Abstract
We describe the case of a patient with confirmed limbic encephalitis associated with leucine-rich glioma-inactivated 1 (LGI1) antibodies. A 59-year-old man presented to the Department of Neurology with bizarre behavior, memory loss, cognitive impairment, visual hallucinations, and myoclonus and faciobrachial dystonic seizures. A brain magnetic resonance imaging (MRI) revealed no hippocampal lesions. Blood tests showed hyponatremia. An electroencephalogram showed disorganization and slowing of background activity. Antiepileptic drugs were ineffective. The patient exhibited considerable improvement following immunotherapy. The diagnosis of limbic encephalitis associated with LGI1 antibodies should be considered in patients with clinical manifestations mimicking psychiatric disorders and in cases of refractory epilepsy especially with faciobrachial dystonic seizures. There is frequently hyponatremia, and cerebral MRI may be normal. Full recovery can be expected with early diagnosis and prompt treatment.
Limbic encephalitis (LE) associated with leucine-rich glioma-inactivated 1 (LGI1) antibodies is a recently described form of autoimmune encephalitis. 1,2 Neuroimaging shows bilateral hyperintense sign in the mesial temporal lobe.1 Hyponatremia occurs in 88% of patients and may be attributed to the expression of LGI1 in the hypothalamus.1,3
Recently, Lai et al2 described that LGI1 is the autoantigen associated with LE previously attributed to voltage-gated potassium channel autoantibodies. The majority of the patients respond favorably to immunotherapy. 2
CASE
A 59-year-old man was received in the Department of Neurology with a 5-month history of gradually worsening behavior disturbances, insomnia, short-term memory impairment, disorientation, visual hallucinations, myoclonus, and faciobrachial dystonic seizures (FBDS) that frequently cause the dropping of objects. His medical history was significant for vitiligo that had appeared 2 years ago on his hands, neck, and back. He also suffered from verruca seborrhoica (Figure 1) on his back and gastroesophageal reflux disease. Diabetes was diagnosed 2 months prior to admission and rapidly required insulin therapy. The patient was a user of neither illicit drugs nor alcohol.
Figure 1.
Verruca seborrheica on the back of the patient.
During the last 3 months, he had been treated with antiepileptic drugs (AEDs) (sodium valproate, lamotrigine, and diazepam), but the treatments were ineffective and the seizures continued.
On admission, he appeared to be confused, drowsy, disoriented in time and place, and had a marked impairment of short-term memory. He scored 15/30 on the Folstein mini-mental state examination (MMSE). During his examination, a notable number of FBDS occurred. They were brief (5 seconds) but very frequent, occurring 30 to 50 times per day. FBDS involved the arm, the ipsilateral face, and occasionally the ipsilateral leg. It was also noted that these seizures were often triggered by severe emotional stress. A neurological exam also revealed asymmetric akinetic-rigid syndrome and bilateral palmomental reflexes.
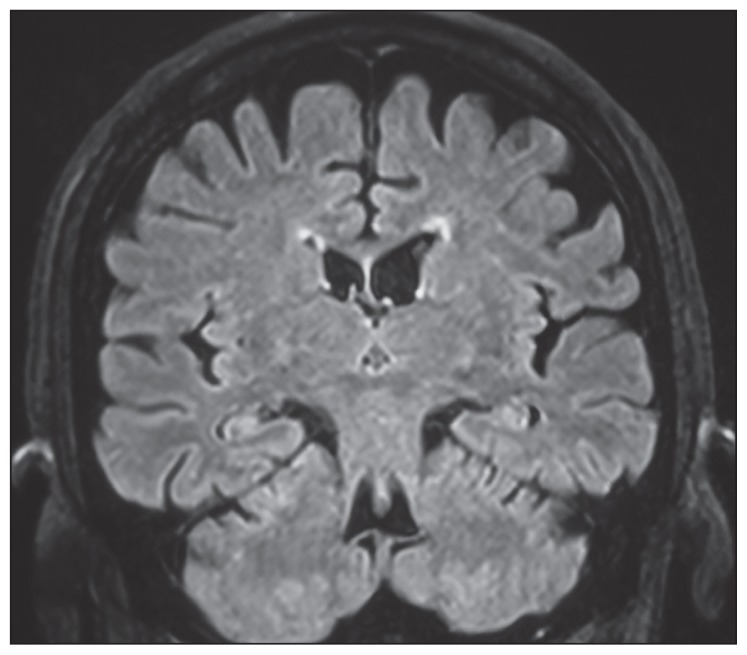
A brain magnetic resonance imaging (MRI) (Figure 2) revealed no hippocampal lesions. The electroencephalogram (EEG) showed disorganization and slowing of background activity without periodic synchronous discharges.
Figure 2.
Brain MRI of the patient, a coronal flair sequence showing a slight atrophy but no hippocampal hyperintensities.
Initially, the cerebrospinal fluid (CSF) findings were normal. A second lumbar puncture, however, showed an elevation of total protein (0.91 g/L) but a normal white cell count (0/μL).
The CSF concentration of protein 14-3-3 was normal. Serologies for human immunodeficiency virus, herpes simplex virus, Epstein-Barr virus, cytomegalo-virus, varicella zoster virus, hepatitis B and C viruses, syphilis, brucellosis, Lyme, as well as mycobacteria in blood and CSF were all negative. Polymerase chain reaction detection for the polyomavirus BK was also negative. Other studies including a cholesterol panel and blood tests to determine homocysteine, folate, and B12 levels as well as thyroid function were all normal.
Hyponatremia (116 mmol/L [normal range 135–145 mmol/L]), hypochloremia (84 mEq/L), hyperglycemia, and elevated glycated hemoglobin were noted. The diagnosis of inappropriate antidiuretic hormone (ADH) release was first suggested. Other causes of hyponatremia were excluded.
Immunologic tests (antinuclear antibody, antineutrophil cytoplasmic antibody, antithyroglobulin antibody, antimicrosomal [antiperoxidase] antibody, anti-endomysial antibody, anticardiolipin antibodies, antiphospholipid antibodies) were all negative. His antineuronal paraneoplastic antibodies (anti-Hu, anti-Yo, anti-Ri, anti-PNMA2, anti-amphiphysin), antibodies to N-methyl-D-aspartate receptors, and antiglutamic acid decarboxylase were also negative. However, high titers of serum anti-voltage-gated potassium channel (VGKC)-complex antibodies were found (678.3 pmol/L [positive > 85 pmol/L]), leading to the definitive diagnosis of VGKC-complex antibody LE. LGI1 antibodies were identified as the specific antigenic target within the VGKC-complex. Anti-contactin-associated protein-like 2 (CASPR2) antibodies were negative.
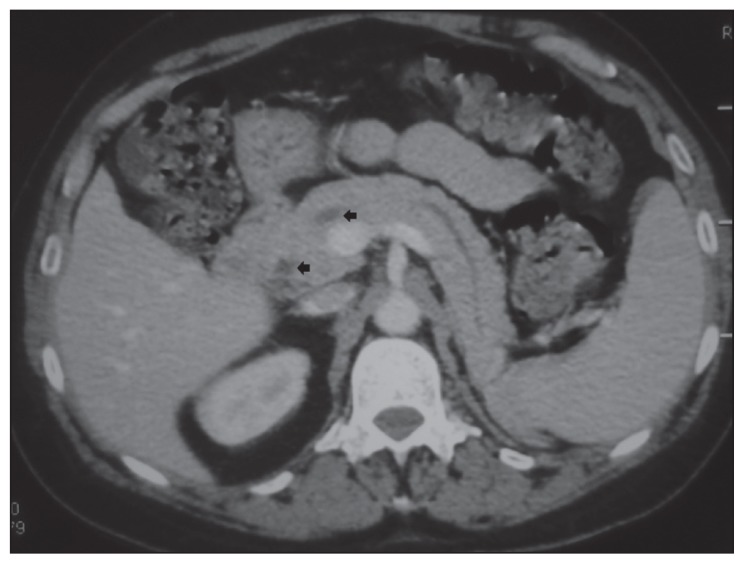
Computed tomography (CT) of the chest, abdomen, and pelvis revealed intraductal papillary mucinous neoplasia of the pancreas (IPMN) with no signs of malignancy (Figure 3) which was confirmed by MRI. Serum carbohydrate antigen 19-9, carcinoembryonic antigen, alpha-fetoprotein, and prostate specific antigen were all normal. Markedly elevated neuron-specific enolase (NSE) was detected (NSE=125.3 μg/L [normal <16.3]). The bronchial fibroscopy was normal.
Figure 3.
Computerized tomography of the abdomen revealing intraductal papillary mucinous neoplasia of the pancreas with no signs of malignancy
The initial treatment consisted of slow correction of the hyponatremia via fluid restriction followed by administration of isotonic saline. It lead to a slight cognitive improvement. Other AEDs were utilized. Phenytoin, piracetam, and levetiracetam proved ineffective. Carbamazepine led to significant side effects of a localized rash appearing on his face and neck. It was then interrupted. Methylprednisolone was contraindicated since his diabetes was not equilibrated.
He was started on immunotherapy with intravenous immunoglobulin (0.4 g/kg daily for 5 days). After 1 month, the patient responded to this treatment becoming seizure free and showing significant improvement in his cognitive state. He achieved an MMSE score of 27/30. Subsequently, the patient was under regular follow-up with no relapse. He was followed during 18 months Antiepileptic treatment was progressively diminished. The gastroenterology service considers his IPMN with high risk of malignant degeneration. They recommended follow-up with annual computed tomography or abdominal MRI. There is no evidence for a causal relationship between IPMN and LE.
DISCUSSION
LE is a rare clinico–neuropathological entity.4 The main etiologies of LE are infections and autoimmune disease. It can be associated with specific anti-VGKC autoantibodies that were first described by Buckley et al. in 2001.5
The antibodies are now understood to bind to different components of VGKC-complexes, such as LGI1 or CASPR2.2,4
Typically, patients with LE and VGKC-complex antibodies present with acute to subacute progression of short-term memory loss (80%), confusion, disorientation, seizures, and agitation.4,5 Psychiatric signs (40%) such as, behavioral and personality changes, hallucinations, temporolimbic seizures, and sleep disturbance, may also occur.3,5 Myoclonic jerks, dystonic facial movements, refractory epilepsy, and dysautonomia have been also reported.3,6 Some patients are erroneously diagnosed at initial presentation with psychosis or cryptogenic epilepsy.4 Our patient exhibited classic signs and symptoms of LE.
Patients are predominately male (2:1 men to women) and many are older than 40 years old with a reported mean age of onset of 64 years (age range 44–74 years).6
The semiology of seizures in LE with VGKC-complex is usually distinctive and predictive of this diagnosis. 1 Various types of seizures have been described. They can be partial, or status epilepticus. They are often refractory and difficult to control despite multiple AEDs.1 FBDS are very suggestive of LE with VGKC-complex. They can occur either during the prodromal stage or during LE. The FBDS are very brief, lasting usually less than 3 seconds. However, they are very frequent, occurring at a median of 50 times per day at their peak.1 FBDS always involve the arm and commonly the ipsilateral face (76%) and leg (34%).1 Hand involvement usually causes many patients to drop items within their grasp. Since they often respond poorly to antiepileptic medication, recognition of these distinctive seizures should prompt serious consideration of immunotherapies.1
In 28% of patients, the FBDS can be triggered by auditory stimuli or by high emotion,1 as in our patient. Unexplained drop attacks, often with backward falls, can occur in 62% of cases and are unrelated to leg dystonia. 1 Our patient had presented with seizures of this kind.
Hyponatremia (<135 mmol/L) is commonly reported and was found in 88% of cases by Irani and al1 Very low sodium concentration in plasma (Between 115 mmol/L and 130 mmol/l) should alert the clinician to the diagnosis of LE with VGKC-complex antibodies. 4 Hyponatremia may aggravate the cognitive impairment and seizures. It is hypothesized to be due to inappropriate antidiuretic hormone (ADH) release.6
The cerebral MRI findings that are characteristic of LE with VGKC-complex are mesial temporal lobe abnormalities (unilaterally or bilaterally) with increased signal on T2 or fluid-attenuated inversion recovery (FLAIR) MRI.4
It is important to note that up to 45% of patients with LE with VGKC-complex antibodies can have normal MRI at onset or throughout the disease course. Therefore, a normal MRI is not dispositive if clinical presentation of the patient suggests otherwise and a determination of serum levels of VGKC-complex antibodies should be pursued.4 Our patient had not displayed the characteristic MRI finding.
Electroencelography usually shows frontotemporal slowing of background activity. Generalized slowing has also been reported.4
CSF findings in LE with VGKC-complex are characterized by slight pleocytosis (41%), mainly consisting of lymphocytes and monocytes, and elevated total protein concentrations (47%).7 CSF findings were normal in 23% of patients.7 VGKC-Ab should, therefore, be determined whenever LE is clinically suspected, irrespective of CSF findings.7
Unlike paraneoplastic encephalitis, anti-VGKC associated encephalitis is potentially treatable and reversible. It responds more favorably to immunotherapy.3,5,6
AEDs are ineffective in the treatment of seizures and they are associated with an unusually high risk of adverse reactions. The immunotherapies are based primarily on intravenous immunoglobulin, steroids, and plasmapheresis. Immunosuppressives can be also used such as mycophenolate mofetil (MMF), azathioprine (AZA), cyclophosphamide, and rituximab.6
Plasma sodium concentrations often normalize and VGKC-complex antibodies are usually undetectable within a few months in patients who are treated adequately. 4 Relapse or chronic evolution can occur, but usually patients having LE with VGKC-complex present with a monophasic disease.6
To our knowledge, our patient represents only the second reported case of LE associated with IPMN.8 Further studies and research are required to determine whether there is a relationship between these 2 diseases or whether their simultaneous occurrence was merely coincidence. The discovery of a biological marker could, for example, help elucidate such a relationship.
This case highlights the need to increase awareness about LE with LGI1 auto-antibodies. Clinicians should be aware of this well-defined entity because of therapeutic implications.
Acknowledgments
We would like to thank Dr. Christian Winchell for his precious help in correcting this manuscript.
REFERENCES
- 1.Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 2.Lai M, Huijbers MGM, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:77685. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70:1883–90. doi: 10.1212/01.wnl.0000312275.04260.a0. [DOI] [PubMed] [Google Scholar]
- 4.Vincent A, Bien CG, Irani SR, Waters P. Auto-antibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759–72. doi: 10.1016/S1474-4422(11)70096-5. [DOI] [PubMed] [Google Scholar]
- 5.Buckley C, Oger J, Clover L, Tüzün E, Carpenter K, Jackson M, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. 2001;50:73–8. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- 6.Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, et al. Potassium channel antibody associated encephalopathy: a potentially immunotherapy responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 7.Jarius S, Hoffmann L, Clover L, Vincent A, Voltz R. CSF findings in patients with voltage gated potassium channel antibody associated limbic encephalitis. J Neurol Sci. 2008;268:74–7. doi: 10.1016/j.jns.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Somers KJ, Sola CL. Voltage-gated potassium channel-complex antibody-associated limbic encephalitis. Psychosomatics. 2011;52:78–81. doi: 10.1016/j.psym.2010.11.002. [DOI] [PubMed] [Google Scholar]