Abstract
Objective
To report patient-reported outcomes of patients with PsA treated with ixekizumab up to 52 weeks.
Methods
In SPIRIT-P1, biologic-naïve patients with active PsA were randomized to ixekizumab 80 mg every 4 weeks (IXEQ4W; N = 107) or every 2 weeks (IXEQ2W; N = 103) following a 160 mg starting dose, adalimumab 40 mg every 2 weeks (ADA; N = 101) or placebo (PBO; N = 106) during the initial 24-week double-blind treatment period. At week 24 (week 16 for inadequate responders), ADA (8-week washout before starting ixekizumab) and PBO patients were re-randomized to IXEQ2W or IXEQ4W. Patients receiving ixekizumab at week 24 received the same dose during the extension period (EP) to week 52. Patients completed measures including the Dermatology Life Quality Index (DLQI), Itch Numeric Rating Scale, 36-Item Short Form Health Survey version 2, European Quality of Life 5 Dimensions Visual Analogue Scale and Work Productivity and Activity Impairment Questionnaire-Specific Health Problem.
Results
The IXEQ4W, IXEQ2W and ADA groups reported significant improvements in DLQI at week 24; 22% (PBO), 53% (IXEQ4W), 63% (IXEQ2W) and 54% (ADA) of patients reported DLQI scores of 0/1. The IXEQ4W, IXEQ2W and ADA groups reported significant improvements in Itch Numeric Rating Scale, 36-Item Short Form Health Survey version 2 physical component summary and some domain scores, and European Quality of Life 5 Dimensions Visual Analogue Scale at weeks 12 and 24; and in three of four Work Productivity and Activity Impairment Questionnaire-Specific Health Problem domains at week 24. Results are also presented through week 52 for the EP.
Conclusion
In biologic-naïve patients with active PsA, ixekizumab significantly improved skin symptoms, health-related quality of life and work productivity.
Trial Registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT01695239; EU Clinical Trials Register, https://www.clinicaltrialsregister.eu, EudraCT2011-002326-49
Keywords: DMARDs (biologic), psoriatic arthritis, treatment, outcomes research, ixekizumab
Rheumatology key messages
Ixekizumab-treated patients with PsA reported improved skin-related symptoms, work productivity and quality of life through 52 weeks.
Introduction
PsA is a progressive, immune-mediated, chronic inflammatory arthritis associated with psoriasis [1]. PsA has several manifestations in addition to psoriasis including peripheral arthritis, spondylitis, enthesitis and dactylitis [1]. Patients report significant pain and functional work impairment [2]. The negative impact of PsA extends to physical and emotional aspects of patients’ lives measured by several patient-reported outcomes (PROs) [3]. Patients with PsA report poorer health-related quality of life (HRQOL) compared with both the general population and patients with psoriasis alone [3–5]. Psoriasis-associated itch can substantially impact HRQOL, even in patients with mild disease [6, 7]. Thus, skin and joint diseases contribute additively to the physical and emotional burden of PsA [8].
The current standard of care for PsA includes NSAIDs, glucocorticosteroids, conventional DMARDs (cDMARDs), biologic DMARDs (bDMARDs) and targeted synthetic DMARDs [9, 10]. TNF inhibitors are frequently used for first-line bDMARD therapy [9, 11, 12], but many patients with PsA experience inadequate response (IR) while others become resistant or intolerant to treatment and continue to accrue further physical impairment. Other currently approved bDMARDs for PsA include IL-12/23- (ustekinumab) and IL-17A- (secukinumab) blocking agents, and a T cell co-stimulation modulator (abatacept) [9, 11–13]. Currently approved bDMARDs control disease activity and improve physical function, HRQOL and work productivity in patients with PsA [14–19]. Other agents that are approved for PsA include apremilast [9, 12] and tofacitinib [20].
Ixekizumab is a high-affinity mAb that selectively targets IL-17A [21] and was recently approved for treatment of PsA. SPIRIT-P1 (NCT01695239; EudraCT 2011-002326-49) is a phase 3 randomized, controlled trial (RCT) of ixekizumab in bDMARD-naïve patients with active PsA [22]. Ixekizumab met the primary end point of a significant proportion of patients achieving an ACR 20 response at week 24 relative to placebo [22]. Additionally, other measures of disease activity and physical function, including the proportions of patients achieving ACR 50 and Psoriasis Area Severity Index (PASI) 75/90/100 responses were significantly improved with ixekizumab at weeks 12 and 24 with significantly less radiographic progression at week 24 compared with placebo [22]. The proportion of patients achieving an ACR 70 response was significantly higher vs placebo at week 24 [22]. Likewise, improvements in 28-Joint DAS using CRP (DAS-28 CRP), HAQ Disability Index (HAQ-DI), the Short Form (36 Items) Health Survey Physical Component Score and the Joint Pain Visual Analogue Scale (VAS) score were observed in ixekizumab compared with placebo at weeks 12 and 24 [22]. The objective of these analyses is to report PROs reflective of the various manifestations of PsA that have not been previously reported, including skin disease, HRQOL and work productivity, in patients with PsA treated with ixekizumab for up to 52 weeks.
Methods
Patients
Biologic DMARD-naïve adults (aged ⩾18 years) with an established diagnosis of PsA for ⩾6 months were eligible [22]. Patients fulfilled Classification Criteria for Psoriatic Arthritis [23], had ⩾3 of 68 tender and ⩾3 of 66 swollen joint counts and had either ⩾1 PsA-related hand or foot joint erosion on centrally read X-rays or CRP > 6 mg/l at baseline [22]. All patients provided written informed consent for study participation.
Study and design
Details of the SPIRIT-P1 Double-blind Treatment Period (DBTP) were previously reported [22]. Briefly, SPIRIT-P1 is a 3-year, phase 3, randomized, double-blind, placebo- and active-controlled trial comparing ixekizumab (Taltz; Eli Lilly and Company) 80 mg every 4 weeks (IXEQ4W), ixekizumab 80 mg every 2 weeks (IXEQ2W) and adalimumab (Humira; AbbVie) 40 mg every 2 weeks (ADA) with placebo (PBO) [22]. Patients in both ixekizumab study arms received a 160 mg starting dose. Patients were randomized 1:1:1:1 (supplementary Fig. S1, available at Rheumatology online) to PBO, ADA, IXEQ4W or IXEQ2W. Randomization was stratified by country and cDMARD use (naïve, past use, current use). The first 24 weeks of SPIRIT-P1 was the DBTP. At week 16, IRs (based on blinded criteria) in any treatment group were required to add or modify concomitant medications. Patients who received ADA and were deemed IR at week 16 were re-randomized 1:1 to IXEQ2W or IXEQ4W and received their first dose of ixekizumab at week 24 (160 mg starting dose), after an 8-week washout period with PBO; they were considered the ADA/IXEQ4W and ADA/IXEQ2W groups in the extension period (EP; from weeks 24 to 52). Patients who received PBO and were deemed IR at week 16 were re-randomized 1:1 to IXEQ2W or IXEQ4W and initiated ixekizumab treatment at week 16 (160 mg starting dose); they were considered the PBO/IXEQ4W and PBO/IXEQ2W groups in the EP.
Patients completing week 24 entered the EP. In this period, patients originally assigned to IXEQ4W and IXEQ2W continued the same dosing regimen during the EP and are referred to as the IXEQ4W/IXEQ4W and IXEQ2W/IXEQ2W groups. Patients who remained on PBO or ADA at week 24 were re-randomized 1:1 to receive IXEQ2W or IXEQ4W during the EP and became part of the PBO/IXEQ4W, PBO/IXEQ2W, ADA/IXEQ4W or ADA/IXEQ2W groups, as appropriate. Patients re-randomized from ADA at week 24 received their first dose of ixekizumab at week 32, after an 8-week washout period, during which time they received PBO. Patients were evaluated for lack of response (failure to demonstrate ⩾20% improvement from baseline in both tender and swollen joint counts) at each study visit beginning at week 32 and discontinued from the study if they demonstrated lack of response.
The RCT was compliant with ethical guidelines including the Declaration of Helsinki and other relevant laws and regulations. The protocol was approved by each site’s ethical review committee/institutional review board and all patients provided written informed consent.
Assessments
Patients were assessed using the Dermatology Life Quality Index (DLQI) [24–26], Itch Numeric Rating Scale (NRS) [27], 36-Item Short Form Health Survey version 2 (SF-36) [28, 29], European Quality of Life 5 Dimensions (EQ-5D) VAS [30] and Work Productivity and Activity Impairment Questionnaire-Specific Health Problem (WPAI-SHP) [31, 32]. For these assessments, reported minimally clinically important differences (MCID) in scores for each PRO are shown in supplementary Table S1, available at Rheumatology online. HAQ-DI and Joint Pain VAS were also assessed [22]. Age- and gender-matched normative values for the SF-36 were derived from the general 1998 US population [33]. The DLQI was not measured beyond week 24 in this study.
Outcome measures previously reported, including improvements in physical function (HAQ-DI) to weeks 24 and 52 [22, 34], Joint Pain VAS to week 24 [22] and SF-36 physical component summary (PCS) score at weeks 12 and 24 [22], are included to provide further context and for completeness given the focus of the current analyses.
Statistical analyses
For the DBTP (weeks 0–24), the intent-to-treat population was analysed; for a subset of this population, patients who had psoriasis over ⩾3% of their body surface area (BSA), skin-related measures were also analysed. For the EP (after weeks 24–52), the population was defined as all patients receiving one or more doses of study treatment during this period. The statistical analyses were performed using SAS Version 9.4 or higher (SAS Institute, Cary, NC, USA).
Baseline was the last non-missing value on or before the date of first injection of study treatment at week 0. Observed data after week 16 for week 16 inadequate responders were excluded during the DBTP. During the DBTP, treatment comparisons of continuous efficacy and categorical variables were made using an analysis of covariance and logistic regression model, respectively, with treatment, geographic region [Europe (Belgium, France, Netherlands, Spain, UK, Bulgaria, Czech Republic, Estonia, Poland, Russia and Ukraine) and the rest of the world (USA, Canada, Mexico and Japan)], baseline value (analysis of covariance model only) and cDMARD experience at baseline in the model. For inadequate responders or patients discontinuing treatment before week 24, missing values were imputed by non-responder imputation for categorical data and last observation carried forward for continuous data. The study was not designed to make comparisons between either ixekizumab group and ADA or to make comparisons between the IXEQ4W and IXEQ2W groups. Descriptive statistics are reported for the EP.
The association between skin clearance (PASI percentage improvement) and improvement in HRQOL (DLQI score of 0/1; Itch NRS = 0) were pre-specified exploratory analyses, investigated by comparing the number and percentage of patients with DLQI 0/1 or Itch NRS = 0 among different levels of PASI improvements using a logistic model with PASI group in the model.
Results
As previously reported, 417 patients were randomized and comprise the intent-to-treat population of the DBTP [22]. Most patients were white (94%), female (54%) and receiving cDMARD therapy (64%) at the time of randomization (Table 1). Of randomized patients, 381 comprised the EP. Patient disposition for the DBTP was previously reported [22].
Table 1.
Baseline characteristics: weeks 0–24 (double-blind treatment period; intent-to-treat population)a
| Characteristics | PBO (N = 106) | ADA (N = 101) | IXEQ4W (N = 107) | IXEQ2W (N = 103) | Total (N = 417) |
|---|---|---|---|---|---|
| Age, years | 50.6 (12.3) | 48.6 (12.4) | 49.1 (10.1) | 49.8 (12.6) | 49.5 (11.9) |
| Male, n (%) | 48 (45.3) | 51 (50.5) | 45 (42.1) | 48 (46.6) | 192 (46.0) |
| Weight, kg | 83.8 (19.6) | 91.6 (21.9) | 85.5 (23.0) | 81.6 (17.5) | 85.6 (20.9) |
| Race, n (%) | |||||
| American Indian/Alaska Native | 2 (1.9) | 3 (3.0) | 2 (1.9) | 2 (1.9) | 9 (2.2) |
| Asian | 5 (4.7) | 3 (3.0) | 2 (1.9) | 5 (4.9) | 15 (3.6) |
| White | 99 (93.4) | 95 (94.1) | 102 (95.3) | 96 (93.2) | 392 (94.0) |
| Multiple | 0 | 0 | 1 (0.9) | 0 | 1 (0.2) |
| Conventional DMARD use, n (%) | |||||
| Naïve | 13 (12.3) | 14 (13.9) | 17 (15.9) | 17 (16.5) | 61 (14.6) |
| Past use | 24 (22.6) | 20 (19.8) | 22 (20.6) | 23 (22.3) | 89 (21.3) |
| Current use | 69 (65.1) | 67 (66.3) | 68 (63.6) | 63 (61.2) | 267 (64.0) |
| Current MTX use, n (%) | 59 (55.7) | 57 (56.4) | 57 (53.3) | 53 (51.5) | 226 (54.2) |
| Time since PsA diagnosis, years | 6.3 (6.9) | 6.9 (7.5) | 6.2 (6.4) | 7.2 (8.0) | 6.7 (7.2) |
| Tender joint count (68 joints) | 19.2 (13.0) | 19.3 (13.0) | 20.5 (13.7) | 21.5 (14.1) | 20.1 (13.4) |
| Swollen joint count (66 joints) | 10.6 (7.3) | 9.9 (6.5) | 11.4 (8.2) | 12.1 (7.2) | 11.0 (7.4) |
| Joint Pain Visual Analogue Scale | 58.5 (23.0) | 58.7 (19.7) | 60.1 (19.4) | 58.4 (21.7) | 58.9 (20.9) |
| HAQ-Disability Index | 1.2 (0.6) | 1.1 (0.6) | 1.2 (0.5) | 1.2 (0.6) | 1.2 (0.6) |
| Patients with current psoriasis, n (%) | 102 (96.2) | 97 (96.0) | 100 (93.5) | 95 (92.2) | 394 (94.5) |
| Body surface area ≥3%, n (%)b | 67 (67.7) | 68 (72.3) | 73 (73.0) | 59 (64.8) | 267 (69.5) |
| Psoriasis Area and Severity Indexc | 6.2 (7.5) | 5.5 (6.5) | 6.9 (6.6) | 6.0 (7.0) | 6.1 (6.9) |
| Dermatology Life Quality Indexb | 7.5 (5.6) | 6.7 (7.0) | 8.0 (6.0) | 8.8 (5.9) | 7.7 (6.2) |
| SF-36 Physical Component Summary | 34.0 (8.3) | 33.9 (8.8) | 32.4 (10.1) | 34.2 (8.7) | 33.6 (9.0) |
| SF-36 Mental Component Summary | 47.4 (12.5) | 46.6 (11.7) | 46.5 (13.4) | 48.0 (9.8) | 47.1 (11.9) |
| SF-36 domain scores | |||||
| Physical functioning | 46.9 (25.5) | 46.0 (25.5) | 41.5 (24.4) | 46.3 (25.7) | 45.1 (25.3) |
| Role physical | 47.3 (23.5) | 45.5 (24.9) | 43.3 (26.0) | 49.8 (25.1) | 46.5 (24.9) |
| Bodily pain | 40.2 (19.1) | 41.3 (18.1) | 37.9 (18.6) | 41.3 (17.8) | 40.2 (18.4) |
| General health | 45.3 (17.7) | 45.2 (18.7) | 44.3 (18.7) | 46.2 (17.6) | 45.2 (18.1) |
| Vitality | 46.2 (21.1) | 42.9 (21.9) | 40.0 (20.5) | 45.2 (19.9) | 43.6 (20.9) |
| Social functioning | 64.6 (27.3) | 65.2 (26.8) | 65.5 (27.4) | 68.4 (22.9) | 65.9 (26.1) |
| Mental health | 65.4 (21.7) | 64.9 (20.3) | 62.0 (22.2) | 66.6 (16.7) | 64.7 (20.4) |
| Role emotional | 74.6 (27.3) | 71.9 (26.6) | 73.3 (26.1) | 74.4 (23.2) | 73.6 (25.8) |
| EQ-5D VAS | 53.9 (22.3) | 56.5 (19.9) | 54.1 (20.6) | 57.1 (19.2) | 55.4 (20.5) |
| Itch Numeric Rating Scaled | 4.6 (2.4) | 4.3 (2.8) | 4.6 (2.5) | 5.1 (2.6) | 4.6 (2.6) |
| WPAI-SH | |||||
| Absenteeism | 8.9 (24.5) | 8.7 (21.4) | 9.2 (21.0) | 7.7 (23.0) | 8.6 (22.3) |
| Presenteeism | 32.4 (21.2) | 37.3 (24.5) | 40.0 (26.7) | 35.8 (21.6) | 36.6 (23.8) |
| Work productivity | 34.6 (23.4) | 40.6 (25.2) | 42.3 (28.5) | 37.4 (21.6) | 38.9 (24.9) |
| Activity impairment | 46.1 (24.7) | 46.9 (26.0) | 47.9 (26.3) | 47.1 (23.4) | 47.0 (25.1) |
Unless indicated, values are mean (s.d.).
Data from: Mease PJ et al. Ann Rheum Dis 2017;76:79–87.
Patients with psoriasis and body surface area measured at baseline.
Patients with psoriasis.
Patients with baseline psoriatic lesions ≥3% of body surface area. ADA: Adalimumab; IXEQ4W/Q2W: ixekizumab every 4 or 2 weeks; EQ-5D VAS: European Quality of Life 5 Dimensions Visual Analogue Scale; N: population size; n: number in group; PBO: placebo; SF-36: 36-Item Short Form Health Survey; WPAI-SH: Work Productivity and Activity Impairment-Specific Health Problem.
Of patients entering the EP, 191 and 190 were assigned to the Total IXEQ4W group (PBO/IXEQ4W, ADA/IXEQ4W and IXEQ4W/IXEQ4W) and the Total IXEQ2W group (PBO/IXEQ2W, ADA/IXEQ2W and IXEQ2W/IXEQ2W), respectively (supplementary Fig. S1 and supplementary Table S2, available at Rheumatology online). Baseline characteristics among the six treatment groups of the EP are shown in supplementary Table S2, available at Rheumatology online.
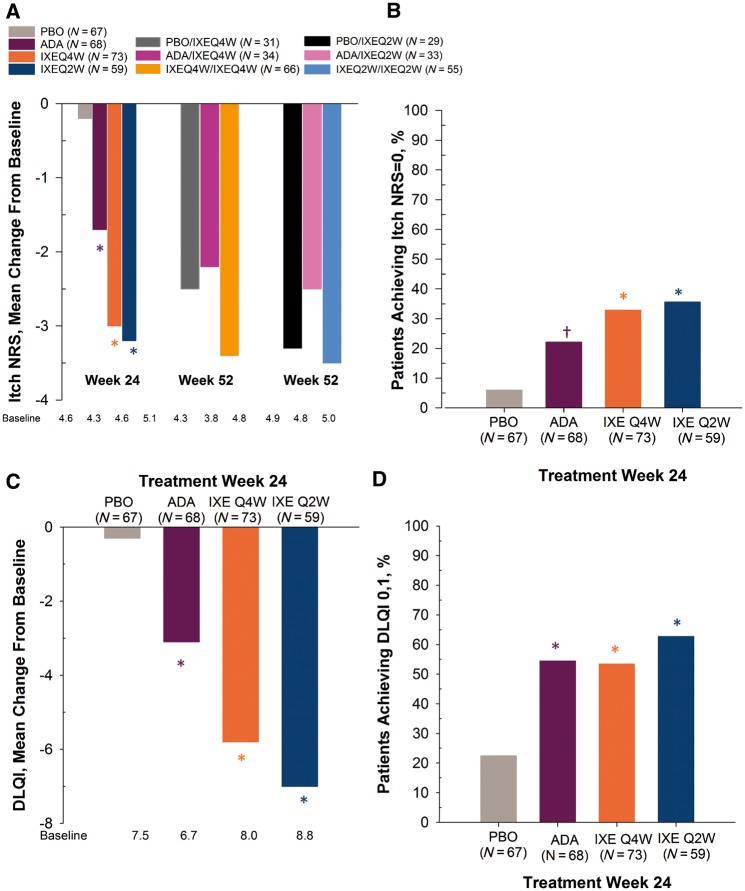
Patient-reported physical functioning (HAQ-DI) and joint pain (VAS) were significantly improved in the ixekizumab groups compared with placebo at week 24 with improvements up to week 52 for HAQ-DI (Table 2) [22, 34]. In the subgroup of patients with psoriasis affecting ⩾3% BSA at baseline, patients in all active treatment groups reported significant improvement relative to placebo in the Itch NRS at week 24 (Fig. 1A and Table 2). Likewise, Itch NRS scores of the IXEQ4W/IXEQ4W and IXEQ2W/IXEQ2W groups were improved relative to baseline at week 52. Week 24 data demonstrates 6, 33, 36 and 22% of patients in PBO, IXEQ4W, IXEQ2W and ADA groups, respectively, reported no itch (Itch NRS = 0) (Fig. 1B and Table 2); 12.2, 54.2, 65.9 and 40.0% of patients (baseline ⩾3% BSA; Itch NRS ⩾4) treated with PBO, IXEQ4W, IXEQ2W and ADA, respectively, reported scores meeting/exceeding the MCID (⩾4 point reduction from baseline; validated in psoriasis but not in PsA) (P ⩽ 0.004 vs PBO) (data not shown).
Table 2.
Efficacy at weeks 12, 24 and 52
| Characteristics | Week 12 (intent-to-treat population) | Week 24 (intent-to-treat population) | Week 52 (extension period population) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBO (N = 106) | ADA (N = 101) | IXEQ4W (N = 107) | IXEQ2W (N = 103) | PBO (N = 106) | ADA (N = 101) | IXEQ4W (N = 107) | IXEQ2W (N = 103) | IXEQ4W/ IXEQ4W (N = 97) | IXEQ2W/ IXEQ2W (N = 96) | |
| Joint Pain Visual Analogue Scalea | −10.8 (21.0) | −27.9 (23.9) | −29.0 (26.2) | −30.0 (27.3) | −13.4 (25.1) | −30.8 (25.1) | −31.0 (28.8) | −33.0 (29.6) | −33.9 (28.3) | −34.4 (27.4) |
| — | <0.001 | <0.001 | <0.001 | — | <0.001 | <0.001 | <0.001 | — | — | |
| HAQ-Disability Indexa,b | −0.13 (0.45) | −0.35 (0.42) | −0.39 (0.51) | −0.48 (0.48) | −0.15 (0.50) | −0.37 (0.44) | −0.44 (0.53) | −0.50 (0.52) | −0.53 (0.56) | −0.55 (0.52) |
| — | <0.001 | <0.001 | <0.001 | — | <0.001 | <0.001 | <0.001 | — | — | |
| Dermatology Life Quality Indexc | NC | NC | NC | NC | −0.3 (6.1) | −3.1 (5.0) | −5.8 (6.3) | −7.0 (6.1) | NC | NC |
| — | — | — | — | — | <0.001 | <0.001 | <0.001 | — | — | |
| Itch Numeric Rating Scalec | 0.1 (2.1) | −1.4 (2.5) | −2.7 (2.6) | −3.2 (3.1) | −0.2 (2.3) | −1.7 (2.6) | −3.0 (2.9) | −3.2 (3.1) | −3.4 (2.5) | −3.5 (3.1) |
| — | <0.001 | <0.001 | <0.001 | — | <0.001 | <0.001 | <0.001 | — | — | |
| EQ-5D VAS | 3.9 (21.2) | 8.6 (22.8) | 12.7 (22.5) | 13.4 (24.5) | 3.3 (21.1) | 9.1 (22.1) | 11.3 (25.8) | 11.9 (25.0) | 14.9 (25.6) | 14.4 (21.2) |
| — | 0.020 | <0.001 | <0.001 | — | 0.013 | 0.004 | <0.001 | — | — | |
| SF-36 Physical Component Summarya | 2.3 (6.8) | 5.4 (7.5) | 6.2 (9.2) | 7.1 (7.6) | 2.7 (7.7) | 6.3 (8.3) | 7.7 (9.8) | 7.7 (8.6) | 9.5 (9.5) | 9.2 (9.4) |
| — | 0.003 | <0.001 | <0.001 | — | 0.002 | <0.001 | <0.001 | — | — | |
| SF-36 Mental Component Summary | 1.8 (9.8) | 4.0 (8.2) | 2.9 (11.4) | 3.2 (8.4) | 1.8 (9.5) | 4.6 (9.5) | 4.6 (11.9) | 3.1 (10.4) | 4.8 (11.9) | 3.4 (9.2) |
| — | 0.128 | 0.632 | 0.220 | — | 0.055 | 0.053 | 0.234 | — | — | |
| SF-36 domain | ||||||||||
| Physical functioning | 4.4 (18.2) | 12.8 (18.6) | 15.5 (21.4) | 16.6 (19.2) | 5.6 (18.4) | 14.4 (19.9) | 18.8 (23.5) | 17.1 (20.8) | 22.6 (22.1) | 20.8 (22.2) |
| — | 0.001 | <0.001 | <0.001 | — | 0.002 | <0.001 | <0.001 | — | — | |
| Role physical | 5.8 (20.6) | 12.3 (17.6) | 14.4 (21.8) | 16.5 (22.0) | 7.8 (20.9) | 16.4 (21.7) | 20.2 (25.3) | 17.2 (23.4) | 24.4 (24.7) | 22.2 (24.9) |
| — | 0.027 | 0.008 | <0.001 | — | 0.007 | <0.001 | <0.001 | — | — | |
| Bodily pain | 7.1 (18.0) | 15.4 (21.4) | 15.8 (22.1) | 17.8 (22.0) | 8.3 (19.8) | 16.8 (21.3) | 20.3 (24.2) | 20.0 (23.3) | 22.8 (25.1) | 22.6 (22.3) |
| — | 0.001 | 0.006 | <0.001 | — | 0.002 | <0.001 | <0.001 | — | — | |
| General health | 3.6 (13.6) | 9.4 (15.9) | 8.4 (17.5) | 9.8 (15.0) | 3.9 (15.8) | 10.4 (14.2) | 9.8 (19.4) | 11.3 (15.9) | 12.4 (18.1) | 11.3 (16.7) |
| — | 0.005 | 0.036 | 0.002 | — | 0.004 | 0.016 | <0.001 | — | — | |
| Vitality | 2.9 (16.4) | 11.2 (18.5) | 9.9 (19.1) | 11.5 (18.3) | 5.1 (17.0) | 11.5 (21.8) | 13.0 (24.7) | 11.4 (19.6) | 16.7 (21.8) | 13.7 (19.7) |
| — | 0.003 | 0.059 | <0.001 | — | 0.071 | 0.074 | 0.043 | — | — | |
| Social functioning | 8.2 (24.8) | 11.5 (22.4) | 9.6 (26.2) | 12.4 (24.6) | 8.9 (26.3) | 14.2 (22.4) | 14.8 (26.1) | 13.3 (23.1) | 17.3 (28.0) | 14.3 (22.5) |
| — | 0.256 | 0.623 | 0.060 | — | 0.051 | 0.025 | 0.031 | — | — | |
| Mental health | 3.5 (16.6) | 7.1 (14.7) | 8.0 (20.5) | 6.0 (14.7) | 4.4 (16.4) | 8.0 (18.6) | 9.4 (22.1) | 6.4 (17.0) | 10.9 (20.5) | 7.1 (15.5) |
| — | 0.098 | 0.154 | 0.181 | — | 0.136 | 0.141 | 0.296 | — | — | |
| Role emotional | 2.9 (20.6) | 9.7 (19.0) | 6.8 (20.6) | 10.1 (20.2) | 2.5 (20.9) | 11.9 (17.0) | 12.8 (23.5) | 9.8 (23.9) | 11.6 (24.9 | 11.5 (20.9) |
| — | 0.021 | 0.228 | 0.005 | — | 0.001 | <0.001 | 0.007 | — | — | |
| WPAI-SHP | ||||||||||
| Absenteeism | 0.4 (5.9) | −4.1 (17.7) | −6.5 (21.5) | −1.7 (10.8) | 1.5 (18.0) | −0.9 (29.3) | −6.9 (20.6) | 1.4 (28.5) | −8.3 (21.3) | −0.4 (22.7) |
| — | 0.267 | 0.074 | 0.252 | — | 0.993 | 0.133 | 0.948 | — | — | |
| Presenteeism | −4.4 (23.0) | −10.4 (18.1) | −17.5 (24.2) | −17.6 (23.5) | −3.1 (21.4) | −14.7 (19.3) | −19.8 (27.3) | −21.6 (24.4) | −23.8 (26.9) | −25.4 (21.3) |
| — | 0.258 | 0.013 | 0.004 | — | 0.016 | <0.001 | <0.001 | — | — | |
| Work Productivity | −5.0 (22.7) | −12.8 (20.6) | −18.2 ( 25.1) | −17.6 (24.3) | −3.7 (23.4) | −15.6 (22.5) | −20.9 (28.3) | −20.1 (26.3) | −25.4 (27.8) | −24.9 (22.9) |
| — | 0.189 | 0.021 | 0.006 | — | 0.036 | <0.001 | <0.001 | — | — | |
| Activity Impairment | −6.5 (22.3) | −13.1 (21.4) | −20.5 (27.4) | −22.9 (27.6) | −6.9 (25.0) | −16.6 (24.3) | −22.4 (32.0) | −25.8 (29.1) | −26.5 (26.7) | −29.1 (24.1) |
| — | 0.050 | <0.001 | <0.001 | — | 0.005 | <0.001 | <0.001 | — | — | |
| HAQ-Disability Index ≥0.35 response rate, %a | 29.3 | 49.4 | 49.0 | 64.4 | 26.1 | 49.4 | 49.0 | 57.8 | 57.1 | 57.1 |
| — | 0.006 | 0.006 | <0.001 | — | 0.001 | <0.001 | <0.001 | — | — | |
| Dermatology Life Quality Index = (0/ 1), %c | NC | NC | NC | NC | 22.4 | 54.4 | 53.4 | 62.7 | NC | NC |
| — | — | — | — | — | <0.001 | <0.001 | <0.001 | — | — | |
| Itch Numeric Rating Scale = 0, %c | 4.5 | 14.7 | 30.1 | 37.3 | 6.0 | 22.1 | 32.9 | 35.6 | NA | NA |
| — | 0.049 | <0.001 | <0.001 | — | 0.008 | <0.001 | <0.001 | — | — | |
Mean (s.d.) change from baseline and P-values unless indicated.
Data from: Mease PJ, et al. Ann Rheum Dis 2017;76:79–87.
Data from: van der Heijde D, et al. J Rheumatol 2018; 45:367–77.
Patients with psoriatic lesions ≥3% of body surface area. ADA: adalimumab; EQ-5D VAS: European Quality of Life 5 Dimensions Visual Analogue Scale; IXEQ4W/Q2W: ixekizumab every 4 or 2 weeks; N: population size; NA: not analysed; NC: not collected; PBO: placebo; SF-36: 36-Item Short Form Health Survey; WPAI-SH: Work Productivity and Activity Impairment-Specific Health Problem.
Fig. 1.
Itch NRS and DLQI in PsA patients with skin lesions affecting ≥3% BSA at baseline
(A) Mean change from baseline: Itch NRS score. (B) Percentage of patients (non-responder imputation) achieving Itch NRS = 0. (C) Mean change from baseline: DLQI total score. (D) Percentage of patients (non-responder imputation) achieving DLQI = 0, 1. Study was not designed to compare active treatment groups. *P < 0.001 vs PBO; †P < 0.01 vs PBO. ADA: adalimumab; BSA: body surface area; DLQI: Dermatology Life Quality Index; IXEQ4W/Q2W: ixekizumab every 4 or 2 weeks; N: number of patients in analysis population; NRS: Numeric Rating Scale; PBO: placebo.
Similarly, in the subgroup of patients with psoriasis affecting ⩾3% BSA at baseline, patients in all active treatment groups reported significant improvements in DLQI at 24 weeks (Fig. 1C and Table 2). Week 24 data demonstrates, 22, 53, 63 and, 54%, of patients in PBO, IXEQ4W, IXEQ2W and ADA groups, respectively, reported DLQI scores of 0/1, meaning that psoriasis had no effect on their lives (Fig. 1D and Table 2). At week 24, the percentages of patients (baseline ⩾3% BSA; DLQI ⩾5) reporting scores meeting/exceeding the MCID (DLQI ⩾5 point improvement from baseline; validated in psoriasis but not in PsA) were 30.2, 61.2, 75.0 and 58.1% in the PBO, IXEQ4W, IXEQ2W and ADA groups, respectively (P ⩽ 0.021 vs PBO) (data not shown). The DLQI was not administered during the EP.
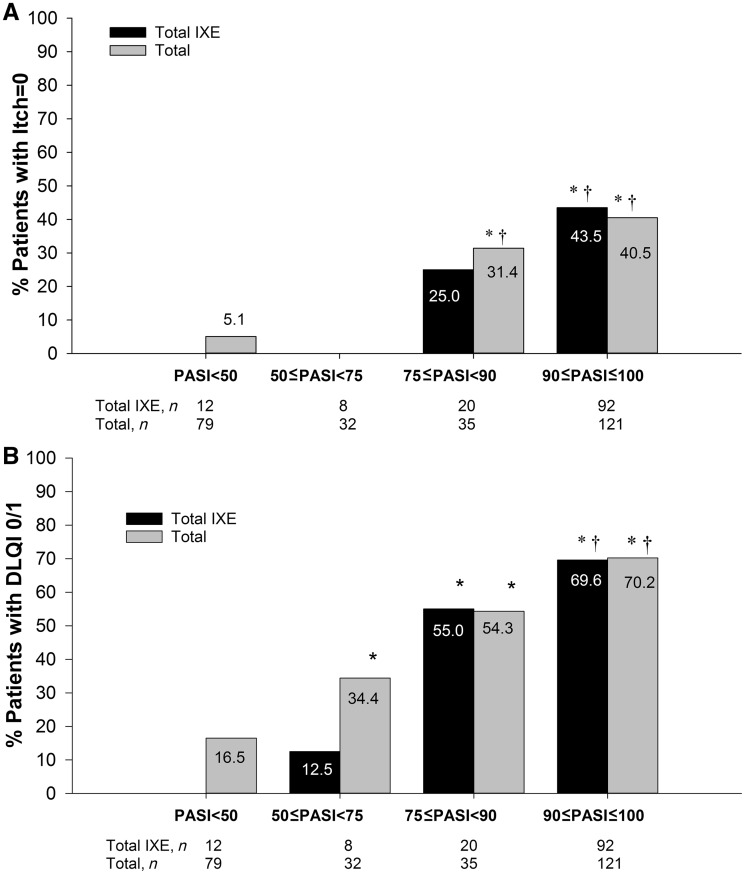
As the level of PASI improvement increased, a greater percentage of patients had no itch (Fig. 2A). Likewise, as the level of PASI improvement increased, a greater percentage of patients reported DLQI 0/1 (Fig. 2B).
Fig. 2.
Association between PASI improvement and Itch or DLQI
(A) Association between PASI improvement and Itch NRS = 0; (B) Association between PASI improvement and DLQI 0/1. Percentage of patients with a response (non-responder imputation). *P < 0.05 vs <50; †P < 0.05 vs 50 to <75. DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index; n: number of patients in each PASI group; Total IXE: both ixekizumab dose groups combined; Total: all groups combined.
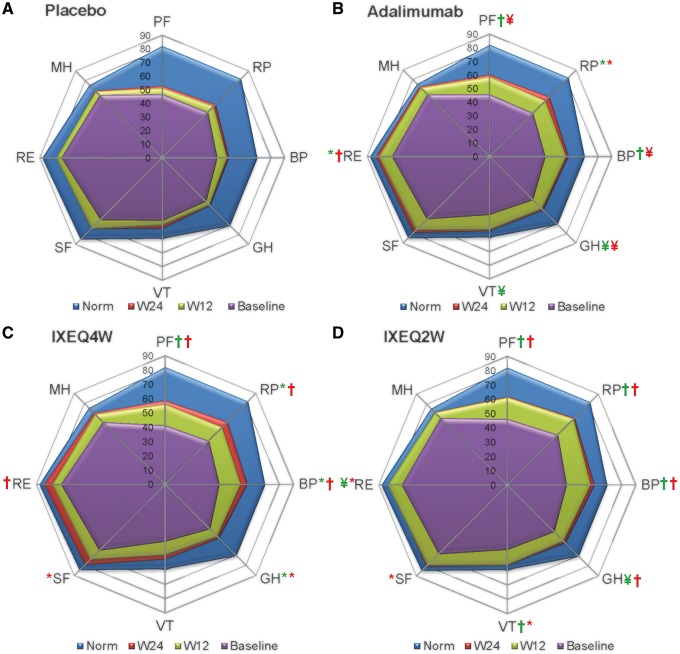
Figure 3 uses spydergrams [35] to depict mean SF-36 0–100 domain scores at baseline, which were similar across active treatment groups. The spydergrams allow comparison with normative scores in an age- and gender-matched US population based on a 1998 survey as well as scores obtained after 12 and 24 weeks of treatment. Following 24 weeks of ixekizumab treatment, the role emotional, mental health and social functioning domain scores were approximately equal to the normative values.
Fig. 3.
SF-36 domain scores
Age and gender matched normative values are shown (blue). The study was not designed to compare active treatment groups. P-values from least squares mean difference comparisons active vs placebo. *P ≤ 0.05; ¥P ≤ 0.005; †P ≤ 0.001. Green, week 12; red, week 24. BP: bodily pain; GH: general health; IXEQ4W/Q2W: ixekizumab every 4 or 2 weeks; MH: mental health; norm: normative values; PF: physical functioning; RE: role emotional; RP: role physical; SF: social functioning; SF-36: 36-item short form health survey; VT: vitality; W12: week 12; W24: week 24.
At week 24, significant improvements compared with PBO were reported in all active treatment groups for SF-36 PCS [22], five of eight domains (excluding vitality, social functioning, mental health) with ADA, six of eight domains (excluding vitality and mental health) with IXEQ4W, and seven of eight domains (excluding mental health) with IXEQ2W (Table 2). Component summaries and domain scores of the IXEQ4W/IXEQ4W and IXEQ2W/IXEQ2W groups were improved relative to baseline at week 52 during the EP (Table 2).
Similarly significant improvements measured by mean changes from baseline in EQ-5D VAS were reported in all active treatment groups compared with PBO at weeks 12 and 24 (Table 2; supplementary Fig. S2, available at Rheumatology online). At week 24, the percentages of patients (baseline EQ-5D VAS ⩽90) reporting scores meeting/exceeding the MCID (⩾10 point improvement from baseline) were 23.2, 42.6, 52.6 and 47.4% in the PBO, IXEQ4W, IXEQ2W and ADA groups, respectively (P ⩽ 0.004 vs PBO) (data not shown). EQ-5D VAS scores for the IXEQ4W/IXEQ4W and IXEQ2W/IXEQ2W groups were improved relative to baseline at week 52 during the EP (Table 2; supplementary Fig. S2, available at Rheumatology online).
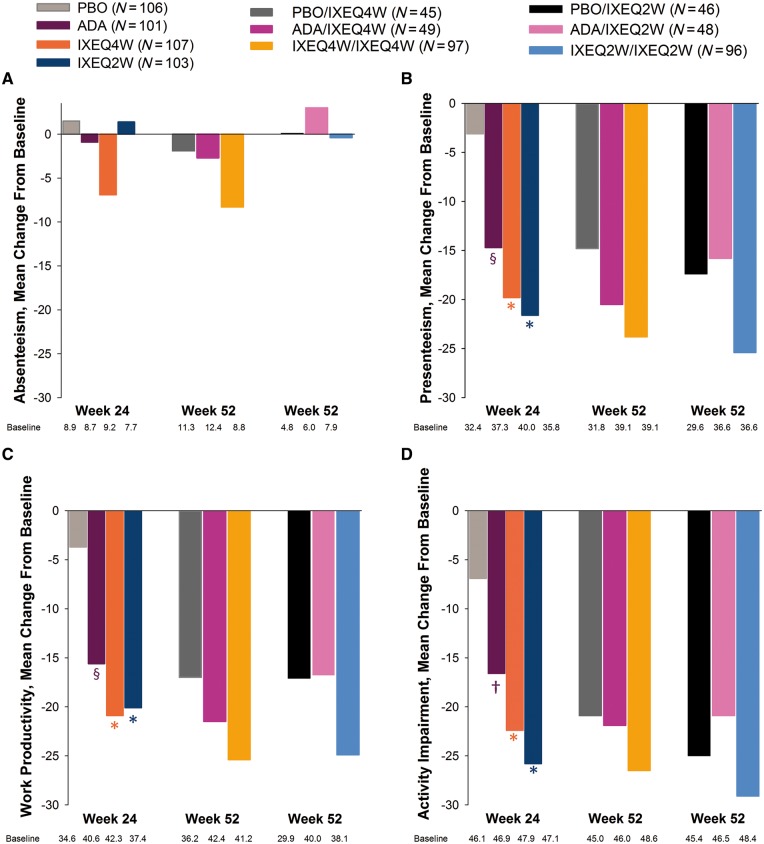
During the DBTP, IXEQ4W- and IXEQ2W-treated patients reported significant improvements in three of four domains of the WPAI-SHP (presenteeism, work productivity and activity impairment) relative to PBO at weeks 12 and 24 (Fig. 4 and Table 2). At week 52, WPAI-SHP scores for the IXEQ4W/IXEQ4W and IXEQ2W/IXEQ2W groups were improved relative to baseline in three of the four WPAI domains (Fig. 4 and Table 2). The week 52 responses of patients in the PBO/IXEQ4W, PBO/IXEQ2W, ADA/IXEQ4W and ADA/IXEQ2W groups were generally of lower magnitude than those in the IXEQ4W/IXEQ4W and IXEQ2W/IXEQ2W groups (Fig. 4 and Table 2; supplementary Table S3, available at Rheumatology online).
Fig. 4.
Work productivity and activity impairment-specific health problem
(A) absenteeism; (B) presenteeism; (C) work productivity; (D) activity impairment. The study was not designed to compare active treatment groups. *P ≤ 0.001 vs PBO; †P < 0.01; §P < 0.05. ADA: adalimumab; IXEQ4W/Q2W: ixekizumab every 4 or 2 weeks; N: number of patients in the analysis population; PBO: placebo; WPAI-SHP: work productivity and activity impairment-specific health problem.
In general, for SF-36 components, SF-36 domain scores and the EQ-5D VAS, patients in the PBO/IXEQ4W, PBO/IXEQ2W, ADA/IXEQ4W and ADA/IXEQ2W groups reported improvements at week 52 generally consistent with the IXEQ4W/IXEQ4W and IXEQ2W/IXEQ2W groups (Table 2, supplementary Table S3 and supplementary Fig. S2, available at Rheumatology online).
Discussion
In SPIRIT-P1, ixekizumab treatment resulted in rapid improvements in the signs and symptoms of PsA in patients who were naïve to biologic therapy [22]. Additionally, improvements in patient-reported components of ACR were reported at 12 and 24 weeks (e.g. HAQ-DI and joint pain VAS) [22]. Here, we show that ixekizumab-treated patients in SPIRIT-P1 reported other improvements in their well-being.
Following ixekizumab treatment, patients reported improvements in both joint-related and physical function-related PROs [22, 34], as well as in general HRQOL and work productivity, up to week 52. We also show here that ixekizumab-treated patients reported improvement in their DLQI scores at week 24 (data not collected at week 52) and itch to week 52. Several bDMARDs that are currently approved for use in PsA also improve patient-reported outcomes [14–19, 36, 37]. In the FUTURE trials, treatment with secukinumab, another IL-17A inhibitor, was shown to positively affect several PROs up to 52 weeks in patients with PsA [19, 36, 37].
In patients with ⩾3% affected BSA, ixekizumab treatment resulted in improved DLQI scores with over half of patients (vs 22% PBO) reporting that their PsA-associated skin symptoms had no effect on their lives (DLQI 0/1) at week 24. Likewise, approximately one-third of ixekizumab-treated patients (vs 6% PBO) reported complete resolution of itch (Itch NRS = 0) at week 24. Among patients with psoriasis, improvements in the severity of skin disease are associated with enhanced HRQOL [38–40]. In the pivotal UNCOVER-2 and UNCOVER-3 studies, incremental improvements in skin disease (PASI) were associated with incremental improvements in DLQI among ixekizumab-treated patients with psoriasis [41]. Likewise, an association between Itch NRS improvement and Psoriasis Area and Severity Index (PASI) improvement was observed in the UNCOVER trials [42]. It is important to note that these previous results were demonstrated in patients with moderate-to-severe psoriasis.
The personal and economic burden of PsA is considerable [43]. The totality of the physical and emotional impact of PsA on HRQOL reflects both joint symptoms and the added burden of psoriasis-associated skin disease [44, 45]. Impaired physical function and work difficulties are associated with joint disease, whereas severity of the skin disease is associated with poor mental functioning [46]. Thus, skin and joint disease contribute additively to the physical and emotional burden of PsA [8]. Here we show that ixekizumab-treated patients reported improvements in itch and the DLQI, complimenting previously published work in regard to joint pain and physical function [22, 34]; these improvements are concomitant with improvements in HRQOL measured by SF-36 and EQ-5D-VAS. By week 24, ixekizumab-treated patients reported significant improvements relative to placebo in SF-36 PCS and in 6 to 7 of 8 domain scores. Collectively, improvements were seen in physical-, mental- and social-related SF-36 domains.
Although the SF-36 is commonly represented as two component summary scores (physical component summary [PCS] and mental component summary [MCS]), these do not completely reflect patterns of change within the individual domains [35]. The PCS and MCS are calculated as a weighted sum of all eight domains (five domains are weighted positively for PCS and three negatively); however, for the MCS, the mental domains are weighted positively and the physical domains negatively. As these summary scores are neither independently weighted nor symmetrical, it is not surprising that one would be significant and the other not. Thus, conclusions should not be drawn solely on PCS and MCS scores without considering individual domain scores, provided one summary score is statistically significant [47].
In addition to physically and mentally impacting patients and increasing healthcare costs, the indirect costs of PsA include disability (short and long term) and lost productivity of paid and unpaid work. Patients with PsA report employment lower than the general population [48]. In our study, ixekizumab-treated patients reported improved work productivity measured by reduced presenteeism (reduced/impaired effectiveness at work), work productivity loss (overall work impairment associated with absenteeism and presenteeism) and activity impairment (activities performed outside of work) at weeks 24 and 52. Patients did not report significant improvements in absenteeism, possibly because the degree of absenteeism was low at baseline compared with the other domains. Effective treatments for PsA will lower the disease burden for patients, their families and society as a whole.
It should be noted that, patients initially treated with placebo or adalimumab who then received ixekizumab thereafter reported generally similar benefits at week 52 to those continuously treated with ixekizumab during the DBTP and EP. Although the trial was not designed to compare the IXEQ4W and IXEQ2W groups, they appeared to have similar health outcome results at weeks 12 and 24. Likewise, the IXEQ4W/IXEQ4W and IXEQ2W/IXEQ2W groups appeared to have similar health outcome results at week 52.
Because the current study was restricted to patients who were biologic-naïve, results cannot be generalized to treatment of patients with a history of failed therapy, loss of efficacy or intolerance to biologics including TNF-inhibitors. Results from the initial 24-week period of SPIRIT-P2, a phase 3 trial investigating the treatment of ixekizumab in patients with an IR to TNF inhibitors, were recently reported [49]. The current RCT was not designed to detect statistical differences between the active treatment groups, but responses in the IXEQ4W and IXEQ2W dose groups appear similar.
In conclusion, ixekizumab-treated patients report improvement in itch and work productivity with concurrent improvements in general measures of HRQOL through 52 weeks of treatment. These improvements complement previously reported improvements in joint pain and physical function [22, 34]. This further confirms the utility of targeting IL-17A in the treatment of both joint and skin diseases in patients with PsA.
Supplementary Material
Acknowledgements
Writing assistance on behalf of Eli Lilly and Company was provided by Lori Kornberg, PhD, who is a full-time employee of Syneos Health, Raleigh, NC, USA and Matthew Hufford, PhD who is a full-time employee of Eli Lilly and Company (Indianapolis, IN, USA).
Funding: This work was supported by Eli Lilly and Company.
Disclosure statement: A.B.G. reports consulting/advisory board agreements with Janssen, Celgene, Bristol Myers Squibb, Beiersdorf, AbbVie, UCB, Novartis, Incyte, Eli Lilly and Company, Dr Reddy’s Labs, Valeant, Dermira, Allergan and Sun Pharmaceutical Industries. She reports research/educational grants from Janssen, Eli Lilly and Company, Novartis, LEO Pharma, Allergan, and Incyte. V.S. reports consulting fees from Eli Lilly and Company, AbbVie, Amgen, Anthera, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celltrion, EMD Serono, Genentech/Roche, GlaxoSmithKline, Janssen, Novartis, Pfizer, Regeneron, Sanofi and UCB. M.K. served on advisory boards of and received honoraria from Eli Lilly and Company. P.M. reports Grant/research support from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, Eli Lilly and Company, Novartis, Pfizer, SUN Pharma, UCB Pharma; he served as a consultant for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Merck, Novartis, Pfizer, SUN Pharma, UCB Pharma; speaker’s bureau for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Genentech, Janssen, Pfizer, UCB Pharma. D.T. has received personal fees and non-financial support from Eli Lilly and Company, received honoraria from AbbVie, Almiral, Amgen, Biogen-Idec, Celgene, Dignity, Dr Reddy’s Laboratory, Galapagos, Galderma, Janssen, LEO Pharma, Maruho, Mitsubishi, Eli Lilly and Company, Novartis, Pfizer, Sandoz-Hexal, Regeneron/Sanofi, UCB and XenoPort for participation in advisory boards, as a speaker, and for consultancy and received research grants from AbbVie, Biogen-Idec, Celgene and Novartis. D.D.G. has consulted for or received grant support from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer and UCB. J.B. and C-Y.L. are full-time employees of Eli Lilly and Company and are minor stockholders. C.L.S. was a full-time employee of Eli Lilly and Company and a minor stockholder at the time this article was written. C.H.L. was an employee of Eli Lilly and Company and a minor stockholder at the time that this article was written. He is currently an employee of Genentech, Inc., and is still a minor stockholder of Eli Lilly and Company.
References
- 1. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P.. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64 (Suppl 2):ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kavanaugh A, Helliwell P, Ritchlin CT.. Psoriatic arthritis and burden of disease: patient perspectives from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Rheumatol Ther 2016;3:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosen CF, Mussani F, Chandran V. et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology 2012;51:571–6. [DOI] [PubMed] [Google Scholar]
- 4. Zachariae H, Zachariae R, Blomqvist K. et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm Venereol 2002;82:108–13. [DOI] [PubMed] [Google Scholar]
- 5. Husted JA, Gladman DD, Farewell VT. et al. Validating the SF-36 health survey questionnaire in patients with psoriatic arthritis. J Rheumatol 1997;24:511–7. [PubMed] [Google Scholar]
- 6. Globe D, Bayliss MS, Harrison DJ.. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes 2009;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strand V, Sharp V, Koenig AS. et al. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis, and psoriasis and effects of etanercept treatment. Ann Rheum Dis 2012;71:1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Betteridge N, Boehncke WH, Bundy C. et al. Promoting patient-centred care in psoriatic arthritis: a multidisciplinary European perspective on improving the patient experience. J Eur Acad Dermatol Venereol 2016;30:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gossec L, Smolen JS, Ramiro S. et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 10. Coates LC, Kavanaugh A, Mease PJ. et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 11. Ash Z, Gaujoux-Viala C, Gossec L. et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26. [DOI] [PubMed] [Google Scholar]
- 12. Ramiro S, Smolen JS, Landewé R. et al. Pharmacological treatment of psoriatic arthritis: a systematic literature review for the 2015 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2016;75:490–8. [DOI] [PubMed] [Google Scholar]
- 13. Mease PJ, Gottlieb AB, van der Heijde D. et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 2017;76:1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boggs RL, Kárpáti S, Li W. et al. Employment is maintained and sick days decreased in psoriasis/psoriatic arthritis patients with etanercept treatment. BMC Dermatol 2014;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kavanaugh A, McInnes IB, Krueger GG. et al. Patient-reported outcomes and the association with clinical response in patients with active psoriatic arthritis treated with golimumab: findings through 2 years of a phase III, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Care Res 2013;65:1666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mease PJ, Ory P, Sharp JT. et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis 2009;68:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mease PJ, Woolley JM, Singh A. et al. Patient-reported outcomes in a randomized trial of etanercept in psoriatic arthritis. J Rheumatol 2010;37:1221–7. [DOI] [PubMed] [Google Scholar]
- 18. Rahman P, Puig L, Gottlieb AB. et al. Ustekinumab treatment and improvement of physical function and health-related quality of life in patients with psoriatic arthritis. Arthritis Care Res 2016;68:1812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strand V, Mease P, Gossec L. et al. Secukinumab improves patient-reported outcomes in subjects with active psoriatic arthritis: results from a randomised phase III trial (FUTURE 1). Ann Rheum Dis 2017;76:203–7. [DOI] [PubMed] [Google Scholar]
- 20. Mease P, Hall S, FitzGerald O. et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017;377:1537–50. [DOI] [PubMed] [Google Scholar]
- 21. Liu L, Lu J, Allan BW. et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res 2016;9:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mease PJ, van der Heijde D, Ritchlin CT. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor W, Gladman D, Helliwell P. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 24. Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY.. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008;159:997–1035. [DOI] [PubMed] [Google Scholar]
- 25. Finlay AY, Khan GK.. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–6. [DOI] [PubMed] [Google Scholar]
- 26. Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY.. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol 2005;125:659–64. [DOI] [PubMed] [Google Scholar]
- 27. Naegeli AN, Flood E, Tucker J, Devlen J, Edson-Heredia E.. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol 2015;54:715–22. [DOI] [PubMed] [Google Scholar]
- 28. Ware JE, Kosinski M, Dewey JE.. How to score version 2 of the SF-36 Health Survey. Lincoln, RI: QualityMetric Incorporated, 2000. [Google Scholar]
- 29. Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 30.EQ-5D. http://www.euroqol.org/ (18 January 2017, date last accessed).
- 31. Reilly Associates Health Outcomes Research. http://www.reillyassociates.net/ (28 June 2017, date last accessed).
- 32. Reilly MC, Zbrozek AS, Dukes EM.. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. [DOI] [PubMed] [Google Scholar]
- 33.SF-36v2 Health Survey 1998 U.S. General Population Norms. http://www.sf-36.org/research/sf98norms.pdf (30 May 2017, date last accessed).
- 34. van der Heijde D, Gladman D, Kishimoto M. et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase 3 study (SPIRIT-P1). J Rheumatol 2018;45:367–377. [DOI] [PubMed] [Google Scholar]
- 35. Strand V, Crawford B, Singh J. et al. Use of “spydergrams” to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann Rheum Dis 2009;68:1800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McInnes IB, Mease PJ, Kirkham B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. [DOI] [PubMed] [Google Scholar]
- 37. Mease PJ, McInnes IB, Kirkham B. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- 38. Edson-Heredia E, Banerjee S, Zhu B. et al. A high level of clinical response is associated with improved patient-reported outcomes in psoriasis: analyses from a phase 2 study in patients treated with ixekizumab. J Eur Acad Dermatol Venereol 2016;30:864–5. [DOI] [PubMed] [Google Scholar]
- 39. Kimball AB, Gordon KB, Fakharzadeh S. et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol 2012;166:861–72. [DOI] [PubMed] [Google Scholar]
- 40. Reich K, Griffiths CE.. The relationship between quality of life and skin clearance in moderate-to-severe psoriasis: lessons learnt from clinical trials with infliximab. Arch Dermatol Res 2008;300:537–44. [DOI] [PubMed] [Google Scholar]
- 41. Griffiths CE, Reich K, Lebwohl M. et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386:541–51. [DOI] [PubMed] [Google Scholar]
- 42. Kimball AB, Luger T, Gottlieb A. et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol 2016;75:1156–61. [DOI] [PubMed] [Google Scholar]
- 43. Lee S, Mendelsohn A, Sarnes E.. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T 2010;35:680–9. [PMC free article] [PubMed] [Google Scholar]
- 44. Sokoll KB, Helliwell PS.. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol 2001;28:1842–6. [PubMed] [Google Scholar]
- 45. Mease PJ, Mittal M, Joshi A. et al. SAT0578 value of treating both skin and joint manifestations of psoriatic arthritis: post-hoc analysis of the adept clinical trial. Ann Rheum Dis 2015;74:870. [Google Scholar]
- 46. Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W.. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes 2009;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taft C, Karlsson J, Sullivan M.. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res 2001;10:395–404. [DOI] [PubMed] [Google Scholar]
- 48. Mau W, Listing J, Huscher D, Zeidler H, Zink A.. Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J Rheumatol 2005;32:721–8. [PubMed] [Google Scholar]
- 49. Nash P, Kirkham B, Okada M. et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017;389:2317–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.