Abstract
Purpose
The aim of this study was to characterize serogroup 19 isolates resistant to macrolides and/or penicillin found among pneumococci recovered from cases of invasive and respiratory tract disease in the Czech Republic in 2014.
Methods
Pneumococcal isolates of serotypes 19A (n=26) and 19F (n=10) that were non-susceptible to penicillin and/or macrolides and had been collected in 2014 were analysed using multi-locus sequence typing (MLST). Four isolates representing the major clones were subjected to whole-genome sequencing (WGS).
Results
The penicillin-susceptible macrolide-resistant isolates of serotype 19A were mainly associated with sequence type (ST) 416 belonging to clonal complex (CC) 199, and the penicillin-resistant isolates were of serotype 19F belonging to ST1464 (CC 320). WGS revealed the presence of pilus 1, in association with pilus 2, in serotype19F isolates belonging to CC 320. Another adhesin, pneumococcal serine-rich protein (PsrP), was only present in serotype 19A isolates of ST416. Analysis of the penicillin-binding proteins (PBPs) of serotype 19F penicillin-resistant isolates (ST1464 and ST271) performed on PBP1a, 2b and 2x identified a large number of mutations in comparison to the reference strain, R6. Both isolates contained a unique PBP profile; however, they were highly similar to PBP sequences of the Taiwan19F-14 reference strain. The Pbp2b sequences of both 19F isolates showed the lowest similarity to those of the Taiwan19F-14 strain (91 % similarity), while they were also found to be distantly related to each other (94 % similarity).
Conclusions
WGS revealed specific virulence factors in antibiotic-resistant pneumococcal clones that spread rapidly in the post-vaccine era in the Czech Republic.
Keywords: Streptoccoccus pneumoniae, MLST, WGS
Introduction
Streptoccoccus pneumoniae is an important pathogen of respiratory tract infections and a leading cause of bacteraemia and meningitis. The occurrence of S. pneumoniae that are resistant to betalactams and other groups of antibiotics complicates the effective treatment of pneumococcal infections [1]. It was predicted that the dissemination of antibiotic-resistant pneumococci would be targeted by the pneumococcal conjugate vaccines, as the serotypes included in the vaccines had accounted for the vast majority of penicillin non-susceptible serotypes [2]. After the implementation of nationwide pneumococcal vaccination for children, the incidence of invasive pneumococcal disease (IPD) has decreased among the vaccinated and, due to the herd effect, it has also decreased in the non-vaccinated population [3, 4]. Although a reduction in invasive disease has been reported, especially in cases caused by penicillin-resistant serotypes, the reduction of intermediately penicillin-resistant strains of vaccine serotypes has been compensated by increased intermediate resistance among non-vaccine serotypes [5–7].
The clone that has the highest impact on the spread of antibiotic resistance in the post-PCV7 era is Taiwan19F-14 [8–10]. This clone, which shows sequence type (ST) 236, was originally detected as having the 19F capsule, and was resistant to betalactams, macrolides and tetracyclines [11]. The surveys following the implementation of pneumococcal vaccination identified isolates belonging to this clone and showing serotype 19A in many countries in Asia and Europe, as well as in the United States [8, 12, 13]. Occasionally, 19A isolates expressing ST320, which is a double-locus variant of ST236, were found in the pre-vaccine era. After the introduction of PCV7, ST320 19A isolates have become the most prevalent multidrug-resistant genotype in the post-vaccine era in the United States and some European countries, and have continued to be identified in the PCV13 era [14–17]. In the Czech Republic, vaccination with PCV10 or PCV13 (covered by health insurance) has been included in the routine childhood immunization programme since January 2010. The use of PCV is voluntary and, according to data from the major health insurance company, 73.9 % of children up to 1 year of age had been vaccinated in 2014. In the post-PCV era, isolates of the 19A serotype that are non-susceptible to penicillin and/or to macrolides and tetracyclines have started to occur in carriage and respiratory samples and have also been found in IPD cases in all age cohorts [18]. The historical pre-vaccine antibiotic-resistant 19A isolates identified in the Czech Republic belonged to two clones, Hungary19A-6 and CSR19A-11 [19]. Isolates of the Taiwan19F-14 clone were not found in our environment in either serotype 19A or 19F isolates, which were mainly associated with ST423 [20]. Since the mid-1990s, serotype 9V, represented by the Spain9V-3 clone, has replaced serotype 19A (the Hungary19A-6 clone), and the total prevalence of serotype 19A declined dramatically [21]. It can be assumed that the implementation of vaccination against pneumococcus has affected the serotype-specific prevalence of pneumococci, including the antibiotic-resistant serotypes, but other factors that have been enhancing the spread of the antibiotic-resistant 19A serotype must be taken into account, as both currently used vaccines (with different 19A coverage) share the market almost equally, and the effect on the replacement by the 19A serotype is therefore limited.
The serotyping of antibiotic-resistant serotypes has shown the increasing prevalence of the 19A serotype following vaccination against pneumococci; however, this information is not sufficient to identify the origin of the strains. To establish the genetic background of the antibiotic-resistant 19A isolates, we used multi-locus sequence typing (MLST) to identify the clonal lineages associated with antibiotic resistance after the implementation of pneumococcal vaccination. The detailed characteristics of the representative isolates of the major serogroup 19 clones have been further identified by whole-genome sequencing (WGS).
Methods
Bacterial isolates
In total, 36 pneumococcal isolates of serotypes 19A (n=26) and 19F (n=10) that were non-susceptible to penicillin and/or macrolides and were collected in 2014 were analysed. Isolates from the blood and cerebrospinal fluid (n=16) were identified among all (n=306) invasive isolates referred to the National Institute of Public Health (NIPH) for antibiotic susceptibility testing and serotyping during national surveillance of IPD. Additional isolates (n=20) were obtained during the surveillance of antibiotic resistance in S. pneumoniae isolates from respiratory tract disease (sinusitis, acute otitis media and community-acquired pneumonia), where only isolates resistant to erythromycin (n=27) are referred to the NIPH.
All isolates were cultured on Columbia agar, and the species identification was confirmed using the deoxycholate test. The serotyping was performed by multiplex PCR using primers and conditions available at the Centers for Disease Control and Prevention (https://www.cdc.gov/streplab/pcr.html). Serotypes were confirmed by the Quellung reaction [22].
Antibiotic susceptibility
The minimum inhibitory concentrations (MICs) were determined for penicillin (PEN), cefotaxime (CTX), tetracycline (TET), erythromycin (ERY), clindamycin (CLI), chloramphenicol (CHL) and trimethoprim/sulfamethoxazole (SXT) by the microdilution broth method [23]. The MICs were interpreted according to the criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST; www.eucast.org). For epidemiological purposes, the microbiological breakpoints of low-level PEN resistance (MIC range from 0.12 to 2 µg m1−1) and high-level PEN resistance (MIC>2 µg m1−1) were used. The resistance to macrolides and tetracyclines was confirmed by PCR amplification of the respective genes. Resistance to macrolides was determined by PCR of internal fragments of the mefA and ermB genes according to the previously described protocols [24, 25]. PCR detection of tetracycline resistance was performed by amplification of the 1862-bp fragment from positions 21 to 1882 of the published sequence of the tetM gene [26].
MLST typing
DNA was isolated according to the manufacturer's instructions using a commercial kit (NA2120, Sigma Aldrich) enriched with 250 U ml−1 of mutanolysin (M9901, Sigma Aldrich). Samples were typed according to a standard protocol [27]. Briefly, the internal fragments of seven housekeeping genes (aroE, gdh, gki, recP, spi, xpt and ddl) were amplified by PCR. The editing and alignment of all the sequences were performed using the publicly available S. pneumoniae MLST database (http://pubmlst.org/spneumoniae/), where new STs were also deposited. Clonal complexes were assigned using the eBURST algorithm (http://eburst.mlst.net). The allelic profiles of the STs of all the isolates were analysed using BioNumerics 7.6 software (Applied Maths, Ghent, East Flanders, Belgium).
WGS library, Illumina sequencing
Four isolates representing the major clones were analysed. The genomic DNA of S. pneumoniae was extracted using the DNA-Sorb-B kit (Sacace Biotechnologies Srl, Como, Italy). The plasmids and chromosomes were sequenced using the Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA). The reads were mapped using the Short Read Sequence Typing 2 (SRST2) tool (version 0.2.0) to assess the presence of virulence and antibiotic resistance genes [28]. The database of virulence genes was obtained from VFDB (http://www.mgc.ac.cn/VFs/), and the database distributed with SRST2 was used to search for resistance genes. The serotypes were determined by mapping reads to the reference cps locus sequences using PneumoCat [29]. The initial reads were quality trimmed using Trimmomatic [30] and assembled via the de Bruijn graph-based de novo assembler SPAdes [31]. The STs of the four de novo assembled genomes were confirmed on http://pubmlst.org/perl/bigsdb/bigsdb.pl?db=pubmlst_spneumoniae_seqdef&page=sequenceQuery. Further analyses of the assembled sequences were performed using Bionumerics 7.6. Gene annotations were carried out by the Bionumerics annotation application using the Taiwan19F-14 element (GenBank accession number CP000921) as the template sequence. Sequences of pbp1a, pbp2b and pbp2x, virulence and antibiotic resistance determinants of two 19A penicillin-susceptible and two 19F penicillin-resistant strains, were aligned to the corresponding sequences of S. pneumoniae R6 (accession number NC_003098.1) and compared in detail. The nucleotide sequences of the pbp genes were compared with the sequences of Taiwan19F-14 (GenBank accession number CP000921), Hungary19A-6 (GenBank accession number CP000936.1) and multidrug-resistant S. pneumoniae strain A026 (GenBank accession number CP006844) [32]. The mutations identified in the nucleotide sequence of pbp2b from the penicillin-resistant isolates were listed and compared with the protein sequence mutations reported by Contreras-Martel [33]. Pairwise clustering was processed by Bionumerics software using its default settings.
Accession numbers
Nucleotide sequences representing each a different pbp type obtained in this study were submitted to GenBank. The nucleotide sequences of pbp2x and pbp2b (isolate 24238) and pbp2x and pbp2b (isolate 25137) were deposited in GenBank under accession numbers MF033177, MF033178, MF033179 and MF033180, respectively.
Results
Antibiotic susceptibility
In 2014, 306 invasive isolates of S. pneumoniae were examined at the National Institute of Public Health, Prague, Czech Republic. Of these, 16 (5.2 %) were non-susceptible to penicillin. Only three isolates belonging to serotype 19F showed high-level resistance to penicillin. The most common serotype among the isolates with low-level resistance (n=13) was serotype 19A (n=4; 30.8 %). Resistance to erythromycin was detected in 22 (7.2 %) isolates and was associated with serotype 19A (n=10; 45.4 %), followed by serotype 19F (n=4; 18.2 %). While serotype 19A was the second most common invasive serotype (n=25; 8.2 %), serotype 19F was far rarer (n=8; 2.6 %). Among the non-invasive respiratory isolates (n=27), resistance to erythromycin was linked with serotypes 19A (n=15; 55.5 %) and 19F (n=6; 22.2 %), and in almost half of the isolates (n=13; 48.1 %) it was combined with decreased susceptibility to penicillin. The median age of patients with serotype 19A was 42 (IQR 65.5–3) years, while it was 63.5 (IQR 70–60.5) years in serotype 19F-infected patients.
With the exception of two non-viable isolates, all serogroup 19 strains recovered in 2014 that were resistant to erythromycin and/or to penicillin (n=36) were further analysed.
Resistance to macrolides was confirmed in all (n=34) erythromycin-resistant isolates. All of them showed the MLSB phenotype and possesed the ermB gene. Dual-macrolide resistance (ermB and mefA genotypes) was found predominantly in 19F isolates (see Table 1). Resistance to tetracycline, which is frequently associated with the MLSB, phenotype, was due to the presence of the tetM genes. All but one of the isolates (serotype 19A, ST 11197) were susceptible to chloramphenicol, and resistance to trimethoprim/sulfamethoxazole was particularly found in 19F isolates.
Table 1. Characteristics of serogroup 19 isolates (n=36) obtained in the Czech Republic in 2014.
| Isolates | Serotype | Source | MIC (µg ml−1) | ATB resistance genes | CC | ST | Allelic profile | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | PEN | CTX | TET | ERY | CLI | CHL | SXT | aroE | gdh | gki | recP | spi | xpt | ddl | PMEN clone | ||||||
| 24 679 | 19A | Blood | 70 | 0.008 | 0.016 | 16.0 | >4.0 | 4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | Netherland15B-37/ST199 |
| 26 584 | 19A | Blood | 60 | 0.008 | 0.016 | 16.0 | >4.0 | >4.0 | 4.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 270 | 19A | CSF | 36 | 0.016 | 0.016 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416† | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 374 | 19A | Ear | 3 | 0.016 | 0.016 | >16.0 | >4.0 | 4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 389 | 19A | Ear | 3 | 0.016 | 0.016 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 417 | 19A | Blood | 64 | 0.016 | 0.016 | 16.0 | >4.0 | 4.0 | 4.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 491 | 19A | Ear | 3 | <0.004 | 0.016 | <0.125 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM* | 199 | 416† | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 630 | 19A | Blood | 68 | 0.016 | 0.016 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 775 | 19A | Ear | Unknown | 0.008 | 0.016 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 776 | 19A | Ear | Unknown | 0.016 | 0.016 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 806 | 19A | Ear | 0 | 0.016 | 0.016 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 844 | 19A | Sputum | 62 | 0.016 | 0.016 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 854 | 19A | Ear | 3 | 0.016 | 0.03 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 27 897 | 19A | Nose | 6 | 0.016 | <0.004 | >16.0 | >4.0 | >4.0 | 2.0 | 0.5 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 28 206 | 19A | Blood | 73 | 0.008 | 0.03 | <0.125 | >4.0 | 2.0 | 2.0 | 0.5 | ermB, tetM* | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 28 240 | 19A | Ear | 0 | 0.008 | 0.03 | 16.0 | >4.0 | >4.0 | 2.0 | 0.5 | ermB, tetM | 199 | 416 | 1 | 13 | 14 | 4 | 17 | 51 | 14 | |
| 24 417 | 19A | Blood | 48 | 0.125 | 0.06 | <0.125 | 0.06 | 0.06 | 2.0 | 0.25 | 199 | 1756 | 8 | 20 | 14 | 4 | 17 | 4 | 14 | ||
| 25 138 | 19A | Blood | 71 | 0.008 | 0.03 | >16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 199 | 11 198 | 1 | 13 | 14 | 4 | 199 | 51 | 14 | |
| 25 137 | 19F | Blood | 57 | 8.0 | 4.0 | 16.0 | >4.0 | >4.0 | 0.5 | >4.0 | ermB, mefA, tetM | 320 | 271† | 4 | 16 | 19 | 15 | 6 | 20 | 26 | Taiwan19F-14/ST236 |
| 27 722 | 19A | Sputum | 67 | 2.0 | 1.0 | 16.0 | >4.0 | >4.0 | 2.0 | >4.0 | ermB, mefA, tetM | 320 | 1464 | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 24 238 | 19F | CSF | 73 | 4.0 | 2.0 | 8.0 | >4.0 | >4.0 | 2.0 | >4.0 | ermB, mefA, tetM | 320 | 1464† | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 24 633 | 19F | Sputum | 56 | 4.0 | 2.0 | 16.0 | >4.0 | >4.0 | 4.0 | >4.0 | ermB, mefA, tetM | 320 | 1464 | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 24 870 | 19F | Sputum | 65 | 4.0 | 2.0 | 16.0 | >4.0 | >4.0 | 2.0 | >4.0 | ermB, mefA, tetM | 320 | 1464 | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 24 993 | 19F | Sputum | 62 | 4.0 | 2.0 | 16.0 | >4.0 | >4.0 | 4.0 | >4.0 | ermB, mefA, tetM | 320 | 1464 | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 27 721 | 19F | Sputum | 60 | 2.0 | 1.0 | 16.0 | >4.0 | >4.0 | 2.0 | >4.0 | ermB, mefA, tetM | 320 | 1464 | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 27 723 | 19F | Sputum | 61 | 2.0 | 1.0 | 16.0 | >4.0 | >4.0 | 2.0 | >4.0 | ermB, mefA, tetM | 320 | 1464 | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 27 992 | 19F | Blood | 67 | 4.0 | 2.0 | 16.0 | >4.0 | >4.0 | 2.0 | >4.0 | ermB, mefA, tetM | 320 | 1464 | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 28 205 | 19F | Sputum | 73 | 4.0 | 2.0 | 16.0 | >4.0 | >4.0 | 2.0 | >4.0 | ermB, mefA, tetM | 320 | 1464 | 4 | 16 | 19 | 15 | 6 | 20 | 106 | |
| 24 493 | 19A | Blood | 21 | 0.25 | 0.25 | 16.0 | >4.0 | >4.0 | 2.0 | 0.125 | ermB, tetM | 230 | 230 | 12 | 19 | 2 | 17 | 6 | 22 | 14 | Denmark14-32/ST230 |
| 28 258 | 19A | Sputum | 69 | 0.5 | 0.125 | 16.0 | >4.0 | 0.5 | 2.0 | 0.125 | ermB, tetM | 230 | 230 | 12 | 19 | 2 | 17 | 6 | 22 | 14 | |
| 27 314 | 19A | Blood | 61 | 2.0 | 1.0 | 16.0 | >4.0 | 0.125 | 2.0 | 1.0 | ermB, tetM | 230 | 276 | 2 | 19 | 2 | 17 | 6 | 22 | 14 | |
| 25 833 | 19A | Blood | 60 | 0.5 | 0.25 | >16.0 | 0.06 | 0.125 | 2.0 | >4.0 | 230 | 2013 | 12 | 19 | 36 | 17 | 6 | 20 | 14 | ||
| 27 227 | 19A | Ear | 3 | 0.016 | 0.016 | >16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 193 | 3863 | 8 | 10 | 211 | 16 | 1 | 26 | 1 | Greece21-30/ST193 |
| 29 469 | 19A | Blood | 4 | 0.016 | 0.016 | 16.0 | >4.0 | >4.0 | 2.0 | 0.25 | ermB, tetM | 193 | 3863 | 8 | 10 | 211 | 16 | 1 | 26 | 1 | |
| 28 087 | 19F | Blood | 79 | 0.125 | 0.06 | 16.0 | 4.0 | >4.0 | 4.0 | 0.5 | ermB, tetM | 177 | 179 | 7 | 14 | 40 | 12 | 1 | 1 | 14 | Portugal19F-21/ST177 |
| 27 102 | 19A | Ear | 5 | <0.004 | 0.008 | >16.0 | >4.0 | 0.125 | 16.0 | 4.0 | ermB, tetM | 66 | 11 197 | 1 | 5 | 41 | 5 | 10 | 616 | 8 | |
PEN, penicilin; CTX, cefotaxime; TET, tetracycline; ERY, erytromycin; CLI, clindamycin; CHL, chloramphenicol; SXT, trimethoprim/sulfamethoxazole; CC, clonal complex; ST, sequence type; PMEN, Pneumococcal Molecular Epidemiology Network.
New sequence types are shown in bold.
*Isolates were found to be phenotypically susceptible due to the deletion of a 26-bp-long segment (from nucleotide position 16 298 to 16323) of the tetM gene.
†Isolates included in the whole-genome sequencing.
MLST analysis
MLST analysis revealed 11 different STs among serogroup 19 (see Table 1). eBURST analysis using the whole MLST database categorized isolates into six CCs. The largest CC, including 18 of the 36 isolates (50 %), was represented by ST416 (n=16), ST11198 and ST1756. ST416 is a subgroup founder in CC199 and is related to the Netherlands15B-37 clone. All isolates within this CC belonged to serotype 19A (18 of 26 isolates; 69.2 %) The second major CC (CC320), which comprised 10 of the 36 isolates (27.8 %), was represented by ST1464 (n=9) and ST271; both are single-locus variants of ST236, the representative of the Taiwan19F-14 clone. This clone was mainly associated with serotype 19F (9 out of 10 isolates; 90 %), while 1 ST1464 isolate was determined to be in serotype 19A. The other minor CCs were CC230, which is related to the Denmark14-32 clone (four isolates of serotype 19A); CC193, which is related to Greece21-30 (two serotype 19A isolates); CC177, which is related to Portugal19F-21 (a 19F isolate); and CC66 (a 19A isolate).
The susceptibility profile was highly consistent with the corresponding CC. With the exception of one ST1756 isolate, CC199 was represented by penicillin-susceptible, but erythromycin-, clindamycin- and tetracycline-resistant isolates. By contrast, all of the CC320 isolates showed high-level penicillin resistance, the MLSB phenotype (due to the double-macrolide resistance mechanism) and resistance to tetracycline and trimethoprim/sulfamethoxazole. Isolates with low-level penicillin resistance were found in two minor CCs: CC230 and CC177.
WGS
Virulence genes
Four representative isolates of two major clones (CC199 represented by ST416 serotype 19A isolates and CC320 represented by ST271 and ST320 serotype 19F isolates) were studied by WGS. To determine the presence of virulence factors, raw sequence reads were mapped against a virulence gene database. The absence of a gene was confirmed by examining the annotated sequence using blast. Apart from the genes involved in capsule biosynthesis, genes encoding virulence factors covering all four main groups of surface proteins [lipoproteins (LPs), leucin–proline–any amino acid–threonine–glycine (LPXTG) proteins, choline-binding proteins (CBPs) and non-classical surface proteins (NCPs)] were found in all of the isolates [34]. Of the 16 described choline-binding proteins, all of the isolates lacked CbpI and CbpM, which have been shown to interact with elastin [35]. Of the 18 LPXTG proteins described, genes coding for metalloprotease ZmpC and MucB were also missing in all of the isolates; moreover, both 19F isolates lacked the psrP gene encoding pneumococcal serine-rich protein (PsrP), which was previously demonstrated to play a role in lung-cell attachment and the invasion of epithelial cells [36]. All of the isolates contained the RlrA islet encoding pilus 1, and CC320 carried pilus 1 in association with pilus 2.
Antibiotic resistance
The genome contained two large transposons. The first one, Tn6002, showing 99.99 % similarity to the Tn6002 complete sequence (GenBank accession number AY898750.1), was found in 19A isolates (CC199). This transposon carried two antibiotic resistance genes: tetM and ermB. However, one 19A isolate (27491) carrying the tetM gene was found to be phenotypically susceptible due to the deletion of a 26-bp-long segment (spanning from nucleotide position 16 298 to 16 323 of the tetM gene), leading to a frameshift mutation. The other transposon was identified as Tn2010, showing 99.99 % similarity to the Tn2010 complete sequence (GenBank accession number AB426620.1). This transposon has been found in both 19F isolates (CC320), and aside from tetM and ermB, it also carries the mefE/mel operon.
Comparison of PBP genes
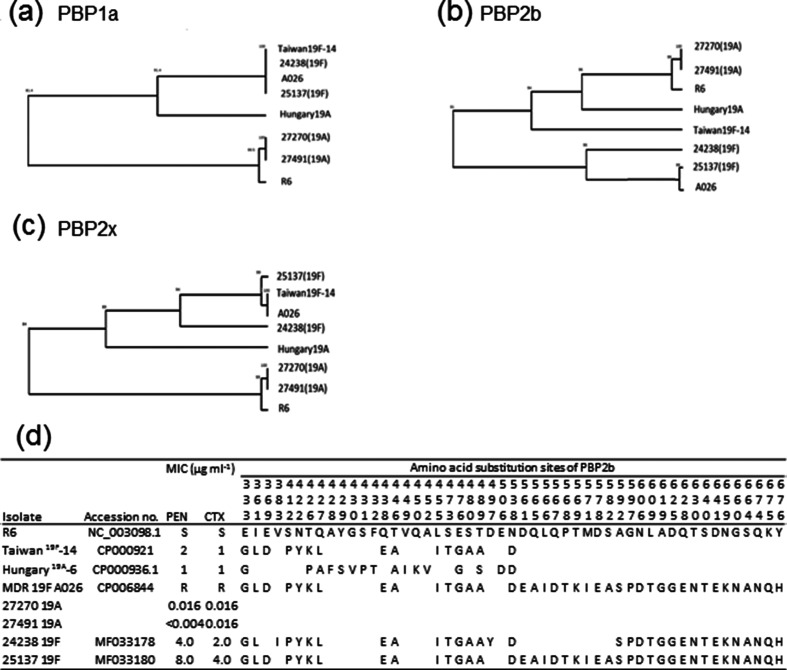
The PBP sequences of pbp2x, pbp2b and pbp1a of penicillin-susceptible 19A isolates were highly identical (99.0 to 100 % similarity) to those of the R6 strain (see Fig. 1). The Pbp sequences of penicillin-resistant 19F isolates were additionally compared to the corresponding sequences of the Taiwan19F-14 and Hungary19A-6 reference strains and the multidrug-resistant A026 pneumococcal strain (GenBank accession number CP006844). The Pbp1a sequences of the two 19F isolates were identical. Moreover, they were 100 % similar to the pbp1a sequence of the Taiwan19F-14 reference strain. The Pbp2x sequence of the 25 137 isolate was also highly similar to the pbp2x sequence of the Taiwan19F-14 reference strain (99 % similarity), but differed from that of the other 19F isolate (94 % similarity). The PBP2x of the 25 137 isolate (ST271) possessed the unique amino acid (aa) substitutions M339F, E378A, M400T and Y595F, which are distinct from those in Taiwan19F-14 but identical to those reported in the ST271 clinical isolates from Hong Kong [37]. Isolate 24 238 (ST1464) possessed an additional five aa substitutions (P268T, D278N, N501K, K505E and A507T) compared to Taiwan19F-14, but none of them has yet been identified as playing a role in resistance to betalactams. Compared to the ST271 isolate, ST1464 lacks three mutations (M339F, M400T and Y595F) associated with cefotaxime resistance [38]. Both isolates contained mutations within the S337TMK motif: T338A in ST1464 and T338A plus M339F in the ST271 isolate. The Pbp2b sequences of both 19F isolates showed the lowest similarity to those of the Taiwan19F-14 strain (91 % similarity), while they were also distantly related to each other (94 % similarity). Comparison of the pbp2b sequences with the R6 sequence revealed the presence of different mosaic blocks in the two 19F isolates. The aa sequence of the 25 137 isolate (ST271) was identical to the sequences of the ST271 isolates of the 19F serotype from Hong Kong. The aa sequence of the other isolate (24238, ST1464) contained relatively short mosaic blocks at positions 333 to 538, which is highly similar to the corresponding Taiwan19F-14 reference aa sequence, but differed from ST271 in three substitutions. The ST1464 isolate possessed two additional mutations (V383I and D497Y) but lacked the E369D mutation conferring high-level resistance to cefotaxime. At positions 561 to 582, the isolates differed in nine aa substitutions (absent in ST1464); however, at positions 592 to 676, the isolates were identical and possessed 16 aa changes that have been reported before in resistant Polish isolates of the Poland23F-16 clone and in multidrug-resistant isolates of serogroup 19 from Hong Kong [37, 39].
Fig. 1.
Genetic relationship of PBP1a, PBP2b and PBP2x amino acid sequences. (a–c) Pair-wise alignments generated by BioNumerics 7.6. showing the relatedness of pbp1a (a), pbp2b (b) and pbp2x (c) nucleotide sequences among penicillin-susceptible R6, multidrug-resistant 19F A026, Taiwan19F-14, Hungary19A-6 and 19A/19F isolates. (d) Amino acid substitution sites of PBP2b. The sequences were aligned according to the corresponding amino acid sequences of R6. PEN, penicillin; CTX, cefotaxime; S, susceptible; R, resistant.
Discussion
Despite the relatively stable low prevalence of antibiotic resistance in invasive pneumococci, an increase in antibiotic-resistant serotype 19A has been observed in the Czech Republic. MLST analysis identified ST416 as the dominant emerging genotype, which was in contrast to previous studies that reported a post-vaccine clonal expansion of the 19A capsular variant of the Taiwan19F-14 clone, but in agreement with findings from other European countries, where clonal expansion of ST416 has been observed [9, 40–42]. It is estimated that the successful spread of serotype 19A is due to its genetic diversity, and various lineages of serotype 19A have emerged in the post-vaccine era [9]. The main drivers of the increasing prevalence of serotype 19A are antibiotic selection pressure and a high propensity for capsular switching. The pathogenicity of pneumococci has been attributed to various structures, most of which are located on its surface [43]. Numerous in vitro studies have identified different pneumococcal proteins as possible protein vaccine candidates [44–46]. Pneumococcal surface proteins are involved in bacterial fitness and pathogen–host interactions. Pneumococci are highly plastic bacteria, as they are able to adapt the expression of certain virulence factors to host niches during various stages of infection [47]. However, the prevalence of some of those surface proteins seems to be clonally related. It has been suggested that the presence of pilus 1 promotes adherence and thereby increases the competitive capacity of piliated strains [48]. Previousr studies have also provided evidence for an association between antibiotic-resistant clones and the presence of pilus 1 [48–51]. In our study, all of the WGS-analysed isolates carried pilus 1. The presence of pilus 1 in 19A isolates of ST416 is of interest, since group founder ST199 isolates are pilus-negative. It is likely that the acquisition of pilus 1 could promote a clonal expansion of this genotype to a significant degree. On the other hand, another adhesin, PsrP, was only present in 19A isolates of ST416. PrsP, which acts as the lung cell adhesin, was found to be significantly associated with serotypes with higher invasive disease potential (including the 19A serotype), and it was hypothesized that PsrP is most probably linked to enhanced virulence of those serotypes [52, 53]. Selva et al. have shown a negative correlation of the simultaneous prevalence of PsrP and pilus 1, suggesting that the production of extremely large proteins might be metabolically expensive and that individual strains either do not support the expression of both proteins, or the function of both adhesins is redundant [36]. However, based on the results of our study, it seems that both virulence factors act synergistically and jointly increase the fitness of the ST416 clonal lineage, which constituted at least 40 % of the serotype 19A population among IPD in 2014. Moreover, the overall prevalence of the 19A serotype increased from 2.7 % in 2010 to 9.9 % in 2015, with 19A being the second most frequently reported invasive serotype in the Czech Republic [54]. A tentative explanation is that the clonal expansion of 19A ST416/CC199, which is becoming more common than 19F ST1464/CC320, is due to the contribution of the co-presence of several serotype-independent factors, which makes a capsular type more prone to occupy the niche left by PCV10 serotypes.
The non-susceptibility to betalactams was mainly associated with serotype 19F of CC320, represented by its single locus variants (SLVs), ST1464 and ST271. These SLVs differed from ST320 in the ddl gene, which is highly variable in penicillin-resistant isolates due to the proximity of the pbp2b gene, which is affected by interspecies recombinational exchanges driven by penicillin usage [55]. Penicillin non-susceptibility in pneumococci is mediated by the recombination of pbp genes (namely pbp1a, pbp2b and pbp2x) leading to aa substitutions at or close to the active site of the transpeptidase domains [56, 57]. The development of penicillin resistance in pneumococci is driven by gene transfer events from related species, especially Streptococcus mitis or Streptococcus oralis [58, 59]. PBP2x and PBP2b are considered to be primary resistance determinants conferring low-level resistance, and an alteration of those PBPs is a prerequisite for the emergence of high-level resistance, together with a concomitant alteration of PBP1a [60]. Although both of the two penicillin-resistant isolates had a unique PBP profile, transpeptidase domains that were identical or related to those previously reported in pneumococci were detected in both isolates [33, 37, 39, 61]. The PBP1a sequence was identical to that of the Taiwan19F-14 strain. Moreover, the same PBP1a aa sequences have been described previously in clinical isolates of serogroup 19 (ST271 and ST320) [37]. It seems that this PBP1a variant is a rather stable characteristic of the Taiwan19F-14 clone, contributing to its typical phenotype. The PBP2x sequence of the ST271 isolate has also been identified in Hong Kong isolates of the corresponding serotype and ST [37]. The aa substitutions in PBP2x, such as T338A, M339F and M400T, were found to be responsible for cefotaxime resistance [38]. The presence of these aa substitutions, together with additional changes (E378A and Y595F), are important for the emergence of high-level resistance to cefotaxime [37, 62]. The presence of mutations was in line with the observed high level of resistance to cefotaxime in the isolate of ST271, while the ST1464 isolate, where three of these aa changes (M339F, M400T and Y595F) were absent, showed low-level resistance (cefotaxime MIC of 2 µg ml−1). Both ST271 and ST1464 possessed aa substitutions of PBP2b involved in enzyme activity (T446A, A619G and Q628E). The Pbp2b sequences contained aa substitutions that were absent in Taiwan19F-14. Whereas ST271 was identical to the ST271 isolates of the 19F serotype reported in Hong Kong, ST1464 lacked 12 aa substitutions inside a block identified previously in the resistant S. mitis strain B22 (EMBL accession number AY187721). The patterns of the two pbp2b were highly similar to those of variants IV (ST271) and V (ST1464) of pbp2b, respectively, shown in Polish isolates of the Poland23F-16 clone [39]. Our results demonstrate the diversity of pbp genes, which occurs even at the subclone level, and corroborate the necessity for a detailed analysis of pbp polymorphism.
In conclusion, after pneumococcal vaccination, new prevailing clones of serotypes 19A and 19F have been identified among antibiotic-resistant invasive pneumococci in the Czech Republic. The success of those clones is probably linked to the presence of specific characteristics (namely pilus 1), but other factors, such as post-vaccine serotype replacement or antibiotic consumption, may have favoured their further spread.
Funding information
This work was supported by the Czech Health Research Council, Ministry of Health of the Czech Republic, grant no. 16-27109A and by MH CZ – DRO (National Institute of Public Health – NIPH, 75010330).
Acknowledgements
This publication made use of the Streptococcus pneumoniae MLST website (https://pubmlst.org/spneumoniae/) located at the University of Oxford (Jolley & Maiden 2010, BMC Bioinformatics, 11 : 595). The development of this site has been funded by the Wellcome Trust.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
This is a retrospective study in which the isolates were used without patient identifiers and hence patient consent has not been obtained.
Footnotes
Abbreviations: aa, amino acid; aroE, shikimate dehydrogenase; BLAST, Basic Local Alignment Search Tool; CBP, choline-binding protein; CC, clonal complex; CHL, chloramphenicol; CLI, clindamycin; CTX, cefotaxime; ddl, D-alanine-D-alanine ligase; ermB, erythromycin resistance methylase B gene; ERY, erythromycin; EUCAST, European Committee on Antimicrobial Susceptibility Testing; gdh, glucose-6-phosphate dehydrogenase; gki, glucose kinase; IPD, invasive pneumococcal disease; LPs, lipoproteins; LPXTG, leucin–proline–any amino acid–threonine–glycine; mefA, macrolide efflux A gene; mefE/mel, two-component macrolide efflux pump operon; MIC, minimum inhibitory concentration; MLSB, macrolide–lincosamide–streptogramin B; MLST, multi-locus sequence typing; MucB, mucin-binding domain B; NCPs, non-classical surface proteins; NIPH, National Institute of Public Health; PBPs, penicillin-binding proteins; PCV7, 7-valent pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PEN, penicillin; PsrP, pneumococcal serine-rich protein; recP, transketolase; RlrA, transcriptional regulator; spi, signal peptidase I; SRST2, Short Read Sequence Typing 2; ST, sequence type; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline; tetM, tetracycline resistance M gene; Tn, transposon; VFDB, virulence factor database; WGS, whole-genome sequencing; xpt, xanthine phosphoribosyltransferase; ZmpC, zinc metalloprotease C; SLV, single locus variant.
Accession numbers: MF033177 (pbp2x isolate No. 24238), MF033178 (pbp2b isolate No. 24238), MF033179 (pbp2x isolate No. 25137) and MF033180 (pbp2b isolate No. 25137).
References
- 1.Obaro S, Adegbola R. The pneumococcus: carriage, disease and conjugate vaccines. J Med Microbiol. 2002;51:98–104. doi: 10.1099/0022-1317-51-2-98. [DOI] [PubMed] [Google Scholar]
- 2.Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343:1917–1924. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 3.Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668–1674. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 4.Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 5.Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 6.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1-11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect. 2009;15:16–20. doi: 10.1111/j.1469-0691.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 8.Brueggemann AB, Pai R, Crook DW, Beall B. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 2007;3:e168. doi: 10.1371/journal.ppat.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore MR, Gertz RE, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016–1027. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 10.Pillai DR, Shahinas D, Buzina A, Pollock RA, Lau R, et al. Genome-wide dissection of globally emergent multi-drug resistant serotype 19A Streptococcus pneumoniae. BMC Genomics. 2009;10:642. doi: 10.1186/1471-2164-10-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi ZY, Enright MC, Wilkinson P, Griffiths D, Spratt BG. Identification of three major clones of multiply antibiotic-resistant Streptococcus pneumoniae in Taiwanese hospitals by multilocus sequence typing. J Clin Microbiol. 1998;36:3514–3519. doi: 10.1128/jcm.36.12.3514-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song JH, Dagan R, Klugman KP, Fritzell B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine. 2012;30:2728–2737. doi: 10.1016/j.vaccine.2012.01.091. [DOI] [PubMed] [Google Scholar]
- 13.Croucher NJ, Chewapreecha C, Hanage WP, Harris SR, McGee L, et al. Evidence for soft selective sweeps in the evolution of pneumococcal multidrug resistance and vaccine escape. Genome Biol Evol. 2014;6:1589–1602. doi: 10.1093/gbe/evu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beall BW, Gertz RE, Hulkower RL, Whitney CG, Moore MR, et al. Shifting genetic structure of invasive serotype 19A pneumococci in the United States. J Infect Dis. 2011;203:1360–1368. doi: 10.1093/infdis/jir052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croucher NJ, Hanage WP, Harris SR, McGee L, van der Linden M, et al. Variable recombination dynamics during the emergence, transmission and 'disarming' of a multidrug-resistant pneumococcal clone. BMC Biol. 2014;12:49. doi: 10.1186/1741-7007-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulten KG, Kaplan SL, Lamberth LB, Barson WJ, Romero JR, et al. Changes in Streptococcus pneumoniae serotype 19A invasive infections in children from 1993 to 2011. J Clin Microbiol. 2013;51:1294–1297. doi: 10.1128/JCM.00058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenoll A, Granizo JJ, Giménez MJ, Yuste J, Aguilar L. Secular trends (1990–2013) in serotypes and associated non-susceptibility of S. pneumoniae isolates causing invasive disease in the pre-/post-era of pneumococcal conjugate vaccines in Spanish regions without universal paediatric pneumococcal vaccination. Vaccine. 2015;33:5691–5699. doi: 10.1016/j.vaccine.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Figueiredo AM, Austrian R, Urbaskova P, Teixeira LA, Tomasz A. Novel penicillin-resistant clones of Streptococcus pneumoniae in the Czech Republic and in Slovakia. Microb Drug Resist. 1995;1:71–78. doi: 10.1089/mdr.1995.1.71. [DOI] [PubMed] [Google Scholar]
- 19.Zemlickova H, Urbaskova P, Jakubu V, Motlova J, Musilek M, et al. Clonal distribution of invasive pneumococci, Czech Republic, 1996-2003. Emerg Infect Dis. 2010;16:287–289. doi: 10.3201/eid1602.080535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zemlicková H, Melter O, Urbásková P. Epidemiological relationships among penicillin non-susceptible Streptococcus pneumoniae strains recovered in the Czech Republic. J Med Microbiol. 2006;55:437–442. doi: 10.1099/jmm.0.46270-0. [DOI] [PubMed] [Google Scholar]
- 21.Vančíková Z, Trojánek M, Zemličková H, Blechová Z, Motlová J, et al. Pneumococcal urinary antigen positivity in healthy colonized children: is it age dependent? Wien Klin Wochenschr. 2013;125:495–500. doi: 10.1007/s00508-013-0405-4. [DOI] [PubMed] [Google Scholar]
- 22.Sørensen UB. Typing of pneumococci by using 12 pooled antisera. J Clin Microbiol. 1993;31:2097–2100. doi: 10.1128/jcm.31.8.2097-2100.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Committee on Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin Microbiol Infect. 2003;9:1–7. [Google Scholar]
- 24.Farrell DJ, Morrissey I, Bakker S, Felmingham D. Detection of macrolide resistance mechanisms in Streptococcus pneumoniae and Streptococcus pyogenes using a multiplex rapid cycle PCR with microwell-format probe hybridization. J Antimicrob Chemother. 2001;48:541–544. doi: 10.1093/jac/48.4.541. [DOI] [PubMed] [Google Scholar]
- 25.Lina G, Quaglia A, Reverdy ME, Leclercq R, Vandenesch F, et al. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43:1062–1066. doi: 10.1128/aac.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty N, Trzcinski K, Pickerill P, Zawadzki P, Dowson CG. Genetic diversity of the tet(M) gene in tetracycline-resistant clonal lineages of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:2979–2984. doi: 10.1128/AAC.44.11.2979-2984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 28.Inouye M, Dashnow H, Raven LA, Schultz MB, Pope BJ, et al. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. Genome Med. 2014;6:90. doi: 10.1186/s13073-014-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapatai G, Sheppard CL, Al-Shahib A, Litt DJ, Underwood AP, et al. Whole genome sequencing of Streptococcus pneumoniae: development, evaluation and verification of targets for serogroup and serotype prediction using an automated pipeline. PeerJ. 2016;4:e2477. doi: 10.7717/peerj.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui Z, Zhou W, Yao K, Liu L, Zhang G, et al. Complete genome sequence of Streptococcus pneumoniae strain A026, a clinical multidrug-resistant isolate carrying Tn2010. Genome Announc. 2013;1:e01034-13. doi: 10.1128/genomeA.01034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contreras-Martel C, Dahout-Gonzalez C, Martins AS, Kotnik M, Dessen A. PBP active site flexibility as the key mechanism for β-lactam resistance in pneumococci. J Mol Biol. 2009;387:899–909. doi: 10.1016/j.jmb.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Dorado I, Galan-Bartual S, Hermoso JA. Pneumococcal surface proteins: when the whole is greater than the sum of its parts. Mol Oral Microbiol. 2012;27:221–245. doi: 10.1111/j.2041-1014.2012.00655.x. [DOI] [PubMed] [Google Scholar]
- 35.Frolet C, Beniazza M, Roux L, Gallet B, Noirclerc-Savoye M, et al. New adhesin functions of surface-exposed pneumococcal proteins. BMC Microbiol. 2010;10:190. doi: 10.1186/1471-2180-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selva L, Ciruela P, Blanchette K, del Amo E, Pallares R, et al. Prevalence and clonal distribution of pcpA, psrP and Pilus-1 among pediatric isolates of Streptococcus pneumoniae. PLoS One. 2012;7:e41587. doi: 10.1371/journal.pone.0041587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ip M, Ang I, Liyanapathirana V, Ma H, Lai R. Genetic analyses of penicillin binding protein determinants in multidrug-resistant Streptococcus pneumoniae serogroup 19 CC320/271 clone with high-level resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 2015;59:4040–4045. doi: 10.1128/AAC.00094-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carapito R, Chesnel L, Vernet T, Zapun A. Pneumococcal β-lactam resistance due to a conformational change in penicillin-binding protein 2x. J Biol Chem. 2006;281:1771–1777. doi: 10.1074/jbc.M511506200. [DOI] [PubMed] [Google Scholar]
- 39.Izdebski R, Rutschmann J, Fiett J, Sadowy E, Gniadkowski M, et al. Highly variable penicillin resistance determinants PBP 2x, PBP 2b, and PBP 1a in isolates of two Streptococcus pneumoniae clonal groups, Poland 23F-16 and Poland 6B-20. Antimicrob Agents Chemother. 2008;52:1021–1027. doi: 10.1128/AAC.01082-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, et al. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis. 2005;192:1988–1995. doi: 10.1086/498043. [DOI] [PubMed] [Google Scholar]
- 41.Del Grosso M, Camilli R, D'Ambrosio F, Petrucci G, Melchiorre S, et al. Increase of pneumococcal serotype 19A in Italy is due to expansion of the piliated clone ST416/CC199. J Med Microbiol. 2013;62:1220–1225. doi: 10.1099/jmm.0.061242-0. [DOI] [PubMed] [Google Scholar]
- 42.van der Linden M, Reinert RR, Kern WV, Imöhl M. Epidemiology of serotype 19A isolates from invasive pneumococcal disease in German children. BMC Infect Dis. 2013;13:70. doi: 10.1186/1471-2334-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev. 2001;65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briles DE, Hollingshead S, Brooks-Walter A, Nabors GS, Ferguson L, et al. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine. 2000;18:1707–1711. doi: 10.1016/S0264-410X(99)00511-3. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed MS, Derbyshire S, Flanagan B, Loh C, Mccormick M, et al. Immune responses to pneumococcal pilus RrgA and RrgB antigens and their relationship with pneumococcal carriage in humans. J Infect. 2014;68:562–571. doi: 10.1016/j.jinf.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Argondizzo APC, Rocha-de-Souza CM, De Almeida Santiago M, Galler R, Reis JN, et al. Pneumococcal predictive proteins selected by microbial genomic approach are serotype cross-reactive and bind to host extracellular matrix proteins. Appl Biochem Biotechnol. 2017;182:1518–1539. doi: 10.1007/s12010-017-2415-6. [DOI] [PubMed] [Google Scholar]
- 47.Overweg K, Pericone CD, Verhoef GG, Weiser JN, Meiring HD, et al. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect Immun. 2000;68:4604–4610. doi: 10.1128/IAI.68.8.4604-4610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjöström K, Blomberg C, Fernebro J, Dagerhamn J, Morfeldt E, et al. Clonal success of piliated penicillin nonsusceptible pneumococci. Proc Natl Acad Sci USA. 2007;104:12907–12912. doi: 10.1073/pnas.0705589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moschioni M, de Angelis G, Melchiorre S, Masignani V, Leibovitz E, et al. Prevalence of pilus-encoding islets among acute otitis media Streptococcus pneumoniae isolates from Israel. Clin Microbiol Infect. 2010;16:1501–1504. doi: 10.1111/j.1469-0691.2010.03105.x. [DOI] [PubMed] [Google Scholar]
- 50.Sadowy E, Kuch A, Gniadkowski M, Hryniewicz W. Expansion and evolution of the Streptococcus pneumoniae Spain9V-ST156 clonal complex in Poland. Antimicrob Agents Chemother. 2010;54:1720–1727. doi: 10.1128/AAC.01340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aguiar SI, Serrano I, Pinto FR, Melo-Cristino J, Ramirez M. The presence of the pilus locus is a clonal property among pneumococcal invasive isolates. BMC Microbiol. 2008;8:41. doi: 10.1186/1471-2180-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shivshankar P, Sanchez C, Rose LF, Orihuela CJ. The Streptococcus pneumoniae adhesin PsrP binds to Keratin 10 on lung cells. Mol Microbiol. 2009;73:663–679. doi: 10.1111/j.1365-2958.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muñoz-Almagro C, Selva L, Sanchez CJ, Esteva C, de Sevilla MF, et al. PsrP, a protective pneumococcal antigen, is highly prevalent in children with pneumonia and is strongly associated with clonal type. Clin Vaccine Immunol. 2010;17:1672–1678. doi: 10.1128/CVI.00271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozakova J, Sebestova H, Krizova P. Invasive pneumococcal disease in the Czech Republic in 2015. Bull Centre Epid Microbiol. 2016;25:100–107. [Google Scholar]
- 55.Enright MC, Spratt BG. Extensive variation in the ddl gene of penicillin-resistant Streptococcus pneumoniae results from a hitchhiking effect driven by the penicillin-binding protein 2b gene. Mol Biol Evol. 1999;16:1687–1695. doi: 10.1093/oxfordjournals.molbev.a026082. [DOI] [PubMed] [Google Scholar]
- 56.Grebe T, Hakenbeck R. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of beta-lactam antibiotics. Antimicrob Agents Chemother. 1996;40:829–834. doi: 10.1128/aac.40.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichmann P, König A, Marton A, Hakenbeck R. Penicillin-binding proteins as resistance determinants in clinical isolates of Streptococcus pneumoniae. Microb Drug Resist. 1996;2:177–181. doi: 10.1089/mdr.1996.2.177. [DOI] [PubMed] [Google Scholar]
- 58.Hakenbeck R, König A, Kern I, van der Linden M, Keck W, et al. Acquisition of five high-Mr penicillin-binding protein variants during transfer of high-level β-lactam resistance from Streptococcus mitis to Streptococcus pneumoniae. J Bacteriol. 1998;180:1831–1840. doi: 10.1128/jb.180.7.1831-1840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dowson CG, Coffey TJ, Kell C, Whiley RA. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol Microbiol. 1993;9:635–643. doi: 10.1111/j.1365-2958.1993.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 60.Sauerbier J, Maurer P, Rieger M, Hakenbeck R. Streptococcus pneumoniae R6 interspecies transformation: genetic analysis of penicillin resistance determinants and genome-wide recombination events. Mol Microbiol. 2012;86:692–706. doi: 10.1111/mmi.12009. [DOI] [PubMed] [Google Scholar]
- 61.Ramalingam J, Vennila J, Subbiah P. Computational studies on the resistance of penicillin-binding protein 2B (PBP2B) of wild-type and mutant strains of Streptococcus pneumoniae against β-lactam antibiotics. Chem Biol Drug Des. 2013;82:275–289. doi: 10.1111/j.1747-0285.2012.01387.x. [DOI] [PubMed] [Google Scholar]
- 62.Smith AM, Klugman KP. Amino acid mutations essential to production of an altered PBP 2X conferring high-level β-lactam resistance in a clinical isolate of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2005;49:4622–4627. doi: 10.1128/AAC.49.11.4622-4627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]