Abstract
BACKGROUND AND OBJECTIVES
Obstructive sleep apnea (OSA) is common in adult population and may cause many adverse clinical results. We aimed to investigate possible changes in cardiopulmonary exercise capacity in OSA patients after positive airway pressure treatment.
DESIGN AND SETTINGS
Patients who were admitted to Gaziantep University Pulmonary Diseases Sleep Center and diagnosed OSA were included. Studies carried out between May 2010 and July 2011. Sixty-five consecutive patients were included in this prospective study.
PATIENTS AND METHODS
Sixty-five adult sleep clinic patients diagnosed with OSA by polysomnography and in whom continuous positive airway pressure (CPAP) ventilation therapy was indicated were included. Cardiopulmonary exercise capacity was assessed by bicycle ergometry during diagnostic workup and at least 4 weeks later.
RESULTS
There were 57 (87.7%) males. The mean age was 45.29 (10.57) years, apnea-hypopnea index 38.02 (23.19 events/h, body mass index 31.72 (4.87) kg/m2. Patients were grouped with respect to compliance with CPAP. The peak oxygen consumption (VO2) did not change in the CPAP compliant group (n=33) (22.52 [6.62] mL/[min.kg] to 21.32 [5.26] mL/[min.kg]; P=.111), and decreased from 21.31 (5.66) mL/(min.kg) to 19.92 (5.40) mL/(min.kg) (P=.05) in the CPAP noncompliant group. Work rate increased from 84.0% to 85.0% in the CPAP compliant group and decreased from 79.6% to 77.1% in the noncompliant group (P=.041). In the group that used the device, ventilation (VE)/VCO2 at anaerobic threshold (AT) declined from 28.42 to 27.36; however, it increased from 27.41 to 27.81 in the group that did not use the device (P=.033).
CONCLUSIONS
Decline in the exercise capacity was prevented in patients with OSA after 4 weeks of CPAP therapy. The changes in VE/VCO2 at AT suggest the reversal of pathophysiologic changes in OSA with the CPAP therapy that may improve cardiac function and cause more efficient ventilation.
Obstructive sleep apnea (OSA) is a common clinical disorder in adult population.1 Repeated episodes of airway closure with resultant decrease in intrathoracic pressure and sympathetic nervous system activation may cause severe complications including cardiovascular system. Continuous positive airway pressure (CPAP) ventilation remains the best treatment option and has been shown to prevent the deterioration of health status.2–4 Cardiopulmonary exercise tests (CPETs) are used for detecting exercise tolerance mechanisms and reasons for intolerance. CPET provides information about cardiopulmonary system, oxygen transport system, muscular system, and metabolic activity of tissues that interact in exercise.5 The aim of this study was to evaluate the impact of OSA and CPAP treatment on cardiovascular, pulmonary, and oxidative metabolism during exercise.
PATIENTS AND METHODS
This prospective study was conducted between May 2010 and July 2011. The ethics committee approval was obtained. The study population consisted of patients diagnosed as OSA, and these patients were admitted to the Gaziantep University Sleep Clinic. Patients with obstructive or restrictive pulmonary disorder, cardiac failure, acute coronary syndrome, or inability to cycle due to orthopedic problems were excluded. Ninety consecutive patients who required CPAP for OSA treatment and were older than 18 years were included.
Body mass index (BMI) was calculated as kg/m2. Epworth sleepiness scale scores were calculated for each subject.6 Polysomnographic data was recorded with Viasys Sleep Screen (Viasys Healthcare, Germany). Sleep studies were evaluated according to American Academy of Sleep Medicine guidelines by the same doctor (MU).7 OSA was classified as mild (apnea-hypopnea index [AHI]≥5–15), moderate (AHI=15–30), and severe (AHI≥30).7
CPAP titration was performed with full-night polysomnographic recordings. Patients were informed about the use of CPAP. Control was conducted after 4 weeks. Patients with more than 4 hours of daily CPAP usage were evaluated as CPAP compliant; other patients were evaluated as the CPAP noncompliant group.
Bicycle ergometry was performed during diagnostic workup and at control visit. Exercise protocol and reference values were based on American Thoracic Society/American College of Chest Physicians guidelines.8 CPET was performed using Vmax Spectra 229 model (Viasys, USA) ergospirometry device. Spirometry and MVV values were obtained before CPET. The exercise consisted of symptom-limited, ramp workload protocol programmed to reach maximum in 10 minutes. The pedal rate was aimed to remain constant at 60 to 70 cycles/min during the procedure. Blood pressures were obtained using a standard sphygmomanometer in 2-minute intervals. Oxygen consumption (VO2), carbon dioxide production (VCO2), anaerobic threshold (AT), heart rate (HR), oxygen saturation, end-tidal O2 and CO2 (PetO2, PetCO2), tidal volume, respiratory rate, and minute ventilation (VE) were recorded.
Data was analyzed with SPSS, version 11.5 (SPSS, Chicago, IL, USA). Repeated measures of ANOVA, student t test, chi-square test, and Pearson correlation test were used as appropriate. Data was shown as mean (SD). P≤.05 was necessary for statistical significance.
RESULTS
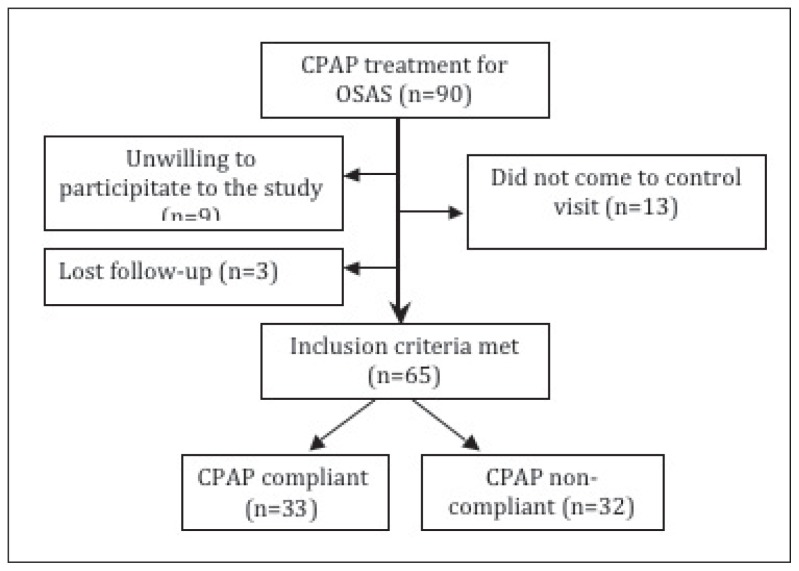
Flow chart for the study group was shown in Figure 1. Sixty-five patients were included in the final analysis (57 males, 8 females). The mean age was 45.3 (10.6) years, AHI 38.0 (23.2), and BMI 31.7 (4.9) kg/m2. Demographic and clinic characteristics of the study population are shown in Table 1.
Figure 1.
Flow chart for patient inclusion.
Table 1.
Demographic and clinical characteristics of patient population (n=65).
| CPAP compliant (n=33) | CPAP noncompliant (n=32) | P | |
|---|---|---|---|
|
| |||
| Age (mean [SD]) | 48.76 (9.2) | 41.72 (10.9) | .006 |
| Gender | |||
| Male | 28 (84.8%) | 29 (90.6%) | .479 |
| Female | 5 (15.2%) | 3 (9.4%) | |
| Smoking history | |||
| No | 9 | 9 | .902 |
| Yes | 12 | 10 | |
| Ex-smoker | 12 | 13 | |
| AHI (mean [SD]) | 40.7 (19.6) | 35.2 (26.4) | .348 |
| BMI (mean [SD]) | 31.3 (3.8) | 32.2 (5.8) | .445 |
| Comorbidity (%) | 73 | 37 | .004 |
| ESS (mean [SD]) | 10.8 (5.2) | 11.5 (4.9) | .555 |
| FEV1(%) (mean [SD]) | 108.3 (16.1) | 99.2 (11.7) | .011 |
| FVC (%) (mean [SD]) | 113.0 (15.2) | 106.0 (10.6) | .034 |
AHI: Apnea-hypopnea index; BMI: body mass index; CPAP: continuous positive airway pressure, ESS: Epworth sleepiness scale; FVC: forced vital capacity; SD: standard deviation.
The mean age was higher among the CPAP compliant group (P=.006); therefore, age was adjusted for comparisons between groups. CPAP compliant group had more co-morbidities also (P=.004), which were hypertension 39.4%, diabetes 6.1%, coronary artery disease 6.1%, hypercholesterolemia 3%, and other 18.2%. Hypertension was present in 12.5%, diabetes in 3.1%, and hypertension and diabetes in 9.4% in the CPAP noncompliant group.
Difference was not detected in mean AHI values with respect to CPAP compliance (P.348). Subgroup analysis with respect to OSA severity was performed. The CPAP compliant group consisted of 9.1% (n=3) mild OSA, 24.2% (n=8) moderate OSA, and 66.7% (n=22) severe OSA patients, whereas the noncompliant group had 21.9% (n=7) mild, 31.2% (n=10) moderate, and 46.9% (n=15) severe sleep apneics. Compliance was detected higher among patients with higher AHI (P=.025, r=0.39), but gender did not affect compliance.
Control visits were performed at 42.1 (16.0) and 35.5 (8.8) days in CPAP compliant and noncompliant groups, respectively (P=.594). CPAP usage was reported as 6.2 (0.9) hours per night in the CPAP compliant group. Cardiopulmonary exercise data with respect to CPAP compliance is shown in Table 2.
Table 2.
Cardiopulmonary exercise test findings with respect to CPAP compliance.
| CPAP compliant (n=33) (mean [SD]) | CPAP noncompliant (n=32) (mean [SD]) | |||||
|---|---|---|---|---|---|---|
| First CPET | Control CPET | Pa | First CPET | Control CPET | Pa | |
|
| ||||||
| VO2 max (mL/min) | 1979.8 (622.2) | 1871.2 (459.9) | .100 | 1957.2 (435.6) | 1839.2 (419.6) | .065 |
| VO2 max. (%) | 87.4 (21.8) | 83.7 (18.6) | .196 | 78.3 (18.3) | 73.2 (16.1) | .053 |
| VO2 max/kg | 22.5 (6.6) | 21.3 (5.3) | .111 | 21.3 (5.7) | 19.9 (5.4) | .050 |
| VO2 max/kg (%) | 68.8 (18.8) | 65.9 (16.4) | .203 | 59.1 (16.5) | 55.0 (13.7) | .050 |
| VO2 (mL/min) | 2610.2 (777.6) | 2541.3 (624.5) | .334 | 2577.5 (602.6) | 2461.7 (597.6) | .143 |
| Work load (watt) | 137.6 (35.1) | 138.8 (33.0) | .601 | 148.0 (32.5) | 143.9 (33.9) | .043 |
| Work load (%) | 84.0 (16.3) | 85.0 (14.6) | .493 | 79.6 (17.9) | 77.1 (17.7) | .028 |
| AT (l/min) | 1.0 (0.3) | 0.9 (0.2) | .106 | 1.0 (0.3) | 0.9 (0.2) | .036 |
| AT (%pred max VO2) | 43.8 (14.2) | 41.1 (9.7) | .162 | 38.7 (12.7) | 34.3 (9.2) | .028 |
| HR (pulse/min) | 158.8 (16.0) | 154.7 (19.5) | .066 | 161.1 (18.2) | 156.3 (17.3) | .005 |
| HR (%) | 89.0 (7.8) | 86.8 (9.9) | .083 | 88.2 (8.2) | 85.4 (7.7) | .002 |
| O2 pulse (mL/pulse) | 12.4 (3.4) | 12.3 (2.9) | .798 | 12.0 (3.0) | 11.8 (2.5) | .505 |
| O2 pulse (%) | 98.6 (23.9) | 98.7 (21.7) | .958 | 88.8 (26.1) | 86.6 (21.8) | .410 |
| HRR | 19.8 (13.7) | 23.7 (17.5) | .088 | 21.6 (14.5) | 26.3 (13.4) | .005 |
| VE max (l/min) | 86.7 (28.4) | 80.3 (22.3) | .024 | 81.1 (21.5) | 76.3 (18.0) | .074 |
| VE max (%) | 74.0 (20.9) | 71.0 (16.8) | .301 | 68.7 (16.0) | 64.3 (13.5) | .046 |
| TV | 1.9 (0.5) | 1.8 (0.4) | .261 | 1.8 (0.5) | 1.8 (0.5) | .721 |
| TV (%) | 79.4 (15.2) | 83.7 (21.0) | .195 | 75.2 (16.7) | 74.2 (16.9) | .635 |
| RR | 42.1 (9.4) | 40.6 (8.2) | .062 | 40.8 (7.2) | 39.0 (7.9) | .030 |
| PetCO2 | 5.0 (0.5) | 5.1 (0.5) | .004 | 5.2 (0.4) | 5.2 (0.4) | .380 |
| PetO2 | 13.8 (0.6) | 13.6 (0.7) | .163 | 13.2 (0.6) | 13.1 (0.6) | .313 |
| VE/VO2 AT | 31.0 (5.2) | 30.2 (3.5) | .198 | 29.9 (2.7) | 30.5 (3.2) | .246 |
| VE/VVO2 AT | 28.4 (3.2) | 27.4 (2.6) | .003 | 27.4 (2.5) | 27.8 (2.7) | .399 |
P values for first and control CPET comparisons.
CPAP: Continuous positive airway pressure ventilation; AT: anaerobic threshold; HR: heart rate; HRR: heart rate reserve; RR: respiratory rate; TV: tidal volume; VE: ventilation.
WR% increased from 84.0 (16.3) to 85.0 (14.6) in the CPAP compliant group. Decrease in WR% was detected in the CPAP noncompliant group from 79.6 (17.9) to 77.1 (17.8) (P=.041) (Figure 2).
The peak VO2 uptake did not change in the CPAP compliant group (n=33) (22.5 [6.6] mL/[min.kg] vs 21.3 (5.3) mL/[min.kg]; P=.111), but decreased from 21.3 (5.7) mL/(min.kg) to 19.9 (5.4) mL/(min.kg) (P=.05) in the CPAP noncompliant group.
AT was detected as 1.0 (0.3) l/min in the initial admission and 0.9 (0.2) l/min in the control visit in the CPAP compliant group (P=.106). AT for the noncompliant group decreased from 1.0 (0.3) l/min in the first and 0.9 (0.2) l/min in the second visit (P=.036). AT as a ratio of the predicted maximum oxygen uptake also decreased significantly in the noncompliant group (from 38.7 [12.7%] to 34.3 [9.2%], P=.028) whereas the difference between the 2 measurements in the CPAP compliant group was not significant (from 43.8 [14.2%] to 41.1 [9.7%], P=.162).
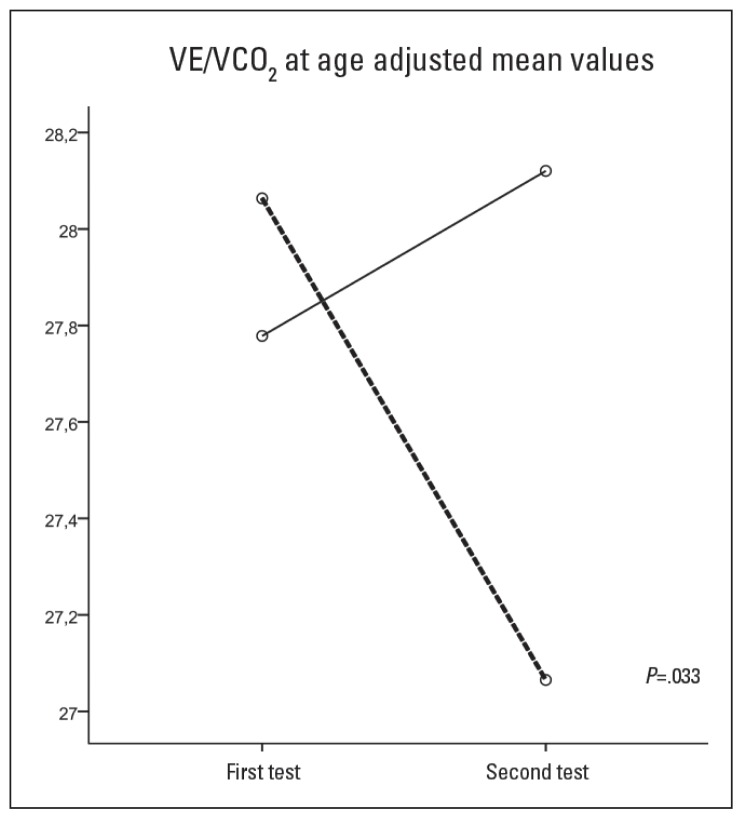
VE/VCO2 at AT decreased from 28.4 (3.2) to 27.4 (2.6) in the CPAP compliant group, whereas this was found to increase from 27.4 (2.5) to 27.8 (2.7) in the CPAP noncompliant group (P=.033) (Figure 3).
Figure 3.
The change in VE/VCO2 at AT from initial test to follow up, broken line (----) CPAP compliant group, straight line (___) CPAP non-compliant group.
Smoking history was not found to be related to exercise parameters among groups. Comparisons between the subgroup of patients with BMI normal+overweight and obese+morbid obese with respect to exercise parameters did not reach statistical significance.
DISCUSSION
In this study, we observed that workload, VO2, and AT decreased in OSA patients without treatment compared to patients using CPAP treatment. VE/VCO2 at AT value decreased in the CPAP compliant group compared to the noncompliant group. Exercise parameters in the CPAP noncompliant group declined over time although they were younger and co-morbidities were fewer. However, exercise parameters in the CPAP compliant group were preserved during the same period. We considered that the treatment was effective in these patients despite co-morbidity and higher age.
Patients with OSA may exhibit respiratory problems as a result of higher BMI. Obesity causes a decrease in functional residual capacity due to impaired expiratory reserve volume and respiratory system compliance. Increased BMI necessitates higher metabolic energy during muscular exercise that causes increased oxygen consumption and CO2 production. Nevertheless, changes in exercise response cannot be solely attributed to obesity because both study groups exhibited similar BMI. Furthermore, studies also display a multitude of abnormalities detected by CPET in OSA patients.
VO2max, HR recovery, and chronotropic exercise reserve were lower in a study consisting of moderate-to-severe OSA patients without cardiac co-morbidity.9 Lin et al found a lower right ventricular ejection fraction, VO2max, VO2 max/kg, AT, workload, and O2 pulse values in 20 moderate-to-severe OSA patients compared to controls.10 We detected lower VO2max/kg and VO2max/kg (%) values in our study group compared to predicted values.
Obese subjects with BMI >35 kg/m2 were included in a study by Vanhecke and coworkers, and patients with OSA (n=42) had a significantly lower oxygen uptake (17.6 mL/[min.kg]) than controls (n=50, 21.1 mL/[min.kg]).11 Increased HR and systolic and diastolic blood pressure were detected at rest, exercise, and recovery period in OSA patients as well.
However, in another study including subjects with BMI lower than 25 mg/m2, VO2max, AT, respiratory exchange ratio, blood pressure, and HR were not different between OSA and controls. OSA may not cause decreased functional capacity in lean patients; however, obesity may contribute to the decrease in cardiovascular capacity.12 We could not evaluate the effect of obesity on exercise parameters in detail because of the insufficient number of patients with normal weight (n=4).
In a study evaluating 23 normotensive, overweight OSA patients and 9 healthy subjects, OSA patients had a lower exercise HR and higher diastolic blood pressure, and a delayed systolic blood pressure recovery.13 Although 26% had hypertension in our study population, all patients showed exaggerated blood pressure response during exercise.
CPAP treatment has been shown to have favorable effects on exercise and functional parameters in patients with OSA. Schlosser et al. found that VO2max/kg increased from 15.3 (4.8) mL/(min.kg) to 18.5 (6.9) mL/(min.kg) after at least 6 months of CPAP treatment in 17 subjects with OSA compared to 8 subjects noncompliant to CPAP.14 In another study consisting of 20 moderate and severe OSA patients, lower respiratory reserve and higher AT and O2 pulse were detected after two months of CPAP treatment. Right ventricle ejection fraction, VO2max, VO2max/kg, and workload values were increased, whereas the decrease in VE/VCO2 did not reach statistical significance.15 We showed a significant increase in workload and decrease in VE/VCO2 despite the shorter period of CPAP treatment. AT, VO2max/kg, and VO2max/kg (%) values were preserved with CPAP; however, longer periods may be necessary for cardiac, vascular, and pulmonary adaptation after treatment. VO2 max/kg and HR remained unchanged after 2 to 3 weeks of CPAP treatment in OSA patients.16 Alonso-Fernández et al showed higher systolic and mean nocturnal blood pressure, higher nocturnal cathecholamine levels, lower cardiac output, and stroke volume response to exercise in consecutive 31 sleep apneics compared to controls. After 3 months of CPAP treatment and sham CPAP crossover, all indices of cardiac function response during exercise, including cardiac output and stroke volume, improved significantly with CPAP.17
We detected decrease in AT VE/VCO2 values in the CPAP compliant group than in the CPAP noncompliant group. VO2max, VCO2, and VE/VO2 remained unchanged. Hargens et al. found that VO2max was similar but VE, VE/VCO2, and VE/VO2 increased in OSA patients compared to controls using bicycle ergometer.18 They explained this effect by repeated hypoxia episodes causing increased ventilatory response to exercise by increasing the release of endothelin- 1 that in turn intensified chemoreflex sensitivity. There are a few mechanisms to explain an increased ventilatory requirement for a given CO2 production: increased dead space and ineffective ventilation, early lactic acidosis, and abnormal activity of chemoreflex receptors.19–23 The elevated VE/VCO2 slope was also associated with leptin levels.24 Lower VE/VCO2 in patients using CPAP may reflect a decrease of chemo-reflex sensitivity and dead space ventilation, and improvement in pulmonary artery pressure and cardiac functions with treatment. Studies also show that VE/VCO2 slope, VE/VCO2 at AT, and lowest VE/VCO2 values may be a good prognostic predictor for chronic pulmonary and cardiac diseases.25–27 AT <11 mL/(min.kg) and VE/VCO2 slope >34 were found to be sensitive in premature death risk in 223 patients with cardiac failure, and the risk increased by 9.6-fold.28 Higher VE/VCO2 nadir was found to be related with a shorter survival in patients suffering from heart failure. 25 We observed a decrease in VE/VCO2 at AT in OSA with CPAP treatment. We suggest that CPAP treatment may improve disease progression, and it has a positive impact on survival.
The main limitation of the study is that CPAP compliance was based on subjective reports by patients, and it is known that patients may overestimate their use of treatment. However, we have questioned about CPAP usage thoroughly and believe that the effect of this on our results is negligible.
In conclusion, we found that OSA patients reached higher workload percentages with CPAP treatment in CPET. The exercise capacity was preserved in OSA with CPAP. We suggest that lower VE/VCO2 in CPAP compliant patients may be caused by a decrease in dead space ventilation, and an improvement in pulmonary artery pressure and cardiac function. Future studies may be necessary to evaluate a long-term impact of CPAP treatment on CPET in OSA patients.
Acknowledgments
We would like to thank Seval Kul for the statistical evaluation of the data.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep disordered breathing among middle aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 3.Phillips CL, McEwen BJ, Morel-Kopp MC, Yee BJ, Sullivan DR, Ward CM, et al. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: a randomised, placebo-controlled crossover study. Thorax. 2012;67(7):639–44. doi: 10.1136/thoraxjnl-2011-200874. [DOI] [PubMed] [Google Scholar]
- 4.Sharma SK, Agrawal S, Damodaran D, Sreenivas V, Kadhiravan T, Lakshmy R, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–86. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 5.Weber KT. Principles and applications of cardio-pulmonary exercise testing. In: Fishman AP, editor. Pulmonary Diseases and Disorders. 3rd ed. Mc Graw-Hill; 1998. pp. 575–588. [Google Scholar]
- 6.Johns MW. A new method for measuring day-time sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 7.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical spesifications. 1st ed. Wenchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 8.American Thoracic Society. American College of Chest Physicians ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–27. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 9.Nanas S, Sakellariou D, Kapsimalakou S, Dimopoulos S, Tassiou A, Tasoulis A, et al. Heart rate recovery and oxygen kinetics after exercise in obstructive sleep apnea syndrome. Clin Cardiol. 2010;33:46–51. doi: 10.1002/clc.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CC, Hsieh WY, Chou CS, Liaw SF. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol. 2006;150:27–34. doi: 10.1016/j.resp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Vanhecke TE, Franklin BA, Zalesin KC, Sangal RB, deJong AT, Agrawal V, McCullough PA. Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest. 2008;134:539–545. doi: 10.1378/chest.08-0567. [DOI] [PubMed] [Google Scholar]
- 12.Rizzi CF, Cintra F, Risso T, Pulz C, Tufik S, de Paola A, Poyares D. Exercise capacity and obstructive sleep apnea in lean subjects. Chest. 2010;137:109–114. doi: 10.1378/chest.09-1201. [DOI] [PubMed] [Google Scholar]
- 13.Kaleth AS, Chittenden TW, Hawkins BJ, Hargens TA, Guill SG, Zedalis D, et al. Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med. 2007;8:160–168. doi: 10.1016/j.sleep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Schlosser BM, Walther JW, Rasche K, Bauer TT, Orth M, de Zeeuw J, et al. Improvement of cardiopulmonary exercise capacity in patients with obstructive sleep apnea syndrome under CPAP therapy. Med Klin. 2006;101:107–113. doi: 10.1007/s00063-006-1015-y. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Lin CK, Wu KM, Chou CS. Effect of treatment by nasal CPAP on cardiopulmonary exercise test in obstructive sleep apnea syndrome. Lung. 2004;182:199–212. doi: 10.1007/s00408-004-2502-7. [DOI] [PubMed] [Google Scholar]
- 16.Przybyłowski T, Bielicki P, Kumor M, Hildebrand K, Maskey-Warzechowska M, Wiwała J, et al. Influence of nasal continuous positive airway pressure on response to exercise in patients with obstructive sleep apnea syndrome. Pneumonol Alergol Pol. 2006;74:39–44. [PubMed] [Google Scholar]
- 17.Alonso-Fernández A, García-Río F, Arias MA, Mediano O, Pino JM, Martínez I, Villamor J. Obstructive sleep apnoea–hypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27:207–215. doi: 10.1093/eurheartj/ehi621. [DOI] [PubMed] [Google Scholar]
- 18.Hargens TA, Guill SG, Aron A, Zedalis D, Gregg JM, Nickols-Richardson SM, Herbert WG. Altered ventilatory responses to exercise testing in young adult men with obstructive sleep apnea. Respir Med. 2009;103:1063–1069. doi: 10.1016/j.rmed.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Guazzi M, Reina G, Tumminello G, Guazzi MD. Exercise ventilation inefficiency and cardiovascular mortality in heart failure: the critical independent prognostic value of the arterial CO2 partial pressure. Eur Heart J. 2005;26:472–480. doi: 10.1093/eurheartj/ehi060. [DOI] [PubMed] [Google Scholar]
- 20.Wasserman K, Zhang YY, Gitt A, Belardinelli R, Koike A, Lubarsky L, Agostoni PG. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96:2221–7. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- 21.Myers J, Salleh A, Buchanan N, Smith D, Neutel J, Bowes E, Froelicher VF. Ventilatory mechanisms of exercise intolerance in chronic heart failure. Am Heart J. 1992;124:710–9. doi: 10.1016/0002-8703(92)90282-z. [DOI] [PubMed] [Google Scholar]
- 22.Tomita T, Takaki H, Hara Y, Sakamaki F, Satoh T, Takagi S, et al. Attenuation of hypercapnic carbon dioxide chemosensitivity after postinfarction exercise training: possible contribution to the improvement in exercise hyperventilation. Heart. 2003;89:404–410. doi: 10.1136/heart.89.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciarka A, Cuylits N, Vachiery JL, Lamotte M, Degaute JP, Naeije R, van de Borne P. Increased peripheral chemoreceptors sensitivity and exercise ventilation in heart transplant recipients. Circulation. 2006;113:252–7. doi: 10.1161/CIRCULATIONAHA.105.560649. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell CP, Tankersley CG, Polotsky VP, Schwartz AR, Smith PL. Leptin, obesity, and respiratory function. Respir Physiol. 2000;119:163–170. doi: 10.1016/s0034-5687(99)00111-5. [DOI] [PubMed] [Google Scholar]
- 25.Myers J, Arena R, Oliveira RB, Bensimhon D, Hsu L, Chase P, et al. The lowest VE/VCO2 ratio during exercise as a predictor of outcomes in patients with heart failure. J Card Fail. 2009;15:756–762. doi: 10.1016/j.cardfail.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palange P, Ward SA, Carlsen KH, Casaburi R, Gallagher CG, Gosselink R, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29:185–209. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 27.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coates AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2) slope and peak VO (2) Eur Heart J. 2000;21:154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 28.Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3079–3084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]