Abstract
In this study, we performed the chemical characterization of Myrcia splendens (Sw.) DC. (Myrtaceae) essential oil from Amazonian Ecuador and the assessment of its bioactivity in terms of cytotoxic, antibacterial, and antioxidant activity as starting point for possible applicative uses. M. splendens essential oil, obtained by hydro-distillation, was analyzed by Gas Chromatography-Mass Spectrometry (GC-MS) and Gas Chromatography-Flame Ionization Detector (GC-FID): the major components were found to be trans-nerolidol (67.81%) and α-bisabolol (17.51%). Furthermore, we assessed the cytotoxic activity against MCF-7 (breast), A549 (lung) human tumor cell lines, and HaCaT (human keratinocytes) non-tumor cell line through 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) test: promising results in terms of selectivity and efficacy against the MCF-7 cell line (IC50 of 5.59 ± 0.13 μg/mL at 48 h) were obtained, mainly due to α-bisabolol. Furthermore, antibacterial activity against Gram positive and negative bacteria were performed through High Performance Thin Layer Chromatography (HPTLC) bioautographic assay and microdilution method: trans-nerolidol and β-cedren-9-one were the main molecules responsible for the low antibacterial effects against human pathogens. Nevertheless, interesting values of Minimum Inhibitory Concentration (MIC) were noticeable against phytopathogen strains. Radical scavenging activity performed by HPTLC bioautographic and spectrophotometric 1,1-diphenyl-2-picrylhydrazyl (DPPH) approaches were negligible. In conclusion, the essential oil revealed a good potential for plant defense and anti-cancer applications.
Keywords: Myrcia splendens, essential oil, cytotoxic activity, antibacterial activity, antioxidant activity, bioautographic assay
1. Introduction
Essential oils are mixtures of complex and fragrant substances, employed in many applicative fields, from aromatherapy to medicine and pharmacy, as well as the agro-food industry and phytoiatry [1]. The applicative importance of essential oils in such diversified fields relies mainly on their chemical characterization as mixtures of low molecular weight lipophilic substances and on their biological properties often due to synergistic effects of two or more constituents [2]. In fact, many research papers about essential oils focus on their chemical portrait and in vitro biological properties in terms of antioxidant, antimicrobial, and cytotoxic activities with the aim of detecting the most performance. One of the most interesting chemical aspects, emerging from all these researches, is their high chemodiversity, i.e., different composition, sometimes even dramatic, among phytocomplex from plants belonging to the same species, but grown in different geographical conditions and biodiversity contexts. This aspect is more evident for those species from areas characterized by high biodiversity, where the interaction among a higher number of different species results in a propulsive tool for secondary metabolites which give rise to remarkable molecular diversity and abundance in plants [3,4]. Biological activities of essential oils, instead, independently of the methodological target, are particularly focused on the detection of the compounds responsible of the activity and on the detection and quantification of possible synergistic evidences [2]. Among several kinds of biological activities, cytotoxic and antioxidant are the most interesting since the results could lead to deepened research about chemical compounds and/or chemical platforms for new pharmaceutical treatments of inflammation-related diseases, such as cancers [5]. Given all these premises, a chemical and bioactivity study has been performed on the essential oil of Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceae) obtained from plants grown in Amazonian Ecuador. Many neotropical Myrtaceae species are known for their traditional health uses, but some of them are scarcely investigated under the chemical point of view and as source of bioactive compounds. Fresh aerial parts of species belonging to Myrcia genus are employed in South American traditional medicine for the treatment of diabetes, hypertension, diarrhea, hemorrhage as infusion, and as a compress to treat inflammation and skin infections [6,7]. Myrcia splendens is a tree found in neotropic regions, distributed from Eastern Mexico to the South-Eastern Brazilian coastal forests. Crude drugs, derivatives, and preparations from Peruvian [8] and Brazilian plants have been studied and interesting cytotoxic activities were evidenced [9]. Since the Amazonian basin is one of the most important biodiversity hotspots world-wide, a first report on chemical characterization and biological properties of the essential oil of M. splendens from Ecuador was performed, with the aim to compare our data with those already reported by related literature.
2. Results
2.1. Chemical Composition of Essential Oil
The distillation of M. splendens leaves gave a colorless essential oil with a yield of 0.11% (w/v), which was lower than previously reported (0.35%) [10], and its density was 0.906 g/mL. The M. splendens essential oil composition is shown in Table 1.
Table 1.
Chemical composition of M. splendens essential oil and its characterization in term of active antioxidant and antibacterial compounds isolated respectively from DPPH-HPTLC (1,1-diphenyl-2-picrylhydrazil-high performance thin layer chromatography) and HPTLC bioautography assays.
| Antioxi. F. 5 | Antibact. F. 6 | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | % Area 1 | Component 2 | AI Exp 3 | AI Lit 4 | Rf = 0.9 | Rf = 0.45–0.6 | Rf = 0.7 | |
| 1 | 2.08 ± 0.10 | α-pinene | 928 | 939 | ||||
| 2 | 0.11 ± 0.01 | β-pinene | 972 | 979 | ||||
| 3 | 0.15 ± 0.02 | α-cubebene | 1350 | 1351 | 1.39 | |||
| 4 | 0.51 ± 0.03 | α-copaene | 1375 | 1377 | 4.21 | |||
| 5 | 0.11 ± 0.02 | β-elemene | 1387 | 1391 | 4.39 | |||
| 6 | 4.21 ± 0.15 | β-caryophyllene | 1409 | 1419 | 36.23 | |||
| 7 | 0.31 ± 0.02 | trans-α-bergamotene | 1430 | 1435 | 3.15 | |||
| 8 | 0.45 ± 0.03 | α-caryophyllene | 1449 | 1455 | 3.94 | |||
| 9 | 0.87 ± 0.06 | trans-β-farnesene | 1453 | 1457 | 7.81 | |||
| 10 | 0.65 ± 0.04 | germacrene D | 1475 | 1485 | 4.62 | |||
| 11 | 0.10 ± 0.01 | cis-β-guaiene | 1485 | 1493 | 1.54 | |||
| 12 | 0.17 ± 0.02 | viridiflorene | 1490 | 1497 | 1.43 | |||
| 13 | 0.11 ± 0.01 | α-muurolene | 1497 | 1499 | 1.27 | |||
| 14 | 0.59 ± 0.03 | α-bisabolene | 1503 | 1507 | 1.24 | |||
| 15 | 0.24 ± 0.02 | cis-γ-bisabolene | 1506 | 1515 | 8.33 | |||
| 16 | 0.53 ± 0.04 | δ-cadinene | 1513 | 1523 | 4.93 | |||
| 17 | 0.16 ± 0.01 | trans-calamenene | 1518 | 1529 | 1.66 | |||
| 18 | 1.03 ± 0.09 | trans-γ-bisabolene | 1523 | 1531 | 10.04 | |||
| 19 | 67.81 ± 2.10 | trans-nerolidol | 1562 | 1563 | 100 | |||
| 20 | 0.15 ± 0.01 | caryophyllene oxide | 1580 | 1583 | ||||
| 21 | Traces | β-cedren-9-one | 1630 | 1631 | 87.34 | |||
| 22 | 17.51 ± 1.01 | α-bisabolol | 1690 | 1686 | ||||
| Total identified | 97.84 | |||||||
1 Relative peak areas ± SEM (standard error media), calculated by GC-FID; 2 Components are listed in order of elution and their nomenclature is in accordance of the NIST (National Institute of Standards and Technology) library; 3 AI exp: arithmetic indices calculated on a Varian VF-5ms column; 4 AI lit: arithmetic indices [11]; 5 Antioxi. F.: fraction of compounds responsible for antioxidant activity. 6 Antibact. F.: fraction of compounds responsible for antibacterial activity.
Twenty-two compounds were characterized, corresponding to 97.84% of the total. Sesquiterpenes were predominant and particularly oxygenated sesquiterpenes, corresponding to 85.47% of the total. Monoterpenes, α- and β-pinene, were present in a lower amount (2.19% as total). Among the sesquiterpenes the most abundant were trans-nerolidol (67.81%), α-bisabolol (17.51%) and β-caryophyllene (4.21%). The predominance of sesquiterpenes is peculiar to the Myrcia genus [12]. M. splendens (syn. M. fallax) essential oil exhibited a different composition compared to related studies on the same species. In fact, Henriques et al. [10] found α-bisabolol (83%) as the main compound, while Nakamura et al. [13] found it to be α-bisabolene (80%); Alarcón et al. [7] stated that guaiol (31%) and carotol (9.9%) were the most abundant constituents. β-elemene was found as the main compound in the study of Lima et al. [14] and α-pinene characterized the essential oil obtained by Pereira et al. [15]. trans-nerolidol was the main compound (80.8%) of M. bracteata essential oil [16]. To the best of our knowledge, trans-nerolidol was found only in the M. splendens species of the present study. β-caryophyllene was already detected in M. glabra, M. multiflora, M. cuprea, and M. tomentosa [17] and α-pinene in M. bombycina and M. myrtifolia [18]. A deep inter- and intraspecific diversity in chemical composition of Myrtaceae essential oils has been reported by various authors. Data obtained on M. splendens essential oil from Ecuador seem to confirm this hypothesis. Previous studies suggested that intraspecific diversity of Myrtaceae terpene profiles could be a response of adaptation to a wide range of environmental conditions, in particular the monoterpene: sesquiterpene ratio seems to be influenced by the efficiency of terpene synthases enzymes [19]. Obtained results indicate that endogenous factors, as high genetic diversity, have more influence on the volatile oil variability of Myrtaceae family than environmental aspects [18]. In particular, the differences observed in chemical composition of M. splendens essential oils could also depend on Amazonian origin of the studied samples. In effect, the high biodiversity of Amazonian region induces plant secondary metabolism to biosynthetic pathways characterized by diversified chemical profile [3].
2.2. Cytotoxic Activity
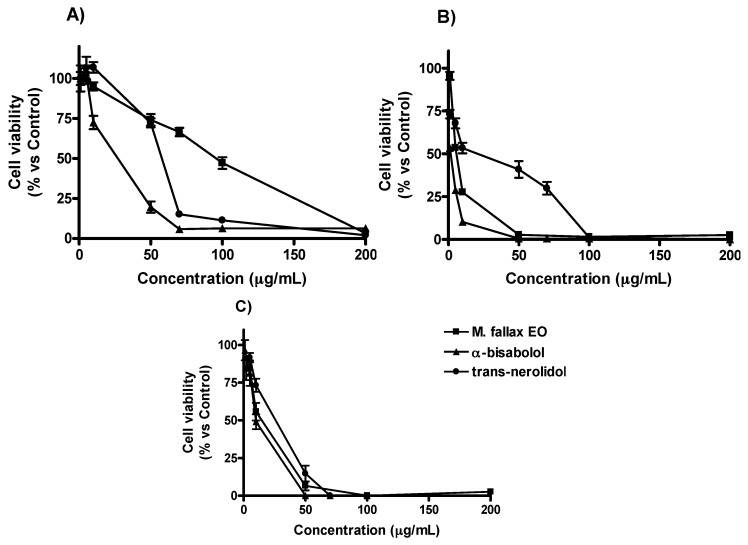
MTT assay was used to evaluate the effect of M. splendens essential oil and its main compounds, α-bisabolol and trans-nerolidol, on the cell viability of A549 (human lung cancer), MCF-7 (human breast adenocarcinoma) tumor lines and HaCaT (human keratinocytes) normal cells, as the reference to assess overall cytotoxic activity. All results showed (Figure 1) a dose-dependent inhibition of cell growth and, in particular, α-bisabolol was the most active component against the three studied cell lines, in the dilutions ranging from 1 to 200 µg/mL.
Figure 1.
Effects of M. splendens essential oil (EO), α-bisabolol and trans-nerolidol on viability of A549 (A), MCF-7 (B) and HaCaT (C) cell lines. Cytotoxicity was assessed by MTT test after 48 h.
Moreover, at the concentration of 10 µg/mL, α-bisabolol decreased the viability of A549, MCF-7, and HaCaT cell lines of 70%, 10% and 50%, respectively, in comparison with the negative control. The results highlighted that inhibition on cell growth was highly dependent on cell type with IC50 values ranging from 1.24 ± 0.03 to 100.99 ± 2.32 μg/mL. In particular, MCF-7 cancer cells showed more growth inhibition than HaCaT cells after 48 h of treatment with α-bisabolol (IC50 = 1.24 ± 0.03 μg/mL vs. IC50 = 10.15 ± 0.35 μg/mL) and essential oil (IC50 = 5.59 ± 0.13 μg/mL vs. IC50 = 21.58 ± 1.26 μg/mL) (Table 2). As reported in other studies, IC50 values lower than 30 μg/mL present a good chemopreventive potential for the essential oil [20]. However, the HaCaT cells showed to be more sensitive, with IC50 values ranging from 10.15 ± 0.35 to 27.76 ± 2.76 μg/mL, than the A549 cell line, with IC50 ranging from 54.28 ± 2.39 to 100.99 ± 2.32 μg/mL. Therefore, the evaluation of the cytotoxic activity revealed promising results in terms of selectivity and efficacy of M. splendens essential oil against the MCF-7 cell line rather than against the A549 (Table 2).
Table 2.
Cytotoxic activity of M. splendens EO, α-bisabolol and trans-nerolidol on three cell lines after 48 h.
| Cell Line (IC50 µg/mL) 1 | |||
|---|---|---|---|
| A549 2 | MCF-7 3 | HaCaT 4 | |
| M. splendens EO | 100.99 ± 2.32 6d | 5.59 ± 0.13 6c | 21.58 ± 1.26 6c |
| α-bisabolol | 27.63 ± 2.01 6b | 1.24 ± 0.03 6a | 10.15 ± 0.35 6b |
| trans-nerolidol | 54.28 ± 2.39 6c | 40.97 ± 5.07 6d | 27.76 ± 2.76 6d |
| doxorubicin 5 | 0.90 ± 0.01 6a | 2.10 ± 0.42 6b | 0.40 ± 0.01 6a |
1 IC50: compound concentrations that affords a 50% cell growth decrease after 48 h. IC50 are the averages of triplicate experiments and represented as mean ± standard deviation; 2 Adenocarcinoma cell line; 3 Breast adenocarcinoma cell line; 4 Keratinocytes cell line; 5 Doxorubicin was used as positive control; 6 Data are presented as mean ± SD, n = 3. Means in each column followed by different letter are significantly different (p < 0.05).
According to these results, M. splendens essential oil may be considered a promissory product to be used for innovative therapeutic or preventive strategies against breast carcinoma. Its cytotoxic activity may depend on the presence of α-bisabolol, which showed a very interesting IC50 value after 48 h (1.24 ± 0.03 µg/mL). Previous studies on α-bisabolol exhibited cytotoxic activity against glioma, pancreatic, ovarian, and kidney carcinoma cells [9,21,22]. Furthermore, it was reported as a chemopreventive agent in rat mammary carcinogenesis [23]. Thus the high content of α-bisabolol (17.51%) in the M. splendens essential oil could explain the cytotoxic activity against MCF-7 cells. On the other hand, data obtained on A549 were less interesting because the IC50 value (27.63 ± 2.01 μg/mL) was very close to 30 μg/mL, the concentration considered as the upper limit for potential chemopreventive activity. In the present study, trans-nerolidol exhibited a cytotoxic activity less interesting than α-bisabolol, nevertheless on A549 cells showed IC50 value at 48 h (54.28 ± 2.39 μg/mL) in line with the one recorded by Sylvestre et al. [24] (66 ± 12 μg/mL). Antineoplastic activity was also recorded on bowel by a mixture of cis- and trans-nerolidol [25], suggesting that deeper studies could be carried out in order to assess cytotoxic activity against other kinds of cancer cell lines.
2.3. Antibacterial Activity: HPTLC-Bioautography and MIC
Antibacterial properties of M. splendens essential oil was assessed against Gram positive and negative bacteria and MIC (Minimum Inhibitory Concentration) was determined by serial microdilution method. M. splendens essential oil exhibited a weak antimicrobial activity compared to chloramphenicol, used as a positive control, against all tested bacteria (Table 3).
Table 3.
Antibacterial activity of M. splendens essential oil expressed as MIC (Minimum Inhibitory Concentration) (μg/mL).
| Strain | M. splendens EO 1 | Antibiotic 2 | |
|---|---|---|---|
| Gram negative | |||
| Agrobacterium tumefaciens | DSM 30207 | 500 3c | 6.25 3c |
| Agrobacterium vitis | DSM 6583 | 2000 3e | 1.56 3a |
| Pseudomonas syringae pv. syringae | DSM 10604 | 250 3b | 3.12 3b |
| Escherichia coli | ATCC 4350 | >2000 3f | 25.00 3d |
| Pseudomonas aeruginosa | ATCC 27853 | >2000 3f | 6.25 3c |
| Gram positive | |||
| Clavibacter michiganensis subsp. nebraskensis | DSM 20400 | 125 3a | 6.25 3c |
| Enterococcus faecalis | ATCC 29212 | 2000 3e | 3.12 3b |
| Listeria grayi | DSM 20601 | 1000 3d | 5.00 3c |
| Staphylococcus aureus | ATCC 29230 | 1000 3d | 3.12 3b |
| Staphylococcus epidermidis | ATCC 14990 | 1000 3d | 3.12 3b |
1 EO: Essential Oil; 2 Chloramphenicol was used as positive control; 3 Means in each column followed by different letters are significantly different (p < 0.05).
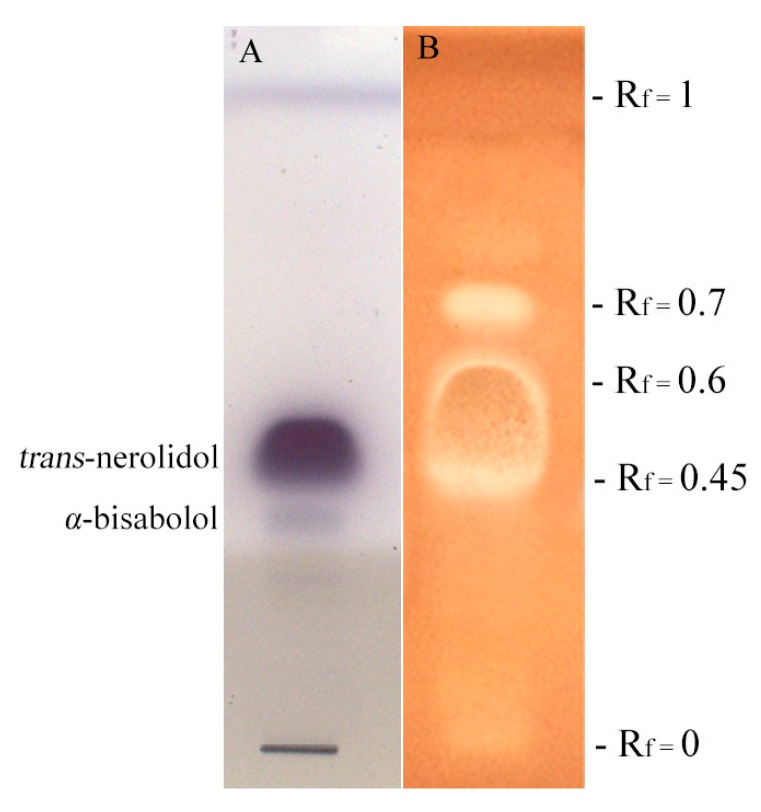
HPTLC-bioautography was performed to find the possible antibacterial fraction of essential oil (Figure 2).
Figure 2.
HPTLC (High Performance Thin Layer Chromatography) bioautographic assay performed for antibacterial activity on S. aureus, of M. splendens essential oil. Rf: retention factor: it represents the ratio between the migration distance of a substance and the migration distance of the solvent front. Rf composition is reported in Table 1. (A) HPTLC derivatized with vanillin-phosphoric acid reagent. (B) HPTLC performed for antibacterial activity on S. aureus.
However, results obtained indicated that phytopathogens strains were more sensitive than human pathogens after treatment with the tested essential oil. In particular, the Gram positive C. michiganensis subsp. nebraskensis resulted the most sensitivity, evidencing the lowest MIC value of 125 μg/mL. Gram negative bacteria showed resistance to M. splendens essential oil except for P. syringae pv. syringae that showed the lowest MIC value of 250 μg/mL. HPTLC-bioautography on S. aureus (Figure 2) exhibited two different areas corresponding to the fractions Rf 0.45–Rf 0.6 and Rf 0.7. The Rf 0.45–Rf 0.6 TLC area appeared as the most bioactive in terms of antibacterial activity and it was totally characterized by trans-nerolidol (100%). The fraction Rf 0.7 was mainly composed by β-cedren-9-one (87.34%). Data obtained suggested that trans-nerolidol is mainly responsible for the antibacterial activity of M. splendens essential oil that is in accordance with the results obtained by various authors. Simoes et al. [26] found that trans-nerolidol in combination with synthetic antibiotics ciprofloxacin, erythromycin, and gentamicin enhances their antibacterial activity against S. aureus and E. coli. Essential oils containing trans-nerolidol have been reported to inhibit the growth of quite a number of bacteria including S. aureus [27,28,29], S. epidermidis [30], E. coli, and P. aeruginosa [28,29]. Nerolidol (a mixture of cis- and trans-nerolidol) is also used as a food flavoring agent [27]. Considering β-cedren-9-one, the main component of Rf 0.7 fraction, few literature references are reported: to the best of our knowledge, this sesquiterpene is the main compound of Artemisias persica root essential oil [31], but no data are available for its antibacterial properties.
2.4. Antioxidant Activity
M. splendens essential oil exhibited antioxidant activity at higher concentration if compared to positive control. In fact, M. splendens essential oil showed a DPPH scavenging activity (IC50 = 43,537 ± 15 μg/mL) which is much lower than vitamin E (IC50 = 7.8 ± 0.5 μg/mL) (Table 4).
Table 4.
Antioxidant activity of M. splendens essential oil compared with the standard (vitamin E).
| Sample | IC50 (μg/mL) 1 |
|---|---|
| M. splendens EO | 43,537.00 ± 15 3b |
| vitamin E 2 | 7.8 ± 0.5 3a |
1 IC50: DPPH scavenging activity of EO was expressed as IC50 (µg/mL) value; 2 Vitamin E was used as positive control; 3 Data are presented as mean ± SD, n = 3. Means in each column followed by different letter are significantly different (p < 0.05).
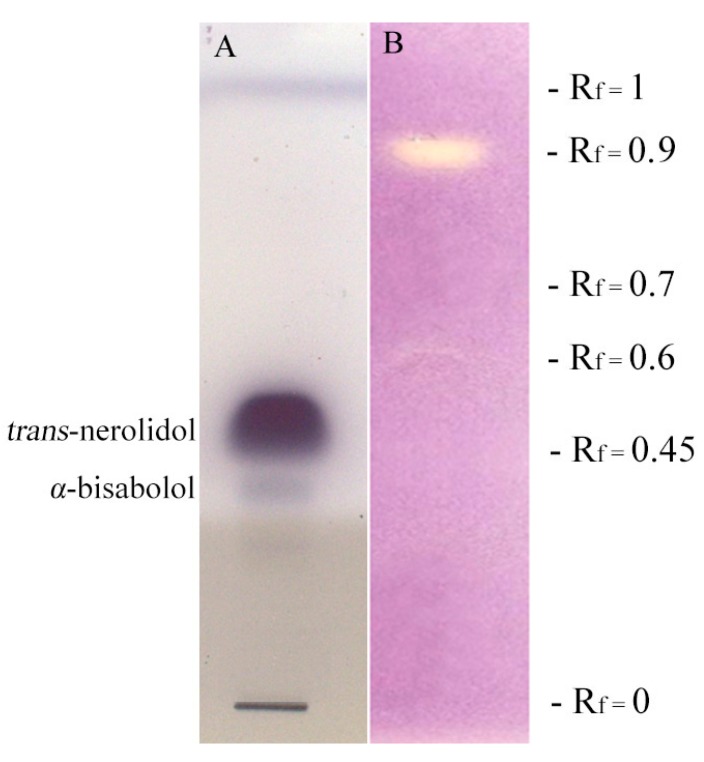
The DPPH-HPTLC (1,1-diphenyl-2-picrylhydrazil—high performance thin layer chromatography) bioautographic assay (Figure 3) indicated that β-caryophyllene (36.23%), trans-γ-bisabolene (10.04%), cis-γ-bisabolene (8.33%), and trans-β-farnesene (7.81%) were the major compounds responsible for the lower scavenging activity at Rf 0.9 (Table 1).
Figure 3.
HPTLC bioautographic assay performed for DPPH activity of M. splendens essential oil. Rf composition is reported in Table 1. (A) HPTLC derivatized with vanillin-phosphoric acid reagent; (B) HPTLC derivatized with solution of DPPH radical.
These sesquiterpenes are present at low amount in the essential oil and that may be the reason for the low antioxidant activity. In addition, other explanation could be that antioxidant activity is reduced by some compounds which exhibits oxidant activity. This hypothesis seems to be confirmed by previous research related to the evaluation of cytotoxic activity of Piper gaudichaudianum Kunth essential oil, rich in trans-nerolidol, against Saccharomyces cerevisiae. The cytotoxic effect increased according to the absence of the superoxide dismutase (SOD), indicating that essential oil and trans-nerolidol developed reactive oxygen species (ROS). ROS production was confirmed by 2′,7′-dichlorofluorescein dictate (DCF-DA) test, on strains that do not produce the SOD enzyme. Cytotoxicity of P. gaudichaudianum essential oil and trans-nerolidol depends on ROS and DNA single strand breaks generated by presence of oxidative lesions [32]. With these premises, the high concentration (67.81%) of trans-nerolidol found in Ecuadorian M. splendens essential oil could explain the weak antioxidant activity in the DPPH assay.
3. Materials and Methods
3.1. Plant Material
The leaves of M. splendens were collected at the CIPCA (Centre for Research, Postgraduate and Conservation of the Amazon) of the Universidad Estatal Amazónica (UEA) (01°14′13″ S, 077°53′25″ W, 570 m) in April 2013, from a wild population in the Amazonian region of Napo, Ecuador. Species authentication was performed by Dr. David Neill. Voucher specimens were deposited at the Herbarium ECUAMZ of the UEA, in Ecuador (voucher specimen: David Neill 17351).
3.2. Isolation of Essential Oil
The essential oil was obtained from fresh leaves by hydrodistillation for 2 h in a stainless steel distiller equipped with a Clevenger-type apparatus. Essential oil yield (0.11%) was calculated on a moisture-free basis and determined as average of three distinct distillations. The oil was dried using anhydrous sodium sulfate and stored in sealed amber vials at 4 °C, for further analysis.
3.3. Chemicals
All chromatographic grade organic solvents, all reference standards and reagents used for GC analysis and for biological activities were purchased from Sigma-Aldrich (Milan, Italy), microbial culture media from Oxoid (Milan, Italy).
3.4. GC and GC-MS Analysis of the Essential Oil
The essential oil was chemically analyzed and the relative peak areas for individual compounds were averaged. For the analysis a ThermoQuest GC-Trace gas-chromatograph (ThermoQuest Italia, Rodano, Italy) equipped with an FID detector and a Varian FactorFour VF-5ms poly-5% phenyl-95%-dimethylsiloxane column (internal diameter, 0.25 mm; length, 30 m; film thickness, 0.15 µm) were used. The following conditions were adopted: injector temperature 300 °C, FID temperature 300 °C, carrier (Helium) flow rate 1 mL/min and split ratio 1:50. Oven temperature was as follow: from 55 to 100 °C at a rate of 1 °C/min, from 100 to 250 °C at a rate of 5 °C/min and then kept constant at 250 °C for 15 min. The volume injected was 1 µL, previously dissolved in CH2Cl2. The oil percentage composition was performed by the normalization method from the GC peak areas, without using correction factors. The chemical characterization of essential oil compounds was performed by a Varian GC-3800 gas chromatograph (Palo Alto, CA, USA) equipped with a Varian MS-4000 mass spectrometer (Palo Alto, CA, USA) using electron impact and hooked to the NIST (National Institute of Standards and Technology) library. The conditions and column were the same described for GC analysis. The mass spectroscopy conditions were as follows: ionization voltage, 70 eV; emission current, 10 µAmp; scan rate, 1 scan/s; mass range, 29–400 Da; trap temperature, 150 °C, transfer line temperature, 300 °C. The essential oil components were characterized by comparing their relative retention time (AI) and the MS fragmentation pattern with those of other known essential oils, with pure compounds and by matching the MS fragmentations patterns and retention indices with the above mentioned mass spectra libraries, and with those in the literature [11]. The Arithmetic Index of the components was determined adding a C8–C32 n-alkanes (Sigma-Aldrich) to the essential oil before injecting in the GC-MS equipment and analyzed under the same conditions reported above [3].
3.5. In Vitro Cytotoxic Activity
The cytotoxic activity was performed on A549 (human lung cancer cell line), MCF-7 (human breast cancer cell line), and HaCaT (human keratinocytes) purchased at “Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia-Romagna” (Brescia, Italy) and it was expressed as the concentration of sample that inhibited 50% of cell growth (IC50). The cell lines were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine and 100 U/mL penicillin/streptomycin. They were grown in 75 cm2 flasks in an air atmosphere characterized by 5% humidity and 95% CO2 at 37 °C, until 80% confluence was reached. Cytotoxic activity was determined by MTT colorimetric assay [33] as reflected by the activity of succinate dehydrogenase. Cells were seeded in 96-well plates at a density of 2 × 104 cells/well in 200 µL DMEM (Dulbecco’s Modified Eagle Medium) complete medium; a period of 24 h was given for ensuring the cell attachment. Then the culture medium was replaced with 200 µL medium containing different concentrations (from 1 to 200 µg/mL) of M. splendens essential oil. In order to understand the role of α-bisabolol and trans-nerolidol in the cytotoxic activity, commercially available standards of those compounds were tested following the same methodology. Negative control was exposed to vehicle only, corresponding to a medium containing 2% FBS. The positive control used was doxorubicin. After 48 h, the culture medium was removed and washed with PBS (phosphate-buffered saline) twice. A lather of 20 μL of MTT (5 mg/mL in PBS) was added in each well and the plates were incubated for 4 h at 37 °C. The medium was removed and replaced with 100 μL of dimethylsulfoxide to dissolve the formazan crystals. The extent of MTT reduction was measured spectrophotometrically at 570 nm using a microplate reader.
3.6. Antibacterial Activity: HPTLC Bioautographic Assay
Bioautographic assay on a high performance thin layer chromatography (HPTLC) plate and the minimum inhibitory concentration (MIC) were performed in order to evaluate antibacterial activity of M. splendens essential oil [34]. Antibacterial activity was assessed against Gram negative Escherichia coli (ATCC 4350), Pseudomonas aeruginosa (ATCC 27853), Agrobacterium tumefaciens (DSM 30207), Agrobacterium vitis (DSM 6583), Pseudomonas syringae pv. syringae (DSM 10604) and Gram positive Listeria grayi (DSM 20601), Staphylococcus aureus (ATCC 29230), Staphylococcus epidermidis (ATCC 14990), Enterococcus faecalis (ATCC 29212), and Clavibacter michiganensis subsp. nebraskensis (DSM 20400).
The HPTLC bioautography was carried out on human pathogen S. aureus, with the aim of defining particular constituents of M. splendens essential oil that are able to determine the antibacterial activity. The bacterium choice was led because of its high performance features on HPTLC plate assay. The assay was performed as follows: 30 μL of a solution of M. splendens essential oil (110 mg/mL in ethanol) were applied on two separate HPTLC plates as 10 mm wide bands with Linomat V (Camag), while 10 μL of a methanol solution of trans-nerolidol (25 mg/mL) and 10 μL of a methanol solution of α-bisabolol (8 mg/mL) were applied on a third one on the same spot, as a 10 mm wide band. Then, all plates were eluted with a solvent solution containing toluene/ethyl acetate/petroleum ether (93/7/20). Finally, the solvent was completely dried maintaining plates for 30 min at room temperature: the first one was disposed in Petri dishes together with Nutrient Agar medium previously added with the bacterial inoculum (105 CFU/50 mL) and 0.25% of a 2,3,5-triphenyl-tetrazolium chloride water solution (20 mg/mL), as a growth indicator. Incubation occurred overnight at 37 °C. Antibacterial compounds, responsible for possible antibacterial properties, looked like yellow spots against a red colored background. The second plate was used to remove active spots on silica and to extract these in methanol solutions that was directly analyzed by GC-MS, while the third one was sprayed with vanillin-phosphoric acid reagent to visualize trans-nerolidol and α-bisabolol as reference standards [35].
3.7. Determination of Minimum Inhibitory Concentration (MIC)
The antibacterial activity was determined as MIC and analyzed by the microdilution method using 96-well micro titer plates [36]. Bacterial cultures were incubated overnight at 37 °C, in Tryptic Soy Broth, while Gram negative A. tumefaciens and A. vitis in Nutrient Broth. One hundred microliters of sterile medium together with 100 μL of serial dilutions of M. splendens essential oil previously dissolved in ethanol (100 mg/mL), were pipetted into all the micro-wells. Serial dilutions were prepared in order to obtain concentration ranges from 27 μg/mL to 2000 µg/mL. One hundred μL of bacterial culture standardized to 2 × 107 CFU/mL was added to the wells and incubated at 37 °C for 16 h and at 26 °C for 24 h, for human and phytopathogens, respectively. After the incubation period, 40 μL of water solution (20 mg/mL) of 2,3,5-triphenyl-tetrazolium chloride was added to each well and then incubated. Microbial growth was checked by a microplate reader at 615 nm, after 30 min of incubation. Chloramphenicol was used as a positive control [34].
3.8. Antioxidant Properties
Antioxidant activity was measured through 1,1-diphenyl-2-picrylhydrazil (DPPH) spectrophotometric assay (using UV-Vis spectroscopy) and DPPH-HPTLC bioautographic assay [34].
3.8.1. Spectrophotometric DPPH Assay
Ten microliters of M. splendens essential oil was dissolved into 900 µL of ethanol, then serial dilutions were assessed in order to obtain different concentrations (0.8–6.67 × 10−4 μL/mL). An aliquot (2.9 mL) of the ethanol solution of DPPH (4 mg/100 mL) was added to the essential oil solution. After a 30 min incubation, in an orbital shaker at 200 rpm, in the dark at room temperature, the mixture was placed in an UV-Vis spectrophotometer and the absorbance was read in triplicate against a blank at 517 nm. The DPPH inhibition in percent was determined by the following formula: IDPPH% = [1 − (A1/A2)] × 100. Where A1 was the DPPH absorbance with the essential oil and A2 without the essential oil. Vitamin E was used as positive control, according to its well-known antioxidant properties [37]. Essential oil antioxidant activity was expressed as IC50 (concentration providing DPPH 50% inhibition), calculated from inhibition curves obtained by plotting inhibition percentage against essential oil concentration. All experiments were assessed in triplicate and values were reported as mean ± SD (Standard Deviation).
3.8.2. DPPH-HPTLC Bioautography
DPPH-HPTLC-bioautography was assessed to determine the active compounds of the essential oil, responsible for possible radical scavenging activity. Twelve microliters of an ethanol solution of M. splendens essential oil (110 mg/mL) were applied twice to a silica gel HPTLC plate as 10 mm wide bands with Linomat V. Then, spots were eluted with a solvent solution (toluene/ethyl acetate/petroleum ether 93/7/20) in a chromatographic chamber. After development, the first chromatogram was sprayed with the DPPH ethanol solution (20 mg/100 mL) to detect possible antioxidant fractions. The active compounds appeared as yellow areas on a violet background. Isolation and identification of antioxidant compounds were carried out removing the TLC areas in the second chromatogram at Rf corresponding to positive spots and then extracting them with methanol. The solutions were analyzed by GC-MS. The third plate obtained from HPTLC bioautographic assay was used to show trans-nerolidol and α-bisabolol as reference standards.
3.9. Statistical Analysis
The experiments were performed in triplicate. IC50 values of anti-proliferative and antioxidant activities were assessed by logarithmic regression curves with 95% confident limits. Relative standard deviations and statistical significance (Student’s t test; p ≤ 0.05) were calculated using software STATISTICA 6.0 (StatSoft Italia srl, Vigonza, Italy).
4. Conclusions
The present study characterized the chemical profile of M. splendens essential oil from Ecuador for the first time and investigated its cytotoxic, antibacterial, and antioxidant properties. The essential oil of this plant revealed a good potential against the MCF-7 cell line and suggested that further deeper investigations of in vitro and in vivo anti-cancer studies are needed. M. splendens essential oil showed negligible antioxidant activity and low antibacterial effects against Gram positive and negative human pathogens, and moderate activity against phytopathogen strains. In addition, it was found that the chemical characterization of M. splendens essential oil from Amazonian Ecuador differs qualitatively and quantitatively from essential oils obtained by plants obtained of the same species but coming from different regions. Moreover, M. splendens essential oil, as a source of trans-nerolidol, should have pharmacological applications because of its enhancing properties on improving skin absorption of a drug with low lipophilic features [38].
Acknowledgments
This research has been supported by an internal grant from the Universidad Estatal Amazónica, Ecuador and grant (Acuerdo Especifico de Cooperacion UPS-UNIFE, 2015) of Universidad Politecnica Salesiana, Ecuador. The authors are grateful to Immacolata Maresca for her contribution to prepare figures for the first revision of manuscript.
Author Contributions
The contributions of authors are as follow: L.S., G.S. and A.Gu. conceived and designed the experiments and analyzed the data; L.S. collected the plant material and performed hydrodistillation; A.Gr. and J.B. performed the antimicrobial experiments and analyzed the data; A.S. and M.T. carried out the cytotoxic and antioxidant assays, and analyzed the data; D.N. collected the plant material and identified the plant; L.S. and A.Gu. wrote the paper. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the essential oil of Myrcia splendens are available from the authors.
References
- 1.Başer K.H.C., Buchbauer G. Introduction. In: Başer K.H.C., Buchbauer G., editors. Handbook of Essential Oils: Science, Technology and Applications. 1st ed. CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2010. pp. 1–2. [Google Scholar]
- 2.Bassolé I.H.N., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrini A., Rossi D., Grandini A., Scalvenzi L., Noriega Rivera P.F., Andreotti E., Tacchini M., Spagnoletti A., Poppi I., Maietti S., et al. Biological and chemo-diverse characterization of Amazonian (Ecuador) Citrus petitgrains. J. Appl. Bot. Food Qual. 2014;87:108–116. doi: 10.5073/JABFQ.2014.087.017. [DOI] [Google Scholar]
- 4.Fattahi B., Nazeri V., Kalantari S., Bonfill M., Fattahi M. Essential oil variation in wild-growing populations of a Salvia reuterana Boiss. collected from Iran: Using GC-MS and multivariate analysis. Ind. Crops Prod. 2016;81:180–190. doi: 10.1016/j.indcrop.2015.11.061. [DOI] [Google Scholar]
- 5.Bhalla Y., Gupta V.K., Jaitak V. Anticancer activity of essential oil: A review. J. Sci. Food Agric. 2013;93:3643–3653. doi: 10.1002/jsfa.6267. [DOI] [PubMed] [Google Scholar]
- 6.Alarcón L.D., Peña A.E., Gonzales de C.N., Quintero A., Meza M., Usubillaga A., Velasco J. Composition and antibacterial activity of the essential oil of Myrcia fallax (Rich.) DC. from Venezuela. Rev. Soc. Quim. Perú. 2009;75:221–227. [Google Scholar]
- 7.Stefanello M.E.A., Pascoal A.C.R.F., Salvador M.J. Essential oils from neotropical Myrtaceae: Chemical diversity and biological properties. Chem. Biodivers. 2011;8:73–94. doi: 10.1002/cbdv.201000098. [DOI] [PubMed] [Google Scholar]
- 8.Hecht S.M., Wofor A.G. Biologically Active Extracts from Myrcia fallax (Myrtaceae) Peru and Method of Obtaining Same. 4,451,459. U.S. Patent. 1984 May 29;
- 9.Stefanello M.E.A., Riva D., Simionatto E.L., De Carvalho J.E., Góis Ruiz A.L., Salvador M.J. Chemical composition and cytotoxic activity of essential oil from Myrcia laruotteana fruits. J. Essent. Oil Res. 2011;23:7–10. doi: 10.1080/10412905.2011.9700473. [DOI] [Google Scholar]
- 10.Henriques A.T., Sobral M., Bridi R., Vérin P., Menut C., Lamaty G., Bessiere J.M. Essential oils from five southern brazilian species of Myrcia (Myrtaceae) J. Essent. Oil Res. 1997;9:13–18. doi: 10.1080/10412905.1997.9700707. [DOI] [Google Scholar]
- 11.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Masspectrometry. 4th ed. Allured Publishing Co.; Carol Stream, IL, USA: 2007. [Google Scholar]
- 12.Stefanello M.E.A., Cervi A.C., Wisniewski A., Jr., Simionatto E.L. Composição e variação sazonal do oleo essencial de Myrcia obtecta (O. Berg.) Kiaersk. var. obtecta, Myrtaceae. Rev. Bras. Farmacogn. 2010;20:82–86. doi: 10.1590/S0102-695X2010000100017. [DOI] [Google Scholar]
- 13.Nakamura M.J., Monteiro S.S., Bizarri C.H.B., Siani A.C., Ramos M.F.S. Essential oils of four Myrtaceae species from the Brazilian southeast. Biochem. Syst. Ecol. 2010;38:1170–1175. doi: 10.1016/j.bse.2010.11.003. [DOI] [Google Scholar]
- 14.Lima G.S.L., Zoghbi M.G.B., Bastos M.N.C., Jardim M.A.G. Óleos Essenciais de Espécies de Eugenia L. e Myrcia DC. ex Guill. (Myrtaceae) Nativas da Restinga da APA de Algodoal-Maiandeua. In: Jardim M.A.G., editor. Diversidade Biológica das Áreas de Proteção Ambiental Ilhas do Combu and Maiandeua–Pará. MPEG; Belem, Brazil: 2009. pp. 405–424. [Google Scholar]
- 15.Pereira R.A., Zoghbi M.G.B., Bastos M.N.C. Essential oils of twelve species of Myrtaceae growing wild in the sandbank of the Resex Maracanã, State of Pará, Brazil. J. Essent. Oil Bear. Plants. 2010;13:440–450. doi: 10.1080/0972060X.2010.10643847. [DOI] [Google Scholar]
- 16.Carreira M.M., Zoghbi M.G.B., Andrade E.H.A., da Silva M.H.L., Maia J.G.S. Essential oils from three Myrcia species. Flav. Fragr. J. 2003;18:421–424. doi: 10.1002/ffj.1242. [DOI] [Google Scholar]
- 17.Sá F.A.S., Borges L.L., Paula J.A.M., Sampaio B.L., Ferri P.H., Paula J.R. Essential oils in aerial parts of Myrcia tomentosa: Composition and variability. Rev. Bras. Farmacogn. 2012;22:1233–1240. doi: 10.1590/S0102-695X2012005000120. [DOI] [Google Scholar]
- 18.Cerqueira M.D., Souza L.C., Passos M.G., Lima E., Poque N.F., Martins D., Guedes M.L.S., Cruz G.F. Seasonal variation and antimicrobial activity of Myrcia myrtifolia essential oils. J. Braz. Chem. Soc. 2007;18:998–1003. doi: 10.1590/S0103-50532007000500018. [DOI] [Google Scholar]
- 19.Padovan A., Keszei A., Külheim C., Foley W.J. The evolution of foliar terpene diversity in Myrtaceae. Phytochem. Rev. 2014;13:695–716. doi: 10.1007/s11101-013-9331-3. [DOI] [Google Scholar]
- 20.Talib W.H., Mahasneh A.M. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci. Pharm. 2010;78:33–45. doi: 10.3797/scipharm.0912-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamatou G.P.P., Viljoen A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010;87:1–7. doi: 10.1007/s11746-009-1483-3. [DOI] [Google Scholar]
- 22.Seki T., Kokuryo T., Yokoyama Y., Suzuki H., Itatsu K., Nakagawa A., Mizutani T., Miyake T., Uno M., Yamauchi K., Nagino M. Antitumor effects of α-bisabolol against pancreatic cancer. Cancer Sci. 2011;102:2199–2205. doi: 10.1111/j.1349-7006.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 23.Costarelli L., Malavolta M., Giacconi R., Cipriano C., Gasparini N., Tesei S., Pierpaoli S., Orlando F., Suzuki H., Perbellini L., et al. In vivo effect of α-bisabolol, a non toxic sesquiterpene alcohol, on the induction of spontaneous mammary tumors in HER-2/neu transgenic mice. Oncol. Res. 2009;18:409–418. doi: 10.3727/096504010X12671222663557. [DOI] [PubMed] [Google Scholar]
- 24.Sylvestre M., Pichette A., Lavoie S., Longtin A., Legault J. Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrina (L.) Coulter. Phytother. Res. 2007;21:536–540. doi: 10.1002/ptr.2095. [DOI] [PubMed] [Google Scholar]
- 25.Wattenberg L.W. Inhibition of azoxymethane-induced neoplasia of the large bowel by 3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene (nerolidol) Carcinogenesis. 1991;12:151–152. doi: 10.1093/carcin/12.1.151. [DOI] [PubMed] [Google Scholar]
- 26.Simoes M., Rocha S., Coimbra M., Vieira M. Enhancement of Escherichia coli and Staphylococcus aureus antibiotic susceptibility using sesquiterpenoids. Med. Chem. 2008;4:616–623. doi: 10.2174/157340608786242016. [DOI] [PubMed] [Google Scholar]
- 27.Braca A., Siciliano T., D’Arrigo M., Germanò M.P. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoterapia. 2008;79:123–125. doi: 10.1016/j.fitote.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Rahman A., Kang S.C. In vitro control of food-borne and food spoilage bacteria by essential oil and ethanol extracts of Lonicera japonica Thunb. Food Chem. 2009;116:670–675. doi: 10.1016/j.foodchem.2009.03.014. [DOI] [Google Scholar]
- 29.Ben Hsouna A., Hamdi N., Ben Halima N. Characterization of essential oil from Citrus aurantium L. flowers: Antimicrobial and antioxidant activities. J. Oleo Sci. 2013;62:763–772. doi: 10.5650/jos.62.763. [DOI] [PubMed] [Google Scholar]
- 30.Khaoukha G., Ben Jemia M., Smain A., Bruno M., Scandolera E., Senatore F. Characterization and antimicrobial activity of the volatile components of the flowers of Magydaris tomentosa (Desf.) DC. collected in Sicily and Algeria. Nat. Prod. Res. 2014;28:1152–1158. doi: 10.1080/14786419.2014.919289. [DOI] [PubMed] [Google Scholar]
- 31.Rustaiyan A., Faridchehr A. A review on constituents and biological activities of further Iranian Artemisia species. Int. J. Pharm. Biol. Chem. Sci. 2014;3:6–14. [Google Scholar]
- 32.Sperotto A.R.M., Moura D.J., Péres V.F., Damasceno F.C., Caramão E.B., Henriques J.A.P., Saffi J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem. Toxicol. 2013;57:57–68. doi: 10.1016/j.fct.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 34.Nostro A., Guerrini A., Marino A., Tacchini M., Di Giulio M., Grandini A., Akin M., Cellini L., Bisignano G., Saraçoğlu H.T. In vitro activity of plants extracts against biofilm-producing food-related bacteria. Int. J. Food Microbiol. 2016;238:33–39. doi: 10.1016/j.ijfoodmicro.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Wagner H., Bladt S. Plant Drug Analysis. A Thin Layer Chromatography Atlas. 2nd ed. Springer; Berlin, Germany: 1996. p. 364. [Google Scholar]
- 36.Clinical Laboratory Standards Institute (CLSI) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 9th ed. CLSI; Wayne, PA, USA: 2012. Approved Standard M07-A9. [Google Scholar]
- 37.Buchbauer G. Biological activities of essential oils. In: Başer K.H.C., Buchbauer G., editors. Handbook of Essential Oils: Science, Technology and Applications. 1st ed. CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2010. pp. 235–280. [Google Scholar]
- 38.Erdal M.S., Peköz A.Y., Aksu B., Araman A. Impacts of chemical enhancers on skin permeation and deposition of terbinafine. Pharm. Dev. Technol. 2014;19:565–570. doi: 10.3109/10837450.2013.813538. [DOI] [PubMed] [Google Scholar]