Abstract
Because of their great structural diversity and multitude of chemical properties, N-heterocyclic carbenes (NHCs) have been utilized in a variety of capacities. Most recently, NHCs have been utilized as carrier molecules for many transition metals in medicinal chemistry. Specifically, Ag(I)-NHCs have been investigated as potent antibacterial agents and chemotherapeutics and have shown great efficacy in both in vitro and in vivo studies. Ag(I)-NHC compounds have been shown to be effective against a wide range of both Gram-positive and Gram-negative bacterial strains. Many compounds have also shown great efficacy as antitumor agents demonstrating comparable or better antitumor activity than standard chemotherapeutics such as cisplatin and 5-fluorouracil. While these compounds have shown great promise, clinical use has remained an unattained goal. Current research has been focused upon synthesis of novel Ag(I)-NHC compounds and further investigations of their antibacterial and antitumor activity. This review will focus on recent advances of Ag(I)-NHCs in medicinal applications.
Keywords: silver, NHC, imidazolium salt, antibacterial, antitumor, chemotherapeutic
1. Introduction
Beginning with the successful isolation of the first stable N-heterocyclic carbene (NHC) by Arduengo in 1991 [1], NHCs have become a staple in research laboratories throughout the world. NHCs started as a mere laboratory curiosity but have evolved into a versatile organic ligand that has been used in a variety of capacities [2]. This is mainly due to their unparalleled structural diversity, as NHCs can be substituted with a variety of functional groups at various positions on the NHC core. They are also relatively easy synthetic targets. Due to the strong σ-donor capabilities as well as the ability to participate in π-back bonding, NHCs offer the synthetic versatility to bind both hard and soft metals [3,4].
Metal-NHC complexes have been primarily used in catalytic chemistry [5], however, more recently NHC ligands have been used as carrier molecules for metals in biological applications. A multitude of transition metals have been used in conjunction with NHCs including copper, silver, gold, platinum, palladium, ruthenium and others. The reported number of NHC-metal complexes is continually growing and several excellent reviews on this topic have previously been published [6,7,8,9,10,11,12,13,14].
While the majority of organometallic pharmaceutical research has been focused upon platinum and gold, the medicinal applications of silver are well documented [15]. Silver is relatively non-toxic to humans, although prolonged exposure can cause rare pigmentation of the skin and eyes [16]. While the mechanism of action of silver based pharmaceuticals has not been fully elucidated, it seems to involve the release of Ag(I) that can enter the cell membrane and disrupt its function [17]. Therefore, employing ligands that can strongly coordinate to the active Ag(I) ions is essential. NHC ligands have been utilized to form more stable Ag(I) compounds because of relatively strong silver-carbon bond and the NHC core provides the ability to be tailored for many different applications. This review will focus on the recent and most relevant advances in the medicinal applications of Ag(I)-NHC complexes including antibacterial and anticancer activity.
2. Antibacterial Activity of Silver NHC Complexes
The antibacterial properties of silver have been used throughout history [18,19]. The use of silver even predates modern civilizations, as early records describe water being stored in silver urns to prevent bacterial growth [20]. As early as 1840 silver nitrate was used to prevent infection in patients with severe burns [18]. Although silver was shown to be effective, with the discovery of penicillin and the production of many other penicillin-based antibiotics, the use of silver-based antimicrobials was significantly decreased. Soon after the wide spread use of penicillin and its derivatives, several bacterial strains that were resistant to these antibiotics emerged, including Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). This led to the resurgence of silver-based antimicrobial research, begun by Moyer in the 1960s [21] and quickly followed by the development by Fox of silver sulfadiazine, also known as Silvadene® (Figure 1) [22]. Silver therapeutics have been used in burn wards and treatment centers since 1968 and continue to be a first line of defense against infections. Although the antimicrobial properties of silver are well known, the mechanism of action has not been fully elucidated. While many reports and reviews indicate that Ag(I) is the bioactive species, studies suggest that Ag(I) ions kill organisms in a variety of ways, contributing to the lack of silver resistance that is observed [20,23,24].
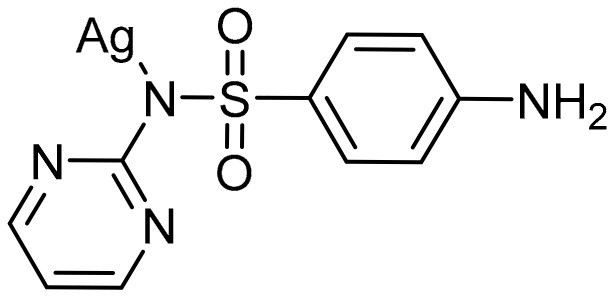
Figure 1.
Structure of silver sulfadiazine.
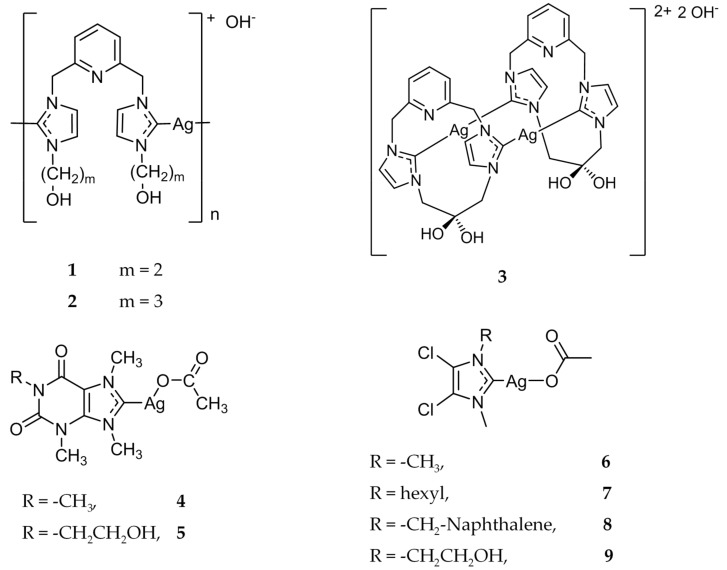
This long history of silver’s uses in the medicinal field encouraged the creation of the first Ag(I)-NHC complex with antimicrobial properties [25]. In 2004, Youngs and coworkers synthesized several pincer Ag(I)-carbene complexes (Figure 2) [26], and tested their antimicrobial properties against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Pseudomonas aeruginosa (P. aeruginosa). The minimum inhibitory concentration (MIC) of compounds 1 and 2 (Figure 2) were evaluated and showed better bacteriostatic activity than AgNO3, even at a much lower concentration. The success of this first Ag(I)-NHC antimicrobial was quickly followed by the development of the dinuclear species, 3 (Figure 2) [27]. The aqueous form of 3 was determined to be two times less effective as an antibiotic when compared to AgNO3. However, the antimicrobial activity of 3 was able to be enhanced via encapsulation in Tecophilic, a family of hydrophilic polyether-based thermoplastic aliphatic polyurethanes that are capable of being electrospun. The prepared nanofibers were again tested against E. coli, S. aureus, and P. aeruginosa for over a week with daily inoculations of freshly grown organisms. The embedded silver complex demonstrated a faster kill rate than Silvadene® and AgNO3, as well as having a prolonged release of the active silver species.
Figure 2.
Structures of Ag(I)-NHC complexes synthesized by Youngs and coworkers [26,27,28,29,34].
Further investigations of biologically active Ag(I)-NHC complexes led to the development of a series of xanthine derivatives, beginning with complex 4 (Figure 2) [28]. The MIC of complex 4 was tested against a variety of Gram-positive and Gram-negative bacteria, including E. Coli J53 strains, with and without the silver resistant plasmid pMG101. Complex 4 showed good activity against all pathogens tested with MIC values ranging from 1 to 8 μg/mL, with the exception of the E. Coli J53 strain with silver resistance (>5000 μg/mL). This further demonstrated that the bioactivity was primarily due to the Ag(I) cation. Complex 4 also showed antimicrobial activity against several strains of Burkholderia cepacia (B. cepacia), a highly resistant respiratory pathogen found in patients with cystic fibrosis. After the initial success of 4, a series of Ag(I)-NHC complexes were synthesized and their bioactivity was evaluated [29,30,31]. Of note, complex 5 showed efficacy comparable to 4, while also demonstrating increased water solubility. Complexes 4 and 5 were also found to be effective against B. cepacia in vivo through nebulization into the lungs of mice [32]. Of the mice that were infected with P. aeruginosa and subsequently treated with nebulized 4, 88% (22/25) survived after 72 h, whereas 62% (16/26) of water treated mice survived. In a separate study, of all the P. aeruginosa infected mice that were treated with nebulized 4, 83% (5/6) survived compared to 17% (1/6) of water treated mice [33]. Several of the Ag(I)-NHC complexes were tested against MRSA, as well as the potential bioterrorism agents Burkholderia pseudomallei, Burkholderia mallei, Bacillus anthracis, and Yersinia pestis [34]. Compounds 6–9 (Figure 2) demonstrated MIC values of <6 μg/mL when tested against B. pseudomallei and B. mallei. Compounds 4 and 6 were also tested against two attenuated strains of Y. pestis, YP1-1 and YP8-1, and showed MIC values of 1 μg/mL.
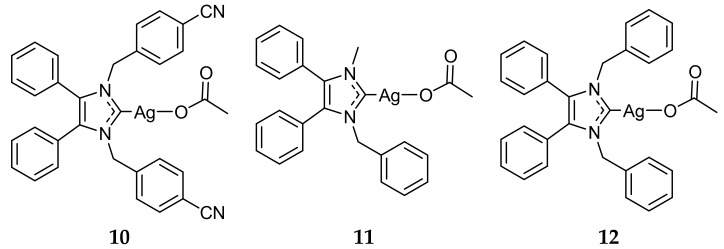
Once the utility of Ag(I)-NHC complexes was established, the synthesis of these compounds garnered much interest abroad. Tacke and coworkers synthesized a vast library of Ag(I)-NHC acetate complexes, the majority containing large lipophilic substituents [35,36,37,38,39,40,41]. The antibacterial properties of many of these complexes were tested against E. coli and S. aureus using the qualitative Kirby-Bauer disk-diffusion method. The majority of the Ag(I)-NHC compounds exhibited low to medium activity (4–7 mm of clearance) against both Gram-positive and Gram-negative bacteria, while their NHC precursors showed significantly lower activity, again indicating the utility of Ag(I) ions in antimicrobial Ag(I)-NHC complexes. Several compounds, including 10 and 11 (Figure 3), showed high antibacterial activity with areas of clearance of 10–12 mm. The improved antibacterial properties were attributed in part to the lipophilicity of these complexes, which allowed for increased penetration through the lipid membrane. This led to the development of the lead antimicrobial compound for the Tacke research group, complex 12 (Figure 3) [42,43]. While 12 exhibited medium activity against E. coli, showing 7 mm area of clearance, the complex was highly active against S. aureus with 15 mm of clearance. Complex 12 was further tested quantitatively against several Gram-positive and Gram-negative bacterial strains and was found to have MIC values ranging from 3.13 to 20 μg/mL, including being active against MRSA with an MIC value of 12.5 μg/mL [44]. The antimicrobial activity of 12 was also evaluated in vivo using Galleria mellonella larvae. Larvae inoculated with S. aureus and Candida albicans and subsequently treated with 12 showed an increased survival rate over those untreated [45].
Figure 3.
Structures of Ag(I)-NHC complexes synthesized by Tacke and coworkers [42,43].
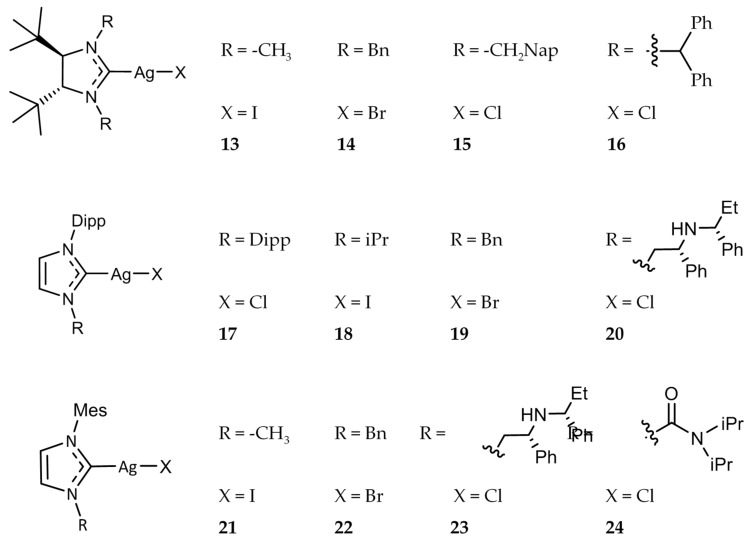
Roland et al. synthesized a series of Ag(I)-NHC halide complexes (Figure 4) and evaluated their antibacterial activity [46]. The compounds were tested against E. coli and S. aureus as well as several resistant strains including S. aureus NorA, a ciprofloxacin resistant strain that overexpresses the multidrug efflux pump NorA, and S. aureus MsrA, which contains the plasmid pUL5054 that gives rise to erythromycin resistance. Complexes 13–16 and 18–24 (Figure 4) demonstrated high activity against E. coli and S. aureus with MIC values ranging from 4 μg/mL to 32 μg/mL. Complex 17 showed no significant activity against E. coli with an MIC value of >128 μg/mL. All of the complexes tested, including 17, were found to have significant activity against both resistant strains as well, with a range of MIC values from 1 μg/mL to 32 μg/mL.
Figure 4.
Structures of Ag(I)-NHC complexes from Roland and Jolivalt [46].
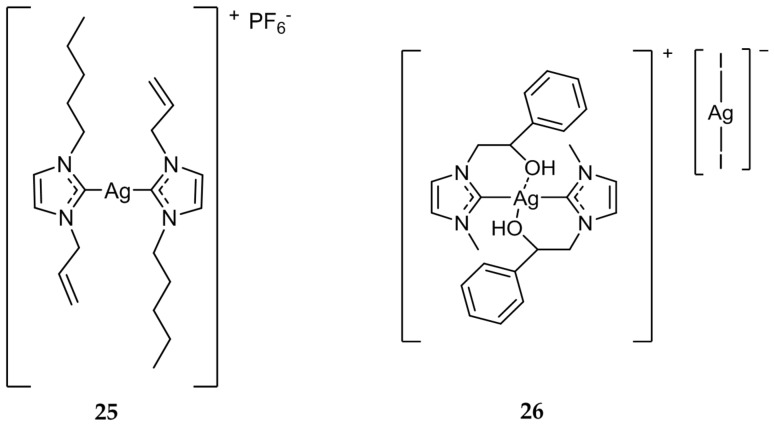
While the majority of the Ag(I)-NHC compounds that have been evaluated are mono-NHC systems, there have been many bis-NHC systems developed as well [47,48,49]. In 2014, Haque et al. synthesized a series of bis-NHC systems and evaluated their antibacterial properties and subsequent nuclease activity [50,51]. Of the compounds synthesized and tested, 25 (Figure 5) was shown to not only be the most effective antibacterial complex (MIC = 12.5 μg/mL), but also was the most efficient in promoting the cleavage of nucleic acids. This correlation could give some indication about the mechanism of action of Ag(I)-NHC antimicrobials.
Figure 5.
Structure of bis-NHC complexes from Haque (25) and Napoli (26) [51,52].
Napoli et al. developed several compounds that contained an alcohol group on an alkyl substituent of one of the two nitrogens of the NHC core [52]. Compound 26 (Figure 5) inhibited the growth of both E. coli and B. subtilis at a concentration of 5 μg/mL, the highest activity of any bis-NHC complex that was evaluated in the study. Compound 26 was also shown to be stable to hydrolysis, which could lead to slow release of bioactive Ag(I) at the wound site, further showing the utility of Ag(I)-NHC compounds as antimicrobial agents.
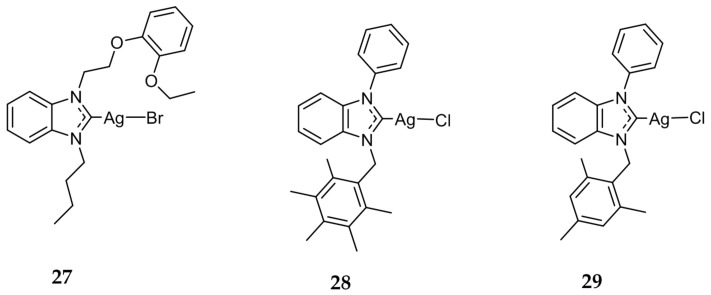
A series of silver benzimidazole compounds were synthesized by Özdemir et al. and tested against several bacterial strains including Enterococcus faecalis, S. aureus, E. coli, and P. aeruginosa [53,54]. While none of the compounds outperformed the standard antimicrobials ampicillin or ciprofloxacin, it was determined that the presence of electron-donating and bulky substituents attached to the nitrogen of the benzimidazole ring increases the antimicrobial activity of the compound. The most lipophilic compound, 27 (Figure 6), was found to be the most active with MIC values ranging from 6.25 μg/mL to 12.5 μg/mL against the series of bacterial strains.
Figure 6.
Structures of benzimidazole Ag(I)-NHC complexes from Özdemir (27) [54] and Gök (28 and 29) [55].
In a separate study performed by Gök et al. in 2014, phenyl-substituted benzimidazole silver complexes were tested for their antibacterial properties against several Gram-positive and Gram-negative bacterial strains [55]. While the Ag(I)-NHC compounds showed increased antimicrobial activity when compared the NHC precursors, they displayed lower activity when compared to the standard tetracycline. Similarly to the previous study conducted by Özdemir et al. the most lipophilic compounds 28 and 29 (Figure 6) were found to be the most active.
3. Antitumor Activity of Silver NHC Complexes
With the serendipitous discovery of cisplatin in the late 1960s, the landscape of cancer chemotherapy was forever changed [56,57]. A research field that was previously dominated by organic compounds and natural products shifted towards the inclusion of metallo-pharmaceuticals. With platinum being at the forefront of research, many other platinum-based compounds were approved for antitumor therapy including carboplatin and oxaliplatin [58]. While the curative effects are well documented, the drawbacks of platinum-based pharmaceuticals are numerous. Many cancer types are not susceptible to platinum drugs and there are many toxic side effects, including gastrointestinal and hematological toxicity [59]. Additionally, many cancers have either acquired or intrinsic resistance to cisplatin and other platinating agents [60]. Because of this, current anticancer research has been devoted to the discovery of novel transition metal compounds. Due to their synthetic versatility and strong, stable metal-ligand bond, NHC-metal complexes have become a vastly increasing class of anticancer agents. While silver was originally investigated because of its advantageous antimicrobial activity, there has been a recent interest in its anticancer properties.
Youngs and coworkers were the first to report the anticancer activity of Ag(I)-NHC compounds with a series of 4,5-dicholorimidazole based Ag(I)-NHC compounds [61]. Compounds 6–8 (Figure 2), previously discussed for their antimicrobial properties, were evaluated for their in vitro antitumor activity against the cancer cell lines OVCAR-3 (ovarian), MB157 (breast), and HeLa (cervical). The concentrations at which the compound inhibits 50% of cell growth (IC50) as compared to a control were compared to both cisplatin and AgNO3. Cisplatin was determined to be the most effective against the ovarian cancer cell line with an IC50 value of 12 μM, compared to 20–25 μM for the silver complexes. Compounds 6–8 were also determined to be ineffective against the HeLa cell line (>200 μM). However, the silver compounds were most active against MB157, having IC50 values of 8 μM, 20 μM, and 10 μM for 6–8 respectively, compared to an IC50 value of 25 μM for cisplatin.
With the in vitro success of these compounds, an in vivo xenograft model was developed utilizing OVCAR-3 and 6. OVCAR-3 cells were injected subcutaneously into the back of nude mice. After tumor growth became visible, 6 was injected at the tumor site. The mice were necropsied to determine the overall effect of 6 on the tumors as well as healthy organs. According to the pathological studies, significant tumor cell death was observed while no ill-effects to the major organs of the mice were detected.
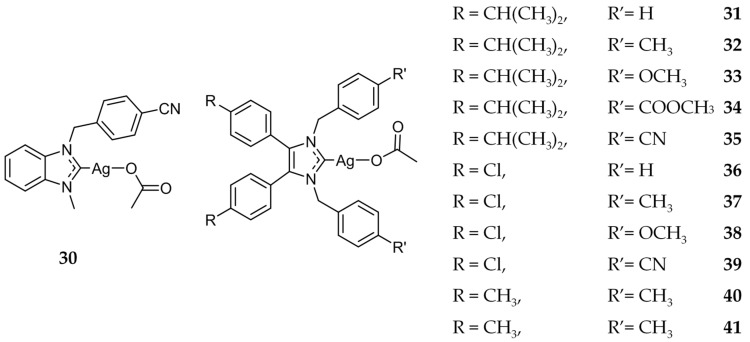
After this initial success of Ag(I)-NHCs, Tacke and coworkers synthesized a series of cyanobenzyl-NHC silver complexes and evaluated their anticancer activity [43,62,63]. The in vitro cytotoxicity of these compounds were evaluated against the human cancerous renal cell line, Caki-1. From these studies, compound 30 (Figure 7) was shown to have an IC50 value of 1.2 ± 0.6 μM, an approximately three-fold increase compared to cisplatin (3.3 μM). It was further shown that 30 was effective in vitro against platinum-resistant cell lines UKF-NB-3 (neuroblastoma) and HCT8 (colon), as well as paclitaxel-resistant cell line PC-3 (prostate) [64]. While these in vitro results were encouraging, in vivo testing of 30 showed no tumor reduction in tumor bearing mice.
Figure 7.
Structures of Ag(I)-NHC complexes from Tacke and coworkers [43,62,63].
Tacke and coworkers also investigated the potential of benzyl bearing NHC compounds as antitumor agents, (compounds 31–41, Figure 7), similar to 12 (Figure 3) discussed previously for its antimicrobial activity [40,41]. Of these compounds, 41 demonstrated the highest in vitro activity with IC50 values of 0.51 ± 0.07 μM and 1.4 ± 0.1 μM against Caki-1 and MCF-7 (breast) cell lines respectively. Additionally, the NHC precursor to 41 was also determined to be highly active against Caki-1 (IC50 = 4.8 ± 0.3 μM), indicating a synergistic effect with the NHC ligand and the silver component.
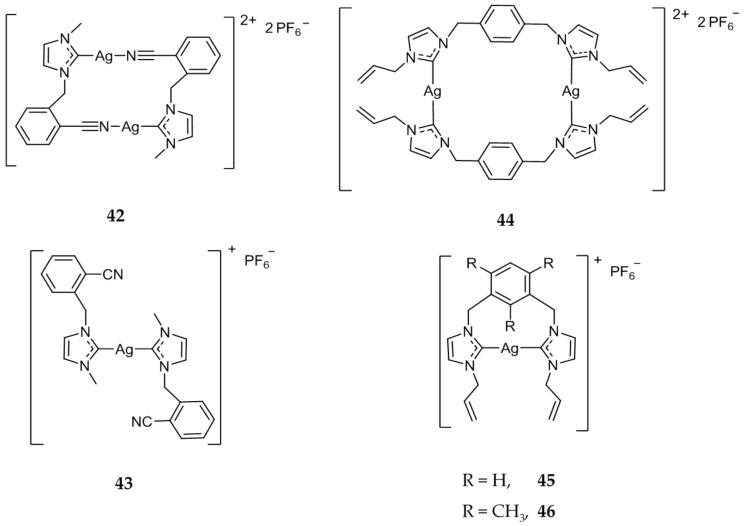
An extensive library of both mono- and dinuclear silver carbene compounds were synthesized and the anticancer efficacy was compared with several standard treatments [47,48,49,50,65,66,67,68,69]. The in vitro activity of 42 (Figure 8), a dinuclear Ag(I)-NHC compound, showed increased activity (IC50 = 1.7 μM) when compared to the mononuclear compound 43 (Figure 8) (IC50 = 6.0 μM) against HCT 116 (colon), attributed to the possible increased stability and subsequent slowed release of Ag(I) ions [70]. Compounds 44–46 (Figure 8) were also tested against HCT 116 cell line and showed high activity with IC50 values of 0.9, 1.3, and 1.1 μM respectively, much more cytotoxic than the standard drug 5-fluorouracil (5-FU) (IC50 = 5 μM) [71].
Figure 8.
Mono- and dinuclear Ag(I)-NHC complexes from Haque and coworkers [70,71].
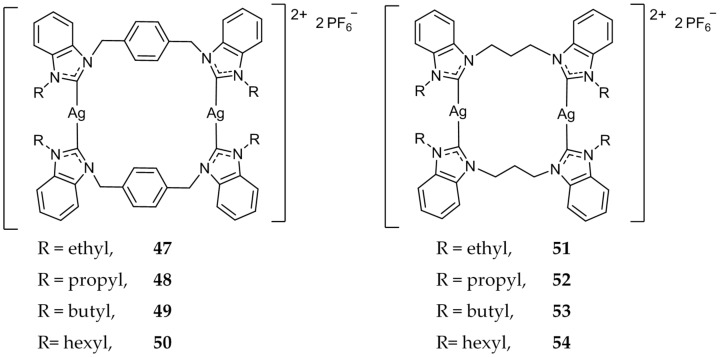
These xylyl-linked systems were further studied and 48 and 49 (Figure 9) showed an increased potency, with IC50 values of 0.4 and 0.01 μM against HCT 116, compared to 19.2 μM for 5-FU [72]. Additionally, several compounds were synthesized with varying lengths of alkyl chains as terminal N-substitutions [73,74,75]. Notably, 50 (Figure 9) was evaluated for cytotoxicity against HCT 116 as well as normal fibroblast (CCD-18Co) cells [76]. While 50 showed potent cytotoxicity against the cancerous cell line HCT 116 (IC50 = 1.7 μM), it did not show any activity (IC50 > 200 μM) against normal colon fibroblasts (CCD-18Co) cells. Compound 50 was also found to induce pro-apoptotic changes in the cellular nucleus via the Hoechst 33342 assay. It was further determined that 50 induced cleavage of caspase-3/7 in HCT 116 cells via FAM-FLICA caspase assay. Most recently, Haque et al. tested the efficacy of propylene linked bis-benzimidazole compounds 51–54 (Figure 9) against MCF-7 [77]. All four compounds showed comparable activity (IC50 = 7 ± 1 μM–18 ± 3 μM) compared to the standard drug Tamoxifen (IC50 = 11 ± 2 μM). The anticancer results showed that all dinuclear Ag(I)-NHC complex were active while their corresponding NHC salts were not.
Figure 9.
Dinuclear benzimidazole Ag(I)-NHC complexes from Haque and coworkers [72,73,74,75,76,77].
Several water soluble Ag(I)-NHC compounds were synthesized by Gandin et al. and their antiproliferative activity was evaluated [78]. The sulfonate functionalized Ag(I)-NHC 55 (Figure 10), was determined to be the most promising compound. The in vitro antitumor activity of 55 was tested against the human tumor cells lines A549, HCT-15, MCF-7, A431 (cervical), and A375 (melanoma) and had comparable activity to cisplatin. Compound 55 had an average IC50 value of 11.63 μM against all five cell lines, compared to an average IC50 of 8.50 μM for cisplatin. Compound 55 was also shown to be effective against cisplatin resistant cells (C13*), with an IC50 value of 11.26 ± 2.11 μM, nearly two fold lower than cisplatin (IC50 = 22.54 ± 2.16 μM). Further mechanistic studies revealed a strong correlation between the high in vitro antiproliferative activity of 55, and its ability to inhibit thioredoxin reductase (TrxR). This inhibition of TrxR led to the activation of the ASK-1 pathway, leading to apoptotic cell death.
Figure 10.
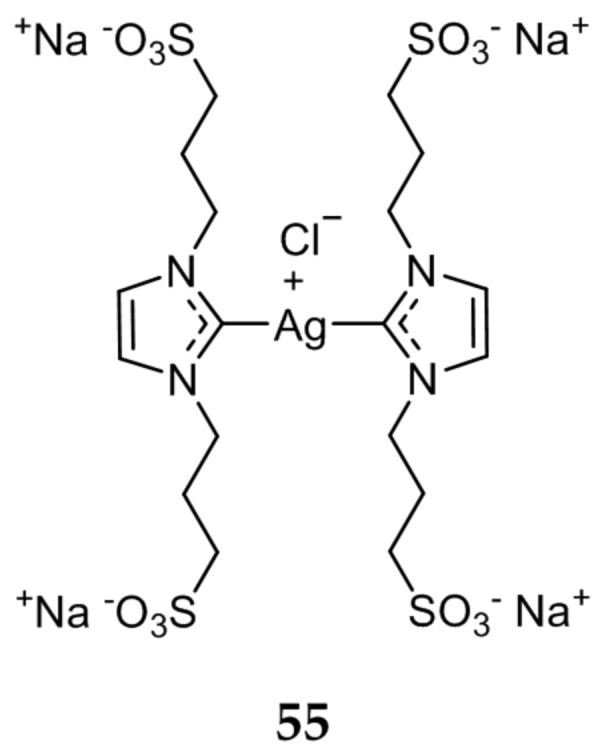
Hydrophilic sulfonated Ag(I)-NHC from Gandin et al. [78].
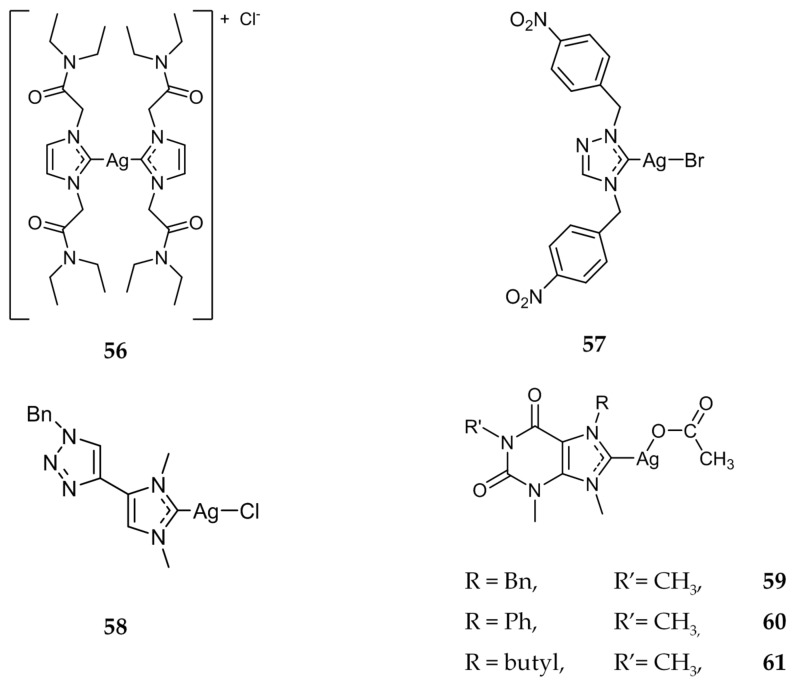
Several other research groups also synthesized, characterized, and investigated the cytotoxic properties of bis-NHC compounds [79,80]. The most promising derivative synthesized by Santini, Dias and coworkers was compound 56 (Figure 11). The IC50 values for 56 against HCT-15 (14.11 ± 2.11 μM) and A549 (lung; IC50 = 16.23 ± 2.31 μM) cell lines were comparable to that of cisplatin with IC50 values of 15.25 ± 2.31 μM and 13.10 ± 1.23 μM respectively. Compound 56 was also subjected to in vitro testing to evaluate its ability to inhibit TrxR. It was found that 56 was able to decrease the TrxR activity by ~80% at a low concentration of 90 nM, further indicating this enzyme as a target for Ag(I)-NHC compounds.
Figure 11.
Structures of Ag(I)-NHC complexes from Santini (56 and 57) [79,80,81], Bellemin-Laponnaz and Tubaro (58) [82], and Willans (59–61) [83].
Santini, Gandin, and coworkers also developed a series of triazolium NHCs, including compound 57 (Figure 11) [81]. When compared to cisplatin, 57 displayed higher activity against several cancerous cell lines, including HCT-15, BxPC3 (pancreatic), A549 (lung), as well as C13*, a cisplatin-resistant ovarian cell line. Similarly to Ag(I)-NHC compounds discussed previously, 57 was also shown to inhibit TrxR. After a 15 min incubation, TrxR was inhibited by ~46% at the IC50 of 7.2 nM. In 2017, another triazole substituted Ag(I)-NHC complex was synthesized, compound 58 (Figure 11) [82]. This compound showed good activity against all three cancer cell lines that were tested; HCT116 (IC50 = 1.38 ± 0.36 μM), MCF7 (IC50 = 2.0 ± 0.2 μM), and PC3 (IC50 = 1.38 ± 0.36 μM). The Ag(I)-NHC compound out performed cisplatin against all three cell lines.
Several xanthine derivatives were synthesized and tested by Willans, Phillips, and coworkers [83]. Compounds 59–61 (Figure 11), as well as compounds 4 and 5, previously discussed for their antimicrobial properties, were evaluated against several cancer cell lines and showed moderate activity, with all IC50 values being in the micromolar range . All the compounds tested were less active than cisplatin. The most cytotoxic silver complex was 60, with the NHC ligand having a phenyl group as a substituent. This phenomenon is attributed by the authors to the phenyl group having a stabilizing effect on the silver NHC bond, slowing the release rate of the silver ions.
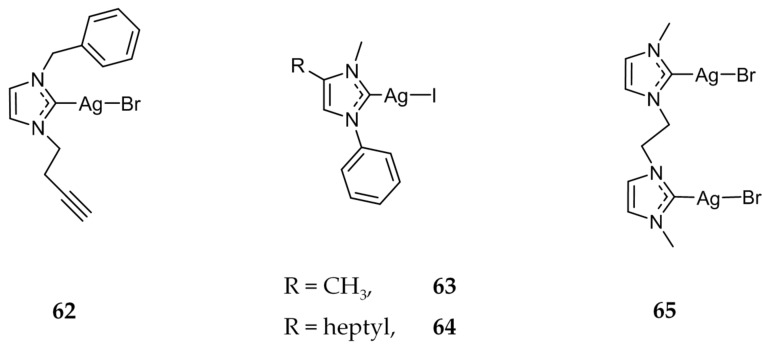
Three alkyne functionalized Ag(I)-NHC compounds were synthesized and evaluated for their applications in biomolecule conjugation [84]. The cytotoxicity of 62 (Figure 12) was found to be in the micromolar range against a variety of human cancer cell lines including DLD-1 (colon; IC50 = 6.8 ± 0.8 μM), Hep-G2 (liver; IC50 = 6.9 ± 0.7 μM), MCF-7 (IC50 =17.1 ± 0.4 μM), and HEK (liver, IC50 = 22.6 ± 0.9 μM).
Figure 12.
Structures of Ag(I)-NHC complexes from Garner et al. (62) [84], Bjørsvik et al. (63 and 64) [85], and Sanchez et al. (65) [86].
The effect of varying the length of an aliphatic chain substituted on the 4-position of the imidazole was also studied for mononuclear 63 and 64 (Figure 12). Bjørsvik et al. compared the activity of 63 and 64 against two leukemia cell lines HL60 and MOLM-13 [85]. Compound 64 was much more active against both cell lines showing IC50 values of 14 μM against HL60 and 27 μM against MOLM-13, compared to 63 with IC50 concentrations of 78 μM and 123 μM respectively. For both compounds, the authors determined cell death to be apoptotic via Hoechst 33342 and Annexin V staining.
Compound 65 (Figure 12) was synthesized and is proposed to interact with DNA mainly through non-covalent interactions as π-π stacking contacts, inducing a conformational change in the structure of DNA [86]. The Ag(I)-NHC complex showed similar activity to cisplatin against prostate (PC3) and colon (HT29) cancerous cell lines.
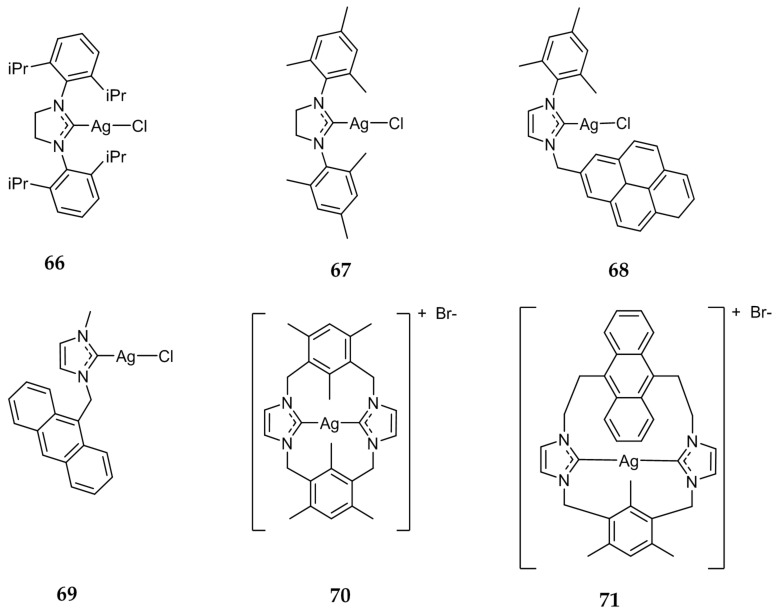
Eloy et al. evaluated a series of Ag(I)-NHC complexes for their antitumor activity and explored their mechanism of action [87]. Compounds 66 and 67 (Figure 13), as well as compounds 13–24 (Figure 4), previously discussed for their antibacterial activity, were tested against a variety of cancer cell lines, including drug-resistant cells, with some compounds exhibiting IC50 values in the nanomolar range. Compounds 17 and 66 were found to be the most active, having a range of IC50 values of 28 ± 1–75 ± 15 nm against HCT 116, MCF-7, and HL60 cell lines. These compounds demonstrated a drastic increase in activity as compared to cisplatin (IC50 = 2.7–5.9 μM). Compounds 17, 66, and 67 were also shown to induce cell death via apoptosis without the involvement of necrosis against HL60 cells. Further studies showed that the apoptotic cell death caused by these Ag(I)-NHC compounds was likely due to the depolarization of the mitochondria membrane potential (∆Ψm), not associated with the overproduction of reactive oxygen species (ROS) nor with caspase-3 activation. Cell death in HL60 cells by 17, 66, and 67 was shown to be caspase-independent apoptosis, triggered by translocation of apoptosis-inducing factor and caspase-12 into the nuclear compartment. Mitochondria was further indicated as a target for Ag(I)-NHC complexes by Eloy et al. by utilizing the fluorescent compound 68.
Figure 13.
Structures of Ag(I)-NHC complexes from Eloy et al. (66–68) [87], Citta et al. (69) [88], and Li et al. (70 and 71) [89].
In 2013, the fluorescent Ag(I)-NHC compound 69 (Figure 13) was synthesized and evaluated by Citta et al. [88]. Compound 69 showed IC50 values in the low micromolar range against cisplatin sensitive A2780S (IC50 = 3.26 ± 0.15 μM), cisplatin resistant A2780R (IC50 = 3.73 ± 0.18 μM), and non-tumorigenic kidney cell line HEK-293T (IC50 = 6.36 ± 0.25 μM). Compound 69 showed an increased selectivity for cancer cells compared to its gold analogue. Further studies demonstrated that 69 inhibited rat cytosolic and mitochondrial thioredoxin reductases as well as the Se-free enzyme glutathione reductase. Fluorescence microscopy studies showed that 69 was distributed intracellularly in tumor cells where they are able to reach the nuclear compartment.
More recently, Li et al. evaluated the antitumor properties of 70 and 71 (Figure 13) [89]. These compounds displayed superior or comparable activity to cisplatin against the cancer cell lines HeLa, A549, MDA-MB-231 (breast), as well as cisplatin resistant A549R. Compounds 70 and 71 also demonstrated lower cytotoxicity against normal liver LO2 cells as compared to cisplatin. Similarly to studies conducted by Eloy et al. 70 and 71 were found to induce apoptosis through modification of ∆Ψm, independent of ROS- and caspase pathways.
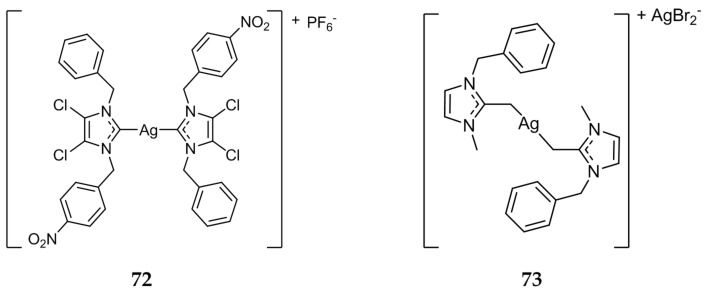
A series of nitrobenzyl substituted mono- and bis-NHC complexes were synthesized and tested against MCF-7 cell line via MTT assay [90]. All compounds tested exhibited IC50 values in the nanomolar range, the bis-NHC complex 72 (Figure 14) showing the highest anticancer potential with an IC50 value of 10.39 nM. The authors indicated, with the exception of 72, mono-NHC compounds displayed better activity than the bis-NHC analogues in this study.
Figure 14.
Structures of Ag(I)-NHC complexes from Sahanini et al. (72) [90] and Allison et al. (73) [91].
In 2017, Allison et al. evaluated the biological activity of compound 73 [91]. Compound 73 (Figure 14) was more active in vitro against A2780, an ovarian cancer cell line, with an IC50 value of 0.44 ± 0.15 μM when compared to cisplatin (IC50 = 0.73 ± 0.30 μM). A significantly increased activity was also demonstrated against the cisplatin resistant strain A2780cis/CP70, with an IC50 value of 0.09 ± 0.01 μM for compound 73 compared to an IC50 of 6.07 ± 1.78 μM for cisplatin. Compound 73 was also found to be a very potent inhibitor of TrxR (IC50 = 2.39 ± 0.59 nM) and was shown to inhibit topoisomerase I in cell-free assays, with complete inhibition at 0.16 μM. The DNA repair enzyme PARP-1 was also inhibited by 73 in vitro, with an IC50 value of 32 ± 7.6 nM, significantly more potent than the known PARP-1 inhibitor NU1025 (IC50 = 2.4 ± 0.27 μM).
4. Antitumor Activity of N-Heterocyclic Carbene Silver Complexes as Compared to Their Precursor Imidazolium Salts
While silver is known to have biological activity, less is known about imidazolium salts. However, by effectively delivering the silver, an imidazolium salt is also released and the therapeutic effect of it should also be of interest. Not only can imidazolium salts be tuned to release silver at the optimal time, they can also be modified so as to better act as anticancer agents themselves. Each type of functional group placed in a different position of the imidazole ring changes the activity of the imidazole and can alter the release of Ag(I) ions.
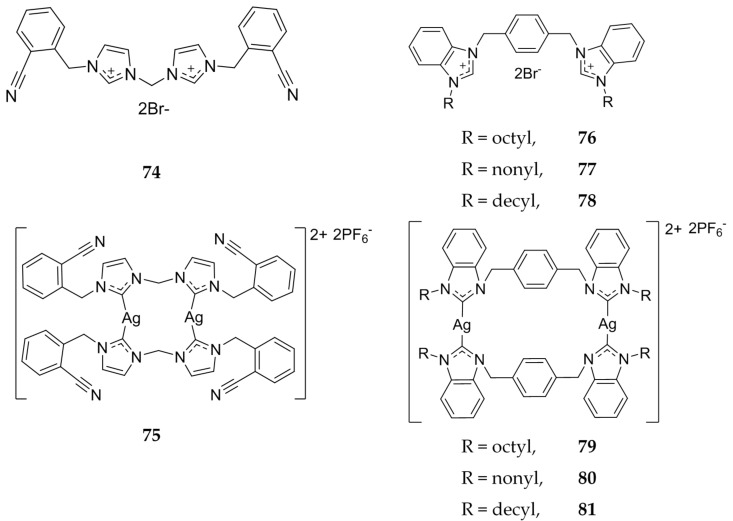
In 2013, Haque et al. evaluated cyanobenzyl bis-imidazolium salts as both the ligand and dinuclear Ag(I)-NHCs (Figure 15) using the MTS assay [75]. Against the human immortalized myelogenous leukemia cell line K562, compound 74 showed no activity (IC50 >200 μM) whereas 75 was shown to have comparable activity to that of 5-FU with IC50 values of 43.7 μM and 35.9 μM, respectively. In addition, para-xylyl linked benzimidazole ligands, 76–78, were compared to the dinuclear silver complexes, 79–81 (Figure 15). Overall, the benzimidazole ligands and silver complexes were more effective against the K562 cell line than the imidazole compounds, with an increase in alkyl chain length from octyl to nonyl to decyl, corresponding to better activity. IC50 values of the ligands alone were 34.9 μM, 8.9 μM, and 9.0 μM, with respect to an increase in alkyl length. The dinuclear silver complexes corresponding to the ligands were shown to be more active with IC50 values of 6.57 μM, 3.67 μM, and 3.37 μM, respectively. From the IC50 values it is clear that the silver complexes are more active than the ligand alone. However, in comparison to 5-FU, the benzimidazole ligands are more effective against leukemia.
Figure 15.
Structures of imidazolium salts and benzimidazolium salts and corresponding Ag(I)-NHCs from Haque and coworkers [75].
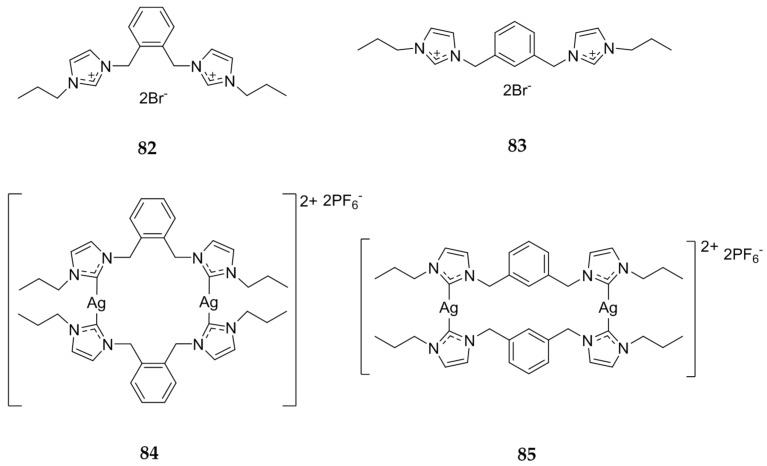
Haque et al. published another paper in 2013 investigating bis-imidazolium salts with either an ortho- or meta-xylyl linkage [92]. Again, the precursor imidazolium salt was directly compared to the Ag(I)-NHC complex using the MTT assay on both HCT 116 and MCF-7 cell lines. The bis-imidazolium salts 82 and 83 (Figure 16) were inactive against the HCT 116 cells (IC50 > 200 μM) while a slight toxicity against the MCF-7 cells was observed (IC50 = 158 to >200 μM). The silver complexes 84 and 85 (Figure 16) showed a significantly greater cytotoxic effect with the meta-xylyl linked compound, 85, which displayed IC50 values of 5.6 μM for HCT 116 and 1.12 μM for MCF-7. This data shows that alone, the bis-benzimidazoles are much more effective than the bis-imidazoles and the silver complexes are even more effective, especially when a larger cage has been achieved with the meta-xylyl linker, as outlined by the authors.
Figure 16.
Structures of imidazolium salts and corresponding Ag(I)-NHCs from Haque and coworkers [92].
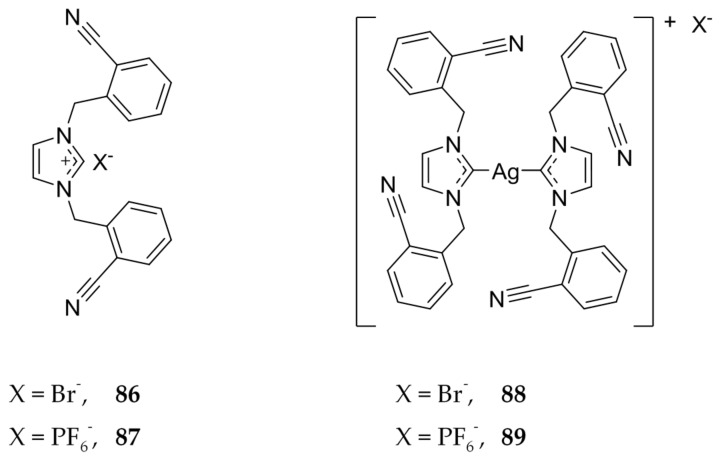
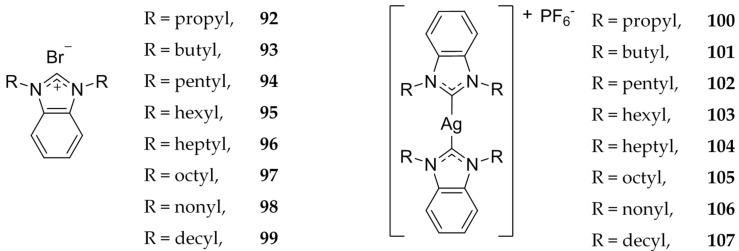
Zulikha et al. also found a ligand that had greater cytotoxicity than 5-FU against HCT 116 cells using the MTT assay (Figure 17) [70]. Interestingly, the active compound, 86 (IC50 = 5.0 ± 0.3 μM), differs from an inactive counterpart, 87 (IC50 > 200 μM), in only the counterion associated with the ligand. A similar trend is seen with the corresponding Ag(I)-NHCs, 88 and 89 (Figure 17), with IC50 values of 1.7 ± 0.2 μM and 27.2 ± 1.1 μM, respectively. Both 86 and 87 were found to have better cytotoxicity than the control, 5-FU. The authors suggest that the PF6− anion contributes to the stability of the silver NHC bond and thus does not allow for a quick release of silver, leading to less cytotoxicity.
Figure 17.
Structures of imidazolium salts and corresponding Ag(I)-NHC complexes from Zulikha et al. [70].
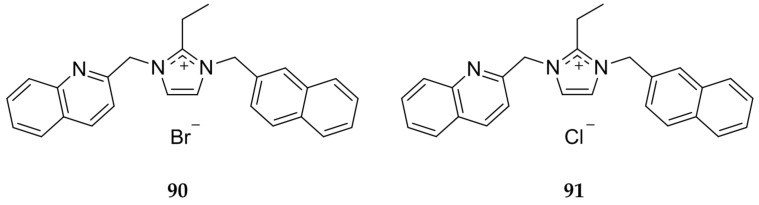
Not only does the anion effect cytotoxicity of imidazolium salts and corresponding Ag(I)-NHCs, it can also alter the solubility as displayed by Youngs and coworkers [93]. The bromide and chloride imidazolium salt, 90 and 91 respectively (Figure 18), were directly compared and it was determined that solubility of the chloride derivative (>10 mM) was at least 2-fold higher than with a bromide anion (~5 mM). This shows that while functional groups substituted on the imidazole core can change activity of both the imidazolium salt and the resulting Ag(I)-NHC, the anion selection is important as well for both stability and solubility.
Figure 18.
Structures of imidazolium salts synthesized by Youngs and coworkers [93].
In 2017, Fatima et al. evaluated a group of benzimidazole compounds with varied alkyl chains off of the nitrogen positions of the ring (Figure 19) [94]. Cytotoxicity was determined by the MTT assay on HCT 116 cells. It was found that increasing the alkyl chain length to 6 carbons or more marked a significant decrease in IC50 value for both the ligand and the Ag(I)-NHC complex. The ligand alone has an IC50 range between 27.3 ± 0.63 and 31.8 ±3.84 μM with less than 6 carbons in the alkyl chain and a range of 1.4 ± 0.15–7.5 ± 2.22 μM with 6 or more in the alkyl chain. Similarly, the silver complex had IC50 ranges of 13.2 ± 1.50–26.8 ± 2.30 μM and 0.02 ± 0.02–3.9 ± 0.62 μM, respectively. In fact, all compounds with 6 or more carbons in the alkyl chain length exhibited greater cytotoxicity than 5-FU (IC50 = 10.2 μM). The silver NHCs in this case have lower IC50 values than the ligands themselves. Authors attribute this to the release of silver. This further establishes the importance of the relationship between lipophilicity and cytotoxicity, as demonstrated in previous studies [95,96,97,98].
Figure 19.
Structure of monobenzimidazolium salts and corresponding bis-imidazolium Ag(I)-NHCs from Haque and coworkers [94].
With the large variety of modifications that can be done on imidazolium salts, compounds to treat many different kinds of cancer can be developed. As displayed above, a delicate balance between lipophilicity, functional groups and associated anion must be found for the best activity of both the imidazolium salt alone and the corresponding silver NHC complexes.
5. Conclusions
Since the beginning of their isolation by Arduengo, NHCs have proved to be a versatile area of research. Their ability to bind different types of metals, as well as the multitude of chemical modifications that exist, makes them useful in many areas of scientific study, including new antibacterial and chemotherapeutic compounds. Specifically, Ag(I)-NHCs have shown promising outcomes in both of these areas.
While the antibacterial properties of silver have been well established, NHCs serve as an efficient way to deliver Ag(I) where needed. Ag(I)-NHCs have been found to be active against both Gram-positive and Gram-negative species, while also being potent enough to act against bioterrorism agents including Burkholderia pseudomallei and Yersinia pestis. Not only have Ag(I)-NHCs shown promise with in vitro studies, some have also increased the survival rate of infected Galleria mellonella larvae and infected mice when tested in vivo.
Recently, Ag(I)-NHCs have also been developed and utilized for the purpose of potential chemotherapeutic drugs as well. Using in vitro assays, Ag(I)-NHCs show toxicity comparable to, or better than, current chemotherapeutics such as cisplatin and 5-fluorouracil in a variety of cancer cell types. Functional groups on the NHC core have been shown to greatly affect toxicity, with lipophilicity and counterion playing an important role. While the mechanism of action of Ag(I)-NHC chemotherapeutics is still unclear, several possible biological targets have been identified. However, further research is still required to fully elucidate the mechanism of action of Ag(I)-NHC complexes.
Acknowledgments
We wish to acknowledge Ashland University and The University of Akron for their support.
Author Contributions
This manuscript was a collaborative effort amongst the three authors listed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Arduengo A.J., Harlow R.L., Kline M. A stable crystalline carbene. J. Am. Chem. Soc. 1991;113:361–363. doi: 10.1021/ja00001a054. [DOI] [Google Scholar]
- 2.Wagers P.O., Panzner M.J., Southerland M.R., DeBord M.A., Deblock M.C., Tessier C.A., Cannon C.L., Youngs W.J. Biologically active N-heterocyclic carbene-metal complexes. In: Diez-Gonzalez S., editor. N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools. Royal Society of Chemistry; Cambridge, UK: 2017. pp. 567–595. [Google Scholar]
- 3.Bourissou D., Guerret O., Gabbai F.P., Bertrand G. Stable Carbenes. Chem. Rev. 2000;100:39–91. doi: 10.1021/cr940472u. [DOI] [PubMed] [Google Scholar]
- 4.Hopkinson M.N., Richter C., Schedler M., Glorius F. An overview of N-heterocyclic carbenes. Nature. 2014;510:485–496. doi: 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]
- 5.Nolan S.P. The development and catalytic uses of N-heterocyclic carbene gold complexes. Acc. Chem. Res. 2011;44:91–100. doi: 10.1021/ar1000764. [DOI] [PubMed] [Google Scholar]
- 6.Hindi K.M., Panzner M.J., Tessier C.A., Cannon C.L., Youngs W.J. The medicinal applications of imidazolium carbene-metal complexes. Chem. Rev. 2009;109:3859–3884. doi: 10.1021/cr800500u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautier A., Cisnetti F. Advances in metal–carbene complexes as potent anti-cancer agents. Metallomics. 2012;4:23–32. doi: 10.1039/C1MT00123J. [DOI] [PubMed] [Google Scholar]
- 8.Cisnetti F., Gautier A. Metal/N-heterocyclic carbene complexes: Opportunities for the development of anticancer metallodrugs. Angew. Chem. Int. Ed. Engl. 2013;52:11976–11978. doi: 10.1002/anie.201306682. [DOI] [PubMed] [Google Scholar]
- 9.Liu W., Gust R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013;42:755–773. doi: 10.1039/C2CS35314H. [DOI] [PubMed] [Google Scholar]
- 10.Aher S.B., Muskawar P.N., Thenmozhi K., Bhagat P.R. Recent developments of metal N-heterocyclic carbenes as anticancer agents. Eur. J. Med. Chem. 2014;81:408–419. doi: 10.1016/j.ejmech.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Liu W., Gust R. Update on metal N-heterocyclic carbene complexes as potential anti-tumor metallodrugs. Coord. Chem. Rev. 2016;329:191–213. doi: 10.1016/j.ccr.2016.09.004. [DOI] [Google Scholar]
- 12.Charra V., de Frémont P., Braunstein P. Multidentate N-heterocyclic carbene complexes of the 3d metals: Synthesis, structure, reactivity and catalysis. Coord. Chem. Rev. 2017;341:53–176. doi: 10.1016/j.ccr.2017.03.007. [DOI] [Google Scholar]
- 13.Wagers P.O., Shelton K.L., Panzner M.J., Tessier C.A., Youngs W.J. Synthesis and Medicinal Properties of Silver–NHC Complexes and Imidazolium Salts. In: Nolan S.P., editor. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis. Wiley-VCH; Weinheim, Germany: 2014. pp. 151–172. [Google Scholar]
- 14.Marinelli M., Santini C., Pellei M. Recent Advances in Medicinal Applications of Coinage-Metal (Cu and Ag) N-Heterocyclic Carbene Complexes. Curr. Top. Med. Chem. 2016;16:2995–3017. doi: 10.2174/1568026616666160506145408. [DOI] [PubMed] [Google Scholar]
- 15.Medici S., Peana M., Crisponi G., Nurchi V.M., Lachowicz J.I., Remelli M., Zoroddu M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016;327–328:349–359. doi: 10.1016/j.ccr.2016.05.015. [DOI] [Google Scholar]
- 16.Drake P.L., Hazelwood K.J. Exposure-related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005;49:575–585. doi: 10.1093/annhyg/mei019. [DOI] [PubMed] [Google Scholar]
- 17.Hartinger C.G., Dyson P.J. Bioorganometallic chemistry—From teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009;38:391–401. doi: 10.1039/B707077M. [DOI] [PubMed] [Google Scholar]
- 18.Klasen H.J. Historical review of the use of silver in the treatment if burns. I. Early uses. Burns. 2000;26:117–130. doi: 10.1016/S0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 19.Klasen H.J. A historical review for the use of silver in the treatment of burns. II. Renew interest in silver. Burns. 2000;26:131–138. doi: 10.1016/S0305-4179(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 20.Russel A.D., Path F.R.C., Hugo W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994;31:351–366. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 21.Moyer C.A., Brentanol L., Gravens D.L., Margraf H.W., Monafo W.W. Treatment of large human burns with 0.5% silver nitrate solution. Arch. Surg. 1965;90:812–867. doi: 10.1001/archsurg.1965.01320120014002. [DOI] [PubMed] [Google Scholar]
- 22.Fox C.L. Silver sulfadiazine—A new topical treatment for Pseudomonas in burns. Arch. Surg. 1968;96:184–188. doi: 10.1001/archsurg.1968.01330200022004. [DOI] [PubMed] [Google Scholar]
- 23.Lansdown A.B.J. Silver I: It’s antibacterial properties and mechanims of action. J. Wound Care. 2002;11:125–130. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 24.Silver S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003;27:341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- 25.Youngs W.J., Knapp A.R., Wagers P.O., Tessier C.A. Nanoparticle encapsulated silvercarbene complexes and their antimicrobial and anticancer properties: A perspective. Dalton Trans. 2012;41:327–336. doi: 10.1039/C1DT11100K. [DOI] [PubMed] [Google Scholar]
- 26.Melaiye A., Simons R.S., Milsted A., Pingitore F., Wesdemiotis C., Tessier C.A., Youngs W.J. Formation of Water-Soluble Pincer Silver(I)-Carbene Complexes: A Novel Antimicrobial Agent. J. Med. Chem. 2004;47:973–977. doi: 10.1021/jm030262m. [DOI] [PubMed] [Google Scholar]
- 27.Melaiye A., Sun Z., Hindi K., Milsted A., Ely D., Reneker D.H., Tessier C.A., Youngs W.J. Silver(I)-imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: Formation of nanosilver particles and antimicrobial activity. J. Am. Chem. Soc. 2005;127:2285–2291. doi: 10.1021/ja040226s. [DOI] [PubMed] [Google Scholar]
- 28.Kascatan-Nebioglu A., Melaiye A., Hindi K., Durmus S., Panzner M.J., Hogue L.A., Mallett R.J., Hovis C.E., Coughenour M., Crosby S.D., et al. Synthesis from caffeine of a mixed N-heterocyclic carbene-silver acetate complex active against resistant respiratory pathogens. J. Med. Chem. 2006;49:6811–6818. doi: 10.1021/jm060711t. [DOI] [PubMed] [Google Scholar]
- 29.Hindi K.M., Siciliano T.J., Durmus S., Panzner M.J., Medvetz D.A., Reddy D.V., Hogue L.A., Hovis C.E., Hilliard J.K., Mallet R.J., et al. Synthesis, stability, and antimicrobial studies of electronically tuned silver acetate N-heterocyclic carbenes. J. Med. Chem. 2008;51:1577–1583. doi: 10.1021/jm0708679. [DOI] [PubMed] [Google Scholar]
- 30.Wright B.D., Shah P.N., McDonald L.J., Shaeffer M.L., Wagers P.O., Panzner M.J., Smolen J., Tagaev J., Tessier C.A., Cannon C.L., et al. Synthesis, characterization, and antimicrobial activity of silver carbene complexes derived from 4,5,6,7-tetrachlorobenzimidazole against antibiotic resistant bacteria. Dalton Trans. 2012;41:6335. doi: 10.1039/c2dt00055e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapp A.R., Panzner M.J., Medvetz D.A., Wright B.D., Tessier C.A., Youngs W.J. Synthesis and antimicrobial studies of silver N-heterocyclic carbene complexes bearing a methyl benzoate substituent. Inorg. Chim. Acta. 2010;364:125–131. doi: 10.1016/j.ica.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cannon C.L., Hogue L.A., Vajravelu R.K., Capps G.H., Ibricevic A., Hindi K.M., Kascatan-Nebioglu A., Walter M.J., Brody S.L., Youngs W.J. In vitro and murine efficacy and toxicity studies of nebulized SCC1, a methylated caffeine-silver(I) complex, for treatment of pulmonary infections. Antimicrob. Agents Chemother. 2009;53:3285–3293. doi: 10.1128/AAC.00314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panzner M.J., Hindi K.M., Wright B.D., Taylor J.B., Han D.S., Youngs W.J., Cannon C.L. A theobromine derived silver N-heterocyclic carbene: Synthesis, characterization, and antimicrobial efficacy studies on cycstic fibrosis relevant pathogens. Dalton Trans. 2009:7308–7313. doi: 10.1039/b907726j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panzner M.J., Deeraksa A., Smith A., Wright B.D., Hindi K.M., Kascatan-Nebioglu A., Torres A.G., Judy B.M., Hovis C.E., Hilliard J.K., et al. Synthesis and in vitro efficacy studies of silver carbene complexes on biosafety level 3 bacteria. Eur. J. Inorg. Chem. 2009:1739–1745. doi: 10.1002/ejic.200801159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil S., Dietrich K., Deally A., Gleeson B., Müller-Bunz H., Paradisi F., Tacke M. Synthesis, cytotoxicity and antibacterial studies of novel symmetrically and nonsymmetrically 4-(methoxycarbonyl)benzyl-substituted n-heterocyclic carbene-silver acetate complexes. Helv. Chim. Acta. 2010;93:2347–2364. doi: 10.1002/hlca.201000310. [DOI] [Google Scholar]
- 36.Patil S., Claffey J., Deally A., Hogan M., Gleeson B., Méndez L.M.M., Müller-Bunz H., Paradisi F., Tacke M. Synthesis, cytotoxicity and antibacterial studies of p-methoxybenzyl- substituted and benzyl-substituted N-heterocyclic carbene-silver complexes. Eur. J. Inorg. Chem. 2010;2010:1020–1031. doi: 10.1002/ejic.200900889. [DOI] [Google Scholar]
- 37.Patil S., Deally A., Gleeson B., Hackenberg F., Müller-Bunz H., Paradisi F., Tacke M. Synthesis, cytotoxicity and antibacterial studies of novel symmetrically and non-symmetrically p-nitrobenzyl-substituted N-heterocyclic carbene-silver(I) acetate complexes. Z. Anorg. Allg. Chem. 2011;637:386–396. doi: 10.1002/zaac.201000395. [DOI] [Google Scholar]
- 38.Hackenberg F., Deally A., Lally G., Malenke S., Müller-Bunz H., Paradisi F., Patil S., Quaglia D., Tacke M. Novel Nonsymmetrically p-Benzyl-Substituted (Benz) imidazole N-Heterocyclic Carbene-Silver(I) Acetate Complexes: Synthesis and Biological Evaluation. Int. J. Inorg. Chem. 2012:1–13. doi: 10.1155/2012/121540. [DOI] [Google Scholar]
- 39.Hackenberg F., Lally G., Müller-Bunz H., Paradisi F., Quaglia D., Streciwilk W., Tacke M. Novel symmetrically p-benzyl-substituted 4,5-diaryl-imidazole N-heterocyclic carbene-silver(I) acetate complexes—Synthesis and biological evaluation. J. Organomet. Chem. 2012;717:123–134. doi: 10.1016/j.jorganchem.2012.07.006. [DOI] [Google Scholar]
- 40.Hackenberg F., Lally G., Müller-Bunz H., Paradisi F., Quaglia D., Streciwilk W., Tacke M. Synthesis and biological evaluation of N-heterocyclic carbene-silver(I) acetate complexes derived from 4,5-ditolyl-imidazole. Inorg. Chim. Acta. 2013;395:135–144. doi: 10.1016/j.ica.2012.10.029. [DOI] [Google Scholar]
- 41.Streciwilk W., Cassidy J., Hackenberg F., Müller-Bunz H., Paradisi F., Tacke M. Synthesis, cytotoxic and antibacterial studies of p-benzyl-substituted NHC-silver(I) acetate compounds derived from 4,5-di-p-diisopropylphenyl- or 4,5-di-p-chlorophenyl-1H-imidazole. J. Organomet. Chem. 2014;749:88–99. doi: 10.1016/j.jorganchem.2013.09.033. [DOI] [Google Scholar]
- 42.Patil S., Deally A., Gleeson B., Muller-Bunz H., Paradisi F., Tacke M. Novel benzyl-substituted N-heterocyclic carbene–silver acetate complexes: Synthesis, cytotoxicity and antibacterial studies. Metallomics. 2011;3:74–88. doi: 10.1039/C0MT00034E. [DOI] [PubMed] [Google Scholar]
- 43.Tacke M. Benzyl-substituted carbene-metal complexes: Potential for novel antibiotics and anticancer drugs. J. Organomet. Chem. 2015;782:17–21. doi: 10.1016/j.jorganchem.2014.09.036. [DOI] [Google Scholar]
- 44.Sharkey M., O’Gara J., Gordon S., Hackenberg F., Healy C., Paradisi F., Patil S., Schaible B., Tacke M. Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3. Antibiotics. 2012;1:25–28. doi: 10.3390/antibiotics1010025. [DOI] [Google Scholar]
- 45.Browne N., Hackenberg F., Streciwilk W., Tacke M., Kavanagh K. Assessment of in vivo antimicrobial activity of the carbene silver(I) acetate derivative SBC3 using Galleria mellonella larvae. Biometals. 2014;27:745–752. doi: 10.1007/s10534-014-9766-z. [DOI] [PubMed] [Google Scholar]
- 46.Roland S., Jolivalt C., Cresteil T., Eloy L., Bouhours P., Hequet A., Mansuy V., Vanucci C., Paris J.M. Investigation of a series of silver-N-Heterocyclic carbenes as antibacterial agents: Activity, synergistic effects, and cytotoxicity. Chem. Eur. J. 2011;17:1442–1446. doi: 10.1002/chem.201002812. [DOI] [PubMed] [Google Scholar]
- 47.Haque R.A., Choo S.Y., Budagumpi S., Abdullah A.A.-A., Khadeer Ahamed M.B., Abdul Majid A.M.S. Synthesis, crystal structures, characterization and biological studies of nitrile-functionalized silver(I) N-heterocyclic carbene complexes. Inorg. Chim. Acta. 2015;433:35–44. doi: 10.1016/j.ica.2015.04.023. [DOI] [Google Scholar]
- 48.Haque R.A., Choo S.Y., Budagumpi S., Iqbal M.A., Al-Ashraf Abdullah A. Silver(I) complexes of mono-and bidentate N-heterocyclic carbene ligands: Synthesis, crystal structures, and in vitro antibacterial and anticancer studies. Eur. J. Med. Chem. 2015;90:82–92. doi: 10.1016/j.ejmech.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Asekunowo P.O., Haque R.A., Razali M.R. Sterically modulated silver(I) complexes of N-benzyl-substituted N-heterocyclic carbenes: Synthesis, crystal structures and bioactivity. Transit. Met. Chem. 2015;40:79–88. doi: 10.1007/s11243-014-9892-z. [DOI] [Google Scholar]
- 50.Haque R.A., Budagumpi S., Choo S.Y., Choong M.K., Lokesh B.E., Sudesh K. Nitrile-functionalized Hg(II)- and Ag(I)-N-heterocyclic carbene complexes: Synthesis, crystal structures, nuclease and DNA binding activities. Appl. Organomet. Chem. 2012;26:689–700. doi: 10.1002/aoc.2912. [DOI] [Google Scholar]
- 51.Haque R.A., Asekunowo P.O., Razali M.R., Mohamad F. NHC–Silver(I) Complexes as Chemical Nucleases; Synthesis, Crystal Structures, and Antibacterial Studies. Heteroatom Chem. 2014;25:194–204. doi: 10.1002/hc.21160. [DOI] [Google Scholar]
- 52.Napoli M., Saturnino C., Cianciulli E. I., Varcamonti M., Zanfardino A., Tommonaro G., Longo P. Silver(I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Organomet. Chem. 2013;725:46–53. doi: 10.1016/j.jorganchem.2012.10.040. [DOI] [Google Scholar]
- 53.Günal S., Kaloğlu N., Özdemir I., Demir S., Özdemir İ. Novel benzimidazolium salts and their silver complexes: Synthesis and antibacterial properties. Inorg. Chem. Commun. 2012;21:142–146. doi: 10.1016/j.inoche.2012.04.033. [DOI] [Google Scholar]
- 54.Kaloğlu M., Kaloğlu N., Özdemir İ., Günal S., Özdemir İ. Novel benzimidazol-2-ylidene carbene precursors and their silver(I) complexes: Potential antimicrobial agents. Bioorg. Med. Chem. 2016;24:3649–3656. doi: 10.1016/j.bmc.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Gök Y., Akkoç S., Albayrak S., Akkurt M., Tahir M.N. N-Phenyl-substituted carbene precursors and their silver complexes: Synthesis, characterization and antimicrobial activities. Appl. Organomet. Chem. 2014;28:244–251. doi: 10.1002/aoc.3116. [DOI] [Google Scholar]
- 56.Rosenberg B., Vancamp L., Trosko J.E., Mansour V.H. Platinum Compounds: A New Class of Potent Antitumor Agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 57.Jung Y., Lippard S.J. Direct Cellular Responses to Platinum-Induced DNA Damage. Chem. Rev. 2007;107:1387–1407. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]
- 58.Wilson J.J., Lippard S.J. Synthetic Methods for the Preparation of Platinum Anticancer Complexes. Chem. Rev. 2014;114:4470–4495. doi: 10.1021/cr4004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabik C.A., Dolan M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galluzzi L., Senovilla L., Vitale I., Michels J., Martins I., Kepp O., Castedo M., Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 61.Medvetz D.A., Hindi K.M., Panzner M.J., Ditto A.J., Yun Y.H., Youngs W.J. Anticancer Activity of Ag(I) N-Heterocyclic Carbene Complexes Derived from 4,5-Dichloro-1H-Imidazole. Met. Based Drugs. 2008;2008:1–7. doi: 10.1155/2008/384010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patil S., Deally A., Gleeson B., Müller-Bunz H., Paradisi F., Tacke M. Synthesis, cytotoxicity and antibacterial studies of symmetrically and non-symmetrically benzyl- or p-cyanobenzyl-substituted N-Heterocyclic carbene—Silver complexes. Appl. Organomet. Chem. 2010;24:781–793. doi: 10.1002/aoc.1702. [DOI] [Google Scholar]
- 63.Hackenberg F., Tacke M. Benzyl-substituted metallocarbene antibiotics and anticancer drugs. Dalton Trans. 2014;43:8144. doi: 10.1039/C4DT00624K. [DOI] [PubMed] [Google Scholar]
- 64.Fichtner I., Cinatl J., Michaelis M., Sanders L.C., Hilger R., Kennedy B.N., Reynolds A.L., Hackenberg F., Lally G., Quinn S.J., et al. In Vitro and In Vivo Investigations into the Carbene Silver Acetate Anticancer Drug Candidate SBC1. Lett. Drug Des. Discov. 2012;9:815–822. doi: 10.2174/157018012803307987. [DOI] [Google Scholar]
- 65.Iqbal M.A., Haque R., Nasri S.F., Majid A.A., Ahamed M.B.K., Farsi E., Fatima T. Potential of silver against human colon cancer: (Synthesis, characterization and crystal structures of xylyl (Ortho, meta, & Para) linked bis-benzimidazolium salts and Ag(I)-NHC complexes: In Vitro anticancer studies) Chem. Cent. J. 2013;7:27. doi: 10.1186/1752-153X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Budagumpi S., Haque R.A., Endud S., Rehman G.U., Salman A.W. Biologically relevant silver(I)-N-heterocyclic carbene complexes: Synthesis, structure, intramolecular interactions, and applications. Eur. J. Inorg. Chem. 2013:4367–4388. doi: 10.1002/ejic.201300483. [DOI] [Google Scholar]
- 67.Haque R.A., Ghdhayeb M.Z., Budagumpi S., Salman A.W., Ahamed M.B.K., Majid A.M.S.A. Non-symmetrically substituted N-heterocyclic carbene-Ag(I) complexes of benzimidazol-2-ylidenes: Synthesis, crystal structures, anticancer activity and transmetallation studies. Inorg. Chim. Acta. 2013;394:519–525. doi: 10.1016/j.ica.2012.09.013. [DOI] [Google Scholar]
- 68.Asif M., Iqbal M.A., Hussein M.A., Oon C.E., Haque R.A., Khadeer Ahamed M.B., Abdul Majid A.S., Abdul Majid A.M.S. Human colon cancer targeted pro-apoptotic, anti-metastatic and cytostatic effects of binuclear Silver(I)-N-Heterocyclic carbene (NHC) complexes. Eur. J. Med. Chem. 2016;108:177–187. doi: 10.1016/j.ejmech.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 69.Ghdhayeb M.Z., Haque R.A., Budagumpi S., Khadeer Ahamed M.B., Abdul Majid A.M.S. Mono- and bis-N-heterocyclic carbene silver(I) and palladium(II) complexes: Synthesis, characterization, crystal structure and in vitro anticancer studies. Polyhedron. 2017;121:222–230. doi: 10.1016/j.poly.2016.09.065. [DOI] [Google Scholar]
- 70.Zulikha H.Z., Haque R.A., Budagumpi S., Abdul A.M.S. Topology control in nitrile-functionalized silver (I)–N-heterocyclic carbene complexes : Synthesis, molecular structures, and in vitro anticancer studies. Inorg. Chim. Acta. 2014;411:40–47. doi: 10.1016/j.ica.2013.11.011. [DOI] [Google Scholar]
- 71.Haque R.A., Ghdhayeb M.Z., Salman A.W., Budagumpi S., Khadeer Ahamed M., Abdul Majid A.M.S. Ag(I)-N-heterocyclic carbene complexes of N-allyl substituted imidazol-2-ylidenes with ortho-, meta- and para-xylyl spacers: Synthesis, crystal structures and in vitro anticancer studies. Inorg. Chem. Commun. 2012;22:113–119. doi: 10.1016/j.inoche.2012.05.037. [DOI] [Google Scholar]
- 72.Iqbal M.A., Haque R.A., Ahamed M.B.K., Majid A.M.S.A., Al-Rawi S.S. Synthesis and anticancer activity of para-xylyl linked bis- benzimidazolium salts and respective Ag(I) N-heterocyclic carbene complexes. Med. Chem. Res. 2013;22:2455–2466. doi: 10.1007/s00044-012-0240-6. [DOI] [Google Scholar]
- 73.Iqbal M.A., Haque R.A., Budagumpi S., Khadeer Ahamed M.B., Abdul Majid A.M.S. Short metal-metal separations and in vitro anticancer studies of a new dinuclear silver(I)-N-heterocyclic carbene complex of para-xylyl-linked bis-benzimidazolium salt. Inorg. Chem. Commun. 2013;28:64–69. doi: 10.1016/j.inoche.2012.11.013. [DOI] [Google Scholar]
- 74.Haque R.A., Iqbal M.A., Asekunowo P., Majid A.M.S.A., Khadeer Ahamed M.B., Umar M.I., Al-Rawi S.S., Al-Suede F.S.R. Synthesis, structure, anticancer, and antioxidant activity of para-xylyl linked bis-benzimidazolium salts and respective dinuclear Ag(I) N-heterocyclic carbene complexes (Part-II) Med. Chem. Res. 2013;22:4663–4676. doi: 10.1007/s00044-012-0461-8. [DOI] [Google Scholar]
- 75.Haque R.A., Hasanudin N., Iqbal M.A., Ahmad A., Hashim S., Majid A.A., Ahamed M.B.K. Synthesis, crystal structures, in vitro anticancer, and in vivo acute oral toxicity studies of bis-imidazolium/benzimidazolium salts and respective dinuclear Ag(I)-N-heterocyclic carbene complexes. J. Coord. Chem. 2013;66:3211–3228. doi: 10.1080/00958972.2013.831406. [DOI] [Google Scholar]
- 76.Iqbal M.A., Umar M.I., Haque R.A., Khadeer Ahamed M.B., Asmawi M.Z., Majid A.M.S.A. Macrophage and colon tumor cells as targets for a binuclear silver(I) N-heterocyclic carbene complex, an anti-inflammatory and apoptosis mediator. J. Inorg. Biochem. 2015;146:1–13. doi: 10.1016/j.jinorgbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Hussaini S.Y., Haque R.A., Asekunowo P.O., Abdul Majid A.M.S., Taleb Agha M., Razali M.R. Synthesis, characterization and anti-proliferative activity of propylene linked bis-benzimidazolium salts and their respective dinuclear Silver(I)-N-heterocyclic carbene complexes. J. Organomet. Chem. 2017;840:56–62. doi: 10.1016/j.jorganchem.2017.04.011. [DOI] [Google Scholar]
- 78.Gandin V., Pellei M., Marinelli M., Marzano C., Dolmella A., Giorgetti M., Santini C. Synthesis and in vitro antitumor activity of water soluble sulfonate- and ester-functionalized silver(I) N-heterocyclic carbene complexes. J. Inorg. Biochem. 2013;129:135–144. doi: 10.1016/j.jinorgbio.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 79.Pellei M., Gandin V., Marinelli M., Marzano C., Yousufuddin M., Dias H.V.R., Santini C. Synthesis and Biological Activity of Ester- and Amide-Functionalized Imidazolium Salts and Related Water-Soluble Coinage Metal N-Heterocyclic Carbene Complexes. Inorg. Chem. 2012;51:9873–9882. doi: 10.1021/ic3013188. [DOI] [PubMed] [Google Scholar]
- 80.Marinelli M., Pellei M., Cimarelli C., Dias H.V.R., Marzano C., Tisato F., Porchia M., Gandin V., Santini C. Novel multicharged silver(I)-NHC complexes derived from zwitterionic 1,3-symmetrically and 1,3-unsymmetrically substituted imidazoles and benzimidazoles: Synthesis and cytotoxic properties. J. Organomet. Chem. 2016;806:45–53. doi: 10.1016/j.jorganchem.2016.01.018. [DOI] [Google Scholar]
- 81.Pellei M., Gandin V., Marinelli M., Orsetti A., Del Bello F., Santini C., Marzano C. Novel triazolium based 11th group NHCs: Synthesis, characterization and cellular response mechanisms. Dalton Trans. 2015;44:21041–21052. doi: 10.1039/C5DT02934A. [DOI] [PubMed] [Google Scholar]
- 82.Monticelli M., Bellemin-Laponnaz S., Tubaro C., Rancan M. Synthesis, Structure and Antitumoural Activity of Triazole-Functionalised NHC–Metal Complexes. Eur. J. Inorg. Chem. 2017:2488–2495. doi: 10.1002/ejic.201700142. [DOI] [Google Scholar]
- 83.Mohamed H.A., Lake B.R.M., Laing T., Phillips R.M., Willans C.E. Synthesis and anticancer activity of silver–N-heterocyclic carbene complexes derived from the natural xanthine products caffeine, theophylline and theobromine. Dalton Trans. 2015;44:7563–7569. doi: 10.1039/C4DT03679D. [DOI] [PubMed] [Google Scholar]
- 84.Garner M.E., Niu W., Chen X., Ghiviriga I., Abboud K., Tan W., Veige A.S. N-heterocyclic carbene gold(I) and silver(I) complexes bearing functional groups for bio-conjugation. Dalton Trans. 2015;44:1914–1923. doi: 10.1039/C4DT02850C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sandtorv A.H., Leitch C., Bedringaas S.L., Gjertsen B.T., Bjørsvik H.R. 4-Alkylated Silver-N-Heterocyclic Carbene (NHC) Complexes with Cytotoxic Effects in Leukemia Cells. ChemMedChem. 2015;10:1522–1527. doi: 10.1002/cmdc.201500234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanchez O., Gonzalez S., Fernandez M., Higuera-Padilla A.R., Leon Y., Coll D., Vidal A., Taylor P., Urdanibia I., Goite M.C., et al. Novel silver(I)- and gold(I)-N-heterocyclic carbene complexes. Synthesis, characterization and evaluation of biological activity against tumor cells. Inorg. Chim. Acta. 2015;437:143–151. doi: 10.1016/j.ica.2015.08.017. [DOI] [Google Scholar]
- 87.Eloy L., Jarrousse A.S., Teyssot M.L., Gautier A., Morel L., Jolivalt C., Cresteil T., Roland S. Anticancer Activity of Silver-N-Heterocyclic Carbene Complexes: Caspase-Independent Induction of Apoptosis via Mitochondrial Apoptosis-Inducing Factor (AIF) ChemMedChem. 2012;7:805–814. doi: 10.1002/cmdc.201200055. [DOI] [PubMed] [Google Scholar]
- 88.Citta A., Schuh E., Mohr F., Folda A., Massimino M.L., Bindoli A., Casini A., Rigobello M.P. Fluorescent silver(I) and gold(I)-N-heterocyclic carbene complexes with cytotoxic properties: Mechanistic insights. Metallomics. 2013;5:1006–1015. doi: 10.1039/c3mt20260g. [DOI] [PubMed] [Google Scholar]
- 89.Li Y., Liu G.-F., Tan C.-P., Ji L.-N., Mao Z.-W. Antitumor properties and mechanisms of mitochondria-targeted Ag(I) and Au(I) complexes containing N-heterocyclic carbenes derived from cyclophanes. Metallomics. 2014;6:1460–1468. doi: 10.1039/C4MT00046C. [DOI] [PubMed] [Google Scholar]
- 90.Shahini C.R., Achar G., Budagumpi S., Tacke M., Patil S.A. Non-symmetrically p-nitrobenzyl-substituted N-heterocyclic carbene-silver(I) complexes as metallopharmaceutical agents. Appl. Organomet. Chem. 2017 doi: 10.1002/aoc.3819. accepted. [DOI] [Google Scholar]
- 91.Allison S.J., Sadiq M., Baronou E., Cooper P.A., Dunnill C., Georgopoulos N.T., Latif A., Shepherd S., Shnyder S.D., Stratford I.J., et al. Preclinical anti-cancer activity and multiple mechanisms of action of a cationic silver complex bearing N-heterocyclic carbene ligands. Cancer Lett. 2017;403:98–107. doi: 10.1016/j.canlet.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 92.Haque R.A., Nasri S.F., Iqbal M.A., Al-Rawi S.S., Jafari S.F., Ahamed M.B.K., Abdul Majid A.M.S. Synthesis, characterization, and crystal structures of bis-imidazolium salts and respective dinuclear Ag(I) N-heterocyclic carbene complexes: In Vitro anticancer studies against “human colon cancer” and “breast cancer”. J. Chem. 2013;2013 doi: 10.1155/2013/804683. [DOI] [Google Scholar]
- 93.DeBord M.A., Wagers P.O., Crabtree S.R., Tessier C.A., Panzner M.J., Youngs W.J. Synthesis, characterization, and in vitro SAR evaluation of N,N’-bis(arylmethyl)-C2-alkyl substituted imidazolium salts. Bioorg. Med. Chem. Lett. 2017;27:196–202. doi: 10.1016/j.bmcl.2016.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fatima T., Haque R.A., Razali M.R., Ahmad A., Asif M., Khadeer Ahamed M.B., Abdul Majid A.M.S. Effect of lipophilicity of wingtip groups on the anticancer potential of mono N-heterocyclic carbene silver(I) complexes: Synthesis, crystal structures and in vitro anticancer study. Appl. Organomet. Chem. 2017 doi: 10.1002/aoc.3735. [DOI] [Google Scholar]
- 95.Shelton K.L., DeBord M.A., Wagers P.O., Southerland M.R., Taraboletti A., Robishaw N.K., Jackson D.P., Tosanovic R., Kofron W.G., Tessier C.A., et al. Synthesis, anti-proliferative activity, and toxicity of C4(C5) substituted N,N′-bis(arylmethyl)imidazolium salts. Tetrahedron. 2016;72:5729–5743. doi: 10.1016/j.tet.2016.07.068. [DOI] [Google Scholar]
- 96.Shelton K.L., DeBord M.A., Wagers P.O., Southerland M.R., Williams T.M., Robishaw N.K., Shriver L.P., Tessier C.A., Panzner M.J., Youngs W.J. Synthesis, anti-proliferative activity, SAR study, and preliminary in vivo toxicity study of substituted N,N′-bis(arylmethyl)benzimidazolium salts against a panel of non-small cell lung cancer cell lines. Bioorg. Med. Chem. 2017;25:421–439. doi: 10.1016/j.bmc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wright B.D., Deblock M.C., Wagers P.O., Duah E., Robishaw N.K., Shelton K.L., Southerland M.R., DeBord M.A., Kersten K.M., McDonald L.J., et al. Anti-tumor activity of lipophilic imidazolium salts on select NSCLC cell lines. Med. Chem. Res. 2015;24:2838–2861. doi: 10.1007/s00044-015-1330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeBord M.A., Southerland M.R., Wagers P.O., Tiemann K.M., Robishaw N.K., Whiddon K.T., Konopka M.C., Tessier C.A., Shriver L.P., Paruchuri S., et al. Synthesis, characterization, in vitro SAR and in vivo evaluation of N,N′bisnaphthylmethyl 2-alkyl substituted imidazolium salts against NSCLC. Bioorg. Med. Chem. Lett. 2017;27:764–775. doi: 10.1016/j.bmcl.2017.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]