Background
Clusters of dengue cases have recently become more frequent in areas of southern France colonised by the vector mosquito Aedes albopictus. In July 2015, a 2-month outbreak of dengue virus serotype 1 (DENV-1) was reported in Nîmes. Aim: We conducted a serosurvey in the affected area at the end of the vector activity period to determine the true extent of dengue transmission. Methods: We collected capillary blood from consenting household members, and information on their medical and travel histories, and exposure to mosquito bites. Recent infections were identified using IgM and IgG anti-DENV ELISA, followed, when positive, by plaque reduction neutralisation tests on serum against DENV 1–4 and West Nile virus. The prevalence estimator was calibrated on reference demographic data. We quantified the spatial clustering of dengue cases within the affected community and inferred the transmission tree. Results: The study participation rate was 39% (564/1,431). Three of 564 participants tested positive for DENV-1 infection (after marginal calibration, 0.41%; 95% confidence interval: 0.00–0.84). The spatial analysis showed that cases were clustered at the household level. Most participants perceived the presence of mosquitos as abundant (83%) and reported frequent mosquito bites (57%). We incidentally identified six past West Nile virus infections (0.9%; 95% CI: 0.2–1.6). Conclusion: This serosurvey confirms the potential for arboviral diseases to cause outbreaks − albeit limited for now − in France and Europe.
Keywords: Dengue outbreak – Seroprevalence – Transmission risk – Aedes albopictus – Europe/France – West Nile virus
Introduction
Dengue is the most common vector-borne viral disease worldwide, affecting 390 million people in tropical and sub-tropical areas each year [1]. The clinical spectrum of dengue ranges from a mild, non-specific febrile syndrome (classic dengue fever) to severe dengue with plasma leakage, haemorrhage or organ impairment [2]. In 20–97% of cases, dengue is clinically inapparent; this proportion varies considerably, across countries and years [3]. Asymptomatic infections may contribute significantly to virus transmission [4].
Aedes aegypti is the main vector of dengue virus in tropical areas [5]. Of more direct concern to continental Europe is a secondary vector, Ae. albopictus. Originally a tree-breeding mosquito of the forests of south-east Asia, Ae. albopictus has dramatically expanded its geographic distribution throughout the world in the past 40 years. Taking advantage of increasing global trade, Ae. albopictus spread to temperate regions in the late 1970s [6]. It was first reported in Europe in 1979 and has since been observed in France and 14 other countries along the Mediterranean. Given the recent establishment and rapid spread of Ae. albopictus in southern France, there is high potential for the emergence of arboviruses such as dengue virus (DENV), chikungunya and Zika viruses [7-10]. Limited local transmission of DENV was reported in southern France in 2010, 2013 and 2014, each cluster involving ≤ 2 cases [11-13]. Recently, from July to September 2015, a dengue outbreak occurred in the neighbourhood of Nîmes, Occitanie region [14]. Epidemiological investigations including door-to-door case finding conducted in August 2015 revealed seven autochthonous cases of dengue serotype 1 (DENV-1) that arose from a likely primary case over a 2-month period [14]. In France, a national surveillance and response plan aims to prevent and control local dissemination of dengue and other Ae. albopictus-transmitted viruses. However, little is known about the drivers and determinants of local transmission of dengue in the European setting, and the national response plan may not be fully effective.
In November 2015 we carried out a seroprevalence survey in order to determine the true extent of the dengue outbreak in Nîmes and the proportion of asymptomatic infections. We also reconstructed the transmission chain to characterise the spatial pattern of dengue transmission and to contribute to improving the performance of the French surveillance and response plan.
Methods
Study design
We conducted a cross-sectional population-based serosurvey in the area affected by dengue in Nîmes. The study population included all members of households located within a 150 m radius around the residences of each case identified in the initial investigation. The study area covers the range where vector control measures were applied in compliance with the French preparedness and response plan [15]. In addition, the 109 households within a 100 m directly adjacent margin of the vector-control range were also eligible for inclusion (Figure 1). When > 50% of the houses in a block fell within the study area, we included the entire block. Inclusion criteria were: (i) residing in the study area since 1 July 2015; (ii) an age of 2 years or older. Patients under anticoagulant therapy were excluded. In total, 512 households were eligible, comprising an estimated 1,471 individuals of whom 1,431 were at least 2 years old (estimates obtained from the French National Institute of Statistics and Economic Studies (INSEE, ‘carroyages 200m’ and RFL2010, 2013); http://www.insee.fr/en/).
Figure 1.
Map of the study area, dengue serosurvey, Nîmes, France, 2015
Source: Ign-BDTopo 2011; SOeS CORINE Land Cover 2015; Santé publique France, 2016.
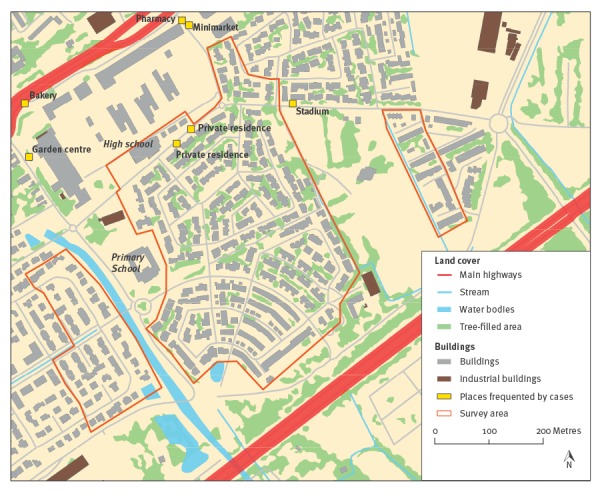
All eligible members of all households within the study area were invited to participate in the study. Enrolment took place between 6 and 20 November 2015, over a period of 9 days excluding holidays and weekends, from 09:00 to 15:00 and 15:00 to 21:00. For unresponsive households, we carried out three additional visits on separate dates and at different times of the day. After four visits without response, the household was considered non-respondent.
Data and blood sample collection
Pairs of trained investigators, comprising at least one medical member of staff, interviewed the study participants, and collected capillary blood samples from fingertips on blotting paper (2–4 drops). They interviewed one adult per household (known henceforth as ‘the householder’) using a standardised questionnaire exploring household characteristics (address, demographics, vector-breeding sites, vegetation, protective measures such as window screens, air conditioning). In addition, the investigators interviewed each consenting household member (or parent for children) using a questionnaire to collect clinical data (medical history, symptoms compatible with dengue infection since 1 July, vaccination against flaviviruses), travel history and recent itineraries, individual risk factors for mosquito bites and dengue infection, knowledge and perception of the disease and its prevention.
Laboratory analysis
Capillary blood samples were tested for serological evidence of dengue infection by in-house capture ELISA at the French National Reference Centre for Arboviruses (NRC) in Marseille [16]. Persons presenting IgM or IgG positive samples were requested to provide an additional blood sample by venepuncture, for plaque reduction neutralisation testing (PRNT) against DENV serotypes 1–4 and West Nile virus (WNV).
Case definitions
A case of recent infection was defined as a person testing positive for anti-DENV IgM and IgG. Samples with isolated IgG and a PRNT positive for DENV (serotype 1 to 4) were classified as past infections.
A symptomatic case was defined as a case of recent infection who had presented with a febrile illness with body temperature ≥ 38 °C, with or without other symptoms, not explained by another medical condition, since 1 July 2015.
A case was classified as imported if the infected person had stayed in an area outside of continental France and Corsica, known at that time for being endemic or epidemic for dengue, in the 15 days preceding the date of onset of symptoms, or in the year before the interview for asymptomatic cases. Cases that did not meet the criteria of an imported case were classified as autochthonous.
The seven confirmed cases detected during the outbreak investigations conducted in August 2015 were all classified as autochthonous, recent dengue infections [14].
Data analysis
Demographic characteristics
We compared the study participants with the reference population (i.e. the 1,431 inhabitants of the neighbourhood aged at least 2 years) in terms of age, sex and socio-professional category using the chi-squared test. We used nine age groups (2–3 years, 4–5 years, 6–10 years, 11–14 years, 15–17 years, 18–24 years, 25–64 years, 65–74 years and 75 years and older) and the eight categories defined by INSEE (farmer, tradesperson, upper white-collar, intermediate occupation, lower white-collar, blue-collar, retired, and never worked) [17]. The marginal distribution of these three variables in the reference population was calculated using information on two infra-communal areas covering the studied neighbourhood. These infra-communal areas, called IRIS (Ilots Regroupés pour l'Information Statistique) are grouped housing blocks created for the purpose of statistical information by INSEE [18].
Estimating prevalence of recent infections
Weights of the study participants, initially equal, were calibrated so that the estimated counts reflected the demographic structure of the reference population in terms of age structure and socio-professional category, as described above.
As the joint distribution of these two variables was not available, we applied a marginal calibration method using the marginal distributions obtained as specified in the previous paragraph. Specifically, we used the raking ratio method [19], implemented in the SAS macro CALMAR [20].
After calibration, the prevalence of recent dengue infections,,was estimated as follows:
where N is the size of the reference population and the weights of the K participants who met the criteria for a case of recent dengue infection.
The variance of was estimated as follows:
where n is the size of the sample of participants. Of note, is called the finite population correction factor.
Finally, we calculated the 95% confidence interval (CI) as follows:
We used SAS version 9.3 (SAS Institute, Cary, North Carolina, United States) to perform the analyses.
Description of the spatial patterns of observed cases and inferring the transmission tree
In this analysis, we used data from all cases identified through the seroprevalence survey and the seven confirmed cases identified through the outbreak investigation conducted in August 2015 [14]. Geographic coordinates of study participants’ residences were located using ArcGIS 10.2.2 software (ESRI Inc). Nine non-infected individuals with missing household coordinates were excluded from the analysis.
We calculated the median, minimum and maximum distances between pairs of cases and pairs of non-infected individuals based on their household locations. We quantified spatial clustering at various distance ranges in the community (same household, 0–50 m, >50–100 m and >100–200 m) as the Relative Risk (RR) of two cases living within a distance range relative to two non-infected individuals living within that distance range [21]. Details of the spatial clustering measure we used are provided in the Supplement. Confidence intervals (95% CI) of the RRs were estimated using normal approximation with small sample adjustment implemented in the epitools package in R.
We developed a statistical model to characterise the spread of DENV in this population and reconstructed the most likely transmission tree. The model jointly analysed data on DENV cases and non-infected individuals in the community and made it possible to test whether the rate of person-to-person transmission declined with distance. We assumed that the serial interval (i.e. time between symptom onset in a case and symptom onset in the persons they infected) of DENV was distributed with a mean of 16 days and a variance of 40 days (based on information about the different infection stages). Technical details are provided in the Supplement. We estimated parameters of the model using Bayesian Markov chain Monte Carlo sampling and report the posterior median with 95% credible intervals (95% CrI). We performed sensitivity analysis on the assumed serial interval distribution and we investigated the effect of potential case under-detection (Supplement).
Ethical approval
The study was carried out with the approval of the French Commission for Data Protection (Commission Nationale de l’Informatique et des Libertés). Participation was voluntary. Signed informed consent was obtained from all participants or their legal guardian for children.
Results
Survey participation and description of the participants
In total, 55% (282/512) of households and 39% (564/1,431) of residents participated in the study. The median age was 45 years (min–max: 2–86 years), with a male-to-female sex ratio of 0.80 (242/303). Of the participating householders 0.6% (3/542) were classified as farmers, 7% (36/542) as tradespeople, 2% (13/564) as upper white-collar, 16% (85/542) as intermediate occupation, 30% (165/542) as lower white-collar, 30% (164/542) as blue-collar, 13% (69/542) as retired and 1% (7/542) as never worked. The sample differed from the study population in terms of socio-professional categories (p < 0.0001) and age groups (p < 0.0001). Their sex distributions did not differ (p = 0.19). The categories of 18–24 years old, and lower white- or blue-collar were over-represented (Table 1).
Table 1. Distribution of age, sex and socio-professional category among study participants and reference populations, dengue serosurvey, Nîmes, France, 2015.
| Participants | Reference population | |||
|---|---|---|---|---|
|
Age group (years) |
Number |
Percentage (%) |
Number |
Percentage (%) |
| 2–3 | 8 | 1.4 | 20 | 1.4 |
| 4–5 | 5 | 0.9a | 38 | 2.7 |
| 6–10 | 33 | 5.9 | 104 | 7.3 |
| 11–14 | 27 | 4.8 a | 109 | 7.6 |
| 15–17 | 13 | 2.3 b | 73 | 5.1 |
| 18–24 | 56 | 10.0 c | 53 | 3.7 |
| 25–64 | 352 | 62.9 | 887 | 62.0 |
| 65–74 | 46 | 8.2 | 110 | 7.7 |
| ≥ 75 | 20 | 3.6 | 37 | 2.6 |
| Total | 564 | 100.0 | 1,431 | 100.0 |
| Sex | ||||
| Males | 256 | 45.5 | 697 | 48.7 |
| Socio-professional category of the householder | ||||
| Farmer | 3 | 0.6 | 10 | 0.7 |
| Tradesperson | 36 | 6.6 | 77 | 5.4 |
| Upper white-collar | 13 | 2.4 c | 183 | 12.8 |
| Intermediate occupation | 85 | 15.7 | 203 | 14.2 |
| Lower white-collar | 165 | 30.4 c | 227 | 15.9 |
| Blue-collar | 164 | 30.3 c | 294 | 20.6 |
| Retired | 69 | 12.7 c | 307 | 21.5 |
| Never worked | 7 | 1.3 c | 130 | 9.1 |
| Total | 542 | 100.0 | 1,431 | 100.0 |
The reference population was estimated for the 512 households eligible for study participation based on data from the French National Institute of Statistics and Economic Studies (INSEE, ‘carroyages 200 m’ and RFL2010, 2013)
a Proportion significantly different from the reference population (chi-squared test, p < 0.05).
b Proportion significantly different from the reference population (chi-squared test, p < 10 − 2).
c Proportion significantly different from the reference population (chi-squared test, p < 10 − 4).
A large proportion of participants reported an abundant presence of mosquitoes in the neighbourhood (83%) and frequent mosquito bites (57%) (Table 2. Most participants’ housing (98%) had a garden or terrace and its inhabitants frequently (88%) reported the presence of potential mosquito breeding sites (automatic watering, ornamental pond, temporary swimming pool, non-covered rainwater collection basin) (Table 2).
Table 2. Participants’ perception and behaviours regarding mosquito bites and their prevention, and household characteristics, dengue serosurvey, Nîmes, France, 2015.
| Number | Percentage (%) | |
|---|---|---|
| Housing characteristics | ||
| Presence of a garden or terrace (n = 269) | 264 | 98.1 |
| Mosquito nets at windows (n = 280) | 99 | 35.4 |
| Use of air conditioning often or sometimes (n = 279) | 91 | 32.6 |
| Reported presence of mosquito breeding sites in the garden or terracea (n = 269) | 237 | 88.1 |
| Windows open during the day often or sometimes (n = 280) | 155 | 55.4 |
| Perception and behaviours regarding mosquito bites and their prevention | ||
| Bitten by mosquito often or sometimes (n = 533) | 304 | 57.0 |
| Presence of mosquitoes perceived as very abundant or abundant (n = 533) | 444 | 83.3 |
| Use of insect repellent often or sometimesb (n = 472) | 248 | 52.5 |
| Wearing long-sleeved shirts and long trousers often or sometimesb (n = 468) | 127 | 27.1 |
a Automatic watering, ornamental pond, temporary swimming pool, non-covered rainwater collection basin.
b Questions asked only of participants aged 15 years and older.
Dengue seroprevalence and proportion of asymptomatic infections
The serosurvey identified three cases of recent autochthonous dengue infection: one tested positive for both anti-DENV IgM and IgG; two tested positive for anti-DENV IgG (these two had already been diagnosed during the outbreak investigation conducted in August 2015) (Figure 2). Of the five cases identified through the outbreak investigation, three cases were absent at the time of study implementation. Two cases in a household of five people refused to participate in the serosurvey on the grounds that they had taken part in the initial investigation. In total, eight autochthonous cases were identified across the two investigations. After calibration, dengue prevalence was estimated at 0.4% (95% CI, 0.0–0.8).
Figure 2.
Biological results and classification of cases, dengue serosurvey, Nîmes, France, 2015
DENV: dengue virus; NT: neutralisation test; PRNT: plaque reduction neutralisation testing; WNV: West Nile virus ; +: positive; -: negative.
a PRNT results: DENV recent infection of indeterminable serotype.
b PRNT results: DENV past infection of indeterminable serotype (2 cases) or Flavivirus past infection (3 cases). All reported a history of travel in a dengue-endemic area. Six participants had IgG antibodies against WNV, confirmed by PRNT. We estimated the prevalence of past WNV infection at 0.9% (95% CI: 0.22–1.57).
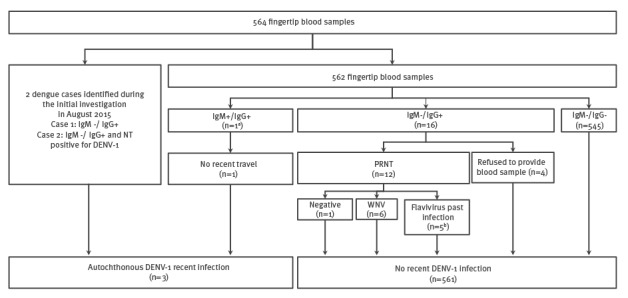
No asymptomatic case was identified. The three cases presented a febrile illness with body temperature ≥ 38 °C (n = 3), retro-orbital pain (n = 2), skin rash (n = 2), headache (n = 2), myalgia (n = 2), arthralgia (n = 2), digestive disorders (n = 1) and asthenia (n = 2).
Spatial case clustering and reconstructed transmission tree
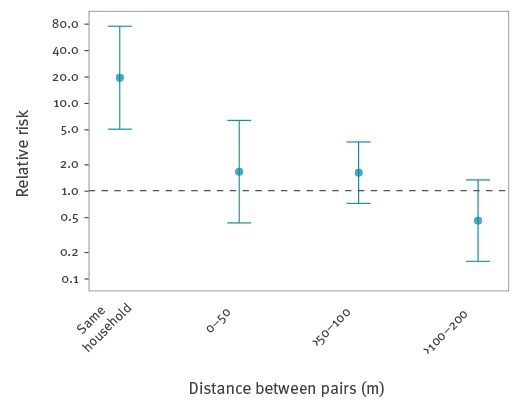
Following the importation of a likely primary case with disease onset on 6 July 2015, eight autochthonous cases developed illness within 68 days (Figure 3 A). The median distance between any two DENV cases (266 m; interquartile range (IQR): 106–333 m; range: 0–384 m) was similar to that observed between any two non-infected individuals recruited in the study (271 m; IQR: 155–385 m; range 0–816 m; Wilcoxon rank-sum test p = 0.064) (Figure 3 B). The spatial distribution of cases and non-infected individuals in the studied area suggested clustering of cases at the household level, where the risk of two DENV cases living in the same household was 18.2 (95% CrI: 4.7–70.2) times higher than the risk of two non-infected individuals living in the same household (Figure 4). There was no significant evidence for clustering at other distances in the study area of ca 800m2.
Figure 3.
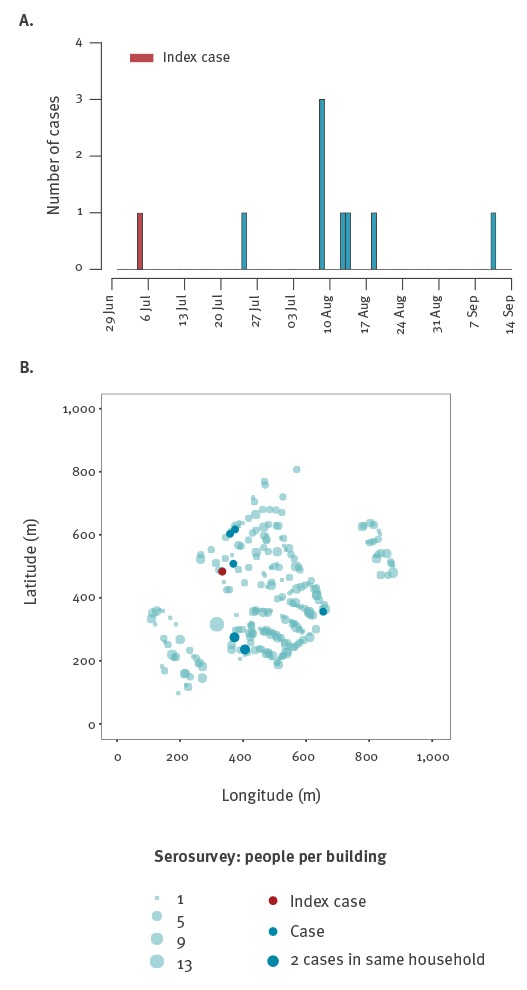
Epidemic curve (A), spatial distribution of cases (B), dengue serosurvey, Nîmes, France, 2015
Figure 4.
Spatial clustering of cases by household distance, dengue serosurvey, Nîmes, France, 2015
Spatial clustering was measured as the relative risk (RR) of two dengue virus cases living within a distance range compared to two non-infected individuals living within that distance range; where the risk of a pair being in that distance range was calculated as number pairs within the range/ number pairs within the range or further apart. A distance of 0 m represents individuals living in the same building but different households.
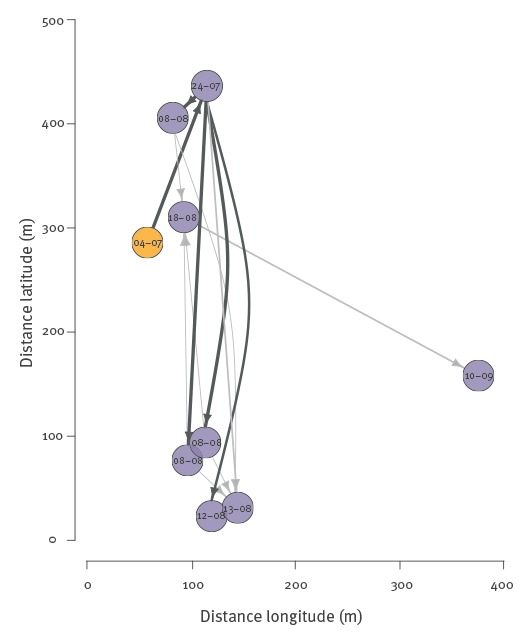
We were able to identify the most likely infector (with > 50% probability) for five of the eight autochthonous cases. The reconstructed transmission tree is shown in Figure 5. The estimated effective reproduction number R (number secondary cases caused by a case) was 0.98 (95% CrI: 0.46–1.74). Cases from the same household most likely belonged to the same generation since their symptom onset dates were very close (0 or 1 day delay). We did not find evidence for a decline in the transmission rate by geographic distance from a case.
Figure 5.
Reconstructed transmission tree, dengue serosurvey, Nîmes, France, 2015
Shown are connections between cases with transmission probabilities ≥0.2; dark grey represents connections with probabilities ≥0.5. The node labels indicate the onset date of disease and node locations represent geographic household locations. Overlapping nodes represent cases who lived in the same household. The index case is indicated in red.
Discussion
In order to determine the extent of the 2015 DENV-1 outbreak in Nîmes, we conducted a serosurvey that identified three recent autochthonous dengue cases, which, together with those already known, led to an estimated dengue seroprevalence of 0.4%. No asymptomatic infection was detected in the affected area. Dengue transmission by local Ae. albopictus mosquitoes remained limited overall to eight autochthonous cases clustered within households.
Similar low-seroprevalence estimates have been documented in other studies carried out in dengue-affected areas in high-income countries: 1% in 2004 in Texas, US, 3% in 2009 in Florida, US, and 0.6% in Croatia in 2012 [22-24].
However, our study is not devoid of limitations inherent to field investigations. We cannot exclude the possibility that additional cases went undetected, since only 55% of the households and 39% of the inhabitants of the neighbourhood participated in the study. But with only one additional case identified by the serosurvey and nearly all of the cases captured by the initial investigation, this should marginally affect our estimate of dengue prevalence in the study area. Study participants had a different age and socio-professional make-up than the reference population. Though only partially, we set out to address the effect of non-response by calibrating our prevalence estimator on reference demographic data. We could not include five cases identified through the outbreak investigation, nor the households to which they belonged. The resulting underestimation of the prevalence of dengue should have remained limited because they represented only a couple of households, surrounded by other dengue-free households.
Our study highlighted a decline in dengue antibody titre 3 months after the onset of fever. Indeed, anti-dengue IgM were undetectable for the two cases already confirmed in August 2015. Theoretically this may have led to misclassification of cases of recent DENV infection among people with anti-DENV IgG but without IgM. This could have been overcome to some extent by carrying out serosurveys sooner after an outbreak. However, we chose to conduct our study in November, in the late-activity season of Ae. albopictus in southern France, to capture any potential residual transmission [25]. Four years after the establishment of Ae. albopictus, vector density has become high enough for dengue transmission in this neighbourhood of Nîmes, mostly composed of small properties with gardens. High vector density was also corroborated by our study findings with high levels of mosquito nuisance reported by the participants.
Physical barriers in the building and landscape features that surrounded the outbreak area, such as large roads, a nearby high school campus and stadium might have contributed to preventing a wider spread of the virus. Vector-control measures applied in the outbreak area in August and September 2015 most likely played a role in breaking the transmission chain, although the exact impact cannot be measured.
Seroprevalence data on the fifth limited dengue cluster observed in France since 2010 suggest that French Ae. albopictus may not be able to sustain explosive DENV transmission. A small number of dengue outbreaks transmitted by Ae. albopictus have been described worldwide in the last decade (Hawaii 2001, Gabon 2007, China 2014, Japan 2014) [26-29]. In Nîmes, transmission of DENV remained limited despite a 2-month long circulation of the virus, from July to September 2015. However, with 160 cases, the 2014 dengue outbreak in Tokyo, Japan, demonstrated that more intensive transmission is possible even in temperate climates and that continued vigilance is needed in areas colonised by Ae. albopictus [29].
The spatial analysis showed that cases clustered at the household level. The two case-pairs in same households developed symptoms either on the same day or within one day; these household members therefore belonged most likely to the same generation of cases. The household clustering may be explained by the common exposure to an infected mosquito in the household. We did not find evidence for a spatial decline in the transmission hazard by distance from a case, estimated on the basis of household locations of cases and non-infected individuals. Several previous studies found evidence for small scale patterns in DENV transmission [30-32]. In our study, the lack of spatial transmission patterns may be due to the small number of observed DENV cases resulting in limited power to detect a significant decline in the transmission rate. The study area and the distances over which DENV cases occurred were also relatively small and spatial effects may have been more apparent if a wider area was investigated or affected. Moreover, though households are generally considered as foci for DENV transmission, individuals may have acquired infections at other locations than their homes.
No asymptomatic cases were identified through the outbreak investigation and serosurvey [14]. Most cases could reasonably be placed on a transmission tree reconstructed only on the basis of time and place of occurrence of symptomatic cases. This suggests that the contribution, if any, of undetected asymptomatic cases was possibly not crucial in this outbreak. All in all, the eight locally acquired cases related to this outbreak reported fever ≥ 38 °C (n = 8), retro-orbital pain (n = 4), skin rash (n = 5), headache (n = 7), myalgia (n = 4), arthralgia (n = 2), digestive disorders (n = 4) and asthenia (n = 6). That no asymptomatic case was identified was unexpected. In the literature, the proportion of inapparent infections varies widely, from 20% to 97% in endemic areas [3]. The active case finding conducted during the initial investigation in August 2015, enhanced awareness and easy access to healthcare in the community might have led us to detect and classify as ‘symptomatic’ any pauci-symptomatic case that would have gone unnoticed elsewhere. The wide clinical spectrum of dengue complicates the determination of the frequency of asymptomatic cases as mentioned in a review of the literature on the extent of inapparent DENV infections [3].
Some studies suggest an association between the initial immunity and the clinical expression with a higher frequency of symptomatic dengue among non-immune people [33,34]. However, the factors associated with the clinical expression of dengue have not been clearly determined. It remains possible that in our study the number of DENV cases was too low to detect asymptomatic cases.
Our results incidentally evidenced low-level circulation of WNV in the study population. A WNV serosurvey, carried out in 2000, presented similar results among blood donors living inside and near Camargue, a regional nature park located 50 km from Nîmes, where WNV re-emerged among horses earlier the same year [35]. Our estimate of 0.9% falls between that of the prevalence of antibodies against WNV in blood donors living in Camargue at 1% and in contiguous regions at 0.2%.
Conclusion
Transmission of DENV during the 2015 outbreak in Nîmes remained of low intensity and clustered within households despite a high perceived density of Ae. albopictus and a 2-month long circulation period of the virus.
The potential for arboviral diseases to cause outbreaks in France should not be underestimated. The geographical distribution of Ae. albopictus is expanding steadily while climate change could increase the potential for outbreaks northward. The intensification of international travel increases the risk of DENV introduction in the naïve population of mainland France. Moreover the competence of Ae. albopictus to transmit other major arboviruses has been demonstrated, in the field, for chikungunya virus and, in the laboratory, for Zika virus
More broadly, this risk is present in several other European countries where new areas are colonised by Ae. albopictus each year.
Our study suggests that surveillance and early outbreak investigation can successfully determine the extent of dengue emergence. Further serosurveys should be conducted around future outbreaks to monitor the evolution of the transmission dynamics of arboviral diseases in Europe. These studies could also further document the proportion of unapparent arboviral infections and their contribution to transmission, with the ultimate aim of adapting the control measures around autochthonous transmission.
Acknowledgements
Laboratoire d'Excellence ‘Integrative Biology of Emerging Infectious Diseases’ (grant n°ANR-10-LABX-62-IBEID).
We thank Béatrice Broche and Isabelle Esteve-Moussion (ARS Occitanie) for supporting the study implementation; Bruno Coignard, Marie-Claire Paty and Jean-Claude Desenclos (SpFrance) for finding funding and improving the manuscript; the staff of the National Reference Centre on Arboviruses (Institut de recherche biomédicale des armées, Marseille) for testing all samples.
We are also grateful to the investigators: Stéphanie Baud, Pascale Berthommé, Dominique Bouillin, Evelyne Dussere-Berard, Mohammed Elarouti, Laurence Laporte, Guy Laruche, Yannick Lecoin, Marie Mas, Jacqueline Maurel, Marie-Brigitte Moyano, Adeline Philippe, Olivier Puech, Purificacion Queimano, Palma Rols, Axel Wiegandt (ARS Occitanie), Davide Tufo (ARS PACA, Marseille, France), Narimane Agoudjil, Siegfried Androllio, Constance Brossier, Sandrine Duron, Joffrey Marchi, Madjid Mokrane, Marc-Antoine Sanchez, Sébastien Sicard, Guillaume Velut (Centre d'épidémiologie et de santé publique des armées, Marseille), Frédéric Jourdain, Yvon Perrin (Centre National d’Expertise sur les vecteurs, Montpellier), François Clinard, Elodie Terrien (Cire Bourgogne-Franche Comté, Dijon), Véronique Servas (Cire Nouvelle Aquitaine, Bordeaux), Cécile Durand, Franck Golliot, Anne Guinard (Cire Occitanie, Toulouse), Alexis Armengaud, Caroline Six, Cyrielle Orenes, Florian Franke, Guillaume Heuzé, Sandra Giron (Cire PACA-Corse, Marseille), Didier Caire, Jean-Baptiste Ferré, Grégory L'Ambert, José Yuste (Interdepartmental Agreement for mosquito control on the Mediterranean coast, Montpellier), Elisabeth Couturier, Damien Pognon (SpFrance).
Finally we thank all study participants and their neighbourhood committee for making this possible.
Conflict of interest: None declared.
Authors’ contributions: Tiphanie Succo and Harold Noël coordinated the study, drafted the initial draft of the manuscript and reviewed final document for accuracy.
Harold Noël drafted the study protocol, participated to the study implementation and data analysis.
Tiphanie Succo performed the analysis for estimating the seroprevalence. Perrine de Crouy-Chanel and Camille Pelat helped with the analysis and reviewed drafts of the manuscript.
Birgit Nikolay, Henrik Salje and Simon Cauchemez designed the spatial case clustering and transmission tree reconstruction analysis. Birgit Nikolay performed this analysis.
Henriette de Valk initiated the study and reviewed and contributed to drafts of the final study protocol and the manuscript for accuracy.
Marianne Maquart and Isabelle Leparc-Goffart conducted the virological analysis and reviewed the manuscript.
Amandine Cochet and Olivier Catelinois developed investigation tools and reviewed drafts of the manuscript.
Cyril Rousseau initiated the study, drafted the manuscript and reviewed the final version.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504-7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: WHO; 2009. Available from: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf [PubMed]
- 3.Grange L, Simon-Loriere E, Sakuntabhai A, Gresh L, Paul R, Harris E. Epidemiological risk factors associated with high global frequency of inapparent dengue virus infections. Front Immunol. 2014;5:280. 10.3389/fimmu.2014.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, Long KC, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci USA. 2015;112(47):14688-93. 10.1073/pnas.1508114112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigau-Pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352(9132):971-7. 10.1016/S0140-6736(97)12483-7 [DOI] [PubMed] [Google Scholar]
- 6.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12(6):435-47. 10.1089/vbz.2011.0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu-Helmersson J, Quam M, Wilder-Smith A, Stenlund H, Ebi K, Massad E, et al. Climate Change and Aedes Vectors: 21st Century Projections for Dengue Transmission in Europe. EBioMedicine. 2016;7:267-77. 10.1016/j.ebiom.2016.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L’Ambert G, Cochet A, et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill. 2015;20(17):21108. 10.2807/1560-7917.ES2015.20.17.21108 [DOI] [PubMed] [Google Scholar]
- 9.Jupille H, Seixas G, Mousson L, Sousa CA, Failloux AB. Zika Virus, a New Threat for Europe? PLoS Negl Trop Dis. 2016;10(8):e0004901. 10.1371/journal.pntd.0004901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. CHIKV study group Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840-6. 10.1016/S0140-6736(07)61779-6 [DOI] [PubMed] [Google Scholar]
- 11.La Ruche G, Souarès Y, Armengaud A, Peloux-Petiot F, Delaunay P, Desprès P, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15(39):19676. [PubMed] [Google Scholar]
- 12.Marchand E, Prat C, Jeannin C, Lafont E, Bergmann T, Flusin O, et al. Autochthonous case of dengue in France, October 2013. Euro Surveill. 2013;18(50):20661. 10.2807/1560-7917.ES2013.18.50.20661 [DOI] [PubMed] [Google Scholar]
- 13.Giron S, Rizzi J, Leparc-Goffart I, Septfons A, Tine R, Cadiou B, et al. (New occurrence of indigenous dengue cases in the Provence-Alpes-Côte d'Azur region, France, August-September 2014) Nouvelles apparitions de cas autochtones de dengue en région Provence-Alpes-Côte d’Azur, France, août-septembre 2014. Bull Epidemiol Hebd (Paris). 2015; (13-14):6. [Google Scholar]
- 14.Succo T, Leparc-Goffart I, Ferré JB, Roiz D, Broche B, Maquart M, et al. Autochthonous dengue outbreak in Nîmes, South of France, July to September 2015. Euro Surveill. 2016;21(21):30240. 10.2807/1560-7917.ES.2016.21.21.30240 [DOI] [PubMed] [Google Scholar]
- 15.Ministère des affaires sociales, de la santé et des droits des femmes, France. Instruction N° DGS/RI1/2015/125 du 16 avril 2015 mettant à jour le guide relatif aux modalités de mise en oeuvre du plan anti-dissémination du chikungunya et de la dengue en métropole. [French Ministry of Social Affairs, Health and Women's Rights, France. Instruction N ° DGS / RI1 / 2015/125 of 16 April 2015 updating the implementation guide of the anti-dissemination plan for chikungunya and dengue fever in metropolitan France]. 2015. French. Available from: http://circulaire.legifrance.gouv.fr/pdf/2015/04/cir_39495.pdf
- 16.D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374(22):2195-8. 10.1056/NEJMc1604449 [DOI] [PubMed] [Google Scholar]
- 17.The National Institute of Statistics and Economic Studies (Insee). Nomenclature des Profession et Catégories Socioprofessionnelles- PCS 2003. [Nomenclature of Occupation and Socio-professional Categories]. Paris: Insee. [Accessed 27 Feb 2017]. Available from: https://www.insee.fr/fr/information/2400059
- 18.The National Institute of Statistics and Economic Studies (Insee). Ilots Regroupés pour l'Information Statistique (IRIS). Definition. [Aggregated geographical units for statistical information. Definition]. Paris: Insee. [Accessed 27 Feb 2017]. French. Available from: https://www.insee.fr/fr/metadonnees/definition/c1523
- 19.Deville J-C, Särndal C-E, Sautory O. Generalized Raking Procedures in Survey Sampling. J Am Stat Assoc. 1993;88(423):1013-20. 10.1080/01621459.1993.10476369 [DOI] [Google Scholar]
- 20.The National Institute of Statistics and Economic Studies (Insee). La macro SAS CALMAR. [The SAS CALMAR macro]. Paris: Insee. [Accessed 23 Feb 2017]. French. Available from: https://www.insee.fr/fr/information/2021902.
- 21.Salje H, Cauchemez S, Alera MT, Rodriguez-Barraquer I, Thaisomboonsuk B, Srikiatkhachorn A, et al. Reconstruction of 60 Years of Chikungunya Epidemiology in the Philippines Demonstrates Episodic and Focal Transmission. J Infect Dis. 2016;213(4):604-10. 10.1093/infdis/jiv470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radke EG, Gregory CJ, Kintziger KW, Sauber-Schatz EK, Hunsperger EA, Gallagher GR, et al. Dengue outbreak in Key West, Florida, USA, 2009. Emerg Infect Dis. 2012;18(1):135-7. 10.3201/eid1801.110130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunkard JM, Robles López JL, Ramirez J, Cifuentes E, Rothenberg SJ, Hunsperger EA, et al. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis. 2007;13(10):1477-83. 10.3201/eid1310.061586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pem-Novosel I, Vilibic-Cavlek T, Gjenero-Margan I, Kaic B, Babic-Erceg A, Merdic E, et al. Dengue virus infection in Croatia: seroprevalence and entomological study. New Microbiol. 2015;38(1):97-100. [PubMed] [Google Scholar]
- 25.Delaunay P, Hubiche T, Blanc V, Perrin Y, Marty P, Del Giudice P. [Aedes albopictus in metropolitan France]. Ann Dermatol Venereol. 2012;139(5):396-401; quiz 395, 402. [DOI] [PubMed]
- 26.Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, et al. Dengue fever, Hawaii, 2001-2002. Emerg Infect Dis. 2005;11(5):742-9. 10.3201/eid1105.041063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010;10(3):259-66. 10.1089/vbz.2009.0005 [DOI] [PubMed] [Google Scholar]
- 28.Shen SQ, Wei HX, Fu YH, Zhang H, Mo QY, Wang XJ, et al. Multiple Sources of Infection and Potential Endemic Characteristics of the Large Outbreak of Dengue in Guangdong in 2014. Sci Rep. 2015;5(1):16913. 10.1038/srep16913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moi ML, Kobayashi D, Isawa H, Sasaki T, Saijo M, Kurane I, et al. Dengue Virus Isolation in Mosquito Aedes albopictus Captured During an Outbreak in Tokyo, 2014, by a Method Relying on Antibody-Dependent Enhancement Mechanism Using FcγR-Expressing BHK Cells. Vector Borne Zoonotic Dis. 2016;16(12):810-2. 10.1089/vbz.2016.1982 [DOI] [PubMed] [Google Scholar]
- 30.Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJ, et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6(7):e1730. 10.1371/journal.pntd.0001730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salje H, Lessler J, Endy TP, Curriero FC, Gibbons RV, Nisalak A, et al. Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci USA. 2012;109(24):9535-8. 10.1073/pnas.1120621109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mammen MP, Pimgate C, Koenraadt CJ, Rothman AL, Aldstadt J, Nisalak A, et al. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5(11):e205. 10.1371/journal.pmed.0050205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson KB, Gibbons RV, Cummings DA, Nisalak A, Green S, Libraty DH, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J Infect Dis. 2014;209(3):360-8. 10.1093/infdis/jit436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alera MT, Srikiatkhachorn A, Velasco JM, Tac-An IA, Lago CB, Clapham HE, et al. Incidence of Dengue Virus Infection in Adults and Children in a Prospective Longitudinal Cohort in the Philippines. PLoS Negl Trop Dis. 2016;10(2):e0004337. 10.1371/journal.pntd.0004337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charrel RN, de Lamballerie X, Durand JP, Gallian P, Attoui H, Biagini P, et al. Prevalence of antibody against West Nile virus in volunteer blood donors living in southeastern France. Transfusion. 2001;41(10):1320-1. 10.1046/j.1537-2995.2001.41101320.x [DOI] [PubMed] [Google Scholar]


