Abstract
Citrus (Citrus reticulate Blanco) is one of the most commonly consumed and widely distributed fruit in the world, which is possessing extensive bioactivities. Present study aimed to fully understand the flavonoids compositions, antioxidant capacities and in vitro anticancer abilities of different citrus resources. Citrus fruits of 35 varieties belonging to 5 types (pummelos, oranges, tangerines, mandarins and hybrids) were collected. Combining li quid chromatography combined with electrospray ionization mass spectrometry (LC-ESI-MS/MS) and ultra-performance liquid chromatography combined with diode array detector (UPLC-DAD), a total of 39 flavonoid compounds were identified, including 4 flavones, 9 flavanones and 26 polymethoxylated flavonoids (PMFs). Each citrus fruit was examined and compared by 4 parts, flavedo, albedo, segment membrane and juice sacs. The juice sacs had the lowest total phenolics, following by the segment membrane. Four antioxidant traits including 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC) and cupric reducing antioxidant capacity (CUPRAC) were applied for the antioxidant capacities evaluation. Three gastric cancer cell lines, SGC-7901, BGC-823 and AGS were applied for the cytotoxicity evaluation. According to the results of correlation analysis, phenolics compounds might be the main contributor to the antioxidant activity of citrus extracts, while PMFs existing only in the flavedo might be closely related to the gastric cancer cell line cytotoxicity of citrus extracts. The results of present study might provide a theoretical guidance for the utilization of citrus resources.
Keywords: Citrus reticulata, phenolics contents, flavonoids composition, antioxidant capacities, anticancer abilities
1. Introduction
Citrus (Citrus reticulate Blanco) is a tropical or subtropical fruit widely distributed around the world. As one of the most consumed fruits it also has great economic importance. Besides its value as a delicious fruit, its nutritional values are also important. Previous studies have reported a variety of bioactivities of citrus fruit, like antioxidant [1], anticancer [2,3], anti-inflammation [4], anti-fat [5] and anti-diabetes properties [6,7]. Many of the bioactivities are attributed to the phenolics and flavonoids that are abundant in citrus fruit [5,8,9].
Citrus can be classified in several types, including mandarins, tangerines, oranges, pummelos, hybrids, lemons, limes, etc. [10]. Zhejiang Province, an important citrus production region in China, is rich in citrus germplasm resources. Several distinctive local characteristic citrus varieties had been found in this region, such as Ougan (OG), Yuhuanyou (YHY), Mantouhong (MTH), Huyou (HY) and Ponkan (PG). Thus, the citrus varieties in this region might provide some very distinctive resources for the bioactivity study of citrus fruits.
The present study aimed to carry out a comprehensive investigation on the flavonoid composition and distribution, antioxidant capacity and gastric tumor cytotoxicity of citrus fruits from Zhejiang Province. A total of 35 varieties belonging to five types of citrus fruits were collected for the study. Ultra-performance liquid chromatography (UPLC) and lipid chromatography combined with electrospray ionization mass spectrometry (LC-ESI-MS/MS) were used for identification and quantification of flavonoid compounds. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC) and cupric reducing antioxidant capacity (CUPRAC), four commonly used antioxidant tests, were applied for the antioxidant capacity evaluation. Gastric tumor cell lines were applied for the cytotoxicity evaluation of citrus flavonoid-rich extracts.
2. Results
2.1. Fruit Basic Quality Index of Different Citrus Varieties
As shown in Table 1, five types (pummelos, oranges, tangerines, mandarins and hybrids) of 35 varieties of citrus fruits were collected and their fruit basic qualities were tested. Significant differences were found in fruit color, weight, edible rate and total soluble solids (TSS) among the 35 tested citrus varieties. MTH showed the highest Citrus Color Index (CCI) value of 16.75, while Qingougan (QOG) showed the lowest CCI at −6.97. Pummelos showed higher weight than the other types of citrus fruits, among which YHY and Mabuwendan (MBWD) had the highest weights at 1754.10 g and 1676.14 g, respectively. Kouzhijin22 (KZJ22) had the lowest weight of 67.97 g. Hongmeiren (HMR) had the highest edible rate of 85.31%, while Zaoxiangyou (ZXY) only had an edible rate of 56.12%. TSS varied from 8.42 to 16.08 °Brix. KZJ22 had the highest TSS value of 16.08 °Brix, followed by Aiyuan31 (AY31), Rinan1 (RN1), Gongchuan (GOC), Mixiagan (MXG), Aiyuan27 (AY27) and Yuanxiaochun (YXC), while QOG and Wuheougan (WHOG) showed the lowest TSS. Correlation analysis showed that CCI and TSS had significant relationships with each other (r = 0.690, p < 0.01), indicating that citrus varieties with better color look may have better taste.
Table 1.
Appearance and taste qualities of Citrus fruits of 35 cultivars. TSS = total soluble solids.
| Cultivar (Abbreviation) | Fruit Type | Color (CCI) | Weight (g) * | Edible Rate (%) | TSS (°Brix) * |
|---|---|---|---|---|---|
| Aiyuan27 (AY27) | Hybrids | 5.09 ± 0.09 | 397.97 ± 27.47 ef | 84.18 | 12.85 ± 0.05 abcdef |
| Aiyuan30 (AY30) | Hybrids | 14.10 ± 0.26 | 114.11 ± 11.15 lmno | 80.06 | 12.51 ± 0.29 bcdefg |
| Aiyuan31 (AY31) | Hybrids | 13.05 ± 0.32 | 385.06 ± 21.19 efg | 84.54 | 14.79 ± 0.04 ab |
| Buzhihuo (BZH) | Hybrids | 4.69 ± 0.38 | 216.62 ± 12.05 ij | 75.95 | 10.91 ± 0.50 cdefghij |
| CaRaCaRa (CR) | Oranges | 5.63 ± 1.85 | 159.36 ± 2.56 ijklm | 75.12 | 11.87 ± 0.60 bcdefghi |
| Chunxiang (CX) | Hybrids | −4.95 ± 0.44 | 200.58 ± 9.23 ijk | 67.34 | 9.17 ± 0.28 ghij |
| Dafen (DF) | Mandarins | 3.29 ± 0.33 | 120.31 ± 12.15 klmno | 79.82 | 10.49 ± 0.28 cdefghij |
| Gaocheng (GAC) | Hybrids | 7.35 ± 0.09 | 343.87 ± 20.94 gh | 74.51 | 12.44 ± 0.25 bcdefgh |
| Gongneiyiyugan (GN) | Hybrids | 2.76 ± 0.66 | 241.55 ± 21.74 ij | 74.14 | 10.21 ± 0.56 cdefghij |
| Gongchuan (GOC) | Mandarins | 8.27 ± 0.33 | 141.94 ± 5.75 jklmno | 82.51 | 13.23 ± 0.45 abcd |
| Hongmeiren (HMR) | Hybrids | 7.59 ± 0.49 | 137.70 ± 4.03 jklmno | 85.31 | 11.10 ± 0.08 cdefghij |
| Huyou (HY) | Pummelos | 1.43 ± 0.80 | 571.61 ± 46.24 d | 67.97 | 9.66 ± 0.08 efghij |
| Kouzhijin22 (KZJ22) | Hybrids | 14.68 ± 0.22 | 67.97 ± 3.80 o | 67.59 | 16.08 ± 0.71 a |
| Mabuwendan (MBWD) | Pummelos | 1.22 ± 0.05 | 1676.14 ± 81.74 a | 59.74 | 8.93 ± 0.08 cdefghij |
| Mantouhong (MTH) | Tangerines | 16.75 ± 0.63 | 84.27 ± 3.26 no | 79.89 | 12.53 ± 0.38 bcdefg |
| Mixiagan (MXG) | Hybrids | 1.97 ± 0.08 | 303.59 ± 18.62 gh | 72.13 | 12.96 ± 0.25 abcde |
| Nangan20 (NG20) | Mandarins | 9.22 ± 0.12 | 100.61 ± 3.60 mno | 74.07 | 11.76 ± 0.15 bcdeghi |
| Newhall (NH) | Oranges | 8.72 ± 0.38 | 168.28 ± 5.15 ijklm | 71.66 | 11.96 ± 0.70 bcdefghi |
| Ougan (OG) | Mandarins | −0.56 ± 1.09 | 175.42 ± 3.55 ijklm | 64.12 | 9.46 ± 0.22 fghij |
| Ponkan (PG) | Tangerines | 4.89 ± 0.94 | 140.61 ± 2.34 jklmno | 74.68 | 9.08 ± 0.12 hij |
| Putaoyou (PTY) | Pummelos | 0.24 ± 1.02 | 442.38 ± 21.67 e | 77.49 | 9.56 ± 0.23 fghij |
| Qingji (QJ) | Hybrids | 6.92 ± 0.20 | 170.73 ± 8.25 ijklm | 73.18 | 10.55 ± 0.41 cdefghij |
| Qingougan (QOG) | Mandarins | −6.97 ± 0.44 | 146.74 ± 3.78 ijklmno | 69.33 | 8.42 ± 0.05 j |
| Rinan1 (RN1) | Mandarins | 8.99 ± 0.08 | 118.75 ± 5.82 klmno | 76.04 | 13.48 ± 0.14 abc |
| Shangyexinxi (SYXX) | Mandarins | 9.09 ± 0.20 | 228.56 ± 5.10 hi | 80.21 | 10.34 ± 0.36 cdefghij |
| Shiwen (SW) | Mandarins | 3.12 ± 0.30 | 74.16 ± 1.31 o | 80.46 | 11.54 ± 0.09 bcdefghi |
| Sijiyou (SJY) | Pummelos | 1.70 ± 0.19 | 1486.37 ± 21.38 b | 65.72 | 9.68 ± 0.30 efghij |
| Tiancao (TC) | Hybrids | 7.32 ± 0.90 | 138.75 ± 2.68 jklmno | 85.24 | 9.89 ± 0.42 defghij |
| Tianchun (TCH) | Hybrids | 2.86 ± 0.21 | 188.85 ± 6.50 ijkl | 69.11 | 11.22 ± 0.18 cdefghij |
| Weizhang (WZ) | Mandarins | 8.39 ± 0.54 | 128.33 ± 4.86 klmno | 73.29 | 10.05 ± 0.22 defghij |
| Wuheougan (WHOG) | Mandarins | −1.09 ± 0.98 | 113.50 ±4.12 lmno | 64.25 | 8.74 ± 0.18 ij |
| Youliang (YL) | Mandarins | 10.45 ± 0.17 | 138.13 ± 12.12 jklmno | 75.32 | 12.22 ± 0.50 bcdefgh |
| Yuanxiaochun (YXC) | Hybrids | 1.34 ± 0.07 | 135.94 ± 6.26 jklmno | 79.54 | 12.76 ± 0.42 abcdef |
| Yuhuanyou (YHY) | Pummelos | 0.68 ± 0.08 | 1754.10 ± 76.73 a | 73.10 | 10.92 ± 0.20 cdefghij |
| Zaoxiangyou (ZXY) | Pummelos | 1.80 ± 0.18 | 1585.77 ± 76.21 b | 56.12 | 11.38 ± 0.09 cdefghij |
* Results were the mean ± SD (n = 15) on a fresh weight (FW) (g) and TSS (°Brix) of citrus fruit. Values within each column followed by different superscript letters were significantly different at p < 0.05 according to Tukey’s tests.
2.2. Total Phenolics and Antioxidant Capacities
Each citrus fruit was divided into four parts from outside to inside: flavedo, albedo, segment membrane and juice sacs. Ultrasonic assistant extraction was used to improve the extraction efficiency according to previous studies [11,12]. As shown in Table 2, Table 3, Table 4 and Table 5, the total phenolics contents varied between the varieties and tissues of citrus fruits.
Table 2.
Total phenolics and antioxidant properties of citrus fruit flavedo of 35 cultivars.
| Cultivars | Total Phenolics | DPPH | FRAP | ORAC | CUPRAC | APC Index * | Rank # |
|---|---|---|---|---|---|---|---|
| AY27 | 15.05 ± 0.56 no | 11.36 ± 0.71 jklm | 9.63 ± 0.44 hijk | 153.73 ± 8.78 kl | 29.35 ± 1.09 pqr | 46.57 | 26 |
| AY30 | 27.55 ± 0.29 a | 20.73 ± 0.12 a | 21.33 ± 1.31 a | 319.01 ± 44.63 bc | 54.80 ± 2.34 a | 92.01 | 1 |
| AY31 | 13.73 ± 0.36 op | 9.99 ± 0.93 mno | 7.12 ± 0.24 lmnop | 188.39 ± 15.19 hijkl | 32.85 ± 3.32 nop | 45.42 | 28 |
| BZH | 19.68 ± 0.58 defg | 16.91 ± 0.24 bc | 12.36 ± 1.65 efg | 221.30 ± 13.70 defghijkl | 42.76 ± 0.59 defg | 66.18 | 7 |
| CR | 18.90 ± 0.45 ghi | 15.34 ± 0.80 bcdef | 11.94 ± 0.48 efgh | 224.91 ± 26.69 defghijk | 42.69 ± 0.59 defg | 63.96 | 10 |
| CX | 11.67 ± 0.40 q | 5.48 ± 0.56 r | 1.94 ± 0.16 s | 162.38 ± 18.82 jkl | 27.62 ± 0.34 qrs | 30.14 | 35 |
| DF | 18.07 ± 0.41 ghijk | 10.29 ± 0.83 lmn | 7.93 ± 0.30 klmno | 206.96 ± 18.53 efghijkl | 37.07 ± 0.65 hijklmn | 49.65 | 24 |
| GAC | 18.09 ± 0.38 ghijk | 17.48 ± 1.04 b | 14.80 ± 0.59 cd | 185.27 ± 27.39 hijkl | 35.14 ± 0.37 klmno | 64.33 | 8 |
| GN | 16.44 ± 0.57 klmn | 10.72 ± 0.23 lmn | 4.85 ± 1.16 pqr | 208.48 ± 30.42 efghijkl | 36.08 ± 2.81 jklmn | 46.19 | 27 |
| GOC | 19.58 ± 0.50 efg | 13.46 ± 0.51 efghij | 11.12 ± 0.30 efghi | 163.88 ± 20.46 jkl | 42.45 ± 1.68 defg | 57.37 | 15 |
| HMR | 24.20 ± 0.69 b | 20.40 ± 0.24 a | 17.23 ± 0.29 b | 270.31 ± 13.88 cdefg | 51.74 ± 0.27 ab | 82.81 | 3 |
| HY | 15.20 ± 0.60 mno | 14.47 ± 0.60 defgh | 8.67 ± 0.28 jklm | 196.63 ± 16.56 ghijkl | 33.55 ± 0.14 nop | 53.40 | 21 |
| KZJ22 | 21.11 ± 1.05 cde | 14.92 ± 0.91 cdefg | 12.51 ± 1.15 def | 357.54 ± 0.53 b | 43.78 ± 1.07 def | 71.69 | 4 |
| MBWD | 13.13 ± 0.18 pq | 9.17 ± 0.94 nopq | 4.92 ± 0.45 pqr | 178.20 ± 22.21 ijkl | 25.62 ± 0.50 rs | 38.01 | 32 |
| MTH | 21.65 ± 0.65 c | 14.77 ± 0.68 cdefg | 12.82 ± 0.48 de | 231.29 ± 39.15 defghij | 41.82 ± 1.70 efgh | 64.25 | 9 |
| MXG | 14.86 ± 0.40 no | 13.04 ± 0.25 ghijk | 9.98 ± 0.96 ghijk | 75.78 ± 35.22 m | 24.50 ± 0.86 s | 42.64 | 30 |
| NG20 | 19.30 ± 0.11 fgh | 13.39 ± 0.64 efghij | 9.43 ± 0.55 ijkl | 288.99 ± 3.61 bcd | 37.61 ± 0.26 hijklmn | 59.76 | 13 |
| NH | 20.60 ± 0.35 cdef | 16.50 ± 0.27 bcd | 12.82 ± 0.73 de | 213.25 ± 26.59 efghijkl | 45.70 ± 1.07 cde | 67.14 | 5 |
| OG | 19.04 ± 0.60 fghi | 11.44 ± 0.83 jklm | 9.59 ± 0.49 hijk | 249.79 ± 21.33 cdefghi | 41.04 ± 0.60 efghi | 57.08 | 16 |
| PG | 18.36 ± 0.22 ghij | 14.39 ± 0.37 defgh | 11.31 ± 1.08 efghi | 250.70 ± 42.77 cdefghi | 39.87 ± 1.39 fghijk | 62.16 | 11 |
| PTY | 17.20 ± 0.76 jkl | 15.54 ± 0.58 bcde | 7.71 ± 0.45 klmno | 201.56 ± 18.04 fghijkl | 34.19 ± 0.78 mno | 54.12 | 19 |
| QJ | 21.45 ± 0.54 c | 16.36 ± 0.06 bcd | 10.36 ± 0.73 fghij | 256.04 ± 37.59 cdefgh | 46.81 ± 2.56 cd | 66.88 | 6 |
| QOG | 18.29 ± 0.36 ghij | 10.62 ± 0.34 lmn | 8.42 ± 0.59 jklmn | 277.70 ± 15.99 cde | 40.36 ± 0.96 fghij | 55.89 | 17 |
| RN1 | 16.33 ± 0.48 lmn | 12.41 ± 0.95 hijkl | 8.52 ± 0.71 jklm | 214.83 ± 20.09 defghijkl | 30.71 ± 1.99 opq | 50.41 | 23 |
| SJY | 12.18 ± 0.32 pq | 7.80 ± 0.55 pq | 3.87 ± 0.07 rs | 149.67 ± 4.81 lm | 24.34 ± 0.32 s | 33.03 | 34 |
| SW | 21.32 ± 0.60 cd | 13.24 ± 0.92 fghij | 10.39 ± 0.76 fghij | 242.22 ± 28.50 defghi | 40.02 ± 1.70 fghij | 59.31 | 14 |
| SYXX | 16.85 ± 0.52 jklm | 11.74 ± 0.81 ijklm | 8.35 ± 0.83 jklmn | 209.35 ± 24.08 efghijkl | 36.65 ± 1.81 ijklmn | 51.83 | 22 |
| TC | 24.80 ± 0.55 b | 19.94 ± 0.30 a | 16.88 ± 1.60 bc | 468.96 ± 34.84 a | 49.16 ± 2.51 bc | 91.26 | 2 |
| TCH | 16.82 ± 0.31 jklm | 10.04 ± 0.50 1mno | 5.57 ± 0.24 opqr | 227.70 ± 11.03 defghijk | 38.84 ± 0.88 ghijklm | 48.49 | 25 |
| WHOG | 17.64 ± 0.37 hijkl | 11.02 ± 0.23 klmn | 7.72 ± 0.62 klmno | 256.28 ± 28.81 cdefgh | 39.12 ± 2.01 fghijkl | 53.85 | 20 |
| WZ | 17.66 ± 0.14 hijkl | 11.77 ± 0.53 ijklm | 9.81 ± 0.53 hijk | 210.70 ± 16.96 efghijkl | 40.07 ± 1.88 fghij | 55.20 | 18 |
| YHY | 13.57 ± 0.17 op | 9.76 ± 0.63 mnop | 6.05 ± 0.53 nopqr | 158.18 ± 8.30 jkl | 26.28 ± 1.21 qrs | 39.28 | 31 |
| YL | 17.57 ± 0.99 ijkl | 13.75 ± 1.56 efghij | 9.22 ± 0.89 ijkl | 275.85 ± 27.24 cdef | 41.02 ± 0.88 efghi | 60.81 | 12 |
| YXC | 15.49 ± 0.73 mn | 7.89 ± 0.80 opq | 6.64 ± 0.04 mnopq | 188.26 ± 8.62 hijkl | 34.69 ± 0.80 lmno | 43.16 | 29 |
| ZXY | 11.52 ± 0.03 q | 7.40 ± 0.13 qr | 4.70 ± 0.08 qr | 158.83 ± 17.20 jkl | 24.44 ± 2.01 s | 34.05 | 33 |
Results were the mean ± SD (n = 3) on a dried weight (g) of citrus basis. Total phenolics were calculated as mg gallic acid equivalents (GAE)/g DW. Antioxidant capacities (DPPH, FRAP, ORAC and CUPRAC) were calculated as mg trolox equivalent antioxidant capacities (TEAC)/g DW. Values within each column followed by different superscript letters were significantly different (p < 0.05) according to Tukey’s tests. * Antioxidant index score = [(sample score/best score) × 100], averaged for all four tests for each cultivar for the antioxidant potency composite (APC) index. # Ranked according to the APC index.
Table 3.
Total phenolics and antioxidant properties of citrus fruit albedo of 35 cultivars.
| Cultivars | Total Phenolics | DPPH | FRAP | ORAC | CUPRAC | APC Index * | Rank # |
|---|---|---|---|---|---|---|---|
| AY27 | 15.19 ± 0.73 fghijklm | 8.22 ± 0.38 defghi | 15.01 ± 0.08 d | 55.49 ± 7.65 m | 18.30 ± 0.83 mnop | 38.40 | 22 |
| AY30 | 19.84 ± 1.30 bcd | 16.53 ± 0.49 a | 18.45 ± 0.88 c | 288.00 ± 39.86 efg | 36.77 ± 0.89 cd | 72.09 | 2 |
| AY31 | 11.68 ± 1.28 lmn | 4.73 ± 0.35 kl | 8.24 ± 0.68 ijkl | 73.31 ± 2.11 lm | 16.26 ± 0.55 opq | 26.31 | 33 |
| BZH | 14.45 ± 2.04 ghijklmn | 10.62 ± 1.57 cd | 11.64 ± 0.92 efg | 220.49 ± 24.87 ghijk | 31.25 ± 1.49 efg | 51.52 | 11 |
| CR | 13.46 ± 0.15 ijklmn | 5.88 ± 0.74 ijkl | 7.28 ± 0.27 jklmno | 232.58 ± 11.48 ghij | 17.42 ± 0.21 nop | 33.49 | 26 |
| CX | 10.35 ± 0.48 n | 4.37 ± 0.69 l | 5.68 ± 0.24 lmno | 177.82 ± 13.67 hijkl | 22.21 ± 1.10 jklm | 30.33 | 32 |
| DF | 13.13 ± 0.44 jklmn | 5.00 ± 0.36 jkl | 7.12 ± 0.03 jklmno | 417.66 ± 22.58 cd | 22.77 ± 0.44 jkl | 41.79 | 20 |
| GAC | 27.15 ± 3.53 a | 17.43 ± 0.93 a | 25.26 ± 2.77 a | 420.79 ± 53.79 cd | 39.34 ± 3.01 bc | 86.39 | 1 |
| GN | 15.74 ± 1.16 defghijkl | 7.00 ± 0.81 fghijk | 7.24 ± 0.34 jklmno | 429.84 ± 62.99 cd | 26.20 ± 1.09 hij | 47.04 | 15 |
| GOC | 16.12 ± 1.71 defghijk | 6.97 ± 0.57 fghijk | 8.06 ± 0.88 jkl | 382.81 ± 24.59 de | 23.24 ± 0.15 jk | 44.48 | 16 |
| HMR | 17.78 ± 1.65 cdefgh | 11.65 ± 0.26 bc | 14.50 ± 0.44 d | 358.36 ± 33.63 def | 33.35 ± 0.31 def | 62.03 | 7 |
| HY | 17.59 ± 0.15 cdefg | 8.60 ± 1.49 defg | 6.80 ± 0.51 jklmno | 424.62 ± 24.53 cd | 25.56 ± 0.60 ij | 48.33 | 14 |
| KZJ22 | 16.39 ± 0.63 defghij | 6.90 ± 0.67 fghijk | 11.84 ± 0.46 ef | 245.27 ± 4.35 g | 23.53 ± 0.75 opq | 43.21 | 17 |
| MBWD | 13.82 ± 0.27 hijklmn | 7.37 ± 0.67 fghij | 8.02 ± 0.19 jkl | 206.45 ± 15.84 ghijk | 14.13 ± 2.06 pqr | 33.60 | 25 |
| MTH | 18.96 ± 0.50 cdef | 13.92 ± 0.55 b | 17.77 ± 1.23 c | 215.75 ± 28.81 ghijk | 34.77 ± 1.47 de | 63.98 | 6 |
| MXG | 14.97 ± 2.42 fghijklm | 5.13 ± 0.06 jkl | 10.71 ± 0.64 fg | 177.63 ± 2.90 hijkl | 12.12 ± 0.39 qr | 30.94 | 31 |
| NG20 | 15.35 ± 0.27 fghijklm | 4.86 ± 0.43 jkl | 9.22 ± 0.17 ghij | 176.15 ± 30.27 hijkl | 20.09 ± 0.61 klmno | 33.32 | 27 |
| NH | 13.73 ± 0.57 hijklmn | 6.55 ± 0.33 ghijkl | 7.67 ± 0.19 jklm | 116.37 ± 1.22 klm | 22.21 ± 1.39 jklm | 33.12 | 29 |
| OG | 20.98 ± 0.60 bc | 8.96 ± 1.10 defg | 13.28 ± 1.25 de | 549.63 ± 73.64 b | 46.43 ± 1.98 a | 71.10 | 4 |
| PG | 15.49 ± 0.57 efghijklm | 17.65 ± 0.26 a | 21.56 ± 1.56 b | 179.13 ± 2.73 hijkl | 34.96 ± 2.78 de | 71.77 | 3 |
| PTY | 24.00 ± 1.50 ab | 12.28 ± 0.33 bc | 11.57 ± 0.34 efgh | 508.19 ± 85.71 bc | 33.86 ± 1.85 def | 65.82 | 5 |
| QJ | 15.49 ± 0.72 efghijklm | 10.23 ± 0.82 cde | 11.51 ± 0.36 efgh | 198.88 ± 12.12 ghijk | 28.14 ± 0.85 g | 48.37 | 13 |
| QOG | 19.69 ± 1.54 cde | 8.49 ± 0.86 defgh | 10.65 ± 0.56 fg | 404.23 ± 4.79 cd | 42.67 ± 1.95 ab | 60.45 | 8 |
| RN1 | 14.18 ± 1.50 higklmn | 8.04 ± 0.78 efghi | 11.89 ± 0.52 ef | 126.91 ± 13.19 jklm | 20.40 ± 0.25 klmno | 38.82 | 21 |
| SJY | 14.37 ± 1.23 higklmn | 7.31 ± 0.74 fghij | 7.53 ± 0.33 jklmn | 224.79 ± 19.36 ghij | 12.41 ± 1.49 qr | 32.78 | 30 |
| SW | 15.54 ± 0.81 efghijklm | 7.35 ± 0.99 fghij | 9.12 ±0.83 ghij | 412.33 ± 29.49 cd | 26.11 ± 0.83 hij | 48.70 | 12 |
| SYXX | 13.41 ± 1.48 ijklmn | 5.22 ± 0.73 jkl | 6.51 ± 0.29 klmno | 360.47 ± 49.12 de | 18.66 ± 1.6l mno | 37.18 | 23 |
| TC | 16.12 ± 1.82 defghijk | 8.56 ± 0.66 defg | 9.04 ± 0.48 hijk | 677.90 ± 51.50 a | 22.61 ± 0.27 jkl | 58.25 | 9 |
| TCH | 11.37 ± 1.58 mn | 4.75 ± 0.44 kl | 5.42 ± 0.60 mno | 253.81 ± 16.73 fg | 22.83 ± 1.49 jkl | 33.75 | 24 |
| WHOG | 18.67 ± 1.05 cdefg | 8.59 ± 0.44 defg | 10.66 ± 0.42 fg | 282.05 ± 78.31 efgh | 41.25 ± 1.21 b | 55.33 | 10 |
| WZ | 11.68 ± 1.24 lmn | 4.72 ± 0.18 kl | 6.33 ± 0.56 lmno | 248.27 ± 30.14 g | 20.81 ± 0.62 klmn | 33.31 | 28 |
| YHY | 16.85 ± 0.57 cdefghij | 4.56 ± 2.19 kl | 4.82 ± 0.37 o | 198.22 ± 10.99 ghijk | 11.92 ± 1.59 r | 24.96 | 35 |
| YL | 13.91 ± 0.64 higklmn | 5.92 ± 0.17 ijkl | 7.31 ± 0.84 jklmno | 436.49 ± 14.06 cd | 21.00 ± 0.18 klmn | 43.02 | 19 |
| YXC | 12.06 ± 1.41 klmn | 9.11 ± 0.70 def | 11.30 ± 1.01 efgh | 76.45 ± 8.52 lm | 30.19 ± 1.02 fgh | 43.16 | 18 |
| ZXY | 14.00 ± 0.39 higklmn | 6.09 ± 0.74 hijkl | 5.07 ± 0.70 no | 168.30 ± 11.89 ijkl | 11.34 ± 1.08 r | 25.96 | 34 |
Results were the mean ± SD (n = 3) on a dried weight (g) of citrus basis. Total phenolics were calculated as mg gallic acid equivalents (GAE)/g DW. Antioxidant capacities (DPPH, FRAP, ORAC and CUPRAC) were calculated as mg trolox equivalent antioxidant capacities (TEAC)/g DW. Values within each column followed by different superscript letters were significantly different (p < 0.05) according to Tukey’s tests. * Antioxidant index score = [(sample score/best score) × 100], averaged for all four tests for each cultivar for the antioxidant potency composite (APC) index. # Ranked according to the APC index.
Table 4.
Total phenolics and antioxidant properties of citrus fruit segment membrane of 35 cultivars.
| Cultivars | Total Phenolics | DPPH | FRAP | ORAC | CUPRAC | APC Index * | Rank # |
|---|---|---|---|---|---|---|---|
| AY27 | 8.84 ± 0.10 cdefgh | 5.12 ± 0.53 defghijk | 15.92 ± 0.77 cd | 49.03 ± 7.40 pq | 5.03 ± 0.20 def | 63.12 | 18 |
| AY30 | 8.59 ± 0.21 efgh | 6.84 ± 0.98 ab | 19.13 ± 0.70 ab | 102.13 ± 6.06 jklmn | 6.13 ± 0.09 ab | 83.70 | 4 |
| AY31 | 7.84 ± 0.38 hi | 3.15 ± 0.29 opq | 10.60 ± 0.08 klm | 69.27 ± 5.44 nopq | 3.16 ± 0.23 lmn | 44.79 | 33 |
| BZH | 8.90 ± 0.16 cdefgh | 5.61 ± 0.15 cdef | 15.41 ± 0.28 cde | 129.18 ± 10.93 fghijk | 4.77 ± 0.26 defghi | 72.72 | 8 |
| CR | 8.18 ± 0.67 gh | 3.62 ± 0.38 lmnopq | 9.40 ± 0.91 mno | 98.24 ± 3.84 klmno | 4.05 ± 0.33 ghijkl | 51.83 | 30 |
| CX | 6.27 ± 0.29 j | 3.55 ± 0.03 mnopq | 8.30 ± 0.27 o | 77.50 ± 5.45 lmnop | 2.57 ± 0.17 n | 41.92 | 34 |
| DF | 8.71 ± 0.18 defgh | 5.25 ± 0.50 defghi | 11.69 ± 0.07 ijkl | 132.53 ± 8.86 efghijk | 3.91 ± 0.30 ijklm | 63.83 | 17 |
| GAC | 12.56 ± 0.70 a | 5.73 ± 0.12 bcde | 19.83 ± 0.90 a | 183.38 ± 5.16 ab | 6.24 ± 0.34 ab | 90.96 | 1 |
| GN | 9.70 ± 0.32 bcdef | 3.67 ± 0.17 lmnopq | 9.96 ± 0.16 lmno | 164.49 ± 10.28 bcdef | 3.30 ± 0.23 klmn | 57.70 | 25 |
| GOC | 9.44 ± 0.43 bcdefg | 5.27 ± 0.17 defgh | 12.83 ± 0.49 ghij | 139.82 ± 14.40 defghi | 3.89 ± 0.25 ijklm | 66.13 | 12 |
| HMR | 10.63 ± 0.33 b | 7.34 ± 0.39 a | 17.32 ± 0.13 ab | 133.80 ± 7.90 efghijk | 5.45 ± 0.21 bcd | 84.24 | 3 |
| HY | 9.88 ± 0.74 bcde | 4.80 ± 0.52 efghijkl | 13.53 ± 0.89 efghi | 171.10 ± 19.05 bcd | 6.37 ± 0.18 a | 78.89 | 5 |
| KZJ22 | 8.55 ± 0.60 efgh | 2.96 ± 0.22 pq | 10.39 ± 0.18 klmn | 98.58 ± 4.77 jklmno | 3.01 ± 0.17 mn | 46.79 | 32 |
| MBWD | 8.67 ± 0.12 defgh | 4.55 ± 0.55 efghijklmn | 8.50 ± 0.94 no | 147.93 ± 12.91 cdefg | 4.60 ± 0.46 defghi | 61.97 | 19 |
| MTH | 7.64 ± 0.34 hij | 5.73 ± 0.05 bcde | 13.26 ± 0.65 ghi | 72.25 ± 5.65 mnopq | 4.13 ± 0.08 ghijk | 61.09 | 20 |
| MXG | 7.77 ± 0.25 hi | 4.34 ± 0.15 hijklmno | 15.78 ± 0.92 cd | 59.10 ± 6.67 pq | 4.85 ± 0.29 defgh | 60.78 | 21 |
| NG20 | 8.84 ± 0.37 cdefgh | 3.42 ± 0.47 nopq | 9.96 ± 0.24 lmno | 139.79 ± 19.17 defghi | 3.43 ± 0.03 jklmn | 54.40 | 28 |
| NH | 9.55 ± 0.63 bcdefg | 4.50 ± 0.54 fghijklmn | 14.00 ± 0.35 defgh | 111.73 ± 2.57 hijkl | 4.64 ± 0.23 defghi | 64.56 | 16 |
| OG | 10.63 ± 0.56 b | 5.29 ± 0.34 defgh | 10.80 ± 0.42 jklm | 141.78 ±12.77 defgh | 2.73 ± 0.09 n | 59.32 | 23 |
| PG | 7.72 ± 0.38 hij | 6.31 ± 0.05 abcd | 14.59 ± 0.83 defg | 71.47 ± 6.54 mnopq | 4.23 ± 0.04 fghij | 65.04 | 14 |
| PTY | 9.66 ± 0.46 bcdef | 3.91 ± 0.17 klmopq | 14.86 ± 0.79 defg | 133.13 ± 6.13 efghijk | 4.60 ± 0.17 defghi | 66.04 | 13 |
| QJ | 8.70 ± 0.39 defgh | 5.64 ± 0.30 bcdef | 13.33 ± 0.13 fghi | 104.83 ± 9.21 ijklm | 5.12 ± 1.03 def | 68.66 | 10 |
| QOG | 9.06 ± 0.71 cdefgh | 4.44 ± 0.47 fghijklmn | 9.52 ± 0.35 mno | 123.99 ± 3.26 ghijk | 3.07 ± 0.13 mn | 54.01 | 29 |
| RN1 | 8.90 ± 0.58 cdefgh | 3.95 ± 0.25 jklmnop | 11.64 ± 0.71 ijkl | 106.21 ± 13.69 ijklm | 3.98 ± 0.28 hijkl | 56.46 | 27 |
| SJY | 8.49 ± 0.57 efgh | 3.52 ± 0.43 mnopq | 9.78 ± 0.63 lmno | 178.73 ± 28.31 abc | 4.88 ± 0.12 defg | 64.86 | 15 |
| SW | 9.01 ± 0.13 cdefgh | 5.57 ± 0.51 cdefg | 14.63 ± 1.00 defg | 152.56 ± 10.44 bcdefg | 4.42 ± 0.09 efghi | 73.02 | 7 |
| SYXX | 9.70 ± 0.18 bcdef | 3.50 ± 0.33 mnopq | 9.51 ± 0.27 mno | 166.46 ± 2.43 bcde | 4.00 ± 0.07 ghijkl | 59.53 | 22 |
| TC | 9.58 ± 0.69 bcdefg | 6.64 ± 0.53 abc | 18.86 ± 1.38 ab | 170.14 ± 2.32 bcd | 6.02 ± 0.08 abc | 90.38 | 2 |
| TCH | 6.42 ± 0.17 ij | 2.72 ± 0.18 q | 9.37 ± 0.27 mno | 64.69 ± 9.16 opq | 2.87 ± 0.10 n | 40.08 | 35 |
| WHOG | 8.44 ± 0.47 efgh | 4.12 ± 0.33 hijklmnop | 9.50 ± 0.83 mno | 101.30 ± 10.04 jklmn | 3.06 ± 0.10 mn | 50.14 | 31 |
| WZ | 8.36 ± 0.57 fgh | 4.65 ± 0.24 efghijklm | 10.00 ± 0.71 lmno | 131.91 ± 17.31 efghijk | 3.36 ± 0.03 jklmn | 57.42 | 26 |
| YHY | 10.10 ± 0.34 bcd | 4.06 ± 0.31 ijklmnop | 10.82 ± 0.61 jklm | 208.86 ± 0.33 a | 5.13 ± 0.12 cde | 72.60 | 9 |
| YL | 10.20 ± 0.24 bc | 4.38 ± 0.21 ghijklmn | 12.24 ± 0.28 hijk | 153.20 ± 25.10 bcdefg | 4.63 ± 0.28 defghi | 66.86 | 11 |
| YXC | 6.66 ± 0.43 ij | 4.82 ± 0.47 efghijkl | 15.33 ± 0.52 cdef | 37.26 ± 3.92 q | 4.66 ± 0.43 defghi | 58.49 | 24 |
| ZXY | 8.96 ± 0.61 cdefgh | 5.14 ± 0.23 defghij | 9.95 ± 0.10 lmno | 179.93 ± 8.53 abc | 6.30 ± 0.19 ab | 76.31 | 6 |
Results were the mean ± SD (n = 3) on a dried weight (g) of citrus basis. Total phenolics were calculated as mg gallic acid equivalents (GAE)/g DW. Antioxidant capacities (DPPH, FRAP, ORAC and CUPRAC) were calculated as mg trolox equivalent antioxidant capacities (TEAC)/g DW. Values within each column followed by different superscript letters were significantly different (p < 0.05) according to Tukey’s tests. * Antioxidant index score = [(sample score/best score) × 100], averaged for all four tests for each cultivar for the antioxidant potency composite (APC) index. # Ranked according to the APC index.
Table 5.
Total phenolics and antioxidant properties of citrus fruit juice sacs of 35 cultivars.
| Cultivars | Total Phenolics | DPPH | FRAP | ORAC | CUPRAC | APC Index * | Rank # |
|---|---|---|---|---|---|---|---|
| AY27 | 6.35 ± 0.13 a | 4.73 ± 0.15 bcd | 13.00 ± 1.09 a | 62.32 ± 7.68 bc | 5.01 ± 0.27 cde | 84.23 | 1 |
| AY30 | 6.10 ± 0.20 abc | 5.02 ± 0.36 abc | 8.54 ± 0.37 defgh | 32.26 ± 2.36 ghijk | 6.04 ± 0.20 ab | 70.68 | 8 |
| AY31 | 4.75 ± 0.21 ghijk | 2.09 ± 0.27 opq | 7.42 ± 0.56 ghijkl | 40.59 ± 2.64 efg | 2.85 ± 0.16 mno | 47.04 | 31 |
| BZH | 5.25 ± 0.13 defghij | 4.29 ± 0.18 cdefgh | 8.36 ± 0.43 defghij | 20.35 ± 0.70 l | 6.32 ± 1.08 ab | 64.22 | 12 |
| CR | 5.84 ± 0.21 abcdef | 4.54 ± 0.12 cdefg | 8.27 ± 0.39 defghij | 53.06 ± 4.85 cd | 4.90 ± 0.20 de | 70.85 | 7 |
| CX | 4.18 ± 0.10 k | 1.66 ± 0.03 pq | 4.28 ± 0.34 o | 27.56 ± 1.35 ijkl | 2.51 ± 0.04 no | 33.60 | 34 |
| DF | 5.89 ± 0.04 abcde | 3.74 ± 0.28 ghijk | 7.07 ± 0.55 hijklm | 38.83 ± 2.42 efgh | 3.97 ± 0.10 fghijkl | 57.01 | 20 |
| GAC | 6.06 ± 0.39 abcd | 4.41 ± 0.18 cdefg | 11.96 ± 0.53 ab | 54.12 ± 4.41 cd | 4.58 ± 0.20 defghi | 76.58 | 5 |
| GN | 5.27 ± 0.05 cdefghij | 3.58 ± 0.26 hijk | 6.60 ± 0.28 jklmn | 28.76 ± 1.54 hijkl | 4.53 ± 0.51 defghij | 54.09 | 26 |
| GOC | 5.42 ± 0.18 bcdefghi | 3.80 ± 0.21 fghij | 6.74 ± 0.46 ijklmn | 29.49 ± 1.13 ghijkl | 3.87 ± 0.23 ghijkl | 53.19 | 28 |
| HMR | 5.27 ± 0.15 cdefghij | 3.23 ± 0.08 ijkl | 7.45 ± 0.61 ghijkl | 28.78 ± 1.70 hijkl | 4.47 ± 0.05 defghij | 53.99 | 27 |
| HY | 5.98 ± 0.31 abcd | 5.40 ± 0.21 ab | 10.04 ± 0.65 cd | 27.64 ± 0.39 hijkl | 6.96 ± 0.09 a | 77.00 | 4 |
| KZJ22 | 5.70 ± 0.29 abcdef | 1.46 ± 0.15 q | 5.63 ± 0.33 mno | 75.63 ± 3.81 a | 2.30 ± 0.19 o | 50.46 | 30 |
| MBWD | 4.80 ± 0.14 ghijk | 4.22 ± 0.20 cdefgh | 7.61 ± 0.29 ghijkl | 20.15 ± 3.74 l | 4.53 ± 0.19 defghij | 55.98 | 22 |
| MTH | 5.39 ± 0.39 bcdefghi | 3.58 ± 0.15 hijk | 8.36 ± 0.43 defghij | 35.82 ± 3.47 ghi | 3.88 ± 0.27 ghijkl | 57.47 | 19 |
| MXG | 5.02 ± 0.11 fghij | 2.41 ± 0.11 mnop | 7.43 ± 0.34 ghijkl | 48.81 ± 3.20 de | 3.17 ± 0.30 lmno | 52.32 | 29 |
| NG20 | 5.44 ± 0.03 bcdefghi | 4.25 ± 0.18 cdefgh | 11.9 ± 1.19 ab | 26.84 ± 0.92 ijkl | 4.77 ± 0.14 defg | 67.43 | 9 |
| NH | 6.39 ± 0.42 a | 4.74 ± 0.51 bcd | 9.13 ± 1.08 cdefg | 57.02 ± 2.10 bcd | 5.87 ± 0.13 bc | 78.17 | 3 |
| OG | 6.18 ± 0.39 ab | 3.25 ± 0.15 ijkl | 5.97 ± 0.81 lmno | 55.16 ± 7.92 bcd | 3.62 ± 0.20 jklm | 56.90 | 21 |
| PG | 6.00 ± 0.23 abcd | 4.17 ± 0.16 defgh | 10.32 ± 0.46 bc | 36.58 ± 1.61 ghi | 4.36 ± 0.23 defghij | 65.79 | 10 |
| PTY | 5.84 ± 0.11 abcedf | 4.28 ± 0.27 cdefgh | 7.57 ± 0.29 ghijkl | 60.32 ± 7.35 bc | 5.02 ± 0.29 cde | 71.20 | 6 |
| QJ | 5.10 ± 0.26 efghij | 4.68 ± 0.22 bcde | 7.82 ± 0.14 fghijk | 22.59 ± 1.15 kl | 5.10 ± 0.30 cd | 61.24 | 14 |
| QOG | 5.91 ± 0.35 abcde | 2.68 ± 0.36 lmno | 6.74 ± 0.12 ijklmn | 48.80 ± 2.53 de | 4.14 ± 0.15 efghijk | 55.66 | 23 |
| RN1 | 4.73 ± 0.42 ghijk | 3.31 ± 0.15 ijkl | 9.04 ± 0.21 cdefg | 37.03 ± 4.50 fghi | 4.15 ± 0.01 efghijk | 58.97 | 16 |
| SJY | 4.62 ± 0.15 ijk | 4.57 ± 0.09 cdef | 8.44 ± 0.39 defghi | 26.43 ± 4.43 ijkl | 4.81 ± 0.31 def | 62.18 | 13 |
| SW | 5.90 ± 0.42 abcde | 4.01 ± 0.20 defghi | 7.95 ± 0.05 efghijk | 35.24 ± 0.94 ghij | 4.63 ± 0.10 defghi | 61.06 | 15 |
| SYXX | 5.49 ± 0.10 bcdefgh | 3.51 ± 0.26 hijk | 6.18 ± 0.24 klmn | 53.77 ± 1.61 cd | 3.80 ± 0.18 ijkl | 58.62 | 17 |
| TC | 6.20 ± 0.17 ab | 5.73 ± 0.62 a | 9.73 ± 0.58 cde | 35.38 ± 5.10 ghi | 6.54 ± 0.22 ab | 78.90 | 2 |
| TCH | 4.63 ± 0.31 hijk | 2.23 ± 0.26 nopq | 5.19 ± 0.51 no | 24.15 ± 1.38 jkl | 3.12 ± 0.20 lmno | 38.90 | 33 |
| WHOG | 6.14 ± 0.46 ab | 3.04 ± 0.03 jklm | 6.29 ± 0.02 klmn | 47.89 ± 1.22 def | 3.84 ± 0.12 hijkl | 54.98 | 24 |
| WZ | 4.67 ± 0.20 ghijkl | 2.98 ± 0.21 klmn | 5.83 ± 0.15 lmno | 23.84 ± 1.42 kl | 3.38 ± 0.04 klmn | 44.23 | 32 |
| YHY | 4.83 ± 0.13 ghijk | 4.61 ± 0.55 bcde | 9.48 ± 1.15 cdef | 27.52 ± 3.15 ijkl | 4.74 ± 0.14 defgh | 64.47 | 11 |
| YL | 5.46 ± 0.28 bcdefghi | 2.97 ± 0.10 klmn | 6.23 ± 0.57 klmn | 65.67 ± 2.39 ab | 3.29 ± 0.26 klmn | 58.46 | 18 |
| YXC | 4.36 ± 0.11 k | 2.79 ± 0.10 lmno | 7.05 ± 0.43 hijklm | 24.17 ± 1.26 jkl | 4.54 ± 0.47 defghij | 50.03 | 35 |
| ZXY | 4.53 ± 0.15 jk | 3.90 ± 0.23 efghi | 6.78 ± 0.28 hijklmn | 27.78 ± 1.10 hijkl | 4.35 ± 0.42 defghij | 54.86 | 25 |
Results were the mean ± SD (n = 3) on a dried weight (g) of citrus basis. Total phenolics were calculated as mg gallic acid equivalents (GAE)/g DW. Antioxidant capacities (DPPH, FRAP, ORAC and CUPRAC) were calculated as mg trolox equivalent antioxidant capacities (TEAC)/g DW. Values within each column followed by different superscript letters were significantly different (p < 0.05) according to Tukey’s tests. * Antioxidant index score = [(sample score/best score) × 100], averaged for all four tests for each cultivar for the antioxidant potency composite (APC) index. # Ranked according to the APC index.
Among the four citrus fruit parts, the juice sacs had the lowest total phenolics contents (4.18 mg gallic acid equivalents (GAE)/g dry weight of extracts (DW) in CX to 6.39 mg GAE/g DW in Newhall (NH)), followed by the segment membrane (6.27 mg GAE/g DW in CX to 12.56 mg GAE/g DW in GAC) in all the 35 varieties. The total phenolics contents in the flavedo ranged from 11.52 mg GAE/g DW in ZXY to 27.55 mg GAE/g DW in AY30. While the total phenolics contents in the albedo ranged from 10.35 mg GAE/g DW in CX to 27.15 mg GAE/g DW in GAC. For the flavedo and albedo part, 12 citrus varieties had higher total phenolics contents in flavedo than in albedo, including six pummelos (HY, YHY, MBWD, Zaoxiangyou (ZXY), Putaoyou (PTY), Sijiyou (SJY)), three hybrids (AY27, GOC, MXG) and three tangerines (OG, QOG, WHOG). Zhang et al. [13] had previously tested the total phenolics of 14 wild mandarin genotypes and two cultivars, which ranged from 29.38 to 51.14 mg GAE/g DW. The total phenolics contents of the sum of flavedo and albedo (22.02 to 47.39 mg GAE/g DW) were close to the results of Zhang et al. [13].
The DPPH radical scavenging activity, FRAP, ORAC and CUPRAC assays were used to measure the antioxidant capacities of the four citrus parts. The mechanisms of these four antioxidant tests can be divided into two types: the DPPH, FRAP and CUPRAC tests are mainly electron transfer type antioxidant methods [14,15,16,17] while ORAC is a hydrogen supply ability type antioxidant method [18,19]. The results showed significant differences among different varieties for the four fruit parts with each measurement method.
DPPH antioxidant tests are commonly used in determining the primary antioxidant capacities. The DPPH radical scavenging mechanisms include two types: the electron transfer type when components dissolve in polar solutions and the hydrogen supply ability type in nonpolar solutions [14,20]. In this study, the DPPH radical scavenging abilities were mainly based on the electron transfer ability of the antioxidant components since the citrus extracts were dissolved in water. DPPH values ranged from 5.48 to 20.73 mg Trolox equivalent antioxidant capacities (TEAC)/g DW in flavedo and from 4.37 to 17.43 mg TEAC/g DW in albedo. AY30 in flavedo and PG in albedo ranked the first, respectively, while CX showed the lowest value in both parts. In the segment membrane, DPPH value varied from 2.72 (TCH) to 7.34 mg TEAC/g DW (HMR). In the juice sacs, TC had the highest DPPH value (5.73 mg TEAC/g DW) and KZJ22 had the lowest (1.46 mg TEAC/g DW).
FRAP is another antioxidant test widely used in the determination of plant antioxidant capacity [15]. FRAP values in flavedo varied from 1.94 to 21.33 mg TEAC/g DW, with the highest and lowest value corresponding to AY30 and CX, respectively, which is similar to the DPPH result. GAC showed the highest FRAP value in both albedo (25.26 mg TEAC/g DW) and segment membrane part (19.83 mg TEAC/g DW) while YHY showed the lowest value in albedo (4.82 mg TEAC/g DW). CX ranked the last in flavedo, segment membrane, juice sacs, which may due to its low total phenolics content. FRAP value of AY27 (13.00 mg TEAC/g DW) ranked the first in juice sacs, which is almost three times higher than CX (4.28 mg TEAC/g DW).
The ORAC method, which was widely used in animal and botany materials, tests the hydrogen atom transfer ability of antioxidant components [18]. The ORAC values were the highest among the four methods, which may due to its unique antioxidant type. TC showed the highest ORAC value in flavedo (468.96 mg TEAC/g DW) and albedo (677.90 mg TEAC/g DW), while NG20 showed the lowest value in flavedo (75.78 mg TEAC/g DW) and AY27 showed the lowest in albedo (55.49 mg TEAC/g DW). In the segment membrane, YHY and YXC showed the highest value (208.86 mg TEAC/g DW) and lowest value (37.26 mg TEAC/g DW), respectively. In juice sacs parts, interestingly, KZJ22 showed the highest ORAC value of 76.53 mg TEAC/g DW, although KZJ22 showed the lowest DPPH value in the same part, suggesting that different antioxidant measurements could show great differences in the antioxidant index.
The CUPRAC assay determines the capacities of tested materials to reduce divalent copper ions to cuprous ions [17]. The CUPRAC value ranking was similar to that of the FRAP value, which may be due to the similar antioxidant mechanisms involved. AY30 showed the highest CUPRAC value in flavedo (54.80 mg TEAC/g DW), OG ranked the first in albedo (46.63 mg TEAC/g DW), while ZXY showed the lowest CUPRAC value in both parts (24.44 mg TEAC/g DW in flavedo and 11.34 mg TEAC/g DW albedo). In segment membrane, HY showed the highest value (6.37 mg TEAC/g DW) and CX showed the lowest value (2.57 mg TEAC/g DW). In juice sacs, HY ranked the first with 6.54 mg TEAC/g DW and KZJ22 ranked the last with CUPRAC value of 2.30 mg TEAC/g DW.
To comprehensively compare the antioxidant capacities, an overall antioxidant potency composite (APC) index was calculated according to the method described by Seeram et al. [21]. The overall APC index showed obvious variations, ranging from 33.03 (SJY) to 92.01 (AY30) in flavedo, from 24.96 (YHY) to 86.39 (GAC) in albedo, from 40.08 (TCH) to 90.96 (GAC) in segment membrane, and from 50.03 (YXC) to 84.23 (AY27) in juice sacs
2.3. Tumor Cytotoxicity
In vitro tumor cytotoxicity of the citrus extracts were measured on three gastric cancer cell lines, i.e., SGC-7901, BGC-823 and AGS. Cell viability assays were performed with a Cell Counting Kit-8 (CCK-8) and the corresponding IC50 values were calculated. Among the four parts, only flavedo extracts of each citrus variety showed significant tumor cytotoxicity, with IC50 values as shown in Table 6, while the other three parts showed no significant cytotoxicity effects (data not shown). Among the three cancer cell lines, the AGS cell line seem to be generally more sensitive to citrus extracts treatments than other two cell lines, which can be inferred from the lower IC50 values. Among the citrus types, pummelo fruits extracts showed the high IC50 value (>100 μg/mL) in all three cell lines, suggested that the tumor cytotoxicity of pummelo fruits to the cancer cells tested was weak. QJ (IC50 value = 20.36 μg/mL), CR (IC50 value = 18.71 μg/mL), NH (IC50 value = 15.77 μg/mL) showed the highest antitumor activity to SGC-7901, BGC-823, AGS cell, respectively. The highest IC50 value for each cell is more than 15 times higher than the lowest.
Table 6.
The IC50 value of citrus flavedo extracts treatment to three gastric cancer cell lines.
| Cultivars | SGC-7901 (μg/mL) | BGC-823 (μg/mL) | AGS (μg/mL) |
|---|---|---|---|
| AY27 | 28.74 ± 0.61 abc | 22.61 ± 1.04 abc | 20.49 ± 0.97 abc |
| AY30 | 27.50 ± 1.34 abc | 28.51 ± 0.81 abcd | 24.32 ± 1.01 abcd |
| AY31 | 62.45 ± 2.49 efg | 68.62 ± 3.07 gh | 53.41 ± 0.78 ef |
| BZH | 40.65 ± 1.86 bcd | 38.90 ± 0.93 cde | 34.64 ± 1.11 bcd |
| CR | 28.50 ± 1.80 abc | 18.71 ± 0.82 a | 24.25 ± 1.32 abcd |
| CX | 80.58 ± 1.12 gh | 83.40 ± 5.94 hijk | 74.89 ± 2.43 gh |
| DF | 64.34 ± 2.31 efg | 42.39 ± 0.90 de | 36.52 ± 0.65 cde |
| GAC | 60.86 ± 1.78 ef | 70.37 ± 3.65 gh | 61.94 ± 2.18 fg |
| GN | 33.81 ± 2.01 abc | 35.80 ± 1.10 abcde | 34.51 ± 0.99 bcd |
| GOC | 55.61 ± 3.35 def | 49.43 ± 2.05 ef | 32.39 ± 1.39 abcd |
| HMR | 100.97 ± 4.35 i | 99.05 ± 1.97 k | 59.95 ± 1.34 fg |
| HY | 164.86 ± 6.31 k | 150.54 ± 7.45 l | 139.8 ± 8.13 j |
| KZJ | 39.07 ± 1.72 abcd | 37.80 ± 1.94 cde | 30.78 ± 1.55 abcd |
| MBWD | 203.51 ± 8.28 l | 153.78 ± 4.51 lm | 114.64 ± 4.46 i |
| MTH | 37.79 ± 1.71 abcd | 37.27 ± 1.36 cde | 29.34 ± 1.40 abcd |
| MXG | 89.16 ± 3.69 hi | 77.75 ± 4.93 ghij | 83.65 ± 2.86 h |
| NG20 | 120.43 ± 6.27 j | 89.07 ± 3.92 ijk | 74.59 ± 2.65 gh |
| NH | 21.65 ± 0.63 ab | 19.88 ± 0.52 ab | 15.77 ± 0.66 a |
| OG | 34.60 ± 0.42 abc | 37.01 ± 1.37 bcde | 32.33 ± 0.64 abcd |
| PG | 37.76 ± 1.14 abcd | 33.21 ± 1.54 abcde | 34.02 ± 1.89 bcd |
| PTY | 207.37 ± 11.72 l | 192.10 ± 3.72 n | 165.46 ± 4.93 k |
| QJ | 20.36 ± 0.81 a | 24.44 ± 1.01 abc | 17.49 ± 0.65 ab |
| QOG | 34.39 ± 3.05 abc | 30.71 ± 0.63 abcd | 23.37 ± 0.57 abcd |
| RN1 | 205.73 ± 5.71 l | 168.73 ± 4.87 m | 134.84 ± 4.77 j |
| SJY | 368.40 ± 20.35 o | 355.32 ± 17.47 p | 311.41 ± 21.54 m |
| SW | 92.66 ± 2.19 hi | 97.75 ± 8.99 k | 79.95 ± 4.45 h |
| SYXX | 81.40 ± 1.75 gh | 74.28 ± 3.77 ghi | 72.35 ± 2.11 gh |
| TC | 35.88 ± 1.39 abc | 33.75 ± 2.10 abcde | 24.24 ± 1.65 abcd |
| TCH | 69.14 ± 3.79 fg | 61.86 ± 2.55 fg | 53.82 ± 0.63 ef |
| WHOG | 26.42 ± 1.72 ab | 30.14 ± 1.59 abcd | 26.24 ± 0.93 abcd |
| WZ | 137.53 ± 6.09 j | 95.04 ± 1.86 jk | 76.47 ± 1.98 gh |
| YHY | 313.09 ± 12.31 n | 253.47 ± 14.60 o | 255.56 ± 18.56 l |
| YL | 81.39 ± 1.85 gh | 73.77 ± 5.29 ghi | 66.24 ± 0.78 fgh |
| YXC | 46.44 ± 2.30 cde | 34.07 ± 0.76 abcde | 39.32 ± 2.39 de |
| ZXY | 291.47 ± 13.15 m | 244.66 ± 9.86 o | 173.76 ± 5.97 k |
Results were the mean ± SD (n = 3) on Half inhibition rate (IC50). Values within each column followed by different superscript letters were significantly different (p < 0.05) according to Tukey’s tests.
2.4. Identification and Quantification of Individual Flavonoid Compound
Further identification and quantification of individual flavonoid in citrus fruits were performed by LC-ESI-MS/MS and UPLC-DAD. A total of 39 flavonoid compounds, including four flavones, nine flavanones and 26 PMFs, were identified. The identification was based on comparison of the retention times and the maximum absorption wavelength of standards, as well as the fragment ion information reported in previous studies (Table 7 and Table S6, Figures S1–S5) [22,23,24].
Table 7.
Determination of flavonoids from citrus fruits by LC-ESI-MS/MS (+ESI mode) and UPLC-DAD.
| Peak No. | RT (min) | λmax (nm) | [M + H]+ (m/z) | Formula | Fragment Ions (m/z) | Tentative Compounds |
|---|---|---|---|---|---|---|
| Flavone C-glycosides | ||||||
| 1 | 2.30 | 240.4, 330.6 | 595.1665 | C27H30O15 | 577, 559, 541, 529, 523, 505 | Vicenin-2 |
| 3 | 3.53 | 255.0, 348.0 | 433.1142 | C21H20O10 | 415, 397, 379, 367, 337, 313 | Apigenin-8-C-glucoside |
| 5 | 4.46 | 272.3 | 463.1234 | C22H22O11 | 445, 427, 397, 380, 367, 343, 313 | Diosmetin-6-C-glucoside |
| Flavanone O-glycosides | ||||||
| 2 | 3.18 | 284.2 | 597.1823 | C27H32O15 | 435, 399, 355, 289, 263, 195 | Eriocitrin |
| 4 | 3.61 | 284.2 | 597.1806 | C27H32O15 | 433, 399, 355, 289, 263, 195 | Neoeriocitrin |
| 6 | 5.02 | 283.0, 329.4 | 581.186 | C27H32O14 | 419, 383, 339, 273, 195, 153 | Narirutin |
| 8 | 5.94 | 283.0, 329.4 | 581.1865 | C27H32O14 | 419, 383, 339, 315, 273, 195 | Naringin |
| 9 | 6.68 | 284.2, 331.8 | 611.1987 | C28H34O15 | 449, 413, 369, 303, 263, 195, 153 | Hesperidin |
| 10 | 7.92 | 284.2 | 611.1974 | C28H34O15 | 413, 369, 303, 263, 195 | Neohesperidin |
| 11 | 10.38 | 283.0 | 595.2019 | C28H34O14 | 377, 353, 329, 287, 263, 195 | Didymin |
| 12 | 10.62 | 328.2 | 595.2017 | C28H34O14 | 463, 379, 287, 263, 153 | Poncirin |
| 15 | 11.68 | 337.7 | 725.2283 | C33H30O31 | 419, 404, 390, 361 | Melitidin |
| Flavone O-glycoside | ||||||
| 7 | 5.65 | 267.0, 334.0 | 579.1704 | C27H30O14 | 433, 315, 271, 195, 153, 127 | Rhoifolin |
| Polymethoxyflavonoids | ||||||
| 13 | 11.02 | 215.6, 324.7 | 329.1019 | C18H16O6 | 314, 299, 271, 228 | Monohydroxytrimethoxyflavone (1) |
| 14 | 11.29 | 328.2 | 359.1114 | C19H18O7 | 344, 329, 314, 286, 257 | Gardenin B |
| 16 | 11.97 | 322.3 | 329.1016 | C18H16O6 | 314, 299, 268, 136 | Monohydroxytrimethoxyflavone (2) |
| 17 | 12.40 | 343.4 | 331.0817 | C17H14O7 | 316, 301, 273, 245, 217, 168 | Trihydroxydimethoxyflavone |
| 18 | 12.57 | 348.0 | 389.1227 | C20H20O8 | 374, 359, 341, 298 | Monohydroxy-pentamethoxyflavone (1) |
| 19 | 12.84 | 323.5 | 373.1277 | C20H20O7 | 358, 343, 315, 287, 181, 153 | Isosinensetin |
| 20 | 13.26 | 281.8 | 359.1129 | C19H18O7 | 344, 329, 314, 301, 163, 147 | Monohydroxytetramethoxyflavone |
| 21 | 13.53 | 335.4 | 389.1227 | C20H20O8 | 374, 359, 341, 298 | Monohydroxypentamethoxyflavone (2) |
| 22 | 13.55 | 351.6 | 403.1389 | C21H22O8 | 388, 373, 359, 327, 183, 163 | Hexamethoxyflavone (1) |
| 23 | 14.25 | 240.4, 330.6 | 373.1289 | C20H20O7 | 357, 343, 329, 312, 297, 153 | Sinensetin |
| 24 | 14.60 | 268.0, 334.0 | 343.118 | C19H18O6 | 328, 313, 285, 257, 181, 153 | Tetramethyl-O-isoscutellarein |
| 25 | 14.80 | 343.4 | 345.0974 | C18H16O7 | 330, 318 | Dihydroxytrimethoxyflavone |
| 26 | 14.96 | 210.0, 336.6 | 403.1392 | C21H22O8 | 388, 373, 359, 327, 183, 163 | Hexa-O-methylgossypetin |
| 27 | 15.17 | 337.7 | 373.1282 | C20H20O7 | 253, 211, 196, 181, 168, 150 | 5,7,3′,4′,5′-Pentamethoxyflavone |
| 28 | 15.78 | 249.8, 334.2 | 403.1388 | C21H22O8 | 388, 373, 358, 355, 327, 211, 183 | Nobiletin |
| 29 | 15.97 | 321.1 | 343.1175 | C19H18O6 | 328, 313, 285, 257, 181, 153 | Tetramethyl-O-scutellarein |
| 30 | 16.32 | 350.4 | 375.1074 | C19H18O8 | 345, 327. 197 | 5,4′-Dihydroxyl-3,7,8,3′-tetramethoxyflavonol |
| 31 | 16.49 | 254.6, 341.2 | 433.1486 | C22H24O9 | 417, 403, 388, 373, 303, 217 | 3,5,6,7,8,3′,4′-Heptamethoxyflavone |
| 32 | 17.42 | 271.1, 323.5 | 373.1281 | C20H20O7 | 358, 343, 328, 297, 211, 183 | Tangeretin |
| 33 | 17.82 | 282.0, 341.0 | 359.1128 | C19H18O7 | 344, 329, 311, 283 | 6-O-Desmethyltangeritin/7-O-desmethyltangeritin |
| 34 | 18.49 | 349.2 | 403.1388 | C21H22O8 | 388, 373, 359, 327, 183, 163 | Hexamethoxyflavone (2) |
| 35 | 18.56 | 283.0, 341.3 | 389.1229 | C20H20O8 | 374, 359, 341, 331, 197 | 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone |
| 36 | 18.99 | 331.8 | 329.1018 | C18H16O6 | 299, 285, 268, 153 | Monohydroxytrimethoxyflavone |
| 37 | 19.36 | 273.5, 357.6 | 389.1232 | C20H20O8 | 374, 359, 341, 298 | Monohydroxypentamethoxyflavone (3) |
| 38 | 19.65 | 346.9 | 419.1326 | C21H22O9 | 404, 389, 372, 218 | Natsudaidai |
| 39 | 20.88 | 290.0, 329.4 | 359.1116 | C19H18O7 | 344, 326, 298, 282, 255, 162 | 5-Hydroxy-7,8,3′,4′-tetramethoxyflavone |
The four flavones included three flavone C-glucosides (vicenin-2, apigenin-8-C-glusoide and diosmetin-6-C-glucoside) and one flavone O-glycoside (rhoifolin). Diosmetin-6-C-glucoside was only found in the Buzhihuo (BZH), NG20, SJY and MXG, with the highest concentration of 9.05 mg/g DW in the albedo part of BZH. Rhoifolin was only found in pomelo type fruit (Table S1 and S3–S5).
The nine flavanone O-glycosides included eriocitrin, neoeriocitrin, narirutin, naringin, hesperidin, neohesperidin, didymin, poncirin and melitidin, in which narirutin, naringin, hesperidin, neohesperidin were the most common flavonoid components in the 35 citrus varieties (Table S1 and 3–S5). Naringin was very abundant in pummelo type fruit. The content of narirutin was generally low in the pomelo type fruit. Melitidin was only found in the flavedo part of MXG, while vicenin-2 was only existed in KZJ22. The content of hesperidin and neohesperidin showed a seesawing like relationship, that is, the varieties with abundant hesperidin tend to have low level of neohesperidin, which may due to the differentiation in synthetic metabolism.
The PMFs were only found in flavedo, including one trihydroxydimethoxyflavone, four trimethoxyflavones, seven tetramethoxyflavones, eight pentamethoxyflavones, five hexa-methoxyflavones and one heptamethoxyflavone (Table S2). Among the 26 PMFs identified, there were 12 monohydroxy PMFs, one dihydroxy PMFs and one trihydroxy PMF. The exact location of the methoxy groups of two monohydroxypentamethoxyflavones, two hexamethoxyflavones and three monohydroxypentamethoxyflavones need further identification and we added a number after their names according to the order they appeared in the UPLC chromatogram. The PMF contents differed among varieties. Trihydroxydimethoxyflavone only existed in HMR (3.78 mg/g DW) and 5,7,3′,4′,5′-pentamethoxyflavone only existed in AY27 (1.22 mg/g DW). Isosinensetin sinensetin, tetramethyl-O-isoscutellarein, nobiletin, tetramethyl-O-scutellarein, 3,5,6,7,8,3′,4′-heptamethoxy-flavone, tangeretin, 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone existed in most citrus varieties. Nobiletin was the maximum PMFs in almost all of the varieties except for TCH, WZ, DF, SYXX, SW and MXG.
2.5. Correlations between Total Phenolics and Bioactivites
Correlation analyses were performed to investigate the relationship between the phenolics content, antioxidant ability and cytotoxicity on gastric cancer cell (Table 8 and Table 9).
Table 8.
Pearson’s correlation coefficients among total phenolics, antioxidant values and in vitro anticancer abilities in citrus flavedo.
| Bioactive Capacities | Total Phenolic | DPPH | FRAP | ORAC | CUPRAC | APC Index | 1/IC50 SGC-7901 | 1/IC50 BGC-823 | 1/IC50 AGS |
|---|---|---|---|---|---|---|---|---|---|
| Total Phenolic | 1 | ||||||||
| DPPH | 0.853 ** | 1 | |||||||
| FRAP | 0.888 ** | 0.923 ** | 1 | ||||||
| ORAC | 0.735 ** | 0.560 ** | 0.563 ** | 1 | |||||
| CUPRAC | 0.936 ** | 0.766 ** | 0.787 ** | 0.741 ** | 1 | ||||
| APC Index | 0.958 ** | 0.919 ** | 0.930 ** | 0.781 ** | 0.916 ** | 1 | |||
| 1/IC50 SGC-7901 | 0.513 ** | 0.375 * | 0.421 ** | 0.366 ** | 0.590 ** | 0.482 ** | 1 | ||
| 1/IC50 BGC-823 | 0.456 ** | 0.316 | 0.395 ** | 0.309 | 0.536 ** | 0.433 ** | 0.947 ** | 1 | |
| 1/IC50 AGS | 0.548 ** | 0.392 ** | 0.456 ** | 0.396 ** | 0.617 ** | 0.514 ** | 0.965 ** | 0.949 ** | 1 |
1/IC50 means the reciprocal value of IC50; One and two asterisks represent statistical significance at p < 0.05 and p < 0.01, respectively.
Table 9.
Pearson’s correlation coefficients among total phenolics, antioxidant values in albedo, segment membrane and juice sacs.
| Bioactive Capacities | Total Phenolic | DPPH | FRAP | ORAC | CUPRAC | APC Index |
|---|---|---|---|---|---|---|
| Albedo | ||||||
| Total Phenolic | 1 | |||||
| DPPH | 0.679 ** | 1 | ||||
| FRAP | 0.645 ** | 0.901 ** | 1 | |||
| ORAC | 0.500 ** | 0.156 | 0.015 | 1 | ||
| CUPRAC | 0.656 ** | 0.677 ** | 0.626 ** | 0.404 ** | 1 | |
| APC Index | 0.804 ** | 0.875 ** | 0.806 ** | 0.534 ** | 0.876 ** | 1 |
| Segment membrane | ||||||
| Total Phenolic | 1 | |||||
| DPPH | 0.322 | 1 | ||||
| FRAP | 0.346 * | 0.741 ** | 1 | |||
| ORAC | 0.725 ** | 0.086 | −0.059 | 1 | ||
| CUPRAC | 0.422 * | 0.589 ** | 0.681 ** | 0.358 | 1 | |
| APC Index | 0.649 ** | 0.779 ** | 0.754 ** | 0.538 ** | 0.884 ** | 1 |
| Juice sacs | ||||||
| Total Phenolic | 1 | |||||
| DPPH | 0.452 ** | 1 | ||||
| FRAP | 0.421 * | 0.696 ** | 1 | |||
| ORAC | 0.576 ** | −0.183 | 0.033 | 1 | ||
| CUPRAC | 0.409 * | 0.896 ** | 0.606 ** | −0.255 | 1 | |
| APC Index | 0.705 ** | 0.853 ** | 0.827 ** | 0.282 | 0.786 ** | 1 |
One and two asterisks represent statistical significance at p < 0.05 and p < 0.01, respectively.
For the 4 antioxidant traits, DPPH, FRAP, CUPRAC showed significant correlation with each other (p < 0.01), indicating that these 3 traits determined the same type of antioxidant capacities. However, ORAC method only showed correlation with other 3 traits in flavedo. In other 3 parts of citrus, ORAC traits showed very weak relation with other 3 methods, suggesting that ORAC value reflected a different type of antioxidant ability. Total phenolics contents in all the 4 parts showed significant correlations with all 4 the antioxidant traits and the APC overall index (Table 8 and Table 9), indicating that phenolics compounds were the principle contributor to antioxidant capacities of citrus. High correlation of total phenolics contents and antioxidant capacities also showed in the peach fruit [25], Chinese bayberry [26], vegetables and grains [27], indicating that this is a common phenomenon in nature.
The cytotoxicity of extracts showed high correlations among 3 cell traits. However, the correlations between antioxidant capacities and anticancer abilities were relatively low, indicating that the in vitro cytotoxicity of citrus extracts may not be caused by its antioxidant ability. In plenty previous anticancer studies, most of the flavanones were reported to be functioned through enhancing the body’s own function which was based on antioxidant capacities of flavanone [28,29,30]. However, the results of present study indicated a different functional mechanism of citrus flavonoids rich extracts.
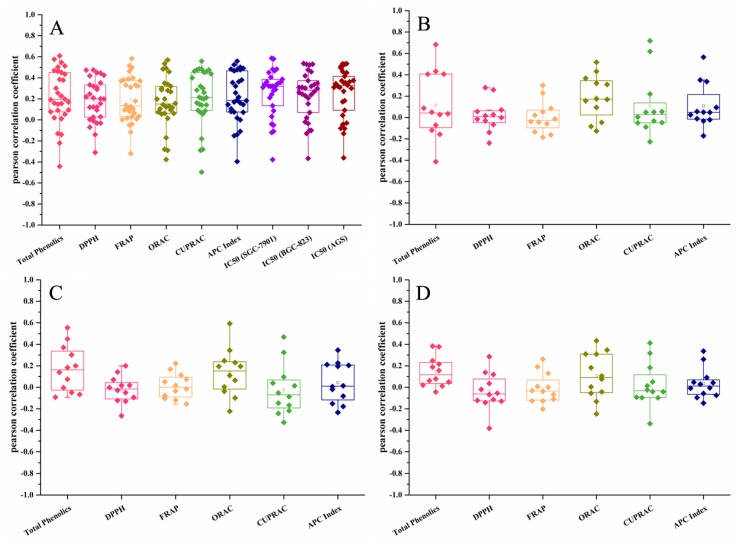
For the cytotoxicity of individual compounds, the contents of 11 PMFs showed significant correlations with cytotoxicity of extracts on all three gastric cancer cell lines, in which nobiletin showed the highest correlation coefficient (r SGC-7901 = 0.587, p < 0.01; r BGC-823 = 0.530, p < 0.01; r AGS = 0.534, p < 0.01) (Figure 1, Tables S7–S10). None of the flavanones or flavones showed significant positive relationships with cytotoxicity and rhoifolin showed a significant negative relationship (r SGC-7901 = −0.378, p < 0.05; r BGC-823 = −0.366, p < 0.05; r AGS = −0.361, p < 0.05). These results suggested that PMFs might be the main contributors to the cytotoxicity of extracts. Similar results were observed in previous studies. PMFs have shown anti-proliferation abilities to many cancers in vitro [31,32] and in vivo [33,34]. They were suggested to be functioned through induction of cell apoptosis [33,35], cell cycle blocking [36,37] and autophagy, etc. [38,39].
Figure 1.
Correlation analysis of bioactive traits and individual flavonoid compounds in flavedo (A); albedo (B); segment membrane (C) and juice sacs (D).
For the antioxidant activity of individual compounds, the didymin showed the positive relationship with APC index and all four antioxidant tests in flavedo. Hesperidin in flavedo (r = 0.558, p < 0.01), neohesperidin in albedo (r = 0.718, p < 0.01), poncirin (r = 0.618, p < 0.01) in albedo showed strong relationship with CUPRAC. Naringin showed significant relationship with ORAC value in segment membrane (r = 0.592, p < 0.01). These results showed that didymin, hesperidin, neohesperidin, poncirin, naringin might played dominant roles in antioxidant ability of citrus extracts.
3. Experimental Section
3.1. Materials
Citrus fruits at commercial maturity were harvested from orchards of Zhejiang Province in December 2015 (Table 1), and transported to the laboratory of Zhejiang University, Hangzhou, within 6 h of harvest. Uniform fruit free from blemishes and mechanical injury was selected for the present study. The fruits were separated into four parts, i.e., flavedo, albedo, segment membrane and juice sacs, and immediately frozen in liquid nitrogen. After freeze-drying (FM 25EL-85, VirTis, Gardiner, NY, USA), all samples were ground into a fine powder and stored at −80 °C for further experiments. The SGC-7901, BGC-823, AGS gastric cancer cell lines were obtained from the Department of Surgery, Second Affiliated Hospital, School of Medicine, Zhejiang University.
3.2. Chemicals and Reagents
All standards and reagents were of HPLC grade. Narirutin, neoeriocitrin, hesperidin, naringenin, poncirin, naringin, hesperidin, neohesperidin, nobiletin, tangeretin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), Trolox, Folin-Ciocalteu reagent, 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), rutin, gallic acid, fluorescein sodium, copper chloride, neocuproine, ammonium acetate, methanol, acetonitrile were purchased from Sigma-Aldrich (St. Louis, MO, USA). Eriocitrin was purchased from Aladdin Industrial Inc. (Shanghai, China). Isosinensetin, and 5-demethylnobiletin were purchased from Biobiopha Co., Ltd. (Kunming, China). Didymin was purchased from J & K Scientific (Shanghai, China). Cell Counting Kit-8 was purchased from Dojindo Molecular Technologies, Inc. (Shanghai, China). Samples for HPLC were filtered through a 0.22 μm membrane before injection. Double-distilled water (ddH2O) was used in all experiment. RPMI 1640 medium, fetal bovine serum (FBS), N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES), trypsin-EDTA were purchased from Gibco (Waltham, MA, USA). Penicillin-streptomycin solution was purchased from Hangzhou Keyi Biotechnology Co., Ltd. (Hangzhou, China). All other solvents and reagents were of analytical grade purchased from Sinopharm Chemical Reagents Co., Ltd. (Shanghai, China).
3.3. Fruit Quality Analysis (Color, Fruit Weight, Edible Proportion and Total Soluble Sugar)
Fruit color measurement was carried out using the Citrus Color Index (CCI) according to a previous report [40]. The raw data were adopted as L*, a* and b* with a MiniScan XE Plus Colorimeter (HunterLab, Reston, VA, USA) and the CCI was calculated as CCI = [1000 × a*/(L* × b*)]. Four evenly distributed equatorial sites were measured for each fruit and a mean value was obtained from the measurement of 15 fruits per variety. The edible rate was calculated as the weight percentage of pulp to the whole fruit. TSS of 15 fruits per variety were measured with a portable digital refractometer (Atago PR-101α, Tokyo, Japan) at 25 °C and the data were expressed as °Brix.
3.4. Extraction and Determination of Total Phenolics
One gram of citrus fruit ground powder was ultrasonically extracted in 20 mL of 95% ethanol at 25 °C in a material-to-solvent ratio of 1:20 (w/v) for three times. The extract was centrifuged at 10,000 ×g for 5 min and the supernatants were evaporated under reduced pressure at 35 °C to remove the ethanol. The phenolics were enriched by solid-phase extraction using a Sep-pak C18 cartridge (12 cc, 2 g sorbent, Waters Corp., Milford, MA, USA). The citrus phenolic-rich extracts were used for further analysis.
Total phenolics contents were measured with a Folin-Ciocalteu method according to previous report [41] with slight modification. In brief, 4 mL of ddH2O and 0.5 mL appropriately-diluted citrus extracts was placed into a test tube, added with 0.5 mL Folin-Ciocalteu (0.5 mol/L) and incubated for 3 min. Then 1 mL of saturated sodium carbonate was added into the mixture following by incubating the reaction for 2 h in 30 °C water bath. The absorbance of the reaction product were measured at 760 nm using a microplate reader (Synergy H1, Biotek, Winooski, VT, USA). Gallic acid was used as the standard and the results were expressed as mg gallic acid equivalent (GAE)/g DW.
3.5. Antioxidant Capacity Assays
The DPPH radical scavenging activity was measured according to our previous publication [42] with some modification. In brief, 2 μL diluted citrus extracts was mixed with 198 μL DPPH solution (60 μM), the mixture was allowed to react for 2 h at room temperature, away from light. Then the absorbance at 515 nm was measured using a microplate reader. Trolox was used as the standard and the results were expressed as mg Trolox equivalent antioxidant capacities (TEAC)/g DW.
The FRAP assay was carried out according to Zhang et al. [43] with modifications. Briefly, the FRAP working solution was prepared by mixing 100 mL acetate buffer (300 mmol/L, pH 3.6), 10 mL TPTZ solution (10 mmol/L in 40 mmol/L HCl) and 10 mL FeCl3 (20 mmol/L). 10 μL appropriately diluted citrus extracts and 90 μL of FRAP working solution was mixed in a 96-well plate and incubated for 5 min. Then the absorbance of 593 nm was recorded with a microplate reader. Trolox was used as the standard and the results were expressed as mg Trolox equivalent antioxidant capacities (TEAC)/g DW.
ORAC antioxidant activity was measured according to previous report [44] with some minor modifications. 25 μL appropriately diluted citrus extraction was placed into a black-walled 96-well plate, and mixed with 150 μL sodium fluorescein (40 nmol/L). The mixture was incubated for 10 min at 37 °C. Then 25 μL AAPH (150 mmol/L) was added and the fluorescence detection was performed immediately with a microplate reader (set with excitation wavelength of 485 nm, emission wavelength of 535 nm, time interval of 2 min for the 2 h detection). Phosphate buffer solution (PBS) was used as a blank control and the final fluorescence measurements were expressed relative to the initial reading (fn). Results were calculated based on the differences in areas under the sodium fluorescein decay curve (AUC) between the blank and samples. Trolox was used as standard and the results were expressed as mg Trolox equivalent antioxidant capacities (TEAC)/g DW. The AUC was calculated as:
| AUC = (f0 + f1 + f2 + … + fn)/f0 |
The CUPARC assay was carried out according to previous report [16] with some modifications. Reaction system consisted of 20 μL appropriately diluted citrus extraction, 50 μL copper chloride (10 mmol/L), 50 μL neocuproine (7 mmol/L), 50 μL ammonium acetate (pH 7) and 50 μL ddH2O was added into a 96-well plate in order. After 30 min of incubation away from light in room temperature, absorbance of 450 nm was measured in a microplate reader. Trolox was used as standard and the results were expressed as mg Trolox equivalent antioxidant capacities (TEAC)/g DW.
An overall APC index was applied to comprehensively evaluated the antioxidant traits of the extracts. For each antioxidant trait, antioxidant index score = [(sample score/best score) × 100]. The APC index was calculated as the average of the antioxidant index scores of the referred 4 methods.
3.6. Cell Culture and Cell Viability Assay
The human gastric cancer cell lines SGC-7901, BGC-823, AGS were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 20 mmol/L HEPEs, at 37 °C in an incubator (Thermo Scientific 3111, Thermo Scientific, Waltham, MA, USA) containing 5% CO2. Cells were passaged every 48 h using trypsin (0.25%)/EDTA (0.02%) solution. Exponentially growing cells were used for experimentation.
Cell viability assay was performed with cell counting kit-8 (CCK-8) analysis according to the methods described in previous study [45]. Briefly, cells (4000 cells per well for SGC-7901, 8000 cells per well for BGC-823 and 9000 cells per well for AGS) were seeded into 96-well plates. After 24 h incubation, the medium were moved and cells were treated with or without citrus extractions in a total volume of 200 μL each well. After 48 h incubation, the supernatant was removed and washed with PBS for 2 times. The cell viability was measured using CCK-8 kit according to the instruction. Taxol was used as positive control. The inhibition ratio was calculated:
| Inhibition ratio = (A450 − A620)/A450. | (1) |
The IC50 value was calculated by probit analysis method using SPSS 19.0 software (IBM, Armonk, NY, USA).
3.7. UPLC-DAD and LC-ESI-MS/MS Analysis of Phonilic Compounds
Individual flavonoid compounds were identified and quantified combining LC-ESI-MS/MS and UPLC-DAD. Flavones and flavanones were detected at 280 nm and polymethoxylated flavonoids were detected at 330 nm. 13 flavanoids, i.e., eriocitrin, neoericitrin, narirutin, naringin, hesperidin, neohesperidin, didmin, poncirin, isosinensetin, sinensitin, nobiletin, tangeretin, 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone, were quantified with their own standard curves according to the retention time and the chromatographic peak area in the UPLC analysis. Other 26 flavonoids were quantified as equivalents of hesperidin at 280 nm. All tests were run in triplicate and data were expressed as mg/g DW.
The flavonoid compounds were determined with a UPLC system (2695 pump, 2996 diode array detector, Waters Corp.) coupled with an BEH C18 analytical column (ACQUITY UPLC, 2.1 × 150 mm, Waters Corp.). The column was operated at a temperature of 25 °C. The injection of sample was 2 μL and the flow rate was 0.3 mL/min. The compounds were detected between 200 and 500 nm. The mobile phase of UPLC consisted waters (Eluent A) and acetonitrile (Eluent B). the gradient program was as follows: 0–5 min, 20% of B; 5–8 min, 20–34% of B; 8–20 min, 34–60% of B; 20–22 min, 60–100% of B; 22–23 min, 100% of B; 23–24 min, 100–20% of B; 24–25 min, 20% of B.
Mass spectrometric analysis were performed according to our previous publication [25]. Briefly, an Agilent 6460 triple quadrupole mass spectrometer equipped with an ESI source (Agilent Technologies, Santa Clara, CA, USA) was used for mass analysis and the analysis were operated in positive ionization mode. The nebulizer pressure was set to 45 psi and drying gas flow rate was 5 L/min. The flow rate and the temperature of the sheath gas was 11 L/min and 350 °C, respectively. Chromatographic separations were done on an BEH C18 analytical column (ACQUITY UPLC, 2.1 × 150 mm) using an Agilent 1290 Infinity UPLC system (Agilent Technologies). The eluent was split and with a rate of 0.3 mL/min going into the mass detector. The data acquisition and processing were performed at an Agilent Mass Hunter Workstation.
3.8. Statistical Analysis
All data were obtained from at least three replications and expressed as the means ± standard deviation. The statistical analyses were carried out using SPSS 19.0 software (IBM, Armonk, NY, USA). Significant differences among the sample were analyzed using one-way ANOVA, followed by Tukey’s test at p < 0.05. Pearson correlation coefficients were calculated at p < 0.05.
4. Conclusions
A total of 39 flavonoids, including four flavones, nine flavanones and 26 PMFs were identified and quantified from 35 varieties of five types of citrus fruit growing in Zhejiang Province of China. Among them, all 39 compounds could be found in the flavedo, three flavones and nine flavanones were found in the albedo, segment membrane and juice sacs, while PMFs were existing only in the flavedo. The flavonoids composition and bioactivity varied depending on the types and tissues of citrus fruit. According to the results of correlation analysis, phenolics were deduced to be the chief contributor for the antioxidant capacity of citrus fruit, in which the individual flavanone compounds including didymin, hesperidin, neohesperidin, poncirin, naringin were the principal contributing components. Phenolics extracts from flavedo showed significant cytotoxicity effects on gastric tumor cell lines, and PMFs were deduced to be the dominant contributors, with the nobiletin as the principle contributing component. The correlation between antioxidant capacities and the cytotoxicity effects was not significant. These results may offer important information for breeding and further utilization of citrus resources.
Acknowledgments
The work was supported by National Key R&D Program of China (2017YFD0400200), the National Natural Science Foundation of China (31571838), the 111 project (B17039), and the Agricultural Outstanding Talents and Innovation Team of the State Agricultural Ministry on Health and Nutrition of Fruit. The authors would like to thank Don Grierson from the University of Nottingham (UK) for discussions, suggestions, and efforts in language editing.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
Yue Wang carried out the experiments and wrote the original manuscript. Jing Qian and Jinping Cao participated in experiments. Dengliang Wang, Chunrong Liu and Rongxi Yang contributed materials. Yue Wang and Jing Qian analyzed the data. Jinping Cao and Xian Li edited and revised the manuscript. Chongde Sun conceived and designed the study. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of citrus powders are not available from the authors.
References
- 1.He J.Z., Shao P., Liu J.H., Ru Q.M. Supercritical Carbon Dioxide Extraction of Flavonoids from Pomelo (Citrus grandis (L.) Osbeck) Peel and Their Antioxidant Activity. Int. J. Mol. Sci. 2012;13:13065–13078. doi: 10.3390/ijms131013065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu X.Y., Luo F.L., Zheng Y.X., Zhang J.K., Huang J.Z., Sun C.D., Li X., Chen K.S. Characterization, Purification of Poncirin from Edible Citrus Ougan (Citrus reticulate cv. Suavissima) and Its Growth Inhibitory Effect on Human Gastric Cancer Cells SGC-7901. Int. J. Mol. Sci. 2013;14:8684–8697. doi: 10.3390/ijms14058684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silalahi J. Anticancer and health protective properties of citrus fruit components. Asia Pac. J. Clin. Nutr. 2002;11:79–84. doi: 10.1046/j.1440-6047.2002.00271.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y.S., Ho S.C. Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel. Food Chem. 2010;119:868–873. doi: 10.1016/j.foodchem.2009.09.092. [DOI] [Google Scholar]
- 5.Nakajima V.M., Macedo G.A., Macedo J.A. Citrus bioactive phenolics: Role in the obesity treatment. LWT Food Sci. Technol. 2014;59:1205–1212. doi: 10.1016/j.lwt.2014.02.060. [DOI] [Google Scholar]
- 6.Campbell J.I.A., Mortensen A., Molgaard P. Tissue lipid lowering-effect of a traditional Nigerian anti-diabetic infusion of Rauwolfia vomitoria foilage and Citrus aurantium fruit. J. Ethnopharmacol. 2006;104:379–386. doi: 10.1016/j.jep.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Garg A., Garg S., Zaneveld L.J.D., Singla A.K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 2001;15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 8.Tripoli E., La Guardia M., Giammanco S., Di Majo D., Giammanco M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007;104:466–479. doi: 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- 9.Benavente-Garcia O., Castillo J. Update on uses and properties of Citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 10.Scora R.W. On the history and origin of Citrus. Bull. Torrey Bot. Club. 1975;102:369–375. doi: 10.2307/2484763. [DOI] [Google Scholar]
- 11.Altemimi A., Choudhary R., Watson D.G., Lightfoot D.A. Effects of ultrasonic treatments on the polyphenol and antioxidant content of spinach extracts. Ultrason. Sonochem. 2015;24:247–255. doi: 10.1016/j.ultsonch.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Dranca F., Oroian M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochem. 2016;31:637–646. doi: 10.1016/j.ultsonch.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Sun Y., Xi W., Shen Y., Qiao L., Zhong L., Ye X., Zhou Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014;145:674–680. doi: 10.1016/j.foodchem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Kumaran A., Karunakaran R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci. Technol. 2007;40:344–352. doi: 10.1016/j.lwt.2005.09.011. [DOI] [Google Scholar]
- 15.Contreras-Calderon J., Calderon-Jaimes L., Guerra-Hernandez E., Garcia-Villanova B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011;44:2047–2053. doi: 10.1016/j.foodres.2010.11.003. [DOI] [Google Scholar]
- 16.Kamiloglu S., Pasli A.A., Ozcelik B., Van Camp J., Capanoglu E. Influence of different processing and storage conditions on in vitro bioaccessibility of polyphenols in black carrot jams and marmalades. Food Chem. 2015;186:74–82. doi: 10.1016/j.foodchem.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 17.Apak R., Guclu K., Ozyurek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 18.Prior R.L., Hoang H., Gu L., Wu X., Bacchiocca M., Howard L., Hampsch-Woodill M., Huang D., Ou B., Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- 19.Rapisarda P., Fabroni S., Peterek S., Russo G., Mock H.P. Juice of New citrus hybrids (Citrus clementina Hort. ex Tan.xC. sinensis L. Osbeck) as a source of natural antioxidants. Food Chem. 2009;117:212–218. doi: 10.1016/j.foodchem.2009.03.101. [DOI] [Google Scholar]
- 20.Villano D., Fernandez-Pachon M.S., Moya M.L., Troncoso A.M., Garcia-Parrilla M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71:230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Seeram N.P., Aviram M., Zhang Y., Henning S.M., Feng L., Dreher M., Heber D. Comparison of antioxidant potency of commonly consumed Polyphenol-Rich beverages in the united states. J. Agric. Food Chem. 2008;56:1415–1422. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J.Y., Zhang Q., Zhang H.X., Ma Q., Lu J.Q., Qiao Y.J. Characterization of Polymethoxylated Flavonoids (PMFs) in the Peels of ‘Shatangju’ Mandarin (Citrus reticulata Blanco) by Online High-Performance Liquid Chromatography Coupled to Photodiode Array Detection and Electrospray Tandem Mass Spectrometry. J. Agric. Food Chem. 2012;60:9023–9034. doi: 10.1021/jf302713c. [DOI] [PubMed] [Google Scholar]
- 23.Duan L., Guo L., Liu K., Liu E.H., Li P. Characterization and classification of seven Citrus herbs by liquid chromatography-quadrupole time-of-flight mass spectrometry and genetic algorithm optimized support vector machines. J. Chromatogr. A. 2014;1339:118–127. doi: 10.1016/j.chroma.2014.02.091. [DOI] [PubMed] [Google Scholar]
- 24.Kim H.G., Kim G.S., Park S., Lee J.H., Seo O.N., Lee S.J., Kim J.H., Shim J.H., Abd El-Aty A.M., Jin J.S., et al. Flavonoid profiling in three citrus varieties native to the Republic of Korea using liquid chromatography coupled with tandem mass spectrometry: Contribution to overall antioxidant activity. Biomed. Chromatogr. 2012;26:464–470. doi: 10.1002/bmc.1688. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X.Y., Zhang W.N., Yin X.R., Su M.S., Sun C.D., Li X., Chen K.S. Phenolic Composition and Antioxidant Properties of Different Peach [Prunus persica (L.) Batsch] Cultivars in China. Int. J. Mol. Sci. 2015;16:5762–5778. doi: 10.3390/ijms16035762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Huang H., Zhang Q., Fan F., Xu C., Sun C., Li X., Chen K. Phytochemical Characterization of Chinese Bayberry (Myrica rubra Sieb. et Zucc.) of 17 Cultivars and Their Antioxidant Properties. Int. J. Mol. Sci. 2015;16:12467–12481. doi: 10.3390/ijms160612467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velioglu Y.S., Mazza G., Gao L., Oomah B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 28.Sakata K., Hara A., Hirose Y., Yamada Y., Kuno T., Katayama M., Yoshida K., Zheng Q., Murakami A., Ohigashi H., et al. Dietary supplementation of the citrus antioxidant auraptene inhibits N,N-diethylnitrosamine-induced rat hepatocarcinogenesis. Oncology. 2004;66:244–252. doi: 10.1159/000078001. [DOI] [PubMed] [Google Scholar]
- 29.Nandakumar N., Balasubramanian M.P. Hesperidin a citrus bioflavonoid modulates hepatic biotransformation enzymes and enhances intrinsic antioxidants in experimental breast cancer rats challenged with 7, 12-dimethylbenz (a) anthracene. J. Exp. Ther. Oncol. 2012;9:321–335. [PubMed] [Google Scholar]
- 30.Kamaraj S., Ramakrishnan G., Anandakumar P., Jagan S., Devaki T. Antioxidant and anticancer efficacy of hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice. Investig. New Drugs. 2009;27:214–222. doi: 10.1007/s10637-008-9159-7. [DOI] [PubMed] [Google Scholar]
- 31.Arafa el S.A., Zhu Q., Barakat B.M., Wani G., Zhao Q., El-Mahdy M.A., Wani A.A. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through downregulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 2009;69:8910–8917. doi: 10.1158/0008-5472.CAN-09-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertens-Talcott S.U., De Castro W.V., Manthey J.A., Derendorf H., Butterweck V. Polymethoxylated flavones and other phenolic derivates from citrus in their inhibitory effects on P-glycoprotein-mediated transport of talinolol in Caco-2 cells. J. Agric. Food Chem. 2007;55:2563–2568. doi: 10.1021/jf063138v. [DOI] [PubMed] [Google Scholar]
- 33.Murakami A., Koshimizu K., Ohigashi H., Kuwahara S., Kuki W., Takahashi Y., Hosotani K., Kawahara S., Matsuoka Y. Characteristic rat tissue accumulation of nobiletin, a chemopreventive polymethoxyflavonoid, in comparison with luteolin. BioFactors. 2002;16:73–82. doi: 10.1002/biof.5520160303. [DOI] [PubMed] [Google Scholar]
- 34.Lam K.H., Alex D., Lam I.K., Tsui S.K.W., Yang Z.F., Lee S.M. Y. Nobiletin, a Polymethoxylated Flavonoid from Citrus, Shows Anti-Angiogenic Activity in a Zebrafish In Vivo Model and HUVEC In Vitro Model. J. Cell. Biochem. 2011;112:3313–3321. doi: 10.1002/jcb.23257. [DOI] [PubMed] [Google Scholar]
- 35.Patil J.R., Murthy K.N.C., Jayaprakasha G.K., Chetti M.B., Patil B.S. Bioactive Compounds from Mexican Lime (Citrus aurantifolia) Juice Induce Apoptosis in Human Pancreatic Cells. J. Agric. Food Chem. 2009;57:10933–10942. doi: 10.1021/jf901718u. [DOI] [PubMed] [Google Scholar]
- 36.Lien L.M., Wang M.J., Chen R.J., Chiu H.C., Wu J.L., Shen M.Y., Chou D.S., Sheu J.R., Lin K.H., Lu W.J. Nobiletin, a Polymethoxylated Flavone, Inhibits Glioma Cell Growth and Migration via Arresting Cell Cycle and Suppressing MAPK and Akt Pathways. Phytother. Res. 2016;30:214–221. doi: 10.1002/ptr.5517. [DOI] [PubMed] [Google Scholar]
- 37.Charoensinphon N., Qiu P.J., Dong P., Zheng J.K., Ngauv P., Cao Y., Li S.M., Ho C.T., Xiao H. 5-Demethyltangeretin inhibits human nonsmall cell lung cancer cell growth by inducing G2/M cell cycle arrest and apoptosis. Mol. Nutr. Food Res. 2013;57:2103–2111. doi: 10.1002/mnfr.201300136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon J.Y., Cho S.K. Nobiletin Induces Protective Autophagy Accompanied by ER-Stress Mediated Apoptosis in Human Gastric Cancer SNU-16 Cells. Molecules. 2016;21:914. doi: 10.3390/molecules21070914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y.R., Li S.M., Ho C.T., Chang Y.H., Tan K.T., Chung T.W., Wang B.Y., Chen Y.K., Lin C.C. Tangeretin derivative, 5-acetyloxy-6,7,8,4-tetramethoxyflavone induces G2/M arrest, apoptosis and autophagy in human non-small cell lung cancer cells in vitro and in vivo. Cancer Biol. Ther. 2016;17:48–64. doi: 10.1080/15384047.2015.1108491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J.Y., Sun C.D., Zhang L.L., Dai X.A., Xu C.J., Chen K.S. Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon. Sci. Hortic. 2010;126:229–235. doi: 10.1016/j.scienta.2010.07.019. [DOI] [Google Scholar]
- 41.Leng F., Lin Q., Wu D., Wang S.P., Wang D.L., Sun C.D. Comparative Transcriptomic Analysis of Grape Berry in Response to Root Restriction during Developmental Stages. Molecules. 2016;21:1431. doi: 10.3390/molecules21111431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun C.D., Zheng Y.X., Chen Q.J., Tang X.L., Jiang M., Zhang J.K., Li X., Chen K.S. Purification and anti-tumour activity of cyanidin-3-O-glucoside from Chinese bayberry fruit. Food Chem. 2012;131:1287–1294. doi: 10.1016/j.foodchem.2011.09.121. [DOI] [Google Scholar]
- 43.Zhang W.S., Li X., Zheng J.T., Wang G.Y., Sun C.D., Ferguson I.B., Chen K.S. Bioactive components and antioxidant capacity of Chinese bayberry (Myrica rubra Sieb. and Zucc.) fruit in relation to fruit maturity and postharvest storage. Eur. Food Res. Technol. 2008;227:1091–1097. doi: 10.1007/s00217-008-0824-z. [DOI] [Google Scholar]
- 44.Roy M.K., Koide M., Rao T.P., Okubo T., Ogasawara Y., Juneja L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010;61:109–124. doi: 10.3109/09637480903292601. [DOI] [PubMed] [Google Scholar]
- 45.Umezu-Goto M., Kishi Y., Taira A., Hama K., Dohmae N., Takio K., Yamori T., Mills G.B., Inoue K., Aoki J., et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.