Abstract
Based on the structural framework of a pyriproxyfen metabolite, nineteen oxime ester derivatives were synthesized via reaction of the carboxylic acids with 4-(2-(2-pyridinyloxy)ethoxy)benzaldehyde oxime. The corresponding structures were comprehensively characterized by 1H-nuclear magnetic resonance (NMR), 13C-NMR, and electrospray ionization high-resolution mass spectrometry (ESI-HRMS). All of the compounds were screened for their insecticidal activities against Plutella xylostella and Myzus persicae, and for their ovicidal activities against Helicoverpa armigera eggs. The results obtained show that most of the oxime ester derivatives displayed moderate to high insecticidal activities and ovicidal activities at a concentration of 600 μg/mL. In particular, the ovicidal activity of compounds 5j, 5o, 5p, 5q, and 5s was determined to be 100%. Importantly, some of the compounds presented even higher biological activities than the reference compound pyriproxyfen. For example, compound 5j displayed an insecticidal activity value of 87.5% against Myzus persicae, whereas the activity value of pyriproxyfen was 68.3% at a concentration of 600 μg/mL. Among the synthesized compounds 5j and 5s exhibited broad biological activity spectra.
Keywords: pyriproxyfen metabolite, oxime ester derivatives, insecticidal activity, ovicidal activity
1. Introduction
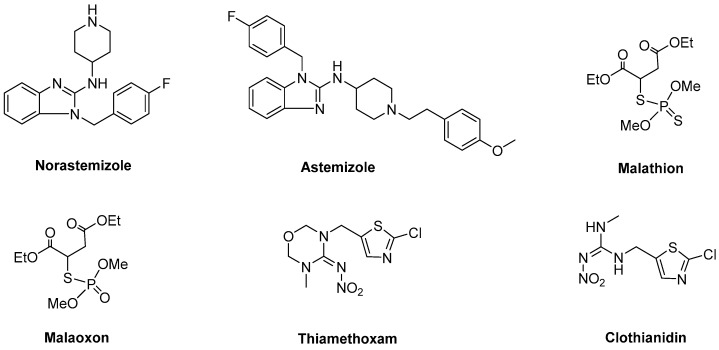
In the 1930s, various research groups reported that prontosil exhibits a suitable therapeutic effect in mice infected with different bacterial strands. However, the results were deemed abnormal with respect to the antibacterial activity of prontosil being absent in vitro [1]. The metabolite of prontosil, sulfanilamide, exhibited a strong antibacterial activity in vivo, indicating that the antibacterial activity of prontosil could mainly be attributed to this metabolite [1,2]. Since then, dozens of sulfonamide species have been developed and used for the treatment of bacterial diseases. Furthermore, the use of drug metabolites as lead compounds has become one of the new drug development approaches widely used in the pharmaceutical industry. For example, norastemizole (Figure 1), stemming from astemizole (Figure 1), was found to increase selectivity in the process of targeting receptors [3].
Figure 1.
Chemical structures of malathion, malaoxon, thiamethoxam, clothianidin, astemizole, and norastemizole.
Concurrently, pesticides also generate a series of pesticide metabolites in organisms through a variety of metabolic pathways. In some cases, these pesticide metabolites may exhibit a good biological activity. For example, malaoxon (Figure 1) represents the metabolite of malathion (Figure 1). However, the inhibitory effect of malaoxon to acetylcholinesterase was found to be increased compared to malathion [4]. A more common example is thiamethoxam (Figure 1) that demonstrates a poor insecticidal activity compared to clothianidin (Figure 1), which is generated by degradation of thiamethoxam [5,6]. In our previous studies, a series of diamide derivatives were designed and synthesized based on a benalaxyl metabolite and the target compounds showed excellent fungicidal activities against Phytophthora capsici and Rhizoctonia solani [7]. From these findings, it can be hypothesized that the chemical structure of the pesticide metabolites may also provide critical information for the future development of pesticide lead compounds.
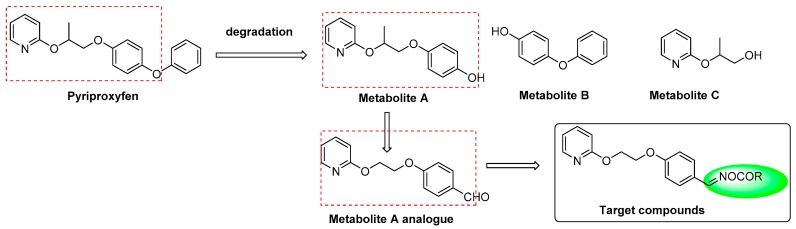
Pyriproxyfen, a juvenile hormone analogue, proves to be highly selective for target organisms with lower mammalian toxicity, and has been clinically used to control the spread of a wide range of arthropods [8,9]. 4-(2-(pyridin-2-yloxy)propoxy)-phenol (metabolite A, Figure 2), 4-phenoxyphenol (metabolite B, Figure 2), and 3-(pyridin-2-yloxy)butan-1-ol (metabolite C, Figure 2) were generated from degradation of pyriproxyfen ex vivo, as well as in animals and plants in vivo [10,11,12,13]. Metabolite A was synthesized and demonstrated a moderate insecticidal activity as well as ovicidal activity. 4-(2-(2-pyridinyloxy)ethoxy)benzaldehyde, a metabolite A analogue, retained the structural skeleton of pyriproxyfen but exhibited potent ovicidal activities against Plutella xylostella, Myzus persicae, and Helicoverpa armigera eggs, with overall activity values of 40.2%, 32.5%, and 75.2%, respectively. Therefore, we hypothesize that 4-(2-(2-pyridinyloxy)ethoxy) benzaldehyde may be used as a lead compound for further studies.
Figure 2.
Molecular structure of the target compounds.
In 1963, tranid, the first oxime ester insecticide, was developed. Thereafter, oxime carbamate insecticides (e.g., methomyl) and oxime phosphate insecticides (e.g., phoxim) have been developed. These oxime ester compounds have attracted considerable attention in the scientific community due to their broad-spectrum biological activities as plant virucides [14], fungicides [15,16], insecticides [17,18,19], and herbicides [20]. In our group, oleanolic acid and avermectin oxime ester derivatives were synthesized and exhibited suitable biological activities, providing further incentive to study the oxime ester group in more detail [16,21]. Continuing this work, nineteen novel oxime ester derivatives were synthesized (cf. Scheme 1) and their ovicidal activities against Plutella xylostella, Myzus persicae, and Helicoverpa armigera eggs were evaluated.
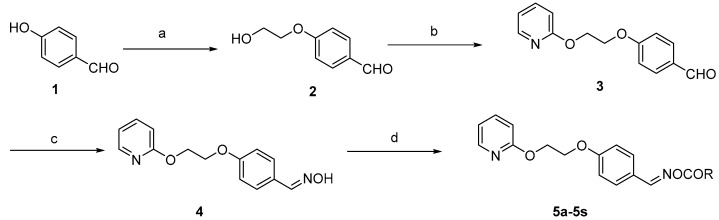
Scheme 1.
General synthetic route for target compounds. Reagents and conditions: (a) BrCH2CH2OH, K2CO3, dimethylformamide (DMF), 100 °C, 12 h; (b) i. NaH, DMF, 0–5 °C; ii. 2-fluoropyridine, DMF, 55 °C, 6 h; (c) NH2OH/NaOH, EtOH/H2O, r.t.; (d) substituted carboxylic acid, dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP), dichloromethane (DCM).
2. Results and Discussion
2.1. Chemistry
As shown in Scheme 1, nineteen oxime ester derivatives were synthesized. Compound 2 was prepared by reacting commercially available 4-hydroxybenzaldehyde with 2-bromoethanol in N,N-dimethylformamide as previously reported in the literature [22]. A subsequent reaction with 2-fluoropyridine in the presence of sodium hydride led to the formation of intermediate 3 [23]. Upon addition of sodium hydride, the mixture was placed in an ice bath due to the release of a large amount of heat as the reaction progressed. Next, intermediate 3 was directly converted to the key intermediate, 4, by treatment with hydroxylamine hydrochloride [24]. Finally, the corresponding target oxime esters (5a–5s) were obtained via intermediate 4 and reactions with carboxylic acid functionalities in the presence of dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP). The physical data of the target compounds 5a–5s are given in Table 1. 1H- and 13C-NMR spectra of all compounds are presented in the Supplementary Materials.
Table 1.
Physical data for compounds 5a–5s.
| Compound | R | Formula | Status | m.p./°C | Yield (%) |
|---|---|---|---|---|---|
| 5a | Ph- | C21H18N2O4 | White solid | 102–104 | 87 |
| 5b | 4-OCH3-Ph- | C22H20N2O5 | White solid | 101–103 | 91 |
| 5c | 2-F-Ph- | C21H17FN2O4 | White solid | 107–109 | 91 |
| 5d | 4-OCH3-Ph-CH2- | C23H22N2O5 | White solid | 97–99 | 90 |
| 5e | 4-CH3-Ph-CH2- | C23H22N2O4 | White solid | 102–104 | 88 |
| 5f | 4-OC2H5-Ph- | C23H22N2O5 | White solid | 103–105 | 83 |
| 5g | 2,4-Cl-Ph-CH2- | C22H18Cl2N2O4 | White solid | 102–104 | 71 |
| 5h | β-Naphthyl-CH2- | C26H22N2O4 | White solid | 112–114 | 94 |
| 5i | 2-(4-chlorophenyl)-3-methylpropyl- | C25H25ClN2O4 | Pale yellow solid | 75–77 | 85 |
| 5j | 2-furyl- | C19H16N2O5 | White solid | 130–132 | 92 |
| 5k | 3-(2-Cl-3,3,3-F-1-propenyl)-2,2-Me-cycloproyl- | C23H22ClF3N2O4 | Yellow solid | 141–143 | 86 |
| 5l | 3-(2-Cl-Py)- | C20H16ClN3O4 | White solid | 133–135 | 90 |
| 5m | 2-(3,6-Cl-Py)- | C20 H15Cl2N3O4 | Yellow solid | 88–90 | 87 |
| 5n | 2,2-Me-3-(2-Me-1-propenyl)-cycloproyl- | C24H28N2O4 | White solid | 99–101 | 85 |
| 5o | CH3CH2CH2- | C18H20N2O4 | White solid | 71–73 | 93 |
| 5p | CH3- | C16H16N2O4 | White solid | 85–87 | 96 |
| 5q | 2-thienyl- | C19H16N2O4S | White solid | 132–134 | 88 |
| 5r | 3-Py- | C20H17N3O4 | White solid | 163–165 | 90 |
| 5s | 2,2,3,3-Me-cyclopropyl- | C22H26N2O4 | Yellow solid | 91–93 | 89 |
Since the oxime group can exist both in the E- or Z-configuration, it was necessary to determine the geometries of all the oxime target compounds 5a–5s. Attempts to obtain X-ray quality single crystals of the oxime intermediates or target products proved unsuccessful in this study. However, the oxime geometry was considered to be in the E-configuration, an assumption that is in agreement with published data [24].
2.2. Biological Activities
2.2.1. Insecticidal Activities
As highlighted in Table 2, the insecticidal activities of all target compounds against Myzus persicae and Plutella xyllostella were evaluated. With the substituent being a phenyl group, the results indicated that compounds 5a–5i possessed 3.6% to 15.7% insecticidal activities against Myzus persicae at a concentration of 600 μg/mL, demonstrating that the substituent species and the position of the phenyl ring had no significant effect on the insecticidal activity. Compounds 5l, 5m, and 5s showed 49.4%, 58.1%, and 25.9% insecticidal activities against Myzus persicae at a concentration of 600 μg/mL. As the substituent was changed to a pyridine group, compounds 5l and 5m, but not compound 5r, exhibited improved insecticidal activities compared to compounds 5a–5i. Compounds 5o and 5p, bearing an alkyl substituent, displayed moderate activity against Myzus persicae. Some of the synthesized compounds exhibited higher insecticidal activities than the reference compound pyriproxyfen. For instance, the activity rates of compounds 5j, 5q and 5s against Myzus persicae were 87.5%, 73.6%, and 72.8% at 600 μg/mL, respectively, whereas pyriproxyfen featured a value no higher than 70% at the same concentration. Moreover, some of the compounds exhibited moderate insecticidal activities against Myzus persicae upon reduction of the concentration to 200 μg/mL; for example, compounds 5j, 5q, and 5s showed insecticidal activities of 53.8%, 36.4%, and 43.1%, respectively, further suggesting that the futyl, thienyl and 2,2,3,3-Me-cyclopropyl groups exhibit a great influence on the activities. Many of the synthesized compounds exhibited no to moderate insecticidal activities against Plutella xyllostella at a concentration of 600 μg/mL. Compounds 5b, 5e, 5f, 5g, 5l, 5n, 5o, and 5p showed no or very weak insecticidal activities against Plutella xyllostella. The activity rates of compounds 5j and 5s were 68.7% and 57.1%, respectively. Considering Plutella xyllostella, any structure–activity relationships were not obvious.
Table 2.
Insecticidal and ovicidal activities of oxime ester derivatives (activity provided in %).
| Compound | Myzus persicae | Plutella xyllostella | Helicoverpa armigera Eggs | ||
|---|---|---|---|---|---|
| 600 μg/mL | 200 μg/mL | 600 μg/mL | 600 μg/mL | 200 μg/mL | |
| 5a | 3.6 ± 0.7 a | 41.7± 1.9 | 20.9 ± 0.7 | ||
| 5b | 13.3 ± 1.6 | 7.7 ± 2.1 | 23.2 ± 0.8 | ||
| 5c | 12.6 ± 2.1 | 38.5 ± 3.0 | 28.8 ± 1.1 | ||
| 5d | 8.7 ± 1.5 | 20.0 ± 1.3 | 15.3 ± 0.4 | ||
| 5e | 11.3 ± 0.4 | 0 ± 0.0 | 47.6 ± 0.1 | ||
| 5f | 15.7 ± 0.2 | 10.0 ± 2.5 | 9.2 ± 1.8 | ||
| 5g | 11.6 ± 1.0 | 0 ± 0.0 | 55.7 ± 3.1 | ||
| 5h | 6.7 ± 0.4 | 40.0 ± 2.0 | 51.6 ± 3.3 | ||
| 5i | 11.3 ± 0.4 | 33.3 ± 1.9 | 45.7 ± 1.7 | ||
| 5j | 87.5 ± 5.3 | 53.8 ± 4.5 | 68.7 ± 3.6 | 100.0 ± 0.0 | 45.3 ± 2.7 |
| 5k | 45.2 ± 2.6 | 26.7 ± 1.8 | 67.6 ± 3.3 | ||
| 5l | 49.4 ± 5.7 | 15.4 ± 0.4 | 62.4 ± 3.1 | ||
| 5m | 58.1 ± 1.9 | 34.3 ± 1.0 | 28.5 ± 0.8 | ||
| 5n | 39.6 ± 2.7 | 15.1 ± 0.8 | 43.3 ± 3.5 | ||
| 5o | 57.7 ± 0.4 | 9.6 ± 2.1 | 100.0 ± 0.0 | 67.8 ± 5.2 | |
| 5p | 53.2 ± 3.6 | 12.8 ± 1.3 | 100.0 ± 0.0 | 79.5 ± 4.2 | |
| 5q | 73.6 ± 2.9 | 36.4 ± 2.1 | 43.1 ± 3.3 | 100.0 ± 0.0 | 37.1 ± 1.6 |
| 5r | 25.9 ± 2.2 | 27.7 ± 1.0 | 73.4 ± 5.1 | ||
| 5s | 72.8 ± 1.2 | 41.3 ± 2.4 | 57.1 ± 1.4 | 100.0 ± 0.0 | 64.3 ± 2.0 |
| Pyriproxyfen | 68.3 ± 4.7 | 38.5 ± 1.3 | 76.0 ± 5.3 | 100.0 ± 0.0 | 54.8 ± 1.7 |
a Each value represents the mean ± the standard error of the mean (SEM) of three replicates.
2.2.2. Ovicidal Activities
Besides insecticidal activities, the ovicidal activities of the new compounds against Helicoverpa armigera eggs were also explored. As shown in Table 2, most of the target compounds exhibited moderate to excellent ovicidal activities against Helicoverpa armigera eggs at a concentration of 600 μg/mL. Compounds 5a, 5b, 5c, 5d, 5f, and 5m showed limited ovicidal activities at 600 μg/mL, with activity values of less than 30%. Compounds 5j, 5o, 5p, 5q, and 5s all featured 100% activity at 600 μg/mL. At a concentration of 200 μg/mL, compounds 5o, 5p, and 5s still exhibited ovicidal activities of 67.8%, 79.5%, and 64.3% against Helicoverpa armigera eggs, values that prove to be higher than that of pyriproxyfen (i.e., 54.8%). Compound 5j featured an ovicidal activity value of 45.3% against Helicoverpa armigera eggs. Similar to that of insecticidal activities against Myzus persicae, compounds 5j, 5q, and 5s exhibited more potent ovicidal activities against Helicoverpa armigera eggs. However, compounds 5o and 5p with moderate insecticidal activities against Myzus persicae, showed the best ovicidal activities against Helicoverpa armigera eggs. The latter finding may indicate that the presence of alkyl groups could improve the overall ovicidal activity.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
All reagents used were of analytical grade and the solvents were dried according to standard procedures. 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance (Bruker Co., Karlsruhe, Germany) 300 spectrometer (300 MHz, 1H; 75 MHz, 13C) in CDCl3 as a solvent and with tetramethylsilane as the internal standard. High-resolution mass spectra (ESI-HRMS) were recorded on a Bruker Daltonics Bio-TOF-Q III mass spectrometer (Bruker Co., Karlsruhe, Germany). The melting points were measured on a YRT-3 apparatus and the values are shown in the uncorrected form. Column chromatographic purification was carried out using 200–300 mesh silica gel. The reactions were monitored by analytical thin-layer chromatography (TLC) carried out on silica gel GF/UV 254 TLC plates.
3.1.2. Synthesis of 4-(2-Hydroxyethoxy)benzaldehyde (2)
To a solution of 4-hydroxybenzaldehyde (10.0 g, 82.0 mmol) in dry DMF (80 mL), K2CO3 (22.6 g, 163.8 mmol) and 2-bromoethanol (12.3 g, 98.3 mmol) were added. The reaction mixture was heated to 100 °C for 12 h with monitoring by TLC. The resulting solution was extracted with ethyl acetate (3 × 80 mL) and dried over anhydrous Na2SO4. The solvents of the combined organic layer were removed under reduced pressure and the residue was purified by flash chromatography on silica gel (200–300 mesh) using petroleum ether and ethyl acetate (v/v = 8:1) as eluents to afford compound 2 as a pale yellow liquid (11.3 g, 83%). 1H-NMR (300 MHz, CDCl3) δ 9.86 (s, 1H, PhCHO), 7.74–7.90 (m, 2H, Ar-H), 6.92–7.10 (m, 2H Ar-H), 4.13–4.20 (m, 2H, PhOCH2), 3.95–4.04 (m, 2H, C H2OH).
3.1.3. Synthesis of 4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde (3)
To an ice-cooled solution of compound 2 (5.0 g, 30.1 mmol) in DMF (40 mL), 60% sodium hydride (1.4 g, 36.1 mmol) was slowly added within 10 min. After stirring for an additional 30 min, 2-fluoropyridine (3.5 g, 36.1 mmol) was added. The resulting mixture was then heated to 55 °C and allowed to react for another 6 h. The reaction was quenched by addition of ice water and the resulting crude product was extracted with ethyl acetate (3 × 50 mL) and dried over anhydrous Na2SO4. The combined organic layers were concentrated in vacuo and then purified by flash chromatography on silica gel (200–300 mesh) using petroleum ether and ethyl acetate (v/v = 10:1) as eluent to provide compound 3 as a white solid (6.4 g, 88%). 1H-NMR (300 MHz, CDCl3): δ 9.86 (s, 1H, PhCHO), 8.11–8.17 (m, 1H, Ar-H), 7.78–7.86 (m, 2H, Ar-H), 7.52–7.60 (m, 1H, Ar-H), 6.99–7.08 (m, 2H Ar-H), 6.84–6.92 (m, 1H Ar-H), 6.74–6.80 (m, 1H Ar-H), 4.58–4.78 (m, 2H, PyOCH2), 4.31–4.45 (m, 2H, PhOCH2).
3.1.4. Synthesis of 4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde oxime (4)
Hydroxylamine hydrochloride (1.5 g, 26.1 mmol) was dissolved in water (10 mL) and neutralized with aqueous sodium hydroxide solution (10%). A solution of compound 3 (4.4 g, 18.1 mmol) in ethanol was slowly added to this mixture. The resulting suspension was stirred at room temperature with monitoring by TLC. After reaction completion, the solvent ethanol was removed in vacuo. The resulting solution was extracted with ethyl acetate (3 × 30 mL) and dried over anhydrous Na2SO4. The solvent of the combined organic layers was removed in vacuo to provide a residue which was then purified by silica gel (200–300 mesh) column chromatography using petroleum ether and ethyl acetate (v/v = 6:1) as eluents to afford compound 4 as a white solid (4.2 g, 90%). m.p.: 81–83 °C; 1H-NMR (300 MHz, CDCl3) δ 8.19 (dd, J = 5.0 Hz, 1.6 Hz, 1H, Ar-H), 8.12 (s, 1H, CH=N), 7.58–7.67 (m, 1H, Ar-H), 7.51–7.57 (m, 2H, Ar-H), 7.48 (s, 1H, NOH), 6.96–7.03 (m, 2H Ar-H), 6.89–6.96 (m, 1H Ar-H), 6.84 (d, J = 8.4 Hz, 1H, Ar-H), 4.65–4.84 (m, 2H, PyOCH2), 4.26–4.51 (m, 2H, PhOCH2). 13C-NMR (75 MHz, CDCl3): 163.18, 159.96, 149.44, 146.49, 138.79, 128.31, 125.03, 116.95, 114.78, 111.40, 66.43, 64.11; HRMS (ESI) calcd. for C14H15N2O4 (M + H)+ 275.1032, found 275.1031.
3.1.5. General Procedure for the Synthesis of 4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde oxime ester derivatives (5a–5s)
A solution of the appropriate carboxylic acid (7.5 mmol) and DCC (7.5 mmol) was stirred in DCM (20 mL) in the presence of DMAP (100 mg). Compound 4 (5.8 mmol) was added to the reaction mixture upon stirring and stirring was continued for another 10 h with monitoring by TLC. The resulting liquid was collected by filtration and evaporated under reduced pressure. The resulting residue was purified by column chromatography on silica (200–300 mesh) using petroleum ether and ethyl acetate (v/v = 4:1~16:1) as eluents.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-benzoyl-oxime (5a). 1H-NMR (300 MHz, CDCl3) δ 8.50 (s, 1H, CH=N), 8.24–8.01 (m, 3H, Ar-H), 7.84–7.68 (m, 2H, Ar-H), 7.65–7.54 (m, 2H, Ar-H), 7.49 (t, J = 7.5 Hz, 2H, Ar-H), 7.05–6.96 (m, 2H, Ar-H), 6.87–6.93 (m, 1H, Ar-H), 6.80 (d, J = 8.4 Hz, 1H, Ar-H), 4.76–4.66 (m, 2H, PyOCH2), 4.45–4.33 (m, 2H, PhOCH2); 13C-NMR (75 MHz, CDCl3) δ 163.31, 161.70, 156.30, 146.73, 138.73, 133.28, 130.23, 129.68, 128.85, 128.52, 122.83, 117.10, 115.08, 111.37, 66.69, 63.86; HRMS (ESI) calcd. for C21H19N2O4 (M + H)+ 363.1339, found 363.1341.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(4-methoxybenzoyl)-oxime (5b). 1H-NMR (300 MHz, CDCl3) δ 8.47 (s, 1H, CH=N), 8.16 (dd, J = 5.0 Hz, 1.5 Hz, 1H, Ar-H), 8.02–8.12 (m, 2H, Ar-H), 7.75 (d, J = 8.8 Hz, 2H, Ar-H), 7.66–7.52 (m, 1H, Ar-H), 7.07–6.87 (m, 5H, Ar-H), 6.80 (d, J = 8.4 Hz, 1H, Ar-H), 4.75–4.65 (m, 2H, PyOCH2), 4.43–4.30 (m, 2H, PhOCH2), 3.87 (s, 3H, OCH3); 13C-NMR (75 MHz, CDCl3) δ 163.84, 163.66, 163.30, 161.58, 155.92, 146.70, 138.71, 131.74, 130.14, 122.97, 121.03, 117.07, 115.03, 113.84, 111.34, 66.66, 63.86, 55.44; HRMS (ESI) calcd. for C22H21N2O5 (M + H)+ 393.1445, found 393.1444.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(2-fluorobenzoyl)-oxime (5c). 1H-NMR (300 MHz, CDCl3) δ 8.48 (s, 1H, CH=N), 8.22–8.011 (m, 1H, Ar-H), 8.07–7.95 (m, 1H, Ar-H), 7.80–7.67 (m, 2H, Ar-H), 7.66–7.51 (m, 2H, Ar-H), 7.26–7.14 (m, 2H, Ar-H), 7.07–6.95 (m, 2H, Ar-H), 6.86–6.92 (m, 1H, Ar-H), 6.80 (d, J = 8.4 Hz, 1H, Ar-H), 4.64–4.78(m, 2H, PyOCH2), 4.33–4.42 (m, 2H, PhOCH2); 13C-NMR (75 MHz, CDCl3) δ 163.29, 161.75, 156.69, 146.71, 138.71, 134.81, 132.30, 130.27, 124.18, 122.67, 117.13, 116.83, 115.07, 111.34, 66.67, 63.83; HRMS (ESI) calcd. for C21H18FN2O4 (M + H)+ 381.1245, found 381.1243.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(2-(4-methoxyphenyl)acetyl)-oxime (5d) 1H-NMR (300 MHz, CDCl3) δ 8.27 (s, 1H, CH=N), 8.20–8.09 (m, 1H, Ar-H), 7.72–7.61 (m, 2H, Ar-H), 7.61–7.51 (m, 1H, Ar-H), 7.30–7.23 (m, 2H, Ar-H), 7.02–6.93 (m, 2H, Ar-H), 6.92–6.85 (m, 3H, Ar-H), 6.78 (d, J = 8.4 Hz, 1H, Ar-H), 4.75–4.62 (m, 2H, PyOCH2), 4.45–4.26 (m, 2H, PhOCH2), 3.79 (s, 3H, OCH3), 3.73 (s, 2H, PhCH2CO); 13C-NMR (75 MHz, CDCl3) δ 169.27, 163.21, 161.54, 158.77, 155.74, 146.64, 138.66, 130.25, 130.02, 125.31, 122.63, 117.02, 114.95, 114.03, 111.25, 66.58, 63.77, 55.17, 39.05; HRMS (ESI) calcd. for C23H23N2O5 (M + H)+ 407.1601, found 407.1601.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(2-(4-methylphenyl)acetyl)-oxime (5e). 1H-NMR (300 MHz, CDCl3) δ 8.27 (s, 1H, CH=N), 8.15 (dd, J = 4.9 Hz, 1.6 Hz, 1H, Ar-H), 7.66 (d, J = 8.8 Hz, 2H, Ar-H), 7.54–7.62 (m, 1H, Ar-H), 7.30–7.18 (m, 2H, Ar-H), 7.15 (d, J = 7.9 Hz, 2H, Ar-H), 6.97 (d, J = 8.8 Hz, 2H, Ar-H), 6.86–6.94 (m, 1H, Ar-H), 6.79 (d, J = 8.3 Hz, 1H, Ar-H), 4.84–4.60 (m, 2H, PyOCH2), 4.44–4.28 (m, 2H, PhOCH2), 3.77 (s, 2H, PhCH2CO), 2.34 (s, 3H, PhCH3); 13C-NMR (75 MHz, CDCl3) δ 16919, 163.29, 161.61, 155.78, 146.72, 138.71, 136.88, 130.26, 130.10, 129.34, 129.13, 122.72, 117.08, 115.01, 111.34, 66.65, 63.83, 39.64, 21.06; HRMS (ESI) calcd. for C23H23N2O4 (M + H)+ 391.1652, found 391.1653.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(4-ethoxybenzoyl)-oxime (5f). 1H-NMR (300 MHz, CDCl3) δ 8.47 (s, 1H, CH=N), 8.16 (dd, J = 5.1 Hz, 1.3 Hz, 1H, Ar-H), 8.06–7.98 (m, 2H, Ar-H), 7.79–7.71 (m, 2H, Ar-H), 7.64–7.51 (m, 1H Ar-H), 7.06–6.84 (m, 5H, Ar-H), 6.80 (d, J = 8.3 Hz, 1H, Ar-H), 4.77–4.60 (m, 2H, PyOCH2), 4.33–4.42 (m, 2H, PhOCH2), 4.09 (q, J = 7.4 Hz, 2H, OCH2CH3), 1.45 (t, J = 7.4 Hz, 3H, OCH2CH3); 13C-NMR (75 MHz, CDCl3) δ 163.90, 163.32, 163.10, 161.59, 155.89, 146.72, 138.73, 131.75, 130.15, 117.08, 115.04, 114.27, 111.37, 66.68, 63.88, 63.76, 14.65; HRMS (ESI) calcd. for C23H23N2O5 (M + H)+ 407.1601, found 407.1604.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(2-(2,4-dichlorobenzoyl))acetyl)-oxime (5g). 1H-NMR (300 MHz, CDCl3) δ 8.27 (s, 1H, CH=N), 8.14–8.17 (m, 1H, Ar-H), 7.64–7.68 (m, 2H, Ar-H), 7.57–7.61 (m, 1H, Ar-H), 7.28–7.32 (m, 3H, Ar-H), 7.02–6.94 (m, 2H, Ar-H), 6.93–6.86 (m, 1H, Ar-H), 6.85–6.69 (m, 1H, Ar-H), 4.76–4.62 (m, 2H, PyOCH2), 4.42–4.29 (m, 2H, PhOCH2), 3.75 (s, 2H, PhCH2CO); 13C-NMR (75 MHz, CDCl3) δ 168.63, 163.26, 161.70, 155.96, 146.69, 138.72, 130.64, 130.12, 128.79, 122.51, 117.08, 115.05, 111.32, 66.65, 63.82, 39.29; HRMS (ESI) calcd. for C22H20ClN2O4 (M + H)+ 411.1106, found 411.1107.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(2-(2-naphthalenyl)acetyl)-oxime (5h). 1H-NMR (300 MHz, CDCl3) δ 8.20 (s, 1H, CH=N), 8.12–8.17 (m, 1H, Ar-H), 8.06 (d, J = 8.4 Hz, 1H, Ar-H), 7.93–7.75 (m, 2H, Ar-H), 7.43–7.64 (m, 7H, Ar-H), 6.95 (d, J = 8.8 Hz, 2H, Ar-H), 6.91–6.84 (m, 1H, Ar-H), 6.75-6.81 (m, 1H, Ar-H), 4.58–4.67 (m, 2H, PyOCH2), 4.40–4.30 (m, 2H, PhOCH2), 4.24 (s, 2H, PhCH2CO); 13C-NMR (75 MHz, CDCl3) δ 168.95, 163.29, 161.63, 155.90, 146.71, 138.71, 130.10, 128.73, 128.24, 128.02, 126.48, 125.84, 125.46, 123.78, 117.08, 115.01, 111.34, 66.64, 63.83, 37.88; HRMS (ESI) calcd. for C26H23N2O4 (M + H)+ 427.1652, found 427.1651.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(2-(4-chlorophenyl)-3-methyl-1-oxobutyl)-oxime (5i). 1H-NMR (300 MHz, CDCl3) δ 8.26 (s, 1H, CH=N), 8.13 (dd, J = 1.9 Hz, 5.0 Hz, 1H, Ar-H), 7.64 (s, 1H, Ar-H), 7.61 (s, 1H, Ar-H), 7.50–7.55 (m, 1H, Ar-H), 7.26–7.35 (m, 4H, Ar-H), 6.95 (s, 1H, Ar-H), 6.92 (s, 1H, Ar-H), 6.82–6.86 (m, 1H, Ar-H), 6.75 (d, J = 8.3 Hz, 1H, Ar-H), 4.56–4.88 (m, 2H, PyOCH2), 4.11–4.50 (m, 2H, PhOCH2), 3.26 (d, J = 10.4 Hz, 1H, CHCO), 2.29–2.45 (m, 1H, CH(CH3)2), 1.11 (d, J = 6.5 Hz, 3H, CHCH3), 0.74 (d, J = 6.7 Hz, 3H, CHCH3); 13C-NMR (75 MHz, CDCl3) δ 170.66, 163.03, 161.45, 155.89, 146.54, 138.51, 136.12, 133.08, 129.96, 129.74, 128.53, 122.44, 116.90, 114.82, 111.08, 66.44, 63.63, 57.55, 31.95, 21.20, 19.95; HRMS (ESI) calcd. for C25H26ClN2O4 (M + H)+ 453.1576, found 453.1575.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(2-furanylcarbonyl)-oxime (5j). 1H-NMR (300 MHz, CDCl3) δ 8.44 (s, 1H, CH=N), 8.15 (dd, J = 1.3 Hz, 5.1 Hz, 1H, Ar-H), 7.69–7.73 (m, 2H, Ar-H), 7.61–7.67 (m, 1H, Ar-H), 7.50–7.61 (m, 1H, Ar-H), 7.30 (dd, J = 0.7 Hz, 3.5 Hz, 1H, Ar-H), 7.00 (s, 1H, Ar-H), 6.98 (s, 1H, Ar-H), 6.86–6.90 (m, 1H, Ar-H), 6.78 (d, J = 8.4 Hz, 1H, Ar-H), 6.55 (dd, J = 1.7 Hz, 3.5 Hz, 1H, Ar-H), 4.44–4.91 (m, 2H, PyOCH2), 4.09–4.46 (m, 2H, PhOCH2); 13C-NMR (75 MHz, CDCl3) δ 163.13, 161.59, 156.31, 156.16, 146.72, 146.60, 142.85, 138.57, 130.09, 122.44, 118.62, 116.95, 114.92, 111.89, 111.14, 66.53, 63.68.; HRMS (ESI) calcd. for C19H17N2O5 (M + H)+ 353.1132, found 353.1133.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-((3-(2-chloro-3,3,3-trifluoro-1-propen-1-yl)-2,2-dimethylcyclop ropyl)carbonyl)-oxime (5k). 1H-NMR (300 MHz, CDCl3) δ 8.28 (s, 1H, CH=N), 8.12 (dd, J = 1.9 Hz, 5.1 Hz, 1H, Ar-H), 7.59–7.75 (m, 2H, Ar-H), 7.52–7.57 (m, 1H, Ar-H), 6.94–7.01 (m, 3H, Ar-H), 6.81–6.89 (m, 1H, ArH), 6.76 (d, J = 8.4 Hz, 1H, CH=C), 4.58–4.79 (m, 2H, PyOCH2), 4.22–4.49 (m, 2H, PhOCH2), 2.26 (t, J = 8.9 Hz, 1H, CH-CH=C), 2.06 (d, J = 8.3 Hz, 1H, CHCO), 1.34, 1.32 (2s, 6H, (CH3)2C); 13C-NMR (75 MHz, CDCl3) δ 167.76, 163.19, 161.60, 155.45, 146.62, 138.61, 130.00, 129.64, 122.58, 118.51, 116.99, 114.96, 111.20, 66.57, 63.72, 31.16, 30.98, 29.10, 28.17, 14.75; HRMS (ESI) calcd. for C23H23ClF3N2O4 (M + H)+ 483.1293, found 483.1290.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-((2-chloro-3-pyridinyl)carbonyl)-oxime (5l). 1H-NMR (300 MHz, CDCl3) δ 8.55 (dd, J = 1.9 Hz, 4.8 Hz, 1H, Ar-H), 8.46 (s, 1H, CH=N), 8.06–8.24 (m, 2H, Ar-H), 7.71–7.75 (m, 2H, Ar-H), 7.55–7.61 (m, 1H, Ar-H), 7.37 (dd, J = 4.8 Hz, 7.7 Hz, 1H, Ar-H), 7.00–7.04 (m, 2H, Ar-H), 6.87–6.91 (m, 1H, Ar-H), 6.79 (d, J = 8.4 Hz, 1H, Ar-H), 4.57–4.83 (m, 2H, PyOCH2), 4.02–4.54 (m, 2H, PhOCH2); 13C-NMR (75 MHz, CDCl3) δ 163.17, 162.38, 161.86, 157.06, 151.95, 149.65, 146.63, 139.99, 138.63, 130.26, 126.21, 122.13, 122.06, 117.00, 115.05, 111.19, 66.61, 63.70; HRMS (ESI) calcd. for C20H17ClN3O4 (M + H)+ 398.0902, found 398.0906.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-((3,6-dichloro-2-pyridinyl)carbonyl)-oxime(5m). 1H-NMR (300 MHz, CDCl3) δ 8.48 (s, 1H, CH=N), 8.15 (dd, J = 1.9 Hz, 5.1 Hz, 1H, Ar-H), 7.80 (d, J = 8.5 Hz, 1H, Ar-H), 7.63–7.75 (m, 2H, Ar-H), 7.52–7.62 (m, 1H, Ar-H), 7.44 (d, J = 8.5 Hz, 1H, Ar-H), 6.98–7.02 (m, 2H, Ar-H), 6.83–6.94 (m, 1H, Ar-H), 6.78 (d, J = 8.4 Hz, 1H, Ar-H), 4.68–4.71 (m, 2H, PyOCH2), 4.36–4.39 (m, 2H, PhOCH2); 13C-NMR (75 MHz, CDCl3) δ 163.11, 161.79, 160.67, 157.34, 148.90, 146.58, 146.51, 140.96, 138.59, 130.24, 129.88, 127.47, 122.03, 116.97, 114.99, 111.14, 66.56, 63.67; HRMS (ESI) calcd. for C20H16Cl2N3O4 (M + H)+ 432.0512, found 432.0510.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-((2,2-dimethyl-3-(2-methyl-1-propen-1-yl)cyclopropyl)carbony l)-oxime (5n). 1H-NMR (300 MHz, CDCl3) δ 8.27 (s, 1H, CH=N), 8.07–8.14 (m, 1H, Ar-H), 7.64 (d, J = 8.8 Hz, 2H, Ar-H), 7.44–7.58 (m, 1H, Ar-H), 6.93 (d, J = 8.8 Hz, 2H, Ar-H), 6.80–6.87 (m, 1H, Ar-H), 6.70–6.79 (m, 1H, Ar-H), 4.80–5.03 (m, 1H, CH=C(CH3)2), 4.51–4.74 (m, 2H, PyOCH2), 4.14–4.42 (m, 2H, PhOCH2), 2.07–2.23 (m, 1H, CH-CH=C), 1.69 (s, 6H, C=C(CH3)2), 1.47 (d, J =5.3 Hz, 1H, CHCO), 1.31, 1.16 (2s, 6H, (CH3)2C); 13C-NMR (75 MHz, CDCl3) δ 169.94, 163.14, 161.31, 154.76, 146.57, 138.54, 135.84, 129.85, 122.95, 120.63, 116.91, 114.83, 111.15, 66.48, 63.69, 33.11, 32.89, 29.18, 25.40, 21.97, 20.25, 18.38; HRMS (ESI) calcd. for C24H29N2O4 (M + H)+ 409.2122, found 409.2122.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(1-oxobutyl)-oxime (5o). 1H-NMR (300 MHz, CDCl3) δ 8.29 (s, 1H, CH=N), 8.13–8.16 (m, 1H, Ar-H), 7.62–7.75 (m, 2H, Ar-H), 7.50–7.61 (m, 1H, Ar-H), 6.93–7.05 (m, 2H, Ar-H), 6.86–6.90 (m, 1H, Ar-H), 6.79 (d, J = 8.4 Hz, 1H, Ar-H), 4.57–4.84 (m, 2H, PyOCH2), 4.24–4.53 (m, 2H, PhOCH2), 2.44 (t, J = 7.4 Hz, CH2CO), 1.71–1.79 (m, 2H, CH3CH2), 1.01 (t, J = 7.4 Hz, 3H, CH3CH2); 13C-NMR (75 MHz, CDCl3) δ 171.10, 163.18, 161.45, 155.33, 146.61, 138.61, 129.94, 122.78, 116.98, 114.90, 111.20, 66.55, 63.73, 34.60, 18.27, 13.56; HRMS (ESI) calcd. for C18H21N2O4 (M + H)+ 329.1496, found 329.1500.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-acetyl-oxime (5p). 1H-NMR (300 MHz, CDCl3) δ 8.29 (s, 1H, CH=N), 8.06–8.22 (m, 1H, Ar-H), 7.62–7.73 (m, 2H, Ar-H), 7.55–7.61 (m, 1H, Ar-H), 6.93–7.04 (m, 2H, Ar-H), 6.87–6.91 (m, 1H, Ar-H), 6.79 (d, J = 8.4 Hz, 1H, Ar-H), 4.56–4.80 (m, 2H, PyOCH2), 4.18–4.45 (m, 2H, PhOCH2), 2.21 (s, 3H, COCH3); 13C-NMR (75 MHz, CDCl3) δ 168.66, 163.17, 161.49, 155.30, 146.59, 138.63, 129.94, 122.67, 116.98, 114.93, 111.20, 66.56, 63.74, 19.47; HRMS (ESI) calcd. for C16H17N2O4 (M + H)+ 301.1183, found 301.1186.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(2-thienylcarbonyl)-oxime (5q). 1H-NMR (300 MHz, CDCl3) δ 8.46 (s, 1H, CH=N), 8.15–8.18 (m, 1H, Ar-H), 7.94 (dd, J = 1.2 Hz, 3.8 Hz, 1H, Ar-H), 7.68–7.80 (m, 2H, Ar-H), 7.49–7.68 (m, 2H, Ar-H), 7.16 (dd, J = 3.8 Hz, 5.0 Hz, 1H, Ar-H), 6.95–7.04 (m, 2H, Ar-H), 6.86–6.95 (m, 1H, Ar-H), 6.81 (d, J = 8.4 Hz, 1H, Ar-H), 4.55–4.94 (m, 2H, PyOCH2), 4.20–4.53 (m, 2H, PhOCH2); 13C-NMR (75 MHz, CDCl3) δ 163.16, 161.57, 159.68, 156.08, 146.61, 138.61, 134.06, 132.87, 131.32, 130.11, 127.78, 122.56, 116.98, 114.95, 111.20, 66.55, 63.72; HRMS (ESI) calcd. for C19H17N2O4S (M + H)+ 369.0904, found 369.0906.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-(3-pyridinylcarbonyl)-oxime (5r). 1H-NMR (300 MHz, CDCl3) δ 9.31–9.36 (m, 1H, Ar-H), 8.85 (dd, J = 4.9 Hz, 1.7 Hz, 1H, Ar-H), 8.53 (s, 1H, CH=N), 8.39–8.43 (m, 1H, Ar-H), 8.17–8.19 (dd, J = 1.3 Hz, 5.1 Hz, 1H, Ar-H), 7.75–7.80 (m, 2H, Ar-H), 7.58–7.64 (m, 1H, Ar-H), 7.47 (dd, J = 4.9 Hz, 8.0 Hz, 1H, Ar-H), 6.97–7.15 (m, 2H, Ar-H), 6.87–6.95 (m, 1H, Ar-H), 6.82 (d, J = 8.4 Hz, 1H, Ar-H), 4.64–4.80 (m, 2H, PyOCH2), 4.33–4.45 (m, 2H, PhOCH2); 13C-NMR (75 MHz, CDCl3) δ 163.25, 162.74, 161.85, 156.80, 153.68, 150.70, 146.69, 138.68, 137.11, 130.28, 124.93, 123.41, 122.39, 117.05, 115.10, 111.28, 66.67, 63.77; HRMS (ESI) calcd. for C20H18N3O4 (M + H)+ 364.1292, found 364.1294.
4-(2-(2-Pyridinyloxy)ethoxy)benzaldehyde-O-((2,2,3,3 tetramethylcyclopropyl)carbonyl)-oxime (5s). 1H-NM R (300 MHz, CDCl3) δ 8.30 (s, 1H, CH=N), 8.16 (dd, J = 1.5 Hz, 5.1 Hz, 1H, Ar-H), 7.63–7.76 (m, 2H, Ar-H), 7.53–7.62 (m, 1H, Ar-H), 6.95–7.01 (m, 2H, Ar-H), 6.85–6.93 (m, 1H, Ar-H), 6.80 (d, J = 8.4 Hz, 1H, Ar-H), 4.59–4.85 (m, 2H, PyOCH2), 4.18–4.54 (m, 2H, PhOCH2), 1.33 (s, 6H, C(CH3)2), 1.29 (s, 1H, CHCO), 1.25 (s, 6H, C(CH3)2); 13C-NMR (75 MHz, CDCl3) δ 169.36, 163.21, 161.28, 154.41, 146.62, 138.61, 129.84, 123.21, 116.97, 114.86, 111.24, 66.54, 63.78, 33.80, 30.97, 23.42, 16.46; HRMS (ESI) calcd. for C22H27N2O4 (M + H)+ 383.1965, found 383.1968.
3.2. Biological Evaluation
3.2.1. Insecticidal Activity against Plutella xylostella
The test samples were dissolved in acetone and then diluted with water to obtain a final concentration of 600 μg/mL. Pyriproxyfen was used as a positive control whereas acetone was used as a negative control. Cabbage leaves were dipped into the obtained solutions for 5 s. The larvae of P. xyllostella were fed with the discs. Cohorts of about 15 P. xyllostella were treated each time and bioassays were repeated in triplicate. After 72 h, the numbers of knocked down larvae were counted and recorded [25,26].
3.2.2. Insecticidal Activity against Myzus persicae
The test samples were dissolved in N,N-dimethylformamide and then diluted to the required concentration with water. Peach leaves with about 40 Myzus persicae were dipped for 5 s into a solution with compound concentrations ranging from 200 to 600 μg/mL. After removing any excess solution on the leaves, Myzus persicae were raised in the leaves at 25 °C and 85% relative humidity for 48 h. Each experiment for every compound was repeated in triplicates [26].
3.2.3. Ovicidal Activity against Helicoverpa armigera Eggs
The test samples were dissolved in N,N-dimethylformamide and then diluted with water to final concentrations of 600 μg/mL and 200 μg/mL. Pieces of gauze with about 60 Helicoverpa armigera eggs were dipped into a corresponding solution for 10 s. After drying, the gauzes were moved into disks. The disks were then kept at 25 °C in plastic Petri dishes with moist filter paper. The number of eggs hatched in the control and treatment samples were recorded and the percentage of ovicidal activity was calculated.
4. Conclusions
In summary, nineteen oxime ester derivatives, 5a–5s, were synthesized. A preliminary evaluation of the ovicidal and insecticidal activities of the target compounds was performed. The bioassay results demonstrated that most of the synthesized compounds displayed moderate insecticidal activities and high ovicidal activities at a concentration of 600 μg/mL. Some derivatives, including compounds 5o and 5p bearing alkyl groups, exhibited respective ovicidal activities of 67.8% and 79.5% against Helicoverpa armigera eggs at a lower concentration of 200 μg/mL, higher than that of the reference compound, pyriproxyfen. Among the synthesized compounds, compounds 5j and 5s demonstrated broad biological activity spectra, with potential insecticidal activities against P. xylostella and Myzus persicae, and satisfactory ovicidal activities against Helicoverpa armigera eggs. Encouraged by the results described above, our laboratory is currently studying similar derivatives based on pesticide metabolites. A report of these advanced studies is currently being written up and will be published in due course.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21172257) and the Program for Innovation and Entrepreneurship of College Students (201710019231).
Supplementary Materials
Supplementary materials are available online. 1H- and 13C-NMR spectra of all compounds are presented in the Supporting Information section.
Author Contributions
Design, Synthesis, experimental work and writing of the manuscript were performed by Guo-Shao Sun, Xin Xu, Le Lin, and Jian-Jun Zhang. Biological evaluations were performed by Le Lin and Shu-Hui Jin. Jian-Jun Zhang supervised the entire project. All authors reviewed and approved the final version of the manuscript before submission.
Conflicts of Interest
All authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of compounds 5a–5s are available from the authors.
References
- 1.Wainwright M., Kristiansen J.E. On the 75th anniversary of Prontosil. Dyes Pigments. 2010;88:231–234. doi: 10.1016/j.dyepig.2010.08.012. [DOI] [Google Scholar]
- 2.Bentley R. Different roads to discovery; Prontosil (hence sulfa drugs) and penicillin (hence β-lactams) J. Ind. Microbiol. Biot. 2009;36:775–786. doi: 10.1007/s10295-009-0553-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Z., Vorperian V.R., Gong Q., Zhang S., January C.T. Block of HERG potassium channels by the antihistamine astemizole and its metabolites desmethylastemizole and norastemizole. J. Cardiovasc. Electrophysiol. 1999;10:836–843. doi: 10.1111/j.1540-8167.1999.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 4.Krstic D., Colovic M., Krinulovic K., Djuric D., Vasic V. Inhibition of AChE by single and simultaneous exposure to malathion and its degradation products. Gen. Physiol. Biophys. 2007;26:247–253. [PubMed] [Google Scholar]
- 5.Karmakar R., Bhattacharya R., Kulshrestha G. Comparative metabolite profiling of the insecticide thiamethoxam in plant and cell suspension culture of tomato. J. Agric. Food Chem. 2009;57:6369–6374. doi: 10.1021/jf9008394. [DOI] [PubMed] [Google Scholar]
- 6.Cavallaro M.C., Morrissey C.A., Headley J.V., Peru K.M., Liber K. Comparative chronic toxicity of imidacloprid, clothianidin, and thiamethoxam to Chironomus dilutus and estimation of toxic equivalency factors. Environ. Toxicol. Chem. 2017;36:372–382. doi: 10.1002/etc.3536. [DOI] [PubMed] [Google Scholar]
- 7.Sun G.S., Wang L.N., Jin S.H., Dong Y.H., Lu H.Z., Zhang J.J. Synthesis and fungicidal activity of diamide compounds based on the metabolite of benalaxyl. Chinese J. Org. Chem. 2017;37:157–165. doi: 10.6023/cjoc201608006. [DOI] [Google Scholar]
- 8.Sihuincha M., ZamoraPerea E., OrellanaRios W., Stancil J.D., LopezSifuentes V., VidalOre C., Devine G.J. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J. Med. Entomol. 2005;42:620–630. doi: 10.1093/jmedent/42.4.620. [DOI] [PubMed] [Google Scholar]
- 9.Shah R.M., Shad S.A., Abbas N. Mechanism, stability and fitness cost of resistance to pyriproxyfen in the house fly, Musca domestica L. (Diptera: Muscidae) Pestic. Biochem. Phys. 2015;119:67–73. doi: 10.1016/j.pestbp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C.T., Liu H., Liu D.H., Wang L.Y., Gao J., Zhou Z.Q., Wang P. Enantiomeric separations of pyriproxyfen and its six chiral metabolites by high-performance liquid chromatography. Chirality. 2016;28:245–252. doi: 10.1002/chir.22568. [DOI] [PubMed] [Google Scholar]
- 11.Kodaka R., Swales S.E., Lewis C., Katagi T. Effect of illumination on degradation of pyriproxyfen in water-sediment system. J. Pestic. Sci. 2011;36:33–40. doi: 10.1584/jpestics.G10-56. [DOI] [Google Scholar]
- 12.Fukushima M., Fujisawa T., katagi T. Tomato metabolism and porphyrin-catalyzed oxidation of pyriproxyfen. J. Agric. Food Chem. 2005;53:5353–5358. doi: 10.1021/jf0503816. [DOI] [PubMed] [Google Scholar]
- 13.Yoshino H., Kaneko H., Nakatsuka I., Yamada H. Metabolism of pyriproxyfen. 3. in vitro metabolism in rats and mice. J. Agric. Food Chem. 1996;44:1578–1581. doi: 10.1021/jf950510q. [DOI] [Google Scholar]
- 14.Ouyang G.P., Chen Z., Cai X.J., Song B.A., Bhadury P.S., Yang S., Jin L.H., Xue W., Hu D.Y., Zeng S., et al. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorg. Med. Chem. 2008;16:9699–9707. doi: 10.1016/j.bmc.2008.09.070. [DOI] [PubMed] [Google Scholar]
- 15.Attia M.I., Zakaria A.S., Almutairi M.S., Ghoneim S.W. In vitro anti-candida activity of certain new 3-(1H-Imidazol-1-yl)propan-1-one oxime esters. Molecules. 2013;18:12208–12221. doi: 10.3390/molecules181012208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao H.Q., Zhou M.J., Duan L.F., Wang W., Zhang J.J., Wang D.Q., Liang X.M. Efficient synthesis and anti-fungal activity of oleanolic acid oxime esters. Molecules. 2013;18:3615–3629. doi: 10.3390/molecules18033615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondal A., Walia S., Shrivastava C., Kumar B., Kumar J. Synthesis and insecticidal activity of karanj ketone oxime and its ester derivatives against the mustard aphid (Lipaphis erysimi) Pestic. Res. J. 2010;22:39–43. [Google Scholar]
- 18.Ma J.A., Huang R.Q., Chai Y.X. Synthesis and insecticidal activities of new pyrethroid acid oxime ester derivatives. Prog. Nat. Sci. 2002;12:271–277. [Google Scholar]
- 19.Yu X., Shi D.F., Zhi X.Y., Li Q., Yao X.J., Xu H. Synthesis and quantitative structure-activity relationship (QSAR) study of C7-oxime ester derivatives of obacunone as insecticidal agents. RSC Adv. 2015;5:31700–31707. doi: 10.1039/C5RA01411E. [DOI] [Google Scholar]
- 20.Li T.G., Liu J.P., Han J.T., Fu B., Wang D.Q., Wang M.A. Synthesis and herbicidal activity of α-phenylsulfonylcyclododecanone oxime esters. Chin. J. Org. Chem. 2009;29:898–903. [Google Scholar]
- 21.Liu X.G., Wu J.P., Liang X.M., Wang D.Q. Synthesis and insecticidal activity of 5-acyloxyimino-5-deoxyavermectin B1 derivatives. Pest Manag. Sci. 2004;6:697–702. doi: 10.1002/ps.859. [DOI] [PubMed] [Google Scholar]
- 22.Chandrika N.T., Shrestha S.K., Ngo H.X., Garneau-Tsodikova S. Synthesis and investigation of novel benzimidazole derivatives as antifungal agents. Bioorg. Med. Chem. 2016;24:3680–3686. doi: 10.1016/j.bmc.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundriyal S., Viswanad B., Ramarao P., Chakraborti A.K., Bharatam P.V. New PPARc ligands based on barbituric acid: Virtual screening, synthesis and receptor binding studies. Bioorg. Med. Chem. Let. 2008;18:4959–4962. doi: 10.1016/j.bmcl.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz L., Girreser U., Clement B. Synthesis and characterization of para-Substituted N,N′-Dihydroxybenzamidines and their derivatives asmodel compounds for a class of prodrugs. Eur. J. Org. Chem. 2014;2014:1961–1975. doi: 10.1002/ejoc.201301622. [DOI] [Google Scholar]
- 25.Sun J.L., Zhou Y.M. Design, synthesis and insecticidal activity of novel phenylurea derivatives. Molecules. 2015;20:5050–5061. doi: 10.3390/molecules20035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai H., Chen J., Li H., Dai B.J., He H.B., Fang Y., Shi Y.J. Synthesis and bioactivities of novel pyrazoleoxime derivatives containing a 5-trifluoromethylpyridyl moiety. Molecules. 2016;21:e276. doi: 10.3390/molecules21030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.