Abstract
A reaction of bis[(2-chlorocarbonyl)phenyl] diselenide with various mono and bisnucleophiles such as aminophenols, phenols, and amines have been studied as a convenient general route to a series of new antimicrobial and antiviral diphenyl diselenides. The compounds, particularly bis[2-(hydroxyphenylcarbamoyl)]phenyl diselenides and reference benzisoselenazol-3(2H)-ones, exhibited high antimicrobial activity against Gram-positive bacterial species (Enterococcus spp., Staphylococcus spp.), and some compounds were also active against Gram-negative E. coli and fungi (Candida spp., A. niger). The majority of compounds demonstrated high activity against human herpes virus type 1 (HHV-1) and moderate activity against encephalomyocarditis virus (EMCV), while they were generally inactive against vesicular stomatitis virus (VSV).
Keywords: ebselen, organoselenium compounds, diselenides, benzisoselenazol-3(2H)-ones, antimicrobial activity, antiviral activity, morpholine, benzohydrazide, 2-hydroxyacetanilide, antimycin A
1. Introduction
Organoselenium chemistry and biology have been areas of continuous research over the last few decades [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Selenium-containing molecules have been of interest as reagents in new synthetic routes [1], promising catalysts of various oxidation reactions [1,2,3,4,5,6,7,18,19,20,21], and bioactive agents [8,9,10,11,12,13,14,15,16,22,23]. In the context of biological applications, organoselenium compounds have received particular attention as glutathione peroxidase (GPx) mimics [9,11,14,24], antioxidant, and anti-inflammatory agents [10,11,13,23]. Furthermore, a number of selenium-containing compounds have been studied as anticancer [12,22,25], anti-angiogenic [26], antiviral [27,28,29,30,31,32,33,34,35], antimicrobial [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], and antileishmanial agents [47].
The development of novel anti-infective agents is an area of intense activity due to the constant appearance of new infections, the emergence of multidrug resistance in common pathogens, as well as the need to eliminate undesirable side effects of available drugs. The urgent need for the next generation of antimicrobials and antivirals perpetuates research into designing novel molecules and investigating their mechanisms of action. The tremendous efforts in this area has led to the identification of many bioactive structures [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Several enzymes, among them thioredoxin reductase [36,48], bacterial urease [49], and plasma membrane H+-ATPase [50,51,52], were suggested as targets for organoselenium compounds with antimicrobial properties. Furthermore, it has been reported that the antiviral effects of organoselenium compounds might be related to the inhibition of hepatitis C virus NS3 helicase [28], HIV-1 capsid dimerization [29], and nucleocapsid protein 7 [30].
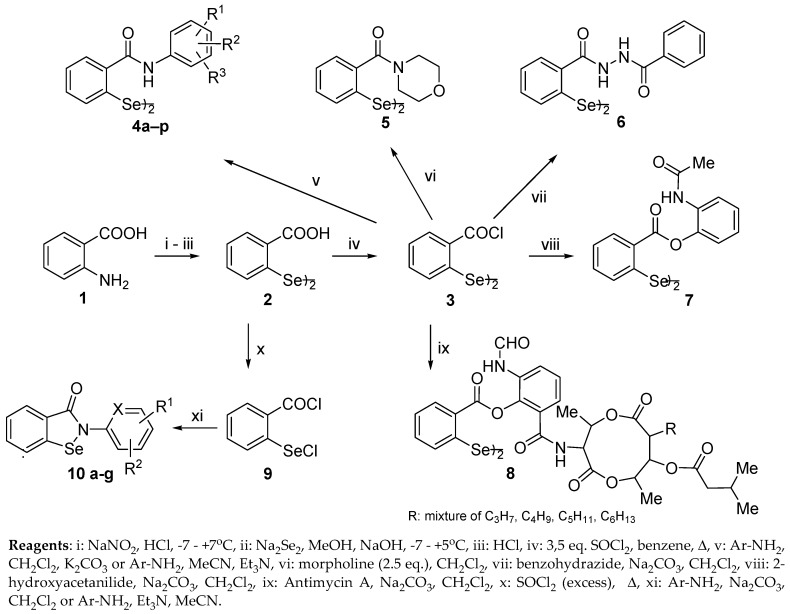
Inspired by the encouraging findings in the area of antimicrobial and antiviral properties of organoselenium compounds and as a continuation of our interest in the chemistry and biology of this class of compounds, we aimed to develop a series of new diphenyl diselenides and investigate their activity against various microbial strains and viruses, along with selected cyclic analogues. It seemed interesting to focus our attention on a group of 2,2’-dicarbamoylphenyl diselenides 4 as the most reduced analogues of ebselen, with a phenyl ring containing hydroxy and/or methoxy moieties. However, the rationale behind studying these compounds was based not only on the fact that the structural modifications in the diphenyl diselenide scaffold seemed to be interesting from the medicinal chemistry perspective; we also aimed to investigated a synthetic route to reduced forms of ebselenols (2-(hydroxyphenyl)-1,2-benzisoselenazol-3(2H)-ones) and its derivatives via the reaction of bis[(2-chlorocarbonyl)phenyl] diselenide 3 with various mono and bisnucleophiles such as aminophenols, phenols, and amines.
Up to the present time, several methods have been applied for the preparation of bis(2-arylcarbamoyl)phenyl diselenides 4. One path is the reductive benzisoselenazolone ring opening of ebselenols and its derivatives [53], which can be obtained from 2-(chloroseleno)benzoyl chloride with moderate to excellent yields [54,55,56]. An alternative route to ebselenols requires metalation-selenenylation of appropriate benzanilides while protecting the hydroxyl group in the first step [57,58]. Although the reductive ring opening reaction has been successfully applied for the reduction of ebselen and its various alkyl analogues [59], in the case of ebselenols, yields were less satisfactory [53]. Only bis[2-(4-hydroxyphenylcarbamoyl)]phenyl diselenide was prepared in 46% yield while bis[2-(2-hydroxyphenylcarbamoyl)]phenyl diselenide and 2-(3-hydroxyphenylcarbamoyl)]phenyl diselenide were obtained in very low yields of 8.3% and 12%, respectively [53]. Another, general route to ebselenol-derived diselenides which we present in this study is the reaction of bis[(2-chlorocarbonyl)phenyl)] diselenide 3 with the corresponding anilines. In the past, this reaction was successfully applied to obtain various 2,2’-dicarbamoyldiphenyl diselenides [60]. However, to our best knowledge, there is only one example of a reaction with an electron-rich arene system bearing a hydroxyl group [26] but with no synthetic details. Thus, we aimed to investigate the behaviour of bis[(2-chlorocarbonyl)phenyl)] diselenide 3 in the reaction with N and O nucleophiles, such as aminophenols, phenols, and amines. The aminophenols were of particular interest as the ones containing two nucleophilic centers in the molecule, with both amine nitrogen and hydroxide oxygen potentially susceptible toward acylation. The reaction of various O and N nucleophiles with another selenium-containing chloride, 2-(chloroseleno)benzoyl chloride 9, showed the preference of a primary amino group toward selenenylation-acylation [55]. As chloride 9 contains both hard and soft electrophilic centers localized on the carbonyl carbon and selenium atoms, respectively, the results of the reaction with O-nucleophiles strongly depended on the nucleophile structure. However, in the absence of the soft electrophilic center, no side reactions should occur. Thus, we expected that the reaction of bis[(2-chlorocarbonyl)phenyl] diselenide 3 with aminophenols could be considered as a convenient alternative for low-efficient reductive ring opening of ebselenols [53] and the preparation from 2,2’-dicarboxydiphenyl diselenide using active ester coupling agents [23,61], where the protection of the hydroxyl group would be recommended.
2. Results and Discussion
2.1. Synthesis
Herein we present our investigation of synthetic route to diselenides 4–8 via a reaction of (bis[(2-chlorocarbonyl)phenyl] diselenide), hereafter called chloride 3, with N and O nucleophiles, such as a series of various aminophenols and their derivatives as well as other specific reactants such as morpholine, benzohydrazide, 2-hydroxyacetanilide, and antimycin A (Scheme 1, Table 1). The key chloride 3 was prepared in a three-step synthesis starting from anthranilic acid 1, which was diazotized and reacted with disodium diselenide in a methanolic alkaline medium to give, after acidification, 2,2’-diselenobisbenzoic acid 2 which was further reacted with thionyl chloride [59,60,62].
Scheme 1.
Synthesis of bis(2-carbamoy)diphenyl diselenides 4a–p, 5 and 6, phenolic esters 7–8, and benzisoselenazol-3(2H)-ones 10a–g.
Table 1.
Characteristics of compounds 4a–p, 5–8, and 10a–g.
| Compound | R1 | R2 | R3 | X | Rx Time (h) | Yield (%) | m.p. (°C) | m.p. lit (°C) |
|---|---|---|---|---|---|---|---|---|
| 4a | 2-OH | - | - | - | 96 | 92 | 233.5–236.5 | 235–238 [53] |
| 4b | 3-OH | - | - | - | 60 | 84 | 285.5–287.0 | 285–286 [53] |
| 4c | 4-OH | - | - | - | 120 | 73 | 271.0–272.0 | 282–284 [53] |
| 4d | 2-OH | 4-Me | - | - | 24 | 60 | 221.0–223.5 | - |
| 4e | 2-OH | 5-Me | - | - | 96 | 89 | 191.5–194.5 | - |
| 4f | 2-OH | 6-Me | - | - | 168 | 88 | 264.0–267.0 | - |
| 4g | 2-OH | 4-Cl | - | - | 96 | 59 | 230.0–234.0 | - |
| 4h | 2-OH | 5-Cl | - | - | 48 | 63 | 219.5–221.5 | - |
| 4i | 2-OH | 5-COOMe | - | - | 72 | 57 | 281.0–282.0 | - |
| 4j | 3-OH | 4-OMe | - | - | 120 | 94 | 232.5–236.5 | - |
| 4k | 2-OMe | - | - | - | 168 | 94 | 211.0–214.0 | 216–217 [53] |
| 4l | 3-OMe | - | - | - | 72 | 98 | 221.0–223.0 | 221–223 [35] |
| 4m | 4-OMe | - | - | - | 48 | 94 | 285.5–287.5 | 290–292 [53] |
| 4n | 2-OMe | 4-Ome | - | - | 168 | 99 | 211.0–213.5 | - |
| 4o | 3-OMe | 4-OMe | - | - | 96 | 72 | 231.5–234.5 | 235 [53] |
| 4p | 3-OMe | 4-OMe | 5-OMe | - | 96 | 76 | 193.0–195.0 | - |
| 5 | - | - | - | - | 120 | 85 | 149.0–152.0 | - |
| 6 | - | - | - | - | 120 | 100 | 296.5 dec. | - |
| 7 | - | - | - | - | 96 | 36 | 178.0–181.0 | - |
| 8 | - | - | - | - | 240 | 97 | >300 | - |
| 10a | 2-OH | - | - | CH | 20 | 40 | 195.5–197.5 | 194–196 [54] |
| 10b | 6-OH | - | - | N | 2 | 65 | 229.0 dec. | 199–201 [55] a |
| 10c | 2-OMe | - | - | CH | 24 | 71 | 189.0–191.5 | 172–174 [63] |
| 10d | 3-OMe | - | - | CH | 2 | 65 | 166.0–168.0 | 165.5–167.5 [35] |
| 10e | 4-OMe | - | - | CH | 20 | 70 | 180.0–181.0 | 180.5–181.5 [35] |
| 10f | 2-OMe | 4-Ome | - | CH | 6 | 65 | 240.5–242.5 | - |
| 10g | 3-OMe | 4-Ome | - | CH | 48 | 68 | 159.0–161.0 | - |
a Phase transfer was observed at 199 °C.
In a first step, reaction of chloride 3 was tested with various free and protected aminophenols, such as monohydroxyanilines, monomethoxyanilines, and their derivatives containing additional substituents in the benzene ring, such as electron-donating methyl and methoxy groups and electron-withdrawing chloro and carboxymethyl groups. As in the case of 2-(chloroseleno)benzoyl chloride 9, which was intensively studied with various bisnucleophiles [55], the reaction occurred on the amine group of both protected (4k–p) and free phenols (4a–j). In the IR spectra, there were observed signals from primary amide bonds at ca. 3300–3500 cm−1 (N–H), carbonyl groups at ca. 1600–1650 cm−1 (C=O), amide groups at ca. 1500–1550 cm−1 (CONH), and in 1H-NMR there were present protons of N–H group at ca. 9.6–10.5 ppm (see the Supplementary Materials for details). All compounds were prepared with very good to excellent yields, significantly higher than those achieved by the reductive ring opening method [53]. The simplest three isomeric aminophenols reacted with 73–92% yield towards 4a–c. Reaction with 2-aminophenols containing electron-withdrawing substituents (Cl and COOMe) at the vicinity carbon atom generally resulted in slightly lower yields (4g–i; 57–63%), when compared to those containing electron-donating ones (4d–f; 60–89%). As expected [35], methoxy derivatives 4j–p were also prepared with very good yields (72–99%). As a result of our studies, seven new open-chain analogues of ebselenols 4d–j and two new methoxy derivatives 4n and 4p were developed.
Reaction of benzoyl chloride 3 with secondary amines such as morpholine also resulted in secondary amide bond formation to produce compound 5 with a very good yield of 85%. A similar compound containing piperazine moiety was prepared with a comparable yield [64].
Another highly nucleophilic benzohydrazide reacted quantitatively with chloride 3 on its terminal nitrogen atom to form bishydrazide 6. Encouraged by this result, we continued our studies with other N-nucleophiles, focusing on hydrazine, ethylene diamine, tri(hydroxymethyl)aminomethane, and isoniazyde (isonicotinic hydrazide). The resulting oligomeric products were, however, difficult to identify.
Further efforts were directed into the investigation of the behavior of aminophenol derivatives containing blocked amine groups such a 2-hydroxyacetanilide and natural antimycin A. In this case, reaction occurred on the free hydroxyl group only, resulting in phenolic ester derivatives 7 and 8, however, highly sterically hindered antimycin A required an extremely long reaction time. In the IR spectrum of compound 7, a characteristic phenolic ester bond was observed at 1760 cm−1. Antimycin A is a mixture of at least four closely related compounds, containing alkyl or acyl substituents of different lengths or degrees of branching (usually C3–C6) in the dilactone portion of the molecule [65]. Thus, the spectroscopic analysis is complicated and only diagnostic signals could be reported in 13C-NMR spectra. Fortunately, in HRMS spectra, seven diagnostic molecular species (M + Na+) were observed with R=C5H11 being a predominant one, which was consistent with the results of the elemental analysis.
Ebselen and several other benzisoselenazol-3(2H)-ones 10a–g, including two new molecules, 10f and 10g, were prepared as a reference for biological tests. The 2,2’-diselenobisbenzoic acid 2 was converted to the 2-(chloroseleno)benzoyl chloride 9 that, upon reaction with corresponding anilines, formed ebselen and benzisoselenazol-3(2H)-ones 10a–g through a tandem selenenylation-acylation sequence (Scheme 1, Table 1) [55,59]. To our best knowledge, compounds 4d–j, 4n, 4p, 5–8, 10f–g are new structures and were not previously described in the literature.
2.2. Antimicrobial Activity
The in vitro antimicrobial activity of compounds 4–7, 10 was screened against four Gram-positive bacterial strains (Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Enterococcus hirae), two Gram-negative bacterial strains (Escherichia coli, Pseudomonas aeruginosa), two yeast-like fungi (Candida albicans, Candida glabrata), and filamentous fungus (Aspergillus niger) in a range of 0.125–128 µg/mL. The minimum inhibitory concentration values (MIC) for organoselenium compounds are summarized in Table 2 and Table 3, along with reference substances.
Table 2.
Antibacterial activity of compounds 4–7, and 10.
| Compound | Gram-Positive Bacteria a | Gram-Negative Bacteria a | ||||
|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | E. faecalis | E. hirae | E. coli | P. aeruginosa | |
| 4a | 16 | 8 | 4 | 8 | >128 | >128 |
| 4b | 16 | 8 | 2 | 8 | >128 | >128 |
| 4c | 16 | 16 | 2 | 8 | >128 | >128 |
| 4d | >128 | 128 | 2 | 4 | >128 | >128 |
| 4e | 16 | 16 | 1 | 2 | 64 | >128 |
| 4f | >128 | >128 | 4 | 8 | >128 | >128 |
| 4g | 128 | 64 | 2 | 4 | >128 | >128 |
| 4h | >128 | 128 | >128 | >128 | >128 | >128 |
| 4i | >128 | 128 | >128 | >128 | >128 | >128 |
| 4j | >128 | 128 | >128 | >128 | >128 | >128 |
| 4k | >128 | >128 | >128 | >128 | >128 | >128 |
| 4l | >128 | 128 | >128 | >128 | >128 | >128 |
| 4m | >128 | >128 | 8 | >128 | >128 | >128 |
| 4n | >128 | 128 | 4 | 64 | >128 | >128 |
| 4o | >128 | 128 | 16 | 64 | >128 | >128 |
| 4p | >128 | 128 | 4 | 32 | >128 | >128 |
| 5 | >128 | 128 | >128 | >128 | >128 | >128 |
| 6 | >128 | 128 | 4 | 8 | >128 | >128 |
| 7 | >128 | >128 | >128 | >128 | >128 | >128 |
| 10a | 8 | 4 | 2 | 4 | 32 | >128 |
| 10b | 8 | 4 | 1 | 2 | >128 | >128 |
| 10c | 32 | 32 | 2 | 8 | 128 | >128 |
| 10d | >128 | 128 | 16 | 32 | >128 | >128 |
| 10e | >128 | 128 | 4 | 32 | >128 | >128 |
| 10f | 64 | 64 | 2 | 4 | >128 | >128 |
| 10g | 64 | 32 | 2 | 8 | >128 | >128 |
| Ebs | 64 | 32 | 2 | 8 | 128 | >128 |
| AMC | >128 | >128 | >128 | >128 | >128 | >128 |
| AMP | 4 | >128 | 16 | >128 | 16 | >128 |
| CHK | >128 | >128 | 128 | 128 | 2 | >128 |
| ERM | >128 | >128 | >128 | >128 | 64 | >128 |
| STR | 32 | 128 | >128 | >128 | 32 | 128 |
| NAT | >128 | >128 | >128 | >128 | 16 | 32 |
a MIC—Minimum Inhibitory Concentration (µg/mL); AMC—antimycin A; AMP—ampicillin; CHK—chloramphenicol; ERM—erythromycin; STR—streptomycin; NAT—nourseothricin.
Table 3.
Antifungal activity of compounds 4–7, and 10.
| Compound | Yeasts a | Fungi a | |
|---|---|---|---|
| C. albicans | C. glabrata | A. niger | |
| 4a | >128 | >128 | >128 |
| 4b | >128 | >128 | >128 |
| 4c | >128 | >128 | >128 |
| 4d | >128 | >128 | >128 |
| 4e | 32 | 32 | 128 |
| 4f | >128 | >128 | >128 |
| 4g | >128 | >128 | >128 |
| 4h | >128 | >128 | >128 |
| 4i | >128 | >128 | >128 |
| 4j | >128 | >128 | >128 |
| 4k | >128 | >128 | >128 |
| 4l | >128 | >128 | >128 |
| 4m | >128 | >128 | >128 |
| 4n | >128 | >128 | >128 |
| 4o | >128 | >128 | >128 |
| 4p | >128 | >128 | >128 |
| 5 | >128 | >128 | - |
| 6 | >128 | >128 | - |
| 7 | >128 | >128 | - |
| 10a | 16 | 16 | >128 |
| 10b | >128 | >128 | >128 |
| 10c | 16 | 32 | 64 |
| 10d | >128 | >128 | - |
| 10e | 64 | >128 | - |
| 10f | 16 | 64 | 64 |
| 10g | 32 | 64 | - |
| Ebselen | 32 | 64 | 16 |
| AMC | >128 | >128 | >128 |
| KLK | >128 | >128 | - |
| ITC | >128 | >128 | - |
| NAT | 64 | 64 | - |
| AMB | 1 | 1 | - |
a MIC—Minimum Inhibitory Concentration (µg/mL); AMC—antimycin A; KLK—ketoconazole; ITC—itraconazole; NAT—nourseothricin; AMB—amphotericin B.
Generally, there is a noticeable tendency that the compounds tested have been more active against Gram-positive bacterial strains than against Gram-negative ones. These results are in line with our previous observations [33,34,35] and might indicate the possible mode of action of the whole group. Gram-positive bacteria are more susceptible to plasma membrane-binding agents, absorbing them directly into the plasma membrane, whereas a Gram-negative outer membrane may capture such agents before they penetrate through a cell wall and bind into the inner membrane, which usually results in subsequent cell permeabilization [66]. Using propidium iodide, no significant plasma membrane permeabilization was observed in S. aureus after treating with ebselen [67], however, membrane binding is not always sufficient for its permeabilization [68]. Membrane binding agents alter plasma membrane biophysics, which may be toxic itself.
The antibacterial activity towards Gram-positive species varied in a range of 1 to >128 µg/mL. Particularly interesting is the strong activity towards E. faecalis and E. hirae. These enterococci display highest vulnerability to most of the tested compounds and only compounds 4h–l, 5 and 7 (as well as 4m in the case of E. hirae) exhibit no activity in the tested range of concentrations. Enterococci seem to be significantly more susceptible towards organoselenium compounds than reference antibiotics, with the exception of 4o and 10d, displaying same MIC as ampicillin towards E. faecalis. This makes organoselenium compounds promising for the treatment of enterococci infections, which although being low virulent bacteria, often cause infections in immunocompromised patients [69]. In the case of staphylococci, it can be noticed that compounds 4a–c and 10a–b, containing hydroxyl substituent in the phenyl ring, were the most active against both S. aureus and S. epidermidis. Benzisoselenazol-3(2H)-ones 10a and 10b were slightly more active than their open-chain analogues 4a–c. Only two compounds, 4e and 10a, showed inhibitory effect against E. coli, similar to commonly known antibiotics. None of the compounds showed activity towards P. aeruginosa in the screening range. However, it is worth pointing out that this microorganism was also not susceptible toward the majority of reference antibiotics in the tested range.
Contrary to their cyclic analogues, diselenides appeared to be practically inactive towards both Candida spp., with the exception of compound 4e, which also displayed a high inhibitory effect against Gram-positive bacteria and moderate effects against Gram-negative E. coli (Table 1). The probable lack of inhibitory effect of the majority of compounds tested might be due to the fact that the antifungal activity of diphenyl diselenides is dependent on the presence of functional groups [40]. However, diphenyl diselenides may still display high activity in reducing germ tube formation, one of the crucial virulent factors of C. albicans [38]. Azoles (ketoconazole and itraconazole), commonly used in antifungal therapy, also did not show activity under the test conditions. However, these two compounds are considered static drugs [70], therefore it is difficult to obtain MIC90 values for them. Thus, it is possible that organoselenium compounds which lack inhibitory effects may still display synergistic effects. Benzisoselenazol-3(2H)-ones 10a, 10c, 10f–g, and 10e (in the case of C. albicans only) inhibited fungal growth in a range of 16–64 µg/mL (Table 2), comparable to nourseothricin. Amphotericin B exhibited a much higher activity than all the benzizsoelenazol-3(2H)-ones tested, however, this highly cytotoxic compound is considered as a drug of choice for the most severe fungal infections, when the patients are close to death [71]. The majority of diselenides have shown no activity against A. niger in the tested range. Only compound 4e showed weak activity against this filamentous fungus. Benzisoselenazol-3(2H)-ones 4c and 4f showed moderate activity towards A. niger, comparable to other previously tested cyclic analogues of ebselen [33,34].
The compound 5, prepared by reaction with a secondary amine, as well as phenolic ester 7 were inactive against all tested microorganisms.
2.3. Antiviral Activity
The antiviral activity of compounds 4, 5, 7, and 10 has been evaluated in vitro towards HHV-1 (human herpes virus type 1, Herpesviridae, enveloped virus), EMCV (encephalomyocarditis virus, Picornaviridae, non-enveloped virus), and VSV (vesicular stomatitis virus, Rhabdoviridae, enveloped virus) in the human cell line A549. The minimum inhibitory concentrations (MICs) for the abovementioned viruses along with cytotoxicity against the testing cell line are summarized in Table 4.
Table 4.
Antiviral activity and cytotoxicity of compounds 4, 5, 7, and 10.
| Compound | TCCD50 a | HHV-1 | EMCV | VSV | |||
|---|---|---|---|---|---|---|---|
| MIC b | I c | MIC b | I c | MIC b | I c | ||
| 4a | 4.9 | 40 | 0.12 | 10 | 0.49 | >1000 | <0.005 |
| 4c | 3.7 | 2 | 1.8 | 60 | 0.06 | >1000 | <0.004 |
| 4d | 4.9 | 2 | 2.5 | >1000 | <0.005 | >1000 | <0.005 |
| 4e | 4.9 | 2 | 2.5 | 6 | 0.82 | >1000 | <0.005 |
| 4f | 9.8 | 4 | 2.5 | >1000 | <0.01 | >1000 | <0.01 |
| 4g | 4.9 | 2 | 2.5 | 40 | 0.12 | >1000 | <0.005 |
| 4h | 4.9 | 6 | 0.82 | >1000 | <0.005 | >1000 | <0.005 |
| 4i | 7.3 | 20 | 0.37 | 20 | 0.37 | >1000 | <0.07 |
| 4j | 4.9 | 4 | 1.2 | 20 | 0.25 | >1000 | <0.005 |
| 4k | 312 | 6 | 52 | 400 | 0.78 | >1000 | <0.31 |
| 4n | 58 | 2 | 29 | 600 | 0.10 | >1000 | <0.06 |
| 4o | 4.9 | 2 | 2.5 | 8 | 0.61 | >1000 | <0.005 |
| 4p | 2.4 | 4 | 0.61 | 8 | 0.30 | 200 | 0.01 |
| 5 | 4.9 | 200 | 0.02 | 10 | 0.49 | >1000 | <0.005 |
| 7 | 15 | 20 | 0.75 | 400 | 0.04 | 600 | 0.03 |
| 10c | 9.8 | 4 | 2.5 | 8 | 1.2 | >1000 | <0.01 |
| 10f | 156 | 2 | 78 | 200 | 0.78 | >1000 | <0.16 |
| Ebselen | 15 | 2 | 7.5 | 10 | 1.5 | >1000 | <0.02 |
a TCCD50—Tissue Culture Cytotoxic Dose (µg/mL); b MIC—Minimum Inhibitory Concentration (µg/mL); c I = TCCD50/MIC.
The compounds exhibited high antiviral activity against HHV-1 and moderate to high activity against EMCV, but in almost all cases were inactive towards VSV (Table 4). A similar tendency was observed in our previous studies [32,33,34,35]. The anti-HHV-1 activity of most diselenides was in a range of 2–40 μg/mL; only the one containing morpholine moiety showed lower activity at 200 μg/mL. The benzisoselenazol-3(2H)-ones also showed high activity against HHV-1, in a range of 2–4 μg/mL, comparable to ebselen. The most promising anti-HHV-1 agents would be compounds 4k, 4n, and 10f with higher chemotherapeutic indices (I), indicating that the concentration active against the virus was lower than that showing a cytotoxic effect. Up to the present time, there were only several organoselenium compounds identified with such high chemotherapeutic indices [33]. Generally, the compounds were less active against EMCV and only compounds 4e, 4o–p, and 10c showed activity below 10 μg/mL.
3. Materials and Methods
3.1. Synthesis
General Information
Melting points were determined on an Electrothermal IA 91100 digital melting-point apparatus using the standard open capillary method. 1H- and 13C-NMR spectra were recorded in CDCl3 or DMSO-d6 on a Bruker DRX 300 Spectrometer (Bruker, Rheinstetten, Germany) (1H: 300.1 MHz, 13C: 75.4 MHz), on a Joel 400 Spectrometer (Joel, Tokyo, Japan) (1H: 399.8 MHz, 13C: 100.5 MHz) or on a Bruker Avance 600 Spectrometer (1H: 600.6 MHz, 13C: 151.0 MHz) at 295 K. Chemical shifts (δ) are given in parts per million (ppm) downfield relative to tetramethylsilane (TMS, Si(CH3)4), and coupling constants (J) are in Hz. Residual solvent central signals were recorded as follows: CDCl3, δH = 7.263, δC = 77.00; DMSO-d6, δH = 2.50, δC = 39.43. FT-IR spectra were recorded between 4000 and 400 cm−1 on a Perkin-Elmer 2000 FT-IR spectrometer. High-resolution mass spectra (HRMS) were recorded on a Waters LCD Premier XE instrument (Waters, Manchester, UK), and only the [M + H]+ or [M + Na]+ diagnostic molecular species are reported. Preparative column chromatography was performed on Merck Si60 silica gel (63–200 μm) (Sigma-Aldrich, Saint Louis, MO, USA) as the stationary phase. Analytical thin-layer chromatography (TLC) was performed on PET foils precoated with silica gel (Merck silica gel, 60 F254) (Sigma-Aldrich, Saint Louis, MO, USA), and visualised using UV light (λmax = 254 nm), or by staining with iodine vapours. Selenium powder (100 mesh) (Sigma-Aldrich, Saint Louis, MO, USA) used for Na2Se2 preparation had a purity of ≥99.5%. Acetonitrile (MeCN) (POCH, Gliwice, Poland) and methylene chloride (CH2Cl2) (POCH, Gliwice, Poland) were distilled over P2O5, and CH2Cl2 was stored over sodium hydride (NaH) pills. Triethylamine (Et3N) (POCH, Gliwice, Poland), distilled over NaOH, was stored over NaOH pellets. Methanol (MeOH) (POCH, Gliwice, Poland) was refluxed over magnesium shavings (Mg) in the presence of elemental iodine (I2) and distilled over Mg(OMe)2 was formed and stored over 3A molecular sieves (Sigma-Aldrich, Saint Louis, MO, USA). Diethyl ether (DEE) (POCH, Gliwice, Poland) was distilled over a CaH2 and LiAlH4 mixture from water batch at ca. 45 °C. Morpholine (POCH, Gliwice, Poland) was distilled over NaOH pellets (POCH, Gliwice, Poland) before use. Anhydrous sodium carbonate (Na2CO3) (POCH, Gliwice, Poland) was ground in a mortar before use. Antimycin A natural product (Sigma-Aldrich, Saint Louis, MO, USA) was used as a mixture of co-crystalized n-alkylated compounds with R = C3–C6 predominantly. 2-Hydroxy-5-carboxymethylaniline was prepared from 3-amino-4-hydroxybenzoic acid (Sigma-Aldrich, Saint Louis, MO, USA) by direct metoksylation in a hot methanol environment and in the presence of H2SO4 (POCH, Gliwice, Poland), as mentioned below. Ebselen was prepared according to the procedure described earlier [59]. Other reagents and starting materials were directly used as obtained commercially. Purity of the products was confirmed by the comparison of their melting point with data given in literature and by spectroscopic methods. The new compounds were fully characterized.
2-Hydroxy-5-carboxymethylaniline. To 2-amino-4-hydroxybenzoic acid (1.53 g, 10 mmol) in dry MeOH (25 mL) was added H2SO4 (1.0 mL, 1.8 g, 19 mmol) portionwise during stirring, and the solution formed was stirred in solvent reflux (+65 °C) for 20 h. The reaction mixture was cooled and poured into 0.5% NaOH (200 mL) and kept in an ice/water bath, NaHCO3 (3.6 g, 44 mmol) was added and the mixture was washed with DEE (100 mL) followed by the addition of 3.5% HCl to a pH of 8.0, and was then extracted with degased DEE. The collected DEE solution was washed with water, dried over Na2SO4, and the solvent was distilled off from a water bath to obtain crude 2-hydroxy-5-carboxymethylaniline. Yield: (1.60 g, 96%); m.p.: 114.5–115.0 °C (110 °C [72]); as prisms (benzene, 40 mL/g). IR (KBr, cm−1) ν 3390, 3300 (NH), 2500–3200 (br OH), 1702 (C=O), 1610, 1429, 1306, 1294, 1284 (C–N), 1201 and 1112 (C–O), 858, 764, 421. 1H-NMR (300 MHz, CDCl3:DMSO-d6, 5:1, v/v): δ 3.81 (s, 3H, OCH3), 4.24 (br s, 2H, NH2), 6.75 (d, J = 8.2 Hz, 1H, Ar-H), 7.24 (dd, J = 8.2 Hz, J = 2.0 Hz, 1H, Ar-H), 7.33 (d, J = 2.0 Hz, 1H, Ar-H), 9.50 (br s, 1H, OH).
2,2’-Diselenobisbenzoic acid 2. The compound was prepared from anthranilic acid 1 using disodium diselenide with a modified literature procedure [59,60,62]. The anthranilic acid 1 (20.5 g, 0.15 mol) was dissolved in a solution of 37% aq. HCl (30 mL) in distilled water (90 mL) at ca. 75 °C. The solution was cooled in an ice-water bath (0–5 °C) to form anthranilic acid hydrochloride precipitate. To the resulting thick suspension, a cold solution of NaNO2 (11.4 g, 0.165 mol) in distilled water (90 mL) at ca. −5 °C was added while stirring for 0.5 h, and the reaction was continued for an additional 15 min. The prepared cold solution of anthranilic acid diazonium chloride was added dropwise for 2.5 h to the freshly prepared methanolic solution of disodium diselenide (NaSeSeNa) at −10–7 °C during stirring with nitrogen evolution accompanied by the formation of red selenium . The reaction mixture was warmed to RT (room temperature) and stored overnight. (NaSeSeNa solution preparation: to the suspension of selenium element (11.2 g, 0.15 mol) in NaOH (18 g, 0.45 mol) solution with MeOH (300 mL) in a round-bottom flask equipped with an oil closure, hydrazine monohydrate (1.9 mL, 0.040 mol) was added and gently stirred at RT for 60 h.) The formed grey selenium was filtered, washed with water, and air-dried to obtain selenium powder (6.20 g, 0.083 mol) with 55% yield, which can be reused. The collected filtrates were acidified with 37% aq. HCl (25 mL), gently heated with mixing and left at RT overnight. The solid was filtered off, washed with hot water (ca. 1 L) (until no odor of salicylic acid in the filtrate was detected), and air-dried. The crude product was recrystallized from hot 1,4-dioxane (400 mL) by slow concentration under ca. 200 mmHg to give acid 2. Yield: (11.1 g, 37%); m.p.: 298–300 °C (300–303 °C [59]); as a pale brick powder (1,4-dioxane).
Bis[(2-chlorocarbonyl)phenyl)] diselenide 3. To 2,2’-diselenobisbenzoic acid (2) (20 g, 50 mmol) suspended in benzene (300 mL) in a round-bottom flask equipped with an oil closure and gas trapping, thionyl chloride (SOCl2) (15.5 g, 0.13 mol) was added. The mixture was gently refluxed at ca. +80 °C with stirring for 4 h and the evolution of gaseous HCl and SO2, following the method previously reported [60]. Benzene and excess of SOCl2 were removed under reduced pressure, then the residue was washed with dry n-hexane (3 × 25 mL) and crystallized from dry CH2Cl2 (15 mL/g) to obtain 3. Yield: (16.4 g, 75%); m.p.: 174–175 °C (m.p.: 174–175 °C [60]); as green yellow prisms.
General procedure for the synthesis of bis(2-carbamoylaryl)phenyldiselenides 4a–p. To a stirred mixture of substituted aniline (1.05 mmol) and sodium carbonate (Na2CO3) powdered in a mortar (0.265 g, 2.5 mmol) in dry CH2Cl2 (5 mL), the solution of bis[(2-chlorocarbonyl)phenyl)] diselenide (3) (0.218 g, 0.50 mmol) in dry CH2Cl2 was added dropwise for 30 min at RT. The reaction progress was monitored using TLC (CHCl3:EtOAc, 4:1, v/v). The stirring was continued for 24–168 h. Over a 3-day period, chloride 3 (10.9 mg, 25.0 μmol) was added. When the reaction was completed, the product was filtered off and washed with CH2Cl2 (2 × 5 mL), water (5 mL), and 3.5% aq. HCl (3 × 5 mL), and air-dried to obtain products 4a–p.
Bis[2-(2-hydroxyphenylcarbamoyl)]phenyl Diselenide 4a. The general procedure starting from 2-hydroxyaniline (0.115 g, 1.05 mmol) at RT was employed, with a 96-h reaction time to obtain 4a. Yield: (0.28 g, 92% calculated on 2-hydroxyaniline used); m.p.: 233.5–236.5 °C (235–238 °C [53]); as a white solid (MeOH, 0.45 g/L). IR (KBr, cm−1) ν 3403 (NH), 3176 (br OH), 1636 (C=O), 1549 (CONH), 1456, 1351, 1286 (C–N), 1235 (C–O), 1194, 1029, 757, 729, 622 (C–Se), 594. 1H-NMR (300 MHz, DMSO-d6): δ 6.86 (ddd, J = 7.8 Hz, J = 7.3 Hz, J = 1.0 Hz, 2H, Ar-H), 6.95 (dd, J = 7.5 Hz, J = 1.0 Hz, 2H, Ar-H), 7.09 (ddd, J = 7.5 Hz, J = 7.3 Hz, J = 1.5 Hz, 2H, Ar-H), 7.40 (ddd, J = 7.3 Hz, J = 6.5 Hz, J = 1.5 Hz, 2H, Ph-H), 7.46 (ddd, J = 7.8 Hz, J = 6.5 Hz, J = 1.5 Hz, 2H, Ph-H), 7.63 (dd, J = 7.8 Hz, J = 1.5 Hz, 2H, Ar-H), 7.79 (dd, J = 7.8 Hz, J = 1.5 Hz, 2H, Ph-H), 8.03 (dd, J = 7.3 Hz, J = 1.5 Hz, 2H, Ph-H), 9.76 (s, 2H, NH), 9.78 (s, 2H, OH). 13C-NMR (75 MHz, DMSO-d6): δ 115.8 (2 × CH), 118.9 (2 × CH), 125.1 (2 × CH), 126.2 (2 × CH), 126.3 (2 × CH), 128.4 (2 × CH), 130.1 (2 × CH), 132.0 (2 × C), 132.1 (2 × CH), 132.2 (2 × C), 133.0 (2 × C), 150.0 (2 × C), 166.1 (2 × C=O). HRMS (TOF MS ESI): m/z for C26H20N2O4Se2 + Na+ calculated: 604.9654; found: 604.9662.
Bis[2-(3-hydroxyphenylcarbamoyl)]phenyl Diselenide 4b. The general procedure starting from 3-hydroxyaniline (109 mg, 1.00 mmol) in anhydrous MeCN (5 mL) and benzoyl chloride 3 in anhydrous MeCN (10 mL) at RT was employed, with a 60-h reaction time followed by the dropwise addition of H2O (10 mL) during stirring. Solid precipitate was filtered off, washed with aq. HCl and EtOH:H2O (19:1, v/v), and air-dried to obtain 4b. Yield: (244 mg, 84%); m.p.: 285.5–287.0 °C (m.p.: 285–286 °C [53]); as a pale yellow powder. 1H-NMR (400 MHz, DMSO-d6): δ 6.51–6.59 (m, 2H, Ar-H), 7.11–7.19 (m, 4H, Ar-H), 7.37–7.39 (m, 2H, Ar-H), 7.40 (ddd, J = 7.5 Hz, J = 7.4 Hz, J = 1.0 Hz, 2H, Ph-H), 7.46 (ddd, J = 7.9 Hz, J = 7.4 Hz, J = 1.4 Hz, 2H, Ph-H), 7.79 (dd, J = 7.9 Hz, J = 1.0 Hz, 2H, Ph-H), 7.93 (dd, J = 7.5 Hz, J = 1.4 Hz, 2H, Ph-H), 9.50 (s, 2H, OH), 10.45 (s, 2H, NH). 13C-NMR (101 MHz, DMSO-d6): δ 107.56 (2 × CH), 111.23 (4 × CH), 126.38 (2 × CH), 128.60 (2 × CH), 129.35 (2 × CH), 130.15 (2 × CH), 131.93 (2 × CH), 131.95 (2 × C), 133.93 (2 × C), 139.65 (2 × C), 157.55 (2 × C), 166.23 (2 × C=O). HRMS (TOF MS ESI): m/z for C26H20N2O4Se2 + Na+ calculated: 604.9654; found: 604.9660. Anal. Calcd. for C26H20N2O4Se2 (582.37): C, 53.62; H, 3.46; N, 4.81. Found: C, 53.87; H, 3.25; N, 4.89.
Bis[2-(4-hydroxyphenylcarbamoyl)]phenyl Diselenide 4c. The general procedure starting from 4-hydroxyaniline (0.115 g, 1.05 mmol) at RT was employed, with a 120-h reaction time to obtain 4c as a beige powder. Yield: (222 mg, 73% calculated on the 4-hydroxyaniline used). An analytically pure sample was obtained by column chromatography on silica gel using a mixture of CHCl3 and EtOAc (4:1, v/v) as an eluent to obtain pure 4c; m.p.: 271–272 °C (282–284 °C [53]); as a pale solid (CH2Cl2). IR (KBr, cm−1) ν 3260 (br NH), 3071 (br OH), 1640 (C=O), 1619, 1589, 1510 (CONH), 1444, 1352, 1280 (C–N), 1235 (C–O), 828, 732, 670 (C–Se), 517. 1H-NMR (300 MHz, DMSO-d6): δ 6.83 (d, J = 8.7 Hz, 4H, Ar-H), 7.35 (d, J = 8.7 Hz, 4H, Ar-H), 7.46 (dd, J = 7.5 Hz, J = 7.2 Hz, 2H, Ph-H), 7.66 (dd, J = 8.0 Hz, J = 7.2 Hz, 2H, Ph-H), 7.87 (d, J = 7.5 Hz, 2H, Ph-H), 8.06 (d, J = 8.0 Hz, 2H, Ph-H), 9.60 (s, 4H, 2 × NH and 2 × OH). 13C-NMR (75 MHz, DMSO-d6): δ 115.49 (4 × CH), 125.67 (2 × CH), 126.04 (2 × CH), 126.70 (4 × CH), 127.74 (2 × CH), 128.21 (2 × C), 130.53 (2 × C), 131.84 (2 × CH), 138.91 (2 × C), 155.66 (2 × C), 164.82 (2 × C=O). HRMS (TOF MS ESI): m/z for C26H20N2O4Se2 + Na+ calculated: 606.9646; found: 606.9676.
Bis[2-(2-hydroxy-4-methylphenylcarbamoyl)]phenyl Diselenide 4d. The general procedure starting from 2-hydroxy-4-methylaniline (129 mg, 1.05 mmol) at RT was employed, with a 24-h reaction time to obtain 4d. Yield: (183 mg, 60% calculated on benzoyl chloride 3 used); m.p.: 221.0–223.5 °C; as a beige powder (CH2Cl2). IR (KBr, cm−1) ν 3394 (NH), 3250 (br OH), 1640 (C=O), 1603, 1541 (CONH), 1288 (C–N), 1228 (C–O), 809, 736, 647 (C–Se), 589. 1H-NMR (300 MHz, DMSO-d6): δ 2.25 (s, 6H, CH3), 6.66 (dd, J = 8.2 Hz, J = 1.2 Hz, 2H, Ar-H), 6.76 (d, J = 1.2 Hz, 2H, Ar-H), 7.39 (ddd, J = 7.2 Hz, J = 7.0 Hz, J = 1.0 Hz, 2H, Ph-H), 7.43 (d, J = 8.2 Hz, 2H, Ar-H), 7.44 (ddd, J = 7.8 Hz, J = 7.0 Hz, J = 1.6 Hz, 2H, Ph-H), 7.77 (dd, J = 7.8 Hz, J = 1.0 Hz, 2H, Ph-H), 8.00 (dd, J = 7.2 Hz, J = 1.6 Hz, 2H, Ph-H), 9.60 (s, 2H, OH), 9.72 (s, 2H, NH). 13C-NMR (75 MHz, DMSO-d6): δ 20.7 (2 × CH3), 116.4 (2 × CH), 119.5 (2 × CH), 122.5 (2 × C), 125.0 (2 × CH), 126.3 (2 × CH), 128.4 (2 × CH), 130.0 (2 × CH), 131.9 (2 × CH), 132.2 (2 × C), 133.0 (2 × C), 135.7 (2 × C), 149.9 (2 × C), 166.1 (2 × C=O). HRMS (TOF MS ESI): m/z for C28H24N2O4Se2 + Na+ calculated: 634.9959; found: 634.9949. Anal. Calcd. for C28H24N2O4Se2 (610.42): C, 55.09; H, 3.96; N, 4.59. Found: C, 55.18; H, 3.82; N, 4.52.
Bis[2-(2-hydroxy-5-methylphenylcarbamoyl)]phenyl Diselenide 4e. The general procedure starting from 2-hydroxy-5-methylaniline (129 mg, 1.05 mmol) and benzyl chloride 3 (240 mg, 0.55 mmol) at RT was employed, with a 96-h reaction time to obtain 4e. Yield: (285 mg, 89% calculated on 2-hydroxy-5-methylaniline); m.p.: 191.5–194.5 °C; as a beige powder (CH2Cl2). IR (KBr, cm−1) ν 3409 (NH), 3235 (br OH), 1640 (C=O), 1599, 1547 (CONH), 1504, 1279 (C–N), 1236 (C–O), 1200, 1027, 809, 740, 619 (C–Se), 452. 1H-NMR (300 MHz, DMSO-d6): δ 2.25 (s, 6H, CH3), 6.83 (d, J = 8.2 Hz, 2H, Ar-H), 6.89 (dd, J = 8.2 Hz, J = 1.7 Hz, 2H, Ar-H), 7.39 (ddd, J = 7.5 Hz, J = 7.1 Hz, J = 1.0 Hz, 2H, Ph-H), 7.46 (d, J = 1.7 Hz, 2H, Ar-H), 7.47 (ddd, J = 7.8 Hz, J = 7.1 Hz, J = 1.4 Hz, 2H, Ph-H), 7.78 (dd, J = 7.8 Hz, J = 1.0 Hz, 2H, Ph-H), 8.00 (dd, J = 7.5 Hz, J = 1.4 Hz, 2H, Ph-H), 9.49 (s, 2H, NH), 9.71 (s, 2H, OH). 13C-NMR (75 MHz, DMSO-d6): δ 20.2 (2 × CH3), 115.7 (2 × CH), 124.8 (2 × C), 125.2 (2 × CH), 126.4 (2 × CH), 126.5 (2 × CH), 127.5 (2 × C), 128.4 (2 × CH), 130.1 (2 × CH), 132.0 (2 × CH), 132.2 (2 × C), 133.0 (2 × C), 147.5 (2 × C), 166.1 (2 × C=O). HRMS (TOF MS ESI): m/z for C28H24N2O4Se2 + Na+ calculated: 632.9967; found: 632.9987. Anal. Calcd. for C28H24N2O4Se2 (610.42): C, 55.09; H, 3.96; N, 4.59. Found: C, 55.01; H, 3.88; N, 4.64.
Bis[2-(2-hydroxy-6-methylphenylcarbamoyl)]phenyl Diselenide 4f. The general procedure starting from 2-hydroxy-6-methylaniline (129 mg, 1.05 mmol) at RT was employed, with a 168-h reaction time to obtain 4f. Yield: (282 mg, 88% calculated on 2-hydroxy-6-methylaniline used); m.p.: 264–267 °C; as a pale beige powder (CH2Cl2). IR (KBr, cm−1) ν 3360 (NH), 3251 (OH), 1622 (C=O), 1592, 1515 (CONH), 1472, 1294 (C–N), 1191 (C–O), 1026, 781, 737, 549 (C–Se). 1H-NMR (300 MHz, DMSO-d6): δ 2.19 (s, 6H, CH3), 6.74 (d, J = 7.7 Hz, 2H, Ar-H), 6.78 (d, J = 8.2 Hz, 2H, Ar-H), 7.05 (dd, J = 8.2 Hz, J = 7.7 Hz, 2H, Ar-H), 7.39 (dd, J = 7.6 Hz, J = 6.0 Hz, 2H, Ph-H), 7.45 (dd, J = 6.8 Hz, J = 6.0 Hz, 2H, Ph-H), 7.77 (d, J = 7.6 Hz, 2H, Ph-H), 8.09 (d, J = 6.8 Hz, 2H, Ph-H), 9.39 (s, 2H, NH), 9.78 (s, 2H, OH). 13C-NMR (75 MHz, DMSO-d6): δ 17.9 (2 × CH3), 113.5 (2 × CH), 120.4 (2 × CH), 123.4 (2 × C), 126.1 (2 × CH), 127.3 (2 × CH), 128.5 (2 × CH), 129.9 (2 × CH), 131.7 (2 × CH), 132.4 (2 × C), 133.0 (2 × C), 136.8 (2 × C), 153.3 (2 × C), 166.5 (2 × C=O). HRMS (TOF MS ESI): m/z for C28H24N2O4Se2 + Na+ calculated: 632.9967; found: 632.9971. Anal. Calcd. for C28H24N2O4Se2 (610.42): C, 55.09; H, 3.96; N, 4.59. Found: C, 55.16; H, 4.09; N, 4.45.
Bis[2-(2-hydroxy-4-chlorophenylcarbamoyl)]phenyl Diselenide 4g. The general procedure starting from 2-hydroxy-4-chloroaniline (151 mg, 1.05 mmol) at RT was employed, with a 96-h reaction time to obtain 4g. Yield: (202 mg, 59% calculated on 2-hydroxy-4-chloroaniline used); m.p.: 230–234 °C; as a brownish gray powder (CH2Cl2). IR (KBr, cm−1) ν 3398 (NH), 3176 (br OH), 1639 (C=O), 1532 (CONH), 1414, 1342, 1265 (C–N), 1226 (C–O), 911, 734, 648, 590 (C–Se), 452. 1H-NMR (300 MHz, DMSO-d6): δ 6.92 (dd, J = 8.5 Hz, J = 2.2 Hz, 2H, Ar-H), 6.98 (d, J = 2.2 Hz, 2H, Ar-H), 7.40 (dd, J = 7.2 Hz, J = 7.0 Hz, 2H, Ph-H), 7.46 (ddd, J = 7.9 Hz, J = 7.0 Hz, J = 1.2 Hz, 2H, Ph-H), 7.63 (d, J = 8.5 Hz, 2H, Ar-H), 7.76 (d, J = 7.9 Hz, 2H, Ph-H), 8.02 (dd, J = 7.2 Hz, J = 1.2 Hz, 2H, Ph-H), 9.86 (s, 2H, NH), 10.37 (s, 2H, OH). 13C-NMR (75 MHz, DMSO-d6): δ 115.5 (2 × CH), 118.7 (2 × CH), 124.3 (2 × C), 126.4 (2 × CH), 126.6 (2 × CH), 128.6 (2 × CH), 129.6 (2 × C), 130.0 (2 × CH), 132.1 (2 × CH), 132.3 (2 × C), 132.7 (2 × C), 151.3 (2 × C), 166.2 (2 × C=O). HRMS (TOF MS ESI): m/z for C26H18Cl2N2O4Se2 + Na+ calculated: 672.8874; 674.8866; found: 672.8876; 674.8884. Anal. Calcd. for C26H18Cl2N2O4Se2 (651.26): C, 47.95; H, 2.79; N, 4.30. Found: C, 48.08; H, 2.54; N, 4.50.
Bis[2-(2-hydroxy-5-chlorophenylcarbamoyl)]phenyl Diselenide 4h. The general procedure starting from 2-hydroxy-5-chloroaniline (151 mg, 1.05 mmol) at RT was employed, with a 48-h reaction time to obtain 4h. Yield: (206 mg, 63% calculated on benzoyl chloride 3); m.p.: 219.5–221.5 °C; as a gray-brown powder (CH2Cl2). IR (KBr, cm−1) ν 3400 (NH), 3218 (br OH), 1638 (C=O), 1596, 1543 (CONH), 1428, 1270, 1239 (C–N), 1195 (C–O), 1027, 731 (C–Cl), 655 (C–Se), 614. 1H-NMR (400 MHz, DMSO-d6): δ 6.96 (d, J = 8.7 Hz, 2H, Ar-H), 7.13 (dd, J = 8.7 Hz, J = 2.6 Hz, 2H, Ar-H), 7.41 (dd, J = 7.6 Hz, J = 7.3 Hz, 2H, Ph-H), 7.47 (dd, J = 7.9 Hz, J = 7.3 Hz, 2H, Ph-H), 7.77 (d, J = 2.6 Hz, 2H, Ar-H), 7.78 (d, J = 7.6 Hz, 2H, Ph-H), 8.01 (d, J = 7.9 Hz, 2H, Ph-H), 9.82 (s, 2H, OH), 10.18 (s, 2H, NH). 13C-NMR (101 MHz, DMSO-d6): δ 116.89 (2 × CH), 121.93 (2 × C), 124.06 (2 × CH), 125.56 (2 × CH), 126.38 (2 × C), 126.41 (2 × CH), 128.61 (2 × CH), 130.10 (2 × CH), 132.18 (2 × CH), 132.30 (2 × C), 132.68 (2 × C), 148.76 (2 × C), 166.19 (2 × C=O). HRMS (TOF MS ESI): m/z for C26H18Cl2N2O4Se2 + Na+ calculated: 674.8866; found: 674.8862. Anal. Calcd. for C26H18Cl2N2O4Se2 (651.26): C, 47.95; H, 2.79; N, 4.30. Found: C, 48.03; H, 2.56; N, 4.25.
Bis[2-(2-hydroxy-5-carboxymethylphenylcarbamoyl)]phenyl Diselenide 4i. The general procedure starting from 2-hydroxy-5-carboxymethylaniline (175 mg, 1.05 mmol) at RT was employed, with a 72-h reaction time to obtain 4i. Yield: (209 mg, 57% calculated on 2-hydroxy-5-carboxymethylaniline used); m.p.: 281–282 °C; as a cream powder (CH2Cl2). IR (KBr, cm−1) ν 3323 (br NH and OH), 1709, 1691 (OC=O), 1639 (NC=O), 1604, 1537 (CONH), 1430, 1298 (C–N), 1236 (C–O), 1120 (C–O), 737, 606 (C–Se). 1H-NMR (400 MHz, DMSO-d6): δ 3.83 (s, 6H, OCH3), 7.05 (d, J = 8.5 Hz, 2H, Ar-H), 7.41 (ddd, J = 7.6 Hz, J = 7.3 Hz, J = 1.2 Hz, 2H, Ph-H), 7.48 (ddd, J = 7.9 Hz, J = 7.3 Hz, J = 1.2 Hz, 2H, Ph-H), 7.74 (dd, J = 8.5 Hz, J = 2.2 Hz, 2H, Ar-H), 7.79 (dd, J = 7.9 Hz, J = 1.2 Hz, 2H, Ph-H), 8.03 (dd, J = 7.6 Hz, J = 1.2 Hz, 2H, Ph-H), 8.31 (d, J = 2.2 Hz, 2H, Ar-H), 9.89 (s, 2H, NH), 10.86 (s, 2H, OH). 13C-NMR (101 MHz, DMSO-d6): δ 51.76 (2 × OCH3), 115.58 (2 × CH), 120.14 (2 × C), 125.09 (2 × C), 126.27 (2 × CH), 126.42 (2 × CH), 127.95 (2 × CH), 128.61 (2 × CH), 130.10 (2 × CH), 132.16 (2 × CH), 132.30 (2 × C), 132.74 (2 × C), 154.64 (2 × C), 165.83 (2 × C=O), 166.25 (2 × C=O). HRMS (TOF MS ESI): m/z for C30H24N2O8Se2 + Na+ calculated: 720.9763; found: 720.9755. Anal. Calcd. for C30H24N2O8Se2 (698.44): C, 51.59; H, 3.46; N, 4.01. Found: C, 51.43; H, 3.60; N, 3.95.
Bis[2-(3-hydroxy-4-methoxyphenylcarbamoyl)]phenyl Diselenide 4j. The general procedure starting from 3-hydroxy-4-methoxyaniline (146 mg, 1.05 mmol) at RT was employed, with a 120-h reaction time to obtain 4j. Yield: (317 mg, 94% calculated on 3-hydroxy-4-methoxyaniline used); m.p.: 232.5–236.5 °C; as a white powder (CH2Cl2). IR (KBr, cm−1) ν 3500 (NH), 3270 (OH), 1670, 1636 (C=O), 1513 (CONH), 1270 (C–N), 1239 (C–O), 1175 (C–O), 1024 (C–O), 738, 565 (C–Se). 1H-NMR (300 MHz, DMSO-d6): δ 3.76 (s, 6H, 2OCH3), 6.91 (d, J = 8.8 Hz, 2H, Ar-H), 7.13 (dd, J = 8.8 Hz, J = 2.5 Hz, 2H, Ar-H), 7.37 (d, J = 2.5 Hz, 2H, Ar-H), 7.36–7.46 (m, 4H, Ph-H), 7.78 (dd, J = 7.7 Hz, J = 1.2 Hz, 2H, Ph-H), 7.93 (dd, J = 7.3 Hz, J = 1.4 Hz, 2H, Ph-H), 7.06 (br s, 2H, OH), 10.32 (s, 2H, NH). 13C-NMR (75 MHz, DMSO-d6): δ 55.8 (2 × OCH3), 108.9 (2 × CH), 111.4 (2 × CH), 112.3 (2 × CH), 126.3 (2 × CH), 128.4 (2 × CH), 130.0 (2 × CH), 131.7 (2 × CH), 131.9 (2 × C), 132.1 (2 × C), 133.9 (2 × C), 144.4 (2 × C), 146.3 (2 × C), 165.8 (2 × C=O). HRMS (TOF MS ESI): m/z for C28H24N2O6Se2 + Na+ calculated: 664.9865; 666.9857; found: 664.9854; 666.9868. Anal. Calcd. for C28H24N2O6Se2 (642.42): C, 52.35; H, 3.77; N, 4.36. Found: C, 52.30; H, 3.52; N, 4.45.
Bis[2-(2-methoxyphenylcarbamoyl)]phenyl Diselenide 4k. The general procedure starting from 2-methoxyaniline (129 mg, 1.05 mmol) at RT was employed, with a 168-h reaction time to obtain 4k. Yield: (0.301 g, 94% calculated on 2-methoxyaniline used); m.p.: 214–215 °C (m.p.: 216–217 °C [53]); as yellow crystals (MeOH, 2 L/g). IR (KBr, cm−1) ν 3411 (NH), 1646 (C=O), 1602, 1528 (CONH), 1462, 1434, 1340, 1289 (C–N), 1251 and 1026 (C–O), 745, 629 (C–Se), 591, 561, 457. 1H-NMR (300 MHz, DMSO-d6): δ 3.84 (s, 6H, OCH3), 7.00 (ddd, J = 7.7 Hz, J = 7.4 Hz, J = 1.0 Hz, 2H, Ar-H), 7.13 (dd, J = 8.3 Hz, J = 1.0 Hz, 2H, Ar-H), 7.24 (ddd, J = 8.3 Hz, J = 7.4 Hz, J = 1.6 Hz, 2H, Ar-H), 7.40 (dd, J = 7.4 Hz, J = 7.4 Hz, 2H, Ph-H), 7.47 (dd, J = 7.9 Hz, J = 7.4 Hz, 2H, Ph-H), 7.73 (dd. J = 7.7 Hz, J = 1.6 Hz, 2H Ar-H), 7.77 (d, J = 7.9 Hz, 2H, Ph-H), 8.01 (d, J = 7.4 Hz, 2H, Ph-H), 9.83 (s, 2H, NH). 13C-NMR (75 MHz, DMSO-d6): δ 55.56 (2 × OCH3), 111.56 (2 × C), 120.17 (2 × C), 125.15 (2 × C), 126.16 (2 × C), 126.39 (2 × C), 126.42 (2 × C), 128.49 (2 × C), 130.06 (2 × C), 132.04 (2 × C), 132.30 (2 × C), 132.96 (2 × C), 152.04 (2 × C), 166.12 (2 × C=O). The HRMS (TOF MS ESI) is in agrement with literature data [26].
Bis[2-(3-methoxyphenylcarbamoyl)]phenyl Diselenide 4l. Preparation according to Reference [35]. The general procedure starting from 3-methoxyaniline (129 mg, 1.05 mmol) at RT was employed, with a 72-h reaction time to obtain 4l. Yield: (0.300 g, 98% calculated on benzoyl chloride 3 used); m.p.: 221–223 °C (221–223 °C [35]); as a colorless powder (CHCl3:EtOAc, 1:2, v/v). Yield of the analytically pure sample: (226 mg, 74%). The 1H-NMR, 13C-NMR, and HRMS (TOF MS ESI) spectra are in agreement with literature data [35].
Bis[2-(4-methoxyphenylcarbamoyl)]phenyl Diselenide 4m. Preparation according to a modified literature procedure [35]. The general procedure starting from 4-methoxyaniline (129 mg, 1.05 mmol) and bis[(2-chlorocarbonyl)phenyl)] diselenide 3 (0.229 g, 0.525 mmol) at RT was employed, with a 48-h reaction time to obtain 4m. Yield: (0.300 g, 94%); m.p.: 285.5–287.5 °C (290–292 °C [53]); as a colorless powder (CHCl3:EtOAc, 1:2, v/v). Yield of the analytically pure sample: (266 mg, 83%). The 1H-NMR, 13C-NMR, and HRMS (TOF MS ESI) spectra are in agreement with literature data [35].
Bis[2-(2,4-dimethoxyphenylcarbamoyl)]phenyl Diselenide 4n. The general procedure starting from 2,4-dimethoxyaniline (161 mg, 1.05 mmol) at RT was employed, with a 168-h reaction time, with separation by acidification with 3.5% HCl to a pH of 2.5, extraction with CH2Cl2, dry with anhydrous Na2SO4, filtration, and solvent evaporation to obtain 4n. Yield: (348 mg, 99% calculated on 2,4-dimethoxyaniline used); m.p.: 211.0–213.5 °C; as a white powder (CH2Cl2). IR (KBr, cm−1) ν 3436 (NH), 1640 (C=O), 1531 (CONH), 1414, 1283 (C–N), 1205, and 1036 (C–O), 831, 732, 578 (C–Se), 453. 1H-NMR (300 MHz, DMSO-d6): δ 3.79 (s, 6H, 2OCH3), 3.82 (s, 6H, 2OCH3), 6.57 (dd, J = 8.8 Hz, J = 2.6 Hz, 2H, Ar-H), 6.69 (d, J = 2.6 Hz, 2H, Ar-H), 7.35–7.48 (m, 4H, Ph-H), 7.47 (d, J = 8.8 Hz, 2H, Ar-H), 7.75 (d, J = 7.3 Hz, 2H, Ph-H), 8.01 (d, J = 7.2 Hz, 2H, Ph-H), 9.76 (s, 2H, NH). 13C-NMR (75 MHz, DMSO-d6): δ 55.33 (2 × OCH3), 55.69 (2 × OCH3), 98.94 (2 × C), 104.25 (2 × C), 119.06 (2 × C), 126.28 (2 × C), 126.79 (2 × C), 128.39 (2 × C), 129.98 (2 × C), 131.88 (2 × C), 132.30 (2 × C), 132.93 (2 × C), 153.74 (2 × C), 158.28 (2 × C), 166.20 (2 × C=O). HRMS (TOF MS ESI): m/z for C30H28N2O6Se2 + Na+ calculated: 693.0178; found: 693.0176. Anal. Calcd. for C30H28N2O6Se2 (670.47): C, 53.74; H, 4.21; N, 4.18. Found: C, 53.62; H, 4.13; N, 4.27.
Bis[2-(3,4-dimethoxyphenylcarbamoyl)]phenyl Diselenide 4o. The general procedure starting from 3,4-dimethoxyaniline (161 mg, 1.05 mmol) at RT was employed, with a 96-h reaction time to obtain 4o. Yield: (253 mg, 72%); m.p.: 231.5–234.5 °C (m.p.: 235 °C [53]); as a pale yellow powder (CH2Cl2). IR (KBr, cm−1) ν 3291 (NH), 1641 (C=O), 1513 (CONH), 1452, 1263 (C–N), 1237 (C–O), 1134, 1026 (C–O), 741, 681, 574 (C–Se), 454. 1H-NMR (300 MHz, DMSO-d6): δ 3.76 (s, 6H, 2OCH3), 3.77 (s, 6H, 2OCH3), 6.96 (d, J = 8.8 Hz, 2H, Ar-H), 7.30–7.45 (m, 6H, Ph-H and Ar-H), 7.47 (s, 2H, Ar-H), 7.83 (s, br., 2H, Ph-H), 7.95 (d, J = 7.7 Hz, 2H, Ph-H), 10.51 (s, 2H, NH). 13C-NMR (75 MHz, DMSO-d6): δ 55.41 (2 × OCH3), 55.67 (2 × OCH3), 105.81 (2 × CH), 111.84, (2 × CH), 112.72 (2 × CH), 126.08 (2 × CH), 128.36 (2 × CH), 130.10 (2 × CH), 131.59 (2 × CH), 132.19 (2 × C), 132.30 (2 × C), 133.63 (2 × C), 145.46 (2 × C), 148.42 (2 × C), 165.78 (2 × C=O). HRMS (TOF MS ESI): m/z for C30H28N2O6Se2 + Na+ calculated: 693.0178. Found: 693.0178.
Bis[2-(3,4,5-trimethoxyphenylcarbamoyl)]phenyl Diselenide 4p. The general procedure starting from 3,4,5-trimethoxyaniline (192 mg, 1.05 mmol) at RT was employed, with a 96-h reaction time to obtain 4p. Yield: (0.291 g, 76%); m.p.: 193–195 °C; as a white powder (CH3OH, 100 g/L). IR (KBr, cm−1) ν 3322 (NH), 1605 (C=O), 1507 (CONH), 1450, 1411, 1313, 1235 (C–N), 1128 (C–O), 1027 (C–O), 1004 (C–O), 738, 623 (C–Se). 1H-NMR (300 MHz, DMSO-d6): δ 3.65 (s, 6H, 2OCH3), 3.77 (s, 12H, 4OCH3), 7.20 (s, 4H, Ar-H), 7.38 (dd, J = 7.3 Hz, 2H, Ph-H), 7.44 (dd, J = 7.3 Hz, 2H, Ph-H), 7.80–8.00 (m, 4H, Ph-H), 10.56 (s, 2H, NH). 1H-NMR (600 MHz, DMSO-d6): δ 3.67 (s, 6H, 2OCH3), 3.80 (s, 12H, 4OCH3), 7.21 (s, 4H, Ar-H), 7.42 (dd, J = 7.3 Hz, J = 6.6 Hz, 2H, Ph-H), 7.46 (dd, J = 7.9 Hz, J = 6.6 Hz, 2H, Ph-H), 7.81 (d, J = 7.9 Hz, 2H, Ph-H), 7.95 (d, J = 7.3 Hz, 2H, Ph-H), 10.47 (s, 2H, NH). 13C-NMR (75 MHz, DMSO-d6): δ 56.33 (4 × OCH3), 60.64 (2 × OCH3), 99.21 (4 × CH), 126.6 (2 × CH), 128.9 (2 × CH), 130.5 (2 × CH), 132.1 (2 × CH, 2 × C), 134.4 (2 × C), 134.6 (2 × C), 135.8 (2 × C), 153.2 (4 × C), 166.5 (2 × C=O). 13C-NMR (151 MHz, DMSO-d6): δ 55.75 (4 × OCH3), 60.10 (2 × OCH3), 98.24 (4 × CH), 126.31 (2 × CH), 128.53 (2 × CH), 130.12 (2 × CH), 131.97 (2 × CH, 2 × C), 133.71 (2 × C), 134.05 (2 × C), 134.75 (2 × C), 152.64 (4 × C), 166.10 (2 × C=O).HRMS (TOF MS ESI): m/z for C32H32N2O8Se2 + Na+ calculated: 755.0381; found: 755.0381. Anal. Calcd. for C32H32N2O8Se2 (730.53): C, 52.61; H, 4.42; N, 3.83. Found: C, 52.46; H, 4.56; N, 3.72.
Bis[2-(N-morpholinecarbamoyl)]phenyl Diselenide 5. To a stirred solution of morpholine (436 mg, 0.44 mL, 5.0 mmol) in dry CH2Cl2 (10 mL), the solution of bis[(2-chlorocarbonyl)phenyl]diselenide 3 (437 mg, 1.0 mmol) in dry CH2Cl2 was added and the reaction was continued at RT (+20 °C) for 120 h until no spot of chloride 3 was observed during TLC control (silica gel, eluent CHCl3:EtOAc, 4:1, v/v). When the reaction was completed, the mixture was poured into water (50 mL), acidified with 3.5% HCl to a pH of 1.0, extracted with CH2Cl2 (3 × 25 mL), and dried with Na2SO4. After evaporation of the solvent, the residue was washed with n-hexane and DEE (25 mL) was added to the oily residue, followed by stirring until the product solidified and was filtered off to obtain 5. Yield: (458 mg, 85% calculated on diselenide 3 used); m.p.: 149–152 °C; as a pale yellow powder (DEE). IR (KBr, cm−1) ν 3059, 2972, 2959, 2902, 2854 (CH), 1625 (C=O), 1608, 1430, 1279, 1248, 1111 (C–N), 1021 (C–O), 1014 (C–O), 757, 596, 559 (C–Se), 461. 1H-NMR (300 MHz, DMSO-d6): δ 3.57 (s, 16H, 8CH2), 7.28–7.37 (m, 4H, Ph-H), 7.39 (ddd, J = 7.7 Hz, J = 6.4 Hz, J = 2.7 Hz, 2H, Ph-H), 7.74 (dd, J = 8.2 Hz, J = 1.2 Hz, 2H, Ph-H). 13C-NMR (75 MHz, DMSO-d6): δ 66.04 (8 × CH2), 126.84 (2 × CH), 127.36 (2 × CH), 129.05 (2 × C), 130.38 (2 × CH), 131.71 (2 × CH), 136.22 (2 × C), 167.48 (2 × C=O). HRMS (TOF MS ESI): m/z for C22H24N2O4Se2 + Na+ calculated: 558.9986; 560.9967; 562.9959; found: 559.0029; 560.9987; 562.9982. Anal. Calcd. for C22H24N2O4Se2 (538.36): C, 49.08; H, 4.49; N, 5.20. Found: C, 48.96; H, 4.62; N, 5.29.
Bis[2-(N-benzamidecarbamoyl)]phenyl Diselenide 6. The general procedure starting from benzhydrazide (143 mg, 1.05 mmol) at RT was employed, with a 120-h reaction time to obtain 6. Yield: (335 mg, 100%); m.p.: 296.5 °C (with decomp.); as a pale yellow wool (AcOH, 1.5 g/L). 1H-NMR (601 MHz, DMSO-d6): δ 7.42 (ddd, J = 7.7 Hz, J = 7.3 Hz, J = 0.8 Hz, 2H, Ph-H), 7.50 (ddd, J = 8.0 Hz, J = 7.3 Hz, J = 1.1 Hz, 2H, Ph-H), 7.54 (dd, J = 7.8 Hz, J = 7.4 Hz, 4H, Ar-H), 7.62 (t, J = 7.3 Hz, 2H, Ar-H), 7.78 (dd, J = 8.0 Hz, J = 0.8 Hz, 2H Ph-H), 7.97 (d, J = 7.8 Hz, 4H, Ar-H), 8.00 (dd, J = 7.7 Hz, J = 1.1 Hz, 2H, Ph-H), 10.68 (s, 2H, NH), 10.83 (s, 2H, NH). 13C-NMR (151 MHz, DMSO-d6): δ 126.40 (2 × CH), 127.45 (4 × CH), 128.30 (2 × CH), 128.51 (4 × CH), 130.12 (2 × CH), 130.70 (2 × C), 131.94 (2 × CH), 132.30 (2 × C), 132.44 (2 × CH), 132.47 (2 × C), 165.77 (2 × C=O), 166.86 (2 × C=O). HRMS (TOF MS ESI): m/z for C28H22N4O4Se2 + Na+ calculated: 656.9891; 658.9872; found: 656.9968; 658.9862. Anal. Calcd. for C28H22N4O4Se2 (636.42): C, 52.84; H, 3.48; N, 8.80. Found: C, 53.02; H, 3.29; N, 8.79.
2-Hydroxyacetanilide phenolic ester of bis(2-carboxy)phenyl diselenide 7. The general procedure starting from 2-hydroxyacetanilide (151 mg, 1.0 mmol) dissolved in dry CH2Cl2 (10 mL) at RT was employed, with a 96-h reaction time to obtain 7. Yield: (119 mg, 36% calculated on 2-hydroxyacetanilide used); m.p.: 178–181 °C; as a pale solid (CH2Cl2). IR (KBr, cm−1) ν 3402 (NH), 1760 (OC=O), 1659 (NC=O), 1545 (CONH), 1455, 1370, 1285 (C–N), 1216 (C–O), 1116, 767, 622 (C–Se), 457. 1H-NMR (300 MHz, DMSO-d6): δ 2.09 (s, 6H, CH3), 6.75 (dd, J = 7.9 Hz, J = 7.1 Hz, 2H, Ph-H), 6.85 (d, J = 7.0 Hz, 2H, Ph-H), 6.93 (dd, J = 7.9 Hz, J = 6.6 Hz, 2H, Ph-H), 7.36 (dd, J = 7.6 Hz, J = 7.0 Hz, 2H, Ph-H), 7.49 (dd, J = 7.2 Hz, J = 7.0 Hz, 2H, Ph-H), 7.66 (d, J = 6.6 Hz, 2H, Ar-H), 7.67 (d, J = 7.9 Hz, 2H, Ph-H), 8.33 (d, J = 7.2 Hz, 2H, Ph-H), 9.72 (s, 2H, NH). HRMS (TOF MS ESI): m/z for C30H24N2O6Se2 + Na+ calculated: 688.9865; 690.9857; found: 688.9866; 690.9855. Anal. Calcd. for C30H24N2O6Se2 (666.44): C, 54.07; H, 3.63; N, 4.20. Found: C, 53.85; H, 3.90; N, 4.06.
(2R,3S,6S,7R,8R)-3-(3-Formamido-2-hydroxybenzamido)-8-n-alkyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl 3-Methylbutanoate (antimycin A) phenolic ester of bis(2-carboxy)phenyl diselenide 8. The general procedure starting from naturally occurred antimycin A (0.21 g, 0.40 mmol) dissolved in dry CH2Cl2 (5 mL), and Na2CO3 (0.16 g, 1.5 mmol), diselenide 3 (87 mg, 0.20 mmol) at RT was employed, with a 10-day reaction time to obtain phenolic ester 8 as a co-crystallizable mixture of different alkyl substituents with R=C5H11 predominantly. Yield: (0.277 g, 97% calculated on antimycin A used); m.p.: >300 °C; as a pale solid. In 13C-NMR, a number of signals is observed and only diagnostic ones are reported 13C-NMR (101 MHz, DMSO-d6): δ 13.88, 17.72, 18.92, 19.00, 19.02, 20.28, 21.85, 21.94, 22.85, 24.43, 25.66, 26.81, 27.18, 28.10 and 30.89 (Caliphatic); 58.05, 58.49, 66.77, 66.80 and 77.35 (CHO and CNO); 107.05, 107.47, 117.11, 117.21, 117.28, 118.94, 124.16, 130.59, 131.94 and 136.79 (CAr, CAr-H); 151.23 (CAr-CON), 166.95, 167.16, 167.26, 167.43, 170.42, 174.84, 174.98, 175.09 and 175.11 (C=O). HRMS (TOF MS ESI): m/z for C64H74N4O20Se2 + Na+ calculated: 1401.3119; found: 1401.3433; for C65H76N4O20Se2 + Na+ calculated: 1411.3291; 1413.3283; found: 1411.3070; 1413.3112; for C66H78N4O20Se2 + Na+ calculated: 1427.3440; 1429.3432; found: 1427.3264; 1429.3787; for C67H80N4O20Se2 + Na+ calculated: 1441.3596; found: 1441.3926; for C68H82N4O20Se2 + Na+ calculated: 1455.3753; 1457.3745; found: 1455.4171; 1457.4158; for C69H84N4O20Se2 + Na+ calculated: 1469.3909; 1471.3902; found: 1469.4357; 1471.4440; for C70H86N4O20Se2 + Na+ calculated: 1481.4085; 1483.4066; 1485.4058; 1486.4092; found: 1481.3927; 1483.4473; 1485.5210; 1486.4746. Anal. Calcd. for C68H82N4O20Se2 (1433.31): C, 56.98; H, 5.77; N, 3.91. Found: C, 57.01; H, 5.82; N, 3.77.
2-(Chloroseleno)benzoyl chloride 9. The compound was prepared from 2,2’-diselenobisbenzoic acid 2 (8.0 g, 20 mmol) using excess of thionyl chloride (6.0 g, 50 mmol) and DMF (1.5 g, 20 mmol) in boiling benzene (150 mL), following the method previously reported [60]. Crude oily product was recrystallized from n-hexane (ca. 15 mL/g) and dried under vacuum (20 mmHg) to give 9. Yield: (8.1 g, 80%); m.p.: 65–67 °C (65–66 °C [60]); as yellow needles (n-hexane).
General synthetic procedure for benzisoselenazol-3(2H)-ones 10a–g. To a stirred solution of substituted aniline (13.0 mmol) in anhydrous CH2Cl2 (5 mL) a solution of (2-chloroseleno)benzoyl chloride 9 (1.02 g, 4.0 mmol) in anhydrous CH2Cl2 (25 mL) was added dropwise. The reaction was continued for 2–24 h. The progress of reaction was controlled by TLC (silica gel, eluent CHCl3:EtOAc, 4:1, v/v). After the reaction was finished, the solvent was evaporated, HCl (3.5%, w/w, 40 mL) was added, and the mixture was stirred at RT overnight, then filtered off and left to dry in the air. Crude products were purified by column chromatography on silica gel (70–230 mesh) eluted with CHCl3 to obtain pure 10a–g.
2-(2-Hydroxyphenyl)-1,2-benzisoselenazol-3(2H)-one 10a. The general procedure starting from 2-hydroxyaniline (0.44 g, 4.0 mmol) and anhydrous Et3N (1.01 g, 1.4 mL, 10 mmol) at RT was employed, with a 20-h reaction time with separation of the product from the crude reaction mixture by column chromatography using EtOAc as an eluent to obtain 10a. Yield: (0.46 g, 40% calculated on benzoyl chloride 9 used); m.p.: 195.5–197.5 °C (194–196 °C [54]); as orange flakes (MeCN:H2O, 84:16, v/v). 1H-NMR (400 MHz, CDCl3): δ 6.99 (ddd, J = 8.0 Hz, J = 7.2 Hz, J = 1.5Hz, 1H, Ar-H), 7.13 (dd, J = 8.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.24–7.30 (m, 2H, Ar-H), 7.47–7.54 (m, 1H, Ph-H), 7.65–7.71 (m, 2H, Ph-H), 8.13 (d, J = 8.1 Hz, 1H, Ar-H), 8.55 (s, 1H, OH) [57]. 13C-NMR (101 MHz, DMSO-d6): δ 116.94 (2 × CH), 119.22 (2 × CH), 125.73 (2 × CH), 125.81 (2 × CH), 125.97 (C), 127.52 (C), 127.76 (CH), 128.76 (CH), 129.32 (CH), 131.82 (CH), 140.50 (C), 153.25 (C), 165.76 (C=O) [57]. The 77Se NMR, and HRMS are in agreement with literature data [26,57].
2-(3-Hydroxypyridin-2-yl)-1,2-benzisoselenazol-3(2H)-one 10b. The general procedure starting from 2-amino-3-hydroxypyridine (0.44 g, 4.0 mmol) and anhydrous Et3N (0.95 g, 1.3 mL, 9.4 mmol) at anhydrous MeCN (40 mL) using benzoyl chloride 9 in anhydrous MeCN was employed for a 2-h reaction time at RT. Solvent was evaporated, water (100 mL) was added portionwise, and the solution was stirred for 1 h. The solid was filtered off, washed with H2O, and air-dried to obtain 10b. Yield: (0.76 g, 65%); m.p.: 229 °C (decomp.) with phase transfer at 199 °C (m.p.: 199–201 °C [55]); as a greenish yellow powder (DMSO). 1H-NMR (DMSO-d6): δ 7.26 (dd, J = 8.0 Hz, J = 4.6 Hz, 1H, Pyr-H), 7.38 (dd, J = 8.0 Hz, J = 1.4 Hz, 1H, Pyr-H), 7.50 (ddd, J = 7.8 Hz, J = 7.2 Hz, J = 0.9 Hz, 1H, Ar-H), 7.72 (ddd, J = 8.0 Hz, J = 7.2 Hz, J = 1.3 Hz, 1H, Ar-H), 7.95 (dd, J = 7.8 Hz, J = 0.9 Hz, 1H Ar-H), 7.98 (dd, J = 4.6 Hz, J = 1.4 Hz, 1H, Pyr-H), 8.09 (dd, J = 8.0 Hz, J = 1.3 Hz, 1H, Ar-H), 12.18 (s, 1H, OH). The 13C-NMR is in agreement with literature data [55].
2-(2-Methoxyphenyl)-1,2-benzisoselenazol-3(2H)-one 10c. The general procedure starting from 2-methoxyaniline (1.60 g, 13 mmol) at RT was employed, with a 24-h reaction time followed by separation by column chromatography to obtain 10a. Yield: (0.86 g, 71% calculated on the benzoyl chloride 9); m.p.: 189.0–191.5 °C (m.p.: 172–174 °C [63]); as yellow prisms (CHCl3). IR (KBr, cm−1) ν 1621 (C=O), 1270 (C–N), 1108 (C–O), 1020 (C–O). 1H-NMR (300 MHz, CDCl3) [63]: δ 3.85 (s, 3H, OCH3), 6.98–7.07 (m, 2H, Ph-H), 7.36 (ddd, J = 8.2 Hz, J = 7.6 Hz, J = 1.7 Hz, 1H, Ph-H), 7.44 (ddd, J = 8.0 Hz, J = 6.4 Hz, J = 1.7 Hz, 1H, Ar-H), 7.48 (dd, J = 8.0 Hz, J = 1.7 Hz, 1H, Ph-H), 7.58–7.70 (m, 2H, Ar-H), 8.13 (d, J = 7.8 Hz, 1H, Ar-H). 13C-NMR (75 MHz, CDCl3): δ 55.80 (OCH3), 112.22 (CH), 120.74 (CH), 123.86 (CH), 126.01 (CH), 126.44 (C), 126.74 (C), 129.21 (CH), 129.66 (CH), 129.89 (CH), 132.17 (CH), 139.31 (C), 155.39 (C), 166.70 (C=O).
2-(3-Methoxyphenyl)-1,2-benzoisoselenazole-3(2H)-one 10d. Prepared according to a modified procedure [35]. The general procedure starting from 3-methoxyaniline (0.493 g, 4.0 mmol) and dry Et3N (1.02 g, 1.4 mL, 10 mmol) in anhydrous MeCN (40 mL) at RT with a 2-h reaction time was employed. Water was added portionwise and the solution was left in the refrigerator for crystallization. Then, the solid was filtered off, washed with water and MeCN, and dried in the air to obtain 10d. Yield: (0.79 g, 65%), m.p: 166–168 °C (165.5–167.5°C [35]); as yellow prisms in (MeCN:H2O, 1:1, v/v). The 1H-NMR and 13C-NMR are in agreement with literature data [35].
2-(4-Methoxyphenyl)-1,2-benzoisoselenazole-3(2H)-one 10e. Prepared according to a modified procedure [35]. The general procedure starting from 3-methoxyaniline (0.49 g, 4.0 mmol) and Et3N (1.4 mL, 1.02 g, 10 mmol) in anhydrous MeCN (40 mL) at RT with a 20-h reaction time was employed. Water (40 mL) was added portionwise and the solution was left in the refrigerator for crystallization. Then, the solid was filtered off, washed with water, dried in the air, and the crude product was recrystalized from EtOAc (30 mL/g) to obtain 10e. Yield: (0.85 g, 70%); m.p.: 180–181 °C (180.5–181.5°C [35]); as yellow prisms (EtOAc). The 1H-NMR and 13C-NMR data are in agreement with literature data [26,35].
2-(2,4-Dimethoxyphenyl)benzisoselenazol-3(2H)-one 10f. The general procedure starting from 2,4-dimethoxyaniline (1.99 g, 13 mmol) at RT was employed, with a 6-h reaction time with separation by column chromatography using CHCl3 as an eluent to obtain 10f. Yield: (0.87 g, 65% calculated on the benzoyl chloride 9); m.p.: 239–240 °C; as pale yellow prisms (CHCl3, 75 mL/g). IR (KBr, cm−1) ν 3050, 1601 (C=O), 1588, 1560, 1510, 1442, 1208 (C–N), 1162 (C–O), 1044 (C–O), 747 (C–Se). 1H-NMR (300 MHz, CDCl3): δ 3.79 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 6.54 (dd, J = 8.6 Hz, J = 2.6 Hz, 1H, Ph-H), 6.59 (d, J = 2.6 Hz, 1H, Ph-H), 7.25 (d, J = 8.6 Hz, 1H, Ph-H), 7.41 (ddd, J = 7.8 Hz, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar-H), 7.60 (ddd, J = 7.8 Hz, J = 7.2 Hz, J = 1.1 Hz, 1H, Ar-H), 7.95 (dd, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar-H), 7.99 (dd, J = 8.0 Hz, J = 1.1 Hz, 1H, Ar-H). 1H-NMR (400 MHz, DMSO-d6): δ 3.75 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 6.58 (dd, J = 8.6 Hz, J = 2.7 Hz, 1H, Ph-H), 6.70 (d, J = 2.7 Hz, 1H, Ph-H), 7.23 (d, J = 8.6 Hz, 1H, Ph-H), 7.45 (ddd, J = 7.6 Hz, J = 7.3 Hz, J = 1.1 Hz, 1H, Ar-H), 7.65 (ddd, J = 7.9 Hz, J = 7.3 Hz, J = 1.5 Hz, 1H, Ar-H), 7.85 (ddd, J = 7.6 Hz, J = 1.5 Hz, J = 0.6 Hz, 1H, Ar-H), 8.05 (ddd, J = 7.9 Hz, J = 1.1 Hz, J = 0.6 Hz, 1H, Ar-H). 13C-NMR (101 MHz, DMSO-d6): δ 55.45 (OCH3), 55.68 (OCH3), 99.39, 104.86, 119.75, 125.74, 125.78, 127.30, 127.74, 130.33, 131.77, 140.29, 156.29, 160.09, 165.76 (C=O). HRMS (TOF MS ESI): m/z for C15H13NO3Se + Na+ calculated: 357.9953; found: 357.9961. Anal. Calcd. for C15H13NO3Se (334.2286): C, 53.90; H, 3.92; N, 4.19. Found: C, 53.94; H, 3.75; N, 4.11.
2-(3,4-Dimethoxyphenyl)-1,2-benzoselenazol-3(2H)-one 10g [53]. The general procedure starting from 3,4-dimethoxyaniline (0.61 g, 4.0 mmol) and anhydrous Et3N (0.81 g, 1.1 mL, 8 mmol) in anhydrous MeCN (30 mL) at RT was employed, with a 48-h reaction time. Distilled water (60 mL) was added portionwise and left to refrigerator overnight to crystallise. The formed solid was filtered off, washed with water, and dried in the air to obtain 10g. Yield: (0.91 g, 68%). The analytically pure sample was obtained by column chromatography using CHCl3:EtOAc in gradient as an eluent with 40% yield; m.p.: 159–161°C; as colorless crystals (EtOAc). 1H-NMR (300 MHz, DMSO-d6): δ 3.78 (s, 6H, 2OCH3), 6.99 (d, J = 8.6 Hz, 1H, Ph-H), 7.03 (dd, J = 8.6 Hz, J = 1.9 Hz, 1H, Ph-H), 7.29 (d, J = 1.9 Hz, 1H, Ph-H), 7.47 (dd, J = 7.8 Hz, J = 7.2 Hz, 1H, Ar-H), 7.67 (dd, J = 8.1 Hz, J = 7.2 Hz, 1H, Ar-H), 7.90 (d, J = 7.8 Hz, 1H, Ar-H), 8.09 (d, J = 8.1 Hz, 1H, Ar-H). 13C-NMR (75 MHz, DMSO-d6): 55.5 (OCH3), 55.6 (OCH3), 109.5 (C–H), 111.8 (C–H), 117.2 (C–H), 125.8 (C–H), 126.1 (C–H), 127.8 (C–H), 128.4 (C), 132.0 (C–H), 132.4 (C), 139.0 (C), 147.1 (C), 148.7 (C), 164.9 (C=O). HRMS (TOF MS ESI): m/z for C15H13NO3Se + Na+ calculated: 357.9953; found: 357.9951. Anal. Calcd. for C15H13NO3Se (334.2282): C, 53.90; H, 3.92; N, 4.19. Found: C, 53.79; H, 4.05; N, 4.08.
3.2. Evaluation of Antimicrobial Activity
The antimicrobial activity of compounds 4–6, 7, 10 was determined by the 96-well microdilution assay, according to Clinical and Laboratory Standards Institute (2008), 3rd ed. M27-A3 [73] with slight modifications, using Gram-positive bacterial strains: Staphylococcus aureus ATCC 6538, Staphylococcus epidermidis ATCC 14990, Enterococcus faecalis ATCC 29212, Enterococcus hirae ATCC 10541; Gram-negative bacterial strains: Escherichia coli ATCC 10536, Pseudomonas aeruginosa ATCC 15442; yeast-like fungi: Candida albicans ATCC 10231, Candida glabrata ATCC 90030; and filamentous fungus Aspergillus niger. Standard strains were supplied by American Type Culture Collection (ATCC), while A. niger was obtained from the Collection of Microorganisms of Wroclaw Medical University. Bacterial strains were cultured in Lysogeny broth (LB) at 37 °C and yeast strains in Sabouraud dextrose broth at 30 °C.
Briefly, stock solutions in DMSO were serially diluted in LB or Sabouraud dextrose broth to obtain a final range of 0.125–128 µg/mL concentration in microdilution sterile plates (Sarstedt, Stare Babice, Poland). Microbial inocula were prepared to obtain a final optical density at λ = 600 nm (OD600) = 0.1 ± 10% in a well. The plates were cultivated for 24 h at 37 °C (bacteria) or 48 h at 30 °C (fungi). Afterwards, OD600 was measured using a microplate reader ASYS UVM 340 (Biogenet, Józefów, Poland). The concentration which resulted in ≥90% growth inhibition was determined as the minimal inhibitory concentration (MIC90; μg/mL).
3.3. Evaluation of Antiviral Activity and Cytotoxicity
The compounds 4–5, 7, 10 at various concentrations were incubated with the following viruses: HHV-1 (human herpes virus type 1), EMCV (encephalomyocarditis virus), and VSV (vesicular stomatitis virus) for 1 h at RT. HHV-1 was used at the dose of 106 TCID50/mL, whereas EMCV and VSV were used at the dose of 108 TCID50/mL. The TCID50 (tissue culture infectious dose) determines the cytopathic effect caused by the virus in about 50% of infected cells. The virus titer was measured in human cell line A549 (human lung adenocarcinoma cell line, ATCC 185) which was observed for cytopathic effect after 48 h. The minimal concentration that caused 1000-fold decrease of virus titer was taken as the minimal inhibitory concentration, MIC (μg/mL).
In parallel, the cytotoxicity of the compounds was determined in the A549 cell line (cell line used in antiviral activity test) by incubation at various concentrations for 48 h at 37 °C in the atmosphere of 5% CO2 in air. The minimal concentration which was toxic to approximately 50% of the cells was taken as the TCCD50 (Tissue Culture Cytotoxic Dose).
Both, cytotoxicity and virus titers were determined using MTT assay. To ensure that colourful compounds did not interfere with MTT reagents, the extent of cell damage was evaluated by microscopic examination prior to MTT assay.
4. Conclusions
The reaction of bis[(2-chlorocarbonyl)phenyl] diselenide 3 with various aminophenols and their derivatives is a convenient synthetic route to bis[2-(hydroxyphenylcarbamoyl)]phenyl diselenides 4 (reduced forms of ebselenols) and other compounds containing protected hydroxyl groups. This general approach allows the production of ebselenol-derived diselenides with higher yields than the alternative method based on the reductive benzisoselenazolone ring opening of ebselenols. The secondary amine such as morpholine and benzohydrazide also reacted on their nitrogen atoms to form bis[2-(N-morpholinecarbamoyl)]phenyl and bis[2-(N-benzamidecarbamoyl)]phenyl diselenides, respectively, while reaction with aminophenol derivatives containing a blocked amine group such a 2-hydroxyacetanilide and natural antimycin A led to corresponding phenolic esters of bis(2-carboxy)phenyl diselenide. As a result of our studies, 15 new structures have been developed, including 13 diselenides and two benzisoselenazol-3(2H)-ones.
The investigation of the antimicrobial and antiviral activities of bis[2-(hydroxyphenylcarbamoyl)]phenyl diselenides and their derivatives as well as cyclic analogues have revealed new biologically active structures. The compounds, particularly bis[2-(hydroxyphenylcarbamoyl)]phenyl diselenides and reference benzisoselenazol-3(2H)-ones, exhibited high antimicrobial activity against Gram-positive bacterial species (Enterococcus spp., Staphylococcus spp.), and some compounds were also active against Gram-negative E. coli and fungi (Candida spp., A. niger). The antiviral activity evaluation allowed the identification of substances with high anti-HHV-1 and moderate anti-EMCV activities. The chemotherapeutic indices for some of these compounds were significantly higher (concentration active against virus was lower than the one showing a cytotoxic effect) than for other organoselenium compounds previously reported in the literature.
As novel molecules with the ability to inhibit microbial and viral functions are in high demand, the results of our studies are encouraging for further exploration of organoselenium compounds as anti-infective agents.
Acknowledgments
This work was supported by the National Science Centre (NCN) through grant no. UMO-2013/09/D/ST5/03814 (evaluation of antimicrobial activity of organoselenium compounds) and a statutory activity of subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wrocław University of Science and Technology (synthesis of organoselenium compounds). The publication fee was supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014–2018. The authors wish to acknowledge the kind help of Kip Hillshafer for his valuable advice during the manuscript preparation.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
M.G. and M.P.-O. conceived and designed the experiments; A.G., J.S. and R.K. performed the experiments; M.G., J.S. and M.P.-O. analyzed the data; M.G., J.S. and M.P.-O. wrote the paper; A.K. and E.P. reviewed the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 4–10 are available from the authors.
References
- 1.Wirth T. Organoselenium Chemistry in Stereoselective Reactions. Angew. Chem. Int. Ed. 2000;39:3740–3749. doi: 10.1002/1521-3773(20001103)39:21<3740::AID-ANIE3740>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 2.Freudendahl D.M., Santoro S., Shahzad S.A., Santi C., Wirth T. Green Chemistry with Selenium Reagents: Development of Efficient Catalytic Reactions. Angew. Chem. Int. Ed. 2009;48:8409–8411. doi: 10.1002/anie.200903893. [DOI] [PubMed] [Google Scholar]
- 3.Back T. Oxidations Catalyzed by Seleninic Acids and Anhydrides, their Precursors and Congeners. Curr. Green Chem. 2016;3:76–91. doi: 10.2174/2213346103666160127003954. [DOI] [Google Scholar]
- 4.Guo R., Huang J., Huang H., Zhao X. Organoselenium-Catalyzed Synthesis of Oxygen- and Nitrogen-Containing Heterocycles. Org. Lett. 2016;18:504–507. doi: 10.1021/acs.orglett.5b03543. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A., Rao G.K., Saleem F., Singh A.K. Organoselenium ligands in catalysis. Dalton Trans. 2012;41:11949–11977. doi: 10.1039/c2dt31198d. [DOI] [PubMed] [Google Scholar]
- 6.Młochowski J., Wójtowicz-Młochowska H. Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents. Molecules. 2015;20:10205–10243. doi: 10.3390/molecules200610205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Młochowski J., Kloc K., Lisiak R., Potaczek P., Wójtowicz H. Developments in the chemistry of selenaheterocyclic compounds of practical importance in synthesis and medicinal biology. Arkivoc. 2007;6:14–46. doi: 10.1002/chin.200711265. [DOI] [Google Scholar]
- 8.Santi C. Organoselenium Chemistry: Between Synthesis and Biochemistry. Bentham Science Publishers; Sharjah, UAE: 2014. [Google Scholar]
- 9.Pacuła A.J., Mangiavacchi F., Sancineto L., Lenardão E.J., Ścianowski J., Santi C. Selenium Containing Compounds from Poison to Drug Candidates: A Review on the GPx-like Activity. Curr. Chem. Biol. 2015;9:97–112. doi: 10.2174/2212796810666160120220725. [DOI] [Google Scholar]
- 10.Soriano-Garcia M. Organoselenium Compounds as Potential Therapeutic and Chemopreventive Agents: A Review. Curr. Med. Chem. 2004;11:1657–1669. doi: 10.2174/0929867043365053. [DOI] [PubMed] [Google Scholar]
- 11.Mugesh G., du Mont W.W., Sies H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001;101:2125–2179. doi: 10.1021/cr000426w. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes A.P., Gandin V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta. 2015;1850:1642–1660. doi: 10.1016/j.bbagen.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Azad G.K., Tomar R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014;41:4865–4879. doi: 10.1007/s11033-014-3417-x. [DOI] [PubMed] [Google Scholar]
- 14.Wirth T. Small Organoselenium Compounds: More than just Glutathione Peroxidase Mimics. Angew. Chem. Int. Ed. 2015;54:10074–10076. doi: 10.1002/anie.201505056. [DOI] [PubMed] [Google Scholar]
- 15.Nogueira C.W., Rocha J.B. Toxicology and pharmacology of selenium: Emphasis on synthetic organoselenium compounds. Arch. Toxicol. 2011;85:1313–1359. doi: 10.1007/s00204-011-0720-3. [DOI] [PubMed] [Google Scholar]
- 16.Ninomiya M., Garud D.R., Koketsu M. Biologically significant selenium-containing heterocycles. Coord. Chem. Rev. 2011;255:2968–2990. doi: 10.1016/j.ccr.2011.07.009. [DOI] [Google Scholar]
- 17.Santoro S., Azeredo J.B., Nascimento V., Sancineto L., Braga A.L., Santi C. The green Side of the Moon: Ecofriendly Aspects of Organoselenium Chemistry. RSC Adv. 2014;4:31521–31535. doi: 10.1039/C4RA04493B. [DOI] [Google Scholar]
- 18.Granda J.M., Piekielska K., Wąsińska M., Kawecka N., Giurg M. Synthesis of 7- and 8-Functionalized 2-Aminophenoxazinones via Cyclocondensation of 2-Aminophenols. Synthesis. 2015;47:3321–3332. doi: 10.1055/s-0034-1381011. [DOI] [Google Scholar]
- 19.Denmark S.E., Chi H.M. Lewis Base Catalyzed, Enantioselective, Intramolecular Sulfenoamination of Olefins. J. Am. Chem. Soc. 2014;136:8915–8918. doi: 10.1021/ja5046296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamata Y., Hashimoto T., Maruoka K. A Chiral Electrophilic Selenium Catalyst for Highly Enantioselective Oxidative Cyclization. J. Am. Chem. Soc. 2016;138:5206–5209. doi: 10.1021/jacs.6b01462. [DOI] [PubMed] [Google Scholar]
- 21.Sancineto L., Tidei C., Bagnoli L., Marini F., Lenardão E.J., Santi C. Selenium Catalyzed Oxidation of Aldehydes: Green Synthesis of Carboxylic Acids and Esters. Molecules. 2015;20:10496–10510. doi: 10.3390/molecules200610496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J., Li D., Xiong K., Ge Y., Jin H., Zhang G., Hong M., Tian Y., Yin J., Zeng H. Inhibition of thioredoxin reductase by a novel series of bis-1,2-benzisoselenazol-3(2H)-ones: Organoselenium compounds for cancer therapy. Bioorg. Med. Chem. 2012;20:3816–3827. doi: 10.1016/j.bmc.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Nascimento V., Ferreira N.L., Canto R.F., Schott K.L., Waczuk E.P., Sancineto L., Santi C., Rocha J.B., Braga A.L. Synthesis and biological evaluation of new nitrogen-containing diselenides. Eur. J. Med. Chem. 2014;87:131–139. doi: 10.1016/j.ejmech.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Shaaban S., Negm A., Sobh M.A., Wessjohann L.A. Organoselenocyanates and symmetrical diselenides redox modulators: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2015;97:190–201. doi: 10.1016/j.ejmech.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Plano D., Karelia D.N., Pandey M.K., Spallholz J.E., Amin S., Sharma A.K. Design, Synthesis, and Biological Evaluation of Novel Selenium (Se-NSAID) Molecules as Anticancer Agents. J. Med. Chem. 2016;59:1946–1959. doi: 10.1021/acs.jmedchem.5b01503. [DOI] [PubMed] [Google Scholar]
- 26.Węglarz-Tomczak E., Burda-Grabowska M., Giurg M., Mucha A. Identification of methionine aminopeptidase 2 as a molecular target of the organoselenium drug ebselen and its derivatives/analogues: Synthesis, inhibitory activity and molecular modeling study. Bioorg. Med. Chem. Lett. 2016;26:5254–5259. doi: 10.1016/j.bmcl.2016.09.050. [DOI] [PubMed] [Google Scholar]
- 27.Sartori G., Jardim N.S., Marcondes Sari M.H., Dobrachinski F., Pesarico A.P., Rodrigues L.C., Jr., Cargnelutti J., Flores E.F., Prigol M., Nogueira C.W. Antiviral Action of Diphenyl Diselenide on Herpes Simplex Virus 2 Infection in Female BALB/c Mice. J. Cell. Biochem. 2016;117:1638–1648. doi: 10.1002/jcb.25457. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S., Weiner W.S., Schroeder C.E., Simpson D.S., Hanson A.M., Sweeney N.L., Marvin R.K., Ndjomou J., Kolli R., Isailovic D., et al. Ebselen inhibits hepatitis C virus NS3 helicase binding to nucleic acid and prevents viral replication. ACS Chem. Biol. 2014;9:2393–2403. doi: 10.1021/cb500512z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thenin-Houssier S., de Vera I.M.S., Pedro-Rosa L., Brady A., Richard A., Konnick B., Opp S., Buffone C., Fuhrmann J., Kota S., et al. Ebselen, a Small-Molecule Capsid Inhibitor of HIV-1 Replication. Antimicrob. Agents Chemother. 2016;60:2195–2208. doi: 10.1128/AAC.02574-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sancineto L., Mariotti A., Bagnoli L., Marini F., Desantis J., Iraci N., Santi C., Pannecouque C., Tabarrini O. Design and Synthesis of DiselenoBisBenzamides (DISeBAs) as Nucleocapsid Protein 7 (NCp7) Inhibitors with anti-HIV Activity. J. Med. Chem. 2015;58:9601–9614. doi: 10.1021/acs.jmedchem.5b01183. [DOI] [PubMed] [Google Scholar]
- 31.Pietka-Ottlik M., Potaczek P., Piasecki E., Mlochowski J. Crucial Role of Selenium in the Virucidal Activity of Benzisoselenazol-3(2H)-ones and Related Diselenides. Molecules. 2010;15:8214–8228. doi: 10.3390/molecules15118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wójtowicz H., Chojnacka M., Młochowski J., Palus J., Syper L., Hudecova D., Uher M., Piasecki E., Rybka M. Functionalized alkyl and aryl diselenides as antimicrobial and antiviral agents: Synthesis and properties. II Farmaco. 2003;58:1235–1242. doi: 10.1016/j.farmac.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Wójtowicz H., Kloc K., Maliszewska I., Młochowski J., Piętka M., Piasecki E. Azaanalogues of ebselen as antimicrobial and antiviral agents: Synthesis and properties. II Farmaco. 2004;59:863–868. doi: 10.1016/j.farmac.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Piętka-Ottlik M., Wójtowicz-Młochowska H., Kołodziejczyk K., Piasecki E., Młochowski J. New Organoselenium Compounds Active against Pathogenic Bacteria, Fungi and Viruses. Chem. Pharm. Bull. 2008;56:1423–1427. doi: 10.1248/cpb.56.1423. [DOI] [PubMed] [Google Scholar]
- 35.Piętka-Ottlik M., Burda-Grabowska M., Woźna M., Waleńska J., Kaleta R., Zaczyńska E., Piasecki E., Giurg M. Synthesis of new alkylated and methoxylated analogues of ebselen with antiviral and antimicrobial properties. Arkivoc. 2017:546–556. doi: 10.3998/ark.5550190.p009.797. [DOI] [Google Scholar]
- 36.Gustafsson T.N., Osman H., Werngren J., Hoffner S., Engman L., Holmgren A. Ebselen and analogs as inhibitors of Bacillus anthracis thioredoxin reductase and bactericidal antibacterials targeting Bacillus species, Staphylococcus aureus and Mycobacterium tuberculosis. Biochim. Biophys. Acta. 2016;1860:1265–1271. doi: 10.1016/j.bbagen.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Xu X.J., Xue Z., Xiao Q., Hou A.X., Liu Y. Antibacterial activities of novel diselenide-bridged bis(porphyrin)s on Staphylococcus aureus investigated by microcalorimetry. Biol. Trace Elem. Res. 2008;125:185–192. doi: 10.1007/s12011-008-8156-1. [DOI] [PubMed] [Google Scholar]
- 38.Rosseti I.B., Wagner C., Fachinetto R., Taube Junior P., Costa M.S. Candida albicans growth and germ tube formation can be inhibited by simple diphenyl diselenides [(PhSe)2, (MeOPhSe)2, (p-Cl-PhSe)2, (F3CPhSe)2] and diphenyl ditelluride. Mycoses. 2011;54:506–513. doi: 10.1111/j.1439-0507.2010.01888.x. [DOI] [PubMed] [Google Scholar]
- 39.Denardi L.B., Mario D.A., de Loreto E.S., Nogueira C.W., Santurio J.M., Alves S.H. Antifungal activities of diphenyl diselenide alone and in combination with fluconazole or amphotericin B against Candida glabrata. Mycopathologia. 2013;176:165–169. doi: 10.1007/s11046-013-9672-x. [DOI] [PubMed] [Google Scholar]
- 40.Loreto E.S., Mario D.A., Santurio J.M., Alves S.H., Nogueira C.W., Zeni G. In vitro antifungal evaluation and structure-activity relationship of diphenyl diselenide and synthetic analogues. Mycoses. 2011;54:572–576. doi: 10.1111/j.1439-0507.2010.01994.x. [DOI] [PubMed] [Google Scholar]
- 41.Chassot F., Pozzebon Venturini T., Baldissera Piasentin F., Morais Santurio J., Estivalet Svidzinski T.I., Hartz Alves S. Antifungal activities of diphenyl diselenide and ebselen against echinocandin-susceptible and -resistant strains of Candida parapsilosis. New Microbiol. 2016;39:301–303. ISN 1121–7138. [PubMed] [Google Scholar]
- 42.Venturini T.P., Chassot F., Loreto É.S., Keller J.T., Azevedo M.I., Zeni G., Santurio J.M., Alves S.H. Antifungal activities of diphenyl diselenide and ebselen alone and in combination with antifungal agents against Fusarium spp. Med. Mycol. 2016;54:550–555. doi: 10.1093/mmy/myv120. [DOI] [PubMed] [Google Scholar]
- 43.Sancineto L., Piccioni M., De Marco S., Pagiotti R., Nascimento V., Braga A.L., Santi C., Pietrella D. Diphenyl diselenide derivatives inhibit microbial biofilm formation involved in wound infection. BMC Microbiol. 2016;16:220–230. doi: 10.1186/s12866-016-0837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pietrella D. Antimicrobial Activity of Organoselenium Compounds. In: Santi C., editor. Organoselenium Chemistry: Between Synthesis and Biochemistry. Bentham Books; Sharjah, UAE: 2014. pp. 328–344. [Google Scholar]
- 45.Pesarico A.P., Sartori G., dos Santos C.F., Neto J.S., Bortolotto V., Santos R.C., Nogueira C.W., Prigol M. 2,2′-Dithienyl diselenide pro-oxidant activity, accounts for antibacterial and antifungal activities. Microbiol. Res. 2013;168:563–568. doi: 10.1016/j.micres.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Rosseti I.B., Taube Junior P., de Campos C.B., da Rocha J.B., Costa M.S. Biofilm formation by Candida albicans is inhibited by 4,4-dichloro diphenyl diselenide (pCl-PhSe)2. Curr. Drug Discov. Technol. 2014;11:234–238. doi: 10.2174/1570163811666140924121758. [DOI] [PubMed] [Google Scholar]
- 47.Plano D., Baquedano Y., Moreno-Mateos D., Font M., Jiménez-Ruiz A., Palop J.A., Sanmartín C. Selenocyanates and diselenides: A new class of potent antileishmanial agents. Eur. J. Med. Chem. 2011;46:3315–3323. doi: 10.1016/j.ejmech.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 48.Lu J., Vlamis-Gardikas A., Kandasamy K., Zhao R., Gustafsson T.N., Engstrand L., Hoffner S., Engman L., Holmgren A. Inhibition of bacterial thioredoxin reductase: An antibiotic mechanism targeting bacteria lacking glutathione. FASEB J. 2013;27:1394–1403. doi: 10.1096/fj.12-223305. [DOI] [PubMed] [Google Scholar]
- 49.Macegoniuk K., Grela E., Palus J., Rudzińska-Szostak E., Grabowiecka A., Biernat M., Berlicki Ł. 1,2-Benzisoselenazol-3(2H)-one Derivatives As a New Class of Bacterial Urease Inhibitors. J. Med. Chem. 2016;59:8125–8133. doi: 10.1021/acs.jmedchem.6b00986. [DOI] [PubMed] [Google Scholar]
- 50.Chan G., Hardej D., Santoro M., Lau-Cam C., Billack B. Evaluation of the antimicrobial activity of ebselen: Role of the yeast plasma membrane H+-ATPase. J. Biochem. Mol. Toxicol. 2007;21:252–264. doi: 10.1002/jbt.20189. [DOI] [PubMed] [Google Scholar]
- 51.Billack B., Pietka-Ottlik M., Santoro M., Nicholson S., Młochowski J., Lau-Cam C. Evaluation of the antifungal and plasma membrane H+-ATPase inhibitory action of ebselen and two ebselen analogs in S. cerevisiae cultures. J. Enzyme Inhib. Med. Chem. 2010;25:312–317. doi: 10.3109/14756360903179419. [DOI] [PubMed] [Google Scholar]
- 52.Orie N.N., Warren A.R., Basaric J., Lau-Cam C., Piętka-Ottlik M., Młochowski J., Billack B. In vitro assessment of the growth and plasma membrane H+ -ATPase inhibitory activity of ebselen and structurally related selenium- and sulfur-containing compounds in Candida albicans. J. Biochem. Mol. Toxicol. 2017 doi: 10.1002/jbt.21892. [DOI] [PubMed] [Google Scholar]
- 53.Welter A., Fischer H., Christiaens L., Wendel A., Etschenberg E., Dereu N., Kuhl P., Graf E. Diselenobis-Benzoic Acid Amides of Primary and Secondary Amines and Processes for the Treatment of Diseases in Humans Caused by a Cell Injury. 4873350. U.S. Patent. 1989 Oct 10;
- 54.Welter A., Christiaens L., Wirtz-Peitz F. Benzisoselenazolones and Process for the Treatment of Rheumatic and Arthritic Diseases Using Them. 4418069. U.S. Patent. 1983 Nov 29;
- 55.Osajda M., Mlochowski J. The reactions of 2-(chloroseleno)benzoyl chloride with nucleophiles. Tetrahedron. 2002;58:7531–7537. doi: 10.1016/S0040-4020(02)00789-5. [DOI] [Google Scholar]
- 56.Bhabak K.P., Mugesh G. Synthesis, Characterization, and Antioxidant Activity of Some Ebselen Analogues. Chem. Eur. J. 2007;13:4594–4601. doi: 10.1002/chem.200601584. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S., Yan J., Poon J.F., Singh V.P., Lu X., Karlsson O.M., Engman L., Kumar S. Multifunctional Antioxidants: Regenerable Radical-Trapping and Hydroperoxide-Decomposing Ebselenols. Angew. Chem. Int. Ed. 2016;55:3729–3733. doi: 10.1002/anie.201510947. [DOI] [PubMed] [Google Scholar]
- 58.Murphy M.P., Smith R.A.J. Mitochondrially Targeted Antioxidants. 0227957. U.S. Patent. 2005 Oct 13;
- 59.Młochowski J., Kloc K., Syper L., Inglot A.D., Piasecki E. Aromatic and Azaaromatic Diselenides, Benzisoselenazolones and Related Compounds as Immunomodulators Active in Humans: Synthesis and Properties. Liebigs Ann. Chem. 1993:1239–1244. doi: 10.1002/jlac.1993199301201. [DOI] [Google Scholar]
- 60.Mlochowski J., Gryglewski R.J., Inglot A.D., Jakubowski A., Juchniewicz L., Kloc K. Synthesis and Properties of 2-Carboxyalkyl-1,2-benzisoselenazol-3(2H)-ones and Related Organoselenium Compounds as Nitric Oxide Synthase Inhibitors and Cytokine Inducers. Eur. J. Org. Chem. 1996;1996:1751–1755. doi: 10.1002/jlac.199619961108. [DOI] [Google Scholar]
- 61.Rafique J., Saba S., Canto R.F., Frizon T.E., Hassan W., Waczuk E.P., Jan M., Back D.F., Da Rocha J.B., Braga A.L. Synthesis and biological evaluation of 2-picolylamide-based diselenides with non-bonded interactions. Molecules. 2015;20:10095–10109. doi: 10.3390/molecules200610095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Küppers J., Schulz-Fincke A.C., Palus J., Giurg M., Skarżewski J., Gütschow M. Convergent Synthesis of Two Fluorescent Ebselen-Coumarin Heterodimers. Pharmaceuticals. 2016;9:43. doi: 10.3390/ph9030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balkrishna S.J., Bhakuni B.S., Kumar S. Copper catalyzed/mediated synthetic methodology for ebselen and related isoselenazolones. Tetrahedron. 2011;67:9565–9575. doi: 10.1016/j.tet.2011.09.141. [DOI] [Google Scholar]
- 64.Lou Z., Li P., Sun Z., Yang S., Wang B., Hana K. A fluorescent probe for rapid detection of thiols and imaging of thiols reducing repair and H2O2 oxidative stress cycles in living cells. Chem. Commun. 2013;49:391–393. doi: 10.1039/C2CC36839K. [DOI] [PubMed] [Google Scholar]
- 65.Rieske J.S. Antimycyn A. In: Gottlieb D., Shaw P.D., editors. Antibiotics. Mechanism of Action. Volume 1. Springer; Berlin/Heidelberg, Germany: New York, NY, USA: 1967. pp. 542–584. [Google Scholar]
- 66.Sheu C.W., Freese E. Lipopolysaccharide Layer Protection of Gram-Negative Bacteria against Inhibition by Long-Chain Fatty Acids. J. Bacteriol. 1973;115:869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ngo H.X., Shrestha S.K., Green K.D., Garneau-Tsodikova S. Development of ebsulfur analogues as potent antibacterials against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2016;24:6298–6306. doi: 10.1016/j.bmc.2016.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong P.T., Schauerte J.A., Wisser K.C., Ding H., Lee E.L., Steel D.G., Gafni A. Amyloid-beta membrane binding and permeabilization are distinct processes influenced separately by membrane charge and fluidity. J. Mol. Biol. 2009;386:81–96. doi: 10.1016/j.jmb.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 69.Warren D.K., Kollef M.H., Seiler S.M., Fridkin S.K. The Epidemiology of Vancomycin-Resistant Enterococcus Colonization in a Medical Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2003;24:257–263. doi: 10.1086/502199. [DOI] [PubMed] [Google Scholar]
- 70.Manavathu E.K., Cutright J.L., Chandrasekar P.H. Organism-Dependent Fungicidal Activities of Azoles. Antimicrob. Agents Chemother. 1998;42:3018–3021. doi: 10.1128/aac.42.11.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gallis H.A., Drew R.H., Pickard W.W. Amphotericin B: 30 Years of Clinical Experience. Rev. Infect. Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Q., Mao Y., Liu Z., Xie K., Zhu Y., Wei Y., Jiang X., Shen J. Synthesis of N-(3-Cyano-7-ethoxy-1,4-dihydro-4-oxoquinolin-6-yl)acetamide. Heterocycles. 2011;83:2851–2856. doi: 10.3987/COM-11-12343. [DOI] [Google Scholar]
- 73.Clinical and Laboratory Standards Institute . 2008 Reference Method for Broth Dilutionantifungal Susceptibility Testing of Yeast Approved Standard. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, NJ, USA: 2008. M27-A3 28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.