Abstract
Aliphatic diazirines have been widely used as prominent photophores for photoaffinity labeling owing to their relatively small size which can reduce the steric effect on the natural interaction between ligands and proteins. Based on our continuous efforts to develop efficient methods for the synthesis of aliphatic diazirines, we present here a comprehensive study about base-mediated one-pot synthesis of aliphatic diazirines. It was found that potassium hydroxide (KOH) can also promote the construction of aliphatic diazirine with good efficiency. Importantly, KOH is cheaper, highly available, and easily handled and stored compared with the previously used base, potassium tert-butoxide (t-BuOK). Gram-scale study showed that it owned great advantages in being used for the large-scale production of aliphatic diazirines. This protocol is highly neat and the desired products can be easily isolated and purified. As the first comprehensive study of the base-mediated one-pot synthesis of aliphatic diazirines, this work provided good insight into the preparation and utilization of diazirine-based photoaffinity labeling probes.
Keywords: photoaffinity labeling, one-pot synthesis, aliphatic diazirine, base-mediated reaction
1. Introduction
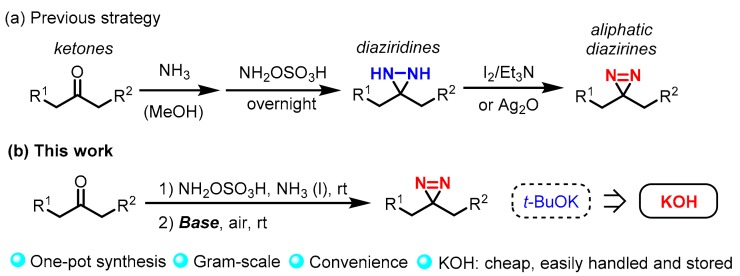
Photoaffinity labeling [1,2,3,4,5] has emerged as a powerful methodology in the field of biology due to its availability for elucidating the interactions between bioactive molecules and proteins. Various photophores—including phenyl azides, benzophenones, and diazirines—are commonly employed in this field [6,7,8,9,10,11]. Under UV irradiation, photophores are converted into highly reactive intermediates which can form covalent bonds between ligands and proteins. The stable links in the components allow for further separation and detection. Among these photophores, aliphatic diazirines [12,13,14,15,16] have attracted tremendous attention due to their relatively small size, thus minimizing the steric effect of the natural interaction between ligands and proteins. For the conventional synthesis procedure of aliphatic diazirines [17,18], the ketones precursor was first treated with ammonia for several hours, then it was reacted with hydroxylamine-O-sulfonic acid (NH2OSO3H) to form diaziridines. After removal of the ammonia, diaziridines were further converted to diazirine in the presence of I2-TEA or Ag2O (Scheme 1a). Despite the wide applications, this protocol suffers many issues such as low yield and tedious procedures. To solve these issues, we have recently developed a novel one-pot synthesis of aliphatic diazirines by using t-BuOK under air [19]. In spite of its good efficiency, t-BuOK is a flammable base and it should be commonly handled and stored under inert gas. Furthermore, its relatively high price also limits the large-scale production of diazirines and the corresponding labeling probes. Herein, for the first time, we carried out a comprehensive study to screen the bases and other conditions, and it was found that KOH also could be used for one-pot synthesis of aliphatic diazirines with great efficiency (Scheme 1b). Significantly, it is cheaper, easily handled, and stored as well as being highly available in many labs. Gram-scale study confirmed its usability for the large-scale production of aliphatic diazirines. Furthermore, a kinetic study to elaborate the one-pot process was also performed on the basis of NMR analysis.
Scheme 1.
Previous (a) and base-mediated one-pot (b) synthesis of aliphatic diazirines.
2. Results and Discussion
2.1. Reaction Optimization
We initiated our studies by using levulinic acid (1a) as a model substrate for reaction optimization, which was treated with 1.1 equivalent of NH2OSO3H in liquid ammonia at room temperature for 12 h to generate the corresponding diaziridine in situ. Then, base (3.3 equiv.) was gradually added into the mixture and the reaction was further stirred at room temperature under air for 2 h. Lithium amide was first tested and it was able to afford aliphatic diazirine (2a) at a 36% yield (Table 1, entry 1). Sodium amide was examined and a slightly higher yield of 2a was obtained (44%, entry 2). Several metal hydrides were able to mediate the construction of diazirine (2a), albeit with moderate yields (entries 3–5). Alkoxides were also utilized to promote the formation of products and the results indicated that a potassium base is preferred to sodium one (entries 6–8). Furthermore, NaOH and KOH [20,21,22,23,24,25] were employed and it was found that KOH was able to afford 2a in 67% yield (entries 9 and 10). Although its efficiency was slightly lower compared to t-BuOK [19], KOH is easily handled, stored, and highly available in many labs. Its relatively low price makes it feasible for large-scale synthesis of aliphatic diazirines. Moreover, the by-products derived from OH anion can easily be removed in this reaction. Thus, we optimized KOH for the subsequent study. The reaction carried out at either low temperature (entry 11) or in methanolic ammonia (entry 12) caused a low yield of product (2a), indicating that both temperature and concentration of ammonia are crucial for the generation of diazirine in this protocol.
Table 1.
Reaction optimization of one-pot synthesis for aliphatic diazirine 2a a.

| Entry | Solvent | Base | Temp | Yield (%) b |
|---|---|---|---|---|
| 1 | NH3 | LiNH2 | RT | 36 |
| 2 | NH3 | NaNH2 | RT | 44 |
| 3 | NH3 | NaH | RT | 47 |
| 4 | NH3 | MgH2 | RT | 20 |
| 5 | NH3 | CaH2 | RT | 17 |
| 6 | NH3 | EtONa | RT | 45 |
| 7 | NH3 | MeONa | RT | 45 |
| 8 | NH3 | MeOK | RT | 63 |
| 9 | NH3 | NaOH | RT | 28 |
| 10 | NH3 | KOH | RT | 67 |
| 11 | NH3 | KOH | 0 °C | 11 |
| 12 | NH3/MeOH c | KOH | RT | 5 |
a Unless otherwise mentioned, 1a (2 mmol) and NH2OSO3H (1.1 equiv.) was added to liquid ammonia (8 mL) at −78 °C and the reaction mixture was stirred at room temperature for 12 h until base (3.3 equiv.) was added. After the addition of base, the reaction was further stirred at room temperature under air for 2 h. b Isolated yields were presented. c Ammonia gas was bubbled into MeOH (8 mL) at 0 °C for 30 min.
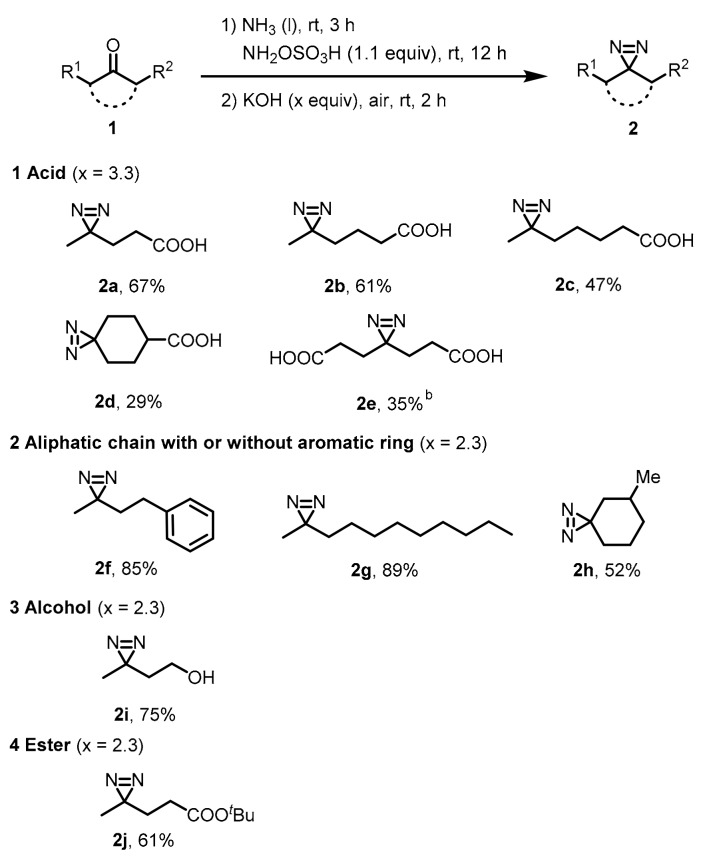
2.2. Scope Investigation
With the optimized condition in hand, the generality of the protocol was evaluated with different ketone procedures. A wide range of substituted ketones—including acid, alcohol, ester, and hexatomic rings—were tolerated under this condition to be converted into the corresponding diazirine products. When monoacid substrates were tested, 3.3 equivalents of KOH were required to create the desired product and the corresponding diazirines were obtained in moderate to good yield (Scheme 2, 2a–d). A binary acid derivative (1e) was subjected to the optimal reaction with 4.3 equivalents of KOH, and the desired product (2e) was isolated in 35% yield. Neutral ketones with aliphatic chain or aromatic ring are good substrates (2f–h) and up to an 89% yield was given for compound (2g). 3-Azibutanol (2i), a widely utilized photophore, was readily synthesized with a 75% yield. Compared to the previously synthetic method for 2i, the KOH-mediated procedure showed a relatively good efficiency. Furthermore, t-butyl alcohol ester was also tolerated in the optimal condition, and the desired product was isolated in 61% yield (2j). The spectroscopic data were summarized in Supplementary Materials.
Scheme 2.
Scope investigation of one-pot synthesis of aliphatic diazirine with KOH a. a Unless otherwise mentioned, 1 (2 mmol) and NH2OSO3H (1.1 equiv.) was added into liquid ammonia (8 mL) at −78 °C and the reaction mixture was stirred at room temperature for 12 h until base was added. After the addition of base, the reaction was further stirred at room temperature under air for 2 h. b 4.3 equivalent of KOH was used.
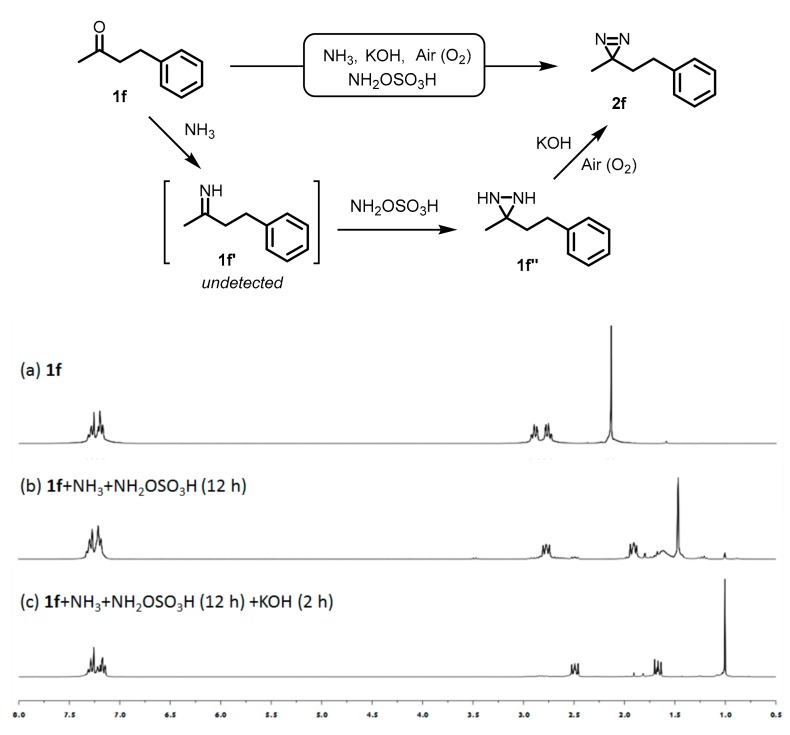
2.3. Kinetic Study of One-Pot Synthesis of Aliphatic Diaizirine
To investigate the one-pot process, we performed a kinetic study based on 1H-NMR analysis. Aliphatic ketone (1f) (Scheme 3a) was initially treated with liquid ammonia at room temperature, while no imine (1f′) was observed although the treatment was continued to 12 h. When 1f was subjected to the reaction with NH2OSO3H in liquid ammonia at room temperature for 12 h, the corresponding diaziridine (1f′′) was detected (Scheme 3b). To be noted, a trace amount of diazirine (2f) was generated at this stage which can be detected by 1H-NMR spectrum. Following with further treatment by KOH for 2 h, the formation of diazirine (2f) was dramatically improved (Scheme 3c). Furthermore, no other by-products were distinctly detected in the spectrum during the formation of diaziridine as well as diazirine. These results indicated that diaziridine as the precursor can be directly converted to diazirine in KOH-mediated one-pot reaction. This method is useful with the isolation and purification of the final products being more convenient.
Scheme 3.
One-pot synthesis of aliphatic diazirine 2f. The reaction stages were monitored using 1H-NMR with CDCl3. (a) starting material 1f; (b) 1f was treated with NH2OSO3H in liquid ammonia for 12 h; (c) The residue was further treated with KOH in liquid ammonia under air for 2 h.
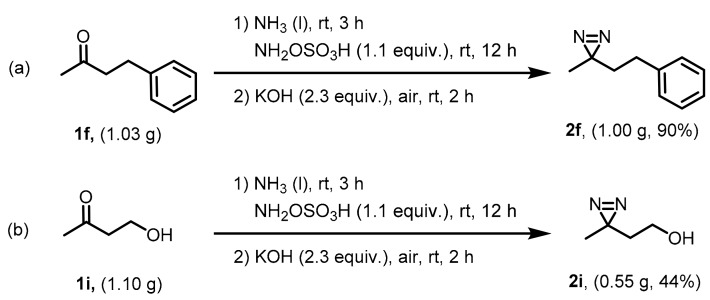
2.4. Gram-Scale Synthesis
To explore the behavior of this protocol for large-scale synthesis of aliphatic diazirines, we designed gram-scale reactions. Compound 1f (1.03 g) was subjected to the optimized conditions and the desired product (2f) can be readily isolated at a 90% yield (Scheme 4a). It undoubtedly confirmed the advantages and applicability of KOH-mediated synthetic strategy for the large-scale synthesis of aliphatic diazirines. 3-Azibutanol (2i) is a widely used photophore that labels active molecules in the field of photoaffinity labeling [26,27,28,29,30]. While many syntheses suffered relatively low yields. By using the KOH-mediated one-pot strategy, we can synthesize 2i in a 75% yield (Scheme 2). It is a great improvement compared with previous synthetic methods as well as the t-BuOK-mediated one. A gram-scale synthesis was carried out and the protocol was able to afford 2i at a 44% yield from gram-scale 1i (Scheme 4b), thus indicating the good applicability of KOH-mediated one-pot strategy in large-scale synthesis.
Scheme 4.
Gram-scale synthesis of 2f (a) and 2i (b).
3. Materials and Methods
3.1. General Procedures
All reactions were carried out in a metal sealed tube (300 mL, 10 MPa, 200 °C). The NMR spectra were measured on JEOL EX-270 (JEOL, Tokyo, Japan). All solvents were of reagent grade and purified using the appropriate methods. HRMS spectra were obtained with a Waters UPLC ESI-TOF mass spectrometer (Waters, Milford, CT, USA).
3.2. Typical Procedures for One-Pot Synthesis of Aliphatic Diazirines from Corresponding Ketones
3.2.1. Acidic Substrates (1a–e)
Ketone (2 mmol) was dissolved in liquid NH3 (8 mL) in a sealed tube at −78 °C. Hydroxylamine-O-sulfonic acid (1.1 equiv.) was carefully added at the same temperature. After closing the tube, the reaction mixture was stirred at room temperature for 12 h, before it was cooled at −78 °C and subsequently opened. KOH (3.3 equiv. for 1a–d and 4.3 equiv. for 1e) was added to liquid NH3 mixture and then the tube was sealed. The reaction mixture was further stirred at room temperature under air for 2 h. The ammonia was gradually removed at room temperature in a fume hood. The residue was dissolved with water (30 mL) which was adjust pH to 2 with HCl aq, then extracted twice with Et2O (30 mL). The organic layer was washed with brine, dried over MgSO4, and evaporated. The residue was subjected to silica-gel column chromatography to afford the diazirinyl products.
3.2.2. Neutral Substrates (1f–j)
Ketone (2 mmol) was dissolved in liquid NH3 (8 mL) in a sealed tube at −78 °C. Hydroxylamine-O-sulfonic acid (1.1 equiv.) was carefully added at same temperature. After closing the tube, the reaction mixture was stirred at room temperature for 12 h, before it was cooled at −78 °C and subsequently opened. KOH (2.3 equiv.) was added to liquid NH3 mixture then the tube was sealed. The reaction mixture was stirred at room temperature under air for 2 h. The ammonia was gradually removed at room temperature under a fume hood. The residue was partitioned between water (30 mL) and Et2O (30 mL). The aqueous layer was extracted twice with Et2O (30 mL). The combined organic layer was washed by brine, dried over MgSO4, and evaporated. The residue was subjected to silica-gel column chromatography to afford the diazirinyl products.
3-(3-Methyl-3H-diazirin-3-yl)propanoic acid (2a): Pale yellow oil, 1H-NMR (270 MHz, CDCl3) δ ppm: 11.88 (s, 1H), 2.26 (t, J = 7.6 Hz, 2H), 1.73 (t, J = 7.6 Hz, 2H), 1.05 (s, 3H); 13C-NMR (68 MHz, CDCl3) 178.3, 29.1, 28.2, 24.8, 19.2; HRMS (ESI) m/z [M + Na]+ calcd. for C5H8N2O2Na 151.0483, found 151.0508.
4-(3-Methyl-3H-diazirin-3-yl)butanoic acid (2b): Pale yellow oil, 1H-NMR (270 MHz, CDCl3) δ ppm: 2.35 (t, J = 7.2 Hz, 2H), 1.48–1.57 (m, 2H), 1.38–1.44 (m, 2H), 1.02 (s, 3H); 13C-NMR (68 MHz, CDCl3) 179.7, 33.4, 33.1, 25.2, 19.5, 19.0; HRMS (ESI) m/z [M + Na]+ calcd. for C6H10N2O2Na 165.0640, found 165.0641.
5-(3-Methyl-3H-diazirin-3-yl)pentanoic acid (2c): Pale yellow oil, 1H-NMR (270 MHz, CDCl3) δ ppm: 2.34 (t, J = 7.4 Hz, 2H), 1.56–1.67 (m, 2H), 1.35–1.41 (m, 2H), 1.19–1.29 (m, 2H), 1.01 (s, 3H); 13C-NMR (68 MHz, CDCl3) 179.9, 33.9, 33.7, 25.5, 24.0, 23.4, 19.6; HRMS (ESI) m/z [M + Na]+ calcd. for C7H12N2O2Na 179.0796, found 179.0778.
1,2-Diazaspiro[2.5]oct-1-ene-6-carboxylic acid (2d): Pale yellow solid, 1H-NMR (270 MHz, CDCl3) δ ppm: 2.47–2.57 (m, 1H), 2.02–2.11 (m, 2H), 1.82–1.92 (m, 2H), 1.61–1.73 (m, 2H), 0.87 (dt, J = 14.1, 3.6 Hz, 2H); 13C-NMR (68 MHz, CDCl3) 181.6, 41.2, 29.8, 27.3, 26.4; HRMS (ESI) m/z [M + H]+ calcd. for C7H11N2O2 155.0821, found 155.0809.
3,3′-(3H-Diazirine-3,3-diyl)dipropanoic acid (2e): Pale yellow solid, 1H-NMR (270 MHz, CDCl3) δ ppm: 2.11 (t, J = 7.5 Hz, 4H), 1.72 (t, J = 7.5 Hz, 4H); 13C-NMR (68 MHz, CDCl3) 176.2, 29.1, 28.9, 28.7; HRMS (ESI) m/z [M + Na]+ calcd. for C7H10N2O4Na 209.0538, found 209.0551.
3-Methyl-3-phenethyldiaziridine (1f′′): Pale yellow oil, 1H-NMR (270 MHz, CDCl3) δ ppm: 7.27–7.32 (m, 2H), 7.18–7.22 (m, 3H), 2.77 (t, J = 7.8 Hz, 2H), 1.91 (t, J = 7.8 Hz, 2H), 1.61–1.70 (m, 2H), 1.47 (s, 3H); 13C-NMR (68 MHz, CDCl3) δ 141.1, 128.5, 128.2, 126.1, 55.0, 40.4, 31.3, 22.9; HRMS (ESI) m/z [M + H]+ calcd. for C10H15N2 163.1235, found 163.1238.
3-Methyl-3-phenethyl-3H-diazirine (2f): Pale yellow oil, 1H-NMR (270 MHz, CDCl3) δ ppm: 7.26–7.31 (m, 2H), 7.15–7.22 (m, 3H), 2.46–2.52 (m, 2H), 1.64–1.70 (m, 2H), 1.00 (s, 3H); 13C-NMR (68 MHz, CDCl3) 140.9, 128.6, 128.3, 126.2, 36.3, 30.0, 25.6, 19.8; HRMS (ESI) m/z [M + H]+ calcd. for C10H13N2 161.1079, found 161.1085.
3-Methyl-3-nonyl-3H-diazirine (2g): Pale yellow oil, 1H-NMR (270 MHz, CDCl3) δ ppm: 1.25–1.35 (m, 16H), 0.99 (s, 3H), 0.88 (t, J = 6.2 Hz, 3H); 13C-NMR (68 MHz, CDCl3) 34.2, 31.8, 29.33, 29.31, 29.2, 29.1, 23.9, 22.5, 19.8, 13.9; HRMS (ESI) m/z [M + H]+ calcd. for C11H23N2 183.1861, found 183.1888.
5-Methyl-1,2-diazaspiro[2.5]oct-1-ene (2h): Pale yellow oil, 1H-NMR (270 MHz, CDCl3) δ ppm: 1.58–1.81 (m, 5H), 1.36–1.45 (m, 1H), 1.00–1.10 (m, 1H), 0.91 (d, J = 6.4 Hz, 3H), 0.50–0.59 (m, 2H); 13C-NMR (68 MHz, CDCl3) 39.8, 33.6, 31.0, 30.9, 28.3, 23.5, 21.8; HRMS (ESI) m/z [M + H]+ calcd. for C7H13N2 125.1079, found 125.1084.
2-(3-Methyl-3H-diazirin-3-yl)ethanol (2i): Pale yellow oil, 1H-NMR (270 MHz, CDCl3) δ ppm: 3.54 (t, J = 6.3 Hz, 2H), 1.65 (t, J = 6.3 Hz, 2H), 1.08 (s, 3H); 13C-NMR (68 MHz, CDCl3) 57.6, 36.9, 24.1, 20.1; HRMS (ESI) m/z [M + H]+ calcd. for C4H9N2O 101.0715, found 101.0738.
Ttert-butyl 3-(3-methyl-3H-diazirin-3-yl)propanoate (2j): 1H-NMR (270 MHz, CDCl3) δ ppm: 2.03 (t, J = 7.7 Hz, 2H), 1.59 (t, J = 7.7 Hz, 2H), 1.38 (s, 9H), 0.95 (s, 3H); 13C-NMR (68 MHz, CDCl3) 171.7, 80.6, 30.0, 29.6, 27.9, 25.1, 19.5; HRMS (ESI) m/z [M + H]+ calcd. for C9H17N2O2 185.1285, found 185.1302.
4. Conclusions
In conclusion, we reported a comprehensive study about the base-mediated one-pot synthesis of aliphatic diazirines. KOH, a cheap, easily-handled and stored base, was optimized for the one-pot reaction. Many precious diazirines were prepared in moderate to excellent yields. Kinetic study showed that diaziridine as the intermediate can be directly converted to diazirine after the addition of KOH under air. Gram-scale synthesis indicated its good applicability in the large-scale synthesis of aliphatic diazirines for photoaffinity labeling. The reactions are pretty neat and products can be easily isolated and purified. This protocol will be helpful for effective synthesis of diazirine-based photoaffinity label ligands to elucidate functional analysis of biological activities.
Acknowledgments
This research was partially supported by the Ministry of Education, Science, Sports, and Culture Grant-in-Aid for Scientific Research (C), (17K0194007 to MH). Part of this work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”.
Supplementary Materials
Copies of the 1H- and 13C-NMR spectra are available online.
Author Contributions
L.W. and M.H. conceived and designed the experiments; L.W., Z.P.T., N.K., and F.O. performed the experiments; L.W. and M.H. analyzed the data; M.H. contributed reagents/materials/analysis tools; Y.S., Y.H., and M.H. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Brunner J. New photolabeling and cross-linking methods. Ann. Rev. Biochem. 1993;62:483–514. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- 2.Ruoho A.E., Kiefer H., Roeder P.E., Singer S.J. The Mechanism of Photoaffinity Labeling. Proc. Natl. Acad. Sci. USA. 1973;70:2567–2571. doi: 10.1073/pnas.70.9.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatanaka Y. Development and leading-edge application of innovative photoaffinity labeling. Chem. Pharm. Bull. 2015;63:1–12. doi: 10.1248/cpb.c14-00645. [DOI] [PubMed] [Google Scholar]
- 4.Tomizawa M., Casida J.E. Molecular recognition of neonicotinoid insecticides: The determinants of life and death. Acc. Chem. Res. 2009;42:260–269. doi: 10.1021/ar800131p. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto M., Hatanaka Y. Recent progress in diazirine-based photoaffinity labeling. Eur. J. Org. Chem. 2008;2008:2513–2523. doi: 10.1002/ejoc.200701069. [DOI] [Google Scholar]
- 6.Fleet G.W.J., Porter R.R., Knowles J.R. Affinity labeling of antibodies with aryl nitrene as reactive group. Nature. 1969;224:511–512. doi: 10.1038/224511a0. [DOI] [Google Scholar]
- 7.Ishida A., Wang L., Tachrim Z.P., Suzuki T., Sakihama Y., Hashidoko Y., Hashimoto M. Comprehensive synthesis of photoreactive phenylthiourea derivatives for the photoaffinity labeling. Chemistryselect. 2017;2:160–164. doi: 10.1002/slct.201601675. [DOI] [Google Scholar]
- 8.Murai Y., Yoshida T., Wang L., Masuda K., Hashidoko Y., Monde K., Hatanaka Y., Hashimoto M. Efficient synthesis of photoreactive 2-propoxyaniline derivatives as artificial sweeteners. Synlett. 2016;27:946–950. doi: 10.1055/s-0035-1561275. [DOI] [Google Scholar]
- 9.Wang L., Yoshida T., Muto Y., Murai Y., Tachrim Z.P., Ishida A., Nakagawa S., Sakihama Y., Hashidoko Y., Masuda K., et al. Synthesis of diazirine based photoreactive saccharin derivatives for the photoaffinity labeling of gustatory receptors. Eur. J. Org. Chem. 2015:3129–3134. doi: 10.1002/ejoc.201500184. [DOI] [Google Scholar]
- 10.Wang L., Hashidoko Y., Hashimoto M. Co-solvent promoted O-benzylation with silver(I) oxide: Synthesis of 1′-benzylated sucrose derivatives, mechanistic studies and scope investigation. J. Org. Chem. 2016;81:4464–4474. doi: 10.1021/acs.joc.6b00144. [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Murai Y., Yoshida T., Ishida A., Masuda K., Sakihama Y., Hashidoko Y., Hatanaka Y., Hashimoto M. Alternative one-pot synthesis of (trifluoromethyl)phenyldiazirines from tosyloxime derivatives: Application for new synthesis of optically pure diazirinylphenylalanines for photoaffinity labeling. Org. Lett. 2015;17:616–619. doi: 10.1021/ol503630z. [DOI] [PubMed] [Google Scholar]
- 12.Das J. Aliphatic diazirines as photoaffinity probes for proteins: Recent developments. Chem. Rev. 2011;111:4405–4417. doi: 10.1021/cr1002722. [DOI] [PubMed] [Google Scholar]
- 13.Thirumurugan P., Matosiuk D., Jozwiak K. Click chemistry for drug development and diverse chemical–biology applications. Chem. Rev. 2013;113:4905–4979. doi: 10.1021/cr200409f. [DOI] [PubMed] [Google Scholar]
- 14.Böttcher T., Pitscheider M., Sieber S.A. Natural products and their biological targets: Proteomic and metabolomic labeling strategies. Angew. Chem. Int. Ed. 2010;49:2680–2698. doi: 10.1002/anie.200905352. [DOI] [PubMed] [Google Scholar]
- 15.Takaoka Y., Ojida A., Hamachi I. Protein organic chemistry and applications for labeling and engineering in live-cell systems. Angew. Chem. Int. Ed. 2013;52:4088–4106. doi: 10.1002/anie.201207089. [DOI] [PubMed] [Google Scholar]
- 16.Wratil P.R., Horstkorte R., Reutter W. Metabolic glycoengineering with N-acyl side chain modified mannosamines. Angew. Chem. Int. Ed. 2016;55:9482–9512. doi: 10.1002/anie.201601123. [DOI] [PubMed] [Google Scholar]
- 17.Church R.F.R., Kende A.S., Weiss M.J. Diazirines. I. Some observations on the scope of the ammonia-hydroxylamine-O-sulfonic acid diaziridine synthesis. The preparation of certain steroid diaziridines and diazirines. J. Am. Chem. Soc. 1965;87:2665–2671. doi: 10.1021/ja01090a025. [DOI] [Google Scholar]
- 18.Church R.F.R., Weiss M.J. Diazirines. II. Synthesis and properties of small functionalized diazirine molecules. Observations on the reaction of a diaziridine with the iodine-iodide ion system. J. Org. Chem. 1970;35:2465–2471. doi: 10.1021/jo00833a001. [DOI] [Google Scholar]
- 19.Wang L., Ishida A., Hashidoko Y., Hashimoto M. Dehydrogenation of NH-NH bond triggered by potassium t-butoxide in liquid NH3. Angew. Chem. Int. Ed. 2017;56:870–873. doi: 10.1002/anie.201610371. [DOI] [PubMed] [Google Scholar]
- 20.Baars H., Beyer A., Kohlhepp S.V., Bolm C. Transition-metal-free synthesis of benzimidazoles mediated by KOH/DMSO. Org. Lett. 2014;16:536–539. doi: 10.1021/ol403414v. [DOI] [PubMed] [Google Scholar]
- 21.Xu J., Zhuang R., Bao L., Tang G., Zhao Y. KOH-mediated transition metal-free synthesis of imines from alcohols and amines. Green Chem. 2012;14:2384–2387. doi: 10.1039/c2gc35714c. [DOI] [Google Scholar]
- 22.Beyer A., Reucher C.M.M., Bolm C. Potassium hydroxide/dimethyl sulfoxide promoted intramolecular cyclization for the synthesis of benzimidazol-2-ones. Org. Lett. 2011;13:2876–2879. doi: 10.1021/ol2008878. [DOI] [PubMed] [Google Scholar]
- 23.Reddy V.P., Kumar A.V., Swapna K., Rao K.R. Copper oxide nanoparticle-catalyzed coupling of diaryl diselenide with aryl halides under ligand-free conditions. Org. Lett. 2009;11:951–953. doi: 10.1021/ol802734f. [DOI] [PubMed] [Google Scholar]
- 24.Anderson K.W., Ikawa T., Tundel R.E., Buchwald S.W. The selective reaction of aryl halides with KOH: Synthesis of phenols, aromatic ethers, and benzofurans. J. Am. Chem. Soc. 2006;128:10694–10695. doi: 10.1021/ja0639719. [DOI] [PubMed] [Google Scholar]
- 25.Kawabata T., Moriyama K., Kawakami S., Tsubaki K. Powdered KOH in DMSO: An efficient base for asymmetric cyclization via memory of chirality at ambient temperature. J. Am. Chem. Soc. 2008;130:4153–4157. doi: 10.1021/ja077684w. [DOI] [PubMed] [Google Scholar]
- 26.Kambe T., Correia B.E., Niphakis M.J., Cravatt B.F. Mapping the protein interaction landscape for fully functionalized small-molecule probes in human cells. J. Am. Chem. Soc. 2014;136:10777–10782. doi: 10.1021/ja505517t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang J., Zhang L., Tan X-L., Qi Y-K., Feng S., Deng H., Yan Y., Zheng J-S., Liu L., Tian C-L. Chemical synthesis of diubiquitin-based photoaffinity probes for selectively profiling ubiquitin-binding proteins. Angew. Chem. Int. Ed. 2017;56:2744–2748. doi: 10.1002/anie.201611659. [DOI] [PubMed] [Google Scholar]
- 28.Arevalo E., Shanmugasundararaj S., Wilkemeyer M.F., Dou X., Chen S., Charness M.E., Miller K.W. An alcohol binding site on the neural cell adhesion molecule L1. Proc. Natl. Acad. Sci. USA. 2008;105:371–375. doi: 10.1073/pnas.0707815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigdel U.K., Zhang J., He C. Diazirine-based DNA photo-cross-linking probes for the study of protein–DNA interactions. Angew. Chem. Int. Ed. 2008;47:90–93. doi: 10.1002/anie.200703625. [DOI] [PubMed] [Google Scholar]
- 30.Morieux P., Salomé C., Park K.D., Stables J.P., Kohn H. The structure-activity relationship of the 3-Oxy site in the anticonvulsant (R)-N-Benzyl 2-acetamido-3-methoxypropionamide. J. Med. Chem. 2010;53:5716–5726. doi: 10.1021/jm100508m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.