Abstract
The genus Alnus (Betulaceae) is comprised of more than 40 species. Many species of this genus have a long history of use in folk medicines. Phytochemical investigations have revealed the presence of diarylheptanoids, polyphenols, flavonoids, terpenoids, steroids and other compounds. Diarylheptanoids, natural products with a 1,7-diphenylheptane structural skeleton, are the dominant constituents in the genus, whose anticancer effect has been brought into focus. Pure compounds and crude extracts from the genus exhibit a wide spectrum of pharmacological activities both in vitro and in vivo. This paper compiles 273 naturally occurring compounds from the genus Alnus along with their structures and pharmacological activities, as reported in 138 references.
Keywords: chemical constituents, biological activities, Alnus, diarylheptanoids
1. Introduction
Alnus is a genus in the family Betulaceae, which comprises more than 40 species mainly distributed in Asia, Africa, Europe and North America. A total of seven species and one variant are distributed in the south and north of China [1]. The plants in the genus are commonly used as traditional medicines [2]. Alnus hirsuta Turcz. indigenously distributed in Korea, China, Japan, and Russia, has been used in Oriental medicine as a remedy for fever, haemorrhages, burn injuries, diarrhea, and alcoholism [3]. It was reported that many bioactive natural components including diarylheptanoids, polyphenols, flavonoids, terpenoids, and steroids, were isolated from the genus [4,5,6,7,8]. Diarylheptanoids comprise a class of natural products formed from 1,7-diphenylheptane, which appears in linear and cyclic forms [9]. They have been regarded as the primary bioactive compounds of Alnus and have drawn attention due to their physiological activities, especially their anticancer and antioxidative activities [10,11].
Alnus japonica Steudel., Alnus hirsuta Turcz. and Alnus glutinosa (L.) Gaertn. have a long-standing medical history and extensive research on their irreplaceable functions has been reported. A. japonica, a famous medical herb in China, Japan and Korea as well as a functional food consumed in health drinks, contains abundant diarylheptanoid derivatives, in addition to methylated and acylated diarylheptanoids and diarylheptanoid glycosides [12,13,14]. It has been known to exert antioxidative, anti-inflammatory, anticancer and hepatoprotective effects and its antioxidative properties are presumed to contribute to its hepatoprotective activity in certain situations [15]. Diarylheptanoids with strong antioxidative activity isolated from A. japonica and A. hirsuta showed significant hepatoprotective effects on t-butyl hydroperoxide (t-BHP)-induced toxicity in primary rat hepatocytes and a human hepatoma cell line (HepG2), respectively [3,16]. Alnus glutinosa (L.) Gaertn., used on the market as a food supplement to help reduce the risk of different chronic dermatological conditions, has increasingly become a research hot-spot for its potent chemo-protective, antioxidant and antimicrobial effects [17,18,19]. Diarylheptanoids from the bark of A. glutinosa may serve as protectors of normal cells during chemotherapy without significantly diminishing the effect of the applied chemotherapeutic agents [4,20].
Sati et al. have summarized 192 chemical constituents and biological activities of genus Alnus [2]. Further studies on the Alnus genus were carried out in recent years, and many chemical components from this genus have been isolated. Therefore, a new comprehensive and systematic review of Alnus genus is much needed. Most of the papers not only covered new chemical components, but also refered to their pharmacology activities, structure-activity relationships and functional mechanisms, especially the anticancer meshanism of hirsutenone [11,21,22,23]. Hirsutenone can sensitize resistant ovarian cancer cells to cisplatin, so that co-treatment with it may have the potential to overcome chemoresistance [24]. Futhermore, an induction of oxidative stress and topo II-mediated DNA damage may play a role in hirsutanone-induced cancer cell death [21]. In this review, we mainly summarized 273 chemical constituents and biological activities of the genus Alnus, based on 138 cited references. It is hoped that the information presented in this paper will be useful for further research and the application of this genus.
2. Chemical Constituents
So far, 273 chemical constituents have been reported from the genus Alnus. These compounds can be classified into five groups: diarylheptanoids (compounds 1–99), polyphenols (compounds 100–137), flavonoids (compounds 138–200), terpenoids and steroids (compounds 201–254), and others (compounds 255–273). Their chemical structures are shown in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15 and Table 16 and Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13, and their names and corresponding plant sources are compiled in Table 17. The occurrence of diarylheptanoids appears to be a characteristic feature of this genus.
Table 1.
Structures of compounds 1–28.

| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 1 | H | H | H | OH (R) | OH (S) | H | H |
| 2 | H | H | H | OH (R) | OH (R) | H | H |
| 3 | H | H | OH (R) | OH (R) | OH (S) | H | H |
| 4 | OH | H | H | OH (R) | OH (R) | H | OH |
| 5 | OH | H | H | OH (R) | H | H | OH |
| 6 | OH | H | H | OH (R) | H | OH | OH |
| 7 | OH | OH | H | OH (R) | H | OH | OH |
| 8 | OH | OH | H | OH (R) | H | H | OH |
| 9 | OH | OH | H | OH (R) | O-xylp (S) | OH | OH |
| 10 | OH | H | H | OH (R) | O-apif(1→6)glcp | H | OH |
| 11 | OH | H | H | O-xylp (R) | H | H | OH |
| 12 | OH | OH | H | O-xylp (R) | H | OH | OH |
| 13 | OH | OH | H | O-xylp (R) | H | H | OH |
| 14 | OH | OH | H | O-glcp (R) | H | OH | OH |
| 15 | OH | H | H | O-glcp (R) | H | OH | OH |
| 16 | OH | OH | H | O-glcp (R) | H | H | OH |
| 17 | OH | H | H | O-glcp (R) | OH | H | OH |
| 18 | OH | H | H | O-glcp (R) | H | H | OH |
| 19 | OH | H | H | O-apif(1→6)glcp (R) | H | H | OH |
| 20 | OH | H | H | O-araf(1→6)glcp (S) | H | H | OH |
| 21 | OH | H | H | O-araf(1→6)glcp (R) | H | H | OH |
| 22 | OH | OH | H | O-glcp(1→3)xylp (R) | H | OH | OH |
| 23 | OH | OH | H | O-apip(1→6)glcp (R) | H | H | OH |
| 24 | OH | OH | H | O-rhap(1→6)glcp (R) | H | H | OH |
| 25 | OH | H | H | O-glcp(1→3)xylp (R) | H | H | OH |
| 26 | OH | OH | H | O-glcp-(E)-DMC (R) | H | OH | OH |
| 27 | OH | OH | H | O-glcp-(Z)-DMC (R) | H | OH | OH |
| 28 | OH | OH | H | O-glcp-(E)-TMC (R) | H | OH | OH |
Table 2.
Structures of compounds 29–71.

| Compound | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| 29 | OH | H | H | H | H | OH |
| 30 | H | H | OH (S) | OH (S) | H | H |
| 31 | H | H | OH (R) | OH (S) | H | H |
| 32 | H | H | H | OH (S) | H | H |
| 33 | OH | OH | H | OH (S) | OH | OH |
| 34 | OH | OH | H | OH (S) | H | OH |
| 35 | OH | H | H | OH (S) | OH | OH |
| 36 | OH | H | H | OH (S) | H | OH |
| 37 | OH | OH | H | OH (R) | OH | OH |
| 38 | OH | OH | H | OCH3 (S) | OH | OH |
| 39 | OH | H | H | OCH3 (S) | OH | OH |
| 40 | OH | H | H | OCH3 (S) | H | OH |
| 41 | OH | OH | H | OCH3 (R) | OH | OH |
| 42 | OH | OH | H | O-nBu (S) | OH | OH |
| 43 | OH | OH | H | O-nBu (S) | OH | OH, △1(E) |
| 44 | OH | H | H | O-nBu (S) | H | OH |
| 45 | OH | H | H | O-xyl (S) | OH | OH |
| 46 | OH | OH | H | O-xyl (S) | H | OH |
| 47 | OH | H | H | O-xyl (S) | H | OH |
| 48 | OH | OH | H | O-xyl (S) | OH | OH |
| 49 | OH | OH | H | O-glc (S) | OH | OH |
| 50 | OH | H | H | O-glc (S) | H | OH |
| 51 | OH | H | H | O-glc (S) | OH | OH |
| 52 | OH | OH | H | O-glc (S) | H | OH |
| 53 | OH | H | H | O-apif(1→6)glcp (S) | H | OH |
| 54 | OH | H | H | O-galloyl-glcp (S) | H | OH |
| 55 | OH | OH | H | O-xylp-p-coumaroyl | OH | OH |
| 56 | OH | OH | H | O-xylp-feruloyl (S) | OH | OH |
| 57 | OH | OH | H | O-galloyl-glcp (S) | OH | OH |
| 58 | OH | OH | H | O-xylp-benzoyl (S) | OH | OH |
| 59 | OH | OH | H | O-xylp-cinnamoyl (S) | OH | OH |
| 60 | OH | OH | H | O-glcp-benzoyl (S) | OH | OH |
| 61 | OH | OH | H | O-glcp-vanilloyl (S) | OH | OH |
| 62 | OH | H | H | O-glcp-coumaroyl (S) | H | OH |
| 63 | OH | H | H | O-glcp-(E)-DMC (S) | H | OH |
| 64 | OH | H | H | O-glcp-(E)-DMC (S) | OH | OH |
| 65 | OH | H | H | O-glcp-(Z)-DMC (S) | H | OH |
| 66 | OH | OH | H | O-glcp-coumaroyl (S) | OH | OH |
| 67 | OH | OH | H | O-glcp-(Z)-DMC (S) | OH | OH |
| 68 | OH | OH | H | O-glcp-(E)-TMC (S) | OH | OH |
| 69 | OH | OH | H | O-glcp-(E)-DMC (S) | OH | OH |
| 70 | OH | OH | H | O-xylp-2-methyl-butanoyl (S) | OH | OH |
| 71 | OH | OH | H | O-R* (S) | OH | OH |
Table 3.
Structures of compounds 72–77.
| Compound | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 72 | H | H | H | H |
| 73 | OH | OH | OH | OH |
| 74 | OH | H | H | OH |
| 75 | OH | H | OCH3 | OH |
| 76 | OH | OH | H | OH |
| 77 | OH | H | OH | OH |
Table 4.
Structures of compounds 100–111.
| Compound | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 100 | OH | H | H | G | G |
| 101 | OG | H | H | G | H |
| 102 | OG | H | H | G | H |
| 103 | OG | G | H | H | H |
| 104 | A | H | H | H | H |
| 105 | OH | H | G | (S) HHDP | |
| 106 | OH | G | G | (S) HHDP | |
| 107 | OG | H | H | (S) HHDP | |
| 108 | OH | (S) HHDP | H | H | |
| 109 | OH | (S) HHDP | (S) HHDP | ||
| 110 | β-OG | (S) HHDP | (S) HHDP | ||
| 111 | OB | (S) HHDP | (S) HHDP | ||
Table 5.
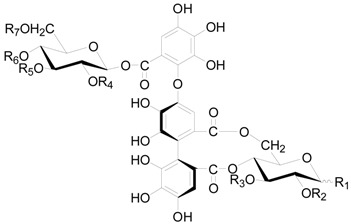
Structures of compounds 121–123.
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 121 | OH | (S) HHDP | G | G | (S) HHDP | ||
| 122 | β-OG | (S) HHDP | G | G | (S) HHDP | ||
| 123 | OH | (S) HHDP | H | H | (S) HHDP | ||
Table 6.
Structures of compounds 124.

| Compound | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 124 | (S) HHDP | (S) HHDP | ||
Table 7.
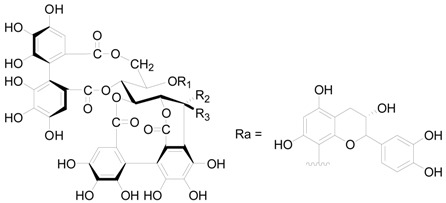
Structures of compounds 125–128.
| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 125 | H | H | OH |
| 126 | G | H | OH |
| 127 | G | OH | H |
| 128 | G | Ra | H |
Table 8.
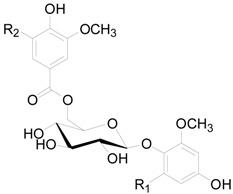
Structures of compounds 129–132.
| Compound | R1 | R2 |
|---|---|---|
| 129 | OCH3 | OCH3 |
| 130 | OCH3 | H |
| 131 | H | OCH3 |
| 132 | H | H |
Table 9.
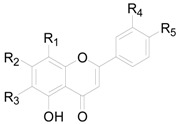
Structures of compounds 138–153.
| Compound | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 138 | H | OH | H | H | H |
| 149 | H | OH | H | H | OH |
| 140 | H | OH | H | OH | OCH3 |
| 141 | H | OH | H | H | OCH3 |
| 142 | H | OCH3 | H | H | H |
| 143 | H | OCH3 | H | H | OH |
| 144 | H | OCH3 | H | OH | OCH3 |
| 145 | H | OCH3 | H | OCH3 | OH |
| 146 | OCH3 | OCH3 | OCH3 | H | H |
| 147 | H | OCH3 | OCH3 | H | OCH3 |
| 148 | H | O-glcp-glcp | H | H | OH |
| 149 | H | OH | H | H | O-glcp-glcp |
| 150 | H | OCH3 | H | H | OCH3 |
| 151 | H | OH | OCH3 | H | OCH3 |
| 152 | H | OH | H | OH | OH |
| 153 | H | O-glc | H | OH | OH |
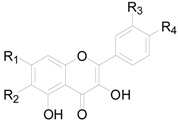
Table 10.
Structures of compounds 154–163.
| Compound | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 154 | OH | H | H | H |
| 155 | OH | H | H | OH |
| 156 | OH | H | H | OCH3 |
| 157 | OH | H | OH | OH |
| 158 | OH | H | OCH3 | OH |
| 159 | OH | OCH3 | H | H |
| 160 | OH | OCH3 | H | OCH3 |
| 161 | OCH3 | H | H | H |
| 162 | OCH3 | H | OH | OH |
| 163 | OCH3 | H | OCH3 | OCH3 |
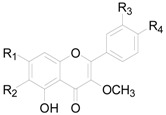
Table 11.
Structures of compounds 164–172.
| Compound | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 164 | OH | H | H | H |
| 165 | OH | H | OCH3 | OH |
| 166 | OH | OCH3 | H | OH |
| 167 | OH | OCH3 | H | OCH3 |
| 168 | OH | OCH3 | OH | OCH3 |
| 169 | OCH3 | H | H | OH |
| 170 | OCH3 | H | OH | OH |
| 171 | OCH3 | H | OH | OCH3 |
| 172 | OCH3 | OCH3 | H | H |
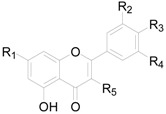
Table 12.
Structures of compounds 173–188.
| Compound | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 173 | OH | OH | OH | H | O-araf |
| 174 | OH | OH | OH | H | O-glcp |
| 175 | OH | H | OH | OH | O-glcp |
| 176 | OH | OH | OH | H | O-rhap |
| 177 | OH | OH | OH | H | O-glucuronide |
| 178 | OH | OH | OH | H | O-rhap(1→6)glcp |
| 179 | OH | OH | OH | H | O-cel |
| 180 | OH | OH | OH | H | O-mal |
| 181 | OH | H | OH | H | O-rha |
| 182 | OH | OH | OH | H | O-galf |
| 183 | OH | H | OH | H | O-rha-rha |
| 184 | OH | OH | OH | H | O-sop |
| 185 | OH | OH | OH | OH | O-galp |
| 186 | OCH3 | OH | OH | H | O-glcp-glcp |
| 187 | OH | OCH3 | OH | H | O-glc |
| 188 | O-rha | OCH3 | OH | H | O-glc |
Table 13.
Structures of compounds 189–195.

| Compound | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 189 | H | OH | H | OH | H |
| 190 | H | OH | H | OH | OH |
| 191 | H | OH | H | OCH3 | H |
| 192 | H | OH | CH3 | OH | H |
| 193 | H | OCH3 | H | OH | H |
| 194 | H | OH | OH | OH | OH |
| 195 | CH3 | OH | CH3 | OH | OH |
Table 14.
Structures of compounds 216–223.

| Compound | R |
|---|---|
| 216 | H |
| 217 | OH |
| 218 | O-xylp |
| 219 | O-glcp |
| 220 | O-arap |
| 221 | O-(2′-OAc)-araf |
| 222 | O-(2′-OAc)-xylp |
| 223 | O-(2′-OAc)-glcp |
Table 15.
Structures of compounds 236–245.

| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 236 | H | OH | CH3 |
| 237 | H | OH | CH2OH |
| 238 | H | O | CH2OH |
| 239 | H | OH | COOH |
| 240 | H | OH | OH |
| 241 | H | OH | CHO |
| 242 | H | OCOCH3 | CHO |
| 243 | H | OCOCH3 | CH3 |
| 244 | H | O | CH3 |
| 245 | OH | O-caffeoyl | CH2OH |
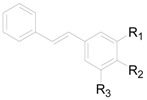
Table 16.
Structures of compounds 255–259.
| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 255 | OH | H | OH |
| 256 | OH | H | OCH3 |
| 257 | OCH3 | OH | OH |
| 258 | H | H | H |
| 259 | OCH3 | H | OCH3 |
Figure 1.
Structures of compounds 78–89.
Figure 2.
Structures of compounds 90–93.
Figure 3.
Structures of compounds 94–99.
Figure 4.
Structures of compounds 112–117.
Figure 5.
Structures of compounds 118 and 119.
Figure 6.
Structures of compounds 120.
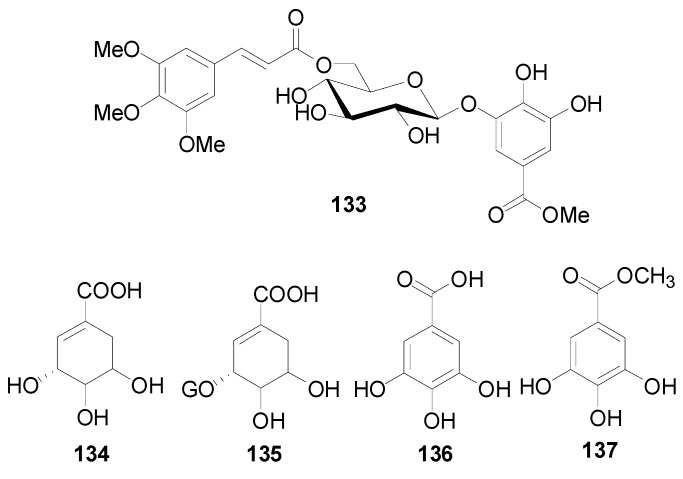
Figure 7.
Structures of compounds 133–137.
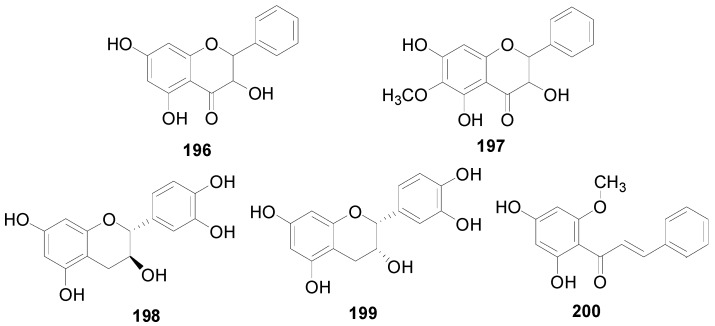
Figure 8.
Structures of compounds 196–200.
Figure 9.
Structures of compounds 201–215.
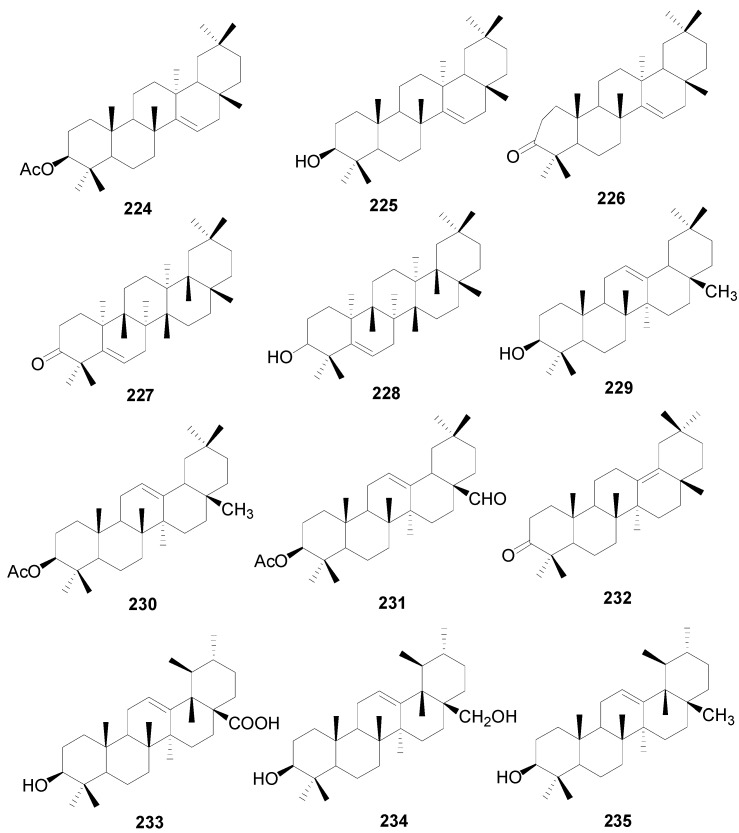
Figure 10.
Structures of compounds 224–235.
Figure 11.
Structures of compounds 246–248.
Figure 12.
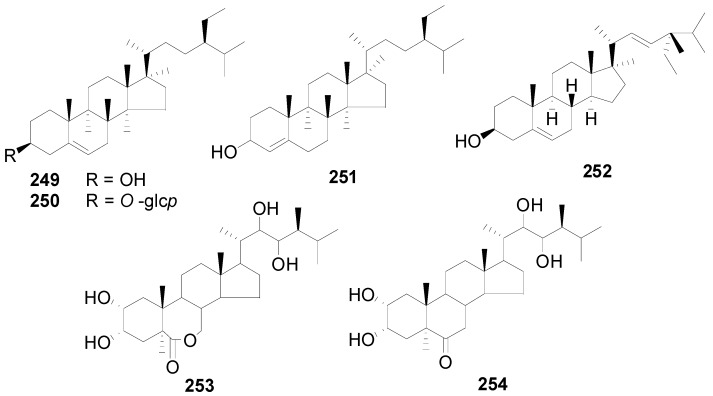
Structures of compounds 249–254.
Figure 13.
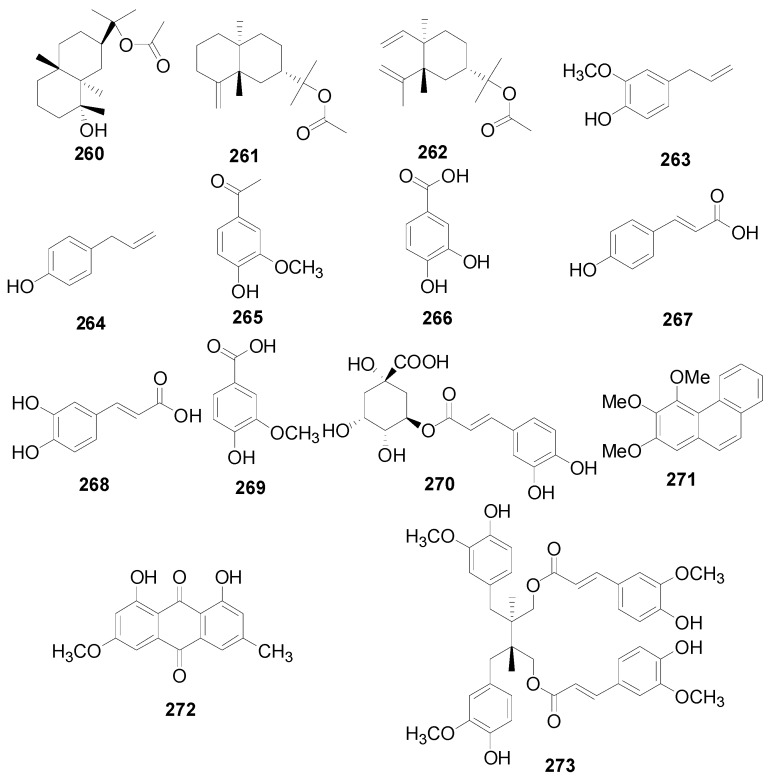
Structures of compounds 260–273.
Table 17.
Chemical Constituents from the Genus Alnus.
| No. | Compound Class and Name | Source | Reference |
|---|---|---|---|
| Diarylheptanoids | |||
| 1 | yashabushidiol A | A. sieboldiana, Alnus fruticosa Rupr., Alnus mandshurica (Callier) Hand.-Mazz | [2,67,68] |
| 2 | yashabushidiol B | A. sieboldiana, A. fruticosa, A. mandshurica | [2,67,68] |
| 3 | yashabushitriol | A. sieboldiana | [2,68] |
| 4 | (+)-hannokinol | A. hirsuta, A. japonica | [35,69] |
| 5 | (−)-centrolobol | Alnus formosana Burk., A. nepalensis, A. acuminata, A. hirsuta | [31,57,70,71] |
| 6 | (±)-7-(3,4-dihydroxyphenyl)-1-(4-hydroxyphenyl)-3-heptanol | A. formosana | [70] |
| 7 | rubranol | A. hirsuta, A. japonica, A. rubra, A. formosana | [2,23,69,70,72] |
| 8 | 1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)-3 (R)-heptanol | A. formosana | [70] |
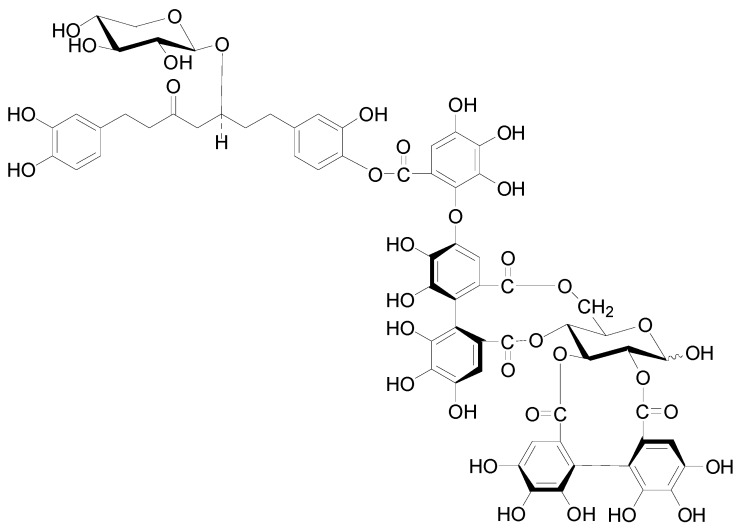
| 9 | (3R,5S)-1,7-bis-(3,4-dihydroxyphenyl)-3-hydroxylheptane-5-O-β-d-xylopyranoside | A. japonica, A. glutinosa, A. incana | [11,73,74] |
| 10 | 5-hydroxy-1,7-bis(4-hydroxyphenyl)heptan-3-yl β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | A. viridis | [42] |
| 11 | 1,7-di(4-hydroxyphenyl)-3(R)-β-d-xylosyloxyheptane. | A. formosana | [70] |
| 12 | rubranoside B | A. hirsuta, A. rubra, A. japonica, A. formosana, A. glutinosa | [2,4,69,70] |
| 13 | alnuside C | A. japonica | [75] |
| 14 | rubranoside A | A. hirsuta, A. japonica, A. rubra, A. incana, A. formosana, A. glutinosa | [2,3,4,70,73,74] |
| 15 | 7-(3,4-dihydroxyphenyl)-1-(4-hydroxyphenyl)-3(R)-β-d-glucosyloxyheptane | A. formosana | [70] |
| 16 | 1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)-3(R)-β-d-glucosyloxyheptane | A. formosana, A. japonica | [70,75] |
| 17 | (1S,3R)-3-hydroxy-5-(4-hydroxyphenyl)-1-[2-(4-hydroxyphenyl)ethyl]pentyl β-d-glucopyranoside | A. viridis | [76] |
| 18 | aceroside VII | A. hirsuta, A. formosana, A. glutinosa, A. viridis | [2,3,4,42,70] |
| 19 | aceroside VIII | A. hirsuta, A. viridis | [42,69] |
| 20 | (1S)-5-(4-hydroxyphenyl)-1-[2-(4-hydroxyphenyl)ethyl]pentyl 6-O-α-L-arabinofuranosyl-β-d-glucopyranoside | A. viridis | [76] |
| 21 | (3R)-1,7-bis(4-hydroxyphenyl)heptan-3-yl α-L-arabinofuranosyl-(1→6)-β-d-glucopyranoside | A. viridis | [42] |
| 22 | rubranoside C | A. japonica, A. hirsuta, A. rubra | [2,3,73] |
| 23 | rubranoside D | A. japonica. A. rubra | [2,73] |
| 24 | alnuside D | A. japonica | [75] |
| 25 | (3R)-1,7-bis-(4-dihydroxyphenyl)-3-heptanol-3-O-β-d-glucopyranosyl(1→3)-β-d-xylopyranoside | A. hirsuta | [2,3] |
| 26 | 3(R)-1,7-di(3,4-dihydroxyphenyl)-3-O-β-d-[6-(E-3,4-dimethoxycinnamoyl glucopyranosyl)] heptane | A. glutinosa | [11] |
| 27 | 3(R)-1,7-di(3,4-dihydroxyphenyl)-5-O-β-d-[6-(Z-3,4-dimethoxycinnamoyl glucopyranosyl)] heptane | A. glutinosa | [11] |
| 28 | 3(R)-1,7-di(3,4-dihydroxyphenyl)-5-O-β-d-[6-(E-3,4,5-trimethoxycinnamoyl glucopyranosyl)] heptane | A. glutinosa | [11] |
| 29 | 1,7-bis-(p-hydroxyphenyl)-3-heptanone | A. nepalensis | [11] |
| 30 | yashabushiketodiol B | A. sieboldiana | [2,68] |
| 31 | yashabushiketodiol A | A. sieboldiana | [2,68] |
| 32 | dihydroyashabushiketol | A. firma, A. sieboldiana, A. maximowiczii | [2,54] |
| 33 | hirsutanonol | A. hirsuta, A. japonica, A. rubra, A. glutinosa, A. formosana, A. acuminata, A. serrulatoides | [11,13,57,70,77,78,79] |
| 34 | 5(S)-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)-5-hydroxyheptane-3-one | A. japonica | [80] |
| 35 | 5(S)-1-(4-dihydroxyphenyl )-7-(3,4-dihydroxyphenyl)-5-hydroxyheptane-3-one | A. japonica, A. nepalensis, A. hirsuta | [32,69,80] |
| 36 | hannokinin | A. japonica, A. nepalensis, A. hirsuta, A. firma | [16,32,41,69] |
| 37 | epihirsutanonol | A. japonica | [80] |
| 38 | 5(S)-O-methylhirsutanonol | A. japonica, A. glutinosa, A. formosana, A. nepalensis | [11,32,70,81] |
| 39 | alunheptanoid A | A. japonica | [63] |
| 40 | 5(S)-O-methylplatyphyllonol | A. japonica | [63] |
| 41 | 5(R)-O-methylhirsutanonol | A. japonica | [63] |
| 42 | 5-O-butylhirusutanonol | A. formosana | [70] |
| 43 | 5(S)-butyloxy-1,7-di(3,4-dihydroxyphenyl)-1(E)-hepten-3-one | A. formosana | [70] |
| 44 | 5(S)-butyloxy-1,7-di(4-hydroxyphenyl)-3-heptanone | A. formosana | [70] |
| 45 | alnuside A | A. japonica, A. serrulatoides, A. hirsuta, A. formosana, A. glutinosa, A. incana | [2,11,28,70,79,82] |
| 46 | alnuside B | A. japonica, A. serrulatoides, A. hirsuta, A. formosana, A. glutinosa, A. incana | [2,11,28,70,79,82] |
| 47 | platyphyllonol-5-O-β-d-xylopyranoside | A. rubra, A. hirsuta, A. japonica, A. glutinosa | [11,69,83,84] |
| 48 | oregonin | A. japonica, A. hirsuta, A. rubra, A. nepalensis, A. glutinosa, A. firma, A. formosana, A. incana, A. serrulatoides, A. pendula, A. tinctoria Sarg. | [2,4,41,70,74,79,85,86,87,88,89] |
| 49 | 5(S)-hirsutanonol-5-O-β-d-glucopyranoside | A. hirsuta, A. japonica, A. rubra, A. incana, A. formosana, A. serrulatoides, A. acuminata, A. nepalensis, A. glutinosa | [2,3,11,57,70,74,79,84,86,88] |
| 50 | platyphylloside | A. japonica, A. hirsuta, A. glutinosa, A. formosana, A. pendula, A. firma, A. incana, A. nepalensis, A. rubra, A. viridis | [2,3,11,24,41,73,84,87,88,90,91] |
| 51 | (5S)-1-(4-hydroxyphenyl)-7-(3,4-dihydroxy-phenyl)-5-O-β-d-glucopyranosyl-heptan-3-one | A. glutinosa | [4] |
| 52 | 1-(3′,4′-dihydroxypheny1)-7-(4′′-hydroxypheny1)-5-O-β-d-glucopyranosylheptan-3-one | A. rubra | [72] |
| 53 | (5S)-5-hydroxy-1,7-bis-(4-hydroxyphenyl)-3-heptanone-5-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | A. hirsuta, A. viridis | [42,69] |
| 54 | (3S)-1,7-bis(4-hydroxyphenyl)-5-oxoheptan-3-yl 6-O-galloyl-β-d-glucopyranoside | A. viridis | [42] |
| 55 | oregonoyl A | A. japonica, A. formosana | [2,70,83] |
| 56 | oregonoyl B | A. japonica | [2,83] |
| 57 | hirsutanonol 5-O-(6-O-galloyl)-β-d-glucopyranoside | A. japonica | [2,92] |
| 58 | 2′′′-O-benzoyl-oregonin | A. formosana | [70] |
| 59 | 2′′′-O-cinnamoyl-oregonin | A. formosana | [70] |
| 60 | oregonoside A | A. rubra | [78] |
| 61 | oregonoside B | A. rubra | [78] |
| 62 | 5(S)-1,7-di(4-hydroxyphenyl)-5-O-β-d-[6-(E-p-coumaroyl glucopyranosyl)]heptane-3-one | A. glutinosa | [11] |
| 63 | 5(S)-1,7-di(4-hydroxyphenyl)-5-O-β-d-[6-(E-3,4-dimethoxycinnamoyl glucopyranosyl)]heptane-3-one | A. glutinosa | [11] |
| 64 | 5(S)-1-(4-hydroxyphenyl)-7-(3,4-dihydroxyphenyl)-5-O-β-d-[6-(E-3,4-dimethoxycinnamoyl glucopyranosyl)]heptane-3-one | A. glutinosa | [11] |
| 65 | 5(S)-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)-5-O-β-d-[6-(Z-3,4-dimethoxycinnamoyl glucopyranosyl)]heptane-3-one | A. glutinosa | [11] |
| 66 | 5(S)-1,7-di(3,4-dihydroxyphenyl)-5-O-β-d-[6-(E-p-coumaroyl glucopyranosyl)]heptane-3-one | A. glutinosa | [11] |
| 67 | 5(S)-1,7-di(3,4-dihydroxyphenyl)-5-O-β-d-[6-(Z-3,4-dimethoxycinnamoyl glucopyranosyl)] heptane-3-one | A. glutinosa | [11] |
| 68 | 5(S)-1,7-di(3,4-dihydroxyphenyl)-5-O-β-d-[6-(E-3,4,5-trimethoxycinnamoyl glucopyranosyl)] heptane-3-one | A. glutinosa | [11] |
| 69 | 5(S)-1,7-di(3,4-dihydroxyphenyl)-5-O-β-d-[6-(E-3,4-dimethoxycinnamoyl glucopyranosyl)] heptane-3-one | A. glutinosa | [11] |
| 70 | 2′′′-O-(2-methylbutanoyl)-oregonin | A. formosana | [70] |
| 71 | 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxy-3-heptanone-5-O-[2-(2-methylbutenoyl)]-β-d-xylopyranoside | A. japonica | [2,28] |
| 72 | 1,7-diphenylhept-3-en-5-one | A. maximowiczii | [2] |
| 73 | hirsutenone | A. japonica, A. hirsuta, A. pendula, A. nepalensis, A. glutinosa, A. firma, A. formosana, A. acuminata | [2,8,11,28,41,57,69,70,87] |
| 74 | platyphyllenone | A. hirsuta, A. japonica, A. formosana, A. rubra A. acuminata, A. viridis | [2,3,16,42,57,70,93] |
| 75 | 1-(4-hydroxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)-4-hepten-3-one | A. hirsuta | [2,30] |
| 76 | 1-(3′,4′-dihydroxyphenyl)-7-(4′′-hydroxyphenyl)-4-hepten-3-one | A. japonica, A. rubra | [2,16,93] |
| 77 | alusenone | A. japonica | [2,13] |
| 78 | nitidone A | Alnus nitida Endl. | [94] |
| 79 | nitidone B | Alnus nitida Endl. | [94] |
| 80 | yashabushiketol | A. firma, A. sieboldiana, A. hirsuta | [2,71,95,96] |
| 81 | (5S)-hydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)-hepta-1E-en-3-one | A. hirsuta | [69] |
| 82 | alnustone | A. pendula, A. japonica | [2,35] |
| 83 | 1,7-bis-(3,4-dihydroxyphenyl)-hepta-4E,6E-dien-3-one | A. hirsuta | [69] |
| 84 | 1,4-hepta-dien-3-one-1,7-bis(3,4-dihydroxyphenyl)-(1E,4E) | A. hirsuta | [69] |
| 85 | 1,7-diphenylheptane-3,5-dione | A. maximowiczii | [2,53] |
| 86 | 1,7-diphenylhept-1-ene-3,5-dione | A. maximowiczii | [2,53] |
| 87 | rhoiptelol B | A. hirsuta | [2,30] |
| 88 | 1,5-epoxy-1-(3′,4′-dihydroxyphenyl)-7-(4′′-hydroxyphenyl)heptane | A. nepalensis | [31] |
| 89 | alnus dimer | A. nepalensis | [32] |
| 90 | trans-rhoiptelol | A. hirsuta | [2,9,30] |
| 91 | myricatomentogenin | A. hirsuta | [2,9,30] |
| 92 | acerogenin L | A. japonica | [2,34] |
| 93 | garugamblin-3 | A. japonica | [2,34] |
| 94 | alnusoxide | A. japonica | [35] |
| 95 | alnusonol | A. japonica, A. hirsuta, A. sieboldiana | [35,71,97] |
| 96 | alnusdiol | A. japonica, A. hirsuta | [35,71] |
| 97 | trideoxysasadanin-8-ene | A. hirsuta | [71] |
| 98 | alnusone | A. japonica, A. hirsuta, A. sieboldiana | [35,71,90,97] |
| 99 | 3,17-dihydroxy-tricyclo[12.3.1.1 2,6]-nonadeca-1(18),2,4,6(19),14, 16-hexaen-9,11-dione | A. sieboldiana | [97] |
| Polyphenols | |||
| 100 | 4,6-di-O-galloyl-d-glucose | A. japonica | [2,5] |
| 101 | 1,4-di-O-galloyl-β-d-glucose | A. japonica | [2,5] |
| 102 | 1,4,6-tri-O-galloyl-β-d-glucose | A. hirsuta | [2,36] |
| 103 | 1,2,6-tri-O-galloyl-β-d-glucose | A. hirsuta, A. sieboldiana | [2,36,37] |
| 104 | gentisic acid 5-O-β-d-(6′-O-galloyl) glucopyranoside | A. hirsuta | [2,36] |
| 105 | gemin D | A. japonica | [2,5] |
| 106 | tellimagrandin I | A. hirsuta, A. sieboldiana | [2,36,37] |
| 107 | strictinin | A. japonica, A. sieboldiana | [5,37] |
| 108 | 2,3-O-(S)-hexahydroxydiphenoyl-d-glucose | A. japonica, A. sieboldiana | [2,5] |
| 109 | pedunculagin | A. japonica, A. sieboldiana, A. hirsuta, A. glutinosa | [2,5,36,38,39] |
| 110 | 1(β)-O-galloylpendunculagin | A. japonica, A. sieboldiana | [2,37] |
| 111 | glutinoin | A. glutinosa | [38] |
| 112 | flosin A | A. japonica | [2,5] |
| 113 | 4,6-(S)-valoneoyl-d-glucose | A. japonica | [2,5] |
| 114 | praecoxin A | A. japonica, A. hirsuta | [2,5,36] |
| 115 | praecoxin D | A. glutinosa | [38] |
| 116 | alnusnins A | A. sieboldiana | [2,37] |
| 117 | alnusnins B | A. sieboldiana | [2,37] |
| 118 | alnusiin | A. sieboldiana | [2,39] |
| 119 | tergallin | A. sieboldiana | [37] |
| 120 | hirsunin | A. hirsuta | [2,36] |
| 121 | 1-desgalloylrugosin F | A. hirsuta | [2,36] |
| 122 | rugosin F | A. hirsuta | [2,36] |
| 123 | alnusjaponins A | A. japonica | [2,5] |
| 124 | alnusjaponins B | A. japonica | [2,5] |
| 125 | casuariin | A. sieboldiana | [2,39] |
| 126 | casuarinin | A. japonica, A. sieboldiana | [2,5,39] |
| 127 | stachyurin | A. japonica, A. sieboldiana | [2,5,37] |
| 128 | stenophyllanin A | A. sieboldiana | [2,37] |
| 129 | 4-hydroxy-2,6-dimethoxyphenyl-6′-O-syringoyl-β-d-glucopyranoside | A. firma | [2,41] |
| 130 | 4-hydroxy-2,6-dimethoxyphenyl-6′-O-vanilloyl-β-d-glucopyranoside | A. firma | [2,41] |
| 131 | 4-hydroxy-2-methoxyphenyl-6′-O-syringoyl-β-d-glucopyranoside | A. firma | [41] |
| 132 | 6′-O-vanilloylisotachioside | A. firma | [41] |
| 133 | methyl 3,4-dihydroxy-5-{[6-O-(3,4,5-trimethoxycinnamoyl) -β-d-glucopyranosyl]oxy}benzoate |
A. viridis | [42] |
| 134 | shikimic acid | A. japonica | [98] |
| 135 | 5-O-galloyl-(−)-shikimic acid | A. japonica | [2,5] |
| 136 | gallic acid | A. nepalensis, A. nitida | [8,99] |
| 137 | methyl gallate | A. sieboldiana | [100] |
| Flavonoids | |||
| 138 | chrysin | A. sieboldiana | [2,49] |
| 139 | apigenin | A. rubra, A. sieboldiana, A. rugosa | [2,44,46,48] |
| 140 | diosmetin | A. rugosa | [48] |
| 141 | acacetin | A. japonica, A. rubra, Alnus koehnei Call. | [2,6,44] |
| 142 | tectochrysin | A. sieboldiana | [2,49] |
| 143 | genkwanin | A. sinuata, A. glutinosa | [2,6,47] |
| 144 | 5,3′-dihydroxy-7,4′-dimethoxyflavone | A. japonica | [2,6] |
| 145 | rhamnazin | A. japonica | [2,6] |
| 146 | 5-hydroxy-6,7,8-tritmethoxyflavone | A. sieboldiana | [2,45,49] |
| 147 | salvigenin | A. japonica, A. rubra, A. koehnei | [2,6,44] |
| 148 | apigenin 7-β-cellobioside | A. sieboldiana | [46] |
| 149 | apigenin 4′-β-cellobioside | A. sieboldiana | [46] |
| 150 | 5-hydroxy-4′,7-dimethoxyflavone | A. japonica, A. acuminata, A. rubra | [2,6,43,44] |
| 151 | scutellarein-6,4′-dimethyl ether | A. japonica, A. rubra | [2,6,44] |
| 152 | luteolin | A. rugosa | [48] |
| 153 | luteolin 7-O-β-glucside | A. rugosa | [48] |
| 154 | galangin | A. sieboldiana, A. pendula, A. viridis | [2,50,54,100] |
| 155 | kaempferol | A. koehnei, A. sieboldiana | [6,46] |
| 156 | kaempferide | A. japonica, A. koehnei | [2,6] |
| 157 | quercetin | A. japonica, A. nepalensis, A. firma, A. formosana, A. sieboldiana | [2,8,91,100,101,102] |
| 158 | isorhamnetin | A. japonica, A. koehnei | [2,6] |
| 159 | alnusin | A. sieboldiana, A. pendula | [2,49,50] |
| 160 | the 6,4′-dimethyl ether of 6-hydroxykaempferol | A. koehnei | [2,6] |
| 161 | izalpinin | A. sieboldiana | [2,50] |
| 162 | rhamnetin | A. koehnei | [2,6] |
| 163 | quercetin-7,3′,4′-trimethyl ether | A. japonica, A. koehnei | [2,6] |
| 164 | galangin 3-methyl ether | A. viridis | [54] |
| 165 | quercetin-3,3′-dimethyl ether | A. koehnei | [2,6] |
| 166 | 3,6-dimethyl ether of 6-hydroxykaempferol | A. koehnei | [2,6] |
| 167 | 3,6,4′-trimethyl ether of 6-hydroxy-kaempferol | A. japonica, A. koehnei | [2,6] |
| 168 | quercetagetin-3,6,4′-trimethyl ether | A. koehnei | [2,6] |
| 169 | kumatakenin | Alnus crispa Pursh., Alnus sinuate Rydbg. | [2,6] |
| 170 | quercetin 3,7-dimethyl ether | A. crispa, A. koehnei, A. sinuata | [2,6] |
| 171 | quercetin-3,7,4′-trimethyl ether (ayanin) | A. crispa | [2,6] |
| 172 | 5-hydroxy-3,6,7-trimethoxyflavone | A. sieboldiana | [2,49] |
| 173 | quercetin-3-O-α-l-arabinofuranoside | A. firma | [2,52] |
| 174 | isoquercitrin | A. firma | [2,52,53] |
| 175 | quercetin-3-O-glucoside | A. formosana, A. nepalensis | [32,91] |
| 176 | quercitrin | A. firma, A. formosana, A. nepalensis, A. japonica | [2,8,28,52,91] |
| 177 | quercetin-3-O-β-d-glucuronide | A. sieboldiana | [2,37] |
| 178 | rutin | A. nitida | [99] |
| 179 | quercetin-3-β-cellobioside | A. sieboldiana | [46] |
| 180 | quercetin-3-β-maltoside | A, sieboldiana | [46] |
| 181 | kaempferol 3-O-rhamnoside | A. japonica, A. formosana | [28,91] |
| 182 | quercetin-3-O-galactoside | A. japonica, A. nepalensis | [8,28] |
| 183 | kaempferol-3-dirhamnoside | A. sieboldiana | [46] |
| 184 | quercetin-3-sophoroside | A. gultinosa, Alnus cordata Loisel. | [2,32] |
| 185 | myricetin-3-O-β-d-galactopyranoside | A. firma | [2] |
| 186 | rhamnetin-3-O-rhamnoside | A. formosana | [91] |
| 187 | isorhamnetin 3-O-β-glucoside | A. rugosa | [48] |
| 188 | isorhamnetin 3-β-O-glucoside-7-O-α-rhamnoside | A. rugosa | [48] |
| 189 | pinocembrin | A. sieboldiana, A. pendula, A. maximowiczii, A. firma | [50,52,53,100] |
| 190 | naringenin | A. sieboldiana | [2,49] |
| 191 | alpinetin | A. pendula, A. firma, A. sieboldiana | [2,49,50] |
| 192 | strobopinin | A. sieboldiana | [2,49] |
| 193 | pinostrobin | A. pendula, A. firma, A. sieboldiana | [2,49,50] |
| 194 | rhododendrin | A. glutinosa | [2,51] |
| 195 | pinobanksin | A. sieboldiana | [2,49] |
| 196 | alnustinol | A. maximowiczii, A. firma, A. sieboldiana, A. pendula | [2,49,50,53] |
| 197 | 3,5,8-trihydroxy-7-methoxyflavone | A. sieboldiana | [45] |
| 198 | (+)-catechin | A. firma, A. viridis | [2,42,52] |
| 199 | (−)-epicatechin | A. firma | [2,52] |
| 200 | 2′,4′-dihydroxy-6′-methoxychalcone | A. viridis | [54] |
| Terpenoids | |||
| 201 | 24-(E)-3-oxodammara-20 (21),24-dien-27-oic acid | A. nepalensis | [32] |
| 202 | mangiferonic acid | A. nepalensis | [8] |
| 203 | alnuserrutriol | A. serrulatoides | [2,55] |
| 204 | alnuserrudiolone | A. sieboldiana, A. serrulatoides | [2,55,103] |
| 205 | alnincanone | A. serrulatoides | [2,55] |
| 206 | alnuserol | A. serrulatoides | [2,55] |
| 207 | alnuseric acid | A. serrulatoides, A. pendula | [2,55,58,104] |
| 208 | alnuselide | A. serrulatoides | [2,55,58] |
| 209 | alnustic acid methyl ester | A. firma | [2,52] |
| 210 | methyl(24E)-3,4-secodammara-4(28),20,24-trien-26-oic acid-3-oate | A. japonica | [2,55] |
| 211 | (24E)-3,4-secodammara-4 (28),20,24-trien-3,26-dioic acid | A. japonica | [2,55] |
| 212 | (20S,24S)-20,24-dihydroxy-3,4-secodammara-4 (28),25-dien-3-oic acid | A. japonica | [2,55] |
| 213 | (23E)-(20S)-20,25-dihydroxy-3,4-secodammara-4 (28),23-dien-3-oic acid | A. japonica | [2,55] |
| 214 | (23E)-(20S)-20,25,26-trihydroxy-3,4-secodammara-4 (28),23-dien-3-oic acid | A. japonica | [2,55] |
| 215 | (23E)-(12R,20S)-12,20,25-trihydroxy-3,4-secodammara-4 (28),23-dien-3-oic acid | A. japonica | [2,55] |
| 216 | (20S)-20-hydroxy-24-methylene-3,4-secodammar-4 (28)-en-3-oic acid | A. pendula | [2,55] |
| 217 | alnustic acid | A. serrulatoides, A. pendula, A. sieboldiana | [2,7,55,103] |
| 218 | alnustic acid-12-O-β-d-xylopyranoside | A. serrulatoides, A. pendula, A. sieboldiana | [2,7,55,103] |
| 219 | alnustic acid-12-O-β-d-glucopyranoside | A. serrulatoides, A. pendula, A. sieboldiana | [2,7,55,103]] |
| 220 | alnustic acid-12-O-α-l-arabinofuranoside | A. serrulatoides, A. pendula, A. sieboldiana | [2,7,55,103] |
| 221 | alnustic acid-12-O-(2′-O-acetyl)-α-l-arabinofuranoside | A. serrulatoides, A. pendula | [2,7,55] |
| 222 | alnustic acid-12-O-(2′-O-acetyl)-β-d-xylopyranoside | A. serrulatoides, A. pendula | [2,7,55] |
| 223 | alnustic acid-12-O-(2′-O-acetyl)-β-d-glucopyranosid | A. serrulatoides, A. pendula | [2,7,55] |
| 224 | taraxeryl acetate | A. japonica, A. hirsuta, A. nepalensis, A. acuminata | [2,8,30,57,92] |
| 225 | taraxerol |
A. japonica, A. hirsuta, A. nepalensis, A. maximowiczii, A. acuminata, A. rubra |
[2,8,30,57,59] |
| 226 | taraxerone | A. japonica, A. rubra, A. nepalensis, A. glutinosa, A. acuminata | [2,56,57] |
| 227 | glutenone | A. japonica, A. rubra, A. fruticosa, A. kamtschatica | [2,72] |
| 228 | glutinol | A. japonica | [2,92] |
| 229 | β-amyrin | A. japonica, A. fruticosa, A. kamtschatica, A. firma, A. glutinosa | [2,52,56] |
| 230 | 3-O-acetyl-β-amyrin | A. japonica, A. firma | [2,52] |
| 231 | 3β-acetoxy-olean-12-ene-28-al | A. acuminata | [57] |
| 232 | δ-amyrone | A. acuminata | [43] |
| 233 | ursolic acid | A. glutinosa | [56] |
| 234 | uvaol | A. glutinosa | [56] |
| 235 | α-amyrin | A. fruticosa, A. kamtschatica | [2] |
| 236 | lupeol |
A. japonica, A. rubra, A. nepalensis, A. glutinosa, Alnus oregona Nutt., A. acuminata |
[2,12,56,57,104] |
| 237 | betulin |
A. hirsuta, A. rubra, A. nepalensis, A. japonica, A. glutinosa, A. maximowiczii, A. oregona |
[2,7,8,12,30,56,59,104] |
| 238 | betulone | A. incana | [105] |
| 239 | betulinic acid | A. japonica, A. hirsuta, A. nepalensis | [2,8,30,63] |
| 240 | 3β,28-dihydroxy-lup-20(29)-ene | A. acuminata | [57] |
| 241 | betulinic aldehyde | A. japonica, A. glutinosa, A. acuminata | [12,56,57] |
| 242 | 3-acetoxybetulinic aldehyde | A. japonica | [12] |
| 243 | lupenylacetate | A. glutinosa | [56] |
| 244 | lupenone | A. japonica, A. rubra, A. fruticosa, A. kamtschatica, A. glutinosa | [2,56] |
| 245 | lup-20(29)en-2,28-diol-3-yl caffeate | A. firma | [61] |
| 246 | 22-hydroxyhopan-3-one | A. nepalensis | [8] |
| 247 | 2-hydroxydiploterol | A. nepalensis | [8] |
| 248 | simiarenol | A. glutinosa | [56] |
| Steroids | |||
| 249 | β-sitosterol |
A. japonica, A. fruticosa, A. rubra, A. nepalensis, A. kamtschatica, A. firma, A. glutinosa, A. acuminata, A. rugosa |
[2,8,48,52,57,63,64,106] |
| 250 | β-sitosterol 3-O-β-d-glucopyranoside | A. japonica, A. nepalensis, A. acuminata, A. rugosa | [2,8,48,57,63,64] |
| 251 | β-rosasterol | A. nepalensis | [64] |
| 252 | stigmasterol | A. nepalensis | [64] |
| 253 | brassinolide | A. glutinosa | [62] |
| 254 | castasterone | A. glutinosa | [62] |
| Others | |||
| 255 | pinosylvin | A. sieboldiana, A. pendula | [2,49,50] |
| 256 | pinosylvin monomethyl ether | A. sieboldiana, A. pendula, A. maximowiczii | [2,49,50,53] |
| 257 | 4′,5′-dihydroxy-3′-methoxy stilbene | A. viridis | [65] |
| 258 | trans-stilbene | A. firma, A. sieboldiana | [2,49,96] |
| 259 | pinosylvin dimethyl ether | A. sieboldiana, A. maximowiczii | [2,49,53] |
| 260 | cryptomeridiol 11-O-monoacetate | A. maximowiczii | [2,53] |
| 261 | β-eudesmol acetate | A. maximowiczii | [2,53] |
| 262 | elemol acetate | A. maximowiczii | [2,53] |
| 263 | eugenol | A. pendula | [2,50] |
| 264 | chavicol | A. pendula | [2,50] |
| 265 | vanillin | A. nepalensis | [64] |
| 266 | protocatechuic acid | A. firma, A. formosana | [91,101] |
| 267 | p-coumaric acid | A. firma | [101] |
| 268 | caffeic acid | A. firma | [101] |
| 269 | vanilic acid | A. japonica | [66] |
| 270 | chlorogenic acid | A. firma | [101] |
| 271 | 2,3,4-trimethoxyphenanthrene | A. maximowiczii | [2,53] |
| 272 | physcion | A. nepalensis | [8] |
| 273 | secoisolariciresinol diferulate | A. japonica | [66] |
2.1. Diarylheptanoids
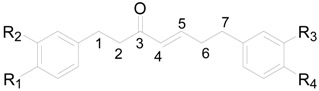
The Alnus genus has abundant diarylheptanoids containing the 1,7-diphenylheptane frame [21]. Diarylheptanoids have drawn attention due to their physiological activities, especially their anticancer activity [11]. A total of 99 diarylheptanoids have been reported from Alnus species. They are categorized into three major groups: linear-type (compounds 1–89), cyclic diphenyl ether-type (compounds 90–93) and cyclic diphenyl-type (compounds 94–99).
Compounds 1–89, the linear-diarylheptanoids shown in Table 1, Table 2 and Table 3 and Figure 1, can be further divided according to the features of the aliphatic carbons. The heptane chains of compounds 1–28 (Table 1) are saturated, and the C-3 or C-5 position is always linked to hydroxyls. Diarylheptanoid derivatives 9–28 possess a monosaccharide or a disaccharide at the C-3 or C-5 position of the heptane chain to form O-glycosides. Moreover, the C-6 position in the glucosyl unit of 26–28 is attached to a cinnamoyl moiety. Compounds 29–71 (Table 2) are classified as 1,7-bis-(p-hydroxyphenyl)-3-heptanones. Compounds 30–37 always contain an oxygen substituent at the C-5 position. The hydroxyl at C-5 of 38–44 is replaced by an aliphatic hydrocarbon. Diarylheptanoids 45–50, 62–69, as well as 7, 9, 14, 18, 33, 38 all could be isolated from the bark of A. glutinosa. Structure-activity analysis revealed a high dependence of their cytotoxic action on the presence of a carbonyl group at C-3, substitution of the heptane chain on C-5 and the number of hydroxyl groups in the aromatic rings [11]. What′s more, in 53–69, the sugar groups attached at the C-5 position in the heptane group are connected with aromatic acyl radical moieties. These compounds are a class of natural product called cinnamic acid sugar ester derivatives (CASEDs), with one or several phenylacrylic moieties such as -coumaroyl, -cinnamoyl, -benzoyl and -vanilloyl, linked with the non-anomeric carbon of a glycosyl skeleton part through ester bonds. Several CASEDs are reported in traditional medicine as compounds to calm the nerves and display anti-depression and neuroprotective activity [25,26,27]. Compound 52 has a disaccharide unit with β-d-apiofuranosyl bonded to β-d-glucopyranose. Compound 69 is an O-(2-methyl)-butanoyl derivative of oregonin (48). In addition, compounds 63–69 were all isolated from the bark of A. glutinosa, and the difference between them is the configuration of the sugar groups in the heptane chain [11]. In alnuside C (71), only isolated from A. japonica, the C-5 hydroxyl on the heptane chain is replaced by a xylose with a methylbutanoyl moiety. The absolute configuration at C-2 of the MeBu unit hasn′t been determined [28].
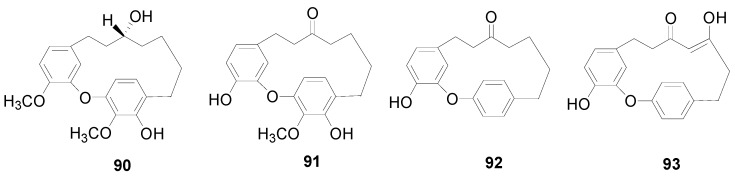
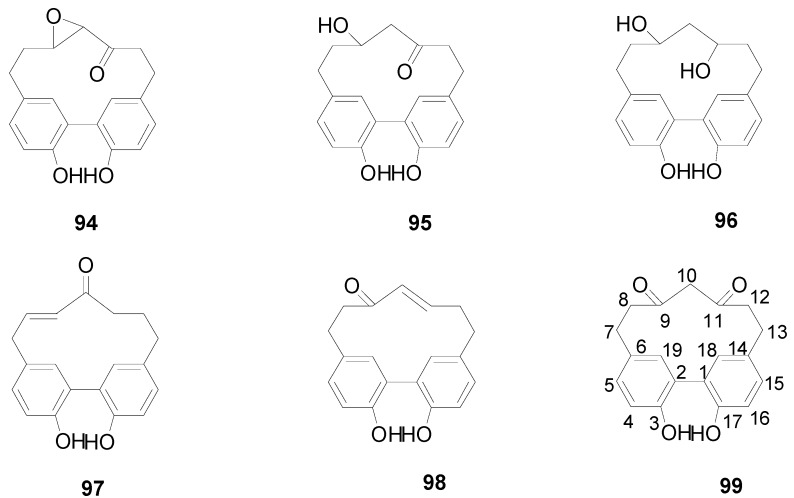
Compounds 72–77 and 78–89 are listed in Table 3 and Figure 1, respectively. There are two double bonds at C-1/C-2, C-4/C-5 or C-6/C-7 and a carbonyl at C-3 of 72–84. Meanwhile, 85 and 86 display two carbonyl groups at C-3 and C-5. Hirsutenone (73) exhibits prominent antioxidant, anti-inflammatory and anticancer effects [16,21,29]. Compounds 87 and 88 from A. hirsuta and Alnus nepalensis D. Don., respectively, both possess a 1,5-oxy bridge in the carbon chain [30,31]. Alnus dimer (89) consists of two units, including a 1,7-bis(3,4-dihydroxyphenyl)-1-(4-hydroxyphenyl)-5-methoxy-heptane-3-one (unit I) and a 1,7-bis (3,4-dihydroxyphenyl)-3-heptanone (unit II), which are connected through a C-C bond between C-3′ of unit I and C-6′ of unit II. Alnus dimer (89) showed potent macrofilaricidal and microfilaricidal activity in vitro [32]. Cyclic diarylheptanoids 90–99 are grouped into metaparacyclophanes 90–93 (Figure 2) and metametacyclophanes 94–99 (Figure 3) according to the position of the phenyl groups connected to each other as well as to the heptane chains. The two phenyl groups of compounds 90–93 are connected as a diaryl ether. Among them, 90 and 91 with a big cyclic ring, showed potent hypoxia-inducible factor-1 (HIF-1) and nuclear factor-κB (NF-κB) inhibitory activity [30,33]. Acerogenin L (92) and garugamblin-3 (93) from the methanol extract of A. japonica, strongly inhibited human low-density lipoprotein oxidation [34]. Compounds 94–99 listed in Figure 3, possess two aryl groups coupled at the meta-position to the side chain moieties and two hydroxyl groups at C-4′ and C-4′′ of two benzene rings. It was reported that the cyclic diarylheptanoids alnusonol (95), alnusdiol (96) and alnusone (98) always co-occur with the corresponding acyclic derivatives hannokinin (36), (+)-hannokinol (4) and platyphyllenone (74). Some workers have proposed a clear explanation of the biosynthetic relationship between the two types of compounds [35].
2.2. Polyphenols
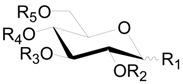
Tannins are the major components of antioxidant polyphenols in genus Alnus, mainly including four groups: gallotannins (compounds 100–104), ellagitannins (compounds 105–120), dimeric ellagitannins (compounds 121–124) and C-glycosidic tannins (compounds 125–128). They are always composed of a galloyl group, a hexahydroxydiphenoyl (HHDP) group and a valoneoyl group with glucose core(s) in which the mode of linkage is different. In addition, some polyphenols and their glycosides are also found in this genus.
Table 4 lists compounds 100–111. 100–104 from A. japonica, A. hirsuta and Alnus sieboldiana Matsum. that belong to the gallotannins, in which the galloyl group is directly attached to the hydroxyl of the glucose moiety through an ester bond [5,36,37]. Compound 104 contains a methylated galloyl group, which is linked to the C-1 of the glucose core. Ellagitannins 105–111 are esterified by one or two HHDP group(s) at C-1, C-2 and/or C-3, C-4 in the glucosyl moiety to form sugar aryl ester linkages, meanwhile, galloyl groups are often present, except in 108 and 109. Glutinoin (111), a novel antioxidative ellagitannin with a glutinoic acid dilactone moiety, was isolated from A. glutinosa cones [38]. The C-1 hydroxyl group of the sugar is coupled with a galloyl group, which is connected to the ellagoyl group via the C-4′ (para) hydroxyl group. The compound with a p-COC-type junction between two units, corresponding to the ellagoyl-galloyl motif in glutinoin, was named glutinoic acid dilactone and the corresponding group was named glutinoyl [38]. To our knowledge, no similar structure has been annotated so far.
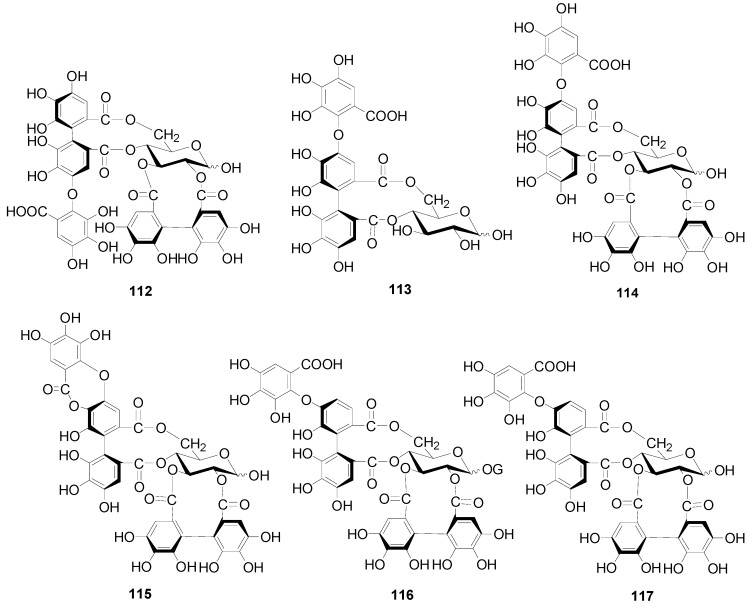
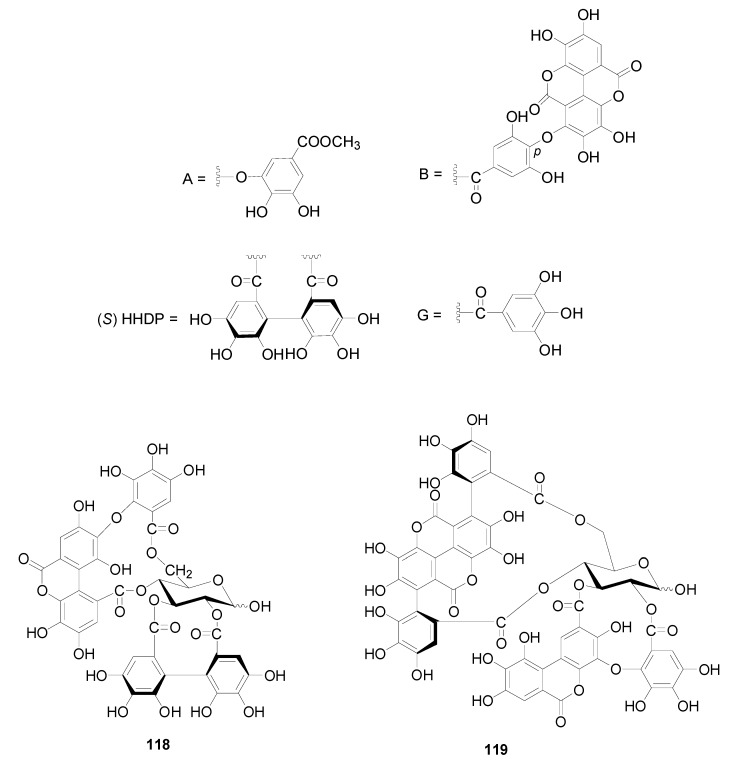
Compounds 112–115 in Figure 4 isolated from the cones of A. glutinosa, the leaves of A. japonica and Alnus hirsuta var. microphylla based have structures with a valoneoyl group and its depsidone form at C-4/C-6 of the glucose core [5,36,38]. Compared with 4,6-(S)-valoneoyl-d-glucose (113), flosin A (112), praecoxin A (114) and praecoxin D (115) all possess a HHDP group at C-2/C-3. Additionally, flosin A (112) and praecoxin A (114) are two isomeric ellagitannins, differing in the orientation of the 4,6-valoneoyl group. Alnusnins A (116) and alnusnins B (117) in Figure 4 contain a tergalloyl group at C-4/C-6 and a HHDP group at C-2/C-3 of the glucose core. Compounds 118 and 119 (Figure 5) from the extracts of fruits and leaves of A. sieboldiana both have a monolactonized tergalloyl group [37,39]. The A, C benzene rings of the tergalloyl group and the HHDP group in alnusiin (118) are linked with C-4/C-6 and C-2/C-3 in glucosyl moiety through ester bonds, respectively, while the A and B rings in tergallin (119) are attached to the C-2/C-3 positions. Additionally, tergallin (119) has a characteristic ellagic acid moiety symmetrically coupled with two galloyl groups at C-4/C-6 by C-C bonds. Hirsunin (120, Figure 6), represents a rare example of a hydrolysable tannin, and consists of diarylheptanoid glycoside (oregonin) and ellagitannin (praecoxin A) moieties. It was isolated from Alnus hirsuta var. microphylla in 1992 and remains the sole example of an ellagitannin with a diarylheptanoid moiety up to now [36,40].
Compounds 121–124 in Table 5 and Table 6 are dimeric ellagitannins consisting of pedunculagin (109) and strictinin (107) units in which the connection position of 124 is different from the other three [5,36]. Furthermore, 1-desgalloylrugosin F (121) and rugosin F (122) differ from each other on account of the galloyl group being present at C-1 attached to the HHDP group or not. Alnusjaponins A (123) and alnusjaponins B (124) occurred in A. japonica are mutually isomeric ellagitannins [5]. C-glucosidic ellagitannins 125–128 (Table 7) contain two HHDP groups hold to the C-2/C-3 and C-4/C-6 positions in an open-chain glucose core, one of which participates in the C-glucosidic linkage forming a phenol-aldehyde coupling. Compounds 126–128 with a galloyl group at C-1 of the glucosyl unit were isolated from A. sieboldiana and A. japonica [5,37,39]. Besides, stenophyllanin A (128), a stachyurin-based congener, is linked with a flavan-3-ol unit through the C-C bond between C-1 of glucose and C-8 of flavan-3-ol. Compounds 129–133 belong to phenolic glycosides. The hydroxyl at C-6 of glucose core is substituted by syringoyl, vanilloyl or trimethoxycinnamoyl group [41,42]. Shikimic acid (134), 5-O-galloyl-(−)-shikimic acid (135), gallic acid (136) and methyl gallate (137) are polyphenols with a low molecular weight.
2.3. Flavonoids
There are 63 flavonoids in this genus, mainly including flavones, flavonols, flavonones, flavanonols, flavanols and one chalcone. A total of 16 flavones (138–153), listed in Table 9, were isolated from eight different Alnus species [2,6,43,44,45,46,47,48,49]. The hydroxy is always replaced by -CH3, -glc, or -glc-glc groups. Flavonols and their derivatives account for a relatively large proportion of the flavonoids in the Alnus genus. Flavonols (compounds 154–163) in Table 10 have a C-3 free OH moiety. Compounds 164–172 (Table 11) have similar structures with a -OMe function at C-3. Table 12 shows chemical constituents 173–188, in which the C-3 hydroxy is substituted by one or two sugar unit(s). Flavonones 189–195 (Table 13) were found in A. sieboldiana, Alnus pendula Matsum., Alnus maximowiczii Call., Alnus firma S.Z. and A. glutinosa [49,50,51,52,53]. Two flavanonols 197 and 198 are shown in Figure 8. (+)-Catechin (198) and (−)-epicatechin (199) in Figure 8 are a couple of stereoisomers. Compound 200 (Figure 8) isolated from buds of Alnus viridis DC., is the only chalcone among the components reported in the genus Alnus [54].
2.4. Triterpenoids and Steroids
A total of 48 triterpenes (compound 201–248) shown in Figure 9, Figure 10 and Figure 11 and Table 14 and Table 15 and six steroids (compounds 249–254) in Figure 12 were obtained from the genus Alnus. Most of triterpenes are isolated from flowers, leaves and barks of Alnus plants [8,30,55,56,57]. They can be assigned into two classes: tetracyclic triterpenes (compounds 201–223) and pentacyclic triterpenes (compounds 224–248).
Triterpenic acids 201 and 202 were both isolated from the leaves of A. nepalensis [8,31]. Particularly, the cycloartane type mangiferonic acid (202), which was not detected in any other Alnus species, has been considered as a specific chemical marker of A. nepalensis from a chemotaxonomical point of view [8]. The tetracyclic triterpenes 203–208 and 216–223, isolated from flowers and leaves of A. sieboldiana, Alnus serrulatoides Call. and A. pendula, are characterized by their C31-dammarane-type and C31-3, 4-seco-dammarane-type skeletons [55]. All the tetracyclic triterpenes 210–215 occurring in A. japonica male flowers were of the C30-3, 4-seco-dammarane-type [55]. In particular, alnuselide (208), structurally similar to alnuseric acid (207), has a lactone ring formed by the 3-carboxyl and the 11-hydroxyl groups [58]. Triterpenoid saponins 218–223 are connected with a sugar moiety at C-12 through an O-glycosidic bond. In addition, positions C-2 in the sugar unit of compounds 221–223 were always linked with an acetyl group.
The isolated pentacyclic triterpenes range from oleananes (compounds 224–232), ursanes (compounds 233–235), lupanes (compounds 236–245), and hopanes (compounds 246–247) to fernanes (248). Taraxerol (225) is widely spread in Alnus species, including A. japonica, A. hirsuta, A. nepalensis, Alnus maximowiczii Call., Alnus acuminata ssp. arguta (Schlecht.) and Alnus rubra Bong. [2,8,30,57,59]. Betulinic acid (239), the lupane-type triterpenoid acid from the methanol extract of A. hisuta, potently inhibited rat liver diacylglycerol acyltransferase enzyme activity and triglyceride synthesis in human HepG2 cells [60]. Lup-20(29)en-2,28-diol-3-yl caffeate (245) is a novel component with a caffeoyl group at the C-3 position that shows therapeutic potential against liver fibrosis [61].
Moreover, six steroids (compounds 249–254) were obtained from the bark of A. nepalensis and A. japonica, the pollen and bark of A. glutinosa, the stem bark of A. acuminata, and the aerial part of A. rugosa L. [48,57,62,63,64].
2.5. Other Compounds
About 19 other compounds were isolated from this genus. Five stilbenes 255–259 (Table 16) were obtained from the leaves and flowers of A. sieboldiana, A. pendula, A. maximowiczii and A. viridis [49,50,53,65]. Compounds 260–262 (Figure 13) linked with a carboxyl group are sesquiterpenoid acetates. Compounds 263–271 (Figure 13) are low molecular weight phenols. The phenanthrene derivative 2,3,4-trimethoxyphenanthrene (271), anthraquinone physcion (272), and phenylpropanoid secoisolariciresinol diferulate (273) were found in A. maximowiczii, A. nepalensis and A. japonica [8,53,66]. Additionally, physcion (272), mangiferonic acid (202) together with 22-hydroxyhopan-3-one (246) are specific chemical markers of A. nepalensis since their distribution has not been detected in any other Alnus species [8].
3. Biological Activities
Many species of the genus Alnus have been used as remedies for diarrhea, dysentery, fever and inflammatory diseases in traditional Chinese and Korean medicine [107]. Alnus species have remarkable anticancer activity, which is the most notable and important pharmacological activity [21]. Beyond that, they also display antioxidant, anti-inflammatory, antimicrobial, antiviral and hepatoprotective activities, etc. [16,52,57,105]. Diarylheptanoids are the the main effective components in the genus Alnus for their remarkable biological activities [2]. Phenolic compounds and flavonoids, which are widely found as secondary metabolites in Alnus plants, are important due to their ability to act as antioxidants [18]. Additionally, several triterpenoids have revealed antitumor activity, HIV-1 viral enzyme inhibition and hepatoprotective effects [52,61,104].
3.1. Anticancer Activity
Many researchers have focused on the pharmacological effects of hirsutenone (73) isolated from A. japonica, A. hirsuta and A. pendula, etc. [13,69,87]. It has a similar chemical structure and anti-cancer properties as curcumin, the most well-known diarylheptanoid in turmeric (Curcuma longa Linn, Zingiberaceae), which has been ranked as a third generation chemoprevention agent by the U.S. National Cancer Institute [21,108,109]. Hirsutenone (73) inhibited the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced upregulation of cyclooxygenase-2 (COX-2) and matrix metalloproteinases-9 (MMP-9) in human breast epithelial cells, which has been implicated in the pathogenesis of different kinds of cancer [110]. Further experiments confirmed the cytotoxic activity of 73 against HT-29 human colon carcinoma cells via the induction of oxidative stress and topo II-mediated DNA damage [21]. It can enhance the apoptotic effect of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) on epithelial ovarian carcinoma cell lines by increasing the activation of the caspase-8- and Bid-dependent pathways and mitochondrial pathway, leading to caspase activation as well [111]. Kang et al. found that hirsutenone suppressed human prostate cancer by targeting Akt1 and 2 as a key component to explain for anti-cancer activity [112]. A research in 2014 indicated that hirsutenone (73) could sensitize chemoresistant ovarian cancer cells to cisplatin via modulation of apoptosis-inducing factor and X-linked inhibitor of apoptosis [24].
Compounds 46, 47, 50, 62, 66 and 73 from A. glutinosa bark exhibited strong anticancer activity compared with other diarylheptanoids from the same species and considerably higher than curcumin, which served as a positive control, in human non-small cell lung carcinoma cell lines. Structure-activity analysis revealed a high dependence of the cytotoxic action on the presence of a carbonyl group at C-3, substitution of a heptane chain on C-5 and the number of hydroxyl groups in the aromatic rings [11]. In addition, Choi et al. found that platyphylloside (50), which has a keto-enol moiety and one hydroxyl group in the aromatic ring, showed the most potent cytotoxic activity on B16 mouse melanoma and human stomachic adenocarcinoma cells. This study also suggested that the ketone or keto-enol moiety and hydroxyl groups in the aromatic rings were essential to the higher cytotoxic activity of diarylheptanoids [73].
A rencent comparative study was performed on structurally analogous diarylheptanoids isolated from the bark of A. viridis and A. glutinosa to address their biological effects and determine structure-activity relationship. (5S)-O-methylplatyphyllonol (40) and platyphyllenone (74) do not possess 3′ and 3′′-OH groups showed significantly higher cytotoxicity compared to that of analogues 5(S)-O-methylhirsutanonol (38) and hirsutenone (73). The C-4/C-5 double bond instead of a methoxy group in compounds 74 and 73 positively influenced cell growth inhibition and pro-apoptotic potential. These results indicated that minor differences in the chemical structure can greatly influence the effect of diarylheptanoids on apoptosis and redox status and determine their selectivity towards cancer cells [76].
Oregonin (48) and hirsutanonol (33) are potential cancer chemopreventive agents. They showed significant inhibitory effects on TPA-induced COX-2 expression in immortalized human breast epithelial MCF10A cells [113]. In addtion, a novel immunomodulator 48 exhibited powerful anticancer activity through augmenting the acttivities of macrophage and natural killer cells [114,115]. Jin et al. isolated six diarylheptanoids from the stem bark of A. hirsuta. Among them, cyclic-type diarylheptanoids 90 and 91 inhibited the HIF-1 activation with IC50 values of 11.2 μM and 12.3 μM while the other diarylheptanoids showed very weak activity with IC50 values greater than 100 μM. It suggested that the big cyclic ring contributes to the strong HIF-1 activity [30]. The leaves, barks, and cones extracts of A. incana and A. viridis showed potent cytotoxic inhibition effect on HeLa cells with IC50 values ranging from 26.02 to 68.5 μg/mL. The most active extract of A. incana bark has been found to contain great amounts of total phenolics (316.2 mg of gallic acid (137)) [116]. Pedunculagin (109), which is an ellagitannin, exhibited dose-dependent cytotoxicity in vitro and a lengthening effect on the lifespan in mice bearing S180 tumors in vivo [117]. Galangin (154), isolated from A. sieboldiana, significantly inhibited tumor necrosis factor-α (TNF-α) gene expression in A549 cells. It may also be useful in cancer prevention [100].
3.2. Antioxidant Activity
Free radical damage is linked to the occurrence of many degenerative diseases, including cancer, cardiovascular disease, cataracts, and aging. Antioxidants can attenuate the damaging effects of reactive oxygen species (ROS) in vitro and have attracted major interest, not only for health care and cosmetics, but also in the food industry [118].
There are many reports that the extracts and isolated compounds from this genus have significant antioxidative activitiy. It has been reported that oregonin (48) and hirsutenone (73) showed prominent ability to scavenge oxygen radicals compared with the positive controls in the ABTS·+ (2,20-azino-bis(3-ethylbenzo-thiazoline)-6-sulphonic acid diammonium salt)-scavenging, superoxide anion radical O2−, and DPPH (1,1-diphenyl-2-picrylhydrazyl) tests, the total oxidant scavenging capacity (TOSC) assay and the oxygen radical absorbance capacity (ORAC) assay [16,74,90]. It is worth mentioning that compounds 48 and 9 showed stronger antioxidative activities than the well-known antioxidant curcumin [90]. Furthermore, many other compounds also exhibited remarkable free-radical-scavenging capacity; e.g., diarylheptanoids 4, 14, 36, 49, polyphenols 109, 111, 115, 134 and flavones 144, 157 [15,38,98,102]. As we all know, polyphenols are natural antioxidants, and the protection against oxidative damage is due to their antioxidant effects. Extracts of A. incana and A. viridis leaves, bark, and cones were found to be strong DPPH free radical scavengers with IC50 values ranging from 3.3 to 18.9 μg/mL. However, correlation with total phenol and tannin contents was not observed. The results showed that the antioxidant effect might be attributed to the presence of other compositions, such as diarylheptanoids and triterpenoids [116]. The ethanolic extracts of A. nitida barks and A. glutinosa stem barks both also possess the radical scavenging capacity [98,119]. Gallic acid (136), rutin (178) and (+)-catechin (198) are the active constituents responsible for the antioxidant activity of A. nitida bark ethanolic extract [119]. Furthermore, the antioxidant properties displayed by the extract of A. glutinosa is linked to a successful reduction in inflammatory processes, and the antioxidant potential of A. nitida bark might protect from liver damage [22,119].
Structurally, hirsutenone (73), hirsutanonol (33), oregonin (48), rubranoside B (12), and rubranoside C (22), which possess two 3,4-dihydroxyphenyl rings, were more active against ROS than alnuside A (45), and alnuside B (46), which have a 3,4-dihydroxyphenyl ring and a 4-hydroxyphenyl ring. Platyphyllone-5-xylose (47), platyphyllone (36), and platyphylloside (50), which have two 4-hydroxyphenyl rings, showed weak activity [16]. From that, we can see that the scavenging capacity against peroxyl radicals is closely related to the phenolic hydroxyls. Some other studies also revealed that the phenolic hydroxyls were essential to the higher antioxidative activity of diarylheptanoids [10,28]. Recently, the combined theoretical and experimental studies confirmed that the catechol moiety as an H-atom donor was very important for the free radical scavenging effect. Thermodynamic descriptors mainly O–H bond dissociation enthalpies (BDEs) establish a clear structure–activity relationship [90]. Oregonin (48) and hirsutenone (73), together with two cyclic diarylheptanoids, acerogenin L (92) and garugamblin-3 (93), exhibited significant human LDL-antioxidant activities in the thiobarbituric acid-reactive substance (TBARS) assays with IC50 values of 3.2, 1.5, 2.9, 1.7 μM, respectively [34,120].
3.3. Anti-Inflammatory Activities
Diarylheptanoids and phenolic glycosides isolated from A. japonica, A. hirsuta, A. firma, A. formosana, A. nitida, A. nepalensis and A. acuminata showed significant anti-inflammatory effect [33,41,57,70,99,121,122].
Kim et al. isolated nine known diarylheptanoids from the barks of A. japonica. Among these diarylheptanoids, oregonin (48) and hirsutenone (73) exhibited apparent inhibitory effects on lipopolysaccharide (LPS)-induced NO production and COX-2 production [121]. An analysis of the structure-activity relationship suggested that the presence of a keto-enol group in the heptane moiety or a caffeoyl group in the aromatic ring was important for the inhibitory activity efficacy [121]. Later on, another study also suggested that the carbonyl group is important for the inhibitory activity of diarylheptanoids against LPS-induced NO production [41]. Lee et al. provided new evidence for the anti-inflammatory actions of oregonin (48), which include the inhibition of inducible nitric oxide synthase (iNOS) gene transcription via suppressing transcriptional activity of NF-κB and activator protein-1 (AP-1), as well as the up regulation of anti-inflammatory molecule HO-1 [123]. In addition, oregonin (48) reduces lipid accumulation, inflammation and ROS production in primary human macrophages, indicating its anti-inflammatory bioactivity [124]. Hirsutenone (73) may exert a preventive effect against microbial endotoxin lipopolysaccharide-induced inflammatory skin diseases through inhibition of extracellular signal-regulated kinase (ERK) pathway-mediated NF-κB activation [29,125].
Compounds 12, 33, 90 and 91 in the bark of A. hirsuta, four phenolic glycosides 129–132 and six diarylheptanoids 36, 41, 45, 48, 50, 53 isolated from A. firma showed significant ability to inhibit LPS-induced inflammation in macrophages or BV2 microglial cells [10,33,41]. Aguilar et al. confirmed the traditional uses of A. acuminata in acute inflammatory conditions and its safety for consumption. Several triterpenoids from the hexane extract and diarylheptanoids from the methanol extract of A. acuminata were isolated and characterized [57]. The methanol extract of A. glutinosa leaves and shikimic acid (134) were found to exhibit remarkable anti-inflammatory effect by “inhibition of acetic acid-induced capillary permeability”, “carrageenan-induced hind paw edema” and “TPA-induced ear edema” assays [98]. A recent study in 2017 evaluated the methanol extract and derived fractions of A. nitida stem bark for anti-inflammatory activity by using in vitro heat induced albumin denaturation assay and various in vivo assays. It suggested that the presence of polyphenols, sterols, terpenoids and other constituents of A. nitida stem bark might contribute towards the anti-inflammatory and analgesic activities [99].
3.4. Antimicrobial and Antiviral Activities
It was reported that the EtOH extract of A. pendula bark had significant antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Oregonin (48) and hirsutenone (73) isolated from the active fractions inhibited MRSA strains with the minimum inhibitory concentrations (MICs) ranged from 31.25 to 250 μg/mL. Moreover, two fold MIC of 73 could completely suppress the growth of MRSA [126]. Later on, the antibacterial evaluation of the fractions by bioautography on Staphylococcus aureus revealed that 48 was the most active, with an antibacterial inhibitory effect comparable to antibiotics [17]. The extracts of cone, leaves, and bark of A. incana and A. viridis showed antimicrobial activities against 15 microorganisms with MIC values ranging from 0.117 to 0.292 mg/mL [116]. Genkwanin (143) isolated from the seeds of A. glutinosa showed high antimicrobial activity against seven strains of Gram-positive and Gram-negative bacteria [47]. Betulin (237), betulone (238) and betulinic acid (240) were identified as the major antimycobacterial constituents in the bark of A. incana. The functionality at C-3 and C-28 of the lupane skeleton was seemed to be important in determining the antimycobacterial activity [105].
Triterpenoids and flavonoids isolated from A. firma were found to inhibit HIV-1 virus replication and controlled its essential enzymes. Alnustic acid methyl ester (209) exhibited significant inhibitory effect to HIV-1 protease with IC50 value of 15.8 μM, and flavonoids 157, 176, 185 inhibited HIV-1 reverse transcriptase all with IC50 of 60 μM [52]. Hirsutenone (73) showed remarkbable inhibitory effect to papain-like protease with IC50 value of 4.1 μM. Furthermore, the authors elucidated that catechol and α,β-unsaturated carbonyl moiety may be responsible for the inhibitory activity [23]. Platyphyllenone (74) and platyphyllonol-5-O-β-d-xylopyranoside (47) showed high anti-viral activity against Influenza A virus H9N2 with EC50 values of 29.9 and 56.1 μM, compared with the positive control, zanamivir (EC50 = 16.9 μg/mL), respectively [83]. Betulinic aldehyde (241) exhibited anti-influenza effect against KBNP-0028 (H9N2) avian influenza virus with an EC50 value of 12.5 μg/mL, compared to a positive control, oseltamivir (EC50 = 0.063 μg/mL) [12].
3.5. Hepatoprotective Activity
The methanolic extract of the bark of A. firma exhibited significant antifibrotic activity. Meawhlie, compounds 73 and 245 isolated from A. firma barks showed potent inhibitory effect on the proliferation of hepatic stellate cell (HSC). The authors determined that the presence of substitution at C-3 and C-5 might be responsible for the inhibitory activity of diarylheptanoids on HSC proliferation [61]. It was reported that A. japonica was used for hepatitis as an endemic species in Korea. There were evidences that the methanol extract of A. japonica stem bark displays hepatoprotective effects against acetaminophen-induced cytotoxicity in cultured rat hepatocytes in vitro [127]. Compounds 12, 14, 22, 33, 37, 38, 45, 46, 48, 49 and 73 isolated from the A. japonica bark and the A. hisuta stem bark showed significant hepatoprotective effects on tert-butyl hydroperoxide (t-BHP) -induced damage to HepG2 cells. According to structure characteristics, the authors considered that the cytoprotective effect was closely related to catechol moiety [3,16,80]. Sajid et al. explored the antioxidant and hepatoprotective properties of A. nitida stem bark crude methanol extract on rats. The study concluded that the hepatoprotective activity of A. nitida bark is likely due to the antioxidant potential [119]. Furthermore, some other studies also suggested that the hepatoprotective effect is closely linked with the antioxidant property [16,127].
3.6. DNA Damage Protection Activity
Novaković et al. isolated twenty-one diarylheptanoids and two polyphenols from the barks of A. glutinosa and A. viridis. All isolated compositions were evaluated for their in vitro protective effects on chromosome aberrations in peripheral human lymphocytes using cytokinesis-block micronucleus (CBMN) assay. Many of them exerted a pronounced effect of decreasing DNA damage of human lymphocytes, acting stronger than the known synthetic protector amifostine [4,42]. Both platyphylloside (50) and 62 isolated from the bark of A. glutinosa could protect HaCaT cells against doxorubicin-induced DNA damage. They showed chemo-protective effects of at multiple subcellular levels, and could be considered as protective agents for non-cancerous dividing cells during chemotherapy [19,20].
3.7. Anti-Adipogenic Activity
Martineau et al. found that the extracts of the inner bark of A. incana strongly inhibited the formation of triglyceride-laden mature adipocytes from 3T3-L1 pre-adipocytes. A. incana extracts acted early in the differentiation process and acted as partial agonists toward peroxisome proliferator activated receptor gamma (PPAR-γ) activity. The diarylheptanoid glycoside oregonin (48) was isolated and confirmed to be the active principle exerting the anti-adipogenic effect in A. incana [82,128]. On the evaluation of antiadipogenic activities of diarylheptanoids isolated from A. hirsuta, eighteen compounds were obtained and most of them could decrease lipid accumulation in 3T3-L1 pre-adipocytes. In the assay system, the most potent compound 47, platyphyllonol-5-O-β-d-xylopyranoside had anti-adipogenic activity mediated by the regulation of PPAR-γ dependent pathway. Furthermore, the ketone functionality at C-3, the substituent at C-5, the double bond in heptanone and the hydroxyl groups in the benzene rings were related to the activity [69]. The lupane-type triterpenoid 239 isolated from the methanol extract of A. hirsuta showed significant inhibitory activity to diacylglycerol acyltransferase with IC50 value of 9.6 μM in the rat liver microsomes. In addition, it also inhibited the triglyceride formation in human HepG2 cells. The results indicated that betulinic acid (239) may be a potential lead agent in the treatment of obesity [60].
3.8. Anti-Atopic Activity
Atopic dermatitis (AD) is a common inflammatory skin disease. Choi and his co-workers did many studies about the anti-atopic dermatitis effect of oregonin (48) and hirsutenone (73). The AD animal models were treated with them via topical application as well as intraperitoneal injection. The Th2-related cytokines IL-4, IL-5, IL-13 levels, IgE inflammatory factors, eosinophil levels in blood and lymphocytes were all reduced in AD-like skin lesions of rat model. These results revealed that the two diarylheptanoids were effective for the treatment of AD [129,130]. The leaves and barks extract from A. japonica was also proved useful in the treatment of atopic dermatitis and other allergic skin diseases in NC/Nga mice [131]. In addition, hirsutenone is an attractive source for developing a topical drug for T cell-based antiatopic dermatitis by its actions as a calcineurin inhibitor [132]. Furthermore, they developed hirsutenone-loaded and oregonin-loaded Tat peptide-admixed elastic liposomal formulations to treat AD, which aid to increasing the skin permeation of medicine [133,134].
3.9. Insecticidal Activity
Research performed by Tung et al. found that the crude extract of A. japonica bark showed significant inhibition effect on the growth of Trypanosoma brucei. Oregonin (48) and hirsutenone (73) displayed obvious inhibitory activities against T. brucei growth in the bloodstream with IC50 of 1.14 and 1.78 μM, respectively. Analysis of their structure–activity relationships revealed that the 3-oxo function of the heptane chain in the diarylheptanoid molecules is necessary for their trypanocidal activity [75]. Compounds 33, 73, 74 and 77 isolated from A. nepalensis exhibited potential antifilarial activity both in vitro and in vivo studies [135]. Alnus dimer (89) and compounds 34 found in A. nepalensis showed potent microfilaricidal (LC100 = 31.25~62.5 μg/mL, IC50 = 11.05~22.10 μg/mL) and macrofilaricidal activities in vitro (LC100 = 15.63 μg/mL, IC50 = 6.57~10.31 μg/mL) [32].
3.10. Other Activities
Apart from the summarized functions above, the constituents or extracts from Alnus plants also have some other activities. Oregonin (48) could improve glucose metabolism and insulin signal transduction of HepG2 cells partly by enhancing the expression level of insulin receptor and insulin receptor substrate-1 [85]. The cyclic diarylheptanoids 95, 98 and 99 isolated from the branch wood of A. sieboldiana were assayed for α-glucosidase inhibitory activities with the IC50 values 2.34, 8.69 and 1.35 μg/mL, respectively. In comparison, they have a stronger inhibitory effect than acarbose (IC50 = 451 μg/mL), a positive control, which is currently used as an antidiabetic agent [97]. Diarylheptanoids 7, 14, 33 and 48 isolated from the bark of A. hirsuta could reduce melanin level and tyrosinase activity in melanoma cell [136]. Furthermore, it was reported that 5(R)-O-methylhirsutanonol (41) and oregonin (48) might be useful in the prevention and treatment of atherosclerosis through attenuation of adhesion molecule expression by inhibition of NF-κB activation [81]. Hirsutenone (73) was able to protect retinal ganglion cells from oxidative stress-induced death. Therefore, it was considered to be a neuroprotective agent for the treatment of neurodegenerative disease, such as glaucoma [137]. The methanol extract of A. glutinosa subsp. glutinosa leaves increased wound tension, contraction capacity and tissue hydroxyproline levels. Shikimic acid (134) was found to be the major compound responsible for the wound healing effect [88]. The methanolic extract of A. rugosa stems showed anticholinesterase activity and it was very interesting for further isolation of acetylcholinesterase inhibitors, which are widely used in the treatment of Alzheimer′s disease [138].
4. Conclusions
This article summarized a total of 273 compounds that have been reported from the genus Alnus, with 138 cited references. Many species in the genus Alnus have been used as traditional herbal medicines in Korea, Japan and China [2]. So far, phytochemical research on the genus has revealed the extensive presence of diarylheptanoids, flavones, polyphenols, terpenoids, steroids and other compound types. The pharmacological activities of pure compounds and crude extract from this genus were mainly focused on anticancer, antioxidant and anti-inflammatory properties. For their significant anticancer activities, diarylheptanoids are a research hotspot in the genus Alnus.
Some researchers have pointed out the anticancer mechanism of hirsutenone (73), which should be more thoroughly tested as a potential anticancer agent in the future. Some experiments involved the structure-function relationships of antioxidant, anticancer, anti-adipogenic and insecticidal activities, but were not deep enough except structure-antioxidant activity relationship [11,69,75,90]. Hence, researchers may need to pay attention to them in future studies. As a whole, the phytochemical and biological investigations were mainly concentrated on the seven Alnus species (A. japonica, A. hirsuta, A. glutinosa, A. incana, A. nepalensis, A. sieboldiana and A. firma), with little or no attention being paid to other folk medical species. In view of this background, plenty of further studies are necessary in order to examine the other plants of the Alnus genus, extend the use of Alnus species and provide clinical rationale for the development of new therapeutic agents from traditional medicine sources. The authors hope this review will provide valuable data for the exploration and advanced research on Alnus species.
Acknowledgments
This project was supported by grants from the National Natural Science Foundation of China (No. 81001697 and 81573692), Beijing Nova Program (No. 2011070), and Self-Selected Topic of Beijing University of Chinese Medicine (No. 2015-JYB-JSMS024, 2015-JYB-XS109 and 2017-JYB-XS-138).
Author Contributions
X.R., T.H., M.S. and G.S. conceived and designed the paper; X.R. wrote the paper; Y.C. and G.S. reviewed the paper. Y.Z., X.C., S.B., and L.W. discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Flora Reipublicae Popularis Sinicae. [(accessed on 23 June 2011)]; Available online: http://frps.eflora.cn/frps/Alnus.
- 2.Sati S.C., Sati N., Sati O.P. Bioactive constituents and medicinal importance of genus Alnus. Phcog. Rev. 2011;5:174–183. doi: 10.4103/0973-7847.91115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park D., Kim H.J., Jung S.Y., Yook C.S., Jin C., Lee Y.S. A new diarylheptanoid glycoside from the stem bark of Alnus hirsuta and protective effects of diarylheptanoid derivatives in human HepG2 cells. Chem. Pharm. Bull. 2010;58:238–241. doi: 10.1248/cpb.58.238. [DOI] [PubMed] [Google Scholar]
- 4.Novaković M., Stanković M., Vučković I., Todorović N., Trifunović S., Tešević V., Vajs V., Milosavljević S. Diarylheptanoids from Alnus glutinosa bark and their chemoprotective effect on human lymphocytes DNA. Planta Med. 2013;79:499–505. doi: 10.1055/s-0032-1328301. [DOI] [PubMed] [Google Scholar]
- 5.Lee M.W., Tanaka T., Nonaka G.I., Nishioka I. Dimeric ellagitannins from Alnus japonica. Phytoehtmistry. 1992;31:2835–2839. doi: 10.1016/0031-9422(92)83642-C. [DOI] [Google Scholar]
- 6.Wollenweber E. Flavonoids from Alnus crispa, A. japonica, A. koehnei and A. sinuata. Phytochemistry. 1974;13:2318–2319. [Google Scholar]
- 7.Suga T., Aoki T., Kawad Y., Ohta S., Ohta E. C31-secodammarane-type triterpenoid saponins from the flowers of Alnus pendula. Phytochemistry. 1984;23:1297–1299. doi: 10.1016/S0031-9422(00)80445-8. [DOI] [Google Scholar]
- 8.Phan M.J., Phan T.S., Truong T.T.C., Matsunami K., Otsuka H. Mangiferonic acid, 22-hydroxyhopan-3-one, and physcion as specific chemical markers for Alnus nepalensis. Biochem. Syst. Ecol. 2010;38:1065–1068. doi: 10.1016/j.bse.2010.09.020. [DOI] [Google Scholar]
- 9.Lv H., She G. Naturally occurring diarylheptanoids. Nat. Prod. Commun. 2010;5:1687–1708. [PubMed] [Google Scholar]
- 10.Hu W.C., Wang M.H. Antioxidative activity and anti-inflammatory effects of diarylheptanoids isolated from Alnus hirsuta. J. Wood Sci. 2011;57:323–330. doi: 10.1007/s10086-010-1170-x. [DOI] [Google Scholar]
- 11.Novaković M., Pešić M., Trifunović S., Vučković I., Todorović N., Podolski-Renić A., Dinić J., Stojković S., Tešević V., Vajs V., et al. Diarylheptanoids from the bark of black alder inhibit the growth of sensitive and multi-drug resistant non-small cell lung carcinoma cells. Phytochemistry. 2014;97:46–54. doi: 10.1016/j.phytochem.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Tung N.H., Kwon H.J., Kim J.H., Ra J.C., Kim J.A., Kim Y.H. An anti-influenza component of the bark of Alnus japonica. Arch. Pharm. Res. 2010;33:363–367. doi: 10.1007/s12272-010-0303-5. [DOI] [PubMed] [Google Scholar]
- 13.Ra J.C., Kim Y.H., Sohn D.H. Antioxidative and Hepatoprotective Compositions Containing Diarylheptanoids from Alnus japonica. 2011/0144039 A1. U.S. Patent. 2011 Jun 16;
- 14.Cho K.J. Method of Making Health Drink for Reducing Hangover Using Alnus japonica Extract and Green Tea Leaf Extract. 2006023093. Korea Patent KR. 2006 Mar 13;
- 15.Lim S.S., Lee M.Y., Ahn H.R., Choi S.J., Lee J.Y., Jung S.H. Preparative isolation and purification of antioxidative diarylheptanoid derivatives from Alnus japonica by high-speed counter-current chromatography. J. Sep. Sci. 2011;34:3344–3352. doi: 10.1002/jssc.201100484. [DOI] [PubMed] [Google Scholar]
- 16.Tung N.H., Kim S.K., Ra G.C., Zhao Y.Z., Sohn D.H., Kim Y.H. Antioxidative and hepatoprotective diarylheptanoids from the bark of Alnus japonica. Planta Med. 2010;76:626–629. doi: 10.1055/s-0029-1240595. [DOI] [PubMed] [Google Scholar]
- 17.Abedini A., Chollet S., Angelis A., Borie N., Nuzillard J.M., Skaltsounis A.L., Reynaud R., Gangloff S.C., Renault J.H., Hubert J. Bioactivity-guided identification of antimicrobial metabolites in Alnus glutinosa bark and optimization of oregonin purification by centrifugal partition chromatography. J. Chromatogr. B. 2016;1029:121–127. doi: 10.1016/j.jchromb.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Dahija S., Čakar J., Vidic D., Maksimović M., Parić A. Total phenolic and flavonoid contents, antioxidant and antimicrobial activities of Alnus glutinosa (L.) Gaertn., Alnus incana (L.) Moench and Alnus viridis (Chaix) DC. extracts. Nat. Prod. Res. 2014;28:2317–2320. doi: 10.1080/14786419.2014.931390. [DOI] [PubMed] [Google Scholar]
- 19.Dinić J., Ranđelović T., Stanković T., Dragoj M., Isaković A., Novaković M., Pešić M. Chemo-protective and regenerative effects of diarylheptanoids from the bark of black alder (Alnus glutinosa) in human normal keratinocytes. Fitoterapia. 2015;105:169–176. doi: 10.1016/j.fitote.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Dinić J., Novaković M., Podolski-Renić A., Stojković S., Mandić B., Tešević V., Vajs V., Isaković A., Pešić M. Antioxidative activity of diarylheptanoids from the bark of black alder (Alnus glutinosa) and their interaction with anticancer drugs. Planta Med. 2014;80:1088–1096. doi: 10.1055/s-0034-1382993. [DOI] [PubMed] [Google Scholar]
- 21.León-Gonzalez A.J., Acero N., Munoz-Mingarro D., López-Lázaro M., Martín-Cordero C. Cytotoxic activity of hirsutanone, a diarylheptanoid isolated from Alnus glutinosa leaves. Phytomedicine. 2014;21:866–870. doi: 10.1016/j.phymed.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Acero N., Mingarro D.M. Effect on tumor necrosis factor-α production and antioxidant ability of black alder, as factors related to its anti-inflammatory properties. J. Med. Food. 2012;15:542–548. doi: 10.1089/jmf.2011.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J.Y., Jeong H.J., Kim J.H., Kim Y.M., Park S.J., Kim D., Park K.H., Lee W.S., Ryu Y.B. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol. Pharm. Bull. 2012;35:2036–2042. doi: 10.1248/bpb.b12-00623. [DOI] [PubMed] [Google Scholar]
- 24.Farrand L., Kim J.Y., Byun B.S., Im-aram A., Lee J., Suh J.Y., Lee K.W., Lee H.J., Tsang B.K. The diarylheptanoid hirsutenone sensitizes chemoresistant ovarian cancer cells to cisplatin via modulation of apoptosis-inducing factor and X-linked inhibitor of apoptosis. J. Biol. Chem. 2014;289:1723–1731. doi: 10.1074/jbc.M113.513879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y., Liao H.B., Guo D.H., Liu P., Wang Y.Y., Rahman K. Antidepressant-like effects of 3, 60-disinapoyl sucrose on hippocampal neuronal plasticity and neurotrophic signal pathway in chronically mild stressed rats. Neurochem. Int. 2010;56:461–465. doi: 10.1016/j.neuint.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Dong X.Z., Huang C.L., Yu B.Y., Hu Y., Mu L.H., Liu P. Effect of Tenuifoliside A isolated from Polygala tenuifolia on the ERK and PI3K pathways in C6 glioma cells. Phytomedicine. 2014;21:1178–1188. doi: 10.1016/j.phymed.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Tu H.H., Liu P., Ma L., Liao H.B., Xie T.T., Mu L.H., Liu Y.M. Study on antidepressant components of sucrose ester from Polygala tenuifolia. Chin. J. Chin. Mater. Med. 2008;33:1278–1280. [PubMed] [Google Scholar]
- 28.Kuroyanagl M., Shimomae M., Nagashima Y., Muto N., Okuda T., Kawahara N., Nakane T., Sano T. New diarylheptanoids from Alnus japonica and their antioxidative activity. Chem. Pham. Bull. 2005;53:1519–1523. doi: 10.1248/cpb.53.1519. [DOI] [PubMed] [Google Scholar]
- 29.Lee C.S., Jang E.R., Kim Y.J., Lee M.S., Seo S.J., Lee M.W. Hirsutenone inhibits lipopolysaccharide-activated NF-κB-induced inflammatory mediator production by suppressing Toll-like receptor 4 and ERK activation. Int. Immunopharmacol. 2010;10:520–525. doi: 10.1016/j.intimp.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Jin W.Y., Cai X.F., Na M.K., Lee J.J., Bae K.H. Triterpenoids and diarylheptanoids from Alnus hirsuta inhibit HIF-1 in AGS cells. Arch. Pharm. Res. 2007;30:412–418. doi: 10.1007/BF02980213. [DOI] [PubMed] [Google Scholar]
- 31.Phan M.G., Truong T.T.C., Phan T.S., Matsunami K., Otsuka K. A new diarylheptanoid and a rare dammarane triterpenoid from Alnus nepalensis. Chem. Nat. Comp. 2011;47:735–737. doi: 10.1007/s10600-011-0046-7. [DOI] [Google Scholar]
- 32.Yadav D., Kushwaha V., Saxena K., Verma R., Murthy P.K., Gupta M.M. Diarylheptanoid compounds from Alnus nepalensis express in vitro and in vivo antifilarial activity. Acta Trop. 2013;218:509–517. doi: 10.1016/j.actatropica.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Jin W.Y., Cai X.F., Na M.K., Lee J.J., Bae K.W. Diarylheptanoids from Alnus hirsuta inhibit the NF-kB activation and NO and TNF-α production. Biol. Pharm. Bul. 2007;30:810–813. doi: 10.1248/bpb.30.810. [DOI] [PubMed] [Google Scholar]
- 34.Kang H.M., Kim J.Y., Jeong T.S., Choi S.G., Ryu Y.H., Taeg Oh G., Baek N.I., Kwon B.M. Cyclic diarylheptanoids inhibit cell mediated low-density lipoprotein oxidation. Nat. Prod. Res. 2006;20:139–143. doi: 10.1080/14786410500045895. [DOI] [PubMed] [Google Scholar]
- 35.Nomura M., Tokoroyama T., Kubota T. Biarylheptanoids and other constituents from wood of Alnus japonica. Phytochemistry. 1981;20:1097–1104. doi: 10.1016/0031-9422(81)83035-X. [DOI] [Google Scholar]
- 36.Lee M.W., Tanaka T., Nonaka G.I., Nishioka I. Hirsunin, an ellagitannin with a diarylheptanoid moiety, from Alnus hirsuta var. microphylla. Phytochemistry. 1992;3:967–970. [Google Scholar]
- 37.Ishimatsu M., Tanaka T., Nonaka G.I., Nishioka I. Alnusnins A and B from the leaves of Alnus sieboldiana. Phytochemistry. 1989;28:3179–3184. doi: 10.1016/0031-9422(89)80302-4. [DOI] [Google Scholar]
- 38.Ivanova S.A., Nomura K., Malfanova I.L., Ptitsyn L.R. Glutinoin, a novel antioxidative ellagitannin from Alnus glutinosa cones with glutinoic acid dilactone moiety. Nat. Prod. Res. 2012;26:1806–1816. doi: 10.1080/14786419.2011.613387. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida T., Yazaki K., Memon M.U., Maruyama I., Kurokawa K., Shingu T., Okuda T. Structure of alnusiin and bicornin, new hydrolyzable tannins, having a monolactonized tergalloyl group. Chem. Pharm. Bull. 1989;37:2655–2660. doi: 10.1248/cpb.37.2655. [DOI] [Google Scholar]
- 40.Lv H., She G. Naturally occurring diarylheptanoids—A supplementary version. Rec. Nat. Prod. 2012;6:321–333. [Google Scholar]
- 41.Lee M.A., Lee H.K., Kim S.H., Kim Y.C., Sung S.H. Chemical constituents of Alnus firma and their inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Planta Med. 2010;76:1007–1010. doi: 10.1055/s-0029-1240911. [DOI] [PubMed] [Google Scholar]
- 42.Novaković M., Stanković M., Vučković I., Todorović N., Trifunović S., Apostolović D., Mandić B., Veljić M., Marin P., Tešević V., et al. Diarylheptanoids from green alder bark and their potential for DNA protection. Chem. Biodivers. 2014;11:872–885. doi: 10.1002/cbdv.201300277. [DOI] [PubMed] [Google Scholar]
- 43.Salama A.M. Anti-inflammatory activity of δ-amyrone and apigenin-4′,7-dimethylether isolated from Alnus acuminata. Rev. Col. Cienc. Quim. Farm. 2005;34:117–121. [Google Scholar]
- 44.Fujinori H., Neil T.G.H. Flavones from Alnus rubra Bong. seed coat. Bull. FFPRI. 2003;2:85–91. [Google Scholar]
- 45.Asakawa Y., Genjida F., Suga T. Four new flavonoids isolated from Alnus sieboldiana. Bull. Chem. Soc. Jpn. 1971;44:297. doi: 10.1246/bcsj.44.297. [DOI] [Google Scholar]
- 46.Ohmoto T., Nikaido T. Constituents of Pollen. IX. Pollen of Alnus sieboldiana Matsum. Shoyakugaku Zasshi. 1980;34:316–320. doi: 10.1248/yakushi1947.97.2_176. [DOI] [PubMed] [Google Scholar]
- 47.Kumarasamy Y., Cox P.J., Jaspars M., Nahar L., Sarker S.D. Bioactivity of hirsutanolol, oregonin and genkwanin isolated from the seeds of Alnus gultinosa (Betulaceae) Nat. Prod. Commun. 2006;1:641–644. [Google Scholar]
- 48.Rashed K., Ćirić A., Glamočlija J., Calhelha R.C., Ferreira I.C.F.R., Soković M. Antimicrobial and cytotoxic activities of Alnus rugosa L. aerial parts and identification of the bioactive components. Ind. Crop. Prod. 2014;59:189–196. doi: 10.1016/j.indcrop.2014.05.017. [DOI] [Google Scholar]
- 49.Asakawa Y. Chemical constituents of Alnus sieboldiana (Betulaceae) II. The isolation and structure of flavonoids and stilbenes. Bull. Chem. Soc. Jpn. 1971;44:2761–2766. doi: 10.1246/bcsj.44.2761. [DOI] [Google Scholar]
- 50.Suga T., Iwata N., Asakawa Y. Chemical constituents of the male flower of Alnus pendula (Betulaceae) Bull. Chem. Soc. Jpn. 1972;45:2058–2060. doi: 10.1246/bcsj.45.2058. [DOI] [Google Scholar]
- 51.Klischies M., Zenk M.H. Stereochemistry of C-methylation in the biosynthesis of rhododendrin in Alnus and Betula. Phytochemistry. 1978;17:1281–1284. doi: 10.1016/S0031-9422(00)94574-6. [DOI] [Google Scholar]
- 52.Yu Y.B., Miyashiro H., Nakamura N., Hattori M., Park J.C. Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Arch. Pharm. Res. 2007;30:820–826. doi: 10.1007/BF02978831. [DOI] [PubMed] [Google Scholar]
- 53.Tori M., Hashimoto A., Hirose K., Asakawa Y. Diarylheptanoids, flavonoids, stilbenoids, sesquiterpenoids and a phenanthrene from Alnus maximowiczii. Phytochemistry. 1995;40:1263–1264. doi: 10.1016/0031-9422(95)00439-E. [DOI] [Google Scholar]
- 54.Wollenweber E. Flavonoid compounds from Alnus virids. Phytochemistry. 1974;13:2618–2619. doi: 10.1016/S0031-9422(00)86948-4. [DOI] [Google Scholar]
- 55.Aoki T., Ohta S., Suga T. Triterpenoids, diarylheptanoids and their glycosides in the flowers of Alnus species. Phytochemistry. 1990;11:3611–3614. doi: 10.1016/0031-9422(90)85286-O. [DOI] [Google Scholar]
- 56.Felföldi-Gáva A., Szarka S., Simándi B., Blazics B., Simon B., Kéry A. Supercritical fluid extraction of Alnus glutinosa (L.) Gaertn. J. Supercrit. Fluids. 2012;61:55–61. doi: 10.1016/j.supflu.2011.10.003. [DOI] [Google Scholar]
- 57.Aguilar M.I., Rovelo R., Verjan J.G., Illescas O., Baeza A.E., Fuente M.D.L., Avila I., Navarrete A. Anti-inflammatory activities, triterpenoids, and diarylheptanoids of Alnus acuminata ssp. arguta. Pharm. Biol. 2011;49:1052–1057. doi: 10.3109/13880209.2011.564634. [DOI] [PubMed] [Google Scholar]
- 58.Suga T., Hirata T. New C31-secodammarane-type triterpenoids, alnuseric acid and alnuselide, in the male flowers of Alnus serrulatoides. Bull. Chem. Soc. Jpn. 1979;52:1153–1155. doi: 10.1246/bcsj.52.1153. [DOI] [Google Scholar]
- 59.Matyukhina L.G., Ryabinin A.A., Saltykova I.A., Shakhvorostova T.B. Triterpenes in plants from the Soviet Far East. Chem. Nat. Comp. 1968;4:387–388. doi: 10.1007/BF00569830. [DOI] [Google Scholar]
- 60.Chung M.Y., Rho M.C., Lee S.W., Park H.R., Kim K., Lee I.A., Kim D.H., Jeune K.H., Lee H.S., Kim Y.K. Inhibition of diacylglycerol acyltransferase by betulinic acid from Alnus hisuta. Planta Med. 2006;72:267–269. doi: 10.1055/s-2005-916178. [DOI] [PubMed] [Google Scholar]
- 61.Lee M., Lee M.K., Kim Y.C., Sung S.H. Antifibrotic constituents of Alnus firma on hepatic stellate cells. Bioorg. Med. Chem. Lett. 2011;21:2906–2910. doi: 10.1016/j.bmcl.2011.03.074. [DOI] [PubMed] [Google Scholar]
- 62.Plattner R., Taylo S.L., Grove M.D. Detection of brassinolide and castasterone in Alnus glutinosa (European Alder) pollen by mass spectrometry/mass spectrometry. J. Nat. Prod. 1986;49:540–545. doi: 10.1021/np50045a033. [DOI] [Google Scholar]
- 63.Ibrahim S.R.M., Fouad M.A., Abdel-Lateff A., Okino T., Mohamed G.A. Alnuheptanoid A: A new diarylheptanoid derivative from Alnus japonica. Nat. Prod. Res. 2014;28:1765–1771. doi: 10.1080/14786419.2014.947489. [DOI] [PubMed] [Google Scholar]
- 64.Chen Z., Li L.J., Zhang J.G., Wan W.C., Tu X.F., Lu L.H., Wu Y.H., Liu W.Q., Li L. Analysis of chemical components from ethy acetate layer of Alnus nepalensis D. Don. Yunnan Chem. Tech. 2011;38:14–17. [Google Scholar]
- 65.Favre-Bonvin J., Jay M., Wollenweber E. A novel stilbene from bud excretion of Alnus viridis. Phytochemistry. 1978;17:821–822. doi: 10.1016/S0031-9422(00)94249-3. [DOI] [Google Scholar]
- 66.Nomura M., Tokoroy T. Further phenolic components from Alnus japonica Steud. JCS Chem. Comm. 1975;131:136–137. doi: 10.1039/c39750000316. [DOI] [Google Scholar]
- 67.Uvarova N.I., Oshitok G.I., Suprunov N.I., Elyakov G.B. Triterpenoids and other constituents from the far-Eastern species of Alnus. Phytoclmnistry. 1972;11:741–743. doi: 10.1016/0031-9422(72)80041-4. [DOI] [Google Scholar]
- 68.Hashimoto T., Tori M., Asakawa Y. Five new diarylheptanoids from the male flowers of Alnus sieboldiana. Chem. Pharm. Bull. 1986;34:1846–1849. doi: 10.1248/cpb.34.1846. [DOI] [Google Scholar]
- 69.Lee M., Song J.Y., Chin Y.W., Sung S.H. Anti-adipogenic diarylheptanoids from Alnus hirsuta f. sibirica on 3T3-L1 cells. Bioorg. Med. Chem. Lett. 2013;23:2069–2073. doi: 10.1016/j.bmcl.2013.01.127. [DOI] [PubMed] [Google Scholar]
- 70.Lai Y.C., Chen C.K., Lin W.W., Lee S.S. A comprehensive investigation of anti-inflammatory diarylheptanoids from the leaves of Alnus formosana. Phytochemistry. 2012;73:84–94. doi: 10.1016/j.phytochem.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 71.Takashi S. Diarylheptanoids of Alnus hirsuta Turcz. (Betulaceae) Volume 42. Research Bulletins of the College Experiment Forests Hokkaido University; Hokkaido, Japan: 1985. pp. 191–205. [Google Scholar]
- 72.Chen J. Ph.D. Thesis. Oregon State University; Corvallis, OR, USA: 1996. Diarylheptanoid Glycosides from Red Alder Bark. [Google Scholar]
- 73.Choi S.E., Kim K.H., Kwon J.H., Kim S.B., Kim H.W., Lee M.W. Cytotoxic activities of diarylheptanoids from Alnus japonica. Arch. Pharm. Res. 2008;31:1287–1289. doi: 10.1007/s12272-001-2108-z. [DOI] [PubMed] [Google Scholar]
- 74.Telysheva G., Dizhbite T., Bikovens O., Ponomarenko J., Janceva S., Krasilnikova J. Structure and antioxidant activity of diarylheptanoids extracted from bark of grey alder (Alnus incana) and potential of biorefinery-based bark processing of European trees. Holzforschung. 2011;65:623–629. doi: 10.1515/hf.2011.096. [DOI] [Google Scholar]
- 75.Tung N.H., Suzuki M., Uto T., Morinaga O., Kwofie K.D., Ammah N., Koram K.A., Aboagye F., Edoh D., Yamashita T., et al. Anti-trypanosomal activity of diarylheptanoids isolated from the bark of Alnus japonica. Am. J. Chin. Med. 2014;42:1245–1260. doi: 10.1142/S0192415X14500785. [DOI] [PubMed] [Google Scholar]
- 76.Dinić J., Novaković M., Renić A.P., Vajs V., Tešević V., Isaković A., Pešić M. Structural differences in diarylheptanoids analogues from Alnus viridis and Alnus glutinosa influence their activity and selectivity towards cancer cells. Chem. Biol. Interact. 2016;249:36–45. doi: 10.1016/j.cbi.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 77.Lee M.W., Kim N.Y., Park M.S., Ahn K.H., Toh S.H., Hahn D.R., Kim Y.C., Chung H.T. Diarylheptanoids with in vitro inducible nitric oxide synthesis inhibitory activity from Alnus hisuta. Planta Med. 2000;66:551–553. doi: 10.1055/s-2000-8606. [DOI] [PubMed] [Google Scholar]
- 78.González-Laredo R.F., Helm R.F., Helm R.F., Chen J., Karchesy J.J. Two acylated diarylheptanoid glycosides from red alder bark. J. Nat. Prod. 1998;61:1292–1294. doi: 10.1021/np980083l. [DOI] [PubMed] [Google Scholar]
- 79.Ohta S., Aoki T., Hirata T., Suga T. The structures of four diarylheptanoid glycosides from the female flowers of Alnus serrulatoides. J. Chem. Soc. Perkin Trans. 1984:1635–1642. doi: 10.1039/p19840001635. [DOI] [Google Scholar]
- 80.Tunga N.H., Ra J.C., Sohnc D.H., Kima Y.H. A new diarylheptanoid from the bark of Alnus japonica. J. Asian Nat. Prod. Res. 2010;12:921–924. doi: 10.1080/10286020.2010.507196. [DOI] [PubMed] [Google Scholar]
- 81.Han J.M., Lee W.S., Kim J.R., Son J., Nam K.H., Choi S.C., Lim J.S., Jeong T.S. Effects of diarylheptanoids on the tumor necrosis factor-α-induced expression of adhesion molecules in human umbilical vein endothelial cells. J. Agric. Food Chem. 2007;55:9457–9464. doi: 10.1021/jf072157h. [DOI] [PubMed] [Google Scholar]
- 82.Martineau L.C., Hervé J., Muhammad A., Saleem A., Harris C.S., Arnsaon J.T., Haddad P.S. Anti-adipogenic activities of Alnus incana and Populus balsamifera bark extracts, Part I: Sites and mechanisms of action. Planta Med. 2010;76:1439–1446. doi: 10.1055/s-0029-1240941. [DOI] [PubMed] [Google Scholar]
- 83.Tung N.H., Kwon N.J., Kim J.H., Ra J.C., Ding Y., Kim J.A., Kim Y.H. Anti-influenza diarylheptanoids from the bark of Alnus japonica. Bioorg. Med. Chem. Lett. 2010;10:1000–1003. doi: 10.1016/j.bmcl.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 84.Chena J., González-Laredo R.F., Karchesy J.J. Minor diarylheptanoid glycosides of Alnus rubra bark. Phytochemistry. 2000;53:971–973. doi: 10.1016/S0031-9422(99)00523-3. [DOI] [PubMed] [Google Scholar]
- 85.Hu W.C., Wang M.H. Diarylheotanoid from Alnus hirsuta improves glucose metabolism via insulin signal transduction in human hepatocarcinoma (HepG2) cells. Biotechnol. Bioproc. Eng. 2011;16:120–126. doi: 10.1007/s12257-010-0311-9. [DOI] [Google Scholar]
- 86.Kim H.J., Kim K.H., Yeom S.H., Kim M.K., Shim J.G., Lim H.W., Lee M.W. New diarylheptanoid from the barks of Alnus japonica Steudel. Chin. Chem. Lett. 2005;16:1337–1340. [Google Scholar]
- 87.Choi S.E., Park K.H., Kim M.H., Song J.H., Jin H.Y., Lee M.W. Diarylheptanoids from the bark of Alnus pendula Matsumura. Nat. Prod. Sci. 2012;18:106–110. [Google Scholar]
- 88.Yadav D., Gupta M.M. Simultaneous quantification of diarylheptanoids in Alnus nepalensis using a validated HPTLC method. J. Chromatogr. Sci. 2013;52:905–910. doi: 10.1093/chromsci/bmt115. [DOI] [PubMed] [Google Scholar]
- 89.Ko E.K., Choi H. Oregonin from the stems and leaves of Korean Alnus species (Betulaceae) J. Chem. Pharm. Res. 2015;7:234–238. [Google Scholar]
- 90.Ponomarenko J., Trouillas P., Martin N., Dizhbite T., Krasilnikova J., Telysheva G. Elucidation of antioxidant properties of wood bark derived saturated diarylheptanoids: A comprehensive (DFT-supported) understanding. Phytochemistry. 2014;103:178–187. doi: 10.1016/j.phytochem.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 91.Lee S.S., Chen S.C., Chen C.K., Chen C.K., Kuo C.M. Chemical constituents from Alnus formosana burk. II. Polar constituents from the leaves. Nat. Prod. Commun. 2006;1:461–464. [Google Scholar]
- 92.Wada H., Tachibana H., Fuchino H., Takana N. Three new diarylheptanoid glycosides from Alnus japonica. Chem. Pharm. Bull. 1998;46:1054–1055. doi: 10.1248/cpb.46.1054. [DOI] [Google Scholar]
- 93.Chen J., Karchesy J.J., González-Laredo R.F. Phenolic diaryheptenones from Alnus rubra bark. Planta Med. 1998;64:74–75. doi: 10.1055/s-2006-957372. [DOI] [PubMed] [Google Scholar]
- 94.Siddiqui I.N., Ahmad V.U., Zahoor A., Ahmed A., Khan S.S., Khan A., Hassan Z. The two diarylheptanoids from Alnus nitida. Nat. Prod. Commun. 2010;5:1787–1788. [PubMed] [Google Scholar]
- 95.Asakawa Y. Chemical constituents of Alnus sieboldiana (Betulaceae). Ш. The synthesis and stereochemistry of yashabushiketols. Bull. Chem. Soc. Jpn. 1972;45:1794–1797. doi: 10.1246/bcsj.45.1794. [DOI] [Google Scholar]
- 96.Asakawa Y. Chemical contituents of Alnus firma (Betuaceae). I. Phenyl propane derivatives isolated from Alnus firma. Bull. Chem. Soc. Jpn. 1970;43:2223–2229. doi: 10.1246/bcsj.43.2223. [DOI] [Google Scholar]
- 97.Chiba K., Ichizawa H., Kawai S., Nishida T. α-Glucosidase inhibition activity by cyclic diarylheptanoids from Alnus sieboldiana. J. Wood Chem. Technol. 2013;33:44–51. doi: 10.1080/02773813.2012.723778. [DOI] [Google Scholar]
- 98.Altınyay C., Süntar I., Altun L., Keleş H., Akkol E.K. Phytochemical and biological studies on Alnus glutinosa subsp. glutinosa, A. orientalis var. orientalis and A. orientalis var. pubescens leaves. J. Ethnopharmacol. 2016;192:148–160. doi: 10.1016/j.jep.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 99.Sajida M., Khana M.R., Shah S.A., Majid M., Ismail H., Maryam S., Batool R., Younis T. Investigations on anti-inflammatory and analgesic activities of Alnus nitida Spach (Endl). stem bark in Sprague Dawley rats. J. Ethnopharmacol. 2017;198:407–416. doi: 10.1016/j.jep.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 100.Ludwiczuk A., Saha A., Kuzuhara T., Asakawa Y. Bioactivity guided isolation of anticancer constituents from leaves of Alnus sieboldiana (Betulaceae) Phytomedicine. 2011;18:491–498. doi: 10.1016/j.phymed.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Kim M.B., Park J.S., Lim S.B. Antioxidant activity and cell toxicity of pressurised liquid extracts from 20 selected plant species in Jeju, Korea. Food Chem. 2010;122:546–552. doi: 10.1016/j.foodchem.2010.03.007. [DOI] [Google Scholar]
- 102.Han H.K., Choi S.S., Kim Y.R., Kim H.J., Kang G.M., Dong M.S., Na C.S., Chung H.S. Diarylheptanoid and flavonoid with antioxidant activity from Alnus japonica Steud on DPPH free radical scavenging assay. J. Food Sci. Nutr. 2006;11:171–175. doi: 10.3746/jfn.2006.11.2.171. [DOI] [Google Scholar]
- 103.Sakamura F., Ohta S., Aoki T., Suga T. Triterpenoids from the female and male flowers of Alnus sieboldiana. Phytochemistry. 1985;24:2744–2745. doi: 10.1016/S0031-9422(00)80715-3. [DOI] [Google Scholar]
- 104.Sheth K., Bianchi E., Wiedhopf R., Cole J.R. Antitumor agents from Alnus oregona (Betulaceae) J. Pharm. Sci. 1973;62:139–140. doi: 10.1002/jps.2600620129. [DOI] [PubMed] [Google Scholar]
- 105.Li H., Webster D., Johnson J.A., Gray C.A. Anti-mycobacterial triterpenes from the Canadian medicinal plant Alnus incana. J. Ethnopharmacol. 2015;165:148–151. doi: 10.1016/j.jep.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 106.Felföldi-Gáva A., Simándi B., Plánder S., Szarka É., Kéry Á. Betulaceae and Platanaceae plants as alternative sources of selected lupane-type triterpenes. Their composition profile and betulin content. Acta Chromatogr. 2009;21:671–681. doi: 10.1556/AChrom.21.2009.4.12. [DOI] [Google Scholar]
- 107.Vo V.C. The Dictionary of Vietnamese Medicinal Plants. Publishing House Medicine; Ho Chi Minh City, Vietnam: 1997. [Google Scholar]
- 108.Kim D.S., Park S.Y., Kim J.K. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1–42) insult. Neurosci. Lett. 2001;303:57–61. doi: 10.1016/S0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- 109.Liu H.T., Wang L.L., Liu L.X. Advances in understanding mechanisms underlying the antitumor activity of curcumin analogue EF24. World Chin. J. Digesto. 2012;20:1853–1857. doi: 10.11569/wcjd.v20.i20.1853. [DOI] [Google Scholar]
- 110.Kim J.H., Lee K.W., Lee M.W., Lee H.J., Kimd S.H., Surha Y.J. Hirsutenone inhibits phorbol ester-induced upregulation of COX-2 and MMP-9 in cultured human mammary epithelial cells: NF-κB as a potential molecular target. FEBS. Lett. 2006;580:385–392. doi: 10.1016/j.febslet.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 111.Lee C.S., Jang E.R., Kim Y.J., Myung S.C., Kim W., Lee M.W. Diarylheptanoid hirsutenone enhances apoptotic effect of TRAIL on epithelial ovarian carcinoma cell lines via activation of death receptor and mitochondrial pathway. Investig. New Drugs. 2012;30:548–557. doi: 10.1007/s10637-010-9601-5. [DOI] [PubMed] [Google Scholar]
- 112.Kang S., Kim J.E., Li Y., Jung S.K., Song N.Y., Thimmegowda N.R., Kim B.Y., Lee H.J., Bode A.M., Dong Z., et al. Hirsutenone in Alnus extract inhibits Akt activity and suppresses prostate cancer cell proliferation. Mol. Carcinogen. 2015;54:1354–1362. doi: 10.1002/mc.22211. [DOI] [PubMed] [Google Scholar]
- 113.Lee M.W., Kim J.H., Jeong D.W., Ahn K.H., Toh S.H., Surh Y.J. Inhibition of clycooxygenase-2 expression by diarylheptanoids from the bark of Alnus hisuta var. sibirica. Biol. Pharm. Bull. 2000;23:517–518. doi: 10.1248/bpb.23.517. [DOI] [PubMed] [Google Scholar]
- 114.Joo S.S., Kim M.S., Oh W.S., Lee D.I. Enhancement of NK cytotoxicity, antimetastasis and elongation effect of survival time in B16-F10 melanoma cells by oregonin. Arch. Pharm. Res. 2002;25:493–499. doi: 10.1007/BF02976608. [DOI] [PubMed] [Google Scholar]
- 115.Joo S.S., Kim H.J., Kwon H.S., Lee D.I. Augmentation of macrophage antitumor activities and nitric oxide production by oregonin. Arch. Pharm. Res. 2002;25:457–462. doi: 10.1007/BF02976602. [DOI] [PubMed] [Google Scholar]
- 116.Stević T., Šavikin K., Zdunić G., Stanojković T., Juranić Z., Janković T., Menković N. Antioxidant, cytotoxic, and antimicrobial activity of Alnus incana (L.) ssp. incana Moench and A. viridis (Chaix) DC ssp. viridis extracts. J. Med. Food. 2010;13:700–704. doi: 10.1089/jmf.2009.0111. [DOI] [PubMed] [Google Scholar]
- 117.Chang J.H., Cho J.H., Kim H.H., Lee K.P., Lee M.W., Han S.S., Lee D.I. Antitumor activity of pedunculagin, one of the ellagitannin. Arch. Pharm. Res. 1995;18:396–401. doi: 10.1007/BF02976342. [DOI] [Google Scholar]
- 118.Poljsak B., Šuput D., Milisav I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sajid M., Khan M.R., Shah N.A., Shah S.A., Ismail H., Younis T., Zahra Z. Phytochemical, antioxidant and hepatoprotective effects of Alnus nitida bark in carbon tetrachloride challenged Sprague Dawley rats. BMC Complem. Altern. Med. 2016;16:268. doi: 10.1186/s12906-016-1245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee W.S., kim J.Y., Im K.R., Cho K.H., Sok D.E., Jeong T.S. Antioxidant effects of diarylheptanoid derivatives from Alnus japonica on human LDL oxidation. Planta Med. 2005;71:295–299. doi: 10.1055/s-2005-864093. [DOI] [PubMed] [Google Scholar]
- 121.Kim H.J., Yeom S.H., Kim M.K., Paek I.N., Lee M.W. Nitric oxide and prostaglandin E2 synthesis inhibitory activities of diarylheptanoids from the barks of Alnus japonica Steudel. Arch. Pharm. Res. 2005;28:177–179. doi: 10.1007/BF02977711. [DOI] [PubMed] [Google Scholar]
- 122.Saxena A., Yadav D., Maurya A.K., Kumar A., Mohanty S., Gupta M.M., Lingaraju M.C., Yatoo M.I., Thakur U.S., Bawankule D.U. Diarylheptanoids from Alnus nepalensis attenuates LPS-induced inflammation in macrophages and endotoxic shock in mice. Int. Immunopharmacol. 2016;30:129–136. doi: 10.1016/j.intimp.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 123.Lee C.J., Lee S.S., Chen S.C., Ho F.M., Lin W.W. Oregonin inhibits lipopolysaccharide-induced iNOS gene transcription and upregulates HO-1 expression in macrophages and microglia. Br. J. Pharmacol. 2005;146:378–388. doi: 10.1038/sj.bjp.0706336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lundqvist A., Magnusson L.U., Ullstrom C., Krasilnikova J., Telysheva T., Dizhbite T., Hulten L.M. Oregonin reduces lipid accumulation and proinflammatory responses in primary human macrophages. Biochem. Bioph. Res. Commun. 2015;458:693–699. doi: 10.1016/j.bbrc.2015.01.161. [DOI] [PubMed] [Google Scholar]
- 125.Lee S.C., Ko H.H., Seo S.J., Choi Y.W., Lee M.W., Myung S.C., Bang H. Diarylheptanoid hirsutenone prevents tumor necrosis factor-α-stimulated production of inflammatory mediators in human keratinocytes through NF-κB inhibition. Int. Immunopharmacol. 2009;9:1097–1104. doi: 10.1016/j.intimp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 126.Choi J.G., Lee M.W., Choi S.E., Kim M.H., Kang O.H., Lee Y.S., Chae H.S., Obiang-Obounou B., OH Y.C., Kim M.R., et al. Antibacterial activity of bark of Alnus pendula against methicillin-resistant Staphylococcus aureus. Eur. Rev. Med. Pharmacol. 2012;16:853–859. [PubMed] [Google Scholar]
- 127.Sang T.K., Jung D.K., Ahn S.H., Ahn G.S., Lee Y.I., Jeong Y.S. Hepatoprotective and antioxidant effects of Alnus japonica extracts on acetaminophen induced hepatotoxicity in rats. Phytother. Res. 2004;18:971–975. doi: 10.1002/ptr.1540. [DOI] [PubMed] [Google Scholar]
- 128.Martineau L.C., Muhammad A., Saleem A., Hervé J., Harris C.S., Arnsaon J.T., Haddad P.S. Anti-adipogenic activities of Alnus incana and Populus balsamifera bark extracts, Part II: bioassay-guided identification of actives salicortin and oregonin. Planta Med. 2010;76:1519–1524. doi: 10.1055/s-0029-1240991. [DOI] [PubMed] [Google Scholar]
- 129.Choi S.E., Jeong M.S., Kang M.J., Lee D.I., Joo S.S., Lee C.S., Bang H., Lee M.K., Myung S.C., Choi Y.W., et al. Effect of topical application and intraperitoneal injection of oregonin on atopic dermatitis in NC/Nga mice. Exp. Dermatol. 2010;19:37–43. doi: 10.1111/j.1600-0625.2009.00961.x. [DOI] [PubMed] [Google Scholar]
- 130.Jeong M.S., Choi S.E., Kim J.Y., Kim J.S., Kim E.J., Park K.H., Lee D.I., Joo S.S., Lee C.S., Bang H., et al. Atopic dermatitis-like skin lesions reduced by topical application and intraperitoneal injection of hirsutenone in NC/Nga mice. Clin. Dev. Immunol. 2010;2010:618517. doi: 10.1155/2010/618517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Choi S.E., Park K.H., Jeong M.S., Kim H.H., Lee D.I., Joo S.S., Lee C.S., Bang H.B., Choi Y.W., Lee M.K., et al. Effect of Alnus japonica extract on a model of atopic dermatitis in NC/Nga mice. J. Ethnopharmacol. 2011;136:406–413. doi: 10.1016/j.jep.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 132.Joo S.S., Kim S.G., Choi S.E., Kim Y.B., Park H.Y., Seo S.J., Choi Y.W., Lee M.W., Lee D.I. Suppression of T cell activation by hirsutenone, isolated from the bark of Alnus japonica, and its therapeutic advantages for atopic dermatitis. Eur. J. Pharmacol. 2009;614:98–105. doi: 10.1016/j.ejphar.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 133.Kang M.J., Eum J.Y., Jeong M.S., Park S.H., Moon K.Y., Kang M.H., Kim M.S., Choi S.E., Lee MW., Lee D.I., et al. Tat peptide-admixed elastic liposomal formulation of hirsutenone for the treatment of atopic dermatitis in NC/Nga mice. Int. J. Nanomed. 2011;6:2459–2467. doi: 10.2147/IJN.S24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kang M.J., Eum J.Y., Jeong M.S., Choi S.E., Park S.H., Cho H.I., Cho C.S., Seo S.J., Lee M.W., Choi Y.W. Facilitated skin permeation of oregonin by elastic liposomal formulations and suppression of atopic dermatitis in NC/Nga mice. Biol. Pharm. Bull. 2010;33:100–106. doi: 10.1248/bpb.33.100. [DOI] [PubMed] [Google Scholar]
- 135.Yadav D., Singh S.C., Verma R.K., Saxena K., Verma R., Murthy P.K., Gupta M.M. Antifilarial diarylheptanoids from Alnus nepalensis leaves growing in high altitude areas of Uttarakhand, India. Phytomedicine. 2013;20:124–132. doi: 10.1016/j.phymed.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 136.Cho S.M., Kwon Y.M., Cho S.M., Kwon Y.M., Lee J.H., Yon K.H., Lee M.W. Melanogenesis inhibitory activities of diarylheptanoids from Alnus hirsuta Turcz in B16 mouse melanoma cell. Arch. Pharm. Res. 2002;25:885–888. doi: 10.1007/BF02977009. [DOI] [PubMed] [Google Scholar]
- 137.Jo H., Choi S.J., Jung S.H. Protective effects of a compound isolated from Alnus japonica on oxidative stress-induced death in transformed retinal ganglion cells. Food Chem. Toxicol. 2013;56:425–435. doi: 10.1016/j.fct.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 138.Rashed K., Sucupira A.C.C., Neto J.M.M., Feitosa C.M. Evaluation of acetylcholinesterase inhibition by Alnus rugosa L. stems methanol extract and phytochemical content. IJBAR. 2013;4:606–609. doi: 10.7439/ijbar.v4i9.456. [DOI] [Google Scholar]