Abstract
An efficient and convenient protocol for the synthesis of 2H-chromenones has been developed. In the presence of tBuOK in DMF, good to excellent yields of various chromenones were obtained from the corresponding salicylaldehydes and arylacetonitriles. No protection of inert gas atmosphere is required here.
Keywords: chromenones synthesis, metal-free, green chemistry, heterocycle synthesis, salicylaldehydes
1. Introduction
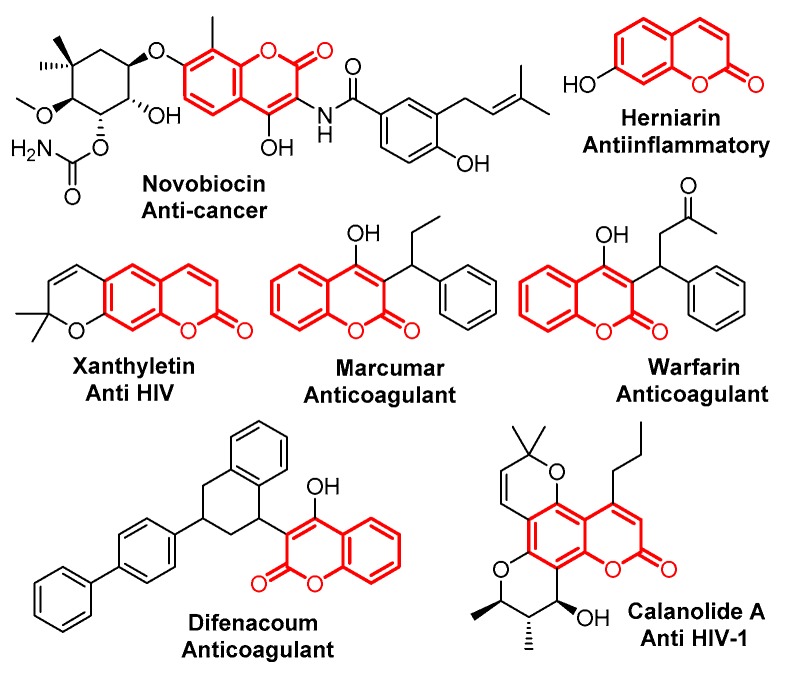
Coumarin is an important class of benzo-fused six-membered heterocycles, which was first isolated as a natural product in 1820, and has been found to have various interesting bioactivities (Figure 1) [1,2,3,4,5,6,7,8]. Due to its importance, many efforts have been made to develop new synthetic procedures for coumarin’s preparation. Classical routes to coumarins based on Pechmann- [9], Knoevenagel- [10], Perkin- [11], Reformatsky- [12] and Wittig- [13] reactions have been extensively investigated. Recently, procedures based on transition metal catalysts, ionic liquids and microwaves have been developed as well [14,15,16,17].
Figure 1.
Selected examples of bioactive chromenones.
In the 21st century, the demands of sustainable development drive organic chemists to pay more attention to the principles of green chemistry in designing their new procedures. Among the various possible directions, the development of new transition metal-free methodologies will be one attractive choice. On one hand, transition metal catalysts are usually considered to be toxic and non-environmentally benign. On the other hand, special attention has to be taken to avoid the problem of transition metal contamination of the final products, especially when in heterocycles synthesis chemistry. With these points in mind and also based on our continual interests in the development of new procedures for the synthesis of heterocycles under transition metal-free conditions [18], we wish to report here a convenient methodology for the construction of coumarins from salicylaldehydes and arylacetonitriles. In the presence of tBuOK in DMF, good to excellent yields of the desired chromenones were obtained and no protection of inert gas atmosphere is required here.
2. Results and Discussion
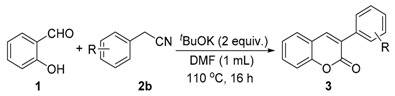
Initially, we choose salicylaldehyde and 2-phenylacetonitrile as the model substrates to establish this reaction system (Table 1). As we expected, with two equivalents of tBuOK as the base in 2 mL of dimethylformamide (DMF) at 110 °C, 77% of the desired product can be isolated (Table 1, entry 1). No better results can be obtained with an increased amount of promotor and similar yield can be observed with a higher concentration (Table 1, entries 2 and 3). Then, several other inorganic bases were screened and none of them could give better results than tBuOK (Table 1, entries 5–10). The reaction temperature was also checked and yields were reduced when the reaction temperature was decreased or increased (Table 1, entries 11 and 12). Subsequently, various solvents were examined but without improved results (Table 1, entries 13–17).
Table 1.
Optimization of the reaction conditions [a].
| Entry | Base | Solvent | T (°C) | Yield (%) [b] |
|---|---|---|---|---|
| 1 | tBuOK | DMF [c] | 110 | 77 |
| 2 | tBuOK [d] | DMF [c] | 110 | 75 |
| 3 | tBuOK | DMF | 110 | 81 |
| 4 | tBuOK | DMF | 110 | 48 |
| 80 | ||||
| 5 | K2CO3 | DMF | 110 | 34 |
| 6 | K3PO4 | DMF | 110 | 55 |
| 7 | KOH | DMF | 110 | 27 |
| 8 | tBuOLi | DMF | 110 | 75 |
| 9 | NaOMe | DMF | 110 | 55 |
| 10 | tBuONa | DMF | 110 | 74 |
| 11 | tBuOK | DMF | 90 | 73 |
| 12 | tBuOK | DMF | 130 | 41 |
| 13 | tBuOK | DMAc | 110 | 54 |
| 14 | tBuOK | DMSO | 110 | 30 |
| 15 | tBuOK | Toluene | 110 | 9 |
| 16 | tBuOK | o-xylene | 110 | 12 |
| 17 | tBuOK | 1,4-dioxane | 110 | 15 |
[a] Reaction conditions: 1 (1 mmol), 2 (1.5 mmol), base (2.0 equiv.), solvent (1 mL), 110 °C, 16 h. [b] Isolated yields. [c] DMF (2 mL). [d] tBuOK (3 equiv.).
With the optimal reaction conditions in hand, several substituted salicylaldehydes were tested and shown in Table 2. Moderate to good yields of 2H-chromenones can be obtained from the corresponding salicylaldehydes and 2-phenylacetonitrile.
Table 2.
Synthesis of chromenones from salicylaldehydes [a].

| Entry | Substrate | Product | Yield (%) [b] |
|---|---|---|---|
| 1 | 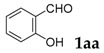 |
 |
81 |
| 2 | 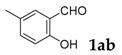 |
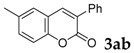 |
77 |
| 3 | 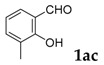 |
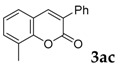 |
65 |
| 4 | 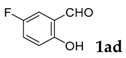 |
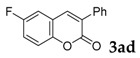 |
93 |
| 5 | 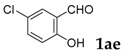 |
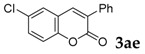 |
66 |
| 6 | 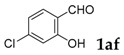 |
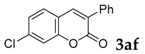 |
96 |
| 7 | 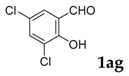 |
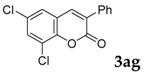 |
52 |
| 8 | 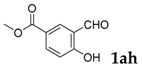 |
 |
40 |
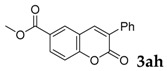
| 9 |  |
 |
90 |
[a] Reaction conditions: 1a (1 mmol), 2 (1.5 mmol), tBuOK (2.0 equiv.), DMF (1 mL), 110 °C, 16 h. [b] Isolated yields.
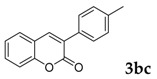
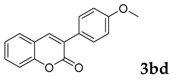
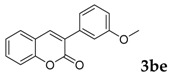
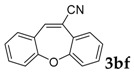
Then, various arylacetonitriles were examined with salicylaldehyde (Table 3). Both electron-donating and electron-withdrawing substituted phenylacetonitriles afforded the corresponding products in moderate to good yields. Notably, when 2-(2-fluorophenyl)acetonitrile and 2-(2-chlorophenyl)acetonitrile were applied as the reaction partner, good yields of dibenzo(b,f)oxepine-10-carbonitrile can be obtained via intermolecular condensation and intramolecular nucleophilic substitution (Table 3, entries 6 and 9) [19]. It is also important to mention that 3-oxo-3-phenylpropanenitrile, 3-phenylpropanenitrile and malononitrile were tested under standard conditions but no desired products can be detected (Table 3, entries 14–16).
Table 3.
Synthesis of chromenones from 2-arylacetonitriles [a].
| Entry | Substrate | Product | Yield (%) [b] |
|---|---|---|---|
| 1 | 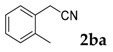 |
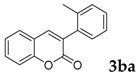 |
81 |
| 2 | 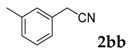 |
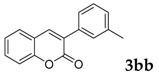 |
77 |
| 3 | 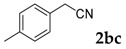 |
 |
65 |
| 4 | 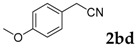 |
 |
70 |
| 5 | 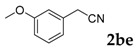 |
 |
70 |
| 6 | 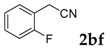 |
 |
86 |
| 7 | 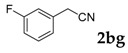 |
 |
78 |
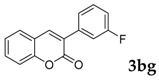
| 8 |  |
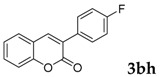 |
93 |
| 9 | 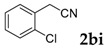 |
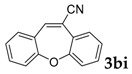 |
68 |
| 10 | 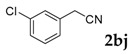 |
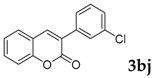 |
66 |
| 11 | 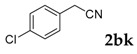 |
 |
70 |
| 12 | 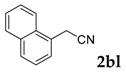 |
 |
40 |
| 13 | 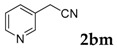 |
 |
51 |
| 14 | 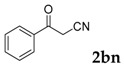 |
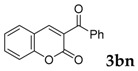 |
0 |
| 15 | 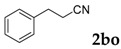 |
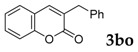 |
0 |
| 16 |  |
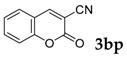 |
0 |
[a] Reaction conditions: 1 (1 mmol), 2b (1.5 mmol), tBuOK (2.0 equiv.), DMF (1 mL), 110 °C, 16 h. [b] Isolated yields.
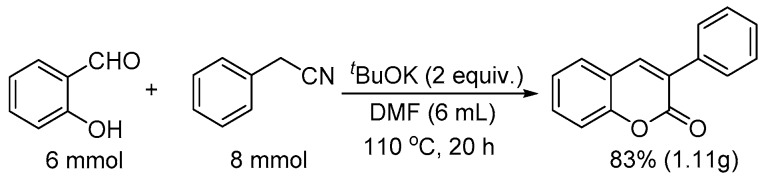
To demonstrate the potential utility of this method, we conducted the reaction in gram scale as well (Scheme 1). Thus, salicylaldehyde (6 mmol) was reacted with phenylacetonitrile (8 mmol) in the presence of two equivalents; for tBuOK at 110 °C for 20 h, 83% yield of 3-phenyl-2H-chromen-2-one was obtained (1.11 g).
Scheme 1.
Gram scale synthesis of 3-phenyl-2H-chromen-2-one.
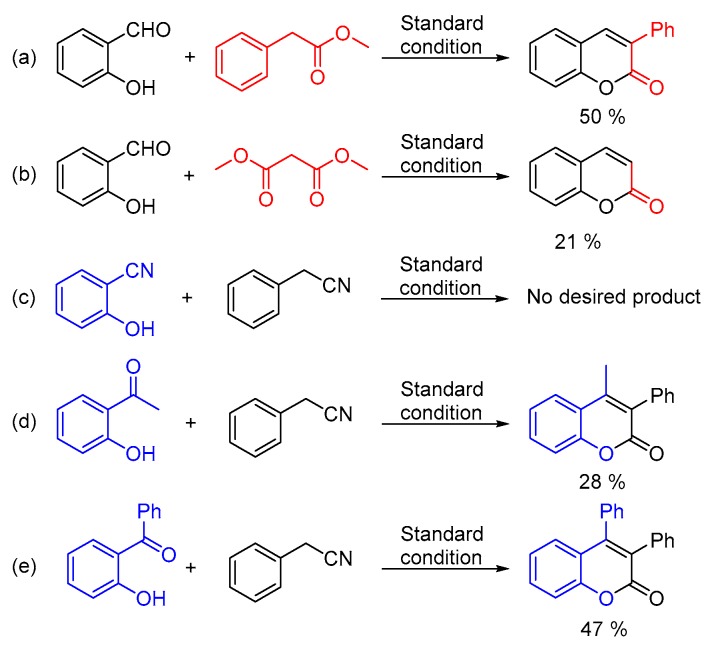
In addition, analogues of the substrates have been tested as well (Scheme 2). Under the standard conditions, 50% of 3-phenyl-2H-chromen-2-one and 21% of 2H-chromen-2-one could be obtained from methyl 2-phenylacetate and dimethyl malonate, respectively (Scheme 2, equation. a and equation. b). Moreover, 2-hydroxybenzonitrile, 2-acetylphenol and 2-hydroxybenzophenone were taken into consideration as well. Unfortunately, no desired product could be detected from 2-hydroxybenzonitrile (Scheme 2, equation. c). Interestingly, 2-acetylphenol could afford acceptable yield of the goal product (Scheme 2, equation. d) and moderate yield of 3,4-diphenyl-2H-chromen-2-one was generated from 2-hydroxybenzophenone and phenylacetonitrile without any further optimization (Scheme 2, equation. e) [20].
Scheme 2.
Substrate analogues testing. (a) methyl 2-phenylacetate (b) dimethyl malonate (c) 2-hydroxybenzonitrile (d) 2-acetylphenol (e) 2-hydroxybenzophenone.
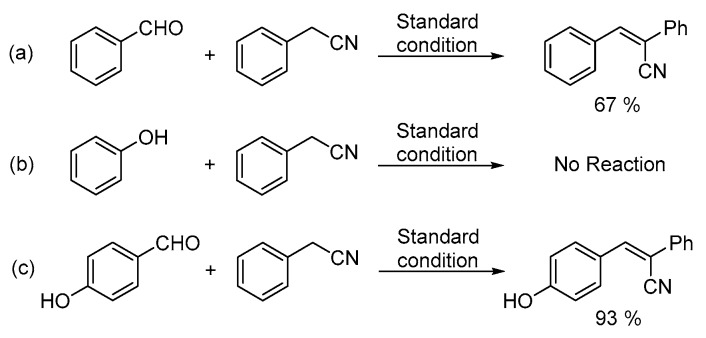
In order to obtain more insight into the reaction pathway, control experiments were performed (Scheme 3). Benzaldehyde, phenol and 4-hydroxybenzaldehyde were reacted with phenylacetonitrile under the standard reaction conditions, respectively. When benzaldehyde was reacted with phenylacetonitrile, 67% of 2,3-diphenylacrylonitrile was obtained while no product could be detected with phenol (Scheme 3, equation. a and equation. b). Compared with salicylaldehyde, 4-hydroxybenzaldehyde is considered as a compound with the same electron properties. Under the same reaction conditions, 93% of 3-(4-hydroxyphenyl)-2-phenylacrylonitrile was generated as the sole product by the reaction of 4-hydroxybenzaldehyde with phenylacetonitrile (Scheme 3, equation. c), which indicated that the first step of this transformation is the intermolecular condensation instead of the nucleophilic addition [21].
Scheme 3.
Control experiments. (a) Benzaldehyde (b) phenol (c) 4-hydroxybenzaldehyde.
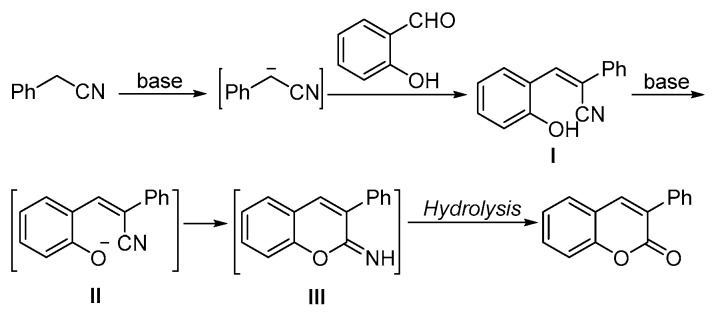
Based on these results, a possible reaction pathway has been proposed (Scheme 4). In the presence of base, phenylacetonitrile transformed into cyano(phenyl)methanide, which subsequently reacted with salicylaldehyde to give intermediate I. With the assistance of the other equivalent base, the hydroxyl group of intermediate I was activated and then reacted with the cyano via an intramolecular addition. The final products will be formed after in situ hydrolysis.
Scheme 4.
Proposed mechanism of the synthesis of chromenone.
3. Materials and Methods
3.1. Materials and General Procedures
3.1.1. Materials
General comments: All reactions were carried out under air. Reactions were monitored by TLC analysis (pre-coated silica gel plates with fluorescent indicator UV254, 0.2 mm) and visualized with 254 nm UV light. Chemicals were purchased from Aldrich (Tianjin, China), Alfa-Aesar (Tianjin, China), TCI (Shanghai, China) and unless otherwise noted were used without further purification. All compounds were characterized by 1H-NMR and 13C-NMR spectroscopy and recorded on Bruker (Beijing, China) AV 300 and AV 400 spectrometers. Gas-chromatography-mass-analysis was performed using an Agilent HP-5890 with an Agilent HP-5973 Mass Selective Detector (EI) and an HP-5-capillary column using helium as a carrier gas.
3.1.2. General Procedures
Salicylaldehyde (1 mmol) and two equivalents of tBuOK were added in a 25 mL tube equipped with a stirring bar. Then, 1 mL of DMF and 2-phenylacetonitrile (1.5 mmol) were injected by syringe. After that, the tube was closed and heated up to 110 °C for 16 h. When the reaction was completed, the reaction mixture was cooled to room temperature. The reaction was quenched with distilled water and the solution was extracted with ethyl acetate. The crude product was purified by column chromatography (ethyl acetate/pentane = 1:25–1:8).
3.2. Synthesis of Adducts (Specific Spectral Reference Supplementary Materials)
3-Phenyl-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.74 (d, J = 0.6 Hz, 1H), 7.66–7.60 (m, 2H), 7.50–7.41 (m, 2H), 7.41–7.32 (m, 3H), 7.29 (dq, J = 7.7, 0.9 Hz, 1H), 7.25–7.19 (m, 1H). 13C-NMR (75 MHz, Chloroform-d) δ 160.55, 153.48, 139.83, 134.67, 131.36, 128.83, 128.49, 128.44, 128.33, 127.87, 124.46, 119.64, 116.42. GC-MS (EI, 70 ev): m/z (%) = 222 (M+, 100), 195 (14), 194 (93), 166 (12), 165 (89), 164 (16), 163 (10), 82 (11).
6-Methyl-3-phenyl-2H-chromen-2-one: 7u1H-NMR (300 MHz, Chloroform-d) δ 7.70 (s, 1H), 7.67–7.58 (m, 2H), 7.48–7.33 (m, 3H), 7.31–7.24 (m, 2H), 7.23–7.14 (m, 1H), 2.36 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 160.74, 151.61, 139.84, 134.81, 134.11, 132.40, 128.71, 128.48, 128.40, 128.14, 127.65, 119.36, 116.11, 20.76. GC-MS (EI, 70 ev): m/z (%) = 236 (M+, 100), 209 (10), 208 (67), 207 (62), 179 (24), 178 (40), 152 (16), 139 (10), 89 (12), 77 (13), 76 (12), 51 (11).
6-Fluoro-3-phenyl-2H-chromen-2-one: 1H-NMR (300 MHz, Chloroform-d) δ 7.75 (s, 1H), 7.73–7.67 (m, 2H), 7.50–7.41 (m, 3H), 7.35 (dddd, J = 8.8, 4.5, 1.8, 1.1 Hz, 1H), 7.29–7.19 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 160.17, 149.63, 138.73, 134.28, 129.52, 129.16, 128.54, 128.53, 120.28, 118.76 (d, J = 24.6 Hz), 118.05, 117.94, 113.05 (d, J = 23.9 Hz). GC-MS (EI, 70 ev): m/z (%) = 240 (M+, 94), 213 (15), 212 (96), 184 (15), 183 (100), 182 (12), 181 (10), 163 (11), 157 (13), 91 (10). HRMS (EI): Calcd. for [[M + H]+: C15H9FO2]+: 241.06593, found: 241.06566.
6-Chloro-3-phenyl-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.73 (t, J = 0.5 Hz, 1H), 7.72–7.66 (m, 2H), 7.53 (d, J = 2.4 Hz, 1H), 7.50–7.41 (m, 4H), 7.31 (dt, J = 8.8, 0.6 Hz, 1H). 13C-NMR (75 MHz, Chloroform-d) δ 160.02, 151.88, 138.45, 134.25, 131.31, 129.75, 128.69–128.43 (m), 129.56, 129.25, 128.58, 127.10, 120.73, 117.93. GC-MS (EI, 70 ev): m/z (%) = 256 (M+, 100), 230 (30), 229 (15), 166 (10), 165 (77), 164 (20), 163 (28), 139 (18), 82 (18), 63 (15).
Methyl-2-oxo-3-phenyl-2H-chromene-6-carboxylate: 7t1H-NMR (300 MHz, Chloroform-d) δ 8.28 (d, J = 2.0 Hz, 1H), 8.19 (dd, J = 8.7, 2.0 Hz, 1H), 7.89–7.84 (m, 1H), 7.76–7.65 (m, 2H), 7.55–7.34 (m, 5H), 3.96 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 165.70, 159.85, 156.21, 139.23, 134.17, 132.28, 129.94, 129.19, 128.56, 128.50, 128.35, 126.59, 119.39, 116.65, 52.47. GC-MS (EI, 70 ev): m/z (%) = 280 (M+, 100), 252 (11), 249 (30), 221 (45), 193 (29), 165 (27), 164 (12), 163 (14), 139 (22), 83 (15).
8-Methyl-3-phenyl-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.79 (s, 1H), 7.75–7.64 (m, 2H), 7.51–7.27 (m, 5H), 7.19 (dd, J = 8.1, 7.0 Hz, 1H), 2.49 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 160.59, 151.78, 140.19, 134.76, 132.61, 129.02, 128.64, 128.43, 128.35, 127.80, 125.78, 125.56, 123.97, 119.29, 15.38. GC-MS (EI, 70 ev): m/z (%) = 236 (M+, 100), 209 (12), 208 (76), 207 (45), 179 (19), 178 (36), 165 (30), 152 (12), 89 (14), 77 (10), 76 (12).
6,8-Dichloro-3-phenyl-2H-chromen-2-one: 7v1H-NMR (300 MHz, Chloroform-d) δ 7.75–7.63 (m, 3H), 7.57 (d, J = 2.3 Hz, 1H), 7.48–7.39 (m, 4H). 13C-NMR (75 MHz, Chloroform-d) δ 158.81, 147.77, 137.89, 133.72, 131.20, 130.23, 129.50, 129.48, 128.60, 128.51, 125.61, 122.27, 121.44. GC-MS (EI, 70 ev): m/z (%) = 291 (M+, 63), 290 (94), 266 (11), 265 (10), 264 (65), 263 (16), 262 (100), 201 (20), 200 (10), 199 (62), 164 (28), 163 (60), 162 (10), 139 (10), 99 (16), 87 (11), 81 (19), 63 (10).
7-Chloro-3-phenyl-2H-chromen-2-one: 1H-NMR (300 MHz, Chloroform-d) δ 7.66 (s, 1H), 7.61–7.52 (m, 2H), 7.44–7.30 (m, 4H), 7.28–7.24 (m, 1H), 7.21–7.09 (m, 1H). 13C-NMR (75 MHz, Chloroform-d) δ 159.84, 153.66, 138.90, 137.23, 134.30, 129.03, 128.64, 128.49, 128.44, 128.26, 125.08, 124.91, 118.21, 116.73. GC-MS (EI, 70 ev): m/z (%) = 256 (M+, 100), 230 (16), 228 (100), 166 (12), 165 (85), 164 (27), 163 (28), 139 (16), 115 (14), 114 (12), 82 (11), 63 (15). HRMS (EI): Calcd. for [[M + H]+: C15H9ClO2]+: 257.03638, found: 257.03614.
2-Phenyl-3H-benzo[f]chromen-3-one: 7u1H-NMR (300 MHz, Chloroform-d) δ 8.36 (d, J = 1.7 Hz, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.83–7.68 (m, 2H), 7.67–7.59 (m, 2H), 7.50 (ddd, J = 8.4, 7.0, 1.4 Hz, 1H), 7.42–7.26 (m, 5H). 13C-NMR (75 MHz, Chloroform-d) δ 160.55, 153.04, 135.60, 135.00, 132.62, 130.23, 129.01, 128.80, 128.50 (d, J = 2.1 Hz), 128.12, 127.10, 125.96, 121.34, 116.58, 113.65. GC-MS (EI, 70 ev): m/z (%) = 272 (M+, 92), 245 (23), 244 (100), 243 (23), 215 (60), 213 (27), 189 (10), 122 (10), 107 (25), 94 (18).
3-(o-Tolyl)-2H-chromen-2-one: 7x1H-NMR (300 MHz, Chloroform-d) δ 7.65 (s, 1H), 7.60–7.46 (m, 2H), 7.39 (ddt, J = 8.2, 1.2, 0.6 Hz, 1H), 7.36–7.28 (m, 3H), 7.28–7.22 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 160.21, 153.80, 141.59, 136.82, 134.66, 131.41, 130.30, 129.73, 128.81, 127.81, 125.85, 124.43, 119.28, 116.55, 19.92. GC-MS (EI, 70 ev): m/z (%) = 236 (M+, 100), 220 (12), 219 (64), 208 (37), 207 (86), 189 (27), 179 (26), 178 (53), 177 (10), 176 (11), 165 (24), 152 (21), 117 (12), 115 (23), 89 (18), 76 (14), 63 (18), 39 (11).
3-(m-Tolyl)-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.76 (s, 1H), 7.55–7.43 (m, 4H), 7.37–7.22 (m, 3H), 7.22–7.16 (m, 1H), 2.39 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 160.55, 153.42, 139.70, 138.03, 134.59, 131.24, 129.59, 129.09, 128.44, 128.32, 127.81, 125.60, 124.40, 119.65, 116.36, 21.45. GC-MS (EI, 70 ev): m/z (%) = 236 (M+, 100), 209 (14), 208 (81), 207 (18), 179 (14), 178 (30), 165 (38), 152 (13), 117 (11), 89 (13), 63 (10).
3-(p-Tolyl)-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.67 (s, 1H), 7.55–7.47 (m, 2H), 7.45–7.35 (m, 2H), 7.25 (dt, J = 7.8, 0.9 Hz, 1H), 7.21–7.09 (m, 3H), 2.29 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 160.60, 153.32, 139.12, 138.82, 131.71, 131.10, 129.09, 128.31, 128.17, 127.74, 124.36, 119.68, 116.30, 21.23. GC-MS (EI, 70 ev): m/z (%) = 236 (M+, 100), 209 (10), 208 (62), 207 (37), 179 (13), 178 (28), 165 (26), 152 (12), 89 (11), 63 (10), 114 (12), 82 (11), 63 (15).
3-(Naphthalen-1-yl)-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.97–7.87 (m, 2H), 7.84–7.76 (m, 2H), 7.64–7.41 (m, 7H), 7.38–7.30 (m, 1H). 13C-NMR (75 MHz, Chloroform-d) δ 160.77, 153.97, 142.77, 133.66, 132.64, 131.65, 131.53, 129.36, 128.53, 128.37, 127.93, 127.63, 126.48, 126.07, 125.23, 124.54, 119.32, 116.68. GC-MS (EI, 70 ev): m/z (%) = 272 (M+, 100), 273 (19), 271 (79), 255 (11), 244 (24), 243 (50), 216 (11), 215 (58), 214 (10), 213 (28), 189 (17), 107 (18), 95 (17), 63 (11).
3-(4-Methoxyphenyl)-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.75 (s, 1H), 7.71–7.63 (m, 2H), 7.56–7.44 (m, 2H), 7.34 (ddd, J = 8.0, 1.3, 0.7 Hz, 1H), 7.31–7.23 (m, 1H), 7.02–6.90 (m, 2H), 3.85 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 160.74, 160.10, 153.24, 138.43, 130.95, 129.78, 127.81, 127.65, 127.02, 124.38, 119.79, 116.32, 113.87, 55.32. GC-MS (EI, 70 ev): m/z (%) = 252 (M+, 100), 224 (10), 210 (10), 209 (65), 181 (41), 152 (35).
3-(3-Methoxyphenyl)-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.81 (s, 1H), 7.53 (td, J = 7.4, 1.6 Hz, 2H), 7.40–7.32 (m, 2H), 7.32–7.25 (m, 3H), 6.95 (ddd, J = 8.1, 2.6, 1.2 Hz, 1H), 3.85 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 160.41, 159.48, 153.45, 139.94, 135.96, 131.40, 129.43, 128.09, 127.89, 124.44, 120.86, 119.55, 116.38, 114.47, 114.16, 55.32. GC-MS (EI, 70 ev): m/z (%) = 252 (M+, 100), 224 (46), 194 (10), 182 (10), 181 (68), 167 (10), 165 (21), 153 (13), 152 (62), 151 (16), 127 (10), 126 (14), 63 (16), 39 (10).
3-(4-Fluorophenyl)-2H-chromen-2-one: 7u1H-NMR (300 MHz, Chloroform-d) δ 7.79 (s, 1H), 7.75–7.64 (m, 2H), 7.54 (ddt, J = 7.6, 6.0, 1.8 Hz, 2H), 7.37 (dt, J = 8.8, 0.8 Hz, 1H), 7.34–7.27 (m, 1H), 7.19–7.06 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 164.70, 160.51, 153.47, 139.65, 131.48, 130.70, 130.39 (d, J = 8.3 Hz), 127.87, 127.30, 124.56, 119.54, 116.47, 115.46 (d, J = 21.6 Hz). GC-MS (EI, 70 ev): m/z (%) = 240 (M+, 93), 212 (100), 184 (14), 183 (81), 181 (14), 157 (19), 107 (12), 106 (21), 92 (12), 91 (13).
3-(3-Fluorophenyl)-2H-chromen-2-one: 7y1H-NMR (300 MHz, Chloroform-d) δ 7.79 (s, 1H), 7.56–7.47 (m, 2H), 7.47–7.38 (m, 2H), 7.38–7.28 (m, 2H), 7.27–7.19 (m, 1H), 7.05 (tdd, J = 8.3, 2.6, 1.1 Hz, 1H). 13C-NMR (75 MHz, Chloroform-d) δ 164.24, 160.57 (d, J = 61.2 Hz), 153.54, 140.38, 136.63 (d, J = 8.1 Hz), 131.78, 129.95 (d, J = 8.4 Hz), 128.04, 126.99 (d, J = 2.4 Hz), 124.61, 124.13 (d, J = 3.1 Hz), 119.36, 116.49, 115.84 (d, J = 7.0 Hz), 115.55 (d, J = 8.9 Hz). GC-MS (EI, 70 ev): m/z (%) = 240 (M+, 80), 212 (90), 183 (100), 157 (10), 63 (10).
Dibenzo[b,f]oxepine-10-carbonitrile: 91H-NMR (300 MHz, Chloroform-d) δ 7.62 (dd, J = 8.1, 1.7 Hz, 1H), 7.54–7.41 (m, 3H), 7.35–7.18 (m, 5H). 13C-NMR (75 MHz, Chloroform-d) δ 158.30, 157.43, 142.37, 132.86, 131.91, 130.42, 128.29, 128.03, 126.17, 125.60, 125.41, 121.91, 121.67, 118.46, 113.99.GC-MS (EI, 70 ev): m/z (%) = 219 (M+, 100), 191 (25), 190 (93), 165 (12), 164 (30), 163 (25), 82 (10), 63 (12).
3-(3-Chlorophenyl)-2H-chromen-2-one: 1H-NMR (300 MHz, Chloroform-d) δ 7.83 (s, 1H), 7.70 (td, J = 1.7, 1.0 Hz, 1H), 7.64–7.59 (m, 1H), 7.59–7.51 (m, 2H), 7.42–7.37 (m, 2H), 7.37–7.28 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 160.15, 153.59, 140.43, 136.35, 134.38, 131.82, 129.68, 128.89, 128.54, 128.05, 126.96, 126.74, 124.63, 119.37, 116.53. GC-MS (EI, 70 ev): m/z (%) = 256 (M+, 100), 230 (27), 229 (17), 228 (95), 166 (10), 165 (80), 164 (22), 163 (27), 139 (12), 110 (10), 82 (13), 75 (12), 63 (12). HRMS (EI): Calcd. for [[M + H]+: C15H9ClO2]+: 257.03638, found: 257.03614.
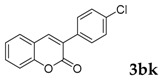
3-(4-Chlorophenyl)-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.82 (d, J = 0.6 Hz, 1H), 7.71–7.62 (m, 2H), 7.59–7.50 (m, 2H), 7.46–7.40 (m, 2H), 7.37 (dt, J = 8.9, 0.8 Hz, 1H), 7.34–7.28 (m, 1H). 13C-NMR (75 MHz, Chloroform-d) δ 160.32, 153.52, 139.91, 134.92, 133.05, 131.66, 129.82, 128.67, 127.95, 127.15, 124.60, 119.46, 116.50. GC-MS (EI, 70 ev): m/z (%) = 256 (M+, 100), 230 (24), 229 (10), 228 (73), 165 (60), 164 (18), 163 (20).
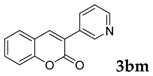
3-(Pyridin-3-yl)-2H-chromen-2-one: 7z1H-NMR (300 MHz, Chloroform-d) δ 8.80 (d, J = 2.4 Hz, 1H), 8.61 (dd, J = 4.9, 1.7 Hz, 1H), 8.05 (d, J = 8.5 Hz, 1H), 7.28–7.22 (m, 1H), 7.44–7.31 (m, 3H), 7.25 (s, 1H), 7.14 (td, J = 7.6, 1.0 Hz, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 153.33, 149.55, 149.14, 136.43, 134.27, 132.25, 130.90, 127.68, 123.61, 122.92, 119.62, 115.38. GC-MS (EI, 70 ev): m/z (%) = 221 (M+, 100), 222 (26), 139 (12).
4-Methyl-3-phenyl-2H-chromen-2-one: 7t1H-NMR (300 MHz, Chloroform-d) δ 7.69 (dd, J = 8.0, 1.5 Hz, 1H), 7.55 (ddd, J = 8.6, 7.2, 1.5 Hz, 1H), 7.50–7.36 (m, 4H), 7.36–7.28 (m, 3H), 2.32 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 160.93, 152.66, 147.59, 134.42, 131.29, 129.99, 128.40, 128.18, 127.33, 125.08, 124.22, 120.54, 116.85, 16.56. GC-MS (EI, 70 ev): m/z (%) = 236 (M+, 96), 235 (82), 208 (60), 207 (100), 179 (24), 178 (62), 177 (11), 176 (13), 165 (22), 152 (22), 151 (10), 139 (15), 131 (28), 115 (20), 102 (12), 89 (23), 77 (21), 75 (10), 63 (21), 51 (17), 50 (10), 39 (15).
3,4-Diphenyl-2H-chromen-2-one: 101H-NMR (300 MHz, Chloroform-d) δ 7.54 (ddd, J = 8.6, 6.6, 2.2 Hz, 1H), 7.44 (ddd, J = 8.3, 1.2, 0.6 Hz, 1H), 7.34–7.28 (m, 3H), 7.23–7.10 (m, 9H). 13C-NMR (75 MHz, Chloroform-d) δ 161.26, 153.22, 151.57, 134.46, 133.84, 131.43, 130.51, 129.35, 128.33, 128.25, 127.78, 127.73, 127.63, 126.99, 124.11, 120.51, 116.76. GC-MS (EI, 70 ev): m/z (%) = 298 (M+, 100), 297 (90), 281 (11), 270 (28), 269 (28), 268 (16), 255 (13), 253 (17), 252 (11), 241 (32), 240 (10), 239 (47), 165 (12), 119 (19).
2H-Chromen-2-one: 7m1H-NMR (300 MHz, Chloroform-d) δ 7.62 (d, J = 9.6 Hz, 1H), 7.50–7.35 (m, 2H), 7.30–7.13 (m, 2H), 6.34 (d, J = 9.5 Hz, 1H). 13C-NMR (75 MHz, Chloroform-d) δ 160.74, 154.03, 143.39, 131.80, 127.83, 124.39, 118.81, 116.88, 116.69. GC-MS (EI, 70 ev): m/z (%) = 146 (M+, 56), 118 (100), 90 (44), 89 (41), 64 (10), 63 (28), 62 (12).
(Z)-2,3-Diphenylacrylonitrile: 111H-NMR (300 MHz, Chloroform-d) δ 7.98–7.85 (m, 2H), 7.73–7.64 (m, 2H), 7.55 (s, 1H), 7.52–7.39 (m, 6H). 13C-NMR (75 MHz, Chloroform-d) δ 142.20, 134.41, 133.66, 130.49, 129.22, 129.16, 129.02, 128.91, 125.95, 117.95, 111.64. GC-MS (EI, 70 ev): m/z (%) = 205 (M+, 100), 204 (92), 203 (26), 190 (52), 178 (23), 177 (27), 176 (24), 165 (13), 151 (13), 102 (12), 89 (14), 88 (11), 77 (11), 76 (16), 75 (11), 63 (13), 51 (22), 50 (14), 39 (11).
(Z)-3-(4-Hydroxyphenyl)-2-phenylacrylonitrile: 111H-NMR (300 MHz, DMSO-d6) δ 10.29 (s, 1H), 7.93–7.76 (m, 3H), 7.75–7.64 (m, 2H), 7.54–7.43 (m, 2H), 7.42–7.31 (m, 1H), 6.92 (d, J = 8.7 Hz, 2H). 13C-NMR (75 MHz, DMSO-d6) δ 160.02, 142.91, 134.37, 131.44, 129.14, 128.61, 125.41, 124.79, 118.64, 115.89, 105.85. GC-MS (EI, 70 ev): m/z (%) = 221 (M+, 100), 206 (18), 204 (10), 203 (11), 202 (24), 192 (11), 191 (14) 190 (19), 177 (11), 165 (40), 164 (13), 63 (12), 51 (16), 39 (10).
4. Conclusions
In summary, a practical procedure for the synthesis of 3-aryl-2H-chromen-2-ones from salicylaldehydes and arylacetonitriles has been established. With tBuOK as the promotor and DMF as the solvent, good to excellent yields of chromenones were obtained. Additionally, no protection of inert gas atmosphere is required here.
Acknowledgments
We gratefully thank the National Natural Science Foundation of China (grant No. 21406207) for support of this program.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
Chengcai Li and Hailin Zhu conceived and designed the experiments; Chengcai Li and Hang Zhang performed the experiments; Chengcai Li and Yongfeng Yang analyzed the data; Feng Wang contributed reagents/materials/analysis tools; Chengcai Li and Hailin Zhu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Musa M.A., Cooperwood J.S., Khan M.O.F. A Review of Coumarin Derivatives in Pharmacotherapy of Breast Cancer. Curr. Med. Chem. 2008;15:2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakur A., Singla R., Jaitak V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015;101:476–495. doi: 10.1016/j.ejmech.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Swarnakar N.K., Jain A.K., Singh R.P. Oral bioavailability, therapeutic efficacy and reactive oxygen species scavenging properties of coenzyme Q10-loaded polymeric nanoparticles. Biomaterials. 2011;32:6860–6874. doi: 10.1016/j.biomaterials.2011.05.079. [DOI] [PubMed] [Google Scholar]
- 4.Matos M.J., Vazquez-Rodriguez S., Santana L., Uriarte E., Fuentes-Edfuf C., Santos Y., Munoz-Crego A. Looking for new targets: Simple coumarins as antibacterial agents. Med. Chem. 2012;8:1140–1145. doi: 10.2174/1573406411208061140. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y., Zhou C.H. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011;42:956–960. doi: 10.1016/j.bmcl.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 6.Choochuay K., Chunhacha P., Pongrakhananon V. Imperatorin sensitizes anoikis and inhibits anchorage-independent growth of lung cancer cells. J. Nat. Med. 2013;67:599–606. doi: 10.1007/s11418-012-0719-y. [DOI] [PubMed] [Google Scholar]
- 7.Basanagouda M., Vishwanath B., Barigidad N., Laxmeshwar S., Devaru S., Venkatesh N. Synthesis, structure–activity relationship of iodinated-4-aryloxymethyl-coumarins as potential anti-cancer and anti-mycobacterial agents. Eur. J. Med. Chem. 2014;74:225–233. doi: 10.1016/j.ejmech.2013.12.061. [DOI] [PubMed] [Google Scholar]
- 8.Kostova I., Raleva S., Genova P. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. 2006;2006:68274. doi: 10.1155/BCA/2006/68274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugino T., Tanaka K. Solvent-Free Coumarin Synthesis. Chem. Lett. 2001;30:110–111. doi: 10.1246/cl.2001.110. [DOI] [Google Scholar]
- 10.Fringuelli F., Pani G., Piermatti O. ChemInform Abstract: Low-Polluting Chemical Processes-Aldol and Allylation Reactions in Water. Cheminform. 1996;27 doi: 10.1002/chin.199620255. [DOI] [Google Scholar]
- 11.Johnson J.R. The Perkin Reaction and Related Reactions. Org. React. 1942;1:210. [Google Scholar]
- 12.Shriner R.L. The reformatsky reaction. Org. React. 1942;1:1. [Google Scholar]
- 13.Yavari I., Hekmat-Shoar R., Zonouzi A. ChemInform Abstract: A New and Efficient Route to 4-Carboxymethylcoumarins Mediated by Vinyltriphenylphosphonium Salt. Tetrahedron Lett. 1998;29:2391–2392. doi: 10.1016/S0040-4039(98)00206-8. [DOI] [Google Scholar]
- 14.Song C.E., Jung D.U., Choung S.Y. Dramatic enhancement of catalytic activity in an ionic liquid: Highly practical Friedel-Crafts alkenylation of arenes with alkynes catalyzed by metal triflates. Angew. Chem. 2004;43:6183–6185. doi: 10.1002/anie.200460292. [DOI] [PubMed] [Google Scholar]
- 15.Reddy M.S., Thirupathi N., Babu M.H. ChemInform Abstract: Synthesis of Substituted 3-Iodocoumarins and 3-Iodobutenolides via Electrophilic Iodocyclization of Ethoxyalkyne Diols. J. Org. Chem. 2013;78:5878–5888. doi: 10.1021/jo400499r. [DOI] [PubMed] [Google Scholar]
- 16.Mi X., Wang C., Huang M. ChemInform Abstract: Preparation of 3-Acyl-4-arylcoumarins via Metal-Free Tandem Oxidative Acylation/Cyclization between Alkynoates with Aldehydes. J. Org. Chem. 2015;80:148–155. doi: 10.1021/jo502220b. [DOI] [PubMed] [Google Scholar]
- 17.Reddy M.S., Thirupathi N., Haribabu M. Tandem aldehyde–alkyne–amine coupling/cycloisomerization: A new synthesis of coumarins. Beilstein. J. Org. Chem. 2013;9:180–184. doi: 10.3762/bjoc.9.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong F., Feng X., Zhang Y. An anion-exchange strategy for 3D hierarchical (BiO)2CO3/amorphous Bi2S3 heterostructures with increased solar absorption and enhanced visible light photocatalysis. RSC Adv. 2015;5:11714–11723. doi: 10.1039/C4RA15798B. [DOI] [Google Scholar]
- 19.Feng J.B., Wu X.F. Oxidative Synthesis of Quinazolinones under Metal-free Conditio. J. Heterocycl. Chem. 2016 doi: 10.1002/jhet.2562. [DOI] [Google Scholar]
- 20.Choi Y.L., Lim H.S., Lim H.J., Heo J.-N. One-Pot Transition-Metal-Free Synthesis of Dibenzo[b,f]oxepins from 2-Halobenzaldehydes. Org. Lett. 2012;14:5102–5105. doi: 10.1021/ol302371s. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., Meng T., Fan R., Wu J. General and efficient route for the synthesis of 3,4-disubstituted coumarins via Pd-catalyzed site-selective cross-coupling reactions. J. Org. Chem. 2007;72:7279–7286. doi: 10.1021/jo071117+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.