Abstract
Possible effects of coenzyme Q10 (CoQ10) supplement on the serum level of high-sensitivity C-reactive protein (hs-CRP) in cardiovascular diseases (CVDs) remains unclear.
Objective:
Therefore, this meta-analysis was conducted to investigate its effects on the serum hs-CRP level in patients with CVDs. A comprehensive search was conducted on the EMBASE, MEDLINE, and PubMed Central databases for pertinent papers in English up to November 2016. All randomized controlled trials (RCTs) that studied the effects of supplementation with CoQ10 on the serum of hs-CRP level in cardiovascular patients were included. We used random-effects models (the DerSimonian–Laird method) to estimate the pooled effect of selected studies and the I2 test to assess the between-study heterogeneity. The subgroup analyses were carried out according to the baseline serum hs-CRP, quality assessment score, supplementation dosage, and duration of intervention. Of 205 studies, five trials were eligible for inclusion in this study with 159 participants in the ntervention and 143 participants in the placebo group. Results of the pooled analysis revealed that the CoQ10 supplementation had no significant effect on the serum level of hs-CRP compared with the placebo group (MD: 0.120; 95% = −0.944, 1.185; P = 0.825). Moreover, the subgroup analyses showed the baseline serum hs-CRP, quality assessment score, and duration of intervention can be sources of heterogeneity. The results of this study demonstrated that the beneficial effect of CoQ10 supplementation for patients with CVDs is observed in those who received this supplement for more than 12 weeks and with the baseline serum hs-CRP >3 mg/L.
Keywords: Cardiovascular diseases, C-reactive protein, ubiquinone
Introduction
Cardiovascular diseases) CVDs) are among the main causes of death around the world.[1] There are multiple risk factors for the development of CVDs such as chronic inflammation. This condition has an important role in the pathogenesis of many other diseases such as diabetes, atherosclerosis, and metabolic syndrome.[2,3,4]
High-sensitivity C-reactive protein (hs-CPR), a fundamental biomarker for inflammation status and an acute phase reactant protein, is synthesized in the liver,[2,5] and increases in numerous CVDs such as acute ischemia and myocardial infarction.[6,7,8] According to several published studies, the elevated serum level of hs-CRP can predict the future risk of CVDs.[9,10,11]
Coenzyme Q10 (ubiquinone), a fat-soluble quinone, has an essential role in the cellular mitochondrial respiratory chain for the synthesis of adenosine triphosphate (ATP).[12,13] Organs such as brain, liver, heart, kidney, and skeletal muscle consume high energy, thus requiring a large amount of this cofactor to synthesize ATP. Ubiquinone does an antioxidant activity that protects lipid in biological membranes from oxidative stress.[14,15] The relationship between coenzyme Q10 (CoQ10) and inflammation in animal[16,17] and cell[18,19] models has been reported in various studies. Although multiple studies have demonstrated a beneficial effect of CoQ10 on inflammatory biomarkers in different groups of participants,[20] the results of studies on patients with CVDs are contradictory. For example, Zhao et al. showed that a 30 mg/day CoQ10 supplementation for 6 months significantly decreased the serum level of hs-CRP in the intervention group.[21] In contrast, Pourmoghaddas et al. showed that a 200 mg/day CoQ10 supplementation for 4 months had no significant effect on the serum level of hs-CRP in the intervention group.[22] Thus, to better understand the potential effect of CoQ10 supplementation on the level of hs-CRP serum in patients with CVDs, we conducted a systematic review and meta-analysis of the published RCTs.
Materials and Methods
Search strategy and selection criteria
We conducted a comprehensive search on the EMBASE, MEDLINE, and PubMed Central databases through Scopus and PubMed for published papers up to November 2016. The used keywords were relevant to the objectives of the study. The search strategy is presented in Supplementary Table 1. A manual search was carried out on the Google scholar search engine and the references were cited in appropriate systematic reviews. The search in PubMed was restricted to humans and the English language, and for Scopus, it was restricted to article, medicine, humans, and the English language. We examined the titles and abstracts of the obtained studies. Finally, the full texts of selected studies were investigated according to the inclusion/exclusion criteria.
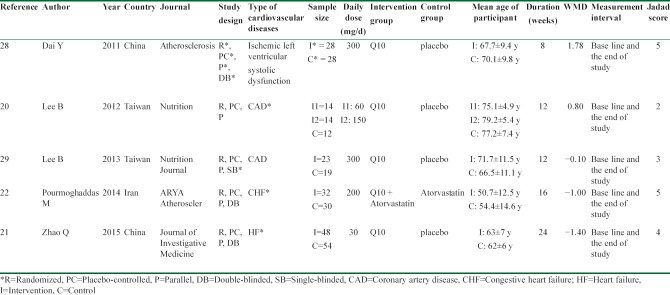
Table 1.
Characteristics of the included studies in this meta-analysis
Eligibility criteria
Studies were included in this meta-analysis if they had the following criteria: (1) the study design was randomized clinical trial (RCT), (2) the intervention was CoQ10 supplementation, (3) the proper outcome was plasma or serum hs-CRP level, (4) the participants were CVD patients.
Studies were excluded if they were (1) Observational studies, (2) review articles, (3) combined CoQ10 supplementation with multivitamins, minerals and carnitine, (4) done without CoQ10 supplementation, (5) duplicate publications, (6) repeated studies (the conclusions of trials were reported in more than one article), and (7) surveys which were conducted on cell and animals.
Quality assessment
The validated Jadad scale form was used to appraise the methodological quality of included RCTs. The questions of this scoring form contain five parts: randomization, details of randomization scheme, level of blinding, reporting of blinding method, and dropouts and withdrawals in the intervention and placebo groups. Each question in the form gets zero or one point. The total score is obtained from the sum of points of all questions. Studies with 0–2 points had low and studies with 3–5 points had high quality.[23,24]
Data extraction and statistical analysis
Two authors (ZA and SF) independently extracted and coded all the data, and disagreements were resolved by consensus and the third author (SS). We extracted the mean and standard deviation levels of hs-CRP at the baseline and end of trial for both intervention and placebo groups. Furthermore, the characteristics of the selected studies included the name of the first author of the study, year of publication, name of journal, the country in which the study was done, the number of individuals in the intervention and placebo groups, type of intervention, type of blinding, dose of supplement, and duration of intervention. The characteristics of participants including sex, mean of age, and mean of body mass index were also collected. We assessed the heterogeneity among the studies using the I2 test that determines the between-study variance and demonstrates whether the observed inconsistency among studies is real or by chance.[25,26,27] There was between-study heterogeneity if I2>50%. The predefined subgroup analyses were applied to recognize the source of heterogeneity. If heterogeneity was significant, a random-effects model (the DerSimonian–Laird estimator) was used to calculate the pooled mean difference (MD). All statistical analyses were performed using Stata software, version 12(Stata Corp, College Station, Texas, USA) and P < 0.05 was considered as significant.
Results
Search results and study selection
The flow diagram of the study is shown in Figure 1. We retrieved 205 studies in the initial search through the PubMed, Scopus database, and manual search. One hundred and fifty-three records remained after excluding duplicates. Of these, 148 records were removed because they did not satisfy our inclusion criteria. Finally, full-texts of five studies were assessed for eligibility and included in the meta-analysis.[20,21,22,28,29] There were two intervention groups in one of the selected studies, so we considered it as two separate studies in the analysis.[20]
Figure 1.
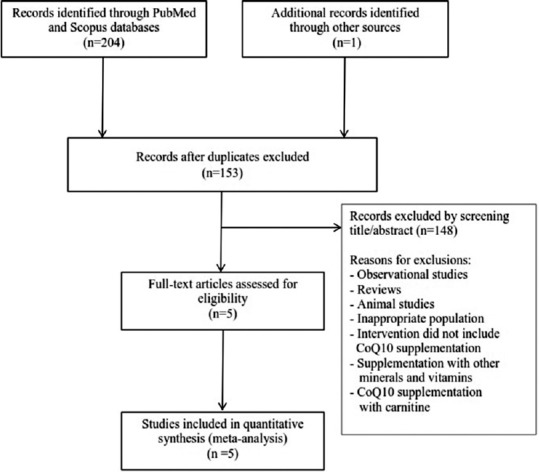
Flow diagram of included trials
Characteristics of the included studies
The characteristics of the included studies are reported in Table 1. The studies were published between 2011 and 2015. Of the five studies, two were carried out in Taiwan,[20,29] two trials were performed in China,[21,28] and one study was done in Iran.[22] All trials had parallel, placebo-controlled designs. Three studies were double-blind,[21,22,28] blinding of one of the trials was single,[29] and the other study did not mention blinding.[20] One study was conducted on patients with ischemic left ventricular systolic dysfunction,[28] two surveys were done on coronary artery disease (CAD) patients,[20,29] and another two studies were carried out on patients with heart failure (HF).[21,22] Duration of intervention in the studies was from 8 to 24 weeks with a median of 12 weeks. The sample size of trials ranged from 40 to 102 participants, and the total sample size of the studies was 302. For the participants of the intervention groups, the mean of age varied from 50.7 to 79.2 years, and in the control groups, it varied from 54.4 to 77.2 years. The daily dosage of supplementation differed from 30 mg/day to 300 mg/day with the median of 175 mg/day. The baseline serum level of hs-CRP in the intervention groups was in the range of 1.2–4.2 mg/L and in the control groups was in the range of 0.8–4.6 mg/L. Based on the Jadad scale, four trials were graded ≥3[21,22,28,29] and only one trial was graded 2.[20]
Meta-analysis
The effect of CoQ10 on the serum hs-CRP level is depicted in Figure 2. The random-effects model (the DerSimonian–Laird method) was used to estimate the pooled MD of the serum of hs-CRP level because there was significant heterogeneity between the studies (test for heterogeneity: P=0.000 and I2= 86.1%). The random-effects model demonstrated that the CoQ10 supplementation had no significant effect on the level of hs-CRP serum (MD: 0.120; 95% = −0.944, 1.185; P = 0.825).
Figure 2.
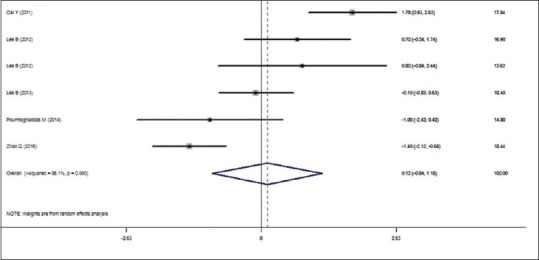
Effect of CoQ10 supplementation on the serum of hs-CRP level in cardiovascular patients
Subgroup analyses
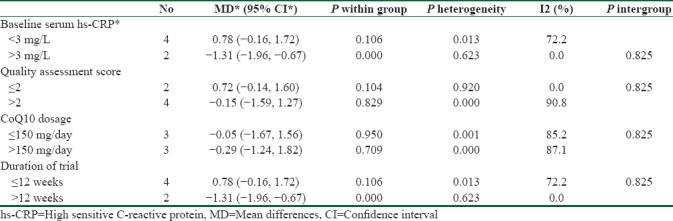
The subgroup analyses were made based on some predefined criteria. The results of the subgroup analyses, presented in Table 2, show that the baseline serum hs-CRP, the quality assessment score, and the duration of trial were the sources of heterogeneity. The serum hs-CRP level decreased significantly in patients with the baseline hs-CRP level >3 mg/L (−1.31 (95% CI = −1.96, −0.67)). The supplementation with dose >150 mg/day caused a greater reduction in the serum level of hs-CRP [−0.29 (95% CI = −1.24, 1.82)] compared to the supplementation with dose ≤150 mg/day [−0.05 (95% CI = −1.67, 1.56)]. Furthermore, the level of hs-CRP serum reduced significantly when the duration of trial was >12 weeks ]−1.31 (95% CI = −1.96, −0.67)].
Table 2.
Subgroup analyses of CoQ10 supplementation on the serum of hs-CRP level
Discussion
To our knowledge, this is the first meta-analysis study that investigated the effect of CoQ10 supplementation on the serum level of hs-CRP in patients with CVDs. The results of this meta-analysis demonstrated that supplementation with CoQ10 had beneficial effects on those who consumed CoQ10 for more than 12 weeks and with the baseline level of hs-CRP greater than 3 mg/L.
In the pathogenesis of CVDs, inflammation plays a key role.[30,31] The serum level of hs-CRP cytokine increases in inflammatory conditions[5] and is related to developing CVDs.[32] In addition, CoQ10 influences inflammatory factors such as cytokines, so it can be useful for treating CVDs.[33,34] Furthermore, Schmelzer et al. indicated that the anti-inflammation effect of ubiquinone could be attributed to the reduction of nuclear factor-κB (NF-κB) dependent gene expression. NF-κB can increase pro-inflammatory cytokines expression. However, antioxidants such as CoQ10 can reduce the activity of this nuclear factor[35,36,37] and in turn decrease the risk of CVDs.
The pooled effect of all studies showed a nonsignificant mean difference for the serum level of hs-CRP in patients with CVDs. Consistent with this study, Lee et al. reported that supplementation with CoQ10 has no significant effect on the serum level of hs-CRP in patients with CVDs.[29] The absence of any significant association could be due to the short duration of the intervention and the insufficient number of individuals with high hs-CRP. Several studies performed on population with CVD showed that there was no significant difference in the serum level of hs-CRP between the intervention and placebo groups.[20,22,28] In line with these studies, Lee et al. demonstrated that a 300 mg/day CoQ10 supplementation for 12 weeks did not significantly decrease the serum level of interleukin-6 (IL-6) in patients with CVDs.[29] In another study, Zhao et al. found that ubiquinone supplementation can cause a significant reduction in the serum level of hs-CRP in heart failure patients. This change was also seen in the placebo group. In this study, the serum levels of IL-6 and tumor necrosis factor alpha (TNF-α) significantly decreased in the intervention and control groups.[21] It appears that supplementation with CoQ10 for a period of 24 weeks has been shown to have the best anti-inflammatory effect compared with other durations. Alehagen et al. also demonstrated that combined selenium and CoQ10 supplementation can reduce the serum level of hs-CRP in elderly individuals.[38] Consistent with this study, Lee et al. showed that that ubiquinone supplementation with the dose of 300 mg/day significantly decreased the serum level of TNF-α.[28]
This meta-analysis showed that there was a greater reduction in the serum level of hs-CRP in patients with supplementation more than 12 weeks. Zhao et al. found a noticeable reduction in the serum level of hs-CRP in the intervention group.[21] The intervention duration in the studies may be well enough to affect this inflammatory factor.
Strengths
The current research has some advantages. First, this is the first meta-analysis that surveyed the effect of CoQ10 supplementation on the serum level of hs-CRP in patients with CVDs. Second, a comprehensive and up-to-date search was performed. Therefore, all randomized trials were included in the study. Third, most studies included in the meta-analysis had high quality, so the results of the present study are assumed to be reliable. Fourth, the study indicated the CoQ10 supplementation may be useful for some patients with CVDs, especially those who receive this supplement for more than 12 weeks and with the baseline hs-CRP level of >3 mg/L.
Limitations
This meta-analysis study had certain limitations. First, the number of RCTs included in the analysis was not large and their sample size was also small. Second, the final amount of the serum level of hs-CRP in the intervention group after 12 months was not reported in one of the studies. Third, information about foods containing ubiquinone and the interaction of this supplement with nutrients and cardiovascular drugs was limited. These possible confounders can impact on the obtained results of the current study. Fourth, there was heterogeneity between the studies included in this meta-analysis, and the effect of this condition was minimized by applying a random-effects model and conducting subgroup analyses. Fifth, the dosages of used CoQ10 in some of the included studies might not be adequate enough to modify the serum level of hs-CRP significantly. Six, studies included in this meta-analysis included patients with various cardiovascular diseases (i.e., ischemic left ventricular systolic dysfunction, CAD, HF). This difference among diseases may affect their hs-CRP level and the association between CoQ10 supplementation and inflammatory state.
Conclusions
In conclusion, this systematic review and meta-analysis revealed that CoQ10 supplementation is effective and useful for CVD patients who take this supplement for more than 12 weeks and with the baseline serum levels of hs-CRP of greater than 3 mg/L.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors received no special grant funding for the research, authorship, and/or publication of this article.
References
- 1.Braunwald E. Cardiovascular medicine at the turn of the millennium: Triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–45. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 3.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–92. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto K, Sera Y, Abe Y, Tominaga T, Horikami K, Hirao K, et al. High serum concentrations of soluble E-selectin correlate with obesity but not fat distribution in patients with type 2 diabetes mellitus. Metabolism. 2002;51:932–4. doi: 10.1053/meta.2002.33354. [DOI] [PubMed] [Google Scholar]
- 5.Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berk BC, Weintraub WS, Alexander RW. Elevation of C-reactive protein in “active” coronary artery disease. Am J Cardiol. 1990;65:168–72. doi: 10.1016/0002-9149(90)90079-g. [DOI] [PubMed] [Google Scholar]
- 7.De Beer F, Hind C, Fox K, Allan R, Maseri A, Pepys M. Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br Heart J. 1982;47:239–43. doi: 10.1136/hrt.47.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietilä K, Harmoinen A, Hermens W, Simoons M, Van de Werf F, Verstraete M. Serum C-reactive protein and infarct size in myocardial infarct patients with a closed versus an open infarct-related coronary artery after thrombolytic therapy. Eur Heart J. 1993;14:915–9. doi: 10.1093/eurheartj/14.7.915. [DOI] [PubMed] [Google Scholar]
- 9.Singh U, Devaraj S. Vitamin E: Inflammation and atherosclerosis. Vitam Horm. 2007;76:519–49. doi: 10.1016/S0083-6729(07)76020-X. [DOI] [PubMed] [Google Scholar]
- 10.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events. Circulation. 2003;108:2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 11.Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003;41:S37–42. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 12.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 13.Bhagavan HN, Chopra RK. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–53. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 14.Singh U, Devaraj S, Jialal I. Coenzyme Q10 supplementation and heart failure. Nutr Rev. 2007;65:286–93. doi: 10.1301/nr.2007.jun.286-293. [DOI] [PubMed] [Google Scholar]
- 15.Alleva R, Tomasetti M, Battino M, Curatola G, Littarru G, Folkers K. The roles of coenzyme Q10 and vitamin E on the peroxidation of human low density lipoprotein subfractions. Proc Natl Acad Sci U S A. 1995;92:9388–91. doi: 10.1073/pnas.92.20.9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XL, Rainwater DL, Mahaney MC, Stocker R. Cosupplementation with vitamin E and coenzyme Q10 reduces circulating markers of inflammation in baboons. Am J Clin Nutr. 2004;80:649–55. doi: 10.1093/ajcn/80.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunitomo M, Yamaguchi Y, Kagota S, Otsubo K. Beneficial effect of coenzyme Q10 on increased oxidative and nitrative stress and inflammation and individual metabolic components developing in a rat model of metabolic syndrome. J Pharmacol Sci. 2008;107:128–37. doi: 10.1254/jphs.fp0072365. [DOI] [PubMed] [Google Scholar]
- 18.Fuller B, Smith D, Howerton A, Kern D. Anti-inflammatory effects of CoQ10 and colorless carotenoids. J Cosmet Dermatol. 2006;5:30–8. doi: 10.1111/j.1473-2165.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmelzer C, Lorenz G, Lindner I, Rimbach G, Niklowitz P, Menke T, et al. Effects of Coenzyme Q_ {10} on TNF-α secretion in human and murine monocytic cell lines. Biofactors. 2007;31:35–41. doi: 10.1002/biof.5520310104. [DOI] [PubMed] [Google Scholar]
- 20.Lee B-J, Huang Y-C, Chen S-J, Lin P-T. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition. 2012;28:767–72. doi: 10.1016/j.nut.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Q, Kebbati AH, Zhang Y, Tang Y, Okello E, Huang C. Effect of coenzyme Q10 on the incidence of atrial fibrillation in patients with heart failure. J Investig Med. 2015;63:735–9. doi: 10.1097/JIM.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 22.Pourmoghaddas M, Rabbani M, Shahabi J, Garakyaraghi M, Khanjani R, Hedayat P. Combination of atorvastatin/coenzyme Q10 as adjunctive treatment in congestive heart failure: A double-blind randomized placebo-controlled clinical trial. ARYA Atheroscler. 2014;10:1–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary.? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ. 2004;328:1–6. doi: 10.1136/bmj.38118.593900.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipsey MW, Wilson DB. Thousand Oaks, California: Sage publications; 2001. Practical Meta-analysis. [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Xu Y, Lu T, Gao F, Mo Z. Metan: Fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 28.Dai Y-L, Luk T-H, Yiu K-H, Wang M, Yip PM, Lee SW, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis. 2011;216:395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Lee B-J, Tseng Y-F, Yen C-H, Lin P-T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr J. 2013;12:1–9. doi: 10.1186/1475-2891-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakamoto N, Hoshino Y, Misaka T, Mizukami H, Suzuki S, Sugimoto K, et al. Serum tenascin-C level is associated with coronary plaque rupture in patients with acute coronary syndrome. Heart Vessels. 2014;29:165–70. doi: 10.1007/s00380-013-0341-2. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura A, Miura SI, Shiga Y, Norimatsu K, Miyase Y, Suematsu Y, et al. Is pentraxin 3 a biomarker, a player, or both in the context of coronary atherosclerosis and metabolic factors.? Heart Vessels. 2015;30:752–61. doi: 10.1007/s00380-014-0553-0. [DOI] [PubMed] [Google Scholar]
- 32.Koenig W, Sund M, Fröhlich M, Fischer H-G, Löwel H, Döring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeldt F, Haas S, Krum H, Hadj A, Ng K, Leong JY, et al. Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J Hum Hypertens. 2007;21:297–306. doi: 10.1038/sj.jhh.1002138. [DOI] [PubMed] [Google Scholar]
- 34.Bagheri Nesami N, Mozaffari-Khosravi H, Najarzadeh A, Salehifar E. The Effect of Coenzyme Q10 Supplementation on Pro-Inflammatory Factors and Adiponectin in Mildly Hypertensive Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Int J Vitam Nutr Res. 2015;85:156–64. doi: 10.1024/0300-9831/a000234. [DOI] [PubMed] [Google Scholar]
- 35.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Doring F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors. 2008;32:179–83. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 36.Schmelzer C, Lorenz G, Rinbach G, Doring F. In vitro effects of the reduced form of coenzyme Q(10) on secretion levels of TNF-alpha and Chemokines in response to LPS in the human monocytic cell line THP-1. J Clin Biochem Nutr. 2009;44:62–6. doi: 10.3164/jcbn.08-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang YP, Eber A, Yuan Y, Yang Z, Rodriguez Y, Levitt RC, et al. Prophylactic and antinociceptive effects of coenzyme Q10 on diabetic neuropathic pain in a mouse model of type 1 diabetes. Anesthesiology. 2013;118:945–54. doi: 10.1097/ALN.0b013e3182829b7b. [DOI] [PubMed] [Google Scholar]
- 38.Alehagen U, Lindahl TL, Aaseth J, Svensson E, Johansson P. Levels of sP-selectin and hs-CRP Decrease with Dietary Intervention with selenium and coenzyme Q10 Combined: A secondary analysis of a randomized clinical trial. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0137680. [DOI] [PMC free article] [PubMed] [Google Scholar]