Abstract
BACKGROUND
Vagus nerve stimulation (VNS) has been approved for the treatment of refractory partial epilepsy in adults and children over 12 years of age. Later on, its application expanded to include younger children and other types of epilepsy. We report our experience with this treatment modality for refractory epilepsy in Saudi Arabia.
DESIGN AND SETTINGS
Open-label, uncontrolled, retrospective study of patients with refractory epilepsy, who were treated with VNS in a tertiary care hospital from January 2010 to June 2013.
PATIENTS AND METHODS
Collected data included 26 patients’ demographics, epilepsy characteristics, seizure frequency, and treatment history. Patients with a follow-up duration of minimum 12 months were included in the analysis. The examined outcome measures were seizure reduction rates, antiepileptic drugs (AEDs) burden, and impact on patients’ quality of life (QOL).
RESULTS
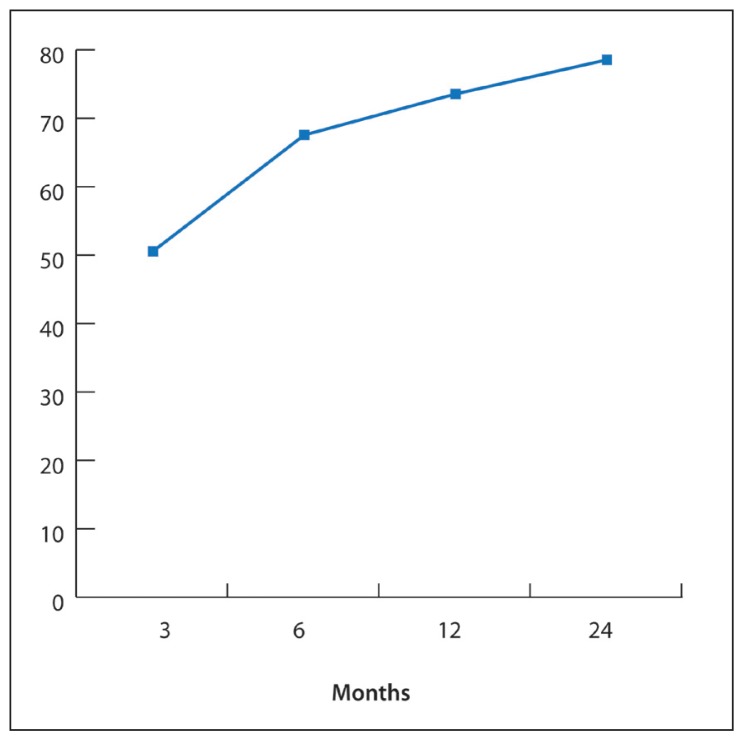
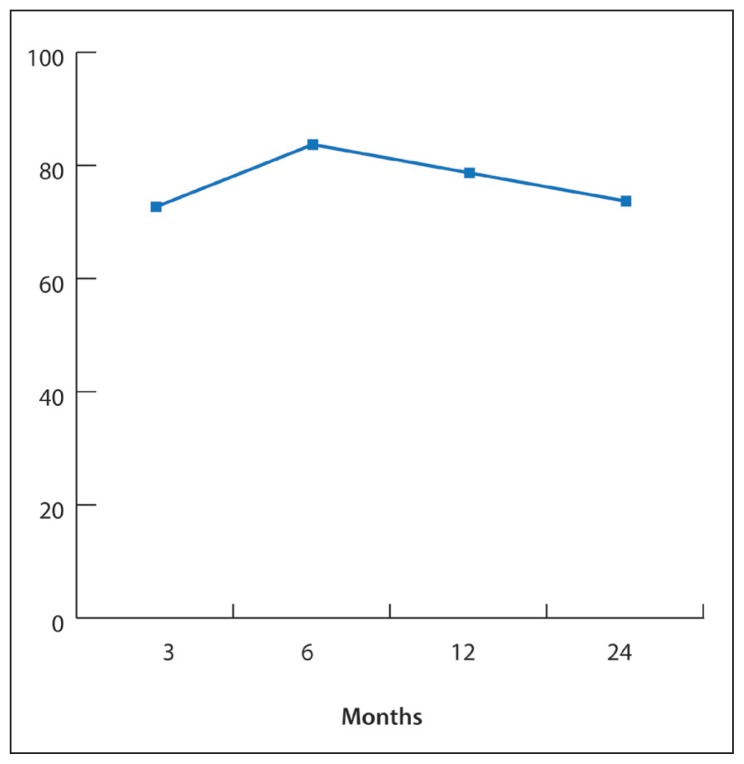
Onset of seizures was from birth to 30 years. Patients’ ages at VNS implantation ranged from 4 to 38 years (18.9 [8.7] years). Epilepsy was classified as focal in 8 patients (30%), multifocal in 9 patients (35%), and generalized in 9 patients (35%). The average number of AEDs failed before VNS was 4.2 (1.4). Greater than 50% seizure reduction was achieved in 50% of patients at 3 months, 67% at 6 months, 73% at 12 months, and 78% at 24 months. There was no significant reduction in AEDs burden during the same period. Subjective QOL improvement was reported by 72% of patients at 3 months, 83% at 6 months, 78% at 12 months, and 73% at 24 months after VNS. Minor adverse effects were reported in 27% of patients. One patient had the device replaced due to malfunction.
CONCLUSION
The experience with VNS in a single center in Saudi Arabia confirms that it is a safe and effective adjunctive therapy for refractory epilepsy in adult and pediatric patients.
Epilepsy is the second most common chronic neurological disorder after stroke affecting approximately 0.5% to 2% of the population.1 Despite adequate treatment with antiepileptic drugs (AEDs), 30% of patients continue to have seizures or experience unacceptable pharmacological side effects.2 For these patients with “medically refractory” epilepsy, resective surgery is a therapeutic alternative when the epileptogenic zone can be identified and renders 40% 90% of patient’s seizure free.3 However, in a substantial number of patients, the epileptogenic zone cannot be identified or is located in an eloquent brain area. Unsuitable candidates for resective surgery have a few options left. Electrical stimulation of the vagus nerve is an efficacious treatment for patients with refractory epilepsy who are not candidates for curative resective surgery or who have experienced insufficient benefit from such a treatment.4
Vagus nerve stimulator (VNS) consists of a generator implanted in the anterior chest wall that delivers intermittent electric stimuli to the brain via a bipolar electrode coiled around the left vagus nerve in the neck. The generator cycles continuously at predetermined parameters. Since the first human implant of the VNS therapy device in 1989, the use of this treatment modality expanded worldwide.5 In the past 25 years, several controlled and open-label studies have established its efficacy and safety for focal seizures both during shortand long-term follow-up. Two pivotal randomized double-blind placebo-controlled trials served as the main evidence for approval of the US and European regulatory boards for the use of VNS in patients with refractory focal seizures.6,7 The E03 and the E05 study, respectively, enrolled 114 and 199 patients with a follow-up of 3 months in both studies. A high stimulation group (500 μs, 30 Hz, 30 seconds/5 minutes) was compared to a low-stimulation group (130 μs, 1 Hz, 30 seconds/3 hours), which was considered a placebo arm. Reduction in mean seizure frequency in the high-stimulation group compared the the low-stimulation group was 24.5% versus 6.1% for the E03 study and 28% versus 15% for the E05 study. Prospective long-term follow-up studies were published a few years later. The E05 study with a follow-up of 1 year in 195 patients reported a responder rate of 35%.8 The E01 to E05 studies with a follow-up of 3 years included 440 patients and reported responder rates of 43%.9 Open-label studies have shown efficacy in other epilepsy types such as generalized epilepsy and Lennox–Gastaut syndrome.10,11 Experience and length of follow-up in pediatric patients is limited. However, the available data are encouraging.12–14 No controlled studies are available, but 1 prospective open-label safety study in 60 children (aged 3–18 years) with different types of seizures showed a 42% reduction in seizure frequency after 18 months of treatment.15
This retrospective study aims to describe our preliminary experience with VNS therapy in a tertiary epilepsy center in Saudi Arabia where VNS has been used in the treatment of refractory epilepsy for a few years.
PATIENTS AND METHODS
This open-label, uncontrolled, retrospective chart review was conducted on 26 adults and pediatric patients who were treated with VNS at a single center between January 2010 and June 2013. All patients were referred to our Epilepsy Clinic for the management of refractory epilepsy. Patients went through careful pre-surgical evaluation including video-electroencephalogram monitoring and neuroimaging and were not found to be candidates for resective surgery. Ketogenic diet was offered as an alternative option for the pediatric cases; however, it was declined by caregivers due to concerns about compliance.
We used NeuroCybernetic Prosthesis 102 and 103 models manufactured by Cyberonics (Houston, TX, USA). Stimulation was turned on 10 to 14 days after surgery. Our standard stimulation protocol starts with initial output current of 0.25 mA, frequency 30 Hz, pulse width 250 μs, on-time 30 s, and off time 5 min. Output current and duty cycle settings were then ramped up gradually every 2 to 4 weeks according to the individual tolerance and the degree of seizure control as per the standard protocol.
Institutional review board approval was obtained for the chart review. Collected data included patient demographics, epilepsy history and characteristics, imaging findings, seizure frequency, and treatment history. Patients with a follow-up duration of minimum 12 months were included in the VNS efficacy analysis. The main outcome measures were as follows: (1) percentage of patients who achieved >50% seizure reduction, (2) the number of prescribed AEDs per patient, and (3) the impact on patients’ quality of life (QOL). These outcome measures were assessed at 3, 6, 12, and 24 months after VNS insertion. The seizure frequency data was obtained from patient/family seizure logs. Seizure load at baseline and each follow-up visit was calculated based on the average of the last 3 months prior to the visit. Changes to the types and numbers of AEDs prescribed were tracked retrospectively in the patients’ charts. The assessment of QOL improvement was subjective based on the feedback provided by patients or caretakers as documented in the follow-up visit notes. We calculated the percentage of patients who reported QOL improvement at each follow-up visit compared with baseline prior to VNS. Data was analyzed using SPSS, version 18.0 (SPSS Inc, Chicago, IL, USA). Descriptive statistics including means, standard deviations, and proportions were calculated.
RESULTS
The charts of 26 patients were reviewed in this study. Table 1 summarizes patient characteristics. The group included 10 females (38%). The median age of epilepsy onset was 8.5 years. Patients’ ages at VNS implantation were 4 to 38 years (median 23). At the time of VNS insertion, 30% of patients were pediatric. Based on seizure semiology and electroencephalogram findings, epilepsy was classified as generalized in 9 patients (35%), multifocal in another 9 patients (35%), and focal in 8 patients (30%). Resective surgery was not considered in patients with focal epilepsy due to high risk of neurological deficit and the lack of lesion on neuroimaging. Etiologies are brain malformation (3), bilateral cortical dysplasia (3), traumatic brain injury (3), post-infectious encephalitis (2), cerebral ischemia (2), brain tumor (1), glutaric acidemia type-II (1), and patients with unknown etiology (11). Follow-up data for 24 months after VNS insertion was available for 18 patients. Greater than 50% seizure reduction was achieved in 50% of patients at 3 months, 67% of patients at 6 months, 73% of patients at 12 months, and 78% of patients at 24 months (Figure 1). One pediatric patient with generalized epilepsy of unknown etiology became seizure free after 6 months of VNS placement.
Table 1.
Patient characteristics.
| Total number | 26 |
| Gender | |
| Male | 16 (62%) |
| Female | 10 (38%) |
| Age at epilepsy onset (yr) | 5.8 (6.7)* |
| Age at VNS insertion (yr) | 18.9 (8.7)* |
| Duration of epilepsy before VNS (yr) | 13.2 (6.6)* |
| Number of AEDs used prior to VNS | 4.2 (1.4)* |
| Epilepsy classification | |
| Focal | 8 (30%) |
| Temporal | 4 |
| Extra-temporal | 4 |
| Multifocal | 9 (35%) |
| Generalized | 9 (35%) |
| Primary | 1 |
| Secondary | 8 |
| Seizure semiology | |
| Generalized seizures | |
| Tonic–clonic | 7 |
| Myoclonic | 8 |
| Clonic | 3 |
| Tonic | 2 |
| Atonic | 4 |
| Absence | 1 |
| Focal seizures | 8 |
| Focal with second generalization | 5 |
These values are the mean (SD).
AEDs: Antiepileptic drugs; yr: years; VNS: vagus nerve stimulation.
Figure 1.
Percentage of patients achieved >50% seizure reduction after VNS insertion.
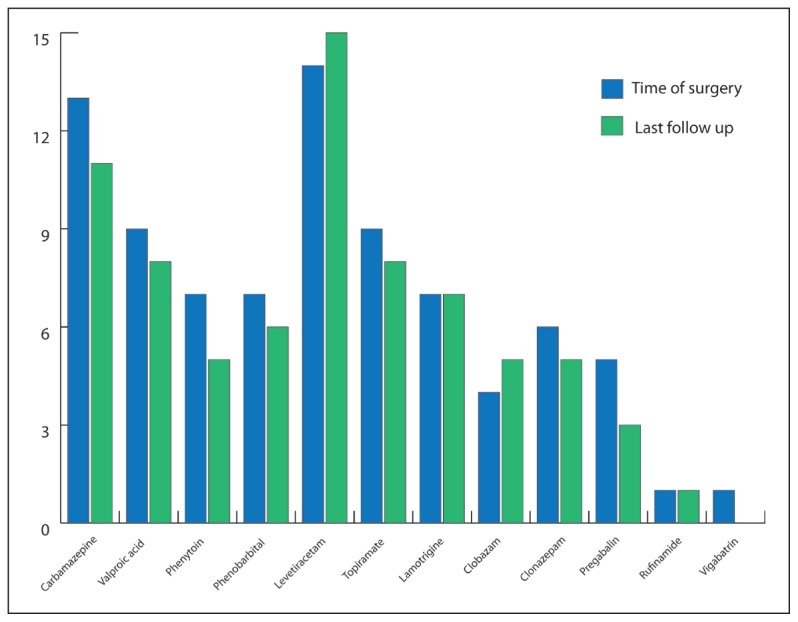
Our patients tried a total of 12 different AEDs (Figure 2). The number of failed AEDs per patient ranged from 3–7 (mean 4.2 [1.4]). At the time of VNS insertion, the average number of AEDs per patient was 3.3 (1.6). No new antiepileptic medications were added in the peri-implantation period. The average number of AEDs prescribed at last follow-up decreased slightly to 2.9 (1.2) per patient. However, this decline was not statistically significant.
Figure 2.
The number of patients taking each AED at the time of VNS surgery and then at the last follow-up visit.
QOL improvement was subjectively reported in 72% of patients at 3 months, 83% at 6 months, 78% at 12 months, and 73% at 24 months after VNS (Figure 3). The reported sources of this improvement were decreased seizure frequency, decreased emergency department visits and hospitalizations, decreased daytime seizures, less or no drop attacks, and improved mood and interaction with surroundings.
Figure 3.
Percentage of patients reported improvement of QOL* after VNS insertion.
Minor adverse effects were reported in 27% of patients; these included hoarseness (5), cough (4), dyspnea (2), and throat paresthesia (1). One patient had the device replaced due to malfunction after 4 months. The device was sent to Cyberonics and was found faulty. No surgical complications were encountered.
DISCUSSION
We reviewed our experience with VNS therapy for refractory epilepsy in adult and pediatric patients over a period of 4 years. To our knowledge, this is a novel report of VNS experience from Saudi Arabia. Seizure reduction of 50% or more was found in 50% to 78% of our patients, with 1 patient being seizure free. This is notably higher than previous studies that reported >50% seizure reduction in 43% to 50% of patients 5, 6, 7, 9, 16 and with a longer follow-up duration in 63% of patients.17 The higher responder rates in our cohort could be attributed to the rather small sample size. Despite this improvement of seizure control, none of our patients could stop all AEDs. Moderate decline of the average number of prescribed AEDs per patient was observed; however, it was not statistically significant.
Symptomatic generalized epilepsies including Lennox–Gastaut syndrome, Dravet syndrome, and myoclonic astatic epilepsy are commonly reported in pediatric VNS studies, compared with mostly focal or multifocal epilepsies in adult studies, as was the case in our study. Identification of responders on the basis of type of epilepsy proves to be difficult. One study reported particular improvement in atypical absences in children with catastrophic epilepsy.18 Another study reported a greater reduction in atonic seizures (drop attacks).19 Casazza et al described a better response to VNS in temporal lobe seizures in comparison to frontal and fronto-central seizures.20 However, no specific seizure type or epilepsy syndrome was consistently reported to have better response to VNS therapy in published reports. In our small cohort, variable seizure types were reported including generalized seizures, focal seizures, and focal with secondary generalization (Table 1). However, we did not find favorable response to VNS in any particular seizure type. The small sample size probably limited our analysis in this regard. In general, identifying predictive factors for response to VNS demands more complex investigations and multicenter large cohort trials.
The responder rate in our study increased steadily from 50% at 3 months to 78% at 24 months followup. This is consistent with the accumulating evidence that seizure improvement does not take place immediately, but it seems to occur in a stepwise manner over the months or years following VNS implantation.8,21–23 In a study of 65 patients treated with VNS for 10 or more years, the authors found that following a “rampup” and accommodation period throughout the initial 24 months after implantation, seizure control improved slightly over the subsequent years of therapy and eventually stabilized.17 As in adult studies, children’s studies show that the effect of VNS on seizure frequency reduction is a slow process that evolves over time.24 These and other studies highlight the need for prolonged follow- up to accurately determine VNS efficacy. This phenomenon of improved seizure control with increasing duration of VNS therapy was reported similarly with anterior thalamic stimulation.25 Hence, there may be an “ongoing effect” of neural stimulation. Another possible explanation is that longer follow-up duration allows for titration of stimulation parameters beyond the narrow settings used in the initial randomized studies that were limited to only 3 months. It is of note that the long period for establishing efficacy of VNS contrasts with AEDs, where a more rapid initial response is seen often, with reduction of efficacy and tolerance over time. Not all patients experience the same steady decline of seizure frequency. Indeed, some patients show decreased seizure severity, abolition of status epilepticus episodes and decreased hospital admissions, decreased daytime seizures and mostly nocturnal seizures, less or no drop attacks, and improved mood and concentration. Therefore, a patient can have marked improvement in QOL.26,27 Similarly, these finding were observed to have a major impact on the QOL in our patients.
Complications of VNS are related primarily to the surgical procedure, and include infection at the site of implantation, electrode fracture, or dislodgement from the device, and battery failure.28,29 The most common side effects of VNS are transient and usually occur during actual stimulation. These include hoarseness, cough, dyspnea, throat pain, and tingling sensations in the throat or chest.7,26,30 These side effects are usually managed by lowering the output current. Infrequent side effects include left vocal cord paralysis.26 Weight loss was also reported in rare cases.31 As in other studies, we found that VNS was well tolerated in most patients and that side effects were mostly transient.
The limitations to our study include its retrospective design and rather small sample. Though this sample is the first to be reported in Saudi Arabia, relatively large cohort of patients treated with VNS in this part of the world. This shows continued spread of VNS utilization as adjunct therapy of refractory epilepsy worldwide. Changes to AEDs regimen were made as needed according to the clinical course, which might have had potential influence on seizure control. However, the overall utilization of AEDs decreased after VNS, which implies an independent positive therapeutic effect of the device. Another limitation is the subjective method of assessing the impact of VNS therapy on QOL, which was based on general feedback from patients/caretakers. A standardized QOL measure applied in a prospective design would yield more objective data.
In conclusion, the experience of our patient series from a single center in Saudi Arabia confirms that VNS therapy is an efficacious and safe treatment for children and adults with refractory epilepsy. Although the overall burden of AEDs decreased after VNS insertion, none of our patients was able to discontinue all antiepileptic medications.
Acknowledgments
We would like to thank our Epilepsy Program supporting staff: Nadia Madani, Ghada Qadi, Alaa Subhi, Ayman Alfaleh and Abdallah Alosaimi.
REFERENCES
- 1.Hauser WA, Hesdorffer DH. Epilepsy: Frequency, Causes and Consequences. New York: Demos Press; 1990. pp. 1–51. [Google Scholar]
- 2.Wong IC, Chadwick DW, Fenwick PB, et al. The long-term use of gabapentin, lamotrigine, and vigabatrin in patients with chronic epilepsy. Epilepsia. 1999;40:1439–45. doi: 10.1111/j.1528-1157.1999.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 3.Boon P, Vandekerckhove T, Achten E, et al. Epilepsy surgery in Belgium, the experience in Gent. Acta Neurologica Belgica. 1999;99:256–65. [PubMed] [Google Scholar]
- 4.Boon P, Vonck K, De Reuck J, Caemaert J. Vagus nerve stimulation for refractory epilepsy. Seizure. 2001;10(6):456–8. doi: 10.1053/seiz.2001.0628. [DOI] [PubMed] [Google Scholar]
- 5.De Herdt V, Boon P, Ceulemans B, et al. Vagus nerve stimulation for refractory epilepsy: a Belgian multicenter study. Eur J Paediatr Neurol. 2007;11(5):261–9. doi: 10.1016/j.ejpn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Menachem E, Manon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: a controlled study of effect on seizures. Epilepsia. 1994;35:616–26. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 7.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 8.DeGiorgio FM, Schachter SC, Handforth A, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41(9):1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Morris GL, Mueller WM The Vagus Nerve Stimulation Study Group EO1–EO5. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology. 1999;53:1731–5. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]
- 10.Holmes MD, Silbergeld DL, Drouhard D, et al. Effect of vagus nerve stimulation on adults with pharmacoresistant generalised epilepsy syndromes. Seizure. 2004;13:340–5. doi: 10.1016/j.seizure.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Hosain S, Nikalov B, Harden C, et al. Vagus nerve stimulation treatment for Lennox–Gastaut Syndrome. J Child Neurol. 2000;15:509–12. doi: 10.1177/088307380001500803. [DOI] [PubMed] [Google Scholar]
- 12.Cukiert A, Cukiert CM, Burattini JA, et al. A prospective long-term study on the outcome after vagus nerve stimulation at maximally tolerated current intensity in a cohort of children with refractory secondary generalized epilepsy. Neuromodulation. 2013;16(6):551–6. doi: 10.1111/j.1525-1403.2012.00522.x. [DOI] [PubMed] [Google Scholar]
- 13.Hauptman JS, Mathern GW. Vagal nerve stimulation for pharmacoresistant epilepsy in children. Surg Neurol Int. 2012;3(Suppl 4):S269–74. doi: 10.4103/2152-7806.103017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terra VC, Furlanetti LL, Nunes AA, et al. Vagus nerve stimulation in pediatric patients: Is it really worthwhile? Epilepsy Behav. 2014;31:329–33. doi: 10.1016/j.yebeh.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Murphy JV Pediatric VNS Study Group. Left vagal nerve stimulation in children with refractory epilepsy. J Pediatr. 1999;134:563–6. doi: 10.1016/s0022-3476(99)70241-6. [DOI] [PubMed] [Google Scholar]
- 16.Vonck K, Thadani V, Gilbert K, et al. Vagus nerve stimulation for refractory epilepsy: a transatlantic experience. J Clin Neurophysiol. 2004;21:283–9. doi: 10.1097/01.wnp.0000139654.32974.4e. [DOI] [PubMed] [Google Scholar]
- 17.Elliott RE, Morsi A, Tanweer O, et al. Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS > 10 years. Epilepsy Behav. 2011;20(3):478–3. doi: 10.1016/j.yebeh.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 18.Majoie HJ, Berfelo MW, Aldenkamp AP, et al. Vagus nerve stimulation in patients with catastrophic childhood epilepsy, a 2-year follow-up study. Seizure. 2005;14:10–18. doi: 10.1016/j.seizure.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Patwardhan RV, Stong B, Bebin EM, et al. Efficacy of vagal nerve stimulation in children with medically refractory epilepsy. Neurosurgery. 2000;47:1353–7. [PubMed] [Google Scholar]
- 20.Casazza M, Avanzini G, Ferroli P, et al. Vagal nerve stimulation: relationship between outcome and electroclinical seizure pattern. Seizure. 2006;15:198–207. doi: 10.1016/j.seizure.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 21.George R, Salinsky M, Kuzniecky R, et al. for the First International Vagus Nerve Stimulation Study Group. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on first 67 patients exiting a controlled study. Epilepsia. 1994;35:637–43. doi: 10.1111/j.1528-1157.1994.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 22.Labar D. Vagus nerve stimulation for 1 year in 269 patients on unchanged antiepileptic drugs. Seizure. 2004;13:392–8. doi: 10.1016/j.seizure.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Uthman BM, Reichl AM, Dean JC, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63:1124–6. doi: 10.1212/01.wnl.0000138499.87068.c0. [DOI] [PubMed] [Google Scholar]
- 24.Valencia I, Holder DL, Helmers SL, et al. Vagus nerve stimulation in pediatric epilepsy: a review. Pediatr Neurol. 2001;25:368–76. doi: 10.1016/s0887-8994(01)00319-8. [DOI] [PubMed] [Google Scholar]
- 25.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 26.Hallbook T, Lundgren J, Stjernqvist K, et al. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; its impact on cognition, quality of life, behavior and mood. Seizure. 2005;14:504–13. doi: 10.1016/j.seizure.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Benifla M, Rutka JT, Logan W, Donner EJ. Vagal nerve stimulation for refractory epilepsy in children: indications and experience at The Hospital for Sick Children. Childs Nerv Syst. 2006;22:1018–26. doi: 10.1007/s00381-006-0123-6. [DOI] [PubMed] [Google Scholar]
- 28.Smyth MD, Tubbs RS, Bebin EM, et al. Complications of chronic vagus nerve stimulation for epilepsy in children. J Neurosurg. 2003;99:500–3. doi: 10.3171/jns.2003.99.3.0500. [DOI] [PubMed] [Google Scholar]
- 29.Helmers SL, Wheless JW, Frost M, et al. Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: retrospective study. J Child Neurol. 2001;16:843–8. doi: 10.1177/08830738010160111101. [DOI] [PubMed] [Google Scholar]
- 30.Murphy JV, Hornig GW, Schallert GS, Tilton CL. Adverse events in children receiving intermittent left vagal nerve stimulation. Pediatr Neurol. 1998;19:42–4. doi: 10.1016/s0887-8994(98)00013-7. [DOI] [PubMed] [Google Scholar]
- 31.Burneo JG, Faught E, Knowlton R, et al. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59:463–4. doi: 10.1212/wnl.59.3.463. [DOI] [PubMed] [Google Scholar]