Background
Over the last decade, the abundant distribution of the Asian tiger mosquito Aedes albopictus in southern Europe and the import of chikungunya virus (CHIKV) by infected travellers has resulted in at least five local outbreaks of chikungunya fever in France and Italy. Considering the ongoing spread of Ae. albopictus to central Europe, we performed an analysis of the Europe-wide spatial risk of CHIKV transmission under different temperature conditions. Methods: Ae. albopictus specimens from Germany and Italy were orally infected with CHIKV from an outbreak in France and kept for two weeks at 18 °C, 21 °C or 24 °C. A salivation assay was conducted to detect infectious CHIKV. Results: Analyses of mosquito saliva for infectious virus particles demonstrated transmission rates (TRs) of > 35%. Highest TRs of 50% for the mosquito population from Germany were detected at 18 °C, while the Italian population had highest TRs of 63% at 18 °C and 21 °C, respectively. Temperature data indicated a potential risk of CHIKV transmission for extended durations, i.e. sufficiently long time periods allowing extrinsic incubation of the virus. This was shown for areas already colonised by Ae. albopictus, as well as for large parts of central Europe that are not colonised. Conclusion: The current risk of CHIKV transmission in Europe is not primarily restricted by temperature, which allows extrinsic incubation of the virus, but rather by the vector distribution. Accordingly, all European countries with established populations of Ae. albopictus should implement respective entomological surveillance and monitoring systems, as basis for suitable control measures.
Keywords: chikungunya virus, Aedes albopictus, transmission rate, risk assessment, Europe
Introduction
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus (Togaviridae family) [1]. The virus was first isolated from humans, as well as from mosquitoes, during an outbreak at the border region between Mozambique and Tanzania in 1952. Human cases of chikungunya fever can cause severe, debilitating and often chronic arthralgia [2]. Several species of the mosquito genus Aedes were found competent to transmit CHIKV. However, Ae. aegypti and Ae. albopictus are considered the most important vectors. In the past, occurrences of CHIKV were restricted to the African and Asian continents, causing local, sporadic epidemics; during the last decade, however, the virus has expanded over a substantial geographical range, invading India, Indian Ocean islands and the Americas, where it has caused millions of human infections [2].
Three phylogenetic CHIKV lineages with distinct antigenic characteristics are known worldwide [2,3]. Two major lineages circulate in Africa (East, Central and Southern Africa region (ECSA) and West Africa) and a third is present in Asia. In Africa, CHIKV is maintained in an enzootic cycle. The transmission cycle includes forest-dwelling mosquito species of the genus Aedes and non-human primates [2]. Anthropophilic mosquito species, Ae. aegypti and Ae. albopictus, play a crucial role in the urbanisation of CHIKV. This is in contrast to Asia, where the virus has historically been maintained in an urban cycle between Ae. aegypti/albopictus and humans.
Ae. aegypti is the primary vector for CHIKV. However, Ae. albopictus is considered to have a high competence to transmit a specific variant of the ECSA lineage. The virus mutant has a single amino acid change from alanine to valine at the E1 envelope glycoprotein amino acid 226 (E1–A226V) [4]. This change leads to a better adaptation of the virus to the species Ae. albopictus, resulting in a 50-fold increase in vector competence in comparison to Ae. aegypti. The ECSA mutant is considered to be the most important factor for CHIKV outbreaks in regions where the primary vector, Ae. aegypti, is absent. This is potentially linked to the spread of the ECSA lineage to the Indian Ocean and the first autochthonous CHIKV transmission in Europe [5]. Thus far, all CHIKV strains isolated during outbreaks in Europe belonged to the ECSA lineage. However, some of the isolates contained the E1-A226V-mutation [6-8], whereas others did not [9,10]. Therefore, other yet undefined mutations in the envelope proteins may also affect the adaptation of CHIKV to Ae. albopictus [9-11].
Autochthonous transmission of CHIKV has been repeatedly observed in mainland Europe. The virus circulated in France in 2010 [9], 2014 [7] and 2017 [8], and two major outbreaks occurred in Italy in 2007 [6] and 2017 [10]. In total, at least 605 human CHIKV cases were reported, the majority in the two epidemics in Italy (n = 575) [8]. This was possible because of the establishment of Ae. albopictus in the region. One of the most invasive mosquito species in the world [12,13], its global spread is driven by transcontinental connectivity through shipping and flight routes. At present, Ae. albopictus has infested more than 25 European countries [12,14], with highest abundances reported from Italy. The species is repeatedly introduced to various locations in central Europe [15], even though regions north of the Alps were previously considered unsuitable for the establishment of Ae. albopictus. Still, overwintering was recently suggested in Germany [16-18]. This included local expansion of populations and detection of larvae already in spring. According to the European Centre for Disease Prevention and Control (ECDC), Solna, Sweden, Ae. albopictus is classified as ‘established’ along the Upper Rhine Valley in Germany and France [14].
There is currently no available vaccine or CHIKV-specific treatment [5]. The control of the disease primarily depends on reduction of the vector population. Further options are individual protection with repellents or behavioural avoidance. Therefore, the evaluation of the vector competence of local mosquito populations is crucial, as the assessment of CHIKV risk transmission allows for the adapting of surveillance and control systems. Vector competence is the ability of a mosquito to acquire a pathogen and subsequently transmit it to a new host [2]. This is commonly evaluated through experimental infection experiments. These studies aim to identify infectious virus particles secreted with the saliva to the vertebrate host. Transmission only becomes possible if the virus can overcome the midgut and salivary glands, which are the most important barriers for infection of the vector and final escape [19]. Vector competence depends on a complex interaction of vector population, virus strain and temperature [20]. Only a suitable combination allows the virus to replicate and disseminate. Invasion of the salivary glands may result in transmission through the next bite.
Vector competence studies using temperatures below 20 °C have become increasingly important since the observation of established populations of the Ae. albopictus north of the Alps [14]. We therefore conducted a vector competence study at three different temperatures, with a CHIKV outbreak strain from France, using Ae. albopictus populations from Germany and Italy, to comprehensively assess the risk of CHIKV transmission in Europe.
Methods
Source, rearing and experimental infection of Aedes albopictus
Ae. albopictus populations were obtained through the collection of eggs during August 2015. Samples originated from a long-standing population in Italy (Mandatoriccio, Calabria) and a recently established population in Germany (Freiburg, Baden-Wuerttemberg). Colonies were reared at 26 °C with a relative humidity of 80% and a 12:12 hour light:dark photoperiod. Mosquitoes of generation F8 to F10 were used for experiments. Between 100 and 150 female mosquitoes (4–10 days old) were sorted in Drosophila breeding vials (Carl Roth, Karlsruhe; 20 mosquitoes each). All specimens were starved for 24 hours before they were fed with artificial, infectious blood meals. Blood meals contained 50% of human blood from the blood bank, 30% fructose at a stock concentration of 8%, 10% filtrated bovine serum (FBS) and 10% of CHIKV stock (strain CNR_24/2014, supplied by the European Virus Archive goes Global project, ECSA lineage, isolated from a human case in France, grown on Vero cells, fifth passage). Blood meals had a final concentration of 106 plaque-forming units of CHIKV per mL. Two droplets of 50 µl per vial were offered for 2 hours. Engorged mosquito specimens were sorted in new Drosophila breeding vials. Groups of 10 to 20 individuals were incubated at either 18 °C, 21 °C or 24 °C with 80% humidity for 14 days. These temperature conditions represent different areas in Europe infested by Ae. albopictus.
The central European areas recently invaded by Ae. albopictus only reach mean daily temperatures of ≥ 18 °C or ≥ 21 °C for 2 weeks per year. Areas with long-standing populations around the Mediterranean Sea can also have 14 days with temperatures ≥ 24 °C.
Collection of infection and transmission rates
Subsequently, mosquitoes were analysed for virus infection and transmission. Infection of mosquito bodies was determined by analyses of head, thorax and abdomen. Legs and wings were removed for demobilisation in the salivation assay. Each body was homogenised in 500 µl of Dulbecco’s Modified Eagle Medium (Sigma-Aldrich, St. Louis, Missouri, United States (US)). Viral RNA was extracted using the 5x MagMax Pathogen RNA/DNA Kit (Thermo Fisher Scientific, Waltham, Massachusetts, US). Detection of CHIKV RNA was conducted with RT-PCR (RealStar Chikungunya RT-PCR Kit 2.0, altona Diagnostics, Hamburg, Germany). The detailed salivation assay to detect transmission was described before [21]. In brief, mosquitoes were anesthetised with CO2 and demobilised. Forced salivation was conducted by placing the proboscis of the mosquito into a filter tip filled with 10 µl of PBS for 30 min. Saliva was incubated on Vero cells for 4 days and cells were checked for cytopathic effect (CPE). The supernatant of CPE-positive cells was harvested. RNA copies were detected using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) and the RealStar Chikungunya RT-PCR Kit 2.0.
Infection rate (IR) is commonly defined as the number of CHIKV-positive mosquito bodies per number of fed females. Different definitions exist for the calculation of transmission rates (TRs). The study did not focus on the mechanistic processes of vector competence (e.g. infection barriers), but aimed to assess the risk for CHIKV transmission in Europe. Therefore, in order to simplify the sample processing, the calculation of TRs followed the definition by Fortuna et al. [22]: number of mosquitoes with CHIKV-positive saliva per number of mosquitoes with CHIKV-positive bodies.
Chikungunya virus transmission risk assessment
The risk map for CHIKV transmission in Europe was constructed by combining the current distribution of Ae. albopictus with temperature data from the infested regions. Current distribution data of Ae. albopictus at the regional administrative level (NUTS3), as at April 2018, were obtained from [14]. Time series of daily mean temperature data (European re-analysis and observations for monitoring, E-OBS v17.0) for the study area were downloaded from http://www.ecad.eu [23]. E-OBS data are available on a 0.25 ° regular latitude-longitude grid and were extracted for a period of 10 years between 2008–2017. For each grid cell, the number of days per year with preceding 14 days having a mean daily temperature ≥ 18 °C were calculated using the programme R [24]. The annual values were then averaged over the 10-year period.
Results
Experimental infection studies showed IRs of 100%, irrespective of whether Ae. albopictus populations from Germany or Italy were used and independent of temperature (18 °C, 21 °C or 24 °C) (Table). Virus titres for both populations were similar. The values ranged from 108.4 to 1011.3 viral RNA copies per specimen, but were substantially higher at 18 °C compared with 24 °C for both populations. Likewise, infectious CHIKV particles were detected in mosquito saliva for all of these combinations, with TRs between 37.5% and 63.3%. Lowest TRs (37.5%) for both populations were observed at the warmest temperature tested (24 °C). In contrast, the highest TR (50%) for the population from Germany was detected at the coolest temperature (18 °C). The population from Italy had similar high TRs of 63.3% at 18 °C and 21 °C, respectively.
Table. Infection rates, transmission rates and virus titres of Aedes albopictus specimens from two different European populations experimentally infected with chikungunya virus and kept at three different temperatures, November 2017–March 2018 (n = 163 mosquitoes).
| Aedes albopictus population origin | T in °C | Infection ratea | Transmission rateb | Titre | |||
|---|---|---|---|---|---|---|---|
| Number of CHIKV-positive specimens/number of analysed specimens | % | Number of specimens with CHIKV-positive saliva/number of specimens with CHIKV-positive body | % | Mean log10
CHIKV RNA copies/specimen |
SD | ||
| Germany | 18 | 32/32 | 100.0 | 16/32 | 50.0 | 11.3 | 0.6 |
| Germany | 21 | 23/23 | 100.0 | 9/23 | 39.1 | 8.4 | 0.8 |
| Germany | 24 | 24/24 | 100.0 | 9/24 | 37.5 | 8.5 | 0.2 |
| Italy | 18 | 30/30 | 100.0 | 19/30 | 63.3 | 10.8 | 1.8 |
| Italy | 21 | 30/30 | 100.0 | 19/30 | 63.3 | 10.4 | 1.6 |
| Italy | 24 | 24/24 | 100.0 | 9/24 | 37.5 | 8.6 | 0.2 |
CHIKV: chikungunya virus; SD: standard deviation; T: temperature.
a Infection rate: number of CHIKV-positive mosquito bodies per number of fed females.
b Transmission rate: number of mosquitoes with CHIKV-positive saliva per number of mosquitoes with CHIKV-positive bodies.
Chikungunya virus transmission risk assessment
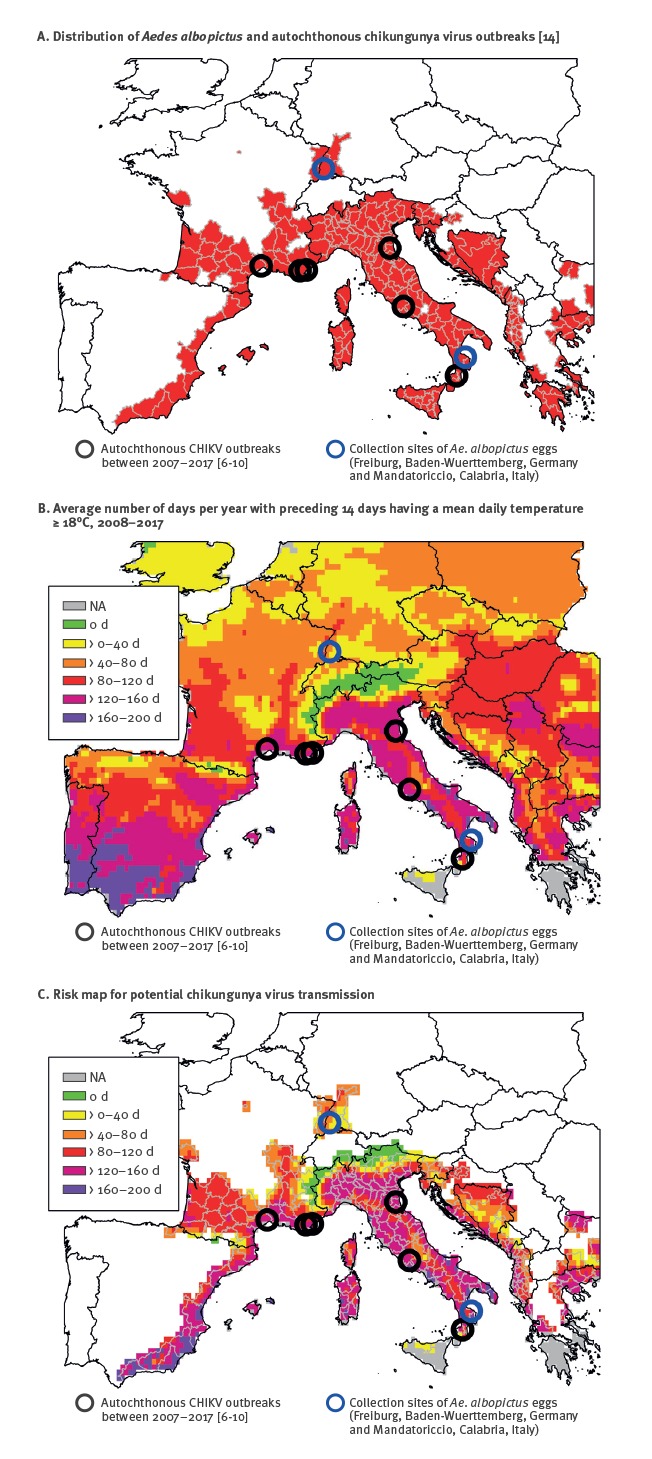
The analysed data indicated that most parts of the vector’s current distribution area allow transmission of CHIKV (Figure). All of these areas have sufficiently long time periods with mean daily temperatures ≥ 18 °C. The only exceptions were regions within the Alps and the Pyrenees; these either have very low vector densities or represent data artefacts, i.e. belonged to a NUTS3 unit infested by Ae. albopictus at lower altitudes. In addition, more parts of central Europe (e.g. Belgium, Poland) have suitable temperature conditions, but are not colonised by Ae. albopictus. The largest time windows with temperatures ≥ 18 °C are found close to the Mediterranean Sea. For example, the coastline of France and large parts of Italy have more than 120 days per year with temperatures that could allow CHIKV transmission. In addition, the temperatures in the Upper Rhine Valley, the northernmost region with established Ae. albopictus populations, theoretically allow extrinsic virus incubation. These areas in Germany and France have periods of more than 40 days per year, on average, that are suitable for CHIKV transmission.
Figure.
(A) Distribution of Aedes albopictus [14], (B) average number of days per year with preceding 14 days having a mean daily temperature ≥ 18 °C, 2008–2017 and (C) risk map for potential chikungunya virus transmission, Europe, 2018
CHIKV: chikungunya virus; d: days; NA: no temperature data available.
Discussion
The extrinsic incubation period (EIP) of CHIKV in Ae. albopictus can be very short, i.e. infectious particles can be present in the saliva within 2 to 3 days after ingestion of an infectious blood meal [25]. This has a direct impact on the epidemiology of CHIKV. It must be considered one of the most important factors allowing for transmission in areas without tropical climates. However, there is a lack of comprehensive knowledge regarding the EIP of CHIKV, especially at low temperatures [26]. Only a few studies have focused on temperate climatic conditions [2]. Such studies are especially important in light of the ongoing spread of Ae. albopictus from source populations around the Mediterranean Sea to central Europe [14-18].
The tested Italian mosquito population, as well as the more recently detected German mosquito population, showed IRs for CHIKV of 100%. TRs of more than 35% were observed for all three tested temperatures. It may appear surprising that the lowest CHIKV TRs (37.5%) for both populations were found at the highest temperature (24 °C) and TRs increased with decreasing temperatures; however, this is in line with previous infection studies with different arboviruses [27]. Some mosquito species have a reduced ability to modulate viral infections under low temperatures. The underlying mechanism might be a temperature-dependent deficiency of antiviral immunity, as RNA silencing is inhibited in mosquitoes subjected to low temperatures. Ae. aegypti specimens reared at cooler temperatures have an impairment of the antiviral immune RNA interference (RNAi) pathway. This pathway is critical to the mosquito’s ability to control viral infections. The exact mechanism between temperature and CHIKV IRs needs to be further explored, even though it is well established that RNAi impairments occur downstream of the initial dicing step [27].
Human CHIKV infections are regularly imported to Europe [28]. The two large outbreaks in Italy also affected visitors (including a German traveller in 2017; personal communication, Christina Frank, Robert Koch-Institute, Germany, November 2017).* Nevertheless, the current risk of CHIKV transmission to humans is predominantly restricted by the presence of Ae. albopictus. Local temperatures allowing extrinsic incubation of the virus only play a minor role. Major parts of the currently infested European areas could allow CHIKV transmission. These areas have periods with 14 consecutive days of mean daily temperatures ≥ 18 °C. This also applies to the Upper Rhine Valley in Germany and France, where recently established populations were documented. Temperature data also illustrate a potential risk for areas in central Europe that are not colonised by Ae. albopictus. This underlines the importance of controlling any further spread of Ae. albopictus in Europe.
Three outbreaks in France and two in Italy between 2007–2017 highlight the risk of autochthonous CHIKV transmission in Europe [6-10]. Notably, these sites were all characterised by time periods of over 120 days allowing CHIKV transmission per year. Results of the study presented here are in line with previous investigations. European populations of Ae. albopictus have vector competence for CHIKV at temperate temperature conditions [5]. In contrast, recent modelling studies identified only a moderate risk for CHIKV transmission in Europe [26,29]. However, as discussed by Tjaden et al. [26], these studies are probably biased by the sparse number of CHIKV records for the region.
The experiments presented in this study used two different European populations of Ae. albopictus at three different temperatures, but only one CHIKV strain. Different CHIKV strains may differ in their potential to be transmitted by Ae. albopictus. The presented infection experiments were conducted with the ECSA strain (CNR_24/2014) that originates from the 2014 CHIKV outbreak in France and carries the E1–A226V mutation, which enhances the vector competence of Ae. albopictus. This might be one explanation for the observation of high IRs and TRs for both Ae. albopictus populations at the various temperatures studied. However, at least two European CHIKV outbreaks in France (2010) and in Italy (2017) were caused by virus strains without this specific mutation [8,10]. Further mutations might have a similar relevance for the probability of transmission in Europe [9,10]. Potential candidates are adaptive mutations in the E2 envelope glycoprotein (e.g. E2-L210Q) [11]. Such mutants were detected in CHIKV isolates from India and might play an important role in the spread and diversification of CHIKV lineages. In addition, one must keep in mind that vector competence is an important parameter of vector capacity, but it is not the only one. Vector capacity is determined by a complex interaction between different factors [30]. Important drivers include the population density and host-feeding patterns. The latter is strongly influenced by both host preference and host availability.
The risk assessment presented here is a conservative scenario. Both tested populations had substantial CHIKV TRs at 18 °C, i.e. 14 days post infection. In addition, temperature data for the risk maps were averaged over a 10-year period. For a more comprehensive and precise evaluation, further vector competence studies investigating lower temperatures and shorter EIPs are required. In addition, this analysis has only focused on Ae. albopictus, though other studies have demonstrated vector competence for CHIKV of further Aedes species [2]. Infection experiments have not identified any other European mosquito species except Ae. albopictus as potential vector for CHIKV, but only a few studies have been done for selected species (e.g. Ae. vexans and Culex pipiens).
In conclusion, Europe offers broad temperature suitability for CHIKV transmission and experiences regular travel-associated virus introduction. Therefore, all European countries with established Ae. albopictus populations should implement entomological surveillance programs as well as monitoring and notification systems for imported human cases to prevent further spread and autochthonous CHIKV transmission in Europe.
Acknowledgements
We thank Branka Žibrat and Annabell Kühl for excellent technical assistance and Esther Schnettler and Anneke Novak-Funk for feedback on an earlier draft of this paper. This publication was supported by the European Virus Archive goes Global (EVAg) project, which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 653316. In addition, we acknowledge the E-OBS dataset from the EU-FP6 project ENSEMBLES (http://ensembles-eu.metoffice.com) and the data providers in the ECA&D project (http://www.ecad.eu). This work was financially supported by the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) through the Federal Environment Agency (UBA), grant number FKZ 3717 48 432 0 and the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE) with the grant number 28-1-91.048-15.
*Authors’ correction
In the Discussion, the sentence “Further spread of the virus was observed during the two large outbreaks in Italy (e.g. to Germany; personal communication, Christina Frank, Robert Koch-Institute, Germany, November 2017)." was replaced by "The two large outbreaks in Italy also affected visitors (including a German traveller in 2017; personal communication, Christina Frank, Robert Koch-Institute, Germany, November 2017)." to avoid possible misinterpretation. The correction was made on 20 July 2018, as requested by the authors.
Conflict of interest: None declared.
Authors’ contributions: Conceived and designed the study: AH, SJ, RL, CK, JSC, ET. Performed the data collection: AH, SJ, MH. Analysed the data: AH, SJ, RL, ET. Provided mosquito specimens: BP, NB. Wrote the paper: AH, SJ, RL, JSC, ET. All authors read and approved the final version of the manuscript.
References
- 1.Lumsden WHR. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. II. General description and epidemiology. Trans R Soc Trop Med Hyg. 1955;49(1):33-57. 10.1016/0035-9203(55)90081-X [DOI] [PubMed] [Google Scholar]
- 2.Coffey LL, Failloux A-B, Weaver SC. Chikungunya virus-vector interactions. Viruses. 2014;6(11):4628-63. 10.3390/v6114628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA, et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84(13):6497-504. 10.1128/JVI.01603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. 10.1371/journal.ppat.0030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vega-Rúa A, Lourenço-de-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yébakima A, et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 2015;9(5):e0003780. 10.1371/journal.pntd.0003780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezza G, Nicoletti L, Angelini R., Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840-46. 10.1016/S0140-6736(07)61779-6 PMID: 18061059 [DOI] [PubMed]
- 7.Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L’Ambert G, Cochet A, et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill. 2015;20(17):21108. 10.2807/1560-7917.ES2015.20.17.21108 [DOI] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control (ECDC). Cluster of autochtonous chikungunya cases in France – First update - 9 October 2017. Stockholm: ECDC; 2017. Available from: https://ecdc.europa.eu/sites/portal/files/documents/RRA-chikungunya-Italy-update-9-Oct-2017.pdf
- 9.Grandadam M, Caro V, Plumet S, Thiberge JM, Souarès Y, Failloux A-B, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17(5):910-3. 10.3201/eid1705.101873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venturi G, Di Luca M, Fortuna C, Remoli ME, Riccardo F, Severini F, et al. Detection of a chikungunya outbreak in Central Italy, August to September 2017. Euro Surveill. 2017;22(39):17-00646. 10.2807/1560-7917.ES.2017.22.39.17-00646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das B, Sahu A, Das M, Patra A, Dwibedi B, Kar SK, et al. Molecular investigations of chikungunya virus during outbreaks in Orissa, Eastern India in 2010. Infect Genet Evol. 2012;12(5):1094-101. 10.1016/j.meegid.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 12.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12(6):435-47. 10.1089/vbz.2011.0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatem AJ, Hay SI, Rogers DJ. Global traffic and disease vector dispersal. Proc Natl Acad Sci USA. 2006;103(16):6242-7. 10.1073/pnas.0508391103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control (ECDC), European Food Safety Authority (EFSA). VectorNet: A European network for sharing data on the geographic distribution of arthropod vectors, transmitting human and animal disease agents. Stockholm: ECDC. [Accessed 24 Apr 2018]. Available from: http://ecdc.europa.eu/en/healthtopics/vectors/VectorNet/Pages/VectorNet.aspx
- 15.Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, et al. Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol Res. 2013;112(4):1787-90. 10.1007/s00436-012-3230-1 [DOI] [PubMed] [Google Scholar]
- 16.Pluskota B, Jöst A, Augsten X, Stelzner L, Ferstl I, Becker N. Successful overwintering of Aedes albopictus in Germany. Parasitol Res. 2016;115(8):3245-7. 10.1007/s00436-016-5078-2 [DOI] [PubMed] [Google Scholar]
- 17.Walther D, Scheuch DE, Kampen H. The invasive Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in Germany: Local reproduction and overwintering. Acta Trop. 2017;166:186-92. 10.1016/j.actatropica.2016.11.024 [DOI] [PubMed] [Google Scholar]
- 18.Becker N, Schön S, Klein AM, Ferstl I, Kizgin A, Tannich E, et al. First mass development of Aedes albopictus (Diptera: Culicidae)-its surveillance and control in Germany. Parasitol Res. 2017;116(3):847-58. 10.1007/s00436-016-5356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28(1):229-62. 10.1146/annurev.en.28.010183.001305 [DOI] [PubMed] [Google Scholar]
- 20.Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge J-M, Lourenco-De-Oliveira R, et al. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc Biol Sci. 2014;281(1792):20141078. http://dx.doi.org/ 10.1098/rspb.2014.1078 PMID:25122228 [DOI] [PMC free article] [PubMed]
- 21.Heitmann A, Jansen S, Lühken R, Leggewie M, Badusche M, Pluskota B, et al. Experimental transmission of Zika virus by mosquitoes from central Europe. Euro Surveill. 2017;22(2):30437. 10.2807/1560-7917.ES.2017.22.2.30437 PMID: 28106528 [DOI] [PMC free article] [PubMed]
- 22.Fortuna C, Remoli ME, Di Luca M, Severini F, Toma L, Benedetti E, et al. Experimental studies on comparison of the vector competence of four Italian Culex pipiens populations for West Nile virus. Parasit Vectors. 2015;8(1):463. 10.1186/s13071-015-1067-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haylock MR, Hofstra N, Klein Tank AMG, Klok EJ, Jones PD, New M. A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J Geophys Res Atmos. 2008;113(D20):D20119 10.1029/2008JD010201 [DOI] [Google Scholar]
- 24.R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. Available from: https://www.R-project.org/
- 25.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux A-B. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One. 2009;4(6):e5895. 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjaden NB, Suk JE, Fischer D, Thomas SM, Beierkuhnlein C, Semenza JC. Modelling the effects of global climate change on Chikungunya transmission in the 21st century. Sci Rep. 2017;7(1):3813. 10.1038/s41598-017-03566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adelman ZN, Anderson MAE, Wiley MR, Murreddu MG, Samuel GH, Morazzani EM, et al. Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl Trop Dis. 2013;7(5):e2239. 10.1371/journal.pntd.0002239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paty MC, Six C, Charlet F, Heuze G, Cochet A, Wiegandt A, et al. Large number of imported chikungunya cases in mainland France, 2014: a challenge for surveillance and response. Euro Surveill. 2014;19(28):20856. 10.2807/1560-7917.ES2014.19.28.20856PMID:25060572 [DOI] [PubMed]
- 29.Nsoesie EO, Kraemer MU, Golding N, Pigott DM, Brady OJ, Moyes CL, et al. Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Euro Surveill. 2016;21(20):30234. 10.2807/1560-7917.ES.2016.21.20.30234 PMID:27239817 [DOI] [PMC free article] [PubMed]
- 30.Kramer LD, Ciota AT. Dissecting vectorial capacity for mosquito-borne viruses. Curr Opin Virol. 2015;15:112-8. 10.1016/j.coviro.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


