Abstract
The aim of this study was to investigate acetylcholinesterase (AChE), monoamine oxidase A (MAO-A), monoamine oxidase B (MAO-B), cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) enzyme inhibitory, and antimicrobial activities of a new series of 2-(4-substituted phenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole derivatives, for their possible use as multi-action therapeutic agents. Target compounds (n = 15) were synthesized under microwave irradiation conditions in two steps, and their structures were elucidated by FT-IR, 1H-NMR, 13C-NMR and high resolution mass spectroscopic analyses. Pharmacological screening studies revealed that two of the compounds (2b and 2j) have inhibitory potential on both COX-1 and COX-2 enzymes. In addition, cytotoxic and genotoxic properties of the compounds 2b, 2j and 2m were investigated via the well-known MTT and Ames tests, which revealed that the mentioned compounds are non-cytotoxic and non-genotoxic. As a concise conclusion, two novel compounds were characterized as potential candidates for treatment of frequently encountered inflammatory diseases.
Keywords: benzimidazoles, morpholines, AChE, MAO, COX
1. Introduction
One of the promising advances in drug discovery is the simultaneous combination of two or more beneficial chemical moieties on the same compound, especially for treatment of a certain disease. Any pharmaceutical benefit that reduces the demand on polypharmacy, e.g., treatment while decreasing side effects, eliminating symptoms, or addition of an adjuvant therapeutic activity is accepted as an ideal starting-point that may lead to the development of novel compounds with multiple pharmacological effects [1]. There is a consensus that multifactorial disorders can originate from many sources, so searching for new hybrid compounds with multiple pharmacological profiles that can be expressed through more than one biochemical pathway would be useful for treating many kinds of diseases [2,3]. Despite the great challenge in the design and optimization of such compounds, this strategy possesses clear advantages over drug mixtures or multicomponent drugs owing to minimization of drug-drug interaction risks [4]. Some available drugs present the capability to modulate more than one bioreceptor, as exemplified by the atypical antipsychotics olanzapine, risperidone and aripiprazole, which act mainly as dopamine and serotonin receptor antagonists and have lower affinity to histamine, cholinergic muscarinic and α-adrenergic receptors [5]. Ladostigil is another example of dual monoamine oxidase B (MAO-B) and acetylcholine esterase (AChE) inhibitor [6] (Figure 1). Despite their great promise in the clinical application, any potential risk of side effects should not be neglected. The dual inhibitors are often characterized by high molecular weight that may reduce the chance of their drug abilities. Therefore, the safety profiles as well as pharmacokinetic properties need to be thoroughly considered in the design of dual inhibitors [7].
Figure 1.
Chemical structures of some agents with dual inhibitory effect.
Benzimidazole derivatives have a prominent position in medicinal chemistry, which are always used as one of the essential starting materials for discovery of new therapeutics. The origin of the special interest towards benzimidazole derivatives has been the 5,6-dimethyl-1-(α-d-ribofuranosyl)-benzimidazole structure, which is a basic part of vitamin B12 [8]. Furthermore, the benzimidazole ring is a structural bioisostere of some of the nucleobases existing in natural nucleotides, which can interact easily with the biopolymers in living systems [9]; this feature is often accepted as the responsible for its biological importance, either alone or as incorporated into different templates. It has been reported to show many pharmacological activities including antimicrobial, MAO or cyclooxygenase (COX) inhibitory, or anticholinesterase [10,11,12,13,14,15,16,17,18]. Besides, albendazole, mebendazole and tiabendazole are some examples of antimicrobial agents that carry the benzimidazole ring (Figure 2). Morpholine is another heterocyclic organic compound, which is also one of the principle building blocks in organic synthesis; several derivatives of morpholines have received attention in the past, due to their significant and numerous pharmacological activities such as antiinflammatory, antidepressant, MAO inhibitor, AChE inhibitor, neuroprotective, antituberculosis, antimalarial and antiparasitic [19,20,21]. Moreover, antibacterial agent linezolid, the MAO-A inhibitor moclobemid, and the antiinflamatory agent emorfazone are some examples of marketed drugs bearing morpholine moieties (Figure 2).
Figure 2.
Chemical structures of some drugs containing benzimidazole or morpholine moieties.
There are several examples in previously published papers in which the strategy described above was successfully applied by combining benzimidazole and morpholine pharmacophores on the same chemical structure; researchers have designed and synthesized many benzimidazole-morpholine compounds and investigate their pharmacological activities such as anticholinesterase [22,23], antimicrobial [24,25,26], antiinflammatory [27,28,29,30] and MAO inhibitory properties [31,32,33,34,35,36,37,38,39].
The antimicrobial and COX inhibitory potential of benzimidazole and morpholine-based compounds may be favorable in the design of dual COX inhibitory-antibacterial agents because inflammation and infection are conditions which are frequently encountered together. A decrease of fever besides elimination of an infectious microorganism or treatment of inflammation besides removal of pain are dual outcomes of such agents [40,41]. Besides, the inhibition potency of benzimidazole and morpholine derivatives against AChE and MAO enzymes may be beneficial in the design of new anti-Alzheimer’s disease agents. Inhibition of AChE increases neurotransmission in the cholinergic synapses and temporally decreases the cognitive deficit. AChE also contributes in other functions related to neuronal development, differentiation, adhesion and β-amyloid protein processing. Additionally, MAO-B inhibition retards further deterioration of cognitive functions. Thus, discovery of a dual inhibitor of these enzymes are expected to have potential for the treatment of Alzheimer’s disease [42].
It is clear that, the benzimidazole-morpholine combination has the potential to serve as a pharmaceutical source for therapy. With the aim of producing safer and more active hybrid compounds, the synthesis and pharmacological evaluation of some novel molecules that include bioactive benzimidazole and morpholine moieties have been presented in this study.
2. Results and Discussion
2.1. Chemistry
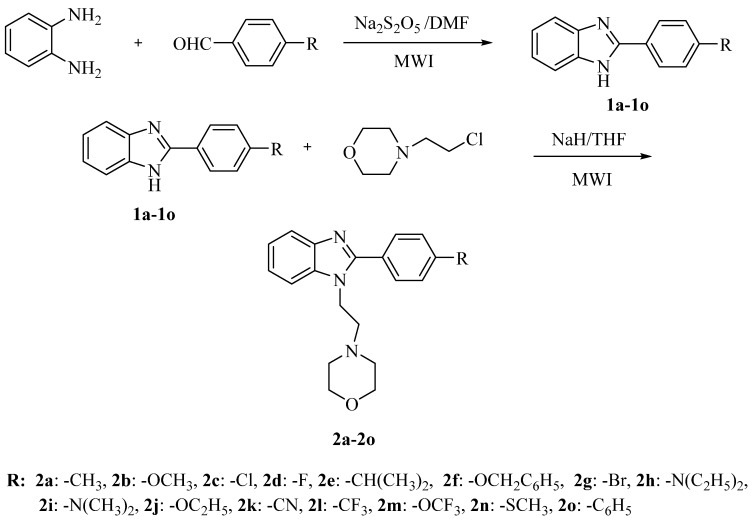
Synthesis of the target compounds 2a–2o is outlined in Scheme 1. Initially, 1,2-phenylenediamine was reacted with various sodium bisulfite adducts of aldehydes under microwave irradiation conditions to obtain the previously reported 2-(4-substitutedphenyl)-1H-benzimidazoles 1a–1o [43,44,45,46,47,48,49,50]. In the second step, compounds 1a–1o were treated with 2-(morpholin-4-yl)ethyl chloride in the presence of NaH, and subjected to microwave irradiation again. In the synthesis, various substituents that may have an impact on biological activity were selected to impart different electronic and hydrophobic natures to the target compounds.
Scheme 1.
Synthesis route to the target compounds 2a–2o.
Structure elucidations of the final compounds 2a–2o were realized by FT-IR, 1H-NMR, 13C-NMR and HRMS methods. The aromatic C-H and aliphatic C-H stretching bands were observed at 3063–3011 cm−1 and 2986–2945 cm−1, respectively in the IR spectra; C=N stretching bands were recorded at 1618–1599 cm−1. The out of plane bands, which were assigned to 1,4-disubstituted benzene, were at 868–818 cm−1. The protons at the positions 3 and 4 of morpholine structure were recorded at about 2.20 ppm as multiplet, in the 1H-NMR spectra. In addition, the protons of ethylene near the morpholine and benzimidazole were observed as a triplet at about 2.50 ppm and 4.40 ppm, respectively. The protons at the positions 1 and 2 of morpholine were assigned as multiplet at about 3.40 ppm. Aromatic protons are appeared between 7.10 ppm and 7.90 ppm, as expected.
In the 13C-NMR spectra, the signals of the ethylene spacer between the benzimidazole and morpholine units appeared between 42.03 ppm and 42.36 ppm, and the other carbons of that chain was recorded between 57.39 ppm and 57.55 ppm. Morpholine carbons had two different peaks at 53.78–53.83 ppm and 66.31–66.50 ppm. The benzimidazole carbon at the position 2 gave a peak between 157.27 ppm and 154.69 ppm, depending on substituent group. The other benzimidazole-carbon peaks were recorded between 111.01 ppm and 143.30 ppm. The chemical shifts of the substituent groups and coupling constant of the fluorinated derivatives were consistent with the theoretical values. HRMS findings were in accordance with the theoretical molecular formula of the compounds 2a–2o.
2.2. Biological Activity Screening
The antimicrobial abilities of the compounds against different human pathogens were evaluated. Escherichia coli ATCC 35218, Escherichia coli ATCC 25922, Pseudomona aeuroginosa ATCC 27853, and Klebsiella pneumoniae ATCC 700603 were used as Gram-negative bacteria. As Gram-positive bacteria Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC29212, and Listeria monocytogenes ATCC 7644 were tested. Candida albicans ATCC 90028, Candida glabrata ATCC 90030, Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 yeasts were also used in antimicrobial activity tests. Chloramphenicol and ketoconazole were used as the reference drugs. Antimicrobial activity results are given in Table 1.
Table 1.
Antimicrobial activity results of test compounds 2a–2o (MIC50 values are given in mg/mL).
| Compound | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2a | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2b | >1 | >1 | >1 | 0.0156 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2c | >1 | >1 | >1 | 0.125 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2d | >1 | >1 | >1 | 0.500 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2e | >1 | >1 | >1 | 0.250 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2f | >1 | >1 | >1 | 0.500 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2g | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2h | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2i | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2j | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2k | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2l | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2m | >1 | >1 | >1 | 0.125 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2n | >1 | >1 | >1 | 0.500 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| 2o | >1 | >1 | >1 | 0.500 | >1 | >1 | >1 | >1 | >1 | >1 | >1 |
| Ref-1 | >1 | >1 | >1 | >1 | >1 | >1 | >1 | 0.0039 | 0.0312 | 0.0019 | 0.0625 |
| Ref-2 | 0.0312 | >1 | 0.0156 | 0.250 | 0.0625 | 0.250 | 0.0312 | >1 | >1 | >1 | >1 |
A: Escherichia coli ATCC 35218; B: Escherichia coli ATCC 25922; C: Staphylococcus aureus ATCC 25923; D: Pseudomona aeuroginosa ATCC 27853; E: Enterococcus faecalis ATCC 29212; F: Klebsiella pneumoniae ATCC 700603; G: Listeria monocytogenes ATCC 7644; H: Candida albicans ATCC 90028; I: Candida glabrata ATCC 90030; J: Candida krusei ATCC 6258; K: Candida parapsilopsis ATCC 22019; Ref-1: Ketoconazole; Ref-2: Chloramphenicol.
Table 1 reveals that the microbial strains tested herein are very resistant to compounds 2a–2o; and only Pseudomona aeuroginosa ATCC 27853 was sensitive to some of the compounds. The compound 2e, which bears an isopropyl substituent, showed equal activity (MIC50 = 250 µg/mL) to the reference drug chloramphenicol against Pseudomona aeuroginosa ATCC 27853. The MIC50 values (MIC50 = 125 µg/mL) of compounds 2c and 2m, which bear trifluoromethoxy and chloro substituents, was 2-fold lower than that of chloramphenicol. The most active compound in the series was 2-(4-methoxyphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2b), which was a methoxy-substituted derivative and showed a 16-fold greater activity than the reference drug with a MIC50 value of 0.0156 µg/mL. These findings show that synthesized compounds do not have broad antibacterial spectrum and suggest that incorporation of methoxy substituent enhances the antibacterial activity against Pseudomona aeuroginosa ATCC 27853.
The inhibitory potencies of the synthesized compounds against AChE, MAO-A, MAO-B, COX-1 and COX-2 enzymes were studied according to previously verified protocols [51,52,53,54]. In all of the assays, the compounds were initially tested at two concentrations (10−3 M and 10−4 M); in case of an inhibition more than 50% for any of the compounds, further studies were performed to determine the IC50, covering a wider range of concentrations (10−5 M and 10−9 M).
Inhibitory activity results of the synthesized compounds on AChE, MAO-A and MAO-B enzymes are summarized in Supplementary Table S1; donepezil, moclobemide and selegiline were used as the reference drugs for AChE, MAO-A and MAO-B inhibition tests, respectively. Relatively low inhibition against the tested enzymes was observed, when compared to the reference drugs; besides, it can be underlined that the compounds possessed better inhibitory profile against MAO-B, in comparison to AChE and MAO-A. Since none of the compounds showed more than 50% inhibition, further assays were not carried out to determine IC50 values. This indicates that the synthesized compounds have no potential as drug candidates for AChE- and MAO-related disorders.
The inhibitory potencies of compounds 2a–2o on COX-1 and COX-2 enzymes are given in Supplementary Table S2; ibuprofen and nimesulide were used as non-selective COX and selective COX-2 inhibitors in these tests, respectively. The compounds 2a, 2b, 2f, 2h, 2j and 2m displayed more than 50% inhibition at the initial concentrations (10−3 M and 10−4 M); consequently, they were assayed at lower concentrations between 10−5 M and 10−9 M, and IC50 values were calculated (Table 2). It has been determined that compounds 2b and 2j have promising inhibitory activity against both COX-1 and COX-2 enzymes. IC50 values of the compound 2b was 8.096 µM and 8.369 µM versus COX-1 and COX-2 enzymes, respectively; their IC50 values were also comparable with those of the reference drugs ibuprofen and nimesulide. Compound 2j possessed 14.18 and 13.09 µM IC50 values on COX-1 and COX-2, respectively.
Table 2.
Inhibitory potencies of selected test compounds (2a, 2b, 2f, 2h, 2j and 2m) at lower concentrations against COX-1 and COX-2 enzymes.
| Comp. | COX 1 Inhibition % | COX 2 Inhibition % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10−5 M | 10−6 M | 10−7 M | 10−8 M | 10−9 M | IC50 µM | 10−5 M | 10−6 M | 10−7 M | 10−8 M | 10−9 M | IC50 µM | |
| 2a | 47.39 ± 1.08 | 40.12 ± 0.92 | 35.68 ± 0.97 | 30.46 ± 0.83 | 28.81 ± 0.79 | 56.08 | 43.72 ± 1.07 | 40.32 ± 1.10 | 38.26 ± 0.92 | 36.20 ± 0.87 | 32.44 ± 0.91 | 58.66 |
| 2b | 59.37 ± 1.24 | 42.75 ± 1.02 | 37.08 ± 0.92 | 26.77 ± 0.87 | 19.56 ± 0.76 | 8.096 | 57.85 ± 1.28 | 43.70 ± 1.12 | 38.12 ± 0.97 | 27.45 ± 0.87 | 21.35 ± 0.74 | 8.369 |
| 2f | 45.38 ± 0.99 | 42.08 ± 0.87 | 34.23 ± 0.76 | 32.68 ± 0.78 | 29.88 ± 0.68 | 43.23 | 46.98 ± 1.13 | 40.22 ± 1.24 | 35.62 ± 0.91 | 31.46 ± 0.84 | 28.74 ± 0.63 | 44.92 |
| 2h | 46.29 ± 1.09 | 40.88 ± 0.99 | 35.62 ± 0.81 | 30.77 ± 0.93 | 24.23 ± 0.72 | 42.97 | 47.54 ± 1.08 | 42.60 ± 1.16 | 31.75 ± 0.99 | 28.35 ± 0.82 | 23.04 ± 0.68 | 51.19 |
| 2j | 51.41 ± 1.15 | 44.45 ± 1.07 | 37.66 ± 0.76 | 27.46 ± 0.82 | 23.44 ± 0.61 | 14.18 | 52.54 ± 1.35 | 43.40 ± 1.27 | 33.98 ± 1.06 | 26.85 ± 0.86 | 20.54 ± 0.76 | 13.09 |
| 2m | 47.36 ± 1.16 | 42.02 ± 0.88 | 36.65 ± 0.92 | 26.19 ± 0.79 | 20.01 ± 0.62 | 21.94 | 46.11 ± 1.13 | 41.23 ± 1.05 | 34.48 ± 0.97 | 28.69 ± 0.83 | 22.30 ± 0.52 | 22.66 |
| Ref-1 | 74.67 ± 1.28 | 45.08 ± 1.16 | 28.23 ± 0.91 | 22.66 ± 0.82 | 14.76 ± 0.60 | 2.435 | 69.51 ± 1.28 | 36.38 ± 0.91 | 29.42 ± 0.84 | 24.03 ± 0.76 | 17.08 ± 0.58 | 5.327 |
| Ref-2 | 68.39 ± 1.26 | 41.91 ± 0.98 | 29.05 ± 0.92 | 20.37 ± 0.81 | 18.84 ± 0.63 | 3.810 | 78.69 ± 1.19 | 48.28 ± 1.09 | 32.97 ± 0.96 | 21.30 ± 0.88 | 14.12 ± 0.61 | 1.683 |
Ref-1: Ibuprofen; Ref-2: Nimesulide.
In terms of structure activity relationships, it is noted that the most active compounds 2b and 2j carry methoxy- and ethoxy- substituents, respectively, at the 4th position of the phenyl on the benzimidazole ring; in addition, when the nature of the substituents was examined, it can be easily recognized that only compounds 2b, 2f, 2j and 2m included alkyloxy groups. Besides 2b and 2j, the compounds 2f and 2m also showed potential activity in initial assays, and were subjected to further tests. Thus, it may be suggested that the substituents including oxygen atoms may cause more inhibition than the other substituents; this phenomenon may be explained by the hydrogen accepting ability of alkyloxy groups.
2.3. Toxicological Studies
It is a well-known fact that a drug candidate should not only possess a beneficial pharmacological activity, but also has to display a low toxicological profile; from this point of view, the MTT cell viability and Ames genotoxicity tests were applied. The cytotoxicity results of the most active compounds (2b, 2j and 2m) against COX enzymes are presented in Table 3. IC50 values of the compounds 2b, 2j and 2m against NIH/3T3 was found as 316 μM, 316 μM and 100 μM, respectively. IC50 of the mentioned compounds against NIH/3T3 is about 5–40 folds higher than their IC50 against COX enzymes. Thus, it can be stated that the compounds are non-toxic at their effective concentrations against COX-1 and COX-2.
Table 3.
IC50 values of selected test compounds (2b, 2j and 2m) against NIH3T3 cell lines and results of Ames MPF test.
| Comp. | Cytotoxicity NIH3T3 Cells IC50 (µM) | Concentration (mg/mL) for Ames Test | REVERTANTS Fold Increase (over Baseline) | |||
|---|---|---|---|---|---|---|
| TA98 | TA100 | |||||
| S9+ | S9− | S9+ | S9− | |||
| 2b | 316 | 0.156 | 0.16 | 0.69 | 0.06 | 1.31 *** |
| 0.3125 | 0.32 | 0.75 | 0.52 | 1.37 *** | ||
| 0.625 | 0.00 | 0.81 | 0.45 | 0.69 | ||
| 1.25 | 0.48 | 0.40 | 0.26 | 0.62 | ||
| 2.5 | 0.80 | 0.40 | 0.26 | 0.25 *** | ||
| 5 | 1.27 | 0.75 | 0.13 | 0.44 * | ||
| 2j | 316 | 0.156 | 0.25 | 0.60 | 0.15 | 0.80 |
| 0.3125 | 0.12 * | 0.94 | 0.00 | 1.00 | ||
| 0.625 | 0.62 | 0.68 | 0.15 | 0.40 | ||
| 1.25 | 0.74 | 0.60 | 0.38 | 0.67 | ||
| 2.5 | 0.12 | 0.94 | 0.31 | 0.67 *** | ||
| 5 | 0.74 * | 0.09 *** | 0.15 | 1.20 | ||
| 2m | 100 | 0.156 | 0.46 | 0.95 | 0.10 | 0.61 |
| 0.3125 | 0.62 | 0.70 | 0.10 | 0.88 | ||
| 0.625 | 0.77 | 0.57 | 0.88 | 0.47 | ||
| 1.25 | 0.46 | 1.08 * | 0.19 | 0.47 | ||
| 2.5 | 1.23 | 0.82 | 0.49 | 0.54 | ||
| 5 | 0.31 | 1.08 * | 0.29 | 0.61 | ||
* t-test p value (unpaired, one-sided) < 0.05. *** t-test p value (unpaired, one-sided) < 0.001.
The Ames assay is a widely used method that use bacteria to test if a compound causes mutations on the DNA of the test microorganism. More formally, it is a biological assay to assess the mutagenic potential of a chemical compound. A positive result indicates that the tested chemical is mutagenic and hence may act as a carcinogen, since cancer is frequently related to mutation. It is a quick and convenient test to evaluate the carcinogenic potential of a compound [55]. Accordingly, this assay was performed to investigate the genotoxicity of compounds 2b, 2j and 2m. In Ames MPF assay, more than 25 positive wells were observed with our positive controls, and negative control wells also showed less than eight positive wells in the presence and absence of S9 with TA98 and TA100; these results are in compliance with previous studies and the requirements for the validation of the Ames MPF [55]. The results are presented in Table 3.
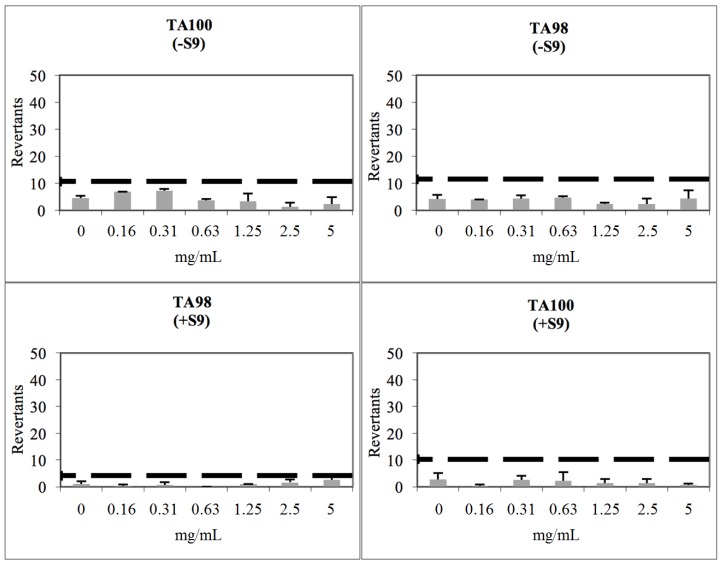
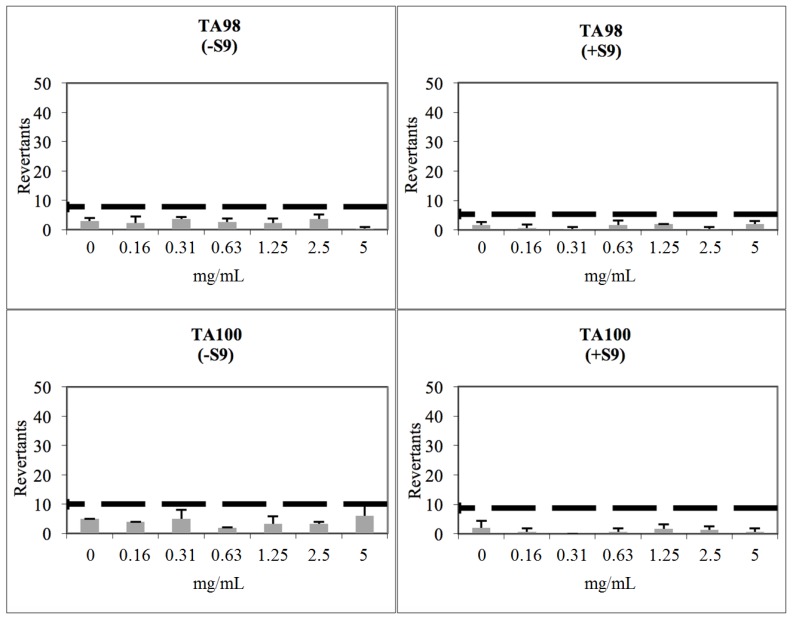
Compound 2b showed a baseline of 5.77 with TA98 in the absence of S9 and 2.10 in the presence of S9. None of the tested concentrations reached the mentioned values above the baseline, and also did not show any significance. Therefore, compound 2b was classified as non-mutagenic against TA98 in the presence/absence of metabolic activation (S9, Figure 3). Compound 2b had a baseline of 5.35 with TA100 in the absence of S9 and 5.14 in the presence of S9. Furthermore, fold inductions over baseline were less than 1.5 in each concentration level of the compounds, and the results did not show a dose-response tendency. Therefore, compound 2b was concluded as non-genotoxic against TA100 with/without metabolic activation (Figure 3).
Figure 3.
Dose-Response curve of compound 2b against TA98 and TA100 in the presence and absence of S9 according to Ames MPF test (the thick black line corresponds to mutagenicity threshold).
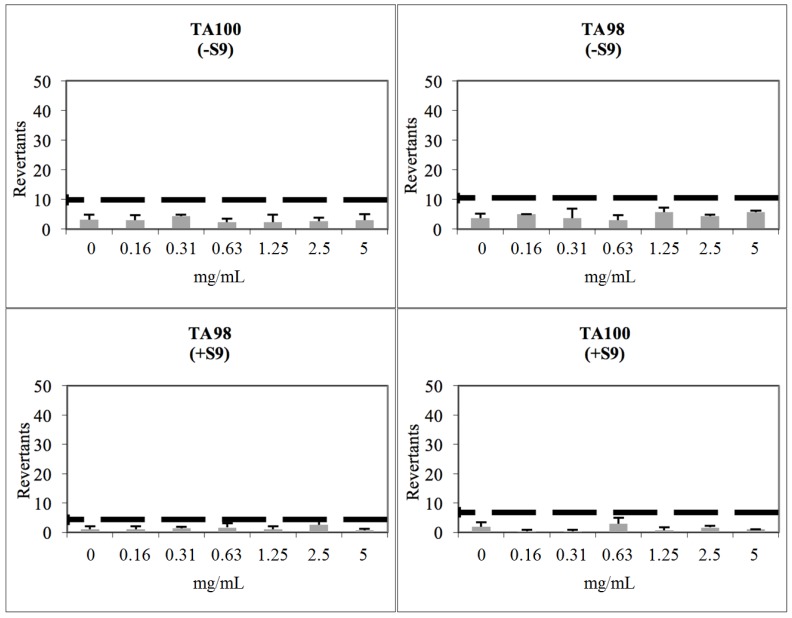
Compound 2j showed a baseline of 2.70 and 3.89 against TA98 with/without S9, respectively. Fold inductions over baseline did not reach values more than 1.5 or 2 and, statistically dissimilar results did not reveal a dose-response tendency. According to these findings, compound 2j accepted as non-mutagenic against TA98 (Figure 4). Compound 2j was found to show a baseline of 4.37 and 5.00 with/without S9 against TA100. Mentioned fold-increases over the baseline according to the criteria were not observed with compound 2j, and significant results did not reach these values, showing a non-monotonic dose-response tendency. Compound 2j was also found to be non-mutagenic against TA100 in the presence/absence of metabolic activation (Figure 4).
Figure 4.
Dose-Response curve of compound 2j against TA98 and TA100 in the presence and absence of S9 according to Ames MPF test (the thick black line corresponds to mutagenicity threshold).
Compound 2m was found to show a baseline of 5.26 and 2.17 in the absence and presence of S9 against TA98, respectively. None of the concentrations reached 1.5 or 2-fold increases over the baseline, according to the criteria. Significantly different results did not show a dose-response tendency and they were also lower than the mentioned fold-increases. Compound 2m showed a baseline of 3.43 and 4.94 with/without S9, respectively against TA100. Fold inductions over baseline were less than 1.5 at each concentration level of the compounds, and there was not any significant difference. Compound 2m was accepted as non-mutagenic against TA98 and TA100 with and without metabolic activation (Figure 5).
Figure 5.
Dose-Response curve of compound 2m against TA98 and TA100 in the presence and absence of S9 according to Ames MPF test (the thick black line corresponds to mutagenicity threshold).
According to the Ames MPF results, compounds were classified as non-mutagenic. As well as cytotoxicity results, genotoxicity findings also increased the value of compounds 2b and 2j as possible COX inhibitors.
2.4. Prediction of ADME and Drug Likeness
Sufficient pharmacological activity and low toxicological profile are not the only prerequisites for a compound to become a drug candidate; in addition, acceptable pharmacokinetic profile is also required. Due to importance of such characteristics, in the current study, ADME properties of the synthesized compounds 2a–2o were also investigated via in-silico routes, and related calculations were realized by using online Molinspiration software [56]. Drug likeness score (DLS) was calculated for all of the compounds 2a–2o and reference drugs using Molsoft’s software [57]. The theoretical calculations of ADME parameters such as molecular weight (MW), logP, topological polar surface are (tPSA), number of hydrogen donors (nON) and acceptors (nOHNH), volume, and DLS are presented in Table 4, along with the violations of Lipinski’s rule. Considering Lipinski’s rule of five, all synthesized compounds have nON smaller than 5, nOHNH smaller than 10 and polar surface area lesser than 140 Å. Besides, MWs of all of the compounds are in accordance with the value smaller than 500 g/mol. Synthesized compounds have logP values of less than 5 except for compounds 2e, 2l and 2o. According to theoretical data, the majority of the compounds were found to obey Lipinski’s rules; only the compounds 2e, 2l and 2o showed minor deviation in one parameter. Furthermore, high DLSs of 1.12 and 0.88 were calculated for the most active compounds 2b and 2m. Thus, it can be stated that synthesized compounds have good pharmacokinetics profile, which improves their biological acceptability.
Table 4.
Some physicochemical parameters of test compounds 2a–2o and reference drugs used in prediction of ADME profiles.
| Compound | MW | logP | tPSA | nON | nOHNH | Volume | Vio | DLS |
|---|---|---|---|---|---|---|---|---|
| 2a | 321.42 | 3.94 | 30.30 | 4 | 0 | 308.95 | 0 | 0.74 |
| 2b | 337.42 | 3.54 | 39.53 | 5 | 0 | 317.94 | 0 | 1.12 |
| 2c | 341.84 | 4.17 | 30.30 | 4 | 0 | 305.93 | 0 | 1.21 |
| 2d | 325.39 | 3.65 | 30.30 | 4 | 0 | 297.32 | 0 | 1.10 |
| 2e | 349.48 | 5.00 | 30.30 | 4 | 0 | 342.34 | 1 | 1.15 |
| 2f | 375.39 | 4.38 | 30.30 | 4 | 0 | 323.69 | 0 | 0.69 |
| 2g | 386.29 | 4.30 | 30.30 | 4 | 0 | 310.28 | 0 | 0.89 |
| 2h | 378.52 | 4.34 | 33.54 | 5 | 0 | 371.90 | 0 | 0.61 |
| 2i | 350.47 | 3.59 | 33.54 | 5 | 0 | 338.30 | 0 | 0.59 |
| 2j | 351.45 | 3.92 | 39.53 | 5 | 0 | 334.74 | 0 | 1.01 |
| 2k | 332.41 | 3.24 | 54.09 | 5 | 0 | 309.25 | 0 | 0.63 |
| 2l | 413.52 | 5.14 | 39.53 | 5 | 0 | 389.59 | 1 | 0.92 |
| 2m | 391.39 | 4.46 | 39.53 | 5 | 0 | 332.67 | 0 | 0.88 |
| 2n | 353.49 | 3.92 | 30.30 | 4 | 0 | 327.08 | 0 | 0.92 |
| 2o | 383.50 | 5.28 | 30.30 | 4 | 0 | 363.80 | 1 | 0.68 |
| Donepezil | 379.50 | 4.10 | 38.78 | 4 | 0 | 367.89 | 0 | 1.76 |
| Moclobemide | 268.74 | 1.69 | 41.57 | 4 | 0 | 240.70 | 0 | 1.36 |
| Selegiline | 187.29 | 2.64 | 3.24 | 1 | 0 | 202.64 | 0 | 1.03 |
| Chloramphenicol | 323.13 | 0.73 | 115.38 | 7 | 3 | 249.16 | 0 | 0.89 |
| Ketoconazole | 531.44 | 3.77 | 69.08 | 8 | 0 | 452.47 | 1 | 1.32 |
| Ibuprofen | 206.28 | 3.46 | 37.30 | 2 | 1 | 211.19 | 0 | 0.96 |
| Nimesulide | 308.31 | 2.81 | 101.23 | 7 | 1 | 248.17 | 0 | −1.40 |
MW: Molecular weight; tPSA: Topological polar surface are; nON: Number of hydrogen donors; nOHNH: Number of hydrogen acceptors; Vio: Violence; DLS: Drug likeness score.
3. Materials and Methods
3.1. General Information
All of the chemicals used in the study were purchased either from Sigma-Aldrich Corp. (St. Louis, MO, USA) or Merck KGaA (Darmstadt, Germany), and used without further chemical or biological purification. Microwave syntheses were realized by using a Monowave 300 high-performance microwave reactor (Anton-Paar, Graz, Austria). Melting points of the synthesized compounds were determined by using a MP90 series automatic melting point determination system (Mettler-Toledo, Columbus, OH, USA) and were presented as uncorrected. 1H- and 13C-NMR spectra were recorded in DMSO-d6 by a Bruker digital FT-NMR spectrometer (Bruker Bioscience, Billerica, MA, USA) at 500 MHz and 75 MHz, respectively (splitting patterns in the NMR spectra were designated as follows: s: singlet; d: doublet; t: triplet; m: multiplet; coupling constants (J) were reported in Hertz). The IR spectra of the compounds were recorded using an IRAffinity-1S Fourier transform IR (FTIR) spectrometer and high resolution mass spectrometric studies were performed using a liquid chromatography mass spectrometry-ion trap-time of flight (LCMS-IT-TOF) instrument, both from Shimadzu (Kyoto, Japan). Chemical purities of the compounds were checked by classical TLC applications performed on silica gel 60 F254 (Merck KGaA); LCMS-IT-TOF chromatograms were also used for the same purpose. The pipetting in the COX-1, COX-2 and AChE assays were performed by using BioTek Precision XS robotic system (BioTek Instruments, Inc., Winooski, VT, USA). Fluorescence intensities were measured by BioTek-Synergy H1 multimode microplate reader (BioTek Instruments, Inc). A model UV-1800 double beam UV-visible spectrophotometer (Shimadzu) was used for spectrophotometric determinations. Statistical calculations were performed via Prism 5 software from GraphPad (La Jolla, IL, USA). Water (ultra-pure, with total organic carbon less than 5 ppb and resistivity of at least 18 MOhm·cm−1) was produced in our laboratory using Milli-Q Synthesis A10 system from Millipore SAS (Molsheim, France) and steam-sterilized before use.
3.2. Chemistry
3.2.1. Microwave-Assisted Synthesis of 2-Substituted-1H-benzimidazole Derivatives 1a–1o
An appropriate aldehyde derivative (0.03 mol), sodium bisulfite (5.7 g, 0.03 mol) and DMF (10 mL) were added into a 30-mL glass vial of microwave synthesis reactor. The vial was located into the reactor and heated at 240 °C under 10 bars of internal pressure for 5 min; consequently, it was cooled down to room temperature (RT), 1,2-phenylenediamine (3.24 g, 0.03 mol) was added and the mixture was subjected to above-mentioned conditions again. After cooling to RT, the mixture was poured into iced-water, the precipitated product was washed using water, dried at RT and recrystallized from ethanol.
3.2.2. Microwave-Assisted Synthesis of 2-Substituted-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole Derivatives 2a–2o
The corresponding 2-substituted-1H-benzimidazole derivative 1a–1o (0.0025 mol), NaH (0.072 g, 0.003 mol) and THF (10 mL) were added into a 30-mL glass vial of microwave synthesis reactor. After addition of 2-(morpholin-4-yl)ethyl chloride (1 mL), the vial was located into the reactor and heated at 170 °C under 10 bars of internal pressure for 30 min. The reaction vial was removed and cooled to RT, and the mixture was poured into iced-water. The precipitated product was washed using water, dried at RT and recrystallized from ethanol.
2-(4-Methylphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2a). Yield: 84%. m.p. 117.7–119.1 °C. FTIR (ATR, cm−1): 3017 (aromatic C-H), 2986 (aliphatic C-H), 1610 (C=N), 828 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.19–2.21 (4H, m, morpholine -CH2-), 2.41 (3H, s, -CH3), 2.51 (2H, t, J = 6.40 Hz, -CH2-), 3.37–3.39 (4H, m, morpholine -CH2-), 4.39 (2H, t, J = 6.40 Hz, -CH2-), 7.22–7.27 (2H, m, benzimidazole H5-H6), 7.29 (2H, d, J = 8.00 Hz, phenyl H3,H′5,), 7.69–7.71 (2H, m, benzimidazole H4,H7), 7.81 (2H, d, J = 8.00 Hz, phenyl H2,H′6). 13C-NMR (DMSO-d6, ppm): δ: 21.40, 42.13, 53.78, 57.43, 66.42, 111.33, 119.51, 122.31, 122.69, 128.30, 129.66, 136.10, 139.69, 143.13, 154.00. HRMS (m/z): [M + H]+ calcd. for C20H23N3O: 322.1914 found: 322.1901.
2-(4-Methoxyphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2b). Yield: 81%. m.p. 132.7–133.9 °C. FTIR (ATR, cm−1): 3019 (aromatic C-H), 2966 (aliphatic C-H), 1611 (C=N), 847 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.20–2.22 (4H, m, morpholine -CH2-), 2.50 (2H, t, J = 6.50 Hz, -CH2-), 3.38–3.40 (4H, m, morpholine -CH2-), 3.85 ( 3H, s, -OCH3), 4.38 (2H, t, J = 6.50 Hz, -CH2-), 7.11 (2H, d, J = 8.00 Hz, phenyl H3,H′5,), 7.21–7.26 (2H, m, benzimidazole H5-H6), 7.61–7.65 (2H, m, benzimidazole H4,H7), 7.76 (2H, d, J = 8.00 Hz, phenyl H2,H′6). 13C-NMR (DMSO-d6, ppm): δ: 42.15, 53.79, 55.78, 57.39, 66.44, 111.25, 114.53, 119.38, 122.27, 122.57, 123.32, 131.21, 136.09, 143.12, 153.88, 160.68. HRMS (m/z): [M + H]+ calcd. for C20H23N3O2: 338.1863 found: 338.1844.
2-(4-Chlorophenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2c). Yield: 69%. m.p. 133.3–134.0 °C. FTIR (ATR, cm−1): 3055 (aromatic C-H), 2953 (aliphatic C-H), 1602 (C=N), 837 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.18–2.19 (4H, m, morpholine -CH2-), 2.58 (2H, t, J = 6.50 Hz, -CH2-), 3.36–3.37 (4H, m, morpholine -CH2-), 4.40 (2H, t, J = 6.50 Hz, -CH2-), 7.23–7.28 (2H, m, benzimidazole H5-H6), 7.62–7.69 (4H, m, benzimidazole H4,H7, phenyl H2,H′6), 7.86–7.88 (2H, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 42.24, 53.82, 57.47, 66.39, 111.49, 119.70, 122.54, 123.02, 129.20, 130.08, 131.59, 134.92, 136.13, 143.08, 152.82. HRMS (m/z): [M + H]+ calcd. for C19H20N3OCl: 342.1368 found: 342.1348.
2-(4-Fluorophenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2d). Yield: 73%. m.p. 91.1–92.0 °C. FTIR (ATR, cm−1): 3055 (aromatic C-H), 2972 (aliphatic C-H), 1605 (C=N), 845 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.15–2.17 (4H, m, morpholine -CH2-), 2.57 (2H, t, J = 6.50 Hz, -CH2-), 3.35–3.36 (4H, m, morpholine -CH2-), 4.38 (2H, t, J = 6.50 Hz, -CH2-), 7.23–7.30 (2H, m, benzimidazole H5-H6), 7.38–7.42 (2H, m, benzimidazole H4,H7), 7.63–7.67 (2H, m, phenyl H2,H′6), 7.86–7.88 (2H, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 42.13, 53.79, 57.43, 66.39, 111.42, 116.15 (d, 2JCF = 21.6 Hz), 119.61, 122.45, 122.89, 127.70 (d, 4JCF = 3.1 Hz), 132.16 (d, 3JCF = 8.5 Hz), 136.03, 143.03, 153.07, 163.26 (d, 1JCF = 245.1 Hz). HRMS (m/z): [M + H]+ calcd. for C19H20N3OF: 326.1663; found: 326.1645.
2-(4-Isopropylphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2e). Yield: 76%. m.p. 123.1–124.0 °C. FTIR (ATR, cm−1): 3050 (aromatic C-H), 2967 (aliphatic C-H), 1611 (C=N), 843 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 1.26 (6H, d, J = 7.00 Hz, -CH3), 2.16–2.18 (4H, m, morpholine -CH2-), 2.51 (2H, t, J = 6.50 Hz, -CH2-), 2.58 (1H, s, -CH-), 3.33–3.35 (4H, m, morpholine -CH2-), 4.41 (2H, t, J = 6.50 Hz, -CH2-), 7.41–7.44 (2H, benzimidazole H5-H6), 7.49 (2H, m, d, J = 8.00 Hz, phenyl H3,H′5), 7.65–7.73 (2H, m, benzimidazole H4,H7), 7.75 (2H, d, J = 8.00 Hz, phenyl H2,H′6). 13C-NMR (DMSO-d6, ppm) δ: 24.22, 33.80, 42.03, 53.78, 57.52, 66.40, 111.31, 119.52, 122.31, 122.69, 127.00, 128.73, 129.80, 136.06, 143.15, 150.44, 154.03.HRMS (m/z): [M + H]+ calcd. for C22H27N3O: 350.2227; found: 350.2199.
2-(4-Benzyloxyphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2f). Yield: 63%. m.p. 160.9–161.9 °C. FTIR (ATR, cm−1): 3051 (aromatic C-H), 2961 (aliphatic -C-H), 1611 (C=N), 831 (para-substituted benzene), 744 (monosubstituted benzene), 702 (monosubstituted benzene).1H-NMR (DMSO-d6, ppm) δ: 2.20 (4H, s, morpholine -CH2-), 2.59 (2H, t, J = 6.50 Hz, -CH2-), 3.38 (4H, s, morpholine -CH2-), 4.38 (2H, t, J = 6.50 Hz, -CH2-), 5.13 (2H, s, -OCH2-), 7.18–7.26 (5H, m, benzyloxy -CH-), 7.35–7.40 (2H, m, benzimidazole H5-H6), 7.48 (2H, d, J = 8.50 Hz, phenyl H2,H′6), 7.61–7.65 (2H, m, benzimidazole H4,H7), 7.76 (2H, d, J = 8.50 Hz, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 42.13, 53.78, 57.39, 66.43, 66.80, 111.26, 115.40, 119.40, 122.27, 122.57, 123.56, 128.22, 128.39, 128.94, 131.22, 136.08, 137.26, 143.13, 153.85, 159.74. HRMS (m/z): [M + H]+ calcd. for C26H27N3O2: 414.2176; found: 414.2156.
2-(4-Bromophenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2g). Yield: 80%. m.p. 166.4–167.3 °C. FTIR (ATR, cm−1): 3053 (aromatic C-H), 2953 (aliphatic C-H), 1608 (C=N), 837 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.18 (4H, s, morpholine -CH2-), 2.58 (2H, t, J = 6.50 Hz, -CH2-), 3.39 (4H, s, morpholine -CH2-), 4.40 (2H, t, J = 6.50 Hz, -CH2-), 7.25–7.30 (2H, m, benzimidazole H5-H6), 7.66–7.68 (2H, m, phenyl H2,H′6), 7.79–7.81 (4H, m, benzimidazole H4,H7, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 42.25, 53.82, 57.48, 66.38, 111.52, 119.69, 122.55, 123.04, 123.66, 130.44, 131.82, 132.13, 136.14, 143.06, 152.89. HRMS (m/z): [M + H]+ calcd. for C19H20N3OBr: 386.0862; found: 386.083.
2-(4-Diethylaminophenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2h). Yield: 79%. m.p. 140.2–141.4 °C. FTIR (ATR, cm−1): 3047 (aromatic C-H), 2970 (aliphatic C-H), 1609 (C=N), 818 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 1.13 (6H, t, J = 7.00 Hz, -CH3), 2.27–2.29 (4H, m, morpholine -CH2-), 2.63 (2H, s, -CH2-), 3.41–3.44 (8H, m, morpholine -CH2-, -CH2-), 4.38 (2H, t, J = 6.50 Hz, -CH2-), 6.78–6.80 (2H, m, phenyl H2,H′6), 7.20 (2H, s, benzimidazole H5-H6), 7.59–7.63 (4H, m, benzimidazole H4,H7, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 12.85, 42.23, 44.15, 53.83, 57.44, 66.49, 110.94, 111.39, 116.81, 118.99, 122.02, 122.06, 130.82, 136.25, 143.30, 148.56, 154.69. HRMS (m/z): [M + H]+ calcd. for C23H30N4O: 379.2492; found: 379.2465.
2-(4-Dimethylaminophenyl)-1-[2-(morpholin-4-yl) ethyl]-1H-benzimidazole (2i). Yield: 72%. m.p. 162.0–163.3 °C. FTIR (ATR, cm−1): 3053 (aromatic C-H), 2955 (aliphatic C-H), 1607 (C=N), 824 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.28 (4H, s, morpholine -CH2-), 2.50 (2H, s, -CH2-), 2.99 (6H, s, -CH3), 3.42–3.44 (4H, m, morpholine -CH2-), 4.38 (2H, t, J = 6.50 Hz, -CH2-), 6.84 (2H, d, J = 8.85 Hz, phenyl H2,H′6), 7.19–7.22 (2H, m, benzimidazole H5-H6), 7.57–7.61 (2H, m, benzimidazole H4,H7), 7.66 ( 2H, d, J = 8.85 Hz, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 40.28, 42.28, 53.83, 57.39, 66.50, 111.01, 112.11, 117.85, 119.06, 122.06, 122.15, 130.53, 136.25, 143.27, 151.36 154.59. HRMS (m/z): [M + H]+ calcd. for C21H26N4O: 351.2179; found: 351.2172.
2-(4-Ethoxyphenyl)-1-[2-(morpholin-4-yl) ethyl]-1H-benzimidazole (2j). Yield: 76%. m.p. 83.4–85.5 °C. FTIR (ATR, cm−1): 3063 (aromatic C-H), 2972 (aliphatic C-H), 1612 (C=N), 854 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 1.37 (3H, t, J = 6.95 Hz, -CH3), 2.21 (4H, s, morpholine -CH2-), 2.50 (2H, s, -CH2-), 3.39 (4H, s, morpholine -CH2-), 4.12 (2H, q, J = 6.95 Hz, -CH2-), 4.38 (2H, t, J = 6.50 Hz, -CH2-), 7.08 (2H, d, J = 8.75 Hz, phenyl H2,H′6), 7.21–7.26 (2H, m, benzimidazole H5-H6), 7.61–7.65 (2H, m, benzimidazole H4,H7), 7.75 (2H, d, J = 8.75 Hz, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 15.05, 42.13, 53.79, 57.39, 63.74 66.44, 111.24, 114.94, 119.37, 122.25, 122.55, 123.17, 131.21, 136.09, 143.13, 153.92, 159.95. HRMS (m/z): [M + H]+ calcd. for C21H25N3O2: 352.2020; found: 352.1985.
2-(4-Cyanophenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2k). Yield: 83%. m.p. 134.9–136.2 °C. FTIR (ATR, cm−1): 3059 (aromatic C-H), 2952 (aliphatic C-H), 2226 (C≡N), 1612 (C=N), 844 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.20 (4H, s, morpholine -CH2-), 2.58 (2H, t, J = 6.50 Hz, -CH2-), 3.38 (4H, s, morpholine -CH2-), 4.43 (2H, t, J = 6.50 Hz, -CH2-), 7.28–7.33 (2H, m, benzimidazole H5-H6), 7.70–7.72 (2H, m, phenyl H2,H′6), 8.08–8.06 (4H, m, benzimidazole H4,H7, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 42.36, 53.82, 57.51, 66.34, 111.69, 112.48, 118.98, 119.96, 122.80, 123.45, 130.64, 133.05, 135.77, 136.23, 143.10, 152.27. HRMS (m/z): [M + H]+ calcd. for C20H20N4O: 333.1710; found: 333.1682.
2-(4-Trifluoromethylphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2l). Yield: 89%. m.p. 124.3–125.2 °C. FTIR (ATR, cm−1): 3052 (aromatic C-H), 2959 (aliphatic C-H), 1618 (C=N), 852 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.15 (4H, s, morpholine -CH2-), 2.57 (2H, t, J = 6.50 Hz, -CH2-), 3.30 (4H, s, morpholine -CH2-), 4.45 (2H, t, J = 6.50 Hz, -CH2-), 7.26–7.33 ( 2H, m, benzimidazole H5-H6), 7.71 (2H, d, J = 7.50 Hz, phenyl H2,H′6), 7.93–7.95 (2H, m, benzimidazole H4,H7), 8.09 (2H, d, J = 7.50 Hz, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 42.28, 53.82, 57.55, 66.31, 111.61, 119.89, 122.69, 123.29, 124.62 (q, 1JCF = 259.2 Hz), 125.98 (q, 3JCF = 3.64 Hz), 130.10 (q, 2JCF = 31.9 Hz), 130.66, 135.33, 136.19, 143.10, 152.49. HRMS (m/z): [M + H]+ calcd. for C20H20N3OF3: 376.1631; found: 376.1605
2-(4-Trifluoromethoxyphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2m). Yield: 71%. m.p. 122.5–123.6 °C. FTIR (ATR, cm−1): 3055 (aromatic C-H), 2945 (aliphatic C-H), 1614 (C=N), 868 (para-substituted benzene). 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 2.12–2.16 (4H, m, morpholine -CH2-), 2.55–2.59 (2H, m, -CH2-), 3.30 (4H, s, morpholine -CH2-), 4.29–4.45 (2H, m, -CH2-), 7.22–7.33 (2H, m, benzimidazole H5-H6), 7.54–7.59 (2H, m, phenyl H2,H′6), 7.65–7.61 (4H, m, benzimidazole H4,H7), 7.95–8.00 (2H, m, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 42.11, 53.79, 57.53, 66.32, 111.48, 119.73, 120.54 (q, 1jC-F = 255.3 Hz), 121.62, 122.55, 123.05, 130.58, 131.95, 136.06, 143.07, 149.52 (q, 2jC-F = 1.5 Hz) 152.68. HRMS (m/z): [M + H]+ calcd. for C20H20N3O2F3: 392.1580; found: 392.1557
2-(4-Methylthiophenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2n). Yield: 77%. m.p. 153.2–154.1 °C. FTIR (ATR, cm−1): 3041 (aromatic C-H), 2951 (aliphatic C-H), 1599 (C=N), 826 (para-substituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.22 (4H, s, morpholine -CH2-), 2.50 (3H, s, -SCH3), 2.61 (2H, t, J = 6.50 Hz, -CH2-), 3.40 (4H, s, morpholine -CH2-), 4.41 (2H, t, J = 6.50 Hz, -CH2-), 7.23–7.26 (2H, m, benzimidazole H5-H6), 7.43 (2H, d, J = 8.50 Hz, phenyl H2,H′6), 7.64–7.66 (2H, m, benzimidazole H4,H7), 7.78 (2H, d, J = 8.50 Hz, phenyl H3,H′5). 13C-NMR (DMSO-d6, ppm) δ: 14.77, 42.25, 53.82, 57.44, 66.43, 111.35, 119.53, 122.39, 122.77, 125.94, 127.29, 130.13, 136.17, 140.93, 143.14, 153.55. HRMS (m/z): [M + H]+ calcd. for C20H23N3O2S: 354.1635; found: 354.1593.
2-(1,1’-Biphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2o). Yield: 83%. m.p. 109.4–111.2 °C. FTIR (ATR, cm−1): 3049 (aromatic C-H), 2955 (aliphatic C-H), 1610 (C=N), 845 (para-substituted benzene), 746 (monosubstituted benzene), 694 (monosubstituted benzene). 1H-NMR (DMSO-d6, ppm) δ: 2.20 (4H, s, morpholine -CH2-), 2.51 (2H, t, J = 6.50 Hz, -CH2-), 3.48 (4H, s, morpholine -CH2-), 4.41 (2H, t, J = 6.50 Hz, -CH2-), 7.25–7.33 (5H, m), 7.40–7.53 (5H, m), 7.66–7.88 (2H, m), 7.67–7.88 (6H, m). 13C-NMR (DMSO-d6, ppm) δ: 42.25, 53.82, 57.53, 66.42, 111.38, 119.69, 122.47, 122.88, 127.24, 128.39, 129.51, 130.19, 130.35, 136.23, 139.69, 141.55, 143.29, 153.63. HRMS (m/z): [M + H]+ calcd. for C25H25N3O: 384.2070; found: 384.2047.
3.3. Antimicrobial Assay
Microbiological studies were performed according to CLSI reference M07-A9 broth microdilution method for bacterial strains [58] and EUCAST definitive method EDef 7.1 for Candida species [59]. Synthesized compounds were tested for their in vitro growth inhibitory activity against Escherichia coli ATCC 35218, Escherichia coli ATCC 25922, Staphylococcus aureus, ATCC 25923, Pseudomona aeuroginosa ATCC 27853, Enterococcus faecalis ATCC29212, Klebsiella pneumoniae ATCC700603 Listeria monocytogenes ATCC7644, Candida albicans ATCC90028, Candida glabrata ATCC 90030, Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019.
The cultures were obtained from Mueller-Hinton broth (Difco) for the bacterial strains and the yeasts were maintained in Roswell Park Memorial Institute (RPMI) medium, after an overnight incubation at 37 °C for both. The inocula of the test microorganisms were adjusted to match an equivalent turbidity of a 0.5 McFarland standard, which was determined using a spectrophotometer; the final inoculum size was determined to be 0.5–2.5 × 105 cfu/mL for antibacterial and antifungal assays. The tests were carried out for both mediums at pH = 7 and two-fold serial dilutions were applied. The last well on the microplates, which was containing only the inoculated broth, was kept as control, and the last well with no growth of microorganism was recorded to represent the minimum inhibitory concentration (MIC50) in μg/mL. For both of the antibacterial and antifungal assays, the test compounds and reference drugs were firstly dissolved in DMSO, and further dilutions were performed to the desired concentrations of 1000, 500, 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9 and 1.95 μg/mL using Mueller–Hinton broth and RPMI medium. The completed plates were incubated for 24 h, and at the end of the incubation, resazurin (20 µg/mL) was added into each well to control the growth in the wells. Final plates including microorganism strains were incubated for 2 h. MIC50 values were determined using microplate reader at 590 nm excitation and 560 nm emission wavelengths; MIC50 readings were performed twice for entire compounds. Chloramphenicol and ketoconazole were used as reference drugs.
3.4. COX-1 and COX-2 Inhibition Assays
Inhibitory potency of the compounds on COX-1 and COX-2 enzymes was determined by using fluorimetric COX-1 and COX-2 inhibition screening kits from BioVision (Milpitas, CA, USA). The experimental protocol as described in the guides provided by the supplier was followed [51,52]. All of the pipetting were performed using a robotized system to increase speed, accuracy and precision. Fluorescence intensities of the samples were kinetically measured at 25 °C for 5–10 min by monitoring emissions at 587 nm after excitation at 535 nm. Two appropriate points (T1 and T2) in the linear range of the plot were chosen and the corresponding fluorescence values (RFU1 and RFU2) were obtained. The slope for all samples including Enzyme Control (EC) was calculated by dividing the net ΔRFU (RFU2 − RFU1) values by the time ΔT (T2 − T1). Percentage of relative inhibition was calculated by using the following equation:
| % Relative inhibition = (Slope of EC − Slope of S/Slope of EC) × 100 |
where S is the term representing the tested compound [51,52]. The initial in vitro assays were performed at 10−3 M and 10−4 M concentrations for all of the test compounds; the ones which showed inhibition above 50% were assayed by the same protocol within a wider range of concentrations (10−5 M and 10−9 M) to determine their IC50 against COX-1 and COX-2 enzymes. The IC50 values were calculated using the plots of the enzyme activity against concentrations by applying regression analyses.
3.5. AChE Inhibition Assay
Inhibition potency of the test compounds against AChE was determined using Ellman’s method [53]. Enzyme solutions were prepared in gelatin solution (1%, w/v), at the concentration of 2.5 units/mL. The synthesized compounds, and donepezil, which was used for reference, were prepared at 10−3 M and 10−4 M concentrations using 2% DMSO solution for initial measurements. AChE solution (20 µL/well) and test solution (20 µL/well) were added to phosphate buffer solution (140 µL/well, pH 8.0 ± 0.1), and incubated at 25 °C for 5 min. The reaction was started by addition of the chromogenic reagent 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB, 20 µL/well, 10 mM) and the substrates acetylthiocholine iodide (ATCI, 10 µL/well, 75 µM) to the enzyme-inhibitor mixture. The production of the yellow anion was recorded by multimode microplate reader for 10 min at 412 nm. As a control, an identical solution of the enzyme without the inhibitor was processed. The control and the inhibitor readings were corrected with blank-readings. All processes were assayed in four independent determinations.
3.6. MAO Inhibition Assay
Enzyme activity assays were performed according to the procedure reported by Matsumoto et al. [54] with slight modifications. In order to prevent the destructive effect of light on MAO enzymes, the assays were performed in 96-well black plates. Phosphate buffer solution (0.1 M, pH = 7.4) was used for preparation of stock solutions (5 mg/mL) of both human recombinant MAO-A and MAO-B enzymes. Enzyme stock solutions were further diluted using assay buffer to achieve a final concentration of 0.006 mg/mL for MAO-A and 0.015 mg/mL for MAO-B. Kynuramine was dissolved in sterile deionized water to prepare stock solution (25 mM) and then diluted using assay buffer to get final concentration of 40 μM for MAO-A enzyme and 20 μM for MAO-B enzyme. Synthesized compounds and reference drugs, which were moclobemide for MAO-A and selegiline for MAO-B, were diluted to 10−3 M and 10−4 M concentrations (100 μL/well) using 2% DMSO. The addition of the test compound solution was followed by addition of either MAO-A or MAO-B enzyme (50 μL/well) solutions. After 10 min of incubation at 37 °C, kynuramine (50 μL/well) was added and enzyme-substrate reaction was initiated. The plate was incubated for 20 min at 37 °C and then reaction was terminated by addition of 2 N NaOH (75 μL/well). The fluorimetric reads from the top was performed at 310 nm excitation and 380 nm emission wavelengths.
3.7. Determination of Cytotoxicity
Cytotoxicity was tested using NIH/3T3 mouse embryonic fibroblast cell line (ATCC® CRL-1658™, London, UK). NIH/3T3 cells were incubated according to the supplier’s recommendations. NIH/3T3 cells were seeded as 1 × 104 cells into each well of 96-well plates. MTT assay was performed as previously described [60,61]. The compounds were tested between 1 mM and 0.000316 mM concentrations (1.0, 0.316, 0.10, 0.0316, 0.01, 0.00316, 0.001, 0.000316 mM). The IC50 values were determined by plotting a dose-response curve of inhibition % versus tested concentrations of the compound [62].
3.8. Determination of Genotoxicity
The genotoxicity of selected compounds (2b, 2j, 2m) was determined by Ames assay using Ames MPF 98/100 mutagenicity assay sample kit (Xenometrix AG, Allschwil, Switzerland) as previously described elsewhere [63]. Salmonella typhimurium strains, TA98 (frameshift mutations) and TA100 (base-pair substitutions) were used in the assay. The concentration of the compounds was between 16 and 5000 μg/mL, in accordance with the guideline [64]. Compounds were prepared in six different concentrations (5.0, 2.5, 1.25, 0.625, 0.3125, 0.156 mg/mL) in DMSO. Mutagenic potential was determined in the absence or presence of Aroclor™-1254 induced male Sprague–Dawley rat liver microsomal enzyme (S9) mix (Xenometrix AG). Positive controls without S9 mix were 2-nitrofluorene (2.0 μg/mL) and 4-nitroquinoline N-oxide (0.1 μg/mL), whereas 1.0 μg/mL and 2.5 μg/mL of 2-aminoanthracene solutions were used as positive controls with S9 against TA98 and TA100, respectively. Solvent control was prepared with 4% DMSO. The end of an experiment was determined by the change of the indicator medium colour to yellow, which was originating from the decrease of pH due to revertant bacteria. Yellow wells were counted as positive, and compared with the negative control. Fold induction over the negative control and fold induction over the baseline were calculated (Fold induction over the negative control is accepted as the ratio of the mean number of positive wells for the dose concentration divided by the mean number of positive wells for the zero dose (negative) control. Fold induction over the baseline is accepted as the ratio of the mean number of positive wells for the dose concentration divided by zero dose baseline. The zero-dose baseline is obtained by adding one standard deviation to the mean number of positive wells of the zero dose control. If the baseline is less than 1, the value is set to 1 for calculation).
Mutagenicity was determined according to the criteria reported previously [55]. For a baseline value of ≤3, significant increases between 2 and 3-fold of the baseline were classified as weak mutagen, and increases ≥3-fold of the baseline were classified as mutagen. For a baseline value of >3, significant increases between 1.5 and 2.5-fold of the baseline were classified as weak mutagen, and increases ≥2.5-fold of the baseline, were classified as mutagen. As a rule, at least two adjacent doses with significant increases or a significant increase at the highest dose level should be observed for a mutagenic compound. All of the doses were compared according to Student’s t-test at p < 0.05 for statistical significance. Compounds that did not possessed any of the characteristics mentioned above were classified as non-mutagenic.
3.9. Prediction of ADME Properties and Drug Likeness
Some physicochemical parameters, which were used to evaluate ADME properties of the compounds (2a–2o) were calculated via online molecular property and bioactivity score calculation software of Molinspiration Cheminformatics (ver. 2017, Molinspiration Cheminformatics, Slovensky Grob, Slovak Republic) [56]. Drug-likeness score of the compounds was assigned by an online drug-likeness and molecular property prediction software (ver. 2017, Molsoft, San Diego, CA, USA) [57].
4. Conclusions
In the present study, synthesis and structural verification of some novel benzimidazole-morpholine compounds were described, and their biological and pharmaceutical value was evaluated by applying multiple biological, pharmacological and toxicological tests. Briefly, it can be concluded that the synthesized compounds did not exceptionally inhibit AChE, MAO-A and MAO-B enzymes. On the other hand, compounds 2b, 2c, 2e and 2m presented acceptable antibacterial activity especially against Pseudomona aeuroginosa ATCC 27853. Furthermore, a promising activity was observed for compounds 2b and 2j against COX-1 and COX-2 enzymes, meanwhile compound 2b was the most active derivative in both antimicrobial and COX inhibition assays. It is a common knowledge that microbial infections generally accompanied with fever and inflammation; thus, among the compounds in the series 2-(4-methoxyphenyl)-1-[2-(morpholin-4-yl)ethyl]-1H-benzimidazole (2b) seems to be most powerful candidate for the treatment of a such disease, with dual activity against Pseudomona aeuroginosa ATCC and COX enzymes.
Acknowledgments
This study was financially supported by Anadolu University Scientific Research Projects Commission, Project no. 1105S101 and 1606S567.
Supplementary Materials
The following are available online. Table S1: Percentage of inhibition of test compounds 2a–2o against AChE, MAO-A and MAO-B enzymes, Table S2: Inhibitory potencies of test compounds 2a–2o at higher concentrations against COX-1 and COX-2 enzymes. The 13C-NMR, 1H-NMR, FTIR and HRMS spectrums of compounds 2a–2o.
Author Contributions
N.Ö.C. and Y.Ö. conceived and designed the experiments; U.A.Ç. and B.N.S. performed the syntheses; N.Ö.C. and Y.Ö. performed the analytical and spectral studies; Ö.A. and M.B. performed the cytotoxicity and genotoxicity tests; Ö.D.C. and Ü.D.Ö. conducted the pharmacological parts; N.Ö.C. and Y.Ö. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1a–1o and 2a–2o are available from the authors.
References
- 1.Kimura I. Medical benefits of using natural compounds and their derivatives having multiple pharmacological actions. Yakugaku Zasshi. 2006;126:133–143. doi: 10.1248/yakushi.126.133. [DOI] [PubMed] [Google Scholar]
- 2.Manssour Fraga C.A., Barreiro E.J. New insights for multifactorial disease therapy: The challenge of the symbiotic drugs. Curr. Drug Ther. 2008;3:1–13. doi: 10.2174/157488508783331225. [DOI] [Google Scholar]
- 3.Gilroy D.W., Lawrence T., Perretti M., Rossi A.G. Inflammatory resolution: New opportunities for drug discovery. Nat. Rev. Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 4.Edwards I.R., Aronson J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 5.Viegas-Junior C., Danuello A., da Silva Bolzani V., Barreiro E.J., Fraga C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007;14:1829–1852. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Sun Y., Guo Y., Wang Z., Huang L., Li X. Dual functional cholinesterase and MAO inhibitors for the treatment of Alzheimer’s disease: Synthesis, pharmacological analysis and molecular modeling of homoisoflavonoid derivatives. J. Enzym. Inhib. Med. Chem. 2016;31:389–397. doi: 10.3109/14756366.2015.1024675. [DOI] [PubMed] [Google Scholar]
- 7.Seo Y.H. Dual inhibitors against topoisomerases and histone deacetylases. J. Cancer Prev. 2015;20:85. doi: 10.15430/JCP.2015.20.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Özkay Y., Tunalı Y., Karaca H., Işıkdağ İ. Antimicrobial activity of a new combination system of benzimidazole and various azoles. Arch. Pharm. 2011;344:264–271. doi: 10.1002/ardp.201000172. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S.V., Subramanian M.R., Chinnaiyan S.K. Synthesis, characterisation and evaluation of N-mannich bases of 2-substituted Benzimidazole derivatives. J. Young Pharm. 2013;5:154–159. doi: 10.1016/j.jyp.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuldipsinh P.B., Stoyanka N., Ivanov I., Manjunath D.G. Novel research strategies of benzimidazole derivatives: A review. Mini Rev. Med. Chem. 2013;13:1421–1447. [PubMed] [Google Scholar]
- 11.Qu Z., Li F., Xing C., Jiang L. Synthesis and antifungal activity of novel benzimidazol-2-ylcyanoketone oxime ethers containing morpholine moiety. Chin. J. Org. Chem. 2015;10:028. doi: 10.6023/cjoc201504018. [DOI] [Google Scholar]
- 12.Vyas M., Swarnkar N., Mehta S., Joshi H., Ameta R., Punjabi P.B. Microwave assisted synthesis and biological assay of 2-substituted [(morpholin-4-yl/4-methylpiperazin-1-yl)methyl]-1H-benzimidazoles using acidic alúmina as solid suport. Afinidad. 2008;65:537. [Google Scholar]
- 13.Shanmugapandiyan P., Denshing K.S., Ilavarasan R., Anbalagan N., Nirmal R. Synthesis and biological activity of 2-(thiazolidin-4-one)phenyl]-1H-phenylbenzimidazoles and 2-[4-(azetidin-2-one)-3-chloro-4-phenyl]-1H-phenyl benzimidazoles. IJPSDR. 2010;2:115–119. [Google Scholar]
- 14.Eisa H.M., Barghash A.E.M., Badr S.M., Farahat A.A. Synthesis and antimicrobial activity of certain benzimidazole and fused benzimidazole derivatives. Indian J. Chem. 2010;49:1515. [Google Scholar]
- 15.Tunçbilek M., Kiper T., Altanlar N. Synthesis and in vitro antimicrobial activity of some novel substituted benzimidazole derivatives having potent activity against MRSA. Eur. J. Med. Chem. 2009;44:1024–1033. doi: 10.1016/j.ejmech.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Patil D.N., Chaturvedi S.C., Kale D.L., Kakde R.B., Dhiker S.B. Synthesis and antimicrobial activity of some benzimidazole derivatives. Cont. J. Pharm. Sci. 2008;2:44–48. [Google Scholar]
- 17.Gill C., Jadhav G., Shaikh M., Kale R., Ghawalkar A., Nagargoje D., Shiradkar M. Clubbed [1,2,3] triazoles by fluorine benzimidazole: A novel approach to H37Rv inhibitors as a potential treatment for tuberculosis. Bioorg. Med. Chem. Lett. 2008;18:6244–6247. doi: 10.1016/j.bmcl.2008.09.096. [DOI] [PubMed] [Google Scholar]
- 18.Wang M., Han X., Zhou Z. New substituted benzimidazole derivatives: A patent review (2013–2014) Expert. Opin. Ther. Pat. 2015;25:595–612. doi: 10.1517/13543776.2015.1015987. [DOI] [PubMed] [Google Scholar]
- 19.Naim M.J., Alam O., Alam M.J., Alam P., Shrivastava N. A review on pharmacological profile of morpholine derivatives. Int. J. Pharmacol. Pharm. Sci. 2015;3:40–51. [Google Scholar]
- 20.Sameem B., Saeedi M., Mahdavi M., Nadri H., Moghadam F.H., Edraki N., Khan M.I., Amini M. Synthesis, docking study and neuroprotective effects of some novel pyrano [3, 2-c] chromene derivatives bearing morpholine/phenylpiperazine moiety. Bioorg. Med. Chem. 2017;25:3980–3988. doi: 10.1016/j.bmc.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Polshettiwar V., Varma R.S. Greener and expeditious synthesis of bioactive heterocycles using microwave irradiation. Pure Appl. Chem. 2008;80:777–790. doi: 10.1351/pac200880040777. [DOI] [Google Scholar]
- 22.Yoon Y.K., Ali M.A., Wei A.C., Choon T.S., Khaw K.Y., Murugaiyah V., Osman H., Masand V.H. Synthesis, characterization, and molecular docking analysis of novel benzimidazole derivatives as cholinesterase inhibitors. Bioorg. Chem. 2013;49:33–39. doi: 10.1016/j.bioorg.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Coban G., Carlino L., Tarikogullari A.H., Parlar S., Sarıkaya G., Alptüzün V., Alpan A.S., Güneş H.S., Erciyas E. 1H-benzimidazole derivatives as butyrylcholinesterase inhibitors: Synthesis and molecular modeling studies. Med. Chem. Res. 2016;25:2005–2014. doi: 10.1007/s00044-016-1648-1. [DOI] [Google Scholar]
- 24.Menteşe E., Ülker S., Kahveci B. Synthesis and study of α-glucosidase inhibitory, antimicrobial and antioxidant activities of some benzimidazole derivatives containing triazole, thiadiazole, oxadiazole, and morpholine rings. Chem. Heterocycl. Compd. 2015;50:1671–1682. doi: 10.1007/s10593-015-1637-1. [DOI] [Google Scholar]
- 25.Zhou S., Li F., Zhang P., Jiang L. Synthesis and antifungal activity of novel 1-(1H-benzoimidazol-1-yl) propan-2-one oxime-ethers containing the morpholine moiety. Res. Chem. Intermed. 2013;39:1735–1743. doi: 10.1007/s11164-012-0708-5. [DOI] [Google Scholar]
- 26.Zhang P., Wan F., Li Y., Li C., Jiang L. Synthesis and antibacterial activity of novel ethyl 2-alkoxyimino-2-benzimidazol-2-yl acetates bearing a morpholine group. Res. Chem. Intermed. 2015;41:3349–3357. doi: 10.1007/s11164-013-1437-0. [DOI] [Google Scholar]
- 27.Achar K.C., Hosamani K.M., Seetharamareddy H.R. In Vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem. 2010;45:2048–2054. doi: 10.1016/j.ejmech.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 28.El-Nezhawy A.O., Biuomy A.R., Hassan F.S., Ismaiel A.K., Omar H.A. Design, synthesis and pharmacological evaluation of omeprazole-like agents with anti-inflammatory activity. Bioorg. Med. Chem. 2013;21:1661–1670. doi: 10.1016/j.bmc.2013.01.070. [DOI] [PubMed] [Google Scholar]
- 29.Jesudason E.P., Sridhar S.K., Malar E.P., Shanmugapandiyan P., Inayathullah M., Arul V., Selvaraj D., Jayakumar R. Synthesis, pharmacological screening, quantum chemical and in vitro permeability studies of N-Mannich bases of benzimidazoles through bovine cornea. Eur. J. Med. Chem. 2009;44:2307–2312. doi: 10.1016/j.ejmech.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 30.Gaba M., Singh D., Singh S., Sharma V., Gaba P. Synthesis and pharmacological evaluation of novel 5-substituted-1-(phenylsulfonyl)-2-methylbenzimidazole derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010;45:2245–2249. doi: 10.1016/j.ejmech.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 31.Reddy B.A. Synthesis, characterization and biological evaluation of 1,2-disubstituted benzimidazole derivatives using Mannich bases. J. Chem. 2010;7:222–226. [Google Scholar]
- 32.Mathew B., Suresh J., Anbazhagan S. Development of novel (1-H) benzimidazole bearing pyrimidine-trione based MAO-A inhibitors: Synthesis, docking studies and antidepressant activity. J. Saudi Chem. Soc. 2016;20(Suppl. 1):S132–S139. doi: 10.1016/j.jscs.2012.09.015. [DOI] [Google Scholar]
- 33.Van den Berg D., Zoellner K.R., Ogunrombi M.O., Malan S.F., Terre’Blanche G., Castagnoli N., Bergh J.J., Petzer J.B. Inhibition of monoamine oxidase B by selected benzimidazole and caffeine analogues. Bioorg. Med. Chem. 2007;15:3692–3702. doi: 10.1016/j.bmc.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 34.Petzer J.P., Steyn S., Castagnoli K.P., Chen J.F., Schwarzschild M.A., Van der Schyf C.J., Castagnoli N. Inhibition of monoamine oxidase B by selective adenosine A 2A receptor antagonists. Bioorg. Med. Chem. 2003;11:1299–1310. doi: 10.1016/S0968-0896(02)00648-X. [DOI] [PubMed] [Google Scholar]
- 35.Grandi T., Sparatore F., Gnerre C., Crivori P., Carrupt P.A., Testa B. Monoamine oxidase inhibitory properties of some benzazoles: Structure-; Activity relationships. AAPS J. 1999;1:1–4. doi: 10.1208/ps010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shukla J.S., Saxena S., Rastogi R. Synthesis of some newer, 1-heterocyclic amino/iminomethyl-2-substituted benzimidazoles as a potent CNS, anticonvulsant and monoamine oxidase inhibitory agents. Curr. Sci. 1982;51:820–822. [Google Scholar]
- 37.Avramova P., Danchev N., Buyukliev R., Bogoslovova T. Synthesis, toxicological, and pharmacological assessment of derivatives of 2-aryl-4-(3-arylpropyl) morpholines. Arch. Pharm. 1998;331:342–346. doi: 10.1002/(SICI)1521-4184(199811)331:11<342::AID-ARDP342>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Ghanbarpour A., Hadizadeh F., Piri F., Rashidi-Ranjbar P. Synthesis, conformational analysis and antidepressant activity of moclobemide new analogues. Pharm. Acta Helv. 1997;72:119–122. doi: 10.1016/S0031-6865(97)00004-6. [DOI] [PubMed] [Google Scholar]
- 39.Avramova P.D., Danchev N.D., Buyukliev R.T. Synthesis, toxicological and pharmacological assessment of esters of carbonic and carbamic acids with 2-aryl-4-hydroxyethylmorpholines. Eur. J. Med. Chem. 1996;31:909–914. doi: 10.1016/S0223-5234(97)89855-8. [DOI] [Google Scholar]
- 40.Bekhit A.A., Abdel-Aziem T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem. 2004;12:1935–1945. doi: 10.1016/j.bmc.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 41.Bekhit A.A., Ashour H.M., Ghany Y.S.A., Bekhit A.E.D.A., Baraka A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur. J. Med. Chem. 2008;43:456–463. doi: 10.1016/j.ejmech.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Yanez M., Vina D. Dual inhibitors of monoamine oxidase and cholinesterase for the treatment of Alzheimer disease. Curr. Top. Med. Chem. 2013;3:1692–1706. doi: 10.2174/15680266113139990120. [DOI] [PubMed] [Google Scholar]
- 43.Secci D., Bolasco A., D’Ascenzio M., della Sala F., Yáñez M., Carradori S. Conventional and microwave-assisted synthesis of benzimidazole derivatives and their in vitro inhibition of human cyclooxygenase. J. Heterocycl. Chem. 2012;49:1187–1195. doi: 10.1002/jhet.1058. [DOI] [Google Scholar]
- 44.Sakram B., Rambabu S., Ashok K., Sonyanaik B., Ravi D. Microwave assisted aqueous phase synthesis of benzothiazoles and benzimidazoles in the presence of Ag2O. Russ. J. Gen. Chem. 2016;86:2737–2743. doi: 10.1134/S1070363216120343. [DOI] [Google Scholar]
- 45.Alp A.S., Kılcıgil G., Özdamar E.D., Çoban T., Eke B. Synthesis and evaluation of antioxidant activities of novel 1,3,4-oxadiazole and imine containing 1H-benzimidazoles. Turk. J. Chem. 2015;39:42–53. doi: 10.3906/kim-1403-44. [DOI] [Google Scholar]
- 46.Kathirvelan D., Yuvaraj P., Babu K., Nagarajan A.S., Reddy B.S. A green synthesis of benzimidazoles. Indian J. Chem. 2013;52:1152–1156. [Google Scholar]
- 47.Hasanpour M., Eshghi H., Bakavoli M., Mirzaeia M. A novel ionic liquid based on imidazolium cation as an efficient and reusable catalyst for the one-pot synthesis of benzoxazoles, benzthiazoles, benzimidazoles and 2-arylsubstituted benzimidazoles. J. Chin. Chem. Soc. 2015;62:412–419. doi: 10.1002/jccs.201400372. [DOI] [Google Scholar]
- 48.Alapati M.L.P.R., Abburi S.R., Mukkamala S.B., Krishnaji Rao M. Simple and efficient one-pot synthesis of 2-substituted benzimidazoles from θ-diaminoarene and aryl aldehydes. Synth. Commun. 2015;45:2436–2443. doi: 10.1080/00397911.2015.1083581. [DOI] [Google Scholar]
- 49.Vinodkumar R., Rajagopal K., Devanna N. Synthesis of highly functionalized 2-(substituted biphenyl) benzimidzoles via suzuki-miyaura cross coupling reaction. J. Heterocycl. Chem. 2007;44:1521–1523. doi: 10.1002/jhet.5570440645. [DOI] [Google Scholar]
- 50.Fathima N., Krishnamurthy M.S., Begum N.S. 2-[4-(Trifluoromethoxy) phenyl]-1H-benzimidazole. Acta Crystallogr. Sect. E Struct. Rep. Online. 2013;69:264. doi: 10.1107/S1600536813001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.BioVision Inc. COX-1 Inhibitor Screening Kit Guide (Fluorometric) BioVision; Milpitas, CA, USA: [(accessed on 17 August 2017)]. Catalog K548-100. Available online: http://www.biovision.com/documentation/datasheets/K548.pdf. [Google Scholar]
- 52.BioVision Inc. COX-2 Inhibitor Screening Kit Guide (Fluorometric) BioVision; Milpitas, CA, USA: [(accessed on 17 August 2017)]. Catalog K547-100. Available online: http://www.biovision.com/documentation/datasheets/K547.pdf. [Google Scholar]
- 53.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto T., Suzuki O., Furuta T., Asai M., Kurokawa Y., Nimura Y., Katsumata Y., Takahashi I. A sensitive fluorometric assay for serum monoamine oxidase with kynuramine as substrate. Clin. Biochem. 1985;18:126–129. doi: 10.1016/S0009-9120(85)80094-1. [DOI] [PubMed] [Google Scholar]
- 55.Flückiger-Isler S., Kamber M. Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames pre-incubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012;747:36–45. doi: 10.1016/j.mrgentox.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Calculation of Molecular Properties and Bioactivity Score Software, Molinspiration Cheminformatics. [(accessed on 17 August 2017)]; Available online: http://www.molinspiration.com/cgi-bin/properties.
- 57.Drug-Likeness and Molecular Property Prediction Software, Molsoft LLC. [(accessed on 17 August 2017)]; Available online: http://molsoft.com/mprop/
- 58.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard-Ninth ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. CLSI document M07-A9. [Google Scholar]
- 59.Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) EUCAST definitive Document EDef 7.1: Method for the Determination of Broth Dilution MICs of Antifungal Agents for Fermentative Yeasts. Clin. Microbiol. Infect. 2008;14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 60.Can Ö.D., Osmaniye D., Demir Özkay Ü., Sağlık B.N., Levent S., Ilgın S., Baysal M., Özkay Y., Kaplancıklı Z.A. MAO enzymes inhibitory activity of new benzimidazole derivatives including hydrazone and propargyl side chains. Eur. J. Med. Chem. 2017;131:92–106. doi: 10.1016/j.ejmech.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Yurttaş L., Özkay Y., Akalın-Çiftçi G., Ulusoylar-Yıldırım Ş. Synthesis and anticancer activity evaluation of N-[4-(2-methylthiazol-4-yl)phenyl] acetamide derivatives containing (benz) azole moiety. J. Enzym. Inhib. Med. Chem. 2014;29:175–184. doi: 10.3109/14756366.2013.763253. [DOI] [PubMed] [Google Scholar]
- 62.Patel S., Gheewala N., Suthar A., Shah A. In Vitro cytotoxicity activity of Solanum nigrum extract against Hela cell line and Vero cell line. Int. J. Pharm. Pharm. Sci. 2009;1:38–46. [Google Scholar]
- 63.Demir Özkay Ü., Can Ö.D., Sağlık B.N., Acar Çevik U., Levent S., Özkay Y., Ilgın S., Atlı Ö. Design, synthesis, and AChE inhibitory activity of new benzothiazole-piperazines. Bioorg. Med. Chem. Lett. 2016;26:5387–5394. doi: 10.1016/j.bmcl.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 64.Chandrasekaran C.V., Sundarajan K., Gupta A., Srikanth H.S., Edwin J., Agarwal A. Evaluation of the genotoxic potential of standardized extract of Glycyrrhiza glabra (GutGard™) Regul. Toxicol. Pharmacol. 2011;61:373–380. doi: 10.1016/j.yrtph.2011.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.