Abstract
Some novel (phenyl-diazenyl)phenols (4a–m) were designed and synthesized to be evaluated for their antibacterial activity. Starting from an active previously-synthesized azobenzene chosen as lead compound, we introduced some modifications and optimization of the structure, in order to improve solubility and drug conveyance. Structures of all newly-synthesized compounds were confirmed by 1H nuclear magnetic resonance (NMR), mass spectrometry, and UV-Vis spectroscopy. Antibacterial activity of the new compounds was tested with the dilution method against the bacteria strains Listeria monocytogenes, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa PAO1. All the compounds were selectively active against Gram-positive bacteria. In particular, compounds 4d, 4h, and 4i showed the highest activity against S. aureus and Listeria monocytogenes, reaching remarkable MIC100 values of 4 μg/mL and 8 μg/mL. The relationship between antimicrobial activity and compound structure has suggested that the presence of hydroxyl groups seems to be essential for antimicrobial activity of phenolic compounds.
Keywords: azo-compound, Gram-positive antibacterial, listeria monocytogenes, synthesis
1. Introduction
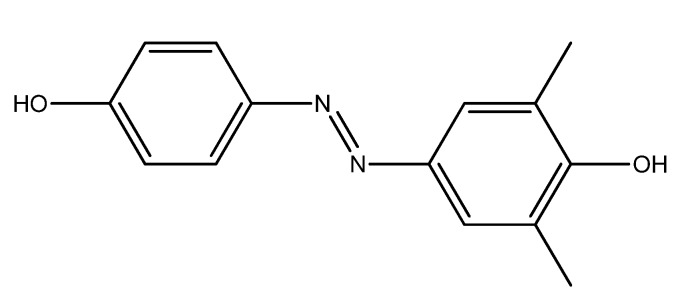
Substituted azobenzene generally have a color ranging from yellow to red, due to the extensive conjugated system and presence of various electron-withdrawing or electron-donating substituents on the aromatic rings. A key feature of azobenzene and other molecules characterized by a high conjugated pattern is its ability to absorb light with a consequent modification of its structure through the transition from cis isomeric form to the trans form. This feature is widely-used in light-responsive materials [1,2,3,4,5], liquid crystals [6,7], electronic devices [8,9,10,11], or in NLO chromophores [12,13]. In recent papers, we reported a series of azobenzene-based molecules with antimicrobial properties [14,15,16,17]. For example, studies carried out in our labs showed that A4 (Figure 1) is one of the most active of the series of compounds synthesized so far, showing antimicrobial activity against Staphylococcus aureus (MIC100 20 μg/mL, the Minimum Inhibitory Concentration required to inhibit the growth of 100% of bacteria), against Candida albicans (MIC0 17 μg/mL, the Minimum Inhibitory Concentration required to inhibit the growth of 100% of fungi), and Listeria monocytogenes (MIC100 25 μg/mL) [16].
Figure 1.
Chemical structure of lead compound A4 [16].
Listeria monocytogenes is one of the most dangerous food pathogens. It shows the ability to survive adverse conditions such as vacuum, low temperature, wide range of pH (4–9), ultraviolet rays and is resistant to conventional pasteurization [18,19,20,21]. Human listeriosis is most often caused by L. monocytogenes serogroup 4 [22,23]. The ingestion of products contaminated with this organism may be a potential health threat to high-risk populations, such as the immune-suppressed, pregnant women, and the elderly [24,25]. Azo-compounds that possess antilisterial properties may therefore be expected to become valuable additives as consumer concern with preventing L. monocytogenes infections increases.
In an attempt to improve the bioavailability of these molecules, their effectiveness and/or specificity of action, various structural modifications have been proposed of the lead compound A4, in order to obtain compounds with better physicochemical properties, while maintaining their antimicrobial activity [26]. In order to evaluate the effect of different substituents on the azobenzene structure, we introduced some new structural changes.
By moving the hydroxyl group from one azobenzene ring to the other and/or adding substituents on both the rings, we obtained compounds with improved antimicrobial activity, with remarkable MIC100 value of 10 μg/mL against S. aureus, and MIC0 of 3 μg/mL against C. albicans [26]. Nevertheless, for some new compounds reported here (like 4l), a tendency to crystallize from aqueous solution was also observed during the period of incubation, and in these cases, it was not possible to reach high concentration of active molecule in the dilution tests. For these molecules, the presence of aromatic rings with hydroxyl groups induces a slow crystallization of the molecule, probably due to the easy packing of the aromatic structures.
Starting from these observations, we decided to improve the activity of these molecules and to achieve a better understanding of the structure-activity relationship of this class of compounds. The changes include the introduction of alkyl derivatives from four to eight carbon atoms such as butyl, isobutyl, neopentyl, isopentyl, and hexyl moieties, in order to reduce lattice energies in the crystals. The main goal was to prevent the formation of highly ordered crystals that can be an obstacle in reaching the most convenient solubility for pharmaceutical applications.
2. Results and Discussion
2.1. Chemistry
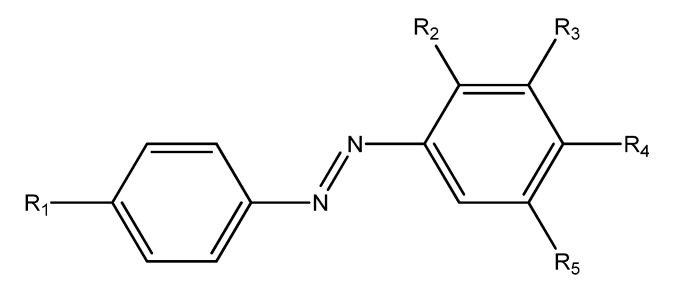
The chemical structures of substituted azobenzene derivatives (4a–m) are reported in Figure 2.
Figure 2.
Substituted-(phenyl-diazenyl) phenols derivatives (4a–m).
| R1 | R2 | R3 | R4 | R5 | |
| 4a | O(CH2)3CH3 | H | CH3 | OH | CH3 |
| 4b | OCH2CH(CH3)2 | H | CH3 | OH | CH3 |
| 4c | OCH2C(CH3)3 | H | CH3 | OH | CH3 |
| 4d | OCH2CH2CH(CH3)2 | H | CH3 | OH | CH3 |
| 4e | OCH2CH(CH2CH3)CH2(CH2)2CH3 | H | CH3 | OH | CH3 |
| 4f | H | H | CH3 | OCH3 | CH3 |
| 4g | OCH2CH(CH3)2 | OH | H | OH | H |
| 4h | OCH2C(CH3)3 | OH | H | OH | H |
| 4i | OCH2CH2CH(CH3)2 | OH | H | OH | H |
| 4j | OCH2CH(CH2CH3)CH2(CH2)2CH3 | OH | H | OH | H |
| 4k | OH | OH | H | OH | H |
| 4l | CH3 | OH | H | OH | H |
| 4m | OCH3 | OH | H | OH | H |
| R1 | R2 | R3 | R4 | R5 | |
| 4a | O(CH2)3CH3 | H | CH3 | OH | CH3 |
| 4b | OCH2CH(CH3)2 | H | CH3 | OH | CH3 |
| 4c | OCH2C(CH3)3 | H | CH3 | OH | CH3 |
| 4d | OCH2CH2CH(CH3)2 | H | CH3 | OH | CH3 |
| 4e | OCH2CH(CH2CH3)CH2(CH2)2CH3 | H | CH3 | OH | CH3 |
| 4f | H | H | CH3 | OCH3 | CH3 |
| 4g | OCH2CH(CH3)2 | OH | H | OH | H |
| 4h | OCH2C(CH3)3 | OH | H | OH | H |
| 4i | OCH2CH2CH(CH3)2 | OH | H | OH | H |
| 4j | OCH2CH(CH2CH3)CH2(CH2)2CH3 | OH | H | OH | H |
| 4k | OH | OH | H | OH | H |
| 4l | CH3 | OH | H | OH | H |
| 4m | OCH3 | OH | H | OH | H |
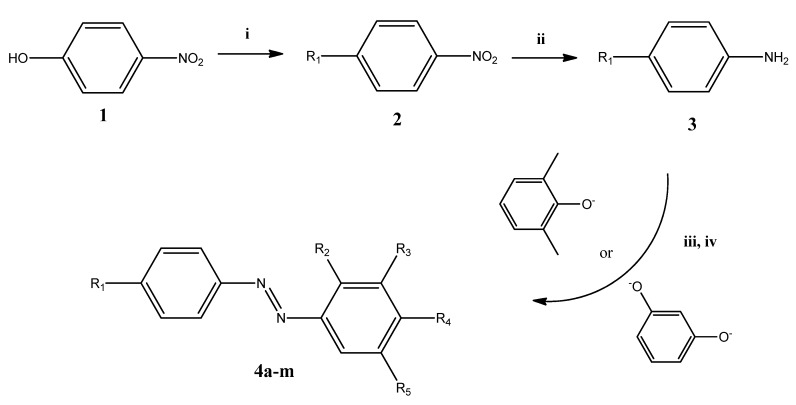
The synthetic scheme of substituted-azobenzene (4a–m) derivatives is reported in Figure 3.
Figure 3.
Synthetic route for compounds 4a–m. i: Alkylhalide, K2CO3, DMF reflux, 3 h; ii: SnCl2, ethanol reflux, 2 h; iii: 0–5 °C, NaNO2 (aq), HCl 37%; iv: 10–15 °C, 1,3-dimethylphenol (for 4a–f) or resorcinol (for 4g–m), NaOH (aq). For Rn moieties, see Figure 2.
2.2. Thermal and Optical Properties
In Table 1, thermal and optical properties of the synthesized compounds are reported. Analogues 4b–m showed only a melting peak in the first heating run and they were not able to crystallize from the melt. Only compound 4a, after the melting peak at 88.7 °C, shows a crystallization phenomenon during the cooling run, at 53.3 °C. When heated in the second run, it showed the same melting peak as the first. Compound 4c showed three melting peaks in the first heating run at 85.0, 116.7, and 126.0 °C, ascribed to a polymorphism, as confirmed by optical observation under polarized light, but it was not possible to observe any crystallization from the melt.
Table 1.
Thermal and optical properties of compounds 4a–m.
| Molecule | Thermal Characterization | Optical Characterization | ||||
|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | λmax (nm) | εmax (L mol−1 cm−1) | |
| 4a | 88.7 | 76.5 | 53.35 | 66.17 | 360 | 26,000 |
| 4b | 126.5 | 74.3 | - | - | 360 | 25,000 |
| 4c | 85.00 | 52.6 | - | - | 360 | 26,100 |
| 116.7 | 19.5 | |||||
| 126.0 | 10.8 | |||||
| 4d | 74.2 | 88.4 | - | - | 361 | 38,000 |
| 4e | 59.2 | 56.1 | - | - | 361 | 33,100 |
| 4f * | - | - | - | - | ||
| 4g | 156.3 | 54.2 | - | - | 384 | 3300 |
| 4h | 179.3 | 80.2 | - | - | 384 | 29,500 |
| 4i | 125.3 | 73.6 | - | - | 384 | 35,700 |
| 4j | 82.6 | 47.2 | - | - | 384 | 3000 |
| 4k | 235 | 75.4 | - | - | 382 | 8300 |
| 4l | 238 | 91.5 | - | - | 447 | 7800 |
| 4m | 160 | 24.8 | - | - | 344 | 4600 |
Tm = melting temperature, from DSC analysis, 10 °C/min, nitrogen flow; Tc = crystallization temperature, from DSC cooling run; Instrument error ±0.5 °C. ΔHm/ΔHc = melting/crystallization enthalpy, evaluated by integration of the peak. Experimental error ±5%. λmax = wavelength at the principal absorption maximum, εmax = molar extinction coefficient at absorption maximum. * Compound 4f is an oil at room temperature.
The spectral region 650–240 nm was investigated by UV-Vis spectrophotometry at a concentration of about 3.0 × 10−5 mol L−1 of azo-compound in acetonitrile solution (Table 1). The UV-visible spectra for 4a–m are mainly dependent only on the azobenzene unit, which is the same for all compounds. The UV-Vis absorption spectra of all the analogues in solution, showed the typical absorption bands of the electronic transitions of the azobenzene chromophore, with absorption maxima ranging from 360 nm to 447 nm.
2.3. Antibacterial Activity
The MIC100 of all synthesized compounds was determined by the microbroth dilution method for Staphylococcus aureus ATCC 29213, a Listeria monocytogenes clinical strain, Pseudomonas aeruginosa PAO1, and Escherichia coli MG1655 reference strains (Table 2).
Table 2.
Antimicrobial activity of 4a–m analogues, expressed as MIC100 (μg/mL).
| MIC100 (μg/mL) after 24 h | ||||
|---|---|---|---|---|
| Staphylococcus aureus | Listeria monocytogenes | Escherichia coli | Pseudomonas aeruginosa | |
| 4a | 32 | >128 | >128 | >128 |
| 4b | 128 | 128 | >128 | >128 |
| 4c | >128 | >128 | >128 | >128 |
| 4d | 4 | 8 | >128 | >128 |
| 4e | 12 | 48 | >128 | >128 |
| 4f | >128 | >128 | >128 | >128 |
| 4g | >128 | 96 | >128 | >128 |
| 4h | 4 | 8 | >128 | >128 |
| 4i | 4 | 8 | >128 | >128 |
| 4j | 16 | 16 | >128 | >128 |
| 4k | >128 | >128 | >128 | >128 |
| 4l | >32 * | >32 * | >32 * | >32 * |
| 4m | 16 | 16 | >128 | >128 |
MIC100: Minimum Inhibitory Concentration required inhibiting the growth of 100% of organisms after 24 h. The values are the geometric mean of at least three determinations. * Dissolution problems: For these compounds, the initial solution was prepared to achieve 128 μg/mL, but in the end, a maximum MIC of 32 μg/mL was reached, before observing a crystallization of the compound.
For six of the analyzed compounds, a strong activity against S. aureus and L. monocytogenes was measured, with MIC100 values up to 4 μg/mL and 8 μg/mL, respectively (for 4d, 4h and 4i). To our knowledge, these MIC100 values are the lowest ever reached for antimicrobial azobenzene compounds. On the contrary, the activity against gram-bacteria E. coli and P. aeruginosa was not detectable for any of the synthesized compound, confirming the trend already observed in our previous reports [16,26]. In particular, the high antibacterial activity against Listeria is particularly noteworthy, because of the ability of this bacterium to survive in several adverse conditions. Since we do not know the mechanism of action, it is not easy to rationalize the activity of all the analogues 4a–m. Nevertheless, some general considerations can be done, regarding their behavior. The presence of long and disordered aliphatic chains produced the expected effect on the tendency to crystallization for many of the obtained compounds, making them more soluble and available in the incubation medium, compared to analogues 4k–m. For these, it was not possible to achieve high concentration in the dilution tests, due to their easy precipitation from the solution. Another consideration is that by completely removing all hydroxyl groups from the azobenzene moiety, we obtained a complete loss of antibacterial activity (compound 4f); this indicates that the possibility of making hydrogen bonds is probably fundamental for the interaction of these antibiotics with their target.
3. Materials and Methods
3.1. General
All reagents and solvents were purchased from Sigma-Aldrich (Milan, Italy) and used without further purification. Optical observations were performed by using a Jenapol (Zeiss S.p.A., Milano, Italy) microscope fitted with a Linkam THMS 600 hot stage (Linkam, Waterfield, Epsom, Tadworth, UK). Phase transition temperatures and enthalpies were measured using a DSC scanning calorimeter Perkin Elmer Pyris 1 (PerkinElmer, Waltham, MA, USA) at a scanning rate of 10 °C/min, under nitrogen flow. UV absorption spectra of the samples were recorded at 25 °C in acetonitrile solution, on a Perkin Elmer Lambda 19 spectrophotometer. The spectral region 650–240 nm was investigated using a cell path length of 1.0 cm. Azobenzene chromophore concentration of about 3.0 × 10−5 mol L−1 was used. 1H-NMR and 13C-NMR spectra were recorded with a Bruker DRX/400 Spectrometer (Bruker, Billerica, MA, USA).
High-resolution mass spectra were acquired on a LTQ-Orbitrap instrument (Thermo-Fisher, Waltham, MA, USA) operating in negative (compounds 4a–e, 4g–m) or positive (compound 4f) ion mode. Each compound was singularly dissolved in methanol at a concentration of 0.1 mg/mL and injected into the MS ion source. Spectra were acquired in the 150–400 m/z range.
3.2. General Method of the Synthesis of (Phenyl-diazenyl)phenols Derivatives 4a–m
3.2.1. Synthesis of 1-Alkyloxy-4-nitrobenzene (2a–e, 2g–j)
A suspension of 0.0216 mol of p-nitrophenol in 25 mL of DMF was prepared. Then, 6.0 g of K2CO3 and 0.0264 mol of alkyliodide or bromide were added, and the solution refluxed for 3 h. The solution was then filtered in water and the crude precipitate was recovered and dried under vacuum. Yield ranged between 60 and 90%.
1-Butoxy-4-nitrobenzene (2a). 1H-NMR CDCl3: (δ, ppm) = 8.15 (d, 2H), 7.25 (d, 2H), 4.06 (t, 2H), 1.76 (m, 2H), 1.47 (m, 2H), 0.99 (t, 3H). Yield 85%.
1-(Isobutoxy)-4-nitrobenzene (2b, 2g). 1H-NMR (DMSO-d6): (δ, ppm) = 8.19 (d, 2H), 7.15 (d, 2H), 3.90 (d, 2H), 2.01 (m, 1H), 0.91 (d, 6H). Yield 80%.
1-(Neopentyloxy)-4-nitrobenzene (2c, 2h). 1H-NMR (DMSO-d6): (δ, ppm) = 8.19 (d, 2H), 7.15 (d, 2H), 3.90 (s, 2H), 1.05 (s, 9H). Yield 60%.
1-(Isopentyloxy)-4-nitrobenzene (2d, 2i). 1H-NMR (DMSO-d6): (δ, ppm) = 8.16 (d, 2H), 7.10 (d, 2H), 4.10 (t, 2H), 1.78 (m, 3H), 0.91 (d, 6H). Yield 60%.
1-((2-Ethylhexyl)oxy)-4-nitrobenzene (2e, 2j). 1H-NMR (DMSO-d6): (δ, ppm) = 8.18 (d, 2H), 7.15 (d, 2H), 4.01 (d, 1H), 3.95 (d, 1H), 1.71 (m, 1H), 1.38 (m, 4H), 1.32 (m, 4H), 0.88 (m, 6H). Yield 65%.
3.2.2. Synthesis of 4-Alkyloxyaniline (3a–e, 3g–j)
The appropriate 1-alkyloxy-4-nitrobenzene (0.0270 mol) was dissolved in 70 mL of ethanol. Tin chloride (0.119 mol) was dissolved in 30 mL of ethanol and was added dropwise. The reaction was set to take place for two hours at 80 °C. The crude product was extracted twice in a mixture of ethyl acetate/water (1:1), in which 60 g of potassium carbonate was dissolved. The organic solvent was evaporated at reduced pressure and the product was obtained as a yellow oil. Yield ranged between 65 and 85%.
4-Butoxyaniline (3a). 1H-NMR (DMSO-d6): (δ, ppm) = 6.64 (d, 2H), 6.52 (d, 2H), 4.56 (s, 2H), 3.58 (t, 2H), 1.75(m, 2H), 1.39 (m, 2H), 0.99 (t, 3H). Yield 65%.
4-Isobutoxyaniline (3b, 3g). 1H-NMR (DMSO-d6): (δ, ppm) = 6.64 (d, 2H), 6.50 (d, 2H), 4.56 (s, 2H), 3.46 (d, 2H), 2.22 (m, 1 H), 0.96 (s, 6H). Yield 65%.
4-Neopentyloxyaniline (3c, 3h). 1H-NMR (DMSO-d6): (δ, ppm) = 6.74 (d, 2H), 6.66 (d, 2H), 4.03 (s, 2H), 3.39 (s, 2H), 1.55 (s, 9H). Yield 85%.
4-Isopentyloxyaniline (3d, 3i). 1H-NMR (DMSO-d6): (δ, ppm) = 8.16 (d, 2H), 7.10 (d, 2H), 4.10 (t, 2H), 3.42 (s, 2H), 1.74 (m, 1H), 1.63 (m, 2H), 0.91 (d, 6H). Yield 83%.
4-((2-Ethylhexyl)oxy)aniline (3e, 3j). 1H-NMR (DMSO-d6): (δ, ppm) = 6.74 (d, 2H), 6.66 (d, 2H), 4.03 (d, 2H), 3.40 (d, 2H), 1.98 (m, 1H), 1.55 (m, 2H), 1.25 (m, 6H), 0.90 (m, 6H). Yield 85%.
3.2.3. Synthesis of Azobenzene Derivatives 4a–m
Azo compounds 4a–m were synthesized according to the classic scheme of diazo coupling reactions, as reported in ref. [16], and illustrated in Figure 3. The general procedure was the following: 0.0052 mol of the selected alkyloxyaniline (3a–m) were suspended in a solution containing 4.0 mL of water and 1.1 mL of HCl 37% (w/w). The solution was cooled at 0–5 °C in a water/ice bath. A solution of 0.42 g of sodium nitrite (0.006 mol) dissolved in 1.2 mL of water was added drop-wise, obtaining a suspension of the diazonium salt (solution A). Separately, a solution containing 1.72 g of NaOH (0.0420 mol) in 16 mL of water with 0.0052 mol of the proper phenol (depending on 4a–m) was prepared (solution B). Solution A was added drop-wise to solution B, under stirring at 12 °C, adjusting the pH at 9–10, with addition of NaOH, if necessary. The system was left reacting for 30 min. Then, 1.2 mL of acetic acid were added, in order to reach a pH value of 5–6, under stirring. A reddish precipitate of the azo compound formed. The crude precipitate was filtered and dried under vacuum. Yields ranged between 45 and 90%.
4-((4-Butoxyphenyl)diazenyl)-2,6-dimethylphenol 4a. 4-Butoxyaniline (3a) and 2-6-dimethylphenol were used as starting reagents. The crude product was crystallized from water/ethanol (10:1) to give pure 4a as gold yellow crystals. Final yield 85%. 1H-NMR (DMSO-d6): (δ, ppm) = 9.01 (broad, 1H), 7.76 (d, 2H), 7.49 (s, 2H), 7.07 (d, 2H), 4.06 (t, 2H), 2.25 (s, 6H), 1.76 (m, 2H), 1.47 (m, 2H), 0.99 (t, 3H). 13C-NMR (DMSO-d6): (δ, ppm) = 163.3; 161.2; 148.5; 146.3; 127.7; 122.7; 116.2; 70.6; 31.3; 20.7; 17.4, 14.7. HR-MS (m/z): 298.17 [M − H]−.
4-((4-Isobutoxyphenyl)diazenyl)-2,6-dimethylphenol 4b. 4-Isobutoxyaniline (3b) and 2-6-dimethylphenol were used as starting reagents. The crude product was crystallized from water/ethanol (10:1) to give pure 4b as yellow crystals. Final yield 90%. 1H-NMR (DMSO-d6): (δ, ppm) = 7.76 (d, 2H), 7.49 (s, 2H), 7.07 (d, 2H), 4.06 (d, 2H), 3.85 (s, 1H), 2.25 (s, 6H), 2.04 (m, 1H), 1.03 (d, 6H). 13C-NMR (DMSO-d6): (δ, ppm) = 163.4; 161.0; 148.6; 146.1; 127.6; 124.6; 124.3; 116.5; 75.0; 27.5; 20.3; 17.2. HR-MS (m/z): 298.17 [M − H]−.
2,6-Dimethyl-4-((4-(neopentyloxy)phenyl)diazenyl)phenol 4c. 4-Neopentyloxyaniline (3c) and 2-6-dimethylphenol were used as starting reagents. The crude product was crystallized from water/ethanol (10:1) to give pure 4c as dark yellow crystals. Final yield 40%. 1H-NMR (acetone-d6): (δ, ppm) = 7.80 (d, 2H), 7.53 (s, 2H), 7.05 (d, 2H), 4.06 (s, 2H), 2.29 (s, 6H), 1.03 (s, 9H). 13C-NMR (DMSO-d6): (δ, ppm) = 163.6; 161.0; 148.9; 146.0; 127.5; 124.5; 124.0; 117.0; 79.5; 32.6; 26.6; 17.1. HR-MS (m/z): 312.18 [M − H]−.
4-((4-(Isopentyloxy)phenyl)diazenyl)-2,6-dimethylphenol 4d. 4-Isopentyloxyaniline (3d) and 2-6-dimethylphenol were used as starting reagents. The crude product was crystallized from water/ethanol (10:1) to give pure 4d as dark yellow crystals. Final yield 85%. 1H-NMR (acetone-d6): (δ, ppm) = 7.83 (d, 2H), 7.56 (s, 2H), 7.05 (d, 2H), 4.14 (t, 2H), 2.32 (s, 6H), 1.87 (m, 1H), 1.72 (m, 2H), 0.98 (d, 6H). 13C-NMR (DMSO-d6): (δ, ppm) = 163.1; 161.0; 148.3; 146.1; 127.6; 124.6; 115.9; 70.8; 38.0; 28.1; 23.3; 17.2. HR-MS (m/z): 311.18 [M − H]−.
4-((4-((2-Ethylhexyl)oxy)phenyl)diazenyl)-2,6-dimethylphenol 4e. 4-((2-Ethylhexyl)oxy)aniline (3e) and 2-6-dimethylphenol were used as starting reagents. The crude product was crystallized from water/ethanol (10:1) to give pure 4e as orange crystals. Final yield 60%. 1H-NMR (acetone-d6): (δ, ppm) = 7.83 (d, 2H), 7.55 (s, 2H), 7.08 (d, 2H), 4.10 (t, 2H), 2.32 (s, 6H), 1.75 (m, 1H), 1.52 (m, 2H), 1.45 (m, 6H), 0.93(m, 6H). 13C-NMR (DMSO-d6): (δ, ppm) = 163.4; 161.0; 148.6; 146.1; 127.6; 124.5; 124.2; 116.4; 73.4; 39.7; 31.8; 30.5; 24.9; 24.0; 17.2; 14.6; 12.2. HR-MS (m/z): 354.23 [M − H]−.
1-(4-Methoxy-3,5-dimethylphenyl)-2-phenyldiazene 4f. The procedure for obtaining 4f was a simple methylation of compound 3b, described in ref [26]. Methyl iodide (0.55 mL) was added to a solution containing 1.00 g of 3b and 1.22 g of potassium carbonate in 15.0 mL of DMF. The reaction was conducted under reflux for 5 h. The final mixture was then poured in 100 mL of cold distilled water. The organic layer was then extracted in chloroform, dried with sodium sulfate, and the solvent was removed under reduced pressure. Final yield 57%. 1H-NMR (DMSO-d6): (δ, ppm) = 7.95 (s, 2H), 7.85 (s, 2H), 7.62 (m, 3H), 3.74 (s, 3H), 2.33 (s, 6H). 13C-NMR (DMSO-d6): (δ, ppm) = 162.6; 153.1; 148.3; 130.7; 130.0; 129.7; 125.8; 123.0; 61.2; 17.1. HR-MS (m/z): 241.13 [M + H]+.
4-((4-Isobutoxyphenyl)diazenyl)benzene-1,3-diol 4g. 4-Isobutoxyaniline (3g) and resorcinol were used as starting reagents. The crude product was purified by column chromatography (ethyl acetate/hexane 3:7) to give pure 4g as red crystals. Final yield 40%. 1H-NMR (Acetone-d6): (δ, ppm) = 7.85 (d, 2H), 7.71 (d, 1H), 7.10 (d, 2H), 6.59 (d, 2H), 6.40 (s, 1H), 3.88 (d, 2H), 1.05 (d, 6H). 13C-NMR (DMSO-d6): (δ, ppm) = 164.5; 163.4; 161.2; 148.6; 134.6; 125.1; 124.2; 116.4; 110.6; 103.7; 75.0; 27.5; 20.3. HR-MS (m/z): 286.13 [M − H]−.
4-((4-(Neopentyloxy)phenyl)diazenyl)benzene-1,3-diol 4h. 4-Neopentyloxyaniline (3h) and resorcinol were used as starting reagents. The crude product was purified by column chromatography (ethyl acetate/hexane 3:7) to give pure 4h as brilliant red crystals. Final yield 40%. 1H-NMR (Acetone-d6): (δ, ppm) = 7.83 (d, 2H), 7.71 (d, 1H), 7.11 (d, 2H), 6.59 (d, 1H), 6.40 (s, 1H), 3.76 (s, 2H), 1.06 (s, 9H). 13C-NMR (DMSO-d6): (δ, ppm) = 164.5; 163.6; 161.2; 148.9; 134.7; 125.1; 124.0; 117.1; 110.6; 103.5; 79.3; 32.4; 26.4. HR-MS (m/z): 300.15 [M − H]−.
4-((4-(Isopentyloxy)phenyl)diazenyl)benzene-1,3-diol 4i. 4-Isopentyloxyaniline (3i) and resorcinol were used as starting reagents. The crude product was purified by column chromatography (ethyl acetate/hexane 3:7) to give pure 4i as dark red crystals. Final yield 45%. 1H-NMR (Acetone-d6): (δ, ppm) = 7.84 (d, 2H), 7.71 (d, 1H), 7.10 (d, 2H), 6.60 (d, 1H), 6.40 (s, 1H), 4.15 (t, 2H), 1.81(m, 1H), 1.71 (m, 2H), 0.98 (d, 6H). 13C-NMR (DMSO-d6): (δ, ppm) = 164.2; 162.7; 160.8; 147.9; 134.3; 124.7; 124.1; 115.5; 110.3; 103.4; 70.4; 37.6; 27.8; 22.9. HR-MS (m/z): 300.15 [M − H]−.
4-((4-((2-Ethylhexyl)oxy)phenyl)diazenyl)benzene-1,3-diol 4j. 4-((2-Ethylhexyl)oxy)aniline (3j) and resorcinol were used as starting reagents. The crude product was purified by column chromatography (ethyl acetate/hexane 3:7) to give pure 4j as red crystals. Final yield 35%. 1H-NMR (Acetone-d6): (δ, ppm) = 7.80 (d, 2H), 7.67 (d, 1H), 7.08 (d, 2H), 6.56 (d, 1H), 6.36 (s, 1H), 3.98 (d, 2H), 2,01(m, 1H), 1.74 (m, 2H), 1.48(m, 2H), 1.32 (m, 4H), 0,90 (m, 6H). 13C-NMR (DMSO-d6): (δ, ppm) = 163.8; 162.8; 160.5; 147.9; 133.9; 124.3; 123.5; 115.9; 110.1; 103.2; 72.6; 39.1; 31.2; 29.9; 24.3; 23.6; 14.1; 11.6. HR-MS (m/z): 342.19 [M − H]−.
4-((4-Hydroxyphenyl)diazenyl)benzene-1,3-diol 4k. 4-Aminophenol and resorcinol were used as starting reagents. The crude product was crystallized from water/methanol (10:1) to give pure 4k as red crystals. Final yield 73%. 1H-NMR (DMSO-d6): (δ, ppm) = 10.37, 10.18, 7.74 (d, 2H), 7.62 (d, 1H), 6.91 (d, 2H), 6.50–6.46 (dd, 1H), 6.32 (s, 1H). 13C-NMR (DMSO-d6): (δ, ppm) = 164.1; 160.9; 160.6; 146.8; 134.1; 124.7; 124.4; 116.9; 110.1; 103.1. HR-MS (m/z): 230.07 [M − H]−.
4-(p-Tolyldiazenyl)benzene-1,3-diol 4l. p-Toluidine and resorcinol were used as starting reagents. The crude product was crystallized from boiling n-octane and then from water/methanol (10:1) to give pure 4l as red crystals. Final yield 81%. 1H-NMR (DMSO-d6): (δ, ppm) = 7.63 (d, 2H); 7.50 (d, 2H); 7.44 (d, 1H); 7.34 (d, 2H); 7.27 (d, 2H); 2.33 (d, 3H). 13C-NMR (DMSO-d6): (δ, ppm) = 163.7; 160.4; 149.2; 140.7; 134.1; 129.7; 124.4; 122.7; 110.1; 103.1; 21.3. HR-MS (m/z): 228.09 [M − H]−.
4-((4-Methoxyphenyl)diazenyl)benzene-1,3-diol 4m. 4-Methoxyaniline and resorcinol were used as starting reagents. The crude product was crystallized from boiling n-octane and then from water/methanol (10:1) to give pure 4m as red crystals. Final yield 45%. 1H-NMR (DMSO-d6): (δ, ppm) = 10.39 (s, 1H); 7.83 (d, 2H); 7.62 (d, 2H); 7.08 (d, 2H); 7.47 (d, 1H); 6.34 (s, 1H); 3.83 (s, 3H). 13C-NMR (DMSO-d6): (δ, ppm) = 163.7; 162.1; 160.4; 147.2; 133.9; 124.4; 114.8; 110.2; 103.2; 56.2. HR-MS (m/z): 244.08 [M − H]−.
3.3. Antimicrobial Tests
Bacterial strains and antimicrobial compounds susceptibility determinations.
Four bacterial species were used as models for susceptibility testing; two Gram-negative: Pseudomonas aeruginosa PAO1 and Escherichia coli MG1655 reference strains; and two Gram-positive: Staphylococcus aureus ATCC 29213 strain and a Listeria monocytogenes clinical strain isolated in Hospital Son Espases, Palma de Mallorca, Spain. These strains were cultured on blood agar plates, at 37 °C the day before susceptibility determination, which was assessed through the Minimum Inhibitory Concentration (MIC). The MICs of the different antimicrobial peptides were determined by the Müller Hinton Broth (MHB) microdilution method, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [27]. Briefly, a stock solution of each compound was prepared, following their particular solubility patterns (DMSO, MilliQ water, etc.). This stock solution was then diluted at least 1:50 in MHB to the appropriate concentration of antimicrobial. The MHB containing the appropriate concentrations of antimicrobial compounds was poured into 96-wells plates (100 μL per well), making 1:2 dilutions with fresh MHB on each column of the plate (concentrations ranging from 128 μg/mL to 0.0625 μg/mL). To inoculate each well, 0.5 McFarland bacterial suspensions were prepared in saline solution. Afterwards, 1:100 dilutions in MHB were obtained from those to yield an approx. concentration of 1 × 106 CFU/mL. Finally, 100 μL of these suspensions were used to inoculate the corresponding wells, mixing them with the previously-added MHB containing the antimicrobial compound. Positive (MHB with bacteria but without antimicrobial compound) and negative (MHB alone) controls were routinely prepared in several wells of the plates. Once inoculated, the plates were incubated overnight at 37 °C, and the lowest antimicrobial concentration well in which the bacteria did not grow was considered the MIC. The experiments were always performed in duplicate, in at least two independent occasions, and median MIC values were considered.
4. Conclusions
Some novel derivatives of (phenyl-diazenyl)phenol (4a–m) were designed, synthesized, and biologically evaluated as antibacterial agents. Many of the synthesized compounds exhibited a significant Gram-positive specific antibacterial activity, in particular against S. aureus and L. monocytogenes, and they were inactive against Gram-negative bacteria. Three of the synthesized analogues show the best antibacterial activity ever measured for azobenzene compounds. 4-((4-(isopentyloxy)phenyl)diazenyl)-2,6-dimethylphenol (4d), 4-((4-(neopentyloxy)phenyl)diazenyl)benzene-1,3-diol (4h), and 4-((4-(isopentyloxy)phenyl)diazenyl)benzene-1,3-diol (4i) were able to inhibit the growth of 100% of S. aureus and L. monocytogenes at concentrations three times lower than the lead compound.
The most active antibacterial compounds contain hydroxyl groups, together with long and branched aliphatic chains in their structure. A complete loss of activity was registered for derivative 4f, which lacks in hydroxyl groups. These observations suggest that the possibility of making hydrogen bonds is probably fundamental for the interaction of these antibiotic molecules with their target receptor.
Thanks to the introduction of particularly bulky and flexible side chains, the molecules presented here could be easily incorporated in DPC micelles, to further increase the drug release. Finally, we could observe that the complete removal of all hydroxyl groups from the azobenzene moiety led to the complete loss of antibacterial activity (compound 4f).
Acknowledgments
This research work was financially supported by the Italian Minister of Instruction, University and Research (MIUR)–300395FRB16. The authors are grateful to Fabrizio dal Piaz for the mass spectral measurements.
Author Contributions
Simona Concilio, Stefano Piotto and Pio Iannelli designed the idea, and the protocol of the study. Simona Concilio and Stefano Piotto helped in synthesizing the compounds and wrote the manuscript. Lucia Sessa, Rosita Diana and Ugo Caruso synthesized and characterized the compounds, wrote the experimental parts and interpreted the experimental data. Gabriel Torrens and Carlos Juan performed the biological studies and wrote the experimental data. All the authors revised the whole manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Pirani F., Angelini A., Frascella F., Rizzo R., Ricciardi S., Descrovi E. Light-driven reversible shaping of individual azopolymeric micro-pillars. Sci. Rep. 2016;6:31702. doi: 10.1038/srep31702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelini A., Pirani F., Frascella F., Ricciardi S., Descrovi E. Light-driven liquid microlenses; Proceedings of the SPIE—The International Society for Optical Engineering; San Francisco, CA, USA. 28 January 2017. [Google Scholar]
- 3.Pirani F., Angelini A., Ricciardi S., Frascella F., Descrovi E. Laser-induced anisotropic wettability on azopolymeric micro-structures. Appl. Phys. Lett. 2017;110:101603. doi: 10.1063/1.4978260. [DOI] [Google Scholar]
- 4.Morvillo P., Diana R., Fontanesi C., Ricciardi R., Lanzi M., Mucci A., Tassinari F., Schenetti L., Minarini C., Parenti F. Low band gap polymers for application in solar cells: Synthesis and characterization of thienothiophene-thiophene copolymers. Polym. Chem. 2014;5:2391–2400. doi: 10.1039/c3py01618h. [DOI] [Google Scholar]
- 5.Parenti F., Morvillo P., Bobeico E., Diana R., Lanzi M., Fontanesi C., Tassinari F., Schenetti L., Mucci A. (Alkylsulfanyl) bithiophene-alt-fluorene: Π-conjugated polymers for organic solar cells. Eur. J. Org. Chem. 2011;2011:5659–5667. doi: 10.1002/ejoc.201100738. [DOI] [Google Scholar]
- 6.Acierno D., Amendola E., Bugatti V., Concilio S., Giorgini L., Iannelli P., Piotto S.P. Synthesis and characterization of segmented liquid crystalline polymers with the azo group in the main chain. Macromolecules. 2004;37:6418–6423. doi: 10.1021/ma049319k. [DOI] [Google Scholar]
- 7.Caruso U., Diana R., Panunzi B., Roviello A., Tingoli M., Tuzi A. Facile synthesis of new pd (II) and cu (II) based metallomesogens from ligands containing thiophene rings. Inorg. Chem. Commun. 2009;12:1135–1138. doi: 10.1016/j.inoche.2009.09.006. [DOI] [Google Scholar]
- 8.Attianese D., Petrosino M., Vacca P., Concilio S., Iannelli P., Rubino A., Bellone S. Switching device based on a thin film of an azo-containing polymer for application in memory cells. IEEE Electron Device Lett. 2008;29:44–46. doi: 10.1109/LED.2007.910792. [DOI] [Google Scholar]
- 9.Angiolini L., Benelli T., Giorgini L., Golemme A., Mazzocchetti L., Termine R. Effect of a chiral substituent on the photochromic and photoconductive properties of a methacrylic polymer bearing side chain azocarbazole moieties. Dyes Pigment. 2014;102:53–62. doi: 10.1016/j.dyepig.2013.10.024. [DOI] [Google Scholar]
- 10.Borbone F., Caruso U., Causà M., Fusco S., Panunzi B., Roviello A., Shikler R., Tuzi A. Series of O, N, O-tridentate ligands zinc (II) complexes with high solid-state photoluminescence quantum yield. Eur. J. Inorg. Chem. 2014;2014:2695–2703. doi: 10.1002/ejic.201400095. [DOI] [Google Scholar]
- 11.Caruso U., Panunzi B., Roviello A., Tuzi A. Fluorescent metallopolymers with Zn (II) in a schiff base/phenoxide coordination environment. Inorg. Chem. Commun. 2013;29:138–140. doi: 10.1016/j.inoche.2012.11.037. [DOI] [Google Scholar]
- 12.Borbone F., Carella A., Caruso U., Roviello G., Tuzi A., Dardano P., Lettieri S., Maddalena P., Barsella A. Large second-order nlo activity in poly (4-vinylpyridine) grafted with pdii and cuii chromophoric complexes with tridentate bent ligands containing heterocycles. Eur. J. Inorg. Chem. 2008;2008:1846–1853. doi: 10.1002/ejic.200701171. [DOI] [Google Scholar]
- 13.Caruso U., Diana R., Fort A., Panunzi B., Roviello A. Macromolecular Symposia. Vol. 234. Wiley Online Library; Naples, Italy: 2006. Synthesis of Polymers Containing Second Order NLO-Active Thiophene and Thiazole Based Chromophores; pp. 87–93. [DOI] [Google Scholar]
- 14.Concilio S., Iannelli P., Sessa L., Olivieri R., Porta A., De Santis F., Pantani R., Piotto S. Biodegradable antimicrobial films based on poly (lactic acid) matrices and active azo compounds. J. Appl. Polym. Sci. 2015;132 doi: 10.1002/app.42357. [DOI] [Google Scholar]
- 15.Piotto S., Concilio S., Sessa L., Iannelli P., Porta A., Calabrese E.C., Galdi M.R., Incarnato L. Novel antimicrobial polymer films active against bacteria and fungi. Polym. Compos. 2013;34:1489–1492. doi: 10.1002/pc.22410. [DOI] [Google Scholar]
- 16.Piotto S., Concilio S., Sessa L., Porta A., Calabrese E.C., Zanfardino A., Varcamonti M., Iannelli P. Small azobenzene derivatives active against bacteria and fungi. Eur. J. Med. Chem. 2013;68:178–184. doi: 10.1016/j.ejmech.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Sessa L., Concilio S., Iannelli P., De Santis F., Porta A., Piotto S. Antimicrobial azobenzene compounds and their potential use in biomaterials; Proceedings of the AIP Conference; Oudeniz, Turkey. 16–19 April 2015. [Google Scholar]
- 18.Farber J.M. Thermal resistance of listeria monocytogenes in foods. Int. J. Food Microbiol. 1989;8:285–291. doi: 10.1016/0168-1605(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 19.Yousef A.H.E., Marth E.L.H. Inactivation of listeria monocytogenes by ultraviolet energy. J. Food Sci. 1988;53:571–573. doi: 10.1111/j.1365-2621.1988.tb07759.x. [DOI] [Google Scholar]
- 20.Gill C.O., Reichel M.P. Growth of the cold-tolerant pathogens yersinia enterocolitica, aeromonas hydrophila and listeria monocytogenes on high-pH beef packaged under vacuum or carbon dioxide. Food Microbiol. 1989;6:223–230. doi: 10.1016/S0740-0020(89)80003-6. [DOI] [Google Scholar]
- 21.Santos M.I., Lima A.I., Monteiro S.A., Ferreira R.M., Pedroso L., Sousa I., Ferreira M.A. Preliminary study on the effect of fermented cheese whey on listeria monocytogenes, escherichia coli O157: H7, and salmonella goldcoast populations inoculated onto fresh organic lettuce. Foodborne Pathog. Dis. 2016;13:423–427. doi: 10.1089/fpd.2015.2079. [DOI] [PubMed] [Google Scholar]
- 22.Gahan C.G.M., Collins J.K. Listeriosis: Biology and implications for the food industry. Trends Food Sci. Technol. 1991;2:89–93. doi: 10.1016/0924-2244(91)90635-V. [DOI] [Google Scholar]
- 23.Farber J., Peterkin P. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swaminathan B., Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9:1236–1243. doi: 10.1016/j.micinf.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Cossart P. Listeriology (1926–2007): The rise of a model pathogen. Microbes Infect. 2007;9:1143–1146. doi: 10.1016/j.micinf.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Sessa L., Petrone A.M., Porta A., Diana R., Iannelli P., Piotto S. Structure modification of an active azo-compound as a route to new antimicrobial compounds. Molecules. 2017;22:875. doi: 10.3390/molecules22060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiegand I., Hilpert K., Hancock R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protoc. 2008;3:163. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]