Abstract
Protein S-sulfhydration is a newly discovered post-translational modification of specific cysteine residue(s) in target proteins, which is involved in a broad range of cellular functions and metabolic pathways. By changing local conformation and the final activity of target proteins, S-sulfhydration is believed to mediate most cellular responses initiated by H2S, a novel gasotransmitter. In comparison to protein S-sulfhydration, nitric oxide-mediated protein S-nitrosylation has been extensively investigated, including its formation, regulation, transfer and metabolism. Although the investigation on the regulatory mechanisms associated with protein S-sulfhydration is still in its infancy, accumulated evidence suggested that protein S-sulfhydration may share similar chemical features with protein S-nitrosylation. Glutathione persulfide acts as a major donor for protein S-sulfhydration. Here, we review the present knowledge on protein S-sulfhydration, and also predict its formation and regulation mechanisms based on the knowledge from protein S-nitrosylation.
Keywords: hydrogen sulfide, nitric oxide, cysteine, S-sulfhydration, S-nitrosylation
1. Introduction
Over the past decades, the modification of cysteine residues by S-nitrosylation has been extensively studied. Similar to phosphorylation, S-nitrosylation is a ubiquitous post-translational modification by selective addition of nitric oxide (NO) moiety to specific cysteine residue(s) in target proteins forming S-nitrosothiol (SNO). So far, many techniques have been developed to identify the nitrosylated cysteine residues. A modified biotin switch assay (BSA) was first utilized to detect S-nitrosylated cysteine through the replacement of labile NO on protein cysteine residues with a stable biotin moiety [1]. In addition, the application of other methods such as tandem mass spectrometry (MS/MS) [1,2] and SNOSID (SNO Site Identification) [3] have been developed to detect and analyze the cysteine residues of S-nitrosylation on proteins. With these experimental approaches, many studies have been trying to explore the chemical features and regulatory mechanisms of cysteine S-nitrosylation [4,5,6,7,8]. The acid–base motif, in particular, offers a potentially promising concept for the specificity of cysteine involved in S-nitrosylation formation [7,8,9]. It also has been demonstrated that S-nitrosylated cysteine may often be flanked by hydrophobic amino acids, showing high surface exposure, high reactivity, and low pKa [8,9].
S-sulfhydration, or S-persulfidation, is a newly discovered protein post-translational modification by yielding a hydropersulfide moiety (–SSH) or polysulfide in the active cysteine residues, which mediates most of the cellular functions induced by hydrogen sulfide (H2S), a novel member in the gasotransmitter family together with NO and carbon monoxide [10,11,12,13,14]. Since the first finding of S-sulfhydration on proteins was described in 2009 [10], many proteins have been reported to be S-sulfhydrated and involved in the physiological and pathological functions of H2S. H2S acts as an endothelium-deriving relaxing factor (EDRF) through S-sulfhydration of potassium channel proteins [15]. S-sulfhydration of Keap1 provides protection against cellular senescence via the regulation of Nrf2 activity [16]. Recently, it was found that S-sulfhydration of MEK1 is associated with repairing damaged DNA inside the cell [17]. eNOS S-sulfhydration regulates eNOS activity through the regulation of eNOS dimerization [18]. In addition, abnormal protein S-sulfhydration has been found to be involved in multiple sclerosis [19], antioxidants [20], neuroprotection [21], and endoplasmic reticulum stress response [22] by altering enzymatic activity, protein localization, protein-protein interactions, and protein stability, etc. Aside from protein modification by S-sulfhydration, H2S redox interaction of heme proteins is another important pathway in sulfide biochemistry [23], which will not be discussed in this review.
In comparison to protein S-sulfhydration, protein S-nitrosylation has been extensively studied, including its formation, regulation, transfer and metabolism [9,24]. The investigation of the regulatory mechanisms associated with protein S-sulfhydration is still in its infancy, little is known about the chemical features and biochemical stability of protein S-sulfhydration. Protein S-sulfhydration and S-nitrosylation share many similarities in terms of their chemical and biological features [11,12,13,25]. In this review, we summarize the present knowledge on protein S-sulfhydration, and also predict its formation and regulation mechanism based on the concepts of protein S-nitrosylation.
2. Potential Forming Mechanisms of Protein S-Sulfhydration
2.1. Protein S-Sulfhydration Detection
It was predicted that one-third of proteins could be modified forming S-sulfhydration, suggesting that S-sulfhydration is a highly prevalent protein post-translational modification [10,11]. S-sulfhydration usually increases the reactivity of target proteins, whereas S-nitrosylation often decreases protein activity [14,25,26]. In terms of its instability and higher nucleophilic characteristic, the detection of cysteine S-sulfhydration is quite challenging. Until now, several methods have been developed to detect S-sulfhydration. Based on the detection method from S-nitrosylation, a modified biotin switch assay was first utilized for detection of S-sulfhydration [10]. In this method, methyl methanethiosulfonate (MMTS) was used to block unmodified cysteine residues, then persulfide group(s) were labeled with biotin. Through purification of biotinylated proteins with streptavidin conjugates and Western blotting with a specific antibody, protein S-sulfhydration can be determined. Later studies found that MMTS can also interact with the persulfide group, which would lead to a reduced signal of protein S-sulfhydration [27,28]. A second method was then established by using fluorescent thiol modifying reagent Cy5-conjugated maleimide to selectively label both modified persulfide and the unmodified free thiol group [29]. Dithiothreitol (DTT) incubation reduces only persulfide but not unmodified cysteines, resulting in a lower intensity of fluorescent signal, which can be quantified to analyze the level of protein S-sulfhydration. A weakness of this method is that maleimide assay would not distinguish S-sulfhydration from other ways of cysteine modification, such as S-nitrosylation and S-glutathionylation [12]. Different with biotin switch assay, a new tag switch assay uses methylsulfonylbenzothiazole (MSBT), an aromatic thiol-blocking reagent, to block both free thiols and persulfides [27]. Afterwards, a nucleophilic tag-switch reagent (cyanoacetic acid nucleophile) was added for only labeling the persulfide groups, which are then enriched using streptavidin conjugates and analyzed by Western blot. A recent improved tag switch assay was reported to select two new cyanoacetic acid derivatives with the fluorescent moiety to increase the sensitivity of detection [28]. Although the selectivity of protein S-sulfhydration detection is higher, with a tag switch assay it is difficult to detect the cross-reactivity of persulfide with other cysteine post-translational modification [28,29]. To detect polysulfide in target protein, two novel and highly specific methods were established, named as polyethylene glycol-conjugated maleimide-labeling gel shift assay (PMSA) and protein persulfide detection protocol (ProPerDp) [30,31], respectively.
2.2. Acid–Base Motif in Protein S-Sulfhydration
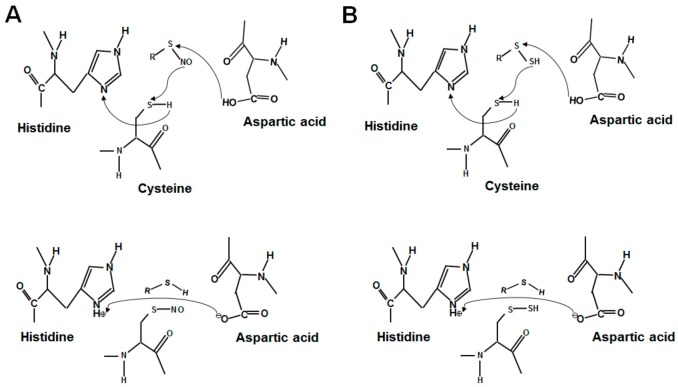
Cysteine plays a number of important roles in regulating cellular functions through its thiol functional group. The number and location of cysteine residues in different proteins is varying. It is a big challenge to determine the specificity of cysteine in target protein for S-sulfhydration [29]. Based on the analysis of NO transfer in many proteins, the acid-base motif was suggested as a site for forming protein S-nitrosylation [7,8,9,32]. Electrostatic interactions of particular cysteine with nearby acid-base amino acids may alter thiol reactivity and confer structural instability. In the acid-base motif, donor molecules are facilitated to form protein S-nitrosylation (Figure 1A). The proposed donor molecules for protein S-nitrosylation are nitrosothiols (RSNO). Nitrosoglutathione (GSNO) is a well-known endogenous RSNO catalyzed by GSNO reductase (GSNOR), and GSNOR/GSNO system is critically involved in NO signaling by maintaining a pool of NO inside the cell [33,34]. Aside from GSNO, nitrosocysteine (CSNO) has also been shown to act as a bioavailable source of NO and to contribute to protein S-nitrosylation [35,36,37]. In addition, Ascenzi et al. proposed that formation of protein S-nitrosylation is dependent on the 3D structure of target S-nitrosylated cysteine residues but not on the linear sequence of amino acids in the protein (Figure 1A) [7].
Figure 1.
The proposed forming mechanisms of protein S-nitrosylation and S-sulfhydration through the acid–base motif. (A) The proposed mechanism of S-nitrosylation in the acid–base motif. (B) The proposed mechanism for S-sulfhydration. The regulatory mechanism for S-nitrosylation and S-sulfhydration is assisted by neighboring acid (histidine) and base (aspartic acid) amino acids.
As of today, the mechanism by which H2S targets specific protein thiols for S-sulfhydration remains unknown in comparison to protein S-nitrosylation. Direct reaction of H2S and free thiol is impossible in consideration of the thermodynamic constrains [25]. Polysulfides recently emerged as potential mediators of H2S signaling [38]. A direct correlation between glutathione persulfide (GSSH) and protein S-sulfhydration has been suggested [28,39,40]. Similar to GSNOR in the regulation of GSNO in the cells, a mitochondrial persulfide dioxygenase enzyme, ETHE1, mediates the generation of GSSH [39,41]. Ida et al. recently confirmed that that GSSH could be an intermediate of the mitochondrial H2S oxidation pathway [40]. The cysteine residues with low pKa exist as thiolate anions under normal conditions, are more easily attacked by various oxidants and are susceptible to S-sulfhydration [25]. Therefore, the acid-base motif might provide a potential explanation for the forming mechanism of protein S-sulfhydration, with GSSH acting as a donor of H2S for protein S-sulfhydration (Figure 1B). Furthermore, the highly efficient formation for protein S-sulfhydration mediated by H2S in vitro has been shown to occur through the addition of sulfane-sulfur from a small molecule of polysulfide, such as GSSH, rather than from SH− as the primary thiol adduct [42]. Sulfane-sulfur is a good target for nucleophilic attack [43]. It is predicted that GSSH or CysSSH have higher nucleophilicity than parental GSH or cysteine. These reactive species improve oxidative stress by scavenging reactive oxygen species (ROS) and electrophiles, etc. [44]. Protein S-sulfhydration or polysulfidation somehow protect protein thiol residues from oxidants and electrophiles-induced damage [44]. It could be further implied that persulfide molecules may be involved in the regulatory mechanism of protein S-sulfhydration through the acid–base motif within spatial proximity to thiol groups [7,32]. The mediation of ATP level, pH value, oxygen level, and surrounding ionic strength may also be involved in the formation and regulation of cysteine S-sulfhydration, which needs to be tested further [26,43]. In addition, with H2S it may be difficult to directly reduce the disulfide-bond inside protein forming S-sulfhydration, since the reaction of disulfides with sulfide is a highly system-specific process from both thermodynamic and kinetic aspects [45].
2.3. Transsulfhydration via Protein–Protein Interaction
Thioredoxin (Trx) is one of the main disulfide reductase systems inside the cells together with thioredoxin reductase and NADPH, and has a wide variety of biological actions [46]. The regulation of protein S-nitrosylation by Trx1 has been reported [47,48,49,50]. It was suggested that Trx1 acts as a denitrosylase and/or transnitrosylase depending on the redox status of different cysteine residues in Trx1 (Table 1). Trx1 mediates denitrosylation of caspase-3, and the denitrosylation activity of Trx1 is dependent on the cysteine 32 and 35 in Trx1 [51]. S-nitrosylation of NF-κB inhibits its activity and Trx1 increases cytokine-induced NF-κB activation through the denitrosylation [46]. Differently, the transnitrosylation activity of Trx1 relies on cysteine 69 and 73 in Trx1. Trx1 itself is basically S-nitrosylated and S-nitrosylation of Trx1 transnitrosylates proteins, such as caspase-3 and apoptosis signal-regulating kinase 1 (ASK1) [52,53]. In this case, cysteine 73 of Trx1 plays an important role for the transnitrosylation of target proteins via direct interaction with Trx1 [51]. Along with Trx1, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) also possesses the activity of transnitrosylation. Many nuclear proteins have been shown to be targeted by GAPDH for transnitrosylation, including the deacetylating enzyme sirtuin-1 (SIRT1), histone deacetylase-2 (HDAC2), and DNA-activated protein kinase (DNA-PK), etc. [54]. Furthermore, transnitrosylation of haemoglobin to the anion exchanger AE1 in the plasma membrane of red blood cells has also been reported [55].
Table 1.
The proteins for transnitrosylation through protein-protein interaction.
| Regulatory Protein | Target Protein | Reference |
|---|---|---|
| Trx1 | Alpha enolase | [51] |
| Heat shock cognate 71 kDa protein | [51] | |
| Peroxiredoxin-1 | [51] | |
| Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) | Deacetylating enzyme sirtuin-1 | [54] |
| Histone deacetylase-2 | [54] | |
| DNA-activated protein kinase | [54] | |
| B23/nucleophosmin | [56] | |
| Haemoglobin | Anion exchanger AE1 | [55] |
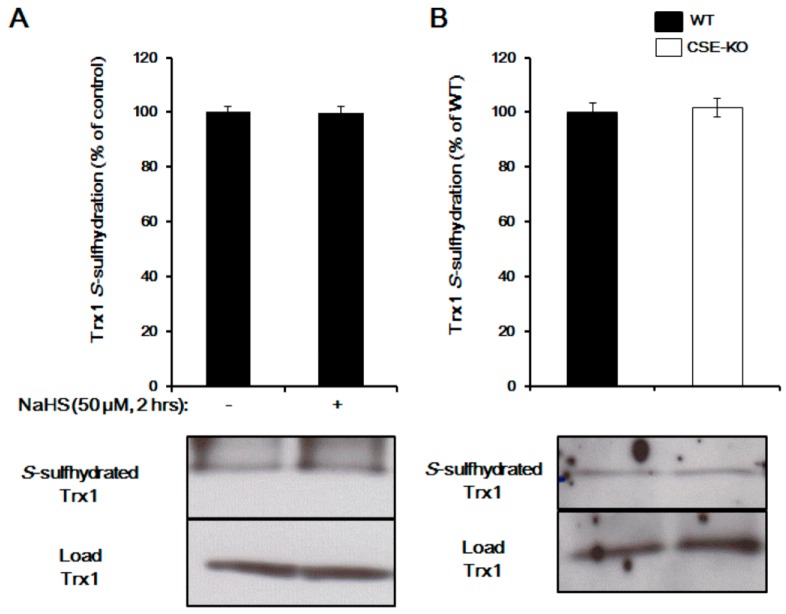
Trx1 regulation of protein S-sulfhydration has also been reported [28,46,57]. By interacting with the two redox active cysteine residues at its active site, Trx1 has the ability to bind with 3-mercaptopyruvate sulfurtransferase (3MST) to generate H2S, pointing to the possibility of Trx1 to break down the double-sulfide bond [46]. A later study proved that Trx1 reduced H2S-stimulated PTP1B S-sulfhydration [22]. Recombinant Trx1 showed a very high reactivity in cleaving protein persulfides and releasing H2S, while inhibition of the Trx1 system caused an increase in intracellular persulfides [28]. We recently provided evidence that Trx1 desulfhydrates pyruvate carboxylase and GAPDH, suggesting that Trx1 indeed acts as a S-desulfhydrase and controls H2S signaling [57]. Trx1 attenuates cysteine S-sulfhydration by direct interaction with S-sulfhydrated proteins at the Trp–Cys32–Gly–Pro–Cys35 motif. Deficiency of TrxR1 in mouse livers markedly elevated persulfide level, further indicating the distinct roles of the Trx systems in regulating protein S-sulfhydration or persulfide [31]. So far, there is no report about protein transsulfhydration activity of Trx1. We found that Trx1 is basically S-sulfhydrated, while Trx1 S-sulfhydration is not altered by exogenously applied NaHS or knockout of cystathionine gamma-lyase (CSE, a H2S-generating gene) (Figure 2), suggesting that Trx1 is not involved in protein transsulfhydration. Future studies need to be performed to find the proteins/enzymes/factors involved in protein transsulfhydration, which would help to understand the biological effect of H2S in both health and disease.
Figure 2.
Trx1 is not a target for S-sulfhydration. (A) Trx1 S-sulfhdyration was measured in NaHS-treated HepG2 cells (50 µM for 2 h) by biotin switch assay. n = 4. (B) Trx1 S-sulfhydration was determined in liver tissues from both wild-type and CSE knockout mice. n = 4. CSE, cystathionine gamma-lyase; KO, knockout; Trx1, thioredoxin 1; WT, wild-type.
2.4. Protein S-Sulfhydration from Oxidized Cysteine (S–OH)
The direct reaction of cysteine and H2S is questioned. Thiols can be easily oxidized, and the presence of reactive oxygen species (ROS) inside the cells can react with the free thiols forming sulfenic (SOH) and sulfinic (SO2H) acids. It is predicted that H2S may first interact with oxidized cysteine in target protein resulting in the reduction of –SOH (S-sulfenylation) to –SSH (S-sulfhydration) [39] (Figure 3). S-sulfenylation, sulfenic acid modifications on cysteine residues in proteins, is reversible [58]. The propensity of cysteine residues to undergo oxidation is influenced mainly by three general factors: thiol nucleophilicity, the surrounding protein microenvironment, and proximity of the target thiol to ROS source [59]. Much evidence has demonstrated that the interaction of target protein with different ROS sources alters thiol status and induces spatial oxidation of cysteine residues [59,60]. The presence of H2O2 in the medium strengthened S-sulfhydration, further supporting this hypothesis [22,28]. Thus, cysteine S-sulfhydration can protect particular proteins from nucleophilic attack. Recent evidence also demonstrated that H2S-induced persulfide formation could not be the consequence of its reaction with protein disulfides, because incubation of the cells with diamide, an inducer of disulfide bond formation, attenuates H2S-induced S-sulfhydration [28]. Furthermore, the presence of different levels of ROS may alter the regulatory role of sulfane sulfur, such as thiosulfate, persulfides, thiosulfonate, polysulfides, polythionates, and elemental sulfur, on protein S-sulfhydration [61]. Further study of the regulatory mechanism will be helpful for understanding the susceptibility of protein thiol modification by S-sulfhydration.
Figure 3.
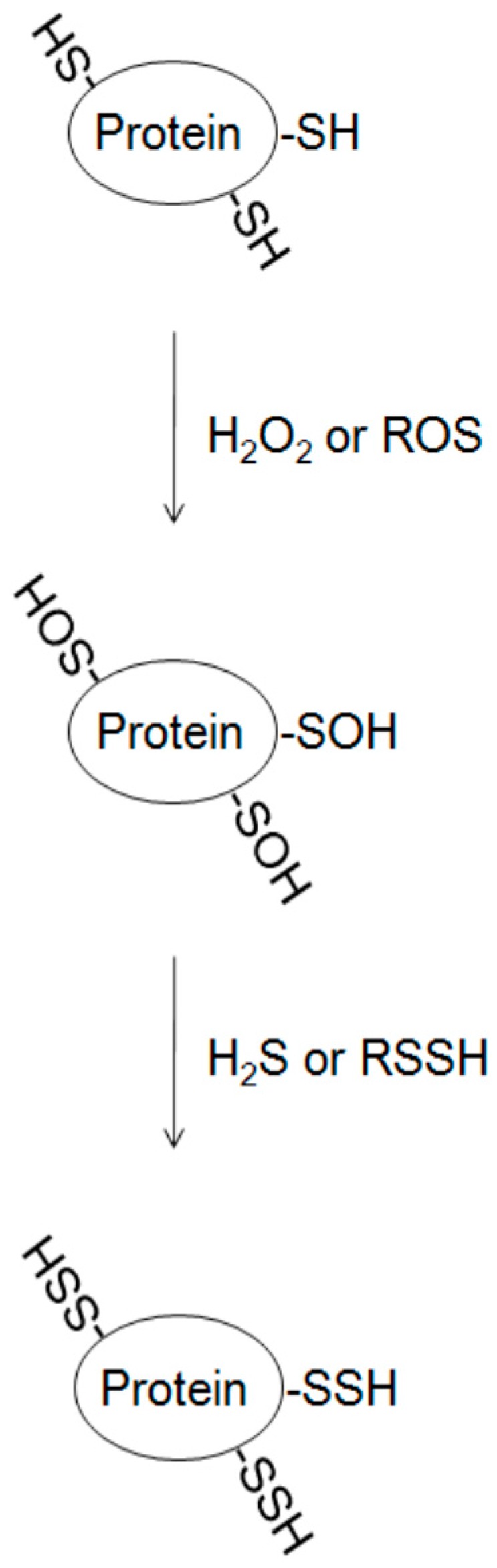
Potential mechanism of protein S-sulfhydration from oxidized cysteine in proteins. H2O2, hydrogen peroxide; H2S, hydrogen sulfide; ROS, reactive oxygen species; RSSH, hydropersulfides.
2.5. Interaction of H2S and NO on Protein Modification
Both NO and H2S are important gasotransmitters and regulate diverse physiological functions through interaction [62,63,64]. H2S influences NO production and its metabolites by affecting NO synthase, and NO is also shown to alter H2S bioavailability by acting on H2S-generating enzymes. The same cysteine residue(s) in the target protein can be either S-nitrosylated or S-sulfhydrated [18]. More directly, H2S has been identified to intertwine with NO or its metabolites, forming various new compounds, such as thionitrous acid (HSNO), sulfinyl nitrite (HSNO2), or nitrosopersulfide (SSNO−) [64,65,66], depending on the concentration of H2S/NO and reaction conditions. The bioactivity of either NO or H2S is governed by concomitant formation of polysulfides and anionic S/N-hybrid species, which would subsequently attack protein for further modification [38].
3. The Donor Molecules for Protein S-Nitrosylation and S-Sulfhydration
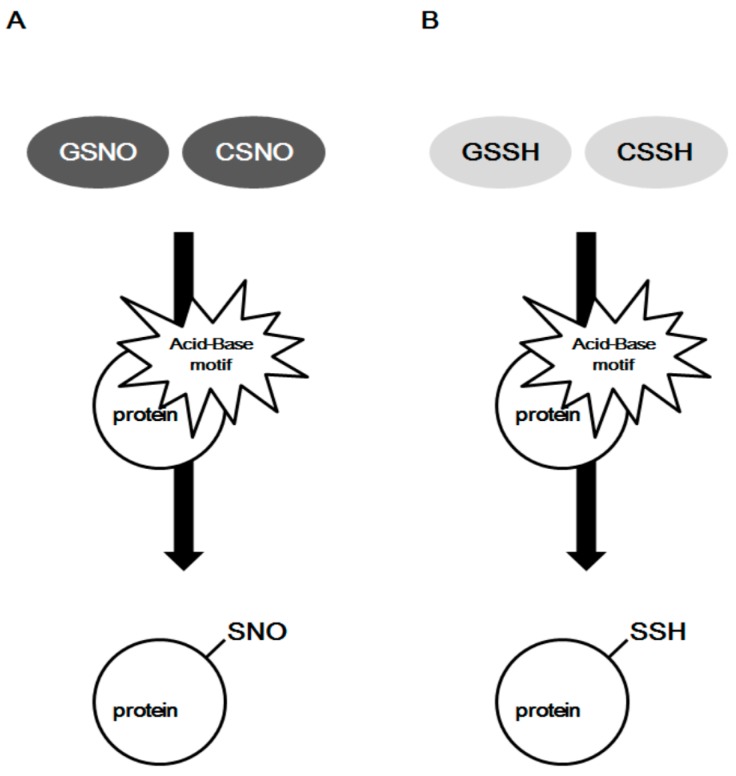
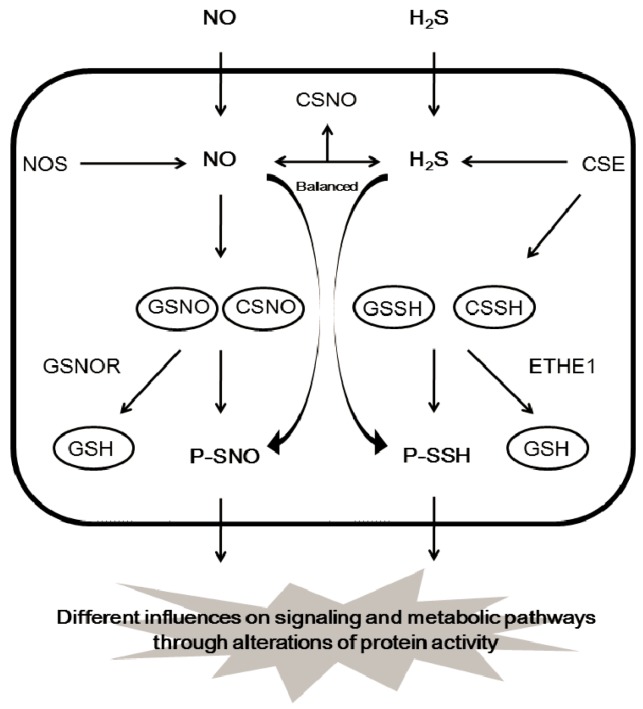
The direct reaction of NO with thiols forming S-nitrosylation is unlikely, and the formation of an SNO is actually aided with higher oxides of NO-containing molecules, such as dinitrogen trioxide (N2O3), S-nitrosothiols, CSNO, and/or GSNO [67,68,69,70]. GSNO and CSNO are often seen to induce protein S-nitrosylation, and both can transfer their NO moiety to protein cysteine residues via transnitrosylation [33,34] (Figure 4A). It is not clear whether protein S-sulfhydration is an enzyme-catalyzed reaction or an automatic redox reaction. Nevertheless, many intermediates must be involved in the formation of protein S-sulfhydration. Persulfide RSSH including GSSH and cysteine persulfide (CSSH) would be the highly potential intermediate for the forming of protein S-sulfhydration due to their high electrophilic features [25,28,39] (Figure 4B). The concentration of GSSH and CSSH inside the cells is reported to be in the high micro molar range, which is positively correlated with H2S level in different tissues, such as brain, kidney, and liver. Under certain conditions, persulfides can release H2S following reduction by another species, including another persulfide, indicating that persulfides may facilitate sulfide storage and transport [71,72,73]. H2S may act as a marker for persulfides and polysulfides [61]. Endogenous GSSH is regulated by H2S-producing enzymes (CSE) or cystathionine beta synthase (CBS), since overexpression of CSE or CBS induces GSSH level and stimulates the formation of protein S-sulfhydration [39,74]. In addition, persulfide formation by CSE- and CBS-mediated CysSSCys metabolism is very active and can act as a source of biological persulfides [28]. In contrast, ETHE1 is reported to metabolize GSSH to GSH with simultaneous oxygen consumption [30]. It is not surprising that the level of S-sulfhydrated proteins, such as GAPDH, pyruvate carboxylase, and eNOS, depends on the expression and activity of H2S-generating enzymes within the cells [10,18,43]. The tissue or cell-specific protein S-sulfhydration may also exist due to the different level of enzymic activity of H2S-generating proteins. It can be predicted that, by mediating the generation of CSSH or GSSH, the persulfide trafficking among different proteins via transsulfhydration can occur (Figure 5).
Figure 4.
The forming mechanisms of protein S-nitrosylation and S-sulfhydration by physiological relevant donors. CSNO, nitrosocystiene; CSSH, cysteine persulfide; GSNO, nitrosoglutathione; GSSH, glutathione persulfide.
Figure 5.
The pool of donors for protein S-nitrosylation and S-sulfhydration. ETHE1, mitochondrial persulfidedioxygenase; GSNOR, GSNO reductase.
4. Prospection
Given the importance of protein S-sulfhydration in diverse cellular functions and pathophysiological responses, the regulatory mechanism of protein S-sulfhydration needs to be clarified. The interaction or competition between cysteine S-sulfhydration and S-nitrosylation in the same protein needs to be determined. Due to the instability and transition, development and improvement in protein S-sulfhydration detection technology and methodology is urgent for a better understanding of its formation and wide biological implications. By targeting protein S-sulfhydration, new drugs or solutions can be quickly developed for preventing and treating a wide range of diseases.
Acknowledgments
This study was supported by Heart and Stroke Foundation of Ontario-Mid-Career Investigator Award to L.W. and a discovery grant from the Natural Sciences and Engineering Research Council of Canada to G.Y.
Abbreviations
3D, 3-dimensional; 3MST, 3-mercaptopyruvate sulfurtransferase; ASK1, apoptosis signal-regulating kinase 1; BSA, biotin switch assay; CBS, cystathionine beta-synthase; CSE, cystathionine gamma-lyase; CSNO, nitrosocystiene; CSSH, cysteine persulfide; DNA-PK, DNA activated protein kinase; DTT, dithiothreitol; EDRF, endothelial-deriving relaxing factor; ETHE1, mitochondrial persulfidedioxygenase; GSNO, nitrosoglutathione; GSNOR, GSNO reductase; GSSH, glutathione persulfide; H2O2, hydrogen peroxide; H2S, hydrogen sulfide; HDAC2, histone deacetylase 2; KO, knockout; MMTS, methyl methanethiosulfonate; MSBT, methylsulfonylbenzothiazole; NF-kB, nuclear factor kappa b; N2O3, dinitrogen trioxide; NO, nitric oxide; NO+, nitrosoniumcation; PTM, post translational modification; ROS, reactive oxygen species; RSNO, nitrosothiol; RSSH, hydropersulfides; SIRT1, deacetylating enzyme sirtuin 1; SNO, S-nitrosothiol; SNOSID, SNO site identification; SOH, sulfenic; SO2H, sulfinic; Trx1, thioredoxin 1; WT, wild-type.
Supplementary Materials
Author Contributions
Y.J. and G.Y. wrote and revised the manuscript. M.F., E.S. and L.W. contributed to the idea and revised the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests regarding the publication of this paper.
References
- 1.Jaffrey S.R., Snyder S.H. The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE. 2001;86 doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 2.Jaffrey S.R., Erdjument-Bromage H., Ferris C.D., Tempst P., Snyder S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 3.Hao G., Derakhshan B., Shi L., Campagne F., Gross S.S. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc. Natl. Acad. Sci. USA. 2006;103:1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B.Q., Hu L.L., Niu S., Cai Y.D., Chou K.C. Predict and analyze S-nitrosylation modification sites with the mRMR and IFS approaches. J. Proteom. 2012;75:1654–1665. doi: 10.1016/j.jprot.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y., Ding J., Wu L.Y., Chou K.C. iSNO-PseAAC: Predict cysteine S-nitrosylation sites in proteins by incorporating position specific amino acid propensity into pseudo amino acid composition. PLoS ONE. 2013;8:e55844. doi: 10.1371/journal.pone.0055844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue Y., Liu Z., Gao X., Jin C., Wen L., Yao X., Ren J. GPS-SNO: Computational prediction of protein S-nitrosylation sites with a modified GPS algorithm. PLoS ONE. 2010;5:e11290. doi: 10.1371/journal.pone.0011290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ascenzi P., Colasanti M., Persichini T., Muolo M., Polticelli F., Venturini G., Bordo D., Bolognesi M. Re-evaluation of amino acid sequence and structural consensus rules for cysteine-nitric oxide reactivity. Biol. Chem. 2000;381:623–627. doi: 10.1515/BC.2000.081. [DOI] [PubMed] [Google Scholar]
- 8.Marino S.M., Gladyshev V.N. Structural analysis of cysteine S-nitrosylation: A modified acid-based motif and the emerging role of trans-nitrosylation. J. Mol. Biol. 2010;395:844–859. doi: 10.1016/j.jmb.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamler J.S., Toone E.J., Lipton S.A., Sucher N.J. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/S0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 10.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul B.D., Snyder S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 12.Paul B.D., Snyder S.H. Protein sulfhydration. Methods Enzymol. 2015;555:79–90. doi: 10.1016/bs.mie.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Paul B.D., Snyder S.H. H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015;40:687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang G. Protein S-sulfhydration as a major source of H2S bioactivity. Recept. Clin. Investig. 2014;1:e337. [Google Scholar]
- 15.Mustafa A.K., Sikka G., Gazi S.K., Steppan J., Jung S.M., Bhunia A.K., Barodka V.M., Gazi F.K., Barrow R.K., Wang R., et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., Khaper N., Wu L., Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 17.Zhao K., Ju Y., Li S., Al Tanny Z., Wang R., Yang G. S-sulfhydration of MEK1 leads to PARP-1 activation and DNA damage repair. EMBO Rep. 2014;15:792–800. doi: 10.1002/embr.201338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altaany Z., Ju Y., Yang G., Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci. Signal. 2014;7:ra87. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 19.Pieragostino D., Del Boccio P., Di Ioia M., Pieroni L., Greco V., De Luca G., D’Aguanno S., Rossi C., Franciotta D., Centonze D., et al. Oxidative modifications of cerebral transthyretin are associated with multiple sclerosis. Proteomics. 2013;13:1002–1009. doi: 10.1002/pmic.201200395. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z.Z., Shi M.M., Xie L., Wu Z.Y., Li G., Hua F., Bian J.S. Sulfhydration of p66Shc at cysteine59 mediates the anti-oxidant effect of Hydrogen Sulfide. Antioxid. Redox Signal. 2014;21:2531–2542. doi: 10.1089/ars.2013.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandiver M.S., Paul B.D., Xu R., Karuppagounder S., Rao F., Snowman A.M., Ko H.S., Lee Y.I., Dawson V.L., Dawson T.M., et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan N., Fu C., Pappin D.J., Tonks N.K. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy P. Mechanistic chemical perspective of hydrogen sulfide signaling. Methods Enzymol. 2015;554:3–29. doi: 10.1016/bs.mie.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 24.Li H., Wan A., Xu G., Ye D. Small changes huge impact: The role of thioredoxin 1 in the regulation of apoptosis by S-nitrosylation. Acta Biochim. Biophys. Sin. 2013;45:153–161. doi: 10.1093/abbs/gms103. [DOI] [PubMed] [Google Scholar]
- 25.Filipovic M.R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol. 2015;230:29–59. doi: 10.1007/978-3-319-18144-8_2. [DOI] [PubMed] [Google Scholar]
- 26.Ju Y., Fu M., Wu L., Yang G. Strategies and tools for detection of protein S-nitrosylation and S-sulfhydration. Biochem. Anal. Biochem. 2015;4:224. [Google Scholar]
- 27.Zhang D., Macinkovic I., Devarie-Baez N.O., Pan J., Park C.M., Carroll K.S., Filipovic M.R., Xian M. Detection of protein S-sulfhydration by a tag-switch technique. Angew. Chem. Int. Ed. Engl. 2014;53:575–581. doi: 10.1002/anie.201305876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedmann R., Onderka C., Wei S., Szijártó I.A., Miljkovic J.L., Mitrovic A., Lange M., Savitsky S., Yadav P.K., Torregrossa R., et al. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem. Sci. 2016;7:3414–3426. doi: 10.1039/C5SC04818D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sen N. Functional and molecular insights of hydrogen sulfide signaling and protein sulfhydration. J. Mol. Biol. 2017;429:543–561. doi: 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung M., Kasamatsu S., Matsunaga T., Akashi S., Ono K., Nishimura A., Morita M., Abdul H.H., Fujii S., Kitamura H., et al. Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem. Biophys. Res. Commun. 2016;480:180–186. doi: 10.1016/j.bbrc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Dóka É., Pader I., Bíró A., Johansson K., Cheng Q., Ballagó K., Prigge J.R., Pastor-Flores D., Dick T.P., Schmidt E.E., et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016;2:e1500968. doi: 10.1126/sciadv.1500968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Irie Y., Keung W.M., Maret W. S-nitrosothiols react preferentially with zinc thiolate clusters of metallothionein III through transnitrosation. Biochemistry. 2002;41:8360–8367. doi: 10.1021/bi020030+. [DOI] [PubMed] [Google Scholar]
- 33.Benhar M., Forrester M.T., Stamler J.S. Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 34.Foster M.W., Hess D.T., Stamler J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayed N., Kim D.D., Fioramonti X., Iwahashi T., Durán W.N., Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ. Res. 2008;103:606–614. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fioramonti X., Deak A., Deshpande S., Carneiro L., Zhou C., Sayed N., Orban B., Berlin J.R., Pénicaud L., Leloup C., et al. Hypothalamic S-nitrosylation contributes to the counter-regulatory response impairment following recurrent hypoglycemia. PLoS ONE. 2013;8:e68709. doi: 10.1371/journal.pone.0068709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu C.X., Li S., Whorton A.R. Redox regulation of PTEN by S-nitrosothiols. Mol. Pharmacol. 2005;68:847–854. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]
- 38.Cortese-Krott M.M., Kuhnle G.G., Dyson A., Fernandez B.O., Grman M., DuMond J.F., Barrow M.P., McLeod G., Nakagawa H., Ondrias K., et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA. 2015;112:4651–4660. doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu C., Kavalier A., Lukyanov E., Gross S.S. S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation: A common paradigm for gasotransmitter signaling by H2S and NO. Methods. 2013;62:177–181. doi: 10.1016/j.ymeth.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ida T., Sawa T., Ihara H., Tsuchiya Y., Watanabe Y., Kumagai Y., Suematsu M., Motohashi H., Fujii S., Matsunaga T., et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabil O., Banerjee R. Characterization of patient mutations in human persulfide dioxygenase (ETHE1) involved in H2S catabolism. J. Biol. Chem. 2012;287:44561–44567. doi: 10.1074/jbc.M112.407411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greiner R., Pálinkás Z., Bäsell K., Becher D., Antelmann H., Nagy P., Dick T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal. 2013;19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ju Y., Untereiner A., Wu L., Yang G. H2S-induced S-sulfhydration of pyruvate carboxylase contributes to gluconeogenesis in liver cells. Biochim. Biophys. Acta. 2015;1850:2293–2303. doi: 10.1016/j.bbagen.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Kasamatsu S., Nishimura A., Morita M., Matsunaga T., Abdul H.H., Akaike T. Redox signaling regulated by cysteine persulfide and protein polysulfidation. Molecules. 2016;21:1721. doi: 10.3390/molecules21121721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasas A., Dóka É., Fábián I., Nagy P. Kinetic and thermodynamic studies on the disulfide-bond reducing potential of hydrogen sulfide. Nitric Oxide. 2015;46:93–101. doi: 10.1016/j.niox.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Mikami Y., Shibuya N., Kimura Y., Nagahara N., Ogasawara Y., Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011;439:479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 47.Haendeler J., Hoffmann J., Tischler V., Berk B.C., Zeiher A.M., Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat. Cell Biol. 2002;4:743–749. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- 48.Kelleher Z.T., Sha Y., Foster M.W., Foster W.M., Forrester M.T., Marshall H.E. Thioredoxin-mediated denitrosylation regulates cytokine-induced nuclear factor κB (NF-κB) activation. J. Biol. Chem. 2014;289:3066–3072. doi: 10.1074/jbc.M113.503938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benhar M., Forrester M.T., Hess D.T., Stamler J.S. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu C., Parrott A.M., Liu T., Beuve A., Li H. Functional proteomics approaches for the identification of transnitrosylase and denitrosylase targets. Methods. 2013;62:151–160. doi: 10.1016/j.ymeth.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu C., Liu T., Chen W., Oka S., Fu C., Jain M.R., Parrott A.M., Baykal A.T., Sadoshima J., Li H. Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol. Cell. Proteom. 2010;9:2262–2275. doi: 10.1074/mcp.M110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumbayev V.V. S-nitrosylation of thioredoxin mediates activation of apoptosis signal-regulating kinase 1. Arch. Biochem. Biophys. 2003;415:133–136. doi: 10.1016/S0003-9861(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell D.A., Morton S.U., Fernhoff N.B., Marletta M.A. Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA. 2007;104:11609–11614. doi: 10.1073/pnas.0704898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kornberg M.D., Sen N., Hara M.R., Juluri K.R., Nguyen J.V., Snowman A.M., Law L., Hester L.D., Snyder S.H. GAPDH mediates nitrosylation of nuclear proteins. Nat. Cell Biol. 2010;12:1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pawloski J.R., Hess D.T., Stamler J.S. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 56.Lee S.B., Kim C.K., Lee K.H., Ahn J.Y. S-nitrosylation of B23/nucleophosmin by GAPDH protects cells from the SIAH1-GAPDH death cascade. J. Cell Biol. 2012;199:65–76. doi: 10.1083/jcb.201205015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ju Y., Wu L., Yang G. Thioredoxin 1 regulation of protein S-desulfhydration. Biochem. Biophys. Rep. 2016;5:27–34. doi: 10.1016/j.bbrep.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray C.I., Van Eyk J.E. Chasing cysteine oxidative modifications: proteomic tools for characterizing cysteine redox status. Circ. Cardiovasc. Genet. 2012;5:591. doi: 10.1161/CIRCGENETICS.111.961425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo Conte M., Carroll K.S. The redox biochemistry of protein sulfenylation and sulfinylation. J. Biol. Chem. 2013;288:26480–26488. doi: 10.1074/jbc.R113.467738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen K., Kirber M.T., Xiao H., Yang Y., Keaney J.F. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Álvarez L., Bianco C.L., Toscano J.P., Lin J., Akaike T., Fukuto J.M. Chemical biology of hydropersulfides and related species: Possible roles in cellular protection and redox signaling. Antioxid. Redox Signal. 2017 doi: 10.1089/ars.2017.7081. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 62.Bianco C.L., Fukuto J.M. Examining the reaction of NO and H2S and the possible cross-talk between the two signaling pathways. Proc. Natl. Acad. Sci. USA. 2015;112:10573–10574. doi: 10.1073/pnas.1513510112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan S., Kevil C.G. Nitric oxide and hydrogen sulfide regulation of ischemic vascular remodeling. Microcirculation. 2016;23:134–145. doi: 10.1111/micc.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolluru G.K., Yuan S., Shen X., Kevil C.G. H2S regulation of nitric oxide metabolism. Methods Enzymol. 2015;554:271–297. doi: 10.1016/bs.mie.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filipovic M.R., Miljkovic J.L., Nauser T., Royzen M., Klos K., Shubina T., Koppenol W.H., Lippard S.J., Ivanović-Burmazović I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cortese-Krott M.M., Fernandez B.O., Santos J.L., Mergia E., Grman M., Nagy P., Kelm M., Butler A., Feelisch M. Nitrosopersulfide (SSNO(−)) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol. 2014;2:234–244. doi: 10.1016/j.redox.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paulsen C.E., Truong T.H., Garcia F.J., Homann A., Gupta V., Leonard S.E., Carroll K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2012;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamotte O., Bertoldo J.B., Besson-Bard A., Rosnoblet C., Aimé S., Hichami S., Terenzi H., Wendehenne D. Protein S-nitrosylation: Specificity and identification strategies in plants. Front. Chem. 2014;2:114. doi: 10.3389/fchem.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 70.Hill B.G., Dranka B.P., Bailey S.M., Lancaster J.R., Darley-Usmar V.M. What part of NO don’t you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 2010;285:19699–19704. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith B.C., Marletta M.A. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr. Opin. Chem. Biol. 2012;16:498–506. doi: 10.1016/j.cbpa.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saund S.S., Sosa V., Henriquez S., Nguyen Q.N., Bianco C.L., Soeda S., Millikin R., White C., Le H., Ono K., et al. The chemical biology of hydropersulfides (RSSH): Chemical stability, reactivity and redox roles. Arch. Biochem. Biophys. 2015;15:15–24. doi: 10.1016/j.abb.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bailey T.S., Pluth M.D. Reactions of isolated persulfides provide insights into the interplay between H2S and persulfide reactivity. Free Radic. Biol. Med. 2015;89:662–667. doi: 10.1016/j.freeradbiomed.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Libiad M., Yadav P.K., Vitvitsky V., Martinov M., Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014;289:30901–30910. doi: 10.1074/jbc.M114.602664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.