND Giardiasis, Leishmaniasis, Malaria, Acanthamoeba, American trypanosomiasis, Toxoplasmosis, AND travel.
Based on this research, the total number of articles accounting for giardiasis was 47, 380 for leishmaniasis, 1362 for malaria, 3988 for Acanthamoeba, 232 for American trypanosomiasis, and 4801 for toxoplasmosis. Articles published other than English language, lacking full-length forms, and those published before 1990 (totally 5507 papers) were excluded from this review. Studies in English language emphazing on ocular manifestation of these infections and their associations with travel were subjected (2763). All searches were combined, and we removed duplicate manuscripts and irrelevant articles by reading their titles and abstracts (122 of 2763). The electronic search was classified using the following phrases: travel, ocular infection, ocular parasitic presentation, diagnosis, treatment, and prevention.
Results
The most important ocular diseases caused by protozoans include giardiasis, leishmaniasis, malaria, Acanthamoeba, American trypanosomiasis, and toxoplasmosis. Treatment and prevention recommendation are summarized in Table 1.
Table 1.
Recommended treatments and preventions for ocular parasitosis caused by protozoan infection during travel
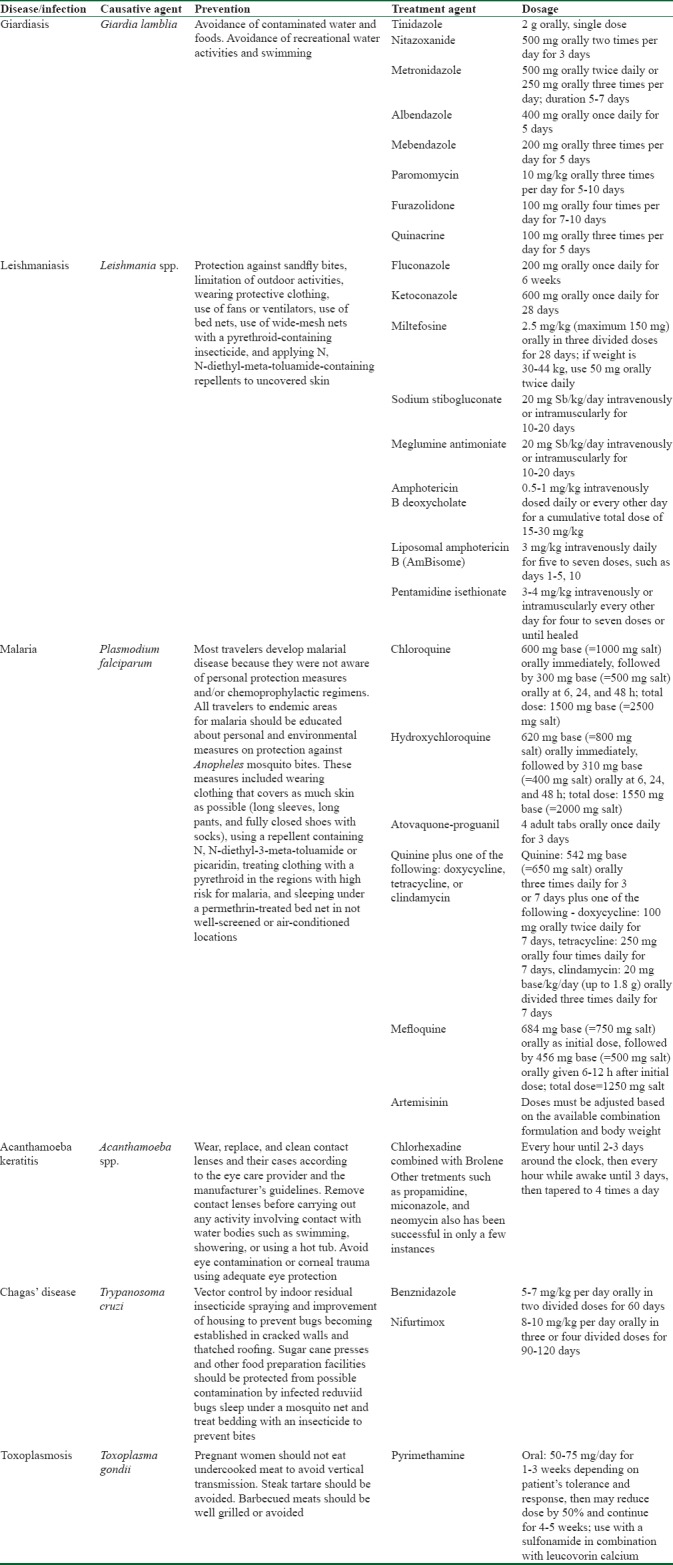
Giardiasis
Giardiasis is caused by an enteric flagellated protozoan parasite of the genus Giardia. Giardia is transmitted through ingestion of cysts in contaminated food or water, or directly through the fecal/oral route.[8]
In one study, the risk of travel-associated giardiasis was estimated at 5.3 per 100,000 travelers with highest incidences in travelers from the Indian Subcontinent, East and West Africa, South and South East Asia, and South and Central America.[9,10,11] In addition, the highest risk was seen in the youngest age group, with a decreasing risk with higher age.[11] Caucasian ethnicity, long travel time (more than 32 days), traveling to South or South East Asia, afebrile presentation, and presenting with gastrointestinal symptoms were all associated with giardiasis in returned tropical travelers.[12] The peak incidence of giardiasis was shown in travelers returning from South Asia, the Middle East and South America.[13]
Extraintestinal manifestations of giardiasis are rarely reported, but in a recent study around 33% of patients affected by giardiasis showed long-term extraintestinal symptoms; hence, these symptoms are not as rare as previously assumed.[14] Ocular complications included iridocyclitis, choroiditis, retinal hemorrhages, salt and pepper degeneration, and severe bilateral anterior uveitis.[15,16,17] Susceptibility to ocular complications with giardiasis seems higher in smaller children.[18] It has been proposed that salt and pepper degeneration may be a consequence of toxic metabolites produced by the parasites, not direct invasion [Figure 1].[15,19]
Figure 1.
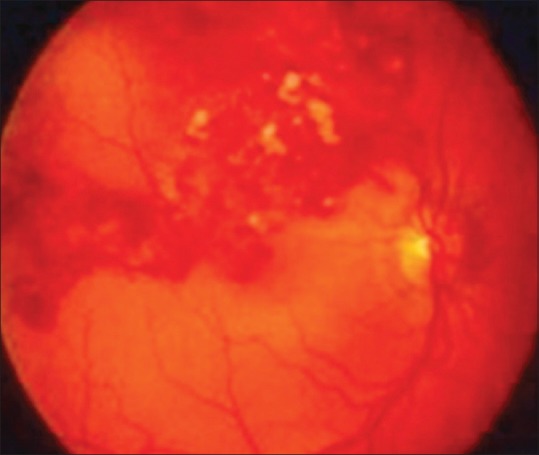
Salt and pepper degeneration of the retina in a child infected with Giardiasis
Several different treatments for giardiasis have been described; first-line therapy for giardiasis is generally tinidazole and metronidizaole. Metronidazole 500 mg orally twice daily or 250 mg orally three times per day is recommended, for a duration of 5–7 days. Also, it is recommended that for children it should be orally divided three times per day for 5–7 days (maximum 250 mg per dose). Another choice for giardiasis treatment approved by the Food and Drug Administration is nitazoxanide.[20] Third-line therapies for pregnant or refractory cases are paromomycin and quinacrine, respectively.[21]
Prevention and recommendations: Giardiasis
Since the main route of transmission of giardiasis is ingestion of contaminated water and foods, our recommendations to travelers for prevention are consumption of only treated water and cooked foods, especially when traveling to locations with high incidences of the parasite. In addition, recreational water activities and swimming should be performed with caution in suspected water in these locations. Suitable treatment for giardiasis is important for reducing ocular complications.
Leishmaniasis
Leishmaniasis is a tropical parasitic disease caused by obligate intramacrophage protozoans. These parasites are transmitted to humans during blood sucking by female sand flies.[22] Travelers to endemic areas are at risk.[23,24,25,26,27,28,29,30] Ocular findings in cutaneous leishmaniasis indicate a local phenomenon due to the initial site of the bite near the eyelids. Ptosis, cicatricial ectropion or entropion, bilateral lagophthalmia, and chronic ulcerative blepharoconjunctivitis have been described.[31,32,33] Extension of the infection to lacrimal ducts may cause dacryocystitis.[34]
Ocular manifestations in visceral leishmaniasis, also known as kala-azar, are predominantly retinal vascular abnormalities, including focal retinal whitening, cotton wool spots, and hemorrhages with increased vessel tortuosity.[35] Bilateral, multifocal retinal hemorrhages can be improved by specific antileishmanial therapy.[7,36,37] Treatment is also summarized in Table 1.
Prevention and recommendation: Leishmaniasis
There is still no available vaccine against leishmaniasis for humans.[38] Travelers to endemic areas must be taught that the only protective methods are those of protection against sandfly bites. These methods include limitation of outdoor activities, especially from sunset to sunrise when sandflies are frequently active; wearing protective clothing; and applying N, N-diethyl-meta-toluamide-containing repellents to uncovered skin. Because of weak flying ability of sandflies, use of fans or ventilators can inhibit their activity. In addition, sleeping in air-conditioned or well-screened areas and indoor spraying is advised.[3] Since the highest activity of sandflies occurs when people are sleeping, the use of bed nets is one of the most important protection methods. The use of wide-mesh nets with a pyrethroid-containing insecticide can decrease sandfly biting rates.[22,39,40] Also, there are some indoor protection such as some topical products which can be applied to the skin. For example, citronella, linalool, and geraniol candles are some effective essential oils for this propose.[41]
Malaria
Malaria is caused by intraerythrocytic protozoa of different Plasmodium species. These parasites are transmitted to humans through the bite of female Anopheles mosquitoes.[42]
Malaria remains endemic in 97 countries.[43] The most important cause of travel-related mortality and morbidity is malaria.[44] In general, all travelers going to sub-Saharan Africa are at risk, unless their travel is entirely restricted to high altitudes, such as Mount Kilimanjaro.[45] However, one study showed that the distribution of malaria is expected to shift with future climate changes with higher risks in African highlands, central Europe, and North America.[46] Traveling to Oceania and West Africa is classified as high-risk travel for developing encephalitis due to malaria that requires chemoprophylaxis.[47]
Ocular complications due to malaria disease are divided into two parts: the effects of the parasite on the eye and the ocular side effects of antimalarial drugs. Ocular involvement in malaria can occur in severe cases.[48] Other studies have shown that retinopathy is associated with mortality in children with cerebral malaria.[49] Malarial retinopathy contains of four distinct components: retinal whitening, retinal vessel wall discoloration to orange or white, hemorrhages, and papilledema [Figure 2].[19,50] The characteristic pattern of retinal whitening and vessel changes appears to be exclusive to malaria in a comatose patient, even if the peripheral blood smear is negative for malarial parasites.[51] Amaurosis fugax, optic neuritis, glaucoma, panuveitis, oculomotor paralysis, and cortical blindness have been described in this disease.[19]
Figure 2.
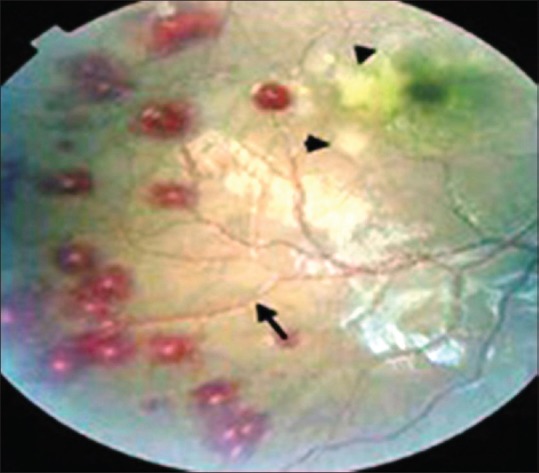
Malaria rethinopathy, showed multiple white centered hemorrhages, macular whitening (black triangles) and vessel wall discoloration to orange (black arrow)
In addition, eyes are affected by antimalarial drugs. Early retinopathy from chloroquine or hydroxychloroquine occurs in 10% and 2.7% of users, respectively.[52] Bilateral pigment changes in the retinal pigment epithelium of the macula and bilateral enlarged blind spots have been seen in mefloquine users.[53,54]
Treatment of systemic infection can cause improvements of ocular manifestations. Because severe or complicated cases of malaria can deteriorate suddenly during the early course of treatment, these patients should be admitted to hospital for at least 24 h.[55] Treatment is also summarized in Table 1.
Prevention and recommendation: Malaria
Most travelers develop malarial disease because they were not aware of personal protection measures and/or chemoprophylactic regimens.[56] All travelers to endemic areas for malaria should be educated about personal and environmental measures on protection against Anopheles mosquito bites. These measures include wearing clothing that covers as much skin as possible (long sleeves, long pants, and fully closed shoes with socks), using a repellent containing N, N-diethyl-3-meta-toluamide or picaridin, treating clothing with a pyrethroid in the regions with high risk for malaria, and sleeping under a permethrin-treated bed net in not well-screened or air-conditioned locations. Since the activity of the Anopheles mosquito is generally between dusk and dawn, limitation of outdoor night activity is advised.[2,57] Chemoprophylaxis is necessary for any person traveling to high-transmission countries, especially in Africa. Multiple regimens for chemoprophylaxis exist and selection of them depends on patient preference, side-effect profile, cost, and drug susceptibility of Plasmodium species in the area.[42] Also, there are some important diseases for travelers which are transmitted by mosquitoes. For example, dengue, filariasis, chikungunya, West Nile virus, yellow fever, dirofilariasis, Japanese encephalitis, tularemia, Saint Louis encephalitis, eastern equine encephalitis, western equine encephalitis, Venezuelan equine encephalitis, Ross River fever, La Crosse encephalitis, Zika fever, and Barmah Forest fever.[58]
Acanthamoeba
Acanthamoeba species are ubiquitous protozoa that have been isolated from various natural environments such as sea water, fresh water lakes, soil, hot spring resorts, bottled water, and air.[59]
Acanthamoeba keratitis (AK) is a severe infection of the cornea that can cause vision loss in some cases. Decreased vision in AK results from corneal ulceration and scarring. Keratitis frequently occurs in immunocompetent contact lens wearers. Contact lens hygiene is very important, but infection has been seen with some commercially available multipurpose solutions, suggesting these solutions are ineffective against Acanthamoeba.[60,61,62] Corneal trauma has been considered to be a risk factor of AK in non-contact lens wearers. Other documented causes include swimming and showering with contact lenses in situ, exposure to contaminated water or soil, and corneal trauma. Some studies showed that AK was more prevalent during warmer seasons, especially during the summer,[63,64,65,66] but others revealed no significant seasonal differences.[67] This difference is perhaps due to more swimming and showering in summer.
The majority of symptoms reported in AK are nonspecific, including pain, eye redness, blurred vision, and photophobia. Early signs of AK include an epitheliopathy with punctate keratopathy and pseudodendrites [Figure 3a]. Ring infiltrate and perineuritis are specific signs in AK and present in only around 50% of patients [Figure 3b].[68,69,70] Clinical outcomes of AK are poor; the most severely infected eyes require keratoplasty or removal of the affected eye.[70,71]
Figure 3.
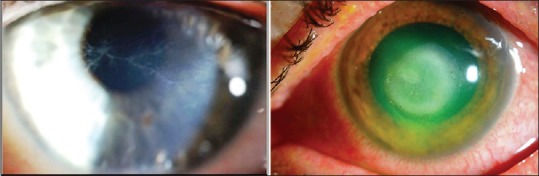
(a) Clinical finding of Acanthamoeba keratitis: A. pseudodendrites keratitis. (b) ring infiltrate
Treatment of amebic keratitis is difficult and disappointing. Long-term topical application of agents such as propamidine, miconazole, and neomycin has been successful in only a few instances.[7] Treatment is also summarized in Table 1.
Prevention and recommendation: Acanthamoeba
Since diagnosis and treatment is difficult in AK, prevention is more effective. These include the following: (1) wear, replace, and clean contact lenses and their cases according to the eye care provider and the manufacturer's guidelines; (2) remove contact lenses before carrying out any activity involving contact with water bodies such as swimming, showering, or using a hot tub; and (3) avoid eye contamination or corneal trauma using adequate eye protection.[72]
American trypanosomiasis (Chagas’ disease)
American trypanosomiasis, also known as kissing bugs and Chagas disease, is only seen on the American continent or among subjects who travel to rural areas of South and Central America. Trypanosoma cruzi, the causative parasite, is transmitted through the bite of reduviid bugs. The reservoir is in humans and domestic mammals, along with armadillos and opossums. Oral transmission through food or sugar cane juice contaminated by infected bugs may occur. Vertical transmission and infection through blood transfusion are important routes of infection and can take place in nonendemic areas.[7,73,74]
Acute infection is rarely symptomatic. Some patients may complain of edema around the bite site or around the eye (Romanna's sign), associated with fever and lymphadenopathy. In the chronic stage, the parasite will infiltrate to other organs, including cardiac conducting tissue, cardiac muscle, and smooth muscle of the gastrointestinal tract.[74,75]
Two medications are available for treatment of American trypanosomiasis, including nifurtimox and benznidazole. Therapy is usually extended for a period of months, and parasitologic cure rates are somewhat disappointing. Both medications carry a long list of significant side effects. Supportive therapy, including surgery where necessary, is given as required for management of related complications. Treatment is also summarized in Table 1.
Prevention and recommendation: American trypanosomiasis
There are very little data regarding prevention of South American trypanosomiasis in travelers. The mainstay of control of the disease is vector control by indoor residual insecticide spraying and improvement of housing to prevent bugs becoming established in cracked walls and thatched roofing.[75] Sugar cane presses and other food preparation facilities should be protected from possible contamination by infected reduviid bugs.
Travelers who are expecting to spend time in rural forested areas, particularly if living or sleeping in local accommodations, should be advised that this will increase their bite risk. It is advisable for travelers in such locations to sleep under a mosquito net and treat bedding with an insecticide to prevent bites.[75]
Toxoplasmosis
Toxoplasma gondii is a protozoan parasite, the lifecycle of which passes through cats. It represents the commonest cause of uveitis worldwide. Human infection occurs through ingestion of food or water contaminated with cat feces. Toxoplasmosis may be acquired at any age but most commonly during childhood.[76]
Humans become infected by this parasite when eating undercooked infected meat and by contamination with cysts following handling of cat litter trays.
Some patients may present with a glandular fever-like systemic febrile illness with adenopathy. Most cases of adult infection will not present with eye signs, but those that do usually present with a focal necrotizing retinitis occasionally associated with vascular occlusion.[77]
The immunocompromized patients and neonates who have been exposed transplacentally by a mother's acute infection are at particularly high risk for ocular complications. Acute infection in newborns and patients infected with HIV may lead to an intense necrotizing chorioretinitis. More commonly, however, chorioretinitis is the result of necrotizing inflammation following the rupture of an older, slowly growing cyst, thus releasing bradyzoites. Ocular symptoms in the patient with congenital ocular toxoplasmosis may include strabismus, nystagmus, and blindness.[7] Acute, acquired toxoplasmosis is associated with scotoma, photophobia, and loss of central vision due to macular involvement. Oculomotor nerve involvement may result in ptosis.
First-line therapy of chorioretinitis in toxoplasmosis includes the use of pyrimethamine with sulfadiazine and folinic acid to prevent bone marrow toxicity.[78]
In addition, clindamycin and azithromycin are commonly used as first-line treatments. Therapy should continue for 1–2 weeks beyond the resolution of symptoms.[7] Treatment is also summarized in Table 1.
Prevention and recommendation: Toxoplasmosis
Since serious consequences may arise if a pregnant woman is infected for the first time during travel, pregnant women should not eat undercooked meat to avoid vertical transmission. In France, steak tartare should be avoided. Barbecued meats should be well grilled or avoided.
Conclusions
Many travel-related eye diseases are preventable by education of health behaviors, using precautionary measures and taking chemoprophylactic medications or appropriate vaccinations for some diseases. The physicians in both endemic and nonendemic areas should become familiar with their clinical presentation and treatment strategies for earlier diagnosis of ocular lesions caused by parasitic infections to reduce costly, inappropriate laboratory evaluations, delayed or ineffective treatments, and severe complications including severe vision loss.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Korzeniewski K. Travel health prevention. Int Marit Health. 2017;68:238–44. doi: 10.5603/IMH.2017.0042. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DO, Chen LH, Kozarsky PE. Medical considerations before international travel. N Engl J Med. 2016;375:247–60. doi: 10.1056/NEJMra1508815. [DOI] [PubMed] [Google Scholar]
- 3.Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032–9. doi: 10.1016/j.ijid.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Gushulak BD, MacPherson DW. Globalization of infectious diseases: The impact of migration. Clin Infect Dis. 2004;38:1742–8. doi: 10.1086/421268. [DOI] [PubMed] [Google Scholar]
- 5.Velayati A, Saburi A. Travel medicine as an important aspect of global health; much still need to be done. Int J Travel Med Global Health. 2014;1:1–2. [Google Scholar]
- 6.Isfeedvajani MS. Travel risks and preventive strategies: An overview. Int J Travel Med Glob Health. 2016;4:39. [Google Scholar]
- 7.Nimir AR, Saliem A, Ibrahim IA. Ophthalmic parasitosis: A review article. Interdiscip Perspect Infect Dis. 2012;2012:587402. doi: 10.1155/2012/587402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cama VA, Mathison BA. Infections by intestinal coccidia and giardia duodenalis. Clin Lab Med. 2015;35:423–44. doi: 10.1016/j.cll.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaminathan A, Torresi J, Schlagenhauf P, Thursky K, Wilder-Smith A, Connor BA, et al. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J Infect. 2009;59:19–27. doi: 10.1016/j.jinf.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: Systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609–14. [PubMed] [Google Scholar]
- 11.Ekdahl K, Andersson Y. Imported giardiasis: Impact of international travel, immigration, and adoption. Am J Trop Med Hyg. 2005;72:825–30. [PubMed] [Google Scholar]
- 12.Takaoka K, Gourtsoyannis Y, Hart JD, Armstrong M, Daniel A, Mewse E, et al. Incidence rate and risk factors for giardiasis and strongyloidiasis in returning UK travellers. J Travel Med. 2016;23:1–6. doi: 10.1093/jtm/taw050. [DOI] [PubMed] [Google Scholar]
- 13.Izadi M, Sadepur A. A closer look to the most frequent travelers’ disease: A systematic update on travelers’ Diarrhea. Int J Travel Med Global Health. 2014;2:95–9. [Google Scholar]
- 14.Halliez MC, Buret AG. Extra-intestinal and long term consequences of giardia duodenalis infections. World J Gastroenterol. 2013;19:8974–85. doi: 10.3748/wjg.v19.i47.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettoello Mantovani M, Giardino I, Magli A, di Martino L, Guandalini S. Intestinal giardiasis associated with ophthalmologic changes. J Pediatr Gastroenterol Nutr. 1990;11:196–200. doi: 10.1097/00005176-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Khalifa E, El-Nouby K, Ali A, El-Mashad A, Negm O. Ocular changes in giardiasis: Human and experimental studies. Tanta Med Sci J. 2007;2:119–31. [Google Scholar]
- 17.Turnbull AM, Lin Z, Matthews BN. Severe bilateral anterior uveitis secondary to giardiasis, initially misdiagnosed as a side effect of metronidazole. Eye (Lond) 2013;27:1225–6. doi: 10.1038/eye.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corsi A, Nucci C, Knafelz D, Bulgarini D, Di Iorio L, Polito A, et al. Ocular changes associated with Giardia lamblia infection in children. Br J Ophthalmol. 1998;82:59–62. doi: 10.1136/bjo.82.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sayed NM, Safar EH. Characterization of the parasite-induced lesions in the posterior segment of the eye. Indian J Ophthalmol. 2015;63:881–7. doi: 10.4103/0301-4738.176028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz JJ, Ayoub A, Gargala G, Chegne NL, Favennec L. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from Northern Peru. Aliment Pharmacol Ther. 2001;15:1409–15. doi: 10.1046/j.1365-2036.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- 21.Mørch K, Hanevik K, Robertson LJ, Strand EA, Langeland N. Treatment-ladder and genetic characterisation of parasites in refractory giardiasis after an outbreak in Norway. J Infect. 2008;56:268–73. doi: 10.1016/j.jinf.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–77. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 23.Mansueto P, Seidita A, Vitale G, Cascio A. Leishmaniasis in travelers: A literature review. Travel Med Infect Dis. 2014;12:563–81. doi: 10.1016/j.tmaid.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharifi I, Aflatoonian MR, Fekri AR, Hakimi Parizi M, Aghaei Afshar A, Khosravi A, et al. A comprehensive review of cutaneous leishmaniasis in kerman province, Southeastern Iran-narrative review article. Iran J Public Health. 2015;44:299–307. [PMC free article] [PubMed] [Google Scholar]
- 26.Lübbert C, Opitz BM, Harms-Zwingenberger G, Nietsch HH. Fever, pancytopenia, and splenomegaly 8 months after a trip to Majorca Island (Spain) Med Klin (Munich) 2008;103:29–35. doi: 10.1007/s00063-008-1003-5. [DOI] [PubMed] [Google Scholar]
- 27.Berens-Riha N, Fleischmann E, Pratlong F, Bretzel G, von Sonnenburg F, Löscher T. Cutaneous leishmaniasis (Leishmania tropica) in a German tourist after travel to greece. J Travel Med. 2009;16:220–2. doi: 10.1111/j.1708-8305.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmid MB, Leichsenring M, Keller C, Hegasy G. Pancytopenia, fever, and splenomegaly in a 2-year-old boy. Dtsch Med Wochenschr. 2009;134:1274–7. doi: 10.1055/s-0029-1225275. [DOI] [PubMed] [Google Scholar]
- 29.Besada E, Njålla RJ, Nossent JC. Imported case of visceral leishmaniasis presenting as pancytopenia in a Norwegian patient treated with methotrexate and etanercept for psoriasis arthritis. Rheumatol Int. 2013;33:2687–9. doi: 10.1007/s00296-012-2483-4. [DOI] [PubMed] [Google Scholar]
- 30.Ehehalt U, Schunk M, Jensenius M, van Genderen PJ, Gkrania-Klotsas E, Chappuis F, et al. Leishmaniasis acquired by travellers to endemic regions in Europe: A EuroTravNet multi-centre study. Travel Med Infect Dis. 2014;12:167–72. doi: 10.1016/j.tmaid.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhry IA, Hylton C, DesMarchais B. Bilateral ptosis and lower eyelid ectropion secondary to cutaneous leishmaniasis. Arch Ophthalmol. 1998;116:1244–5. [PubMed] [Google Scholar]
- 32.Satici A, Gurler B, Gurel MS, Aslan G, Oguz H. Mechanical ptosis and lagophthalmos in cutaneous leishmaniasis. Br J Ophthalmol. 1998;82:975. doi: 10.1136/bjo.82.8.974a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayele FA, Wolde YA, Hagos T, Diro E. Ocular leishmaniasis presenting as chronic ulcerative blepharoconjunctivitis: A case report. J Clin Exp Ophthalmol. 2015;6:1–2. [Google Scholar]
- 34.Durdu M, Gökçe S, Bagirova M, Yalaz M, Allahverdiyev AM, Uzun S. Periocular involvement in cutaneous leishmaniasis. J Eur Acad Dermatol Venereol. 2007;21:214–8. doi: 10.1111/j.1468-3083.2006.01903.x. [DOI] [PubMed] [Google Scholar]
- 35.Maude RJ, Ahmed BU, Rahman AH, Rahman R, Majumder MI, Menezes DB, et al. Retinal changes in visceral leishmaniasis by retinal photography. BMC Infect Dis. 2014;14:527. doi: 10.1186/1471-2334-14-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montero JA, Ruiz-Moreno JM, Sanchis E. Intraretinal hemorrhage associated with leishmaniasis. Ophthalmic Surg Lasers Imaging. 2003;34:212–4. [PubMed] [Google Scholar]
- 37.Sundar S, Chakravarty J. An update on pharmacotherapy for leishmaniasis. Expert Opin Pharmacother. 2015;16:237–52. doi: 10.1517/14656566.2015.973850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar R, Engwerda C. Vaccines to prevent leishmaniasis. Clin Transl Immunology. 2014;3:e13. doi: 10.1038/cti.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: New approaches to disease control. BMJ. 2003;326:377–82. doi: 10.1136/bmj.326.7385.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroeger A, Avila EV, Morison L. Insecticide impregnated curtains to control domestic transmission of cutaneous leishmaniasis in Venezuela: Cluster randomised trial. BMJ. 2002;325:810–3. doi: 10.1136/bmj.325.7368.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller GC, Junnila A, Kravchenko VD, Revay EE, Butlers J, Schlein Y. Indoor protection against mosquito and sand fly bites: A comparison between citronella, linalool, and geraniol candles. J Am Mosq Control Assoc. 2008;24:150–3. doi: 10.2987/8756-971X(2008)24[150:IPAMAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Hahn WO, Pottinger PS. Malaria in the traveler: How to manage before departure and evaluate upon return. Med Clin North Am. 2016;100:289–302. doi: 10.1016/j.mcna.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Geneva: World Health Organization; 2015. Malaria Rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs: Round 6 (2014-2015) [Google Scholar]
- 44.Leder K, Torresi J, Libman MD, Cramer JP, Castelli F, Schlagenhauf P, et al. GeoSentinel surveillance of illness in returned travelers, 2007-2011. Ann Intern Med. 2013;158:456–68. doi: 10.7326/0003-4819-158-6-201303190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elliott JH, O'Brien D, Leder K, Kitchener S, Schwartz E, Weld L, et al. Imported plasmodium vivax malaria: Demographic and clinical features in nonimmune travelers. J Travel Med. 2004;11:213–7. doi: 10.2310/7060.2004.19004. [DOI] [PubMed] [Google Scholar]
- 46.Caminade C, Kovats S, Rocklov J, Tompkins AM, Morse AP, Colón-González FJ, et al. Impact of climate change on global malaria distribution. Proc Natl Acad Sci U S A. 2014;111:3286–91. doi: 10.1073/pnas.1302089111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izadi M, Is'haqi A, Is'haqi MA, Jonaidi Jafari N, Rahamaty F, Banki A. An overview of travel-associated central nervous system infectious diseases: Risk assessment, general considerations and future directions. Asian Pac J Trop Biomed. 2014;4:589–96. doi: 10.12980/APJTB.4.2014APJTB-2014-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrera V, Hiscott PS, Craig AG, White VA, Milner DA, Beare NA, et al. Severity of retinopathy parallels the degree of parasite sequestration in the eyes and brains of Malawian children with fatal cerebral malaria. J Infect Dis. 2015;211:1977–86. doi: 10.1093/infdis/jiu592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh J, Verma R, Tiwari A, Mishra D, Singh HP. Retinopathy as a prognostic marker in cerebral malaria. Indian Pediatr. 2016;53:315–7. doi: 10.1007/s13312-016-0844-x. [DOI] [PubMed] [Google Scholar]
- 50.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: A newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–7. [PMC free article] [PubMed] [Google Scholar]
- 51.Beare NA, Lewallen S, Taylor TE, Molyneux ME. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol. 2011;6:349–55. doi: 10.2217/fmb.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.AlKadi HO. Antimalarial drug toxicity: A review. Chemotherapy. 2007;53:385–91. doi: 10.1159/000109767. [DOI] [PubMed] [Google Scholar]
- 53.Walker RA, Colleaux KM. Maculopathy associated with mefloquine (Lariam) therapy for malaria prophylaxis. Can J Ophthalmol. 2007;42:125–6. [PubMed] [Google Scholar]
- 54.Melo Mde M, Ciriano JP, van Genderen PJ. Narrow vision after view-broadening travel. J Travel Med. 2008;15:278–80. doi: 10.1111/j.1708-8305.2008.00222.x. [DOI] [PubMed] [Google Scholar]
- 55.Lalloo DG, Shingadia D, Bell DJ, Beeching NJ, Whitty CJ, Chiodini PL, et al. UK malaria treatment guidelines 2016. J Infect. 2016;72:635–49. doi: 10.1016/j.jinf.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ladhani S, Aibara RJ, Riordan FA, Shingadia D. Imported malaria in children: A review of clinical studies. Lancet Infect Dis. 2007;7:349–57. doi: 10.1016/S1473-3099(07)70110-X. [DOI] [PubMed] [Google Scholar]
- 57.Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359:603–12. doi: 10.1056/NEJMcp0803572. [DOI] [PubMed] [Google Scholar]
- 58.Mirzaian E, Durham MJ, Hess K, Goad JA. Mosquito-borne illnesses in travelers: A review of risk and prevention. Pharmacotherapy. 2010;30:1031–43. doi: 10.1592/phco.30.10.1031. [DOI] [PubMed] [Google Scholar]
- 59.Marciano-Cabral F, Cabral G. Acanthamoeba spp. As agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zanetti S, Fiori PL, Pinna A, Usai S, Carta F, Fadda G. Susceptibility of Acanthamoeba castellanii to contact lens disinfecting solutions. Antimicrob Agents Chemother. 1995;39:1596–8. doi: 10.1128/aac.39.7.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hiti K, Walochnik J, Haller-Schober EM, Faschinger C, Aspöck H. Viability of Acanthamoeba after exposure to a multipurpose disinfecting contact lens solution and two hydrogen peroxide systems. Br J Ophthalmol. 2002;86:144–6. doi: 10.1136/bjo.86.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba. Parasit Vectors. 2012;5:6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joslin CE, Tu EY, McMahon TT, Passaro DJ, Stayner LT, Sugar J, et al. Epidemiological characteristics of a Chicago-area Acanthamoeba keratitis outbreak. Am J Ophthalmol. 2006;142:212–7. doi: 10.1016/j.ajo.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 64.McAllum P, Bahar I, Kaiserman I, Srinivasan S, Slomovic A, Rootman D, et al. Temporal and seasonal trends in Acanthamoeba keratitis. Cornea. 2009;28:7–10. doi: 10.1097/ICO.0b013e318181a863. [DOI] [PubMed] [Google Scholar]
- 65.Rahimi F, Rafizadeh SM, Beheshtnejad AH, Hashemian MN, Zare MA, Siatiri H, et al. Acanthamoeba keratitis and its associated risk factors in Farabi eye hospital of Tehran. Iranian Journal of Ophthalmology. 2013;25:297–303. [Google Scholar]
- 66.Yamazoe K, Yamamoto Y, Shimazaki-Den S, Shimazaki J. Visual outcome in Japanese patients with Acanthamoeba keratitis. Eye (Lond) 2012;26:517–22. doi: 10.1038/eye.2011.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang C, Sun X, Wang Z, Zhang Y. Acanthamoeba keratitis: Clinical characteristics and management. Ocul Surf. 2015;13:164–8. doi: 10.1016/j.jtos.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Bacon AS, Dart JK, Ficker LA, Matheson MM, Wright P. Acanthamoeba keratitis. The value of early diagnosis. Ophthalmology. 1993;100:1238–43. doi: 10.1016/s0161-6420(93)31499-5. [DOI] [PubMed] [Google Scholar]
- 69.Ross J, Roy SL, Mathers WD, Ritterband DC, Yoder JS, Ayers T, et al. Clinical characteristics of Acanthamoeba keratitis infections in 28 states, 2008 to 2011. Cornea. 2014;33:161–8. doi: 10.1097/ICO.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 70.Maycock NJ, Jayaswal R. Update on Acanthamoeba keratitis: Diagnosis, treatment, and outcomes. Cornea. 2016;35:713–20. doi: 10.1097/ICO.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 71.Tanhehco T, Colby K. The clinical experience of Acanthamoeba keratitis at a tertiary care eye hospital. Cornea. 2010;29:1005–10. doi: 10.1097/ICO.0b013e3181cf9949. [DOI] [PubMed] [Google Scholar]
- 72.Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, Arnalich-Montiel F, Piñero JE, Valladares B. Acanthamoeba keratitis: An emerging disease gathering importance worldwide? Trends Parasitol. 2013;29:181–7. doi: 10.1016/j.pt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Moncayo A, Silveira AC. Current epidemiological trends for chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):17–30. doi: 10.1590/s0074-02762009000900005. [DOI] [PubMed] [Google Scholar]
- 74.Moncayo A, Ortiz Yanine MI. An update on chagas disease (human American trypanosomiasis) Ann Trop Med Parasitol. 2006;100:663–77. doi: 10.1179/136485906X112248. [DOI] [PubMed] [Google Scholar]
- 75.Diazgranados CA, Saavedra-Trujillo CH, Mantilla M, Valderrama SL, Alquichire C, Franco-Paredes C. Chagasic encephalitis in HIV patients: Common presentation of an evolving epidemiological and clinical association. Lancet Infect Dis. 2009;9:324–30. doi: 10.1016/S1473-3099(09)70088-X. [DOI] [PubMed] [Google Scholar]
- 76.McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA community-based uveitis study group. Am J Ophthalmol. 1996;121:35–46. doi: 10.1016/s0002-9394(14)70532-x. [DOI] [PubMed] [Google Scholar]
- 77.Rathinam SR, Cunningham ET., Jr Infectious causes of uveitis in the developing world. Int Ophthalmol Clin. 2000;40:137–52. doi: 10.1097/00004397-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 78.Engstrom RE, Jr, Holland GN, Nussenblatt RB, Jabs DA. Current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 1991;111:601–10. doi: 10.1016/s0002-9394(14)73706-7. [DOI] [PubMed] [Google Scholar]


