Abstract
Emerging evidence supports the importance of ghrelin to defend against starvation-induced hypoglycemia. This effect may be mediated by inhibition of glucose-stimulated insulin secretion as well as reduced insulin sensitivity. However, administration of ghrelin during meal consumption also stimulates the release of glucagon-like peptide 1 (GLP-1), an incretin important in nutrient disposition. The objective of this study was to evaluate the interaction between ghrelin and GLP-1 on parameters of glucose tolerance following a mixed-nutrient meal. Fifteen healthy men and women completed the study. Each consumed a standard meal on four separate occasions with a superimposed infusion of 1) saline, 2) ghrelin, 3) the GLP-1 receptor antagonist exendin(9-39) (Ex9), or 4) combined ghrelin and Ex9. Similar to previous studies, infusion of ghrelin caused glucose intolerance, whereas Ex9 had a minimal effect. However, combined ghrelin and Ex9 resulted in greater postprandial glycemia than either alone, and this effect was associated with impaired β-cell function and decreased glucose clearance. These findings suggest that in the fed state, stimulation of GLP-1 mitigates some of the effect of ghrelin on glucose tolerance. This novel interaction between gastrointestinal hormones suggests a system that balances insulin secretion and glucose disposal in the fed and fasting states.
Introduction
The importance of the enteroendocrine system in nutrient sensing and assimilation is increasingly recognized. Throughout a meal, the gastrointestinal (GI) hormones act in concert to regulate gastric acid secretion, GI motility, glucose homeostasis, appetite, and food intake. Each of the more than 20 known GI hormones was initially thought to be produced by specific enteroendocrine cells, but it is now understood that these cells are plastic and express a range of peptide precursors (1). This finding suggests flexibility and coordination of the GI endocrine system to regulate biological functions.
Two gut peptides that have received considerable attention in recent years are glucagon-like peptide 1 (GLP-1) and ghrelin. GLP-1, a posttranslational product of proglucagon, is secreted by the L cells of the distal gut during meal ingestion. GLP-1 reduces postprandial glucose excursions through its combined effects to stimulate insulin release, inhibit glucagon secretion, and delay gastric emptying (2). This suite of activities promotes glucose tolerance and is sufficiently potent that GLP-1 receptor (GLP-1R) agonists are now applied to the treatment of type 2 diabetes. Ghrelin is an orexigenic hormone produced by neuroendocrine cells in the stomach (3). The ghrelin receptor, also termed the growth hormone secretagogue receptor 1a (GHSR-1a), is highly expressed in the pituitary gland, islet δ-cells, and the GI tract, including the L cells (4,5). Ghrelin levels peak before an anticipated meal and decline with nutrient intake, as GLP-1 levels rise (2,4). Accordingly, many of the functions of ghrelin are the inverse of GLP-1: ghrelin accelerates GI motility, suppresses glucose-stimulated insulin secretion (GSIS), and impairs glucose tolerance (4,6,7).
Previous studies in humans and rodents indicate that ghrelin, given at pharmacological doses, stimulates GLP-1 secretion during a meal (5,8). These findings suggest an interaction between ghrelin and GLP-1 on glucose metabolism. We hypothesized that stimulation of GLP-1 release by ghrelin would attenuate the effects of ghrelin to limit insulin secretion and impair glucose tolerance. To test this hypothesis, healthy men and women were given ghrelin and/or the GLP-1R antagonist exendin(9-39) (Ex9) during standard mixed-nutrient meals.
Research Design and Methods
Subjects
Healthy men and women aged 18–45 years with a BMI of 18–29 kg/m2 were recruited from the greater Cincinnati area. Subjects with a personal or family history of diabetes or active medical problems including renal, hepatic, and endocrine disorders or who were on medications known to alter glucose metabolism were excluded.
All study procedures were conducted in the Clinical Translational Research Center at the Cincinnati Children’s Hospital Medical Center (CCHMC). All study participants gave informed consent for the study by signing a form approved by University of Cincinnati and CCHMC institutional review boards.
Synthetic human acyl ghrelin and Ex9 were obtained from CSBio (Menlo Park, CA). 6,6-[2H2]-glucose and [U-13C]-glucose were obtained from Cambridge Isotope Laboratories (Tewksbury, MA). Synthetic acyl ghrelin (active ghrelin) and Ex9 were used under research Investigational New Drug applications (79,009 and 65,837) from the U.S. Food and Drug Administration.
Experimental Protocol
Subjects arrived at the CCHMC Clinical Translational Research Center between 0730 and 0800 after a 10–12 h fast on four occasions separated by at least 1 week. Intravenous catheters were placed in veins of both forearms for blood sampling and infusion of test substances. The arm with the sampling catheter was placed in a 55°C chamber to arterialize venous blood.
On the morning of the four study days, a priming dose of 6,6-[2H2]-glucose (22 μmol/kg) was given at −120 min, followed by a continuous infusion at 0.22 μmol/kg/min throughout a 240-min mixed-meal tolerance test (MTT). A bolus injection of 0.28 μg/kg of synthetic human acyl ghrelin, 25 μg/kg of Ex9, combined acyl ghrelin and Ex9, or 5 mL of 0.9% saline was given intravenously at −30 min, followed by continuous infusion of acyl ghrelin at 2 μg/kg/h (0.6 nmol/kg/h), Ex9 at 0.15 mg/kg/h (750 pmol/kg/h), combined acyl ghrelin and Ex9, or 0.9% saline; these primed peptide infusions were designed to achieve steady state prior to the MTT based on previous studies. We have previously shown that the 2 μg/kg/h dose of ghrelin suppresses intravenous GSIS (9), whereas 0.15 mg/kg/h of Ex9 blocks 90% of supraphysiological GLP-1–induced insulin release (10). The infusions were continued throughout the MTT, which occurred on four separate occasions in a random order. Following 30 min of saline, ghrelin, and/or Ex9 infusion, subjects consumed a mixed meal consisting of 50 g of glucose solution, 1 g of [U-13C]-glucose, and 50 g of eggs and cheese (585 kcal, 51% carbohydrates, 31% fat, 18% protein) within 10 min. Blood samples were collected at 10–30 min intervals during the 300-min tracer infusion period.
Blood samples for tracer activity and insulin measurement were collected in EDTA tubes. Anti-protease cocktail containing aprotinin and EDTA were added to tubes for C-peptide measurement. Blood samples were placed on ice and plasma separated by centrifugation within 1 h; plasma or serum was stored at −80°C until assay. Vital signs were monitored every 15 min during the study procedure.
Assays
Details of biochemical assays were described previously (6,11). Briefly, blood glucose concentrations were determined at the bedside using a glucose analyzer (YSI 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH). Plasma immunoreactive insulin and C-peptide levels were measured by ELISA, as we have reported in the past (10). Plasma enrichment of [U-13C]-glucose and 6,6-[2H2]-glucose was measured by gas chromatography–mass spectrometry as described previously (12). All samples were run in duplicate, and all specimens from a given participant were run in the same assay.
Calculations
Fasting/baseline values of insulin and glucose were taken as the mean of samples drawn at −10 and −1 min before the MTT. Integrated values of glucose and insulin were expressed as area under the response curve (AUC) over the level before meal ingestion. Insulin secretion rate (ISR) was derived from plasma C-peptide concentrations using a deconvolution model with population estimates of C-peptide clearance (13) using MLAB (Civilized Software Inc., Bethesda, MD). To correct for differences in postprandial glucose among the four conditions, incremental insulin levels in response to the meal were summed as ΔAUC insulin/ΔAUC glucose0–240 min (ΔAUC I/ΔAUC G0–240 min). Insulin sensitivity during the meal was computed with the oral glucose insulin sensitivity (OGIS) index (14,15) and the Matsuda index (16). The disposition index (DI) was calculated as (ΔAUC I/ΔAUC G0–240 min) × Matsuda index0–240 min.
β-Cell function during the MTT was studied using a previously validated model (15,17) that expresses ISR as the sum of two components: 1) rate sensitivity Kd, which accounts for the effect of the rate of change in glucose concentrations on ISR and represents principally early insulin release, and 2) glucose sensitivity (the slope of ISR and blood glucose concentration), which accounts for the impact of changes in glucose concentration on ISR. The glucose sensitivity was computed separately for the first part of the MTT, as glucose levels increased to peak values, and for the latter part, as they declined toward fasting levels (12).
Total rate of postprandial glucose appearance (total Ra) was calculated by modeling 6,6-[2H2]-glucose enrichment using mathematical models as previously described (11). Fasting endogenous glucose production (EGP) was calculated as the ratio of 6,6-[2H2]-glucose infusion rate to plasma tracer enrichment (tracer-to-tracee ratio 6,6) from measurements obtained in the last 20 min of the basal tracer equilibration period, when plasma glucose concentration and 6,6-[2H2]-glucose enrichment are stable. The contribution of meal (exogenous) glucose appearance (Ra meal) and EGP to total Ra during the MTT was calculated using the [U-13C]-glucose enrichment data. Peripheral glucose disposal (Rd) was calculated by subtracting the rate of change of plasma glucose mass from total Ra. Glucose clearance was estimated during the MTT using Rd divided by glucose concentration (mL/kg/kg). Insulin clearance was calculated for both fasting and fed states by dividing ISR basal by fasting insulin and AUC ISR0–240 min by AUC I0–240 min, respectively (18).
Statistical Analysis
The primary analysis was the effects of the treatments on glucose tolerance using comparison of postprandial glucose AUC. Secondary analyses included ghrelin effects on postprandial insulin secretion and clearance and glucose fluxes. Primary and secondary comparisons were made using repeated-measures ANOVA to compare the treatment measures. Comparisons were made between saline and ghrelin infusions as well as ghrelin and combined ghrelin and Ex9 infusions, unless otherwise specified. A nonparametric variation of the ANOVA model (Friedman test) was used when there was a significant departure of the data from parametric assumptions. Holm-Šidák and Dunn multiple comparison tests were used for post hoc analysis of parametric and nonparametric data, respectively. Data were analyzed using GraphPad Prism, version 7.0 (GraphPad Software) and Statview (SAS). All results are expressed as mean ± SD unless otherwise noted.
Results
Subject Characteristics
The fifteen volunteers (9 males and 6 females) were aged mean ± SD 26.0 ± 5.7 years (range 20–44 years) and had a BMI of 25.2 ± 3.0 kg/m2 (range 18.9–29.2 kg/m2). The fasting blood glucose for the group was 88.9 ± 5.8 mg/dL; the fasting plasma insulin was 6.6 ± 4.4 mU/L.
Effects of Exogenous Ghrelin and GLP-1R Blockade on Glucose Tolerance and Insulin Secretion
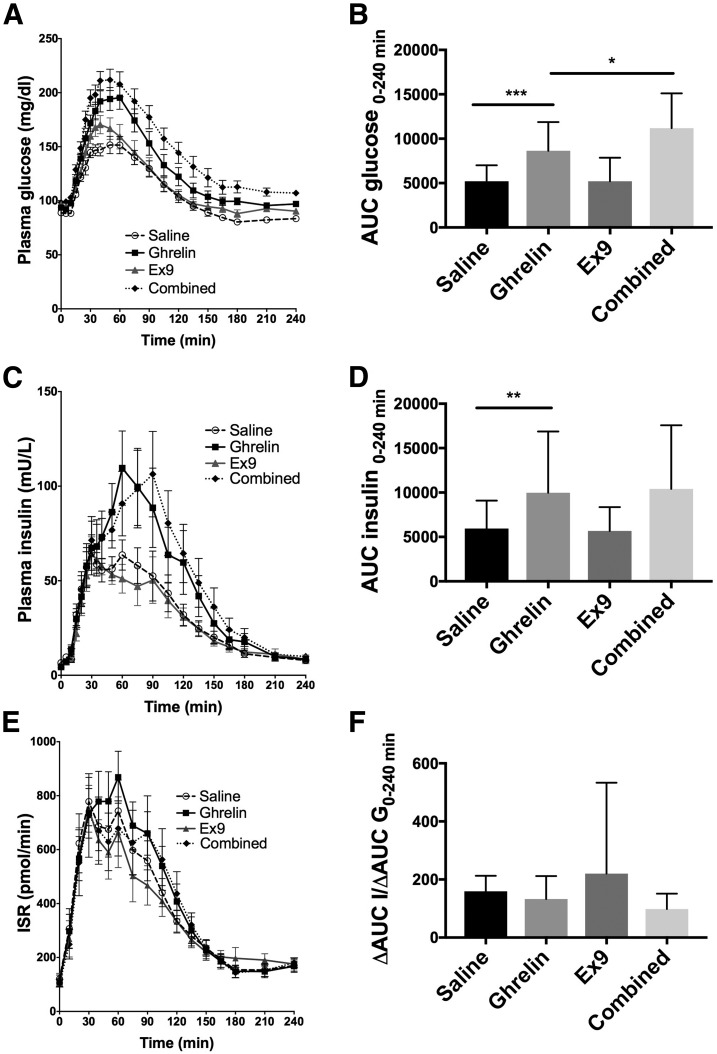
Intravenous ghrelin increased postprandial glucose AUC compared with saline (P = 0.0005) (Fig. 1A and B and Table 1). The addition of Ex9 to ghrelin caused postprandial glucose to be significantly elevated compared with ghrelin alone (P = 0.03). This interaction supports an effect of GLP-1 to attenuate ghrelin action and provide a counterbalance to the negative effects of ghrelin on glucose tolerance after eating.
Figure 1.
Effects of ghrelin, Ex9, and combined ghrelin and Ex9 treatment on plasma glucose (A and B), insulin (C and D), ISR (E), and insulin corrected for glucose (F) during the 240-min MTT. Statistical comparisons were made between saline and ghrelin treatments and ghrelin and combined treatments. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 1.
The effects of ghrelin, Ex9, and combined ghrelin and Ex9 treatment on glucose tolerance, insulin secretion, insulin sensitivity, insulin clearance, and postprandial glucose flux in healthy men and women
| Saline | Ghrelin | Ex9 | Combined | P | |
|---|---|---|---|---|---|
| AUC G0–240 min (mg/dL) |
5,201 ± 1,806 |
8,634 ± 3,238* |
5,209 ± 2,647 |
11,196 ± 3,912† |
<0.0001 |
| AUC I0–240 min (mU/L) |
5,949 ± 3,138 |
9,967 ± 6,907* |
5,678 ± 2,673 |
10,408 ± 7,161 |
0.0002 |
| ΔAUC I/ΔAUC G0–240 min |
159.2 ± 53.5 |
132.8 ± 78.8 |
220.3 ± 312.8 |
98.2 ± 53 |
0.0523 |
| AUC ISR0–240 min (pmol/min) |
90,710 ± 41,439 |
98,229 ± 38,765 |
85,724 ± 35,692 |
93,337 ± 57,799 |
0.2572 |
| Slope (pmol/min/mmol/L) |
164.5 ± 83.9 |
127.1 ± 93.3 |
149 ± 102.3 |
86 ± 66.4† |
0.0002 |
| Kd (pmol/mmol/L) |
1,290 ± 1,197 |
642.6 ± 1,091* |
1,154 ± 875.3 |
668.1 ± 765.9 |
0.0078 |
| Matsuda index |
10.25 ± 9 |
8.8 ± 5.3 |
8 ± 3 |
6.6 ± 3.7 |
0.0356 |
| OGIS0–180 min (mL/min/kg) |
10.2 ± 1.8 |
8.7 ± 1.7* |
9.4 ± 1.2 |
8.5 ± 1.8 |
<0.0001 |
| DI |
1,478 ± 1,096 |
1,015 ± 561 |
1,971 ± 3,438 |
533 ± 244† |
<0.0001 |
| Fasting insulin clearance (mL/min) |
2.35 ± 1.5 |
2.75 ± 1.5 |
2.29 ± 1.05 |
1.83 ± 0.66 |
0.0476 |
| Meal insulin clearance (mL/min) |
1.29 ± 0.8 |
1.01 ± 0.605* |
1.17 ± 0.427 |
0.817 ± 0.398 |
0.0011 |
| AUC PYY (pg/mL) |
2,936 ± 1,187 |
4,337 ± 1,573* |
4,765 ± 2,029 |
7,587 ± 2,189† |
<0.0001 |
| AUC total Ra0–240 min (μmol/min/kg) |
3,864 ± 485.5 |
4,156 ± 632.6 |
3,916 ± 425.6 |
4,130 ± 506 |
0.1718 |
| AUC Ra meal0–120 min (μmol/min/kg) |
1,981 ± 306.4 |
2,294 ± 485.1* |
1,821 ± 461.8 |
2,228 ± 330.7 |
<0.0001 |
| AUC EGP0–240 min (μmol/min/kg) |
1,126 ± 395.7 |
1,407 ± 568.6 |
1,243 ± 429.6 |
1,428 ± 423.6 |
0.0208 |
| AUC glucose clearance0–240 min (mL/min/kg) | 674.1 ± 102.1 | 589.4 ± 107.6* | 646 ± 97.5 | 528.7 ± 88.3† | <0.0001 |
Data are mean ± SD. Comparisons were made between 1) ghrelin and saline and 2) ghrelin and combined treatments.
*P < 0.05 acyl ghrelin vs. saline;
†P < 0.05 acyl ghrelin vs. combined.
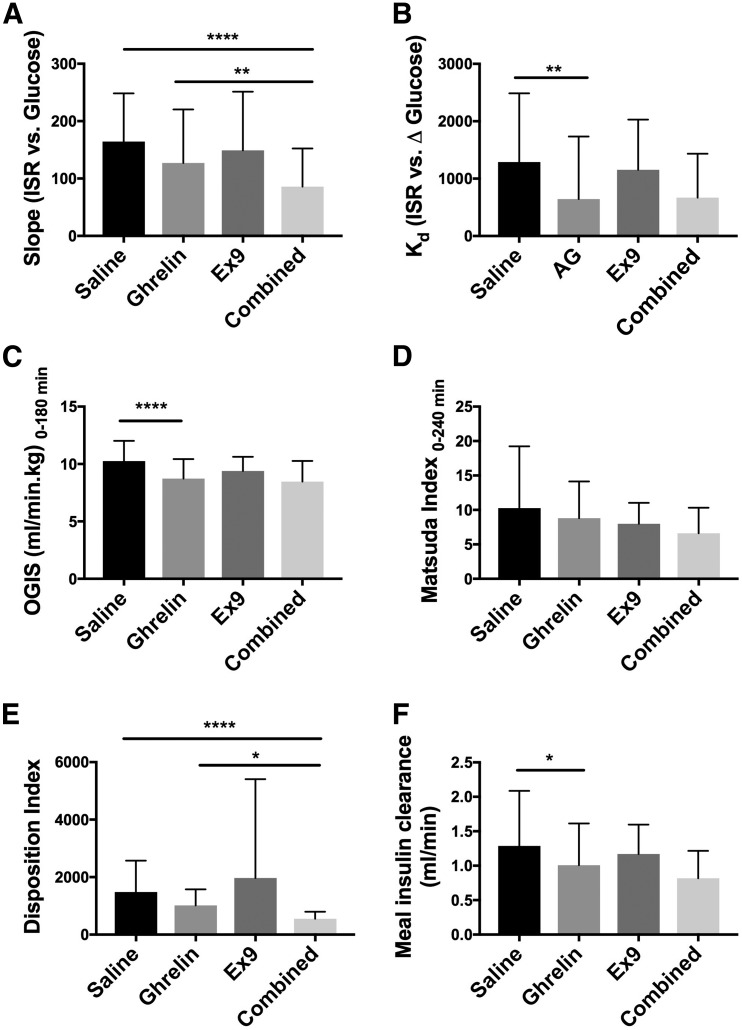
Plasma insulin AUC during the MTT was higher with the ghrelin infusion compared with the saline infusion (P = 0.005) (Fig. 1C and D and Table 1). However, when the relative hyperglycemia was taken into account (ΔAUC I/ΔAUC G0–240 min), there was no difference between the two treatments (P = 0.052) (Fig. 1F and Table 1). Meal-induced insulin secretion during the Ex9 infusion did not differ from saline (P > 0.9999), and the addition of Ex9 to ghrelin did not alter insulin secretion compared with ghrelin alone (P > 0.9999). The ISR, derived from C-peptide, was not significantly different among the four treatment groups (P = 0.26) (Fig. 1E and Table 1). Similarly, β-cell sensitivity to glucose (slope) was unaffected by ghrelin alone but decreased with combined ghrelin and Ex9 compared with saline (P ≤ 0.0001) (Fig. 2A and Table 1). The rate sensitivity (Kd) was decreased by ghrelin infusion compared with saline (P = 0.006), but the addition of Ex9 did not further reduce Kd (P > 0.9999) (Fig. 2B and Table 1).
Figure 2.
Effects of ghrelin, Ex9, and combined ghrelin and Ex9 treatments on slope (A), Kd (B), insulin sensitivity (C and D), DI (E), and meal insulin clearance (F). AG, acyl ghrelin. With the exception of slope and DI, statistical comparisons were made between saline and ghrelin treatments and ghrelin and combined treatments. For slope and disposition index, an additional analysis was performed comparing saline and combined treatment in A and E. *P < 0.05; **P < 0.01; ****P < 0.0001.
Effects of Exogenous Ghrelin and GLP-1R Blockade on Insulin Sensitivity and DI
Compared with the saline control, ghrelin decreased insulin sensitivity during the MTT as estimated by OGIS0–180 min (P < 0.0001) (Fig. 2C and Table 1). Ex9 had no effect on OGIS compared with saline. Insulin sensitivity, estimated by the Matsuda index, was not different between the ghrelin and saline treatments (P > 0.9999) (Fig. 2D and Table 1). The DI was not affected by ghrelin alone, but the addition of Ex9 to ghrelin caused significantly lower β-cell function compared with saline (P < 0.0001) (Fig. 2E and Table 1). Ghrelin treatment did not alter fasting insulin clearance but decreased meal-stimulated insulin clearance compared with saline (P = 0.039) (Fig. 2F and Table 1). Similarly, combined ghrelin and Ex9 treatment decreased insulin clearance during the MTT (P = 0.0038).
Effects of Exogenous Ghrelin and GLP-1R Blockade on the GI Hormone Peptide YY
Ghrelin infusion increased postprandial peptide YY (PYY) secretion compared with saline (P = 0.0021) (Supplementary Fig. 1 and Table 1). GLP-1R blockade had similar effects to ghrelin, elevating PYY levels versus saline (P = 0.0004). Combined ghrelin and Ex9 treatment further raised PYY levels compared with the ghrelin infusion alone (P = 0.0028).
Effects of Exogenous Ghrelin and GLP-1R Blockade on Postprandial Glucose Turnover
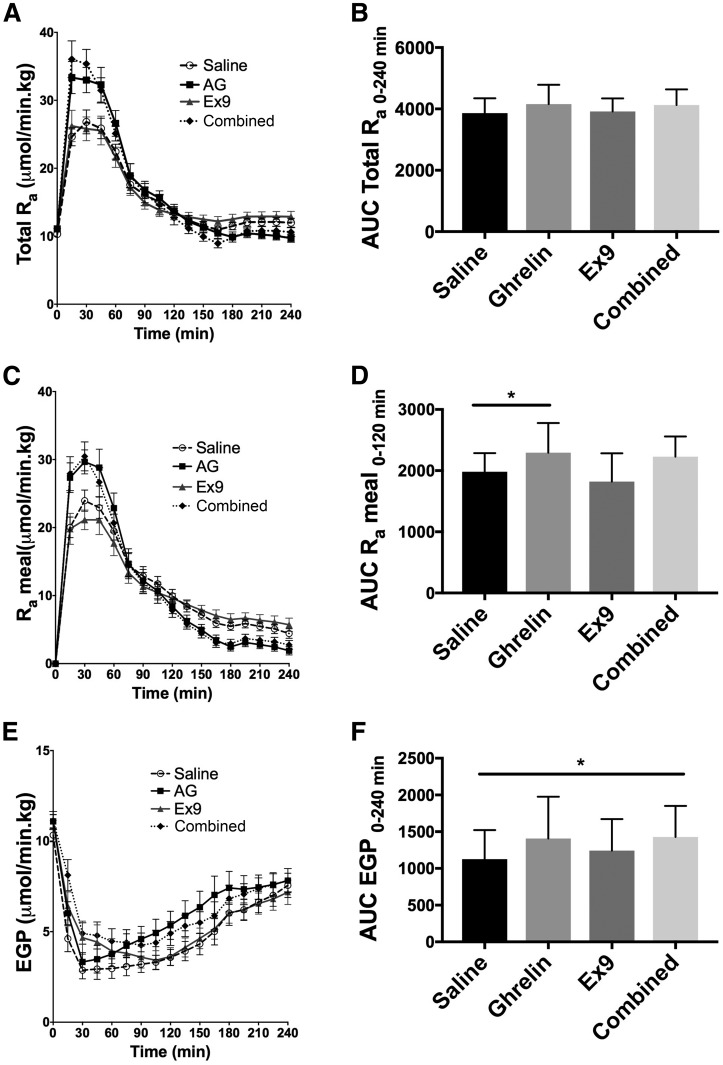
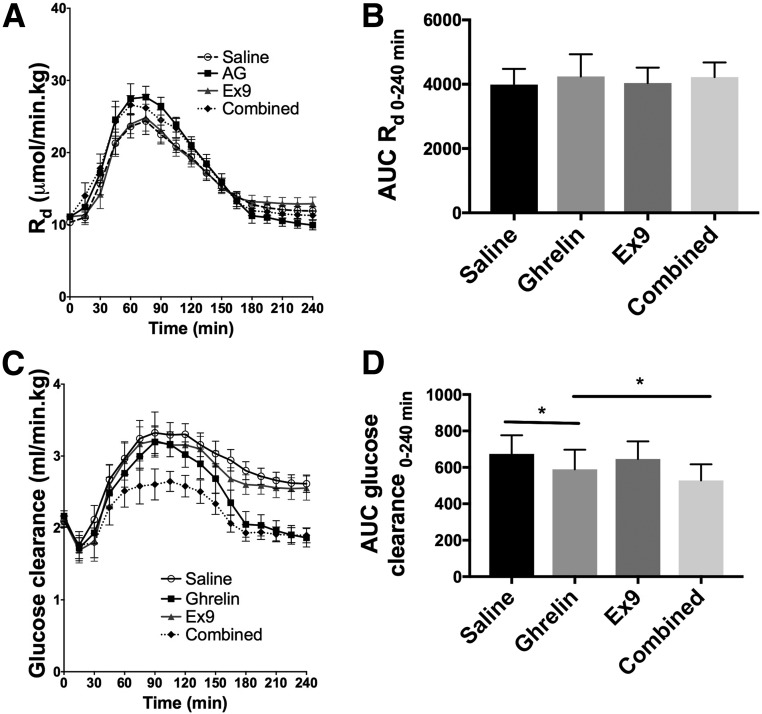
Total Ra during the test meal did not differ among the four treatments (Fig. 3A and B and Table 1). However, when the contributions of exogenous and endogenous glucose were evaluated separately, ghrelin increased Ra meal (Ra 0–120 min) compared with saline (P = 0.02) (Fig. 3C and D and Table 1). EGP was increased by the combined ghrelin and Ex9 treatment as compared with saline (P = 0.04) (Fig. 3E and F and Table 1). There was no difference in Rd among the four treatments (Fig. 4A and B). Ghrelin decreased glucose clearance compared with saline (P = 0.023), and the combined ghrelin and Ex9 treatment further decreased this parameter compared with ghrelin alone (P = 0.023) (Fig. 4C and D and Table 1).
Figure 3.
Effects of ghrelin, Ex9, and combined ghrelin and Ex9 treatments on total Ra (A and B), exogenous Ra meal (C and D), and EGP (E and F) during the MTT. AG, acyl ghrelin. With the exception of EGP, statistical comparisons were made between saline and ghrelin treatments and ghrelin and combined treatments. For EGP (F) we performed an additional analysis comparing saline and combined treatment. *P < 0.05.
Figure 4.
Effects of ghrelin, Ex9, and combined ghrelin and Ex9 treatments on Rd (A and B) and clearance (C and D) during the MTT. AG, acyl ghrelin. Statistical comparisons were made between saline and ghrelin treatments and ghrelin and combined treatments. *P < 0.05.
Discussion
The optimization of nutrient disposal and the maintenance of normal glucose tolerance require the coordinated actions of multiple factors. The role of GI hormones in this process is generally established, but there is little information about interactions among them. Because ghrelin and GLP-1 have primarily opposing actions on glucose metabolism but ghrelin stimulates GLP-1 secretion (5,8) we hypothesized an interaction. The current study demonstrates that ghrelin-induced GLP-1 dampens the effects of ghrelin to suppress β-cell function and worsen glucose tolerance. This effect was mediated by β-cell glucose sensitivity and hepatic glucose production. These findings suggest that ghrelin, which is elevated in the fasting state and at the onset of meals, engages GLP-1, and possibly other gut hormones such as PYY, in the regulation of glucose homeostasis during feeding.
The major effects of ghrelin, such as enhancing olfactory sensitivity and promoting food intake, increasing growth hormone secretion and lipolysis, restricting peripheral glucose uptake, and restraining insulin secretion to prevent hypoglycemia, can all be linked as a protective mechanism against starvation (19–21). Specifically, during caloric restriction, the presence of ghrelin is essential for prolonging survival by regulating islet hormone and ghrelin secretion and promoting hepatic gluconeogenesis to maintain euglycemia (22,23). During short-term fasting, elevated endogenous ghrelin decreases insulin secretion from β-cells indirectly by stimulating somatostatin release from δ-cells within the pancreatic islet (24,25). Ghrelin also enhances lipolysis and decreases insulin action by both counterregulatory hormone–dependent (4,22,26) and –independent mechanisms (8,27). In the current study, we demonstrate that, in fed state, supraphysiological levels of ghrelin decreased insulin sensitivity and glucose clearance impairing glucose tolerance, consistent with our previous reports (8).
We and others have shown that ghrelin stimulates postprandial GLP-1 secretion (5,8). In mice, an intraperitoneal injection of ghrelin 15 min before an oral glucose tolerance test not only stimulated GLP-1 secretion but also increased GSIS and improved glucose tolerance (5). These effects were lost in mice coinjected with the GLP-1R antagonist exendin-4 and not seen in the GLP-1R knockout mice. Thus, this elegant study by Gagnon et al. (5) demonstrated that the improved glucose tolerance with preprandial ghrelin injection was due to the enhanced GLP-1 secretion and the activation of the GLP-1R. In healthy humans, continuous infusion of ghrelin throughout a MTT increased total GLP-1 secretion nearly threefold but still caused glucose intolerance (8). Thus, it seemed that any positive effects of elevated GLP-1 on glucose tolerance were overwhelmed by ghrelin-induced insulin resistance and impaired β-cell function. However, we could not determine the relative contribution of increased GLP-1 to those parameters. In the current study, we attempted to answer whether ghrelin-stimulated GLP-1 secretion can offset some of the negative effects of ghrelin on prandial glucose metabolism. By blocking GLP-1 action with Ex9 during ghrelin infusion and an MTT, we report herein further impairment of glucose tolerance, decreased β-cell function, increased EGP, and reduced glucose clearance compared with ghrelin treatment alone. These results indicate a balance between ghrelin and GLP-1 effects in our experiment. Removing the effects of GLP-1, through GLP-1R blockade, allows the negative effect of ghrelin on β-cell function to become more apparent. We cannot make any conclusions as to whether and how much a ghrelin–GLP-1 interaction occurs during normal feeding, when there is endogenous secretion of ghrelin. However, these findings provide a proof of principle for a regulation of the effects of one gut hormone by another.
In the current study, we also found that ghrelin infusion enhanced postprandial PYY secretion. Similar to GLP-1, PYY is an intestinal hormone that rises after meals, slows gastric emptying, and decreases appetite (28,29). Consistent with these common effects, PYY is colocalized and cosecreted with GLP-1 from human colonic L cells (30). Given that ghrelin stimulates GLP-1 secretion, the finding that PYY release is also enhanced by ghrelin is novel but not totally surprising. GLP-1R blockade also increased PYY levels compared with saline. Previous studies have shown increased postprandial GLP-1 release during Ex9 infusion (31); thus, we suspect the increased PYY secretion was again related to cosecretion with GLP-1. Notably, the combined ghrelin and Ex9 treatment resulted in further enhancement in PYY secretion compared with ghrelin alone. PYY can affect parameters such as insulin secretion and sensitivity. We cannot delineate the contributions of increased PYY to these parameters in our current study, but our findings highlight the complexity of GI hormone interactions affecting glucose metabolism.
Ghrelin treatment alone did not have a significant impact on EGP, a finding consistent with previous observations made by Vestergaard et al. (27) and Tamboli et al. (32). However, the addition of GLP-1R blockade to ghrelin increased EGP. This result agrees with prior studies that have demonstrated that GLP-1 reduces EGP by both islet-dependent (33,34) and islet-independent mechanisms (35,36). In addition to fasting, pathological states such as anorexia nervosa yield a pattern of GI hormones similar to those in our study, with elevated circulating ghrelin and attenuated GLP-1 (37,38). Decreased insulin levels resulting from high ghrelin and low GLP-1 promotes lipolysis, glycogenolysis, and gluconeogenesis, which are clearly beneficial in that condition (38). The physiological relevance of these findings and the interplay between ghrelin and GLP-1 in clinical states is worthy of further investigation.
Ghrelin is structurally similar to motilin (39), and ghrelin’s effect to enhance GI motility and gastric acid production (7,40) is consistent with a role to prepare the gut for incoming nutrients and it complements the orexigenic action for which ghrelin is best known. The therapeutic potential of using ghrelin mimetics to treat postoperative ileus and gastroparesis has been explored (40,41), but the effect of ghrelin on glucose flux during a meal has not, to our knowledge, been studied previously. In contrast to ghrelin, GLP-1 is known to delay gastric emptying and decrease oral Ra, and blockade with Ex9 treatment accelerates gastric emptying (42). As mentioned previously, PYY also slows gastric emptying (28). Using glucose tracers, we observed that supraphysiological amounts of ghrelin increased Ra meal. This finding is best explained by the well-known effect of ghrelin to speed gastric emptying in healthy individuals and those with type 2 diabetes (4,43). Notably, rapid gastric emptying likely accounts for much of the effect of ghrelin on postprandial glucose in our subjects, with diminished glucose clearance constituting the remainder. We were initially surprised that the combined ghrelin and GLP-1R blockade did not further augment Ra meal. However, the combined treatment enhanced PYY secretion to a greater extent than ghrelin alone, which may have curbed additional acceleration of gastric emptying by Ex9.
Insulin clearance is an important determinant of circulating insulin concentrations. In humans, approximately 40%–80% of insulin is extracted during the first pass through the liver (44–46), and most of the remainder is cleared by the kidney. The rate of hepatic insulin clearance is positively correlated with pulsatile insulin secretion (46) and inversely associated with insulin sensitivity (47). When insulin resistance was induced by dietary fat, normal dogs responded by enhancing β-cell function by approximately 20% and reducing insulin clearance by approximately 50%; the decreased insulin clearance preceded β-cell compensation (48). Therefore, decreased insulin clearance is hypothesized to be an important compensatory mechanism for insulin-resistant states, such as type 2 diabetes, as it reduces the burden on β-cells to produce additional insulin (49,50). In our study, ghrelin, with or without Ex9, decreased meal-related insulin clearance and insulin sensitivity (OGIS) compared with saline. Although the mechanism by which this occurs is not apparent from our data, the finding raises the question as to whether ghrelin modulates insulin clearance in pathological conditions such as insulin resistance, type 2 diabetes, anorexia nervosa, or Prader-Willi syndrome.
There are several limitations to our study. First, the levels of ghrelin used were supraphysiological and remained elevated in the postprandial period, when ghrelin typically falls in healthy humans. We chose the dose of ghrelin based on our previous study to allow us to specifically test the additive effect of Ex9 and thereby the role of a ghrelin–GLP-1 interaction during the MTT. By comparing the ghrelin and combined ghrelin and Ex9 treatments to saline, the study highlights the importance of the postprandial fall of ghrelin and rise of GLP-1 on glucose tolerance. However, we acknowledge that further work will be necessary to test the interplay of ghrelin and GLP-1 in a more physiological setting. Second, we were unable to differentiate the effect of ghrelin on hepatic versus extrahepatic insulin clearance with the current study design. Last, even with 15 subjects, which is a reasonable sample size for a physiological study, we may have been underpowered for some observations based on the lack of a significant effect of ghrelin alone to suppress ISR and DI in contrast to our previous findings (8).
In summary, we have demonstrated additive effects of ghrelin and GLP-1R blockade during feeding. These findings indicate that ghrelin stimulation of GLP-1 has significant effects on several parameters of glucose tolerance, including DI, β-cell glucose sensitivity, EGP, and glucose clearance. Overall, our results suggest a model whereby the effects of ghrelin to protect against hypoglycemia during starvation can be quickly and at least partially reversed when food becomes available.
Supplementary Material
Article Information
Acknowledgments. The authors thank the study participants and the research staff at the Clinical Translational Research Center at CCHMC. The authors also appreciate the excellent technical assistance of Dr. Radha Krishna (Duke Molecular Physiology Institute, Duke University).
Funding. This study was supported by funding from National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (R01DK097550 to J.T. and R01DK101991 to D.A.D.). L.C.P. was supported by NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development grants 4T32HD043029-14 and 5T32HD043029-15 (Duke Research Training Program for Pediatricians). The study is supported in part by the NIH National Center for Research Resources (U.S. Public Health Service grant UL1 RR026314).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Authors Contributions. L.C.P., A.G., and J.T. analyzed the data. L.C.P., D.A.D., and J.T. wrote the manuscript. S.M.G. contributed to data collection and edited the manuscript. J.T. conceived, designed, and performed the experiment. J.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01729299, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0451/-/DC1.
References
- 1.Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest 2015;125:908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 3.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000;141:4255–4261 [DOI] [PubMed] [Google Scholar]
- 4.Müller TD, Nogueiras R, Andermann ML, et al. Ghrelin. Mol Metab 2015;4:437–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagnon J, Baggio LL, Drucker DJ, Brubaker PL. Ghrelin is a novel regulator of GLP-1 secretion. Diabetes 2015;64:1513–1521 [DOI] [PubMed] [Google Scholar]
- 6.Tong J, Prigeon RL, Davis HW, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010;59:2145–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin F, Edholm T, Schmidt PT, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab 2006;91:3296–3302 [DOI] [PubMed] [Google Scholar]
- 8.Tong J, Davis HW, Gastaldelli A, D’Alessio D. Ghrelin impairs prandial glucose tolerance and insulin secretion in healthy humans despite increasing GLP-1. J Clin Endocrinol Metab 2016;101:2405–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Tschöp MH, D’Alessio D. Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans. J Clin Endocrinol Metab 2013;98:2536–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salehi M, Vahl TP, D’Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab 2008;93:4909–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011;54:2093–2102 [DOI] [PubMed] [Google Scholar]
- 12.Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014;146:669–680.e2 [DOI] [PMC free article] [PubMed]
- 13.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 14.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001;24:539–548 [DOI] [PubMed] [Google Scholar]
- 15.Gastaldelli A, Brodows RG, D’Alessio D. The effect of chronic twice daily exenatide treatment on β-cell function in new onset type 2 diabetes. Clin Endocrinol (Oxf) 2014;80:545–553 [DOI] [PubMed] [Google Scholar]
- 16.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 17.Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of beta-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab 2002;283:E1159–E1166 [DOI] [PubMed] [Google Scholar]
- 18.Tillil H, Shapiro ET, Miller MA, et al. Dose-dependent effects of oral and intravenous glucose on insulin secretion and clearance in normal humans. Am J Physiol 1988;254:E349–E357 [DOI] [PubMed] [Google Scholar]
- 19.Heppner KM, Tong J. Mechanisms in endocrinology: regulation of glucose metabolism by the ghrelin system: multiple players and multiple actions. Eur J Endocrinol 2014;171:R21–R32 [DOI] [PubMed] [Google Scholar]
- 20.Tong J, Mannea E, Aimé P, et al. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci 2011;31:5841–5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Druce MR, Wren AM, Park AJ, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes 2005;29:1130–1136 [DOI] [PubMed] [Google Scholar]
- 22.Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem 2012;287:17942–17950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Fang F, Goldstein JL, Brown MS, Zhao TJ. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proc Natl Acad Sci U S A 2015;112:1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiGruccio MR, Mawla AM, Donaldson CJ, et al. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab 2016;5:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adriaenssens AE, Svendsen B, Lam BY, et al. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 2016;59:2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagaya N, Kojima M, Uematsu M, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol 2001;280:R1483–R1487 [DOI] [PubMed] [Google Scholar]
- 27.Vestergaard ET, Jessen N, Møller N, Jørgensen JO. Acyl ghrelin induces insulin resistance independently of GH, cortisol, and free fatty acids. Sci Rep 2017;7:42706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987;28:166–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev 2006;7:163–182 [DOI] [PubMed] [Google Scholar]
- 30.Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013;56:1413–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamboli RA, Antoun J, Sidani RM, et al. Metabolic responses to exogenous ghrelin in obesity and early after Roux-en-Y gastric bypass in humans. Diabetes Obes Metab 2017;19:1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hvidberg A, Nielsen MT, Hilsted J, Orskov C, Holst JJ. Effect of glucagon-like peptide-1 (proglucagon 78-107amide) on hepatic glucose production in healthy man. Metabolism 1994;43:104–108 [DOI] [PubMed] [Google Scholar]
- 34.Larsson H, Holst JJ, Ahrén B. Glucagon-like peptide-1 reduces hepatic glucose production indirectly through insulin and glucagon in humans. Acta Physiol Scand 1997;160:413–422 [DOI] [PubMed] [Google Scholar]
- 35.Seghieri M, Rebelos E, Gastaldelli A, et al. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia 2013;56:156–161 [DOI] [PubMed] [Google Scholar]
- 36.Prigeon RL, Quddusi S, Paty B, D’Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 2003;285:E701–E707 [DOI] [PubMed] [Google Scholar]
- 37.Tomasik PJ, Sztefko K, Malek A. GLP-1 as a satiety factor in children with eating disorders. Horm Metab Res 2002;34:77–80 [DOI] [PubMed] [Google Scholar]
- 38.Misra M, Klibanski A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol 2014;2:581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomasetto C, Karam SM, Ribieras S, et al. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology 2000;119:395–405 [DOI] [PubMed] [Google Scholar]
- 40.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut 2005;54:1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popescu I, Fleshner PR, Pezzullo JC, Charlton PA, Kosutic G, Senagore AJ. The Ghrelin agonist TZP-101 for management of postoperative ileus after partial colectomy: a randomized, dose-ranging, placebo-controlled clinical trial. Dis Colon Rectum 2010;53:126–134 [DOI] [PubMed] [Google Scholar]
- 42.Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab 2010;95:215–221 [DOI] [PubMed] [Google Scholar]
- 43.Gonlachanvit S, Hsu CW, Boden GH, et al. Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig Dis Sci 2003;48:488–497 [DOI] [PubMed] [Google Scholar]
- 44.Eaton RP, Allen RC, Schade DS. Hepatic removal of insulin in normal man: dose response to endogenous insulin secretion. J Clin Endocrinol Metab 1983;56:1294–1300 [DOI] [PubMed] [Google Scholar]
- 45.Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes 1984;33:486–494 [DOI] [PubMed] [Google Scholar]
- 46.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005;54:1649–1656 [DOI] [PubMed] [Google Scholar]
- 47.Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes 2016;65:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response. Diabetes 2000;49:2116–2125 [DOI] [PubMed] [Google Scholar]
- 49.Erdmann J, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metab 2008;294:E568–E575 [DOI] [PubMed] [Google Scholar]
- 50.Kaga H, Tamura Y, Takeno K, et al. Correlates of insulin clearance in apparently healthy non-obese Japanese men. Sci Rep 2017;7:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.