Abstract
Phytopathogenic fungi can lead to significant cereal yield losses, also producing mycotoxins dangerous for human and animal health. The fungal control based on the use of synthetic fungicides can be complemented by "green" methods for crop protection, based on the use of natural products. In this frame, the antifungal activities of bergamot and lemon essential oils and of five natural compounds recurrent in essential oils (citronellal, citral, cinnamaldehyde, cuminaldehyde and limonene) have been evaluated against three species of mycotoxigenic fungi (Fusarium sporotrichioides, F. graminearum and F. langsethiae) responsible for Fusarium Head Blight in small-grain cereals. The natural products concentrations effective for reducing or inhibiting the in vitro fungal growth were determined for each fungal species and the following scale of potency was found: cinnamaldehyde > cuminaldehyde > citral > citronellal > bergamot oil > limonene > lemon oil. Moreover, the in vitro mycotoxin productions of the three Fusaria strains exposed to sub-lethal concentrations of the seven products was evaluated. The three fungal species showed variability in response to the treatments, both in terms of inhibition of mycelial growth and in terms of modulation of mycotoxin production that can be enhanced by sub-lethal concentrations of some natural products. This last finding must be taken into account in the frame of an open field application of some plant-derived fungicides.
Keywords: natural products, Fusarium graminearum, Fusarium sporotrichioides, Fusarium langsethiae, T-2, HT-2 toxins, deoxynivalenol (DON)
1. Introduction
Cereals are the most important food source worldwide, with a global 2016 production of 2600 million tonnes [1]. Cereal productions can be negatively affected by several diseases, sustained by fungi, bacteria and viruses. Among fungi that infect cereals in field, Fusaria are successful pathogens, characterized by considerable morphological and physiological variability, and capable of occupying many ecological niches in very different cereal cultivation environments [2,3]. In small-grain cereals, several Fusaria species are etiologic agents of Fusarium Head Blight (FHB), a complex disease resulting in relevant yield losses and in the contamination of the grains with mycotoxins, fungal secondary metabolites dangerous for human and animal health [4]. In particular, the three fungal species F. sporotrichioides, F. graminearum and F. langsethiae are involved in FHB in a wide range of cultivation environments, including Mediterranean ones, and are responsible for the production of A and B trichothecenes [5]. These sesquiterpenoid molecules contain a tetracyclic 12,13-epoxy ring responsible for the toxicological properties: group A includes T-2, HT-2 toxins, whereas group B includes deoxynivalenol (DON). Type-B trichothecenes seem to be prevalent in several cereal cultivation areas, but type-A trichothecenes have higher toxicity than those of the type-B group [6]. In particular, T-2 and HT-2 toxins are the most toxic among trichothecenes and therefore deserve special attention.
The control and the reduction of such contaminations are high priorities for the cereal production chain. Several different strategies can be adopted to mitigate the problem, including breeding resistant varieties, fungal populations monitoring, adoption of suitable agronomic practices and protocols for crop protection [7]. With reference to this latter point, demethylation inhibitors are among the synthetic fungicides effective in controlling FHB: molecules such as tebuconazole, protoconazole and metaconazole are widely used, alone or in combination [8]. Limitations of these molecules are their high cost, the fact that their effectiveness is strictly dependent on the timely application and on the evolution of resistant fungal populations. This latter possibility suggests that, to overcome multidrug resistance evolution, the range of synthetic fungicides should be widened and complemented with natural fungicides produced by the secondary metabolism of different plant species, such as essential oils and their components. Essential oils, in nature, play an important role in plant-pathogen interaction and in plant protection thanks to their antibacterial, antiviral, antifungal and insecticidal action. It has long been known that many plant essential oils have microbicidal properties [9], which has suggested their use in traditional medicines and in pharmacology, as reviewed by Sharifi-Rad et al. [10]. Several studies have been focused on evaluating the use of essential oils in food conservation [11] and, to a lesser extent, in crop protection [12]. Some of these natural compounds are now principal constituents of antifungal formulations, commercially available and used to inhibit storage fungi and to control various plant diseases [13,14]. Moreover, plant essential oils have been demonstrated to be a useful tool for the control of mycotoxigenic moulds, as reviewed by Prakash et al. [15].
In our study, we have considered seven natural products (bergamot and lemon essential oils, citronellal, citral, cinnamaldehyde, cuminaldehyde and limonene) that are recurrent in medicinal and aromatic plants and were selected taking into account their commercial availability, the fact that they are considered as GRAS (Generally Recognized As Safe) and their low costs.
Bergamot essential oil is extracted from pericarp and mesocarp of Citrus aurantium ssp. bergamia (Risso & Poiteau) fresh fruit and has limonene, linalyl acetate, linalool, γ-terpinene, and β-pinene as major components [16,17]. Its antimicrobial action has been demonstrated against a wide range of pathogens, including some plant pathogen fungi, such as Fusarium solani, F. sporotrichioides and F. oxysporum, and against food spoilage fungi belonging to the Aspergillus genus [18].
Lemon essential oil is derived from Citrus limon L. and is characterized by limonene, γ-terpinene, β-pinene, α-pinene, citral and myrcene as major components. Antifungal properties of lemon oil have been demonstrated against several phytopatogens, such as Aspergillus, Penicillium, Fusarium spp., Rhizopus spp. and Botrytis (reviewed by Jing et al.) [19].
Bergamot and lemon essential oils are mixtures of several components that exert antimicrobial action through complex and not yet completely understood molecular mechanisms, resulting in cell permeability alteration, cytoplasmic membranes damage, microbial cell death (reviewed by Calo et al.) [20].
Citronellal is an aldehyde and is the major component of Cymbopogon, lemon-scented gum (Eucalyptus citriodora), and lemon-scented teatree (Leptospermum petersonii) essential oils. Citronellal has been demonstrated effective for the control of rice pathogens, such as Rhizoctonia and Helminthosporium oryzae [21] and its antifungal activity results in plasma membrane damage, as demonstrated in Penicillium digitatum by Wang et al. [22].
Citral is a mixture of the monoterpenic aldehydes geranial and neral and is present in the essential oil of several plants, among others lemon myrtle, Litsea citrata, Litsea cubeba, lemongrass, lemon tea-tree, Ocimum gratissimum, Lindera citriodora, Calypranthes parriculata. Antifungal properties of citral have been demonstrated in six plant pathogenic fungi (Magnaporthe grisea, Gibberella zeae, Fusarium oxysporum, Valsa mali, Botrytis cinerea and Rhizoctonia solani) by Li et al. [23]. These researchers found that citral negatively affects the mycelia reducing sugar, soluble protein, chitinase activity, pyruvate content and MDA content of Magnaporthe grisea, even if the exact molecular mechanism of action still remains to be determined.
Cinnamaldehyde occurs in the essential oil present in bark of species belonging to the Cinnamomum genus and is currently widely used in cosmetic, food and pharmaceutical industries. Cinnamaldehyde interferes with enzymatic reactions of fungal cell wall synthesis, affecting the morphogenesis and growth in F. verticillioides, as demonstrated by Xing et al. [24]. In Aspergillus flavus, cinnamaldehyde inhibits radial growth, spore production, mycelium formation, and aflatoxin biosynthesis in a dose-dependent manner [25].
Cuminaldehyde is present in the essential oils of Cumin cyminum, Eucalyptus, Cinnamomum cassia and others medicinal plants. Marei et al. [26] demonstrated its strong antifungal property against the four plant pathogenic fungi Rhizoctonia solani, Fusarium oxysporum, Penicillium digitatum and Aspergillus niger.
These same fungi are inhibited even by limonene, which inhibits fungal enzymes such as pectin methyl esterase, cellulase and polyphenol oxidase, leading to cytoplasm granulation and cytoplasmic membrane damage [26]. Even spoilage mycotoxigenic moulds, such as Aspergillus flavus and A. parasiticus, are inhibited by limonene treatment [27]. Limonene is a monoterpene cyclic hydrocarbon present in the essential oils of several aromatic and medicinal plants, e.g., Citrus, Juniperus, Pinus, but is mainly produced as side product from the citrus juice industry.
The antifungal properties of several natural compounds are known; however, very limited knowledge is available on the effect of their sub-lethal concentrations on fungal populations. This aspect is of relevance in pathogen control strategies: in the case of demethylation-inhibiting fungicides, a major consequence of their sub-optimal applications has been identified in the development of quantitative resistance [28]. In the case of mycotoxigenic fungi, there are evidences that sub-optimal amounts of azole fungicides can increase mycotoxin production [29,30,31].
The aim of this work was to investigate the influence of seven natural products, including both essential oils and components of essential oils, on the three mycotoxigenic species F. sporotrichioides, F. graminearum and F. langsethiae. The biological activity of the seven compounds was evaluated measuring their impact on in vitro fungal mycelium growth and on mycotoxin production. In particular, the impact of sub-lethal concentrations of the natural products on the in vitro mycotoxin production was studied.
2. Results and Discussion
Bergamot oil, lemon oil, citronellal, citral, cinnamaldehyde, cuminaldehyde and limonene are all inhibitor of Fusaria growth in vitro, as reported in Table 1.
Table 1.
EC50 * and EC100 ** (expressed as mL of compounds per 100 mL medium) of the seven products on in vitro growth of F. sporotrichioides, F. graminearum and F. langsethiae.
| Fusarium sporotrichioides | Fusarium graminearum | Fusarium langsethiae | ||||
|---|---|---|---|---|---|---|
| EC50 | EC100 | EC50 | EC100 | EC50 | EC100 | |
| cuminaldehyde | 0.031 | 0.075 | 0.046 | 0.075 | 0.015 | 0.025 |
| cinnamaldehyde | 0.036 | 0.05 | 0.02 | 0.025 | 0.02 | 0.05 |
| lemon oil | 1.5 | >1.5 | 1.3 | >1.5 | 0.95 | >1.5 |
| citral | 0.067 | 0.15 | 0.05 | 0.1 | 0.027 | 0.05 |
| limonene | 1 | >1.5 | 0.5 | 0.75 | 0.5 | >1.5 |
| bergamot oil | 0.12 | 0.25 | 0.22 | 0.7 | 0.23 | 0.7 |
| citronellal | 0.32 | 0.5 | 0.12 | 0.25 | 0.11 | 0.5 |
* Effective Concentration (EC) for 50% fungal growth inhibition; ** Effective Concentration for 100% fungal growth inhibition.
The three fungal species showed different levels of sensitivity to the different treatments. The data obtained indicated that, among the different compounds, there is variability in the concentrations effective to reduce the mycelial growth of the three fungi.
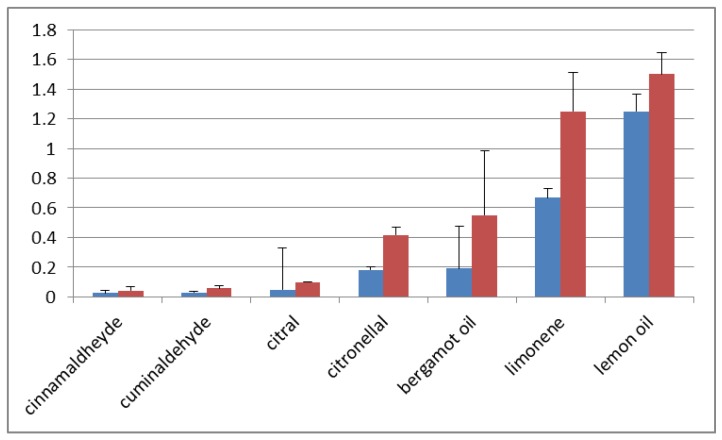
Figure 1 reports the mean concentrations able to reduce 50% and 100% fungal growth, calculated starting from the values obtained in all the fungal strains. The different natural products have different potency and the following scale can be indicated:
| cinnamaldehyde > cuminaldehyde > citral > citronellal > bergamot oil > limonene > lemon oil |
The group of aldehydes has therefore stronger antifungal activity in comparison with the hydrocarbon limonene and with the bergamot and lemon essential oils, in which hydrocarbons are the major components.
Figure 1.
Inhibitory concentrations of the natural products on Fusaria mycelial growth. The mean concentrations (expressed as mL of compounds/100mL medium) that give a 50% inhibition (blue bars) and a 100% inhibition (red bars) of the mycelium growth in vitro are shown. The values are calculated as means of the three Fusaria species.
This finding is in agreement with the scale of potency indicated by Kurita and Koike [32], further confirmed by Morcia et al. [33]:
| phenols > alcohols > aldehydes > ketones > ethers > hydrocarbons. |
According to our study, aldehydes are confirmed to be antifungal molecules characterized by higher level of potency in comparison with hydrocarbons. Lu et al. [34] agree that essential oils containing phenolic and aldehyde components (thymol, cinnamaldehyde, cuminaldehyde and citral-a) have higher level of antimicrobial activity against P. parasitica var. nicotianae than other essential oils containing alcohol and terpene compounds. Lee et al. [35], studing the antifungal activity of Myrtaceae essential oils, found that aldehyde and alcohol compounds are more toxic for Phytophtora infestans, Cryponectria parasitica and Fusarium circinatum than hydrocarbons. In agreement with our results, these authors observed that, among the aldehyde compounds evaluated, citral is more active than citronellal.
Our results confirm that the seven compounds tested are effective in contrasting Fusaria growth in vitro. A number of works report that several plant essential oils, at proper concentrations, inhibit both the fungal growth and the mycotoxin production, as reviewed by Prakash et al. [14]. However, the impact of sub-lethal concentrations on mycotoxin synthesis has not been so deeply studied. Therefore, we have quantified the in vitro mycotoxin productions of the three Fusaria strains when the fungi are growing in presence of sub-lethal concentrations of the seven products.
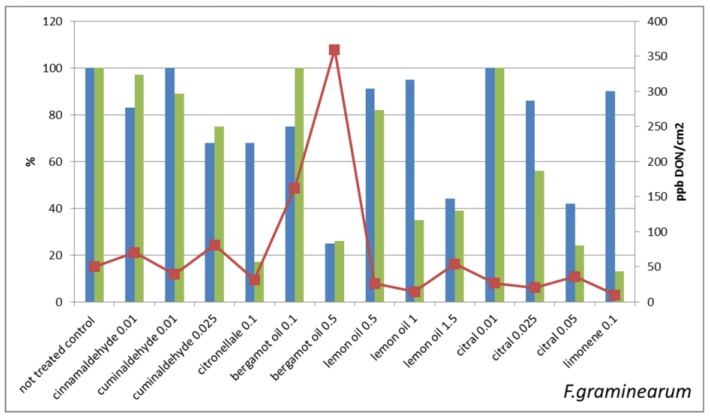
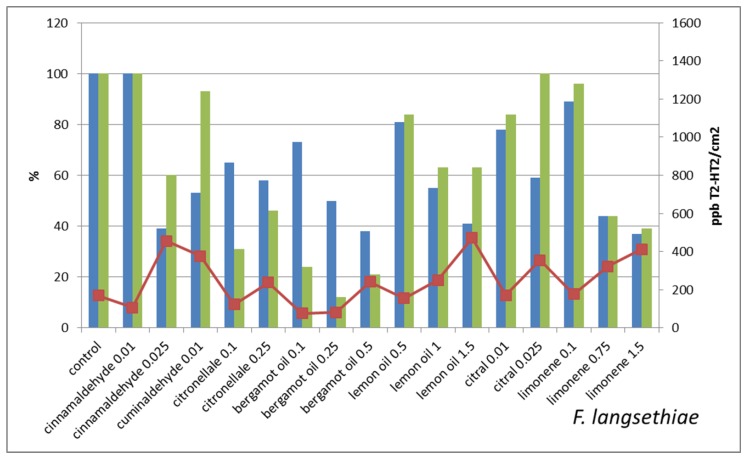
Figure 2 reports the mycelium growth of Fusarium graminearum (expressed as percentage of growth respect to control) and the DON production (expressed both as percentage of total DON production respect to control and as ppb of DON per mycelium unit area (cm2)). The correlation between the reduction of mycelium growth and of total mycotoxin production (r = 0.53) is significant at 0.05 level. In Fusarium langsethiae (Figure 3) the correlation between reduction of mycelium growth and of T-2,HT-2 production is significant at 0.05 level (r = 0.59).
Figure 2.
Impact of sub-lethal product concentrations on F. graminearum growth and mycotoxin production. Blue bars indicate the mean values of mycelium in vitro growth (expressed as percentage of growth respect to untreated control) of Fusarium graminearum treated with sub-lethal concentrations of the indicated natural products. Green bars represent the mycotoxin production (expressed as percentage of deoxynivalenol (DON) respect to untreated control) and the line chart indicates the DON concentration (expressed in ppb) per mycelium unit area (expressed in cm2).
Figure 3.
Impact of sub-lethal product concentrations on F. langsethiae growth and mycotoxin production. Blue bars indicate the mean values of mycelium in vitro growth (expressed as percentage of growth respect to untreated control) of Fusarium langsethiae treated with sub-lethal concentrations of the indicated natural products. Green bars represent the mycotoxin production (expressed as percentage of T-2, HT-2 respect to untreated control) and the line chart indicates the T-2, HT-2 concentration (expressed in ppb) per mycelium unit area (expressed in cm2).
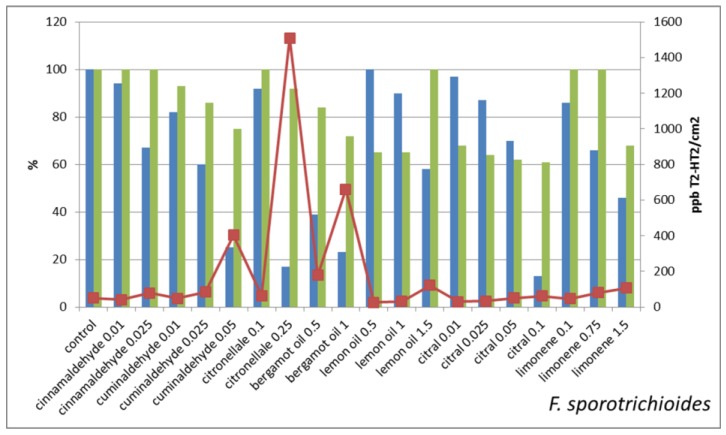
On the contrary, in Fusarium sporotrichioides (Figure 4) the correlation between reduction of mycelium growth and of T-2, HT-2 production is not significant at 0.05 level (r = 0.19).
Figure 4.
Impact of sub-lethal product concentrations on F. sporotrichioides growth and mycotoxin production. Blue bars indicate the mean values of mycelium in vitro growth (expressed as percentage of growth respect to untreated control) of Fusarium sporotrichioides treated with sub-lethal concentrations of the indicated natural products. Green bars represent the mycotoxin production (expressed as percentage of T-2, HT-2 respect to untreated control) and the line chart indicates the T-2, HT-2 concentration (expressed in ppb) per mycelium unit area (expressed in cm2).
These results suggest that the concomitant reduction of mycelium growth and of mycotoxin synthesis is not always occurring: some of the sub-lethal treatments seem to trigger mycotoxin synthesis. This is evident when the mycotoxin concentrations per cm2 of mycelium are considered. More in details, the Fusaria strains that we have treated with sub-lethal concentrations of antifungal molecules show different behaviors that can be schematized in four classes, i.e.:
the mycotoxin concentration/cm2 in treated is lower than in untreated control;
the mycotoxin concentration/cm2 in treated is very close to untreated control;
the mycotoxin concentration/cm2 is two–three times higher in treated in comparison with untreated control;
the mycotoxin concentration/cm2 in treated is orders of magnitude higher than in untreated control
The treatments that strongly reduce mycelium growth all belong to this last class, e.g., F. graminearum treated with 0.5% bergamot oil and F. sporotrichioides treated with 0.25% citronellal and with 1% bergamot oil. On the contrary, treatments that slightly reduce the mycelium growth result in lowering the mycotoxin production in comparison with untreated control, e.g., F. graminearum treated with cuminaldehyde 0.01% or with citral 0.01% or limonene 0.1% and F. sporotrichioides treated with lemon oil 0.5%.
The modulation of in vitro mycotoxin synthesis by sub-lethal concentrations of synthetic fungicides has been evaluated in several studies, obtaining contrasting results [36]. For example, inhibition or reduction of 3-ADON and of diacetoxyscirpenol have been observed in F. graminearum strains grown in presence of dicloram (500 μg/mL) or vinclozolin (250 μg/mL) [37] and of tebuconazole (1 μg/mL), thiabendazole (1 μg/mL), benomyl (1 μg/mL), or prochloraz (5 μg/mL) [38]. On the contrary, tebuconazole (0.1 μg/mL) and difenoconazole (0.1 μg/mL) increase the production of 3-ADON in cultures of F. culmorum [39]. Fusarium sporotrichioides in presence of sub-lethal level of tridemorph (30 to 50 μg/mL) [40] or carbendazim (5 μg/mL) [41] increases the T-2 toxin production. Magan et al. [42] reported that sub-optimal levels of fungicides stimulated DON production by F. culmorum in wheat grain. Kulik et al. [43] reported that sub-lethal concentrations of azoles induce tri genes transcription, resulting in increased level of trichothecenes in F. graminearum. Ochiai et al. [29] found that a sub-lethal dose of tebuconazole increases tri transcript level in F. asiaticum, and Becher et al. [44] observed higher level of NIV production in F. graminearum NIV chemotype adapted to sub-lethal concentrations of tebuconazole.
In general, mycelia growth retardation or inhibition due to essential oils treatments significantly decrease mycotoxin production [45]. However, the fungal growth inhibition and toxins production inhibition do not always occur together. Sumalan et al. [46] found that Thymus vulgaris, Mentha piperita and Cinnamomum zeylanicum produced the higher mycotoxin inhibition in Fusaria, but the most fungicidal effect was recorded for Salvia officinalis. Hope et al. [47] found that sub-optimal cinnamon oil concentration can significatively reduce the growth of F. culmorum and F. graminearum, but enhance their toxins production. Prakash et al. [15] found that low concentration of P. betle essential oil induces overproduction of AFB1 in A. flavus. Stimulation of aflatoxigenesis in Aspergilla has been observed even by Garcia et al. [48] in presence of E. arvense and S. rebaudiana extracts.
It is known that adverse environmental conditions, among others oxidative stress and N-starvation stress, can trigger mycotoxin production in fungi [49]. It can therefore be hypothesized that low fungicide doses can be a stress condition which increases the toxin synthesis as a defense mechanism by the fungus [17,24]. Moreover, the independency of the mycelium growth and toxin production cellular processes has been postulated by Ferruz et al. [50]. These authors in fact demonstrated a different modulation of fungal growth and T-2, HT-2 production in Fusarium langsethiae and F. sporotrichioides treated with different concentrations of phenolic acids. The regulation of toxin synthesis is in fact at transcriptional level and independent from that of fungal growth.
3. Material and Methods
3.1. Fungal Strains
Fusarium sporotrichioides (ITEM Collection n. 692), F. langsethiae (ITEM Collection n. 11020) and F. graminearum (ITEM Collection n. 6477) monosporic reference strains were kindly provided by Dr. Antonio Moretti, ISPA-CNR (Institute of Sciences for Food Production, National Research Council, Bari, Italy) and belong to the toxigenic fungi ISPA ITEM Collection.
3.2. Chemicals
The following compounds were purchased from Sigma-Aldrich S.r.l. (Milan, Italy): (+/−)- citronellal (code 27470); bergamot oil (code W215309); lemon oil (code W262528); citral (code W230316); cinnamaldehyde, (code W228613); cuminaldehyde (code 135178) and (R)–(+)–limonene (code 62118).
3.3. Evaluation of the Effect of Compounds on In Vitro Fungal Growth
Colony plugs (0.5 cm diameter) were extracted with a cork borer from the margin of fungal colonies grown on Potato Dextrose Agar (PDA) medium (Liofilchem, Teramo, Italy) for 1 week at 24 °C. The inocula were placed in the center of Petri dishes (one plug per plate) amended with 0.5% Tween 20 and with the products at percentages ranging from 0 to 1.5%. The plates were then sealed with Parafilm and incubated at 24 °C under fluorescent light (12 h photoperiod). The diameters of fungal colonies were measured in two perpendicular directions up to 6 days post-inoculation. All the experiments were conducted twice in triplicate. The effect of different molecules and of different concentrations of compounds was expressed as percentage inhibition, calculated according to the formula: I = [(C − T)/C] × 100, where I is the percentage of inhibition, C is the control plate colony diameter in mm, and T is the treated plate colony diameter in mm. The EC50 values were evaluated after probit/logit data linearization [51].
3.4. Evaluation of the Effect of Compounds on In Vitro Mycotoxin Production
The whole mycelium, including the underlying PDA, was collected from control and treated plates six days after inoculation with Fusaria.
T-2,HT-2 content was determined in plates inoculated with Fusarium sporotrichioides and Fusarium langsethiae, whereas DON content was determined in plates inoculated with Fusarium graminearum.
The collected samples were extracted with 5 volumes of 70% methanol for the analysis of T-2,HT-2 and 5 volumes of distilled water for the analysis of DON and vigorously shaken for 15 min before filtering.
The amount of T-2,HT-2 toxins (as sum of toxins) was determined using the kit Veratox® for T-2,HT-2, (Product code 8230, Neogen Corporation, Lansing, Michigan, USA) a competitive direct enzyme-linked immunosorbent assay (ELISA). The photometric reading was done at 630 nm, according to the manufacturer’s instructions.
The amount of DON was determined using the kit RIDASCREEN® DON (Product code R5906, R-Biopharm AG, Darmstadt, Germany), a competitive enzyme immunoassay (ELISA). The photometric reading was done at 450 nm, according to the manufacturer’s instructions.
The photometric readings were done with a ChroMate Model 4300 microplate reader (Awareness Technology INC. Palm City, FL, USA) and the toxin concentrations were calculated using the RIDA® SOFT Win. net software (code Z9996, R-Biopharm AG, Darmstadt, Germany).
4. Conclusions
We have demonstrated that cinnamaldehyde, cuminaldehyde, citral, citronellal, bergamot oil, limonene and lemon oil, at proper concentrations, can inhibit the in vitro mycelium growth of three Fusaria species responsible for mycotoxin contamination of cereals. However, at sub-lethal concentrations some of the treatments can increase mycotoxin production. This specific effect, observed only in some of the product–fungus combinations evaluated, deserves deeper study, in view of the practical application of such molecules for crop protection.
Acknowledgments
This work was supported by the “Molecole naturali per una cerealicoltura sostenibile” MAE project and partially funded by MIC CERES project.
Author Contributions
Valeria Terzi, Caterina Morcia and Roberta Ghizzoni conceived and designed the experiments; Roberta Ghizzoni and Assetou Bara performed the experiments; Giorgio Tumino analyzed the data; Valeria Terzi, Roberta Ghizzoni, Caterina Morcia and Nesrine Salhi wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest
Footnotes
Sample Availability: Samples of the fungal isolates are available from the authors.
References
- 1.Ward T.J., Clear R.M., Rooney A.P., O’Donnell K., Gaba D., Patrick S., Starkey D.E., Gilbert J., Geiser D.M., Nowicki T.W. An adaptive evolutionary shift in Fusarium Head Blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in north America. Fungal Genet. Biol. 2008;45:473–484. doi: 10.1016/j.fgb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Bottalico A., Perrone G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Path. 2002;108:611–624. doi: 10.1023/A:1020635214971. [DOI] [Google Scholar]
- 3.De Ruyck K., de Boevre M., Huybrechts I., De Saeger S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mut. Res./Rev. Mut. Res. 2015;766:32–41. doi: 10.1016/j.mrrev.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Foroud N.A., Eudes F. Trichothecenes in cereal grains. Int. J. Mol. Sci. 2009;10:147–173. doi: 10.3390/ijms10010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desjardins A.E., Hohn T.M., McCormick S.P. Trichothecene biosynthesis in Fusarium. Microbiol. Mol. Biol. Rev. 1993;57:595–604. doi: 10.1128/mr.57.3.595-604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzi V., Tumino G., Stanca A.M., Morcia C. Reducing the incidence of cereal head infection and mycotoxins in small grain cereal species. J. Cereal Sci. 2014;59:284–293. doi: 10.1016/j.jcs.2013.10.005. [DOI] [Google Scholar]
- 7.Mesterhazy Á. Control of Fusarium Head Blight of wheat by fungicides. In: Leonard K.J., Bushnell W.R., editors. Fusarium Head Blight of Wheat and Barley. The American Phytopathological Society; St. Paul, MN, USA: 2003. pp. 363–380. [Google Scholar]
- 8.Lang G., Buchbauer G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. Flavour Fragr. J. 2012;27:13–39. doi: 10.1002/ffj.2082. [DOI] [Google Scholar]
- 9.Sharifi-Rad J., Sureda A., Tenore G.C., Daglia M., Sharifi-Rad M., Valussi M., Tundis R., Sharifi-Rad M., Loizzo M.R., Ademiluyi A.O., et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules. 2017;22:70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burt S. Essential oils: Their antibacterial properties and potential applications in foods: A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Morcia C., Spini M., Malnati M., Stanca A.M., Terzi V. Essential oils and their components for the control of phytopathogenic fungi that affect plant health and agri-food quality and safety. In: Rai M., Chikindas M., editors. Natural Antimicrobials for Food Safety and Food Quality. CABI Press; Oxford, UK: 2011. pp. 224–241. [Google Scholar]
- 12.Dayan F.E., Cantrell C.L., Duke S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009;17:4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Isman M.B., Machial C.M. Pesticides based on plant essential oils: from traditional practice to commercialization. In: Rai M., Carpinella M.C., editors. Naturally Occurring Bioactive Compounds. Elsevier BV; Amsterdam, the Netherland: 2016. pp. 29–44. [Google Scholar]
- 14.Prakash B., Kedia A., Mishra P.K., Dubey N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities–Potential and challenges. Food Contr. 2015;47:381–391. doi: 10.1016/j.foodcont.2014.07.023. [DOI] [Google Scholar]
- 15.Avila-Sosa R., Navarro-Cruz A.R., Sosa-Morales M.E., Lopez-Malo A., Palou E. Bergamot (Citrus bergamia) Oils. In: Preedy V.R., editor. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; London, UK: 2016. pp. 247–252. [Google Scholar]
- 16.Dugo G., Bonaccorsi I. Citrus Bergamia: Bergamot and Its Derivatives. CCR Press; Boka Raton, FL, USA: 2013. [Google Scholar]
- 17.Stević T., Berić T., Šavikin K., Soković M., Gođevac D., Dimkić I., Stanković S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crops Prod. 2014;55:116–122. doi: 10.1016/j.indcrop.2014.02.011. [DOI] [Google Scholar]
- 18.Jing L., Lei Z., Li L., Xie R., Xi W., Guan Y., Zhou Z. Antifungal activity of citrus essential oils. J. Agric. Food Chem. 2014;62:3011–3033. doi: 10.1021/jf5006148. [DOI] [PubMed] [Google Scholar]
- 19.Calo J.R., Crandall P.G., O’Bryan C.A., Ricke S.C. Essential oils as antimicrobials in food systems–A review. Food Cont. 2015;54:111–119. doi: 10.1016/j.foodcont.2014.12.040. [DOI] [Google Scholar]
- 20.Ramezani H., Singh H.P., Batish D.R., Kohli R.K., Dargan J.S. Fungicidal effect of volatile oils from Eucalyptus citriodora and its major constituent citronellal. New Zealand Plant Prot. 2002;55:327–330. [Google Scholar]
- 21.Wu Y., OuYang Q., Tao N. Plasma Membrane Damage Contributes to Antifungal Activity of Citronellal Against Penicillium Digitatum. J. Food Sci. Technol. 2016;53:3853–3858. doi: 10.1007/s13197-016-2358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R.-Y., Wu X.-M., Yin X.-H., Long Y.-H., Li M. Naturally produced citral can significantly inhibit normal physiology and induce cytotoxicity on Magnaporthe grisea. Pest. Biochem. Physiol. 2015;118:19–25. doi: 10.1016/j.pestbp.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Xing F., Hua H., Selvaraj J.N., Zhao Y., Zhou L., Liu X., Liu Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Cont. 2014;46:343–350. doi: 10.1016/j.foodcont.2014.04.037. [DOI] [Google Scholar]
- 24.Sun Q., Shang B., Wang L., Lu Z., Liu Y. Cinnamaldehyde inhibits fungal growth and aflatoxin B1 biosynthesis by modulating the oxidative stress response of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2016;100:1355–1364. doi: 10.1007/s00253-015-7159-z. [DOI] [PubMed] [Google Scholar]
- 25.Marei G.I.K., Rasoul M.A.A., Abdelgaleil S.A. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pest. Biochem. Physiol. 2012;103:56–61. doi: 10.1016/j.pestbp.2012.03.004. [DOI] [Google Scholar]
- 26.Adegoke G.O., Evwiehuroma F.O., Afolabi M.O. African cardamom (Aframomum danielli) oils. In: Preedy V.R., editor. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; London, UK: 2016. pp. 163–171. [Google Scholar]
- 27.Deising H.B., Reimann S., Pascholati S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008;39:286–295. doi: 10.1590/S1517-83822008000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochiai N., Tokai T., Takahashi-Ando N., Fujimura M., Kimura M. Genetically engineered Fusarium as a tool to evaluate the effects of environmental factors on initiation of trichothecenes biosynthesis. FEMS Microbiol. Lett. 2007;275:53–61. doi: 10.1111/j.1574-6968.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 29.Audenart K., Callewaert E., Höfte M., De saeger S., Gaesaert G. Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Microbiol. 2010;10:112. doi: 10.1186/1471-2180-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popiel D., Dawidziuk A., Koczyk G., Mackowiak A. Multiple factes of response to fungicides-the influence of azole treatment on expression of key mycotoxin biosynthetic genes and candidate resistance factors in the control of resistant Fusarium strains. Eur. J. Plant Pathol. 2017;147:773–785. doi: 10.1007/s10658-016-1042-3. [DOI] [Google Scholar]
- 31.Kurita N., Koike S. Synergistic antimicrobial effect of ethanol, sodium chloride, acetic acid and essential oil components. Agric. Biol. Chem. 1983;47:67–75. [Google Scholar]
- 32.Morcia C., Malnati M., Terzi V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Add. Contam. 2012;3:415–422. doi: 10.1080/19440049.2011.643458. [DOI] [PubMed] [Google Scholar]
- 33.Lu M., Han Z., Yao L. In vitro and in vivo antimicrobial efficacy of essential oils and individual compounds against Phytophthora parasitica var. nicotianae. J. Appl. Microbiol. 2013;115:187–198. doi: 10.1111/jam.12208. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y.S., Kim J., Shin S.C., Lee S.G., Park I.K. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Frag. J. 2008;23:23–28. doi: 10.1002/ffj.1850. [DOI] [Google Scholar]
- 35.Edwards S.G., Pirgozliev S.R., Hare M.C., Jenkinson P. Quantification of trichothecene-producing Fusarium species in harvested grain by competitive PCR to determine efficacies of fungicides against Fusarium Head Blight of winter wheat. Appl. Environ. Microbiol. 2001;67:1575–1580. doi: 10.1128/AEM.67.4.1575-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasan H.A.H. Fungicide inhibition of aflatoxins, diacetoxyscirpenol and zearalenone production. Folia Microbiol. 1993;38:295–298. doi: 10.1007/BF02898597. [DOI] [PubMed] [Google Scholar]
- 37.Matthies A., Buchenauer H. Investigations on the action of different active ingredients on the biosynthesis of mycotoxins in Fusarium culmorum and Fusarium graminearum. In: Lyr H., Russell P.E., Sisler H.D., editors. Modern Fungicides and Antifungal Compounds. Intercept Ltd.; Andover, UK: 1996. pp. 199–204. [Google Scholar]
- 38.D’Mello J.P.F., Macdonald A.M.C., Postel D., Dijksma W.T.P., Dujardin A., Placinta C.M. Pesticide use and mycotoxin production in Fusarium and Aspergillus phytopathogens. Eur. J. Plant Pathol. 1998;104:741–751. doi: 10.1023/A:1008621505708. [DOI] [Google Scholar]
- 39.Moss M.O., Frank J.M. Influence of the fungicide tridemorph on T2 toxin production of Fusarium sporotrichioides. Trans. Br. Mycol. Soc. 1985;84:585–590. doi: 10.1016/S0007-1536(85)80111-X. [DOI] [Google Scholar]
- 40.Placinta C.M., Macdonald A.M.C., D’Mello J.B.F., Harling R. Proceedings of the Brighton Crop Protection Conference 1996: Pests and Diseases, Brighton, UK, 18–21 November 1996. British Crop Protection Council; Farnham, UK: 1996. The Influence of Carbendazim on Mycotoxin Production in Fusarium sporotrichioides; pp. 415–416. [Google Scholar]
- 41.Magan N., Hope R., Colleate A., Baxter E.S. Relationship between Growth and Mycotoxin Production by Fusarium species, Biocides and Environment. Eur. J. Plant Pathol. 2002;108:685–690. doi: 10.1023/A:1020618728175. [DOI] [Google Scholar]
- 42.Kulik T., Loiko M., Jestoi M., Perkowski J. Sublethal concentrations of azoles induce tri transcript levels and trichothecene production in Fusarium graminearum. FEMS Microbiol. Lett. 2012;335:58–67. doi: 10.1111/j.1574-6968.2012.02637.x. [DOI] [PubMed] [Google Scholar]
- 43.Becher R., Hettwer U., Karlovsky P., Deising H.B., Wirsel S.G. Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology. 2010;100:444–453. doi: 10.1094/PHYTO-100-5-0444. [DOI] [PubMed] [Google Scholar]
- 44.Da Cruz Cabral L., Fernández Pinto V., Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013;166:1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 45.Sumalan R.-M., Alexa E., Poiana M.-A. Assessment of inhibitory potential of essential oils on natural mycoflora and Fusarium mycotoxins production in wheat. Chem. Central J. 2013;7:32. doi: 10.1186/1752-153X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hope R., Cairns-Fuller V., Aldred D., Magan N. Use of antioxidants and essential oils for controlling mycotoxins in grain. BCPC Crop Sci. Technol. 2005;5B:429–436. [Google Scholar]
- 47.Garcia D., Ramos A.J., Sanchis V., Marí S. Effect of Equisetum arvense and Stevia rebaudiana extracts on growth and mycotoxin production by Aspergillus flavus and Fusarium verticillioides in maize seeds as affected by water activity. Int. J. Food Microbiol. 2012;153:21–27. doi: 10.1016/j.ijfoodmicro.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Reverberi M., Ricelli A., Zjalic S., Fabbri A.A., Fanelli C. Natural function of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010;87:899–911. doi: 10.1007/s00253-010-2657-5. [DOI] [PubMed] [Google Scholar]
- 49.Ferruz E., Atanasova-Pénichon V., Bonnin-Verdal M., Marchegay G., Pinson-Gadais L., Ducos C., Lorán S., Ariño A., Barreau C., Richard-Forget F. Effects of Phenolic Acids on the Growth and Production of T-2 and HT-2 Toxins by Fusarium langsethiae and F. sporotrichioides. Molecules. 2016;21:449. doi: 10.3390/molecules21040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finney D.J. Probit Analysis. Cambridge University Press; Cambridge, UK: 1971. [Google Scholar]
- 51.Crop Prospects and Food Situation. [(accessed on 28 July 2017)]; Available online: http://www.fao.org/3/a-i6903e.pdf.