Abstract
In order to develop novel chemotherapeutic agents with potent anticancer activities, a series of dehydroabietic acid (DHA) derivatives bearing an acylhydrazone moiety were designed and synthesized by the condensation between dehydroabietic acylhydrazide (3) and a variety of substituted arylaldehydes. The inhibitory activities of these compounds against CNE-2 (nasopharynx), HepG2 (liver), HeLa (epithelial cervical), and BEL-7402 (liver) human carcinoma cell lines were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay in vitro. The screening results revealed that many of the compounds showed moderate to high levels of anticancer activities against the tested cancer cell lines and some displayed similar potent inhibitory activities to the commercial anticancer drug cisplatin, while they exhibited lower cytotoxicity against normal human liver cell (HL-7702). Particularly, compound 4w, N’-(3,5-difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide, with an IC50 (50% inhibitory concentration) value of 2.21 μM against HeLa cell, was about 17-fold more active than that of the parent compound, and showed remarkable cytotoxicity with an IC50 value of 14.46 μM against BEL-7402 cell. These results provide an encouraging framework that could lead to the development of potent novel anticancer agents.
Keywords: dehydroabietic acid, acylhydrazone, anticancer activity, synthesis
1. Introduction
Cancer is among the leading causes of death on a global scale and currently the mortality rate has shown an increase in the recent past [1,2,3]. Chemotherapy represents one of the most important therapeutic strategies against various kinds of cancer; however, the available anticancer drugs usually cause toxicity to non-malignant tissues and lead to the development of resistance. Therefore, there is an urgent need for novel molecules with higher selectivity and more potent anticancer activities. Natural products have played a dominant role in drug discovery, especially since around 60% of all anticancer approved drugs are derived from natural resources [4]. Modification of the biologically active natural products has led to the development of potentially important bioactive molecules, leads, and drugs [5]. Encouraged by these results, our interest in investigating natural products for their potential therapeutic effects has recently encouraged us to examine the influences of dehydroabietic acid (DHA) derivatives for their anticancer activity.
DHA is a naturally occurring tricyclic diterpenic resin acid, which can easily be obtained from Pinus rosin or commercial disproportionated rosin [6]. Recently, DHA and its derivatives were claimed to possess a wide range of biological and pharmacological activities, such as antiprotozoal [7], antiulcer [8], anti-inflammatory [9], immunomodulatory, antiviral [10], antimicrobial [11,12], antifungal [13], anxiolytic [14], anti-aging [15], gastroprotective [16], and BK channel-opening [17] activities. In addition, in recent years a number of DHA derivatives have been reported to have anticancer activity in many human cancer cells such as cervical carcinoma cells, hepatocarcinoma cells, lung cancer cells, prostate cancer cells, ovarian cancer cells, and breast cancer cells [18,19,20]. These results suggest that dehydroabietic acid is a promising starting material for the synthesis of new anticancer agents.
On the other hand, acylhydrazone derivatives have been claimed to possess various bioactivities. 8-Hydroxyquinoline containing an acylhydrazone subunit has been reported as a definite potential therapeutic agent against Parkinson’s disease [21]. Some acylhydrazone derivatives bearing 1,3,4-oxadiazole showed excellent antiproliferative activity [22]. N′-(4-Arylidene)-1H-benzo[d]imidazole-2-carbohydrazides demonstrated in vitro inhibitory activity against human tumor cell lines in the low micromolar range [23]. Salicylaldehyde 1-arylmethyl-3-1H-pyrazole-5-carbohydrazide hydrazine derivatives behaved as iron chelators especially in A549 lung cancer cells and this was correlated to their cytotoxic activity [24]. In particular, (E)-2-hydroxybenzaldehyde-5-(2,4-difluorophenyl)-2-furoyl hydrazone (50% inhibitory concentration (IC50) = 16.4 μM) exhibited better anticancer activity than doxorubicin (IC50 = 53.3 μM) against human promyelocytic leukemic cells (HL-60) [25].
Furthermore, a series of dehydroabietic acid and α-pinene derivatives were synthesized and some of them exhibited good antifungal [26,27,28], herbicidal [29,30], and insecticidal [31] activities. Prompted by the aforementioned findings and in continuation of our ongoing research in the field of design, synthesis, and biological evaluation of DHA derivatives, we present in this paper the synthesis and evaluation of a new series of acylhydrazone derivatives from DHA as potential anticancer agents against CNE-2 (Nasopharynx), HepG2 (liver), HeLa (epithelial cervical), and BEL-7402 (liver) human cancer cell lines and HL-7702 normal human liver cell line.
2. Results and Discussion
2.1. Synthesis
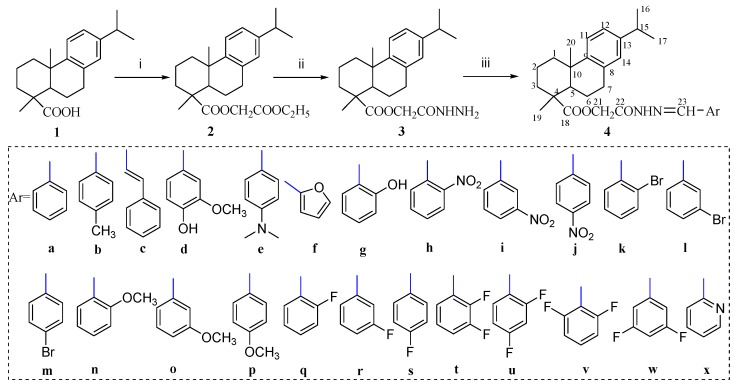
The synthesis of new DHA derivatives 4a–x was carried out according to the protocol shown in Scheme 1. All these derivatives are new compounds not previously reported. Esterification of dehydroabietic acid (1) with ethyl chloroacetate in the presence of potassium carbonate in dimethylformamide (DMF) gave dehydroabietic ethyl acetate (2). High yields of acylhydrazide (3) were achieved upon aminolysis in an ethanolic solution of 2 and hydrazine hydrate by stirring at 80 °C for 3 h. Acylhydrazones 4a–x were obtained in good to excellent yield by coupling the acylhydrazide 3 with the appropriate arylaldehydes in ethanol.
Scheme 1.
Synthesis of target compounds. Reagents and conditions: (i) ClCH2COOC2H5, K2CO3, Dimethylformamide (DMF), 40 °C, 4 h, yield 85%; (ii) NH2NH2·H2O, EtOH, 80 °C, 3 h, yield 77%; (iii) Ar-CHO, EtOH, HOAc, 80 °C, 6 h, yield 54–90%.
2.2. Evaluation of Anticancer Activity
Compounds 4a–x were evaluated for their cytotoxic activity in vitro against the human cancer cell lines, CNE-2, HepG2, HeLa, and BEL-7402 by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay. Cisplatin was included in the experiments as a positive control, and the IC50 of DHA is also presented to compare the anticancer activities. The calculated IC50 values were reported differently according to the different cancer cells. The results are summarized in Table 1.
Table 1.
Effect of compounds 4a–x against cell viability of different cell lines.
| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| CNE-2 | HepG2 | HeLa | BEL-7402 | HL-7702 | |
| 4a | 28.20 ± 0.43 | 27.81 ± 0.58 | 17.89 ± 0.13 | 36.70 ± 0.34 | >100 |
| 4b | 17.22 ± 0.21 | 12.44 ± 0.31 | 6.72 ± 0.22 | 15.38 ± 0.08 | 68.58 ± 2.14 |
| 4c | 15.82 ± 0.56 | 27.60 ± 0.23 | 10.84 ± 0.43 | 21.03 ± 0.09 | 53.22 ± 1.36 |
| 4d | 38.68 ± 0.49 | 35.26 ± 0.11 | 12.22 ± 0.08 | 33.59 ± 0.38 | 50.74 ± 1.82 |
| 4e | 48.85 ± 0.05 | 7.62 ± 0.32 | 34.92 ± 0.16 | 15.60 ± 0.44 | 63.04 ± 3.06 |
| 4f | 51.39 ± 0.34 | 25.61 ± 0.35 | 33.23 ± 0.21 | 16.90 ± 0.41 | 61.90 ± 1.34 |
| 4g | 60.42 ± 1.02 | 12.50 ± 0.46 | 27.26 ± 0.50 | 36.40 ± 0.74 | >100 |
| 4h | 98.78 ± 0.45 | 44.64 ± 0.92 | 15.20 ± 0.29 | 38.53 ± 0.52 | >100 |
| 4i | 12.61 ± 0.24 | 57.84 ± 0.75 | 13.91 ± 0.41 | 44.65 ± 0.21 | 77.08 ± 0.96 |
| 4j | 93.26 ± 0.52 | 39.50 ± 0.82 | 13.06 ± 0.08 | 27.45 ± 0.17 | >100 |
| 4k | 47.70 ± 0.23 | 8.47 ± 0.55 | 5.53 ± 0.04 | 48.16 ± 0.32 | 65.94 ± 1.48 |
| 4l | 11.45 ± 0.11 | 35.05 ± 0.96 | 25.14 ± 0.10 | 19.95 ± 0.27 | 86.44 ± 3.12 |
| 4m | 89.66 ± 0.27 | 9.94 ± 0.59 | 12.84 ± 0.15 | 23.08 ± 0.33 | >100 |
| 4n | 26.08 ± 0.05 | 50.08 ± 0.74 | 45.13 ± 0.09 | 23.79 ± 0.51 | 70.37 ± 2.23 |
| 4o | 86.46 ± 0.13 | 51.28 ± 0.63 | 32.06 ± 0.21 | 14.77 ± 0.38 | >100 |
| 4p | 56.98 ± 0.08 | 48.59 ± 0.09 | 34.33 ± 0.32 | 55.29 ± 0.44 | 91.75 ± 1.57 |
| 4q | 27.44 ± 0.72 | 93.76 ± 0.39 | 27.71 ± 0.14 | 22.79 ± 0.34 | >100 |
| 4r | 51.02 ± 0.94 | 31.57 ± 0.47 | 12.82 ± 0.13 | 25.86 ± 0.53 | 68.59 ± 1.25 |
| 4s | 49.81 ± 0.49 | 90.08 ± 0.33 | 28.79 ± 0.24 | 25.22 ± 0.22 | >100 |
| 4t | 33.34 ± 0.56 | 22.89 ± 0.04 | 11.14 ± 0.15 | 18.78 ± 0.59 | 72.04 ± 1.66 |
| 4u | 32.83 ± 0.16 | 25.36 ± 0.54 | 5.86 ± 0.09 | 18.64 ± 0.37 | 55.80 ± 1.38 |
| 4v | 26.77 ± 0.46 | 16.90 ± 0.18 | 14.96 ± 0.21 | 20.52 ± 0.53 | 44.63 ± 2.02 |
| 4w | 15.44 ± 0.21 | 12.07 ± 0.07 | 2.21 ± 0.04 | 14.46 ± 0.22 | 66.08 ± 1.84 |
| 4x | 12.96 ± 0.13 | 8.07 ± 0.25 | 4.94 ± 0.13 | 15.83 ± 0.18 | 58.62 ± 2.62 |
| DHA | 88.64 ± 0.73 | 80.36 ± 0.84 | 37.40 ± 0.64 | 46.70 ± 0.55 | >100 |
| Cisplatin | 8.75 ± 0.24 | 6.42 ± 0.18 | 1.94 ± 0.20 | 12.68 ± 0.33 | 20.76 ± 0.83 |
As shown in Table 1, most of the target compounds showed moderate to high anticancer activity, revealing that introduction of the acylhydrazone on the skeleton of DHA could markedly improve the anticancer activity. In CNE-2 cells, most of the compounds (such as 4a–g, 4i, 4k–l, and 4n–x) displayed better cytotoxicity than DHA (IC50 = 88.64 μM) with IC50 in the range of 11.45–86.46 μM. Among these compounds, compound 4l showed the best cytotoxicity, with IC50 of 11.45 μM. It was noted that changing Ar = phenyl compound 4a to 2-pyridyl (4x) markedly increased this activity. The substituents in phenyl of compound 4 have important influence on the cytotoxic inhibition and the introduction of electron donor substituents may result in the decrease of cytotoxicity.
In HepG2 cells, most of the compounds (except 4q and 4s) exhibited better inhibition than DHA (IC50 = 80.36 μM) with IC50 in the range of 7.62–57.84 μM. From the data, compounds 4e, 4k, and 4x showed the best inhibition with IC50 of 7.62, 8.47 and 8.07, respectively. We found that introduction of methyl, dimethylamino, hydroxyl, and difluoro groups into benzene group of acylhydrazone moiety showed a positive influence on antitumor activities in the HepG2 assay, while nitro, methoxyl, and fluoro groups exhibited a negative effect.
In HeLa cells, all compounds showed better cytotoxicity than DHA (IC50 = 37.40 μM), with IC50 in the range of 2.21–34.92 μM. In particular, compound 4w showed the highest cytotoxicity with the lowest IC50 value of 2.21 μM on HeLa cells, which possessed almost the same potency as that of positive drug cisplatin (IC50 = 1.94 μM) and was 16.92 times more active than DHA. The antitumor activities of tested were found to be in the order of cisplatin > 4w > 4x > 4k > 4u > 4b > 4c > 4t > 4d > 4r > 4m > 4j > 4i > 4v > 4h > 4a > 4l > 4g > 4q > 4s > 4o > 4f > 4p > 4e > DHA > 4n. Evidently, methyl at para, bromo at ortho, as well as bifluoro at ortho and para or at meta position in benzene group of acylhydrazone moiety may result in the enhancement of antitumor activity, while the presence of dimethylamino, hydroxyl, and methoxyl in benzene lead to the decrease of antitumor activity. In addition, displacement of benzene with pyridine appeared to have a positive influence on the antitumor activity in this assay.
In BEL-7402 cells, IC50 values of all compounds ranged from 14.46 to 48.16 μM, while the IC50 value of the parent compound DHA is 46.70 μM. Compounds 4o and 4w displayed similar potent inhibitory activities (IC50 = 14.77 and 14.46 μM) compared with positive control cisplatin (IC50 = 12.68 μM). Based on the results, it could be summarized that methyl and dimethylamino at para, and methoxyl and bifluoro at meta positions in the benzene group of the acylhydrazone moiety improve the antitumor activity against the BEL-7402 cell line, while the presence of a nitro group in benzene showed a negative effect.
3. Experimental Section
3.1. General Information
All the chemicals and reagents were commercially available and used without further purification. Routine thin-layer chromatography (TLC) was performed on silica gel plates (silica gel GF254 from Qingdao Haiyang Chemical Co., Ltd., Qingdao, China), preparative flash column chromatography was performed on the 200–300 mesh silica gel (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). Melting points were recorded on an X-4 microscope melting point apparatus (Beijing Tech Instrument Co., Ltd., Beijing, China) without calibration. 1H- and 13C-NMR spectra were recorded on a Bruker Avance III HD 600 MHz spectrometer (Bruker Co., Ltd., Zurich, Switzerland) at room temperature with tetramethylsilane (TMS) as an internal standard and CDCl3 as solvents. Chemical shifts are expressed in (ppm) and coupling constants (J) in Hz. Mass spectra were recorded with a liquid chromatograph mass spectrometer (Shimazu Co., Ltd., Kyoto, Japan). Infrared spectra (IR) were performed on Prestige-21 FTIR spectrometer (Shimadzu Co., Ltd., Nakagyo-ku, Japan). NMR, IR, and mass spectra of compounds 2, 3, and 4a–x can be found at Supplementary Materials.
3.2. Synthesis of 2-(Dehydroabietyloxy) Aceticether (2)
To a solution of dehydroabietic acid (3.00 g, 10.0 mmol) and ethyl chloroacetate (2 mL, 14.1 mmol) in DMF (20 mL), anhydrous potassium carbonate 2.8 g (4.0 mmol) was added, and the reaction mixture was stirred at 40 °C for 4 h. The progress of the reaction was monitored by TLC. Upon completion, the reaction mixture was poured into iced water and extracted with ethyl acetate (3 × 40 mL). The separated organic phase was washed with brine solution, dried over anhydrous sodium sulfate, and then concentrated under reduced pressure. The crude products were purified by silica gel column chromatography (PET/EtOAc = 10:1, v/v) to obtain colorless oily liquid compound 2. Yield 85%. IR (KBr, cm−1): 2954, 2872, 1762 (C=O), 1735 (C=O), 1498, 1463, 1382, 1209, 1168, 1120, 1029, 823. 1H-NMR (CDCl3, 600 MHz): δ 7.18 (d, J = 8.4 Hz, 1H, H-11), 7.00 (d, J = 8.4 Hz, 1H, H-12), 6.91 (s, 1H, H-14), 4.62 (d, J = 14.0 Hz, 1H, H-21), 4.55 (d, J = 14.0 Hz, 1H, H-21), 4.22 (q, J =7.0 Hz, 2H, OCH2), 2.81–3.00 (m, 3H, H-9 and H-17), 2.32 (d, J = 12.0 Hz, 2H, H-6 and He-4), 1.52–1.87 (m, 7H, H-2, H-3, Ha-4 and H-10), 1.34 (s, 3H, CH3, H-19), 1.29 (t, J = 6.0 Hz 3H, CH2CH3), 1.24 (s, 6H, H-16 and H-17), 1.23 (s, 3H, H-20). 13C-NMR (151 MHz, CDCl3) δ 179.44 (C=O), 169.40 (C=O), 148.27, 147.12, 136.26, 128.35, 125.54, 125.28, 62.71, 62.21, 49.01, 46.04, 39.35, 38.33, 38.04, 34.89, 31.42, 26.61, 25.43, 23.00, 19.97, 17.93, 15.55.
3.3. Synthesis of 2-(Dehydroabietyloxy) Acetohydrazide (3)
A mixture of 2-(dehydroabietyloxy) aceticether (1.93 g, 5.0 mmol), and 80% hydrazine hydrate (2 mL, 32.9 mmol) in EtOH (20 mL) was refluxed for 3 h. After cooling, the formed precipitate was filtered off, washed with water and then recrystallized from ethanol to give white solid. Yield 77%. m.p. 115.2–116.2 °C. IR (KBr, cm−1): 3464, 3190 (N–H), 3122, 2956, 1732 (C=O), 1680 (C=O), 1421, 1238, 1170, 1124, 823. 1H-NMR (600 MHz, CDCl3) δ 9.46 (s, 1H, CONH), 7.18 (d, J = 8.2 Hz, 1H, H-11), 7.00 (d, J = 9.3 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 4.62–5.00 (m, 2H, H-21), 2.91–2.99 (m, 1H, H-15), 2.81–2.89 (m, 2H, H-7), 2.31–2.35 (m, 2H, He-1, H-5), 2.00 (s, 2H, NH2), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.33 (s, 3H, H-19), 1.24 (s, 6H, H-16 and H-17), 1.23 (s, 3H, H-20). 13C-NMR (151 MHz, CDCl3) δ 178.40 (C=O), 169.54 (C=O), 147.10, 145.78, 135.09, 127.09, 124.30, 123.96, 61.74, 47.73, 44.77, 38.10, 37.08, 33.62, 30.22, 25.53, 25.39, 24.17, 21.74, 18.76, 16.73. MS (ESI) m/z 356.84 ([M − H]−).
3.4. General Procedure for the Synthesis of Dehydroabietic Acid-Based Acylhydrazones 4a–x
A mixture of acylhydrazide 3 (2 mmol), the appropriate arylaldehyde (2.2 mmol) and glacial acetic acid (0.25 mL) in EtOH (20 mL) was refluxed for 6 h. After cooling, the formed precipitate was filtered off and purified by crystallization from anhydrous ethanol to afford the acylhydrazone derivatives.
N’-(Benzylidene)-2-(dehydroabietyloxy)acetohydrazide (4a): White solid; yield 87%, m.p. 155.1–156.7 °C. IR (KBr, cm−1): 3446, 3197 (N–H), 2951, 2864, 2366, 1732 (C=O), 1697 (C=O), 1614, 1492, 1411, 1296, 1232, 1174, 1118, 1022, 947, 819, 754, 690. 1H-NMR (600 MHz, CDCl3) δ 10.70 (s, 1H, CONH), 7.84 (s, 1H, N=CH), 7.64 (d, J = 9.2 Hz, 2H, H-2′ and H-6′), 7.40 (d, J = 6.2 Hz, 3H, H-3′, H-4′ and H-5′), 7.22 (d, J = 8.2 Hz, 1H, H-11), 7.03 (d, J = 8.2 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 5.19 (q, J = 16.0 Hz, 2H, H-21), 3.05 (m, 1H, H-15), 2.82–2.91 (m, 2H, H-7), 2.42 (dd, J = 12.3, 1.7 Hz, 1H, He-1), 2.36 (d, J = 12.9 Hz, 1H, H-5), 1.52–1.93 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.21 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.17 (C=O), 147.08, 145.85, 145.52, 135.02, 133.66, 130.53, 128.95, 127.37, 127.13, 124.36, 124.04, 61.54, 47.86, 44.88, 38.14, 37.12, 36.98, 33.62, 30.32, 25.46, 24.18, 21.82, 18.81, 16.81. MS (ESI) m/z 458.86 ([M − H]−).
N’-(4-Methylbenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4b): White solid; yield 89%, m.p. 170.0–172.1 °C. IR (KBr, cm−1): 2951, 2929, 1737 (C=O), 1691 (C=O), 1610, 1498, 1417, 1311, 1236, 1172, 1124, 954, 891, 813. 1H-NMR (600 MHz, CDCl3) δ 10.62 (s, 1H, CONH), 7.80 (s, 1H, N=CH), 7.53 (d, J = 8.1 Hz, 2H, H-2′ and H-6′), 7.21 (d, J = 12.4 Hz, 3H, H-3′, H-5′ and H-11), 7.03 (d, J = 8.1 Hz, 1H, H-12), 6.88 (s, 1H, H-14), 5.17 (q, J = 16.0 Hz, 2H, H-21), 3.01 (d, J = 7.1 Hz, 1H, H-15), 2.79–2.92 (m, 2H, H-7), 2.42 (dd, J = 12.5, 2.0 Hz, 1H, He-1), 2.40 (s, 3H, Ph-CH3), 2.35 (d, J = 13.2 Hz, 1H, H-5), 1.53–2.01 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.21 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.50 (C=O), 170.00 (C=O), 147.09, 145.82, 145.57, 140.83, 135.05, 130.97, 129.67, 127.33, 127.13, 124.34, 124.01, 61.56, 47.85, 44.88, 38.13, 37.12, 36.97, 33.61, 30.32, 25.46, 24.16, 21.81, 21.70, 18.81, 16.81. MS (ESI) m/z 472.88 ([M − H]−).
N’-(3-Phenylallylidene)-2-(dehydroabietyloxy)acetohydrazide (4c): light yellow solid; yield 89%, m.p. 185.7–187.3 °C. IR (KBr, cm−1): 2924, 1734 (C=O), 1687 (C=O), 1496, 1417, 1296, 1238, 1170, 1124, 972, 750, 690, 599. 1H-NMR (600 MHz, CDCl3) δ 10.45 (s, 1H, CONH), 7.63 (d, J = 7.1 Hz, 1H, 1H, N=CH), 7.46 (d, J = 7.4 Hz, 2H, H-2′ and H-6′), 7.38 (t, 2H, H-3′ and H-5’), 7.34 (d, J = 7.1 Hz, 1H, H-4′), 7.21 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.9 Hz, 1H, H-12), 6.90 (s, 1H, H-14), 6.85 (s, 2H, HC=CH), 5.09 (q, J = 15.9 Hz, 2H, H-21), 2.99–3.05 (m, 1H, H-15), 2.81–2.93 (m, 2H, H-7), 2.41 (dd, J = 12.5, 2.0 Hz, 1H, He-1), 2.36 (d, J = 12.9 Hz, 1H, H-5), 1.54–1.99 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.22 (d, J = 6.9, 2.0 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.47 (C=O), 169.84 (C=O), 147.47, 147.06, 145.86, 139.99, 135.86, 135.00, 129.28, 129.03, 127.25, 127.14, 124.68, 124.33, 124.03, 61.47, 47.85, 44.88, 38.13, 37.11, 36.95, 33.61, 30.30, 25.44, 24.15, 21.82, 18.81, 16.81. MS (ESI) m/z 484.91 ([M − H]−).
N’-(3-Methoxy-4-hydroxylbenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4d): yellow solid; yield 85%, m.p. 117.6–119.0 °C. IR (KBr, cm−1): 3446, 3230 (N–H), 2954, 1730 (C=O), 1687 (C=O), 1598, 1516, 1462, 1425, 1282, 1238, 1170, 1122, 1029, 950, 862, 819, 756, 623. 1H-NMR (600 MHz, CDCl3) δ 10.35 (s, 1H, CONH), 7.72 (s, 1H, N=CH), 7.23 (s, 1H, H-6’), 7.19 (d, J = 8.2 Hz, 1H, H-2’), 7.01 (d, J = 8.0 Hz, 2H, H-5′ and H-11), 6.90 (d, J = 8.1 Hz, 1H, H-12), 6.87 (s, 1H, H-14), 5.15 (q, J = 15.9 Hz, 2H, H-21), 3.93 (s, 3H, OCH3), 2.98–3.02 (m, 1H, H-15), 2.76–2.93 (m, 2H, H-7), 2.39 (dd, J = 12.3, 1.5 Hz, 1H, He-1), 2.33 (d, J = 12.7 Hz, 1H, H-5), 1.48–2.03 (m, 7H, Ha-1, H-2, H-3, H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.58 (C=O), 169.65 (C=O), 148.24, 147.18, 147.04, 145.85, 145.66, 134.99, 127.10, 126.11, 124.31, 124.01, 122.89, 114.63, 107.78, 61.53, 56.11, 53.70, 47.86, 38.10, 37.09, 36.96, 33.60, 30.25, 25.42, 24.15, 21.81, 18.78, 16.79. MS (ESI) m/z 504.87 ([M − H]−).
N’-(4-Dimethylaminobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4e): brown red solid, yield 66%, m.p. 163.4–164.7 °C. IR (KBr, cm−1): 2953, 1735 (C=O), 1687 (C=O), 1606, 1531, 1417, 1359, 1298, 1234, 1174, 1116, 1047, 948, 815. 1H-NMR (600 MHz, CDCl3) δ 10.00 (s, 1H, CONH), 7.69 (s, 1H, N=CH), 7.50 (d, J = 8.5 Hz, 2H, H-2’ and H-6’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.2 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 6.70 (d, J = 8.5 Hz, 2H, H-3’ and H-5’), 5.14 (q, J =16.2 Hz, 2H, H-21), 3.03 (s, 6H, N(CH3)2), 2.94–3.00 (m, 1H, H-15), 2.80–2.90 (m, 2H, H-7), 2.40 (dd, J = 12.3, 1.4 Hz, 1H, He-1), 2.31 (d, J = 12.7 Hz, 1H, H-5), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3, H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.22 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.48 (C=O), 169.22 (C=O), 147.12, 146.94, 145.91, 145.76, 134.86, 128.77, 127.14, 124.35, 124.31, 123.94, 112.10, 61.64, 47.83, 44.78, 40.49, 38.04, 37.11, 36.92, 33.62, 30.30, 25.42, 24.16, 21.78, 18.74, 16.68. MS (ESI) m/z 501.89 ([M − H]−).
N’-((Furan-2-yl)methylene)-2-(dehydroabietyloxy)acetohydrazide (4f): yellow solid; yield 58%, m.p. 125.7–126.4 °C. IR (KBr, cm−1): 2968, 2868, 1730 (C=O), 1683 (C=O), 1625, 1541, 1469, 1419, 1386, 1334, 1284, 1228, 1170, 1124, 1012, 974, 937, 883, 837, 750. 1H-NMR (600 MHz, CDCl3) δ 10.68 (s, 1H, CONH), 7.71 (s, 1H, N=CH), 7.50 (s, 1H, H-4’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.01 (d, J = 8.1 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 6.67 (d, J = 3.4 Hz, 1H, H-4’), 6.48 (s, 1H, H-4’), 5.12 (q, J = 16.1 Hz, 2H, H-21), 2.96–3.02 (m, 1H, H-15), 2.81–2.91 (m, 2H, H-7), 2.39 (dd, J = 12.3, 1.6 Hz, 1H, He-1), 2.34 (d, J = 12.9 Hz, 1H, H-5), 1.52–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.44 (C=O), 170.16 (C=O), 149.15, 147.07, 145.83, 144.78, 135.24, 135.03, 127.11, 124.34, 124.01, 113.35, 112.09, 61.44, 47.82, 44.85, 38.11, 37.09, 36.89, 33.61, 30.27, 25.43, 24.16, 21.76, 18.78, 16.76. MS (ESI) m/z 448.86 ([M − H]−).
N’-(2-Hydroxylbenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4g): yellow solid; yield 73%, m.p. 214.9–215.6 °C. IR (KBr, cm−1): 3251 (N–H), 2953, 2870, 1726 (C=O), 1678 (C=O), 1616, 1552, 1292, 1390, 1274, 1220, 1166, 1124, 974, 896, 759. 1H-NMR (600 MHz, CDCl3) δ 10.81 (s, 1H, CONH), 9.87 (s, 1H, OH), 8.12 (s, 1H, N=CH), 7.34 (m, 1H, H-6’), 7.20 (d, J = 8.2 Hz, 1H and H-4’), 7.15 (d, J = 6.8 Hz, 1H, H-5’), 6.96–7.04 (m, 2H, H-11 and H-14), 6.84–6.91 (m, 7.4 Hz, 2H, H-12 and H-3’), 5.05 (q, J = 15.5 Hz, 2H, H-21), 2.95–3.02 (m, 1H, H-15), 2.78–2.90 (m, 2H, H-7), 2.38 (dd, J = 12.4, 1.2 Hz, 1H, He-1), 2.34 (d, J = 12.8 Hz, 1H, H-5),1.47–2.01 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.43 (C=O), 168.98 (C=O), 163.35, 157.91, 146.96, 145.91, 134.88, 132.39, 131.42, 127.12, 124.32, 124.07, 120.15, 119.53, 117.14, 60.92, 47.88, 44.83, 38.07, 37.08, 36.97, 33.61, 30.22, 25.40, 24.14, 21.85, 18.74, 16.77. MS (ESI) m/z 474.85 ([M − H]−).
N’-(2-Nitrobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4h): yellow solid; yield 62%; m.p. 94.9–96.7 °C. IR (KBr, cm−1): 3442, 3207 (N–H), 3101, 2954, 2927, 2868, 1728 (C=O), 1691 (C=O), 1597, 1527, 1463, 1417, 1346, 1301, 1238, 1170, 1126, 970, 935, 821, 783, 742. 1H-NMR (600 MHz, CDCl3) δ 10.48 (s, 1H, CONH), 8.38 (s, 1H, N=CH), 8.03 (d, J = 8.2 Hz, 2H, H-3’ and H-6’), 7.65 (t, J = 7.8 Hz, 1H, H-5’), 7.55 (t, J = 7.8 Hz, 1H, H-4’), 7.18 (d, J = 8.2 Hz, 1H, H-11), 7.00 (d, J = 8.1 Hz, 1H, H-12), 6.87 (s, 1H, H-14), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.95 (m, 1H, H-15), 2.85 (m, 2H, H-7), 2.35 (dd, J = 12.5, 1.7 Hz, 1H, He-1), 2.32 (d, J = 13.1 Hz, 1H, H-5), 1.49–1.93 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.59 (C=O), 169.97 (C=O), 148.30, 147.11, 145.77, 140.59, 135.06, 133.55, 130.64, 128.71, 128.49, 127.08, 125.02, 124.31, 123.96, 61.38, 47.85, 44.80, 38.07, 37.08, 36.92, 33.60, 30.19, 25.42, 24.16, 21.78, 18.71, 16.71. MS (ESI) m/z 503.88 ([M − H]−).
N’-(3-Nitrobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4i): light yellow solid; yield 82%, m.p. 99.6–101.9 °C. IR (KBr, cm−1): 3190, 2926, 1734 (C=O), 1693 (C=O), 1579, 1533, 1463, 1348, 1238, 1172, 1126, 981, 825, 734, 678. 1H-NMR (600 MHz, CDCl3) δ 10.85 (s, 1H, CONH), 8.46 (s, 1H, N=CH), 8.24 (s, 1H, H-2′), 7.92 (s, 2H, H-5′ and H-6′), 7.55 (s, 1H, H-3′), 7.19 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 9.3 Hz, 1H, H-14), 6.86 (s, 1H, H-12), 5.18 (q, J = 16.0 Hz, 2H, H-21), 2.96–3.02 (m, 1H, H-15), 2.77–2.91 (m, 2H, H-7), 2.31–2.42 (m, 2H, He-1 and H-5), 1.49–2.01 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.21 (d, J = 6.9, Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.60 (C=O), 170.44 (C=O), 148.76, 147.00, 145.90, 142.97, 135.39, 134.84, 132.84, 130.05, 127.07, 124.82, 124.34, 124.08, 121.84, 61.45, 47.86, 44.88, 38.10, 37.09, 36.93, 33.59, 30.23, 25.39, 24.15, 21.82, 18.74, 16.80. MS (ESI) m/z 503.87 ([M − H]−).
N’-(4-Nitrobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4j): yellow solid; yield 54%, m.p. 177.2–179.8 °C. IR (KBr, cm−1): 3099, 2947, 1739 (C=O), 1691 (C=O), 1591, 1523, 1463, 1413, 1342, 1234, 1170, 1111, 1014, 943, 833, 745. 1H-NMR (600 MHz, CDCl3) δ 10.63 (s, 1H, CONH), 8.24 (s, 2H, H-3’ and H-5’), 7.87 (s, 1H, N=CH), 7.75 (d, J = 8.6 Hz, 2H, H-2’ and H-6’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 6.86 (s, 1H, H-12), 7.02 (d, J = 9.3 Hz, 1H, H-14), 5.17 (q, J = 16.0 Hz, 2H, H-21), 2.95–3.01 (m, 1H, H-15), 2.77–2.91 (m, 2H, H-7), 2.34–2.39 (m, 2H, He-1 and H-5), 1.48–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.20 (d, J = 6.9, Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.53 (C=O), 170.36 (C=O), 148.71, 146.96, 145.96, 134.92, 132.51, 132.28, 128.70, 127.09, 124.79, 124.34, 124.06, 61.39, 47.86, 44.91, 38.09, 37.13, 36.93, 33.58, 30.26, 25.40, 24.13, 21.80, 18.77, 16.79. MS (ESI) m/z 503.87 ([M − H]−).
N’-(2-Bromobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4k): White solid; yield 70%; m.p. 184.9–185.7 °C. IR (KBr, cm−1): 3246 (N–H), 2935, 1730 (C=O), 1699 (C=O), 1668, 1602, 1496, 1462, 1402, 1234, 1172, 1128, 945, 877, 754, 711, 638. 1H-NMR (600 MHz, CDCl3) δ 10.54 (s, 1H, CONH), 8.21 (s, 1H, N=CH), 7.92 (d, J = 7.8 Hz, 1H, H-6’), 7.58 (d, J = 7.9 Hz, 1H, H-3’), 7.34 (t, J = 7.6 Hz, 1H, H-5’), 7.23–7.27 (m, 1H, H-4’), 7.19 (d, J = 8.2 Hz, 1H, H-11), 7.01 (d, J = 9.3 Hz, 1H, H-14), 6.87 (s, 1H, H-12), 5.16 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.00 (m, 1H, H-15), 2.80–2.89 (m, 2H, H-7), 2.32–2.39 (m, 2H, He-1, H-5), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.00 (C=O), 147.07, 145.80, 144.04, 135.06, 133.38, 132.63, 131.60, 127.78, 127.71, 127.11, 124.38, 124.32, 123.98, 61.48, 47.84, 44.81, 38.10, 37.10, 37.00, 33.61, 30.25, 25.42, 24.16, 21.90, 18.80, 16.78. MS (ESI) m/z 538.72 ([M − H]−).
N’-(3-Bromobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4l): yellow solid; yield 61%; m.p. 178.3–179.8 °C. IR (KBr, cm−1): 3554, 3414, 3194 (N–H), 3111, 2947, 1730 (C=O), 1691 (C=O), 1560, 1409, 1236, 1170, 1120, 960, 877, 821, 734, 678. 1H-NMR (600 MHz, CDCl3) δ 9.85 (s, 1H, CONH), 7.64 (s, 1H, N=CH), 7.50 (dd, J = 21.1, 7.6 Hz, 1H, H-6’), 7.41 (d, J = 14.0 Hz, 1H, H-2’), 7.24 (s, 1H, H-4’), 7.20 (d, J = 8.1 Hz, 1H, H-5’), 7.02 (d, J = 7.9 Hz, 1H, H-11), 6.90 (s, 1H, H-14), 6.82 (s, 1H, H-12), 5.11–5.15 (m, 2H, H-21), 2.95–2.98 (m, 1H, H-15), 2.80–2.93 (m, 2H, H-7), 2.38 (d, J = 12.3 Hz, 1H, He-1), 2.34 (d, J = 12.7 Hz, 1H, H-5), 1.47–1.96 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.22 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.38 (C=O), 170.05 (C=O), 149.58, 147.07, 145.85, 138.47, 135.04, 132.22, 130.45, 129.89, 127.11, 125.69, 124.33, 124.02, 123.15, 61.80, 47.79, 44.80, 38.13, 37.10, 36.93, 33.61, 30.26, 25.42, 24.18, 21.82, 18.80, 16.78. MS (ESI) m/z 536.77 ([M − H]−).
N’-(4-Bromobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4m): White solid; yield 90%, m.p. 188.1–188.6 °C. IR (KBr, cm−1): 3201 (N–H), 3101, 2949, 1734 (C=O), 1691 (C=O), 1415, 1309, 1236, 1118, 1064, 1006, 952, 889, 817. 1H-NMR (600 MHz, CDCl3) δ 10.51 (s, 1H, CONH), 7.75 (s, 1H, N=CH), 7.51 (d, J = 8.5 Hz, 2H, H-3’ and H-5’), 7.46 (d, J = 8.5 Hz, 2H, H-2’ and H-6’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 9.3 Hz, 1H, H-12), 6.86 (s, 1H, H-14), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.02 (m, 1H, H-15), 2.79–2.90 (m, 2H, H-7), 2.38 (dd, J = 12.4, 1.7 Hz, 1H, He-1), 2.34 (d, J = 12.8 Hz, 1H, H-5), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.22 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.50 (C=O), 170.04 (C=O), 147.01, 145.88, 144.20, 134.92, 132.51, 132.28, 128.70, 127.09, 124.79, 124.34, 124.06, 61.47, 47.84, 44.88, 38.10, 37.10, 36.93, 33.60, 30.28, 25.43, 24.15, 21.80, 18.77, 16.79. MS (ESI) m/z 538.73 ([M − H]−).
N’-(2-Methoxybenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4n): White solid; yield 84%; m.p. 190.3–191.8 °C. IR (KBr, cm−1): 3442, 3217 (N-H), 3084, 2958, 1730 (C=O), 1691 (C=O), 1666, 1600, 1462, 1361, 1298, 1247, 1176, 1126, 1080, 1024, 968, 819, 761, 644. 1H-NMR (600 MHz, CDCl3) δ 10.38 (s, 1H, CONH), 8.24 (s, 1H, N=CH), 7.88 (d, J = 7.6 Hz, 1H, H-6’), 7.38 (t, J = 7.8 Hz, 1H, H-4’), 7.20 (d, J = 8.2 Hz, 1H, H-5’), 6.97–7.05 (d, 2H, H-11 and H-3’), 6.91 (d, J = 8.4 Hz, 1H, H-14), 6.87 (s, 1H, H-12), 5.18 (s, 2H, H-21), 3.85 (s, 3H, OCH3), 2.96–3.06 (m, 1H, H-15), 2.79–2.83 (m, 2H, H-7), 2.40 (d, J = 11.9 Hz, 1H, He-1), 2.34 (d, J = 12.5 Hz, 1H, H-5), 1.48–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.49 (C=O), 169.74 (C=O), 158.21, 147.09, 145.79 141.27, 135.19, 131.75, 127.15, 126.40, 124.31, 123.94, 122.21, 120.96, 111.18, 61.63, 55.62, 47.84, 44.91, 38.15, 37.12, 36.89, 33.61, 30.24, 25.47, 24.17, 21.76, 18.83 (C-6), 16.77. MS (ESI) m/z 488.86 ([M − H]−).
N’-(3-Methoxybenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4o): light yellow solid; yield 81%; m.p. 136.7–137.9 °C. IR (KBr, cm−1): 3412, 3080, 2958, 2927, 2870, 1726 (C=O), 1685 (C=O), 1573, 1458, 1419, 1311, 1286, 1249, 1130, 1043, 948, 873, 823, 775, 688. 1H-NMR (600 MHz, CDCl3) δ 10.71 (s, 1H, CONH), 7.80 (s, 1H, N=CH), 7.31 (t, 1H, H-6’), 7.21 (d, J = 13.2 Hz, 2H, H-2’ and H-5’), 7.17 (d, J = 7.6 Hz, 1H, H-11), 7.03 (d, J = 8.0 Hz, 1H, H-12), 6.97 (d, J = 10.5 Hz, 1H, H-4’), 6.89 (s, 1H, H-14), 5.18 (q, J = 16.0 Hz, 2H, H-21), 3.85 (s, 3H, OCH3), 2.99–3.05 (s, 1H, H-15), 2.81–2.92 (m, 2H, H-7), 2.42 (dd, J = 12.3, 1.8 Hz, 1H, He-1), 2.36 (d, J = 12.8 Hz, 1H, H-5), 1.50–2.00 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.15 (C=O), 160.02, 147.06, 145.85, 145.41, 135.01, 129.98, 127.13, 124.34, 124.02, 120.55, 116.66, 111.53, 61.51, 55.47, 47.86, 44.86, 38.13, 37.11, 36.99, 33.62, 30.30, 25.45, 24.16, 21.83, 18.81, 16.81. MS (ESI) m/z 488.91 ([M − H]−).
N’-(4-Methoxybenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4p): White solid; yield 90%; m.p. 174.8–175.5 °C. IR (KBr, cm−1): 2931, 1737 (C=O), 1689 (C=O), 1604, 1504, 1419, 1303, 1247, 1168, 1124, 1035, 954, 893, 837. 1H-NMR (600 MHz, CDCl3) δ 10.49 (s, 1H, CONH), 7.76 (s, 1H, N=CH), 7.56 (d, 2H, H-2’, H-6’), 7.20 (d, 1H, H-11), 7.01 (d, 1H, H-12), 6.92 (d, 2H, H-3’, H-5’), 6.88 (s, 1H, H-14), 5.16 (q, J = 15.9 Hz, 2H, H-21), 3.85 (s, 3H, OCH3), 2.98–3.04 (m, 1H, H-15), 2.81–2.90 (m, 2H, H-7), 2.41 (d, J = 11.8 Hz, 1H, He-1), 2.35 (d, J = 12.8 Hz, 1H, H-5), 1.53–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9, Hz, 6H, H-16 and H-17).13C-NMR (151 MHz, CDCl3) δ 178.49 (C=O), 169.80 (C=O), 161.51, 147.08, 145.82, 145.18, 135.05, 128.90, 127.12, 126.41, 124.34, 124.00, 114.38, 61.56, 55.53, 47.84, 44.88, 38.13, 37.11, 36.95, 33.61, 30.31, 25.45, 24.16, 21.80, 18.80, 16.80. MS (ESI) m/z 488.88 ([M − H]−).
Dehydroabietic acid-based 2-fluorophenyl acylhydrazone (4q): light yellow solid; yield 72%; m.p. 130.2–130.5 °C. IR (KBr, cm−1): 2954, 2868, 1730 (C=O), 1691 (C=O), 1415, 1311, 1240, 1170, 1126, 1053, 877, 823, 756, 630. 1H-NMR (600 MHz, CDCl3) δ 10.86 (s, 1H, CONH), 8.10 (s, 1H, N=CH), 7.89 (d, J = 7.8 Hz, 1H, H-6’), 7.38 (d, J = 7.9 Hz, 1H, H-3’), 7.21 (d, J = 7.6 Hz, 1H, H-5’), 7.18 (d, J = 8.9 Hz, 1H, H-11), 7.12 (d, J = 8.2 Hz, 1H, H-4’), 7.02 (d, J = 9.3 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 5.17 (q, J = 16.0 Hz, 2H, H-21), 2.95–3.05 (m, 1H, H-15), 2.80–2.93 (m, 2H, H-7), 2.41 (dd, J = 12.4, 1.7 Hz, 1H, He-1), 2.35 (d, J = 12.9 Hz, 1H, H-5), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.59 (C=O), 170.29 (C=O), 162.42, 147.14, 145.78, 138.62, 135.14, 132.02, 127.12, 126.72, 124.59, 124.34, 123.97, 121.60, 116.17, 61.44, 47.87, 44.78, 38.13, 37.10, 37.01, 33.62, 30.23, 25.43, 24.18, 21.79, 18.77, 16.72. MS (ESI) m/z 476.88 ([M − H]−).
N’-(3-Fluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4r): White solid; yield 82%, m.p. 166.0–167.3 °C. IR (KBr, cm−1): 3414, 3107, 2954, 1732 (C=O), 1697 (C=O), 1610, 1579, 1450, 1411, 1236, 1124, 943, 866, 785, 684. 1H-NMR (600 MHz, CDCl3) δ 10.75 (s, 1H, CONH), 7.80 (s, 1H, N=CH), 7.39 (d, J = 9.2 Hz, 1H, H-5’), 7.35 (d, J = 5.3 Hz, 1H, H-2’), 7.34 (s, 1H, H-4’), 7.21 (d, J = 8.2 Hz, 1H, H-11), 7.10 (t, J = 7.7 Hz, 1H, H-5’), 7.02 (d, J = 7.9 Hz, 1H, H-12), 6.88 (s, 1H, H-14), 5.16 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.07 (m, 1H, H-15), 2.78–2.93 (m, 2H, H-7), 2.40 (dd, J = 12.2, 1.2 Hz, 1H, He-1), 2.36 (d, J = 12.9 Hz, 1H, H-5), 1.51–2.00 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.22 (d, J = 6.8 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.31 (C=O), 163.96, 147.04, 145.88, 144.17, 135.92, 134.94, 130.58, 127.10, 124.35, 124.06, 123.67, 117.45, 113.20, 61.46, 47.85, 44.87, 38.11, 37.10, 36.96, 33.61, 30.29, 25.43, 24.13, 21.81, 18.78, 16.80. MS (ESI) m/z 476.89 ([M − H]−).
N’-(4-Fluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4s): White solid; yield 83%; m.p. 169.2–170.5 °C. IR (KBr, cm−1): 3224 (N–H), 3070, 2866, 1726 (C=O), 1687 (C=O), 1598, 1550, 1417, 1238, 1172, 1128, 1080, 945, 837, 723. 1H-NMR (600 MHz, CDCl3) δ 10.70 (s, 1H, CONH), 7.80 (s, 1H, N=CH), 7.61 (t, J = 8.3 Hz, 2H, H-2’ and H-6’), 7.21 (d, J = 8.1 Hz, 1H, H-11), 7.07 (t, 2H, J = 8.2 Hz, H-3’ and H-5’), 7.03 (d, J = 8.2 Hz, 1H, H-12), 6.87 (s, 1H, H-14), 5.16 (q, J = 16.0 Hz, 2H, H-21), 2.95–3.06 (m, 1H, H-15), 2.78–2.90 (m, 2H, H-7), 2.40 (d, J = 12.3 Hz, 1H, He-1), 2.35 (d, J = 12.9 Hz, 1H, H-5), 1.49–2.00 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.13 (C=O), 164.94, 147.04, 145.89, 144.37, 134.94, 129.89, 129.24, 127.10, 124.35, 124.07, 116.21, 61.50, 47.84, 44.90, 38.12, 37.11, 36.96, 33.61, 30.30, 25.44, 24.13, 21.81, 18.79, 16.80. MS (ESI) m/z 476.84 ([M − H]−).
N’-(2,3-Difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4t): White solid; yield 62%; m.p. 150.2–153.5 °C. IR (KBr, cm−1): 3097, 2966, 2868, 1732 (C=O), 1697 (C=O), 1612, 1487, 1415, 1305, 1238, 1170, 1124, 989, 786. 1H-NMR (600 MHz, CDCl3) δ 10.00 (s, 1H, CONH), 8.02 (s, 1H, H-23), 7.63 (dd, J = 7.5, 6.2 Hz, 1H, H-6’), 7.22 (dd, J = 20.1, 8.8 Hz, 2H, H-4’ and H-5’), 7.13 (dd, J = 12.4, 7.9 Hz, 1H, H-11), 7.02 (dd, J = 8.1, 1.5 Hz, 1H, H-12), 6.90 (s, 1H, H-14), 5.15 (q, J = 16.0 Hz, 2H, H-21), 2.96–3.04 (m, 1H, H-15), 2.80–2.93 (m, 2H, H-7), 2.39 (dd, J = 12.4, 1.9 Hz, 1H, He-1), 2.34 (d, J = 12.9 Hz, 1H, H-5), 1.53–1.95 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.24 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.36 (C=O), 169.52 (C=O), 151.49, 150.36, 148.76, 146.95, 145.67, 136.87, 134.91, 126.95, 124.35, 124.15, 123.83, 121.39, 118.73, 61.23, 47.73, 44.68, 37.97, 36.96, 36.80, 33.46, 30.05, 25.23, 23.97, 21.64, 18.59, 16.57. MS (ESI) m/z 495.1 ([M − H]−).
N’-(2,4-Difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4u): White solid; yield 77%; m.p. 181.9–184.5 °C. IR (KBr, cm−1): 3072, 2949, 2870, 1737 (C=O), 1691 (C=O), 1608, 1494, 1419, 1298, 1274, 1230, 1170, 1126, 962, 854, 794. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H, CONH), 7.97 (s, 1H, H-23), 7.89 (dd, J = 14.9, 8.4 Hz, 1H and H-6’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.0 Hz, 1H, H-12), 6.95 (t, J = 9.2 Hz, 1H, C-14), 6.92–6.83 (m, 2H, H-3’and H-5’), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.04 (m, 1H, H-15), 2.78–2.93 (m, 2H, C-7), 2.39 (dd, J = 12.3, 1.7 Hz, 1H, He-1), 2.34 (d, J = 13.0 Hz, 1H, H-5), 2.00–1.53 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.24 (d, J = 6.9 Hz, 6H, H-16 and H-17), 13C-NMR (151 MHz, CDCl3) δ 178.34 (C=O), 169.28 (C=O), 162.43, 160.74, 154.86, 146.95, 145.67, 137.02, 134.94, 126.95, 124.15, 123.83, 117.72, 112.44, 104.29, 61.26, 47.72, 44.68, 37.97, 36.96, 36.78, 33.46, 30.06, 25.24, 23.99, 21.63, 18.60, 16.57. MS (ESI) m/z 495.2 ([M − H]−).
N’-(2,6-Difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4v): Brown solid;yield 93%; m.p. 107.3–110.0 °C. IR (KBr, cm−1): 3101, 2954, 2868, 1728, 1693, 1620, 1467, 1415, 1240, 1170, 1126, 1012, 881, 785. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H, CONH), 7.97 (s, 1H, H-23), 7.89 (dd, J = 14.9, 8.4 Hz, 1H, H-4’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.0 Hz, 1H, H-12), 6.95 (t, J = 9.2 Hz, 1H, H-14), 6.88 (q, J = 8.5 Hz, 2H, H-3’ and H-5’), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.04 (m, 1H, H-15), 2.78–2.94 (m, 2H, H-7), 2.39 (dd, J = 12.3, 1.7 Hz, 1H, He-1), 2.34 (d, J = 13.0 Hz, 1H, H-5), 1.54–2.00 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.27 (d, J = 7.1 Hz, 3H, H-20), 1.24 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.29 (C=O), 170.00 (C=O), 162.04, 160.34, 153.60, 147.00, 145.62, 134.94, 131.20, 126.96, 124.14, 123.78, 117.72, 112.44, 104.29, 61.27, 47.68, 44.67, 37.98, 36.96, 36.78, 33.46, 30.06, 25.25, 23.97, 21.59, 18.61, 16.57. MS (ESI) m/z 495.2 ([M − H]−).
N’-(3,5-Difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4w): White solid; yield 72%; m.p. 182.6–186.6 °C. IR (KBr,cm−1): 3091, 2949, 2885, 1724 (C=O), 1703 (C=O), 1612, 1583, 1436, 1415, 1363, 1298, 1240, 1174, 1122, 983, 850, 752.1H-NMR (600 MHz, CDCl3) δ 10.02 (s, 1H, CONH), 7.71 (s, 1H, H-23), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.16 (d, J = 5.8 Hz, 2H, H-2’ and H-6’), 7.02 (d, J = 8.0 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 6.87 (t, J = 8.6 Hz, 1H, H-4’), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.96 –3.05 (m, 1H, H-15), 2.80–2.93 (m, 2H, H-7), 2.39 (d, J = 12.6, Hz, 1H, He-1), 2.36 (d, J = 13.0 Hz, 1H, H-5),1.53–1.97 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.31 (C=O), 169.65 (C=O), 164.00, 162.43, 162.35, 146.88, 145.74, 142.28, 136.58, 134.79, 126.94, 124.15, 123.89, 109.95, 105.67, 61.22, 47.72, 44.73, 37.97, 36.96, 36.77, 33.45, 30.08, 25.24, 23.97, 21.64, 18.60, 16.62. MS (ESI) m/z 495.1 ([M − H]−).
N’-((Pyridine-3-yl)methylene)-2-(dehydroabietyloxy)acetohydrazide (4x): White solid; yield 85%; m.p. 191.9–193.8 °C. IR (KBr, cm−1): 3084, 2956, 2866, 1723 (C=O), 1710 (C=O), 1614, 1462, 1402, 1288, 1240, 1170, 1126, 887, 704. 1H-NMR (600 MHz, CDCl3) δ 10.34 (s, 1H, CONH), 8.80 (s, 1H, H-2’), 8.65 (d, J = 4.1 Hz, 1H, H-4’), 8.00 (d, J = 7.9 Hz, 1H, H-6’), 7.83 (s, 1H, H-23), 7.35 (dd, J = 7.8, 4.9 Hz, 1H, H-5’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.1 Hz, 1H, H-12), 6.88 (s, 1H, H-14), 5.16 (q, J = 16.0 Hz, 2H, H-21), 2.97–3.04 (m, 1H, H-15), 2.80–2.92 (m, 2H, H-7), 2.39 (d, J = 11.2 Hz, 1H, He-1), 2.35 (d, J = 12.8 Hz, 1H, H-5), 1.53–1.94 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.34 (C=O), 169.75 (C=O), 151.13, 149.01, 146.89, 145.73, 141.81, 134.78, 133.50, 129.41, 126.94, 124.16, 123.89, 123.77, 61.26, 47.72, 44.73, 37.97, 36.96, 36.80, 33.46, 30.09, 25.24, 23.99, 21.66, 18.62, 16.63. MS (ESI) m/z 460.2 ([M − H]−).
3.5. Cytotoxicity Assay In Vitro
The CNE-2, HepG2, HeLa, and BEL-7402 cell lines used in this work were all purchased from the Institute of Biochemistry and Cell Biology, China Academy of Sciences. All were supplemented with 10% heat-inactivated fetal bovine serum in a humidified atmosphere of 5% CO2/95% air at 37 °C. In order to investigate the potential of compounds 4, cisplatin, a commercial classical anticancer drug was used as a reference drug. CNE-2, HepG2, HeLa, and BEL-7402 cells were seeded into 96-well microculture plates and allowed to adhere for 24 h, respectively. After cells were exposed to compounds at concentrations from 100 to 0.1 μM for 48 h, medium was aspirated and replenished with complete medium. IC50 values was evaluated by MTT tetrazolium dye assay. All the tests were repeated in at least three independent experiments. The IC50 values of the compounds were calculated using SPSS Version 10 software (IBM, New York, NY, USA), which defined the IC50 value as the concentration required to inhibit cell growth by 50%.
4. Conclusions
This study started with the aim to explore the potential anticancer activity of dualistic molecules bearing a combination of the dehydroabietic acid and acylhydrazone moieties. Their cytotoxic activities against human nasopharyngeal carcinoma (CNE-2), human liver carcinoma (HepG2), human cervix carcinoma (HeLa), and human hepatocellular carcinoma (BEL-7402) cells lines, along with HL-7702, a normal human liver cell line, were investigated. A number of compounds showed moderate to high anticancer activity and the results revealed that the introduction of acylhydrazone on the skeleton of DHA markedly improved the anticancer activity. In particular, compounds 4k and 4x inhibited the growth of HepG2 and HeLa cell lines with low (<10 μM) micromolar IC50 values. Compound 4w was found to exhibit anticancer activity against HeLa and BEL-7402 cell lines compared to cisplatin as reference drug. In addition, compounds 4i and 4l displayed good cytotoxic activities against CNE-2 cells (IC50 values of 12.61 μM and 11.45 μM, respectively). The results highlight these novel dehydroabietic acid derivatives as potential leads for the further investigation for new anticancer drug candidates.
Acknowledgments
This research was financially supported by the Natural Science Foundation of Guangxi Province of China (No. 2015GXNSFAA139035), the Science and Technology Research Projects for Universities in Guangxi Province of China (No. YB2014278), and the National Natural Science Foundation of China (No. 31060100).
Supplementary Materials
Supplementary materials are available online.
Author Contributions
Fang-Yao Li designed and carried out the experimental and wrote the paper; Xiu Wang supervised and directed the biological assay; Wen-Gui Duan constructed the target compound structure, designed the experimental scheme, contributed with valuable discussions and revised the paper. Gui-Shan Lin participated in the discussion of evaluation of anticancer activity. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 2, 3, and 4a–x are available from the authors.
References
- 1.Xue S.A., Stauss H.J. Enhancing immune responses for cancer therapy. Cell. Mol. Immunol. 2007;4:173–184. [PubMed] [Google Scholar]
- 2.Malvezzi M., Carioli G., Rodriguez T., Negri E., La Vecchia C. Global trends and predictions in ovarian cancer mortality. Ann. Oncol. 2016;27:2017–2025. doi: 10.1093/annonc/mdw306. [DOI] [PubMed] [Google Scholar]
- 3.Chen W.Q., Zheng R.S., Baade P.D., Zhang S.W., Zeng H.M., Bray F., Jemal A., Yu X.Q., He J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Newman D.J., Cragg G.M., Snader K.M. Natural products as source of new drugs over the period. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.H. Discovery and development of natural product derived chemo-therapeutic agents based on a medicinal chemistry approach. J. Nat. Prod. 2010;73:500–516. doi: 10.1021/np900821e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chyu C.F., Lin H.C., Kuo Y.H. New abietane and seco-abietane diterpenes from the roots of Taiwania cryptomerioides. Chem. Pharm. Bull. 2005;53:11–14. doi: 10.1248/cpb.53.11. [DOI] [PubMed] [Google Scholar]
- 7.Pertino M.W., Vega C., Rolón M., Coronel C., Arias A.R., Schmeda-irschmann G. Antiprotozoal activity of triazole derivatives of dehydroabietic acid and oleanolic acid. Molecules. 2017;22:369. doi: 10.3390/molecules22030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada H., Kodato S., Kawamori M., Morikawa T., Nakai H., Takeda M., Saito S., Onoda Y., Tamaki H. Antiulcer activity of dehydroabietic acid derivatives. Chem. Pharm. Bull. 1985;33:1472–1487. doi: 10.1248/cpb.33.1472. [DOI] [PubMed] [Google Scholar]
- 9.Kang M.S., Hirai S., Goto T., Kuroyanagi K., Lee J.Y., Uemura T., Ezaki Y., Takahashi N., Kawada T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem. Biophys. Res. Commun. 2008;369:333–338. doi: 10.1016/j.bbrc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Zapata B., Rojas M., Betancur-Galvis L., Mesa-Arango A.C., Pérez-Guaitac D., González M.A. Cytotoxic, immunomodulatory, antimycotic, and antiviral activities of semisynthetic 14-hydroxyabietane derivatives and triptoquinone C-4 epimers. Med. Chem. Commun. 2013;4:1239–1246. doi: 10.1039/c3md00151b. [DOI] [Google Scholar]
- 11.Zhang W.M., Yang T., Pan X.Y., Liu X.L., Lin H.X., Gao Z.B., Yang C.G., Cui Y.M. The synthesis and antistaphylococcal activity of dehydroabietic acid derivatives: Modifications at C12 and C7. Eur. J. Med. Chem. 2017;127:917–927. doi: 10.1016/j.ejmech.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Helfenstein A., Vahermo M., Nawrot D.A., Demirci F., Iscan G., Krogerus S., Yli-Kauhaluoma J., Moreira V.M., Tammela P. Antibacterial profiling of abietane-type diterpenoids. Bioorg. Med. Chem. 2017;25:132–137. doi: 10.1016/j.bmc.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Kusumoto1 N., Zhao T., Swedjemark G., Ashitani T., Takahashi K., Borg-Karlson A.K. Antifungal properties of terpenoids in Picea abies against Heterobasidion parviporum. For. Path. 2014;44:353–361. doi: 10.1111/efp.12106. [DOI] [Google Scholar]
- 14.Tolmacheva I.A., Tarantin A.V., Boteva A.A., Anikina L.V., Vikharev Y.B., Grishko V.V., Tolstikov A.G. Synthesis and biological activity of nitrogen-containing derivatives of methyl dehydroabietate. Pharm. Chem. J. 2006;40:27–31. doi: 10.1007/s11094-006-0161-0. [DOI] [Google Scholar]
- 15.Kim J., Kang Y.G., Lee J.Y., Choi D.H., Cho Y.U., Shin J.M., Park J.S., Lee J.H., Kima W.G., Seo D.B., et al. The natural phytochemical dehydroabietic acid is an anti-aging reagent that mediates the direct activation of SIRT1. Mol. Cell. Endocrinol. 2015;412:216–225. doi: 10.1016/j.mce.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Sepúlveda B., Astudillo L., Rodríguez J.A., Yáñez T., Theoduloz C., Schmeda-Hirschmann G. Gastroprotective and cytotoxic effect of dehydroabietic acid derivatives. Pharmacol. Res. 2005;52:429–437. doi: 10.1016/j.phrs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Cui Y.M., Liu X.L., Zhang W.M., Lin H.X., Ohwada T., Ido K., Sawada K. The synthesis and BK channel-opening activity of N-acylaminoalkyloxime derivatives of dehydroabietic acid. Bioorg. Med. Chem. Lett. 2016;26:283–287. doi: 10.1016/j.bmcl.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Xu H.T., Liu L.L., Fan X.T., Zhang G.J., Li Y.C., Jiang B. Identification of a diverse synthetic abietane diterpenoid library for anticancer activity. Bioorg. Med. Chem. Lett. 2017;27:505–510. doi: 10.1016/j.bmcl.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Wen G., Miao T.T., Hua D.W., Jin X.Y., Tao X.B., Huang C.B., Wang S.F. Synthesis and in vitro cytotoxic evaluation of new 1H-benzo[d]imidazole derivatives of dehydroabietic acid. Bioorg. Med. Chem. Lett. 2017;27:1296–1300. doi: 10.1016/j.bmcl.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Huang X.C., Huang R.Z., Liao Z.X., Pan Y.M., Gou S.H., Wang H.S. Synthesis and pharmacological evaluation of dehydroabietic acid thiourea derivatives containing bisphosphonate moiety as an inducer of apoptosis. Eur. J. Med. Chem. 2016;108:381–391. doi: 10.1016/j.ejmech.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Cukierman D.S., Pinheiro A.B., Castiñeiras-Filho S.L., Silva A.S., Miotto M.C., De Falco A., de P., Ribeiro T., Maisonette S., da Cunha A.L., Hauser-Davis R.A., et al. A moderate metal-binding hydrazone meets the criteria for a bioinorganic approach towards Parkinson’s disease: Therapeutic potential, blood-brain barrier crossing evaluation and preliminary toxicological studies. J. Inorg. Biochem. 2017;170:160–168. doi: 10.1016/j.jinorgbio.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F., Wang X.L., Shi J., Wang S.F., Yin Y., Yang Y.S., Zhang W.M., Zhu H.L. Synthesis, molecular modeling and biological evaluation of N-benzylidene-2-((5-(pyridin-4-yl)-1,3,4-oxadiazol-2-yl)thio)acetohydrazide derivatives as potential anticancer agents. Bioorg. Med. Chem. 2014;22:468–477. doi: 10.1016/j.bmc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Onnis V., Demurtas M., Deplano A., Balboni G., Baldisserotto A., Manfredini S., Pacifico S., Liekens S., Balzarini J. Design, synthesis and evaluation of antiproliferative activity of new benzimidazolehydrazones. Molecules. 2016;21:579. doi: 10.3390/molecules21050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Y., Fan C.D., Zhao B.X., Shin D.S., Miao J.Y. Synthesis and structure–activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone derivatives as potential agents against A549 lung cancer cells. Eur. J. Med. Chem. 2008;43:2347–2353. doi: 10.1016/j.ejmech.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Cui Z.N., Li Y., Ling Y., Huang J., Cui J.R., Wang R.Q., Yang X.L. New class of potent antitumor acylhydrazone derivatives containing furan. Eur. J. Med. Chem. 2010;45:5576–5584. doi: 10.1016/j.ejmech.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Chen N.Y., Duan W.G., Lin G.S., Liu L.Z., Zhang R., Li D.P. Synthesis and antifungal activity of dehydroabietic acid-based 1,3,4-thiadiazole-thiazolidinone compounds. Mol. Divers. 2016;20:897–905. doi: 10.1007/s11030-016-9691-x. [DOI] [PubMed] [Google Scholar]
- 27.Chen N.Y., Duan W.G., Liu L.Z., Li F.Y., Lu M.P., Liu B.M. Synthesis and antifungal activity of dehydroabietic acid-based thiadiazole-phosphonates. Holzforschung. 2015;69:1069–1075. doi: 10.1515/hf-2014-0315. [DOI] [Google Scholar]
- 28.Lin G.S., Duan W.G., Yang L.X., Huang M., Lei F.H. Synthesis and antifungal activity of novel myrtenal-based 4-methyl-1,2,4-triazole-thioethers. Molecules. 2017;22:193. doi: 10.3390/molecules22020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan W.G., Ma X.L., Mo Q.J., Huang J.X., Cen B., Xu X.T., Lei F.H. Synthesis and herbicidal activity of 5-dehydroabietyl-1,3,4-oxadiazolederivatives. Holzforschung. 2011;65:191–197. doi: 10.1515/hf.2011.016. [DOI] [Google Scholar]
- 30.Mo Q.J., Duan W.G., Li X.R., Huang D.P., Luo Z.J. Synthesis and herbicidal activity of 2-substituted amino-5-dehydroabietyl-1,3,4-oxadiazole derivatives. Chin. J. Org. Chem. 2011;31:1114–1121. [Google Scholar]
- 31.Li F.Y., Mo Q.J., Duan W.G., Lin G.S., Cen B., Chen N.Y., Yang Z.Q. Synthesis and insecticidal activities of N-(5-dehydroabietyl-1,3,4-thiadiazol-2-yl)-benzene-sulfonamides. Med. Chem. Res. 2014;23:4420–4426. doi: 10.1007/s00044-014-1009-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.