ABSTRACT
The aim of this study was to assess the possible relationship between AAION (arteritic anterior ischemic optic neuropathy) and NAION (non-arteritic anterior ischemic optic neuropathy) with blood platelet parameters and NLR (neutrophil-to-lymphocyte ratio). The medical records of 12 patients with AAION, 33 patients with NAION, and 35 healthy subjects were examined. MPV, PDW, and PCT values showed marked elevation in AAION and NAION groups compared with control group. The mean NLR was statistically significantly higher only in AAION group compared to the NAION and control groups, suggesting that platelet function plays an important role in AIONs and NLR might be used to differentiate AAION from NAION.
KEYWORDS: Anterior ischemic optic neuropathy, mean platelet volume, neutrophil to lymphocyte ratio, platelet distribution width, plateletcrit
Introduction
Anterior ischemic optic neuropathies (AIONs) describe a group of diseases that specifically target the optic nerve and result in sudden vision loss. These include non-arteritic and arteritic anterior ischemic optic neuropathy (NAION and AAION).
NAION is the most common form of AIONs in people over the age of 50 years and is characterized by a sudden, painless, monocular or binocular impairment of vision, initially accompanied by segmental or diffuse optic disc swelling and subsequently optic disc atrophy with retinal arteriolar narrowing.1–3 It is thought that circulatory insufficiency within the optic nerve head (ONH) causes NAION; however, the exact mechanism and location of the vasculopathy are still unclear.3 It is thought that an initial ischemic event, secondary optic nerve damage caused by compartment syndrome, and macular oedema and/or subretinal fluid collection cause visual loss in patients with NAION.4,5 Moreover, there is evidence that secondary inflammation at the site of the ischemia may contribute to or produce more visual loss.4–6
AAION is a vision and life threatening condition that almost always is caused by giant cell arteritis (GCA), a medium and large-vessel systemic vasculitis affecting elderly patients. The estimated annual incidence of AAION is 0.36 per 100 000 population in patients over the age of 50 years.7 Sudden vision loss in AAION occurs most often due to an inflammatory thrombosis of the short posterior ciliary arteries that supplies the ONH. When these vessels become thrombosed with inflammation, an optic disk stroke occurs. The two most important laboratory findings that help to make the diagnosis of GCA are erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). It was reported that the combination of ESR and CRP is 97% specific for the diagnosis of GCA8, ESR had a sensitivity of 76–86% and CRP had a sensitivity of 97.5%.9 Additionally, Kyle et al.10 reported that During the relapses of GCA, ESR is normal in 48% of cases and CRP in 56%. Other markers, including thrombocytosis, normocytic anaemia, and leucocytosis may also be helpful in diagnosing GCA; however, these findings are associated with a sensitivity below 60%.11–13
The mean platelet volume (MPV) and the platelet distribution width (PDW) are markers of subclinical platelet activation and play an important role in the pathophysiology of trombotic events, such as myocardial infarction, cerebral ischemia and coronary and peripheral artery diseases.14,15 Plateletcrit (PCT) is an indicator of the number of circulating platelets in a unit volume of blood and may act as a biomarker for several diseases.16,17
It is well established that the systemic inflammatory response is associated with alterations in circulating white blood cells, specifically the presence of neutrophilia with relative lymphocytopaenia.18,19 The neutrophile-to-lymphocyte ratio (NLR), which is calculated by dividing the peripheral neutrophil count by the lymphocyte count, is considered an important inflammatory marker in several diseases such as colorectal cancer, cardiovascular diseases and type 2 diabetes mellitus (DM).20–22 The NLR was also investigated in a kind of ocular diseases and found that it can be useful as biomarkers in patients with primary open angle glaucoma23, dry eye24, and idiopathic acute anterior uveitis.25
To the best of our knowledge, blood platelet parameters and NLR values have not been previously investigated and compared in the types of AIONs. This retrospective study was conducted to assess the possible relationship between AAION and NAION with blood platelet parameters and NLR. In addition, we investigated the relationship between AIONs and inflammation by analysing NLR. We also tried to find out if the platelet indices and NLR can be used as predictive factors and be new biomarkers for differentiation of AAION from NAION.
Methods
Study design and patient selection
This clinical retrospective comparative study was performed at the neuroophthalmology department of a tertiary Eye Training and Research Hospital. The study protocol was approved by the Ethics Committee and the study was carried out in accordance with the Declaration of Helsinki. The study was designed as a retrospective study involving three groups: AAION, NAION, and control.
The AAION group consisted of 12 patients who were evaluated in the neuroophthalmology department, between January 2012 and July 2016. The inclusion criteria for the patients to be admitted to the study for AAION were: unilateral disc swelling associated with clinical features consistent with AAION observed in the acute phase, temporal artery biopsy that confirmed GCA, and no other posterior segment ocular pathology except AAION (e.g. diabetes, age-related macular degeneration, and glaucoma).
The NAION group consisted of 33 patients and the diagnosis of NAION was made based on the presence of the following: the sudden onset of painless vision loss, relative afferent pupillary defect, visual field defect compatible with optic neuropathy, characteristic fundus changes consistent with optic neuropathy, and a lack of clinical findings suggesting any other diseases.
The control group consisted of 35 age- and sex-matched healthy volunteers with similar risk factors for systemic disorders including hypertension (HT) and DM. They were selected from outpatient clinic of ophthalmology with simple refractive errors.
Participants with any of the following conditions were excluded: any systemic disease except HT and DM, hepatic or renal failure, atrial fibrillation, inflammatory diseases, chronic smoking, alcohol abuse, a history or clinical evidence of retinal diseases (such as diabetic and/or hypertensive retinopathy), glaucoma, an intraocular pressure greater than 21 mmHg, a neurological disease or other diseases of the visual pathways, and a history of previous intraocular surgery or trauma. In addition, perioperative subjects and patients using drugs known to be associated with changes in platelet count (PC) and/or function (such as phosphodiesterase inhibitors). When the medical records of participants were examined, it was seen that no patient received steroid treatment at the time of the blood draw, and steroid treatment was started after taking blood for necessary cases.
All patients underwent a comprehensive ophthalmic examination including the best-corrected visual acuity tests using the Snellen chart (20 feet), intraocular pressure measurements, visual field test, slit lamp biomicroscopy, and dilated fundoscopy.
Laboratory data
In our clinical practice, peripheral blood samples are drawn from the antecubital vein while patients are in an upright position and the samples are collected with minimal stasis, drawn into EDTA vacutainer tubes, and studied within 1 hour to prevent in vitro platelet activation as standard clinical care. Complete blood counts are measured using laser-based flow cytometric impedance. Hemoglobin (Hb), PC, MPV, PDW, and PCT values were recorded from medical records of patients. Neutrophil and lymphocyte counts were also taken into account to calculate the NLR. The reference ranges in our haematology laboratory are as follows: Hb, 11.2–16.2 g/dL; PC, 150 × 109/L–450 × 109/L; MPV, 6.8–10.8 fL; PDW, 9.6–15.2 fL and PCT, 0.19–0.39%.
Statistical analysis
The data obtained from the study were entered into the computer and analysed using the Statistical Package for Social Sciences (SPSS) version 22.0 for Windows (SPSS Inc., Chicago, IL). Descriptive statistics are presented as mean ± standard deviations, frequency distributions, and percentages. Chi-square test was used in the analysis of categorical variables. Normal distribution of the variables was tested using visual (histogram and probability graphs) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilk Test). Equality of variances was checked by the Levene test. The one-way analysis of variance, Welch analysis of variance, and Kruskal–Wallis tests were used to determine if there were any significant differences between the three groups. Post-hoc tests for pairwise comparisons were also performed. A probability level of p < 0.05 was considered statistically significant. The diagnostic value of the NLR as a biomarker for differentiation of AAION from NAION was determined by a receiver operating characteristic (ROC) analysis.
Results
This study included 80 subjects; 12 of them were in the AAION group, 33 of them were in NAION group, and the remaining 35 were in the control group. The mean ages of the patients with AAION, NAION and that of matched controls were 66.66 ± 7.85 (range: 57–81) years, 63.84 ± 8.38 (range: 45–82) years, and 61.00 ± 9.25 (range: 45–84) years, respectively. Although the patients with AAION were slightly older than patients with NAION group and the control group, there were no statistically significant differences regarding the ages, sexes, and systemic disorders of the patients between the groups. The characteristics of participants are displayed in Table 1.
Table 1.
Demographic characteristics and systemic disorders of all participants.
| AAION (n = 12) |
NAION (n = 33) |
CONTROL (n = 35) |
p | |
|---|---|---|---|---|
| Age, years (mean±SD) | 66.66 ± 7.85 | 63.84 ± 8.38 | 61.00 ± 9.25 | 0.734a |
| Male/female (n/n) | 5/7 | 17/16 | 18/17 | 0.911b |
| HT (n) | 4 | 11 | 8 | 0.590b |
| DM (n) | 1 | 6 | 6 | 0.718b |
| HT and DM (n) | 1 | 3 | 2 | 0.864b |
AAION: arteritic anterior ischemic optic neuropathy; NAION: non-arteritic anterior ischemic optic neuropathy; HT: hypertension; DM: diabetes mellitus.
SD, Standard deviation
aSignificance in analysis of variance (comparison among three groups).
bChi-square test.
The blood samples showed a marked elevation in MPV, PCT, and PDW levels in patients with AAION and NAION compared with control subjects. The mean serum levels of MPV, PCT, and PDW were 8.50 ± 1.32 fL, 0.21 ± 0.03%, and 15.16 ± 0.88 fL in the AAION group; 8.24 ± 0.39 fL, 0.20 ± 0.03%, ND 14.85 ± 0.88 fL in the NAION group; and 7.24 ± 0.31 fL, 0.18 ± 0.03%, and 14.32 ± 0.72 fL in the control group, respectively. However, pairwise comparisons of the AAION group and the NAION group revealed that there were no significant differences between these two groups regarding MPV, PCT, and PDW. On the other hand, the mean NLR values were 2.72 ± 0.84 in the AAION group, 2.04 ± 0.42 in the NAION group and 1.97 ± 0.31 in the control group. It was statistically significantly higher only in subjects with AAION compared with the NAION and control groups (p < 0.001). Namely, there was no statistically significant difference between the NAION group and the control group regarding the mean NLR of the patients. Moreover, the mean values of Hb and PC were similar in all groups. Comparison of blood parameters in the study groups are shown in Table 2.
Table 2.
Comparison of blood parameters in the groups.
| AAION (n = 12) Mean±SD |
NAION (n = 33) Mean±SD |
CONTROL (n = 35) Mean±SD |
p | |
|---|---|---|---|---|
| Hb (g/dl) | 12.76 ± 1.02 | 13.15 ± 1.00 | 13.63 ± 1.45 | 0.077a |
| PC (103/μL) | 300.75 ± 52.82 | 282.60 ± 36.50 | 270.57 ± 58.05 | 0.182a |
| MPV (fL) | 8.50 ± 1.32 | 8.24 ± 0.39 | 7.24 ± 0.31 | <0.001a |
| 0.305b, <0.001c, <0.001d | ||||
| PDW (fL) | 15.16 ± 0.88 | 14.85 ± 0.88 | 14.32 ± 0.72 | 0.003a |
| 0.297b, 0.002c, 0.009d | ||||
| PCT (%) | 0.21 ± 0.03 | 0.20 ± 0.03 | 0.18 ± 0.03 | 0.015a |
| 0.411b, 0.011c, 0.015d | ||||
| NLR | 2.72 ± 0.84 | 2.04 ± 0.42 | 1.97 ± 0.31 | <0.001a |
| 0.001b, <0.001c, 0.417d |
Data are presented as mean±standard deviation. AAION: arteritic anterior ischemic optic neuropathy; NAION: non-arteritic anterior ischemic optic neuropathy; Hb: haemoglobin; PC: platelet count; MPV: mean platelet volume; PDW, platelet distribution width; PCT: plateletcrit; NLR: the neutrophil to lymphocyte ratio, fL: femtoliter.
aSignificance in analysis of variance (comparison among three groups)
bSignificance between AAION and NAION group (pairwise comparison)
cSignificance between AAION and control group (pairwise comparison)
dSignificance between NAION and control group (pairwise comparison)
The ROC analysis was performed to differentiate AAION and NAION. In the ROC curve analysis of the NLR, the area under the ROC (AUROC) value of the NLR was found to be 0.721. The best cut-off value was 2.255, with a sensitivity of 66% and a specificity of 73% (Figure 1).
Figure 1.
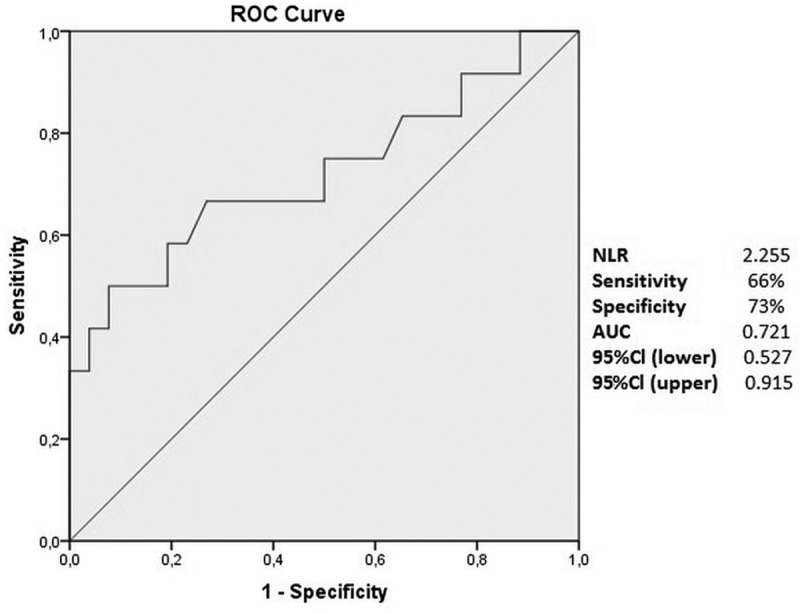
Receiver operator characteristic curve of NLR to distinguish AAION and NAION.
Discussion
This study investigated the blood PC and function including MPV, PDW, and PCT in patients with AAION and NAION, as well as NLR and their relationships with the types of AION. According to our knowledge, this is the first study that has evaluated the blood platelet parameters and NLR in patients with the types of AION.
The PC has been regarded as a marker of haemostasis. Meanwhile, the evidence suggests that it is platelet size, rather than the count itself, that is associated with platelet function and activation.26 MPV reflects the average size of the platelets, whereas variations and heterogeneity of platelets are expressed by PDW.27 Large platelets contain more dense granules and are metabolically more active than small platelets and they express higher levels of prothrombotic substance, including thromboxane B2, serotonin, and β-thromboglobulin.28 High MPV is linked to various established risk factors, cardio- and cerebrovascular diseases, and low-grade inflammatory conditions prone to arterial and venous thrombosis.29 It has also been shown that an increased MPV value is associated with several ocular vascular diseases such as retinal artery occlusion, diabetic retinopathy, and hypertensive retinopathy.30–32 Sahin et al.32 found a significant increase in the MPV values in patients with retinal artery occlusion and in another study they found an increased MPV in patients with NAION.33 Although both the MPV and PDW are markers of platelet activation, Vagdatli et al.34 showed that the PDW seems to be a more specific marker of platelet activation than the MPV, which was not found to be elevated during single platelet distension caused by platelet swelling. The combined use of measuring the MPV and the PDW could help to predict coagulation activation more efficiently. In our study, the levels of both the MPV and the PDW values were significantly higher in AAION and NAION groups than in the controls whereas there were no significant differences of the mean MPV and PDW values among arteritic and non-arteritic AIONs.
The PCT could be defined as the percentage of blood volume occupied by platelets and can be calculated as follows: PC × MPV/107.35 Actually it shows the amount of the number of circulating platelets in a unit volume of blood, and in this sense it is similar to the haematocrit for erythrocytes. During the last decade, the PCT was evaluated in various studies including different disorders; e.g. coronary artery disease, DM, pulmonary tuberculosis, obstructive sleep apnoea, and inflammation.36–39 Moreover, Sahin et al.40 showed that PCT is correlated with CRP, an inflammatory marker in patients with chronic inflammatory diseases. In the present study the PCT was significantly higher in patients in AIONs than the control group and there was no difference among arteritic and non-arteritic AIONs. Significantly increased PCT values were thought to be associated with increased microvascular blood flow resistance and the circulatory insufficiency of the ONH in patients with AION.
Previous studies have shown some correlations between inflammation markers and AAION. ESR and CRP are the most extensively studied inflammatory markers.41 In recent years, there has been a focus on white blood cell subtypes such as neutrophil, lymphocyte, and NLR as the predictors of inflammatory conditions. NLR may be more preferable because of two factors: First, although some conditions such as exercise and dehydration may increase the absolute number of neutrophils and lymphocytes, NLR is less commonly affected; Second, and more importantly, it is a ratio calculated from the counts of products of two different but complementary immune pathways.42 Neutrophils are effective in ongoing active non-specific inflammation through the secretion of various inflammatory enzymes and mediators including elastase, myeloperoxidase, and free-oxygen radicals. In contrast to the phagocytic and killing effects of neutrophils, lymphocytes perform the regulatory function of the immune system.42,43 Because the NLR reflects both immune pathways and is presumably less affected by confounding conditions, it may be more predictive than either parameter alone.42 Karaca et al.44 found increased NLR values in patients with progressive keratoconus. Numerous studies also researched the NLR in patients with diabetic retinopathy.45,46
In our study, we found that NLR values were statistically significantly higher only in AAION group compared with the NAION and the control groups. Despite AAION and NAION both being ischemic disorders, the pathogenesis of these two conditions differ. In NAION, there is no occlusion of the posterior ciliary artery (embolic NAION occurs rarely). It is thought that an initial ischemic event, secondary optic nerve damage from a compartment syndrome and macular oedema and/or subretinal fluid collection cause visual loss in patients with NAION. Moreover, there is evidence that secondary inflammation at the site of the ischemia may contribute to or produce more visual loss. Unlike the progressive oedema and compartment syndrome associated with NAION, AAION results from sudden vascular dysfunction due to “inflammation-induced” thrombosis of the medium-sized arteries supplying the optic nerve. AAION occurs because of thrombotic occlusion of the short posterior ciliary arteries, resulting in infarction of the ONH.5,47,48 Thus, we believe that although both vascular and inflammatory changes are prominent factors in the pathogenesis of NAION, alterations in platelet function and microvascular blood flow are more important than the inflammatory reactions in the pathogenesis of NAION. NLR may be used as an additional parameter to differentiate AAION from NAION, which is sometimes quite difficult.
There are some limitations of our study. First, the present study was performed retrospectively. Further, the number of patients in the AAION group was less than that of the NAION and control groups, which did not allow a fair comparison, but AAION is a very rare disease. We also used spot MPV, PDW, PCT, and NLR values for analysis, rather than follow-up values. Although there were no significant differences between the groups in terms of systemic disorders (including HT and DM), unknown and/or undetected systemic disorders and probable differences in body mass indices may have affected the blood platelet parameters and thus acted as confounding factors.
As a result, inflammatory reaction beside ischemic changes may play a role in the etiopathogenesis of AAION. A complete blood count is a relatively routine, inexpensive, practical and easy examination that supplies additional information such as MPV, PDW PCT, and NLR could help us show subclinical platelet activation and systemic inflammation. Since GCA is an ocular emergency, it is essential to differentiate arteritic from non-arteritic AION at the earliest possible moment, to enable us to start corticosteroid therapy immediately in order to prevent any further loss of vision and the NLR may provide additional information to differentiate AAION from NAION. The cut-off values described in this study could be useful in differentiation of AAION from NAION. However, these data should be interpreted with caution, because of the relatively small sample size. Further prospective researches involving a large number of patients would be needed to utility of NLR as a new biomarker for differentiation of AAION from NAION. We believe that our study can encourage researchers to investigate CRP, ESR, blood platelet markers, and NLR in patients with AION by prospective multicentre randomized clinical studies.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the writing and content of the article.
References
- [1].Miller NR, Arnold AC.. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye (Lond). 2015;29:65–79. doi: 10.1038/eye.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003;23:157–163. doi: 10.1097/00041327-200306000-00012. [DOI] [PubMed] [Google Scholar]
- [3].Miller NR. Current concepts in the diagnosis, pathogenesis, and management of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2011;31:1–3. doi: 10.1097/WNO.0b013e31821f955c. [DOI] [PubMed] [Google Scholar]
- [4].Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009;28:34–62. doi: 10.1016/j.preteyeres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- [5].Hayreh SS, Baines JA. Occlusion of the posterior ciliary artery. 3. Effects on the optic nerve head. Br J Ophthalmol. 1972;56:754–764. doi: 10.1136/bjo.56.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hayreh SS. Ischaemic optic neuropathy. Indian J Ophthalmol. 2000;48:171–194. [PubMed] [Google Scholar]
- [7].Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy. Population-based study in the state of Missouri and Los Angeles County, California. J Neuroophthalmol. 1994;141:38–44. [PubMed] [Google Scholar]
- [8].Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123:285–296. doi: 10.1016/S0002-9394(14)70123-0. [DOI] [PubMed] [Google Scholar]
- [9].Parikh M, Miller NR, Lee AG, et al. Prevalence of a normal C-reactive protein with an elevated erythrocyte sedimentation rate in biopsy-proven giant cell arteritis. Ophthalmology. 2006;113:1842–1845. doi: 10.1016/j.ophtha.2006.05.020. [DOI] [PubMed] [Google Scholar]
- [10].Kyle V, Cawston TE, Hazleman BL. Erythocyte sedimentation rate and C reactive protein in the assessment of polymyalgia rheumatica/giant cell arteritis on presentation and during follow up. Ann Rheum Dis. 1989;48:667–671. doi: 10.1136/ard.48.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Costello F, Zimmerman MB, Podhajsky PA, Hayreh SS. Role of thrombocytosis in diagnosis of giant cell arteritis and differentiation of arteritic from non-arteritic anterior ischemic optic neuropathy. Eur J Ophthalmol. 2004;14:245–257. [DOI] [PubMed] [Google Scholar]
- [12].Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation rate and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum. 2012;41:866–871. doi: 10.1016/j.semarthrit.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Foroozan R, Danesh-Meyer H, Savino PJ, Gamble G, Mekari-Sabbagh ON, Sergott RC. Thrombocytosis in patients with biopsy-proven giant cell arteritis. Ophthalmology. 2002;109:1267–1271. doi: 10.1016/S0161-6420(02)01076-X. [DOI] [PubMed] [Google Scholar]
- [14].Sansanayudh N, Anothaisintawee T, Muntham D, McEvoy M, Attia J, Thakkinstian A. Mean platelet volume and coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2014;175:433–440. doi: 10.1016/j.ijcard.2014.06.028. [DOI] [PubMed] [Google Scholar]
- [15].Berger JS, Eraso LH, Xie D, Sha D, Mohler ER 3rd.. Mean platelet volume and prevalence of peripheral artery disease, the National Health and Nutrition Examination Survey, 1999–2004. Atherosclerosis. 2010;213:586–591. doi: 10.1016/j.atherosclerosis.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wiwanitkit V. Plateletcrit, mean platelet volume, platelet distribution width: its expected values and correlation with parallel red blood cell parameters. Clin Appl Thromb Hemost. 2004;10:175–178. doi: 10.1177/107602960401000208. [DOI] [PubMed] [Google Scholar]
- [17].Tang J, Gao X, Zhi M, et al. Plateletcrit: a sensitive biomarker for evaluating disease activity in Crohn’s disease with low hs-CRP. J Dig Dis. 2015;16:118–124. doi: 10.1111/1751-2980.12225. [DOI] [PubMed] [Google Scholar]
- [18].Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- [19].Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- [20].Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/(ISSN)1096-9098. [DOI] [PubMed] [Google Scholar]
- [21].Horne BD, Anderson JL, Intermountain Heart Collaborative Study Group, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- [22].Guo X, Zhang S, Zhang Q, et al. Neutrophil:lymphocyteratio is positively related to type 2 diabetes in a large-scale adult population: a Tianjin chronic low-grade systemic inflammation and health cohort study. Eur J Endocrinol. 2015;173:217–225. doi: 10.1530/EJE-15-0176. [DOI] [PubMed] [Google Scholar]
- [23].Ozgonul C, Sertoglu E, Mumcuoglu T, Kucukevcilioglu M. Neutrophil-to-Lymphocyte ratio and Platelet-to-Lymphocyte ratio as novel biomarkers of primary open-angle glaucoma. J Glaucoma. 2016;25:815–820. doi: 10.1097/IJG.0000000000000392. [DOI] [PubMed] [Google Scholar]
- [24].Celik T. Assessment of neutrophil-to-Lymphocyte ratio and platelet-to-lymphocyte ratio in patients with dry eye disease. Ocul Immunol Inflamm. September 14, 2017; 1–4. [Epub ahead of print]. doi: 10.1080/09273948.2017.1340486. [DOI] [PubMed] [Google Scholar]
- [25].Ozgonul C, Sertoglu E, Ayyildiz O, et al. Novel biomarkers for patients with idiopathic acute anterior uveitis: neutrophil to lymphocyteratio and platelet to lymphocyte ratio. Int J Ophthalmol. 2017;10:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. 2003;9:177–190. doi: 10.1177/107602960300900301. [DOI] [PubMed] [Google Scholar]
- [27].Zhang Z, Xu X, Ni H, Deng H. Platelet indices are novel predictors of hospital mortality in intensive care unit patients. J Crit Care. 2014;29:885.e1–e6. doi: 10.1016/j.jcrc.2014.04.020. [DOI] [PubMed] [Google Scholar]
- [28].Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- [29].Yilmaz T, Yilmaz A. Altered platelet morphological parameters in patients with retinal vein occlusion. Eur Rev Med Pharmacol Sci. 2016;20:1934–1939. [PubMed] [Google Scholar]
- [30].Citirik M, Beyazyildiz E, Simsek M, Beyazyildiz O, Haznedaroglu IC. MPV may reflect subcinical platelet activation in diabetic patients with and without diabetic retinopathy. Eye (Lond). 2015;29:376–379. doi: 10.1038/eye.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bhatti AB, Ali F, Satti SA. Relationship of hypertensive retinopathy with mean platelet volume among hypertensive patients. Cureus. 2015;7:e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sahin M, Sahin A, Elbey B, Yüksel H, Türkcü FM, Cingü AK. Mean platelet volume in patients with nonarteritic anterior ischemic optic neuropathy. J Ophthalmol. 2016;2016:1051572. doi: 10.1155/2016/1051572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sahin M, Sahin A, Yuksel H, Türkcü FM, Yıldırım A. Mean platelet volume in patients with retinal artery occlusion. Arq Bras Oftalmol. 2016;79:12–14. doi: 10.5935/0004-2749.20160005. [DOI] [PubMed] [Google Scholar]
- [34].Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28–32. [PMC free article] [PubMed] [Google Scholar]
- [35].Chandrashekar V. Plateletcrit as a screening tool for detection of platelet quantitative disorders. J Hematol. 2013;2:22–26. [Google Scholar]
- [36].Tetikoğlu M, Aktas S, Sagdık HM, Yigitoglu ST, Özcura F. Mean platelet volume is associated with diabetic macular edema in patients with type-2 diabetes mellitus. Semin Ophthalmol.2017;32(5):651–654. [DOI] [PubMed] [Google Scholar]
- [37].Santimone I, DI Castelnuovo A, De Curtis A, et al. MOLI-SANIProject investigators. White blood cell count, sex and age are major determinants of heterogeneity of platelet indices in an adult general population: results from the MOLI-SANI project. Haematologica. 2011;96:1180–1188. doi: 10.3324/haematol.2011.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nena E, Papanas N, Steiropoulos P, et al. Mean platelet volume and platelet distribution width in non-diabetic subjects with obstructive sleep apnea syndrome: new indices of severity? Platelets. 2012;23:447–454. doi: 10.3109/09537104.2011.632031. [DOI] [PubMed] [Google Scholar]
- [39].Dalamaga M, Karmanıolas K, Lekka A, et al. Platelet markers correlate with glycemic indices in diabetic, but not diabetic-myelodysplastic patients with normal platelet count. Dis Markers. 2010;29:55–61. doi: 10.1155/2010/284041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sahin F, Yazar E, Yıldız P. Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidiscip Respir Med. 2012;7:38. doi: 10.1186/2049-6958-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Eshagian J, Goeken JA. C-reactive protein in giant cell (cranial, temporal) arteritis. Ophthalmology. 1980;87:1160–1166. doi: 10.1016/S0161-6420(80)35110-5. [DOI] [PubMed] [Google Scholar]
- [42].Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- [43].Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91:1872–1885. doi: 10.1161/01.CIR.91.6.1872. [DOI] [PubMed] [Google Scholar]
- [44].Karaca EE, Özmen MC, Ekici F, Yüksel E, Türkoğlu Z. Neutrophil-to-lymphocyte ratio may predict progression in patients with keratoconus. Cornea. 2014;33:1168–1173. doi: 10.1097/ICO.0000000000000260. [DOI] [PubMed] [Google Scholar]
- [45].Ulu SM, Dogan M, Ahsen A, et al. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopthy. Diabetes Technol Ther. 2013;15:942–947. doi: 10.1089/dia.2013.0097. [DOI] [PubMed] [Google Scholar]
- [46].Wang RT, Zhang JR, Li Y, Liu T, Yu KJ. Neutrophil-Lymphocyte ratio is associated with arterial stiffness in diabetic retinopathy in type 2 diabetes. J Diabetes Complications. 2015;29:245–249. doi: 10.1016/j.jdiacomp.2014.11.006. [DOI] [PubMed] [Google Scholar]
- [47].Hayreh SS. Anterior ischaemic optic neuropathy. 2. Fundus on ophthalmoscopy and fluorescein angiography. Br J Ophthalmol. 1974;58:964–980. doi: 10.1136/bjo.58.12.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Henkind P, Charles NC, Pearson J. Histopathology of ischemic optic neuropathy. Am J Ophthalmol. 1970;69:78–90. doi: 10.1016/0002-9394(70)91859-3. [DOI] [PubMed] [Google Scholar]


